User login
Official Newspaper of the American College of Surgeons
Perioperative fluid levels increase ileus risk
SAN FRANCISCO – Too much or too little IV fluid on the day of surgery was associated with a 10%-12% increased risk for postoperative ileus in a retrospective study of 84,722 patients undergoing colon surgery.
Patients who received more than 5 liters of IV fluid on the day of surgery had a 10% increased risk of postoperative ileus and patients who received no more than 1.7 L on the surgery had a 12% increased risk of postoperative ileus, compared with patients who received 1.71-5 L of fluid, Dr. Julie K. Marosky Thacker and her associates reported at the annual clinical congress of the American College of Surgeons.
“This is one of the first studies to show that in a U.S.-based review, we have a significant number of patients getting over 5 L of fluid on the day of colon operation,” and that both high and low fluids increase the risk of postoperative ileus, Dr. Thacker said. “Observed fluid use is not compliant with the recommendations that are widespread and described in the principles of Enhance Recovery After Surgery.”
Perhaps optimizing fluids could decrease postoperative ileus and improve outcomes, she added.
The observational study of data on adults undergoing colon surgery at 524 U.S. hospitals found a wide variation in the amount of IV fluids used on the day of surgery, ranging from none to more than 8 L, with a median of 3.1 L, Dr. Thacker of Duke University, Durham, N.C., said. The researchers defined excessive fluids as the highest quartile of fluid levels and low fluids as the lowest quartile.
Overall, 18% of patients developed postoperative ileus. The higher risk for ileus with low or high IV fluids on the day of surgery was seen in open and laparoscopic procedures.
Patients with ileus had significantly longer hospitalizations, higher costs, and increased likelihood of readmission, compared with patients without ileus. The hospital length of stay averaged 10 days with ileus and 6 days without ileus. Total costs averaged $20,734 per patient with ileus and $13,865 without ileus. Among patients with ileus, readmission rates were 14% within 30 days, 17% within 60 days, and 20% within 90 days. Among patients without ileus, readmission rates were 9%, 12%, and 14% at those time points, respectively.
Data for the study came from the Premier Data research database of a nationally representative sample of adult patients having colon surgery from January 1, 2008 through June 30, 2012. Procedures included laparoscopic partial excision of the large intestine, isolation of a segment of the large intestine, open and other partial excisions of the large intestine, total intra-abdominal colectomy, anastomosis of the small intestine to the rectal stump, and other small-to-large intestinal anastomoses.
Patients had a mean age of 62 years, 46% were male, and 73% were white. Forty-six percent of patients were covered by Medicare and 36% by managed care plans. Sixty-one percent of hospitals were nonteaching hospitals, and 89% were in an urban location.
The analysis adjusted for the influence of multiple other factors that may be associated with the risk of ileus, she said.
Deltex Medical, which markets fluid monitoring systems, funded the study. Dr. Thacker has been a consultant for Deltex and for Premier Data Inc., which acquired the data.
On Twitter @sherryboschert
SAN FRANCISCO – Too much or too little IV fluid on the day of surgery was associated with a 10%-12% increased risk for postoperative ileus in a retrospective study of 84,722 patients undergoing colon surgery.
Patients who received more than 5 liters of IV fluid on the day of surgery had a 10% increased risk of postoperative ileus and patients who received no more than 1.7 L on the surgery had a 12% increased risk of postoperative ileus, compared with patients who received 1.71-5 L of fluid, Dr. Julie K. Marosky Thacker and her associates reported at the annual clinical congress of the American College of Surgeons.
“This is one of the first studies to show that in a U.S.-based review, we have a significant number of patients getting over 5 L of fluid on the day of colon operation,” and that both high and low fluids increase the risk of postoperative ileus, Dr. Thacker said. “Observed fluid use is not compliant with the recommendations that are widespread and described in the principles of Enhance Recovery After Surgery.”
Perhaps optimizing fluids could decrease postoperative ileus and improve outcomes, she added.
The observational study of data on adults undergoing colon surgery at 524 U.S. hospitals found a wide variation in the amount of IV fluids used on the day of surgery, ranging from none to more than 8 L, with a median of 3.1 L, Dr. Thacker of Duke University, Durham, N.C., said. The researchers defined excessive fluids as the highest quartile of fluid levels and low fluids as the lowest quartile.
Overall, 18% of patients developed postoperative ileus. The higher risk for ileus with low or high IV fluids on the day of surgery was seen in open and laparoscopic procedures.
Patients with ileus had significantly longer hospitalizations, higher costs, and increased likelihood of readmission, compared with patients without ileus. The hospital length of stay averaged 10 days with ileus and 6 days without ileus. Total costs averaged $20,734 per patient with ileus and $13,865 without ileus. Among patients with ileus, readmission rates were 14% within 30 days, 17% within 60 days, and 20% within 90 days. Among patients without ileus, readmission rates were 9%, 12%, and 14% at those time points, respectively.
Data for the study came from the Premier Data research database of a nationally representative sample of adult patients having colon surgery from January 1, 2008 through June 30, 2012. Procedures included laparoscopic partial excision of the large intestine, isolation of a segment of the large intestine, open and other partial excisions of the large intestine, total intra-abdominal colectomy, anastomosis of the small intestine to the rectal stump, and other small-to-large intestinal anastomoses.
Patients had a mean age of 62 years, 46% were male, and 73% were white. Forty-six percent of patients were covered by Medicare and 36% by managed care plans. Sixty-one percent of hospitals were nonteaching hospitals, and 89% were in an urban location.
The analysis adjusted for the influence of multiple other factors that may be associated with the risk of ileus, she said.
Deltex Medical, which markets fluid monitoring systems, funded the study. Dr. Thacker has been a consultant for Deltex and for Premier Data Inc., which acquired the data.
On Twitter @sherryboschert
SAN FRANCISCO – Too much or too little IV fluid on the day of surgery was associated with a 10%-12% increased risk for postoperative ileus in a retrospective study of 84,722 patients undergoing colon surgery.
Patients who received more than 5 liters of IV fluid on the day of surgery had a 10% increased risk of postoperative ileus and patients who received no more than 1.7 L on the surgery had a 12% increased risk of postoperative ileus, compared with patients who received 1.71-5 L of fluid, Dr. Julie K. Marosky Thacker and her associates reported at the annual clinical congress of the American College of Surgeons.
“This is one of the first studies to show that in a U.S.-based review, we have a significant number of patients getting over 5 L of fluid on the day of colon operation,” and that both high and low fluids increase the risk of postoperative ileus, Dr. Thacker said. “Observed fluid use is not compliant with the recommendations that are widespread and described in the principles of Enhance Recovery After Surgery.”
Perhaps optimizing fluids could decrease postoperative ileus and improve outcomes, she added.
The observational study of data on adults undergoing colon surgery at 524 U.S. hospitals found a wide variation in the amount of IV fluids used on the day of surgery, ranging from none to more than 8 L, with a median of 3.1 L, Dr. Thacker of Duke University, Durham, N.C., said. The researchers defined excessive fluids as the highest quartile of fluid levels and low fluids as the lowest quartile.
Overall, 18% of patients developed postoperative ileus. The higher risk for ileus with low or high IV fluids on the day of surgery was seen in open and laparoscopic procedures.
Patients with ileus had significantly longer hospitalizations, higher costs, and increased likelihood of readmission, compared with patients without ileus. The hospital length of stay averaged 10 days with ileus and 6 days without ileus. Total costs averaged $20,734 per patient with ileus and $13,865 without ileus. Among patients with ileus, readmission rates were 14% within 30 days, 17% within 60 days, and 20% within 90 days. Among patients without ileus, readmission rates were 9%, 12%, and 14% at those time points, respectively.
Data for the study came from the Premier Data research database of a nationally representative sample of adult patients having colon surgery from January 1, 2008 through June 30, 2012. Procedures included laparoscopic partial excision of the large intestine, isolation of a segment of the large intestine, open and other partial excisions of the large intestine, total intra-abdominal colectomy, anastomosis of the small intestine to the rectal stump, and other small-to-large intestinal anastomoses.
Patients had a mean age of 62 years, 46% were male, and 73% were white. Forty-six percent of patients were covered by Medicare and 36% by managed care plans. Sixty-one percent of hospitals were nonteaching hospitals, and 89% were in an urban location.
The analysis adjusted for the influence of multiple other factors that may be associated with the risk of ileus, she said.
Deltex Medical, which markets fluid monitoring systems, funded the study. Dr. Thacker has been a consultant for Deltex and for Premier Data Inc., which acquired the data.
On Twitter @sherryboschert
AT THE ACS CLINICAL CONGRESS
Key clinical point: Giving no more than 1.7 liters or more than 5 liters of IV fluids on the day of surgery increased the risk of ileus.
Major finding: The ileus risk was 10% higher with excessive IV fluids and 12% higher with low fluids.
Data source: A retrospective observational cohort study of data on 84,722 patients undergoing colon surgery.
Disclosures: Deltex Medical, which markets fluid monitoring systems, funded the study. Dr. Thacker has been a consultant for Deltex and for Premier Data Inc., which acquired the data.
FDA warns against laparoscopic power morcellation
The laparoscopic power morcellator, in wide use for years, should not be used in the “vast majority” of women undergoing a hysterectomy or myomectomy, the Food and Drug Administration announced.
The updated safety communication regarding the use of laparoscopic power morcellators to treat uterine fibroids includes an Immediately in Effect guidance to manufacturers of the devices that the labels should include “specific safety statements” in the form of a boxed warning and two contraindications on proper usage.
“The FDA’s primary concern is the safety and well-being of patients and taking these steps will help the agency’s safety recommendations to be implemented as quickly as possible,” Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, said in a statement. “Updating the device label with a boxed warning and contraindications will provide clinicians and patients with critical information about the risk of spreading cancerous tissue when these procedures are performed.”
According to the FDA announcement, the boxed warning on future labels should say, “Uterine tissue may contain unsuspected cancer. The use of laparoscopic power morcellators during fibroid surgery may spread cancer and decrease the long-term survival of patients. This information should be shared with patients when considering surgery with the use of these devices.”
The first of the two contraindications, both of which also would be included on all future laparoscopic power morcellator labels, warns against using the devices for removal of uterine tissue that possibly contains fibroids in patients who are perimenopausal or postmenopausal, and candidates for en bloc tissue removal through the vagina or minilaparotomy incision, as these groups of women “represent the majority of women with fibroids who undergo hysterectomy and myomectomy.” The second contraindication cautions against using laparoscopic power morcellators “in gynecologic surgery in which the tissue to be morcellated is known or suspected to be cancerous.”
