User login
AHA: Consider obesity as CVD risk factor in children
The American Heart Association has included obesity and severe obesity in its updated scientific statement outlining risk factors and considerations for cardiovascular risk reduction in high-risk pediatric patients.
The scientific statement is an update to a 2006 American Heart Association (AHA) statement, adding details about obesity as an at-risk condition and severe obesity as a moderate-risk condition. Other additions include classifying type 2 diabetes as a high-risk condition and expanding on new risk factors for cardiovascular disease (CVD) among patients who received treatment for childhood cancer.
The AHA said the statement is aimed at pediatric cardiologists, primary care physicians, and subspecialists who care for at-risk pediatric patients, as well as providers who will care for these patients as they transition to adult life.
Obesity
In the AHA scientific statement, Sarah de Ferranti, MD, MPH, of Boston Children’s Hospital, chair of the writing group, and her colleagues, highlighted a 2016 study that identified a twofold to threefold higher risk of CVD-related mortality among patients who were overweight or obese, compared with patients of normal weight (Diabetes Care. 2016 Nov;39[11]:1996-2003).
Patients with obesity and severe obesity are at increased risk of aortic or coronary fatty streaks, dyslipidemia, high blood pressure, hyperglycemia, and insulin resistance, as well as inflammatory and oxidative stress, the AHA writing group noted.
They estimated that approximately 6% of U.S. children aged 2-19 years old are considered severely obese.
After identifying patients with obesity, the writing group said, a “multimodal and graduated approach to treatment” for these patients is generally warranted, with a focus on dietary and lifestyle changes, and use of pharmacotherapy and bariatric surgery if indicated.
However, the authors said therapeutic life change modification “is limited in severe obesity because of small effect size and difficulty with sustainability,” while use of pharmacotherapy for treatment of pediatric obesity remains understudied and medications such as orlistat and metformin offer only modest weight loss.
Bariatric surgery, “the only treatment for severe pediatric obesity consistently associated with clinically meaningful and durable weight loss,” is not consistently offered to patients under 12 years old, they added.
Diabetes
The AHA statement also addresses risks from type 1 (T1D) and type 2 diabetes (T2D). Children with T1D and T2D are at increased risk for dyslipidemia, hypertension, microalbuminuria, and obesity. Annual screening for these patients is indicated, and cardiovascular risk factor reduction can be achieved by managing hyperglycemia, controlling weight gain as a result of medication, and implementing therapeutic lifestyle changes, when possible.
Childhood cancer
As survival rates from childhood cancer have improved, there is a need to address the increased risk of cardiovascular-related mortality (estimated at 8-10 times higher than the general population) as well as cancer relapse, according to the writing group.
Among patients recruited to the Childhood Cancer Survivor Study, there was a 9-fold increase in cerebrovascular accident, 10-fold increased risk of coronary artery disease, and 15-fold increase in heart failure for childhood cancer survivors, compared with their siblings who were cancer free.
Cancer treatments such as radiation exposure are linked to increased rates of myocardial infarction, heart failure, valvular abnormalities, and pericardial disease at a twofold to sixfold higher rate when administered at a greater than 1,500 centigray dose, compared to cancer survivors who did not receive radiation, the authors wrote.
Anthracycline treatment is associated with a dose-dependent increase in the risk of dilated cardiomyopathy, while hematopoietic stem cell transplantation may increase the risk of CVD-related mortality from heart failure, cerebrovascular accident, cardiomyopathy, coronary artery disease, and rhythm disorders.
In treating childhood cancer survivors for CVD risk factors, “a low threshold should be used when considering the initiation of pharmacological agents because of the high risk of these youth,” and standard pharmacotherapies can be used, the authors said. “Treatment of cardiovascular risk factors should consider the cancer therapies the patient has received previously.”
In the AHA statement, Dr. de Ferranti and her colleagues also outlined epidemiology, screening, and treatment data for other cardiovascular risk factors such as familial hypercholesterolemia, Lipoprotein(a), hypertension, chronic kidney disease, congenital heart disease, Kawasaki disease, and heart transplantation.
Some members of the writing group reported research grants from Amgen, Sanofi, the Wisconsin Partnership Program, and the National Institutes of Health. One author reported unpaid consultancies with Novo Nordisk, Orexigen, and Vivus.
SOURCE: de Ferranti SD et al. Circulation. 2019 Feb 25. doi: 10.1161/CIR.0000000000000618.
The American Heart Association has included obesity and severe obesity in its updated scientific statement outlining risk factors and considerations for cardiovascular risk reduction in high-risk pediatric patients.
The scientific statement is an update to a 2006 American Heart Association (AHA) statement, adding details about obesity as an at-risk condition and severe obesity as a moderate-risk condition. Other additions include classifying type 2 diabetes as a high-risk condition and expanding on new risk factors for cardiovascular disease (CVD) among patients who received treatment for childhood cancer.
The AHA said the statement is aimed at pediatric cardiologists, primary care physicians, and subspecialists who care for at-risk pediatric patients, as well as providers who will care for these patients as they transition to adult life.
Obesity
In the AHA scientific statement, Sarah de Ferranti, MD, MPH, of Boston Children’s Hospital, chair of the writing group, and her colleagues, highlighted a 2016 study that identified a twofold to threefold higher risk of CVD-related mortality among patients who were overweight or obese, compared with patients of normal weight (Diabetes Care. 2016 Nov;39[11]:1996-2003).
Patients with obesity and severe obesity are at increased risk of aortic or coronary fatty streaks, dyslipidemia, high blood pressure, hyperglycemia, and insulin resistance, as well as inflammatory and oxidative stress, the AHA writing group noted.
They estimated that approximately 6% of U.S. children aged 2-19 years old are considered severely obese.
After identifying patients with obesity, the writing group said, a “multimodal and graduated approach to treatment” for these patients is generally warranted, with a focus on dietary and lifestyle changes, and use of pharmacotherapy and bariatric surgery if indicated.
However, the authors said therapeutic life change modification “is limited in severe obesity because of small effect size and difficulty with sustainability,” while use of pharmacotherapy for treatment of pediatric obesity remains understudied and medications such as orlistat and metformin offer only modest weight loss.
Bariatric surgery, “the only treatment for severe pediatric obesity consistently associated with clinically meaningful and durable weight loss,” is not consistently offered to patients under 12 years old, they added.
Diabetes
The AHA statement also addresses risks from type 1 (T1D) and type 2 diabetes (T2D). Children with T1D and T2D are at increased risk for dyslipidemia, hypertension, microalbuminuria, and obesity. Annual screening for these patients is indicated, and cardiovascular risk factor reduction can be achieved by managing hyperglycemia, controlling weight gain as a result of medication, and implementing therapeutic lifestyle changes, when possible.
Childhood cancer
As survival rates from childhood cancer have improved, there is a need to address the increased risk of cardiovascular-related mortality (estimated at 8-10 times higher than the general population) as well as cancer relapse, according to the writing group.
Among patients recruited to the Childhood Cancer Survivor Study, there was a 9-fold increase in cerebrovascular accident, 10-fold increased risk of coronary artery disease, and 15-fold increase in heart failure for childhood cancer survivors, compared with their siblings who were cancer free.
Cancer treatments such as radiation exposure are linked to increased rates of myocardial infarction, heart failure, valvular abnormalities, and pericardial disease at a twofold to sixfold higher rate when administered at a greater than 1,500 centigray dose, compared to cancer survivors who did not receive radiation, the authors wrote.
Anthracycline treatment is associated with a dose-dependent increase in the risk of dilated cardiomyopathy, while hematopoietic stem cell transplantation may increase the risk of CVD-related mortality from heart failure, cerebrovascular accident, cardiomyopathy, coronary artery disease, and rhythm disorders.
In treating childhood cancer survivors for CVD risk factors, “a low threshold should be used when considering the initiation of pharmacological agents because of the high risk of these youth,” and standard pharmacotherapies can be used, the authors said. “Treatment of cardiovascular risk factors should consider the cancer therapies the patient has received previously.”
In the AHA statement, Dr. de Ferranti and her colleagues also outlined epidemiology, screening, and treatment data for other cardiovascular risk factors such as familial hypercholesterolemia, Lipoprotein(a), hypertension, chronic kidney disease, congenital heart disease, Kawasaki disease, and heart transplantation.
Some members of the writing group reported research grants from Amgen, Sanofi, the Wisconsin Partnership Program, and the National Institutes of Health. One author reported unpaid consultancies with Novo Nordisk, Orexigen, and Vivus.
SOURCE: de Ferranti SD et al. Circulation. 2019 Feb 25. doi: 10.1161/CIR.0000000000000618.
The American Heart Association has included obesity and severe obesity in its updated scientific statement outlining risk factors and considerations for cardiovascular risk reduction in high-risk pediatric patients.
The scientific statement is an update to a 2006 American Heart Association (AHA) statement, adding details about obesity as an at-risk condition and severe obesity as a moderate-risk condition. Other additions include classifying type 2 diabetes as a high-risk condition and expanding on new risk factors for cardiovascular disease (CVD) among patients who received treatment for childhood cancer.
The AHA said the statement is aimed at pediatric cardiologists, primary care physicians, and subspecialists who care for at-risk pediatric patients, as well as providers who will care for these patients as they transition to adult life.
Obesity
In the AHA scientific statement, Sarah de Ferranti, MD, MPH, of Boston Children’s Hospital, chair of the writing group, and her colleagues, highlighted a 2016 study that identified a twofold to threefold higher risk of CVD-related mortality among patients who were overweight or obese, compared with patients of normal weight (Diabetes Care. 2016 Nov;39[11]:1996-2003).
