User login
Better endografts mean fewer reinterventions for endovascular AAA
INDIANAPOLIS – Reintervention rates following endovascular repair of abdominal aortic aneurysms have fallen steadily with the introduction of each successive generation of endografts, while reintervention rates after open surgical repair remained stable during a recent 15-year period.
This was among the key findings from the first in-depth analysis of reinterventions occurring in contemporaneous cohorts of abdominal aortic aneurysm (AAA) patients undergoing endovascular aneurysm repair (EVAR) or open repair. The large single-center retrospective study demonstrated major differences between the two treatment strategies in terms of the incidence, nature, timing, and mortality associated with complications requiring reintervention, Dr. Mustafa Al-Jubouri said at the annual meeting of the American Surgical Association.
Dr. Al-Jubouri of Jobst Vascular Institute, Toledo, Ohio, reported on the 1,144 patients who underwent AAA repair there during 1996-2011. Forty-nine percent had EVAR, 51% open surgical repair. Beginning in 2003, more EVARs than open repairs were done annually at the Toledo institute, consistent with the experience at many major centers in the United States and elsewhere, where EVAR has become the first-line treatment based upon evidence that it offers lower operative mortality, less blood loss, and shorter ICU and hospital lengths of stay.
These advantages come at a cost, however: namely, a greater rate of secondary interventions, mainly due to device migration, failure, or endoleaks. The purpose of Dr. Al-Jubouri’s study was to evaluate the rates and reasons for reintervention over time in the two cohorts, as well as the impact of reintervention on long-term survival.
Reintervention was required in 13.6% of the EVAR group during a mean follow-up of 4.58 years, and in 5.1% of the open surgery group during 6.58 years. A single reintervention occurred in 7.9% of EVAR patients and 3.6% of the open repair group. More than one reintervention was required in 5.8% of EVAR patients compared to just 1.6% of the open repair group.
The types and timing of complications leading to reintervention were very different in the two groups. Sixty-eight percent of reinterventions in the EVAR group were for treatment of endoleaks. Another 11.5% were to address device migration, and an equal number were for occlusion.
In contrast, the three most frequent causes of reintervention in the open repair group were colonic ischemia, accounting for 30.4% of reintervention procedures; severe bleeding, 21.7%; and incisional hernia, which triggered another 21.7% of reinterventions.
Notably, 60% of all reinterventions in the open repair group occurred during the initial hospitalization, while less than 7% of reinterventions in the EVAR patients happened within 1 month of the index procedure and only one-third within the first year, the surgeon continued.
Thirty-day mortality in EVAR patients who underwent reintervention within the first month was zero, compared to a 23.3% mortality rate in open repair patients requiring reintervention within 1 month. However, when patients did not require early reintervention, 30-day mortality rates in the two groups did not differ significantly: 1.9% in EVAR group and in the open repair group. That means when patients in the open surgery group required early reintervention, their mortality rate shot up sevenfold.
After the first 30 days post-index procedure, long-term survival rates in the two groups were similar.
Need for reintervention in the open repair group was strongly related to larger aneurysm size. In contrast, reintervention rates were similar in the EVAR group regardless of aneurysm size.
A first reintervention after EVAR occurred in 23.7% of patients who received a first-generation endograft, such as the Ancure or Talent; in 16.2% of those who got the second-generation AneuRx endograft; and in 9.1% with a third-generation endograft, such as the Excluder, Endurant, Powerlink, or Zenith. The annualized rate of reintervention during the first 3 years of follow-up was 6.8% per year with first-generation devices, 7.2% per year with second-generation endografts, and significantly lower at 3.4% per year with the third-generation.
One major reason reintervention rates in EVAR patients have declined over time is that each newer generation of endograft is lower-profile, easier to deploy, and more durable. Also, many of the surgeons now putting in third-generation endografts were performing EVAR 15 years ago; they’re very experienced operators, Dr. Al-Jubouri noted.
Discussant Dr. James R. Debord proposed another explanation for the decrease in EVAR reinterventions over time.
"Isn’t it much more likely that it’s due to recognition of the fact that many of these type 2 endoleaks that we used to intervene on early on don’t require reintervention unless there’s sac enlargement?" commented Dr. Debord, professor of clinical surgery and chief of vascular surgery at the University of Illinois at Peoria.
Dr. Al-Jubouri concurred that this is an important factor in the declining rate of EVAR reinterventions.
"We saw a significant decrease in reinterventions for type 2 endoleaks between the first, second, and third generations," he said.
Asked how his study findings have changed the follow-up protocols at Jobst Vascular Institute, the surgeon replied that in the early years of the series EVAR patients got a CT scan at 6 weeks, 6 months, 1 year, and annually thereafter. This evaluation has evolved over time. Now EVAR patients get a CT scan at 6-12 weeks, and duplex ultrasounds at 6 months, 1 year, and annually thereafter.
"There is no standardized follow-up for open repair patients. However, most [patients] get an annual duplex ultrasound for their follow-up. A CT scan is not part of the follow-up of patients with open repair. But most if not all of the complications that developed in the open repair group were symptomatic," he explained.
He reported having no financial conflicts.
Dr. Mustafa Al-Jubouri and his colleagues assessed reinterventions and outcomes after EVAR and open AAA repair over a long time period, and found decreasing rates of reintervention after EVAR, which they attribute to improvements in technology from first to third and later-generation devices. I would concur with the one discussant, that some of the decrease may also be due to the understanding that not all type II endoleaks require repair. Further, much of the decrease may be due to physician experience – both with appropriate patient and device selection, and technical expertise, including with deployment. However, regardless of the underlying reason for the improvement in the reintervention rate, it is heartening that reintervention is decreasing as physicians become more facile, and industry provides technological improvements to the devices.
Dr. Linda Harris, FACS, is division chief and program director of vascular surgery at State University of New York, Buffalo. Dr. Harris has no disclosures
Dr. Mustafa Al-Jubouri and his colleagues assessed reinterventions and outcomes after EVAR and open AAA repair over a long time period, and found decreasing rates of reintervention after EVAR, which they attribute to improvements in technology from first to third and later-generation devices. I would concur with the one discussant, that some of the decrease may also be due to the understanding that not all type II endoleaks require repair. Further, much of the decrease may be due to physician experience – both with appropriate patient and device selection, and technical expertise, including with deployment. However, regardless of the underlying reason for the improvement in the reintervention rate, it is heartening that reintervention is decreasing as physicians become more facile, and industry provides technological improvements to the devices.
Dr. Linda Harris, FACS, is division chief and program director of vascular surgery at State University of New York, Buffalo. Dr. Harris has no disclosures
Dr. Mustafa Al-Jubouri and his colleagues assessed reinterventions and outcomes after EVAR and open AAA repair over a long time period, and found decreasing rates of reintervention after EVAR, which they attribute to improvements in technology from first to third and later-generation devices. I would concur with the one discussant, that some of the decrease may also be due to the understanding that not all type II endoleaks require repair. Further, much of the decrease may be due to physician experience – both with appropriate patient and device selection, and technical expertise, including with deployment. However, regardless of the underlying reason for the improvement in the reintervention rate, it is heartening that reintervention is decreasing as physicians become more facile, and industry provides technological improvements to the devices.
Dr. Linda Harris, FACS, is division chief and program director of vascular surgery at State University of New York, Buffalo. Dr. Harris has no disclosures
INDIANAPOLIS – Reintervention rates following endovascular repair of abdominal aortic aneurysms have fallen steadily with the introduction of each successive generation of endografts, while reintervention rates after open surgical repair remained stable during a recent 15-year period.
This was among the key findings from the first in-depth analysis of reinterventions occurring in contemporaneous cohorts of abdominal aortic aneurysm (AAA) patients undergoing endovascular aneurysm repair (EVAR) or open repair. The large single-center retrospective study demonstrated major differences between the two treatment strategies in terms of the incidence, nature, timing, and mortality associated with complications requiring reintervention, Dr. Mustafa Al-Jubouri said at the annual meeting of the American Surgical Association.
Dr. Al-Jubouri of Jobst Vascular Institute, Toledo, Ohio, reported on the 1,144 patients who underwent AAA repair there during 1996-2011. Forty-nine percent had EVAR, 51% open surgical repair. Beginning in 2003, more EVARs than open repairs were done annually at the Toledo institute, consistent with the experience at many major centers in the United States and elsewhere, where EVAR has become the first-line treatment based upon evidence that it offers lower operative mortality, less blood loss, and shorter ICU and hospital lengths of stay.
These advantages come at a cost, however: namely, a greater rate of secondary interventions, mainly due to device migration, failure, or endoleaks. The purpose of Dr. Al-Jubouri’s study was to evaluate the rates and reasons for reintervention over time in the two cohorts, as well as the impact of reintervention on long-term survival.
Reintervention was required in 13.6% of the EVAR group during a mean follow-up of 4.58 years, and in 5.1% of the open surgery group during 6.58 years. A single reintervention occurred in 7.9% of EVAR patients and 3.6% of the open repair group. More than one reintervention was required in 5.8% of EVAR patients compared to just 1.6% of the open repair group.
The types and timing of complications leading to reintervention were very different in the two groups. Sixty-eight percent of reinterventions in the EVAR group were for treatment of endoleaks. Another 11.5% were to address device migration, and an equal number were for occlusion.
In contrast, the three most frequent causes of reintervention in the open repair group were colonic ischemia, accounting for 30.4% of reintervention procedures; severe bleeding, 21.7%; and incisional hernia, which triggered another 21.7% of reinterventions.
Notably, 60% of all reinterventions in the open repair group occurred during the initial hospitalization, while less than 7% of reinterventions in the EVAR patients happened within 1 month of the index procedure and only one-third within the first year, the surgeon continued.
Thirty-day mortality in EVAR patients who underwent reintervention within the first month was zero, compared to a 23.3% mortality rate in open repair patients requiring reintervention within 1 month. However, when patients did not require early reintervention, 30-day mortality rates in the two groups did not differ significantly: 1.9% in EVAR group and in the open repair group. That means when patients in the open surgery group required early reintervention, their mortality rate shot up sevenfold.
After the first 30 days post-index procedure, long-term survival rates in the two groups were similar.
Need for reintervention in the open repair group was strongly related to larger aneurysm size. In contrast, reintervention rates were similar in the EVAR group regardless of aneurysm size.
A first reintervention after EVAR occurred in 23.7% of patients who received a first-generation endograft, such as the Ancure or Talent; in 16.2% of those who got the second-generation AneuRx endograft; and in 9.1% with a third-generation endograft, such as the Excluder, Endurant, Powerlink, or Zenith. The annualized rate of reintervention during the first 3 years of follow-up was 6.8% per year with first-generation devices, 7.2% per year with second-generation endografts, and significantly lower at 3.4% per year with the third-generation.
One major reason reintervention rates in EVAR patients have declined over time is that each newer generation of endograft is lower-profile, easier to deploy, and more durable. Also, many of the surgeons now putting in third-generation endografts were performing EVAR 15 years ago; they’re very experienced operators, Dr. Al-Jubouri noted.
Discussant Dr. James R. Debord proposed another explanation for the decrease in EVAR reinterventions over time.
"Isn’t it much more likely that it’s due to recognition of the fact that many of these type 2 endoleaks that we used to intervene on early on don’t require reintervention unless there’s sac enlargement?" commented Dr. Debord, professor of clinical surgery and chief of vascular surgery at the University of Illinois at Peoria.
Dr. Al-Jubouri concurred that this is an important factor in the declining rate of EVAR reinterventions.
"We saw a significant decrease in reinterventions for type 2 endoleaks between the first, second, and third generations," he said.
Asked how his study findings have changed the follow-up protocols at Jobst Vascular Institute, the surgeon replied that in the early years of the series EVAR patients got a CT scan at 6 weeks, 6 months, 1 year, and annually thereafter. This evaluation has evolved over time. Now EVAR patients get a CT scan at 6-12 weeks, and duplex ultrasounds at 6 months, 1 year, and annually thereafter.
"There is no standardized follow-up for open repair patients. However, most [patients] get an annual duplex ultrasound for their follow-up. A CT scan is not part of the follow-up of patients with open repair. But most if not all of the complications that developed in the open repair group were symptomatic," he explained.
He reported having no financial conflicts.
INDIANAPOLIS – Reintervention rates following endovascular repair of abdominal aortic aneurysms have fallen steadily with the introduction of each successive generation of endografts, while reintervention rates after open surgical repair remained stable during a recent 15-year period.
This was among the key findings from the first in-depth analysis of reinterventions occurring in contemporaneous cohorts of abdominal aortic aneurysm (AAA) patients undergoing endovascular aneurysm repair (EVAR) or open repair. The large single-center retrospective study demonstrated major differences between the two treatment strategies in terms of the incidence, nature, timing, and mortality associated with complications requiring reintervention, Dr. Mustafa Al-Jubouri said at the annual meeting of the American Surgical Association.
Dr. Al-Jubouri of Jobst Vascular Institute, Toledo, Ohio, reported on the 1,144 patients who underwent AAA repair there during 1996-2011. Forty-nine percent had EVAR, 51% open surgical repair. Beginning in 2003, more EVARs than open repairs were done annually at the Toledo institute, consistent with the experience at many major centers in the United States and elsewhere, where EVAR has become the first-line treatment based upon evidence that it offers lower operative mortality, less blood loss, and shorter ICU and hospital lengths of stay.
These advantages come at a cost, however: namely, a greater rate of secondary interventions, mainly due to device migration, failure, or endoleaks. The purpose of Dr. Al-Jubouri’s study was to evaluate the rates and reasons for reintervention over time in the two cohorts, as well as the impact of reintervention on long-term survival.
Reintervention was required in 13.6% of the EVAR group during a mean follow-up of 4.58 years, and in 5.1% of the open surgery group during 6.58 years. A single reintervention occurred in 7.9% of EVAR patients and 3.6% of the open repair group. More than one reintervention was required in 5.8% of EVAR patients compared to just 1.6% of the open repair group.
The types and timing of complications leading to reintervention were very different in the two groups. Sixty-eight percent of reinterventions in the EVAR group were for treatment of endoleaks. Another 11.5% were to address device migration, and an equal number were for occlusion.
In contrast, the three most frequent causes of reintervention in the open repair group were colonic ischemia, accounting for 30.4% of reintervention procedures; severe bleeding, 21.7%; and incisional hernia, which triggered another 21.7% of reinterventions.
Notably, 60% of all reinterventions in the open repair group occurred during the initial hospitalization, while less than 7% of reinterventions in the EVAR patients happened within 1 month of the index procedure and only one-third within the first year, the surgeon continued.
