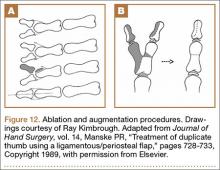
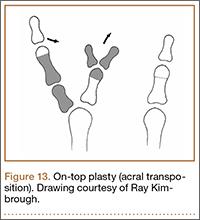
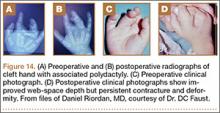
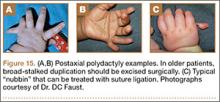
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Risk Factors for In-Hospital Myocardial Infarction After Shoulder Arthroplasty
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
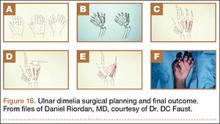
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
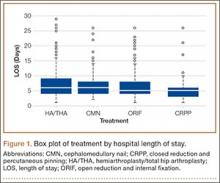
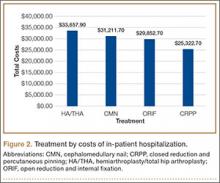
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
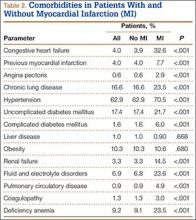
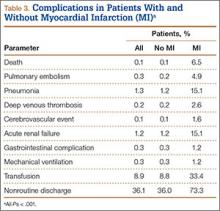
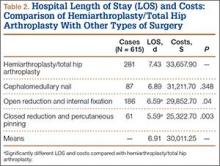
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
The incidence of shoulder arthroplasty in the United States is increasing annually,1-3 and the majority of these operations occur in older patients.4-6 Elderly patients with cardiovascular, pulmonary, cerebral, renal, and hepatic disease are increasingly susceptible to numerous surgical complications.4 Myocardial infarction (MI) is a complication that occurs in 0.7% of noncardiac surgeries. This figure increases to 1.1% in patients with coronary artery disease.7-11 Perioperative MI increases morbidity and mortality,8 and perioperative cardiac morbidity is the leading cause of death after anesthesia and surgery.12 The financial effects of perioperative cardiac morbidity and mortality must also be considered. A 2009 claims analysis study estimated charges associated with a perioperative MI at $15,000 and the cost of cardiac death at $21,909.13
Cardiovascular complications are associated with a significant degree of morbidity and mortality in patients who undergo arthroplasty.14-16 Although studies have elucidated 30- and 90-day morbidity and mortality rates after shoulder arthroplasty, in hip and knee arthroplasty17-19 little has been done to determine predictors of perioperative MI in a representative database of patients. Given the increasing incidence of shoulder arthroplasty in the United States, the elective nature of this procedure, and the percentage of the US population with cardiovascular risk factors,20 it is important to establish predictors of perioperative MI to ensure patients and physicians have the necessary resources to make informed decisions.
We conducted a study to examine the risk factors for perioperative MI in a large cohort of patients admitted for shoulder arthroplasty to US hospitals. We wanted to evaluate the association between perioperative MI and shoulder arthroplasty with respect to demographics, primary diagnosis, medical comorbidities, and perioperative complications. Specifically, we tested the null hypothesis that, among patients undergoing shoulder arthroplasty, and accounting for confounding variables, there would be no difference in risk factors for patients who have a perioperative MI.
Materials and Methods
This study was exempt from approval by our institutional review board. All data used in this project were deidentified before use.
Nationwide Inpatient Sample (NIS)
The Nationwide Inpatient Sample (NIS), an annual survey of hospitals, is conducted by the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality (AHRQ). This database is the largest publicly available all-payer inpatient discharge database in the United States.21 Sampling 8 million hospital stays each year, NIS includes information from a representative batch of 20% of US hospitals. In 2011, 46 states and 1045 hospitals contributed information to the database, representing 97% of the US population.22 This large sample allows researchers to analyze a robust set of medical conditions and uncommon treatments. The survey, conducted each year since 1988, includes demographic, clinical, and resource use data.23 Discharge weight files are provided by NIS to arrive at valid national estimates.
This database is particularly useful because it provides information on up to 25 medical diagnoses and 15 procedures, which are recorded with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Researchers can use this database to analyze patient and hospital characteristics as well as inpatient outcomes.24,25 Numerous studies have used NIS to address pertinent queries across the medical landscape.22,26
Patient Selection and Analysis
We used NIS to isolate a population of 422,371 adults (≥18 years old) who underwent total shoulder arthroplasty (TSA) or hemi–shoulder arthroplasty (HSA) between January 1, 2002 and December 31, 2011. We then placed the patients in this population into 1 of 2 cohorts. The first cohort had an acute MI during the perioperative period after TSA, and the second, larger cohort did not have an acute MI after TSA. Acute MI was identified using ICD-9-CM code 410.xx. To identify a population of shoulder arthroplasty patients, we included discharges with an ICD-9-CM procedure code of 81.80 or 81.88 (both TSA) or 81.81 (HSA) in the sample. We then considered the degree to which each of 5 variables—primary diagnosis, age, sex, race, and select medical comorbidities—was predictive of in-hospital MI after TSA.
Statistical Analysis
Given the large sample used in this study, normal distribution of data was assumed. Using bivariate analysis, Pearson χ2 test for categorical data, and independent-samples t test for continuous data, we compared the nonacute MI and acute MI groups. Multivariable binary logistic regression analyses allowed us to isolate the extent that primary diagnosis, age, sex, race, and medical comorbidities were predictors of acute MI after shoulder arthroplasty. Statistical significance was set at P < .05. SPSS Version 22.0 (SPSS, Chicago, Illinois) was used for all statistical analyses and data modeling.
Results
Between January 1, 2002 and December 31, 2011, an estimated total of 422,371 patients underwent shoulder arthroplasty (59.3% TSA, 40.7% HSA). Of these patients, 1174 (0.28%) had a perioperative MI, and 421,197 (99.72%) did not (Table 1). Patients with a primary diagnosis of proximal humerus fracture (33.8% vs 16.6%; P < .001) or rotator cuff arthropathy (10.1% vs 9.9%; P < .001) were more likely than patients with other diagnoses to have an in-hospital MI.
Our review of the demographics found that patients who underwent shoulder arthroplasty and had a perioperative MI were likely older (75±8.9 years vs 69±11 years; P < .001), Caucasian (94.2% vs 91.9%; P = .002), male (43.2% vs 39.7%; P = .013), in the highest median household income bracket of $63,000 or more (30.8% vs 25.6%; P < .001), and using Medicare (80.9% vs 66.3%; P < .001). They were more likely to be treated in a medical center of medium size (25.6% vs 23.7%; P = .042) or larger (61.8% vs 61.2%; P = .042). MIs occurred more often in urban environments (91.4% vs 88.5%; P = .002) and in HSA patients (55% vs 40.6%; P < .001), resulting in longer hospital stays (9.4±7.9 days vs 2.7±2.5 days; P < .001) and higher probability of death (6.5% vs 0.1%; P < .001).
We then analyzed the 2 cohorts for medical comorbidities (Table 2). Patients in the MI cohort presented with a significantly higher incidence of congestive heart failure, previous MI, angina pectoris, chronic lung disease, hypertension, diabetes, renal failure, fluid and electrolyte disorders, pulmonary circulatory disease, coagulopathy, and deficiency anemia (P < .001) but not liver disease and obesity. Bivariate analysis of perioperative outcomes (Table 3) indicated that these patients also had a statistically higher rate of numerous other complications: pulmonary embolism (4.9% vs 0.2%; P < .001), pneumonia (15.1% vs 1.2%; P < .001), deep venous thrombosis (2.6% vs 0.2%; P < .001), cerebrovascular event (1.6% vs 0.1%; P < .001), acute renal failure (15.1% vs 1.2%; P < .001), gastrointestinal complication (1.2% vs 0.3%; P < .001), mechanical ventilation (1.2% vs 0.3%; P < .001), transfusion (33.4% vs 8.8%; P < .001), and nonroutine discharge (73.3% vs 36.0%; P < .001).
Multivariable logistic regression analysis was performed to determine independent predictors of perioperative MI after shoulder arthroplasty (Table 4). Patients with a primary diagnosis of proximal humerus fracture (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.15-1.65; P < .001) were more likely than patients with a primary diagnosis of osteoarthritis to have an MI. The odds of postoperative MI increased with age (OR, 1.04 per year; 95% CI, 1.03-1.05; P < .001) and were higher in males (OR, 1.72; 95% CI, 1.52-1.96; P < .001). Compared with Caucasians, African Americans (OR, 0.19; 95% CI, 0.09-0.40; P < .001) were less likely to have an in-hospital MI after shoulder arthroplasty. After shoulder arthroplasty, the odds of MI in the perioperative period increased with each subsequent day of care (OR, 1.10; 95% CI, 1.10-1.11; P < .001).
Regarding independent comorbidities, multivariable logistic regression analysis also determined that history of congestive heart failure (OR, 4.86; 95% CI, 4.20-5.61; P < .001), angina pectoris (OR, 2.90; 95% CI, 2.02-4.17; P < .001), complicated diabetes (OR, 1.96; 95% CI, 1.49-2.57; P < .001), renal failure (OR, 1.42; 95% CI, 1.17-1.72; P < .001), fluid and electrolyte disorders (OR, 1.42; 95% CI, 1.21-1.67; P < .001), and deficiency anemia (OR, 1.62; 95% CI, 1.40-1.88; P < .001) were significant predictors of perioperative MI after shoulder arthroplasty.
Discussion
Results of other studies have elucidated 30- and 90-day mortality rates and postoperative complications after shoulder arthroplasty, but, relative to hip and knee arthroplasty,17-19 little has been done to determine predictors of perioperative MI in a large sample of shoulder arthroplasty patients. Given the increasing rates of shoulder arthroplasty1-3 and the demographics of this population,4-6 it is likely that postoperative cardiovascular events will increase in frequency. We found that, in order of decreasing significance, the top 4 risk predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and a primary diagnosis of proximal humerus fracture. The rate of acute MI in patients who were older than 75 years when they underwent HSA for proximal humerus fracture was 0.80%.
Demographics
We found that patients who had an acute MI after shoulder arthroplasty were likely older, male, and Caucasian. Age and male sex are well-established risk factors for increased cardiac complications after arthroplasty.27-29 Previous studies have indicated that the rate of cardiac events increases in arthroplasty patients older than 65 years.19,28,29 In our study, more than 50% of the patients who had an acute perioperative MI were older than 85 years. Less explainable is the increased occurrence of acute MI in Caucasian patients and wealthy patients, given that minorities in the United States have higher rates of cardiovascular disease.30 Shoulder arthroplasty is an elective procedure, more likely to be undertaken by Caucasians. Therefore, at-risk minority groups and financially challenged groups may be less likely to have this procedure.
Primary Diagnosis
In this series, patients with a primary diagnosis of proximal humerus fracture were more likely to have an in-hospital MI. This finding is consistent with previous studies indicating a higher rate of complications for proximal humerus fracture patients than for shoulder arthroplasty patients.31,32 Given that more than 75% of patients who present with a proximal humerus fracture are older than 70 years, it would be prudent to examine operative indications after this diagnosis,33 particularly as benefit from surgery for fractures has not been definitively demonstrated.34-37
Comorbidities
Many of the patients in our MI cohort presented with congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, or deficiency anemia. This is in keeping with other studies indicating that preexisting cardiovascular morbidity increases the rate of MI after various forms of arthroplasty.7-11 Patients in our MI cohort were also susceptible to a variety of post-MI perioperative complications, including pulmonary embolism, pneumonia, deep venous thrombosis, cerebrovascular event, acute renal failure, gastrointestinal complication, mechanical ventilation, transfusion, and nonroutine discharge, and their incidence of death was higher. These findings are consistent with reports that postoperative cardiovascular complications increase the degree of morbidity and mortality in arthroplasty patients.14-16 It is also worth noting that the odds of MI in the perioperative period increase with each subsequent day of care. This is understandable given that patients presenting with numerous comorbidities are at increased risk for perioperative complications38 resulting in hospital readmission.39
The literature indicates that MI occurs as a complication in 0.7% of patients who undergo noncardiac surgery,7 though some series have shown it is more prevalent after arthroplasty procedures.28,40 MI significantly increases the rate of perioperative morbidity and mortality,8 and perioperative cardiac morbidity is a leading cause of death after anesthesia and surgery.12 Furthermore, the most common cause of death after lower extremity arthroplasty is cardiovascular-related.41,42 In patients who presented for elective hip arthroplasty, cardiorespiratory disease was one of the main risk factors (with older age and male sex) shown to increase perioperative mortality.43
Perioperative cardiovascular complications increase postoperative morbidity and mortality.12 The rate of cardiovascular complications after shoulder arthroplasty ranges from 0.8% to 2.6%, and the incidence of MI hovers between 0.3% and 0.9%.17,19,28,40,44 A recent study in 793 patients found that, over a 30-day period, cardiovascular complications accounted for more than one-fourth of all complications.17 Singh and colleagues19 analyzed cardiopulmonary complications after primary shoulder arthroplasty in a total of 3480 patients (4019 arthroplasties) and found this group had a 90-day cardiac morbidity (MI, congestive heart failure, arrhythmia) rate of 2.6%. In that study, a Deyo-Charlson index of 1 or more was a significant independent risk factor for cardiac complications following surgery. Scores on this weighted index of 17 comorbidities are used to assess the complexities of a patient population. Given the severity of cardiovascular perioperative complications, it is important to preoperatively identify high-risk population groups and sufficiently study and optimize patients before shoulder arthroplasty.
There is much debate about the effectiveness of perioperative β-blockers in reducing perioperative cardiac morbidity and mortality.45-48 Such a discussion is outside of the scope of this article, but it may be prudent to seek a cardiology consultation for patients presenting with risk factors for perioperative MI. β-Blockers may prove useful in reducing cardiac morbidity in high-risk patients after noncardiac surgery.45,49
Many limitations are inherent in studies that use a nationally represented database such as NIS, which we used in this study. It is highly likely that NIS does not capture all potential postoperative complications, as this database is very large and subject to errors in data entry and clinical coding. In addition, detailed clinical information (eg, severity of certain comorbid diseases before shoulder arthroplasty, details about the intraoperative course) was not readily available for analysis. Another limitation, which may have led to an underestimate of complication rates, was our not being able to obtain information about postdischarge complications.
Despite these limitations, NIS and other databases have helped researchers answer questions about low-incidence conditions and generalize findings to a national population. In the present study, we analyzed 2 cohorts, patients with and without acute MI after shoulder arthroplasty, to determine predictors for and complications of postarthroplasty MI. We identified numerous predictors for acute MI: congestive heart failure, angina pectoris, complicated diabetes, renal failure, fluid and electrolyte disorders, and deficiency anemia prior to arthroplasty. As perioperative MI is associated with significant morbidity,14-16 it would be wise to screen patients for such comorbid conditions, assess the severity of these conditions, and offer shoulder arthroplasty with prudence.
Conclusion
The top 4 predictors for acute MI after shoulder arthroplasty were congestive heart failure, angina pectoralis, complicated diabetes mellitus, and male sex. Other pertinent risk factors included older age, Caucasian ethnicity, and primary diagnosis of proximal humerus fracture. Surgeons and patients must be aware of predictors for adverse surgical outcomes such as perioperative MI and understand the extent to which these events increase perioperative morbidity and mortality.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
3. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop. 2009;467(10):2606-2612.
4. Boettcher WG. Total hip arthroplasties in the elderly. Morbidity, mortality, and cost effectiveness. Clin Orthop. 1992;(274):30-34.
5. Greenfield S, Apolone G, McNeil BJ, Cleary PD. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care. 1993;31(2):141-154.
6. Kreder HJ, Williams JI, Jaglal S, Hu R, Axcell T, Stephen D. Are complication rates for elective primary total hip arthroplasty in Ontario related to surgeon and hospital volumes? A preliminary investigation. Can J Surg. 1998;41(6):431-437.
7. Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120(3):564-578.
8. Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323(26):1781-1788.
9. Tarhan S, Moffitt EA, Taylor WF, Giuliani ER. Myocardial infarction after general anesthesia. JAMA. 1972;220(11):1451-1454.
10. Landesberg G, Mosseri M, Zahger D, et al. Myocardial infarction after vascular surgery: the role of prolonged stress-induced, ST depression-type ischemia. J Am Coll Cardiol. 2001;37(7):1839-1845.
11. van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271.
12. Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72(1):153-184.
13. Fleisher LA, Corbett W, Berry C, Poldermans D. Cost-effectiveness of differing perioperative beta-blockade strategies in vascular surgery patients. J Cardiothorac Vasc Anesth. 2004;18(1):7-13.
14. Aynardi M, Pulido L, Parvizi J, Sharkey PF, Rothman RH. Early mortality after modern total hip arthroplasty. Clin Orthop. 2009;467(1):213-218.
15. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
16. Baser O, Supina D, Sengupta N, Wang L, Kwong L. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm. 2010;67(17):1438-1445.
17. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop. 2010;468(3):717-722.
18. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop. 2007;(455):183-189.
19. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
20. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28-e292.
21. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036.
22. Maynard C, Sales AE. Changes in the use of coronary artery revascularization procedures in the Department of Veterans Affairs, the National Hospital Discharge Survey, and the Nationwide Inpatient Sample, 1991–1999. BMC Health Serv Res. 2003;3(1):12.
23. Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Obstructive sleep apnea as a risk factor after shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(12):e6-e9.
24. Hambright D, Henderson RA, Cook C, Worrell T, Moorman CT, Bolognesi MP. A comparison of perioperative outcomes in patients with and without rheumatoid arthritis after receiving a total shoulder replacement arthroplasty. J Shoulder Elbow Surg. 2011;20(1):77-85.
25. Odum SM, Troyer JL, Kelly MP, Dedini RD, Bozic KJ. A cost-utility analysis comparing the cost-effectiveness of simultaneous and staged bilateral total knee arthroplasty. J Bone Joint Surg Am. 2013;95(16):1441-1449.
26. Ponce BA, Menendez ME, Oladeji LO, Soldado F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):671-678.
27. Alfonso DT, Toussaint RJ, Alfonso BD, Strauss EJ, Steiger DT, Di Cesare PE. Nonsurgical complications after total hip and knee arthroplasty. Am J Orthop. 2006;35(11):503-510.
28. Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology. 2002;96(5):1140-1146.
29. Singh JA, Jensen MR, Harmsen WS, Gabriel SE, Lewallen DG. Cardiac and thromboembolic complications and mortality in patients undergoing total hip and total knee arthroplasty. Ann Rheum Dis. 2011;70(12):2082-2088.
30. Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143-152.
31. Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop. 2014;472(8):2317-2324.
32. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
33. de Kruijf M, Vroemen JP, de Leur K, van der Voort EA, Vos DI, Van der Laan L. Proximal fractures of the humerus in patients older than 75 years of age: should we consider operative treatment? J Orthop Traumatol. 2014;15(2):111-115.
34. Hauschild O, Konrad G, Audige L, et al. Operative versus non-operative treatment for two-part surgical neck fractures of the proximal humerus. Arch Orthop Trauma Surg. 2013;133(10):1385-1393.
35. Hanson B, Neidenbach P, de Boer P, Stengel D. Functional outcomes after nonoperative management of fractures of the proximal humerus. J Shoulder Elbow Surg. 2009;18(4):612-621.
36. Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database Syst Rev. 2012;12:CD000434.
37. Court-Brown CM, Cattermole H, McQueen MM. Impacted valgus fractures (B1.1) of the proximal humerus. The results of non-operative treatment. J Bone Joint Surg Br. 2002;84(4):504-508.
38. Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860.
39. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
40. Khan SK, Malviya A, Muller SD, et al. Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop. 2014;85(1):26-31.
41. Paavolainen P, Pukkala E, Pulkkinen P, Visuri T. Causes of death after total hip arthroplasty: a nationwide cohort study with 24,638 patients. J Arthroplasty. 2002;17(3):274-281.
