User login
62-year-old woman • dysuria • dyspareunia • urinary incontinence • Dx?
THE CASE
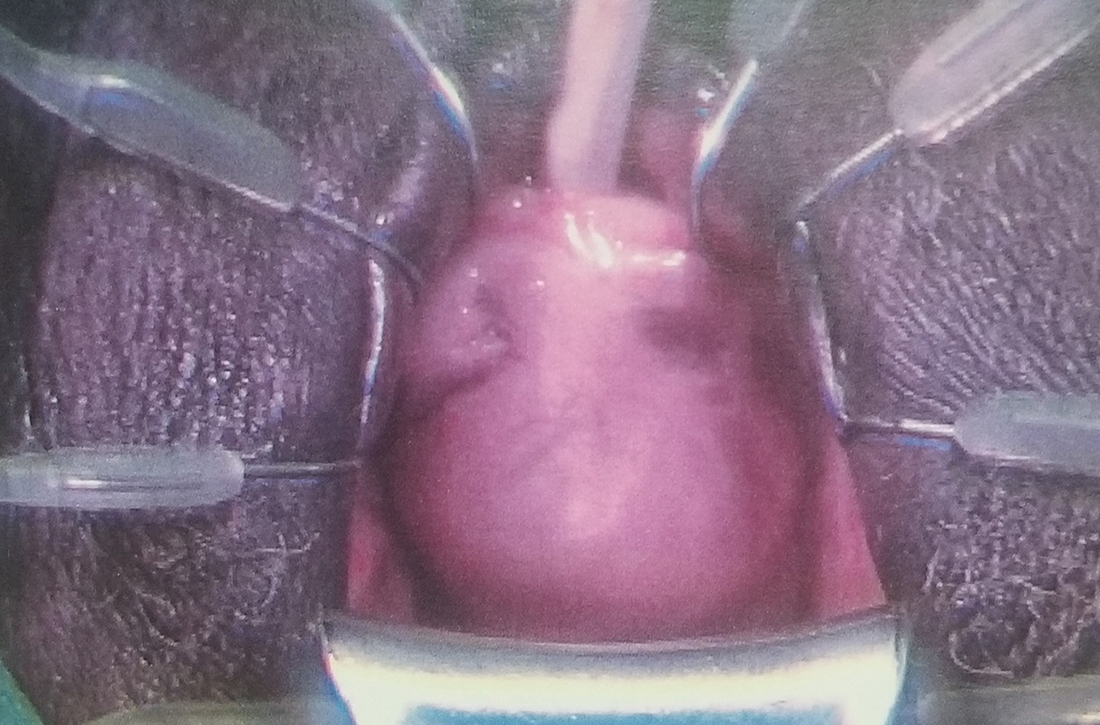
A 62-year-old postmenopausal woman presented to the clinic as a new patient for her annual physical examination. She reported a 9-year history of symptoms including dysuria, post-void dribbling, dyspareunia, and urinary incontinence on review of systems. Her physical examination revealed an anterior vaginal wall bulge (FIGURE). Results of a urinalysis were negative. The patient was referred to Urology for further evaluation.
THE DIAGNOSIS
A pelvic magnetic resonance imaging (MRI) scan revealed a large periurethral diverticulum with a horseshoe shape.
DISCUSSION
Women are more likely than men to develop urethral diverticulum, and it can manifest at any age, usually in the third through seventh decade.4,5 It was once thought to be more common in Black women, although the literature does not support this.6 Black women are 3 times more likely to be operated on than White women to treat urethral diverticula.7
Unknown origin. Most cases of urethral diverticulum are acquired; the etiology is uncertain.8,9 The assumption is that urethral diverticulum occurs as a result of repeated infection of the periurethral glands with subsequent obstruction, abscess formation, and chronic inflammation.1,2,4 Childbirth trauma, iatrogenic causes, and urethral instrumentation have also been implicated.3,4 In rare cases of congenital urethral diverticula, the diverticula are thought to be remnants of Gartner duct cysts, and yet, incidence in the pediatric population is low.8
Diagnosis is confirmed through physical exam and imaging
The urethral diverticulum manifests anteriorly and palpation of the anterior vaginal wall may reveal a painful mass.10 A split-speculum is used for careful inspection and palpation of the anterior vaginal wall.9 If the diverticulum is found to be firm on palpation, or there is bloody urethral drainage, malignancy (although rare) must be ruled out.4,5 Refer such patients to a urologist or urogynecologist.
Radiologic imaging (eg, ultrasound,
Continue to: Nonspecific symptoms may lead to misdiagnosis
Nonspecific symptoms may lead to misdiagnosis. The symptoms associated with urethral diverticulum are diverse and linked to several differential diagnoses (TABLE).3,4,12 The most common signs and symptoms are pelvic pain, urethral mass, dyspareunia, dysuria, urinary incontinence, and post-void dribbling—all of which are considered nonspecific.3,10,11 These nonspecific symptoms (or even an absence of symptoms), along with a physician’s lack of familiarity with urethral diverticulum, can result in a misdiagnosis or even a delayed diagnosis (up to 5.2 years).3,10

Managing symptoms vs preventing recurrence
Conservative management with antibiotics, anticholinergics, and/or observation is acceptable for patients with mild symptoms and those who are pregnant or who have a current infection or serious comorbidities that preclude surgery.3,9 Complete excision of the urethral diverticulum with reconstruction is considered the most effective surgical management for symptom relief and recurrence prevention.3,4,11,14
Our patient underwent a successful transvaginal suburethral diverticulectomy.
THE TAKEAWAY
The diagnosis of female urethral diverticulum is often delayed or misdiagnosed because symptoms are diverse and nonspecific. One should have a high degree of suspicion for urethral diverticulum in patients with dysuria, dyspareunia, pelvic pain, urinary incontinence, and irritative voiding symptoms who are not responding to conservative management. Ultrasound is an appropriate first-line imaging modality. However, a pelvic MRI is the most sensitive and specific in diagnosing urethral diverticulum.12
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 720 Westview Drive, Atlanta, GA 30310; [email protected]
1. Billow M, James R, Resnick K, et al. An unusual presentation of a urethral diverticulum as a vaginal wall mass: a case report. J Med Case Rep. 2013;7:171. doi: 10.1186/1752-1947-7-171
2. El-Nashar SA, Bacon MM, Kim-Fine S, et al. Incidence of female urethral diverticulum: a population-based analysis and literature review. Int Urogynecol J. 2014;25:73-79. doi: 10.1007/s00192-013-2155-2
3. Cameron AP. Urethral diverticulum in the female: a meta-analysis of modern series. Minerva Ginecol. 2016;68:186-210.
4. Greiman AK, Rolef J, Rovner ES. Urethral diverticulum: a systematic review. Arab J Urol. 2019;17:49-57. doi: 10.1080/2090598X.2019.1589748
5. Allen D, Mishra V, Pepper W, et al. A single-center experience of symptomatic male urethral diverticula. Urology. 2007;70:650-653. doi: 10.1016/j.urology.2007.06.1111
6. O’Connor E, Iatropoulou D, Hashimoto S, et al. Urethral diverticulum carcinoma in females—a case series and review of the English and Japanese literature. Transl Androl Urol. 2018;7:703-729. doi: 10.21037/tau.2018.07.08
7. Burrows LJ, Howden NL, Meyn L, et al. Surgical procedures for urethral diverticula in women in the United States, 1979-1997. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:158-161. doi: 10.1007/s00192-004-1145-9
8. Riyach O, Ahsaini M, Tazi MF, et al. Female urethral diverticulum: cases report and literature. Ann Surg Innov Res. 2014;8:1. doi: 10.1186/1750-1164-8-1
9. Antosh DD, Gutman RE. Diagnosis and management of female urethral diverticulum. Female Pelvic Med Reconstr Surg. 2011;17:264-271. doi: 10.1097/SPV.0b013e318234a242
10. Romanzi LJ, Groutz A, Blaivas JG. Urethral diverticulum in women: diverse presentations resulting in diagnostic delay and mismanagement. J Urol. 2000;164:428-433.
11. Reeves FA, Inman RD, Chapple CR. Management of symptomatic urethral diverticula in women: a single-centre experience. Eur Urol. 2014;66:164-172. doi: 10.1016/j.eururo.2014.02.041
12. Dwarkasing RS, Dinkelaar W, Hop WCJ, et al. MRI evaluation of urethral diverticula and differential diagnosis in symptomatic women. AJR Am J Roentgenol. 2011;197:676-682. doi: 10.2214/AJR.10.6144
13. Porten S, Kielb S. Diagnosis of female diverticula using magnetic resonance imaging. Adv Urol. 2008;2008:213516. doi: 10.1155/2008/213516
14. Ockrim JL, Allen DJ, Shah PJ, et al. A tertiary experience of urethral diverticulectomy: diagnosis, imaging and surgical outcomes. BJU Int. 2009;103:1550-1554. doi: 10.1111/j.1464-410X.2009.08348.x
THE CASE
A 62-year-old postmenopausal woman presented to the clinic as a new patient for her annual physical examination. She reported a 9-year history of symptoms including dysuria, post-void dribbling, dyspareunia, and urinary incontinence on review of systems. Her physical examination revealed an anterior vaginal wall bulge (FIGURE). Results of a urinalysis were negative. The patient was referred to Urology for further evaluation.

THE DIAGNOSIS
A pelvic magnetic resonance imaging (MRI) scan revealed a large periurethral diverticulum with a horseshoe shape.
DISCUSSION
Women are more likely than men to develop urethral diverticulum, and it can manifest at any age, usually in the third through seventh decade.4,5 It was once thought to be more common in Black women, although the literature does not support this.6 Black women are 3 times more likely to be operated on than White women to treat urethral diverticula.7
Unknown origin. Most cases of urethral diverticulum are acquired; the etiology is uncertain.8,9 The assumption is that urethral diverticulum occurs as a result of repeated infection of the periurethral glands with subsequent obstruction, abscess formation, and chronic inflammation.1,2,4 Childbirth trauma, iatrogenic causes, and urethral instrumentation have also been implicated.3,4 In rare cases of congenital urethral diverticula, the diverticula are thought to be remnants of Gartner duct cysts, and yet, incidence in the pediatric population is low.8
Diagnosis is confirmed through physical exam and imaging
The urethral diverticulum manifests anteriorly and palpation of the anterior vaginal wall may reveal a painful mass.10 A split-speculum is used for careful inspection and palpation of the anterior vaginal wall.9 If the diverticulum is found to be firm on palpation, or there is bloody urethral drainage, malignancy (although rare) must be ruled out.4,5 Refer such patients to a urologist or urogynecologist.
Radiologic imaging (eg, ultrasound,
Continue to: Nonspecific symptoms may lead to misdiagnosis
Nonspecific symptoms may lead to misdiagnosis. The symptoms associated with urethral diverticulum are diverse and linked to several differential diagnoses (TABLE).3,4,12 The most common signs and symptoms are pelvic pain, urethral mass, dyspareunia, dysuria, urinary incontinence, and post-void dribbling—all of which are considered nonspecific.3,10,11 These nonspecific symptoms (or even an absence of symptoms), along with a physician’s lack of familiarity with urethral diverticulum, can result in a misdiagnosis or even a delayed diagnosis (up to 5.2 years).3,10

Managing symptoms vs preventing recurrence
Conservative management with antibiotics, anticholinergics, and/or observation is acceptable for patients with mild symptoms and those who are pregnant or who have a current infection or serious comorbidities that preclude surgery.3,9 Complete excision of the urethral diverticulum with reconstruction is considered the most effective surgical management for symptom relief and recurrence prevention.3,4,11,14
Our patient underwent a successful transvaginal suburethral diverticulectomy.
THE TAKEAWAY
The diagnosis of female urethral diverticulum is often delayed or misdiagnosed because symptoms are diverse and nonspecific. One should have a high degree of suspicion for urethral diverticulum in patients with dysuria, dyspareunia, pelvic pain, urinary incontinence, and irritative voiding symptoms who are not responding to conservative management. Ultrasound is an appropriate first-line imaging modality. However, a pelvic MRI is the most sensitive and specific in diagnosing urethral diverticulum.12
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 720 Westview Drive, Atlanta, GA 30310; [email protected]
THE CASE
A 62-year-old postmenopausal woman presented to the clinic as a new patient for her annual physical examination. She reported a 9-year history of symptoms including dysuria, post-void dribbling, dyspareunia, and urinary incontinence on review of systems. Her physical examination revealed an anterior vaginal wall bulge (FIGURE). Results of a urinalysis were negative. The patient was referred to Urology for further evaluation.

THE DIAGNOSIS
A pelvic magnetic resonance imaging (MRI) scan revealed a large periurethral diverticulum with a horseshoe shape.
DISCUSSION
Women are more likely than men to develop urethral diverticulum, and it can manifest at any age, usually in the third through seventh decade.4,5 It was once thought to be more common in Black women, although the literature does not support this.6 Black women are 3 times more likely to be operated on than White women to treat urethral diverticula.7
Unknown origin. Most cases of urethral diverticulum are acquired; the etiology is uncertain.8,9 The assumption is that urethral diverticulum occurs as a result of repeated infection of the periurethral glands with subsequent obstruction, abscess formation, and chronic inflammation.1,2,4 Childbirth trauma, iatrogenic causes, and urethral instrumentation have also been implicated.3,4 In rare cases of congenital urethral diverticula, the diverticula are thought to be remnants of Gartner duct cysts, and yet, incidence in the pediatric population is low.8
Diagnosis is confirmed through physical exam and imaging
The urethral diverticulum manifests anteriorly and palpation of the anterior vaginal wall may reveal a painful mass.10 A split-speculum is used for careful inspection and palpation of the anterior vaginal wall.9 If the diverticulum is found to be firm on palpation, or there is bloody urethral drainage, malignancy (although rare) must be ruled out.4,5 Refer such patients to a urologist or urogynecologist.
Radiologic imaging (eg, ultrasound,
Continue to: Nonspecific symptoms may lead to misdiagnosis
Nonspecific symptoms may lead to misdiagnosis. The symptoms associated with urethral diverticulum are diverse and linked to several differential diagnoses (TABLE).3,4,12 The most common signs and symptoms are pelvic pain, urethral mass, dyspareunia, dysuria, urinary incontinence, and post-void dribbling—all of which are considered nonspecific.3,10,11 These nonspecific symptoms (or even an absence of symptoms), along with a physician’s lack of familiarity with urethral diverticulum, can result in a misdiagnosis or even a delayed diagnosis (up to 5.2 years).3,10