Thirty-day mortality in EVAR patients who underwent reintervention within the first month was zero, compared to a 23.3% mortality rate in open repair patients requiring reintervention within 1 month. However, when patients did not require early reintervention, 30-day mortality rates in the two groups did not differ significantly: 1.9% in EVAR group and in the open repair group. That means when patients in the open surgery group required early reintervention, their mortality rate shot up sevenfold.
After the first 30 days post-index procedure, long-term survival rates in the two groups were similar.
Need for reintervention in the open repair group was strongly related to larger aneurysm size. In contrast, reintervention rates were similar in the EVAR group regardless of aneurysm size.
A first reintervention after EVAR occurred in 23.7% of patients who received a first-generation endograft, such as the Ancure or Talent; in 16.2% of those who got the second-generation AneuRx endograft; and in 9.1% with a third-generation endograft, such as the Excluder, Endurant, Powerlink, or Zenith. The annualized rate of reintervention during the first 3 years of follow-up was 6.8% per year with first-generation devices, 7.2% per year with second-generation endografts, and significantly lower at 3.4% per year with the third-generation.
One major reason reintervention rates in EVAR patients have declined over time is that each newer generation of endograft is lower-profile, easier to deploy, and more durable. Also, many of the surgeons now putting in third-generation endografts were performing EVAR 15 years ago; they’re very experienced operators, Dr. Al-Jubouri noted.
Discussant Dr. James R. Debord proposed another explanation for the decrease in EVAR reinterventions over time.
"Isn’t it much more likely that it’s due to recognition of the fact that many of these type 2 endoleaks that we used to intervene on early on don’t require reintervention unless there’s sac enlargement?" commented Dr. Debord, professor of clinical surgery and chief of vascular surgery at the University of Illinois at Peoria.
Dr. Al-Jubouri concurred that this is an important factor in the declining rate of EVAR reinterventions.
"We saw a significant decrease in reinterventions for type 2 endoleaks between the first, second, and third generations," he said.
Asked how his study findings have changed the follow-up protocols at Jobst Vascular Institute, the surgeon replied that in the early years of the series EVAR patients got a CT scan at 6 weeks, 6 months, 1 year, and annually thereafter. This evaluation has evolved over time. Now EVAR patients get a CT scan at 6-12 weeks, and duplex ultrasounds at 6 months, 1 year, and annually thereafter.
"There is no standardized follow-up for open repair patients. However, most [patients] get an annual duplex ultrasound for their follow-up. A CT scan is not part of the follow-up of patients with open repair. But most if not all of the complications that developed in the open repair group were symptomatic," he explained.
He reported having no financial conflicts.
AT THE ASA ANNUAL MEETING
Major Finding: Reintervention rates were markedly higher following endovascular repair compared with open surgical repair of abdominal aortic aneurysms, but the adverse effects associated with reintervention after open repair were far more serious.
Data Source: A retrospective study of the 15-year experience at a large-volume vascular surgery. It encompassed 1,144 patients who underwent abdominal aortic aneurysm repair and their subsequent reintervention rates.
Disclosures: The presenter reported having no conflicts of interest.
Endovascular AAA repair superior for kidney disease patients
INDIANAPOLIS – Contrary to conventional wisdom, endovascular aneurysm repair (EVAR) provides outcomes superior to those achieved with open surgical repair of abdominal aortic aneurysm in patients with chronic renal insufficiency, a large study indicates.
"EVAR should be the first-line therapy in the patient with chronic renal insufficiency when the patient has the appropriate anatomy. However, in patients with severe renal impairment, a higher threshold should be applied for repair because the risks of both open repair and EVAR are significantly higher," Dr. Bao-Ngoc H. Nguyen declared at the annual meeting of the American Surgical Association.
"Chronic renal failure is quite prevalent in patients with abdominal aortic aneurysm: up to 30%. It is quite worrisome because any further decline in renal function in these patients could push them toward dialysis. More than that, postoperative renal failure is a predictor for early and late mortality," noted Dr. Nguyen of George Washington University, Washington.
She presented a retrospective study in patients with abdominal aortic aneurysm and chronic kidney disease. The aim, she explained, was to answer a key question: "Which one of these two treatment modalities is the lesser of two evils?"
For answers, Dr. Nguyen and coinvestigators turned to the American College of Surgeons National Quality Improvement Program (NSQIP) database for 2005-2010. They identified 3,523 patients with moderate chronic renal insufficiency, defined as an estimated glomerular filtration rate (eGFR) of 30-60 mL/minute, who underwent EVAR for abdominal aortic aneurysm and 1,117 treated via open surgical repair. Another 363 EVAR patients had severe chronic renal insufficiency, with an eGFR of less than 30 mL/minute, as did 139 patients who underwent open repair. Vascular surgeons performed all procedures in this study.
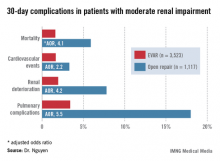
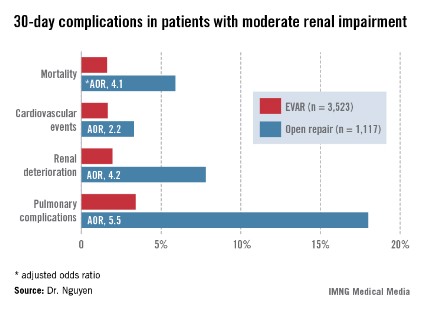
Patients with moderate renal insufficiency who underwent EVAR had markedly lower 30-day rates of mortality, pulmonary complications, cardiovascular events, and postoperative renal dysfunction, including acute kidney injury, than did those who had open surgical repair. One or more adverse events occurred in 6% of the EVAR group, compared with 24.1% of open repair patients. In a multivariate analysis controlled for preoperative differences in the patient groups, those undergoing open repair had an adjusted 4.1-fold greater risk of mortality as well as a 2.2-fold increased risk of cardiovascular events, a 4.2-fold increased risk of renal deterioration including a 5.2-fold greater risk of dialysis, and additional hazards.
In contrast, among the much smaller population of patients with baseline severe chronic renal insufficiency, there was no significant difference between the two treatment groups in terms of 30-day mortality, postoperative renal deterioration, or cardiovascular complications, although pulmonary complications were an adjusted fivefold more likely in the open surgery than among EVAR patients. Of note, rates of all adverse outcomes were markedly higher in both groups than in those with moderate chronic renal insufficiency, such that one or more adverse events occurred in 16.9% of EVAR patients and 42.5% of the open repair patients with severe chronic renal insufficiency.
Discussant Dr. Michael Watkins commented that this study has one glaring shortcoming resulting from a limitation of the NSQIP database.
While NSQIP contains only validated data entered by unbiased, well-trained professionals and NSQIP is "far superior" to the various administrative databases commonly used in evaluating outcomes, it doesn’t include key details about patients’ presenting anatomy, observed Dr. Watkins, director of the vascular research laboratory at Massachusetts General Hospital, Boston.
"Was the anatomy really similar in the two groups, or were patients who underwent open repair not candidates for EVAR?" he asked.
Dr. Nguyen conceded that this constitutes a major study limitation, adding that she agrees with Dr. Watkins that anatomy should be the first and foremost factor considered in deciding upon the surgical approach in abdominal aortic aneurysm repair.
She reported having no financial conflicts.
INDIANAPOLIS – Contrary to conventional wisdom, endovascular aneurysm repair (EVAR) provides outcomes superior to those achieved with open surgical repair of abdominal aortic aneurysm in patients with chronic renal insufficiency, a large study indicates.
"EVAR should be the first-line therapy in the patient with chronic renal insufficiency when the patient has the appropriate anatomy. However, in patients with severe renal impairment, a higher threshold should be applied for repair because the risks of both open repair and EVAR are significantly higher," Dr. Bao-Ngoc H. Nguyen declared at the annual meeting of the American Surgical Association.
"Chronic renal failure is quite prevalent in patients with abdominal aortic aneurysm: up to 30%. It is quite worrisome because any further decline in renal function in these patients could push them toward dialysis. More than that, postoperative renal failure is a predictor for early and late mortality," noted Dr. Nguyen of George Washington University, Washington.
She presented a retrospective study in patients with abdominal aortic aneurysm and chronic kidney disease. The aim, she explained, was to answer a key question: "Which one of these two treatment modalities is the lesser of two evils?"
For answers, Dr. Nguyen and coinvestigators turned to the American College of Surgeons National Quality Improvement Program (NSQIP) database for 2005-2010. They identified 3,523 patients with moderate chronic renal insufficiency, defined as an estimated glomerular filtration rate (eGFR) of 30-60 mL/minute, who underwent EVAR for abdominal aortic aneurysm and 1,117 treated via open surgical repair. Another 363 EVAR patients had severe chronic renal insufficiency, with an eGFR of less than 30 mL/minute, as did 139 patients who underwent open repair. Vascular surgeons performed all procedures in this study.
Patients with moderate renal insufficiency who underwent EVAR had markedly lower 30-day rates of mortality, pulmonary complications, cardiovascular events, and postoperative renal dysfunction, including acute kidney injury, than did those who had open surgical repair. One or more adverse events occurred in 6% of the EVAR group, compared with 24.1% of open repair patients. In a multivariate analysis controlled for preoperative differences in the patient groups, those undergoing open repair had an adjusted 4.1-fold greater risk of mortality as well as a 2.2-fold increased risk of cardiovascular events, a 4.2-fold increased risk of renal deterioration including a 5.2-fold greater risk of dialysis, and additional hazards.
In contrast, among the much smaller population of patients with baseline severe chronic renal insufficiency, there was no significant difference between the two treatment groups in terms of 30-day mortality, postoperative renal deterioration, or cardiovascular complications, although pulmonary complications were an adjusted fivefold more likely in the open surgery than among EVAR patients. Of note, rates of all adverse outcomes were markedly higher in both groups than in those with moderate chronic renal insufficiency, such that one or more adverse events occurred in 16.9% of EVAR patients and 42.5% of the open repair patients with severe chronic renal insufficiency.
Discussant Dr. Michael Watkins commented that this study has one glaring shortcoming resulting from a limitation of the NSQIP database.
While NSQIP contains only validated data entered by unbiased, well-trained professionals and NSQIP is "far superior" to the various administrative databases commonly used in evaluating outcomes, it doesn’t include key details about patients’ presenting anatomy, observed Dr. Watkins, director of the vascular research laboratory at Massachusetts General Hospital, Boston.
"Was the anatomy really similar in the two groups, or were patients who underwent open repair not candidates for EVAR?" he asked.
Dr. Nguyen conceded that this constitutes a major study limitation, adding that she agrees with Dr. Watkins that anatomy should be the first and foremost factor considered in deciding upon the surgical approach in abdominal aortic aneurysm repair.
She reported having no financial conflicts.
INDIANAPOLIS – Contrary to conventional wisdom, endovascular aneurysm repair (EVAR) provides outcomes superior to those achieved with open surgical repair of abdominal aortic aneurysm in patients with chronic renal insufficiency, a large study indicates.
"EVAR should be the first-line therapy in the patient with chronic renal insufficiency when the patient has the appropriate anatomy. However, in patients with severe renal impairment, a higher threshold should be applied for repair because the risks of both open repair and EVAR are significantly higher," Dr. Bao-Ngoc H. Nguyen declared at the annual meeting of the American Surgical Association.
"Chronic renal failure is quite prevalent in patients with abdominal aortic aneurysm: up to 30%. It is quite worrisome because any further decline in renal function in these patients could push them toward dialysis. More than that, postoperative renal failure is a predictor for early and late mortality," noted Dr. Nguyen of George Washington University, Washington.
She presented a retrospective study in patients with abdominal aortic aneurysm and chronic kidney disease. The aim, she explained, was to answer a key question: "Which one of these two treatment modalities is the lesser of two evils?"
For answers, Dr. Nguyen and coinvestigators turned to the American College of Surgeons National Quality Improvement Program (NSQIP) database for 2005-2010. They identified 3,523 patients with moderate chronic renal insufficiency, defined as an estimated glomerular filtration rate (eGFR) of 30-60 mL/minute, who underwent EVAR for abdominal aortic aneurysm and 1,117 treated via open surgical repair. Another 363 EVAR patients had severe chronic renal insufficiency, with an eGFR of less than 30 mL/minute, as did 139 patients who underwent open repair. Vascular surgeons performed all procedures in this study.
Patients with moderate renal insufficiency who underwent EVAR had markedly lower 30-day rates of mortality, pulmonary complications, cardiovascular events, and postoperative renal dysfunction, including acute kidney injury, than did those who had open surgical repair. One or more adverse events occurred in 6% of the EVAR group, compared with 24.1% of open repair patients. In a multivariate analysis controlled for preoperative differences in the patient groups, those undergoing open repair had an adjusted 4.1-fold greater risk of mortality as well as a 2.2-fold increased risk of cardiovascular events, a 4.2-fold increased risk of renal deterioration including a 5.2-fold greater risk of dialysis, and additional hazards.
In contrast, among the much smaller population of patients with baseline severe chronic renal insufficiency, there was no significant difference between the two treatment groups in terms of 30-day mortality, postoperative renal deterioration, or cardiovascular complications, although pulmonary complications were an adjusted fivefold more likely in the open surgery than among EVAR patients. Of note, rates of all adverse outcomes were markedly higher in both groups than in those with moderate chronic renal insufficiency, such that one or more adverse events occurred in 16.9% of EVAR patients and 42.5% of the open repair patients with severe chronic renal insufficiency.
Discussant Dr. Michael Watkins commented that this study has one glaring shortcoming resulting from a limitation of the NSQIP database.
While NSQIP contains only validated data entered by unbiased, well-trained professionals and NSQIP is "far superior" to the various administrative databases commonly used in evaluating outcomes, it doesn’t include key details about patients’ presenting anatomy, observed Dr. Watkins, director of the vascular research laboratory at Massachusetts General Hospital, Boston.
"Was the anatomy really similar in the two groups, or were patients who underwent open repair not candidates for EVAR?" he asked.
Dr. Nguyen conceded that this constitutes a major study limitation, adding that she agrees with Dr. Watkins that anatomy should be the first and foremost factor considered in deciding upon the surgical approach in abdominal aortic aneurysm repair.
She reported having no financial conflicts.
AT THE ASA ANNUAL MEETING
Major Finding: Patients with moderate chronic renal insufficiency who underwent open surgical repair of abdominal aortic aneurysm had a 4.2-fold greater risk of postoperative renal deterioration than did those who had an endovascular aneurysm repair.
