User login
Gastroparesis
To the Editor: We read with great pleasure the article by Sharayah et al about acute gastroparesis in a patient with diabetic ketoacidosis.1 However, in the case description, the authors reached a diagnosis of gastroparesis secondary to diabetic ketoacidosis without aptly ruling out some of its most common causes such as hypokalemia and other electrolyte imbalances seen in diabetic patients (in the setting of recurrent vomiting).
The authors also did not include the patient’s duration of diabetes or hemoglobin A1c level, both of which are linked with gastroparesis in diabetic patients.2 Pertinent biochemical information that can help readers formulate a rational approach and journey to making a diagnosis appears elusive in their article.
- Sharayah AM, Hajjaj N, Osman R, Livornese D. Gastroparesis in a patient with diabetic ketoacidosis. Cleve Clin J Med 2019; 86(4):238–239. doi:10.3949/ccjm.86a.18116
- Bharucha AE, Kudva Y, Basu A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol 2015; 13(3):466–476.e461. doi:10.1016/j.cgh.2014.06.034
To the Editor: We read with great pleasure the article by Sharayah et al about acute gastroparesis in a patient with diabetic ketoacidosis.1 However, in the case description, the authors reached a diagnosis of gastroparesis secondary to diabetic ketoacidosis without aptly ruling out some of its most common causes such as hypokalemia and other electrolyte imbalances seen in diabetic patients (in the setting of recurrent vomiting).
The authors also did not include the patient’s duration of diabetes or hemoglobin A1c level, both of which are linked with gastroparesis in diabetic patients.2 Pertinent biochemical information that can help readers formulate a rational approach and journey to making a diagnosis appears elusive in their article.
To the Editor: We read with great pleasure the article by Sharayah et al about acute gastroparesis in a patient with diabetic ketoacidosis.1 However, in the case description, the authors reached a diagnosis of gastroparesis secondary to diabetic ketoacidosis without aptly ruling out some of its most common causes such as hypokalemia and other electrolyte imbalances seen in diabetic patients (in the setting of recurrent vomiting).
The authors also did not include the patient’s duration of diabetes or hemoglobin A1c level, both of which are linked with gastroparesis in diabetic patients.2 Pertinent biochemical information that can help readers formulate a rational approach and journey to making a diagnosis appears elusive in their article.
- Sharayah AM, Hajjaj N, Osman R, Livornese D. Gastroparesis in a patient with diabetic ketoacidosis. Cleve Clin J Med 2019; 86(4):238–239. doi:10.3949/ccjm.86a.18116
- Bharucha AE, Kudva Y, Basu A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol 2015; 13(3):466–476.e461. doi:10.1016/j.cgh.2014.06.034
- Sharayah AM, Hajjaj N, Osman R, Livornese D. Gastroparesis in a patient with diabetic ketoacidosis. Cleve Clin J Med 2019; 86(4):238–239. doi:10.3949/ccjm.86a.18116
- Bharucha AE, Kudva Y, Basu A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol 2015; 13(3):466–476.e461. doi:10.1016/j.cgh.2014.06.034
In reply: Gastroparesis
In Reply: We thank the readers for their letter. Our patient’s laboratory values at the time of presentation were as follows:
- Corrected sodium 142 mmol/L
- Potassium 5.5 mmol/L
- Phosphorus 6.6 mmol/L.
The rest of the electrolyte levels were within normal limits.
These reported electrolyte levels were unlikely to cause such gastroparesis. The patient’s hemoglobin A1c was 8.7% at the time of presentation, with no previous values available. However, since abdominal computed tomography done 1 year before this presentation did not show stomach dilation and the patient was asymptomatic, his gastroparesis was presumed to be acute.
In Reply: We thank the readers for their letter. Our patient’s laboratory values at the time of presentation were as follows:
- Corrected sodium 142 mmol/L
- Potassium 5.5 mmol/L
- Phosphorus 6.6 mmol/L.
The rest of the electrolyte levels were within normal limits.
These reported electrolyte levels were unlikely to cause such gastroparesis. The patient’s hemoglobin A1c was 8.7% at the time of presentation, with no previous values available. However, since abdominal computed tomography done 1 year before this presentation did not show stomach dilation and the patient was asymptomatic, his gastroparesis was presumed to be acute.
In Reply: We thank the readers for their letter. Our patient’s laboratory values at the time of presentation were as follows:
- Corrected sodium 142 mmol/L
- Potassium 5.5 mmol/L
- Phosphorus 6.6 mmol/L.
The rest of the electrolyte levels were within normal limits.
These reported electrolyte levels were unlikely to cause such gastroparesis. The patient’s hemoglobin A1c was 8.7% at the time of presentation, with no previous values available. However, since abdominal computed tomography done 1 year before this presentation did not show stomach dilation and the patient was asymptomatic, his gastroparesis was presumed to be acute.
What we know—and don’t—about non-nutritive sweeteners
An estimated 93.3 million Americans (roughly 40% of the US population) were obese in 2015-2016, and most of them had at least 1 chronic disease.1 As a result, patient education focused on lifestyle modification, including healthy nutrition and physical activity, has become an integral part of our everyday practice.
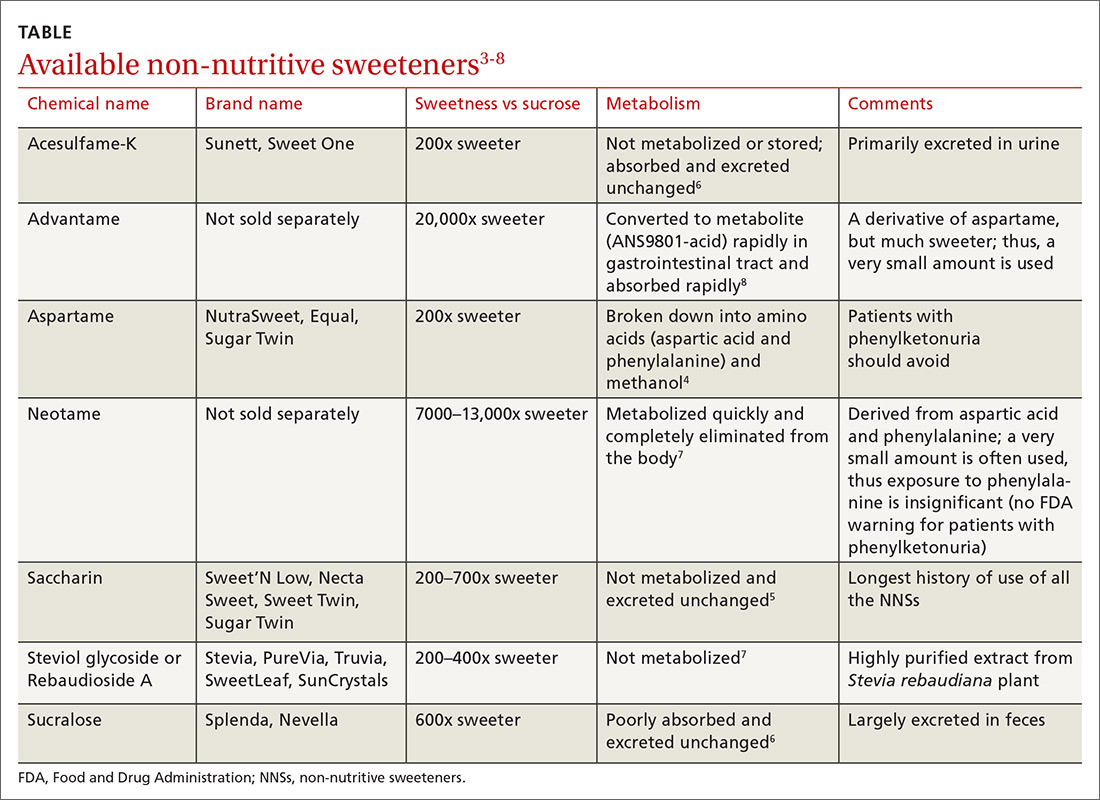
At the same time, the most recent dietary guidelines recommend that added sugar make up < 10% of daily calories.2 In the United States, low-calorie food and beverages containing non-nutritive sweeteners (NNSs; TABLE3-8) have become a popular means of keeping the sweetness in our diet without the health ramifications associated with sugar. These NNSs (aka, artificial sweeteners, high-intensity sweeteners, and non-caloric sweeteners) are ubiquitous in soft drinks, processed grains (including breads, cereals, and granola bars), and dairy products (including yogurts, flavored milk, and ice cream). As examples, NNSs are present in 42% of flavored waters, 33% of yogurts, and all diet beverages.9,10 They can even be found in medications, multivitamins, toothpaste, and mouthwash.
Business is booming
Global NNS consumption has been growing more than 5% per year, meaning that by 2020, NNSs are expected to be a $2.2 billion industry.11 One study using data from the National Health and Nutrition Examination Survey (NHANES) found that the use of NNSs in the United States increased from 21.1% in 2003 to 24.9% in 2009-2010 among adults and increased from 7.8% to 18.9% over the same time period among children.12
The main increase in the consumption of NNSs across all age groups has been via the consumption of beverages. Approximately 11% of healthy weight, 19% of overweight, and 22% of obese adults consume diet beverages.13,14 Consumption of diet beverages or NNSs increases with age12 and is especially common among women with higher levels of education and income.15
However, concerns remain about the safety of these agents and their effect on weight, appetite, and the body’s glycemic response. This article reviews the available research and current recommendations regarding the use of NNSs.
WHAT EFFECT DO NNS s HAVE ON WEIGHT?
The data on NNSs and weight are inconsistent. One randomized controlled trial(RCT) compared weight loss over the course of 1 year (12-week weight loss phase; 9-month weight maintenance phase) when 303 participants consumed either water or drinks sweetened with NNSs.16 Weight loss was significantly greater in the NNS drink group when compared with the water group.16
Observational studies have revealed similar findings.17,18 Data from NHANES revealed that US adults (n = 14,098) during 2 nonconsecutive 24-hour dietary recall periods demonstrated lower total energy (calorie) intake if they consumed NNSs vs no NNSs.19 Another study using 2011-2016 NHANES data on adolescents (n = 7026) found no difference in energy intake between those who consumed beverages containing NNSs vs those who consumed beverages containing sugar.20
Continue to: Other lines of investigation...
Other lines of investigation, including animal studies, have shown that long-term use of NNSs is associated with numerous metabolic derangements including weight gain.21 The negative effects of NNSs appear to be the greatest in males and those who are obese and have high-calorie diets.21
A 2017 meta-analysis concluded that evidence from RCTs does not support a benefit of NNSs on weight management, and that routine consumption of NNSs may be associated with increased body mass index (BMI) and cardiometabolic risk.22 Another systematic review and meta-analysis found that there was a higher pooled risk for obesity among those who drank beverages containing NNSs vs those who drank sugar-containing beverages.23
Based on the most current literature, we conclude that NNSs are not beneficial for weight loss. While there is concern about weight gain through psychological effects (stimulation of sweetness receptors without satiety), further well-designed research is needed to explore whether this concern has merit.
WHAT IS THE EFFECT OF NNSs ON APPETITE?
There appears to be no effect. While original studies seemed to indicate there was an effect, later studies leaned to the contrary.
The notion that NNSs might enhance appetite and food intake was advanced in the 1980s by John Blundell and his research team.24 The hypothesis was that since NNSs uncouple sweet taste and calories, they do not exert the normal post-ingestive inhibitory influence that real sugar does. This, in turn, disrupts appetite control mechanisms.25-27
Continue to: However, subsequent research studies...
However, subsequent research studies found no relationship between the use of NNSs and appetite.28-30 Mattes and colleagues hypothesized that such a difference in findings could result from the fact that earlier studies focused on isolating NNSs from other energy-yielding products, which emphasized an association with heightened hunger.29 Subsequent studies showed that when NNSs were incorporated into energy-yielding products, there was no association between NNSs and increased hunger or appetite.
DO NNSs INCREASE THE RISK FOR TYPE 2 DIABETES MELLITUS?
The data are mixed. One study of women participating in the Nurses’ Health Study II showed that those who consumed caffeinated, artificially-sweetened beverages had a 35% higher risk of developing type 2 diabetes mellitus (T2DM); however, this risk was no longer significant after adjusting for BMI and energy intake.31
The Health Professionals Follow-Up Trial studied more than 40,000 men for more than 20 years and found that NNS consumption increased the risk of developing T2DM by 40%.32 However, this finding lost statistical significance after adjusting for BMI.32
These results make it difficult to determine whether there is any association between NNSs and T2DM; rather NNS-containing beverages are likely consumed more often by those who have higher BMIs and by those trying to lose weight.
A 2017 randomized crossover study involving 10 healthy men looked at the effects of a variety of caloric and non-caloric sweeteners on 24-hour glucose profiles and found no differences.33 Another study, a randomized, double-blind, crossover trial involving 60 non-obese adults without diabetes who did not consume NNSs, randomized the participants one-to-one to drink either 2 cans per day of either a beverage containing aspartame and acesulfame K or an unsweetened, no-calorie beverage for 12 weeks.34
Continue to: After a 4-week washout period...
After a 4-week washout period, the participants then switched to the opposite beverage for 12 weeks. The study concluded that consumption of 2 cans of a beverage containing aspartame and acesulfame K over 12 weeks had no significant effect on insulin sensitivity or secretion in nondiabetic adults.34
Similar results were obtained from a study involving 100 non-obese adults.35 The researchers found that aspartame ingested at 2 different doses (350 or 1050 mg/d) in beverages over 12 weeks had no effect on a 240-minute oral glucose tolerance test, blood pressure, appetite, or body weight.35
A 2016 systematic review critically evaluated the effect of NNSs on both glucose absorption and appetite.36 The review included 14 observational prospective trials, 28 RCTs, and 2 meta-analyses. The sweeteners studied included aspartame, sucralose, saccharin, acesulfame K, and stevia.36 The studies were focused largely on single-exposure outcomes (20 trials), but a minority of the studies (8 trials) looked at longer exposures from 1 to 18 weeks. Only some of the studies controlled for critical variables, such as BMI. In the end, there was no consistent pattern of increased or decreased risk for insulin resistance or diabetes.36
Two meta-analyses tried to determine if an association exists between consumption of beverages containing NNSs and the development of T2DM.37,38 The first meta-analysis with 4 studies showed a slight, but significant, relative risk (RR) of 1.13 (95% confidence interval [CI], 1.02-1.25) for those who consumed beverages containing NNSs.37 In the second meta-analysis (10 studies), NNS consumption had an RR of 1.48 (95% CI, 1.35-1.62), but the risk was lower (and no longer significant) after adjusting for BMI.38 A study of 98 Hispanic adolescents who were overweight or obese found that chronic users (n = 9) of NNSs had higher HbA1c levels 1 year later than did controls (n = 75) and people who initiated use of NNSs between the baseline and 1-year visit (n = 14).39
The American Diabetes Association (ADA) and American Heart Association joint position statement on NNSs, first published in 2012, says that NNSs can be utilized to reduce caloric and carbohydrate consumption for overall diabetes control and to obtain a healthy body weight.40 These principles were reaffirmed in the ADA Standards of Care in 2019.41
Continue to: The 2015 US Scientific Reports on Dietary Guidelines...