42. Sharrock NE, Cazan MG, Hargett MJ, Williams-Russo P, Wilson PD Jr. Changes in mortality after total hip and knee arthroplasty over a ten-year period. Anesth Analg. 1995;80(2):242-248.
43. Parvizi J, Johnson BG, Rowland C, Ereth MH, Lewallen DG. Thirty-day mortality after elective total hip arthroplasty. J Bone Joint Surg Am. 2001;83(10):1524-1528.
44. Morris MJ, Molli RG, Berend KR, Lombardi AV Jr. Mortality and perioperative complications after unicompartmental knee arthroplasty. Knee. 2013;20(3):218-220.
45. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
46. Wijeysundera DN, Beattie WS, Wijeysundera HC, Yun L, Austin PC, Ko DT. Duration of preoperative beta-blockade and outcomes after major elective noncardiac surgery. Can J Cardiol. 2014;30(2):217-223.
47. Andersson C, Merie C, Jorgensen M, et al. Association of beta-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing noncardiac surgery: a Danish nationwide cohort study. JAMA Int Med. 2014;174(3):336-344.
48. Bakker EJ, Ravensbergen NJ, Poldermans D. Perioperative cardiac evaluation, monitoring, and risk reduction strategies in noncardiac surgery patients. Curr Opin Crit Care. 2011;17(5):409-415.
49. Auerbach AD, Goldman L. Beta-blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA. 2002;287(11):1435-1444.
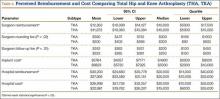
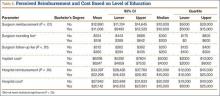
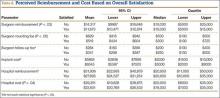
Patients’ Perceptions of the Costs of Total Hip and Knee Arthroplasty
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
Medical economics has been a major sociopolitical issue in the United States for the past 20 years, with concerns focused on increasing medical spending. These costs are projected to continue to rise, from 15.3% of gross domestic product in 2002 to 19.6% in 2017.1
Multiple steps have been taken to help reduce the cost of health care, many of which center on physician reimbursement. The Balanced Budget Act of 1997 worked to control Medicare spending by increasing reimbursement for clinic visits by setting reductions for procedural reimbursements. This specifically affects orthopedic surgeons, who between 1991 and 2002 experienced a 28% reduction in reimbursement, after inflation, for commonly performed orthopedic procedures, including hip and knee arthroplasty.2 Unfortunately, this system does not take into account the value of services as perceived by patients.
Total hip and knee arthroplasty (THA, TKA) are well-established surgical treatments for advanced osteoarthritis of the hip and knee, respectively. Much research has been done on patient satisfaction with these procedures and on their long-term results and cost-effectiveness. These procedures rank among the highest in patient satisfaction, and improvements in technique and technology have steadily improved long-term results. THA and TKA have proved to be cost-effective in appropriately indicated patients.
The demand for THA and TKA is projected to increase by 174% and 673%, respectively, from 2005 to 2030.3 Legislators, payers, health care providers, and patients are understandably concerned about the rising cost of health care and the implications for access to elective surgical procedures. In a recent study by Foran and colleagues,4 surveyed postoperative patients indicated that Medicare reimbursement was “much lower” for arthroplasty than it should be. In addition, they overestimated (compared with national averages) what Medicare reimburses for hip and knee arthroplasty. Many raised concerns that orthopedic surgeons might drop Medicare entirely.4
These misconceptions about reimbursement may stem partly from the inaccessibility of health care cost information. Rosenthal and colleagues5 recently queried a random selection of US hospitals and demonstrated the difficulty in obtaining THA pricing information.
In a system in which consumers and payers are often not one and the same, it is unclear if consumers understand the cost of their health care. We conducted a study to assess patients’ perceptions of the cost of total joint arthroplasty (TJA) and gain insight into their understanding of health care costs and their sense of the value of this elective surgical procedure.
Materials and Methods
After obtaining institutional review board approval and informed consent for this study, we surveyed 284 consecutive patients who underwent THA or TKA at an academic medical center. Patients had either primary or revision surgery performed (by Dr. Hallstrom or Dr. Urquhart) and were surveyed during their first (2-week) postoperative visit, between March 1, 2012 and December 20, 2012.
Surveys were labeled with patient identifiers to facilitate abstraction of data from electronic medical records. Operative reports and discharge summaries were reviewed for data that included sex, age, diagnosis, procedure, surgeon, implant, admission date, and length of stay.
The survey asked for demographic information, including level of education, insurance coverage, and annual household income, and included a question to verify the surgical procedure and a question to determine if the patient had reviewed a hospital billing statement pertaining to the patient’s admission. The survey also included these questions about reimbursement and cost:
- How much do you feel your orthopedic surgeon was reimbursed for your surgery? (EXCLUDING payments to the hospital)
- How much do you think your surgeon gets reimbursed to see you IN THE HOSPITAL after surgery?
- How much do you think your surgeon gets reimbursed per visit to see you IN CLINIC for follow-up during the first 3 months after surgery?
- How much do you think the implant used in your surgery cost?
- How much do you think the hospital was reimbursed for your surgery and admission to the hospital after surgery? (EXCLUDING payments to the surgeon)
- How much do you think it cost the hospital to provide your surgery and admission to the hospital after surgery?
Responses were limited to numeric currency format using a response area as shown in Figure 1. Overall patient satisfaction was elicited with use of a 5-point scale ranging from 1 (very unsatisfied) to 5 (very satisfied). Regarding type of implant used, patients could select from 6 prominent vendors or indicate “other” or “don’t know.” They were also asked which of several factors should primarily determine surgeon reimbursement: overall patient satisfaction, technical difficulty, amount of risk/possible harm, duration/amount of time, and rate of complications. A free-response comments section was provided at the end of the survey.
Data from the survey and the electronic medical records were collected using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, Tennessee). Statistical analysis was performed with SAS Version 9.3 (SAS Institute, Cary, North Carolina). Data were screened before further analysis. Patients who provided nonnumeric responses in numeric response fields were excluded from further analysis. Numeric ranges were applied in subsequent analysis using the mean of the range. Implausible responses resulted in the removal of the entire encounter from subsequent analysis.
Demographic data used to define categories for further subgroup analysis are presented as percentages of the group. Medians, means, and interquartile ranges were calculated for all responses regarding reimbursement and cost. Differences in perceptions of reimbursement and cost based on subgroups, including procedure type, diagnosis, education level, and satisfaction, were calculated. Independent-samples Student t tests were used to determine the statistical significance of the differences detected.
Results
Of the 400 eligible patients seen at the first postoperative follow-up, 284 (71%) were enrolled in the study. Mean (SD) age was 62.6 (12.6) years. Of the 284 patients enrolled, 154 (54%) were female. Of the participants who reported their education and income, 125 (44%) had a bachelor’s degree or higher degree, and 68 (23.9%) reported income of more than $100,000 per year. The largest payers reported by patients were private insurance (80%) and Medicare (46%). Additional demographic details are listed in Table 1.
Of the 284 patients enrolled in the study, 159 (56%) had THA, and 88 (31%) had TKA (Table 2). Thirty-seven patients (13%) underwent revision procedures. Only 5 patients (2%) indicated they had reviewed their hospital billing statement from their most recent admission. Two hundred forty-two patients (85%) were satisfied or very satisfied with their procedure.
Regarding the implant used in their surgery, 216 patients (76%) indicated they did not know which company manufactured it. Of the 68 patients (24%) who named a manufacturer, 53 (78%) were correct in their selection (intraoperative records were checked). Patients indicated they thought the implant used in their surgery cost $6447 on average (95% CI, $5581-$7312).
On average, patients thought their surgeon was reimbursed $12,014 (95% CI, $10,845-$13,183) for their procedure, and they estimated that the hospital was reimbursed $28,392 (95% CI, $25,271-$31,512) for their perioperative care and that it cost the hospital $24,389 (95% CI, $21,612-$27,165) to provide it. Means, confidence intervals, medians, and interquartile ranges for parameters of reimbursement and cost are listed in Table 3. Seventy-one patients (25%) thought on average that the hospital took a net loss for each TJA performed, and 146 patients (51%) thought on average that the hospital generated a net profit for each TJA.
On average, patients thought surgeons were reimbursed $11,872 for a THA and $12,263 for a TKA. Patients also estimated a higher hospital cost (THA, $22,981; TKA, $26,998) and reimbursement (THA, $27,366; TKA, $30,230) after TKA than THA. These differences in perceptions of cost and reimbursement for THA and TKA appear in Table 4 and Figure 2.
Statistically significant differences were also found in perceptions of cost and reimbursement based on level of education and overall patient satisfaction. Patients with a bachelor’s degree or higher estimated physician reimbursement at $11,006, whereas patients with a lower level of education estimated reimbursement at $12,890. In addition, patients with a lower level of education gave estimates of hospital cost and reimbursement that were $7698 and $10,799 higher, respectively, than the estimates given by patients with a higher level of education (Table 5, Figure 3). Patients who were satisfied or very satisfied with their overall TJA experience estimated surgeon reimbursement at $11,673. Patients who indicated they were unsatisfied, very unsatisfied, or neutral regarding their overall experience gave a higher estimate of surgeon reimbursement: $14,317 (Table 6, Figure 4).
Because of the small number of enrolled patients who had revision surgery and the high variability in patient responses, there were no meaningful or statistically significant differences in perceptions of cost and reimbursement based on revision or primary surgery.
Patients also estimated substantial additional reimbursements to physicians for services included at no additional charge with the global surgical package. Median estimates were $300 for reimbursement to a physician making rounds in the hospital and $250 for reimbursement for an outpatient follow-up. Only 47 patients (17%) and 35 patients (12%) correctly indicated there is no additional payment for making rounds and outpatient follow-up, respectively. Estimates of these reimbursements varied by education level, procedure, and overall satisfaction (Tables 4–6).
Discussion
The sustainable growth rate (SGR) formula, part of the Balanced Budget Act of 1997, was constructed to manage health care costs in the context of overall economic growth. By 2001, Medicare health care expenditures had begun to outpace economic growth, and the SGR formula dictated a reduction in reimbursement to physicians. Each year over the past decade, Congress has passed legislation providing a temporary reprieve, staving off a drastic reduction of as much as 25% in 2010.6 Despite these adjustments, reimbursement continues to decrease because of overall inflation.
More worrisome is that “more than half of the nearly trillion dollar price tag for expanding coverage under the Affordable Care Act (ACA) will be paid by decreasing spending for the more than 46.3 million individuals covered by Medicare.”7 ACA provisions will also create an Independent Payment Advisory Board (IPAB) to oversee health care costs and reduce Medicare spending when it is expected to exceed target levels.8 As IPAB cannot recommend increasing revenues or changing benefits, and because it is initially prohibited from recommending decreasing payments to hospitals, the decreases will likely have the greatest impact on physician reimbursement.7-9
Health care policy has been a major campaign issue during recent US elections. The public and popular media remain engaged in this important discussion. Although patients, policymakers, and physicians are understandably concerned about cost and access to health care, it is unclear if patients understand the distribution of health care cost and reimbursement.
Other authors have studied patients’ perceptions of physician reimbursement for TJA. Hayden and colleagues10 surveyed 1000 residents of a Texas city. The 121 who responded to the survey thought that fair compensation for performing a TKA was $5080, on average.10 Although this was significantly higher than the actual Medicare reimbursement at the time, a later study, by Foran and colleagues,4 found patients’ estimates of both fair reimbursement and Medicare reimbursement for TJA to be even higher. Foran and colleagues4 surveyed 1120 patients who thought surgeons deserved to be paid $14,358 for THA and $13,322 for TKA, on average. These reimbursement values are nearly an order of magnitude higher than actual reimbursements. For Medicare payments, patients lowered their estimates to $8212 for THA and $7196 for TKA.4
To our knowledge, the present study is the first to use a “postconsumer” survey to assess patients’ perceptions of THA and TKA costs. Our results confirmed that patients substantially overestimated reimbursement for THA and TKA at $11,872 and $12,263, respectively, relative to the average Medicare reimbursements of $1467 and $1530, respectively.11 We also found that patients overestimated both hospital cost and reimbursement for THA at $22,981 and $27,366, respectively, relative to recently published hospital economic analyses showing THA cost and reimbursement to be $11,688 and $15,789, respectively.12 Few patients enrolled in our study demonstrated an understanding of the services included in the global surgical package. Only about 12% of patients correctly indicated there was no additional payment to the physician for initial follow-up appointments. However, patients were fairly accurate in their estimates of implant cost. On average, patients who underwent THA priced their implant at $6823, which is only about 9% higher than the reported median cost of $6072 to $6400.13,14
We also found significant differences in perceptions of cost based on level of education, joint replaced, and overall level of satisfaction. On average, patients with a bachelor’s degree or higher gave estimates of cost and reimbursement that were lower than those given by patients with a lower level of education. Estimates of physician reimbursement and hospital reimbursement and cost were higher from patients who had TKA than from patients who had THA.
Comparing perceptions of reimbursement for appendectomy and coronary artery bypass with perceptions for TJA, Foran and colleagues4 found that patients understood the relative complexity of each procedure, as evidenced by their estimates of fair reimbursement for each. However, in comparing patient estimates for the different components of cost and reimbursement for TJA, we found great variability in understanding. Patients in our study overestimated payments to the hospital by 73% but overestimated the cost of the THA implant by only 9%. However, the same patients overestimated physician reimbursement for THA by about 800%. If these patients’ estimates of reimbursement are considered surrogates for relative value, then physicians, based on actual payments, are grossly undervalued relative to implant manufacturers.
Our study had several limitations. First, the enrolled patients were all seen at one medical center, in Ann Arbor, Michigan, and our results may not be generalizable outside the region. Second, the survey respondents were postoperative patients who had an established relationship with the study’s principal investigators—a relationship that may have been a source of bias in the consideration of reimbursement as a function of value. Third, despite our efforts to carefully design a survey with open-ended responses, the order in which the survey questions were presented may have influenced patient responses. Fourth, the open-ended question design may have had an impact on responses where the correct answer would have required entering 0.00.
Despite these limitations, our study results demonstrated general public misconceptions about cost and reimbursement for common orthopedic procedures. Although more transparency in health care cost information may not immediately result in a more well-informed population,15 our patients, given the opportunity to develop an understanding of the economics of their own medical treatment, may become better prepared to make informed choices regarding changes in health care policy.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
1. Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366-388.
2. Hariri S, Bozic KJ, Lavernia C, Prestipino A, Rubash HE. Medicare physician reimbursement: past, present, and future. J Bone Joint Surg Am. 2007;89(11):2536-2546.
3. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
4. Foran JR, Sheth NP, Ward SR, et al. Patient perception of physician reimbursement in elective total hip and knee arthroplasty. J Arthroplasty. 2012;27(5):703-709.
5. Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
6. US Senate Committee on Finance. H.R. 4994: the Medicare and Medicaid Extenders Act of 2010. http://www.finance.senate.gov/legislation/details/?id=9f97aa2e-5056-a032-52d4-8db158b12b11. Accessed March 25, 2015.
7. Zinberg JM. When patients call, will physicians respond? JAMA. 2011;305(19):2011-2012.
8. Jost TS. The Independent Payment Advisory Board. N Engl J Med. 2010;363(2):103-105.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Estimated financial effects of the “Patient Protection and Affordable Care Act,” as amended. 2010. http://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/downloads/PPACA_2010-04-22.pdf. Accessed March 25, 2015.
10. Hayden SA, Hayden D, White LW. The U.S. public’s perceived value of the surgeon’s fee for total knee replacement. Abstract presented at: 75th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 5-9, 2008; San Francisco, CA. Abstract 214.
11. Centers for Medicare & Medicaid Services. Physician Fee Schedule Search Tool. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 25, 2015.
12. Rana AJ, Iorio R, Healy WL. Hospital economics of primary THA decreasing reimbursement and increasing cost, 1990 to 2008. Clin Orthop. 2011;469(2):355-361.
13. Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18(1):23-27.
14. Robinson JC, Pozen A, Tseng S, Bozic KJ. Variability in costs associated with total hip and knee replacement implants. J Bone Joint Surg Am. 2012;94(18):1693-1698.
15. Smolders JM, Van Loon CJ, Rijnberg WJ, Van Susante JL. Patients poorly estimate the overall costs of a total knee arthroplasty and strongly overestimate the surgeon’s fee. Acta Orthop Belg. 2007;73(3):339-344.
Polydactyly of the Hand
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
Polydactyly is the presence of extra digits. Its incidence is likely underestimated because many practitioners treat simple “nubbins” without referring them to orthopedic specialists.1-3 Polydactyly can be detected by ultrasound as early as 14 weeks’ gestational age, with partial autoamputation seen in most isolated polydactylies.4 The thumb, responsible for 40% of hand function, must be able to oppose the other digits with a stable pinch.5 Polydactyly encumbers this motion when the duplicated digits deviate from normal alignment. Ezaki6 noted that the anatomy is better described as “split” than “duplicated.” There are many dichotomous ways to classify polydactyly: preaxial (radial) versus postaxial (ulnar), thumb versus triphalangeal, simple versus complex (Figure 1). Mixed polydactyly is defined as the presence of preaxial and postaxial polydactyly.7 Surgical management seeks to allow normal hand function and to restore cosmesis.
Epidemiology
Sun and colleagues8 reported the overall polydactyly incidence as 2 per 1000 live births in China from 1998 to 2009, with a slight male predominance; polydactyly was also 3 times more common than syndactyly in this population. Ivy,9 in a 5-year audit of Pennsylvania Department of Health records, found polydactyly to be the fourth most common congenital anomaly after clubfoot, cleft lip/palate, and spina bifida. Thumb duplication occurs in 0.08 to 1.4 per 1000 live births and is more common in American Indians and Asians than in other races.5,10 It occurs in a male-to-female ratio of 2.5 to 1 and is most often unilateral.5 Postaxial polydactyly is predominant in black infants; it is most often inherited in an autosomal dominant fashion, if isolated, or in an autosomal recessive pattern, if syndromic.1 A prospective San Diego study of 11,161 newborns found postaxial type B polydactyly in 1 per 531 live births (1 per 143 black infants, 1 per 1339 white infants); 76% of cases were bilateral, and 86% had a positive family history.3 In patients of non-African descent, it is associated with anomalies in other organs. Central duplication is rare and often autosomal dominant.5,10
Genetics and Development
As early as 1896, the heritability of polydactyly was noted.11 As of 2010, polydactyly has been associated with 310 diseases.12 Ninety-nine genes, most involved in regulation of anterior-posterior formation of the limb bud, have been implicated.12,13
The upper limb begins to form at day 26 in utero.14 Apoptosis in the interdigital necrotic zones results in the formation of individual digits. It is presumed that, in polydactyly, the involved tissue is hypoplastic because of an abnormal interaction between mesoderm and ectoderm.5 Presence of an apical ectodermal ridge determines the formation of a limb bud, and on it the zone of polarizing activity (ZPA) dictates preaxial and postaxial alignment.14,15 The ZPA is located on the posterior zone of the developing limb bud. The levels of GLI3, a zinc finger-containing DNA-binding protein, are highest in the anterior area, and HAND2, a basic helix-loop-helix DNA-binding protein, is found in the ZPA. This polarity promotes sonic hedgehog (Shh) gene expression in the posterior region, which in turn prevents GLI3 cleavage into its repressed form. GLI3R (repressed) and GLI3A (active) concentrations are highest, therefore, in the anterior and posterior portions of the bud, respectively. The GLI3A:GLI3R ratio is responsible for the identity and number of digits in the hand (ie, the thumb develops in regions of high GLI3R). GLI and Shh mutations lead to polydactylous hands with absent thumbs (Figure 2).16
Ciliopathies have also been shown to cause postaxial polydactyly, possibly because of the role that nonmotile cilia play in hedgehog signaling.17 Mutations in Shh genomic regulators cause preaxial polydactyly.18 HoxD activates Shh in the ZPA; HoxD13 mutations are associated with synpolydactyly.16,19 In each of these mutations, Shh production is altered, and some form of polydactyly results.
