User login
Paclitaxel-coated balloon boosts femoropopliteal angioplasty patency
For patients who have femoropopliteal peripheral artery disease, percutaneous transluminal angioplasty with a paclitaxel-coated balloon achieves better 1-year patency than does using a standard balloon, according to a report published online June 24 in the New England Journal of Medicine.
Angioplasty initially restores blood flow in most patients with this type of PAD, but more than 60% develop restenosis from vessel recoil and neointimal hyperplasia within 1 year. The LEVANT2 (Lutonix Paclitaxel-Coated Balloon for the Prevention of Femoropopliteal Restenosis) clinical trial assessed the performance of a drug-coated balloon (316 patients) against a standard balloon (160 patients) in participants treated at 54 sites in the U.S. and Europe, said Dr. Kenneth Rosenfield of Massachusetts General Hospital, Boston, and his associates.
The primary efficacy endpoint – the rate of patency of the target lesion at 1 year – was significantly higher with the paclitaxel-coated balloon (65.2%) than with the standard balloon (52.6%), the investigators said (N. Engl. J. Med. 2015 June 24 [doi:10.1056/NEJMoa1406235]).
However, secondary efficacy endpoints including the rates of event-free survival (86.7% vs. 81.5%), target-lesion revascularizations (12.3% vs. 16.8%), overall mortality (2.4% vs. 2.8%), amputation (0.3% vs. 0.0%) and thrombosis (0.4% vs. 0.7%) were not significantly different between the two study groups. Scores on a measure of walking distance improved significantly more with the paclitaxel-coated balloon, but ankle-brachial index and Rutherford scores measuring pain and symptoms of intermittent claudication did not differ significantly between the two study groups.
The primary safety endpoint – a composite of the proportion of patients free from perioperative death from any cause plus the proportion free from amputation, reintervention, or PAD-associated death at 1 year – was 83.9% with the paclitaxel-coated balloon and 79.0% with the standard balloon. This met the criterion for noninferiority.
“Our trial does not provide definitive guidance concerning the potential role of this paclitaxel-coated balloon in clinical practice. Although the findings are encouraging, long-term follow-up will be useful in determining whether the benefit of this intervention is sustained, increased, or attenuated over time,” Dr. Rosenfield and his associates said.
This study was funded by Lutonix-Bard, maker of the paclitaxel-coated balloon. Dr. Rosenfield reported ties to Lutonix/Bard, Cordis, Atrium, Abbott Vascular, and VIVA Physicians; his associates reported ties to numerous industry sources.
For patients who have femoropopliteal peripheral artery disease, percutaneous transluminal angioplasty with a paclitaxel-coated balloon achieves better 1-year patency than does using a standard balloon, according to a report published online June 24 in the New England Journal of Medicine.
Angioplasty initially restores blood flow in most patients with this type of PAD, but more than 60% develop restenosis from vessel recoil and neointimal hyperplasia within 1 year. The LEVANT2 (Lutonix Paclitaxel-Coated Balloon for the Prevention of Femoropopliteal Restenosis) clinical trial assessed the performance of a drug-coated balloon (316 patients) against a standard balloon (160 patients) in participants treated at 54 sites in the U.S. and Europe, said Dr. Kenneth Rosenfield of Massachusetts General Hospital, Boston, and his associates.
The primary efficacy endpoint – the rate of patency of the target lesion at 1 year – was significantly higher with the paclitaxel-coated balloon (65.2%) than with the standard balloon (52.6%), the investigators said (N. Engl. J. Med. 2015 June 24 [doi:10.1056/NEJMoa1406235]).
However, secondary efficacy endpoints including the rates of event-free survival (86.7% vs. 81.5%), target-lesion revascularizations (12.3% vs. 16.8%), overall mortality (2.4% vs. 2.8%), amputation (0.3% vs. 0.0%) and thrombosis (0.4% vs. 0.7%) were not significantly different between the two study groups. Scores on a measure of walking distance improved significantly more with the paclitaxel-coated balloon, but ankle-brachial index and Rutherford scores measuring pain and symptoms of intermittent claudication did not differ significantly between the two study groups.
The primary safety endpoint – a composite of the proportion of patients free from perioperative death from any cause plus the proportion free from amputation, reintervention, or PAD-associated death at 1 year – was 83.9% with the paclitaxel-coated balloon and 79.0% with the standard balloon. This met the criterion for noninferiority.
“Our trial does not provide definitive guidance concerning the potential role of this paclitaxel-coated balloon in clinical practice. Although the findings are encouraging, long-term follow-up will be useful in determining whether the benefit of this intervention is sustained, increased, or attenuated over time,” Dr. Rosenfield and his associates said.
This study was funded by Lutonix-Bard, maker of the paclitaxel-coated balloon. Dr. Rosenfield reported ties to Lutonix/Bard, Cordis, Atrium, Abbott Vascular, and VIVA Physicians; his associates reported ties to numerous industry sources.
For patients who have femoropopliteal peripheral artery disease, percutaneous transluminal angioplasty with a paclitaxel-coated balloon achieves better 1-year patency than does using a standard balloon, according to a report published online June 24 in the New England Journal of Medicine.
Angioplasty initially restores blood flow in most patients with this type of PAD, but more than 60% develop restenosis from vessel recoil and neointimal hyperplasia within 1 year. The LEVANT2 (Lutonix Paclitaxel-Coated Balloon for the Prevention of Femoropopliteal Restenosis) clinical trial assessed the performance of a drug-coated balloon (316 patients) against a standard balloon (160 patients) in participants treated at 54 sites in the U.S. and Europe, said Dr. Kenneth Rosenfield of Massachusetts General Hospital, Boston, and his associates.
The primary efficacy endpoint – the rate of patency of the target lesion at 1 year – was significantly higher with the paclitaxel-coated balloon (65.2%) than with the standard balloon (52.6%), the investigators said (N. Engl. J. Med. 2015 June 24 [doi:10.1056/NEJMoa1406235]).
However, secondary efficacy endpoints including the rates of event-free survival (86.7% vs. 81.5%), target-lesion revascularizations (12.3% vs. 16.8%), overall mortality (2.4% vs. 2.8%), amputation (0.3% vs. 0.0%) and thrombosis (0.4% vs. 0.7%) were not significantly different between the two study groups. Scores on a measure of walking distance improved significantly more with the paclitaxel-coated balloon, but ankle-brachial index and Rutherford scores measuring pain and symptoms of intermittent claudication did not differ significantly between the two study groups.
The primary safety endpoint – a composite of the proportion of patients free from perioperative death from any cause plus the proportion free from amputation, reintervention, or PAD-associated death at 1 year – was 83.9% with the paclitaxel-coated balloon and 79.0% with the standard balloon. This met the criterion for noninferiority.
“Our trial does not provide definitive guidance concerning the potential role of this paclitaxel-coated balloon in clinical practice. Although the findings are encouraging, long-term follow-up will be useful in determining whether the benefit of this intervention is sustained, increased, or attenuated over time,” Dr. Rosenfield and his associates said.
This study was funded by Lutonix-Bard, maker of the paclitaxel-coated balloon. Dr. Rosenfield reported ties to Lutonix/Bard, Cordis, Atrium, Abbott Vascular, and VIVA Physicians; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A paclitaxel-coated balloon confers better 1-year patency than a standard balloon in femoropopliteal angioplasty.
Major finding: The primary efficacy endpoint – the rate of patency of the target lesion at 1 year – was significantly higher with the paclitaxel-coated balloon (65.2%) than with the standard balloon (52.6%).
Data source: An industry-sponsored multicenter prospective randomized controlled trial comparing paclitaxel-coated against standard balloons in 476 patients undergoing femoropopliteal angioplasty who were followed for 1 year.
Disclosures: This study was funded by Lutonix-Bard, maker of the paclitaxel-coated balloon. Dr. Rosenfield reported ties to Lutonix/Bard, Cordis, Atrium, Abbott Vascular, and VIVA Physicians; his associates reported ties to numerous industry sources.
Idarucizumab reverses dabigatran’s anticoagulant effects
TORONTO – Idarucizumab is a promising agent that quickly and safely reverses the anticoagulant effects of dabigatran whether the goal is to control serious bleeding or to permit urgent surgery, according to interim results of a multicenter trial.
Idarucizumab is a monoclonal antibody that binds to dabigatran to reverse its activity. The data, presented by Dr. V. Charles Pollack Jr. at the International Society on Thrombosis and Haemostasis congress, involved the first 90 patients of an ongoing trial with a planned enrollment of 300. The data from this trial, called REVERSE-AD, were published online simultaneously with the June 22 presentation at the congress (N. Engl. J. Med 2015 [doi:10.1056/NEJMoa1502000]).
“Non–vitamin K antagonist oral anticoagulants (NOACs) are generally safer than warfarin, and provide similar or improved efficacy in the prevention of stroke in patients with nonvalvular atrial fibrillation and in the prevention and treatment of venous thromboembolism,” Dr. Pollack said in an interview. “Nonetheless, serious bleeding events may occur with NOAC use, and patients taking one of these agents occasionally require urgent surgery or other intervention for which normal hemostasis is required,” added Dr. Pollack, chair of the department of emergency medicine at Pennsylvania Hospital in Philadelphia.
In RE-VERSE AD (a study of the reversal effects of idarucizumab on active dabigatran), the first 90 patients were divided into two distinct groups. Group A, with 51 patients, included those on dabigatran with serious bleeding. Group B, with 39 patients, required reversal of dabigatran for urgent or emergent procedures. In both, idarucizumab provided a median maximum reversal of 100% (95% confidence interval, 100-100) of the anticoagulation effect within 4 hours.
Clotting assays were normalized almost immediately in almost 90% of patients, and the effect was durable, with 80% having measured dabigatran levels reflecting no significant anticoagulation 24 hours later, Dr. Pollack said.
“Clinical outcomes were quite good in this multimorbid patient population, with restoration of hemostasis as reported by local investigators achieved in less than 12 hours when assessable, and with 92% of surgical patients being reported as having normal hemostasis at the time of the procedure,” he said.
Idarucizumab was generally well tolerated in the patient population. “There were no serious adverse events related to the reversal agent ... and only one patient experienced a thrombotic complication within 72 hours, and that patient had not been restarted on any antithrombotic medications,” Dr. Pollack said.
“The study is ongoing,” he added, “but these interim results show rather convincingly that idarucizumab completely and safely reverses the anticoagulant effects of dabigatran within minutes.”
In addition, Dr. Pollack said the availability of a specific reversal agent for dabigatran would enhance its safety margin, and thus alleviate the fears of providers who may hesitate to use a NOAC because of the lack of an “antidote.”
“In fact, most such cases can already be successfully and safely managed with general support and ‘tincture of time’ (the half-life of dabigatran is much shorter than that of warfarin), but having a specific ‘go-to’ option could streamline the care of the most significantly compromised patients,” he said.
Dr. Pollack emphasized, however, that idarucizumab is a specific reversal agent for dabigatran, not an antidote. “To me, the latter would imply that idarucizumab immediately stops bleeding associated with active use of dabigatran,” he said.
Providers should realize that while idarucizumab seems capable of removing dabigatran-induced coagulopathy from the list of concerns when managing a patient with serious bleeding or before a “sharp” procedure, bleeding is a multifaceted issue that also may be due to traumatized blood vessels, other causes of coagulopathy such as liver disease, or concurrent use of antiplatelet medications, he said.
“The patient with a serious or life-threatening bleed on dabigatran will likely need additional care to investigate and manage such concerns,” Dr. Pollack said. “But at least idarucizumab can specifically, safely, and rapidly address the primary consideration.
“The safety of anticoagulation therapy with dabigatran is further enhanced with idarucizumab, a specific reversal agent that won’t need to be used often, but the availability of which would be reassuring to prescribers,” he concluded.
Boehringer Ingelheim sponsored RE-VERSE AD. Idarucizumab was given a fast-track status by the Food and Drug Administration, and BI submitted a new drug application in March 2015, according to the company.
Dr. Pollack reported receiving personal fees from BI, Janssen, Daiichi-Sankyo, Bristol-Myers Squibb, and Pfizer. Disclosures for all the investigators are available at NEJM.org.
TORONTO – Idarucizumab is a promising agent that quickly and safely reverses the anticoagulant effects of dabigatran whether the goal is to control serious bleeding or to permit urgent surgery, according to interim results of a multicenter trial.
Idarucizumab is a monoclonal antibody that binds to dabigatran to reverse its activity. The data, presented by Dr. V. Charles Pollack Jr. at the International Society on Thrombosis and Haemostasis congress, involved the first 90 patients of an ongoing trial with a planned enrollment of 300. The data from this trial, called REVERSE-AD, were published online simultaneously with the June 22 presentation at the congress (N. Engl. J. Med 2015 [doi:10.1056/NEJMoa1502000]).
“Non–vitamin K antagonist oral anticoagulants (NOACs) are generally safer than warfarin, and provide similar or improved efficacy in the prevention of stroke in patients with nonvalvular atrial fibrillation and in the prevention and treatment of venous thromboembolism,” Dr. Pollack said in an interview. “Nonetheless, serious bleeding events may occur with NOAC use, and patients taking one of these agents occasionally require urgent surgery or other intervention for which normal hemostasis is required,” added Dr. Pollack, chair of the department of emergency medicine at Pennsylvania Hospital in Philadelphia.
In RE-VERSE AD (a study of the reversal effects of idarucizumab on active dabigatran), the first 90 patients were divided into two distinct groups. Group A, with 51 patients, included those on dabigatran with serious bleeding. Group B, with 39 patients, required reversal of dabigatran for urgent or emergent procedures. In both, idarucizumab provided a median maximum reversal of 100% (95% confidence interval, 100-100) of the anticoagulation effect within 4 hours.
Clotting assays were normalized almost immediately in almost 90% of patients, and the effect was durable, with 80% having measured dabigatran levels reflecting no significant anticoagulation 24 hours later, Dr. Pollack said.
“Clinical outcomes were quite good in this multimorbid patient population, with restoration of hemostasis as reported by local investigators achieved in less than 12 hours when assessable, and with 92% of surgical patients being reported as having normal hemostasis at the time of the procedure,” he said.
Idarucizumab was generally well tolerated in the patient population. “There were no serious adverse events related to the reversal agent ... and only one patient experienced a thrombotic complication within 72 hours, and that patient had not been restarted on any antithrombotic medications,” Dr. Pollack said.
“The study is ongoing,” he added, “but these interim results show rather convincingly that idarucizumab completely and safely reverses the anticoagulant effects of dabigatran within minutes.”
In addition, Dr. Pollack said the availability of a specific reversal agent for dabigatran would enhance its safety margin, and thus alleviate the fears of providers who may hesitate to use a NOAC because of the lack of an “antidote.”
“In fact, most such cases can already be successfully and safely managed with general support and ‘tincture of time’ (the half-life of dabigatran is much shorter than that of warfarin), but having a specific ‘go-to’ option could streamline the care of the most significantly compromised patients,” he said.
Dr. Pollack emphasized, however, that idarucizumab is a specific reversal agent for dabigatran, not an antidote. “To me, the latter would imply that idarucizumab immediately stops bleeding associated with active use of dabigatran,” he said.
Providers should realize that while idarucizumab seems capable of removing dabigatran-induced coagulopathy from the list of concerns when managing a patient with serious bleeding or before a “sharp” procedure, bleeding is a multifaceted issue that also may be due to traumatized blood vessels, other causes of coagulopathy such as liver disease, or concurrent use of antiplatelet medications, he said.
“The patient with a serious or life-threatening bleed on dabigatran will likely need additional care to investigate and manage such concerns,” Dr. Pollack said. “But at least idarucizumab can specifically, safely, and rapidly address the primary consideration.
“The safety of anticoagulation therapy with dabigatran is further enhanced with idarucizumab, a specific reversal agent that won’t need to be used often, but the availability of which would be reassuring to prescribers,” he concluded.
Boehringer Ingelheim sponsored RE-VERSE AD. Idarucizumab was given a fast-track status by the Food and Drug Administration, and BI submitted a new drug application in March 2015, according to the company.
Dr. Pollack reported receiving personal fees from BI, Janssen, Daiichi-Sankyo, Bristol-Myers Squibb, and Pfizer. Disclosures for all the investigators are available at NEJM.org.
TORONTO – Idarucizumab is a promising agent that quickly and safely reverses the anticoagulant effects of dabigatran whether the goal is to control serious bleeding or to permit urgent surgery, according to interim results of a multicenter trial.
Idarucizumab is a monoclonal antibody that binds to dabigatran to reverse its activity. The data, presented by Dr. V. Charles Pollack Jr. at the International Society on Thrombosis and Haemostasis congress, involved the first 90 patients of an ongoing trial with a planned enrollment of 300. The data from this trial, called REVERSE-AD, were published online simultaneously with the June 22 presentation at the congress (N. Engl. J. Med 2015 [doi:10.1056/NEJMoa1502000]).
“Non–vitamin K antagonist oral anticoagulants (NOACs) are generally safer than warfarin, and provide similar or improved efficacy in the prevention of stroke in patients with nonvalvular atrial fibrillation and in the prevention and treatment of venous thromboembolism,” Dr. Pollack said in an interview. “Nonetheless, serious bleeding events may occur with NOAC use, and patients taking one of these agents occasionally require urgent surgery or other intervention for which normal hemostasis is required,” added Dr. Pollack, chair of the department of emergency medicine at Pennsylvania Hospital in Philadelphia.
In RE-VERSE AD (a study of the reversal effects of idarucizumab on active dabigatran), the first 90 patients were divided into two distinct groups. Group A, with 51 patients, included those on dabigatran with serious bleeding. Group B, with 39 patients, required reversal of dabigatran for urgent or emergent procedures. In both, idarucizumab provided a median maximum reversal of 100% (95% confidence interval, 100-100) of the anticoagulation effect within 4 hours.
Clotting assays were normalized almost immediately in almost 90% of patients, and the effect was durable, with 80% having measured dabigatran levels reflecting no significant anticoagulation 24 hours later, Dr. Pollack said.
“Clinical outcomes were quite good in this multimorbid patient population, with restoration of hemostasis as reported by local investigators achieved in less than 12 hours when assessable, and with 92% of surgical patients being reported as having normal hemostasis at the time of the procedure,” he said.
Idarucizumab was generally well tolerated in the patient population. “There were no serious adverse events related to the reversal agent ... and only one patient experienced a thrombotic complication within 72 hours, and that patient had not been restarted on any antithrombotic medications,” Dr. Pollack said.
“The study is ongoing,” he added, “but these interim results show rather convincingly that idarucizumab completely and safely reverses the anticoagulant effects of dabigatran within minutes.”
In addition, Dr. Pollack said the availability of a specific reversal agent for dabigatran would enhance its safety margin, and thus alleviate the fears of providers who may hesitate to use a NOAC because of the lack of an “antidote.”
“In fact, most such cases can already be successfully and safely managed with general support and ‘tincture of time’ (the half-life of dabigatran is much shorter than that of warfarin), but having a specific ‘go-to’ option could streamline the care of the most significantly compromised patients,” he said.
Dr. Pollack emphasized, however, that idarucizumab is a specific reversal agent for dabigatran, not an antidote. “To me, the latter would imply that idarucizumab immediately stops bleeding associated with active use of dabigatran,” he said.
Providers should realize that while idarucizumab seems capable of removing dabigatran-induced coagulopathy from the list of concerns when managing a patient with serious bleeding or before a “sharp” procedure, bleeding is a multifaceted issue that also may be due to traumatized blood vessels, other causes of coagulopathy such as liver disease, or concurrent use of antiplatelet medications, he said.
“The patient with a serious or life-threatening bleed on dabigatran will likely need additional care to investigate and manage such concerns,” Dr. Pollack said. “But at least idarucizumab can specifically, safely, and rapidly address the primary consideration.
“The safety of anticoagulation therapy with dabigatran is further enhanced with idarucizumab, a specific reversal agent that won’t need to be used often, but the availability of which would be reassuring to prescribers,” he concluded.
Boehringer Ingelheim sponsored RE-VERSE AD. Idarucizumab was given a fast-track status by the Food and Drug Administration, and BI submitted a new drug application in March 2015, according to the company.
Dr. Pollack reported receiving personal fees from BI, Janssen, Daiichi-Sankyo, Bristol-Myers Squibb, and Pfizer. Disclosures for all the investigators are available at NEJM.org.
AT 2015 ISTH CONGRESS
Key clinical point: The investigational monoclonal antibody idarucizumab reversed the anticoagulant effects of dabigatran.
Major finding: Idarucizumab provided a median maximum dabigatran reversal of 100% (95% CI, 100-100) of the anticoagulation effect within 4 hours in an interim analysis.
Data source: RE-VERSE AD, a prospective cohort study in which 90 patients treated with dabigatran who had uncontrolled bleeding or required emergency surgery or procedures were given 5.0 g idarucizumab.
Disclosures: Boehringer Ingelheim sponsored RE-VERSE AD. Dr. Pollack reported receiving personal fees from Boehringer Ingelheim, Janssen, Daiichi-Sankyo, Bristol-Myers Squibb, and Pfizer. Disclosures for all the investigators are available at NEJM.org.
Decompressive brain surgery carries high complication risk
VIENNA – Decompressive hemicraniectomy for malignant middle cerebral artery infarction was associated with high rates of in-hospital and late complications in a clinical practice setting, according to research reported at the annual European Stroke Conference.
The retrospective findings showed that 88.1% of the 48 patients who underwent the surgery experienced complications such as intracranial hemorrhage (ICH) or symptomatic epilepsy while hospitalized, and 89.5% experienced complications in the later months of their recovery.