“The FDA strongly encourages doctors to inform their patients of the risk of spreading unsuspected cancer from the use of these devices in fibroid surgery and discuss the benefits and risks associated with all treatment options,” Dr. Maisel said in a statement.
This announcement appears to be stronger than the previous statement, “perhaps geared to those select few who have not ceased using the laparoscopic power morcellator,” Dr. David Jaspan, chairman of the department of obstetrics and gynecology at Einstein Medical Center, Philadelphia, said in an interview. “Gynecologic surgeons must collaborate with medical engineers to create equipment that will enable women to once again enjoy the advantages of minimally invasive techniques that we have worked so hard to develop,” he added. “We have seen an increased number of laparotomies, and thus increased women’s risk for postoperative morbidity. I am hopeful that novel approaches will soon be made available for our patients.”
This latest set of warnings is an update of guidelines originally issued by the FDA in April 2014, in which the federal agency stated that use of laparoscopic power morcellators for hysterectomy or myomectomy can significantly increase the risk of spreading “unsuspected” cancer found in the tissue of the uterine wall. A quantitative analysis released by the FDA estimated that 1 in 350 women who undergo hysterectomy or myomectomy for fibroids is found to have an unsuspected uterine sarcoma.
The agency announced that its Immediately in Effect (IIE) guidance applies to all new and currently marketed laparoscopic power morcellators used for “general and specific gynecological indications.”
The campaign to highlight the risks of morcellators has been led by Dr. Hooman Noorchashm, a cardiothoracic surgeon, and his wife, Dr. Amy Reed, an anesthesiologist who was diagnosed with stage 4 leiomyosarcoma after undergoing a hysterectomy with morcellation at the age of 40 for what was thought to be benign fibroids. Dr. Noorchashm and Dr. Reed have called for a ban on the use of laparoscopic power morcellators.
Asked to comment on the FDA announcement, Dr. Noorchashm said the decision represents “ a massive and historic regulatory failure on the part of FDA Center for Devices and Radiological Health.”
In an interview, he added, “The evidence of avoidable deadly harm was beyond doubt in this case. But the FDA CDRH could not bring itself to fully protect those at risk. This demonstrates that the FDA is a ‘captured agency’ with more loyalty to corporate and industry interests than to patient safety. I think the United States Congress needs to conduct a hearing and overhaul and clarify the FDA’s overall mission. And in particular medical device safety legislation. Corporations have power, people don’t. And without cogent federal government, lives are left exposed.”
--Elizabeth Mechcatie contributed to this article.
*This article was updated November 24, 2014.
The laparoscopic power morcellator, in wide use for years, should not be used in the “vast majority” of women undergoing a hysterectomy or myomectomy, the Food and Drug Administration announced.
The updated safety communication regarding the use of laparoscopic power morcellators to treat uterine fibroids includes an Immediately in Effect guidance to manufacturers of the devices that the labels should include “specific safety statements” in the form of a boxed warning and two contraindications on proper usage.
“The FDA’s primary concern is the safety and well-being of patients and taking these steps will help the agency’s safety recommendations to be implemented as quickly as possible,” Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, said in a statement. “Updating the device label with a boxed warning and contraindications will provide clinicians and patients with critical information about the risk of spreading cancerous tissue when these procedures are performed.”
According to the FDA announcement, the boxed warning on future labels should say, “Uterine tissue may contain unsuspected cancer. The use of laparoscopic power morcellators during fibroid surgery may spread cancer and decrease the long-term survival of patients. This information should be shared with patients when considering surgery with the use of these devices.”
The first of the two contraindications, both of which also would be included on all future laparoscopic power morcellator labels, warns against using the devices for removal of uterine tissue that possibly contains fibroids in patients who are perimenopausal or postmenopausal, and candidates for en bloc tissue removal through the vagina or minilaparotomy incision, as these groups of women “represent the majority of women with fibroids who undergo hysterectomy and myomectomy.” The second contraindication cautions against using laparoscopic power morcellators “in gynecologic surgery in which the tissue to be morcellated is known or suspected to be cancerous.”
“The FDA strongly encourages doctors to inform their patients of the risk of spreading unsuspected cancer from the use of these devices in fibroid surgery and discuss the benefits and risks associated with all treatment options,” Dr. Maisel said in a statement.
This announcement appears to be stronger than the previous statement, “perhaps geared to those select few who have not ceased using the laparoscopic power morcellator,” Dr. David Jaspan, chairman of the department of obstetrics and gynecology at Einstein Medical Center, Philadelphia, said in an interview. “Gynecologic surgeons must collaborate with medical engineers to create equipment that will enable women to once again enjoy the advantages of minimally invasive techniques that we have worked so hard to develop,” he added. “We have seen an increased number of laparotomies, and thus increased women’s risk for postoperative morbidity. I am hopeful that novel approaches will soon be made available for our patients.”
This latest set of warnings is an update of guidelines originally issued by the FDA in April 2014, in which the federal agency stated that use of laparoscopic power morcellators for hysterectomy or myomectomy can significantly increase the risk of spreading “unsuspected” cancer found in the tissue of the uterine wall. A quantitative analysis released by the FDA estimated that 1 in 350 women who undergo hysterectomy or myomectomy for fibroids is found to have an unsuspected uterine sarcoma.
The agency announced that its Immediately in Effect (IIE) guidance applies to all new and currently marketed laparoscopic power morcellators used for “general and specific gynecological indications.”
The campaign to highlight the risks of morcellators has been led by Dr. Hooman Noorchashm, a cardiothoracic surgeon, and his wife, Dr. Amy Reed, an anesthesiologist who was diagnosed with stage 4 leiomyosarcoma after undergoing a hysterectomy with morcellation at the age of 40 for what was thought to be benign fibroids. Dr. Noorchashm and Dr. Reed have called for a ban on the use of laparoscopic power morcellators.
Asked to comment on the FDA announcement, Dr. Noorchashm said the decision represents “ a massive and historic regulatory failure on the part of FDA Center for Devices and Radiological Health.”
In an interview, he added, “The evidence of avoidable deadly harm was beyond doubt in this case. But the FDA CDRH could not bring itself to fully protect those at risk. This demonstrates that the FDA is a ‘captured agency’ with more loyalty to corporate and industry interests than to patient safety. I think the United States Congress needs to conduct a hearing and overhaul and clarify the FDA’s overall mission. And in particular medical device safety legislation. Corporations have power, people don’t. And without cogent federal government, lives are left exposed.”
--Elizabeth Mechcatie contributed to this article.
*This article was updated November 24, 2014.
The laparoscopic power morcellator, in wide use for years, should not be used in the “vast majority” of women undergoing a hysterectomy or myomectomy, the Food and Drug Administration announced.
The updated safety communication regarding the use of laparoscopic power morcellators to treat uterine fibroids includes an Immediately in Effect guidance to manufacturers of the devices that the labels should include “specific safety statements” in the form of a boxed warning and two contraindications on proper usage.
“The FDA’s primary concern is the safety and well-being of patients and taking these steps will help the agency’s safety recommendations to be implemented as quickly as possible,” Dr. William Maisel, deputy director for science and chief scientist at the FDA’s Center for Devices and Radiological Health, said in a statement. “Updating the device label with a boxed warning and contraindications will provide clinicians and patients with critical information about the risk of spreading cancerous tissue when these procedures are performed.”
According to the FDA announcement, the boxed warning on future labels should say, “Uterine tissue may contain unsuspected cancer. The use of laparoscopic power morcellators during fibroid surgery may spread cancer and decrease the long-term survival of patients. This information should be shared with patients when considering surgery with the use of these devices.”
The first of the two contraindications, both of which also would be included on all future laparoscopic power morcellator labels, warns against using the devices for removal of uterine tissue that possibly contains fibroids in patients who are perimenopausal or postmenopausal, and candidates for en bloc tissue removal through the vagina or minilaparotomy incision, as these groups of women “represent the majority of women with fibroids who undergo hysterectomy and myomectomy.” The second contraindication cautions against using laparoscopic power morcellators “in gynecologic surgery in which the tissue to be morcellated is known or suspected to be cancerous.”
“The FDA strongly encourages doctors to inform their patients of the risk of spreading unsuspected cancer from the use of these devices in fibroid surgery and discuss the benefits and risks associated with all treatment options,” Dr. Maisel said in a statement.
This announcement appears to be stronger than the previous statement, “perhaps geared to those select few who have not ceased using the laparoscopic power morcellator,” Dr. David Jaspan, chairman of the department of obstetrics and gynecology at Einstein Medical Center, Philadelphia, said in an interview. “Gynecologic surgeons must collaborate with medical engineers to create equipment that will enable women to once again enjoy the advantages of minimally invasive techniques that we have worked so hard to develop,” he added. “We have seen an increased number of laparotomies, and thus increased women’s risk for postoperative morbidity. I am hopeful that novel approaches will soon be made available for our patients.”
This latest set of warnings is an update of guidelines originally issued by the FDA in April 2014, in which the federal agency stated that use of laparoscopic power morcellators for hysterectomy or myomectomy can significantly increase the risk of spreading “unsuspected” cancer found in the tissue of the uterine wall. A quantitative analysis released by the FDA estimated that 1 in 350 women who undergo hysterectomy or myomectomy for fibroids is found to have an unsuspected uterine sarcoma.
The agency announced that its Immediately in Effect (IIE) guidance applies to all new and currently marketed laparoscopic power morcellators used for “general and specific gynecological indications.”
The campaign to highlight the risks of morcellators has been led by Dr. Hooman Noorchashm, a cardiothoracic surgeon, and his wife, Dr. Amy Reed, an anesthesiologist who was diagnosed with stage 4 leiomyosarcoma after undergoing a hysterectomy with morcellation at the age of 40 for what was thought to be benign fibroids. Dr. Noorchashm and Dr. Reed have called for a ban on the use of laparoscopic power morcellators.
Asked to comment on the FDA announcement, Dr. Noorchashm said the decision represents “ a massive and historic regulatory failure on the part of FDA Center for Devices and Radiological Health.”
In an interview, he added, “The evidence of avoidable deadly harm was beyond doubt in this case. But the FDA CDRH could not bring itself to fully protect those at risk. This demonstrates that the FDA is a ‘captured agency’ with more loyalty to corporate and industry interests than to patient safety. I think the United States Congress needs to conduct a hearing and overhaul and clarify the FDA’s overall mission. And in particular medical device safety legislation. Corporations have power, people don’t. And without cogent federal government, lives are left exposed.”
