User login
Reattaching intercostals fails to squelch spinal cord ischemia in TAAA repairs
CHICAGO – Intercostal artery reimplantation fails to significantly reduce spinal cord injury following thoracoabdominal aortic aneurysm surgery, results of a large retrospective study show.
“Although there was a small decrease in spinal cord ischemia with ICAR, reattaching the intercostals did not produce a statistically significant reduction in spinal cord ischemia, even in the highest risk patients,” Dr. Charles W. Acher of the University of Wisconsin–Madison, said at the annual meeting of the Midwestern Vascular Surgical Society.
Intercostal artery reimplantation (ICAR) is one of several strategies that have been used to prevent spinal cord ischemia (SCI), paraplegia, and paraparesis that occurs from the interruption of the blood supply to intercostal arteries (ICAs) during thoracoabdominal aortic aneurysm (TAAA) repair.
Surgeons at UW–Madison adopted the ICAR strategy in 2005and now reimplant open ICAs located at T7-L2 in all Type I, II, and III TAAAs, using a previously published technique (J Surg Res. 2009;154:99-104).
Using a prospectively maintained database, the current analysis sought to compare outcomes between 540 patients who had TAAA surgery during 1989-2004 when open ICAs were ligated and 265 patients who had surgery during 2005-2013 with ICAR.The surgical technique for both groups was cross clamp without assisted circulation. The anesthetic technique was also uniform during the study period and included moderate systemic hypothermia (32° - 33° C); spinal fluid drainage (spinal fluid pressure less than 5 mm Hg); naloxone 1 mcg/kg per hour; use of mannitol, methylprednisolone, and barbiturate burst suppression; goal-directed therapy for a mean arterial pressure of 90-100 mm Hg and cardiac index of 2.5 L per minute/meter2; and proactive component blood therapy to avoid anemia, hypovolemia, and hypertension.
Aneurysm extent, acuity, mortality, renal failure, and pulmonary failure were the same in both groups.
The incidence of SCI was similar in all TAAAs at 5.25% without ICAR and 3.4% with ICAR (P = .23) and in the subset of patients with Type I, II, and III aneurysms (8.8% vs. 5.1%; P = .152), Dr. Acher reported on behalf of lead author and his colleague, Dr. Martha M. Wynn.
Interestingly, ICAR patients had more dissections than did the open ICA ligation patients (18% vs. 15%; P = .0016), more previous aortic surgery (47% vs. 31%; P = .0004), and longer renal ischemia time (61 minutes vs. 53 minutes; P = .0001), but had a shorter length of stay (14 days vs. 22 days; P = .0001) and were younger (mean age, 66 years vs. 70 years; P = .0001).
In a multivariate model of all TAAAs, significant predictors of spinal cord ischemia/injury were type II TAAA (odds ratio, 7.59; P = .0001), dissection (OR, 4.25; P = .0015), age as a continuous variable (P = .0085), and acute TAAA (OR, 2.1; P = .0525), Dr. Acher said. Time period of surgery, and therefore ICAR, was not significant (OR, 0.78; P = .55).
ICAR also failed to achieve significance as an SCI predictor in a subanalysis restricted to the highest-risk patients, defined as those having Type II TAAA, dissection, and acute surgery (OR, 0.67; P = .3387).
“Interrupting blood supply to the spinal cord causes spinal cord ischemia that can be mitigated almost entirely by physiologic interventions that increase spinal cord ischemic tolerance and collateral network perfusion during and after surgery,” Dr. Acher said. “Although the cause of SCI in TAAA surgery is anatomic, prevention of the injury is largely physiologic.”
During a discussion of the study, Dr. Acher surprised the audience by saying the findings have not changed current practice at the university. He cited several reasons, observing that there were more dissections in the ICAR group, and most of the ischemia in the ICAR group was delayed, suggesting that more patients could be rescued. In addition, there was a slight downward trend in spinal cord injury and immediate paraplegia with ICAR, however, these were not statistically significant.
“Because of those things, I still think it’s valuable, particularly in patients that are at highest risk, which are the dissections, with lots of open intercostals, but the emphasis should still be on physiologic parameters,” he said. “If you want to salvage patients, that’s the most important thing.
“Even if ICAR were ever shown to be statistically significant in a larger patient population, any role it has in reducing spinal cord injury would be extremely small,” he added in an interview.
The authors reported having no conflicts of interest.
Spinal cord ischemia is a rare but devastating complication of thoracoabdominal aneurysm repair. Crawford and his colleagues documented in 1993 an incidence of spinal cord ischemia (SCI) as high as 30% for extensive thoracoabdominal repairs. Efforts to diminish the risk of SCI were concentrated in identifying and preserving the direct arterial perfusion to the spinal cord from segmental arteries but continued experimental and clinical experience have suggested that multiple factors contribute to SCI.

|
Dr. Luis A. Sanchez |
Some generally accepted principles for minimizing SCI include hypothermia, distal aortic perfusion with atriofemoral bypass or partial cardiopulmonary bypass, cerebrospinal fluid drainage, and avoidance of hemodynamic instability. Reimplantation of intercostal branches has been suggested as an adjunct to these techniques by some investigators with limited data to support its generalized application. More recently, a growing body of evidence supports the concept of a collateral network that can support the perfusion to the spinal cord after interruption of multiple intercostal arteries and the importance of the hypogastric and subclavian arteries as critical branches that perfuse the spinal collateral network.
The retrospective review of the extensive experience at the University of Wisconsin in Madison supports the concept that “physiologic interventions that increase spinal cord tolerance and collateral network perfusion during and after surgery” are more important than the reimplantation of intercostal vessels during this complex procedure, even in patients considered at the highest risk for SCI. Intercostal artery reimplantation failed to achieve significance as an SCI predictor when comparing two large cohorts of patients (540 vs. 265) treated with intercostal ligation vs. reimplantation. Increasingly, available data support the concept of a collateral network that maintains perfusion to the spinal cord after intercostal artery occlusion.
Additional new concepts and techniques including a two-stage approach for extensive thoracoabdominal repair, preliminary occlusion of some segmental arteries, and the use of hybrid and endovascular techniques may further decrease the incidence of SCI by taking advantage of the collateral network and allow some preconditioning of the spinal cord. Fortunately for these challenging patients, significant advances continue to be made to better understand and prevent spinal cord ischemia.
Dr. Luis A. Sanchez is Chief, Section of Vascular Surgery and the Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Department of Surgery, Washington University in St. Louis.
Spinal cord ischemia is a rare but devastating complication of thoracoabdominal aneurysm repair. Crawford and his colleagues documented in 1993 an incidence of spinal cord ischemia (SCI) as high as 30% for extensive thoracoabdominal repairs. Efforts to diminish the risk of SCI were concentrated in identifying and preserving the direct arterial perfusion to the spinal cord from segmental arteries but continued experimental and clinical experience have suggested that multiple factors contribute to SCI.

|
Dr. Luis A. Sanchez |
Some generally accepted principles for minimizing SCI include hypothermia, distal aortic perfusion with atriofemoral bypass or partial cardiopulmonary bypass, cerebrospinal fluid drainage, and avoidance of hemodynamic instability. Reimplantation of intercostal branches has been suggested as an adjunct to these techniques by some investigators with limited data to support its generalized application. More recently, a growing body of evidence supports the concept of a collateral network that can support the perfusion to the spinal cord after interruption of multiple intercostal arteries and the importance of the hypogastric and subclavian arteries as critical branches that perfuse the spinal collateral network.
The retrospective review of the extensive experience at the University of Wisconsin in Madison supports the concept that “physiologic interventions that increase spinal cord tolerance and collateral network perfusion during and after surgery” are more important than the reimplantation of intercostal vessels during this complex procedure, even in patients considered at the highest risk for SCI. Intercostal artery reimplantation failed to achieve significance as an SCI predictor when comparing two large cohorts of patients (540 vs. 265) treated with intercostal ligation vs. reimplantation. Increasingly, available data support the concept of a collateral network that maintains perfusion to the spinal cord after intercostal artery occlusion.
Additional new concepts and techniques including a two-stage approach for extensive thoracoabdominal repair, preliminary occlusion of some segmental arteries, and the use of hybrid and endovascular techniques may further decrease the incidence of SCI by taking advantage of the collateral network and allow some preconditioning of the spinal cord. Fortunately for these challenging patients, significant advances continue to be made to better understand and prevent spinal cord ischemia.
Dr. Luis A. Sanchez is Chief, Section of Vascular Surgery and the Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Department of Surgery, Washington University in St. Louis.
Spinal cord ischemia is a rare but devastating complication of thoracoabdominal aneurysm repair. Crawford and his colleagues documented in 1993 an incidence of spinal cord ischemia (SCI) as high as 30% for extensive thoracoabdominal repairs. Efforts to diminish the risk of SCI were concentrated in identifying and preserving the direct arterial perfusion to the spinal cord from segmental arteries but continued experimental and clinical experience have suggested that multiple factors contribute to SCI.

|
Dr. Luis A. Sanchez |
Some generally accepted principles for minimizing SCI include hypothermia, distal aortic perfusion with atriofemoral bypass or partial cardiopulmonary bypass, cerebrospinal fluid drainage, and avoidance of hemodynamic instability. Reimplantation of intercostal branches has been suggested as an adjunct to these techniques by some investigators with limited data to support its generalized application. More recently, a growing body of evidence supports the concept of a collateral network that can support the perfusion to the spinal cord after interruption of multiple intercostal arteries and the importance of the hypogastric and subclavian arteries as critical branches that perfuse the spinal collateral network.
The retrospective review of the extensive experience at the University of Wisconsin in Madison supports the concept that “physiologic interventions that increase spinal cord tolerance and collateral network perfusion during and after surgery” are more important than the reimplantation of intercostal vessels during this complex procedure, even in patients considered at the highest risk for SCI. Intercostal artery reimplantation failed to achieve significance as an SCI predictor when comparing two large cohorts of patients (540 vs. 265) treated with intercostal ligation vs. reimplantation. Increasingly, available data support the concept of a collateral network that maintains perfusion to the spinal cord after intercostal artery occlusion.
Additional new concepts and techniques including a two-stage approach for extensive thoracoabdominal repair, preliminary occlusion of some segmental arteries, and the use of hybrid and endovascular techniques may further decrease the incidence of SCI by taking advantage of the collateral network and allow some preconditioning of the spinal cord. Fortunately for these challenging patients, significant advances continue to be made to better understand and prevent spinal cord ischemia.
Dr. Luis A. Sanchez is Chief, Section of Vascular Surgery and the Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Department of Surgery, Washington University in St. Louis.
CHICAGO – Intercostal artery reimplantation fails to significantly reduce spinal cord injury following thoracoabdominal aortic aneurysm surgery, results of a large retrospective study show.
“Although there was a small decrease in spinal cord ischemia with ICAR, reattaching the intercostals did not produce a statistically significant reduction in spinal cord ischemia, even in the highest risk patients,” Dr. Charles W. Acher of the University of Wisconsin–Madison, said at the annual meeting of the Midwestern Vascular Surgical Society.
Intercostal artery reimplantation (ICAR) is one of several strategies that have been used to prevent spinal cord ischemia (SCI), paraplegia, and paraparesis that occurs from the interruption of the blood supply to intercostal arteries (ICAs) during thoracoabdominal aortic aneurysm (TAAA) repair.
Surgeons at UW–Madison adopted the ICAR strategy in 2005and now reimplant open ICAs located at T7-L2 in all Type I, II, and III TAAAs, using a previously published technique (J Surg Res. 2009;154:99-104).
Using a prospectively maintained database, the current analysis sought to compare outcomes between 540 patients who had TAAA surgery during 1989-2004 when open ICAs were ligated and 265 patients who had surgery during 2005-2013 with ICAR.The surgical technique for both groups was cross clamp without assisted circulation. The anesthetic technique was also uniform during the study period and included moderate systemic hypothermia (32° - 33° C); spinal fluid drainage (spinal fluid pressure less than 5 mm Hg); naloxone 1 mcg/kg per hour; use of mannitol, methylprednisolone, and barbiturate burst suppression; goal-directed therapy for a mean arterial pressure of 90-100 mm Hg and cardiac index of 2.5 L per minute/meter2; and proactive component blood therapy to avoid anemia, hypovolemia, and hypertension.
Aneurysm extent, acuity, mortality, renal failure, and pulmonary failure were the same in both groups.
The incidence of SCI was similar in all TAAAs at 5.25% without ICAR and 3.4% with ICAR (P = .23) and in the subset of patients with Type I, II, and III aneurysms (8.8% vs. 5.1%; P = .152), Dr. Acher reported on behalf of lead author and his colleague, Dr. Martha M. Wynn.
Interestingly, ICAR patients had more dissections than did the open ICA ligation patients (18% vs. 15%; P = .0016), more previous aortic surgery (47% vs. 31%; P = .0004), and longer renal ischemia time (61 minutes vs. 53 minutes; P = .0001), but had a shorter length of stay (14 days vs. 22 days; P = .0001) and were younger (mean age, 66 years vs. 70 years; P = .0001).
In a multivariate model of all TAAAs, significant predictors of spinal cord ischemia/injury were type II TAAA (odds ratio, 7.59; P = .0001), dissection (OR, 4.25; P = .0015), age as a continuous variable (P = .0085), and acute TAAA (OR, 2.1; P = .0525), Dr. Acher said. Time period of surgery, and therefore ICAR, was not significant (OR, 0.78; P = .55).
ICAR also failed to achieve significance as an SCI predictor in a subanalysis restricted to the highest-risk patients, defined as those having Type II TAAA, dissection, and acute surgery (OR, 0.67; P = .3387).
“Interrupting blood supply to the spinal cord causes spinal cord ischemia that can be mitigated almost entirely by physiologic interventions that increase spinal cord ischemic tolerance and collateral network perfusion during and after surgery,” Dr. Acher said. “Although the cause of SCI in TAAA surgery is anatomic, prevention of the injury is largely physiologic.”
During a discussion of the study, Dr. Acher surprised the audience by saying the findings have not changed current practice at the university. He cited several reasons, observing that there were more dissections in the ICAR group, and most of the ischemia in the ICAR group was delayed, suggesting that more patients could be rescued. In addition, there was a slight downward trend in spinal cord injury and immediate paraplegia with ICAR, however, these were not statistically significant.
“Because of those things, I still think it’s valuable, particularly in patients that are at highest risk, which are the dissections, with lots of open intercostals, but the emphasis should still be on physiologic parameters,” he said. “If you want to salvage patients, that’s the most important thing.
“Even if ICAR were ever shown to be statistically significant in a larger patient population, any role it has in reducing spinal cord injury would be extremely small,” he added in an interview.
The authors reported having no conflicts of interest.
CHICAGO – Intercostal artery reimplantation fails to significantly reduce spinal cord injury following thoracoabdominal aortic aneurysm surgery, results of a large retrospective study show.
“Although there was a small decrease in spinal cord ischemia with ICAR, reattaching the intercostals did not produce a statistically significant reduction in spinal cord ischemia, even in the highest risk patients,” Dr. Charles W. Acher of the University of Wisconsin–Madison, said at the annual meeting of the Midwestern Vascular Surgical Society.
Intercostal artery reimplantation (ICAR) is one of several strategies that have been used to prevent spinal cord ischemia (SCI), paraplegia, and paraparesis that occurs from the interruption of the blood supply to intercostal arteries (ICAs) during thoracoabdominal aortic aneurysm (TAAA) repair.
Surgeons at UW–Madison adopted the ICAR strategy in 2005and now reimplant open ICAs located at T7-L2 in all Type I, II, and III TAAAs, using a previously published technique (J Surg Res. 2009;154:99-104).
Using a prospectively maintained database, the current analysis sought to compare outcomes between 540 patients who had TAAA surgery during 1989-2004 when open ICAs were ligated and 265 patients who had surgery during 2005-2013 with ICAR.The surgical technique for both groups was cross clamp without assisted circulation. The anesthetic technique was also uniform during the study period and included moderate systemic hypothermia (32° - 33° C); spinal fluid drainage (spinal fluid pressure less than 5 mm Hg); naloxone 1 mcg/kg per hour; use of mannitol, methylprednisolone, and barbiturate burst suppression; goal-directed therapy for a mean arterial pressure of 90-100 mm Hg and cardiac index of 2.5 L per minute/meter2; and proactive component blood therapy to avoid anemia, hypovolemia, and hypertension.
Aneurysm extent, acuity, mortality, renal failure, and pulmonary failure were the same in both groups.
The incidence of SCI was similar in all TAAAs at 5.25% without ICAR and 3.4% with ICAR (P = .23) and in the subset of patients with Type I, II, and III aneurysms (8.8% vs. 5.1%; P = .152), Dr. Acher reported on behalf of lead author and his colleague, Dr. Martha M. Wynn.
Interestingly, ICAR patients had more dissections than did the open ICA ligation patients (18% vs. 15%; P = .0016), more previous aortic surgery (47% vs. 31%; P = .0004), and longer renal ischemia time (61 minutes vs. 53 minutes; P = .0001), but had a shorter length of stay (14 days vs. 22 days; P = .0001) and were younger (mean age, 66 years vs. 70 years; P = .0001).
In a multivariate model of all TAAAs, significant predictors of spinal cord ischemia/injury were type II TAAA (odds ratio, 7.59; P = .0001), dissection (OR, 4.25; P = .0015), age as a continuous variable (P = .0085), and acute TAAA (OR, 2.1; P = .0525), Dr. Acher said. Time period of surgery, and therefore ICAR, was not significant (OR, 0.78; P = .55).
ICAR also failed to achieve significance as an SCI predictor in a subanalysis restricted to the highest-risk patients, defined as those having Type II TAAA, dissection, and acute surgery (OR, 0.67; P = .3387).
“Interrupting blood supply to the spinal cord causes spinal cord ischemia that can be mitigated almost entirely by physiologic interventions that increase spinal cord ischemic tolerance and collateral network perfusion during and after surgery,” Dr. Acher said. “Although the cause of SCI in TAAA surgery is anatomic, prevention of the injury is largely physiologic.”
During a discussion of the study, Dr. Acher surprised the audience by saying the findings have not changed current practice at the university. He cited several reasons, observing that there were more dissections in the ICAR group, and most of the ischemia in the ICAR group was delayed, suggesting that more patients could be rescued. In addition, there was a slight downward trend in spinal cord injury and immediate paraplegia with ICAR, however, these were not statistically significant.
“Because of those things, I still think it’s valuable, particularly in patients that are at highest risk, which are the dissections, with lots of open intercostals, but the emphasis should still be on physiologic parameters,” he said. “If you want to salvage patients, that’s the most important thing.
“Even if ICAR were ever shown to be statistically significant in a larger patient population, any role it has in reducing spinal cord injury would be extremely small,” he added in an interview.
The authors reported having no conflicts of interest.
AT MIDWESTERN VASCULAR 2015
Key clinical point: Intercostal artery reimplantation (ICAR) did not produce a significant reduction in spinal cord ischemia following thoracoabdominal aortic aneurysm repair, even in the highest risk patients.
Major finding: ICAR was not a significant predictor of spinal cord ischemia (OR, 0.78; P = .55).
Data source: Retrospective analysis of 805 patients undergoing TAAA with or without ICAR.
Disclosures: The authors reported having no conflicts of interest.