Data Source: A retrospective study of a large national surgical database.
Disclosures: The presenter reported having no conflicts of interest.
The diabetic foot: Intervene for vascular disease
MIAMI BEACH – The extent of vascular disease, not the presence of diabetes or the cause of the ulceration, should drive the decision to intervene in a patient with a "diabetic foot," according to Dr. Joshua Beckman.
"When do I intervene on a diabetic foot? When I intervene on all feet: when there’s inadequate blood flow to heal the lesion," he said at the International Symposium on Endovascular Therapy 2013.
When lesions are detected in diabetic patients with PAD, assess for ankle pressures and determine ankle brachial index regardless of whether the lesion is neuropathic, he advised, explaining that, "even if you have a neuropathic initiation for your ulcer, if you have a low perfusion pressure, particularly under 50 mmHg, you’re not going to heal that lesion no matter how it was started."
"I always assess patients, whether I think the lesion is a neuropathic one or not, for the ankle pressures and do ankle brachial index and, assuming it’s low, I refer for revascularization based on the same criteria I would everybody else," said Dr. Beckman of Harvard Medical School, Boston.
That said, up to one-third of patients with diabetes have peripheral artery disease, and its presence is an independent predictor of adverse outcomes. "In fact, the combination of PAD and diabetes represents the group of people in the United States that has the most amputations, and their risk may be 20-fold that of people without both PAD and diabetes," he noted.
Indeed, the risk of amputation is much greater in diabetic patients, regardless of the care they receive, Dr. Beckman said, citing a study of 136 lower-extremity angiograms in patients with PAD. The odds ratios for amputation and death at 4.5 years’ follow-up were 5.4 for those with diabetes and 3.1 for those without diabetes (Diabetes Care 2001;24:1433-7).
Additionally, in a study of 1,244 men with claudication, the risk of ischemic ulceration was 4-fold higher in diabetic patients than in nondiabetic patients. Ankle brachial indices ranged from 0.9 to 0.1, and there was no point at which a diabetic patient was not worse off than a nondiabetic patient, he said. The risk in a diabetic patient with an ankle brachial index of 0.9 was equal to the risk in a nondiabetic patient with an ankle brachial index of 0.1 (J. Vasc. Surg. 2001;34:962-70).
Diabetic patients also can have a number of other initiators of diabetic foot ulceration, including neuropathy, bone deformity, callus, penetrating injury, and ill-fitting shoes. In combination, these factors can be especially problematic, he said.
Also, the extensor muscle abnormality that can result from motor neuropathy can change foot morphology, thereby increasing pressure on areas such as the ball of the foot, where ulcers are common.
Furthermore, a patient with neuropathy can walk around for a long time without recognizing a problem that an individual with normal nerve function wouldn’t tolerate. "A diabetic patient can have a bad shoe that rubs all day and not appreciate it," Dr. Beckman said.
With PAD, there are a "whole host of issues we never deal with in the nondiabetic patient that really accelerate the pathway toward ischemic ulceration and critical limb ischemia," he said. "Diabetes is a risk multiplier. It makes things worse, and it’s far more involved in the initiation [of ulceration] than in the decision whether to fix somebody."
Further underscoring the importance of such follow-up is a study of 533 diabetic patients hospitalized for critical limb ischemia. Nearly 50% of them developed critical limb ischemia in the contralateral limb at 6-year follow-up (Diabetes Res. Clin. Prac. 2007;77:445-50).
"So this is a group of people who need not only exquisite care, but exquisite surveillance, because it is very likely that, over time, they will come back to your office with the same problem in the other foot. I’m not recommending intervention or surgery in the asymptomatic foot, but I am suggesting you should probably never let them go, and have routine follow-ups where you look at their feet every time," he said.
Dr. Beckman reported having no disclosures relevant to his talk.
MIAMI BEACH – The extent of vascular disease, not the presence of diabetes or the cause of the ulceration, should drive the decision to intervene in a patient with a "diabetic foot," according to Dr. Joshua Beckman.
"When do I intervene on a diabetic foot? When I intervene on all feet: when there’s inadequate blood flow to heal the lesion," he said at the International Symposium on Endovascular Therapy 2013.
When lesions are detected in diabetic patients with PAD, assess for ankle pressures and determine ankle brachial index regardless of whether the lesion is neuropathic, he advised, explaining that, "even if you have a neuropathic initiation for your ulcer, if you have a low perfusion pressure, particularly under 50 mmHg, you’re not going to heal that lesion no matter how it was started."
"I always assess patients, whether I think the lesion is a neuropathic one or not, for the ankle pressures and do ankle brachial index and, assuming it’s low, I refer for revascularization based on the same criteria I would everybody else," said Dr. Beckman of Harvard Medical School, Boston.
That said, up to one-third of patients with diabetes have peripheral artery disease, and its presence is an independent predictor of adverse outcomes. "In fact, the combination of PAD and diabetes represents the group of people in the United States that has the most amputations, and their risk may be 20-fold that of people without both PAD and diabetes," he noted.
Indeed, the risk of amputation is much greater in diabetic patients, regardless of the care they receive, Dr. Beckman said, citing a study of 136 lower-extremity angiograms in patients with PAD. The odds ratios for amputation and death at 4.5 years’ follow-up were 5.4 for those with diabetes and 3.1 for those without diabetes (Diabetes Care 2001;24:1433-7).
Additionally, in a study of 1,244 men with claudication, the risk of ischemic ulceration was 4-fold higher in diabetic patients than in nondiabetic patients. Ankle brachial indices ranged from 0.9 to 0.1, and there was no point at which a diabetic patient was not worse off than a nondiabetic patient, he said. The risk in a diabetic patient with an ankle brachial index of 0.9 was equal to the risk in a nondiabetic patient with an ankle brachial index of 0.1 (J. Vasc. Surg. 2001;34:962-70).
Diabetic patients also can have a number of other initiators of diabetic foot ulceration, including neuropathy, bone deformity, callus, penetrating injury, and ill-fitting shoes. In combination, these factors can be especially problematic, he said.
Also, the extensor muscle abnormality that can result from motor neuropathy can change foot morphology, thereby increasing pressure on areas such as the ball of the foot, where ulcers are common.
Furthermore, a patient with neuropathy can walk around for a long time without recognizing a problem that an individual with normal nerve function wouldn’t tolerate. "A diabetic patient can have a bad shoe that rubs all day and not appreciate it," Dr. Beckman said.
With PAD, there are a "whole host of issues we never deal with in the nondiabetic patient that really accelerate the pathway toward ischemic ulceration and critical limb ischemia," he said. "Diabetes is a risk multiplier. It makes things worse, and it’s far more involved in the initiation [of ulceration] than in the decision whether to fix somebody."
Further underscoring the importance of such follow-up is a study of 533 diabetic patients hospitalized for critical limb ischemia. Nearly 50% of them developed critical limb ischemia in the contralateral limb at 6-year follow-up (Diabetes Res. Clin. Prac. 2007;77:445-50).
"So this is a group of people who need not only exquisite care, but exquisite surveillance, because it is very likely that, over time, they will come back to your office with the same problem in the other foot. I’m not recommending intervention or surgery in the asymptomatic foot, but I am suggesting you should probably never let them go, and have routine follow-ups where you look at their feet every time," he said.
Dr. Beckman reported having no disclosures relevant to his talk.
MIAMI BEACH – The extent of vascular disease, not the presence of diabetes or the cause of the ulceration, should drive the decision to intervene in a patient with a "diabetic foot," according to Dr. Joshua Beckman.
"When do I intervene on a diabetic foot? When I intervene on all feet: when there’s inadequate blood flow to heal the lesion," he said at the International Symposium on Endovascular Therapy 2013.
When lesions are detected in diabetic patients with PAD, assess for ankle pressures and determine ankle brachial index regardless of whether the lesion is neuropathic, he advised, explaining that, "even if you have a neuropathic initiation for your ulcer, if you have a low perfusion pressure, particularly under 50 mmHg, you’re not going to heal that lesion no matter how it was started."
"I always assess patients, whether I think the lesion is a neuropathic one or not, for the ankle pressures and do ankle brachial index and, assuming it’s low, I refer for revascularization based on the same criteria I would everybody else," said Dr. Beckman of Harvard Medical School, Boston.
That said, up to one-third of patients with diabetes have peripheral artery disease, and its presence is an independent predictor of adverse outcomes. "In fact, the combination of PAD and diabetes represents the group of people in the United States that has the most amputations, and their risk may be 20-fold that of people without both PAD and diabetes," he noted.
Indeed, the risk of amputation is much greater in diabetic patients, regardless of the care they receive, Dr. Beckman said, citing a study of 136 lower-extremity angiograms in patients with PAD. The odds ratios for amputation and death at 4.5 years’ follow-up were 5.4 for those with diabetes and 3.1 for those without diabetes (Diabetes Care 2001;24:1433-7).
Additionally, in a study of 1,244 men with claudication, the risk of ischemic ulceration was 4-fold higher in diabetic patients than in nondiabetic patients. Ankle brachial indices ranged from 0.9 to 0.1, and there was no point at which a diabetic patient was not worse off than a nondiabetic patient, he said. The risk in a diabetic patient with an ankle brachial index of 0.9 was equal to the risk in a nondiabetic patient with an ankle brachial index of 0.1 (J. Vasc. Surg. 2001;34:962-70).
Diabetic patients also can have a number of other initiators of diabetic foot ulceration, including neuropathy, bone deformity, callus, penetrating injury, and ill-fitting shoes. In combination, these factors can be especially problematic, he said.
Also, the extensor muscle abnormality that can result from motor neuropathy can change foot morphology, thereby increasing pressure on areas such as the ball of the foot, where ulcers are common.
Furthermore, a patient with neuropathy can walk around for a long time without recognizing a problem that an individual with normal nerve function wouldn’t tolerate. "A diabetic patient can have a bad shoe that rubs all day and not appreciate it," Dr. Beckman said.
With PAD, there are a "whole host of issues we never deal with in the nondiabetic patient that really accelerate the pathway toward ischemic ulceration and critical limb ischemia," he said. "Diabetes is a risk multiplier. It makes things worse, and it’s far more involved in the initiation [of ulceration] than in the decision whether to fix somebody."
Further underscoring the importance of such follow-up is a study of 533 diabetic patients hospitalized for critical limb ischemia. Nearly 50% of them developed critical limb ischemia in the contralateral limb at 6-year follow-up (Diabetes Res. Clin. Prac. 2007;77:445-50).
"So this is a group of people who need not only exquisite care, but exquisite surveillance, because it is very likely that, over time, they will come back to your office with the same problem in the other foot. I’m not recommending intervention or surgery in the asymptomatic foot, but I am suggesting you should probably never let them go, and have routine follow-ups where you look at their feet every time," he said.
Dr. Beckman reported having no disclosures relevant to his talk.
EXPERT ANALYSIS FROM ISET 2013
Endurant stent proves durable at 2 years
MIAMI BEACH – At 2 years, the Endurant stent graft was durable for abdominal aortic aneurysm, based on findings from a registry of nearly 1,300 patients.
Freedom from aneurysm-related death was 98% in the 500 registry participants who were followed for at least 2 years, reported Dr. Dittmar Böckler, of University Hospital Heidelberg, Germany.
Procedural success was nearly 98% in 1,263 participants in the ENGAGE Global Registry of patients treated with Medtronic’s Endurant system. The findings offer encouragement about "real world" endovascular aneurysm repair (EVAR) and new-generation EVAR devices, Dr. Böckler said at the International Symposium on Endovascular Therapy 2013.
Procedural success was based on a composite of technical success (99%), freedom from intraoperative death (100%), and freedom from type I and III endoleaks (98.6%). Also, there was freedom from reinterventions at 1 year (95%) in all 1,236 patients and at 2 years (93%) in the 500 patients who have been followed that long.
The risk of any type of second procedure was 5.6% at 1 year and 1.6% at 2 years. The risk of a second procedure for an endoleak was 1.4% at 1 year and 1.8% at 2 years. The total endoleak rate was a "remarkably low" 9.7% at 1 year and 9.1% at 2 years, he said.
No stent migration occurred at either time point. Sac enlargement, which is known to increase the long-term risk of aneurysm rupture, was rare, occurring in 3.4% of patients at 1 year and 2.9% at 2 years, Dr. Böckler said.
As for EVAR treatment failure, the conversion rate was "very acceptable" at 0.6% and 0.8% at 1 and 2 years, respectively, and the rupture rate was 0.2% at 1 year and remained the same at 2 years, he noted.
Stent graft occlusion occurred in 3.5% at 1 year and in 2.7% at 2 years. Similar patency was seen in 150 patients from the Endurant U.S. Investigational Device Exemption (IDE) trial; at 2 years, 3.1% of patients in that trial had occlusions.
The ENGAGE findings compare favorably with those of other studies of EVAR, including the DREAM (Dutch Randomized Endovascular Aneurysm Management) study and the OVER (Open Vs. Endovascular Repair) trial, he said.
Of note, the need for secondary intervention was halved with the Endurant system; it was 6.4% in ENGAGE and 6.1% in Endurant U.S. IDE, compared with 13.7% in OVER and 12% in DREAM.
"The clinical effectiveness of EVAR is well established," Dr. Böckler said, citing the outcomes of numerous EVAR trials. The 2-year outcomes begin to address the remaining concerns and questions, including whether outcomes are durable, whether performance outside of controlled trials will match that seen in trials, whether newer technology will perform as well as or better than older technology, and whether it will perform as well in real-world settings and in different populations and practices.
The registry – a multicenter, postmarket, noninterventional, nonrandomized prospective study – includes 1,263 patients enrolled between March 2009 and April 2011 to assess real-world safety and clinical performance of the Endurant stent graft system. Patients will be followed for 5 years. ENGAGE is the largest contemporary EVAR registry for a single manufacturer’s stent, and it includes a database that can be pooled and compared with other available stent graft data, according to Dr. Böckler.
Of note, the registry participants include a challenging patient population: 16% had symptomatic abdominal aortic aneurysms, 10.6% were classed as ASA (American Society of Anesthesiologists) IV (having severe systemic disease that is a constant threat to life), and 10.5% were female patients with narrow access arteries.
Although more patients and additional follow-up are needed to prove long-term efficacy, these 2-year findings demonstrate that real-world EVAR practice with the Endurant stent graft system provides very good and durable results, Dr. Böckler said. "EVAR is getting better with the new-generation devices."
Dr. Böckler has served as a consultant, advisory board member, or speaker for Endologix, Endomax, Gore, Medtronic, Siemens, and Maquet. He has received research or grant support from Gore, Maquet, Medtronic, and Siemens.
