User login
Official Newspaper of the American College of Surgeons
LAW & MEDICINE: ‘Defective and unreasonably dangerous’
Question: After statins had been in use for several years, data began to accumulate purporting to show that they increase the risk of diabetes. When Mrs. Smith learned that her recent diagnosis of diabetes might have something to do with the drug, she consulted a lawyer who began advertising for similar cases to consolidate them into a class action lawsuit. The legal theory (theories) seeking to prove product liability will be based on:
A. Contract law and breach of warranty.
B. Negligence in tort law.
C. Strict liability without requiring proof of fault.
D. A defective product that is unreasonably dangerous.
E. All of the above.
Answer:E. Should a prescription drug lead to harm, an injured party can sue the manufacturer who had placed it into the stream of commerce. The law of products liability governs this cause of action, wherein recovery is based on a number of legal theories, specifically negligence, breach of warranty, and strict liability. The latter is the most favored, as there is no need to prove fault or warranty. Products liability law also covers defective medical devices. The recent multimillion-dollar settlements and jury verdicts with Endo, Johnson & Johnson, Bard, and other manufacturers over their vaginal mesh devices are good examples.
In products liability, injured plaintiffs frequently claim a failure to warn of known risks, such as cardiovascular deaths caused by Vioxx, a nonsteroidal anti-inflammatory drug that was withdrawn in 2004. Merck, its manufacturer, has thus far won 11 and lost 3 of the cases that have gone to trial. Some of these judgments are under appeal; most notably, a Texas Court of Appeals recently reversed a $253 million award initially won by plaintiff Robert Ernst in the very first trial. However, the company has proposed $4.85 billion to settle tens of thousands of similar pending lawsuits. Other recent examples alleging failure to warn are heart attacks linked to the diabetes drug rosiglitazone and bladder cancer associated with the diabetes drug pioglitazone.
In 1963, the California Supreme Court bypassed the law of contracts and warranty in a seminal case of product-related injury, and introduced the notion of strict liability, which goes beyond simple negligence (Greenman v Yuba Power Products Inc., 377 P.2d 897 [Cal. 1963]). The strict liability approach centers on whether a product is defective and unreasonably dangerous, and it has now been adopted in virtually all jurisdictions.
The theory holds that a professional supplier who sells a product that is both defective and unreasonably dangerous is strictly liable to foreseeable plaintiffs. “Defective” is usually defined as product quality that is less than what a reasonable consumer expects. “Unreasonably dangerous” is a conclusion that the risks that result from its condition outweigh the product’s advantages.
Strict liability is not about negligence or fault, but about a social policy that shifts to the manufacturer the cost of compensating the injured consumer. To prevail, the plaintiff must show proximate cause, and assumption of risk is still a valid defense.
Statins, which are powerful HMG-CoA reductase inhibitors widely used to treat hypercholesterolemia, are currently at the center of pharmaceutical products litigation.
Pfizer, the manufacturer of Lipitor (atorvastatin) has become the target of numerous lawsuits alleging that the drug causes diabetes. Lipitor is the best-selling prescription drug ever, with sales reaching $130 billion since it was approved in 1996. In the United States alone, more than 29 million people have been prescribed this medication. The drug is highly effective in lowering serum cholesterol and is proven to reduce cardiovascular deaths.
A meta-analysis in 2010 revealed an increased risk of diabetes in patients taking statins (Lancet 2010;375:735-42). Statin therapy was associated with a 9% increased risk for incident diabetes; it was calculated that treatment of 255 patients with statins for 4 years resulted in 1 extra case of diabetes. An earlier smaller study had rejected this conclusion, but other studies were in support.
In 2012, the Food and Drug Administration (FDA) required the revision of the package insert of Lipitor and other statins to warn that their use had been linked to a small increased risk of diabetes.
In 2013, a large Canadian study confirmed the increased incidence of new-onset diabetes in patients taking atorvastatin (hazard ratio, 1.22) and simvastatin (hazard ratio, 1.10). This population-based cohort study involved nondiabetic patients age 66 years or older who started statins between 1997 and 2010 (BMJ 2013;346:f2610).
These and other results, coupled with the FDA-mandated revised labeling, have spawned the filing of nearly 1,000 lawsuits by patients who developed diabetes while taking statins, especially postmenopausal women. The rapid increase in the number of lawsuits may be related to the recent decision of a federal judicial panel on multidistrict litigation to consolidate all Lipitor diabetes lawsuits into a single federal courtroom in Charleston, S.C., as a class-action suit. The first case has yet to go to trial, but is expected to do so in 2015.
Previous products liability cases implicating statins have famously involved cerivastatin (Baycol), a one-time rival to Lipitor, for causing rhabdomyolysis. The drug was pulled from the market in 2001 after it reportedly caused 31 deaths. Bayer, its manufacturer, paid about $1 billion in 2005 to settle some 3,000 cases. An example of a medication causing diabetes is quetiapine (Seroquel), an antipsychotic drug manufactured by AstraZeneca, which in 2011 agreed to pay $647 million to settle more than 28,000 lawsuits.
However, the upcoming Lipitor litigation may be more difficult for the plaintiffs to win. Among some of the medico-legal questions to be addressed are:
1) Was there prior company knowledge of the risk and a failure to warn?
2) Were the patients harmed by the drug, given that diabetes is a very common disease and may be linked more to genetics and/or an underlying metabolic syndrome in those who are hyperlipidemic, hypertensive, or obese – the very same patients likely to be on a statin?
3) Is Lipitor a defective product, and is it unreasonably dangerous?
Despite the FDA-directed change in labeling, a number of scientists and the FDA itself have emphasized that the cardiac benefits of a statin drug are greater than any small increased risk of developing diabetes.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: After statins had been in use for several years, data began to accumulate purporting to show that they increase the risk of diabetes. When Mrs. Smith learned that her recent diagnosis of diabetes might have something to do with the drug, she consulted a lawyer who began advertising for similar cases to consolidate them into a class action lawsuit. The legal theory (theories) seeking to prove product liability will be based on:
A. Contract law and breach of warranty.
B. Negligence in tort law.
C. Strict liability without requiring proof of fault.
D. A defective product that is unreasonably dangerous.
E. All of the above.
Answer:E. Should a prescription drug lead to harm, an injured party can sue the manufacturer who had placed it into the stream of commerce. The law of products liability governs this cause of action, wherein recovery is based on a number of legal theories, specifically negligence, breach of warranty, and strict liability. The latter is the most favored, as there is no need to prove fault or warranty. Products liability law also covers defective medical devices. The recent multimillion-dollar settlements and jury verdicts with Endo, Johnson & Johnson, Bard, and other manufacturers over their vaginal mesh devices are good examples.
In products liability, injured plaintiffs frequently claim a failure to warn of known risks, such as cardiovascular deaths caused by Vioxx, a nonsteroidal anti-inflammatory drug that was withdrawn in 2004. Merck, its manufacturer, has thus far won 11 and lost 3 of the cases that have gone to trial. Some of these judgments are under appeal; most notably, a Texas Court of Appeals recently reversed a $253 million award initially won by plaintiff Robert Ernst in the very first trial. However, the company has proposed $4.85 billion to settle tens of thousands of similar pending lawsuits. Other recent examples alleging failure to warn are heart attacks linked to the diabetes drug rosiglitazone and bladder cancer associated with the diabetes drug pioglitazone.
In 1963, the California Supreme Court bypassed the law of contracts and warranty in a seminal case of product-related injury, and introduced the notion of strict liability, which goes beyond simple negligence (Greenman v Yuba Power Products Inc., 377 P.2d 897 [Cal. 1963]). The strict liability approach centers on whether a product is defective and unreasonably dangerous, and it has now been adopted in virtually all jurisdictions.
The theory holds that a professional supplier who sells a product that is both defective and unreasonably dangerous is strictly liable to foreseeable plaintiffs. “Defective” is usually defined as product quality that is less than what a reasonable consumer expects. “Unreasonably dangerous” is a conclusion that the risks that result from its condition outweigh the product’s advantages.
Strict liability is not about negligence or fault, but about a social policy that shifts to the manufacturer the cost of compensating the injured consumer. To prevail, the plaintiff must show proximate cause, and assumption of risk is still a valid defense.
Statins, which are powerful HMG-CoA reductase inhibitors widely used to treat hypercholesterolemia, are currently at the center of pharmaceutical products litigation.
Pfizer, the manufacturer of Lipitor (atorvastatin) has become the target of numerous lawsuits alleging that the drug causes diabetes. Lipitor is the best-selling prescription drug ever, with sales reaching $130 billion since it was approved in 1996. In the United States alone, more than 29 million people have been prescribed this medication. The drug is highly effective in lowering serum cholesterol and is proven to reduce cardiovascular deaths.
A meta-analysis in 2010 revealed an increased risk of diabetes in patients taking statins (Lancet 2010;375:735-42). Statin therapy was associated with a 9% increased risk for incident diabetes; it was calculated that treatment of 255 patients with statins for 4 years resulted in 1 extra case of diabetes. An earlier smaller study had rejected this conclusion, but other studies were in support.
In 2012, the Food and Drug Administration (FDA) required the revision of the package insert of Lipitor and other statins to warn that their use had been linked to a small increased risk of diabetes.
In 2013, a large Canadian study confirmed the increased incidence of new-onset diabetes in patients taking atorvastatin (hazard ratio, 1.22) and simvastatin (hazard ratio, 1.10). This population-based cohort study involved nondiabetic patients age 66 years or older who started statins between 1997 and 2010 (BMJ 2013;346:f2610).
These and other results, coupled with the FDA-mandated revised labeling, have spawned the filing of nearly 1,000 lawsuits by patients who developed diabetes while taking statins, especially postmenopausal women. The rapid increase in the number of lawsuits may be related to the recent decision of a federal judicial panel on multidistrict litigation to consolidate all Lipitor diabetes lawsuits into a single federal courtroom in Charleston, S.C., as a class-action suit. The first case has yet to go to trial, but is expected to do so in 2015.
