User login
Official Newspaper of the American College of Surgeons
Ablation during mitral valve surgery offers up mixed results
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
SAN DIEGO – Surgical ablation of atrial fibrillation at the time of mitral valve surgery provides significantly greater rhythm control than mitral valve surgery alone, a study showed.
Freedom from atrial fibrillation (AF) at both 6 months and 1 year was 63% in patients undergoing mitral valve surgery (MVS) plus ablation and 29% in those undergoing MVS alone, a statistically significant difference.
However, patients who had ablation plus MVS were 2.5 times more likely to have a permanent pacemaker implanted than were those who had MVS alone, at 21.5% and 8.1%, respectively, also a significant difference.
Ablation did not increase mortality or major adverse cardiac or cerebrovascular events, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Preoperative AF is present in up to 50% of patients undergoing mitral valve operations and is associated with an increased risk of death and stroke.
The study enrolled 260 relatively elderly patients (mean age 69 years) with AF that was persistent (non–self-terminating for at least 7 days) or long-standing persistent (continuous for at least a year), in addition to mitral valve disease. A total of 133 patients were randomly assigned to MVS plus ablation and 127 to MVS alone. The ablation group was further randomized to pulmonary vein isolation or a biatrial maze procedure; all underwent closure of the left atrial appendage.
There was no significant difference in freedom from AF at 6 months and 1 year between patients who had pulmonary vein isolation or a biatrial maze procedure, at 61% and 66%, respectively, said Dr. Gillinov, a cardiac surgeon at Cleveland Clinic.
One-year mortality was similar among all patients undergoing MVS plus ablation vs. MVS alone, at 6.8% and 8.7%.
The two groups also had similar Short Form-12 questionnaire scores for physical function and mental function, although AF occurring at least once daily was significantly less common with ablation, at 19.8%, compared with 45.2% in the MVS-alone patients, he said.
The heart rhythm endpoint was “stringent,” with 3-day Holter monitors obtained at both 6 and 12 months and repeat ablation procedures and death considered treatment failures, Dr. Gillinov said.
He acknowledged that 20% of patients did not have data for the primary endpoint and that the endpoint was not a clinical one, but said a trial with mortality or stroke as the endpoint would require more than 1,000 patients and many years follow-up.
Regarding whether ablation should now be performed routinely, “the glass is half full or half empty,” remarked discussant Dr. Bernard Gersh of Mayo Clinic in Rochester, Minn. “On one hand, you have shown less atrial fibrillation [with ablation], but no effect on quality of life, and the price to be paid was a higher rate of pacemaker implantation,” he said.
The pacemaker implantation rate was higher than expected – 17% in-hospital – and does represent a potential cost, but he would routinely do a maze procedure, Dr. Gillinov said.
Discussant Dr. Alice Jacobs of the Cardiovascular Center at Boston Medical Center, said she expected Dr. Gillinov to say the procedure should not be used in everyone given the lack of benefit in stroke, probably because they tied off the left atrium appendage, and the increase in pacemaker implantations.
About half of the pacemaker implantations were due to atrioventricular block, possibly a consequence of the valve surgery, and one-third to sinus-node dysfunction, which is common in elderly patients, Dr. Gillinov explained.
The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
AT ACC 15
Key clinical point: Surgical ablation of atrial fibrillation during mitral valve surgery decreases AF at 6 months and 1 year, but increases pacemaker implantations.
Major finding: Freedom from AF at both 6 months and 1 year was 63% with mitral valve surgery plus ablation and 29% for MVS alone.
Data source: Prospective, randomized study in 260 patients with persistent or longstanding persistent AF who required mitral valve surgery.
Disclosures: The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research. Dr. Gillinov reported serving as a consultant/speaker for AtriCure, Medtronic, On-X, Edwards, and Tendyne; research funding from St. Jude Medical; an equity interest in Clear Catheter; and that his institution receives royalties from AtriCure for a left atrial appendage occlusion device.
Circumcision, appendectomy most common pediatric surgeries
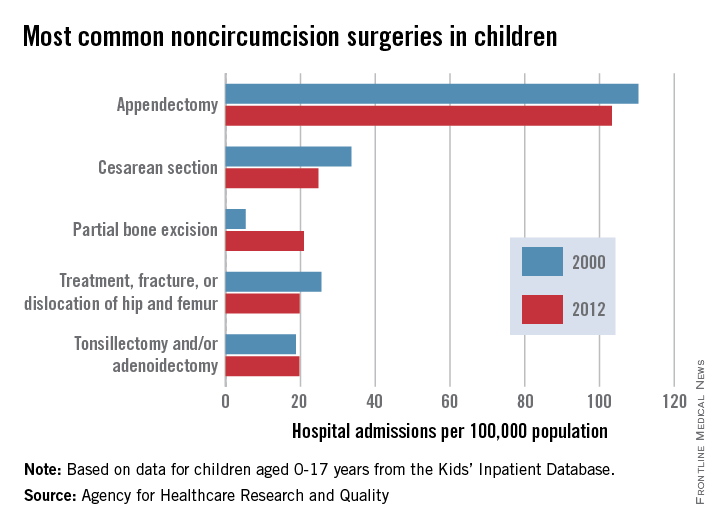
Appendectomies were the most common noncircumcision surgery performed during hospital inpatient stays on children aged 0-17 years in 2012, according to a report from the Agency for Healthcare Research and Quality.
With a rate of 103/100,000 population in 2012, appendectomy procedures were just over four times more common than the next procedure, cesarean section (25/100,000). The procedure rate for partial bone excision was 21/100,000, and treatment of a fractured or dislocated hip or femur and tonsillectomy and/or adenoidectomy were both 20.
The number of cesarean sections, appendectomies, and fractured or dislocated hip or femur surgeries decreased significantly from their 2000 level; tonsillectomy/adenoidectomy procedures remained steady. The number of partial bone excisions increased dramatically, however, rising more than 300% from 2000 to 2012.
Circumcisions were, by far, the most common surgery performed on children, however – a rate of 1,441 hospital stays per 100,000 in 2012, nearly 14 times higher than that of appendectomies. The circumcision rate represents a nearly 10% drop from 2000, though, when it was 1,593/100,000.
The AHRQ report used data collected by the Healthcare Cost and Utilization Project Kids’ Inpatient Database.
|
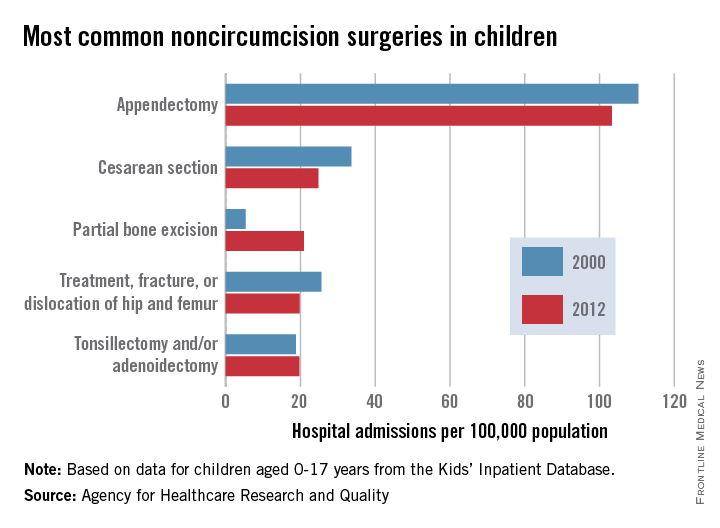
Appendectomies were the most common noncircumcision surgery performed during hospital inpatient stays on children aged 0-17 years in 2012, according to a report from the Agency for Healthcare Research and Quality.
With a rate of 103/100,000 population in 2012, appendectomy procedures were just over four times more common than the next procedure, cesarean section (25/100,000). The procedure rate for partial bone excision was 21/100,000, and treatment of a fractured or dislocated hip or femur and tonsillectomy and/or adenoidectomy were both 20.
The number of cesarean sections, appendectomies, and fractured or dislocated hip or femur surgeries decreased significantly from their 2000 level; tonsillectomy/adenoidectomy procedures remained steady. The number of partial bone excisions increased dramatically, however, rising more than 300% from 2000 to 2012.
Circumcisions were, by far, the most common surgery performed on children, however – a rate of 1,441 hospital stays per 100,000 in 2012, nearly 14 times higher than that of appendectomies. The circumcision rate represents a nearly 10% drop from 2000, though, when it was 1,593/100,000.
The AHRQ report used data collected by the Healthcare Cost and Utilization Project Kids’ Inpatient Database.

|
Appendectomies were the most common noncircumcision surgery performed during hospital inpatient stays on children aged 0-17 years in 2012, according to a report from the Agency for Healthcare Research and Quality.
With a rate of 103/100,000 population in 2012, appendectomy procedures were just over four times more common than the next procedure, cesarean section (25/100,000). The procedure rate for partial bone excision was 21/100,000, and treatment of a fractured or dislocated hip or femur and tonsillectomy and/or adenoidectomy were both 20.
The number of cesarean sections, appendectomies, and fractured or dislocated hip or femur surgeries decreased significantly from their 2000 level; tonsillectomy/adenoidectomy procedures remained steady. The number of partial bone excisions increased dramatically, however, rising more than 300% from 2000 to 2012.
Circumcisions were, by far, the most common surgery performed on children, however – a rate of 1,441 hospital stays per 100,000 in 2012, nearly 14 times higher than that of appendectomies. The circumcision rate represents a nearly 10% drop from 2000, though, when it was 1,593/100,000.
The AHRQ report used data collected by the Healthcare Cost and Utilization Project Kids’ Inpatient Database.

|
Biomarker is linked to rucaparib benefit in ovarian cancer
CHICAGO – Women whose ovarian cancers harbor a BRCA mutation or have a similar genomic signature are most likely to benefit from the investigational agent rucaparib, suggest interim results of a phase II trial reported at the annual meeting of the Society of Gynecologic Oncology.
