User login
Does stenting of severe renal artery stenosis improve outomes compared with medical therapy alone?
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
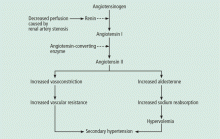
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
Stenting may benefit select patients with severe renal artery stenosis
In their article in this issue of the Cleveland Clinic Journal of Medicine, Kabach et al answer no to the question of whether stenting of severe renal artery stenosis improves outcomes compared with medical therapy alone.1 They review the findings of four key studies2–5 published between 2003 and 2014 and conclude that, in patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting with medical therapy can improve blood pressure control but has no significant impact on cardiovascular or mortality outcomes.1
Furthermore, the authors state that in view of the risk of complications associated with stenting, medical management should continue to be the first-line therapy.1 Indeed, the ASTRAL study (Angioplasty and Stenting for Renal Artery Lesions) investigators found substantial risks without evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.3
Nevertheless, I believe that this procedure may benefit certain patients.
MAYO CLINIC COHORT STUDY
In 2008, our group at Mayo Clinic Health system in Eau Claire, Wisconsin, published the results of a prospective cohort study in 26 patients with renal artery stenosis and chronic kidney disease who presented with rapidly worsening renal failure (defined as an increase in serum creatinine of ≥ 25%) while receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).6,7
These drugs—inhibitors of the renin-angiotensin-aldosterone system—slow the progression of chronic kidney disease but can acutely worsen renal function, especially in patients with renal artery stenosis, and withdrawing them in this situation was the focus of our study.
The patients (10 men and 16 women) ranged in age from 63 to 87 (mean age 75.3).
At enrollment, the ACE inhibitors and ARBs were discontinued, standard nephrologic care was applied, and the glomerular filtration rate (estimated by the Modification of Diet in Renal Disease Study equation) was monitored. After at least 2 weeks, percutaneous renal angioplasty with stent placement was considered if the patient met any of the following criteria:
- Persistence of renal failure
- Flash pulmonary edema
- Uncontrolled hypertension despite the use of at least three antihypertensive medications.
Nine patients underwent percutaneous angioplasty and stenting and 17 did not. The procedure was done on one renal artery in 8 patients and both renal arteries in 1. Indications for the procedure were recent worsening of renal failure in 8 patients and recent worsening renal failure together with symptomatic flash pulmonary edema in 1 patient. (Flash pulmonary edema is the only class I recommendation for percutaneous renal angioplasty in the 2006 joint guidelines of the American College of Cardiology and the American Heart Association.8) As noted above, all the patients were experiencing acute exacerbation of chronic kidney disease at the time.
We found clear evidence of additional improved and sustained renal function in the patients who underwent percutaneous renal angioplasty and stenting compared with the patients who did not (Figures 1 and 2).6,7
In an editorial9 following publication of the ASTRAL study, our group reported a subsequent 82-month analysis of our 26-patient cohort completed in June 2009. In the 7 surviving patients who had undergone percutaneous renal angioplasty and stenting, the estimated glomerular filtration rate had increased from 27.4 mL/min/1.73 m2 (± 12.7, range 11–47) at baseline to 50.3 (± 21.7, range 23–68) (P = .018) after 46.9 months.
PATIENT NUMBER 13
To illustrate how percutaneous renal angioplasty and stenting can reverse recently worsening renal failure in renal artery stenosis, I would like to discuss in greater detail a patient from our two previous reports.6,7
Patient 13, a 67-year-old woman with hypertension, was referred to our nephrology service in September 2004 to consider starting hemodialysis for symptomatic renal failure. Her serum creatinine had increased to 3.4 mg/dL from a previous baseline level of 2.0 mg/dL, and she also had worsening anemia and hyperkalemia. She had been taking lisinopril 10 mg/day for the last 12 months. Magnetic resonance angiography revealed high-grade (> 95%) bilateral renal artery stenosis with an atrophic left kidney.
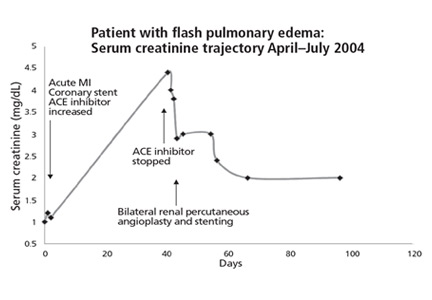
Lisinopril was promptly discontinued, and within 2 weeks her serum creatinine level had decreased by more than 0.5 mg/dL. In mid-November 2004, she underwent right renal artery angioplasty with stent placement. After that, her serum creatinine decreased further, and 3 months later it had dropped to 1.6 mg/dL. The value continued to improve, with the lowest measurement of 1.1 mg/dL, equivalent to an estimated glomerular filtration rate of 51 mL/min/1.73 m2. This was in May 2006, 19 months after stopping lisinopril and 17 months after angioplasty and stenting. The last available serum creatinine level (August 2006) was 1.2 mg/dL, equivalent to an estimated glomerular filtration rate of 45 mL/min/1.73 m2 (Figure 1). Unfortunately, the patient died of metastatic lung cancer in December of that year.
Also of note, the patient who underwent angioplasty because of recurrent flash pulmonary edema had no recurrences of it afterward.
We concluded that, in patients with hemodynamically significant renal artery stenosis presenting with acutely worsening renal failure, renal angioplasty with stenting has the potential to reverse renal failure, improve blood pressure control, and stop flash pulmonary edema.6–8
Notably, all patients in our study who underwent renal angioplasty with stenting had new-onset acute renal injury as defined by an increase in the baseline serum creatinine of more than 25% during the 3 months before stent placement.6–8 Patients in the STAR,2 HERCULES,4 and CORAL5 studies had renal artery stenosis but otherwise stable chronic kidney disease at the time of enrollment. In the ASTRAL study,3 12% of the patients had acute kidney injury on study enrollment, defined as an increase in the serum creatinine level of more than 20% or of more than 1.13 mg/dL.3
While we strongly agree with aggressive yet monitored medical therapy for patients with hemodynamically significant renal artery stenosis, I posit that selected patients do indeed derive significant clinical benefits from renal angioplasty and stenting. Our group’s prospective individual-patient-level data support the paradigm that angioplasty with stenting is useful in patients with renal artery stenosis who experience “acute-on-chronic” kidney disease.
The pathophysiology of renal artery stenosis and the progression of chronic kidney disease are complex, and many factors affect patient outcomes and response to treatment. Thus, the message is that treatment of severe renal artery stenosis must be individualized.9–11 No one treatment fits all.10,11
- Kabach A, Agha OQ, Baibars M, Alraiyes AH, Alraies MC. Does stenting of severe renal artery stenosis improve outcomes compared with medical therapy alone? Cleve Clin J Med 2015; 82:491–494.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Jaff MR, Bates M, Sullivan T, et al; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv 2012; 80:343–350.
- Cooper CJ, Murphy TP, Cutlip DE, et al; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22.
- Onuigbo MAC, Onuigbo NTC. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo Health System clinic analysis. QJM 2008; 101:519–527.
- Onuigbo MA, Onuigbo NT. Renal failure and concurrent RAAS blockade in older CKD patients with renal artery stenosis: an extended Mayo Clinic prospective 63-month experience. Ren Fail 2008; 30:363–371.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Onuigbo M, Frenandes R, Nijhawan V. The ASTRAL trial results revisited—to stent or not to stent in renal artery stenosis? QJM 2010; 103:357.
- Singh M, Kovacs DF, Singh A, Dhaliwal P, Khosla S. ACE inhibition and renal artery stenosis: what lessons have we learnt? A 21st century perspective. In: Onuigbo MAC, ed. ACE inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 2. New York, NY: NOVA Publishers; 2013:203–218.
- Onuigbo MA, Onuigbo C, Onuigbo V, et al. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: the introduction of the new 'CKD express©' IT software program. In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 1. New York, NY: NOVA Publishers; 2013: 41–56.
In their article in this issue of the Cleveland Clinic Journal of Medicine, Kabach et al answer no to the question of whether stenting of severe renal artery stenosis improves outcomes compared with medical therapy alone.1 They review the findings of four key studies2–5 published between 2003 and 2014 and conclude that, in patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting with medical therapy can improve blood pressure control but has no significant impact on cardiovascular or mortality outcomes.1
Furthermore, the authors state that in view of the risk of complications associated with stenting, medical management should continue to be the first-line therapy.1 Indeed, the ASTRAL study (Angioplasty and Stenting for Renal Artery Lesions) investigators found substantial risks without evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.3
Nevertheless, I believe that this procedure may benefit certain patients.
MAYO CLINIC COHORT STUDY
In 2008, our group at Mayo Clinic Health system in Eau Claire, Wisconsin, published the results of a prospective cohort study in 26 patients with renal artery stenosis and chronic kidney disease who presented with rapidly worsening renal failure (defined as an increase in serum creatinine of ≥ 25%) while receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).6,7
These drugs—inhibitors of the renin-angiotensin-aldosterone system—slow the progression of chronic kidney disease but can acutely worsen renal function, especially in patients with renal artery stenosis, and withdrawing them in this situation was the focus of our study.
The patients (10 men and 16 women) ranged in age from 63 to 87 (mean age 75.3).
At enrollment, the ACE inhibitors and ARBs were discontinued, standard nephrologic care was applied, and the glomerular filtration rate (estimated by the Modification of Diet in Renal Disease Study equation) was monitored. After at least 2 weeks, percutaneous renal angioplasty with stent placement was considered if the patient met any of the following criteria:
- Persistence of renal failure
- Flash pulmonary edema
- Uncontrolled hypertension despite the use of at least three antihypertensive medications.
Nine patients underwent percutaneous angioplasty and stenting and 17 did not. The procedure was done on one renal artery in 8 patients and both renal arteries in 1. Indications for the procedure were recent worsening of renal failure in 8 patients and recent worsening renal failure together with symptomatic flash pulmonary edema in 1 patient. (Flash pulmonary edema is the only class I recommendation for percutaneous renal angioplasty in the 2006 joint guidelines of the American College of Cardiology and the American Heart Association.8) As noted above, all the patients were experiencing acute exacerbation of chronic kidney disease at the time.
We found clear evidence of additional improved and sustained renal function in the patients who underwent percutaneous renal angioplasty and stenting compared with the patients who did not (Figures 1 and 2).6,7
In an editorial9 following publication of the ASTRAL study, our group reported a subsequent 82-month analysis of our 26-patient cohort completed in June 2009. In the 7 surviving patients who had undergone percutaneous renal angioplasty and stenting, the estimated glomerular filtration rate had increased from 27.4 mL/min/1.73 m2 (± 12.7, range 11–47) at baseline to 50.3 (± 21.7, range 23–68) (P = .018) after 46.9 months.
PATIENT NUMBER 13
To illustrate how percutaneous renal angioplasty and stenting can reverse recently worsening renal failure in renal artery stenosis, I would like to discuss in greater detail a patient from our two previous reports.6,7
Patient 13, a 67-year-old woman with hypertension, was referred to our nephrology service in September 2004 to consider starting hemodialysis for symptomatic renal failure. Her serum creatinine had increased to 3.4 mg/dL from a previous baseline level of 2.0 mg/dL, and she also had worsening anemia and hyperkalemia. She had been taking lisinopril 10 mg/day for the last 12 months. Magnetic resonance angiography revealed high-grade (> 95%) bilateral renal artery stenosis with an atrophic left kidney.
Lisinopril was promptly discontinued, and within 2 weeks her serum creatinine level had decreased by more than 0.5 mg/dL. In mid-November 2004, she underwent right renal artery angioplasty with stent placement. After that, her serum creatinine decreased further, and 3 months later it had dropped to 1.6 mg/dL. The value continued to improve, with the lowest measurement of 1.1 mg/dL, equivalent to an estimated glomerular filtration rate of 51 mL/min/1.73 m2. This was in May 2006, 19 months after stopping lisinopril and 17 months after angioplasty and stenting. The last available serum creatinine level (August 2006) was 1.2 mg/dL, equivalent to an estimated glomerular filtration rate of 45 mL/min/1.73 m2 (Figure 1). Unfortunately, the patient died of metastatic lung cancer in December of that year.
Also of note, the patient who underwent angioplasty because of recurrent flash pulmonary edema had no recurrences of it afterward.
We concluded that, in patients with hemodynamically significant renal artery stenosis presenting with acutely worsening renal failure, renal angioplasty with stenting has the potential to reverse renal failure, improve blood pressure control, and stop flash pulmonary edema.6–8
Notably, all patients in our study who underwent renal angioplasty with stenting had new-onset acute renal injury as defined by an increase in the baseline serum creatinine of more than 25% during the 3 months before stent placement.6–8 Patients in the STAR,2 HERCULES,4 and CORAL5 studies had renal artery stenosis but otherwise stable chronic kidney disease at the time of enrollment. In the ASTRAL study,3 12% of the patients had acute kidney injury on study enrollment, defined as an increase in the serum creatinine level of more than 20% or of more than 1.13 mg/dL.3
While we strongly agree with aggressive yet monitored medical therapy for patients with hemodynamically significant renal artery stenosis, I posit that selected patients do indeed derive significant clinical benefits from renal angioplasty and stenting. Our group’s prospective individual-patient-level data support the paradigm that angioplasty with stenting is useful in patients with renal artery stenosis who experience “acute-on-chronic” kidney disease.
The pathophysiology of renal artery stenosis and the progression of chronic kidney disease are complex, and many factors affect patient outcomes and response to treatment. Thus, the message is that treatment of severe renal artery stenosis must be individualized.9–11 No one treatment fits all.10,11
In their article in this issue of the Cleveland Clinic Journal of Medicine, Kabach et al answer no to the question of whether stenting of severe renal artery stenosis improves outcomes compared with medical therapy alone.1 They review the findings of four key studies2–5 published between 2003 and 2014 and conclude that, in patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting with medical therapy can improve blood pressure control but has no significant impact on cardiovascular or mortality outcomes.1
Furthermore, the authors state that in view of the risk of complications associated with stenting, medical management should continue to be the first-line therapy.1 Indeed, the ASTRAL study (Angioplasty and Stenting for Renal Artery Lesions) investigators found substantial risks without evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.3
Nevertheless, I believe that this procedure may benefit certain patients.