The 2015 US Scientific Reports on Dietary Guidelines provided a consensus statement saying, “Future experimental studies should examine the relationship between artificially sweetened soft drinks and biomarkers of insulin resistance and other diabetes markers.”42
DO NNSs HAVE ANY ADVERSE HEALTH EFFECTS?
Maybe. Many individuals avoid NNSs due to fear of developing cancer. While rat studies have previously shown a dose-dependent increased risk of developing cancer, epidemiologic studies in humans have not confirmed an association.43 The National Cancer Institute reports that carcinogenicity studies of NNSs have not shown an association with cancer in humans.44
A prospective study—the Nurses’ Health Study, which followed over 88,000 women for 24 years—found that consumption of > 2 diet sodas per day was associated with an increased risk for coronary heart disease (CHD) and chronic kidney disease (CKD) compared with consumption of < 1 diet soda per month.45 However, other prospective studies have shown that these specific negative health effects may not be present when controlling for weight.45,46
While the prospective studies found some associations between medical conditions (eg, CHD and CKD) and NNS consumption, the literature is limited to intake from beverages and does not include NNS-containing foods. More studies are needed to determine the relationship between NNSs and potential adverse health events, since the current literature is observational and cannot predict causation.
A 2019 study explored the associations between long-term consumption of sugar-sweetened beverages and artificially sweetened beverages (ASBs) and the risk of mortality in the United States.47 This study included 37,716 men from the Health Professionals Follow-up Study and 80,647 women from the Nurses’ Health Study. Subjects who had the highest consumption of ASBs had higher risks for total and cardiovascular disease mortality.47 Cohort-specific analyses showed that an association between ASB consumption and mortality was observed in the participants from the Nurses’ Health Study but not in those from the Health Professionals Follow-up Study, warranting further investigation.47 Cancer mortality and ASB consumption were not shown to have an association in this study.
Continue to: WHY ARE THE DATA INCONCLUSIVE?
WHY ARE THE DATA INCONCLUSIVE?
Nutritional studies are hard to complete accurately outside of the laboratory setting. Also, the science of NNSs is new and evolving.
With regard to obesity and NNSs, it is possible that findings have been due to reverse causation. People who are overweight or obese are more likely to consume low-calorie foods and beverages; they are also at greater risk for developing diseases, such as T2DM.48,49
HOW SAFE ARE NNSs?
They appear to be safe, but more data are needed. Each of the 7 FDA-approved NNSs has passed extensive laboratory, animal, and human testing, and appears to cause no harm in the human body when consumed.49 But clearly the data are incomplete. As we continue to gain a greater understanding of the metabolism of NNSs, we may need to revisit the issue of safety.
ARE THERE ANY NNSs THAT SOME PEOPLE SHOULD AVOID?
Yes. People with phenylketonuria, who have difficulty metabolizing phenylalanine (a component of aspartame), should avoid consumption of aspartame.50
In addition, NNSs have been found to be present in breast milk.51 While the significance of this finding is yet to be determined, we warn against the use of NNSs by women who are breastfeeding.51
WHAT EFFECT—IF ANY—DO NNSs HAVE ON GUT MICROBIOTA?
We don’t know. Disruptions in the gut microbiome have been linked to numerous metabolic abnormalities, including obesity, insulin resistance, and diabetes, as well as cardiovascular disorders.52,53 Diet is a main determinant of balance in the gut microbiota.54 The gut microbiota are centrally involved in energy harvest, and studies have suggested that low gut bacterial diversity is associated with increased adiposity, insulin resistance, and low-grade inflammation.55-60 Whether NNSs have a relationship with abnormal changes in gut microbiota requires further study.
CORRESPONDENCE
Clipper F. Young, PharmD, MPH, CDE, BC-ADM, BCGP, Touro University California, College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; [email protected].
1. Adult obesity facts. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/obesity/data/adult.html. Reviewed August 13, 2018. Accessed July 15, 2019.
2. Dietary guidelines for Americans 2015-2020: answers to your questions. USDA ChooseMyPlate.gov Web site. https://www.choosemyplate.gov/2015-2020-dietary-guidelines-answers-your-questions. Accessed July 15, 2019.
3. Additional information about high-intensity sweeteners permitted for use in food in the United States. US Food and Drug Administration Web site. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Published February 8, 2018. Accessed July 15, 2019.
4. Magnuson B, for the Aspartame Expert Work Group. Nutritive and non-nutritive sweeteners. NNNS: aspartame, methanol and formaldehyde relationships (2011). https://www.foodsweeteners.com/wp-content/uploads/2015/08/Aspartame-Methanol-and-Formaldehyde-Relationships.pdf. Accessed July 15, 2019.
5. Jo JH, Kim S, Jeon TW, et al. Investigation of the regulatory effects of saccharin on cytochrome P450s in male ICR mice. Toxicol Res. 2017;33:25-30.
6. Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweetener: where are we today? Diabetes Spectrum. 2012;25:104-110.
7. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners – a review. J Food Sci Technol. 2014;51:611-621.
8. EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA Journal. 2013;11:3301.
9. Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
10. Ng SW, Slining MM, Popkin BM. Use of caloric and non-caloric sweeteners in US consumer packaged foods, 2005-2009. J Acad Nutr Diet. 2012;112:1828-1834.
11. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446-450.
12. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294-306.
13. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640-646.
14. Bleich SN, Wolfson JA, Vine S, et al. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104:e72-e78.
15. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr. 2015;69:1035-1041.
16. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014;22:1415-1421.
17. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22:1415-1421.
18. Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691-700.
19. Malek AM, Hunt KJ, DellaValle DM, et al. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007-2012. Curr Dev Nutr. 2018;2:nzy054.
20. Sylvetsky AC, Figueroa J, Zimmerman T, et al. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr Obes. 2019;2:e12535.
21. Fowler SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517-523.
22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189: E929-E939.
23. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially-sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110:513-520.
24. Blundell JE, Rogers PJ, Hill AJ. Uncoupling sweetness and calories: methodological aspects of laboratory studies on appetite control. Appetite. 1988;11(Suppl 1):54-61.
25. Bellisle F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr Obes Rep. 2015;4:106-110.
26. Bryant CE, Wasse LK, Astbury N, et al. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68:629-631.
27. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159-1164.
28. Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C. Functional roles of low calorie sweeteners on gut function. Physiol Behav. 2016;164(Pt B):479-481.
29. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1-14.
30. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155-166.
31. Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927-934.
32. de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and the risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321-1327.
33. Tey SL, Salleh NB, Henry CJ, et al. Effect of non-nutritive (artificial vs natural) sweeteners on 24-hour glucose profile. Eur J Clin Nutr. 2017;71:1129-1132.
34. Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148:1293-1299.
35. Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148:650-657.
36. Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, et al. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PloS One. 2016;11:e0161264.
37. Greenwood DC, Threspleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112:725-734.
38. Imamura F, O’Conner L, Ye M, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
39. Davis JN, Asigbee FM, Markowitz AK, et al. Consumption of artificial sweetened beverages associated with adiposity and increasing HbA1c in Hispanic youth. Clin Obes. 2018;8:236-243.
40. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35:1798-1808.
41. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S1-S183.
42. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Office of Disease Prevention and Health Promotion Web site. https://health.gov/dietaryguidelines/2015-scientific-report/.Published February 2015. Accessed July 15, 2019.
43. Aune D. Soft drinks, aspartame, and the risk of cancer and cardiovascular disease. Am J Clin Nutr. 2012;96:1249-1251.
44. Artificial sweeteners and cancer. National Cancer Institute Web site. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet. Reviewed August 10, 2016. Accessed July 15, 2019.
45. Fung TT, Malik V, Rexrode KM, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037-1042.
46. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160-166.
47. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113-2125.
48. Gardener H, Rundek T, Markert M, et al. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Inten Med. 2012;27:1120-1126.
49. Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
50. US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm#Aspartame. Accessed May 26, 2019.
51. Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78:1029-1032.
52. Rajani C, Jia W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin Sci (Lond). 2018;132:791-811.
53. Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
54. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance. Physiol Behav. 2016;164(Pt B):488-493.
55. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484.
56. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
57. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
58. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415-1429.
59. Anderson RL. Effect of saccharin ingestion on stool composition in relation to caecal enlargement and increased stool hydration. Food Chem Toxicol. 1983;21:255-257.
60. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186.
An estimated 93.3 million Americans (roughly 40% of the US population) were obese in 2015-2016, and most of them had at least 1 chronic disease.1 As a result, patient education focused on lifestyle modification, including healthy nutrition and physical activity, has become an integral part of our everyday practice.
At the same time, the most recent dietary guidelines recommend that added sugar make up < 10% of daily calories.2 In the United States, low-calorie food and beverages containing non-nutritive sweeteners (NNSs; TABLE3-8) have become a popular means of keeping the sweetness in our diet without the health ramifications associated with sugar. These NNSs (aka, artificial sweeteners, high-intensity sweeteners, and non-caloric sweeteners) are ubiquitous in soft drinks, processed grains (including breads, cereals, and granola bars), and dairy products (including yogurts, flavored milk, and ice cream). As examples, NNSs are present in 42% of flavored waters, 33% of yogurts, and all diet beverages.9,10 They can even be found in medications, multivitamins, toothpaste, and mouthwash.

Business is booming
Global NNS consumption has been growing more than 5% per year, meaning that by 2020, NNSs are expected to be a $2.2 billion industry.11 One study using data from the National Health and Nutrition Examination Survey (NHANES) found that the use of NNSs in the United States increased from 21.1% in 2003 to 24.9% in 2009-2010 among adults and increased from 7.8% to 18.9% over the same time period among children.12
The main increase in the consumption of NNSs across all age groups has been via the consumption of beverages. Approximately 11% of healthy weight, 19% of overweight, and 22% of obese adults consume diet beverages.13,14 Consumption of diet beverages or NNSs increases with age12 and is especially common among women with higher levels of education and income.15
However, concerns remain about the safety of these agents and their effect on weight, appetite, and the body’s glycemic response. This article reviews the available research and current recommendations regarding the use of NNSs.
WHAT EFFECT DO NNS s HAVE ON WEIGHT?
The data on NNSs and weight are inconsistent. One randomized controlled trial(RCT) compared weight loss over the course of 1 year (12-week weight loss phase; 9-month weight maintenance phase) when 303 participants consumed either water or drinks sweetened with NNSs.16 Weight loss was significantly greater in the NNS drink group when compared with the water group.16
Observational studies have revealed similar findings.17,18 Data from NHANES revealed that US adults (n = 14,098) during 2 nonconsecutive 24-hour dietary recall periods demonstrated lower total energy (calorie) intake if they consumed NNSs vs no NNSs.19 Another study using 2011-2016 NHANES data on adolescents (n = 7026) found no difference in energy intake between those who consumed beverages containing NNSs vs those who consumed beverages containing sugar.20
Continue to: Other lines of investigation...
Other lines of investigation, including animal studies, have shown that long-term use of NNSs is associated with numerous metabolic derangements including weight gain.21 The negative effects of NNSs appear to be the greatest in males and those who are obese and have high-calorie diets.21
A 2017 meta-analysis concluded that evidence from RCTs does not support a benefit of NNSs on weight management, and that routine consumption of NNSs may be associated with increased body mass index (BMI) and cardiometabolic risk.22 Another systematic review and meta-analysis found that there was a higher pooled risk for obesity among those who drank beverages containing NNSs vs those who drank sugar-containing beverages.23
Based on the most current literature, we conclude that NNSs are not beneficial for weight loss. While there is concern about weight gain through psychological effects (stimulation of sweetness receptors without satiety), further well-designed research is needed to explore whether this concern has merit.
WHAT IS THE EFFECT OF NNSs ON APPETITE?
There appears to be no effect. While original studies seemed to indicate there was an effect, later studies leaned to the contrary.
The notion that NNSs might enhance appetite and food intake was advanced in the 1980s by John Blundell and his research team.24 The hypothesis was that since NNSs uncouple sweet taste and calories, they do not exert the normal post-ingestive inhibitory influence that real sugar does. This, in turn, disrupts appetite control mechanisms.25-27
Continue to: However, subsequent research studies...
However, subsequent research studies found no relationship between the use of NNSs and appetite.28-30 Mattes and colleagues hypothesized that such a difference in findings could result from the fact that earlier studies focused on isolating NNSs from other energy-yielding products, which emphasized an association with heightened hunger.29 Subsequent studies showed that when NNSs were incorporated into energy-yielding products, there was no association between NNSs and increased hunger or appetite.
DO NNSs INCREASE THE RISK FOR TYPE 2 DIABETES MELLITUS?
The data are mixed. One study of women participating in the Nurses’ Health Study II showed that those who consumed caffeinated, artificially-sweetened beverages had a 35% higher risk of developing type 2 diabetes mellitus (T2DM); however, this risk was no longer significant after adjusting for BMI and energy intake.31
The Health Professionals Follow-Up Trial studied more than 40,000 men for more than 20 years and found that NNS consumption increased the risk of developing T2DM by 40%.32 However, this finding lost statistical significance after adjusting for BMI.32
These results make it difficult to determine whether there is any association between NNSs and T2DM; rather NNS-containing beverages are likely consumed more often by those who have higher BMIs and by those trying to lose weight.
