User login
Official Newspaper of the American College of Surgeons
Sigmoidoscopy may not be enough for older patients
WASHINGTON – Colon cancer screening with sigmoidoscopy alone could miss up to 50% of colon polyps in older patients.
As people age, polyps seem to develop more and more proximally, Dr. Victor Tsirline said at the annual clinical congress of the American College of Surgeons. His review of more than 120,000 colonoscopies found that a flexible sigmoidoscopy alone could miss 44% of polyps in patients aged 60-69 years and 50% in those aged 70-79 years.
"We found that proximal colon polyps are more frequent with advanced age than previously considered," said Dr. Tsirline of Carolinas Medical Center, Charlotte, N.C. "So if this is true, what happens if we use sigmoidoscopy instead of colonoscopy? If we had, we would have missed 22,800 polyps, and 16,800 of those would have been adenomatous. In [patients 59 and younger] 32%-36% would be missed and in the older patients, 45%-50%."
Dr. Tsirline obtained his data from the Provation MD endoscopy transcription system. He obtained information on 120,365 colonoscopies that were performed from 2003 to 2011.
He cross-referenced this with CoPathPlus, a pathology reporting system. This allowed him to cross-reference polyp pathology (adenoma vs. hyperplasia) by computer algorithm. There was complete information available on 43,833 polyps.
Because of the large sample size, he set his level of statistical significance at P = less than 0.01.
The patients in the study were aged 20-90 years. Of the entire group of procedures, 53,492 colonoscopies (44%) identified polyps. Most studies (64%) found a single polyp; 25% found two, and 11% found three or more. A subset of the colonoscopies was only for average risk screening (44,806). Of these, 46% identified polyps.
Overall, 48% of polyps were adenomatous; 37% were hyperplastic. Pathology was not available for the remainder.
The polyps were fairly evenly distributed throughout the colon: rectum, 18%; sigmoid, 26%; descending, 14%; transverse, 16%; ascending, 15%; cecum, 11%.
However, when broken down by patient age, the distribution changed significantly. With every advancing decade of life, patients were:
• 22% less likely to have polyps in the rectum or sigmoid.
• 7% more likely to have polyps in the descending colon.
• 19% more likely to have polyps in the transverse colon.
• 30% more likely to have polyps in the ascending colon.
• 22% more likely to have polyps in the cecum.
All of these risks were statistically significant, and they held for both adenomatous and hyperplastic polyps.
The findings led Dr. Tsirline to conclude that flexible sigmoidoscopy should not be relied upon as an effective colon cancer screening method in patients older than 60 years. The U.S. Preventive Services Task Force states that sigmoidoscopy every 5 years combined with high-sensitivity fecal occult blood testing every 3 years is an adequate screening alternative.
"From this study, it’s pretty apparent that sigmoidoscopy should not be used for colon cancer screening in older patients," he said.
During a discussion, Dr. Tsirline fielded a question about screening the very elderly – patients in their 80s and 90s. The study group did include a small number of these patients, he said.
"I think the argument for not screening older individuals is based on the question of whether finding a colon cancer would change anything. Most people think the risks of screening and treatment would outweigh the benefits. Yes, you may find anything, but what are you going to do about it?"
Dr. Tsirline had no financial disclosures.
WASHINGTON – Colon cancer screening with sigmoidoscopy alone could miss up to 50% of colon polyps in older patients.
As people age, polyps seem to develop more and more proximally, Dr. Victor Tsirline said at the annual clinical congress of the American College of Surgeons. His review of more than 120,000 colonoscopies found that a flexible sigmoidoscopy alone could miss 44% of polyps in patients aged 60-69 years and 50% in those aged 70-79 years.
"We found that proximal colon polyps are more frequent with advanced age than previously considered," said Dr. Tsirline of Carolinas Medical Center, Charlotte, N.C. "So if this is true, what happens if we use sigmoidoscopy instead of colonoscopy? If we had, we would have missed 22,800 polyps, and 16,800 of those would have been adenomatous. In [patients 59 and younger] 32%-36% would be missed and in the older patients, 45%-50%."
Dr. Tsirline obtained his data from the Provation MD endoscopy transcription system. He obtained information on 120,365 colonoscopies that were performed from 2003 to 2011.
He cross-referenced this with CoPathPlus, a pathology reporting system. This allowed him to cross-reference polyp pathology (adenoma vs. hyperplasia) by computer algorithm. There was complete information available on 43,833 polyps.
Because of the large sample size, he set his level of statistical significance at P = less than 0.01.
The patients in the study were aged 20-90 years. Of the entire group of procedures, 53,492 colonoscopies (44%) identified polyps. Most studies (64%) found a single polyp; 25% found two, and 11% found three or more. A subset of the colonoscopies was only for average risk screening (44,806). Of these, 46% identified polyps.
Overall, 48% of polyps were adenomatous; 37% were hyperplastic. Pathology was not available for the remainder.
The polyps were fairly evenly distributed throughout the colon: rectum, 18%; sigmoid, 26%; descending, 14%; transverse, 16%; ascending, 15%; cecum, 11%.
However, when broken down by patient age, the distribution changed significantly. With every advancing decade of life, patients were:
• 22% less likely to have polyps in the rectum or sigmoid.
• 7% more likely to have polyps in the descending colon.
• 19% more likely to have polyps in the transverse colon.
• 30% more likely to have polyps in the ascending colon.
• 22% more likely to have polyps in the cecum.
All of these risks were statistically significant, and they held for both adenomatous and hyperplastic polyps.
The findings led Dr. Tsirline to conclude that flexible sigmoidoscopy should not be relied upon as an effective colon cancer screening method in patients older than 60 years. The U.S. Preventive Services Task Force states that sigmoidoscopy every 5 years combined with high-sensitivity fecal occult blood testing every 3 years is an adequate screening alternative.
"From this study, it’s pretty apparent that sigmoidoscopy should not be used for colon cancer screening in older patients," he said.
During a discussion, Dr. Tsirline fielded a question about screening the very elderly – patients in their 80s and 90s. The study group did include a small number of these patients, he said.
"I think the argument for not screening older individuals is based on the question of whether finding a colon cancer would change anything. Most people think the risks of screening and treatment would outweigh the benefits. Yes, you may find anything, but what are you going to do about it?"
Dr. Tsirline had no financial disclosures.
WASHINGTON – Colon cancer screening with sigmoidoscopy alone could miss up to 50% of colon polyps in older patients.
As people age, polyps seem to develop more and more proximally, Dr. Victor Tsirline said at the annual clinical congress of the American College of Surgeons. His review of more than 120,000 colonoscopies found that a flexible sigmoidoscopy alone could miss 44% of polyps in patients aged 60-69 years and 50% in those aged 70-79 years.
"We found that proximal colon polyps are more frequent with advanced age than previously considered," said Dr. Tsirline of Carolinas Medical Center, Charlotte, N.C. "So if this is true, what happens if we use sigmoidoscopy instead of colonoscopy? If we had, we would have missed 22,800 polyps, and 16,800 of those would have been adenomatous. In [patients 59 and younger] 32%-36% would be missed and in the older patients, 45%-50%."
Dr. Tsirline obtained his data from the Provation MD endoscopy transcription system. He obtained information on 120,365 colonoscopies that were performed from 2003 to 2011.
He cross-referenced this with CoPathPlus, a pathology reporting system. This allowed him to cross-reference polyp pathology (adenoma vs. hyperplasia) by computer algorithm. There was complete information available on 43,833 polyps.
Because of the large sample size, he set his level of statistical significance at P = less than 0.01.
The patients in the study were aged 20-90 years. Of the entire group of procedures, 53,492 colonoscopies (44%) identified polyps. Most studies (64%) found a single polyp; 25% found two, and 11% found three or more. A subset of the colonoscopies was only for average risk screening (44,806). Of these, 46% identified polyps.
Overall, 48% of polyps were adenomatous; 37% were hyperplastic. Pathology was not available for the remainder.
The polyps were fairly evenly distributed throughout the colon: rectum, 18%; sigmoid, 26%; descending, 14%; transverse, 16%; ascending, 15%; cecum, 11%.
However, when broken down by patient age, the distribution changed significantly. With every advancing decade of life, patients were:
• 22% less likely to have polyps in the rectum or sigmoid.
• 7% more likely to have polyps in the descending colon.
• 19% more likely to have polyps in the transverse colon.
• 30% more likely to have polyps in the ascending colon.
• 22% more likely to have polyps in the cecum.
All of these risks were statistically significant, and they held for both adenomatous and hyperplastic polyps.
The findings led Dr. Tsirline to conclude that flexible sigmoidoscopy should not be relied upon as an effective colon cancer screening method in patients older than 60 years. The U.S. Preventive Services Task Force states that sigmoidoscopy every 5 years combined with high-sensitivity fecal occult blood testing every 3 years is an adequate screening alternative.
"From this study, it’s pretty apparent that sigmoidoscopy should not be used for colon cancer screening in older patients," he said.
During a discussion, Dr. Tsirline fielded a question about screening the very elderly – patients in their 80s and 90s. The study group did include a small number of these patients, he said.
"I think the argument for not screening older individuals is based on the question of whether finding a colon cancer would change anything. Most people think the risks of screening and treatment would outweigh the benefits. Yes, you may find anything, but what are you going to do about it?"
Dr. Tsirline had no financial disclosures.
AT THE ACS CLINICAL CONGRESS
Major finding: Because people are 30% more likely to have polyps in the ascending colon and 22% more likely to have polyps in the cecum as they age, sigmoidoscopy may not be an adequate screening method.
Data source: Study of 120,000 colonoscopies.
Disclosures: Dr. Victor Tsirline had no financial disclosures.
Delays in esophagectomy yield more postop complications
WASHINGTON – Delays in esophageal cancer surgery after a course of neoadjuvant chemotherapy and radiation were associated with more surgical complications and worse survival, based on the results of a retrospective study presented at the annual clinical congress of the American College of Surgeons.
Clinicians should focus on "prehabilitating" their patients, completing their neoadjuvant therapy and recommending esophagectomy as soon as clinically feasible, Dr. Nicholas Teman said.
Dr. Teman and his colleagues at the University of Michigan, Ann Arbor, reviewed prospectively collected data from the period of 1999-2010 on 457 patients treated at a single site. All patients underwent neoadjuvant chemotherapy and radiation with a subsequent esophagectomy; patients who underwent salvage esophagectomies were excluded from the analysis.
Outcome measures included postoperative pulmonary adverse events, anastomotic leaks, pathologic response, and mortality.
The mean time to surgery after chemotherapy and radiation was 50 days, ranging between 10 and 523 days. The most common reasons for surgical delays were patient deconditioning, noncompliance, seeking a second opinion, and complications stemming from neoadjuvant therapies.
When the time from completion of neoadjuvant therapy to surgery was analyzed as a continuous variable, there were no differences in postoperative complications and mortality. Similarly, postoperative staging and pathologic response were not significantly different.
Additionally, outcomes did not significantly differ for those who had surgery within 8 weeks of completing chemotherapy and radiation and those who had surgery after 8 weeks.
However, when time to surgery was used to place patients into quintiles of 8 weeks or less (n = 345), 9-12 weeks (n = 58), 13-16 weeks (n = 27), 17-26 weeks (n = 19), and 27 or more weeks (n = 8), there were significant differences in pulmonary complications (P = .05) and anastomotic leaks (P = .02), and a trend toward worse mortality between the quintiles (P = .09). No significant differences in pathologic response were noted in the quintiles.
Predictors of higher long-term mortality were lower pretreatment weight (P = .04), tobacco use (P = .05), higher pretreatment stage (P = .004), and failure to complete neoadjuvant treatment (P = .003).
One limitation of the study was that the neoadjuvant therapies were not standardized. A shorter time to surgery was predicted if chemotherapy included cisplatin (P = .04) or taxol (P = .001), and if there were increasing chemotherapy cycles. Chemotherapy that included 5-flourouracil was associated with longer times to surgery (P less than .001).
Dr. Teman and his associates reported no relevant disclosures.
WASHINGTON – Delays in esophageal cancer surgery after a course of neoadjuvant chemotherapy and radiation were associated with more surgical complications and worse survival, based on the results of a retrospective study presented at the annual clinical congress of the American College of Surgeons.
Clinicians should focus on "prehabilitating" their patients, completing their neoadjuvant therapy and recommending esophagectomy as soon as clinically feasible, Dr. Nicholas Teman said.
Dr. Teman and his colleagues at the University of Michigan, Ann Arbor, reviewed prospectively collected data from the period of 1999-2010 on 457 patients treated at a single site. All patients underwent neoadjuvant chemotherapy and radiation with a subsequent esophagectomy; patients who underwent salvage esophagectomies were excluded from the analysis.
Outcome measures included postoperative pulmonary adverse events, anastomotic leaks, pathologic response, and mortality.
The mean time to surgery after chemotherapy and radiation was 50 days, ranging between 10 and 523 days. The most common reasons for surgical delays were patient deconditioning, noncompliance, seeking a second opinion, and complications stemming from neoadjuvant therapies.
When the time from completion of neoadjuvant therapy to surgery was analyzed as a continuous variable, there were no differences in postoperative complications and mortality. Similarly, postoperative staging and pathologic response were not significantly different.
Additionally, outcomes did not significantly differ for those who had surgery within 8 weeks of completing chemotherapy and radiation and those who had surgery after 8 weeks.
However, when time to surgery was used to place patients into quintiles of 8 weeks or less (n = 345), 9-12 weeks (n = 58), 13-16 weeks (n = 27), 17-26 weeks (n = 19), and 27 or more weeks (n = 8), there were significant differences in pulmonary complications (P = .05) and anastomotic leaks (P = .02), and a trend toward worse mortality between the quintiles (P = .09). No significant differences in pathologic response were noted in the quintiles.