Managing symptoms vs preventing recurrence
Conservative management with antibiotics, anticholinergics, and/or observation is acceptable for patients with mild symptoms and those who are pregnant or who have a current infection or serious comorbidities that preclude surgery.3,9 Complete excision of the urethral diverticulum with reconstruction is considered the most effective surgical management for symptom relief and recurrence prevention.3,4,11,14
Our patient underwent a successful transvaginal suburethral diverticulectomy.
THE TAKEAWAY
The diagnosis of female urethral diverticulum is often delayed or misdiagnosed because symptoms are diverse and nonspecific. One should have a high degree of suspicion for urethral diverticulum in patients with dysuria, dyspareunia, pelvic pain, urinary incontinence, and irritative voiding symptoms who are not responding to conservative management. Ultrasound is an appropriate first-line imaging modality. However, a pelvic MRI is the most sensitive and specific in diagnosing urethral diverticulum.12
CORRESPONDENCE
Folashade Omole, MD, FAAFP, 720 Westview Drive, Atlanta, GA 30310; [email protected]
1. Billow M, James R, Resnick K, et al. An unusual presentation of a urethral diverticulum as a vaginal wall mass: a case report. J Med Case Rep. 2013;7:171. doi: 10.1186/1752-1947-7-171
2. El-Nashar SA, Bacon MM, Kim-Fine S, et al. Incidence of female urethral diverticulum: a population-based analysis and literature review. Int Urogynecol J. 2014;25:73-79. doi: 10.1007/s00192-013-2155-2
3. Cameron AP. Urethral diverticulum in the female: a meta-analysis of modern series. Minerva Ginecol. 2016;68:186-210.
4. Greiman AK, Rolef J, Rovner ES. Urethral diverticulum: a systematic review. Arab J Urol. 2019;17:49-57. doi: 10.1080/2090598X.2019.1589748
5. Allen D, Mishra V, Pepper W, et al. A single-center experience of symptomatic male urethral diverticula. Urology. 2007;70:650-653. doi: 10.1016/j.urology.2007.06.1111
6. O’Connor E, Iatropoulou D, Hashimoto S, et al. Urethral diverticulum carcinoma in females—a case series and review of the English and Japanese literature. Transl Androl Urol. 2018;7:703-729. doi: 10.21037/tau.2018.07.08
7. Burrows LJ, Howden NL, Meyn L, et al. Surgical procedures for urethral diverticula in women in the United States, 1979-1997. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:158-161. doi: 10.1007/s00192-004-1145-9
8. Riyach O, Ahsaini M, Tazi MF, et al. Female urethral diverticulum: cases report and literature. Ann Surg Innov Res. 2014;8:1. doi: 10.1186/1750-1164-8-1
9. Antosh DD, Gutman RE. Diagnosis and management of female urethral diverticulum. Female Pelvic Med Reconstr Surg. 2011;17:264-271. doi: 10.1097/SPV.0b013e318234a242
10. Romanzi LJ, Groutz A, Blaivas JG. Urethral diverticulum in women: diverse presentations resulting in diagnostic delay and mismanagement. J Urol. 2000;164:428-433.
11. Reeves FA, Inman RD, Chapple CR. Management of symptomatic urethral diverticula in women: a single-centre experience. Eur Urol. 2014;66:164-172. doi: 10.1016/j.eururo.2014.02.041
12. Dwarkasing RS, Dinkelaar W, Hop WCJ, et al. MRI evaluation of urethral diverticula and differential diagnosis in symptomatic women. AJR Am J Roentgenol. 2011;197:676-682. doi: 10.2214/AJR.10.6144
13. Porten S, Kielb S. Diagnosis of female diverticula using magnetic resonance imaging. Adv Urol. 2008;2008:213516. doi: 10.1155/2008/213516
14. Ockrim JL, Allen DJ, Shah PJ, et al. A tertiary experience of urethral diverticulectomy: diagnosis, imaging and surgical outcomes. BJU Int. 2009;103:1550-1554. doi: 10.1111/j.1464-410X.2009.08348.x
1. Billow M, James R, Resnick K, et al. An unusual presentation of a urethral diverticulum as a vaginal wall mass: a case report. J Med Case Rep. 2013;7:171. doi: 10.1186/1752-1947-7-171
2. El-Nashar SA, Bacon MM, Kim-Fine S, et al. Incidence of female urethral diverticulum: a population-based analysis and literature review. Int Urogynecol J. 2014;25:73-79. doi: 10.1007/s00192-013-2155-2
3. Cameron AP. Urethral diverticulum in the female: a meta-analysis of modern series. Minerva Ginecol. 2016;68:186-210.
4. Greiman AK, Rolef J, Rovner ES. Urethral diverticulum: a systematic review. Arab J Urol. 2019;17:49-57. doi: 10.1080/2090598X.2019.1589748
5. Allen D, Mishra V, Pepper W, et al. A single-center experience of symptomatic male urethral diverticula. Urology. 2007;70:650-653. doi: 10.1016/j.urology.2007.06.1111
6. O’Connor E, Iatropoulou D, Hashimoto S, et al. Urethral diverticulum carcinoma in females—a case series and review of the English and Japanese literature. Transl Androl Urol. 2018;7:703-729. doi: 10.21037/tau.2018.07.08
7. Burrows LJ, Howden NL, Meyn L, et al. Surgical procedures for urethral diverticula in women in the United States, 1979-1997. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:158-161. doi: 10.1007/s00192-004-1145-9
8. Riyach O, Ahsaini M, Tazi MF, et al. Female urethral diverticulum: cases report and literature. Ann Surg Innov Res. 2014;8:1. doi: 10.1186/1750-1164-8-1
9. Antosh DD, Gutman RE. Diagnosis and management of female urethral diverticulum. Female Pelvic Med Reconstr Surg. 2011;17:264-271. doi: 10.1097/SPV.0b013e318234a242
10. Romanzi LJ, Groutz A, Blaivas JG. Urethral diverticulum in women: diverse presentations resulting in diagnostic delay and mismanagement. J Urol. 2000;164:428-433.
11. Reeves FA, Inman RD, Chapple CR. Management of symptomatic urethral diverticula in women: a single-centre experience. Eur Urol. 2014;66:164-172. doi: 10.1016/j.eururo.2014.02.041
12. Dwarkasing RS, Dinkelaar W, Hop WCJ, et al. MRI evaluation of urethral diverticula and differential diagnosis in symptomatic women. AJR Am J Roentgenol. 2011;197:676-682. doi: 10.2214/AJR.10.6144
13. Porten S, Kielb S. Diagnosis of female diverticula using magnetic resonance imaging. Adv Urol. 2008;2008:213516. doi: 10.1155/2008/213516
14. Ockrim JL, Allen DJ, Shah PJ, et al. A tertiary experience of urethral diverticulectomy: diagnosis, imaging and surgical outcomes. BJU Int. 2009;103:1550-1554. doi: 10.1111/j.1464-410X.2009.08348.x
How to better identify and manage women with elevated breast cancer risk
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
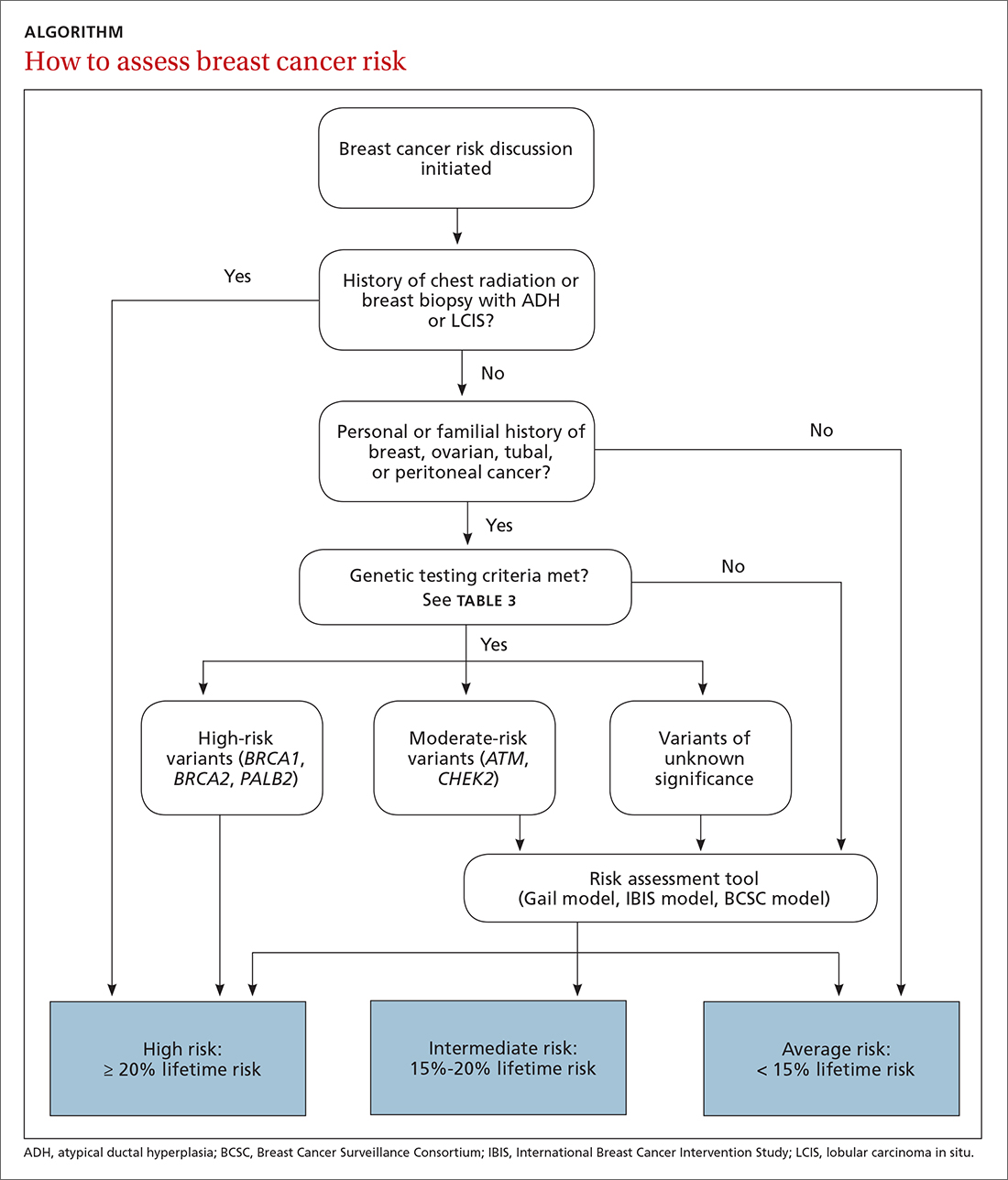
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.
CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
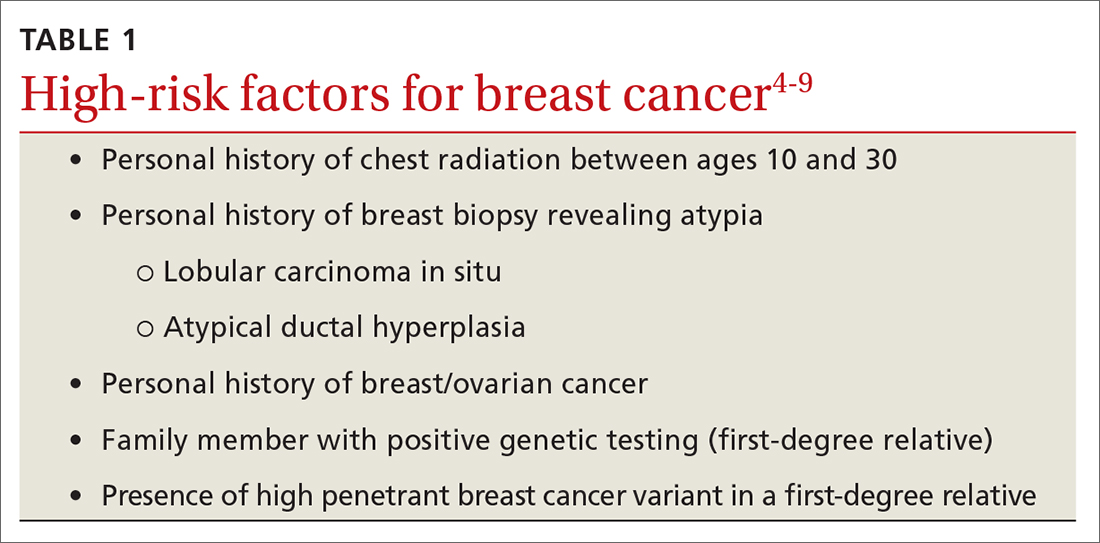
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9
In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
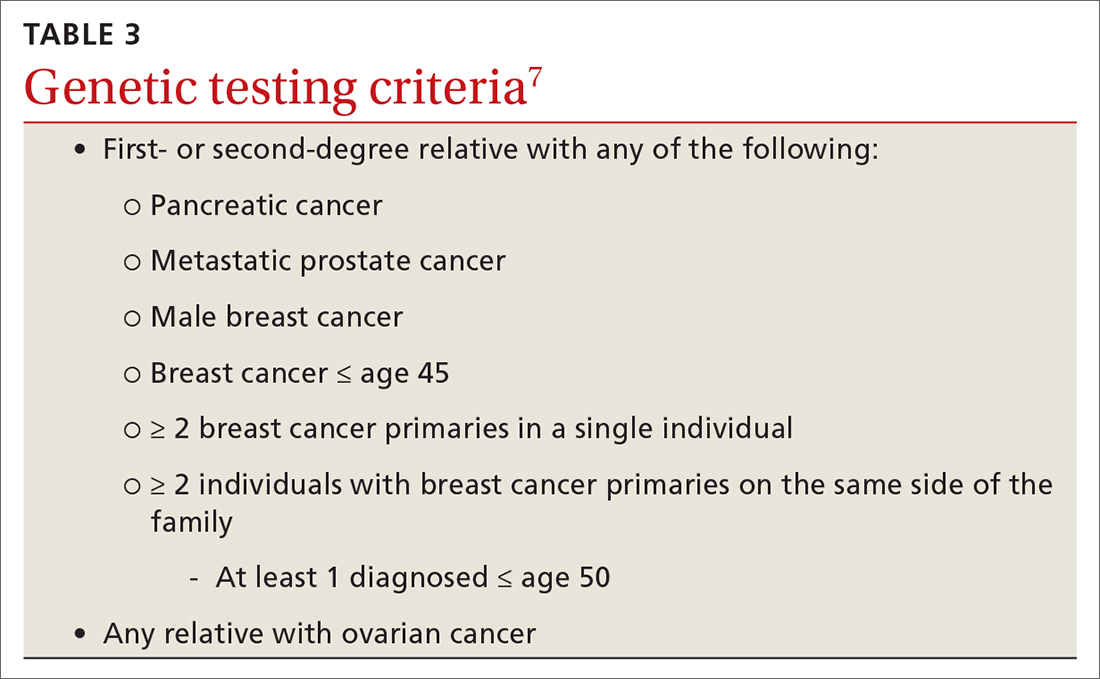
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.
Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
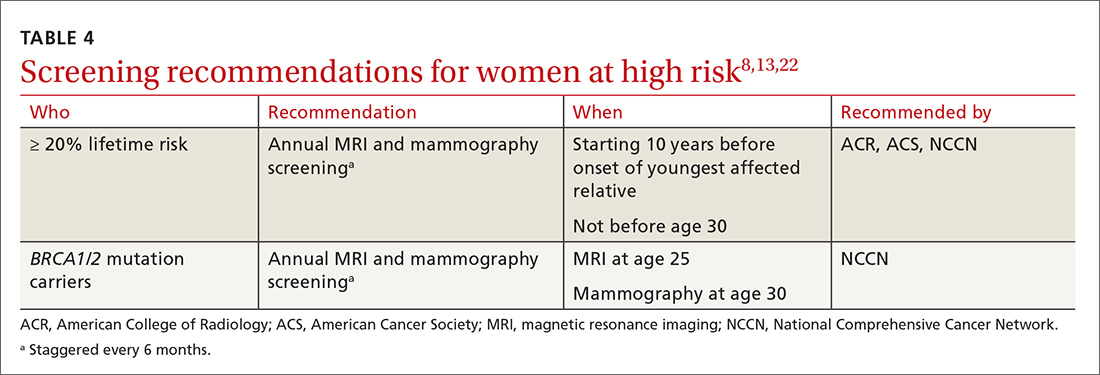
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.
Risk-reduction strategies
Chemoprophylaxis
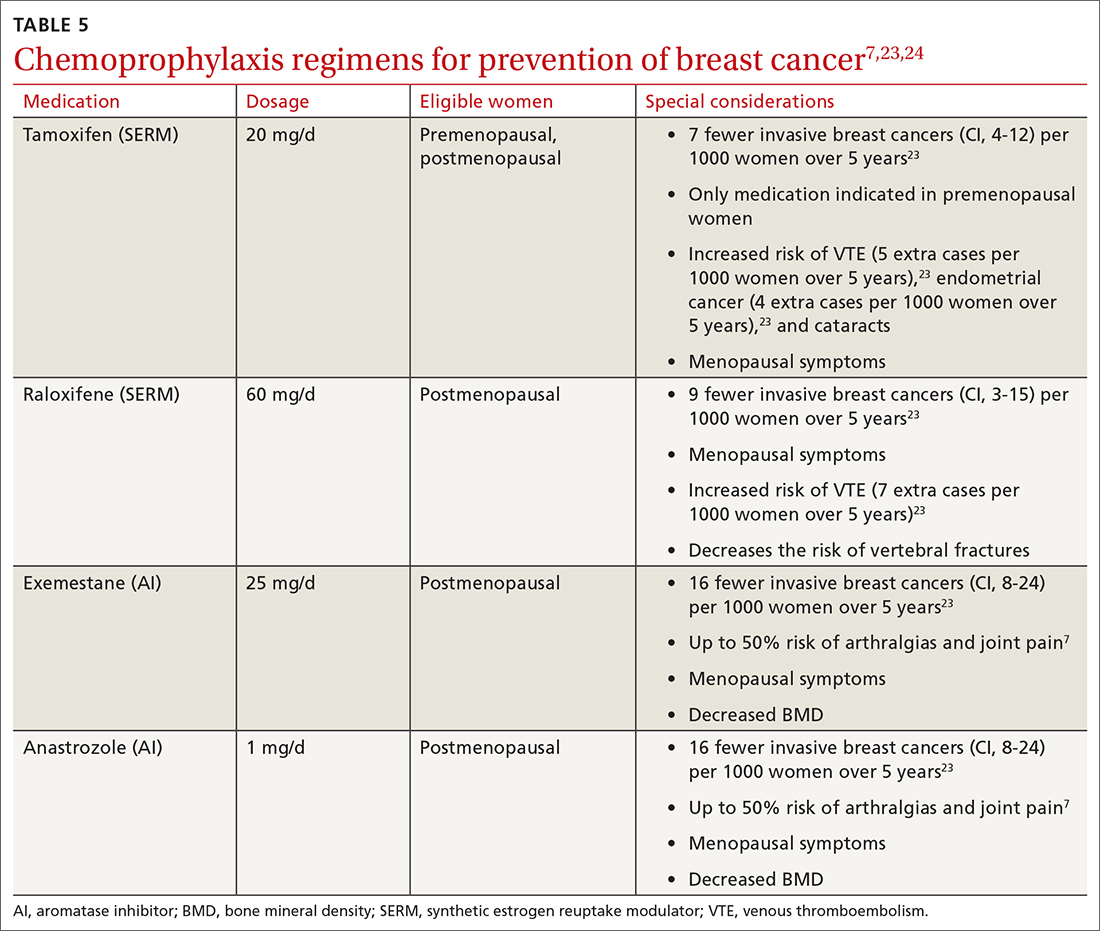
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.
Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.

CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9

In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.

Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.

Risk-reduction strategies
Chemoprophylaxis
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.

Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
Breast cancer is the most common invasive cancer in women in the United States; it is estimated that there will be 287,850 new cases of breast cancer in the United States during 2022 with 43,250 deaths.1 Lives are extended and saved every day because of a robust arsenal of treatments and interventions available to those who have been given a diagnosis of breast cancer. And, of course, lives are also extended and saved when we identify women at risk and provide early interventions. But in busy offices where time is short and there are competing demands on our time, proper assessment of a woman’s risk of breast cancer does not always happen. As a result, women with a higher risk of breast cancer may not be getting appropriate management.2,3
Familiarizing yourself with several risk-assessment tools and knowing when genetic testing is needed can make a big difference. Knowing the timing of mammograms and magnetic resonance imaging (MRI) for women deemed to be at high risk is also key. The following review employs a case-based approach (with an accompanying ALGORITHM) to illustrate how best to identify women who are at heightened risk of breast cancer and maximize their care. We also discuss the chemoprophylaxis regimens that may be used for those at increased risk.

CASE
Rachel P, age 37, presents to establish care. She has an Ashkenazi Jewish background and wonders if she should start doing breast cancer screening before age 40. She has 2 children, ages 4 years and 2 years. Her maternal aunt had unilateral breast cancer at age 54, and her maternal grandmother died of ovarian cancer at age 65.
Risk assessment
The risk assessment process (see ALGORITHM) must start with either the clinician or the patient initiating the discussion about breast cancer risk. The clinician may initiate the discussion with a new patient or at an annual physical examination. The patient may start the discussion because they are experiencing new breast symptoms, have anxiety about developing breast cancer, or have a family member with a new cancer diagnosis.
Risk factors. There are single factors that convey enough risk to automatically designate the patient as high risk (see TABLE 14-9). These factors include having a history of chest radiation between the ages of 10 and 30, a history of breast biopsy with either lobular carcinoma in situ (LCIS) or atypical ductal hyperplasia (ADH), past breast and/or ovarian cancer, and either a family or personal history of a high penetrant genetic variant for breast cancer.4-9

In women with previous chest radiation, breast cancer risk correlates with the total dose of radiation.5 For women with a personal history of breast cancer, the younger the age at diagnosis, the higher the risk of contralateral breast cancer.5 Precancerous changes such as ADH, LCIS, and ductal carcinoma in situ (DCIS) also confer moderate increases in risk. Women with these diagnoses will commonly have follow-up with specialists.
Risk assessment tools. There are several models available to assess a woman’s breast cancer risk (see TABLE 210-12). The Gail model (https://bcrisktool.cancer.gov/) is the oldest, quickest, and most widely known. However, the Gail model only accounts for first-degree relatives diagnosed with breast cancer, may underpredict risk in women with a more extensive family history, and has not been studied in women younger than 35. The International Breast Cancer Intervention Study (IBIS) Risk Evaluation Tool (https://ibis-risk-calculator.magview.com/), commonly referred to as the Tyrer-Cuzick model, incorporates second-degree relatives into the prediction model—although women may not know their full family history. Both the IBIS and the Breast Cancer Surveillance Consortium (BCSC) model (https://tools.bcsc-scc.org/BC5yearRisk/intro.htm) include breast density in the prediction algorithm. The choice of tool depends on clinician comfort and individual patient risk factors. There is no evidence that one model is better than another.10-12

Continue to: CASE
CASE
Ms. P’s clinician starts with an assessment using the Gail model. However, when the result comes back with average risk, the clinician decides to follow up with the Tyrer-Cuzick model in order to incorporate Ms. P’s multiple second-degree relatives with breast and ovarian cancer. (The BCSC model was not used because it only includes first-degree relatives.)
Genetic testing
The National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing if a woman has a first- or second-degree relative with pancreatic cancer, metastatic prostate cancer, male breast cancer, breast cancer at age 45 or younger, 2 or more breast cancers in a single person, 2 or more people on the same side of the family with at least 1 diagnosed at age 50 or younger, or any relative with ovarian cancer (see TABLE 3).7 Before ordering genetic testing, it is useful to refer the patient to a genetic counselor for a thorough discussion of options.

Results of genetic testing may include high-risk variants, moderate-risk variants, and variants of unknown significance (VUS), or be negative for any variants. High-risk variants for breast cancer include BRCA1, BRCA2, PALB2, and cancer syndrome variants such as TP53, PTEN, STK11, and CDH1.5,6,9,13-15 These high-risk variants confer sufficient risk that women with these mutations are automatically categorized in the high-risk group. It is estimated that high-risk variants account for only 25% of the genetic risk for breast cancer.16
BRCA1/2 and PTEN mutations confer greater than 80% lifetime risk, while other high-risk variants such as TP53, CDH1, and STK11 confer risks between 25% and 40%. These variants are also associated with cancers of other organs, depending on the mutation.17
Moderate-risk variants—ATM and CHEK2—do not confer sufficient risk to elevate women into the high-risk group. However, they do qualify these intermediate-risk women to participate in a specialized management strategy.5,9,13,18
VUS are those for which the associated risk is unclear, but more research may be done to categorize the risk.9 The clinical management of women with VUS usually entails close monitoring.
In an effort to better characterize breast cancer risk using a combination of pathogenic variants found in broad multi-gene cancer predisposition panels, researchers have developed a method to combine risks in a “polygenic risk score” (PRS) that can be used to counsel women (see “What is a polygenic risk score for breast cancer?” on page 203).19-21PRS predicts an additional 18% of genetic risk in women of European descent.21
SIDEBAR
What is a polygenic risk score for breast cancer?
- A polygenic risk score (PRS) is a mathematical method to combine results from a variety of different single nucleotide polymorphisms (SNPs; ie, single base pair variants) into a prediction tool that can estimate a woman’s lifetime risk of breast cancer.
- A PRS may be most accurate in determining risk for women with intermediate pathogenic variants, such as ATM and CHEK2. 19,20
- PRS has not been studied in non-White women.21
Continue to: CASE
CASE
Using the assessment results, the clinician talks to Ms. P about her lifetime risk for breast cancer. The Gail model indicates her lifetime risk is 13.3%, just slightly higher than the average (12.5%), and her 5-year risk is 0.5% (average, 0.4%). The IBIS or Tyrer-Cuzick model, which takes into account her second-degree relatives with breast and ovarian cancer and her Ashkenazi ethnicity (which confers increased risk due to elevated risk of BRCA mutations), predicts her lifetime risk of breast cancer to be 20.4%. This categorizes Ms. P as high risk.
Enhanced screening recommendations for women at high risk
TABLE 48,13,22 summarizes screening recommendations for women deemed to be at high risk for breast cancer. The American Cancer Society (ACS), NCCN, and the American College of Radiology (ACR) recommend that women with at least a 20% lifetime risk have yearly magnetic resonance imaging (MRI) and mammography (staggered so that the patient has 1 test every 6 months) starting 10 years before the age of onset for the youngest affected relative but not before age 30.8 For carriers of high-risk (as well as intermediate-risk) genes, NCCN recommends annual MRI screening starting at age 40.13BRCA1/2 screening includes annual MRI starting at age 25 and annual mammography every 6 months starting at age 30.22 Clinicians should counsel women with moderate risk factors (elevated breast density; personal history of ADH, LCIS, or DCIS) about the potential risks and benefits of enhanced screening and chemoprophylaxis.

Risk-reduction strategies
Chemoprophylaxis
The US Preventive Services Task Force (USPSTF) recommends that all women at increased risk for breast cancer consider chemoprophylaxis (B recommendation)23 based on convincing evidence that 5 years of treatment with either a synthetic estrogen reuptake modulator (SERM) or an aromatase inhibitor (AI) decreases the incidence of estrogen receptor positive breast cancers. (See TABLE 57,23,24 for absolute risk reduction.) There is no benefit for chemoprophylaxis in women at average risk (D recommendation).23 It is unclear whether chemoprophylaxis is indicated in women with moderate increased risk (ie, who do not meet the 20% lifetime risk criteria). Chemoprophylaxis may not be effective in women with BRCA1 mutations, as they often develop triple-negative breast cancers.