--Elizabeth Mechcatie contributed to this article.
*This article was updated November 24, 2014.
VIDEO: AMA President on the ACA, narrow networks, and Medicaid expansion
WASHINGTON– With enrollment now open for the second year of Affordable Care Act coverage, what kinds of concerns do physicians have?
American Medical Association President Robert M. Wah discusses in this video interview the latest round of enrollment, and potential pluses and minuses for physicians, including the 90-day grace period. That aspect of the law gives patients who enroll in health plans through ACA marketplaces up to 90 days to pay premiums. Although insurers are liable for services rendered in the first month, physicians might be left without reimbursement for care given in the ensuing 60 days.
Another looming question as enrollment gets underway: Will patients be able to determine that their physicians are participating in a given network? The AMA and others have been pressuring the federal government and insurers to do a better job of updating network information. At its interim policy-making meeting in November, the AMA adopted a new policy urging any and all changes to be made before enrollment begins. The organization’s delegates also said that state regulators should be the primary enforcers of ensuring network adequacy.
Dr. Wah also discussed the AMA’s approach to ensuring that more Americans get health coverage through Medicaid, building on another policy passed by the delegates at the interim meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
WASHINGTON– With enrollment now open for the second year of Affordable Care Act coverage, what kinds of concerns do physicians have?
American Medical Association President Robert M. Wah discusses in this video interview the latest round of enrollment, and potential pluses and minuses for physicians, including the 90-day grace period. That aspect of the law gives patients who enroll in health plans through ACA marketplaces up to 90 days to pay premiums. Although insurers are liable for services rendered in the first month, physicians might be left without reimbursement for care given in the ensuing 60 days.
Another looming question as enrollment gets underway: Will patients be able to determine that their physicians are participating in a given network? The AMA and others have been pressuring the federal government and insurers to do a better job of updating network information. At its interim policy-making meeting in November, the AMA adopted a new policy urging any and all changes to be made before enrollment begins. The organization’s delegates also said that state regulators should be the primary enforcers of ensuring network adequacy.
Dr. Wah also discussed the AMA’s approach to ensuring that more Americans get health coverage through Medicaid, building on another policy passed by the delegates at the interim meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
WASHINGTON– With enrollment now open for the second year of Affordable Care Act coverage, what kinds of concerns do physicians have?
American Medical Association President Robert M. Wah discusses in this video interview the latest round of enrollment, and potential pluses and minuses for physicians, including the 90-day grace period. That aspect of the law gives patients who enroll in health plans through ACA marketplaces up to 90 days to pay premiums. Although insurers are liable for services rendered in the first month, physicians might be left without reimbursement for care given in the ensuing 60 days.
Another looming question as enrollment gets underway: Will patients be able to determine that their physicians are participating in a given network? The AMA and others have been pressuring the federal government and insurers to do a better job of updating network information. At its interim policy-making meeting in November, the AMA adopted a new policy urging any and all changes to be made before enrollment begins. The organization’s delegates also said that state regulators should be the primary enforcers of ensuring network adequacy.
Dr. Wah also discussed the AMA’s approach to ensuring that more Americans get health coverage through Medicaid, building on another policy passed by the delegates at the interim meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
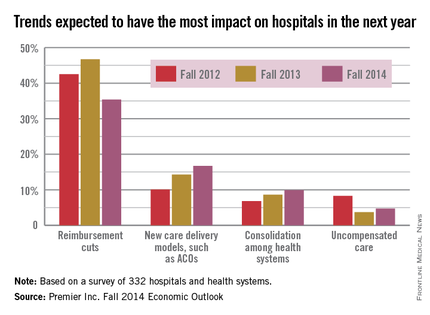
Hospitals showed less concern over ACA in 2014 than 2013
While reimbursement cuts related to the Affordable Care Act remain the most pressing issue hospitals expect to face in 2015, other trends are receiving more attention, according to a survey from health care improvement company Premier Inc.
While just over 35% of hospitals felt that ACA-related reimbursements cuts would impact them most in the next year, this is down from this time in 2013, when 47% of hospitals considered it the most important issue. New care delivery models were the most important concern for 17% of hospitals this year, up from only 10% in fall 2012. Health system consolidation and uncompensated care also became more important to hospitals, according to the survey.
Fewer hospitals are listing ACA compliance as the biggest driver of health care cost; 23% of hospitals cite it as their biggest issue, but this is down from a high of 36% in the spring of 2012. Labor costs are another very significant concern, with 20% of hospitals considering it their biggest driver of costs, Premier reported.
Premiere is an alliance of 3,400 hospitals and 110,000 other providers. The company’s fall 2014 Economic Outlook survey involved 332 hospitals and health systems from 42 states.
While reimbursement cuts related to the Affordable Care Act remain the most pressing issue hospitals expect to face in 2015, other trends are receiving more attention, according to a survey from health care improvement company Premier Inc.
While just over 35% of hospitals felt that ACA-related reimbursements cuts would impact them most in the next year, this is down from this time in 2013, when 47% of hospitals considered it the most important issue. New care delivery models were the most important concern for 17% of hospitals this year, up from only 10% in fall 2012. Health system consolidation and uncompensated care also became more important to hospitals, according to the survey.
Fewer hospitals are listing ACA compliance as the biggest driver of health care cost; 23% of hospitals cite it as their biggest issue, but this is down from a high of 36% in the spring of 2012. Labor costs are another very significant concern, with 20% of hospitals considering it their biggest driver of costs, Premier reported.
Premiere is an alliance of 3,400 hospitals and 110,000 other providers. The company’s fall 2014 Economic Outlook survey involved 332 hospitals and health systems from 42 states.

While reimbursement cuts related to the Affordable Care Act remain the most pressing issue hospitals expect to face in 2015, other trends are receiving more attention, according to a survey from health care improvement company Premier Inc.
While just over 35% of hospitals felt that ACA-related reimbursements cuts would impact them most in the next year, this is down from this time in 2013, when 47% of hospitals considered it the most important issue. New care delivery models were the most important concern for 17% of hospitals this year, up from only 10% in fall 2012. Health system consolidation and uncompensated care also became more important to hospitals, according to the survey.
Fewer hospitals are listing ACA compliance as the biggest driver of health care cost; 23% of hospitals cite it as their biggest issue, but this is down from a high of 36% in the spring of 2012. Labor costs are another very significant concern, with 20% of hospitals considering it their biggest driver of costs, Premier reported.
Premiere is an alliance of 3,400 hospitals and 110,000 other providers. The company’s fall 2014 Economic Outlook survey involved 332 hospitals and health systems from 42 states.

Insurance commissioners publish first draft of network adequacy model law
Model legislation that aims to ensure the adequacy, accessibility, and quality of health insurers’ provider networks does not address a number of concerns voiced by physicians.
The draft legislation, released by the National Association of Insurance Commissioners (NAIC), provides that a managed care plan shall maintain a network sufficient in numbers and types of providers to ensure that all services to covered persons will be accessible without unreasonable delay. Determining adequacy includes – but is not limited to – provider-covered person ratios by specialty; geographic accessibility; waiting times for appointments; hours of operation; new health delivery options, such as telemedicine; and the volume of technological and specialty services available.
The model law applies to all forms of health insurance, including qualified health plans, health maintenance organizations, and preferred provider organizations, J.P. Weiske, chair of NAIC’s network adequacy model review (B) subgroup said in a Nov. 19 webinar hosted by the Alliance for Health Reform.
“One of the biggest decisions we made is it would apply to all forms of health insurance that’s sold,” said Mr. Weiske, who serves as legislative liaison for Wisconsin Insurance Commissioner Ted Nickel.
Once finalized, the model law could be used as a basis for legislation to reform health insurance that could be introduced in any or all state legislatures.
The model legislation arrives amid ongoing physician criticism that many plans being sold on the Affordable Care Act’s insurance exchanges have narrow networks that limit access to primary care doctors, area hospitals, and specialists.
On Nov. 17, more than 115 health care organizations, including the American Medical Association, sent a letter to NAIC calling for network adequacy for patients, greater regulatory oversight in the hands of commissioners, and increased transparency for health insurers. The AMA also passed a new policy at its Nov. 11 interim meeting that supports strengthening the monitoring and enforcement of network adequacy at the federal and state levels.
In their letter, the groups requested that NAIC tackle several critical issues within its model act, recommending that provider networks include a full range of primary, specialty, and subspecialty providers to ensure that consumers have access to all covered services; that regulators actively review and monitor all networks using appropriate quantitative standards; that appeals processes are fair, timely, and transparent; and that the use of tiered provider networks be regulated.
NAIC’s model legislation addresses such issues as provider directories, telemedicine, and the transparency of health networks. It would require that a health insurer post online a current provider directory for each of its network plans and update each directory at least monthly. It also calls on insurers to prepare an access plan prior to offering a new managed care network plan and include:
• How the use of telemedicine or other technology may be used to meet access standards.
• The health insurer’s procedures for making and authorizing referrals within and outside its network, if applicable.
• The health insurer’s process for monitoring and ensuring the sufficiency of the network to meet the health care needs of populations that enroll in managed care network plans.
But the model law does not include standards related to some prevalent concerns by doctors, such as certain appeals, rules related to tiers, and time and distance standards.
For instance, the model does not include provisions about an appeals process for providers unhappy about being excluded from a network or how close geographically a network’s providers should be to patients they are serving. In regard to tiered networks, the model act includes only a sidebar noting that “states may want to pay particular attention to network sufficiency, marketing, and disclosure issues that may arise with respect to certain health carrier network designs, such as so-called tiered networks.” NAIC may revisit the issue of tiered networks at a later date.
“The problem that we had with tiered networks is that they’re relatively new,” Mr. Weiske said during the webinar. “There’s not a lot of similarity between them, but there are some significant regulatory concerns. We decided to essentially put regulators on notice that this is something they should look at.”
Mr. Weiske noted that it made sense to include broad flexibility in the model legislation to allow state regulators to dictate the details that best fit their patient populations and health care systems.
If enacted, the proposed law could end up being very different in different states, added Dr. John J. Reilly Jr., senior medical director of population health for the University of Pittsburgh Medical Center’s health services division and chair of the university’s department of medicine.
“The draft we’ve seen is very broad,” he said during the webinar. “It does not mean the way that states implement it is going to be broad. They can be very specific in their language. For insurers or clinical systems that cross state lines, there will be the potential for having different regulations across their networks because of different implementation of this model draft by different state regulatory agencies.”