Predictors of higher long-term mortality were lower pretreatment weight (P = .04), tobacco use (P = .05), higher pretreatment stage (P = .004), and failure to complete neoadjuvant treatment (P = .003).
One limitation of the study was that the neoadjuvant therapies were not standardized. A shorter time to surgery was predicted if chemotherapy included cisplatin (P = .04) or taxol (P = .001), and if there were increasing chemotherapy cycles. Chemotherapy that included 5-flourouracil was associated with longer times to surgery (P less than .001).
Dr. Teman and his associates reported no relevant disclosures.
WASHINGTON – Delays in esophageal cancer surgery after a course of neoadjuvant chemotherapy and radiation were associated with more surgical complications and worse survival, based on the results of a retrospective study presented at the annual clinical congress of the American College of Surgeons.
Clinicians should focus on "prehabilitating" their patients, completing their neoadjuvant therapy and recommending esophagectomy as soon as clinically feasible, Dr. Nicholas Teman said.
Dr. Teman and his colleagues at the University of Michigan, Ann Arbor, reviewed prospectively collected data from the period of 1999-2010 on 457 patients treated at a single site. All patients underwent neoadjuvant chemotherapy and radiation with a subsequent esophagectomy; patients who underwent salvage esophagectomies were excluded from the analysis.
Outcome measures included postoperative pulmonary adverse events, anastomotic leaks, pathologic response, and mortality.
The mean time to surgery after chemotherapy and radiation was 50 days, ranging between 10 and 523 days. The most common reasons for surgical delays were patient deconditioning, noncompliance, seeking a second opinion, and complications stemming from neoadjuvant therapies.
When the time from completion of neoadjuvant therapy to surgery was analyzed as a continuous variable, there were no differences in postoperative complications and mortality. Similarly, postoperative staging and pathologic response were not significantly different.
Additionally, outcomes did not significantly differ for those who had surgery within 8 weeks of completing chemotherapy and radiation and those who had surgery after 8 weeks.
However, when time to surgery was used to place patients into quintiles of 8 weeks or less (n = 345), 9-12 weeks (n = 58), 13-16 weeks (n = 27), 17-26 weeks (n = 19), and 27 or more weeks (n = 8), there were significant differences in pulmonary complications (P = .05) and anastomotic leaks (P = .02), and a trend toward worse mortality between the quintiles (P = .09). No significant differences in pathologic response were noted in the quintiles.
Predictors of higher long-term mortality were lower pretreatment weight (P = .04), tobacco use (P = .05), higher pretreatment stage (P = .004), and failure to complete neoadjuvant treatment (P = .003).
One limitation of the study was that the neoadjuvant therapies were not standardized. A shorter time to surgery was predicted if chemotherapy included cisplatin (P = .04) or taxol (P = .001), and if there were increasing chemotherapy cycles. Chemotherapy that included 5-flourouracil was associated with longer times to surgery (P less than .001).
Dr. Teman and his associates reported no relevant disclosures.
AT THE ACS CLINICAL CONGRESS
Major finding: When time to surgery was used to place patients into quintiles of 8 weeks or less (n = 345), 9-12 weeks (n = 58), 13-16 weeks (n = 27), 17-26 weeks (n = 19), and 27 or more weeks (n = 8), there were significant differences in pulmonary complications (P = .05) and anastomotic leaks (P = .02), and a trend toward worse mortality between the quintiles (P = .09).
Data source: Retrospective review of prospective data on 457 patients treated from 1999-2010 at a single surgical site.
Disclosures: Dr. Teman and his associates reported no relevant disclosures.
Experts call for broad sharing of clinical trial data
Clinical trial data should be shared as broadly as possible to help spur scientific innovation and answer questions of importance to public health.
"The question is not whether, but how, these data should be broadly shared," wrote Michelle M. Mello and her colleagues from Harvard University, Boston, the Pharmaceutical Research and Manufacturers of America, and several consulting companies, in an article published online Oct. 21 in the New England Journal of Medicine.
At a minimum, data-sharing should be available for trials of all approved prescription drugs, devices, and biologics in any country that has adequate intellectual property protection. A system has to ensure responsible use of data, protect privacy of research participants, and treat "all qualified data requesters and trial sponsors evenhandedly," requiring both generators and requesters to work according to the same rigorous scientific principles (N. Engl. J. Med. 2013 Oct. 21 [doi: 10.1056/NEJMhle1309073]).
The demand for more data from and about clinical trials – including protocol designs, results summaries, and more recently, raw input data – has grown over the past 15 years. Some medical journals have pushed for disclosure of more data upon request. The Food and Drug Administration increasingly has been requiring disclosure, and pharmaceutical manufacturers in the United States and Europe have made commitments to making more information public. Beginning in March 2014, the European Medicines Agency will require disclosure of some raw data, individual case report forms, and other data.
The authors envision at least four potential models for sharing data. With purely open access, everything would be available for download for free. This is the riskiest model, they said, since it would provide the least accountability.
Another model: The data generator would keep the data but answer very specific requests. A third model would have the clinical trial sponsor review data requests and decide whether and how to release the data.
In the last model, an independent review board would determine whether the data should be released. The board would collect the data from the sponsor and issue it to the requester, on a limited, need-to-know basis.
This model would likely best balance all of the competing needs, according to the authors. The independent board promises "to ensure accountability on the part of data generators and users and allow trial sponsors a voice while precluding them from denying access to data for reasons the public would not consider legitimate," they wrote.
An independent board also would help protect research participants and make sure that the playing field is level among all stakeholders, they added.
The authors noted many benefits to allowing wider access to patient-level data, such as independent analyses of safety and effectiveness, new lines of inquiry that could expose product flaws or trial design flaws, and the potential to answer questions that might affect public health but that weren’t explored in the original study.
The biggest downside is that individual participants’ privacy could be compromised, according to the authors. The risk of exposure "raises critical questions about how to ensure that participants understand the potential ramifications of data sharing," they wrote.
Mandatory disclosure could also discourage investment in research and development if manufacturers believe that the data could be used by competitors. Wider data-sharing could also lead to second-guessing of approvals by regulatory agencies.
Clinical trial data-sharing received attention from the World Medical Association in its most recent update of the Declaration of Helsinki, published online Oct. 19 in JAMA (doi: 10.1001/jama.2013.281053). Three general principles addressed data sharing:
• Number 9: It is the duty of physicians who are involved in medical research to protect the life, health, dignity, integrity, right to self-determination, privacy, and confidentiality of personal information of research subjects. The responsibility for the protection of research subjects must always rest with the physician or other health care professionals and never with the research subjects, even though they have given consent.
• Number 24. Every precaution must be taken to protect the privacy of research subjects and the confidentiality of their personal information.
• Number 32. For medical research using identifiable human material or data, such as research on material or data contained in biobanks or similar repositories, physicians must seek informed consent for its collection, storage and/or reuse. There may be exceptional situations where consent would be impossible or impracticable to obtain for such research. In such situations the research may be done only after consideration and approval of a research ethics committee.
Several of the authors of the paper published in the New England Journal of Medicine disclosed that they work for consulting companies that receive fees from various manufacturers and from academic medical centers. The working group was convened through the Multi-Regional Clinical Trials Center at Harvard University, which receives funds from pharmaceutical companies and not-for-profit entities.
On Twitter @aliciaault
Contrary to industry fears, we argue that access to full – though appropriately deidentified – data sets from clinical trials will benefit the research-based biopharmaceutical industry. We predict that it will help to increase the efficiency of drug development, improve cost-effectiveness, improve comparative-effectiveness analysis, and reduce duplication of effort among trial sponsors.
A managed-release environment that allows sharing of patient-level data while ensuring patient privacy would create a level playing field for all stakeholders. What is sometimes labeled as "free riding" may ultimately pay dividends for innovative companies and for public health. It is ironic that the organizations that most resist wider access to data are the ones that stand to benefit so much from greater transparency.
Dr. Hans-Georg Eichler, Frank Petavy, Dr. Francesco Pignatti, and Dr. Guido Rasi are all with the European Medicines Agency in London. Their remarks are taken from an accompanying editorial(N. Eng. J. Med. 2013 Oct. 21 [doi: 10.1056/NEJMp1310771]).
Contrary to industry fears, we argue that access to full – though appropriately deidentified – data sets from clinical trials will benefit the research-based biopharmaceutical industry. We predict that it will help to increase the efficiency of drug development, improve cost-effectiveness, improve comparative-effectiveness analysis, and reduce duplication of effort among trial sponsors.
A managed-release environment that allows sharing of patient-level data while ensuring patient privacy would create a level playing field for all stakeholders. What is sometimes labeled as "free riding" may ultimately pay dividends for innovative companies and for public health. It is ironic that the organizations that most resist wider access to data are the ones that stand to benefit so much from greater transparency.
Dr. Hans-Georg Eichler, Frank Petavy, Dr. Francesco Pignatti, and Dr. Guido Rasi are all with the European Medicines Agency in London. Their remarks are taken from an accompanying editorial(N. Eng. J. Med. 2013 Oct. 21 [doi: 10.1056/NEJMp1310771]).
Contrary to industry fears, we argue that access to full – though appropriately deidentified – data sets from clinical trials will benefit the research-based biopharmaceutical industry. We predict that it will help to increase the efficiency of drug development, improve cost-effectiveness, improve comparative-effectiveness analysis, and reduce duplication of effort among trial sponsors.
A managed-release environment that allows sharing of patient-level data while ensuring patient privacy would create a level playing field for all stakeholders. What is sometimes labeled as "free riding" may ultimately pay dividends for innovative companies and for public health. It is ironic that the organizations that most resist wider access to data are the ones that stand to benefit so much from greater transparency.
Dr. Hans-Georg Eichler, Frank Petavy, Dr. Francesco Pignatti, and Dr. Guido Rasi are all with the European Medicines Agency in London. Their remarks are taken from an accompanying editorial(N. Eng. J. Med. 2013 Oct. 21 [doi: 10.1056/NEJMp1310771]).
Clinical trial data should be shared as broadly as possible to help spur scientific innovation and answer questions of importance to public health.
"The question is not whether, but how, these data should be broadly shared," wrote Michelle M. Mello and her colleagues from Harvard University, Boston, the Pharmaceutical Research and Manufacturers of America, and several consulting companies, in an article published online Oct. 21 in the New England Journal of Medicine.
At a minimum, data-sharing should be available for trials of all approved prescription drugs, devices, and biologics in any country that has adequate intellectual property protection. A system has to ensure responsible use of data, protect privacy of research participants, and treat "all qualified data requesters and trial sponsors evenhandedly," requiring both generators and requesters to work according to the same rigorous scientific principles (N. Engl. J. Med. 2013 Oct. 21 [doi: 10.1056/NEJMhle1309073]).
The demand for more data from and about clinical trials – including protocol designs, results summaries, and more recently, raw input data – has grown over the past 15 years. Some medical journals have pushed for disclosure of more data upon request. The Food and Drug Administration increasingly has been requiring disclosure, and pharmaceutical manufacturers in the United States and Europe have made commitments to making more information public. Beginning in March 2014, the European Medicines Agency will require disclosure of some raw data, individual case report forms, and other data.
The authors envision at least four potential models for sharing data. With purely open access, everything would be available for download for free. This is the riskiest model, they said, since it would provide the least accountability.
Another model: The data generator would keep the data but answer very specific requests. A third model would have the clinical trial sponsor review data requests and decide whether and how to release the data.
In the last model, an independent review board would determine whether the data should be released. The board would collect the data from the sponsor and issue it to the requester, on a limited, need-to-know basis.
This model would likely best balance all of the competing needs, according to the authors. The independent board promises "to ensure accountability on the part of data generators and users and allow trial sponsors a voice while precluding them from denying access to data for reasons the public would not consider legitimate," they wrote.
An independent board also would help protect research participants and make sure that the playing field is level among all stakeholders, they added.
The authors noted many benefits to allowing wider access to patient-level data, such as independent analyses of safety and effectiveness, new lines of inquiry that could expose product flaws or trial design flaws, and the potential to answer questions that might affect public health but that weren’t explored in the original study.
The biggest downside is that individual participants’ privacy could be compromised, according to the authors. The risk of exposure "raises critical questions about how to ensure that participants understand the potential ramifications of data sharing," they wrote.
Mandatory disclosure could also discourage investment in research and development if manufacturers believe that the data could be used by competitors. Wider data-sharing could also lead to second-guessing of approvals by regulatory agencies.
Clinical trial data-sharing received attention from the World Medical Association in its most recent update of the Declaration of Helsinki, published online Oct. 19 in JAMA (doi: 10.1001/jama.2013.281053). Three general principles addressed data sharing:
• Number 9: It is the duty of physicians who are involved in medical research to protect the life, health, dignity, integrity, right to self-determination, privacy, and confidentiality of personal information of research subjects. The responsibility for the protection of research subjects must always rest with the physician or other health care professionals and never with the research subjects, even though they have given consent.
• Number 24. Every precaution must be taken to protect the privacy of research subjects and the confidentiality of their personal information.
• Number 32. For medical research using identifiable human material or data, such as research on material or data contained in biobanks or similar repositories, physicians must seek informed consent for its collection, storage and/or reuse. There may be exceptional situations where consent would be impossible or impracticable to obtain for such research. In such situations the research may be done only after consideration and approval of a research ethics committee.
Several of the authors of the paper published in the New England Journal of Medicine disclosed that they work for consulting companies that receive fees from various manufacturers and from academic medical centers. The working group was convened through the Multi-Regional Clinical Trials Center at Harvard University, which receives funds from pharmaceutical companies and not-for-profit entities.
On Twitter @aliciaault
Clinical trial data should be shared as broadly as possible to help spur scientific innovation and answer questions of importance to public health.