Genitourinary manifestations of sickle cell disease
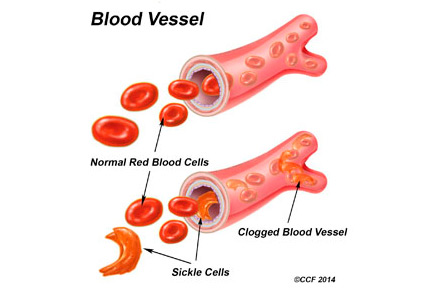
Sickle cell disease is a common genetic disorder in the United States that disproportionately affects people of African ancestry. The characteristic sickling of red blood cells under conditions of reduced oxygen tension leads to intravascular hemolysis and vaso-occlusive events, which in turn cause tissue ischemia-reperfusion injury affecting multiple organs, including the genitourinary system.1–3
In this paper, we review the genitourinary effects of sickle cell disease, focusing on sickle cell nephropathy, priapism, and renal medullary carcinoma.
THE WIDE-RANGING EFFECTS OF SICKLE CELL DISEASE
In the United States, sickle cell disease affects 1 of every 500 blacks and 1 of every 36,000 Hispanics.1 The term describes hemoglobinopathies associated with sickling of red blood cells.
Sickling of red blood cells results from a single base-pair change in the beta-globin gene from glutamic acid to valine at position 6, causing abnormal hemoglobin (hemoglobin S), which polymerizes under conditions of reduced oxygen tension and alters the biconcave disk shape into a rigid, irregular, unstable cell. The sickle-shaped cells are prone to intravascular hemolysis,2 causing intermittent vaso-occlusive events that result in tissue ischemia-reperfusion injury. Genitourinary problems include impaired ability to concentrate urine, hematuria, renal medullary carcinoma, and increased frequency of urinary tract infection.
SICKLE CELL NEPHROPATHY
The kidney is one of the most frequently affected organs in sickle cell disease. Renal manifestations begin to appear in early childhood, with impaired medullary concentrating ability and ischemic damage to the tubular cells caused by sickling within the vasa recta renis precipitated by the acidic, hypoxic, and hypertonic environment in the renal medulla.
As in early diabetic nephropathy, renal blood flow is enhanced and the glomerular filtration rate (GFR) is increased. Increased cardiac output as a result of anemia, localized release of prostaglandins, and a hypoxia-induced increase in nitric oxide synthesis all play a role in the increase in GFR.4,5
Oxidative stress, an increase in markers of inflammation, and local activation of the renin-angiotensin system contribute to renal damage in sickle cell disease.5–7 The resulting hyperfiltration injury leads to microalbuminuria, which occurs in 20% to 40% of children with sickle cell anemia8,9 and in as many as 60% of adults.
The glomerular lesions associated with sickle cell disease vary from glomerulopathy in the early stages to secondary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, and glomerular thrombotic microangiopathy.10
Clinical presentations and workup
Clinical presentations are not limited to glomerular disease but include hyperchloremic metabolic acidosis and hyperkalemia resulting from defects in potassium secretion and renal acidification.
Hyperphosphatemia—a result of increased reabsorption of phosphorus, increased secretion of uric acid, and increased creatinine clearance—is seen in patients with sickle cell disease.11,12 About 10% of patients can develop an acute kidney injury as a result of volume depletion, rhabdomyolysis, renal vein thrombosis, papillary necrosis, and urinary tract obstruction secondary to blood clots.11,13
Up to 30% of adult patients with sickle cell disease develop chronic kidney disease. Predictors include severe anemia, hypertension, proteinuria, nephrotic syndrome, and microscopic hematuria.14 From 4% to 12% of patients go on to develop end-stage renal disease, but with a 1-year mortality rate three times higher than in patients without sickle cell disease.15
In general, patients with sickle cell anemia have blood pressures below those of age- and sex-matched individuals, but elevated blood pressure and low GFR are not uncommon in affected children. In a cohort of 48 children ages 3 to 18, 8.3% had an estimated GFR less than 90 mL/min/1.73 m2, and 16.7% had elevated blood pressure (prehypertension and hypertension).16
In patients with sickle cell disease, evaluation of proteinuria, hematuria, hypertension, and renal failure should take into consideration the unique renal physiologic and pathologic processes involved. Recent evidence17,18 suggests that the Chronic Kidney Disease Epidemiology Collaboration equation provides a better estimate of GFR than the Modification of Diet in Renal Disease and Cockcroft-Gault equations, although all three creatinine-based methods overestimate GFR in patients with sickle cell disease when compared with GFR measured with technetium-99m-labeled diethylenetriamine penta-acetic acid renal scanning.
Treatment options
Treatment of sickle cell nephropathy includes adequate fluid intake (given the loss of concentrating ability), adequate blood pressure control, use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in patients who have microalbuminuria or proteinuria (or both)9,11,19 and hydroxyurea. Treatment with enalapril has been shown to decrease proteinuria in patients with sickle cell nephropathy.9 In a cohort of children with sickle cell disease, four of nine patients treated with an ACE inhibitor developed hyperkalemia, leading to discontinuation of the drug in three patients.9
ACE inhibitors and ARBs must be used cautiously in these patients because they have defects in potassium secretion. Hydroxyurea has also been shown to decrease hyperfiltration and microalbuminuria in recent studies,20,21 and this could protect against the development of overt nephropathy.
Higher mortality rates have been reported in patients with sickle cell disease who developed end-stage renal disease than in patients with end-stage renal disease without sickle cell disease. Sickle cell disease also increases the risk of pulmonary hypertension and the vaso-occlusive complication known as acute chest syndrome, contributing to increased mortality rates. Of note, in a study that looked at the association between mortality rates and pre-end-stage care of renal disease using data from the Centers for Medicare and Medicaid Services, patients with sickle cell disease who had had predialysis nephrology care had lower mortality rates.15
Treatments for end-stage renal disease are also effective in patients with sickle cell disease and include hemodialysis, peritoneal dialysis, and renal transplantation. Data from the Organ Procurement and Transplantation Network and the United Network for Organ Sharing show that from 2000 to 2011, African American kidney recipients with sickle cell disease had better survival rates than patients who had undergone transplantation from 1988 to 1999, although rates of long-term survival and graft survival were lower than in transplant recipients with other diagnoses.22
It is important to note that complications as a result of vaso-occlusive events and thrombosis can lead to graft loss; therefore, sickle cell crisis after transplantation requires careful management.
Take-home messages
- Loss of urine-concentrating ability and hyperfiltration are the earliest pathologic changes in sickle cell disease.
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- ACE inhibitors or ARBs should be used with caution, given the heightened risk of hyperkalemia in sickle cell disease.
- Recent results with hydroxyurea in decreasing hyperfiltration and microalbuminuria are encouraging.
- Early referral for predialysis nephrologic care is needed in sickle cell patients with chronic kidney disease.
PRIAPISM IN SICKLE CELL DISEASE
Priapism was formerly defined as a full, painful erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm. But priapism is now recognized as two separate disorders—ischemic (veno-occlusive, low-flow) priapism and nonischemic (arterial, high-flow) priapism. The new definition includes both disorders: ie, a full or partial erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm.
Ischemic priapism
Hematologic disorders are major contributors to ischemic priapism and include sickle cell disease, multiple myeloma, fat emboli (hyperalimentation),23 glucose-6-phosphate dehydrogenase deficiency, and hemoglobin Olmsted variant.24
Ischemic priapism is often seen in sickle cell disease and is considered an emergency. It is characterized by an abnormally rigid erection not involving the glans penis. Entrapment of blood in the corpora cavernosa leads to hypoxia, hypercarbia, and acidosis, which in turn leads to a painful compartment syndrome that, if untreated, results in smooth muscle necrosis and subsequent fibrosis. The results are a smaller penis and erectile dysfunction that is unresponsive to any treatment other than implantation of a penile prosthesis. However, scarring of the corpora cavernosa can make this procedure exceedingly difficult, requiring advanced techniques such as corporeal excavation.25
Men with a subtype of ischemic priapism called “stuttering” priapism26 suffer recurrent prolonged erections during sleep. The patient awakens with a painful erection that usually subsides, but sometimes only after several hours. Patients with this disorder suffer from sleep deprivation. Stuttering priapism may lead to full-blown ischemic priapism that does not resolve without intervention.
Nonischemic priapism
In nonischemic priapism, the corpora are engorged but not rigid. The condition results from unregulated arterial inflow and thus is not painful and does not result in damage to the corporeal smooth muscle.
Most cases of nonischemic priapism follow blunt perineal trauma or trauma associated with needle insertion into the corpora. This form of priapism is not associated with sickle cell disease. Because tissue damage does not occur, nonischemic or arterial priapism is not considered an emergency.
Treatment guidelines
Differentiating ischemic from nonischemic priapism is usually straightforward, based on the history, physical examination, corporeal blood gases, and duplex ultrasonography.27
Ischemic priapism is an emergency. After needle aspiration of blood from the corpora cavernosa, phenylephrine is diluted with normal saline to a concentration of 100 to 500 µg/mL and is injected in 1-mL amounts repeatedly at 3- to 5-minute intervals until the erection subsides or until a 1-hour time limit is reached. Blood pressure and pulse are monitored during these injections. If aspiration and phenylephrine irrigation fail, surgical shunting is performed.27
Measures to treat sickle cell disease (hydration, oxygen, exchange transfusions) may be employed simultaneously but should never delay aspiration and phenylephrine injections.25
As nonischemic priapism is not considered an emergency, management begins with observation. Patients eventually become dissatisfied with their constant partial erection, and they then present for treatment. Most cases resolve after selective catheterization of the internal pudendal artery and embolization of the fistula with absorbable material. If this fails, surgical exploration with ligation of the vessels leading to the fistula is indicated.
Prevalence in sickle cell trait vs sickle cell disease
Ischemic priapism is uncommon in men with sickle cell trait, but prevalence rates in men with sickle cell disease are as high as 42%.28 In a study of 130 men with sickle cell disease, 35% had a history of prolonged ischemic priapism, 72% had a history of stuttering priapism, and 75% of men with stuttering priapism had their first episode before age 20.29
Rates of erectile dysfunction increase with the duration of ischemic episodes and range from 20% to 90%.28,30 In childhood, sickle cell disease accounts for 63% of the cases of ischemic priapism, and in adults it accounts for 23% of cases.31
Take-home messages
- Sickle cell disease accounts for two-thirds of cases of ischemic priapism in children, and one-fourth of adult cases.
- Ischemic priapism is a medical emergency.
- Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease (hydration, oxygen, exchange transfusions).
OTHER UROLOGIC COMPLICATIONS OF SICKLE CELL DISEASE
Other urologic complications of sickle cell trait and sickle cell disease include microscopic hematuria, gross hematuria, and renal colic. A formal evaluation of any patient with persistent microscopic hematuria or gross hematuria should consist of urinalysis, computed tomography, and cystoscopy. This approach assesses the upper and lower genitourinary system for treatable causes. Renal ultrasonography can be used instead of computed tomography but tends to provide less information.
Special considerations
In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis. In papillary hypertrophy, friable blood vessels can rupture, resulting in microscopic and gross hematuria. In papillary necrosis, the papilla can slough off and become lodged in the ureter.
Nevertheless, hematuria and renal colic in patients with sickle cell disease or trait are most often attributable to common causes such as infection and stones. A finding of hydronephrosis in the absence of a stone, however, suggests obstruction due to a clot or a sloughed papilla. Ureteroscopy, fulguration, and ureteral stent placement can stop the bleeding and alleviate obstruction in these cases.
Renal medullary carcinoma
Another important reason to order imaging in patients with sickle cell disease or trait who present with urologic symptoms is to rule out renal medullary carcinoma, a rare but aggressive cancer that arises from the collecting duct epithelium. This cancer is twice as likely to occur in males than in females; it has been reported in patients ranging in age from 10 to 40, with a median age at presentation of 26.32
When patients present with symptomatic renal medullary cancer, in most cases the cancer has already metastasized.
On computed tomography, the tumor tends to occupy a central location in the kidney and appears to infiltrate and replace adjacent kidney tissue. Retroperitoneal lymphadenopathy and metastasis are common.
Treatment typically entails radical nephrectomy, chemotherapy, and in some circumstances, radiotherapy. Case reports have shown promising tumor responses to carboplatin and paclitaxel regimens.33,34 Also, a low threshold for imaging in patients with sickle cell disease and trait may increase the odds of early detection of this aggressive cancer.
- Centers for Disease Control and Prevention (CDC). Sickle cell disease (SCD). Data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed August 18, 2015.
- Paulin L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–548.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84:363–376.
- Haymann JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5:756–761.
- da Silva GB Jr, Libório AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 2011; 90:1371–1379.
- Emokpae MA, Uadia PO, Gadzama AA. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med 2010; 9:141–146.
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012; 64:72–80.
- Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70:307–309.
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326:910–915.
- Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010; 89:18–27.
- Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br J Haematol 2011; 155:287–297.
- Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 1982; 72:188–192.
- Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 1990; 13:347–351.
- Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115:614–620.
- McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 2012; 159:360–367.
- Bodas P, Huang A, O Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14:237.
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine based estimating equations. PLoS One 2013; 8:e69922.
- Arlet JB, Ribeil JA, Chatellier G, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012; 13:83.
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29:140–144.
- Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29:1211–1218.
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88:116–119.
- Huang E, Parke C, Mehrnia A, et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol Dial Transplant 2013; 28:1039–1046.
- Klein EA, Montague DK, Steiger E. Priapism associated with the use of intravenous fat emulsion: case reports and postulated pathogenesis. J Urol May 1985; 133:857–859.
- Thuret I, Bardakdjian J, Badens C, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu-->Arg. Am J Hematol 1996; 51:133–136.
- Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology 2006; 67:1072–1075.
- Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl 2012; 14:156–163.
- Montague DK, Jarow J, Broderick GA, et al; Members of the Erectile Dysfunction Guideline Update Panel; American Urological Association. American Urological Association guideline on the management of priapism. J Urol 2003; 170:1318–1324.
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980; 140:1434–1437.
- Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002; 90:898–902.
- Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med 2004; 1:116–120.
- Nelson JH, 3rd, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977; 117:455–458.
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368–374.
- Gangireddy VG, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134–139.
- Walsh AM, Fiveash JB, Reddy AT, Friedman GK. Response to radiation in renal medullary carcinoma. Rare Tumors 2011; 3:e32.
Sickle cell disease is a common genetic disorder in the United States that disproportionately affects people of African ancestry. The characteristic sickling of red blood cells under conditions of reduced oxygen tension leads to intravascular hemolysis and vaso-occlusive events, which in turn cause tissue ischemia-reperfusion injury affecting multiple organs, including the genitourinary system.1–3
In this paper, we review the genitourinary effects of sickle cell disease, focusing on sickle cell nephropathy, priapism, and renal medullary carcinoma.
THE WIDE-RANGING EFFECTS OF SICKLE CELL DISEASE
In the United States, sickle cell disease affects 1 of every 500 blacks and 1 of every 36,000 Hispanics.1 The term describes hemoglobinopathies associated with sickling of red blood cells.
Sickling of red blood cells results from a single base-pair change in the beta-globin gene from glutamic acid to valine at position 6, causing abnormal hemoglobin (hemoglobin S), which polymerizes under conditions of reduced oxygen tension and alters the biconcave disk shape into a rigid, irregular, unstable cell. The sickle-shaped cells are prone to intravascular hemolysis,2 causing intermittent vaso-occlusive events that result in tissue ischemia-reperfusion injury. Genitourinary problems include impaired ability to concentrate urine, hematuria, renal medullary carcinoma, and increased frequency of urinary tract infection.
SICKLE CELL NEPHROPATHY
The kidney is one of the most frequently affected organs in sickle cell disease. Renal manifestations begin to appear in early childhood, with impaired medullary concentrating ability and ischemic damage to the tubular cells caused by sickling within the vasa recta renis precipitated by the acidic, hypoxic, and hypertonic environment in the renal medulla.
As in early diabetic nephropathy, renal blood flow is enhanced and the glomerular filtration rate (GFR) is increased. Increased cardiac output as a result of anemia, localized release of prostaglandins, and a hypoxia-induced increase in nitric oxide synthesis all play a role in the increase in GFR.4,5
Oxidative stress, an increase in markers of inflammation, and local activation of the renin-angiotensin system contribute to renal damage in sickle cell disease.5–7 The resulting hyperfiltration injury leads to microalbuminuria, which occurs in 20% to 40% of children with sickle cell anemia8,9 and in as many as 60% of adults.
The glomerular lesions associated with sickle cell disease vary from glomerulopathy in the early stages to secondary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, and glomerular thrombotic microangiopathy.10
Clinical presentations and workup
Clinical presentations are not limited to glomerular disease but include hyperchloremic metabolic acidosis and hyperkalemia resulting from defects in potassium secretion and renal acidification.
Hyperphosphatemia—a result of increased reabsorption of phosphorus, increased secretion of uric acid, and increased creatinine clearance—is seen in patients with sickle cell disease.11,12 About 10% of patients can develop an acute kidney injury as a result of volume depletion, rhabdomyolysis, renal vein thrombosis, papillary necrosis, and urinary tract obstruction secondary to blood clots.11,13
Up to 30% of adult patients with sickle cell disease develop chronic kidney disease. Predictors include severe anemia, hypertension, proteinuria, nephrotic syndrome, and microscopic hematuria.14 From 4% to 12% of patients go on to develop end-stage renal disease, but with a 1-year mortality rate three times higher than in patients without sickle cell disease.15
In general, patients with sickle cell anemia have blood pressures below those of age- and sex-matched individuals, but elevated blood pressure and low GFR are not uncommon in affected children. In a cohort of 48 children ages 3 to 18, 8.3% had an estimated GFR less than 90 mL/min/1.73 m2, and 16.7% had elevated blood pressure (prehypertension and hypertension).16
In patients with sickle cell disease, evaluation of proteinuria, hematuria, hypertension, and renal failure should take into consideration the unique renal physiologic and pathologic processes involved. Recent evidence17,18 suggests that the Chronic Kidney Disease Epidemiology Collaboration equation provides a better estimate of GFR than the Modification of Diet in Renal Disease and Cockcroft-Gault equations, although all three creatinine-based methods overestimate GFR in patients with sickle cell disease when compared with GFR measured with technetium-99m-labeled diethylenetriamine penta-acetic acid renal scanning.
Treatment options
Treatment of sickle cell nephropathy includes adequate fluid intake (given the loss of concentrating ability), adequate blood pressure control, use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in patients who have microalbuminuria or proteinuria (or both)9,11,19 and hydroxyurea. Treatment with enalapril has been shown to decrease proteinuria in patients with sickle cell nephropathy.9 In a cohort of children with sickle cell disease, four of nine patients treated with an ACE inhibitor developed hyperkalemia, leading to discontinuation of the drug in three patients.9
ACE inhibitors and ARBs must be used cautiously in these patients because they have defects in potassium secretion. Hydroxyurea has also been shown to decrease hyperfiltration and microalbuminuria in recent studies,20,21 and this could protect against the development of overt nephropathy.
