User login
Official Newspaper of the American College of Surgeons
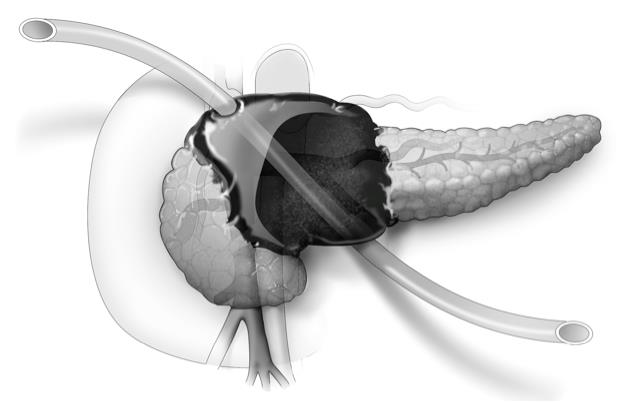
Rectal preservation feasible after cancer clinical remission
HOUSTON – Patients who achieve complete clinical responses after neoadjuvant therapy for locally advanced rectal cancer may, in many cases, be safely spared the trauma and morbidity of total mesorectal excision.
That’s the opinion of investigators at Memorial Sloan Kettering Cancer Center, New York, who found that nearly two-thirds of patients followed with nonoperative management (NOM) had durable complete clinical remissions (cCR) for at least 4 years.
Disease-specific survival and overall survival rates among patients who had nonoperative management were similar to those seen in patients with pathologic complete responses (pCR), defined as no viable tumor cells in the resected specimen.
“We know that there is a very good overall and disease-free survival associated with a [pCR]. Clinical complete response is also associated with pathologic complete response. In that regard, this brings up the question: Is an operation always necessary in these patients?” Dr. Jesse Joshua Smith of the cancer center said at the annual Society of Surgical Oncology Cancer Symposium.
Dr. Smith and colleagues conducted a review of their center’s experience to date with NOM for patients with locally advanced rectal cancer, asking whether the data support the approach as oncologically safe and effective for organ preservation.
They identified 442 patients with rectal cancer treated with neoadjuvant chemotherapy from 2006 through 2014 and compared results for 73 who achieved a cCR and were followed with nonoperative management with those of 72 patients who had a pCR following total mesorectal excision.
Demographic and clinical characteristics between the groups were generally similar, although patients in the pCR group were significantly younger (58 years vs. 65 years, P = .01), had a greater tumor distance from the anal verge (median of 6 cm vs. 5.25 cm, P = .02), and higher proportions of clinical stage II (32% vs. 24%) and III (66% vs. 62%, P = .02).
Among the 73 patients managed with NOM, 54 had durable cCR at 4 years. Of the remaining 19 with local tumor regrowth, 2 had local excisions with no further recurrence and 17 went on to have rectal resections. The total number of patients with rectal preservation in this group was 56 (77%).
Of the 19 patients in the conservatively managed group who had local regrowths, all but three of the recurrences were detected within 13 months.
As noted before, neither disease-specific survival nor overall survival were significantly different between patients managed with NOM or with total mesorectal excision.
There were numerically more distant recurrences at both 1 and 4 years among patients treated with NOM compared with total mesorectal excision (7% vs. 2%, and 17% vs. 9%, respectively), but the differences were not statistically significant, the authors found.
Dr. Smith noted that patients who are offered the option of NOM have a discussion with the surgeon emphasizing that the practice is nonstandard management, carries about a 25% risk of local regrowth, and requires increased endoscopic and radiographic surveillance. Patients also are informed about the risks of salvage abdominoperineal resection or extended resections, and about the potential risk of compromising cure.
The study was supported in part by the Berezuk Colorectal Cancer Fund. Dr. Smith reported having no disclosures.
HOUSTON – Patients who achieve complete clinical responses after neoadjuvant therapy for locally advanced rectal cancer may, in many cases, be safely spared the trauma and morbidity of total mesorectal excision.
That’s the opinion of investigators at Memorial Sloan Kettering Cancer Center, New York, who found that nearly two-thirds of patients followed with nonoperative management (NOM) had durable complete clinical remissions (cCR) for at least 4 years.
Disease-specific survival and overall survival rates among patients who had nonoperative management were similar to those seen in patients with pathologic complete responses (pCR), defined as no viable tumor cells in the resected specimen.
“We know that there is a very good overall and disease-free survival associated with a [pCR]. Clinical complete response is also associated with pathologic complete response. In that regard, this brings up the question: Is an operation always necessary in these patients?” Dr. Jesse Joshua Smith of the cancer center said at the annual Society of Surgical Oncology Cancer Symposium.
Dr. Smith and colleagues conducted a review of their center’s experience to date with NOM for patients with locally advanced rectal cancer, asking whether the data support the approach as oncologically safe and effective for organ preservation.
They identified 442 patients with rectal cancer treated with neoadjuvant chemotherapy from 2006 through 2014 and compared results for 73 who achieved a cCR and were followed with nonoperative management with those of 72 patients who had a pCR following total mesorectal excision.
Demographic and clinical characteristics between the groups were generally similar, although patients in the pCR group were significantly younger (58 years vs. 65 years, P = .01), had a greater tumor distance from the anal verge (median of 6 cm vs. 5.25 cm, P = .02), and higher proportions of clinical stage II (32% vs. 24%) and III (66% vs. 62%, P = .02).
Among the 73 patients managed with NOM, 54 had durable cCR at 4 years. Of the remaining 19 with local tumor regrowth, 2 had local excisions with no further recurrence and 17 went on to have rectal resections. The total number of patients with rectal preservation in this group was 56 (77%).
Of the 19 patients in the conservatively managed group who had local regrowths, all but three of the recurrences were detected within 13 months.
As noted before, neither disease-specific survival nor overall survival were significantly different between patients managed with NOM or with total mesorectal excision.
There were numerically more distant recurrences at both 1 and 4 years among patients treated with NOM compared with total mesorectal excision (7% vs. 2%, and 17% vs. 9%, respectively), but the differences were not statistically significant, the authors found.
Dr. Smith noted that patients who are offered the option of NOM have a discussion with the surgeon emphasizing that the practice is nonstandard management, carries about a 25% risk of local regrowth, and requires increased endoscopic and radiographic surveillance. Patients also are informed about the risks of salvage abdominoperineal resection or extended resections, and about the potential risk of compromising cure.
The study was supported in part by the Berezuk Colorectal Cancer Fund. Dr. Smith reported having no disclosures.
HOUSTON – Patients who achieve complete clinical responses after neoadjuvant therapy for locally advanced rectal cancer may, in many cases, be safely spared the trauma and morbidity of total mesorectal excision.
That’s the opinion of investigators at Memorial Sloan Kettering Cancer Center, New York, who found that nearly two-thirds of patients followed with nonoperative management (NOM) had durable complete clinical remissions (cCR) for at least 4 years.
Disease-specific survival and overall survival rates among patients who had nonoperative management were similar to those seen in patients with pathologic complete responses (pCR), defined as no viable tumor cells in the resected specimen.
“We know that there is a very good overall and disease-free survival associated with a [pCR]. Clinical complete response is also associated with pathologic complete response. In that regard, this brings up the question: Is an operation always necessary in these patients?” Dr. Jesse Joshua Smith of the cancer center said at the annual Society of Surgical Oncology Cancer Symposium.
Dr. Smith and colleagues conducted a review of their center’s experience to date with NOM for patients with locally advanced rectal cancer, asking whether the data support the approach as oncologically safe and effective for organ preservation.
They identified 442 patients with rectal cancer treated with neoadjuvant chemotherapy from 2006 through 2014 and compared results for 73 who achieved a cCR and were followed with nonoperative management with those of 72 patients who had a pCR following total mesorectal excision.
Demographic and clinical characteristics between the groups were generally similar, although patients in the pCR group were significantly younger (58 years vs. 65 years, P = .01), had a greater tumor distance from the anal verge (median of 6 cm vs. 5.25 cm, P = .02), and higher proportions of clinical stage II (32% vs. 24%) and III (66% vs. 62%, P = .02).
Among the 73 patients managed with NOM, 54 had durable cCR at 4 years. Of the remaining 19 with local tumor regrowth, 2 had local excisions with no further recurrence and 17 went on to have rectal resections. The total number of patients with rectal preservation in this group was 56 (77%).
Of the 19 patients in the conservatively managed group who had local regrowths, all but three of the recurrences were detected within 13 months.
As noted before, neither disease-specific survival nor overall survival were significantly different between patients managed with NOM or with total mesorectal excision.
There were numerically more distant recurrences at both 1 and 4 years among patients treated with NOM compared with total mesorectal excision (7% vs. 2%, and 17% vs. 9%, respectively), but the differences were not statistically significant, the authors found.
Dr. Smith noted that patients who are offered the option of NOM have a discussion with the surgeon emphasizing that the practice is nonstandard management, carries about a 25% risk of local regrowth, and requires increased endoscopic and radiographic surveillance. Patients also are informed about the risks of salvage abdominoperineal resection or extended resections, and about the potential risk of compromising cure.
The study was supported in part by the Berezuk Colorectal Cancer Fund. Dr. Smith reported having no disclosures.
Key clinical point: Patients with complete clinical response after neoadjuvant chemotherapy for rectal cancer may be able to be safely followed with nonoperative management.
Major finding: There were no significant differences at 4 years in either disease-specific or overall survival among patients with rectal cancer managed conservatively or with total mesorectal excision.
Data source: Review of prospectively collected data on 145 patients with locally advanced rectal cancer.
Disclosures: The study was supported in part by the Berezuk Colorectal Cancer Fund. Dr. Smith reported having no disclosures.
CMS: SGR repeal equals less pay in long-term
Despite being billed as a permanent solution to the annual threat of Medicare payment cuts to physicians, a bill repealing the Sustainable Growth Rate formula will not ensure adequate physician payments in the long term, according to actuaries at the Centers for Medicare & Medicaid Services.
In an April 9 report outlining the effect of the Medicare Access and CHIP Reauthorization Act (H.R. 2), the CMS Office of the Actuary said it anticipates “that physician payment rates under H.R. 2 would be lower than scheduled under the current SGR formula by 2048 and would continue to worsen thereafter.
“Absent a change in the method or level of update by subsequent legislation, we expect access to Medicare-participating physicians to become a significant issue in the long term under H.R. 2,” the report’s authors noted.
The actuarial report attributes the long-term issues to a number of the bill’s provisions. First, it notes the expiration in 2025 of updates totaling $500 million per year and a 5% annual bonus, which will result in a payment reduction for most physicians.
Second, “this bill specifies the physician payment update amounts for all years in the future, and these amounts do not vary based on underlying economic conditions, nor are they expected to keep pace with the average rate of physician cost increases,” the report warned. That will result in payments that will be inadequate when rates of inflation are higher or when price updates are not enough to cover cost increases.
Compared with costs under current law, the CMS actuaries estimated the legislation’s net cost to the federal government from 2015 through 2025 would be $102.8 billion.
The SGR repeal bill, which also reauthorizes the Children’s Health Insurance Program for 2 more years, passed in the House March 26 with overwhelming bipartisan support. The bill is expected to pass the Senate after members return April 13 from their spring recess.
Despite being billed as a permanent solution to the annual threat of Medicare payment cuts to physicians, a bill repealing the Sustainable Growth Rate formula will not ensure adequate physician payments in the long term, according to actuaries at the Centers for Medicare & Medicaid Services.
In an April 9 report outlining the effect of the Medicare Access and CHIP Reauthorization Act (H.R. 2), the CMS Office of the Actuary said it anticipates “that physician payment rates under H.R. 2 would be lower than scheduled under the current SGR formula by 2048 and would continue to worsen thereafter.
“Absent a change in the method or level of update by subsequent legislation, we expect access to Medicare-participating physicians to become a significant issue in the long term under H.R. 2,” the report’s authors noted.
The actuarial report attributes the long-term issues to a number of the bill’s provisions. First, it notes the expiration in 2025 of updates totaling $500 million per year and a 5% annual bonus, which will result in a payment reduction for most physicians.
Second, “this bill specifies the physician payment update amounts for all years in the future, and these amounts do not vary based on underlying economic conditions, nor are they expected to keep pace with the average rate of physician cost increases,” the report warned. That will result in payments that will be inadequate when rates of inflation are higher or when price updates are not enough to cover cost increases.
Compared with costs under current law, the CMS actuaries estimated the legislation’s net cost to the federal government from 2015 through 2025 would be $102.8 billion.