MAYO CLINIC COHORT STUDY
In 2008, our group at Mayo Clinic Health system in Eau Claire, Wisconsin, published the results of a prospective cohort study in 26 patients with renal artery stenosis and chronic kidney disease who presented with rapidly worsening renal failure (defined as an increase in serum creatinine of ≥ 25%) while receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).6,7
These drugs—inhibitors of the renin-angiotensin-aldosterone system—slow the progression of chronic kidney disease but can acutely worsen renal function, especially in patients with renal artery stenosis, and withdrawing them in this situation was the focus of our study.
The patients (10 men and 16 women) ranged in age from 63 to 87 (mean age 75.3).
At enrollment, the ACE inhibitors and ARBs were discontinued, standard nephrologic care was applied, and the glomerular filtration rate (estimated by the Modification of Diet in Renal Disease Study equation) was monitored. After at least 2 weeks, percutaneous renal angioplasty with stent placement was considered if the patient met any of the following criteria:
- Persistence of renal failure
- Flash pulmonary edema
- Uncontrolled hypertension despite the use of at least three antihypertensive medications.
Nine patients underwent percutaneous angioplasty and stenting and 17 did not. The procedure was done on one renal artery in 8 patients and both renal arteries in 1. Indications for the procedure were recent worsening of renal failure in 8 patients and recent worsening renal failure together with symptomatic flash pulmonary edema in 1 patient. (Flash pulmonary edema is the only class I recommendation for percutaneous renal angioplasty in the 2006 joint guidelines of the American College of Cardiology and the American Heart Association.8) As noted above, all the patients were experiencing acute exacerbation of chronic kidney disease at the time.
We found clear evidence of additional improved and sustained renal function in the patients who underwent percutaneous renal angioplasty and stenting compared with the patients who did not (Figures 1 and 2).6,7
In an editorial9 following publication of the ASTRAL study, our group reported a subsequent 82-month analysis of our 26-patient cohort completed in June 2009. In the 7 surviving patients who had undergone percutaneous renal angioplasty and stenting, the estimated glomerular filtration rate had increased from 27.4 mL/min/1.73 m2 (± 12.7, range 11–47) at baseline to 50.3 (± 21.7, range 23–68) (P = .018) after 46.9 months.
PATIENT NUMBER 13
To illustrate how percutaneous renal angioplasty and stenting can reverse recently worsening renal failure in renal artery stenosis, I would like to discuss in greater detail a patient from our two previous reports.6,7
Patient 13, a 67-year-old woman with hypertension, was referred to our nephrology service in September 2004 to consider starting hemodialysis for symptomatic renal failure. Her serum creatinine had increased to 3.4 mg/dL from a previous baseline level of 2.0 mg/dL, and she also had worsening anemia and hyperkalemia. She had been taking lisinopril 10 mg/day for the last 12 months. Magnetic resonance angiography revealed high-grade (> 95%) bilateral renal artery stenosis with an atrophic left kidney.
Lisinopril was promptly discontinued, and within 2 weeks her serum creatinine level had decreased by more than 0.5 mg/dL. In mid-November 2004, she underwent right renal artery angioplasty with stent placement. After that, her serum creatinine decreased further, and 3 months later it had dropped to 1.6 mg/dL. The value continued to improve, with the lowest measurement of 1.1 mg/dL, equivalent to an estimated glomerular filtration rate of 51 mL/min/1.73 m2. This was in May 2006, 19 months after stopping lisinopril and 17 months after angioplasty and stenting. The last available serum creatinine level (August 2006) was 1.2 mg/dL, equivalent to an estimated glomerular filtration rate of 45 mL/min/1.73 m2 (Figure 1). Unfortunately, the patient died of metastatic lung cancer in December of that year.
Also of note, the patient who underwent angioplasty because of recurrent flash pulmonary edema had no recurrences of it afterward.
We concluded that, in patients with hemodynamically significant renal artery stenosis presenting with acutely worsening renal failure, renal angioplasty with stenting has the potential to reverse renal failure, improve blood pressure control, and stop flash pulmonary edema.6–8
Notably, all patients in our study who underwent renal angioplasty with stenting had new-onset acute renal injury as defined by an increase in the baseline serum creatinine of more than 25% during the 3 months before stent placement.6–8 Patients in the STAR,2 HERCULES,4 and CORAL5 studies had renal artery stenosis but otherwise stable chronic kidney disease at the time of enrollment. In the ASTRAL study,3 12% of the patients had acute kidney injury on study enrollment, defined as an increase in the serum creatinine level of more than 20% or of more than 1.13 mg/dL.3
While we strongly agree with aggressive yet monitored medical therapy for patients with hemodynamically significant renal artery stenosis, I posit that selected patients do indeed derive significant clinical benefits from renal angioplasty and stenting. Our group’s prospective individual-patient-level data support the paradigm that angioplasty with stenting is useful in patients with renal artery stenosis who experience “acute-on-chronic” kidney disease.
The pathophysiology of renal artery stenosis and the progression of chronic kidney disease are complex, and many factors affect patient outcomes and response to treatment. Thus, the message is that treatment of severe renal artery stenosis must be individualized.9–11 No one treatment fits all.10,11
- Kabach A, Agha OQ, Baibars M, Alraiyes AH, Alraies MC. Does stenting of severe renal artery stenosis improve outcomes compared with medical therapy alone? Cleve Clin J Med 2015; 82:491–494.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Jaff MR, Bates M, Sullivan T, et al; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv 2012; 80:343–350.
- Cooper CJ, Murphy TP, Cutlip DE, et al; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22.
- Onuigbo MAC, Onuigbo NTC. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo Health System clinic analysis. QJM 2008; 101:519–527.
- Onuigbo MA, Onuigbo NT. Renal failure and concurrent RAAS blockade in older CKD patients with renal artery stenosis: an extended Mayo Clinic prospective 63-month experience. Ren Fail 2008; 30:363–371.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Onuigbo M, Frenandes R, Nijhawan V. The ASTRAL trial results revisited—to stent or not to stent in renal artery stenosis? QJM 2010; 103:357.
- Singh M, Kovacs DF, Singh A, Dhaliwal P, Khosla S. ACE inhibition and renal artery stenosis: what lessons have we learnt? A 21st century perspective. In: Onuigbo MAC, ed. ACE inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 2. New York, NY: NOVA Publishers; 2013:203–218.
- Onuigbo MA, Onuigbo C, Onuigbo V, et al. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: the introduction of the new 'CKD express©' IT software program. In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 1. New York, NY: NOVA Publishers; 2013: 41–56.
- Kabach A, Agha OQ, Baibars M, Alraiyes AH, Alraies MC. Does stenting of severe renal artery stenosis improve outcomes compared with medical therapy alone? Cleve Clin J Med 2015; 82:491–494.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Jaff MR, Bates M, Sullivan T, et al; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv 2012; 80:343–350.
- Cooper CJ, Murphy TP, Cutlip DE, et al; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22.
- Onuigbo MAC, Onuigbo NTC. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo Health System clinic analysis. QJM 2008; 101:519–527.
- Onuigbo MA, Onuigbo NT. Renal failure and concurrent RAAS blockade in older CKD patients with renal artery stenosis: an extended Mayo Clinic prospective 63-month experience. Ren Fail 2008; 30:363–371.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Onuigbo M, Frenandes R, Nijhawan V. The ASTRAL trial results revisited—to stent or not to stent in renal artery stenosis? QJM 2010; 103:357.
- Singh M, Kovacs DF, Singh A, Dhaliwal P, Khosla S. ACE inhibition and renal artery stenosis: what lessons have we learnt? A 21st century perspective. In: Onuigbo MAC, ed. ACE inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 2. New York, NY: NOVA Publishers; 2013:203–218.
- Onuigbo MA, Onuigbo C, Onuigbo V, et al. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: the introduction of the new 'CKD express©' IT software program. In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 1. New York, NY: NOVA Publishers; 2013: 41–56.
SVS: AAA reimbursement needs to take anatomic complexity into account
CHICAGO – Anatomic complexity should be factored into reimbursements for abdominal aortic aneurysm repairs, University of Rochester (N.Y.) investigators concluded after they compared costs to complexity in 33 open and 107 endovascular repairs during 2007-2010.
They found that complex aneurysms – especially ones with Anatomic Severity Grade (ASG) scores above 15 – need more adjunctive procedures and cost more to repair, although at the moment, payers don’t usually take complexity directly into account.
It’s the first study to show a direct relationship between anatomic complexity and hospital cost. “Preoperative assessment with ASG scores can delineate patients at greater risk for increased resource utilization. A critical examination of the relationship between anatomic complexity and finances is required within the context of aggressive endovascular treatment strategies and shifts towards value-based reimbursement. Anatomy is related to cost. [Complexity] should be considered as a factor when calculating limited bundle reimbursements,” said investigator Dr. Khurram Rasheed, a vascular surgery resident in Rochester.
Developed by the Society for Vascular Surgery, the ASG is an assessment of the aortic neck, aneurysm body, iliac arteries, and pelvic perfusion for 16 parameters, including angles, calcifications, and tortuosity. Each parameter is scored from 0-3. Higher scores mean greater complexity, with 48 being the highest possible score (J. Vasc. Surg. 2002;35:1061-6).
An ASG of 15 proved to be a handy marker for when complexity starts to affect the bottom line. A score of 15 or higher correlated with increased costs and increased propensity for requiring intraoperative adjuncts such as renal artery stenting (odds ratio, 5.75; 95% confidence interval, 1.82-18.19). It also correlated with chronic kidney disease and end-stage renal disease, meaning that sicker patients were likely to have worse anatomy and cost more to repair, Dr. Rasheed reported at the meeting hosted by the Society for Special Surgery.
All the cases in the study were elective, and the majority of the patients were elderly white men.
The mean total-cost of endovascular aortic repair (EVAR) was $24,701, mean length of stay (LOS) of 3.0 days, and mean ASG score of 15.9. Cases below an ASG score of 15 cost a mean of $22,020 and had a mean LOS of 2.93 days. Above 15, the mean cost was $26,574 and mean LOS was 3.07 days.
About a quarter of EVAR patients required intraoperative adjuncts, most above an ASG score of 15; their cases cost a mean of $31,509, with a mean ASG score of 18.48 and LOS of 3.85 days.
For open repair, the mean total cost was $38,310, LOS of 13.5 days, and ASG score of 18.1. When five patients with unusually long hospital stays were excluded, open repair cost less than EVAR, which is consistent with previous reports. Just two open-repair patients (6%) needed adjunct procedures.
Open-repair cases with an ASG score below 15 cost a mean of $24,508 and had a mean LOS of 10 days. Cases with a higher score cost a mean of $41,071 and stayed in the hospital an average of 14.2 days. Despite trends, the ASG score differences in cost and LOS for open-repair cases did not reach statistical significance; type II error was probably to blame, Dr. Rasheed said.
The investigators have no disclosures.
CHICAGO – Anatomic complexity should be factored into reimbursements for abdominal aortic aneurysm repairs, University of Rochester (N.Y.) investigators concluded after they compared costs to complexity in 33 open and 107 endovascular repairs during 2007-2010.
They found that complex aneurysms – especially ones with Anatomic Severity Grade (ASG) scores above 15 – need more adjunctive procedures and cost more to repair, although at the moment, payers don’t usually take complexity directly into account.
It’s the first study to show a direct relationship between anatomic complexity and hospital cost. “Preoperative assessment with ASG scores can delineate patients at greater risk for increased resource utilization. A critical examination of the relationship between anatomic complexity and finances is required within the context of aggressive endovascular treatment strategies and shifts towards value-based reimbursement. Anatomy is related to cost. [Complexity] should be considered as a factor when calculating limited bundle reimbursements,” said investigator Dr. Khurram Rasheed, a vascular surgery resident in Rochester.
Developed by the Society for Vascular Surgery, the ASG is an assessment of the aortic neck, aneurysm body, iliac arteries, and pelvic perfusion for 16 parameters, including angles, calcifications, and tortuosity. Each parameter is scored from 0-3. Higher scores mean greater complexity, with 48 being the highest possible score (J. Vasc. Surg. 2002;35:1061-6).
An ASG of 15 proved to be a handy marker for when complexity starts to affect the bottom line. A score of 15 or higher correlated with increased costs and increased propensity for requiring intraoperative adjuncts such as renal artery stenting (odds ratio, 5.75; 95% confidence interval, 1.82-18.19). It also correlated with chronic kidney disease and end-stage renal disease, meaning that sicker patients were likely to have worse anatomy and cost more to repair, Dr. Rasheed reported at the meeting hosted by the Society for Special Surgery.
All the cases in the study were elective, and the majority of the patients were elderly white men.
The mean total-cost of endovascular aortic repair (EVAR) was $24,701, mean length of stay (LOS) of 3.0 days, and mean ASG score of 15.9. Cases below an ASG score of 15 cost a mean of $22,020 and had a mean LOS of 2.93 days. Above 15, the mean cost was $26,574 and mean LOS was 3.07 days.
About a quarter of EVAR patients required intraoperative adjuncts, most above an ASG score of 15; their cases cost a mean of $31,509, with a mean ASG score of 18.48 and LOS of 3.85 days.
For open repair, the mean total cost was $38,310, LOS of 13.5 days, and ASG score of 18.1. When five patients with unusually long hospital stays were excluded, open repair cost less than EVAR, which is consistent with previous reports. Just two open-repair patients (6%) needed adjunct procedures.
Open-repair cases with an ASG score below 15 cost a mean of $24,508 and had a mean LOS of 10 days. Cases with a higher score cost a mean of $41,071 and stayed in the hospital an average of 14.2 days. Despite trends, the ASG score differences in cost and LOS for open-repair cases did not reach statistical significance; type II error was probably to blame, Dr. Rasheed said.
The investigators have no disclosures.
CHICAGO – Anatomic complexity should be factored into reimbursements for abdominal aortic aneurysm repairs, University of Rochester (N.Y.) investigators concluded after they compared costs to complexity in 33 open and 107 endovascular repairs during 2007-2010.