Investigators assessed activity of this novel oral PARP (poly-ADP-ribose) inhibitor in ARIEL2, A Study of Rucaparib in Patients With Platinum-Sensitive, Relapsed, High-Grade Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. The study was sponsored by Clovis Oncology, manufacturer of rucaparib.
They also tested a biomarker of homologous recombination deficiency (HRD), which confers susceptibility to PARP inhibitors and is commonly seen in BRCA-mutated tumors, said Dr. Elizabeth Swisher, medical director of the breast and ovarian cancer prevention program at the Seattle Cancer Care Alliance and a professor at the University of Washington, Seattle.
Tumors were genomically profiled with next-generation sequencing, which picks up the loss of heterozygosity caused by HRD, whether specifically due to a BRCA mutation or to other molecular aberrations that give tumors a similar genomic signature.
Results in the first 121 evaluable patients showed that 25% had tumors with a BRCA mutation (a percentage capped in the study) and 42% had tumors that lacked such mutation but nonetheless had a BRCA-like signature, while 33% had tumors negative for the biomarker, Dr. Swisher reported.
The response rate to rucaparib according to RECIST (Response Evaluation Criteria In Solid Tumors) was 43% among 61 evaluable patients overall. But it varied significantly by HRD biomarker status: It was 65% in those with BRCA-mutated tumors and 40% in those with a BRCA-like signature, but only 8% in those with tumors negative for the biomarker (P less than .001).
The most common treatment-related adverse events were nausea, fatigue, and transient elevation of liver function tests. None of the patients stopped treatment because of toxicity.
“Rucaparib is active and well tolerated in high-grade ovarian cancer,” summarized Dr. Swisher.
“Comprehensive genomic analysis of the tumor based on a next-generation sequencing platform can prospectively identify ovarian cancer patients who respond to rucaparib, and it identifies both relevant BRCA mutations and a BRCA-like signature in one test,” she said. “The BRCA-like signature could have utility in other cancer types beyond ovarian cancer, potentially.”
She noted that ARIEL2 has recently been expanded to be a registration study for the treatment of patients with ovarian cancer who have had failure of three prior therapies, regardless of BRCA mutational status, and remains open for enrollment.
Investigators with the ARIEL program are refining the HRD biomarker, according to Dr. Swisher. “We will look at different cutoffs to see if we can get an even better cutoff for defining the BRCA-like cases. That biomarker will be locked down and tested prospectively in ARIEL3, which is the pivotal phase III, randomized, placebo-controlled study – recurrent, maintenance, platinum sensitive – that hopefully will be a registration study,” she elaborated.
Dr. Swisher disclosed that she had no relevant conflicts of interest. The trial was sponsored by Clovis Oncology.
CHICAGO – Women whose ovarian cancers harbor a BRCA mutation or have a similar genomic signature are most likely to benefit from the investigational agent rucaparib, suggest interim results of a phase II trial reported at the annual meeting of the Society of Gynecologic Oncology.
Investigators assessed activity of this novel oral PARP (poly-ADP-ribose) inhibitor in ARIEL2, A Study of Rucaparib in Patients With Platinum-Sensitive, Relapsed, High-Grade Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. The study was sponsored by Clovis Oncology, manufacturer of rucaparib.
They also tested a biomarker of homologous recombination deficiency (HRD), which confers susceptibility to PARP inhibitors and is commonly seen in BRCA-mutated tumors, said Dr. Elizabeth Swisher, medical director of the breast and ovarian cancer prevention program at the Seattle Cancer Care Alliance and a professor at the University of Washington, Seattle.
Tumors were genomically profiled with next-generation sequencing, which picks up the loss of heterozygosity caused by HRD, whether specifically due to a BRCA mutation or to other molecular aberrations that give tumors a similar genomic signature.
Results in the first 121 evaluable patients showed that 25% had tumors with a BRCA mutation (a percentage capped in the study) and 42% had tumors that lacked such mutation but nonetheless had a BRCA-like signature, while 33% had tumors negative for the biomarker, Dr. Swisher reported.
The response rate to rucaparib according to RECIST (Response Evaluation Criteria In Solid Tumors) was 43% among 61 evaluable patients overall. But it varied significantly by HRD biomarker status: It was 65% in those with BRCA-mutated tumors and 40% in those with a BRCA-like signature, but only 8% in those with tumors negative for the biomarker (P less than .001).
The most common treatment-related adverse events were nausea, fatigue, and transient elevation of liver function tests. None of the patients stopped treatment because of toxicity.
“Rucaparib is active and well tolerated in high-grade ovarian cancer,” summarized Dr. Swisher.
“Comprehensive genomic analysis of the tumor based on a next-generation sequencing platform can prospectively identify ovarian cancer patients who respond to rucaparib, and it identifies both relevant BRCA mutations and a BRCA-like signature in one test,” she said. “The BRCA-like signature could have utility in other cancer types beyond ovarian cancer, potentially.”
She noted that ARIEL2 has recently been expanded to be a registration study for the treatment of patients with ovarian cancer who have had failure of three prior therapies, regardless of BRCA mutational status, and remains open for enrollment.
Investigators with the ARIEL program are refining the HRD biomarker, according to Dr. Swisher. “We will look at different cutoffs to see if we can get an even better cutoff for defining the BRCA-like cases. That biomarker will be locked down and tested prospectively in ARIEL3, which is the pivotal phase III, randomized, placebo-controlled study – recurrent, maintenance, platinum sensitive – that hopefully will be a registration study,” she elaborated.
Dr. Swisher disclosed that she had no relevant conflicts of interest. The trial was sponsored by Clovis Oncology.
CHICAGO – Women whose ovarian cancers harbor a BRCA mutation or have a similar genomic signature are most likely to benefit from the investigational agent rucaparib, suggest interim results of a phase II trial reported at the annual meeting of the Society of Gynecologic Oncology.
Investigators assessed activity of this novel oral PARP (poly-ADP-ribose) inhibitor in ARIEL2, A Study of Rucaparib in Patients With Platinum-Sensitive, Relapsed, High-Grade Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. The study was sponsored by Clovis Oncology, manufacturer of rucaparib.
They also tested a biomarker of homologous recombination deficiency (HRD), which confers susceptibility to PARP inhibitors and is commonly seen in BRCA-mutated tumors, said Dr. Elizabeth Swisher, medical director of the breast and ovarian cancer prevention program at the Seattle Cancer Care Alliance and a professor at the University of Washington, Seattle.
Tumors were genomically profiled with next-generation sequencing, which picks up the loss of heterozygosity caused by HRD, whether specifically due to a BRCA mutation or to other molecular aberrations that give tumors a similar genomic signature.
Results in the first 121 evaluable patients showed that 25% had tumors with a BRCA mutation (a percentage capped in the study) and 42% had tumors that lacked such mutation but nonetheless had a BRCA-like signature, while 33% had tumors negative for the biomarker, Dr. Swisher reported.
The response rate to rucaparib according to RECIST (Response Evaluation Criteria In Solid Tumors) was 43% among 61 evaluable patients overall. But it varied significantly by HRD biomarker status: It was 65% in those with BRCA-mutated tumors and 40% in those with a BRCA-like signature, but only 8% in those with tumors negative for the biomarker (P less than .001).
The most common treatment-related adverse events were nausea, fatigue, and transient elevation of liver function tests. None of the patients stopped treatment because of toxicity.
“Rucaparib is active and well tolerated in high-grade ovarian cancer,” summarized Dr. Swisher.
“Comprehensive genomic analysis of the tumor based on a next-generation sequencing platform can prospectively identify ovarian cancer patients who respond to rucaparib, and it identifies both relevant BRCA mutations and a BRCA-like signature in one test,” she said. “The BRCA-like signature could have utility in other cancer types beyond ovarian cancer, potentially.”
She noted that ARIEL2 has recently been expanded to be a registration study for the treatment of patients with ovarian cancer who have had failure of three prior therapies, regardless of BRCA mutational status, and remains open for enrollment.
Investigators with the ARIEL program are refining the HRD biomarker, according to Dr. Swisher. “We will look at different cutoffs to see if we can get an even better cutoff for defining the BRCA-like cases. That biomarker will be locked down and tested prospectively in ARIEL3, which is the pivotal phase III, randomized, placebo-controlled study – recurrent, maintenance, platinum sensitive – that hopefully will be a registration study,” she elaborated.
Dr. Swisher disclosed that she had no relevant conflicts of interest. The trial was sponsored by Clovis Oncology.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: A biomarker identified women with ovarian cancer most likely to have a response to rucaparib.
Major finding: The response rate was 65% in women whose tumors had a BRCA mutation and 40% in women whose tumors had a BRCA-like genomic signature.
Data source: An interim analysis of a phase II trial with data from 121 women with high-grade platinum-sensitive ovarian cancer.
Disclosures: Dr. Swisher disclosed that she had no relevant conflicts of interest. The trial was sponsored by Clovis Oncology.
Expert blasts robotic hysterectomy evidence
ORLANDO – The proportion of hysterectomies performed with robotic assistance has been growing steadily in the absence of objective evidence that this approach is superior to alternatives, according to Dr. David Grimes, an ob.gyn. at the University of North Carolina at Chapel Hill.
As part of his keynote lecture at the annual scientific meeting of the Society of Gynecologic Surgeons, Dr. Grimes cautioned surgeons that there are few randomized controlled trials of robotic hysterectomy, and published evidence has not established any use for robotic surgery in gynecology.
“Robotic hysterectomy is an expensive, unproven technology that is associated with a further degradation in the surgical training that our residents now get, and it is replacing the preferred means of hysterectomy, which is vaginal,” said Dr. Grimes.
He pointed to a 2009 committee opinion from the American College of Obstetricians and Gynecologists that cites vaginal hysterectomy as the approach of choice because it is the safest and most cost-effective way to remove a noncancerous uterus (Obstet. Gynecol. 2009;114;1156-8).
