User login
Official Newspaper of the American College of Surgeons
Flu vaccine administered to 75% of health care personnel in 2013-2014
About 75% of health care personnel received an influenza vaccination during the 2013-2014 flu season, up slightly from the 72% who were vaccinated during the 2012-2013 season, the Centers for Disease Control and Prevention reported.
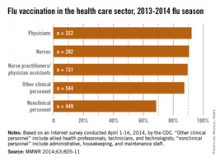
With a vaccination rate of 92.2%, physicians had the highest coverage of the various health care occupations measured. Nurses were second at 90.5%, followed by nurse practitioners/physician assistants at 89.6%. Clinical personnel such as allied health professionals, technicians, and technologists had a vaccination rate of 87.4%, and nonclinical personnel – including administrative staff or managers, food service workers, housekeeping or maintenance staff, and laundry workers – had a rate of 68.6%, the CDC said (MMWR 2014;63:805-11).
|
Coverage among physicians was nearly the same as in the 2012-2013 flu season, when the flu vaccination rate was 92.3%, but coverage among nurses was up from 84.8% last season, and coverage among other clinical personnel increased from 81.9%. Coverage for nonclinical personnel increased by almost 4 percentage points over the 2012-2013 flu season, while nurse practitioners/physician assistants had an increase of slightly more than 1 percentage point, the CDC data showed.
The CDC analysis was based on an Internet survey conducted April 1-16, 2014, with 1,882 eligible responses received.
About 75% of health care personnel received an influenza vaccination during the 2013-2014 flu season, up slightly from the 72% who were vaccinated during the 2012-2013 season, the Centers for Disease Control and Prevention reported.
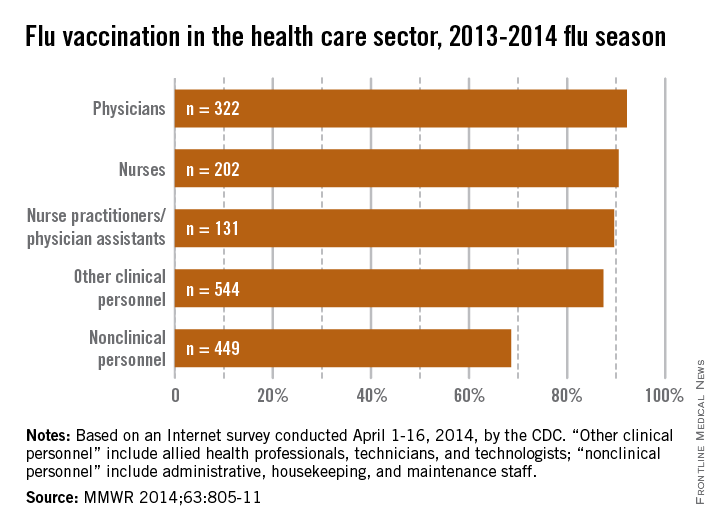
With a vaccination rate of 92.2%, physicians had the highest coverage of the various health care occupations measured. Nurses were second at 90.5%, followed by nurse practitioners/physician assistants at 89.6%. Clinical personnel such as allied health professionals, technicians, and technologists had a vaccination rate of 87.4%, and nonclinical personnel – including administrative staff or managers, food service workers, housekeeping or maintenance staff, and laundry workers – had a rate of 68.6%, the CDC said (MMWR 2014;63:805-11).
|
Coverage among physicians was nearly the same as in the 2012-2013 flu season, when the flu vaccination rate was 92.3%, but coverage among nurses was up from 84.8% last season, and coverage among other clinical personnel increased from 81.9%. Coverage for nonclinical personnel increased by almost 4 percentage points over the 2012-2013 flu season, while nurse practitioners/physician assistants had an increase of slightly more than 1 percentage point, the CDC data showed.
The CDC analysis was based on an Internet survey conducted April 1-16, 2014, with 1,882 eligible responses received.
About 75% of health care personnel received an influenza vaccination during the 2013-2014 flu season, up slightly from the 72% who were vaccinated during the 2012-2013 season, the Centers for Disease Control and Prevention reported.
With a vaccination rate of 92.2%, physicians had the highest coverage of the various health care occupations measured. Nurses were second at 90.5%, followed by nurse practitioners/physician assistants at 89.6%. Clinical personnel such as allied health professionals, technicians, and technologists had a vaccination rate of 87.4%, and nonclinical personnel – including administrative staff or managers, food service workers, housekeeping or maintenance staff, and laundry workers – had a rate of 68.6%, the CDC said (MMWR 2014;63:805-11).
|
Coverage among physicians was nearly the same as in the 2012-2013 flu season, when the flu vaccination rate was 92.3%, but coverage among nurses was up from 84.8% last season, and coverage among other clinical personnel increased from 81.9%. Coverage for nonclinical personnel increased by almost 4 percentage points over the 2012-2013 flu season, while nurse practitioners/physician assistants had an increase of slightly more than 1 percentage point, the CDC data showed.
The CDC analysis was based on an Internet survey conducted April 1-16, 2014, with 1,882 eligible responses received.
FROM MMWR
Radiation therapy for early breast cancer did not increase lymphedema risk
SAN FRANCISCO – Directing radiation therapy to lymph nodes in the breast or chest wall as part of treatment for early node-negative breast cancer does not increase lymphedema risk, according to a secondary analysis of the National Surgical Adjuvant Breast and Bowel Project’s B-32 trial.
“There was no evidence to suggest a detrimental impact of nonregional nodal breast or chest wall radiation on the risk of lymphedema beyond surgery,” lead author Dr. Susan A. McCloskey of the University of California, Los Angeles, said at the annual scientific meeting of the American Society for Radiation Oncology.
The results also showed that subjectively perceived lymphedema was less common than objectively measured lymphedema, and the two were poorly correlated. “Additional analyses of our objective data are currently in progress to evaluate quantification methods that may better correlate with the subjective assessment,” she said.
In the trial, women with clinically node-negative breast cancer were randomized to sentinel node resection followed by routine axillary lymph node dissection (ALND) – the standard when the trial began – or to sentinel node resection followed by ALND only if that node was positive.
Previously reported results showed that the two strategies yielded statistically equivalent overall survival, disease-free survival, and regional control (Lancet Oncol. 2010;11:927-933) and that ALND increased the risk of lymphedema (J. Surg. Oncol. 2010;102:111-8).
“In large part, on the basis of these findings, sentinel node resection alone became [the] standard of care for women presenting with clinically negative axillary nodes,” Dr. McCloskey noted.
The new analysis compared lymphedema outcomes according to receipt of radiation among the 3,894 women with pathologically negative sentinel nodes. Most underwent breast-conserving surgery, and 83% received radiation therapy as part of their treatment, nearly always breast or chest wall–only radiation (that is, nonregional nodal radiation).
The women were evaluated for the presence of lymphedema every 6 months. Subjective lymphedema, assessed with a questionnaire, was defined as a report that swelling was somewhat, quite, or very bothersome. Objective lymphedema, assessed with a water displacement test, was defined as a relative difference in volumes between arms exceeding 10%.
During 36 months of follow-up, there was no significant difference at any time point between women who did and did not receive radiation in the adjusted rate of lymphedema, whether it was assessed subjectively or objectively, reported Dr. McCloskey. The findings were the same when women were stratified by the extent of nodal surgery.
The rate of subjective lymphedema was consistently lower than the rate of objective lymphedema, both among women who had only sentinel node resection (averaging roughly 2.5% vs. 7.5%) and among women who had sentinel node resection followed by ALND (averaging roughly 10% vs. 15%). Overall, there was poor agreement between objectively and subjectively measured lymphedema, with kappa values ranging from just 0.02 to 0.21, where 1.0 would represent perfect agreement.
“Some considerations that we view as hypothesis generating at this point are that a relative arm volume difference of less than 10% is bothersome to some women, and conversely, a relative arm volume difference of greater than 10% is not bothersome to all women. So there may be issues of body habitus or handedness that may affect these metrics,” Dr. McCloskey commented. “Also, I think the jury is still out on exactly what the best metric is to measure lymphedema, both in terms of water displacement and the relative arm volume difference equation.”
In a related press briefing, she said that the findings could affect treatment decisions, given that some women with early breast cancer opt for mastectomy in part because of fears about radiation therapy.
“Where I practice, we run a multidisciplinary breast clinic where all women who are newly diagnosed come to see a team of physicians at the time of their diagnosis. I find that one of the most feared topics for discussion is radiation, and many women will talk about a litany of potential side effects that they are fearful of. And as many of you know, there have been dramatic increases in rates of mastectomy in the United States,” she said. “So it’s an opportunity, I think, to reassure women who are particularly fearful of lymphedema that yes, there is still a risk from the surgery and the type of surgery that’s done, but it doesn’t appear that choice of breast conservation and having routine breast radiation is going to impact that risk beyond the surgery. So I think it can affect what women choose.”
The findings are good news when it comes to quality of life after breast cancer treatment, agreed Dr. Tracy Balboni, a radiation oncologist at the Dana-Farber Cancer Institute in Boston and moderator of the press briefing. The evidence suggesting that conventional radiation therapy doesn’t add to the risk of lymphedema for patients “help[s] us feel assured that we’re not going to be reducing the quality of life of our cancer patients through the addition of conventional radiation therapy to the whole breast,” she said.
Dr. McCloskey disclosed no relevant conflicts of interest.
SAN FRANCISCO – Directing radiation therapy to lymph nodes in the breast or chest wall as part of treatment for early node-negative breast cancer does not increase lymphedema risk, according to a secondary analysis of the National Surgical Adjuvant Breast and Bowel Project’s B-32 trial.
“There was no evidence to suggest a detrimental impact of nonregional nodal breast or chest wall radiation on the risk of lymphedema beyond surgery,” lead author Dr. Susan A. McCloskey of the University of California, Los Angeles, said at the annual scientific meeting of the American Society for Radiation Oncology.
The results also showed that subjectively perceived lymphedema was less common than objectively measured lymphedema, and the two were poorly correlated. “Additional analyses of our objective data are currently in progress to evaluate quantification methods that may better correlate with the subjective assessment,” she said.
In the trial, women with clinically node-negative breast cancer were randomized to sentinel node resection followed by routine axillary lymph node dissection (ALND) – the standard when the trial began – or to sentinel node resection followed by ALND only if that node was positive.
Previously reported results showed that the two strategies yielded statistically equivalent overall survival, disease-free survival, and regional control (Lancet Oncol. 2010;11:927-933) and that ALND increased the risk of lymphedema (J. Surg. Oncol. 2010;102:111-8).
“In large part, on the basis of these findings, sentinel node resection alone became [the] standard of care for women presenting with clinically negative axillary nodes,” Dr. McCloskey noted.
The new analysis compared lymphedema outcomes according to receipt of radiation among the 3,894 women with pathologically negative sentinel nodes. Most underwent breast-conserving surgery, and 83% received radiation therapy as part of their treatment, nearly always breast or chest wall–only radiation (that is, nonregional nodal radiation).
The women were evaluated for the presence of lymphedema every 6 months. Subjective lymphedema, assessed with a questionnaire, was defined as a report that swelling was somewhat, quite, or very bothersome. Objective lymphedema, assessed with a water displacement test, was defined as a relative difference in volumes between arms exceeding 10%.
During 36 months of follow-up, there was no significant difference at any time point between women who did and did not receive radiation in the adjusted rate of lymphedema, whether it was assessed subjectively or objectively, reported Dr. McCloskey. The findings were the same when women were stratified by the extent of nodal surgery.
The rate of subjective lymphedema was consistently lower than the rate of objective lymphedema, both among women who had only sentinel node resection (averaging roughly 2.5% vs. 7.5%) and among women who had sentinel node resection followed by ALND (averaging roughly 10% vs. 15%). Overall, there was poor agreement between objectively and subjectively measured lymphedema, with kappa values ranging from just 0.02 to 0.21, where 1.0 would represent perfect agreement.
“Some considerations that we view as hypothesis generating at this point are that a relative arm volume difference of less than 10% is bothersome to some women, and conversely, a relative arm volume difference of greater than 10% is not bothersome to all women. So there may be issues of body habitus or handedness that may affect these metrics,” Dr. McCloskey commented. “Also, I think the jury is still out on exactly what the best metric is to measure lymphedema, both in terms of water displacement and the relative arm volume difference equation.”
In a related press briefing, she said that the findings could affect treatment decisions, given that some women with early breast cancer opt for mastectomy in part because of fears about radiation therapy.