They found that complex aneurysms – especially ones with Anatomic Severity Grade (ASG) scores above 15 – need more adjunctive procedures and cost more to repair, although at the moment, payers don’t usually take complexity directly into account.
It’s the first study to show a direct relationship between anatomic complexity and hospital cost. “Preoperative assessment with ASG scores can delineate patients at greater risk for increased resource utilization. A critical examination of the relationship between anatomic complexity and finances is required within the context of aggressive endovascular treatment strategies and shifts towards value-based reimbursement. Anatomy is related to cost. [Complexity] should be considered as a factor when calculating limited bundle reimbursements,” said investigator Dr. Khurram Rasheed, a vascular surgery resident in Rochester.
Developed by the Society for Vascular Surgery, the ASG is an assessment of the aortic neck, aneurysm body, iliac arteries, and pelvic perfusion for 16 parameters, including angles, calcifications, and tortuosity. Each parameter is scored from 0-3. Higher scores mean greater complexity, with 48 being the highest possible score (J. Vasc. Surg. 2002;35:1061-6).
An ASG of 15 proved to be a handy marker for when complexity starts to affect the bottom line. A score of 15 or higher correlated with increased costs and increased propensity for requiring intraoperative adjuncts such as renal artery stenting (odds ratio, 5.75; 95% confidence interval, 1.82-18.19). It also correlated with chronic kidney disease and end-stage renal disease, meaning that sicker patients were likely to have worse anatomy and cost more to repair, Dr. Rasheed reported at the meeting hosted by the Society for Special Surgery.
All the cases in the study were elective, and the majority of the patients were elderly white men.
The mean total-cost of endovascular aortic repair (EVAR) was $24,701, mean length of stay (LOS) of 3.0 days, and mean ASG score of 15.9. Cases below an ASG score of 15 cost a mean of $22,020 and had a mean LOS of 2.93 days. Above 15, the mean cost was $26,574 and mean LOS was 3.07 days.
About a quarter of EVAR patients required intraoperative adjuncts, most above an ASG score of 15; their cases cost a mean of $31,509, with a mean ASG score of 18.48 and LOS of 3.85 days.
For open repair, the mean total cost was $38,310, LOS of 13.5 days, and ASG score of 18.1. When five patients with unusually long hospital stays were excluded, open repair cost less than EVAR, which is consistent with previous reports. Just two open-repair patients (6%) needed adjunct procedures.
Open-repair cases with an ASG score below 15 cost a mean of $24,508 and had a mean LOS of 10 days. Cases with a higher score cost a mean of $41,071 and stayed in the hospital an average of 14.2 days. Despite trends, the ASG score differences in cost and LOS for open-repair cases did not reach statistical significance; type II error was probably to blame, Dr. Rasheed said.
The investigators have no disclosures.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Get the hang of calculating Anatomic Severity Grade scores on your triple A cases; it might one day increase your reimbursements.
Major finding: EVAR cases below an ASG score of 15 cost a mean of $22,020 and had a length of stay of 2.93 days. Above 15, the cost was $26,574 and mean LOS was 3.07 days.
Data source: Review of 33 open and 107 endovascular repairs at the University of Rochester in New York.
Disclosures: The investigators have no disclosures.
SVS: Beta-blockers cut stroke, death risk in carotid stenting
CHICAGO – Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand, according to a review of 5,248 stent cases during 2005-2014.
“Compared to non-users, patients on long-term beta-blockers are at 34% less risk of stroke and death after carotid artery stenting [odds ratio, 0.66; 95% confidence interval, 0.46-0.95; P = .025], and this risk reduction is amplified to 65% in patients with postop hypertension [OR, 0.35; 95% CI, 0.17-0.73; P = .005]. Beta-blockers significantly reduce the stroke and death risk ... and should be investigated prospectively for potential use during” carotid artery stenting (CAS), said senior investigator Dr. Mahmoud Malas, director of endovascular surgery and associate professor of surgery at Johns Hopkins Bayview Medical Center in Baltimore.
In the study, long-term beta-blocker use was not associated with post-procedure hypotension in the study. Among patients who developed it, however, beta-blockers were associated with a 48% reduction in the risk of stroke or death at 30 days (OR, 0.52; 95% CI, 0.28-0.98; P = .43).
“We think” the benefits are due to “up-regulation of adrenergic receptors. We think also there is better baroreceptor reflex sensitivity.” Long-term use of beta-blockers reduces heart rate variability, as well, and decreases the risk of hyperperfusion fourfold, Dr. Malas said at the meeting hosted by the Society for Vascular Surgery.
The researchers looked into the issue because they are trying to find a way to make CAS safer in the wake of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and others that have shown increased risk compared with carotid endarterectomy.
The subjects were all captured in SVS’s Vascular Quality Initiative database; 2,152 were not on beta-blockers before CAS, 259 were on them for less than 30 days, and 2,837 were on them for more than 30 days. There were no statistical between-group differences in lesion sites, approach (femoral in almost all the cases), or contrast volume used in surgery, a marker of case complexity.
Long-term users had more diabetes, hypertension, coronary artery disease, and congestive heart failure, whereas short term users were more symptomatic; those and other differences were controlled for on multivariate analysis. Aspirin, clopidogrel, and statin use were similar between the groups. About two-thirds of the subjects were men, and the average age in the study was about 70 years old.
Overall, the 30-day stroke and death rate was 3.4% (minor stroke 1.5%, major 0.9%, and death 1.2%).
Predictors of postoperative stroke or death at 30 days included symptomatic status, age, diabetes, and perioperative hypotension and hypertension. Prior carotid endarterectomy and distal embolic protection were both protective.
The investigators have no disclosures.
CHICAGO – Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand, according to a review of 5,248 stent cases during 2005-2014.
“Compared to non-users, patients on long-term beta-blockers are at 34% less risk of stroke and death after carotid artery stenting [odds ratio, 0.66; 95% confidence interval, 0.46-0.95; P = .025], and this risk reduction is amplified to 65% in patients with postop hypertension [OR, 0.35; 95% CI, 0.17-0.73; P = .005]. Beta-blockers significantly reduce the stroke and death risk ... and should be investigated prospectively for potential use during” carotid artery stenting (CAS), said senior investigator Dr. Mahmoud Malas, director of endovascular surgery and associate professor of surgery at Johns Hopkins Bayview Medical Center in Baltimore.
In the study, long-term beta-blocker use was not associated with post-procedure hypotension in the study. Among patients who developed it, however, beta-blockers were associated with a 48% reduction in the risk of stroke or death at 30 days (OR, 0.52; 95% CI, 0.28-0.98; P = .43).
“We think” the benefits are due to “up-regulation of adrenergic receptors. We think also there is better baroreceptor reflex sensitivity.” Long-term use of beta-blockers reduces heart rate variability, as well, and decreases the risk of hyperperfusion fourfold, Dr. Malas said at the meeting hosted by the Society for Vascular Surgery.
The researchers looked into the issue because they are trying to find a way to make CAS safer in the wake of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and others that have shown increased risk compared with carotid endarterectomy.
The subjects were all captured in SVS’s Vascular Quality Initiative database; 2,152 were not on beta-blockers before CAS, 259 were on them for less than 30 days, and 2,837 were on them for more than 30 days. There were no statistical between-group differences in lesion sites, approach (femoral in almost all the cases), or contrast volume used in surgery, a marker of case complexity.
Long-term users had more diabetes, hypertension, coronary artery disease, and congestive heart failure, whereas short term users were more symptomatic; those and other differences were controlled for on multivariate analysis. Aspirin, clopidogrel, and statin use were similar between the groups. About two-thirds of the subjects were men, and the average age in the study was about 70 years old.
Overall, the 30-day stroke and death rate was 3.4% (minor stroke 1.5%, major 0.9%, and death 1.2%).
Predictors of postoperative stroke or death at 30 days included symptomatic status, age, diabetes, and perioperative hypotension and hypertension. Prior carotid endarterectomy and distal embolic protection were both protective.
The investigators have no disclosures.
CHICAGO – Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand, according to a review of 5,248 stent cases during 2005-2014.
“Compared to non-users, patients on long-term beta-blockers are at 34% less risk of stroke and death after carotid artery stenting [odds ratio, 0.66; 95% confidence interval, 0.46-0.95; P = .025], and this risk reduction is amplified to 65% in patients with postop hypertension [OR, 0.35; 95% CI, 0.17-0.73; P = .005]. Beta-blockers significantly reduce the stroke and death risk ... and should be investigated prospectively for potential use during” carotid artery stenting (CAS), said senior investigator Dr. Mahmoud Malas, director of endovascular surgery and associate professor of surgery at Johns Hopkins Bayview Medical Center in Baltimore.
In the study, long-term beta-blocker use was not associated with post-procedure hypotension in the study. Among patients who developed it, however, beta-blockers were associated with a 48% reduction in the risk of stroke or death at 30 days (OR, 0.52; 95% CI, 0.28-0.98; P = .43).
“We think” the benefits are due to “up-regulation of adrenergic receptors. We think also there is better baroreceptor reflex sensitivity.” Long-term use of beta-blockers reduces heart rate variability, as well, and decreases the risk of hyperperfusion fourfold, Dr. Malas said at the meeting hosted by the Society for Vascular Surgery.
The researchers looked into the issue because they are trying to find a way to make CAS safer in the wake of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and others that have shown increased risk compared with carotid endarterectomy.
The subjects were all captured in SVS’s Vascular Quality Initiative database; 2,152 were not on beta-blockers before CAS, 259 were on them for less than 30 days, and 2,837 were on them for more than 30 days. There were no statistical between-group differences in lesion sites, approach (femoral in almost all the cases), or contrast volume used in surgery, a marker of case complexity.
Long-term users had more diabetes, hypertension, coronary artery disease, and congestive heart failure, whereas short term users were more symptomatic; those and other differences were controlled for on multivariate analysis. Aspirin, clopidogrel, and statin use were similar between the groups. About two-thirds of the subjects were men, and the average age in the study was about 70 years old.
Overall, the 30-day stroke and death rate was 3.4% (minor stroke 1.5%, major 0.9%, and death 1.2%).
Predictors of postoperative stroke or death at 30 days included symptomatic status, age, diabetes, and perioperative hypotension and hypertension. Prior carotid endarterectomy and distal embolic protection were both protective.
The investigators have no disclosures.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Carotid artery stenting is safer if patients have been on beta-blockers for at least a month beforehand.
Major finding: The risk of stroke or death after carotid artery stenting is reduced by 34% when patients are on long-term beta-blockers preoperatively.
Data source: Review of 5,248 stent cases during 2005-2014
Disclosures: The investigators have no disclosures.
SVS: Stroke reduction outweighs bleeding risk of dual antiplatelet therapy in CEA
CHICAGO – Don’t automatically discontinue dual antiplatelet therapy for carotid endarterectomy because the neuroprotective effects may outweigh the bleeding risks, researchers concluded after a review of more than 28,000 patients who underwent the procedure during 2003-2014.
They found in the study that the 7,059 patients on perioperative dual antiplatelet therapy with clopidogrel (Plavix) and aspirin had about a 40% reduction in transient ischemic attacks (TIAs), strokes, and stroke-related deaths when compared with the 21,624 patients on aspirin alone.
The investigators found on multivariate analysis that bleeding bad enough for a return trip to the operating room was more common in their dual antiplatelet group (odds ratio, 1.73; P < .01), but they felt the neuroprotective effect was probably worth the “slightly increased bleeding risk.” Earlier research suggests that about half of vascular surgeons will discontinue clopidogrel a week or so before carotid endarterectomy (CEA) because of bleeding risks (Eur. J. Vasc. Endovasc. Surg. 2009;38:402-7).
“Although dual therapy increases perioperative bleeding, it confers an overall benefit by reducing stroke and death. Patients taking dual therapy at the time of CEA should continue treatment preoperatively. This study also suggests that initiating dual therapy is beneficial for asymptomatic patients,” lead investigator Dr. Douglas Jones of the New York Presbyterian Hospital in New York said at the meeting hosted by the Society for Vascular Surgery.
The team used the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative database. Patients were about 70 years old on average and about 60% were men. Dual-therapy patients had more coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and diabetes.
On multivariate analysis to control for those differences, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62; P = .04); and stroke death (OR, 0.65; P = .03). It did not protect against myocardial infarction.
“More than 95% of patients received heparin for these cases,” said Dr. Jones, noting that protamine-reversal after the case “had the greatest protective effect” against major bleeding, which is consistent with previous reports. Protamine reversal reduced it by more than 50% (OR, 0.44; P < .01).
The results, for the most part, were similar on propensity matching of 4,548 patients on dual therapy to 4,548 on aspirin alone, all of whom had CEA after 2010. Dual-therapy patients were about twice as likely to return to the operating room for bleeding (1.3% vs. 0.7%), but also had fewer thrombotic complications (for instance, stroke 0.6% vs. 1.0% in the aspirin cohort).
Asymptomatic patients on dual therapy were again about twice as likely to return to surgery for major bleeding, but half as likely to have a stroke. Bleeding was more common in symptomatic dual therapy patients, as well, but for reasons that aren’t clear, a trend toward fewer thrombotic events in symptomatic patients on propensity matching did not reach statistical significance. “The protective effect was greatest among asymptomatic patients,” Dr. Jones said.
Patients on dual therapy were also more likely to have a drain placed, but drain placement did not protect against reoperation for bleeding (OR, 1.06; P = .75).
Dr. Jones has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
CHICAGO – Don’t automatically discontinue dual antiplatelet therapy for carotid endarterectomy because the neuroprotective effects may outweigh the bleeding risks, researchers concluded after a review of more than 28,000 patients who underwent the procedure during 2003-2014.
They found in the study that the 7,059 patients on perioperative dual antiplatelet therapy with clopidogrel (Plavix) and aspirin had about a 40% reduction in transient ischemic attacks (TIAs), strokes, and stroke-related deaths when compared with the 21,624 patients on aspirin alone.
The investigators found on multivariate analysis that bleeding bad enough for a return trip to the operating room was more common in their dual antiplatelet group (odds ratio, 1.73; P < .01), but they felt the neuroprotective effect was probably worth the “slightly increased bleeding risk.” Earlier research suggests that about half of vascular surgeons will discontinue clopidogrel a week or so before carotid endarterectomy (CEA) because of bleeding risks (Eur. J. Vasc. Endovasc. Surg. 2009;38:402-7).