Previous products liability cases implicating statins have famously involved cerivastatin (Baycol), a one-time rival to Lipitor, for causing rhabdomyolysis. The drug was pulled from the market in 2001 after it reportedly caused 31 deaths. Bayer, its manufacturer, paid about $1 billion in 2005 to settle some 3,000 cases. An example of a medication causing diabetes is quetiapine (Seroquel), an antipsychotic drug manufactured by AstraZeneca, which in 2011 agreed to pay $647 million to settle more than 28,000 lawsuits.
However, the upcoming Lipitor litigation may be more difficult for the plaintiffs to win. Among some of the medico-legal questions to be addressed are:
1) Was there prior company knowledge of the risk and a failure to warn?
2) Were the patients harmed by the drug, given that diabetes is a very common disease and may be linked more to genetics and/or an underlying metabolic syndrome in those who are hyperlipidemic, hypertensive, or obese – the very same patients likely to be on a statin?
3) Is Lipitor a defective product, and is it unreasonably dangerous?
Despite the FDA-directed change in labeling, a number of scientists and the FDA itself have emphasized that the cardiac benefits of a statin drug are greater than any small increased risk of developing diabetes.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Question: After statins had been in use for several years, data began to accumulate purporting to show that they increase the risk of diabetes. When Mrs. Smith learned that her recent diagnosis of diabetes might have something to do with the drug, she consulted a lawyer who began advertising for similar cases to consolidate them into a class action lawsuit. The legal theory (theories) seeking to prove product liability will be based on:
A. Contract law and breach of warranty.
B. Negligence in tort law.
C. Strict liability without requiring proof of fault.
D. A defective product that is unreasonably dangerous.
E. All of the above.
Answer:E. Should a prescription drug lead to harm, an injured party can sue the manufacturer who had placed it into the stream of commerce. The law of products liability governs this cause of action, wherein recovery is based on a number of legal theories, specifically negligence, breach of warranty, and strict liability. The latter is the most favored, as there is no need to prove fault or warranty. Products liability law also covers defective medical devices. The recent multimillion-dollar settlements and jury verdicts with Endo, Johnson & Johnson, Bard, and other manufacturers over their vaginal mesh devices are good examples.
In products liability, injured plaintiffs frequently claim a failure to warn of known risks, such as cardiovascular deaths caused by Vioxx, a nonsteroidal anti-inflammatory drug that was withdrawn in 2004. Merck, its manufacturer, has thus far won 11 and lost 3 of the cases that have gone to trial. Some of these judgments are under appeal; most notably, a Texas Court of Appeals recently reversed a $253 million award initially won by plaintiff Robert Ernst in the very first trial. However, the company has proposed $4.85 billion to settle tens of thousands of similar pending lawsuits. Other recent examples alleging failure to warn are heart attacks linked to the diabetes drug rosiglitazone and bladder cancer associated with the diabetes drug pioglitazone.
In 1963, the California Supreme Court bypassed the law of contracts and warranty in a seminal case of product-related injury, and introduced the notion of strict liability, which goes beyond simple negligence (Greenman v Yuba Power Products Inc., 377 P.2d 897 [Cal. 1963]). The strict liability approach centers on whether a product is defective and unreasonably dangerous, and it has now been adopted in virtually all jurisdictions.
The theory holds that a professional supplier who sells a product that is both defective and unreasonably dangerous is strictly liable to foreseeable plaintiffs. “Defective” is usually defined as product quality that is less than what a reasonable consumer expects. “Unreasonably dangerous” is a conclusion that the risks that result from its condition outweigh the product’s advantages.
Strict liability is not about negligence or fault, but about a social policy that shifts to the manufacturer the cost of compensating the injured consumer. To prevail, the plaintiff must show proximate cause, and assumption of risk is still a valid defense.
Statins, which are powerful HMG-CoA reductase inhibitors widely used to treat hypercholesterolemia, are currently at the center of pharmaceutical products litigation.
Pfizer, the manufacturer of Lipitor (atorvastatin) has become the target of numerous lawsuits alleging that the drug causes diabetes. Lipitor is the best-selling prescription drug ever, with sales reaching $130 billion since it was approved in 1996. In the United States alone, more than 29 million people have been prescribed this medication. The drug is highly effective in lowering serum cholesterol and is proven to reduce cardiovascular deaths.
A meta-analysis in 2010 revealed an increased risk of diabetes in patients taking statins (Lancet 2010;375:735-42). Statin therapy was associated with a 9% increased risk for incident diabetes; it was calculated that treatment of 255 patients with statins for 4 years resulted in 1 extra case of diabetes. An earlier smaller study had rejected this conclusion, but other studies were in support.
In 2012, the Food and Drug Administration (FDA) required the revision of the package insert of Lipitor and other statins to warn that their use had been linked to a small increased risk of diabetes.
In 2013, a large Canadian study confirmed the increased incidence of new-onset diabetes in patients taking atorvastatin (hazard ratio, 1.22) and simvastatin (hazard ratio, 1.10). This population-based cohort study involved nondiabetic patients age 66 years or older who started statins between 1997 and 2010 (BMJ 2013;346:f2610).
These and other results, coupled with the FDA-mandated revised labeling, have spawned the filing of nearly 1,000 lawsuits by patients who developed diabetes while taking statins, especially postmenopausal women. The rapid increase in the number of lawsuits may be related to the recent decision of a federal judicial panel on multidistrict litigation to consolidate all Lipitor diabetes lawsuits into a single federal courtroom in Charleston, S.C., as a class-action suit. The first case has yet to go to trial, but is expected to do so in 2015.
Previous products liability cases implicating statins have famously involved cerivastatin (Baycol), a one-time rival to Lipitor, for causing rhabdomyolysis. The drug was pulled from the market in 2001 after it reportedly caused 31 deaths. Bayer, its manufacturer, paid about $1 billion in 2005 to settle some 3,000 cases. An example of a medication causing diabetes is quetiapine (Seroquel), an antipsychotic drug manufactured by AstraZeneca, which in 2011 agreed to pay $647 million to settle more than 28,000 lawsuits.
However, the upcoming Lipitor litigation may be more difficult for the plaintiffs to win. Among some of the medico-legal questions to be addressed are:
1) Was there prior company knowledge of the risk and a failure to warn?
2) Were the patients harmed by the drug, given that diabetes is a very common disease and may be linked more to genetics and/or an underlying metabolic syndrome in those who are hyperlipidemic, hypertensive, or obese – the very same patients likely to be on a statin?
3) Is Lipitor a defective product, and is it unreasonably dangerous?
Despite the FDA-directed change in labeling, a number of scientists and the FDA itself have emphasized that the cardiac benefits of a statin drug are greater than any small increased risk of developing diabetes.
Dr. Tan is professor emeritus of medicine and former adjunct professor of law at the University of Hawaii, and currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Some of the articles in this series are adapted from the author’s 2006 book, “Medical Malpractice: Understanding the Law, Managing the Risk,” and his 2012 Halsbury treatise, “Medical Negligence and Professional Misconduct.” For additional information, readers may contact the author at [email protected].
Medicare begins bundled hospital outpatient payments in 2015
After a year-long delay, Medicare officials are implementing bundled payments for a number of services performed in hospital outpatient departments.
The new payments, which were finalized in the 2015 Medicare Hospital Outpatient Prospective Payment System rule, take effect on Jan. 1, 2015.
The Centers for Medicare & Medicaid Services selected 25 primary services and their adjunctive services and supplies as comprehensive ambulatory payment classifications (C-APCs) and will provide a single payment under Medicare Part B. Medicare beneficiaries will pay a single copayment for the C-APC services.
The 25 C-APCs fall within 12 clinical families:
• automatic implantable cardiac defibrillators, pacemakers, and related devices.
• breast surgery.
• ENT procedures.
• cardiac electrophysiology.
• ophthalmic surgery.
• gastrointestinal procedures.
• neurostimulators.
• orthopedic surgery.
• implantable drug delivery systems.
• radiation oncology.
• urogenital procedures.
• vascular procedures.
For instance, Medicare has designated implantation of a drug infusion device as a C-APC (0227) with a bundled Part B Medicare payment of $15,566.
The final rule, which was released by CMS on Oct. 31, also reversed the CMS policy of requiring physician certification for all Medicare Part A hospital admissions.
Starting Jan. 1, a formal physician certification is required only for long-stay cases of 20 days or more, or in costly outlier cases. For most patients, the admission order, medical record, and progress notes will contain “sufficient information to support the medical necessity of an inpatient admission without a separate requirement of an additional, formal, physician certification,” according to the final rule.
The revision of the physician certification policy does not remove any of the requirements associated with Medicare’s controversial 2-midnight policy, which governs short-stay hospitalizations.
The final rule will be published in the Federal Register on Nov. 10.
On Twitter @maryellenny
After a year-long delay, Medicare officials are implementing bundled payments for a number of services performed in hospital outpatient departments.
The new payments, which were finalized in the 2015 Medicare Hospital Outpatient Prospective Payment System rule, take effect on Jan. 1, 2015.
The Centers for Medicare & Medicaid Services selected 25 primary services and their adjunctive services and supplies as comprehensive ambulatory payment classifications (C-APCs) and will provide a single payment under Medicare Part B. Medicare beneficiaries will pay a single copayment for the C-APC services.
The 25 C-APCs fall within 12 clinical families:
• automatic implantable cardiac defibrillators, pacemakers, and related devices.
• breast surgery.
• ENT procedures.
• cardiac electrophysiology.
• ophthalmic surgery.
• gastrointestinal procedures.
• neurostimulators.
• orthopedic surgery.
• implantable drug delivery systems.
• radiation oncology.
• urogenital procedures.
• vascular procedures.
For instance, Medicare has designated implantation of a drug infusion device as a C-APC (0227) with a bundled Part B Medicare payment of $15,566.
The final rule, which was released by CMS on Oct. 31, also reversed the CMS policy of requiring physician certification for all Medicare Part A hospital admissions.
Starting Jan. 1, a formal physician certification is required only for long-stay cases of 20 days or more, or in costly outlier cases. For most patients, the admission order, medical record, and progress notes will contain “sufficient information to support the medical necessity of an inpatient admission without a separate requirement of an additional, formal, physician certification,” according to the final rule.