“Where I practice, we run a multidisciplinary breast clinic where all women who are newly diagnosed come to see a team of physicians at the time of their diagnosis. I find that one of the most feared topics for discussion is radiation, and many women will talk about a litany of potential side effects that they are fearful of. And as many of you know, there have been dramatic increases in rates of mastectomy in the United States,” she said. “So it’s an opportunity, I think, to reassure women who are particularly fearful of lymphedema that yes, there is still a risk from the surgery and the type of surgery that’s done, but it doesn’t appear that choice of breast conservation and having routine breast radiation is going to impact that risk beyond the surgery. So I think it can affect what women choose.”
The findings are good news when it comes to quality of life after breast cancer treatment, agreed Dr. Tracy Balboni, a radiation oncologist at the Dana-Farber Cancer Institute in Boston and moderator of the press briefing. The evidence suggesting that conventional radiation therapy doesn’t add to the risk of lymphedema for patients “help[s] us feel assured that we’re not going to be reducing the quality of life of our cancer patients through the addition of conventional radiation therapy to the whole breast,” she said.
Dr. McCloskey disclosed no relevant conflicts of interest.
SAN FRANCISCO – Directing radiation therapy to lymph nodes in the breast or chest wall as part of treatment for early node-negative breast cancer does not increase lymphedema risk, according to a secondary analysis of the National Surgical Adjuvant Breast and Bowel Project’s B-32 trial.
“There was no evidence to suggest a detrimental impact of nonregional nodal breast or chest wall radiation on the risk of lymphedema beyond surgery,” lead author Dr. Susan A. McCloskey of the University of California, Los Angeles, said at the annual scientific meeting of the American Society for Radiation Oncology.
The results also showed that subjectively perceived lymphedema was less common than objectively measured lymphedema, and the two were poorly correlated. “Additional analyses of our objective data are currently in progress to evaluate quantification methods that may better correlate with the subjective assessment,” she said.
In the trial, women with clinically node-negative breast cancer were randomized to sentinel node resection followed by routine axillary lymph node dissection (ALND) – the standard when the trial began – or to sentinel node resection followed by ALND only if that node was positive.
Previously reported results showed that the two strategies yielded statistically equivalent overall survival, disease-free survival, and regional control (Lancet Oncol. 2010;11:927-933) and that ALND increased the risk of lymphedema (J. Surg. Oncol. 2010;102:111-8).
“In large part, on the basis of these findings, sentinel node resection alone became [the] standard of care for women presenting with clinically negative axillary nodes,” Dr. McCloskey noted.
The new analysis compared lymphedema outcomes according to receipt of radiation among the 3,894 women with pathologically negative sentinel nodes. Most underwent breast-conserving surgery, and 83% received radiation therapy as part of their treatment, nearly always breast or chest wall–only radiation (that is, nonregional nodal radiation).
The women were evaluated for the presence of lymphedema every 6 months. Subjective lymphedema, assessed with a questionnaire, was defined as a report that swelling was somewhat, quite, or very bothersome. Objective lymphedema, assessed with a water displacement test, was defined as a relative difference in volumes between arms exceeding 10%.
During 36 months of follow-up, there was no significant difference at any time point between women who did and did not receive radiation in the adjusted rate of lymphedema, whether it was assessed subjectively or objectively, reported Dr. McCloskey. The findings were the same when women were stratified by the extent of nodal surgery.
The rate of subjective lymphedema was consistently lower than the rate of objective lymphedema, both among women who had only sentinel node resection (averaging roughly 2.5% vs. 7.5%) and among women who had sentinel node resection followed by ALND (averaging roughly 10% vs. 15%). Overall, there was poor agreement between objectively and subjectively measured lymphedema, with kappa values ranging from just 0.02 to 0.21, where 1.0 would represent perfect agreement.
“Some considerations that we view as hypothesis generating at this point are that a relative arm volume difference of less than 10% is bothersome to some women, and conversely, a relative arm volume difference of greater than 10% is not bothersome to all women. So there may be issues of body habitus or handedness that may affect these metrics,” Dr. McCloskey commented. “Also, I think the jury is still out on exactly what the best metric is to measure lymphedema, both in terms of water displacement and the relative arm volume difference equation.”
In a related press briefing, she said that the findings could affect treatment decisions, given that some women with early breast cancer opt for mastectomy in part because of fears about radiation therapy.
“Where I practice, we run a multidisciplinary breast clinic where all women who are newly diagnosed come to see a team of physicians at the time of their diagnosis. I find that one of the most feared topics for discussion is radiation, and many women will talk about a litany of potential side effects that they are fearful of. And as many of you know, there have been dramatic increases in rates of mastectomy in the United States,” she said. “So it’s an opportunity, I think, to reassure women who are particularly fearful of lymphedema that yes, there is still a risk from the surgery and the type of surgery that’s done, but it doesn’t appear that choice of breast conservation and having routine breast radiation is going to impact that risk beyond the surgery. So I think it can affect what women choose.”
The findings are good news when it comes to quality of life after breast cancer treatment, agreed Dr. Tracy Balboni, a radiation oncologist at the Dana-Farber Cancer Institute in Boston and moderator of the press briefing. The evidence suggesting that conventional radiation therapy doesn’t add to the risk of lymphedema for patients “help[s] us feel assured that we’re not going to be reducing the quality of life of our cancer patients through the addition of conventional radiation therapy to the whole breast,” she said.
Dr. McCloskey disclosed no relevant conflicts of interest.
AT THE ASTRO ANNUAL MEETING
Key clinical point:The rate of lymphedema did not differ between breast cancer patients with pathologically negative sentinel nodes who did and did not receive radiation therapy.
Major finding:During 36 months of follow-up, there was no significant difference at any time point between women who did and did not receive radiation in the adjusted rate of lymphedema, whether it was assessed subjectively or objectively.
Data source: A secondary analysis of 3,894 women with early breast cancer from a phase III randomized trial.
Disclosures: Dr. McCloskey disclosed no relevant financial conflicts.
VIDEO: Waiting for long-term data on pCR could be disservice to some breast cancer patients, expert says
SAN FRANCISCO – Only a fraction of patients with breast cancer who are eligible for neoadjuvant therapy are getting it, partly because of confusion around the significance of achieving a pathologic complete response, Dr. William M. Sikov said in an interview at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Dr. Sikov of Brown University, Providence, R.I., explained why it’s been difficult for researchers to show improved outcomes even after a pathologic complete response (pCR) is obtained, but argued that waiting for long-term outcomes data for neoadjuvant therapy could be a disservice to some patients with breast cancer.
Breast cancer surgeons at his own institution have become converts in favor of neoadjuvant therapy, and Dr. Sikov explained why in this video report.
He reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN FRANCISCO – Only a fraction of patients with breast cancer who are eligible for neoadjuvant therapy are getting it, partly because of confusion around the significance of achieving a pathologic complete response, Dr. William M. Sikov said in an interview at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Dr. Sikov of Brown University, Providence, R.I., explained why it’s been difficult for researchers to show improved outcomes even after a pathologic complete response (pCR) is obtained, but argued that waiting for long-term outcomes data for neoadjuvant therapy could be a disservice to some patients with breast cancer.
Breast cancer surgeons at his own institution have become converts in favor of neoadjuvant therapy, and Dr. Sikov explained why in this video report.
He reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN FRANCISCO – Only a fraction of patients with breast cancer who are eligible for neoadjuvant therapy are getting it, partly because of confusion around the significance of achieving a pathologic complete response, Dr. William M. Sikov said in an interview at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Dr. Sikov of Brown University, Providence, R.I., explained why it’s been difficult for researchers to show improved outcomes even after a pathologic complete response (pCR) is obtained, but argued that waiting for long-term outcomes data for neoadjuvant therapy could be a disservice to some patients with breast cancer.
Breast cancer surgeons at his own institution have become converts in favor of neoadjuvant therapy, and Dr. Sikov explained why in this video report.
He reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Whole blood autotransfusion linked to fewer fluids, lower costs
PHILADELPHIA – Early whole-blood autotransfusion of severely injured trauma victims’ own blood, while employed frequently in the military, has been rare in civilian populations, but a recent trial has found it to be safe, effective, and less costly than customary allogeneic transfusions.
Dr. Peter Rhee, chief of trauma, critical care, burns, and emergency surgery at the University of Arizona Medical Center, Tucson, reported on results from a 6-year retrospective study at the annual meeting of the American Association for the Surgery of Trauma. The study evaluated 272 trauma patients in two centers in Tucson and Los Angeles County who required transfusions upon presentation in the emergency department.
“We did this because there is a lot of concern in animal and also human ex vivo laboratory type experiments [regarding] complications from coagulopathy and possibly even increasing the inflammatory processes. So we looked at those types of complications and found that there were no clinically significant complications we could identify in regard to coagulopathy or inflammatory processes with AT [autotransfusion],” Dr. Rhee said.
“But what we also found was that since getting a patient’s own blood back costs less, hospital costs would also be less.”
Patients who underwent AT vs. no AT received significantly less allogeneic packed red blood cells (10.3 vs. 12.1 units), platelets (5.2 vs. 7.9 units), and fresh frozen plasma (6.1 vs. 8.2 units) than patients who did not undergo transfusion.
The investigators reported that AT cost approximately $8,794 per patient vs. $10,7427 for allogeneic transfusions. Hospital costs ($42,156 vs. $43,963) were also lower without any appreciable difference in outcomes.
The trial population was split evenly between those receiving AT and those who did not. Demographics and injury characteristics were similar between the two groups.
“Autologous autotransfusion of blood collected through the chest tube was safe, is associated reduced allogeneic transfusion, and is associated with decreased hospital costs,” Dr. Rhee said. “I think this trial provides safety data for us to go on and do a larger prospective, multicenter study.”
Dr. Rhee reported having no relevant financial disclosures.
PHILADELPHIA – Early whole-blood autotransfusion of severely injured trauma victims’ own blood, while employed frequently in the military, has been rare in civilian populations, but a recent trial has found it to be safe, effective, and less costly than customary allogeneic transfusions.
Dr. Peter Rhee, chief of trauma, critical care, burns, and emergency surgery at the University of Arizona Medical Center, Tucson, reported on results from a 6-year retrospective study at the annual meeting of the American Association for the Surgery of Trauma. The study evaluated 272 trauma patients in two centers in Tucson and Los Angeles County who required transfusions upon presentation in the emergency department.
“We did this because there is a lot of concern in animal and also human ex vivo laboratory type experiments [regarding] complications from coagulopathy and possibly even increasing the inflammatory processes. So we looked at those types of complications and found that there were no clinically significant complications we could identify in regard to coagulopathy or inflammatory processes with AT [autotransfusion],” Dr. Rhee said.
“But what we also found was that since getting a patient’s own blood back costs less, hospital costs would also be less.”
Patients who underwent AT vs. no AT received significantly less allogeneic packed red blood cells (10.3 vs. 12.1 units), platelets (5.2 vs. 7.9 units), and fresh frozen plasma (6.1 vs. 8.2 units) than patients who did not undergo transfusion.
The investigators reported that AT cost approximately $8,794 per patient vs. $10,7427 for allogeneic transfusions. Hospital costs ($42,156 vs. $43,963) were also lower without any appreciable difference in outcomes.
The trial population was split evenly between those receiving AT and those who did not. Demographics and injury characteristics were similar between the two groups.
“Autologous autotransfusion of blood collected through the chest tube was safe, is associated reduced allogeneic transfusion, and is associated with decreased hospital costs,” Dr. Rhee said. “I think this trial provides safety data for us to go on and do a larger prospective, multicenter study.”
Dr. Rhee reported having no relevant financial disclosures.
PHILADELPHIA – Early whole-blood autotransfusion of severely injured trauma victims’ own blood, while employed frequently in the military, has been rare in civilian populations, but a recent trial has found it to be safe, effective, and less costly than customary allogeneic transfusions.
Dr. Peter Rhee, chief of trauma, critical care, burns, and emergency surgery at the University of Arizona Medical Center, Tucson, reported on results from a 6-year retrospective study at the annual meeting of the American Association for the Surgery of Trauma. The study evaluated 272 trauma patients in two centers in Tucson and Los Angeles County who required transfusions upon presentation in the emergency department.
“We did this because there is a lot of concern in animal and also human ex vivo laboratory type experiments [regarding] complications from coagulopathy and possibly even increasing the inflammatory processes. So we looked at those types of complications and found that there were no clinically significant complications we could identify in regard to coagulopathy or inflammatory processes with AT [autotransfusion],” Dr. Rhee said.
“But what we also found was that since getting a patient’s own blood back costs less, hospital costs would also be less.”