While these complication rates are higher than those seen in the randomized controlled clinical studies, the operation still proved life saving for many, with in-hospital and overall mortality rates of 12.5% and 14.6%, respectively, which is similar to the mortality rate seen in the DESTINY trial (Stroke 2007;38:2518-25) after 6 months.
“Patients who underwent [decompressive hemicraniectomy] are a complication-prone collective”, said Dr. Hans-Werner Pledl, resident physician at the department of neurology, UniversitätsMedizin Mannheim, University of Heidelberg (Germany). “Especially in the elderly, recovery stays limited in relevant factors such as ambulation and conversation for self-sufficiency,” he added.
To date, four clinical trials – DECIMAL (Stroke 2007;38:2506-17), HAMLET (Lancet Neurol 2009;8:326-33) and DESTINY and DESTINY II (Int J Stroke 2011;6:79-86) – have looked at the efficacy and safety of DHC in small numbers of patients with life-threatening middle cerebral artery (MCA) infarction. Of these, only DESTINY II included patients over 60 years of age so while there was evidence that the pressure-relieving surgery reduced mortality if performed early, albeit with an increase in functional disability, experience in older patients was less clear. To look at the complication rates in a real-world practice setting, Dr. Pledl of University Hospital Mannheim’s stroke unit, examined the medical records of 48 patients with MCA infarction who underwent DHC between 2008 and 2014. At the time of admission, the 21 male and 27 female patients were aged 28 to 70 years, with the mean age being 57 years. Dr. Pledl noted that two out of every five (41.7%) patients was over the age of 60 years.
On average, patients were referred to the stroke unit within 3 hours and 44 minutes of the incident event, but some were seen within 30 minutes and others within 5 days. A total of 43.8% of patients had an MCA infarction involving the dominant hemisphere and just under 60% received thrombolytic therapy with rtPA. The median time to surgery was 1.3 days, with just over one-fifth (21.7%) of patients undergoing DHC more than 48 hours after their stroke.
The median National Institutes of Health Stroke Scale scores at admission and discharge were 19 and 18, respectively, while the modified Rankin Scale (mRS) score was 5 at both time points. The Barthel Index was 0 at admission, signifying that the patient was heavily dependent on a carer to perform basic living activities, and 7.5 at discharge, indicating some only marginal improvement in patients’ independence.
The majority (75%) of patients achieved reasonable recovery with early (phase B) rehabilitation, 44% with continued poststroke (phase C) rehabilitation, and 6% were able to become self-sufficient and some even returning to work (phase D). “Remarkably, nearly half (48.9%) of patients return home after rehabilitation and do not stay in a clinical or institutional care facility,” Dr. Pledl said.
In-hospital neurological or psychiatric complications included ICH (seven patients), symptomatic epilepsy (six patients), and delirium (five patients). Perioperative complications included meningitis (three patients), wound healing disorders (three patients), and two patients had epidural hemorrhage (EDH). Common infections included pneumonia (13 patients) and urinary tract infections (UTI, eight patients), and other complications included anemia (14 patients) and cardiac complications (nine patients).
During the recovery phase, the most common neurological or psychiatric complications were central pain syndrome and symptomatic epilepsy, affecting nine patients each. Patients again experienced EDH (five patients), with some cases of hydrocephalus (four patients) and wound-healing problems (three patients). UTIs were the most common type of infection, seen in 14 patients. Other late complications included dysphagia (41.7%) and tracheostomy (35.4%), and post-rehab depression (54.2%).
Dr. Pledl suggested that the findings could be used to help better inform patients and their carers so they can have “realistic expectations” of the procedure’s likely outcomes and decide whether or not to have the surgery performed. These “real world” data could also help physicians to be more aware of the likely complications and perhaps address them in some way so that they have minimal impact on patients’ quality of life.
Although patients who experienced complications in this study were not asked if they regretted the decision to undergo the surgery, there is evidence to show that patients and carers can accept a significant level of disability without having significantly impaired quality of life. Nevertheless, the decision on whether DHC should be performed should be made on an individual case basis, especially in older patients, Dr. Pledl concluded.
The next step is to see if there are any subgroups of patients who might fare better or worse after DHC and hopefully identify some predictive imaging markers that could help the decision-making process.
Dr. Pledl reported no conflicts.
VIENNA – Decompressive hemicraniectomy for malignant middle cerebral artery infarction was associated with high rates of in-hospital and late complications in a clinical practice setting, according to research reported at the annual European Stroke Conference.
The retrospective findings showed that 88.1% of the 48 patients who underwent the surgery experienced complications such as intracranial hemorrhage (ICH) or symptomatic epilepsy while hospitalized, and 89.5% experienced complications in the later months of their recovery.
While these complication rates are higher than those seen in the randomized controlled clinical studies, the operation still proved life saving for many, with in-hospital and overall mortality rates of 12.5% and 14.6%, respectively, which is similar to the mortality rate seen in the DESTINY trial (Stroke 2007;38:2518-25) after 6 months.
“Patients who underwent [decompressive hemicraniectomy] are a complication-prone collective”, said Dr. Hans-Werner Pledl, resident physician at the department of neurology, UniversitätsMedizin Mannheim, University of Heidelberg (Germany). “Especially in the elderly, recovery stays limited in relevant factors such as ambulation and conversation for self-sufficiency,” he added.
To date, four clinical trials – DECIMAL (Stroke 2007;38:2506-17), HAMLET (Lancet Neurol 2009;8:326-33) and DESTINY and DESTINY II (Int J Stroke 2011;6:79-86) – have looked at the efficacy and safety of DHC in small numbers of patients with life-threatening middle cerebral artery (MCA) infarction. Of these, only DESTINY II included patients over 60 years of age so while there was evidence that the pressure-relieving surgery reduced mortality if performed early, albeit with an increase in functional disability, experience in older patients was less clear. To look at the complication rates in a real-world practice setting, Dr. Pledl of University Hospital Mannheim’s stroke unit, examined the medical records of 48 patients with MCA infarction who underwent DHC between 2008 and 2014. At the time of admission, the 21 male and 27 female patients were aged 28 to 70 years, with the mean age being 57 years. Dr. Pledl noted that two out of every five (41.7%) patients was over the age of 60 years.
On average, patients were referred to the stroke unit within 3 hours and 44 minutes of the incident event, but some were seen within 30 minutes and others within 5 days. A total of 43.8% of patients had an MCA infarction involving the dominant hemisphere and just under 60% received thrombolytic therapy with rtPA. The median time to surgery was 1.3 days, with just over one-fifth (21.7%) of patients undergoing DHC more than 48 hours after their stroke.
The median National Institutes of Health Stroke Scale scores at admission and discharge were 19 and 18, respectively, while the modified Rankin Scale (mRS) score was 5 at both time points. The Barthel Index was 0 at admission, signifying that the patient was heavily dependent on a carer to perform basic living activities, and 7.5 at discharge, indicating some only marginal improvement in patients’ independence.
The majority (75%) of patients achieved reasonable recovery with early (phase B) rehabilitation, 44% with continued poststroke (phase C) rehabilitation, and 6% were able to become self-sufficient and some even returning to work (phase D). “Remarkably, nearly half (48.9%) of patients return home after rehabilitation and do not stay in a clinical or institutional care facility,” Dr. Pledl said.
In-hospital neurological or psychiatric complications included ICH (seven patients), symptomatic epilepsy (six patients), and delirium (five patients). Perioperative complications included meningitis (three patients), wound healing disorders (three patients), and two patients had epidural hemorrhage (EDH). Common infections included pneumonia (13 patients) and urinary tract infections (UTI, eight patients), and other complications included anemia (14 patients) and cardiac complications (nine patients).
During the recovery phase, the most common neurological or psychiatric complications were central pain syndrome and symptomatic epilepsy, affecting nine patients each. Patients again experienced EDH (five patients), with some cases of hydrocephalus (four patients) and wound-healing problems (three patients). UTIs were the most common type of infection, seen in 14 patients. Other late complications included dysphagia (41.7%) and tracheostomy (35.4%), and post-rehab depression (54.2%).
Dr. Pledl suggested that the findings could be used to help better inform patients and their carers so they can have “realistic expectations” of the procedure’s likely outcomes and decide whether or not to have the surgery performed. These “real world” data could also help physicians to be more aware of the likely complications and perhaps address them in some way so that they have minimal impact on patients’ quality of life.
Although patients who experienced complications in this study were not asked if they regretted the decision to undergo the surgery, there is evidence to show that patients and carers can accept a significant level of disability without having significantly impaired quality of life. Nevertheless, the decision on whether DHC should be performed should be made on an individual case basis, especially in older patients, Dr. Pledl concluded.
The next step is to see if there are any subgroups of patients who might fare better or worse after DHC and hopefully identify some predictive imaging markers that could help the decision-making process.
Dr. Pledl reported no conflicts.
VIENNA – Decompressive hemicraniectomy for malignant middle cerebral artery infarction was associated with high rates of in-hospital and late complications in a clinical practice setting, according to research reported at the annual European Stroke Conference.
The retrospective findings showed that 88.1% of the 48 patients who underwent the surgery experienced complications such as intracranial hemorrhage (ICH) or symptomatic epilepsy while hospitalized, and 89.5% experienced complications in the later months of their recovery.
While these complication rates are higher than those seen in the randomized controlled clinical studies, the operation still proved life saving for many, with in-hospital and overall mortality rates of 12.5% and 14.6%, respectively, which is similar to the mortality rate seen in the DESTINY trial (Stroke 2007;38:2518-25) after 6 months.
“Patients who underwent [decompressive hemicraniectomy] are a complication-prone collective”, said Dr. Hans-Werner Pledl, resident physician at the department of neurology, UniversitätsMedizin Mannheim, University of Heidelberg (Germany). “Especially in the elderly, recovery stays limited in relevant factors such as ambulation and conversation for self-sufficiency,” he added.
To date, four clinical trials – DECIMAL (Stroke 2007;38:2506-17), HAMLET (Lancet Neurol 2009;8:326-33) and DESTINY and DESTINY II (Int J Stroke 2011;6:79-86) – have looked at the efficacy and safety of DHC in small numbers of patients with life-threatening middle cerebral artery (MCA) infarction. Of these, only DESTINY II included patients over 60 years of age so while there was evidence that the pressure-relieving surgery reduced mortality if performed early, albeit with an increase in functional disability, experience in older patients was less clear. To look at the complication rates in a real-world practice setting, Dr. Pledl of University Hospital Mannheim’s stroke unit, examined the medical records of 48 patients with MCA infarction who underwent DHC between 2008 and 2014. At the time of admission, the 21 male and 27 female patients were aged 28 to 70 years, with the mean age being 57 years. Dr. Pledl noted that two out of every five (41.7%) patients was over the age of 60 years.
On average, patients were referred to the stroke unit within 3 hours and 44 minutes of the incident event, but some were seen within 30 minutes and others within 5 days. A total of 43.8% of patients had an MCA infarction involving the dominant hemisphere and just under 60% received thrombolytic therapy with rtPA. The median time to surgery was 1.3 days, with just over one-fifth (21.7%) of patients undergoing DHC more than 48 hours after their stroke.
The median National Institutes of Health Stroke Scale scores at admission and discharge were 19 and 18, respectively, while the modified Rankin Scale (mRS) score was 5 at both time points. The Barthel Index was 0 at admission, signifying that the patient was heavily dependent on a carer to perform basic living activities, and 7.5 at discharge, indicating some only marginal improvement in patients’ independence.
The majority (75%) of patients achieved reasonable recovery with early (phase B) rehabilitation, 44% with continued poststroke (phase C) rehabilitation, and 6% were able to become self-sufficient and some even returning to work (phase D). “Remarkably, nearly half (48.9%) of patients return home after rehabilitation and do not stay in a clinical or institutional care facility,” Dr. Pledl said.
In-hospital neurological or psychiatric complications included ICH (seven patients), symptomatic epilepsy (six patients), and delirium (five patients). Perioperative complications included meningitis (three patients), wound healing disorders (three patients), and two patients had epidural hemorrhage (EDH). Common infections included pneumonia (13 patients) and urinary tract infections (UTI, eight patients), and other complications included anemia (14 patients) and cardiac complications (nine patients).
During the recovery phase, the most common neurological or psychiatric complications were central pain syndrome and symptomatic epilepsy, affecting nine patients each. Patients again experienced EDH (five patients), with some cases of hydrocephalus (four patients) and wound-healing problems (three patients). UTIs were the most common type of infection, seen in 14 patients. Other late complications included dysphagia (41.7%) and tracheostomy (35.4%), and post-rehab depression (54.2%).
Dr. Pledl suggested that the findings could be used to help better inform patients and their carers so they can have “realistic expectations” of the procedure’s likely outcomes and decide whether or not to have the surgery performed. These “real world” data could also help physicians to be more aware of the likely complications and perhaps address them in some way so that they have minimal impact on patients’ quality of life.
Although patients who experienced complications in this study were not asked if they regretted the decision to undergo the surgery, there is evidence to show that patients and carers can accept a significant level of disability without having significantly impaired quality of life. Nevertheless, the decision on whether DHC should be performed should be made on an individual case basis, especially in older patients, Dr. Pledl concluded.
The next step is to see if there are any subgroups of patients who might fare better or worse after DHC and hopefully identify some predictive imaging markers that could help the decision-making process.
Dr. Pledl reported no conflicts.
AT THE EUROPEAN STROKE CONFERENCE
Key clinical point: The high risk of complications associated with decompressive hemicraniectomy for malignant middle cerebral artery infarction warrants appropriate counseling and individualized therapeutic decision-making.
Major finding: The in-hospital and late complication rates associated with decompressive hemicraniectomy for malignant middle cerebral artery infarction were 88.1% and 89.5%, respectively.
Data source: Retrospective, observational, single-center study of 48 patients who underwent decompressive hemicrainiectomy between 2008 and 2014.
Disclosures: Dr. Pledl reported no conflicts.
Stricter DVT prophylaxis guidelines needed for cardiac and vascular surgery
Cardiac and vascular surgery patients should receive deep vein thrombosis (DVT) prophylaxis before and after surgery, say researchers who found a high incidence of postoperative DVT in these patients compared to general surgery patients.
The retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database found that 18,670 patients developed a DVT within 30 days of the operation.
The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery, reported Dr. Faisal Aziz and his colleagues at Pennsylvania State University (Ann. Vasc. Surg. 2015; 29: 661-9).
Vascular surgery patients were at 1.5 times the risk of a postop DVT and cardiac surgery patients were at 3 times the risk compared with general surgery patients, a significant difference.
Preoperative factors associated with increased risk of developing DVT in the postoperative period included inpatient admission status (OR 7.8), general anesthesia (OR 2), and dyspnea at rest (OR 5).
“Despite the fact that most arterial surgery operations involve administration of therapeutic doses of anticoagulation therapy during the operations, incidence of postoperative DVT is high in these patients,” the study authors wrote.
“Intraoperative anticoagulation is not protective against development of DVT in the postoperative period” they said.
“Physicians should ensure adequate DVT prophylaxis in postoperative vascular surgery and cardiac surgery patients, according to established evidence based guidelines,” they concluded.
The authors did not report any financial disclosures.
Cardiac and vascular surgery patients should receive deep vein thrombosis (DVT) prophylaxis before and after surgery, say researchers who found a high incidence of postoperative DVT in these patients compared to general surgery patients.
The retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database found that 18,670 patients developed a DVT within 30 days of the operation.
The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery, reported Dr. Faisal Aziz and his colleagues at Pennsylvania State University (Ann. Vasc. Surg. 2015; 29: 661-9).
Vascular surgery patients were at 1.5 times the risk of a postop DVT and cardiac surgery patients were at 3 times the risk compared with general surgery patients, a significant difference.
Preoperative factors associated with increased risk of developing DVT in the postoperative period included inpatient admission status (OR 7.8), general anesthesia (OR 2), and dyspnea at rest (OR 5).
“Despite the fact that most arterial surgery operations involve administration of therapeutic doses of anticoagulation therapy during the operations, incidence of postoperative DVT is high in these patients,” the study authors wrote.
“Intraoperative anticoagulation is not protective against development of DVT in the postoperative period” they said.
“Physicians should ensure adequate DVT prophylaxis in postoperative vascular surgery and cardiac surgery patients, according to established evidence based guidelines,” they concluded.
The authors did not report any financial disclosures.
Cardiac and vascular surgery patients should receive deep vein thrombosis (DVT) prophylaxis before and after surgery, say researchers who found a high incidence of postoperative DVT in these patients compared to general surgery patients.
The retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database found that 18,670 patients developed a DVT within 30 days of the operation.
The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery, reported Dr. Faisal Aziz and his colleagues at Pennsylvania State University (Ann. Vasc. Surg. 2015; 29: 661-9).
Vascular surgery patients were at 1.5 times the risk of a postop DVT and cardiac surgery patients were at 3 times the risk compared with general surgery patients, a significant difference.
Preoperative factors associated with increased risk of developing DVT in the postoperative period included inpatient admission status (OR 7.8), general anesthesia (OR 2), and dyspnea at rest (OR 5).
“Despite the fact that most arterial surgery operations involve administration of therapeutic doses of anticoagulation therapy during the operations, incidence of postoperative DVT is high in these patients,” the study authors wrote.
“Intraoperative anticoagulation is not protective against development of DVT in the postoperative period” they said.
“Physicians should ensure adequate DVT prophylaxis in postoperative vascular surgery and cardiac surgery patients, according to established evidence based guidelines,” they concluded.
The authors did not report any financial disclosures.
FROM ANNALS OF VASCULAR SURGERY
Key clinical point: Intraoperative anticoagulation alone does not prevent DVT in patients undergoing vascular and cardiac surgery.
Major finding: The incidence of DVT according to the type of surgery was 2% for cardiac surgery, 0.99% for vascular surgery and 0.66% for general surgery.
Data source: Retrospective study of 2,669,772 surgery patients from the American College of Surgeons National Surgical Quality Improvement Program database.
Disclosures: The authors did not report any financial disclosures.
Decision Making in Venous Thromboembolism
From the Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, MA.
Abstract
- Objective: To review the diagnosis and management of venous thromboembolism (VTE).
- Methods: Review of the literature.
- Results: VTE and its associated complications account for significant morbidity and mortality. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. Clinical decision rules have been developed that can help determine which patients warrant further testing. Anticoagulation, the mainstay of VTE treatment, increases bleeding risk, necessitating tailored treatment strategies that must incorporate etiology, risk, benefit, cost, and patient preference.
- Conclusion: Further study is needed to understand individual patient risks and to identify treatments that will lead to improved patient outcomes.
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 in Western countries develop VTE. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of those patients diagnosed with thrombotic events will die within the first month after diagnosis [1].PE is a common consequence of DVT; 40% of patients who are diagnosed with DVT will be subsequently found to have PE upon further imaging. This high rate of association is also seen in those who present with PE, 70% of whom will also be found to have concomitant DVT [2,3].
Anatomic risk factors include Paget-Schroetter syndrome (compression of upper extremity veins due to abnormalities at the thoracic outlet), May-Thurner syndrome (significant compression of the left common iliac vein by the right common iliac artery), and abnormalities of the inferior vena cava [14–16].Medications that are associated with increased risk of VTE include but are not limited to estrogen (both in oral contraceptives as well as hormone replacement therapy) [17,18],the selective estrogen receptor modulator tamoxifen [19],testosterone [20],and glucocorticoids [21].It is important to note that many patients with VTE have more than one acquired risk factor for thrombosis [22],and also that acquired risk factors are more likely to lead to VTE in the setting of underlying inherited thrombophilic conditions [23].
Pathogenesis
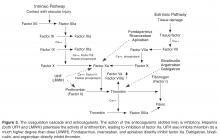
Abnormalities in both coagulation factors and the vascular bed are at the core of the pathogenesis of VTE. The multifaceted etiology of thrombosis was first described in 1856 by Virchow, who defined a triad of defects in the vessel wall, platelets, and coagulation proteins [24].Usually the vessel wall is lined with endothelial cells that provide a nonthrombotic surface and limit platelet aggregation through release of prostacyclins and nitric oxide. When the endothelial lining is compromised, the homeostatic surveillance system is disturbed and platelet activation and the coagulation system are initiated. Tissue factor exposure in the damaged area of the vessel leads to activation of the coagulation cascade. Collagen that is present in the area of the wound is also exposed and can activate platelets, which provide the phospholipid surface upon which the coagulation cascade occurs. Platelets initially tether to the exposed collagen through binding of glycoprotein Ib-V-IX in association with von Willebrand factor [25].The thrombus is initiated as more platelets are recruited to exposed collagen of the injured endothelium through aggregation in response to the binding of GPIIIb/IIa with fibrinogen. This process is self-perpetuating as these activated platelets release additional proteins such as adenosine diphosphate (ADP), serotonin, and thromboxane A2, all of which fuel the recruitment and activation of additional platelets [26].
Diagnosis
The key to decreasing the morbidity and mortality associated with VTE is timely diagnosis and early initiation of therapy. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. DVT is usually associated with pain in calf or thigh, unilateral swelling, tenderness, and redness. PE can present as chest pain, shortness of breath, syncope, hemoptysis, and/or cardiac palpitations.
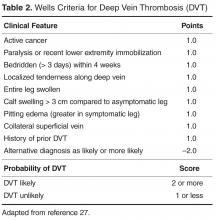
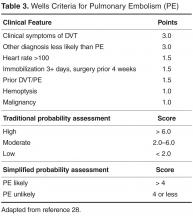
Decision Rules
Clinical decision rules based on signs, symptoms, and risk factors have been developed to estimate the pretest probability of PE or DVT and to help determine which patients warrant further testing. These clinical decision rules include the Wells criteria (separate rules for DVT and PE) [27,28],as well as the Geneva score [29],which is focused on identifying patients with a likelihood of having a PE. In general, these clinical rules are applied at presentation to predict the risk of VTE, and patients who score high are evaluated by imaging modalities, while those with lower scores should be considered for further stratification based on D-dimer testing. The goal of clinical assessment and use of a decision rule is to identify patients at low risk of VTE to reduce the number of imaging studies performed. Most of the decision rules focus on the use of noninvasive evaluations that are easily implemented, including clinical history and presentation, abnormalities in oxygen saturation, chest radiography findings, and electro-cardiography.