The revision of the physician certification policy does not remove any of the requirements associated with Medicare’s controversial 2-midnight policy, which governs short-stay hospitalizations.
The final rule will be published in the Federal Register on Nov. 10.
On Twitter @maryellenny
After a year-long delay, Medicare officials are implementing bundled payments for a number of services performed in hospital outpatient departments.
The new payments, which were finalized in the 2015 Medicare Hospital Outpatient Prospective Payment System rule, take effect on Jan. 1, 2015.
The Centers for Medicare & Medicaid Services selected 25 primary services and their adjunctive services and supplies as comprehensive ambulatory payment classifications (C-APCs) and will provide a single payment under Medicare Part B. Medicare beneficiaries will pay a single copayment for the C-APC services.
The 25 C-APCs fall within 12 clinical families:
• automatic implantable cardiac defibrillators, pacemakers, and related devices.
• breast surgery.
• ENT procedures.
• cardiac electrophysiology.
• ophthalmic surgery.
• gastrointestinal procedures.
• neurostimulators.
• orthopedic surgery.
• implantable drug delivery systems.
• radiation oncology.
• urogenital procedures.
• vascular procedures.
For instance, Medicare has designated implantation of a drug infusion device as a C-APC (0227) with a bundled Part B Medicare payment of $15,566.
The final rule, which was released by CMS on Oct. 31, also reversed the CMS policy of requiring physician certification for all Medicare Part A hospital admissions.
Starting Jan. 1, a formal physician certification is required only for long-stay cases of 20 days or more, or in costly outlier cases. For most patients, the admission order, medical record, and progress notes will contain “sufficient information to support the medical necessity of an inpatient admission without a separate requirement of an additional, formal, physician certification,” according to the final rule.
The revision of the physician certification policy does not remove any of the requirements associated with Medicare’s controversial 2-midnight policy, which governs short-stay hospitalizations.
The final rule will be published in the Federal Register on Nov. 10.
On Twitter @maryellenny
Vagal stimulation advances for heart failure
LAS VEGAS – A novel device-based approach to the treatment of heart failure via vagal nerve stimulation resulted in improvements in objectively measurable cardiac function as well as in subjective heart failure symptoms in the ANTHEM-HF trial.
The improvements seen in ANTHEM-HF (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure) were similar regardless of whether participants were randomized to stimulation of the left or right vagal nerve. That’s an important finding because left-sided vagal nerve stimulation (VNS) technology could readily be combined with implantable cardioverter-defibrillators and cardiac resynchronization therapy devices, which are routinely placed on the left side of the thorax, Dr. Inder S. Anand observed in presenting the study findings at the annual meeting of the Heart Failure Society of America.
“We believe that if this technology pans out and proves effective, it will be introduced into devices very easily. That’s the advantage of left-sided stimulation,” explained Dr. Anand, professor of medicine at the University of Minnesota, Minneapolis.
He was quick to add, however, that “There needs to be a lot more work before autonomic regulation therapy is ready for prime time.” That’s because ANTHEM-HF was a relatively small prospective study – just 60 patients – and it was uncontrolled and unblinded.
Left-sided VNS is a well-established treatment for epilepsy, with more than 100,000 patients having received devices in the last several decades. It’s also seeing increasing use in refractory depression.
The approach is still investigational for heart failure, where autonomic imbalance with increased sympathetic activity and decreased parasympathetic tone is associated with heart failure progression and worse clinical outcomes, the cardiologist explained.
In ANTHEM-HF, 60 patients on optimal medical therapy for heart failure with reduced ejection fraction were randomized to left- or right-sided implantation of the Cyberonics VNS device. Once activated, the VNS intensity was titrated over 10 weeks to the maximum tolerable current that remained below the threshold of heart rate change, side effects, and patient sensation. The chronic intermittent stimulation to the vagus nerve was delivered at 10 Hz, a 250 microsec pulse width, and an average stimulation current of 2.0 mA. The stimulation cycle was 14 seconds on, 66 seconds off.
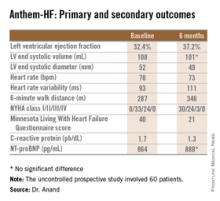
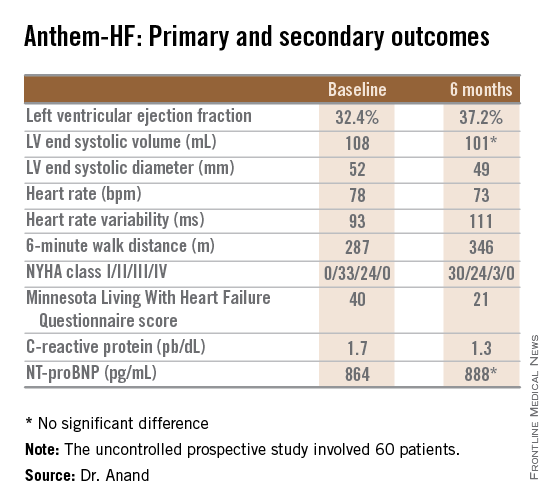
The primary study endpoint was change in LVEF over the course of 6 months. From a baseline of 32.4% it improved significantly to 37.2%, which Dr. Anand deemed “very impressive” for just 6 months of therapy. The improvement was similar regardless of whether the VNS was left or right sided.
He said the study didn’t raise any safety concerns. The only serious treatment-related adverse event was a fatal perioperative embolic stroke in a recipient of a left-sided device.
The most common adverse events were hoarseness or other voice alterations, which occurred in 19 patients. Thirteen patients reported a new cough. These side effects mirror the experience in patients treated with the VNS device for epilepsy or depression.
Discussant Jagmeet P. Singh emphasized that “it’s important to interpret the ANTHEM-HF results with some degree of caution.” That’s because the usual variability within LVEF measurements can be in the 5% range, and the 4.8% improvement seen in this study wasn’t accompanied by a significant improvement in LV end systolic volume. Moreover, the improvements seen in secondary endpoints – 6-minute walk distance, NYHA class, and the Minnesota Living With Heart Failure qualify-of-life score – are all potentially open to bias since neither patients nor physicians were blinded.
Dr. Singh noted that the NECTAR-HF study, a blinded, sham-controlled clinical trial of VNS presented earlier at the annual congress of the European Society of Cardiology, was a negative study that showed no improvement in functional measures. While NECTAR-HF caused some skeptics to question the whole concept of VNS as a useful therapeutic strategy, the stimulation protocols used in NECTAR-HF and ANTHEM-HF were quite different, and it’s likely that the stimulation amplitude employed in NECTAR-HF wasn’t sufficient to affect the target subset of vagal nerve fibers, according to Dr. Singh, director of the cardiac resynchronization therapy program and the Holter laboratory at Massachusetts General Hospital, Boston.
He considers the ANTHEM-HF finding that left-sided VNS is comparable to right to be an important advance that “really moves the field forward.” Yet many critical questions about autonomic regulation therapy for heart failure remain unanswered. These include whether it truly is safe and effective, and if so, the optimal target dose and frequency of stimulation. Answers should be forthcoming from the ongoing INOVATE-HF study, a randomized, controlled, 650-patient study, sponsored by BioControl Medical. The trial features hard clinical endpoints and higher target-dose stimulation protocols than in prior studies.
“This trial will probably put an end to the debate as to whether vagal nerve stimulation has an impact on heart failure or not,” the cardiologist predicted.
“Strategies to nonpharmacologically modulate the autonomic nervous system is an area of immense interest and investigation, and it’s going to be extended,” he observed. “I think that this is the autonomic era, and so it’s really important that we get it right. I think we’ve learned from the renal denervation experience that we need to be really careful in selecting patients for any form of autonomic manipulation. I don’t think we should select patients just off their LVEF. We should have some parameter that can quantify autonomic dysregulation prior to initiation of therapy.”
It will also be important to be able to individualize VNS therapy, just as physicians now do with beta blockers and other pharmacotherapies, he added.
The ANTHEM-HF trial was sponsored by Cyberonics. Dr. Anand reported serving as a consultant to and/or recipient of research grants from Cyberonics, Amgen, Critical Diagnostics, Novartis, and Zensun. Dr. Singh has received research support and/or served on speakers bureaus for Boston Scientific, St. Jude Medical, and the Sorin Group.
LAS VEGAS – A novel device-based approach to the treatment of heart failure via vagal nerve stimulation resulted in improvements in objectively measurable cardiac function as well as in subjective heart failure symptoms in the ANTHEM-HF trial.
The improvements seen in ANTHEM-HF (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure) were similar regardless of whether participants were randomized to stimulation of the left or right vagal nerve. That’s an important finding because left-sided vagal nerve stimulation (VNS) technology could readily be combined with implantable cardioverter-defibrillators and cardiac resynchronization therapy devices, which are routinely placed on the left side of the thorax, Dr. Inder S. Anand observed in presenting the study findings at the annual meeting of the Heart Failure Society of America.
“We believe that if this technology pans out and proves effective, it will be introduced into devices very easily. That’s the advantage of left-sided stimulation,” explained Dr. Anand, professor of medicine at the University of Minnesota, Minneapolis.
He was quick to add, however, that “There needs to be a lot more work before autonomic regulation therapy is ready for prime time.” That’s because ANTHEM-HF was a relatively small prospective study – just 60 patients – and it was uncontrolled and unblinded.
Left-sided VNS is a well-established treatment for epilepsy, with more than 100,000 patients having received devices in the last several decades. It’s also seeing increasing use in refractory depression.
The approach is still investigational for heart failure, where autonomic imbalance with increased sympathetic activity and decreased parasympathetic tone is associated with heart failure progression and worse clinical outcomes, the cardiologist explained.
In ANTHEM-HF, 60 patients on optimal medical therapy for heart failure with reduced ejection fraction were randomized to left- or right-sided implantation of the Cyberonics VNS device. Once activated, the VNS intensity was titrated over 10 weeks to the maximum tolerable current that remained below the threshold of heart rate change, side effects, and patient sensation. The chronic intermittent stimulation to the vagus nerve was delivered at 10 Hz, a 250 microsec pulse width, and an average stimulation current of 2.0 mA. The stimulation cycle was 14 seconds on, 66 seconds off.