Patients with obesity and severe obesity are at increased risk of aortic or coronary fatty streaks, dyslipidemia, high blood pressure, hyperglycemia, and insulin resistance, as well as inflammatory and oxidative stress, the AHA writing group noted.
They estimated that approximately 6% of U.S. children aged 2-19 years old are considered severely obese.
After identifying patients with obesity, the writing group said, a “multimodal and graduated approach to treatment” for these patients is generally warranted, with a focus on dietary and lifestyle changes, and use of pharmacotherapy and bariatric surgery if indicated.
However, the authors said therapeutic life change modification “is limited in severe obesity because of small effect size and difficulty with sustainability,” while use of pharmacotherapy for treatment of pediatric obesity remains understudied and medications such as orlistat and metformin offer only modest weight loss.
Bariatric surgery, “the only treatment for severe pediatric obesity consistently associated with clinically meaningful and durable weight loss,” is not consistently offered to patients under 12 years old, they added.
Diabetes
The AHA statement also addresses risks from type 1 (T1D) and type 2 diabetes (T2D). Children with T1D and T2D are at increased risk for dyslipidemia, hypertension, microalbuminuria, and obesity. Annual screening for these patients is indicated, and cardiovascular risk factor reduction can be achieved by managing hyperglycemia, controlling weight gain as a result of medication, and implementing therapeutic lifestyle changes, when possible.
Childhood cancer
As survival rates from childhood cancer have improved, there is a need to address the increased risk of cardiovascular-related mortality (estimated at 8-10 times higher than the general population) as well as cancer relapse, according to the writing group.
Among patients recruited to the Childhood Cancer Survivor Study, there was a 9-fold increase in cerebrovascular accident, 10-fold increased risk of coronary artery disease, and 15-fold increase in heart failure for childhood cancer survivors, compared with their siblings who were cancer free.
Cancer treatments such as radiation exposure are linked to increased rates of myocardial infarction, heart failure, valvular abnormalities, and pericardial disease at a twofold to sixfold higher rate when administered at a greater than 1,500 centigray dose, compared to cancer survivors who did not receive radiation, the authors wrote.
Anthracycline treatment is associated with a dose-dependent increase in the risk of dilated cardiomyopathy, while hematopoietic stem cell transplantation may increase the risk of CVD-related mortality from heart failure, cerebrovascular accident, cardiomyopathy, coronary artery disease, and rhythm disorders.
In treating childhood cancer survivors for CVD risk factors, “a low threshold should be used when considering the initiation of pharmacological agents because of the high risk of these youth,” and standard pharmacotherapies can be used, the authors said. “Treatment of cardiovascular risk factors should consider the cancer therapies the patient has received previously.”
In the AHA statement, Dr. de Ferranti and her colleagues also outlined epidemiology, screening, and treatment data for other cardiovascular risk factors such as familial hypercholesterolemia, Lipoprotein(a), hypertension, chronic kidney disease, congenital heart disease, Kawasaki disease, and heart transplantation.
Some members of the writing group reported research grants from Amgen, Sanofi, the Wisconsin Partnership Program, and the National Institutes of Health. One author reported unpaid consultancies with Novo Nordisk, Orexigen, and Vivus.
SOURCE: de Ferranti SD et al. Circulation. 2019 Feb 25. doi: 10.1161/CIR.0000000000000618.
FROM CIRCULATION
FDA clears first-of-its-kind phone app for insulin management
The Food and Drug Administration has granted 501(k) clearance to a phone app for managing insulin in patients with type 2 diabetes.
The app will enhance Hygieia’s d-Nav Insulin Guidance Service, which uses Cloud-based technology and a small group of health care professionals to support physicians and help patients with diabetes achieve better glycemic control by providing personalized insulin adjustments. The system has been shown, in a 90-day clinical study, to reduce both costs and levels of glycosylated hemoglobin. Patients can use the app to enter glucose-event data and receive a recommended insulin dose.
“Insulin therapy is critical to the health of more than 8 million people in the United States, but it is often ineffective, largely because it requires a continual, personalized adjustment that is not practical for physicians and not manageable for patients. The d-Nav service, including the user-friendly phone app, makes insulin therapy more effective, less time intensive, and less costly for everyone involved,” Eran Bashan, CEO of Hygieia, said in a press release.
This is the first insulin-mangement app that can titrate individualized doses for all insulin regimens and the first that can connect to any glucose meter that shares information with the cloud. It is available for both Android and iOS.
Find the full press release on the Hygieia website.
The Food and Drug Administration has granted 501(k) clearance to a phone app for managing insulin in patients with type 2 diabetes.
The app will enhance Hygieia’s d-Nav Insulin Guidance Service, which uses Cloud-based technology and a small group of health care professionals to support physicians and help patients with diabetes achieve better glycemic control by providing personalized insulin adjustments. The system has been shown, in a 90-day clinical study, to reduce both costs and levels of glycosylated hemoglobin. Patients can use the app to enter glucose-event data and receive a recommended insulin dose.
“Insulin therapy is critical to the health of more than 8 million people in the United States, but it is often ineffective, largely because it requires a continual, personalized adjustment that is not practical for physicians and not manageable for patients. The d-Nav service, including the user-friendly phone app, makes insulin therapy more effective, less time intensive, and less costly for everyone involved,” Eran Bashan, CEO of Hygieia, said in a press release.
This is the first insulin-mangement app that can titrate individualized doses for all insulin regimens and the first that can connect to any glucose meter that shares information with the cloud. It is available for both Android and iOS.
Find the full press release on the Hygieia website.
The Food and Drug Administration has granted 501(k) clearance to a phone app for managing insulin in patients with type 2 diabetes.
The app will enhance Hygieia’s d-Nav Insulin Guidance Service, which uses Cloud-based technology and a small group of health care professionals to support physicians and help patients with diabetes achieve better glycemic control by providing personalized insulin adjustments. The system has been shown, in a 90-day clinical study, to reduce both costs and levels of glycosylated hemoglobin. Patients can use the app to enter glucose-event data and receive a recommended insulin dose.
“Insulin therapy is critical to the health of more than 8 million people in the United States, but it is often ineffective, largely because it requires a continual, personalized adjustment that is not practical for physicians and not manageable for patients. The d-Nav service, including the user-friendly phone app, makes insulin therapy more effective, less time intensive, and less costly for everyone involved,” Eran Bashan, CEO of Hygieia, said in a press release.
This is the first insulin-mangement app that can titrate individualized doses for all insulin regimens and the first that can connect to any glucose meter that shares information with the cloud. It is available for both Android and iOS.
Find the full press release on the Hygieia website.
Diabetic kidney disease, retinopathy associated with PAD in patients with foot ulcers
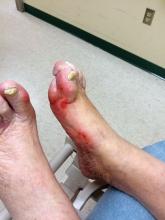
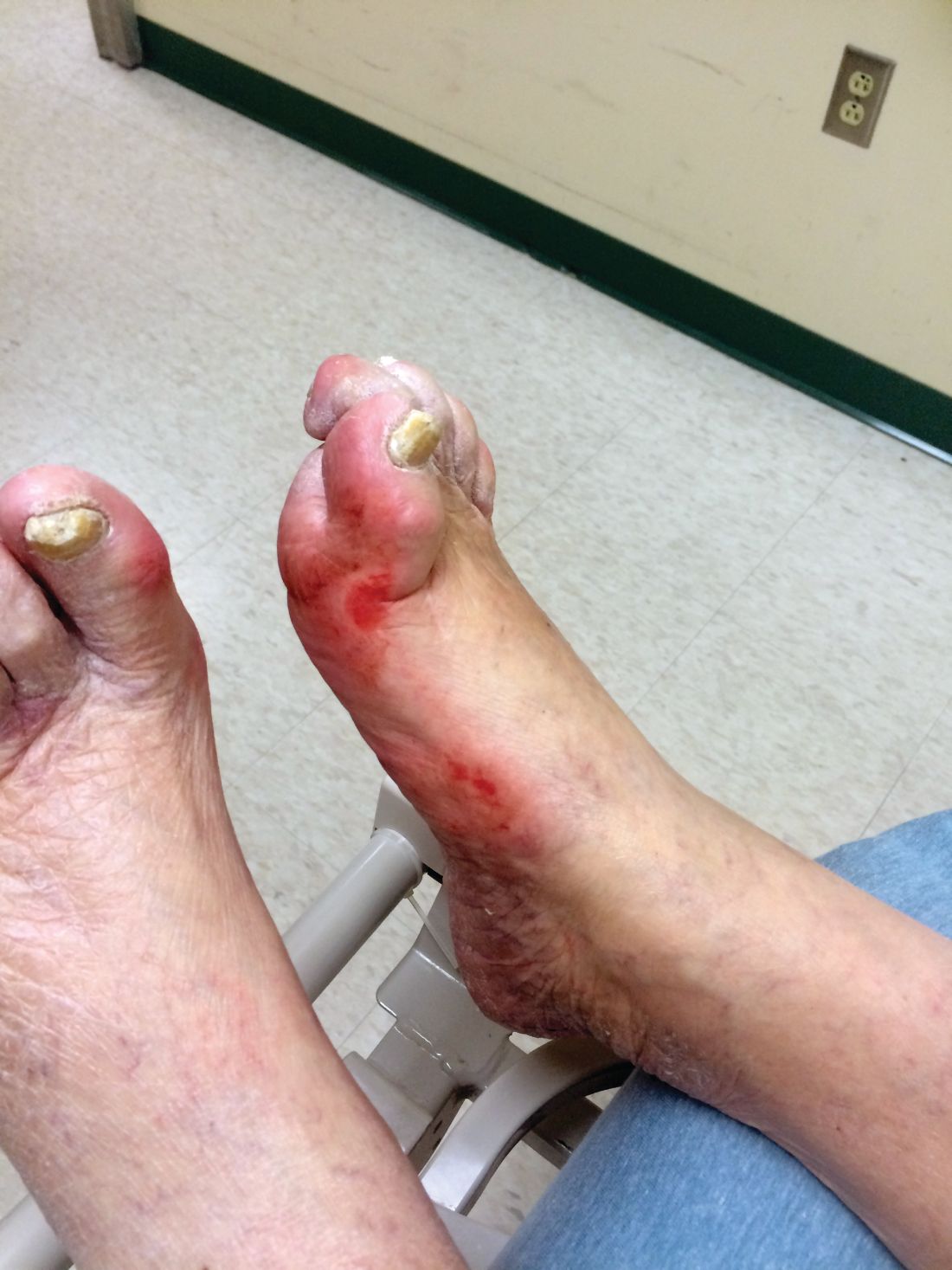
Patients with diabetic foot ulcers have a high incidence of associated chronic vascular disease, including kidney disease, retinopathy, and peripheral artery disease (PAD). In addition, according to a study reported by Magdy H. Megallaa, MD, and colleagues at Alexandria (Egypt) University.