Patients who underwent AT vs. no AT received significantly less allogeneic packed red blood cells (10.3 vs. 12.1 units), platelets (5.2 vs. 7.9 units), and fresh frozen plasma (6.1 vs. 8.2 units) than patients who did not undergo transfusion.
The investigators reported that AT cost approximately $8,794 per patient vs. $10,7427 for allogeneic transfusions. Hospital costs ($42,156 vs. $43,963) were also lower without any appreciable difference in outcomes.
The trial population was split evenly between those receiving AT and those who did not. Demographics and injury characteristics were similar between the two groups.
“Autologous autotransfusion of blood collected through the chest tube was safe, is associated reduced allogeneic transfusion, and is associated with decreased hospital costs,” Dr. Rhee said. “I think this trial provides safety data for us to go on and do a larger prospective, multicenter study.”
Dr. Rhee reported having no relevant financial disclosures.
Key clinical point: Autotransfusion appears to be safe and cost-effective in trauma patients.
Major finding: Patients who underwent autotransfusion (AT) vs. no AT received significantly less allogeneic packed red blood cells (10.3 vs. 12.1 units), platelets (5.2 vs. 7.9 units), and fresh frozen plasma (6.1 vs. 8.2 units) than patients who did not undergo transfusion. Therapy expenses ($8,794 vs. $10,427) and hospital costs ($42,156 vs. $43,963) were also lower without any appreciable difference in outcomes.
Data source: Six-year, multi-institutional, retrospective study of 272 trauma patients, evenly divided between receiving AT and not receiving AT.
Disclosures: Dr. Rhee reported having no relevant financial disclosures.
Same-day combined ERCP and cholecystectomy: achievable and cost effective
“Same-day procedures decreased length of stay by 2 days and led to an approximately $30,000 cost savings with no difference in conversion rates or complications between the two cohorts. The success rate of operative ERCP was 100%,” Dr. Jeffrey Wild, of Geisinger Health System in Northeastern Pennsylvania, reported at the annual meeting of the American Association for the Surgery of Trauma.
The Geisinger study validated the findings of two previous European studies (Endoscopy 2006;38:779-86; Am. Surg. 2013;79:1243-7). “These studies found decreased length of stay by 2 to 3 days, they found no difference in the incidence of retained stones, no difference in conversion rates to open cholecystectomy, and there was no difference in complications between the two groups,” Dr. Wild said.
The Geisinger investigators conducted the single-center, retrospective study of 240 patients from 2010 to 2014 comparing same-day and separate-day approaches for patients with choledocholithiasis and cholecystitis. In all, 65 patients had same-day procedures, with an average length of stay of 3 days vs. 5 days for patients who had ERCP and cholecystectomy on separate days, Dr. Wild said.
Like the European studies, the Geisinger experience found no statistical difference in conversion rates to open operation (12% for same-day vs. 14% for separate-day procedures) while the rate of discharge to a skilled nursing facility was half in the same-day cohort: 10% vs. 20% for separate-day patients, Dr. Wild said.
The goal of the Geisinger gallbladder pathway is to facilitate early operations in patients with cholecystitis. “Patients who present with cholecystitis should undergo cholecystectomy within 24 hours of presentation, if appropriate,” Dr. Wild said. “If there is evidence of biliary obstruction and the need for further work-up, our goal is to have gastroenterology work-up and management of the patient and have cholecystectomy done within the first 48 hours.”
The study noted some slight variations between the same-day and separate-day approaches, Dr. Wild said. The success rate of the endoscopist to cannulate the ampulla and perform ERCP was 95% in the same-day group and 100% in the separate-day cohort. ERCP was positive in identifying common bile duct stones or sludge in 97% of the same-day group vs. 91% in separate-day patients. More patients in the separate-day cohort required a second ERCP, usually 3 or 4 weeks after discharge and for removal of biliary stents, Dr. Wild said. Demographics in both groups were similar.
Operating room times varied between the two groups, and even within the same-day group depending on the setting for the ERCP, according to Dr. Wild. For patients in the separate-day group, average operative time was 1 hour, 42 minutes; same-day patients who had ERCP in the endoscopy suite and then transferred to the OR for cholecystectomy averaged 1 hour, 34 minutes; while the same-day cohort who had both ERCP and cholecystectomy done in the OR averaged 2 hours, 12 minutes.
Same-day care required coordination between different departments, Dr. Wild said. “Patients in the same-day group required coordination between the acute care surgical service, anesthesia, and gastroenterology to make sure both procedures could be performed under the same general anesthesia,” he said. The same-day group was almost evenly split between having ERCP in endoscopy before moving to the OR and having both done in the OR, Dr. Wild said.
“The findings of this study are, Number 1, intuitively obvious and easily predicted; and, Number 2, why didn’t I think of that myself?” said discussant Dr. Michael Chang of Wake Forest University Baptist Medical Center, Winston-Salem, N.C.
Dr. Chang also noted that the study provides an example of how to restructure care organizations. “Grouping practitioners by disease process, as opposed to what board they’re certified by or what department they live in, is thought to be a more patient-centered approach to provide cost-effective care,” he said.
Dr. Chang and others asked how the Geisinger surgeons overcame institutional barriers in creating their care model. “Most institutions are still dependent on the gastroenterologists, and lining up that procedure with another service can be difficult,” Dr. Donald Reed Jr. of Lutheran Medical Group in Fort Wayne, Ind., noted.
Dr. Wild acknowledged at first the pathway encountered some resistance. “But what really started this process was that the endoscopy suite was closed on weekends and all the ERCPs were performed in OR,” he said. “And then we were taking patients the following morning back to the OR to take out their gallbladder. Some of my senior partners questioned why aren’t we doing these at the same time.”
At this point, gastroenterology is “very willing” to embrace ERCP in the OR before gallbladder removal, Dr. Wild said.
Dr. Wild reported having no relevant financial disclosures.
“Same-day procedures decreased length of stay by 2 days and led to an approximately $30,000 cost savings with no difference in conversion rates or complications between the two cohorts. The success rate of operative ERCP was 100%,” Dr. Jeffrey Wild, of Geisinger Health System in Northeastern Pennsylvania, reported at the annual meeting of the American Association for the Surgery of Trauma.
The Geisinger study validated the findings of two previous European studies (Endoscopy 2006;38:779-86; Am. Surg. 2013;79:1243-7). “These studies found decreased length of stay by 2 to 3 days, they found no difference in the incidence of retained stones, no difference in conversion rates to open cholecystectomy, and there was no difference in complications between the two groups,” Dr. Wild said.
The Geisinger investigators conducted the single-center, retrospective study of 240 patients from 2010 to 2014 comparing same-day and separate-day approaches for patients with choledocholithiasis and cholecystitis. In all, 65 patients had same-day procedures, with an average length of stay of 3 days vs. 5 days for patients who had ERCP and cholecystectomy on separate days, Dr. Wild said.
Like the European studies, the Geisinger experience found no statistical difference in conversion rates to open operation (12% for same-day vs. 14% for separate-day procedures) while the rate of discharge to a skilled nursing facility was half in the same-day cohort: 10% vs. 20% for separate-day patients, Dr. Wild said.
The goal of the Geisinger gallbladder pathway is to facilitate early operations in patients with cholecystitis. “Patients who present with cholecystitis should undergo cholecystectomy within 24 hours of presentation, if appropriate,” Dr. Wild said. “If there is evidence of biliary obstruction and the need for further work-up, our goal is to have gastroenterology work-up and management of the patient and have cholecystectomy done within the first 48 hours.”
The study noted some slight variations between the same-day and separate-day approaches, Dr. Wild said. The success rate of the endoscopist to cannulate the ampulla and perform ERCP was 95% in the same-day group and 100% in the separate-day cohort. ERCP was positive in identifying common bile duct stones or sludge in 97% of the same-day group vs. 91% in separate-day patients. More patients in the separate-day cohort required a second ERCP, usually 3 or 4 weeks after discharge and for removal of biliary stents, Dr. Wild said. Demographics in both groups were similar.
Operating room times varied between the two groups, and even within the same-day group depending on the setting for the ERCP, according to Dr. Wild. For patients in the separate-day group, average operative time was 1 hour, 42 minutes; same-day patients who had ERCP in the endoscopy suite and then transferred to the OR for cholecystectomy averaged 1 hour, 34 minutes; while the same-day cohort who had both ERCP and cholecystectomy done in the OR averaged 2 hours, 12 minutes.
Same-day care required coordination between different departments, Dr. Wild said. “Patients in the same-day group required coordination between the acute care surgical service, anesthesia, and gastroenterology to make sure both procedures could be performed under the same general anesthesia,” he said. The same-day group was almost evenly split between having ERCP in endoscopy before moving to the OR and having both done in the OR, Dr. Wild said.
“The findings of this study are, Number 1, intuitively obvious and easily predicted; and, Number 2, why didn’t I think of that myself?” said discussant Dr. Michael Chang of Wake Forest University Baptist Medical Center, Winston-Salem, N.C.
Dr. Chang also noted that the study provides an example of how to restructure care organizations. “Grouping practitioners by disease process, as opposed to what board they’re certified by or what department they live in, is thought to be a more patient-centered approach to provide cost-effective care,” he said.
Dr. Chang and others asked how the Geisinger surgeons overcame institutional barriers in creating their care model. “Most institutions are still dependent on the gastroenterologists, and lining up that procedure with another service can be difficult,” Dr. Donald Reed Jr. of Lutheran Medical Group in Fort Wayne, Ind., noted.
Dr. Wild acknowledged at first the pathway encountered some resistance. “But what really started this process was that the endoscopy suite was closed on weekends and all the ERCPs were performed in OR,” he said. “And then we were taking patients the following morning back to the OR to take out their gallbladder. Some of my senior partners questioned why aren’t we doing these at the same time.”
At this point, gastroenterology is “very willing” to embrace ERCP in the OR before gallbladder removal, Dr. Wild said.
Dr. Wild reported having no relevant financial disclosures.
“Same-day procedures decreased length of stay by 2 days and led to an approximately $30,000 cost savings with no difference in conversion rates or complications between the two cohorts. The success rate of operative ERCP was 100%,” Dr. Jeffrey Wild, of Geisinger Health System in Northeastern Pennsylvania, reported at the annual meeting of the American Association for the Surgery of Trauma.
The Geisinger study validated the findings of two previous European studies (Endoscopy 2006;38:779-86; Am. Surg. 2013;79:1243-7). “These studies found decreased length of stay by 2 to 3 days, they found no difference in the incidence of retained stones, no difference in conversion rates to open cholecystectomy, and there was no difference in complications between the two groups,” Dr. Wild said.
The Geisinger investigators conducted the single-center, retrospective study of 240 patients from 2010 to 2014 comparing same-day and separate-day approaches for patients with choledocholithiasis and cholecystitis. In all, 65 patients had same-day procedures, with an average length of stay of 3 days vs. 5 days for patients who had ERCP and cholecystectomy on separate days, Dr. Wild said.
Like the European studies, the Geisinger experience found no statistical difference in conversion rates to open operation (12% for same-day vs. 14% for separate-day procedures) while the rate of discharge to a skilled nursing facility was half in the same-day cohort: 10% vs. 20% for separate-day patients, Dr. Wild said.
The goal of the Geisinger gallbladder pathway is to facilitate early operations in patients with cholecystitis. “Patients who present with cholecystitis should undergo cholecystectomy within 24 hours of presentation, if appropriate,” Dr. Wild said. “If there is evidence of biliary obstruction and the need for further work-up, our goal is to have gastroenterology work-up and management of the patient and have cholecystectomy done within the first 48 hours.”
The study noted some slight variations between the same-day and separate-day approaches, Dr. Wild said. The success rate of the endoscopist to cannulate the ampulla and perform ERCP was 95% in the same-day group and 100% in the separate-day cohort. ERCP was positive in identifying common bile duct stones or sludge in 97% of the same-day group vs. 91% in separate-day patients. More patients in the separate-day cohort required a second ERCP, usually 3 or 4 weeks after discharge and for removal of biliary stents, Dr. Wild said. Demographics in both groups were similar.
Operating room times varied between the two groups, and even within the same-day group depending on the setting for the ERCP, according to Dr. Wild. For patients in the separate-day group, average operative time was 1 hour, 42 minutes; same-day patients who had ERCP in the endoscopy suite and then transferred to the OR for cholecystectomy averaged 1 hour, 34 minutes; while the same-day cohort who had both ERCP and cholecystectomy done in the OR averaged 2 hours, 12 minutes.
