User login
Official Newspaper of the American College of Surgeons
Doctors support malpractice provision in SGR bill
A little-noticed provision in legislation to repeal the Medicare Sustainable Growth Rate formula would protect doctors from lawsuits based on their performance on federal quality measures.
Language contained in H.R. 2, the Medicare Access and CHIP Reauthorization Act specifies that the development, recognition, or implementation of any federal health care guideline or standard shall not be construed to establish a duty of care in medical malpractice claims.
The provision helps distinguish government quality guidelines and payment rules from medical liability standards, according to Brian K. Atchinson, president and CEO of PIAA, a national trade association for medical malpractice liability insurers.
“None of these rules or guidelines were created with the intent to establish a legal standard for negligence, and so it makes sense for Congress to clarify that fact,” Mr. Atchinson said in an interview. “The standard of care provision in the SGR fix bill does just that, and nothing more. It does not shift the playing field to either plaintiffs or defendants. Instead, it ensures that these federal rules are not misused for purposes for which they were never intended.”
The language was originally included in the Affordable Care Act, but was removed by the Senate. If the SGR repeal legislation is enacted, the provision would prohibit plaintiffs from using a doctor’s performance in a quality improvement program as the sole basis for a medical liability lawsuit or to prove negligence. For example, a physician who missed earning an incentive under the Physician Quality Reporting System could not have that fact raised in a malpractice action to build the plaintiff’s case.
Federal guidelines and quality criteria intended to measure the impact of health care delivery and payment systems should not be exploited to invent new legal actions against physicians, said Dr. Robert M. Wah, president of the American Medical Association.
“These guidelines cannot be inflated into claims of physician negligence,” Dr. Wah said in a statement. “Nor can it be assumed that failure to report under these programs is an indication of substandard care. It is clear that explicit protections are needed to hold the line against a medical liability system that invites abuse. The potential for new liability exposure is not the way to encourage physician engagement in the development and implementation of new strategies to improve the quality and efficiency of care.”
Officials at the American Congress of Obstetricians and Gynecologists said the bill’s provision is one step toward better legal protection for physicians who participate in federal quality programs. However, they stressed the need for further protection, such safeguards incorporated in H.R. 4106, the Saving Lives, Saving Costs Act introduced in the last Congress by Rep. Andy Barr (R-Ky.) and Rep. Ami Bera, (D-Calif.). The bill would provide safe harbor protection to doctors who are sued if they followed evidence-based clinical guidelines.
“ACOG is pleased that a provision in the SGR package was included to address standard of care protection and continues to support prompt passage of SGR repeal legislation,” according to a statement from the organization. “However, while we support the provision in the SGR package, our work will not stop once that legislation passes. We will continue to seek comprehensive and alternative medical liability reforms, and we hope that Reps. Barr and Bera reintroduce their safe harbor bill soon. [The legislation] would improve quality of care by promoting physician adherence to clinical practice guidelines and would also help to avoid frivolous lawsuits, lowering overall health care costs, and ensuring that physicians can continue to treat their patients.”
Under the safe harbor legislation, clinical guidelines developed by professional medical organizations would be used to determine whether a plaintiff’s lawsuit could continue against a physician defendant. If a doctor adhered to the approved guidelines during the time of the alleged malpractice event, the case would be removed from court proceedings, while a medical review panel investigated the claim. The bill also would allow for relevant cases to be moved from state to federal court if they involved federal dollars such as Medicare.
The bill has yet to be introduced in the current Congress.
The Medicare Access and CHIP Reauthorization Act meanwhile, awaits action by the Senate, which returns from a recess on April 13. The House on March 26 overwhelming passed the bill, which would repeal the SGR, reauthorize the Children’s Health Insurance Program for 2 years, and reform Medicare.
Medicare physician pay was cut by approximately 21% effective April 1, because of the expiration of the last temporary SGR fix. However, the Centers for Medicare & Medicaid Services announced that it would hold Medicare payments for 2 weeks, allowing Congress to complete action on the issue.
Without a legislative fix, CMS will begin processing claims with a 21% reduction in the physician’s rate beginning April 15. Should the SGR repeal legislation be signed into law, CMS will reprocess any claims processed at the lower rate.
On Twitter @legal_med
A little-noticed provision in legislation to repeal the Medicare Sustainable Growth Rate formula would protect doctors from lawsuits based on their performance on federal quality measures.
Language contained in H.R. 2, the Medicare Access and CHIP Reauthorization Act specifies that the development, recognition, or implementation of any federal health care guideline or standard shall not be construed to establish a duty of care in medical malpractice claims.
The provision helps distinguish government quality guidelines and payment rules from medical liability standards, according to Brian K. Atchinson, president and CEO of PIAA, a national trade association for medical malpractice liability insurers.
“None of these rules or guidelines were created with the intent to establish a legal standard for negligence, and so it makes sense for Congress to clarify that fact,” Mr. Atchinson said in an interview. “The standard of care provision in the SGR fix bill does just that, and nothing more. It does not shift the playing field to either plaintiffs or defendants. Instead, it ensures that these federal rules are not misused for purposes for which they were never intended.”
The language was originally included in the Affordable Care Act, but was removed by the Senate. If the SGR repeal legislation is enacted, the provision would prohibit plaintiffs from using a doctor’s performance in a quality improvement program as the sole basis for a medical liability lawsuit or to prove negligence. For example, a physician who missed earning an incentive under the Physician Quality Reporting System could not have that fact raised in a malpractice action to build the plaintiff’s case.
Federal guidelines and quality criteria intended to measure the impact of health care delivery and payment systems should not be exploited to invent new legal actions against physicians, said Dr. Robert M. Wah, president of the American Medical Association.
“These guidelines cannot be inflated into claims of physician negligence,” Dr. Wah said in a statement. “Nor can it be assumed that failure to report under these programs is an indication of substandard care. It is clear that explicit protections are needed to hold the line against a medical liability system that invites abuse. The potential for new liability exposure is not the way to encourage physician engagement in the development and implementation of new strategies to improve the quality and efficiency of care.”
Officials at the American Congress of Obstetricians and Gynecologists said the bill’s provision is one step toward better legal protection for physicians who participate in federal quality programs. However, they stressed the need for further protection, such safeguards incorporated in H.R. 4106, the Saving Lives, Saving Costs Act introduced in the last Congress by Rep. Andy Barr (R-Ky.) and Rep. Ami Bera, (D-Calif.). The bill would provide safe harbor protection to doctors who are sued if they followed evidence-based clinical guidelines.
“ACOG is pleased that a provision in the SGR package was included to address standard of care protection and continues to support prompt passage of SGR repeal legislation,” according to a statement from the organization. “However, while we support the provision in the SGR package, our work will not stop once that legislation passes. We will continue to seek comprehensive and alternative medical liability reforms, and we hope that Reps. Barr and Bera reintroduce their safe harbor bill soon. [The legislation] would improve quality of care by promoting physician adherence to clinical practice guidelines and would also help to avoid frivolous lawsuits, lowering overall health care costs, and ensuring that physicians can continue to treat their patients.”
Under the safe harbor legislation, clinical guidelines developed by professional medical organizations would be used to determine whether a plaintiff’s lawsuit could continue against a physician defendant. If a doctor adhered to the approved guidelines during the time of the alleged malpractice event, the case would be removed from court proceedings, while a medical review panel investigated the claim. The bill also would allow for relevant cases to be moved from state to federal court if they involved federal dollars such as Medicare.
The bill has yet to be introduced in the current Congress.
The Medicare Access and CHIP Reauthorization Act meanwhile, awaits action by the Senate, which returns from a recess on April 13. The House on March 26 overwhelming passed the bill, which would repeal the SGR, reauthorize the Children’s Health Insurance Program for 2 years, and reform Medicare.
Medicare physician pay was cut by approximately 21% effective April 1, because of the expiration of the last temporary SGR fix. However, the Centers for Medicare & Medicaid Services announced that it would hold Medicare payments for 2 weeks, allowing Congress to complete action on the issue.
Without a legislative fix, CMS will begin processing claims with a 21% reduction in the physician’s rate beginning April 15. Should the SGR repeal legislation be signed into law, CMS will reprocess any claims processed at the lower rate.
On Twitter @legal_med
A little-noticed provision in legislation to repeal the Medicare Sustainable Growth Rate formula would protect doctors from lawsuits based on their performance on federal quality measures.
Language contained in H.R. 2, the Medicare Access and CHIP Reauthorization Act specifies that the development, recognition, or implementation of any federal health care guideline or standard shall not be construed to establish a duty of care in medical malpractice claims.
The provision helps distinguish government quality guidelines and payment rules from medical liability standards, according to Brian K. Atchinson, president and CEO of PIAA, a national trade association for medical malpractice liability insurers.
“None of these rules or guidelines were created with the intent to establish a legal standard for negligence, and so it makes sense for Congress to clarify that fact,” Mr. Atchinson said in an interview. “The standard of care provision in the SGR fix bill does just that, and nothing more. It does not shift the playing field to either plaintiffs or defendants. Instead, it ensures that these federal rules are not misused for purposes for which they were never intended.”
The language was originally included in the Affordable Care Act, but was removed by the Senate. If the SGR repeal legislation is enacted, the provision would prohibit plaintiffs from using a doctor’s performance in a quality improvement program as the sole basis for a medical liability lawsuit or to prove negligence. For example, a physician who missed earning an incentive under the Physician Quality Reporting System could not have that fact raised in a malpractice action to build the plaintiff’s case.
Federal guidelines and quality criteria intended to measure the impact of health care delivery and payment systems should not be exploited to invent new legal actions against physicians, said Dr. Robert M. Wah, president of the American Medical Association.
“These guidelines cannot be inflated into claims of physician negligence,” Dr. Wah said in a statement. “Nor can it be assumed that failure to report under these programs is an indication of substandard care. It is clear that explicit protections are needed to hold the line against a medical liability system that invites abuse. The potential for new liability exposure is not the way to encourage physician engagement in the development and implementation of new strategies to improve the quality and efficiency of care.”
Officials at the American Congress of Obstetricians and Gynecologists said the bill’s provision is one step toward better legal protection for physicians who participate in federal quality programs. However, they stressed the need for further protection, such safeguards incorporated in H.R. 4106, the Saving Lives, Saving Costs Act introduced in the last Congress by Rep. Andy Barr (R-Ky.) and Rep. Ami Bera, (D-Calif.). The bill would provide safe harbor protection to doctors who are sued if they followed evidence-based clinical guidelines.
“ACOG is pleased that a provision in the SGR package was included to address standard of care protection and continues to support prompt passage of SGR repeal legislation,” according to a statement from the organization. “However, while we support the provision in the SGR package, our work will not stop once that legislation passes. We will continue to seek comprehensive and alternative medical liability reforms, and we hope that Reps. Barr and Bera reintroduce their safe harbor bill soon. [The legislation] would improve quality of care by promoting physician adherence to clinical practice guidelines and would also help to avoid frivolous lawsuits, lowering overall health care costs, and ensuring that physicians can continue to treat their patients.”
Under the safe harbor legislation, clinical guidelines developed by professional medical organizations would be used to determine whether a plaintiff’s lawsuit could continue against a physician defendant. If a doctor adhered to the approved guidelines during the time of the alleged malpractice event, the case would be removed from court proceedings, while a medical review panel investigated the claim. The bill also would allow for relevant cases to be moved from state to federal court if they involved federal dollars such as Medicare.
The bill has yet to be introduced in the current Congress.
The Medicare Access and CHIP Reauthorization Act meanwhile, awaits action by the Senate, which returns from a recess on April 13. The House on March 26 overwhelming passed the bill, which would repeal the SGR, reauthorize the Children’s Health Insurance Program for 2 years, and reform Medicare.
Medicare physician pay was cut by approximately 21% effective April 1, because of the expiration of the last temporary SGR fix. However, the Centers for Medicare & Medicaid Services announced that it would hold Medicare payments for 2 weeks, allowing Congress to complete action on the issue.
Without a legislative fix, CMS will begin processing claims with a 21% reduction in the physician’s rate beginning April 15. Should the SGR repeal legislation be signed into law, CMS will reprocess any claims processed at the lower rate.
On Twitter @legal_med
Clinical doc improvement ups income, quality
HOUSTON – Results of a pilot program suggest that a surgeon-led Clinical Documentation Improvement (CDI) program can improve the accuracy of diagnostic coding, validate the quality of care delivered, and help ensure that hospitals are fairly compensated for the complexity of care they provide.
A comparison of outcomes of cases from four surgical oncologists from the periods before and after implementation of a CDI suggested that the actual case mix index (CMI) was 9% higher than the original charts indicated, a change that would translate into a more than $700,000 increase in reimbursement, said Dr. Keith Gray from the University of Tennessee Medical Center in Knoxville.
“CDI is low-hanging fruit in the era of pay for quality and dwindling hospital margins. Physicians and hospitals can benefit, and surgical oncologists are the natural physicians in the hospital to lead this process, “ Dr. Gray said at the Society of Surgical Oncology 2015 Cancer Symposium.
CDI programs are collaborative efforts between clinicians and health information management professionals, designed to document the quality of care the institution delivers through improved diagnostic coding, he explained.
The benefits of CDI include more accurate reflection of the severity of illness of patients, better sharing of data among caregivers, optimizing of claims processing, and a stronger bottom line.
“We all think we have the sickest patients in the country, and that’s why our results don’t match up. Clinical documentation is an opportunity for you to prove that,” he said.
In the study, a physician extender trained in CDI audited and update all inpatient diagnoses made by four surgical oncologists in a hospital-based practice from November 2012 through May 2013. The diagnoses were listed as being present on admission or recorded during the inpatient stay.
The investigators looked at data on the CMI, average mortality risk, and average severity of illness for 489 patients treated during the study period. These data were compared with a control set from 482 patients treated from March 2011 through October 2012, the period immediately prior to the implementation of the CDI.