Stakeholders have until Jan. 12, 2015, to provide comments on the first draft of the model law. The draft will then move through several NAIC committees before becoming a final model and ready for state legislators to review.
On Twitter @legal_med
Model legislation that aims to ensure the adequacy, accessibility, and quality of health insurers’ provider networks does not address a number of concerns voiced by physicians.
The draft legislation, released by the National Association of Insurance Commissioners (NAIC), provides that a managed care plan shall maintain a network sufficient in numbers and types of providers to ensure that all services to covered persons will be accessible without unreasonable delay. Determining adequacy includes – but is not limited to – provider-covered person ratios by specialty; geographic accessibility; waiting times for appointments; hours of operation; new health delivery options, such as telemedicine; and the volume of technological and specialty services available.
The model law applies to all forms of health insurance, including qualified health plans, health maintenance organizations, and preferred provider organizations, J.P. Weiske, chair of NAIC’s network adequacy model review (B) subgroup said in a Nov. 19 webinar hosted by the Alliance for Health Reform.
“One of the biggest decisions we made is it would apply to all forms of health insurance that’s sold,” said Mr. Weiske, who serves as legislative liaison for Wisconsin Insurance Commissioner Ted Nickel.
Once finalized, the model law could be used as a basis for legislation to reform health insurance that could be introduced in any or all state legislatures.
The model legislation arrives amid ongoing physician criticism that many plans being sold on the Affordable Care Act’s insurance exchanges have narrow networks that limit access to primary care doctors, area hospitals, and specialists.
On Nov. 17, more than 115 health care organizations, including the American Medical Association, sent a letter to NAIC calling for network adequacy for patients, greater regulatory oversight in the hands of commissioners, and increased transparency for health insurers. The AMA also passed a new policy at its Nov. 11 interim meeting that supports strengthening the monitoring and enforcement of network adequacy at the federal and state levels.
In their letter, the groups requested that NAIC tackle several critical issues within its model act, recommending that provider networks include a full range of primary, specialty, and subspecialty providers to ensure that consumers have access to all covered services; that regulators actively review and monitor all networks using appropriate quantitative standards; that appeals processes are fair, timely, and transparent; and that the use of tiered provider networks be regulated.
NAIC’s model legislation addresses such issues as provider directories, telemedicine, and the transparency of health networks. It would require that a health insurer post online a current provider directory for each of its network plans and update each directory at least monthly. It also calls on insurers to prepare an access plan prior to offering a new managed care network plan and include:
• How the use of telemedicine or other technology may be used to meet access standards.
• The health insurer’s procedures for making and authorizing referrals within and outside its network, if applicable.
• The health insurer’s process for monitoring and ensuring the sufficiency of the network to meet the health care needs of populations that enroll in managed care network plans.
But the model law does not include standards related to some prevalent concerns by doctors, such as certain appeals, rules related to tiers, and time and distance standards.
For instance, the model does not include provisions about an appeals process for providers unhappy about being excluded from a network or how close geographically a network’s providers should be to patients they are serving. In regard to tiered networks, the model act includes only a sidebar noting that “states may want to pay particular attention to network sufficiency, marketing, and disclosure issues that may arise with respect to certain health carrier network designs, such as so-called tiered networks.” NAIC may revisit the issue of tiered networks at a later date.
“The problem that we had with tiered networks is that they’re relatively new,” Mr. Weiske said during the webinar. “There’s not a lot of similarity between them, but there are some significant regulatory concerns. We decided to essentially put regulators on notice that this is something they should look at.”
Mr. Weiske noted that it made sense to include broad flexibility in the model legislation to allow state regulators to dictate the details that best fit their patient populations and health care systems.
If enacted, the proposed law could end up being very different in different states, added Dr. John J. Reilly Jr., senior medical director of population health for the University of Pittsburgh Medical Center’s health services division and chair of the university’s department of medicine.
“The draft we’ve seen is very broad,” he said during the webinar. “It does not mean the way that states implement it is going to be broad. They can be very specific in their language. For insurers or clinical systems that cross state lines, there will be the potential for having different regulations across their networks because of different implementation of this model draft by different state regulatory agencies.”
Stakeholders have until Jan. 12, 2015, to provide comments on the first draft of the model law. The draft will then move through several NAIC committees before becoming a final model and ready for state legislators to review.
On Twitter @legal_med
Model legislation that aims to ensure the adequacy, accessibility, and quality of health insurers’ provider networks does not address a number of concerns voiced by physicians.
The draft legislation, released by the National Association of Insurance Commissioners (NAIC), provides that a managed care plan shall maintain a network sufficient in numbers and types of providers to ensure that all services to covered persons will be accessible without unreasonable delay. Determining adequacy includes – but is not limited to – provider-covered person ratios by specialty; geographic accessibility; waiting times for appointments; hours of operation; new health delivery options, such as telemedicine; and the volume of technological and specialty services available.
The model law applies to all forms of health insurance, including qualified health plans, health maintenance organizations, and preferred provider organizations, J.P. Weiske, chair of NAIC’s network adequacy model review (B) subgroup said in a Nov. 19 webinar hosted by the Alliance for Health Reform.
“One of the biggest decisions we made is it would apply to all forms of health insurance that’s sold,” said Mr. Weiske, who serves as legislative liaison for Wisconsin Insurance Commissioner Ted Nickel.
Once finalized, the model law could be used as a basis for legislation to reform health insurance that could be introduced in any or all state legislatures.
The model legislation arrives amid ongoing physician criticism that many plans being sold on the Affordable Care Act’s insurance exchanges have narrow networks that limit access to primary care doctors, area hospitals, and specialists.
On Nov. 17, more than 115 health care organizations, including the American Medical Association, sent a letter to NAIC calling for network adequacy for patients, greater regulatory oversight in the hands of commissioners, and increased transparency for health insurers. The AMA also passed a new policy at its Nov. 11 interim meeting that supports strengthening the monitoring and enforcement of network adequacy at the federal and state levels.
In their letter, the groups requested that NAIC tackle several critical issues within its model act, recommending that provider networks include a full range of primary, specialty, and subspecialty providers to ensure that consumers have access to all covered services; that regulators actively review and monitor all networks using appropriate quantitative standards; that appeals processes are fair, timely, and transparent; and that the use of tiered provider networks be regulated.
NAIC’s model legislation addresses such issues as provider directories, telemedicine, and the transparency of health networks. It would require that a health insurer post online a current provider directory for each of its network plans and update each directory at least monthly. It also calls on insurers to prepare an access plan prior to offering a new managed care network plan and include:
• How the use of telemedicine or other technology may be used to meet access standards.
• The health insurer’s procedures for making and authorizing referrals within and outside its network, if applicable.
• The health insurer’s process for monitoring and ensuring the sufficiency of the network to meet the health care needs of populations that enroll in managed care network plans.
But the model law does not include standards related to some prevalent concerns by doctors, such as certain appeals, rules related to tiers, and time and distance standards.
For instance, the model does not include provisions about an appeals process for providers unhappy about being excluded from a network or how close geographically a network’s providers should be to patients they are serving. In regard to tiered networks, the model act includes only a sidebar noting that “states may want to pay particular attention to network sufficiency, marketing, and disclosure issues that may arise with respect to certain health carrier network designs, such as so-called tiered networks.” NAIC may revisit the issue of tiered networks at a later date.
“The problem that we had with tiered networks is that they’re relatively new,” Mr. Weiske said during the webinar. “There’s not a lot of similarity between them, but there are some significant regulatory concerns. We decided to essentially put regulators on notice that this is something they should look at.”
Mr. Weiske noted that it made sense to include broad flexibility in the model legislation to allow state regulators to dictate the details that best fit their patient populations and health care systems.
If enacted, the proposed law could end up being very different in different states, added Dr. John J. Reilly Jr., senior medical director of population health for the University of Pittsburgh Medical Center’s health services division and chair of the university’s department of medicine.
“The draft we’ve seen is very broad,” he said during the webinar. “It does not mean the way that states implement it is going to be broad. They can be very specific in their language. For insurers or clinical systems that cross state lines, there will be the potential for having different regulations across their networks because of different implementation of this model draft by different state regulatory agencies.”
Stakeholders have until Jan. 12, 2015, to provide comments on the first draft of the model law. The draft will then move through several NAIC committees before becoming a final model and ready for state legislators to review.
On Twitter @legal_med
FDA approves extended-release hydrocodone with abuse-deterrent features
An extended-release formulation of hydrocodone with properties that are “expected to reduce, but not totally prevent” abuse has been approved, the Food and Drug Administration announced on Nov. 20.
The hydrocodone-only product is indicated for treating pain “severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate,” according to the FDA statement. It is not approved for as-needed pain relief, and because of its risks for abuse, misuse, and addiction, “should only be prescribed to people for whom alternative treatment options are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient pain management,” the FDA statement said.
The product will be marketed as Hysingla ER, by Purdue Pharma, the manufacturer of extended-release oxycodone marketed as OxyContin.
Hysingla ER comes in 20-mg, 30-mg, 40-mg, 60-mg , 100-mg, and 120-mg strengths, taken once a day; daily doses of 80 mg or more should not be prescribed to people who have not previously been treated with an opioid. These amounts are higher than immediate-release hydrocodone combination products, but the range is “comparable” to currently available extended-release opioids, the statement points out.
The tablet has properties that make it difficult to crush, break, or dissolve. It also forms a thick gel when put in liquid, which “resists passage through a hypodermic needle,” according to the prescribing information. While the product’s physical and chemical properties are expected to make abuse by these routes difficult, abuse by these routes is still possible, the FDA statement said.
As part of the FDA’s Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting opioids, Purdue is required to provide health care professionals with information on how to safely prescribe the drug and to provide documents to patients, including a medication guide with each prescription, about how to safely use, store, and dispose of these products.
The company is also required to conduct postmarketing studies to evaluate the impact of the abuse-deterrent properties on the risk of abuse and the impact of that abuse in the community, according to the statement.
“While the science of abuse deterrence is still evolving, the development of opioids that are harder to abuse is helpful in addressing the public health crisis of prescription drug abuse in the U.S.,” Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, said in the statement. “Encouraging the development of opioids with abuse-deterrent properties is just one component of a broader approach to reducing abuse and misuse and will better enable the agency to balance addressing this problem with ensuring that patients have access to appropriate treatments for pain,” she added.
In October, hydrocodone was switched from a schedule III to the stricter schedule II category.
