User login
Official Newspaper of the American College of Surgeons
Survival no better after primary tumor removal in metastatic breast cancer
SAN ANTONIO – Surgical removal of the primary tumor and affected lymph nodes afforded no overall survival benefit in women who had metastatic breast cancer at initial presentation, according to a pair of randomized trials presented at the San Antonio Breast Cancer Symposium.
In a first trial, conducted among 350 women in India who had responded to initial chemotherapy, about 20% of the women were still alive at 5 years regardless of whether they had surgery or just received more systemic therapy, first author Dr. Rajendra Badwe, director of the Tata Memorial Hospital in Mumbai, India, reported in a session and at a press briefing.
Superior locoregional control conferred by surgery was canceled out by a higher risk of progression in distant sites, lending support to more than 20-year-old preclinical data by Dr. Bernard Fisher and his colleagues suggesting that an intact primary suppresses growth of distant metastases (Cancer Res. 1989;49:1996-2001).
"Locoregional treatment of the primary tumor in women presenting with metastatic breast cancer did not result in any overall survival benefit and hence should not be offered as a routine practice," Dr. Badwe commented.
"The biological fallout of this study is that surgical removal of the primary tumor in these women appears to confer a growth advantage on distant metastases. ... This is the first time that we have evidence in human studies that locoregional treatment has a great kinetic effect on distant metastases," he added.
In a second trial, conducted among 293 women in Turkey with untreated de novo metastatic breast cancer, median estimated survival was statistically indistinguishable, at about 3.5 years, regardless of whether women had up-front surgery or simply systemic therapy, first author Dr. Atilla Soran reported in a session on behalf of the Turkish Federation of Societies for Breast Diseases.
Subgroup analyses suggested that surgery conferred a survival advantage among women with solitary bone metastases but a survival disadvantage among women with multiple liver or lung metastases.
The rate of locoregional progression was one-fifth as high with surgery as with systemic therapy.
"There was no statistically significant difference in overall survival at early follow-up, but we need longer follow-up," Dr. Soran commented. "There were potentially important subgroup differences."
The two trials have implications – both for other ongoing trials and for patient care – according to invited discussant Dr. Seema A. Khan, a professor of surgery and Bluhm Family Professor of Cancer Research at Northwestern University, Chicago, and a physician at the university’s Lynn Sage Breast Center.
"A large benefit of primary site local therapy seems unlikely based on the data we saw today," she maintained. "The assumptions we have used in our ongoing trial designs will need to be reassessed. Preplanned pooled analyses may yield sufficient power to detect smaller differences."
As for patient care, "it is pretty clear that at this point in time, we have to make sure that our patients understand that there is really no proven survival advantage to primary site local therapy," Dr. Khan said. "So I don’t think this treatment should be offered to patients with asymptomatic tumors unless they are participating in a clinical trial. There may be a local control advantage; we need to see more data on that."
Indian trial
Participants in the Indian trial, conducted between 2005 and 2013, were women with de novo metastatic breast cancer who had had a complete or partial response to anthracycline chemotherapy alone or with a taxane.
They were randomized evenly to receive locoregional therapy (lumpectomy or mastectomy with axillary lymph node dissection, plus radiation therapy to the chest wall or breast and lymph nodes) or no locoregional therapy as a control. Both groups received hormonal therapy if indicated.
In the control group, about 10% of women underwent a palliative mastectomy because of impending fungation or pain in the breast, as permitted by study protocol, according to Dr. Badwe.
The patients thus received contemporary therapy, he said, except that the 16% with HER2-positive disease did not receive the targeted agent trastuzumab (Herceptin).
Results showed that median overall survival was about 18 months, and the 5-year rate was 19.2% with locoregional therapy and 20.5% without it, a nonsignificant difference. "Uniformly, there was no difference at all in any subsets," Dr. Badwe reported.
The study had a one-sided superiority design, he noted. "We wouldn’t have been able to see a 2.5- or 3-month difference, or a 4% detriment" in overall survival.
The surgery group had a lower risk of local progression-free survival events (hazard ratio, 0.16; P = .00), but a higher risk of distant progression-free survival events (HR, 1.42; P = .01).
Several theories might explain why removal of the primary would accelerate growth of metastases, according to Dr. Badwe.
"The first and the foremost is that the act of surgery itself might elaborate some growth factors which might allow metastatic disease to grow. The second possibility, which was suggested by Dr. Fisher (Cancer Res. 1989;49:1996-2001) is that the primary tumor, which predates the onset of distant metastases, elaborates some inhibitory factors, and they are not there once the primary tumor is removed, bestowing autonomy of growth on the distant metastases," he explained. "And the third possibility is the act of surgery might induce some more metastatic processes by dissemination and create new disease."
Session attendee Dr. Steven Vogl, an oncologist in the Bronx, N.Y., said, "The ascertainment of disease progression requires disease that you can follow. A woman with bone-only metastases with her cancer in place, you can tell when her cancer is getting worse because the primary site or the axillary nodes are getting bigger. It’s much more difficult if you’ve taken those off and irradiated the chest wall. Have you looked at your data to see if that’s what was going on, why you had more distant metastases, because they couldn’t progress locally? This is a medical trial explanation that contradicts Fisher’s biologic hypothesis."
"There was a fixed time duration at which systemic investigations were performed to assess whether the distant metastases progressed," Dr. Badwe replied. If anything, the patients who did not have surgery had more assessments of their distant metastases, he said.
Dr. Tari A. King, a session attendee from the Memorial Sloan-Kettering Cancer Center, New York, noted that a lack of HER2 therapy in the trial may have had a large effect.
"We do have prospective registry data here [abstract 18-09, presented in a poster session] from a trial that we completed in the United States sponsored by the Translational Breast Cancer Research Consortium, and the patients in our study, whether they received surgery or not, their 2-year overall survival is far superior to what you’ve just showed us," she commented. "So I’m not sure that we can really apply your data to the modern targeted therapy regimens that we see in the United States."
Turkish trial
The Turkish trial, known as the MF07-01 trial, was conduced between 2008 and 2012 among treatment-naïve patients.
They were randomized evenly to receive either systemic therapy alone or surgery for the primary tumor (with or without axillary dissection) followed by radiation therapy if indicated, plus systemic therapy.
All patients received hormonal therapy as needed, and those with HER2-positive disease received trastuzumab.
With a median follow-up of 18 months, the median overall survival was 46 months with initial surgery and 42 months with initial systemic therapy, a nonsignificant difference, reported Dr. Soran, who disclosed no relevant conflicts of interest.
In unplanned subgroup analyses, the findings were similar for most subgroups of patients. However, surgery yielded superior survival in patients with solitary bone metastases (not reached vs. 42 months, P = .02) and inferior survival in patients having multiple liver or pulmonary metastases (16 months vs. not reached, P = .02).
The rate of locoregional progression was much lower with initial surgery than with initial systemic therapy (0.7% vs. 3.6%).
Dr. Soran emphasized that the trial’s planned median follow-up is 36 months, so the presented results are only preliminary. Quality of life and morbidity analyses are ongoing.
Dr. Badwe and Dr. Soran disclosed no relevant conflicts of interest.
SAN ANTONIO – Surgical removal of the primary tumor and affected lymph nodes afforded no overall survival benefit in women who had metastatic breast cancer at initial presentation, according to a pair of randomized trials presented at the San Antonio Breast Cancer Symposium.
In a first trial, conducted among 350 women in India who had responded to initial chemotherapy, about 20% of the women were still alive at 5 years regardless of whether they had surgery or just received more systemic therapy, first author Dr. Rajendra Badwe, director of the Tata Memorial Hospital in Mumbai, India, reported in a session and at a press briefing.
Superior locoregional control conferred by surgery was canceled out by a higher risk of progression in distant sites, lending support to more than 20-year-old preclinical data by Dr. Bernard Fisher and his colleagues suggesting that an intact primary suppresses growth of distant metastases (Cancer Res. 1989;49:1996-2001).
"Locoregional treatment of the primary tumor in women presenting with metastatic breast cancer did not result in any overall survival benefit and hence should not be offered as a routine practice," Dr. Badwe commented.
"The biological fallout of this study is that surgical removal of the primary tumor in these women appears to confer a growth advantage on distant metastases. ... This is the first time that we have evidence in human studies that locoregional treatment has a great kinetic effect on distant metastases," he added.
In a second trial, conducted among 293 women in Turkey with untreated de novo metastatic breast cancer, median estimated survival was statistically indistinguishable, at about 3.5 years, regardless of whether women had up-front surgery or simply systemic therapy, first author Dr. Atilla Soran reported in a session on behalf of the Turkish Federation of Societies for Breast Diseases.
Subgroup analyses suggested that surgery conferred a survival advantage among women with solitary bone metastases but a survival disadvantage among women with multiple liver or lung metastases.
The rate of locoregional progression was one-fifth as high with surgery as with systemic therapy.
"There was no statistically significant difference in overall survival at early follow-up, but we need longer follow-up," Dr. Soran commented. "There were potentially important subgroup differences."