The primary study endpoint was change in LVEF over the course of 6 months. From a baseline of 32.4% it improved significantly to 37.2%, which Dr. Anand deemed “very impressive” for just 6 months of therapy. The improvement was similar regardless of whether the VNS was left or right sided.
He said the study didn’t raise any safety concerns. The only serious treatment-related adverse event was a fatal perioperative embolic stroke in a recipient of a left-sided device.
The most common adverse events were hoarseness or other voice alterations, which occurred in 19 patients. Thirteen patients reported a new cough. These side effects mirror the experience in patients treated with the VNS device for epilepsy or depression.
Discussant Jagmeet P. Singh emphasized that “it’s important to interpret the ANTHEM-HF results with some degree of caution.” That’s because the usual variability within LVEF measurements can be in the 5% range, and the 4.8% improvement seen in this study wasn’t accompanied by a significant improvement in LV end systolic volume. Moreover, the improvements seen in secondary endpoints – 6-minute walk distance, NYHA class, and the Minnesota Living With Heart Failure qualify-of-life score – are all potentially open to bias since neither patients nor physicians were blinded.
Dr. Singh noted that the NECTAR-HF study, a blinded, sham-controlled clinical trial of VNS presented earlier at the annual congress of the European Society of Cardiology, was a negative study that showed no improvement in functional measures. While NECTAR-HF caused some skeptics to question the whole concept of VNS as a useful therapeutic strategy, the stimulation protocols used in NECTAR-HF and ANTHEM-HF were quite different, and it’s likely that the stimulation amplitude employed in NECTAR-HF wasn’t sufficient to affect the target subset of vagal nerve fibers, according to Dr. Singh, director of the cardiac resynchronization therapy program and the Holter laboratory at Massachusetts General Hospital, Boston.
He considers the ANTHEM-HF finding that left-sided VNS is comparable to right to be an important advance that “really moves the field forward.” Yet many critical questions about autonomic regulation therapy for heart failure remain unanswered. These include whether it truly is safe and effective, and if so, the optimal target dose and frequency of stimulation. Answers should be forthcoming from the ongoing INOVATE-HF study, a randomized, controlled, 650-patient study, sponsored by BioControl Medical. The trial features hard clinical endpoints and higher target-dose stimulation protocols than in prior studies.
“This trial will probably put an end to the debate as to whether vagal nerve stimulation has an impact on heart failure or not,” the cardiologist predicted.
“Strategies to nonpharmacologically modulate the autonomic nervous system is an area of immense interest and investigation, and it’s going to be extended,” he observed. “I think that this is the autonomic era, and so it’s really important that we get it right. I think we’ve learned from the renal denervation experience that we need to be really careful in selecting patients for any form of autonomic manipulation. I don’t think we should select patients just off their LVEF. We should have some parameter that can quantify autonomic dysregulation prior to initiation of therapy.”
It will also be important to be able to individualize VNS therapy, just as physicians now do with beta blockers and other pharmacotherapies, he added.
The ANTHEM-HF trial was sponsored by Cyberonics. Dr. Anand reported serving as a consultant to and/or recipient of research grants from Cyberonics, Amgen, Critical Diagnostics, Novartis, and Zensun. Dr. Singh has received research support and/or served on speakers bureaus for Boston Scientific, St. Jude Medical, and the Sorin Group.
LAS VEGAS – A novel device-based approach to the treatment of heart failure via vagal nerve stimulation resulted in improvements in objectively measurable cardiac function as well as in subjective heart failure symptoms in the ANTHEM-HF trial.
The improvements seen in ANTHEM-HF (Autonomic Neural Regulation Therapy to Enhance Myocardial Function in Heart Failure) were similar regardless of whether participants were randomized to stimulation of the left or right vagal nerve. That’s an important finding because left-sided vagal nerve stimulation (VNS) technology could readily be combined with implantable cardioverter-defibrillators and cardiac resynchronization therapy devices, which are routinely placed on the left side of the thorax, Dr. Inder S. Anand observed in presenting the study findings at the annual meeting of the Heart Failure Society of America.
“We believe that if this technology pans out and proves effective, it will be introduced into devices very easily. That’s the advantage of left-sided stimulation,” explained Dr. Anand, professor of medicine at the University of Minnesota, Minneapolis.
He was quick to add, however, that “There needs to be a lot more work before autonomic regulation therapy is ready for prime time.” That’s because ANTHEM-HF was a relatively small prospective study – just 60 patients – and it was uncontrolled and unblinded.
Left-sided VNS is a well-established treatment for epilepsy, with more than 100,000 patients having received devices in the last several decades. It’s also seeing increasing use in refractory depression.
The approach is still investigational for heart failure, where autonomic imbalance with increased sympathetic activity and decreased parasympathetic tone is associated with heart failure progression and worse clinical outcomes, the cardiologist explained.
In ANTHEM-HF, 60 patients on optimal medical therapy for heart failure with reduced ejection fraction were randomized to left- or right-sided implantation of the Cyberonics VNS device. Once activated, the VNS intensity was titrated over 10 weeks to the maximum tolerable current that remained below the threshold of heart rate change, side effects, and patient sensation. The chronic intermittent stimulation to the vagus nerve was delivered at 10 Hz, a 250 microsec pulse width, and an average stimulation current of 2.0 mA. The stimulation cycle was 14 seconds on, 66 seconds off.
The primary study endpoint was change in LVEF over the course of 6 months. From a baseline of 32.4% it improved significantly to 37.2%, which Dr. Anand deemed “very impressive” for just 6 months of therapy. The improvement was similar regardless of whether the VNS was left or right sided.
He said the study didn’t raise any safety concerns. The only serious treatment-related adverse event was a fatal perioperative embolic stroke in a recipient of a left-sided device.
The most common adverse events were hoarseness or other voice alterations, which occurred in 19 patients. Thirteen patients reported a new cough. These side effects mirror the experience in patients treated with the VNS device for epilepsy or depression.
Discussant Jagmeet P. Singh emphasized that “it’s important to interpret the ANTHEM-HF results with some degree of caution.” That’s because the usual variability within LVEF measurements can be in the 5% range, and the 4.8% improvement seen in this study wasn’t accompanied by a significant improvement in LV end systolic volume. Moreover, the improvements seen in secondary endpoints – 6-minute walk distance, NYHA class, and the Minnesota Living With Heart Failure qualify-of-life score – are all potentially open to bias since neither patients nor physicians were blinded.
Dr. Singh noted that the NECTAR-HF study, a blinded, sham-controlled clinical trial of VNS presented earlier at the annual congress of the European Society of Cardiology, was a negative study that showed no improvement in functional measures. While NECTAR-HF caused some skeptics to question the whole concept of VNS as a useful therapeutic strategy, the stimulation protocols used in NECTAR-HF and ANTHEM-HF were quite different, and it’s likely that the stimulation amplitude employed in NECTAR-HF wasn’t sufficient to affect the target subset of vagal nerve fibers, according to Dr. Singh, director of the cardiac resynchronization therapy program and the Holter laboratory at Massachusetts General Hospital, Boston.
He considers the ANTHEM-HF finding that left-sided VNS is comparable to right to be an important advance that “really moves the field forward.” Yet many critical questions about autonomic regulation therapy for heart failure remain unanswered. These include whether it truly is safe and effective, and if so, the optimal target dose and frequency of stimulation. Answers should be forthcoming from the ongoing INOVATE-HF study, a randomized, controlled, 650-patient study, sponsored by BioControl Medical. The trial features hard clinical endpoints and higher target-dose stimulation protocols than in prior studies.
“This trial will probably put an end to the debate as to whether vagal nerve stimulation has an impact on heart failure or not,” the cardiologist predicted.
“Strategies to nonpharmacologically modulate the autonomic nervous system is an area of immense interest and investigation, and it’s going to be extended,” he observed. “I think that this is the autonomic era, and so it’s really important that we get it right. I think we’ve learned from the renal denervation experience that we need to be really careful in selecting patients for any form of autonomic manipulation. I don’t think we should select patients just off their LVEF. We should have some parameter that can quantify autonomic dysregulation prior to initiation of therapy.”
It will also be important to be able to individualize VNS therapy, just as physicians now do with beta blockers and other pharmacotherapies, he added.
The ANTHEM-HF trial was sponsored by Cyberonics. Dr. Anand reported serving as a consultant to and/or recipient of research grants from Cyberonics, Amgen, Critical Diagnostics, Novartis, and Zensun. Dr. Singh has received research support and/or served on speakers bureaus for Boston Scientific, St. Jude Medical, and the Sorin Group.
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Autonomic regulation therapy via a vagal nerve stimulation device shows promise for the treatment of heart failure with reduced ejection fraction.
Major finding: During 6 months of vagal nerve stimulation, patients’ mean LVEF rose significantly from 32.4% to 37.2%, with similar results regardless of whether the stimulation was left- or right-sided.
Data source: The ANTHEM-HF study was a prospective, unblinded, and uncontrolled study involving 60 patients who received vagal nerve stimulation for 6 months.
Disclosures: The study was sponsored by Cyberonics. The presenter has received research grants from and served as a consultant to that and other companies.
Four-antigen vaccine boosts S. aureus antibodies
PHILADELPHIA – A single injection of the investigational 4-antigen SA4Ag vaccine was well tolerated and induced robust antibody responses against Staphylococcusaureus in healthy adults in a phase I/II study.
“The very quick development of antibodies suggest that the vaccine could be used pre-operatively [2-3 weeks] for elective surgeries that are at higher risk of Staph. infection, such as thoracotomy or insertion of rods for scoliosis or spinal fusion,” Dr. Robert W. Frenck Jr. said in an interview at Infectious Diseases Week 2014.
As to the durability of responses, antibody levels for all four antigens in the vaccine rose rapidly and then decayed slowly over the next 12 months, he said. “The results support the continued development of SA4Ag for the prevention of invasive S. aureus disease in at-risk adults, including those undergoing elective surgery.”