D-Dimer Testing
D-dimer testing is at the core of all predictive models for VTE. D-dimer is a fibrin degradation product that is detectable in the blood during active fibrinolysis and occurs after clot formation. The concentration of D-dimer increases in patients with active clot. D-dimer testing is usually performed as a quantitative ELISA or automated turbidometric assay and is highly sensitive (> 95%) in excluding a diagnosis of VTE if results are in the normal range [30].The presence of a normal D-dimer and a low probability based on clinical assessment criteria can be integrated to determine which patients have a low (generally < 99%) likelihood of having VTE [31].It should be noted that other factors can lead to an increased D-dimer, including malignancy, trauma, critical illness, disseminated intravascular coagulation, pregnancy, infection, and postoperative status, which can produce false-positive results and cloud the utility of the test in excluding those at low risk of VTE from undergoing imaging [32–34].Additionally, D-dimer values naturally increase with age and recent work has shown utility of an age-adjusted D-dimer threshold, though this method is not yet widespread in clinical practice [35,36].
Imaging
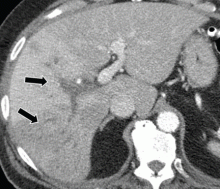
After application of a clinical prediction rule, the mainstay of diagnosis of VTE is imaging. For DVT the use of ultrasonography is considered the gold standard, with both high sensitivity (89–100%) and specificity (86–100%), especially when the DVT is located proximally [37–39].We generally recommend compression ultrasound starting with the proximal veins but expanding to include the whole leg if the proximal studies are negative [40–42].Other diagnostic options include computed tomography (CT) venography, which is not first line as it is highly invasive and exposes the patient to iodine-based contrast dyes, and magnetic resonance venography (MRV), which offers superb visualization for diagnosis of pelvic vein thrombosis but is limited because of availability and cost issues.
Helical CT pulmonary angiography (CTPA) is the diagnostic test of choice in PE, with high sensitivity (96%) and specificity (95%), and has replaced conventional ventilation perfusion (VQ) scanning or other methods such as magnetic resonance pulmonary angiography in most settings [43,44].CTPA should be avoided in patients who have severe chronic kidney disease or a contrast allergy, and is often avoided in patients who are pregnant due to potential risk of radiation exposure, and in such situations VQ scanning may be employed.
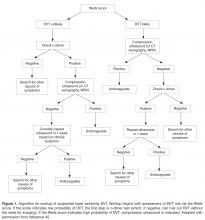
Algorithmic Approach to Workup
Of note, there are multiple clinical situations in which the application of a clinical prediction rule followed by D-dimer testing and/or imaging cannot be “standardized” with such algorithms. These include situations where D-dimer may be falsely positive (as above), situations in which alternative imaging strategies should be used to avoid contrast exposure in workup of PE (as above), and workup of suspected upper extremity DVT. Upper extremity ultrasound comprises about 10% of all DVT and frequently occurs in the setting of risk factors such as central venous catheters or pacemakers; specific upper-extremity risk-assessment rules have been developed [47,48].
Acute Treatment Options
The first step in treatment is identification of patients who are at high risk of
In standard cases of DVT and PE without hemodynamic compromise, the current standard of care is to initiate parenteral anticoagulation. The immediate goal of therapy is to treat rapidly with anticoagulants to prevent the thrombus from propagating further and to prevent DVT from embolization to the lungs or other vascular beds. The initial treatment of VTE has been extensively discussed and guidelines have been established with recommendations for initiation of anticoagulation; the American College of Chest Physicians (ACCP) released the 9th edition of their guidelines in 2012 based on consensus agreements derived from primary data [51].
Heparin-based drugs are the mainstay of initial treatment. These drugs act by potentiating antithrombin and therefore inactivating thrombin and other coagulation factors such as Xa. Unfractionated heparin (UFH) can be administered as an initial bolus followed by a continuous infusion with dosing being based on weight and titrated to activated partial thromboplastin time (aPTT) or the anti-factor Xa level. Alternatively, patients may be treated with a low molecular weight heparin (LMWH) administered subcutaneously in fixed weight-adjusted doses, which obviates the need for monitoring in most cases [52].LMWHs work in a similar manner to UFH but have more anti-Xa activity in comparison to anti-thrombin activity. LMWH appears to be more effective than UFH for initial treatment of VTE and has been associated with lower risk of major hemorrhage [53].The options for treatment of VTE have expanded in recent years with the approval of fondparinux, a pentasaccharide specifically targeted to inhibit factor Xa. Fondaparinux has been shown to have similar efficacy to LMWH in patients with DVT [54],and while it has not been evaluated directly against LMWH for initial treatment of PE it has been shown to be at least as effective and safe as UFH [55].
Both LMWH and fondaparinux are cleared renally and therefore have increased bleeding risk in patients with renal impairment. In patients with creatinine clearance of less than 30 mL/min, dose reduction or lengthening of dosing interval may be appropriate. Anti-factor Xa activity can be used as a functional assay to monitor and titrate the level of anticoagulation in patients treated with UFH, LMWH, and fondaparinux. Monitoring is useful in the setting of impaired renal function (as above) in addition to extremes of body weight and pregnancy. When used for monitoring of UFH, the anti-factor Xa activity can be measured at any time during administration with a therapeutic goal range of 0.3–0.7 international units (IU)/mL. When used for LMWH, a “peak” anti-factor Xa should be measured approximately 4 hours after dosing, with therapeutic goals depending on preparation and schedule of treatment but generally between 0.6 to 1.0 IU/mL for twice daily and around 1.0 -2.0 IU/mL for once-daily [56].For patients on dialysis, we generally use intravenous UFH for acute treatment of VTE, though recent work has shown that enoxaparin (doses of 0.4 to 1 mg/kg/day) was as safe as UFH with respect to bleeding and was associated with shorter hospital length of stay [57].For long-term treatment of VTE, warfarin is generally preferred based on clinical experience with this agent, though small studies have suggested that parenteral agents may be useful alternatives to warfarin [58].
In many patients who are clinically stable without significant medical comorbidities, outpatient administration of these medications without hospitalization is considered safe. Patients with DVT are often safe to manage as outpatients unless significant clot burden is present and thrombolysis is being considered. For PE, the pulmonary embolism severity index (PESI) and simplified index (sPESI) may be useful to risk-stratify patients and identify those at low risk of complications who may be suitable for outpatient treatment [59,60].Studies have shown that hemodynamically stable patients who did not require supplemental oxygenation or have contraindications to LMWH therapy were safely managed as outpatients with low risk of recurrent VTE and bleeding [61,62].One exception may be patients with intermediate risk PE, who are hemodynamically stable but have evidence of right ventricular dysfunction and may be better served by an initial in-hospital observation period, especially if thrombolysis is being considered.
Most patients who present with VTE are transitioned to warfarin for long-term therapy. Warfarin can be started on the same day as parenteral anticoagulation. Both drugs are overlapped for at least 5 days, with a target INR of 2.0–3.0. Patients may achieve the target INR level quickly because factor VII has a short half-life and the level drops quickly; however, the overlap of 5 days is essential even when the INR is in the target range because a full anticoagulant affect is not achieved until prothrombin levels decline, and this is a slow process due to the long half-life of prothrombin. Warfarin also causes rapid decrease in levels of natural anticoagulants such as protein C and protein S, which further exacerbates the net hypercoagulable state in the short-term. Warfarin without a bridging parenteral agent carries a risk of warfarin-induced skin necrosis [63]and is not effective as an initial anticoagulant treatment in acute VTE as there is a relatively high risk of symptomatic clot extension or recurrent VTE compared to warfarin with use of a bridging agent [64].In specific cases such as cancer-associated VTE (see discussion below), LMWH is preferred to warfarin for long-term active therapy.
Long-Term Active Therapy After Acute Treatment
Duration of Anticoagulation
Recommended duration of anticoagulation depends on a myriad of factors including severity of VTE, risk of recurrence, bleeding risk, and lifestyle modification issues, as well as on the safety and availability of alternative therapies such as low-intensity warfarin, aspirin, or the new oral anticoagulants. The decision tree for length of treatment starts with whether the VTE was a provoked or a spontaneous event. Provoked events occur when the event is associated with an identifiable risk factor, such as immobilization from prolonged medical illness or surgical intervention, pregnancy or oral contraceptive use, and prolonged air travel.
Consensus guidelines suggest that 3 months of anti-coagulation are generally sufficient treatment for a provoked VTE [51,65,66]. Data from multiple studies and a meta-analysis suggests that less than 3 months of anticoagulation (4 to 6 weeks in most trials) is associated with an approximately 1.5-fold higher risk of recurrent VTE than 3 months [67,68].However, data from this meta-analysis also suggests that anticoagulation for longer than 3 months (6 to 12 months in most trials) is not associated with higher rates of recurrent VTE. We generally anticoagulate for 3 months in patients with provoked VTE.
Determining the duration of anticoagulation is more complex in patients with idiopathic/unprovoked VTE. Kearon and colleagues found that in patients with first idiopathic VTE, patients who were anticoagulated for 24 months versus 3 months had lower risk of recurrent VTE (1.3% per patient-year with 24 months versus 27.4% per patient-year with 3 months) [69].Similar studies and meta-analyses have demonstrated decreased recurrence rates in patients anticoagulated for a prolonged period of time. However, one study of prolonged anticoagulation revealed that at 3 years there was no difference in recurrence rate in patients with PE who were anticoagulated for 6 months versus 1 year [70].The likelihood of recurrent DVT in patients with first episode of idiopathic proximal DVT treated with either 3 months or 12 months of warfarin was similar after treatment was discontinued [71].Prolonged periods of anticoagulation do not directly influence risk of recurrence but instead may only delay occurrence of a second event [72].For that reason, the decision is essentially whether to anticoagulate for 3 months or to continue therapy indefinitely [73]. Current guidelines recommend continuing anticoagulation for 3 months in those at high risk of bleeding, and continuing for an extended duration in those at low or moderate bleeding risk [51]. Patients' values and perferences should be entertained and decisions made on a patient-by-patient basis.
For patients at high risk of recurrent VTE, we generally recommend indefinite anticoagulation unless the patient has a significantly elevated bleeding risk or strongly prefers to discontinue anticoagulation and compliance concerns are evident. High-risk patients are those who have suffered from multiple episodes of recurrent VTE, those who have clotted while being anticoagulated, and those with acquired risk factors, such as antiphospholipid antibodies and malignancy. Other high-risk groups are those with high-risk thrombophilias such as deficiency of protein S, protein C, or antithrombin, homozygous factor V Leiden or prothrombin gene mutations, and compound heterozygous factor V Leiden/prothrombin gene mutation in the setting of an unprovoked event. Further discussion of models for risk assessment of recurrence is provided below.
Assessment of Bleeding Risk
The bleeding risk associated with the use of anticoagulation must be weighed against the risk of clotting events when determining duration of anticoagulation, especially in those patients for whom indefinite anticoagulation is a consideration. Risk of bleeding while on anticoagulation is approximately 1–3% per 100 patient-years [74],but concomitant medical conditions such as renal failure, diabetes-related cerebrovascular disease, malignancy, advanced age, and use of antiplatelet agents all increase the risk of bleeding. Bleeding risk is highest when patients first initiate anticoagulation and is approximately 10 times the risk in the first month of therapy than after the first year of therapy [75].
Risk assessment models such as the RIETE score may be helpful when indefinite anticoagulation is a possibility [76].The RIETE score encompasses 6 risk factors (age > 75 years, recent bleeding, cancer, creatinine level > 1.2 mg/dL, anemia, or PE at baseline) to categorize patients into low risk (0 points, 0.3% risk of bleeding), intermediate risk (1–4 points, 2.6% risk of bleeding) and high risk (> 4 points, 6.2% risk of bleeding) within 3 months of anticoagulant therapy. The ACCP has developed a more extensive list of 17 potential risk factors for bleeding to categorize patients into low risk (no risk factors, 0.8%/year risk of bleeding), intermediate risk (1 risk factor, 1.6%/year risk of bleeding) and high risk (2 or more risk factors, >6.5%/year risk of bleeding) categories [77].The RIETE score is simpler to use but was not developed for assessing risk of bleeding during indefinite therapy, while the ACCP risk categorization predicts a yearly risk and is therefore applicable for long-term risk assessment but is more cumbersome to use. In practice, we generally use a clinical gestalt of a patient’s clinical risk factors (particularly age, renal or hepatic dysfunction, and frequent falls) to assess if they may be at high risk of bleeding and if the risk of indefinite anticoagulation may thus outweigh the potential benefit.
We also note that several scoring systems (HAS-BLED, HEMORR2HAGES, and ATRIA scores) have been developed to predict those at high risk of bleeding on anticoagulation for atrial fibrillation [78–80].These scores generally include similar clinical risk factors to those in the RIETE and ACCP scoring systems. Several studies have compared the HAS-BLED, HEMORR2HAGES, and ATRIA scores and a systematic review and meta-analysis concluded that the HAS-BLED score is recommended, due to increased sensitivity and ease of application [81].However, as these scores have not been validated for anticoagulation in the setting of VTE, we do not use them in this capacity.
Risk Stratification for Recurrent VTE
When predicting risk of recurrent VTE, clinical risk factors including obesity, male gender, and underlying thrombophilia (including the “high risk” inherited thrombophilias identified above) must taken into consideration. Location of the thrombus must also be considered; it has also been demonstrated that patients with DVT involving the iliofemoral veins are at higher risk of recurrence than those without iliac involvement [82].Other factors that may be useful in risk stratification include D-dimer level and ultrasound to search for residual venous thrombosis.
D-dimer Levels
D-dimer levels are one of the more promising methods for assessing the risk of recurrent VTE after cessation of anticoagulation, especially in the case of idiopathic VTE where indefinite anticoagulation should be considered but may pose either risk of bleeding or significant inconvenience to patients. A normal D-dimer measured 1 month after cessation of anticoagulation offers a high negative predictive value for risk of recurrence [83].A number of studies have demonstrated that patients with elevated D-dimer 1 month after anticoagulation cessation are at increased risk for a recurrent event [84–86].Two predictive models that have been developed incorporate D-dimer testing into decision making [87,88].The DASH predictive model relies on the D-dimer result in addition to age, male sex, and use of hormone therapy as a method of risk stratification for recurrent VTE in patients with a first unprovoked event. Using this scoring system, patients with a score of 0 or 1 had a recurrence rate of 3.1%, those with a score of 2 a recurrence rate of 6.4%, and those with a score of 3 or greater a recurrence rate of 12.3%. The authors postulate that by using this assessment scheme they can avoid lifelong anticoagulation in 51% of patients. The Vienna prediction model uses male sex, location of VTE (proximal DVT and PE are at higher risk), and D-dimer level to predict risk of recurrent VTE. This model has recently been updated to include a “dynamic” component to predict risk of recurrence of VTE from multiple random time points [89].
Overall, D-dimer may be useful for risk stratification. We often employ the method of stopping anticoagulation in patients with unprovoked VTE after 3 months (if the patient has no identifiable clinical risk factors that place them at high risk of recurrence) and testing D-dimer 1 month after cessation of anticoagulation. An elevated D-dimer is a solid reason to restart anticoagulation (potentially on an indefinite basis), while a negative D-dimer provides support for withholding further anticoagulation in the absence of other significant risk factors for recurrence. However, lack of agreement regarding assay cut-points as well as multiple reasons other than VTE for D-dimer elevation may limit widespread use of this method. We generally use a cutpoint of 250 ug/L as “negative,” though at least one study showed that cut-points of 250 ug/L versus 500 ug/L did not change the utility of this method [90].In our practice, risk prediction models are most useful to provide patients with additional information and a visual presentation to support our recommendation. This is particularly true of the Vienna prediction rule, which is available in a printable nomogram which can be distributed to patients and completed together during the clinic visit.
Imaging Analysis
Imaging analysis may also assist with risk stratification. Clinical assessment modules have been developed that incorporate repeat imaging studies for assessment of recannulization of affected veins. In patients with residual vein thrombosis (RVT) at the time anticoagulation was stopped, the hazard ratio for recurrence was 2.4 compared to those without RVT [91].There are a number of ways RVT could impact recurrence, including inpaired venous flow leading to stasis and activation of the coagulation cascade. Subsequent studies used serial ultrasound to determine when to stop anticoagulation. In one study, patients were anticoagulated for 3 months and for those that had RVT, anticoagulation was continued for up to 9 months for provoked and 21 months for unprovoked VTE. In comparison to fixed dosing of 6 months of anti-coagulation, those who had their length of anticoagulation tailored to ultrasonography findings had a lower rate of recurrent VTE [92].Limitations to using RVT in clinical decision-making include lack of a standard definition of RVT and variability in both timing of ultrasound (operator variability) and interpretation of results [93].
Other Options
Another option in patients who are being considered for indefinite anticoagulation is to decrease the intensity of anticoagulation. Since this would theoretically lower the risk of bleeding, the perceived benefit of long-term, low-intensity anticoagulation would be reduction in both bleeding and clotting risk. The PREVENT trial randomized patients who had received full-dose anticoagulation for a median of 6.5 months to either low-intensity warfarin (INR goal of 1.5-2.0 instead of 2.0-3.0) or placebo. In the anticoagulation group, there was a 64% risk reduction in recurrent VTE (hazard ratio 0.36, 95% CI 0.19 to 0.67) but an increased risk of bleeding (hazard ratio 1.92, 95% CI 1.26 to 2.93) [94].The ELATE study randomized patients with unprovoked VTE who had completed 3 or more months of full-intensity warfarin therapy (target INR 2.0–3.0) to continue therapy with either low-intensity warfarin (target INR 1.5–2.0) or full-intensity warfarin (target INR 2.0-3.0). Compared to the low-intensity group, the conventional-intensity group had lower rates of recurrent VTE and no increased rates of major bleeding [95].This study, however, has been criticized because of its overall low bleeding rate in both treatment groups.
Aspirin is an option in patients in whom long-term anticoagulation is untenable. The ASPIRE trial demonstrated that in patients with unprovoked VTE who had completed a course of initial anticoagulation, aspirin 100 mg daily reduced the risk of major vascular events compared to placebo with no increase in bleeding [96].However, aspirin was not associated with a significant reduction in risk of VTE alone (only the composite vascular event endpoint). The WARFASA trial, however, demonstrated that aspirin 100 mg daily was associated with a significant reduction in recurrent VTE compared to placebo after 6 to 18 months of anticoagulation without an increase in major bleeding [97].The absolute risk of recurrence was 11% in the placebo group and 5.9% in the aspirin group. More recently, the INSPIRE collaboration analyzed data from both trials and found that aspirin after initial anticoagulation reduced the risk of recurrent VTE by approximately 42% with a low rate of major bleeding [98].The absolute risk reduction was even larger in men and older patients. For this reason, we recommend aspirin to those patients in whom indefinite anticoagulation may be warranted from the standpoint of reducing risk of recurrent VTE but in whom the risk of bleeding precludes its use.
Hypercoagulable States In Specific Populations
Inherited Thrombophilias
Patients with a hereditary thrombophilia are at increased risk for incident VTE [99].These inherited mutations result in either a loss of normal anticoagulant function or gain of a prothrombotic state. Hereditary disorders associated with VTE include deficiency of antithrombin, protein C, or protein S, or the presence of factor V Leiden and/or prothrombin G20210A mutations. Although deficiency of protein C, protein S, or antithrombin is uncommon and affects only 0.5% of the population, these states have been associated with a 10-fold increased risk of thrombosis in comparison to the general population. Factor V Leiden and prothrombin gene mutation are less likely to be associated with incident thrombosis (2 to 5-fold increased risk of VTE) and are more prevalent in the Caucasian population [100].Though these hereditary thrombophilias increase risk of VTE, prophylactic anti-coagulation prior to a first VTE is not generally indicated.
Data regarding the impact of the inherited thrombophilias on risk of recurrent VTE is less well defined. While some data suggest that inherited thrombophilias are associated with increased risk of recurrent VTE, the degree of impact may be clinically modest especially in those with heterozygous factor V Leiden or prothrombin gene mutations [101].Ideally, a clinical trial would be designed to assess whether hereditary thrombophilia testing is beneficial for patients with VTE in decision-making regarding length of anticoagulation, type of anticoagulation, and risk of recurrence. If a patient with a low-risk inherited thrombophilia has a DVT in the setting of an additional provoking risk factor (surgery, pregnancy, etc), a 3-month course of anticoagulation followed by D-dimer assessment as above is reasonable. If a patient with an inherited thrombophilia experiences an idiopathic VTE, or if a patient with a “high-risk” thrombophilia as described above experiences any type of VTE, we generally recommend indefinite anticoagulation in the absence of high bleeding risk, though again this is a very patient-dependent choice.
Acquired Thrombophilias
Antiphospholipid Syndrome
Antibodies directed against proteins that bind phospho-lipids are associated with an acquired hypercoagulable state. The autoantibodies are categorized as antiphospho-lipid antibodies (APLAs), which include anticardiolipin antibodies (IgG and IgM), beta-2 glycoprotein 1 antibodies (anti-B2 GP), and lupus anticoagulant. These antibodies can form autonomously, as seen in primary disorders, or in association with autoimmune disease as a secondary disorder.