The SGR repeal bill, which also reauthorizes the Children’s Health Insurance Program for 2 more years, passed in the House March 26 with overwhelming bipartisan support. The bill is expected to pass the Senate after members return April 13 from their spring recess.
Despite being billed as a permanent solution to the annual threat of Medicare payment cuts to physicians, a bill repealing the Sustainable Growth Rate formula will not ensure adequate physician payments in the long term, according to actuaries at the Centers for Medicare & Medicaid Services.
In an April 9 report outlining the effect of the Medicare Access and CHIP Reauthorization Act (H.R. 2), the CMS Office of the Actuary said it anticipates “that physician payment rates under H.R. 2 would be lower than scheduled under the current SGR formula by 2048 and would continue to worsen thereafter.
“Absent a change in the method or level of update by subsequent legislation, we expect access to Medicare-participating physicians to become a significant issue in the long term under H.R. 2,” the report’s authors noted.
The actuarial report attributes the long-term issues to a number of the bill’s provisions. First, it notes the expiration in 2025 of updates totaling $500 million per year and a 5% annual bonus, which will result in a payment reduction for most physicians.
Second, “this bill specifies the physician payment update amounts for all years in the future, and these amounts do not vary based on underlying economic conditions, nor are they expected to keep pace with the average rate of physician cost increases,” the report warned. That will result in payments that will be inadequate when rates of inflation are higher or when price updates are not enough to cover cost increases.
Compared with costs under current law, the CMS actuaries estimated the legislation’s net cost to the federal government from 2015 through 2025 would be $102.8 billion.
The SGR repeal bill, which also reauthorizes the Children’s Health Insurance Program for 2 more years, passed in the House March 26 with overwhelming bipartisan support. The bill is expected to pass the Senate after members return April 13 from their spring recess.
Neoadjuvant therapy facilitates breast conservation
HOUSTON – For some women with breast cancer, neoadjuvant therapy can increase the likelihood of breast-conserving treatment and may limit the extent of axillary dissection, a breast cancer researcher says.
“Neoadjuvant chemotherapy has long been used in the management of inflammatory breast cancer, in patients with locally advanced, or inoperable disease, and it’s increasingly being used in patients who have operable breast cancer,” said Dr. Elizabeth A. Mittendorf of the University of Texas MD Anderson Cancer Center, Houston.
A meta-analysis published in 2007 suggested that neoadjuvant therapy in patients with operable breast cancer reduced the mastectomy rate by 17%, a figure that Dr. Mittendorf said likely underestimates the benefit, because many of the trials included in the analysis did not require patients to be considered for breast conservation at presentation.
The meta-analysis also showed that local recurrence rates did not differ from those seen with mastectomy when patients treated with neoadjuvant therapy were downstaged to breast-conserving therapy, and that there were no differences in local recurrence rates for neoadjuvant vs. adjuvant chemotherapy stratified by type of surgery, Dr. Mittendorf said at the annual Society of Surgical Oncology Cancer Symposium.
Key clinical trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, showed that neoadjuvant chemotherapy did not have an effect on either disease-free or overall survival compared with adjuvant chemotherapy, Dr. Mittendorf noted.
Response to neoadjuvant chemotherapy is also a good predictor of prognosis, she said, pointing to a pooled analysis of 12 studies published in 2014 in The Lancet. The authors of the analysis reported that patients with a pathologic complete response (pCR; no invasive disease in either the breast or axilla) after neoadjuvant chemotherapy had significantly improved survival, with the greatest prognostic values seen in patients with aggressive tumor subtypes.
Factors to consider when selecting neoadjuvant chemotherapy include:
• Tumor size.
• Lymph node status.
• Estrogen, progesterone, and/or HER2 status.
• Treatment sensitivity (as measured by Ki-67 or other markers).
• Pathologic complete response rates.
Chemo for HR-positive?
“With respect to hormone receptor–positive breast cancer, I think the most important question for these patients is do they even need chemotherapy?” Dr. Mittendorf said.
Hormone receptor–positive (HR-positive) breast cancers have been shown to be less responsive to neoadjuvant chemotherapy, and pCR is less prognostic of outcome in this tumor subtype. Older patients with HR-positive cancers who are borderline candidates for breast-conserving therapy might benefit from neoadjuvant therapy with an aromatase inhibitor, she noted.
HER2-positive disease
For patients with HER2-positive breast cancers, it may be possible to tailor neoadjuvant therapy, so that patients who achieve a pCR with neoadjuvant trastuzumab (Herceptin) might be spared an additional 6 months of adjuvant therapy. Dr. Mittendorf’s group published a recent study
Combination anti-HER2 therapies (trastuzumab and pertuzumab [Perjeta] as used in the NeoSphere Trial may help to improve pCR rates and outcomes in patients with HER2-positive tumors, Dr. Mittendorf said.
Triple negative disease
Among patients with triple-negative breast cancer (tumors lacking hormonal receptors and HER2), those who have residual cancer after neoadjuvant chemotherapy have a poor prognosis. At MD Anderson, patients with localized triple-negative breast cancer who are scheduled to receive neoadjuvant chemotherapy first have a biopsy with molecular profiling, and are immediately started on an anthracycline-based regimen.
Patients who have a response to the chemotherapy proceed to receive a taxane, while nonresponders will be triaged onto phase II studies based on the subtype of triple-negative breast cancer. Patients who are positive for BRCA mutations will be started on a carboplatin/paclitaxel regimen, while those with mesenchymal tumor subtypes will be started on a phosphoinositide 3-kinase (PI3K) inhibitor, and those with basal-like tumors will be started on an immunotherapy protocol.Better understanding of the biology of different tumor subtypes may also help to reduce the extent of axillary surgery, by helping clinicians to identify those patients who are likely to have a nodal pCR, Dr. Mittendorf said.
HOUSTON – For some women with breast cancer, neoadjuvant therapy can increase the likelihood of breast-conserving treatment and may limit the extent of axillary dissection, a breast cancer researcher says.
“Neoadjuvant chemotherapy has long been used in the management of inflammatory breast cancer, in patients with locally advanced, or inoperable disease, and it’s increasingly being used in patients who have operable breast cancer,” said Dr. Elizabeth A. Mittendorf of the University of Texas MD Anderson Cancer Center, Houston.
A meta-analysis published in 2007 suggested that neoadjuvant therapy in patients with operable breast cancer reduced the mastectomy rate by 17%, a figure that Dr. Mittendorf said likely underestimates the benefit, because many of the trials included in the analysis did not require patients to be considered for breast conservation at presentation.
The meta-analysis also showed that local recurrence rates did not differ from those seen with mastectomy when patients treated with neoadjuvant therapy were downstaged to breast-conserving therapy, and that there were no differences in local recurrence rates for neoadjuvant vs. adjuvant chemotherapy stratified by type of surgery, Dr. Mittendorf said at the annual Society of Surgical Oncology Cancer Symposium.
Key clinical trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, showed that neoadjuvant chemotherapy did not have an effect on either disease-free or overall survival compared with adjuvant chemotherapy, Dr. Mittendorf noted.
Response to neoadjuvant chemotherapy is also a good predictor of prognosis, she said, pointing to a pooled analysis of 12 studies published in 2014 in The Lancet. The authors of the analysis reported that patients with a pathologic complete response (pCR; no invasive disease in either the breast or axilla) after neoadjuvant chemotherapy had significantly improved survival, with the greatest prognostic values seen in patients with aggressive tumor subtypes.
Factors to consider when selecting neoadjuvant chemotherapy include:
• Tumor size.
• Lymph node status.
• Estrogen, progesterone, and/or HER2 status.
• Treatment sensitivity (as measured by Ki-67 or other markers).
• Pathologic complete response rates.
Chemo for HR-positive?
“With respect to hormone receptor–positive breast cancer, I think the most important question for these patients is do they even need chemotherapy?” Dr. Mittendorf said.
Hormone receptor–positive (HR-positive) breast cancers have been shown to be less responsive to neoadjuvant chemotherapy, and pCR is less prognostic of outcome in this tumor subtype. Older patients with HR-positive cancers who are borderline candidates for breast-conserving therapy might benefit from neoadjuvant therapy with an aromatase inhibitor, she noted.
HER2-positive disease
For patients with HER2-positive breast cancers, it may be possible to tailor neoadjuvant therapy, so that patients who achieve a pCR with neoadjuvant trastuzumab (Herceptin) might be spared an additional 6 months of adjuvant therapy. Dr. Mittendorf’s group published a recent study
Combination anti-HER2 therapies (trastuzumab and pertuzumab [Perjeta] as used in the NeoSphere Trial may help to improve pCR rates and outcomes in patients with HER2-positive tumors, Dr. Mittendorf said.
Triple negative disease
Among patients with triple-negative breast cancer (tumors lacking hormonal receptors and HER2), those who have residual cancer after neoadjuvant chemotherapy have a poor prognosis. At MD Anderson, patients with localized triple-negative breast cancer who are scheduled to receive neoadjuvant chemotherapy first have a biopsy with molecular profiling, and are immediately started on an anthracycline-based regimen.
Patients who have a response to the chemotherapy proceed to receive a taxane, while nonresponders will be triaged onto phase II studies based on the subtype of triple-negative breast cancer. Patients who are positive for BRCA mutations will be started on a carboplatin/paclitaxel regimen, while those with mesenchymal tumor subtypes will be started on a phosphoinositide 3-kinase (PI3K) inhibitor, and those with basal-like tumors will be started on an immunotherapy protocol.Better understanding of the biology of different tumor subtypes may also help to reduce the extent of axillary surgery, by helping clinicians to identify those patients who are likely to have a nodal pCR, Dr. Mittendorf said.
HOUSTON – For some women with breast cancer, neoadjuvant therapy can increase the likelihood of breast-conserving treatment and may limit the extent of axillary dissection, a breast cancer researcher says.
“Neoadjuvant chemotherapy has long been used in the management of inflammatory breast cancer, in patients with locally advanced, or inoperable disease, and it’s increasingly being used in patients who have operable breast cancer,” said Dr. Elizabeth A. Mittendorf of the University of Texas MD Anderson Cancer Center, Houston.
A meta-analysis published in 2007 suggested that neoadjuvant therapy in patients with operable breast cancer reduced the mastectomy rate by 17%, a figure that Dr. Mittendorf said likely underestimates the benefit, because many of the trials included in the analysis did not require patients to be considered for breast conservation at presentation.
The meta-analysis also showed that local recurrence rates did not differ from those seen with mastectomy when patients treated with neoadjuvant therapy were downstaged to breast-conserving therapy, and that there were no differences in local recurrence rates for neoadjuvant vs. adjuvant chemotherapy stratified by type of surgery, Dr. Mittendorf said at the annual Society of Surgical Oncology Cancer Symposium.
Key clinical trials, including the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 trials, showed that neoadjuvant chemotherapy did not have an effect on either disease-free or overall survival compared with adjuvant chemotherapy, Dr. Mittendorf noted.
Response to neoadjuvant chemotherapy is also a good predictor of prognosis, she said, pointing to a pooled analysis of 12 studies published in 2014 in The Lancet. The authors of the analysis reported that patients with a pathologic complete response (pCR; no invasive disease in either the breast or axilla) after neoadjuvant chemotherapy had significantly improved survival, with the greatest prognostic values seen in patients with aggressive tumor subtypes.
Factors to consider when selecting neoadjuvant chemotherapy include:
• Tumor size.
• Lymph node status.
• Estrogen, progesterone, and/or HER2 status.
• Treatment sensitivity (as measured by Ki-67 or other markers).
• Pathologic complete response rates.
Chemo for HR-positive?
“With respect to hormone receptor–positive breast cancer, I think the most important question for these patients is do they even need chemotherapy?” Dr. Mittendorf said.
Hormone receptor–positive (HR-positive) breast cancers have been shown to be less responsive to neoadjuvant chemotherapy, and pCR is less prognostic of outcome in this tumor subtype. Older patients with HR-positive cancers who are borderline candidates for breast-conserving therapy might benefit from neoadjuvant therapy with an aromatase inhibitor, she noted.
HER2-positive disease
For patients with HER2-positive breast cancers, it may be possible to tailor neoadjuvant therapy, so that patients who achieve a pCR with neoadjuvant trastuzumab (Herceptin) might be spared an additional 6 months of adjuvant therapy. Dr. Mittendorf’s group published a recent study
Combination anti-HER2 therapies (trastuzumab and pertuzumab [Perjeta] as used in the NeoSphere Trial may help to improve pCR rates and outcomes in patients with HER2-positive tumors, Dr. Mittendorf said.
