User login
Official Newspaper of the American College of Surgeons
Earlier epilepsy surgery may have reproductive benefits for women
WASHINGTON – Younger age at the time of surgery, and receipt of fewer medications prior to surgery were associated with higher rates of pregnancy and birth after surgery in a study of women with epilepsy.
The findings, from a retrospective review of the charts of 113 women who underwent surgery involving cortical resection between 1997 and 2008 for intractable focal epilepsy, suggest that earlier may be better when it comes to surgical intervention for young women with epilepsy who desire pregnancy, Dr. Rachel R. Fabris reported at the annual meeting of the American Epilepsy Society.
For the women included in this analysis, the mean age was 13.3 years at epilepsy onset and the mean age was 30.5 years at time of surgery at the Mayo Clinic, Rochester, Minn. They had an average of 5.57 medication trials, 42% had at least monthly seizures, and 21% had daily seizures. They were followed for a mean of 5.7 years after surgery, and 75% had Engel Class I disease after surgery, said Dr. Fabris of the Mayo Clinic.
Prior to surgery, the women had an average of 0.93 pregnancies each, and an average of 0.73 births; after surgery, a total of 17 women had a total of 35 pregnancies and 25 births, for an average of 1.27 pregnancies and 0.96 births each.
One patient reported infertility after surgery.
Younger patients experienced the greatest increases in the number of pregnancies and births (P = .0036 and .0060, respectively), and those taking fewer medications also experienced a significant change in the number of births after surgery (P = .0362). The former finding is likely not meaningful, because younger women have higher reproductive rates than do older women, in general; the latter finding, however, may suggest a role for earlier surgical intervention in women with epilepsy, Dr. Fabris said.
These preliminary findings provide additional evidence of the importance of early surgery for intractable focal epilepsy, but their significance with respect to reproduction is uncertain given the small number of patients, she said. Dr. Fabris said she believes this to be the first study to evaluate the effect of surgery on reproductive outcomes.
"We already know from former studies that women with epilepsy have lower birth rates than women in the general population, and multiple studies have shown this to be a multifactorial process that involves an interplay of endocrine as well as societal factors," she said during a press briefing at the conference.
Since antiepileptic medications also are known to have adverse effects on fertility, it may be that this effect is contributing to the current findings, she noted, concluding that additional study with more patients is needed to provide more meaningful results. There were no relevant financial disclosures.
WASHINGTON – Younger age at the time of surgery, and receipt of fewer medications prior to surgery were associated with higher rates of pregnancy and birth after surgery in a study of women with epilepsy.
The findings, from a retrospective review of the charts of 113 women who underwent surgery involving cortical resection between 1997 and 2008 for intractable focal epilepsy, suggest that earlier may be better when it comes to surgical intervention for young women with epilepsy who desire pregnancy, Dr. Rachel R. Fabris reported at the annual meeting of the American Epilepsy Society.
For the women included in this analysis, the mean age was 13.3 years at epilepsy onset and the mean age was 30.5 years at time of surgery at the Mayo Clinic, Rochester, Minn. They had an average of 5.57 medication trials, 42% had at least monthly seizures, and 21% had daily seizures. They were followed for a mean of 5.7 years after surgery, and 75% had Engel Class I disease after surgery, said Dr. Fabris of the Mayo Clinic.
Prior to surgery, the women had an average of 0.93 pregnancies each, and an average of 0.73 births; after surgery, a total of 17 women had a total of 35 pregnancies and 25 births, for an average of 1.27 pregnancies and 0.96 births each.
One patient reported infertility after surgery.
Younger patients experienced the greatest increases in the number of pregnancies and births (P = .0036 and .0060, respectively), and those taking fewer medications also experienced a significant change in the number of births after surgery (P = .0362). The former finding is likely not meaningful, because younger women have higher reproductive rates than do older women, in general; the latter finding, however, may suggest a role for earlier surgical intervention in women with epilepsy, Dr. Fabris said.
These preliminary findings provide additional evidence of the importance of early surgery for intractable focal epilepsy, but their significance with respect to reproduction is uncertain given the small number of patients, she said. Dr. Fabris said she believes this to be the first study to evaluate the effect of surgery on reproductive outcomes.
"We already know from former studies that women with epilepsy have lower birth rates than women in the general population, and multiple studies have shown this to be a multifactorial process that involves an interplay of endocrine as well as societal factors," she said during a press briefing at the conference.
Since antiepileptic medications also are known to have adverse effects on fertility, it may be that this effect is contributing to the current findings, she noted, concluding that additional study with more patients is needed to provide more meaningful results. There were no relevant financial disclosures.
WASHINGTON – Younger age at the time of surgery, and receipt of fewer medications prior to surgery were associated with higher rates of pregnancy and birth after surgery in a study of women with epilepsy.
The findings, from a retrospective review of the charts of 113 women who underwent surgery involving cortical resection between 1997 and 2008 for intractable focal epilepsy, suggest that earlier may be better when it comes to surgical intervention for young women with epilepsy who desire pregnancy, Dr. Rachel R. Fabris reported at the annual meeting of the American Epilepsy Society.
For the women included in this analysis, the mean age was 13.3 years at epilepsy onset and the mean age was 30.5 years at time of surgery at the Mayo Clinic, Rochester, Minn. They had an average of 5.57 medication trials, 42% had at least monthly seizures, and 21% had daily seizures. They were followed for a mean of 5.7 years after surgery, and 75% had Engel Class I disease after surgery, said Dr. Fabris of the Mayo Clinic.
Prior to surgery, the women had an average of 0.93 pregnancies each, and an average of 0.73 births; after surgery, a total of 17 women had a total of 35 pregnancies and 25 births, for an average of 1.27 pregnancies and 0.96 births each.
One patient reported infertility after surgery.
Younger patients experienced the greatest increases in the number of pregnancies and births (P = .0036 and .0060, respectively), and those taking fewer medications also experienced a significant change in the number of births after surgery (P = .0362). The former finding is likely not meaningful, because younger women have higher reproductive rates than do older women, in general; the latter finding, however, may suggest a role for earlier surgical intervention in women with epilepsy, Dr. Fabris said.
These preliminary findings provide additional evidence of the importance of early surgery for intractable focal epilepsy, but their significance with respect to reproduction is uncertain given the small number of patients, she said. Dr. Fabris said she believes this to be the first study to evaluate the effect of surgery on reproductive outcomes.
"We already know from former studies that women with epilepsy have lower birth rates than women in the general population, and multiple studies have shown this to be a multifactorial process that involves an interplay of endocrine as well as societal factors," she said during a press briefing at the conference.
Since antiepileptic medications also are known to have adverse effects on fertility, it may be that this effect is contributing to the current findings, she noted, concluding that additional study with more patients is needed to provide more meaningful results. There were no relevant financial disclosures.
AT AES 2013
Major finding: Prior to surgery, the women had an average of 0.93 pregnancies each, and an average of 0.73 births; after surgery, a total of 17 women had a total of 35 pregnancies and 25 births, for an average of 1.27 pregnancies and 0.96 births each.
Data source: A chart review involving 113 patients.
Disclosures: There were no relevant financial disclosures.
Routinely screen relatives when thoracic aortic aneurysm disease presents before age 60
DALLAS – Routine screening is warranted for the first-degree relatives of patients who present with thoracic aortic disease before age 60 years in the absence of predisposing conditions such as hypertension, Marfan syndrome, or bicuspid aortic valve, Dr. Elizabeth N. Robertson said at the American Heart Association scientific sessions.
"We’ve shown that screening of first-degree relatives for familial thoracic aortic aneurysm disease is essential, as we detected an average of two additional affected individuals per initial patient," noted Dr. Robertson of Royal Prince Alfred Hospital in Camperdown, Australia.

Thoracic aortic aneurysm and dissection (TAAD) is more common than previously recognized. It accounted for one in seven cases of thoracic aortic disease in a series of 1,276 patients who presented with thoracic aortic disease to the tertiary center during a recent 12-year period.
TAAD is an asymptomatic progressive dilatation of the thoracic aorta characterized by cystic medial necrosis. It has no evident predisposing cause. It can occur sporadically or in a familial form, which has been linked to multiple gene mutations transmitted in autosomal dominant fashion in large family studies. Unlike Marfan syndrome, Ehler-Danlos syndrome, and other genetic causes of thoracic aortic disease, TAAD has no characteristic external physical features. The clinical signs of TAAD are minimal and nonpathognomonic: an aortic flow murmur or a prominent A2 second heart sound.
"The most common first indication of a problem is occurrence of aortic dissection, by which time it’s often too late," she said.
Dr. Robertson and her coinvestigators did a retrospective review of 1,276 patients who presented with thoracic aortic disease at the Royal Prince Alfred Hospital cardiovascular myopathy service during 2000-2012. TAAD was seen in 178. TAAD was defined as aortic dilatation or dissection before age 60 years with no predisposing condition and with confirmation of cystic medial necrosis whenever possible. TAAD was sporadic in 93 patients. The other 85 had familial TAAD based upon their history of having one or more affected family members. Screening was offered to all first-degree relatives of the patients with familial TAAD.
Two-dimensional echocardiographic screening of 383 first-degree family members identified an additional 181 affected individuals, bringing the total study population with familial TAAD to 266. When the screened patients were added to the 93 patients with sporadic TAAD, thoracic aortic aneurysm and dissection became the second most common cause of thoracic aortic dilatation or dissection seen at the hospital during the study period.
The median age at diagnosis was 46 years both in the sporadic and familial TAAD groups. However, the detection rate was steady from the teen years through old age, underscoring the importance of surveillance of younger at-risk individuals. Most of the at-risk population was male, 73% of the familial and 86% of the sporadic cases. The aortic diameter at diagnosis varied, but it was 50 mm or greater in 25% of the subjects in the familial and sporadic groups. Under current guidelines, this measure warrants semi-urgent surgical intervention.
Of the first-degree relatives identified through screening, 26% experienced aortic dissection, as did more than 60% of the initial 85 probands with familial TAAD. The familial phenotype appears to be more aggressive: 40% of patients with familial TAAD who had an aortic dissection died as a result, compared with less than 15% of patients with sporadic TAAD who had aortic dissection.
Dissection in patients with familial TAAD frequently occurred at smaller aortic diameters, including less than 40 mm. Dr. Robertson noted this width would not typically be flagged as a problematic in routine screening.
The rate of progression of dilatation in patients with familial TAAD was 0.5 mm per year, which is roughly half the rate typically seen in patients with Marfan syndrome, according to Dr. Robertson.
Session cochair Dr. Brendan M. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston, called Dr. Robertson’s study "fascinating" and thanked her for bringing to his attention a serious and relatively common condition he was hitherto unaware of. Given that aortic size is age-, gender-, and body surface area–dependent, what’s the best threshold aortic diameter in defining a positive screening test in first-degree relatives?, he asked.
Because of those associations, Dr. Robertson replied, the best definition of a positive screening test is a Z score greater than 2, rather than simply relying upon a given aortic diameter measurement.
Dr. Robertson’s study was conducted free of commercial support. She reported having no financial conflicts of interest.
DALLAS – Routine screening is warranted for the first-degree relatives of patients who present with thoracic aortic disease before age 60 years in the absence of predisposing conditions such as hypertension, Marfan syndrome, or bicuspid aortic valve, Dr. Elizabeth N. Robertson said at the American Heart Association scientific sessions.
"We’ve shown that screening of first-degree relatives for familial thoracic aortic aneurysm disease is essential, as we detected an average of two additional affected individuals per initial patient," noted Dr. Robertson of Royal Prince Alfred Hospital in Camperdown, Australia.

Thoracic aortic aneurysm and dissection (TAAD) is more common than previously recognized. It accounted for one in seven cases of thoracic aortic disease in a series of 1,276 patients who presented with thoracic aortic disease to the tertiary center during a recent 12-year period.
TAAD is an asymptomatic progressive dilatation of the thoracic aorta characterized by cystic medial necrosis. It has no evident predisposing cause. It can occur sporadically or in a familial form, which has been linked to multiple gene mutations transmitted in autosomal dominant fashion in large family studies. Unlike Marfan syndrome, Ehler-Danlos syndrome, and other genetic causes of thoracic aortic disease, TAAD has no characteristic external physical features. The clinical signs of TAAD are minimal and nonpathognomonic: an aortic flow murmur or a prominent A2 second heart sound.
"The most common first indication of a problem is occurrence of aortic dissection, by which time it’s often too late," she said.
Dr. Robertson and her coinvestigators did a retrospective review of 1,276 patients who presented with thoracic aortic disease at the Royal Prince Alfred Hospital cardiovascular myopathy service during 2000-2012. TAAD was seen in 178. TAAD was defined as aortic dilatation or dissection before age 60 years with no predisposing condition and with confirmation of cystic medial necrosis whenever possible. TAAD was sporadic in 93 patients. The other 85 had familial TAAD based upon their history of having one or more affected family members. Screening was offered to all first-degree relatives of the patients with familial TAAD.
Two-dimensional echocardiographic screening of 383 first-degree family members identified an additional 181 affected individuals, bringing the total study population with familial TAAD to 266. When the screened patients were added to the 93 patients with sporadic TAAD, thoracic aortic aneurysm and dissection became the second most common cause of thoracic aortic dilatation or dissection seen at the hospital during the study period.