Associations
Many syndromes have been associated with polydactyly. Not all polydactyly is associated with other disorders, but the more complex the polydactyly, the more likely that other anomalies are present. Every patient who presents with polydactyly should have a full history taken and a physical examination performed (Figure 3). Any patient with syndromic findings or atypical presentations (eg, triphalangism, postaxial polydactyly in a patient of non-African descent, central and index polydactyly) should be referred to a geneticist.
Classifications
The Wassel20 classification describes the anatomical presentation of thumb duplication on the basis of 70 cases in Iowa (Figures 4, 5; Table 1). Because some duplications fall outside the Wassel classification, many researchers have proposed modifications (Figure 6).21-25
The Temtamy and McKusick10 classification, which is the product of geneticists, classifies duplications by grouping genetically related presentations (Table 2). It provides the most commonly used postaxial classification, with type A being a fully developed digit and type B a rudimentary and pedunculated digit, informally referred to as a nubbin. Type B is more common than type A. Given inheritance patterns, it is assumed that type A is likely multifactorial and type B autosomal dominant.10 Thumb polydactyly inheritance is still unclear. The other types of preaxial polydactyly and high degrees of polydactyly are rare but seem to be passed on in an autosomal dominant fashion on pedigree analysis.10
The Stelling and Turek classification presents the duplication from a tissue perspective: Type I duplication is a rudimentary mass devoid of other tissue elements; type II is a subtotal duplication with some normal structures; and type III is a duplication of the entire “osteoarticular column,” including the metacarpal.1 It is interesting to note that histology of type I duplications shows neuroma-like tissue.26-28 Again, normal is a relative term because, in polydactyly, the duplications are hypoplastic and deviated, with anomalous anatomy.
The Rayan classification describes ulnar polydactyly and was derived from a case study series of 148 patients in Oklahoma (Table 3).29
There are also some complex polydactylies that are not easily classified: ulnar dimelia, cleft hand, pentadactyly, and hyperphalangism. Ulnar dimelia, also known as “mirror hand,” is typically 7 digits with no thumb, but other variations are seen. The radius is often absent, and the elbow is abnormal. There is some debate about whether it is a fusion of 2 hands. Pentadactyly, or the 5-fingered hand, appears as 5 triphalangeal digits with no thumb (Figure 7).
Isolated thumb triphalangism might appear similar to pentadactyly. Miura30,31 pointed out that the radial digit in the pentadactylous hand may be opposable (thumb-like) or nonopposable; in his studies, the patients with the opposable thumb had a metacarpal with a proximal epiphysis (Figure 8). Consequently, the triphalangeal thumb metacarpal with a distal epiphysis is true pentadactyly, whereas that with a proximal epiphysis is hyperphalangism (Figure 9). Treatment of these complex polydactylies involves the same underlying principles as for preaxial and postaxial polydactyly, albeit with additional proximal upper extremity considerations.
When to Operate (Timing)
Ezaki6 recommended surgical intervention at age 6 to 9 months, before fine motor skills have developed with the abnormal anatomy. Cortical learning occurs as the child begins prehensile activities before 6 months, but the risks of anesthesia outweigh functional benefits until the child is older. Waiting until 1 year of age is not uncommon, though surgery at an earlier age may be beneficial if the polydactyly affects hand function.32 It is not uncommon to wait with the more balanced thumb polydactylies to assess thumb function. Hypoplasia might also delay surgical intervention until there is enough tissue inventory for reconstruction. Wassel20 noted that surgical intervention ideally occurs before the supernumerary elements displace the normal elements, as tends to happen with growth. Suture ligation is an option in the neonatal unit for some pedunculated digits.33 Studies have shown satisfactory results in adults treated for polydactyly, if the patient presents later than expected.34
Surgical Considerations
Knavel recommended simple excision, stating that “ablation requires no ingenuity and creates no problems.”5 This belief, though true for some duplications, will not lead to the best outcome for more complex polydactylies. The goal of surgery is a stable and well-aligned thumb for pinch and prehensile activity, as well as a cosmetically pleasing hand. Incisions should not be made linearly along the axis of the digit, as the scar will cause deviation with growth.24
Wassel type I polydactyly might appear incidentally as a broad thumb, in which case it requires no intervention (Figure 10). However, in Wassel types I and II polydactyly with deformity, the Bilhaut-Cloquet procedure is useful for both bifid and duplicated phalanges (Figure 11).5,6,30,32,35 Collateral ligaments may need to be released in type II because of difficulty in opposing the tissue. Cosmetic results with Bilhaut-Cloquet are unpredictable. The original technique required symmetrically sized digits; results today have been improved with microtechniques and preservation of an entire nail.36 Another option is ablation of the more hypoplastic osseous element and soft-tissue augmentation of the residual digit. The theme of ablation and augmentation is seen throughout the literature for the surgical treatment of polydactyly (Figure 12).1
For type III polydactyly, the bifid proximal phalanx is narrowed by resection and realigned with osteotomy of the remaining diaphysis. Type IV polydactyly, the most common thumb duplication, often requires advancement of the abductor pollicis brevis to the base of the proximal phalanx to aid in metacarpophalangeal (MCP) stabilization, abduction, and opposition. The metacarpal head, if broad and with 2 facets, can be shaped to form a single articulating surface. The metacarpal, occasionally with the proximal phalanx, often requires realignment by closing wedge osteotomy. Last, tendons on the resected bony elements should be rebalanced on the remaining digit, and anomalous slips must be released. For instance, given a radial insertion of the long flexor tendon on the distal phalanx, the tendon should be moved centrally. A strong flexor or extensor tendon on the amputated digit should be transferred to the remaining digit.24
Types V and VI are treated similarly to type IV, with the addition of a first web space Z-plasty or web widening if there is thenar eminence contracture. Acral transposition has also been described, with transposition of the tip of the ablated digit in place of the tip of the kept digit; this technique is ideal if one digit has a more normal proximal part while the other has a more normal distal part (Figure 13).35
Type VII thumb polydactyly, the type most likely inherited and associated with other disorders, should be treated like type VI. The nail should be preserved; amputation of the distal phalanx is not advised. Resection of the delta phalanx or 1 interphalangeal (IP) joint is an option. Articular surfaces will remodel if done before the age of 1 year. If the thenar eminence is hypoplastic, then Huber transfer of the abductor digiti minimi should be considered.37 Resection of the triphalangeal thumb is also advised, even if the biphalangeal thumb is more hypoplastic, with transfer of the ligaments and tendons, as described earlier.5,6,24,30,32,35
Thumb triphalangism, if isolated, and hyperphalangism in the other digits, can be treated with resection of the delta phalanx or one of the IP joints if it is affecting function or cosmesis.1,6 Wood and Flatt23 recommended early resection of a thumb delta phalanx because of the likelihood of deviation that impedes thumb function. For children, they recommended delta phalanx resection and Kirschner wire fixation for 6 weeks; for adults, they recommended resection or fusion of the joint, with osteotomy as needed for deviation.23,24 For thumb triphalangism, multiple surgeries are the norm, as Wood24 reported in his study of 21 patients who underwent 78 operations in total.
Index polydactyly may present as a simple pedunculated skin tag, which can be simply excised, or as a more complex musculoskeletal duplication. More complex presentations can be treated with procedures similar to those used for the thumb. Typically, the additional digit is radially deviated and angulated, eventually leading to impingement of thumb pinch and the first web space. Ray amputation is also an option if no reconstructive surgery will produce the stable, sensate radial pinch that is essential to hand function.32
Ring-finger polydactyly and long-finger polydactyly are often complicated by some element of syndactyly, resulting in a relative paucity of skin (Figure 14). There is failure of both formation (hypoplasia) and differentiation (syndactyly). The hypoplasia particularly affects the function of these digits by tethering them; multiple surgeries to restore proper hand function are the norm.1 Reconstructive surgery for these digits requires preoperative tissue inventory followed by resection and augmentation; as in syndactyly, skin for coverage is at a premium. Creation of a 3-fingered hand is an option.23
Temtamy and McKusick10 type A little-finger polydactyly is treated similarly to the thumb, with the caveat that hypothenar and intrinsic muscles that insert on the resected little finger are transferred to the remaining digit. In contrast to thumb polydactyly, the extrinsic musculature tends to be in good position. Suture ligation of type B polydactyly, as described by Flatt, is likely more common than orthopedists appreciate, as pediatricians and neonatal unit practitioners commonly perform this procedure in the nursery.1-3 It has been described with 2-0 Vicryl3 (Ethicon, Somerville, New Jersey) and 4-0 silk sutures,32 with the goal of necrosis and autoamputation. Parents should be told the finger generally falls off about 10 days (range, 4-21 days) after ligation.3 Multiple authors have cited a report of exsanguination from suture ligation, but we could not locate the primary source. It is advisable to wait until a patient is 6 months of age if planning to resect the nubbin in the operating room, given the anesthesia risk and the lack of functional impairment. Katz and Linder33 indicated they remove type B polydactyly in the nursery suite used for circumcisions; they use anesthetizing cream on the skin, and sharp excision with a scalpel, followed by direct pressure and Steri-Strip (3M, St. Paul, Minnesota) application. Suture ligation is recommended only if there is a narrow, thin (<2 mm) soft-tissue stalk; any broad or bony stalk necessitates surgical removal to avoid neuroma formation and failure of autonecrosis (Figure 15).27 Other options are a single swipe of a scalpel and elliptical excision; sharp transaction of the digital nerve with subsequent retraction is advised to avoid neuroma formation.2
Barton described ulnar dimelia operations as “spare parts surgery.”1 Extra digits are ablated and a thumb created (Figure 16). The hand might have a digit in relatively good rotational position for thumbplasty, or the principles of pollicization may need to be used. If the patient is already using the hand, the surgeon should note which finger the patient uses as a thumb.24 Any accompanying wrist flexion contracture must be corrected with careful attention to musculotendinous balancing. Because the forearm and elbow, and occasionally even the more proximal limb, will be abnormal in this disorder, multiple surgeries are again the norm.1
Pentadactyly is treated like thumb hypoplasia, with first web space creation.1
Complications
In polydactyly, a reoperation rate of up to 25% has been reported, with most reoperations performed because of residual or subsequent deformity.5,30,31,38 Risk factors for reoperation are type IV thumb duplication, preoperative “zigzag” deformity, and radially deviated thumb elements at presentation.5 The delta phalanx may not show on radiographs until the patient is 18 months old, but functional deformity will worsen as long as it is present. Zigzag deformity may be due to the delta phalanx or to musculotendinous imbalance, such as a radially inserted flexor pollicis longus (FPL) or lack of stable MCP abduction. Miura31 found that careful reconstruction of the joint capsule and thenar muscles from the ablated digit to the remnant digit is the key to a successful initial surgery. Lee and colleagues39 defined zigzag deformity as more than 20° MCP and IP angulation; for cases present before surgery, they recommended FPL relocation by the pullout technique in addition to osteotomies to prevent further interphalangeal deviation (Figures 17, 18).
Abnormal physeal growth, joint instability, and stiffness can all occur. Stiffness is particularly difficult to treat but seldom presents a functional problem. Joint enlargement, which is not uncommon, results from either broad articular surfaces or retained cartilage from the perichondral ring after resection that later ossifies.5,38 Nubbin-type duplications may not fall off after suture ligation, necessitating further excision, and a cosmetic bump is seen after 40% of suture ligations.3 Patillo and Rayan28 and Rayan and Frey29 warned against suture ligation unless the nubbin has a small stalk because of the possibility of infection and gangrene. The excised nubbin tissue is histologically nervous, and there have been reports of painful neuromas in the remaining scar of a ligated nubbin that respond well to excision.26,27,40 It is thought that these painful lesions form because the ligature prevents the digital nerves to the vestigial digit from retracting.27 Nail deformity and IP joint stiffness are seen with the Bilhaut-Cloquet procedure, though often finger function remains satisfactory.
Conclusion
Polydactyly is a common congenital hand abnormality. Its true incidence is unknown because of inconsistent documentation. Surgeons must strive for a functional, cosmetic hand, given a diverse set of possible anomalies. Hypoplasia is the rule; tissue should be ablated and augmented as necessary. Musculotendinous insertions may need to be centralized. Patients’ family members should always be counseled that more surgery may be needed in the future, as further deformity can occur with growth. Surgically corrected thumb duplications will be stiffer, shorter, and thinner than their normal counterparts. Nail ridges are common. However, it should be noted that 88% of these patients are satisfied with their results.41 Some amount of contracture and abnormal function should be expected with index-, long-, and ring-finger duplications. The only remnant of type B postaxial duplications may be a slight discoloration or bump, though stiffness and deformity can happen with a type A deformity. A “duplicated” digit that requires surgical correction will never be completely normal, but acceptable function is routinely achievable.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
1. Graham TJ, Ress AM. Finger polydactyly. Hand Clin. 1998;14(1):49-64.
2. Abzug JM, Kozin SH. Treatment of postaxial polydactyly type B. J Hand Surg Am. 2013;38(6):1223-1225.
3. Watson BT, Hennrikus WL. Postaxial type-B polydactyly—prevalence and treatment. J Bone Joint Surg Am. 1997;79(1):65-68.
4. Zimmer EZ, Bronshtein M. Fetal polydactyly diagnosis during early pregnancy: clinical applications. Am J Obstet Gynecol. 2000;183(3):755-758.
5. Cohen MS. Thumb duplication. Hand Clin. 1998;14(1):17-27.
6. Ezaki M. Radial polydactyly. Hand Clin. 1990;6(4):577-588.
7. Nathan PA, Keniston RC. Crossed polydactyly: case report and review of the literature. J Bone Joint Surg Am. 1975;57(6):847-849.
8. Sun G, Xu ZM, Liang JF, Li L, Tang DX. Twelve-year prevalence of common neonatal congenital malformations in Zhejiang Province, China. World J Pediatr. 2011;7(4):331-336.
9. Ivy RH. Congenital anomalies as recorded on birth certificates in the Division of Vital Statistics of the Pennsylvania Department of Health, for the period of 1951–1955, inclusive. Plast Reconstr Surg. 1957;20(5):400-411.
10. Temtamy SA, McKusick VA. Polydactyly as a part of syndromes. In: Bergsma D, ed. Mudge JR, Paul NW, Conde Greene S, associate eds. The Genetics of Hand Malformations. New York, NY: Liss. Birth Defects Original Article Series. 1978;14(3):364-439.
11. Gould W, Pyle L. Anomalies and Curiosities of Medicine. New York, NY: Bell; 1896.
12. Biesecker LG. Polydactyly: how many disorders and how many genes: 2010 update. Dev Dyn. 2011;250(5):931-942.
13. Grzeschik K. Human limb malformations; an approach to the molecular basis of development. Int J Dev Biol. 2001;46(7):983-991.
14. Zaleske DJ. Development of the upper limb. Hand Clin. 1985;1(3):383-390.
15. Beatty E. Upper limb tissue differentiation in the human embryo. Hand Clin. 1985;1(3):391-404.
16. Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of hedgehog signaling. Trends Genet. 2012;28(8):364-373.
17. Ware SM, Aygun MG, Heldebrandt F. Spectrum of clinical diseases caused by disorders of primary cilia. Proc Am Thorac Soc. 2011;8(5):444-450.
18. Lettice LA, Hill RE. Preaxial polydactyly: a model for defective long-range regulation in congenital abnormalities. Curr Opin Genet Dev. 2005;15(3):294-300.
19. Al-Qattan MA. Type II familial synpolydactyly: report on two families with an emphasis on variations of expression. Eur J Hum Genet. 2011;19(1):112-114.
20. Wassel HD. The results of surgery for polydactyly of the thumb. Clin Orthop. 1969;(64):175-193.
21. Blauth W, Olason AT. Classification of polydactyly of the hands and feet. Arch Orthop Trauma Surg. 1988;107(6):334-344.
22. Wood VE. Super digit. Hand Clin. 1990;6(4):673-684.
23. Wood VE, Flatt AE. Congenital triangular bones in the hand. J Hand Surg Am. 1977;2(3):179-193.
24. Wood VE. Polydactyly and the triphalangeal thumb. J Hand Surg Am. 1978;3(5):436-444.
25. Zuidam JM, Selles RW, Ananta M, Runia J, Hovius SER. A classification system of radial polydactyly: inclusion of triphalangeal thumb and triplication. J Hand Surg Am. 2008;33(3):373-377.
26. Leber GE, Gosain AK. Surgical excision of pedunculated supernumerary digits prevents traumatic amputation neuromas. Pediatr Dermatol. 2003;20(2):108-112.
27. Mullick S, Borschel GH. A selective approach to treatment of ulnar polydactyly: preventing painful neuroma and incomplete excision. Pediatr Dermatol. 2001;27(1):39-42.
28. Patillo D, Rayan GM. Complications of suture ligation ablation for ulnar polydactyly: a report of two cases. Hand (N Y). 2011;6(1):102-105.
29. Rayan GM, Frey B. Ulnar polydactyly. Plastic Reconstr Surg. 2001;107(6):1449-1454.
30. Miura T. Triphalangeal thumb. Plastic Reconstr Surg. 1976;58(5):587-594.
31. Miura T. Duplicated thumb. Plastic Reconstr Surg. 1982;69(3):470-481.
32. Simmons BP. Polydactyly. Hand Clin. 1985;1(3):545-566.
33. Katz K, Linder N. Postaxial type B polydactyly treated by excision in the neonatal nursery. J Pediatr Orthop. 2011;31(4):448-449.
34. Manohar A, Beard AJ. Outcome of reconstruction for duplication of the thumb in adults aged over 40. Hand Surg. 2011;16(2):207-210.
35. Watt AJ, Chung KC. Duplication. Hand Clin. 2009;25(2):215-228.
36. Tonkin MA. Thumb duplication: concepts and techniques. Clin Orthop Surg. 2012;4(1):1-17.
37. Huber E. Relief operation in the case of paralysis of the median nerve. J Hand Surg Eur. 2004;29(1):35-37.
38. Mih AD. Complications of duplicate thumb reconstruction. Hand Clin. 1998;14(1):143-149.
39. Lee CC, Park HY, Yoon JO, Lee KW. Correction of Wassel type IV thumb duplication with zigzag deformity: results of a new method of flexor pollicis longus tendon relocation. J Hand Surg Eur. 2013;38(3):272-280.
40. Hare PJ. Rudimentary polydactyly. Br J Dermatol. 1954;66(11):402-408.
41. Yen CH, Chan WL, Leung HB, Mak KH. Thumb polydactyly: clinical outcome after reconstruction. J Orthop Surg (Hong Kong). 2006;14(3):295-302.
42. Edmunds JO. A tribute to Daniel C. Riordan, MD (1917–2012). Tulane University School of Medicine, Department of Orthopaedics website. http://tulane.edu/som/departments/orthopaedics/news-and-events/danriordantribute.cfm. Accessed March 31, 2015.
43. Faust DC, Herms R. Daniel C. Riordan, MD, 1917–2012. J Hand Surg Am. 2013;38(1):202-205.
A Blood Test for Osteoarthritis?
The first blood test to detect rheumatoid arthritis and osteoarthritis may soon be developed, according to a study published March 19 in Scientific Reports. The research findings could potentially lead to patients being tested for rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms.
Lead researcher Dr. Naila Rabbani, Reader of Experimental Systems Biology at the University of Warwick in Coventry United Kingdom, and colleagues have identified a biomarker that is linked to both rheumatoid arthritis and osteoarthritis. While there are established tests for rheumatoid arthritis, the newly identified biomarker could lead to one that can diagnose rheumatoid arthritis and osteoarthritis.