Triple negative disease
Among patients with triple-negative breast cancer (tumors lacking hormonal receptors and HER2), those who have residual cancer after neoadjuvant chemotherapy have a poor prognosis. At MD Anderson, patients with localized triple-negative breast cancer who are scheduled to receive neoadjuvant chemotherapy first have a biopsy with molecular profiling, and are immediately started on an anthracycline-based regimen.
Patients who have a response to the chemotherapy proceed to receive a taxane, while nonresponders will be triaged onto phase II studies based on the subtype of triple-negative breast cancer. Patients who are positive for BRCA mutations will be started on a carboplatin/paclitaxel regimen, while those with mesenchymal tumor subtypes will be started on a phosphoinositide 3-kinase (PI3K) inhibitor, and those with basal-like tumors will be started on an immunotherapy protocol.Better understanding of the biology of different tumor subtypes may also help to reduce the extent of axillary surgery, by helping clinicians to identify those patients who are likely to have a nodal pCR, Dr. Mittendorf said.
SLN mapping found accurate in endometrial cancer
CHICAGO – Sentinel lymph node mapping alone may suffice for initial staging in women with clinical high-risk endometrial cancer, according to a prospective cohort study reported at the annual meeting of the Society of Gynecologic Oncology.
“While the majority of women with endometrial cancer do not have nodal involvement, positive nodes are an important prognostic factor and often guide postoperative adjuvant therapy. The use of SLN [sentinel lymph node] mapping could potentially maximize the identification of positive nodes while minimizing the risk of full lymphadenectomy,” noted Dr. Pamela T. Soliman, Center Medical Director of the Gynecologic Medical Center at The University of Texas MD Anderson Cancer Center, Houston.

|
| Susan London/Frontline Medical News |
Dr. Pamela Soliman |
She and her colleagues studied 60 women who had clinical stage II disease with high-risk features on preoperative biopsy. All women underwent preoperative PET-CT, then SLN mapping, and finally a full surgical staging with pelvic and para-aortic lymphadenectomy to the renal vessels.
Results showed that 92% of women had at least one SLN detected and 61% had SLNs detected bilaterally. “These findings are consistent with others reported in the literature suggesting that this technique is reproducible,” Dr. Soliman maintained.
Importantly, she said, all of the patients ultimately found to have stage IIIc disease had at least one positive SLN identified, translating to a sensitivity of SLN mapping of 100% and a false-negative rate of zero.
“While we do not expect that the true false-negative rate is zero in this patient population, our results are very promising, and we are hopeful that with our study and other studies presented here, we can potentially change the way we manage patients with high-risk endometrial cancer,” she said.
“If we continue to successfully identify the women with positive lymph nodes using the mapping technique, potentially an SLN biopsy alone could be considered for women with high-risk endometrial cancer. It’s unclear, however, how this change in practice will affect our postoperative management as well as our long-term survival,” Dr. Soliman added.
The SLN mapping in the study was done with a cervical injection of dye, both superficial and deep, at the 3 and 9 o’clock positions, with the surgical approach (laparotomy, laparoscopy, robot-assisted, or a combination) and type of dye used for mapping left up to the surgeon. Indocyanine green was used in about two-thirds of cases.
The high SLN detection rate was consistent across different surgical approaches and types of dye used, reported Dr. Soliman, who disclosed that she had no relevant conflicts of interest.
A total of 146 SLNs were identified in 56 women. Seventeen of these SLNs – all external iliac or obturator nodes – in 10 patients were positive; in half of these patients, the SLNs were the only histologically positive nodes found.
One woman had a negative SLN on the right, although the non-SLNs on that side were positive. “She was not considered a false-negative because her SLN biopsy on the other side was positive,” Dr. Soliman explained.
CHICAGO – Sentinel lymph node mapping alone may suffice for initial staging in women with clinical high-risk endometrial cancer, according to a prospective cohort study reported at the annual meeting of the Society of Gynecologic Oncology.
“While the majority of women with endometrial cancer do not have nodal involvement, positive nodes are an important prognostic factor and often guide postoperative adjuvant therapy. The use of SLN [sentinel lymph node] mapping could potentially maximize the identification of positive nodes while minimizing the risk of full lymphadenectomy,” noted Dr. Pamela T. Soliman, Center Medical Director of the Gynecologic Medical Center at The University of Texas MD Anderson Cancer Center, Houston.

|
| Susan London/Frontline Medical News |
Dr. Pamela Soliman |
She and her colleagues studied 60 women who had clinical stage II disease with high-risk features on preoperative biopsy. All women underwent preoperative PET-CT, then SLN mapping, and finally a full surgical staging with pelvic and para-aortic lymphadenectomy to the renal vessels.
Results showed that 92% of women had at least one SLN detected and 61% had SLNs detected bilaterally. “These findings are consistent with others reported in the literature suggesting that this technique is reproducible,” Dr. Soliman maintained.
Importantly, she said, all of the patients ultimately found to have stage IIIc disease had at least one positive SLN identified, translating to a sensitivity of SLN mapping of 100% and a false-negative rate of zero.
“While we do not expect that the true false-negative rate is zero in this patient population, our results are very promising, and we are hopeful that with our study and other studies presented here, we can potentially change the way we manage patients with high-risk endometrial cancer,” she said.
“If we continue to successfully identify the women with positive lymph nodes using the mapping technique, potentially an SLN biopsy alone could be considered for women with high-risk endometrial cancer. It’s unclear, however, how this change in practice will affect our postoperative management as well as our long-term survival,” Dr. Soliman added.
The SLN mapping in the study was done with a cervical injection of dye, both superficial and deep, at the 3 and 9 o’clock positions, with the surgical approach (laparotomy, laparoscopy, robot-assisted, or a combination) and type of dye used for mapping left up to the surgeon. Indocyanine green was used in about two-thirds of cases.
The high SLN detection rate was consistent across different surgical approaches and types of dye used, reported Dr. Soliman, who disclosed that she had no relevant conflicts of interest.
A total of 146 SLNs were identified in 56 women. Seventeen of these SLNs – all external iliac or obturator nodes – in 10 patients were positive; in half of these patients, the SLNs were the only histologically positive nodes found.
One woman had a negative SLN on the right, although the non-SLNs on that side were positive. “She was not considered a false-negative because her SLN biopsy on the other side was positive,” Dr. Soliman explained.
CHICAGO – Sentinel lymph node mapping alone may suffice for initial staging in women with clinical high-risk endometrial cancer, according to a prospective cohort study reported at the annual meeting of the Society of Gynecologic Oncology.
“While the majority of women with endometrial cancer do not have nodal involvement, positive nodes are an important prognostic factor and often guide postoperative adjuvant therapy. The use of SLN [sentinel lymph node] mapping could potentially maximize the identification of positive nodes while minimizing the risk of full lymphadenectomy,” noted Dr. Pamela T. Soliman, Center Medical Director of the Gynecologic Medical Center at The University of Texas MD Anderson Cancer Center, Houston.

|
| Susan London/Frontline Medical News |
Dr. Pamela Soliman |
She and her colleagues studied 60 women who had clinical stage II disease with high-risk features on preoperative biopsy. All women underwent preoperative PET-CT, then SLN mapping, and finally a full surgical staging with pelvic and para-aortic lymphadenectomy to the renal vessels.
Results showed that 92% of women had at least one SLN detected and 61% had SLNs detected bilaterally. “These findings are consistent with others reported in the literature suggesting that this technique is reproducible,” Dr. Soliman maintained.
Importantly, she said, all of the patients ultimately found to have stage IIIc disease had at least one positive SLN identified, translating to a sensitivity of SLN mapping of 100% and a false-negative rate of zero.
“While we do not expect that the true false-negative rate is zero in this patient population, our results are very promising, and we are hopeful that with our study and other studies presented here, we can potentially change the way we manage patients with high-risk endometrial cancer,” she said.
“If we continue to successfully identify the women with positive lymph nodes using the mapping technique, potentially an SLN biopsy alone could be considered for women with high-risk endometrial cancer. It’s unclear, however, how this change in practice will affect our postoperative management as well as our long-term survival,” Dr. Soliman added.
The SLN mapping in the study was done with a cervical injection of dye, both superficial and deep, at the 3 and 9 o’clock positions, with the surgical approach (laparotomy, laparoscopy, robot-assisted, or a combination) and type of dye used for mapping left up to the surgeon. Indocyanine green was used in about two-thirds of cases.
The high SLN detection rate was consistent across different surgical approaches and types of dye used, reported Dr. Soliman, who disclosed that she had no relevant conflicts of interest.
A total of 146 SLNs were identified in 56 women. Seventeen of these SLNs – all external iliac or obturator nodes – in 10 patients were positive; in half of these patients, the SLNs were the only histologically positive nodes found.
One woman had a negative SLN on the right, although the non-SLNs on that side were positive. “She was not considered a false-negative because her SLN biopsy on the other side was positive,” Dr. Soliman explained.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: SLN mapping had a false-negative rate of zero.
Major finding: The SLN mapping procedure identified all cases of pathologic stage IIIc disease.
Data source: A prospective cohort study of 60 women with clinical stage II high-risk endometrial cancer.
Disclosures: Dr. Soliman disclosed that she had no conflicts of interest.
Failure to warn or report
Question: A patient who is a school bus driver is also an alcoholic, but he continues to drive despite his physician’s warning against drinking and driving. He already has had one prior driving under the influence (DUI) conviction. Fearing a potential vehicular accident with injuries to the schoolchildren, the physician, with permission, informed the patient’s wife of the situation; but that did not make a difference.
Under the circumstances, which of the following is best?
A. The physician has discharged his professional duty by warning the patient and informing his wife.
B. It is unethical for the physician to report this to the employer, because it violates the principle of confidentiality.
C. The physician may have an ethical and legal duty to report, because the risk of harm to the school children is foreseeable and outweighs other considerations.
D. A and B.
E. A and C.
Answer: C. The issue in this hypothetical revolves around the failure to warn and report when a doctor has determined that his or her patient poses a significant societal risk. It places the health care provider in a dilemma, because it pits patient confidentiality against the public interest in safety.
There does not appear to be an appellate case precisely on point, but the well known case of Tarasoff1 offers useful insight.
In Tarasoff, a California court imposed a legal duty on a college psychologist to warn an intended victim of harm, even though that meant breaching confidentiality in a professional relationship. A jilted patient had confided in the university psychologist his intention to kill his ex-girlfriend. The information, though shared with campus security, was not released to the intended victim, the girlfriend, whom the patient stabbed to death 2 months later.
The California court found the psychologist and the University of California (as the psychologist’s employer) liable, reasoning that the protection of public safety was more important than the sanctity of the doctor-patient confidentiality relationship.
The court wrote: “We recognize the public interest in supporting effective treatment of mental illness and in protecting the rights of patients to privacy and the consequent public importance of safeguarding the confidential character of psychotherapeutic communication. Against this interest, however, we must weigh the public interest in safety from violent assault. ... In this risk-infested society, we can hardly tolerate the further exposure to danger that would result from a concealed knowledge of the therapist that his patient was lethal.”
However, the duty to warn a third party may not be as clear cut where there is no readily identifiable victim.2
Further, the law is unsettled regarding a physician’s duty to report negligent conduct that poses a significant risk to public safety, e.g., where a patient insists on operating a vehicle against medical advice. Obviously, the doctor should spare no effort in trying to persuade such a patient to discontinue driving. When this fails, however, and depending on the circumstances, the physician should seriously consider breaching patent confidentiality by notifying the driving licensing bureau.
This opinion is in accord with the AMA Code of Medical Ethics: “Physicians should use their best judgment when determining when to report impairments that could limit a patient’s ability to drive safely. In situations where clear evidence of substantial driving impairment implies a strong threat to patient and public safety, and where the physician’s advice to discontinue driving privileges is ignored, it is desirable and ethical to notify the department of motor vehicles.”3
What about informing the patient’s employer if the risk of harm arises in the work setting?
The duty to report is clearer if the physician is a company doctor specifically charged with the task of determining whether an employee is fit for work. However, a patient’s personal physician may be a different matter, because he or she does not share the same contractual obligation as a company employed doctor.
Still, the circumstances may well dictate the need to report. In the hypothetical scenario posed above, the patient is refusing to heed the advice of physician and spouse, and given the history of a DUI, can be said to be placing innocent children and others at risk. The physician may need to go beyond warning the patient and wife, even though revealing a patient’s diagnosis violates the principle of confidentiality.
Arguably controversial, the physician’s justification is that there is a higher ethical and legal duty to report, as the risk of harm to the school children is both unreasonable and foreseeable, outweighing other considerations.