The two trials have implications – both for other ongoing trials and for patient care – according to invited discussant Dr. Seema A. Khan, a professor of surgery and Bluhm Family Professor of Cancer Research at Northwestern University, Chicago, and a physician at the university’s Lynn Sage Breast Center.
"A large benefit of primary site local therapy seems unlikely based on the data we saw today," she maintained. "The assumptions we have used in our ongoing trial designs will need to be reassessed. Preplanned pooled analyses may yield sufficient power to detect smaller differences."
As for patient care, "it is pretty clear that at this point in time, we have to make sure that our patients understand that there is really no proven survival advantage to primary site local therapy," Dr. Khan said. "So I don’t think this treatment should be offered to patients with asymptomatic tumors unless they are participating in a clinical trial. There may be a local control advantage; we need to see more data on that."
Indian trial
Participants in the Indian trial, conducted between 2005 and 2013, were women with de novo metastatic breast cancer who had had a complete or partial response to anthracycline chemotherapy alone or with a taxane.
They were randomized evenly to receive locoregional therapy (lumpectomy or mastectomy with axillary lymph node dissection, plus radiation therapy to the chest wall or breast and lymph nodes) or no locoregional therapy as a control. Both groups received hormonal therapy if indicated.
In the control group, about 10% of women underwent a palliative mastectomy because of impending fungation or pain in the breast, as permitted by study protocol, according to Dr. Badwe.
The patients thus received contemporary therapy, he said, except that the 16% with HER2-positive disease did not receive the targeted agent trastuzumab (Herceptin).
Results showed that median overall survival was about 18 months, and the 5-year rate was 19.2% with locoregional therapy and 20.5% without it, a nonsignificant difference. "Uniformly, there was no difference at all in any subsets," Dr. Badwe reported.
The study had a one-sided superiority design, he noted. "We wouldn’t have been able to see a 2.5- or 3-month difference, or a 4% detriment" in overall survival.
The surgery group had a lower risk of local progression-free survival events (hazard ratio, 0.16; P = .00), but a higher risk of distant progression-free survival events (HR, 1.42; P = .01).
Several theories might explain why removal of the primary would accelerate growth of metastases, according to Dr. Badwe.
"The first and the foremost is that the act of surgery itself might elaborate some growth factors which might allow metastatic disease to grow. The second possibility, which was suggested by Dr. Fisher (Cancer Res. 1989;49:1996-2001) is that the primary tumor, which predates the onset of distant metastases, elaborates some inhibitory factors, and they are not there once the primary tumor is removed, bestowing autonomy of growth on the distant metastases," he explained. "And the third possibility is the act of surgery might induce some more metastatic processes by dissemination and create new disease."
Session attendee Dr. Steven Vogl, an oncologist in the Bronx, N.Y., said, "The ascertainment of disease progression requires disease that you can follow. A woman with bone-only metastases with her cancer in place, you can tell when her cancer is getting worse because the primary site or the axillary nodes are getting bigger. It’s much more difficult if you’ve taken those off and irradiated the chest wall. Have you looked at your data to see if that’s what was going on, why you had more distant metastases, because they couldn’t progress locally? This is a medical trial explanation that contradicts Fisher’s biologic hypothesis."
"There was a fixed time duration at which systemic investigations were performed to assess whether the distant metastases progressed," Dr. Badwe replied. If anything, the patients who did not have surgery had more assessments of their distant metastases, he said.
Dr. Tari A. King, a session attendee from the Memorial Sloan-Kettering Cancer Center, New York, noted that a lack of HER2 therapy in the trial may have had a large effect.
"We do have prospective registry data here [abstract 18-09, presented in a poster session] from a trial that we completed in the United States sponsored by the Translational Breast Cancer Research Consortium, and the patients in our study, whether they received surgery or not, their 2-year overall survival is far superior to what you’ve just showed us," she commented. "So I’m not sure that we can really apply your data to the modern targeted therapy regimens that we see in the United States."
Turkish trial
The Turkish trial, known as the MF07-01 trial, was conduced between 2008 and 2012 among treatment-naïve patients.
They were randomized evenly to receive either systemic therapy alone or surgery for the primary tumor (with or without axillary dissection) followed by radiation therapy if indicated, plus systemic therapy.
All patients received hormonal therapy as needed, and those with HER2-positive disease received trastuzumab.
With a median follow-up of 18 months, the median overall survival was 46 months with initial surgery and 42 months with initial systemic therapy, a nonsignificant difference, reported Dr. Soran, who disclosed no relevant conflicts of interest.
In unplanned subgroup analyses, the findings were similar for most subgroups of patients. However, surgery yielded superior survival in patients with solitary bone metastases (not reached vs. 42 months, P = .02) and inferior survival in patients having multiple liver or pulmonary metastases (16 months vs. not reached, P = .02).
The rate of locoregional progression was much lower with initial surgery than with initial systemic therapy (0.7% vs. 3.6%).
Dr. Soran emphasized that the trial’s planned median follow-up is 36 months, so the presented results are only preliminary. Quality of life and morbidity analyses are ongoing.
Dr. Badwe and Dr. Soran disclosed no relevant conflicts of interest.
SAN ANTONIO – Surgical removal of the primary tumor and affected lymph nodes afforded no overall survival benefit in women who had metastatic breast cancer at initial presentation, according to a pair of randomized trials presented at the San Antonio Breast Cancer Symposium.
In a first trial, conducted among 350 women in India who had responded to initial chemotherapy, about 20% of the women were still alive at 5 years regardless of whether they had surgery or just received more systemic therapy, first author Dr. Rajendra Badwe, director of the Tata Memorial Hospital in Mumbai, India, reported in a session and at a press briefing.
Superior locoregional control conferred by surgery was canceled out by a higher risk of progression in distant sites, lending support to more than 20-year-old preclinical data by Dr. Bernard Fisher and his colleagues suggesting that an intact primary suppresses growth of distant metastases (Cancer Res. 1989;49:1996-2001).
"Locoregional treatment of the primary tumor in women presenting with metastatic breast cancer did not result in any overall survival benefit and hence should not be offered as a routine practice," Dr. Badwe commented.
"The biological fallout of this study is that surgical removal of the primary tumor in these women appears to confer a growth advantage on distant metastases. ... This is the first time that we have evidence in human studies that locoregional treatment has a great kinetic effect on distant metastases," he added.
In a second trial, conducted among 293 women in Turkey with untreated de novo metastatic breast cancer, median estimated survival was statistically indistinguishable, at about 3.5 years, regardless of whether women had up-front surgery or simply systemic therapy, first author Dr. Atilla Soran reported in a session on behalf of the Turkish Federation of Societies for Breast Diseases.
Subgroup analyses suggested that surgery conferred a survival advantage among women with solitary bone metastases but a survival disadvantage among women with multiple liver or lung metastases.
The rate of locoregional progression was one-fifth as high with surgery as with systemic therapy.
"There was no statistically significant difference in overall survival at early follow-up, but we need longer follow-up," Dr. Soran commented. "There were potentially important subgroup differences."
The two trials have implications – both for other ongoing trials and for patient care – according to invited discussant Dr. Seema A. Khan, a professor of surgery and Bluhm Family Professor of Cancer Research at Northwestern University, Chicago, and a physician at the university’s Lynn Sage Breast Center.
"A large benefit of primary site local therapy seems unlikely based on the data we saw today," she maintained. "The assumptions we have used in our ongoing trial designs will need to be reassessed. Preplanned pooled analyses may yield sufficient power to detect smaller differences."
As for patient care, "it is pretty clear that at this point in time, we have to make sure that our patients understand that there is really no proven survival advantage to primary site local therapy," Dr. Khan said. "So I don’t think this treatment should be offered to patients with asymptomatic tumors unless they are participating in a clinical trial. There may be a local control advantage; we need to see more data on that."
Indian trial
Participants in the Indian trial, conducted between 2005 and 2013, were women with de novo metastatic breast cancer who had had a complete or partial response to anthracycline chemotherapy alone or with a taxane.
They were randomized evenly to receive locoregional therapy (lumpectomy or mastectomy with axillary lymph node dissection, plus radiation therapy to the chest wall or breast and lymph nodes) or no locoregional therapy as a control. Both groups received hormonal therapy if indicated.
In the control group, about 10% of women underwent a palliative mastectomy because of impending fungation or pain in the breast, as permitted by study protocol, according to Dr. Badwe.
The patients thus received contemporary therapy, he said, except that the 16% with HER2-positive disease did not receive the targeted agent trastuzumab (Herceptin).
Results showed that median overall survival was about 18 months, and the 5-year rate was 19.2% with locoregional therapy and 20.5% without it, a nonsignificant difference. "Uniformly, there was no difference at all in any subsets," Dr. Badwe reported.
The study had a one-sided superiority design, he noted. "We wouldn’t have been able to see a 2.5- or 3-month difference, or a 4% detriment" in overall survival.
The surgery group had a lower risk of local progression-free survival events (hazard ratio, 0.16; P = .00), but a higher risk of distant progression-free survival events (HR, 1.42; P = .01).
Several theories might explain why removal of the primary would accelerate growth of metastases, according to Dr. Badwe.