The median age at diagnosis was 46 years both in the sporadic and familial TAAD groups. However, the detection rate was steady from the teen years through old age, underscoring the importance of surveillance of younger at-risk individuals. Most of the at-risk population was male, 73% of the familial and 86% of the sporadic cases. The aortic diameter at diagnosis varied, but it was 50 mm or greater in 25% of the subjects in the familial and sporadic groups. Under current guidelines, this measure warrants semi-urgent surgical intervention.
Of the first-degree relatives identified through screening, 26% experienced aortic dissection, as did more than 60% of the initial 85 probands with familial TAAD. The familial phenotype appears to be more aggressive: 40% of patients with familial TAAD who had an aortic dissection died as a result, compared with less than 15% of patients with sporadic TAAD who had aortic dissection.
Dissection in patients with familial TAAD frequently occurred at smaller aortic diameters, including less than 40 mm. Dr. Robertson noted this width would not typically be flagged as a problematic in routine screening.
The rate of progression of dilatation in patients with familial TAAD was 0.5 mm per year, which is roughly half the rate typically seen in patients with Marfan syndrome, according to Dr. Robertson.
Session cochair Dr. Brendan M. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston, called Dr. Robertson’s study "fascinating" and thanked her for bringing to his attention a serious and relatively common condition he was hitherto unaware of. Given that aortic size is age-, gender-, and body surface area–dependent, what’s the best threshold aortic diameter in defining a positive screening test in first-degree relatives?, he asked.
Because of those associations, Dr. Robertson replied, the best definition of a positive screening test is a Z score greater than 2, rather than simply relying upon a given aortic diameter measurement.
Dr. Robertson’s study was conducted free of commercial support. She reported having no financial conflicts of interest.
DALLAS – Routine screening is warranted for the first-degree relatives of patients who present with thoracic aortic disease before age 60 years in the absence of predisposing conditions such as hypertension, Marfan syndrome, or bicuspid aortic valve, Dr. Elizabeth N. Robertson said at the American Heart Association scientific sessions.
"We’ve shown that screening of first-degree relatives for familial thoracic aortic aneurysm disease is essential, as we detected an average of two additional affected individuals per initial patient," noted Dr. Robertson of Royal Prince Alfred Hospital in Camperdown, Australia.

Thoracic aortic aneurysm and dissection (TAAD) is more common than previously recognized. It accounted for one in seven cases of thoracic aortic disease in a series of 1,276 patients who presented with thoracic aortic disease to the tertiary center during a recent 12-year period.
TAAD is an asymptomatic progressive dilatation of the thoracic aorta characterized by cystic medial necrosis. It has no evident predisposing cause. It can occur sporadically or in a familial form, which has been linked to multiple gene mutations transmitted in autosomal dominant fashion in large family studies. Unlike Marfan syndrome, Ehler-Danlos syndrome, and other genetic causes of thoracic aortic disease, TAAD has no characteristic external physical features. The clinical signs of TAAD are minimal and nonpathognomonic: an aortic flow murmur or a prominent A2 second heart sound.
"The most common first indication of a problem is occurrence of aortic dissection, by which time it’s often too late," she said.
Dr. Robertson and her coinvestigators did a retrospective review of 1,276 patients who presented with thoracic aortic disease at the Royal Prince Alfred Hospital cardiovascular myopathy service during 2000-2012. TAAD was seen in 178. TAAD was defined as aortic dilatation or dissection before age 60 years with no predisposing condition and with confirmation of cystic medial necrosis whenever possible. TAAD was sporadic in 93 patients. The other 85 had familial TAAD based upon their history of having one or more affected family members. Screening was offered to all first-degree relatives of the patients with familial TAAD.
Two-dimensional echocardiographic screening of 383 first-degree family members identified an additional 181 affected individuals, bringing the total study population with familial TAAD to 266. When the screened patients were added to the 93 patients with sporadic TAAD, thoracic aortic aneurysm and dissection became the second most common cause of thoracic aortic dilatation or dissection seen at the hospital during the study period.
The median age at diagnosis was 46 years both in the sporadic and familial TAAD groups. However, the detection rate was steady from the teen years through old age, underscoring the importance of surveillance of younger at-risk individuals. Most of the at-risk population was male, 73% of the familial and 86% of the sporadic cases. The aortic diameter at diagnosis varied, but it was 50 mm or greater in 25% of the subjects in the familial and sporadic groups. Under current guidelines, this measure warrants semi-urgent surgical intervention.
Of the first-degree relatives identified through screening, 26% experienced aortic dissection, as did more than 60% of the initial 85 probands with familial TAAD. The familial phenotype appears to be more aggressive: 40% of patients with familial TAAD who had an aortic dissection died as a result, compared with less than 15% of patients with sporadic TAAD who had aortic dissection.
Dissection in patients with familial TAAD frequently occurred at smaller aortic diameters, including less than 40 mm. Dr. Robertson noted this width would not typically be flagged as a problematic in routine screening.
The rate of progression of dilatation in patients with familial TAAD was 0.5 mm per year, which is roughly half the rate typically seen in patients with Marfan syndrome, according to Dr. Robertson.
Session cochair Dr. Brendan M. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston, called Dr. Robertson’s study "fascinating" and thanked her for bringing to his attention a serious and relatively common condition he was hitherto unaware of. Given that aortic size is age-, gender-, and body surface area–dependent, what’s the best threshold aortic diameter in defining a positive screening test in first-degree relatives?, he asked.
Because of those associations, Dr. Robertson replied, the best definition of a positive screening test is a Z score greater than 2, rather than simply relying upon a given aortic diameter measurement.
Dr. Robertson’s study was conducted free of commercial support. She reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: One in seven patients presenting with thoracic aortic disease in a large series had thoracic aortic aneurysm and dissection, or TAAD, an under-recognized, asymptomatic condition characterized by cystic medial necrosis and a strong genetic component.
Data source: This was a review of 1,276 patients who presented to a tertiary center with thoracic aortic disease. Systemic screening of first-degree relatives of patients with familial TAAD turned up two additional cases per proband.
Disclosures: The study was conducted free of commercial support. The presenter reported having no financial conflicts.
Genotyping adds little to optimized warfarin dosing
DALLAS – Genotyping to guide the starting dosage of warfarin treatment showed no added value above tailoring treatment with a panel of clinical features in a randomized, controlled U.S. trial of more than 1,000 patients.
The resounding null result from adding genotype information should spell the end of this practice, said Dr. Stephen E. Kimmel, lead investigator for the study, and professor and director of cardiovascular epidemiology at the University of Pennsylvania in Philadelphia.
"Based on what we’ve seen, I don’t believe there is sufficient evidence to add genetic information on top of the available clinical algorithms," Dr. Kimmel said at the American Heart Association scientific sessions. "I don’t think we’ll see another genetics trial in warfarin treatment. I think this is it."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Good outcomes in the comparison group largely accounted for the failure of genotyping to significantly improve the percentage of time that patients’ international normalized ratios (INRs) were in the target range of 2.0-3.0. The amount of time in the therapeutic INR range (PTTR) averaged 45% in patients in the comparison arm during the first 4 weeks of warfarin treatment.
"When the comparison group does so well, it’s more difficult for genotyping to have an effect," said Dr. Elaine M. Hylek, professor of medicine and an anticoagulant specialist at Boston University.
That limitation, coupled with the shifting anticoagulant landscape, knock genotyping out of the picture, she agreed. "It will be difficult funding [another study of genotyping] with all the anticoagulant alternatives that are now out there" for preventing thrombosis in patients with atrial fibrillation or a recent venous thromboembolism.
The Clarification of Optimal Anticoagulant through Genetics (COAG) trial enrolled 1,015 patients initiating warfarin therapy at 18 U.S. centers during September 2009 to April 2013. The study randomized patients to two different ways to calculate their warfarin dosage during the first 5 days of treatment. Half had their dosage calculated by a formula that took into account seven clinical and demographic factors, including age, race, smoking status, and body surface area. The others had their dosage calculated with the same formula and factors plus added information on the patient’s genotype for two genes that affect warfarin activity, CYP2C9 and VKORC1. Patients’ average age was 58 years; just over a quarter were African American.
During the first 4 weeks on treatment, the average PTTR was 45% in both arms of the study, the trial’s primary endpoint. Adding genotyping information led to a bigger improvement in the PTTR among the non–African American patients compared with those who were African American, but did not produce a significantly increased PTTR in the non–African American subgroup.
Dr. Munir Pirmohamed reported results from a similar study that enrolled 455 patients starting warfarin therapy at any of five centers in the United Kingdom and Sweden. The EU-Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin study mainly differed from the COAG study by the background method used to calculate a starting warfarin dosage over the first 3 days of treatment. In EU-PACT, the warfarin dosage of all patients was adjusted for age but not for other factors. In the intervention arm, the starting dosages were adjusted for the status of the same two genes, CYP2C9 and VKORC1.
During the first 12 weeks after starting warfarin, the cumulative average PTTR was 67% in the patients whose dose was adjusted by genotype and 60% in patients who were not genotyped, a statistically significant difference for this study’s primary endpoint, reported Dr. Pirmohamed, professor and head of molecular and clinical pharmacology at the University of Liverpool, England.
Taken together, the findings of the two studies highlight that patients should not start warfarin treatment on a fixed dosage, said Dr. Patrick T. Ellinor, a cardiologist and arrhythmia specialist at Massachusetts General Hospital, Boston, and designated discussant for both reports at the meeting. The findings support use of a clinical algorithm that takes into account several clinical factors, he added.
Concurrently with the meeting, the reports were published online for COAG (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1310669]) and for EU-PACT (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1311386]).
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed and Dr. Ellinor had no disclosures.
Warfarin on top despite competition
Warfarin remains the most widely used anticoagulant in the United States. Among patients with atrial fibrillation taking anticoagulation treatment, 72% used warfarin during the third quarter of 2013, data from a large registry show.
The new anticoagulants on the U.S. market – dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto) – are gaining ground and widely acknowledged to be better, safer, and easier to manage. But warfarin clings to the market largely because of familiarity and low price, according to several experts.
"There is no doubt that the new anticoagulants look better compared with warfarin, but the clinical fact is that it’s what you know versus what you don’t know, and warfarin has been used for 60 years," Dr. Kimmel said at the press conference.
"The new drugs perform better than warfarin does; they get patients to where they need to be more quickly, but warfarin has been around a long time. We know its interactions and risks, there is the ability to reverse its effect, and the cost to patients is a real issue. If the new anticoagulants were the same price as warfarin then I think you’d see a lot more patients get a new drug," Dr. Ellinor said in an interview.
Data on current U.S. uptake of the new oral anticoagulants came from the 148,320 unique patients with atrial fibrillation included during June to September of 2013 in the PINNACLE Registry database run by the American College of Cardiology. Among these patients, about 83,000 (56%) were on some anticoagulant treatment, and within this subgroup, 72% were on warfarin, 27% on a new anticoagulant, and the remainder on different treatment, said Dr. John Gordon Harold, ACC president and a cardiologist at Cedars-Sinai Heart Institute in Los Angeles.
"In my own practice the new anticoagulants are being used with increasing frequency, mainly driven by direct-to-consumer advertising," Dr. Harold said in an interview. "We have patients who are completely stable on warfarin. (They) come in because of a consumer ad and they ask if they should switch drugs. When patients are stable I don’t encourage them to switch, but we have a shared decision making conversation and go over the pros and cons, the cost, and the outcomes data. A lot of patients prefer to pay the difference" and switch to a new anticoagulant.
Dr. Harold said he also recommends that patients switch off warfarin if they have problems with compliance and variability in their international normalized ratio (INR).
"If you can keep a patient on warfarin in their INR target range 80% or more of the time then I wouldn’t change, but most patients on warfarin have a very hard time maintaining an INR of 2-3," said Dr. Mark S. Link, professor and codirector of the cardiac arrhythmia center at Tufts Medical Center, Boston. But he said cost is a major factor keeping many patients on warfarin.
The new anticoagulants "are better than warfarin, but we are often forced by insurers to start with the cheaper drug," Dr. Link said during the news conference.
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed, Dr. Ellinor, Dr. Harold, and Dr. Link had no relevant disclosures.
On Twitter @mitchelzoler
DALLAS – Genotyping to guide the starting dosage of warfarin treatment showed no added value above tailoring treatment with a panel of clinical features in a randomized, controlled U.S. trial of more than 1,000 patients.
The resounding null result from adding genotype information should spell the end of this practice, said Dr. Stephen E. Kimmel, lead investigator for the study, and professor and director of cardiovascular epidemiology at the University of Pennsylvania in Philadelphia.
"Based on what we’ve seen, I don’t believe there is sufficient evidence to add genetic information on top of the available clinical algorithms," Dr. Kimmel said at the American Heart Association scientific sessions. "I don’t think we’ll see another genetics trial in warfarin treatment. I think this is it."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Good outcomes in the comparison group largely accounted for the failure of genotyping to significantly improve the percentage of time that patients’ international normalized ratios (INRs) were in the target range of 2.0-3.0. The amount of time in the therapeutic INR range (PTTR) averaged 45% in patients in the comparison arm during the first 4 weeks of warfarin treatment.