The same analysis concerning reporting may be applied in uncooperative patients with certain medical diagnoses (e.g., uncontrolled epilepsy, serious mental illness, or disabling cardiac or cerebrovascular disease, to name a few) and in those taking mind-altering prescription medications or illicit drugs.
A key inquiry is whether the patient’s work activity is potentially hazardous, as in those who are entrusted with public transportation, e.g., bus drivers, pilots and train/ship engineers, or those who operate heavy machinery.
The most familiar example of the failure to warn (as opposed to report) is of course in informed consent litigation, where the physician is faulted for not warning the patient of the risk of harm and the patient in fact suffers that harm. It is framed as the failure to disclose material risks. The California Supreme Court has stated that “material information is that which the physician knows or should know would be regarded as significant by a reasonable person in the patient’s position when deciding to accept or reject the recommended medical procedure”; that “a (material) fact must also be one which is not commonly appreciated”; and the scope of disclosure may be expanded in patients with “unique concerns or lack of familiarity with medical procedures.”4 There is, however, no legal requirement to deliver a “mini-course in medical science.”5
Another situation where there may have been a failure to warn is in medical products liability. Here, the plaintiff alleges that the defective product has caused injuries because the manufacturer has issued no prior warning to the health care provider or end user. A prime example is the current Lipitor and diabetes class action lawsuit against Pfizer.
In failing to warn a patient, a doctor may incur liability not only for injuries to the patient but also to nonpatient third parties.
In a 2002 Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter.6 The driver alleged that the prescription medication prazosin caused him to develop hypotension and lose control of the car, and that the treating physician was negligent in failing to warn of this potential side effect.
The Hawaii Supreme Court held that physicians have a duty to their patients to warn of potential adverse effects, and this responsibility should therefore extend to third parties.
Court decisions elsewhere have also found liability where, for public policy reasons, a “special relationship” is deemed to exist between the doctor and the injured third party. Foreseeability is one important factor in construing its existence, notwithstanding the absence of the traditional doctor-patient relationship.
References
1. Tarasoff v. Regents of University of California, 551 P.2d 334 (Cal. 1976).
2. Furr v. Spring Grove State Hospital, 454 A.2d 414 (Md. 1983).
3. AMA Code of Medical Ethics [2.24 (3), 2012-2013 ed.].
4. Truman v. Thomas, 27 Cal.3d 285 (1980).
5. Cobbs v. Grant, 8 Cal.3d 229 (1972).
6. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii. He currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
Question: A patient who is a school bus driver is also an alcoholic, but he continues to drive despite his physician’s warning against drinking and driving. He already has had one prior driving under the influence (DUI) conviction. Fearing a potential vehicular accident with injuries to the schoolchildren, the physician, with permission, informed the patient’s wife of the situation; but that did not make a difference.
Under the circumstances, which of the following is best?
A. The physician has discharged his professional duty by warning the patient and informing his wife.
B. It is unethical for the physician to report this to the employer, because it violates the principle of confidentiality.
C. The physician may have an ethical and legal duty to report, because the risk of harm to the school children is foreseeable and outweighs other considerations.
D. A and B.
E. A and C.
Answer: C. The issue in this hypothetical revolves around the failure to warn and report when a doctor has determined that his or her patient poses a significant societal risk. It places the health care provider in a dilemma, because it pits patient confidentiality against the public interest in safety.
There does not appear to be an appellate case precisely on point, but the well known case of Tarasoff1 offers useful insight.
In Tarasoff, a California court imposed a legal duty on a college psychologist to warn an intended victim of harm, even though that meant breaching confidentiality in a professional relationship. A jilted patient had confided in the university psychologist his intention to kill his ex-girlfriend. The information, though shared with campus security, was not released to the intended victim, the girlfriend, whom the patient stabbed to death 2 months later.
The California court found the psychologist and the University of California (as the psychologist’s employer) liable, reasoning that the protection of public safety was more important than the sanctity of the doctor-patient confidentiality relationship.
The court wrote: “We recognize the public interest in supporting effective treatment of mental illness and in protecting the rights of patients to privacy and the consequent public importance of safeguarding the confidential character of psychotherapeutic communication. Against this interest, however, we must weigh the public interest in safety from violent assault. ... In this risk-infested society, we can hardly tolerate the further exposure to danger that would result from a concealed knowledge of the therapist that his patient was lethal.”
However, the duty to warn a third party may not be as clear cut where there is no readily identifiable victim.2
Further, the law is unsettled regarding a physician’s duty to report negligent conduct that poses a significant risk to public safety, e.g., where a patient insists on operating a vehicle against medical advice. Obviously, the doctor should spare no effort in trying to persuade such a patient to discontinue driving. When this fails, however, and depending on the circumstances, the physician should seriously consider breaching patent confidentiality by notifying the driving licensing bureau.
This opinion is in accord with the AMA Code of Medical Ethics: “Physicians should use their best judgment when determining when to report impairments that could limit a patient’s ability to drive safely. In situations where clear evidence of substantial driving impairment implies a strong threat to patient and public safety, and where the physician’s advice to discontinue driving privileges is ignored, it is desirable and ethical to notify the department of motor vehicles.”3
What about informing the patient’s employer if the risk of harm arises in the work setting?
The duty to report is clearer if the physician is a company doctor specifically charged with the task of determining whether an employee is fit for work. However, a patient’s personal physician may be a different matter, because he or she does not share the same contractual obligation as a company employed doctor.
Still, the circumstances may well dictate the need to report. In the hypothetical scenario posed above, the patient is refusing to heed the advice of physician and spouse, and given the history of a DUI, can be said to be placing innocent children and others at risk. The physician may need to go beyond warning the patient and wife, even though revealing a patient’s diagnosis violates the principle of confidentiality.
Arguably controversial, the physician’s justification is that there is a higher ethical and legal duty to report, as the risk of harm to the school children is both unreasonable and foreseeable, outweighing other considerations.
The same analysis concerning reporting may be applied in uncooperative patients with certain medical diagnoses (e.g., uncontrolled epilepsy, serious mental illness, or disabling cardiac or cerebrovascular disease, to name a few) and in those taking mind-altering prescription medications or illicit drugs.
A key inquiry is whether the patient’s work activity is potentially hazardous, as in those who are entrusted with public transportation, e.g., bus drivers, pilots and train/ship engineers, or those who operate heavy machinery.
The most familiar example of the failure to warn (as opposed to report) is of course in informed consent litigation, where the physician is faulted for not warning the patient of the risk of harm and the patient in fact suffers that harm. It is framed as the failure to disclose material risks. The California Supreme Court has stated that “material information is that which the physician knows or should know would be regarded as significant by a reasonable person in the patient’s position when deciding to accept or reject the recommended medical procedure”; that “a (material) fact must also be one which is not commonly appreciated”; and the scope of disclosure may be expanded in patients with “unique concerns or lack of familiarity with medical procedures.”4 There is, however, no legal requirement to deliver a “mini-course in medical science.”5
Another situation where there may have been a failure to warn is in medical products liability. Here, the plaintiff alleges that the defective product has caused injuries because the manufacturer has issued no prior warning to the health care provider or end user. A prime example is the current Lipitor and diabetes class action lawsuit against Pfizer.
In failing to warn a patient, a doctor may incur liability not only for injuries to the patient but also to nonpatient third parties.
In a 2002 Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter.6 The driver alleged that the prescription medication prazosin caused him to develop hypotension and lose control of the car, and that the treating physician was negligent in failing to warn of this potential side effect.
The Hawaii Supreme Court held that physicians have a duty to their patients to warn of potential adverse effects, and this responsibility should therefore extend to third parties.
Court decisions elsewhere have also found liability where, for public policy reasons, a “special relationship” is deemed to exist between the doctor and the injured third party. Foreseeability is one important factor in construing its existence, notwithstanding the absence of the traditional doctor-patient relationship.
References
1. Tarasoff v. Regents of University of California, 551 P.2d 334 (Cal. 1976).
2. Furr v. Spring Grove State Hospital, 454 A.2d 414 (Md. 1983).
3. AMA Code of Medical Ethics [2.24 (3), 2012-2013 ed.].
4. Truman v. Thomas, 27 Cal.3d 285 (1980).
5. Cobbs v. Grant, 8 Cal.3d 229 (1972).
6. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii. He currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
Question: A patient who is a school bus driver is also an alcoholic, but he continues to drive despite his physician’s warning against drinking and driving. He already has had one prior driving under the influence (DUI) conviction. Fearing a potential vehicular accident with injuries to the schoolchildren, the physician, with permission, informed the patient’s wife of the situation; but that did not make a difference.
Under the circumstances, which of the following is best?
A. The physician has discharged his professional duty by warning the patient and informing his wife.
B. It is unethical for the physician to report this to the employer, because it violates the principle of confidentiality.
C. The physician may have an ethical and legal duty to report, because the risk of harm to the school children is foreseeable and outweighs other considerations.
D. A and B.
E. A and C.
Answer: C. The issue in this hypothetical revolves around the failure to warn and report when a doctor has determined that his or her patient poses a significant societal risk. It places the health care provider in a dilemma, because it pits patient confidentiality against the public interest in safety.
There does not appear to be an appellate case precisely on point, but the well known case of Tarasoff1 offers useful insight.
In Tarasoff, a California court imposed a legal duty on a college psychologist to warn an intended victim of harm, even though that meant breaching confidentiality in a professional relationship. A jilted patient had confided in the university psychologist his intention to kill his ex-girlfriend. The information, though shared with campus security, was not released to the intended victim, the girlfriend, whom the patient stabbed to death 2 months later.
The California court found the psychologist and the University of California (as the psychologist’s employer) liable, reasoning that the protection of public safety was more important than the sanctity of the doctor-patient confidentiality relationship.
The court wrote: “We recognize the public interest in supporting effective treatment of mental illness and in protecting the rights of patients to privacy and the consequent public importance of safeguarding the confidential character of psychotherapeutic communication. Against this interest, however, we must weigh the public interest in safety from violent assault. ... In this risk-infested society, we can hardly tolerate the further exposure to danger that would result from a concealed knowledge of the therapist that his patient was lethal.”
However, the duty to warn a third party may not be as clear cut where there is no readily identifiable victim.2
Further, the law is unsettled regarding a physician’s duty to report negligent conduct that poses a significant risk to public safety, e.g., where a patient insists on operating a vehicle against medical advice. Obviously, the doctor should spare no effort in trying to persuade such a patient to discontinue driving. When this fails, however, and depending on the circumstances, the physician should seriously consider breaching patent confidentiality by notifying the driving licensing bureau.
This opinion is in accord with the AMA Code of Medical Ethics: “Physicians should use their best judgment when determining when to report impairments that could limit a patient’s ability to drive safely. In situations where clear evidence of substantial driving impairment implies a strong threat to patient and public safety, and where the physician’s advice to discontinue driving privileges is ignored, it is desirable and ethical to notify the department of motor vehicles.”3
What about informing the patient’s employer if the risk of harm arises in the work setting?
The duty to report is clearer if the physician is a company doctor specifically charged with the task of determining whether an employee is fit for work. However, a patient’s personal physician may be a different matter, because he or she does not share the same contractual obligation as a company employed doctor.
Still, the circumstances may well dictate the need to report. In the hypothetical scenario posed above, the patient is refusing to heed the advice of physician and spouse, and given the history of a DUI, can be said to be placing innocent children and others at risk. The physician may need to go beyond warning the patient and wife, even though revealing a patient’s diagnosis violates the principle of confidentiality.
Arguably controversial, the physician’s justification is that there is a higher ethical and legal duty to report, as the risk of harm to the school children is both unreasonable and foreseeable, outweighing other considerations.
The same analysis concerning reporting may be applied in uncooperative patients with certain medical diagnoses (e.g., uncontrolled epilepsy, serious mental illness, or disabling cardiac or cerebrovascular disease, to name a few) and in those taking mind-altering prescription medications or illicit drugs.
A key inquiry is whether the patient’s work activity is potentially hazardous, as in those who are entrusted with public transportation, e.g., bus drivers, pilots and train/ship engineers, or those who operate heavy machinery.