Higher mortality rates have been reported in patients with sickle cell disease who developed end-stage renal disease than in patients with end-stage renal disease without sickle cell disease. Sickle cell disease also increases the risk of pulmonary hypertension and the vaso-occlusive complication known as acute chest syndrome, contributing to increased mortality rates. Of note, in a study that looked at the association between mortality rates and pre-end-stage care of renal disease using data from the Centers for Medicare and Medicaid Services, patients with sickle cell disease who had had predialysis nephrology care had lower mortality rates.15
Treatments for end-stage renal disease are also effective in patients with sickle cell disease and include hemodialysis, peritoneal dialysis, and renal transplantation. Data from the Organ Procurement and Transplantation Network and the United Network for Organ Sharing show that from 2000 to 2011, African American kidney recipients with sickle cell disease had better survival rates than patients who had undergone transplantation from 1988 to 1999, although rates of long-term survival and graft survival were lower than in transplant recipients with other diagnoses.22
It is important to note that complications as a result of vaso-occlusive events and thrombosis can lead to graft loss; therefore, sickle cell crisis after transplantation requires careful management.
Take-home messages
- Loss of urine-concentrating ability and hyperfiltration are the earliest pathologic changes in sickle cell disease.
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- ACE inhibitors or ARBs should be used with caution, given the heightened risk of hyperkalemia in sickle cell disease.
- Recent results with hydroxyurea in decreasing hyperfiltration and microalbuminuria are encouraging.
- Early referral for predialysis nephrologic care is needed in sickle cell patients with chronic kidney disease.
PRIAPISM IN SICKLE CELL DISEASE
Priapism was formerly defined as a full, painful erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm. But priapism is now recognized as two separate disorders—ischemic (veno-occlusive, low-flow) priapism and nonischemic (arterial, high-flow) priapism. The new definition includes both disorders: ie, a full or partial erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm.
Ischemic priapism
Hematologic disorders are major contributors to ischemic priapism and include sickle cell disease, multiple myeloma, fat emboli (hyperalimentation),23 glucose-6-phosphate dehydrogenase deficiency, and hemoglobin Olmsted variant.24
Ischemic priapism is often seen in sickle cell disease and is considered an emergency. It is characterized by an abnormally rigid erection not involving the glans penis. Entrapment of blood in the corpora cavernosa leads to hypoxia, hypercarbia, and acidosis, which in turn leads to a painful compartment syndrome that, if untreated, results in smooth muscle necrosis and subsequent fibrosis. The results are a smaller penis and erectile dysfunction that is unresponsive to any treatment other than implantation of a penile prosthesis. However, scarring of the corpora cavernosa can make this procedure exceedingly difficult, requiring advanced techniques such as corporeal excavation.25
Men with a subtype of ischemic priapism called “stuttering” priapism26 suffer recurrent prolonged erections during sleep. The patient awakens with a painful erection that usually subsides, but sometimes only after several hours. Patients with this disorder suffer from sleep deprivation. Stuttering priapism may lead to full-blown ischemic priapism that does not resolve without intervention.
Nonischemic priapism
In nonischemic priapism, the corpora are engorged but not rigid. The condition results from unregulated arterial inflow and thus is not painful and does not result in damage to the corporeal smooth muscle.
Most cases of nonischemic priapism follow blunt perineal trauma or trauma associated with needle insertion into the corpora. This form of priapism is not associated with sickle cell disease. Because tissue damage does not occur, nonischemic or arterial priapism is not considered an emergency.
Treatment guidelines
Differentiating ischemic from nonischemic priapism is usually straightforward, based on the history, physical examination, corporeal blood gases, and duplex ultrasonography.27
Ischemic priapism is an emergency. After needle aspiration of blood from the corpora cavernosa, phenylephrine is diluted with normal saline to a concentration of 100 to 500 µg/mL and is injected in 1-mL amounts repeatedly at 3- to 5-minute intervals until the erection subsides or until a 1-hour time limit is reached. Blood pressure and pulse are monitored during these injections. If aspiration and phenylephrine irrigation fail, surgical shunting is performed.27
Measures to treat sickle cell disease (hydration, oxygen, exchange transfusions) may be employed simultaneously but should never delay aspiration and phenylephrine injections.25
As nonischemic priapism is not considered an emergency, management begins with observation. Patients eventually become dissatisfied with their constant partial erection, and they then present for treatment. Most cases resolve after selective catheterization of the internal pudendal artery and embolization of the fistula with absorbable material. If this fails, surgical exploration with ligation of the vessels leading to the fistula is indicated.
Prevalence in sickle cell trait vs sickle cell disease
Ischemic priapism is uncommon in men with sickle cell trait, but prevalence rates in men with sickle cell disease are as high as 42%.28 In a study of 130 men with sickle cell disease, 35% had a history of prolonged ischemic priapism, 72% had a history of stuttering priapism, and 75% of men with stuttering priapism had their first episode before age 20.29
Rates of erectile dysfunction increase with the duration of ischemic episodes and range from 20% to 90%.28,30 In childhood, sickle cell disease accounts for 63% of the cases of ischemic priapism, and in adults it accounts for 23% of cases.31
Take-home messages
- Sickle cell disease accounts for two-thirds of cases of ischemic priapism in children, and one-fourth of adult cases.
- Ischemic priapism is a medical emergency.
- Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease (hydration, oxygen, exchange transfusions).
OTHER UROLOGIC COMPLICATIONS OF SICKLE CELL DISEASE
Other urologic complications of sickle cell trait and sickle cell disease include microscopic hematuria, gross hematuria, and renal colic. A formal evaluation of any patient with persistent microscopic hematuria or gross hematuria should consist of urinalysis, computed tomography, and cystoscopy. This approach assesses the upper and lower genitourinary system for treatable causes. Renal ultrasonography can be used instead of computed tomography but tends to provide less information.
Special considerations
In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis. In papillary hypertrophy, friable blood vessels can rupture, resulting in microscopic and gross hematuria. In papillary necrosis, the papilla can slough off and become lodged in the ureter.
Nevertheless, hematuria and renal colic in patients with sickle cell disease or trait are most often attributable to common causes such as infection and stones. A finding of hydronephrosis in the absence of a stone, however, suggests obstruction due to a clot or a sloughed papilla. Ureteroscopy, fulguration, and ureteral stent placement can stop the bleeding and alleviate obstruction in these cases.
Renal medullary carcinoma
Another important reason to order imaging in patients with sickle cell disease or trait who present with urologic symptoms is to rule out renal medullary carcinoma, a rare but aggressive cancer that arises from the collecting duct epithelium. This cancer is twice as likely to occur in males than in females; it has been reported in patients ranging in age from 10 to 40, with a median age at presentation of 26.32
When patients present with symptomatic renal medullary cancer, in most cases the cancer has already metastasized.
On computed tomography, the tumor tends to occupy a central location in the kidney and appears to infiltrate and replace adjacent kidney tissue. Retroperitoneal lymphadenopathy and metastasis are common.
Treatment typically entails radical nephrectomy, chemotherapy, and in some circumstances, radiotherapy. Case reports have shown promising tumor responses to carboplatin and paclitaxel regimens.33,34 Also, a low threshold for imaging in patients with sickle cell disease and trait may increase the odds of early detection of this aggressive cancer.
Sickle cell disease is a common genetic disorder in the United States that disproportionately affects people of African ancestry. The characteristic sickling of red blood cells under conditions of reduced oxygen tension leads to intravascular hemolysis and vaso-occlusive events, which in turn cause tissue ischemia-reperfusion injury affecting multiple organs, including the genitourinary system.1–3
In this paper, we review the genitourinary effects of sickle cell disease, focusing on sickle cell nephropathy, priapism, and renal medullary carcinoma.
THE WIDE-RANGING EFFECTS OF SICKLE CELL DISEASE
In the United States, sickle cell disease affects 1 of every 500 blacks and 1 of every 36,000 Hispanics.1 The term describes hemoglobinopathies associated with sickling of red blood cells.
Sickling of red blood cells results from a single base-pair change in the beta-globin gene from glutamic acid to valine at position 6, causing abnormal hemoglobin (hemoglobin S), which polymerizes under conditions of reduced oxygen tension and alters the biconcave disk shape into a rigid, irregular, unstable cell. The sickle-shaped cells are prone to intravascular hemolysis,2 causing intermittent vaso-occlusive events that result in tissue ischemia-reperfusion injury. Genitourinary problems include impaired ability to concentrate urine, hematuria, renal medullary carcinoma, and increased frequency of urinary tract infection.
SICKLE CELL NEPHROPATHY
The kidney is one of the most frequently affected organs in sickle cell disease. Renal manifestations begin to appear in early childhood, with impaired medullary concentrating ability and ischemic damage to the tubular cells caused by sickling within the vasa recta renis precipitated by the acidic, hypoxic, and hypertonic environment in the renal medulla.
As in early diabetic nephropathy, renal blood flow is enhanced and the glomerular filtration rate (GFR) is increased. Increased cardiac output as a result of anemia, localized release of prostaglandins, and a hypoxia-induced increase in nitric oxide synthesis all play a role in the increase in GFR.4,5
Oxidative stress, an increase in markers of inflammation, and local activation of the renin-angiotensin system contribute to renal damage in sickle cell disease.5–7 The resulting hyperfiltration injury leads to microalbuminuria, which occurs in 20% to 40% of children with sickle cell anemia8,9 and in as many as 60% of adults.
The glomerular lesions associated with sickle cell disease vary from glomerulopathy in the early stages to secondary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, and glomerular thrombotic microangiopathy.10
Clinical presentations and workup
Clinical presentations are not limited to glomerular disease but include hyperchloremic metabolic acidosis and hyperkalemia resulting from defects in potassium secretion and renal acidification.
Hyperphosphatemia—a result of increased reabsorption of phosphorus, increased secretion of uric acid, and increased creatinine clearance—is seen in patients with sickle cell disease.11,12 About 10% of patients can develop an acute kidney injury as a result of volume depletion, rhabdomyolysis, renal vein thrombosis, papillary necrosis, and urinary tract obstruction secondary to blood clots.11,13
Up to 30% of adult patients with sickle cell disease develop chronic kidney disease. Predictors include severe anemia, hypertension, proteinuria, nephrotic syndrome, and microscopic hematuria.14 From 4% to 12% of patients go on to develop end-stage renal disease, but with a 1-year mortality rate three times higher than in patients without sickle cell disease.15
In general, patients with sickle cell anemia have blood pressures below those of age- and sex-matched individuals, but elevated blood pressure and low GFR are not uncommon in affected children. In a cohort of 48 children ages 3 to 18, 8.3% had an estimated GFR less than 90 mL/min/1.73 m2, and 16.7% had elevated blood pressure (prehypertension and hypertension).16
In patients with sickle cell disease, evaluation of proteinuria, hematuria, hypertension, and renal failure should take into consideration the unique renal physiologic and pathologic processes involved. Recent evidence17,18 suggests that the Chronic Kidney Disease Epidemiology Collaboration equation provides a better estimate of GFR than the Modification of Diet in Renal Disease and Cockcroft-Gault equations, although all three creatinine-based methods overestimate GFR in patients with sickle cell disease when compared with GFR measured with technetium-99m-labeled diethylenetriamine penta-acetic acid renal scanning.
Treatment options
Treatment of sickle cell nephropathy includes adequate fluid intake (given the loss of concentrating ability), adequate blood pressure control, use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in patients who have microalbuminuria or proteinuria (or both)9,11,19 and hydroxyurea. Treatment with enalapril has been shown to decrease proteinuria in patients with sickle cell nephropathy.9 In a cohort of children with sickle cell disease, four of nine patients treated with an ACE inhibitor developed hyperkalemia, leading to discontinuation of the drug in three patients.9
ACE inhibitors and ARBs must be used cautiously in these patients because they have defects in potassium secretion. Hydroxyurea has also been shown to decrease hyperfiltration and microalbuminuria in recent studies,20,21 and this could protect against the development of overt nephropathy.
Higher mortality rates have been reported in patients with sickle cell disease who developed end-stage renal disease than in patients with end-stage renal disease without sickle cell disease. Sickle cell disease also increases the risk of pulmonary hypertension and the vaso-occlusive complication known as acute chest syndrome, contributing to increased mortality rates. Of note, in a study that looked at the association between mortality rates and pre-end-stage care of renal disease using data from the Centers for Medicare and Medicaid Services, patients with sickle cell disease who had had predialysis nephrology care had lower mortality rates.15
Treatments for end-stage renal disease are also effective in patients with sickle cell disease and include hemodialysis, peritoneal dialysis, and renal transplantation. Data from the Organ Procurement and Transplantation Network and the United Network for Organ Sharing show that from 2000 to 2011, African American kidney recipients with sickle cell disease had better survival rates than patients who had undergone transplantation from 1988 to 1999, although rates of long-term survival and graft survival were lower than in transplant recipients with other diagnoses.22
It is important to note that complications as a result of vaso-occlusive events and thrombosis can lead to graft loss; therefore, sickle cell crisis after transplantation requires careful management.
Take-home messages
- Loss of urine-concentrating ability and hyperfiltration are the earliest pathologic changes in sickle cell disease.
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- ACE inhibitors or ARBs should be used with caution, given the heightened risk of hyperkalemia in sickle cell disease.
- Recent results with hydroxyurea in decreasing hyperfiltration and microalbuminuria are encouraging.
- Early referral for predialysis nephrologic care is needed in sickle cell patients with chronic kidney disease.
PRIAPISM IN SICKLE CELL DISEASE
Priapism was formerly defined as a full, painful erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm. But priapism is now recognized as two separate disorders—ischemic (veno-occlusive, low-flow) priapism and nonischemic (arterial, high-flow) priapism. The new definition includes both disorders: ie, a full or partial erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm.
Ischemic priapism
Hematologic disorders are major contributors to ischemic priapism and include sickle cell disease, multiple myeloma, fat emboli (hyperalimentation),23 glucose-6-phosphate dehydrogenase deficiency, and hemoglobin Olmsted variant.24
Ischemic priapism is often seen in sickle cell disease and is considered an emergency. It is characterized by an abnormally rigid erection not involving the glans penis. Entrapment of blood in the corpora cavernosa leads to hypoxia, hypercarbia, and acidosis, which in turn leads to a painful compartment syndrome that, if untreated, results in smooth muscle necrosis and subsequent fibrosis. The results are a smaller penis and erectile dysfunction that is unresponsive to any treatment other than implantation of a penile prosthesis. However, scarring of the corpora cavernosa can make this procedure exceedingly difficult, requiring advanced techniques such as corporeal excavation.25
Men with a subtype of ischemic priapism called “stuttering” priapism26 suffer recurrent prolonged erections during sleep. The patient awakens with a painful erection that usually subsides, but sometimes only after several hours. Patients with this disorder suffer from sleep deprivation. Stuttering priapism may lead to full-blown ischemic priapism that does not resolve without intervention.
Nonischemic priapism
In nonischemic priapism, the corpora are engorged but not rigid. The condition results from unregulated arterial inflow and thus is not painful and does not result in damage to the corporeal smooth muscle.
Most cases of nonischemic priapism follow blunt perineal trauma or trauma associated with needle insertion into the corpora. This form of priapism is not associated with sickle cell disease. Because tissue damage does not occur, nonischemic or arterial priapism is not considered an emergency.
Treatment guidelines
Differentiating ischemic from nonischemic priapism is usually straightforward, based on the history, physical examination, corporeal blood gases, and duplex ultrasonography.27
Ischemic priapism is an emergency. After needle aspiration of blood from the corpora cavernosa, phenylephrine is diluted with normal saline to a concentration of 100 to 500 µg/mL and is injected in 1-mL amounts repeatedly at 3- to 5-minute intervals until the erection subsides or until a 1-hour time limit is reached. Blood pressure and pulse are monitored during these injections. If aspiration and phenylephrine irrigation fail, surgical shunting is performed.27
Measures to treat sickle cell disease (hydration, oxygen, exchange transfusions) may be employed simultaneously but should never delay aspiration and phenylephrine injections.25
As nonischemic priapism is not considered an emergency, management begins with observation. Patients eventually become dissatisfied with their constant partial erection, and they then present for treatment. Most cases resolve after selective catheterization of the internal pudendal artery and embolization of the fistula with absorbable material. If this fails, surgical exploration with ligation of the vessels leading to the fistula is indicated.
Prevalence in sickle cell trait vs sickle cell disease
Ischemic priapism is uncommon in men with sickle cell trait, but prevalence rates in men with sickle cell disease are as high as 42%.28 In a study of 130 men with sickle cell disease, 35% had a history of prolonged ischemic priapism, 72% had a history of stuttering priapism, and 75% of men with stuttering priapism had their first episode before age 20.29
Rates of erectile dysfunction increase with the duration of ischemic episodes and range from 20% to 90%.28,30 In childhood, sickle cell disease accounts for 63% of the cases of ischemic priapism, and in adults it accounts for 23% of cases.31
Take-home messages
- Sickle cell disease accounts for two-thirds of cases of ischemic priapism in children, and one-fourth of adult cases.
- Ischemic priapism is a medical emergency.
- Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease (hydration, oxygen, exchange transfusions).
OTHER UROLOGIC COMPLICATIONS OF SICKLE CELL DISEASE
Other urologic complications of sickle cell trait and sickle cell disease include microscopic hematuria, gross hematuria, and renal colic. A formal evaluation of any patient with persistent microscopic hematuria or gross hematuria should consist of urinalysis, computed tomography, and cystoscopy. This approach assesses the upper and lower genitourinary system for treatable causes. Renal ultrasonography can be used instead of computed tomography but tends to provide less information.
Special considerations
In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis. In papillary hypertrophy, friable blood vessels can rupture, resulting in microscopic and gross hematuria. In papillary necrosis, the papilla can slough off and become lodged in the ureter.
Nevertheless, hematuria and renal colic in patients with sickle cell disease or trait are most often attributable to common causes such as infection and stones. A finding of hydronephrosis in the absence of a stone, however, suggests obstruction due to a clot or a sloughed papilla. Ureteroscopy, fulguration, and ureteral stent placement can stop the bleeding and alleviate obstruction in these cases.
Renal medullary carcinoma
Another important reason to order imaging in patients with sickle cell disease or trait who present with urologic symptoms is to rule out renal medullary carcinoma, a rare but aggressive cancer that arises from the collecting duct epithelium. This cancer is twice as likely to occur in males than in females; it has been reported in patients ranging in age from 10 to 40, with a median age at presentation of 26.32
When patients present with symptomatic renal medullary cancer, in most cases the cancer has already metastasized.
On computed tomography, the tumor tends to occupy a central location in the kidney and appears to infiltrate and replace adjacent kidney tissue. Retroperitoneal lymphadenopathy and metastasis are common.
Treatment typically entails radical nephrectomy, chemotherapy, and in some circumstances, radiotherapy. Case reports have shown promising tumor responses to carboplatin and paclitaxel regimens.33,34 Also, a low threshold for imaging in patients with sickle cell disease and trait may increase the odds of early detection of this aggressive cancer.
- Centers for Disease Control and Prevention (CDC). Sickle cell disease (SCD). Data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed August 18, 2015.
- Paulin L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–548.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84:363–376.
- Haymann JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5:756–761.
- da Silva GB Jr, Libório AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 2011; 90:1371–1379.
- Emokpae MA, Uadia PO, Gadzama AA. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med 2010; 9:141–146.
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012; 64:72–80.
- Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70:307–309.
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326:910–915.
- Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010; 89:18–27.
- Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br J Haematol 2011; 155:287–297.
- Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 1982; 72:188–192.
- Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 1990; 13:347–351.
- Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115:614–620.
- McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 2012; 159:360–367.
- Bodas P, Huang A, O Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14:237.
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine based estimating equations. PLoS One 2013; 8:e69922.
- Arlet JB, Ribeil JA, Chatellier G, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012; 13:83.
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29:140–144.
- Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29:1211–1218.
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88:116–119.
- Huang E, Parke C, Mehrnia A, et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol Dial Transplant 2013; 28:1039–1046.
- Klein EA, Montague DK, Steiger E. Priapism associated with the use of intravenous fat emulsion: case reports and postulated pathogenesis. J Urol May 1985; 133:857–859.
- Thuret I, Bardakdjian J, Badens C, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu-->Arg. Am J Hematol 1996; 51:133–136.
- Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology 2006; 67:1072–1075.
- Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl 2012; 14:156–163.
- Montague DK, Jarow J, Broderick GA, et al; Members of the Erectile Dysfunction Guideline Update Panel; American Urological Association. American Urological Association guideline on the management of priapism. J Urol 2003; 170:1318–1324.
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980; 140:1434–1437.
- Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002; 90:898–902.
- Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med 2004; 1:116–120.
- Nelson JH, 3rd, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977; 117:455–458.
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368–374.
- Gangireddy VG, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134–139.
- Walsh AM, Fiveash JB, Reddy AT, Friedman GK. Response to radiation in renal medullary carcinoma. Rare Tumors 2011; 3:e32.
- Centers for Disease Control and Prevention (CDC). Sickle cell disease (SCD). Data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed August 18, 2015.
- Paulin L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–548.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84:363–376.
- Haymann JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5:756–761.
- da Silva GB Jr, Libório AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 2011; 90:1371–1379.
- Emokpae MA, Uadia PO, Gadzama AA. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med 2010; 9:141–146.
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012; 64:72–80.
- Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70:307–309.
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326:910–915.
- Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010; 89:18–27.
- Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br J Haematol 2011; 155:287–297.
- Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 1982; 72:188–192.
- Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 1990; 13:347–351.
- Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115:614–620.
- McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 2012; 159:360–367.
- Bodas P, Huang A, O Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14:237.
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine based estimating equations. PLoS One 2013; 8:e69922.
- Arlet JB, Ribeil JA, Chatellier G, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012; 13:83.
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29:140–144.
- Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29:1211–1218.
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88:116–119.
- Huang E, Parke C, Mehrnia A, et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol Dial Transplant 2013; 28:1039–1046.
- Klein EA, Montague DK, Steiger E. Priapism associated with the use of intravenous fat emulsion: case reports and postulated pathogenesis. J Urol May 1985; 133:857–859.
- Thuret I, Bardakdjian J, Badens C, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu-->Arg. Am J Hematol 1996; 51:133–136.
- Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology 2006; 67:1072–1075.
- Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl 2012; 14:156–163.
- Montague DK, Jarow J, Broderick GA, et al; Members of the Erectile Dysfunction Guideline Update Panel; American Urological Association. American Urological Association guideline on the management of priapism. J Urol 2003; 170:1318–1324.
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980; 140:1434–1437.
- Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002; 90:898–902.
- Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med 2004; 1:116–120.
- Nelson JH, 3rd, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977; 117:455–458.
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368–374.
- Gangireddy VG, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134–139.
- Walsh AM, Fiveash JB, Reddy AT, Friedman GK. Response to radiation in renal medullary carcinoma. Rare Tumors 2011; 3:e32.
KEY POINTS
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- Ischemic priapism is a medical emergency. Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease.
- In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis.
Upper-limb deep vein thrombosis in Paget-Schroetter syndrome
A 43-year-old man with no medical history presented with pain and swelling in his left arm for 2 weeks. He was a regular weight lifter, and his exercise routine included repetitive hyperextension and hyperabduction of his arms while lifting heavy weights.
He had no history of recent trauma or venous cannulation of the left arm. His family history was negative for thrombophilic disorders. Physical examination revealed a swollen and erythematous left arm and visible venous collaterals at the neck, shoulder, and chest. There was no evidence of arterial insufficiency.
Duplex ultrasonography confirmed thrombosis of the left brachial, axillary, and subclavian veins. Further evaluation with computed tomography showed no intrathoracic mass but revealed several subsegmental pulmonary thrombi in the right lung. A screen for thrombophilia was negative. Venography confirmed complete thrombotic occlusion of the subclavian, axillary, and brachial veins (Figure 1).
Catheter-directed thrombolysis with tissue plasminogen activator resulted in complete resolution of the thrombosis, but venography after 3 days of thrombolysis showed 50% residual stenosis of the left subclavian vein where it passes under the first rib (Figure 2). The redness and swelling had markedly improved 2 days after thrombolytic therapy. He was discharged home on rivaroxaban 20 mg daily.
Follow-up venography 2 months later (Figure 3), with the patient performing hyperabduction of the arms, showed a patent subclavian vein with no thrombosis, but dynamic compression and occlusion of the subclavian vein where it passes the first rib. Magnetic resonance imaging (MRI) of the neck showed no cervical (ie, extra) rib and no soft-tissue abnormalities of the scalene triangle.
Following this, the patient underwent resection of the left first rib for decompression of the venous thoracic outlet, which resulted in resolution of his symptoms. He remained asymptomatic at 6-month follow-up.
PAGET-SCHROETTER SYNDROME
Paget-Schroetter syndrome, also referred to as effort-induced or effort thrombosis, is thrombosis of the axillary or subclavian vein associated with strenuous and repetitive activity of the arms. Anatomic abnormalities at the thoracic outlet—cervical rib, congenital bands, hypertrophy of scalene tendons, abnormal insertion of the costoclavicular ligament—and repetitive trauma to the endothelium of the subclavian vein are key factors in its initiation and progression.
The condition is seen primarily in young people who participate in strenuous activities such as rowing, weight lifting, and baseball pitching. It is estimated to be the cause of 40% of cases of primary upper-extremity deep vein thrombosis in the absence of an obvious risk factor or trigger such as a central venous catheter, pacemaker, port, or occult malignancy.1
A provocative test such as the Adson test or hyperabduction test during MRI or venography helps confirm thoracic outlet obstruction by demonstrating dynamic obstruction.2
TREATMENT CONSIDERATIONS
There are no universal guidelines for the treatment of Paget-Schroetter syndrome. However, the available data3–5 suggest a multimodal approach that involves early catheter-directed thrombolysis and subsequent surgical decompression of the thoracic outlet. This can restore venous patency and reduce the risk of long-term complications such as rethrombosis and postthrombotic syndrome.3–5
Surgical treatment includes resection of the first rib and division of the scalene muscles and the costoclavicular ligament. MRI with provocative testing helps guide the surgical approach. Anticoagulation therapy alone—ie, without thrombolysis and surgical decompression—is inadequate as it leads to recurrence of thrombosis and residual symptoms.6
Paget-Schroetter syndrome should not be managed the same as lower-extremity deep vein thrombosis because the cause and the exacerbating factors are different.
Unanswered questions
Because we have no data from randomized controlled trials, questions about management remain. What should be the duration of anticoagulation, especially in the absence of coexisting thrombophilia? Is thrombophilia screening useful? What is the optimal timing for starting thrombolytic therapy?
A careful history and heightened suspicion are required to make this diagnosis. If undiagnosed, it carries a risk of significant long-term morbidity and death. Dynamic obstruction during venography, in addition to MRI, can help identify an anatomic obstruction.
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost 2006; 32:729–736.
- Demirbag D, Unlu E, Ozdemir F, et al. The relationship between magnetic resonance imaging findings and postural maneuver and physical examination tests in patients with thoracic outlet syndrome: results of a double-blind, controlled study. Arch Phys Med Rehabil 2007; 88:844–851.
- Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med 2010; 11:358–362.
- Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg 2007; 45:328–334.
- Thompson RW. Comprehensive management of subclavian vein effort thrombosis. Semin Intervent Radiol 2012; 29:44–51.
- AbuRahma AF, Robinson PA. Effort subclavian vein thrombosis: evolution of management. J Endovasc Ther 2000; 7:302–308.
A 43-year-old man with no medical history presented with pain and swelling in his left arm for 2 weeks. He was a regular weight lifter, and his exercise routine included repetitive hyperextension and hyperabduction of his arms while lifting heavy weights.
He had no history of recent trauma or venous cannulation of the left arm. His family history was negative for thrombophilic disorders. Physical examination revealed a swollen and erythematous left arm and visible venous collaterals at the neck, shoulder, and chest. There was no evidence of arterial insufficiency.
Duplex ultrasonography confirmed thrombosis of the left brachial, axillary, and subclavian veins. Further evaluation with computed tomography showed no intrathoracic mass but revealed several subsegmental pulmonary thrombi in the right lung. A screen for thrombophilia was negative. Venography confirmed complete thrombotic occlusion of the subclavian, axillary, and brachial veins (Figure 1).
Catheter-directed thrombolysis with tissue plasminogen activator resulted in complete resolution of the thrombosis, but venography after 3 days of thrombolysis showed 50% residual stenosis of the left subclavian vein where it passes under the first rib (Figure 2). The redness and swelling had markedly improved 2 days after thrombolytic therapy. He was discharged home on rivaroxaban 20 mg daily.
Follow-up venography 2 months later (Figure 3), with the patient performing hyperabduction of the arms, showed a patent subclavian vein with no thrombosis, but dynamic compression and occlusion of the subclavian vein where it passes the first rib. Magnetic resonance imaging (MRI) of the neck showed no cervical (ie, extra) rib and no soft-tissue abnormalities of the scalene triangle.
Following this, the patient underwent resection of the left first rib for decompression of the venous thoracic outlet, which resulted in resolution of his symptoms. He remained asymptomatic at 6-month follow-up.
PAGET-SCHROETTER SYNDROME
Paget-Schroetter syndrome, also referred to as effort-induced or effort thrombosis, is thrombosis of the axillary or subclavian vein associated with strenuous and repetitive activity of the arms. Anatomic abnormalities at the thoracic outlet—cervical rib, congenital bands, hypertrophy of scalene tendons, abnormal insertion of the costoclavicular ligament—and repetitive trauma to the endothelium of the subclavian vein are key factors in its initiation and progression.
The condition is seen primarily in young people who participate in strenuous activities such as rowing, weight lifting, and baseball pitching. It is estimated to be the cause of 40% of cases of primary upper-extremity deep vein thrombosis in the absence of an obvious risk factor or trigger such as a central venous catheter, pacemaker, port, or occult malignancy.1
A provocative test such as the Adson test or hyperabduction test during MRI or venography helps confirm thoracic outlet obstruction by demonstrating dynamic obstruction.2
TREATMENT CONSIDERATIONS
There are no universal guidelines for the treatment of Paget-Schroetter syndrome. However, the available data3–5 suggest a multimodal approach that involves early catheter-directed thrombolysis and subsequent surgical decompression of the thoracic outlet. This can restore venous patency and reduce the risk of long-term complications such as rethrombosis and postthrombotic syndrome.3–5
Surgical treatment includes resection of the first rib and division of the scalene muscles and the costoclavicular ligament. MRI with provocative testing helps guide the surgical approach. Anticoagulation therapy alone—ie, without thrombolysis and surgical decompression—is inadequate as it leads to recurrence of thrombosis and residual symptoms.6
Paget-Schroetter syndrome should not be managed the same as lower-extremity deep vein thrombosis because the cause and the exacerbating factors are different.
Unanswered questions
Because we have no data from randomized controlled trials, questions about management remain. What should be the duration of anticoagulation, especially in the absence of coexisting thrombophilia? Is thrombophilia screening useful? What is the optimal timing for starting thrombolytic therapy?
A careful history and heightened suspicion are required to make this diagnosis. If undiagnosed, it carries a risk of significant long-term morbidity and death. Dynamic obstruction during venography, in addition to MRI, can help identify an anatomic obstruction.
A 43-year-old man with no medical history presented with pain and swelling in his left arm for 2 weeks. He was a regular weight lifter, and his exercise routine included repetitive hyperextension and hyperabduction of his arms while lifting heavy weights.
He had no history of recent trauma or venous cannulation of the left arm. His family history was negative for thrombophilic disorders. Physical examination revealed a swollen and erythematous left arm and visible venous collaterals at the neck, shoulder, and chest. There was no evidence of arterial insufficiency.
Duplex ultrasonography confirmed thrombosis of the left brachial, axillary, and subclavian veins. Further evaluation with computed tomography showed no intrathoracic mass but revealed several subsegmental pulmonary thrombi in the right lung. A screen for thrombophilia was negative. Venography confirmed complete thrombotic occlusion of the subclavian, axillary, and brachial veins (Figure 1).
Catheter-directed thrombolysis with tissue plasminogen activator resulted in complete resolution of the thrombosis, but venography after 3 days of thrombolysis showed 50% residual stenosis of the left subclavian vein where it passes under the first rib (Figure 2). The redness and swelling had markedly improved 2 days after thrombolytic therapy. He was discharged home on rivaroxaban 20 mg daily.
Follow-up venography 2 months later (Figure 3), with the patient performing hyperabduction of the arms, showed a patent subclavian vein with no thrombosis, but dynamic compression and occlusion of the subclavian vein where it passes the first rib. Magnetic resonance imaging (MRI) of the neck showed no cervical (ie, extra) rib and no soft-tissue abnormalities of the scalene triangle.
Following this, the patient underwent resection of the left first rib for decompression of the venous thoracic outlet, which resulted in resolution of his symptoms. He remained asymptomatic at 6-month follow-up.
PAGET-SCHROETTER SYNDROME
Paget-Schroetter syndrome, also referred to as effort-induced or effort thrombosis, is thrombosis of the axillary or subclavian vein associated with strenuous and repetitive activity of the arms. Anatomic abnormalities at the thoracic outlet—cervical rib, congenital bands, hypertrophy of scalene tendons, abnormal insertion of the costoclavicular ligament—and repetitive trauma to the endothelium of the subclavian vein are key factors in its initiation and progression.
The condition is seen primarily in young people who participate in strenuous activities such as rowing, weight lifting, and baseball pitching. It is estimated to be the cause of 40% of cases of primary upper-extremity deep vein thrombosis in the absence of an obvious risk factor or trigger such as a central venous catheter, pacemaker, port, or occult malignancy.1
A provocative test such as the Adson test or hyperabduction test during MRI or venography helps confirm thoracic outlet obstruction by demonstrating dynamic obstruction.2
TREATMENT CONSIDERATIONS
There are no universal guidelines for the treatment of Paget-Schroetter syndrome. However, the available data3–5 suggest a multimodal approach that involves early catheter-directed thrombolysis and subsequent surgical decompression of the thoracic outlet. This can restore venous patency and reduce the risk of long-term complications such as rethrombosis and postthrombotic syndrome.3–5
Surgical treatment includes resection of the first rib and division of the scalene muscles and the costoclavicular ligament. MRI with provocative testing helps guide the surgical approach. Anticoagulation therapy alone—ie, without thrombolysis and surgical decompression—is inadequate as it leads to recurrence of thrombosis and residual symptoms.6
Paget-Schroetter syndrome should not be managed the same as lower-extremity deep vein thrombosis because the cause and the exacerbating factors are different.
Unanswered questions
Because we have no data from randomized controlled trials, questions about management remain. What should be the duration of anticoagulation, especially in the absence of coexisting thrombophilia? Is thrombophilia screening useful? What is the optimal timing for starting thrombolytic therapy?
A careful history and heightened suspicion are required to make this diagnosis. If undiagnosed, it carries a risk of significant long-term morbidity and death. Dynamic obstruction during venography, in addition to MRI, can help identify an anatomic obstruction.
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost 2006; 32:729–736.
- Demirbag D, Unlu E, Ozdemir F, et al. The relationship between magnetic resonance imaging findings and postural maneuver and physical examination tests in patients with thoracic outlet syndrome: results of a double-blind, controlled study. Arch Phys Med Rehabil 2007; 88:844–851.
- Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med 2010; 11:358–362.
- Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg 2007; 45:328–334.
- Thompson RW. Comprehensive management of subclavian vein effort thrombosis. Semin Intervent Radiol 2012; 29:44–51.
- AbuRahma AF, Robinson PA. Effort subclavian vein thrombosis: evolution of management. J Endovasc Ther 2000; 7:302–308.
- Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost 2006; 32:729–736.
- Demirbag D, Unlu E, Ozdemir F, et al. The relationship between magnetic resonance imaging findings and postural maneuver and physical examination tests in patients with thoracic outlet syndrome: results of a double-blind, controlled study. Arch Phys Med Rehabil 2007; 88:844–851.
- Alla VM, Natarajan N, Kaushik M, Warrier R, Nair CK. Paget-Schroetter syndrome: review of pathogenesis and treatment of effort thrombosis. West J Emerg Med 2010; 11:358–362.
- Molina JE, Hunter DW, Dietz CA. Paget-Schroetter syndrome treated with thrombolytics and immediate surgery. J Vasc Surg 2007; 45:328–334.
- Thompson RW. Comprehensive management of subclavian vein effort thrombosis. Semin Intervent Radiol 2012; 29:44–51.
- AbuRahma AF, Robinson PA. Effort subclavian vein thrombosis: evolution of management. J Endovasc Ther 2000; 7:302–308.
Readmissions rise with endovascular lower limb procedures
CHICAGO – Endovascular lower-extremity procedures were not associated with lower 30-day readmission rates compared with open surgery in a retrospective review of 7,089 patients.
All-cause, 30-day readmissions were actually higher with an endovascular approach at 12.3% vs. 9.6% for open procedures (Relative risk, 1.28; P = .0003).
Among all patients, an index diagnosis of gangrene was most predictive of readmission (RR, 1.89; P less than .0001), Dr. Todd R. Vogel said at the annual meeting of the Midwestern Vascular Surgical Society.
The data were compiled from 7,089 patients in the Cerner Health Facts database who were admitted for peripheral artery disease and elective lower extremity procedures (3,615 open; 3,474 endo) between September 2008 and March 2014. Their average age was 67.7 years, 44.7% were aged 70 years or older, 60% were men, and 21% were African American.
Older patients and men were significantly more likely to receive endovascular procedures (P less than .0001), said Dr. Vogel, chief of vascular surgery, University of Missouri Health System in Columbia.
Overall, 767 patients (11%) were readmitted (344 open; 423 endo), with gangrene accounting for 21.7% of readmissions.
Other index diagnoses associated with higher 30-day readmissions for all lower extremity procedures were fluid and electrolyte disorders, chronic anemia, lower extremity infection, heart failure, chronic kidney disease, and chronic pulmonary disease.
When stratified by procedure type, the reasons for readmission were very different within the same population of patients based on procedure type, Dr. Vogel said.
Patients who underwent an open procedure were more likely to be readmitted if they had heart failure (RR, 1.78; P less than .0001) or posthemorrhagic anemia (RR, 1.54: P = .006).
Infections – be they lower extremity infection, other infection, postoperative infection, or sepsis – were not predictive of readmission when documented at the index admission for the open cohort.