MIAMI BEACH – At 2 years, the Endurant stent graft was durable for abdominal aortic aneurysm, based on findings from a registry of nearly 1,300 patients.
Freedom from aneurysm-related death was 98% in the 500 registry participants who were followed for at least 2 years, reported Dr. Dittmar Böckler, of University Hospital Heidelberg, Germany.
Procedural success was nearly 98% in 1,263 participants in the ENGAGE Global Registry of patients treated with Medtronic’s Endurant system. The findings offer encouragement about "real world" endovascular aneurysm repair (EVAR) and new-generation EVAR devices, Dr. Böckler said at the International Symposium on Endovascular Therapy 2013.
Procedural success was based on a composite of technical success (99%), freedom from intraoperative death (100%), and freedom from type I and III endoleaks (98.6%). Also, there was freedom from reinterventions at 1 year (95%) in all 1,236 patients and at 2 years (93%) in the 500 patients who have been followed that long.
The risk of any type of second procedure was 5.6% at 1 year and 1.6% at 2 years. The risk of a second procedure for an endoleak was 1.4% at 1 year and 1.8% at 2 years. The total endoleak rate was a "remarkably low" 9.7% at 1 year and 9.1% at 2 years, he said.
No stent migration occurred at either time point. Sac enlargement, which is known to increase the long-term risk of aneurysm rupture, was rare, occurring in 3.4% of patients at 1 year and 2.9% at 2 years, Dr. Böckler said.
As for EVAR treatment failure, the conversion rate was "very acceptable" at 0.6% and 0.8% at 1 and 2 years, respectively, and the rupture rate was 0.2% at 1 year and remained the same at 2 years, he noted.
Stent graft occlusion occurred in 3.5% at 1 year and in 2.7% at 2 years. Similar patency was seen in 150 patients from the Endurant U.S. Investigational Device Exemption (IDE) trial; at 2 years, 3.1% of patients in that trial had occlusions.
The ENGAGE findings compare favorably with those of other studies of EVAR, including the DREAM (Dutch Randomized Endovascular Aneurysm Management) study and the OVER (Open Vs. Endovascular Repair) trial, he said.
Of note, the need for secondary intervention was halved with the Endurant system; it was 6.4% in ENGAGE and 6.1% in Endurant U.S. IDE, compared with 13.7% in OVER and 12% in DREAM.
"The clinical effectiveness of EVAR is well established," Dr. Böckler said, citing the outcomes of numerous EVAR trials. The 2-year outcomes begin to address the remaining concerns and questions, including whether outcomes are durable, whether performance outside of controlled trials will match that seen in trials, whether newer technology will perform as well as or better than older technology, and whether it will perform as well in real-world settings and in different populations and practices.
The registry – a multicenter, postmarket, noninterventional, nonrandomized prospective study – includes 1,263 patients enrolled between March 2009 and April 2011 to assess real-world safety and clinical performance of the Endurant stent graft system. Patients will be followed for 5 years. ENGAGE is the largest contemporary EVAR registry for a single manufacturer’s stent, and it includes a database that can be pooled and compared with other available stent graft data, according to Dr. Böckler.
Of note, the registry participants include a challenging patient population: 16% had symptomatic abdominal aortic aneurysms, 10.6% were classed as ASA (American Society of Anesthesiologists) IV (having severe systemic disease that is a constant threat to life), and 10.5% were female patients with narrow access arteries.
Although more patients and additional follow-up are needed to prove long-term efficacy, these 2-year findings demonstrate that real-world EVAR practice with the Endurant stent graft system provides very good and durable results, Dr. Böckler said. "EVAR is getting better with the new-generation devices."
Dr. Böckler has served as a consultant, advisory board member, or speaker for Endologix, Endomax, Gore, Medtronic, Siemens, and Maquet. He has received research or grant support from Gore, Maquet, Medtronic, and Siemens.
MIAMI BEACH – At 2 years, the Endurant stent graft was durable for abdominal aortic aneurysm, based on findings from a registry of nearly 1,300 patients.
Freedom from aneurysm-related death was 98% in the 500 registry participants who were followed for at least 2 years, reported Dr. Dittmar Böckler, of University Hospital Heidelberg, Germany.
Procedural success was nearly 98% in 1,263 participants in the ENGAGE Global Registry of patients treated with Medtronic’s Endurant system. The findings offer encouragement about "real world" endovascular aneurysm repair (EVAR) and new-generation EVAR devices, Dr. Böckler said at the International Symposium on Endovascular Therapy 2013.
Procedural success was based on a composite of technical success (99%), freedom from intraoperative death (100%), and freedom from type I and III endoleaks (98.6%). Also, there was freedom from reinterventions at 1 year (95%) in all 1,236 patients and at 2 years (93%) in the 500 patients who have been followed that long.
The risk of any type of second procedure was 5.6% at 1 year and 1.6% at 2 years. The risk of a second procedure for an endoleak was 1.4% at 1 year and 1.8% at 2 years. The total endoleak rate was a "remarkably low" 9.7% at 1 year and 9.1% at 2 years, he said.
No stent migration occurred at either time point. Sac enlargement, which is known to increase the long-term risk of aneurysm rupture, was rare, occurring in 3.4% of patients at 1 year and 2.9% at 2 years, Dr. Böckler said.
As for EVAR treatment failure, the conversion rate was "very acceptable" at 0.6% and 0.8% at 1 and 2 years, respectively, and the rupture rate was 0.2% at 1 year and remained the same at 2 years, he noted.
Stent graft occlusion occurred in 3.5% at 1 year and in 2.7% at 2 years. Similar patency was seen in 150 patients from the Endurant U.S. Investigational Device Exemption (IDE) trial; at 2 years, 3.1% of patients in that trial had occlusions.
The ENGAGE findings compare favorably with those of other studies of EVAR, including the DREAM (Dutch Randomized Endovascular Aneurysm Management) study and the OVER (Open Vs. Endovascular Repair) trial, he said.
Of note, the need for secondary intervention was halved with the Endurant system; it was 6.4% in ENGAGE and 6.1% in Endurant U.S. IDE, compared with 13.7% in OVER and 12% in DREAM.
"The clinical effectiveness of EVAR is well established," Dr. Böckler said, citing the outcomes of numerous EVAR trials. The 2-year outcomes begin to address the remaining concerns and questions, including whether outcomes are durable, whether performance outside of controlled trials will match that seen in trials, whether newer technology will perform as well as or better than older technology, and whether it will perform as well in real-world settings and in different populations and practices.
The registry – a multicenter, postmarket, noninterventional, nonrandomized prospective study – includes 1,263 patients enrolled between March 2009 and April 2011 to assess real-world safety and clinical performance of the Endurant stent graft system. Patients will be followed for 5 years. ENGAGE is the largest contemporary EVAR registry for a single manufacturer’s stent, and it includes a database that can be pooled and compared with other available stent graft data, according to Dr. Böckler.
Of note, the registry participants include a challenging patient population: 16% had symptomatic abdominal aortic aneurysms, 10.6% were classed as ASA (American Society of Anesthesiologists) IV (having severe systemic disease that is a constant threat to life), and 10.5% were female patients with narrow access arteries.
Although more patients and additional follow-up are needed to prove long-term efficacy, these 2-year findings demonstrate that real-world EVAR practice with the Endurant stent graft system provides very good and durable results, Dr. Böckler said. "EVAR is getting better with the new-generation devices."
Dr. Böckler has served as a consultant, advisory board member, or speaker for Endologix, Endomax, Gore, Medtronic, Siemens, and Maquet. He has received research or grant support from Gore, Maquet, Medtronic, and Siemens.
AT ISET 2013
Major finding: Procedural success was nearly 98% in 1,263 participants in the ENGAGE Global Registry.
Data source: ENGAGE is a multicenter, postmarket, noninterventional, nonrandomized prospective registry.
Disclosures: Dr. Böckler has served as a consultant, advisory board member, or speaker for Endologix, Endomax, Gore, Medtronic, Siemens, and Maquet. He has received research or grant support from Gore, Maquet, Medtronic, and Siemens.
Crossing devices offer solutions for failed recanalization
MIAMI BEACH – When the "wire and catheter" approach fails, crossing devices come into play for recanalizing vessels during endovascular interventions, according to Dr. John Rundback.
Specialized crossing devices may improve the ability to treat chronic total occlusions (CTOs), including calcified and long, complex lesions that can be very difficult to cross, particularly in the infrapopliteal region, said Dr. Rundback, medical director of the Interventional Institute at Holy Name Medical Center, Briarcliff Manor, N.Y.
When it comes to specialized crossing devices, there is a whole spectrum available, and many are relatively new on the market, so experience with them is limited, Dr. Rundback said at the International Symposium on Endovascular Therapy 2013.
The goal with each, however, is to remain intraluminal and to maximize the interventional options, he said, noting that goal is particularly relevant with the advent of drug-eluting balloons.
Although the data are sparse, and these devices – which are generally used in patients who have failed traditional wire and catheter crossing – have not been compared to wire and catheter techniques in a rigorous fashion, it is nonetheless clear that there are cases in which these devices will be needed.
"You have to sort of pick one or two and keep them in your lab, and gain familiarity," he said.
The approved and emerging devices he discussed include the Viance and Enteer peripheral CTO crossing devices (Covidien), the Crosser CTO device (Bard Peripheral Vascular), the Wildcat and Kittycat CTO devices (Avinger), and the TruePath crossing device (Boston Scientific).
Viance and Enteer
The Viance crossing catheter is a high-speed rotating recanalization device, and the Enteer reentry system is a unique reentry catheter. The two were studied together as a novel overall strategy, Dr. Rundback explained.
In a study involving 66 patients, which led to the recent approval of the device, CTO lesion lengths were reasonably long, much like those Dr. Runback said he sees in his practice. However, moderate to severe calcification was present in only 42% of patients, which is less than he generally sees, and a fair amount of tortuosity was present in 50%-60% of patients.
About two-thirds of the cases involved the superficial femoral artery (SFA), and the remaining cases were in the tibial circulation. Overall, the approach was safe, and the success rate was 85%, Dr. Rundback said.
The Crosser
The latest version of this device, approved in the United States for both coronary and peripheral indications, involves a dedicated hydraulic vibrational system that provides translational force through the lumen.
"It’s the one we tend to use the most in our practice, and our junior associates have had great success with this device," Dr. Rundback noted.
The Crosser device is unique in that it establishes a luminal plane where you often don’t see anything, and moves quite smoothly and easily through the lumen, he said.
In the PATRIOT (Peripheral Approach to Recanalization in Occluded Totals) study of this device, 85 guide wire–refractory peripheral CTO patients were treated with a high technical success rate of 84% and no perforations.
Most cases involved the SFA, but about a third were popliteal or below. Lesion length was reasonable (average, 117.5 mm), and about 75% of patients had old, calcified lesions.
Treatment was quick, taking only about 2 minutes on average.
"That has been our experience as well. These actually work very quickly to reestablish straight-line flow," he said, noting that it is important to be cautious, nonetheless.
"You can get extraluminal without knowing it. [The technique] requires a certain amount of practice and tactile feedback to become familiar with the utility of these devices," he said.
Wildcat and Kittycat
These devices are rotating crossing devices (Kittycat is a small-vessel device) that have shown promise in trials.
In the CONNECT (Chronic Total Occlusion Crossing with the Wildcat Catheter) trial, the technical success rate was 89%, and safety was greater than 95% in patients with an average lesion length of 174 mm, about half of whom had moderately calcified lesions.
The newest incarnations of these rotating crossing devices use optical coherence technology that allows visualization of the lumen as the occlusion is traversed.
Dr. Rundback said he has no personal experience with these devices, but said that the prospect of visualizing the position within the lumen "does have some sort of empirical appeal and may provide real, true benefit in terms of staying in the lumen."
TruePath
This FDA-approved crossing device uses a high-speed, rotating diamond-studded burr to advance through lesions.
It is entirely self-contained and easy to use, Dr. Rundback said, noting that the device uses a feedback system involving red lights and beeping sounds that are activated when resistance is encountered in the system. This provides audible, visible, and tactile feedback to help avoid going extraluminal.
In the ReOpen study of 85 patients with a mean occlusion length of 166 mm who failed guide-wire treatment, the technical success rate was 80% and the device was safe, he said.
Dr. Rundback reported having no relevant financial disclosures.
MIAMI BEACH – When the "wire and catheter" approach fails, crossing devices come into play for recanalizing vessels during endovascular interventions, according to Dr. John Rundback.
Specialized crossing devices may improve the ability to treat chronic total occlusions (CTOs), including calcified and long, complex lesions that can be very difficult to cross, particularly in the infrapopliteal region, said Dr. Rundback, medical director of the Interventional Institute at Holy Name Medical Center, Briarcliff Manor, N.Y.
When it comes to specialized crossing devices, there is a whole spectrum available, and many are relatively new on the market, so experience with them is limited, Dr. Rundback said at the International Symposium on Endovascular Therapy 2013.
The goal with each, however, is to remain intraluminal and to maximize the interventional options, he said, noting that goal is particularly relevant with the advent of drug-eluting balloons.
Although the data are sparse, and these devices – which are generally used in patients who have failed traditional wire and catheter crossing – have not been compared to wire and catheter techniques in a rigorous fashion, it is nonetheless clear that there are cases in which these devices will be needed.
"You have to sort of pick one or two and keep them in your lab, and gain familiarity," he said.
The approved and emerging devices he discussed include the Viance and Enteer peripheral CTO crossing devices (Covidien), the Crosser CTO device (Bard Peripheral Vascular), the Wildcat and Kittycat CTO devices (Avinger), and the TruePath crossing device (Boston Scientific).
Viance and Enteer
The Viance crossing catheter is a high-speed rotating recanalization device, and the Enteer reentry system is a unique reentry catheter. The two were studied together as a novel overall strategy, Dr. Rundback explained.
In a study involving 66 patients, which led to the recent approval of the device, CTO lesion lengths were reasonably long, much like those Dr. Runback said he sees in his practice. However, moderate to severe calcification was present in only 42% of patients, which is less than he generally sees, and a fair amount of tortuosity was present in 50%-60% of patients.
About two-thirds of the cases involved the superficial femoral artery (SFA), and the remaining cases were in the tibial circulation. Overall, the approach was safe, and the success rate was 85%, Dr. Rundback said.
The Crosser
The latest version of this device, approved in the United States for both coronary and peripheral indications, involves a dedicated hydraulic vibrational system that provides translational force through the lumen.