A 2017 randomized crossover study involving 10 healthy men looked at the effects of a variety of caloric and non-caloric sweeteners on 24-hour glucose profiles and found no differences.33 Another study, a randomized, double-blind, crossover trial involving 60 non-obese adults without diabetes who did not consume NNSs, randomized the participants one-to-one to drink either 2 cans per day of either a beverage containing aspartame and acesulfame K or an unsweetened, no-calorie beverage for 12 weeks.34
Continue to: After a 4-week washout period...
After a 4-week washout period, the participants then switched to the opposite beverage for 12 weeks. The study concluded that consumption of 2 cans of a beverage containing aspartame and acesulfame K over 12 weeks had no significant effect on insulin sensitivity or secretion in nondiabetic adults.34
Similar results were obtained from a study involving 100 non-obese adults.35 The researchers found that aspartame ingested at 2 different doses (350 or 1050 mg/d) in beverages over 12 weeks had no effect on a 240-minute oral glucose tolerance test, blood pressure, appetite, or body weight.35
A 2016 systematic review critically evaluated the effect of NNSs on both glucose absorption and appetite.36 The review included 14 observational prospective trials, 28 RCTs, and 2 meta-analyses. The sweeteners studied included aspartame, sucralose, saccharin, acesulfame K, and stevia.36 The studies were focused largely on single-exposure outcomes (20 trials), but a minority of the studies (8 trials) looked at longer exposures from 1 to 18 weeks. Only some of the studies controlled for critical variables, such as BMI. In the end, there was no consistent pattern of increased or decreased risk for insulin resistance or diabetes.36
Two meta-analyses tried to determine if an association exists between consumption of beverages containing NNSs and the development of T2DM.37,38 The first meta-analysis with 4 studies showed a slight, but significant, relative risk (RR) of 1.13 (95% confidence interval [CI], 1.02-1.25) for those who consumed beverages containing NNSs.37 In the second meta-analysis (10 studies), NNS consumption had an RR of 1.48 (95% CI, 1.35-1.62), but the risk was lower (and no longer significant) after adjusting for BMI.38 A study of 98 Hispanic adolescents who were overweight or obese found that chronic users (n = 9) of NNSs had higher HbA1c levels 1 year later than did controls (n = 75) and people who initiated use of NNSs between the baseline and 1-year visit (n = 14).39
The American Diabetes Association (ADA) and American Heart Association joint position statement on NNSs, first published in 2012, says that NNSs can be utilized to reduce caloric and carbohydrate consumption for overall diabetes control and to obtain a healthy body weight.40 These principles were reaffirmed in the ADA Standards of Care in 2019.41
Continue to: The 2015 US Scientific Reports on Dietary Guidelines...
The 2015 US Scientific Reports on Dietary Guidelines provided a consensus statement saying, “Future experimental studies should examine the relationship between artificially sweetened soft drinks and biomarkers of insulin resistance and other diabetes markers.”42
DO NNSs HAVE ANY ADVERSE HEALTH EFFECTS?
Maybe. Many individuals avoid NNSs due to fear of developing cancer. While rat studies have previously shown a dose-dependent increased risk of developing cancer, epidemiologic studies in humans have not confirmed an association.43 The National Cancer Institute reports that carcinogenicity studies of NNSs have not shown an association with cancer in humans.44
A prospective study—the Nurses’ Health Study, which followed over 88,000 women for 24 years—found that consumption of > 2 diet sodas per day was associated with an increased risk for coronary heart disease (CHD) and chronic kidney disease (CKD) compared with consumption of < 1 diet soda per month.45 However, other prospective studies have shown that these specific negative health effects may not be present when controlling for weight.45,46
While the prospective studies found some associations between medical conditions (eg, CHD and CKD) and NNS consumption, the literature is limited to intake from beverages and does not include NNS-containing foods. More studies are needed to determine the relationship between NNSs and potential adverse health events, since the current literature is observational and cannot predict causation.
A 2019 study explored the associations between long-term consumption of sugar-sweetened beverages and artificially sweetened beverages (ASBs) and the risk of mortality in the United States.47 This study included 37,716 men from the Health Professionals Follow-up Study and 80,647 women from the Nurses’ Health Study. Subjects who had the highest consumption of ASBs had higher risks for total and cardiovascular disease mortality.47 Cohort-specific analyses showed that an association between ASB consumption and mortality was observed in the participants from the Nurses’ Health Study but not in those from the Health Professionals Follow-up Study, warranting further investigation.47 Cancer mortality and ASB consumption were not shown to have an association in this study.
Continue to: WHY ARE THE DATA INCONCLUSIVE?
WHY ARE THE DATA INCONCLUSIVE?
Nutritional studies are hard to complete accurately outside of the laboratory setting. Also, the science of NNSs is new and evolving.
With regard to obesity and NNSs, it is possible that findings have been due to reverse causation. People who are overweight or obese are more likely to consume low-calorie foods and beverages; they are also at greater risk for developing diseases, such as T2DM.48,49
HOW SAFE ARE NNSs?
They appear to be safe, but more data are needed. Each of the 7 FDA-approved NNSs has passed extensive laboratory, animal, and human testing, and appears to cause no harm in the human body when consumed.49 But clearly the data are incomplete. As we continue to gain a greater understanding of the metabolism of NNSs, we may need to revisit the issue of safety.
ARE THERE ANY NNSs THAT SOME PEOPLE SHOULD AVOID?
Yes. People with phenylketonuria, who have difficulty metabolizing phenylalanine (a component of aspartame), should avoid consumption of aspartame.50
In addition, NNSs have been found to be present in breast milk.51 While the significance of this finding is yet to be determined, we warn against the use of NNSs by women who are breastfeeding.51
WHAT EFFECT—IF ANY—DO NNSs HAVE ON GUT MICROBIOTA?
We don’t know. Disruptions in the gut microbiome have been linked to numerous metabolic abnormalities, including obesity, insulin resistance, and diabetes, as well as cardiovascular disorders.52,53 Diet is a main determinant of balance in the gut microbiota.54 The gut microbiota are centrally involved in energy harvest, and studies have suggested that low gut bacterial diversity is associated with increased adiposity, insulin resistance, and low-grade inflammation.55-60 Whether NNSs have a relationship with abnormal changes in gut microbiota requires further study.
CORRESPONDENCE
Clipper F. Young, PharmD, MPH, CDE, BC-ADM, BCGP, Touro University California, College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; [email protected].
An estimated 93.3 million Americans (roughly 40% of the US population) were obese in 2015-2016, and most of them had at least 1 chronic disease.1 As a result, patient education focused on lifestyle modification, including healthy nutrition and physical activity, has become an integral part of our everyday practice.
At the same time, the most recent dietary guidelines recommend that added sugar make up < 10% of daily calories.2 In the United States, low-calorie food and beverages containing non-nutritive sweeteners (NNSs; TABLE3-8) have become a popular means of keeping the sweetness in our diet without the health ramifications associated with sugar. These NNSs (aka, artificial sweeteners, high-intensity sweeteners, and non-caloric sweeteners) are ubiquitous in soft drinks, processed grains (including breads, cereals, and granola bars), and dairy products (including yogurts, flavored milk, and ice cream). As examples, NNSs are present in 42% of flavored waters, 33% of yogurts, and all diet beverages.9,10 They can even be found in medications, multivitamins, toothpaste, and mouthwash.

Business is booming
Global NNS consumption has been growing more than 5% per year, meaning that by 2020, NNSs are expected to be a $2.2 billion industry.11 One study using data from the National Health and Nutrition Examination Survey (NHANES) found that the use of NNSs in the United States increased from 21.1% in 2003 to 24.9% in 2009-2010 among adults and increased from 7.8% to 18.9% over the same time period among children.12
The main increase in the consumption of NNSs across all age groups has been via the consumption of beverages. Approximately 11% of healthy weight, 19% of overweight, and 22% of obese adults consume diet beverages.13,14 Consumption of diet beverages or NNSs increases with age12 and is especially common among women with higher levels of education and income.15
However, concerns remain about the safety of these agents and their effect on weight, appetite, and the body’s glycemic response. This article reviews the available research and current recommendations regarding the use of NNSs.
WHAT EFFECT DO NNS s HAVE ON WEIGHT?
The data on NNSs and weight are inconsistent. One randomized controlled trial(RCT) compared weight loss over the course of 1 year (12-week weight loss phase; 9-month weight maintenance phase) when 303 participants consumed either water or drinks sweetened with NNSs.16 Weight loss was significantly greater in the NNS drink group when compared with the water group.16
Observational studies have revealed similar findings.17,18 Data from NHANES revealed that US adults (n = 14,098) during 2 nonconsecutive 24-hour dietary recall periods demonstrated lower total energy (calorie) intake if they consumed NNSs vs no NNSs.19 Another study using 2011-2016 NHANES data on adolescents (n = 7026) found no difference in energy intake between those who consumed beverages containing NNSs vs those who consumed beverages containing sugar.20
Continue to: Other lines of investigation...
Other lines of investigation, including animal studies, have shown that long-term use of NNSs is associated with numerous metabolic derangements including weight gain.21 The negative effects of NNSs appear to be the greatest in males and those who are obese and have high-calorie diets.21
A 2017 meta-analysis concluded that evidence from RCTs does not support a benefit of NNSs on weight management, and that routine consumption of NNSs may be associated with increased body mass index (BMI) and cardiometabolic risk.22 Another systematic review and meta-analysis found that there was a higher pooled risk for obesity among those who drank beverages containing NNSs vs those who drank sugar-containing beverages.23
Based on the most current literature, we conclude that NNSs are not beneficial for weight loss. While there is concern about weight gain through psychological effects (stimulation of sweetness receptors without satiety), further well-designed research is needed to explore whether this concern has merit.
WHAT IS THE EFFECT OF NNSs ON APPETITE?
There appears to be no effect. While original studies seemed to indicate there was an effect, later studies leaned to the contrary.
The notion that NNSs might enhance appetite and food intake was advanced in the 1980s by John Blundell and his research team.24 The hypothesis was that since NNSs uncouple sweet taste and calories, they do not exert the normal post-ingestive inhibitory influence that real sugar does. This, in turn, disrupts appetite control mechanisms.25-27
Continue to: However, subsequent research studies...
However, subsequent research studies found no relationship between the use of NNSs and appetite.28-30 Mattes and colleagues hypothesized that such a difference in findings could result from the fact that earlier studies focused on isolating NNSs from other energy-yielding products, which emphasized an association with heightened hunger.29 Subsequent studies showed that when NNSs were incorporated into energy-yielding products, there was no association between NNSs and increased hunger or appetite.
DO NNSs INCREASE THE RISK FOR TYPE 2 DIABETES MELLITUS?
The data are mixed. One study of women participating in the Nurses’ Health Study II showed that those who consumed caffeinated, artificially-sweetened beverages had a 35% higher risk of developing type 2 diabetes mellitus (T2DM); however, this risk was no longer significant after adjusting for BMI and energy intake.31
The Health Professionals Follow-Up Trial studied more than 40,000 men for more than 20 years and found that NNS consumption increased the risk of developing T2DM by 40%.32 However, this finding lost statistical significance after adjusting for BMI.32
These results make it difficult to determine whether there is any association between NNSs and T2DM; rather NNS-containing beverages are likely consumed more often by those who have higher BMIs and by those trying to lose weight.
A 2017 randomized crossover study involving 10 healthy men looked at the effects of a variety of caloric and non-caloric sweeteners on 24-hour glucose profiles and found no differences.33 Another study, a randomized, double-blind, crossover trial involving 60 non-obese adults without diabetes who did not consume NNSs, randomized the participants one-to-one to drink either 2 cans per day of either a beverage containing aspartame and acesulfame K or an unsweetened, no-calorie beverage for 12 weeks.34
Continue to: After a 4-week washout period...
After a 4-week washout period, the participants then switched to the opposite beverage for 12 weeks. The study concluded that consumption of 2 cans of a beverage containing aspartame and acesulfame K over 12 weeks had no significant effect on insulin sensitivity or secretion in nondiabetic adults.34
Similar results were obtained from a study involving 100 non-obese adults.35 The researchers found that aspartame ingested at 2 different doses (350 or 1050 mg/d) in beverages over 12 weeks had no effect on a 240-minute oral glucose tolerance test, blood pressure, appetite, or body weight.35
A 2016 systematic review critically evaluated the effect of NNSs on both glucose absorption and appetite.36 The review included 14 observational prospective trials, 28 RCTs, and 2 meta-analyses. The sweeteners studied included aspartame, sucralose, saccharin, acesulfame K, and stevia.36 The studies were focused largely on single-exposure outcomes (20 trials), but a minority of the studies (8 trials) looked at longer exposures from 1 to 18 weeks. Only some of the studies controlled for critical variables, such as BMI. In the end, there was no consistent pattern of increased or decreased risk for insulin resistance or diabetes.36
Two meta-analyses tried to determine if an association exists between consumption of beverages containing NNSs and the development of T2DM.37,38 The first meta-analysis with 4 studies showed a slight, but significant, relative risk (RR) of 1.13 (95% confidence interval [CI], 1.02-1.25) for those who consumed beverages containing NNSs.37 In the second meta-analysis (10 studies), NNS consumption had an RR of 1.48 (95% CI, 1.35-1.62), but the risk was lower (and no longer significant) after adjusting for BMI.38 A study of 98 Hispanic adolescents who were overweight or obese found that chronic users (n = 9) of NNSs had higher HbA1c levels 1 year later than did controls (n = 75) and people who initiated use of NNSs between the baseline and 1-year visit (n = 14).39
The American Diabetes Association (ADA) and American Heart Association joint position statement on NNSs, first published in 2012, says that NNSs can be utilized to reduce caloric and carbohydrate consumption for overall diabetes control and to obtain a healthy body weight.40 These principles were reaffirmed in the ADA Standards of Care in 2019.41
Continue to: The 2015 US Scientific Reports on Dietary Guidelines...
The 2015 US Scientific Reports on Dietary Guidelines provided a consensus statement saying, “Future experimental studies should examine the relationship between artificially sweetened soft drinks and biomarkers of insulin resistance and other diabetes markers.”42
DO NNSs HAVE ANY ADVERSE HEALTH EFFECTS?