In contrast, chronic conditions were the major predictors of readmission for patients undergoing endovascular procedures, he said. They included chronic anemia (RR, 1.58; P less than .0001), chronic airway obstruction (RR, 1.36; P = .0095), chronic heart disease (RR, 1.33; P = .0019), chronic kidney disease (RR, 1.37; P = .0013), diabetes (RR, 1.34; P = .0012), and hypertension (RR, 1.27; P = .023).
Fluid and electrolyte disorders (RR, 1.65, P less than .0001) and lower extremity infections (RR, 1.57, P = .0016) were also significant predictors of readmission in the endovascular group.
To ensure there were no disparities between index and readmission diagnoses, a final analysis was performed by procedure type in the 767 readmissions. It confirmed that for the endovascular procedures, chronic problems are bringing patients back to the hospital and not necessarily complications from the procedure, whereas infections, device complications, and hemorrhage are the reasons open surgery patients return, Dr. Vogel said.
“The question is are chronic conditions associated with readmissions the fault of the intervention? As physicians can we hope to curb this in patients who have chronic problems and are then readmitted?” he said.
Some audience members argued that no matter if the patient had a chronic condition or not preoperatively, the responsibility rests with the surgeon because he or she opted to put the patient through an elective endovascular procedure and now they’re returning with chronic heart failure, for example.
Dr. Vogel said this was the first pass at the data and trying to understand what drives readmissions and that it’s possible an endovascular procedure could exacerbate a chronic condition, but that surgeons should take steps to mitigate readmission risk in those with known chronic conditions.
Other attendees questioned how many of the readmissions were planned, hinting that the readmissions may not be directly related to the endovascular technique.
Dr. Vogel said it was difficult using only the ICD-9 codes in the database to determine exactly how many readmissions were planned, but noted that further analyses are intended.
“Reasons for readmission can be exacerbation of chronic patient issues, as seen in the endovascular group, or may be secondary to later complications of the procedure such as wound infections and device complications, as seen after open bypass procedures,” he said in an interview. “Identifying patients with increased risk for readmission after vascular procedures may lead to more effective and higher quality care during the index hospitalization. Our future studies will focus on a more detailed, granular evaluation of these high-risk diagnoses groups through use of the electronic medical record.”
Dr. Vogel reported having no financial disclosures.
On Twitter @pwendl
CHICAGO – Endovascular lower-extremity procedures were not associated with lower 30-day readmission rates compared with open surgery in a retrospective review of 7,089 patients.
All-cause, 30-day readmissions were actually higher with an endovascular approach at 12.3% vs. 9.6% for open procedures (Relative risk, 1.28; P = .0003).
Among all patients, an index diagnosis of gangrene was most predictive of readmission (RR, 1.89; P less than .0001), Dr. Todd R. Vogel said at the annual meeting of the Midwestern Vascular Surgical Society.
The data were compiled from 7,089 patients in the Cerner Health Facts database who were admitted for peripheral artery disease and elective lower extremity procedures (3,615 open; 3,474 endo) between September 2008 and March 2014. Their average age was 67.7 years, 44.7% were aged 70 years or older, 60% were men, and 21% were African American.
Older patients and men were significantly more likely to receive endovascular procedures (P less than .0001), said Dr. Vogel, chief of vascular surgery, University of Missouri Health System in Columbia.
Overall, 767 patients (11%) were readmitted (344 open; 423 endo), with gangrene accounting for 21.7% of readmissions.
Other index diagnoses associated with higher 30-day readmissions for all lower extremity procedures were fluid and electrolyte disorders, chronic anemia, lower extremity infection, heart failure, chronic kidney disease, and chronic pulmonary disease.
When stratified by procedure type, the reasons for readmission were very different within the same population of patients based on procedure type, Dr. Vogel said.
Patients who underwent an open procedure were more likely to be readmitted if they had heart failure (RR, 1.78; P less than .0001) or posthemorrhagic anemia (RR, 1.54: P = .006).
Infections – be they lower extremity infection, other infection, postoperative infection, or sepsis – were not predictive of readmission when documented at the index admission for the open cohort.
In contrast, chronic conditions were the major predictors of readmission for patients undergoing endovascular procedures, he said. They included chronic anemia (RR, 1.58; P less than .0001), chronic airway obstruction (RR, 1.36; P = .0095), chronic heart disease (RR, 1.33; P = .0019), chronic kidney disease (RR, 1.37; P = .0013), diabetes (RR, 1.34; P = .0012), and hypertension (RR, 1.27; P = .023).
Fluid and electrolyte disorders (RR, 1.65, P less than .0001) and lower extremity infections (RR, 1.57, P = .0016) were also significant predictors of readmission in the endovascular group.
To ensure there were no disparities between index and readmission diagnoses, a final analysis was performed by procedure type in the 767 readmissions. It confirmed that for the endovascular procedures, chronic problems are bringing patients back to the hospital and not necessarily complications from the procedure, whereas infections, device complications, and hemorrhage are the reasons open surgery patients return, Dr. Vogel said.
“The question is are chronic conditions associated with readmissions the fault of the intervention? As physicians can we hope to curb this in patients who have chronic problems and are then readmitted?” he said.
Some audience members argued that no matter if the patient had a chronic condition or not preoperatively, the responsibility rests with the surgeon because he or she opted to put the patient through an elective endovascular procedure and now they’re returning with chronic heart failure, for example.
Dr. Vogel said this was the first pass at the data and trying to understand what drives readmissions and that it’s possible an endovascular procedure could exacerbate a chronic condition, but that surgeons should take steps to mitigate readmission risk in those with known chronic conditions.
Other attendees questioned how many of the readmissions were planned, hinting that the readmissions may not be directly related to the endovascular technique.
Dr. Vogel said it was difficult using only the ICD-9 codes in the database to determine exactly how many readmissions were planned, but noted that further analyses are intended.
“Reasons for readmission can be exacerbation of chronic patient issues, as seen in the endovascular group, or may be secondary to later complications of the procedure such as wound infections and device complications, as seen after open bypass procedures,” he said in an interview. “Identifying patients with increased risk for readmission after vascular procedures may lead to more effective and higher quality care during the index hospitalization. Our future studies will focus on a more detailed, granular evaluation of these high-risk diagnoses groups through use of the electronic medical record.”
Dr. Vogel reported having no financial disclosures.
On Twitter @pwendl
CHICAGO – Endovascular lower-extremity procedures were not associated with lower 30-day readmission rates compared with open surgery in a retrospective review of 7,089 patients.
All-cause, 30-day readmissions were actually higher with an endovascular approach at 12.3% vs. 9.6% for open procedures (Relative risk, 1.28; P = .0003).
Among all patients, an index diagnosis of gangrene was most predictive of readmission (RR, 1.89; P less than .0001), Dr. Todd R. Vogel said at the annual meeting of the Midwestern Vascular Surgical Society.
The data were compiled from 7,089 patients in the Cerner Health Facts database who were admitted for peripheral artery disease and elective lower extremity procedures (3,615 open; 3,474 endo) between September 2008 and March 2014. Their average age was 67.7 years, 44.7% were aged 70 years or older, 60% were men, and 21% were African American.
Older patients and men were significantly more likely to receive endovascular procedures (P less than .0001), said Dr. Vogel, chief of vascular surgery, University of Missouri Health System in Columbia.
Overall, 767 patients (11%) were readmitted (344 open; 423 endo), with gangrene accounting for 21.7% of readmissions.
Other index diagnoses associated with higher 30-day readmissions for all lower extremity procedures were fluid and electrolyte disorders, chronic anemia, lower extremity infection, heart failure, chronic kidney disease, and chronic pulmonary disease.
When stratified by procedure type, the reasons for readmission were very different within the same population of patients based on procedure type, Dr. Vogel said.
Patients who underwent an open procedure were more likely to be readmitted if they had heart failure (RR, 1.78; P less than .0001) or posthemorrhagic anemia (RR, 1.54: P = .006).
Infections – be they lower extremity infection, other infection, postoperative infection, or sepsis – were not predictive of readmission when documented at the index admission for the open cohort.
In contrast, chronic conditions were the major predictors of readmission for patients undergoing endovascular procedures, he said. They included chronic anemia (RR, 1.58; P less than .0001), chronic airway obstruction (RR, 1.36; P = .0095), chronic heart disease (RR, 1.33; P = .0019), chronic kidney disease (RR, 1.37; P = .0013), diabetes (RR, 1.34; P = .0012), and hypertension (RR, 1.27; P = .023).
Fluid and electrolyte disorders (RR, 1.65, P less than .0001) and lower extremity infections (RR, 1.57, P = .0016) were also significant predictors of readmission in the endovascular group.
To ensure there were no disparities between index and readmission diagnoses, a final analysis was performed by procedure type in the 767 readmissions. It confirmed that for the endovascular procedures, chronic problems are bringing patients back to the hospital and not necessarily complications from the procedure, whereas infections, device complications, and hemorrhage are the reasons open surgery patients return, Dr. Vogel said.
“The question is are chronic conditions associated with readmissions the fault of the intervention? As physicians can we hope to curb this in patients who have chronic problems and are then readmitted?” he said.
Some audience members argued that no matter if the patient had a chronic condition or not preoperatively, the responsibility rests with the surgeon because he or she opted to put the patient through an elective endovascular procedure and now they’re returning with chronic heart failure, for example.
Dr. Vogel said this was the first pass at the data and trying to understand what drives readmissions and that it’s possible an endovascular procedure could exacerbate a chronic condition, but that surgeons should take steps to mitigate readmission risk in those with known chronic conditions.
Other attendees questioned how many of the readmissions were planned, hinting that the readmissions may not be directly related to the endovascular technique.
Dr. Vogel said it was difficult using only the ICD-9 codes in the database to determine exactly how many readmissions were planned, but noted that further analyses are intended.
“Reasons for readmission can be exacerbation of chronic patient issues, as seen in the endovascular group, or may be secondary to later complications of the procedure such as wound infections and device complications, as seen after open bypass procedures,” he said in an interview. “Identifying patients with increased risk for readmission after vascular procedures may lead to more effective and higher quality care during the index hospitalization. Our future studies will focus on a more detailed, granular evaluation of these high-risk diagnoses groups through use of the electronic medical record.”
Dr. Vogel reported having no financial disclosures.
On Twitter @pwendl
AT MIDWESTERN VASCULAR 2015
Key clinical point: Endovascular procedures were not superior to open surgery in reducing 30-day readmissions in patients undergoing lower extremity procedures.
Major finding: All-cause 30-day readmissions were 12.3% for endovascular and 9.6% for open (P = .0003).
Data source: Retrospective study in 7,089 patients undergoing elective lower extremity procedures.
Disclosures: The research was supported by an award from the Agency for Healthcare Research and Quality. Dr. Vogel reported having no conflicts of interest.
Sunshine Act shows vascular surgeons reap more industry payments
CHICAGO – Drug- and device makers paid $3.4 billion to U.S. physicians and hospitals in the last 5 months of 2013, according to first-year data from the Centers for Medicare & Medicaid Services (CMS) Open Payments program, Dr. John Blebea reported at the annual meeting of the Midwestern Vascular Surgical Society.
The Open Payments program is the first step by the federal government toward transparency on the financial relationships between physicians and drug- and device makers and is charged with providing data that is both understandable by the public and searchable for individual physicians.
Under the Physician Payments Sunshine Act, a provision of the Affordable Care Act, manufacturers of drugs, medical devices, and biologics that participate in Medicare and Medicaid are required to report any payments or transfers of items with a $10 onetime value or $100 cumulative annual value to nonresident physicians and teaching hospitals.
Dr. Blebea and his colleagues at the University of Oklahoma in Tulsa sought to examine payments made to vascular specialists during the first year of the Open Payments program using data available from August 2013 to December 2013.
Nationally, 1,347 companies paid $2.9 billion (85%) to 470,000 physicians and $599 million (15%) to 1,019 hospitals during that period. Almost half of payments to physicians ($1.19 billion) was for research; $735 million was for food, travel, honoraria, and consulting services, and about one-third ($908 million) was in stock ownership or investments, Dr. Blebea said.
The investigators also looked at data from New York alone, where payments varied widely among specialties. Four vascular surgeons and one cardiologist reported ownership or investment interests totaling $1,092,025 and $98,689, respectively, but the data were skewed because one vascular surgeon had investment stock valued at $1,033,728, Dr. Blebea said.
Research grants were uncommon among the 223 vascular surgeons, 229 interventional cardiologists, and 88 radiologists and valued at just $4,250, $5,372, and $8,532.
General payments were significantly different between the three groups ($1,808,890 vs. $534,688 vs. $73,492; P less than .0001), he said. This averaged $3,196 per vascular surgeon, $1,889 per cardiologist, and $738 per radiologist. But, again there were broad variations in the data, resulting in medians of $279, $99, and $116, respectively.
One could argue that $279 isn’t a lot in terms of payments for services made or received, but a small number of vascular surgeons did receive what one could argue is a significant amount of money, Dr. Blebea said. Specifically, 8% received more than $5,000 over the 5 months, and three received more than $100,000.
“So you could ask the question: ‘Could this induce bias in scientific presentations?’ and you could answer, ‘Maybe yes, maybe no,’ ” he said. “But what about the three individuals who received more than $100,000? The answer there is that they are probably more likely to be consciously or unconsciously biased in their presentations.”
Dr. Iraklis Pipinos of the University of Nebraska, Omaha, questioned the number of specialists in the New York analysis, noting that he would expect the number of cardiologists to be four to five times that of vascular specialists.
“It’s an important point and I share your concern,” Dr. Blebea responded. “In actual fact, how people are reported in terms of their specialties is how the companies categorize you, so the data may not be completely accurate. It’s one of the challenges.”
Industry groups and professional societies have raised concerns about the incompleteness of the Open Payments data and argued that inaccuracies could harm reputations and undermine trust between patients and their physicians.
Physicians have 45 days after the data submission period to review their Open Payments data and dispute errors before the information is released publicly. Errors can be contested after the deadline has passed, with corrections made in the next reporting cycle.
Still, of the 4.3 million payments made nationally in the last 5 months of 2013, only 1,145 payments (0.02%) worth just $6.25 million were contested, Dr. Blebea reported.
“So it’s either accurate or most physicians didn’t bother to contest inaccuracies,” he said, adding, “I certainly did [contest the data] because there was an inaccuracy in what was reported for me and that was corrected, but how many people will correct these in the future? I hope everybody does.”
Of the $6.49 billion paid to physicians and hospitals in 2014, physicians have disputed only $5.06 million in general payments and $13.16 million in research payments, according to 2014 data reported by the CMS .
Dr. Daniel G. Clair, chair of vascular surgery at the Cleveland Clinic, commented that contrary to what the analysis suggests, it isn’t easy to distinguish between research dollars and nonresearch dollars and between payments made to an institution versus those made to an individual.
“I work for a facility where I am a salaried professional and contracts for some of these things are negotiated between the institution and the company. I’m completely left out of it, but because I happen to be the individual who provides services, it looks like that money is coming to me,” he said.
To provide more transparency in payments, Dr. Blebea said he would recommend quantitative disclosure of industry payments at scientific meetings and in publications with reporting of a range of payments, such as less than $1,000, $1,000-$5,000, $5,001-$10,000, and more than $10,000, rather than specific amounts.
Dr. Blebea and Dr. Pipinos reported having no relevant financial disclosures. Dr. Clair reported serving on the data and safety monitoring board for Bard, as an advisory board member for Boston Scientific and Medtronic, and as a consultant for Endologix.
CHICAGO – Drug- and device makers paid $3.4 billion to U.S. physicians and hospitals in the last 5 months of 2013, according to first-year data from the Centers for Medicare & Medicaid Services (CMS) Open Payments program, Dr. John Blebea reported at the annual meeting of the Midwestern Vascular Surgical Society.
The Open Payments program is the first step by the federal government toward transparency on the financial relationships between physicians and drug- and device makers and is charged with providing data that is both understandable by the public and searchable for individual physicians.
Under the Physician Payments Sunshine Act, a provision of the Affordable Care Act, manufacturers of drugs, medical devices, and biologics that participate in Medicare and Medicaid are required to report any payments or transfers of items with a $10 onetime value or $100 cumulative annual value to nonresident physicians and teaching hospitals.
Dr. Blebea and his colleagues at the University of Oklahoma in Tulsa sought to examine payments made to vascular specialists during the first year of the Open Payments program using data available from August 2013 to December 2013.
Nationally, 1,347 companies paid $2.9 billion (85%) to 470,000 physicians and $599 million (15%) to 1,019 hospitals during that period. Almost half of payments to physicians ($1.19 billion) was for research; $735 million was for food, travel, honoraria, and consulting services, and about one-third ($908 million) was in stock ownership or investments, Dr. Blebea said.
The investigators also looked at data from New York alone, where payments varied widely among specialties. Four vascular surgeons and one cardiologist reported ownership or investment interests totaling $1,092,025 and $98,689, respectively, but the data were skewed because one vascular surgeon had investment stock valued at $1,033,728, Dr. Blebea said.
Research grants were uncommon among the 223 vascular surgeons, 229 interventional cardiologists, and 88 radiologists and valued at just $4,250, $5,372, and $8,532.
General payments were significantly different between the three groups ($1,808,890 vs. $534,688 vs. $73,492; P less than .0001), he said. This averaged $3,196 per vascular surgeon, $1,889 per cardiologist, and $738 per radiologist. But, again there were broad variations in the data, resulting in medians of $279, $99, and $116, respectively.
One could argue that $279 isn’t a lot in terms of payments for services made or received, but a small number of vascular surgeons did receive what one could argue is a significant amount of money, Dr. Blebea said. Specifically, 8% received more than $5,000 over the 5 months, and three received more than $100,000.
“So you could ask the question: ‘Could this induce bias in scientific presentations?’ and you could answer, ‘Maybe yes, maybe no,’ ” he said. “But what about the three individuals who received more than $100,000? The answer there is that they are probably more likely to be consciously or unconsciously biased in their presentations.”
Dr. Iraklis Pipinos of the University of Nebraska, Omaha, questioned the number of specialists in the New York analysis, noting that he would expect the number of cardiologists to be four to five times that of vascular specialists.
“It’s an important point and I share your concern,” Dr. Blebea responded. “In actual fact, how people are reported in terms of their specialties is how the companies categorize you, so the data may not be completely accurate. It’s one of the challenges.”
Industry groups and professional societies have raised concerns about the incompleteness of the Open Payments data and argued that inaccuracies could harm reputations and undermine trust between patients and their physicians.
Physicians have 45 days after the data submission period to review their Open Payments data and dispute errors before the information is released publicly. Errors can be contested after the deadline has passed, with corrections made in the next reporting cycle.
Still, of the 4.3 million payments made nationally in the last 5 months of 2013, only 1,145 payments (0.02%) worth just $6.25 million were contested, Dr. Blebea reported.
“So it’s either accurate or most physicians didn’t bother to contest inaccuracies,” he said, adding, “I certainly did [contest the data] because there was an inaccuracy in what was reported for me and that was corrected, but how many people will correct these in the future? I hope everybody does.”
Of the $6.49 billion paid to physicians and hospitals in 2014, physicians have disputed only $5.06 million in general payments and $13.16 million in research payments, according to 2014 data reported by the CMS .
Dr. Daniel G. Clair, chair of vascular surgery at the Cleveland Clinic, commented that contrary to what the analysis suggests, it isn’t easy to distinguish between research dollars and nonresearch dollars and between payments made to an institution versus those made to an individual.