The most familiar example of the failure to warn (as opposed to report) is of course in informed consent litigation, where the physician is faulted for not warning the patient of the risk of harm and the patient in fact suffers that harm. It is framed as the failure to disclose material risks. The California Supreme Court has stated that “material information is that which the physician knows or should know would be regarded as significant by a reasonable person in the patient’s position when deciding to accept or reject the recommended medical procedure”; that “a (material) fact must also be one which is not commonly appreciated”; and the scope of disclosure may be expanded in patients with “unique concerns or lack of familiarity with medical procedures.”4 There is, however, no legal requirement to deliver a “mini-course in medical science.”5
Another situation where there may have been a failure to warn is in medical products liability. Here, the plaintiff alleges that the defective product has caused injuries because the manufacturer has issued no prior warning to the health care provider or end user. A prime example is the current Lipitor and diabetes class action lawsuit against Pfizer.
In failing to warn a patient, a doctor may incur liability not only for injuries to the patient but also to nonpatient third parties.
In a 2002 Hawaii case, a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter.6 The driver alleged that the prescription medication prazosin caused him to develop hypotension and lose control of the car, and that the treating physician was negligent in failing to warn of this potential side effect.
The Hawaii Supreme Court held that physicians have a duty to their patients to warn of potential adverse effects, and this responsibility should therefore extend to third parties.
Court decisions elsewhere have also found liability where, for public policy reasons, a “special relationship” is deemed to exist between the doctor and the injured third party. Foreseeability is one important factor in construing its existence, notwithstanding the absence of the traditional doctor-patient relationship.
References
1. Tarasoff v. Regents of University of California, 551 P.2d 334 (Cal. 1976).
2. Furr v. Spring Grove State Hospital, 454 A.2d 414 (Md. 1983).
3. AMA Code of Medical Ethics [2.24 (3), 2012-2013 ed.].
4. Truman v. Thomas, 27 Cal.3d 285 (1980).
5. Cobbs v. Grant, 8 Cal.3d 229 (1972).
6. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii. He currently directs the St. Francis International Center for Healthcare Ethics in Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
Heart surgeons get serious about RCTs
SAN DIEGO – The days when cardiologists could look down their noses at cardiac surgeons as primitive when it comes to conducting high-quality clinical research have come and gone.
“Cardiologists have been much more sophisticated than we have in doing randomized controlled trials. But cardiac surgeons are finally making strong progress in conducting randomized clinical trials, an area we’re not typically known for,” Dr. Vinod H. Thourani asserted at the annual meeting of the American College of Cardiology.
Much of this progress can be credited to the relatively recent creation of the Cardiothoracic Surgical Trials Network (CSTN), funded by the U.S. National Institutions of Health and the Canadian Institutes of Health Research. This surgical network is carrying out cutting-edge RCTs that will change the practice of cardiology as well as heart surgery, said Dr. Thourani, professor of surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
To illustrate the breadth of current research in cardiothoracic surgery, he presented thumbnail sketches of RCTs due to report findings as early as this spring and no later than the latter part of next year or early 2017. The trials he selected, some conducted by the CSTN and others with industry sponsorship, address the aortic valve, the mitral valve, postoperative atrial fibrillation, revascularization in patients with unprotected left main coronary artery disease, and advanced heart failure.
• Heart failure: Outcomes of the ENDURANCE destination trial are due to be presented this spring at the annual meeting of the International Society for Heart and Lung Transplantation in Nice, France. This study will compare 12-month outcomes of continuous-flow ventricular assist devices as destination therapy in advanced heart failure. In this HeartWare-sponsored study, 310 patients received the investigational HeartWare ventricular assist system and 155 controls were implanted with the FDA-approved HeartMate II device marketed by Thoratec.
• Atrial fibrillation: The Rate Control Versus Rhythm Control for Postoperative Atrial Fibrillation trial compares the two management strategies in patients with new-onset AF following cardiac surgery. This CSTN-conducted study, to be presented next year, looks at which treatment approach results in fewer days in the hospital, as well as heart rhythm at discharge and through 60 days of follow-up, economic costs, and the incidence of postoperative clinical events.
• Coronary artery disease: The EXCEL trial has randomized 2,600 patients with unprotected left main coronary artery disease to coronary artery bypass surgery or percutaneous intervention with a XIENCE everolimus-eluting stent. The primary outcome is the composite of all-cause mortality, acute MI, or stroke. First results of this Abbott Vascular–sponsored trial are due to be reported next year.“This is a study that will clearly be impactful for you,” Dr. Thourani observed.
• Aortic valve disease: Two major RCTs are looking at the impact of extending transcatheter aortic valve replacement (TAVR) to an intermediate-surgical-risk population of patients with symptomatic severe aortic stenosis. The PARTNER II trial compares transfemoral or transapical/transaortic TAVR with a SAPIEN XT valve to surgical aortic valve replacement in patients with a Society of Thoracic Surgeons mortality risk score of 4%-8%. The primary endpoint is all-cause mortality and disabling stroke at 2 years. Results of this Edwards Lifesciences–sponsored trial will be presented by early 2016.
The Medtronic-sponsored SURTAVI trial compares TAVR using the company’s CoreValve alone or with PCI if revascularization is indicated versus surgical aortic valve replacement alone or with coronary artery bypass grafting if revascularization is indicated. This is a randomized trial involving 2,500 intermediate-risk patients. Of note, the SURTAVI investigators have revised the study protocol to open the trial to patients who are age 75 years or older or have an STS score of 2%-10%, which really redefines the concept of intermediate risk, Dr. Thourani said. Results will be presented by early 2017.
• Mitral valve disease: The Evaluation of Outcomes Following Mitral Valve Repair/Replacement in Severe Chronic Ischemic Mitral Regurgitation trial, carried out by the CSTN, will present 2-year outcome data later this year or in early 2016. The 1-year results caused major consternation in the surgical world. The eye opener was that 33% of patients in the repair group had moderate or severe mitral regurgitation at 12 months, compared with just 2% in the replacement group (N. Engl. J. Med. 2014;370:23-32). Mitral valve repair has traditionally been by far the more popular strategy. If the 2-year results show a growing disparity in terms of rates of severe mitral regurgitation, that may change.
Another CSTN study, the Surgical Intervention in Moderate Ischemic Mitral Regurgitation trial, found no demonstrable clinical benefit in adding a mitral valve repair operation to CABG surgery at 1 year of follow-up. The incidence of moderate or severe mitral regurgitation was lower at 1 year with concomitant valve repair and CABG, but this was offset by more neurologic events, longer ICU and total hospital stays, and no differences in the degree of reverse remodeling, mortality, or quality of life (N. Engl. J. Med. 2014;371:2178-88).
“Two-year follow-up is ongoing. It really becomes very important that later this year or next year we’re going to have the results available for you to determine if the lower incidence of moderate or severe mitral regurgitation at 1 year translates into a net clinical benefit for patients undergoing CABG and mitral repair. This study has big implications for the practice of thoracic surgery and for how cardiologists refer patients,” according to Dr. Thourani.
The COAPT trial is randomizing patients with symptomatic functional mitral regurgitation and very high surgical risk to percutaneous catheter-based treatment with the MitraClip or to a standard-care control group. One-year outcomes will be presented in 2016, and follow-up out to 5 years is planned.
“You can see now that in cardiac surgery there’s a lot going on,” Dr. Thourani concluded. “It’s not only good for us, but it’s good for you. As a cardiovascular community, we need to work together more.”
He reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott Medical, Boston Scientific, Medtronic, and Sorin.
SAN DIEGO – The days when cardiologists could look down their noses at cardiac surgeons as primitive when it comes to conducting high-quality clinical research have come and gone.
“Cardiologists have been much more sophisticated than we have in doing randomized controlled trials. But cardiac surgeons are finally making strong progress in conducting randomized clinical trials, an area we’re not typically known for,” Dr. Vinod H. Thourani asserted at the annual meeting of the American College of Cardiology.
Much of this progress can be credited to the relatively recent creation of the Cardiothoracic Surgical Trials Network (CSTN), funded by the U.S. National Institutions of Health and the Canadian Institutes of Health Research. This surgical network is carrying out cutting-edge RCTs that will change the practice of cardiology as well as heart surgery, said Dr. Thourani, professor of surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
To illustrate the breadth of current research in cardiothoracic surgery, he presented thumbnail sketches of RCTs due to report findings as early as this spring and no later than the latter part of next year or early 2017. The trials he selected, some conducted by the CSTN and others with industry sponsorship, address the aortic valve, the mitral valve, postoperative atrial fibrillation, revascularization in patients with unprotected left main coronary artery disease, and advanced heart failure.
• Heart failure: Outcomes of the ENDURANCE destination trial are due to be presented this spring at the annual meeting of the International Society for Heart and Lung Transplantation in Nice, France. This study will compare 12-month outcomes of continuous-flow ventricular assist devices as destination therapy in advanced heart failure. In this HeartWare-sponsored study, 310 patients received the investigational HeartWare ventricular assist system and 155 controls were implanted with the FDA-approved HeartMate II device marketed by Thoratec.
• Atrial fibrillation: The Rate Control Versus Rhythm Control for Postoperative Atrial Fibrillation trial compares the two management strategies in patients with new-onset AF following cardiac surgery. This CSTN-conducted study, to be presented next year, looks at which treatment approach results in fewer days in the hospital, as well as heart rhythm at discharge and through 60 days of follow-up, economic costs, and the incidence of postoperative clinical events.
• Coronary artery disease: The EXCEL trial has randomized 2,600 patients with unprotected left main coronary artery disease to coronary artery bypass surgery or percutaneous intervention with a XIENCE everolimus-eluting stent. The primary outcome is the composite of all-cause mortality, acute MI, or stroke. First results of this Abbott Vascular–sponsored trial are due to be reported next year.“This is a study that will clearly be impactful for you,” Dr. Thourani observed.
• Aortic valve disease: Two major RCTs are looking at the impact of extending transcatheter aortic valve replacement (TAVR) to an intermediate-surgical-risk population of patients with symptomatic severe aortic stenosis. The PARTNER II trial compares transfemoral or transapical/transaortic TAVR with a SAPIEN XT valve to surgical aortic valve replacement in patients with a Society of Thoracic Surgeons mortality risk score of 4%-8%. The primary endpoint is all-cause mortality and disabling stroke at 2 years. Results of this Edwards Lifesciences–sponsored trial will be presented by early 2016.
The Medtronic-sponsored SURTAVI trial compares TAVR using the company’s CoreValve alone or with PCI if revascularization is indicated versus surgical aortic valve replacement alone or with coronary artery bypass grafting if revascularization is indicated. This is a randomized trial involving 2,500 intermediate-risk patients. Of note, the SURTAVI investigators have revised the study protocol to open the trial to patients who are age 75 years or older or have an STS score of 2%-10%, which really redefines the concept of intermediate risk, Dr. Thourani said. Results will be presented by early 2017.
• Mitral valve disease: The Evaluation of Outcomes Following Mitral Valve Repair/Replacement in Severe Chronic Ischemic Mitral Regurgitation trial, carried out by the CSTN, will present 2-year outcome data later this year or in early 2016. The 1-year results caused major consternation in the surgical world. The eye opener was that 33% of patients in the repair group had moderate or severe mitral regurgitation at 12 months, compared with just 2% in the replacement group (N. Engl. J. Med. 2014;370:23-32). Mitral valve repair has traditionally been by far the more popular strategy. If the 2-year results show a growing disparity in terms of rates of severe mitral regurgitation, that may change.
Another CSTN study, the Surgical Intervention in Moderate Ischemic Mitral Regurgitation trial, found no demonstrable clinical benefit in adding a mitral valve repair operation to CABG surgery at 1 year of follow-up. The incidence of moderate or severe mitral regurgitation was lower at 1 year with concomitant valve repair and CABG, but this was offset by more neurologic events, longer ICU and total hospital stays, and no differences in the degree of reverse remodeling, mortality, or quality of life (N. Engl. J. Med. 2014;371:2178-88).
“Two-year follow-up is ongoing. It really becomes very important that later this year or next year we’re going to have the results available for you to determine if the lower incidence of moderate or severe mitral regurgitation at 1 year translates into a net clinical benefit for patients undergoing CABG and mitral repair. This study has big implications for the practice of thoracic surgery and for how cardiologists refer patients,” according to Dr. Thourani.
The COAPT trial is randomizing patients with symptomatic functional mitral regurgitation and very high surgical risk to percutaneous catheter-based treatment with the MitraClip or to a standard-care control group. One-year outcomes will be presented in 2016, and follow-up out to 5 years is planned.
“You can see now that in cardiac surgery there’s a lot going on,” Dr. Thourani concluded. “It’s not only good for us, but it’s good for you. As a cardiovascular community, we need to work together more.”
He reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott Medical, Boston Scientific, Medtronic, and Sorin.
SAN DIEGO – The days when cardiologists could look down their noses at cardiac surgeons as primitive when it comes to conducting high-quality clinical research have come and gone.