There is no licensed vaccine that prevents invasive S. aureus infection, although several multi-antigen vaccines are in development.
SA4Ag vaccine is a second-generation vaccine to Pfizer’s 3-antigen SA3AG candidate vaccine and was granted Fast Track designation by the U.S. Food and Drug Administration in February 2014, according to the manufacturer.
SA4Ag contains capsular polysaccharides serotypes 5 and 8 (CP5 and CP8) individually conjugated to CRM₁₉₇, a recombinant surface protein clumping factor A (rmClfA), with a recombinant manganese transporter protein C known as rP305A. Each component was selected based on the virulence of these antigens in S. aureus infections, Dr. Frenck Jr., professor of pediatrics and interim director of infectious diseases, at Cincinnati (Ohio) Children’s Hospital Medical Center, said.
The study stratified 456 healthy adults by age (18-49 years and 50-64 years) and then randomly assigned them to receive a single injection of placebo or one of three formulations of SA4Ag: fixed doses of 30 μg CP5-CRM₁₉₇, 30 μg CP8-CRM₁₉₇, and 60 μg rmClfA, and either a low- (20 μg), mid- (60 μg), or high- (200 μg) rP305A dose.
The participants average age was 45 years, 57% were female, 73% were white, and 87.5% completed the study through month 12.
Local reactions reported through day 14 were mild or moderate, and systemic events and other adverse events were comparable across all groups, Dr. Frenck Jr. said. No vaccine-related serious adverse events or deaths were reported.
At day 29, all participants vaccinated with SA4Ag achieved the CP5 opsonophagocytic activity (OPA) threshold (≥ 1,000 titers) and 96%–99% met the CP8 OPA threshold (≥ 2,000 titers).
“The percentage of subjects reaching the threshold is equivalent for each one of the vaccine doses, indicating that the rP305A did not affect the immune response to the other components in the vaccine,” Dr. Frenck Jr. said.
Less than 25% of patients given placebo achieved the CP5 or CP8 OPA thresholds.
Immune responses to ClfA were robust by day 15 and did not vary by the dose of rP305A, again suggesting that rP305A does not affect the other three antigens in the vaccine, he observed.
Immune responses to the rP305A antigen were dose dependent, with the percentage of patients with a threshold response increasing step-wise from the low (47%), mid (63.2%), and high (83%) doses.
By day 29, there was a very brisk rise in geometric mean titers for CP5. Responses did not differ by rP305A dose or across age groups, with a similar pattern observed for CP8 and ClfA, Dr. Fenck Jr. said.
Session co-moderator Dr. Walter Orenstein, from Emory University in Atlanta, commented that the rate of decay post-vaccination was promising, particularly compared with that observed with meningococcal vaccines.
The vaccine “program is exciting because it not only relies on just antibody, but it actually looks at functional responses and those functional responses have some correlate with protection against staphylococcal disease,” he said in an interview. High-risk populations, like renal dialysis patients, were not studied and it would be interesting to see how well they would respond. “Previous studies have shown transient benefits for those types of populations.”
PHILADELPHIA – A single injection of the investigational 4-antigen SA4Ag vaccine was well tolerated and induced robust antibody responses against Staphylococcusaureus in healthy adults in a phase I/II study.
“The very quick development of antibodies suggest that the vaccine could be used pre-operatively [2-3 weeks] for elective surgeries that are at higher risk of Staph. infection, such as thoracotomy or insertion of rods for scoliosis or spinal fusion,” Dr. Robert W. Frenck Jr. said in an interview at Infectious Diseases Week 2014.
As to the durability of responses, antibody levels for all four antigens in the vaccine rose rapidly and then decayed slowly over the next 12 months, he said. “The results support the continued development of SA4Ag for the prevention of invasive S. aureus disease in at-risk adults, including those undergoing elective surgery.”
There is no licensed vaccine that prevents invasive S. aureus infection, although several multi-antigen vaccines are in development.
SA4Ag vaccine is a second-generation vaccine to Pfizer’s 3-antigen SA3AG candidate vaccine and was granted Fast Track designation by the U.S. Food and Drug Administration in February 2014, according to the manufacturer.
SA4Ag contains capsular polysaccharides serotypes 5 and 8 (CP5 and CP8) individually conjugated to CRM₁₉₇, a recombinant surface protein clumping factor A (rmClfA), with a recombinant manganese transporter protein C known as rP305A. Each component was selected based on the virulence of these antigens in S. aureus infections, Dr. Frenck Jr., professor of pediatrics and interim director of infectious diseases, at Cincinnati (Ohio) Children’s Hospital Medical Center, said.
The study stratified 456 healthy adults by age (18-49 years and 50-64 years) and then randomly assigned them to receive a single injection of placebo or one of three formulations of SA4Ag: fixed doses of 30 μg CP5-CRM₁₉₇, 30 μg CP8-CRM₁₉₇, and 60 μg rmClfA, and either a low- (20 μg), mid- (60 μg), or high- (200 μg) rP305A dose.
The participants average age was 45 years, 57% were female, 73% were white, and 87.5% completed the study through month 12.
Local reactions reported through day 14 were mild or moderate, and systemic events and other adverse events were comparable across all groups, Dr. Frenck Jr. said. No vaccine-related serious adverse events or deaths were reported.
At day 29, all participants vaccinated with SA4Ag achieved the CP5 opsonophagocytic activity (OPA) threshold (≥ 1,000 titers) and 96%–99% met the CP8 OPA threshold (≥ 2,000 titers).
“The percentage of subjects reaching the threshold is equivalent for each one of the vaccine doses, indicating that the rP305A did not affect the immune response to the other components in the vaccine,” Dr. Frenck Jr. said.
Less than 25% of patients given placebo achieved the CP5 or CP8 OPA thresholds.
Immune responses to ClfA were robust by day 15 and did not vary by the dose of rP305A, again suggesting that rP305A does not affect the other three antigens in the vaccine, he observed.
Immune responses to the rP305A antigen were dose dependent, with the percentage of patients with a threshold response increasing step-wise from the low (47%), mid (63.2%), and high (83%) doses.
By day 29, there was a very brisk rise in geometric mean titers for CP5. Responses did not differ by rP305A dose or across age groups, with a similar pattern observed for CP8 and ClfA, Dr. Fenck Jr. said.
Session co-moderator Dr. Walter Orenstein, from Emory University in Atlanta, commented that the rate of decay post-vaccination was promising, particularly compared with that observed with meningococcal vaccines.
The vaccine “program is exciting because it not only relies on just antibody, but it actually looks at functional responses and those functional responses have some correlate with protection against staphylococcal disease,” he said in an interview. High-risk populations, like renal dialysis patients, were not studied and it would be interesting to see how well they would respond. “Previous studies have shown transient benefits for those types of populations.”
PHILADELPHIA – A single injection of the investigational 4-antigen SA4Ag vaccine was well tolerated and induced robust antibody responses against Staphylococcusaureus in healthy adults in a phase I/II study.
“The very quick development of antibodies suggest that the vaccine could be used pre-operatively [2-3 weeks] for elective surgeries that are at higher risk of Staph. infection, such as thoracotomy or insertion of rods for scoliosis or spinal fusion,” Dr. Robert W. Frenck Jr. said in an interview at Infectious Diseases Week 2014.
As to the durability of responses, antibody levels for all four antigens in the vaccine rose rapidly and then decayed slowly over the next 12 months, he said. “The results support the continued development of SA4Ag for the prevention of invasive S. aureus disease in at-risk adults, including those undergoing elective surgery.”
There is no licensed vaccine that prevents invasive S. aureus infection, although several multi-antigen vaccines are in development.
SA4Ag vaccine is a second-generation vaccine to Pfizer’s 3-antigen SA3AG candidate vaccine and was granted Fast Track designation by the U.S. Food and Drug Administration in February 2014, according to the manufacturer.
SA4Ag contains capsular polysaccharides serotypes 5 and 8 (CP5 and CP8) individually conjugated to CRM₁₉₇, a recombinant surface protein clumping factor A (rmClfA), with a recombinant manganese transporter protein C known as rP305A. Each component was selected based on the virulence of these antigens in S. aureus infections, Dr. Frenck Jr., professor of pediatrics and interim director of infectious diseases, at Cincinnati (Ohio) Children’s Hospital Medical Center, said.
The study stratified 456 healthy adults by age (18-49 years and 50-64 years) and then randomly assigned them to receive a single injection of placebo or one of three formulations of SA4Ag: fixed doses of 30 μg CP5-CRM₁₉₇, 30 μg CP8-CRM₁₉₇, and 60 μg rmClfA, and either a low- (20 μg), mid- (60 μg), or high- (200 μg) rP305A dose.
The participants average age was 45 years, 57% were female, 73% were white, and 87.5% completed the study through month 12.
Local reactions reported through day 14 were mild or moderate, and systemic events and other adverse events were comparable across all groups, Dr. Frenck Jr. said. No vaccine-related serious adverse events or deaths were reported.
At day 29, all participants vaccinated with SA4Ag achieved the CP5 opsonophagocytic activity (OPA) threshold (≥ 1,000 titers) and 96%–99% met the CP8 OPA threshold (≥ 2,000 titers).
“The percentage of subjects reaching the threshold is equivalent for each one of the vaccine doses, indicating that the rP305A did not affect the immune response to the other components in the vaccine,” Dr. Frenck Jr. said.
Less than 25% of patients given placebo achieved the CP5 or CP8 OPA thresholds.
Immune responses to ClfA were robust by day 15 and did not vary by the dose of rP305A, again suggesting that rP305A does not affect the other three antigens in the vaccine, he observed.
Immune responses to the rP305A antigen were dose dependent, with the percentage of patients with a threshold response increasing step-wise from the low (47%), mid (63.2%), and high (83%) doses.
By day 29, there was a very brisk rise in geometric mean titers for CP5. Responses did not differ by rP305A dose or across age groups, with a similar pattern observed for CP8 and ClfA, Dr. Fenck Jr. said.