Dr. Grimes also cited a recently published joint committee opinion from ACOG and SGS that reviewed data suggesting that robotic hysterectomy has higher costs but no advantage in regard to morbidity, when compared with laparotomy for benign hysterectomies. For gynecologic malignancies, robotic surgery was found less expensive than open hysterectomy across multiple studies because of shorter hospital stays (Obstet. Gynecol. 2015;125:760-7).
Data supporting the benefits of robotic surgery remain sparse, according to Dr. Grimes. He cited a Cochrane Review based on six studies that found there is only “low-quality” evidence on which to conclude that robotic hysterectomy is as safe as conventional laparotomy and only “moderate-quality” evidence that it reduces hospital stays (Cochrane Database Syst. Rev. 2014 Dec. 10;12:CD011422). The authors of the review emphasized that more research is needed.
The United Kingdom’s National Health Service and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists have drawn similar conclusions, according to Dr. Grimes.
But even without clear evidence, the rates of robotic hysterectomy in the United States have been climbing at the same time that rates of vaginal hysterectomy have fallen, said Dr. Grimes, who estimated that approximately 2,500 U.S. hospitals now offer robotic surgery. The growth, he said, is due largely to “aggressive marketing.”
Dr. Grimes proposed that to protect patient safety, the use of robotic surgery should be restricted to formal clinical trial protocols approved by an institutional review board.
“Any use of the robot today off protocol is uncontrolled human experimentation,” he said at the meeting, which is jointly sponsored by the American College of Surgeons.
Dr. Charles R. Rardin, chairman of the SGS Program Committee and director of the robotic surgery program at Women & Infants Hospital of Rhode Island, said “the audience very much appreciated the candor and dedication” expressed by Dr. Grimes for high-quality evidence, but he did not offer a direct endorsement from SGS of the characterization of robotic hysterectomy as experimental.
“SGS has always hosted a vigorous and academic dialogue regarding the pros and cons of robotic surgery,” Dr. Rardin said. “We advocate well-designed research to determine which patients are likely to benefit from robotic surgery. We emphasize that case selection should be based on the best available relevant data as well as expert opinion, and surgical consent should include risks related to the robotic approach.”
Dr. Grimes and Dr. Rardin reported having no relevant financial disclosures.
ORLANDO – The proportion of hysterectomies performed with robotic assistance has been growing steadily in the absence of objective evidence that this approach is superior to alternatives, according to Dr. David Grimes, an ob.gyn. at the University of North Carolina at Chapel Hill.
As part of his keynote lecture at the annual scientific meeting of the Society of Gynecologic Surgeons, Dr. Grimes cautioned surgeons that there are few randomized controlled trials of robotic hysterectomy, and published evidence has not established any use for robotic surgery in gynecology.
“Robotic hysterectomy is an expensive, unproven technology that is associated with a further degradation in the surgical training that our residents now get, and it is replacing the preferred means of hysterectomy, which is vaginal,” said Dr. Grimes.
He pointed to a 2009 committee opinion from the American College of Obstetricians and Gynecologists that cites vaginal hysterectomy as the approach of choice because it is the safest and most cost-effective way to remove a noncancerous uterus (Obstet. Gynecol. 2009;114;1156-8).
Dr. Grimes also cited a recently published joint committee opinion from ACOG and SGS that reviewed data suggesting that robotic hysterectomy has higher costs but no advantage in regard to morbidity, when compared with laparotomy for benign hysterectomies. For gynecologic malignancies, robotic surgery was found less expensive than open hysterectomy across multiple studies because of shorter hospital stays (Obstet. Gynecol. 2015;125:760-7).
Data supporting the benefits of robotic surgery remain sparse, according to Dr. Grimes. He cited a Cochrane Review based on six studies that found there is only “low-quality” evidence on which to conclude that robotic hysterectomy is as safe as conventional laparotomy and only “moderate-quality” evidence that it reduces hospital stays (Cochrane Database Syst. Rev. 2014 Dec. 10;12:CD011422). The authors of the review emphasized that more research is needed.
The United Kingdom’s National Health Service and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists have drawn similar conclusions, according to Dr. Grimes.
But even without clear evidence, the rates of robotic hysterectomy in the United States have been climbing at the same time that rates of vaginal hysterectomy have fallen, said Dr. Grimes, who estimated that approximately 2,500 U.S. hospitals now offer robotic surgery. The growth, he said, is due largely to “aggressive marketing.”
Dr. Grimes proposed that to protect patient safety, the use of robotic surgery should be restricted to formal clinical trial protocols approved by an institutional review board.
“Any use of the robot today off protocol is uncontrolled human experimentation,” he said at the meeting, which is jointly sponsored by the American College of Surgeons.
Dr. Charles R. Rardin, chairman of the SGS Program Committee and director of the robotic surgery program at Women & Infants Hospital of Rhode Island, said “the audience very much appreciated the candor and dedication” expressed by Dr. Grimes for high-quality evidence, but he did not offer a direct endorsement from SGS of the characterization of robotic hysterectomy as experimental.
“SGS has always hosted a vigorous and academic dialogue regarding the pros and cons of robotic surgery,” Dr. Rardin said. “We advocate well-designed research to determine which patients are likely to benefit from robotic surgery. We emphasize that case selection should be based on the best available relevant data as well as expert opinion, and surgical consent should include risks related to the robotic approach.”
Dr. Grimes and Dr. Rardin reported having no relevant financial disclosures.
ORLANDO – The proportion of hysterectomies performed with robotic assistance has been growing steadily in the absence of objective evidence that this approach is superior to alternatives, according to Dr. David Grimes, an ob.gyn. at the University of North Carolina at Chapel Hill.
As part of his keynote lecture at the annual scientific meeting of the Society of Gynecologic Surgeons, Dr. Grimes cautioned surgeons that there are few randomized controlled trials of robotic hysterectomy, and published evidence has not established any use for robotic surgery in gynecology.
“Robotic hysterectomy is an expensive, unproven technology that is associated with a further degradation in the surgical training that our residents now get, and it is replacing the preferred means of hysterectomy, which is vaginal,” said Dr. Grimes.
He pointed to a 2009 committee opinion from the American College of Obstetricians and Gynecologists that cites vaginal hysterectomy as the approach of choice because it is the safest and most cost-effective way to remove a noncancerous uterus (Obstet. Gynecol. 2009;114;1156-8).
Dr. Grimes also cited a recently published joint committee opinion from ACOG and SGS that reviewed data suggesting that robotic hysterectomy has higher costs but no advantage in regard to morbidity, when compared with laparotomy for benign hysterectomies. For gynecologic malignancies, robotic surgery was found less expensive than open hysterectomy across multiple studies because of shorter hospital stays (Obstet. Gynecol. 2015;125:760-7).
Data supporting the benefits of robotic surgery remain sparse, according to Dr. Grimes. He cited a Cochrane Review based on six studies that found there is only “low-quality” evidence on which to conclude that robotic hysterectomy is as safe as conventional laparotomy and only “moderate-quality” evidence that it reduces hospital stays (Cochrane Database Syst. Rev. 2014 Dec. 10;12:CD011422). The authors of the review emphasized that more research is needed.
The United Kingdom’s National Health Service and the Royal Australian and New Zealand College of Obstetricians and Gynaecologists have drawn similar conclusions, according to Dr. Grimes.
But even without clear evidence, the rates of robotic hysterectomy in the United States have been climbing at the same time that rates of vaginal hysterectomy have fallen, said Dr. Grimes, who estimated that approximately 2,500 U.S. hospitals now offer robotic surgery. The growth, he said, is due largely to “aggressive marketing.”
Dr. Grimes proposed that to protect patient safety, the use of robotic surgery should be restricted to formal clinical trial protocols approved by an institutional review board.
“Any use of the robot today off protocol is uncontrolled human experimentation,” he said at the meeting, which is jointly sponsored by the American College of Surgeons.
Dr. Charles R. Rardin, chairman of the SGS Program Committee and director of the robotic surgery program at Women & Infants Hospital of Rhode Island, said “the audience very much appreciated the candor and dedication” expressed by Dr. Grimes for high-quality evidence, but he did not offer a direct endorsement from SGS of the characterization of robotic hysterectomy as experimental.
“SGS has always hosted a vigorous and academic dialogue regarding the pros and cons of robotic surgery,” Dr. Rardin said. “We advocate well-designed research to determine which patients are likely to benefit from robotic surgery. We emphasize that case selection should be based on the best available relevant data as well as expert opinion, and surgical consent should include risks related to the robotic approach.”
Dr. Grimes and Dr. Rardin reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM SGS 2015
Survival similar with bioprosthetic and mechanical valves
Among younger patients who underwent mitral valve replacement, 15-year survival was not significantly different between those who received bioprosthetic valves and those who received mechanical devices, based on data from a retrospective study published online April 14 in JAMA.
However, “there is a tradeoff between the incremental risk of reoperation associated with bioprosthetic valves and the greater long-term risk of stroke and major bleeding with mechanical prosthetic valves,” said Dr. Joanna Chikwe of Mount Sinai Hospital, New York, and her associates.
“Even though [our] findings suggest bioprosthetic valve replacement may be a reasonable alternative to mechanical prosthetic valve replacement in patients aged 50-69 years, the 15-year follow-up was insufficient to fully assess lifetime risks, particularly of reoperation,” the researchers noted (JAMA 2015;313:1435-42 [doi:10.1001/jama.2015.3164]).
The choice between bioprosthetic and mechanical mitral valves is controversial in patients younger than 70 years. Bioprosthetics are much more likely to degenerate over time and require reoperation, but mechanical valves put recipients at increased risk of thromboembolism and hemorrhage and require lifelong anticoagulation. In this age group, the use of bioprosthetic devices has steadily and markedly increased in the past decade, from a small fraction of patients to the majority of patients. Yet until now, no large-scale studies have compared long-term survival and other outcomes between the two valves in this patient population.