"It’s the one we tend to use the most in our practice, and our junior associates have had great success with this device," Dr. Rundback noted.
The Crosser device is unique in that it establishes a luminal plane where you often don’t see anything, and moves quite smoothly and easily through the lumen, he said.
In the PATRIOT (Peripheral Approach to Recanalization in Occluded Totals) study of this device, 85 guide wire–refractory peripheral CTO patients were treated with a high technical success rate of 84% and no perforations.
Most cases involved the SFA, but about a third were popliteal or below. Lesion length was reasonable (average, 117.5 mm), and about 75% of patients had old, calcified lesions.
Treatment was quick, taking only about 2 minutes on average.
"That has been our experience as well. These actually work very quickly to reestablish straight-line flow," he said, noting that it is important to be cautious, nonetheless.
"You can get extraluminal without knowing it. [The technique] requires a certain amount of practice and tactile feedback to become familiar with the utility of these devices," he said.
Wildcat and Kittycat
These devices are rotating crossing devices (Kittycat is a small-vessel device) that have shown promise in trials.
In the CONNECT (Chronic Total Occlusion Crossing with the Wildcat Catheter) trial, the technical success rate was 89%, and safety was greater than 95% in patients with an average lesion length of 174 mm, about half of whom had moderately calcified lesions.
The newest incarnations of these rotating crossing devices use optical coherence technology that allows visualization of the lumen as the occlusion is traversed.
Dr. Rundback said he has no personal experience with these devices, but said that the prospect of visualizing the position within the lumen "does have some sort of empirical appeal and may provide real, true benefit in terms of staying in the lumen."
TruePath
This FDA-approved crossing device uses a high-speed, rotating diamond-studded burr to advance through lesions.
It is entirely self-contained and easy to use, Dr. Rundback said, noting that the device uses a feedback system involving red lights and beeping sounds that are activated when resistance is encountered in the system. This provides audible, visible, and tactile feedback to help avoid going extraluminal.
In the ReOpen study of 85 patients with a mean occlusion length of 166 mm who failed guide-wire treatment, the technical success rate was 80% and the device was safe, he said.
Dr. Rundback reported having no relevant financial disclosures.
MIAMI BEACH – When the "wire and catheter" approach fails, crossing devices come into play for recanalizing vessels during endovascular interventions, according to Dr. John Rundback.
Specialized crossing devices may improve the ability to treat chronic total occlusions (CTOs), including calcified and long, complex lesions that can be very difficult to cross, particularly in the infrapopliteal region, said Dr. Rundback, medical director of the Interventional Institute at Holy Name Medical Center, Briarcliff Manor, N.Y.
When it comes to specialized crossing devices, there is a whole spectrum available, and many are relatively new on the market, so experience with them is limited, Dr. Rundback said at the International Symposium on Endovascular Therapy 2013.
The goal with each, however, is to remain intraluminal and to maximize the interventional options, he said, noting that goal is particularly relevant with the advent of drug-eluting balloons.
Although the data are sparse, and these devices – which are generally used in patients who have failed traditional wire and catheter crossing – have not been compared to wire and catheter techniques in a rigorous fashion, it is nonetheless clear that there are cases in which these devices will be needed.
"You have to sort of pick one or two and keep them in your lab, and gain familiarity," he said.
The approved and emerging devices he discussed include the Viance and Enteer peripheral CTO crossing devices (Covidien), the Crosser CTO device (Bard Peripheral Vascular), the Wildcat and Kittycat CTO devices (Avinger), and the TruePath crossing device (Boston Scientific).
Viance and Enteer
The Viance crossing catheter is a high-speed rotating recanalization device, and the Enteer reentry system is a unique reentry catheter. The two were studied together as a novel overall strategy, Dr. Rundback explained.
In a study involving 66 patients, which led to the recent approval of the device, CTO lesion lengths were reasonably long, much like those Dr. Runback said he sees in his practice. However, moderate to severe calcification was present in only 42% of patients, which is less than he generally sees, and a fair amount of tortuosity was present in 50%-60% of patients.
About two-thirds of the cases involved the superficial femoral artery (SFA), and the remaining cases were in the tibial circulation. Overall, the approach was safe, and the success rate was 85%, Dr. Rundback said.
The Crosser
The latest version of this device, approved in the United States for both coronary and peripheral indications, involves a dedicated hydraulic vibrational system that provides translational force through the lumen.
"It’s the one we tend to use the most in our practice, and our junior associates have had great success with this device," Dr. Rundback noted.
The Crosser device is unique in that it establishes a luminal plane where you often don’t see anything, and moves quite smoothly and easily through the lumen, he said.
In the PATRIOT (Peripheral Approach to Recanalization in Occluded Totals) study of this device, 85 guide wire–refractory peripheral CTO patients were treated with a high technical success rate of 84% and no perforations.
Most cases involved the SFA, but about a third were popliteal or below. Lesion length was reasonable (average, 117.5 mm), and about 75% of patients had old, calcified lesions.
Treatment was quick, taking only about 2 minutes on average.
"That has been our experience as well. These actually work very quickly to reestablish straight-line flow," he said, noting that it is important to be cautious, nonetheless.
"You can get extraluminal without knowing it. [The technique] requires a certain amount of practice and tactile feedback to become familiar with the utility of these devices," he said.
Wildcat and Kittycat
These devices are rotating crossing devices (Kittycat is a small-vessel device) that have shown promise in trials.
In the CONNECT (Chronic Total Occlusion Crossing with the Wildcat Catheter) trial, the technical success rate was 89%, and safety was greater than 95% in patients with an average lesion length of 174 mm, about half of whom had moderately calcified lesions.
The newest incarnations of these rotating crossing devices use optical coherence technology that allows visualization of the lumen as the occlusion is traversed.
Dr. Rundback said he has no personal experience with these devices, but said that the prospect of visualizing the position within the lumen "does have some sort of empirical appeal and may provide real, true benefit in terms of staying in the lumen."
TruePath
This FDA-approved crossing device uses a high-speed, rotating diamond-studded burr to advance through lesions.
It is entirely self-contained and easy to use, Dr. Rundback said, noting that the device uses a feedback system involving red lights and beeping sounds that are activated when resistance is encountered in the system. This provides audible, visible, and tactile feedback to help avoid going extraluminal.
In the ReOpen study of 85 patients with a mean occlusion length of 166 mm who failed guide-wire treatment, the technical success rate was 80% and the device was safe, he said.
Dr. Rundback reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM ISET 2013
SMART stent shows 75% superficial femoral patency at 2 years
MIAMI BEACH – At 2-year follow-up, the SMART stent was associated with a 75% primary patency rate in patients with obstructive superficial femoral artery disease.
The findings were noted in the STROLL study (SMART Vascular Stent Systems in the Treatment of Obstructive Superficial Femoral Artery Disease). The primary patency rate was 82% at 12 months and 75% at 24 months in 250 patients aged 30 years and older who were enrolled in the single-arm, multicenter study, Dr. William Gray reported at the International Symposium on Endovascular Therapy 2013.
At 12 months, 87% were free from target lesion revascularization; at 24 months, 80% were free from target lesion revascularization, said Dr. Gray, director of endovascular services at Columbia University Medical Center, New York.
No major adverse events were noted at 30 days after the index procedure, and the rate of stent fractures was 2% at 12 months, with no additional stent fractures occurring between 12 and 24 months.
All stent fractures were type 1, with no incidents of type II-V fractures, he reported.
Additionally, more than 80% of patients had improvement or normalization of peripheral artery disease outcomes as measured using Rutherford-Becker classification and ankle brachial index, he noted.
Study participants had de novo or restenotic native superficial femoral artery lesions or total occlusions of 4-15 cm (mean, 77 mm) in length, and reference vessel diameters of 4.0-6.0 mm. Nearly 24% had total occlusions, and 47% had diabetes.
All were treated using the SMART Control nitinol self-expanding stent system manufactured by Cordis. SMART stents have been approved for peripheral indication in international markets since 1999. In 2012, the SMART Control nitinol stent system, which was previously approved for use in the iliac arteries, was also approved by the U.S. Food and Drug Administration for use in the superficial femoral artery and/or the proximal popliteal artery.
The findings "speak well of the efficacy of the SMART stent," Dr. Gray said. Follow-up will continue until 3 years post procedure.
The STROLL study was sponsored by Cordis. Dr. Gray was a principal investigator for the study and serves as a paid advisory board member and consultant for Cordis.
MIAMI BEACH – At 2-year follow-up, the SMART stent was associated with a 75% primary patency rate in patients with obstructive superficial femoral artery disease.
The findings were noted in the STROLL study (SMART Vascular Stent Systems in the Treatment of Obstructive Superficial Femoral Artery Disease). The primary patency rate was 82% at 12 months and 75% at 24 months in 250 patients aged 30 years and older who were enrolled in the single-arm, multicenter study, Dr. William Gray reported at the International Symposium on Endovascular Therapy 2013.
At 12 months, 87% were free from target lesion revascularization; at 24 months, 80% were free from target lesion revascularization, said Dr. Gray, director of endovascular services at Columbia University Medical Center, New York.
No major adverse events were noted at 30 days after the index procedure, and the rate of stent fractures was 2% at 12 months, with no additional stent fractures occurring between 12 and 24 months.
All stent fractures were type 1, with no incidents of type II-V fractures, he reported.
Additionally, more than 80% of patients had improvement or normalization of peripheral artery disease outcomes as measured using Rutherford-Becker classification and ankle brachial index, he noted.
Study participants had de novo or restenotic native superficial femoral artery lesions or total occlusions of 4-15 cm (mean, 77 mm) in length, and reference vessel diameters of 4.0-6.0 mm. Nearly 24% had total occlusions, and 47% had diabetes.
All were treated using the SMART Control nitinol self-expanding stent system manufactured by Cordis. SMART stents have been approved for peripheral indication in international markets since 1999. In 2012, the SMART Control nitinol stent system, which was previously approved for use in the iliac arteries, was also approved by the U.S. Food and Drug Administration for use in the superficial femoral artery and/or the proximal popliteal artery.
The findings "speak well of the efficacy of the SMART stent," Dr. Gray said. Follow-up will continue until 3 years post procedure.
The STROLL study was sponsored by Cordis. Dr. Gray was a principal investigator for the study and serves as a paid advisory board member and consultant for Cordis.
MIAMI BEACH – At 2-year follow-up, the SMART stent was associated with a 75% primary patency rate in patients with obstructive superficial femoral artery disease.
The findings were noted in the STROLL study (SMART Vascular Stent Systems in the Treatment of Obstructive Superficial Femoral Artery Disease). The primary patency rate was 82% at 12 months and 75% at 24 months in 250 patients aged 30 years and older who were enrolled in the single-arm, multicenter study, Dr. William Gray reported at the International Symposium on Endovascular Therapy 2013.
At 12 months, 87% were free from target lesion revascularization; at 24 months, 80% were free from target lesion revascularization, said Dr. Gray, director of endovascular services at Columbia University Medical Center, New York.
No major adverse events were noted at 30 days after the index procedure, and the rate of stent fractures was 2% at 12 months, with no additional stent fractures occurring between 12 and 24 months.
All stent fractures were type 1, with no incidents of type II-V fractures, he reported.
Additionally, more than 80% of patients had improvement or normalization of peripheral artery disease outcomes as measured using Rutherford-Becker classification and ankle brachial index, he noted.
Study participants had de novo or restenotic native superficial femoral artery lesions or total occlusions of 4-15 cm (mean, 77 mm) in length, and reference vessel diameters of 4.0-6.0 mm. Nearly 24% had total occlusions, and 47% had diabetes.
All were treated using the SMART Control nitinol self-expanding stent system manufactured by Cordis. SMART stents have been approved for peripheral indication in international markets since 1999. In 2012, the SMART Control nitinol stent system, which was previously approved for use in the iliac arteries, was also approved by the U.S. Food and Drug Administration for use in the superficial femoral artery and/or the proximal popliteal artery.
The findings "speak well of the efficacy of the SMART stent," Dr. Gray said. Follow-up will continue until 3 years post procedure.
The STROLL study was sponsored by Cordis. Dr. Gray was a principal investigator for the study and serves as a paid advisory board member and consultant for Cordis.
AT ISET 2013
Major finding: The primary patency rate in patients with obstructive superficial femoral artery disease was 75% at 24 months.
Data source: Prospective, nonrandomized, multicenter, single-arm study involving 250 patients.
Disclosures: The STROLL study was sponsored by Cordis. Dr. Gray serves as a paid consultant for Cordis.
High patency seen for interwoven nitinol stents in femoropopliteal lesions
MIAMI BEACH – Patency was superior with interwoven nitinol stents as compared with laser-cut nitinol stents and balloon angioplasty when used for the treatment of high-grade obstructive disease in the femoropopliteal region, based on the findings from a review of 100 consecutive cases.
The stent patency rate was 92% at a mean of about 15 months follow-up for the SUPERA 500 stents (IDEV Technologies) used in the SAKE trial (SUPERA Interwoven Nitinol Stent Outcomes in Above-Knee Interventions: A Single Center Experience). That patency rate is consistent with rates seen in multiple other prospective and retrospective studies of the SUPERA stent, Dr. Nemalan Selvaraj reported during a late-breaking abstract session at the International Symposium on Endovascular Therapy 2013.
In the SAKE trial, stent patency was defined by the need for revascularization based on clinical indications, including a fall in ankle brachial index or recurrent symptoms. In this series, ankle brachial index improved significantly from 0.57 at baseline to 0.81 at 12 months, said Dr. Salvaraj of Deborah Heart and Lung Center, Browns Mills, N.J.
Patients included in the study had Rutherford class 2-5 disease and were treated between March 2010 and September 2011. Mean lesion length was 144 mm (range, 40-460; median of 120 mm); 36% of the patients had diabetes and 50% were smokers.
In superficial femoral artery interventions, long-term patency rates are poor with balloon angioplasty alone. Laser-cut nitinol stents have improved patency rates, but stent fracture remains a problem, he noted.
The stent fracture rate with the interwoven nitinol stents has been 0% across numerous studies.
The study’s findings are limited by its clinically driven analysis. Duplex ultrasound confirmation was not used to confirm patency in many of the patients. Nonetheless, the findings suggest that interwoven nitinol stents are an option for treating high-grade obstructive disease above the knee, including cases involving complex anatomy, flexion points, and very long lesions, Dr. Salvaraj said.
Given the unique characteristics of these stents, further study in areas typically considered "no-stent zones," such as the common femoral artery and the popliteal artery at the level of knee flexion, is warranted, he concluded.