Their cross-sectional study, published in Diabetes & Metabolic Syndrome: Clinical Research and Reviews, comprised 180 type 2 diabetic patients (aged 30-70 years) with diabetic foot ulcers (DFUs) who attended an outpatient clinic between July and December 2017.
The prevalence of diabetic kidney disease and diabetic retinopathy was 86.1% and 90.0%, respectively, with 86.7% of patients having neuropathic DFUs, 11.1% having ischemic DFUs, and 2.2% having neuroischemic DFUs. The prevalence of peripheral neuropathy and PAD was 82% and 20%, respectively.
Using albuminuria as a measure of diabetic kidney disease, the researchers found that 86.1% of the patients had albuminuria and that there was a statistically significant association between albuminuria and the patient’s vibration reception threshold (VPT), a measure of diabetic neuropathy (P less than .001), and the ankle brachial index (ABI), a measure of PAD (P less than .031).
In addition, there was a statistically significant association between diabetic retinopathy and VPT (P less than .008) and between diabetic retinopathy and ABI (P less than .001).
“Albuminuria, diabetic retinopathy and peripheral neuropathy are very common among those patients and strongly associated with risk factors of diabetic foot ulceration,” the researchers concluded.
They reported that they had no conflicts.
SOURCE: Megallaa MH et al. Diabetes Metab Syndr. 2019 Mar-Apr;13(2):1287-92.
Patients with diabetic foot ulcers have a high incidence of associated chronic vascular disease, including kidney disease, retinopathy, and peripheral artery disease (PAD). In addition, according to a study reported by Magdy H. Megallaa, MD, and colleagues at Alexandria (Egypt) University.
Their cross-sectional study, published in Diabetes & Metabolic Syndrome: Clinical Research and Reviews, comprised 180 type 2 diabetic patients (aged 30-70 years) with diabetic foot ulcers (DFUs) who attended an outpatient clinic between July and December 2017.
The prevalence of diabetic kidney disease and diabetic retinopathy was 86.1% and 90.0%, respectively, with 86.7% of patients having neuropathic DFUs, 11.1% having ischemic DFUs, and 2.2% having neuroischemic DFUs. The prevalence of peripheral neuropathy and PAD was 82% and 20%, respectively.
Using albuminuria as a measure of diabetic kidney disease, the researchers found that 86.1% of the patients had albuminuria and that there was a statistically significant association between albuminuria and the patient’s vibration reception threshold (VPT), a measure of diabetic neuropathy (P less than .001), and the ankle brachial index (ABI), a measure of PAD (P less than .031).
In addition, there was a statistically significant association between diabetic retinopathy and VPT (P less than .008) and between diabetic retinopathy and ABI (P less than .001).
“Albuminuria, diabetic retinopathy and peripheral neuropathy are very common among those patients and strongly associated with risk factors of diabetic foot ulceration,” the researchers concluded.
They reported that they had no conflicts.
SOURCE: Megallaa MH et al. Diabetes Metab Syndr. 2019 Mar-Apr;13(2):1287-92.
Patients with diabetic foot ulcers have a high incidence of associated chronic vascular disease, including kidney disease, retinopathy, and peripheral artery disease (PAD). In addition, according to a study reported by Magdy H. Megallaa, MD, and colleagues at Alexandria (Egypt) University.
Their cross-sectional study, published in Diabetes & Metabolic Syndrome: Clinical Research and Reviews, comprised 180 type 2 diabetic patients (aged 30-70 years) with diabetic foot ulcers (DFUs) who attended an outpatient clinic between July and December 2017.
The prevalence of diabetic kidney disease and diabetic retinopathy was 86.1% and 90.0%, respectively, with 86.7% of patients having neuropathic DFUs, 11.1% having ischemic DFUs, and 2.2% having neuroischemic DFUs. The prevalence of peripheral neuropathy and PAD was 82% and 20%, respectively.
Using albuminuria as a measure of diabetic kidney disease, the researchers found that 86.1% of the patients had albuminuria and that there was a statistically significant association between albuminuria and the patient’s vibration reception threshold (VPT), a measure of diabetic neuropathy (P less than .001), and the ankle brachial index (ABI), a measure of PAD (P less than .031).
In addition, there was a statistically significant association between diabetic retinopathy and VPT (P less than .008) and between diabetic retinopathy and ABI (P less than .001).
“Albuminuria, diabetic retinopathy and peripheral neuropathy are very common among those patients and strongly associated with risk factors of diabetic foot ulceration,” the researchers concluded.
They reported that they had no conflicts.
SOURCE: Megallaa MH et al. Diabetes Metab Syndr. 2019 Mar-Apr;13(2):1287-92.
FROM DIABETES & METABOLIC SYNDROME: CLINICAL RESEARCH & REVIEWS
AACE/ACE algorithm provides practical clinical guidance on managing diabetes
Leading endocrinology societies have copublished an algorithm offering updated, specific clinical guidance on lifestyle therapy, management of hypertension and dyslipidemia, and glucose control in patients with type 2 diabetes.
This update from the American Association of Clinical Endocrinologists and the American College of Endocrinology, published in Endocrine Practice, also highlights obesity and prediabetes as underlying risk factors for development of diabetes.
The algorithm, based on new and “comprehensive clinical data” on type 2 diabetes management, is designed as a supplement 2015 AACE/ACE clinical practice guidelines, according to Alan J. Garber, MD, PhD, chair of the Diabetes Management Algorithm Task Force.
“It’s intended to provide clinicians with a practical guide that prompts them to look for factors or influences in the patient’s lifestyle or health that may be a factor in identifying the best treatment approach or medication,” he said in a statement.
Lifestyle medication is critical for all patients with diabetes, according to Dr. Garber and the algorithm coauthors, who recommended a “primarily plant-based meal plan” that limits intake of saturated fatty acids and avoids trans fats. They said overweight patients should restrict caloric intake with a goal of reducing body weight by up to 10%.
Physical activity should include at least 150 minutes per week of activities such as brisk walking or weight training, they said, adding that patients should be advised to sleep 7 hours per night, on average.
Weight loss medications might be needed along with lifestyle modification for patients with body mass index (BMI) over 27 kg/m2 with complications, and for all patients with BMI over 30 regardless of whether they have complications, according to the AACE/ACE committee members who drafted the report.
Bariatric surgery might be considered in patients with BMI over 35 and comorbidities, particularly if patients fail to achieve weight loss goals using other means, they added.
The primary goal of prediabetes management is weight loss, wrote the authors. While there are no Food and Drug Administration–approved agents for prediabetes management, they said, antihyperglycemic agents such as metformin and acarbose have been shown to reduce risk of diabetes by 25%-30% in patients with prediabetes.
While pressure control needs to be individualized, but a goal of less than 130/80 mm Hg is warranted for most patients with diabetes, according to the authors, who note that most patients will require medication to reach their goal.
“Because angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers can slow progression of nephropathy and retinopathy, they are preferred for patients with type 2 diabetes,” said Dr. Garber and his coauthors in the executive summary accompanying the algorithm.
Early and intensive management of dyslipidemia is important to reduce the significant risk of atherosclerotic cardiovascular disease in patients with diabetes, according to the authors, who say diabetes patients should be classified as high risk, very high risk, or extreme risk. They recommended LDL cholesterol targets of less than 100 mg/dL for high-risk patients, less than 70 mg/dL for very-high-risk patients, and less than 55 mg/dL for the extreme-risk group.
Statins should be considered first-line treatment for lowering cholesterol, unless contraindicated, with other lipid-modifying agents added as needed to reach lipid targets.
Inhibitors of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) address a “large unmet need” for more aggressive lipid lowering in patients with clinical atherosclerotic disease and diabetes, the authors noted.
Added to maximal statin therapy, PCSK9 inhibitors reduce LDL cholesterol by about 50% while also raising HDL cholesterol and having positive effects on other lipids, according to the authors.