Criteria have been developed to distinguish antiphospholipid-associated clotting disorders from other forms of thrombophilia. The updated Sapporo criteria depend on both laboratory and clinical diagnostic criteria [102].The laboratory diagnosis of APLAs requires the presence of lupus anticoagulants, anticardiolipin antibodies, or anti-B2 GP on at least 2 assays at least 12 weeks apart with elevation above the 99th percentile of the testing laboratory’s normal distribution [103].Testing for lupus anticoagulant is based on 3 stages, the first of which is inhibition of phospholipid-dependent coagulation tests with prolonged clotting time (eg, aPTT or dilute Russell’s viper venom time). The diagnosis is confirmed by a secondary test in which excess hexagonal phase phospholipids are added to incubate with the patient’s plasma to absorb the APLA [104].The presence of anticardiolipin antibodies and anti beta-2 GP antibodies is determined using ELISA based immunoassays. Unlike most other thrombophilias, antiphospholipid syndrome is associated with both arterial and venous thromboembolic events and may be an indication for lifelong anticoagulation after a first thrombotic event. We generally recommend indefinite anticoagulation in the absence of significant bleeding risk.
Cancer-Associated Hypercoagulable State
Patients with cancer have a propensity for thromboembolic events. The underlying mechanisms responsible for cancer-associated clotting events are multifactorial and an area of intense research. Tumor cells can initiate activation of the clotting cascade through release of tissue factor and other pro-coagulant molecules [105].Type and stage of cancer impact risk of VTE, and the tumor itself can compress vasculature leading to venous stasis. Furthermore, chemotherapy, hormone therapy, antiangiogenic drugs, erythropoietin agents, and indwelling central venous catheters all are associated with increased risk of thrombotic events. Approximately 25% of all cancer patients will experience a thrombotic event during the course of their disease [106]. In fact, the presence of a spontaneous clot may be a harbinger of underlying malignancy [107].Approximately 10% of patients who present with an idiopathic VTE are diagnosed with cancer in the next 1 to 2 years.
The utility of extensive cancer screening in patients with spontaneous clotting events is often debated. The small studies that have addressed cancer associated clots have not demonstrated any mortality benefit with extensive screening. A prospective cohort study addressed the utility of limited versus extensive screening [108].In this study, all patients underwent a series of basic screening tests such as history taking, physical examination, chest radiograph, and basic laboratory parameters. Approximately half of the patients underwent additional testing (CT of chest and abdomen and mammography for women). Screening did not result in increased survival or fewer cancer-related deaths. 3.5 % of patients in the extensive screening group were diagnosed with malignancy in comparison to 2.4% in the limited screening group. During follow-up, cancer was diagnosed in 3.7% and 5.0% in the extensive and limited screening groups, respectively. The authors concluded that the low yield of extensive screening and lack of survival benefit did not warrant routinely ordering cancer screening tests above and beyond age-appropriate screening in patients with idiopathic VTE. However, it is known that identification of occult malignancy at an earlier stage of disease is beneficial, and cancer diagnosed within one year of an episode of VTE is generally more advanced and associated with a poorer prognosis [109].It is our practice to take a through history from patients with unprovoked clots particularly focusing on symptoms suggestive of an underlying cancer. We recommend that patients be up to date with all age-appropriate cancer screening.
Heparin-based products (rather than warfarin) are recommended for long-term treatment of cancer-associated DVT. Several trials, most prominently the CLOT trial, have demonstrated that LMWH is associated with reduced risk of recurrent VTE compared with warfarin in cancer patients [110].Fondaparinux may be a reasonable alternative if a patient is unable to tolerate a LMWH. In terms of treatment duration, patients with cancer-associated VTE should be anticoagulated indefinitely as long as they continue to have evidence of active malignancy and/or remain on antineoplastic treatment [111].
Heparin has potential anticancer effects beyond its anticoagulation properties. It is believed that heparin use in patients with cancer can influence cancer progression by acting as an antimetastatic agent. The molecular mechanisms underlying this significant observation are not completely understood, although the first documented benefit of these drugs dates back to the 1970s [112].Overall, LMWH have been associated with improved overall survival in cancer patients and this effect appears to be distinct from its ability to prevent life-threatening VTE episodes [113].
Estrogen-Related Thromboembolic Disease
Pregnancy is a well-established acquired hypercoagulable state, and thromboembolic disease accounts for significant morbidity and mortality in pregnancy and the postpartum period. Approximately 1 in 1000 women will suffer from a thrombotic event during pregnancy or shortly after delivery [8]. The etiology of the tendency to clot during pregnancy is multifactorial and mainly reflects venous stasis due to vasculature compression by the uterus, changes in coagulation factors as the pregnancy progresses, and endothelial damage during delivery, especially Cesarean section. Both factor VIII and von Willebrand factor levels increase, especially in the final months of pregnancy. Simultaneously, levels of the natural anticoagulant protein S diminish, leading to an acquired resistance to activated protein C which results in increased thrombin generation and therefore a hypercoagulable state [114].The risk of thrombosis in pregnancy is clearly heightened in women with inherited thrombophilias, especially in the postpartum period [115].
Similarly to pregnancy, hormone-based contraceptive agents and estrogen replacement therapies are also associated with increased thrombotic risk. Over the years, drug manufacturers have tried to mitigate the clotting risk associated with these drugs by reducing the amount of estrogen and altering the type of progesterone used, yet a risk still remains, resulting in a VTE incidence 2 to 7 times higher in this population [116].The risk is highest in the first 4 months of use and is unaffected by duration of use; risk extends for 3 months after cessation of estrogen-containing therapy. Patients who develop VTE while taking an oral contraceptive are generally instructed to stop the contraceptive and consider an alternative form of birth control. Although routine screening for thrombophilia is not offered to women before prescribing oral contraceptives, a thorough personal and family history regarding venous and arterial thrombotic events as well as recurrent pregnancy loss in women should be taken to evaluate thromboembolic risk factors. We generally avoid use of oral contraceptives in patients with a known hereditary thrombophilia, and consider screening prior to initiation of therapy in those with a strong family history of VTE.
Superficial VTE
Although the main disorders that comprise VTE are DVT and PE, another common presentation is superficial venous thromboembolism (SVT). The risk factors for developing an SVT are similar to those for DVT. In addition, varicose veins also increase the incidence of developing SVT [117].SVT is not associated with excessive mortality, and the main concern with it is progression to DVT. About 25% of patients diagnosed with SVT may have DVT or PE at the time of diagnosis and about 3% without DVT or PE at time of diagnosis developed one of these complications over the following 3 months; clot propagation is another common complication [118].Ultrasound may be of utility in diagnosing occult DVT in patients who initially diagnosed with SVT [119].
For patients who have only SVT at baseline without concomitant DVT or PE, it is difficult to determine which patients are at risk for developing DVT. Some risk stratification models include clot location. Since SVT clots usually develop in the saphenous vein, the clot would need to either progress from the sapheno-femoral junction to the common femoral vein; thus, any clots located near the sapheno-femoral junction are at risk of progressing into the deep vasculature [120].Clots within 3 cm of the junction may be more likely to progress to DVT [121].Chengelis and colleagues feel that proximal saphenous vein thrombosis should likely be treated with anticoagulation [122].Others have taken a more general approach, stating that all clots above the knee or in the thigh area should be treated aggressively [123].
There are solid data for the use of anticoagulation in SVT. In the STEFLUX (Superficial ThromboEmbolism and Fluxum) study, participants received the LMWH parnaparin at one of 3 doses: 8500 IU once daily for 10 days followed by placebo for 20 days, 8500 IU once daily for 10 days and then 6400 IU once daily for 20 days, or 4250 IU once daily for 30 days. Those who received the intermediate dosing had lower rates of DVT, PE, and relapse/SVT recurrence in the first 33 days [124].In the CALISTO trial, fondaparinux 2.5mg per day for 45 days effectively reduced the risk of symptomatic DVT, PE, or SVT recurrence or extension and was not associated with any increased major bleeding compared to placebo [125].A Cochrane review included 30 studies involving over 6500 participants with SVT of the lower extremities. The treatments used in these studies included fondaparinux, LMWH, UFH, non-steriodal anti-inflammatory agents, topical treatment, and surgery. According to the findings, use of fondaparinux at prophylactic dosing for 6 weeks is considered a valid therapeutic option for SVT [126].It is our practice to consider the use of anticoagulants (generally LMWH or fondaparinux) as part of the treatment regimen for SVT.
Target-Specific Oral Anticoagulants And Treatment of VTE
The direct thrombin inhibitor dabigatran directly binds to thrombin in a concentration-dependent manner [127].Peak plasma concentration is achieved within 0.5 to 2.0 hours after ingestion, and its half-life is 12 to 17 hours. Use of dabigatran in both primary and secondary prevention of VTE has been extensively studied, especially in orthopedic surgery where there have been 4 main trials (RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE I and II). While RE-MOBILIZE showed that dabigatran 220 mg or 150 mg once daily was inferior to enoxaparin 30 mg twice daily in preventing VTE after total knee arthroplasty, RE-MODEL and RE-NOVATE I and II demonstrated that dabigatran 150 mg or 220 mg once daily was noninferior to enoxaparin 40 mg once daily for prevention of VTE in patients undergoing total knee replacement and hip replacement [128–131].The side effect profile was also promising, with no significant differences in the frequency of major bleeding between dabigatran and enoxaparin. Pooled data and meta-analyses from these trials have demonstrated that for prevention of VTE associated with hip or knee surgery, dabigatran 220 mg or 150 mg once daily is as effective as 40 mg of enoxaparin given daily or 30 mg given twice a day, with a similar bleeding profile [132,133].
More recently, dabigatran been used in the acute treatment and secondary prevention of VTE. In the RE-COVER trial, dabigatran 150 mg twice daily was compared to warfarin (INR 2–3) in the treatment of acute VTE for 6 months, after an initial treatment period of up to 9 days with LMWH or UFH. Dabigatran was noninferior to warfarin with respect to 6-month incidence of recurrent symptomatic objectively confirmed VTE and related deaths, and was not associated with increased bleeding [134].In the RE-MEDY and RE-SONATE trials of extended anticoagulation, dabigatran was as effective as warfarin for prevention of recurrent VTE when continued after 3 months of initial anticoagulation and associated with less bleeding, and was more effective than placebo in preventing recurrent VTE but associated with a higher risk of bleeding [135].Unexpectedly, the risk of acute coronary syndrome was slightly higher in the dabigatran group than the warfarin group, as seen in other studies.
Rivaroxaban, a TSOAC that targets factor Xa, has also shown efficacy in preventing VTE after knee or hip surgery. The RE-CORD 1-4 studies all focused on the use of rivaroxaban in comparison to enoxaparin and found that rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily in prevention of VTE in total knee and total hip arthroplasty [136–138].Meta-analysis of multiple rivaroxaban VTE prophylaxis trials also demonstrated that rivaroxaban significantly lowered the risk of VTE in these surgical patients in comparison to the use of enoxaparin [139].Prophylactic use of rivaroxaban was also studied in acutely ill hospitalized patients in the MAGELLAN trial. Rivaroxaban 10 mg daily for 35 days was compared to enoxaparin 40 mg daily for 10 days followed by placebo and was found to be noninferior to enoxaparin in reduction of VTE risk at day 10 and superior to placebo at day 35 [140].However, the rate of bleeding, although low in both arms, was higher in the rivaroxaban arm.
Rivaroxaban has been studied in randomized clinical trials for acute treatment of DVT and PE and for extended prophylaxis for recurrent VTE (EINSTEIN-DVT, EINSTEIN-PE and EINSTEIN-Extension, respectively). The treatment strategy for use of rivaroxaban differed from that of dabigatran (in the RE-COVER trial), as rivaroxaban was used upfront as initial anticoagulation rather than after an initial period of parenteral therapy with LMWH or UFH. In both the DVT and PE trials, rivaroxaban was noninferior to standard treatment with enoxaparin followed by warfarin therapy, with no significant difference in major bleeding at 6 months of treatment [141,142].The extension trial also demonstrated that use of rivaroxaban in comparison to placebo for an additional 6 or 12 months after standard therapy was associated with significantly fewer recurrent VTE [141]. These studies led to FDA approval for rivaroxaban for primary prevention of VTE in patients undergoing elective total hip or knee repair surgery, for treatment of acute DVT or PE, and for extended prophylaxis in patients following initial treatment.
The anti-factor Xa TSOAC apixaban has been studied in similar fashion as rivaroxaban. In the AMPLIFY study, apixaban was given at a dose of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months (as monotherapy, without initial parenteral agent) and compared to enoxaparin followed by warfarin for treatment of acute VTE. Apixaban was as effective as warfarin in terms of recurrent symptomatic VTE or VTE-related death, and was associated with significantly fewer bleeding events [143].Extended-duration apixaban given at treatment dose (5 mg twice daily) or at prophylactic dose (2.5 mg twice daily) for 12 months after completion of treatment-dose apixaban for VTE demonstrated superiority to placebo for extended prophylaxis in AMPLIFY-EXT, and there was no increase in major bleeding compared to placebo [144].Apixaban was recently approved by the FDA for both treatment and secondary prophylaxis of VTE.
More recently, a third anti-factor Xa TSOAC edoxaban demonstrated noninferiority to warfarin in prevention of recurrent symptomatic VTE when administered to patients with DVT or PE at 60 mg once daily for 3 to 12 months [145].Edoxaban also led to significantly less bleeding than warfarin. Edoxaban was recently approved by the FDA for treatment of VTE.
These TSOACs show promise in treatment and prevention of VTE but should be used in patients who meet appropriate criteria for renal function, age, and bleeding risk, as there are currently no available antidotes to reverse their effects. If significant bleeding occurs and cannot be controlled by usual maneuvers such as mechanical compression or surgical intervention, there is little data to guide the use of pharmacologic interventions. Plasma dabigatran levels can be reduced through the use of hemodialysis [146].Antibodies capable of neutralizing dabigatran have been developed, and one specific antibody, idarucizumab, was well-tolerated and showed immediate and complete reversal of dabigatran in subjects of different age and renal function [147,148].Andexanet, a modified recombinant derivative of factor Xa with no catalytic activty, acts as a “decoy receptor” with higher affinity to factor Xa inhibitors than natural factor Xa. Phase II studies in healthy volunteers demonstrated that andexanet immediately reversed the anticoagulation activity of apixaban, rivaroxaban, enoxaparin, and most recently edoxaban without thrombotic consequences [149].Two randomized, double-blind, placebo-controlled phase III studies (ANNEXA-A, looking at the reversal of apixaban, and ANNEXA-R, looking at reversal of rivaroxaban) are underway, and preliminary results show that a single intravenous bolus of andexanet demonstrated almost complete reversal [150].Finally, aripazine (PER977), a synthetic small molecule that binds to heparins as well as all TSOACs, was shown in a phase II trial to decrease blood clotting time to within 10% above baseline value in 10 minutes or less with an effect lasting for 24 hours [151].
Some have advocated for use of prothrombin complex concentrate (PCC) or recombinant factor VIIa for reversal of TSOAC-associated bleeding. Rivaroxaban was demonstrated to be partially reversible by PCC, whereas this approach was not as successful for dabigatran in healthy volunteers [152].In vitro evidence, however, showed that PCC did not significantly change aPTT [153].At present, the use of nonspecific hemostatic agents (including recombinant factor VIIa, 4-factor prothrombin complex concentrate, and activated prothrombin complex concentrates) is suggested for reversal of TSOACs in patients who present with life-threatening bleeding [154,155].
Conclusion
Patients with VTE present with a wide range of findings and factors that impact management. Decision making in VTE management is a fluid process that should be re-evaluated as new data emerge and individual circumstances change. There is more focus on VTE prevention and treatment today than there was even a decade ago. Diagnostic algorithms, identification of new risk factors, refinement in understanding of the pathogenesis of thrombosis, and identification of new anticoagulants with more favorable risk-benefit profiles will all ultimately contribute to improved patient care.
Corresponding author: Jean M. Connors, MD, Brigham and Women's Hospital, 75 Francis St., Boston, MA 02215.
1. White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(Suppl 1):I4-8.
2. Moster KM and Fedullo PF. The diagnosis of deep-vein thrombosis. N Engl J Med 1994;330:863–4.
3. Kearon C. Natural history of venous thromboembolism. Circulation 2003;(Suppl 1):122–30.
4. Heijboer H, Brandjes DP, Buller HR, et al. Deficiencies of coagulation-inhibiting and fibrinolytic proteins in outpatients with deep-vein thrombosis. N Engl J Med 1990;323:1512–6.
5. Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:441–51.
6. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334–49.
7. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
9. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102.
10. Mahmoodi BK, Gansevoort RT, Naess IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964–71.
11. Landolfi R, Marchioli R, Patrono C. Mechanisms of bleeding and thrombosis in myeloproliferative disorders. Thromb Haemost 1997;78:617–21.
12. Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:1253–8.
13.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63.
14. Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008;6:1262–6.
15. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an aymptomatic patient population. J Vasc Surg 2004;39:937–43.
16. Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878–80.
17. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298.
18. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–80.
19. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88.
20. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost 2014;20:244–9.
21. Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52.
22. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722–7.
23. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost 2013;11:116-23.
24. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (Suppl 1): I9–16.
25. Clemetson KJ. Platelet GPIb-V-IX complex. Thromb Haemost 1997;78:266–70.
26. Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood 1989;74:1181–95.
27. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
28. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost 2000;416–20.
29. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–71.
30. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004;140:589–602.
31. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res 2010;125:e123–27.
32. Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med 2005;31:48–55.
33. Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med 2009;122:1050–3.
34. Chan WS, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
35. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357–61.
36. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 2014; 146:1444–51.
37. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004; 109 (Suppl 1): I9–14.
38. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102–10.
39. Tapson VF, Carroll BA, Davidson BL, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999;160:1043–66.
40. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e351S–418S.
41. Pezzullo JA, Perkins AB, Cronan JJ. Symptomatic deep vein thrombosis: diagnosis with limited compression US. Radiology 1996;198:67–70.
42. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations Radiology 1996;199:45–7.
43. Remy-Jardin M, Remy J, Deschidre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996;200:699–706.
44. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27.
45. Goodacre S. In the clinic. Deep venous thrombosis. Ann Intern Med 2008;149:ITC3–1.
46. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266–74.
47. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9.
48. Constans J, Salmi LR, Sevestre-Pietri MA, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost 2008;99:202–7.
49. Meyer G, Vicault E, Danasys T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11.
50. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-bline, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459–68.
51. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):7S–47S.
52. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998;3:41–6.
53. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2010;9:CD001100.
54. Buller HR, Davidson BL, Decousus H, et al. Fondaprinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 2004;140: 867–73.
55. Buller HR, Davidson BL, Decousus H, et al. Subcutaenous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–702.
56. Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112:e53–60.
57. Pon TK, Dager WE, Roberts AJ, White RH. Subcutaneous enoxaparin for therapeutic anticoagulation in hemodialysis patients. Thromb Res 2014;133:1023–8.
58. Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A. Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 2013;28:3090–5.
59. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–6.
60. Jiminez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–9.
61. Schulman S. Advances in the management of venous thromboembolism. Best Pract Res Clin Haematol 2012;25:361–77.
62. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost 2010;8:2412–7.
63. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–72.
64. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9.
65. Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007;146:211–22.
66. Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553–62.
67. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study group. N Engl J Med 1995;332:1661–5.
68. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036.
69. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-07.
70. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19–25.
71. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal duration Italian trial investigators. N Engl J Med 2001;345:165–9.
72. Bauer KA. Long-term management of venous thromboembolism: a 61-year old woman with unprovoked venous thromboembolism. JAMA 2011;305:1336–45.
73. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:
1794–801.
74. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):257S–298S.
75. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993;95:315–28.
76. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost 2008;100:26–31.
77. Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e419S–94S.
78. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010;138:1093–1100.
79. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9.
80. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
81. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–84.
82. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110:515–9.
83. Palareti G, Legnani C, Cosmi B, et al. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002; 87:7–12.
84. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9.
85. Bruinstroop E, Klok FA, Van De Ree MA, et al. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost 2009;611–8.
86. Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149:481–90.
87. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25.
88. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna Prediction Model. Circulation2010; 121:1630–6.
89. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014; 3: e000467.
90. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31.
91. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
92. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577–85.
93. Marcucci M, Iorio A, Douketis J. Management of patients with unprovoked venous thromboembolism: an evidence-based and practical approach. Curr Treat Options Cardiovasc Med 2013;15:224–39.
94. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425–34.
95. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631–9.
96. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979–87.
97. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67.
98. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130:1062–71.
99. Makris M. Thrombophilia: grading the risk. Blood 2009;113:5038–9.
100. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;30:2472.
101. Ho WK, Hankey GH, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006;166:729–36.
102. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
103. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2009; 7:1737–40.
104. Brandt JT, Barna LK, Triplett DA. Laboratory identification of lupus anticoagulants: results of the Second International Workship for identification of lupus anticoagulants. On behalf of the subcommittee on lupus anticoagulants/antiphospholipid antibodies of the ISTH. Thromb Haemost 1995;74: 1597–603.
105. Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol 2001;106:25-32.
106. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23.
107. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (Suppl 1): I17–21.
108. Van Doormaal FF, Terpstra W, Van Der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost 2011;9:79–84.
109. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50.
110. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
111. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402-29.
112. Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: “clinical study.” J Surg Oncol 1973;5:189–93.
113. Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost 2007;5:729–37.
114. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153–68.
115. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
116. Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol 2012;24:235-40.
117. Marchiori A, Mosena L, Prandoni P. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost 2006;32:737–43.
118. Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med 2010;152:218–24.
119. Galanaud JP, Genty C, Sevestre MA, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost 2011;105:31–9.
120. Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg 2001;193:556–62.
121. Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg 1992;164:269–75.