Hysingla ER is expected to be available in early 2015, according to a statement by Purdue.
In August 2014, hydrocodone was switched from a schedule III to a schedule II controlled substance.
The prescribing information is available at http://www.purduepharma.com/wp-content/uploads/hysinglaerpi.pdf.
An extended-release formulation of hydrocodone with properties that are “expected to reduce, but not totally prevent” abuse has been approved, the Food and Drug Administration announced on Nov. 20.
The hydrocodone-only product is indicated for treating pain “severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate,” according to the FDA statement. It is not approved for as-needed pain relief, and because of its risks for abuse, misuse, and addiction, “should only be prescribed to people for whom alternative treatment options are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient pain management,” the FDA statement said.
The product will be marketed as Hysingla ER, by Purdue Pharma, the manufacturer of extended-release oxycodone marketed as OxyContin.
Hysingla ER comes in 20-mg, 30-mg, 40-mg, 60-mg , 100-mg, and 120-mg strengths, taken once a day; daily doses of 80 mg or more should not be prescribed to people who have not previously been treated with an opioid. These amounts are higher than immediate-release hydrocodone combination products, but the range is “comparable” to currently available extended-release opioids, the statement points out.
The tablet has properties that make it difficult to crush, break, or dissolve. It also forms a thick gel when put in liquid, which “resists passage through a hypodermic needle,” according to the prescribing information. While the product’s physical and chemical properties are expected to make abuse by these routes difficult, abuse by these routes is still possible, the FDA statement said.
As part of the FDA’s Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting opioids, Purdue is required to provide health care professionals with information on how to safely prescribe the drug and to provide documents to patients, including a medication guide with each prescription, about how to safely use, store, and dispose of these products.
The company is also required to conduct postmarketing studies to evaluate the impact of the abuse-deterrent properties on the risk of abuse and the impact of that abuse in the community, according to the statement.
“While the science of abuse deterrence is still evolving, the development of opioids that are harder to abuse is helpful in addressing the public health crisis of prescription drug abuse in the U.S.,” Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, said in the statement. “Encouraging the development of opioids with abuse-deterrent properties is just one component of a broader approach to reducing abuse and misuse and will better enable the agency to balance addressing this problem with ensuring that patients have access to appropriate treatments for pain,” she added.
In October, hydrocodone was switched from a schedule III to the stricter schedule II category.
Hysingla ER is expected to be available in early 2015, according to a statement by Purdue.
In August 2014, hydrocodone was switched from a schedule III to a schedule II controlled substance.
The prescribing information is available at http://www.purduepharma.com/wp-content/uploads/hysinglaerpi.pdf.
An extended-release formulation of hydrocodone with properties that are “expected to reduce, but not totally prevent” abuse has been approved, the Food and Drug Administration announced on Nov. 20.
The hydrocodone-only product is indicated for treating pain “severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate,” according to the FDA statement. It is not approved for as-needed pain relief, and because of its risks for abuse, misuse, and addiction, “should only be prescribed to people for whom alternative treatment options are ineffective, not tolerated, or would be otherwise inadequate to provide sufficient pain management,” the FDA statement said.
The product will be marketed as Hysingla ER, by Purdue Pharma, the manufacturer of extended-release oxycodone marketed as OxyContin.
Hysingla ER comes in 20-mg, 30-mg, 40-mg, 60-mg , 100-mg, and 120-mg strengths, taken once a day; daily doses of 80 mg or more should not be prescribed to people who have not previously been treated with an opioid. These amounts are higher than immediate-release hydrocodone combination products, but the range is “comparable” to currently available extended-release opioids, the statement points out.
The tablet has properties that make it difficult to crush, break, or dissolve. It also forms a thick gel when put in liquid, which “resists passage through a hypodermic needle,” according to the prescribing information. While the product’s physical and chemical properties are expected to make abuse by these routes difficult, abuse by these routes is still possible, the FDA statement said.
As part of the FDA’s Risk Evaluation and Mitigation Strategy (REMS) for extended-release and long-acting opioids, Purdue is required to provide health care professionals with information on how to safely prescribe the drug and to provide documents to patients, including a medication guide with each prescription, about how to safely use, store, and dispose of these products.
The company is also required to conduct postmarketing studies to evaluate the impact of the abuse-deterrent properties on the risk of abuse and the impact of that abuse in the community, according to the statement.
“While the science of abuse deterrence is still evolving, the development of opioids that are harder to abuse is helpful in addressing the public health crisis of prescription drug abuse in the U.S.,” Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, said in the statement. “Encouraging the development of opioids with abuse-deterrent properties is just one component of a broader approach to reducing abuse and misuse and will better enable the agency to balance addressing this problem with ensuring that patients have access to appropriate treatments for pain,” she added.
In October, hydrocodone was switched from a schedule III to the stricter schedule II category.
Hysingla ER is expected to be available in early 2015, according to a statement by Purdue.
In August 2014, hydrocodone was switched from a schedule III to a schedule II controlled substance.
The prescribing information is available at http://www.purduepharma.com/wp-content/uploads/hysinglaerpi.pdf.
FROM THE FDA
Radioactive iodine may boost survival in papillary thyroid cancer
CORONADO, CALIF. – The use of radioactive iodine in patients with papillary thyroid cancer showed a small but statistically significant survival benefit for all tumor size categories, a long-term analysis of national data suggested.
“The incidence of papillary thyroid carcinoma is rapidly rising, but the survival advantage of radioactive iodine ablation has not been confirmed,” Dr. Paritosh Suman said at the annual meeting of the American Thyroid Association.
To investigate the effect of radioactive iodine (RAI) on papillary thyroid cancer mortality, Dr. Suman and his associates identified 108,565 patients from the National Cancer Data Base who were diagnosed with papillary thyroid cancer and underwent total or near total thyroidectomy between 1998 and 2006.
The investigators classified tumors into one of four groups by diameter size: 10 mm or less, 11-20 mm, 21-40 mm, and greater than 40 mm. The study researchers used Cox regression analysis to quantify the effect of radioactive iodine, adjusting for clinicopathologic, demographic, and socioeconomic variables. A total of 52% of the patients were older than 45 years, 77% were female, and 54% received RAI.
Factors predicting the use of RAI were being male, having positive margins, having cervical lymph node involvement, and having a tumor size greater than 4 cm in diameter, said Dr. Suman, an endocrinology surgery fellow at North Shore University Health System in Evanston, Ill. The 10-year overall survival rate was 90% in those who received RAI, compared with 87.4% among those who did not, for a small but statistically significant survival advantage (P < .0001).
Among patients who received RAI, the 10-year survival advantage by diameter of tumor was 3.4% for those with tumors 10 mm or less; 2.8% with 11-20 mm; 3.3% with 21-40 mm, and 5.7% with greater than 40 mm.
Age also played a factor, with a 10-year survival advantage of 0.5% for those aged 18-35 years; 1.5% for those aged 36-45 years; 0.9% for those aged 46-55 years; 0.6% for those aged 56-65 years; and 2.1% for those older than 65 years. Both men and women who received RAI had a statistically significant survival advantage at 10 years (3.9% and 1.6%, respectively).
When the researchers assessed the 10-year survival advantage by lymph node category of patients who received RAI, the rates were 2.9% for N-0, 4.2% for N-1, 5.2% for N-1A, and 5.8% for N-1B. By margin status, the 10-year survival advantage was 3.1% for negative margins, 3.6% for positive margins, 3.5% for microscopic margins, and 8.8% for gross margins.
In an analysis of all patients with very low-risk, low-risk, and high-risk papillary thyroid carcinoma according to ATA guidelines for the use of RAI, the study authors found a 10-year survival advantage of RAI in each of the three categories.
Among patients with very low-risk papillary thyroid carcinoma, the rate of 10-year survival was 92.2% among those who received RAI, compared with 89% among those who did not (hazard ratio, 0.74). Similar associations were observed in those with low-risk carcinoma (91.8% vs. 89%; HR, 0.80) and in those with high-risk carcinoma (86.2% vs. 79.2%; HR, 0.71).
“RAI is associated with a statistically significant but small overall survival advantage for most patients,” Dr. Suman said. “High-risk patients, defined by large tumor size, lymph node involvement, and gross margins, achieve the greatest benefit with RAI ablation.”
Dr. Suman reported having no relevant financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – The use of radioactive iodine in patients with papillary thyroid cancer showed a small but statistically significant survival benefit for all tumor size categories, a long-term analysis of national data suggested.
“The incidence of papillary thyroid carcinoma is rapidly rising, but the survival advantage of radioactive iodine ablation has not been confirmed,” Dr. Paritosh Suman said at the annual meeting of the American Thyroid Association.
To investigate the effect of radioactive iodine (RAI) on papillary thyroid cancer mortality, Dr. Suman and his associates identified 108,565 patients from the National Cancer Data Base who were diagnosed with papillary thyroid cancer and underwent total or near total thyroidectomy between 1998 and 2006.
The investigators classified tumors into one of four groups by diameter size: 10 mm or less, 11-20 mm, 21-40 mm, and greater than 40 mm. The study researchers used Cox regression analysis to quantify the effect of radioactive iodine, adjusting for clinicopathologic, demographic, and socioeconomic variables. A total of 52% of the patients were older than 45 years, 77% were female, and 54% received RAI.
Factors predicting the use of RAI were being male, having positive margins, having cervical lymph node involvement, and having a tumor size greater than 4 cm in diameter, said Dr. Suman, an endocrinology surgery fellow at North Shore University Health System in Evanston, Ill. The 10-year overall survival rate was 90% in those who received RAI, compared with 87.4% among those who did not, for a small but statistically significant survival advantage (P < .0001).
Among patients who received RAI, the 10-year survival advantage by diameter of tumor was 3.4% for those with tumors 10 mm or less; 2.8% with 11-20 mm; 3.3% with 21-40 mm, and 5.7% with greater than 40 mm.
Age also played a factor, with a 10-year survival advantage of 0.5% for those aged 18-35 years; 1.5% for those aged 36-45 years; 0.9% for those aged 46-55 years; 0.6% for those aged 56-65 years; and 2.1% for those older than 65 years. Both men and women who received RAI had a statistically significant survival advantage at 10 years (3.9% and 1.6%, respectively).
When the researchers assessed the 10-year survival advantage by lymph node category of patients who received RAI, the rates were 2.9% for N-0, 4.2% for N-1, 5.2% for N-1A, and 5.8% for N-1B. By margin status, the 10-year survival advantage was 3.1% for negative margins, 3.6% for positive margins, 3.5% for microscopic margins, and 8.8% for gross margins.