The authors found that with the clinical documentation program in place, the CMI, risk of mortality estimates, and severity of illness index all increased.
The practice’s mean CMI, for example, increased from 2.38 to 2.59 (P < .001), a 9% relative increase. Dr. Gray noted that every 0.1 change in CMI represents a $700/patient difference in reimbursement. Therefore, the change would translate into a $718,830 relative increase in reimbursement.
Similarly, the severity of illness index, based on patient comorbidities, age, procedures, and principal diagnosis also increased, from a mean of 2.32 for controls to 2.54 during the study period, translating into a 9.5% increase (P < .001).
Risk of mortality estimates – the likelihood of in-hospital death based on comorbidities, age, procedures, and principal diagnosis – also increased, from a mean 1.88 to 2.07, a 10% increase (P < .001).
Although the CMI, risk of mortality, and severity of illness all increased during the study period, compared with the control period, the percentage of cases above the average length of stay, a measure of quality care, declined significantly, from 45.6% pre-CDI to 31.1% after CDI was implemented (P = .0001). Other measures of quality such as the observed to expected mortality ratio, length of stay ratio, and percentage of cases above the average cost also improved, but the changes were not statistically significant.
“CDI is relatively easy to implement with the resources that we have in place, and there’s minimal additional training to become efficient in this process,” Dr. Gray said.
The study was internally funded. Dr. Gray did not report potential conflicts of interest.
HOUSTON – Results of a pilot program suggest that a surgeon-led Clinical Documentation Improvement (CDI) program can improve the accuracy of diagnostic coding, validate the quality of care delivered, and help ensure that hospitals are fairly compensated for the complexity of care they provide.
A comparison of outcomes of cases from four surgical oncologists from the periods before and after implementation of a CDI suggested that the actual case mix index (CMI) was 9% higher than the original charts indicated, a change that would translate into a more than $700,000 increase in reimbursement, said Dr. Keith Gray from the University of Tennessee Medical Center in Knoxville.
“CDI is low-hanging fruit in the era of pay for quality and dwindling hospital margins. Physicians and hospitals can benefit, and surgical oncologists are the natural physicians in the hospital to lead this process, “ Dr. Gray said at the Society of Surgical Oncology 2015 Cancer Symposium.
CDI programs are collaborative efforts between clinicians and health information management professionals, designed to document the quality of care the institution delivers through improved diagnostic coding, he explained.
The benefits of CDI include more accurate reflection of the severity of illness of patients, better sharing of data among caregivers, optimizing of claims processing, and a stronger bottom line.
“We all think we have the sickest patients in the country, and that’s why our results don’t match up. Clinical documentation is an opportunity for you to prove that,” he said.
In the study, a physician extender trained in CDI audited and update all inpatient diagnoses made by four surgical oncologists in a hospital-based practice from November 2012 through May 2013. The diagnoses were listed as being present on admission or recorded during the inpatient stay.
The investigators looked at data on the CMI, average mortality risk, and average severity of illness for 489 patients treated during the study period. These data were compared with a control set from 482 patients treated from March 2011 through October 2012, the period immediately prior to the implementation of the CDI.
The authors found that with the clinical documentation program in place, the CMI, risk of mortality estimates, and severity of illness index all increased.
The practice’s mean CMI, for example, increased from 2.38 to 2.59 (P < .001), a 9% relative increase. Dr. Gray noted that every 0.1 change in CMI represents a $700/patient difference in reimbursement. Therefore, the change would translate into a $718,830 relative increase in reimbursement.
Similarly, the severity of illness index, based on patient comorbidities, age, procedures, and principal diagnosis also increased, from a mean of 2.32 for controls to 2.54 during the study period, translating into a 9.5% increase (P < .001).
Risk of mortality estimates – the likelihood of in-hospital death based on comorbidities, age, procedures, and principal diagnosis – also increased, from a mean 1.88 to 2.07, a 10% increase (P < .001).
Although the CMI, risk of mortality, and severity of illness all increased during the study period, compared with the control period, the percentage of cases above the average length of stay, a measure of quality care, declined significantly, from 45.6% pre-CDI to 31.1% after CDI was implemented (P = .0001). Other measures of quality such as the observed to expected mortality ratio, length of stay ratio, and percentage of cases above the average cost also improved, but the changes were not statistically significant.
“CDI is relatively easy to implement with the resources that we have in place, and there’s minimal additional training to become efficient in this process,” Dr. Gray said.
The study was internally funded. Dr. Gray did not report potential conflicts of interest.
HOUSTON – Results of a pilot program suggest that a surgeon-led Clinical Documentation Improvement (CDI) program can improve the accuracy of diagnostic coding, validate the quality of care delivered, and help ensure that hospitals are fairly compensated for the complexity of care they provide.
A comparison of outcomes of cases from four surgical oncologists from the periods before and after implementation of a CDI suggested that the actual case mix index (CMI) was 9% higher than the original charts indicated, a change that would translate into a more than $700,000 increase in reimbursement, said Dr. Keith Gray from the University of Tennessee Medical Center in Knoxville.
“CDI is low-hanging fruit in the era of pay for quality and dwindling hospital margins. Physicians and hospitals can benefit, and surgical oncologists are the natural physicians in the hospital to lead this process, “ Dr. Gray said at the Society of Surgical Oncology 2015 Cancer Symposium.
CDI programs are collaborative efforts between clinicians and health information management professionals, designed to document the quality of care the institution delivers through improved diagnostic coding, he explained.
The benefits of CDI include more accurate reflection of the severity of illness of patients, better sharing of data among caregivers, optimizing of claims processing, and a stronger bottom line.
“We all think we have the sickest patients in the country, and that’s why our results don’t match up. Clinical documentation is an opportunity for you to prove that,” he said.
In the study, a physician extender trained in CDI audited and update all inpatient diagnoses made by four surgical oncologists in a hospital-based practice from November 2012 through May 2013. The diagnoses were listed as being present on admission or recorded during the inpatient stay.
The investigators looked at data on the CMI, average mortality risk, and average severity of illness for 489 patients treated during the study period. These data were compared with a control set from 482 patients treated from March 2011 through October 2012, the period immediately prior to the implementation of the CDI.
The authors found that with the clinical documentation program in place, the CMI, risk of mortality estimates, and severity of illness index all increased.
The practice’s mean CMI, for example, increased from 2.38 to 2.59 (P < .001), a 9% relative increase. Dr. Gray noted that every 0.1 change in CMI represents a $700/patient difference in reimbursement. Therefore, the change would translate into a $718,830 relative increase in reimbursement.
Similarly, the severity of illness index, based on patient comorbidities, age, procedures, and principal diagnosis also increased, from a mean of 2.32 for controls to 2.54 during the study period, translating into a 9.5% increase (P < .001).
Risk of mortality estimates – the likelihood of in-hospital death based on comorbidities, age, procedures, and principal diagnosis – also increased, from a mean 1.88 to 2.07, a 10% increase (P < .001).
Although the CMI, risk of mortality, and severity of illness all increased during the study period, compared with the control period, the percentage of cases above the average length of stay, a measure of quality care, declined significantly, from 45.6% pre-CDI to 31.1% after CDI was implemented (P = .0001). Other measures of quality such as the observed to expected mortality ratio, length of stay ratio, and percentage of cases above the average cost also improved, but the changes were not statistically significant.
“CDI is relatively easy to implement with the resources that we have in place, and there’s minimal additional training to become efficient in this process,” Dr. Gray said.
The study was internally funded. Dr. Gray did not report potential conflicts of interest.
AT SSO 2015
Key clinical point: A surgeon-led clinical documentation program can increase revenue by demonstrating case-mix severity.
Major finding: An audit of previous cases showed that proper documentation would yield $718,830 in additional reimbursements.
Data source: Pilot program and retrospective case audit involving cases of 489 patients and 482 controls.
Disclosures: The study was internally funded. Dr. Gray did not report potential conflicts of interest.
‘Fresh’ no better than standard red cells
Compared with standard-issue red cells, transfusion of “fresh” red cells stored for fewer than 8 days failed to improve 90-day mortality in a large international study of adult ICU patients, which was reported online April 9 in the New England Journal of Medicine.
“Current regulations permit the storage of red cells for up to 42 days, but prolonged storage has been associated with changes that may render cells ineffective as oxygen carriers and that lead to the accumulation of substances that have untoward biologic effects,” said Dr. Jacques Lacroix of the University of Montreal and his associates.
A recent meta-analysis suggested that transfusion of older red cells was associated with a 16% increase in mortality among critically ill patients, but several randomized trials have failed to document any adverse effects on oxygenation, immunologic, or coagulation factors. Dr. Lacroix and his associates performed the Age of Blood Evaluation (ABLE) study, a prospective, blinded clinical trial involving ICU patients enrolled during a 5-year period at 64 medical centers in Canada, the United Kingdom, France, the Netherlands, and Belgium. A total of 1,211 participants were randomly assigned to receive “fresh” blood stored for an average of 6 days and 1,219 to receive standard blood stored for an average of 22 days – a difference that the investigators deemed statistically and clinically significant.
The primary outcome measure, 90-day all-cause mortality, was 37% in the fresh-blood group and 35% in the standard-blood group, a nonsignificant difference. There also were no significant differences in mortality between the two study groups in any of several subgroup analyses based on patient age; the number of units transfused; baseline APACHE II scores; major comorbidities; duration of respiratory, hemodynamic, or renal support; length of ICU stay; or length of hospital stay, Dr Lacroix and his associates said (N. Engl. J. Med. 2015 April 9 [doi:10.1056/NEJMoa1500704]).
“These findings have important implications for the critical care and blood transfusion communities. We surmise that the use of fresh red cells is not justified at this time. We might also infer that changes to red cells or the storage medium that have been documented in many laboratory studies may have limited clinical consequences,” they noted.
This trial was supported by the Canadian Institutes of Health Research, several other Canadian and French government agencies, and Sanquin Blood Supply. Dr. Lacroix reported having no financial disclosures; his associates reported ties to AKPA Pharma, Roche Diagnostics, GlaxoSmithKline, Novartis, and Amgen.
Compared with standard-issue red cells, transfusion of “fresh” red cells stored for fewer than 8 days failed to improve 90-day mortality in a large international study of adult ICU patients, which was reported online April 9 in the New England Journal of Medicine.
“Current regulations permit the storage of red cells for up to 42 days, but prolonged storage has been associated with changes that may render cells ineffective as oxygen carriers and that lead to the accumulation of substances that have untoward biologic effects,” said Dr. Jacques Lacroix of the University of Montreal and his associates.
A recent meta-analysis suggested that transfusion of older red cells was associated with a 16% increase in mortality among critically ill patients, but several randomized trials have failed to document any adverse effects on oxygenation, immunologic, or coagulation factors. Dr. Lacroix and his associates performed the Age of Blood Evaluation (ABLE) study, a prospective, blinded clinical trial involving ICU patients enrolled during a 5-year period at 64 medical centers in Canada, the United Kingdom, France, the Netherlands, and Belgium. A total of 1,211 participants were randomly assigned to receive “fresh” blood stored for an average of 6 days and 1,219 to receive standard blood stored for an average of 22 days – a difference that the investigators deemed statistically and clinically significant.
The primary outcome measure, 90-day all-cause mortality, was 37% in the fresh-blood group and 35% in the standard-blood group, a nonsignificant difference. There also were no significant differences in mortality between the two study groups in any of several subgroup analyses based on patient age; the number of units transfused; baseline APACHE II scores; major comorbidities; duration of respiratory, hemodynamic, or renal support; length of ICU stay; or length of hospital stay, Dr Lacroix and his associates said (N. Engl. J. Med. 2015 April 9 [doi:10.1056/NEJMoa1500704]).
“These findings have important implications for the critical care and blood transfusion communities. We surmise that the use of fresh red cells is not justified at this time. We might also infer that changes to red cells or the storage medium that have been documented in many laboratory studies may have limited clinical consequences,” they noted.
This trial was supported by the Canadian Institutes of Health Research, several other Canadian and French government agencies, and Sanquin Blood Supply. Dr. Lacroix reported having no financial disclosures; his associates reported ties to AKPA Pharma, Roche Diagnostics, GlaxoSmithKline, Novartis, and Amgen.
Compared with standard-issue red cells, transfusion of “fresh” red cells stored for fewer than 8 days failed to improve 90-day mortality in a large international study of adult ICU patients, which was reported online April 9 in the New England Journal of Medicine.
“Current regulations permit the storage of red cells for up to 42 days, but prolonged storage has been associated with changes that may render cells ineffective as oxygen carriers and that lead to the accumulation of substances that have untoward biologic effects,” said Dr. Jacques Lacroix of the University of Montreal and his associates.
A recent meta-analysis suggested that transfusion of older red cells was associated with a 16% increase in mortality among critically ill patients, but several randomized trials have failed to document any adverse effects on oxygenation, immunologic, or coagulation factors. Dr. Lacroix and his associates performed the Age of Blood Evaluation (ABLE) study, a prospective, blinded clinical trial involving ICU patients enrolled during a 5-year period at 64 medical centers in Canada, the United Kingdom, France, the Netherlands, and Belgium. A total of 1,211 participants were randomly assigned to receive “fresh” blood stored for an average of 6 days and 1,219 to receive standard blood stored for an average of 22 days – a difference that the investigators deemed statistically and clinically significant.
The primary outcome measure, 90-day all-cause mortality, was 37% in the fresh-blood group and 35% in the standard-blood group, a nonsignificant difference. There also were no significant differences in mortality between the two study groups in any of several subgroup analyses based on patient age; the number of units transfused; baseline APACHE II scores; major comorbidities; duration of respiratory, hemodynamic, or renal support; length of ICU stay; or length of hospital stay, Dr Lacroix and his associates said (N. Engl. J. Med. 2015 April 9 [doi:10.1056/NEJMoa1500704]).