"The question is not whether, but how, these data should be broadly shared," wrote Michelle M. Mello and her colleagues from Harvard University, Boston, the Pharmaceutical Research and Manufacturers of America, and several consulting companies, in an article published online Oct. 21 in the New England Journal of Medicine.
At a minimum, data-sharing should be available for trials of all approved prescription drugs, devices, and biologics in any country that has adequate intellectual property protection. A system has to ensure responsible use of data, protect privacy of research participants, and treat "all qualified data requesters and trial sponsors evenhandedly," requiring both generators and requesters to work according to the same rigorous scientific principles (N. Engl. J. Med. 2013 Oct. 21 [doi: 10.1056/NEJMhle1309073]).
The demand for more data from and about clinical trials – including protocol designs, results summaries, and more recently, raw input data – has grown over the past 15 years. Some medical journals have pushed for disclosure of more data upon request. The Food and Drug Administration increasingly has been requiring disclosure, and pharmaceutical manufacturers in the United States and Europe have made commitments to making more information public. Beginning in March 2014, the European Medicines Agency will require disclosure of some raw data, individual case report forms, and other data.
The authors envision at least four potential models for sharing data. With purely open access, everything would be available for download for free. This is the riskiest model, they said, since it would provide the least accountability.
Another model: The data generator would keep the data but answer very specific requests. A third model would have the clinical trial sponsor review data requests and decide whether and how to release the data.
In the last model, an independent review board would determine whether the data should be released. The board would collect the data from the sponsor and issue it to the requester, on a limited, need-to-know basis.
This model would likely best balance all of the competing needs, according to the authors. The independent board promises "to ensure accountability on the part of data generators and users and allow trial sponsors a voice while precluding them from denying access to data for reasons the public would not consider legitimate," they wrote.
An independent board also would help protect research participants and make sure that the playing field is level among all stakeholders, they added.
The authors noted many benefits to allowing wider access to patient-level data, such as independent analyses of safety and effectiveness, new lines of inquiry that could expose product flaws or trial design flaws, and the potential to answer questions that might affect public health but that weren’t explored in the original study.
The biggest downside is that individual participants’ privacy could be compromised, according to the authors. The risk of exposure "raises critical questions about how to ensure that participants understand the potential ramifications of data sharing," they wrote.
Mandatory disclosure could also discourage investment in research and development if manufacturers believe that the data could be used by competitors. Wider data-sharing could also lead to second-guessing of approvals by regulatory agencies.
Clinical trial data-sharing received attention from the World Medical Association in its most recent update of the Declaration of Helsinki, published online Oct. 19 in JAMA (doi: 10.1001/jama.2013.281053). Three general principles addressed data sharing:
• Number 9: It is the duty of physicians who are involved in medical research to protect the life, health, dignity, integrity, right to self-determination, privacy, and confidentiality of personal information of research subjects. The responsibility for the protection of research subjects must always rest with the physician or other health care professionals and never with the research subjects, even though they have given consent.
• Number 24. Every precaution must be taken to protect the privacy of research subjects and the confidentiality of their personal information.
• Number 32. For medical research using identifiable human material or data, such as research on material or data contained in biobanks or similar repositories, physicians must seek informed consent for its collection, storage and/or reuse. There may be exceptional situations where consent would be impossible or impracticable to obtain for such research. In such situations the research may be done only after consideration and approval of a research ethics committee.
Several of the authors of the paper published in the New England Journal of Medicine disclosed that they work for consulting companies that receive fees from various manufacturers and from academic medical centers. The working group was convened through the Multi-Regional Clinical Trials Center at Harvard University, which receives funds from pharmaceutical companies and not-for-profit entities.
On Twitter @aliciaault
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Obama addresses health exchange website woes
President Obama acknowledged that ongoing problems with the healthcare.gov website – the health insurance exchange for Americans in most states – were giving consumers pause, making supporters nervous, and helping adversaries discredit the Affordable Care Act.
"There’s no sugarcoating it. The website has been too slow," President Obama said during an Oct. 21 White House speech. He said that consumers were having trouble logging on to the website and have gotten bogged down during the application process.
"Nobody’s more frustrated by that than I am," he said, adding that "I want the cash registers to work; I want the checkout lines to be smooth. So I want people to be able to get this great product."
That product, according to the president, is high-quality health insurance that’s offered at a good price.
He said there has been "massive demand," citing nearly 20 million visitors to healthcare.gov. Thirty-four states are directing their residents to buy coverage through that federal website. The president also said there was a lot of demand for plans offered through the exchanges being run by 16 states and the District of Columbia.
Overall, half a million consumers have submitted applications through the federal and state websites, President Obama said. "People don’t just want it; they’re showing up to buy it," he said.
For consumers who have difficulty in accessing the website, President Obama referred them to a toll-free phone number, 800-318-2596. He also said that people could apply in-person at various locations.
"Nobody’s madder than me about the fact that the website isn’t working as well as it should, which means it’s going to get fixed," he said.
On Oct. 20, Health and Human Services department officials said that some of those fixes were underway. The site has been sluggish because "the initial wave of interest stressed the account service," according to HHS officials. But they said that the "data hub" was working, meaning that consumers would be told if they were eligible for subsidies. They also noted that the agency is bringing in technical help to fix the website – something President Obama repeated in his speech.
But the president also said he was frustrated that there was so much focus on the website, given that it was only 3 weeks into a 6-month enrollment process, and that many of the ACA’s benefits were already in place.
He addressed what he called "some of the politics that have swirled around the Affordable Care Act," noting Republican efforts to defund or repeal the ACA.
"And I’m sure that, given the problems with the website so far, they’re going to be looking to go after it even harder," he said.
But, he said, "We did not wage this long and contentious battle just around a website." The battle was "to make sure that millions of Americans in the wealthiest nation on Earth finally have the same chance to get the same security of affordable quality health care as anybody else."
Speaker of the House John Boehner (R-Ohio) was not impressed. "If the president is frustrated by the mounting failures of his health care law, it wasn’t apparent today," said Rep. Boehner, in a statement. "Instead of answers, we got well-worn talking points. Instead of explanations, we got excuses."
He added that "the House’s oversight of this failure is just beginning."
On Oct. 24, the House Energy and Commerce Committee will hold the first of what is likely to be many hearings on the website rollout. HHS Secretary Kathleen Sebelius was invited to testify, but declined because of a scheduling conflict.
Rep. Boehner said that "Secretary Sebelius must change her mind and appear at this week’s hearing."
On Twitter @aliciaault
*This story was updated 10/22/13.
President Obama acknowledged that ongoing problems with the healthcare.gov website – the health insurance exchange for Americans in most states – were giving consumers pause, making supporters nervous, and helping adversaries discredit the Affordable Care Act.
"There’s no sugarcoating it. The website has been too slow," President Obama said during an Oct. 21 White House speech. He said that consumers were having trouble logging on to the website and have gotten bogged down during the application process.
"Nobody’s more frustrated by that than I am," he said, adding that "I want the cash registers to work; I want the checkout lines to be smooth. So I want people to be able to get this great product."
That product, according to the president, is high-quality health insurance that’s offered at a good price.
He said there has been "massive demand," citing nearly 20 million visitors to healthcare.gov. Thirty-four states are directing their residents to buy coverage through that federal website. The president also said there was a lot of demand for plans offered through the exchanges being run by 16 states and the District of Columbia.
Overall, half a million consumers have submitted applications through the federal and state websites, President Obama said. "People don’t just want it; they’re showing up to buy it," he said.
For consumers who have difficulty in accessing the website, President Obama referred them to a toll-free phone number, 800-318-2596. He also said that people could apply in-person at various locations.
"Nobody’s madder than me about the fact that the website isn’t working as well as it should, which means it’s going to get fixed," he said.
On Oct. 20, Health and Human Services department officials said that some of those fixes were underway. The site has been sluggish because "the initial wave of interest stressed the account service," according to HHS officials. But they said that the "data hub" was working, meaning that consumers would be told if they were eligible for subsidies. They also noted that the agency is bringing in technical help to fix the website – something President Obama repeated in his speech.
But the president also said he was frustrated that there was so much focus on the website, given that it was only 3 weeks into a 6-month enrollment process, and that many of the ACA’s benefits were already in place.
He addressed what he called "some of the politics that have swirled around the Affordable Care Act," noting Republican efforts to defund or repeal the ACA.
"And I’m sure that, given the problems with the website so far, they’re going to be looking to go after it even harder," he said.
But, he said, "We did not wage this long and contentious battle just around a website." The battle was "to make sure that millions of Americans in the wealthiest nation on Earth finally have the same chance to get the same security of affordable quality health care as anybody else."
Speaker of the House John Boehner (R-Ohio) was not impressed. "If the president is frustrated by the mounting failures of his health care law, it wasn’t apparent today," said Rep. Boehner, in a statement. "Instead of answers, we got well-worn talking points. Instead of explanations, we got excuses."
He added that "the House’s oversight of this failure is just beginning."
On Oct. 24, the House Energy and Commerce Committee will hold the first of what is likely to be many hearings on the website rollout. HHS Secretary Kathleen Sebelius was invited to testify, but declined because of a scheduling conflict.
Rep. Boehner said that "Secretary Sebelius must change her mind and appear at this week’s hearing."
On Twitter @aliciaault
*This story was updated 10/22/13.
President Obama acknowledged that ongoing problems with the healthcare.gov website – the health insurance exchange for Americans in most states – were giving consumers pause, making supporters nervous, and helping adversaries discredit the Affordable Care Act.
"There’s no sugarcoating it. The website has been too slow," President Obama said during an Oct. 21 White House speech. He said that consumers were having trouble logging on to the website and have gotten bogged down during the application process.
"Nobody’s more frustrated by that than I am," he said, adding that "I want the cash registers to work; I want the checkout lines to be smooth. So I want people to be able to get this great product."
That product, according to the president, is high-quality health insurance that’s offered at a good price.
He said there has been "massive demand," citing nearly 20 million visitors to healthcare.gov. Thirty-four states are directing their residents to buy coverage through that federal website. The president also said there was a lot of demand for plans offered through the exchanges being run by 16 states and the District of Columbia.
Overall, half a million consumers have submitted applications through the federal and state websites, President Obama said. "People don’t just want it; they’re showing up to buy it," he said.
For consumers who have difficulty in accessing the website, President Obama referred them to a toll-free phone number, 800-318-2596. He also said that people could apply in-person at various locations.
"Nobody’s madder than me about the fact that the website isn’t working as well as it should, which means it’s going to get fixed," he said.
On Oct. 20, Health and Human Services department officials said that some of those fixes were underway. The site has been sluggish because "the initial wave of interest stressed the account service," according to HHS officials. But they said that the "data hub" was working, meaning that consumers would be told if they were eligible for subsidies. They also noted that the agency is bringing in technical help to fix the website – something President Obama repeated in his speech.
But the president also said he was frustrated that there was so much focus on the website, given that it was only 3 weeks into a 6-month enrollment process, and that many of the ACA’s benefits were already in place.
He addressed what he called "some of the politics that have swirled around the Affordable Care Act," noting Republican efforts to defund or repeal the ACA.
"And I’m sure that, given the problems with the website so far, they’re going to be looking to go after it even harder," he said.
But, he said, "We did not wage this long and contentious battle just around a website." The battle was "to make sure that millions of Americans in the wealthiest nation on Earth finally have the same chance to get the same security of affordable quality health care as anybody else."
Speaker of the House John Boehner (R-Ohio) was not impressed. "If the president is frustrated by the mounting failures of his health care law, it wasn’t apparent today," said Rep. Boehner, in a statement. "Instead of answers, we got well-worn talking points. Instead of explanations, we got excuses."
He added that "the House’s oversight of this failure is just beginning."
On Oct. 24, the House Energy and Commerce Committee will hold the first of what is likely to be many hearings on the website rollout. HHS Secretary Kathleen Sebelius was invited to testify, but declined because of a scheduling conflict.
Rep. Boehner said that "Secretary Sebelius must change her mind and appear at this week’s hearing."
On Twitter @aliciaault
*This story was updated 10/22/13.
RYGB showed better nutritional outcomes than duodenal switch
WASHINGTON – Despite better excess weight loss outcomes from the biliopancreatic diversion with duodenal switch, the Roux-en-Y gastric bypass procedure was associated with better nutritional outcomes in the superobese, according to the results of a prospective cohort study presented at this year’s American College of Surgeons Clinical Congress.
Investigators retrospectively analyzed data collected prospectively from 350 consecutive superobese patients, who underwent either biliopancreatic diversion with duodenal switch (BPD/DS; n = 198) or Roux-en-Y gastric bypass surgery (RYGB; n = 152), and compared long-term nutritional outcomes in each cohort. The research was conducted by Dr. Marc Ward and his colleagues at the University of Chicago, who presented the results.
The cohorts were self-selected and equally distributed across the sexes; each group’s mean age was just under 41 years. The mean body mass index (BMI) in the BPD/DS group was 59 kg/m2 and was 56 kg/m2 in RYGB. The preoperative body weight in the BPD/DS group was higher than that in the RYGB group (range, 267 lbs to 597 lbs. vs. 240 lbs to 505 lbs, respectively).
Although the BPD/DS had higher morbidity and mortality rates than did the RYGB, as well as more complications, such as altered bowel habits, the BPD/DS is associated with better comorbidity resolution independent of weight loss, and up to 20% greater excess weight loss in superobese patients. Superobesity is defined as having a BMI of 50 kg/m2 and above.
Because the reduction in intestinal absorptive surface area in BPD/DS is greater than in RYGB, the researchers theorized that the resultant nutritional deficiencies might be clinically significant enough to consider when counseling patients on procedure selection.