"When the comparison group does so well, it’s more difficult for genotyping to have an effect," said Dr. Elaine M. Hylek, professor of medicine and an anticoagulant specialist at Boston University.
That limitation, coupled with the shifting anticoagulant landscape, knock genotyping out of the picture, she agreed. "It will be difficult funding [another study of genotyping] with all the anticoagulant alternatives that are now out there" for preventing thrombosis in patients with atrial fibrillation or a recent venous thromboembolism.
The Clarification of Optimal Anticoagulant through Genetics (COAG) trial enrolled 1,015 patients initiating warfarin therapy at 18 U.S. centers during September 2009 to April 2013. The study randomized patients to two different ways to calculate their warfarin dosage during the first 5 days of treatment. Half had their dosage calculated by a formula that took into account seven clinical and demographic factors, including age, race, smoking status, and body surface area. The others had their dosage calculated with the same formula and factors plus added information on the patient’s genotype for two genes that affect warfarin activity, CYP2C9 and VKORC1. Patients’ average age was 58 years; just over a quarter were African American.
During the first 4 weeks on treatment, the average PTTR was 45% in both arms of the study, the trial’s primary endpoint. Adding genotyping information led to a bigger improvement in the PTTR among the non–African American patients compared with those who were African American, but did not produce a significantly increased PTTR in the non–African American subgroup.
Dr. Munir Pirmohamed reported results from a similar study that enrolled 455 patients starting warfarin therapy at any of five centers in the United Kingdom and Sweden. The EU-Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin study mainly differed from the COAG study by the background method used to calculate a starting warfarin dosage over the first 3 days of treatment. In EU-PACT, the warfarin dosage of all patients was adjusted for age but not for other factors. In the intervention arm, the starting dosages were adjusted for the status of the same two genes, CYP2C9 and VKORC1.
During the first 12 weeks after starting warfarin, the cumulative average PTTR was 67% in the patients whose dose was adjusted by genotype and 60% in patients who were not genotyped, a statistically significant difference for this study’s primary endpoint, reported Dr. Pirmohamed, professor and head of molecular and clinical pharmacology at the University of Liverpool, England.
Taken together, the findings of the two studies highlight that patients should not start warfarin treatment on a fixed dosage, said Dr. Patrick T. Ellinor, a cardiologist and arrhythmia specialist at Massachusetts General Hospital, Boston, and designated discussant for both reports at the meeting. The findings support use of a clinical algorithm that takes into account several clinical factors, he added.
Concurrently with the meeting, the reports were published online for COAG (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1310669]) and for EU-PACT (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1311386]).
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed and Dr. Ellinor had no disclosures.
Warfarin on top despite competition
Warfarin remains the most widely used anticoagulant in the United States. Among patients with atrial fibrillation taking anticoagulation treatment, 72% used warfarin during the third quarter of 2013, data from a large registry show.
The new anticoagulants on the U.S. market – dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto) – are gaining ground and widely acknowledged to be better, safer, and easier to manage. But warfarin clings to the market largely because of familiarity and low price, according to several experts.
"There is no doubt that the new anticoagulants look better compared with warfarin, but the clinical fact is that it’s what you know versus what you don’t know, and warfarin has been used for 60 years," Dr. Kimmel said at the press conference.
"The new drugs perform better than warfarin does; they get patients to where they need to be more quickly, but warfarin has been around a long time. We know its interactions and risks, there is the ability to reverse its effect, and the cost to patients is a real issue. If the new anticoagulants were the same price as warfarin then I think you’d see a lot more patients get a new drug," Dr. Ellinor said in an interview.
Data on current U.S. uptake of the new oral anticoagulants came from the 148,320 unique patients with atrial fibrillation included during June to September of 2013 in the PINNACLE Registry database run by the American College of Cardiology. Among these patients, about 83,000 (56%) were on some anticoagulant treatment, and within this subgroup, 72% were on warfarin, 27% on a new anticoagulant, and the remainder on different treatment, said Dr. John Gordon Harold, ACC president and a cardiologist at Cedars-Sinai Heart Institute in Los Angeles.
"In my own practice the new anticoagulants are being used with increasing frequency, mainly driven by direct-to-consumer advertising," Dr. Harold said in an interview. "We have patients who are completely stable on warfarin. (They) come in because of a consumer ad and they ask if they should switch drugs. When patients are stable I don’t encourage them to switch, but we have a shared decision making conversation and go over the pros and cons, the cost, and the outcomes data. A lot of patients prefer to pay the difference" and switch to a new anticoagulant.
Dr. Harold said he also recommends that patients switch off warfarin if they have problems with compliance and variability in their international normalized ratio (INR).
"If you can keep a patient on warfarin in their INR target range 80% or more of the time then I wouldn’t change, but most patients on warfarin have a very hard time maintaining an INR of 2-3," said Dr. Mark S. Link, professor and codirector of the cardiac arrhythmia center at Tufts Medical Center, Boston. But he said cost is a major factor keeping many patients on warfarin.
The new anticoagulants "are better than warfarin, but we are often forced by insurers to start with the cheaper drug," Dr. Link said during the news conference.
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed, Dr. Ellinor, Dr. Harold, and Dr. Link had no relevant disclosures.
On Twitter @mitchelzoler
DALLAS – Genotyping to guide the starting dosage of warfarin treatment showed no added value above tailoring treatment with a panel of clinical features in a randomized, controlled U.S. trial of more than 1,000 patients.
The resounding null result from adding genotype information should spell the end of this practice, said Dr. Stephen E. Kimmel, lead investigator for the study, and professor and director of cardiovascular epidemiology at the University of Pennsylvania in Philadelphia.
"Based on what we’ve seen, I don’t believe there is sufficient evidence to add genetic information on top of the available clinical algorithms," Dr. Kimmel said at the American Heart Association scientific sessions. "I don’t think we’ll see another genetics trial in warfarin treatment. I think this is it."
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Good outcomes in the comparison group largely accounted for the failure of genotyping to significantly improve the percentage of time that patients’ international normalized ratios (INRs) were in the target range of 2.0-3.0. The amount of time in the therapeutic INR range (PTTR) averaged 45% in patients in the comparison arm during the first 4 weeks of warfarin treatment.
"When the comparison group does so well, it’s more difficult for genotyping to have an effect," said Dr. Elaine M. Hylek, professor of medicine and an anticoagulant specialist at Boston University.
That limitation, coupled with the shifting anticoagulant landscape, knock genotyping out of the picture, she agreed. "It will be difficult funding [another study of genotyping] with all the anticoagulant alternatives that are now out there" for preventing thrombosis in patients with atrial fibrillation or a recent venous thromboembolism.
The Clarification of Optimal Anticoagulant through Genetics (COAG) trial enrolled 1,015 patients initiating warfarin therapy at 18 U.S. centers during September 2009 to April 2013. The study randomized patients to two different ways to calculate their warfarin dosage during the first 5 days of treatment. Half had their dosage calculated by a formula that took into account seven clinical and demographic factors, including age, race, smoking status, and body surface area. The others had their dosage calculated with the same formula and factors plus added information on the patient’s genotype for two genes that affect warfarin activity, CYP2C9 and VKORC1. Patients’ average age was 58 years; just over a quarter were African American.
During the first 4 weeks on treatment, the average PTTR was 45% in both arms of the study, the trial’s primary endpoint. Adding genotyping information led to a bigger improvement in the PTTR among the non–African American patients compared with those who were African American, but did not produce a significantly increased PTTR in the non–African American subgroup.
Dr. Munir Pirmohamed reported results from a similar study that enrolled 455 patients starting warfarin therapy at any of five centers in the United Kingdom and Sweden. The EU-Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin study mainly differed from the COAG study by the background method used to calculate a starting warfarin dosage over the first 3 days of treatment. In EU-PACT, the warfarin dosage of all patients was adjusted for age but not for other factors. In the intervention arm, the starting dosages were adjusted for the status of the same two genes, CYP2C9 and VKORC1.
During the first 12 weeks after starting warfarin, the cumulative average PTTR was 67% in the patients whose dose was adjusted by genotype and 60% in patients who were not genotyped, a statistically significant difference for this study’s primary endpoint, reported Dr. Pirmohamed, professor and head of molecular and clinical pharmacology at the University of Liverpool, England.
Taken together, the findings of the two studies highlight that patients should not start warfarin treatment on a fixed dosage, said Dr. Patrick T. Ellinor, a cardiologist and arrhythmia specialist at Massachusetts General Hospital, Boston, and designated discussant for both reports at the meeting. The findings support use of a clinical algorithm that takes into account several clinical factors, he added.
Concurrently with the meeting, the reports were published online for COAG (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1310669]) and for EU-PACT (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1311386]).
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed and Dr. Ellinor had no disclosures.
Warfarin on top despite competition
Warfarin remains the most widely used anticoagulant in the United States. Among patients with atrial fibrillation taking anticoagulation treatment, 72% used warfarin during the third quarter of 2013, data from a large registry show.
The new anticoagulants on the U.S. market – dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto) – are gaining ground and widely acknowledged to be better, safer, and easier to manage. But warfarin clings to the market largely because of familiarity and low price, according to several experts.
"There is no doubt that the new anticoagulants look better compared with warfarin, but the clinical fact is that it’s what you know versus what you don’t know, and warfarin has been used for 60 years," Dr. Kimmel said at the press conference.
"The new drugs perform better than warfarin does; they get patients to where they need to be more quickly, but warfarin has been around a long time. We know its interactions and risks, there is the ability to reverse its effect, and the cost to patients is a real issue. If the new anticoagulants were the same price as warfarin then I think you’d see a lot more patients get a new drug," Dr. Ellinor said in an interview.
Data on current U.S. uptake of the new oral anticoagulants came from the 148,320 unique patients with atrial fibrillation included during June to September of 2013 in the PINNACLE Registry database run by the American College of Cardiology. Among these patients, about 83,000 (56%) were on some anticoagulant treatment, and within this subgroup, 72% were on warfarin, 27% on a new anticoagulant, and the remainder on different treatment, said Dr. John Gordon Harold, ACC president and a cardiologist at Cedars-Sinai Heart Institute in Los Angeles.
"In my own practice the new anticoagulants are being used with increasing frequency, mainly driven by direct-to-consumer advertising," Dr. Harold said in an interview. "We have patients who are completely stable on warfarin. (They) come in because of a consumer ad and they ask if they should switch drugs. When patients are stable I don’t encourage them to switch, but we have a shared decision making conversation and go over the pros and cons, the cost, and the outcomes data. A lot of patients prefer to pay the difference" and switch to a new anticoagulant.
Dr. Harold said he also recommends that patients switch off warfarin if they have problems with compliance and variability in their international normalized ratio (INR).
"If you can keep a patient on warfarin in their INR target range 80% or more of the time then I wouldn’t change, but most patients on warfarin have a very hard time maintaining an INR of 2-3," said Dr. Mark S. Link, professor and codirector of the cardiac arrhythmia center at Tufts Medical Center, Boston. But he said cost is a major factor keeping many patients on warfarin.
The new anticoagulants "are better than warfarin, but we are often forced by insurers to start with the cheaper drug," Dr. Link said during the news conference.
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed, Dr. Ellinor, Dr. Harold, and Dr. Link had no relevant disclosures.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Patients starting warfarin with dosing based on a formula that took into account seven clinical and demographic factors averaged 45% of the time in therapeutic range regardless of whether the starting dosage was adjusted based on genotype results.
Data source: COAG, a randomized trial with 1,015 patients starting warfarin therapy at 18 U.S. centers.
Disclosures: The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed and Dr. Ellinor had no disclosures.
Zenith AAA fenestrated graft: 30-day outcomes match those seen in trial
CHICAGO – Real-world 30-day outcomes with the Zenith fenestrated endovascular graft compared well with those in the U.S. fenestrated trial for the treatment of juxtarenal aortic aneurysms, based on a multicenter, retrospective study of 57 patients.
The good results were seen despite higher rates of comorbidities and more challenging anatomy in the patients treated post approval. But "these are short-term outcomes and, ultimately, we’re going to need to know the long-term durability of this type of repair," Dr. Chandu Vemuri said at the annual meeting of the Midwestern Vascular Surgical Society.
The Zenith fenestrated AAA endovascular graft (zFEN) has been commercially available outside the United States since November 2002, and it gained federal approval in April 2012 based on the results of the pivotal U.S. fenestrated trial (USFT). The graft is indicated for the treatment of patients with abdominal aortic or aortoiliac aneurysms that have an infrarenal aortic neck at least 4 mm in length.
Notably, 47% of patients in the current analysis did not meet the USFT anatomic criteria of a 4- to 15-mm infrarenal neck. There were also significantly more mesenteric stents used in this group than in the USFT (13 vs. 0; P < .05), reflecting the higher anatomic complexity of these patients, said Dr. Vemuri, of Washington University, St. Louis.
Compared with the 42 USFT patients, study patients also had significantly higher rates of preoperative coronary artery disease (79% vs. 52%), myocardial infarction (60% vs. 24%), and renal insufficiency (26% vs. 9.5%); all P < .05).