“Cardiologists have been much more sophisticated than we have in doing randomized controlled trials. But cardiac surgeons are finally making strong progress in conducting randomized clinical trials, an area we’re not typically known for,” Dr. Vinod H. Thourani asserted at the annual meeting of the American College of Cardiology.
Much of this progress can be credited to the relatively recent creation of the Cardiothoracic Surgical Trials Network (CSTN), funded by the U.S. National Institutions of Health and the Canadian Institutes of Health Research. This surgical network is carrying out cutting-edge RCTs that will change the practice of cardiology as well as heart surgery, said Dr. Thourani, professor of surgery and codirector of the Structural Heart and Valve Center at Emory University, Atlanta.
To illustrate the breadth of current research in cardiothoracic surgery, he presented thumbnail sketches of RCTs due to report findings as early as this spring and no later than the latter part of next year or early 2017. The trials he selected, some conducted by the CSTN and others with industry sponsorship, address the aortic valve, the mitral valve, postoperative atrial fibrillation, revascularization in patients with unprotected left main coronary artery disease, and advanced heart failure.
• Heart failure: Outcomes of the ENDURANCE destination trial are due to be presented this spring at the annual meeting of the International Society for Heart and Lung Transplantation in Nice, France. This study will compare 12-month outcomes of continuous-flow ventricular assist devices as destination therapy in advanced heart failure. In this HeartWare-sponsored study, 310 patients received the investigational HeartWare ventricular assist system and 155 controls were implanted with the FDA-approved HeartMate II device marketed by Thoratec.
• Atrial fibrillation: The Rate Control Versus Rhythm Control for Postoperative Atrial Fibrillation trial compares the two management strategies in patients with new-onset AF following cardiac surgery. This CSTN-conducted study, to be presented next year, looks at which treatment approach results in fewer days in the hospital, as well as heart rhythm at discharge and through 60 days of follow-up, economic costs, and the incidence of postoperative clinical events.
• Coronary artery disease: The EXCEL trial has randomized 2,600 patients with unprotected left main coronary artery disease to coronary artery bypass surgery or percutaneous intervention with a XIENCE everolimus-eluting stent. The primary outcome is the composite of all-cause mortality, acute MI, or stroke. First results of this Abbott Vascular–sponsored trial are due to be reported next year.“This is a study that will clearly be impactful for you,” Dr. Thourani observed.
• Aortic valve disease: Two major RCTs are looking at the impact of extending transcatheter aortic valve replacement (TAVR) to an intermediate-surgical-risk population of patients with symptomatic severe aortic stenosis. The PARTNER II trial compares transfemoral or transapical/transaortic TAVR with a SAPIEN XT valve to surgical aortic valve replacement in patients with a Society of Thoracic Surgeons mortality risk score of 4%-8%. The primary endpoint is all-cause mortality and disabling stroke at 2 years. Results of this Edwards Lifesciences–sponsored trial will be presented by early 2016.
The Medtronic-sponsored SURTAVI trial compares TAVR using the company’s CoreValve alone or with PCI if revascularization is indicated versus surgical aortic valve replacement alone or with coronary artery bypass grafting if revascularization is indicated. This is a randomized trial involving 2,500 intermediate-risk patients. Of note, the SURTAVI investigators have revised the study protocol to open the trial to patients who are age 75 years or older or have an STS score of 2%-10%, which really redefines the concept of intermediate risk, Dr. Thourani said. Results will be presented by early 2017.
• Mitral valve disease: The Evaluation of Outcomes Following Mitral Valve Repair/Replacement in Severe Chronic Ischemic Mitral Regurgitation trial, carried out by the CSTN, will present 2-year outcome data later this year or in early 2016. The 1-year results caused major consternation in the surgical world. The eye opener was that 33% of patients in the repair group had moderate or severe mitral regurgitation at 12 months, compared with just 2% in the replacement group (N. Engl. J. Med. 2014;370:23-32). Mitral valve repair has traditionally been by far the more popular strategy. If the 2-year results show a growing disparity in terms of rates of severe mitral regurgitation, that may change.
Another CSTN study, the Surgical Intervention in Moderate Ischemic Mitral Regurgitation trial, found no demonstrable clinical benefit in adding a mitral valve repair operation to CABG surgery at 1 year of follow-up. The incidence of moderate or severe mitral regurgitation was lower at 1 year with concomitant valve repair and CABG, but this was offset by more neurologic events, longer ICU and total hospital stays, and no differences in the degree of reverse remodeling, mortality, or quality of life (N. Engl. J. Med. 2014;371:2178-88).
“Two-year follow-up is ongoing. It really becomes very important that later this year or next year we’re going to have the results available for you to determine if the lower incidence of moderate or severe mitral regurgitation at 1 year translates into a net clinical benefit for patients undergoing CABG and mitral repair. This study has big implications for the practice of thoracic surgery and for how cardiologists refer patients,” according to Dr. Thourani.
The COAPT trial is randomizing patients with symptomatic functional mitral regurgitation and very high surgical risk to percutaneous catheter-based treatment with the MitraClip or to a standard-care control group. One-year outcomes will be presented in 2016, and follow-up out to 5 years is planned.
“You can see now that in cardiac surgery there’s a lot going on,” Dr. Thourani concluded. “It’s not only good for us, but it’s good for you. As a cardiovascular community, we need to work together more.”
He reported serving as a consultant to Edwards Lifesciences and St. Jude Medical and receiving research grants from Abbott Medical, Boston Scientific, Medtronic, and Sorin.
EXPERT ANALYSIS FROM ACC 15
New melanoma therapies may break the bank
HOUSTON – Newer systemic therapies for metastatic malignant melanoma have resulted in significant gains in survival, but at a cost that may be unsustainable in the near future, according to Dr. Jeffrey E. Gershenwald.
Up to one-half of all expenses related to the treatment of malignant melanoma are accounted for by the care of patients with advanced disease, yet patients with distant metastases (stage IV disease) account for only about 2% of all patients, said Dr. Gershenwald, professor of surgical oncology at the University of Texas M.D. Anderson Cancer Center, Houston.
“How best can we achieve the right therapy for the right patient at the right time, and as we learn more and more about some of the therapies, particularly in melanoma, for the right length of time? We can’t really afford to give treatments in perpetuity, so we need to know how long they actually need to be delivered in order to have optimal value for the patient,” he said at the annual Society of Surgical Oncology Cancer Symposium.
Over the last 3 decades, and particularly over the last 5 years, there have been tremendous forward strides in therapy. In 1975, when dacarbazine became the standard of care for metastatic melanoma, it was associated with response rates of only about 6%-15%, durable responses in only 5%-15% of patients, and a median overall survival of about 6-9 months, Dr. Gershenwald reported.
Treatment toxicities, but not response rates, increased with the introduction of interleukin-2 in 1998, which for want of a better drug became the new preferred treatment.
But with the introduction of new systemic therapies, such as immune checkpoint inhibitors (ipilimumab [Yervoy], nivolumab [Opdivo], and pembrolizumab [Keytruda]) and targeted agents (vemurafenib [Zelboraf], dabrafenib [Tafinlar], and trametinib [Mekinist]), response rates have soared, resulting in an improvement in 1-year survival rates from about 30% to 35% in 1970 to as high as 80% in clinical trials in 2014.
Increased survival, higher costs
Dr. Gershenwald pointed to a recently published cost-effectiveness analysis of treatment strategies for BRAF-mutated metastatic melanoma. In it, the authors noted that vemurafenib costs $13,000 per month, translating into $207,000 for a patient with median survival. Patients for whom vemurafenib fails are often put on ipilimumab, at $150,000 per course.
The authors calculated that the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was nearly $354,993 per quality-adjusted life-year (QALY) gained, a figure that is more than threefold higher than widely accepted thresholds for cost-effective treatment ($50,000-$100,000 per QALY gained).
The ICER for firstline vemurafenib followed by ipilimumab was $158,139, still well above the accepted limits.
The authors of the cost analysis noted that the treatments could become cost effective if drug prices were to drop significantly, or if clinical trials could establish whether it was possible to achieve a durable response without continued therapy.
Going forward, clinicians will need to consider disease burden, including both the extent and growth rate of the disease, as well as the risk of recurrence, in deciding whether to use adjuvant therapies, Dr. Gershenwald said.
In addition, clinical choices will be based on disease biology, predictors of response (although few such predictors currently exist), the likelihood of resistance, and drug toxicities, quality of life, and ease of administration, he said.
Dr. Gershenwald disclosed serving on a Merck advisory board.
HOUSTON – Newer systemic therapies for metastatic malignant melanoma have resulted in significant gains in survival, but at a cost that may be unsustainable in the near future, according to Dr. Jeffrey E. Gershenwald.
Up to one-half of all expenses related to the treatment of malignant melanoma are accounted for by the care of patients with advanced disease, yet patients with distant metastases (stage IV disease) account for only about 2% of all patients, said Dr. Gershenwald, professor of surgical oncology at the University of Texas M.D. Anderson Cancer Center, Houston.
“How best can we achieve the right therapy for the right patient at the right time, and as we learn more and more about some of the therapies, particularly in melanoma, for the right length of time? We can’t really afford to give treatments in perpetuity, so we need to know how long they actually need to be delivered in order to have optimal value for the patient,” he said at the annual Society of Surgical Oncology Cancer Symposium.
Over the last 3 decades, and particularly over the last 5 years, there have been tremendous forward strides in therapy. In 1975, when dacarbazine became the standard of care for metastatic melanoma, it was associated with response rates of only about 6%-15%, durable responses in only 5%-15% of patients, and a median overall survival of about 6-9 months, Dr. Gershenwald reported.
Treatment toxicities, but not response rates, increased with the introduction of interleukin-2 in 1998, which for want of a better drug became the new preferred treatment.
But with the introduction of new systemic therapies, such as immune checkpoint inhibitors (ipilimumab [Yervoy], nivolumab [Opdivo], and pembrolizumab [Keytruda]) and targeted agents (vemurafenib [Zelboraf], dabrafenib [Tafinlar], and trametinib [Mekinist]), response rates have soared, resulting in an improvement in 1-year survival rates from about 30% to 35% in 1970 to as high as 80% in clinical trials in 2014.
Increased survival, higher costs
Dr. Gershenwald pointed to a recently published cost-effectiveness analysis of treatment strategies for BRAF-mutated metastatic melanoma. In it, the authors noted that vemurafenib costs $13,000 per month, translating into $207,000 for a patient with median survival. Patients for whom vemurafenib fails are often put on ipilimumab, at $150,000 per course.
The authors calculated that the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was nearly $354,993 per quality-adjusted life-year (QALY) gained, a figure that is more than threefold higher than widely accepted thresholds for cost-effective treatment ($50,000-$100,000 per QALY gained).
The ICER for firstline vemurafenib followed by ipilimumab was $158,139, still well above the accepted limits.
The authors of the cost analysis noted that the treatments could become cost effective if drug prices were to drop significantly, or if clinical trials could establish whether it was possible to achieve a durable response without continued therapy.
Going forward, clinicians will need to consider disease burden, including both the extent and growth rate of the disease, as well as the risk of recurrence, in deciding whether to use adjuvant therapies, Dr. Gershenwald said.
In addition, clinical choices will be based on disease biology, predictors of response (although few such predictors currently exist), the likelihood of resistance, and drug toxicities, quality of life, and ease of administration, he said.
Dr. Gershenwald disclosed serving on a Merck advisory board.
HOUSTON – Newer systemic therapies for metastatic malignant melanoma have resulted in significant gains in survival, but at a cost that may be unsustainable in the near future, according to Dr. Jeffrey E. Gershenwald.
Up to one-half of all expenses related to the treatment of malignant melanoma are accounted for by the care of patients with advanced disease, yet patients with distant metastases (stage IV disease) account for only about 2% of all patients, said Dr. Gershenwald, professor of surgical oncology at the University of Texas M.D. Anderson Cancer Center, Houston.
“How best can we achieve the right therapy for the right patient at the right time, and as we learn more and more about some of the therapies, particularly in melanoma, for the right length of time? We can’t really afford to give treatments in perpetuity, so we need to know how long they actually need to be delivered in order to have optimal value for the patient,” he said at the annual Society of Surgical Oncology Cancer Symposium.
Over the last 3 decades, and particularly over the last 5 years, there have been tremendous forward strides in therapy. In 1975, when dacarbazine became the standard of care for metastatic melanoma, it was associated with response rates of only about 6%-15%, durable responses in only 5%-15% of patients, and a median overall survival of about 6-9 months, Dr. Gershenwald reported.