122. Chengelis DL, Benedick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
123. Verlato F, Zuccetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the leg. J Vasc Surg 1999;30:1113–5.
124. Cosmi B, Filippini M, Tonti D, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost 2012;10:1026–35.
125. Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32.
126. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
127. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303.
128. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9.
129. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85.
130. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007;370:949–56.
131. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, non-inferiority trial. Thromb Haemost2011;105:721–9.
132. Friedman RJ, Dahl OE, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res 2010;126:175–82.
133. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009;101:77–85.
134. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;36:2342–52.
135. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18.
136. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75.
137. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomized controlled trial. Lancet 2008;372:31–9.
138. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86.
139. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2010;66:1099–108.
140. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23.
141. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510.
142. EINSTEIN-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97.
143. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808.
144. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708.
145. Hokusai-VTE investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15.
146. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596–605.
147. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
148. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete, and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at the American Society of Hematology 2014 Annual Meeting; San Francisco, CA.
149. Crowther MA, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Abstract #4269. Presented at the American Society of Hematology 2014 Annual Meeting, San Francisco, CA.
150. Crowther M, Levy GG, Lu G, et al. ANNEXATM-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT 064445), a universal antidote for factor Xa (fa) inhibitors. Presented at the American Heart Association 2014 Annual Meeting, Chicago, IL.
151. Ansell JE, Bakhru SH, Lauliche BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141–2.
152. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9.
153. Korber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost 2014;20:735–40.
154. Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013;80:443–51.
155. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014;123:1152–8.
From the Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, MA.
Abstract
- Objective: To review the diagnosis and management of venous thromboembolism (VTE).
- Methods: Review of the literature.
- Results: VTE and its associated complications account for significant morbidity and mortality. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. Clinical decision rules have been developed that can help determine which patients warrant further testing. Anticoagulation, the mainstay of VTE treatment, increases bleeding risk, necessitating tailored treatment strategies that must incorporate etiology, risk, benefit, cost, and patient preference.
- Conclusion: Further study is needed to understand individual patient risks and to identify treatments that will lead to improved patient outcomes.
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 in Western countries develop VTE. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of those patients diagnosed with thrombotic events will die within the first month after diagnosis [1].PE is a common consequence of DVT; 40% of patients who are diagnosed with DVT will be subsequently found to have PE upon further imaging. This high rate of association is also seen in those who present with PE, 70% of whom will also be found to have concomitant DVT [2,3].
Anatomic risk factors include Paget-Schroetter syndrome (compression of upper extremity veins due to abnormalities at the thoracic outlet), May-Thurner syndrome (significant compression of the left common iliac vein by the right common iliac artery), and abnormalities of the inferior vena cava [14–16].Medications that are associated with increased risk of VTE include but are not limited to estrogen (both in oral contraceptives as well as hormone replacement therapy) [17,18],the selective estrogen receptor modulator tamoxifen [19],testosterone [20],and glucocorticoids [21].It is important to note that many patients with VTE have more than one acquired risk factor for thrombosis [22],and also that acquired risk factors are more likely to lead to VTE in the setting of underlying inherited thrombophilic conditions [23].
Pathogenesis
Abnormalities in both coagulation factors and the vascular bed are at the core of the pathogenesis of VTE. The multifaceted etiology of thrombosis was first described in 1856 by Virchow, who defined a triad of defects in the vessel wall, platelets, and coagulation proteins [24].Usually the vessel wall is lined with endothelial cells that provide a nonthrombotic surface and limit platelet aggregation through release of prostacyclins and nitric oxide. When the endothelial lining is compromised, the homeostatic surveillance system is disturbed and platelet activation and the coagulation system are initiated. Tissue factor exposure in the damaged area of the vessel leads to activation of the coagulation cascade. Collagen that is present in the area of the wound is also exposed and can activate platelets, which provide the phospholipid surface upon which the coagulation cascade occurs. Platelets initially tether to the exposed collagen through binding of glycoprotein Ib-V-IX in association with von Willebrand factor [25].The thrombus is initiated as more platelets are recruited to exposed collagen of the injured endothelium through aggregation in response to the binding of GPIIIb/IIa with fibrinogen. This process is self-perpetuating as these activated platelets release additional proteins such as adenosine diphosphate (ADP), serotonin, and thromboxane A2, all of which fuel the recruitment and activation of additional platelets [26].
Diagnosis
The key to decreasing the morbidity and mortality associated with VTE is timely diagnosis and early initiation of therapy. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. DVT is usually associated with pain in calf or thigh, unilateral swelling, tenderness, and redness. PE can present as chest pain, shortness of breath, syncope, hemoptysis, and/or cardiac palpitations.
Decision Rules
Clinical decision rules based on signs, symptoms, and risk factors have been developed to estimate the pretest probability of PE or DVT and to help determine which patients warrant further testing. These clinical decision rules include the Wells criteria (separate rules for DVT and PE) [27,28],as well as the Geneva score [29],which is focused on identifying patients with a likelihood of having a PE. In general, these clinical rules are applied at presentation to predict the risk of VTE, and patients who score high are evaluated by imaging modalities, while those with lower scores should be considered for further stratification based on D-dimer testing. The goal of clinical assessment and use of a decision rule is to identify patients at low risk of VTE to reduce the number of imaging studies performed. Most of the decision rules focus on the use of noninvasive evaluations that are easily implemented, including clinical history and presentation, abnormalities in oxygen saturation, chest radiography findings, and electro-cardiography.
D-Dimer Testing
D-dimer testing is at the core of all predictive models for VTE. D-dimer is a fibrin degradation product that is detectable in the blood during active fibrinolysis and occurs after clot formation. The concentration of D-dimer increases in patients with active clot. D-dimer testing is usually performed as a quantitative ELISA or automated turbidometric assay and is highly sensitive (> 95%) in excluding a diagnosis of VTE if results are in the normal range [30].The presence of a normal D-dimer and a low probability based on clinical assessment criteria can be integrated to determine which patients have a low (generally < 99%) likelihood of having VTE [31].It should be noted that other factors can lead to an increased D-dimer, including malignancy, trauma, critical illness, disseminated intravascular coagulation, pregnancy, infection, and postoperative status, which can produce false-positive results and cloud the utility of the test in excluding those at low risk of VTE from undergoing imaging [32–34].Additionally, D-dimer values naturally increase with age and recent work has shown utility of an age-adjusted D-dimer threshold, though this method is not yet widespread in clinical practice [35,36].
Imaging
After application of a clinical prediction rule, the mainstay of diagnosis of VTE is imaging. For DVT the use of ultrasonography is considered the gold standard, with both high sensitivity (89–100%) and specificity (86–100%), especially when the DVT is located proximally [37–39].We generally recommend compression ultrasound starting with the proximal veins but expanding to include the whole leg if the proximal studies are negative [40–42].Other diagnostic options include computed tomography (CT) venography, which is not first line as it is highly invasive and exposes the patient to iodine-based contrast dyes, and magnetic resonance venography (MRV), which offers superb visualization for diagnosis of pelvic vein thrombosis but is limited because of availability and cost issues.
Helical CT pulmonary angiography (CTPA) is the diagnostic test of choice in PE, with high sensitivity (96%) and specificity (95%), and has replaced conventional ventilation perfusion (VQ) scanning or other methods such as magnetic resonance pulmonary angiography in most settings [43,44].CTPA should be avoided in patients who have severe chronic kidney disease or a contrast allergy, and is often avoided in patients who are pregnant due to potential risk of radiation exposure, and in such situations VQ scanning may be employed.
Algorithmic Approach to Workup
Of note, there are multiple clinical situations in which the application of a clinical prediction rule followed by D-dimer testing and/or imaging cannot be “standardized” with such algorithms. These include situations where D-dimer may be falsely positive (as above), situations in which alternative imaging strategies should be used to avoid contrast exposure in workup of PE (as above), and workup of suspected upper extremity DVT. Upper extremity ultrasound comprises about 10% of all DVT and frequently occurs in the setting of risk factors such as central venous catheters or pacemakers; specific upper-extremity risk-assessment rules have been developed [47,48].
Acute Treatment Options
The first step in treatment is identification of patients who are at high risk of
In standard cases of DVT and PE without hemodynamic compromise, the current standard of care is to initiate parenteral anticoagulation. The immediate goal of therapy is to treat rapidly with anticoagulants to prevent the thrombus from propagating further and to prevent DVT from embolization to the lungs or other vascular beds. The initial treatment of VTE has been extensively discussed and guidelines have been established with recommendations for initiation of anticoagulation; the American College of Chest Physicians (ACCP) released the 9th edition of their guidelines in 2012 based on consensus agreements derived from primary data [51].
Heparin-based drugs are the mainstay of initial treatment. These drugs act by potentiating antithrombin and therefore inactivating thrombin and other coagulation factors such as Xa. Unfractionated heparin (UFH) can be administered as an initial bolus followed by a continuous infusion with dosing being based on weight and titrated to activated partial thromboplastin time (aPTT) or the anti-factor Xa level. Alternatively, patients may be treated with a low molecular weight heparin (LMWH) administered subcutaneously in fixed weight-adjusted doses, which obviates the need for monitoring in most cases [52].LMWHs work in a similar manner to UFH but have more anti-Xa activity in comparison to anti-thrombin activity. LMWH appears to be more effective than UFH for initial treatment of VTE and has been associated with lower risk of major hemorrhage [53].The options for treatment of VTE have expanded in recent years with the approval of fondparinux, a pentasaccharide specifically targeted to inhibit factor Xa. Fondaparinux has been shown to have similar efficacy to LMWH in patients with DVT [54],and while it has not been evaluated directly against LMWH for initial treatment of PE it has been shown to be at least as effective and safe as UFH [55].
Both LMWH and fondaparinux are cleared renally and therefore have increased bleeding risk in patients with renal impairment. In patients with creatinine clearance of less than 30 mL/min, dose reduction or lengthening of dosing interval may be appropriate. Anti-factor Xa activity can be used as a functional assay to monitor and titrate the level of anticoagulation in patients treated with UFH, LMWH, and fondaparinux. Monitoring is useful in the setting of impaired renal function (as above) in addition to extremes of body weight and pregnancy. When used for monitoring of UFH, the anti-factor Xa activity can be measured at any time during administration with a therapeutic goal range of 0.3–0.7 international units (IU)/mL. When used for LMWH, a “peak” anti-factor Xa should be measured approximately 4 hours after dosing, with therapeutic goals depending on preparation and schedule of treatment but generally between 0.6 to 1.0 IU/mL for twice daily and around 1.0 -2.0 IU/mL for once-daily [56].For patients on dialysis, we generally use intravenous UFH for acute treatment of VTE, though recent work has shown that enoxaparin (doses of 0.4 to 1 mg/kg/day) was as safe as UFH with respect to bleeding and was associated with shorter hospital length of stay [57].For long-term treatment of VTE, warfarin is generally preferred based on clinical experience with this agent, though small studies have suggested that parenteral agents may be useful alternatives to warfarin [58].
In many patients who are clinically stable without significant medical comorbidities, outpatient administration of these medications without hospitalization is considered safe. Patients with DVT are often safe to manage as outpatients unless significant clot burden is present and thrombolysis is being considered. For PE, the pulmonary embolism severity index (PESI) and simplified index (sPESI) may be useful to risk-stratify patients and identify those at low risk of complications who may be suitable for outpatient treatment [59,60].Studies have shown that hemodynamically stable patients who did not require supplemental oxygenation or have contraindications to LMWH therapy were safely managed as outpatients with low risk of recurrent VTE and bleeding [61,62].One exception may be patients with intermediate risk PE, who are hemodynamically stable but have evidence of right ventricular dysfunction and may be better served by an initial in-hospital observation period, especially if thrombolysis is being considered.
Most patients who present with VTE are transitioned to warfarin for long-term therapy. Warfarin can be started on the same day as parenteral anticoagulation. Both drugs are overlapped for at least 5 days, with a target INR of 2.0–3.0. Patients may achieve the target INR level quickly because factor VII has a short half-life and the level drops quickly; however, the overlap of 5 days is essential even when the INR is in the target range because a full anticoagulant affect is not achieved until prothrombin levels decline, and this is a slow process due to the long half-life of prothrombin. Warfarin also causes rapid decrease in levels of natural anticoagulants such as protein C and protein S, which further exacerbates the net hypercoagulable state in the short-term. Warfarin without a bridging parenteral agent carries a risk of warfarin-induced skin necrosis [63]and is not effective as an initial anticoagulant treatment in acute VTE as there is a relatively high risk of symptomatic clot extension or recurrent VTE compared to warfarin with use of a bridging agent [64].In specific cases such as cancer-associated VTE (see discussion below), LMWH is preferred to warfarin for long-term active therapy.
Long-Term Active Therapy After Acute Treatment
Duration of Anticoagulation
Recommended duration of anticoagulation depends on a myriad of factors including severity of VTE, risk of recurrence, bleeding risk, and lifestyle modification issues, as well as on the safety and availability of alternative therapies such as low-intensity warfarin, aspirin, or the new oral anticoagulants. The decision tree for length of treatment starts with whether the VTE was a provoked or a spontaneous event. Provoked events occur when the event is associated with an identifiable risk factor, such as immobilization from prolonged medical illness or surgical intervention, pregnancy or oral contraceptive use, and prolonged air travel.
Consensus guidelines suggest that 3 months of anti-coagulation are generally sufficient treatment for a provoked VTE [51,65,66]. Data from multiple studies and a meta-analysis suggests that less than 3 months of anticoagulation (4 to 6 weeks in most trials) is associated with an approximately 1.5-fold higher risk of recurrent VTE than 3 months [67,68].However, data from this meta-analysis also suggests that anticoagulation for longer than 3 months (6 to 12 months in most trials) is not associated with higher rates of recurrent VTE. We generally anticoagulate for 3 months in patients with provoked VTE.
Determining the duration of anticoagulation is more complex in patients with idiopathic/unprovoked VTE. Kearon and colleagues found that in patients with first idiopathic VTE, patients who were anticoagulated for 24 months versus 3 months had lower risk of recurrent VTE (1.3% per patient-year with 24 months versus 27.4% per patient-year with 3 months) [69].Similar studies and meta-analyses have demonstrated decreased recurrence rates in patients anticoagulated for a prolonged period of time. However, one study of prolonged anticoagulation revealed that at 3 years there was no difference in recurrence rate in patients with PE who were anticoagulated for 6 months versus 1 year [70].The likelihood of recurrent DVT in patients with first episode of idiopathic proximal DVT treated with either 3 months or 12 months of warfarin was similar after treatment was discontinued [71].Prolonged periods of anticoagulation do not directly influence risk of recurrence but instead may only delay occurrence of a second event [72].For that reason, the decision is essentially whether to anticoagulate for 3 months or to continue therapy indefinitely [73]. Current guidelines recommend continuing anticoagulation for 3 months in those at high risk of bleeding, and continuing for an extended duration in those at low or moderate bleeding risk [51]. Patients' values and perferences should be entertained and decisions made on a patient-by-patient basis.
For patients at high risk of recurrent VTE, we generally recommend indefinite anticoagulation unless the patient has a significantly elevated bleeding risk or strongly prefers to discontinue anticoagulation and compliance concerns are evident. High-risk patients are those who have suffered from multiple episodes of recurrent VTE, those who have clotted while being anticoagulated, and those with acquired risk factors, such as antiphospholipid antibodies and malignancy. Other high-risk groups are those with high-risk thrombophilias such as deficiency of protein S, protein C, or antithrombin, homozygous factor V Leiden or prothrombin gene mutations, and compound heterozygous factor V Leiden/prothrombin gene mutation in the setting of an unprovoked event. Further discussion of models for risk assessment of recurrence is provided below.
Assessment of Bleeding Risk
The bleeding risk associated with the use of anticoagulation must be weighed against the risk of clotting events when determining duration of anticoagulation, especially in those patients for whom indefinite anticoagulation is a consideration. Risk of bleeding while on anticoagulation is approximately 1–3% per 100 patient-years [74],but concomitant medical conditions such as renal failure, diabetes-related cerebrovascular disease, malignancy, advanced age, and use of antiplatelet agents all increase the risk of bleeding. Bleeding risk is highest when patients first initiate anticoagulation and is approximately 10 times the risk in the first month of therapy than after the first year of therapy [75].
Risk assessment models such as the RIETE score may be helpful when indefinite anticoagulation is a possibility [76].The RIETE score encompasses 6 risk factors (age > 75 years, recent bleeding, cancer, creatinine level > 1.2 mg/dL, anemia, or PE at baseline) to categorize patients into low risk (0 points, 0.3% risk of bleeding), intermediate risk (1–4 points, 2.6% risk of bleeding) and high risk (> 4 points, 6.2% risk of bleeding) within 3 months of anticoagulant therapy. The ACCP has developed a more extensive list of 17 potential risk factors for bleeding to categorize patients into low risk (no risk factors, 0.8%/year risk of bleeding), intermediate risk (1 risk factor, 1.6%/year risk of bleeding) and high risk (2 or more risk factors, >6.5%/year risk of bleeding) categories [77].The RIETE score is simpler to use but was not developed for assessing risk of bleeding during indefinite therapy, while the ACCP risk categorization predicts a yearly risk and is therefore applicable for long-term risk assessment but is more cumbersome to use. In practice, we generally use a clinical gestalt of a patient’s clinical risk factors (particularly age, renal or hepatic dysfunction, and frequent falls) to assess if they may be at high risk of bleeding and if the risk of indefinite anticoagulation may thus outweigh the potential benefit.
We also note that several scoring systems (HAS-BLED, HEMORR2HAGES, and ATRIA scores) have been developed to predict those at high risk of bleeding on anticoagulation for atrial fibrillation [78–80].These scores generally include similar clinical risk factors to those in the RIETE and ACCP scoring systems. Several studies have compared the HAS-BLED, HEMORR2HAGES, and ATRIA scores and a systematic review and meta-analysis concluded that the HAS-BLED score is recommended, due to increased sensitivity and ease of application [81].However, as these scores have not been validated for anticoagulation in the setting of VTE, we do not use them in this capacity.
Risk Stratification for Recurrent VTE
When predicting risk of recurrent VTE, clinical risk factors including obesity, male gender, and underlying thrombophilia (including the “high risk” inherited thrombophilias identified above) must taken into consideration. Location of the thrombus must also be considered; it has also been demonstrated that patients with DVT involving the iliofemoral veins are at higher risk of recurrence than those without iliac involvement [82].Other factors that may be useful in risk stratification include D-dimer level and ultrasound to search for residual venous thrombosis.
D-dimer Levels
D-dimer levels are one of the more promising methods for assessing the risk of recurrent VTE after cessation of anticoagulation, especially in the case of idiopathic VTE where indefinite anticoagulation should be considered but may pose either risk of bleeding or significant inconvenience to patients. A normal D-dimer measured 1 month after cessation of anticoagulation offers a high negative predictive value for risk of recurrence [83].A number of studies have demonstrated that patients with elevated D-dimer 1 month after anticoagulation cessation are at increased risk for a recurrent event [84–86].Two predictive models that have been developed incorporate D-dimer testing into decision making [87,88].The DASH predictive model relies on the D-dimer result in addition to age, male sex, and use of hormone therapy as a method of risk stratification for recurrent VTE in patients with a first unprovoked event. Using this scoring system, patients with a score of 0 or 1 had a recurrence rate of 3.1%, those with a score of 2 a recurrence rate of 6.4%, and those with a score of 3 or greater a recurrence rate of 12.3%. The authors postulate that by using this assessment scheme they can avoid lifelong anticoagulation in 51% of patients. The Vienna prediction model uses male sex, location of VTE (proximal DVT and PE are at higher risk), and D-dimer level to predict risk of recurrent VTE. This model has recently been updated to include a “dynamic” component to predict risk of recurrence of VTE from multiple random time points [89].
Overall, D-dimer may be useful for risk stratification. We often employ the method of stopping anticoagulation in patients with unprovoked VTE after 3 months (if the patient has no identifiable clinical risk factors that place them at high risk of recurrence) and testing D-dimer 1 month after cessation of anticoagulation. An elevated D-dimer is a solid reason to restart anticoagulation (potentially on an indefinite basis), while a negative D-dimer provides support for withholding further anticoagulation in the absence of other significant risk factors for recurrence. However, lack of agreement regarding assay cut-points as well as multiple reasons other than VTE for D-dimer elevation may limit widespread use of this method. We generally use a cutpoint of 250 ug/L as “negative,” though at least one study showed that cut-points of 250 ug/L versus 500 ug/L did not change the utility of this method [90].In our practice, risk prediction models are most useful to provide patients with additional information and a visual presentation to support our recommendation. This is particularly true of the Vienna prediction rule, which is available in a printable nomogram which can be distributed to patients and completed together during the clinic visit.
Imaging Analysis
Imaging analysis may also assist with risk stratification. Clinical assessment modules have been developed that incorporate repeat imaging studies for assessment of recannulization of affected veins. In patients with residual vein thrombosis (RVT) at the time anticoagulation was stopped, the hazard ratio for recurrence was 2.4 compared to those without RVT [91].There are a number of ways RVT could impact recurrence, including inpaired venous flow leading to stasis and activation of the coagulation cascade. Subsequent studies used serial ultrasound to determine when to stop anticoagulation. In one study, patients were anticoagulated for 3 months and for those that had RVT, anticoagulation was continued for up to 9 months for provoked and 21 months for unprovoked VTE. In comparison to fixed dosing of 6 months of anti-coagulation, those who had their length of anticoagulation tailored to ultrasonography findings had a lower rate of recurrent VTE [92].Limitations to using RVT in clinical decision-making include lack of a standard definition of RVT and variability in both timing of ultrasound (operator variability) and interpretation of results [93].