Dr. Vemuri was quick to point out, however, that the results of the study may not broadly translate. The trial was conducted at institutions that had a high aortic volume, and involved highly experienced surgeons, many of whom took part in the USFT and were familiar with the zFEN device.
The technical success rate, with regard to aneurysm exclusion, was 100% in both the study and the USFT. In two study patients, the left renal artery (RA) fenestration was not able to be aligned. One patient had a kinked renal stent that was successfully restented, and one patient was converted to a unibody prosthesis with femoral-femoral bypass and an iliac plug, he said.
Other 30-day outcomes were similar between the study and USFT patients, including mortality (1 vs. 0), renal insufficiency (4 vs. 0), postoperative dialysis (1 vs. 0), and endoleak (10 vs. 9). Two of the 10 endoleaks were type III and 8 were type II.
The most common graft configuration was a scallop of the superior mesenteric artery (SMA) and bilateral RA fenestration in 40 patients, followed by bilateral RA fenestrations alone in 8, and a celiac scallop, SMA fenestration, and bilateral RA fenestration in 3, Dr. Vemuri observed.
One patient each had the following: right RA scallop plus left RA fenestration, right RA fenestration, right accessory RA fenestration, left RA fenestration, celiac scallop and SMA fenestration with a right RA fenestration, and an SMA scallop with left RA fenestration.
Total operative time averaged 250 minutes.
Audience members pressed Dr. Vemuri for details on the 47% of patients who did not meet the USFT anatomic criteria and whether the current cohort was really anatomically challenging or just consisted of on-label cases that were harder than usual.
Dr. Vemuri responded that the intervention was off-label in 27% of patients who did not have the minimum 4-mm infrarenal neck. One graft configuration was more complex than in the device’s instructions for use (IFU) document. All of the patients had a sufficient infrarenal neck for appropriate seal.
"I don’t think the purpose of this study is to endorse use outside the IFU of the device, but to report that when people do, this is what happens," he added.
Dr. Vemuri reported having no financial disclosures. His coauthors reported relationships including serving as consultants or researchers for Cook, which produces the Zenith graft.
CHICAGO – Real-world 30-day outcomes with the Zenith fenestrated endovascular graft compared well with those in the U.S. fenestrated trial for the treatment of juxtarenal aortic aneurysms, based on a multicenter, retrospective study of 57 patients.
The good results were seen despite higher rates of comorbidities and more challenging anatomy in the patients treated post approval. But "these are short-term outcomes and, ultimately, we’re going to need to know the long-term durability of this type of repair," Dr. Chandu Vemuri said at the annual meeting of the Midwestern Vascular Surgical Society.
The Zenith fenestrated AAA endovascular graft (zFEN) has been commercially available outside the United States since November 2002, and it gained federal approval in April 2012 based on the results of the pivotal U.S. fenestrated trial (USFT). The graft is indicated for the treatment of patients with abdominal aortic or aortoiliac aneurysms that have an infrarenal aortic neck at least 4 mm in length.
Notably, 47% of patients in the current analysis did not meet the USFT anatomic criteria of a 4- to 15-mm infrarenal neck. There were also significantly more mesenteric stents used in this group than in the USFT (13 vs. 0; P < .05), reflecting the higher anatomic complexity of these patients, said Dr. Vemuri, of Washington University, St. Louis.
Compared with the 42 USFT patients, study patients also had significantly higher rates of preoperative coronary artery disease (79% vs. 52%), myocardial infarction (60% vs. 24%), and renal insufficiency (26% vs. 9.5%); all P < .05).
Dr. Vemuri was quick to point out, however, that the results of the study may not broadly translate. The trial was conducted at institutions that had a high aortic volume, and involved highly experienced surgeons, many of whom took part in the USFT and were familiar with the zFEN device.
The technical success rate, with regard to aneurysm exclusion, was 100% in both the study and the USFT. In two study patients, the left renal artery (RA) fenestration was not able to be aligned. One patient had a kinked renal stent that was successfully restented, and one patient was converted to a unibody prosthesis with femoral-femoral bypass and an iliac plug, he said.
Other 30-day outcomes were similar between the study and USFT patients, including mortality (1 vs. 0), renal insufficiency (4 vs. 0), postoperative dialysis (1 vs. 0), and endoleak (10 vs. 9). Two of the 10 endoleaks were type III and 8 were type II.
The most common graft configuration was a scallop of the superior mesenteric artery (SMA) and bilateral RA fenestration in 40 patients, followed by bilateral RA fenestrations alone in 8, and a celiac scallop, SMA fenestration, and bilateral RA fenestration in 3, Dr. Vemuri observed.
One patient each had the following: right RA scallop plus left RA fenestration, right RA fenestration, right accessory RA fenestration, left RA fenestration, celiac scallop and SMA fenestration with a right RA fenestration, and an SMA scallop with left RA fenestration.
Total operative time averaged 250 minutes.
Audience members pressed Dr. Vemuri for details on the 47% of patients who did not meet the USFT anatomic criteria and whether the current cohort was really anatomically challenging or just consisted of on-label cases that were harder than usual.
Dr. Vemuri responded that the intervention was off-label in 27% of patients who did not have the minimum 4-mm infrarenal neck. One graft configuration was more complex than in the device’s instructions for use (IFU) document. All of the patients had a sufficient infrarenal neck for appropriate seal.
"I don’t think the purpose of this study is to endorse use outside the IFU of the device, but to report that when people do, this is what happens," he added.
Dr. Vemuri reported having no financial disclosures. His coauthors reported relationships including serving as consultants or researchers for Cook, which produces the Zenith graft.
CHICAGO – Real-world 30-day outcomes with the Zenith fenestrated endovascular graft compared well with those in the U.S. fenestrated trial for the treatment of juxtarenal aortic aneurysms, based on a multicenter, retrospective study of 57 patients.
The good results were seen despite higher rates of comorbidities and more challenging anatomy in the patients treated post approval. But "these are short-term outcomes and, ultimately, we’re going to need to know the long-term durability of this type of repair," Dr. Chandu Vemuri said at the annual meeting of the Midwestern Vascular Surgical Society.
The Zenith fenestrated AAA endovascular graft (zFEN) has been commercially available outside the United States since November 2002, and it gained federal approval in April 2012 based on the results of the pivotal U.S. fenestrated trial (USFT). The graft is indicated for the treatment of patients with abdominal aortic or aortoiliac aneurysms that have an infrarenal aortic neck at least 4 mm in length.
Notably, 47% of patients in the current analysis did not meet the USFT anatomic criteria of a 4- to 15-mm infrarenal neck. There were also significantly more mesenteric stents used in this group than in the USFT (13 vs. 0; P < .05), reflecting the higher anatomic complexity of these patients, said Dr. Vemuri, of Washington University, St. Louis.
Compared with the 42 USFT patients, study patients also had significantly higher rates of preoperative coronary artery disease (79% vs. 52%), myocardial infarction (60% vs. 24%), and renal insufficiency (26% vs. 9.5%); all P < .05).
Dr. Vemuri was quick to point out, however, that the results of the study may not broadly translate. The trial was conducted at institutions that had a high aortic volume, and involved highly experienced surgeons, many of whom took part in the USFT and were familiar with the zFEN device.
The technical success rate, with regard to aneurysm exclusion, was 100% in both the study and the USFT. In two study patients, the left renal artery (RA) fenestration was not able to be aligned. One patient had a kinked renal stent that was successfully restented, and one patient was converted to a unibody prosthesis with femoral-femoral bypass and an iliac plug, he said.
Other 30-day outcomes were similar between the study and USFT patients, including mortality (1 vs. 0), renal insufficiency (4 vs. 0), postoperative dialysis (1 vs. 0), and endoleak (10 vs. 9). Two of the 10 endoleaks were type III and 8 were type II.
The most common graft configuration was a scallop of the superior mesenteric artery (SMA) and bilateral RA fenestration in 40 patients, followed by bilateral RA fenestrations alone in 8, and a celiac scallop, SMA fenestration, and bilateral RA fenestration in 3, Dr. Vemuri observed.
One patient each had the following: right RA scallop plus left RA fenestration, right RA fenestration, right accessory RA fenestration, left RA fenestration, celiac scallop and SMA fenestration with a right RA fenestration, and an SMA scallop with left RA fenestration.
Total operative time averaged 250 minutes.
Audience members pressed Dr. Vemuri for details on the 47% of patients who did not meet the USFT anatomic criteria and whether the current cohort was really anatomically challenging or just consisted of on-label cases that were harder than usual.
Dr. Vemuri responded that the intervention was off-label in 27% of patients who did not have the minimum 4-mm infrarenal neck. One graft configuration was more complex than in the device’s instructions for use (IFU) document. All of the patients had a sufficient infrarenal neck for appropriate seal.
"I don’t think the purpose of this study is to endorse use outside the IFU of the device, but to report that when people do, this is what happens," he added.
Dr. Vemuri reported having no financial disclosures. His coauthors reported relationships including serving as consultants or researchers for Cook, which produces the Zenith graft.
AT MIDWESTERN VASCULAR 2013
Major finding: The technical success rate was 100%.
Data source: Retrospective study of 57 patients with juxtarenal aortic aneurysms treated post approval with the Zenith fenestrated endovascular graft.
Disclosures: Dr. Vemuri reported having no financial disclosures. His coauthors reported relationships including serving as consultants or researchers for Cook, which produces the Zenith graft.
President signs budget deal, short-term SGR fix
Physicians will get a 0.5% raise in Medicare pay on Jan. 1, thanks to a last-minute legislative fix to the Sustainable Growth Rate formula signed into law by President Obama on Dec. 26.
The Pathway for SGR Reform Act of 2013 was attached as an amendment to the Bipartisan Budget Agreement of 2013. The budget deal, brokered by Sen. Patty Murray (D-Wash.) and Rep. Paul Ryan (R-Wisc.), passed the House on Dec. 12 and the Senate on Dec. 18. The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. It would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
The law also extends the 2% sequestration cut to Medicare payments by 2 years, to 2023.
It encourages the Centers for Medicare and Medicaid Services to simplify physicians’ administrative burden by trying to more closely coordinate quality measure requirements and give doctors more timely feedback on those measures.
The law extends funding for a variety of other health-related federal programs for an additional 3 months, including for Area Agencies on Aging.
Congress is expected to start consideration again of a permanent replacement for the SGR when it returns on Jan. 6.
On Twitter @aliciaault
*Correction 2/05/14: The following passage was deleted from a previous version of this story as the provision was not included in the law as signed.
Finally, the law delays enforcement of the "two-midnight rule" until Oct. 2014. The goal of the two-midnight policy, which was included in the fiscal 2014 Inpatient Prospective Payment System final rule, is to cut down on hospitals using observation status to keep from admitting patients. Hospitals now have until Oct. 1, 2014 to adjust to the new policy, which requires patients to be admitted if physicians think they will need a stay longer than 48 hours.
Physicians will get a 0.5% raise in Medicare pay on Jan. 1, thanks to a last-minute legislative fix to the Sustainable Growth Rate formula signed into law by President Obama on Dec. 26.
The Pathway for SGR Reform Act of 2013 was attached as an amendment to the Bipartisan Budget Agreement of 2013. The budget deal, brokered by Sen. Patty Murray (D-Wash.) and Rep. Paul Ryan (R-Wisc.), passed the House on Dec. 12 and the Senate on Dec. 18. The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. It would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
The law also extends the 2% sequestration cut to Medicare payments by 2 years, to 2023.
It encourages the Centers for Medicare and Medicaid Services to simplify physicians’ administrative burden by trying to more closely coordinate quality measure requirements and give doctors more timely feedback on those measures.
The law extends funding for a variety of other health-related federal programs for an additional 3 months, including for Area Agencies on Aging.
Congress is expected to start consideration again of a permanent replacement for the SGR when it returns on Jan. 6.
On Twitter @aliciaault
*Correction 2/05/14: The following passage was deleted from a previous version of this story as the provision was not included in the law as signed.
Finally, the law delays enforcement of the "two-midnight rule" until Oct. 2014. The goal of the two-midnight policy, which was included in the fiscal 2014 Inpatient Prospective Payment System final rule, is to cut down on hospitals using observation status to keep from admitting patients. Hospitals now have until Oct. 1, 2014 to adjust to the new policy, which requires patients to be admitted if physicians think they will need a stay longer than 48 hours.
Physicians will get a 0.5% raise in Medicare pay on Jan. 1, thanks to a last-minute legislative fix to the Sustainable Growth Rate formula signed into law by President Obama on Dec. 26.
The Pathway for SGR Reform Act of 2013 was attached as an amendment to the Bipartisan Budget Agreement of 2013. The budget deal, brokered by Sen. Patty Murray (D-Wash.) and Rep. Paul Ryan (R-Wisc.), passed the House on Dec. 12 and the Senate on Dec. 18. The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. It would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
The law also extends the 2% sequestration cut to Medicare payments by 2 years, to 2023.
It encourages the Centers for Medicare and Medicaid Services to simplify physicians’ administrative burden by trying to more closely coordinate quality measure requirements and give doctors more timely feedback on those measures.
The law extends funding for a variety of other health-related federal programs for an additional 3 months, including for Area Agencies on Aging.
Congress is expected to start consideration again of a permanent replacement for the SGR when it returns on Jan. 6.