“Although dual therapy increases perioperative bleeding, it confers an overall benefit by reducing stroke and death. Patients taking dual therapy at the time of CEA should continue treatment preoperatively. This study also suggests that initiating dual therapy is beneficial for asymptomatic patients,” lead investigator Dr. Douglas Jones of the New York Presbyterian Hospital in New York said at the meeting hosted by the Society for Vascular Surgery.
The team used the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative database. Patients were about 70 years old on average and about 60% were men. Dual-therapy patients had more coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and diabetes.
On multivariate analysis to control for those differences, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62; P = .04); and stroke death (OR, 0.65; P = .03). It did not protect against myocardial infarction.
“More than 95% of patients received heparin for these cases,” said Dr. Jones, noting that protamine-reversal after the case “had the greatest protective effect” against major bleeding, which is consistent with previous reports. Protamine reversal reduced it by more than 50% (OR, 0.44; P < .01).
The results, for the most part, were similar on propensity matching of 4,548 patients on dual therapy to 4,548 on aspirin alone, all of whom had CEA after 2010. Dual-therapy patients were about twice as likely to return to the operating room for bleeding (1.3% vs. 0.7%), but also had fewer thrombotic complications (for instance, stroke 0.6% vs. 1.0% in the aspirin cohort).
Asymptomatic patients on dual therapy were again about twice as likely to return to surgery for major bleeding, but half as likely to have a stroke. Bleeding was more common in symptomatic dual therapy patients, as well, but for reasons that aren’t clear, a trend toward fewer thrombotic events in symptomatic patients on propensity matching did not reach statistical significance. “The protective effect was greatest among asymptomatic patients,” Dr. Jones said.
Patients on dual therapy were also more likely to have a drain placed, but drain placement did not protect against reoperation for bleeding (OR, 1.06; P = .75).
Dr. Jones has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
CHICAGO – Don’t automatically discontinue dual antiplatelet therapy for carotid endarterectomy because the neuroprotective effects may outweigh the bleeding risks, researchers concluded after a review of more than 28,000 patients who underwent the procedure during 2003-2014.
They found in the study that the 7,059 patients on perioperative dual antiplatelet therapy with clopidogrel (Plavix) and aspirin had about a 40% reduction in transient ischemic attacks (TIAs), strokes, and stroke-related deaths when compared with the 21,624 patients on aspirin alone.
The investigators found on multivariate analysis that bleeding bad enough for a return trip to the operating room was more common in their dual antiplatelet group (odds ratio, 1.73; P < .01), but they felt the neuroprotective effect was probably worth the “slightly increased bleeding risk.” Earlier research suggests that about half of vascular surgeons will discontinue clopidogrel a week or so before carotid endarterectomy (CEA) because of bleeding risks (Eur. J. Vasc. Endovasc. Surg. 2009;38:402-7).
“Although dual therapy increases perioperative bleeding, it confers an overall benefit by reducing stroke and death. Patients taking dual therapy at the time of CEA should continue treatment preoperatively. This study also suggests that initiating dual therapy is beneficial for asymptomatic patients,” lead investigator Dr. Douglas Jones of the New York Presbyterian Hospital in New York said at the meeting hosted by the Society for Vascular Surgery.
The team used the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative database. Patients were about 70 years old on average and about 60% were men. Dual-therapy patients had more coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and diabetes.
On multivariate analysis to control for those differences, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62; P = .04); and stroke death (OR, 0.65; P = .03). It did not protect against myocardial infarction.
“More than 95% of patients received heparin for these cases,” said Dr. Jones, noting that protamine-reversal after the case “had the greatest protective effect” against major bleeding, which is consistent with previous reports. Protamine reversal reduced it by more than 50% (OR, 0.44; P < .01).
The results, for the most part, were similar on propensity matching of 4,548 patients on dual therapy to 4,548 on aspirin alone, all of whom had CEA after 2010. Dual-therapy patients were about twice as likely to return to the operating room for bleeding (1.3% vs. 0.7%), but also had fewer thrombotic complications (for instance, stroke 0.6% vs. 1.0% in the aspirin cohort).
Asymptomatic patients on dual therapy were again about twice as likely to return to surgery for major bleeding, but half as likely to have a stroke. Bleeding was more common in symptomatic dual therapy patients, as well, but for reasons that aren’t clear, a trend toward fewer thrombotic events in symptomatic patients on propensity matching did not reach statistical significance. “The protective effect was greatest among asymptomatic patients,” Dr. Jones said.
Patients on dual therapy were also more likely to have a drain placed, but drain placement did not protect against reoperation for bleeding (OR, 1.06; P = .75).
Dr. Jones has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Strokes are less likely after CEA if patients are on perioperative clopidogrel and aspirin.
Major finding: On multivariate analysis, dual therapy was protective against TIA or stroke (OR, 0.60; P < .01); ipsilateral TIA or stroke (OR, 0.68; P = .05); stroke (OR, 0.62, P = .04); and stroke death (OR, 0.65; P = .03).
Data source: Review of more than 28,000 carotid endarterectomy patients
Disclosures: The presenter has no disclosures. Other investigators disclosed consulting fees from Medtronic, Volcano, Bard, and AnGes.
SVS: Choose endarterectomy over stenting for complex carotid lesions
CHICAGO – Carotid endarterectomy is safer than stenting when lesions have greater complexity based on length, location, and presence of sequential lesions, according to a subanalysis of the CREST study.
Previous studies have shown that carotid artery stenting (CAS) is also risky in patients with type 3 aortic arches; atherosclerotic aortic arches; internal carotid artery tortuosity; circumferential calcification; and ulcerated lesions.
Taken together, “we can now begin to populate a list of conditions that are high risk for carotid artery stenting. For patients with these factors, we would strongly recommend that carotid endarterectomy be employed rather than carotid artery stenting,” said CREST (Carotid Revascularization Endarterectomy versus Stent Trial) investigator Dr. Wesley Moore, professor and chief, emeritus, of the division of vascular surgery at the University of California, Los Angeles.
“However, in the absence of these higher risk characteristics, carotid artery stenting should yield results equivalent to carotid endarterectomy,” he said at a meeting hosted by the Society for Vascular Surgery.
CREST demonstrated that carotid artery stenting carries about twice the risk of stroke and death (4.4%) as carotid endarterectomy (2.3%); the investigators revisited their subjects’ preop angiograms to see if lesion characteristics were to blame in a subanalysis of the trial results.
In CREST, 438 patients had angiograms before carotid endarterectomies (CEA), about a third of the total number of CEA patients, while preop angiograms were done in all of the 1,262 CAS patients. There were no statistically significant differences in age, gender, stenosis symptoms, smoking history, arrhythmias, and left ventricular hypertrophy between CEA and CAS patients.
For lesions longer than 12.85 mm – the median length in CREST – the combined outcome of strokes and death occurred in 1.9% of CEA and 6.1% of CAS patients (CAS odds ratio, 3.45; 95% confidence interval, 1.21-9.83). For sequential lesions, strokes and death occurred in 0.7% of CEA and 5.8% of CAS patients (CAS OR, 9.21; 95% CI, 1.23-68.94).
With long, sequential lesions distal to the carotid bulb, stroke and death occurred in 6.3% of CAS patients but no CEA patients, giving an “infinite odds ratio in favor of CEA,” Dr. Moore said.
Two-thirds of all the patients in CREST had lesion risk factors for CAS, which might help explain the original findings.
CREST also found that stenting was riskier in older people and women, but it seems likely now that age and gender were surrogates for adverse lesion characteristics.
“The fact of the matter is that older patients have more complex lesions, so age tended to be a surrogate for lesion complexity. If I have an 80-year-old with a short, isolated lesion, I don’t think the fact that they are 80 represents higher risk. I think the lesion being short puts them in the same low risk [category] as other favorable CAS characteristics. I think that’s also true for gender,” Dr. Moore said.
Angiograms almost always underestimate the length of carotid lesions. CT and MRI do a better job, but “ultrasound may be even better than those two,” he noted.
CREST was funded by the National Institutes of Health and Abbott Vascular Solutions. Dr. Moore has no disclosures. Other authors reported relationships with Abbott, Medtronic, Boston Scientific, and other companies.
CHICAGO – Carotid endarterectomy is safer than stenting when lesions have greater complexity based on length, location, and presence of sequential lesions, according to a subanalysis of the CREST study.
Previous studies have shown that carotid artery stenting (CAS) is also risky in patients with type 3 aortic arches; atherosclerotic aortic arches; internal carotid artery tortuosity; circumferential calcification; and ulcerated lesions.
Taken together, “we can now begin to populate a list of conditions that are high risk for carotid artery stenting. For patients with these factors, we would strongly recommend that carotid endarterectomy be employed rather than carotid artery stenting,” said CREST (Carotid Revascularization Endarterectomy versus Stent Trial) investigator Dr. Wesley Moore, professor and chief, emeritus, of the division of vascular surgery at the University of California, Los Angeles.
“However, in the absence of these higher risk characteristics, carotid artery stenting should yield results equivalent to carotid endarterectomy,” he said at a meeting hosted by the Society for Vascular Surgery.
CREST demonstrated that carotid artery stenting carries about twice the risk of stroke and death (4.4%) as carotid endarterectomy (2.3%); the investigators revisited their subjects’ preop angiograms to see if lesion characteristics were to blame in a subanalysis of the trial results.
In CREST, 438 patients had angiograms before carotid endarterectomies (CEA), about a third of the total number of CEA patients, while preop angiograms were done in all of the 1,262 CAS patients. There were no statistically significant differences in age, gender, stenosis symptoms, smoking history, arrhythmias, and left ventricular hypertrophy between CEA and CAS patients.
For lesions longer than 12.85 mm – the median length in CREST – the combined outcome of strokes and death occurred in 1.9% of CEA and 6.1% of CAS patients (CAS odds ratio, 3.45; 95% confidence interval, 1.21-9.83). For sequential lesions, strokes and death occurred in 0.7% of CEA and 5.8% of CAS patients (CAS OR, 9.21; 95% CI, 1.23-68.94).
With long, sequential lesions distal to the carotid bulb, stroke and death occurred in 6.3% of CAS patients but no CEA patients, giving an “infinite odds ratio in favor of CEA,” Dr. Moore said.
Two-thirds of all the patients in CREST had lesion risk factors for CAS, which might help explain the original findings.
CREST also found that stenting was riskier in older people and women, but it seems likely now that age and gender were surrogates for adverse lesion characteristics.
“The fact of the matter is that older patients have more complex lesions, so age tended to be a surrogate for lesion complexity. If I have an 80-year-old with a short, isolated lesion, I don’t think the fact that they are 80 represents higher risk. I think the lesion being short puts them in the same low risk [category] as other favorable CAS characteristics. I think that’s also true for gender,” Dr. Moore said.
Angiograms almost always underestimate the length of carotid lesions. CT and MRI do a better job, but “ultrasound may be even better than those two,” he noted.
CREST was funded by the National Institutes of Health and Abbott Vascular Solutions. Dr. Moore has no disclosures. Other authors reported relationships with Abbott, Medtronic, Boston Scientific, and other companies.
CHICAGO – Carotid endarterectomy is safer than stenting when lesions have greater complexity based on length, location, and presence of sequential lesions, according to a subanalysis of the CREST study.
Previous studies have shown that carotid artery stenting (CAS) is also risky in patients with type 3 aortic arches; atherosclerotic aortic arches; internal carotid artery tortuosity; circumferential calcification; and ulcerated lesions.
Taken together, “we can now begin to populate a list of conditions that are high risk for carotid artery stenting. For patients with these factors, we would strongly recommend that carotid endarterectomy be employed rather than carotid artery stenting,” said CREST (Carotid Revascularization Endarterectomy versus Stent Trial) investigator Dr. Wesley Moore, professor and chief, emeritus, of the division of vascular surgery at the University of California, Los Angeles.
“However, in the absence of these higher risk characteristics, carotid artery stenting should yield results equivalent to carotid endarterectomy,” he said at a meeting hosted by the Society for Vascular Surgery.
CREST demonstrated that carotid artery stenting carries about twice the risk of stroke and death (4.4%) as carotid endarterectomy (2.3%); the investigators revisited their subjects’ preop angiograms to see if lesion characteristics were to blame in a subanalysis of the trial results.
In CREST, 438 patients had angiograms before carotid endarterectomies (CEA), about a third of the total number of CEA patients, while preop angiograms were done in all of the 1,262 CAS patients. There were no statistically significant differences in age, gender, stenosis symptoms, smoking history, arrhythmias, and left ventricular hypertrophy between CEA and CAS patients.
For lesions longer than 12.85 mm – the median length in CREST – the combined outcome of strokes and death occurred in 1.9% of CEA and 6.1% of CAS patients (CAS odds ratio, 3.45; 95% confidence interval, 1.21-9.83). For sequential lesions, strokes and death occurred in 0.7% of CEA and 5.8% of CAS patients (CAS OR, 9.21; 95% CI, 1.23-68.94).
With long, sequential lesions distal to the carotid bulb, stroke and death occurred in 6.3% of CAS patients but no CEA patients, giving an “infinite odds ratio in favor of CEA,” Dr. Moore said.
Two-thirds of all the patients in CREST had lesion risk factors for CAS, which might help explain the original findings.
CREST also found that stenting was riskier in older people and women, but it seems likely now that age and gender were surrogates for adverse lesion characteristics.
“The fact of the matter is that older patients have more complex lesions, so age tended to be a surrogate for lesion complexity. If I have an 80-year-old with a short, isolated lesion, I don’t think the fact that they are 80 represents higher risk. I think the lesion being short puts them in the same low risk [category] as other favorable CAS characteristics. I think that’s also true for gender,” Dr. Moore said.
Angiograms almost always underestimate the length of carotid lesions. CT and MRI do a better job, but “ultrasound may be even better than those two,” he noted.
CREST was funded by the National Institutes of Health and Abbott Vascular Solutions. Dr. Moore has no disclosures. Other authors reported relationships with Abbott, Medtronic, Boston Scientific, and other companies.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Carotid endarterectomy had a lower rate of the combined outcome of stroke or death in patients with greater lesion complexity, which may be a better marker than age or gender for determining the best treatment modality.