Dr. Chikwe and her associates reviewed data from a New York state database of all inpatient hospitalizations. They included 3,433 patients aged 50-69 years at baseline who underwent mitral valve replacement with bioprosthetic (23.2%) or mechanical (76.8%) devices from 1997 through 2007. They also assessed a subset of 664 patient pairs who were propensity matched.
After a median follow-up of 8.2 years (maximum, 16.8 years), there was no significant difference in long-term survival between the two groups. Actuarial 15-year survival was 59.9% with bioprosthetic valves and 57.5% with mechanical devices, the investigators said.
This lack of survival difference “refocuses the emphasis” onto major complications and quality of life, the researchers noted. Regarding these secondary outcomes, the incidence of stroke was significantly higher with mechanical mitral valves (14.0% vs 6.8%) and carried a high mortality (8.5%). The incidence of serious bleeding events also was significantly higher with mechanical valves (14.9% vs 9.0%), and also carried a high (7.4%) mortality. Such risks should “be a major consideration in any discussion of prosthesis choice,” Dr. Chikwe and her associates noted.
Conversely, the incidence of mitral valve reoperation was significantly lower with mechanical devices (5.0% vs 11.1%), and related mortality was 5.3%.
These findings differ from those of recent single-center retrospective series, which reported a long-term survival benefit with mechanical valves in younger patients. Those studies, however, were much smaller and had methodological flaws such as failure to control for competing causes of death, the investigators noted.
This study was supported in part by the Mount Sinai School of Medicine, New York, which receives royalties from Edwards Lifesciences and Medtronic for heart valve devices. Dr. Chikwe reported having no relevant financial disclosures; one of her associates reported ties to Medtronic.
Among younger patients who underwent mitral valve replacement, 15-year survival was not significantly different between those who received bioprosthetic valves and those who received mechanical devices, based on data from a retrospective study published online April 14 in JAMA.
However, “there is a tradeoff between the incremental risk of reoperation associated with bioprosthetic valves and the greater long-term risk of stroke and major bleeding with mechanical prosthetic valves,” said Dr. Joanna Chikwe of Mount Sinai Hospital, New York, and her associates.
“Even though [our] findings suggest bioprosthetic valve replacement may be a reasonable alternative to mechanical prosthetic valve replacement in patients aged 50-69 years, the 15-year follow-up was insufficient to fully assess lifetime risks, particularly of reoperation,” the researchers noted (JAMA 2015;313:1435-42 [doi:10.1001/jama.2015.3164]).
The choice between bioprosthetic and mechanical mitral valves is controversial in patients younger than 70 years. Bioprosthetics are much more likely to degenerate over time and require reoperation, but mechanical valves put recipients at increased risk of thromboembolism and hemorrhage and require lifelong anticoagulation. In this age group, the use of bioprosthetic devices has steadily and markedly increased in the past decade, from a small fraction of patients to the majority of patients. Yet until now, no large-scale studies have compared long-term survival and other outcomes between the two valves in this patient population.
Dr. Chikwe and her associates reviewed data from a New York state database of all inpatient hospitalizations. They included 3,433 patients aged 50-69 years at baseline who underwent mitral valve replacement with bioprosthetic (23.2%) or mechanical (76.8%) devices from 1997 through 2007. They also assessed a subset of 664 patient pairs who were propensity matched.
After a median follow-up of 8.2 years (maximum, 16.8 years), there was no significant difference in long-term survival between the two groups. Actuarial 15-year survival was 59.9% with bioprosthetic valves and 57.5% with mechanical devices, the investigators said.
This lack of survival difference “refocuses the emphasis” onto major complications and quality of life, the researchers noted. Regarding these secondary outcomes, the incidence of stroke was significantly higher with mechanical mitral valves (14.0% vs 6.8%) and carried a high mortality (8.5%). The incidence of serious bleeding events also was significantly higher with mechanical valves (14.9% vs 9.0%), and also carried a high (7.4%) mortality. Such risks should “be a major consideration in any discussion of prosthesis choice,” Dr. Chikwe and her associates noted.
Conversely, the incidence of mitral valve reoperation was significantly lower with mechanical devices (5.0% vs 11.1%), and related mortality was 5.3%.
These findings differ from those of recent single-center retrospective series, which reported a long-term survival benefit with mechanical valves in younger patients. Those studies, however, were much smaller and had methodological flaws such as failure to control for competing causes of death, the investigators noted.
This study was supported in part by the Mount Sinai School of Medicine, New York, which receives royalties from Edwards Lifesciences and Medtronic for heart valve devices. Dr. Chikwe reported having no relevant financial disclosures; one of her associates reported ties to Medtronic.
Among younger patients who underwent mitral valve replacement, 15-year survival was not significantly different between those who received bioprosthetic valves and those who received mechanical devices, based on data from a retrospective study published online April 14 in JAMA.
However, “there is a tradeoff between the incremental risk of reoperation associated with bioprosthetic valves and the greater long-term risk of stroke and major bleeding with mechanical prosthetic valves,” said Dr. Joanna Chikwe of Mount Sinai Hospital, New York, and her associates.
“Even though [our] findings suggest bioprosthetic valve replacement may be a reasonable alternative to mechanical prosthetic valve replacement in patients aged 50-69 years, the 15-year follow-up was insufficient to fully assess lifetime risks, particularly of reoperation,” the researchers noted (JAMA 2015;313:1435-42 [doi:10.1001/jama.2015.3164]).
The choice between bioprosthetic and mechanical mitral valves is controversial in patients younger than 70 years. Bioprosthetics are much more likely to degenerate over time and require reoperation, but mechanical valves put recipients at increased risk of thromboembolism and hemorrhage and require lifelong anticoagulation. In this age group, the use of bioprosthetic devices has steadily and markedly increased in the past decade, from a small fraction of patients to the majority of patients. Yet until now, no large-scale studies have compared long-term survival and other outcomes between the two valves in this patient population.
Dr. Chikwe and her associates reviewed data from a New York state database of all inpatient hospitalizations. They included 3,433 patients aged 50-69 years at baseline who underwent mitral valve replacement with bioprosthetic (23.2%) or mechanical (76.8%) devices from 1997 through 2007. They also assessed a subset of 664 patient pairs who were propensity matched.
After a median follow-up of 8.2 years (maximum, 16.8 years), there was no significant difference in long-term survival between the two groups. Actuarial 15-year survival was 59.9% with bioprosthetic valves and 57.5% with mechanical devices, the investigators said.
This lack of survival difference “refocuses the emphasis” onto major complications and quality of life, the researchers noted. Regarding these secondary outcomes, the incidence of stroke was significantly higher with mechanical mitral valves (14.0% vs 6.8%) and carried a high mortality (8.5%). The incidence of serious bleeding events also was significantly higher with mechanical valves (14.9% vs 9.0%), and also carried a high (7.4%) mortality. Such risks should “be a major consideration in any discussion of prosthesis choice,” Dr. Chikwe and her associates noted.
Conversely, the incidence of mitral valve reoperation was significantly lower with mechanical devices (5.0% vs 11.1%), and related mortality was 5.3%.
These findings differ from those of recent single-center retrospective series, which reported a long-term survival benefit with mechanical valves in younger patients. Those studies, however, were much smaller and had methodological flaws such as failure to control for competing causes of death, the investigators noted.
This study was supported in part by the Mount Sinai School of Medicine, New York, which receives royalties from Edwards Lifesciences and Medtronic for heart valve devices. Dr. Chikwe reported having no relevant financial disclosures; one of her associates reported ties to Medtronic.
FROM JAMA
Key clinical point: 15-year survival was not significantly different between younger patients who received a mechanical mitral valve and those who received a bioprosthetic valve.
Major finding: Actuarial 15-year survival was 59.9% with bioprosthetic valves and 57.5% with mechanical mitral valves.
Data source: A retrospective cohort study comparing long-term outcomes after mitral valve replacement in 3,433 patients aged 50-69 years living in New York.
Disclosures: This study was supported in part by the Mount Sinai School of Medicine, New York, which receives royalties from Edwards Lifesciences and Medtronic for heart valve devices. Dr. Chikwe reported having no relevant financial disclosures; one of her associates reported ties to Medtronic.
Model accurately spots low-risk endometrial cancer
CHICAGO – A preoperative risk model accurately identifies women with endometrial cancer who are unlikely to have lymph node metastases, finds a prospective cohort study reported at the annual Meeting of the Society of Gynecologic Oncology.
The model – which uses favorable MRI features, endometrioid histology on biopsy, and a cancer antigen 125 (CA125) level of 35 U/mL or lower to define a low-risk group – had a negative predictive value of 97% when tested in 529 Asian women.
“Using [our model], we can reliably identify patients with a low risk for lymph node metastasis before surgery,” said Dr. Sokbom Kang, director of the division of gynecologic oncology at the National Cancer Center, Goyang, Korea.
“In the clinic, our preoperative risk assessment may be useful in patient counseling. By sharing this risk information with our patients, we may improve their decision-making process about their surgery,” he added. “Not only does it help patient counseling and surgical planning, but it also may be useful in patient selection for future surgical trials.”
Some guidelines have stopped recommending routine lymphadenectomy in patients with endometrial cancer, according to Dr. Kang. “However, some experts still endorse this procedure, even in low-risk patients. Their argument is, low-risk patients cannot be accurately identified because preoperative tests are inaccurate.”
The risk model was developed by the Korean Gynecologic Oncology group (J. Clin. Oncol. 2012;30:1329-34) and has since been validated in smaller single-nationality cohorts.
In the new study, known as PALME (Preoperative Risk Assessment for Lymph Node Metastasis in Endometrial Cancer), it was tested among consecutive women from 25 hospitals in Korea, Japan, and China who had a histologic diagnosis of endometrial cancer. Those with squamous cell carcinoma or sarcoma histologies were excluded.
The women underwent MRI and CA125 testing in the 4 weeks before surgery. They had surgical staging with pelvic lymphadenectomy, and para-aortic lymphadenectomy was recommended. The median number of nodes removed was 23.