Initially, the research's focus was on citrullinated proteins, a biomarker suspected to be present in the blood of patients with early stage rheumatoid arthritis. It had previously been established that patients with rheumatoid arthritis have citrullinated protein antibodies, but it was not believed that the same held true for people with osteoarthritis. However, investigators found that there was an increase in citrullinated protein levels in both early-stage osteoarthritis and rheumatoid arthritis.
Study authors then produced an algorithm of 3 biomarkers, plasma/serum citrullinated protein, 4-hydroxyproline, and anti-cyclic citrullinated peptide. Based on this algorithm, the researchers found that with a single test they could potentially detect and discriminate between the major types of arthritis at the early stages, before joint damage has occurred.
“Detection of early stage osteoarthritis made the study very promising and we would have been satisfied with this only, but beyond this we also found we could detect and discriminate early-stage rheumatoid arthritis and other inflammatory joint diseases at the same,” said Dr. Rabbani.
“This discovery raises the potential of a blood test that can help diagnose both rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms,” Dr. Rabbani stated.
Suggested Reading
Ahmed U, Anwar A, Savage RS, et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci Rep. 2015 Mar 19;5:9259.
The first blood test to detect rheumatoid arthritis and osteoarthritis may soon be developed, according to a study published March 19 in Scientific Reports. The research findings could potentially lead to patients being tested for rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms.
Lead researcher Dr. Naila Rabbani, Reader of Experimental Systems Biology at the University of Warwick in Coventry United Kingdom, and colleagues have identified a biomarker that is linked to both rheumatoid arthritis and osteoarthritis. While there are established tests for rheumatoid arthritis, the newly identified biomarker could lead to one that can diagnose rheumatoid arthritis and osteoarthritis.
Initially, the research's focus was on citrullinated proteins, a biomarker suspected to be present in the blood of patients with early stage rheumatoid arthritis. It had previously been established that patients with rheumatoid arthritis have citrullinated protein antibodies, but it was not believed that the same held true for people with osteoarthritis. However, investigators found that there was an increase in citrullinated protein levels in both early-stage osteoarthritis and rheumatoid arthritis.
Study authors then produced an algorithm of 3 biomarkers, plasma/serum citrullinated protein, 4-hydroxyproline, and anti-cyclic citrullinated peptide. Based on this algorithm, the researchers found that with a single test they could potentially detect and discriminate between the major types of arthritis at the early stages, before joint damage has occurred.
“Detection of early stage osteoarthritis made the study very promising and we would have been satisfied with this only, but beyond this we also found we could detect and discriminate early-stage rheumatoid arthritis and other inflammatory joint diseases at the same,” said Dr. Rabbani.
“This discovery raises the potential of a blood test that can help diagnose both rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms,” Dr. Rabbani stated.
The first blood test to detect rheumatoid arthritis and osteoarthritis may soon be developed, according to a study published March 19 in Scientific Reports. The research findings could potentially lead to patients being tested for rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms.
Lead researcher Dr. Naila Rabbani, Reader of Experimental Systems Biology at the University of Warwick in Coventry United Kingdom, and colleagues have identified a biomarker that is linked to both rheumatoid arthritis and osteoarthritis. While there are established tests for rheumatoid arthritis, the newly identified biomarker could lead to one that can diagnose rheumatoid arthritis and osteoarthritis.
Initially, the research's focus was on citrullinated proteins, a biomarker suspected to be present in the blood of patients with early stage rheumatoid arthritis. It had previously been established that patients with rheumatoid arthritis have citrullinated protein antibodies, but it was not believed that the same held true for people with osteoarthritis. However, investigators found that there was an increase in citrullinated protein levels in both early-stage osteoarthritis and rheumatoid arthritis.
Study authors then produced an algorithm of 3 biomarkers, plasma/serum citrullinated protein, 4-hydroxyproline, and anti-cyclic citrullinated peptide. Based on this algorithm, the researchers found that with a single test they could potentially detect and discriminate between the major types of arthritis at the early stages, before joint damage has occurred.
“Detection of early stage osteoarthritis made the study very promising and we would have been satisfied with this only, but beyond this we also found we could detect and discriminate early-stage rheumatoid arthritis and other inflammatory joint diseases at the same,” said Dr. Rabbani.
“This discovery raises the potential of a blood test that can help diagnose both rheumatoid arthritis and osteoarthritis several years before the onset of physical symptoms,” Dr. Rabbani stated.
Suggested Reading
Ahmed U, Anwar A, Savage RS, et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci Rep. 2015 Mar 19;5:9259.
Suggested Reading
Ahmed U, Anwar A, Savage RS, et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci Rep. 2015 Mar 19;5:9259.
Twin Study Offers New Insights Into the Link Between Back Pain and Depression
Genetic factors help to explain the common association between low back pain and depression, according to a large study of twins published in the March issue of Pain.
Marina B. Pinheiro, MSc, and her research colleagues at the University of Sydney in Australia, analyzed data from the Murcia Twin Registry of nearly 2,150 Spanish twins. Questionnaire responses were assessed to determine whether participants with symptoms of depression had a higher prevalence of back pain. A series of statistical analyses were then performed to clarify genetic factors and to determine how an environment that is shared early on can contribute to the linkage between depression and back pain.
The results showed a significant association between symptoms of depression and low back pain. On the initial analysis, which considered the participants as individuals, the odds of having back pain were about 1.6 higher for those with symptoms of depression and anxiety.
For the analysis of twin pairs, which controlled for genetic and familial factors that could influence the relationship between depression and back pain, there was a 1.7 increase in odds. The association was even stronger—more than a 2.3 increase in odds of low back pain associated with depression and anxiety—on the analysis of dizygotic twins.
Upon further analysis of monozygotic twins, the association between symptoms of depression and low back pain disappeared. This suggested that the strong association found in non-identical twins resulted from the confounding effects of common genetic factors influencing both conditions.
Overall, the finding that the association between symptoms of depression and low back pain disappears after fully adjusting for genetics and familial confounders in identical twins suggests that genetics is the main confounder of the relationship between depression and back pain.
Suggested Reading
Pinheiro MB, Ferreira ML, Refshauge K, et al. Genetics and the environment affect the relationship between depression and low back pain: a co-twin control study of Spanish twins. Pain. 2015;156(3):496-503.
Genetic factors help to explain the common association between low back pain and depression, according to a large study of twins published in the March issue of Pain.
Marina B. Pinheiro, MSc, and her research colleagues at the University of Sydney in Australia, analyzed data from the Murcia Twin Registry of nearly 2,150 Spanish twins. Questionnaire responses were assessed to determine whether participants with symptoms of depression had a higher prevalence of back pain. A series of statistical analyses were then performed to clarify genetic factors and to determine how an environment that is shared early on can contribute to the linkage between depression and back pain.
The results showed a significant association between symptoms of depression and low back pain. On the initial analysis, which considered the participants as individuals, the odds of having back pain were about 1.6 higher for those with symptoms of depression and anxiety.
For the analysis of twin pairs, which controlled for genetic and familial factors that could influence the relationship between depression and back pain, there was a 1.7 increase in odds. The association was even stronger—more than a 2.3 increase in odds of low back pain associated with depression and anxiety—on the analysis of dizygotic twins.
Upon further analysis of monozygotic twins, the association between symptoms of depression and low back pain disappeared. This suggested that the strong association found in non-identical twins resulted from the confounding effects of common genetic factors influencing both conditions.
Overall, the finding that the association between symptoms of depression and low back pain disappears after fully adjusting for genetics and familial confounders in identical twins suggests that genetics is the main confounder of the relationship between depression and back pain.
Genetic factors help to explain the common association between low back pain and depression, according to a large study of twins published in the March issue of Pain.
Marina B. Pinheiro, MSc, and her research colleagues at the University of Sydney in Australia, analyzed data from the Murcia Twin Registry of nearly 2,150 Spanish twins. Questionnaire responses were assessed to determine whether participants with symptoms of depression had a higher prevalence of back pain. A series of statistical analyses were then performed to clarify genetic factors and to determine how an environment that is shared early on can contribute to the linkage between depression and back pain.
The results showed a significant association between symptoms of depression and low back pain. On the initial analysis, which considered the participants as individuals, the odds of having back pain were about 1.6 higher for those with symptoms of depression and anxiety.
For the analysis of twin pairs, which controlled for genetic and familial factors that could influence the relationship between depression and back pain, there was a 1.7 increase in odds. The association was even stronger—more than a 2.3 increase in odds of low back pain associated with depression and anxiety—on the analysis of dizygotic twins.
Upon further analysis of monozygotic twins, the association between symptoms of depression and low back pain disappeared. This suggested that the strong association found in non-identical twins resulted from the confounding effects of common genetic factors influencing both conditions.
Overall, the finding that the association between symptoms of depression and low back pain disappears after fully adjusting for genetics and familial confounders in identical twins suggests that genetics is the main confounder of the relationship between depression and back pain.
Suggested Reading
Pinheiro MB, Ferreira ML, Refshauge K, et al. Genetics and the environment affect the relationship between depression and low back pain: a co-twin control study of Spanish twins. Pain. 2015;156(3):496-503.
Suggested Reading
Pinheiro MB, Ferreira ML, Refshauge K, et al. Genetics and the environment affect the relationship between depression and low back pain: a co-twin control study of Spanish twins. Pain. 2015;156(3):496-503.
Common OTC Analgesic Proven Inefficacious for Treating Low Back Pain
Paracetamol (acetaminophen) is ineffective for the treatment of spinal pain and provides negligible benefits for low back pain or osteoarthritis of the hip or knee, its usage also may affect the liver, according to a study published March 31 in BMJ.
Lead study author Gustavo Machado, a PhD student from The George Institute for Global Health at the University of Sydney in Australia, and his research colleagues conducted a systematic review and meta-analysis to examine the efficacy and safety of paracetamol for lower back pain and osteoarthritis of the hip or knee. The reduction of pain intensity, improvement of disability, quality of life, safety, and patient adherence were analyzed in this trial.
The study included 13 randomized controlled trials that examined the effects of paracetamol use compared with placebo. Ten trials included 3,541 patients and evaluated the use of paracetamol for osteoarthritis of the hip or knee, and 3 trials included 1,825 patients that were evaluated for the use of paracetamol for lower back pain.
Among the study’s findings:
• For lower back pain, paracetamol had no effect and did not reduce disability or improve quality of life compared with placebo.
• Paracetamol use for osteoarthritis was shown to increase the likelihood of receiving abnormal results on liver function tests by almost 4 times compared with placebo.
• For osteoarthritis, the researchers found small, but not clinically important benefits in the reduction of pain and disability compared with placebo.
“This latest research, the most comprehensive systematic review of its kind, reaffirms this with an even larger, global patient base, and has for the first time also established that the effects of paracetamol for knee and hip osteoarthritis are too small to be of clinical importance,” Mr. Machado stated.
The study also found that adverse side effects varied across all of the trials. But no differences were found in the number of patients using paracetamol reporting these effects or being withdrawn from studies because of adverse events compared with those using a placebo. The adherence to treatment schedule rates was similar among patients taking paracetamol compared with those taking placebo.
“Use of paracetamol for low back pain or osteoarthritis was also shown to be associated with higher risk of liver toxicity in patients," Mr. Machado said. “Patients were nearly 4 times more likely to have abnormal results on liver function tests compared to those taking placebo pills.”
“World-wide, paracetamol is the most widely used over-the counter medicine for musculoskeletal conditions, so it is important to reconsider treatment recommendations given this new evidence,” stated Mr. Machado.
Suggested Reading
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015 Mar 31;350:h1225.
Paracetamol (acetaminophen) is ineffective for the treatment of spinal pain and provides negligible benefits for low back pain or osteoarthritis of the hip or knee, its usage also may affect the liver, according to a study published March 31 in BMJ.
Lead study author Gustavo Machado, a PhD student from The George Institute for Global Health at the University of Sydney in Australia, and his research colleagues conducted a systematic review and meta-analysis to examine the efficacy and safety of paracetamol for lower back pain and osteoarthritis of the hip or knee. The reduction of pain intensity, improvement of disability, quality of life, safety, and patient adherence were analyzed in this trial.
The study included 13 randomized controlled trials that examined the effects of paracetamol use compared with placebo. Ten trials included 3,541 patients and evaluated the use of paracetamol for osteoarthritis of the hip or knee, and 3 trials included 1,825 patients that were evaluated for the use of paracetamol for lower back pain.
Among the study’s findings:
• For lower back pain, paracetamol had no effect and did not reduce disability or improve quality of life compared with placebo.
• Paracetamol use for osteoarthritis was shown to increase the likelihood of receiving abnormal results on liver function tests by almost 4 times compared with placebo.
• For osteoarthritis, the researchers found small, but not clinically important benefits in the reduction of pain and disability compared with placebo.
“This latest research, the most comprehensive systematic review of its kind, reaffirms this with an even larger, global patient base, and has for the first time also established that the effects of paracetamol for knee and hip osteoarthritis are too small to be of clinical importance,” Mr. Machado stated.
The study also found that adverse side effects varied across all of the trials. But no differences were found in the number of patients using paracetamol reporting these effects or being withdrawn from studies because of adverse events compared with those using a placebo. The adherence to treatment schedule rates was similar among patients taking paracetamol compared with those taking placebo.
“Use of paracetamol for low back pain or osteoarthritis was also shown to be associated with higher risk of liver toxicity in patients," Mr. Machado said. “Patients were nearly 4 times more likely to have abnormal results on liver function tests compared to those taking placebo pills.”
“World-wide, paracetamol is the most widely used over-the counter medicine for musculoskeletal conditions, so it is important to reconsider treatment recommendations given this new evidence,” stated Mr. Machado.
Paracetamol (acetaminophen) is ineffective for the treatment of spinal pain and provides negligible benefits for low back pain or osteoarthritis of the hip or knee, its usage also may affect the liver, according to a study published March 31 in BMJ.
Lead study author Gustavo Machado, a PhD student from The George Institute for Global Health at the University of Sydney in Australia, and his research colleagues conducted a systematic review and meta-analysis to examine the efficacy and safety of paracetamol for lower back pain and osteoarthritis of the hip or knee. The reduction of pain intensity, improvement of disability, quality of life, safety, and patient adherence were analyzed in this trial.
The study included 13 randomized controlled trials that examined the effects of paracetamol use compared with placebo. Ten trials included 3,541 patients and evaluated the use of paracetamol for osteoarthritis of the hip or knee, and 3 trials included 1,825 patients that were evaluated for the use of paracetamol for lower back pain.
Among the study’s findings:
• For lower back pain, paracetamol had no effect and did not reduce disability or improve quality of life compared with placebo.
• Paracetamol use for osteoarthritis was shown to increase the likelihood of receiving abnormal results on liver function tests by almost 4 times compared with placebo.
• For osteoarthritis, the researchers found small, but not clinically important benefits in the reduction of pain and disability compared with placebo.
“This latest research, the most comprehensive systematic review of its kind, reaffirms this with an even larger, global patient base, and has for the first time also established that the effects of paracetamol for knee and hip osteoarthritis are too small to be of clinical importance,” Mr. Machado stated.
The study also found that adverse side effects varied across all of the trials. But no differences were found in the number of patients using paracetamol reporting these effects or being withdrawn from studies because of adverse events compared with those using a placebo. The adherence to treatment schedule rates was similar among patients taking paracetamol compared with those taking placebo.
“Use of paracetamol for low back pain or osteoarthritis was also shown to be associated with higher risk of liver toxicity in patients," Mr. Machado said. “Patients were nearly 4 times more likely to have abnormal results on liver function tests compared to those taking placebo pills.”
“World-wide, paracetamol is the most widely used over-the counter medicine for musculoskeletal conditions, so it is important to reconsider treatment recommendations given this new evidence,” stated Mr. Machado.
Suggested Reading
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015 Mar 31;350:h1225.
Suggested Reading
Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015 Mar 31;350:h1225.
Phone Counseling Bolsters Recovery and Reduces Pain Following Spinal Surgery
Participating in a short series of phone conversations with trained counselors can substantially boost recovery and reduce pain in patients after spinal surgery, according to a study published online ahead of print March 28 in Archives of Physical Medicine and Rehabilitation.
The phone calls were designed to enhance standard pre- and post-operative care by reinforcing the value of continuing with physical therapy and back-strengthening exercise regimens.
“Phone counseling appears to be an easy, low-cost strategy that yields meaningful results by improving patient engagement in physical therapy and at-home exercise programs that are so vital for their recovery,” said lead study author Richard Skolasky Jr., ScD, Associate Professor of Orthopedic Surgery at the Johns Hopkins University School of Medicine in Baltimore.
The study included 122 patients ages 46 to 72, who underwent surgery at Johns Hopkins University between 2009 and 2012 to correct spinal stenosis. Each patient was assigned either home exercise programs or physical therapy to help accelerate their recovery time. About half of the patients also received a series of phone counseling sessions from a trained spinal surgery counselor to discuss the importance of exercise in their recovery. The first and most detailed phone session took place a few weeks before the patients had their surgeries. Two follow-up sessions occurred at 6 weeks and at 3 months after the operation was performed.
The study found that patients who received phone calls participated in physical therapy and home exercise at higher rates, and had less pain and less disability 6 months after their surgery, compared with the standard-approach group. Six months after surgery, 74% of patients who received phone counseling experienced significant improvements on standard measures of physical functioning and self-reported measures of pain, compared with 41% of people who did not receive phone calls.
“Modern orthopedic science has made great strides in surgical techniques to correct spinal deformities and achieved significant progress in developing physical therapies that boost the benefits of surgery, but we have not been all that good at motivating and engaging patients to partake in such post-surgical recovery programs,” said co-investigator Stephen Wegener, PhD, Associate Professor of Physical Medicine and Rehabilitation at Johns Hopkins University.
“The findings of our research suggest we may have found a way to add that missing ingredient that draws patients to be more active participants in their physical rehabilitation and recovery,” stated Dr. Wegener.
Suggested Reading
Skolasky RL, Maggard AM, Li D, et al. Health behavior change counseling in surgery for degenerative lumbar spinal stenosis. part I: improvement in rehabilitation engagement and functional outcomes. Arch Phys Med Rehabil. 2015 Mar 28 [Epub ahead of print].
Participating in a short series of phone conversations with trained counselors can substantially boost recovery and reduce pain in patients after spinal surgery, according to a study published online ahead of print March 28 in Archives of Physical Medicine and Rehabilitation.
The phone calls were designed to enhance standard pre- and post-operative care by reinforcing the value of continuing with physical therapy and back-strengthening exercise regimens.
“Phone counseling appears to be an easy, low-cost strategy that yields meaningful results by improving patient engagement in physical therapy and at-home exercise programs that are so vital for their recovery,” said lead study author Richard Skolasky Jr., ScD, Associate Professor of Orthopedic Surgery at the Johns Hopkins University School of Medicine in Baltimore.
The study included 122 patients ages 46 to 72, who underwent surgery at Johns Hopkins University between 2009 and 2012 to correct spinal stenosis. Each patient was assigned either home exercise programs or physical therapy to help accelerate their recovery time. About half of the patients also received a series of phone counseling sessions from a trained spinal surgery counselor to discuss the importance of exercise in their recovery. The first and most detailed phone session took place a few weeks before the patients had their surgeries. Two follow-up sessions occurred at 6 weeks and at 3 months after the operation was performed.
The study found that patients who received phone calls participated in physical therapy and home exercise at higher rates, and had less pain and less disability 6 months after their surgery, compared with the standard-approach group. Six months after surgery, 74% of patients who received phone counseling experienced significant improvements on standard measures of physical functioning and self-reported measures of pain, compared with 41% of people who did not receive phone calls.