Same-day care required coordination between different departments, Dr. Wild said. “Patients in the same-day group required coordination between the acute care surgical service, anesthesia, and gastroenterology to make sure both procedures could be performed under the same general anesthesia,” he said. The same-day group was almost evenly split between having ERCP in endoscopy before moving to the OR and having both done in the OR, Dr. Wild said.
“The findings of this study are, Number 1, intuitively obvious and easily predicted; and, Number 2, why didn’t I think of that myself?” said discussant Dr. Michael Chang of Wake Forest University Baptist Medical Center, Winston-Salem, N.C.
Dr. Chang also noted that the study provides an example of how to restructure care organizations. “Grouping practitioners by disease process, as opposed to what board they’re certified by or what department they live in, is thought to be a more patient-centered approach to provide cost-effective care,” he said.
Dr. Chang and others asked how the Geisinger surgeons overcame institutional barriers in creating their care model. “Most institutions are still dependent on the gastroenterologists, and lining up that procedure with another service can be difficult,” Dr. Donald Reed Jr. of Lutheran Medical Group in Fort Wayne, Ind., noted.
Dr. Wild acknowledged at first the pathway encountered some resistance. “But what really started this process was that the endoscopy suite was closed on weekends and all the ERCPs were performed in OR,” he said. “And then we were taking patients the following morning back to the OR to take out their gallbladder. Some of my senior partners questioned why aren’t we doing these at the same time.”
At this point, gastroenterology is “very willing” to embrace ERCP in the OR before gallbladder removal, Dr. Wild said.
Dr. Wild reported having no relevant financial disclosures.
FROM THE AAST ANNUAL MEETING
Key clinical point: Scheduling both ERCP and cholecystectomy on the same day reduces hospital stays and saves money.
Major finding: Patients who had preoperative endoscopic retrograde cholangiopancreatography (ERCP) on the same day as cholecystectomy had 3-day hospital stays compared to 5 days for patients who had the procedures on separate days.
Data source: Single-center retrospective study of 240 patients from 2010 to 2014.
Disclosures: Dr. Wild reported having no relevant financial disclosures.
Protocol for small-bowel obstruction
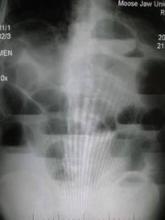
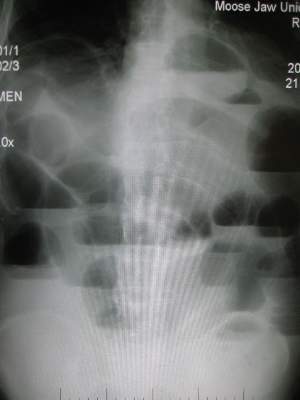
PHILADELPHIA – Closely monitoring patients admitted for small bowel obstruction every 4 hours and starting them on intravenous fluids, bowel rest, and nasogastric tube decompression may aid in quickly differentiating partial and complete SBO and direct them into targeted treatment earlier, according to investigators at the University of Florida Health, Gainesville.
“Despite the prevalence of small bowel obstruction and the fact that we’ve been dealing with it for more than a century, we still do not have a general consensus on how to differentiate between partial bowel obstruction and complete obstruction,” Dr. Janeen Jordan said at the annual meeting of the American Association for the Surgery of Trauma. Dr. Jordan reported on a study involving 91 patients admitted for SBO over 1 year at the University of Florida Health.
Early differentiation of partial vs. complete bowel obstruction and early intervention were goals of the protocol, Dr. Jordan said. “We really wanted to get them to surgery within 3 days with hopes of decreasing our bowel resection rate, hospital length of stay and, of course, mortality,” she said.
She outlined the protocol her institution used in symptomatic patients with x-ray findings positive for SBO: admission for intravenous fluid resuscitation, bowel rest, nasogastric tube decompression, and exams every 4 hours. Once stabilized, patients received a CT scan with only intravenous contrast to confirm the diagnosis. “If there was any suggestion of bowel compromise such as pneumatosis, mesenteric edema, or suggestion of a closed-loop obstruction, they were evaluated immediately for operation,” Dr. Jordan said. “If patients had none of those findings or had fecalization, which suggests a chronic problem instead of an acute issue, those patients were admitted for bowel decompression and IV fluid resuscitation.”
After resuscitation, patients with peritonitis or CT imaging positive for bowel compromise had exploratory surgery. All other patients received an osmotically active, water-soluble contrast agent and diagnostic imaging at 4, 8, 12 and 24 hours. If contrast did not reach the colon in 24 hours, the patient underwent exploratory surgery.
Twenty-six patients went directly to the OR without entering the protocol, 58% of whom required bowel resection, Dr. Jordan said. Among the 75 patients in the protocol, 43% required surgery. The average time to surgery was within 1 day for those not on the protocol and 2 days for those managed with contrast. Demographics between the two groups were similar, although the nonprotocol patients were more likely to have bowel wall thickening on CT scan.
The analysis also compared patients who received contrast and those who did not, and further broke that down into patients who had surgery vs. nonoperative management in each group. “Giving them contrast didn’t increase hospital length of stay,” Dr. Jordan said. “And the patients who didn’t get surgery had an overall hospital length of stay of 3-4 days; if they had surgery, regardless whether or not they received contrast, their hospital length of stay was approximately 10 days.” Additionally, the length of time for the contrast to reach the colon was predictive of who would fail at eating or drinking by mouth or have recurrent symptoms before discharge.
Discussant Dr. Clay Cothren Burlew of Denver Health said she found the concept of a protocol for patients with symptoms of SBO “incredibly appealing.” However, she added, “Patients without overt clinical signs of peritonitis mandating operative intervention are often fraught with challenges. Questions of the timing of intervention to bring about resolution have never really been met. It’s a gray zone in surgery.”
Dr. Martin Schreiber of Oregon Health & Science University, Portland, questioned the cost involved in imaging without contrast. “Instead of doing a CT scan without enteral contrast and then doing an upper GI, why not do the CT with enteral contrast and then do your follow-up x-rays to see if contrast reaches the colon? Skip a step and save lots of money.”
Dr. Ronald Maier of the University of Washington, Seattle, pointed out the protocol seeks to rule out surgery for a patient population that’s unlikely to have surgery anyway. “We know, as many studies have presented, 85% of these people – if you never operate on them – go home without an operation and they do fine,” he said. “So you’re hunting for the 15%, and yet in your protocol you operate on nearly half of them.”
Previously published studies that linked interventions after 3 days in the hospital with higher bowel resection and mortality determined the timing of surgery in the protocol, Dr. Jordan said. However, she admitted that the rate of surgery was lower at the end of the study period than at the beginning.
Dr. Jordan reported having no financial disclosures.
PHILADELPHIA – Closely monitoring patients admitted for small bowel obstruction every 4 hours and starting them on intravenous fluids, bowel rest, and nasogastric tube decompression may aid in quickly differentiating partial and complete SBO and direct them into targeted treatment earlier, according to investigators at the University of Florida Health, Gainesville.
“Despite the prevalence of small bowel obstruction and the fact that we’ve been dealing with it for more than a century, we still do not have a general consensus on how to differentiate between partial bowel obstruction and complete obstruction,” Dr. Janeen Jordan said at the annual meeting of the American Association for the Surgery of Trauma. Dr. Jordan reported on a study involving 91 patients admitted for SBO over 1 year at the University of Florida Health.
Early differentiation of partial vs. complete bowel obstruction and early intervention were goals of the protocol, Dr. Jordan said. “We really wanted to get them to surgery within 3 days with hopes of decreasing our bowel resection rate, hospital length of stay and, of course, mortality,” she said.
She outlined the protocol her institution used in symptomatic patients with x-ray findings positive for SBO: admission for intravenous fluid resuscitation, bowel rest, nasogastric tube decompression, and exams every 4 hours. Once stabilized, patients received a CT scan with only intravenous contrast to confirm the diagnosis. “If there was any suggestion of bowel compromise such as pneumatosis, mesenteric edema, or suggestion of a closed-loop obstruction, they were evaluated immediately for operation,” Dr. Jordan said. “If patients had none of those findings or had fecalization, which suggests a chronic problem instead of an acute issue, those patients were admitted for bowel decompression and IV fluid resuscitation.”
After resuscitation, patients with peritonitis or CT imaging positive for bowel compromise had exploratory surgery. All other patients received an osmotically active, water-soluble contrast agent and diagnostic imaging at 4, 8, 12 and 24 hours. If contrast did not reach the colon in 24 hours, the patient underwent exploratory surgery.
Twenty-six patients went directly to the OR without entering the protocol, 58% of whom required bowel resection, Dr. Jordan said. Among the 75 patients in the protocol, 43% required surgery. The average time to surgery was within 1 day for those not on the protocol and 2 days for those managed with contrast. Demographics between the two groups were similar, although the nonprotocol patients were more likely to have bowel wall thickening on CT scan.
The analysis also compared patients who received contrast and those who did not, and further broke that down into patients who had surgery vs. nonoperative management in each group. “Giving them contrast didn’t increase hospital length of stay,” Dr. Jordan said. “And the patients who didn’t get surgery had an overall hospital length of stay of 3-4 days; if they had surgery, regardless whether or not they received contrast, their hospital length of stay was approximately 10 days.” Additionally, the length of time for the contrast to reach the colon was predictive of who would fail at eating or drinking by mouth or have recurrent symptoms before discharge.
Discussant Dr. Clay Cothren Burlew of Denver Health said she found the concept of a protocol for patients with symptoms of SBO “incredibly appealing.” However, she added, “Patients without overt clinical signs of peritonitis mandating operative intervention are often fraught with challenges. Questions of the timing of intervention to bring about resolution have never really been met. It’s a gray zone in surgery.”
Dr. Martin Schreiber of Oregon Health & Science University, Portland, questioned the cost involved in imaging without contrast. “Instead of doing a CT scan without enteral contrast and then doing an upper GI, why not do the CT with enteral contrast and then do your follow-up x-rays to see if contrast reaches the colon? Skip a step and save lots of money.”
Dr. Ronald Maier of the University of Washington, Seattle, pointed out the protocol seeks to rule out surgery for a patient population that’s unlikely to have surgery anyway. “We know, as many studies have presented, 85% of these people – if you never operate on them – go home without an operation and they do fine,” he said. “So you’re hunting for the 15%, and yet in your protocol you operate on nearly half of them.”
Previously published studies that linked interventions after 3 days in the hospital with higher bowel resection and mortality determined the timing of surgery in the protocol, Dr. Jordan said. However, she admitted that the rate of surgery was lower at the end of the study period than at the beginning.
Dr. Jordan reported having no financial disclosures.
PHILADELPHIA – Closely monitoring patients admitted for small bowel obstruction every 4 hours and starting them on intravenous fluids, bowel rest, and nasogastric tube decompression may aid in quickly differentiating partial and complete SBO and direct them into targeted treatment earlier, according to investigators at the University of Florida Health, Gainesville.
“Despite the prevalence of small bowel obstruction and the fact that we’ve been dealing with it for more than a century, we still do not have a general consensus on how to differentiate between partial bowel obstruction and complete obstruction,” Dr. Janeen Jordan said at the annual meeting of the American Association for the Surgery of Trauma. Dr. Jordan reported on a study involving 91 patients admitted for SBO over 1 year at the University of Florida Health.
Early differentiation of partial vs. complete bowel obstruction and early intervention were goals of the protocol, Dr. Jordan said. “We really wanted to get them to surgery within 3 days with hopes of decreasing our bowel resection rate, hospital length of stay and, of course, mortality,” she said.
She outlined the protocol her institution used in symptomatic patients with x-ray findings positive for SBO: admission for intravenous fluid resuscitation, bowel rest, nasogastric tube decompression, and exams every 4 hours. Once stabilized, patients received a CT scan with only intravenous contrast to confirm the diagnosis. “If there was any suggestion of bowel compromise such as pneumatosis, mesenteric edema, or suggestion of a closed-loop obstruction, they were evaluated immediately for operation,” Dr. Jordan said. “If patients had none of those findings or had fecalization, which suggests a chronic problem instead of an acute issue, those patients were admitted for bowel decompression and IV fluid resuscitation.”
After resuscitation, patients with peritonitis or CT imaging positive for bowel compromise had exploratory surgery. All other patients received an osmotically active, water-soluble contrast agent and diagnostic imaging at 4, 8, 12 and 24 hours. If contrast did not reach the colon in 24 hours, the patient underwent exploratory surgery.