"The first and the foremost is that the act of surgery itself might elaborate some growth factors which might allow metastatic disease to grow. The second possibility, which was suggested by Dr. Fisher (Cancer Res. 1989;49:1996-2001) is that the primary tumor, which predates the onset of distant metastases, elaborates some inhibitory factors, and they are not there once the primary tumor is removed, bestowing autonomy of growth on the distant metastases," he explained. "And the third possibility is the act of surgery might induce some more metastatic processes by dissemination and create new disease."
Session attendee Dr. Steven Vogl, an oncologist in the Bronx, N.Y., said, "The ascertainment of disease progression requires disease that you can follow. A woman with bone-only metastases with her cancer in place, you can tell when her cancer is getting worse because the primary site or the axillary nodes are getting bigger. It’s much more difficult if you’ve taken those off and irradiated the chest wall. Have you looked at your data to see if that’s what was going on, why you had more distant metastases, because they couldn’t progress locally? This is a medical trial explanation that contradicts Fisher’s biologic hypothesis."
"There was a fixed time duration at which systemic investigations were performed to assess whether the distant metastases progressed," Dr. Badwe replied. If anything, the patients who did not have surgery had more assessments of their distant metastases, he said.
Dr. Tari A. King, a session attendee from the Memorial Sloan-Kettering Cancer Center, New York, noted that a lack of HER2 therapy in the trial may have had a large effect.
"We do have prospective registry data here [abstract 18-09, presented in a poster session] from a trial that we completed in the United States sponsored by the Translational Breast Cancer Research Consortium, and the patients in our study, whether they received surgery or not, their 2-year overall survival is far superior to what you’ve just showed us," she commented. "So I’m not sure that we can really apply your data to the modern targeted therapy regimens that we see in the United States."
Turkish trial
The Turkish trial, known as the MF07-01 trial, was conduced between 2008 and 2012 among treatment-naïve patients.
They were randomized evenly to receive either systemic therapy alone or surgery for the primary tumor (with or without axillary dissection) followed by radiation therapy if indicated, plus systemic therapy.
All patients received hormonal therapy as needed, and those with HER2-positive disease received trastuzumab.
With a median follow-up of 18 months, the median overall survival was 46 months with initial surgery and 42 months with initial systemic therapy, a nonsignificant difference, reported Dr. Soran, who disclosed no relevant conflicts of interest.
In unplanned subgroup analyses, the findings were similar for most subgroups of patients. However, surgery yielded superior survival in patients with solitary bone metastases (not reached vs. 42 months, P = .02) and inferior survival in patients having multiple liver or pulmonary metastases (16 months vs. not reached, P = .02).
The rate of locoregional progression was much lower with initial surgery than with initial systemic therapy (0.7% vs. 3.6%).
Dr. Soran emphasized that the trial’s planned median follow-up is 36 months, so the presented results are only preliminary. Quality of life and morbidity analyses are ongoing.
Dr. Badwe and Dr. Soran disclosed no relevant conflicts of interest.
AT SABCS 2013
Major finding: There was no significant difference in overall survival with surgery vs. systemic therapy in either the Indian trial (5-year rate, 19.2% vs. 20.5%) or the Turkish trial (median, 46 vs. 42 months).
Data source: A randomized trial among 350 women in India with de novo metastatic breast cancer who had had a response to chemotherapy, and a randomized trial in 293 treatment-naïve patients in Turkey with untreated de novo metastatic breast cancer.
Disclosures: Dr. Badwe and Dr. Soran disclosed no relevant conflicts of interest.
Novel treatment promising for chronic neuropathic postmastectomy pain
SAN ANTONIO – Perineural injection of bupivacaine and dexamethasone was a simple and effective treatment for chronic neuropathic pain following mastectomy, based on results of a pilot study.
The effectiveness of this novel therapy strongly suggests the source of this common pain syndrome is damage to the T4 and T5 sensory nerves during surgery, rather than damage to the intercostobrachial nerve, as traditionally thought, according to Dr. Cathy J. Tang of the University of California, San Francisco.
The T4 and T5 sensory nerves come off the chest wall and enter the breast accompanied by a blood vessel. When these nerves are cut and cauterized during mastectomy, the resultant nerve damage can manifest as neuroma formation and neuropathic pain along the two dermatomes, she said at the San Antonio Breast Cancer Symposium.
Chronic postmastectomy breast pain is commonly referred to as postmastectomy pain syndrome. Published estimates of its incidence after mastectomy range from 20% to 68%. The pain can start in the immediate postoperative period, or onset can be delayed up to 6 months or more post mastectomy. The pain is typically experienced as a shooting or burning pain, with point tenderness. It persists well after the expected healing period.
The intervention involves identifying a patient’s points of maximum pain or tenderness, usually located laterally along the midaxillary line or at the inframammary fold directly below the nipple. These points are injected at the level of the chest wall. Each injection consists of 2 mL of an equal ratio of 0.5% bupivacaine plus 4 mg/mL of dexamethasone followed by a minute or two of massage to enhance infiltration of the area.
Dr. Tang reported on 19 patients who developed postmastectomy pain syndrome after either partial mastectomy, total mastectomy with immediate reconstruction, or lateral core biopsy in one case. A total of 29 points of maximum tenderness were identified and treated. All patients had pain relief within minutes, with point pain scores on a 0-10 scale falling from 8-9 to 0-1. Long-term pain relief was experienced after 17 of the 29 initial injections (59%) in 11 patients. Pain was resolved at another nine sites after a second injection. A third injection at one recalcitrant site led to long-term pain relief. Thus, perineural injections alleviated pain at 27 of 29 treated sites, or 93%, at a mean of 10.7 months of follow-up.
In light of how simple and safe this treatment is, Dr. Tang urged routine inquiry about postmastectomy neuropathic pain. Patients with postmastectomy pain often report an inability to lie on the affected side or to wear a bra.
The study also indicates the importance of careful dissection of the T4 and T5 sensory nerves during mastectomy in order to minimize the risk of postoperative neuroma formation.
Dr. Tang reported having no financial conflicts regarding this unfunded study.
SAN ANTONIO – Perineural injection of bupivacaine and dexamethasone was a simple and effective treatment for chronic neuropathic pain following mastectomy, based on results of a pilot study.
The effectiveness of this novel therapy strongly suggests the source of this common pain syndrome is damage to the T4 and T5 sensory nerves during surgery, rather than damage to the intercostobrachial nerve, as traditionally thought, according to Dr. Cathy J. Tang of the University of California, San Francisco.
The T4 and T5 sensory nerves come off the chest wall and enter the breast accompanied by a blood vessel. When these nerves are cut and cauterized during mastectomy, the resultant nerve damage can manifest as neuroma formation and neuropathic pain along the two dermatomes, she said at the San Antonio Breast Cancer Symposium.
Chronic postmastectomy breast pain is commonly referred to as postmastectomy pain syndrome. Published estimates of its incidence after mastectomy range from 20% to 68%. The pain can start in the immediate postoperative period, or onset can be delayed up to 6 months or more post mastectomy. The pain is typically experienced as a shooting or burning pain, with point tenderness. It persists well after the expected healing period.
The intervention involves identifying a patient’s points of maximum pain or tenderness, usually located laterally along the midaxillary line or at the inframammary fold directly below the nipple. These points are injected at the level of the chest wall. Each injection consists of 2 mL of an equal ratio of 0.5% bupivacaine plus 4 mg/mL of dexamethasone followed by a minute or two of massage to enhance infiltration of the area.
Dr. Tang reported on 19 patients who developed postmastectomy pain syndrome after either partial mastectomy, total mastectomy with immediate reconstruction, or lateral core biopsy in one case. A total of 29 points of maximum tenderness were identified and treated. All patients had pain relief within minutes, with point pain scores on a 0-10 scale falling from 8-9 to 0-1. Long-term pain relief was experienced after 17 of the 29 initial injections (59%) in 11 patients. Pain was resolved at another nine sites after a second injection. A third injection at one recalcitrant site led to long-term pain relief. Thus, perineural injections alleviated pain at 27 of 29 treated sites, or 93%, at a mean of 10.7 months of follow-up.
In light of how simple and safe this treatment is, Dr. Tang urged routine inquiry about postmastectomy neuropathic pain. Patients with postmastectomy pain often report an inability to lie on the affected side or to wear a bra.
The study also indicates the importance of careful dissection of the T4 and T5 sensory nerves during mastectomy in order to minimize the risk of postoperative neuroma formation.
Dr. Tang reported having no financial conflicts regarding this unfunded study.
SAN ANTONIO – Perineural injection of bupivacaine and dexamethasone was a simple and effective treatment for chronic neuropathic pain following mastectomy, based on results of a pilot study.
The effectiveness of this novel therapy strongly suggests the source of this common pain syndrome is damage to the T4 and T5 sensory nerves during surgery, rather than damage to the intercostobrachial nerve, as traditionally thought, according to Dr. Cathy J. Tang of the University of California, San Francisco.