Accurate risk assessment and shared decision-making enable the clinician and patient to discuss the potential risks and benefits of chemoprophylaxis.7,24 The USPSTF did not find that any 1 risk prediction tool was better than another to identify women who should be counseled about chemoprophylaxis. Clinicians should counsel all women taking AIs about optimizing bone health with adequate calcium and vitamin D intake and routine bone density tests.
Surgical risk reduction
The NCCN guidelines state that risk-reducing bilateral mastectomy is reserved for individuals with high-risk gene variants and individuals with prior chest radiation between ages 10 and 30.25 NCCN also recommends discussing risk-reducing mastectomy with all women with BRCA mutations.22
Bilateral mastectomy is the most effective method to reduce breast cancer risk and should be discussed after age 25 in women with BRCA mutations and at least 8 years after chest radiation is completed.26 There is a reduction in breast cancer incidence of 90%.25 Breast imaging for screening (mammography or MRI) is not indicated after risk-reducing mastectomy. However, clinical breast examinations of the surgical site are important, because there is a small risk of developing breast cancer in that area.26
Risk-reducing oophorectomy is the standard of care for women with BRCA mutations to reduce the risk of ovarian cancer. It can also reduce the risk of breast cancer in women with BRCA mutations.27
Continue to: CASE
CASE
Based on her risk assessment results, family history, and genetic heritage, Ms. P qualifies for referral to a genetic counselor for discussion of BRCA testing. The clinician discusses adding annual MRI to Ms. P’s breast cancer screening regimen, based on ACS, NCCN, and ACR recommendations, due to her 20.4% lifetime risk. Discussion of whether and when to start chemoprophylaxis is typically based on breast cancer risk, projected benefit, and the potential impact of medication adverse effects. A high-risk woman is eligible for 5 years of chemoprophylaxis (tamoxifen if premenopausal) based on her lifetime risk. The clinician discusses timing with Ms. P, and even though she is finished with childbearing, she would like to wait until she is age 45, which is before the age at which her aunt was given a diagnosis of breast cancer.
Conclusion
Primary care clinicians are well positioned to identify women with an elevated risk of breast cancer and refer them for enhanced screening and chemoprophylaxis (see ALGORITHM). Shared decision-making with the inclusion of patient decision aids (https://decisionaid.ohri.ca/AZsearch.php?criteria=breast+cancer) about genetic testing, chemoprophylaxis, and prophylactic mastectomy or oophorectomy may help women at intermediate or high risk of breast cancer feel empowered to make decisions about their breast—and overall—health.
CORRESPONDENCE
Sarina Schrager, MD, MS, Professor, Department of Family Medicine and Community Health, University of Wisconsin, 1100 Delaplaine Court, Madison, WI 53715; [email protected]
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
1. National Cancer Institute. Cancer stat facts: female breast cancer. Accessed May 13, 2022. https://seer.cancer.gov/statfacts/html/breast.html
2. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279. doi:10.3122/jabfm.2009.03.080153
3. Hamilton JG, Abdiwahab E, Edwards HM, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315-324. doi:10.1007/s11606-016-3943-4
4. Eden KB, Ivlev I, Bensching KL, et al. Use of an online breast cancer risk assessment and patient decision aid in primary care practices. J Womens Health (Larchmt). 2020;29:763-769. doi: 10.1089/jwh.2019.8143
5. Kleibl Z, Kristensen VN. Women at high risk of breast cancer: molecular characteristics, clinical presentation and management. Breast. 2016;28:136-44. doi: 10.1016/j.breast.2016.05.006
6. Sciaraffa T, Guido B, Khan SA, et al. Breast cancer risk assessment and management programs: a practical guide. Breast J. 2020;26:1556-1564. doi: 10.1111/tbj.13967
7. Farkas A, Vanderberg R, Merriam S, et al. Breast cancer chemoprevention: a practical guide for the primary care provider. J Womens Health (Larchmt). 2020;29:46-56. doi: 10.1089/jwh.2018.7643
8. McClintock AH, Golob AL, Laya MB. Breast cancer risk assessment: a step-wise approach for primary care providers on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275. doi: 10.1016/j.mayocp.2020.04.017
9. Catana A, Apostu AP, Antemie RG. Multi gene panel testing for hereditary breast cancer - is it ready to be used? Med Pharm Rep. 2019;92:220-225. doi: 10.15386/mpr-1083
10. Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin North Am. 2017;55:457-474. doi: 10.1016/j.rcl.2016.12.013
11. Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: a review of risk assessment models. J Natl Cancer Inst. 2010;102:680-91. doi: 10.1093/jnci/djq088
12. Kim G, Bahl M. Assessing risk of breast cancer: a review of risk prediction models. J Breast Imaging. 2021;3:144-155. doi: 10.1093/jbi/wbab001
13. Narod SA. Which genes for hereditary breast cancer? N Engl J Med. 2021;384:471-473. doi: 10.1056/NEJMe2035083
14. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190-1196. doi: 10.1001/jamaoncol.2017.0424
15. Obeid EI, Hall MJ, Daly MB. Multigene panel testing and breast cancer risk: is it time to scale down? JAMA Oncol. 2017;3:1176-1177. doi: 10.1001/jamaoncol.2017.0342
16. Michailidou K, Lindström S, Dennis J, et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92-94. doi: 10.1038/nature24284
17. Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol. 2015;26:1291-1299. doi: 10.1093/annonc/mdv022
18. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384:440-451. doi: 10.1056/NEJMoa2005936
19. Gao C, Polley EC, Hart SN, et al. Risk of breast cancer among carriers of pathogenic variants in breast cancer predisposition genes varies by polygenic risk score. J Clin Oncol. 2021;39:2564-2573. doi: 10.1200/JCO.20.01992
20. Gallagher S, Hughes E, Wagner S, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3:e208501. doi: 10.1001/jamanetworkopen.2020.8501
21. Yanes T, Young MA, Meiser B, et al. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21. doi: 10.1186/s13058-020-01260-3
22. Schrager S, Torell E, Ledford K, et al. Managing a woman with BRCA mutations? Shared decision-making is key. J Fam Pract. 2020;69:237-243
23. US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867. doi: 10.1001/jama.2019.11885
24. Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol 2015;22:3230-3235. doi: 10.1245/s10434-015-4715-9
25. Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417-436. doi: 10.1038/s41568-020-0266-x
26. Jatoi I, Kemp Z. Risk-reducing mastectomy. JAMA. 2021;325:1781-1782. doi: 10.1001/jama.2020.22414
27. Choi Y, Terry MB, Daly MB, et al. Association of risk-reducing salpingo-oophorectomy with breast cancer risk in women with BRCA1 and BRCA2 pathogenic variants. JAMA Oncol. 2021;7:585-592. doi:10.1001/jamaoncol.2020.7995
PRACTICE RECOMMENDATIONS
› Assess breast cancer risk in all women starting at age 35. C
› Perform enhanced screening in all women with a lifetime risk of breast cancer > 20%. A
› Discuss chemoprevention for all women at elevated risk for breast cancer. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Double morning-after pill dose for women with obesity not effective
Emergency contraception is more likely to fail in women with obesity, but simply doubling the dose of levonorgestrel (LNG)-based contraception does not appear to be effective according to the results of a randomized, controlled trial.
Alison B. Edelman, MD, MPH, of the department of obstetrics & gynecology at Oregon Health & Science University, Portland, led the study published online in Obstetrics & Gynecology.
The researchers included healthy women ages 18-35 with regular menstrual cycles, body mass index (BMI) higher than 30 kg/m2, and weight at least 176 pounds in a randomized study.
After confirming ovulation, researchers monitored participants with transvaginal ultrasonography and blood sampling for progesterone, luteinizing hormone, and estradiol every other day until a dominant follicle 15 mm or greater was seen.
At that point the women received either LNG 1.5 mg or 3 mg and returned for daily monitoring up to 7 days.
Emergency contraception with LNG works by preventing the luteinizing hormone surge, blocking follicle rupture. The researchers had hypothesized that women with obesity might not be getting enough LNG to block the surge after oral dosing.
Previous trials had shown women with obesity had a fourfold higher risk of pregnancy, compared with women with normal BMI taking emergency contraception.
The primary outcome in this trial was whether women had follicle rupture 5 days after dosing.
The authors wrote: “The study had 80% power to detect a 30% difference in the proportion of cycles with at least a 5-day delay in follicle rupture (50% decrease).”
A total of 70 women completed study procedures. The two groups (35 women in each) had similar demographics (mean age, 28 years; BMI, 38).
No differences found between groups
“We found no difference between groups in the proportion of participants without follicle rupture,” the researchers wrote.
More than 5 days after dosing, 51.4% in the lower-dose group did not experience follicle rupture. In the double-dose group 68.6% did not experience rupture but the difference was not significant (P = .14).
Among participants with follicle rupture before 5 days, the time to rupture – the secondary endpoint – also did not differ between groups.
The researchers concluded that more research on the failures of hormonal emergency contraception in women with obesity is needed.
Eve Espey, MD, MPH, distinguished professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said in an interview that the study was well designed and the results “form a strong basis for clinical recommendations.”
“Providers should not recommend a higher dose of LNG emergency contraception for patients who are overweight or obese, but rather should counsel patients on the superior effectiveness of ulipristal acetate for those seeking oral emergency contraception as well as the longer time period after unprotected sex – 5 days – that ulipristal maintains its effectiveness.”
“Providers should also counsel patients on the most effective emergency contraception methods, the copper or LNG intrauterine device,” she said.
She said the unique study design of a pharmacodynamic randomized controlled trial adds weight to the findings.
She and the authors noted a limitation is the use of a surrogate outcome, ovulation delay, for ethical and feasibility reasons, instead of the outcome of interest, pregnancy.
The trial was conducted at Oregon Health & Science University and Eastern Virginia Medical School, Norfolk, from June 2017 to February 2021.
Study enrollees were compensated for their time. They were required not to be at risk for pregnancy (abstinent or using a nonhormonal method of contraception).
Dr. Edelman reported receiving honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from UpToDate. Several coauthors have received payments for consulting from multiple pharmaceutical companies. These companies and organizations may have a commercial or financial interest in the results of this research and technology. Another was involved in this study as a private consultant and is employed by Gilead Sciences, which was not involved in this research.
Emergency contraception is more likely to fail in women with obesity, but simply doubling the dose of levonorgestrel (LNG)-based contraception does not appear to be effective according to the results of a randomized, controlled trial.
Alison B. Edelman, MD, MPH, of the department of obstetrics & gynecology at Oregon Health & Science University, Portland, led the study published online in Obstetrics & Gynecology.
The researchers included healthy women ages 18-35 with regular menstrual cycles, body mass index (BMI) higher than 30 kg/m2, and weight at least 176 pounds in a randomized study.
After confirming ovulation, researchers monitored participants with transvaginal ultrasonography and blood sampling for progesterone, luteinizing hormone, and estradiol every other day until a dominant follicle 15 mm or greater was seen.
At that point the women received either LNG 1.5 mg or 3 mg and returned for daily monitoring up to 7 days.
Emergency contraception with LNG works by preventing the luteinizing hormone surge, blocking follicle rupture. The researchers had hypothesized that women with obesity might not be getting enough LNG to block the surge after oral dosing.
Previous trials had shown women with obesity had a fourfold higher risk of pregnancy, compared with women with normal BMI taking emergency contraception.
The primary outcome in this trial was whether women had follicle rupture 5 days after dosing.
The authors wrote: “The study had 80% power to detect a 30% difference in the proportion of cycles with at least a 5-day delay in follicle rupture (50% decrease).”
A total of 70 women completed study procedures. The two groups (35 women in each) had similar demographics (mean age, 28 years; BMI, 38).
No differences found between groups
“We found no difference between groups in the proportion of participants without follicle rupture,” the researchers wrote.
More than 5 days after dosing, 51.4% in the lower-dose group did not experience follicle rupture. In the double-dose group 68.6% did not experience rupture but the difference was not significant (P = .14).
Among participants with follicle rupture before 5 days, the time to rupture – the secondary endpoint – also did not differ between groups.
The researchers concluded that more research on the failures of hormonal emergency contraception in women with obesity is needed.
Eve Espey, MD, MPH, distinguished professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said in an interview that the study was well designed and the results “form a strong basis for clinical recommendations.”
“Providers should not recommend a higher dose of LNG emergency contraception for patients who are overweight or obese, but rather should counsel patients on the superior effectiveness of ulipristal acetate for those seeking oral emergency contraception as well as the longer time period after unprotected sex – 5 days – that ulipristal maintains its effectiveness.”
“Providers should also counsel patients on the most effective emergency contraception methods, the copper or LNG intrauterine device,” she said.
She said the unique study design of a pharmacodynamic randomized controlled trial adds weight to the findings.
She and the authors noted a limitation is the use of a surrogate outcome, ovulation delay, for ethical and feasibility reasons, instead of the outcome of interest, pregnancy.
The trial was conducted at Oregon Health & Science University and Eastern Virginia Medical School, Norfolk, from June 2017 to February 2021.
Study enrollees were compensated for their time. They were required not to be at risk for pregnancy (abstinent or using a nonhormonal method of contraception).
Dr. Edelman reported receiving honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from UpToDate. Several coauthors have received payments for consulting from multiple pharmaceutical companies. These companies and organizations may have a commercial or financial interest in the results of this research and technology. Another was involved in this study as a private consultant and is employed by Gilead Sciences, which was not involved in this research.
Emergency contraception is more likely to fail in women with obesity, but simply doubling the dose of levonorgestrel (LNG)-based contraception does not appear to be effective according to the results of a randomized, controlled trial.
Alison B. Edelman, MD, MPH, of the department of obstetrics & gynecology at Oregon Health & Science University, Portland, led the study published online in Obstetrics & Gynecology.
The researchers included healthy women ages 18-35 with regular menstrual cycles, body mass index (BMI) higher than 30 kg/m2, and weight at least 176 pounds in a randomized study.
After confirming ovulation, researchers monitored participants with transvaginal ultrasonography and blood sampling for progesterone, luteinizing hormone, and estradiol every other day until a dominant follicle 15 mm or greater was seen.
At that point the women received either LNG 1.5 mg or 3 mg and returned for daily monitoring up to 7 days.
Emergency contraception with LNG works by preventing the luteinizing hormone surge, blocking follicle rupture. The researchers had hypothesized that women with obesity might not be getting enough LNG to block the surge after oral dosing.
Previous trials had shown women with obesity had a fourfold higher risk of pregnancy, compared with women with normal BMI taking emergency contraception.
The primary outcome in this trial was whether women had follicle rupture 5 days after dosing.
The authors wrote: “The study had 80% power to detect a 30% difference in the proportion of cycles with at least a 5-day delay in follicle rupture (50% decrease).”
A total of 70 women completed study procedures. The two groups (35 women in each) had similar demographics (mean age, 28 years; BMI, 38).
No differences found between groups
“We found no difference between groups in the proportion of participants without follicle rupture,” the researchers wrote.
More than 5 days after dosing, 51.4% in the lower-dose group did not experience follicle rupture. In the double-dose group 68.6% did not experience rupture but the difference was not significant (P = .14).
Among participants with follicle rupture before 5 days, the time to rupture – the secondary endpoint – also did not differ between groups.
The researchers concluded that more research on the failures of hormonal emergency contraception in women with obesity is needed.
Eve Espey, MD, MPH, distinguished professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said in an interview that the study was well designed and the results “form a strong basis for clinical recommendations.”
“Providers should not recommend a higher dose of LNG emergency contraception for patients who are overweight or obese, but rather should counsel patients on the superior effectiveness of ulipristal acetate for those seeking oral emergency contraception as well as the longer time period after unprotected sex – 5 days – that ulipristal maintains its effectiveness.”
“Providers should also counsel patients on the most effective emergency contraception methods, the copper or LNG intrauterine device,” she said.
She said the unique study design of a pharmacodynamic randomized controlled trial adds weight to the findings.
She and the authors noted a limitation is the use of a surrogate outcome, ovulation delay, for ethical and feasibility reasons, instead of the outcome of interest, pregnancy.
The trial was conducted at Oregon Health & Science University and Eastern Virginia Medical School, Norfolk, from June 2017 to February 2021.
Study enrollees were compensated for their time. They were required not to be at risk for pregnancy (abstinent or using a nonhormonal method of contraception).
Dr. Edelman reported receiving honoraria and travel reimbursement from the American College of Obstetricians and Gynecologists, the World Health Organization, and Gynuity for committee activities and honoraria for peer review from the Karolinska Institute. She receives royalties from UpToDate. Several coauthors have received payments for consulting from multiple pharmaceutical companies. These companies and organizations may have a commercial or financial interest in the results of this research and technology. Another was involved in this study as a private consultant and is employed by Gilead Sciences, which was not involved in this research.