“Modern orthopedic science has made great strides in surgical techniques to correct spinal deformities and achieved significant progress in developing physical therapies that boost the benefits of surgery, but we have not been all that good at motivating and engaging patients to partake in such post-surgical recovery programs,” said co-investigator Stephen Wegener, PhD, Associate Professor of Physical Medicine and Rehabilitation at Johns Hopkins University.
“The findings of our research suggest we may have found a way to add that missing ingredient that draws patients to be more active participants in their physical rehabilitation and recovery,” stated Dr. Wegener.
Participating in a short series of phone conversations with trained counselors can substantially boost recovery and reduce pain in patients after spinal surgery, according to a study published online ahead of print March 28 in Archives of Physical Medicine and Rehabilitation.
The phone calls were designed to enhance standard pre- and post-operative care by reinforcing the value of continuing with physical therapy and back-strengthening exercise regimens.
“Phone counseling appears to be an easy, low-cost strategy that yields meaningful results by improving patient engagement in physical therapy and at-home exercise programs that are so vital for their recovery,” said lead study author Richard Skolasky Jr., ScD, Associate Professor of Orthopedic Surgery at the Johns Hopkins University School of Medicine in Baltimore.
The study included 122 patients ages 46 to 72, who underwent surgery at Johns Hopkins University between 2009 and 2012 to correct spinal stenosis. Each patient was assigned either home exercise programs or physical therapy to help accelerate their recovery time. About half of the patients also received a series of phone counseling sessions from a trained spinal surgery counselor to discuss the importance of exercise in their recovery. The first and most detailed phone session took place a few weeks before the patients had their surgeries. Two follow-up sessions occurred at 6 weeks and at 3 months after the operation was performed.
The study found that patients who received phone calls participated in physical therapy and home exercise at higher rates, and had less pain and less disability 6 months after their surgery, compared with the standard-approach group. Six months after surgery, 74% of patients who received phone counseling experienced significant improvements on standard measures of physical functioning and self-reported measures of pain, compared with 41% of people who did not receive phone calls.
“Modern orthopedic science has made great strides in surgical techniques to correct spinal deformities and achieved significant progress in developing physical therapies that boost the benefits of surgery, but we have not been all that good at motivating and engaging patients to partake in such post-surgical recovery programs,” said co-investigator Stephen Wegener, PhD, Associate Professor of Physical Medicine and Rehabilitation at Johns Hopkins University.
“The findings of our research suggest we may have found a way to add that missing ingredient that draws patients to be more active participants in their physical rehabilitation and recovery,” stated Dr. Wegener.
Suggested Reading
Skolasky RL, Maggard AM, Li D, et al. Health behavior change counseling in surgery for degenerative lumbar spinal stenosis. part I: improvement in rehabilitation engagement and functional outcomes. Arch Phys Med Rehabil. 2015 Mar 28 [Epub ahead of print].
Suggested Reading
Skolasky RL, Maggard AM, Li D, et al. Health behavior change counseling in surgery for degenerative lumbar spinal stenosis. part I: improvement in rehabilitation engagement and functional outcomes. Arch Phys Med Rehabil. 2015 Mar 28 [Epub ahead of print].
21st-Century Patient Collections: Implement a Point-of-Service Collections Program Now
An 8-surgeon group in the Southeast had a history of high patient receivables, the result of a long-held culture of “We’ll submit to your insurance and bill you after insurance pays.”
The billing and collections staff worked in the basement—far away and out of sight of the patients who showed up for their postoperative visits owing big bucks.
In a flash of wisdom, the administrator agreed to move the patient-balance collector into a converted closet near the check-out area, and provided the information, tools, and training that enabled her to speak with patients about their balances when they came in for an appointment. In her first month in this role and location, this employee collected more than her annual salary from patients.
It Takes a Program
This is one of our favorite client success stories, and it illustrates a key point: point-of-service (POS) collections do not have to be complicated. But the process does have to be deliberate and coordinated. Practices cannot simply update the financial policy and hope the staff members magically begin collecting. If this is your strategy, we promise that it will fail.
Successful POS collecting requires a program approach. And this approach starts at the front-end of the billing cycle, not “after insurance pays.”
POS collections have never been more important. Health insurance exchanges and payers are increasing deductibles and coinsurances. Physicians are opting out of network. Given these realities, POS collections are vital to your cash flow and effective receivables management.
If you are starting practice, you have a perfect opportunity to open with POS collecting in place. A solo surgeon whom we set up in practice did so, and has collected up-front for office services, scans, and surgeries from his first day in practice. Today, the practice’s only outstanding patient receivables are those of patients on payment plans—and these are less than 1% of total accounts receivable.
We also converted the “after insurance pays” philosophy of a surgeon in the South, implementing both POS collections and surgical deposits. In the first month, his patient payments increased by 40%. Another solo orthopedist reported an increased take-home salary of $90,000 in the first year after we helped his staff collect surgery deposits.
Six POS Program Elements
In 30 years of implementing or training staff to implement POS collections, we have come to recognize the following 6 key elements to include in your program approach: Policies + Procedures + Technology + Training + Monitoring + Coaching.
At a high level, here are the actions your practice will need to take:
1. Update the financial policy with 1 written standard for all physicians.
2. Develop granular procedures driven by the policy; these are the “how-tos” that enable the staff to collect successfully.
3. Implement new technologies, such as cost estimators, recurring payments, and online bill pay.
4. Schedule formal training to ensure that staff members know how to ask for money. (Do not assume they are, can, or will without training.)
5. Measure and monitor the outcome of patient collections and staff performance.
6. Provide ongoing coaching and oversight to maintain motivation and skills.
A blueprint for addressing each one of these actions follows.
1. Update the financial policy
The policy is the set of expectations on which to build all procedures and training. Dust off this document, and review it as a group with the practice administrator. First, strike old language that says the patient will be balance-billed, or will only be asked in the office for his visit copay. Next, strive for clarity. “You will be asked to pay your financial responsibility at the time of service,” really says nothing. Instead, the policy should be direct:
If you are recommended for surgery, our staff will calculate your coinsurance and unmet deductible amounts: 50% of this amount will be collected as a surgery deposit, and the remaining 50% is due on or before the day of surgery. Payment plans are available.
For office visits and services, break down the policy by coverage type. We find that a table such as the one shown makes expectations clear.
Finally, strive for 1 standard policy for all providers. If every provider is allowed to create his or her own set of collection policies, the practice is setting staff up for complexity overload, and collections will suffer.
2. Develop granular procedures
Few practices take the time to translate the financial policy into written procedures that can be followed by staff. The policy establishes the rules, but the procedures tell staff what to do to implement those rules. For instance:
Create a “POS Playbook” that contains information such as procedures, cost-quotation worksheets, US Poverty guidelines1, and financing brochures. As old-school as it sounds, a 3-ring binder is great for this information, and makes information access and updates easy.
3. Implement collection technologies
Modern practices use inexpensive (and often free) tools that increase patient convenience and staff efficiency. Implement at least 2 of these useful technologies and watch your POS collections increase:
Reports from your practice management system (PMS). Use the technology you already have. There are 2 standard reports in your PMS or clearinghouse that give front-desk staff the data to ask patients for money. Eligibility status and past-due balance reports indicate amounts owed, unmet deductibles, and the ineligible patients they can collect from when they come in for their appointment.
Online cost estimators. These free, online tools are offered by payers and provide staff with real-time data about a patient’s unmet deductible and coinsurance. When staff members enter Current Procedural Terminology (CPT) codes and the patient’s benefit information into the online cost estimator, they can access valuable information. Many insurance plans offer cost estimators on their web sites. Others deliver the data through statewide or regional portals, such as Availity (www.availity.com). The accuracy of cost-estimator data can vary by region and depends on the data links with payers. Ask your team to evaluate which estimators are best for you based on your payer mix.
Online bill pay. Everyone appreciates the convenience of paying bills online. Most patient portals offer this feature. If yours does not or you do not have a portal, you can offer PayPal (www.paypal.com) on your practice website, or use a system such as Intuit Health (www.intuithealth.com).
Recurring billing. Recurring billing is how you pay for services, such as Netflix, Pandora, or your gym membership: it is automatically billed to a credit card each month. Offer this option to patients as a payment plan method, and staff will no longer need to send costly statements, post monthly check payments, or follow up when a patient is delinquent. Plus, it guarantees payment every month; patients can no longer say, “I forgot.”
TransFirst (www.transfirstassociation.com) and a-claim (www.a-claim.com) offer recurring billing through a “virtual terminal” that staff logs in to at checkout, or during the preprocedure patient counseling process. Both vendors also offer the option of automatically charging a patient’s credit card after their insurance pays, speeding patient account pay-off and negating the need for statements.
Real-time collections scripts based on payer rules. Patient Access, offered by Availity, combines real-time payer data with financial policies that are entered during set-up to create instant, patient-specific scripts that staff members read to the patient in front of them.
4. Schedule formal training
Just because someone can collect a copay does not mean he or she is comfortable with or capable of asking patients for past-due balances, surgical deposits, or large coinsurances. It is the rare staff person who is a “natural” at asking patients for money in a polished and professional manner.
That’s why training staff how to ask patients for money is vital. A front-office supervisor or manager should conduct several training sessions to cover policies and procedures. Training materials should include talking points and scenarios for collecting for office services and past-due balances, and calculating what patients owe, using technology tools. Use role-playing to ensure staff can explain payment plan options and how to apply for patient financing or financial assistance.
Few practices can skip this part of the POS program and still be successful. If your manager or supervisor is not capable of training, it is worth the investment to hire an outside expert. Without thorough training, staff efforts will be suboptimal or, at worst, fail because the staff members will not know how or what to collect.
5. Measure and monitor the outcome
The Hawthorne effect is a psychological phenomenon that says people perform better and make more positive changes as a result of increased attention.2 In other words, staff members will perform better, and collect more, if they know someone is paying attention. Trust us on this one.
Employees respect what management inspects. So even if the implementation of POS collections has been a big success, do not take your eyes off the ball.
Stop by the front desk or surgery coordinator’s office a few times a month and ask how much has been collected. Randomly review daily over-the-counter collections logs. And always put POS collections performance on the monthly partner meeting agenda; review a graph that shows monthly collections at checkout and surgery deposits. Keeping tabs on performance enables the practice to take action quickly when collections drop, and before that decline becomes acute.
6. Provide ongoing coaching and oversight
Most practices train once, then wonder why staff motivation (and collections too) fall off after a while. Like that new couch you bought: it was all you could talk about the week after it was delivered. Now, it is only a comfy place to sit. It is the same with collections efforts. When the newness wears off, staff motivation does too, and training principles can be forgotten. That’s human nature. Conduct role-playing in staff meetings each quarter and discuss best practices for handling patient objections. Encourage peer-to-peer observation and coaching to address knowledge gaps and missed collection opportunities. Ongoing training and coaching will tease out training needs and boost your team’s collection confidence and success.
1. 2015 Poverty Guidelines. US Department of Health and Human Services website. http://aspe.hhs.gov/poverty/15poverty.cfm. Accessed March 25, 2015.
2. The Hawthorne effect. The Economist website. http://www.economist.com/node/12510632. Published November 3, 2008. Accessed March 25, 2015.
An 8-surgeon group in the Southeast had a history of high patient receivables, the result of a long-held culture of “We’ll submit to your insurance and bill you after insurance pays.”
The billing and collections staff worked in the basement—far away and out of sight of the patients who showed up for their postoperative visits owing big bucks.
In a flash of wisdom, the administrator agreed to move the patient-balance collector into a converted closet near the check-out area, and provided the information, tools, and training that enabled her to speak with patients about their balances when they came in for an appointment. In her first month in this role and location, this employee collected more than her annual salary from patients.
It Takes a Program
This is one of our favorite client success stories, and it illustrates a key point: point-of-service (POS) collections do not have to be complicated. But the process does have to be deliberate and coordinated. Practices cannot simply update the financial policy and hope the staff members magically begin collecting. If this is your strategy, we promise that it will fail.
Successful POS collecting requires a program approach. And this approach starts at the front-end of the billing cycle, not “after insurance pays.”
POS collections have never been more important. Health insurance exchanges and payers are increasing deductibles and coinsurances. Physicians are opting out of network. Given these realities, POS collections are vital to your cash flow and effective receivables management.
If you are starting practice, you have a perfect opportunity to open with POS collecting in place. A solo surgeon whom we set up in practice did so, and has collected up-front for office services, scans, and surgeries from his first day in practice. Today, the practice’s only outstanding patient receivables are those of patients on payment plans—and these are less than 1% of total accounts receivable.
We also converted the “after insurance pays” philosophy of a surgeon in the South, implementing both POS collections and surgical deposits. In the first month, his patient payments increased by 40%. Another solo orthopedist reported an increased take-home salary of $90,000 in the first year after we helped his staff collect surgery deposits.
Six POS Program Elements
In 30 years of implementing or training staff to implement POS collections, we have come to recognize the following 6 key elements to include in your program approach: Policies + Procedures + Technology + Training + Monitoring + Coaching.
At a high level, here are the actions your practice will need to take:
1. Update the financial policy with 1 written standard for all physicians.
2. Develop granular procedures driven by the policy; these are the “how-tos” that enable the staff to collect successfully.
3. Implement new technologies, such as cost estimators, recurring payments, and online bill pay.
4. Schedule formal training to ensure that staff members know how to ask for money. (Do not assume they are, can, or will without training.)
5. Measure and monitor the outcome of patient collections and staff performance.
6. Provide ongoing coaching and oversight to maintain motivation and skills.
A blueprint for addressing each one of these actions follows.
1. Update the financial policy
The policy is the set of expectations on which to build all procedures and training. Dust off this document, and review it as a group with the practice administrator. First, strike old language that says the patient will be balance-billed, or will only be asked in the office for his visit copay. Next, strive for clarity. “You will be asked to pay your financial responsibility at the time of service,” really says nothing. Instead, the policy should be direct:
If you are recommended for surgery, our staff will calculate your coinsurance and unmet deductible amounts: 50% of this amount will be collected as a surgery deposit, and the remaining 50% is due on or before the day of surgery. Payment plans are available.
For office visits and services, break down the policy by coverage type. We find that a table such as the one shown makes expectations clear.
Finally, strive for 1 standard policy for all providers. If every provider is allowed to create his or her own set of collection policies, the practice is setting staff up for complexity overload, and collections will suffer.
2. Develop granular procedures
Few practices take the time to translate the financial policy into written procedures that can be followed by staff. The policy establishes the rules, but the procedures tell staff what to do to implement those rules. For instance:
Create a “POS Playbook” that contains information such as procedures, cost-quotation worksheets, US Poverty guidelines1, and financing brochures. As old-school as it sounds, a 3-ring binder is great for this information, and makes information access and updates easy.
3. Implement collection technologies
Modern practices use inexpensive (and often free) tools that increase patient convenience and staff efficiency. Implement at least 2 of these useful technologies and watch your POS collections increase:
Reports from your practice management system (PMS). Use the technology you already have. There are 2 standard reports in your PMS or clearinghouse that give front-desk staff the data to ask patients for money. Eligibility status and past-due balance reports indicate amounts owed, unmet deductibles, and the ineligible patients they can collect from when they come in for their appointment.
Online cost estimators. These free, online tools are offered by payers and provide staff with real-time data about a patient’s unmet deductible and coinsurance. When staff members enter Current Procedural Terminology (CPT) codes and the patient’s benefit information into the online cost estimator, they can access valuable information. Many insurance plans offer cost estimators on their web sites. Others deliver the data through statewide or regional portals, such as Availity (www.availity.com). The accuracy of cost-estimator data can vary by region and depends on the data links with payers. Ask your team to evaluate which estimators are best for you based on your payer mix.
Online bill pay. Everyone appreciates the convenience of paying bills online. Most patient portals offer this feature. If yours does not or you do not have a portal, you can offer PayPal (www.paypal.com) on your practice website, or use a system such as Intuit Health (www.intuithealth.com).
Recurring billing. Recurring billing is how you pay for services, such as Netflix, Pandora, or your gym membership: it is automatically billed to a credit card each month. Offer this option to patients as a payment plan method, and staff will no longer need to send costly statements, post monthly check payments, or follow up when a patient is delinquent. Plus, it guarantees payment every month; patients can no longer say, “I forgot.”
TransFirst (www.transfirstassociation.com) and a-claim (www.a-claim.com) offer recurring billing through a “virtual terminal” that staff logs in to at checkout, or during the preprocedure patient counseling process. Both vendors also offer the option of automatically charging a patient’s credit card after their insurance pays, speeding patient account pay-off and negating the need for statements.
Real-time collections scripts based on payer rules. Patient Access, offered by Availity, combines real-time payer data with financial policies that are entered during set-up to create instant, patient-specific scripts that staff members read to the patient in front of them.
4. Schedule formal training
Just because someone can collect a copay does not mean he or she is comfortable with or capable of asking patients for past-due balances, surgical deposits, or large coinsurances. It is the rare staff person who is a “natural” at asking patients for money in a polished and professional manner.
That’s why training staff how to ask patients for money is vital. A front-office supervisor or manager should conduct several training sessions to cover policies and procedures. Training materials should include talking points and scenarios for collecting for office services and past-due balances, and calculating what patients owe, using technology tools. Use role-playing to ensure staff can explain payment plan options and how to apply for patient financing or financial assistance.
Few practices can skip this part of the POS program and still be successful. If your manager or supervisor is not capable of training, it is worth the investment to hire an outside expert. Without thorough training, staff efforts will be suboptimal or, at worst, fail because the staff members will not know how or what to collect.
5. Measure and monitor the outcome
The Hawthorne effect is a psychological phenomenon that says people perform better and make more positive changes as a result of increased attention.2 In other words, staff members will perform better, and collect more, if they know someone is paying attention. Trust us on this one.
Employees respect what management inspects. So even if the implementation of POS collections has been a big success, do not take your eyes off the ball.
Stop by the front desk or surgery coordinator’s office a few times a month and ask how much has been collected. Randomly review daily over-the-counter collections logs. And always put POS collections performance on the monthly partner meeting agenda; review a graph that shows monthly collections at checkout and surgery deposits. Keeping tabs on performance enables the practice to take action quickly when collections drop, and before that decline becomes acute.
6. Provide ongoing coaching and oversight
Most practices train once, then wonder why staff motivation (and collections too) fall off after a while. Like that new couch you bought: it was all you could talk about the week after it was delivered. Now, it is only a comfy place to sit. It is the same with collections efforts. When the newness wears off, staff motivation does too, and training principles can be forgotten. That’s human nature. Conduct role-playing in staff meetings each quarter and discuss best practices for handling patient objections. Encourage peer-to-peer observation and coaching to address knowledge gaps and missed collection opportunities. Ongoing training and coaching will tease out training needs and boost your team’s collection confidence and success.
An 8-surgeon group in the Southeast had a history of high patient receivables, the result of a long-held culture of “We’ll submit to your insurance and bill you after insurance pays.”
The billing and collections staff worked in the basement—far away and out of sight of the patients who showed up for their postoperative visits owing big bucks.
In a flash of wisdom, the administrator agreed to move the patient-balance collector into a converted closet near the check-out area, and provided the information, tools, and training that enabled her to speak with patients about their balances when they came in for an appointment. In her first month in this role and location, this employee collected more than her annual salary from patients.
It Takes a Program
This is one of our favorite client success stories, and it illustrates a key point: point-of-service (POS) collections do not have to be complicated. But the process does have to be deliberate and coordinated. Practices cannot simply update the financial policy and hope the staff members magically begin collecting. If this is your strategy, we promise that it will fail.
Successful POS collecting requires a program approach. And this approach starts at the front-end of the billing cycle, not “after insurance pays.”
POS collections have never been more important. Health insurance exchanges and payers are increasing deductibles and coinsurances. Physicians are opting out of network. Given these realities, POS collections are vital to your cash flow and effective receivables management.