The T4 and T5 sensory nerves come off the chest wall and enter the breast accompanied by a blood vessel. When these nerves are cut and cauterized during mastectomy, the resultant nerve damage can manifest as neuroma formation and neuropathic pain along the two dermatomes, she said at the San Antonio Breast Cancer Symposium.
Chronic postmastectomy breast pain is commonly referred to as postmastectomy pain syndrome. Published estimates of its incidence after mastectomy range from 20% to 68%. The pain can start in the immediate postoperative period, or onset can be delayed up to 6 months or more post mastectomy. The pain is typically experienced as a shooting or burning pain, with point tenderness. It persists well after the expected healing period.
The intervention involves identifying a patient’s points of maximum pain or tenderness, usually located laterally along the midaxillary line or at the inframammary fold directly below the nipple. These points are injected at the level of the chest wall. Each injection consists of 2 mL of an equal ratio of 0.5% bupivacaine plus 4 mg/mL of dexamethasone followed by a minute or two of massage to enhance infiltration of the area.
Dr. Tang reported on 19 patients who developed postmastectomy pain syndrome after either partial mastectomy, total mastectomy with immediate reconstruction, or lateral core biopsy in one case. A total of 29 points of maximum tenderness were identified and treated. All patients had pain relief within minutes, with point pain scores on a 0-10 scale falling from 8-9 to 0-1. Long-term pain relief was experienced after 17 of the 29 initial injections (59%) in 11 patients. Pain was resolved at another nine sites after a second injection. A third injection at one recalcitrant site led to long-term pain relief. Thus, perineural injections alleviated pain at 27 of 29 treated sites, or 93%, at a mean of 10.7 months of follow-up.
In light of how simple and safe this treatment is, Dr. Tang urged routine inquiry about postmastectomy neuropathic pain. Patients with postmastectomy pain often report an inability to lie on the affected side or to wear a bra.
The study also indicates the importance of careful dissection of the T4 and T5 sensory nerves during mastectomy in order to minimize the risk of postoperative neuroma formation.
Dr. Tang reported having no financial conflicts regarding this unfunded study.
AT SABCS 2013
Major finding: Injection of a combination of bupivacaine and dexamethasone at well-defined sites of maximum pain and tenderness resolved pain at 93% of treated sites in patients with chronic neuropathic postmastectomy pain.
Data source: A prospective case series involving 19 patients with postmastectomy pain syndrome. A total of 29 sites of maximum pain and tenderness were treated.
Disclosures: The study was conducted free of commercial support. The presenter reported having no relevant financial conflicts.
New health IT czar takes over in January
Dr. Karen B. DeSalvo has been tapped to become the federal government’s next national coordinator for health information technology, the Health and Human Services Department announced.
Dr. DeSalvo, who helped modernize the New Orleans health care infrastructure after Hurricane Katrina, will play a central role in setting policy around the Medicare and Medicaid Electronic Health Record Incentive Programs, which include "meaningful use" requirements for the use of health IT.
Her first day in the new post will be Jan. 13.
"Dr. DeSalvo’s hands-on experience with health delivery system reform and HIT and its potential to improve health care and public health will be invaluable assets to the Office of the National Coordinator and the [Health and Human Services] department," HHS Secretary Kathleen Sebelius said in an e-mail to ONC staff on Dec. 19.
Dr. DeSalvo, an internist, also currently serves as a senior health policy adviser to New Orleans Mayor Mitch Landrieu. Over the course of her career, she has been an advocate for increasing the use of health IT in primary care and public health, and as part of emergency preparedness efforts. She has also led the planning of the city’s new public hospital, which includes a fully integrated health IT network.
The Healthcare Information and Management Systems Society praised Dr. DeSalvo for her "deep understanding of the value of informatics, as well as the challenges and promise of interoperability."
Dr. DeSalvo replaces Dr. Jacob Reider, the acting national coordinator, who will return to his former role as chief medical officer at the Office of the National Coordinator for Health IT. Dr. Reider took over the top job in the fall, after the departure of Dr. Farzad Mostashari.
On Twitter @MaryEllenNY
Dr. Karen B. DeSalvo has been tapped to become the federal government’s next national coordinator for health information technology, the Health and Human Services Department announced.
Dr. DeSalvo, who helped modernize the New Orleans health care infrastructure after Hurricane Katrina, will play a central role in setting policy around the Medicare and Medicaid Electronic Health Record Incentive Programs, which include "meaningful use" requirements for the use of health IT.
Her first day in the new post will be Jan. 13.
"Dr. DeSalvo’s hands-on experience with health delivery system reform and HIT and its potential to improve health care and public health will be invaluable assets to the Office of the National Coordinator and the [Health and Human Services] department," HHS Secretary Kathleen Sebelius said in an e-mail to ONC staff on Dec. 19.
Dr. DeSalvo, an internist, also currently serves as a senior health policy adviser to New Orleans Mayor Mitch Landrieu. Over the course of her career, she has been an advocate for increasing the use of health IT in primary care and public health, and as part of emergency preparedness efforts. She has also led the planning of the city’s new public hospital, which includes a fully integrated health IT network.
The Healthcare Information and Management Systems Society praised Dr. DeSalvo for her "deep understanding of the value of informatics, as well as the challenges and promise of interoperability."
Dr. DeSalvo replaces Dr. Jacob Reider, the acting national coordinator, who will return to his former role as chief medical officer at the Office of the National Coordinator for Health IT. Dr. Reider took over the top job in the fall, after the departure of Dr. Farzad Mostashari.
On Twitter @MaryEllenNY
Dr. Karen B. DeSalvo has been tapped to become the federal government’s next national coordinator for health information technology, the Health and Human Services Department announced.
Dr. DeSalvo, who helped modernize the New Orleans health care infrastructure after Hurricane Katrina, will play a central role in setting policy around the Medicare and Medicaid Electronic Health Record Incentive Programs, which include "meaningful use" requirements for the use of health IT.
Her first day in the new post will be Jan. 13.
"Dr. DeSalvo’s hands-on experience with health delivery system reform and HIT and its potential to improve health care and public health will be invaluable assets to the Office of the National Coordinator and the [Health and Human Services] department," HHS Secretary Kathleen Sebelius said in an e-mail to ONC staff on Dec. 19.
Dr. DeSalvo, an internist, also currently serves as a senior health policy adviser to New Orleans Mayor Mitch Landrieu. Over the course of her career, she has been an advocate for increasing the use of health IT in primary care and public health, and as part of emergency preparedness efforts. She has also led the planning of the city’s new public hospital, which includes a fully integrated health IT network.
The Healthcare Information and Management Systems Society praised Dr. DeSalvo for her "deep understanding of the value of informatics, as well as the challenges and promise of interoperability."
Dr. DeSalvo replaces Dr. Jacob Reider, the acting national coordinator, who will return to his former role as chief medical officer at the Office of the National Coordinator for Health IT. Dr. Reider took over the top job in the fall, after the departure of Dr. Farzad Mostashari.
On Twitter @MaryEllenNY
Social media liability
Question: Which of the following is incorrect?
A. Medical malpractice lawsuits arising out of social media interactions are still uncommon.
B. Comments shared by an ex-employee with friends on Facebook may breach doctor-patient confidentiality, with liability imputed to the doctor-employer.
C. Using the same platform, a doctor must promptly rebut disparaging comments on Yelp in order to protect his or her reputation.
D. An employment contract should cover matters concerning confidentiality and privacy.
E. Staff should use office computers only for work-related activities.
Answer: C. Physicians’ widespread use of social media sites such as Facebook, LinkedIn, and Twitter has spawned novel issues of professional liability. Use of such media, augmented by ubiquitous mobile devices such as smartphones and tablets, typically involves physician-to-physician and physician-to-patient communications but may also be personal in nature. Many patients have approached their doctors to "friend" them on Facebook. About a third of all doctors are said to have received such requests, and about a quarter have accepted. Other doctors are regular or occasional bloggers, offering views both medical and nonmedical.
While embracing the immense value of social media, the physician must remain mindful of the legal and ethical risks that such networking poses. State medical boards are facing increasing complaints of online professional breach, and civil lawsuits can be expected to mount.
Allegations of medical malpractice can arise if there is a showing that negligent conduct has caused an injury of some kind. Though currently uncommon, one can expect such lawsuits to proliferate. To be sure, there will be arguments about whether there exists a doctor-patient relationship from which a duty of care arises (Internal Medicine News, "Liability in the Internet Age," April 15, 2011, p. 74), but liability can come about in unexpected ways.
In a recent Massachusetts case, a pediatrician faced a malpractice suit that alleged a failure to diagnose diabetes and diabetic ketoacidosis. An offer to settle followed quickly once it was realized that the plaintiff’s attorney had discovered the defendant’s publicly blogged details about his deposition and trial preparation (American Medical News, "Internet won't protect your secret identity," Aug. 13, 2007) Lesson: Use the blogosphere to educate, not vent; and never presume to successfully hide behind the veil of anonymity.
There are other legal issues. For example, some state employment laws forbid navigating the Internet in search of an applicant’s medical or criminal history, as such searches are permissible only after a tentative job offer has been made.