Other Options
Another option in patients who are being considered for indefinite anticoagulation is to decrease the intensity of anticoagulation. Since this would theoretically lower the risk of bleeding, the perceived benefit of long-term, low-intensity anticoagulation would be reduction in both bleeding and clotting risk. The PREVENT trial randomized patients who had received full-dose anticoagulation for a median of 6.5 months to either low-intensity warfarin (INR goal of 1.5-2.0 instead of 2.0-3.0) or placebo. In the anticoagulation group, there was a 64% risk reduction in recurrent VTE (hazard ratio 0.36, 95% CI 0.19 to 0.67) but an increased risk of bleeding (hazard ratio 1.92, 95% CI 1.26 to 2.93) [94].The ELATE study randomized patients with unprovoked VTE who had completed 3 or more months of full-intensity warfarin therapy (target INR 2.0–3.0) to continue therapy with either low-intensity warfarin (target INR 1.5–2.0) or full-intensity warfarin (target INR 2.0-3.0). Compared to the low-intensity group, the conventional-intensity group had lower rates of recurrent VTE and no increased rates of major bleeding [95].This study, however, has been criticized because of its overall low bleeding rate in both treatment groups.
Aspirin is an option in patients in whom long-term anticoagulation is untenable. The ASPIRE trial demonstrated that in patients with unprovoked VTE who had completed a course of initial anticoagulation, aspirin 100 mg daily reduced the risk of major vascular events compared to placebo with no increase in bleeding [96].However, aspirin was not associated with a significant reduction in risk of VTE alone (only the composite vascular event endpoint). The WARFASA trial, however, demonstrated that aspirin 100 mg daily was associated with a significant reduction in recurrent VTE compared to placebo after 6 to 18 months of anticoagulation without an increase in major bleeding [97].The absolute risk of recurrence was 11% in the placebo group and 5.9% in the aspirin group. More recently, the INSPIRE collaboration analyzed data from both trials and found that aspirin after initial anticoagulation reduced the risk of recurrent VTE by approximately 42% with a low rate of major bleeding [98].The absolute risk reduction was even larger in men and older patients. For this reason, we recommend aspirin to those patients in whom indefinite anticoagulation may be warranted from the standpoint of reducing risk of recurrent VTE but in whom the risk of bleeding precludes its use.
Hypercoagulable States In Specific Populations
Inherited Thrombophilias
Patients with a hereditary thrombophilia are at increased risk for incident VTE [99].These inherited mutations result in either a loss of normal anticoagulant function or gain of a prothrombotic state. Hereditary disorders associated with VTE include deficiency of antithrombin, protein C, or protein S, or the presence of factor V Leiden and/or prothrombin G20210A mutations. Although deficiency of protein C, protein S, or antithrombin is uncommon and affects only 0.5% of the population, these states have been associated with a 10-fold increased risk of thrombosis in comparison to the general population. Factor V Leiden and prothrombin gene mutation are less likely to be associated with incident thrombosis (2 to 5-fold increased risk of VTE) and are more prevalent in the Caucasian population [100].Though these hereditary thrombophilias increase risk of VTE, prophylactic anti-coagulation prior to a first VTE is not generally indicated.
Data regarding the impact of the inherited thrombophilias on risk of recurrent VTE is less well defined. While some data suggest that inherited thrombophilias are associated with increased risk of recurrent VTE, the degree of impact may be clinically modest especially in those with heterozygous factor V Leiden or prothrombin gene mutations [101].Ideally, a clinical trial would be designed to assess whether hereditary thrombophilia testing is beneficial for patients with VTE in decision-making regarding length of anticoagulation, type of anticoagulation, and risk of recurrence. If a patient with a low-risk inherited thrombophilia has a DVT in the setting of an additional provoking risk factor (surgery, pregnancy, etc), a 3-month course of anticoagulation followed by D-dimer assessment as above is reasonable. If a patient with an inherited thrombophilia experiences an idiopathic VTE, or if a patient with a “high-risk” thrombophilia as described above experiences any type of VTE, we generally recommend indefinite anticoagulation in the absence of high bleeding risk, though again this is a very patient-dependent choice.
Acquired Thrombophilias
Antiphospholipid Syndrome
Antibodies directed against proteins that bind phospho-lipids are associated with an acquired hypercoagulable state. The autoantibodies are categorized as antiphospho-lipid antibodies (APLAs), which include anticardiolipin antibodies (IgG and IgM), beta-2 glycoprotein 1 antibodies (anti-B2 GP), and lupus anticoagulant. These antibodies can form autonomously, as seen in primary disorders, or in association with autoimmune disease as a secondary disorder.
Criteria have been developed to distinguish antiphospholipid-associated clotting disorders from other forms of thrombophilia. The updated Sapporo criteria depend on both laboratory and clinical diagnostic criteria [102].The laboratory diagnosis of APLAs requires the presence of lupus anticoagulants, anticardiolipin antibodies, or anti-B2 GP on at least 2 assays at least 12 weeks apart with elevation above the 99th percentile of the testing laboratory’s normal distribution [103].Testing for lupus anticoagulant is based on 3 stages, the first of which is inhibition of phospholipid-dependent coagulation tests with prolonged clotting time (eg, aPTT or dilute Russell’s viper venom time). The diagnosis is confirmed by a secondary test in which excess hexagonal phase phospholipids are added to incubate with the patient’s plasma to absorb the APLA [104].The presence of anticardiolipin antibodies and anti beta-2 GP antibodies is determined using ELISA based immunoassays. Unlike most other thrombophilias, antiphospholipid syndrome is associated with both arterial and venous thromboembolic events and may be an indication for lifelong anticoagulation after a first thrombotic event. We generally recommend indefinite anticoagulation in the absence of significant bleeding risk.
Cancer-Associated Hypercoagulable State
Patients with cancer have a propensity for thromboembolic events. The underlying mechanisms responsible for cancer-associated clotting events are multifactorial and an area of intense research. Tumor cells can initiate activation of the clotting cascade through release of tissue factor and other pro-coagulant molecules [105].Type and stage of cancer impact risk of VTE, and the tumor itself can compress vasculature leading to venous stasis. Furthermore, chemotherapy, hormone therapy, antiangiogenic drugs, erythropoietin agents, and indwelling central venous catheters all are associated with increased risk of thrombotic events. Approximately 25% of all cancer patients will experience a thrombotic event during the course of their disease [106]. In fact, the presence of a spontaneous clot may be a harbinger of underlying malignancy [107].Approximately 10% of patients who present with an idiopathic VTE are diagnosed with cancer in the next 1 to 2 years.
The utility of extensive cancer screening in patients with spontaneous clotting events is often debated. The small studies that have addressed cancer associated clots have not demonstrated any mortality benefit with extensive screening. A prospective cohort study addressed the utility of limited versus extensive screening [108].In this study, all patients underwent a series of basic screening tests such as history taking, physical examination, chest radiograph, and basic laboratory parameters. Approximately half of the patients underwent additional testing (CT of chest and abdomen and mammography for women). Screening did not result in increased survival or fewer cancer-related deaths. 3.5 % of patients in the extensive screening group were diagnosed with malignancy in comparison to 2.4% in the limited screening group. During follow-up, cancer was diagnosed in 3.7% and 5.0% in the extensive and limited screening groups, respectively. The authors concluded that the low yield of extensive screening and lack of survival benefit did not warrant routinely ordering cancer screening tests above and beyond age-appropriate screening in patients with idiopathic VTE. However, it is known that identification of occult malignancy at an earlier stage of disease is beneficial, and cancer diagnosed within one year of an episode of VTE is generally more advanced and associated with a poorer prognosis [109].It is our practice to take a through history from patients with unprovoked clots particularly focusing on symptoms suggestive of an underlying cancer. We recommend that patients be up to date with all age-appropriate cancer screening.
Heparin-based products (rather than warfarin) are recommended for long-term treatment of cancer-associated DVT. Several trials, most prominently the CLOT trial, have demonstrated that LMWH is associated with reduced risk of recurrent VTE compared with warfarin in cancer patients [110].Fondaparinux may be a reasonable alternative if a patient is unable to tolerate a LMWH. In terms of treatment duration, patients with cancer-associated VTE should be anticoagulated indefinitely as long as they continue to have evidence of active malignancy and/or remain on antineoplastic treatment [111].
Heparin has potential anticancer effects beyond its anticoagulation properties. It is believed that heparin use in patients with cancer can influence cancer progression by acting as an antimetastatic agent. The molecular mechanisms underlying this significant observation are not completely understood, although the first documented benefit of these drugs dates back to the 1970s [112].Overall, LMWH have been associated with improved overall survival in cancer patients and this effect appears to be distinct from its ability to prevent life-threatening VTE episodes [113].
Estrogen-Related Thromboembolic Disease
Pregnancy is a well-established acquired hypercoagulable state, and thromboembolic disease accounts for significant morbidity and mortality in pregnancy and the postpartum period. Approximately 1 in 1000 women will suffer from a thrombotic event during pregnancy or shortly after delivery [8]. The etiology of the tendency to clot during pregnancy is multifactorial and mainly reflects venous stasis due to vasculature compression by the uterus, changes in coagulation factors as the pregnancy progresses, and endothelial damage during delivery, especially Cesarean section. Both factor VIII and von Willebrand factor levels increase, especially in the final months of pregnancy. Simultaneously, levels of the natural anticoagulant protein S diminish, leading to an acquired resistance to activated protein C which results in increased thrombin generation and therefore a hypercoagulable state [114].The risk of thrombosis in pregnancy is clearly heightened in women with inherited thrombophilias, especially in the postpartum period [115].
Similarly to pregnancy, hormone-based contraceptive agents and estrogen replacement therapies are also associated with increased thrombotic risk. Over the years, drug manufacturers have tried to mitigate the clotting risk associated with these drugs by reducing the amount of estrogen and altering the type of progesterone used, yet a risk still remains, resulting in a VTE incidence 2 to 7 times higher in this population [116].The risk is highest in the first 4 months of use and is unaffected by duration of use; risk extends for 3 months after cessation of estrogen-containing therapy. Patients who develop VTE while taking an oral contraceptive are generally instructed to stop the contraceptive and consider an alternative form of birth control. Although routine screening for thrombophilia is not offered to women before prescribing oral contraceptives, a thorough personal and family history regarding venous and arterial thrombotic events as well as recurrent pregnancy loss in women should be taken to evaluate thromboembolic risk factors. We generally avoid use of oral contraceptives in patients with a known hereditary thrombophilia, and consider screening prior to initiation of therapy in those with a strong family history of VTE.
Superficial VTE
Although the main disorders that comprise VTE are DVT and PE, another common presentation is superficial venous thromboembolism (SVT). The risk factors for developing an SVT are similar to those for DVT. In addition, varicose veins also increase the incidence of developing SVT [117].SVT is not associated with excessive mortality, and the main concern with it is progression to DVT. About 25% of patients diagnosed with SVT may have DVT or PE at the time of diagnosis and about 3% without DVT or PE at time of diagnosis developed one of these complications over the following 3 months; clot propagation is another common complication [118].Ultrasound may be of utility in diagnosing occult DVT in patients who initially diagnosed with SVT [119].
For patients who have only SVT at baseline without concomitant DVT or PE, it is difficult to determine which patients are at risk for developing DVT. Some risk stratification models include clot location. Since SVT clots usually develop in the saphenous vein, the clot would need to either progress from the sapheno-femoral junction to the common femoral vein; thus, any clots located near the sapheno-femoral junction are at risk of progressing into the deep vasculature [120].Clots within 3 cm of the junction may be more likely to progress to DVT [121].Chengelis and colleagues feel that proximal saphenous vein thrombosis should likely be treated with anticoagulation [122].Others have taken a more general approach, stating that all clots above the knee or in the thigh area should be treated aggressively [123].
There are solid data for the use of anticoagulation in SVT. In the STEFLUX (Superficial ThromboEmbolism and Fluxum) study, participants received the LMWH parnaparin at one of 3 doses: 8500 IU once daily for 10 days followed by placebo for 20 days, 8500 IU once daily for 10 days and then 6400 IU once daily for 20 days, or 4250 IU once daily for 30 days. Those who received the intermediate dosing had lower rates of DVT, PE, and relapse/SVT recurrence in the first 33 days [124].In the CALISTO trial, fondaparinux 2.5mg per day for 45 days effectively reduced the risk of symptomatic DVT, PE, or SVT recurrence or extension and was not associated with any increased major bleeding compared to placebo [125].A Cochrane review included 30 studies involving over 6500 participants with SVT of the lower extremities. The treatments used in these studies included fondaparinux, LMWH, UFH, non-steriodal anti-inflammatory agents, topical treatment, and surgery. According to the findings, use of fondaparinux at prophylactic dosing for 6 weeks is considered a valid therapeutic option for SVT [126].It is our practice to consider the use of anticoagulants (generally LMWH or fondaparinux) as part of the treatment regimen for SVT.
Target-Specific Oral Anticoagulants And Treatment of VTE
The direct thrombin inhibitor dabigatran directly binds to thrombin in a concentration-dependent manner [127].Peak plasma concentration is achieved within 0.5 to 2.0 hours after ingestion, and its half-life is 12 to 17 hours. Use of dabigatran in both primary and secondary prevention of VTE has been extensively studied, especially in orthopedic surgery where there have been 4 main trials (RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE I and II). While RE-MOBILIZE showed that dabigatran 220 mg or 150 mg once daily was inferior to enoxaparin 30 mg twice daily in preventing VTE after total knee arthroplasty, RE-MODEL and RE-NOVATE I and II demonstrated that dabigatran 150 mg or 220 mg once daily was noninferior to enoxaparin 40 mg once daily for prevention of VTE in patients undergoing total knee replacement and hip replacement [128–131].The side effect profile was also promising, with no significant differences in the frequency of major bleeding between dabigatran and enoxaparin. Pooled data and meta-analyses from these trials have demonstrated that for prevention of VTE associated with hip or knee surgery, dabigatran 220 mg or 150 mg once daily is as effective as 40 mg of enoxaparin given daily or 30 mg given twice a day, with a similar bleeding profile [132,133].
More recently, dabigatran been used in the acute treatment and secondary prevention of VTE. In the RE-COVER trial, dabigatran 150 mg twice daily was compared to warfarin (INR 2–3) in the treatment of acute VTE for 6 months, after an initial treatment period of up to 9 days with LMWH or UFH. Dabigatran was noninferior to warfarin with respect to 6-month incidence of recurrent symptomatic objectively confirmed VTE and related deaths, and was not associated with increased bleeding [134].In the RE-MEDY and RE-SONATE trials of extended anticoagulation, dabigatran was as effective as warfarin for prevention of recurrent VTE when continued after 3 months of initial anticoagulation and associated with less bleeding, and was more effective than placebo in preventing recurrent VTE but associated with a higher risk of bleeding [135].Unexpectedly, the risk of acute coronary syndrome was slightly higher in the dabigatran group than the warfarin group, as seen in other studies.
Rivaroxaban, a TSOAC that targets factor Xa, has also shown efficacy in preventing VTE after knee or hip surgery. The RE-CORD 1-4 studies all focused on the use of rivaroxaban in comparison to enoxaparin and found that rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily in prevention of VTE in total knee and total hip arthroplasty [136–138].Meta-analysis of multiple rivaroxaban VTE prophylaxis trials also demonstrated that rivaroxaban significantly lowered the risk of VTE in these surgical patients in comparison to the use of enoxaparin [139].Prophylactic use of rivaroxaban was also studied in acutely ill hospitalized patients in the MAGELLAN trial. Rivaroxaban 10 mg daily for 35 days was compared to enoxaparin 40 mg daily for 10 days followed by placebo and was found to be noninferior to enoxaparin in reduction of VTE risk at day 10 and superior to placebo at day 35 [140].However, the rate of bleeding, although low in both arms, was higher in the rivaroxaban arm.
Rivaroxaban has been studied in randomized clinical trials for acute treatment of DVT and PE and for extended prophylaxis for recurrent VTE (EINSTEIN-DVT, EINSTEIN-PE and EINSTEIN-Extension, respectively). The treatment strategy for use of rivaroxaban differed from that of dabigatran (in the RE-COVER trial), as rivaroxaban was used upfront as initial anticoagulation rather than after an initial period of parenteral therapy with LMWH or UFH. In both the DVT and PE trials, rivaroxaban was noninferior to standard treatment with enoxaparin followed by warfarin therapy, with no significant difference in major bleeding at 6 months of treatment [141,142].The extension trial also demonstrated that use of rivaroxaban in comparison to placebo for an additional 6 or 12 months after standard therapy was associated with significantly fewer recurrent VTE [141]. These studies led to FDA approval for rivaroxaban for primary prevention of VTE in patients undergoing elective total hip or knee repair surgery, for treatment of acute DVT or PE, and for extended prophylaxis in patients following initial treatment.
The anti-factor Xa TSOAC apixaban has been studied in similar fashion as rivaroxaban. In the AMPLIFY study, apixaban was given at a dose of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months (as monotherapy, without initial parenteral agent) and compared to enoxaparin followed by warfarin for treatment of acute VTE. Apixaban was as effective as warfarin in terms of recurrent symptomatic VTE or VTE-related death, and was associated with significantly fewer bleeding events [143].Extended-duration apixaban given at treatment dose (5 mg twice daily) or at prophylactic dose (2.5 mg twice daily) for 12 months after completion of treatment-dose apixaban for VTE demonstrated superiority to placebo for extended prophylaxis in AMPLIFY-EXT, and there was no increase in major bleeding compared to placebo [144].Apixaban was recently approved by the FDA for both treatment and secondary prophylaxis of VTE.
More recently, a third anti-factor Xa TSOAC edoxaban demonstrated noninferiority to warfarin in prevention of recurrent symptomatic VTE when administered to patients with DVT or PE at 60 mg once daily for 3 to 12 months [145].Edoxaban also led to significantly less bleeding than warfarin. Edoxaban was recently approved by the FDA for treatment of VTE.
These TSOACs show promise in treatment and prevention of VTE but should be used in patients who meet appropriate criteria for renal function, age, and bleeding risk, as there are currently no available antidotes to reverse their effects. If significant bleeding occurs and cannot be controlled by usual maneuvers such as mechanical compression or surgical intervention, there is little data to guide the use of pharmacologic interventions. Plasma dabigatran levels can be reduced through the use of hemodialysis [146].Antibodies capable of neutralizing dabigatran have been developed, and one specific antibody, idarucizumab, was well-tolerated and showed immediate and complete reversal of dabigatran in subjects of different age and renal function [147,148].Andexanet, a modified recombinant derivative of factor Xa with no catalytic activty, acts as a “decoy receptor” with higher affinity to factor Xa inhibitors than natural factor Xa. Phase II studies in healthy volunteers demonstrated that andexanet immediately reversed the anticoagulation activity of apixaban, rivaroxaban, enoxaparin, and most recently edoxaban without thrombotic consequences [149].Two randomized, double-blind, placebo-controlled phase III studies (ANNEXA-A, looking at the reversal of apixaban, and ANNEXA-R, looking at reversal of rivaroxaban) are underway, and preliminary results show that a single intravenous bolus of andexanet demonstrated almost complete reversal [150].Finally, aripazine (PER977), a synthetic small molecule that binds to heparins as well as all TSOACs, was shown in a phase II trial to decrease blood clotting time to within 10% above baseline value in 10 minutes or less with an effect lasting for 24 hours [151].
Some have advocated for use of prothrombin complex concentrate (PCC) or recombinant factor VIIa for reversal of TSOAC-associated bleeding. Rivaroxaban was demonstrated to be partially reversible by PCC, whereas this approach was not as successful for dabigatran in healthy volunteers [152].In vitro evidence, however, showed that PCC did not significantly change aPTT [153].At present, the use of nonspecific hemostatic agents (including recombinant factor VIIa, 4-factor prothrombin complex concentrate, and activated prothrombin complex concentrates) is suggested for reversal of TSOACs in patients who present with life-threatening bleeding [154,155].
Conclusion
Patients with VTE present with a wide range of findings and factors that impact management. Decision making in VTE management is a fluid process that should be re-evaluated as new data emerge and individual circumstances change. There is more focus on VTE prevention and treatment today than there was even a decade ago. Diagnostic algorithms, identification of new risk factors, refinement in understanding of the pathogenesis of thrombosis, and identification of new anticoagulants with more favorable risk-benefit profiles will all ultimately contribute to improved patient care.
Corresponding author: Jean M. Connors, MD, Brigham and Women's Hospital, 75 Francis St., Boston, MA 02215.
From the Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, MA.
Abstract
- Objective: To review the diagnosis and management of venous thromboembolism (VTE).
- Methods: Review of the literature.
- Results: VTE and its associated complications account for significant morbidity and mortality. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. Clinical decision rules have been developed that can help determine which patients warrant further testing. Anticoagulation, the mainstay of VTE treatment, increases bleeding risk, necessitating tailored treatment strategies that must incorporate etiology, risk, benefit, cost, and patient preference.
- Conclusion: Further study is needed to understand individual patient risks and to identify treatments that will lead to improved patient outcomes.
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 in Western countries develop VTE. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of those patients diagnosed with thrombotic events will die within the first month after diagnosis [1].PE is a common consequence of DVT; 40% of patients who are diagnosed with DVT will be subsequently found to have PE upon further imaging. This high rate of association is also seen in those who present with PE, 70% of whom will also be found to have concomitant DVT [2,3].
Anatomic risk factors include Paget-Schroetter syndrome (compression of upper extremity veins due to abnormalities at the thoracic outlet), May-Thurner syndrome (significant compression of the left common iliac vein by the right common iliac artery), and abnormalities of the inferior vena cava [14–16].Medications that are associated with increased risk of VTE include but are not limited to estrogen (both in oral contraceptives as well as hormone replacement therapy) [17,18],the selective estrogen receptor modulator tamoxifen [19],testosterone [20],and glucocorticoids [21].It is important to note that many patients with VTE have more than one acquired risk factor for thrombosis [22],and also that acquired risk factors are more likely to lead to VTE in the setting of underlying inherited thrombophilic conditions [23].