Session co-moderator Dr. Walter Orenstein, from Emory University in Atlanta, commented that the rate of decay post-vaccination was promising, particularly compared with that observed with meningococcal vaccines.
The vaccine “program is exciting because it not only relies on just antibody, but it actually looks at functional responses and those functional responses have some correlate with protection against staphylococcal disease,” he said in an interview. High-risk populations, like renal dialysis patients, were not studied and it would be interesting to see how well they would respond. “Previous studies have shown transient benefits for those types of populations.”
AT ID WEEK 2014
Key clinical point: A vaccine to prevent invasive Staphylococcus aureus infections is in the early stages of testing.
Major finding: At day 29, all participants who received the active vaccine achieved the CP5 opsonophagocytic activity threshold and 96%–99% met the CP8 threshold.
Data source: Double-blind phase I/II study in 456 healthy adults.
Disclosures: The study was funded by Pfizer. Dr. Frenck Jr. reported receiving grant support from Pfizer to conduct the study. Three co-authors are Pfizer employees.
In critically ill patients, dalteparin is more cost-effective for VTE prevention
The low molecular weight heparin dalteparin and unfractionated heparin are associated with similar rates of thrombosis and major bleeding, but dalteparin is associated with lower rates of pulmonary embolus and heparin-induced thrombocytopenia, based on results from a prospective randomized study.
Given for prevention of venous thromboembolism, median hospital costs per patient were $39,508 for dalteparin users and $40,805 for unfractionated heparin users. Dalteparin remained the least costly strategy until its acquisition costs rose from $8 per dose to $179, as reported online 1 November in the Journal of the American Medical Association [doi:10.1001/jama.2014.15101].
The economic analysis—conducted alongside the multi-centre, randomized PROTECT trial in 2344 critically-ill medical-surgical patients— showed no matter how low the acquisition cost of unfractionated heparin, there was no threshold that favored that form of prophylaxis, according to data also presented at the Critical Care Canada Forum.
“From a health care payer perspective, VTE prophylaxis with the LMWH [low molecular weight heparin] dalteparin in critically ill medical-surgical patients was more effective and had similar or lower costs than the use of UFH [unfractionated heparin],” wrote Dr. Robert A. Fowler, from the Sunnybrook Health Sciences Centre, University of Toronto, and colleagues.The E-PROTECT study was funded by the Heart and Stroke Foundation (Ontario, Canada), the University of Toronto, and the Canadian Intensive Care Foundation. PROTECT was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation (Canada), and the Australian and New Zealand College of Anesthetists Research Foundation. Some authors reported fees, support, and consultancies from the pharmaceutical industry.
The low molecular weight heparin dalteparin and unfractionated heparin are associated with similar rates of thrombosis and major bleeding, but dalteparin is associated with lower rates of pulmonary embolus and heparin-induced thrombocytopenia, based on results from a prospective randomized study.
Given for prevention of venous thromboembolism, median hospital costs per patient were $39,508 for dalteparin users and $40,805 for unfractionated heparin users. Dalteparin remained the least costly strategy until its acquisition costs rose from $8 per dose to $179, as reported online 1 November in the Journal of the American Medical Association [doi:10.1001/jama.2014.15101].
The economic analysis—conducted alongside the multi-centre, randomized PROTECT trial in 2344 critically-ill medical-surgical patients— showed no matter how low the acquisition cost of unfractionated heparin, there was no threshold that favored that form of prophylaxis, according to data also presented at the Critical Care Canada Forum.
“From a health care payer perspective, VTE prophylaxis with the LMWH [low molecular weight heparin] dalteparin in critically ill medical-surgical patients was more effective and had similar or lower costs than the use of UFH [unfractionated heparin],” wrote Dr. Robert A. Fowler, from the Sunnybrook Health Sciences Centre, University of Toronto, and colleagues.The E-PROTECT study was funded by the Heart and Stroke Foundation (Ontario, Canada), the University of Toronto, and the Canadian Intensive Care Foundation. PROTECT was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation (Canada), and the Australian and New Zealand College of Anesthetists Research Foundation. Some authors reported fees, support, and consultancies from the pharmaceutical industry.
The low molecular weight heparin dalteparin and unfractionated heparin are associated with similar rates of thrombosis and major bleeding, but dalteparin is associated with lower rates of pulmonary embolus and heparin-induced thrombocytopenia, based on results from a prospective randomized study.
Given for prevention of venous thromboembolism, median hospital costs per patient were $39,508 for dalteparin users and $40,805 for unfractionated heparin users. Dalteparin remained the least costly strategy until its acquisition costs rose from $8 per dose to $179, as reported online 1 November in the Journal of the American Medical Association [doi:10.1001/jama.2014.15101].
The economic analysis—conducted alongside the multi-centre, randomized PROTECT trial in 2344 critically-ill medical-surgical patients— showed no matter how low the acquisition cost of unfractionated heparin, there was no threshold that favored that form of prophylaxis, according to data also presented at the Critical Care Canada Forum.
“From a health care payer perspective, VTE prophylaxis with the LMWH [low molecular weight heparin] dalteparin in critically ill medical-surgical patients was more effective and had similar or lower costs than the use of UFH [unfractionated heparin],” wrote Dr. Robert A. Fowler, from the Sunnybrook Health Sciences Centre, University of Toronto, and colleagues.The E-PROTECT study was funded by the Heart and Stroke Foundation (Ontario, Canada), the University of Toronto, and the Canadian Intensive Care Foundation. PROTECT was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation (Canada), and the Australian and New Zealand College of Anesthetists Research Foundation. Some authors reported fees, support, and consultancies from the pharmaceutical industry.
FROM JAMA
Key clinical point: Dalteparin is more cost-effective than unfractionated heparin in the prevention of venous thromboembolism.
Major finding: Dalteparin is as effective as unfractionated heparin in reducing thrombosis, for the same cost, but with less pulmonary embolus and heparin-induced thrombocytopenia.
Data source: Economic analysis of a prospective randomized controlled trial of low molecular weight heparin dalteparin versus unfractionated heparin in 2344 critically-ill medical-surgical patients
Disclosures: The E-PROTECT study was funded by the Heart and Stroke Foundation (Ontario, Canada), the University of Toronto, and the Canadian Intensive Care Foundation. PROTECT was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation (Canada), and the Australian and New Zealand College of Anesthetists Research Foundation. Some authors reported fees, support, and consultancies to the pharmaceutical industry.
Medicare finalizes $40 per month payments for patient care coordination
Time spent on care coordination outside of the face-to-face visit will receive pay recognition in 2015, as a result of a regulation from the Centers for Medicare & Medicaid Services.
On Oct. 31, CMS officials released the final 2015 Medicare Physician Fee Schedule rule, which lays out how much, and under what conditions, payment will be made for providing care coordination services to Medicare patients with multiple chronic conditions.
Starting Jan. 1, about $40 per patient per month can be earned for non-face-to-face chronic care management services, such as developing and revising the care plan, communicating with other treating providers, and managing medications.
The billing codes – CPT codes 99490 and 99487 and 99489 – apply only to Medicare patients with two or more significant, chronic conditions. CMS plans to make a bundled payment for codes 99487 and 99489, according to the rule.
CMS will also allow more “flexibility” in the supervision of clinical staff who provide care coordination services. Under the rule, physicians can bill “incident to” services provided by clinical staff members, even if they are not direct employees and are under general, but not direct, supervision.
CMS said the rules for “incident to” services are somewhat looser than usual because of the nature of non-face-to-face care coordination, which often involves after-hours contact with nurses and coordination with providers who are not direct employees of the physician practice.
The fee schedule final rule also expands access to telehealth services for Medicare beneficiaries. Medicare will now allow annual wellness visits, psychoanalysis, psychotherapy, and prolonged evaluation and management services to be performed via telemedicine.
CMS is also finalizing changes to the Open Payments Program, which aims to increase transparency by requiring drug, device, and biological manufacturers to report on their payments to physicians and teaching hospitals. CMS published the first round of payment data on Sept. 30, 2014.
The most significant change for physicians is the elimination of the continuing education exclusion. Currently, manufacturers do not have to report payments made indirectly to speakers at continuing medication education (CME) courses, provided the events are organized by certain accredited groups (the Accreditation Council for Continuing Medical Education, the American Academy of Family Physicians, the American Medical Association, or the American Osteopathic Association) and that the manufacturer has not had a role in recruiting or influencing the speakers.
Under the change finalized in the rule, CMS would no longer provide the exemption only for CME events run by accredited groups. Instead, indirect CME payments will be excluded from reporting across the board, provided the payments meet other rules about lack of industry interference. The new rules will take effect Jan. 1, 2016.
The CME change “will create a more consistent reporting requirement, and will also be more consistent for consumers who will ultimately have access to the reported data,” CMS wrote in a fact sheet on the rule.
The agency will also require manufacturers to report stocks, stock options, or any other ownership interest as distinct categories. The changes aren’t expected to be implemented until the 2016 data collection period, according to CMS.
The final fee schedule rule also reminds physicians of the looming Sustainable Growth Rate cut. Unless Congress acts to avert the cut before March 31, 2015, physicians will face on average a 21.2% across-the-board Medicare fee reduction.
The final rule will be published in the Federal Register on Nov. 13.
Time spent on care coordination outside of the face-to-face visit will receive pay recognition in 2015, as a result of a regulation from the Centers for Medicare & Medicaid Services.
On Oct. 31, CMS officials released the final 2015 Medicare Physician Fee Schedule rule, which lays out how much, and under what conditions, payment will be made for providing care coordination services to Medicare patients with multiple chronic conditions.
Starting Jan. 1, about $40 per patient per month can be earned for non-face-to-face chronic care management services, such as developing and revising the care plan, communicating with other treating providers, and managing medications.
The billing codes – CPT codes 99490 and 99487 and 99489 – apply only to Medicare patients with two or more significant, chronic conditions. CMS plans to make a bundled payment for codes 99487 and 99489, according to the rule.
CMS will also allow more “flexibility” in the supervision of clinical staff who provide care coordination services. Under the rule, physicians can bill “incident to” services provided by clinical staff members, even if they are not direct employees and are under general, but not direct, supervision.