Another legal issue involves staff who use office computers or mobile devices for personal activities. This should be pointedly forbidden, as any negligence may be imputed to the doctor under the doctrine of vicarious liability. Current or former staff may unwittingly or even intentionally disclose confidential details of patients. So, as a risk-management strategy, employment contracts should address all of these matters proactively.
In addition to civil suits by an aggrieved patient and/or family, the doctor may face civil and criminal sanctions under the federal Health Insurance Portability and Accountability Act (HIPAA) and other statutes. All professional liability carriers are keen to assist their insured members in formulating office policies and procedures that govern privacy, confidentiality, and disclosure, and practitioners should take advantage of this service.
The ethics surrounding social media typically center on privacy, for example, should a liver transplant physician use social media to ferret out a patient’s recent drinking habits?
Where there is professional misconduct arising out of Internet postings, a state medical board may launch an investigation. A preliminary study indicates that the most common violations are inappropriate patient communication of a sexual nature, Internet prescribing for unknown individuals, and online misrepresentation of credentials (JAMA 2012;307:1141-2).
In a recent illustrative article, the same authors posed 10 hypothetical scenarios to determine the need for disciplinary action (Ann. Int. Med. 2013;158:124-30). The evaluators deemed 4 of the 10 definitely worthy of investigation: misleading information about clinical outcomes, using images without consent, misrepresenting credentials, and inappropriately contacting patients. Other vignettes thought to be probably reprehensible were the depiction of alcohol intoxication, violating patient confidentiality, and using discriminatory speech. There was even concern raised regarding derogatory speech toward patients, showing alcohol use without intoxication, and providing clinical narratives without violating confidentiality.
Errant behavior may be observed early in one’s training. Most medical schools have identified instances of unprofessional student online postings such as breaching patient confidentiality, using profane or discriminatory language, depiction of intoxication, and sexually suggestive material (JAMA 2009;302:1309-15).
It may be impossible to separate personal from professional use of social media, so it has been suggested that ethical guidelines be framed in terms of appropriateness rather than boundaries (JAMA 2013;310:581-2).
Physicians must remain mindful that their online postings are searchable and permanent, notwithstanding the façade of anonymity. Venting of frustration or work stress is rarely justified in the public domain of the Internet.
One doctor, reportedly with some 3,000 followers, gained recent notoriety – and criticism – with his sarcasm, profanity, and patient-bashing through his tweets. Another was fined $500 and lost hospital privileges for posting information traceable to a specific person, despite not divulging the patient’s identity (American Medical News, "Anonymous posts: Liberating or unprofessional?" July 11, 2011).
Recognizing the growing prevalence of doctors’ participation on social media, a growing number of professional organizations – including the American Medical Association, the American College of Physicians, and the Mayo Clinic, among others – have offered guidelines in this area. Most relevantly, the Federation of State Medical Boards, a national nonprofit organization representing the 70 medical and osteopathic boards of the United States and its territories, has published a reader-friendly report entitled, "Model Policy Guidelines for the Appropriate Use of Social Media and Social Networking in Medical Practice."
Then there is the patient who posts negative comments about his or her doctor, say, on Yelp. Occasionally, these comments are derogatory, even defamatory. Such online attacks are difficult to counter, but engaging in an online war is more likely to be aggravating than salutary and adds unwanted publicity.
The preferred way is to attempt to identify the source and to request that the material be removed from the website, either by the poster or the domain host. If a simple request fails, an attorney’s letter, a subpoena, or a judge’s restraining order may be warranted. Occasionally, a defamation suit, even if time consuming and expensive, may prove necessary – and successful.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. It is adapted from the author’s book, "Medical Malpractice: Understanding the Law, Managing the Risk" (2006). For additional information, readers may contact the author at [email protected].
Question: Which of the following is incorrect?
A. Medical malpractice lawsuits arising out of social media interactions are still uncommon.
B. Comments shared by an ex-employee with friends on Facebook may breach doctor-patient confidentiality, with liability imputed to the doctor-employer.
C. Using the same platform, a doctor must promptly rebut disparaging comments on Yelp in order to protect his or her reputation.
D. An employment contract should cover matters concerning confidentiality and privacy.
E. Staff should use office computers only for work-related activities.
Answer: C. Physicians’ widespread use of social media sites such as Facebook, LinkedIn, and Twitter has spawned novel issues of professional liability. Use of such media, augmented by ubiquitous mobile devices such as smartphones and tablets, typically involves physician-to-physician and physician-to-patient communications but may also be personal in nature. Many patients have approached their doctors to "friend" them on Facebook. About a third of all doctors are said to have received such requests, and about a quarter have accepted. Other doctors are regular or occasional bloggers, offering views both medical and nonmedical.
While embracing the immense value of social media, the physician must remain mindful of the legal and ethical risks that such networking poses. State medical boards are facing increasing complaints of online professional breach, and civil lawsuits can be expected to mount.
Allegations of medical malpractice can arise if there is a showing that negligent conduct has caused an injury of some kind. Though currently uncommon, one can expect such lawsuits to proliferate. To be sure, there will be arguments about whether there exists a doctor-patient relationship from which a duty of care arises (Internal Medicine News, "Liability in the Internet Age," April 15, 2011, p. 74), but liability can come about in unexpected ways.
In a recent Massachusetts case, a pediatrician faced a malpractice suit that alleged a failure to diagnose diabetes and diabetic ketoacidosis. An offer to settle followed quickly once it was realized that the plaintiff’s attorney had discovered the defendant’s publicly blogged details about his deposition and trial preparation (American Medical News, "Internet won't protect your secret identity," Aug. 13, 2007) Lesson: Use the blogosphere to educate, not vent; and never presume to successfully hide behind the veil of anonymity.
There are other legal issues. For example, some state employment laws forbid navigating the Internet in search of an applicant’s medical or criminal history, as such searches are permissible only after a tentative job offer has been made.
Another legal issue involves staff who use office computers or mobile devices for personal activities. This should be pointedly forbidden, as any negligence may be imputed to the doctor under the doctrine of vicarious liability. Current or former staff may unwittingly or even intentionally disclose confidential details of patients. So, as a risk-management strategy, employment contracts should address all of these matters proactively.
In addition to civil suits by an aggrieved patient and/or family, the doctor may face civil and criminal sanctions under the federal Health Insurance Portability and Accountability Act (HIPAA) and other statutes. All professional liability carriers are keen to assist their insured members in formulating office policies and procedures that govern privacy, confidentiality, and disclosure, and practitioners should take advantage of this service.
The ethics surrounding social media typically center on privacy, for example, should a liver transplant physician use social media to ferret out a patient’s recent drinking habits?
Where there is professional misconduct arising out of Internet postings, a state medical board may launch an investigation. A preliminary study indicates that the most common violations are inappropriate patient communication of a sexual nature, Internet prescribing for unknown individuals, and online misrepresentation of credentials (JAMA 2012;307:1141-2).
In a recent illustrative article, the same authors posed 10 hypothetical scenarios to determine the need for disciplinary action (Ann. Int. Med. 2013;158:124-30). The evaluators deemed 4 of the 10 definitely worthy of investigation: misleading information about clinical outcomes, using images without consent, misrepresenting credentials, and inappropriately contacting patients. Other vignettes thought to be probably reprehensible were the depiction of alcohol intoxication, violating patient confidentiality, and using discriminatory speech. There was even concern raised regarding derogatory speech toward patients, showing alcohol use without intoxication, and providing clinical narratives without violating confidentiality.
Errant behavior may be observed early in one’s training. Most medical schools have identified instances of unprofessional student online postings such as breaching patient confidentiality, using profane or discriminatory language, depiction of intoxication, and sexually suggestive material (JAMA 2009;302:1309-15).
It may be impossible to separate personal from professional use of social media, so it has been suggested that ethical guidelines be framed in terms of appropriateness rather than boundaries (JAMA 2013;310:581-2).
Physicians must remain mindful that their online postings are searchable and permanent, notwithstanding the façade of anonymity. Venting of frustration or work stress is rarely justified in the public domain of the Internet.
One doctor, reportedly with some 3,000 followers, gained recent notoriety – and criticism – with his sarcasm, profanity, and patient-bashing through his tweets. Another was fined $500 and lost hospital privileges for posting information traceable to a specific person, despite not divulging the patient’s identity (American Medical News, "Anonymous posts: Liberating or unprofessional?" July 11, 2011).
Recognizing the growing prevalence of doctors’ participation on social media, a growing number of professional organizations – including the American Medical Association, the American College of Physicians, and the Mayo Clinic, among others – have offered guidelines in this area. Most relevantly, the Federation of State Medical Boards, a national nonprofit organization representing the 70 medical and osteopathic boards of the United States and its territories, has published a reader-friendly report entitled, "Model Policy Guidelines for the Appropriate Use of Social Media and Social Networking in Medical Practice."
Then there is the patient who posts negative comments about his or her doctor, say, on Yelp. Occasionally, these comments are derogatory, even defamatory. Such online attacks are difficult to counter, but engaging in an online war is more likely to be aggravating than salutary and adds unwanted publicity.