Maybe. Many individuals avoid NNSs due to fear of developing cancer. While rat studies have previously shown a dose-dependent increased risk of developing cancer, epidemiologic studies in humans have not confirmed an association.43 The National Cancer Institute reports that carcinogenicity studies of NNSs have not shown an association with cancer in humans.44
A prospective study—the Nurses’ Health Study, which followed over 88,000 women for 24 years—found that consumption of > 2 diet sodas per day was associated with an increased risk for coronary heart disease (CHD) and chronic kidney disease (CKD) compared with consumption of < 1 diet soda per month.45 However, other prospective studies have shown that these specific negative health effects may not be present when controlling for weight.45,46
While the prospective studies found some associations between medical conditions (eg, CHD and CKD) and NNS consumption, the literature is limited to intake from beverages and does not include NNS-containing foods. More studies are needed to determine the relationship between NNSs and potential adverse health events, since the current literature is observational and cannot predict causation.
A 2019 study explored the associations between long-term consumption of sugar-sweetened beverages and artificially sweetened beverages (ASBs) and the risk of mortality in the United States.47 This study included 37,716 men from the Health Professionals Follow-up Study and 80,647 women from the Nurses’ Health Study. Subjects who had the highest consumption of ASBs had higher risks for total and cardiovascular disease mortality.47 Cohort-specific analyses showed that an association between ASB consumption and mortality was observed in the participants from the Nurses’ Health Study but not in those from the Health Professionals Follow-up Study, warranting further investigation.47 Cancer mortality and ASB consumption were not shown to have an association in this study.
Continue to: WHY ARE THE DATA INCONCLUSIVE?
WHY ARE THE DATA INCONCLUSIVE?
Nutritional studies are hard to complete accurately outside of the laboratory setting. Also, the science of NNSs is new and evolving.
With regard to obesity and NNSs, it is possible that findings have been due to reverse causation. People who are overweight or obese are more likely to consume low-calorie foods and beverages; they are also at greater risk for developing diseases, such as T2DM.48,49
HOW SAFE ARE NNSs?
They appear to be safe, but more data are needed. Each of the 7 FDA-approved NNSs has passed extensive laboratory, animal, and human testing, and appears to cause no harm in the human body when consumed.49 But clearly the data are incomplete. As we continue to gain a greater understanding of the metabolism of NNSs, we may need to revisit the issue of safety.
ARE THERE ANY NNSs THAT SOME PEOPLE SHOULD AVOID?
Yes. People with phenylketonuria, who have difficulty metabolizing phenylalanine (a component of aspartame), should avoid consumption of aspartame.50
In addition, NNSs have been found to be present in breast milk.51 While the significance of this finding is yet to be determined, we warn against the use of NNSs by women who are breastfeeding.51
WHAT EFFECT—IF ANY—DO NNSs HAVE ON GUT MICROBIOTA?
We don’t know. Disruptions in the gut microbiome have been linked to numerous metabolic abnormalities, including obesity, insulin resistance, and diabetes, as well as cardiovascular disorders.52,53 Diet is a main determinant of balance in the gut microbiota.54 The gut microbiota are centrally involved in energy harvest, and studies have suggested that low gut bacterial diversity is associated with increased adiposity, insulin resistance, and low-grade inflammation.55-60 Whether NNSs have a relationship with abnormal changes in gut microbiota requires further study.
CORRESPONDENCE
Clipper F. Young, PharmD, MPH, CDE, BC-ADM, BCGP, Touro University California, College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; [email protected].
1. Adult obesity facts. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/obesity/data/adult.html. Reviewed August 13, 2018. Accessed July 15, 2019.
2. Dietary guidelines for Americans 2015-2020: answers to your questions. USDA ChooseMyPlate.gov Web site. https://www.choosemyplate.gov/2015-2020-dietary-guidelines-answers-your-questions. Accessed July 15, 2019.
3. Additional information about high-intensity sweeteners permitted for use in food in the United States. US Food and Drug Administration Web site. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Published February 8, 2018. Accessed July 15, 2019.
4. Magnuson B, for the Aspartame Expert Work Group. Nutritive and non-nutritive sweeteners. NNNS: aspartame, methanol and formaldehyde relationships (2011). https://www.foodsweeteners.com/wp-content/uploads/2015/08/Aspartame-Methanol-and-Formaldehyde-Relationships.pdf. Accessed July 15, 2019.
5. Jo JH, Kim S, Jeon TW, et al. Investigation of the regulatory effects of saccharin on cytochrome P450s in male ICR mice. Toxicol Res. 2017;33:25-30.
6. Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweetener: where are we today? Diabetes Spectrum. 2012;25:104-110.
7. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners – a review. J Food Sci Technol. 2014;51:611-621.
8. EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA Journal. 2013;11:3301.
9. Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
10. Ng SW, Slining MM, Popkin BM. Use of caloric and non-caloric sweeteners in US consumer packaged foods, 2005-2009. J Acad Nutr Diet. 2012;112:1828-1834.
11. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446-450.
12. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294-306.
13. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640-646.
14. Bleich SN, Wolfson JA, Vine S, et al. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104:e72-e78.
15. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr. 2015;69:1035-1041.
16. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014;22:1415-1421.
17. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22:1415-1421.
18. Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691-700.
19. Malek AM, Hunt KJ, DellaValle DM, et al. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007-2012. Curr Dev Nutr. 2018;2:nzy054.
20. Sylvetsky AC, Figueroa J, Zimmerman T, et al. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr Obes. 2019;2:e12535.
21. Fowler SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517-523.
22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189: E929-E939.
23. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially-sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110:513-520.
24. Blundell JE, Rogers PJ, Hill AJ. Uncoupling sweetness and calories: methodological aspects of laboratory studies on appetite control. Appetite. 1988;11(Suppl 1):54-61.
25. Bellisle F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr Obes Rep. 2015;4:106-110.
26. Bryant CE, Wasse LK, Astbury N, et al. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68:629-631.
27. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159-1164.
28. Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C. Functional roles of low calorie sweeteners on gut function. Physiol Behav. 2016;164(Pt B):479-481.
29. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1-14.
30. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155-166.
31. Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927-934.
32. de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and the risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321-1327.
33. Tey SL, Salleh NB, Henry CJ, et al. Effect of non-nutritive (artificial vs natural) sweeteners on 24-hour glucose profile. Eur J Clin Nutr. 2017;71:1129-1132.
34. Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148:1293-1299.
35. Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148:650-657.
36. Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, et al. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PloS One. 2016;11:e0161264.
37. Greenwood DC, Threspleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112:725-734.
38. Imamura F, O’Conner L, Ye M, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
39. Davis JN, Asigbee FM, Markowitz AK, et al. Consumption of artificial sweetened beverages associated with adiposity and increasing HbA1c in Hispanic youth. Clin Obes. 2018;8:236-243.
40. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35:1798-1808.
41. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S1-S183.
42. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Office of Disease Prevention and Health Promotion Web site. https://health.gov/dietaryguidelines/2015-scientific-report/.Published February 2015. Accessed July 15, 2019.
43. Aune D. Soft drinks, aspartame, and the risk of cancer and cardiovascular disease. Am J Clin Nutr. 2012;96:1249-1251.
44. Artificial sweeteners and cancer. National Cancer Institute Web site. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet. Reviewed August 10, 2016. Accessed July 15, 2019.
45. Fung TT, Malik V, Rexrode KM, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037-1042.
46. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160-166.
47. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113-2125.
48. Gardener H, Rundek T, Markert M, et al. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Inten Med. 2012;27:1120-1126.
49. Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
50. US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm#Aspartame. Accessed May 26, 2019.
51. Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78:1029-1032.
52. Rajani C, Jia W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin Sci (Lond). 2018;132:791-811.
53. Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
54. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance. Physiol Behav. 2016;164(Pt B):488-493.
55. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484.
56. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
57. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
58. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415-1429.
59. Anderson RL. Effect of saccharin ingestion on stool composition in relation to caecal enlargement and increased stool hydration. Food Chem Toxicol. 1983;21:255-257.
60. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186.
1. Adult obesity facts. Centers for Disease Control and Prevention Web site. https://www.cdc.gov/obesity/data/adult.html. Reviewed August 13, 2018. Accessed July 15, 2019.
2. Dietary guidelines for Americans 2015-2020: answers to your questions. USDA ChooseMyPlate.gov Web site. https://www.choosemyplate.gov/2015-2020-dietary-guidelines-answers-your-questions. Accessed July 15, 2019.
3. Additional information about high-intensity sweeteners permitted for use in food in the United States. US Food and Drug Administration Web site. https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states. Published February 8, 2018. Accessed July 15, 2019.
4. Magnuson B, for the Aspartame Expert Work Group. Nutritive and non-nutritive sweeteners. NNNS: aspartame, methanol and formaldehyde relationships (2011). https://www.foodsweeteners.com/wp-content/uploads/2015/08/Aspartame-Methanol-and-Formaldehyde-Relationships.pdf. Accessed July 15, 2019.
5. Jo JH, Kim S, Jeon TW, et al. Investigation of the regulatory effects of saccharin on cytochrome P450s in male ICR mice. Toxicol Res. 2017;33:25-30.
6. Shwide-Slavin C, Swift C, Ross T. Nonnutritive sweetener: where are we today? Diabetes Spectrum. 2012;25:104-110.
7. Chattopadhyay S, Raychaudhuri U, Chakraborty R. Artificial sweeteners – a review. J Food Sci Technol. 2014;51:611-621.
8. EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific opinion on the safety of advantame for the proposed uses as a food additive. EFSA Journal. 2013;11:3301.
9. Fitch C, Keim KS; Academy of Nutrition and Dietetics. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
10. Ng SW, Slining MM, Popkin BM. Use of caloric and non-caloric sweeteners in US consumer packaged foods, 2005-2009. J Acad Nutr Diet. 2012;112:1828-1834.
11. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav. 2016;164(Pt B):446-450.
12. Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes. 2013;8:294-306.
13. Sylvetsky AC, Welsh JA, Brown RJ, et al. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr. 2012;96:640-646.
14. Bleich SN, Wolfson JA, Vine S, et al. Diet-beverage consumption and caloric intake among US adults, overall and by body weight. Am J Public Health. 2014;104:e72-e78.
15. Drewnowski A, Rehm CD. Socio-demographic correlates and trends in low-calorie sweetener use among adults in the United States from 1999 to 2008. Eur J Clin Nutr. 2015;69:1035-1041.
16. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014;22:1415-1421.
17. Peters JC, Wyatt HR, Foster GD, et al. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity (Silver Spring). 2014;22:1415-1421.
18. Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691-700.
19. Malek AM, Hunt KJ, DellaValle DM, et al. Reported consumption of low-calorie sweetener in foods, beverages, and food and beverage additions by US adults: NHANES 2007-2012. Curr Dev Nutr. 2018;2:nzy054.
20. Sylvetsky AC, Figueroa J, Zimmerman T, et al. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr Obes. 2019;2:e12535.
21. Fowler SPG. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav. 2016;164(Pt B):517-523.
22. Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189: E929-E939.
23. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, et al. Sugar and artificially-sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110:513-520.
24. Blundell JE, Rogers PJ, Hill AJ. Uncoupling sweetness and calories: methodological aspects of laboratory studies on appetite control. Appetite. 1988;11(Suppl 1):54-61.
25. Bellisle F. Intense sweeteners, appetite for the sweet taste, and relationship to weight management. Curr Obes Rep. 2015;4:106-110.
26. Bryant CE, Wasse LK, Astbury N, et al. Non-nutritive sweeteners: no class effect on the glycaemic or appetite responses to ingested glucose. Eur J Clin Nutr. 2014;68:629-631.
27. Canty DJ, Chan MM. Effects of consumption of caloric vs noncaloric sweet drinks on indices of hunger and food consumption in normal adults. Am J Clin Nutr. 1991;53:1159-1164.
28. Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C. Functional roles of low calorie sweeteners on gut function. Physiol Behav. 2016;164(Pt B):479-481.
29. Mattes RD, Popkin BM. Nonnutritive sweetener consumption in humans: effects on appetite and food intake and their putative mechanisms. Am J Clin Nutr. 2009;89:1-14.
30. Bhupathiraju SN, Pan A, Malik VS, et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr. 2013;97:155-166.
31. Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927-934.
32. de Koning L, Malik VS, Rimm EB, et al. Sugar-sweetened and artificially sweetened beverage consumption and the risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93:1321-1327.
33. Tey SL, Salleh NB, Henry CJ, et al. Effect of non-nutritive (artificial vs natural) sweeteners on 24-hour glucose profile. Eur J Clin Nutr. 2017;71:1129-1132.
34. Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148:1293-1299.
35. Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148:650-657.
36. Romo-Romo A, Aguilar-Salinas CA, Brito-Cordova GX, et al. Effects of the non-nutritive sweeteners on glucose metabolism and appetite regulating hormones: systematic review of observational prospective studies and clinical trials. PloS One. 2016;11:e0161264.
37. Greenwood DC, Threspleton DE, Evans CE, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112:725-734.
38. Imamura F, O’Conner L, Ye M, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576.
39. Davis JN, Asigbee FM, Markowitz AK, et al. Consumption of artificial sweetened beverages associated with adiposity and increasing HbA1c in Hispanic youth. Clin Obes. 2018;8:236-243.
40. Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives. a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2012;35:1798-1808.
41. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S1-S183.
42. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Health and Human Services and the Secretary of Agriculture. Office of Disease Prevention and Health Promotion Web site. https://health.gov/dietaryguidelines/2015-scientific-report/.Published February 2015. Accessed July 15, 2019.
43. Aune D. Soft drinks, aspartame, and the risk of cancer and cardiovascular disease. Am J Clin Nutr. 2012;96:1249-1251.
44. Artificial sweeteners and cancer. National Cancer Institute Web site. https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet. Reviewed August 10, 2016. Accessed July 15, 2019.
45. Fung TT, Malik V, Rexrode KM, et al. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009;89:1037-1042.
46. Lin J, Curhan GC. Associations of sugar and artificially sweetened soda with albuminuria and kidney function decline in women. Clin J Am Soc Nephrol. 2011;6:160-166.