CMS said the rules for “incident to” services are somewhat looser than usual because of the nature of non-face-to-face care coordination, which often involves after-hours contact with nurses and coordination with providers who are not direct employees of the physician practice.
The fee schedule final rule also expands access to telehealth services for Medicare beneficiaries. Medicare will now allow annual wellness visits, psychoanalysis, psychotherapy, and prolonged evaluation and management services to be performed via telemedicine.
CMS is also finalizing changes to the Open Payments Program, which aims to increase transparency by requiring drug, device, and biological manufacturers to report on their payments to physicians and teaching hospitals. CMS published the first round of payment data on Sept. 30, 2014.
The most significant change for physicians is the elimination of the continuing education exclusion. Currently, manufacturers do not have to report payments made indirectly to speakers at continuing medication education (CME) courses, provided the events are organized by certain accredited groups (the Accreditation Council for Continuing Medical Education, the American Academy of Family Physicians, the American Medical Association, or the American Osteopathic Association) and that the manufacturer has not had a role in recruiting or influencing the speakers.
Under the change finalized in the rule, CMS would no longer provide the exemption only for CME events run by accredited groups. Instead, indirect CME payments will be excluded from reporting across the board, provided the payments meet other rules about lack of industry interference. The new rules will take effect Jan. 1, 2016.
The CME change “will create a more consistent reporting requirement, and will also be more consistent for consumers who will ultimately have access to the reported data,” CMS wrote in a fact sheet on the rule.
The agency will also require manufacturers to report stocks, stock options, or any other ownership interest as distinct categories. The changes aren’t expected to be implemented until the 2016 data collection period, according to CMS.
The final fee schedule rule also reminds physicians of the looming Sustainable Growth Rate cut. Unless Congress acts to avert the cut before March 31, 2015, physicians will face on average a 21.2% across-the-board Medicare fee reduction.
The final rule will be published in the Federal Register on Nov. 13.
Time spent on care coordination outside of the face-to-face visit will receive pay recognition in 2015, as a result of a regulation from the Centers for Medicare & Medicaid Services.
On Oct. 31, CMS officials released the final 2015 Medicare Physician Fee Schedule rule, which lays out how much, and under what conditions, payment will be made for providing care coordination services to Medicare patients with multiple chronic conditions.
Starting Jan. 1, about $40 per patient per month can be earned for non-face-to-face chronic care management services, such as developing and revising the care plan, communicating with other treating providers, and managing medications.
The billing codes – CPT codes 99490 and 99487 and 99489 – apply only to Medicare patients with two or more significant, chronic conditions. CMS plans to make a bundled payment for codes 99487 and 99489, according to the rule.
CMS will also allow more “flexibility” in the supervision of clinical staff who provide care coordination services. Under the rule, physicians can bill “incident to” services provided by clinical staff members, even if they are not direct employees and are under general, but not direct, supervision.
CMS said the rules for “incident to” services are somewhat looser than usual because of the nature of non-face-to-face care coordination, which often involves after-hours contact with nurses and coordination with providers who are not direct employees of the physician practice.
The fee schedule final rule also expands access to telehealth services for Medicare beneficiaries. Medicare will now allow annual wellness visits, psychoanalysis, psychotherapy, and prolonged evaluation and management services to be performed via telemedicine.
CMS is also finalizing changes to the Open Payments Program, which aims to increase transparency by requiring drug, device, and biological manufacturers to report on their payments to physicians and teaching hospitals. CMS published the first round of payment data on Sept. 30, 2014.
The most significant change for physicians is the elimination of the continuing education exclusion. Currently, manufacturers do not have to report payments made indirectly to speakers at continuing medication education (CME) courses, provided the events are organized by certain accredited groups (the Accreditation Council for Continuing Medical Education, the American Academy of Family Physicians, the American Medical Association, or the American Osteopathic Association) and that the manufacturer has not had a role in recruiting or influencing the speakers.
Under the change finalized in the rule, CMS would no longer provide the exemption only for CME events run by accredited groups. Instead, indirect CME payments will be excluded from reporting across the board, provided the payments meet other rules about lack of industry interference. The new rules will take effect Jan. 1, 2016.
The CME change “will create a more consistent reporting requirement, and will also be more consistent for consumers who will ultimately have access to the reported data,” CMS wrote in a fact sheet on the rule.
The agency will also require manufacturers to report stocks, stock options, or any other ownership interest as distinct categories. The changes aren’t expected to be implemented until the 2016 data collection period, according to CMS.
The final fee schedule rule also reminds physicians of the looming Sustainable Growth Rate cut. Unless Congress acts to avert the cut before March 31, 2015, physicians will face on average a 21.2% across-the-board Medicare fee reduction.
The final rule will be published in the Federal Register on Nov. 13.
VIDEO: How to negotiate the robotic surgery learning curve
SAN FRANCISCO– Before attempting robotic surgery, it’s important to be comfortable with both the open and laparoscopic versions of the procedure, according to Dr. Kenneth Meredith, director of robotic surgery at the University of Wisconsin, Madison.
In experienced hands, robotic results can be good. In a case series of 138 robotic-assisted Ivor Lewis esophagectomies at the university for esophageal cancer, the median intensive care unit stay was 2 days and median hospital stay 9 days, Dr. Meredith. Complications occurred in about a quarter of patients.
In a video interview at the American College of Surgeons Clinical Congress, Dr. Meredith explained the significance of the findings and gave tips on how to negotiate the robotic surgery learning curve.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO– Before attempting robotic surgery, it’s important to be comfortable with both the open and laparoscopic versions of the procedure, according to Dr. Kenneth Meredith, director of robotic surgery at the University of Wisconsin, Madison.
In experienced hands, robotic results can be good. In a case series of 138 robotic-assisted Ivor Lewis esophagectomies at the university for esophageal cancer, the median intensive care unit stay was 2 days and median hospital stay 9 days, Dr. Meredith. Complications occurred in about a quarter of patients.
In a video interview at the American College of Surgeons Clinical Congress, Dr. Meredith explained the significance of the findings and gave tips on how to negotiate the robotic surgery learning curve.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO– Before attempting robotic surgery, it’s important to be comfortable with both the open and laparoscopic versions of the procedure, according to Dr. Kenneth Meredith, director of robotic surgery at the University of Wisconsin, Madison.
In experienced hands, robotic results can be good. In a case series of 138 robotic-assisted Ivor Lewis esophagectomies at the university for esophageal cancer, the median intensive care unit stay was 2 days and median hospital stay 9 days, Dr. Meredith. Complications occurred in about a quarter of patients.
In a video interview at the American College of Surgeons Clinical Congress, Dr. Meredith explained the significance of the findings and gave tips on how to negotiate the robotic surgery learning curve.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AMERICAN COLLEGE OF SURGEONS CLINICAL CONGRESS
VIDEO: Nonclinical factors affect lung resection survival
SAN FRANCISCO– A study of data from more than 200,000 patients identified several nonclinical factors ssociated with 30-day survival after lung resection for non–small cell lung cancer, Dr. Manu S. Sancheti reported at the annual clinical congress of the American College of Surgeons.
The analysis by Dr. Sancheti and his associates won the top prize for poster presentations at the congress.
In an interview at the award presentation, Dr. Sancheti of Emory University, Atlanta, described his results and ideas about how physicians and health care systems might use this information to improve care.
He reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN FRANCISCO– A study of data from more than 200,000 patients identified several nonclinical factors ssociated with 30-day survival after lung resection for non–small cell lung cancer, Dr. Manu S. Sancheti reported at the annual clinical congress of the American College of Surgeons.
The analysis by Dr. Sancheti and his associates won the top prize for poster presentations at the congress.
In an interview at the award presentation, Dr. Sancheti of Emory University, Atlanta, described his results and ideas about how physicians and health care systems might use this information to improve care.
He reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
SAN FRANCISCO– A study of data from more than 200,000 patients identified several nonclinical factors ssociated with 30-day survival after lung resection for non–small cell lung cancer, Dr. Manu S. Sancheti reported at the annual clinical congress of the American College of Surgeons.
The analysis by Dr. Sancheti and his associates won the top prize for poster presentations at the congress.
In an interview at the award presentation, Dr. Sancheti of Emory University, Atlanta, described his results and ideas about how physicians and health care systems might use this information to improve care.
He reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @sherryboschert
AT THE ACS CLINICAL CONGRESS
Comborbidities likely explain opioid + sleep apnea mortality risk
AUSTIN, TEX. – Any association between opioid use and death in patients with sleep apnea cannot be explained by sleep apnea alone, according to a retrospective analysis of data from the prospective observational DREAM study.
In 1,867 patients with moderate or severe sleep apnea who were on an opioid medication, no association was seen between opioid use and severity of sleep-disordered breathing, even with increasing doses, Dr. Husham Sharifi reported at the annual meeting of the American College of Chest Physicians.
Further, when opioid use was analyzed as an unadjusted variable, it was associated with an increase in mortality (odds ratio, 1.53), but this effect was attenuated by adjustment for sleep apnea (OR, 1.52), and was further attenuated – to the point where it was no longer statistically significant – by adjustment for both sleep apnea and Charlson Comorbidity Index (OR, 1.37), said Dr. Sharifi, who was an attending physician at Brigham and Women’s Hospital, Boston, at the time he completed this research. He is now a fellow at Stanford (Calif.) University.
Sleep apnea remained an independent predictor of mortality even after adjustment for opioid use and Charlson Comorbidity Index, he said.
The DREAM study, which stands for Determining Risk of Vascular Events by Apnea Monitoring, includes a well-defined cohort of patients at three Veterans Administration sleep centers who were referred for overnight polysomnography for suspected sleep-disordered breathing between Jan. 1, 2000, and Dec. 31, 2004. All patients had an Apnea-Hypopnea Index score greater than 15, indicating moderate to severe sleep apnea, and all were on opioid medication. The patients were followed for between 3 and 10 years, with follow-up ending Dec. 31, 2010.
Opioid use has increased dramatically over the past 20 years – by more than 700%, Dr. Sharifi said.