“I work for a facility where I am a salaried professional and contracts for some of these things are negotiated between the institution and the company. I’m completely left out of it, but because I happen to be the individual who provides services, it looks like that money is coming to me,” he said.
To provide more transparency in payments, Dr. Blebea said he would recommend quantitative disclosure of industry payments at scientific meetings and in publications with reporting of a range of payments, such as less than $1,000, $1,000-$5,000, $5,001-$10,000, and more than $10,000, rather than specific amounts.
Dr. Blebea and Dr. Pipinos reported having no relevant financial disclosures. Dr. Clair reported serving on the data and safety monitoring board for Bard, as an advisory board member for Boston Scientific and Medtronic, and as a consultant for Endologix.
CHICAGO – Drug- and device makers paid $3.4 billion to U.S. physicians and hospitals in the last 5 months of 2013, according to first-year data from the Centers for Medicare & Medicaid Services (CMS) Open Payments program, Dr. John Blebea reported at the annual meeting of the Midwestern Vascular Surgical Society.
The Open Payments program is the first step by the federal government toward transparency on the financial relationships between physicians and drug- and device makers and is charged with providing data that is both understandable by the public and searchable for individual physicians.
Under the Physician Payments Sunshine Act, a provision of the Affordable Care Act, manufacturers of drugs, medical devices, and biologics that participate in Medicare and Medicaid are required to report any payments or transfers of items with a $10 onetime value or $100 cumulative annual value to nonresident physicians and teaching hospitals.
Dr. Blebea and his colleagues at the University of Oklahoma in Tulsa sought to examine payments made to vascular specialists during the first year of the Open Payments program using data available from August 2013 to December 2013.
Nationally, 1,347 companies paid $2.9 billion (85%) to 470,000 physicians and $599 million (15%) to 1,019 hospitals during that period. Almost half of payments to physicians ($1.19 billion) was for research; $735 million was for food, travel, honoraria, and consulting services, and about one-third ($908 million) was in stock ownership or investments, Dr. Blebea said.
The investigators also looked at data from New York alone, where payments varied widely among specialties. Four vascular surgeons and one cardiologist reported ownership or investment interests totaling $1,092,025 and $98,689, respectively, but the data were skewed because one vascular surgeon had investment stock valued at $1,033,728, Dr. Blebea said.
Research grants were uncommon among the 223 vascular surgeons, 229 interventional cardiologists, and 88 radiologists and valued at just $4,250, $5,372, and $8,532.
General payments were significantly different between the three groups ($1,808,890 vs. $534,688 vs. $73,492; P less than .0001), he said. This averaged $3,196 per vascular surgeon, $1,889 per cardiologist, and $738 per radiologist. But, again there were broad variations in the data, resulting in medians of $279, $99, and $116, respectively.
One could argue that $279 isn’t a lot in terms of payments for services made or received, but a small number of vascular surgeons did receive what one could argue is a significant amount of money, Dr. Blebea said. Specifically, 8% received more than $5,000 over the 5 months, and three received more than $100,000.
“So you could ask the question: ‘Could this induce bias in scientific presentations?’ and you could answer, ‘Maybe yes, maybe no,’ ” he said. “But what about the three individuals who received more than $100,000? The answer there is that they are probably more likely to be consciously or unconsciously biased in their presentations.”
Dr. Iraklis Pipinos of the University of Nebraska, Omaha, questioned the number of specialists in the New York analysis, noting that he would expect the number of cardiologists to be four to five times that of vascular specialists.
“It’s an important point and I share your concern,” Dr. Blebea responded. “In actual fact, how people are reported in terms of their specialties is how the companies categorize you, so the data may not be completely accurate. It’s one of the challenges.”
Industry groups and professional societies have raised concerns about the incompleteness of the Open Payments data and argued that inaccuracies could harm reputations and undermine trust between patients and their physicians.
Physicians have 45 days after the data submission period to review their Open Payments data and dispute errors before the information is released publicly. Errors can be contested after the deadline has passed, with corrections made in the next reporting cycle.
Still, of the 4.3 million payments made nationally in the last 5 months of 2013, only 1,145 payments (0.02%) worth just $6.25 million were contested, Dr. Blebea reported.
“So it’s either accurate or most physicians didn’t bother to contest inaccuracies,” he said, adding, “I certainly did [contest the data] because there was an inaccuracy in what was reported for me and that was corrected, but how many people will correct these in the future? I hope everybody does.”
Of the $6.49 billion paid to physicians and hospitals in 2014, physicians have disputed only $5.06 million in general payments and $13.16 million in research payments, according to 2014 data reported by the CMS .
Dr. Daniel G. Clair, chair of vascular surgery at the Cleveland Clinic, commented that contrary to what the analysis suggests, it isn’t easy to distinguish between research dollars and nonresearch dollars and between payments made to an institution versus those made to an individual.
“I work for a facility where I am a salaried professional and contracts for some of these things are negotiated between the institution and the company. I’m completely left out of it, but because I happen to be the individual who provides services, it looks like that money is coming to me,” he said.
To provide more transparency in payments, Dr. Blebea said he would recommend quantitative disclosure of industry payments at scientific meetings and in publications with reporting of a range of payments, such as less than $1,000, $1,000-$5,000, $5,001-$10,000, and more than $10,000, rather than specific amounts.
Dr. Blebea and Dr. Pipinos reported having no relevant financial disclosures. Dr. Clair reported serving on the data and safety monitoring board for Bard, as an advisory board member for Boston Scientific and Medtronic, and as a consultant for Endologix.
AT MIDWESTERN VASCULAR 2015
The pros and cons of novel anticoagulants
Novel anticoagulants will likely replace need for vitamin K antagonists
BY MADHUKAR S. PATEL, M.D., AND ELLIOT L. CHAIKOF, M.D.
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation, that Campbell & Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs), began.
Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice.
Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared to VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs.
NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low-molecular-weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities that may influence drug effect and clearance. Lastly, it should be mentioned that the pharmacologic benefits of NOACs apply not only from a patient perspective, but also from a health care systems standpoint, as their use may provide an opportunity to deliver more cost-effective care.
Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared to warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs to VKAs or placebos for the management of nonvalvular atrial fibrillation and venous thromboembolism, and as adjunctive therapy for patients with acute coronary syndrome.6
Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation, where NOACs yield significant reductions in stroke, intracranial hemorrhage, and all-cause mortality compared to warfarin, while displaying variable effects with regard to gastrointestinal bleeding.6,7 In patients with VTE, NOACs have been found to have efficacy similar to that of VKAs with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6
Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon which class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life threatening bleeding complications.
Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monoclonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently under phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9
Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable compared to VKAs and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusion
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism, where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the healthcare system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Dr. Patel is a research fellow and Dr. Chaikof is surgeon-in-chief, both at the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported no conflicts of interest.
References
1. J Am Vet Med Assoc. 1924;64:553-75 (See Br J Haematol 2008 Mar 18;141[6]:757-63).
2. J Biol Chem. 1941;138:21-33 (See Nutr Rev. 1974 Aug;32[8]:244-6).
3. Am Soc Hematol Educ Program. 2013;2013:464-70.
4. Eur Heart J. 2013 Jul;34(27):2094-2106.
5. Stroke. 2013 Jun;44(6):1676-81.
6. Nat Rev Cardiol. 2014 Dec;11(12):693-703.
7. Lancet. 2014 Mar 15;383(9921):955-62.
8. N Engl J Med. 2015;373(6):511-20.
9. N Engl J Med. 2014;371(22):2141-2.
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
BY THOMAS WAKEFIELD, M.D., ANDREA OBI, M.D., AND DAWN COLEMAN, M.D.
Recently, several new oral anticoagulants have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once- or twice-daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute venous thromboembolism (VTE) and atrial fibrillation (AF).
Dabigatran and edoxaban
As with warfarin, dabigatran and edoxaban require the use of a low-molecular-weight heparin (LMWH) or unfractionated heparin “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent that can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique among the NOACs, have been tested for extended therapy of acute DVT after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding compared to placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding compared to warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels and to reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects.
With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published a recommendation7 that lists these scenarios:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL/min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.
Currently, there exists no commercially available reversal agent for any of the NOACs and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials is lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however, drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
There are no national guidelines nor large scale studies to guide bridging NOACs for procedures. The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
Conclusion
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that for all the benefits, they each carry a risk of bleeding as they all target portions of the coagulation mechanism. We believe, that as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Dr. Wakefield is director of the Samuel and Jean Frankel Cardiovascular Center, Dr. Obi is a vascular surgery fellow, and Dr. Coleman is program director, section of vascular surgery, at the University of Michigan, Ann Arbor. They reported no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-52.
2. J Vasc Surg: Venous Lymphat Disord. 2013;1:418-26.
3. N Engl J Med. 2013;369:1406-15.
4. N Engl J Med. 2010;363:2499-2510.
5. N Engl J Med. 2013;368:699-708.
6. Arterioscler Thromb Vasc Biol. 2015;35:1056-65.
7. J Thromb Haemost. 2013;11:756-60.
Novel anticoagulants will likely replace need for vitamin K antagonists
BY MADHUKAR S. PATEL, M.D., AND ELLIOT L. CHAIKOF, M.D.
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation, that Campbell & Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs), began.
Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice.
Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared to VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs.
NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low-molecular-weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities that may influence drug effect and clearance. Lastly, it should be mentioned that the pharmacologic benefits of NOACs apply not only from a patient perspective, but also from a health care systems standpoint, as their use may provide an opportunity to deliver more cost-effective care.
Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared to warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs to VKAs or placebos for the management of nonvalvular atrial fibrillation and venous thromboembolism, and as adjunctive therapy for patients with acute coronary syndrome.6
Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation, where NOACs yield significant reductions in stroke, intracranial hemorrhage, and all-cause mortality compared to warfarin, while displaying variable effects with regard to gastrointestinal bleeding.6,7 In patients with VTE, NOACs have been found to have efficacy similar to that of VKAs with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6
Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon which class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life threatening bleeding complications.
Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monoclonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently under phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9
Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable compared to VKAs and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusion
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism, where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the healthcare system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Dr. Patel is a research fellow and Dr. Chaikof is surgeon-in-chief, both at the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported no conflicts of interest.
References
1. J Am Vet Med Assoc. 1924;64:553-75 (See Br J Haematol 2008 Mar 18;141[6]:757-63).
2. J Biol Chem. 1941;138:21-33 (See Nutr Rev. 1974 Aug;32[8]:244-6).
3. Am Soc Hematol Educ Program. 2013;2013:464-70.
4. Eur Heart J. 2013 Jul;34(27):2094-2106.
5. Stroke. 2013 Jun;44(6):1676-81.
6. Nat Rev Cardiol. 2014 Dec;11(12):693-703.
7. Lancet. 2014 Mar 15;383(9921):955-62.
8. N Engl J Med. 2015;373(6):511-20.
9. N Engl J Med. 2014;371(22):2141-2.
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
BY THOMAS WAKEFIELD, M.D., ANDREA OBI, M.D., AND DAWN COLEMAN, M.D.
Recently, several new oral anticoagulants have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once- or twice-daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute venous thromboembolism (VTE) and atrial fibrillation (AF).
Dabigatran and edoxaban
As with warfarin, dabigatran and edoxaban require the use of a low-molecular-weight heparin (LMWH) or unfractionated heparin “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent that can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique among the NOACs, have been tested for extended therapy of acute DVT after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding compared to placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding compared to warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels and to reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects.
With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published a recommendation7 that lists these scenarios:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL/min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.
Currently, there exists no commercially available reversal agent for any of the NOACs and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials is lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however, drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
There are no national guidelines nor large scale studies to guide bridging NOACs for procedures. The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
Conclusion
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that for all the benefits, they each carry a risk of bleeding as they all target portions of the coagulation mechanism. We believe, that as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Dr. Wakefield is director of the Samuel and Jean Frankel Cardiovascular Center, Dr. Obi is a vascular surgery fellow, and Dr. Coleman is program director, section of vascular surgery, at the University of Michigan, Ann Arbor. They reported no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-52.
2. J Vasc Surg: Venous Lymphat Disord. 2013;1:418-26.
3. N Engl J Med. 2013;369:1406-15.
4. N Engl J Med. 2010;363:2499-2510.
5. N Engl J Med. 2013;368:699-708.
6. Arterioscler Thromb Vasc Biol. 2015;35:1056-65.
7. J Thromb Haemost. 2013;11:756-60.
Novel anticoagulants will likely replace need for vitamin K antagonists
BY MADHUKAR S. PATEL, M.D., AND ELLIOT L. CHAIKOF, M.D.
The discovery of oral anticoagulants began in 1924, when Schofield linked the death of grazing cattle from internal hemorrhage to the consumption of spoiled sweet clover hay.1 It was not until 1941, however, while trying to understand this observation, that Campbell & Link were able to identify the dicoumarol anticoagulant, which formed as a result of the spoiling process.2 Ultimately, after noting that vitamin K led to reversal of the dicoumarol effect, synthesis of the first class of oral anticoagulants, known as vitamin K antagonists (VKAs), began.
Despite the numerous challenges associated with managing patients using this class of anticoagulants, VKAs have become the mainstay of oral anticoagulation therapy for the past 70 years. Over the past 5 years, however, new oral anticoagulants (NOACs) have emerged and are changing clinical practice.
Mechanistically, these medications are targeted therapies and work as either direct thrombin inhibitors (dabigatran etexilate) or direct factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban). Given their favorable pharmacologic design, NOACs have the potential to replace VKAs as they not only have an encouraging safety profile, but also are therapeutically equivalent or even superior to VKAs when used in certain patient populations.
Pharmacologic design
The targeted drug design of NOACs provides many pharmacologic advantages. Compared to VKAs, NOACs have a notably more predictable pharmacologic profile and relatively wide therapeutic window, which allows for fixed dosing, a rapid onset and offset, and fewer drug interactions.3 These characteristics eliminate the need for the routine dose monitoring and serial dose adjustments frequently associated with VKAs.
NOACs less commonly require bridging therapy with parenteral unfractionated heparin or low-molecular-weight heparins (LMWH) while awaiting therapeutic drug levels, as these levels are reached sooner and more predictably than with VKAs.4 As with any medication, however, appropriate consideration should to be given to specific patient populations such as those who are older or have significant comorbidities that may influence drug effect and clearance. Lastly, it should be mentioned that the pharmacologic benefits of NOACs apply not only from a patient perspective, but also from a health care systems standpoint, as their use may provide an opportunity to deliver more cost-effective care.
Specifically, economic models using available clinical trial data for stroke prevention in nonvalvular atrial fibrillation have shown that NOACs (apixaban, dabigatran, and rivaroxaban) are cost-effective alternatives when compared to warfarin.5 Although the results from such economic analyses are limited by the modeling assumptions they rely upon, these findings suggest that at least initially, cost should not be used as a prohibitive reason for adopting these new therapeutics.
Patient selection
The decision to institute oral anticoagulation therapy depends on each patient’s individualized bleeding risk to benefit of ischemia prevention ratio. A major determinant of this ratio is the clinical indication for which anticoagulation is begun. Numerous phase III clinical trials have been conducted comparing the use of NOACs to VKAs or placebos for the management of nonvalvular atrial fibrillation and venous thromboembolism, and as adjunctive therapy for patients with acute coronary syndrome.6
Meta-analyses of randomized trials have shown the most significant benefit to be in patients with nonvalvular atrial fibrillation, where NOACs yield significant reductions in stroke, intracranial hemorrhage, and all-cause mortality compared to warfarin, while displaying variable effects with regard to gastrointestinal bleeding.6,7 In patients with VTE, NOACs have been found to have efficacy similar to that of VKAs with regard to the prevention of VTE or VTE-related death, and have been noted to have a better safety profile.6
Lastly, when studied as an adjunctive agent to dual antiplatelet therapy in patients with acute coronary syndrome, NOACs have been associated with an increased bleeding risk without a significant decrease in thrombosis risk.6 Taken together, these data suggest that the primary indication for instituting NOAC therapy should be considered strongly when deciding upon which class of anticoagulant to use.
Overcoming challenges
Since the introduction of NOACs, there has been concern over the lack of specific antidotes to therapy, especially when administered in patients with impaired clearance, a high likelihood of need for an urgent or emergent procedure, or those presenting with life threatening bleeding complications.
Most recently, however, interim analysis from clinical trial data has shown complete reversal of the direct thrombin inhibitor dabigatran with the humanized monoclonal antibody idarucizumab within minutes of administration in greater than 88% of patients studied.8 Similarly, agents such as a PER977 are currently under phase II clinical trials as they have been shown to form noncovalent hydrogen bonds and charge-charge interactions with oral factor Xa inhibitors as well as oral thrombin inhibitors leading to their reversal.9
Given these promising findings, it likely will not be long until reversal agents for NOACs become clinically available. Until that time, it is encouraging that the bleeding profile of these drugs has been found to be favorable compared to VKAs and their short half-life allows for a relatively expeditious natural reversal of their anticoagulant effect as the drug is eliminated.
Conclusion
Unlike the serendipitous path leading to the discovery of the first class of oral anticoagulants (VKAs), NOACs have been specifically designed to provide targeted anticoagulation and to address the shortcomings of VKAs. To this end, NOACs are becoming increasingly important in the management of patients with specific clinical conditions such as nonvalvular atrial fibrillation and venous thromboembolism, where they have been shown to provide a larger net clinical benefit relative to the available alternatives. Furthermore, with economic analyses providing evidence that NOACs are cost-effective for the healthcare system and clinical trial results suggesting progress in the development of antidotes for reversal, it is likely that with growing experience, these agents will replace VKAs as the mainstay for prophylactic and therapeutic oral anticoagulation in targeted patient populations.
Dr. Patel is a research fellow and Dr. Chaikof is surgeon-in-chief, both at the department of surgery, Beth Israel Deaconess Medical Center, Boston. They reported no conflicts of interest.
References
1. J Am Vet Med Assoc. 1924;64:553-75 (See Br J Haematol 2008 Mar 18;141[6]:757-63).
2. J Biol Chem. 1941;138:21-33 (See Nutr Rev. 1974 Aug;32[8]:244-6).
3. Am Soc Hematol Educ Program. 2013;2013:464-70.
4. Eur Heart J. 2013 Jul;34(27):2094-2106.
5. Stroke. 2013 Jun;44(6):1676-81.
6. Nat Rev Cardiol. 2014 Dec;11(12):693-703.
7. Lancet. 2014 Mar 15;383(9921):955-62.
8. N Engl J Med. 2015;373(6):511-20.
9. N Engl J Med. 2014;371(22):2141-2.
What the doctor didn’t order: unintended consequences and pitfalls of NOACs
BY THOMAS WAKEFIELD, M.D., ANDREA OBI, M.D., AND DAWN COLEMAN, M.D.
Recently, several new oral anticoagulants have gained FDA approval to replace warfarin, capturing the attention of popular media. These include dabigatran, rivaroxaban, apixaban, and edoxaban. Dabigatran targets activated factor II (factor IIa), while rivaroxaban, apixaban, and edoxaban target activated factor X (factor Xa). Easy to take with a once- or twice-daily pill, with no cumbersome monitoring, they represent a seemingly ideal treatment for the chronically anticoagulated patient. All agents are currently FDA approved in the United States for treatment of acute venous thromboembolism (VTE) and atrial fibrillation (AF).