In an analysis of all patients with very low-risk, low-risk, and high-risk papillary thyroid carcinoma according to ATA guidelines for the use of RAI, the study authors found a 10-year survival advantage of RAI in each of the three categories.
Among patients with very low-risk papillary thyroid carcinoma, the rate of 10-year survival was 92.2% among those who received RAI, compared with 89% among those who did not (hazard ratio, 0.74). Similar associations were observed in those with low-risk carcinoma (91.8% vs. 89%; HR, 0.80) and in those with high-risk carcinoma (86.2% vs. 79.2%; HR, 0.71).
“RAI is associated with a statistically significant but small overall survival advantage for most patients,” Dr. Suman said. “High-risk patients, defined by large tumor size, lymph node involvement, and gross margins, achieve the greatest benefit with RAI ablation.”
Dr. Suman reported having no relevant financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – The use of radioactive iodine in patients with papillary thyroid cancer showed a small but statistically significant survival benefit for all tumor size categories, a long-term analysis of national data suggested.
“The incidence of papillary thyroid carcinoma is rapidly rising, but the survival advantage of radioactive iodine ablation has not been confirmed,” Dr. Paritosh Suman said at the annual meeting of the American Thyroid Association.
To investigate the effect of radioactive iodine (RAI) on papillary thyroid cancer mortality, Dr. Suman and his associates identified 108,565 patients from the National Cancer Data Base who were diagnosed with papillary thyroid cancer and underwent total or near total thyroidectomy between 1998 and 2006.
The investigators classified tumors into one of four groups by diameter size: 10 mm or less, 11-20 mm, 21-40 mm, and greater than 40 mm. The study researchers used Cox regression analysis to quantify the effect of radioactive iodine, adjusting for clinicopathologic, demographic, and socioeconomic variables. A total of 52% of the patients were older than 45 years, 77% were female, and 54% received RAI.
Factors predicting the use of RAI were being male, having positive margins, having cervical lymph node involvement, and having a tumor size greater than 4 cm in diameter, said Dr. Suman, an endocrinology surgery fellow at North Shore University Health System in Evanston, Ill. The 10-year overall survival rate was 90% in those who received RAI, compared with 87.4% among those who did not, for a small but statistically significant survival advantage (P < .0001).
Among patients who received RAI, the 10-year survival advantage by diameter of tumor was 3.4% for those with tumors 10 mm or less; 2.8% with 11-20 mm; 3.3% with 21-40 mm, and 5.7% with greater than 40 mm.
Age also played a factor, with a 10-year survival advantage of 0.5% for those aged 18-35 years; 1.5% for those aged 36-45 years; 0.9% for those aged 46-55 years; 0.6% for those aged 56-65 years; and 2.1% for those older than 65 years. Both men and women who received RAI had a statistically significant survival advantage at 10 years (3.9% and 1.6%, respectively).
When the researchers assessed the 10-year survival advantage by lymph node category of patients who received RAI, the rates were 2.9% for N-0, 4.2% for N-1, 5.2% for N-1A, and 5.8% for N-1B. By margin status, the 10-year survival advantage was 3.1% for negative margins, 3.6% for positive margins, 3.5% for microscopic margins, and 8.8% for gross margins.
In an analysis of all patients with very low-risk, low-risk, and high-risk papillary thyroid carcinoma according to ATA guidelines for the use of RAI, the study authors found a 10-year survival advantage of RAI in each of the three categories.
Among patients with very low-risk papillary thyroid carcinoma, the rate of 10-year survival was 92.2% among those who received RAI, compared with 89% among those who did not (hazard ratio, 0.74). Similar associations were observed in those with low-risk carcinoma (91.8% vs. 89%; HR, 0.80) and in those with high-risk carcinoma (86.2% vs. 79.2%; HR, 0.71).
“RAI is associated with a statistically significant but small overall survival advantage for most patients,” Dr. Suman said. “High-risk patients, defined by large tumor size, lymph node involvement, and gross margins, achieve the greatest benefit with RAI ablation.”
Dr. Suman reported having no relevant financial disclosures.
On Twitter @dougbrunk
AT THE ATA ANNUAL MEETING
Key clinical point: The use of radioactive iodine is associated with a small but statistically significant increase in overall survival in most patients with papillary thyroid carcinoma.
Major finding: The 10-year overall survival rate was 90% in patients who received RAI, compared with 87.4% among those who did not, a small but statistically significant difference (P < .0001).
Data source: An analysis of 108,565 patients from the National Cancer Data Base who were diagnosed with papillary thyroid cancer and underwent total or near total thyroidectomy between 1998 and 2006.
Disclosures: Dr. Suman reported having no relevant financial disclosures.
VIDEO: AMA President Wah talks SGR, Medicaid parity, and IPAB
WASHINGTON – The American Medical Association is hoping – along with many other medical societies and their physician members – to convince Congress that it should repeal Medicare’s Sustainable Growth Rate formula before the end of the year.
The cost to do so is a relative bargain, and physicians have grown tired of trying to plan around a process that is predictable, yet unpredictable, according to AMA President Robert M. Wah. Why not move now, he wondered, given that there was so much progress in this Congress, including a bill that was passed by the House?
In an exclusive video interview, Dr. Wah talked about the pressing need for the SGR replacement, along with what Republican majorities in both the House and Senate in the incoming Congress might mean to physicians.
Some Republicans have said that they will press for a partial or total repeal of the Affordable Care Act. Dr. Wah touched on whether the AMA supports any part of that notion, including getting rid of the Independent Payment Advisory Board, known as the IPAB.
Dr. Wah also discussed another urgent topic: What to do about the expiring element of the ACA that gave primary care physicians who treat Medicaid patients the same rate of pay as those who serve Medicare beneficiaries. The AMA recently reiterated its position that the so-called pay bump – which ends Dec. 31 – should be extended, and that it also should include obstetricians and gynecologists, who were left out of the initial policy.
On Twitter @aliciaault
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – The American Medical Association is hoping – along with many other medical societies and their physician members – to convince Congress that it should repeal Medicare’s Sustainable Growth Rate formula before the end of the year.
The cost to do so is a relative bargain, and physicians have grown tired of trying to plan around a process that is predictable, yet unpredictable, according to AMA President Robert M. Wah. Why not move now, he wondered, given that there was so much progress in this Congress, including a bill that was passed by the House?
In an exclusive video interview, Dr. Wah talked about the pressing need for the SGR replacement, along with what Republican majorities in both the House and Senate in the incoming Congress might mean to physicians.
Some Republicans have said that they will press for a partial or total repeal of the Affordable Care Act. Dr. Wah touched on whether the AMA supports any part of that notion, including getting rid of the Independent Payment Advisory Board, known as the IPAB.
Dr. Wah also discussed another urgent topic: What to do about the expiring element of the ACA that gave primary care physicians who treat Medicaid patients the same rate of pay as those who serve Medicare beneficiaries. The AMA recently reiterated its position that the so-called pay bump – which ends Dec. 31 – should be extended, and that it also should include obstetricians and gynecologists, who were left out of the initial policy.
On Twitter @aliciaault
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – The American Medical Association is hoping – along with many other medical societies and their physician members – to convince Congress that it should repeal Medicare’s Sustainable Growth Rate formula before the end of the year.
The cost to do so is a relative bargain, and physicians have grown tired of trying to plan around a process that is predictable, yet unpredictable, according to AMA President Robert M. Wah. Why not move now, he wondered, given that there was so much progress in this Congress, including a bill that was passed by the House?
In an exclusive video interview, Dr. Wah talked about the pressing need for the SGR replacement, along with what Republican majorities in both the House and Senate in the incoming Congress might mean to physicians.
Some Republicans have said that they will press for a partial or total repeal of the Affordable Care Act. Dr. Wah touched on whether the AMA supports any part of that notion, including getting rid of the Independent Payment Advisory Board, known as the IPAB.
Dr. Wah also discussed another urgent topic: What to do about the expiring element of the ACA that gave primary care physicians who treat Medicaid patients the same rate of pay as those who serve Medicare beneficiaries. The AMA recently reiterated its position that the so-called pay bump – which ends Dec. 31 – should be extended, and that it also should include obstetricians and gynecologists, who were left out of the initial policy.
On Twitter @aliciaault
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Palliative tumor removal extends survival
Palliative resection of the primary tumor actually extends survival in patients with metastatic colorectal adenocarcinoma, according to a report published online Nov. 4 in Annals of Surgery.
In what the investigators described as the first population-based study to assess trends in cancer-specific and overall survival among U.S. patients who did or did not undergo palliative removal of the primary tumor, the resection consistently conferred statistically significant and clinically meaningful survival benefits among 37,793 patients treated during a 12-year period.
“There is a heated debate in the medical and surgical oncology community regarding whether or not an asymptomatic primary tumor should be removed in patients with unresectable, synchronous cancer metastases,” wrote Dr. Ignazio Tarantino of the department of surgery, Kantonsspital St. Gallen (Switzerland) and the department of general, abdominal, and transplant surgery, University of Heidelberg (Germany) and his associates.
Current National Comprehensive Cancer Network guidelines recommend against palliative surgery in this setting, primarily because of evidence that leaving the primary tumor in situ seldom leads to life-threatening complications such as bleeding or bowel obstruction, while resection can cause complications and is not strictly necessary in terminally ill patients. But given these new findings of a significant survival benefit, “the dogma that [such tumors] never should be resected ... must be questioned,” the investigators wrote.
Dr. Tarantino and his associates analyzed Surveillance, Epidemiology, and End Results (SEER) data for 23,004 patients (60.9% of the total study population) who underwent primary tumor resection and 14,789 (39.1%) who did not. The percentage of patients who had the surgery steadily declined throughout the study period.
Palliative removal of the primary tumor was a significant protective factor for overall survival (HR of death, 0.49) and for cancer-specific survival (HR of cancer death, 0.49) in both the primary data analysis and a proportional hazard regression analysis.
Patients undergoing resection tended to be younger and healthier than those who did not have the procedure, so the researchers performed a propensity-score matching analysis to account for baseline differences between the two study groups. After adjustment for numerous potential confounders, palliative primary tumor resection continued to exert a significant protective effect for overall and cancer-specific survival, with HRs of 0.40 and 0.39, respectively. The survival benefit also persisted, with identical hazard ratios, in two further sensitivity analyses of the data, Dr. Tarantino and his associates noted (Ann. Surg. 2014 Nov. 4 [doi: 10.1097/SLA.0000000000000860]).