47. Malik VS, Li Y, Pan A, et al. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139:2113-2125.
48. Gardener H, Rundek T, Markert M, et al. Diet soft drink consumption is associated with an increased risk of vascular events in the Northern Manhattan Study. J Gen Inten Med. 2012;27:1120-1126.
49. Fitch C, Keim KS. Position of the Academy of Nutrition and Dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet. 2012;112:739-758.
50. US Food and Drug Administration. Additional information about high-intensity sweeteners permitted for use in food in the United States. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm#Aspartame. Accessed May 26, 2019.
51. Sylvetsky AC, Gardner AL, Bauman V, et al. Nonnutritive sweeteners in breast milk. J Toxicol Environ Health. 2015;78:1029-1032.
52. Rajani C, Jia W. Disruptions in gut microbial-host co-metabolism and the development of metabolic disorders. Clin Sci (Lond). 2018;132:791-811.
53. Kho ZY, Lal SK. The human gut microbiome—a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
54. Nettleton JE, Reimer RA, Shearer J. Reshaping the gut microbiota: impact of low calorie sweeteners and the link to insulin resistance. Physiol Behav. 2016;164(Pt B):488-493.
55. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480-484.
56. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585-588.
57. Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546.
58. Abou-Donia MB, El-Masry EM, Abdel-Rahman AA, et al. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J Toxicol Environ Health A. 2008;71:1415-1429.
59. Anderson RL. Effect of saccharin ingestion on stool composition in relation to caecal enlargement and increased stool hydration. Food Chem Toxicol. 1983;21:255-257.
60. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186.
PRACTICE RECOMMENDATIONS
› Advise patients who are trying to lose weight that non-nutritive sweeteners (NNSs) are not beneficial for weight loss. A
› Reassure patients that NNSs do not appear to cause, or increase the risk of, developing type 2 diabetes mellitus. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
GLP-1 agonists, SGLT2 inhibitors offer more options in diabetes management
ORLANDO – The big news in diabetes management this year is “happy cardiologists and nephrologists.”
According to Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, founder of Metabolic Medicine Associates in King George, Va., these specialists are happy because the American College of Cardiology and the American Diabetes Association both recently updated their respective societies’ guidelines to include evidence that treating patients with type 2 diabetes with glucagonlike peptide-1 (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, or metformin can lower risk of cardiovascular disease and chronic kidney disease.
“Finally, the ACC is aligned with the ADA,” Ms. Kessler said in her presentation. “This is amazing, and it’s good news.”
Recent innovations in diabetes management technology, such as continuous glucose monitors, are also helping to make diabetes management easier. “If you’re not using some of this technology in your primary care practice, it’s coming to you, and it’s amazing the data it can provide to us,” said Ms. Kessler at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
In endocrinology, diabetes is thought of in terms of macrovascular and microvascular disease, she said. Macrovascular disease is cardiovascular disease and stroke, while microvascular disease is nephropathy, neuropathy, and retinopathy. Diabetes is a cardiovascular risk factor and puts patients at higher risk for cardiovascular death, all-cause mortality, and hospitalization because of myocardial infarction or stroke, compared with patients who do not have type 2 diabetes. There is also a higher risk of kidney disease, nerve damage, blindness, nonalcoholic fatty liver disease, depression, complications during pregnancy, periodontal disease, and erectile dysfunction, said Ms. Kessler, who also is a nurse practitioner and researcher.
However, including getting enough sleep, dietary interventions that target weight loss and blood glucose control, and increasing physical activity that has cardiopulmonary benefits. Clinicians should also treat underlying conditions that contribute to increased cardiovascular risk, such as obesity, dyslipidemia, hypertension, and nonalcoholic fatty liver disease.
Addressing insulin resistance and hyperglycemia are also important, but patients must avoid hypoglycemia. “Any patient with diabetes, we don’t want to drive them there because that’s a cardiac risk,” said Ms. Kessler. The endothelial microvascular and macrovascular damage is believed to be caused by glycemic swings, she added.
For pharmacologic therapy, patients with type 2 diabetes should stay on metformin if they are already on the drug, and it can even be used in cases where patients have reduced kidney function, with a glomerular filtration rate (GFR) between 30 and 60 mL/min per 1.73 m2, with a lower dose used between 30 and 45 mL/min per 1.73 m2. To treat patients with atherosclerotic cardiovascular disease, recent evidence has shown GLP-1 agonists are beneficial and can also promote appetite satiety, prandial support, and reduce a patient’s weight, but the drug is expensive, and about 15% of patients will not see therapeutic benefit while on the medication, said Ms. Kessler. Clinicians should also watch for increased risk of pancreatitis while patients use GLP-1 agonists, and it should not be prescribed in patients with a history of thyroid medullary cancer or multiple endocrine neoplasia type 2 (MEN2).
SGLT2 inhibitors can benefit type 1 diabetes and type 2 diabetes patients with heart failure and diabetic kidney disease, but should be the second or third choice in therapy. The dosage of SGLT2 inhibitors should be cut in half when used with insulin and sulfonylurea, and the drug can also increase LDL cholesterol.
Ms. Kessler noted that while GLP-1 agonists and SGLT2 inhibitors prevent or reduce cardiovascular risk, they are not currently approved to treat cardiovascular disease.
Ms. Kessler reports being an advisor and speaker for Novo Nordisk on the subject of obesity. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – The big news in diabetes management this year is “happy cardiologists and nephrologists.”
According to Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, founder of Metabolic Medicine Associates in King George, Va., these specialists are happy because the American College of Cardiology and the American Diabetes Association both recently updated their respective societies’ guidelines to include evidence that treating patients with type 2 diabetes with glucagonlike peptide-1 (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, or metformin can lower risk of cardiovascular disease and chronic kidney disease.
“Finally, the ACC is aligned with the ADA,” Ms. Kessler said in her presentation. “This is amazing, and it’s good news.”
Recent innovations in diabetes management technology, such as continuous glucose monitors, are also helping to make diabetes management easier. “If you’re not using some of this technology in your primary care practice, it’s coming to you, and it’s amazing the data it can provide to us,” said Ms. Kessler at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
In endocrinology, diabetes is thought of in terms of macrovascular and microvascular disease, she said. Macrovascular disease is cardiovascular disease and stroke, while microvascular disease is nephropathy, neuropathy, and retinopathy. Diabetes is a cardiovascular risk factor and puts patients at higher risk for cardiovascular death, all-cause mortality, and hospitalization because of myocardial infarction or stroke, compared with patients who do not have type 2 diabetes. There is also a higher risk of kidney disease, nerve damage, blindness, nonalcoholic fatty liver disease, depression, complications during pregnancy, periodontal disease, and erectile dysfunction, said Ms. Kessler, who also is a nurse practitioner and researcher.
However, including getting enough sleep, dietary interventions that target weight loss and blood glucose control, and increasing physical activity that has cardiopulmonary benefits. Clinicians should also treat underlying conditions that contribute to increased cardiovascular risk, such as obesity, dyslipidemia, hypertension, and nonalcoholic fatty liver disease.
Addressing insulin resistance and hyperglycemia are also important, but patients must avoid hypoglycemia. “Any patient with diabetes, we don’t want to drive them there because that’s a cardiac risk,” said Ms. Kessler. The endothelial microvascular and macrovascular damage is believed to be caused by glycemic swings, she added.
For pharmacologic therapy, patients with type 2 diabetes should stay on metformin if they are already on the drug, and it can even be used in cases where patients have reduced kidney function, with a glomerular filtration rate (GFR) between 30 and 60 mL/min per 1.73 m2, with a lower dose used between 30 and 45 mL/min per 1.73 m2. To treat patients with atherosclerotic cardiovascular disease, recent evidence has shown GLP-1 agonists are beneficial and can also promote appetite satiety, prandial support, and reduce a patient’s weight, but the drug is expensive, and about 15% of patients will not see therapeutic benefit while on the medication, said Ms. Kessler. Clinicians should also watch for increased risk of pancreatitis while patients use GLP-1 agonists, and it should not be prescribed in patients with a history of thyroid medullary cancer or multiple endocrine neoplasia type 2 (MEN2).
SGLT2 inhibitors can benefit type 1 diabetes and type 2 diabetes patients with heart failure and diabetic kidney disease, but should be the second or third choice in therapy. The dosage of SGLT2 inhibitors should be cut in half when used with insulin and sulfonylurea, and the drug can also increase LDL cholesterol.
Ms. Kessler noted that while GLP-1 agonists and SGLT2 inhibitors prevent or reduce cardiovascular risk, they are not currently approved to treat cardiovascular disease.
Ms. Kessler reports being an advisor and speaker for Novo Nordisk on the subject of obesity. Global Academy for Medical Education and this news organization are owned by the same parent company.
ORLANDO – The big news in diabetes management this year is “happy cardiologists and nephrologists.”
According to Christine Kessler, MN, ANP-C, CNS, BC-ADM, FAANP, founder of Metabolic Medicine Associates in King George, Va., these specialists are happy because the American College of Cardiology and the American Diabetes Association both recently updated their respective societies’ guidelines to include evidence that treating patients with type 2 diabetes with glucagonlike peptide-1 (GLP-1) agonists, sodium-glucose cotransporter 2 (SGLT2) inhibitors, or metformin can lower risk of cardiovascular disease and chronic kidney disease.
“Finally, the ACC is aligned with the ADA,” Ms. Kessler said in her presentation. “This is amazing, and it’s good news.”
Recent innovations in diabetes management technology, such as continuous glucose monitors, are also helping to make diabetes management easier. “If you’re not using some of this technology in your primary care practice, it’s coming to you, and it’s amazing the data it can provide to us,” said Ms. Kessler at the Cardiovascular & Respiratory Summit by Global Academy for Medical Education.
In endocrinology, diabetes is thought of in terms of macrovascular and microvascular disease, she said. Macrovascular disease is cardiovascular disease and stroke, while microvascular disease is nephropathy, neuropathy, and retinopathy. Diabetes is a cardiovascular risk factor and puts patients at higher risk for cardiovascular death, all-cause mortality, and hospitalization because of myocardial infarction or stroke, compared with patients who do not have type 2 diabetes. There is also a higher risk of kidney disease, nerve damage, blindness, nonalcoholic fatty liver disease, depression, complications during pregnancy, periodontal disease, and erectile dysfunction, said Ms. Kessler, who also is a nurse practitioner and researcher.
However, including getting enough sleep, dietary interventions that target weight loss and blood glucose control, and increasing physical activity that has cardiopulmonary benefits. Clinicians should also treat underlying conditions that contribute to increased cardiovascular risk, such as obesity, dyslipidemia, hypertension, and nonalcoholic fatty liver disease.
Addressing insulin resistance and hyperglycemia are also important, but patients must avoid hypoglycemia. “Any patient with diabetes, we don’t want to drive them there because that’s a cardiac risk,” said Ms. Kessler. The endothelial microvascular and macrovascular damage is believed to be caused by glycemic swings, she added.
For pharmacologic therapy, patients with type 2 diabetes should stay on metformin if they are already on the drug, and it can even be used in cases where patients have reduced kidney function, with a glomerular filtration rate (GFR) between 30 and 60 mL/min per 1.73 m2, with a lower dose used between 30 and 45 mL/min per 1.73 m2. To treat patients with atherosclerotic cardiovascular disease, recent evidence has shown GLP-1 agonists are beneficial and can also promote appetite satiety, prandial support, and reduce a patient’s weight, but the drug is expensive, and about 15% of patients will not see therapeutic benefit while on the medication, said Ms. Kessler. Clinicians should also watch for increased risk of pancreatitis while patients use GLP-1 agonists, and it should not be prescribed in patients with a history of thyroid medullary cancer or multiple endocrine neoplasia type 2 (MEN2).
SGLT2 inhibitors can benefit type 1 diabetes and type 2 diabetes patients with heart failure and diabetic kidney disease, but should be the second or third choice in therapy. The dosage of SGLT2 inhibitors should be cut in half when used with insulin and sulfonylurea, and the drug can also increase LDL cholesterol.
Ms. Kessler noted that while GLP-1 agonists and SGLT2 inhibitors prevent or reduce cardiovascular risk, they are not currently approved to treat cardiovascular disease.
Ms. Kessler reports being an advisor and speaker for Novo Nordisk on the subject of obesity. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM CARPS 2019
Endocrine Society advises on diabetes care for older adults
according to a new guideline on diabetes care for older adults issued by the Endocrine Society.
“The prevalence of diabetes in the United States is projected to increase dramatically during the next 3 decades as the population ages, the numbers of higher-risk minority groups increase, and people with diabetes live longer because of decreasing rates of cardiovascular deaths,” wrote Derek LeRoith, MD, of Icahn School of Medicine at Mount Sinai, New York, and his writing committee colleagues. They said their goal was to provide health care providers with guidance for the management of type 1 or type 2 diabetes in older patients, with a focus on simplifying medication regimens and management strategies to avoid “unnecessary and/or harmful adverse effects.”
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, is based mainly on evidence from controlled trials in two systematic reviews that specifically focused on adults aged 65 years and older. The guideline addresses six areas of consideration for this patient population:
- Role of the endocrinologist and diabetes care specialist.
- Screening for diabetes and prediabetes, and diabetes prevention.
- Assessment of older patients with diabetes.
- Treatment of hyperglycemia.
- Treating complications of diabetes.
- Special settings and populations.
Partnerships and screening
The guideline recommends that primary care providers partner with an endocrinologist or diabetes specialist in the care of patients aged 65 and older with newly diagnosed diabetes, and that the specialist take primary responsibility for diabetes care of patients with type 1 diabetes or those who need more complex intervention to achieve treatment goals.
Screening for diabetes in adults aged 65 years and older using fasting plasma glucose and/or hemoglobin A1c should occur every 2 years, but that schedule should be adjusted based on shared decision making with the patient, the committee said. Providers are advised to assess the patient’s overall health and personal values before settling on treatment goals and strategies. The writing group also recommends periodic cognitive screening and that medication regimens be simplified as much as possible.
Tackling hyperglycemia
For treatment of hyperglycemia, the guideline recommends outpatient strategies to minimize hypoglycemia and periodic or continuous glucose monitoring. The strategies include lifestyle modifications as a first-line intervention for ambulatory patients, as well as nutritional assessment. A high-protein diet is recommended for older patients with frailty, but no restrictions on diet are advised for patients who cannot meet glycemic targets with lifestyle modification and who are at risk for malnutrition.