“These findings have important implications for the critical care and blood transfusion communities. We surmise that the use of fresh red cells is not justified at this time. We might also infer that changes to red cells or the storage medium that have been documented in many laboratory studies may have limited clinical consequences,” they noted.
This trial was supported by the Canadian Institutes of Health Research, several other Canadian and French government agencies, and Sanquin Blood Supply. Dr. Lacroix reported having no financial disclosures; his associates reported ties to AKPA Pharma, Roche Diagnostics, GlaxoSmithKline, Novartis, and Amgen.
Key clinical point: Transfusing “fresher” red blood cells didn’t decrease 90-day mortality in ICU patients, compared with standard transfusions.
Major finding: The primary outcome measure, 90-day all-cause mortality, was 37% in the fresh-blood group and 35% in the standard-blood group.
Data source: A 5-year international, randomized, blinded clinical trial involving 2,430 adult ICU patients.
Disclosures: This trial was supported by the Canadian Institutes of Health Research, several other Canadian and French government agencies, and Sanquin Blood Supply. Dr. Lacroix reported having no financial disclosures; his associates reported ties to AKPA Pharma, Roche Diagnostics, GlaxoSmithKline, Novartis, and Amgen.
Bundled gynecologic surgery payments modified on appeal
ORLANDO – At least some coding edits introduced by the National Correct Coding Initiative that eliminated billing for additional gynecological surgeries performed at the time of vaginal hysterectomy have been effectively challenged by a group of professional organizations led by the American Urogynecologic Society.
In an update at the annual scientific meeting of the Society of Gynecologic Surgeons (SGS), which was among the organizations contributing to the effort, surgeons were told that some of the National Correct Coding Initiative (NCCI) bundling of procedures introduced on Oct. 1, 2014, will be modified to allow separate billing beginning April 1, 2015, including retroactively billing for procedures performed before the modification.
“The NCCI enacted wide sweeping pair edits that limited the types of additional procedures that could be billed at the time of vaginal hysterectomy. For the reconstructive vaginal surgeon, this eliminated the ability to bill for additional procedures, such as combined colporrhaphy and apical vaginal suspensions,” reported Dr. Marc Toglia, who served as vice chair of the Committee for Coding and Health Policy for American Urogynecologic Society (AUGS) that led the challenge.
The bundled procedures proposed by the NCCI are part of a larger effort to avoid paying surgeons twice for surgeries that are commonly performed together without significantly increasing operating time, according to Dr. Toglia. He reported that these particular coding edits were enacted by the Centers for Medicaid & Medicare Services despite strong opposition from AUGS, SGS, the American College of Obstetricians and Gynecologists (ACOG), and others.
“While pair edits are not uncommon – for example, you cannot bill separately for cystoscopy at the time that a pubovaginal sling is performed for urinary incontinence – AUGS felt that NCCI was incorrectly combining procedures performed for different indications and requiring substantially more work than the base procedure,” Dr. Toglia explained. “The NCCI seemed focused on the fact that procedures commonly performed at the same time of vaginal hysterectomy were routinely part of this procedure.”
The NCCI revisited the Oct. 1, 2014, coding edits in the face of the continued opposition led by AUGS. As a result, modifiers can be used to allow billing for some procedures, such as colporrhaphy, done at the same time as vaginal hysterectomy or to bill for complex procedures that required substantial additional work. However, not all the coding edits have yet to be successfully challenged. A set of six bundling codes planned for implementation on April 1 have so far only been postponed until July 1.
Referring to the modifiers, Dr. Toglia, who is chief of female pelvic medicine and reconstructive surgery for the Main Line Health System in Philadelphia, explained that “the edits were not changed. Rather, there is now a work-around.”
Practical information about how to properly employ the coding modifications can be obtained at the AUGS website. The website also has more information about initiatives to challenge other coding modifications that have been proposed and are now being challenged by AUGS.
The efforts by Dr. Toglia were strongly endorsed by Dr. Andrew J. Walter, who was installed as the new president of SGS immediately after the coding initiatives were described. In an interview, Dr. Walter, who is in private practice in Roseville, Calif., suggested that it is not just a question of protecting income but avoiding disincentives. He believes surgeons should not be discouraged from combining procedures when the goal is to improve outcome and patient well being.
“SGS, AUGS, and other professional societies need to work together to ensure that reimbursement is fair and serves the interest of excellent medical care,” Dr. Walter said.
Dr. Toglia and Dr. Walter reported no relevant financial disclosures.
ORLANDO – At least some coding edits introduced by the National Correct Coding Initiative that eliminated billing for additional gynecological surgeries performed at the time of vaginal hysterectomy have been effectively challenged by a group of professional organizations led by the American Urogynecologic Society.
In an update at the annual scientific meeting of the Society of Gynecologic Surgeons (SGS), which was among the organizations contributing to the effort, surgeons were told that some of the National Correct Coding Initiative (NCCI) bundling of procedures introduced on Oct. 1, 2014, will be modified to allow separate billing beginning April 1, 2015, including retroactively billing for procedures performed before the modification.
“The NCCI enacted wide sweeping pair edits that limited the types of additional procedures that could be billed at the time of vaginal hysterectomy. For the reconstructive vaginal surgeon, this eliminated the ability to bill for additional procedures, such as combined colporrhaphy and apical vaginal suspensions,” reported Dr. Marc Toglia, who served as vice chair of the Committee for Coding and Health Policy for American Urogynecologic Society (AUGS) that led the challenge.
The bundled procedures proposed by the NCCI are part of a larger effort to avoid paying surgeons twice for surgeries that are commonly performed together without significantly increasing operating time, according to Dr. Toglia. He reported that these particular coding edits were enacted by the Centers for Medicaid & Medicare Services despite strong opposition from AUGS, SGS, the American College of Obstetricians and Gynecologists (ACOG), and others.
“While pair edits are not uncommon – for example, you cannot bill separately for cystoscopy at the time that a pubovaginal sling is performed for urinary incontinence – AUGS felt that NCCI was incorrectly combining procedures performed for different indications and requiring substantially more work than the base procedure,” Dr. Toglia explained. “The NCCI seemed focused on the fact that procedures commonly performed at the same time of vaginal hysterectomy were routinely part of this procedure.”
The NCCI revisited the Oct. 1, 2014, coding edits in the face of the continued opposition led by AUGS. As a result, modifiers can be used to allow billing for some procedures, such as colporrhaphy, done at the same time as vaginal hysterectomy or to bill for complex procedures that required substantial additional work. However, not all the coding edits have yet to be successfully challenged. A set of six bundling codes planned for implementation on April 1 have so far only been postponed until July 1.
Referring to the modifiers, Dr. Toglia, who is chief of female pelvic medicine and reconstructive surgery for the Main Line Health System in Philadelphia, explained that “the edits were not changed. Rather, there is now a work-around.”
Practical information about how to properly employ the coding modifications can be obtained at the AUGS website. The website also has more information about initiatives to challenge other coding modifications that have been proposed and are now being challenged by AUGS.
The efforts by Dr. Toglia were strongly endorsed by Dr. Andrew J. Walter, who was installed as the new president of SGS immediately after the coding initiatives were described. In an interview, Dr. Walter, who is in private practice in Roseville, Calif., suggested that it is not just a question of protecting income but avoiding disincentives. He believes surgeons should not be discouraged from combining procedures when the goal is to improve outcome and patient well being.
“SGS, AUGS, and other professional societies need to work together to ensure that reimbursement is fair and serves the interest of excellent medical care,” Dr. Walter said.
Dr. Toglia and Dr. Walter reported no relevant financial disclosures.
ORLANDO – At least some coding edits introduced by the National Correct Coding Initiative that eliminated billing for additional gynecological surgeries performed at the time of vaginal hysterectomy have been effectively challenged by a group of professional organizations led by the American Urogynecologic Society.
In an update at the annual scientific meeting of the Society of Gynecologic Surgeons (SGS), which was among the organizations contributing to the effort, surgeons were told that some of the National Correct Coding Initiative (NCCI) bundling of procedures introduced on Oct. 1, 2014, will be modified to allow separate billing beginning April 1, 2015, including retroactively billing for procedures performed before the modification.
“The NCCI enacted wide sweeping pair edits that limited the types of additional procedures that could be billed at the time of vaginal hysterectomy. For the reconstructive vaginal surgeon, this eliminated the ability to bill for additional procedures, such as combined colporrhaphy and apical vaginal suspensions,” reported Dr. Marc Toglia, who served as vice chair of the Committee for Coding and Health Policy for American Urogynecologic Society (AUGS) that led the challenge.
The bundled procedures proposed by the NCCI are part of a larger effort to avoid paying surgeons twice for surgeries that are commonly performed together without significantly increasing operating time, according to Dr. Toglia. He reported that these particular coding edits were enacted by the Centers for Medicaid & Medicare Services despite strong opposition from AUGS, SGS, the American College of Obstetricians and Gynecologists (ACOG), and others.
“While pair edits are not uncommon – for example, you cannot bill separately for cystoscopy at the time that a pubovaginal sling is performed for urinary incontinence – AUGS felt that NCCI was incorrectly combining procedures performed for different indications and requiring substantially more work than the base procedure,” Dr. Toglia explained. “The NCCI seemed focused on the fact that procedures commonly performed at the same time of vaginal hysterectomy were routinely part of this procedure.”
The NCCI revisited the Oct. 1, 2014, coding edits in the face of the continued opposition led by AUGS. As a result, modifiers can be used to allow billing for some procedures, such as colporrhaphy, done at the same time as vaginal hysterectomy or to bill for complex procedures that required substantial additional work. However, not all the coding edits have yet to be successfully challenged. A set of six bundling codes planned for implementation on April 1 have so far only been postponed until July 1.
Referring to the modifiers, Dr. Toglia, who is chief of female pelvic medicine and reconstructive surgery for the Main Line Health System in Philadelphia, explained that “the edits were not changed. Rather, there is now a work-around.”
Practical information about how to properly employ the coding modifications can be obtained at the AUGS website. The website also has more information about initiatives to challenge other coding modifications that have been proposed and are now being challenged by AUGS.
The efforts by Dr. Toglia were strongly endorsed by Dr. Andrew J. Walter, who was installed as the new president of SGS immediately after the coding initiatives were described. In an interview, Dr. Walter, who is in private practice in Roseville, Calif., suggested that it is not just a question of protecting income but avoiding disincentives. He believes surgeons should not be discouraged from combining procedures when the goal is to improve outcome and patient well being.
“SGS, AUGS, and other professional societies need to work together to ensure that reimbursement is fair and serves the interest of excellent medical care,” Dr. Walter said.
Dr. Toglia and Dr. Walter reported no relevant financial disclosures.
EXPERT ANALYSIS FROM SGS 2015
Smaller tubes take bite out of blood draws in critically ill
CHICAGO – Switching from conventional to small-volume phlebotomy tubes is an easy step toward reducing iatrogenic blood loss in critically ill adults, a new study suggests.
“We were looking at the amount of blood we were drawing off these patients and when we asked the nurses, the numbers were crazy. It could be as high as 20 mL per time that they drew off the patient and we felt we had to do better. The common sense dictum is the more blood you draw off, the more harm you are causing the patient,” principal investigator Dr. Heather Dolman from Detroit Receiving Hospital, Wayne State University, said in an interview.
For patients staying only a day or 2 at the hospital, the type of blood tube used may not make a difference. But for the critically ill, who studies suggest can have an average of 5 to more than 24 samples drawn a day, the cumulative blood loss over an extended stay can be sizable.
Clinicians are also inclined to order more diagnostic tests as the severity of illness increases, thus putting their sickest patients at the greatest risk of iatrogenic anemia and transfusion. Anemia secondary to phlebotomy accounts for up to 40% of packed red blood cells transfused, Dr. Dolman noted at the annual meeting of the Central Surgical Association.
The process of blood sampling itself also involves a fair amount of waste. Conventional arterial line systems require that an initial blood sample be removed to “clear the line.” This typically results in 2-10 mL of blood being discarded before a second sample of undiluted blood can be obtained.
Some hospitals have turned to closed blood sampling devices that avoid the need for a second sample. The impact of blood-conserving devices on transfusion rates has been underwhelming, with only one study showing a positive impact leading to reduced blood product use.
As part of their blood-conserving strategy, Dr. Dolman and her colleagues asked the hospital to invest in small-volume phlebotomy tubes (SVTs), which are sized somewhere between a conventional-volume tube (CVT) and a pediatric blood tube.
SVTs reduce the amount of blood needed from 8.5 mL with a conventional tube to 5.0 mL for a basic metabolic panel, from 6.0 mL to 2.0 mL for a complete blood count (CBC) or cross-matching, and from 2.7 mL to 1.8 mL for a prothrombin time /internationalized ratio/partial thromboplastin time, Dr. Dolman said. The cost of an SVT is the same as a CVT, as is the cost of the machinery needed to analyze the samples.
“Everyone is worried about missing out on data, but if you look at the research on the amount of blood the machine really needs, it is only 0.1 mL, that’s less than a cc,” she said. “The technology has been there for a while, I just think the common sense aspect of all this, no one has ever thought of.”
The investigators then retrospectively compared 248 critically ill patients in the ICU, of whom 116 had blood drawn with an SVT and 132 with a CVT. The two groups were well matched with respect to age (55 years vs. 57 years), admission to the emergency surgery/trauma service (63% vs. 64%), and mean APACHE II scores (14.1 vs. 12.7).
Transfusion was at the discretion of the primary team using a restrictive hemoglobin threshold of < 7.0 gm/dL, unless hemodynamic instability or active bleeding were present.
Utilizing an SVT significantly reduced daily blood loss from phlebotomy from 31.7 mL with a CVT to 22.5 mL (P < .0001) and overall phlebotomy blood loss from 299 mL to 174 mL (P < .001), Dr. Dolman reported.