Pharmacotherapy for type 2 diabetes requires a “nuanced approach” that takes into account factors such as age, comorbidities, and risk of hypoglycemia, the authors wrote, noting that the AACE supports a hemoglobin A1c target of 6.5% or less for most patients.
The algorithm for glycemic control lists glucose-lowering agents in order of recommended usage. For example, in patients with an entry HbA1c less than 7.5%, the strongest recommendations for were monotherapy with metformin, followed by GLP1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
If insulin becomes necessary, the recommended approach is to add a single daily dose of basal insulin, and if a basal insulin regimen fails to control glucose, it may help to add a GLP1 receptor agonist or dipeptidyl peptidase 4 (DPP4) inhibitor, according to the algorithm.
Avoiding hypoglycemia is important, and one possible “safety measure” to prevent that is using a continuous glucose monitoring device that provides real-time glucose data. “Significant advances have been made in accuracy and availability of CGM devices,” the authors wrote.
Current expert consensus is that clinical CGM devices should be considered if patients have not achieved their glycemic target after 3 months or if they need a treatment that puts them at risk for hypoglycemia, according to Dr. Garber and his colleagues.
Dr. Garber reported that he had no financial relationships relevant to the consensus statement and algorithm. Coauthors of the report provided disclosures related to Novo Nordisk, Eli Lilly, Janssen Pharmaceuticals, Abbott, Sanofi-Aventis, and other pharmaceutical companies.
SOURCE: Garber AJ et al. Endocr Pract. 2019 Jan;25(1):91-120.
Leading endocrinology societies have copublished an algorithm offering updated, specific clinical guidance on lifestyle therapy, management of hypertension and dyslipidemia, and glucose control in patients with type 2 diabetes.
This update from the American Association of Clinical Endocrinologists and the American College of Endocrinology, published in Endocrine Practice, also highlights obesity and prediabetes as underlying risk factors for development of diabetes.
The algorithm, based on new and “comprehensive clinical data” on type 2 diabetes management, is designed as a supplement 2015 AACE/ACE clinical practice guidelines, according to Alan J. Garber, MD, PhD, chair of the Diabetes Management Algorithm Task Force.
“It’s intended to provide clinicians with a practical guide that prompts them to look for factors or influences in the patient’s lifestyle or health that may be a factor in identifying the best treatment approach or medication,” he said in a statement.
Lifestyle medication is critical for all patients with diabetes, according to Dr. Garber and the algorithm coauthors, who recommended a “primarily plant-based meal plan” that limits intake of saturated fatty acids and avoids trans fats. They said overweight patients should restrict caloric intake with a goal of reducing body weight by up to 10%.
Physical activity should include at least 150 minutes per week of activities such as brisk walking or weight training, they said, adding that patients should be advised to sleep 7 hours per night, on average.
Weight loss medications might be needed along with lifestyle modification for patients with body mass index (BMI) over 27 kg/m2 with complications, and for all patients with BMI over 30 regardless of whether they have complications, according to the AACE/ACE committee members who drafted the report.
Bariatric surgery might be considered in patients with BMI over 35 and comorbidities, particularly if patients fail to achieve weight loss goals using other means, they added.
The primary goal of prediabetes management is weight loss, wrote the authors. While there are no Food and Drug Administration–approved agents for prediabetes management, they said, antihyperglycemic agents such as metformin and acarbose have been shown to reduce risk of diabetes by 25%-30% in patients with prediabetes.
While pressure control needs to be individualized, but a goal of less than 130/80 mm Hg is warranted for most patients with diabetes, according to the authors, who note that most patients will require medication to reach their goal.
“Because angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers can slow progression of nephropathy and retinopathy, they are preferred for patients with type 2 diabetes,” said Dr. Garber and his coauthors in the executive summary accompanying the algorithm.
Early and intensive management of dyslipidemia is important to reduce the significant risk of atherosclerotic cardiovascular disease in patients with diabetes, according to the authors, who say diabetes patients should be classified as high risk, very high risk, or extreme risk. They recommended LDL cholesterol targets of less than 100 mg/dL for high-risk patients, less than 70 mg/dL for very-high-risk patients, and less than 55 mg/dL for the extreme-risk group.
Statins should be considered first-line treatment for lowering cholesterol, unless contraindicated, with other lipid-modifying agents added as needed to reach lipid targets.
Inhibitors of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) address a “large unmet need” for more aggressive lipid lowering in patients with clinical atherosclerotic disease and diabetes, the authors noted.
Added to maximal statin therapy, PCSK9 inhibitors reduce LDL cholesterol by about 50% while also raising HDL cholesterol and having positive effects on other lipids, according to the authors.
Pharmacotherapy for type 2 diabetes requires a “nuanced approach” that takes into account factors such as age, comorbidities, and risk of hypoglycemia, the authors wrote, noting that the AACE supports a hemoglobin A1c target of 6.5% or less for most patients.
The algorithm for glycemic control lists glucose-lowering agents in order of recommended usage. For example, in patients with an entry HbA1c less than 7.5%, the strongest recommendations for were monotherapy with metformin, followed by GLP1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
If insulin becomes necessary, the recommended approach is to add a single daily dose of basal insulin, and if a basal insulin regimen fails to control glucose, it may help to add a GLP1 receptor agonist or dipeptidyl peptidase 4 (DPP4) inhibitor, according to the algorithm.
Avoiding hypoglycemia is important, and one possible “safety measure” to prevent that is using a continuous glucose monitoring device that provides real-time glucose data. “Significant advances have been made in accuracy and availability of CGM devices,” the authors wrote.
Current expert consensus is that clinical CGM devices should be considered if patients have not achieved their glycemic target after 3 months or if they need a treatment that puts them at risk for hypoglycemia, according to Dr. Garber and his colleagues.
Dr. Garber reported that he had no financial relationships relevant to the consensus statement and algorithm. Coauthors of the report provided disclosures related to Novo Nordisk, Eli Lilly, Janssen Pharmaceuticals, Abbott, Sanofi-Aventis, and other pharmaceutical companies.
SOURCE: Garber AJ et al. Endocr Pract. 2019 Jan;25(1):91-120.
Leading endocrinology societies have copublished an algorithm offering updated, specific clinical guidance on lifestyle therapy, management of hypertension and dyslipidemia, and glucose control in patients with type 2 diabetes.
This update from the American Association of Clinical Endocrinologists and the American College of Endocrinology, published in Endocrine Practice, also highlights obesity and prediabetes as underlying risk factors for development of diabetes.
The algorithm, based on new and “comprehensive clinical data” on type 2 diabetes management, is designed as a supplement 2015 AACE/ACE clinical practice guidelines, according to Alan J. Garber, MD, PhD, chair of the Diabetes Management Algorithm Task Force.
“It’s intended to provide clinicians with a practical guide that prompts them to look for factors or influences in the patient’s lifestyle or health that may be a factor in identifying the best treatment approach or medication,” he said in a statement.
Lifestyle medication is critical for all patients with diabetes, according to Dr. Garber and the algorithm coauthors, who recommended a “primarily plant-based meal plan” that limits intake of saturated fatty acids and avoids trans fats. They said overweight patients should restrict caloric intake with a goal of reducing body weight by up to 10%.
Physical activity should include at least 150 minutes per week of activities such as brisk walking or weight training, they said, adding that patients should be advised to sleep 7 hours per night, on average.
Weight loss medications might be needed along with lifestyle modification for patients with body mass index (BMI) over 27 kg/m2 with complications, and for all patients with BMI over 30 regardless of whether they have complications, according to the AACE/ACE committee members who drafted the report.
Bariatric surgery might be considered in patients with BMI over 35 and comorbidities, particularly if patients fail to achieve weight loss goals using other means, they added.
The primary goal of prediabetes management is weight loss, wrote the authors. While there are no Food and Drug Administration–approved agents for prediabetes management, they said, antihyperglycemic agents such as metformin and acarbose have been shown to reduce risk of diabetes by 25%-30% in patients with prediabetes.
While pressure control needs to be individualized, but a goal of less than 130/80 mm Hg is warranted for most patients with diabetes, according to the authors, who note that most patients will require medication to reach their goal.
“Because angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers can slow progression of nephropathy and retinopathy, they are preferred for patients with type 2 diabetes,” said Dr. Garber and his coauthors in the executive summary accompanying the algorithm.
Early and intensive management of dyslipidemia is important to reduce the significant risk of atherosclerotic cardiovascular disease in patients with diabetes, according to the authors, who say diabetes patients should be classified as high risk, very high risk, or extreme risk. They recommended LDL cholesterol targets of less than 100 mg/dL for high-risk patients, less than 70 mg/dL for very-high-risk patients, and less than 55 mg/dL for the extreme-risk group.
Statins should be considered first-line treatment for lowering cholesterol, unless contraindicated, with other lipid-modifying agents added as needed to reach lipid targets.
Inhibitors of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) address a “large unmet need” for more aggressive lipid lowering in patients with clinical atherosclerotic disease and diabetes, the authors noted.
Added to maximal statin therapy, PCSK9 inhibitors reduce LDL cholesterol by about 50% while also raising HDL cholesterol and having positive effects on other lipids, according to the authors.
Pharmacotherapy for type 2 diabetes requires a “nuanced approach” that takes into account factors such as age, comorbidities, and risk of hypoglycemia, the authors wrote, noting that the AACE supports a hemoglobin A1c target of 6.5% or less for most patients.