At seven postoperative follow-up points between 6 months and 8 years, the investigators obtained a variety of nutritional parameters from each group. Patients were given nutritional supplementation as clinically indicated.
Dr. Ward said that while he and his colleagues expected the BPD/DS group to have lower nutritional values, "We didn’t expect that 75% of our patients would have, at 4 years out, a below-normal level of vitamin A, compared to 23% in the RYGB patients."
There were similar surprises for other nutritional markers: At all time points, the BPD/DS group also had significantly more nutritional deficiencies than did the RYGB group in fat-soluble vitamins D and E, and in minerals selenium and zinc. Between years 1 and 3, iron values were near parity at about 20%, although the BPD/DS group was still more deficient, and at year 8 had more than double the rate of iron deficiency as RYGB patients.
Values for albumin, vitamin B12, ferritin, folate, and parathyroid hormone, however, were not significantly different between the two groups. Dr. Ward said that low nutritional values in patients, "does not necessarily mean they are developing symptoms or can’t be treated with supplementation."
Only one RYGB patient underwent revision because of insufficient weight loss, whereas five BPD/DS patients underwent revision, all for malnutrition.
"It’s absolutely crucial for people who elect to have a duodenal switch operation to have long-term, life-long nutritional follow-up," Dr. Ward told the audience. He also said that clinicians should closely evaluate their patients’ level of commitment to compliance over the long-term when discussing bariatric procedures.
WASHINGTON – Despite better excess weight loss outcomes from the biliopancreatic diversion with duodenal switch, the Roux-en-Y gastric bypass procedure was associated with better nutritional outcomes in the superobese, according to the results of a prospective cohort study presented at this year’s American College of Surgeons Clinical Congress.
Investigators retrospectively analyzed data collected prospectively from 350 consecutive superobese patients, who underwent either biliopancreatic diversion with duodenal switch (BPD/DS; n = 198) or Roux-en-Y gastric bypass surgery (RYGB; n = 152), and compared long-term nutritional outcomes in each cohort. The research was conducted by Dr. Marc Ward and his colleagues at the University of Chicago, who presented the results.
The cohorts were self-selected and equally distributed across the sexes; each group’s mean age was just under 41 years. The mean body mass index (BMI) in the BPD/DS group was 59 kg/m2 and was 56 kg/m2 in RYGB. The preoperative body weight in the BPD/DS group was higher than that in the RYGB group (range, 267 lbs to 597 lbs. vs. 240 lbs to 505 lbs, respectively).
Although the BPD/DS had higher morbidity and mortality rates than did the RYGB, as well as more complications, such as altered bowel habits, the BPD/DS is associated with better comorbidity resolution independent of weight loss, and up to 20% greater excess weight loss in superobese patients. Superobesity is defined as having a BMI of 50 kg/m2 and above.
Because the reduction in intestinal absorptive surface area in BPD/DS is greater than in RYGB, the researchers theorized that the resultant nutritional deficiencies might be clinically significant enough to consider when counseling patients on procedure selection.
At seven postoperative follow-up points between 6 months and 8 years, the investigators obtained a variety of nutritional parameters from each group. Patients were given nutritional supplementation as clinically indicated.
Dr. Ward said that while he and his colleagues expected the BPD/DS group to have lower nutritional values, "We didn’t expect that 75% of our patients would have, at 4 years out, a below-normal level of vitamin A, compared to 23% in the RYGB patients."
There were similar surprises for other nutritional markers: At all time points, the BPD/DS group also had significantly more nutritional deficiencies than did the RYGB group in fat-soluble vitamins D and E, and in minerals selenium and zinc. Between years 1 and 3, iron values were near parity at about 20%, although the BPD/DS group was still more deficient, and at year 8 had more than double the rate of iron deficiency as RYGB patients.
Values for albumin, vitamin B12, ferritin, folate, and parathyroid hormone, however, were not significantly different between the two groups. Dr. Ward said that low nutritional values in patients, "does not necessarily mean they are developing symptoms or can’t be treated with supplementation."
Only one RYGB patient underwent revision because of insufficient weight loss, whereas five BPD/DS patients underwent revision, all for malnutrition.
"It’s absolutely crucial for people who elect to have a duodenal switch operation to have long-term, life-long nutritional follow-up," Dr. Ward told the audience. He also said that clinicians should closely evaluate their patients’ level of commitment to compliance over the long-term when discussing bariatric procedures.
WASHINGTON – Despite better excess weight loss outcomes from the biliopancreatic diversion with duodenal switch, the Roux-en-Y gastric bypass procedure was associated with better nutritional outcomes in the superobese, according to the results of a prospective cohort study presented at this year’s American College of Surgeons Clinical Congress.
Investigators retrospectively analyzed data collected prospectively from 350 consecutive superobese patients, who underwent either biliopancreatic diversion with duodenal switch (BPD/DS; n = 198) or Roux-en-Y gastric bypass surgery (RYGB; n = 152), and compared long-term nutritional outcomes in each cohort. The research was conducted by Dr. Marc Ward and his colleagues at the University of Chicago, who presented the results.
The cohorts were self-selected and equally distributed across the sexes; each group’s mean age was just under 41 years. The mean body mass index (BMI) in the BPD/DS group was 59 kg/m2 and was 56 kg/m2 in RYGB. The preoperative body weight in the BPD/DS group was higher than that in the RYGB group (range, 267 lbs to 597 lbs. vs. 240 lbs to 505 lbs, respectively).
Although the BPD/DS had higher morbidity and mortality rates than did the RYGB, as well as more complications, such as altered bowel habits, the BPD/DS is associated with better comorbidity resolution independent of weight loss, and up to 20% greater excess weight loss in superobese patients. Superobesity is defined as having a BMI of 50 kg/m2 and above.
Because the reduction in intestinal absorptive surface area in BPD/DS is greater than in RYGB, the researchers theorized that the resultant nutritional deficiencies might be clinically significant enough to consider when counseling patients on procedure selection.
At seven postoperative follow-up points between 6 months and 8 years, the investigators obtained a variety of nutritional parameters from each group. Patients were given nutritional supplementation as clinically indicated.
Dr. Ward said that while he and his colleagues expected the BPD/DS group to have lower nutritional values, "We didn’t expect that 75% of our patients would have, at 4 years out, a below-normal level of vitamin A, compared to 23% in the RYGB patients."
There were similar surprises for other nutritional markers: At all time points, the BPD/DS group also had significantly more nutritional deficiencies than did the RYGB group in fat-soluble vitamins D and E, and in minerals selenium and zinc. Between years 1 and 3, iron values were near parity at about 20%, although the BPD/DS group was still more deficient, and at year 8 had more than double the rate of iron deficiency as RYGB patients.
Values for albumin, vitamin B12, ferritin, folate, and parathyroid hormone, however, were not significantly different between the two groups. Dr. Ward said that low nutritional values in patients, "does not necessarily mean they are developing symptoms or can’t be treated with supplementation."
Only one RYGB patient underwent revision because of insufficient weight loss, whereas five BPD/DS patients underwent revision, all for malnutrition.
"It’s absolutely crucial for people who elect to have a duodenal switch operation to have long-term, life-long nutritional follow-up," Dr. Ward told the audience. He also said that clinicians should closely evaluate their patients’ level of commitment to compliance over the long-term when discussing bariatric procedures.
AT THE ACS CLINICAL CONGRESS
Major finding: A significantly greater proportion of deficient nutritional values were found in duodenal switch patients at multiple postoperative points than in RYGB patients.
Data source: Retrospective review of prospective database study of 350 consecutive superobese patients tested at clinical follow-up post duodenal switch or RYGB surgery between 2002 and 2005.
Disclosures: Dr. Ward and his colleagues reported no relevant disclosures.
Mixed results with angiography for splenic injuries
SAN FRANCISCO – Trauma centers that nonselectively performed angiography on patients with high-grade blunt splenic injury did not significantly reduce the likelihood of delayed splenectomy in a retrospective analysis of data on 6,870 patients treated at 267 hospitals.
On an individual patient level, however, use of angiography was associated with a reduced risk of delayed splenectomy (more than 6 hours after admission) after researchers controlled for the influence of multiple other factors, Dr. Ben L. Zarzaur and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
These somewhat conflicting findings suggest that "nonselective protocol-driven use of angiography at the hospital in the setting of high-grade blunt splenic injury does not benefit in terms of splenic salvage. Angiography use should be tailored to the individual patient," said Dr. Zarzaur of the University of Tennessee, Memphis.
"Attention should be paid to overall injury severity and splenic injury severity" because more severe injuries were associated with delayed splenectomy in the study, he said, adding, "Particular attention should be considered for screening for splenic vascular abnormalities."
The investigators used data from the National Trauma Data Bank (NTDB) on adults treated for high-grade blunt splenic injury at Level I or II trauma centers that admitted at least 10 such patients in 2007-2010, with high-grade injury defined as Abbreviated Injury Scale grade 3 or higher. They stratified hospital angiography use as none, low (in less than 20% of patients with high-grade blunt splenic injury), or high (in 20% or more of these patients).
Approximately 30% of patients at high-angiography centers underwent urgent splenectomy, compared with 33%-36% at hospitals with no or low-angiography use, a difference that was statistically significant. While the likelihood of a delayed splenectomy was 33% higher at low-angiography hospitals and 49% higher at hospitals without angiography, compared with high-angiography hospitals, these differences were not significant, Dr. Zarzaur reported.
The investigators used the classification of hospitals – no-, low-, or high-angiography use – to represent the three schools of thought that have developed over the past few decades regarding angiography for patients with blunt splenic injury who do not undergo immediate urgent splenectomy. The minimalist school of thought recommends using observation, not angiography for blunt splenic injury. The maximalist school of thought favors protocol-driven use of angiography for patients with certain grades of spleen injury. In between, physicians who favor a selective strategy use CT or clinical criteria or both to try and identify patients at high risk for delayed splenectomy, and reserve the risks of angiography for those patients, he said.
They chose a cutoff of 20% angiography use in patients with high-grade blunt splenic injury to discriminate between low- and high-angiography use because that represented the 90th percentile for all trauma centers in the study.
Nine percent of patients were treated at hospitals that did not use angiography for blunt splenic injury, 66% at low-angiography hospitals, and 25% at high-angiography hospitals.
Patients with grade 5 blunt splenic injury were more than twice as likely to need delayed splenectomy, compared with patients with grade 3 or 4 injury. Higher overall Injury Severity Scores (10 or higher) also doubled the risk for delayed splenectomy.
Patients with grades 4 or 5 blunt splenic injury were significantly more likely to undergo angiography at high-angiography centers than at low-angiography centers. High-angiography centers were more likely than were low-angiography centers to remove spleens with grade 5 injury after angiography, though this difference did not reach statistical significance.
Continuing controversy around the use of angiography for blunt splenic injury is illustrated by a 2011 survey of members of the American Association for the Surgery of Trauma. Members favored observation, not angiography, for grades 1 and 2 spleen injuries but showed no consensus on higher-grade injuries (J. Trauma 2011;70:1026-31).
A recent study of 1,275 patients treated for blunt splenic injury at four trauma centers that showed a significantly better chance of saving the spleen at hospitals with higher use of splenic artery embolization, especially in patients with higher-grade splenic injury (J. Trauma Acute Care Surg. 2013;75:69-74).
The current study excluded patients who died on arrival at the hospital, patients who were admitted more than 24 hours after injury, and patients who underwent splenectomy within 6 hours of admission (early splenectomy).
Dr. Zarzaur reported having no financial disclosures.
On Twitter @sherryboschert
Almost 20 years after the initial description and 30 years after we began using splenic angiography in the management of blunt splenic injury, why is it that we simply can’t settle this question? When is splenic angiography and/or catheter therapy useful in high-grade injuries?
In this study, the authors have reviewed the National Trauma Data Bank (NTDB) and have demonstrated that, at hospitals that use angiography more frequently than other hospitals, the rate of delayed splenectomy in high-grade splenic injury (defined as grades 3-5) is not different. They suggest that angiography should be selective, with particular attention to screening for vascular abnormalities.

|
|
The authors collected information on both angiography and angiography with embolization. However, in the manuscript and the talk, they only refer to high-angiography centers. This seems to me to be a fundamental problem. This is particularly true because recent data from Jacksonville suggest that embolization of truly high-grade injury such as grade 4 injuries (even in the absence of blush) improves the salvage rate of nonoperative management.
In addition, the authors selected 20% as their cutoff for high- and low-angiography centers because 20% represented the 90th percentile of centers with regard to angiography use. I think that’s French for "it made the data analyzable." However, 20% is relatively low. If the authors wish to look at nonselective angiography they should look at us. We’re maximalists. We do angiography on 100% of patients with grades 3, 4 and 5 injuries. That’s nonselective use of angiography.
The intelligent use of this technique requires interpretation of CT and then the details of the patient presentation. For instance, a grade 4 splenic injury with or without blush but no hemoperitoneum and a totally stable patient is, in my mind, amenable to catheter therapy. Another patient with a grade 4 injury and reactive extravasation outside of the spleen and a huge peritoneum is probably best served by operative exploration. Both are grade 4 injuries, but the patients are fundamentally different.
The authors’ conclusions suggest that there is a relationship between splenic vascular injury identified on CT and success or failure of nonoperative management. This is clearly the authors’ prejudice, as they have published these findings a number of times. We’ve known for years, thanks to work from the authors’ institution, that expectant management of a patient with blush on CT fails 70% of the time and a significant number of the blushes are seen on day 3 but not day 1. Since the authors have absolutely no information on the presence or absence of blush in this data set, I fail to see how that can be one of their conclusions.