Twenty-six patients went directly to the OR without entering the protocol, 58% of whom required bowel resection, Dr. Jordan said. Among the 75 patients in the protocol, 43% required surgery. The average time to surgery was within 1 day for those not on the protocol and 2 days for those managed with contrast. Demographics between the two groups were similar, although the nonprotocol patients were more likely to have bowel wall thickening on CT scan.
The analysis also compared patients who received contrast and those who did not, and further broke that down into patients who had surgery vs. nonoperative management in each group. “Giving them contrast didn’t increase hospital length of stay,” Dr. Jordan said. “And the patients who didn’t get surgery had an overall hospital length of stay of 3-4 days; if they had surgery, regardless whether or not they received contrast, their hospital length of stay was approximately 10 days.” Additionally, the length of time for the contrast to reach the colon was predictive of who would fail at eating or drinking by mouth or have recurrent symptoms before discharge.
Discussant Dr. Clay Cothren Burlew of Denver Health said she found the concept of a protocol for patients with symptoms of SBO “incredibly appealing.” However, she added, “Patients without overt clinical signs of peritonitis mandating operative intervention are often fraught with challenges. Questions of the timing of intervention to bring about resolution have never really been met. It’s a gray zone in surgery.”
Dr. Martin Schreiber of Oregon Health & Science University, Portland, questioned the cost involved in imaging without contrast. “Instead of doing a CT scan without enteral contrast and then doing an upper GI, why not do the CT with enteral contrast and then do your follow-up x-rays to see if contrast reaches the colon? Skip a step and save lots of money.”
Dr. Ronald Maier of the University of Washington, Seattle, pointed out the protocol seeks to rule out surgery for a patient population that’s unlikely to have surgery anyway. “We know, as many studies have presented, 85% of these people – if you never operate on them – go home without an operation and they do fine,” he said. “So you’re hunting for the 15%, and yet in your protocol you operate on nearly half of them.”
Previously published studies that linked interventions after 3 days in the hospital with higher bowel resection and mortality determined the timing of surgery in the protocol, Dr. Jordan said. However, she admitted that the rate of surgery was lower at the end of the study period than at the beginning.
Dr. Jordan reported having no financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point: Protocol clarifies targeted treatment in SBO management.
Major finding: A quarter of patients with small bowel obstruction went directly to surgery based on imaging or exam findings, half of whom required bowel resection. The remainder were managed on a protocol to differentiate partial and complete bowl obstruction, 45% of whom needed surgery. Those in the protocol went to surgery within 2 days vs. 1 day for those not in the protocol.
Data source: Single-institution experience involved 101 admissions admitted for SBO over 1 year.
Disclosures: Dr. Jordan reported having no financial disclosures.
Congress poised to act on 2015 meaningful use full year reporting requirement
WASHINGTON – Members of Congress are moving to push legislation through that would shorten the reporting period for stage 2 meaningful use in 2015 if the Centers for Medicare & Medicaid Services does not act.
At issue is the requirement that eligible physicians, hospitals, and other providers must attest that they meet stage 2 requirements for the full 365-day period in 2015 (beginning Oct. 1, 2014, for hospitals and Jan 1, 2015, for physicians) or face a 1% reduction in Medicare payments as a penalty for not adopting the stage 2 requirements.
“Only 9% of hospitals in this country right now are up to the [stage 2] mandate on meaningful use,” Rep. Renee Ellmers (R-N.C.) said Sept. 18 at the HIMSS Policy Summit. “There’s only 1% of physician offices in this country that are up to the meaningful use mandate.”
Rep. Ellmers and Rep. Jim Matheson (D-Utah), have introduced the Flexibility in Health IT Reporting Act (H.R. 5481), which would alter those reporting requirements so eligible providers and hospitals would have to attest that they met stage 2 requirements for a 3-month period for 2015 rather than meeting the current regulations, which call for a full year. This would give doctors and hospitals extra time to ensure electronic health record systems are properly upgraded for the next level of requirements.
“My hope is that CMS is going to hear from so many of you, so many from industry that they will actually end up making the change on their own without us actually having to vote on it,” Rep. Ellmers said. “If we do have to vote on it, it will be when we come back in the lame-duck session. I don’t want to have to wait that long.”
She suggested that hospitals could question whether it is worth it to press for stage 2 attestation or just take the 1% penalty.
“There will already have been $24 billion of hard-earned taxpayer dollars that have been invested in this,” she said. “That is going by the wayside if hospitals are not participating in meaningful use,”
Robert Tennant, senior policy adviser at the Medical Group Management Association, made a similar observation about the physician community. He noted that physicians who are eligible to receive incentive bonuses under the meaningful use program and have been participating since the program began in 2011 have already received $38,000 of the $44,000 maximum bonus.
“To move to stage 2 will only allow you to receive a maximum of $6,000, so the money is somewhat less now and so what we don’t want to do is add further discouragement for [eligible providers] to move to stage 2 of the program,” Mr. Tennant said in an interview.
The American Medical Association voiced its support for the bill in a letter to Rep. Ellmers.
At a separate press conference Sept. 18 as part of National Health IT Week, Rep. Phil Gingrey (R-Ga.), also voiced his support for the bill.
WASHINGTON – Members of Congress are moving to push legislation through that would shorten the reporting period for stage 2 meaningful use in 2015 if the Centers for Medicare & Medicaid Services does not act.
At issue is the requirement that eligible physicians, hospitals, and other providers must attest that they meet stage 2 requirements for the full 365-day period in 2015 (beginning Oct. 1, 2014, for hospitals and Jan 1, 2015, for physicians) or face a 1% reduction in Medicare payments as a penalty for not adopting the stage 2 requirements.
“Only 9% of hospitals in this country right now are up to the [stage 2] mandate on meaningful use,” Rep. Renee Ellmers (R-N.C.) said Sept. 18 at the HIMSS Policy Summit. “There’s only 1% of physician offices in this country that are up to the meaningful use mandate.”
Rep. Ellmers and Rep. Jim Matheson (D-Utah), have introduced the Flexibility in Health IT Reporting Act (H.R. 5481), which would alter those reporting requirements so eligible providers and hospitals would have to attest that they met stage 2 requirements for a 3-month period for 2015 rather than meeting the current regulations, which call for a full year. This would give doctors and hospitals extra time to ensure electronic health record systems are properly upgraded for the next level of requirements.
“My hope is that CMS is going to hear from so many of you, so many from industry that they will actually end up making the change on their own without us actually having to vote on it,” Rep. Ellmers said. “If we do have to vote on it, it will be when we come back in the lame-duck session. I don’t want to have to wait that long.”
She suggested that hospitals could question whether it is worth it to press for stage 2 attestation or just take the 1% penalty.
“There will already have been $24 billion of hard-earned taxpayer dollars that have been invested in this,” she said. “That is going by the wayside if hospitals are not participating in meaningful use,”
Robert Tennant, senior policy adviser at the Medical Group Management Association, made a similar observation about the physician community. He noted that physicians who are eligible to receive incentive bonuses under the meaningful use program and have been participating since the program began in 2011 have already received $38,000 of the $44,000 maximum bonus.
“To move to stage 2 will only allow you to receive a maximum of $6,000, so the money is somewhat less now and so what we don’t want to do is add further discouragement for [eligible providers] to move to stage 2 of the program,” Mr. Tennant said in an interview.
The American Medical Association voiced its support for the bill in a letter to Rep. Ellmers.
At a separate press conference Sept. 18 as part of National Health IT Week, Rep. Phil Gingrey (R-Ga.), also voiced his support for the bill.
WASHINGTON – Members of Congress are moving to push legislation through that would shorten the reporting period for stage 2 meaningful use in 2015 if the Centers for Medicare & Medicaid Services does not act.
At issue is the requirement that eligible physicians, hospitals, and other providers must attest that they meet stage 2 requirements for the full 365-day period in 2015 (beginning Oct. 1, 2014, for hospitals and Jan 1, 2015, for physicians) or face a 1% reduction in Medicare payments as a penalty for not adopting the stage 2 requirements.
“Only 9% of hospitals in this country right now are up to the [stage 2] mandate on meaningful use,” Rep. Renee Ellmers (R-N.C.) said Sept. 18 at the HIMSS Policy Summit. “There’s only 1% of physician offices in this country that are up to the meaningful use mandate.”
Rep. Ellmers and Rep. Jim Matheson (D-Utah), have introduced the Flexibility in Health IT Reporting Act (H.R. 5481), which would alter those reporting requirements so eligible providers and hospitals would have to attest that they met stage 2 requirements for a 3-month period for 2015 rather than meeting the current regulations, which call for a full year. This would give doctors and hospitals extra time to ensure electronic health record systems are properly upgraded for the next level of requirements.
“My hope is that CMS is going to hear from so many of you, so many from industry that they will actually end up making the change on their own without us actually having to vote on it,” Rep. Ellmers said. “If we do have to vote on it, it will be when we come back in the lame-duck session. I don’t want to have to wait that long.”
She suggested that hospitals could question whether it is worth it to press for stage 2 attestation or just take the 1% penalty.
“There will already have been $24 billion of hard-earned taxpayer dollars that have been invested in this,” she said. “That is going by the wayside if hospitals are not participating in meaningful use,”
Robert Tennant, senior policy adviser at the Medical Group Management Association, made a similar observation about the physician community. He noted that physicians who are eligible to receive incentive bonuses under the meaningful use program and have been participating since the program began in 2011 have already received $38,000 of the $44,000 maximum bonus.
“To move to stage 2 will only allow you to receive a maximum of $6,000, so the money is somewhat less now and so what we don’t want to do is add further discouragement for [eligible providers] to move to stage 2 of the program,” Mr. Tennant said in an interview.
The American Medical Association voiced its support for the bill in a letter to Rep. Ellmers.
At a separate press conference Sept. 18 as part of National Health IT Week, Rep. Phil Gingrey (R-Ga.), also voiced his support for the bill.
CTA before visceral arteriography improves bleed identification, localization
PHILADELPHIA – A protocol that employed CT angiography instead of or along with nuclear bleeding scans before visceral arteriography in patients with lower gastrointestinal hemorrhage helped localize bleeds more accurately and reduced the need for subsequent imaging studies, compared with VA alone, a single-center study found.
“CTA rather than a nuclear bleeding scan [NBS] prior to visceral arteriography is associated with fewer imaging studies but better localization of bleeding; if patients do receive more contrast, we did not observe any change in the renal function as a result,” Dr. Christina Jacovides of the University of Pennsylvania, Philadelphia, reported at the annual meeting of the American Association for the Surgery of Trauma.
The study evaluated 161 patients over an 8-year observation period: 78 from 2005 to 2009, before the protocol was implemented; and 83 under the protocol.
“We saw that this protocol effectively changed the pre-VA approach from bleeding scans to CTA,” Dr. Jacovides said.
Lower gastrointestinal hemorrhage carries “substantial” morbidity, in the words of the study authors, so developing tools that can locate the bleeding and target treatment is critical in reducing deaths and complications, Dr. Jacovides said. “Bleeding scans have been demonstrated to be highly sensitive but have had poor localization, whereas CTA has also been demonstrated to be highly sensitive and provides localization similar to that seen with VA,” she said.
In the study, obtaining visceral arteriography without any imaging beforehand identified bleeding in 62% of cases, versus 94% with pre-VA imaging consisting of either CTA, NBS, or both. CTA only before VA resulted in more imaging studies than VA alone (2.5 vs. 1.3), but was also more than twice as likely to find bleeding (92% vs. 43%). Bleed localization rates were similar between VA only and pre-VA CTA.
When compared to NBS, CTA only before VA resulted in fewer imaging studies (2.1 vs. 2.5 for NBS) and similar rates of finding bleeding (around 94% for both), but significantly higher rates of bleed localization on VA (45.7% for CTA vs. 26.4% for NBS), according to the study findings. Embolization rates among the different protocols also varied, from 23% for NBS to 40% for CTA only before VA. The study did not evaluate costs.
How the university’s protocol affected renal function drew the attention of discussant Dr. Leslie Kobayashi,of the University of California, San Diego, who noted that creatinine levels were actually higher in the VA-only group. “This would suggest to me that patient factors such as severity of hemorrhage, presence of shock, and location of comorbidities are most associated with increases in creatinine rather than the actual contrast bolus or contrast dose,” she said. However, the study did not include a multivariate analysis to determine if CT contrast was associated with a rise in creatinine, Dr. Jacovides said.
Dr. Hasan Alam of the University of Michigan Health System, Ann Arbor, acknowledged that CTA before VA may be viable in hemodynamically stable patients, but not so for those with more critical injuries. “The problem is sometimes logistics, because you have to go to two different places for the CTA and VA, so a time factor is involved,” he said. “So if you have a sick patient who is robustly or briskly bleeding, who’s hypotensive, for those 5%-10% of patients, that causes a delay.”