Major finding: For lesions longer than 12.85 mm, the combined outcome of strokes and death occurred in 1.9% of carotid endarterectomy patients and 6.1% of carotid stent patients (CAS OR, 3.45; 95% CI, 1.21-9.83). Subsanalysis of the CREST study
Data source: Subsanalysis of the CREST study
Disclosures: CREST was funded by the National Institutes of Health and Abbott Vascular Solutions. The presenter has no disclosures. Other authors reported relationships with Abbott, Medtronic, Boston Scientific, and other companies.
SVS: Opt for early repair of PDA/GDA splanchnic aneurysms
CHICAGO – Pancreaticoduodenal and gastroduodenal artery aneurysms should be repaired at diagnosis, according to Dr. Michael Corey, a vascular surgeon at Massachusetts General Hospital in Boston.
The reason is “they rupture at small sizes. Most other small splanchnic artery aneurysms” – below 25 mm – “do not grow or rupture over time and can safely undergo surveillance imaging every 3 years,” he said at a meeting hosted by the Society for Vascular Surgery.
The insights come from Dr. Corey’s review of 264 splanchnic artery aneurysms (SAAs) treated at Massachusetts General Hospital from 1994 to 2014 .
Pancreaticoduodenal (PDA) and gastroduodenal (GDA) artery aneurysms were the most likely to cause trouble. Almost all of the 36 in the study were associated with high-grade celiac axis stenosis, and 12 (33%) were symptomatic at presentation, including 7 (19%) that had ruptured at a mean size of 27.4 mm, range 15-48 mm.
Those 7 accounted for more than half of the 13 ruptures in the study. There were also five ruptures among 95 splenic artery aneurysms – the most common aneurysm type in the study – at a mean of 42 mm, and one among 34 hepatic artery aneurysms at 40 mm. Thirty-day morbidity after rupture repair was 54% and mortality 8%.
Pancreaticoduodenal (odds ratio, 14.41; 95% confidence interval, 3.5-59.9; P = .0002) and gastroduodenal artery aneurysms (OR, 6.95; 95% CI, 1.1-45.1; P = .042) were far more predictive of rupture than aneurysm size (OR, 1.04; 95% CI, 1.01-1.08; P = .0042). The strongest predictor was type 4 Ehlers-Danlos syndrome (OR, 34.09; 95% CI, 2.4-479.8; P = .0089). Calcification, meanwhile, did not predict rupture, growth, or thrombus burden.
Dr. Corey and his colleagues reviewed Massachusetts General’s experience with SAAs because “no strong consensus exists in the literature concerning the indications for treatment; 2 cm is currently the indication for surgical treatment of asymptomatic lesions,” he said.
Two centimeters might be too aggressive in some cases. Among 176 aneurysms put under surveillance for a mean of 36.1 months, the mean aneurysm size was 16.3 mm but ranged up to 40 mm. Even so, none of them ruptured. Just 12 aneurysms grew during surveillance, and only 8 eventually needed intervention. Perhaps most “small asymptomatic lesions do not affect longevity,” Dr. Corey said. The mean aneurysm size was 31.1 mm in the 88 patients repaired within 6 months of diagnosis. Splenic, pancreaticoduodenal, gastroduodenal, and hepatic aneurysms were the most likely to be repaired early, the majority by coil embolization and other endovascular techniques. Thirty-day morbidity for intact repair was 13% and mortality 3%.
Most of the splenic artery aneurysms were asymptomatic at presentation. In the half that were watched, just six grew.
Similarly, 78 celiac artery aneurysms – the second most common in the study – all presented without symptoms. Just 3 of the 60 under surveillance grew over a mean of 43.6 months. “These aneurysms rarely change,” Dr. Corey said.
Most of the 34 hepatic artery aneurysms and 17 superior mesenteric artery (SMA) aneurysms were asymptomatic. Between both groups, 20 aneurysms were put under surveillance; growth was noted in 1, an SMA lesion.
Although there was a shift from open to endovascular repair during the study period, there were no statistically significant differences in morbidity or mortality between the two approaches.
Dr. Corey has no disclosures.
CHICAGO – Pancreaticoduodenal and gastroduodenal artery aneurysms should be repaired at diagnosis, according to Dr. Michael Corey, a vascular surgeon at Massachusetts General Hospital in Boston.
The reason is “they rupture at small sizes. Most other small splanchnic artery aneurysms” – below 25 mm – “do not grow or rupture over time and can safely undergo surveillance imaging every 3 years,” he said at a meeting hosted by the Society for Vascular Surgery.
The insights come from Dr. Corey’s review of 264 splanchnic artery aneurysms (SAAs) treated at Massachusetts General Hospital from 1994 to 2014 .
Pancreaticoduodenal (PDA) and gastroduodenal (GDA) artery aneurysms were the most likely to cause trouble. Almost all of the 36 in the study were associated with high-grade celiac axis stenosis, and 12 (33%) were symptomatic at presentation, including 7 (19%) that had ruptured at a mean size of 27.4 mm, range 15-48 mm.
Those 7 accounted for more than half of the 13 ruptures in the study. There were also five ruptures among 95 splenic artery aneurysms – the most common aneurysm type in the study – at a mean of 42 mm, and one among 34 hepatic artery aneurysms at 40 mm. Thirty-day morbidity after rupture repair was 54% and mortality 8%.
Pancreaticoduodenal (odds ratio, 14.41; 95% confidence interval, 3.5-59.9; P = .0002) and gastroduodenal artery aneurysms (OR, 6.95; 95% CI, 1.1-45.1; P = .042) were far more predictive of rupture than aneurysm size (OR, 1.04; 95% CI, 1.01-1.08; P = .0042). The strongest predictor was type 4 Ehlers-Danlos syndrome (OR, 34.09; 95% CI, 2.4-479.8; P = .0089). Calcification, meanwhile, did not predict rupture, growth, or thrombus burden.
Dr. Corey and his colleagues reviewed Massachusetts General’s experience with SAAs because “no strong consensus exists in the literature concerning the indications for treatment; 2 cm is currently the indication for surgical treatment of asymptomatic lesions,” he said.
Two centimeters might be too aggressive in some cases. Among 176 aneurysms put under surveillance for a mean of 36.1 months, the mean aneurysm size was 16.3 mm but ranged up to 40 mm. Even so, none of them ruptured. Just 12 aneurysms grew during surveillance, and only 8 eventually needed intervention. Perhaps most “small asymptomatic lesions do not affect longevity,” Dr. Corey said. The mean aneurysm size was 31.1 mm in the 88 patients repaired within 6 months of diagnosis. Splenic, pancreaticoduodenal, gastroduodenal, and hepatic aneurysms were the most likely to be repaired early, the majority by coil embolization and other endovascular techniques. Thirty-day morbidity for intact repair was 13% and mortality 3%.
Most of the splenic artery aneurysms were asymptomatic at presentation. In the half that were watched, just six grew.
Similarly, 78 celiac artery aneurysms – the second most common in the study – all presented without symptoms. Just 3 of the 60 under surveillance grew over a mean of 43.6 months. “These aneurysms rarely change,” Dr. Corey said.
Most of the 34 hepatic artery aneurysms and 17 superior mesenteric artery (SMA) aneurysms were asymptomatic. Between both groups, 20 aneurysms were put under surveillance; growth was noted in 1, an SMA lesion.
Although there was a shift from open to endovascular repair during the study period, there were no statistically significant differences in morbidity or mortality between the two approaches.
Dr. Corey has no disclosures.
CHICAGO – Pancreaticoduodenal and gastroduodenal artery aneurysms should be repaired at diagnosis, according to Dr. Michael Corey, a vascular surgeon at Massachusetts General Hospital in Boston.
The reason is “they rupture at small sizes. Most other small splanchnic artery aneurysms” – below 25 mm – “do not grow or rupture over time and can safely undergo surveillance imaging every 3 years,” he said at a meeting hosted by the Society for Vascular Surgery.
The insights come from Dr. Corey’s review of 264 splanchnic artery aneurysms (SAAs) treated at Massachusetts General Hospital from 1994 to 2014 .
Pancreaticoduodenal (PDA) and gastroduodenal (GDA) artery aneurysms were the most likely to cause trouble. Almost all of the 36 in the study were associated with high-grade celiac axis stenosis, and 12 (33%) were symptomatic at presentation, including 7 (19%) that had ruptured at a mean size of 27.4 mm, range 15-48 mm.
Those 7 accounted for more than half of the 13 ruptures in the study. There were also five ruptures among 95 splenic artery aneurysms – the most common aneurysm type in the study – at a mean of 42 mm, and one among 34 hepatic artery aneurysms at 40 mm. Thirty-day morbidity after rupture repair was 54% and mortality 8%.
Pancreaticoduodenal (odds ratio, 14.41; 95% confidence interval, 3.5-59.9; P = .0002) and gastroduodenal artery aneurysms (OR, 6.95; 95% CI, 1.1-45.1; P = .042) were far more predictive of rupture than aneurysm size (OR, 1.04; 95% CI, 1.01-1.08; P = .0042). The strongest predictor was type 4 Ehlers-Danlos syndrome (OR, 34.09; 95% CI, 2.4-479.8; P = .0089). Calcification, meanwhile, did not predict rupture, growth, or thrombus burden.
Dr. Corey and his colleagues reviewed Massachusetts General’s experience with SAAs because “no strong consensus exists in the literature concerning the indications for treatment; 2 cm is currently the indication for surgical treatment of asymptomatic lesions,” he said.
Two centimeters might be too aggressive in some cases. Among 176 aneurysms put under surveillance for a mean of 36.1 months, the mean aneurysm size was 16.3 mm but ranged up to 40 mm. Even so, none of them ruptured. Just 12 aneurysms grew during surveillance, and only 8 eventually needed intervention. Perhaps most “small asymptomatic lesions do not affect longevity,” Dr. Corey said. The mean aneurysm size was 31.1 mm in the 88 patients repaired within 6 months of diagnosis. Splenic, pancreaticoduodenal, gastroduodenal, and hepatic aneurysms were the most likely to be repaired early, the majority by coil embolization and other endovascular techniques. Thirty-day morbidity for intact repair was 13% and mortality 3%.
Most of the splenic artery aneurysms were asymptomatic at presentation. In the half that were watched, just six grew.
Similarly, 78 celiac artery aneurysms – the second most common in the study – all presented without symptoms. Just 3 of the 60 under surveillance grew over a mean of 43.6 months. “These aneurysms rarely change,” Dr. Corey said.
Most of the 34 hepatic artery aneurysms and 17 superior mesenteric artery (SMA) aneurysms were asymptomatic. Between both groups, 20 aneurysms were put under surveillance; growth was noted in 1, an SMA lesion.
Although there was a shift from open to endovascular repair during the study period, there were no statistically significant differences in morbidity or mortality between the two approaches.
Dr. Corey has no disclosures.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Pancreaticoduodenal and gastroduodenal artery aneurysms rupture at smaller sizes than do other visceral aneurysms.
Major finding: Almost all of the 36 aneurysms in the study were associated with high-grade celiac axis stenosis, and 12 (33%) were symptomatic at presentation, including 7 (19%) that had ruptured at a mean size of 27.4 mm (range, 15-48 mm).
Data source: Review of 264 splanchnic artery aneurysms treated at Massachusetts General Hospital from 1994 to 2014.
Disclosures: The lead investigator has no relevant disclosures.
SVS: Don’t let TPA delay urgent carotid interventions for mild and moderate strokes
CHICAGO – Urgent carotid interventions were safe after thrombolysis for acute mild to moderate strokes, according to a review of 165 patients at the Ochsner Clinic in New Orleans.
“Our data support the practice of not denying a patient an urgent carotid intervention simply because of TPA [tissue plasminogen activator] administration during the acute stroke period,” said lead investigator Dr. Nicolas Zea, an Ochsner vascular surgeon.
“Urgent carotid endarterectomy [CEA] or coronary artery stenting [CAS] can be safely undertaken in minor to moderate strokes with NIH stroke scale scores less than 10; TPA itself does not appear to be a contraindication, even within 72 hours,” he added.
Urgent carotid interventions are becoming more common after ischemic strokes to prevent recurrences. The approach is most effective within 2 weeks of the index event, but there have been concerns that intracranial hemorrhages (ICH) and other complications might be more likely if patients have had TPA.
Dr. Ochsner and his colleagues conducted their review because, “as vascular surgeons, we are going to encounter a lot more of these patients in the very near future,” Dr. Zea said a meeting hosted by the Society for Vascular Surgery.
From January 2009 to January 2015, 31 patients at Ochsner had carotid interventions – 25 CEA, 6 CAS – a mean of 2.1 days after receiving TPA for transient ischemic attacks (TIA) or ischemic strokes. The patients’ mean National Institutes of Health Stroke Scale (NIHSS) score was 6.6.
Over the same period, 134 patients who had not received TPA had urgent carotid interventions – 110 CEA, 24 CAS – a mean of 2.6 days after TIA or ischemic stroke presentation. Their mean NIHSS score was 6.1.
There were no statistically significant demographic or comorbidity differences between the TPA and no-TPA groups; patients were about 70 years old, on average, and the majority were men. Most had ipsilateral carotid stenosis greater than 70%, or acute occlusions.
The 30-day overall complication rate was 12.9% in the TPA group and 6.7% in the non-TPA group, a nonsignificant difference (P = 0.35).
In the TPA group, there was one (3.2%) ICH, one (3.2%) neck hematoma, and two (6.4%) deaths. In the no-TPA group, there were two (1.5%) ICHs, two (1.5%) neck hematomas, one (0.7%) ischemic stroke, two (1.5%) myocardial infarctions, and two (1.5%) deaths.
In both groups, ICH patients had stroke scores greater than 10. Also, although the rate of death was higher in the TPA group, the deaths “were not necessarily related to thrombolysis,” Dr. Zea noted. One death was from pulmonary embolism, the second from unknown causes. Deaths were due to acute mesenteric ischemia and ICH in the no-TPA group, Dr. Zea said.