If you are starting practice, you have a perfect opportunity to open with POS collecting in place. A solo surgeon whom we set up in practice did so, and has collected up-front for office services, scans, and surgeries from his first day in practice. Today, the practice’s only outstanding patient receivables are those of patients on payment plans—and these are less than 1% of total accounts receivable.
We also converted the “after insurance pays” philosophy of a surgeon in the South, implementing both POS collections and surgical deposits. In the first month, his patient payments increased by 40%. Another solo orthopedist reported an increased take-home salary of $90,000 in the first year after we helped his staff collect surgery deposits.
Six POS Program Elements
In 30 years of implementing or training staff to implement POS collections, we have come to recognize the following 6 key elements to include in your program approach: Policies + Procedures + Technology + Training + Monitoring + Coaching.
At a high level, here are the actions your practice will need to take:
1. Update the financial policy with 1 written standard for all physicians.
2. Develop granular procedures driven by the policy; these are the “how-tos” that enable the staff to collect successfully.
3. Implement new technologies, such as cost estimators, recurring payments, and online bill pay.
4. Schedule formal training to ensure that staff members know how to ask for money. (Do not assume they are, can, or will without training.)
5. Measure and monitor the outcome of patient collections and staff performance.
6. Provide ongoing coaching and oversight to maintain motivation and skills.
A blueprint for addressing each one of these actions follows.
1. Update the financial policy
The policy is the set of expectations on which to build all procedures and training. Dust off this document, and review it as a group with the practice administrator. First, strike old language that says the patient will be balance-billed, or will only be asked in the office for his visit copay. Next, strive for clarity. “You will be asked to pay your financial responsibility at the time of service,” really says nothing. Instead, the policy should be direct:
If you are recommended for surgery, our staff will calculate your coinsurance and unmet deductible amounts: 50% of this amount will be collected as a surgery deposit, and the remaining 50% is due on or before the day of surgery. Payment plans are available.
For office visits and services, break down the policy by coverage type. We find that a table such as the one shown makes expectations clear.
Finally, strive for 1 standard policy for all providers. If every provider is allowed to create his or her own set of collection policies, the practice is setting staff up for complexity overload, and collections will suffer.
2. Develop granular procedures
Few practices take the time to translate the financial policy into written procedures that can be followed by staff. The policy establishes the rules, but the procedures tell staff what to do to implement those rules. For instance:
Create a “POS Playbook” that contains information such as procedures, cost-quotation worksheets, US Poverty guidelines1, and financing brochures. As old-school as it sounds, a 3-ring binder is great for this information, and makes information access and updates easy.
3. Implement collection technologies
Modern practices use inexpensive (and often free) tools that increase patient convenience and staff efficiency. Implement at least 2 of these useful technologies and watch your POS collections increase:
Reports from your practice management system (PMS). Use the technology you already have. There are 2 standard reports in your PMS or clearinghouse that give front-desk staff the data to ask patients for money. Eligibility status and past-due balance reports indicate amounts owed, unmet deductibles, and the ineligible patients they can collect from when they come in for their appointment.
Online cost estimators. These free, online tools are offered by payers and provide staff with real-time data about a patient’s unmet deductible and coinsurance. When staff members enter Current Procedural Terminology (CPT) codes and the patient’s benefit information into the online cost estimator, they can access valuable information. Many insurance plans offer cost estimators on their web sites. Others deliver the data through statewide or regional portals, such as Availity (www.availity.com). The accuracy of cost-estimator data can vary by region and depends on the data links with payers. Ask your team to evaluate which estimators are best for you based on your payer mix.
Online bill pay. Everyone appreciates the convenience of paying bills online. Most patient portals offer this feature. If yours does not or you do not have a portal, you can offer PayPal (www.paypal.com) on your practice website, or use a system such as Intuit Health (www.intuithealth.com).
Recurring billing. Recurring billing is how you pay for services, such as Netflix, Pandora, or your gym membership: it is automatically billed to a credit card each month. Offer this option to patients as a payment plan method, and staff will no longer need to send costly statements, post monthly check payments, or follow up when a patient is delinquent. Plus, it guarantees payment every month; patients can no longer say, “I forgot.”
TransFirst (www.transfirstassociation.com) and a-claim (www.a-claim.com) offer recurring billing through a “virtual terminal” that staff logs in to at checkout, or during the preprocedure patient counseling process. Both vendors also offer the option of automatically charging a patient’s credit card after their insurance pays, speeding patient account pay-off and negating the need for statements.
Real-time collections scripts based on payer rules. Patient Access, offered by Availity, combines real-time payer data with financial policies that are entered during set-up to create instant, patient-specific scripts that staff members read to the patient in front of them.
4. Schedule formal training
Just because someone can collect a copay does not mean he or she is comfortable with or capable of asking patients for past-due balances, surgical deposits, or large coinsurances. It is the rare staff person who is a “natural” at asking patients for money in a polished and professional manner.
That’s why training staff how to ask patients for money is vital. A front-office supervisor or manager should conduct several training sessions to cover policies and procedures. Training materials should include talking points and scenarios for collecting for office services and past-due balances, and calculating what patients owe, using technology tools. Use role-playing to ensure staff can explain payment plan options and how to apply for patient financing or financial assistance.
Few practices can skip this part of the POS program and still be successful. If your manager or supervisor is not capable of training, it is worth the investment to hire an outside expert. Without thorough training, staff efforts will be suboptimal or, at worst, fail because the staff members will not know how or what to collect.
5. Measure and monitor the outcome
The Hawthorne effect is a psychological phenomenon that says people perform better and make more positive changes as a result of increased attention.2 In other words, staff members will perform better, and collect more, if they know someone is paying attention. Trust us on this one.
Employees respect what management inspects. So even if the implementation of POS collections has been a big success, do not take your eyes off the ball.
Stop by the front desk or surgery coordinator’s office a few times a month and ask how much has been collected. Randomly review daily over-the-counter collections logs. And always put POS collections performance on the monthly partner meeting agenda; review a graph that shows monthly collections at checkout and surgery deposits. Keeping tabs on performance enables the practice to take action quickly when collections drop, and before that decline becomes acute.
6. Provide ongoing coaching and oversight
Most practices train once, then wonder why staff motivation (and collections too) fall off after a while. Like that new couch you bought: it was all you could talk about the week after it was delivered. Now, it is only a comfy place to sit. It is the same with collections efforts. When the newness wears off, staff motivation does too, and training principles can be forgotten. That’s human nature. Conduct role-playing in staff meetings each quarter and discuss best practices for handling patient objections. Encourage peer-to-peer observation and coaching to address knowledge gaps and missed collection opportunities. Ongoing training and coaching will tease out training needs and boost your team’s collection confidence and success.
1. 2015 Poverty Guidelines. US Department of Health and Human Services website. http://aspe.hhs.gov/poverty/15poverty.cfm. Accessed March 25, 2015.
2. The Hawthorne effect. The Economist website. http://www.economist.com/node/12510632. Published November 3, 2008. Accessed March 25, 2015.
1. 2015 Poverty Guidelines. US Department of Health and Human Services website. http://aspe.hhs.gov/poverty/15poverty.cfm. Accessed March 25, 2015.
2. The Hawthorne effect. The Economist website. http://www.economist.com/node/12510632. Published November 3, 2008. Accessed March 25, 2015.
Total Hip Arthroplasty After Contralateral Hip Disarticulation: A Challenging “Simple Primary”
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
Patients with lower limb amputation have a high incidence of hip and knee osteoarthritis (OA) in the residual limb as well as the contralateral limb. A radical surgery, hip disarticulation is generally performed in younger patients after malignancy or trauma. Compliance is poor with existing prostheses, resulting in increased dependency on and use of the remaining sound limb.
In this case report, a crutch-walking 51-year-old woman presented with severe left hip arthritis 25 years after a right hip disarticulation. She underwent total hip arthroplasty (THA), a challenging procedure in a person without a contralateral hip joint. The many complex technical considerations associated with her THA included precise perioperative planning, the selection of appropriate prostheses and bearing surfaces, and the preoperative and intraoperative assessment of limb length and offset. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman presented to our service with a 3-year history of debilitating left hip pain. Twenty-five years earlier, she had been diagnosed with synovial sarcoma of the right knee and underwent limb-sparing surgery, followed by a true hip disarticulation performed for local recurrence. After her surgery, she declined the use of a prosthesis and mobilized with the use of 2 crutches. She has remained otherwise healthy and active, and runs her own business, which involves some lifting and carrying of objects. During the 3 years prior to presentation, she developed progressively debilitating left hip and groin pain, which radiated to the medial aspect of her left knee. Her mobilization distance had reduced to a few hundred meters, and she experienced significant night pain, and start-up pain. Activity modification, weight loss, and nonsteroidal anti-inflammatory medication afforded no relief. She denied any back pain or radicular symptoms.
Clinical examination showed a well-healed scar and pristine stump under her right hemipelvis. Passive range of movement of her left hip was painful for all movements, reduced at flexion (90º) and internal (10º) and external rotation (5º). Examination of her left knee was normal, with a full range of movement and no joint-line tenderness. A high body mass index (>30) was noted. Radiographic imaging confirmed significant OA of the hip joint (Figure 1). Informed consent was obtained for THA. The implants were selected—an uncemented collared Corail Stem (DePuy, Warsaw, Indiana) with a stainless steel dual mobility (DM) Novae SunFit acetabular cup (Serf, Decines, France), with bearing components of ceramic on polyethylene. A preoperative computed tomography (CT) scan of the left hip was performed (Figure 2) to aid templating, which was accomplished using plain films and CT images, with reference to the proximal femur for deciding level of neck cut, planning stem size, and optimizing length and offset, while determining cup size, depth, inclination, and height for the acetabular component.
Prior to surgery, the patient was positioned in the lateral decubitus position, using folded pillows under the medial aspect of her left proximal and distal thigh in lieu of her amputated limb. Pillows were secured to the table with elastic bandage tape. Standard pubic symphysis, lumbosacral, and midthoracic padded bolsters stabilized the pelvis in the normal fashion, with additional elastic bandage tape to further secure the pelvis brim to the table and reduce intraoperative motion. A posterior approach was used. A capsulotomy was performed with the hip in extension and slight abduction, with meticulous preservation of the capsule as the guide for the patient’s native length and offset. Reaming of the acetabulum was line to line, with insertion of an uncemented DM metal-back press-fit hydroxyapatite-coated shell placed in a standard fashion parallel with the transverse acetabular ligament, as described by Archbold and colleagues.1 The femur was sequentially reamed with broaches until press fit was achieved, and a calcar reamer was used to optimize interface with the collared implant. The surgeon’s standard 4 clinical tests were performed with trial implants after reduction to gauge hip tension, length, and offset. These tests are positive shuck test with hip and knee extension, lack of shuck in hip extension with knee flexion, lack of kick sign in hip extension and knee flexion, and palpation of gluteus medius belly to determine tension. Finally, with the hip returned to the extended and slightly abducted position, the capsule was tested for length and tension. The definitive stem implant was inserted, final testing with trial heads was repeated prior to definitive neck length and head selection, and final reduction was performed. A layered closure was performed, after generous washout. Pillows were taped together and positioned from the bed railing across the midline of the bed to prevent abduction, in the fashion of an abduction pillow.
The patient was mobilized the day after surgery and permitted full weight-bearing. Recovery was uneventful, and the patient returned to work within 6 weeks of surgery after her scheduled appointment and radiographic examination (Figure 3). Ongoing regular clinical and radiologic surveillance are planned.
Discussion
Hip and knee OA in the residual limb is more common for amputees than for the general population.2,3 THA for OA in amputees has been reported after below-knee amputation in both the ipsilateral and the contralateral hip.4 A true hip disarticulation is a rarely performed radical surgical procedure, involving the removal of the entire femur, and is most often related to surgical oncologic treatment or combat-related injuries, both being more common in younger people. Like many patients who have had a hip disarticulation,5 our patient declined a prosthesis, finding the design cosmetically unappealing and uncomfortable, in favor of crutch-walking. This accelerated wear of the remaining hip, and is a sobering reminder of the high demand on the bearing surfaces of the implants after her procedure.
The implants chosen for this procedure are critical. We use implants which are proven and reliable. Our institution uses the Corail Stem, an uncemented collared stem with an Orthopaedic Data Evaluation Panel (ODEP) 10A rating,6 widely used for THA.7 For the acetabulum, we chose the Novae SunFit, a modern version based on Bousquet’s 1976 DM design. The DM cup is a tripolar cup with a fixed porous-coated or cemented metal cup, which articulates with a large mobile polyethylene liner. A standard head in either metal or ceramic is inserted into this liner. The articulation between the head and the liner is constrained, while the articulation between the liner and the metal cup is unconstrained. This interposition of a mobile insert increases the effective head diameter, and the favorable head-neck ratio allows increased range of motion while avoiding early femoral neck impingement with a fixed liner or metal cup. A growing body of evidence indicates that DM cups reduce dislocation rates in primary and revision total knee arthroplasty and, when used with prudence, in selected tumor cases.8 A study of 1905 hips, using second-generation DM cups, reported cumulative survival rate of 98.6% at 12.2 years,9 with favorable outcomes compared with standard prostheses in the medium term for younger patients,10 and in the longer term,11 without increasing polyethylene wear.12
We use DM cups for 2 patient cohorts: first, for all patients older than 75 years because, in this age group, the risk of dislocation is higher than the risk of revision for wear-induced lysis; and second, in younger patients with any neuromuscular, cognitive, or mechanical risk factors that would excessively increase the risk of dislocation. This reflects the balance of risks in arthroplasty, with the ever-present trade-off between polyethylene-induced osteolysis and stability. Dislocation of the remaining sound limb for this young, active, agile patient would be a catastrophic complication. Given our patient’s risk factors for dislocation—female, an amputee with a high risk of falling, high body mass index, and lack of a contralateral limb to restrict adduction—the balance of risks favored hip stability over wear. We chose, therefore, a DM cup, using a ceramic-head-on-polyethylene-insert surface-bearing combination.
CT scanning is routinely performed in our institution to optimize preoperative templating. The preoperative CT images enable accurate planning, notably for the extramedullary reconstruction,13 and are used in addition to acetates and standard radiographs. This encourages preservation of acetabular bone stock by selecting the smallest suitable cup, reduces the risk of femoral fracture by giving an accurate prediction of the stem size, and ensures accuracy of restoring the patient’s offset and length. Although limb-length discrepancy was not an issue for this patient with a single sound limb, the sequalae of excessively increasing offset or length (eg, gluteus medius tendinopathy and trochanteric bursitis) would arguably be more debilitating than for someone who could offload weight to the “good hip.” For these reasons, marrying the preoperative templating with on-table testing with trial prostheses and restoring the native capsular tension is vital.
The importance of on-table positioning for proximal amputees undergoing hip arthroplasty has been highlighted.14 Lacking the normal bony constraints increases the risk of intraoperative on-table movement, which, in turn, risks reducing the accuracy of implant positioning. Crude limb-length checking using the contralateral knee is not possible. In addition, the lack of a contralateral hip joint causes a degree of compensatory pelvic tilt, which raises the option of increasing the coverage to compensate for obligate adduction during single-leg, crutch-walking gait. Lacking established guidelines to accommodate these variables, we inserted the cup in a standard fashion, at 45º, referencing acetabular version using the transverse acetabular ligament,1 and used the smallest stable cup after line-to-line reaming.
This case of THA in a young, crutch-walking patient with a contralateral true hip disarticulation highlights the importance of meticulous preoperative planning, implant selection appropriate for the patient in question, perioperative positioning, and the technical and operative challenges of restoring the patient’s normal hip architecture.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
1. Archbold HA, Mockford B, Molloy D, McConway J, Ogonda L, Beverland D. The transverse acetabular ligament: an aid to orientation of the acetabular component during primary total hip replacement: a preliminary study of 1000 cases investigating postoperative stability. J Bone Joint Surg Br. 2006;88(7):883-886.
2. Kulkarni J, Adams J, Thomas E, Silman A. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil. 1998;12(4):348-353.
3. Struyf PA, van Heugten CM, Hitters MW, Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch Phys Med Rehabil. 2009;90(3):440-446.
4. Nejat EJ, Meyer A, Sánchez PM, Schaefer SH, Westrich GH. Total hip arthroplasty and rehabilitation in ambulatory lower extremity amputees--a case series. Iowa Orthop J. 2005;25:38-41.
5. Zaffer SM, Braddom RL, Conti A, Goff J, Bokma D. Total hip disarticulation prosthesis with suction socket: report of two cases. Am J Phys Med Rehabil. 1999;78(2):160-162.
6. Lewis P. ODEP [Orthopaedic Data Evaluation Panel]. NHS Supply Chain website. http://www.supplychain.nhs.uk/odep. Accessed April 2, 2015.
7. National Joint Registry for England and Wales. 8th Annual Report, 2011. National Joint Registry website. www.njrcentre.org.uk/NjrCentre/Portals/0/Documents/NJR%208th%20Annual%20Report%202011.pdf. Accessed April 2, 2015.
8. Grazioli A, Ek ET, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36(12):2411-2418.
9. Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop. 2012;470(7):1932-1940.
10. Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29(6):1323-1328.
11. Philippot R, Meucci JF, Boyer B, Farizon F. Modern dual-mobility cup implanted with an uncemented stem: about 100 cases with 12-year follow-up. Surg Technol Int. 2013;23:208-212.
12. Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37(12):2345-2350.
13. Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009;91(13):333-340.
14. Bong MR, Kaplan KM, Jaffe WL. Total hip arthroplasty in a patient with contralateral hemipelvectomy. J Arthroplasty. 2006;21(5):762-764.
Operative Intervention for Geriatric Hip Fracture: Does Type of Surgery Affect Hospital Length of Stay?
Hip fractures, the most severe and costly fall-related fractures, account for 350,000 hospital admissions per year.1 The majority of hip fractures result from low-impact falls, typically in patients over age 60 years. In fact, the increase in hip fracture with age is nearly exponential.2,3 With the predicted aging of our population, hip fractures will continue to increase in volume. Between 2000 and 2050, the elderly US population will increase by 135%,4 proportionately increasing the number of projected hip fractures. Considering that hip fractures account for 72% of total costs in terms of orthopedic fracture care in the elderly, the dramatic rise in hip fractures is of great concern for future costs of health care delivery in this field.5-7
In an effort to move toward a value-based system in which costs are reduced while quality of care is maintained, Medicare recently unveiled a new bundled payment system of reimbursement. Through this system, hospitals will be reimbursed for treatment provided to Medicare beneficiaries based on the expected costs of care, instead of through the traditional fee-for-service model. Given this development, orthopedic surgeons will need to develop interventions that reduce costs while maintaining quality of care after hip fracture surgery.
One of the most significant ramifications of a value-based system is that reimbursement for hip fractures may be standardized based on a single diagnosis regardless of the actual costs associated with treatment.8 In hip fracture cases, however, a wide range of factors, including degree of communition of the bone, presence of medical comorbidities,9 and amount of soft-tissue injury, can dramatically increase recovery time. In fact, one of the most important determinants of treatment costs related to hospital length of stay (LOS) is whether the fracture is a femoral neck or intertrochanteric fracture.10,11 Type of fracture is a significant determinant of surgical options, and these can dramatically change patient outcomes and costs of surgical care.12-16 In addition, hospital recovery time or LOS can vary widely based on type of surgery. As hospitalization costs account for 44% of the direct medical costs for hip fractures,17 differences in LOS can have major financial implications in a value-based system of reimbursement in which all forms of hip fracture are reimbursed a standard amount.
We conducted a study to analyze differences in hospital LOS for different forms of hip fracture repair to determine the potential financial repercussions of a bundled payment model of reimbursement. By performing a retrospective chart review at a large, level I trauma center, we were able to compare LOS and associated costs for total hip arthroplasty (THA), hemiarthroplasty (HA), cephalomedullary nailing (CMN), open reduction and internal fixation (ORIF), and closed reduction and percutaneous pinning (CRPP).