On Twitter @aliciaault
*Correction 2/05/14: The following passage was deleted from a previous version of this story as the provision was not included in the law as signed.
Finally, the law delays enforcement of the "two-midnight rule" until Oct. 2014. The goal of the two-midnight policy, which was included in the fiscal 2014 Inpatient Prospective Payment System final rule, is to cut down on hospitals using observation status to keep from admitting patients. Hospitals now have until Oct. 1, 2014 to adjust to the new policy, which requires patients to be admitted if physicians think they will need a stay longer than 48 hours.
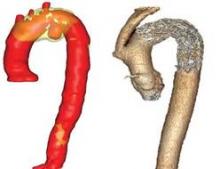
Aortic arch repair outcomes held acceptable
CHICAGO – Repair of the isolated nontraumatic aortic arch aneurysm can be performed with acceptable early and late results, based on a 20-year review of 137 patients.
Early mortality was seen in nine patients (6.6%), and was similarly split between endovascular and open repair (7.3% vs. 6.5%, respectively).
Five-year survival did not differ between the endovascular- and open-repair groups (86% vs. 70%; P = .57), although the risk of reintervention was significantly higher with an endovascular strategy (94% vs. 77.5%; P = .02).
"These data support ongoing efforts to develop branched endografts specifically tailored for complex and difficult-to-treat pathology to potentially reduce the morbidity of therapy for this population," Dr. Himanshu J. Patel said at the annual meeting of the Midwestern Vascular Surgical Society.
Marginal landing zones and the need for complex arch debranching procedures to extend landing zones and facilitate repair can hinder endovascular repair, though open aortic repair is challenged by the frequent need for hypothermic circulatory arrest. Early and late results with either option are ill defined for this pathology, observed Dr. Patel, with the Cardiovascular Center, University of Michigan, Ann Arbor.
To investigate these outcomes, the authors evaluated data from 1993 to 2013 for 137 patients with nontraumatic aortic aneurysms located between the left and right pulmonary artery; 93 patients underwent open repair and 44 had an endovascular or hybrid repair.
The pathology was saccular in 39%, dissection in 11%, pseudoaneurysm in 18%, and anomalous arch in 11%. Rupture was identified in 14%. The average patient age was 59 years, and 46% were male.
In the open-repair group, 84 patients underwent a posterolateral thoracotomy and 9 had a median sternotomy. Extracorporeal perfusion was used in all, and 80 required deep hypothermic arrest.
In the endovascular group, the proximal extent of surgery was classified as Ishimaru zone 0 in 10 patients, zone 1 in 1, and zone 2 in 33. Treatment included full arch debranching and stent graft repair for zone 0 and zone 2 patients, respectively. A chimney stent of the carotid artery and left subclavian arterial bypass was used for the zone 1 patient.
Although the institutional preference is to utilize left subclavian artery (LSCA) revascularization for all, 76% of endovascular patients requiring LSCA coverage underwent preoperative bypass, Dr. Patel observed.
Significant differences existed at baseline between the two groups, with the endovascular group almost a full decade older than the open group (65 vs. 56 years). The endovascular group was also more likely to have saccular pathology (57% vs. 30%), and less likely to have a history of tobacco use (12% vs. 84%) or prior arch repair (16% vs. 36%).
Early morbidity included stroke in 6.6%, spinal cord ischemia in 0.7%, need for dialysis in 3.6%, and tracheostomy in 7.2%, Dr. Patel said.
The composite endpoint of stroke or early mortality was independently predicted by increasing age (odds ratio, 1.05; P = .055) and use of a hybrid procedure (OR, 6.4; P = .01).
Two-year freedom from aortic rupture or reintervention was 78% in the open group and 94% in the endovascular group (P = .02).
After a mean follow-up of 66 months, four patients had died of stroke, two from myocardial infarction, one from a proximal anastomosis disruption/rupture, and one from a type A dissection/rupture, Dr. Patel said.
The average survival was 145 months and 5-year survival rate was 59%.
Independent predictors of late mortality were increasing age (hazard ratio, 1.06; P = .001), peripheral vascular disease (HR, 2.4; P = .024), postoperative stroke (HR, 6.4; P less than .001), and postoperative dialysis (HR, 11.4; P less than .001). Notably, repair type was not predictive (P = .22), Dr. Patel said.
He cautioned the audience, however, that "cerebrovascular accidents remain an important cause of early and late mortality."
Dr. Patel reported consulting fees from W.L. Gore and Terumo.
CHICAGO – Repair of the isolated nontraumatic aortic arch aneurysm can be performed with acceptable early and late results, based on a 20-year review of 137 patients.
Early mortality was seen in nine patients (6.6%), and was similarly split between endovascular and open repair (7.3% vs. 6.5%, respectively).
Five-year survival did not differ between the endovascular- and open-repair groups (86% vs. 70%; P = .57), although the risk of reintervention was significantly higher with an endovascular strategy (94% vs. 77.5%; P = .02).
"These data support ongoing efforts to develop branched endografts specifically tailored for complex and difficult-to-treat pathology to potentially reduce the morbidity of therapy for this population," Dr. Himanshu J. Patel said at the annual meeting of the Midwestern Vascular Surgical Society.
Marginal landing zones and the need for complex arch debranching procedures to extend landing zones and facilitate repair can hinder endovascular repair, though open aortic repair is challenged by the frequent need for hypothermic circulatory arrest. Early and late results with either option are ill defined for this pathology, observed Dr. Patel, with the Cardiovascular Center, University of Michigan, Ann Arbor.
To investigate these outcomes, the authors evaluated data from 1993 to 2013 for 137 patients with nontraumatic aortic aneurysms located between the left and right pulmonary artery; 93 patients underwent open repair and 44 had an endovascular or hybrid repair.
The pathology was saccular in 39%, dissection in 11%, pseudoaneurysm in 18%, and anomalous arch in 11%. Rupture was identified in 14%. The average patient age was 59 years, and 46% were male.
In the open-repair group, 84 patients underwent a posterolateral thoracotomy and 9 had a median sternotomy. Extracorporeal perfusion was used in all, and 80 required deep hypothermic arrest.
In the endovascular group, the proximal extent of surgery was classified as Ishimaru zone 0 in 10 patients, zone 1 in 1, and zone 2 in 33. Treatment included full arch debranching and stent graft repair for zone 0 and zone 2 patients, respectively. A chimney stent of the carotid artery and left subclavian arterial bypass was used for the zone 1 patient.
Although the institutional preference is to utilize left subclavian artery (LSCA) revascularization for all, 76% of endovascular patients requiring LSCA coverage underwent preoperative bypass, Dr. Patel observed.
Significant differences existed at baseline between the two groups, with the endovascular group almost a full decade older than the open group (65 vs. 56 years). The endovascular group was also more likely to have saccular pathology (57% vs. 30%), and less likely to have a history of tobacco use (12% vs. 84%) or prior arch repair (16% vs. 36%).
Early morbidity included stroke in 6.6%, spinal cord ischemia in 0.7%, need for dialysis in 3.6%, and tracheostomy in 7.2%, Dr. Patel said.
The composite endpoint of stroke or early mortality was independently predicted by increasing age (odds ratio, 1.05; P = .055) and use of a hybrid procedure (OR, 6.4; P = .01).
Two-year freedom from aortic rupture or reintervention was 78% in the open group and 94% in the endovascular group (P = .02).
After a mean follow-up of 66 months, four patients had died of stroke, two from myocardial infarction, one from a proximal anastomosis disruption/rupture, and one from a type A dissection/rupture, Dr. Patel said.
The average survival was 145 months and 5-year survival rate was 59%.
Independent predictors of late mortality were increasing age (hazard ratio, 1.06; P = .001), peripheral vascular disease (HR, 2.4; P = .024), postoperative stroke (HR, 6.4; P less than .001), and postoperative dialysis (HR, 11.4; P less than .001). Notably, repair type was not predictive (P = .22), Dr. Patel said.
He cautioned the audience, however, that "cerebrovascular accidents remain an important cause of early and late mortality."
Dr. Patel reported consulting fees from W.L. Gore and Terumo.
CHICAGO – Repair of the isolated nontraumatic aortic arch aneurysm can be performed with acceptable early and late results, based on a 20-year review of 137 patients.
Early mortality was seen in nine patients (6.6%), and was similarly split between endovascular and open repair (7.3% vs. 6.5%, respectively).
Five-year survival did not differ between the endovascular- and open-repair groups (86% vs. 70%; P = .57), although the risk of reintervention was significantly higher with an endovascular strategy (94% vs. 77.5%; P = .02).
"These data support ongoing efforts to develop branched endografts specifically tailored for complex and difficult-to-treat pathology to potentially reduce the morbidity of therapy for this population," Dr. Himanshu J. Patel said at the annual meeting of the Midwestern Vascular Surgical Society.
Marginal landing zones and the need for complex arch debranching procedures to extend landing zones and facilitate repair can hinder endovascular repair, though open aortic repair is challenged by the frequent need for hypothermic circulatory arrest. Early and late results with either option are ill defined for this pathology, observed Dr. Patel, with the Cardiovascular Center, University of Michigan, Ann Arbor.
To investigate these outcomes, the authors evaluated data from 1993 to 2013 for 137 patients with nontraumatic aortic aneurysms located between the left and right pulmonary artery; 93 patients underwent open repair and 44 had an endovascular or hybrid repair.
The pathology was saccular in 39%, dissection in 11%, pseudoaneurysm in 18%, and anomalous arch in 11%. Rupture was identified in 14%. The average patient age was 59 years, and 46% were male.
In the open-repair group, 84 patients underwent a posterolateral thoracotomy and 9 had a median sternotomy. Extracorporeal perfusion was used in all, and 80 required deep hypothermic arrest.
In the endovascular group, the proximal extent of surgery was classified as Ishimaru zone 0 in 10 patients, zone 1 in 1, and zone 2 in 33. Treatment included full arch debranching and stent graft repair for zone 0 and zone 2 patients, respectively. A chimney stent of the carotid artery and left subclavian arterial bypass was used for the zone 1 patient.
Although the institutional preference is to utilize left subclavian artery (LSCA) revascularization for all, 76% of endovascular patients requiring LSCA coverage underwent preoperative bypass, Dr. Patel observed.
Significant differences existed at baseline between the two groups, with the endovascular group almost a full decade older than the open group (65 vs. 56 years). The endovascular group was also more likely to have saccular pathology (57% vs. 30%), and less likely to have a history of tobacco use (12% vs. 84%) or prior arch repair (16% vs. 36%).
Early morbidity included stroke in 6.6%, spinal cord ischemia in 0.7%, need for dialysis in 3.6%, and tracheostomy in 7.2%, Dr. Patel said.
The composite endpoint of stroke or early mortality was independently predicted by increasing age (odds ratio, 1.05; P = .055) and use of a hybrid procedure (OR, 6.4; P = .01).
Two-year freedom from aortic rupture or reintervention was 78% in the open group and 94% in the endovascular group (P = .02).
After a mean follow-up of 66 months, four patients had died of stroke, two from myocardial infarction, one from a proximal anastomosis disruption/rupture, and one from a type A dissection/rupture, Dr. Patel said.
The average survival was 145 months and 5-year survival rate was 59%.
Independent predictors of late mortality were increasing age (hazard ratio, 1.06; P = .001), peripheral vascular disease (HR, 2.4; P = .024), postoperative stroke (HR, 6.4; P less than .001), and postoperative dialysis (HR, 11.4; P less than .001). Notably, repair type was not predictive (P = .22), Dr. Patel said.
He cautioned the audience, however, that "cerebrovascular accidents remain an important cause of early and late mortality."
Dr. Patel reported consulting fees from W.L. Gore and Terumo.
AT MIDWESTERN VASCULAR 2013
Major finding: Five-year survival was 86% in the endovascular group and 70% in the open group (P = .57).
Data source: A retrospective study of 137 patients undergoing isolated aortic arch repair between 1993 and 2013.
Disclosures: Dr. Patel reported consulting fees from W.L. Gore and Terumo.
Renal injury taints proximal AAA repair
CHICAGO – Proximal abdominal aortic aneurysm repair can be achieved with a low perioperative mortality of about 3%, although renal dysfunction remains an Achilles’ heel, according to a 27-year review involving 245 patients.
In all, 60% of patients had postoperative acute kidney injury (AKI), 28% had persistent AKI at discharge, and 1.6% were discharged on hemodialysis. Persistent AKI at discharge was also associated with reduced long-term survival, Dr. Loay Kabbani said at the annual meeting of the Midwestern Vascular Surgical Society.
AKI stage was based on the Risk, Injury, Failure, Loss, and End-stage (RIFLE) kidney disease/Acute Kidney Injury Network (AKIN) classification system, with a change in serum creatinine of more than 0.3 mg/dL considered positive for injury. This is a much more sensitive criterion than what was used in previous studies, but is the new standard for reporting renal injury.
"This data should assist in establishing a benchmark for endovascular repair of these complex aneurysms," said Dr. Kabbani, a vascular surgeon at Henry Ford Hospital in Detroit.