FROM OBSTETRICS & GYNECOLOGY
PCOS comes with high morbidity, medication use into late 40s
Women with polycystic ovary syndrome (PCOS) have an increased risk for several diseases and symptoms, many independent of body mass index (BMI), new research indicates.
Some diseases are linked for the first time to PCOS in this study, the authors wrote.
Researchers, led by Linda Kujanpää, MD, of the research unit for pediatrics, dermatology, clinical genetics, obstetrics, and gynecology at University of Oulu (Finland), found the morbidity risk is evident through the late reproductive years.
The paper was published online in Acta Obstetricia et Gynecologica Scandinavica.
This population-based follow-up study investigated comorbidities and medication and health care services use among women with PCOS in Finland at age 46 years via answers to a questionnaire.
The whole PCOS population (n = 280) consisted of women who reported both hirsutism and oligo/amenorrhea at age 31 (4.1%) and/or polycystic ovary morphology/PCOS at age 46 (3.1%), of which 246 replied to the 46-year questionnaire. They were compared with a control group of 1,573 women without PCOS.
Overall morbidity risk was 35% higher than for women without PCOS (risk ratio, 1.35; 95% confidence interval, 1.16-1.57). Medication use was 27% higher (RR, 1.27; 95% CI, 1.08-1.50), and the risk remained after adjusting for BMI.
Diagnoses with increased prevalence in women with PCOS were osteoarthritis, migraine, hypertension, tendinitis, and endometriosis. PCOS was also associated with autoimmune diseases and recurrent upper respiratory tract infections.
“BMI seems not to be solely responsible for the increased morbidity,” the researchers found. The average morbidity score of women with PCOS with a BMI of 25 kg/m2 or higher was similar to that of women with PCOS and lower BMI.
Mindy Christianson, MD, medical director at Johns Hopkins Fertility Center and associate professor of gynecology and obstetrics at Johns Hopkins University, both in Baltimore, said in an interview that the links to diseases independent of BMI are interesting because there’s so much focus on counseling women with PCOS to lose weight.
While that message is still important, it’s important to realize that some related diseases and conditions – such as autoimmune diseases and migraine – are not driven by BMI.
“It really drives home the point that polycystic ovary syndrome is really a chronic medical condition and puts patients at risk for a number of health conditions,” she said. “Having a good primary care physician is important to help them with their overall health.”
Women with PCOS said their health was poor or very poor almost three times more often than did women in the control group.
Surprisingly few studies have looked at overall comorbidity in women with PCOS, the authors wrote.
“This should be of high priority given the high cost to society resulting from PCOS-related morbidity,” they added. As an example, they pointed out that PCOS-related type 2 diabetes alone costs an estimated $1.77 billion in the United States and £237 million ($310 million) each year in the United Kingdom.
Additionally, the focus in previous research has typically been on women in their early or mid-reproductive years, and morbidity burden data in late reproductive years are scarce.
The study population was pulled from the longitudinal Northern Finland Birth Cohort 1966 and included all pregnancies with estimated date of delivery during 1966 in two provinces of Finland (5,889 women).
Dr. Christianson said she hopes this study will spur more research on PCOS, which has been severely underfunded, especially in the United States.
Part of the reason for that is there is a limited number of subspecialists in the country who work with patients with PCOS and do research in the area. PCOS often gets lost in the research priorities of infertility, diabetes, and thyroid disease.
The message in this study that PCOS is not just a fertility issue or an obesity issue but an overall health issue with a substantial cost to the health system may help raise awareness, Dr. Christianson said.
This study was supported by grants from The Finnish Medical Foundation, The Academy of Finland, The Sigrid Juselius Foundation, The Finnish Cultural Foundation, The Jalmari and Rauha Ahokas Foundation, The Päivikki and Sakari Sohlberg Foundation, Genesis Research Trust, The Medical Research Council, University of Oulu, Oulu University Hospital, Ministry of Health and Social Affairs, National Institute for Health and Welfare, Regional Institute of Occupational Health, and the European Regional Development Fund. The Study authors and Dr. Christianson reported no relevant financial relationships.
Women with polycystic ovary syndrome (PCOS) have an increased risk for several diseases and symptoms, many independent of body mass index (BMI), new research indicates.
Some diseases are linked for the first time to PCOS in this study, the authors wrote.
Researchers, led by Linda Kujanpää, MD, of the research unit for pediatrics, dermatology, clinical genetics, obstetrics, and gynecology at University of Oulu (Finland), found the morbidity risk is evident through the late reproductive years.
The paper was published online in Acta Obstetricia et Gynecologica Scandinavica.
This population-based follow-up study investigated comorbidities and medication and health care services use among women with PCOS in Finland at age 46 years via answers to a questionnaire.
The whole PCOS population (n = 280) consisted of women who reported both hirsutism and oligo/amenorrhea at age 31 (4.1%) and/or polycystic ovary morphology/PCOS at age 46 (3.1%), of which 246 replied to the 46-year questionnaire. They were compared with a control group of 1,573 women without PCOS.
Overall morbidity risk was 35% higher than for women without PCOS (risk ratio, 1.35; 95% confidence interval, 1.16-1.57). Medication use was 27% higher (RR, 1.27; 95% CI, 1.08-1.50), and the risk remained after adjusting for BMI.
Diagnoses with increased prevalence in women with PCOS were osteoarthritis, migraine, hypertension, tendinitis, and endometriosis. PCOS was also associated with autoimmune diseases and recurrent upper respiratory tract infections.
“BMI seems not to be solely responsible for the increased morbidity,” the researchers found. The average morbidity score of women with PCOS with a BMI of 25 kg/m2 or higher was similar to that of women with PCOS and lower BMI.
Mindy Christianson, MD, medical director at Johns Hopkins Fertility Center and associate professor of gynecology and obstetrics at Johns Hopkins University, both in Baltimore, said in an interview that the links to diseases independent of BMI are interesting because there’s so much focus on counseling women with PCOS to lose weight.
While that message is still important, it’s important to realize that some related diseases and conditions – such as autoimmune diseases and migraine – are not driven by BMI.
“It really drives home the point that polycystic ovary syndrome is really a chronic medical condition and puts patients at risk for a number of health conditions,” she said. “Having a good primary care physician is important to help them with their overall health.”
Women with PCOS said their health was poor or very poor almost three times more often than did women in the control group.
Surprisingly few studies have looked at overall comorbidity in women with PCOS, the authors wrote.
“This should be of high priority given the high cost to society resulting from PCOS-related morbidity,” they added. As an example, they pointed out that PCOS-related type 2 diabetes alone costs an estimated $1.77 billion in the United States and £237 million ($310 million) each year in the United Kingdom.
Additionally, the focus in previous research has typically been on women in their early or mid-reproductive years, and morbidity burden data in late reproductive years are scarce.
The study population was pulled from the longitudinal Northern Finland Birth Cohort 1966 and included all pregnancies with estimated date of delivery during 1966 in two provinces of Finland (5,889 women).
Dr. Christianson said she hopes this study will spur more research on PCOS, which has been severely underfunded, especially in the United States.
Part of the reason for that is there is a limited number of subspecialists in the country who work with patients with PCOS and do research in the area. PCOS often gets lost in the research priorities of infertility, diabetes, and thyroid disease.
The message in this study that PCOS is not just a fertility issue or an obesity issue but an overall health issue with a substantial cost to the health system may help raise awareness, Dr. Christianson said.
This study was supported by grants from The Finnish Medical Foundation, The Academy of Finland, The Sigrid Juselius Foundation, The Finnish Cultural Foundation, The Jalmari and Rauha Ahokas Foundation, The Päivikki and Sakari Sohlberg Foundation, Genesis Research Trust, The Medical Research Council, University of Oulu, Oulu University Hospital, Ministry of Health and Social Affairs, National Institute for Health and Welfare, Regional Institute of Occupational Health, and the European Regional Development Fund. The Study authors and Dr. Christianson reported no relevant financial relationships.
Women with polycystic ovary syndrome (PCOS) have an increased risk for several diseases and symptoms, many independent of body mass index (BMI), new research indicates.
Some diseases are linked for the first time to PCOS in this study, the authors wrote.
Researchers, led by Linda Kujanpää, MD, of the research unit for pediatrics, dermatology, clinical genetics, obstetrics, and gynecology at University of Oulu (Finland), found the morbidity risk is evident through the late reproductive years.
The paper was published online in Acta Obstetricia et Gynecologica Scandinavica.
This population-based follow-up study investigated comorbidities and medication and health care services use among women with PCOS in Finland at age 46 years via answers to a questionnaire.
The whole PCOS population (n = 280) consisted of women who reported both hirsutism and oligo/amenorrhea at age 31 (4.1%) and/or polycystic ovary morphology/PCOS at age 46 (3.1%), of which 246 replied to the 46-year questionnaire. They were compared with a control group of 1,573 women without PCOS.
Overall morbidity risk was 35% higher than for women without PCOS (risk ratio, 1.35; 95% confidence interval, 1.16-1.57). Medication use was 27% higher (RR, 1.27; 95% CI, 1.08-1.50), and the risk remained after adjusting for BMI.
Diagnoses with increased prevalence in women with PCOS were osteoarthritis, migraine, hypertension, tendinitis, and endometriosis. PCOS was also associated with autoimmune diseases and recurrent upper respiratory tract infections.
“BMI seems not to be solely responsible for the increased morbidity,” the researchers found. The average morbidity score of women with PCOS with a BMI of 25 kg/m2 or higher was similar to that of women with PCOS and lower BMI.
Mindy Christianson, MD, medical director at Johns Hopkins Fertility Center and associate professor of gynecology and obstetrics at Johns Hopkins University, both in Baltimore, said in an interview that the links to diseases independent of BMI are interesting because there’s so much focus on counseling women with PCOS to lose weight.
While that message is still important, it’s important to realize that some related diseases and conditions – such as autoimmune diseases and migraine – are not driven by BMI.
“It really drives home the point that polycystic ovary syndrome is really a chronic medical condition and puts patients at risk for a number of health conditions,” she said. “Having a good primary care physician is important to help them with their overall health.”
Women with PCOS said their health was poor or very poor almost three times more often than did women in the control group.
Surprisingly few studies have looked at overall comorbidity in women with PCOS, the authors wrote.
“This should be of high priority given the high cost to society resulting from PCOS-related morbidity,” they added. As an example, they pointed out that PCOS-related type 2 diabetes alone costs an estimated $1.77 billion in the United States and £237 million ($310 million) each year in the United Kingdom.
Additionally, the focus in previous research has typically been on women in their early or mid-reproductive years, and morbidity burden data in late reproductive years are scarce.
The study population was pulled from the longitudinal Northern Finland Birth Cohort 1966 and included all pregnancies with estimated date of delivery during 1966 in two provinces of Finland (5,889 women).
Dr. Christianson said she hopes this study will spur more research on PCOS, which has been severely underfunded, especially in the United States.
Part of the reason for that is there is a limited number of subspecialists in the country who work with patients with PCOS and do research in the area. PCOS often gets lost in the research priorities of infertility, diabetes, and thyroid disease.
The message in this study that PCOS is not just a fertility issue or an obesity issue but an overall health issue with a substantial cost to the health system may help raise awareness, Dr. Christianson said.
This study was supported by grants from The Finnish Medical Foundation, The Academy of Finland, The Sigrid Juselius Foundation, The Finnish Cultural Foundation, The Jalmari and Rauha Ahokas Foundation, The Päivikki and Sakari Sohlberg Foundation, Genesis Research Trust, The Medical Research Council, University of Oulu, Oulu University Hospital, Ministry of Health and Social Affairs, National Institute for Health and Welfare, Regional Institute of Occupational Health, and the European Regional Development Fund. The Study authors and Dr. Christianson reported no relevant financial relationships.
FROM ACTA OBSTETRICIA ET GYNECOLOGICA SCANDINAVICA
Pregnant women with monkeypox advised to have C-section
The risk of monkeypox infection remains low for the general public, the authors wrote, though cases continue to grow worldwide, particularly in the United Kingdom.
“We are aware infants and children are at greater risk of becoming seriously ill if they do catch monkeypox,” Edward Morris, MBBS, one of the authors and president of the Royal College of Obstetricians and Gynecologists, said in a statement.
“Therefore, to minimize the risk of a baby contracting the virus, we recommend health care professionals discuss the benefits and risks of having a cesarean birth with a pregnant woman or person who has or is suspected of having the virus,” he said.
Dr. Morris and colleagues pulled together existing evidence on monkeypox diagnosis, treatment, and recommended modes of birth for mothers and babies.
“The World Health Organization states there could be adverse consequences for pregnant women and babies if they become infected, including congenital monkeypox, miscarriage, or stillbirth, which is why we have provided clear guidance for health care professionals in this paper,” Dr. Morris said.
The monkeypox virus typically spreads through direct contact, droplets, or contaminated surfaces and objects. But some limited evidence shows that the virus can be passed from a mother to a baby via the placenta, which can lead to congenital monkeypox.
What’s more, mothers may be able to transmit the virus during or after birth. Although no evidence exists around the optimal mode of birth, a pregnant woman with an active monkeypox infection may choose to avoid vaginal delivery to reduce direct contact.
“If genital lesions are identified on a pregnant woman, then a cesarean birth will be recommended,” the authors wrote. “If a pregnant woman or person has suspected or confirmed monkeypox, a caesarean birth will be offered following discussion of the possible risk of neonatal infection, which may be serious.”
After giving birth, close contact can spread the virus as well. To minimize the risk, the authors recommend isolating the baby from family members who have confirmed or suspected monkeypox and carefully monitoring for infection.
Mothers with an active monkeypox infection should also avoid breastfeeding to lower the risk of spreading the virus to their newborn, the authors wrote. But to support breastfeeding after infection, mothers can express and discard milk until the isolation period has passed.
Pregnant women who become infected may also consider getting vaccinated, the authors wrote. Vaccination up to 14 days after exposure doesn’t prevent the disease but can reduce the severity of symptoms. In the current outbreak, public health organizations advised doctors to vaccinate contacts of confirmed cases, including pregnant people.
The data for monkeypox vaccine use in pregnant women is small, the authors wrote, including fewer than 300 women. In previous studies, no adverse outcomes were found. The vaccine is also considered safe for breastfeeding.
“The decision whether to have the vaccine in pregnancy should be a personal choice,” the authors wrote. “Pregnant women and people should be encouraged to discuss the risks and benefits of vaccination, including possible side effects, with a health care professional before making their final decision.”
A version of this article first appeared on Medscape.com.
The risk of monkeypox infection remains low for the general public, the authors wrote, though cases continue to grow worldwide, particularly in the United Kingdom.
“We are aware infants and children are at greater risk of becoming seriously ill if they do catch monkeypox,” Edward Morris, MBBS, one of the authors and president of the Royal College of Obstetricians and Gynecologists, said in a statement.
“Therefore, to minimize the risk of a baby contracting the virus, we recommend health care professionals discuss the benefits and risks of having a cesarean birth with a pregnant woman or person who has or is suspected of having the virus,” he said.
Dr. Morris and colleagues pulled together existing evidence on monkeypox diagnosis, treatment, and recommended modes of birth for mothers and babies.
“The World Health Organization states there could be adverse consequences for pregnant women and babies if they become infected, including congenital monkeypox, miscarriage, or stillbirth, which is why we have provided clear guidance for health care professionals in this paper,” Dr. Morris said.
The monkeypox virus typically spreads through direct contact, droplets, or contaminated surfaces and objects. But some limited evidence shows that the virus can be passed from a mother to a baby via the placenta, which can lead to congenital monkeypox.
What’s more, mothers may be able to transmit the virus during or after birth. Although no evidence exists around the optimal mode of birth, a pregnant woman with an active monkeypox infection may choose to avoid vaginal delivery to reduce direct contact.
“If genital lesions are identified on a pregnant woman, then a cesarean birth will be recommended,” the authors wrote. “If a pregnant woman or person has suspected or confirmed monkeypox, a caesarean birth will be offered following discussion of the possible risk of neonatal infection, which may be serious.”