Dr. Salvaraj, who presented this data on behalf of principal investigator Dr. Richard C. Kovach, reported having no disclosures. Dr. Kovach has received grant or research funding from and/or been a speaker, trainer, consultant, investigator, or medical advisory board member for Spectranetics, Boston Scientific, IDEV, Medtronic, Angioscore, Lutonix, AngelMed/St. Jude, Avinger, Gore, Abbott, Bard, and Ostialcorp.
MIAMI BEACH – Patency was superior with interwoven nitinol stents as compared with laser-cut nitinol stents and balloon angioplasty when used for the treatment of high-grade obstructive disease in the femoropopliteal region, based on the findings from a review of 100 consecutive cases.
The stent patency rate was 92% at a mean of about 15 months follow-up for the SUPERA 500 stents (IDEV Technologies) used in the SAKE trial (SUPERA Interwoven Nitinol Stent Outcomes in Above-Knee Interventions: A Single Center Experience). That patency rate is consistent with rates seen in multiple other prospective and retrospective studies of the SUPERA stent, Dr. Nemalan Selvaraj reported during a late-breaking abstract session at the International Symposium on Endovascular Therapy 2013.
In the SAKE trial, stent patency was defined by the need for revascularization based on clinical indications, including a fall in ankle brachial index or recurrent symptoms. In this series, ankle brachial index improved significantly from 0.57 at baseline to 0.81 at 12 months, said Dr. Salvaraj of Deborah Heart and Lung Center, Browns Mills, N.J.
Patients included in the study had Rutherford class 2-5 disease and were treated between March 2010 and September 2011. Mean lesion length was 144 mm (range, 40-460; median of 120 mm); 36% of the patients had diabetes and 50% were smokers.
In superficial femoral artery interventions, long-term patency rates are poor with balloon angioplasty alone. Laser-cut nitinol stents have improved patency rates, but stent fracture remains a problem, he noted.
The stent fracture rate with the interwoven nitinol stents has been 0% across numerous studies.
The study’s findings are limited by its clinically driven analysis. Duplex ultrasound confirmation was not used to confirm patency in many of the patients. Nonetheless, the findings suggest that interwoven nitinol stents are an option for treating high-grade obstructive disease above the knee, including cases involving complex anatomy, flexion points, and very long lesions, Dr. Salvaraj said.
Given the unique characteristics of these stents, further study in areas typically considered "no-stent zones," such as the common femoral artery and the popliteal artery at the level of knee flexion, is warranted, he concluded.
Dr. Salvaraj, who presented this data on behalf of principal investigator Dr. Richard C. Kovach, reported having no disclosures. Dr. Kovach has received grant or research funding from and/or been a speaker, trainer, consultant, investigator, or medical advisory board member for Spectranetics, Boston Scientific, IDEV, Medtronic, Angioscore, Lutonix, AngelMed/St. Jude, Avinger, Gore, Abbott, Bard, and Ostialcorp.
MIAMI BEACH – Patency was superior with interwoven nitinol stents as compared with laser-cut nitinol stents and balloon angioplasty when used for the treatment of high-grade obstructive disease in the femoropopliteal region, based on the findings from a review of 100 consecutive cases.
The stent patency rate was 92% at a mean of about 15 months follow-up for the SUPERA 500 stents (IDEV Technologies) used in the SAKE trial (SUPERA Interwoven Nitinol Stent Outcomes in Above-Knee Interventions: A Single Center Experience). That patency rate is consistent with rates seen in multiple other prospective and retrospective studies of the SUPERA stent, Dr. Nemalan Selvaraj reported during a late-breaking abstract session at the International Symposium on Endovascular Therapy 2013.
In the SAKE trial, stent patency was defined by the need for revascularization based on clinical indications, including a fall in ankle brachial index or recurrent symptoms. In this series, ankle brachial index improved significantly from 0.57 at baseline to 0.81 at 12 months, said Dr. Salvaraj of Deborah Heart and Lung Center, Browns Mills, N.J.
Patients included in the study had Rutherford class 2-5 disease and were treated between March 2010 and September 2011. Mean lesion length was 144 mm (range, 40-460; median of 120 mm); 36% of the patients had diabetes and 50% were smokers.
In superficial femoral artery interventions, long-term patency rates are poor with balloon angioplasty alone. Laser-cut nitinol stents have improved patency rates, but stent fracture remains a problem, he noted.
The stent fracture rate with the interwoven nitinol stents has been 0% across numerous studies.
The study’s findings are limited by its clinically driven analysis. Duplex ultrasound confirmation was not used to confirm patency in many of the patients. Nonetheless, the findings suggest that interwoven nitinol stents are an option for treating high-grade obstructive disease above the knee, including cases involving complex anatomy, flexion points, and very long lesions, Dr. Salvaraj said.
Given the unique characteristics of these stents, further study in areas typically considered "no-stent zones," such as the common femoral artery and the popliteal artery at the level of knee flexion, is warranted, he concluded.
Dr. Salvaraj, who presented this data on behalf of principal investigator Dr. Richard C. Kovach, reported having no disclosures. Dr. Kovach has received grant or research funding from and/or been a speaker, trainer, consultant, investigator, or medical advisory board member for Spectranetics, Boston Scientific, IDEV, Medtronic, Angioscore, Lutonix, AngelMed/St. Jude, Avinger, Gore, Abbott, Bard, and Ostialcorp.
AT ISET 2013
Major finding: Stent patency was 92% at a mean follow-up of about 15 months.
Data source: Review of 100 consecutive cases with high-grade obstructive disease in the femoropopliteal region.
Disclosures: Dr. Selvaraj, who presented this data on behalf of principal investigator Dr. Richard C. Kovach, reported having no disclosures. Dr. Kovach disclosed grant or research funding from a wide range of device makers, including IDEV Technologies, the maker of SUPERA.
Vascular surgeons get superior outcomes in aortic aneurysm repair
INDIANAPOLIS – Major outcomes in patients undergoing endovascular repair of abdominal aortic aneurysm are superior in terms of mortality, length of stay, and total hospital charges when the procedure is done by vascular surgeons rather than cardiologists or interventional radiologists, according to an analysis of a comprehensive national hospital database.
"Obviously these are striking findings," Dr. Mark A. Talamini noted in presenting the study results at the annual meeting of the American Surgical Association. "We believe that health policy in support of selective referrals for aneurysm repair, or integrating interventionalists and vascular surgeons more effectively, should be considered."
He presented an outcomes analysis involving 28,094 patients who underwent endovascular implantation of a graft for an abdominal aortic aneurysm within the Nationwide Inpatient Sample during 2001-2009. This database, sponsored by the Agency for Healthcare Research and Quality, receives input from a representative cross-section composed of 20% of U.S. hospitals. Dr. Talamini and coworkers were able to reliably determine whether an operator was a vascular surgeon, a cardiologist, or an interventional radiologist. Vascular surgeons performed 78.1% of the cases, while nonsurgeon interventionalists did the rest. Ninety-seven percent of patients presented with a nonruptured aneurysm.
The unadjusted differences in key outcomes between vascular surgeons and interventionalists were striking. Perhaps even more impressive were the differences following adjustment for operator volume, comorbid conditions, aneurysm rupture status, patient demographics and socioeconomic status, and hospital location and teaching status. The interventionalists’ patients had a 39% greater risk of mortality, an average of $20,000 more in total hospital charges, and a 1.4-day longer length of stay, reported Dr. Talamini, a nonvascular surgeon who is professor and chairman of the department of surgery at the University of California, San Diego.
Additional findings of interest were that the patients of high-volume operators (defined as those who performed more than 10 cases per year) had a 31% reduction in mortality risk regardless of operator specialty. In addition, high-volume operators averaged $10,000 per patient less in total hospital charges and shorter hospital length of stay by 1 full day. Undergoing aneurysm repair in a teaching hospital had no impact upon mortality or total charges, but was associated with an average 0.4-day greater length of stay, he continued.
Dr. Talamini offered two potential explanations for the disparate outcomes. One is that perhaps the patient populations of vascular surgeons and interventionalists differ in ways that were not accounted for in the multivariate analysis. The other possibility is that vascular surgeons achieve better outcomes because their training and experience are superior, allowing them to make better judgments about treatment than those of interventionalists.
"Obviously, this is the ‘we’re better than they are’ argument, and I hardly think we can assume that this is the case until we exhaust all other potential explanations. Further work using longitudinal databases with more detail hopefully will allow us to do just that," said Dr. Talamini.
Discussant Dr. K. Craig Kent called the study findings "very provocative."
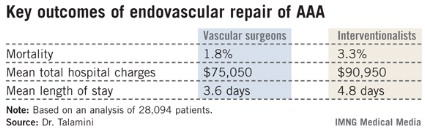
"The moral of the story is expertise in disease is far more important than expertise in technology," declared Dr. Kent, professor and chairman of the department of surgery at the University of Wisconsin, Madison.
"When I first became a vascular surgeon 25 years ago it was difficult to recruit to the specialty. There were few that wanted to care for a group of patients for whom procedures were long and tedious, reoperations were common, and outcomes weren’t always favorable," Dr. Kent recalled. "Fast forward to 2013, where everybody wants to be a vascular surgeon: cardiologists, interventional radiologists, nephrologists, dermatologists, vascular medicine physicians, and many others. Why the dramatic change? For the nonsurgeons, the reason is the development of minimally invasive technology that has allowed any specialist with catheter-based skills to participate in vascular care. But is it appropriate for nonsurgical specialists to treat vascular patients? The answer from this study is a resounding no."
Dr. Samuel E. Wilson, a vascular surgeon who was Dr. Talamini’s coinvestigator in the study, said he thinks patient selection is the key to understanding the outcome disparities.
"If you think about it, the vascular surgeon in his office has the ability to make an elective decision, carefully considered, and decide whether or not he’s going to actually do the procedure. The hospital-based radiologist may not have that opportunity; he receives a call, a procedure on an inpatient is requested, and he feels obligated to proceed. Another key aspect may be postoperative care. Vascular surgery patients receive their postoperative care under the direction of the surgeon," observed Dr. Wilson of the University of California, Irvine.
It’s worth noting, he added, that the outcomes for both vascular surgeons and interventionalists improved over the years of the study. The results are coming closer together over time, although significant differences remain.
Dr. Robert S. Rhodes said that general surgeons should be included in any further comparative effectiveness studies focused on endovascular repair of abdominal aortic aneurysms.
"Our data at the American Board of Surgery suggests that general surgeons who perform vascular surgery actually do so in substantial volume, so it may be that they’ve also acquired endovascular skills," said Dr. Rhodes, associate executive director for vascular surgery at the ABS.
None of the speakers reported having any financial conflicts.
INDIANAPOLIS – Major outcomes in patients undergoing endovascular repair of abdominal aortic aneurysm are superior in terms of mortality, length of stay, and total hospital charges when the procedure is done by vascular surgeons rather than cardiologists or interventional radiologists, according to an analysis of a comprehensive national hospital database.
"Obviously these are striking findings," Dr. Mark A. Talamini noted in presenting the study results at the annual meeting of the American Surgical Association. "We believe that health policy in support of selective referrals for aneurysm repair, or integrating interventionalists and vascular surgeons more effectively, should be considered."
He presented an outcomes analysis involving 28,094 patients who underwent endovascular implantation of a graft for an abdominal aortic aneurysm within the Nationwide Inpatient Sample during 2001-2009. This database, sponsored by the Agency for Healthcare Research and Quality, receives input from a representative cross-section composed of 20% of U.S. hospitals. Dr. Talamini and coworkers were able to reliably determine whether an operator was a vascular surgeon, a cardiologist, or an interventional radiologist. Vascular surgeons performed 78.1% of the cases, while nonsurgeon interventionalists did the rest. Ninety-seven percent of patients presented with a nonruptured aneurysm.
The unadjusted differences in key outcomes between vascular surgeons and interventionalists were striking. Perhaps even more impressive were the differences following adjustment for operator volume, comorbid conditions, aneurysm rupture status, patient demographics and socioeconomic status, and hospital location and teaching status. The interventionalists’ patients had a 39% greater risk of mortality, an average of $20,000 more in total hospital charges, and a 1.4-day longer length of stay, reported Dr. Talamini, a nonvascular surgeon who is professor and chairman of the department of surgery at the University of California, San Diego.
Additional findings of interest were that the patients of high-volume operators (defined as those who performed more than 10 cases per year) had a 31% reduction in mortality risk regardless of operator specialty. In addition, high-volume operators averaged $10,000 per patient less in total hospital charges and shorter hospital length of stay by 1 full day. Undergoing aneurysm repair in a teaching hospital had no impact upon mortality or total charges, but was associated with an average 0.4-day greater length of stay, he continued.
Dr. Talamini offered two potential explanations for the disparate outcomes. One is that perhaps the patient populations of vascular surgeons and interventionalists differ in ways that were not accounted for in the multivariate analysis. The other possibility is that vascular surgeons achieve better outcomes because their training and experience are superior, allowing them to make better judgments about treatment than those of interventionalists.
"Obviously, this is the ‘we’re better than they are’ argument, and I hardly think we can assume that this is the case until we exhaust all other potential explanations. Further work using longitudinal databases with more detail hopefully will allow us to do just that," said Dr. Talamini.
Discussant Dr. K. Craig Kent called the study findings "very provocative."

"The moral of the story is expertise in disease is far more important than expertise in technology," declared Dr. Kent, professor and chairman of the department of surgery at the University of Wisconsin, Madison.
"When I first became a vascular surgeon 25 years ago it was difficult to recruit to the specialty. There were few that wanted to care for a group of patients for whom procedures were long and tedious, reoperations were common, and outcomes weren’t always favorable," Dr. Kent recalled. "Fast forward to 2013, where everybody wants to be a vascular surgeon: cardiologists, interventional radiologists, nephrologists, dermatologists, vascular medicine physicians, and many others. Why the dramatic change? For the nonsurgeons, the reason is the development of minimally invasive technology that has allowed any specialist with catheter-based skills to participate in vascular care. But is it appropriate for nonsurgical specialists to treat vascular patients? The answer from this study is a resounding no."
Dr. Samuel E. Wilson, a vascular surgeon who was Dr. Talamini’s coinvestigator in the study, said he thinks patient selection is the key to understanding the outcome disparities.