There was one (3.2%) hemorrhagic conversion in the TPA group and two (1.5%) in the no-TPA group. Similarly, one (3.2%) TPA patient and two (1.5%) no-TPA patients had complications from access site bleeding. The differences were not statistically significant.
In the TPA group, it didn’t seem to matter if intervention came within 72 hours of administration – as in about half the cases – or afterward, when TPA risks have largely passed. There was one death and one ICH in patients in the earlier group, and one death in the later group, a nonsignificant difference.
There was no outside funding for the study. Dr. Zea had no disclosures. One of the coinvestigators is a consultant for Lutonix.
CHICAGO – Urgent carotid interventions were safe after thrombolysis for acute mild to moderate strokes, according to a review of 165 patients at the Ochsner Clinic in New Orleans.
“Our data support the practice of not denying a patient an urgent carotid intervention simply because of TPA [tissue plasminogen activator] administration during the acute stroke period,” said lead investigator Dr. Nicolas Zea, an Ochsner vascular surgeon.
“Urgent carotid endarterectomy [CEA] or coronary artery stenting [CAS] can be safely undertaken in minor to moderate strokes with NIH stroke scale scores less than 10; TPA itself does not appear to be a contraindication, even within 72 hours,” he added.
Urgent carotid interventions are becoming more common after ischemic strokes to prevent recurrences. The approach is most effective within 2 weeks of the index event, but there have been concerns that intracranial hemorrhages (ICH) and other complications might be more likely if patients have had TPA.
Dr. Ochsner and his colleagues conducted their review because, “as vascular surgeons, we are going to encounter a lot more of these patients in the very near future,” Dr. Zea said a meeting hosted by the Society for Vascular Surgery.
From January 2009 to January 2015, 31 patients at Ochsner had carotid interventions – 25 CEA, 6 CAS – a mean of 2.1 days after receiving TPA for transient ischemic attacks (TIA) or ischemic strokes. The patients’ mean National Institutes of Health Stroke Scale (NIHSS) score was 6.6.
Over the same period, 134 patients who had not received TPA had urgent carotid interventions – 110 CEA, 24 CAS – a mean of 2.6 days after TIA or ischemic stroke presentation. Their mean NIHSS score was 6.1.
There were no statistically significant demographic or comorbidity differences between the TPA and no-TPA groups; patients were about 70 years old, on average, and the majority were men. Most had ipsilateral carotid stenosis greater than 70%, or acute occlusions.
The 30-day overall complication rate was 12.9% in the TPA group and 6.7% in the non-TPA group, a nonsignificant difference (P = 0.35).
In the TPA group, there was one (3.2%) ICH, one (3.2%) neck hematoma, and two (6.4%) deaths. In the no-TPA group, there were two (1.5%) ICHs, two (1.5%) neck hematomas, one (0.7%) ischemic stroke, two (1.5%) myocardial infarctions, and two (1.5%) deaths.
In both groups, ICH patients had stroke scores greater than 10. Also, although the rate of death was higher in the TPA group, the deaths “were not necessarily related to thrombolysis,” Dr. Zea noted. One death was from pulmonary embolism, the second from unknown causes. Deaths were due to acute mesenteric ischemia and ICH in the no-TPA group, Dr. Zea said.
There was one (3.2%) hemorrhagic conversion in the TPA group and two (1.5%) in the no-TPA group. Similarly, one (3.2%) TPA patient and two (1.5%) no-TPA patients had complications from access site bleeding. The differences were not statistically significant.
In the TPA group, it didn’t seem to matter if intervention came within 72 hours of administration – as in about half the cases – or afterward, when TPA risks have largely passed. There was one death and one ICH in patients in the earlier group, and one death in the later group, a nonsignificant difference.
There was no outside funding for the study. Dr. Zea had no disclosures. One of the coinvestigators is a consultant for Lutonix.
CHICAGO – Urgent carotid interventions were safe after thrombolysis for acute mild to moderate strokes, according to a review of 165 patients at the Ochsner Clinic in New Orleans.
“Our data support the practice of not denying a patient an urgent carotid intervention simply because of TPA [tissue plasminogen activator] administration during the acute stroke period,” said lead investigator Dr. Nicolas Zea, an Ochsner vascular surgeon.
“Urgent carotid endarterectomy [CEA] or coronary artery stenting [CAS] can be safely undertaken in minor to moderate strokes with NIH stroke scale scores less than 10; TPA itself does not appear to be a contraindication, even within 72 hours,” he added.
Urgent carotid interventions are becoming more common after ischemic strokes to prevent recurrences. The approach is most effective within 2 weeks of the index event, but there have been concerns that intracranial hemorrhages (ICH) and other complications might be more likely if patients have had TPA.
Dr. Ochsner and his colleagues conducted their review because, “as vascular surgeons, we are going to encounter a lot more of these patients in the very near future,” Dr. Zea said a meeting hosted by the Society for Vascular Surgery.
From January 2009 to January 2015, 31 patients at Ochsner had carotid interventions – 25 CEA, 6 CAS – a mean of 2.1 days after receiving TPA for transient ischemic attacks (TIA) or ischemic strokes. The patients’ mean National Institutes of Health Stroke Scale (NIHSS) score was 6.6.
Over the same period, 134 patients who had not received TPA had urgent carotid interventions – 110 CEA, 24 CAS – a mean of 2.6 days after TIA or ischemic stroke presentation. Their mean NIHSS score was 6.1.
There were no statistically significant demographic or comorbidity differences between the TPA and no-TPA groups; patients were about 70 years old, on average, and the majority were men. Most had ipsilateral carotid stenosis greater than 70%, or acute occlusions.
The 30-day overall complication rate was 12.9% in the TPA group and 6.7% in the non-TPA group, a nonsignificant difference (P = 0.35).
In the TPA group, there was one (3.2%) ICH, one (3.2%) neck hematoma, and two (6.4%) deaths. In the no-TPA group, there were two (1.5%) ICHs, two (1.5%) neck hematomas, one (0.7%) ischemic stroke, two (1.5%) myocardial infarctions, and two (1.5%) deaths.
In both groups, ICH patients had stroke scores greater than 10. Also, although the rate of death was higher in the TPA group, the deaths “were not necessarily related to thrombolysis,” Dr. Zea noted. One death was from pulmonary embolism, the second from unknown causes. Deaths were due to acute mesenteric ischemia and ICH in the no-TPA group, Dr. Zea said.
There was one (3.2%) hemorrhagic conversion in the TPA group and two (1.5%) in the no-TPA group. Similarly, one (3.2%) TPA patient and two (1.5%) no-TPA patients had complications from access site bleeding. The differences were not statistically significant.
In the TPA group, it didn’t seem to matter if intervention came within 72 hours of administration – as in about half the cases – or afterward, when TPA risks have largely passed. There was one death and one ICH in patients in the earlier group, and one death in the later group, a nonsignificant difference.
There was no outside funding for the study. Dr. Zea had no disclosures. One of the coinvestigators is a consultant for Lutonix.
AT THE 2015 VASCULAR ANNUAL MEETING
Key clinical point: Tissue plasminogen activator does not contraindicate urgent carotid endarterectomies or stenting.
Major finding: The 30-day overall complication rate was 12.9% in the TPA group and 6.7% in the no-TPA group, a nonsignificant difference (P = 0.35).
Data source: A retrospective study of 165 patients.
Disclosures: There was no outside funding for the study. Dr. Zea had no disclosures. One of the coinvestigators is a consultant for Lutonix.
A continuous cardiac murmur
A 45-year-old woman presents with shortness of breath that has been progressively worsening for 3 weeks. She has no history of medical conditions and is taking no medications. Her blood pressure is 132/68 mm Hg, pulse 90 beats per minute, respirations 14 per minute, and oxygen saturation 95% on room air by pulse oximetry.
Physical examination reveals clear lung fields and no jugular venous distention or peripheral edema. However, she has a grade 3 of 6 continuous murmur audible over the entire precordium that does not change in intensity with respiration.
1. Which of the following is the likely cause of this patient’s cardiac murmur?
- Ventricular septal defect
- Atrial septal defect
- Ruptured sinus of Valsalva aneurysm
- Aortic regurgitation
- Patent ductus arteriosus
- Pulmonic stenosis
Table 1 summarizes the characteristics of the murmurs caused by these various cardiac defects.
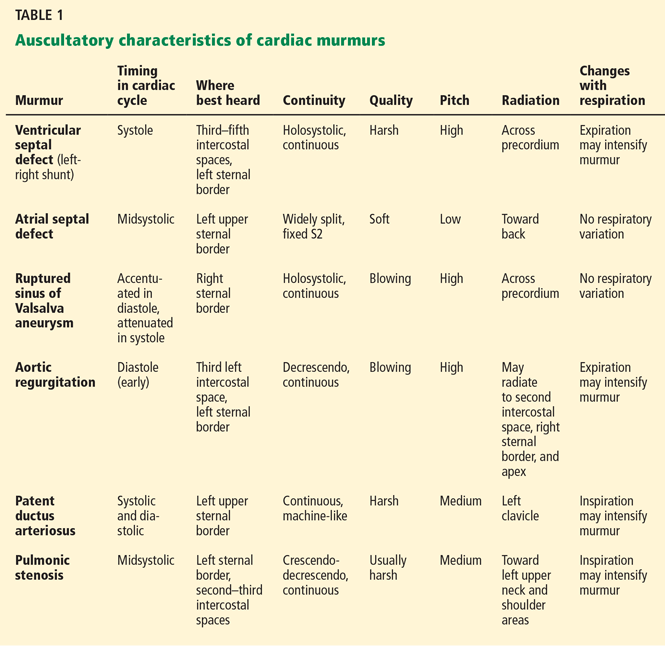
Ventricular septal defect causes murmurs that are characteristically holosystolic and heard best at the lower left sternal border with radiation to the right lower sternal border, which overlies the defect.
The murmur of restrictive ventricular septal defect is most often holosystolic because the pressure difference between the ventricles is generated almost instantly at the onset of systole with a left-to-right shunt continuing throughout ventricular contraction. In contrast, nonrestrictive ventricular septal defects generally do not generate a murmur, since pressure is equalized across the defect. This left-to-right shunting may lead to right ventricular volume overload, resulting in delayed closure of the pulmonary valve and a widely split S2. Irreversible pulmonary hypertension with shunt reversal may occur if the defect remains untreated.1
Atrial septal defect. The most characteristic feature of atrial septal defect is a fixed split S2 resulting from right ventricular volume overload due to left-to-right atrial shunting of blood flow. As flow is shunted from the left to the right atrium and subsequently into the right ventricle, ejection of excess blood through the pulmonary valve produces a midsystolic flow murmur, heard best over the left upper sternal border, that may radiate to the back.
Ruptured sinus of Valsalva aneurysm. The pressure is higher in the aorta than in the right atrium throughout the cardiac cycle, and if a shunt is created between the two structures by a ruptured sinus of Valsalva aneurysm, the blood flow across this shunt throughout the cardiac cycle produces a continuous murmur. In contrast, if a sinus of Valsalva aneurysm ruptures into the right ventricle, the murmur is accentuated in diastole and attenuated in systole, and is often associated with pounding pulses and a thrill along either the left or right sternal border.1
Aortic regurgitation causes a diastolic murmur as blood flows retrograde into the left ventricle through the incompetent aortic valve. This murmur is usually described as a blowing, decrescendo murmur heard best at the third left intercostal space.
Patent ductus arteriosus is a communication between the descending thoracic aorta and the pulmonary artery that fails to close at birth. The hallmark murmur associated with this defect is a continuous “machine-like” murmur located at the upper left sternal border, often radiating down the left side of the sternum into the back. Of note, increasing the systemic pressure by the Valsalva maneuver or handgrip exercise will increase the diastolic component of the continuous murmur associated with ruptured sinus of Valsalva aneurysm, helping to differentiate it from patent ductus arteriosus.2
Pulmonic stenosis causes a systolic murmur heard best at the second intercostal space along the left sternal border and having a crescendo-decrescendo intensity and harsh quality. As the right ventricle takes longer to eject its blood volume through the stenotic pulmonary valve, the delay in closure between the aortic and pulmonary valve is widened, resulting in a significant splitting of the S2. In addition, any maneuver that increases preload will also increase the intensity of the murmur.3
Our patient has a murmur that is continuous, is heard across the entire precordium, and has no respiratory variation. These features are most consistent with a sinus of Valsalva aneurysm that has ruptured into the right atrium.
The 2008 update of the joint American College of Cardiology and American Heart Association guidelines4 recommends further evaluation of diastolic or continuous murmurs with echocardiography, as these murmurs are most often signs of a pathologic condition. In addition, echocardiography is warranted to evaluate grade 3 or higher systolic murmurs and those that are holosystolic.4
SINUS OF VALSALVA ANEURYSM
Sinus of Valsalva aneurysm is rare, with an incidence of 0.09% to 0.15%. From 65% to 85% are in the right coronary cusp, 10% to 30% are in the noncoronary cusp, and fewer than 5% are in the left coronary cusp.5
This condition is most often congenital, accounting for up to 3.5% of congenital cardiac anomalies, though it can be acquired. Formation of the aneurysm is generally related to weakening of elastic fibers and muscular tissues that progresses over time.
Many cases of sinus of Valsalva aneurysm are associated with additional cardiac defects.1 Ventricular septal defect is the most common coexisting congenital anomaly, occurring in up to 53% of patients and frequently associated with aneurysms involving the right coronary cusp and with sinus of Valsalva aneurysm.6 Other congenital anomalies often accompanying sinus of Valsalva aneurysm include pulmonary stenosis, atrial septal defect, bicuspid aortic valve, tetralogy of Fallot, patent ductus arteriosus, coarctation of the aorta, and subaortic stenosis. Another associated condition is aortic regurgitation, for which more than half of affected patients eventually require aortic valve replacement.2
Acquired sinus of Valsalva aneurysm can be the result of endocarditis, trauma, surgery, cardiac catheterization, or inflammatory or degenerative processes including, rarely, tertiary syphilis.3
Sinus of Valsalva aneurysm often remains asymptomatic, but symptoms may arise if the aneurysm ruptures, resulting in intracardiac shunting or aneurysm-associated compression of adjacent cardiac structures such as coronary arteries. Rupture may be spontaneous, secondary to chest trauma or excess exertion, or iatrogenic.