After giving birth, close contact can spread the virus as well. To minimize the risk, the authors recommend isolating the baby from family members who have confirmed or suspected monkeypox and carefully monitoring for infection.
Mothers with an active monkeypox infection should also avoid breastfeeding to lower the risk of spreading the virus to their newborn, the authors wrote. But to support breastfeeding after infection, mothers can express and discard milk until the isolation period has passed.
Pregnant women who become infected may also consider getting vaccinated, the authors wrote. Vaccination up to 14 days after exposure doesn’t prevent the disease but can reduce the severity of symptoms. In the current outbreak, public health organizations advised doctors to vaccinate contacts of confirmed cases, including pregnant people.
The data for monkeypox vaccine use in pregnant women is small, the authors wrote, including fewer than 300 women. In previous studies, no adverse outcomes were found. The vaccine is also considered safe for breastfeeding.
“The decision whether to have the vaccine in pregnancy should be a personal choice,” the authors wrote. “Pregnant women and people should be encouraged to discuss the risks and benefits of vaccination, including possible side effects, with a health care professional before making their final decision.”
A version of this article first appeared on Medscape.com.
The risk of monkeypox infection remains low for the general public, the authors wrote, though cases continue to grow worldwide, particularly in the United Kingdom.
“We are aware infants and children are at greater risk of becoming seriously ill if they do catch monkeypox,” Edward Morris, MBBS, one of the authors and president of the Royal College of Obstetricians and Gynecologists, said in a statement.
“Therefore, to minimize the risk of a baby contracting the virus, we recommend health care professionals discuss the benefits and risks of having a cesarean birth with a pregnant woman or person who has or is suspected of having the virus,” he said.
Dr. Morris and colleagues pulled together existing evidence on monkeypox diagnosis, treatment, and recommended modes of birth for mothers and babies.
“The World Health Organization states there could be adverse consequences for pregnant women and babies if they become infected, including congenital monkeypox, miscarriage, or stillbirth, which is why we have provided clear guidance for health care professionals in this paper,” Dr. Morris said.
The monkeypox virus typically spreads through direct contact, droplets, or contaminated surfaces and objects. But some limited evidence shows that the virus can be passed from a mother to a baby via the placenta, which can lead to congenital monkeypox.
What’s more, mothers may be able to transmit the virus during or after birth. Although no evidence exists around the optimal mode of birth, a pregnant woman with an active monkeypox infection may choose to avoid vaginal delivery to reduce direct contact.
“If genital lesions are identified on a pregnant woman, then a cesarean birth will be recommended,” the authors wrote. “If a pregnant woman or person has suspected or confirmed monkeypox, a caesarean birth will be offered following discussion of the possible risk of neonatal infection, which may be serious.”
After giving birth, close contact can spread the virus as well. To minimize the risk, the authors recommend isolating the baby from family members who have confirmed or suspected monkeypox and carefully monitoring for infection.
Mothers with an active monkeypox infection should also avoid breastfeeding to lower the risk of spreading the virus to their newborn, the authors wrote. But to support breastfeeding after infection, mothers can express and discard milk until the isolation period has passed.
Pregnant women who become infected may also consider getting vaccinated, the authors wrote. Vaccination up to 14 days after exposure doesn’t prevent the disease but can reduce the severity of symptoms. In the current outbreak, public health organizations advised doctors to vaccinate contacts of confirmed cases, including pregnant people.
The data for monkeypox vaccine use in pregnant women is small, the authors wrote, including fewer than 300 women. In previous studies, no adverse outcomes were found. The vaccine is also considered safe for breastfeeding.
“The decision whether to have the vaccine in pregnancy should be a personal choice,” the authors wrote. “Pregnant women and people should be encouraged to discuss the risks and benefits of vaccination, including possible side effects, with a health care professional before making their final decision.”
A version of this article first appeared on Medscape.com.
FROM ULTRASOUND IN OBSTETRICS & GYNECOLOGY
In utero COVID exposure tied to developmental differences in infants
suggests a small-scale analysis that points to the need for further study and monitoring during pregnancy.
The study included 24 pregnant women, half of whom had COVID-19 during pregnancy, and their offspring. It showed impairments at 6 weeks of age on the social interactive dimension of a neonatal assessment.
“Not all babies born to mothers infected with COVID show neurodevelopmental differences, but our data show that their risk is increased in comparison to those not exposed to COVID in the womb. We need a bigger study to confirm the exact extent of the difference,” said lead researcher Rosa Ayesa Arriola, PhD, Valdecilla Research Institute (IDIVAL), Hospital Universitario Marqués de Valdecilla, Santander, Spain, in a release.
The findings were presented at the virtual European Psychiatric Association 2022 Congress.
Differing responses to cuddling
Coauthor Águeda Castro Quintas, PhD student, Network Centre for Biomedical Research in Mental Health, University of Barcelona, explained that the tests showed the children born to mothers who had COVID-19 during pregnancy reacted “slightly differently to being held, or cuddled.”
“We need to note that these are preliminary results, but this is part of a project following a larger sample of 100 mothers and their babies,” she added. The authors plan to compare their results with those from a similar study.
The group will also monitor infant language and motor development aged between 18 and 42 months.
“This is an ongoing project, and we are at an early stage,” Ms. Castro Quintas said. “We don’t know if these effects will result in any longer-term issues,” but longer-term observation “may help us understand this.”
“Of course, in babies who are so young, there are several things we just can’t measure, such as language skills or cognition,” added coinvestigator Nerea San Martín González, department of evolutionary biology, ecology and environmental sciences, University of Barcelona.
While emphasizing the need for larger sample sizes, she said that “in the meantime, we need to stress the importance of medical monitoring to facilitate a healthy pregnancy.”
The researchers note that the consequences of the COVID-19 pandemic for the newborns of affected mothers remain “unknown.”
However, previous studies of other infections during pregnancy suggest that offspring could be “especially vulnerable,”as the pathophysiological mechanisms of the infection, such as cytokine storms and microcoagulation, “could clearly compromise fetal neurodevelopment.”
To investigate further, they examined the neurodevelopment of infants born both immediately before and during the COVID-19 pandemic, from 2017 to 2021.
Twenty-one women who had COVID-19 during pregnancy were matched with 21 healthy controls. They were studied both during pregnancy and in the postpartum period, completing hormonal and other biochemical tests, salivary tests, movement assessments, and psychological questionnaires, adjusted for various factors.
The team also administered the Brazelton Neonatal Behavioural Assessment Scale (NBAS) to the offspring at 6 weeks of age to evaluate neurologic, social, and behavioral aspects of function.
“We have been especially sensitive in how we have conducted these tests,” said Ms. Castro Quintas. “Each mother and baby were closely examined by clinicians with expert training in the field and in the tests.”
Among those offspring exposed to COVID-19 during pregnancy, there was a significant decrease in scores on the social interactive dimension of the NBAS, particularly if infection occurred before week 20 of gestation.
Other NBAS subscales were not associated with maternal COVID-19 during pregnancy.
More research needed
Commenting on the findings, Livio Provenzi, PhD, a psychologist and researcher in developmental psychobiology at the University of Pavia (Italy), noted there is a “great need” to study the direct and indirect effect of the COVID-19 pandemic on parents and their children. “Pregnancy is a period of life which shapes much of our subsequent development, and exposure to adversity in pregnancy can leave long-lasting biological footprints.”
Dr. Provenzi, who was not involved in the study, added in the release that the findings reinforce “evidence of epigenetic alterations in infants born from mothers exposed to pandemic-related stress during pregnancy.
“It shows we need more large-scale, international research to allow us to understand the developmental effects of this health emergency and to deliver better quality of care to parents and infants.”
The study was funded by the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III through the University of Barcelona multicenter project and the Government of Cantabria. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
suggests a small-scale analysis that points to the need for further study and monitoring during pregnancy.
The study included 24 pregnant women, half of whom had COVID-19 during pregnancy, and their offspring. It showed impairments at 6 weeks of age on the social interactive dimension of a neonatal assessment.
“Not all babies born to mothers infected with COVID show neurodevelopmental differences, but our data show that their risk is increased in comparison to those not exposed to COVID in the womb. We need a bigger study to confirm the exact extent of the difference,” said lead researcher Rosa Ayesa Arriola, PhD, Valdecilla Research Institute (IDIVAL), Hospital Universitario Marqués de Valdecilla, Santander, Spain, in a release.
The findings were presented at the virtual European Psychiatric Association 2022 Congress.
Differing responses to cuddling
Coauthor Águeda Castro Quintas, PhD student, Network Centre for Biomedical Research in Mental Health, University of Barcelona, explained that the tests showed the children born to mothers who had COVID-19 during pregnancy reacted “slightly differently to being held, or cuddled.”
“We need to note that these are preliminary results, but this is part of a project following a larger sample of 100 mothers and their babies,” she added. The authors plan to compare their results with those from a similar study.
The group will also monitor infant language and motor development aged between 18 and 42 months.
“This is an ongoing project, and we are at an early stage,” Ms. Castro Quintas said. “We don’t know if these effects will result in any longer-term issues,” but longer-term observation “may help us understand this.”
“Of course, in babies who are so young, there are several things we just can’t measure, such as language skills or cognition,” added coinvestigator Nerea San Martín González, department of evolutionary biology, ecology and environmental sciences, University of Barcelona.
While emphasizing the need for larger sample sizes, she said that “in the meantime, we need to stress the importance of medical monitoring to facilitate a healthy pregnancy.”
The researchers note that the consequences of the COVID-19 pandemic for the newborns of affected mothers remain “unknown.”
However, previous studies of other infections during pregnancy suggest that offspring could be “especially vulnerable,”as the pathophysiological mechanisms of the infection, such as cytokine storms and microcoagulation, “could clearly compromise fetal neurodevelopment.”
To investigate further, they examined the neurodevelopment of infants born both immediately before and during the COVID-19 pandemic, from 2017 to 2021.
Twenty-one women who had COVID-19 during pregnancy were matched with 21 healthy controls. They were studied both during pregnancy and in the postpartum period, completing hormonal and other biochemical tests, salivary tests, movement assessments, and psychological questionnaires, adjusted for various factors.
The team also administered the Brazelton Neonatal Behavioural Assessment Scale (NBAS) to the offspring at 6 weeks of age to evaluate neurologic, social, and behavioral aspects of function.
“We have been especially sensitive in how we have conducted these tests,” said Ms. Castro Quintas. “Each mother and baby were closely examined by clinicians with expert training in the field and in the tests.”
Among those offspring exposed to COVID-19 during pregnancy, there was a significant decrease in scores on the social interactive dimension of the NBAS, particularly if infection occurred before week 20 of gestation.
Other NBAS subscales were not associated with maternal COVID-19 during pregnancy.
More research needed
Commenting on the findings, Livio Provenzi, PhD, a psychologist and researcher in developmental psychobiology at the University of Pavia (Italy), noted there is a “great need” to study the direct and indirect effect of the COVID-19 pandemic on parents and their children. “Pregnancy is a period of life which shapes much of our subsequent development, and exposure to adversity in pregnancy can leave long-lasting biological footprints.”
Dr. Provenzi, who was not involved in the study, added in the release that the findings reinforce “evidence of epigenetic alterations in infants born from mothers exposed to pandemic-related stress during pregnancy.
“It shows we need more large-scale, international research to allow us to understand the developmental effects of this health emergency and to deliver better quality of care to parents and infants.”
The study was funded by the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III through the University of Barcelona multicenter project and the Government of Cantabria. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
suggests a small-scale analysis that points to the need for further study and monitoring during pregnancy.
The study included 24 pregnant women, half of whom had COVID-19 during pregnancy, and their offspring. It showed impairments at 6 weeks of age on the social interactive dimension of a neonatal assessment.
“Not all babies born to mothers infected with COVID show neurodevelopmental differences, but our data show that their risk is increased in comparison to those not exposed to COVID in the womb. We need a bigger study to confirm the exact extent of the difference,” said lead researcher Rosa Ayesa Arriola, PhD, Valdecilla Research Institute (IDIVAL), Hospital Universitario Marqués de Valdecilla, Santander, Spain, in a release.
The findings were presented at the virtual European Psychiatric Association 2022 Congress.
Differing responses to cuddling
Coauthor Águeda Castro Quintas, PhD student, Network Centre for Biomedical Research in Mental Health, University of Barcelona, explained that the tests showed the children born to mothers who had COVID-19 during pregnancy reacted “slightly differently to being held, or cuddled.”
“We need to note that these are preliminary results, but this is part of a project following a larger sample of 100 mothers and their babies,” she added. The authors plan to compare their results with those from a similar study.
The group will also monitor infant language and motor development aged between 18 and 42 months.
“This is an ongoing project, and we are at an early stage,” Ms. Castro Quintas said. “We don’t know if these effects will result in any longer-term issues,” but longer-term observation “may help us understand this.”
“Of course, in babies who are so young, there are several things we just can’t measure, such as language skills or cognition,” added coinvestigator Nerea San Martín González, department of evolutionary biology, ecology and environmental sciences, University of Barcelona.
While emphasizing the need for larger sample sizes, she said that “in the meantime, we need to stress the importance of medical monitoring to facilitate a healthy pregnancy.”
The researchers note that the consequences of the COVID-19 pandemic for the newborns of affected mothers remain “unknown.”
However, previous studies of other infections during pregnancy suggest that offspring could be “especially vulnerable,”as the pathophysiological mechanisms of the infection, such as cytokine storms and microcoagulation, “could clearly compromise fetal neurodevelopment.”
To investigate further, they examined the neurodevelopment of infants born both immediately before and during the COVID-19 pandemic, from 2017 to 2021.
Twenty-one women who had COVID-19 during pregnancy were matched with 21 healthy controls. They were studied both during pregnancy and in the postpartum period, completing hormonal and other biochemical tests, salivary tests, movement assessments, and psychological questionnaires, adjusted for various factors.
The team also administered the Brazelton Neonatal Behavioural Assessment Scale (NBAS) to the offspring at 6 weeks of age to evaluate neurologic, social, and behavioral aspects of function.
“We have been especially sensitive in how we have conducted these tests,” said Ms. Castro Quintas. “Each mother and baby were closely examined by clinicians with expert training in the field and in the tests.”
Among those offspring exposed to COVID-19 during pregnancy, there was a significant decrease in scores on the social interactive dimension of the NBAS, particularly if infection occurred before week 20 of gestation.
Other NBAS subscales were not associated with maternal COVID-19 during pregnancy.
More research needed
Commenting on the findings, Livio Provenzi, PhD, a psychologist and researcher in developmental psychobiology at the University of Pavia (Italy), noted there is a “great need” to study the direct and indirect effect of the COVID-19 pandemic on parents and their children. “Pregnancy is a period of life which shapes much of our subsequent development, and exposure to adversity in pregnancy can leave long-lasting biological footprints.”
Dr. Provenzi, who was not involved in the study, added in the release that the findings reinforce “evidence of epigenetic alterations in infants born from mothers exposed to pandemic-related stress during pregnancy.
“It shows we need more large-scale, international research to allow us to understand the developmental effects of this health emergency and to deliver better quality of care to parents and infants.”
The study was funded by the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III through the University of Barcelona multicenter project and the Government of Cantabria. No relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM EPA 2022
Biologics, Women, and Pregnancy: What’s Known?
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
As the and the child’s development.
“I get asked a lot about fertility,” Vivian Shi, MD, associate professor of dermatology at the University of Arkansas, Little Rock, said at MedscapeLive’s Women’s and Pediatric Dermatology Seminar. Patients want to know, she said, if they go on a specific drug, whether it will affect their chances of conceiving and what else they need to know about safety.
She told the audience what she tells her patients: The answers are not complete but are evolving at a steady pace.