The mechanism by which palliative resection imparts a survival benefit is not yet known, they added.
Major advances in systemic treatment of metastatic colorectal cancer were achieved during the study period, and survival improved accordingly across both groups of patients over time. “Because of the improvement in systemic treatment, we anticipated that the differences in survival between the subsets of patients who did and who did not undergo palliative primary tumor resection would decrease over time. However – against our a priori hypothesis – our analysis demonstrates the contrary,” the investigators noted.
No financial or material support was provided for this study. Dr. Tarantino and his associates reported having no financial disclosures.
Palliative resection of the primary tumor actually extends survival in patients with metastatic colorectal adenocarcinoma, according to a report published online Nov. 4 in Annals of Surgery.
In what the investigators described as the first population-based study to assess trends in cancer-specific and overall survival among U.S. patients who did or did not undergo palliative removal of the primary tumor, the resection consistently conferred statistically significant and clinically meaningful survival benefits among 37,793 patients treated during a 12-year period.
“There is a heated debate in the medical and surgical oncology community regarding whether or not an asymptomatic primary tumor should be removed in patients with unresectable, synchronous cancer metastases,” wrote Dr. Ignazio Tarantino of the department of surgery, Kantonsspital St. Gallen (Switzerland) and the department of general, abdominal, and transplant surgery, University of Heidelberg (Germany) and his associates.
Current National Comprehensive Cancer Network guidelines recommend against palliative surgery in this setting, primarily because of evidence that leaving the primary tumor in situ seldom leads to life-threatening complications such as bleeding or bowel obstruction, while resection can cause complications and is not strictly necessary in terminally ill patients. But given these new findings of a significant survival benefit, “the dogma that [such tumors] never should be resected ... must be questioned,” the investigators wrote.
Dr. Tarantino and his associates analyzed Surveillance, Epidemiology, and End Results (SEER) data for 23,004 patients (60.9% of the total study population) who underwent primary tumor resection and 14,789 (39.1%) who did not. The percentage of patients who had the surgery steadily declined throughout the study period.
Palliative removal of the primary tumor was a significant protective factor for overall survival (HR of death, 0.49) and for cancer-specific survival (HR of cancer death, 0.49) in both the primary data analysis and a proportional hazard regression analysis.
Patients undergoing resection tended to be younger and healthier than those who did not have the procedure, so the researchers performed a propensity-score matching analysis to account for baseline differences between the two study groups. After adjustment for numerous potential confounders, palliative primary tumor resection continued to exert a significant protective effect for overall and cancer-specific survival, with HRs of 0.40 and 0.39, respectively. The survival benefit also persisted, with identical hazard ratios, in two further sensitivity analyses of the data, Dr. Tarantino and his associates noted (Ann. Surg. 2014 Nov. 4 [doi: 10.1097/SLA.0000000000000860]).
The mechanism by which palliative resection imparts a survival benefit is not yet known, they added.
Major advances in systemic treatment of metastatic colorectal cancer were achieved during the study period, and survival improved accordingly across both groups of patients over time. “Because of the improvement in systemic treatment, we anticipated that the differences in survival between the subsets of patients who did and who did not undergo palliative primary tumor resection would decrease over time. However – against our a priori hypothesis – our analysis demonstrates the contrary,” the investigators noted.
No financial or material support was provided for this study. Dr. Tarantino and his associates reported having no financial disclosures.
Palliative resection of the primary tumor actually extends survival in patients with metastatic colorectal adenocarcinoma, according to a report published online Nov. 4 in Annals of Surgery.
In what the investigators described as the first population-based study to assess trends in cancer-specific and overall survival among U.S. patients who did or did not undergo palliative removal of the primary tumor, the resection consistently conferred statistically significant and clinically meaningful survival benefits among 37,793 patients treated during a 12-year period.
“There is a heated debate in the medical and surgical oncology community regarding whether or not an asymptomatic primary tumor should be removed in patients with unresectable, synchronous cancer metastases,” wrote Dr. Ignazio Tarantino of the department of surgery, Kantonsspital St. Gallen (Switzerland) and the department of general, abdominal, and transplant surgery, University of Heidelberg (Germany) and his associates.
Current National Comprehensive Cancer Network guidelines recommend against palliative surgery in this setting, primarily because of evidence that leaving the primary tumor in situ seldom leads to life-threatening complications such as bleeding or bowel obstruction, while resection can cause complications and is not strictly necessary in terminally ill patients. But given these new findings of a significant survival benefit, “the dogma that [such tumors] never should be resected ... must be questioned,” the investigators wrote.
Dr. Tarantino and his associates analyzed Surveillance, Epidemiology, and End Results (SEER) data for 23,004 patients (60.9% of the total study population) who underwent primary tumor resection and 14,789 (39.1%) who did not. The percentage of patients who had the surgery steadily declined throughout the study period.
Palliative removal of the primary tumor was a significant protective factor for overall survival (HR of death, 0.49) and for cancer-specific survival (HR of cancer death, 0.49) in both the primary data analysis and a proportional hazard regression analysis.
Patients undergoing resection tended to be younger and healthier than those who did not have the procedure, so the researchers performed a propensity-score matching analysis to account for baseline differences between the two study groups. After adjustment for numerous potential confounders, palliative primary tumor resection continued to exert a significant protective effect for overall and cancer-specific survival, with HRs of 0.40 and 0.39, respectively. The survival benefit also persisted, with identical hazard ratios, in two further sensitivity analyses of the data, Dr. Tarantino and his associates noted (Ann. Surg. 2014 Nov. 4 [doi: 10.1097/SLA.0000000000000860]).
The mechanism by which palliative resection imparts a survival benefit is not yet known, they added.
Major advances in systemic treatment of metastatic colorectal cancer were achieved during the study period, and survival improved accordingly across both groups of patients over time. “Because of the improvement in systemic treatment, we anticipated that the differences in survival between the subsets of patients who did and who did not undergo palliative primary tumor resection would decrease over time. However – against our a priori hypothesis – our analysis demonstrates the contrary,” the investigators noted.
No financial or material support was provided for this study. Dr. Tarantino and his associates reported having no financial disclosures.
Key clinical point: Palliative removal of the primary tumor extends survival in patients with metastatic colorectal cancer.
Major finding: Palliative resection of the primary tumor was a significant protective factor for overall survival (HR of death, 0.49) and for cancer-specific survival (HR of cancer death, 0.49) in both the primary data analysis and a proportional hazard regression analysis.
Data source: A population-based study of the duration of survival in 23,004 patients with metastatic colorectal cancer who had palliative removal of the primary tumor and 14,789 who did not during a 12-year period.
Disclosures: No financial or material support was provided for this study. Dr. Tarantino and his associates reported having no disclosures.
Mastectomies, reconstruction, on the rise for women with early stage disease
Significant increases in the mastectomy rate among women with early breast cancer who were candidates for breast conservation surgery during a recent 14-year period in the United States were accompanied by increases in breast reconstruction and bilateral mastectomies, in a retrospective cohort study that tracked national trends in this group of women.
The results, based on outcomes of about 1.2 million women with early breast cancer in a national oncology outcomes database, “are generally consistent with trends noted in other state, regional, and national studies,” reported Dr. Kristy Kummerow of the division of surgical oncology and endocrine surgery, Vanderbilt University, Nashville, Tenn., and her associates. While they speculated on some of the reasons behind these findings, “further research is needed to understand patient, provider, policy, and social factors associated with these trends,” they concluded in the study, which was published online Nov. 19 in JAMA Surgery (doi:10.1001/jamasurg.2014.2895).
While the use of breast conservation surgery (BCS) as an alternative to mastectomy for early-stage breast cancer increased steadily after studies showed the two approaches had equal outcomes, and after endorsement by a National Institutes of Health Consensus Conference in 1990, the authors noted that there has been evidence that the trend is reversing.
Using data from the National Cancer Data Base, which collects outcomes data on about 70% of the patients diagnosed with cancer in the United States, they evaluated trends in mastectomies among adult women newly diagnosed with early (unilateral) breast cancer from January 1998 through December 2011. Women were included if TNM stage information was available, tumors were 5 cm or less with nine or fewer involved axillary lymph nodes based on clinical staging, and they underwent BCS (lumpectomy, segmental mastectomy, or re-excision of the biopsy site) or mastectomy (subcutaneous, total, modified radical or radical).
Over the 14-year period, among the approximately 1.2 million women who met the criteria, 64.5% had BCS and 35.5% had a mastectomy. The women who had a mastectomy were slightly younger (mean age 59.6 years vs. 61.6 years), and the proportion of racial and ethnic minorities was lower in this group.
The proportion of women eligible for BCS who underwent a mastectomy increased from 34.3% in 1998 to 37.8% in 2011, a statistically significant increase, “with steeper increases” seen in women who had node-negative and noninvasive disease, the authors reported. For the most recent 8-year period – 2003 to 2011 – the rate increased by 34%, “with the most notable rise in mastectomy rates occurring after 2006,” and the highest increases seen among women with clinically node-negative disease. Age and tumor size were “the most influential covariates,” with younger women “more likely to undergo mastectomy irrespective of tumor size, while in older women mastectomy was strongly associated with tumor size greater than 2 cm.”
During the period studied, there were significant increases in breast reconstruction and bilateral mastectomies among women who had mastectomies, which were secondary outcome measures. The proportion of women who underwent breast reconstruction increased from 11.6% in 1998 to 36.4% in 2011, and the proportion of women who had a bilateral mastectomy for unilateral disease increased from 1.9% to 11.2% during this period. The trend toward more reconstruction surgery could be due to 1998 legislation mandating insurance coverage of reconstructive surgery after mastectomy, the authors wrote.
Study limitations included missing clinical staging information for a large proportion of the women, no information on BRCA status or triple-negative tumors, and an inability to determine why the mastectomy was performed in individual cases, they noted.
None of the authors had disclosures to report. The study is based on work supported by the Office of Academic Affiliations, Department of Veterans Affairs, the VA National Quality Scholars Program, and with the use of facilities at the VA Tennessee Valley Healthcare System, Nashville.