The investigators identified 245 patients who underwent proximal AAA repair between 1986 and February 2013 at Henry Ford Hospital for juxtarenal (127), suprarenal (68), or type IV thoracoabdominal AAAs (50). The average aneurysm size was 6.4 cm (range, 3.1-14 cm), and mean preoperative estimated glomerular filtration rate was 62 mL/min per 1.73 m2 (range, 14-166 mL/min per 1.73 m2).
Most patients were male (69%), white (88.5%), and hypertensive (84%); 55% had chronic kidney disease based on a preoperative estimated glomerular filtration rate below 60 mL/min per 1.73 m2. Their average age was 71 years.
For most patients, the approach was retroperitoneal (48%) or thoracoabdominal (30%); the clamp was placed supraceliac (58%); and tube grafts were used (64%).
In-hospital mortality was 2.9% (7 patients) and 30-day mortality 3.3% (8 patients), Dr. Kabbani said.
Postoperative complications were AKI in 144 patients (60%), major pulmonary complications in 55 (22%), myocardial infarction in 9 (4%), return to the operating room for postoperative bleeding in 7 (3%), neurologic complications in 7 (3%), and bowel ischemia in 5 (2%).
Specifically, postoperative AKI was stage 1 (mild) in 35%, stage 2 (moderate) in 15%, and stage 3 (severe) in 10%, improving to 12%, 4%, and 3%, respectively, at discharge.
Though preoperative comorbidities were similar between patients with juxtarenal, suprarenal, and type IV aneurysms, postoperative complications increased significantly as the aneurysm extended more proximally (31% vs. 50% vs. 72%; P < .0001), Dr. Kabbani said.
Rates of AKI at discharge also followed the same pattern (18.2% vs. 35.4% vs. 43.8%; P less than .0012).
After a median follow-up of 54 months, the 5-year survival estimate was 70% and 10-year survival 43% based on a review of electronic medical records and the Social Security Death Index.
Cox regression analyses revealed no difference in long-term survival based on aneurysm type, but significantly worse survival based on increased aneurysm size (hazard ratio, 1.1; P = .01), preoperative chronic obstructive pulmonary disease (HR, 1.8; P = .002), congestive heart failure (HR, 3.5; P less than .001), history of stroke (HR, 1.9; P = .04), and persistent stage 2 (HR, 5.29; P = .001) and stage 3 AKI at discharge (HR, 2.71; P = .014), he said.
During a discussion of the results, Dr. Kabbani said the hospital usually uses mannitol (12.5-25 g) for renal protection and cold renal perfusion in select patients, although the latter did not jump out as a risk factor for CKI. The data were not broken down to see whether use of renal bypass during surgery affected outcomes for specific AAA types. Some attendees countered that renal protection should be used for all patients undergoing proximal AAA repair.
Dr. Kabbani reported having no financial disclosures.
CHICAGO – Proximal abdominal aortic aneurysm repair can be achieved with a low perioperative mortality of about 3%, although renal dysfunction remains an Achilles’ heel, according to a 27-year review involving 245 patients.
In all, 60% of patients had postoperative acute kidney injury (AKI), 28% had persistent AKI at discharge, and 1.6% were discharged on hemodialysis. Persistent AKI at discharge was also associated with reduced long-term survival, Dr. Loay Kabbani said at the annual meeting of the Midwestern Vascular Surgical Society.
AKI stage was based on the Risk, Injury, Failure, Loss, and End-stage (RIFLE) kidney disease/Acute Kidney Injury Network (AKIN) classification system, with a change in serum creatinine of more than 0.3 mg/dL considered positive for injury. This is a much more sensitive criterion than what was used in previous studies, but is the new standard for reporting renal injury.
"This data should assist in establishing a benchmark for endovascular repair of these complex aneurysms," said Dr. Kabbani, a vascular surgeon at Henry Ford Hospital in Detroit.
The investigators identified 245 patients who underwent proximal AAA repair between 1986 and February 2013 at Henry Ford Hospital for juxtarenal (127), suprarenal (68), or type IV thoracoabdominal AAAs (50). The average aneurysm size was 6.4 cm (range, 3.1-14 cm), and mean preoperative estimated glomerular filtration rate was 62 mL/min per 1.73 m2 (range, 14-166 mL/min per 1.73 m2).
Most patients were male (69%), white (88.5%), and hypertensive (84%); 55% had chronic kidney disease based on a preoperative estimated glomerular filtration rate below 60 mL/min per 1.73 m2. Their average age was 71 years.
For most patients, the approach was retroperitoneal (48%) or thoracoabdominal (30%); the clamp was placed supraceliac (58%); and tube grafts were used (64%).
In-hospital mortality was 2.9% (7 patients) and 30-day mortality 3.3% (8 patients), Dr. Kabbani said.
Postoperative complications were AKI in 144 patients (60%), major pulmonary complications in 55 (22%), myocardial infarction in 9 (4%), return to the operating room for postoperative bleeding in 7 (3%), neurologic complications in 7 (3%), and bowel ischemia in 5 (2%).
Specifically, postoperative AKI was stage 1 (mild) in 35%, stage 2 (moderate) in 15%, and stage 3 (severe) in 10%, improving to 12%, 4%, and 3%, respectively, at discharge.
Though preoperative comorbidities were similar between patients with juxtarenal, suprarenal, and type IV aneurysms, postoperative complications increased significantly as the aneurysm extended more proximally (31% vs. 50% vs. 72%; P < .0001), Dr. Kabbani said.
Rates of AKI at discharge also followed the same pattern (18.2% vs. 35.4% vs. 43.8%; P less than .0012).
After a median follow-up of 54 months, the 5-year survival estimate was 70% and 10-year survival 43% based on a review of electronic medical records and the Social Security Death Index.
Cox regression analyses revealed no difference in long-term survival based on aneurysm type, but significantly worse survival based on increased aneurysm size (hazard ratio, 1.1; P = .01), preoperative chronic obstructive pulmonary disease (HR, 1.8; P = .002), congestive heart failure (HR, 3.5; P less than .001), history of stroke (HR, 1.9; P = .04), and persistent stage 2 (HR, 5.29; P = .001) and stage 3 AKI at discharge (HR, 2.71; P = .014), he said.
During a discussion of the results, Dr. Kabbani said the hospital usually uses mannitol (12.5-25 g) for renal protection and cold renal perfusion in select patients, although the latter did not jump out as a risk factor for CKI. The data were not broken down to see whether use of renal bypass during surgery affected outcomes for specific AAA types. Some attendees countered that renal protection should be used for all patients undergoing proximal AAA repair.
Dr. Kabbani reported having no financial disclosures.
CHICAGO – Proximal abdominal aortic aneurysm repair can be achieved with a low perioperative mortality of about 3%, although renal dysfunction remains an Achilles’ heel, according to a 27-year review involving 245 patients.
In all, 60% of patients had postoperative acute kidney injury (AKI), 28% had persistent AKI at discharge, and 1.6% were discharged on hemodialysis. Persistent AKI at discharge was also associated with reduced long-term survival, Dr. Loay Kabbani said at the annual meeting of the Midwestern Vascular Surgical Society.
AKI stage was based on the Risk, Injury, Failure, Loss, and End-stage (RIFLE) kidney disease/Acute Kidney Injury Network (AKIN) classification system, with a change in serum creatinine of more than 0.3 mg/dL considered positive for injury. This is a much more sensitive criterion than what was used in previous studies, but is the new standard for reporting renal injury.
"This data should assist in establishing a benchmark for endovascular repair of these complex aneurysms," said Dr. Kabbani, a vascular surgeon at Henry Ford Hospital in Detroit.
The investigators identified 245 patients who underwent proximal AAA repair between 1986 and February 2013 at Henry Ford Hospital for juxtarenal (127), suprarenal (68), or type IV thoracoabdominal AAAs (50). The average aneurysm size was 6.4 cm (range, 3.1-14 cm), and mean preoperative estimated glomerular filtration rate was 62 mL/min per 1.73 m2 (range, 14-166 mL/min per 1.73 m2).
Most patients were male (69%), white (88.5%), and hypertensive (84%); 55% had chronic kidney disease based on a preoperative estimated glomerular filtration rate below 60 mL/min per 1.73 m2. Their average age was 71 years.
For most patients, the approach was retroperitoneal (48%) or thoracoabdominal (30%); the clamp was placed supraceliac (58%); and tube grafts were used (64%).
In-hospital mortality was 2.9% (7 patients) and 30-day mortality 3.3% (8 patients), Dr. Kabbani said.
Postoperative complications were AKI in 144 patients (60%), major pulmonary complications in 55 (22%), myocardial infarction in 9 (4%), return to the operating room for postoperative bleeding in 7 (3%), neurologic complications in 7 (3%), and bowel ischemia in 5 (2%).
Specifically, postoperative AKI was stage 1 (mild) in 35%, stage 2 (moderate) in 15%, and stage 3 (severe) in 10%, improving to 12%, 4%, and 3%, respectively, at discharge.
Though preoperative comorbidities were similar between patients with juxtarenal, suprarenal, and type IV aneurysms, postoperative complications increased significantly as the aneurysm extended more proximally (31% vs. 50% vs. 72%; P < .0001), Dr. Kabbani said.
Rates of AKI at discharge also followed the same pattern (18.2% vs. 35.4% vs. 43.8%; P less than .0012).
After a median follow-up of 54 months, the 5-year survival estimate was 70% and 10-year survival 43% based on a review of electronic medical records and the Social Security Death Index.
Cox regression analyses revealed no difference in long-term survival based on aneurysm type, but significantly worse survival based on increased aneurysm size (hazard ratio, 1.1; P = .01), preoperative chronic obstructive pulmonary disease (HR, 1.8; P = .002), congestive heart failure (HR, 3.5; P less than .001), history of stroke (HR, 1.9; P = .04), and persistent stage 2 (HR, 5.29; P = .001) and stage 3 AKI at discharge (HR, 2.71; P = .014), he said.
During a discussion of the results, Dr. Kabbani said the hospital usually uses mannitol (12.5-25 g) for renal protection and cold renal perfusion in select patients, although the latter did not jump out as a risk factor for CKI. The data were not broken down to see whether use of renal bypass during surgery affected outcomes for specific AAA types. Some attendees countered that renal protection should be used for all patients undergoing proximal AAA repair.
Dr. Kabbani reported having no financial disclosures.
AT MIDWESTERN VASCULAR 2013
Major finding: Perioperative mortality was 2.9%, but 60% of patients had postoperative acute kidney injury.
Data source: A retrospective analysis of 245 patients undergoing open proximal abdominal aortic aneurysm repair.
Disclosures: Dr. Kabbani reported having no financial disclosures.
Insurer disputes fueling more lawsuits by doctors
Fed up with unfair practices by some insurance companies, more physicians are heading to the courtroom to resolve conflicts with insurers, litigation experts say. In the last year, doctors in various states took legal action against insurers for such allegations as claim denials, underpayments, and network exclusions.
"Providers are becoming increasingly frustrated [with insurers] on a number of different areas," said David Doyle, founder and CEO of CRT Medical Systems, a medical billing and practice-management company based in Novi, Mich. "Physicians are saying, ‘Enough.’ They’re taking the offensive position and going after the payers."
Recent lawsuits highlight this growing trend. In Connecticut, the medical associations of Fairfield and Hartford counties in November issued a legal challenge against UnitedHealthcare for terminating more than 2,000 doctors from its Medicare Advantage plan network. Physicians claim the terminations were made without cause and will severely harm patient care. UnitedHealthcare has said the restructuring was made to encourage higher quality health care coverage. A district court on Dec. 5 temporarily halted the terminations after the medical associations sought emergency relief.
In the fall, the U.S. District Court for the Northern District of Illinois said a lawsuit brought by an Illinois dermatologist could stand against Humana Insurance Co. The doctor claims the insurer informed his patients falsely that he was no longer in the insurer’s network and to find another doctor. The district court found the doctor had alleged sufficient facts to support his claim of tortious contract interference.
Meanwhile, a Los Angeles Superior Court judge ruled on Dec. 9 that a suit by the California Medical Association and several dozen doctors to move forward against Aetna. The CMA sued Aetna in 2012 for allegedly underpaying out-of-network physicians and failing to approve some out-of-network services.
Aetna moved to dismiss the suit, but the court ruled the doctors pled valid claims under the state’s unfair business practice law. A similar lawsuit over payment denials by the Los Angeles County Medical Association is pending against insurer Health Net.
One reason for the increase in lawsuits is that physicians are better at recognizing insurers’ errors than they were in the past, according to Elizabeth Woodcock, founder of Woodcock & Assoc., a medical practice consulting firm in Atlanta.
"As we’ve gotten more electronic systems, [payment issues] become more identified," she said. "We’ve become better at picking these things up."
Historically, doctors primary struggles with insurance companies consisted of late payments and retroactive denials related to eligibility issues, according to Mark *S. Kopson, a Michigan health law attorney and vice chair of membership for the American Health Lawyers Association’s Payers, Plans and Managed Care Practice Group. Today, insurance battles more often stem from disputes over medically necessary treatment and disagreements over pay for performance measures, he said.
"Those are becoming more prevalent and that’s largely due to the change in reimbursement methods," he said. "The Affordable Care Act has had an impact on the issue. There’s a significant increase in performance-based reimbursement."