This translated into a nonsignificant trend for fewer units of packed red blood cells transfused in the SVT group (mean 4.4 vs. 6.0; P = .16).
The same pattern was observed in the 158 patients admitted to the emergency surgery/trauma service, with SVT also leading to significantly fewer episodes of severe anemia (6 vs. 20; P = .01) and a trend toward shorter ICU stays (9.2 days vs. 10.6 days; P = .46), she said.
Patients with an APACHE score of at least 20, a group one would anticipate to derive greater advantage from a blood-conserving strategy, did not benefit from use of an SVT vs. a CVT, but the number of patients was very low at just 27 and 19, respectively, Dr. Dolman noted.
Anemia, however, had a profound impact on the critically ill cohort. Patients with severe anemia were significantly more likely than those with a hemoglobin level of at least 7 gm/dL to have longer ICU stays (16 days vs. 7.7 days; P < .001), longer hospital stays (23.3 days vs. 13.6 days; P < .001), and to die in the hospital (29% vs. 13%; P = .01).
Using a small-volume tube cut the number of patients with more than one episode of severe anemia from 22 to 11 (P = .01) and those with more than two episodes from 6 to 4 (P = .53).
“Anemia in the critically ill is a significant problem,” Dr. Dolman said. “Phlebotomy waste contributes to anemia and should be recorded to decrease this hidden loss.”
The impact of transfusion vs. no transfusion was less pronounced with respect to ICU stay (12 days vs. 6 days; P < .001), hospital stay (19 days vs. 11 days; P = .44), and in-hospital mortality (17% vs. 15%; P = .60), but can lead to other negative sequelae such as increased risk of infection, circulatory overload transfusion reactions, and immune modulation, she added.
Detroit Receiving Hospital continues to use conventional tubes in its ICU and other units, although a switch to small-volume tubes is expected to be considered following peer review of the full results, Dr. Dolman said.
Dr. Dolman reported having no financial disclosures.
On Twitter @pwendl
“This is something that should be replicated at institutions across the country,” discussant William C. Cirocco said in an interview. “Why not? It may not have clinical implications for the patient who is only in the hospital for 2 or 3 days, but for the ICU patient, it will have big impact. It’s a no-brainer.”
Dr. William C. Cirocco is a professor of surgery at Ohio State University in Columbus. He reported no relevant conflicts of interest.
“This is something that should be replicated at institutions across the country,” discussant William C. Cirocco said in an interview. “Why not? It may not have clinical implications for the patient who is only in the hospital for 2 or 3 days, but for the ICU patient, it will have big impact. It’s a no-brainer.”
Dr. William C. Cirocco is a professor of surgery at Ohio State University in Columbus. He reported no relevant conflicts of interest.
“This is something that should be replicated at institutions across the country,” discussant William C. Cirocco said in an interview. “Why not? It may not have clinical implications for the patient who is only in the hospital for 2 or 3 days, but for the ICU patient, it will have big impact. It’s a no-brainer.”
Dr. William C. Cirocco is a professor of surgery at Ohio State University in Columbus. He reported no relevant conflicts of interest.
CHICAGO – Switching from conventional to small-volume phlebotomy tubes is an easy step toward reducing iatrogenic blood loss in critically ill adults, a new study suggests.
“We were looking at the amount of blood we were drawing off these patients and when we asked the nurses, the numbers were crazy. It could be as high as 20 mL per time that they drew off the patient and we felt we had to do better. The common sense dictum is the more blood you draw off, the more harm you are causing the patient,” principal investigator Dr. Heather Dolman from Detroit Receiving Hospital, Wayne State University, said in an interview.
For patients staying only a day or 2 at the hospital, the type of blood tube used may not make a difference. But for the critically ill, who studies suggest can have an average of 5 to more than 24 samples drawn a day, the cumulative blood loss over an extended stay can be sizable.
Clinicians are also inclined to order more diagnostic tests as the severity of illness increases, thus putting their sickest patients at the greatest risk of iatrogenic anemia and transfusion. Anemia secondary to phlebotomy accounts for up to 40% of packed red blood cells transfused, Dr. Dolman noted at the annual meeting of the Central Surgical Association.
The process of blood sampling itself also involves a fair amount of waste. Conventional arterial line systems require that an initial blood sample be removed to “clear the line.” This typically results in 2-10 mL of blood being discarded before a second sample of undiluted blood can be obtained.
Some hospitals have turned to closed blood sampling devices that avoid the need for a second sample. The impact of blood-conserving devices on transfusion rates has been underwhelming, with only one study showing a positive impact leading to reduced blood product use.
As part of their blood-conserving strategy, Dr. Dolman and her colleagues asked the hospital to invest in small-volume phlebotomy tubes (SVTs), which are sized somewhere between a conventional-volume tube (CVT) and a pediatric blood tube.
SVTs reduce the amount of blood needed from 8.5 mL with a conventional tube to 5.0 mL for a basic metabolic panel, from 6.0 mL to 2.0 mL for a complete blood count (CBC) or cross-matching, and from 2.7 mL to 1.8 mL for a prothrombin time /internationalized ratio/partial thromboplastin time, Dr. Dolman said. The cost of an SVT is the same as a CVT, as is the cost of the machinery needed to analyze the samples.
“Everyone is worried about missing out on data, but if you look at the research on the amount of blood the machine really needs, it is only 0.1 mL, that’s less than a cc,” she said. “The technology has been there for a while, I just think the common sense aspect of all this, no one has ever thought of.”
The investigators then retrospectively compared 248 critically ill patients in the ICU, of whom 116 had blood drawn with an SVT and 132 with a CVT. The two groups were well matched with respect to age (55 years vs. 57 years), admission to the emergency surgery/trauma service (63% vs. 64%), and mean APACHE II scores (14.1 vs. 12.7).
Transfusion was at the discretion of the primary team using a restrictive hemoglobin threshold of < 7.0 gm/dL, unless hemodynamic instability or active bleeding were present.
Utilizing an SVT significantly reduced daily blood loss from phlebotomy from 31.7 mL with a CVT to 22.5 mL (P < .0001) and overall phlebotomy blood loss from 299 mL to 174 mL (P < .001), Dr. Dolman reported.
This translated into a nonsignificant trend for fewer units of packed red blood cells transfused in the SVT group (mean 4.4 vs. 6.0; P = .16).
The same pattern was observed in the 158 patients admitted to the emergency surgery/trauma service, with SVT also leading to significantly fewer episodes of severe anemia (6 vs. 20; P = .01) and a trend toward shorter ICU stays (9.2 days vs. 10.6 days; P = .46), she said.
Patients with an APACHE score of at least 20, a group one would anticipate to derive greater advantage from a blood-conserving strategy, did not benefit from use of an SVT vs. a CVT, but the number of patients was very low at just 27 and 19, respectively, Dr. Dolman noted.
Anemia, however, had a profound impact on the critically ill cohort. Patients with severe anemia were significantly more likely than those with a hemoglobin level of at least 7 gm/dL to have longer ICU stays (16 days vs. 7.7 days; P < .001), longer hospital stays (23.3 days vs. 13.6 days; P < .001), and to die in the hospital (29% vs. 13%; P = .01).
Using a small-volume tube cut the number of patients with more than one episode of severe anemia from 22 to 11 (P = .01) and those with more than two episodes from 6 to 4 (P = .53).
“Anemia in the critically ill is a significant problem,” Dr. Dolman said. “Phlebotomy waste contributes to anemia and should be recorded to decrease this hidden loss.”
The impact of transfusion vs. no transfusion was less pronounced with respect to ICU stay (12 days vs. 6 days; P < .001), hospital stay (19 days vs. 11 days; P = .44), and in-hospital mortality (17% vs. 15%; P = .60), but can lead to other negative sequelae such as increased risk of infection, circulatory overload transfusion reactions, and immune modulation, she added.
Detroit Receiving Hospital continues to use conventional tubes in its ICU and other units, although a switch to small-volume tubes is expected to be considered following peer review of the full results, Dr. Dolman said.
Dr. Dolman reported having no financial disclosures.
On Twitter @pwendl
CHICAGO – Switching from conventional to small-volume phlebotomy tubes is an easy step toward reducing iatrogenic blood loss in critically ill adults, a new study suggests.
“We were looking at the amount of blood we were drawing off these patients and when we asked the nurses, the numbers were crazy. It could be as high as 20 mL per time that they drew off the patient and we felt we had to do better. The common sense dictum is the more blood you draw off, the more harm you are causing the patient,” principal investigator Dr. Heather Dolman from Detroit Receiving Hospital, Wayne State University, said in an interview.
For patients staying only a day or 2 at the hospital, the type of blood tube used may not make a difference. But for the critically ill, who studies suggest can have an average of 5 to more than 24 samples drawn a day, the cumulative blood loss over an extended stay can be sizable.
Clinicians are also inclined to order more diagnostic tests as the severity of illness increases, thus putting their sickest patients at the greatest risk of iatrogenic anemia and transfusion. Anemia secondary to phlebotomy accounts for up to 40% of packed red blood cells transfused, Dr. Dolman noted at the annual meeting of the Central Surgical Association.
The process of blood sampling itself also involves a fair amount of waste. Conventional arterial line systems require that an initial blood sample be removed to “clear the line.” This typically results in 2-10 mL of blood being discarded before a second sample of undiluted blood can be obtained.
Some hospitals have turned to closed blood sampling devices that avoid the need for a second sample. The impact of blood-conserving devices on transfusion rates has been underwhelming, with only one study showing a positive impact leading to reduced blood product use.
As part of their blood-conserving strategy, Dr. Dolman and her colleagues asked the hospital to invest in small-volume phlebotomy tubes (SVTs), which are sized somewhere between a conventional-volume tube (CVT) and a pediatric blood tube.
SVTs reduce the amount of blood needed from 8.5 mL with a conventional tube to 5.0 mL for a basic metabolic panel, from 6.0 mL to 2.0 mL for a complete blood count (CBC) or cross-matching, and from 2.7 mL to 1.8 mL for a prothrombin time /internationalized ratio/partial thromboplastin time, Dr. Dolman said. The cost of an SVT is the same as a CVT, as is the cost of the machinery needed to analyze the samples.
“Everyone is worried about missing out on data, but if you look at the research on the amount of blood the machine really needs, it is only 0.1 mL, that’s less than a cc,” she said. “The technology has been there for a while, I just think the common sense aspect of all this, no one has ever thought of.”
The investigators then retrospectively compared 248 critically ill patients in the ICU, of whom 116 had blood drawn with an SVT and 132 with a CVT. The two groups were well matched with respect to age (55 years vs. 57 years), admission to the emergency surgery/trauma service (63% vs. 64%), and mean APACHE II scores (14.1 vs. 12.7).
Transfusion was at the discretion of the primary team using a restrictive hemoglobin threshold of < 7.0 gm/dL, unless hemodynamic instability or active bleeding were present.
Utilizing an SVT significantly reduced daily blood loss from phlebotomy from 31.7 mL with a CVT to 22.5 mL (P < .0001) and overall phlebotomy blood loss from 299 mL to 174 mL (P < .001), Dr. Dolman reported.
This translated into a nonsignificant trend for fewer units of packed red blood cells transfused in the SVT group (mean 4.4 vs. 6.0; P = .16).
The same pattern was observed in the 158 patients admitted to the emergency surgery/trauma service, with SVT also leading to significantly fewer episodes of severe anemia (6 vs. 20; P = .01) and a trend toward shorter ICU stays (9.2 days vs. 10.6 days; P = .46), she said.
Patients with an APACHE score of at least 20, a group one would anticipate to derive greater advantage from a blood-conserving strategy, did not benefit from use of an SVT vs. a CVT, but the number of patients was very low at just 27 and 19, respectively, Dr. Dolman noted.
Anemia, however, had a profound impact on the critically ill cohort. Patients with severe anemia were significantly more likely than those with a hemoglobin level of at least 7 gm/dL to have longer ICU stays (16 days vs. 7.7 days; P < .001), longer hospital stays (23.3 days vs. 13.6 days; P < .001), and to die in the hospital (29% vs. 13%; P = .01).
Using a small-volume tube cut the number of patients with more than one episode of severe anemia from 22 to 11 (P = .01) and those with more than two episodes from 6 to 4 (P = .53).
“Anemia in the critically ill is a significant problem,” Dr. Dolman said. “Phlebotomy waste contributes to anemia and should be recorded to decrease this hidden loss.”
The impact of transfusion vs. no transfusion was less pronounced with respect to ICU stay (12 days vs. 6 days; P < .001), hospital stay (19 days vs. 11 days; P = .44), and in-hospital mortality (17% vs. 15%; P = .60), but can lead to other negative sequelae such as increased risk of infection, circulatory overload transfusion reactions, and immune modulation, she added.
Detroit Receiving Hospital continues to use conventional tubes in its ICU and other units, although a switch to small-volume tubes is expected to be considered following peer review of the full results, Dr. Dolman said.
Dr. Dolman reported having no financial disclosures.
On Twitter @pwendl
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Utilizing small-volume phlebotomy tubes minimizes diagnostic blood loss in the critically ill.
Major finding: Small tubes vs. conventional tubes reduced overall phlebotomy blood loss (174 mL vs. 299 mL; P < .001) and transfused packed RBCs (mean 4.4 units vs. 6.0 units; P = .16).
Data source: Retrospective case cohort in 248 critically ill patients.
Disclosures: Dr. Dolman reported having no financial disclosures.
Lateral neck dissection morbidity high, but transient
CHICAGO – Lateral neck dissection for thyroid cancer is associated with significant early postoperative morbidity of 20%, even in the hands of experienced endocrine surgeons at a high-volume medical center.
Among 99 procedures, 20 patients had 26 complications, including surgical site infection in 10, chyle leak in 7, spinal accessory nerve dysfunction in 7, and seroma in 2.