Materials and Methods
After receiving institutional review board approval for this study, we retrospectively reviewed all hip fracture cases treated at a level I trauma center between January 2000 and December 2009. Current Procedural Terminology (CPT) codes were searched for cases of low-energy falls that caused hip fractures that were resolved with THA, HA, CMN, ORIF, or CRPP. Patients who underwent HA or THA were grouped for analysis. Patients who were over age 60 years and had acetabular, proximal femoral, trochanteric, or femoral neck fractures were included in our search. Patients who had incomplete medical records or did not meet the age criterion were excluded from analysis.
We reviewed patient charts in our institutional electronic medical records database to collect these data: date of birth, age, sex, date of admission, date of discharge, American Society of Anesthesiologists (ASA) Physical Status score, complications, height, weight, start and stop times of procedure, whether or not the procedure was an emergent procedure, days from admission to surgery, 90-day readmissions, days from surgery to discharge, and general category of operation. We also recorded individual comorbidities, including prior myocardial infarction, dysrhythmia, atrial fibrillation, congestive heart failure, heart block, cerebrovascular disease, chronic obstructive pulmonary disease, emphysema, current smoking status, smoking history, renal disease, dialysis, cancer, and diabetes. Duration of surgery was calculated from recorded start and stop times. Body mass index was calculated using height and weight recorded during initial stay. LOS was recorded as the difference between the admission and discharge dates.
Mean total cost to the hospital ($4530/d patient was hospitalized) was obtained from the institution’s financial services. All fractional LOS values were rounded to the nearest whole number and multiplied by the per diem cost. Student t test was used to compare mean LOS and costs of HA/THA with those of all the other procedures. Additional tests were run to analyze differences in LOS and type of surgeries performed throughout the 9-year period. A multivariate regression model controlling for ASA score, body mass index, age, sex, and comorbidities was developed to analyze differences in LOS and costs for patients who underwent HA/THA versus CMN, ORIF, and CRPP. Significance was set at P = .05.
Results
Our search identified 720 patients who were over age 60 years and underwent operative fixation for hip fracture at our level I trauma center between 2000 and 2009. Of these 720 patients, 105 who had incomplete charts or did not meet the age criteria were excluded, leaving 615 patients (with complete records of isolated low-energy hip fractures) for analysis.
Table 1 lists the demographics of our patient population. The majority of patients had undergone ORIF (30.24%) or HA/THA (45.69%). CRPP was the least common procedure (9.92%) after CMN (14.15%). Mean age was 78.4 years; the majority of patients were between 75 and 89 years of age. Mean hospital LOS was 6.91 days. The majority of patients (n = 414; 67.32%) were female. ASA scores had a narrow distribution, with most patients assigned a score of 3. The readmission rate was significantly higher for HA/THA (39.1%) than for ORIF (28.5%; P = .02) and CRPP (24.6%; P = .04).
Table 2 lists mean LOS and associated costs for each procedure compared with HA/THA. Mean LOS for all patients was 6.91 days, with associated hospitalization costs of $30,011.25. Patients who underwent HA/THA had the longest mean LOS (7.43 days) and highest mean hospitalization costs ($33,657.90). In comparison, patients who underwent ORIF had a mean LOS of 6.59 days with $29,852.70 in costs (P = .04). CRPP also had a significantly (P < .003) shorter LOS (5.59 days) and lower costs ($25,322.70). Although CMN had a mean LOS of 6.89 days and $31,211.70 in costs, the difference in LOS was not significantly different from that of HA/THA. The proportion of surgeries that were HA/THA, CMN, ORIF, and CRPP did not change significantly through the 9-year period (P = .19). Similarly, mean LOS did not change significantly for any of the types of surgery through this period (Table 3).
Figure 1 provides the distribution of LOS for all 4 procedures. The interquartile range (IQR) for patients who underwent HA/THA was 4 to 9 days (median, 6 days). Patients who underwent CMN also had a median LOS of 6 days and an IQR of 4 to 8 days. Both ORIF (IQR, 4-8 days) and CRPP (IQR, 3-6 days) were associated with a median LOS of 5 days.
Figure 2 shows mean hospitalization costs based on type of procedure. HA/THA had the highest mean cost, $33,657.90, or $8335.20 more than CRPP ($25,322.70). Patients who underwent CMN had a mean cost of $31,211.70, versus $29,852.70 for patients who underwent ORIF.
Table 4 summarizes the multivariate analysis results. After ASA score, sex, age, and comorbidities were controlled for, there was an overall significant relationship involving surgical treatment, LOS, and associated hospitalization costs for HA/THA, ORIF, and CRPP. Compared with HA/THA, ORIF had $3805.20 less in costs (P = .042) and 0.84 fewer hospital days. Patients who underwent CRPP were hospitalized for significantly fewer days (1.63) and associated costs ($7383.90) (P = .0076). There was no significant difference in LOS and costs between HA/THA and CMN. Of the controlled variables, only ASA score (P < .001) and male sex (P = .001) were significantly associated with changes in LOS and costs. There was no significant association with comorbidities, LOS, or costs.
Discussion
In this study of surgical intervention in patients with hip fractures, we determined that HA/THA was associated with significantly increased hospital LOS and costs than ORIF and CRPP. Although arthroplasty had an increased mean LOS compared with CMN, the difference was not statistically significant. In addition to type of procedure, both male sex (P = .001) and preoperative ASA score (P < .001) were significant predictors of LOS and costs. These findings are supported by other studies in which preoperative functioning was found to be a strong predictor of increased LOS and costs among hip fracture patients,18 most likely because of increased risk for complications.19
Although our study was the first to directly compare LOS and costs for HA/THA and CMN, other investigators have analyzed the effect of surgical complications on LOS for patients treated with THA, HA, and CMN. In a study on the effects of surgical complications on LOS after hip fracture surgery, Foss and colleagues17 reported that the proportion of CMN patients (31%) with complications was larger than that of HA patients (19%) and THA patients (0%). They also reported that surgical complications were associated with significantly increased LOS during primary admission. Similarly, Edwards and colleagues20 found that the infection risk was higher with CMN (3.1%) than with THA (0%) and HA (0%-2.3%) and that infections were associated with increased LOS (P > .001). However, further statistical analysis revealed that the odds of developing an infection were not significantly higher with CMN than with other studies.20 Similarly, other studies have reported low rates of complications, including nonunion, with CMN.21,22 In our study, we found no significant difference in LOS and costs for CMN and HA/THA after controlling for ASA score, which is known to be associated with a higher risk for complications.18,19
The largest difference in LOS and costs after controlling for potential confounding variables was between HA/THA and CRPP ($7383.90). To our knowledge, only one study has performed a comparative analysis of LOS for CRPP and other surgical treatments for hip fractures. For femoral neck fractures treated between 1990 and 1994, Fekete and colleagues23 found that LOS was 14.9 days for ORIF cases and 12.1 days for CRPP cases—a difference of 2.8 days. In comparison, we found a 1-day difference in mean LOS between ORIF cases (6.59 days) and CRPP cases (5.59 days).
Other studies of LOS and associated costs over a 2-year period have found that ORIF is overall more costly than HA/THA. For example, Keating and colleagues13 compared total costs of care, including LOS, for healthy older patients with displaced intracapsular hip fractures treated with ORIF, bipolar HA, or THA. Although ORIF was initially less costly than HA/THA, overall ORIF costs over 2 years were significantly higher because of readmissions, which increased overall LOS. Similarly, in cases of displaced femoral fractures, Iorio and colleagues15 found that LOS was 6.4 days for ORIF, 4.9 days for unipolar HA, 6.2 days for bipolar HA, and 5.5 days for cemented and hybrid THA. However, when overall projected costs were estimated, including the costs of rehabilitation and of (probable) revision arthroplasty, ORIF was estimated to cost more over a 2-year period because of the need for additional care and in-patient stays. In contrast, we found that hospitalization costs were $3805.20 lower for ORIF than for HA/THA, even after adjusting for comorbidities, and that ORIF had a lower overall readmission rate. Early discharge of patients who are at risk for subsequent complications may have played a significant role in increasing readmission rates for arthroplasty patients. These findings indicate the complexities involved in a bundled payment system of reimbursement, in which a single payment for both initial stay and related readmissions will force orthopedists to consider long-term hospitalization costs when deciding on length of postoperative care and the most cost-effective surgical treatment.
One of the limitations of this study is its retrospective design. Although selection of our sample from a single level I trauma center reduced differences in cost and patient care protocols between institutions, it also reduced the generalizability of our actual costs. In addition, for some patients, LOS may have increased because of delays in surgery or discharge, lack of operating room availability, or need for further medical clearance for additional procedures. Day of admission could also have significantly affected LOS. However, the effects of these confounding factors were reduced because of the large sample analyzed. As stated earlier, overall LOS depends on both initial in-patient stays and readmissions. Therefore, long-term prospective studies that compare LOS and associated costs for patients with hip fractures treated with ORIF, CRPP, HA/THA, and CMN are needed.
Conclusion
It has been recently suggested that hip fracture repair be included in the National Pilot Program on Payment Bundling, which will potentially reimburse orthopedic surgeons a standardized amount for hip fracture surgery regardless of actual treatment costs.8 In this model, it will be essential to understand how type of fracture and surgical procedure can influence LOS and therefore hip fracture treatment costs. We found that, based on these factors, mean LOS ranged from 5.59 to 7.43 days, which translates to a cost range of $25,322.70 to $33,657.90. Before a standardized bundled payment system is implemented, further studies are needed to identify other factors that can significantly affect the cost of hip fracture repair.
1. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
2. Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham study. Am J Public Health. 2002;92(5):858-862.
3. Scott JC. Osteoporosis and hip fractures. Rheum Dis Clin North Am. 1990;16(3):717-740.
4. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776-781.
5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465-475.
6. Burge RT, King AB, Balda E, Worley D. Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health. 2003;6(5):574-583.
7. Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605-615.
8. Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff. 2011;30(9):1708-1717.
9. Shah A, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop. 2002;(399):28-34.
10. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
11. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
12. Carroll C, Stevenson M, Scope A, Evans P, Buckley S. Hemiarthroplasty and total hip arthroplasty for treating primary intracapsular fracture of the hip: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15(36):1-74.
13. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
14. Rogmark C, Carlsson A, Johnell O, Sembo I. Costs of internal fixation and arthroplasty for displaced femoral neck fractures: a randomized study of 68 patients. Acta Orthop Scand. 2003;74(3):293-298.
15. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop. 2001;(383):229-242.
16. Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24(6):854-860.
17. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
18. Garcia AE, Bonnaig JV, Yoneda ZT. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
19. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
20. Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770-777.
21. Jain P, Maini L, Mishra P, Upadhyay A, Agarwal A. Cephalomedullary interlocked nail for ipsilateral hip and femoral shaft fractures. Injury. 2004;35(10):1031-1038.
22. Matre K, Havelin LI, Gjertsen JE, Espehaug B, Fevang JM. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop. 2013;471(4):1379-1386.
23. Fekete K, Manninger J, Kazár G, Cserháti P, Bosch U. Percutaneous internal fixation of femoral neck fractures with cannulated screws and a small tension band plate. Orthop Traumatol. 2000;8(4):250-263.
Hip fractures, the most severe and costly fall-related fractures, account for 350,000 hospital admissions per year.1 The majority of hip fractures result from low-impact falls, typically in patients over age 60 years. In fact, the increase in hip fracture with age is nearly exponential.2,3 With the predicted aging of our population, hip fractures will continue to increase in volume. Between 2000 and 2050, the elderly US population will increase by 135%,4 proportionately increasing the number of projected hip fractures. Considering that hip fractures account for 72% of total costs in terms of orthopedic fracture care in the elderly, the dramatic rise in hip fractures is of great concern for future costs of health care delivery in this field.5-7
In an effort to move toward a value-based system in which costs are reduced while quality of care is maintained, Medicare recently unveiled a new bundled payment system of reimbursement. Through this system, hospitals will be reimbursed for treatment provided to Medicare beneficiaries based on the expected costs of care, instead of through the traditional fee-for-service model. Given this development, orthopedic surgeons will need to develop interventions that reduce costs while maintaining quality of care after hip fracture surgery.
One of the most significant ramifications of a value-based system is that reimbursement for hip fractures may be standardized based on a single diagnosis regardless of the actual costs associated with treatment.8 In hip fracture cases, however, a wide range of factors, including degree of communition of the bone, presence of medical comorbidities,9 and amount of soft-tissue injury, can dramatically increase recovery time. In fact, one of the most important determinants of treatment costs related to hospital length of stay (LOS) is whether the fracture is a femoral neck or intertrochanteric fracture.10,11 Type of fracture is a significant determinant of surgical options, and these can dramatically change patient outcomes and costs of surgical care.12-16 In addition, hospital recovery time or LOS can vary widely based on type of surgery. As hospitalization costs account for 44% of the direct medical costs for hip fractures,17 differences in LOS can have major financial implications in a value-based system of reimbursement in which all forms of hip fracture are reimbursed a standard amount.
We conducted a study to analyze differences in hospital LOS for different forms of hip fracture repair to determine the potential financial repercussions of a bundled payment model of reimbursement. By performing a retrospective chart review at a large, level I trauma center, we were able to compare LOS and associated costs for total hip arthroplasty (THA), hemiarthroplasty (HA), cephalomedullary nailing (CMN), open reduction and internal fixation (ORIF), and closed reduction and percutaneous pinning (CRPP).
Materials and Methods
After receiving institutional review board approval for this study, we retrospectively reviewed all hip fracture cases treated at a level I trauma center between January 2000 and December 2009. Current Procedural Terminology (CPT) codes were searched for cases of low-energy falls that caused hip fractures that were resolved with THA, HA, CMN, ORIF, or CRPP. Patients who underwent HA or THA were grouped for analysis. Patients who were over age 60 years and had acetabular, proximal femoral, trochanteric, or femoral neck fractures were included in our search. Patients who had incomplete medical records or did not meet the age criterion were excluded from analysis.
We reviewed patient charts in our institutional electronic medical records database to collect these data: date of birth, age, sex, date of admission, date of discharge, American Society of Anesthesiologists (ASA) Physical Status score, complications, height, weight, start and stop times of procedure, whether or not the procedure was an emergent procedure, days from admission to surgery, 90-day readmissions, days from surgery to discharge, and general category of operation. We also recorded individual comorbidities, including prior myocardial infarction, dysrhythmia, atrial fibrillation, congestive heart failure, heart block, cerebrovascular disease, chronic obstructive pulmonary disease, emphysema, current smoking status, smoking history, renal disease, dialysis, cancer, and diabetes. Duration of surgery was calculated from recorded start and stop times. Body mass index was calculated using height and weight recorded during initial stay. LOS was recorded as the difference between the admission and discharge dates.
Mean total cost to the hospital ($4530/d patient was hospitalized) was obtained from the institution’s financial services. All fractional LOS values were rounded to the nearest whole number and multiplied by the per diem cost. Student t test was used to compare mean LOS and costs of HA/THA with those of all the other procedures. Additional tests were run to analyze differences in LOS and type of surgeries performed throughout the 9-year period. A multivariate regression model controlling for ASA score, body mass index, age, sex, and comorbidities was developed to analyze differences in LOS and costs for patients who underwent HA/THA versus CMN, ORIF, and CRPP. Significance was set at P = .05.
Results
Our search identified 720 patients who were over age 60 years and underwent operative fixation for hip fracture at our level I trauma center between 2000 and 2009. Of these 720 patients, 105 who had incomplete charts or did not meet the age criteria were excluded, leaving 615 patients (with complete records of isolated low-energy hip fractures) for analysis.
Table 1 lists the demographics of our patient population. The majority of patients had undergone ORIF (30.24%) or HA/THA (45.69%). CRPP was the least common procedure (9.92%) after CMN (14.15%). Mean age was 78.4 years; the majority of patients were between 75 and 89 years of age. Mean hospital LOS was 6.91 days. The majority of patients (n = 414; 67.32%) were female. ASA scores had a narrow distribution, with most patients assigned a score of 3. The readmission rate was significantly higher for HA/THA (39.1%) than for ORIF (28.5%; P = .02) and CRPP (24.6%; P = .04).
Table 2 lists mean LOS and associated costs for each procedure compared with HA/THA. Mean LOS for all patients was 6.91 days, with associated hospitalization costs of $30,011.25. Patients who underwent HA/THA had the longest mean LOS (7.43 days) and highest mean hospitalization costs ($33,657.90). In comparison, patients who underwent ORIF had a mean LOS of 6.59 days with $29,852.70 in costs (P = .04). CRPP also had a significantly (P < .003) shorter LOS (5.59 days) and lower costs ($25,322.70). Although CMN had a mean LOS of 6.89 days and $31,211.70 in costs, the difference in LOS was not significantly different from that of HA/THA. The proportion of surgeries that were HA/THA, CMN, ORIF, and CRPP did not change significantly through the 9-year period (P = .19). Similarly, mean LOS did not change significantly for any of the types of surgery through this period (Table 3).
Figure 1 provides the distribution of LOS for all 4 procedures. The interquartile range (IQR) for patients who underwent HA/THA was 4 to 9 days (median, 6 days). Patients who underwent CMN also had a median LOS of 6 days and an IQR of 4 to 8 days. Both ORIF (IQR, 4-8 days) and CRPP (IQR, 3-6 days) were associated with a median LOS of 5 days.
Figure 2 shows mean hospitalization costs based on type of procedure. HA/THA had the highest mean cost, $33,657.90, or $8335.20 more than CRPP ($25,322.70). Patients who underwent CMN had a mean cost of $31,211.70, versus $29,852.70 for patients who underwent ORIF.
Table 4 summarizes the multivariate analysis results. After ASA score, sex, age, and comorbidities were controlled for, there was an overall significant relationship involving surgical treatment, LOS, and associated hospitalization costs for HA/THA, ORIF, and CRPP. Compared with HA/THA, ORIF had $3805.20 less in costs (P = .042) and 0.84 fewer hospital days. Patients who underwent CRPP were hospitalized for significantly fewer days (1.63) and associated costs ($7383.90) (P = .0076). There was no significant difference in LOS and costs between HA/THA and CMN. Of the controlled variables, only ASA score (P < .001) and male sex (P = .001) were significantly associated with changes in LOS and costs. There was no significant association with comorbidities, LOS, or costs.
Discussion
In this study of surgical intervention in patients with hip fractures, we determined that HA/THA was associated with significantly increased hospital LOS and costs than ORIF and CRPP. Although arthroplasty had an increased mean LOS compared with CMN, the difference was not statistically significant. In addition to type of procedure, both male sex (P = .001) and preoperative ASA score (P < .001) were significant predictors of LOS and costs. These findings are supported by other studies in which preoperative functioning was found to be a strong predictor of increased LOS and costs among hip fracture patients,18 most likely because of increased risk for complications.19
Although our study was the first to directly compare LOS and costs for HA/THA and CMN, other investigators have analyzed the effect of surgical complications on LOS for patients treated with THA, HA, and CMN. In a study on the effects of surgical complications on LOS after hip fracture surgery, Foss and colleagues17 reported that the proportion of CMN patients (31%) with complications was larger than that of HA patients (19%) and THA patients (0%). They also reported that surgical complications were associated with significantly increased LOS during primary admission. Similarly, Edwards and colleagues20 found that the infection risk was higher with CMN (3.1%) than with THA (0%) and HA (0%-2.3%) and that infections were associated with increased LOS (P > .001). However, further statistical analysis revealed that the odds of developing an infection were not significantly higher with CMN than with other studies.20 Similarly, other studies have reported low rates of complications, including nonunion, with CMN.21,22 In our study, we found no significant difference in LOS and costs for CMN and HA/THA after controlling for ASA score, which is known to be associated with a higher risk for complications.18,19
The largest difference in LOS and costs after controlling for potential confounding variables was between HA/THA and CRPP ($7383.90). To our knowledge, only one study has performed a comparative analysis of LOS for CRPP and other surgical treatments for hip fractures. For femoral neck fractures treated between 1990 and 1994, Fekete and colleagues23 found that LOS was 14.9 days for ORIF cases and 12.1 days for CRPP cases—a difference of 2.8 days. In comparison, we found a 1-day difference in mean LOS between ORIF cases (6.59 days) and CRPP cases (5.59 days).