Network exclusions and terminations are also a growing catalyst for litigation by doctors, legal experts said. Plans are moving to more restrictive networks to leverage physicians into accepting lower contract rates, and to maintain profits and price competitiveness in the market, said Dr. Myles Riner, a managed care consultant and past president of the American College of Emergency Physicians California chapter.
In addition, insurers often attempt to boot physicians who are viewed as "complainers," said Andrew H. Selesnick, a partner at Los Angeles-based Michelman & Robinson, and chair of the firm’s Healthcare Law Department. Mr. Selesnick recently represented a client who received a network termination letter after the doctor successfully advocated for a Medicare patient to receive coverage for a treatment. The insurer withdrew the termination after Mr. Selesnick argued the move appeared retaliatory and warned of pursuing an injunction.
"For the most part, [network] terminations are profit or retaliation motivated," he said. "You really need to fight those bogus terminations."
Physicians’ success suing over insurer conduct has been mixed. For example, In April, jurors awarded Dr. Jeffrey B. Nordella, a California family physician, $3.8 million in damages after Anthem Blue Cross excluded him from its network. Dr. Nordella claimed the insurer was retaliating against him for his advocacy of patients who were denied coverage.
But in May, a Washington state appeals court threw out a lawsuit by the Washington State Medical Association over fair payments for out-of-network emergency services. The WSMA had sued state Insurance Commissioner Mike Kreidler for allegedly failing to enforce a state law compelling insurers to pay the cost of patients’ out-of-network emergency treatment. The court said the association had no grounds to sue Mr. Kreidler.
"In the major litigation, we’ve seen victories both on the provider and on the plan side," Mr. Kopson said. "One thing that is abundantly clear: This type of litigation is extremely complex and it is extremely expensive."
For this reason, it helps to have multiple health providers involved in a class action against insurers or a medical organization that supports the plaintiff physician, he said. Considering the value of the claim is also important before suing, Ms. Woodcock said. In some cases, the cost of attorney and legal expenses far exceed the disputed payment amount, she noted.
Legal standing is another major consideration, Mr. Selesnick said. Doctors should determine whether they have grounds to sue insurers, with an eye toward legal rules and contract terms. Legal counsel is key, Mr. Selesnick said.
Of course, resolving questions and conflicts with insurers early can prevent lawsuits all together and save both sides significant time and expense, Mr. Kopson said.
"Health plans and providers are undeniably in a symbiotic relationship," he said. "It means you have to pursue a collaborative – as opposed to an adversarial – posture. The best way to avoid litigation is to engage in vigorous discussion and negotiation up front before the contract is signed."
*Correction 2/26/14: A previous version of this story gave the incorrect middle initial of Mr. Mark S. Kopson. This version has been updated.
Fed up with unfair practices by some insurance companies, more physicians are heading to the courtroom to resolve conflicts with insurers, litigation experts say. In the last year, doctors in various states took legal action against insurers for such allegations as claim denials, underpayments, and network exclusions.
"Providers are becoming increasingly frustrated [with insurers] on a number of different areas," said David Doyle, founder and CEO of CRT Medical Systems, a medical billing and practice-management company based in Novi, Mich. "Physicians are saying, ‘Enough.’ They’re taking the offensive position and going after the payers."
Recent lawsuits highlight this growing trend. In Connecticut, the medical associations of Fairfield and Hartford counties in November issued a legal challenge against UnitedHealthcare for terminating more than 2,000 doctors from its Medicare Advantage plan network. Physicians claim the terminations were made without cause and will severely harm patient care. UnitedHealthcare has said the restructuring was made to encourage higher quality health care coverage. A district court on Dec. 5 temporarily halted the terminations after the medical associations sought emergency relief.
In the fall, the U.S. District Court for the Northern District of Illinois said a lawsuit brought by an Illinois dermatologist could stand against Humana Insurance Co. The doctor claims the insurer informed his patients falsely that he was no longer in the insurer’s network and to find another doctor. The district court found the doctor had alleged sufficient facts to support his claim of tortious contract interference.
Meanwhile, a Los Angeles Superior Court judge ruled on Dec. 9 that a suit by the California Medical Association and several dozen doctors to move forward against Aetna. The CMA sued Aetna in 2012 for allegedly underpaying out-of-network physicians and failing to approve some out-of-network services.
Aetna moved to dismiss the suit, but the court ruled the doctors pled valid claims under the state’s unfair business practice law. A similar lawsuit over payment denials by the Los Angeles County Medical Association is pending against insurer Health Net.
One reason for the increase in lawsuits is that physicians are better at recognizing insurers’ errors than they were in the past, according to Elizabeth Woodcock, founder of Woodcock & Assoc., a medical practice consulting firm in Atlanta.
"As we’ve gotten more electronic systems, [payment issues] become more identified," she said. "We’ve become better at picking these things up."
Historically, doctors primary struggles with insurance companies consisted of late payments and retroactive denials related to eligibility issues, according to Mark *S. Kopson, a Michigan health law attorney and vice chair of membership for the American Health Lawyers Association’s Payers, Plans and Managed Care Practice Group. Today, insurance battles more often stem from disputes over medically necessary treatment and disagreements over pay for performance measures, he said.
"Those are becoming more prevalent and that’s largely due to the change in reimbursement methods," he said. "The Affordable Care Act has had an impact on the issue. There’s a significant increase in performance-based reimbursement."
Network exclusions and terminations are also a growing catalyst for litigation by doctors, legal experts said. Plans are moving to more restrictive networks to leverage physicians into accepting lower contract rates, and to maintain profits and price competitiveness in the market, said Dr. Myles Riner, a managed care consultant and past president of the American College of Emergency Physicians California chapter.
In addition, insurers often attempt to boot physicians who are viewed as "complainers," said Andrew H. Selesnick, a partner at Los Angeles-based Michelman & Robinson, and chair of the firm’s Healthcare Law Department. Mr. Selesnick recently represented a client who received a network termination letter after the doctor successfully advocated for a Medicare patient to receive coverage for a treatment. The insurer withdrew the termination after Mr. Selesnick argued the move appeared retaliatory and warned of pursuing an injunction.
"For the most part, [network] terminations are profit or retaliation motivated," he said. "You really need to fight those bogus terminations."
Physicians’ success suing over insurer conduct has been mixed. For example, In April, jurors awarded Dr. Jeffrey B. Nordella, a California family physician, $3.8 million in damages after Anthem Blue Cross excluded him from its network. Dr. Nordella claimed the insurer was retaliating against him for his advocacy of patients who were denied coverage.
But in May, a Washington state appeals court threw out a lawsuit by the Washington State Medical Association over fair payments for out-of-network emergency services. The WSMA had sued state Insurance Commissioner Mike Kreidler for allegedly failing to enforce a state law compelling insurers to pay the cost of patients’ out-of-network emergency treatment. The court said the association had no grounds to sue Mr. Kreidler.
"In the major litigation, we’ve seen victories both on the provider and on the plan side," Mr. Kopson said. "One thing that is abundantly clear: This type of litigation is extremely complex and it is extremely expensive."
For this reason, it helps to have multiple health providers involved in a class action against insurers or a medical organization that supports the plaintiff physician, he said. Considering the value of the claim is also important before suing, Ms. Woodcock said. In some cases, the cost of attorney and legal expenses far exceed the disputed payment amount, she noted.
Legal standing is another major consideration, Mr. Selesnick said. Doctors should determine whether they have grounds to sue insurers, with an eye toward legal rules and contract terms. Legal counsel is key, Mr. Selesnick said.
Of course, resolving questions and conflicts with insurers early can prevent lawsuits all together and save both sides significant time and expense, Mr. Kopson said.
"Health plans and providers are undeniably in a symbiotic relationship," he said. "It means you have to pursue a collaborative – as opposed to an adversarial – posture. The best way to avoid litigation is to engage in vigorous discussion and negotiation up front before the contract is signed."
*Correction 2/26/14: A previous version of this story gave the incorrect middle initial of Mr. Mark S. Kopson. This version has been updated.
Fed up with unfair practices by some insurance companies, more physicians are heading to the courtroom to resolve conflicts with insurers, litigation experts say. In the last year, doctors in various states took legal action against insurers for such allegations as claim denials, underpayments, and network exclusions.
"Providers are becoming increasingly frustrated [with insurers] on a number of different areas," said David Doyle, founder and CEO of CRT Medical Systems, a medical billing and practice-management company based in Novi, Mich. "Physicians are saying, ‘Enough.’ They’re taking the offensive position and going after the payers."
Recent lawsuits highlight this growing trend. In Connecticut, the medical associations of Fairfield and Hartford counties in November issued a legal challenge against UnitedHealthcare for terminating more than 2,000 doctors from its Medicare Advantage plan network. Physicians claim the terminations were made without cause and will severely harm patient care. UnitedHealthcare has said the restructuring was made to encourage higher quality health care coverage. A district court on Dec. 5 temporarily halted the terminations after the medical associations sought emergency relief.
In the fall, the U.S. District Court for the Northern District of Illinois said a lawsuit brought by an Illinois dermatologist could stand against Humana Insurance Co. The doctor claims the insurer informed his patients falsely that he was no longer in the insurer’s network and to find another doctor. The district court found the doctor had alleged sufficient facts to support his claim of tortious contract interference.
Meanwhile, a Los Angeles Superior Court judge ruled on Dec. 9 that a suit by the California Medical Association and several dozen doctors to move forward against Aetna. The CMA sued Aetna in 2012 for allegedly underpaying out-of-network physicians and failing to approve some out-of-network services.
Aetna moved to dismiss the suit, but the court ruled the doctors pled valid claims under the state’s unfair business practice law. A similar lawsuit over payment denials by the Los Angeles County Medical Association is pending against insurer Health Net.
One reason for the increase in lawsuits is that physicians are better at recognizing insurers’ errors than they were in the past, according to Elizabeth Woodcock, founder of Woodcock & Assoc., a medical practice consulting firm in Atlanta.
"As we’ve gotten more electronic systems, [payment issues] become more identified," she said. "We’ve become better at picking these things up."
Historically, doctors primary struggles with insurance companies consisted of late payments and retroactive denials related to eligibility issues, according to Mark *S. Kopson, a Michigan health law attorney and vice chair of membership for the American Health Lawyers Association’s Payers, Plans and Managed Care Practice Group. Today, insurance battles more often stem from disputes over medically necessary treatment and disagreements over pay for performance measures, he said.
"Those are becoming more prevalent and that’s largely due to the change in reimbursement methods," he said. "The Affordable Care Act has had an impact on the issue. There’s a significant increase in performance-based reimbursement."
Network exclusions and terminations are also a growing catalyst for litigation by doctors, legal experts said. Plans are moving to more restrictive networks to leverage physicians into accepting lower contract rates, and to maintain profits and price competitiveness in the market, said Dr. Myles Riner, a managed care consultant and past president of the American College of Emergency Physicians California chapter.
In addition, insurers often attempt to boot physicians who are viewed as "complainers," said Andrew H. Selesnick, a partner at Los Angeles-based Michelman & Robinson, and chair of the firm’s Healthcare Law Department. Mr. Selesnick recently represented a client who received a network termination letter after the doctor successfully advocated for a Medicare patient to receive coverage for a treatment. The insurer withdrew the termination after Mr. Selesnick argued the move appeared retaliatory and warned of pursuing an injunction.
"For the most part, [network] terminations are profit or retaliation motivated," he said. "You really need to fight those bogus terminations."
Physicians’ success suing over insurer conduct has been mixed. For example, In April, jurors awarded Dr. Jeffrey B. Nordella, a California family physician, $3.8 million in damages after Anthem Blue Cross excluded him from its network. Dr. Nordella claimed the insurer was retaliating against him for his advocacy of patients who were denied coverage.
But in May, a Washington state appeals court threw out a lawsuit by the Washington State Medical Association over fair payments for out-of-network emergency services. The WSMA had sued state Insurance Commissioner Mike Kreidler for allegedly failing to enforce a state law compelling insurers to pay the cost of patients’ out-of-network emergency treatment. The court said the association had no grounds to sue Mr. Kreidler.
"In the major litigation, we’ve seen victories both on the provider and on the plan side," Mr. Kopson said. "One thing that is abundantly clear: This type of litigation is extremely complex and it is extremely expensive."
For this reason, it helps to have multiple health providers involved in a class action against insurers or a medical organization that supports the plaintiff physician, he said. Considering the value of the claim is also important before suing, Ms. Woodcock said. In some cases, the cost of attorney and legal expenses far exceed the disputed payment amount, she noted.
Legal standing is another major consideration, Mr. Selesnick said. Doctors should determine whether they have grounds to sue insurers, with an eye toward legal rules and contract terms. Legal counsel is key, Mr. Selesnick said.
Of course, resolving questions and conflicts with insurers early can prevent lawsuits all together and save both sides significant time and expense, Mr. Kopson said.
"Health plans and providers are undeniably in a symbiotic relationship," he said. "It means you have to pursue a collaborative – as opposed to an adversarial – posture. The best way to avoid litigation is to engage in vigorous discussion and negotiation up front before the contract is signed."
*Correction 2/26/14: A previous version of this story gave the incorrect middle initial of Mr. Mark S. Kopson. This version has been updated.