How, then, can we make sense of this? I believe the answer is in the manuscript’s last paragraph, which begins, "Another limitation of this study stems from the limitations of the NTDB." There’s little doubt that the NTDB can record an accurate snapshot of practice in the United States, but in my mind, it lacks the specificity to really answer the question, when is splenic angiography useful in high-grade injuries?
The authors have no information on presence of absence of blush, hemodynamic status other than at admission, blood transfusion rate, or technique of embolization, and they recognize that some of the data may not be accurate. I just don’t believe that the NTDB can actually answer this question.
In the end, rules are rarely helpful in the care of patients. Intelligent application of innovative techniques cannot solely be governed by rules. Perhaps the take-home message here is that the use of angiography and embolization to treat higher-grade splenic injuries is perhaps not something that everybody should be using. It may be that this technique is best preserved for high-volume centers with a real interest and a real expertise in this subject.
Dr. Thomas M. Scalea is a professor of surgery at the University of Maryland, Baltimore. These are excerpts of his remarks as a discussant of the study at the meeting. He reported having no financial disclosures.
Almost 20 years after the initial description and 30 years after we began using splenic angiography in the management of blunt splenic injury, why is it that we simply can’t settle this question? When is splenic angiography and/or catheter therapy useful in high-grade injuries?
In this study, the authors have reviewed the National Trauma Data Bank (NTDB) and have demonstrated that, at hospitals that use angiography more frequently than other hospitals, the rate of delayed splenectomy in high-grade splenic injury (defined as grades 3-5) is not different. They suggest that angiography should be selective, with particular attention to screening for vascular abnormalities.

|
|
The authors collected information on both angiography and angiography with embolization. However, in the manuscript and the talk, they only refer to high-angiography centers. This seems to me to be a fundamental problem. This is particularly true because recent data from Jacksonville suggest that embolization of truly high-grade injury such as grade 4 injuries (even in the absence of blush) improves the salvage rate of nonoperative management.
In addition, the authors selected 20% as their cutoff for high- and low-angiography centers because 20% represented the 90th percentile of centers with regard to angiography use. I think that’s French for "it made the data analyzable." However, 20% is relatively low. If the authors wish to look at nonselective angiography they should look at us. We’re maximalists. We do angiography on 100% of patients with grades 3, 4 and 5 injuries. That’s nonselective use of angiography.
The intelligent use of this technique requires interpretation of CT and then the details of the patient presentation. For instance, a grade 4 splenic injury with or without blush but no hemoperitoneum and a totally stable patient is, in my mind, amenable to catheter therapy. Another patient with a grade 4 injury and reactive extravasation outside of the spleen and a huge peritoneum is probably best served by operative exploration. Both are grade 4 injuries, but the patients are fundamentally different.
The authors’ conclusions suggest that there is a relationship between splenic vascular injury identified on CT and success or failure of nonoperative management. This is clearly the authors’ prejudice, as they have published these findings a number of times. We’ve known for years, thanks to work from the authors’ institution, that expectant management of a patient with blush on CT fails 70% of the time and a significant number of the blushes are seen on day 3 but not day 1. Since the authors have absolutely no information on the presence or absence of blush in this data set, I fail to see how that can be one of their conclusions.
How, then, can we make sense of this? I believe the answer is in the manuscript’s last paragraph, which begins, "Another limitation of this study stems from the limitations of the NTDB." There’s little doubt that the NTDB can record an accurate snapshot of practice in the United States, but in my mind, it lacks the specificity to really answer the question, when is splenic angiography useful in high-grade injuries?
The authors have no information on presence of absence of blush, hemodynamic status other than at admission, blood transfusion rate, or technique of embolization, and they recognize that some of the data may not be accurate. I just don’t believe that the NTDB can actually answer this question.
In the end, rules are rarely helpful in the care of patients. Intelligent application of innovative techniques cannot solely be governed by rules. Perhaps the take-home message here is that the use of angiography and embolization to treat higher-grade splenic injuries is perhaps not something that everybody should be using. It may be that this technique is best preserved for high-volume centers with a real interest and a real expertise in this subject.
Dr. Thomas M. Scalea is a professor of surgery at the University of Maryland, Baltimore. These are excerpts of his remarks as a discussant of the study at the meeting. He reported having no financial disclosures.
Almost 20 years after the initial description and 30 years after we began using splenic angiography in the management of blunt splenic injury, why is it that we simply can’t settle this question? When is splenic angiography and/or catheter therapy useful in high-grade injuries?
In this study, the authors have reviewed the National Trauma Data Bank (NTDB) and have demonstrated that, at hospitals that use angiography more frequently than other hospitals, the rate of delayed splenectomy in high-grade splenic injury (defined as grades 3-5) is not different. They suggest that angiography should be selective, with particular attention to screening for vascular abnormalities.

|
|
The authors collected information on both angiography and angiography with embolization. However, in the manuscript and the talk, they only refer to high-angiography centers. This seems to me to be a fundamental problem. This is particularly true because recent data from Jacksonville suggest that embolization of truly high-grade injury such as grade 4 injuries (even in the absence of blush) improves the salvage rate of nonoperative management.
In addition, the authors selected 20% as their cutoff for high- and low-angiography centers because 20% represented the 90th percentile of centers with regard to angiography use. I think that’s French for "it made the data analyzable." However, 20% is relatively low. If the authors wish to look at nonselective angiography they should look at us. We’re maximalists. We do angiography on 100% of patients with grades 3, 4 and 5 injuries. That’s nonselective use of angiography.
The intelligent use of this technique requires interpretation of CT and then the details of the patient presentation. For instance, a grade 4 splenic injury with or without blush but no hemoperitoneum and a totally stable patient is, in my mind, amenable to catheter therapy. Another patient with a grade 4 injury and reactive extravasation outside of the spleen and a huge peritoneum is probably best served by operative exploration. Both are grade 4 injuries, but the patients are fundamentally different.
The authors’ conclusions suggest that there is a relationship between splenic vascular injury identified on CT and success or failure of nonoperative management. This is clearly the authors’ prejudice, as they have published these findings a number of times. We’ve known for years, thanks to work from the authors’ institution, that expectant management of a patient with blush on CT fails 70% of the time and a significant number of the blushes are seen on day 3 but not day 1. Since the authors have absolutely no information on the presence or absence of blush in this data set, I fail to see how that can be one of their conclusions.
How, then, can we make sense of this? I believe the answer is in the manuscript’s last paragraph, which begins, "Another limitation of this study stems from the limitations of the NTDB." There’s little doubt that the NTDB can record an accurate snapshot of practice in the United States, but in my mind, it lacks the specificity to really answer the question, when is splenic angiography useful in high-grade injuries?
The authors have no information on presence of absence of blush, hemodynamic status other than at admission, blood transfusion rate, or technique of embolization, and they recognize that some of the data may not be accurate. I just don’t believe that the NTDB can actually answer this question.
In the end, rules are rarely helpful in the care of patients. Intelligent application of innovative techniques cannot solely be governed by rules. Perhaps the take-home message here is that the use of angiography and embolization to treat higher-grade splenic injuries is perhaps not something that everybody should be using. It may be that this technique is best preserved for high-volume centers with a real interest and a real expertise in this subject.
Dr. Thomas M. Scalea is a professor of surgery at the University of Maryland, Baltimore. These are excerpts of his remarks as a discussant of the study at the meeting. He reported having no financial disclosures.
SAN FRANCISCO – Trauma centers that nonselectively performed angiography on patients with high-grade blunt splenic injury did not significantly reduce the likelihood of delayed splenectomy in a retrospective analysis of data on 6,870 patients treated at 267 hospitals.
On an individual patient level, however, use of angiography was associated with a reduced risk of delayed splenectomy (more than 6 hours after admission) after researchers controlled for the influence of multiple other factors, Dr. Ben L. Zarzaur and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
These somewhat conflicting findings suggest that "nonselective protocol-driven use of angiography at the hospital in the setting of high-grade blunt splenic injury does not benefit in terms of splenic salvage. Angiography use should be tailored to the individual patient," said Dr. Zarzaur of the University of Tennessee, Memphis.
"Attention should be paid to overall injury severity and splenic injury severity" because more severe injuries were associated with delayed splenectomy in the study, he said, adding, "Particular attention should be considered for screening for splenic vascular abnormalities."
The investigators used data from the National Trauma Data Bank (NTDB) on adults treated for high-grade blunt splenic injury at Level I or II trauma centers that admitted at least 10 such patients in 2007-2010, with high-grade injury defined as Abbreviated Injury Scale grade 3 or higher. They stratified hospital angiography use as none, low (in less than 20% of patients with high-grade blunt splenic injury), or high (in 20% or more of these patients).
Approximately 30% of patients at high-angiography centers underwent urgent splenectomy, compared with 33%-36% at hospitals with no or low-angiography use, a difference that was statistically significant. While the likelihood of a delayed splenectomy was 33% higher at low-angiography hospitals and 49% higher at hospitals without angiography, compared with high-angiography hospitals, these differences were not significant, Dr. Zarzaur reported.
The investigators used the classification of hospitals – no-, low-, or high-angiography use – to represent the three schools of thought that have developed over the past few decades regarding angiography for patients with blunt splenic injury who do not undergo immediate urgent splenectomy. The minimalist school of thought recommends using observation, not angiography for blunt splenic injury. The maximalist school of thought favors protocol-driven use of angiography for patients with certain grades of spleen injury. In between, physicians who favor a selective strategy use CT or clinical criteria or both to try and identify patients at high risk for delayed splenectomy, and reserve the risks of angiography for those patients, he said.
They chose a cutoff of 20% angiography use in patients with high-grade blunt splenic injury to discriminate between low- and high-angiography use because that represented the 90th percentile for all trauma centers in the study.
Nine percent of patients were treated at hospitals that did not use angiography for blunt splenic injury, 66% at low-angiography hospitals, and 25% at high-angiography hospitals.
Patients with grade 5 blunt splenic injury were more than twice as likely to need delayed splenectomy, compared with patients with grade 3 or 4 injury. Higher overall Injury Severity Scores (10 or higher) also doubled the risk for delayed splenectomy.
Patients with grades 4 or 5 blunt splenic injury were significantly more likely to undergo angiography at high-angiography centers than at low-angiography centers. High-angiography centers were more likely than were low-angiography centers to remove spleens with grade 5 injury after angiography, though this difference did not reach statistical significance.
Continuing controversy around the use of angiography for blunt splenic injury is illustrated by a 2011 survey of members of the American Association for the Surgery of Trauma. Members favored observation, not angiography, for grades 1 and 2 spleen injuries but showed no consensus on higher-grade injuries (J. Trauma 2011;70:1026-31).
A recent study of 1,275 patients treated for blunt splenic injury at four trauma centers that showed a significantly better chance of saving the spleen at hospitals with higher use of splenic artery embolization, especially in patients with higher-grade splenic injury (J. Trauma Acute Care Surg. 2013;75:69-74).
The current study excluded patients who died on arrival at the hospital, patients who were admitted more than 24 hours after injury, and patients who underwent splenectomy within 6 hours of admission (early splenectomy).
Dr. Zarzaur reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Trauma centers that nonselectively performed angiography on patients with high-grade blunt splenic injury did not significantly reduce the likelihood of delayed splenectomy in a retrospective analysis of data on 6,870 patients treated at 267 hospitals.
On an individual patient level, however, use of angiography was associated with a reduced risk of delayed splenectomy (more than 6 hours after admission) after researchers controlled for the influence of multiple other factors, Dr. Ben L. Zarzaur and his associates reported at the annual meeting of the American Association for the Surgery of Trauma.
These somewhat conflicting findings suggest that "nonselective protocol-driven use of angiography at the hospital in the setting of high-grade blunt splenic injury does not benefit in terms of splenic salvage. Angiography use should be tailored to the individual patient," said Dr. Zarzaur of the University of Tennessee, Memphis.
"Attention should be paid to overall injury severity and splenic injury severity" because more severe injuries were associated with delayed splenectomy in the study, he said, adding, "Particular attention should be considered for screening for splenic vascular abnormalities."
The investigators used data from the National Trauma Data Bank (NTDB) on adults treated for high-grade blunt splenic injury at Level I or II trauma centers that admitted at least 10 such patients in 2007-2010, with high-grade injury defined as Abbreviated Injury Scale grade 3 or higher. They stratified hospital angiography use as none, low (in less than 20% of patients with high-grade blunt splenic injury), or high (in 20% or more of these patients).
Approximately 30% of patients at high-angiography centers underwent urgent splenectomy, compared with 33%-36% at hospitals with no or low-angiography use, a difference that was statistically significant. While the likelihood of a delayed splenectomy was 33% higher at low-angiography hospitals and 49% higher at hospitals without angiography, compared with high-angiography hospitals, these differences were not significant, Dr. Zarzaur reported.
The investigators used the classification of hospitals – no-, low-, or high-angiography use – to represent the three schools of thought that have developed over the past few decades regarding angiography for patients with blunt splenic injury who do not undergo immediate urgent splenectomy. The minimalist school of thought recommends using observation, not angiography for blunt splenic injury. The maximalist school of thought favors protocol-driven use of angiography for patients with certain grades of spleen injury. In between, physicians who favor a selective strategy use CT or clinical criteria or both to try and identify patients at high risk for delayed splenectomy, and reserve the risks of angiography for those patients, he said.
They chose a cutoff of 20% angiography use in patients with high-grade blunt splenic injury to discriminate between low- and high-angiography use because that represented the 90th percentile for all trauma centers in the study.
Nine percent of patients were treated at hospitals that did not use angiography for blunt splenic injury, 66% at low-angiography hospitals, and 25% at high-angiography hospitals.
Patients with grade 5 blunt splenic injury were more than twice as likely to need delayed splenectomy, compared with patients with grade 3 or 4 injury. Higher overall Injury Severity Scores (10 or higher) also doubled the risk for delayed splenectomy.