The preferred way is to attempt to identify the source and to request that the material be removed from the website, either by the poster or the domain host. If a simple request fails, an attorney’s letter, a subpoena, or a judge’s restraining order may be warranted. Occasionally, a defamation suit, even if time consuming and expensive, may prove necessary – and successful.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. It is adapted from the author’s book, "Medical Malpractice: Understanding the Law, Managing the Risk" (2006). For additional information, readers may contact the author at [email protected].
Question: Which of the following is incorrect?
A. Medical malpractice lawsuits arising out of social media interactions are still uncommon.
B. Comments shared by an ex-employee with friends on Facebook may breach doctor-patient confidentiality, with liability imputed to the doctor-employer.
C. Using the same platform, a doctor must promptly rebut disparaging comments on Yelp in order to protect his or her reputation.
D. An employment contract should cover matters concerning confidentiality and privacy.
E. Staff should use office computers only for work-related activities.
Answer: C. Physicians’ widespread use of social media sites such as Facebook, LinkedIn, and Twitter has spawned novel issues of professional liability. Use of such media, augmented by ubiquitous mobile devices such as smartphones and tablets, typically involves physician-to-physician and physician-to-patient communications but may also be personal in nature. Many patients have approached their doctors to "friend" them on Facebook. About a third of all doctors are said to have received such requests, and about a quarter have accepted. Other doctors are regular or occasional bloggers, offering views both medical and nonmedical.
While embracing the immense value of social media, the physician must remain mindful of the legal and ethical risks that such networking poses. State medical boards are facing increasing complaints of online professional breach, and civil lawsuits can be expected to mount.
Allegations of medical malpractice can arise if there is a showing that negligent conduct has caused an injury of some kind. Though currently uncommon, one can expect such lawsuits to proliferate. To be sure, there will be arguments about whether there exists a doctor-patient relationship from which a duty of care arises (Internal Medicine News, "Liability in the Internet Age," April 15, 2011, p. 74), but liability can come about in unexpected ways.
In a recent Massachusetts case, a pediatrician faced a malpractice suit that alleged a failure to diagnose diabetes and diabetic ketoacidosis. An offer to settle followed quickly once it was realized that the plaintiff’s attorney had discovered the defendant’s publicly blogged details about his deposition and trial preparation (American Medical News, "Internet won't protect your secret identity," Aug. 13, 2007) Lesson: Use the blogosphere to educate, not vent; and never presume to successfully hide behind the veil of anonymity.
There are other legal issues. For example, some state employment laws forbid navigating the Internet in search of an applicant’s medical or criminal history, as such searches are permissible only after a tentative job offer has been made.
Another legal issue involves staff who use office computers or mobile devices for personal activities. This should be pointedly forbidden, as any negligence may be imputed to the doctor under the doctrine of vicarious liability. Current or former staff may unwittingly or even intentionally disclose confidential details of patients. So, as a risk-management strategy, employment contracts should address all of these matters proactively.
In addition to civil suits by an aggrieved patient and/or family, the doctor may face civil and criminal sanctions under the federal Health Insurance Portability and Accountability Act (HIPAA) and other statutes. All professional liability carriers are keen to assist their insured members in formulating office policies and procedures that govern privacy, confidentiality, and disclosure, and practitioners should take advantage of this service.
The ethics surrounding social media typically center on privacy, for example, should a liver transplant physician use social media to ferret out a patient’s recent drinking habits?
Where there is professional misconduct arising out of Internet postings, a state medical board may launch an investigation. A preliminary study indicates that the most common violations are inappropriate patient communication of a sexual nature, Internet prescribing for unknown individuals, and online misrepresentation of credentials (JAMA 2012;307:1141-2).
In a recent illustrative article, the same authors posed 10 hypothetical scenarios to determine the need for disciplinary action (Ann. Int. Med. 2013;158:124-30). The evaluators deemed 4 of the 10 definitely worthy of investigation: misleading information about clinical outcomes, using images without consent, misrepresenting credentials, and inappropriately contacting patients. Other vignettes thought to be probably reprehensible were the depiction of alcohol intoxication, violating patient confidentiality, and using discriminatory speech. There was even concern raised regarding derogatory speech toward patients, showing alcohol use without intoxication, and providing clinical narratives without violating confidentiality.
Errant behavior may be observed early in one’s training. Most medical schools have identified instances of unprofessional student online postings such as breaching patient confidentiality, using profane or discriminatory language, depiction of intoxication, and sexually suggestive material (JAMA 2009;302:1309-15).
It may be impossible to separate personal from professional use of social media, so it has been suggested that ethical guidelines be framed in terms of appropriateness rather than boundaries (JAMA 2013;310:581-2).
Physicians must remain mindful that their online postings are searchable and permanent, notwithstanding the façade of anonymity. Venting of frustration or work stress is rarely justified in the public domain of the Internet.
One doctor, reportedly with some 3,000 followers, gained recent notoriety – and criticism – with his sarcasm, profanity, and patient-bashing through his tweets. Another was fined $500 and lost hospital privileges for posting information traceable to a specific person, despite not divulging the patient’s identity (American Medical News, "Anonymous posts: Liberating or unprofessional?" July 11, 2011).
Recognizing the growing prevalence of doctors’ participation on social media, a growing number of professional organizations – including the American Medical Association, the American College of Physicians, and the Mayo Clinic, among others – have offered guidelines in this area. Most relevantly, the Federation of State Medical Boards, a national nonprofit organization representing the 70 medical and osteopathic boards of the United States and its territories, has published a reader-friendly report entitled, "Model Policy Guidelines for the Appropriate Use of Social Media and Social Networking in Medical Practice."
Then there is the patient who posts negative comments about his or her doctor, say, on Yelp. Occasionally, these comments are derogatory, even defamatory. Such online attacks are difficult to counter, but engaging in an online war is more likely to be aggravating than salutary and adds unwanted publicity.
The preferred way is to attempt to identify the source and to request that the material be removed from the website, either by the poster or the domain host. If a simple request fails, an attorney’s letter, a subpoena, or a judge’s restraining order may be warranted. Occasionally, a defamation suit, even if time consuming and expensive, may prove necessary – and successful.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. It is adapted from the author’s book, "Medical Malpractice: Understanding the Law, Managing the Risk" (2006). For additional information, readers may contact the author at [email protected].
Senate passes SGR and budget bill; White House next
The Senate has passed a budget package that includes a 3-month delay in physician pay cuts mandated by the Medicare Sustainable Growth Rate formula but also extends other overall Medicare budget cuts for 2 years.
The Senate voted 64-36 on Dec. 18 to approve the Bipartisan Budget Act of 2013 crafted by Senate Budget Committee Chairman Patty Murray (D-Wash.) and House Budget Committee Chairman Paul Ryan (R-Wisc.). Nine Republican senators voted in favor of the deal, joining 55 Democrats.
The 3-month reprieve from the SGR cuts was added to the budget deal by the House before it voted to approve the package on Dec. 12.
Now, if President Obama signs the budget bill, which is expected, physicians will see 0.5% a month pay increase through Mar. 31.
The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. It would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
The bill also would extend the 2% sequestration cut to Medicare payments by 2 years, to 2023.
The House has recessed and is not due back until Jan. 7. The Senate has not set its adjournment date yet, but is expected to reconvene on Jan. 6.
Congress is expected to start consideration again of a permanent replacement for the SGR when it returns.
On Twitter @aliciaault
The Senate has passed a budget package that includes a 3-month delay in physician pay cuts mandated by the Medicare Sustainable Growth Rate formula but also extends other overall Medicare budget cuts for 2 years.
The Senate voted 64-36 on Dec. 18 to approve the Bipartisan Budget Act of 2013 crafted by Senate Budget Committee Chairman Patty Murray (D-Wash.) and House Budget Committee Chairman Paul Ryan (R-Wisc.). Nine Republican senators voted in favor of the deal, joining 55 Democrats.
The 3-month reprieve from the SGR cuts was added to the budget deal by the House before it voted to approve the package on Dec. 12.
Now, if President Obama signs the budget bill, which is expected, physicians will see 0.5% a month pay increase through Mar. 31.
The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. It would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
The bill also would extend the 2% sequestration cut to Medicare payments by 2 years, to 2023.
The House has recessed and is not due back until Jan. 7. The Senate has not set its adjournment date yet, but is expected to reconvene on Jan. 6.
Congress is expected to start consideration again of a permanent replacement for the SGR when it returns.
On Twitter @aliciaault
The Senate has passed a budget package that includes a 3-month delay in physician pay cuts mandated by the Medicare Sustainable Growth Rate formula but also extends other overall Medicare budget cuts for 2 years.
The Senate voted 64-36 on Dec. 18 to approve the Bipartisan Budget Act of 2013 crafted by Senate Budget Committee Chairman Patty Murray (D-Wash.) and House Budget Committee Chairman Paul Ryan (R-Wisc.). Nine Republican senators voted in favor of the deal, joining 55 Democrats.
The 3-month reprieve from the SGR cuts was added to the budget deal by the House before it voted to approve the package on Dec. 12.
Now, if President Obama signs the budget bill, which is expected, physicians will see 0.5% a month pay increase through Mar. 31.