Long-term complications were rare, however, occurring in just one patient with a spinal accessory nerve injury, Dr. Jason A. Glenn said at the annual meeting of the Central Surgical Association.
Using a prospectively collected thyroid database, the investigators reviewed 96 patients who underwent lateral neck dissection (LND) for suspicion of initial or recurrent lateral neck metastases by one of four experienced endocrine surgeons at the Medical College of Wisconsin in Milwaukee.
Three patients had reoperations during the study period of February 2009 and June 2014, resulting in 99 procedures and 198 lateral necks evaluated preoperatively. Most patients were women (73%) and their median age was 45 years.
LND was performed on 127 necks and metastatic disease was confirmed in 111 (87%). This included all 82 patients who had positive preoperative fine needle aspiration (FNA), 25 of 37 patients operated on without FNA, and 4 of 8 patients with a negative or nondiagnostic FNA, Dr. Glenn said.
The median number of lymph nodes excised was 22 (range 1-122), with a median of 3 (range 0-39) malignant nodes per lateral neck.
“FNA is an important adjunct in the preoperative evaluation, especially when it returns a positive result,” he said. “However, when FNA is negative, not available, or not performed, you really must consider the entire clinical picture, as 64% of these patients were found to have lymph node metastases in our study.”
Surgical drains were placed in 94% of the 127 lateral neck dissections and remained in place for a median of 6 days. The median length of stay was 1 day.
There was no association between drain duration and surgical site infection, although chyle leak was associated with a significantly longer median drain duration (12 days vs. 6 days; P value < .01), Dr. Glenn said.
Two of the seven patients with chyle leak, defined by drain output that was milky white and/or exceeded 1,000 cc in 24 hours, underwent reoperation with ligation of the cervical thoracic duct and fibrin sealant application. Both leaks resolved and patients were discharge on postoperative day 2.
“Surgical drains allow for early leak recognition and monitoring of leak resolution,” he said. “Most of these complications were diagnosed and managed on an outpatient basis, highlighting the importance of continuity of care between the inpatient and outpatient setting for the treatment of thyroid cancer.”
Discussant Janice L. Pasieka, head of general surgery and a clinical professor of surgery and oncology at the University of Calgary (Alberta), said the retrospective review is a very valuable contribution to the literature because of its comprehensive follow-up.
“Today, most patients with this type of procedure are discharged within the 23 hours, and as such, complications such as nerve palsies, chyle leaks, and surgical site infections are not apparent for the majority of patients during their hospital stay,” Dr. Pasieka said. “Many times, the true incidences are lost unless the patient re-presents to the health care system, thus introducing your bias of only those significant enough to require intervention.”
Dr. Glenn and his coauthors reported no financial disclosures.
CHICAGO – Lateral neck dissection for thyroid cancer is associated with significant early postoperative morbidity of 20%, even in the hands of experienced endocrine surgeons at a high-volume medical center.
Among 99 procedures, 20 patients had 26 complications, including surgical site infection in 10, chyle leak in 7, spinal accessory nerve dysfunction in 7, and seroma in 2.
Long-term complications were rare, however, occurring in just one patient with a spinal accessory nerve injury, Dr. Jason A. Glenn said at the annual meeting of the Central Surgical Association.
Using a prospectively collected thyroid database, the investigators reviewed 96 patients who underwent lateral neck dissection (LND) for suspicion of initial or recurrent lateral neck metastases by one of four experienced endocrine surgeons at the Medical College of Wisconsin in Milwaukee.
Three patients had reoperations during the study period of February 2009 and June 2014, resulting in 99 procedures and 198 lateral necks evaluated preoperatively. Most patients were women (73%) and their median age was 45 years.
LND was performed on 127 necks and metastatic disease was confirmed in 111 (87%). This included all 82 patients who had positive preoperative fine needle aspiration (FNA), 25 of 37 patients operated on without FNA, and 4 of 8 patients with a negative or nondiagnostic FNA, Dr. Glenn said.
The median number of lymph nodes excised was 22 (range 1-122), with a median of 3 (range 0-39) malignant nodes per lateral neck.
“FNA is an important adjunct in the preoperative evaluation, especially when it returns a positive result,” he said. “However, when FNA is negative, not available, or not performed, you really must consider the entire clinical picture, as 64% of these patients were found to have lymph node metastases in our study.”
Surgical drains were placed in 94% of the 127 lateral neck dissections and remained in place for a median of 6 days. The median length of stay was 1 day.
There was no association between drain duration and surgical site infection, although chyle leak was associated with a significantly longer median drain duration (12 days vs. 6 days; P value < .01), Dr. Glenn said.
Two of the seven patients with chyle leak, defined by drain output that was milky white and/or exceeded 1,000 cc in 24 hours, underwent reoperation with ligation of the cervical thoracic duct and fibrin sealant application. Both leaks resolved and patients were discharge on postoperative day 2.
“Surgical drains allow for early leak recognition and monitoring of leak resolution,” he said. “Most of these complications were diagnosed and managed on an outpatient basis, highlighting the importance of continuity of care between the inpatient and outpatient setting for the treatment of thyroid cancer.”
Discussant Janice L. Pasieka, head of general surgery and a clinical professor of surgery and oncology at the University of Calgary (Alberta), said the retrospective review is a very valuable contribution to the literature because of its comprehensive follow-up.
“Today, most patients with this type of procedure are discharged within the 23 hours, and as such, complications such as nerve palsies, chyle leaks, and surgical site infections are not apparent for the majority of patients during their hospital stay,” Dr. Pasieka said. “Many times, the true incidences are lost unless the patient re-presents to the health care system, thus introducing your bias of only those significant enough to require intervention.”
Dr. Glenn and his coauthors reported no financial disclosures.
CHICAGO – Lateral neck dissection for thyroid cancer is associated with significant early postoperative morbidity of 20%, even in the hands of experienced endocrine surgeons at a high-volume medical center.
Among 99 procedures, 20 patients had 26 complications, including surgical site infection in 10, chyle leak in 7, spinal accessory nerve dysfunction in 7, and seroma in 2.
Long-term complications were rare, however, occurring in just one patient with a spinal accessory nerve injury, Dr. Jason A. Glenn said at the annual meeting of the Central Surgical Association.
Using a prospectively collected thyroid database, the investigators reviewed 96 patients who underwent lateral neck dissection (LND) for suspicion of initial or recurrent lateral neck metastases by one of four experienced endocrine surgeons at the Medical College of Wisconsin in Milwaukee.
Three patients had reoperations during the study period of February 2009 and June 2014, resulting in 99 procedures and 198 lateral necks evaluated preoperatively. Most patients were women (73%) and their median age was 45 years.
LND was performed on 127 necks and metastatic disease was confirmed in 111 (87%). This included all 82 patients who had positive preoperative fine needle aspiration (FNA), 25 of 37 patients operated on without FNA, and 4 of 8 patients with a negative or nondiagnostic FNA, Dr. Glenn said.
The median number of lymph nodes excised was 22 (range 1-122), with a median of 3 (range 0-39) malignant nodes per lateral neck.
“FNA is an important adjunct in the preoperative evaluation, especially when it returns a positive result,” he said. “However, when FNA is negative, not available, or not performed, you really must consider the entire clinical picture, as 64% of these patients were found to have lymph node metastases in our study.”
Surgical drains were placed in 94% of the 127 lateral neck dissections and remained in place for a median of 6 days. The median length of stay was 1 day.
There was no association between drain duration and surgical site infection, although chyle leak was associated with a significantly longer median drain duration (12 days vs. 6 days; P value < .01), Dr. Glenn said.
Two of the seven patients with chyle leak, defined by drain output that was milky white and/or exceeded 1,000 cc in 24 hours, underwent reoperation with ligation of the cervical thoracic duct and fibrin sealant application. Both leaks resolved and patients were discharge on postoperative day 2.
“Surgical drains allow for early leak recognition and monitoring of leak resolution,” he said. “Most of these complications were diagnosed and managed on an outpatient basis, highlighting the importance of continuity of care between the inpatient and outpatient setting for the treatment of thyroid cancer.”
Discussant Janice L. Pasieka, head of general surgery and a clinical professor of surgery and oncology at the University of Calgary (Alberta), said the retrospective review is a very valuable contribution to the literature because of its comprehensive follow-up.
“Today, most patients with this type of procedure are discharged within the 23 hours, and as such, complications such as nerve palsies, chyle leaks, and surgical site infections are not apparent for the majority of patients during their hospital stay,” Dr. Pasieka said. “Many times, the true incidences are lost unless the patient re-presents to the health care system, thus introducing your bias of only those significant enough to require intervention.”
Dr. Glenn and his coauthors reported no financial disclosures.
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Lateral neck dissections for thyroid cancer are associated with high early morbidity but few long-term complications.
Major finding: The overall complication rate was 20%, however, most were transient.
Data source: Retrospective observational series of 96 patients undergoing lateral neck dissection.
Disclosures: Dr. Glenn and his coauthors reported no financial disclosures.
Risk scale predicts mortality after gastric cancer surgery
HOUSTON – A simple preoperative scale can accurately predict a patient’s risk for near-term death following surgery for gastric cancer, investigators say.
The scale accounts for both patient and hospital factors, and is useful as a clinical tool for preoperative counseling of patients, reported Dr. Cristina Harnsberger of the University of California San Diego.
“Male gender, increasing age, and comorbid disease increase risk of in-hospital mortality for patients who undergo gastric resection for malignancy. Additionally, low hospital volume was an independent risk factor,” she said at the annual Society of Surgical Oncology Cancer Symposium.
The scale was able to accurately classify patients as being at low or high risk, and the observed and expected mortality rates for each risk score were well correlated, she said.
Weighing risks
Perioperative mortality rates following resection for gastric malignancies range from 0.6% to 15%. Risk scales and nomograms are intended to help clinicians predict risks for individual patients, but most incorporate postoperative data rather than preoperative or hospital data, Dr. Harnsberger said.
She and her colleagues conducted a study to determine whether a simple preoperative scale based on patient and hospital factors could accurately predict risk for death following gastric resection for malignancy.
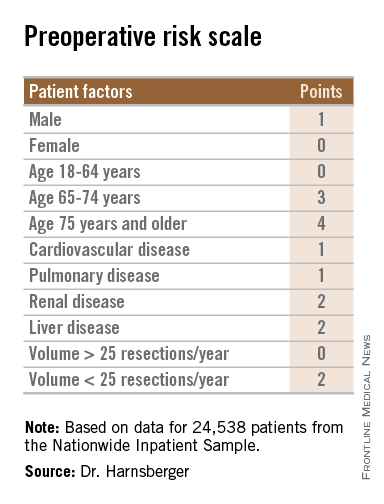
They drew on data from the Nationwide Inpatient Sample database to identify adult patients with a diagnosis of gastric cancer who underwent potentially curative partial or total gastrectomy from 1998 through 2011.
They identified a total 24,538 patients, based on International Classification of Diseases, Revision 9 (ICD-9) diagnosis and procedure codes.
They then created multivariate logistic regression models to identify independent predictors of mortality, create a predictive model, and construct a risk scale. The models controlled for sex, age, race, comorbidities, insurance status, hospital volume (less than 25 vs. 25 or more gastric resections for malignancy per year), laparoscopic vs. open approach, poverty level, alcohol abuse, tobacco use, diabetes mellitus, and year of procedure.
The mean length of stay for the patient sample was 11 days. The overall in-hospital mortality was 5.5%.
The models identified three patient-related factors and one hospital-related factor that were predictive of mortality and when combined in a risk scale proved to be accurate.
The patient factors were male sex, age 65 and older, and comorbid disease, specifically cardiovascular, pulmonary, renal, and/or hepatic.
The hospital factor, expressed as protective, was 25 or more gastric resections for cancer per year.
The maximum possible score is 13. Patients with scores lower than 6 are at low risk for perioperative mortality, while those with scores 6 and higher are at high risk. Among patients with a score of 0-5, the perioperative death rate ranged from 1.3% to 4.5%. In contrast, patients with higher scores had death rates ranging from 6.0% to 23.1%.
Clinical applications for the bedside risk scale include perioperative patient counseling, aiding in informed consent discussions, and as an adjunct to postoperative risk calculators, Dr. Harnsberger said.The study funding source was not disclosed. Dr. Harnsberger reported having no disclosures.
HOUSTON – A simple preoperative scale can accurately predict a patient’s risk for near-term death following surgery for gastric cancer, investigators say.
The scale accounts for both patient and hospital factors, and is useful as a clinical tool for preoperative counseling of patients, reported Dr. Cristina Harnsberger of the University of California San Diego.
“Male gender, increasing age, and comorbid disease increase risk of in-hospital mortality for patients who undergo gastric resection for malignancy. Additionally, low hospital volume was an independent risk factor,” she said at the annual Society of Surgical Oncology Cancer Symposium.
The scale was able to accurately classify patients as being at low or high risk, and the observed and expected mortality rates for each risk score were well correlated, she said.
Weighing risks
Perioperative mortality rates following resection for gastric malignancies range from 0.6% to 15%. Risk scales and nomograms are intended to help clinicians predict risks for individual patients, but most incorporate postoperative data rather than preoperative or hospital data, Dr. Harnsberger said.
She and her colleagues conducted a study to determine whether a simple preoperative scale based on patient and hospital factors could accurately predict risk for death following gastric resection for malignancy.

They drew on data from the Nationwide Inpatient Sample database to identify adult patients with a diagnosis of gastric cancer who underwent potentially curative partial or total gastrectomy from 1998 through 2011.
They identified a total 24,538 patients, based on International Classification of Diseases, Revision 9 (ICD-9) diagnosis and procedure codes.