The algorithm for glycemic control lists glucose-lowering agents in order of recommended usage. For example, in patients with an entry HbA1c less than 7.5%, the strongest recommendations for were monotherapy with metformin, followed by GLP1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
If insulin becomes necessary, the recommended approach is to add a single daily dose of basal insulin, and if a basal insulin regimen fails to control glucose, it may help to add a GLP1 receptor agonist or dipeptidyl peptidase 4 (DPP4) inhibitor, according to the algorithm.
Avoiding hypoglycemia is important, and one possible “safety measure” to prevent that is using a continuous glucose monitoring device that provides real-time glucose data. “Significant advances have been made in accuracy and availability of CGM devices,” the authors wrote.
Current expert consensus is that clinical CGM devices should be considered if patients have not achieved their glycemic target after 3 months or if they need a treatment that puts them at risk for hypoglycemia, according to Dr. Garber and his colleagues.
Dr. Garber reported that he had no financial relationships relevant to the consensus statement and algorithm. Coauthors of the report provided disclosures related to Novo Nordisk, Eli Lilly, Janssen Pharmaceuticals, Abbott, Sanofi-Aventis, and other pharmaceutical companies.
SOURCE: Garber AJ et al. Endocr Pract. 2019 Jan;25(1):91-120.
FROM ENDOCRINE PRACTICE
Lower prices drive OTC insulin sales at Walmart
than the OTC insulin sold elsewhere, according to a national survey of pharmacy employees.
The survey was undertaken by Jennifer N. Goldstein, MD, of Christiana Care Health System in Newark, Del., and her colleagues and published online Feb. 18 in JAMA Internal Medicine.
As Walmart does not make its sales data public, Dr. Goldstein and her colleagues conducted a national telephone-based survey of Walmart and chain pharmacies in the 49 states where OTC insulin is available. They administered a five-item questionnaire that sought to determine the frequency of OTC insulin sales.
Of the 561 pharmacies that completed the questionnaire, 500 (89.1%) responded that they did sell OTC insulin; this included 284 of 292 Walmart pharmacies. Among those Walmart pharmacies, 247 (87%) said sales of OTC insulin occurred daily; 31 (10.9%) sold it weekly; and 3 (1.1%) sold it monthly.
The chains (CVS, Walgreens, Rite Aid) reported far less frequent sales of OTC insulin; 100 out of 216 (46.3%) pharmacies said they made sales only “a few times a year,” and none reported daily sales.
A majority of respondents (54.9%) also answered yes when asked if they believed patients purchased OTC insulin because they could not afford the copayment on prescription insulin; 70.1% of those positive responses came from Walmart pharmacies.
The coauthors acknowledged that their survey represents the impressions of pharmacy employees as opposed to actual sales data. The numbers, however, do “support an estimate of daily sales of more than 18,000 vials of over-the-counter insulin at Walmart pharmacies.” As for next steps, they noted that “further studies should explore clinical and safety outcomes related to the use of over-the-counter insulin.”
One author was supported by a grant from the National Institutes of Health. No conflicts of interest were reported.
SOURCE: Goldstein JN et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7279
than the OTC insulin sold elsewhere, according to a national survey of pharmacy employees.
The survey was undertaken by Jennifer N. Goldstein, MD, of Christiana Care Health System in Newark, Del., and her colleagues and published online Feb. 18 in JAMA Internal Medicine.
As Walmart does not make its sales data public, Dr. Goldstein and her colleagues conducted a national telephone-based survey of Walmart and chain pharmacies in the 49 states where OTC insulin is available. They administered a five-item questionnaire that sought to determine the frequency of OTC insulin sales.
Of the 561 pharmacies that completed the questionnaire, 500 (89.1%) responded that they did sell OTC insulin; this included 284 of 292 Walmart pharmacies. Among those Walmart pharmacies, 247 (87%) said sales of OTC insulin occurred daily; 31 (10.9%) sold it weekly; and 3 (1.1%) sold it monthly.
The chains (CVS, Walgreens, Rite Aid) reported far less frequent sales of OTC insulin; 100 out of 216 (46.3%) pharmacies said they made sales only “a few times a year,” and none reported daily sales.
A majority of respondents (54.9%) also answered yes when asked if they believed patients purchased OTC insulin because they could not afford the copayment on prescription insulin; 70.1% of those positive responses came from Walmart pharmacies.
The coauthors acknowledged that their survey represents the impressions of pharmacy employees as opposed to actual sales data. The numbers, however, do “support an estimate of daily sales of more than 18,000 vials of over-the-counter insulin at Walmart pharmacies.” As for next steps, they noted that “further studies should explore clinical and safety outcomes related to the use of over-the-counter insulin.”
One author was supported by a grant from the National Institutes of Health. No conflicts of interest were reported.
SOURCE: Goldstein JN et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7279
than the OTC insulin sold elsewhere, according to a national survey of pharmacy employees.
The survey was undertaken by Jennifer N. Goldstein, MD, of Christiana Care Health System in Newark, Del., and her colleagues and published online Feb. 18 in JAMA Internal Medicine.
As Walmart does not make its sales data public, Dr. Goldstein and her colleagues conducted a national telephone-based survey of Walmart and chain pharmacies in the 49 states where OTC insulin is available. They administered a five-item questionnaire that sought to determine the frequency of OTC insulin sales.
Of the 561 pharmacies that completed the questionnaire, 500 (89.1%) responded that they did sell OTC insulin; this included 284 of 292 Walmart pharmacies. Among those Walmart pharmacies, 247 (87%) said sales of OTC insulin occurred daily; 31 (10.9%) sold it weekly; and 3 (1.1%) sold it monthly.
The chains (CVS, Walgreens, Rite Aid) reported far less frequent sales of OTC insulin; 100 out of 216 (46.3%) pharmacies said they made sales only “a few times a year,” and none reported daily sales.
A majority of respondents (54.9%) also answered yes when asked if they believed patients purchased OTC insulin because they could not afford the copayment on prescription insulin; 70.1% of those positive responses came from Walmart pharmacies.
The coauthors acknowledged that their survey represents the impressions of pharmacy employees as opposed to actual sales data. The numbers, however, do “support an estimate of daily sales of more than 18,000 vials of over-the-counter insulin at Walmart pharmacies.” As for next steps, they noted that “further studies should explore clinical and safety outcomes related to the use of over-the-counter insulin.”
One author was supported by a grant from the National Institutes of Health. No conflicts of interest were reported.
SOURCE: Goldstein JN et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7279
FROM JAMA INTERNAL MEDICINE
Key clinical point: Patients who purchase over-the-counter insulin typically do so at Walmart, likely because they charge much less than chain pharmacies.
Major finding: 87% of Walmart pharmacies report daily sales of over-the-counter insulin, compared with 0% of chain pharmacies.
Study details: A national telephone-based survey of Walmart and chain pharmacies in the 49 states where over-the-counter insulin is sold.
Disclosures: One author was supported by a grant from the National Institutes of Health. No conflicts of interest were reported.
Source: Goldstein JN et al. JAMA Intern Med. 2019 Feb 18. doi: 10.1001/jamainternmed.2018.7279
FDA approves first interoperable insulin infusion pump
, for insulin delivery in children and adults with diabetes.
The pump delivers insulin under the skin at a variable or fixed rate. It can function on its own, or it can be digitally connected to automatically communicate with and receive drug-dosing commands from other diabetes management devices, such as automated insulin-dosing systems, the agency announced.
The approval was based on a review of performance data demonstrating that the device can deliver insulin accurately and reliably and at the rates and volumes programmed by the user. The agency also assessed the pump’s ability to connect reliably with other devices, as well as its cybersecurity and fail-safe modes.
Risks associated with the device were similar to those of other infusion pumps and include infection, bleeding, pain, or skin irritations. Blockages and air bubbles can occur in the tubing, which will affect drug delivery. Risks associated with incorrect drug delivery include hypo- and hyperglycemia as well as diabetic ketoacidosis.
“The marketing authorization of the [pump] has the potential to aid patients who seek more individualized diabetes therapy systems and opens the door for developers of future connected diabetes devices to get other safe and effective products to patients more efficiently,” FDA Commissioner Scott Gottlieb, MD, said in the announcement.
, for insulin delivery in children and adults with diabetes.
The pump delivers insulin under the skin at a variable or fixed rate. It can function on its own, or it can be digitally connected to automatically communicate with and receive drug-dosing commands from other diabetes management devices, such as automated insulin-dosing systems, the agency announced.
The approval was based on a review of performance data demonstrating that the device can deliver insulin accurately and reliably and at the rates and volumes programmed by the user. The agency also assessed the pump’s ability to connect reliably with other devices, as well as its cybersecurity and fail-safe modes.
Risks associated with the device were similar to those of other infusion pumps and include infection, bleeding, pain, or skin irritations. Blockages and air bubbles can occur in the tubing, which will affect drug delivery. Risks associated with incorrect drug delivery include hypo- and hyperglycemia as well as diabetic ketoacidosis.
“The marketing authorization of the [pump] has the potential to aid patients who seek more individualized diabetes therapy systems and opens the door for developers of future connected diabetes devices to get other safe and effective products to patients more efficiently,” FDA Commissioner Scott Gottlieb, MD, said in the announcement.
, for insulin delivery in children and adults with diabetes.
The pump delivers insulin under the skin at a variable or fixed rate. It can function on its own, or it can be digitally connected to automatically communicate with and receive drug-dosing commands from other diabetes management devices, such as automated insulin-dosing systems, the agency announced.
The approval was based on a review of performance data demonstrating that the device can deliver insulin accurately and reliably and at the rates and volumes programmed by the user. The agency also assessed the pump’s ability to connect reliably with other devices, as well as its cybersecurity and fail-safe modes.
Risks associated with the device were similar to those of other infusion pumps and include infection, bleeding, pain, or skin irritations. Blockages and air bubbles can occur in the tubing, which will affect drug delivery. Risks associated with incorrect drug delivery include hypo- and hyperglycemia as well as diabetic ketoacidosis.
“The marketing authorization of the [pump] has the potential to aid patients who seek more individualized diabetes therapy systems and opens the door for developers of future connected diabetes devices to get other safe and effective products to patients more efficiently,” FDA Commissioner Scott Gottlieb, MD, said in the announcement.
Study Design, Population, and Key Outcomes of DECLARE-TIMI 58 Compared With Other Cardiovascular Outcomes Trials for Sodium-Glucose Cotransporter-2 Inhibitors
Click here to read the supplement
Topics Include:
- The cardiovascular (CV) safety and efficacy of the sodium-glucose cotransporter-2 inhibitor (SGLT-2i) dapagliflozin was evaluated in the DECLARE-TIMI 58 study.
- The study design and patient population of DECLARE-TIMI 58 differed from those of other CV outcomes trials of SGLT-2is (EMPA-REG OUTCOME and CANVAS)
- Key outcome results between the 3 CV outcomes trials of SGLT-2is may have been affected by variations in the study designs and patient populations, given that DECLARE-TIMI 58 evaluated a larger population of patients with type 2 diabetes (T2D) without prior CV events than the EMPA-REG OUTCOME or CANVAS studies, potentially allowing for a broader generalizability of the study results to patients with T2D in real-world clinical practice.
Click here to read the supplement
About the Author:
Bulent S. Atac, MD
Albert Einstein College of
Medicine and Montefiore Medical Center,
Bronx, New York
Click here to read the supplement
Topics Include:
- The cardiovascular (CV) safety and efficacy of the sodium-glucose cotransporter-2 inhibitor (SGLT-2i) dapagliflozin was evaluated in the DECLARE-TIMI 58 study.
- The study design and patient population of DECLARE-TIMI 58 differed from those of other CV outcomes trials of SGLT-2is (EMPA-REG OUTCOME and CANVAS)
- Key outcome results between the 3 CV outcomes trials of SGLT-2is may have been affected by variations in the study designs and patient populations, given that DECLARE-TIMI 58 evaluated a larger population of patients with type 2 diabetes (T2D) without prior CV events than the EMPA-REG OUTCOME or CANVAS studies, potentially allowing for a broader generalizability of the study results to patients with T2D in real-world clinical practice.
Click here to read the supplement
About the Author:
Bulent S. Atac, MD
Albert Einstein College of
Medicine and Montefiore Medical Center,
Bronx, New York
Click here to read the supplement
Topics Include:
- The cardiovascular (CV) safety and efficacy of the sodium-glucose cotransporter-2 inhibitor (SGLT-2i) dapagliflozin was evaluated in the DECLARE-TIMI 58 study.
- The study design and patient population of DECLARE-TIMI 58 differed from those of other CV outcomes trials of SGLT-2is (EMPA-REG OUTCOME and CANVAS)
- Key outcome results between the 3 CV outcomes trials of SGLT-2is may have been affected by variations in the study designs and patient populations, given that DECLARE-TIMI 58 evaluated a larger population of patients with type 2 diabetes (T2D) without prior CV events than the EMPA-REG OUTCOME or CANVAS studies, potentially allowing for a broader generalizability of the study results to patients with T2D in real-world clinical practice.
Click here to read the supplement
About the Author:
Bulent S. Atac, MD
Albert Einstein College of
Medicine and Montefiore Medical Center,
Bronx, New York
What comes first with SGLT2 inhibitors – diabetes or heart failure?
This week in MDedge Cardiocast: Most tPA-eligible stroke patients now get treated within an hour, atrial fib patients with prior hemorrhagic stroke who get their LAA closed can be safely treated, , and the MESA cardiovascular disease risk calculator can be an improvement on the ACC/AHA version.
This week in MDedge Cardiocast: Most tPA-eligible stroke patients now get treated within an hour, atrial fib patients with prior hemorrhagic stroke who get their LAA closed can be safely treated, , and the MESA cardiovascular disease risk calculator can be an improvement on the ACC/AHA version.
This week in MDedge Cardiocast: Most tPA-eligible stroke patients now get treated within an hour, atrial fib patients with prior hemorrhagic stroke who get their LAA closed can be safely treated, , and the MESA cardiovascular disease risk calculator can be an improvement on the ACC/AHA version.
Retrospective Analysis of Liraglutide as Add-On Therapy in Type 2 Diabetes Mellitus: Quantifying the Changes in Insulin Requirements
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
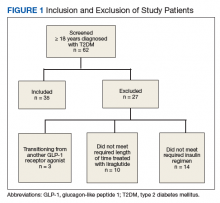
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
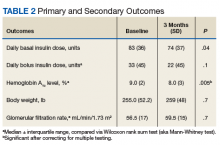
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
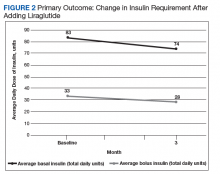
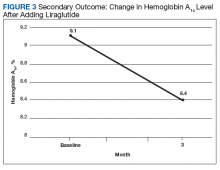
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Clinical pharmacists in VA primary care pharmacy clinics can effectively and safely use liraglutide to reduce hemoglobin A1c and insulin requirements in veterans.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
Diabetes mellitus (DM) was the third most common medical diagnosis in 2016.1 Uncontrolled DM can lead to cardiovascular disease, nephropathy, neuropathy, and retinopathy. It is estimated that only 52.5% of patients with DM have achieved their goal hemoglobin A1c (HbA1c) level. The 2018 American Diabetes Association (ADA) clinical guidelines lack strong recommendations on sequential therapy for patients who have received a diagnosis of type 2 diabetes mellitus (T2DM) and have been unable to achieve their goal HbA1c level with lifestyle changes and maximum-dose metformin.2 Although those guidelines support treatment intensification with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), prescribing patterns for T2DM most commonly include adding insulin to try to control blood glucose and reduce long-term comorbidities.2,3
Related:
Insulin therapy is known for its ability to effectively lower blood glucose and HbA1c levels but comes with many limitations. Mealtime insulin has the highest risk of hypoglycemia, causes significant weight gain, requires several additional injections per day, and additional monitoring of blood glucose.4,5 The 2018 ADA guidelines state that hypoglycemia is the major limiting factor in the management of insulin-treated T2DM.2
Compared with mealtime insulin, GLP-1 RAs have the benefit of reducing the risk of hypoglycemia, weight gain, and number of daily injections.5 In addition, compared with insulin alone, GLP-1 RAs have the advantage of reducing glycemic variability.6 These advantages are especially attractive in the treatment of geriatric patients. Given its mechanism of action, liraglutide is expected to have an effect on both fasting and postprandial blood glucose. There are no recommendations on how to empirically reduce the dose of insulin when starting liraglutide.7
Background
GLP-1 is an incretin hormone that is secreted in response to meal ingestion. GLP-1 stimulates insulin release, suppresses elevated glucagon levels, and delays gastric emptying. Patients with a DM diagnosis have impaired secretion of GLP-1.8
The GLP-1 RA liraglutide was approved by the FDA in January 2010 as a once-daily injection for patients with uncontrolled T2DM despite lifestyle changes and metformin monotherapy. Because of its intermediate half-life, liraglutide has an effect on both fasting and postprandial blood glucose.7 GLP-1 RAs are associated with reduced hypoglycemic episodes—an association attributable to the mechanism of action and potentially to improved pancreatic α-cell function.3,4 In July 2016, results of the LEADER trial showed that liraglutide therapy had a cardiovascular benefit in high-risk patients.8 In October 2017, liraglutide was FDAapproved for reducing 3-point major adverse cardiac events.7
Xultophy (Novo Nordisk, Plainsboro, NJ) is a fixed-dose medication combining degludec, a long-acting basal insulin analog, with liraglutide. As seen in the DUAL trials, Xultophy was more beneficial in reducing HbA1c levels than each component alone, and minimized hypoglycemic events, weight gain, and complexity of insulin treatment intensification.9-11 Therapy that combines basal insulin and a GLP-1 RA may be more effective than either agent as monotherapy and may have a significant impact on cardiovascular risk because of the synergistic vasodilatory, anti-inflammatory, and antioxidant properties of insulin and GLP-1 RA.6 In addition, combination therapy offers many benefits over traditional basal and bolus insulin regimens. These benefits include fewer daily injections, additional weight reduction resulting from the reduced insulin requirement, and fewer episodes of hypoglycemia. Reported gastrointestinal adverse effects have been transient and were not augmented when a GLP-1 RA was used in combination with basal insulin.11
Methods
We performed a retrospective chart analysis to quantify the benefit of using liraglutide as an add-on therapy to basal and bolus insulin regimens in veterans treated at VA Boston Healthcare System (VABHS). The analysis evaluated changes in insulin doses and HbA1c levels when liraglutide was added to these regimens. Patients identified for the study had electronic medication orders for concurrent therapy with liraglutide, insulin glargine, and insulin aspart filled through outpatient VABHS campus pharmacies for at least 3 months between January 2010 and December 2016. Sixty-nine patients who were on basal-bolus insulin for T2DM and who were prescribed liraglutide for treatment intensification were screened for inclusion and exclusion criteria. Data were analyzed at baseline and 3 months after liraglutide treatment.
Study Protocol
The inclusion criteria were patients aged ≥ 18 years, T2DM diagnosis, and therapy with insulin glargine and insulin aspart for at least 3 months before treatment intensi fication with liraglutide. Exclusion criteria were diagnosis of type 1 DM. To accurately quantify mean change in number of insulin units used, the study included patients only if they had been prescribed insulin glargine and insulin aspart before starting liraglutide. All other insulin regimens were excluded. To detect the true change that occurs when liraglutide is added to basal-bolus insulin, the study also excluded patients if they had been previously prescribed another GLP-1 RA. Patients with contraindications to liraglutide, insulin aspart, or insulin glargine were excluded as well. In addition, patients were excluded from the exposed arm if they were injecting < 1.2 mg of liraglutide once daily or if they had been on liraglutide for < 3 months.
Study Outcomes
All 35 patients who met the inclusion and exclusion criteria were included in this retrospective chart review. The primary outcome was determined by changes in HbA1c level and number of insulin doses 3 months after treatment with liraglutide. For each patient, a chart review was performed to determine the amount of insulin added or reduced during the study period. Data were collected at baseline and 3 months after initiation of liraglutide.
Statistical Analysis
Statistical analyses were performed with SPSS Version 20.0 (IBM, Armonk, NY). Population characteristics and study outcomes with normal distribution were compared using a paired t test and are reported as means with standard deviations. Nonnormally distributed variables (bolus insulin, HbA1c level) were compared using the nonparametric Wilcoxon rank sum test and are reported as median values with interquartile ranges. Normality was tested with the Shapiro-Wilk test. The primary outcome evaluated was change in number of insulin units used. Secondary outcomes included change in HbA1c level and change in body weight. A Bonferroni correction for multiple comparisons was used to prevent type I error. Significance at the Bonferroni-corrected level of .01 (.05/5 = .01) is indicated.
Results
Patients were included if they were previously on insulin glargine and insulin aspart before starting liraglutide for treatment intensification.
As Table 1 indicates, 100% of patients were male, and mean (SD) age was 65.5 (9.3) years.
After 3 months of therapy with liraglutide, HbA1c levels were reduced by a mean of 1.0% (P = .005) (Table 2).
Discussion
After 3 months of treatment with liraglutide, patients experienced a significant decrease in HbA1c levels. Insulin doses also decreased, but this finding was not statistically significant after correcting for multiple testing. These results are similar with those in larger studies of the effectiveness of liraglutide and the addition of liraglutide to insulin therapy. 6,8,12,13 Liraglutide has been shown to decrease HbA1c levels, lower rates of progression of kidney failure, decrease weight, and provide cardiovascular benefit.
In a prospective, randomized controlled trial evaluating the effect of adding liraglutide to insulin therapy, 21 of the 37 patients who had T2DM and required more than 100 total units of basal-bolus insulin daily were initiated on liraglutide, and changes in HbA1c level, body weight, and glycemic variability were compared. Results showed statistically significant improvement in all 3 outcomes in the group treated with liraglutide.6 Our findings, in conjunction with those of the larger studies, suggest that many of these results are generalizable to our local veteran population. Importantly, liraglutide was successfully started in pharmacy clinics—an indication that this treatment need not be initiated by an endocrine specialist.
Limitations
Given the lack of gender and racial diversity in this study population, our findings have limited generalizability to other populations. It is possible that, with a larger sample size, these results regarding reduced basal insulin doses would be significant. It has been hypothesized that patients experience fewer episodes of hypoglycemia when insulin doses are reduced, but we were unable to measure the frequency of these episodes. Other study limitations include inability to assess adherence and inability to account for concurrent regimens and/or for lifestyle changes that may have been made during the study period. Further, the study did not collect data on changes made to current DM medication regimens during the study period, and these changes may have influenced outcomes.
Conclusion
Patients who require treatment intensification for insulin-dependent T2DM may benefit from having liraglutide added to their basal-bolus insulin regimen. Liraglutide may prove to be more favorable than bolus insulin when choosing add-on therapy to basal insulin. Benefits include reductions in insulin doses, HbA1c levels, number of daily injections, and body weight. Therefore, we suggest that empirically reducing basal insulin by 10% to 25% and bolus insulin by 25% to 50% will avoid relative hypoglycemia. Prescribers must keep in mind patient-specific factors when adjusting insulin doses, if these doses are adjusted at all. Follow-up of 2 to 4 weeks is recommended for review of home monitoring of glucose for further insulin adjustments.
This study has important clinical implications. First, the finding of a reduction in HbA1c levels supports use of liraglutide therapy for HbA1c reduction in veterans. Second, the number of veterans who were successfully initiated on liraglutide therapy by nonphysician providers indicates that liraglutide can be effectively and safely started in primary care pharmacy clinics, increasing access to the medication.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
1. Centers for Medicare & Medicaid Services. ICD-10. https://www.cms.gov/medicare/coding/icd10. Accessed July 26, 2018.
2. American Diabetes Association. Introduction: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S2.
3. Combination therapy with insulins and GLP-1 receptor agonists. http://www.powerpak.com/course/content/113275. Updated 2018. Accessed July 26, 2018.
4. Carris NW, Taylor JR, Gums JG. Combining a GLP-1 receptor agonist and basal insulin: study evidence and practical considerations. Drugs. 2014;74(18):2141-2152.
5. Young LA, Buse JB, Weaver MA, et al; Monitor Trial Group. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017;177(7):920-929.
6. Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827-832.
7. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; August 2017.
8. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
9. Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926-2933
10. Glough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its component given alone: results of a phase 3, open-label, randomized, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
11. Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized controlled trial. JAMA. 2016;315(9):898-907.
12. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, anti-inflammatory and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37(7):1938-1943.
13. Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Torffvit O, Pehrsson NG. Design and methods of a randomised double-blind trial of adding liraglutide to control HbA1c in patients with type 2 diabetes with impaired glycaemic control treated with multiple daily insulin injections (MDI-Liraglutide trial). Prim Care Diabetes. 2015;9(1):15-22.
Consider adopting the MESA 10-year CHD risk calculator
SNOWMASS, COLO. – The Multi-Ethnic Study of Atherosclerosis–based 10-year coronary heart disease risk calculator offers significant advantages over the far more widely used Atherosclerotic Cardiovascular Disease risk estimator based upon the pooled cohort equations recommended in the American College of Cardiology/American Heart Association guidelines, Robert A. Vogel, MD, said at the Annual Cardiovascular Conference at Snowmass.
“I don’t use the PCE very much. I use the MESA [Multi-Ethnic Study of Atherosclerosis]. I like it because it gives me options I don’t have with the PCE [pooled cohort equations],” Dr. Vogel, a preventive cardiology expert at the University of Colorado at Denver, Aurora, said at the meeting sponsored by the American College of Cardiology.
Those added options include, importantly, the ability to plug in a patient’s coronary artery calcium score. MESA, a landmark longitudinal National Institutes of Health–sponsored study, generated a great deal of the data that established the prognostic value of measuring coronary artery calcium.
“You can do the MESA worksheet with or without coronary artery calcium, either way,” Dr. Vogel noted.
Another big advantage for the MESA risk calculator: It asks a yes/no question about family history of heart attack in first-degree relatives. That’s truly risk-altering information, and yet it’s absent from the ACC/AHA ASCVD (Atherosclerotic Cardiovascular Disease) risk calculator, the cardiologist continued.
Plus, the MESA risk calculator includes four ethnic options: Caucasian, African American, Chinese, or Hispanic, while the ACC/AHA’s PCE-based tool includes only the categories of white, African American, or other.
Dr. Vogel offered a case example for purposes of comparison and contrast of the two risk calculators: a 48-year-old white man with no history or symptoms of cardiovascular disease whose father had an MI at age 52. He wants to know whether he should start taking a statin. The patient is a former smoker who quit 3 years ago. His physical exam is normal. He has a body mass index of 29 kg/m2, an LDL cholesterol of 134 mg/dL, a total cholesterol of 194 mg/dL, a triglyceride level of 92 mg/dL, blood pressure of 128/78 mm Hg, an HDL cholesterol of 42 mg/dL, and a hemoglobin A1c of 5.9%.
Using the PCE-based risk calculator, the man’s 10-year risk of cardiovascular disease is only 3.3%, which falls below the ACC/AHA guideline-recommended threshold for statin therapy for primary prevention. But with the MESA score, as a result of the additional information regarding the family history of premature cardiovascular disease, the patient’s risk jumps to 4.9%, even without including a coronary artery calcium score.
“This shows you how big a factor the family history is. I think it’s unfortunate that the PCE doesn’t put it in,” Dr. Vogel said.
Indeed, in an analysis from MESA, a 60-year-old man with a lipid and blood pressure profile similar to that of Dr. Vogel’s patient would have a 10-year cardiovascular disease risk of 6% with a negative family history and a 9% risk with a positive history, he noted.
In the 48-year-old patient used as an example by Dr. Vogel, plugging into the MESA risk calculator a coronary artery calcium score of, say, 50, 100, or 150 Agatston units would boost the 10-year cardiovascular disease risk to 7.5%, 8.9%, and 9.9%, respectively.
Both the MESA and the ACC/AHA risk calculators incorporate diabetes as a simple yes/no item. It’s either present or absent. This is too crude a dichotomy for Dr. Vogel’s liking in light of data showing that individuals with impaired glucose tolerance have a cumulative risk of cardiovascular mortality that’s intermediate between diabetic and normoglycemic individuals. And of course, the patient in his case example had a HbA1c of 5.9%. So what is a physician to do?
“I fudge the numbers,” he said. “
He also adjusts a former smoker’s estimated 10-year cardiovascular risk based upon how long ago the smoker quit. An ex-smoker’s hazard ratio for coronary heart disease doesn’t drop to that of a never-smoker until 8 years after quitting. And since the patient in this example has been tobacco-free for only 3 years, Dr. Vogel bumps up that individual’s risk level once again. So at this point, even without the additional information that would be provided by a coronary calcium score, the patient’s adjusted MESA 10-year risk is in the 10% range, compared with the 3.3% figure derived using the PCE-based risk calculator.
He reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center, receiving research grants from Sanofi, and serving on speakers bureaus for Regeneron and Sanofi.
SNOWMASS, COLO. – The Multi-Ethnic Study of Atherosclerosis–based 10-year coronary heart disease risk calculator offers significant advantages over the far more widely used Atherosclerotic Cardiovascular Disease risk estimator based upon the pooled cohort equations recommended in the American College of Cardiology/American Heart Association guidelines, Robert A. Vogel, MD, said at the Annual Cardiovascular Conference at Snowmass.
“I don’t use the PCE very much. I use the MESA [Multi-Ethnic Study of Atherosclerosis]. I like it because it gives me options I don’t have with the PCE [pooled cohort equations],” Dr. Vogel, a preventive cardiology expert at the University of Colorado at Denver, Aurora, said at the meeting sponsored by the American College of Cardiology.
Those added options include, importantly, the ability to plug in a patient’s coronary artery calcium score. MESA, a landmark longitudinal National Institutes of Health–sponsored study, generated a great deal of the data that established the prognostic value of measuring coronary artery calcium.
“You can do the MESA worksheet with or without coronary artery calcium, either way,” Dr. Vogel noted.
Another big advantage for the MESA risk calculator: It asks a yes/no question about family history of heart attack in first-degree relatives. That’s truly risk-altering information, and yet it’s absent from the ACC/AHA ASCVD (Atherosclerotic Cardiovascular Disease) risk calculator, the cardiologist continued.
Plus, the MESA risk calculator includes four ethnic options: Caucasian, African American, Chinese, or Hispanic, while the ACC/AHA’s PCE-based tool includes only the categories of white, African American, or other.
Dr. Vogel offered a case example for purposes of comparison and contrast of the two risk calculators: a 48-year-old white man with no history or symptoms of cardiovascular disease whose father had an MI at age 52. He wants to know whether he should start taking a statin. The patient is a former smoker who quit 3 years ago. His physical exam is normal. He has a body mass index of 29 kg/m2, an LDL cholesterol of 134 mg/dL, a total cholesterol of 194 mg/dL, a triglyceride level of 92 mg/dL, blood pressure of 128/78 mm Hg, an HDL cholesterol of 42 mg/dL, and a hemoglobin A1c of 5.9%.
Using the PCE-based risk calculator, the man’s 10-year risk of cardiovascular disease is only 3.3%, which falls below the ACC/AHA guideline-recommended threshold for statin therapy for primary prevention. But with the MESA score, as a result of the additional information regarding the family history of premature cardiovascular disease, the patient’s risk jumps to 4.9%, even without including a coronary artery calcium score.
“This shows you how big a factor the family history is. I think it’s unfortunate that the PCE doesn’t put it in,” Dr. Vogel said.
Indeed, in an analysis from MESA, a 60-year-old man with a lipid and blood pressure profile similar to that of Dr. Vogel’s patient would have a 10-year cardiovascular disease risk of 6% with a negative family history and a 9% risk with a positive history, he noted.
In the 48-year-old patient used as an example by Dr. Vogel, plugging into the MESA risk calculator a coronary artery calcium score of, say, 50, 100, or 150 Agatston units would boost the 10-year cardiovascular disease risk to 7.5%, 8.9%, and 9.9%, respectively.
Both the MESA and the ACC/AHA risk calculators incorporate diabetes as a simple yes/no item. It’s either present or absent. This is too crude a dichotomy for Dr. Vogel’s liking in light of data showing that individuals with impaired glucose tolerance have a cumulative risk of cardiovascular mortality that’s intermediate between diabetic and normoglycemic individuals. And of course, the patient in his case example had a HbA1c of 5.9%. So what is a physician to do?
“I fudge the numbers,” he said. “
He also adjusts a former smoker’s estimated 10-year cardiovascular risk based upon how long ago the smoker quit. An ex-smoker’s hazard ratio for coronary heart disease doesn’t drop to that of a never-smoker until 8 years after quitting. And since the patient in this example has been tobacco-free for only 3 years, Dr. Vogel bumps up that individual’s risk level once again. So at this point, even without the additional information that would be provided by a coronary calcium score, the patient’s adjusted MESA 10-year risk is in the 10% range, compared with the 3.3% figure derived using the PCE-based risk calculator.
He reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center, receiving research grants from Sanofi, and serving on speakers bureaus for Regeneron and Sanofi.
SNOWMASS, COLO. – The Multi-Ethnic Study of Atherosclerosis–based 10-year coronary heart disease risk calculator offers significant advantages over the far more widely used Atherosclerotic Cardiovascular Disease risk estimator based upon the pooled cohort equations recommended in the American College of Cardiology/American Heart Association guidelines, Robert A. Vogel, MD, said at the Annual Cardiovascular Conference at Snowmass.
“I don’t use the PCE very much. I use the MESA [Multi-Ethnic Study of Atherosclerosis]. I like it because it gives me options I don’t have with the PCE [pooled cohort equations],” Dr. Vogel, a preventive cardiology expert at the University of Colorado at Denver, Aurora, said at the meeting sponsored by the American College of Cardiology.
Those added options include, importantly, the ability to plug in a patient’s coronary artery calcium score. MESA, a landmark longitudinal National Institutes of Health–sponsored study, generated a great deal of the data that established the prognostic value of measuring coronary artery calcium.
“You can do the MESA worksheet with or without coronary artery calcium, either way,” Dr. Vogel noted.
Another big advantage for the MESA risk calculator: It asks a yes/no question about family history of heart attack in first-degree relatives. That’s truly risk-altering information, and yet it’s absent from the ACC/AHA ASCVD (Atherosclerotic Cardiovascular Disease) risk calculator, the cardiologist continued.
Plus, the MESA risk calculator includes four ethnic options: Caucasian, African American, Chinese, or Hispanic, while the ACC/AHA’s PCE-based tool includes only the categories of white, African American, or other.
Dr. Vogel offered a case example for purposes of comparison and contrast of the two risk calculators: a 48-year-old white man with no history or symptoms of cardiovascular disease whose father had an MI at age 52. He wants to know whether he should start taking a statin. The patient is a former smoker who quit 3 years ago. His physical exam is normal. He has a body mass index of 29 kg/m2, an LDL cholesterol of 134 mg/dL, a total cholesterol of 194 mg/dL, a triglyceride level of 92 mg/dL, blood pressure of 128/78 mm Hg, an HDL cholesterol of 42 mg/dL, and a hemoglobin A1c of 5.9%.
Using the PCE-based risk calculator, the man’s 10-year risk of cardiovascular disease is only 3.3%, which falls below the ACC/AHA guideline-recommended threshold for statin therapy for primary prevention. But with the MESA score, as a result of the additional information regarding the family history of premature cardiovascular disease, the patient’s risk jumps to 4.9%, even without including a coronary artery calcium score.
“This shows you how big a factor the family history is. I think it’s unfortunate that the PCE doesn’t put it in,” Dr. Vogel said.
Indeed, in an analysis from MESA, a 60-year-old man with a lipid and blood pressure profile similar to that of Dr. Vogel’s patient would have a 10-year cardiovascular disease risk of 6% with a negative family history and a 9% risk with a positive history, he noted.
In the 48-year-old patient used as an example by Dr. Vogel, plugging into the MESA risk calculator a coronary artery calcium score of, say, 50, 100, or 150 Agatston units would boost the 10-year cardiovascular disease risk to 7.5%, 8.9%, and 9.9%, respectively.
Both the MESA and the ACC/AHA risk calculators incorporate diabetes as a simple yes/no item. It’s either present or absent. This is too crude a dichotomy for Dr. Vogel’s liking in light of data showing that individuals with impaired glucose tolerance have a cumulative risk of cardiovascular mortality that’s intermediate between diabetic and normoglycemic individuals. And of course, the patient in his case example had a HbA1c of 5.9%. So what is a physician to do?
“I fudge the numbers,” he said. “
He also adjusts a former smoker’s estimated 10-year cardiovascular risk based upon how long ago the smoker quit. An ex-smoker’s hazard ratio for coronary heart disease doesn’t drop to that of a never-smoker until 8 years after quitting. And since the patient in this example has been tobacco-free for only 3 years, Dr. Vogel bumps up that individual’s risk level once again. So at this point, even without the additional information that would be provided by a coronary calcium score, the patient’s adjusted MESA 10-year risk is in the 10% range, compared with the 3.3% figure derived using the PCE-based risk calculator.
He reported serving as a paid consultant to the National Football League and the Pritikin Longevity Center, receiving research grants from Sanofi, and serving on speakers bureaus for Regeneron and Sanofi.
REPORTING FROM ACC SNOWMASS 2019