Metformin is the first-choice recommendation for patients with diabetes aged 65 and older who need medical management in addition to lifestyle modification, but it is not recommended for individuals with impaired kidney function or gastrointestinal intolerance, according to the guideline. Oral and injectable drugs and/or insulin are recommended if metformin and lifestyle changes are insufficient to meet glycemic targets, the writers noted.
Managing complications
Hypertension is among the diabetes-related complications that need to be managed in older adults, and the guideline recommends a target blood pressure of 140/90 mm Hg, but other targets – based on patient-provider shared decision making – may be considered for patients in high-risk groups.
The guideline calls for management of hyperlipidemia with statin therapy and “use of an annual lipid profile to achieve the recommended levels for reducing absolute cardiovascular disease events and all-cause mortality.” The committee does not specify low-density lipoprotein cholesterol targets because of insufficient evidence, but recommends alternative treatments, including ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors, if statin therapy is not enough to help the patients meet goals. The writers also advocate fish oil and/or fenofibrate for patients with fasting triglycerides of more than 500 mg/dL.
To manage congestive heart failure in older patients with diabetes, the guideline recommends following standard clinical practice guidelines for the condition, and cautious use of oral hypoglycemic agents, including glinides, rosiglitazone, pioglitazone, and dipeptidyl peptidase–4 inhibitors. The writers noted that low-dose aspirin is recommended for patients with diabetes with a history of atherosclerotic cardiovascular disease.
The committee also recommends an annual comprehensive eye exam for patients with diabetes aged 65 years and older to identify retinal disease and suggests that actions, such as physical therapy and reduced use of sedatives, be taken to minimize the risk of falls in patients with neuropathy or problems with balance and gait.
Older patients with diabetes also should be screened annually for chronic kidney disease, and the dosage of diabetes medications should be adjusted to minimize side effects in patients with kidney problems.
Tailoring care to setting
Finally, the guideline addresses special settings and populations, including managing diabetes in hospitals or nursing homes, or in patients who are transitioning to homes or long-term care facilities. Recommendations in this category include simplifying medications for older adults with terminal illness or severe comorbidities, as well as setting glycemic targets as part of a hospital discharge plan.
“The most important aspect of successful transition is effective, detailed, and thorough bidirectional communication between the discharging and receiving teams of health care providers,” the writers emphasized.
The guideline is cosponsored by the European Society of Endocrinology, the Gerontological Society of America, and the Obesity Society. The chair of the committee had no relevant financial conflicts to disclose, and at least 50% of the committee members were free of relevant conflicts of interest.
SOURCE: LeRoith D et al. J Clin Endocrinol Metab. 2019;104:1520-74.
according to a new guideline on diabetes care for older adults issued by the Endocrine Society.
“The prevalence of diabetes in the United States is projected to increase dramatically during the next 3 decades as the population ages, the numbers of higher-risk minority groups increase, and people with diabetes live longer because of decreasing rates of cardiovascular deaths,” wrote Derek LeRoith, MD, of Icahn School of Medicine at Mount Sinai, New York, and his writing committee colleagues. They said their goal was to provide health care providers with guidance for the management of type 1 or type 2 diabetes in older patients, with a focus on simplifying medication regimens and management strategies to avoid “unnecessary and/or harmful adverse effects.”
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, is based mainly on evidence from controlled trials in two systematic reviews that specifically focused on adults aged 65 years and older. The guideline addresses six areas of consideration for this patient population:
- Role of the endocrinologist and diabetes care specialist.
- Screening for diabetes and prediabetes, and diabetes prevention.
- Assessment of older patients with diabetes.
- Treatment of hyperglycemia.
- Treating complications of diabetes.
- Special settings and populations.
Partnerships and screening
The guideline recommends that primary care providers partner with an endocrinologist or diabetes specialist in the care of patients aged 65 and older with newly diagnosed diabetes, and that the specialist take primary responsibility for diabetes care of patients with type 1 diabetes or those who need more complex intervention to achieve treatment goals.
Screening for diabetes in adults aged 65 years and older using fasting plasma glucose and/or hemoglobin A1c should occur every 2 years, but that schedule should be adjusted based on shared decision making with the patient, the committee said. Providers are advised to assess the patient’s overall health and personal values before settling on treatment goals and strategies. The writing group also recommends periodic cognitive screening and that medication regimens be simplified as much as possible.
Tackling hyperglycemia
For treatment of hyperglycemia, the guideline recommends outpatient strategies to minimize hypoglycemia and periodic or continuous glucose monitoring. The strategies include lifestyle modifications as a first-line intervention for ambulatory patients, as well as nutritional assessment. A high-protein diet is recommended for older patients with frailty, but no restrictions on diet are advised for patients who cannot meet glycemic targets with lifestyle modification and who are at risk for malnutrition.
Metformin is the first-choice recommendation for patients with diabetes aged 65 and older who need medical management in addition to lifestyle modification, but it is not recommended for individuals with impaired kidney function or gastrointestinal intolerance, according to the guideline. Oral and injectable drugs and/or insulin are recommended if metformin and lifestyle changes are insufficient to meet glycemic targets, the writers noted.
Managing complications
Hypertension is among the diabetes-related complications that need to be managed in older adults, and the guideline recommends a target blood pressure of 140/90 mm Hg, but other targets – based on patient-provider shared decision making – may be considered for patients in high-risk groups.
The guideline calls for management of hyperlipidemia with statin therapy and “use of an annual lipid profile to achieve the recommended levels for reducing absolute cardiovascular disease events and all-cause mortality.” The committee does not specify low-density lipoprotein cholesterol targets because of insufficient evidence, but recommends alternative treatments, including ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors, if statin therapy is not enough to help the patients meet goals. The writers also advocate fish oil and/or fenofibrate for patients with fasting triglycerides of more than 500 mg/dL.
To manage congestive heart failure in older patients with diabetes, the guideline recommends following standard clinical practice guidelines for the condition, and cautious use of oral hypoglycemic agents, including glinides, rosiglitazone, pioglitazone, and dipeptidyl peptidase–4 inhibitors. The writers noted that low-dose aspirin is recommended for patients with diabetes with a history of atherosclerotic cardiovascular disease.
The committee also recommends an annual comprehensive eye exam for patients with diabetes aged 65 years and older to identify retinal disease and suggests that actions, such as physical therapy and reduced use of sedatives, be taken to minimize the risk of falls in patients with neuropathy or problems with balance and gait.
Older patients with diabetes also should be screened annually for chronic kidney disease, and the dosage of diabetes medications should be adjusted to minimize side effects in patients with kidney problems.
Tailoring care to setting
Finally, the guideline addresses special settings and populations, including managing diabetes in hospitals or nursing homes, or in patients who are transitioning to homes or long-term care facilities. Recommendations in this category include simplifying medications for older adults with terminal illness or severe comorbidities, as well as setting glycemic targets as part of a hospital discharge plan.
“The most important aspect of successful transition is effective, detailed, and thorough bidirectional communication between the discharging and receiving teams of health care providers,” the writers emphasized.
The guideline is cosponsored by the European Society of Endocrinology, the Gerontological Society of America, and the Obesity Society. The chair of the committee had no relevant financial conflicts to disclose, and at least 50% of the committee members were free of relevant conflicts of interest.
SOURCE: LeRoith D et al. J Clin Endocrinol Metab. 2019;104:1520-74.
according to a new guideline on diabetes care for older adults issued by the Endocrine Society.
“The prevalence of diabetes in the United States is projected to increase dramatically during the next 3 decades as the population ages, the numbers of higher-risk minority groups increase, and people with diabetes live longer because of decreasing rates of cardiovascular deaths,” wrote Derek LeRoith, MD, of Icahn School of Medicine at Mount Sinai, New York, and his writing committee colleagues. They said their goal was to provide health care providers with guidance for the management of type 1 or type 2 diabetes in older patients, with a focus on simplifying medication regimens and management strategies to avoid “unnecessary and/or harmful adverse effects.”
The guideline, published in the Journal of Clinical Endocrinology & Metabolism, is based mainly on evidence from controlled trials in two systematic reviews that specifically focused on adults aged 65 years and older. The guideline addresses six areas of consideration for this patient population:
- Role of the endocrinologist and diabetes care specialist.
- Screening for diabetes and prediabetes, and diabetes prevention.
- Assessment of older patients with diabetes.
- Treatment of hyperglycemia.
- Treating complications of diabetes.
- Special settings and populations.
Partnerships and screening
The guideline recommends that primary care providers partner with an endocrinologist or diabetes specialist in the care of patients aged 65 and older with newly diagnosed diabetes, and that the specialist take primary responsibility for diabetes care of patients with type 1 diabetes or those who need more complex intervention to achieve treatment goals.
Screening for diabetes in adults aged 65 years and older using fasting plasma glucose and/or hemoglobin A1c should occur every 2 years, but that schedule should be adjusted based on shared decision making with the patient, the committee said. Providers are advised to assess the patient’s overall health and personal values before settling on treatment goals and strategies. The writing group also recommends periodic cognitive screening and that medication regimens be simplified as much as possible.
Tackling hyperglycemia
For treatment of hyperglycemia, the guideline recommends outpatient strategies to minimize hypoglycemia and periodic or continuous glucose monitoring. The strategies include lifestyle modifications as a first-line intervention for ambulatory patients, as well as nutritional assessment. A high-protein diet is recommended for older patients with frailty, but no restrictions on diet are advised for patients who cannot meet glycemic targets with lifestyle modification and who are at risk for malnutrition.
Metformin is the first-choice recommendation for patients with diabetes aged 65 and older who need medical management in addition to lifestyle modification, but it is not recommended for individuals with impaired kidney function or gastrointestinal intolerance, according to the guideline. Oral and injectable drugs and/or insulin are recommended if metformin and lifestyle changes are insufficient to meet glycemic targets, the writers noted.
Managing complications
Hypertension is among the diabetes-related complications that need to be managed in older adults, and the guideline recommends a target blood pressure of 140/90 mm Hg, but other targets – based on patient-provider shared decision making – may be considered for patients in high-risk groups.
The guideline calls for management of hyperlipidemia with statin therapy and “use of an annual lipid profile to achieve the recommended levels for reducing absolute cardiovascular disease events and all-cause mortality.” The committee does not specify low-density lipoprotein cholesterol targets because of insufficient evidence, but recommends alternative treatments, including ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors, if statin therapy is not enough to help the patients meet goals. The writers also advocate fish oil and/or fenofibrate for patients with fasting triglycerides of more than 500 mg/dL.
To manage congestive heart failure in older patients with diabetes, the guideline recommends following standard clinical practice guidelines for the condition, and cautious use of oral hypoglycemic agents, including glinides, rosiglitazone, pioglitazone, and dipeptidyl peptidase–4 inhibitors. The writers noted that low-dose aspirin is recommended for patients with diabetes with a history of atherosclerotic cardiovascular disease.
The committee also recommends an annual comprehensive eye exam for patients with diabetes aged 65 years and older to identify retinal disease and suggests that actions, such as physical therapy and reduced use of sedatives, be taken to minimize the risk of falls in patients with neuropathy or problems with balance and gait.
Older patients with diabetes also should be screened annually for chronic kidney disease, and the dosage of diabetes medications should be adjusted to minimize side effects in patients with kidney problems.
Tailoring care to setting
Finally, the guideline addresses special settings and populations, including managing diabetes in hospitals or nursing homes, or in patients who are transitioning to homes or long-term care facilities. Recommendations in this category include simplifying medications for older adults with terminal illness or severe comorbidities, as well as setting glycemic targets as part of a hospital discharge plan.
“The most important aspect of successful transition is effective, detailed, and thorough bidirectional communication between the discharging and receiving teams of health care providers,” the writers emphasized.
The guideline is cosponsored by the European Society of Endocrinology, the Gerontological Society of America, and the Obesity Society. The chair of the committee had no relevant financial conflicts to disclose, and at least 50% of the committee members were free of relevant conflicts of interest.
SOURCE: LeRoith D et al. J Clin Endocrinol Metab. 2019;104:1520-74.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
No increase in UTI risk with SGLT-2 inhibitors
Sodium-glucose cotransporter 2 inhibitors do not appear to increase the risk of urinary tract infections, compared with other antidiabetic medications, new research has found.
In a paper published in Annals of Internal Medicine, researchers reported the outcomes of a population-based, propensity-matched cohort study in which the analyzed data from 235,730 individuals newly prescribed sodium-glucose cotransporter 2 (SGLT-2) inhibitors, dipeptidyl peptidase–4 (DPP-4) inhibitors, and glucagonlike peptide–1 receptor (GLP-1) agonists.
In the first cohort, comparing SGLT-2 inhibitors with DDP-4 inhibitors, there were no significant differences between the two groups in the incidence rate of severe urinary tract infections (UTIs) (adjusted hazard ratio, 0.98; 95% confidence interval, 0.68-1.41).
In the second cohort, which compared patients taking GLP-1 receptor agonists with those taking SGLT-2 inhibitors, researchers saw a slightly lower incidence of severe UTIs among individuals on SGLT-2 inhibitors (HR, 0.72; 95% CI, 0.53-0.99; P = .04).
The analysis also failed to find any evidence that SGLT-2 inhibitors were associated with an increase in the risk of hospitalization or outpatient treatment for a UTI.
Chintan V. Dave, PharmD – now at Rutgers University, New Brunswick, N.J. – and colleagues from Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote that the findings have significant clinical implications.
“Patients who may be good candidates to receive SGLT-2 inhibitors for diabetes control but who have a history of recurrent UTIs may be precluded from being prescribed these agents; because UTIs are highly prevalent in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications that has been shown to decrease risk for major cardiovascular events and death,” they wrote.
The researchers stressed that “other factors beyond risk for UTI events should be considered in decisions about whether to prescribe SGLT-2 therapy for patients with diabetes.”
The authors did note that their study was subject to the usual limitations of observational studies, such as susceptibility to confounding, and was also limited to people with health insurance.
An accompanying editorial by Kristian B. Filion, PhD, and Oriana H. Yu, MD, of McGill University, Montreal, commented that the data provided reassuring, real-world evidence on the potential safety issue of UTI risk with SGLT-2 inhibitors.