Results showed that the model classified 51% of the patients as having a low risk of lymph node metastases, reported Dr. Kang, who disclosed that he had no relevant conflicts of interest.
On the basis of surgical findings, the model had a negative predictive value of 97%, corresponding to a false-negative predictive rate of just 3%, which was in line with earlier results seen in the smaller validation studies.
In a receiver operating characteristic curve analysis, the model had a summarized sensitivity of 91% and a summarized specificity of 54%.
The performance was similar when tumor grade was substituted for CA125 level, except that specificity decreased significantly. “The lower specificity means fewer patients will benefit from our selective lymphadenectomy strategy, so it impairs the cost-effectiveness of our strategy,” Dr. Kang said.
The model performed similarly as well as a model using features of the primary tumor drawn from the final pathology report. “This proves our preoperative risk assessment has similar accuracy to the postoperative risk assessment for identifying a low risk of lymph node metastasis,” he concluded.
CHICAGO – A preoperative risk model accurately identifies women with endometrial cancer who are unlikely to have lymph node metastases, finds a prospective cohort study reported at the annual Meeting of the Society of Gynecologic Oncology.
The model – which uses favorable MRI features, endometrioid histology on biopsy, and a cancer antigen 125 (CA125) level of 35 U/mL or lower to define a low-risk group – had a negative predictive value of 97% when tested in 529 Asian women.
“Using [our model], we can reliably identify patients with a low risk for lymph node metastasis before surgery,” said Dr. Sokbom Kang, director of the division of gynecologic oncology at the National Cancer Center, Goyang, Korea.
“In the clinic, our preoperative risk assessment may be useful in patient counseling. By sharing this risk information with our patients, we may improve their decision-making process about their surgery,” he added. “Not only does it help patient counseling and surgical planning, but it also may be useful in patient selection for future surgical trials.”
Some guidelines have stopped recommending routine lymphadenectomy in patients with endometrial cancer, according to Dr. Kang. “However, some experts still endorse this procedure, even in low-risk patients. Their argument is, low-risk patients cannot be accurately identified because preoperative tests are inaccurate.”
The risk model was developed by the Korean Gynecologic Oncology group (J. Clin. Oncol. 2012;30:1329-34) and has since been validated in smaller single-nationality cohorts.
In the new study, known as PALME (Preoperative Risk Assessment for Lymph Node Metastasis in Endometrial Cancer), it was tested among consecutive women from 25 hospitals in Korea, Japan, and China who had a histologic diagnosis of endometrial cancer. Those with squamous cell carcinoma or sarcoma histologies were excluded.
The women underwent MRI and CA125 testing in the 4 weeks before surgery. They had surgical staging with pelvic lymphadenectomy, and para-aortic lymphadenectomy was recommended. The median number of nodes removed was 23.
Results showed that the model classified 51% of the patients as having a low risk of lymph node metastases, reported Dr. Kang, who disclosed that he had no relevant conflicts of interest.
On the basis of surgical findings, the model had a negative predictive value of 97%, corresponding to a false-negative predictive rate of just 3%, which was in line with earlier results seen in the smaller validation studies.
In a receiver operating characteristic curve analysis, the model had a summarized sensitivity of 91% and a summarized specificity of 54%.
The performance was similar when tumor grade was substituted for CA125 level, except that specificity decreased significantly. “The lower specificity means fewer patients will benefit from our selective lymphadenectomy strategy, so it impairs the cost-effectiveness of our strategy,” Dr. Kang said.
The model performed similarly as well as a model using features of the primary tumor drawn from the final pathology report. “This proves our preoperative risk assessment has similar accuracy to the postoperative risk assessment for identifying a low risk of lymph node metastasis,” he concluded.
CHICAGO – A preoperative risk model accurately identifies women with endometrial cancer who are unlikely to have lymph node metastases, finds a prospective cohort study reported at the annual Meeting of the Society of Gynecologic Oncology.
The model – which uses favorable MRI features, endometrioid histology on biopsy, and a cancer antigen 125 (CA125) level of 35 U/mL or lower to define a low-risk group – had a negative predictive value of 97% when tested in 529 Asian women.
“Using [our model], we can reliably identify patients with a low risk for lymph node metastasis before surgery,” said Dr. Sokbom Kang, director of the division of gynecologic oncology at the National Cancer Center, Goyang, Korea.
“In the clinic, our preoperative risk assessment may be useful in patient counseling. By sharing this risk information with our patients, we may improve their decision-making process about their surgery,” he added. “Not only does it help patient counseling and surgical planning, but it also may be useful in patient selection for future surgical trials.”
Some guidelines have stopped recommending routine lymphadenectomy in patients with endometrial cancer, according to Dr. Kang. “However, some experts still endorse this procedure, even in low-risk patients. Their argument is, low-risk patients cannot be accurately identified because preoperative tests are inaccurate.”
The risk model was developed by the Korean Gynecologic Oncology group (J. Clin. Oncol. 2012;30:1329-34) and has since been validated in smaller single-nationality cohorts.
In the new study, known as PALME (Preoperative Risk Assessment for Lymph Node Metastasis in Endometrial Cancer), it was tested among consecutive women from 25 hospitals in Korea, Japan, and China who had a histologic diagnosis of endometrial cancer. Those with squamous cell carcinoma or sarcoma histologies were excluded.
The women underwent MRI and CA125 testing in the 4 weeks before surgery. They had surgical staging with pelvic lymphadenectomy, and para-aortic lymphadenectomy was recommended. The median number of nodes removed was 23.
Results showed that the model classified 51% of the patients as having a low risk of lymph node metastases, reported Dr. Kang, who disclosed that he had no relevant conflicts of interest.
On the basis of surgical findings, the model had a negative predictive value of 97%, corresponding to a false-negative predictive rate of just 3%, which was in line with earlier results seen in the smaller validation studies.
In a receiver operating characteristic curve analysis, the model had a summarized sensitivity of 91% and a summarized specificity of 54%.
The performance was similar when tumor grade was substituted for CA125 level, except that specificity decreased significantly. “The lower specificity means fewer patients will benefit from our selective lymphadenectomy strategy, so it impairs the cost-effectiveness of our strategy,” Dr. Kang said.
The model performed similarly as well as a model using features of the primary tumor drawn from the final pathology report. “This proves our preoperative risk assessment has similar accuracy to the postoperative risk assessment for identifying a low risk of lymph node metastasis,” he concluded.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: A preoperative risk model may help identify women with endometrial cancer who can skip lymphadenectomy.
Major finding: The model had a negative predictive value of 97%.
Data source: A prospective cohort study of 529 Asian women with endometrial cancer.
Disclosures: Dr. Kang disclosed that he had no relevant conflicts of interest.
SSIs a factor in postop colon cancer survival
HOUSTON – Surgical-site infections occurring in patients who underwent curative resection for localized colon cancer were associated with worse overall survival in a large retrospective study.
Among nearly 10,000 patients with nonmetastatic colon cancer who underwent surgery with curative intent, surgical-site infections (SSIs) were associated with both worse overall survival and a reduced likelihood of receiving adjuvant chemotherapy, reported Dr. Gala Markia Barden, a surgical resident at Baylor College of Medicine, Houston.
Both SSIs and failure to receive adjuvant chemotherapy are independently associated with worse overall survival, she said at the annual Society of Surgical Oncology Cancer Symposium.
“Future studies and practice guidelines should focus on target areas for improving these potentially preventable problems, including active surveillance for and early recognition of surgical-site infections, as well as vigilant follow-up to ensure treatment completion and to improve the transition between the surgical and medical oncology teams to mitigate losses to follow-up,” she said.
Tapping into the merged Veterans Affairs Surgical Quality Improvement Program and VA Central Cancer Registry (VASQIP-VA) databases, the authors identified 9,946 patients aged 18 years and older who underwent radical resection for colon cancer from 1999 through 2009. Patients with rectal cancers or early postoperative deaths (within 90 days of surgery) were excluded.
The investigators examined the relationships between SSIs and both 5-year overall survival and receipt of adjuvant chemotherapy, which has been documented to improve survival in patients with stage III colon cancer. Delivery of adjuvant chemotherapy in these patients is considered to be a measure of the quality of cancer care, Dr. Barden noted.
Of the 9,946 patients included in the study, 1,340 (13.5%) developed SSIs. These patients were slightly but significantly younger (P < .001), had worse functional status (P = .002), and had higher American Society of Anesthesiologists (ASA) physical status scores (P < .001).
In univariate analysis, the investigators found that, in the entire cohort, SSIs were associated with worse overall survival (OS); in multivariate analysis controlling for sex, nutrition, functional status, ASA score, and number of lymph nodes resected, they saw that SSI was associated with a hazard ratio (HR) for worse overall survival of 1.35 (P < .0001).
When they looked at the association of SSI and OS stratified by cancer stage, however, they found that it was significant only for stage III disease. Patients with stage III who developed an SSI had a median OS of 29 months, compared with 33 months for those with no site infections (P< .001).
Dr. Barden and her associates also found that 42% of patients with infections did not receive adjuvant chemotherapy, compared with 34% of patients without SSIs (P = .002).
To see whether the worse survival among patients with SSI was primarily driven by the failure to deliver chemotherapy, they created a model adjusted for cancer risk factors, which showed that patients with stage III disease who developed an SSI and did not undergo adjuvant chemotherapy had an HR of worse overall survival of 1.59 (P < .0001).
They then added into the model those patients with SSIs who did receive adjuvant therapy but, contrary to their expectations, saw that the HR was only slightly reduced (1.56) and remained significant (P < .0001). The model also confirmed that failure to deliver chemotherapy was associated with worse survival (HR 1.52, P <.0001)
Dr. Barden acknowledged that the study was limited by the retrospective design, predominantly male VA cohort, and the lack of information in the databases about why patients did not receive adjuvant therapy.