They then created multivariate logistic regression models to identify independent predictors of mortality, create a predictive model, and construct a risk scale. The models controlled for sex, age, race, comorbidities, insurance status, hospital volume (less than 25 vs. 25 or more gastric resections for malignancy per year), laparoscopic vs. open approach, poverty level, alcohol abuse, tobacco use, diabetes mellitus, and year of procedure.
The mean length of stay for the patient sample was 11 days. The overall in-hospital mortality was 5.5%.
The models identified three patient-related factors and one hospital-related factor that were predictive of mortality and when combined in a risk scale proved to be accurate.
The patient factors were male sex, age 65 and older, and comorbid disease, specifically cardiovascular, pulmonary, renal, and/or hepatic.
The hospital factor, expressed as protective, was 25 or more gastric resections for cancer per year.
The maximum possible score is 13. Patients with scores lower than 6 are at low risk for perioperative mortality, while those with scores 6 and higher are at high risk. Among patients with a score of 0-5, the perioperative death rate ranged from 1.3% to 4.5%. In contrast, patients with higher scores had death rates ranging from 6.0% to 23.1%.
Clinical applications for the bedside risk scale include perioperative patient counseling, aiding in informed consent discussions, and as an adjunct to postoperative risk calculators, Dr. Harnsberger said.The study funding source was not disclosed. Dr. Harnsberger reported having no disclosures.
HOUSTON – A simple preoperative scale can accurately predict a patient’s risk for near-term death following surgery for gastric cancer, investigators say.
The scale accounts for both patient and hospital factors, and is useful as a clinical tool for preoperative counseling of patients, reported Dr. Cristina Harnsberger of the University of California San Diego.
“Male gender, increasing age, and comorbid disease increase risk of in-hospital mortality for patients who undergo gastric resection for malignancy. Additionally, low hospital volume was an independent risk factor,” she said at the annual Society of Surgical Oncology Cancer Symposium.
The scale was able to accurately classify patients as being at low or high risk, and the observed and expected mortality rates for each risk score were well correlated, she said.
Weighing risks
Perioperative mortality rates following resection for gastric malignancies range from 0.6% to 15%. Risk scales and nomograms are intended to help clinicians predict risks for individual patients, but most incorporate postoperative data rather than preoperative or hospital data, Dr. Harnsberger said.
She and her colleagues conducted a study to determine whether a simple preoperative scale based on patient and hospital factors could accurately predict risk for death following gastric resection for malignancy.

They drew on data from the Nationwide Inpatient Sample database to identify adult patients with a diagnosis of gastric cancer who underwent potentially curative partial or total gastrectomy from 1998 through 2011.
They identified a total 24,538 patients, based on International Classification of Diseases, Revision 9 (ICD-9) diagnosis and procedure codes.
They then created multivariate logistic regression models to identify independent predictors of mortality, create a predictive model, and construct a risk scale. The models controlled for sex, age, race, comorbidities, insurance status, hospital volume (less than 25 vs. 25 or more gastric resections for malignancy per year), laparoscopic vs. open approach, poverty level, alcohol abuse, tobacco use, diabetes mellitus, and year of procedure.
The mean length of stay for the patient sample was 11 days. The overall in-hospital mortality was 5.5%.
The models identified three patient-related factors and one hospital-related factor that were predictive of mortality and when combined in a risk scale proved to be accurate.
The patient factors were male sex, age 65 and older, and comorbid disease, specifically cardiovascular, pulmonary, renal, and/or hepatic.
The hospital factor, expressed as protective, was 25 or more gastric resections for cancer per year.
The maximum possible score is 13. Patients with scores lower than 6 are at low risk for perioperative mortality, while those with scores 6 and higher are at high risk. Among patients with a score of 0-5, the perioperative death rate ranged from 1.3% to 4.5%. In contrast, patients with higher scores had death rates ranging from 6.0% to 23.1%.
Clinical applications for the bedside risk scale include perioperative patient counseling, aiding in informed consent discussions, and as an adjunct to postoperative risk calculators, Dr. Harnsberger said.The study funding source was not disclosed. Dr. Harnsberger reported having no disclosures.
AT SSO 2015
Key clinical point: The bedside risk scale can be used in patient counseling prior to surgery for gastric malignancies.
Major finding: Patients with scores of 0-5 had perioperative death rates of 1.3%-4.5%. Patients with higher scores had death rates ranging from 6.0% to 23.1%.
Data source: Review of retrospective data on 24,538 adults who underwent partial or total gastric resection for malignancies.
Disclosures: The study funding source was not disclosed. Dr. Harnsberger reported having no disclosures.
Medicare at 50: High-price therapeutics put Medicare at a crossroad
High-cost advances in diagnosis and treatment are entering medicine each and every month. As Medicare turns 50 this year, how can the program – with its growing number of beneficiaries and their advancing age – cope with the onslaught?
“When we look at the price of something in health care, very often we just look at one bucket,” Amy Miller, Ph.D., executive vice president at the Personalized Medicine Coalition, said in an interview. “We look at the drug bucket or we look at the diagnostic bucket or we look at the hospital bucket or the doctor visit bucket, but when we are talking about the targeted therapy that is more or less known to work for a particular patient with a particular condition, we need to think about the price more broadly. We need to think about the systemic cost savings of getting that drug to the right patient the first time, without failing first on other drugs.”
For some high-priced drugs, that appears to be the case. Take for example the direct-acting antiviral agents (DAA) recently approved to treat hepatitis C (HCV) infection.
“If you have a drug like [sofosbuvir], which is extremely expensive at face value, it may not have such a big long-term effect because [sofosbuvir] is a cure,” said Dr. Soeren Mattke, senior scientist in RAND Corp. in Boston. “So if you take patients with hepatitis C that have a certain trajectory of spending over the next decade or so – treatment, liver transplantation, and the like – it basically wipes out the infection. In the long run, even though the drug is very, very expensive, it may not be such a bad deal for Medicare.”
And while some physicians would like to see these new DAAs prescribed to all appropriate patients with HCV, even they acknowledge that the high price tag can be fiscally constraining in the long run and can handle some restrictions to ease the financial burden.
“If you have a 70-year-old patient who has no evidence of any liver fibrosis and they have lived with hepatitis C for 30 or 40 years, I don’t think it’s unreasonable to suggest that maybe they wait for their therapy until an even less expensive option comes along,” said Dr. Sean Koppe, director of hepatology at University of Illinois at Chicago. “I think if the payer is going to be a little bit restrictive but still allow us to treat the majority of our patients who are showing some signs of fibrosis, I wouldn’t be too bothered by that approach.”
Oncology, another area where high-priced treatments are prevalent, is not as cut and dried in terms of medical outcomes as HCV.
“Some of the oncology drugs [have a cost of] $50,000-$60,000 per treatment course, but you extend life expectancy of a terminal cancer patient by weeks,” Dr. Mattke said. “So while you are looking at this drug, and they cost practically the same [as some DAAs], the impact is quite different.”
But one bright spot that can potentially help alleviate pricing pressures is the growing emphasis on personalized or precision medicine.
“When we talk about a high-priced therapeutic, we have to remember that not too long ago, when a drug came to market, it was marketed to everyone with a particular condition,” Dr. Miller said. “But when [crizotinib] hit the market, it only treated 4% of those with non–small-cell lung cancer initially based on its approved label.”
Identifying the right users will be key to moderating the impact of high-priced therapeutics.
“But if diagnostics aren’t adequately covered or reimbursed or if a particular therapy is on a higher tier or there’s more risk for the physician, giving that drug to a patient, even if diagnostics indicate it’s the right one, then the models might not work,” Dr. Miller said, adding that she is “encouraged” that the federal government is talking about more active use of precision medicine.
But, according to Dr. Mattke, there are pitfalls to precision medicine, too.
“If personalized medicine means that you are able to design a drug that targets the very specific molecular [structure] of a particular cancer and reverses it, this could be a very, very expensive drug, but it could be totally worth it,” he said. “If personalized medicine means there’s a highly differentiated range of drugs out there that all are so-so effective, you may end up with some marginally valuable drugs at extremely high prices, and yet force Medicare to pay for it because they cannot take cost into consideration.”
When it comes to costly medications and treatments, Medicare’s fee-for-service design isn’t helping either.
“We have a payment system that works through a third party payer, so the person who needs the care is not usually the person who is paying for the care,” said Dr. Jeffery Ward, an oncologist who serves on the clinical practice committee of the American Society of Clinical Oncology. “Prices and the fees are set based on what you do. I don’t get paid better for doing a good job than I would get for doing a cruddy job. [Once,] that served medicine and Medicare well, but now we have a health care crisis.”
Dr. Ward added that incentives are misaligned in a manner that rewards doctors for choosing more expensive drugs and procedures for their patients.
As oncologists, “we are going to have get over our addiction to [being compensated on the] margin on drugs,” Dr. Ward said. “We’re going to have to be able to develop a system and have faith in a system that will pay us fairly for what we do instead of paying us based on what drugs we choose.”
However, Dr. Steven Allen, who chairs the American Society of Hematology committee on practice, said that he doesn’t believe that is a key issue.
“I think you are really only referring to a very small percentage of physicians,” Dr. Allen said. “I think the vast majority of physicians do what’s right for their patients. ... They will choose the best drug for their patients regardless of the reimbursement the physician may receive given that drug.”
Dr. Ward said that to address the need to cover these potent, high-cost treatments, “I think what Medicare is going to have to do at its 50th birthday is figure out how to begin to reward physicians for doing the right thing and for providing quality care instead of simply paying for quantity.”
The federal government is moving in that direction. In January, Health and Human Services Secretary Sylvia Burwell announced a new goal for Medicare: Fifty percent of all payments should be value based by 2018 (N. Engl. J. Med. 2015;372:897-9 [doi 10.1056.NEJMp1500445]). But what exactly does value mean? While that point is debated on a broad scale, one thing that is obvious is that it will require a culture shift on a many levels.
High-cost advances in diagnosis and treatment are entering medicine each and every month. As Medicare turns 50 this year, how can the program – with its growing number of beneficiaries and their advancing age – cope with the onslaught?
“When we look at the price of something in health care, very often we just look at one bucket,” Amy Miller, Ph.D., executive vice president at the Personalized Medicine Coalition, said in an interview. “We look at the drug bucket or we look at the diagnostic bucket or we look at the hospital bucket or the doctor visit bucket, but when we are talking about the targeted therapy that is more or less known to work for a particular patient with a particular condition, we need to think about the price more broadly. We need to think about the systemic cost savings of getting that drug to the right patient the first time, without failing first on other drugs.”
For some high-priced drugs, that appears to be the case. Take for example the direct-acting antiviral agents (DAA) recently approved to treat hepatitis C (HCV) infection.
“If you have a drug like [sofosbuvir], which is extremely expensive at face value, it may not have such a big long-term effect because [sofosbuvir] is a cure,” said Dr. Soeren Mattke, senior scientist in RAND Corp. in Boston. “So if you take patients with hepatitis C that have a certain trajectory of spending over the next decade or so – treatment, liver transplantation, and the like – it basically wipes out the infection. In the long run, even though the drug is very, very expensive, it may not be such a bad deal for Medicare.”
And while some physicians would like to see these new DAAs prescribed to all appropriate patients with HCV, even they acknowledge that the high price tag can be fiscally constraining in the long run and can handle some restrictions to ease the financial burden.
“If you have a 70-year-old patient who has no evidence of any liver fibrosis and they have lived with hepatitis C for 30 or 40 years, I don’t think it’s unreasonable to suggest that maybe they wait for their therapy until an even less expensive option comes along,” said Dr. Sean Koppe, director of hepatology at University of Illinois at Chicago. “I think if the payer is going to be a little bit restrictive but still allow us to treat the majority of our patients who are showing some signs of fibrosis, I wouldn’t be too bothered by that approach.”
Oncology, another area where high-priced treatments are prevalent, is not as cut and dried in terms of medical outcomes as HCV.
“Some of the oncology drugs [have a cost of] $50,000-$60,000 per treatment course, but you extend life expectancy of a terminal cancer patient by weeks,” Dr. Mattke said. “So while you are looking at this drug, and they cost practically the same [as some DAAs], the impact is quite different.”
But one bright spot that can potentially help alleviate pricing pressures is the growing emphasis on personalized or precision medicine.
“When we talk about a high-priced therapeutic, we have to remember that not too long ago, when a drug came to market, it was marketed to everyone with a particular condition,” Dr. Miller said. “But when [crizotinib] hit the market, it only treated 4% of those with non–small-cell lung cancer initially based on its approved label.”
Identifying the right users will be key to moderating the impact of high-priced therapeutics.
“But if diagnostics aren’t adequately covered or reimbursed or if a particular therapy is on a higher tier or there’s more risk for the physician, giving that drug to a patient, even if diagnostics indicate it’s the right one, then the models might not work,” Dr. Miller said, adding that she is “encouraged” that the federal government is talking about more active use of precision medicine.
But, according to Dr. Mattke, there are pitfalls to precision medicine, too.
“If personalized medicine means that you are able to design a drug that targets the very specific molecular [structure] of a particular cancer and reverses it, this could be a very, very expensive drug, but it could be totally worth it,” he said. “If personalized medicine means there’s a highly differentiated range of drugs out there that all are so-so effective, you may end up with some marginally valuable drugs at extremely high prices, and yet force Medicare to pay for it because they cannot take cost into consideration.”
When it comes to costly medications and treatments, Medicare’s fee-for-service design isn’t helping either.
“We have a payment system that works through a third party payer, so the person who needs the care is not usually the person who is paying for the care,” said Dr. Jeffery Ward, an oncologist who serves on the clinical practice committee of the American Society of Clinical Oncology. “Prices and the fees are set based on what you do. I don’t get paid better for doing a good job than I would get for doing a cruddy job. [Once,] that served medicine and Medicare well, but now we have a health care crisis.”
Dr. Ward added that incentives are misaligned in a manner that rewards doctors for choosing more expensive drugs and procedures for their patients.