Pathogenesis
Abnormalities in both coagulation factors and the vascular bed are at the core of the pathogenesis of VTE. The multifaceted etiology of thrombosis was first described in 1856 by Virchow, who defined a triad of defects in the vessel wall, platelets, and coagulation proteins [24].Usually the vessel wall is lined with endothelial cells that provide a nonthrombotic surface and limit platelet aggregation through release of prostacyclins and nitric oxide. When the endothelial lining is compromised, the homeostatic surveillance system is disturbed and platelet activation and the coagulation system are initiated. Tissue factor exposure in the damaged area of the vessel leads to activation of the coagulation cascade. Collagen that is present in the area of the wound is also exposed and can activate platelets, which provide the phospholipid surface upon which the coagulation cascade occurs. Platelets initially tether to the exposed collagen through binding of glycoprotein Ib-V-IX in association with von Willebrand factor [25].The thrombus is initiated as more platelets are recruited to exposed collagen of the injured endothelium through aggregation in response to the binding of GPIIIb/IIa with fibrinogen. This process is self-perpetuating as these activated platelets release additional proteins such as adenosine diphosphate (ADP), serotonin, and thromboxane A2, all of which fuel the recruitment and activation of additional platelets [26].
Diagnosis
The key to decreasing the morbidity and mortality associated with VTE is timely diagnosis and early initiation of therapy. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. DVT is usually associated with pain in calf or thigh, unilateral swelling, tenderness, and redness. PE can present as chest pain, shortness of breath, syncope, hemoptysis, and/or cardiac palpitations.
Decision Rules
Clinical decision rules based on signs, symptoms, and risk factors have been developed to estimate the pretest probability of PE or DVT and to help determine which patients warrant further testing. These clinical decision rules include the Wells criteria (separate rules for DVT and PE) [27,28],as well as the Geneva score [29],which is focused on identifying patients with a likelihood of having a PE. In general, these clinical rules are applied at presentation to predict the risk of VTE, and patients who score high are evaluated by imaging modalities, while those with lower scores should be considered for further stratification based on D-dimer testing. The goal of clinical assessment and use of a decision rule is to identify patients at low risk of VTE to reduce the number of imaging studies performed. Most of the decision rules focus on the use of noninvasive evaluations that are easily implemented, including clinical history and presentation, abnormalities in oxygen saturation, chest radiography findings, and electro-cardiography.
D-Dimer Testing
D-dimer testing is at the core of all predictive models for VTE. D-dimer is a fibrin degradation product that is detectable in the blood during active fibrinolysis and occurs after clot formation. The concentration of D-dimer increases in patients with active clot. D-dimer testing is usually performed as a quantitative ELISA or automated turbidometric assay and is highly sensitive (> 95%) in excluding a diagnosis of VTE if results are in the normal range [30].The presence of a normal D-dimer and a low probability based on clinical assessment criteria can be integrated to determine which patients have a low (generally < 99%) likelihood of having VTE [31].It should be noted that other factors can lead to an increased D-dimer, including malignancy, trauma, critical illness, disseminated intravascular coagulation, pregnancy, infection, and postoperative status, which can produce false-positive results and cloud the utility of the test in excluding those at low risk of VTE from undergoing imaging [32–34].Additionally, D-dimer values naturally increase with age and recent work has shown utility of an age-adjusted D-dimer threshold, though this method is not yet widespread in clinical practice [35,36].
Imaging
After application of a clinical prediction rule, the mainstay of diagnosis of VTE is imaging. For DVT the use of ultrasonography is considered the gold standard, with both high sensitivity (89–100%) and specificity (86–100%), especially when the DVT is located proximally [37–39].We generally recommend compression ultrasound starting with the proximal veins but expanding to include the whole leg if the proximal studies are negative [40–42].Other diagnostic options include computed tomography (CT) venography, which is not first line as it is highly invasive and exposes the patient to iodine-based contrast dyes, and magnetic resonance venography (MRV), which offers superb visualization for diagnosis of pelvic vein thrombosis but is limited because of availability and cost issues.
Helical CT pulmonary angiography (CTPA) is the diagnostic test of choice in PE, with high sensitivity (96%) and specificity (95%), and has replaced conventional ventilation perfusion (VQ) scanning or other methods such as magnetic resonance pulmonary angiography in most settings [43,44].CTPA should be avoided in patients who have severe chronic kidney disease or a contrast allergy, and is often avoided in patients who are pregnant due to potential risk of radiation exposure, and in such situations VQ scanning may be employed.
Algorithmic Approach to Workup
Of note, there are multiple clinical situations in which the application of a clinical prediction rule followed by D-dimer testing and/or imaging cannot be “standardized” with such algorithms. These include situations where D-dimer may be falsely positive (as above), situations in which alternative imaging strategies should be used to avoid contrast exposure in workup of PE (as above), and workup of suspected upper extremity DVT. Upper extremity ultrasound comprises about 10% of all DVT and frequently occurs in the setting of risk factors such as central venous catheters or pacemakers; specific upper-extremity risk-assessment rules have been developed [47,48].
Acute Treatment Options
The first step in treatment is identification of patients who are at high risk of
In standard cases of DVT and PE without hemodynamic compromise, the current standard of care is to initiate parenteral anticoagulation. The immediate goal of therapy is to treat rapidly with anticoagulants to prevent the thrombus from propagating further and to prevent DVT from embolization to the lungs or other vascular beds. The initial treatment of VTE has been extensively discussed and guidelines have been established with recommendations for initiation of anticoagulation; the American College of Chest Physicians (ACCP) released the 9th edition of their guidelines in 2012 based on consensus agreements derived from primary data [51].
Heparin-based drugs are the mainstay of initial treatment. These drugs act by potentiating antithrombin and therefore inactivating thrombin and other coagulation factors such as Xa. Unfractionated heparin (UFH) can be administered as an initial bolus followed by a continuous infusion with dosing being based on weight and titrated to activated partial thromboplastin time (aPTT) or the anti-factor Xa level. Alternatively, patients may be treated with a low molecular weight heparin (LMWH) administered subcutaneously in fixed weight-adjusted doses, which obviates the need for monitoring in most cases [52].LMWHs work in a similar manner to UFH but have more anti-Xa activity in comparison to anti-thrombin activity. LMWH appears to be more effective than UFH for initial treatment of VTE and has been associated with lower risk of major hemorrhage [53].The options for treatment of VTE have expanded in recent years with the approval of fondparinux, a pentasaccharide specifically targeted to inhibit factor Xa. Fondaparinux has been shown to have similar efficacy to LMWH in patients with DVT [54],and while it has not been evaluated directly against LMWH for initial treatment of PE it has been shown to be at least as effective and safe as UFH [55].
Both LMWH and fondaparinux are cleared renally and therefore have increased bleeding risk in patients with renal impairment. In patients with creatinine clearance of less than 30 mL/min, dose reduction or lengthening of dosing interval may be appropriate. Anti-factor Xa activity can be used as a functional assay to monitor and titrate the level of anticoagulation in patients treated with UFH, LMWH, and fondaparinux. Monitoring is useful in the setting of impaired renal function (as above) in addition to extremes of body weight and pregnancy. When used for monitoring of UFH, the anti-factor Xa activity can be measured at any time during administration with a therapeutic goal range of 0.3–0.7 international units (IU)/mL. When used for LMWH, a “peak” anti-factor Xa should be measured approximately 4 hours after dosing, with therapeutic goals depending on preparation and schedule of treatment but generally between 0.6 to 1.0 IU/mL for twice daily and around 1.0 -2.0 IU/mL for once-daily [56].For patients on dialysis, we generally use intravenous UFH for acute treatment of VTE, though recent work has shown that enoxaparin (doses of 0.4 to 1 mg/kg/day) was as safe as UFH with respect to bleeding and was associated with shorter hospital length of stay [57].For long-term treatment of VTE, warfarin is generally preferred based on clinical experience with this agent, though small studies have suggested that parenteral agents may be useful alternatives to warfarin [58].
In many patients who are clinically stable without significant medical comorbidities, outpatient administration of these medications without hospitalization is considered safe. Patients with DVT are often safe to manage as outpatients unless significant clot burden is present and thrombolysis is being considered. For PE, the pulmonary embolism severity index (PESI) and simplified index (sPESI) may be useful to risk-stratify patients and identify those at low risk of complications who may be suitable for outpatient treatment [59,60].Studies have shown that hemodynamically stable patients who did not require supplemental oxygenation or have contraindications to LMWH therapy were safely managed as outpatients with low risk of recurrent VTE and bleeding [61,62].One exception may be patients with intermediate risk PE, who are hemodynamically stable but have evidence of right ventricular dysfunction and may be better served by an initial in-hospital observation period, especially if thrombolysis is being considered.
Most patients who present with VTE are transitioned to warfarin for long-term therapy. Warfarin can be started on the same day as parenteral anticoagulation. Both drugs are overlapped for at least 5 days, with a target INR of 2.0–3.0. Patients may achieve the target INR level quickly because factor VII has a short half-life and the level drops quickly; however, the overlap of 5 days is essential even when the INR is in the target range because a full anticoagulant affect is not achieved until prothrombin levels decline, and this is a slow process due to the long half-life of prothrombin. Warfarin also causes rapid decrease in levels of natural anticoagulants such as protein C and protein S, which further exacerbates the net hypercoagulable state in the short-term. Warfarin without a bridging parenteral agent carries a risk of warfarin-induced skin necrosis [63]and is not effective as an initial anticoagulant treatment in acute VTE as there is a relatively high risk of symptomatic clot extension or recurrent VTE compared to warfarin with use of a bridging agent [64].In specific cases such as cancer-associated VTE (see discussion below), LMWH is preferred to warfarin for long-term active therapy.
Long-Term Active Therapy After Acute Treatment
Duration of Anticoagulation
Recommended duration of anticoagulation depends on a myriad of factors including severity of VTE, risk of recurrence, bleeding risk, and lifestyle modification issues, as well as on the safety and availability of alternative therapies such as low-intensity warfarin, aspirin, or the new oral anticoagulants. The decision tree for length of treatment starts with whether the VTE was a provoked or a spontaneous event. Provoked events occur when the event is associated with an identifiable risk factor, such as immobilization from prolonged medical illness or surgical intervention, pregnancy or oral contraceptive use, and prolonged air travel.
Consensus guidelines suggest that 3 months of anti-coagulation are generally sufficient treatment for a provoked VTE [51,65,66]. Data from multiple studies and a meta-analysis suggests that less than 3 months of anticoagulation (4 to 6 weeks in most trials) is associated with an approximately 1.5-fold higher risk of recurrent VTE than 3 months [67,68].However, data from this meta-analysis also suggests that anticoagulation for longer than 3 months (6 to 12 months in most trials) is not associated with higher rates of recurrent VTE. We generally anticoagulate for 3 months in patients with provoked VTE.
Determining the duration of anticoagulation is more complex in patients with idiopathic/unprovoked VTE. Kearon and colleagues found that in patients with first idiopathic VTE, patients who were anticoagulated for 24 months versus 3 months had lower risk of recurrent VTE (1.3% per patient-year with 24 months versus 27.4% per patient-year with 3 months) [69].Similar studies and meta-analyses have demonstrated decreased recurrence rates in patients anticoagulated for a prolonged period of time. However, one study of prolonged anticoagulation revealed that at 3 years there was no difference in recurrence rate in patients with PE who were anticoagulated for 6 months versus 1 year [70].The likelihood of recurrent DVT in patients with first episode of idiopathic proximal DVT treated with either 3 months or 12 months of warfarin was similar after treatment was discontinued [71].Prolonged periods of anticoagulation do not directly influence risk of recurrence but instead may only delay occurrence of a second event [72].For that reason, the decision is essentially whether to anticoagulate for 3 months or to continue therapy indefinitely [73]. Current guidelines recommend continuing anticoagulation for 3 months in those at high risk of bleeding, and continuing for an extended duration in those at low or moderate bleeding risk [51]. Patients' values and perferences should be entertained and decisions made on a patient-by-patient basis.
For patients at high risk of recurrent VTE, we generally recommend indefinite anticoagulation unless the patient has a significantly elevated bleeding risk or strongly prefers to discontinue anticoagulation and compliance concerns are evident. High-risk patients are those who have suffered from multiple episodes of recurrent VTE, those who have clotted while being anticoagulated, and those with acquired risk factors, such as antiphospholipid antibodies and malignancy. Other high-risk groups are those with high-risk thrombophilias such as deficiency of protein S, protein C, or antithrombin, homozygous factor V Leiden or prothrombin gene mutations, and compound heterozygous factor V Leiden/prothrombin gene mutation in the setting of an unprovoked event. Further discussion of models for risk assessment of recurrence is provided below.
Assessment of Bleeding Risk
The bleeding risk associated with the use of anticoagulation must be weighed against the risk of clotting events when determining duration of anticoagulation, especially in those patients for whom indefinite anticoagulation is a consideration. Risk of bleeding while on anticoagulation is approximately 1–3% per 100 patient-years [74],but concomitant medical conditions such as renal failure, diabetes-related cerebrovascular disease, malignancy, advanced age, and use of antiplatelet agents all increase the risk of bleeding. Bleeding risk is highest when patients first initiate anticoagulation and is approximately 10 times the risk in the first month of therapy than after the first year of therapy [75].
Risk assessment models such as the RIETE score may be helpful when indefinite anticoagulation is a possibility [76].The RIETE score encompasses 6 risk factors (age > 75 years, recent bleeding, cancer, creatinine level > 1.2 mg/dL, anemia, or PE at baseline) to categorize patients into low risk (0 points, 0.3% risk of bleeding), intermediate risk (1–4 points, 2.6% risk of bleeding) and high risk (> 4 points, 6.2% risk of bleeding) within 3 months of anticoagulant therapy. The ACCP has developed a more extensive list of 17 potential risk factors for bleeding to categorize patients into low risk (no risk factors, 0.8%/year risk of bleeding), intermediate risk (1 risk factor, 1.6%/year risk of bleeding) and high risk (2 or more risk factors, >6.5%/year risk of bleeding) categories [77].The RIETE score is simpler to use but was not developed for assessing risk of bleeding during indefinite therapy, while the ACCP risk categorization predicts a yearly risk and is therefore applicable for long-term risk assessment but is more cumbersome to use. In practice, we generally use a clinical gestalt of a patient’s clinical risk factors (particularly age, renal or hepatic dysfunction, and frequent falls) to assess if they may be at high risk of bleeding and if the risk of indefinite anticoagulation may thus outweigh the potential benefit.
We also note that several scoring systems (HAS-BLED, HEMORR2HAGES, and ATRIA scores) have been developed to predict those at high risk of bleeding on anticoagulation for atrial fibrillation [78–80].These scores generally include similar clinical risk factors to those in the RIETE and ACCP scoring systems. Several studies have compared the HAS-BLED, HEMORR2HAGES, and ATRIA scores and a systematic review and meta-analysis concluded that the HAS-BLED score is recommended, due to increased sensitivity and ease of application [81].However, as these scores have not been validated for anticoagulation in the setting of VTE, we do not use them in this capacity.
Risk Stratification for Recurrent VTE
When predicting risk of recurrent VTE, clinical risk factors including obesity, male gender, and underlying thrombophilia (including the “high risk” inherited thrombophilias identified above) must taken into consideration. Location of the thrombus must also be considered; it has also been demonstrated that patients with DVT involving the iliofemoral veins are at higher risk of recurrence than those without iliac involvement [82].Other factors that may be useful in risk stratification include D-dimer level and ultrasound to search for residual venous thrombosis.
D-dimer Levels
D-dimer levels are one of the more promising methods for assessing the risk of recurrent VTE after cessation of anticoagulation, especially in the case of idiopathic VTE where indefinite anticoagulation should be considered but may pose either risk of bleeding or significant inconvenience to patients. A normal D-dimer measured 1 month after cessation of anticoagulation offers a high negative predictive value for risk of recurrence [83].A number of studies have demonstrated that patients with elevated D-dimer 1 month after anticoagulation cessation are at increased risk for a recurrent event [84–86].Two predictive models that have been developed incorporate D-dimer testing into decision making [87,88].The DASH predictive model relies on the D-dimer result in addition to age, male sex, and use of hormone therapy as a method of risk stratification for recurrent VTE in patients with a first unprovoked event. Using this scoring system, patients with a score of 0 or 1 had a recurrence rate of 3.1%, those with a score of 2 a recurrence rate of 6.4%, and those with a score of 3 or greater a recurrence rate of 12.3%. The authors postulate that by using this assessment scheme they can avoid lifelong anticoagulation in 51% of patients. The Vienna prediction model uses male sex, location of VTE (proximal DVT and PE are at higher risk), and D-dimer level to predict risk of recurrent VTE. This model has recently been updated to include a “dynamic” component to predict risk of recurrence of VTE from multiple random time points [89].
Overall, D-dimer may be useful for risk stratification. We often employ the method of stopping anticoagulation in patients with unprovoked VTE after 3 months (if the patient has no identifiable clinical risk factors that place them at high risk of recurrence) and testing D-dimer 1 month after cessation of anticoagulation. An elevated D-dimer is a solid reason to restart anticoagulation (potentially on an indefinite basis), while a negative D-dimer provides support for withholding further anticoagulation in the absence of other significant risk factors for recurrence. However, lack of agreement regarding assay cut-points as well as multiple reasons other than VTE for D-dimer elevation may limit widespread use of this method. We generally use a cutpoint of 250 ug/L as “negative,” though at least one study showed that cut-points of 250 ug/L versus 500 ug/L did not change the utility of this method [90].In our practice, risk prediction models are most useful to provide patients with additional information and a visual presentation to support our recommendation. This is particularly true of the Vienna prediction rule, which is available in a printable nomogram which can be distributed to patients and completed together during the clinic visit.
Imaging Analysis
Imaging analysis may also assist with risk stratification. Clinical assessment modules have been developed that incorporate repeat imaging studies for assessment of recannulization of affected veins. In patients with residual vein thrombosis (RVT) at the time anticoagulation was stopped, the hazard ratio for recurrence was 2.4 compared to those without RVT [91].There are a number of ways RVT could impact recurrence, including inpaired venous flow leading to stasis and activation of the coagulation cascade. Subsequent studies used serial ultrasound to determine when to stop anticoagulation. In one study, patients were anticoagulated for 3 months and for those that had RVT, anticoagulation was continued for up to 9 months for provoked and 21 months for unprovoked VTE. In comparison to fixed dosing of 6 months of anti-coagulation, those who had their length of anticoagulation tailored to ultrasonography findings had a lower rate of recurrent VTE [92].Limitations to using RVT in clinical decision-making include lack of a standard definition of RVT and variability in both timing of ultrasound (operator variability) and interpretation of results [93].
Other Options
Another option in patients who are being considered for indefinite anticoagulation is to decrease the intensity of anticoagulation. Since this would theoretically lower the risk of bleeding, the perceived benefit of long-term, low-intensity anticoagulation would be reduction in both bleeding and clotting risk. The PREVENT trial randomized patients who had received full-dose anticoagulation for a median of 6.5 months to either low-intensity warfarin (INR goal of 1.5-2.0 instead of 2.0-3.0) or placebo. In the anticoagulation group, there was a 64% risk reduction in recurrent VTE (hazard ratio 0.36, 95% CI 0.19 to 0.67) but an increased risk of bleeding (hazard ratio 1.92, 95% CI 1.26 to 2.93) [94].The ELATE study randomized patients with unprovoked VTE who had completed 3 or more months of full-intensity warfarin therapy (target INR 2.0–3.0) to continue therapy with either low-intensity warfarin (target INR 1.5–2.0) or full-intensity warfarin (target INR 2.0-3.0). Compared to the low-intensity group, the conventional-intensity group had lower rates of recurrent VTE and no increased rates of major bleeding [95].This study, however, has been criticized because of its overall low bleeding rate in both treatment groups.
Aspirin is an option in patients in whom long-term anticoagulation is untenable. The ASPIRE trial demonstrated that in patients with unprovoked VTE who had completed a course of initial anticoagulation, aspirin 100 mg daily reduced the risk of major vascular events compared to placebo with no increase in bleeding [96].However, aspirin was not associated with a significant reduction in risk of VTE alone (only the composite vascular event endpoint). The WARFASA trial, however, demonstrated that aspirin 100 mg daily was associated with a significant reduction in recurrent VTE compared to placebo after 6 to 18 months of anticoagulation without an increase in major bleeding [97].The absolute risk of recurrence was 11% in the placebo group and 5.9% in the aspirin group. More recently, the INSPIRE collaboration analyzed data from both trials and found that aspirin after initial anticoagulation reduced the risk of recurrent VTE by approximately 42% with a low rate of major bleeding [98].The absolute risk reduction was even larger in men and older patients. For this reason, we recommend aspirin to those patients in whom indefinite anticoagulation may be warranted from the standpoint of reducing risk of recurrent VTE but in whom the risk of bleeding precludes its use.
Hypercoagulable States In Specific Populations
Inherited Thrombophilias
Patients with a hereditary thrombophilia are at increased risk for incident VTE [99].These inherited mutations result in either a loss of normal anticoagulant function or gain of a prothrombotic state. Hereditary disorders associated with VTE include deficiency of antithrombin, protein C, or protein S, or the presence of factor V Leiden and/or prothrombin G20210A mutations. Although deficiency of protein C, protein S, or antithrombin is uncommon and affects only 0.5% of the population, these states have been associated with a 10-fold increased risk of thrombosis in comparison to the general population. Factor V Leiden and prothrombin gene mutation are less likely to be associated with incident thrombosis (2 to 5-fold increased risk of VTE) and are more prevalent in the Caucasian population [100].Though these hereditary thrombophilias increase risk of VTE, prophylactic anti-coagulation prior to a first VTE is not generally indicated.