Dr. Jacovides noted that at least among the patients who received pre-VA imaging, the protocol at the University of Pennsylvania actually got those patients to treatment quicker. “We did find that among patients who underwent CTA only instead of NBS, the time from the first scan to first VA was significantly reduced; it was about 1,200 minutes on bleeding scans and about 530 minutes with CTA on average,” she said.
Dr. Jacovides reported having no relevant financial disclosures. She is a surgical intern at Thomas Jefferson University Hospital, Philadelphia, and completed this research as a medical student at the University of Pennsylvania, where the senior authors are in the division of traumatology, surgical critical care, and emergency surgery.
PHILADELPHIA – A protocol that employed CT angiography instead of or along with nuclear bleeding scans before visceral arteriography in patients with lower gastrointestinal hemorrhage helped localize bleeds more accurately and reduced the need for subsequent imaging studies, compared with VA alone, a single-center study found.
“CTA rather than a nuclear bleeding scan [NBS] prior to visceral arteriography is associated with fewer imaging studies but better localization of bleeding; if patients do receive more contrast, we did not observe any change in the renal function as a result,” Dr. Christina Jacovides of the University of Pennsylvania, Philadelphia, reported at the annual meeting of the American Association for the Surgery of Trauma.
The study evaluated 161 patients over an 8-year observation period: 78 from 2005 to 2009, before the protocol was implemented; and 83 under the protocol.
“We saw that this protocol effectively changed the pre-VA approach from bleeding scans to CTA,” Dr. Jacovides said.
Lower gastrointestinal hemorrhage carries “substantial” morbidity, in the words of the study authors, so developing tools that can locate the bleeding and target treatment is critical in reducing deaths and complications, Dr. Jacovides said. “Bleeding scans have been demonstrated to be highly sensitive but have had poor localization, whereas CTA has also been demonstrated to be highly sensitive and provides localization similar to that seen with VA,” she said.
In the study, obtaining visceral arteriography without any imaging beforehand identified bleeding in 62% of cases, versus 94% with pre-VA imaging consisting of either CTA, NBS, or both. CTA only before VA resulted in more imaging studies than VA alone (2.5 vs. 1.3), but was also more than twice as likely to find bleeding (92% vs. 43%). Bleed localization rates were similar between VA only and pre-VA CTA.
When compared to NBS, CTA only before VA resulted in fewer imaging studies (2.1 vs. 2.5 for NBS) and similar rates of finding bleeding (around 94% for both), but significantly higher rates of bleed localization on VA (45.7% for CTA vs. 26.4% for NBS), according to the study findings. Embolization rates among the different protocols also varied, from 23% for NBS to 40% for CTA only before VA. The study did not evaluate costs.
How the university’s protocol affected renal function drew the attention of discussant Dr. Leslie Kobayashi,of the University of California, San Diego, who noted that creatinine levels were actually higher in the VA-only group. “This would suggest to me that patient factors such as severity of hemorrhage, presence of shock, and location of comorbidities are most associated with increases in creatinine rather than the actual contrast bolus or contrast dose,” she said. However, the study did not include a multivariate analysis to determine if CT contrast was associated with a rise in creatinine, Dr. Jacovides said.
Dr. Hasan Alam of the University of Michigan Health System, Ann Arbor, acknowledged that CTA before VA may be viable in hemodynamically stable patients, but not so for those with more critical injuries. “The problem is sometimes logistics, because you have to go to two different places for the CTA and VA, so a time factor is involved,” he said. “So if you have a sick patient who is robustly or briskly bleeding, who’s hypotensive, for those 5%-10% of patients, that causes a delay.”
Dr. Jacovides noted that at least among the patients who received pre-VA imaging, the protocol at the University of Pennsylvania actually got those patients to treatment quicker. “We did find that among patients who underwent CTA only instead of NBS, the time from the first scan to first VA was significantly reduced; it was about 1,200 minutes on bleeding scans and about 530 minutes with CTA on average,” she said.
Dr. Jacovides reported having no relevant financial disclosures. She is a surgical intern at Thomas Jefferson University Hospital, Philadelphia, and completed this research as a medical student at the University of Pennsylvania, where the senior authors are in the division of traumatology, surgical critical care, and emergency surgery.
PHILADELPHIA – A protocol that employed CT angiography instead of or along with nuclear bleeding scans before visceral arteriography in patients with lower gastrointestinal hemorrhage helped localize bleeds more accurately and reduced the need for subsequent imaging studies, compared with VA alone, a single-center study found.
“CTA rather than a nuclear bleeding scan [NBS] prior to visceral arteriography is associated with fewer imaging studies but better localization of bleeding; if patients do receive more contrast, we did not observe any change in the renal function as a result,” Dr. Christina Jacovides of the University of Pennsylvania, Philadelphia, reported at the annual meeting of the American Association for the Surgery of Trauma.
The study evaluated 161 patients over an 8-year observation period: 78 from 2005 to 2009, before the protocol was implemented; and 83 under the protocol.
“We saw that this protocol effectively changed the pre-VA approach from bleeding scans to CTA,” Dr. Jacovides said.
Lower gastrointestinal hemorrhage carries “substantial” morbidity, in the words of the study authors, so developing tools that can locate the bleeding and target treatment is critical in reducing deaths and complications, Dr. Jacovides said. “Bleeding scans have been demonstrated to be highly sensitive but have had poor localization, whereas CTA has also been demonstrated to be highly sensitive and provides localization similar to that seen with VA,” she said.
In the study, obtaining visceral arteriography without any imaging beforehand identified bleeding in 62% of cases, versus 94% with pre-VA imaging consisting of either CTA, NBS, or both. CTA only before VA resulted in more imaging studies than VA alone (2.5 vs. 1.3), but was also more than twice as likely to find bleeding (92% vs. 43%). Bleed localization rates were similar between VA only and pre-VA CTA.
When compared to NBS, CTA only before VA resulted in fewer imaging studies (2.1 vs. 2.5 for NBS) and similar rates of finding bleeding (around 94% for both), but significantly higher rates of bleed localization on VA (45.7% for CTA vs. 26.4% for NBS), according to the study findings. Embolization rates among the different protocols also varied, from 23% for NBS to 40% for CTA only before VA. The study did not evaluate costs.
How the university’s protocol affected renal function drew the attention of discussant Dr. Leslie Kobayashi,of the University of California, San Diego, who noted that creatinine levels were actually higher in the VA-only group. “This would suggest to me that patient factors such as severity of hemorrhage, presence of shock, and location of comorbidities are most associated with increases in creatinine rather than the actual contrast bolus or contrast dose,” she said. However, the study did not include a multivariate analysis to determine if CT contrast was associated with a rise in creatinine, Dr. Jacovides said.
Dr. Hasan Alam of the University of Michigan Health System, Ann Arbor, acknowledged that CTA before VA may be viable in hemodynamically stable patients, but not so for those with more critical injuries. “The problem is sometimes logistics, because you have to go to two different places for the CTA and VA, so a time factor is involved,” he said. “So if you have a sick patient who is robustly or briskly bleeding, who’s hypotensive, for those 5%-10% of patients, that causes a delay.”
Dr. Jacovides noted that at least among the patients who received pre-VA imaging, the protocol at the University of Pennsylvania actually got those patients to treatment quicker. “We did find that among patients who underwent CTA only instead of NBS, the time from the first scan to first VA was significantly reduced; it was about 1,200 minutes on bleeding scans and about 530 minutes with CTA on average,” she said.
Dr. Jacovides reported having no relevant financial disclosures. She is a surgical intern at Thomas Jefferson University Hospital, Philadelphia, and completed this research as a medical student at the University of Pennsylvania, where the senior authors are in the division of traumatology, surgical critical care, and emergency surgery.
AT THE AAST ANNUAL MEETING
Key clinical point: In patients with lower GI hemorrhage, performing CT angiography before visceral arteriography can improve diagnostic accuracy and reduce radiation exposure.
Major finding: CTA before VA vs. VA alone was more than twice as likely to find bleeds and reduce fluoroscopy times without affecting kidney function, and without subjecting patients to the radiation of a nuclear bleeding scan. The ability of CTA before VA to find bleeding was comparable to that of an NBS, with an ability to localize bleeding about 60% better than that of an NBS.
Data source: Single-center review of an interventional radiology database of 161 patients after implementation of institutional policy.
Disclosures: Dr. Jacovides reported having no relevant financial disclosures. She is a surgical intern at Thomas Jefferson University Hospital, Philadelphia, and completed this research as a medical student at the University of Pennsylvania, where the senior authors are in the division of traumatology, surgical critical care, and emergency surgery.
Transanal extraction found effective in rectal cancer surgery
Patients who underwent laparoscopic total mesorectal excision with coloanal anastomosis for rectal cancer had similar rates of mortality and morbidity, regardless of whether the extraction was performed transanally or transabdominally, a long-term single-center study showed.
“There are few data of full laparoscopic coloanal anastomosis for rectal cancer, including small series and short follow-up,” authors led by Dr. Quentin Denost of the department of surgery at Saint-André Hospital, Bordeau, France, wrote. “Moreover, the risk of anastomotic or perineal recurrence induced by transanal extraction of the rectal specimen is not known.” In addition, they continued, functional outcomes of laparoscopic coloanal anastomosis “have never been reported, and therefore the potential risk of anal incontinence related to transanal specimen extraction has never been discussed.”
In an effort to investigate the long-term outcome of laparoscopic coloanal anastomosis for rectal cancer, the researchers evaluated records of 220 patients who underwent laparoscopic total mesorectal excision and coloanal anastomosis for rectal cancer at Saint-André Hospital between 2000 and 2010 (Ann. Surg. 2014 Sept. 1 [doi:10.1097/SLA.0000000000000855]). Study endpoints of interest were circumferential margin, mesorectal grade, local recurrence, survival, and functional outcome obtained by a questionnaire sent to patients free of disease with at least 1 year of follow-up after stoma closure.
More than half of the patients (63%) were male, their median age was 64, and their median body mass index was 25 kg/m2. The tumors were a median of 4 cm from the anal verge and 1 cm from the anal ring, and 82% of the patients had stage T3 or T4 disease.
The authors reported that the overall mortality and surgical morbidity rates were 0.5% and 17%, respectively, the rate of positive circumferential resection margin was 9%, and the median anal continence score was 6 (range, 0-20). After a median follow-up of 51 months, the local recurrence rate was 4%, while at 5 years, the overall survival and disease-free survival rates were 83% and 70%, respectively.
When the authors evaluated results by extraction site, no significant differences were observed between the transanal extraction and transabdomonal extraction groups in the rate of overall mortality (0.8% vs. 0%, respectively; P = 1.000), overall morbidity (34% vs. 43%), positive circumferential margin (7% vs. 11%; P = .324), mesorectal grade, local recurrence (4% vs. 5%; P = .98), and disease-free survival (72% vs. 68%; P = .63). The median continence score was 6 in both groups (P = .92).
The findings demonstrated that pelvic control and survival “were not compromised by the association between mini invasive surgery and ultralow sphincter preservation,” the authors concluded. “Moreover, we demonstrated the safety and efficacy of transanal extraction of the rectal specimen with similar oncologic and functional outcome than the conventional abdominal extraction. Because of the wound advantages of transanal extraction, in terms of abdominal wall preservation, transanal extraction can be recommended in laparoscopic surgical management of low rectal cancer.”
They acknowledged certain limitations of the study, including the fact that BMI was slightly lower in the transanal group, compared with the transabdomonal group (24.3 vs. 25.8 kg/m2, respectively; P = .01). This suggests “that some obese patients probably received transabdominal instead of transanal extraction,” Dr. Denost and associates wrote. “Therefore, as we recommend preventing excessive stretching of the anal sphincter during rectal extraction, we also recommend to be cautious when performing transanal extraction in obese patients with wide mesorectal specimen, especially to avoid mesorectal injury and tumor spillage.”
The authors reported having no relevant financial disclosures.
On Twitter @dougbrunk
Patients who underwent laparoscopic total mesorectal excision with coloanal anastomosis for rectal cancer had similar rates of mortality and morbidity, regardless of whether the extraction was performed transanally or transabdominally, a long-term single-center study showed.
“There are few data of full laparoscopic coloanal anastomosis for rectal cancer, including small series and short follow-up,” authors led by Dr. Quentin Denost of the department of surgery at Saint-André Hospital, Bordeau, France, wrote. “Moreover, the risk of anastomotic or perineal recurrence induced by transanal extraction of the rectal specimen is not known.” In addition, they continued, functional outcomes of laparoscopic coloanal anastomosis “have never been reported, and therefore the potential risk of anal incontinence related to transanal specimen extraction has never been discussed.”