“Putting this talk together was kind of like a scavenger hunt,” said Dr. Shi, who gathered data from pregnancy exposure registries, published research, the Food and Drug Administration, and other sources on biologics. As more studies emerge each year, she said, recommendations will become stronger for considering treatment by certain biological drugs, taking into account effects on fertility, pregnancy, lactation, and the infant.
Among the biologics commonly used in dermatology are:
- Tumor necrosis factor (TNF) inhibitors (etanercept, adalimumab, infliximab, certolizumab).
- Interleukin (IL)–12 and -23 antagonist (ustekinumab).
- IL-17 antagonists (ixekizumab, secukinumab, brodalumab).
- IL-23 antagonists (risankizumab, tildrakizumab, guselkumab).
- IL-4, -13 antagonist (dupilumab) and IL-13 antagonist (tralokinumab).
- CD20-directed cytolytic antibody (rituximab).
To help with decision-making, Dr. Shi discussed the relatively new FDA labeling regulations as well as pregnancy exposure registries, research studies, and recommendations.
FDA pregnancy risk summaries
Under the previous system of classification of drugs in pregnancy, the FDA rated drugs as A, B, C, D, X. These categories ranged from showing no risks to the fetus to clear risk, but were oversimplistic and confusing, Dr. Shi said. Category C was especially confusing, as a drug with no animal or human data was put in the same category as a drug with adverse fetal effects on animals, she noted.
However, effective June 30, 2015, the FDA replaced pregnancy categories with risk summaries by medication. As of June, 2020, all prescription drugs were to remove pregnancy letter labeling. The risk summaries note human data when they are available and also note when no data are available. This information, Dr. Shi said, originates from many sources, including studies published in the medical literature, postmarketing studies conducted by companies, and pregnancy exposure registries, conducted by some companies and others. The FDA does not endorse any specific registries, but does post a list of such registries. Another helpful resource, she said, is Mother to Baby, a service of the nonprofit Organization of Teratology Information Specialists (OTIS).
Known, not known
Citing published literature, Dr. Shi said that TNF inhibitors have the most robust safety data from preconception to after birth. Less is known, she said, about the reproductive safety effects of other biologics used for dermatologic conditions, as they are newer than the anti-TNF medicines.
She reviewed a variety of research studies evaluating the safety of biologics during pregnancy and beyond. Highlights include results from a large registry, the Psoriasis Longitudinal Assessment and Registry (PSOLAR), of 298 pregnancies in about 220 women from 2007 to 2019, looking at 13 different biologics. The overall and live-birth outcomes in the women on biologics for psoriasis were similar to those for the general population and the rate of congenital anomalies was 0.8%, researchers reported in 2021, lower than the generally cited annual figure of U.S. births.
Studies evaluating biologics for nondermatologic conditions suggest safety. A prospective cohort study of women who took adalimumab in pregnancy (for rheumatoid arthritis or Crohn’s disease) found no increased risk for birth defects. In another study looking at women who were breastfeeding, researchers found no increased risk of infections or delay in developmental milestones in the children of women taking biologics for inflammatory bowel disease, compared with those not on the medications.
A report using data from the World Health Organization concludes that dupilumab appears to be safe during pregnancy, based on an evaluation of 36 pregnancy-related reports among more than 37,000 unique adverse event reports related to dupilumab in a global database.
Recommendations about biologic use from different organizations don’t always mesh, Dr. Shi said, noting that European guidelines tend to be stricter, as some reviews show.
If a mother is exposed to any biologic therapy other than certolizumab during the third trimester, after 27 weeks, Dr. Shi said, “you want to consider avoiding a live vaccine for the first 6 months of the baby’s life.” It turns out, she said, the only recommended live vaccine during that period is the rotavirus vaccine, and she suggests doctors recommend postponing that one until the babies are older if women have been on biologics other than certolizumab.
Her other take-home messages: TNF inhibitors have the most robust safety data from before conception through lactation. Under current guidelines, certolizumab is viewed as the safest to use throughout pregnancy. Dr. Shi’s message to her colleagues fielding the same questions she gets from patients: “There is more data coming out every year. Ultimately, we will have better information to inform our patients.”
At the conference, Lawrence F. Eichenfield, MD, a course director and professor of dermatology and pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego, encouraged Dr. Shi to write up her presentation as a resource for other dermatologists – which she said is in progress.
Medscape Live and this news organization are owned by the same parent company. Dr. Shi disclosed consulting and investigative and research funding from several pharmaceutical firms, but not directly related to the content of her presentation.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
‘Medical maximizers’ dole out unneeded antibiotics for ASB
So why did you get that prescription?
The Infectious Diseases Society of America recommends against antibiotics in this scenario, with exceptions for patients who are pregnant or undergoing certain urologic procedures.
Antibiotics for asymptomatic bacteriuria (ASB) generally do not help; are costly; and can cause side effects, Clostridioides difficile infection, and antibiotic resistance.
Still, antibiotic treatment for asymptomatic bacteriuria remains common, despite guidelines.
And when researchers recently surveyed 551 primary care clinicians to see which ones would inappropriately prescribe antibiotics for a positive urine culture, the answer was most of them: 71%.
“Regardless of years in practice, training background, or professional degree, most clinicians indicated that they would prescribe antibiotics for asymptomatic bacteriuria,” the researchers reported in JAMA Network Open.
Some groups of clinicians seemed especially likely to prescribe antibiotics unnecessarily.
“Medical maximizers” – clinicians who prefer treatment even when its value is ambiguous – and family medicine clinicians were more likely to prescribe antibiotics in response to a hypothetical case.
On the other hand, resident physicians and clinicians in the U.S. Pacific Northwest were less likely to provide antibiotics inappropriately, the researchers found.
Study author Jonathan D. Baghdadi, MD, PhD, with the department of epidemiology and public health at the University of Maryland and the Veterans Affairs Maryland Healthcare System in Baltimore, summed up the findings on Twitter: “ ... who prescribes antibiotics for asymptomatic bacteriuria? The answer is most primary care clinicians in every category, but it’s more common among clinicians who want to ‘do everything.’ ”
Dr. Baghdadi said the gaps reflect problems with the medical system rather than individual clinicians.
“I don’t believe that individual clinicians knowingly choose to prescribe inappropriate antibiotics in defiance of guidelines,” Dr. Baghdadi told this news organization. “Clinical decision-making is complicated, and the decision to prescribe inappropriate antibiotics depends on patient expectations, clinician perception of patient expectations, time pressure in the clinic, regional variation in medical practice, the culture of antibiotic use, and likely in some cases the perception that doing more is better.”
In addition, researchers have used various definitions of ASB over time and in different contexts, he said.
What to do for Mr. Williams?
To examine clinician attitudes and characteristics associated with prescribing antibiotics for asymptomatic bacteriuria, Dr. Baghdadi and his colleagues analyzed survey responses from 490 physicians and 61 advanced practice clinicians.
Study participants completed tests that measure numeracy, risk-taking preferences, burnout, and tendency to maximize care. They were presented with four hypothetical clinical scenarios, including a case of asymptomatic bacteriuria: “Mr. Williams, a 65-year-old man, comes to the office for follow-up of his osteoarthritis. He has noted foul-smelling urine and no pain or difficulty with urination. A urine dipstick shows trace blood. He has no particular preference for testing and wants your advice.”
Clinicians who had been in practice for at least 10 years were more likely to prescribe antibiotics (82%) to “Mr. Williams” than were those with 3-9 years in practice (73%) or less than 3 years in practice (64%).
Of 120 clinicians with a background in family medicine, 85% said they would have prescribed antibiotics, versus 62% of 207 clinicians with a background in internal medicine.
Nurse practitioners and physician assistants were more likely to prescribe antibiotics (90%) than were attending (78%) and resident physicians (63%).
In one analysis, a background in family medicine was associated with nearly three times higher odds of prescribing antibiotics. And a high “medical maximizer” score was associated with about twice the odds of prescribing the medications.
Meanwhile, resident physicians and clinicians in the Pacific Northwest had a lower likelihood of prescribing antibiotics, with odds ratios of 0.57 and 0.49, respectively.
The respondents who prescribed antibiotics estimated a 90% probability of UTI, whereas those who did not prescribe antibiotics estimated a 15% probability of the condition.
Breaking a habit
Some prescribers may know not to treat asymptomatic bacteriuria but mistakenly consider certain findings to be symptoms of UTI.
Bradley Langford, PharmD, an antimicrobial stewardship expert with Public Health Ontario, said in his experience, most clinicians who say they know not to treat ASB incorrectly believe that cloudy urine, altered cognition, and other nonspecific symptoms indicate a UTI.
“The fact that most clinicians would treat ASB suggests that there is still a lot of work to do to improve antimicrobial stewardship, particularly outside of the hospital setting,” Dr. Langford told this news organization.
Avoiding unnecessary antibiotics is important not just because of the lack of benefit, but also because of the potential harms, said Dr. Langford. He has created a list of rebuttals for commonly given reasons for testing and treating asymptomatic bacteriuria.
“Using antibiotics for ASB can counterintuitively increase the risk for symptomatic UTI due to the disruption of protective local microflora, allowing for the growth of more pathogenic/resistant organisms,” he said.
One approach to addressing the problem: Don’t test urine in the first place if patients are asymptomatic. Virtual learning sessions have been shown to reduce urine culturing and urinary antibiotic prescribing in long-term care homes, Dr. Langford noted.
Updated training for health care professionals from the outset may also be key, and the lower rate of prescribing intent among resident physicians is reassuring, he said.
A role for patients
Patients could also help decrease the inappropriate use of antibiotics.
“Be clear with your doctor about your expectations for the health care interaction, including whether you are expecting to receive antibiotics,” Dr. Baghdadi said. “Your doctor may assume you contacted them because you wanted a prescription. If you are not expecting antibiotics, you should feel free to say so. And if you are asymptomatic, you may not need antibiotics, even if the urine culture is positive.”
The study was funded by a grant from the National Institutes of Health, and Dr. Baghdadi received grant support from the University of Maryland, Baltimore Institute for Clinical and Translational Research. Coauthors disclosed government grants and ties to Memorial Sloan Kettering Cancer Center, Vedanta Biosciences, Opentrons, and Fimbrion. Dr. Langford reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
So why did you get that prescription?
The Infectious Diseases Society of America recommends against antibiotics in this scenario, with exceptions for patients who are pregnant or undergoing certain urologic procedures.
Antibiotics for asymptomatic bacteriuria (ASB) generally do not help; are costly; and can cause side effects, Clostridioides difficile infection, and antibiotic resistance.
Still, antibiotic treatment for asymptomatic bacteriuria remains common, despite guidelines.
And when researchers recently surveyed 551 primary care clinicians to see which ones would inappropriately prescribe antibiotics for a positive urine culture, the answer was most of them: 71%.
“Regardless of years in practice, training background, or professional degree, most clinicians indicated that they would prescribe antibiotics for asymptomatic bacteriuria,” the researchers reported in JAMA Network Open.
Some groups of clinicians seemed especially likely to prescribe antibiotics unnecessarily.
“Medical maximizers” – clinicians who prefer treatment even when its value is ambiguous – and family medicine clinicians were more likely to prescribe antibiotics in response to a hypothetical case.
On the other hand, resident physicians and clinicians in the U.S. Pacific Northwest were less likely to provide antibiotics inappropriately, the researchers found.
Study author Jonathan D. Baghdadi, MD, PhD, with the department of epidemiology and public health at the University of Maryland and the Veterans Affairs Maryland Healthcare System in Baltimore, summed up the findings on Twitter: “ ... who prescribes antibiotics for asymptomatic bacteriuria? The answer is most primary care clinicians in every category, but it’s more common among clinicians who want to ‘do everything.’ ”
Dr. Baghdadi said the gaps reflect problems with the medical system rather than individual clinicians.
“I don’t believe that individual clinicians knowingly choose to prescribe inappropriate antibiotics in defiance of guidelines,” Dr. Baghdadi told this news organization. “Clinical decision-making is complicated, and the decision to prescribe inappropriate antibiotics depends on patient expectations, clinician perception of patient expectations, time pressure in the clinic, regional variation in medical practice, the culture of antibiotic use, and likely in some cases the perception that doing more is better.”
In addition, researchers have used various definitions of ASB over time and in different contexts, he said.
What to do for Mr. Williams?
To examine clinician attitudes and characteristics associated with prescribing antibiotics for asymptomatic bacteriuria, Dr. Baghdadi and his colleagues analyzed survey responses from 490 physicians and 61 advanced practice clinicians.
Study participants completed tests that measure numeracy, risk-taking preferences, burnout, and tendency to maximize care. They were presented with four hypothetical clinical scenarios, including a case of asymptomatic bacteriuria: “Mr. Williams, a 65-year-old man, comes to the office for follow-up of his osteoarthritis. He has noted foul-smelling urine and no pain or difficulty with urination. A urine dipstick shows trace blood. He has no particular preference for testing and wants your advice.”
Clinicians who had been in practice for at least 10 years were more likely to prescribe antibiotics (82%) to “Mr. Williams” than were those with 3-9 years in practice (73%) or less than 3 years in practice (64%).
Of 120 clinicians with a background in family medicine, 85% said they would have prescribed antibiotics, versus 62% of 207 clinicians with a background in internal medicine.
Nurse practitioners and physician assistants were more likely to prescribe antibiotics (90%) than were attending (78%) and resident physicians (63%).
In one analysis, a background in family medicine was associated with nearly three times higher odds of prescribing antibiotics. And a high “medical maximizer” score was associated with about twice the odds of prescribing the medications.
Meanwhile, resident physicians and clinicians in the Pacific Northwest had a lower likelihood of prescribing antibiotics, with odds ratios of 0.57 and 0.49, respectively.
The respondents who prescribed antibiotics estimated a 90% probability of UTI, whereas those who did not prescribe antibiotics estimated a 15% probability of the condition.
Breaking a habit
Some prescribers may know not to treat asymptomatic bacteriuria but mistakenly consider certain findings to be symptoms of UTI.
Bradley Langford, PharmD, an antimicrobial stewardship expert with Public Health Ontario, said in his experience, most clinicians who say they know not to treat ASB incorrectly believe that cloudy urine, altered cognition, and other nonspecific symptoms indicate a UTI.
“The fact that most clinicians would treat ASB suggests that there is still a lot of work to do to improve antimicrobial stewardship, particularly outside of the hospital setting,” Dr. Langford told this news organization.
Avoiding unnecessary antibiotics is important not just because of the lack of benefit, but also because of the potential harms, said Dr. Langford. He has created a list of rebuttals for commonly given reasons for testing and treating asymptomatic bacteriuria.
“Using antibiotics for ASB can counterintuitively increase the risk for symptomatic UTI due to the disruption of protective local microflora, allowing for the growth of more pathogenic/resistant organisms,” he said.
One approach to addressing the problem: Don’t test urine in the first place if patients are asymptomatic. Virtual learning sessions have been shown to reduce urine culturing and urinary antibiotic prescribing in long-term care homes, Dr. Langford noted.
Updated training for health care professionals from the outset may also be key, and the lower rate of prescribing intent among resident physicians is reassuring, he said.
A role for patients
Patients could also help decrease the inappropriate use of antibiotics.
“Be clear with your doctor about your expectations for the health care interaction, including whether you are expecting to receive antibiotics,” Dr. Baghdadi said. “Your doctor may assume you contacted them because you wanted a prescription. If you are not expecting antibiotics, you should feel free to say so. And if you are asymptomatic, you may not need antibiotics, even if the urine culture is positive.”
The study was funded by a grant from the National Institutes of Health, and Dr. Baghdadi received grant support from the University of Maryland, Baltimore Institute for Clinical and Translational Research. Coauthors disclosed government grants and ties to Memorial Sloan Kettering Cancer Center, Vedanta Biosciences, Opentrons, and Fimbrion. Dr. Langford reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
So why did you get that prescription?
The Infectious Diseases Society of America recommends against antibiotics in this scenario, with exceptions for patients who are pregnant or undergoing certain urologic procedures.
Antibiotics for asymptomatic bacteriuria (ASB) generally do not help; are costly; and can cause side effects, Clostridioides difficile infection, and antibiotic resistance.
Still, antibiotic treatment for asymptomatic bacteriuria remains common, despite guidelines.