Patients with grades 4 or 5 blunt splenic injury were significantly more likely to undergo angiography at high-angiography centers than at low-angiography centers. High-angiography centers were more likely than were low-angiography centers to remove spleens with grade 5 injury after angiography, though this difference did not reach statistical significance.
Continuing controversy around the use of angiography for blunt splenic injury is illustrated by a 2011 survey of members of the American Association for the Surgery of Trauma. Members favored observation, not angiography, for grades 1 and 2 spleen injuries but showed no consensus on higher-grade injuries (J. Trauma 2011;70:1026-31).
A recent study of 1,275 patients treated for blunt splenic injury at four trauma centers that showed a significantly better chance of saving the spleen at hospitals with higher use of splenic artery embolization, especially in patients with higher-grade splenic injury (J. Trauma Acute Care Surg. 2013;75:69-74).
The current study excluded patients who died on arrival at the hospital, patients who were admitted more than 24 hours after injury, and patients who underwent splenectomy within 6 hours of admission (early splenectomy).
Dr. Zarzaur reported having no financial disclosures.
On Twitter @sherryboschert
AT THE AAST ANNUAL MEETING
Major finding: The likelihood of delayed splenectomy was 33% higher at centers without angiography and 49% higher at low-angiography centers, compared with high-angiography centers, but the differences were not statistically significant.
Data source: Retrospective analysis of data from the National Trauma Data Bank on 6,870 patients treated for blunt splenic injury at 267 hospitals.
Disclosures: Dr. Zarzaur reported having no financial disclosures.
Early walking and eating improve outcomes for bowel surgery patients
WASHINGTON – A program to enhance recovery from bowel surgery significantly decreased the incidence rate of both postoperative ileus and 30-day readmission.
Early walking and postoperative alimentation, beginning with three times on postop day 1, drove the improved outcomes, Dr. Terrence Loftus reported at the annual clinical congress of the American College of Surgeons.
His 3-year retrospective study compared outcomes before and after the program was implemented. In addition to confirming the benefits, the study says something about how change becomes part of a systemwide culture, said Dr. Loftus, medical director of surgical services and clinical resources for Banner Health, Phoenix.
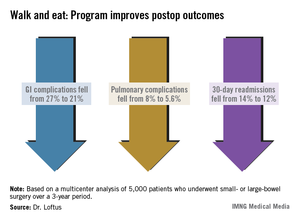
"When you see a before-and-after picture, it gives you the sense that change is like clicking on a light switch, and then everything starts happening. In reality, it’s not. It’s something that catches on over time. It’s a social process that gets passed along. It’s not Dr. Loftus coming in and showing a few slides and saying, ‘Okay, now this is what you have to do.’ It’s surgeons having conversations in the lounge and saying, ‘I tried this and it worked. You should try it too.’ "
In 2010, Banner Health System’s Surgery Clinical Consensus Group decided to tackle the system’s less-than-stellar rates of postoperative ileus and readmission among patients who had bowel surgery.
While there are a number of published enhanced-care pathways, Dr. Loftus found a wide variety of applications over Banner Health’s large medical system. "In some places, people didn’t even know about them, and in others they were using their own version," he said. "We needed a systemwide approach to recovery – something that would be useful over a large, heterogeneous health care system."
The Banner consensus group came up with the Bowel Surgery Strategic Initiative, a program implemented in 19 hospitals spread over six states, covered by 119 surgeons. The system was widely diversified, including everything from an 18-bed critical access unit to a large 600-bed level I trauma center.
"The surgeons ranged from solo private practice, to group practices, hospital employed, and locum tenens, so you can begin to understand the wide diversity of practices and environments we were trying to implement this in," Dr. Loftus said.
The first step was to develop a literature-based GI care pathway that could be widely implemented. The group’s first version had 80 steps – vastly too many.
The final program was based on just two drivers: early alimentation and early ambulation. Eating began with the earliest possible removal of the nasogastric tube (if present), 200-250 cc of oral intake on postoperative day 1, and a progressively advancing diet. Ambulation started with getting up and walking three times on postop day 1, with progressively increasing physical activity.
The team studied a 3-year period spanning 18 months on either side of the implementation date of July 2011. The analysis included 5,000 patients who had undergone elective small- or large-bowel surgery during that time period.
Dr. Loftus said there was a significant increase in both ambulation and alimentation. Before the program, the mean ambulation rate was 53%; that increased to 73% afterward. Alimentation increased from 51% to 71%. The composite of both drivers increased from 53% to 72%.
There were associated improvements in most of the clinical outcomes assessed. Overall, the complication rate decreased significantly, from 36% to 29%. Gastrointestinal complications fell from 27% to 21%, with the incidence of postoperative ileus declining from 27% to 21%. Pulmonary complications also decreased significantly, from 8% to 5.6%.
Thirty-day readmission declined from 14% to 12% – also a significant decrease. This was expected, Dr. Loftus said, because most of the readmissions were due to paralytic ileus.
Length of stay, however, was not significantly affected. There was no change in mortality.
The project also illuminated the importance of a facility’s own professional culture. Chart audits found that some facilities were only ambulating patients about a third of the time that it was ordered – even though nursing estimated the rate at 80% and surgeons at 70%.
Facilities that had particularly good postintervention outcomes were already ahead of the game before the project. "These already had a culture of activity. Nursing was getting patients up on day 1, and they set up their schedules in a way that says, ‘These patients will get up. We are going to do this no matter what.’ "
Dr. Loftus had no financial disclosures.
WASHINGTON – A program to enhance recovery from bowel surgery significantly decreased the incidence rate of both postoperative ileus and 30-day readmission.
Early walking and postoperative alimentation, beginning with three times on postop day 1, drove the improved outcomes, Dr. Terrence Loftus reported at the annual clinical congress of the American College of Surgeons.
His 3-year retrospective study compared outcomes before and after the program was implemented. In addition to confirming the benefits, the study says something about how change becomes part of a systemwide culture, said Dr. Loftus, medical director of surgical services and clinical resources for Banner Health, Phoenix.

"When you see a before-and-after picture, it gives you the sense that change is like clicking on a light switch, and then everything starts happening. In reality, it’s not. It’s something that catches on over time. It’s a social process that gets passed along. It’s not Dr. Loftus coming in and showing a few slides and saying, ‘Okay, now this is what you have to do.’ It’s surgeons having conversations in the lounge and saying, ‘I tried this and it worked. You should try it too.’ "
In 2010, Banner Health System’s Surgery Clinical Consensus Group decided to tackle the system’s less-than-stellar rates of postoperative ileus and readmission among patients who had bowel surgery.
While there are a number of published enhanced-care pathways, Dr. Loftus found a wide variety of applications over Banner Health’s large medical system. "In some places, people didn’t even know about them, and in others they were using their own version," he said. "We needed a systemwide approach to recovery – something that would be useful over a large, heterogeneous health care system."
The Banner consensus group came up with the Bowel Surgery Strategic Initiative, a program implemented in 19 hospitals spread over six states, covered by 119 surgeons. The system was widely diversified, including everything from an 18-bed critical access unit to a large 600-bed level I trauma center.
"The surgeons ranged from solo private practice, to group practices, hospital employed, and locum tenens, so you can begin to understand the wide diversity of practices and environments we were trying to implement this in," Dr. Loftus said.
The first step was to develop a literature-based GI care pathway that could be widely implemented. The group’s first version had 80 steps – vastly too many.
The final program was based on just two drivers: early alimentation and early ambulation. Eating began with the earliest possible removal of the nasogastric tube (if present), 200-250 cc of oral intake on postoperative day 1, and a progressively advancing diet. Ambulation started with getting up and walking three times on postop day 1, with progressively increasing physical activity.
The team studied a 3-year period spanning 18 months on either side of the implementation date of July 2011. The analysis included 5,000 patients who had undergone elective small- or large-bowel surgery during that time period.
Dr. Loftus said there was a significant increase in both ambulation and alimentation. Before the program, the mean ambulation rate was 53%; that increased to 73% afterward. Alimentation increased from 51% to 71%. The composite of both drivers increased from 53% to 72%.
There were associated improvements in most of the clinical outcomes assessed. Overall, the complication rate decreased significantly, from 36% to 29%. Gastrointestinal complications fell from 27% to 21%, with the incidence of postoperative ileus declining from 27% to 21%. Pulmonary complications also decreased significantly, from 8% to 5.6%.
Thirty-day readmission declined from 14% to 12% – also a significant decrease. This was expected, Dr. Loftus said, because most of the readmissions were due to paralytic ileus.
Length of stay, however, was not significantly affected. There was no change in mortality.
The project also illuminated the importance of a facility’s own professional culture. Chart audits found that some facilities were only ambulating patients about a third of the time that it was ordered – even though nursing estimated the rate at 80% and surgeons at 70%.
Facilities that had particularly good postintervention outcomes were already ahead of the game before the project. "These already had a culture of activity. Nursing was getting patients up on day 1, and they set up their schedules in a way that says, ‘These patients will get up. We are going to do this no matter what.’ "
Dr. Loftus had no financial disclosures.
WASHINGTON – A program to enhance recovery from bowel surgery significantly decreased the incidence rate of both postoperative ileus and 30-day readmission.
Early walking and postoperative alimentation, beginning with three times on postop day 1, drove the improved outcomes, Dr. Terrence Loftus reported at the annual clinical congress of the American College of Surgeons.
His 3-year retrospective study compared outcomes before and after the program was implemented. In addition to confirming the benefits, the study says something about how change becomes part of a systemwide culture, said Dr. Loftus, medical director of surgical services and clinical resources for Banner Health, Phoenix.

"When you see a before-and-after picture, it gives you the sense that change is like clicking on a light switch, and then everything starts happening. In reality, it’s not. It’s something that catches on over time. It’s a social process that gets passed along. It’s not Dr. Loftus coming in and showing a few slides and saying, ‘Okay, now this is what you have to do.’ It’s surgeons having conversations in the lounge and saying, ‘I tried this and it worked. You should try it too.’ "
In 2010, Banner Health System’s Surgery Clinical Consensus Group decided to tackle the system’s less-than-stellar rates of postoperative ileus and readmission among patients who had bowel surgery.
While there are a number of published enhanced-care pathways, Dr. Loftus found a wide variety of applications over Banner Health’s large medical system. "In some places, people didn’t even know about them, and in others they were using their own version," he said. "We needed a systemwide approach to recovery – something that would be useful over a large, heterogeneous health care system."
The Banner consensus group came up with the Bowel Surgery Strategic Initiative, a program implemented in 19 hospitals spread over six states, covered by 119 surgeons. The system was widely diversified, including everything from an 18-bed critical access unit to a large 600-bed level I trauma center.
"The surgeons ranged from solo private practice, to group practices, hospital employed, and locum tenens, so you can begin to understand the wide diversity of practices and environments we were trying to implement this in," Dr. Loftus said.
The first step was to develop a literature-based GI care pathway that could be widely implemented. The group’s first version had 80 steps – vastly too many.
The final program was based on just two drivers: early alimentation and early ambulation. Eating began with the earliest possible removal of the nasogastric tube (if present), 200-250 cc of oral intake on postoperative day 1, and a progressively advancing diet. Ambulation started with getting up and walking three times on postop day 1, with progressively increasing physical activity.
The team studied a 3-year period spanning 18 months on either side of the implementation date of July 2011. The analysis included 5,000 patients who had undergone elective small- or large-bowel surgery during that time period.
Dr. Loftus said there was a significant increase in both ambulation and alimentation. Before the program, the mean ambulation rate was 53%; that increased to 73% afterward. Alimentation increased from 51% to 71%. The composite of both drivers increased from 53% to 72%.
There were associated improvements in most of the clinical outcomes assessed. Overall, the complication rate decreased significantly, from 36% to 29%. Gastrointestinal complications fell from 27% to 21%, with the incidence of postoperative ileus declining from 27% to 21%. Pulmonary complications also decreased significantly, from 8% to 5.6%.
Thirty-day readmission declined from 14% to 12% – also a significant decrease. This was expected, Dr. Loftus said, because most of the readmissions were due to paralytic ileus.
Length of stay, however, was not significantly affected. There was no change in mortality.
The project also illuminated the importance of a facility’s own professional culture. Chart audits found that some facilities were only ambulating patients about a third of the time that it was ordered – even though nursing estimated the rate at 80% and surgeons at 70%.
Facilities that had particularly good postintervention outcomes were already ahead of the game before the project. "These already had a culture of activity. Nursing was getting patients up on day 1, and they set up their schedules in a way that says, ‘These patients will get up. We are going to do this no matter what.’ "
Dr. Loftus had no financial disclosures.
AT THE ACS CLINICAL CONGRESS
Major finding: A recovery program based on early ambulation and alimentation for patients with bowel surgery decreased readmission from 14% to 12%, and postoperative complications from 36% to 29%.
Data source: The retrospective study contained 5,000 patients who had elective small- or large-bowel surgery.
Disclosures: Dr. Loftus had no financial disclosures.
Congress approves deal to open government, leaves health reform intact
Congress has approved a deal to fully reopen the federal government and keep the nation from defaulting on its debt. The agreement does not delay or defund the Affordable Care Act.
On Oct. 16 – 16 days into the government shutdown and about a day before the United States was set to breach its debt ceiling – the Senate voted 81-18 to approve a bipartisan compromise brokered by Senate Majority Leader Harry Reid (D-Nev.) and Minority Leader Mitch McConnell (R-Ky.). Later that night, the House approved the bill 285-144. The president signed the legislation in the early hours of Oct. 17.