Other studies of LOS and associated costs over a 2-year period have found that ORIF is overall more costly than HA/THA. For example, Keating and colleagues13 compared total costs of care, including LOS, for healthy older patients with displaced intracapsular hip fractures treated with ORIF, bipolar HA, or THA. Although ORIF was initially less costly than HA/THA, overall ORIF costs over 2 years were significantly higher because of readmissions, which increased overall LOS. Similarly, in cases of displaced femoral fractures, Iorio and colleagues15 found that LOS was 6.4 days for ORIF, 4.9 days for unipolar HA, 6.2 days for bipolar HA, and 5.5 days for cemented and hybrid THA. However, when overall projected costs were estimated, including the costs of rehabilitation and of (probable) revision arthroplasty, ORIF was estimated to cost more over a 2-year period because of the need for additional care and in-patient stays. In contrast, we found that hospitalization costs were $3805.20 lower for ORIF than for HA/THA, even after adjusting for comorbidities, and that ORIF had a lower overall readmission rate. Early discharge of patients who are at risk for subsequent complications may have played a significant role in increasing readmission rates for arthroplasty patients. These findings indicate the complexities involved in a bundled payment system of reimbursement, in which a single payment for both initial stay and related readmissions will force orthopedists to consider long-term hospitalization costs when deciding on length of postoperative care and the most cost-effective surgical treatment.
One of the limitations of this study is its retrospective design. Although selection of our sample from a single level I trauma center reduced differences in cost and patient care protocols between institutions, it also reduced the generalizability of our actual costs. In addition, for some patients, LOS may have increased because of delays in surgery or discharge, lack of operating room availability, or need for further medical clearance for additional procedures. Day of admission could also have significantly affected LOS. However, the effects of these confounding factors were reduced because of the large sample analyzed. As stated earlier, overall LOS depends on both initial in-patient stays and readmissions. Therefore, long-term prospective studies that compare LOS and associated costs for patients with hip fractures treated with ORIF, CRPP, HA/THA, and CMN are needed.
Conclusion
It has been recently suggested that hip fracture repair be included in the National Pilot Program on Payment Bundling, which will potentially reimburse orthopedic surgeons a standardized amount for hip fracture surgery regardless of actual treatment costs.8 In this model, it will be essential to understand how type of fracture and surgical procedure can influence LOS and therefore hip fracture treatment costs. We found that, based on these factors, mean LOS ranged from 5.59 to 7.43 days, which translates to a cost range of $25,322.70 to $33,657.90. Before a standardized bundled payment system is implemented, further studies are needed to identify other factors that can significantly affect the cost of hip fracture repair.
Hip fractures, the most severe and costly fall-related fractures, account for 350,000 hospital admissions per year.1 The majority of hip fractures result from low-impact falls, typically in patients over age 60 years. In fact, the increase in hip fracture with age is nearly exponential.2,3 With the predicted aging of our population, hip fractures will continue to increase in volume. Between 2000 and 2050, the elderly US population will increase by 135%,4 proportionately increasing the number of projected hip fractures. Considering that hip fractures account for 72% of total costs in terms of orthopedic fracture care in the elderly, the dramatic rise in hip fractures is of great concern for future costs of health care delivery in this field.5-7
In an effort to move toward a value-based system in which costs are reduced while quality of care is maintained, Medicare recently unveiled a new bundled payment system of reimbursement. Through this system, hospitals will be reimbursed for treatment provided to Medicare beneficiaries based on the expected costs of care, instead of through the traditional fee-for-service model. Given this development, orthopedic surgeons will need to develop interventions that reduce costs while maintaining quality of care after hip fracture surgery.
One of the most significant ramifications of a value-based system is that reimbursement for hip fractures may be standardized based on a single diagnosis regardless of the actual costs associated with treatment.8 In hip fracture cases, however, a wide range of factors, including degree of communition of the bone, presence of medical comorbidities,9 and amount of soft-tissue injury, can dramatically increase recovery time. In fact, one of the most important determinants of treatment costs related to hospital length of stay (LOS) is whether the fracture is a femoral neck or intertrochanteric fracture.10,11 Type of fracture is a significant determinant of surgical options, and these can dramatically change patient outcomes and costs of surgical care.12-16 In addition, hospital recovery time or LOS can vary widely based on type of surgery. As hospitalization costs account for 44% of the direct medical costs for hip fractures,17 differences in LOS can have major financial implications in a value-based system of reimbursement in which all forms of hip fracture are reimbursed a standard amount.
We conducted a study to analyze differences in hospital LOS for different forms of hip fracture repair to determine the potential financial repercussions of a bundled payment model of reimbursement. By performing a retrospective chart review at a large, level I trauma center, we were able to compare LOS and associated costs for total hip arthroplasty (THA), hemiarthroplasty (HA), cephalomedullary nailing (CMN), open reduction and internal fixation (ORIF), and closed reduction and percutaneous pinning (CRPP).
Materials and Methods
After receiving institutional review board approval for this study, we retrospectively reviewed all hip fracture cases treated at a level I trauma center between January 2000 and December 2009. Current Procedural Terminology (CPT) codes were searched for cases of low-energy falls that caused hip fractures that were resolved with THA, HA, CMN, ORIF, or CRPP. Patients who underwent HA or THA were grouped for analysis. Patients who were over age 60 years and had acetabular, proximal femoral, trochanteric, or femoral neck fractures were included in our search. Patients who had incomplete medical records or did not meet the age criterion were excluded from analysis.
We reviewed patient charts in our institutional electronic medical records database to collect these data: date of birth, age, sex, date of admission, date of discharge, American Society of Anesthesiologists (ASA) Physical Status score, complications, height, weight, start and stop times of procedure, whether or not the procedure was an emergent procedure, days from admission to surgery, 90-day readmissions, days from surgery to discharge, and general category of operation. We also recorded individual comorbidities, including prior myocardial infarction, dysrhythmia, atrial fibrillation, congestive heart failure, heart block, cerebrovascular disease, chronic obstructive pulmonary disease, emphysema, current smoking status, smoking history, renal disease, dialysis, cancer, and diabetes. Duration of surgery was calculated from recorded start and stop times. Body mass index was calculated using height and weight recorded during initial stay. LOS was recorded as the difference between the admission and discharge dates.
Mean total cost to the hospital ($4530/d patient was hospitalized) was obtained from the institution’s financial services. All fractional LOS values were rounded to the nearest whole number and multiplied by the per diem cost. Student t test was used to compare mean LOS and costs of HA/THA with those of all the other procedures. Additional tests were run to analyze differences in LOS and type of surgeries performed throughout the 9-year period. A multivariate regression model controlling for ASA score, body mass index, age, sex, and comorbidities was developed to analyze differences in LOS and costs for patients who underwent HA/THA versus CMN, ORIF, and CRPP. Significance was set at P = .05.
Results
Our search identified 720 patients who were over age 60 years and underwent operative fixation for hip fracture at our level I trauma center between 2000 and 2009. Of these 720 patients, 105 who had incomplete charts or did not meet the age criteria were excluded, leaving 615 patients (with complete records of isolated low-energy hip fractures) for analysis.
Table 1 lists the demographics of our patient population. The majority of patients had undergone ORIF (30.24%) or HA/THA (45.69%). CRPP was the least common procedure (9.92%) after CMN (14.15%). Mean age was 78.4 years; the majority of patients were between 75 and 89 years of age. Mean hospital LOS was 6.91 days. The majority of patients (n = 414; 67.32%) were female. ASA scores had a narrow distribution, with most patients assigned a score of 3. The readmission rate was significantly higher for HA/THA (39.1%) than for ORIF (28.5%; P = .02) and CRPP (24.6%; P = .04).
Table 2 lists mean LOS and associated costs for each procedure compared with HA/THA. Mean LOS for all patients was 6.91 days, with associated hospitalization costs of $30,011.25. Patients who underwent HA/THA had the longest mean LOS (7.43 days) and highest mean hospitalization costs ($33,657.90). In comparison, patients who underwent ORIF had a mean LOS of 6.59 days with $29,852.70 in costs (P = .04). CRPP also had a significantly (P < .003) shorter LOS (5.59 days) and lower costs ($25,322.70). Although CMN had a mean LOS of 6.89 days and $31,211.70 in costs, the difference in LOS was not significantly different from that of HA/THA. The proportion of surgeries that were HA/THA, CMN, ORIF, and CRPP did not change significantly through the 9-year period (P = .19). Similarly, mean LOS did not change significantly for any of the types of surgery through this period (Table 3).
Figure 1 provides the distribution of LOS for all 4 procedures. The interquartile range (IQR) for patients who underwent HA/THA was 4 to 9 days (median, 6 days). Patients who underwent CMN also had a median LOS of 6 days and an IQR of 4 to 8 days. Both ORIF (IQR, 4-8 days) and CRPP (IQR, 3-6 days) were associated with a median LOS of 5 days.
Figure 2 shows mean hospitalization costs based on type of procedure. HA/THA had the highest mean cost, $33,657.90, or $8335.20 more than CRPP ($25,322.70). Patients who underwent CMN had a mean cost of $31,211.70, versus $29,852.70 for patients who underwent ORIF.
Table 4 summarizes the multivariate analysis results. After ASA score, sex, age, and comorbidities were controlled for, there was an overall significant relationship involving surgical treatment, LOS, and associated hospitalization costs for HA/THA, ORIF, and CRPP. Compared with HA/THA, ORIF had $3805.20 less in costs (P = .042) and 0.84 fewer hospital days. Patients who underwent CRPP were hospitalized for significantly fewer days (1.63) and associated costs ($7383.90) (P = .0076). There was no significant difference in LOS and costs between HA/THA and CMN. Of the controlled variables, only ASA score (P < .001) and male sex (P = .001) were significantly associated with changes in LOS and costs. There was no significant association with comorbidities, LOS, or costs.
Discussion
In this study of surgical intervention in patients with hip fractures, we determined that HA/THA was associated with significantly increased hospital LOS and costs than ORIF and CRPP. Although arthroplasty had an increased mean LOS compared with CMN, the difference was not statistically significant. In addition to type of procedure, both male sex (P = .001) and preoperative ASA score (P < .001) were significant predictors of LOS and costs. These findings are supported by other studies in which preoperative functioning was found to be a strong predictor of increased LOS and costs among hip fracture patients,18 most likely because of increased risk for complications.19
Although our study was the first to directly compare LOS and costs for HA/THA and CMN, other investigators have analyzed the effect of surgical complications on LOS for patients treated with THA, HA, and CMN. In a study on the effects of surgical complications on LOS after hip fracture surgery, Foss and colleagues17 reported that the proportion of CMN patients (31%) with complications was larger than that of HA patients (19%) and THA patients (0%). They also reported that surgical complications were associated with significantly increased LOS during primary admission. Similarly, Edwards and colleagues20 found that the infection risk was higher with CMN (3.1%) than with THA (0%) and HA (0%-2.3%) and that infections were associated with increased LOS (P > .001). However, further statistical analysis revealed that the odds of developing an infection were not significantly higher with CMN than with other studies.20 Similarly, other studies have reported low rates of complications, including nonunion, with CMN.21,22 In our study, we found no significant difference in LOS and costs for CMN and HA/THA after controlling for ASA score, which is known to be associated with a higher risk for complications.18,19
The largest difference in LOS and costs after controlling for potential confounding variables was between HA/THA and CRPP ($7383.90). To our knowledge, only one study has performed a comparative analysis of LOS for CRPP and other surgical treatments for hip fractures. For femoral neck fractures treated between 1990 and 1994, Fekete and colleagues23 found that LOS was 14.9 days for ORIF cases and 12.1 days for CRPP cases—a difference of 2.8 days. In comparison, we found a 1-day difference in mean LOS between ORIF cases (6.59 days) and CRPP cases (5.59 days).
Other studies of LOS and associated costs over a 2-year period have found that ORIF is overall more costly than HA/THA. For example, Keating and colleagues13 compared total costs of care, including LOS, for healthy older patients with displaced intracapsular hip fractures treated with ORIF, bipolar HA, or THA. Although ORIF was initially less costly than HA/THA, overall ORIF costs over 2 years were significantly higher because of readmissions, which increased overall LOS. Similarly, in cases of displaced femoral fractures, Iorio and colleagues15 found that LOS was 6.4 days for ORIF, 4.9 days for unipolar HA, 6.2 days for bipolar HA, and 5.5 days for cemented and hybrid THA. However, when overall projected costs were estimated, including the costs of rehabilitation and of (probable) revision arthroplasty, ORIF was estimated to cost more over a 2-year period because of the need for additional care and in-patient stays. In contrast, we found that hospitalization costs were $3805.20 lower for ORIF than for HA/THA, even after adjusting for comorbidities, and that ORIF had a lower overall readmission rate. Early discharge of patients who are at risk for subsequent complications may have played a significant role in increasing readmission rates for arthroplasty patients. These findings indicate the complexities involved in a bundled payment system of reimbursement, in which a single payment for both initial stay and related readmissions will force orthopedists to consider long-term hospitalization costs when deciding on length of postoperative care and the most cost-effective surgical treatment.
One of the limitations of this study is its retrospective design. Although selection of our sample from a single level I trauma center reduced differences in cost and patient care protocols between institutions, it also reduced the generalizability of our actual costs. In addition, for some patients, LOS may have increased because of delays in surgery or discharge, lack of operating room availability, or need for further medical clearance for additional procedures. Day of admission could also have significantly affected LOS. However, the effects of these confounding factors were reduced because of the large sample analyzed. As stated earlier, overall LOS depends on both initial in-patient stays and readmissions. Therefore, long-term prospective studies that compare LOS and associated costs for patients with hip fractures treated with ORIF, CRPP, HA/THA, and CMN are needed.
Conclusion
It has been recently suggested that hip fracture repair be included in the National Pilot Program on Payment Bundling, which will potentially reimburse orthopedic surgeons a standardized amount for hip fracture surgery regardless of actual treatment costs.8 In this model, it will be essential to understand how type of fracture and surgical procedure can influence LOS and therefore hip fracture treatment costs. We found that, based on these factors, mean LOS ranged from 5.59 to 7.43 days, which translates to a cost range of $25,322.70 to $33,657.90. Before a standardized bundled payment system is implemented, further studies are needed to identify other factors that can significantly affect the cost of hip fracture repair.
1. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
2. Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham study. Am J Public Health. 2002;92(5):858-862.
3. Scott JC. Osteoporosis and hip fractures. Rheum Dis Clin North Am. 1990;16(3):717-740.
4. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776-781.
5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465-475.
6. Burge RT, King AB, Balda E, Worley D. Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health. 2003;6(5):574-583.
7. Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605-615.
8. Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff. 2011;30(9):1708-1717.
9. Shah A, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop. 2002;(399):28-34.
10. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
11. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
12. Carroll C, Stevenson M, Scope A, Evans P, Buckley S. Hemiarthroplasty and total hip arthroplasty for treating primary intracapsular fracture of the hip: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15(36):1-74.
13. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
14. Rogmark C, Carlsson A, Johnell O, Sembo I. Costs of internal fixation and arthroplasty for displaced femoral neck fractures: a randomized study of 68 patients. Acta Orthop Scand. 2003;74(3):293-298.
15. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop. 2001;(383):229-242.
16. Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24(6):854-860.
17. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
18. Garcia AE, Bonnaig JV, Yoneda ZT. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
19. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
20. Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770-777.
21. Jain P, Maini L, Mishra P, Upadhyay A, Agarwal A. Cephalomedullary interlocked nail for ipsilateral hip and femoral shaft fractures. Injury. 2004;35(10):1031-1038.
22. Matre K, Havelin LI, Gjertsen JE, Espehaug B, Fevang JM. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop. 2013;471(4):1379-1386.
23. Fekete K, Manninger J, Kazár G, Cserháti P, Bosch U. Percutaneous internal fixation of femoral neck fractures with cannulated screws and a small tension band plate. Orthop Traumatol. 2000;8(4):250-263.
1. American Academy of Orthopaedic Surgeons. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
2. Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham study. Am J Public Health. 2002;92(5):858-862.
3. Scott JC. Osteoporosis and hip fractures. Rheum Dis Clin North Am. 1990;16(3):717-740.
4. Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31(4):776-781.
5. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465-475.
6. Burge RT, King AB, Balda E, Worley D. Methodology for estimating current and future burden of osteoporosis in state populations: application to Florida in 2000 through 2025. Value Health. 2003;6(5):574-583.
7. Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605-615.
8. Sood N, Huckfeldt PJ, Escarce JJ, Grabowski DC, Newhouse JP. Medicare’s bundled payment pilot for acute and postacute care: analysis and recommendations on where to begin. Health Aff. 2011;30(9):1708-1717.
9. Shah A, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop. 2002;(399):28-34.
10. Sund R, Riihimäki J, Mäkelä M, et al. Modeling the length of the care episode after hip fracture: does the type of fracture matter? Scand J Surg. 2009;98(3):169-174.
11. Fox KM, Magaziner J, Hebel JR, Kenzora JE, Kashner TM. Intertrochanteric versus femoral neck hip fractures: differential characteristics, treatment, and sequelae. J Gerontol A Biol Sci Med Sci. 1999;54(12):M635-M640.
12. Carroll C, Stevenson M, Scope A, Evans P, Buckley S. Hemiarthroplasty and total hip arthroplasty for treating primary intracapsular fracture of the hip: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15(36):1-74.
13. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
14. Rogmark C, Carlsson A, Johnell O, Sembo I. Costs of internal fixation and arthroplasty for displaced femoral neck fractures: a randomized study of 68 patients. Acta Orthop Scand. 2003;74(3):293-298.
15. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop. 2001;(383):229-242.
16. Slover J, Hoffman MV, Malchau H, Tosteson AN, Koval KJ. A cost-effectiveness analysis of the arthroplasty options for displaced femoral neck fractures in the active, healthy, elderly population. J Arthroplasty. 2009;24(6):854-860.
17. Foss NB, Palm H, Krasheninnikoff M, Kehlet H, Gebuhr P. Impact of surgical complications on length of stay after hip fracture surgery. Injury. 2007;38(7):780-784.
18. Garcia AE, Bonnaig JV, Yoneda ZT. Patient variables which may predict length of stay and hospital costs in elderly patients with hip fracture. J Orthop Trauma. 2012;26(11):620-623.
19. Donegan DJ, Gay AN, Baldwin K, Morales EE, Esterhai JL Jr, Mehta S. Use of medical comorbidities to predict complications after hip fracture surgery in the elderly. J Bone Joint Surg Am. 2010;92(4):807-813.
20. Edwards C, Counsell A, Boulton C, Moran CG. Early infection after hip fracture surgery: risk factors, costs and outcome. J Bone Joint Surg Br. 2008;90(6):770-777.
21. Jain P, Maini L, Mishra P, Upadhyay A, Agarwal A. Cephalomedullary interlocked nail for ipsilateral hip and femoral shaft fractures. Injury. 2004;35(10):1031-1038.
22. Matre K, Havelin LI, Gjertsen JE, Espehaug B, Fevang JM. Intramedullary nails result in more reoperations than sliding hip screws in two-part intertrochanteric fractures. Clin Orthop. 2013;471(4):1379-1386.
23. Fekete K, Manninger J, Kazár G, Cserháti P, Bosch U. Percutaneous internal fixation of femoral neck fractures with cannulated screws and a small tension band plate. Orthop Traumatol. 2000;8(4):250-263.