The study was internally supported. Dr. Barden reported having no conflicts of interest.
HOUSTON – Surgical-site infections occurring in patients who underwent curative resection for localized colon cancer were associated with worse overall survival in a large retrospective study.
Among nearly 10,000 patients with nonmetastatic colon cancer who underwent surgery with curative intent, surgical-site infections (SSIs) were associated with both worse overall survival and a reduced likelihood of receiving adjuvant chemotherapy, reported Dr. Gala Markia Barden, a surgical resident at Baylor College of Medicine, Houston.
Both SSIs and failure to receive adjuvant chemotherapy are independently associated with worse overall survival, she said at the annual Society of Surgical Oncology Cancer Symposium.
“Future studies and practice guidelines should focus on target areas for improving these potentially preventable problems, including active surveillance for and early recognition of surgical-site infections, as well as vigilant follow-up to ensure treatment completion and to improve the transition between the surgical and medical oncology teams to mitigate losses to follow-up,” she said.
Tapping into the merged Veterans Affairs Surgical Quality Improvement Program and VA Central Cancer Registry (VASQIP-VA) databases, the authors identified 9,946 patients aged 18 years and older who underwent radical resection for colon cancer from 1999 through 2009. Patients with rectal cancers or early postoperative deaths (within 90 days of surgery) were excluded.
The investigators examined the relationships between SSIs and both 5-year overall survival and receipt of adjuvant chemotherapy, which has been documented to improve survival in patients with stage III colon cancer. Delivery of adjuvant chemotherapy in these patients is considered to be a measure of the quality of cancer care, Dr. Barden noted.
Of the 9,946 patients included in the study, 1,340 (13.5%) developed SSIs. These patients were slightly but significantly younger (P < .001), had worse functional status (P = .002), and had higher American Society of Anesthesiologists (ASA) physical status scores (P < .001).
In univariate analysis, the investigators found that, in the entire cohort, SSIs were associated with worse overall survival (OS); in multivariate analysis controlling for sex, nutrition, functional status, ASA score, and number of lymph nodes resected, they saw that SSI was associated with a hazard ratio (HR) for worse overall survival of 1.35 (P < .0001).
When they looked at the association of SSI and OS stratified by cancer stage, however, they found that it was significant only for stage III disease. Patients with stage III who developed an SSI had a median OS of 29 months, compared with 33 months for those with no site infections (P< .001).
Dr. Barden and her associates also found that 42% of patients with infections did not receive adjuvant chemotherapy, compared with 34% of patients without SSIs (P = .002).
To see whether the worse survival among patients with SSI was primarily driven by the failure to deliver chemotherapy, they created a model adjusted for cancer risk factors, which showed that patients with stage III disease who developed an SSI and did not undergo adjuvant chemotherapy had an HR of worse overall survival of 1.59 (P < .0001).
They then added into the model those patients with SSIs who did receive adjuvant therapy but, contrary to their expectations, saw that the HR was only slightly reduced (1.56) and remained significant (P < .0001). The model also confirmed that failure to deliver chemotherapy was associated with worse survival (HR 1.52, P <.0001)
Dr. Barden acknowledged that the study was limited by the retrospective design, predominantly male VA cohort, and the lack of information in the databases about why patients did not receive adjuvant therapy.
The study was internally supported. Dr. Barden reported having no conflicts of interest.
HOUSTON – Surgical-site infections occurring in patients who underwent curative resection for localized colon cancer were associated with worse overall survival in a large retrospective study.
Among nearly 10,000 patients with nonmetastatic colon cancer who underwent surgery with curative intent, surgical-site infections (SSIs) were associated with both worse overall survival and a reduced likelihood of receiving adjuvant chemotherapy, reported Dr. Gala Markia Barden, a surgical resident at Baylor College of Medicine, Houston.
Both SSIs and failure to receive adjuvant chemotherapy are independently associated with worse overall survival, she said at the annual Society of Surgical Oncology Cancer Symposium.
“Future studies and practice guidelines should focus on target areas for improving these potentially preventable problems, including active surveillance for and early recognition of surgical-site infections, as well as vigilant follow-up to ensure treatment completion and to improve the transition between the surgical and medical oncology teams to mitigate losses to follow-up,” she said.
Tapping into the merged Veterans Affairs Surgical Quality Improvement Program and VA Central Cancer Registry (VASQIP-VA) databases, the authors identified 9,946 patients aged 18 years and older who underwent radical resection for colon cancer from 1999 through 2009. Patients with rectal cancers or early postoperative deaths (within 90 days of surgery) were excluded.
The investigators examined the relationships between SSIs and both 5-year overall survival and receipt of adjuvant chemotherapy, which has been documented to improve survival in patients with stage III colon cancer. Delivery of adjuvant chemotherapy in these patients is considered to be a measure of the quality of cancer care, Dr. Barden noted.
Of the 9,946 patients included in the study, 1,340 (13.5%) developed SSIs. These patients were slightly but significantly younger (P < .001), had worse functional status (P = .002), and had higher American Society of Anesthesiologists (ASA) physical status scores (P < .001).
In univariate analysis, the investigators found that, in the entire cohort, SSIs were associated with worse overall survival (OS); in multivariate analysis controlling for sex, nutrition, functional status, ASA score, and number of lymph nodes resected, they saw that SSI was associated with a hazard ratio (HR) for worse overall survival of 1.35 (P < .0001).
When they looked at the association of SSI and OS stratified by cancer stage, however, they found that it was significant only for stage III disease. Patients with stage III who developed an SSI had a median OS of 29 months, compared with 33 months for those with no site infections (P< .001).
Dr. Barden and her associates also found that 42% of patients with infections did not receive adjuvant chemotherapy, compared with 34% of patients without SSIs (P = .002).
To see whether the worse survival among patients with SSI was primarily driven by the failure to deliver chemotherapy, they created a model adjusted for cancer risk factors, which showed that patients with stage III disease who developed an SSI and did not undergo adjuvant chemotherapy had an HR of worse overall survival of 1.59 (P < .0001).
They then added into the model those patients with SSIs who did receive adjuvant therapy but, contrary to their expectations, saw that the HR was only slightly reduced (1.56) and remained significant (P < .0001). The model also confirmed that failure to deliver chemotherapy was associated with worse survival (HR 1.52, P <.0001)
Dr. Barden acknowledged that the study was limited by the retrospective design, predominantly male VA cohort, and the lack of information in the databases about why patients did not receive adjuvant therapy.
The study was internally supported. Dr. Barden reported having no conflicts of interest.
AT SSO 2015
Key clinical point: Surgical site infections in patients with colon cancer are associated with both worse overall survival and lower chance of receiving adjuvant chemotherapy.
Major finding: Median overall survival for stage III patients with SSIs was 29 months, vs. 33 for no SSIs.
Data source: Retrospective cohort study of 9,946 patients who underwent radical colon cancer resection with curative intent.
Disclosures: The study was internally supported. Dr. Barden reported having no conflicts of interest.
Evidence builds for complete revascularization in STEMI
SAN DIEGO– Complete revascularization of multivessel disease in patients hospitalized for ST-segment elevation MI improves long-term outcomes, although PCI of the culprit lesion only remains an option for some, the DANAMI3-PRIMULTI trial results suggest.
At 1 year, fractional flow reserve–guided complete revascularization significantly reduced the risk of all-cause death, nonfatal MI, and repeat revascularization, from 22% with infarct-only percutaneous coronary intervention (PCI) to 13%.
The reduction in the primary composite endpoint, however, was driven only by the need for fewer repeat revascularizations of non–infarct-related artery (IRA) lesions and not by hard endpoints.
“Therefore, although complete revascularization should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy of IRA PCI only,” principal investigator Thomas Engstrøm said at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
Current guidelines support IRA-only PCI, although two contemporary studies – PRAMI and CvLPRIT – suggest that a preventive strategy of revascularization of all lesions in the coronary arteries improves outcomes, he noted.
DANAMI3-PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: Primary PCI in Multivessel Disease) is the largest trial to date to examine this issue.
Investigators at two centers randomized 2,239 patients within 12 hours of STEMI to conventional primary PCI, ischemic postconditioning, or deferred stenting. Among 2,212 patients who had successful infarct-related artery PCI, 627 had multivessel disease and were further randomized to IRA PCI only or fractional flow reserve-guided complete revascularization. Multivessel disease was defined as greater than 50% stenosis in a non-IRA artery greater than 2 mm suitable for PCI.
Nonfatal MI occurred in 5% of patients in both groups, and all-cause death occurred in 4% of IRA-only patients and 5% of complete revascularization patients, reported Dr. Engstrøm, consultant cardiologist, Rigshospitalet, University of Copenhagen.
Ischemia-driven revascularizations were significantly more common in the IRA-only group, at 17%, compared with 5% in complete revascularization group, a significant difference. Notably, 40% of these repeat revascularizations were urgent on the basis of unstable angina, he said.
Panelist Sunil V. Rao, from Duke University in Durham, N.C., asked whether the knowledge that residual disease was left behind in half of the patients in the unblinded trial could potentially bias against the IRA-only arm.
Dr. Engstrøm acknowledged that “there may be a bias when the patients leave the hospital and know they have stenoses that are untreated,” but said great care was taken in the design of the trial because “we wanted to stress quite precisely the wording of the guidelines, which state that repeat revascularization could be either due to subjective or objective ischemia and to answer this question.”
DANAMI3-PRIMULTI’s modest patient population sets the stage for larger, more conclusive studies, but in the meantime will have an interesting impact on the U.S. guideline recommendation, currently a class III recommendation and suggestive of harm for treating additional lesions in the setting of acute MI, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said.
Dr. Kandzari echoed concerns that awareness of residual disease may have influenced the likelihood of repeat revascularizations, but also complimented the investigators on a thoughtful trial design that selected lesions based on clinically significant criteria and allowed non-infarct–related arteries to be treated in a staged fashion rather than mandating treatment at the time of PCI.