The law funds the federal government at its current levels until Jan. 15, 2014, keeping the sequestration cuts in effect. It also allows the government to continue to borrow money to meet its financial obligations until Feb. 7, 2014. In the meantime, lawmakers have agreed to convene a budget conference to work out a long-range budget plan by mid-December.
Although the bill does not fundamentally change the Affordable Care Act (ACA), it does require the Department of Health and Human Services to beef up its income verification for individuals seeking subsidies to purchase insurance on the exchanges.
Last July, the agency announced that it would not verify the income of every applicant who applied for federal subsidies through the exchanges. Instead, federal officials planned to audit a sample of applications to ensure that individuals were getting the appropriate federal insurance subsidies. GOP lawmakers objected to this approach, saying that relying too heavily on the honor system in determining federal subsidies would leave the government open to fraud.
Under the new law, HHS must submit a report to Congress on its verification procedures by Jan. 1, 2014, and the HHS Inspector General must submit a progress report by July 2014 on the effectiveness of the new procedures.
Although the new law doesn’t do anything to hamper the implementation of the ACA, opponents have not given up. Sen. McConnell, who negotiated the deal with Democrats, said that Republicans will keep trying to repeal the ACA.
"This law is ravaging our economy, killing jobs, driving up premiums, and driving people off the health care plans they have and like in droves," he said on the Senate floor. "Its disastrous rollout is a sign of even worse things to come. And the Democrat refusal to delay it reflects a stubborn ideological obsession that will do untold damage to our country."
On Twitter @MaryEllenNY
Congress has approved a deal to fully reopen the federal government and keep the nation from defaulting on its debt. The agreement does not delay or defund the Affordable Care Act.
On Oct. 16 – 16 days into the government shutdown and about a day before the United States was set to breach its debt ceiling – the Senate voted 81-18 to approve a bipartisan compromise brokered by Senate Majority Leader Harry Reid (D-Nev.) and Minority Leader Mitch McConnell (R-Ky.). Later that night, the House approved the bill 285-144. The president signed the legislation in the early hours of Oct. 17.
The law funds the federal government at its current levels until Jan. 15, 2014, keeping the sequestration cuts in effect. It also allows the government to continue to borrow money to meet its financial obligations until Feb. 7, 2014. In the meantime, lawmakers have agreed to convene a budget conference to work out a long-range budget plan by mid-December.
Although the bill does not fundamentally change the Affordable Care Act (ACA), it does require the Department of Health and Human Services to beef up its income verification for individuals seeking subsidies to purchase insurance on the exchanges.
Last July, the agency announced that it would not verify the income of every applicant who applied for federal subsidies through the exchanges. Instead, federal officials planned to audit a sample of applications to ensure that individuals were getting the appropriate federal insurance subsidies. GOP lawmakers objected to this approach, saying that relying too heavily on the honor system in determining federal subsidies would leave the government open to fraud.
Under the new law, HHS must submit a report to Congress on its verification procedures by Jan. 1, 2014, and the HHS Inspector General must submit a progress report by July 2014 on the effectiveness of the new procedures.
Although the new law doesn’t do anything to hamper the implementation of the ACA, opponents have not given up. Sen. McConnell, who negotiated the deal with Democrats, said that Republicans will keep trying to repeal the ACA.
"This law is ravaging our economy, killing jobs, driving up premiums, and driving people off the health care plans they have and like in droves," he said on the Senate floor. "Its disastrous rollout is a sign of even worse things to come. And the Democrat refusal to delay it reflects a stubborn ideological obsession that will do untold damage to our country."
On Twitter @MaryEllenNY
Congress has approved a deal to fully reopen the federal government and keep the nation from defaulting on its debt. The agreement does not delay or defund the Affordable Care Act.
On Oct. 16 – 16 days into the government shutdown and about a day before the United States was set to breach its debt ceiling – the Senate voted 81-18 to approve a bipartisan compromise brokered by Senate Majority Leader Harry Reid (D-Nev.) and Minority Leader Mitch McConnell (R-Ky.). Later that night, the House approved the bill 285-144. The president signed the legislation in the early hours of Oct. 17.
The law funds the federal government at its current levels until Jan. 15, 2014, keeping the sequestration cuts in effect. It also allows the government to continue to borrow money to meet its financial obligations until Feb. 7, 2014. In the meantime, lawmakers have agreed to convene a budget conference to work out a long-range budget plan by mid-December.
Although the bill does not fundamentally change the Affordable Care Act (ACA), it does require the Department of Health and Human Services to beef up its income verification for individuals seeking subsidies to purchase insurance on the exchanges.
Last July, the agency announced that it would not verify the income of every applicant who applied for federal subsidies through the exchanges. Instead, federal officials planned to audit a sample of applications to ensure that individuals were getting the appropriate federal insurance subsidies. GOP lawmakers objected to this approach, saying that relying too heavily on the honor system in determining federal subsidies would leave the government open to fraud.
Under the new law, HHS must submit a report to Congress on its verification procedures by Jan. 1, 2014, and the HHS Inspector General must submit a progress report by July 2014 on the effectiveness of the new procedures.
Although the new law doesn’t do anything to hamper the implementation of the ACA, opponents have not given up. Sen. McConnell, who negotiated the deal with Democrats, said that Republicans will keep trying to repeal the ACA.
"This law is ravaging our economy, killing jobs, driving up premiums, and driving people off the health care plans they have and like in droves," he said on the Senate floor. "Its disastrous rollout is a sign of even worse things to come. And the Democrat refusal to delay it reflects a stubborn ideological obsession that will do untold damage to our country."
On Twitter @MaryEllenNY
Alvimopan reduces ileus, cuts hospital time in bowel surgery
WASHINGTON – The nonopioid pain reliever alvimopan significantly decreased the incidence of postoperative ileus and shortened hospital stay among bowel surgery patients who took the drug as part of an existing accelerated recovery program.
Compared with patients who took placebo, those who took alvimopan left the hospital about 1 day sooner. In addition to the decrease in ileus, patients experienced a quicker return to normal bowel function, including time to first flatus and first bowel movement, Dr. Robert Moesinger said at the annual clinical congress of the American College of Surgeons.
"Alvimopan augmented the already improved outcomes we had seen with our validated recovery pathway, in both laparoscopic- and open-surgery patients," said Dr. Moesinger of Intermountain Healthcare, Salt Lake City. "Given the very diverse nature of our health care system, with multiple types of hospitals and surgeons, we feel these data are widely applicable and we are very comfortable recommending its routine use for patients having elective bowel surgery."
Dr. Moesinger and his colleagues conducted a randomized, placebo-controlled trial of alvimopan in 248 such patients. Those taking the study drug received 12 mg before surgery; after surgery, they received 12 mg twice a day until discharge.
The primary endpoint was postoperative length of stay. Secondary endpoints included the incidence of postoperative ileus, time to first flatus and first bowel movement, tolerance of solid food, total hospital and pharmacy costs, nasogastric tube reinsertion, 30-day readmission rates, and anastomotic leak.
The patients’ mean age was 61 years. There were no significant differences in any of their baseline demographics or clinical characteristics. Because of a statistical fluke, the placebo group did contain significantly more open-surgery patients than the alvimopan group (34% vs. 20%). The rest of the patients had laparoscopic surgery. Patients had an average of nine doses of the study drug.
The mean length of stay was 4 days in the alvimopan group and 5 in the placebo group – a significant difference.
There was significantly less postoperative ileus in the alvimopan group (2% vs. 10%). Significantly fewer of those taking the study drug needed a nasogastric tube reinserted (2% vs. 9%). The rates of 30-day readmission, reoperation, and anastomotic leak were similar in both groups. The mean time to first bowel movement was about 1 day sooner in the alvimopan group.
The median hospital cost was $10,832 for the alvimopan group and $11,924 for the placebo group – a significant difference. The median total pharmacy cost was $476 vs. $501; this difference was not significant.
Because Cubist Pharmaceuticals, which makes alvimopan, provided the study drug at no cost, the overall cost analysis cannot be considered complete, Dr. Moesinger noted. However, the pharmacy cost for a similar course of the drug would be $84. Figuring that cost into the total saved for each admission ($1,686) still yielded a net financial benefit of $930 per patient, he said.
Dr. Moesinger had no financial disclosures.
Alvimopan (Entereg, Cubist Pharmaceuticals) is an orally administered, peripherally acting mu-opioid receptor antagonist that does not cross the blood-brain barrier. It is designed to reverse opioid-induced changes in the gastrointestinal tract without adversely affecting opioid-induced analgesia.

|
| Dr. Brian E. Lacy |
Alvimopan, the first FDA-approved medication for the treatment of postoperative ileus (POI), was approved in May 2008 with a Risk Evaluation and Mitigation Strategy in place, due to concerns over an increased number of myocardial infarctions in one research study.
Postoperative ileus is the impairment of gastrointestinal motility after abdominal or pelvic surgery, and is an expected complication of major abdominal surgery. It can affect all segments of the gastrointestinal tract and may lead to symptoms of nausea, vomiting, bloating, distention, constipation, and inability to evacuate flatus. Several studies have demonstrated that POI delays hospital discharges, increases the rate of hospital readmission after abdominal surgery, and increases the risk of postoperative complications. As such, POI imposes a significant economic impact on the health care system.
The current study confirms earlier studies involving over 2,000 patients that led to the approval of alvimopan. The current study was a single-center, randomized, double-blind study comparing placebo to alvimopan. Patients randomized to alvimopan (12 mg preoperatively; 12 mg twice daily until discharge) were discharged from the hospital 1 day earlier, were less likely to have a nasogastric tube reinserted, and had a bowel movement 1 day earlier, compared with those patients receiving placebo. The authors reported a significant cost savings per patient.*
These findings are important given the absence of other FDA-approved medications for the treatment of POI and the lack of other effective treatments. Alvimopan is available only to patients who are in hospitals that are registered to use this medication under the EASE (Entereg Access Support and Education) program. This recent study should prompt nonparticipating hospitals to consider routine use of alvimopan in all patients undergoing elective and emergent surgeries at risk for development of POI.
Dr. Brian E. Lacy is a professor of medicine at the Geisel School of Medicine at Dartmouth, Hanover, N.H., and chief of the section of gastroenterology and hepatology at Dartmouth-Hitchcock Medical Center. He has no relevant conflicts of interest.
*CORRECTION 11/21/13: The original version of this story misstated the cost savings per patient.
Alvimopan (Entereg, Cubist Pharmaceuticals) is an orally administered, peripherally acting mu-opioid receptor antagonist that does not cross the blood-brain barrier. It is designed to reverse opioid-induced changes in the gastrointestinal tract without adversely affecting opioid-induced analgesia.

|
| Dr. Brian E. Lacy |
Alvimopan, the first FDA-approved medication for the treatment of postoperative ileus (POI), was approved in May 2008 with a Risk Evaluation and Mitigation Strategy in place, due to concerns over an increased number of myocardial infarctions in one research study.
Postoperative ileus is the impairment of gastrointestinal motility after abdominal or pelvic surgery, and is an expected complication of major abdominal surgery. It can affect all segments of the gastrointestinal tract and may lead to symptoms of nausea, vomiting, bloating, distention, constipation, and inability to evacuate flatus. Several studies have demonstrated that POI delays hospital discharges, increases the rate of hospital readmission after abdominal surgery, and increases the risk of postoperative complications. As such, POI imposes a significant economic impact on the health care system.
The current study confirms earlier studies involving over 2,000 patients that led to the approval of alvimopan. The current study was a single-center, randomized, double-blind study comparing placebo to alvimopan. Patients randomized to alvimopan (12 mg preoperatively; 12 mg twice daily until discharge) were discharged from the hospital 1 day earlier, were less likely to have a nasogastric tube reinserted, and had a bowel movement 1 day earlier, compared with those patients receiving placebo. The authors reported a significant cost savings per patient.*
These findings are important given the absence of other FDA-approved medications for the treatment of POI and the lack of other effective treatments. Alvimopan is available only to patients who are in hospitals that are registered to use this medication under the EASE (Entereg Access Support and Education) program. This recent study should prompt nonparticipating hospitals to consider routine use of alvimopan in all patients undergoing elective and emergent surgeries at risk for development of POI.
Dr. Brian E. Lacy is a professor of medicine at the Geisel School of Medicine at Dartmouth, Hanover, N.H., and chief of the section of gastroenterology and hepatology at Dartmouth-Hitchcock Medical Center. He has no relevant conflicts of interest.
*CORRECTION 11/21/13: The original version of this story misstated the cost savings per patient.
Alvimopan (Entereg, Cubist Pharmaceuticals) is an orally administered, peripherally acting mu-opioid receptor antagonist that does not cross the blood-brain barrier. It is designed to reverse opioid-induced changes in the gastrointestinal tract without adversely affecting opioid-induced analgesia.

|
| Dr. Brian E. Lacy |
Alvimopan, the first FDA-approved medication for the treatment of postoperative ileus (POI), was approved in May 2008 with a Risk Evaluation and Mitigation Strategy in place, due to concerns over an increased number of myocardial infarctions in one research study.
Postoperative ileus is the impairment of gastrointestinal motility after abdominal or pelvic surgery, and is an expected complication of major abdominal surgery. It can affect all segments of the gastrointestinal tract and may lead to symptoms of nausea, vomiting, bloating, distention, constipation, and inability to evacuate flatus. Several studies have demonstrated that POI delays hospital discharges, increases the rate of hospital readmission after abdominal surgery, and increases the risk of postoperative complications. As such, POI imposes a significant economic impact on the health care system.