However, they also stressed that the study excluded individuals at high risk or with a history of UTIs, and that these were subgroups who required further investigation.
The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
SOURCE: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
Sodium-glucose cotransporter 2 inhibitors do not appear to increase the risk of urinary tract infections, compared with other antidiabetic medications, new research has found.
In a paper published in Annals of Internal Medicine, researchers reported the outcomes of a population-based, propensity-matched cohort study in which the analyzed data from 235,730 individuals newly prescribed sodium-glucose cotransporter 2 (SGLT-2) inhibitors, dipeptidyl peptidase–4 (DPP-4) inhibitors, and glucagonlike peptide–1 receptor (GLP-1) agonists.
In the first cohort, comparing SGLT-2 inhibitors with DDP-4 inhibitors, there were no significant differences between the two groups in the incidence rate of severe urinary tract infections (UTIs) (adjusted hazard ratio, 0.98; 95% confidence interval, 0.68-1.41).
In the second cohort, which compared patients taking GLP-1 receptor agonists with those taking SGLT-2 inhibitors, researchers saw a slightly lower incidence of severe UTIs among individuals on SGLT-2 inhibitors (HR, 0.72; 95% CI, 0.53-0.99; P = .04).
The analysis also failed to find any evidence that SGLT-2 inhibitors were associated with an increase in the risk of hospitalization or outpatient treatment for a UTI.
Chintan V. Dave, PharmD – now at Rutgers University, New Brunswick, N.J. – and colleagues from Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote that the findings have significant clinical implications.
“Patients who may be good candidates to receive SGLT-2 inhibitors for diabetes control but who have a history of recurrent UTIs may be precluded from being prescribed these agents; because UTIs are highly prevalent in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications that has been shown to decrease risk for major cardiovascular events and death,” they wrote.
The researchers stressed that “other factors beyond risk for UTI events should be considered in decisions about whether to prescribe SGLT-2 therapy for patients with diabetes.”
The authors did note that their study was subject to the usual limitations of observational studies, such as susceptibility to confounding, and was also limited to people with health insurance.
An accompanying editorial by Kristian B. Filion, PhD, and Oriana H. Yu, MD, of McGill University, Montreal, commented that the data provided reassuring, real-world evidence on the potential safety issue of UTI risk with SGLT-2 inhibitors.
However, they also stressed that the study excluded individuals at high risk or with a history of UTIs, and that these were subgroups who required further investigation.
The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
SOURCE: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
Sodium-glucose cotransporter 2 inhibitors do not appear to increase the risk of urinary tract infections, compared with other antidiabetic medications, new research has found.
In a paper published in Annals of Internal Medicine, researchers reported the outcomes of a population-based, propensity-matched cohort study in which the analyzed data from 235,730 individuals newly prescribed sodium-glucose cotransporter 2 (SGLT-2) inhibitors, dipeptidyl peptidase–4 (DPP-4) inhibitors, and glucagonlike peptide–1 receptor (GLP-1) agonists.
In the first cohort, comparing SGLT-2 inhibitors with DDP-4 inhibitors, there were no significant differences between the two groups in the incidence rate of severe urinary tract infections (UTIs) (adjusted hazard ratio, 0.98; 95% confidence interval, 0.68-1.41).
In the second cohort, which compared patients taking GLP-1 receptor agonists with those taking SGLT-2 inhibitors, researchers saw a slightly lower incidence of severe UTIs among individuals on SGLT-2 inhibitors (HR, 0.72; 95% CI, 0.53-0.99; P = .04).
The analysis also failed to find any evidence that SGLT-2 inhibitors were associated with an increase in the risk of hospitalization or outpatient treatment for a UTI.
Chintan V. Dave, PharmD – now at Rutgers University, New Brunswick, N.J. – and colleagues from Brigham and Women’s Hospital and Harvard Medical School, Boston, wrote that the findings have significant clinical implications.
“Patients who may be good candidates to receive SGLT-2 inhibitors for diabetes control but who have a history of recurrent UTIs may be precluded from being prescribed these agents; because UTIs are highly prevalent in patients with diabetes, this could exclude a substantial number of patients from receiving an entire class of medications that has been shown to decrease risk for major cardiovascular events and death,” they wrote.
The researchers stressed that “other factors beyond risk for UTI events should be considered in decisions about whether to prescribe SGLT-2 therapy for patients with diabetes.”
The authors did note that their study was subject to the usual limitations of observational studies, such as susceptibility to confounding, and was also limited to people with health insurance.
An accompanying editorial by Kristian B. Filion, PhD, and Oriana H. Yu, MD, of McGill University, Montreal, commented that the data provided reassuring, real-world evidence on the potential safety issue of UTI risk with SGLT-2 inhibitors.
However, they also stressed that the study excluded individuals at high risk or with a history of UTIs, and that these were subgroups who required further investigation.
The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
SOURCE: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Sodium-glucose cotransporter 2 inhibitors are not associated with a greater risk of urinary tract infections, compared with dipeptidyl peptidase-4 inhibitors or glucagonlike peptide–1 receptor agonists.
Major finding: The incidence of severe urinary tract infections is similar among patients taking sodium-glucose cotransporter 2 inhibitors, dipeptidyl peptidase–4, inhibitors or glucagonlike peptide–1 receptor agonists.
Study details: A population-based, propensity-matched cohort study in 235,730 individuals with type 2 diabetes.
Disclosures: The study was supported by the Harvard Medical School and the National Institute on Aging. Three authors reported support from private industry outside the submitted work.
Source: Dave CV et al. Ann Intern Med. 2019 Jul 29. doi: 10.7326/M18-3136.
Polypharmacy in the Elderly
FDA approves Baqsimi nasal powder for emergency hypoglycemia treatment
in patients aged 4 years and older.
Injectable glucagon has been approved in the United States for several decades.
The safety and efficacy of the Baqsimi powder was assessed in two studies with adults with diabetes and one with pediatric patients. In all three studies, a single dose of Baqsimi was compared with a single dose of glucagon injection, and Baqsimi adequately raised blood sugar levels in response to insulin-induced hypoglycemia.
The most common adverse events associated with Baqsimi include nausea, vomiting, headache, upper respiratory tract irritation, watery eyes, redness of eyes, and itchiness. The safety profile is similar to that of injectable glucagon, with the addition of nasal- and eye-related symptoms because of the method of delivery.
“There are many products on the market for those who need insulin, but until now, people suffering from a severe hypoglycemic episode had to be treated with a glucagon injection that first had to be mixed in a several-step process. This new way to administer glucagon may simplify the process, which can be critical during an episode, especially since the patient may have lost consciousness or may be having a seizure. In those situations, we want the process to treat the suffering person to be as simple as possible,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
in patients aged 4 years and older.
Injectable glucagon has been approved in the United States for several decades.
The safety and efficacy of the Baqsimi powder was assessed in two studies with adults with diabetes and one with pediatric patients. In all three studies, a single dose of Baqsimi was compared with a single dose of glucagon injection, and Baqsimi adequately raised blood sugar levels in response to insulin-induced hypoglycemia.
The most common adverse events associated with Baqsimi include nausea, vomiting, headache, upper respiratory tract irritation, watery eyes, redness of eyes, and itchiness. The safety profile is similar to that of injectable glucagon, with the addition of nasal- and eye-related symptoms because of the method of delivery.
“There are many products on the market for those who need insulin, but until now, people suffering from a severe hypoglycemic episode had to be treated with a glucagon injection that first had to be mixed in a several-step process. This new way to administer glucagon may simplify the process, which can be critical during an episode, especially since the patient may have lost consciousness or may be having a seizure. In those situations, we want the process to treat the suffering person to be as simple as possible,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
in patients aged 4 years and older.
Injectable glucagon has been approved in the United States for several decades.
The safety and efficacy of the Baqsimi powder was assessed in two studies with adults with diabetes and one with pediatric patients. In all three studies, a single dose of Baqsimi was compared with a single dose of glucagon injection, and Baqsimi adequately raised blood sugar levels in response to insulin-induced hypoglycemia.
The most common adverse events associated with Baqsimi include nausea, vomiting, headache, upper respiratory tract irritation, watery eyes, redness of eyes, and itchiness. The safety profile is similar to that of injectable glucagon, with the addition of nasal- and eye-related symptoms because of the method of delivery.
“There are many products on the market for those who need insulin, but until now, people suffering from a severe hypoglycemic episode had to be treated with a glucagon injection that first had to be mixed in a several-step process. This new way to administer glucagon may simplify the process, which can be critical during an episode, especially since the patient may have lost consciousness or may be having a seizure. In those situations, we want the process to treat the suffering person to be as simple as possible,” Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said in the press release.
Find the full press release on the FDA website.
Inadequate glycemic control in type 1 diabetes leads to increased fracture risk
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
A single percentage increase in the level of hemoglobin A1c (HbA1c) in patients with newly diagnosed type 1 diabetes is significantly associated with an increase in fracture risk, according to findings in a study published in Diabetic Medicine.
To determine the effect of glycemic control on fracture risk, Rasiah Thayakaran, PhD, of the University of Birmingham (England) and colleagues analyzed data from 5,368 patients with newly diagnosed type 1 diabetes in the United Kingdom. HbA1c measurements were collected until either fracture or the end of the study, and were then converted from percentages to mmol/mol. Patient age ranged between 1 and 60 years, and the mean age was 22 years.
During 37,830 person‐years of follow‐up, 525 fractures were observed, with an incidence rate of 14 per 1,000 person‐years. The rate among men was 15 per 1,000 person‐years, compared with 12 per 1,000 person‐years among women. There was a significant association between hemoglobin level and risk of fractures (adjusted hazard ratio, 1.007 mmol/mol; 95% confidence interval, 1.002-1.011 mmol/mol), representing an increase of 7% in risk for fracture for each percentage increase in hemoglobin level.
“When assessing an individual with newly diagnosed type 1 diabetes and high HbA1c, increased clinical awareness about the fracture risk may be incorporated in decision‐making regarding the clinical management and even in prompting early antiosteoporotic intervention,” Dr. Thayakaran and coauthors wrote.
The researchers acknowledged the study’s limitations, including a possibility of residual confounding because of their use of observational data. In addition, they could not confirm whether the increase in fracture risk should be attributed to bone fragility or to increased risk of falls. Finally, though they noted using a comprehensive list of codes to identify fractures, they could not verify “completeness of recording ... and therefore reported overall fracture incidence should be interpreted with caution.”
The study was not funded. The authors reported no conflicts of interest.
SOURCE: Thayakaran R et al. Diab Med. 2019 Mar 8. doi: 10.1111/dme.13945.
FROM DIABETIC MEDICINE
DECLARE-TIMI58 shows improved kidney function with dapagliflozin
SAN FRANCISCO – and it showed reductions in the relative risk of renal-specific and cardiorenal outcomes of 47% and 24%, respectively, over 4 years, according to an analysis of the DECLARE-TIMI58 trial presented at the annual scientific sessions of the American Diabetes Association.
The findings add to a message physicians are hearing more and more frequently – that SGLT2 inhibitors, which also include empagliflozin (Jardiance) and canagliflozin (Invokana), could be useful for the early prevention of chronic kidney disease in patients who have type 2 diabetes. The main difference between the DECLARE-TIMI58 and previous study data was that most of its population did not have chronic kidney disease.
The drug should be given “at a very early stage of disease – it can reverse the disease and its complications [and] change the outcomes of patients,” said investigator Itamar Raz, MD, of Hebrew University, Jerusalem, in presenting the findings, which were published simultaneously in the Lancet Diabetes & Endocrinology (2019 Jun 10. doi: 10.1016/S2213-8587[19]30180-9).
What’s new in this renal analysis of DECLARE-TIMI58 is that patients were relatively healthy – 60% of them were without established cardiovascular disease and had much better renal function at baseline, compared with patients in other studies. About half of the patients started out with an estimated glomerular filtration rate (eGFR) more than 90 mL/min per 1.73 m2, which means that they had normal kidney function, and most of the rest of the patients had normal to near-normal renal function or mild renal failure. Findings from previous trials with SGLT2 inhibitors that showed renal protection generally included patients with established cardiovascular disease who started out with greater kidney impairment.
Previous findings have also demonstrated cardioprotective effects with SGLT2 inhibitors in patients with type 2 diabetes. For instance, in earlier results from the DECLARE-TIMI58 trial, dapagliflozin reduced the frequency of cardiovascular death or hospitalization for heart failure, compared with placebo (4.9% vs. 5.8%, respectively; New Engl J Med. 2019;380:347-57), although it did not significantly reduce the frequency of stroke, heart attack, or all-cause death, unlike the results from glucagonlike peptide–1 receptor agonists in much sicker patients (Circulation. 2019;139[17]:2022-31).
Taken as a whole, Dr. Raz said that findings for SGLT2 inhibitors, which, like the glucagonlike peptide–1 receptor agonists, are indicated as second-line therapy in type 2 diabetes after metformin, suggest that they should be used sooner in type 2 disease, perhaps in patients with a hemoglobin A1c level as low at 6.5%.
The absolute benefits of dapagliflozin, compared with placebo, notwithstanding, there are safety concerns with SGLT2s, including genitourinary infections, acute kidney injury, Fournier gangrene, diabetic ketoacidosis, bone fractures, and leg amputations (the latter two only with canagliflozin), many of which have been subject to warnings from the Food and Drug Administration.
Safety outcomes for dapagliflozin, compared with placebo, were not tabulated in the new renal report, but the authors noted a previously reported decrease of 30% in risk for acute kidney injury and major hypoglycemia with dapagliflozin over placebo. There was 1 case of Fournier gangrene with the drug versus 5 with placebo; 27 cases of diabetic ketoacidosis versus 12, respectively; 123 amputations versus 113; and 76 genital infections versus 9.
Matthew Riddle, MD, of Oregon Health and Science University, Portland, said SGLT2 studies “have shown important short-term benefit, but we have no information on long-term safety. These drugs are not physiologic; they do something powerful, but it’s nothing you see in nature. We don’t really know what they do yet; the physiology is still being worked out.”