In an accompanying editorial, Dr. Bonnie Sun and Dr. Michael Zenilman wrote that the “surprising rise” in the mastectomy rate for women with early stage breast cancers in this study raises various questions, including whether all the patients were candidates for BCS, which is difficult to determine “without accounting for findings on magnetic resonance imaging, family history, clinical stage, and tumor to breast ratio.” Another question is why the women chose a mastectomy and whether the reasons for that choice were valid. “While the choice to pursue mastectomy over BCS is never wrong, it must be made for the right reasons,” they said, adding, “when presenting these surgical options, we must ensure that decisions are not based on misconceptions.” This study “ should at least serve as a wake-up call that as we fulfill that responsibility, and use every modality of care to give patients the best quality of life and survival advantage, the guidelines may need to change again,” they wrote (JAMA Surgery 2014 Nov. 19 [doi:10.1001/jamasurg.2014.2902)].
Dr. Bonnie Sun and Dr. Michael E. Zenilman are in the department of surgery, Johns Hopkins Medicine, Bethesda, Md. Dr. Zenilman is a consultant for Champions in Oncology; Dr. Sun had no disclosures to report.
In an accompanying editorial, Dr. Bonnie Sun and Dr. Michael Zenilman wrote that the “surprising rise” in the mastectomy rate for women with early stage breast cancers in this study raises various questions, including whether all the patients were candidates for BCS, which is difficult to determine “without accounting for findings on magnetic resonance imaging, family history, clinical stage, and tumor to breast ratio.” Another question is why the women chose a mastectomy and whether the reasons for that choice were valid. “While the choice to pursue mastectomy over BCS is never wrong, it must be made for the right reasons,” they said, adding, “when presenting these surgical options, we must ensure that decisions are not based on misconceptions.” This study “ should at least serve as a wake-up call that as we fulfill that responsibility, and use every modality of care to give patients the best quality of life and survival advantage, the guidelines may need to change again,” they wrote (JAMA Surgery 2014 Nov. 19 [doi:10.1001/jamasurg.2014.2902)].
Dr. Bonnie Sun and Dr. Michael E. Zenilman are in the department of surgery, Johns Hopkins Medicine, Bethesda, Md. Dr. Zenilman is a consultant for Champions in Oncology; Dr. Sun had no disclosures to report.
In an accompanying editorial, Dr. Bonnie Sun and Dr. Michael Zenilman wrote that the “surprising rise” in the mastectomy rate for women with early stage breast cancers in this study raises various questions, including whether all the patients were candidates for BCS, which is difficult to determine “without accounting for findings on magnetic resonance imaging, family history, clinical stage, and tumor to breast ratio.” Another question is why the women chose a mastectomy and whether the reasons for that choice were valid. “While the choice to pursue mastectomy over BCS is never wrong, it must be made for the right reasons,” they said, adding, “when presenting these surgical options, we must ensure that decisions are not based on misconceptions.” This study “ should at least serve as a wake-up call that as we fulfill that responsibility, and use every modality of care to give patients the best quality of life and survival advantage, the guidelines may need to change again,” they wrote (JAMA Surgery 2014 Nov. 19 [doi:10.1001/jamasurg.2014.2902)].
Dr. Bonnie Sun and Dr. Michael E. Zenilman are in the department of surgery, Johns Hopkins Medicine, Bethesda, Md. Dr. Zenilman is a consultant for Champions in Oncology; Dr. Sun had no disclosures to report.
Significant increases in the mastectomy rate among women with early breast cancer who were candidates for breast conservation surgery during a recent 14-year period in the United States were accompanied by increases in breast reconstruction and bilateral mastectomies, in a retrospective cohort study that tracked national trends in this group of women.
The results, based on outcomes of about 1.2 million women with early breast cancer in a national oncology outcomes database, “are generally consistent with trends noted in other state, regional, and national studies,” reported Dr. Kristy Kummerow of the division of surgical oncology and endocrine surgery, Vanderbilt University, Nashville, Tenn., and her associates. While they speculated on some of the reasons behind these findings, “further research is needed to understand patient, provider, policy, and social factors associated with these trends,” they concluded in the study, which was published online Nov. 19 in JAMA Surgery (doi:10.1001/jamasurg.2014.2895).
While the use of breast conservation surgery (BCS) as an alternative to mastectomy for early-stage breast cancer increased steadily after studies showed the two approaches had equal outcomes, and after endorsement by a National Institutes of Health Consensus Conference in 1990, the authors noted that there has been evidence that the trend is reversing.
Using data from the National Cancer Data Base, which collects outcomes data on about 70% of the patients diagnosed with cancer in the United States, they evaluated trends in mastectomies among adult women newly diagnosed with early (unilateral) breast cancer from January 1998 through December 2011. Women were included if TNM stage information was available, tumors were 5 cm or less with nine or fewer involved axillary lymph nodes based on clinical staging, and they underwent BCS (lumpectomy, segmental mastectomy, or re-excision of the biopsy site) or mastectomy (subcutaneous, total, modified radical or radical).
Over the 14-year period, among the approximately 1.2 million women who met the criteria, 64.5% had BCS and 35.5% had a mastectomy. The women who had a mastectomy were slightly younger (mean age 59.6 years vs. 61.6 years), and the proportion of racial and ethnic minorities was lower in this group.
The proportion of women eligible for BCS who underwent a mastectomy increased from 34.3% in 1998 to 37.8% in 2011, a statistically significant increase, “with steeper increases” seen in women who had node-negative and noninvasive disease, the authors reported. For the most recent 8-year period – 2003 to 2011 – the rate increased by 34%, “with the most notable rise in mastectomy rates occurring after 2006,” and the highest increases seen among women with clinically node-negative disease. Age and tumor size were “the most influential covariates,” with younger women “more likely to undergo mastectomy irrespective of tumor size, while in older women mastectomy was strongly associated with tumor size greater than 2 cm.”
During the period studied, there were significant increases in breast reconstruction and bilateral mastectomies among women who had mastectomies, which were secondary outcome measures. The proportion of women who underwent breast reconstruction increased from 11.6% in 1998 to 36.4% in 2011, and the proportion of women who had a bilateral mastectomy for unilateral disease increased from 1.9% to 11.2% during this period. The trend toward more reconstruction surgery could be due to 1998 legislation mandating insurance coverage of reconstructive surgery after mastectomy, the authors wrote.
Study limitations included missing clinical staging information for a large proportion of the women, no information on BRCA status or triple-negative tumors, and an inability to determine why the mastectomy was performed in individual cases, they noted.
None of the authors had disclosures to report. The study is based on work supported by the Office of Academic Affiliations, Department of Veterans Affairs, the VA National Quality Scholars Program, and with the use of facilities at the VA Tennessee Valley Healthcare System, Nashville.
Significant increases in the mastectomy rate among women with early breast cancer who were candidates for breast conservation surgery during a recent 14-year period in the United States were accompanied by increases in breast reconstruction and bilateral mastectomies, in a retrospective cohort study that tracked national trends in this group of women.
The results, based on outcomes of about 1.2 million women with early breast cancer in a national oncology outcomes database, “are generally consistent with trends noted in other state, regional, and national studies,” reported Dr. Kristy Kummerow of the division of surgical oncology and endocrine surgery, Vanderbilt University, Nashville, Tenn., and her associates. While they speculated on some of the reasons behind these findings, “further research is needed to understand patient, provider, policy, and social factors associated with these trends,” they concluded in the study, which was published online Nov. 19 in JAMA Surgery (doi:10.1001/jamasurg.2014.2895).
While the use of breast conservation surgery (BCS) as an alternative to mastectomy for early-stage breast cancer increased steadily after studies showed the two approaches had equal outcomes, and after endorsement by a National Institutes of Health Consensus Conference in 1990, the authors noted that there has been evidence that the trend is reversing.
Using data from the National Cancer Data Base, which collects outcomes data on about 70% of the patients diagnosed with cancer in the United States, they evaluated trends in mastectomies among adult women newly diagnosed with early (unilateral) breast cancer from January 1998 through December 2011. Women were included if TNM stage information was available, tumors were 5 cm or less with nine or fewer involved axillary lymph nodes based on clinical staging, and they underwent BCS (lumpectomy, segmental mastectomy, or re-excision of the biopsy site) or mastectomy (subcutaneous, total, modified radical or radical).
Over the 14-year period, among the approximately 1.2 million women who met the criteria, 64.5% had BCS and 35.5% had a mastectomy. The women who had a mastectomy were slightly younger (mean age 59.6 years vs. 61.6 years), and the proportion of racial and ethnic minorities was lower in this group.
The proportion of women eligible for BCS who underwent a mastectomy increased from 34.3% in 1998 to 37.8% in 2011, a statistically significant increase, “with steeper increases” seen in women who had node-negative and noninvasive disease, the authors reported. For the most recent 8-year period – 2003 to 2011 – the rate increased by 34%, “with the most notable rise in mastectomy rates occurring after 2006,” and the highest increases seen among women with clinically node-negative disease. Age and tumor size were “the most influential covariates,” with younger women “more likely to undergo mastectomy irrespective of tumor size, while in older women mastectomy was strongly associated with tumor size greater than 2 cm.”
During the period studied, there were significant increases in breast reconstruction and bilateral mastectomies among women who had mastectomies, which were secondary outcome measures. The proportion of women who underwent breast reconstruction increased from 11.6% in 1998 to 36.4% in 2011, and the proportion of women who had a bilateral mastectomy for unilateral disease increased from 1.9% to 11.2% during this period. The trend toward more reconstruction surgery could be due to 1998 legislation mandating insurance coverage of reconstructive surgery after mastectomy, the authors wrote.
Study limitations included missing clinical staging information for a large proportion of the women, no information on BRCA status or triple-negative tumors, and an inability to determine why the mastectomy was performed in individual cases, they noted.
None of the authors had disclosures to report. The study is based on work supported by the Office of Academic Affiliations, Department of Veterans Affairs, the VA National Quality Scholars Program, and with the use of facilities at the VA Tennessee Valley Healthcare System, Nashville.
FROM JAMA SURGERY
Key clinical point: Significantly more women with early breast cancer are undergoing mastectomy, despite being eligible for breast conservation surgery.
Major finding: The proportion of women with early breast cancer eligible for BCS who had a mastectomy increased from 34.3% in 1998 to 37.8% in 2011, a statistically significant increase.
Data source: A retrospective cohort study of trends during 1998-2011 in mastectomies among 1.2 million women with early breast cancer who were eligible for BCS and enrolled in the National Cancer Data Base.
Disclosures:None of the authors had disclosures to report. The study is based on work supported by the Office of Academic Affiliations, Department of Veterans Affairs , the VA National Quality Scholars Program, and with the use of facilities at the VA Tennessee Valley Healthcare System, Nashville.