The current study confirms earlier studies involving over 2,000 patients that led to the approval of alvimopan. The current study was a single-center, randomized, double-blind study comparing placebo to alvimopan. Patients randomized to alvimopan (12 mg preoperatively; 12 mg twice daily until discharge) were discharged from the hospital 1 day earlier, were less likely to have a nasogastric tube reinserted, and had a bowel movement 1 day earlier, compared with those patients receiving placebo. The authors reported a significant cost savings per patient.*
These findings are important given the absence of other FDA-approved medications for the treatment of POI and the lack of other effective treatments. Alvimopan is available only to patients who are in hospitals that are registered to use this medication under the EASE (Entereg Access Support and Education) program. This recent study should prompt nonparticipating hospitals to consider routine use of alvimopan in all patients undergoing elective and emergent surgeries at risk for development of POI.
Dr. Brian E. Lacy is a professor of medicine at the Geisel School of Medicine at Dartmouth, Hanover, N.H., and chief of the section of gastroenterology and hepatology at Dartmouth-Hitchcock Medical Center. He has no relevant conflicts of interest.
*CORRECTION 11/21/13: The original version of this story misstated the cost savings per patient.
WASHINGTON – The nonopioid pain reliever alvimopan significantly decreased the incidence of postoperative ileus and shortened hospital stay among bowel surgery patients who took the drug as part of an existing accelerated recovery program.
Compared with patients who took placebo, those who took alvimopan left the hospital about 1 day sooner. In addition to the decrease in ileus, patients experienced a quicker return to normal bowel function, including time to first flatus and first bowel movement, Dr. Robert Moesinger said at the annual clinical congress of the American College of Surgeons.
"Alvimopan augmented the already improved outcomes we had seen with our validated recovery pathway, in both laparoscopic- and open-surgery patients," said Dr. Moesinger of Intermountain Healthcare, Salt Lake City. "Given the very diverse nature of our health care system, with multiple types of hospitals and surgeons, we feel these data are widely applicable and we are very comfortable recommending its routine use for patients having elective bowel surgery."
Dr. Moesinger and his colleagues conducted a randomized, placebo-controlled trial of alvimopan in 248 such patients. Those taking the study drug received 12 mg before surgery; after surgery, they received 12 mg twice a day until discharge.
The primary endpoint was postoperative length of stay. Secondary endpoints included the incidence of postoperative ileus, time to first flatus and first bowel movement, tolerance of solid food, total hospital and pharmacy costs, nasogastric tube reinsertion, 30-day readmission rates, and anastomotic leak.
The patients’ mean age was 61 years. There were no significant differences in any of their baseline demographics or clinical characteristics. Because of a statistical fluke, the placebo group did contain significantly more open-surgery patients than the alvimopan group (34% vs. 20%). The rest of the patients had laparoscopic surgery. Patients had an average of nine doses of the study drug.
The mean length of stay was 4 days in the alvimopan group and 5 in the placebo group – a significant difference.
There was significantly less postoperative ileus in the alvimopan group (2% vs. 10%). Significantly fewer of those taking the study drug needed a nasogastric tube reinserted (2% vs. 9%). The rates of 30-day readmission, reoperation, and anastomotic leak were similar in both groups. The mean time to first bowel movement was about 1 day sooner in the alvimopan group.
The median hospital cost was $10,832 for the alvimopan group and $11,924 for the placebo group – a significant difference. The median total pharmacy cost was $476 vs. $501; this difference was not significant.
Because Cubist Pharmaceuticals, which makes alvimopan, provided the study drug at no cost, the overall cost analysis cannot be considered complete, Dr. Moesinger noted. However, the pharmacy cost for a similar course of the drug would be $84. Figuring that cost into the total saved for each admission ($1,686) still yielded a net financial benefit of $930 per patient, he said.
Dr. Moesinger had no financial disclosures.
WASHINGTON – The nonopioid pain reliever alvimopan significantly decreased the incidence of postoperative ileus and shortened hospital stay among bowel surgery patients who took the drug as part of an existing accelerated recovery program.
Compared with patients who took placebo, those who took alvimopan left the hospital about 1 day sooner. In addition to the decrease in ileus, patients experienced a quicker return to normal bowel function, including time to first flatus and first bowel movement, Dr. Robert Moesinger said at the annual clinical congress of the American College of Surgeons.
"Alvimopan augmented the already improved outcomes we had seen with our validated recovery pathway, in both laparoscopic- and open-surgery patients," said Dr. Moesinger of Intermountain Healthcare, Salt Lake City. "Given the very diverse nature of our health care system, with multiple types of hospitals and surgeons, we feel these data are widely applicable and we are very comfortable recommending its routine use for patients having elective bowel surgery."
Dr. Moesinger and his colleagues conducted a randomized, placebo-controlled trial of alvimopan in 248 such patients. Those taking the study drug received 12 mg before surgery; after surgery, they received 12 mg twice a day until discharge.
The primary endpoint was postoperative length of stay. Secondary endpoints included the incidence of postoperative ileus, time to first flatus and first bowel movement, tolerance of solid food, total hospital and pharmacy costs, nasogastric tube reinsertion, 30-day readmission rates, and anastomotic leak.
The patients’ mean age was 61 years. There were no significant differences in any of their baseline demographics or clinical characteristics. Because of a statistical fluke, the placebo group did contain significantly more open-surgery patients than the alvimopan group (34% vs. 20%). The rest of the patients had laparoscopic surgery. Patients had an average of nine doses of the study drug.
The mean length of stay was 4 days in the alvimopan group and 5 in the placebo group – a significant difference.
There was significantly less postoperative ileus in the alvimopan group (2% vs. 10%). Significantly fewer of those taking the study drug needed a nasogastric tube reinserted (2% vs. 9%). The rates of 30-day readmission, reoperation, and anastomotic leak were similar in both groups. The mean time to first bowel movement was about 1 day sooner in the alvimopan group.
The median hospital cost was $10,832 for the alvimopan group and $11,924 for the placebo group – a significant difference. The median total pharmacy cost was $476 vs. $501; this difference was not significant.
Because Cubist Pharmaceuticals, which makes alvimopan, provided the study drug at no cost, the overall cost analysis cannot be considered complete, Dr. Moesinger noted. However, the pharmacy cost for a similar course of the drug would be $84. Figuring that cost into the total saved for each admission ($1,686) still yielded a net financial benefit of $930 per patient, he said.
Dr. Moesinger had no financial disclosures.
AT THE ACS CLINICAL CONGRESS
Major finding: Compared with placebo, alvimopan given before and after elective bowel surgery reduced the length of stay by 1 day and significantly decreased the incidence of postoperative ileus (2% vs. 10%).
Data source: The randomized placebo-controlled trial comprised 248 patients.
Disclosures: Dr. Moesinger had no financial disclosures.
Surgeons as proficient as gastroenterologists at colonoscopy
WASHINGTON (IMNG) – A British study suggests that it is experience, not specialty, that makes the endoscopist.
The review of more than 2,000 colonoscopies performed in 1 year determined that gastroenterologists and surgeons had identical colonoscopy completion and adverse-event outcomes, Dr. Camille Yvon reported at the annual congress of the American College of Surgeons.
Dr. Yvon, of the Medway Maritime NHS Hospital in Gillingham, England, reported the results of an audit conducted at the Kent facility from March 2011 to March 2012. It was based on prospectively collected data and was designed to compare clinical outcomes among a group of 10 physicians who were performing endoscopies during that time. Included were five consultant colorectal surgeons, one senior trust grade surgeon, and four consultant gastroenterologists.
All of the clinicians were certified by the U.K. Joint Advisory Group on Endoscopy (JAG). The group requires clinicians to complete a mentored training period and to meet specific safety and quality standards, including the following:
• 90% completion rate for colonoscopies (entering the cecum).
• Adenoma detection rate more than 10%.
• Polyp recovery more than 90%.
• Good-quality bowel prep more than 90%.
• Colonoscopy perforation rate less than 1/1,000.
• Postpolypectomy bleeding requiring transfusion less than 1/100.
• Postpolypectomy perforation rate less than 1/500.
• Flexible sigmoidoscopy perforation rate less than 1/5,000.
To become JAG accredited, physicians must be supervised by an accredited physician while performing at least 200 procedures, 100 of which were in the past year; 75% must have been colonoscopies. Certification assessment is done by two different people on two different occasions.
The audit comprised 2,058 colonoscopies. The measured outcomes were number of colonoscopies per clinician, cecal intubation rate of at least 90%, perforation rate of less than 1/1,000, and a less than 1/100 rate of postpolypectomy bleeding requiring a transfusion.
Of the total number of colonoscopies, 1,237 were done by the surgeons and the rest by the gastroenterologists. Clinicians performed an average of 205 procedures per year (range, 105-448 per year). Gastroenterologists performed an average of 205 per year, while surgeons performed an average of 206.
The overall cecal intubation rate was 94%. The average rate for both surgeons and gastroenterologists was also 94%.
There were no perforations during the study period. There were two instances of severe postpolypectomy bleeding (0.09%). However, Dr. Yvon reported, the number was too small for any meaningful statistical analysis.
Dr. Yvon had no financial disclosures.
WASHINGTON (IMNG) – A British study suggests that it is experience, not specialty, that makes the endoscopist.
The review of more than 2,000 colonoscopies performed in 1 year determined that gastroenterologists and surgeons had identical colonoscopy completion and adverse-event outcomes, Dr. Camille Yvon reported at the annual congress of the American College of Surgeons.
Dr. Yvon, of the Medway Maritime NHS Hospital in Gillingham, England, reported the results of an audit conducted at the Kent facility from March 2011 to March 2012. It was based on prospectively collected data and was designed to compare clinical outcomes among a group of 10 physicians who were performing endoscopies during that time. Included were five consultant colorectal surgeons, one senior trust grade surgeon, and four consultant gastroenterologists.
All of the clinicians were certified by the U.K. Joint Advisory Group on Endoscopy (JAG). The group requires clinicians to complete a mentored training period and to meet specific safety and quality standards, including the following:
• 90% completion rate for colonoscopies (entering the cecum).
• Adenoma detection rate more than 10%.
• Polyp recovery more than 90%.
• Good-quality bowel prep more than 90%.
• Colonoscopy perforation rate less than 1/1,000.
• Postpolypectomy bleeding requiring transfusion less than 1/100.
• Postpolypectomy perforation rate less than 1/500.
• Flexible sigmoidoscopy perforation rate less than 1/5,000.
To become JAG accredited, physicians must be supervised by an accredited physician while performing at least 200 procedures, 100 of which were in the past year; 75% must have been colonoscopies. Certification assessment is done by two different people on two different occasions.
The audit comprised 2,058 colonoscopies. The measured outcomes were number of colonoscopies per clinician, cecal intubation rate of at least 90%, perforation rate of less than 1/1,000, and a less than 1/100 rate of postpolypectomy bleeding requiring a transfusion.
Of the total number of colonoscopies, 1,237 were done by the surgeons and the rest by the gastroenterologists. Clinicians performed an average of 205 procedures per year (range, 105-448 per year). Gastroenterologists performed an average of 205 per year, while surgeons performed an average of 206.
The overall cecal intubation rate was 94%. The average rate for both surgeons and gastroenterologists was also 94%.
There were no perforations during the study period. There were two instances of severe postpolypectomy bleeding (0.09%). However, Dr. Yvon reported, the number was too small for any meaningful statistical analysis.
Dr. Yvon had no financial disclosures.
WASHINGTON (IMNG) – A British study suggests that it is experience, not specialty, that makes the endoscopist.
The review of more than 2,000 colonoscopies performed in 1 year determined that gastroenterologists and surgeons had identical colonoscopy completion and adverse-event outcomes, Dr. Camille Yvon reported at the annual congress of the American College of Surgeons.
Dr. Yvon, of the Medway Maritime NHS Hospital in Gillingham, England, reported the results of an audit conducted at the Kent facility from March 2011 to March 2012. It was based on prospectively collected data and was designed to compare clinical outcomes among a group of 10 physicians who were performing endoscopies during that time. Included were five consultant colorectal surgeons, one senior trust grade surgeon, and four consultant gastroenterologists.
All of the clinicians were certified by the U.K. Joint Advisory Group on Endoscopy (JAG). The group requires clinicians to complete a mentored training period and to meet specific safety and quality standards, including the following:
• 90% completion rate for colonoscopies (entering the cecum).
• Adenoma detection rate more than 10%.
• Polyp recovery more than 90%.
• Good-quality bowel prep more than 90%.
• Colonoscopy perforation rate less than 1/1,000.
• Postpolypectomy bleeding requiring transfusion less than 1/100.
• Postpolypectomy perforation rate less than 1/500.
• Flexible sigmoidoscopy perforation rate less than 1/5,000.
To become JAG accredited, physicians must be supervised by an accredited physician while performing at least 200 procedures, 100 of which were in the past year; 75% must have been colonoscopies. Certification assessment is done by two different people on two different occasions.
The audit comprised 2,058 colonoscopies. The measured outcomes were number of colonoscopies per clinician, cecal intubation rate of at least 90%, perforation rate of less than 1/1,000, and a less than 1/100 rate of postpolypectomy bleeding requiring a transfusion.
Of the total number of colonoscopies, 1,237 were done by the surgeons and the rest by the gastroenterologists. Clinicians performed an average of 205 procedures per year (range, 105-448 per year). Gastroenterologists performed an average of 205 per year, while surgeons performed an average of 206.
The overall cecal intubation rate was 94%. The average rate for both surgeons and gastroenterologists was also 94%.
There were no perforations during the study period. There were two instances of severe postpolypectomy bleeding (0.09%). However, Dr. Yvon reported, the number was too small for any meaningful statistical analysis.
Dr. Yvon had no financial disclosures.
AT THE ACS CLINICAL CONGRESS
Major finding: Gastroenterologists and surgeons both completed an average of 94% of colonoscopies they performed during a 1-year audit period.
Data source: The audit examined outcomes of more than 2,000 colonoscopies.
Disclosures: Dr. Yvon had no financial disclosures.