As oncologists, “we are going to have get over our addiction to [being compensated on the] margin on drugs,” Dr. Ward said. “We’re going to have to be able to develop a system and have faith in a system that will pay us fairly for what we do instead of paying us based on what drugs we choose.”
However, Dr. Steven Allen, who chairs the American Society of Hematology committee on practice, said that he doesn’t believe that is a key issue.
“I think you are really only referring to a very small percentage of physicians,” Dr. Allen said. “I think the vast majority of physicians do what’s right for their patients. ... They will choose the best drug for their patients regardless of the reimbursement the physician may receive given that drug.”
Dr. Ward said that to address the need to cover these potent, high-cost treatments, “I think what Medicare is going to have to do at its 50th birthday is figure out how to begin to reward physicians for doing the right thing and for providing quality care instead of simply paying for quantity.”
The federal government is moving in that direction. In January, Health and Human Services Secretary Sylvia Burwell announced a new goal for Medicare: Fifty percent of all payments should be value based by 2018 (N. Engl. J. Med. 2015;372:897-9 [doi 10.1056.NEJMp1500445]). But what exactly does value mean? While that point is debated on a broad scale, one thing that is obvious is that it will require a culture shift on a many levels.
High-cost advances in diagnosis and treatment are entering medicine each and every month. As Medicare turns 50 this year, how can the program – with its growing number of beneficiaries and their advancing age – cope with the onslaught?
“When we look at the price of something in health care, very often we just look at one bucket,” Amy Miller, Ph.D., executive vice president at the Personalized Medicine Coalition, said in an interview. “We look at the drug bucket or we look at the diagnostic bucket or we look at the hospital bucket or the doctor visit bucket, but when we are talking about the targeted therapy that is more or less known to work for a particular patient with a particular condition, we need to think about the price more broadly. We need to think about the systemic cost savings of getting that drug to the right patient the first time, without failing first on other drugs.”
For some high-priced drugs, that appears to be the case. Take for example the direct-acting antiviral agents (DAA) recently approved to treat hepatitis C (HCV) infection.
“If you have a drug like [sofosbuvir], which is extremely expensive at face value, it may not have such a big long-term effect because [sofosbuvir] is a cure,” said Dr. Soeren Mattke, senior scientist in RAND Corp. in Boston. “So if you take patients with hepatitis C that have a certain trajectory of spending over the next decade or so – treatment, liver transplantation, and the like – it basically wipes out the infection. In the long run, even though the drug is very, very expensive, it may not be such a bad deal for Medicare.”
And while some physicians would like to see these new DAAs prescribed to all appropriate patients with HCV, even they acknowledge that the high price tag can be fiscally constraining in the long run and can handle some restrictions to ease the financial burden.
“If you have a 70-year-old patient who has no evidence of any liver fibrosis and they have lived with hepatitis C for 30 or 40 years, I don’t think it’s unreasonable to suggest that maybe they wait for their therapy until an even less expensive option comes along,” said Dr. Sean Koppe, director of hepatology at University of Illinois at Chicago. “I think if the payer is going to be a little bit restrictive but still allow us to treat the majority of our patients who are showing some signs of fibrosis, I wouldn’t be too bothered by that approach.”
Oncology, another area where high-priced treatments are prevalent, is not as cut and dried in terms of medical outcomes as HCV.
“Some of the oncology drugs [have a cost of] $50,000-$60,000 per treatment course, but you extend life expectancy of a terminal cancer patient by weeks,” Dr. Mattke said. “So while you are looking at this drug, and they cost practically the same [as some DAAs], the impact is quite different.”
But one bright spot that can potentially help alleviate pricing pressures is the growing emphasis on personalized or precision medicine.
“When we talk about a high-priced therapeutic, we have to remember that not too long ago, when a drug came to market, it was marketed to everyone with a particular condition,” Dr. Miller said. “But when [crizotinib] hit the market, it only treated 4% of those with non–small-cell lung cancer initially based on its approved label.”
Identifying the right users will be key to moderating the impact of high-priced therapeutics.
“But if diagnostics aren’t adequately covered or reimbursed or if a particular therapy is on a higher tier or there’s more risk for the physician, giving that drug to a patient, even if diagnostics indicate it’s the right one, then the models might not work,” Dr. Miller said, adding that she is “encouraged” that the federal government is talking about more active use of precision medicine.
But, according to Dr. Mattke, there are pitfalls to precision medicine, too.
“If personalized medicine means that you are able to design a drug that targets the very specific molecular [structure] of a particular cancer and reverses it, this could be a very, very expensive drug, but it could be totally worth it,” he said. “If personalized medicine means there’s a highly differentiated range of drugs out there that all are so-so effective, you may end up with some marginally valuable drugs at extremely high prices, and yet force Medicare to pay for it because they cannot take cost into consideration.”
When it comes to costly medications and treatments, Medicare’s fee-for-service design isn’t helping either.
“We have a payment system that works through a third party payer, so the person who needs the care is not usually the person who is paying for the care,” said Dr. Jeffery Ward, an oncologist who serves on the clinical practice committee of the American Society of Clinical Oncology. “Prices and the fees are set based on what you do. I don’t get paid better for doing a good job than I would get for doing a cruddy job. [Once,] that served medicine and Medicare well, but now we have a health care crisis.”
Dr. Ward added that incentives are misaligned in a manner that rewards doctors for choosing more expensive drugs and procedures for their patients.
As oncologists, “we are going to have get over our addiction to [being compensated on the] margin on drugs,” Dr. Ward said. “We’re going to have to be able to develop a system and have faith in a system that will pay us fairly for what we do instead of paying us based on what drugs we choose.”
However, Dr. Steven Allen, who chairs the American Society of Hematology committee on practice, said that he doesn’t believe that is a key issue.
“I think you are really only referring to a very small percentage of physicians,” Dr. Allen said. “I think the vast majority of physicians do what’s right for their patients. ... They will choose the best drug for their patients regardless of the reimbursement the physician may receive given that drug.”
Dr. Ward said that to address the need to cover these potent, high-cost treatments, “I think what Medicare is going to have to do at its 50th birthday is figure out how to begin to reward physicians for doing the right thing and for providing quality care instead of simply paying for quantity.”
The federal government is moving in that direction. In January, Health and Human Services Secretary Sylvia Burwell announced a new goal for Medicare: Fifty percent of all payments should be value based by 2018 (N. Engl. J. Med. 2015;372:897-9 [doi 10.1056.NEJMp1500445]). But what exactly does value mean? While that point is debated on a broad scale, one thing that is obvious is that it will require a culture shift on a many levels.
Vesicovaginal fistulas after hysterectomy linked to urinary tract injury
ORLANDO – Injuring the bladder during a hysterectomy is associated with a greater likelihood of developing a postoperative vesicovaginal fistula, according to a retrospective analysis presented at the annual scientific meeting of the Society of Gynecologic Surgeons.
In data captured from 641,056 hysterectomies performed in California, New York, and Florida in 2005-2010, the odds ratio of a vesicovaginal fistula was nearly 19 times higher if a urinary tract injury was sustained at the time of hysterectomy, a complication that increased in frequency during the 5-year study period, reported Dr. Rony A. Adam, professor of obstetrics and gynecology in the division of female pelvic and reconstructive surgery at Vanderbilt University Medical Center, Nashville, Tenn.
“Although we do not know all the factors that impact formation of vesicovaginal fistula post hysterectomy, it is clear that bladder injury at the time of hysterectomy even when identified and repaired is significantly associated [with this complication],” Dr. Adam reported at the meeting, jointly sponsored by the American College of Surgeons.
The statistical analyses were conducted with the inpatient and ambulatory surgery databases from the Healthcare Cost and Utilization Project (HCUP) for the three states. The large geographically diverse populations were considered by the authors to be nationally representative.
For this analysis, vesicovaginal fistulas and urinary tract injuries were tracked for total abdominal hysterectomy, subtotal abdominal hysterectomy, and total vaginal hysterectomy with or without laparoscopic assistance. Over the 5-year study period, urinary tract injuries climbed steadily in all three groups. When the last year of analysis was compared with the first, a greater increase in odds ratio was observed in the total abdominal group (1.88) than in the subtotal (1.27) or the vaginal (1.26) groups, but each increase was significant.
“The uniformly increasing bladder injury rate may be explained by the increasing cesarean section rates,” according to Dr. Adam, who cited evidence suggesting that cesarean section increases risk of urinary tract injuries in subsequent hysterectomy.
The rate of vesicovaginal fistulas was 21.07 per 1,000 women when a urinary tract injury was incurred during hysterectomy versus 0.95 per 1,000 women when it was not (odds ratio, 18.91). The overall rate of vesicovaginal injury increased in the last year of the study relative to the first (OR, 1.28), although this increase fell just short of statistical significant (P = .059).
“It is possible that surgeons have gotten better at detecting and repairing urinary tract injury, which could explain why the vesicovaginal fistula rate has remained stable in the face of an increasing rate of urinary tract injuries,” Dr. Adam reported.
Not only did the rate of urinary tract injuries climb faster over the study period in those undergoing total abdominal hysterectomy, but also there was a stronger association in patients undergoing this form of hysterectomy between urinary tract injury and vesicovaginal fistula formation, said Dr. Adam. Overall, the OR for vesicovaginal fistula after urinary tract injury was about half as great when either subtotal or vaginal hysterectomy was compared to total abdominal hysterectomy
Although Dr. Adam emphasized that a retrospective study of this type can only establish an association and cannot confirm causation, he said that this study suggests urinary tract injury may be a useful quality-of-care measure for performance of hysterectomy. The SGS-invited discussant Dr. Blair Washington, a urogynecologist at Virginia Mason Hospital and Medical Center, Seattle, agreed.
“Characterizing morbidity associated with hysterectomy is increasingly important as we define benchmarks for quality outcomes in the changing health care economy,” Dr. Washington said. Calling this study “outstanding,” she suggested these data are potentially helpful for counseling patients about risks of hysterectomy, and identifying and evaluating strategies that will help to improve outcomes.
Dr. Adam reported no relevant financial disclosures.
ORLANDO – Injuring the bladder during a hysterectomy is associated with a greater likelihood of developing a postoperative vesicovaginal fistula, according to a retrospective analysis presented at the annual scientific meeting of the Society of Gynecologic Surgeons.
In data captured from 641,056 hysterectomies performed in California, New York, and Florida in 2005-2010, the odds ratio of a vesicovaginal fistula was nearly 19 times higher if a urinary tract injury was sustained at the time of hysterectomy, a complication that increased in frequency during the 5-year study period, reported Dr. Rony A. Adam, professor of obstetrics and gynecology in the division of female pelvic and reconstructive surgery at Vanderbilt University Medical Center, Nashville, Tenn.
“Although we do not know all the factors that impact formation of vesicovaginal fistula post hysterectomy, it is clear that bladder injury at the time of hysterectomy even when identified and repaired is significantly associated [with this complication],” Dr. Adam reported at the meeting, jointly sponsored by the American College of Surgeons.
The statistical analyses were conducted with the inpatient and ambulatory surgery databases from the Healthcare Cost and Utilization Project (HCUP) for the three states. The large geographically diverse populations were considered by the authors to be nationally representative.
For this analysis, vesicovaginal fistulas and urinary tract injuries were tracked for total abdominal hysterectomy, subtotal abdominal hysterectomy, and total vaginal hysterectomy with or without laparoscopic assistance. Over the 5-year study period, urinary tract injuries climbed steadily in all three groups. When the last year of analysis was compared with the first, a greater increase in odds ratio was observed in the total abdominal group (1.88) than in the subtotal (1.27) or the vaginal (1.26) groups, but each increase was significant.
“The uniformly increasing bladder injury rate may be explained by the increasing cesarean section rates,” according to Dr. Adam, who cited evidence suggesting that cesarean section increases risk of urinary tract injuries in subsequent hysterectomy.
The rate of vesicovaginal fistulas was 21.07 per 1,000 women when a urinary tract injury was incurred during hysterectomy versus 0.95 per 1,000 women when it was not (odds ratio, 18.91). The overall rate of vesicovaginal injury increased in the last year of the study relative to the first (OR, 1.28), although this increase fell just short of statistical significant (P = .059).
“It is possible that surgeons have gotten better at detecting and repairing urinary tract injury, which could explain why the vesicovaginal fistula rate has remained stable in the face of an increasing rate of urinary tract injuries,” Dr. Adam reported.
Not only did the rate of urinary tract injuries climb faster over the study period in those undergoing total abdominal hysterectomy, but also there was a stronger association in patients undergoing this form of hysterectomy between urinary tract injury and vesicovaginal fistula formation, said Dr. Adam. Overall, the OR for vesicovaginal fistula after urinary tract injury was about half as great when either subtotal or vaginal hysterectomy was compared to total abdominal hysterectomy
Although Dr. Adam emphasized that a retrospective study of this type can only establish an association and cannot confirm causation, he said that this study suggests urinary tract injury may be a useful quality-of-care measure for performance of hysterectomy. The SGS-invited discussant Dr. Blair Washington, a urogynecologist at Virginia Mason Hospital and Medical Center, Seattle, agreed.
“Characterizing morbidity associated with hysterectomy is increasingly important as we define benchmarks for quality outcomes in the changing health care economy,” Dr. Washington said. Calling this study “outstanding,” she suggested these data are potentially helpful for counseling patients about risks of hysterectomy, and identifying and evaluating strategies that will help to improve outcomes.
Dr. Adam reported no relevant financial disclosures.
ORLANDO – Injuring the bladder during a hysterectomy is associated with a greater likelihood of developing a postoperative vesicovaginal fistula, according to a retrospective analysis presented at the annual scientific meeting of the Society of Gynecologic Surgeons.