Treatment toxicities, but not response rates, increased with the introduction of interleukin-2 in 1998, which for want of a better drug became the new preferred treatment.
But with the introduction of new systemic therapies, such as immune checkpoint inhibitors (ipilimumab [Yervoy], nivolumab [Opdivo], and pembrolizumab [Keytruda]) and targeted agents (vemurafenib [Zelboraf], dabrafenib [Tafinlar], and trametinib [Mekinist]), response rates have soared, resulting in an improvement in 1-year survival rates from about 30% to 35% in 1970 to as high as 80% in clinical trials in 2014.
Increased survival, higher costs
Dr. Gershenwald pointed to a recently published cost-effectiveness analysis of treatment strategies for BRAF-mutated metastatic melanoma. In it, the authors noted that vemurafenib costs $13,000 per month, translating into $207,000 for a patient with median survival. Patients for whom vemurafenib fails are often put on ipilimumab, at $150,000 per course.
The authors calculated that the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was nearly $354,993 per quality-adjusted life-year (QALY) gained, a figure that is more than threefold higher than widely accepted thresholds for cost-effective treatment ($50,000-$100,000 per QALY gained).
The ICER for firstline vemurafenib followed by ipilimumab was $158,139, still well above the accepted limits.
The authors of the cost analysis noted that the treatments could become cost effective if drug prices were to drop significantly, or if clinical trials could establish whether it was possible to achieve a durable response without continued therapy.
Going forward, clinicians will need to consider disease burden, including both the extent and growth rate of the disease, as well as the risk of recurrence, in deciding whether to use adjuvant therapies, Dr. Gershenwald said.
In addition, clinical choices will be based on disease biology, predictors of response (although few such predictors currently exist), the likelihood of resistance, and drug toxicities, quality of life, and ease of administration, he said.
Dr. Gershenwald disclosed serving on a Merck advisory board.
AT SSO 2015
Key clinical point: Immunotherapies and targeted agents for metastatic melanoma are effective but very costly.
Major finding: The incremental cost-effectiveness ratio for vemurafenib, compared with dacarbazine, was nearly $354,993 per quality-adjusted life-year gained.
Data source: A review of data on the efficacy and costs of therapy for metastatic malignant melanoma.
Disclosures: Dr. Gershenwald disclosed serving on a Merck advisory board.
U-tube drainage adds option for necrotizing pancreatitis
CHICAGO – Placement of a U-tube drain provides enhanced percutaneous drainage and minimizes catheter-related complications in patients with complicated or infected necrotizing pancreatitis, new research suggests.
The U-tube method uses a large, 20-French Silastic tube with numerous large holes in the middle. One exit must be anterior through the peritoneal cavity and the other can be posterior in the retroperitoneum, Dr. Daniel E. Abbott said at the annual meeting of the Central Surgical Association.
The novel drainage system allows bidirectional flushing, greater interface with large fluid collections leading to more rapid resolution of retroperitoneal necrosis, and less risk of dislodgement resulting in fewer catheter exchanges or replacements. The system also creates a large-bore fistula tract to fall back on should subsequent fistulojejunostomy be needed, said Dr. Abbott of the University of Cincinnati Medical Center.
He reported on the largest clinical experience with primary U-tube drainage to date, involving 22 patients with necrotizing pancreatitis (NP) treated from 2011 to 2014. In 7 patients, (32%) no surgical procedure was ultimately required.
Of the others, 13 required further surgical intervention for a disrupted duct, and (59%) 2 patients died (9.1% ), Dr. Abbott said. Among eight patients who underwent fistulojejunostomy and five who underwent distal pancreatectomy and/or splenectomy, NP resolved in all but one patient with a recurrent amylase-rich fluid leak.
This compares favorably with a 20% mortality rate and 80% NP resolution among five institutional controls treated with open necrosectomy, he said.
Dr. Abbott acknowledged that the study was limited by small numbers and an insufficient control group for direct comparison.
Other studies have shown mortality rates in NP ranging from 39% with open necrosectomy to 4.5% with focused open necrosectomy, but their median length of stays were 54.5 days and 57 days, respectively. LOS was trimmed to just 19 days in one open necrosectomy study (Ann. Surg. 2008;247:294-9), but mortality was 11.4%, Dr. Abbott observed.
Dr. Abbott’s center is currently placing one to two U-tubes per month in patients with symptomatic NP (nausea, vomiting, weight loss, or infection on radiographic imaging) amenable to drainage and plans to update the analysis with prospectively collected data, including costs, in 2-3 years, he said.
“The U-tube obviously seems to work very well, but ... our radiologists sometimes have a hard time placing just one single tube. That [U-]tube, in particular, has to come anterior and out posterior and other organs can potentially get in the way,” said Dr. Michael Ujiki, director of minimally invasive surgery at NorthShore University Health System, Evanston, Ill.
The morbidity and mortality rates are certainly better, but the improvements could be the result of improved ICU care or getting away from antibiotics, said Dr. Ujiki, a discussant at the meeting.
Dr. Abbott noted that “we are operating on people when they’re generally well instead of operating when they’re generally sick. When you’re doing an open necrosectomy, not only might there be multisystem organ failure, but [patients’] nutritional status is undoubtedly poor as well.”
Radiologists have yet to be unable to place a U-tube, but have delayed placement in stable, normotensive patients until fluid collections get larger to provide a better target, he said. The U-tube also can be placed at separate interventions. The two things patients complain most about are the size of the large tubes and when enzymatic fluid leaks onto their skin if the tube become clogged.
CHICAGO – Placement of a U-tube drain provides enhanced percutaneous drainage and minimizes catheter-related complications in patients with complicated or infected necrotizing pancreatitis, new research suggests.
The U-tube method uses a large, 20-French Silastic tube with numerous large holes in the middle. One exit must be anterior through the peritoneal cavity and the other can be posterior in the retroperitoneum, Dr. Daniel E. Abbott said at the annual meeting of the Central Surgical Association.
The novel drainage system allows bidirectional flushing, greater interface with large fluid collections leading to more rapid resolution of retroperitoneal necrosis, and less risk of dislodgement resulting in fewer catheter exchanges or replacements. The system also creates a large-bore fistula tract to fall back on should subsequent fistulojejunostomy be needed, said Dr. Abbott of the University of Cincinnati Medical Center.
He reported on the largest clinical experience with primary U-tube drainage to date, involving 22 patients with necrotizing pancreatitis (NP) treated from 2011 to 2014. In 7 patients, (32%) no surgical procedure was ultimately required.
Of the others, 13 required further surgical intervention for a disrupted duct, and (59%) 2 patients died (9.1% ), Dr. Abbott said. Among eight patients who underwent fistulojejunostomy and five who underwent distal pancreatectomy and/or splenectomy, NP resolved in all but one patient with a recurrent amylase-rich fluid leak.
This compares favorably with a 20% mortality rate and 80% NP resolution among five institutional controls treated with open necrosectomy, he said.
Dr. Abbott acknowledged that the study was limited by small numbers and an insufficient control group for direct comparison.
Other studies have shown mortality rates in NP ranging from 39% with open necrosectomy to 4.5% with focused open necrosectomy, but their median length of stays were 54.5 days and 57 days, respectively. LOS was trimmed to just 19 days in one open necrosectomy study (Ann. Surg. 2008;247:294-9), but mortality was 11.4%, Dr. Abbott observed.
Dr. Abbott’s center is currently placing one to two U-tubes per month in patients with symptomatic NP (nausea, vomiting, weight loss, or infection on radiographic imaging) amenable to drainage and plans to update the analysis with prospectively collected data, including costs, in 2-3 years, he said.
“The U-tube obviously seems to work very well, but ... our radiologists sometimes have a hard time placing just one single tube. That [U-]tube, in particular, has to come anterior and out posterior and other organs can potentially get in the way,” said Dr. Michael Ujiki, director of minimally invasive surgery at NorthShore University Health System, Evanston, Ill.
The morbidity and mortality rates are certainly better, but the improvements could be the result of improved ICU care or getting away from antibiotics, said Dr. Ujiki, a discussant at the meeting.
Dr. Abbott noted that “we are operating on people when they’re generally well instead of operating when they’re generally sick. When you’re doing an open necrosectomy, not only might there be multisystem organ failure, but [patients’] nutritional status is undoubtedly poor as well.”
Radiologists have yet to be unable to place a U-tube, but have delayed placement in stable, normotensive patients until fluid collections get larger to provide a better target, he said. The U-tube also can be placed at separate interventions. The two things patients complain most about are the size of the large tubes and when enzymatic fluid leaks onto their skin if the tube become clogged.
CHICAGO – Placement of a U-tube drain provides enhanced percutaneous drainage and minimizes catheter-related complications in patients with complicated or infected necrotizing pancreatitis, new research suggests.
The U-tube method uses a large, 20-French Silastic tube with numerous large holes in the middle. One exit must be anterior through the peritoneal cavity and the other can be posterior in the retroperitoneum, Dr. Daniel E. Abbott said at the annual meeting of the Central Surgical Association.
The novel drainage system allows bidirectional flushing, greater interface with large fluid collections leading to more rapid resolution of retroperitoneal necrosis, and less risk of dislodgement resulting in fewer catheter exchanges or replacements. The system also creates a large-bore fistula tract to fall back on should subsequent fistulojejunostomy be needed, said Dr. Abbott of the University of Cincinnati Medical Center.
He reported on the largest clinical experience with primary U-tube drainage to date, involving 22 patients with necrotizing pancreatitis (NP) treated from 2011 to 2014. In 7 patients, (32%) no surgical procedure was ultimately required.
Of the others, 13 required further surgical intervention for a disrupted duct, and (59%) 2 patients died (9.1% ), Dr. Abbott said. Among eight patients who underwent fistulojejunostomy and five who underwent distal pancreatectomy and/or splenectomy, NP resolved in all but one patient with a recurrent amylase-rich fluid leak.
This compares favorably with a 20% mortality rate and 80% NP resolution among five institutional controls treated with open necrosectomy, he said.
Dr. Abbott acknowledged that the study was limited by small numbers and an insufficient control group for direct comparison.
Other studies have shown mortality rates in NP ranging from 39% with open necrosectomy to 4.5% with focused open necrosectomy, but their median length of stays were 54.5 days and 57 days, respectively. LOS was trimmed to just 19 days in one open necrosectomy study (Ann. Surg. 2008;247:294-9), but mortality was 11.4%, Dr. Abbott observed.
Dr. Abbott’s center is currently placing one to two U-tubes per month in patients with symptomatic NP (nausea, vomiting, weight loss, or infection on radiographic imaging) amenable to drainage and plans to update the analysis with prospectively collected data, including costs, in 2-3 years, he said.
“The U-tube obviously seems to work very well, but ... our radiologists sometimes have a hard time placing just one single tube. That [U-]tube, in particular, has to come anterior and out posterior and other organs can potentially get in the way,” said Dr. Michael Ujiki, director of minimally invasive surgery at NorthShore University Health System, Evanston, Ill.
The morbidity and mortality rates are certainly better, but the improvements could be the result of improved ICU care or getting away from antibiotics, said Dr. Ujiki, a discussant at the meeting.
Dr. Abbott noted that “we are operating on people when they’re generally well instead of operating when they’re generally sick. When you’re doing an open necrosectomy, not only might there be multisystem organ failure, but [patients’] nutritional status is undoubtedly poor as well.”
Radiologists have yet to be unable to place a U-tube, but have delayed placement in stable, normotensive patients until fluid collections get larger to provide a better target, he said. The U-tube also can be placed at separate interventions. The two things patients complain most about are the size of the large tubes and when enzymatic fluid leaks onto their skin if the tube become clogged.
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: U-tube drainage may eliminate the need for surgery in severe necrotizing pancreatitis.
Major finding: Disease-specific mortality occurred in 2 of 22 patients.
Data source: Retrospective study of 22 patients with symptomatic and/or infected necrotizing pancreatitis.
Disclosures: The researchers reported having no financial conflicts.
Study: More DVTs than expected in patients who had varicose vein surgeries
A retrospective study of patients who underwent varicose vein surgeries with a tourniquet found a greater incidence of deep vein thromboses (DVTs) than previous studies.
Within the first 3 postoperative days, 113 (7.7%) of the 1,461 patients had DVTs. The researchers also found that DVTs occurred significantly more often in patients with gastrocnemius vein dilation (GVD). A total of 410 (28%) of the study’s participants had GVTs, and the incidence of DVTs was significantly greater in individuals with GVD compared to those without such a symptom. GVD had a higher predictive power for postoperative DVT than did all of the other risk factors examined in univariate and multivariate analyses.