"If you think about it, the vascular surgeon in his office has the ability to make an elective decision, carefully considered, and decide whether or not he’s going to actually do the procedure. The hospital-based radiologist may not have that opportunity; he receives a call, a procedure on an inpatient is requested, and he feels obligated to proceed. Another key aspect may be postoperative care. Vascular surgery patients receive their postoperative care under the direction of the surgeon," observed Dr. Wilson of the University of California, Irvine.
It’s worth noting, he added, that the outcomes for both vascular surgeons and interventionalists improved over the years of the study. The results are coming closer together over time, although significant differences remain.
Dr. Robert S. Rhodes said that general surgeons should be included in any further comparative effectiveness studies focused on endovascular repair of abdominal aortic aneurysms.
"Our data at the American Board of Surgery suggests that general surgeons who perform vascular surgery actually do so in substantial volume, so it may be that they’ve also acquired endovascular skills," said Dr. Rhodes, associate executive director for vascular surgery at the ABS.
None of the speakers reported having any financial conflicts.
INDIANAPOLIS – Major outcomes in patients undergoing endovascular repair of abdominal aortic aneurysm are superior in terms of mortality, length of stay, and total hospital charges when the procedure is done by vascular surgeons rather than cardiologists or interventional radiologists, according to an analysis of a comprehensive national hospital database.
"Obviously these are striking findings," Dr. Mark A. Talamini noted in presenting the study results at the annual meeting of the American Surgical Association. "We believe that health policy in support of selective referrals for aneurysm repair, or integrating interventionalists and vascular surgeons more effectively, should be considered."
He presented an outcomes analysis involving 28,094 patients who underwent endovascular implantation of a graft for an abdominal aortic aneurysm within the Nationwide Inpatient Sample during 2001-2009. This database, sponsored by the Agency for Healthcare Research and Quality, receives input from a representative cross-section composed of 20% of U.S. hospitals. Dr. Talamini and coworkers were able to reliably determine whether an operator was a vascular surgeon, a cardiologist, or an interventional radiologist. Vascular surgeons performed 78.1% of the cases, while nonsurgeon interventionalists did the rest. Ninety-seven percent of patients presented with a nonruptured aneurysm.
The unadjusted differences in key outcomes between vascular surgeons and interventionalists were striking. Perhaps even more impressive were the differences following adjustment for operator volume, comorbid conditions, aneurysm rupture status, patient demographics and socioeconomic status, and hospital location and teaching status. The interventionalists’ patients had a 39% greater risk of mortality, an average of $20,000 more in total hospital charges, and a 1.4-day longer length of stay, reported Dr. Talamini, a nonvascular surgeon who is professor and chairman of the department of surgery at the University of California, San Diego.
Additional findings of interest were that the patients of high-volume operators (defined as those who performed more than 10 cases per year) had a 31% reduction in mortality risk regardless of operator specialty. In addition, high-volume operators averaged $10,000 per patient less in total hospital charges and shorter hospital length of stay by 1 full day. Undergoing aneurysm repair in a teaching hospital had no impact upon mortality or total charges, but was associated with an average 0.4-day greater length of stay, he continued.
Dr. Talamini offered two potential explanations for the disparate outcomes. One is that perhaps the patient populations of vascular surgeons and interventionalists differ in ways that were not accounted for in the multivariate analysis. The other possibility is that vascular surgeons achieve better outcomes because their training and experience are superior, allowing them to make better judgments about treatment than those of interventionalists.
"Obviously, this is the ‘we’re better than they are’ argument, and I hardly think we can assume that this is the case until we exhaust all other potential explanations. Further work using longitudinal databases with more detail hopefully will allow us to do just that," said Dr. Talamini.
Discussant Dr. K. Craig Kent called the study findings "very provocative."

"The moral of the story is expertise in disease is far more important than expertise in technology," declared Dr. Kent, professor and chairman of the department of surgery at the University of Wisconsin, Madison.
"When I first became a vascular surgeon 25 years ago it was difficult to recruit to the specialty. There were few that wanted to care for a group of patients for whom procedures were long and tedious, reoperations were common, and outcomes weren’t always favorable," Dr. Kent recalled. "Fast forward to 2013, where everybody wants to be a vascular surgeon: cardiologists, interventional radiologists, nephrologists, dermatologists, vascular medicine physicians, and many others. Why the dramatic change? For the nonsurgeons, the reason is the development of minimally invasive technology that has allowed any specialist with catheter-based skills to participate in vascular care. But is it appropriate for nonsurgical specialists to treat vascular patients? The answer from this study is a resounding no."
Dr. Samuel E. Wilson, a vascular surgeon who was Dr. Talamini’s coinvestigator in the study, said he thinks patient selection is the key to understanding the outcome disparities.
"If you think about it, the vascular surgeon in his office has the ability to make an elective decision, carefully considered, and decide whether or not he’s going to actually do the procedure. The hospital-based radiologist may not have that opportunity; he receives a call, a procedure on an inpatient is requested, and he feels obligated to proceed. Another key aspect may be postoperative care. Vascular surgery patients receive their postoperative care under the direction of the surgeon," observed Dr. Wilson of the University of California, Irvine.
It’s worth noting, he added, that the outcomes for both vascular surgeons and interventionalists improved over the years of the study. The results are coming closer together over time, although significant differences remain.
Dr. Robert S. Rhodes said that general surgeons should be included in any further comparative effectiveness studies focused on endovascular repair of abdominal aortic aneurysms.
"Our data at the American Board of Surgery suggests that general surgeons who perform vascular surgery actually do so in substantial volume, so it may be that they’ve also acquired endovascular skills," said Dr. Rhodes, associate executive director for vascular surgery at the ABS.
None of the speakers reported having any financial conflicts.
AT THE ASA ANNUAL MEETING
Major Finding: The mortality rate in patients undergoing endovascular repair of an abdominal aortic aneurysm was 1.8% when vascular surgeons did the procedure compared to 3.3% when it was performed by an interventional radiologist or cardiologist.
Data Source: An analysis of 28,094 patients who underwent endovascular repair of an abdominal aortic aneurysm in 2001-2009 and were included in the Nationwide Inpatient Sample.
Disclosures: The Nationwide Inpatient Sample is sponsored by the Agency for Healthcare Research and Quality. The presenter reported having no conflicts of interest.
'Liberation therapy' may make MS worse
SAN DIEGO – Percutaneous transluminal venous angioplasty – also known as "liberation therapy" – doesn’t help people with multiple sclerosis and may increase MS brain activity in the short term, according to a small, randomized, sham-controlled trial from the State University of New York at Buffalo, the first randomized trial to investigate the procedure.
It "was ineffective in correcting" chronic cerebrospinal venous insufficiency (CCSVI), the recently described condition it targets. "The results ... caution against widespread adoption of venous angioplasty in the management of patients with MS outside of rigorous clinical trials," the investigators concluded.
The findings follow a recent Food and Drug Administration warning that PTVA (percutaneous transluminal venous angioplasty) can cause deaths and injuries, including strokes, damage to the treated vein, blood clots, cranial nerve damage, abdominal bleeding, and detachment and migration of stents.
The idea is to use balloon angioplasty and stents to widen veins in the chests and necks that appear to be narrowed in some MS patients. Proponents of the procedure say that those narrowed veins impair blood flow and lead to disease progression. The researchers who discovered the problem dubbed it CCSVI. A cottage industry has since sprung up to offer PTVA to MS patients.
The FDA noted in its warning that there have been no "controlled ... rigorously conducted, properly targeted" studies of the issue; that may have changed when Dr. Robert Zivadinov, a professor in the department of neurology at SUNY-Buffalo, presented his team’s findings at the annual meeting of the American Academy of Neurology.
"When you reopened those veins in the neck, I think something happened in reperfusing the brain and re-exacerbating disease activity. The message of this is clear. The majority of patients who are relapsing-remitting should not undergo this treatment," he said in an interview.
Ten patients got PTVA in the first phase of the study. The second phase randomized 9 to PTVA and 10 to a sham intervention. Most had relapsing-remitting MS.
There were no MS relapses in the first phase, but PTVA patients had more relapses (4 vs. 1; P = .389) and more MRI disease activity (cumulative number of new contrast-enhancing lesions (19 vs. 3; P = .062) and new T2 lesions (17 vs. 3; P = .066) in the 6 months following treatment in phase II.
PTVA patients also didn’t fare any better on Expanded Disability Status Scale (EDSS) scores, Multiple Sclerosis Functional Composite scores, 6-minute walk tests, or measures of cognition and quality of life.
"We chose very active patients who had one relapse in the previous year or [gadolinium-] enhancing lesions in the 3 months before. The sample size is small, but [more than half] of patients in the treatment group showed increased activity," Dr. Zivadinov said.
The majority of the subjects were women. On average, they were about 45 years old, had been diagnosed with MS for 11 years, and were mildly to moderately disabled (mean EDSS score about 4). Most were on interferon, glatiramer acetate, or both.
Venous angioplasty didn’t cause any serious complications, and it restored venous outflow to at least 50% of normal in most patients. Phase I patients had a better than 75% improvement overall. Phase II patients had less benefit; there were no differences in venous hemodynamic insufficiency scores between treated and sham patients.
The treatment "failed to provide any sustained improvement in venous outflow as measured through duplex and/or clinical and MRI outcomes," and "more sizable changes in venous outflow [were] associated with increased disease activity primarily noted on MRI," Dr. Zivadinov and his colleagues concluded.
The work was funded primarily by SUNY-Buffalo’s Neuroimaging Analysis Center and Baird MS Research Center. Dr. Zivadinov receives personal compensation from Teva Pharmaceuticals, Biogen Idec, EMD Serono, Bayer, Genzyme-Sanofi, Novartis, Bracco Imaging, and Questcor Pharmaceuticals.
SAN DIEGO – Percutaneous transluminal venous angioplasty – also known as "liberation therapy" – doesn’t help people with multiple sclerosis and may increase MS brain activity in the short term, according to a small, randomized, sham-controlled trial from the State University of New York at Buffalo, the first randomized trial to investigate the procedure.
It "was ineffective in correcting" chronic cerebrospinal venous insufficiency (CCSVI), the recently described condition it targets. "The results ... caution against widespread adoption of venous angioplasty in the management of patients with MS outside of rigorous clinical trials," the investigators concluded.
The findings follow a recent Food and Drug Administration warning that PTVA (percutaneous transluminal venous angioplasty) can cause deaths and injuries, including strokes, damage to the treated vein, blood clots, cranial nerve damage, abdominal bleeding, and detachment and migration of stents.
The idea is to use balloon angioplasty and stents to widen veins in the chests and necks that appear to be narrowed in some MS patients. Proponents of the procedure say that those narrowed veins impair blood flow and lead to disease progression. The researchers who discovered the problem dubbed it CCSVI. A cottage industry has since sprung up to offer PTVA to MS patients.
The FDA noted in its warning that there have been no "controlled ... rigorously conducted, properly targeted" studies of the issue; that may have changed when Dr. Robert Zivadinov, a professor in the department of neurology at SUNY-Buffalo, presented his team’s findings at the annual meeting of the American Academy of Neurology.
"When you reopened those veins in the neck, I think something happened in reperfusing the brain and re-exacerbating disease activity. The message of this is clear. The majority of patients who are relapsing-remitting should not undergo this treatment," he said in an interview.
Ten patients got PTVA in the first phase of the study. The second phase randomized 9 to PTVA and 10 to a sham intervention. Most had relapsing-remitting MS.
There were no MS relapses in the first phase, but PTVA patients had more relapses (4 vs. 1; P = .389) and more MRI disease activity (cumulative number of new contrast-enhancing lesions (19 vs. 3; P = .062) and new T2 lesions (17 vs. 3; P = .066) in the 6 months following treatment in phase II.
PTVA patients also didn’t fare any better on Expanded Disability Status Scale (EDSS) scores, Multiple Sclerosis Functional Composite scores, 6-minute walk tests, or measures of cognition and quality of life.
"We chose very active patients who had one relapse in the previous year or [gadolinium-] enhancing lesions in the 3 months before. The sample size is small, but [more than half] of patients in the treatment group showed increased activity," Dr. Zivadinov said.
The majority of the subjects were women. On average, they were about 45 years old, had been diagnosed with MS for 11 years, and were mildly to moderately disabled (mean EDSS score about 4). Most were on interferon, glatiramer acetate, or both.
Venous angioplasty didn’t cause any serious complications, and it restored venous outflow to at least 50% of normal in most patients. Phase I patients had a better than 75% improvement overall. Phase II patients had less benefit; there were no differences in venous hemodynamic insufficiency scores between treated and sham patients.
The treatment "failed to provide any sustained improvement in venous outflow as measured through duplex and/or clinical and MRI outcomes," and "more sizable changes in venous outflow [were] associated with increased disease activity primarily noted on MRI," Dr. Zivadinov and his colleagues concluded.
The work was funded primarily by SUNY-Buffalo’s Neuroimaging Analysis Center and Baird MS Research Center. Dr. Zivadinov receives personal compensation from Teva Pharmaceuticals, Biogen Idec, EMD Serono, Bayer, Genzyme-Sanofi, Novartis, Bracco Imaging, and Questcor Pharmaceuticals.
SAN DIEGO – Percutaneous transluminal venous angioplasty – also known as "liberation therapy" – doesn’t help people with multiple sclerosis and may increase MS brain activity in the short term, according to a small, randomized, sham-controlled trial from the State University of New York at Buffalo, the first randomized trial to investigate the procedure.
It "was ineffective in correcting" chronic cerebrospinal venous insufficiency (CCSVI), the recently described condition it targets. "The results ... caution against widespread adoption of venous angioplasty in the management of patients with MS outside of rigorous clinical trials," the investigators concluded.
The findings follow a recent Food and Drug Administration warning that PTVA (percutaneous transluminal venous angioplasty) can cause deaths and injuries, including strokes, damage to the treated vein, blood clots, cranial nerve damage, abdominal bleeding, and detachment and migration of stents.
The idea is to use balloon angioplasty and stents to widen veins in the chests and necks that appear to be narrowed in some MS patients. Proponents of the procedure say that those narrowed veins impair blood flow and lead to disease progression. The researchers who discovered the problem dubbed it CCSVI. A cottage industry has since sprung up to offer PTVA to MS patients.
The FDA noted in its warning that there have been no "controlled ... rigorously conducted, properly targeted" studies of the issue; that may have changed when Dr. Robert Zivadinov, a professor in the department of neurology at SUNY-Buffalo, presented his team’s findings at the annual meeting of the American Academy of Neurology.
"When you reopened those veins in the neck, I think something happened in reperfusing the brain and re-exacerbating disease activity. The message of this is clear. The majority of patients who are relapsing-remitting should not undergo this treatment," he said in an interview.