The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. It would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
The bill also would extend the 2% sequestration cut to Medicare payments by 2 years, to 2023.
The House has recessed and is not due back until Jan. 7. The Senate has not set its adjournment date yet, but is expected to reconvene on Jan. 6.
Congress is expected to start consideration again of a permanent replacement for the SGR when it returns.
On Twitter @aliciaault
Physicians, vendors behind schedule on ICD-10
Physicians, vendors, and health insurance plans are moving forward with their preparations for the ICD-10 coding set, but they aren’t moving fast enough, a survey has shown.
Unless the industry speeds up its work in early 2014, there won’t be adequate time for end-to-end testing and payments could be disrupted when the new code set – formally known as the International Statistical Classification of Diseases and Related Health Problems, 10th Revision – takes effect on Oct. 1, 2014, according to the survey from the Workgroup for Electronic Data Interchange (WEDI).
"Based on the survey results, it is clear the industry continues to make slow progress, but not the amount of progress that is needed for a smooth transition," Jim Daley, chairman of the WEDI, a public-private health information technology group that advises the Health and Human Services department, wrote in a letter to the agency.
The WEDI has been surveying the industry since 2009 to gauge progress toward ICD-10 implementation. The latest results come from an October 2013 survey of providers, vendors, and health plans.
Nearly a quarter of 196 physician practices, hospitals, and health systems surveyed planned to begin their own internal testing of business processes and systems changes by the end of 2013, and just under half said they would conduct internal testing in the first half of 2014. The remaining providers surveyed planned to begin internal testing later in 2014 or did not know when it would occur.
This is far behind the goals set in the WEDI/NCHICA timeline, which call for completing internal testing in July 2013 to leave time for external testing with payers throughout 2014.
Health plans and vendors also have some catching up to do, according to the survey results. Of the 59 vendors surveyed, about one-fifth said they were halfway or less than halfway complete with product development, a task that was supposed to be done by the end of 2011, according to the suggested timeline. And about one-third of the 98 health insurance plans that responded said they have already begun or expect to begin external testing by the end of 2013, leaving most health plans less than 9 months to complete external testing.
On Twitter @MaryEllenNY
Physicians, vendors, and health insurance plans are moving forward with their preparations for the ICD-10 coding set, but they aren’t moving fast enough, a survey has shown.
Unless the industry speeds up its work in early 2014, there won’t be adequate time for end-to-end testing and payments could be disrupted when the new code set – formally known as the International Statistical Classification of Diseases and Related Health Problems, 10th Revision – takes effect on Oct. 1, 2014, according to the survey from the Workgroup for Electronic Data Interchange (WEDI).
"Based on the survey results, it is clear the industry continues to make slow progress, but not the amount of progress that is needed for a smooth transition," Jim Daley, chairman of the WEDI, a public-private health information technology group that advises the Health and Human Services department, wrote in a letter to the agency.
The WEDI has been surveying the industry since 2009 to gauge progress toward ICD-10 implementation. The latest results come from an October 2013 survey of providers, vendors, and health plans.
Nearly a quarter of 196 physician practices, hospitals, and health systems surveyed planned to begin their own internal testing of business processes and systems changes by the end of 2013, and just under half said they would conduct internal testing in the first half of 2014. The remaining providers surveyed planned to begin internal testing later in 2014 or did not know when it would occur.
This is far behind the goals set in the WEDI/NCHICA timeline, which call for completing internal testing in July 2013 to leave time for external testing with payers throughout 2014.
Health plans and vendors also have some catching up to do, according to the survey results. Of the 59 vendors surveyed, about one-fifth said they were halfway or less than halfway complete with product development, a task that was supposed to be done by the end of 2011, according to the suggested timeline. And about one-third of the 98 health insurance plans that responded said they have already begun or expect to begin external testing by the end of 2013, leaving most health plans less than 9 months to complete external testing.
On Twitter @MaryEllenNY
Physicians, vendors, and health insurance plans are moving forward with their preparations for the ICD-10 coding set, but they aren’t moving fast enough, a survey has shown.
Unless the industry speeds up its work in early 2014, there won’t be adequate time for end-to-end testing and payments could be disrupted when the new code set – formally known as the International Statistical Classification of Diseases and Related Health Problems, 10th Revision – takes effect on Oct. 1, 2014, according to the survey from the Workgroup for Electronic Data Interchange (WEDI).
"Based on the survey results, it is clear the industry continues to make slow progress, but not the amount of progress that is needed for a smooth transition," Jim Daley, chairman of the WEDI, a public-private health information technology group that advises the Health and Human Services department, wrote in a letter to the agency.
The WEDI has been surveying the industry since 2009 to gauge progress toward ICD-10 implementation. The latest results come from an October 2013 survey of providers, vendors, and health plans.
Nearly a quarter of 196 physician practices, hospitals, and health systems surveyed planned to begin their own internal testing of business processes and systems changes by the end of 2013, and just under half said they would conduct internal testing in the first half of 2014. The remaining providers surveyed planned to begin internal testing later in 2014 or did not know when it would occur.
This is far behind the goals set in the WEDI/NCHICA timeline, which call for completing internal testing in July 2013 to leave time for external testing with payers throughout 2014.
Health plans and vendors also have some catching up to do, according to the survey results. Of the 59 vendors surveyed, about one-fifth said they were halfway or less than halfway complete with product development, a task that was supposed to be done by the end of 2011, according to the suggested timeline. And about one-third of the 98 health insurance plans that responded said they have already begun or expect to begin external testing by the end of 2013, leaving most health plans less than 9 months to complete external testing.
On Twitter @MaryEllenNY
Breast MRI screening finds undetected cancers in 11 per 1,000 average-risk women
SAN ANTONIO – MRI screening of women who were at average risk of breast cancer and had a negative screening mammogram resulted in the diagnosis of 11 cases of cancer per 1,000 women screened, Dr. Simone Schrading said at the San Antonio Breast Cancer Symposium.
"The additional cancer detection rate is high, even in heavily prescreened women. In experienced hands, the positive predictive value of MRI screening in this average-risk cohort was comparable to that seen in mammographic screening programs or in MRI high-risk screening cohorts," said Dr. Schrading, of the University of Aachen, Germany.
While MRI is well established as a screening method for women at high familial risk of breast cancer, there have been no data to support its use in average-risk women.
Dr. Schrading reported on a prospective, single-center study evaluating the additional cancer yield and accuracy of breast MRI screening in 1,725 women at average risk for breast cancer. All had normal clinical breast examinations and digital screening mammograms. In addition, 89% of the women had undergone high-frequency breast ultrasound screening, which was normal in all cases. None of the subjects had a personal or family history of breast or ovarian cancer, and none had been diagnosed with breast proliferative changes or atypia. Their mean age was 55 years (range, 42-71 years).
MRI screening detected breast cancers in 18 of these patients, for a detection rate of 11 per 1,000 screened. Seven malignancies were ductal carcinoma in situ, and the other 11 were invasive breast cancer. The mean size of the invasive cancers was 11 mm. Five of the 7 ductal carcinomas in situ and 6 of 11 invasive cancers were high-grade cancers. On the other hand, the stage distribution of the invasive cancers was favorable: All were staged as pN0, M0.
Nearly 91% of screening MRIs were negative as defined by a BIRADS 1 or 2 rating. Another 5.9% were rated BIRADS 3; follow-up MRIs in this group of patients showed no breast cancers. Suspicious lesions – that is, BIRADS 4 or 5 – were noted in 54 patients, or 3.2% of the study group, and they were evaluated by MRI-guided biopsy. The lesions proved benign in 28 of 54 cases, high risk in 8, and malignant in 18.
Thus, the positive predictive value of breast MRI screening in this average-risk, extensively prescreened population was 33% if only BIRADS 4 and 5 cancers were defined as true positives, and 48% if BIRADS 4 and 5 high-risk lesions were also counted.
The age distribution of the patients with MRI-detected breast cancers was similar to that of the total study population. Mammographic breast density did not predict the likelihood of identifying breast cancer by MRI.
Dr. Laura J. Esserman rose from the audience to question the cost-benefit ratio of introducing MRI screening for the vast population of average-risk women.
The aggregate cost of breast cancer screening in the United States is already at least $8 billion annually. Utilizing MRI to screen average-risk women would push that figure up by an order of magnitude, observed Dr. Esserman, professor of surgery and of radiology and director of the Carol Franc Buck Breast Care Center at the University of California, San Francisco.
"MRI screening is still very expensive, it’s true," Dr. Schrading replied. "The main reason for the high cost of MRI is the long acquisition time and the long reading time. The long acquisition time is because the MRI protocols we have today are designed for diagnostic purposes, not for the purpose of screening. Our goal is to use a screening protocol for MRI, where the acquisition time is only 3 minutes and the reading time is less than 30 seconds. This might be a way to reduce the cost of MRI," she said.
The study was conducted free of commercial support. Dr. Schrading declared having no financial conflicts of interest.