Imaging studies such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging are essential in diagnosing and managing sinus of Valsalva aneurysm and identifying coexisting cardiac anomalies.
Rupture occurs most commonly into the right ventricle, followed in frequency by the right atrium or left atrium. Once rupture occurs, median survival is 1 to 2 years if left untreated, with death often secondary to congestive heart failure or infective endocarditis.7
Surgery remains the preferred approach to the treatment of ruptured sinus of Valsalva aneurysm. Operative risk is reasonably low and long-term outcomes are good. The appropriate therapy for unruptured and asymptomatic sinus of Valsalva aneurysm remains less clear.
Successful transcatheter closure of ruptured sinus of Valsalva aneurysm has been described using Amplatzer devices, a procedure that avoids sternotomy and cardiopulmonary bypass. Despite advances in percutaneous techniques, open surgery with or without aortic valve replacement remains the current standard of care.8
BACK TO OUR PATIENT
In the case described above, the initial diagnostic study done to evaluate the patient’s dyspnea and murmur was transthoracic echocardiography, which demonstrated a relatively preserved ejection fraction with mild aortic regurgitation and an aneurysmal structure extending from the aortic root toward the right atrium.
Transesophageal echocardiography confirmed this finding (Figure 1). Cross-sectional imaging of the aortic valve (Figure 2) showed the aneurysm arising from the noncoronary cusp and communicating with the right atrium. Color flow Doppler (Figure 3) confirmed continuous flow between the aneurysmal sinus and right atrium throughout the cardiac cycle, consistent with the continuous murmur noted on physical examination.
The aneurysm was also noted on aortography (Figure 4) obtained before the patient underwent surgery to correct it. The surgery was successful, no complications occurred, and the murmur and associated dyspnea had completely resolved at subsequent follow-up.
This case highlights the importance of imaging studies such as echocardiography in diagnosing and managing sinus of Valsalva aneurysm, and also the importance of physical examination in guiding the diagnostic evaluation and differentiating this condition from other cardiac disorders.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2011:1411–1468.
- Topi B, John J, Agarwal A, et al. An uncommon cause of a continuous murmur. Exp Clin Cardiol 2012; 17:148–149.
- Constant J. Bedside Cardiology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:268–320.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg 2008; 135:1153–1158.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 2010; 18:209–211.
- Moustafa S, Mookadam F, Cooper L, et al. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol 2007; 99:1159–1164.
- Zhao SH, Yan CW, Zhu XY, et al. Transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with an Amplatzer duct occluder. Int J Cardiol 2008; 129:81–85.
A 45-year-old woman presents with shortness of breath that has been progressively worsening for 3 weeks. She has no history of medical conditions and is taking no medications. Her blood pressure is 132/68 mm Hg, pulse 90 beats per minute, respirations 14 per minute, and oxygen saturation 95% on room air by pulse oximetry.
Physical examination reveals clear lung fields and no jugular venous distention or peripheral edema. However, she has a grade 3 of 6 continuous murmur audible over the entire precordium that does not change in intensity with respiration.
1. Which of the following is the likely cause of this patient’s cardiac murmur?
- Ventricular septal defect
- Atrial septal defect
- Ruptured sinus of Valsalva aneurysm
- Aortic regurgitation
- Patent ductus arteriosus
- Pulmonic stenosis
Table 1 summarizes the characteristics of the murmurs caused by these various cardiac defects.

Ventricular septal defect causes murmurs that are characteristically holosystolic and heard best at the lower left sternal border with radiation to the right lower sternal border, which overlies the defect.
The murmur of restrictive ventricular septal defect is most often holosystolic because the pressure difference between the ventricles is generated almost instantly at the onset of systole with a left-to-right shunt continuing throughout ventricular contraction. In contrast, nonrestrictive ventricular septal defects generally do not generate a murmur, since pressure is equalized across the defect. This left-to-right shunting may lead to right ventricular volume overload, resulting in delayed closure of the pulmonary valve and a widely split S2. Irreversible pulmonary hypertension with shunt reversal may occur if the defect remains untreated.1
Atrial septal defect. The most characteristic feature of atrial septal defect is a fixed split S2 resulting from right ventricular volume overload due to left-to-right atrial shunting of blood flow. As flow is shunted from the left to the right atrium and subsequently into the right ventricle, ejection of excess blood through the pulmonary valve produces a midsystolic flow murmur, heard best over the left upper sternal border, that may radiate to the back.
Ruptured sinus of Valsalva aneurysm. The pressure is higher in the aorta than in the right atrium throughout the cardiac cycle, and if a shunt is created between the two structures by a ruptured sinus of Valsalva aneurysm, the blood flow across this shunt throughout the cardiac cycle produces a continuous murmur. In contrast, if a sinus of Valsalva aneurysm ruptures into the right ventricle, the murmur is accentuated in diastole and attenuated in systole, and is often associated with pounding pulses and a thrill along either the left or right sternal border.1
Aortic regurgitation causes a diastolic murmur as blood flows retrograde into the left ventricle through the incompetent aortic valve. This murmur is usually described as a blowing, decrescendo murmur heard best at the third left intercostal space.
Patent ductus arteriosus is a communication between the descending thoracic aorta and the pulmonary artery that fails to close at birth. The hallmark murmur associated with this defect is a continuous “machine-like” murmur located at the upper left sternal border, often radiating down the left side of the sternum into the back. Of note, increasing the systemic pressure by the Valsalva maneuver or handgrip exercise will increase the diastolic component of the continuous murmur associated with ruptured sinus of Valsalva aneurysm, helping to differentiate it from patent ductus arteriosus.2
Pulmonic stenosis causes a systolic murmur heard best at the second intercostal space along the left sternal border and having a crescendo-decrescendo intensity and harsh quality. As the right ventricle takes longer to eject its blood volume through the stenotic pulmonary valve, the delay in closure between the aortic and pulmonary valve is widened, resulting in a significant splitting of the S2. In addition, any maneuver that increases preload will also increase the intensity of the murmur.3
Our patient has a murmur that is continuous, is heard across the entire precordium, and has no respiratory variation. These features are most consistent with a sinus of Valsalva aneurysm that has ruptured into the right atrium.
The 2008 update of the joint American College of Cardiology and American Heart Association guidelines4 recommends further evaluation of diastolic or continuous murmurs with echocardiography, as these murmurs are most often signs of a pathologic condition. In addition, echocardiography is warranted to evaluate grade 3 or higher systolic murmurs and those that are holosystolic.4
SINUS OF VALSALVA ANEURYSM
Sinus of Valsalva aneurysm is rare, with an incidence of 0.09% to 0.15%. From 65% to 85% are in the right coronary cusp, 10% to 30% are in the noncoronary cusp, and fewer than 5% are in the left coronary cusp.5
This condition is most often congenital, accounting for up to 3.5% of congenital cardiac anomalies, though it can be acquired. Formation of the aneurysm is generally related to weakening of elastic fibers and muscular tissues that progresses over time.
Many cases of sinus of Valsalva aneurysm are associated with additional cardiac defects.1 Ventricular septal defect is the most common coexisting congenital anomaly, occurring in up to 53% of patients and frequently associated with aneurysms involving the right coronary cusp and with sinus of Valsalva aneurysm.6 Other congenital anomalies often accompanying sinus of Valsalva aneurysm include pulmonary stenosis, atrial septal defect, bicuspid aortic valve, tetralogy of Fallot, patent ductus arteriosus, coarctation of the aorta, and subaortic stenosis. Another associated condition is aortic regurgitation, for which more than half of affected patients eventually require aortic valve replacement.2
Acquired sinus of Valsalva aneurysm can be the result of endocarditis, trauma, surgery, cardiac catheterization, or inflammatory or degenerative processes including, rarely, tertiary syphilis.3
Sinus of Valsalva aneurysm often remains asymptomatic, but symptoms may arise if the aneurysm ruptures, resulting in intracardiac shunting or aneurysm-associated compression of adjacent cardiac structures such as coronary arteries. Rupture may be spontaneous, secondary to chest trauma or excess exertion, or iatrogenic.
Imaging studies such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging are essential in diagnosing and managing sinus of Valsalva aneurysm and identifying coexisting cardiac anomalies.
Rupture occurs most commonly into the right ventricle, followed in frequency by the right atrium or left atrium. Once rupture occurs, median survival is 1 to 2 years if left untreated, with death often secondary to congestive heart failure or infective endocarditis.7
Surgery remains the preferred approach to the treatment of ruptured sinus of Valsalva aneurysm. Operative risk is reasonably low and long-term outcomes are good. The appropriate therapy for unruptured and asymptomatic sinus of Valsalva aneurysm remains less clear.
Successful transcatheter closure of ruptured sinus of Valsalva aneurysm has been described using Amplatzer devices, a procedure that avoids sternotomy and cardiopulmonary bypass. Despite advances in percutaneous techniques, open surgery with or without aortic valve replacement remains the current standard of care.8
BACK TO OUR PATIENT
In the case described above, the initial diagnostic study done to evaluate the patient’s dyspnea and murmur was transthoracic echocardiography, which demonstrated a relatively preserved ejection fraction with mild aortic regurgitation and an aneurysmal structure extending from the aortic root toward the right atrium.
Transesophageal echocardiography confirmed this finding (Figure 1). Cross-sectional imaging of the aortic valve (Figure 2) showed the aneurysm arising from the noncoronary cusp and communicating with the right atrium. Color flow Doppler (Figure 3) confirmed continuous flow between the aneurysmal sinus and right atrium throughout the cardiac cycle, consistent with the continuous murmur noted on physical examination.



The aneurysm was also noted on aortography (Figure 4) obtained before the patient underwent surgery to correct it. The surgery was successful, no complications occurred, and the murmur and associated dyspnea had completely resolved at subsequent follow-up.

This case highlights the importance of imaging studies such as echocardiography in diagnosing and managing sinus of Valsalva aneurysm, and also the importance of physical examination in guiding the diagnostic evaluation and differentiating this condition from other cardiac disorders.
A 45-year-old woman presents with shortness of breath that has been progressively worsening for 3 weeks. She has no history of medical conditions and is taking no medications. Her blood pressure is 132/68 mm Hg, pulse 90 beats per minute, respirations 14 per minute, and oxygen saturation 95% on room air by pulse oximetry.
Physical examination reveals clear lung fields and no jugular venous distention or peripheral edema. However, she has a grade 3 of 6 continuous murmur audible over the entire precordium that does not change in intensity with respiration.
1. Which of the following is the likely cause of this patient’s cardiac murmur?
- Ventricular septal defect
- Atrial septal defect
- Ruptured sinus of Valsalva aneurysm
- Aortic regurgitation
- Patent ductus arteriosus
- Pulmonic stenosis
Table 1 summarizes the characteristics of the murmurs caused by these various cardiac defects.

Ventricular septal defect causes murmurs that are characteristically holosystolic and heard best at the lower left sternal border with radiation to the right lower sternal border, which overlies the defect.
The murmur of restrictive ventricular septal defect is most often holosystolic because the pressure difference between the ventricles is generated almost instantly at the onset of systole with a left-to-right shunt continuing throughout ventricular contraction. In contrast, nonrestrictive ventricular septal defects generally do not generate a murmur, since pressure is equalized across the defect. This left-to-right shunting may lead to right ventricular volume overload, resulting in delayed closure of the pulmonary valve and a widely split S2. Irreversible pulmonary hypertension with shunt reversal may occur if the defect remains untreated.1
Atrial septal defect. The most characteristic feature of atrial septal defect is a fixed split S2 resulting from right ventricular volume overload due to left-to-right atrial shunting of blood flow. As flow is shunted from the left to the right atrium and subsequently into the right ventricle, ejection of excess blood through the pulmonary valve produces a midsystolic flow murmur, heard best over the left upper sternal border, that may radiate to the back.
Ruptured sinus of Valsalva aneurysm. The pressure is higher in the aorta than in the right atrium throughout the cardiac cycle, and if a shunt is created between the two structures by a ruptured sinus of Valsalva aneurysm, the blood flow across this shunt throughout the cardiac cycle produces a continuous murmur. In contrast, if a sinus of Valsalva aneurysm ruptures into the right ventricle, the murmur is accentuated in diastole and attenuated in systole, and is often associated with pounding pulses and a thrill along either the left or right sternal border.1
Aortic regurgitation causes a diastolic murmur as blood flows retrograde into the left ventricle through the incompetent aortic valve. This murmur is usually described as a blowing, decrescendo murmur heard best at the third left intercostal space.
Patent ductus arteriosus is a communication between the descending thoracic aorta and the pulmonary artery that fails to close at birth. The hallmark murmur associated with this defect is a continuous “machine-like” murmur located at the upper left sternal border, often radiating down the left side of the sternum into the back. Of note, increasing the systemic pressure by the Valsalva maneuver or handgrip exercise will increase the diastolic component of the continuous murmur associated with ruptured sinus of Valsalva aneurysm, helping to differentiate it from patent ductus arteriosus.2
Pulmonic stenosis causes a systolic murmur heard best at the second intercostal space along the left sternal border and having a crescendo-decrescendo intensity and harsh quality. As the right ventricle takes longer to eject its blood volume through the stenotic pulmonary valve, the delay in closure between the aortic and pulmonary valve is widened, resulting in a significant splitting of the S2. In addition, any maneuver that increases preload will also increase the intensity of the murmur.3
Our patient has a murmur that is continuous, is heard across the entire precordium, and has no respiratory variation. These features are most consistent with a sinus of Valsalva aneurysm that has ruptured into the right atrium.
The 2008 update of the joint American College of Cardiology and American Heart Association guidelines4 recommends further evaluation of diastolic or continuous murmurs with echocardiography, as these murmurs are most often signs of a pathologic condition. In addition, echocardiography is warranted to evaluate grade 3 or higher systolic murmurs and those that are holosystolic.4
SINUS OF VALSALVA ANEURYSM
Sinus of Valsalva aneurysm is rare, with an incidence of 0.09% to 0.15%. From 65% to 85% are in the right coronary cusp, 10% to 30% are in the noncoronary cusp, and fewer than 5% are in the left coronary cusp.5
This condition is most often congenital, accounting for up to 3.5% of congenital cardiac anomalies, though it can be acquired. Formation of the aneurysm is generally related to weakening of elastic fibers and muscular tissues that progresses over time.