In an effort to investigate the long-term outcome of laparoscopic coloanal anastomosis for rectal cancer, the researchers evaluated records of 220 patients who underwent laparoscopic total mesorectal excision and coloanal anastomosis for rectal cancer at Saint-André Hospital between 2000 and 2010 (Ann. Surg. 2014 Sept. 1 [doi:10.1097/SLA.0000000000000855]). Study endpoints of interest were circumferential margin, mesorectal grade, local recurrence, survival, and functional outcome obtained by a questionnaire sent to patients free of disease with at least 1 year of follow-up after stoma closure.
More than half of the patients (63%) were male, their median age was 64, and their median body mass index was 25 kg/m2. The tumors were a median of 4 cm from the anal verge and 1 cm from the anal ring, and 82% of the patients had stage T3 or T4 disease.
The authors reported that the overall mortality and surgical morbidity rates were 0.5% and 17%, respectively, the rate of positive circumferential resection margin was 9%, and the median anal continence score was 6 (range, 0-20). After a median follow-up of 51 months, the local recurrence rate was 4%, while at 5 years, the overall survival and disease-free survival rates were 83% and 70%, respectively.
When the authors evaluated results by extraction site, no significant differences were observed between the transanal extraction and transabdomonal extraction groups in the rate of overall mortality (0.8% vs. 0%, respectively; P = 1.000), overall morbidity (34% vs. 43%), positive circumferential margin (7% vs. 11%; P = .324), mesorectal grade, local recurrence (4% vs. 5%; P = .98), and disease-free survival (72% vs. 68%; P = .63). The median continence score was 6 in both groups (P = .92).
The findings demonstrated that pelvic control and survival “were not compromised by the association between mini invasive surgery and ultralow sphincter preservation,” the authors concluded. “Moreover, we demonstrated the safety and efficacy of transanal extraction of the rectal specimen with similar oncologic and functional outcome than the conventional abdominal extraction. Because of the wound advantages of transanal extraction, in terms of abdominal wall preservation, transanal extraction can be recommended in laparoscopic surgical management of low rectal cancer.”
They acknowledged certain limitations of the study, including the fact that BMI was slightly lower in the transanal group, compared with the transabdomonal group (24.3 vs. 25.8 kg/m2, respectively; P = .01). This suggests “that some obese patients probably received transabdominal instead of transanal extraction,” Dr. Denost and associates wrote. “Therefore, as we recommend preventing excessive stretching of the anal sphincter during rectal extraction, we also recommend to be cautious when performing transanal extraction in obese patients with wide mesorectal specimen, especially to avoid mesorectal injury and tumor spillage.”
The authors reported having no relevant financial disclosures.
On Twitter @dougbrunk
Patients who underwent laparoscopic total mesorectal excision with coloanal anastomosis for rectal cancer had similar rates of mortality and morbidity, regardless of whether the extraction was performed transanally or transabdominally, a long-term single-center study showed.
“There are few data of full laparoscopic coloanal anastomosis for rectal cancer, including small series and short follow-up,” authors led by Dr. Quentin Denost of the department of surgery at Saint-André Hospital, Bordeau, France, wrote. “Moreover, the risk of anastomotic or perineal recurrence induced by transanal extraction of the rectal specimen is not known.” In addition, they continued, functional outcomes of laparoscopic coloanal anastomosis “have never been reported, and therefore the potential risk of anal incontinence related to transanal specimen extraction has never been discussed.”
In an effort to investigate the long-term outcome of laparoscopic coloanal anastomosis for rectal cancer, the researchers evaluated records of 220 patients who underwent laparoscopic total mesorectal excision and coloanal anastomosis for rectal cancer at Saint-André Hospital between 2000 and 2010 (Ann. Surg. 2014 Sept. 1 [doi:10.1097/SLA.0000000000000855]). Study endpoints of interest were circumferential margin, mesorectal grade, local recurrence, survival, and functional outcome obtained by a questionnaire sent to patients free of disease with at least 1 year of follow-up after stoma closure.
More than half of the patients (63%) were male, their median age was 64, and their median body mass index was 25 kg/m2. The tumors were a median of 4 cm from the anal verge and 1 cm from the anal ring, and 82% of the patients had stage T3 or T4 disease.
The authors reported that the overall mortality and surgical morbidity rates were 0.5% and 17%, respectively, the rate of positive circumferential resection margin was 9%, and the median anal continence score was 6 (range, 0-20). After a median follow-up of 51 months, the local recurrence rate was 4%, while at 5 years, the overall survival and disease-free survival rates were 83% and 70%, respectively.
When the authors evaluated results by extraction site, no significant differences were observed between the transanal extraction and transabdomonal extraction groups in the rate of overall mortality (0.8% vs. 0%, respectively; P = 1.000), overall morbidity (34% vs. 43%), positive circumferential margin (7% vs. 11%; P = .324), mesorectal grade, local recurrence (4% vs. 5%; P = .98), and disease-free survival (72% vs. 68%; P = .63). The median continence score was 6 in both groups (P = .92).
The findings demonstrated that pelvic control and survival “were not compromised by the association between mini invasive surgery and ultralow sphincter preservation,” the authors concluded. “Moreover, we demonstrated the safety and efficacy of transanal extraction of the rectal specimen with similar oncologic and functional outcome than the conventional abdominal extraction. Because of the wound advantages of transanal extraction, in terms of abdominal wall preservation, transanal extraction can be recommended in laparoscopic surgical management of low rectal cancer.”
They acknowledged certain limitations of the study, including the fact that BMI was slightly lower in the transanal group, compared with the transabdomonal group (24.3 vs. 25.8 kg/m2, respectively; P = .01). This suggests “that some obese patients probably received transabdominal instead of transanal extraction,” Dr. Denost and associates wrote. “Therefore, as we recommend preventing excessive stretching of the anal sphincter during rectal extraction, we also recommend to be cautious when performing transanal extraction in obese patients with wide mesorectal specimen, especially to avoid mesorectal injury and tumor spillage.”
The authors reported having no relevant financial disclosures.
On Twitter @dougbrunk
FROM ANNALS OF SURGERY
Key clinical point: Transanal extraction can be recommended in the laparoscopic surgical management of low rectal cancer.
Major finding: During laparoscopic total mesorectal excision, no significant differences were observed between patients who underwent transanal extraction or transabdomonal extraction in the rate of overall mortality (0.8% vs. 0%, respectively; P = 1.000), overall morbidity (34% vs. 43%), or local recurrence (4% vs. 5%; P = .98).
Data source: A single-center study of 220 patients who underwent laparoscopic total mesorectal excision and coloanal anastomosis for rectal cancer at Saint-André Hospital in Bordeaux, France, between 2000 and 2010.
Disclosures: The authors reported having no relevant financial disclosures.
Death by discontinuity of care
The story
SJ was a 66-year-old woman with a history of ulcerative colitis (UC) who was recently status post laparoscopic proctocolectomy with ileoanal J pouch and diverting ileostomy 2 weeks ago at Hospital A. At the time of her surgical discharge, she was tolerating an oral diet, but over the next 2 weeks her oral intake declined, she reported feeling light-headed with movement, and she had an increase in abdominal pain despite oral analgesia. SJ was at her surgical follow-up appointment when she passed out in the waiting room. She awoke spontaneously, but she was hypotensive and was taken by ambulance to the emergency room of Hospital B. On examination SJ was very orthostatic. She had blood drawn, and she had an ECG, an abdominal radiograph, and a CT scan of the abdomen and pelvis performed. Her ECG and abdominal imaging were unremarkable. She was found to have an elevated lipase (910 U/dL) and low hemoglobin (9.9 mg/dL), although her anemia was not significantly different from 2 weeks ago. SJ was sent from Hospital B to Hospital C and admitted by Dr. Hospitalist 1 (nighttime, weekend coverage) for dehydration and possible pancreatitis. Dr. Hospitalist 1 initiated intravenous fluids and ordered an ultrasound of the abdomen. Intermittent pneumatic compression devices were ordered for deep vein thrombosis prophylaxis.
The following morning, SJ was seen by Dr. Hospitalist 2 (daytime, weekend coverage). On examination, SJ was noted to have bilateral lower extremity edema. She remained orthostatic despite several liters of saline. Dr. Hospitalist 2 ordered a CT scan of the chest with a PE protocol along with ultrasonography of the legs. SJ’s morning hemoglobin was 8.4 mg/dL and Dr. Hospitalist 2 ordered a blood transfusion. The results of the imaging returned the next day and both the CT and lower extremity ultrasounds were normal. However, the abdominal ultrasound ordered by Dr. Hospitalist 1 incidentally identified an inferior vena cava filter (IVCF) with a small amount of adherent clot.
The next day, SJ was seen by Dr. Hospitalist 3 (daytime, weekday attending). SJ’s hemoglobin was now 10.4 mg/dL and her lipase was normal. Dr. Hospitalist 3 documented that SJ was doing “better,” and that the plan was to wean IV fluids, work with physical therapy, and discharge soon. But SJ continued to complain of abdominal tightness, burning in her legs, and light-headedness with activity. On hospital day 4, Dr. Hospitalist 3 ordered oral antibiotics for possible leg cellulitis. On hospital day 5, SJ passed out briefly during physical therapy and Dr. Hospitalist 3 increased her IV fluids. Over the next 3 days, Dr. Hospitalist 3 stopped and restarted the IV fluids several times.
On hospital day 8, SJ was seen by Dr. Hospitalist 4 (daytime, weekend coverage). SJ remained orthostatic. Dr. Hospitalist 4 ordered a CT of the abdomen to evaluate the IVCF, which identified thrombus material within the IVCF and the entire caudal vena cava, iliac, and femoral vessels. Full-dose anticoagulation was initiated with low-molecular-weight heparin. On hospital day 10, SJ collapsed in physical therapy and lost her pulse. A full code blue response, including systemic TPA administration, failed to revive her and she was pronounced dead. An autopsy was performed and determined pulmonary embolism as the cause of death.
Complaint
SJ’s husband had difficulty reconciling the fact that SJ died so recently after her surgical discharge and that she had been considered “well on her way” to a full recovery. The case was referred to an attorney and subsequent review supported medical negligence and a complaint was filed. The complaint alleged that the Hospitalists (specifically 1, 2, and 3) failed to recognize SJ’s increased risk for thrombosis, failed to diagnose her IVC obstruction, and failed to initiate appropriate treatment in the form of therapeutic anticoagulation. Had the standard of care been followed, the complaint alleged, SJ would not have died.
Scientific principles
Inferior vena cava obstruction has been reported in 3%-30% of patients following IVC filter placement related to new local thrombus formation, thrombogenicity of the device, trapped embolus, or extension of a more distal DVT cephalad. Patients with inferior vena caval thrombosis (IVCT) may present with a spectrum of signs and symptoms and this variability is a significant part of the challenge of diagnosis. The classic presentation of IVCT includes bilateral lower extremity edema with dilated, visible superficial abdominal veins.
Complaint rebuttal and discussion
The Hospitalists defended themselves by providing reasonable alternatives to the actual diagnosis. SJ had a new ileostomy and orthostasis is common in such patients. Yet SJ did not have documented high stoma outputs and her electrolytes and renal function were inconsistent with hypovolemia.
Defense experts also pointed to SJ’s anemia and orthostasis and opined that anticoagulation would be contraindicated until hemorrhage could be ruled out. Yet SJ’s anemia was not significantly different from her surgical discharge and SJ was on anticoagulant DVT prophylaxis her entire surgical hospitalization with even lower levels of hemoglobin.
Plaintiff experts asserted that the Hospitalists should have contacted SJ’s colorectal surgeon if they were reluctant to use anticoagulants to further inform the risks and benefits. Ultimately, the defense had little explanation for the Hospitalists’ collective failure to follow-up on the abdominal ultrasound that demonstrated a small amount of adherent clot.
Conclusion
SJ was at two different hospitals and had four different Hospitalist s in 10 days.
Dr. Hospitalist 1 never saw the radiology films from Hospital B that showed an IVCF. When Dr. Hospitalist 2 began caring for SJ, he was unaware that SJ even had an IVCF or that she had a prior history of PE. Over the weekend, Dr. Hospitalist 2 did not access the labs from Hospital A to see if SJ’s anemia was new or not. Dr. Hospitalist 3 did not know that Dr. Hospitalist 1 ordered an abdominal ultrasound on admission and because the result was not flagged as “abnormal” the small adherent clot on the IVCF was not integrated into SJ’s clinical presentation.