First-in-man bioengineered graft proves enduring for vascular access
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.

|
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.

|
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
This work is exciting. The early patency, thrombosis, and infection rates are encouraging.
The unmet clinical need for better ways to provide vascular access for hemodialysis is huge. There are 450,000 U.S. patients with end-stage renal disease on long-term hemodialysis. In this population, hemodialysis access morbidity costs more than $1 billion per year. Although the preferred means of vascular access is an arteriovenous fistula, many hemodialysis patients don’t have suitable veins. And 60% of fistulas become unusable within 6 months.

|
|
We’ve got a conundrum where PTFE grafts have their problems and fistulas have their own problems. We don’t have a good clinical armamentarium.
Synthetic grafts most often lose patency because of venous outflow tract stenosis due to intimal hyperplasia. Balloon angioplasty of the stenotic anastomosis has been the conventional treatment to restore patency, but a landmark randomized trial carried out several years ago (N. Engl. J. Med. 2010;362:494-503) showed the patency rate was a mere 23%, significantly worse than the 51% patency rate with a PTFE-covered stent graft – and even that 51% patency rate, is abysmal.
Dr. Sanjay Misra is professor of radiology at the Mayo Clinic in Rochester, Minn. He was the invited discussant of the paper at the meeting and declared having no relevant financial disclosures.
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
DALLAS – An investigational tissue-engineered vascular graft has enduring potential for vascular access for hemodialysis in patients with end-stage renal disease, based on early clinical results.
Moreover, other potential uses are on the horizon. The big picture involves subsequent extrapolation of this technology from the large-diameter, high-flow bioengineered vessels required for hemodialysis to the creation of small-diameter, low-flow vessels for coronary artery and peripheral arterial graft surgery, Dr. Jeffrey H. Lawson explained at the American Heart Association scientific sessions.
"Our goal is to make a tissue-engineered conduit that could be used widely throughout the body," said Dr. Lawson, professor of surgery and of pathology at Duke University Medical Center, Durham, N.C.
He presented the results from the first-in-man, ongoing phase I clinical experience with the Humacyte graft, which to date has been implanted to provide vascular access for hemodialysis in 28 patients, with 6-month patency as the primary study endpoint. This was a challenging study population, with an average of 4.1 previous access procedure failures per patient. The presentation at the AHA was the first public disclosure of the results of a project Dr. Lawson has been working on for more than 15 years. His surgical colleagues from Poland, who have done the implantations in patients with end-stage renal disease, were in attendance.
The overall 6-month patency was 100%, with no infections, no sign of an immune response, and no aneurysms or other indication of structural degeneration, he said.
Of the 28 patients, 20 had no further interventions, yielding a primary unassisted 6-month patency rate of 71%. Eight patients collectively underwent 10 interventions to maintain patency: eight had thrombectomies for graft- or surgically related thrombosis and two had venous anastomoses. Flow rates have remained suitable for dialysis in all patients, and the grafts are being used for dialysis three times per week. Dr. Lawson described the grafts as easy to cannulate via standard techniques.
He characterized these initial results as "quite remarkable" compared with the outcomes in two large studies of the current benchmark technologies, which are synthetic grafts made of PTFE (polytetrafluoroethyline). In those studies, the primary patency rate at 6 months was less than 50%, with a secondary patency rate of 77% and a 10% infection rate. In other studies, 30%-40% of PTFE grafts are abandoned within 12 months due to loss of patency.
The process of creating the bioengineered grafts begins with harvesting human aortic vascular smooth muscle cells, seeding them on a biodegradable matrix, then culturing them under pulsatile conditions. When the biodegradable matrix melts away, what remains is a tube comprised of vascular smooth muscle cells and extracellular matrix. This is then decellularized, yielding a tube of extracellular matrix that can be shipped off the shelf and around the world.
In primate models, the implanted bioengineered graft has been shown to repopulate with the host’s own vascular smooth muscle cells lined intimally by endothelium.
"Where we implanted an acellular structure, it appears to now be a living tissue, suggesting [the graft] has become their tissue, not ours," Dr. Lawson said.
To date, none of the bioengineered grafts implanted in patients has been explanted, so it’s unknown whether the favorable histologic changes seen in primates’ grafts also occur in humans. Larger clinical trials with longer follow-up are planned in order to assess the bioengineered graft’s durability.
Dr. Lawson’s study is funded by a Department of Defense research grant and by Humacyte. He serves as a consultant to the company.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: The 6-month enduring patency rate of an investigational tissue-engineered vascular graft for hemodialysis access was 100%, markedly better than rates achievable with synthetic PTFE grafts, the current benchmark technology.
Data source: An initial report from an ongoing prospective first-in-man study in which, to date, 28 patients with end-stage renal disease have been implanted with a novel tissue-engineered vascular graft for use as a hemodialysis access.
Disclosures: The study was funded by the Department of Defense and Humacyte. The presenter is a consultant to the company.
Outpatient diverticulitis therapy cures patients, saves money
Discharging patients with uncomplicated diverticulitis from the emergency department on antibiotics was just as effective as hospitalization – and cost about a third as much, based on a study conducted in Spain.
Dr. Sebastiano Biondi and his colleagues conducted their randomized, parallel-group study at five hospitals. The cohort consisted of 132 adults, mean age 56 years, who presented to the emergency department with localized abdominal pain. All had an abdominal CT scan and received intravenous antibiotics, either an initial IV infusion of 1 g of amoxicillin per 125 mg of clavulanic acid, or, in the case of those with a penicillin allergy, ciprofloxacin 200 mg and metronidazole 500 mg.
Half of the patients were then assigned to either inpatient or outpatient antibiotic treatment. The admitted patients continued to receive IV antibiotics and fluids for 36-48 hours until they tolerated oral feeding. The outpatients were discharged on oral amoxicillin and clavulanic acid (875 mg per 125 mg every 8 hours) or, in those with penicillin allergy, the combination of ciprofloxacin (500 mg every 12 hours) and metronidazole (500 mg every 8 hours).
The study’s main endpoint was treatment failure rate (persistence, increase, or recurrence of abdominal pain and/or fever; inflammatory bowel obstruction; or the need for radiological abscess drainage or immediate surgery due to complicated diverticulitis). Patients were followed every day for 5 days, and then interviewed on day 14. Before the final follow-up at 60 days, patients underwent a colonoscopy to rule out malignancy.
Seven patients (5%) were readmitted due to treatment failure: 4 (6%) in the inpatient group and three (4.5%) in the outpatient group. The difference was not statistically significant. No one needed emergency surgery as part of readmission, and there were no deaths.
A quality of life assessment found that hospitalized patients reported a significantly higher level of physical health during the first 2 weeks of treatment, but that difference disappeared after 14 days.
Total treatment costs were significantly less in the outpatient group, with an average savings of $1,840 per patient ($895 for outpatient care compared with $2,735 for inpatient care). Almost all of that cost difference was due to hospital bed cost, with an average stay of 4 days.
"The outpatient protocol of this study is applicable to a selected group of patients with uncomplicated diverticulitis," Dr. Biondi reported in the January issue of Annals of Surgery (doi: 10.1097/SLA.0b013e3182965a11).
Widespread adoption of outpatient treatment could have a profound effect on the cost of treating diverticulitis, an ever more common gastrointestinal problem, said Dr. Biondi of the University of Barcelona and his coauthors. "According to data from the National Hospital Discharge Survey, diverticular disease is responsible for 314,000 hospital admissions per year in the United States, and the estimated annual cost in 1998 was about $2.6 billion."
The Spanish Ministry of Health funded the trial. The authors had no financial conflicts.
Discharging patients with uncomplicated diverticulitis from the emergency department on antibiotics was just as effective as hospitalization – and cost about a third as much, based on a study conducted in Spain.
Dr. Sebastiano Biondi and his colleagues conducted their randomized, parallel-group study at five hospitals. The cohort consisted of 132 adults, mean age 56 years, who presented to the emergency department with localized abdominal pain. All had an abdominal CT scan and received intravenous antibiotics, either an initial IV infusion of 1 g of amoxicillin per 125 mg of clavulanic acid, or, in the case of those with a penicillin allergy, ciprofloxacin 200 mg and metronidazole 500 mg.
Half of the patients were then assigned to either inpatient or outpatient antibiotic treatment. The admitted patients continued to receive IV antibiotics and fluids for 36-48 hours until they tolerated oral feeding. The outpatients were discharged on oral amoxicillin and clavulanic acid (875 mg per 125 mg every 8 hours) or, in those with penicillin allergy, the combination of ciprofloxacin (500 mg every 12 hours) and metronidazole (500 mg every 8 hours).
The study’s main endpoint was treatment failure rate (persistence, increase, or recurrence of abdominal pain and/or fever; inflammatory bowel obstruction; or the need for radiological abscess drainage or immediate surgery due to complicated diverticulitis). Patients were followed every day for 5 days, and then interviewed on day 14. Before the final follow-up at 60 days, patients underwent a colonoscopy to rule out malignancy.
Seven patients (5%) were readmitted due to treatment failure: 4 (6%) in the inpatient group and three (4.5%) in the outpatient group. The difference was not statistically significant. No one needed emergency surgery as part of readmission, and there were no deaths.
A quality of life assessment found that hospitalized patients reported a significantly higher level of physical health during the first 2 weeks of treatment, but that difference disappeared after 14 days.
Total treatment costs were significantly less in the outpatient group, with an average savings of $1,840 per patient ($895 for outpatient care compared with $2,735 for inpatient care). Almost all of that cost difference was due to hospital bed cost, with an average stay of 4 days.
"The outpatient protocol of this study is applicable to a selected group of patients with uncomplicated diverticulitis," Dr. Biondi reported in the January issue of Annals of Surgery (doi: 10.1097/SLA.0b013e3182965a11).
Widespread adoption of outpatient treatment could have a profound effect on the cost of treating diverticulitis, an ever more common gastrointestinal problem, said Dr. Biondi of the University of Barcelona and his coauthors. "According to data from the National Hospital Discharge Survey, diverticular disease is responsible for 314,000 hospital admissions per year in the United States, and the estimated annual cost in 1998 was about $2.6 billion."
The Spanish Ministry of Health funded the trial. The authors had no financial conflicts.
Discharging patients with uncomplicated diverticulitis from the emergency department on antibiotics was just as effective as hospitalization – and cost about a third as much, based on a study conducted in Spain.
Dr. Sebastiano Biondi and his colleagues conducted their randomized, parallel-group study at five hospitals. The cohort consisted of 132 adults, mean age 56 years, who presented to the emergency department with localized abdominal pain. All had an abdominal CT scan and received intravenous antibiotics, either an initial IV infusion of 1 g of amoxicillin per 125 mg of clavulanic acid, or, in the case of those with a penicillin allergy, ciprofloxacin 200 mg and metronidazole 500 mg.
Half of the patients were then assigned to either inpatient or outpatient antibiotic treatment. The admitted patients continued to receive IV antibiotics and fluids for 36-48 hours until they tolerated oral feeding. The outpatients were discharged on oral amoxicillin and clavulanic acid (875 mg per 125 mg every 8 hours) or, in those with penicillin allergy, the combination of ciprofloxacin (500 mg every 12 hours) and metronidazole (500 mg every 8 hours).
The study’s main endpoint was treatment failure rate (persistence, increase, or recurrence of abdominal pain and/or fever; inflammatory bowel obstruction; or the need for radiological abscess drainage or immediate surgery due to complicated diverticulitis). Patients were followed every day for 5 days, and then interviewed on day 14. Before the final follow-up at 60 days, patients underwent a colonoscopy to rule out malignancy.
Seven patients (5%) were readmitted due to treatment failure: 4 (6%) in the inpatient group and three (4.5%) in the outpatient group. The difference was not statistically significant. No one needed emergency surgery as part of readmission, and there were no deaths.
A quality of life assessment found that hospitalized patients reported a significantly higher level of physical health during the first 2 weeks of treatment, but that difference disappeared after 14 days.
Total treatment costs were significantly less in the outpatient group, with an average savings of $1,840 per patient ($895 for outpatient care compared with $2,735 for inpatient care). Almost all of that cost difference was due to hospital bed cost, with an average stay of 4 days.
"The outpatient protocol of this study is applicable to a selected group of patients with uncomplicated diverticulitis," Dr. Biondi reported in the January issue of Annals of Surgery (doi: 10.1097/SLA.0b013e3182965a11).
Widespread adoption of outpatient treatment could have a profound effect on the cost of treating diverticulitis, an ever more common gastrointestinal problem, said Dr. Biondi of the University of Barcelona and his coauthors. "According to data from the National Hospital Discharge Survey, diverticular disease is responsible for 314,000 hospital admissions per year in the United States, and the estimated annual cost in 1998 was about $2.6 billion."
The Spanish Ministry of Health funded the trial. The authors had no financial conflicts.
FROM ANNALS OF SURGERY
Major finding: Compared with inpatients, those with uncomplicated diverticulitis treated as outpatients had similarly low rates of treatment failure (4.5% vs. 6%), with a cost savings of $1,840 for each outpatient treatment.
Data source: The randomized, parallel-group study comprised 132 patients.
Disclosures: The Spanish Ministry of Health funded the study. The authors had no financial conflicts.