Dabigatran and edoxaban
As with warfarin, dabigatran and edoxaban require the use of a low-molecular-weight heparin (LMWH) or unfractionated heparin “bridge” when therapy is beginning, while rivaroxaban and apixaban are instituted as monotherapy without such a bridge. Dabigatran etexilate (PradaxaR, Boehringer Ingelheim) has the longest half-life of all of the NOACs at 12-17 hours, and this half-life is prolonged with increasing age and decreasing renal function.1 It is the only new agent that can be at least partially reversed with dialysis.2 Edoxaban (SavaysaR, Daiichi Sankyo) carries a boxed warning stating that this agent is less effective in AF patients with a creatinine clearance greater than 95 mL/min, and that kidney function should be assessed prior to starting treatment: Such patients have a greater risk of stroke compared with similar patients treated with warfarin. Edoxaban is the only agent specifically tested at a lower dose in patients at significantly increased risk of bleeding complications (low body weight and/or decreased creatinine clearance).3
Rivaroxaban and apixaban
Rivaroxaban (XareltoR, Bayer and Janssen), and apixaban (EliquisR, Bristol Myers-Squibb), unique among the NOACs, have been tested for extended therapy of acute DVT after treatment of 6-12 months. They were found to result in a significant decrease in recurrent VTE without an increase in major bleeding compared to placebo.4,5 Rivaroxaban has once-daily dosing and apixaban has twice-daily dosing; both are immediate monotherapy, making them quite convenient for patients. Apixaban is the only agent among the NOACs to have a slight decrease in gastrointestinal bleeding compared to warfarin.6
Consequences and pitfalls with NOACs
Problems with these new drugs, which may diminish our current level of enthusiasm for these agents to totally replace warfarin, include the inability to reliably follow their levels and to reverse their anticoagulant effects, the lack of data available on bridging when other procedures need to be performed, their short half-lives, and the lack of data on their anti-inflammatory effects.
With regard to monitoring of anticoagulation, the International Society of Thrombosis and Hemostasis (ISTH) has published a recommendation7 that lists these scenarios:
• When a patient is bleeding.
• Before surgery or an invasive procedure when the patient has taken the drug in the previous 24 hours, or longer if creatinine clearance (CrCl) is less than 50 mL/min.
• Identification of subtherapeutic or supratherapeutic levels in patients taking other drugs that are known to affect pharmacokinetics.
• Identification of subtherapeutic or supratherapeutic levels in patients at body weight extremes.
• Patients with deteriorating renal function.
• During perioperative management.
• During reversal of anticoagulation.
• When there is suspicion of overdose.
• Assessment of compliance in patients suffering thrombotic events while on treatment.
Currently, there exists no commercially available reversal agent for any of the NOACs and existing reversal agents for traditional anticoagulants are of limited, if any, use. Drugs under development include agents for the factor Xa inhibitors and for the thrombin inhibitor. Until the time that specific reversal agents exist, supportive care is the mainstay of therapy. In cases of trauma or severe or life-threatening bleeding, administration of concentrated clotting factors (prothrombin complex concentrate) or dialysis (dabigatran only) may be utilized. However, data from large clinical trials is lacking. A recent study of 90 patients receiving an antibody directed against dabigatran has revealed that the anticoagulant effects of dabigatran were reversed safely within minutes of administration; however, drug levels were not consistently suppressed at 24 hours in 20% of the cohort.8
There are no national guidelines nor large scale studies to guide bridging NOACs for procedures. The relatively short half-life for these agents makes it likely that traditional bridging as is practiced for warfarin is not necessary.9 However, this represents a double edged sword; withholding anticoagulation for two doses (such as if a patient becomes ill or a clinician is overly cautious around the time of a procedure) may leave the patient unprotected.
The final question with the new agents is their anti-inflammatory effects. We know that heparin and LMWH have significant pleiotropic effects that are not necessarily related to their anticoagulant effects. These effects are important to decrease the inflammatory nature of the thrombus and its effect on the vein wall. We do not know if the new oral agents have similar effects, as this has never fully been tested. In view of the fact that two of the agents are being used as monotherapy agents without any heparin/LMWH bridge, the anti-inflammatory properties of these new agents should be defined to make sure that such a bridge is not necessary.
Conclusion
So, in summary, although these agents have much to offer, there are many questions that remain to be addressed and answered before they totally replace traditional approaches to anticoagulation, in the realm of VTE. It must not be overlooked that for all the benefits, they each carry a risk of bleeding as they all target portions of the coagulation mechanism. We believe, that as with any “gift horse,” physicians should perhaps examine the data more closely and proceed with caution.
Dr. Wakefield is director of the Samuel and Jean Frankel Cardiovascular Center, Dr. Obi is a vascular surgery fellow, and Dr. Coleman is program director, section of vascular surgery, at the University of Michigan, Ann Arbor. They reported no conflicts of interest.
References
1. N Engl J Med. 2009;361:2342-52.
2. J Vasc Surg: Venous Lymphat Disord. 2013;1:418-26.
3. N Engl J Med. 2013;369:1406-15.
4. N Engl J Med. 2010;363:2499-2510.
5. N Engl J Med. 2013;368:699-708.
6. Arterioscler Thromb Vasc Biol. 2015;35:1056-65.
7. J Thromb Haemost. 2013;11:756-60.
Scoring tool points to postop ventilator dependence
CHICAGO – A new preoperative risk scoring tool may help identify patients at high risk for requiring mechanical ventilation for more than 48 hours in the 30 days after surgery, a study suggests.
The risk score is based on seven measures: whether patients have had a small bowel procedure, have had an esophageal procedure, are current smokers, have severe chronic obstructive pulmonary disease, have hypoalbuminemia, are older than age 60 years, or have signs of systemic inflammatory response syndrome or sepsis.
The score was validated via the American College of Surgeons (ACS)/National Surgical Quality Improvement Program (NSQIP) database to identify patients who underwent nonemergent general or vascular surgery at Thomas Jefferson University Hospital between 2006 and 2013, Dr. Adam P. Johnson, study coauthor, reported at the ACS/NSQIP National Conference.
The risk score assigned 1 point each for a small bowel procedure, current smoking, severe chronic obstructive pulmonary disease, and hypoalbuminemia (less than 3.5 mg/dL); 2 points each for age over 60 years and signs of systemic inflammatory response syndrome or sepsis; and 3 points for esophageal procedures. Total risk scores ranged from 0 to 7 points for the population.
The median score was 2 for patients who did not need a ventilator after surgery and 3 for those who did, Dr. Johnson said.
Notably, patients with a risk score of more than 3 comprised the 20%-30% of patients who experienced 60%-70% of adverse events. A cutoff value of 3 identified the top 20% of patients at highest risk for ventilator dependence, with a ventilator dependence rate of 5.4% (P less than .01).
The risk factors and scoring system are specific to Thomas Jefferson University Hospital, where many patients with advanced gastrointestinal malignancies are treated. However, other institutions should be able to use the methodology and framework to identify ventilator risk factors in their own patients, Dr. Johnson suggested.
Future steps include evaluating how the risk tool performs when compared with risk scores derived from national datasets, automating the best performing risk score, and using the score in the preadmission testing of every patient undergoing elective general surgery or vascular operations. Once identified, high-risk patients would then be entered into an aggressive pre-, intra-, and postoperative pulmonary optimization pathway.
“The pathway might be resource intensive for all patients, but we might be able to hone in and use it more effectively for patients at greatest risk,” Dr. Johnson said in a statement.
Although ventilator dependence occurs in only about 1-3% of patients, the consequences are nonetheless significant, increasing mortality and health care costs, said Dr. Scott W. Cowan, senior study author and Jefferson’s NSQIP Surgeon Champion.
CHICAGO – A new preoperative risk scoring tool may help identify patients at high risk for requiring mechanical ventilation for more than 48 hours in the 30 days after surgery, a study suggests.
The risk score is based on seven measures: whether patients have had a small bowel procedure, have had an esophageal procedure, are current smokers, have severe chronic obstructive pulmonary disease, have hypoalbuminemia, are older than age 60 years, or have signs of systemic inflammatory response syndrome or sepsis.
The score was validated via the American College of Surgeons (ACS)/National Surgical Quality Improvement Program (NSQIP) database to identify patients who underwent nonemergent general or vascular surgery at Thomas Jefferson University Hospital between 2006 and 2013, Dr. Adam P. Johnson, study coauthor, reported at the ACS/NSQIP National Conference.
The risk score assigned 1 point each for a small bowel procedure, current smoking, severe chronic obstructive pulmonary disease, and hypoalbuminemia (less than 3.5 mg/dL); 2 points each for age over 60 years and signs of systemic inflammatory response syndrome or sepsis; and 3 points for esophageal procedures. Total risk scores ranged from 0 to 7 points for the population.
The median score was 2 for patients who did not need a ventilator after surgery and 3 for those who did, Dr. Johnson said.
Notably, patients with a risk score of more than 3 comprised the 20%-30% of patients who experienced 60%-70% of adverse events. A cutoff value of 3 identified the top 20% of patients at highest risk for ventilator dependence, with a ventilator dependence rate of 5.4% (P less than .01).
The risk factors and scoring system are specific to Thomas Jefferson University Hospital, where many patients with advanced gastrointestinal malignancies are treated. However, other institutions should be able to use the methodology and framework to identify ventilator risk factors in their own patients, Dr. Johnson suggested.
Future steps include evaluating how the risk tool performs when compared with risk scores derived from national datasets, automating the best performing risk score, and using the score in the preadmission testing of every patient undergoing elective general surgery or vascular operations. Once identified, high-risk patients would then be entered into an aggressive pre-, intra-, and postoperative pulmonary optimization pathway.
“The pathway might be resource intensive for all patients, but we might be able to hone in and use it more effectively for patients at greatest risk,” Dr. Johnson said in a statement.
Although ventilator dependence occurs in only about 1-3% of patients, the consequences are nonetheless significant, increasing mortality and health care costs, said Dr. Scott W. Cowan, senior study author and Jefferson’s NSQIP Surgeon Champion.
CHICAGO – A new preoperative risk scoring tool may help identify patients at high risk for requiring mechanical ventilation for more than 48 hours in the 30 days after surgery, a study suggests.
The risk score is based on seven measures: whether patients have had a small bowel procedure, have had an esophageal procedure, are current smokers, have severe chronic obstructive pulmonary disease, have hypoalbuminemia, are older than age 60 years, or have signs of systemic inflammatory response syndrome or sepsis.
The score was validated via the American College of Surgeons (ACS)/National Surgical Quality Improvement Program (NSQIP) database to identify patients who underwent nonemergent general or vascular surgery at Thomas Jefferson University Hospital between 2006 and 2013, Dr. Adam P. Johnson, study coauthor, reported at the ACS/NSQIP National Conference.
The risk score assigned 1 point each for a small bowel procedure, current smoking, severe chronic obstructive pulmonary disease, and hypoalbuminemia (less than 3.5 mg/dL); 2 points each for age over 60 years and signs of systemic inflammatory response syndrome or sepsis; and 3 points for esophageal procedures. Total risk scores ranged from 0 to 7 points for the population.
The median score was 2 for patients who did not need a ventilator after surgery and 3 for those who did, Dr. Johnson said.
Notably, patients with a risk score of more than 3 comprised the 20%-30% of patients who experienced 60%-70% of adverse events. A cutoff value of 3 identified the top 20% of patients at highest risk for ventilator dependence, with a ventilator dependence rate of 5.4% (P less than .01).
The risk factors and scoring system are specific to Thomas Jefferson University Hospital, where many patients with advanced gastrointestinal malignancies are treated. However, other institutions should be able to use the methodology and framework to identify ventilator risk factors in their own patients, Dr. Johnson suggested.
Future steps include evaluating how the risk tool performs when compared with risk scores derived from national datasets, automating the best performing risk score, and using the score in the preadmission testing of every patient undergoing elective general surgery or vascular operations. Once identified, high-risk patients would then be entered into an aggressive pre-, intra-, and postoperative pulmonary optimization pathway.
“The pathway might be resource intensive for all patients, but we might be able to hone in and use it more effectively for patients at greatest risk,” Dr. Johnson said in a statement.
Although ventilator dependence occurs in only about 1-3% of patients, the consequences are nonetheless significant, increasing mortality and health care costs, said Dr. Scott W. Cowan, senior study author and Jefferson’s NSQIP Surgeon Champion.
AT THE ACS NSQIP NATIONAL CONFERENCE
Key clinical point: A preoperative risk score can help identify patients at highest risk for postoperative ventilator dependence.
Major finding: A risk score greater than 3 identified the top 20%-30% of patients experiencing 60%-70% of postop ventilator dependence events.
Data source: Retrospective analysis of 7,473 elective general and vascular surgical patients.
Disclosures: The authors reported having no financial disclosures.
Percutaneous thrombectomy reduces risk of postthrombotic syndrome
Adding a mechanical suction technique to local thrombolysis to break up and remove blood clots reduced postthrombotic syndrome (PTS) after deep vein thrombosis (DVT) without causing increased complications, according to a small retrospective study.
Dr. Chun-Yang Huang of the National Yang Ming University (Taipei, Taiwan) and colleagues examined patients diagnosed with acute proximal lower limb DVT. Patients received either thrombolysis alone via a catheter-directed thrombolysis (CDT), or percutaneous mechanical thrombectomy (PMT) by a combination of pharmacologic thrombolysis and suction; both techniques were accompanied by systemic anticoagulation. Though both treatment groups fared well during treatment and for the 12-month follow-up period, the PMT group had a significantly lower incidence of PTS 1 year after treatment (Ann. Vascular Surg. 2015. doi: 10.1016/j.avsg.2015.01.014).
For those with DVT, parenteral anticoagulation prevents propagation of the clot and minimizes risk of pulmonary embolism (PE); however, anticoagulation does not accelerate dissolution of the existing clot. According to study authors, 30%-40% of those with proximal leg DVTs will go on to develop PTS, with the prolonged distal venous stasis from an undisturbed clot causing loss of valvular competence and resultant chronic venous insufficiency. PTS can involve leg swelling, discomfort, skin changes, and ulceration, with significant impact on quality of life and health care costs.
Techniques such as CDT and PMT can increase the rate of clot dissolution, thus restoring patency sooner and minimizing risk for PTS. However, these methods also can carry increased risk of bleeding and infection, considerations that must be balanced against potential benefit.
Investigators reviewed records for 39 patients who were diagnosed with ultrasound- or CT-confirmed acute proximal lower limb DVT and received either CDT or PMT during the period from November 2010 to November 2013. Patients were not randomized to treatment arms but were assigned using clinical judgment and patient preference. During the 12-month follow-up, three participants died of malignancy and two were lost to follow-up. Analysis was completed for the remaining 34 patients.
Overall, patient characteristics did not differ significantly between groups, with mean ages of 62.75 for the PMT group (n = 16) and 64.17 for the CDT group (n = 18). In all, 13/34 participants were female. Patients in both treatment groups fared well, with no 30-day mortality, and no episodes of major bleeding, PE, or renal failure. Ten patients in the PMT group and six in the CDT group required stenting of the common iliac vein to maintain patency, a nonsignificant difference. Just one participant in the CDE group experienced a minor bleeding event.
Turning to outcomes, study authors assessed postprocedure patency, finding improved patency for both procedures (P less than .001 for both, compared with preoperation patency scores), with no significant difference between the two groups post procedure. Thrombus scores were also significantly better for both treatment arms post procedure (P less than .001). Clot burden tended to improve more rapidly over the 12-month follow-up period for the PMT group, though the difference between groups was just short of statistically significant.
At 12 months, though the amount of venous reflux did not differ significantly between groups, those who had received PMT had significantly fewer signs and symptoms of PTS. This assessment used the Villalta scale, a standardized assessment and scoring system for PTS, where higher numbers indicate worse PTS. The PMT group’s Villalta score was 2.06 +/–2.95, compared with 5.06 +/–4.07 for the CDT group (P = .030).
Study limitations included the small study size, retrospective study design, and lack of randomization. Acknowledging these limitations, Dr. Huang and coauthors called for larger, multicenter, randomized controlled studies of PMT. The personal and economic costs of PTS, they argue, warrant exploring whether PMT may help minimize total thrombolysis dose, reduce hospital stays, and decrease costs while minimizing the risks of chronic venous insufficiency post DVT.
Dr. Huang and coauthors reported no conflicts of interest.
Adding a mechanical suction technique to local thrombolysis to break up and remove blood clots reduced postthrombotic syndrome (PTS) after deep vein thrombosis (DVT) without causing increased complications, according to a small retrospective study.
Dr. Chun-Yang Huang of the National Yang Ming University (Taipei, Taiwan) and colleagues examined patients diagnosed with acute proximal lower limb DVT. Patients received either thrombolysis alone via a catheter-directed thrombolysis (CDT), or percutaneous mechanical thrombectomy (PMT) by a combination of pharmacologic thrombolysis and suction; both techniques were accompanied by systemic anticoagulation. Though both treatment groups fared well during treatment and for the 12-month follow-up period, the PMT group had a significantly lower incidence of PTS 1 year after treatment (Ann. Vascular Surg. 2015. doi: 10.1016/j.avsg.2015.01.014).
For those with DVT, parenteral anticoagulation prevents propagation of the clot and minimizes risk of pulmonary embolism (PE); however, anticoagulation does not accelerate dissolution of the existing clot. According to study authors, 30%-40% of those with proximal leg DVTs will go on to develop PTS, with the prolonged distal venous stasis from an undisturbed clot causing loss of valvular competence and resultant chronic venous insufficiency. PTS can involve leg swelling, discomfort, skin changes, and ulceration, with significant impact on quality of life and health care costs.
Techniques such as CDT and PMT can increase the rate of clot dissolution, thus restoring patency sooner and minimizing risk for PTS. However, these methods also can carry increased risk of bleeding and infection, considerations that must be balanced against potential benefit.
Investigators reviewed records for 39 patients who were diagnosed with ultrasound- or CT-confirmed acute proximal lower limb DVT and received either CDT or PMT during the period from November 2010 to November 2013. Patients were not randomized to treatment arms but were assigned using clinical judgment and patient preference. During the 12-month follow-up, three participants died of malignancy and two were lost to follow-up. Analysis was completed for the remaining 34 patients.
Overall, patient characteristics did not differ significantly between groups, with mean ages of 62.75 for the PMT group (n = 16) and 64.17 for the CDT group (n = 18). In all, 13/34 participants were female. Patients in both treatment groups fared well, with no 30-day mortality, and no episodes of major bleeding, PE, or renal failure. Ten patients in the PMT group and six in the CDT group required stenting of the common iliac vein to maintain patency, a nonsignificant difference. Just one participant in the CDE group experienced a minor bleeding event.
Turning to outcomes, study authors assessed postprocedure patency, finding improved patency for both procedures (P less than .001 for both, compared with preoperation patency scores), with no significant difference between the two groups post procedure. Thrombus scores were also significantly better for both treatment arms post procedure (P less than .001). Clot burden tended to improve more rapidly over the 12-month follow-up period for the PMT group, though the difference between groups was just short of statistically significant.