And when researchers recently surveyed 551 primary care clinicians to see which ones would inappropriately prescribe antibiotics for a positive urine culture, the answer was most of them: 71%.
“Regardless of years in practice, training background, or professional degree, most clinicians indicated that they would prescribe antibiotics for asymptomatic bacteriuria,” the researchers reported in JAMA Network Open.
Some groups of clinicians seemed especially likely to prescribe antibiotics unnecessarily.
“Medical maximizers” – clinicians who prefer treatment even when its value is ambiguous – and family medicine clinicians were more likely to prescribe antibiotics in response to a hypothetical case.
On the other hand, resident physicians and clinicians in the U.S. Pacific Northwest were less likely to provide antibiotics inappropriately, the researchers found.
Study author Jonathan D. Baghdadi, MD, PhD, with the department of epidemiology and public health at the University of Maryland and the Veterans Affairs Maryland Healthcare System in Baltimore, summed up the findings on Twitter: “ ... who prescribes antibiotics for asymptomatic bacteriuria? The answer is most primary care clinicians in every category, but it’s more common among clinicians who want to ‘do everything.’ ”
Dr. Baghdadi said the gaps reflect problems with the medical system rather than individual clinicians.
“I don’t believe that individual clinicians knowingly choose to prescribe inappropriate antibiotics in defiance of guidelines,” Dr. Baghdadi told this news organization. “Clinical decision-making is complicated, and the decision to prescribe inappropriate antibiotics depends on patient expectations, clinician perception of patient expectations, time pressure in the clinic, regional variation in medical practice, the culture of antibiotic use, and likely in some cases the perception that doing more is better.”
In addition, researchers have used various definitions of ASB over time and in different contexts, he said.
What to do for Mr. Williams?
To examine clinician attitudes and characteristics associated with prescribing antibiotics for asymptomatic bacteriuria, Dr. Baghdadi and his colleagues analyzed survey responses from 490 physicians and 61 advanced practice clinicians.
Study participants completed tests that measure numeracy, risk-taking preferences, burnout, and tendency to maximize care. They were presented with four hypothetical clinical scenarios, including a case of asymptomatic bacteriuria: “Mr. Williams, a 65-year-old man, comes to the office for follow-up of his osteoarthritis. He has noted foul-smelling urine and no pain or difficulty with urination. A urine dipstick shows trace blood. He has no particular preference for testing and wants your advice.”
Clinicians who had been in practice for at least 10 years were more likely to prescribe antibiotics (82%) to “Mr. Williams” than were those with 3-9 years in practice (73%) or less than 3 years in practice (64%).
Of 120 clinicians with a background in family medicine, 85% said they would have prescribed antibiotics, versus 62% of 207 clinicians with a background in internal medicine.
Nurse practitioners and physician assistants were more likely to prescribe antibiotics (90%) than were attending (78%) and resident physicians (63%).
In one analysis, a background in family medicine was associated with nearly three times higher odds of prescribing antibiotics. And a high “medical maximizer” score was associated with about twice the odds of prescribing the medications.
Meanwhile, resident physicians and clinicians in the Pacific Northwest had a lower likelihood of prescribing antibiotics, with odds ratios of 0.57 and 0.49, respectively.
The respondents who prescribed antibiotics estimated a 90% probability of UTI, whereas those who did not prescribe antibiotics estimated a 15% probability of the condition.
Breaking a habit
Some prescribers may know not to treat asymptomatic bacteriuria but mistakenly consider certain findings to be symptoms of UTI.
Bradley Langford, PharmD, an antimicrobial stewardship expert with Public Health Ontario, said in his experience, most clinicians who say they know not to treat ASB incorrectly believe that cloudy urine, altered cognition, and other nonspecific symptoms indicate a UTI.
“The fact that most clinicians would treat ASB suggests that there is still a lot of work to do to improve antimicrobial stewardship, particularly outside of the hospital setting,” Dr. Langford told this news organization.
Avoiding unnecessary antibiotics is important not just because of the lack of benefit, but also because of the potential harms, said Dr. Langford. He has created a list of rebuttals for commonly given reasons for testing and treating asymptomatic bacteriuria.
“Using antibiotics for ASB can counterintuitively increase the risk for symptomatic UTI due to the disruption of protective local microflora, allowing for the growth of more pathogenic/resistant organisms,” he said.
One approach to addressing the problem: Don’t test urine in the first place if patients are asymptomatic. Virtual learning sessions have been shown to reduce urine culturing and urinary antibiotic prescribing in long-term care homes, Dr. Langford noted.
Updated training for health care professionals from the outset may also be key, and the lower rate of prescribing intent among resident physicians is reassuring, he said.
A role for patients
Patients could also help decrease the inappropriate use of antibiotics.
“Be clear with your doctor about your expectations for the health care interaction, including whether you are expecting to receive antibiotics,” Dr. Baghdadi said. “Your doctor may assume you contacted them because you wanted a prescription. If you are not expecting antibiotics, you should feel free to say so. And if you are asymptomatic, you may not need antibiotics, even if the urine culture is positive.”
The study was funded by a grant from the National Institutes of Health, and Dr. Baghdadi received grant support from the University of Maryland, Baltimore Institute for Clinical and Translational Research. Coauthors disclosed government grants and ties to Memorial Sloan Kettering Cancer Center, Vedanta Biosciences, Opentrons, and Fimbrion. Dr. Langford reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Hope for quicker and more accurate endometriosis diagnosis
A new imaging study hopes to make diagnosing endometriosis quicker, more accurate and reduce the need for invasive surgery.
In October 2020 the All Party Parliamentary Group on Endometriosis published a report that included within its recommendations “a commitment to drive down diagnosis times” for women with the condition. On average, it takes around 8 years for a woman to get a diagnosis of endometriosis, a figure, said the authors of the report, that had “not improved in the last decade.”
Indeed, in its report the APPG said that it was seeking a commitment from Governments in all four nations to reduce average diagnosis times with “targets of 4 years or less by 2025, and a year or less by 2030.”
Surgery often needed for endometriosis diagnosis
Endometriosis affects 1 in 10 women between puberty and menopause – 1.5 million in the United Kingdom – often results in multiple general practitioner and accident and emergency department visits, multiple scans, and often laparoscopic surgery to confirm the diagnosis, as there is currently no simple diagnostic test for the condition. One of the main reasons for the delay in diagnosis is the lack of noninvasive tests capable of detecting all endometriosis subtypes – ovarian, superficial, and deep disease.
Now, experts at the Endometriosis CaRe Centre and Nuffield Department of Women’s and Reproductive Health, University of Oxford (England), in collaboration with British life sciences company Serac Healthcare, hope to establish a faster process for diagnosing endometriosis.
Christian Becker, codirector of the Endometriosis CaRe Centre in Oxford, and a study lead, said: “There is an urgent unmet clinical need for a noninvasive marker to identify or rule out endometriosis as it is such a very common disease affecting more than 190 million women worldwide.”
In the study, researchers will investigate whether a 20-minute imaging scan can detect the most common types of endometriosis, which currently require surgery to diagnose. In turn, they hope that earlier diagnosis of the condition will allow women to seek appropriate treatment sooner. They will use an experimental imaging marker – 99mTc-maraciclatide – that binds to areas of inflammation and that can be used in endometriosis to visualize the disease on a scan. The imaging marker has already been used for detecting inflammation in conditions such as rheumatoid arthritis.
Between 2 and 7 days before planned surgery for suspected endometriosis, participants will be invited for an imaging scan, and the team will compare the suspected locations of disease detected on the scan with those seen during surgery to confirm whether this imaging test could be an effective noninvasive method of detecting all endometriosis subtypes.
Doctor visits and repeated investigations reduced
The researchers commented that the potential strengths of the scan lie in the way the imaging marker binds to areas of inflammation, which may allow doctors to distinguish between new and old lesions and detect endometriosis in areas not easily seen during surgery, such as the lung.
They added that the development of a 20-minute imaging test would reduce the need for repeated visits to doctors, for repeated investigations, and for invasive surgery to obtain a diagnosis. This would ultimately “reduce the time taken to confirm or exclude endometriosis,” they pointed out.
Following the publication of the APPG report in October 2020 the group’s then chair, the late Sir David Amess, said: “Without investment in research, a reduction in diagnosis time, and appropriate NHS pathways, those with endometriosis will continue to face huge barriers in accessing the appropriate support at the right time.”
Krina Zondervan, head of department at the Nuffield Department of Women’s and Reproductive Health, University of Oxford, and a study lead, said: “This study highlights that close collaborations between academics, clinicians and industry are important to combine and accelerate discovery and innovation in addressing high-priority areas in women’s health such as endometriosis.”
David Hail, CEO of Serac Healthcare, said: “We are excited about the potential of 99mTc-maraciclatide to diagnose endometriosis noninvasively and delighted to be working with the internationally renowned team at Oxford on this important first study.”
A version of this article first appeared on Medscape UK.
A new imaging study hopes to make diagnosing endometriosis quicker, more accurate and reduce the need for invasive surgery.
In October 2020 the All Party Parliamentary Group on Endometriosis published a report that included within its recommendations “a commitment to drive down diagnosis times” for women with the condition. On average, it takes around 8 years for a woman to get a diagnosis of endometriosis, a figure, said the authors of the report, that had “not improved in the last decade.”
Indeed, in its report the APPG said that it was seeking a commitment from Governments in all four nations to reduce average diagnosis times with “targets of 4 years or less by 2025, and a year or less by 2030.”
Surgery often needed for endometriosis diagnosis
Endometriosis affects 1 in 10 women between puberty and menopause – 1.5 million in the United Kingdom – often results in multiple general practitioner and accident and emergency department visits, multiple scans, and often laparoscopic surgery to confirm the diagnosis, as there is currently no simple diagnostic test for the condition. One of the main reasons for the delay in diagnosis is the lack of noninvasive tests capable of detecting all endometriosis subtypes – ovarian, superficial, and deep disease.
Now, experts at the Endometriosis CaRe Centre and Nuffield Department of Women’s and Reproductive Health, University of Oxford (England), in collaboration with British life sciences company Serac Healthcare, hope to establish a faster process for diagnosing endometriosis.
Christian Becker, codirector of the Endometriosis CaRe Centre in Oxford, and a study lead, said: “There is an urgent unmet clinical need for a noninvasive marker to identify or rule out endometriosis as it is such a very common disease affecting more than 190 million women worldwide.”
In the study, researchers will investigate whether a 20-minute imaging scan can detect the most common types of endometriosis, which currently require surgery to diagnose. In turn, they hope that earlier diagnosis of the condition will allow women to seek appropriate treatment sooner. They will use an experimental imaging marker – 99mTc-maraciclatide – that binds to areas of inflammation and that can be used in endometriosis to visualize the disease on a scan. The imaging marker has already been used for detecting inflammation in conditions such as rheumatoid arthritis.
Between 2 and 7 days before planned surgery for suspected endometriosis, participants will be invited for an imaging scan, and the team will compare the suspected locations of disease detected on the scan with those seen during surgery to confirm whether this imaging test could be an effective noninvasive method of detecting all endometriosis subtypes.
Doctor visits and repeated investigations reduced
The researchers commented that the potential strengths of the scan lie in the way the imaging marker binds to areas of inflammation, which may allow doctors to distinguish between new and old lesions and detect endometriosis in areas not easily seen during surgery, such as the lung.
They added that the development of a 20-minute imaging test would reduce the need for repeated visits to doctors, for repeated investigations, and for invasive surgery to obtain a diagnosis. This would ultimately “reduce the time taken to confirm or exclude endometriosis,” they pointed out.
Following the publication of the APPG report in October 2020 the group’s then chair, the late Sir David Amess, said: “Without investment in research, a reduction in diagnosis time, and appropriate NHS pathways, those with endometriosis will continue to face huge barriers in accessing the appropriate support at the right time.”
Krina Zondervan, head of department at the Nuffield Department of Women’s and Reproductive Health, University of Oxford, and a study lead, said: “This study highlights that close collaborations between academics, clinicians and industry are important to combine and accelerate discovery and innovation in addressing high-priority areas in women’s health such as endometriosis.”
David Hail, CEO of Serac Healthcare, said: “We are excited about the potential of 99mTc-maraciclatide to diagnose endometriosis noninvasively and delighted to be working with the internationally renowned team at Oxford on this important first study.”
A version of this article first appeared on Medscape UK.
A new imaging study hopes to make diagnosing endometriosis quicker, more accurate and reduce the need for invasive surgery.
In October 2020 the All Party Parliamentary Group on Endometriosis published a report that included within its recommendations “a commitment to drive down diagnosis times” for women with the condition. On average, it takes around 8 years for a woman to get a diagnosis of endometriosis, a figure, said the authors of the report, that had “not improved in the last decade.”
Indeed, in its report the APPG said that it was seeking a commitment from Governments in all four nations to reduce average diagnosis times with “targets of 4 years or less by 2025, and a year or less by 2030.”
Surgery often needed for endometriosis diagnosis
Endometriosis affects 1 in 10 women between puberty and menopause – 1.5 million in the United Kingdom – often results in multiple general practitioner and accident and emergency department visits, multiple scans, and often laparoscopic surgery to confirm the diagnosis, as there is currently no simple diagnostic test for the condition. One of the main reasons for the delay in diagnosis is the lack of noninvasive tests capable of detecting all endometriosis subtypes – ovarian, superficial, and deep disease.
Now, experts at the Endometriosis CaRe Centre and Nuffield Department of Women’s and Reproductive Health, University of Oxford (England), in collaboration with British life sciences company Serac Healthcare, hope to establish a faster process for diagnosing endometriosis.
Christian Becker, codirector of the Endometriosis CaRe Centre in Oxford, and a study lead, said: “There is an urgent unmet clinical need for a noninvasive marker to identify or rule out endometriosis as it is such a very common disease affecting more than 190 million women worldwide.”
In the study, researchers will investigate whether a 20-minute imaging scan can detect the most common types of endometriosis, which currently require surgery to diagnose. In turn, they hope that earlier diagnosis of the condition will allow women to seek appropriate treatment sooner. They will use an experimental imaging marker – 99mTc-maraciclatide – that binds to areas of inflammation and that can be used in endometriosis to visualize the disease on a scan. The imaging marker has already been used for detecting inflammation in conditions such as rheumatoid arthritis.
Between 2 and 7 days before planned surgery for suspected endometriosis, participants will be invited for an imaging scan, and the team will compare the suspected locations of disease detected on the scan with those seen during surgery to confirm whether this imaging test could be an effective noninvasive method of detecting all endometriosis subtypes.
Doctor visits and repeated investigations reduced
The researchers commented that the potential strengths of the scan lie in the way the imaging marker binds to areas of inflammation, which may allow doctors to distinguish between new and old lesions and detect endometriosis in areas not easily seen during surgery, such as the lung.
They added that the development of a 20-minute imaging test would reduce the need for repeated visits to doctors, for repeated investigations, and for invasive surgery to obtain a diagnosis. This would ultimately “reduce the time taken to confirm or exclude endometriosis,” they pointed out.
Following the publication of the APPG report in October 2020 the group’s then chair, the late Sir David Amess, said: “Without investment in research, a reduction in diagnosis time, and appropriate NHS pathways, those with endometriosis will continue to face huge barriers in accessing the appropriate support at the right time.”
Krina Zondervan, head of department at the Nuffield Department of Women’s and Reproductive Health, University of Oxford, and a study lead, said: “This study highlights that close collaborations between academics, clinicians and industry are important to combine and accelerate discovery and innovation in addressing high-priority areas in women’s health such as endometriosis.”
David Hail, CEO of Serac Healthcare, said: “We are excited about the potential of 99mTc-maraciclatide to diagnose endometriosis noninvasively and delighted to be working with the internationally renowned team at Oxford on this important first study.”
A version of this article first appeared on Medscape UK.
Women are not being warned that anesthetic may reduce birth pill efficacy
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
FROM EUROANAESTHESIA