Many cases of sinus of Valsalva aneurysm are associated with additional cardiac defects.1 Ventricular septal defect is the most common coexisting congenital anomaly, occurring in up to 53% of patients and frequently associated with aneurysms involving the right coronary cusp and with sinus of Valsalva aneurysm.6 Other congenital anomalies often accompanying sinus of Valsalva aneurysm include pulmonary stenosis, atrial septal defect, bicuspid aortic valve, tetralogy of Fallot, patent ductus arteriosus, coarctation of the aorta, and subaortic stenosis. Another associated condition is aortic regurgitation, for which more than half of affected patients eventually require aortic valve replacement.2
Acquired sinus of Valsalva aneurysm can be the result of endocarditis, trauma, surgery, cardiac catheterization, or inflammatory or degenerative processes including, rarely, tertiary syphilis.3
Sinus of Valsalva aneurysm often remains asymptomatic, but symptoms may arise if the aneurysm ruptures, resulting in intracardiac shunting or aneurysm-associated compression of adjacent cardiac structures such as coronary arteries. Rupture may be spontaneous, secondary to chest trauma or excess exertion, or iatrogenic.
Imaging studies such as echocardiography, cardiac computed tomography, and cardiac magnetic resonance imaging are essential in diagnosing and managing sinus of Valsalva aneurysm and identifying coexisting cardiac anomalies.
Rupture occurs most commonly into the right ventricle, followed in frequency by the right atrium or left atrium. Once rupture occurs, median survival is 1 to 2 years if left untreated, with death often secondary to congestive heart failure or infective endocarditis.7
Surgery remains the preferred approach to the treatment of ruptured sinus of Valsalva aneurysm. Operative risk is reasonably low and long-term outcomes are good. The appropriate therapy for unruptured and asymptomatic sinus of Valsalva aneurysm remains less clear.
Successful transcatheter closure of ruptured sinus of Valsalva aneurysm has been described using Amplatzer devices, a procedure that avoids sternotomy and cardiopulmonary bypass. Despite advances in percutaneous techniques, open surgery with or without aortic valve replacement remains the current standard of care.8
BACK TO OUR PATIENT
In the case described above, the initial diagnostic study done to evaluate the patient’s dyspnea and murmur was transthoracic echocardiography, which demonstrated a relatively preserved ejection fraction with mild aortic regurgitation and an aneurysmal structure extending from the aortic root toward the right atrium.
Transesophageal echocardiography confirmed this finding (Figure 1). Cross-sectional imaging of the aortic valve (Figure 2) showed the aneurysm arising from the noncoronary cusp and communicating with the right atrium. Color flow Doppler (Figure 3) confirmed continuous flow between the aneurysmal sinus and right atrium throughout the cardiac cycle, consistent with the continuous murmur noted on physical examination.



The aneurysm was also noted on aortography (Figure 4) obtained before the patient underwent surgery to correct it. The surgery was successful, no complications occurred, and the murmur and associated dyspnea had completely resolved at subsequent follow-up.

This case highlights the importance of imaging studies such as echocardiography in diagnosing and managing sinus of Valsalva aneurysm, and also the importance of physical examination in guiding the diagnostic evaluation and differentiating this condition from other cardiac disorders.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2011:1411–1468.
- Topi B, John J, Agarwal A, et al. An uncommon cause of a continuous murmur. Exp Clin Cardiol 2012; 17:148–149.
- Constant J. Bedside Cardiology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:268–320.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg 2008; 135:1153–1158.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 2010; 18:209–211.
- Moustafa S, Mookadam F, Cooper L, et al. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol 2007; 99:1159–1164.
- Zhao SH, Yan CW, Zhu XY, et al. Transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with an Amplatzer duct occluder. Int J Cardiol 2008; 129:81–85.
- Bonow RO, Mann DL, Zipes DP, Libby P. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2011:1411–1468.
- Topi B, John J, Agarwal A, et al. An uncommon cause of a continuous murmur. Exp Clin Cardiol 2012; 17:148–149.
- Constant J. Bedside Cardiology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999:268–320.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008; 118:e523–e661.
- Jung SH, Yun TJ, Im YM, et al. Ruptured sinus of Valsalva aneurysm: transaortic repair may cause sinus of Valsalva distortion and aortic regurgitation. J Thorac Cardiovasc Surg 2008; 135:1153–1158.
- Post MC, Braam RL, Groenemeijer BE, Nicastia D, Rensing BJ, Schepens MA. Rupture of right coronary sinus of Valsalva aneurysm into right ventricle. Neth Heart J 2010; 18:209–211.
- Moustafa S, Mookadam F, Cooper L, et al. Sinus of Valsalva aneurysms—47 years of a single center experience and systematic overview of published reports. Am J Cardiol 2007; 99:1159–1164.
- Zhao SH, Yan CW, Zhu XY, et al. Transcatheter occlusion of the ruptured sinus of Valsalva aneurysm with an Amplatzer duct occluder. Int J Cardiol 2008; 129:81–85.
SVS: Four easy preop variables predict mortality in ruptured AAAs
CHICAGO – Age greater than 76 years, plus preoperative creatinine greater than 2 mg/dL, blood pH less than 7.2, and systolic pressure at any point below 70 mm Hg collectively predicted 100% mortality with open or endovascular repair of ruptured abdominal aortic aneurysms, according to a new mortality risk score from Harborview Medical Center in Seattle.
Meeting all four criteria gives the maximum score of 4. Any one of the factors alone – a score of 1 – predicted 30% mortality with open repair and 9% with endovascular aneurysm repair (EVAR); a 2 predicted 80% mortality with open repair and 37% with EVAR; and a 3 predicted 82% mortality with open repair and 70% with EVAR.
Vascular surgeons at Harborview developed the system so they’d know whether to recommend transport or comfort care for ruptured abdominal aortic aneurysms (AAAs). The Level 1 trauma center serves more than a quarter of the U.S. landmass, and handles about 30-40 ruptured AAA’s annually. It’s not uncommon for patients to be flown in from Alaska.
Existing risk scores haven’t been validated for EVAR or rely on intraoperative variables, so they aren’t much help when counseling patients and referring physicians on what to do.
“Our ruptured AAA mortality risk score is based on four variables readily assessed preoperatively, allows accurate prediction of in-hospital mortality after repair of ruptured AAAs in the EVAR-first era, and does so better than any score thus published. It’s clinically relevant to the decision to transport and helps guide difficult discussions with patients and their families,” said investigator Dr. Ty Garland, chief vascular surgery resident at the University of Washington, Seattle.
When using the new risk score. “we don’t ever block transfer, but we have discussions with referring providers and in several cases with patients and their families over the telephone.” When the situation is hopeless, “we explain the data.” Twice in the past 6 months, patients have opted to spend their last hours at home with their families, Dr. Garland said at the Society for Vascular Surgery’s annual meeting.
To develop their system, the investigators culled through 37,000 variables from 303 ruptured AAA patients treated at Harborview from 2002-2013. Fifteen patients died in the emergency department, en route to surgery, or after choosing comfort care. Overall, 30-day mortality was 54% for open repair and 22% for EVAR.
On multivariate analysis, the team isolated the four preoperative variables most predictive of death. Preoperative creatinine greater than 2 mg/dL almost quadrupled the risk (odds ratio 3.7; P < .001); systolic blood pressure less than 70 mm Hg nearly tripled it (OR 2.7; P = .002); and pH less than 7.2 (OR 2.6; P = .009) and age greater than 76 years (OR 2.1; P = .011) more than doubled it.
The investigators then checked their results against the Vascular Study Group of New England Cardiac Index, Glasgow Aneurysm Score, and Edinburgh Ruptured Aneurysm Score. “Our preoperative risk score was most predictive of death, with an area under the curve of 0.76,” Dr. Garland said.
There was no outside funding for the work and Dr. Garland has no disclosures.
CHICAGO – Age greater than 76 years, plus preoperative creatinine greater than 2 mg/dL, blood pH less than 7.2, and systolic pressure at any point below 70 mm Hg collectively predicted 100% mortality with open or endovascular repair of ruptured abdominal aortic aneurysms, according to a new mortality risk score from Harborview Medical Center in Seattle.
Meeting all four criteria gives the maximum score of 4. Any one of the factors alone – a score of 1 – predicted 30% mortality with open repair and 9% with endovascular aneurysm repair (EVAR); a 2 predicted 80% mortality with open repair and 37% with EVAR; and a 3 predicted 82% mortality with open repair and 70% with EVAR.
Vascular surgeons at Harborview developed the system so they’d know whether to recommend transport or comfort care for ruptured abdominal aortic aneurysms (AAAs). The Level 1 trauma center serves more than a quarter of the U.S. landmass, and handles about 30-40 ruptured AAA’s annually. It’s not uncommon for patients to be flown in from Alaska.
Existing risk scores haven’t been validated for EVAR or rely on intraoperative variables, so they aren’t much help when counseling patients and referring physicians on what to do.
“Our ruptured AAA mortality risk score is based on four variables readily assessed preoperatively, allows accurate prediction of in-hospital mortality after repair of ruptured AAAs in the EVAR-first era, and does so better than any score thus published. It’s clinically relevant to the decision to transport and helps guide difficult discussions with patients and their families,” said investigator Dr. Ty Garland, chief vascular surgery resident at the University of Washington, Seattle.
When using the new risk score. “we don’t ever block transfer, but we have discussions with referring providers and in several cases with patients and their families over the telephone.” When the situation is hopeless, “we explain the data.” Twice in the past 6 months, patients have opted to spend their last hours at home with their families, Dr. Garland said at the Society for Vascular Surgery’s annual meeting.
To develop their system, the investigators culled through 37,000 variables from 303 ruptured AAA patients treated at Harborview from 2002-2013. Fifteen patients died in the emergency department, en route to surgery, or after choosing comfort care. Overall, 30-day mortality was 54% for open repair and 22% for EVAR.
On multivariate analysis, the team isolated the four preoperative variables most predictive of death. Preoperative creatinine greater than 2 mg/dL almost quadrupled the risk (odds ratio 3.7; P < .001); systolic blood pressure less than 70 mm Hg nearly tripled it (OR 2.7; P = .002); and pH less than 7.2 (OR 2.6; P = .009) and age greater than 76 years (OR 2.1; P = .011) more than doubled it.
The investigators then checked their results against the Vascular Study Group of New England Cardiac Index, Glasgow Aneurysm Score, and Edinburgh Ruptured Aneurysm Score. “Our preoperative risk score was most predictive of death, with an area under the curve of 0.76,” Dr. Garland said.
There was no outside funding for the work and Dr. Garland has no disclosures.
CHICAGO – Age greater than 76 years, plus preoperative creatinine greater than 2 mg/dL, blood pH less than 7.2, and systolic pressure at any point below 70 mm Hg collectively predicted 100% mortality with open or endovascular repair of ruptured abdominal aortic aneurysms, according to a new mortality risk score from Harborview Medical Center in Seattle.
Meeting all four criteria gives the maximum score of 4. Any one of the factors alone – a score of 1 – predicted 30% mortality with open repair and 9% with endovascular aneurysm repair (EVAR); a 2 predicted 80% mortality with open repair and 37% with EVAR; and a 3 predicted 82% mortality with open repair and 70% with EVAR.
Vascular surgeons at Harborview developed the system so they’d know whether to recommend transport or comfort care for ruptured abdominal aortic aneurysms (AAAs). The Level 1 trauma center serves more than a quarter of the U.S. landmass, and handles about 30-40 ruptured AAA’s annually. It’s not uncommon for patients to be flown in from Alaska.
Existing risk scores haven’t been validated for EVAR or rely on intraoperative variables, so they aren’t much help when counseling patients and referring physicians on what to do.
“Our ruptured AAA mortality risk score is based on four variables readily assessed preoperatively, allows accurate prediction of in-hospital mortality after repair of ruptured AAAs in the EVAR-first era, and does so better than any score thus published. It’s clinically relevant to the decision to transport and helps guide difficult discussions with patients and their families,” said investigator Dr. Ty Garland, chief vascular surgery resident at the University of Washington, Seattle.
When using the new risk score. “we don’t ever block transfer, but we have discussions with referring providers and in several cases with patients and their families over the telephone.” When the situation is hopeless, “we explain the data.” Twice in the past 6 months, patients have opted to spend their last hours at home with their families, Dr. Garland said at the Society for Vascular Surgery’s annual meeting.
To develop their system, the investigators culled through 37,000 variables from 303 ruptured AAA patients treated at Harborview from 2002-2013. Fifteen patients died in the emergency department, en route to surgery, or after choosing comfort care. Overall, 30-day mortality was 54% for open repair and 22% for EVAR.
On multivariate analysis, the team isolated the four preoperative variables most predictive of death. Preoperative creatinine greater than 2 mg/dL almost quadrupled the risk (odds ratio 3.7; P < .001); systolic blood pressure less than 70 mm Hg nearly tripled it (OR 2.7; P = .002); and pH less than 7.2 (OR 2.6; P = .009) and age greater than 76 years (OR 2.1; P = .011) more than doubled it.
The investigators then checked their results against the Vascular Study Group of New England Cardiac Index, Glasgow Aneurysm Score, and Edinburgh Ruptured Aneurysm Score. “Our preoperative risk score was most predictive of death, with an area under the curve of 0.76,” Dr. Garland said.
There was no outside funding for the work and Dr. Garland has no disclosures.
AT SVS 2015
Key clinical point: You can rely on preoperative variables to recommend surgery or comfort care for ruptured AAAs.
Major finding: Age greater than 76 years, plus preop creatinine greater than 2 mg/dL, blood pH less than 7.2, and systolic pressure at any point below 70 mm Hg predicted 100% mortality with open or endovascular repair of ruptured abdominal aortic aneurysms.
Data source: More than 300 ruptured AAA patients treated at Harborview Medical Center in Seattle from 2002-2013
Disclosures: There was no outside funding for the work, and the presenting investigator has no relevant disclosures.