At 12 months, though the amount of venous reflux did not differ significantly between groups, those who had received PMT had significantly fewer signs and symptoms of PTS. This assessment used the Villalta scale, a standardized assessment and scoring system for PTS, where higher numbers indicate worse PTS. The PMT group’s Villalta score was 2.06 +/–2.95, compared with 5.06 +/–4.07 for the CDT group (P = .030).
Study limitations included the small study size, retrospective study design, and lack of randomization. Acknowledging these limitations, Dr. Huang and coauthors called for larger, multicenter, randomized controlled studies of PMT. The personal and economic costs of PTS, they argue, warrant exploring whether PMT may help minimize total thrombolysis dose, reduce hospital stays, and decrease costs while minimizing the risks of chronic venous insufficiency post DVT.
Dr. Huang and coauthors reported no conflicts of interest.
Adding a mechanical suction technique to local thrombolysis to break up and remove blood clots reduced postthrombotic syndrome (PTS) after deep vein thrombosis (DVT) without causing increased complications, according to a small retrospective study.
Dr. Chun-Yang Huang of the National Yang Ming University (Taipei, Taiwan) and colleagues examined patients diagnosed with acute proximal lower limb DVT. Patients received either thrombolysis alone via a catheter-directed thrombolysis (CDT), or percutaneous mechanical thrombectomy (PMT) by a combination of pharmacologic thrombolysis and suction; both techniques were accompanied by systemic anticoagulation. Though both treatment groups fared well during treatment and for the 12-month follow-up period, the PMT group had a significantly lower incidence of PTS 1 year after treatment (Ann. Vascular Surg. 2015. doi: 10.1016/j.avsg.2015.01.014).
For those with DVT, parenteral anticoagulation prevents propagation of the clot and minimizes risk of pulmonary embolism (PE); however, anticoagulation does not accelerate dissolution of the existing clot. According to study authors, 30%-40% of those with proximal leg DVTs will go on to develop PTS, with the prolonged distal venous stasis from an undisturbed clot causing loss of valvular competence and resultant chronic venous insufficiency. PTS can involve leg swelling, discomfort, skin changes, and ulceration, with significant impact on quality of life and health care costs.
Techniques such as CDT and PMT can increase the rate of clot dissolution, thus restoring patency sooner and minimizing risk for PTS. However, these methods also can carry increased risk of bleeding and infection, considerations that must be balanced against potential benefit.
Investigators reviewed records for 39 patients who were diagnosed with ultrasound- or CT-confirmed acute proximal lower limb DVT and received either CDT or PMT during the period from November 2010 to November 2013. Patients were not randomized to treatment arms but were assigned using clinical judgment and patient preference. During the 12-month follow-up, three participants died of malignancy and two were lost to follow-up. Analysis was completed for the remaining 34 patients.
Overall, patient characteristics did not differ significantly between groups, with mean ages of 62.75 for the PMT group (n = 16) and 64.17 for the CDT group (n = 18). In all, 13/34 participants were female. Patients in both treatment groups fared well, with no 30-day mortality, and no episodes of major bleeding, PE, or renal failure. Ten patients in the PMT group and six in the CDT group required stenting of the common iliac vein to maintain patency, a nonsignificant difference. Just one participant in the CDE group experienced a minor bleeding event.
Turning to outcomes, study authors assessed postprocedure patency, finding improved patency for both procedures (P less than .001 for both, compared with preoperation patency scores), with no significant difference between the two groups post procedure. Thrombus scores were also significantly better for both treatment arms post procedure (P less than .001). Clot burden tended to improve more rapidly over the 12-month follow-up period for the PMT group, though the difference between groups was just short of statistically significant.
At 12 months, though the amount of venous reflux did not differ significantly between groups, those who had received PMT had significantly fewer signs and symptoms of PTS. This assessment used the Villalta scale, a standardized assessment and scoring system for PTS, where higher numbers indicate worse PTS. The PMT group’s Villalta score was 2.06 +/–2.95, compared with 5.06 +/–4.07 for the CDT group (P = .030).
Study limitations included the small study size, retrospective study design, and lack of randomization. Acknowledging these limitations, Dr. Huang and coauthors called for larger, multicenter, randomized controlled studies of PMT. The personal and economic costs of PTS, they argue, warrant exploring whether PMT may help minimize total thrombolysis dose, reduce hospital stays, and decrease costs while minimizing the risks of chronic venous insufficiency post DVT.
Dr. Huang and coauthors reported no conflicts of interest.
Key clinical point: Both percutaneous PMT and catheter-directed thrombolysis (CDT) were safe and effective, but PMT reduced risk of postthrombotic syndrome.
Major finding: In a small retrospective analysis of patients with deep vein thrombosis (DVT), both PMT and CDT were safe and effective when used in combination with systemic anticoagulation; however, postthrombotic syndrome (PTS) scoring was significantly better for those receiving PMT (Villalta score 2.1 +/- 3.0 vs. 5.1 +/- 4.1, P = .030).
Data source: Retrospective study of 39 patients who were diagnosed with acute proximal DVT of the lower limb between November 2010 and November 2013 at a Taiwanese hospital.
Disclosures: The authors reported that they had no conflicts of interest; funding source was not provided.
Endovascular stents effective for iliofemoral obstructions in patients with PTS
Endovascular stenting is a safe and effective way to treat iliofemoral obstructions in patients with postthrombotic syndrome, according to Dr. M. Yin of Shanghai (China) JiaoTong University, and associates.
The stenting process was achieved without major complications in 95% of cases. Cumulative primary, assisted primary, and secondary patency rates after 3 years were 69%, 79%, and 92%, respectively. Patients with severe postthrombotic syndrome (PTS) saw a significant drop in their Villalta score, compared with patients treated with elastic compression stockings (ECS) therapy, though scores were similar in patients with moderate PTS in both groups. The 24-month recurrence-free ulcer healing rate was significantly higher in the stenting group (87% vs. 71%).
“ECS therapy shows equal clinical effects with stent placement in patients with moderate PTS,” but the stented patients did not have to wear stockings after the procedure, the researchers wrote.
Find the full study in the European Journal of Vascular & Endovascular Surgery (doi: 10.1016/j.ejvs.2015.03.029).
Endovascular stenting is a safe and effective way to treat iliofemoral obstructions in patients with postthrombotic syndrome, according to Dr. M. Yin of Shanghai (China) JiaoTong University, and associates.
The stenting process was achieved without major complications in 95% of cases. Cumulative primary, assisted primary, and secondary patency rates after 3 years were 69%, 79%, and 92%, respectively. Patients with severe postthrombotic syndrome (PTS) saw a significant drop in their Villalta score, compared with patients treated with elastic compression stockings (ECS) therapy, though scores were similar in patients with moderate PTS in both groups. The 24-month recurrence-free ulcer healing rate was significantly higher in the stenting group (87% vs. 71%).
“ECS therapy shows equal clinical effects with stent placement in patients with moderate PTS,” but the stented patients did not have to wear stockings after the procedure, the researchers wrote.
Find the full study in the European Journal of Vascular & Endovascular Surgery (doi: 10.1016/j.ejvs.2015.03.029).
Endovascular stenting is a safe and effective way to treat iliofemoral obstructions in patients with postthrombotic syndrome, according to Dr. M. Yin of Shanghai (China) JiaoTong University, and associates.
The stenting process was achieved without major complications in 95% of cases. Cumulative primary, assisted primary, and secondary patency rates after 3 years were 69%, 79%, and 92%, respectively. Patients with severe postthrombotic syndrome (PTS) saw a significant drop in their Villalta score, compared with patients treated with elastic compression stockings (ECS) therapy, though scores were similar in patients with moderate PTS in both groups. The 24-month recurrence-free ulcer healing rate was significantly higher in the stenting group (87% vs. 71%).
“ECS therapy shows equal clinical effects with stent placement in patients with moderate PTS,” but the stented patients did not have to wear stockings after the procedure, the researchers wrote.
Find the full study in the European Journal of Vascular & Endovascular Surgery (doi: 10.1016/j.ejvs.2015.03.029).
SVS: AAA surveillance comes at an emotional cost
CHICAGO – For some patients, surveillance of low-risk abdominal aortic aneurysms is so stressful that early repair might be a better option.
Until now, though, it’s been hard to know who those patients are. There hasn’t been a way to quantify the impact of abdominal aortic aneurysm (AAA) surveillance on quality of life.
Dr. Bjoern Suckow, a vascular surgeon at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and his colleagues at the University of Massachusetts and elsewhere are working to fix that problem. “I do believe that there is a certain subset of patients who we know are” at low risk for rupture “who are so consumed by fear and anxiety during surveillance that the impact on quality of life might make us want to repair them slightly sooner. I hope this will help us weed out who that subgroup might be,” Dr. Suckow said at a meeting hosted by the Society for Vascular Surgery.
With the help of patient and physician focus groups and interviews, the team developed AAA-specific quality of life (QOL) surveys and administered them to 351 patients under surveillance for aneurysms below about 5.5 cm, and 657 who had undergone mostly endovascular AAA repair at six United States institutions.
The surveys included nine questions to assess concerns about rupture, surgery, costs, and death. The responses were averaged to give an emotional impact score (EIS) ranging from 0 to 100, with higher scores indicating worse emotional QOL. The survey also included 10 questions to assess changes in heavy lifting, strenuous activity, travel habits, and other behaviors. Those results were averaged to give a behavioral change score (BCS) that also ranged from 0 to 100, with higher scores indicating greater negative impact.
A significant portion of the surveillance patients thought it was “very likely” their aneurysm would rupture within a year; their EIS was 45 and BCS 30; patients who thought rupture was unlikely had an EIS of 12 and BCS of 13 (P less than .001). Overall, patients under surveillance had worse emotional impact sores than did those who had undergone repair.
“We routinely counsel patients with small aneurysms that the rupture risk is low” – less than 5% – “and outweighed by the higher risk of repair. We were surprised that even though we feel we do a great job counseling and educating our patients, some of them do not understand or retain what we mean.” Eventually, surveys could be used in the clinic to identify patients with “less understanding, so [we can] spend more time with them,” Dr. Suckow said.
In general, “the range of impact on QOL by AAA surveillance is broad. For most patients, the impact is minimal, but for some, especially those with a greater perceived rupture risk, it is severe. Overall, surveillance has a persistent negative impact on QOL, particularly emotional QOL. This impact appears to diminish following either open or endovascular repair,” he said.
The respondents were about 76 years old, on average. Most were white men, and about half were high school graduates.
Dr. Suckow has no relevant financial conflicts. The work was funded by the National Institutes of Health and career development awards from the Society for Vascular Surgery and the American College of Surgeons.
The diagnosis of a small aortic aneurysm, whether by screening or as an incidental finding, causes anxiety in our patients. The risk of rupture of small AAA has been demonstrated to be low – less than 1% per year below 5.0 cm in males (Health Technol. Assess. 2013;41:1-108) . Therefore, appropriate counseling and surveillance intervals should optimize the management of AAA patients. This study highlights the adverse effects of a diagnosis of small AAA on a proportion of our patients, despite appropriate explanation. Frequently patients know someone who died of AAA rupture and many do not understand the risk when it is explained in routine consultations. Perhaps we should all ensure that a member of our team contacts patients with small AAA post review and perform a short Quality of Life questionnaire by phone so that we can identify those who are suffering a negative impact on their QOL. We could then intensify our counseling and reassurance for this cohort of patients. This study should make us all reflect on whether our surveillance programs need to be modified, to ensure that our patients are not adversely affected by a diagnosis of small AAA.
Dr. Robert Fitridge is professor of vascular surgery, University of Adelaide, Australia, and associate medical editor of Vascular Specialist.
The diagnosis of a small aortic aneurysm, whether by screening or as an incidental finding, causes anxiety in our patients. The risk of rupture of small AAA has been demonstrated to be low – less than 1% per year below 5.0 cm in males (Health Technol. Assess. 2013;41:1-108) . Therefore, appropriate counseling and surveillance intervals should optimize the management of AAA patients. This study highlights the adverse effects of a diagnosis of small AAA on a proportion of our patients, despite appropriate explanation. Frequently patients know someone who died of AAA rupture and many do not understand the risk when it is explained in routine consultations. Perhaps we should all ensure that a member of our team contacts patients with small AAA post review and perform a short Quality of Life questionnaire by phone so that we can identify those who are suffering a negative impact on their QOL. We could then intensify our counseling and reassurance for this cohort of patients. This study should make us all reflect on whether our surveillance programs need to be modified, to ensure that our patients are not adversely affected by a diagnosis of small AAA.
Dr. Robert Fitridge is professor of vascular surgery, University of Adelaide, Australia, and associate medical editor of Vascular Specialist.
The diagnosis of a small aortic aneurysm, whether by screening or as an incidental finding, causes anxiety in our patients. The risk of rupture of small AAA has been demonstrated to be low – less than 1% per year below 5.0 cm in males (Health Technol. Assess. 2013;41:1-108) . Therefore, appropriate counseling and surveillance intervals should optimize the management of AAA patients. This study highlights the adverse effects of a diagnosis of small AAA on a proportion of our patients, despite appropriate explanation. Frequently patients know someone who died of AAA rupture and many do not understand the risk when it is explained in routine consultations. Perhaps we should all ensure that a member of our team contacts patients with small AAA post review and perform a short Quality of Life questionnaire by phone so that we can identify those who are suffering a negative impact on their QOL. We could then intensify our counseling and reassurance for this cohort of patients. This study should make us all reflect on whether our surveillance programs need to be modified, to ensure that our patients are not adversely affected by a diagnosis of small AAA.
Dr. Robert Fitridge is professor of vascular surgery, University of Adelaide, Australia, and associate medical editor of Vascular Specialist.
CHICAGO – For some patients, surveillance of low-risk abdominal aortic aneurysms is so stressful that early repair might be a better option.
Until now, though, it’s been hard to know who those patients are. There hasn’t been a way to quantify the impact of abdominal aortic aneurysm (AAA) surveillance on quality of life.
Dr. Bjoern Suckow, a vascular surgeon at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and his colleagues at the University of Massachusetts and elsewhere are working to fix that problem. “I do believe that there is a certain subset of patients who we know are” at low risk for rupture “who are so consumed by fear and anxiety during surveillance that the impact on quality of life might make us want to repair them slightly sooner. I hope this will help us weed out who that subgroup might be,” Dr. Suckow said at a meeting hosted by the Society for Vascular Surgery.
With the help of patient and physician focus groups and interviews, the team developed AAA-specific quality of life (QOL) surveys and administered them to 351 patients under surveillance for aneurysms below about 5.5 cm, and 657 who had undergone mostly endovascular AAA repair at six United States institutions.
The surveys included nine questions to assess concerns about rupture, surgery, costs, and death. The responses were averaged to give an emotional impact score (EIS) ranging from 0 to 100, with higher scores indicating worse emotional QOL. The survey also included 10 questions to assess changes in heavy lifting, strenuous activity, travel habits, and other behaviors. Those results were averaged to give a behavioral change score (BCS) that also ranged from 0 to 100, with higher scores indicating greater negative impact.
A significant portion of the surveillance patients thought it was “very likely” their aneurysm would rupture within a year; their EIS was 45 and BCS 30; patients who thought rupture was unlikely had an EIS of 12 and BCS of 13 (P less than .001). Overall, patients under surveillance had worse emotional impact sores than did those who had undergone repair.
“We routinely counsel patients with small aneurysms that the rupture risk is low” – less than 5% – “and outweighed by the higher risk of repair. We were surprised that even though we feel we do a great job counseling and educating our patients, some of them do not understand or retain what we mean.” Eventually, surveys could be used in the clinic to identify patients with “less understanding, so [we can] spend more time with them,” Dr. Suckow said.
In general, “the range of impact on QOL by AAA surveillance is broad. For most patients, the impact is minimal, but for some, especially those with a greater perceived rupture risk, it is severe. Overall, surveillance has a persistent negative impact on QOL, particularly emotional QOL. This impact appears to diminish following either open or endovascular repair,” he said.
The respondents were about 76 years old, on average. Most were white men, and about half were high school graduates.
Dr. Suckow has no relevant financial conflicts. The work was funded by the National Institutes of Health and career development awards from the Society for Vascular Surgery and the American College of Surgeons.
CHICAGO – For some patients, surveillance of low-risk abdominal aortic aneurysms is so stressful that early repair might be a better option.
Until now, though, it’s been hard to know who those patients are. There hasn’t been a way to quantify the impact of abdominal aortic aneurysm (AAA) surveillance on quality of life.
Dr. Bjoern Suckow, a vascular surgeon at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and his colleagues at the University of Massachusetts and elsewhere are working to fix that problem. “I do believe that there is a certain subset of patients who we know are” at low risk for rupture “who are so consumed by fear and anxiety during surveillance that the impact on quality of life might make us want to repair them slightly sooner. I hope this will help us weed out who that subgroup might be,” Dr. Suckow said at a meeting hosted by the Society for Vascular Surgery.
With the help of patient and physician focus groups and interviews, the team developed AAA-specific quality of life (QOL) surveys and administered them to 351 patients under surveillance for aneurysms below about 5.5 cm, and 657 who had undergone mostly endovascular AAA repair at six United States institutions.
The surveys included nine questions to assess concerns about rupture, surgery, costs, and death. The responses were averaged to give an emotional impact score (EIS) ranging from 0 to 100, with higher scores indicating worse emotional QOL. The survey also included 10 questions to assess changes in heavy lifting, strenuous activity, travel habits, and other behaviors. Those results were averaged to give a behavioral change score (BCS) that also ranged from 0 to 100, with higher scores indicating greater negative impact.
A significant portion of the surveillance patients thought it was “very likely” their aneurysm would rupture within a year; their EIS was 45 and BCS 30; patients who thought rupture was unlikely had an EIS of 12 and BCS of 13 (P less than .001). Overall, patients under surveillance had worse emotional impact sores than did those who had undergone repair.
“We routinely counsel patients with small aneurysms that the rupture risk is low” – less than 5% – “and outweighed by the higher risk of repair. We were surprised that even though we feel we do a great job counseling and educating our patients, some of them do not understand or retain what we mean.” Eventually, surveys could be used in the clinic to identify patients with “less understanding, so [we can] spend more time with them,” Dr. Suckow said.
In general, “the range of impact on QOL by AAA surveillance is broad. For most patients, the impact is minimal, but for some, especially those with a greater perceived rupture risk, it is severe. Overall, surveillance has a persistent negative impact on QOL, particularly emotional QOL. This impact appears to diminish following either open or endovascular repair,” he said.
The respondents were about 76 years old, on average. Most were white men, and about half were high school graduates.
Dr. Suckow has no relevant financial conflicts. The work was funded by the National Institutes of Health and career development awards from the Society for Vascular Surgery and the American College of Surgeons.
AT The 2015 Vascular Annual Meeting
Key clinical point: Check with your AAA surveillance patients to make sure they know their rupture risk is low.
Major finding: Surveillance patients who thought it was “very likely” their aneurysm would rupture within a year had an emotional impact score of 45. Patients who thought rupture was unlikely had a sore of 12 (P less than .001).
Data source: Surveys of 1,008 AAA patients at six U.S. medical centers.
Disclosures: There was no outside funding for the work, and the lead investigator has no relevant disclosures.