There was only one death between the index procedure and the additional PCI and this was caused by a cardiac rupture, and thus merely a result of the disease itself and not the staged approach, Dr. Engstrøm said in an interview.
“Of course, you can argue that full revascularization at the time of the index lesion may support even the IRA territory resulting in smaller final infarcts,” he added. “We find this, however, unlikely since both groups ended up with quite small infarct sequelae: LVEF 50% in both groups.”
Panelist Theodore A. Bass, chief of cardiology at the University of Florida in Jacksonville, said the trial “further confirms emerging data that in the same setting, or at least the same hospitalization, more aggressive treatment may be warranted in certain patients.”
“Our data support that complete revascularization can be done without harm and with a very good outcome,” Dr. Engstrøm said. “So you might argue: Why wait for the readmission either in the case of stable angina or in the indication of unstable angina with a need for urgent PCI?”
Dr. Engstrøm reported having no financial disclosures. Dr. Rao reported consulting fees/honoraria from Terumo Medical and the Medicines Company and research grants from Bellerophon Therapeutics. Dr. Kandzari reported consultant fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and Thoratec. Dr. Bass reported consulting fees/honoraria from Merck.
SAN DIEGO– Complete revascularization of multivessel disease in patients hospitalized for ST-segment elevation MI improves long-term outcomes, although PCI of the culprit lesion only remains an option for some, the DANAMI3-PRIMULTI trial results suggest.
At 1 year, fractional flow reserve–guided complete revascularization significantly reduced the risk of all-cause death, nonfatal MI, and repeat revascularization, from 22% with infarct-only percutaneous coronary intervention (PCI) to 13%.
The reduction in the primary composite endpoint, however, was driven only by the need for fewer repeat revascularizations of non–infarct-related artery (IRA) lesions and not by hard endpoints.
“Therefore, although complete revascularization should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy of IRA PCI only,” principal investigator Thomas Engstrøm said at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
Current guidelines support IRA-only PCI, although two contemporary studies – PRAMI and CvLPRIT – suggest that a preventive strategy of revascularization of all lesions in the coronary arteries improves outcomes, he noted.
DANAMI3-PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: Primary PCI in Multivessel Disease) is the largest trial to date to examine this issue.
Investigators at two centers randomized 2,239 patients within 12 hours of STEMI to conventional primary PCI, ischemic postconditioning, or deferred stenting. Among 2,212 patients who had successful infarct-related artery PCI, 627 had multivessel disease and were further randomized to IRA PCI only or fractional flow reserve-guided complete revascularization. Multivessel disease was defined as greater than 50% stenosis in a non-IRA artery greater than 2 mm suitable for PCI.
Nonfatal MI occurred in 5% of patients in both groups, and all-cause death occurred in 4% of IRA-only patients and 5% of complete revascularization patients, reported Dr. Engstrøm, consultant cardiologist, Rigshospitalet, University of Copenhagen.
Ischemia-driven revascularizations were significantly more common in the IRA-only group, at 17%, compared with 5% in complete revascularization group, a significant difference. Notably, 40% of these repeat revascularizations were urgent on the basis of unstable angina, he said.
Panelist Sunil V. Rao, from Duke University in Durham, N.C., asked whether the knowledge that residual disease was left behind in half of the patients in the unblinded trial could potentially bias against the IRA-only arm.
Dr. Engstrøm acknowledged that “there may be a bias when the patients leave the hospital and know they have stenoses that are untreated,” but said great care was taken in the design of the trial because “we wanted to stress quite precisely the wording of the guidelines, which state that repeat revascularization could be either due to subjective or objective ischemia and to answer this question.”
DANAMI3-PRIMULTI’s modest patient population sets the stage for larger, more conclusive studies, but in the meantime will have an interesting impact on the U.S. guideline recommendation, currently a class III recommendation and suggestive of harm for treating additional lesions in the setting of acute MI, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said.
Dr. Kandzari echoed concerns that awareness of residual disease may have influenced the likelihood of repeat revascularizations, but also complimented the investigators on a thoughtful trial design that selected lesions based on clinically significant criteria and allowed non-infarct–related arteries to be treated in a staged fashion rather than mandating treatment at the time of PCI.
There was only one death between the index procedure and the additional PCI and this was caused by a cardiac rupture, and thus merely a result of the disease itself and not the staged approach, Dr. Engstrøm said in an interview.
“Of course, you can argue that full revascularization at the time of the index lesion may support even the IRA territory resulting in smaller final infarcts,” he added. “We find this, however, unlikely since both groups ended up with quite small infarct sequelae: LVEF 50% in both groups.”
Panelist Theodore A. Bass, chief of cardiology at the University of Florida in Jacksonville, said the trial “further confirms emerging data that in the same setting, or at least the same hospitalization, more aggressive treatment may be warranted in certain patients.”
“Our data support that complete revascularization can be done without harm and with a very good outcome,” Dr. Engstrøm said. “So you might argue: Why wait for the readmission either in the case of stable angina or in the indication of unstable angina with a need for urgent PCI?”
Dr. Engstrøm reported having no financial disclosures. Dr. Rao reported consulting fees/honoraria from Terumo Medical and the Medicines Company and research grants from Bellerophon Therapeutics. Dr. Kandzari reported consultant fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and Thoratec. Dr. Bass reported consulting fees/honoraria from Merck.
SAN DIEGO– Complete revascularization of multivessel disease in patients hospitalized for ST-segment elevation MI improves long-term outcomes, although PCI of the culprit lesion only remains an option for some, the DANAMI3-PRIMULTI trial results suggest.
At 1 year, fractional flow reserve–guided complete revascularization significantly reduced the risk of all-cause death, nonfatal MI, and repeat revascularization, from 22% with infarct-only percutaneous coronary intervention (PCI) to 13%.
The reduction in the primary composite endpoint, however, was driven only by the need for fewer repeat revascularizations of non–infarct-related artery (IRA) lesions and not by hard endpoints.
“Therefore, although complete revascularization should be recommended, any condition that makes complex PCI unattractive may support a more conservative strategy of IRA PCI only,” principal investigator Thomas Engstrøm said at the American College of Cardiology/Cardiovascular Research Foundation Innovation in Intervention Summit.
Current guidelines support IRA-only PCI, although two contemporary studies – PRAMI and CvLPRIT – suggest that a preventive strategy of revascularization of all lesions in the coronary arteries improves outcomes, he noted.
DANAMI3-PRIMULTI (Third Danish Study of Optimal Acute Treatment of Patients With ST-Segment Elevation Myocardial Infarction: Primary PCI in Multivessel Disease) is the largest trial to date to examine this issue.
Investigators at two centers randomized 2,239 patients within 12 hours of STEMI to conventional primary PCI, ischemic postconditioning, or deferred stenting. Among 2,212 patients who had successful infarct-related artery PCI, 627 had multivessel disease and were further randomized to IRA PCI only or fractional flow reserve-guided complete revascularization. Multivessel disease was defined as greater than 50% stenosis in a non-IRA artery greater than 2 mm suitable for PCI.
Nonfatal MI occurred in 5% of patients in both groups, and all-cause death occurred in 4% of IRA-only patients and 5% of complete revascularization patients, reported Dr. Engstrøm, consultant cardiologist, Rigshospitalet, University of Copenhagen.
Ischemia-driven revascularizations were significantly more common in the IRA-only group, at 17%, compared with 5% in complete revascularization group, a significant difference. Notably, 40% of these repeat revascularizations were urgent on the basis of unstable angina, he said.
Panelist Sunil V. Rao, from Duke University in Durham, N.C., asked whether the knowledge that residual disease was left behind in half of the patients in the unblinded trial could potentially bias against the IRA-only arm.
Dr. Engstrøm acknowledged that “there may be a bias when the patients leave the hospital and know they have stenoses that are untreated,” but said great care was taken in the design of the trial because “we wanted to stress quite precisely the wording of the guidelines, which state that repeat revascularization could be either due to subjective or objective ischemia and to answer this question.”
DANAMI3-PRIMULTI’s modest patient population sets the stage for larger, more conclusive studies, but in the meantime will have an interesting impact on the U.S. guideline recommendation, currently a class III recommendation and suggestive of harm for treating additional lesions in the setting of acute MI, Dr. David Kandzari, director of interventional cardiology at the Piedmont Heart Center in Atlanta, said.
Dr. Kandzari echoed concerns that awareness of residual disease may have influenced the likelihood of repeat revascularizations, but also complimented the investigators on a thoughtful trial design that selected lesions based on clinically significant criteria and allowed non-infarct–related arteries to be treated in a staged fashion rather than mandating treatment at the time of PCI.
There was only one death between the index procedure and the additional PCI and this was caused by a cardiac rupture, and thus merely a result of the disease itself and not the staged approach, Dr. Engstrøm said in an interview.
“Of course, you can argue that full revascularization at the time of the index lesion may support even the IRA territory resulting in smaller final infarcts,” he added. “We find this, however, unlikely since both groups ended up with quite small infarct sequelae: LVEF 50% in both groups.”
Panelist Theodore A. Bass, chief of cardiology at the University of Florida in Jacksonville, said the trial “further confirms emerging data that in the same setting, or at least the same hospitalization, more aggressive treatment may be warranted in certain patients.”
“Our data support that complete revascularization can be done without harm and with a very good outcome,” Dr. Engstrøm said. “So you might argue: Why wait for the readmission either in the case of stable angina or in the indication of unstable angina with a need for urgent PCI?”
Dr. Engstrøm reported having no financial disclosures. Dr. Rao reported consulting fees/honoraria from Terumo Medical and the Medicines Company and research grants from Bellerophon Therapeutics. Dr. Kandzari reported consultant fees/honoraria from Boston Scientific, Medtronic, Micell Technologies, and Thoratec. Dr. Bass reported consulting fees/honoraria from Merck.
AT ACC/CRFi2
Key clinical point: Complete revascularization of multivessel disease in patients with STEMI improved long-term outcomes, but may not be the optimal strategy for all.