In data captured from 641,056 hysterectomies performed in California, New York, and Florida in 2005-2010, the odds ratio of a vesicovaginal fistula was nearly 19 times higher if a urinary tract injury was sustained at the time of hysterectomy, a complication that increased in frequency during the 5-year study period, reported Dr. Rony A. Adam, professor of obstetrics and gynecology in the division of female pelvic and reconstructive surgery at Vanderbilt University Medical Center, Nashville, Tenn.
“Although we do not know all the factors that impact formation of vesicovaginal fistula post hysterectomy, it is clear that bladder injury at the time of hysterectomy even when identified and repaired is significantly associated [with this complication],” Dr. Adam reported at the meeting, jointly sponsored by the American College of Surgeons.
The statistical analyses were conducted with the inpatient and ambulatory surgery databases from the Healthcare Cost and Utilization Project (HCUP) for the three states. The large geographically diverse populations were considered by the authors to be nationally representative.
For this analysis, vesicovaginal fistulas and urinary tract injuries were tracked for total abdominal hysterectomy, subtotal abdominal hysterectomy, and total vaginal hysterectomy with or without laparoscopic assistance. Over the 5-year study period, urinary tract injuries climbed steadily in all three groups. When the last year of analysis was compared with the first, a greater increase in odds ratio was observed in the total abdominal group (1.88) than in the subtotal (1.27) or the vaginal (1.26) groups, but each increase was significant.
“The uniformly increasing bladder injury rate may be explained by the increasing cesarean section rates,” according to Dr. Adam, who cited evidence suggesting that cesarean section increases risk of urinary tract injuries in subsequent hysterectomy.
The rate of vesicovaginal fistulas was 21.07 per 1,000 women when a urinary tract injury was incurred during hysterectomy versus 0.95 per 1,000 women when it was not (odds ratio, 18.91). The overall rate of vesicovaginal injury increased in the last year of the study relative to the first (OR, 1.28), although this increase fell just short of statistical significant (P = .059).
“It is possible that surgeons have gotten better at detecting and repairing urinary tract injury, which could explain why the vesicovaginal fistula rate has remained stable in the face of an increasing rate of urinary tract injuries,” Dr. Adam reported.
Not only did the rate of urinary tract injuries climb faster over the study period in those undergoing total abdominal hysterectomy, but also there was a stronger association in patients undergoing this form of hysterectomy between urinary tract injury and vesicovaginal fistula formation, said Dr. Adam. Overall, the OR for vesicovaginal fistula after urinary tract injury was about half as great when either subtotal or vaginal hysterectomy was compared to total abdominal hysterectomy
Although Dr. Adam emphasized that a retrospective study of this type can only establish an association and cannot confirm causation, he said that this study suggests urinary tract injury may be a useful quality-of-care measure for performance of hysterectomy. The SGS-invited discussant Dr. Blair Washington, a urogynecologist at Virginia Mason Hospital and Medical Center, Seattle, agreed.
“Characterizing morbidity associated with hysterectomy is increasingly important as we define benchmarks for quality outcomes in the changing health care economy,” Dr. Washington said. Calling this study “outstanding,” she suggested these data are potentially helpful for counseling patients about risks of hysterectomy, and identifying and evaluating strategies that will help to improve outcomes.
Dr. Adam reported no relevant financial disclosures.
AT SGS 2015
Key clinical point: Vesicovaginal fistulas following hysterectomy are strongly related to urinary tract injuries incurred during surgery, according to data from > 600,000 hysterectomies.
Major finding: When the urinary tract is injured during hysterectomy, a complication that increased steadily over the recent study period, the odds of a vesicovaginal fistula increased almost 19-fold.
Data source: Retrospective database analysis of 641,056 hysterectomies.
Disclosures: Dr. Adam reported no relevant financial disclosures.
Penalties for high infection rates expected to be unfair
ORLANDO – Financial penalties designed to induce hospitals with high surgical site infection rates to improve this aspect of quality of care are likely to be distributed unfairly, according to an analysis presented at the annual scientific meeting of the Society of Gynecologic Surgeons.
The plan, to be initiated in 2016 as part of the Hospital-Acquired Condition (HAC) Reduction Program of the Centers for Medicare & Medicaid, is to levy a 1% penalty for all hospitals that fall into the quartile with the greatest rate of surgical site infections (SSI), but only a proportion of these appear to be outliers, reported Dr. Daniel M. Morgan, an ob.gyn. at the University of Michigan, Ann Arbor.
Leaving aside the question of whether all hospitals in the bottom 25% for avoiding SSI are true outliers and, therefore, deserve a penalty, the study in Michigan suggested that the methodology proposed to rank SSI rates does not appear to be properly adjusted for risk.
In this study, SSI associated with hysterectomy was evaluated in 49 hospitals participating in a statewide surgical quality collaborative in which at least 10 hysterectomies were performed. Using data from the 16,000 hysterectomies in this database, hospitals were stratified by SSI rates using the National Healthcare Safety Network (NHSN) protocol (Infect. Control Hosp. Epidemiol. 2011;32:970-86). This is the methodology planned for the HAC reduction program.
While risk adjustment with the NHSN model was restricted to age, American Society of Anesthesiologists (ASA) class, surgical time, use of laparoscopy, and bed size, the Michigan initiative used a multivariate mixed logistic regression model to identify other factors found to significantly influence SSI rates. These included body mass index (BMI) >30, a gynecologic cancer diagnosis, and payment for services through Medicaid.
Using a quartile stratification, 12 of the 49 hospitals would warrant a penalty under the proposed HAC reduction program, but using the Michigan risk adjustment, 8 of these hospitals, or two-thirds of the total, would not have SSI rates significantly different from the mean and would be penalized unfairly.
Several of the hospitals changed quartiles when the Michigan risk adjustment methodology used the additional risk modifiers over those employed in the NHSN protocol. A change in ranking was more common in smaller hospitals relative to those with more than 500 beds, Dr. Morgan said at the meeting, jointly sponsored by the American College of Surgeons.
These data predict “some serious deficiencies in the planned protocol” that will result in “inappropriate targeting of some hospitals that fall into the bottom quartile,” Dr. Morgan said.
It is reasonable to target SSI rates as a strategy to improve quality of care, according to the SGS-invited discussant for this study, Dr. Kristen Matteson, an ob.gyn. at Brown University, Providence, R.I. She said that SSI is an important cause of morbidity and a significant driver of increased costs and it is appropriate to target those with ineffective or substandard processes for preventing infection. However, defining the outliers, “as demonstrated by these authors, it is a complicated process,” she said.
In an interview, Dr. Matteson suggested that it is not only developing a methodology for accurate risk adjustment but also confirming that the bottom 25% actually have rates that are clinically different than higher quartiles. Mathematically, there is always a bottom 25% on any scale, so it makes more sense to develop a cut-off that establishes true outliers rather than those that happen to fall in the bottom quartile.
These policies are going to be implemented soon, and data such as those in this study suggest that they may target hospitals that do not deserve to be targeted, Dr. Matteson said.
Dr. Morgan reported no relevant financial disclosures.
ORLANDO – Financial penalties designed to induce hospitals with high surgical site infection rates to improve this aspect of quality of care are likely to be distributed unfairly, according to an analysis presented at the annual scientific meeting of the Society of Gynecologic Surgeons.
The plan, to be initiated in 2016 as part of the Hospital-Acquired Condition (HAC) Reduction Program of the Centers for Medicare & Medicaid, is to levy a 1% penalty for all hospitals that fall into the quartile with the greatest rate of surgical site infections (SSI), but only a proportion of these appear to be outliers, reported Dr. Daniel M. Morgan, an ob.gyn. at the University of Michigan, Ann Arbor.
Leaving aside the question of whether all hospitals in the bottom 25% for avoiding SSI are true outliers and, therefore, deserve a penalty, the study in Michigan suggested that the methodology proposed to rank SSI rates does not appear to be properly adjusted for risk.
In this study, SSI associated with hysterectomy was evaluated in 49 hospitals participating in a statewide surgical quality collaborative in which at least 10 hysterectomies were performed. Using data from the 16,000 hysterectomies in this database, hospitals were stratified by SSI rates using the National Healthcare Safety Network (NHSN) protocol (Infect. Control Hosp. Epidemiol. 2011;32:970-86). This is the methodology planned for the HAC reduction program.
While risk adjustment with the NHSN model was restricted to age, American Society of Anesthesiologists (ASA) class, surgical time, use of laparoscopy, and bed size, the Michigan initiative used a multivariate mixed logistic regression model to identify other factors found to significantly influence SSI rates. These included body mass index (BMI) >30, a gynecologic cancer diagnosis, and payment for services through Medicaid.
Using a quartile stratification, 12 of the 49 hospitals would warrant a penalty under the proposed HAC reduction program, but using the Michigan risk adjustment, 8 of these hospitals, or two-thirds of the total, would not have SSI rates significantly different from the mean and would be penalized unfairly.
Several of the hospitals changed quartiles when the Michigan risk adjustment methodology used the additional risk modifiers over those employed in the NHSN protocol. A change in ranking was more common in smaller hospitals relative to those with more than 500 beds, Dr. Morgan said at the meeting, jointly sponsored by the American College of Surgeons.
These data predict “some serious deficiencies in the planned protocol” that will result in “inappropriate targeting of some hospitals that fall into the bottom quartile,” Dr. Morgan said.
It is reasonable to target SSI rates as a strategy to improve quality of care, according to the SGS-invited discussant for this study, Dr. Kristen Matteson, an ob.gyn. at Brown University, Providence, R.I. She said that SSI is an important cause of morbidity and a significant driver of increased costs and it is appropriate to target those with ineffective or substandard processes for preventing infection. However, defining the outliers, “as demonstrated by these authors, it is a complicated process,” she said.
In an interview, Dr. Matteson suggested that it is not only developing a methodology for accurate risk adjustment but also confirming that the bottom 25% actually have rates that are clinically different than higher quartiles. Mathematically, there is always a bottom 25% on any scale, so it makes more sense to develop a cut-off that establishes true outliers rather than those that happen to fall in the bottom quartile.
These policies are going to be implemented soon, and data such as those in this study suggest that they may target hospitals that do not deserve to be targeted, Dr. Matteson said.
Dr. Morgan reported no relevant financial disclosures.
ORLANDO – Financial penalties designed to induce hospitals with high surgical site infection rates to improve this aspect of quality of care are likely to be distributed unfairly, according to an analysis presented at the annual scientific meeting of the Society of Gynecologic Surgeons.
The plan, to be initiated in 2016 as part of the Hospital-Acquired Condition (HAC) Reduction Program of the Centers for Medicare & Medicaid, is to levy a 1% penalty for all hospitals that fall into the quartile with the greatest rate of surgical site infections (SSI), but only a proportion of these appear to be outliers, reported Dr. Daniel M. Morgan, an ob.gyn. at the University of Michigan, Ann Arbor.
Leaving aside the question of whether all hospitals in the bottom 25% for avoiding SSI are true outliers and, therefore, deserve a penalty, the study in Michigan suggested that the methodology proposed to rank SSI rates does not appear to be properly adjusted for risk.
In this study, SSI associated with hysterectomy was evaluated in 49 hospitals participating in a statewide surgical quality collaborative in which at least 10 hysterectomies were performed. Using data from the 16,000 hysterectomies in this database, hospitals were stratified by SSI rates using the National Healthcare Safety Network (NHSN) protocol (Infect. Control Hosp. Epidemiol. 2011;32:970-86). This is the methodology planned for the HAC reduction program.
While risk adjustment with the NHSN model was restricted to age, American Society of Anesthesiologists (ASA) class, surgical time, use of laparoscopy, and bed size, the Michigan initiative used a multivariate mixed logistic regression model to identify other factors found to significantly influence SSI rates. These included body mass index (BMI) >30, a gynecologic cancer diagnosis, and payment for services through Medicaid.
Using a quartile stratification, 12 of the 49 hospitals would warrant a penalty under the proposed HAC reduction program, but using the Michigan risk adjustment, 8 of these hospitals, or two-thirds of the total, would not have SSI rates significantly different from the mean and would be penalized unfairly.
Several of the hospitals changed quartiles when the Michigan risk adjustment methodology used the additional risk modifiers over those employed in the NHSN protocol. A change in ranking was more common in smaller hospitals relative to those with more than 500 beds, Dr. Morgan said at the meeting, jointly sponsored by the American College of Surgeons.
These data predict “some serious deficiencies in the planned protocol” that will result in “inappropriate targeting of some hospitals that fall into the bottom quartile,” Dr. Morgan said.
It is reasonable to target SSI rates as a strategy to improve quality of care, according to the SGS-invited discussant for this study, Dr. Kristen Matteson, an ob.gyn. at Brown University, Providence, R.I. She said that SSI is an important cause of morbidity and a significant driver of increased costs and it is appropriate to target those with ineffective or substandard processes for preventing infection. However, defining the outliers, “as demonstrated by these authors, it is a complicated process,” she said.
In an interview, Dr. Matteson suggested that it is not only developing a methodology for accurate risk adjustment but also confirming that the bottom 25% actually have rates that are clinically different than higher quartiles. Mathematically, there is always a bottom 25% on any scale, so it makes more sense to develop a cut-off that establishes true outliers rather than those that happen to fall in the bottom quartile.
These policies are going to be implemented soon, and data such as those in this study suggest that they may target hospitals that do not deserve to be targeted, Dr. Matteson said.
Dr. Morgan reported no relevant financial disclosures.
AT SGS 2015
Key clinical point: A plan to penalize those hospitals with the highest surgical site infection rates appears likely to levy fines unfairly, according to risk-adjusted calculations on a representative hospital sample in Michigan.
Major finding: Lowering surgical site infections is part of an effort to improve quality of care, but at least half of hospitals to be penalized under current plans for high infection rates after hysterectomy will not differ significantly from the mean.
Data source: Database analysis.
Disclosures: Dr. Morgan reported no relevant financial disclosures.