Ten patients got PTVA in the first phase of the study. The second phase randomized 9 to PTVA and 10 to a sham intervention. Most had relapsing-remitting MS.
There were no MS relapses in the first phase, but PTVA patients had more relapses (4 vs. 1; P = .389) and more MRI disease activity (cumulative number of new contrast-enhancing lesions (19 vs. 3; P = .062) and new T2 lesions (17 vs. 3; P = .066) in the 6 months following treatment in phase II.
PTVA patients also didn’t fare any better on Expanded Disability Status Scale (EDSS) scores, Multiple Sclerosis Functional Composite scores, 6-minute walk tests, or measures of cognition and quality of life.
"We chose very active patients who had one relapse in the previous year or [gadolinium-] enhancing lesions in the 3 months before. The sample size is small, but [more than half] of patients in the treatment group showed increased activity," Dr. Zivadinov said.
The majority of the subjects were women. On average, they were about 45 years old, had been diagnosed with MS for 11 years, and were mildly to moderately disabled (mean EDSS score about 4). Most were on interferon, glatiramer acetate, or both.
Venous angioplasty didn’t cause any serious complications, and it restored venous outflow to at least 50% of normal in most patients. Phase I patients had a better than 75% improvement overall. Phase II patients had less benefit; there were no differences in venous hemodynamic insufficiency scores between treated and sham patients.
The treatment "failed to provide any sustained improvement in venous outflow as measured through duplex and/or clinical and MRI outcomes," and "more sizable changes in venous outflow [were] associated with increased disease activity primarily noted on MRI," Dr. Zivadinov and his colleagues concluded.
The work was funded primarily by SUNY-Buffalo’s Neuroimaging Analysis Center and Baird MS Research Center. Dr. Zivadinov receives personal compensation from Teva Pharmaceuticals, Biogen Idec, EMD Serono, Bayer, Genzyme-Sanofi, Novartis, Bracco Imaging, and Questcor Pharmaceuticals.
AT THE 2013 AAN ANNUAL MEETING
Major finding: A total of 19 new contrast-enhancing MRI lesions were observed in 9 "liberation therapy" MS patients within 6 months of treatment, compared with 3 lesions in 10 control patients.
Date Source: A randomized, sham-controlled trial with 29 MS patients
Disclosures: The work was funded primarily by SUNY–Buffalo’s Neuroimaging Analysis Center and Baird MS Research Center. Dr. Zivadinov receives personal compensation from Teva Pharmaceuticals, Biogen Idec, EMD Serono, Bayer, Genzyme-Sanofi, Novartis, Bracco Imaging, and Questcor Pharmaceuticals.
Pooled data allow fine-tuning of surveillance intervals for AAA
Information pooled in a meta-analysis of 18 data sets may allow clinicians to fine-tune the ultrasonographic surveillance of small abdominal aortic aneurysms, according to a report in the Feb. 27 issue of JAMA.
"Currently, there is no consensus regarding appropriate surveillance intervals for patients with (AAAs) [abdominal aortic aneurysms]. By pooling results from 18 studies, we quantified AAA growth rates and the AAA rupture risk as a function of aortic diameter. Our intent was to provide an objective basis for selecting surveillance intervals for patients with small AAAs," said Simon G. Thompson, D.Sc., of the cardiovascular epidemiology unit, department of public health and primary care, University of Cambridge (England), and his associates.
Their findings suggest that the overall number and frequency of surveillance scans can be reduced, at least for men. However, the picture is still unclear for women, and more research is required before specific recommendations can be made, the investigators said.
Dr. Thompson and his colleagues reviewed randomized trials, observational studies, and papers presented at vascular surgical conferences that included data sets with 100 or more patients who underwent repeated ultrasound measurements of the diameter of AAAs over time. This yielded 18 data sets that included 15,471 subjects with AAA diameters between 3.0 and 5.4 cm, because 5.5 cm is usually the threshold at which surgical repair of the lesion is recommended.
The 13,728 men and 1,743 women were followed for an average of 1-8 years. There were "relatively few" AAA ruptures: 178 among men and 50 among women.
Among men, a 3-cm AAA took a mean of 7.4 years to have a 10% chance of reaching 5.5 cm, a 4-cm AAA took a mean of 3.2 years to have a 10% chance of reaching 5.5 cm, and a 5-cm AAA took a mean of 0.7 years to have a 10% chance of reaching 5.5 cm.
Similarly, rupture rates among men approximately doubled for every 0.5-cm increase in AAA diameter. For AAAs with diameters of 3.0-4.5 cm, the average time to reach a rupture risk of 1% was at least 2 years.
These data can be used to guide the decision of how often to perform ultrasound surveillance of AAAs in men. Based on the lower 95% confidence limits, the risk of rupture in men would be less than 1% if surveillance intervals were extended to 3 years for AAAs measuring 3.0-3.9 cm, to 2 years for those measuring 4.0-4.4 cm, and to 1 year for those measuring 4.5-5.4 cm.
"For a U.S. patient with a 3.0-cm AAA detected by screening, this would reduce the average number of surveillance scans from approximately 15 to 7," the investigators wrote (JAMA 2013;309:806-13).
However, if the lower 95% prediction limits of the estimates were applied (to acknowledge that the population in each study might have different growth and rupture rates), the risk of rupture in men would be less than 1% if surveillance intervals were extended to 2 years for AAAs measuring 3.0-3.9 cm, to 1 year for those measuring 4.0-4.9 cm, and to 6 months for those measuring 5.0-5.4 cm. This would result in a lesser average reduction in the number of surveillance scans from 15 to 10.
The rate of rupture was four times higher for women than for men, even though the rate of AAA growth was similar. The higher risk of AAA rupture in women is well known, although the reasons for this discrepancy are not yet certain. Researchers have proposed that differences in anatomy, structure, sex hormones, and smoking habits all may play a role.
"The clinical implication is that a lower AAA diameter threshold for surgery should be adopted for women, a recommendation already made by the joint council of the American Association for Vascular Surgery and the Society for Vascular Surgery," Dr. Thompson and his associates wrote.
A threshold of 4.5 cm rather than 5.5 cm might be more appropriate for women, but this decision must be weighed against the evidence that women have a higher operative mortality than men and a poorer outcome at postoperative hospital discharge, the investigators noted.
This study was supported by the U.K. National Institute for Health Research’s Health Technology Assessment Programme. The authors reported that they had no relevant financial conflicts, although individual members had participated in aneurysm clinical trials.
Information pooled in a meta-analysis of 18 data sets may allow clinicians to fine-tune the ultrasonographic surveillance of small abdominal aortic aneurysms, according to a report in the Feb. 27 issue of JAMA.
"Currently, there is no consensus regarding appropriate surveillance intervals for patients with (AAAs) [abdominal aortic aneurysms]. By pooling results from 18 studies, we quantified AAA growth rates and the AAA rupture risk as a function of aortic diameter. Our intent was to provide an objective basis for selecting surveillance intervals for patients with small AAAs," said Simon G. Thompson, D.Sc., of the cardiovascular epidemiology unit, department of public health and primary care, University of Cambridge (England), and his associates.
Their findings suggest that the overall number and frequency of surveillance scans can be reduced, at least for men. However, the picture is still unclear for women, and more research is required before specific recommendations can be made, the investigators said.
Dr. Thompson and his colleagues reviewed randomized trials, observational studies, and papers presented at vascular surgical conferences that included data sets with 100 or more patients who underwent repeated ultrasound measurements of the diameter of AAAs over time. This yielded 18 data sets that included 15,471 subjects with AAA diameters between 3.0 and 5.4 cm, because 5.5 cm is usually the threshold at which surgical repair of the lesion is recommended.
The 13,728 men and 1,743 women were followed for an average of 1-8 years. There were "relatively few" AAA ruptures: 178 among men and 50 among women.
Among men, a 3-cm AAA took a mean of 7.4 years to have a 10% chance of reaching 5.5 cm, a 4-cm AAA took a mean of 3.2 years to have a 10% chance of reaching 5.5 cm, and a 5-cm AAA took a mean of 0.7 years to have a 10% chance of reaching 5.5 cm.
Similarly, rupture rates among men approximately doubled for every 0.5-cm increase in AAA diameter. For AAAs with diameters of 3.0-4.5 cm, the average time to reach a rupture risk of 1% was at least 2 years.
These data can be used to guide the decision of how often to perform ultrasound surveillance of AAAs in men. Based on the lower 95% confidence limits, the risk of rupture in men would be less than 1% if surveillance intervals were extended to 3 years for AAAs measuring 3.0-3.9 cm, to 2 years for those measuring 4.0-4.4 cm, and to 1 year for those measuring 4.5-5.4 cm.
"For a U.S. patient with a 3.0-cm AAA detected by screening, this would reduce the average number of surveillance scans from approximately 15 to 7," the investigators wrote (JAMA 2013;309:806-13).
However, if the lower 95% prediction limits of the estimates were applied (to acknowledge that the population in each study might have different growth and rupture rates), the risk of rupture in men would be less than 1% if surveillance intervals were extended to 2 years for AAAs measuring 3.0-3.9 cm, to 1 year for those measuring 4.0-4.9 cm, and to 6 months for those measuring 5.0-5.4 cm. This would result in a lesser average reduction in the number of surveillance scans from 15 to 10.
The rate of rupture was four times higher for women than for men, even though the rate of AAA growth was similar. The higher risk of AAA rupture in women is well known, although the reasons for this discrepancy are not yet certain. Researchers have proposed that differences in anatomy, structure, sex hormones, and smoking habits all may play a role.
"The clinical implication is that a lower AAA diameter threshold for surgery should be adopted for women, a recommendation already made by the joint council of the American Association for Vascular Surgery and the Society for Vascular Surgery," Dr. Thompson and his associates wrote.
A threshold of 4.5 cm rather than 5.5 cm might be more appropriate for women, but this decision must be weighed against the evidence that women have a higher operative mortality than men and a poorer outcome at postoperative hospital discharge, the investigators noted.
This study was supported by the U.K. National Institute for Health Research’s Health Technology Assessment Programme. The authors reported that they had no relevant financial conflicts, although individual members had participated in aneurysm clinical trials.
Information pooled in a meta-analysis of 18 data sets may allow clinicians to fine-tune the ultrasonographic surveillance of small abdominal aortic aneurysms, according to a report in the Feb. 27 issue of JAMA.
"Currently, there is no consensus regarding appropriate surveillance intervals for patients with (AAAs) [abdominal aortic aneurysms]. By pooling results from 18 studies, we quantified AAA growth rates and the AAA rupture risk as a function of aortic diameter. Our intent was to provide an objective basis for selecting surveillance intervals for patients with small AAAs," said Simon G. Thompson, D.Sc., of the cardiovascular epidemiology unit, department of public health and primary care, University of Cambridge (England), and his associates.
Their findings suggest that the overall number and frequency of surveillance scans can be reduced, at least for men. However, the picture is still unclear for women, and more research is required before specific recommendations can be made, the investigators said.
Dr. Thompson and his colleagues reviewed randomized trials, observational studies, and papers presented at vascular surgical conferences that included data sets with 100 or more patients who underwent repeated ultrasound measurements of the diameter of AAAs over time. This yielded 18 data sets that included 15,471 subjects with AAA diameters between 3.0 and 5.4 cm, because 5.5 cm is usually the threshold at which surgical repair of the lesion is recommended.
The 13,728 men and 1,743 women were followed for an average of 1-8 years. There were "relatively few" AAA ruptures: 178 among men and 50 among women.
Among men, a 3-cm AAA took a mean of 7.4 years to have a 10% chance of reaching 5.5 cm, a 4-cm AAA took a mean of 3.2 years to have a 10% chance of reaching 5.5 cm, and a 5-cm AAA took a mean of 0.7 years to have a 10% chance of reaching 5.5 cm.
Similarly, rupture rates among men approximately doubled for every 0.5-cm increase in AAA diameter. For AAAs with diameters of 3.0-4.5 cm, the average time to reach a rupture risk of 1% was at least 2 years.
These data can be used to guide the decision of how often to perform ultrasound surveillance of AAAs in men. Based on the lower 95% confidence limits, the risk of rupture in men would be less than 1% if surveillance intervals were extended to 3 years for AAAs measuring 3.0-3.9 cm, to 2 years for those measuring 4.0-4.4 cm, and to 1 year for those measuring 4.5-5.4 cm.
"For a U.S. patient with a 3.0-cm AAA detected by screening, this would reduce the average number of surveillance scans from approximately 15 to 7," the investigators wrote (JAMA 2013;309:806-13).
However, if the lower 95% prediction limits of the estimates were applied (to acknowledge that the population in each study might have different growth and rupture rates), the risk of rupture in men would be less than 1% if surveillance intervals were extended to 2 years for AAAs measuring 3.0-3.9 cm, to 1 year for those measuring 4.0-4.9 cm, and to 6 months for those measuring 5.0-5.4 cm. This would result in a lesser average reduction in the number of surveillance scans from 15 to 10.
The rate of rupture was four times higher for women than for men, even though the rate of AAA growth was similar. The higher risk of AAA rupture in women is well known, although the reasons for this discrepancy are not yet certain. Researchers have proposed that differences in anatomy, structure, sex hormones, and smoking habits all may play a role.
"The clinical implication is that a lower AAA diameter threshold for surgery should be adopted for women, a recommendation already made by the joint council of the American Association for Vascular Surgery and the Society for Vascular Surgery," Dr. Thompson and his associates wrote.
A threshold of 4.5 cm rather than 5.5 cm might be more appropriate for women, but this decision must be weighed against the evidence that women have a higher operative mortality than men and a poorer outcome at postoperative hospital discharge, the investigators noted.
This study was supported by the U.K. National Institute for Health Research’s Health Technology Assessment Programme. The authors reported that they had no relevant financial conflicts, although individual members had participated in aneurysm clinical trials.
FROM JAMA
Major Finding: The risk of AAA rupture in men would be less than 1% if surveillance intervals were extended to 3 years for AAAs measuring 3.0-3.9 cm, to 2 years for those measuring 4.0-4.4 cm, and to 1 year for those measuring 4.5-5.4 cm.
Data Source: A meta-analysis of 18 studies each involving at least 100 patients who had AAAs of 3.0-5.4 cm in diameter and who had serial ultrasound measurements of the lesions for an average of 1-8 years.
Disclosures: This study was supported by the U.K. National Institute for Health Research’s Health Technology Assessment Programme. The authors reported that they had no relevant financial conflicts, although individual members had participated in aneurysm clinical trials.