Major finding: Complete revascularization reduced the risk of the primary endpoint from 22% with infarct-only PCI to 13%.
Data source: Randomized trial in 627 STEMI patients with multivessel disease.
Disclosures: The presenter had no financial disclosures.
CMS formally proposes changes to EHR reporting period for 2015
Physicians and other health care professionals would need to attest to meeting criteria for the meaningful use of electronic health records for 90 days in 2015, under a proposed change announced by the Centers for Medicare & Medicaid Services April 10.
The agency included a number of other changes in the proposed rule, including reducing the attestation period for those new to the meaningful use program in 2015 and 2016 to 90 days. The proposed rule is scheduled to be published April 15 in the Federal Register.
The proposed rule is designed to align the stage 1 and stage 2 meaningful use criteria with the proposed stage 3 criteria issued earlier this year.
Other changes include moving the hospital meaningful use attestation period to a calendar year for 2015, away from the current fiscal year period. This would give hospitals an additional 3 months to attest to meeting the meaningful use criteria in 2015.
The proposed rule also would reduce the number of patients who must access their patient portal from 5% of patients to “equal to or greater than 1.” The measure tracking secure messaging would be changed from a percentage-based measure to attesting that the secure messaging function is “fully enabled.”
Comments on the proposed rule can be made at www.regulations.gov are due June 15.
Physicians and other health care professionals would need to attest to meeting criteria for the meaningful use of electronic health records for 90 days in 2015, under a proposed change announced by the Centers for Medicare & Medicaid Services April 10.
The agency included a number of other changes in the proposed rule, including reducing the attestation period for those new to the meaningful use program in 2015 and 2016 to 90 days. The proposed rule is scheduled to be published April 15 in the Federal Register.
The proposed rule is designed to align the stage 1 and stage 2 meaningful use criteria with the proposed stage 3 criteria issued earlier this year.
Other changes include moving the hospital meaningful use attestation period to a calendar year for 2015, away from the current fiscal year period. This would give hospitals an additional 3 months to attest to meeting the meaningful use criteria in 2015.
The proposed rule also would reduce the number of patients who must access their patient portal from 5% of patients to “equal to or greater than 1.” The measure tracking secure messaging would be changed from a percentage-based measure to attesting that the secure messaging function is “fully enabled.”
Comments on the proposed rule can be made at www.regulations.gov are due June 15.
Physicians and other health care professionals would need to attest to meeting criteria for the meaningful use of electronic health records for 90 days in 2015, under a proposed change announced by the Centers for Medicare & Medicaid Services April 10.
The agency included a number of other changes in the proposed rule, including reducing the attestation period for those new to the meaningful use program in 2015 and 2016 to 90 days. The proposed rule is scheduled to be published April 15 in the Federal Register.
The proposed rule is designed to align the stage 1 and stage 2 meaningful use criteria with the proposed stage 3 criteria issued earlier this year.
Other changes include moving the hospital meaningful use attestation period to a calendar year for 2015, away from the current fiscal year period. This would give hospitals an additional 3 months to attest to meeting the meaningful use criteria in 2015.
The proposed rule also would reduce the number of patients who must access their patient portal from 5% of patients to “equal to or greater than 1.” The measure tracking secure messaging would be changed from a percentage-based measure to attesting that the secure messaging function is “fully enabled.”
Comments on the proposed rule can be made at www.regulations.gov are due June 15.
Analysts predict swift passage of SGR repeal
Despite some lingering objections, observers in Washington expect the Senate will move quickly to pass legislation that repeals the Medicare Sustainable Growth Rate formula.
Some tense moments could happen early in the process as it is expected the Senate will allow amendments to H.R. 2, the Medicare Access and CHIP Reauthorization Act.
The Senate is expected to take up the bill shortly after members return from spring recess on April 13.
They are under the gun to act quickly as the Centers for Medicare & Medicaid Services has been holding claims submitted since the last temporary SGR fix expired on April 1. Unless a legislative fix is completed, the agency will begin to pay those claims – reduced by 21% because of the SGR – on April 15.
The first hurdle the bill faces is a waiving of the rules that require a bill’s costs to be fully offset, something that will require 60 votes. G. William Hoagland, senior vice president at the Bipartisan Policy Center, expects that to be cleared.
Even those who see the bill as being bad for Medicare and for the deficit long term believe the bill will pass.
“I think there are concerns in the Senate about what this means in terms of the long-term deficit, but I suspect that it has enough bipartisan support to patch together the votes it needs,” Michael D. Tanner, senior fellow at the Cato Institute, said in an interview.
Mr. Tanner said that he supports the SGR because it has forced Congress to continually examine and refine the Medicare program .
“The threat of the SGR cuts every couple of years has served to force changes within the Medicare system,” he said. Congress has “had to come up with substitute cuts every time they played with this, and that has had an effect in holding down the growth rate in Medicare and holding down the budget deficit. By removing that threat ... I think in the future they are going to find that the budget deficit increases.”
Dan Mendelson, CEO of the health consultancy AvalereHealth, challenged that assertion. “There’s really nothing good about the SGR policy,” he said in an interview. “It didn’t work to control costs. It didn’t introduce a quality-based payment system, and it’s a relic. I think it’s also fair to say that the discussions around the SGR every year were really not productive in moving the health care system forward.”
Among the amendments that could be offered would be to reauthorize the Children’s Health Insurance Program for 4 years instead of the 2-year reauthorization included in the House bill. Mr. Hoagland said he does not expect any amendments will be passed to alter the current form of H.R. 2, thus allowing it to move directly to the White House. President Obama already has voiced his support of the bill.
Despite some lingering objections, observers in Washington expect the Senate will move quickly to pass legislation that repeals the Medicare Sustainable Growth Rate formula.
Some tense moments could happen early in the process as it is expected the Senate will allow amendments to H.R. 2, the Medicare Access and CHIP Reauthorization Act.
The Senate is expected to take up the bill shortly after members return from spring recess on April 13.
They are under the gun to act quickly as the Centers for Medicare & Medicaid Services has been holding claims submitted since the last temporary SGR fix expired on April 1. Unless a legislative fix is completed, the agency will begin to pay those claims – reduced by 21% because of the SGR – on April 15.
The first hurdle the bill faces is a waiving of the rules that require a bill’s costs to be fully offset, something that will require 60 votes. G. William Hoagland, senior vice president at the Bipartisan Policy Center, expects that to be cleared.
Even those who see the bill as being bad for Medicare and for the deficit long term believe the bill will pass.
“I think there are concerns in the Senate about what this means in terms of the long-term deficit, but I suspect that it has enough bipartisan support to patch together the votes it needs,” Michael D. Tanner, senior fellow at the Cato Institute, said in an interview.
Mr. Tanner said that he supports the SGR because it has forced Congress to continually examine and refine the Medicare program .
“The threat of the SGR cuts every couple of years has served to force changes within the Medicare system,” he said. Congress has “had to come up with substitute cuts every time they played with this, and that has had an effect in holding down the growth rate in Medicare and holding down the budget deficit. By removing that threat ... I think in the future they are going to find that the budget deficit increases.”
Dan Mendelson, CEO of the health consultancy AvalereHealth, challenged that assertion. “There’s really nothing good about the SGR policy,” he said in an interview. “It didn’t work to control costs. It didn’t introduce a quality-based payment system, and it’s a relic. I think it’s also fair to say that the discussions around the SGR every year were really not productive in moving the health care system forward.”
Among the amendments that could be offered would be to reauthorize the Children’s Health Insurance Program for 4 years instead of the 2-year reauthorization included in the House bill. Mr. Hoagland said he does not expect any amendments will be passed to alter the current form of H.R. 2, thus allowing it to move directly to the White House. President Obama already has voiced his support of the bill.
Despite some lingering objections, observers in Washington expect the Senate will move quickly to pass legislation that repeals the Medicare Sustainable Growth Rate formula.
Some tense moments could happen early in the process as it is expected the Senate will allow amendments to H.R. 2, the Medicare Access and CHIP Reauthorization Act.
The Senate is expected to take up the bill shortly after members return from spring recess on April 13.
They are under the gun to act quickly as the Centers for Medicare & Medicaid Services has been holding claims submitted since the last temporary SGR fix expired on April 1. Unless a legislative fix is completed, the agency will begin to pay those claims – reduced by 21% because of the SGR – on April 15.
The first hurdle the bill faces is a waiving of the rules that require a bill’s costs to be fully offset, something that will require 60 votes. G. William Hoagland, senior vice president at the Bipartisan Policy Center, expects that to be cleared.
Even those who see the bill as being bad for Medicare and for the deficit long term believe the bill will pass.
“I think there are concerns in the Senate about what this means in terms of the long-term deficit, but I suspect that it has enough bipartisan support to patch together the votes it needs,” Michael D. Tanner, senior fellow at the Cato Institute, said in an interview.
Mr. Tanner said that he supports the SGR because it has forced Congress to continually examine and refine the Medicare program .
“The threat of the SGR cuts every couple of years has served to force changes within the Medicare system,” he said. Congress has “had to come up with substitute cuts every time they played with this, and that has had an effect in holding down the growth rate in Medicare and holding down the budget deficit. By removing that threat ... I think in the future they are going to find that the budget deficit increases.”
Dan Mendelson, CEO of the health consultancy AvalereHealth, challenged that assertion. “There’s really nothing good about the SGR policy,” he said in an interview. “It didn’t work to control costs. It didn’t introduce a quality-based payment system, and it’s a relic. I think it’s also fair to say that the discussions around the SGR every year were really not productive in moving the health care system forward.”
Among the amendments that could be offered would be to reauthorize the Children’s Health Insurance Program for 4 years instead of the 2-year reauthorization included in the House bill. Mr. Hoagland said he does not expect any amendments will be passed to alter the current form of H.R. 2, thus allowing it to move directly to the White House. President Obama already has voiced his support of the bill.