All Hospitalist groups struggle to provide continuity in a system of discontinuity. In this case, important details were missed and it led to a delay in diagnosis and ultimately treatment.
This case was settled for an undisclosed amount on behalf of the plaintiff.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
The story
SJ was a 66-year-old woman with a history of ulcerative colitis (UC) who was recently status post laparoscopic proctocolectomy with ileoanal J pouch and diverting ileostomy 2 weeks ago at Hospital A. At the time of her surgical discharge, she was tolerating an oral diet, but over the next 2 weeks her oral intake declined, she reported feeling light-headed with movement, and she had an increase in abdominal pain despite oral analgesia. SJ was at her surgical follow-up appointment when she passed out in the waiting room. She awoke spontaneously, but she was hypotensive and was taken by ambulance to the emergency room of Hospital B. On examination SJ was very orthostatic. She had blood drawn, and she had an ECG, an abdominal radiograph, and a CT scan of the abdomen and pelvis performed. Her ECG and abdominal imaging were unremarkable. She was found to have an elevated lipase (910 U/dL) and low hemoglobin (9.9 mg/dL), although her anemia was not significantly different from 2 weeks ago. SJ was sent from Hospital B to Hospital C and admitted by Dr. Hospitalist 1 (nighttime, weekend coverage) for dehydration and possible pancreatitis. Dr. Hospitalist 1 initiated intravenous fluids and ordered an ultrasound of the abdomen. Intermittent pneumatic compression devices were ordered for deep vein thrombosis prophylaxis.
The following morning, SJ was seen by Dr. Hospitalist 2 (daytime, weekend coverage). On examination, SJ was noted to have bilateral lower extremity edema. She remained orthostatic despite several liters of saline. Dr. Hospitalist 2 ordered a CT scan of the chest with a PE protocol along with ultrasonography of the legs. SJ’s morning hemoglobin was 8.4 mg/dL and Dr. Hospitalist 2 ordered a blood transfusion. The results of the imaging returned the next day and both the CT and lower extremity ultrasounds were normal. However, the abdominal ultrasound ordered by Dr. Hospitalist 1 incidentally identified an inferior vena cava filter (IVCF) with a small amount of adherent clot.
The next day, SJ was seen by Dr. Hospitalist 3 (daytime, weekday attending). SJ’s hemoglobin was now 10.4 mg/dL and her lipase was normal. Dr. Hospitalist 3 documented that SJ was doing “better,” and that the plan was to wean IV fluids, work with physical therapy, and discharge soon. But SJ continued to complain of abdominal tightness, burning in her legs, and light-headedness with activity. On hospital day 4, Dr. Hospitalist 3 ordered oral antibiotics for possible leg cellulitis. On hospital day 5, SJ passed out briefly during physical therapy and Dr. Hospitalist 3 increased her IV fluids. Over the next 3 days, Dr. Hospitalist 3 stopped and restarted the IV fluids several times.
On hospital day 8, SJ was seen by Dr. Hospitalist 4 (daytime, weekend coverage). SJ remained orthostatic. Dr. Hospitalist 4 ordered a CT of the abdomen to evaluate the IVCF, which identified thrombus material within the IVCF and the entire caudal vena cava, iliac, and femoral vessels. Full-dose anticoagulation was initiated with low-molecular-weight heparin. On hospital day 10, SJ collapsed in physical therapy and lost her pulse. A full code blue response, including systemic TPA administration, failed to revive her and she was pronounced dead. An autopsy was performed and determined pulmonary embolism as the cause of death.
Complaint
SJ’s husband had difficulty reconciling the fact that SJ died so recently after her surgical discharge and that she had been considered “well on her way” to a full recovery. The case was referred to an attorney and subsequent review supported medical negligence and a complaint was filed. The complaint alleged that the Hospitalists (specifically 1, 2, and 3) failed to recognize SJ’s increased risk for thrombosis, failed to diagnose her IVC obstruction, and failed to initiate appropriate treatment in the form of therapeutic anticoagulation. Had the standard of care been followed, the complaint alleged, SJ would not have died.
Scientific principles
Inferior vena cava obstruction has been reported in 3%-30% of patients following IVC filter placement related to new local thrombus formation, thrombogenicity of the device, trapped embolus, or extension of a more distal DVT cephalad. Patients with inferior vena caval thrombosis (IVCT) may present with a spectrum of signs and symptoms and this variability is a significant part of the challenge of diagnosis. The classic presentation of IVCT includes bilateral lower extremity edema with dilated, visible superficial abdominal veins.
Complaint rebuttal and discussion
The Hospitalists defended themselves by providing reasonable alternatives to the actual diagnosis. SJ had a new ileostomy and orthostasis is common in such patients. Yet SJ did not have documented high stoma outputs and her electrolytes and renal function were inconsistent with hypovolemia.
Defense experts also pointed to SJ’s anemia and orthostasis and opined that anticoagulation would be contraindicated until hemorrhage could be ruled out. Yet SJ’s anemia was not significantly different from her surgical discharge and SJ was on anticoagulant DVT prophylaxis her entire surgical hospitalization with even lower levels of hemoglobin.
Plaintiff experts asserted that the Hospitalists should have contacted SJ’s colorectal surgeon if they were reluctant to use anticoagulants to further inform the risks and benefits. Ultimately, the defense had little explanation for the Hospitalists’ collective failure to follow-up on the abdominal ultrasound that demonstrated a small amount of adherent clot.
Conclusion
SJ was at two different hospitals and had four different Hospitalist s in 10 days.
Dr. Hospitalist 1 never saw the radiology films from Hospital B that showed an IVCF. When Dr. Hospitalist 2 began caring for SJ, he was unaware that SJ even had an IVCF or that she had a prior history of PE. Over the weekend, Dr. Hospitalist 2 did not access the labs from Hospital A to see if SJ’s anemia was new or not. Dr. Hospitalist 3 did not know that Dr. Hospitalist 1 ordered an abdominal ultrasound on admission and because the result was not flagged as “abnormal” the small adherent clot on the IVCF was not integrated into SJ’s clinical presentation.
All Hospitalist groups struggle to provide continuity in a system of discontinuity. In this case, important details were missed and it led to a delay in diagnosis and ultimately treatment.
This case was settled for an undisclosed amount on behalf of the plaintiff.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.
The story
SJ was a 66-year-old woman with a history of ulcerative colitis (UC) who was recently status post laparoscopic proctocolectomy with ileoanal J pouch and diverting ileostomy 2 weeks ago at Hospital A. At the time of her surgical discharge, she was tolerating an oral diet, but over the next 2 weeks her oral intake declined, she reported feeling light-headed with movement, and she had an increase in abdominal pain despite oral analgesia. SJ was at her surgical follow-up appointment when she passed out in the waiting room. She awoke spontaneously, but she was hypotensive and was taken by ambulance to the emergency room of Hospital B. On examination SJ was very orthostatic. She had blood drawn, and she had an ECG, an abdominal radiograph, and a CT scan of the abdomen and pelvis performed. Her ECG and abdominal imaging were unremarkable. She was found to have an elevated lipase (910 U/dL) and low hemoglobin (9.9 mg/dL), although her anemia was not significantly different from 2 weeks ago. SJ was sent from Hospital B to Hospital C and admitted by Dr. Hospitalist 1 (nighttime, weekend coverage) for dehydration and possible pancreatitis. Dr. Hospitalist 1 initiated intravenous fluids and ordered an ultrasound of the abdomen. Intermittent pneumatic compression devices were ordered for deep vein thrombosis prophylaxis.
The following morning, SJ was seen by Dr. Hospitalist 2 (daytime, weekend coverage). On examination, SJ was noted to have bilateral lower extremity edema. She remained orthostatic despite several liters of saline. Dr. Hospitalist 2 ordered a CT scan of the chest with a PE protocol along with ultrasonography of the legs. SJ’s morning hemoglobin was 8.4 mg/dL and Dr. Hospitalist 2 ordered a blood transfusion. The results of the imaging returned the next day and both the CT and lower extremity ultrasounds were normal. However, the abdominal ultrasound ordered by Dr. Hospitalist 1 incidentally identified an inferior vena cava filter (IVCF) with a small amount of adherent clot.
The next day, SJ was seen by Dr. Hospitalist 3 (daytime, weekday attending). SJ’s hemoglobin was now 10.4 mg/dL and her lipase was normal. Dr. Hospitalist 3 documented that SJ was doing “better,” and that the plan was to wean IV fluids, work with physical therapy, and discharge soon. But SJ continued to complain of abdominal tightness, burning in her legs, and light-headedness with activity. On hospital day 4, Dr. Hospitalist 3 ordered oral antibiotics for possible leg cellulitis. On hospital day 5, SJ passed out briefly during physical therapy and Dr. Hospitalist 3 increased her IV fluids. Over the next 3 days, Dr. Hospitalist 3 stopped and restarted the IV fluids several times.
On hospital day 8, SJ was seen by Dr. Hospitalist 4 (daytime, weekend coverage). SJ remained orthostatic. Dr. Hospitalist 4 ordered a CT of the abdomen to evaluate the IVCF, which identified thrombus material within the IVCF and the entire caudal vena cava, iliac, and femoral vessels. Full-dose anticoagulation was initiated with low-molecular-weight heparin. On hospital day 10, SJ collapsed in physical therapy and lost her pulse. A full code blue response, including systemic TPA administration, failed to revive her and she was pronounced dead. An autopsy was performed and determined pulmonary embolism as the cause of death.
Complaint
SJ’s husband had difficulty reconciling the fact that SJ died so recently after her surgical discharge and that she had been considered “well on her way” to a full recovery. The case was referred to an attorney and subsequent review supported medical negligence and a complaint was filed. The complaint alleged that the Hospitalists (specifically 1, 2, and 3) failed to recognize SJ’s increased risk for thrombosis, failed to diagnose her IVC obstruction, and failed to initiate appropriate treatment in the form of therapeutic anticoagulation. Had the standard of care been followed, the complaint alleged, SJ would not have died.
Scientific principles
Inferior vena cava obstruction has been reported in 3%-30% of patients following IVC filter placement related to new local thrombus formation, thrombogenicity of the device, trapped embolus, or extension of a more distal DVT cephalad. Patients with inferior vena caval thrombosis (IVCT) may present with a spectrum of signs and symptoms and this variability is a significant part of the challenge of diagnosis. The classic presentation of IVCT includes bilateral lower extremity edema with dilated, visible superficial abdominal veins.
Complaint rebuttal and discussion
The Hospitalists defended themselves by providing reasonable alternatives to the actual diagnosis. SJ had a new ileostomy and orthostasis is common in such patients. Yet SJ did not have documented high stoma outputs and her electrolytes and renal function were inconsistent with hypovolemia.
Defense experts also pointed to SJ’s anemia and orthostasis and opined that anticoagulation would be contraindicated until hemorrhage could be ruled out. Yet SJ’s anemia was not significantly different from her surgical discharge and SJ was on anticoagulant DVT prophylaxis her entire surgical hospitalization with even lower levels of hemoglobin.
Plaintiff experts asserted that the Hospitalists should have contacted SJ’s colorectal surgeon if they were reluctant to use anticoagulants to further inform the risks and benefits. Ultimately, the defense had little explanation for the Hospitalists’ collective failure to follow-up on the abdominal ultrasound that demonstrated a small amount of adherent clot.
Conclusion
SJ was at two different hospitals and had four different Hospitalist s in 10 days.
Dr. Hospitalist 1 never saw the radiology films from Hospital B that showed an IVCF. When Dr. Hospitalist 2 began caring for SJ, he was unaware that SJ even had an IVCF or that she had a prior history of PE. Over the weekend, Dr. Hospitalist 2 did not access the labs from Hospital A to see if SJ’s anemia was new or not. Dr. Hospitalist 3 did not know that Dr. Hospitalist 1 ordered an abdominal ultrasound on admission and because the result was not flagged as “abnormal” the small adherent clot on the IVCF was not integrated into SJ’s clinical presentation.
All Hospitalist groups struggle to provide continuity in a system of discontinuity. In this case, important details were missed and it led to a delay in diagnosis and ultimately treatment.
This case was settled for an undisclosed amount on behalf of the plaintiff.
Dr. Michota is director of academic affairs in the hospital medicine department at the Cleveland Clinic and medical editor of Hospitalist News. He has been involved in peer review both within and outside the legal system. Read past columns at eHospitalist news.com/Lessons.