SAN ANTONIO – MRI screening of women who were at average risk of breast cancer and had a negative screening mammogram resulted in the diagnosis of 11 cases of cancer per 1,000 women screened, Dr. Simone Schrading said at the San Antonio Breast Cancer Symposium.
"The additional cancer detection rate is high, even in heavily prescreened women. In experienced hands, the positive predictive value of MRI screening in this average-risk cohort was comparable to that seen in mammographic screening programs or in MRI high-risk screening cohorts," said Dr. Schrading, of the University of Aachen, Germany.
While MRI is well established as a screening method for women at high familial risk of breast cancer, there have been no data to support its use in average-risk women.
Dr. Schrading reported on a prospective, single-center study evaluating the additional cancer yield and accuracy of breast MRI screening in 1,725 women at average risk for breast cancer. All had normal clinical breast examinations and digital screening mammograms. In addition, 89% of the women had undergone high-frequency breast ultrasound screening, which was normal in all cases. None of the subjects had a personal or family history of breast or ovarian cancer, and none had been diagnosed with breast proliferative changes or atypia. Their mean age was 55 years (range, 42-71 years).
MRI screening detected breast cancers in 18 of these patients, for a detection rate of 11 per 1,000 screened. Seven malignancies were ductal carcinoma in situ, and the other 11 were invasive breast cancer. The mean size of the invasive cancers was 11 mm. Five of the 7 ductal carcinomas in situ and 6 of 11 invasive cancers were high-grade cancers. On the other hand, the stage distribution of the invasive cancers was favorable: All were staged as pN0, M0.
Nearly 91% of screening MRIs were negative as defined by a BIRADS 1 or 2 rating. Another 5.9% were rated BIRADS 3; follow-up MRIs in this group of patients showed no breast cancers. Suspicious lesions – that is, BIRADS 4 or 5 – were noted in 54 patients, or 3.2% of the study group, and they were evaluated by MRI-guided biopsy. The lesions proved benign in 28 of 54 cases, high risk in 8, and malignant in 18.
Thus, the positive predictive value of breast MRI screening in this average-risk, extensively prescreened population was 33% if only BIRADS 4 and 5 cancers were defined as true positives, and 48% if BIRADS 4 and 5 high-risk lesions were also counted.
The age distribution of the patients with MRI-detected breast cancers was similar to that of the total study population. Mammographic breast density did not predict the likelihood of identifying breast cancer by MRI.
Dr. Laura J. Esserman rose from the audience to question the cost-benefit ratio of introducing MRI screening for the vast population of average-risk women.
The aggregate cost of breast cancer screening in the United States is already at least $8 billion annually. Utilizing MRI to screen average-risk women would push that figure up by an order of magnitude, observed Dr. Esserman, professor of surgery and of radiology and director of the Carol Franc Buck Breast Care Center at the University of California, San Francisco.
"MRI screening is still very expensive, it’s true," Dr. Schrading replied. "The main reason for the high cost of MRI is the long acquisition time and the long reading time. The long acquisition time is because the MRI protocols we have today are designed for diagnostic purposes, not for the purpose of screening. Our goal is to use a screening protocol for MRI, where the acquisition time is only 3 minutes and the reading time is less than 30 seconds. This might be a way to reduce the cost of MRI," she said.
The study was conducted free of commercial support. Dr. Schrading declared having no financial conflicts of interest.
SAN ANTONIO – MRI screening of women who were at average risk of breast cancer and had a negative screening mammogram resulted in the diagnosis of 11 cases of cancer per 1,000 women screened, Dr. Simone Schrading said at the San Antonio Breast Cancer Symposium.
"The additional cancer detection rate is high, even in heavily prescreened women. In experienced hands, the positive predictive value of MRI screening in this average-risk cohort was comparable to that seen in mammographic screening programs or in MRI high-risk screening cohorts," said Dr. Schrading, of the University of Aachen, Germany.
While MRI is well established as a screening method for women at high familial risk of breast cancer, there have been no data to support its use in average-risk women.
Dr. Schrading reported on a prospective, single-center study evaluating the additional cancer yield and accuracy of breast MRI screening in 1,725 women at average risk for breast cancer. All had normal clinical breast examinations and digital screening mammograms. In addition, 89% of the women had undergone high-frequency breast ultrasound screening, which was normal in all cases. None of the subjects had a personal or family history of breast or ovarian cancer, and none had been diagnosed with breast proliferative changes or atypia. Their mean age was 55 years (range, 42-71 years).
MRI screening detected breast cancers in 18 of these patients, for a detection rate of 11 per 1,000 screened. Seven malignancies were ductal carcinoma in situ, and the other 11 were invasive breast cancer. The mean size of the invasive cancers was 11 mm. Five of the 7 ductal carcinomas in situ and 6 of 11 invasive cancers were high-grade cancers. On the other hand, the stage distribution of the invasive cancers was favorable: All were staged as pN0, M0.
Nearly 91% of screening MRIs were negative as defined by a BIRADS 1 or 2 rating. Another 5.9% were rated BIRADS 3; follow-up MRIs in this group of patients showed no breast cancers. Suspicious lesions – that is, BIRADS 4 or 5 – were noted in 54 patients, or 3.2% of the study group, and they were evaluated by MRI-guided biopsy. The lesions proved benign in 28 of 54 cases, high risk in 8, and malignant in 18.
Thus, the positive predictive value of breast MRI screening in this average-risk, extensively prescreened population was 33% if only BIRADS 4 and 5 cancers were defined as true positives, and 48% if BIRADS 4 and 5 high-risk lesions were also counted.
The age distribution of the patients with MRI-detected breast cancers was similar to that of the total study population. Mammographic breast density did not predict the likelihood of identifying breast cancer by MRI.
Dr. Laura J. Esserman rose from the audience to question the cost-benefit ratio of introducing MRI screening for the vast population of average-risk women.
The aggregate cost of breast cancer screening in the United States is already at least $8 billion annually. Utilizing MRI to screen average-risk women would push that figure up by an order of magnitude, observed Dr. Esserman, professor of surgery and of radiology and director of the Carol Franc Buck Breast Care Center at the University of California, San Francisco.
"MRI screening is still very expensive, it’s true," Dr. Schrading replied. "The main reason for the high cost of MRI is the long acquisition time and the long reading time. The long acquisition time is because the MRI protocols we have today are designed for diagnostic purposes, not for the purpose of screening. Our goal is to use a screening protocol for MRI, where the acquisition time is only 3 minutes and the reading time is less than 30 seconds. This might be a way to reduce the cost of MRI," she said.
The study was conducted free of commercial support. Dr. Schrading declared having no financial conflicts of interest.
AT SABCS 2013
Major finding: Breast MRI screening of women who were at average risk for breast cancer and already had a negative mammogram had a breast cancer detection rate of 11 cases per 1,000 women screened.
Data source: This was a prospective, uncontrolled, single-center study of breast MRI screening in 1,725 women with no personal or family history of breast or ovarian cancer, no diagnosis of proliferative changes, and a mean age of 55 years.
Disclosures: The study was conducted free of commercial support. Dr. Schrading declared having no financial conflicts of interest.
Meeting attendees identify top studies presented at SABCS
Live from the 2013 San Antonio Breast Cancer Symposium, our onsite reporters checked in with meeting attendees to find out the "most interesting thing" they had learned so far. From identifying predictive signatures via genomic data to treating postmenopausal women with bisphosphonates, passing oncologists shared their favorite, potentially practice-changing highlights.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Live from the 2013 San Antonio Breast Cancer Symposium, our onsite reporters checked in with meeting attendees to find out the "most interesting thing" they had learned so far. From identifying predictive signatures via genomic data to treating postmenopausal women with bisphosphonates, passing oncologists shared their favorite, potentially practice-changing highlights.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Live from the 2013 San Antonio Breast Cancer Symposium, our onsite reporters checked in with meeting attendees to find out the "most interesting thing" they had learned so far. From identifying predictive signatures via genomic data to treating postmenopausal women with bisphosphonates, passing oncologists shared their favorite, potentially practice-changing highlights.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
'Vogl, New York' offers San Antonio perspectives
In an interview with Frontline Medical News, New York oncologist and San Antonio Breast Cancer Symposium star gadfly Dr. Steven Vogl gives his quick take on several of the studies making news at this year's meeting.
In an interview with Frontline Medical News, New York oncologist and San Antonio Breast Cancer Symposium star gadfly Dr. Steven Vogl gives his quick take on several of the studies making news at this year's meeting.
In an interview with Frontline Medical News, New York oncologist and San Antonio Breast Cancer Symposium star gadfly Dr. Steven Vogl gives his quick take on several of the studies making news at this year's meeting.
New mega-review underscores mammography’s benefits
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.
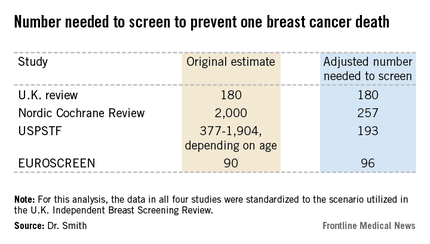
But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.

But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.

But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
EXPERT OPINION FROM SABCS 2013