DECLARE-TIMI58 randomized 8,582 patients with type 2 diabetes to receive dapagliflozin 10 mg orally daily and 8,578 patients to receive placebo. The patients remained on routine diabetes and cardiovascular care. Inclusion criteria included either established atherosclerotic cardiovascular disease (41% of patients) or cardiovascular risk factors (almost 60%), and creatinine clearance of at least 60 mL/min. Median follow-up was 4.2 years.
Overall, 4.2% of patients receiving dapagliflozin and 5.3% of those receiving placebo, met a prespecified secondary cardiorenal composite outcome of end-stage renal disease; death from renal or cardiovascular causes; or a decline of at least 40% in eGFR to less than 60 mL/min per 1.73 m2 according to two tests at least 4 weeks apart (hazard ratio, 0.76; P less than .0001).
Similarly, 1.5% of dapagliflozin patients and 2.6% of those on placebo met a prespecified renal-specific composite of those factors minus death from cardiovascular causes (HR, 0.53; P less than .0001).
Among placebo-treated patients, 2.5% had a sustained decline in eGFR of at least 40% to less than 60 mL/min per 1.73 m2, compared with 1.4% in the dapagliflozin group (HR, 0.54; P less than .0001). There were 11 cases of end-stage renal disease or renal death with dapagliflozin (0.1%), compared with 27 (0.3%) with placebo (HR, 0.41; P = .012).
Among patients who entered the trial with significant renal impairment – an eGFR of less than 60 mL/min per 1.73 m2 – the difference in further renal decline with dapagliflozin was not statistically significant against placebo because of the small number of patients, but Dr. Raz said the drug should still be used earlier in type 2 disease.
Dapagliflozin patients fared worse on renal measurements at 6 months, but caught up by year 2, and surpassed placebo at years 3 and 4, the authors wrote in the latest report.
Just more than 60% of participants were men; patients were in their 60s, on average, and overweight. About 80% of the patients were white.
AstraZeneca, which makes dapagliflozin, was involved with study design, data collection, data analysis, interpretation, and writing of the report. Four authors were employees of the company, and all but two of the 17 others, including Dr. Raz, disclosed personal payments from the company and/or research funding. Dr. Riddle disclosed receiving research funding from AstraZeneca.
SAN FRANCISCO – and it showed reductions in the relative risk of renal-specific and cardiorenal outcomes of 47% and 24%, respectively, over 4 years, according to an analysis of the DECLARE-TIMI58 trial presented at the annual scientific sessions of the American Diabetes Association.
The findings add to a message physicians are hearing more and more frequently – that SGLT2 inhibitors, which also include empagliflozin (Jardiance) and canagliflozin (Invokana), could be useful for the early prevention of chronic kidney disease in patients who have type 2 diabetes. The main difference between the DECLARE-TIMI58 and previous study data was that most of its population did not have chronic kidney disease.
The drug should be given “at a very early stage of disease – it can reverse the disease and its complications [and] change the outcomes of patients,” said investigator Itamar Raz, MD, of Hebrew University, Jerusalem, in presenting the findings, which were published simultaneously in the Lancet Diabetes & Endocrinology (2019 Jun 10. doi: 10.1016/S2213-8587[19]30180-9).
What’s new in this renal analysis of DECLARE-TIMI58 is that patients were relatively healthy – 60% of them were without established cardiovascular disease and had much better renal function at baseline, compared with patients in other studies. About half of the patients started out with an estimated glomerular filtration rate (eGFR) more than 90 mL/min per 1.73 m2, which means that they had normal kidney function, and most of the rest of the patients had normal to near-normal renal function or mild renal failure. Findings from previous trials with SGLT2 inhibitors that showed renal protection generally included patients with established cardiovascular disease who started out with greater kidney impairment.
Previous findings have also demonstrated cardioprotective effects with SGLT2 inhibitors in patients with type 2 diabetes. For instance, in earlier results from the DECLARE-TIMI58 trial, dapagliflozin reduced the frequency of cardiovascular death or hospitalization for heart failure, compared with placebo (4.9% vs. 5.8%, respectively; New Engl J Med. 2019;380:347-57), although it did not significantly reduce the frequency of stroke, heart attack, or all-cause death, unlike the results from glucagonlike peptide–1 receptor agonists in much sicker patients (Circulation. 2019;139[17]:2022-31).
Taken as a whole, Dr. Raz said that findings for SGLT2 inhibitors, which, like the glucagonlike peptide–1 receptor agonists, are indicated as second-line therapy in type 2 diabetes after metformin, suggest that they should be used sooner in type 2 disease, perhaps in patients with a hemoglobin A1c level as low at 6.5%.
The absolute benefits of dapagliflozin, compared with placebo, notwithstanding, there are safety concerns with SGLT2s, including genitourinary infections, acute kidney injury, Fournier gangrene, diabetic ketoacidosis, bone fractures, and leg amputations (the latter two only with canagliflozin), many of which have been subject to warnings from the Food and Drug Administration.
Safety outcomes for dapagliflozin, compared with placebo, were not tabulated in the new renal report, but the authors noted a previously reported decrease of 30% in risk for acute kidney injury and major hypoglycemia with dapagliflozin over placebo. There was 1 case of Fournier gangrene with the drug versus 5 with placebo; 27 cases of diabetic ketoacidosis versus 12, respectively; 123 amputations versus 113; and 76 genital infections versus 9.
Matthew Riddle, MD, of Oregon Health and Science University, Portland, said SGLT2 studies “have shown important short-term benefit, but we have no information on long-term safety. These drugs are not physiologic; they do something powerful, but it’s nothing you see in nature. We don’t really know what they do yet; the physiology is still being worked out.”
DECLARE-TIMI58 randomized 8,582 patients with type 2 diabetes to receive dapagliflozin 10 mg orally daily and 8,578 patients to receive placebo. The patients remained on routine diabetes and cardiovascular care. Inclusion criteria included either established atherosclerotic cardiovascular disease (41% of patients) or cardiovascular risk factors (almost 60%), and creatinine clearance of at least 60 mL/min. Median follow-up was 4.2 years.
Overall, 4.2% of patients receiving dapagliflozin and 5.3% of those receiving placebo, met a prespecified secondary cardiorenal composite outcome of end-stage renal disease; death from renal or cardiovascular causes; or a decline of at least 40% in eGFR to less than 60 mL/min per 1.73 m2 according to two tests at least 4 weeks apart (hazard ratio, 0.76; P less than .0001).
Similarly, 1.5% of dapagliflozin patients and 2.6% of those on placebo met a prespecified renal-specific composite of those factors minus death from cardiovascular causes (HR, 0.53; P less than .0001).
Among placebo-treated patients, 2.5% had a sustained decline in eGFR of at least 40% to less than 60 mL/min per 1.73 m2, compared with 1.4% in the dapagliflozin group (HR, 0.54; P less than .0001). There were 11 cases of end-stage renal disease or renal death with dapagliflozin (0.1%), compared with 27 (0.3%) with placebo (HR, 0.41; P = .012).
Among patients who entered the trial with significant renal impairment – an eGFR of less than 60 mL/min per 1.73 m2 – the difference in further renal decline with dapagliflozin was not statistically significant against placebo because of the small number of patients, but Dr. Raz said the drug should still be used earlier in type 2 disease.
Dapagliflozin patients fared worse on renal measurements at 6 months, but caught up by year 2, and surpassed placebo at years 3 and 4, the authors wrote in the latest report.
Just more than 60% of participants were men; patients were in their 60s, on average, and overweight. About 80% of the patients were white.
AstraZeneca, which makes dapagliflozin, was involved with study design, data collection, data analysis, interpretation, and writing of the report. Four authors were employees of the company, and all but two of the 17 others, including Dr. Raz, disclosed personal payments from the company and/or research funding. Dr. Riddle disclosed receiving research funding from AstraZeneca.
SAN FRANCISCO – and it showed reductions in the relative risk of renal-specific and cardiorenal outcomes of 47% and 24%, respectively, over 4 years, according to an analysis of the DECLARE-TIMI58 trial presented at the annual scientific sessions of the American Diabetes Association.
The findings add to a message physicians are hearing more and more frequently – that SGLT2 inhibitors, which also include empagliflozin (Jardiance) and canagliflozin (Invokana), could be useful for the early prevention of chronic kidney disease in patients who have type 2 diabetes. The main difference between the DECLARE-TIMI58 and previous study data was that most of its population did not have chronic kidney disease.
The drug should be given “at a very early stage of disease – it can reverse the disease and its complications [and] change the outcomes of patients,” said investigator Itamar Raz, MD, of Hebrew University, Jerusalem, in presenting the findings, which were published simultaneously in the Lancet Diabetes & Endocrinology (2019 Jun 10. doi: 10.1016/S2213-8587[19]30180-9).
What’s new in this renal analysis of DECLARE-TIMI58 is that patients were relatively healthy – 60% of them were without established cardiovascular disease and had much better renal function at baseline, compared with patients in other studies. About half of the patients started out with an estimated glomerular filtration rate (eGFR) more than 90 mL/min per 1.73 m2, which means that they had normal kidney function, and most of the rest of the patients had normal to near-normal renal function or mild renal failure. Findings from previous trials with SGLT2 inhibitors that showed renal protection generally included patients with established cardiovascular disease who started out with greater kidney impairment.
Previous findings have also demonstrated cardioprotective effects with SGLT2 inhibitors in patients with type 2 diabetes. For instance, in earlier results from the DECLARE-TIMI58 trial, dapagliflozin reduced the frequency of cardiovascular death or hospitalization for heart failure, compared with placebo (4.9% vs. 5.8%, respectively; New Engl J Med. 2019;380:347-57), although it did not significantly reduce the frequency of stroke, heart attack, or all-cause death, unlike the results from glucagonlike peptide–1 receptor agonists in much sicker patients (Circulation. 2019;139[17]:2022-31).
Taken as a whole, Dr. Raz said that findings for SGLT2 inhibitors, which, like the glucagonlike peptide–1 receptor agonists, are indicated as second-line therapy in type 2 diabetes after metformin, suggest that they should be used sooner in type 2 disease, perhaps in patients with a hemoglobin A1c level as low at 6.5%.
The absolute benefits of dapagliflozin, compared with placebo, notwithstanding, there are safety concerns with SGLT2s, including genitourinary infections, acute kidney injury, Fournier gangrene, diabetic ketoacidosis, bone fractures, and leg amputations (the latter two only with canagliflozin), many of which have been subject to warnings from the Food and Drug Administration.
Safety outcomes for dapagliflozin, compared with placebo, were not tabulated in the new renal report, but the authors noted a previously reported decrease of 30% in risk for acute kidney injury and major hypoglycemia with dapagliflozin over placebo. There was 1 case of Fournier gangrene with the drug versus 5 with placebo; 27 cases of diabetic ketoacidosis versus 12, respectively; 123 amputations versus 113; and 76 genital infections versus 9.
Matthew Riddle, MD, of Oregon Health and Science University, Portland, said SGLT2 studies “have shown important short-term benefit, but we have no information on long-term safety. These drugs are not physiologic; they do something powerful, but it’s nothing you see in nature. We don’t really know what they do yet; the physiology is still being worked out.”
DECLARE-TIMI58 randomized 8,582 patients with type 2 diabetes to receive dapagliflozin 10 mg orally daily and 8,578 patients to receive placebo. The patients remained on routine diabetes and cardiovascular care. Inclusion criteria included either established atherosclerotic cardiovascular disease (41% of patients) or cardiovascular risk factors (almost 60%), and creatinine clearance of at least 60 mL/min. Median follow-up was 4.2 years.
Overall, 4.2% of patients receiving dapagliflozin and 5.3% of those receiving placebo, met a prespecified secondary cardiorenal composite outcome of end-stage renal disease; death from renal or cardiovascular causes; or a decline of at least 40% in eGFR to less than 60 mL/min per 1.73 m2 according to two tests at least 4 weeks apart (hazard ratio, 0.76; P less than .0001).
Similarly, 1.5% of dapagliflozin patients and 2.6% of those on placebo met a prespecified renal-specific composite of those factors minus death from cardiovascular causes (HR, 0.53; P less than .0001).
Among placebo-treated patients, 2.5% had a sustained decline in eGFR of at least 40% to less than 60 mL/min per 1.73 m2, compared with 1.4% in the dapagliflozin group (HR, 0.54; P less than .0001). There were 11 cases of end-stage renal disease or renal death with dapagliflozin (0.1%), compared with 27 (0.3%) with placebo (HR, 0.41; P = .012).
Among patients who entered the trial with significant renal impairment – an eGFR of less than 60 mL/min per 1.73 m2 – the difference in further renal decline with dapagliflozin was not statistically significant against placebo because of the small number of patients, but Dr. Raz said the drug should still be used earlier in type 2 disease.
Dapagliflozin patients fared worse on renal measurements at 6 months, but caught up by year 2, and surpassed placebo at years 3 and 4, the authors wrote in the latest report.
Just more than 60% of participants were men; patients were in their 60s, on average, and overweight. About 80% of the patients were white.
AstraZeneca, which makes dapagliflozin, was involved with study design, data collection, data analysis, interpretation, and writing of the report. Four authors were employees of the company, and all but two of the 17 others, including Dr. Raz, disclosed personal payments from the company and/or research funding. Dr. Riddle disclosed receiving research funding from AstraZeneca.
REPORTING FROM ADA 2019
Key clinical point: The sodium-glucose transporter 2 inhibitor dapagliflozin slowed progression of kidney disease in patients with type 2 diabetes.
Major finding: Overall, 4.2% of dapagliflozin and 5.3% of placebo patients met a prespecified secondary cardiorenal composite outcome of end-stage renal disease, death from renal or cardiovascular causes, or a decline of at least 40% in estimated glomerular filtration rate to less than 60 mL/min per 1.73m2 (HR, 0.76; P less than .0001).
Study details: Placebo-controlled trial in more than 17,000 patients with type 2 diabetes
Disclosures: AstraZeneca, the maker of dapagliflozin, funded and conducted the study. Four authors were employees of the company, and all but two of the remaining 17, including Dr. Raz, disclosed personal payments from the company and/or research funding. Dr. Riddle reported receiving research funding from AstraZeneca.
Source: Mosenzon O et al. Lancet Diabetes Endocrinol. 2019 Jun 10. doi: 10.1016/S2213-8587(19)30180-9.