While there does not appear to be a significant impact of opioid use on daytime respiration, there are some limited data suggesting that it may be associated with sleep-disordered breathing, he said.
The current findings, though limited by a number of factors including the observational nature of the study and possible selection bias as the DREAM cohort is a referral population, suggest that the relationship between opioid use and sleep apnea death is likely explained not by sleep apnea, but by an increased prevalence of known risk factors for morbidity and mortality in patients who take opioids and have sleep apnea, he concluded.
Dr. Sharifi reported having no disclosures.
AUSTIN, TEX. – Any association between opioid use and death in patients with sleep apnea cannot be explained by sleep apnea alone, according to a retrospective analysis of data from the prospective observational DREAM study.
In 1,867 patients with moderate or severe sleep apnea who were on an opioid medication, no association was seen between opioid use and severity of sleep-disordered breathing, even with increasing doses, Dr. Husham Sharifi reported at the annual meeting of the American College of Chest Physicians.
Further, when opioid use was analyzed as an unadjusted variable, it was associated with an increase in mortality (odds ratio, 1.53), but this effect was attenuated by adjustment for sleep apnea (OR, 1.52), and was further attenuated – to the point where it was no longer statistically significant – by adjustment for both sleep apnea and Charlson Comorbidity Index (OR, 1.37), said Dr. Sharifi, who was an attending physician at Brigham and Women’s Hospital, Boston, at the time he completed this research. He is now a fellow at Stanford (Calif.) University.
Sleep apnea remained an independent predictor of mortality even after adjustment for opioid use and Charlson Comorbidity Index, he said.
The DREAM study, which stands for Determining Risk of Vascular Events by Apnea Monitoring, includes a well-defined cohort of patients at three Veterans Administration sleep centers who were referred for overnight polysomnography for suspected sleep-disordered breathing between Jan. 1, 2000, and Dec. 31, 2004. All patients had an Apnea-Hypopnea Index score greater than 15, indicating moderate to severe sleep apnea, and all were on opioid medication. The patients were followed for between 3 and 10 years, with follow-up ending Dec. 31, 2010.
Opioid use has increased dramatically over the past 20 years – by more than 700%, Dr. Sharifi said.
While there does not appear to be a significant impact of opioid use on daytime respiration, there are some limited data suggesting that it may be associated with sleep-disordered breathing, he said.
The current findings, though limited by a number of factors including the observational nature of the study and possible selection bias as the DREAM cohort is a referral population, suggest that the relationship between opioid use and sleep apnea death is likely explained not by sleep apnea, but by an increased prevalence of known risk factors for morbidity and mortality in patients who take opioids and have sleep apnea, he concluded.
Dr. Sharifi reported having no disclosures.
AUSTIN, TEX. – Any association between opioid use and death in patients with sleep apnea cannot be explained by sleep apnea alone, according to a retrospective analysis of data from the prospective observational DREAM study.
In 1,867 patients with moderate or severe sleep apnea who were on an opioid medication, no association was seen between opioid use and severity of sleep-disordered breathing, even with increasing doses, Dr. Husham Sharifi reported at the annual meeting of the American College of Chest Physicians.
Further, when opioid use was analyzed as an unadjusted variable, it was associated with an increase in mortality (odds ratio, 1.53), but this effect was attenuated by adjustment for sleep apnea (OR, 1.52), and was further attenuated – to the point where it was no longer statistically significant – by adjustment for both sleep apnea and Charlson Comorbidity Index (OR, 1.37), said Dr. Sharifi, who was an attending physician at Brigham and Women’s Hospital, Boston, at the time he completed this research. He is now a fellow at Stanford (Calif.) University.
Sleep apnea remained an independent predictor of mortality even after adjustment for opioid use and Charlson Comorbidity Index, he said.
The DREAM study, which stands for Determining Risk of Vascular Events by Apnea Monitoring, includes a well-defined cohort of patients at three Veterans Administration sleep centers who were referred for overnight polysomnography for suspected sleep-disordered breathing between Jan. 1, 2000, and Dec. 31, 2004. All patients had an Apnea-Hypopnea Index score greater than 15, indicating moderate to severe sleep apnea, and all were on opioid medication. The patients were followed for between 3 and 10 years, with follow-up ending Dec. 31, 2010.
Opioid use has increased dramatically over the past 20 years – by more than 700%, Dr. Sharifi said.
While there does not appear to be a significant impact of opioid use on daytime respiration, there are some limited data suggesting that it may be associated with sleep-disordered breathing, he said.
The current findings, though limited by a number of factors including the observational nature of the study and possible selection bias as the DREAM cohort is a referral population, suggest that the relationship between opioid use and sleep apnea death is likely explained not by sleep apnea, but by an increased prevalence of known risk factors for morbidity and mortality in patients who take opioids and have sleep apnea, he concluded.
Dr. Sharifi reported having no disclosures.
Key clinical point: Comorbid conditions may explain the relationship between opioid use, sleep apnea, and death.
Major finding: Opioid use was not significantly associated with mortality after adjustment for sleep apnea and comorbidities (odds ratio, 1.37).
Data source: A retrospective analysis of data for 1,867 patients from the prospective observational DREAM cohort.
Disclosures: Dr. Sharifi reported having no disclosures.
ACA implementation hinges on election outcomes
The outcome of the 2014 midterm election could impact continued implementation of the Affordable Care Act.
“Even though health care is only one of a number of top issues in the 2014 election, the majority party in Congress will claim it has a mandate for its priorities on what is still a controversial national health care issue,” according to an analysis of public opinion polls by Robert Blendon, Sc.D., and John Benson of the Harvard School of Public Health published Oct. 29 in the New England Journal of Medicine.
Even if Republicans take control of the Senate and maintain control of the House of Representatives, they are unlikely to be successful at repealing the ACA, but they “could … reduce federal support for health insurance subsidies, Medicaid expansion, public health grants, innovation investments, program administrative information and outlays for research,” Dr. Blendon and Mr. Benson suggested.
A Republican-controlled Congress also would probably delay employer and individual mandates and reduce taxes on employers, insurers, and health care companies.
If Democrats maintain their majority in the Senate, the authors predicted continued ACA implementation and more states to expand Medicaid services.
The investigators looked at 27 public opinion polls from 14 organizations (N. Engl. J. Med. 2014 Oct. 29 doi:10.1056/NEJMsr1412118). They noted that “a larger proportion of likely voters say they are less likely to vote for a congressional candidate who supports the ACA (40%) than say that they are more likely to vote for such a candidate (31%).” Twenty-seven percent say that a candidate’s stance on the ACA will not impact their vote.
More independent voters say that they are less likely to vote for an anti-ACA candidate (43%) than a pro-ACA candidate (25%).
Finally, Dr. Blendon and Mr. Benson wrote that, while a majority of voters do not favor outright repeal of the health care reform law, many favor either repealing the ACA (31%) or scaling it back (23%).
The outcome of the 2014 midterm election could impact continued implementation of the Affordable Care Act.
“Even though health care is only one of a number of top issues in the 2014 election, the majority party in Congress will claim it has a mandate for its priorities on what is still a controversial national health care issue,” according to an analysis of public opinion polls by Robert Blendon, Sc.D., and John Benson of the Harvard School of Public Health published Oct. 29 in the New England Journal of Medicine.
Even if Republicans take control of the Senate and maintain control of the House of Representatives, they are unlikely to be successful at repealing the ACA, but they “could … reduce federal support for health insurance subsidies, Medicaid expansion, public health grants, innovation investments, program administrative information and outlays for research,” Dr. Blendon and Mr. Benson suggested.
A Republican-controlled Congress also would probably delay employer and individual mandates and reduce taxes on employers, insurers, and health care companies.
If Democrats maintain their majority in the Senate, the authors predicted continued ACA implementation and more states to expand Medicaid services.
The investigators looked at 27 public opinion polls from 14 organizations (N. Engl. J. Med. 2014 Oct. 29 doi:10.1056/NEJMsr1412118). They noted that “a larger proportion of likely voters say they are less likely to vote for a congressional candidate who supports the ACA (40%) than say that they are more likely to vote for such a candidate (31%).” Twenty-seven percent say that a candidate’s stance on the ACA will not impact their vote.
More independent voters say that they are less likely to vote for an anti-ACA candidate (43%) than a pro-ACA candidate (25%).
Finally, Dr. Blendon and Mr. Benson wrote that, while a majority of voters do not favor outright repeal of the health care reform law, many favor either repealing the ACA (31%) or scaling it back (23%).
The outcome of the 2014 midterm election could impact continued implementation of the Affordable Care Act.
“Even though health care is only one of a number of top issues in the 2014 election, the majority party in Congress will claim it has a mandate for its priorities on what is still a controversial national health care issue,” according to an analysis of public opinion polls by Robert Blendon, Sc.D., and John Benson of the Harvard School of Public Health published Oct. 29 in the New England Journal of Medicine.
Even if Republicans take control of the Senate and maintain control of the House of Representatives, they are unlikely to be successful at repealing the ACA, but they “could … reduce federal support for health insurance subsidies, Medicaid expansion, public health grants, innovation investments, program administrative information and outlays for research,” Dr. Blendon and Mr. Benson suggested.
A Republican-controlled Congress also would probably delay employer and individual mandates and reduce taxes on employers, insurers, and health care companies.
If Democrats maintain their majority in the Senate, the authors predicted continued ACA implementation and more states to expand Medicaid services.
The investigators looked at 27 public opinion polls from 14 organizations (N. Engl. J. Med. 2014 Oct. 29 doi:10.1056/NEJMsr1412118). They noted that “a larger proportion of likely voters say they are less likely to vote for a congressional candidate who supports the ACA (40%) than say that they are more likely to vote for such a candidate (31%).” Twenty-seven percent say that a candidate’s stance on the ACA will not impact their vote.
More independent voters say that they are less likely to vote for an anti-ACA candidate (43%) than a pro-ACA candidate (25%).
Finally, Dr. Blendon and Mr. Benson wrote that, while a majority of voters do not favor outright repeal of the health care reform law, many favor either repealing the ACA (31%) or scaling it back (23%).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE