User login
SGLT2 inhibitors morph into HF drugs
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Intensive insulin added no benefit for hyperglycemia after ischemic stroke
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
REPORTING FROM ISC 2019
Key clinical point: Aggressive insulin management of hyperglycemia following an ischemic stroke gave no clinical benefit, compared with a standard approach.
Major finding: After 90 days, favorable outcomes occurred in 21% of patients on aggressive insulin treatment and 22% on standard treatment.
Study details: SHINE, a multicenter, randomized trial with 1,151 acute ischemic stroke patients.
Disclosures: SHINE received no commercial funding. Dr. Johnston had no disclosures.
Source: Johnston KC et al. ISC 2019, Abstract LB1.
Roux-en-Y achieves diabetes remission in majority of patients
Around three-quarters of people with type 2 diabetes mellitus (T2DM) who undergo Roux-en-Y gastric bypass experience remission of their disease within a year of the surgery, according to published findings from a population-based observational study. However, one in four of those people will have relapsed by 5 years, the authors noted.
Researchers looked at the effect of Roux-en-Y gastric bypass (RYGB) in 1,111 individuals with T2DM, compared with 1,074 controls who also had T2DM but did not undergo gastric bypass.
By 6 months after surgery, 65% of those who had undergone RYGB met the criteria for remission – defined as no use of glucose-lowering drugs and an HbA1c below 48 mmol/mol (less than 6.5%) or metformin monotherapy with HbA1c below 42 mmol/mol (less than 6.0%).
By 1 year, 74% of those who had surgery had achieved remission, and 73% of those remained in remission 5 years after surgery. However, at 2 years, 6% of those who had achieved remission in the first year had already relapsed; by 3 years, 12% had relapsed; and by 4 years, 18% had relapsed. By 5 years after surgery, a total of 27% of those who originally achieved remission in the first year had relapsed.
The overall prevalence of remission remained at 70% for every 6-month period during the duration of the study, which suggests that, although some achieved remission early and then relapsed, others achieved remission later.
Individuals who were aged 50-60 years were 12% less likely to achieve remission, compared with those who were younger than 40 years, whereas those aged 60 years or more were 17% less likely to achieve remission.
A longer duration of diabetes was also associated with a lower likelihood of achieving remission after RYGB; individuals who had had diabetes for 8 years or more had a 27% lower likelihood of remission, compared with those who had had the disease for less than 2 years.
A higher HbA1c (greater than 53 mmol/mol) was associated with a 19% lower likelihood of remission, and individuals using insulin had a 43% lower likelihood of remission.
“Overall, our findings add evidence to the importance of regular check-ups following RYGB, despite initial diabetes remission, and also suggest that timing of RYGB is important (i.e., consider RYGB while there are still functional pancreatic beta cells),” wrote Lene R. Madsen, MD, from the department of endocrinology and internal medicine at Aarhus (Denmark) University Hospital and her colleagues.
The study also examined the effect of RYGB on microvascular and macrovascular diabetes complications. This revealed that the incidence of diabetic retinopathy was nearly halved among individuals who had undergone gastric bypass, the incidence of hospital-coded diabetic kidney disease was 46% lower, and the incidence of diabetic neuropathy was 16% lower.
In particular, individuals who achieved remission in the first year after surgery had a 57% lower incidence of microvascular events, compared with those who did not have surgery.
The authors noted that individuals who did not reach the threshold for diabetes remission after surgery still showed signs of better glycemic control, compared with individuals who had not undergone surgery.
“This aligns with the theory of ‘metabolic memory’ introduced by Coleman et al. [Diabetes Care. 2016;39(8):1400-07], suggesting that time spent in diabetes remission after RYGB is not spent in vain when it comes to reducing the risk of subsequent microvascular complications,” they wrote.
The surgery was also associated with a 46% reduction in the incidence of ischemic heart disease. In the first 30 days after surgery, 7.5% of patients were readmitted to hospital for any surgical complication, but the 90-day mortality rate after surgery was less than 0.5%.
The study was supported by the Health Research Fund of Central Denmark, the Novo Nordisk Foundation, and the A.P. Møller Foundation. The authors reported no conflicts of interest.
SOURCE: Madsen LR et al. Diabetologia. 2019, Feb 6. doi: 10.1007/s00125-019-4816-2.
Around three-quarters of people with type 2 diabetes mellitus (T2DM) who undergo Roux-en-Y gastric bypass experience remission of their disease within a year of the surgery, according to published findings from a population-based observational study. However, one in four of those people will have relapsed by 5 years, the authors noted.
Researchers looked at the effect of Roux-en-Y gastric bypass (RYGB) in 1,111 individuals with T2DM, compared with 1,074 controls who also had T2DM but did not undergo gastric bypass.
By 6 months after surgery, 65% of those who had undergone RYGB met the criteria for remission – defined as no use of glucose-lowering drugs and an HbA1c below 48 mmol/mol (less than 6.5%) or metformin monotherapy with HbA1c below 42 mmol/mol (less than 6.0%).
By 1 year, 74% of those who had surgery had achieved remission, and 73% of those remained in remission 5 years after surgery. However, at 2 years, 6% of those who had achieved remission in the first year had already relapsed; by 3 years, 12% had relapsed; and by 4 years, 18% had relapsed. By 5 years after surgery, a total of 27% of those who originally achieved remission in the first year had relapsed.
The overall prevalence of remission remained at 70% for every 6-month period during the duration of the study, which suggests that, although some achieved remission early and then relapsed, others achieved remission later.
Individuals who were aged 50-60 years were 12% less likely to achieve remission, compared with those who were younger than 40 years, whereas those aged 60 years or more were 17% less likely to achieve remission.
A longer duration of diabetes was also associated with a lower likelihood of achieving remission after RYGB; individuals who had had diabetes for 8 years or more had a 27% lower likelihood of remission, compared with those who had had the disease for less than 2 years.
A higher HbA1c (greater than 53 mmol/mol) was associated with a 19% lower likelihood of remission, and individuals using insulin had a 43% lower likelihood of remission.
“Overall, our findings add evidence to the importance of regular check-ups following RYGB, despite initial diabetes remission, and also suggest that timing of RYGB is important (i.e., consider RYGB while there are still functional pancreatic beta cells),” wrote Lene R. Madsen, MD, from the department of endocrinology and internal medicine at Aarhus (Denmark) University Hospital and her colleagues.
The study also examined the effect of RYGB on microvascular and macrovascular diabetes complications. This revealed that the incidence of diabetic retinopathy was nearly halved among individuals who had undergone gastric bypass, the incidence of hospital-coded diabetic kidney disease was 46% lower, and the incidence of diabetic neuropathy was 16% lower.
In particular, individuals who achieved remission in the first year after surgery had a 57% lower incidence of microvascular events, compared with those who did not have surgery.
The authors noted that individuals who did not reach the threshold for diabetes remission after surgery still showed signs of better glycemic control, compared with individuals who had not undergone surgery.
“This aligns with the theory of ‘metabolic memory’ introduced by Coleman et al. [Diabetes Care. 2016;39(8):1400-07], suggesting that time spent in diabetes remission after RYGB is not spent in vain when it comes to reducing the risk of subsequent microvascular complications,” they wrote.
The surgery was also associated with a 46% reduction in the incidence of ischemic heart disease. In the first 30 days after surgery, 7.5% of patients were readmitted to hospital for any surgical complication, but the 90-day mortality rate after surgery was less than 0.5%.
The study was supported by the Health Research Fund of Central Denmark, the Novo Nordisk Foundation, and the A.P. Møller Foundation. The authors reported no conflicts of interest.
SOURCE: Madsen LR et al. Diabetologia. 2019, Feb 6. doi: 10.1007/s00125-019-4816-2.
Around three-quarters of people with type 2 diabetes mellitus (T2DM) who undergo Roux-en-Y gastric bypass experience remission of their disease within a year of the surgery, according to published findings from a population-based observational study. However, one in four of those people will have relapsed by 5 years, the authors noted.
Researchers looked at the effect of Roux-en-Y gastric bypass (RYGB) in 1,111 individuals with T2DM, compared with 1,074 controls who also had T2DM but did not undergo gastric bypass.
By 6 months after surgery, 65% of those who had undergone RYGB met the criteria for remission – defined as no use of glucose-lowering drugs and an HbA1c below 48 mmol/mol (less than 6.5%) or metformin monotherapy with HbA1c below 42 mmol/mol (less than 6.0%).
By 1 year, 74% of those who had surgery had achieved remission, and 73% of those remained in remission 5 years after surgery. However, at 2 years, 6% of those who had achieved remission in the first year had already relapsed; by 3 years, 12% had relapsed; and by 4 years, 18% had relapsed. By 5 years after surgery, a total of 27% of those who originally achieved remission in the first year had relapsed.
The overall prevalence of remission remained at 70% for every 6-month period during the duration of the study, which suggests that, although some achieved remission early and then relapsed, others achieved remission later.
Individuals who were aged 50-60 years were 12% less likely to achieve remission, compared with those who were younger than 40 years, whereas those aged 60 years or more were 17% less likely to achieve remission.
A longer duration of diabetes was also associated with a lower likelihood of achieving remission after RYGB; individuals who had had diabetes for 8 years or more had a 27% lower likelihood of remission, compared with those who had had the disease for less than 2 years.
A higher HbA1c (greater than 53 mmol/mol) was associated with a 19% lower likelihood of remission, and individuals using insulin had a 43% lower likelihood of remission.
“Overall, our findings add evidence to the importance of regular check-ups following RYGB, despite initial diabetes remission, and also suggest that timing of RYGB is important (i.e., consider RYGB while there are still functional pancreatic beta cells),” wrote Lene R. Madsen, MD, from the department of endocrinology and internal medicine at Aarhus (Denmark) University Hospital and her colleagues.
The study also examined the effect of RYGB on microvascular and macrovascular diabetes complications. This revealed that the incidence of diabetic retinopathy was nearly halved among individuals who had undergone gastric bypass, the incidence of hospital-coded diabetic kidney disease was 46% lower, and the incidence of diabetic neuropathy was 16% lower.
In particular, individuals who achieved remission in the first year after surgery had a 57% lower incidence of microvascular events, compared with those who did not have surgery.
The authors noted that individuals who did not reach the threshold for diabetes remission after surgery still showed signs of better glycemic control, compared with individuals who had not undergone surgery.
“This aligns with the theory of ‘metabolic memory’ introduced by Coleman et al. [Diabetes Care. 2016;39(8):1400-07], suggesting that time spent in diabetes remission after RYGB is not spent in vain when it comes to reducing the risk of subsequent microvascular complications,” they wrote.
The surgery was also associated with a 46% reduction in the incidence of ischemic heart disease. In the first 30 days after surgery, 7.5% of patients were readmitted to hospital for any surgical complication, but the 90-day mortality rate after surgery was less than 0.5%.
The study was supported by the Health Research Fund of Central Denmark, the Novo Nordisk Foundation, and the A.P. Møller Foundation. The authors reported no conflicts of interest.
SOURCE: Madsen LR et al. Diabetologia. 2019, Feb 6. doi: 10.1007/s00125-019-4816-2.
FROM DIABETOLOGIA
Key clinical point: Diabetes remission was achieved in three-quarters of Roux-en-Y surgical patients.
Major finding: The incidence of diabetes remission 1 year after Roux-en-Y gastric bypass was 74%.
Study details: A population-based cohort study in 1,111 individuals with type 2 diabetes mellitus who underwent Roux-en-Y gastric bypass, compared with 1,074 nonsurgical controls with diabetes.
Disclosures: The study was supported by the Health Research Fund of Central Denmark, the Novo Nordisk Foundation, and the A.P. Møller Foundation. The authors reported no conflicts of interest.
Source: Madsen LR et al. Diabetologia. 2019, Feb 6. doi: 10.1007/s00125-019-4816-2.
Gastric Electric Stimulation for Refractory Gastroparesis
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.
Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.
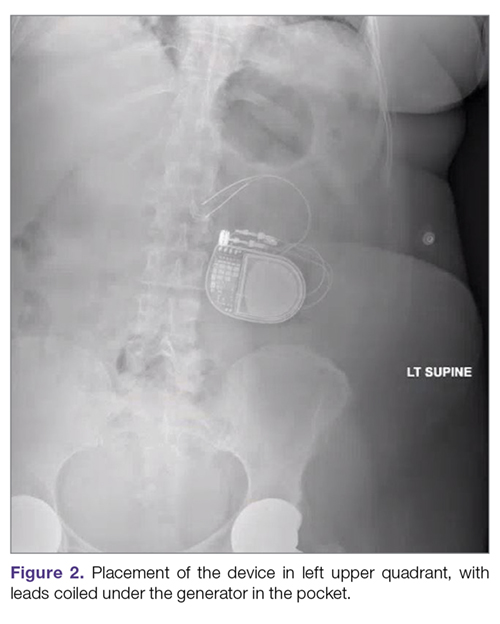
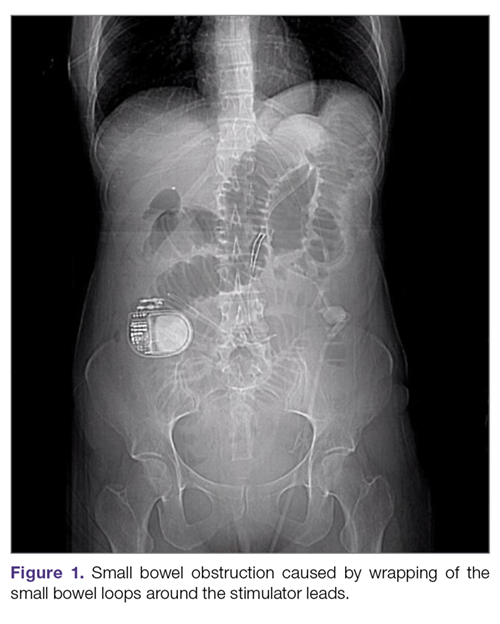
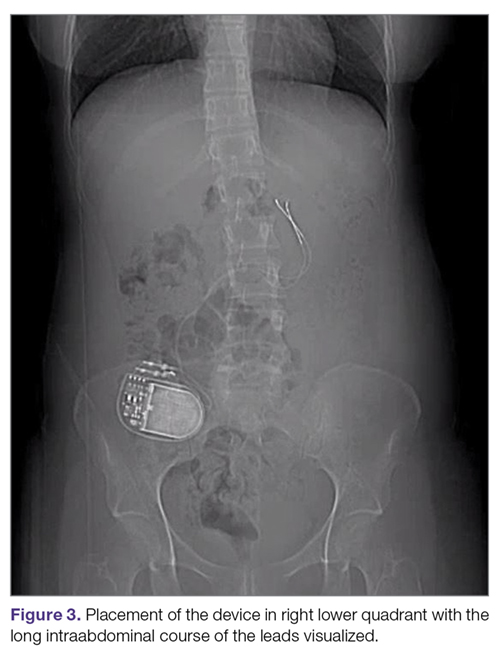
Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.
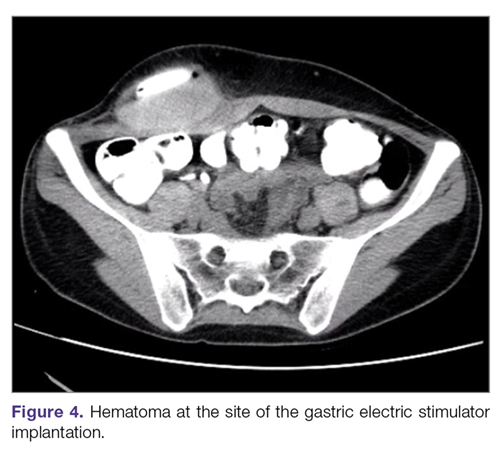
Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.
Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
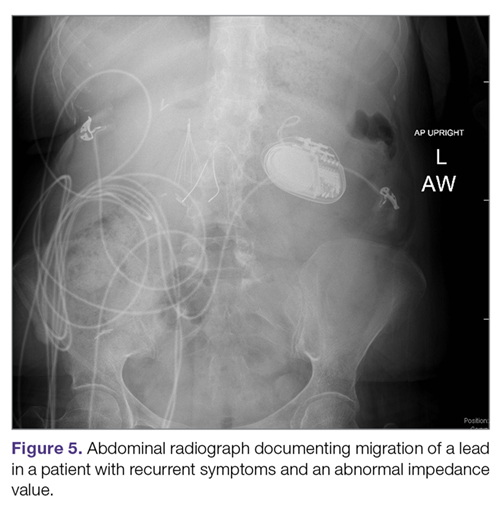
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29
Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
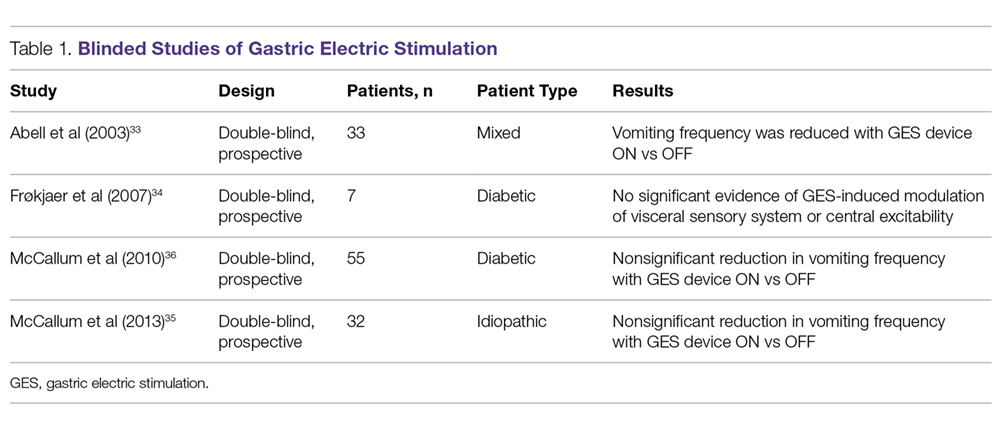
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38
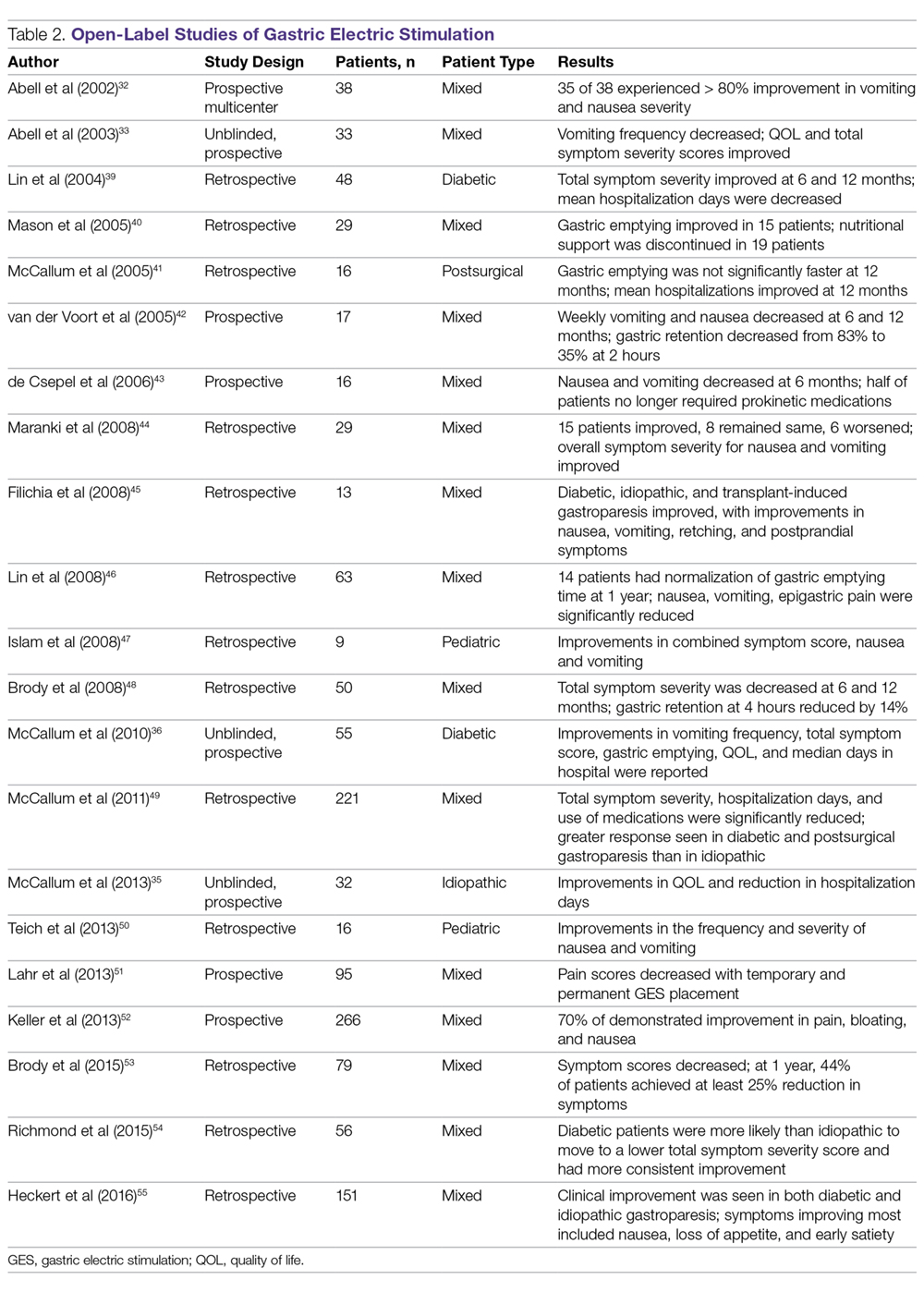
In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.
In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.

Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.

Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.

Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.

Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29

Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38

In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.

In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
From Temple University School of Medicine, Philadelphia, PA.
Abstract
- Objective: To outline the use and utility of gastric electric stimulation (GES) as a therapeutic intervention for gastroparesis.
- Methods: Review of the literature.
- Results: Gastroparesis is characterized by delayed gastric emptying, with symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Some patients with gastroparesis do not respond to medical intervention, and for these patients surgical intervention may be warranted. GES utilizes high-frequency gastric neurostimulation to facilitate gastric emptying and reduce symptoms of gastroparesis. It is indicated for patients with idiopathic and diabetic gastroparesis who have nausea and vomiting as their primary symptoms and who have not responded to medical therapy. GES has also been used in postsurgical and pediatric gastroparesis patients. Optimizing the outcome of this surgical treatment through proper patient selection and meticulous surgical technique is essential as there are inherent risks to the procedure. Nonblinded studies of GES for medically refractory gastroparesis have demonstrated therapeutic symptomatic benefit, whereas randomized controlled trials have not. New interventions such as pyloromyotomy and pyloroplasty are reasonable alternatives or addendums to GES.
- Conclusion: GES may be considered among the therapies available for treating patients with refractory symptoms of gastroparesis. More studies, specifically those comparing GES, pyloromyotomy, GES combined with pyloromyotomy, and placebo, are needed to help guide therapy selection for refractory gastroparesis.
Keywords: diabetes; gastroparesis; dysmotility; gastric emptying; electric stimulation.
Gastroparesis is a chronic dysmotility disorder characterized by delayed gastric emptying with associated symptoms of nausea, vomiting, early satiety, postprandial fullness, and abdominal pain. Medical treatments for gastroparesis include dietary modifications, glucose control in those with diabetes, prokinetic medications, antiemetic medications, and symptom modulators, but unfortunately patients frequently do not respond to these treatments. In patients refractory to medical therapy, surgical treatments can be considered.
Gastric electric stimulation (GES; Enterra [Medtronic, Minneapolis, MN]) was approved via a Food and Drug Administration (FDA) Humanitarian Use Device (HUD) exemption for the treatment of medically refractory gastroparesis in 2000. Understanding the indications, risks, outcomes, and alternatives to GES is essential to providing appropriate care for patients with medically refractory gastroparesis. This article outlines the use and utility of GES as a therapeutic intervention for gastroparesis.
Types of Gastroparesis
Gastroparesis is a chronic symptomatic disorder of the stomach manifested by delayed gastric emptying without evidence of gastric outlet obstruction or ulceration.1 The pathophysiology of gastroparesis appears to involve abnormalities in functioning of several elements including the autonomic nervous system, especially the vagus nerve, smooth muscle cells, enteric neurons, and interstitial cells of Cajal.
Idiopathic gastroparesis and diabetic gastroparesis are the 2 most common types of gastroparesis.2 Symptomatic delayed gastric emptying with no primary underlying abnormality predisposing to gastroparesis is categorized as idiopathic gastroparesis.3 A small subset of patients with idiopathic gastroparesis report an initial infectious prodrome such as gastroenteritis or respiratory infection. It has been suggested that this postinfectious gastroparesis results from viral injury to the neural innervation of the stomach or the interstitial cells of Cajal in the stomach.4 Viruses that have been implicated in the development of gastroparesis include cytomegalovirus, Epstein-Barr virus, Norwalk virus, rotavirus, herpes zoster, and varicella zoster.5-9
Diabetic gastroparesis is characterized as onset of symptoms of gastroparesis in patients with diabetes, with concomitant delayed gastric emptying. It is often attributed to chronic hyperglycemia-induced damage to the vagus nerve, and is frequently observed in association with other diabetic complications such as neuropathy, retinopathy, and nephropathy.10
Gastroparesis that develops following surgery is classified as postsurgical gastroparesis. In the past, this form of gastroparesis most commonly occurred after ulcer surgery, often performed with vagotomy. These types of surgeries are performed less frequently in the era of proton pump inhibitor therapy and treatments for Helicobacter pylori. Presently, Nissen fundoplication and bariatric surgery are the more common surgical procedures associated with gastroparesis.3 Long-term use of medications that delay gastric emptying, such as opiate narcotic medications, can lead to gastroparesis and represent another form of iatrogenic gastroparesis. Other forms of gastroparesis (atypical gastroparesis) arise due to various underlying etiologies, including neurological disorders (eg, Parkinson disease, multiple sclerosis), metabolic or endocrine conditions (eg, hypothyroidism), autoimmune disorders, connective tissue and collagen vascular disorders (eg, systemic lupus erythematosus, scleroderma, Sjögren syndrome, Ehlers-Danlos syndrome), or eating disorders (eg, anorexia, bulimia).3
Epidemiology
There is a female preponderance in patients with gastroparesis. Data from the Rochester Epidemiology Project, a database of linked medical records for residents of Olmsted County, MN, showed that the age-adjusted prevalence of definite gastroparesis per 100,000 inhabitants was 37.8 for women and 9.6 for men.11 More recent estimates have suggested a much higher prevalence of probable gastroparesis (approximately 1.8%) in the general population using symptoms suggestive of gastroparesis.12 Hospitalization rates for gastroparesis have increased since 2000, which could reflect rising prevalence and/or the effects of heightened awareness about and better identification of gastroparesis.13 This increase may also be due in part to the rising rate of diabetes leading to more cases of diabetic gastroparesis; withdrawal of some gastroparesis treatments from the market (cisapride, tegaserod) leading to hospitalizations for symptoms not adequately being treated; and hospitalizations needed for insertion of the gastric electric stimulator.
Gastroparesis Symptoms
The main symptoms of gastroparesis are early satiety, postprandial fullness, bloating, nausea, and vomiting.14 Nausea (> 90% of patients) and early satiety (60% of patients) are the most common symptoms.15 Abdominal pain is often present in patients with gastroparesis but is usually not the predominant symptom. The pain can be multifactorial, with somatic, visceral, and neuropathic components.16-18 Moderate to severe abdominal pain has been found more often in patients with idiopathic gastroparesis and in association with opiate use.16 Symptoms of gastroparesis may be persistent or present as episodic flares. Due to the symptoms, some patients will experience weight loss and malnutrition and, in severe cases, dehydration.19
Although the definition of gastroparesis is a delay in gastric emptying along with symptoms, symptoms correlate poorly with the degree of delayed gastric emptying. The symptoms that appear to have the strongest correlation with gastric emptying are nausea, vomiting, early satiety, and postprandial fullness, whereas symptoms such as abdominal pain and bloating have little correlation. Furthermore, improving gastric emptying does not necessarily lead to improved symptoms, and symptom improvement does not always lead to improved gastric emptying times.20 Between 5% and 12% of patients with diabetes report symptoms consistent with gastroparesis, though many of these patients have normal gastric emptying. The symptoms of gastroparesis overlap with those of functional dyspepsia, as both may have motor and sensory alterations.21
The Gastroparesis Cardinal Symptom Index (GCSI), a subset of the Patient Assessment of Gastrointestinal Disorders Symptom Severity Index (PAGI-SYM), is a questionnaire that is commonly used to establish symptom severity in patients with gastroparesis. It is comprised of 3 subscales—nausea and vomiting, postprandial fullness and early satiety, and bloating—which are averaged to provide a total GCSI score. Symptoms over the 2 weeks prior to administration of the questionnaire are assessed and rated from 0 (none) to 5 (very severe).22 Grading the severity of gastroparesis may take into account symptoms, quality of life, and gastric emptying. One commonly used grading system assigns a grade from 1 to 3, with grade 1 being mild gastroparesis, grade 2 being compensated gastroparesis, and grade 3 being gastric failure with refractory symptoms that are uncontrolled.18,23 Quality-of-life surveys also suggest that gastroparesis independent of other factors leads to a worse quality of life.24
Indications for GES
Gastric electric stimulator implantation is a surgical procedure with inherent risks and complications and is reserved for patients with intractable symptoms of gastroparesis who remain symptomatic despite treatment attempts with dietary management, antiemetic agents (eg, compazine, phenergan, and ondansetron), and prokinetic agents (eg, metoclopramide, erythromycin, and domperidone). Symptom modulators such as nortriptyline and mirtazapine are occasionally tried.
Surgical intervention can be considered upon failure of medical treatment measures. At least a year of documented care provided by a physician specializing in gastroparesis is suggested for surgical consideration. The gastric electric neurostimulator is approved by the FDA as a HUD for the care of patients with idiopathic and diabetic gastroparesis, performed on a compassionate basis. GES implantation requires Institutional Review Board approval at the institution, and patients are required to have documented delayed gastric emptying.
It is important to remember that the GES device is incompatible with magnetic resonance imaging (MRI) and explantation of the device is necessary prior to MRI. As such, in patients with anticipated need of frequent MRI, such as those with multiple sclerosis, serious consideration should be given to alternative strategies prior to focusing on this modality.
Device Placement
GES was devised to improve gastric emptying. The Enterra GES system uses high-frequency, low-energy electric stimulation. An alternative method is true gastric pacing that uses high-energy, low-frequency stimulation to entrain the gastric slow waves and subsequent contractions at 3 cycles per minute (cpm). Gastric pacing has greater energy requirements than GES, which makes the size of the stimulator too large to be practical. In pilot animal studies, GES produced an accelerating effect on gastric emptying, but in human studies GES had an inconsistent effect on gastric emptying. Studies have suggested that GES influences the proximal stomach, with a reduction of gastric tone,25 and also that GES has an afferent modulatory mechanism.26
The Enterra GES is placed surgically under general anesthesia, commonly via laparotomy or minimal access surgical techniques (laparoscopically or robotically assisted). Preoperative intravenous antibiotics are given. The system consists of a pair of electrodes connected to a pulse generator. The 2 stimulation leads are inserted into the gastric muscularis propria 1 cm apart along the greater curvature 10 cm proximal to the pylorus. Upper endoscopy is performed to ensure that the leads do not penetrate through the mucosa into the stomach lumen; if this occurs, repositioning of the lead is necessary. A horizontal incision through the skin is made, and the distal ends of the stimulating wires are tunneled through the abdominal wall and connected to the pulse generator. The impedance (resistance) between the wires is measured to ensure the appropriate range (200-800 Ohms). The neurostimulator with the distal ends of the stimulating wires is then placed into the subcutaneous pocket and sutured to the underlying fascia. The pulse generator delivers a high-frequency, low-energy, 0.1-second train of pulses at a frequency of 12 cpm. Within each pulse train, individual pulses oscillate at a frequency of 14 cycles per second. The voltage of the stimulations is set to provide a current of 5 milliamps (mA; remembering that voltage = current × resistance).
Patients are often hospitalized with a recovery time of 1 to 3 days. Immediate postoperative care usually includes intravenous fluids, controlling any postoperative ileus, advancing diet, and providing analgesic pain medications. Hospital length of stay can be impacted by surgical technique.25 Patients are seen several weeks after discharge for assessment of the incision and toleration of diet. Medications for gastroparesis that patients were taking prior to the GES implantation are usually continued postoperatively, with a goal of reducing these medications over time. Patients are then followed every 3 to 12 months, depending on their clinical condition.
At follow-up visits, medications are reviewed and new treatments can be added if appropriate. The gastric stimulator is interrogated to determine if changes in resistance occurred; if necessary, minor readjustments can be made to keep the current at desired levels (5 mA). For persistent symptoms with GES treatment, the stimulator parameters can be adjusted after 3 months of follow up, typically first increasing the current from 5 to 7.5 mA and then to 10 mA. After this, the frequency can be increased from 14 Hz to 28 Hz, and then to 55 Hz. Rarely, the ON duration is increased from 0.1 to 1 second. Increasing the ON time can worsen symptoms in some patients, cause abdominal pain, and decrease the battery life from the usual 7 years.
Complications of GES
In an analysis of the Manufacturer and User Facility Device Experience (MAUDE) databank, Bielefeldt identified 1587 reports of adverse effects related to the gastric electric stimulator from January 2001 to October 2015.27 The most common adverse effects are reviewed here.
Skin erosion/wound dehiscence is one of the most common reported complications; it may be related to superficial placement or inadequate securing of the device to the fascia. Abscess can develop postoperatively due to hematogenous seeding or may be a sign of lead erosion into the lumen, tracking along the leads into subcutaneous tissue.28 It is important to warn patients to protect the area over the device from needle injections as this also can lead to hematoma formation and direct contamination of the device. If the device gets infected, it cannot be salvaged and requires explantation. Implantation of a new device can be attempted once all wound issues resolve.
Device migration/flipping most often occurs because the device is inadequately fixed to the underlying fascia, but occasionally it can occur from patients flipping the device around. Flipping can occur due to superficial pocket location within subcutaneous tissue, especially in obese patients. Migration/flipping can lead to prominence of the contour of the device and discomfort, ultimately requiring surgical correction.

Perforation and erosion of the leads. With time, leads can erode into the stomach, although this is rare. Usually erosion is associated with loss of device function. Endoscopy confirms this finding. In rare cases, infection can track proximally along the lead and present as a surgical site infection at the pulse generator. This complication often requires explantation of the neurostimulator leads and pulse generator.

Intestinal obstruction. Although rare, the intestines can get wrapped around the leads of the device, causing different degrees of obstruction (Figure 1). Positioning the device in the left upper quadrant minimizes the intraabdominal length of the leads and pulls them maximally out, coiling under the device (Figure 2). In cases where other locations are used either due to a hostile upper abdominal region (skin infection, presence of gastrostomy or other devices) or surgeon’s preference, the GES device can be implanted in the lower abdomen (Figure 3). In these circumstances, carefully draping the omentum over the bowels might help to prevent this complication. Tacking of the leads to the parietal peritoneum with sutures can also be preventative. In cases of obstruction requiring intervention by laparotomy or minimal access techniques (laparoscopy or robotic assisted surgery), all efforts are made to preserve the neurostimulator leads. In cases that require bowel resection, lead contamination is a serious concern, but lead explantation is not mandatory. Close postoperative monitoring for the development of lead infection is required.

Hematoma and seroma. Postoperative hematomas can occur from inadequate hemostasis, and seromas can occur in the stimulator pocket. Small hematomas may be observed if not complicated (Figure 4). In cases of large hematomas with skin compromise or dehiscence, prompt washout and drainage is required. In ideal cases, the device can be preserved. Relocation to another site might be required if skin necrosis develops. The possibility of device contamination also must be considered; after resolution of wound issues, implantation of a new device may be tried. Seromas at the generator pocket site are a frequent occurrence but are often benign, self-limiting, and generally resolve over 4 to 6 weeks.

Incisional hernia. Hernias can develop after any abdominal surgery and are not unique to GES implantation. Use of minimally invasive technique for the GES implantation minimizes this complication.
Electric shock sensations may occur from breakage of the plastic lining covering the stimulator wires or from fluid buildup around the insertion of the wires into the stimulator. Shocks can also occur due to shortening of the leads on the muscles of the abdominal wall. Patients describe periodic muscle cramps with the frequency of the device (every 5 seconds). To prevent this complication, freshly implanted leads should be covered by an omental flap to isolate them from the abdominal wall. In patients who continue to feel shocks despite all efforts, the possibility of visceral hypersensitivity should be considered. A trial of symptom modulators such as nortriptyline and lowering of the output amperage below the minimal recommended setting of 5 mA can be undertaken. If these interventions do not work, the device must be turned off for a period of time. Occasionally, replacement of the leads or explantation of the device must be considered.
Lack of effect/persistent symptoms. If a patient presents with lack of improvement after device implantation, a thorough workup should be undertaken to ensure that the device is functioning properly. In the case of abnormal impedance values, an abdominal x-ray study can be performed to rule out lead migration (Figure 5). If no abnormalities are detected, the output of the device can be increased. After adjusting device settings, the patient should be assessed for improvement over at least a 1- to 3-month period. One report suggests that in patients not responding to GES, repositioning the location of the stimulator leads on the stomach can be helpful.29

Outcomes of GES
Study results of investigative GES models in animals and select patients were published in 1997.30,31 Following these reports, 2 large multicenter studies were conducted to demonstrate the efficacy of GES for the treatment of refractory gastroparesis. The Gastric Electrical Mechanical Stimulation Study (GEMS) was an open-label, multicenter study of 38 patients who received percutaneous and later permanent GES devices.32 Marked reduction in weekly vomiting and nausea was observed at 4 weeks, with a 90% reduction in nausea and vomiting frequency at 11 months. Following this, a second multicenter study (Worldwide Anti-Vomiting Electrical Stimulation Study [WAVES]) involving a double-blind sham stimulation controlled trial with 33 idiopathic and diabetic gastroparesis patients was performed.33 During the blinded portion of this study, there was a noticeable decrease in vomiting frequency, particularly in the patients with diabetic gastroparesis. Patient preference was for the stimulator ON as compared to OFF. The FDA’s HUD exemption for the Enterra GES device in 2000 was based on these studies.
Four independent double-blind studies of GES have been conducted (Table 1).33-37 It has been difficult to demonstrate improvement during the double-blind period with gastric stimulation compared to no stimulation. Despite total symptom severity improvement and individual symptom improvements in these studies, a recent meta-analysis demonstrated a summative insignificant difference between the GES ON versus OFF states.38

In contrast to the double-blind studies, numerous open-label studies have demonstrated clinical improvements in patients with diabetic and idiopathic gastroparesis (Table 2),32,33,35,36,39-55 leading some to question whether the demonstrable efficacy reflects a placebo effect or regression to the mean. Patients may perceive an operative, aggressive intervention as likely to be effectual in comparison to incremental medication efforts, thus creating a placebo effect. It should also be noted that not all open-label studies have demonstrated improvement with GES. Indeed, Jones et al reported no significant difference in nausea and vomiting at 6-month follow-up, and recommended that physicians exercise caution with GES as a therapeutic strategy given the cost and lack of confirmed demonstrable effect.56 Thus, the clinical successes demonstrated in open-label studies must be weighed not only against the lack of unequivocal improvement, but also against the potential deleterious effects of the surgery.

In an open-label study that employed the GCSI to follow symptoms of gastroparesis, 29 patients underwent GES implantation over an 18-month period, with follow-up in 28 patients.44 GES resulted in clinical improvement in 50% of patients with refractory gastroparesis. The overall GCSI significantly decreased, with improvement in the nausea/vomiting subscore and the post-prandial fullness subscore, but no improvement in the bloating subscore or abdominal pain. The decrease in GCSI was greater for patients with diabetic versus idiopathic gastroparesis. Patients with the main symptom of nausea/vomiting had a greater improvement than patients with the main symptom of abdominal pain. Patients taking narcotic analgesics at the time of implant had a poorer response compared to patients who were not. In this study, 3 clinical parameters were associated with a favorable clinical response: (1) diabetic rather than idiopathic gastroparesis, (2) nausea/vomiting rather than abdominal pain as the primary symptom, and (3) independence from narcotic analgesics prior to stimulator implantation. Knowledge of these 3 factors may allow improved patient selection for GES.
A large prospective study by Heckert et al detailed marked improvements with GES and the patterns of those improvements.55 Nausea, vomiting, loss of appetite, and early satiety improved significantly with stimulator use, with a greater improvement in vomiting in patients with diabetic gastroparesis than in those with the idiopathic form. Although GES improved symptoms in 75% of all patients, patients with diabetes had a post-GES Clinical Patient Grading Assessment score that was statistically higher than the score among patients with idiopathic gastroparesis. This difference is thought to be due to the neuromolecular mechanism of diabetic gastroparesis, where blunting of the enteric nervous system may contribute to symptomatology.
Several studies have demonstrated a clinical response to GES in patients with postsurgical gastroparesis. A study by Oubre et al showed that GES led to weekly vomiting improvements as well as a reduction in total symptom severity score.57 A study by McCallum et al further demonstrated improved symptoms, quality of life, nutritional status, and hospitalization requirements.58 GES has also been shown to improve gastroparesis symptoms in pediatric populations.47,59 Thus, although not a direct indication, GES has been shown to be beneficial in various subtypes of gastroparesis.
Additionally, irrespective of gastroparesis type, the improved symptomatology with GES appears to be durable, with one study showing persistent clinical improvements up to 8 years after device placement.60 The improvements were persistent and incremental. Likewise, McCallum et al showed that continued reductions in total symptom severity scores were evident in all gastroparesis types up to 10 years after stimulator implantation.61 The success of the procedures in part comes from careful selection of patients. Clinical parameters that are associated with favorable clinical response include diabetic gastroparesis subtype, nausea/vomiting predominance, and independence from narcotic analgesics prior to stimulator placement.62
GES has also been noted to improve other patient care metrics besides symptomatology, including nutritional status, reduced need for nutritional supplementation, and improved HbA1c.63-65 Additionally, a study by Cutts et al established that health care resource utilization significantly improved at 12, 24, and 36 months following GES placement, as compared to patients receiving standard medical therapy.66 This decreased resource utilization was also reflected in decreased costs in the GES group compared with the standard care group.
Surgical Alternatives to GES
Pyloric interventions such as pyloroplasty and pyloromyotomy are other surgical treatment modalities offered for gastroparesis. Whereas GES uses neurostimulation to facilitate gastric emptying and potentially improve fundic accommodation, pyloric interventions are intended to increase gastric emptying by reducing outflow resistance from the pyloric sphincter.
Pyloric Interventions
Various studies have shown significant improvements with pyloric interventions, similar to the improvements seen with GES. One such study involving 177 patients demonstrated an 86% improvement in gastric emptying, with symptom severity scores for nausea, vomiting, bloating, abdominal pain, and early satiety decreasing significantly at 3 months following pyloroplasty.67 A significant advantage of pyloric interventions is that pyloromyotomy can be performed endoscopically (gastric peroral endoscopic pyloromyotomy [G-POEM] or peroral pyloromyotomy [POP]), thus minimizing the risks of open surgery. A recent review that included a pooled analysis of 7 studies of G-POEM for gastroparesis demonstrated 100% technical success, with clinical efficacy in 81.5% of the procedures as assessed by the GCSI.68 Additionally, the intraoperative and perioperative complication rates were 6.6% and 7.6%, respectively, suggesting that G-POEM is a safe and clinically beneficial therapeutic option. Few studies comparing the outcomes of pyloric interventions to GES have been performed.
Recently, GES has been combined with pyloric interventions to maximize therapeutic potential. This allows simultaneous neurologic and functional interventions to expedite gastric emptying and improve patient symptomatology. Davis et al demonstrated significant improvement in 21 patients who underwent GES placement and pyloroplasty, with 71% improvement in total symptom severity.69 Notably, dual surgery did not increase the incidence of infection or adverse surgical outcomes. Although this study did not directly compare dual surgery to GES alone, the results are nonetheless favorable. GES provides a strong antiemetic and anti-nausea effect, whereas the pyloromyotomy provides improvement in gastric emptying.
Feeding/Venting Tubes
Feeding jejunostomy tubes and venting gastrostomy tubes can be used alone or in combination with GES. Feeding jejunostomy is performed for malnutrition and weight loss that accompanies the refractory symptoms of early satiety, nausea, and vomiting. Venting gastrostomy tubes allow for removal of retained gastric contents that may cause distension, nausea, and vomiting. Gastrojejunostomy tubes can also be placed endoscopically or by interventional radiology.
Gastrectomy
Gastrectomy can provide therapeutic benefit through elimination of the gastric reservoir function and consequent removal of afferent neural impulses. In select patient populations, outcomes of gastrectomy have compared favorably with those of GES. For example, one study demonstrated favorable outcomes of Roux-en-Y gastrectomy in morbidly obese patients with gastroparesis.70 In another study, favorable outcomes were reported in a cohort of 103 patients, with gastrectomy demonstrating 87% symptom improvement (nausea, vomiting, epigastric pain) compared to just 63% improvement with GES.71 However, the dramatic impact on anatomy and physiology and the invasiveness of the procedure need to be weighed against the therapeutic benefit. For example, in the same study, the 30-day morbidity was 23% for gastrectomy versus just 8% for the GES implant.71
When to Use GES
The gastric electrical neurostimulator (Enterra; Medtronic, Inc.) is approved for treatment of idiopathic and diabetic gastroparesis that is refractory to medical treatment, performed on a compassionate basis. Patients with diabetic gastroparesis respond to GES better than do patients with the idiopathic form. Of the symptoms of gastroparesis, primarily nausea and vomiting improve. Thus, GES favors patients with diabetic gastroparesis who have primarily nausea and vomiting, rather than, for instance, patients with idiopathic gastroparesis who have primarily abdominal pain and may be taking narcotics. Some centers provide GES for postsurgical patients and children with gastroparesis.
The 3 main surgical interventions for medically refractory gastroparesis are GES, pyloric intervention (pyloroplasty or pyloromyotomy), and gastrectomy. Of the 3 interventions, gastrectomy is the most radical given its dramatic effect on anatomy and is thus not preferred. The clinical decision then becomes: GES, pyloric intervention, or both? There are limited data to support a definitive answer to this question.
In a single-center retrospective analysis of prospective data (electronic medical record), Arthur et al compared outcomes of GES patients with medically refractory gastroparesis who received various surgical interventions.72 In total, 33 stimulator, 7 pyloroplasty, 2 gastrectomy, and 16 combined stimulator and pyloroplasty patients were analyzed for postoperative symptom improvement. Pyloroplasty alone demonstrated the least symptom improvement, combination GES and pyloroplasty demonstrated increased improvement, and GES alone demonstrated the most improvement. The results of this study suggest that barring contraindication, placement of a gastric stimulator as the initial treatment is best, with pyloroplasty reserved for patients who do not achieve adequate symptom control. Limitations of the study include its single-center design and low patient numbers for pyloroplasty in isolation.
In contrast, a recent retrospective systematic review synthesized the outcomes of various studies of GES and pyloric interventions for medically refractory gastroparesis.73 A therapeutic effect was found for each surgical intervention, with pyloric surgery patients demonstrating a greater response to intervention than GES patients. Unfortunately, attempts to analyze combination interventions were hindered by a lack of power.
Conclusion
Initial management of gastroparesis is medical (lifestyle and diet changes), with antiemetic and prokinetic agents used in refractory cases. Following failure of this therapy, placement of a GES device is a surgical intervention that has been approved under FDA humanitarian device exemption to help ameliorate symptomatology. Improvement with GES has been demonstrated in nonblinded studies, but the lack of randomized controlled trials demonstrating benefit suggests the possibility of an underlying placebo effect. Additionally, new medical procedures such as G-POEM complicate the decision of which intervention should be attempted first. More studies, specifically comparing GES, pyloric interventions, and combined GES with pyloric intervention to placebo, are needed to fully understand what therapy is best for refractory gastroparesis.
Corresponding author: Henry P. Parkman, MD, Gastroenterology Section, Temple University School of Medicine, 3401 North Broad Street, Philadelphia, PA 19140; [email protected].
Financial disclosures: None.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
1. Camilleri M, Parkman HP, Shafi MA, et al. Clinical Guideline: Management of gastroparesis. Am J Gastroenterol. 2013;108:18-37.
2. Jehangir A, Parkman HP. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig Dis Sci. 2018;63:2231-2243.
3. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592-1622.
4. Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101-115.
5. Bityutskiy LP, Soykan I, McCallum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis--clinical characteristics and long-term outcomes. Am J Gastroenterol. 1997;92:1501-1504.
6. Kebede D, Barthel JS, Singh A. Transient gastroparesis associated with cutaneous herpes zoster. Dig Dis Sci. 1987;32:318-322.
7. Meeroff JC, Schreiber DS, Trier JS, Blacklow NR. Abnormal gastric motor function in viral gastroenteritis. Ann Intern Med. 1980;92:370-373.
8. Paliwal M, Prasanna KS, Saraswat VA, et al. Varicella zoster cranial polyneuropathy presenting with dysphagia, esophagitis and gastroparesis. J Neurogastroenterol Motil. 2011;17:192-194.
9. Sigurdsson L, Flores A, Putnam PE, et al. Postviral gastroparesis: presentation, treatment, and outcome. J Pediatr. 1997;131:751-754.
10. Kockar MC, Kayahan IK, Bavbek N. Diabetic gastroparesis in association with autonomic neuropathy and microvasculopathy. Acta Med Okayama. 2002;56:237-243.
11. Jung HK, Choung RS, Locke GR III, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233.
12. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34-42.
13. Wang YR, Fisher RS. Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995-2004. Am J Gastroenterol. 2008;103:313-322.
14. Parkman HP, Camilleri M, Farrugia G, et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol Motil. 2010;22:113-133.
15. Soykan I, Sivri B, Sarosiek I, et al. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398-2404.
16. Cherian D, Sachdeva P, Fisher RS, Parkman HP. Abdominal pain is a frequent symptom of gastroparesis. Clin Gastroenterol Hepatol. 2010;8:676-681.
17. Hasler WL, Wilson LA, Parkman HP, et al. Factors related to abdominal pain in gastroparesis: contrast to patients with predominant nausea and vomiting. Neurogastroenterol Motil. 2013;25:427-438.
18. Jehangir A, Abdallah RT, Parkman HP. Characterizing abdominal pain in patients with gastroparesis into neuropathic and nociceptive components. J Clin Gastroenterol. 2018 May 18. doi: 10.1097/MCG.0000000000001059.
19. Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am. 2015;44:1-7.
20. Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol. 2018;14:140-145.
21. Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. 2014;63:1972-1978.
22. Revicki DA, Camilleri M, Kuo B, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670-680.
23. Revicki DA, Camilleri M, Kuo B, et al. Evaluating symptom outcomes in gastroparesis clinical trials: validity and responsiveness of the Gastroparesis Cardinal Symptom Index-Daily Diary (GCSI-DD). Neurogastroenterol Motil. 2012;24:456-463.
24. Bharucha AE. Epidemiology and natural history of gastroparesis. Gastroenterol Clin North Am. 2015;44:9-19.
25. Soffer E, Abell T, Lin Z, et al. Review article: Gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681-694.
26. Qin C, Chen JD, Zhang J, Foreman RD. Modulatory effects and afferent pathways of gastric electrical stimulation on rat thoracic spinal neurons receiving input from the stomach. Neurosci Res. 2007;57:29-39
27. Bielefeldt K. Adverse events of gastric electrical stimulators recorded in the Manufacturer and User Device Experience (MAUDE) Registry. Auton Neurosci. 2017;202:40-44
28. Liu RC, Sabnis AA, Chand B. Erosion of gastric electrical stimulator electrodes: evaluation, management, and laparoscopic techniques. Surg Laparosc Endosc Percutan Tech. 2007;17:438-441.
29. Harrison NS, Williams PA, Walker MR, et al. Evaluation and treatment of gastric stimulator failure in patients with gastroparesis. Surg Innov. 2014;21:244-249.
30. Familoni BO, Abell TL, Nemoto D, et al. Electrical stimulation at a frequency higher than basal rate in human stomach. Dig Dis Sci. 1997;42:885-891.
31. Familoni BO, Abell TL, Nemoto D, et al. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892-897.
32. Abell TL, Van Cutsem E, Abrahamsson H, et al. Gastric electrical stimulation in intractable symptomatic gastroparesis. Digestion. 2002;66:204-212.
33. Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421-428.
34. Frøkjaer JB, Ejskjaer N, Rask P, et al. Central neuronal mechanisms of gastric electrical stimulation in diabetic gastroparesis. Scand J Gastroenterol. 2008;43:1066-1075.
35. McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-836.
36. McCallum RW, Snape W, Brody F, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947-954.
37. Abell TL, Johnson WD, Kedar A, et al. A double-masked, randomized, placebo-controlled trial of temporary endoscopic mucosal gastric electrical stimulation for gastroparesis. Gastrointest Endosc. 2011;74:496-503.
38. Levinthal DJ. Systematic review and meta-analysis: Gastric electrical stimulation for gastroparesis. Auton Neurosci. 2017;202:45-55.
39. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
40. Mason RJ, Lipham J, Eckerling G, et al. Gastric electrical stimulation: An alternative surgical therapy for patients with gastroparesis. Arch Surg. 2005;140:841-846.
41. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
42. van der Voort IR, Becker JC, Dietl KH, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38-42.
43. de Csepel J, Goldfarb B, Shapsis A, et al. Electrical stimulation for gastroparesis. gastric motility restored. Surg Endosc. 2006;20:302-306.
44. Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072-2078.
45. Filichia LA, Cendan CJ. Small case series of gastric stimulation for the management of transplant-induced gastroparesis. J Surg Res. 2008;148:90-93.
46. Lin Z, Hou Q, Sarosiek I, et al. Association between changes in symptoms and gastric emptying in gastroparetic patients treated with gastric electrical stimulation. Neurogastroenterol Motil. 2008;20:464-470.
47. Islam S, Vick LR, Runnels MJ, et al. Gastric electrical stimulation for children with intractable nausea and gastroparesis. J Pediatr Surg. 2008;43:437-442.
48. Brody F, Vaziri K, Saddler A, et al. Gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2008;207:533-538.
49. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
50. Teich S, Mousa HM, Punati J, Di Lorenzo C. Efficacy of permanent gastric electrical stimulation for the treatment of gastroparesis and functional dyspepsia in children and adolescents. J Pediatr Surg. 2013;48:178-183.
51. Lahr CJ, Griffith J, Subramony C, et al. Gastric electrical stimulation for abdominal pain in patients with symptoms of gastroparesis. Am Surg. 2013;79:457-464.
52. Keller DS, Parkman HP, Boucek DO, et al. Surgical outcomes after gastric electric stimulator placement for refractory gastroparesis. J Gastrointest Surg. 2013;17:620-626.
53. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
54. Richmond B, Chong B, Modak A, et al. Gastric electrical stimulation for refractory gastroparesis: Predictors of response and redefining a successful outcome. Am Surg. 2015;81:467-471.
55. Heckert J, Sankineni A, Hughes WB, et al. Gastric electric stimulation for refractory gastroparesis: A prospective analysis of 151 patients at a single center. Dig Dis Sci. 2016;61:168-175.
56. Jones MP, Ebert CC, Murayama K. Enterra for gastroparesis. Am J Gastroenterol. 2003;98:2578.
57. Oubre B, Luo J, Al-Juburi A, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: Long-term outcome. South Med J. 2005;98:693-697.
58. McCallum R, Lin Z, Wetzel P, et al. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49-54.
59. Islam S, McLaughlin J, Pierson J, et al. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71.
60. Brody F, Zettervall SL, Richards NG, et al. Follow-up after gastric electrical stimulation for gastroparesis. J Am Coll Surg. 2015;220:57-63.
61. McCallum RW, Lin Z, Forster J, et al. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314-319.
62. Maranki J, Lytes V, Meilahn JE, et al. Dig Dis Sci. 2008 53:2072-2078.
63. Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. JPEN J Parenter Enteral Nutr. 2003;27:277-281.
64. Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071-1076.
65. Lin Z, McElhinney C, Sarosiek I, et al. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328-1334.
66. Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35-43.
67. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326-1332.
68. Khoury T, Mizrahi M, Mahamid M, et al. State of the art review with literature summary on gastric peroral endoscopic pyloromyotomy for gastroparesis. J Gastroenterol Hepatol. 2018;33:1829-1833.
69. Davis BR, Sarosiek I, Bashashati M, et al. The long-term efficacy and safety of pyloroplasty combined with gastric electrical stimulation therapy in gastroparesis. J Gastrointest Surg. 2017;21:222-227.
70. Sun Z, Rodriguez J, McMichael J, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683-2689.
71. Zehetner J, Ravari F, Ayazi S, et al. Minimally invasive surgical approach for the treatment of gastroparesis. Surg Endosc. 2013;27:61-66.
72. Arthur LE, Slattery L, Richardson W. Tailored approach to gastroparesis significantly improves symptoms. Surg Endosc. 2017;32:977-982.
73. Zoll B, Zhao H, Edwards MA, et al. Outcomes of surgical intervention for refractory gastroparesis: A systematic review. J Surg Res. 2018;231:263-269.
Insulin may be toxic to the placenta in early pregnancy
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
FROM FERTILITY & STERILITY
Key clinical point: Trophoblasts cultured during the first trimester of pregnancy exposed to insulin were more likely to have increased apoptosis, DNA damage, and decreased cell survival, while pretreatment with metformin prior to exposure with insulin prevented these effects.
Major finding: DNA damage and rate of apoptosis increased in trophoblast cells exposed to 1 nmol of insulin, and cell survival decreased, compared with primary lung fibroblast cells; treating the cells with metformin prior to exposure with insulin resulted in prevention of these effects.
Study details: An experimental in vitro study of first trimester trophoblast cells exposed to insulin and metformin.
Disclosures: This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported they had no relevant financial disclosures.
Source: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
SGLT inhibitor still possible for T1DM, despite FDA committee vote
on recommendation for approval from the Food and Drug Administration’s Endocrinologic and Metabolic Drugs Advisory Committee.
In the company’s three trials, involving about 3,000 insulin-dependent adults treated for up to a year, the drug lowered hemoglobin A1c a respectable 0.5% without increasing hypoglycemia risk; reduced glucose variability; and increased time in range, with some modest benefits in both weight loss and lower blood pressure. There was no sign of the increased amputation risk that has bedeviled the sodium-glucose cotransporter 2 (SGLT2) inhibitor canagliflozin (Invokana), already on the market for type 2 diabetes mellitus.
The fly in the ointment was diabetic ketoacidosis (DKA); the drug increased the risk eightfold versus placebo, and, although there were no DKA deaths and over 60% of patients resumed sotagliflozin after recovering, the cases were serious and sometimes occurred in patients with glucose levels as low as 150 mg/dL. Younger people and women seemed to be at higher risk, according to the data.
DKA risk was 4 cases per 100 patients/year, a 4% risk, and that was in the ideal setting of a trial, not everyday practice. The annual background risk of DKA is 1% or less in type 1 diabetes mellitus (T1DM).
“It’s got to be safer than this,” said committee chair Peter Wilson, MD, professor of cardiology and public health at Emory University, Atlanta.
Dr. Wilson voted to recommend approval but with the major caveat that Sanofi have a strong risk mitigation program in place, perhaps based on ketone monitoring to catch emerging DKA before people end up in the ED. That was a universal request among others who voted for recommendation; among those who voted against, the concern in large part was that, even with such a program, the risk of DKA was still too high.
“If they had already developed a mitigation program that had been piloted, and they showed us some data, there would have been more enthusiasm, but we didn’t have that,” he said in an interview after the hearing.
Sanofi did suggest possible risk mitigation strategies during the meeting. In a statement afterwards, spokesman Nicolas Kressmann said, “While we acknowledge the increase in incidence of DKA observed with the addition of sotagliflozin to insulin, we believe that the risks may be mitigated and managed with proper patient selection and education regarding appropriate ketone monitoring. We will continue to work with the FDA to ensure the agency has the data it needs to evaluate the safety and efficacy of sotagliflozin when used as an oral treatment together with insulin by adults with T1DM. We are confident in the data of our T1DM clinical program.”
Meanwhile, the company’s development for T2DMs is ongoing, with results from a number of trials expected later in 2019. Sotagliflozin would join canagliflozin and two other SGLT2 inhibitors already on the market for T2DM, none of which have been approved for T1DM disease. The approved drugs work by increasing renal glucose excretion.
A significant proportion of DKA cases in sotagliflozin’s T1DM trials were preceded by infections and other well-known triggers, “but there were a proportion of patients where they couldn’t identify the cause; it just kind of came out of the blue. Something about the medication lowers the threshold,” said panelist and endocrinologist Cecilia Low Wang, MD, director of the glucose management team at the University of Colorado Anschutz Medical Campus, Aurora, who voted against recommending approval.
“There’s definitely an increased risk” with other SGLT2 inhibitors, as well, when used off label for T1DM. “No one really knows why,” she said.
Dr. Wilson was also concerned that insulin wasn’t more tightly titrated in the placebo groups, which might have led to the 0.5% improvement seen with sotagliflozin, but “they wanted to have trials that were likely to be beneficial, so it’s reasonable to do what they did,” he said.
Overall, “we don’t really have many options for type 1, and many of us were sympathetic to the idea of increasing options.” In T1DM, “you can lose your concentration” on insulin dosing for a couple hours, “and the next thing you know you are going too high or too low and going off the road. These pills help smooth out your ups and downs. I would like to think [sotagliflozin] might be approved for a restricted group, for which we’ve really sorted out the ketone data,” he said.
Dr. Wilson and Dr. Low Wang did not have any disclosures.
on recommendation for approval from the Food and Drug Administration’s Endocrinologic and Metabolic Drugs Advisory Committee.
In the company’s three trials, involving about 3,000 insulin-dependent adults treated for up to a year, the drug lowered hemoglobin A1c a respectable 0.5% without increasing hypoglycemia risk; reduced glucose variability; and increased time in range, with some modest benefits in both weight loss and lower blood pressure. There was no sign of the increased amputation risk that has bedeviled the sodium-glucose cotransporter 2 (SGLT2) inhibitor canagliflozin (Invokana), already on the market for type 2 diabetes mellitus.
The fly in the ointment was diabetic ketoacidosis (DKA); the drug increased the risk eightfold versus placebo, and, although there were no DKA deaths and over 60% of patients resumed sotagliflozin after recovering, the cases were serious and sometimes occurred in patients with glucose levels as low as 150 mg/dL. Younger people and women seemed to be at higher risk, according to the data.
DKA risk was 4 cases per 100 patients/year, a 4% risk, and that was in the ideal setting of a trial, not everyday practice. The annual background risk of DKA is 1% or less in type 1 diabetes mellitus (T1DM).
“It’s got to be safer than this,” said committee chair Peter Wilson, MD, professor of cardiology and public health at Emory University, Atlanta.
Dr. Wilson voted to recommend approval but with the major caveat that Sanofi have a strong risk mitigation program in place, perhaps based on ketone monitoring to catch emerging DKA before people end up in the ED. That was a universal request among others who voted for recommendation; among those who voted against, the concern in large part was that, even with such a program, the risk of DKA was still too high.
“If they had already developed a mitigation program that had been piloted, and they showed us some data, there would have been more enthusiasm, but we didn’t have that,” he said in an interview after the hearing.
Sanofi did suggest possible risk mitigation strategies during the meeting. In a statement afterwards, spokesman Nicolas Kressmann said, “While we acknowledge the increase in incidence of DKA observed with the addition of sotagliflozin to insulin, we believe that the risks may be mitigated and managed with proper patient selection and education regarding appropriate ketone monitoring. We will continue to work with the FDA to ensure the agency has the data it needs to evaluate the safety and efficacy of sotagliflozin when used as an oral treatment together with insulin by adults with T1DM. We are confident in the data of our T1DM clinical program.”
Meanwhile, the company’s development for T2DMs is ongoing, with results from a number of trials expected later in 2019. Sotagliflozin would join canagliflozin and two other SGLT2 inhibitors already on the market for T2DM, none of which have been approved for T1DM disease. The approved drugs work by increasing renal glucose excretion.
A significant proportion of DKA cases in sotagliflozin’s T1DM trials were preceded by infections and other well-known triggers, “but there were a proportion of patients where they couldn’t identify the cause; it just kind of came out of the blue. Something about the medication lowers the threshold,” said panelist and endocrinologist Cecilia Low Wang, MD, director of the glucose management team at the University of Colorado Anschutz Medical Campus, Aurora, who voted against recommending approval.
“There’s definitely an increased risk” with other SGLT2 inhibitors, as well, when used off label for T1DM. “No one really knows why,” she said.
Dr. Wilson was also concerned that insulin wasn’t more tightly titrated in the placebo groups, which might have led to the 0.5% improvement seen with sotagliflozin, but “they wanted to have trials that were likely to be beneficial, so it’s reasonable to do what they did,” he said.
Overall, “we don’t really have many options for type 1, and many of us were sympathetic to the idea of increasing options.” In T1DM, “you can lose your concentration” on insulin dosing for a couple hours, “and the next thing you know you are going too high or too low and going off the road. These pills help smooth out your ups and downs. I would like to think [sotagliflozin] might be approved for a restricted group, for which we’ve really sorted out the ketone data,” he said.
Dr. Wilson and Dr. Low Wang did not have any disclosures.
on recommendation for approval from the Food and Drug Administration’s Endocrinologic and Metabolic Drugs Advisory Committee.
In the company’s three trials, involving about 3,000 insulin-dependent adults treated for up to a year, the drug lowered hemoglobin A1c a respectable 0.5% without increasing hypoglycemia risk; reduced glucose variability; and increased time in range, with some modest benefits in both weight loss and lower blood pressure. There was no sign of the increased amputation risk that has bedeviled the sodium-glucose cotransporter 2 (SGLT2) inhibitor canagliflozin (Invokana), already on the market for type 2 diabetes mellitus.
The fly in the ointment was diabetic ketoacidosis (DKA); the drug increased the risk eightfold versus placebo, and, although there were no DKA deaths and over 60% of patients resumed sotagliflozin after recovering, the cases were serious and sometimes occurred in patients with glucose levels as low as 150 mg/dL. Younger people and women seemed to be at higher risk, according to the data.
DKA risk was 4 cases per 100 patients/year, a 4% risk, and that was in the ideal setting of a trial, not everyday practice. The annual background risk of DKA is 1% or less in type 1 diabetes mellitus (T1DM).
“It’s got to be safer than this,” said committee chair Peter Wilson, MD, professor of cardiology and public health at Emory University, Atlanta.
Dr. Wilson voted to recommend approval but with the major caveat that Sanofi have a strong risk mitigation program in place, perhaps based on ketone monitoring to catch emerging DKA before people end up in the ED. That was a universal request among others who voted for recommendation; among those who voted against, the concern in large part was that, even with such a program, the risk of DKA was still too high.
“If they had already developed a mitigation program that had been piloted, and they showed us some data, there would have been more enthusiasm, but we didn’t have that,” he said in an interview after the hearing.
Sanofi did suggest possible risk mitigation strategies during the meeting. In a statement afterwards, spokesman Nicolas Kressmann said, “While we acknowledge the increase in incidence of DKA observed with the addition of sotagliflozin to insulin, we believe that the risks may be mitigated and managed with proper patient selection and education regarding appropriate ketone monitoring. We will continue to work with the FDA to ensure the agency has the data it needs to evaluate the safety and efficacy of sotagliflozin when used as an oral treatment together with insulin by adults with T1DM. We are confident in the data of our T1DM clinical program.”
Meanwhile, the company’s development for T2DMs is ongoing, with results from a number of trials expected later in 2019. Sotagliflozin would join canagliflozin and two other SGLT2 inhibitors already on the market for T2DM, none of which have been approved for T1DM disease. The approved drugs work by increasing renal glucose excretion.
A significant proportion of DKA cases in sotagliflozin’s T1DM trials were preceded by infections and other well-known triggers, “but there were a proportion of patients where they couldn’t identify the cause; it just kind of came out of the blue. Something about the medication lowers the threshold,” said panelist and endocrinologist Cecilia Low Wang, MD, director of the glucose management team at the University of Colorado Anschutz Medical Campus, Aurora, who voted against recommending approval.
“There’s definitely an increased risk” with other SGLT2 inhibitors, as well, when used off label for T1DM. “No one really knows why,” she said.
Dr. Wilson was also concerned that insulin wasn’t more tightly titrated in the placebo groups, which might have led to the 0.5% improvement seen with sotagliflozin, but “they wanted to have trials that were likely to be beneficial, so it’s reasonable to do what they did,” he said.
Overall, “we don’t really have many options for type 1, and many of us were sympathetic to the idea of increasing options.” In T1DM, “you can lose your concentration” on insulin dosing for a couple hours, “and the next thing you know you are going too high or too low and going off the road. These pills help smooth out your ups and downs. I would like to think [sotagliflozin] might be approved for a restricted group, for which we’ve really sorted out the ketone data,” he said.
Dr. Wilson and Dr. Low Wang did not have any disclosures.
(Sponsored) Video Roundtable: Basal Insulin Pharmacology

Pharmacokinetic (PK) and pharmacodynamic (PD) properties of drugs form the basis for the development of drugs used in everyday clinical practice, such as commonly used insulin products. PK measures the concentration of a drug in the body, and reflects the rates and amounts absorbed and processed. PD is the biologic effect of a drug in the body, including the time-course of when the biologic effect starts, peaks, and ends. While the determination of PK/PD parameters is important and foundational for the development of different insulin products, studies are often complex and can be difficult to translate into real-world clinical practice. In this roundtable, the speakers discuss PK/PD concepts, focusing on the differentiation of basal insulin analogs and their use in individualized diabetes therapy.
First, the speakers discuss the euglycemic glucose clamp methodology—the standard technique for evaluating PK/PD of insulin—including how it is performed, what parameters it measures (and how they can be interpreted), and its limitations.
Next, the speakers discuss how PK/PD impacts drug development, with particular focus on PK/PD studies used in the development of the second-generation basal insulin analogs insulin glargine 300 U/mL (Gla-300) and insulin degludec.
Finally, the speakers discuss how PK/PD data translate into clinical practice, including the relationship between PK/PD and drug efficacy and safety, and how it influences dosing strategies, hypoglycemia risk, and patient education. Further, the speakers discuss how the PK/PD profile of basal insulins can inform primary care providers when selecting appropriate individualized therapy for patients.
Financial support provided by Sanofi US, Inc.

Pharmacokinetic (PK) and pharmacodynamic (PD) properties of drugs form the basis for the development of drugs used in everyday clinical practice, such as commonly used insulin products. PK measures the concentration of a drug in the body, and reflects the rates and amounts absorbed and processed. PD is the biologic effect of a drug in the body, including the time-course of when the biologic effect starts, peaks, and ends. While the determination of PK/PD parameters is important and foundational for the development of different insulin products, studies are often complex and can be difficult to translate into real-world clinical practice. In this roundtable, the speakers discuss PK/PD concepts, focusing on the differentiation of basal insulin analogs and their use in individualized diabetes therapy.
First, the speakers discuss the euglycemic glucose clamp methodology—the standard technique for evaluating PK/PD of insulin—including how it is performed, what parameters it measures (and how they can be interpreted), and its limitations.
Next, the speakers discuss how PK/PD impacts drug development, with particular focus on PK/PD studies used in the development of the second-generation basal insulin analogs insulin glargine 300 U/mL (Gla-300) and insulin degludec.
Finally, the speakers discuss how PK/PD data translate into clinical practice, including the relationship between PK/PD and drug efficacy and safety, and how it influences dosing strategies, hypoglycemia risk, and patient education. Further, the speakers discuss how the PK/PD profile of basal insulins can inform primary care providers when selecting appropriate individualized therapy for patients.
Financial support provided by Sanofi US, Inc.

Pharmacokinetic (PK) and pharmacodynamic (PD) properties of drugs form the basis for the development of drugs used in everyday clinical practice, such as commonly used insulin products. PK measures the concentration of a drug in the body, and reflects the rates and amounts absorbed and processed. PD is the biologic effect of a drug in the body, including the time-course of when the biologic effect starts, peaks, and ends. While the determination of PK/PD parameters is important and foundational for the development of different insulin products, studies are often complex and can be difficult to translate into real-world clinical practice. In this roundtable, the speakers discuss PK/PD concepts, focusing on the differentiation of basal insulin analogs and their use in individualized diabetes therapy.
First, the speakers discuss the euglycemic glucose clamp methodology—the standard technique for evaluating PK/PD of insulin—including how it is performed, what parameters it measures (and how they can be interpreted), and its limitations.
Next, the speakers discuss how PK/PD impacts drug development, with particular focus on PK/PD studies used in the development of the second-generation basal insulin analogs insulin glargine 300 U/mL (Gla-300) and insulin degludec.
Finally, the speakers discuss how PK/PD data translate into clinical practice, including the relationship between PK/PD and drug efficacy and safety, and how it influences dosing strategies, hypoglycemia risk, and patient education. Further, the speakers discuss how the PK/PD profile of basal insulins can inform primary care providers when selecting appropriate individualized therapy for patients.
Financial support provided by Sanofi US, Inc.
Lessons learned from the PCSK9 inhibitor trials
LOS ANGELES – , according to the best available data.
In addition, there does not seem to be a floor of achieved LDL-C levels where cardiovascular outcomes are not decreased.
Those are two key lessons from recent trials of PCSK9 inhibitors that Norman E. Lepor, MD, highlighted at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. Anti-PCSK9 antibodies have shown a significant lipid-lowering effect, lowering LDL-C by 45%-55% from baseline regardless of whether patients are on statins or ezetimibe. More than 70% of high-risk patients are able to achieve an LDL-C level less than 70 mg/dL.
In two randomized trials known as SPIRE-1 and SPIRE-2, researchers compared the PCSK9 inhibitor bococizumab with placebo (N Engl J Med 2017;376:1527-39). The main difference between these two trials was the baseline cardiovascular risk was higher in SPIRE-2, particularly the baseline LDL levels. “Both trials were stopped early because of the increased incidence of antibodies against bococizumab as well as adverse events associated with that, particularly injection site reactions,” said Dr. Lepor, a cardiologist who is professor of medicine at the Geffen School of Medicine at UCLA.
SPIRE-1 did not show a significant difference within a short time of that trial, but SPIRE-2 showed a profound reduction in cardiovascular events within a short time. “That led to the conclusion that the higher the LDL, the higher risk you are, the more likely that you’re going to attain a more robust reduction of cardiovascular events,” Dr. Lepor said. Bococizumab, a partially humanized antibody, did not come to market because of the high incidence of anti-drug antibody and associated diminution of therapeutic effect, he added.
Next came the FOURIER trial of evolocumab, a randomized, double-blind, placebo-controlled trial involving 27,564 patients with atherosclerotic cardiovascular disease and LDL cholesterol levels of 70 mg/dL or higher who were receiving statin therapy. Study participants received evolocumab (either 140 mg every 2 weeks or 420 mg monthly) or matching placebo as subcutaneous injections (N Engl J Med. 2017;376:1713-22). “The reduction in LDL was very robust and occurred quite early, and there was consistency in terms of long-term effect,” said Dr. Lepor, a past president of the California chapter of the American College of Cardiology. Specifically, researchers observed a 15% reduction in the primary endpoints of a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization (P less than .001), and a 20% reduction in the key secondary endpoints of a composite of cardiovascular death, MI, or stroke.
“It’s important to note that these are patients who were already well treated on statins,” he said. “There really does not seem to be a floor of LDL levels where we can say there is no further benefit. Achieved LDL-C at 4 weeks did lead to the ability to prognosticate benefit. We’re not seeing an increase in situations like diabetes, neurocognitive effects, and myalgias. The incidence of adverse events are similar to placebo. We feel very good that there do not seem to be safety issues for these agents, particularly in a population of patients with very low LDLs.” Dr. Lepor went on to note that FOURIER substudies have shown that there did not seem to be any increased incidence of neurocognitive disorders in patients taking evolocumab and that diabetes “tends to be a disease amplifier.”
In a more recent trial known as ODYSSEY OUTCOMES, 18,536 acute coronary syndrome (ACS) patients were randomized to alirocumab 75 mg once every 2 weeks or placebo (N Engl J Med. 2018;379:2097-107). The dose of alirocumab was increased to 150 mg every 2 weeks if the LDL was not lowered to less than 50 mg/dL. “The trial had other interesting aspects to it,” Dr. Lepor said. “If you had an LDL during the trial of less than 25 mg/dL, you were down-titrated from the 150-mg dose to the 75-mg dose, or if you were on the 75-mg dose with two consecutive LDLs less than 15 mg/dL, the alirocumab was stopped, all in blinded fashion.” The primary outcome was time to first occurrence of CHD death, nonfatal MI, ischemic stroke, or hospitalization for unstable angina.
At baseline, patients had a median age of 58 years, 25% were women, their median LDL was 92 mg/dL. About 89% of patients were on high dose atorvastatin/rosuvastatin. Time from index ACS to randomization was a median of 2.6 months.
The primary efficacy endpoint was major adverse cardiac events including CHD death, nonfatal MI, ischemic stroke, or unstable angina requiring hospitalization. After a median follow-up of 2.8 years, the researchers observed highly significant 15% reduction of the primary endpoint with alirocumab compared with placebo (hazard ratio, 0.85; P = .0001), and a 15% reduction of death from any cause. In patients with a baseline LDL-C of 100 mg/dL or greater, the benefits of alirocumab were further amplified. “The higher your LDL, the higher your risk,” Dr. Lepor said. “The higher your risk, the greater your benefit from therapeutic intervention.”
He concluded his presentation by likening the treatment approach with PCSK9 inhibitors in ACS patients to that of chemotherapy in cancer patients. “In patients who have the very high risk of recurrence, those are the ones we want to attack with tougher chemotherapy,” he said. “Who are the patients who benefit most from PCSK9 inhibitors? ACS patients and those who are breaking through statin therapy with cardiovascular events, those with diabetes, CKD, and peripheral vascular disease.”
Dr. Lepor disclosed that he serves on the advisory board for Sanofi/Regeneron and is on the speakers bureau for Amgen and Sanofi/Regeneron.
LOS ANGELES – , according to the best available data.
In addition, there does not seem to be a floor of achieved LDL-C levels where cardiovascular outcomes are not decreased.
Those are two key lessons from recent trials of PCSK9 inhibitors that Norman E. Lepor, MD, highlighted at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. Anti-PCSK9 antibodies have shown a significant lipid-lowering effect, lowering LDL-C by 45%-55% from baseline regardless of whether patients are on statins or ezetimibe. More than 70% of high-risk patients are able to achieve an LDL-C level less than 70 mg/dL.
In two randomized trials known as SPIRE-1 and SPIRE-2, researchers compared the PCSK9 inhibitor bococizumab with placebo (N Engl J Med 2017;376:1527-39). The main difference between these two trials was the baseline cardiovascular risk was higher in SPIRE-2, particularly the baseline LDL levels. “Both trials were stopped early because of the increased incidence of antibodies against bococizumab as well as adverse events associated with that, particularly injection site reactions,” said Dr. Lepor, a cardiologist who is professor of medicine at the Geffen School of Medicine at UCLA.
SPIRE-1 did not show a significant difference within a short time of that trial, but SPIRE-2 showed a profound reduction in cardiovascular events within a short time. “That led to the conclusion that the higher the LDL, the higher risk you are, the more likely that you’re going to attain a more robust reduction of cardiovascular events,” Dr. Lepor said. Bococizumab, a partially humanized antibody, did not come to market because of the high incidence of anti-drug antibody and associated diminution of therapeutic effect, he added.
Next came the FOURIER trial of evolocumab, a randomized, double-blind, placebo-controlled trial involving 27,564 patients with atherosclerotic cardiovascular disease and LDL cholesterol levels of 70 mg/dL or higher who were receiving statin therapy. Study participants received evolocumab (either 140 mg every 2 weeks or 420 mg monthly) or matching placebo as subcutaneous injections (N Engl J Med. 2017;376:1713-22). “The reduction in LDL was very robust and occurred quite early, and there was consistency in terms of long-term effect,” said Dr. Lepor, a past president of the California chapter of the American College of Cardiology. Specifically, researchers observed a 15% reduction in the primary endpoints of a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization (P less than .001), and a 20% reduction in the key secondary endpoints of a composite of cardiovascular death, MI, or stroke.
“It’s important to note that these are patients who were already well treated on statins,” he said. “There really does not seem to be a floor of LDL levels where we can say there is no further benefit. Achieved LDL-C at 4 weeks did lead to the ability to prognosticate benefit. We’re not seeing an increase in situations like diabetes, neurocognitive effects, and myalgias. The incidence of adverse events are similar to placebo. We feel very good that there do not seem to be safety issues for these agents, particularly in a population of patients with very low LDLs.” Dr. Lepor went on to note that FOURIER substudies have shown that there did not seem to be any increased incidence of neurocognitive disorders in patients taking evolocumab and that diabetes “tends to be a disease amplifier.”
In a more recent trial known as ODYSSEY OUTCOMES, 18,536 acute coronary syndrome (ACS) patients were randomized to alirocumab 75 mg once every 2 weeks or placebo (N Engl J Med. 2018;379:2097-107). The dose of alirocumab was increased to 150 mg every 2 weeks if the LDL was not lowered to less than 50 mg/dL. “The trial had other interesting aspects to it,” Dr. Lepor said. “If you had an LDL during the trial of less than 25 mg/dL, you were down-titrated from the 150-mg dose to the 75-mg dose, or if you were on the 75-mg dose with two consecutive LDLs less than 15 mg/dL, the alirocumab was stopped, all in blinded fashion.” The primary outcome was time to first occurrence of CHD death, nonfatal MI, ischemic stroke, or hospitalization for unstable angina.
At baseline, patients had a median age of 58 years, 25% were women, their median LDL was 92 mg/dL. About 89% of patients were on high dose atorvastatin/rosuvastatin. Time from index ACS to randomization was a median of 2.6 months.
The primary efficacy endpoint was major adverse cardiac events including CHD death, nonfatal MI, ischemic stroke, or unstable angina requiring hospitalization. After a median follow-up of 2.8 years, the researchers observed highly significant 15% reduction of the primary endpoint with alirocumab compared with placebo (hazard ratio, 0.85; P = .0001), and a 15% reduction of death from any cause. In patients with a baseline LDL-C of 100 mg/dL or greater, the benefits of alirocumab were further amplified. “The higher your LDL, the higher your risk,” Dr. Lepor said. “The higher your risk, the greater your benefit from therapeutic intervention.”
He concluded his presentation by likening the treatment approach with PCSK9 inhibitors in ACS patients to that of chemotherapy in cancer patients. “In patients who have the very high risk of recurrence, those are the ones we want to attack with tougher chemotherapy,” he said. “Who are the patients who benefit most from PCSK9 inhibitors? ACS patients and those who are breaking through statin therapy with cardiovascular events, those with diabetes, CKD, and peripheral vascular disease.”
Dr. Lepor disclosed that he serves on the advisory board for Sanofi/Regeneron and is on the speakers bureau for Amgen and Sanofi/Regeneron.
LOS ANGELES – , according to the best available data.
In addition, there does not seem to be a floor of achieved LDL-C levels where cardiovascular outcomes are not decreased.
Those are two key lessons from recent trials of PCSK9 inhibitors that Norman E. Lepor, MD, highlighted at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease. Anti-PCSK9 antibodies have shown a significant lipid-lowering effect, lowering LDL-C by 45%-55% from baseline regardless of whether patients are on statins or ezetimibe. More than 70% of high-risk patients are able to achieve an LDL-C level less than 70 mg/dL.
In two randomized trials known as SPIRE-1 and SPIRE-2, researchers compared the PCSK9 inhibitor bococizumab with placebo (N Engl J Med 2017;376:1527-39). The main difference between these two trials was the baseline cardiovascular risk was higher in SPIRE-2, particularly the baseline LDL levels. “Both trials were stopped early because of the increased incidence of antibodies against bococizumab as well as adverse events associated with that, particularly injection site reactions,” said Dr. Lepor, a cardiologist who is professor of medicine at the Geffen School of Medicine at UCLA.
SPIRE-1 did not show a significant difference within a short time of that trial, but SPIRE-2 showed a profound reduction in cardiovascular events within a short time. “That led to the conclusion that the higher the LDL, the higher risk you are, the more likely that you’re going to attain a more robust reduction of cardiovascular events,” Dr. Lepor said. Bococizumab, a partially humanized antibody, did not come to market because of the high incidence of anti-drug antibody and associated diminution of therapeutic effect, he added.
Next came the FOURIER trial of evolocumab, a randomized, double-blind, placebo-controlled trial involving 27,564 patients with atherosclerotic cardiovascular disease and LDL cholesterol levels of 70 mg/dL or higher who were receiving statin therapy. Study participants received evolocumab (either 140 mg every 2 weeks or 420 mg monthly) or matching placebo as subcutaneous injections (N Engl J Med. 2017;376:1713-22). “The reduction in LDL was very robust and occurred quite early, and there was consistency in terms of long-term effect,” said Dr. Lepor, a past president of the California chapter of the American College of Cardiology. Specifically, researchers observed a 15% reduction in the primary endpoints of a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization (P less than .001), and a 20% reduction in the key secondary endpoints of a composite of cardiovascular death, MI, or stroke.
“It’s important to note that these are patients who were already well treated on statins,” he said. “There really does not seem to be a floor of LDL levels where we can say there is no further benefit. Achieved LDL-C at 4 weeks did lead to the ability to prognosticate benefit. We’re not seeing an increase in situations like diabetes, neurocognitive effects, and myalgias. The incidence of adverse events are similar to placebo. We feel very good that there do not seem to be safety issues for these agents, particularly in a population of patients with very low LDLs.” Dr. Lepor went on to note that FOURIER substudies have shown that there did not seem to be any increased incidence of neurocognitive disorders in patients taking evolocumab and that diabetes “tends to be a disease amplifier.”
In a more recent trial known as ODYSSEY OUTCOMES, 18,536 acute coronary syndrome (ACS) patients were randomized to alirocumab 75 mg once every 2 weeks or placebo (N Engl J Med. 2018;379:2097-107). The dose of alirocumab was increased to 150 mg every 2 weeks if the LDL was not lowered to less than 50 mg/dL. “The trial had other interesting aspects to it,” Dr. Lepor said. “If you had an LDL during the trial of less than 25 mg/dL, you were down-titrated from the 150-mg dose to the 75-mg dose, or if you were on the 75-mg dose with two consecutive LDLs less than 15 mg/dL, the alirocumab was stopped, all in blinded fashion.” The primary outcome was time to first occurrence of CHD death, nonfatal MI, ischemic stroke, or hospitalization for unstable angina.
At baseline, patients had a median age of 58 years, 25% were women, their median LDL was 92 mg/dL. About 89% of patients were on high dose atorvastatin/rosuvastatin. Time from index ACS to randomization was a median of 2.6 months.
The primary efficacy endpoint was major adverse cardiac events including CHD death, nonfatal MI, ischemic stroke, or unstable angina requiring hospitalization. After a median follow-up of 2.8 years, the researchers observed highly significant 15% reduction of the primary endpoint with alirocumab compared with placebo (hazard ratio, 0.85; P = .0001), and a 15% reduction of death from any cause. In patients with a baseline LDL-C of 100 mg/dL or greater, the benefits of alirocumab were further amplified. “The higher your LDL, the higher your risk,” Dr. Lepor said. “The higher your risk, the greater your benefit from therapeutic intervention.”
He concluded his presentation by likening the treatment approach with PCSK9 inhibitors in ACS patients to that of chemotherapy in cancer patients. “In patients who have the very high risk of recurrence, those are the ones we want to attack with tougher chemotherapy,” he said. “Who are the patients who benefit most from PCSK9 inhibitors? ACS patients and those who are breaking through statin therapy with cardiovascular events, those with diabetes, CKD, and peripheral vascular disease.”
Dr. Lepor disclosed that he serves on the advisory board for Sanofi/Regeneron and is on the speakers bureau for Amgen and Sanofi/Regeneron.
EXPERT ANALYSIS FROM WCIRDC 2018
Oral GLP-1 receptor agonist pioneered in T2DM trial
BERLIN – An investigational oral formulation of the glucagonlike peptide–1 receptor agonist (GLP-1 RA) semaglutide reduced glycated hemoglobin (HbA1c) to a greater extent than did placebo at all doses tested in patients with type 2 diabetes mellitus (T2DM) in the phase 3a PIONEER 1 trial.
The estimated mean change in HbA1c from baseline to week 26 – the primary endpoint – using an on-treatment analysis was –0.8% with a once-daily dose of 3 mg, –1.3% with a once-daily dose of 7 mg, and –1.5% with a once-daily dose of 14 mg. The corresponding value for placebo was –0.1%, with all comparisons statistically significant (P less than .001).
The on-treatment analysis evaluated treatment effects for all randomized patients (n = 703) and assumed that all subjects remained on-treatment and excluded the effect of any rescue medication. Results for an intention-to-treat (ITT) analysis provided similar results, however, with estimated mean changes in HbA1c of –0.9%, –1.2%, and –1.4% for the three respective semaglutide doses and –0.3% for placebo.
“There was a very nice dose-dependent decrease in HbA1c, which was superior to placebo for all doses of semaglutide” said study investigator Martin Haluzík, MD, at the annual meeting of the European Association for the Study of Diabetes.
“I think it’s quite important to stress the magnitude of the decrease of HbA1c, because with the highest dose it was –1.5% from a baseline of 8%, which I believe is something that hasn’t ever really been seen with any other oral antidiabetic medication,” added Dr. Haluzík, professor of internal medicine at the 1st Faculty of Medicine at Charles University and deputy head of the Institute for Clinical and Experimental Medicine, both in Prague.
Currently, GLP-1 RAs are available only in a subcutaneous formulation, Dr. Haluzík reminded his audience, adding that oral semaglutide was the first GLP-1 RA to be developed in a tablet formulation and was in the late stages of clinical development.
PIONEER 1 is the first of 10 phase 3a trials with oral semaglutide to be reported. “Additional studies, across the full spectrum of diabetes care, in special populations, comparing it with active comparators with varied trial duration, have been completed or will be completed in 2018,” said coinvestigator for the study Vanita Aroda, MD, during a separate presentation at a dedicated symposium on the PIONEER program.
Dr. Aroda, the director of the diabetes research program at Brigham and Women’s Hospital in Boston, observed that oral semaglutide was being evaluated from early care as monotherapy in the PIONEER 1 study. The other trials, such as PIONEER 2, PIONEER 3, PIONEER 4, and PIONEER 7 were looking at oral semaglutide in combination with oral antidiabetic agents versus various active comparators; PIONEER 5 and PIONEER 6 were in special populations; and PIONEER 8 was looking at its use on top of basal insulin. Two further trials are also part of the study program.
“I think this is the first time that we actually have completed data of an entire program, including cardiovascular data, all within the same year,” Dr. Aroda said. “All of the studies are in the process of data analysis or data reporting.”
PIONEER 1 was a multicenter, randomized, double-blind, placebo-controlled trial examining efficacy and safety of semaglutide versus placebo in 703 adults with drug-naive T2DM who were being treated with diet and exercise only.
Three doses of oral semaglutide – 3 mg, 7 mg, and 14 mg – were assessed and compared with placebo. There was a fixed 4-week dose escalation period, with all patients starting treatment with 3 mg of semaglutide and then increasing to 7 mg by week 4 and 14 mg by week 8. This was to try to reduce the risk of gastrointestinal side effects, which are known to occur with GLP-1 RAs.
Secondary outcome measures were change in body weight, fasting plasma glucose, HbA1c below a target of 7% (53 mmol/mol), which were all measured from baseline to week 26; adverse events, including severe and blood glucose–confirmed symptomatic hypoglycemic episodes, were assessed out to week 31.
The average age of patients in the trial was around 55 years, around half were female, and the starting HbA1c was approximately 8%.The mean body weight ranged from 86.9 kg to 89 kg in the different treatment groups, with a similar body mass index of about 32 kg/m2.
Clinically meaningful weight loss, compared with placebo, was only achieved with the highest dose of oral semaglutide, with a –4.1 kg reduction versus –1.5 kg for placebo from baseline to week 26 (P less than .001, on-treatment analysis). Reductions in body weight for the 3-mg and 7-mg oral semaglutide doses were a respective –1.7 kg and –2.5 kg. ITT results were again similar, with weight losses of –1.5, –2.3, and –3.7 kg for the 3-, 7-, and 14-mg doses of oral semaglutide, respectively, and –1.4 kg for placebo.
More patients treated with oral semaglutide 3, 7, or 14 mg versus placebo achieved an HbA1c of below 7% (59.1%, 71.9%, and 80.3%, respectively, vs. 33.8%) or a body weight loss of 5% or more (21.3%, 28.7%, and 44.3% vs. 15.7%).
Furthermore, more patients treated with oral semaglutide achieved an HbA1c of or below 7% without hypoglycemic episodes or body weight gain than did those given placebo. There were also more patients who achieved an HbA1c reduction of at least 1% and a weight loss of 3% or higher.
“Oral semaglutide demonstrated a safety and tolerability profile consistent with that of [injectable] GLP-1 RAs,” Dr. Haluzík reported. Adverse events were seen in 57.7%, 53.1%, and 56.6%of patients treated with 3, 7, and 14 mg of oral semaglutide and 55.6% of those treated with placebo.
The most common adverse events seen with oral semaglutide affected the gastrointestinal tract, with nausea affecting 8%, 5.1%, and 16% of 3, 7, and 14 mg–treated patients versus 5.6% of placebo-treated patients. Vomiting affected a respective 2.9%, 4.6%, 6.9%, and 2.1%, and diarrhea a respective 8.6%, 5.1%, 5.1%, and 2.2%.
Severe or blood glucose–confirmed, symptomatic hypoglycemia was reported in 2.9%, 1.1%, and 0.6%of those treated with 3, 7, 14 mg of oral semaglutide and 0.6% of placebo-treated patients.
PIONEER 1 represents a “step change in GLP-1 receptor agonist therapy”, said Cliff Bailey, MD, who discussed the trial aa the EASD’s independent commentator during a symposium on the PIONEER program. These data are “leading to a new delivery route for GLP-1 receptor agonists, from injection to oral,” and “this can be done with good metabolic efficacy, with substantial reductions in A1c and body weight, and with a safety profile that’s comparable to the subcutaneous injection.”
Dr. Bailey, who is professor of clinical science at Aston University in Birmingham, England, noted, however, the oral dosing of semaglutide “requires patient commitment because it needs to be taken before breakfast time, and it may also, to some extent, affect the timing of some of the other medications.”
The study was sponsored by Novo Nordisk. Dr. Haluzík disclosed acting as a consultant to AstraZeneca, Eli Lilly, Johnson & Johnson, Mundipharma, Novatin, Novo Nordisk, and Sanofi Aventis. Dr. Aroda was an investigator in the PIONEER 1 study and disclosed acting as a consultant to Novo Nordisk; she also disclosed relationships with multiple other pharmaceutical companies, including AstraZeneca, BMS, Calbra, Eisai, Elcelyx Therapeutics, Janssen, and Sanofi Aventis. Dr. Bailey acknowledged attending advisory boards, undertaking ad hoc consultancy work, and receiving research and travel support from several pharmaceutical companies that included Novo Nordisk.
SOURCES: Haluzík M et al. EASD 2018, Abstract 38; Aroda V. EASD 2018, Session S18 - PIONEER Trial; Bailey C. EASD 2018, Session S18 - PIONEER Trial.
BERLIN – An investigational oral formulation of the glucagonlike peptide–1 receptor agonist (GLP-1 RA) semaglutide reduced glycated hemoglobin (HbA1c) to a greater extent than did placebo at all doses tested in patients with type 2 diabetes mellitus (T2DM) in the phase 3a PIONEER 1 trial.
The estimated mean change in HbA1c from baseline to week 26 – the primary endpoint – using an on-treatment analysis was –0.8% with a once-daily dose of 3 mg, –1.3% with a once-daily dose of 7 mg, and –1.5% with a once-daily dose of 14 mg. The corresponding value for placebo was –0.1%, with all comparisons statistically significant (P less than .001).
The on-treatment analysis evaluated treatment effects for all randomized patients (n = 703) and assumed that all subjects remained on-treatment and excluded the effect of any rescue medication. Results for an intention-to-treat (ITT) analysis provided similar results, however, with estimated mean changes in HbA1c of –0.9%, –1.2%, and –1.4% for the three respective semaglutide doses and –0.3% for placebo.
“There was a very nice dose-dependent decrease in HbA1c, which was superior to placebo for all doses of semaglutide” said study investigator Martin Haluzík, MD, at the annual meeting of the European Association for the Study of Diabetes.
“I think it’s quite important to stress the magnitude of the decrease of HbA1c, because with the highest dose it was –1.5% from a baseline of 8%, which I believe is something that hasn’t ever really been seen with any other oral antidiabetic medication,” added Dr. Haluzík, professor of internal medicine at the 1st Faculty of Medicine at Charles University and deputy head of the Institute for Clinical and Experimental Medicine, both in Prague.
Currently, GLP-1 RAs are available only in a subcutaneous formulation, Dr. Haluzík reminded his audience, adding that oral semaglutide was the first GLP-1 RA to be developed in a tablet formulation and was in the late stages of clinical development.
PIONEER 1 is the first of 10 phase 3a trials with oral semaglutide to be reported. “Additional studies, across the full spectrum of diabetes care, in special populations, comparing it with active comparators with varied trial duration, have been completed or will be completed in 2018,” said coinvestigator for the study Vanita Aroda, MD, during a separate presentation at a dedicated symposium on the PIONEER program.
Dr. Aroda, the director of the diabetes research program at Brigham and Women’s Hospital in Boston, observed that oral semaglutide was being evaluated from early care as monotherapy in the PIONEER 1 study. The other trials, such as PIONEER 2, PIONEER 3, PIONEER 4, and PIONEER 7 were looking at oral semaglutide in combination with oral antidiabetic agents versus various active comparators; PIONEER 5 and PIONEER 6 were in special populations; and PIONEER 8 was looking at its use on top of basal insulin. Two further trials are also part of the study program.
“I think this is the first time that we actually have completed data of an entire program, including cardiovascular data, all within the same year,” Dr. Aroda said. “All of the studies are in the process of data analysis or data reporting.”
PIONEER 1 was a multicenter, randomized, double-blind, placebo-controlled trial examining efficacy and safety of semaglutide versus placebo in 703 adults with drug-naive T2DM who were being treated with diet and exercise only.
Three doses of oral semaglutide – 3 mg, 7 mg, and 14 mg – were assessed and compared with placebo. There was a fixed 4-week dose escalation period, with all patients starting treatment with 3 mg of semaglutide and then increasing to 7 mg by week 4 and 14 mg by week 8. This was to try to reduce the risk of gastrointestinal side effects, which are known to occur with GLP-1 RAs.
Secondary outcome measures were change in body weight, fasting plasma glucose, HbA1c below a target of 7% (53 mmol/mol), which were all measured from baseline to week 26; adverse events, including severe and blood glucose–confirmed symptomatic hypoglycemic episodes, were assessed out to week 31.
The average age of patients in the trial was around 55 years, around half were female, and the starting HbA1c was approximately 8%.The mean body weight ranged from 86.9 kg to 89 kg in the different treatment groups, with a similar body mass index of about 32 kg/m2.
Clinically meaningful weight loss, compared with placebo, was only achieved with the highest dose of oral semaglutide, with a –4.1 kg reduction versus –1.5 kg for placebo from baseline to week 26 (P less than .001, on-treatment analysis). Reductions in body weight for the 3-mg and 7-mg oral semaglutide doses were a respective –1.7 kg and –2.5 kg. ITT results were again similar, with weight losses of –1.5, –2.3, and –3.7 kg for the 3-, 7-, and 14-mg doses of oral semaglutide, respectively, and –1.4 kg for placebo.
More patients treated with oral semaglutide 3, 7, or 14 mg versus placebo achieved an HbA1c of below 7% (59.1%, 71.9%, and 80.3%, respectively, vs. 33.8%) or a body weight loss of 5% or more (21.3%, 28.7%, and 44.3% vs. 15.7%).
Furthermore, more patients treated with oral semaglutide achieved an HbA1c of or below 7% without hypoglycemic episodes or body weight gain than did those given placebo. There were also more patients who achieved an HbA1c reduction of at least 1% and a weight loss of 3% or higher.
“Oral semaglutide demonstrated a safety and tolerability profile consistent with that of [injectable] GLP-1 RAs,” Dr. Haluzík reported. Adverse events were seen in 57.7%, 53.1%, and 56.6%of patients treated with 3, 7, and 14 mg of oral semaglutide and 55.6% of those treated with placebo.
The most common adverse events seen with oral semaglutide affected the gastrointestinal tract, with nausea affecting 8%, 5.1%, and 16% of 3, 7, and 14 mg–treated patients versus 5.6% of placebo-treated patients. Vomiting affected a respective 2.9%, 4.6%, 6.9%, and 2.1%, and diarrhea a respective 8.6%, 5.1%, 5.1%, and 2.2%.
Severe or blood glucose–confirmed, symptomatic hypoglycemia was reported in 2.9%, 1.1%, and 0.6%of those treated with 3, 7, 14 mg of oral semaglutide and 0.6% of placebo-treated patients.
PIONEER 1 represents a “step change in GLP-1 receptor agonist therapy”, said Cliff Bailey, MD, who discussed the trial aa the EASD’s independent commentator during a symposium on the PIONEER program. These data are “leading to a new delivery route for GLP-1 receptor agonists, from injection to oral,” and “this can be done with good metabolic efficacy, with substantial reductions in A1c and body weight, and with a safety profile that’s comparable to the subcutaneous injection.”
Dr. Bailey, who is professor of clinical science at Aston University in Birmingham, England, noted, however, the oral dosing of semaglutide “requires patient commitment because it needs to be taken before breakfast time, and it may also, to some extent, affect the timing of some of the other medications.”
The study was sponsored by Novo Nordisk. Dr. Haluzík disclosed acting as a consultant to AstraZeneca, Eli Lilly, Johnson & Johnson, Mundipharma, Novatin, Novo Nordisk, and Sanofi Aventis. Dr. Aroda was an investigator in the PIONEER 1 study and disclosed acting as a consultant to Novo Nordisk; she also disclosed relationships with multiple other pharmaceutical companies, including AstraZeneca, BMS, Calbra, Eisai, Elcelyx Therapeutics, Janssen, and Sanofi Aventis. Dr. Bailey acknowledged attending advisory boards, undertaking ad hoc consultancy work, and receiving research and travel support from several pharmaceutical companies that included Novo Nordisk.
SOURCES: Haluzík M et al. EASD 2018, Abstract 38; Aroda V. EASD 2018, Session S18 - PIONEER Trial; Bailey C. EASD 2018, Session S18 - PIONEER Trial.
BERLIN – An investigational oral formulation of the glucagonlike peptide–1 receptor agonist (GLP-1 RA) semaglutide reduced glycated hemoglobin (HbA1c) to a greater extent than did placebo at all doses tested in patients with type 2 diabetes mellitus (T2DM) in the phase 3a PIONEER 1 trial.
The estimated mean change in HbA1c from baseline to week 26 – the primary endpoint – using an on-treatment analysis was –0.8% with a once-daily dose of 3 mg, –1.3% with a once-daily dose of 7 mg, and –1.5% with a once-daily dose of 14 mg. The corresponding value for placebo was –0.1%, with all comparisons statistically significant (P less than .001).
The on-treatment analysis evaluated treatment effects for all randomized patients (n = 703) and assumed that all subjects remained on-treatment and excluded the effect of any rescue medication. Results for an intention-to-treat (ITT) analysis provided similar results, however, with estimated mean changes in HbA1c of –0.9%, –1.2%, and –1.4% for the three respective semaglutide doses and –0.3% for placebo.
“There was a very nice dose-dependent decrease in HbA1c, which was superior to placebo for all doses of semaglutide” said study investigator Martin Haluzík, MD, at the annual meeting of the European Association for the Study of Diabetes.
“I think it’s quite important to stress the magnitude of the decrease of HbA1c, because with the highest dose it was –1.5% from a baseline of 8%, which I believe is something that hasn’t ever really been seen with any other oral antidiabetic medication,” added Dr. Haluzík, professor of internal medicine at the 1st Faculty of Medicine at Charles University and deputy head of the Institute for Clinical and Experimental Medicine, both in Prague.
Currently, GLP-1 RAs are available only in a subcutaneous formulation, Dr. Haluzík reminded his audience, adding that oral semaglutide was the first GLP-1 RA to be developed in a tablet formulation and was in the late stages of clinical development.
PIONEER 1 is the first of 10 phase 3a trials with oral semaglutide to be reported. “Additional studies, across the full spectrum of diabetes care, in special populations, comparing it with active comparators with varied trial duration, have been completed or will be completed in 2018,” said coinvestigator for the study Vanita Aroda, MD, during a separate presentation at a dedicated symposium on the PIONEER program.
Dr. Aroda, the director of the diabetes research program at Brigham and Women’s Hospital in Boston, observed that oral semaglutide was being evaluated from early care as monotherapy in the PIONEER 1 study. The other trials, such as PIONEER 2, PIONEER 3, PIONEER 4, and PIONEER 7 were looking at oral semaglutide in combination with oral antidiabetic agents versus various active comparators; PIONEER 5 and PIONEER 6 were in special populations; and PIONEER 8 was looking at its use on top of basal insulin. Two further trials are also part of the study program.
“I think this is the first time that we actually have completed data of an entire program, including cardiovascular data, all within the same year,” Dr. Aroda said. “All of the studies are in the process of data analysis or data reporting.”
PIONEER 1 was a multicenter, randomized, double-blind, placebo-controlled trial examining efficacy and safety of semaglutide versus placebo in 703 adults with drug-naive T2DM who were being treated with diet and exercise only.
Three doses of oral semaglutide – 3 mg, 7 mg, and 14 mg – were assessed and compared with placebo. There was a fixed 4-week dose escalation period, with all patients starting treatment with 3 mg of semaglutide and then increasing to 7 mg by week 4 and 14 mg by week 8. This was to try to reduce the risk of gastrointestinal side effects, which are known to occur with GLP-1 RAs.
Secondary outcome measures were change in body weight, fasting plasma glucose, HbA1c below a target of 7% (53 mmol/mol), which were all measured from baseline to week 26; adverse events, including severe and blood glucose–confirmed symptomatic hypoglycemic episodes, were assessed out to week 31.
The average age of patients in the trial was around 55 years, around half were female, and the starting HbA1c was approximately 8%.The mean body weight ranged from 86.9 kg to 89 kg in the different treatment groups, with a similar body mass index of about 32 kg/m2.
Clinically meaningful weight loss, compared with placebo, was only achieved with the highest dose of oral semaglutide, with a –4.1 kg reduction versus –1.5 kg for placebo from baseline to week 26 (P less than .001, on-treatment analysis). Reductions in body weight for the 3-mg and 7-mg oral semaglutide doses were a respective –1.7 kg and –2.5 kg. ITT results were again similar, with weight losses of –1.5, –2.3, and –3.7 kg for the 3-, 7-, and 14-mg doses of oral semaglutide, respectively, and –1.4 kg for placebo.
More patients treated with oral semaglutide 3, 7, or 14 mg versus placebo achieved an HbA1c of below 7% (59.1%, 71.9%, and 80.3%, respectively, vs. 33.8%) or a body weight loss of 5% or more (21.3%, 28.7%, and 44.3% vs. 15.7%).
Furthermore, more patients treated with oral semaglutide achieved an HbA1c of or below 7% without hypoglycemic episodes or body weight gain than did those given placebo. There were also more patients who achieved an HbA1c reduction of at least 1% and a weight loss of 3% or higher.
“Oral semaglutide demonstrated a safety and tolerability profile consistent with that of [injectable] GLP-1 RAs,” Dr. Haluzík reported. Adverse events were seen in 57.7%, 53.1%, and 56.6%of patients treated with 3, 7, and 14 mg of oral semaglutide and 55.6% of those treated with placebo.
The most common adverse events seen with oral semaglutide affected the gastrointestinal tract, with nausea affecting 8%, 5.1%, and 16% of 3, 7, and 14 mg–treated patients versus 5.6% of placebo-treated patients. Vomiting affected a respective 2.9%, 4.6%, 6.9%, and 2.1%, and diarrhea a respective 8.6%, 5.1%, 5.1%, and 2.2%.
Severe or blood glucose–confirmed, symptomatic hypoglycemia was reported in 2.9%, 1.1%, and 0.6%of those treated with 3, 7, 14 mg of oral semaglutide and 0.6% of placebo-treated patients.
PIONEER 1 represents a “step change in GLP-1 receptor agonist therapy”, said Cliff Bailey, MD, who discussed the trial aa the EASD’s independent commentator during a symposium on the PIONEER program. These data are “leading to a new delivery route for GLP-1 receptor agonists, from injection to oral,” and “this can be done with good metabolic efficacy, with substantial reductions in A1c and body weight, and with a safety profile that’s comparable to the subcutaneous injection.”
Dr. Bailey, who is professor of clinical science at Aston University in Birmingham, England, noted, however, the oral dosing of semaglutide “requires patient commitment because it needs to be taken before breakfast time, and it may also, to some extent, affect the timing of some of the other medications.”
The study was sponsored by Novo Nordisk. Dr. Haluzík disclosed acting as a consultant to AstraZeneca, Eli Lilly, Johnson & Johnson, Mundipharma, Novatin, Novo Nordisk, and Sanofi Aventis. Dr. Aroda was an investigator in the PIONEER 1 study and disclosed acting as a consultant to Novo Nordisk; she also disclosed relationships with multiple other pharmaceutical companies, including AstraZeneca, BMS, Calbra, Eisai, Elcelyx Therapeutics, Janssen, and Sanofi Aventis. Dr. Bailey acknowledged attending advisory boards, undertaking ad hoc consultancy work, and receiving research and travel support from several pharmaceutical companies that included Novo Nordisk.
SOURCES: Haluzík M et al. EASD 2018, Abstract 38; Aroda V. EASD 2018, Session S18 - PIONEER Trial; Bailey C. EASD 2018, Session S18 - PIONEER Trial.
REPORTING FROM EASD 2018
Key clinical point: A novel oral formulation of semaglutide proved better than placebo for diabetes control and weight loss in patients with T2DM.
Major finding: Mean change in hemoglobin A1c (baseline to week 26) – the primary endpoint – using an on-treatment analysis was –0.8% with a once-daily dose of 3 mg, –1.3% with a once-daily dose of 7 mg, and –1.5% with a once-daily dose of 14 mg. The corresponding value for placebo was –0.1%.
Study details: Phase 3a, multicenter, randomized, double-blind, placebo-controlled trial examining efficacy and safety of semaglutide versus placebo in 703 individuals with T2DM being treated with diet and exercise only.
Disclosures: The study was sponsored by Novo Nordisk. Dr. Haluzík disclosed acting as a consultant to AstraZeneca, Eli Lilly, Johnson & Johnson, Mundipharma, Novatin, Novo Nordisk, and Sanofi Aventis. Dr. Aroda was an investigator in the PIONEER 1 study and disclosed acting as a consultant to Novo Nordisk. She also disclosed relationships with multiple other pharmaceutical companies, including AstraZeneca, BMS, Calbra, Eisai, Elcelyx Therapeutics, Janssen, and Sanofi Aventis. Dr. Bailey acknowledged attending advisory boards of, undertaking ad hoc consultancy work for, and receiving research and travel support from several pharmaceutical companies that included Novo Nordisk.
Source: Haluzík M et al. EASD 2018, Abstract 38; Aroda V. EASD 2018, Session S18 – PIONEER Trial; Bailey C. EASD 2018, Session S18 – PIONEER Trial.
Metformin associated with acidosis only in patients with eGFR 30 mL/min per 1.73 m 2
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.
Clinical question: Does metformin increase the risk of lactic acidosis in chronic kidney disease (CKD)?
Background: Metformin is first-line therapy for type 2 diabetes mellitus (DM) because of its low cost, safety, and potential cardiovascular benefit, but fear of lactic acidosis has limited its use in CKD. The risk of acidosis in CKD patients with varying levels of renal function has not been clearly defined.
Study design: Retrospective community-based cohort study.
Setting: Geisinger Health System in Pennsylvania.
Synopsis: A total of 75,413 patients were identified with diagnostic codes or medication prescriptions indicating DM. Forty-five percent of patients were taking metformin at enrollment, increasing by 18% over the 5.7 years of median follow-up. The primary outcome was inpatient acidosis, defined by an ICD-9-CM code capturing multiple forms of acidosis but excluding diabetic ketoacidosis.
When metformin users and nonusers were compared, risk of acidosis was similar for the entire cohort and for subgroups of patients with an estimated glomerular filtration rate (eGFR) greater than 90, 60-89, 45-59, and 30-44. Conversely, metformin use was associated with a higher risk of acidosis in patients with eGFR less than 30 (adjusted hazard ratio, 2.07; 95% confidence interval, 1.33-3.22). Metformin not increasing the risk of acidosis at eGFR greater than 30 also was noted in an additional analysis using sulfonylurea medications as an active comparator and was replicated in a separate database with 82,000 patients from 350 private health systems. As with all observational studies, this study is limited by the potential for residual confounding.
Bottom line: Metformin appears to be safe in CKD patients with eGFR above 30 mL/min per 1.73 m2.
Citation: Lazarus B et al. Association of metformin use with risk of lactic acidosis across the range of kidney function: A community- based cohort study. JAMA Int Med. 2018;178(7):903-10.
Dr. Wanner is director, hospital medicine section, and associate chief, division of general internal medicine, University of Utah, Salt Lake City.