The vast majority of the DVTs diagnosed were isolated distal. While 94 patients suffered from this kind of DVT, the remaining 19 DVTs were proximal. According to Dr. Chen Kai of Wenzhou (China) Medical University, and colleagues, proximal DVTs were nearly always asymptomatic and a larger percentage of them took more time to disappear than did the distal DVTs. Within 6 months following anticoagulant therapy, 94.3% of the distal DVTs exhibited thrombus resolution and 55.6% of the proximal DVTs were thrombus free. None of the study’s participants had died because of DVT or pulmonary embolus during the 6 months following their surgeries.
This study’s “present data reflect a higher incidence of postoperative DVT than previous studies, and we also identify GVD as a significant risk factor. Larger prospective studies will be needed to evaluate this issue precisely and to understand the clinical relevance of these results,” wrote the researchers.Find the full study in Thombosis Research (doi: 10.1016/j.thromres.2015.03.008).
A retrospective study of patients who underwent varicose vein surgeries with a tourniquet found a greater incidence of deep vein thromboses (DVTs) than previous studies.
Within the first 3 postoperative days, 113 (7.7%) of the 1,461 patients had DVTs. The researchers also found that DVTs occurred significantly more often in patients with gastrocnemius vein dilation (GVD). A total of 410 (28%) of the study’s participants had GVTs, and the incidence of DVTs was significantly greater in individuals with GVD compared to those without such a symptom. GVD had a higher predictive power for postoperative DVT than did all of the other risk factors examined in univariate and multivariate analyses.
The vast majority of the DVTs diagnosed were isolated distal. While 94 patients suffered from this kind of DVT, the remaining 19 DVTs were proximal. According to Dr. Chen Kai of Wenzhou (China) Medical University, and colleagues, proximal DVTs were nearly always asymptomatic and a larger percentage of them took more time to disappear than did the distal DVTs. Within 6 months following anticoagulant therapy, 94.3% of the distal DVTs exhibited thrombus resolution and 55.6% of the proximal DVTs were thrombus free. None of the study’s participants had died because of DVT or pulmonary embolus during the 6 months following their surgeries.
This study’s “present data reflect a higher incidence of postoperative DVT than previous studies, and we also identify GVD as a significant risk factor. Larger prospective studies will be needed to evaluate this issue precisely and to understand the clinical relevance of these results,” wrote the researchers.Find the full study in Thombosis Research (doi: 10.1016/j.thromres.2015.03.008).
A retrospective study of patients who underwent varicose vein surgeries with a tourniquet found a greater incidence of deep vein thromboses (DVTs) than previous studies.
Within the first 3 postoperative days, 113 (7.7%) of the 1,461 patients had DVTs. The researchers also found that DVTs occurred significantly more often in patients with gastrocnemius vein dilation (GVD). A total of 410 (28%) of the study’s participants had GVTs, and the incidence of DVTs was significantly greater in individuals with GVD compared to those without such a symptom. GVD had a higher predictive power for postoperative DVT than did all of the other risk factors examined in univariate and multivariate analyses.
The vast majority of the DVTs diagnosed were isolated distal. While 94 patients suffered from this kind of DVT, the remaining 19 DVTs were proximal. According to Dr. Chen Kai of Wenzhou (China) Medical University, and colleagues, proximal DVTs were nearly always asymptomatic and a larger percentage of them took more time to disappear than did the distal DVTs. Within 6 months following anticoagulant therapy, 94.3% of the distal DVTs exhibited thrombus resolution and 55.6% of the proximal DVTs were thrombus free. None of the study’s participants had died because of DVT or pulmonary embolus during the 6 months following their surgeries.
This study’s “present data reflect a higher incidence of postoperative DVT than previous studies, and we also identify GVD as a significant risk factor. Larger prospective studies will be needed to evaluate this issue precisely and to understand the clinical relevance of these results,” wrote the researchers.Find the full study in Thombosis Research (doi: 10.1016/j.thromres.2015.03.008).
VTE with transient risk factors is being overtreated
After a first episode of venous thromboembolism, more than 40% of patients with transient risk factors underwent anticoagulation therapy for 12 months or longer – a duration at least four times longer than the period recommended in guidelines, said authors of a large prospective cohort study.
Patients with VTE associated with surgery had about a 0.7% risk/patient-year of recurrence after 3 months of anticoagulation therapy. Patients with transient nonsurgical risk factors had about a 4% risk/patient-year of VTE recurrence.
Further, these patients were more likely to have major bleeding events than recurrent VTEs and were more likely to die of a fatal bleed than from a recurrent pulmonary embolism. Additionally, 38% of major bleeds among patients with transient risk factors occurred during the first 3 months of anticoagulation therapy.
“Our data suggest that in real life, physicians appear to be more concerned about the risk of recurrent VTE after discontinuing therapy than about the risk of bleeding,” said Dr. Walter Ageno at the University of Insubria in Varese, Italy, and his associates. “Clinicians base their treatment decisions on individual risk stratification, taking into account the location of VTE and the presence of additional risk factors for recurrence and bleeding. However, before adequately validated clinical prediction rules become available, this approach may expose a substantial proportion of patients, in particular those with VTE secondary to transient risk factors, to a possibly unnecessary risk of bleeding.”
The American College of Chest Physicians recommends 3 months of anticoagulation therapy for patients with VTE secondary to surgery or a transient, nonsurgical risk factor, and extended (possibly indefinite) anticoagulation for patients with unprovoked VTE or VTE caused by cancer. To look at real-world practice, the researchers carried out a prospective cohort study of 6,944 VTE patients in Italy, Spain, and Belgium. In all, 32% of patients had transient risk factors, 41% had unprovoked VTE, and 27% had cancer (Thrombosis Res. 2015;135:666-72). After excluding patients who died within a year after VTE, 42% of patients with transient risk factors such as recent surgery, pregnancy, or prolonged travel were treated with anticoagulants for more than 12 months, the researchers reported. Significant predictors of extended anticoagulation treatment including being older than 65 years old, having chronic heart failure, pulmonary embolism at presentation, and recurrent VTE during anticoagulation, the researchers also reported. Patients who weighed less than 75 kg, had anemia, or had transient risk factors for VTE were less likely to undergo prolonged treatment than were other patients.
“There is still uncertainty among experts on the optimal duration of secondary prevention of VTE,” concluded the investigators. “This decision should be taken by balancing the risk of recurrence after stopping treatment with the risk of bleeding if treatment is continued.”
Adequately validated clinical prediction rules are needed to make those decisions, the researchers said. Until such tools are validated, a substantial proportion of patients with transiet and secondary risk factors for VTE may be exposed to a possibly unnecessary risk of bleeding, they concluded.
Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
After a first episode of venous thromboembolism, more than 40% of patients with transient risk factors underwent anticoagulation therapy for 12 months or longer – a duration at least four times longer than the period recommended in guidelines, said authors of a large prospective cohort study.
Patients with VTE associated with surgery had about a 0.7% risk/patient-year of recurrence after 3 months of anticoagulation therapy. Patients with transient nonsurgical risk factors had about a 4% risk/patient-year of VTE recurrence.
Further, these patients were more likely to have major bleeding events than recurrent VTEs and were more likely to die of a fatal bleed than from a recurrent pulmonary embolism. Additionally, 38% of major bleeds among patients with transient risk factors occurred during the first 3 months of anticoagulation therapy.
“Our data suggest that in real life, physicians appear to be more concerned about the risk of recurrent VTE after discontinuing therapy than about the risk of bleeding,” said Dr. Walter Ageno at the University of Insubria in Varese, Italy, and his associates. “Clinicians base their treatment decisions on individual risk stratification, taking into account the location of VTE and the presence of additional risk factors for recurrence and bleeding. However, before adequately validated clinical prediction rules become available, this approach may expose a substantial proportion of patients, in particular those with VTE secondary to transient risk factors, to a possibly unnecessary risk of bleeding.”
The American College of Chest Physicians recommends 3 months of anticoagulation therapy for patients with VTE secondary to surgery or a transient, nonsurgical risk factor, and extended (possibly indefinite) anticoagulation for patients with unprovoked VTE or VTE caused by cancer. To look at real-world practice, the researchers carried out a prospective cohort study of 6,944 VTE patients in Italy, Spain, and Belgium. In all, 32% of patients had transient risk factors, 41% had unprovoked VTE, and 27% had cancer (Thrombosis Res. 2015;135:666-72). After excluding patients who died within a year after VTE, 42% of patients with transient risk factors such as recent surgery, pregnancy, or prolonged travel were treated with anticoagulants for more than 12 months, the researchers reported. Significant predictors of extended anticoagulation treatment including being older than 65 years old, having chronic heart failure, pulmonary embolism at presentation, and recurrent VTE during anticoagulation, the researchers also reported. Patients who weighed less than 75 kg, had anemia, or had transient risk factors for VTE were less likely to undergo prolonged treatment than were other patients.
“There is still uncertainty among experts on the optimal duration of secondary prevention of VTE,” concluded the investigators. “This decision should be taken by balancing the risk of recurrence after stopping treatment with the risk of bleeding if treatment is continued.”
Adequately validated clinical prediction rules are needed to make those decisions, the researchers said. Until such tools are validated, a substantial proportion of patients with transiet and secondary risk factors for VTE may be exposed to a possibly unnecessary risk of bleeding, they concluded.
Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
After a first episode of venous thromboembolism, more than 40% of patients with transient risk factors underwent anticoagulation therapy for 12 months or longer – a duration at least four times longer than the period recommended in guidelines, said authors of a large prospective cohort study.
Patients with VTE associated with surgery had about a 0.7% risk/patient-year of recurrence after 3 months of anticoagulation therapy. Patients with transient nonsurgical risk factors had about a 4% risk/patient-year of VTE recurrence.
Further, these patients were more likely to have major bleeding events than recurrent VTEs and were more likely to die of a fatal bleed than from a recurrent pulmonary embolism. Additionally, 38% of major bleeds among patients with transient risk factors occurred during the first 3 months of anticoagulation therapy.
“Our data suggest that in real life, physicians appear to be more concerned about the risk of recurrent VTE after discontinuing therapy than about the risk of bleeding,” said Dr. Walter Ageno at the University of Insubria in Varese, Italy, and his associates. “Clinicians base their treatment decisions on individual risk stratification, taking into account the location of VTE and the presence of additional risk factors for recurrence and bleeding. However, before adequately validated clinical prediction rules become available, this approach may expose a substantial proportion of patients, in particular those with VTE secondary to transient risk factors, to a possibly unnecessary risk of bleeding.”
The American College of Chest Physicians recommends 3 months of anticoagulation therapy for patients with VTE secondary to surgery or a transient, nonsurgical risk factor, and extended (possibly indefinite) anticoagulation for patients with unprovoked VTE or VTE caused by cancer. To look at real-world practice, the researchers carried out a prospective cohort study of 6,944 VTE patients in Italy, Spain, and Belgium. In all, 32% of patients had transient risk factors, 41% had unprovoked VTE, and 27% had cancer (Thrombosis Res. 2015;135:666-72). After excluding patients who died within a year after VTE, 42% of patients with transient risk factors such as recent surgery, pregnancy, or prolonged travel were treated with anticoagulants for more than 12 months, the researchers reported. Significant predictors of extended anticoagulation treatment including being older than 65 years old, having chronic heart failure, pulmonary embolism at presentation, and recurrent VTE during anticoagulation, the researchers also reported. Patients who weighed less than 75 kg, had anemia, or had transient risk factors for VTE were less likely to undergo prolonged treatment than were other patients.
“There is still uncertainty among experts on the optimal duration of secondary prevention of VTE,” concluded the investigators. “This decision should be taken by balancing the risk of recurrence after stopping treatment with the risk of bleeding if treatment is continued.”
Adequately validated clinical prediction rules are needed to make those decisions, the researchers said. Until such tools are validated, a substantial proportion of patients with transiet and secondary risk factors for VTE may be exposed to a possibly unnecessary risk of bleeding, they concluded.
Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.
FROM THROMBOSIS RESEARCH
Key clinical point: After venous thromboembolism, patients with transient or removable risk factors for VTE often underwent unneeded, prolonged anticoagulation therapy.
Major finding: Of patients with transient VTE risk factors, 42% underwent anticoagulation therapy for 12 months or longer.
Data source: Prospective cohort study of 6,944 patients with VTE.
Disclosures: Sanofi Spain and Bayer Pharma AG funded the study. The investigators reported having no relevant conflicts of interest.