Data regarding the impact of the inherited thrombophilias on risk of recurrent VTE is less well defined. While some data suggest that inherited thrombophilias are associated with increased risk of recurrent VTE, the degree of impact may be clinically modest especially in those with heterozygous factor V Leiden or prothrombin gene mutations [101].Ideally, a clinical trial would be designed to assess whether hereditary thrombophilia testing is beneficial for patients with VTE in decision-making regarding length of anticoagulation, type of anticoagulation, and risk of recurrence. If a patient with a low-risk inherited thrombophilia has a DVT in the setting of an additional provoking risk factor (surgery, pregnancy, etc), a 3-month course of anticoagulation followed by D-dimer assessment as above is reasonable. If a patient with an inherited thrombophilia experiences an idiopathic VTE, or if a patient with a “high-risk” thrombophilia as described above experiences any type of VTE, we generally recommend indefinite anticoagulation in the absence of high bleeding risk, though again this is a very patient-dependent choice.
Acquired Thrombophilias
Antiphospholipid Syndrome
Antibodies directed against proteins that bind phospho-lipids are associated with an acquired hypercoagulable state. The autoantibodies are categorized as antiphospho-lipid antibodies (APLAs), which include anticardiolipin antibodies (IgG and IgM), beta-2 glycoprotein 1 antibodies (anti-B2 GP), and lupus anticoagulant. These antibodies can form autonomously, as seen in primary disorders, or in association with autoimmune disease as a secondary disorder.
Criteria have been developed to distinguish antiphospholipid-associated clotting disorders from other forms of thrombophilia. The updated Sapporo criteria depend on both laboratory and clinical diagnostic criteria [102].The laboratory diagnosis of APLAs requires the presence of lupus anticoagulants, anticardiolipin antibodies, or anti-B2 GP on at least 2 assays at least 12 weeks apart with elevation above the 99th percentile of the testing laboratory’s normal distribution [103].Testing for lupus anticoagulant is based on 3 stages, the first of which is inhibition of phospholipid-dependent coagulation tests with prolonged clotting time (eg, aPTT or dilute Russell’s viper venom time). The diagnosis is confirmed by a secondary test in which excess hexagonal phase phospholipids are added to incubate with the patient’s plasma to absorb the APLA [104].The presence of anticardiolipin antibodies and anti beta-2 GP antibodies is determined using ELISA based immunoassays. Unlike most other thrombophilias, antiphospholipid syndrome is associated with both arterial and venous thromboembolic events and may be an indication for lifelong anticoagulation after a first thrombotic event. We generally recommend indefinite anticoagulation in the absence of significant bleeding risk.
Cancer-Associated Hypercoagulable State
Patients with cancer have a propensity for thromboembolic events. The underlying mechanisms responsible for cancer-associated clotting events are multifactorial and an area of intense research. Tumor cells can initiate activation of the clotting cascade through release of tissue factor and other pro-coagulant molecules [105].Type and stage of cancer impact risk of VTE, and the tumor itself can compress vasculature leading to venous stasis. Furthermore, chemotherapy, hormone therapy, antiangiogenic drugs, erythropoietin agents, and indwelling central venous catheters all are associated with increased risk of thrombotic events. Approximately 25% of all cancer patients will experience a thrombotic event during the course of their disease [106]. In fact, the presence of a spontaneous clot may be a harbinger of underlying malignancy [107].Approximately 10% of patients who present with an idiopathic VTE are diagnosed with cancer in the next 1 to 2 years.
The utility of extensive cancer screening in patients with spontaneous clotting events is often debated. The small studies that have addressed cancer associated clots have not demonstrated any mortality benefit with extensive screening. A prospective cohort study addressed the utility of limited versus extensive screening [108].In this study, all patients underwent a series of basic screening tests such as history taking, physical examination, chest radiograph, and basic laboratory parameters. Approximately half of the patients underwent additional testing (CT of chest and abdomen and mammography for women). Screening did not result in increased survival or fewer cancer-related deaths. 3.5 % of patients in the extensive screening group were diagnosed with malignancy in comparison to 2.4% in the limited screening group. During follow-up, cancer was diagnosed in 3.7% and 5.0% in the extensive and limited screening groups, respectively. The authors concluded that the low yield of extensive screening and lack of survival benefit did not warrant routinely ordering cancer screening tests above and beyond age-appropriate screening in patients with idiopathic VTE. However, it is known that identification of occult malignancy at an earlier stage of disease is beneficial, and cancer diagnosed within one year of an episode of VTE is generally more advanced and associated with a poorer prognosis [109].It is our practice to take a through history from patients with unprovoked clots particularly focusing on symptoms suggestive of an underlying cancer. We recommend that patients be up to date with all age-appropriate cancer screening.
Heparin-based products (rather than warfarin) are recommended for long-term treatment of cancer-associated DVT. Several trials, most prominently the CLOT trial, have demonstrated that LMWH is associated with reduced risk of recurrent VTE compared with warfarin in cancer patients [110].Fondaparinux may be a reasonable alternative if a patient is unable to tolerate a LMWH. In terms of treatment duration, patients with cancer-associated VTE should be anticoagulated indefinitely as long as they continue to have evidence of active malignancy and/or remain on antineoplastic treatment [111].
Heparin has potential anticancer effects beyond its anticoagulation properties. It is believed that heparin use in patients with cancer can influence cancer progression by acting as an antimetastatic agent. The molecular mechanisms underlying this significant observation are not completely understood, although the first documented benefit of these drugs dates back to the 1970s [112].Overall, LMWH have been associated with improved overall survival in cancer patients and this effect appears to be distinct from its ability to prevent life-threatening VTE episodes [113].
Estrogen-Related Thromboembolic Disease
Pregnancy is a well-established acquired hypercoagulable state, and thromboembolic disease accounts for significant morbidity and mortality in pregnancy and the postpartum period. Approximately 1 in 1000 women will suffer from a thrombotic event during pregnancy or shortly after delivery [8]. The etiology of the tendency to clot during pregnancy is multifactorial and mainly reflects venous stasis due to vasculature compression by the uterus, changes in coagulation factors as the pregnancy progresses, and endothelial damage during delivery, especially Cesarean section. Both factor VIII and von Willebrand factor levels increase, especially in the final months of pregnancy. Simultaneously, levels of the natural anticoagulant protein S diminish, leading to an acquired resistance to activated protein C which results in increased thrombin generation and therefore a hypercoagulable state [114].The risk of thrombosis in pregnancy is clearly heightened in women with inherited thrombophilias, especially in the postpartum period [115].
Similarly to pregnancy, hormone-based contraceptive agents and estrogen replacement therapies are also associated with increased thrombotic risk. Over the years, drug manufacturers have tried to mitigate the clotting risk associated with these drugs by reducing the amount of estrogen and altering the type of progesterone used, yet a risk still remains, resulting in a VTE incidence 2 to 7 times higher in this population [116].The risk is highest in the first 4 months of use and is unaffected by duration of use; risk extends for 3 months after cessation of estrogen-containing therapy. Patients who develop VTE while taking an oral contraceptive are generally instructed to stop the contraceptive and consider an alternative form of birth control. Although routine screening for thrombophilia is not offered to women before prescribing oral contraceptives, a thorough personal and family history regarding venous and arterial thrombotic events as well as recurrent pregnancy loss in women should be taken to evaluate thromboembolic risk factors. We generally avoid use of oral contraceptives in patients with a known hereditary thrombophilia, and consider screening prior to initiation of therapy in those with a strong family history of VTE.
Superficial VTE
Although the main disorders that comprise VTE are DVT and PE, another common presentation is superficial venous thromboembolism (SVT). The risk factors for developing an SVT are similar to those for DVT. In addition, varicose veins also increase the incidence of developing SVT [117].SVT is not associated with excessive mortality, and the main concern with it is progression to DVT. About 25% of patients diagnosed with SVT may have DVT or PE at the time of diagnosis and about 3% without DVT or PE at time of diagnosis developed one of these complications over the following 3 months; clot propagation is another common complication [118].Ultrasound may be of utility in diagnosing occult DVT in patients who initially diagnosed with SVT [119].
For patients who have only SVT at baseline without concomitant DVT or PE, it is difficult to determine which patients are at risk for developing DVT. Some risk stratification models include clot location. Since SVT clots usually develop in the saphenous vein, the clot would need to either progress from the sapheno-femoral junction to the common femoral vein; thus, any clots located near the sapheno-femoral junction are at risk of progressing into the deep vasculature [120].Clots within 3 cm of the junction may be more likely to progress to DVT [121].Chengelis and colleagues feel that proximal saphenous vein thrombosis should likely be treated with anticoagulation [122].Others have taken a more general approach, stating that all clots above the knee or in the thigh area should be treated aggressively [123].
There are solid data for the use of anticoagulation in SVT. In the STEFLUX (Superficial ThromboEmbolism and Fluxum) study, participants received the LMWH parnaparin at one of 3 doses: 8500 IU once daily for 10 days followed by placebo for 20 days, 8500 IU once daily for 10 days and then 6400 IU once daily for 20 days, or 4250 IU once daily for 30 days. Those who received the intermediate dosing had lower rates of DVT, PE, and relapse/SVT recurrence in the first 33 days [124].In the CALISTO trial, fondaparinux 2.5mg per day for 45 days effectively reduced the risk of symptomatic DVT, PE, or SVT recurrence or extension and was not associated with any increased major bleeding compared to placebo [125].A Cochrane review included 30 studies involving over 6500 participants with SVT of the lower extremities. The treatments used in these studies included fondaparinux, LMWH, UFH, non-steriodal anti-inflammatory agents, topical treatment, and surgery. According to the findings, use of fondaparinux at prophylactic dosing for 6 weeks is considered a valid therapeutic option for SVT [126].It is our practice to consider the use of anticoagulants (generally LMWH or fondaparinux) as part of the treatment regimen for SVT.
Target-Specific Oral Anticoagulants And Treatment of VTE
The direct thrombin inhibitor dabigatran directly binds to thrombin in a concentration-dependent manner [127].Peak plasma concentration is achieved within 0.5 to 2.0 hours after ingestion, and its half-life is 12 to 17 hours. Use of dabigatran in both primary and secondary prevention of VTE has been extensively studied, especially in orthopedic surgery where there have been 4 main trials (RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE I and II). While RE-MOBILIZE showed that dabigatran 220 mg or 150 mg once daily was inferior to enoxaparin 30 mg twice daily in preventing VTE after total knee arthroplasty, RE-MODEL and RE-NOVATE I and II demonstrated that dabigatran 150 mg or 220 mg once daily was noninferior to enoxaparin 40 mg once daily for prevention of VTE in patients undergoing total knee replacement and hip replacement [128–131].The side effect profile was also promising, with no significant differences in the frequency of major bleeding between dabigatran and enoxaparin. Pooled data and meta-analyses from these trials have demonstrated that for prevention of VTE associated with hip or knee surgery, dabigatran 220 mg or 150 mg once daily is as effective as 40 mg of enoxaparin given daily or 30 mg given twice a day, with a similar bleeding profile [132,133].
More recently, dabigatran been used in the acute treatment and secondary prevention of VTE. In the RE-COVER trial, dabigatran 150 mg twice daily was compared to warfarin (INR 2–3) in the treatment of acute VTE for 6 months, after an initial treatment period of up to 9 days with LMWH or UFH. Dabigatran was noninferior to warfarin with respect to 6-month incidence of recurrent symptomatic objectively confirmed VTE and related deaths, and was not associated with increased bleeding [134].In the RE-MEDY and RE-SONATE trials of extended anticoagulation, dabigatran was as effective as warfarin for prevention of recurrent VTE when continued after 3 months of initial anticoagulation and associated with less bleeding, and was more effective than placebo in preventing recurrent VTE but associated with a higher risk of bleeding [135].Unexpectedly, the risk of acute coronary syndrome was slightly higher in the dabigatran group than the warfarin group, as seen in other studies.
Rivaroxaban, a TSOAC that targets factor Xa, has also shown efficacy in preventing VTE after knee or hip surgery. The RE-CORD 1-4 studies all focused on the use of rivaroxaban in comparison to enoxaparin and found that rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily in prevention of VTE in total knee and total hip arthroplasty [136–138].Meta-analysis of multiple rivaroxaban VTE prophylaxis trials also demonstrated that rivaroxaban significantly lowered the risk of VTE in these surgical patients in comparison to the use of enoxaparin [139].Prophylactic use of rivaroxaban was also studied in acutely ill hospitalized patients in the MAGELLAN trial. Rivaroxaban 10 mg daily for 35 days was compared to enoxaparin 40 mg daily for 10 days followed by placebo and was found to be noninferior to enoxaparin in reduction of VTE risk at day 10 and superior to placebo at day 35 [140].However, the rate of bleeding, although low in both arms, was higher in the rivaroxaban arm.
Rivaroxaban has been studied in randomized clinical trials for acute treatment of DVT and PE and for extended prophylaxis for recurrent VTE (EINSTEIN-DVT, EINSTEIN-PE and EINSTEIN-Extension, respectively). The treatment strategy for use of rivaroxaban differed from that of dabigatran (in the RE-COVER trial), as rivaroxaban was used upfront as initial anticoagulation rather than after an initial period of parenteral therapy with LMWH or UFH. In both the DVT and PE trials, rivaroxaban was noninferior to standard treatment with enoxaparin followed by warfarin therapy, with no significant difference in major bleeding at 6 months of treatment [141,142].The extension trial also demonstrated that use of rivaroxaban in comparison to placebo for an additional 6 or 12 months after standard therapy was associated with significantly fewer recurrent VTE [141]. These studies led to FDA approval for rivaroxaban for primary prevention of VTE in patients undergoing elective total hip or knee repair surgery, for treatment of acute DVT or PE, and for extended prophylaxis in patients following initial treatment.
The anti-factor Xa TSOAC apixaban has been studied in similar fashion as rivaroxaban. In the AMPLIFY study, apixaban was given at a dose of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months (as monotherapy, without initial parenteral agent) and compared to enoxaparin followed by warfarin for treatment of acute VTE. Apixaban was as effective as warfarin in terms of recurrent symptomatic VTE or VTE-related death, and was associated with significantly fewer bleeding events [143].Extended-duration apixaban given at treatment dose (5 mg twice daily) or at prophylactic dose (2.5 mg twice daily) for 12 months after completion of treatment-dose apixaban for VTE demonstrated superiority to placebo for extended prophylaxis in AMPLIFY-EXT, and there was no increase in major bleeding compared to placebo [144].Apixaban was recently approved by the FDA for both treatment and secondary prophylaxis of VTE.
More recently, a third anti-factor Xa TSOAC edoxaban demonstrated noninferiority to warfarin in prevention of recurrent symptomatic VTE when administered to patients with DVT or PE at 60 mg once daily for 3 to 12 months [145].Edoxaban also led to significantly less bleeding than warfarin. Edoxaban was recently approved by the FDA for treatment of VTE.
These TSOACs show promise in treatment and prevention of VTE but should be used in patients who meet appropriate criteria for renal function, age, and bleeding risk, as there are currently no available antidotes to reverse their effects. If significant bleeding occurs and cannot be controlled by usual maneuvers such as mechanical compression or surgical intervention, there is little data to guide the use of pharmacologic interventions. Plasma dabigatran levels can be reduced through the use of hemodialysis [146].Antibodies capable of neutralizing dabigatran have been developed, and one specific antibody, idarucizumab, was well-tolerated and showed immediate and complete reversal of dabigatran in subjects of different age and renal function [147,148].Andexanet, a modified recombinant derivative of factor Xa with no catalytic activty, acts as a “decoy receptor” with higher affinity to factor Xa inhibitors than natural factor Xa. Phase II studies in healthy volunteers demonstrated that andexanet immediately reversed the anticoagulation activity of apixaban, rivaroxaban, enoxaparin, and most recently edoxaban without thrombotic consequences [149].Two randomized, double-blind, placebo-controlled phase III studies (ANNEXA-A, looking at the reversal of apixaban, and ANNEXA-R, looking at reversal of rivaroxaban) are underway, and preliminary results show that a single intravenous bolus of andexanet demonstrated almost complete reversal [150].Finally, aripazine (PER977), a synthetic small molecule that binds to heparins as well as all TSOACs, was shown in a phase II trial to decrease blood clotting time to within 10% above baseline value in 10 minutes or less with an effect lasting for 24 hours [151].
Some have advocated for use of prothrombin complex concentrate (PCC) or recombinant factor VIIa for reversal of TSOAC-associated bleeding. Rivaroxaban was demonstrated to be partially reversible by PCC, whereas this approach was not as successful for dabigatran in healthy volunteers [152].In vitro evidence, however, showed that PCC did not significantly change aPTT [153].At present, the use of nonspecific hemostatic agents (including recombinant factor VIIa, 4-factor prothrombin complex concentrate, and activated prothrombin complex concentrates) is suggested for reversal of TSOACs in patients who present with life-threatening bleeding [154,155].
Conclusion
Patients with VTE present with a wide range of findings and factors that impact management. Decision making in VTE management is a fluid process that should be re-evaluated as new data emerge and individual circumstances change. There is more focus on VTE prevention and treatment today than there was even a decade ago. Diagnostic algorithms, identification of new risk factors, refinement in understanding of the pathogenesis of thrombosis, and identification of new anticoagulants with more favorable risk-benefit profiles will all ultimately contribute to improved patient care.
Corresponding author: Jean M. Connors, MD, Brigham and Women's Hospital, 75 Francis St., Boston, MA 02215.
1. White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(Suppl 1):I4-8.
2. Moster KM and Fedullo PF. The diagnosis of deep-vein thrombosis. N Engl J Med 1994;330:863–4.
3. Kearon C. Natural history of venous thromboembolism. Circulation 2003;(Suppl 1):122–30.
4. Heijboer H, Brandjes DP, Buller HR, et al. Deficiencies of coagulation-inhibiting and fibrinolytic proteins in outpatients with deep-vein thrombosis. N Engl J Med 1990;323:1512–6.
5. Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:441–51.
6. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334–49.
7. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
9. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102.
10. Mahmoodi BK, Gansevoort RT, Naess IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964–71.
11. Landolfi R, Marchioli R, Patrono C. Mechanisms of bleeding and thrombosis in myeloproliferative disorders. Thromb Haemost 1997;78:617–21.
12. Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:1253–8.
13.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63.
14. Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008;6:1262–6.
15. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an aymptomatic patient population. J Vasc Surg 2004;39:937–43.
16. Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878–80.
17. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298.
18. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–80.
19. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88.
20. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost 2014;20:244–9.
21. Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52.
22. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722–7.
23. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost 2013;11:116-23.
24. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (Suppl 1): I9–16.
25. Clemetson KJ. Platelet GPIb-V-IX complex. Thromb Haemost 1997;78:266–70.
26. Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood 1989;74:1181–95.
27. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
28. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost 2000;416–20.
29. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–71.
30. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004;140:589–602.
31. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res 2010;125:e123–27.
32. Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med 2005;31:48–55.
33. Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med 2009;122:1050–3.
34. Chan WS, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
35. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357–61.
36. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 2014; 146:1444–51.
37. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004; 109 (Suppl 1): I9–14.
38. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102–10.
39. Tapson VF, Carroll BA, Davidson BL, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999;160:1043–66.
40. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e351S–418S.
41. Pezzullo JA, Perkins AB, Cronan JJ. Symptomatic deep vein thrombosis: diagnosis with limited compression US. Radiology 1996;198:67–70.
42. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations Radiology 1996;199:45–7.
43. Remy-Jardin M, Remy J, Deschidre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996;200:699–706.
44. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27.
45. Goodacre S. In the clinic. Deep venous thrombosis. Ann Intern Med 2008;149:ITC3–1.
46. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266–74.
47. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9.
48. Constans J, Salmi LR, Sevestre-Pietri MA, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost 2008;99:202–7.
49. Meyer G, Vicault E, Danasys T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11.
50. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-bline, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459–68.
51. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):7S–47S.
52. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998;3:41–6.
53. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2010;9:CD001100.
54. Buller HR, Davidson BL, Decousus H, et al. Fondaprinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 2004;140: 867–73.
55. Buller HR, Davidson BL, Decousus H, et al. Subcutaenous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–702.
56. Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112:e53–60.
57. Pon TK, Dager WE, Roberts AJ, White RH. Subcutaneous enoxaparin for therapeutic anticoagulation in hemodialysis patients. Thromb Res 2014;133:1023–8.
58. Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A. Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 2013;28:3090–5.
59. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–6.
60. Jiminez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–9.
61. Schulman S. Advances in the management of venous thromboembolism. Best Pract Res Clin Haematol 2012;25:361–77.
62. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost 2010;8:2412–7.
63. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–72.
64. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9.
65. Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007;146:211–22.
66. Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553–62.
67. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study group. N Engl J Med 1995;332:1661–5.
68. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036.
69. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-07.
70. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19–25.
71. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal duration Italian trial investigators. N Engl J Med 2001;345:165–9.
72. Bauer KA. Long-term management of venous thromboembolism: a 61-year old woman with unprovoked venous thromboembolism. JAMA 2011;305:1336–45.
73. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:
1794–801.
74. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):257S–298S.
75. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993;95:315–28.
76. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost 2008;100:26–31.
77. Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e419S–94S.
78. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010;138:1093–1100.
79. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9.
80. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
81. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–84.
82. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110:515–9.
83. Palareti G, Legnani C, Cosmi B, et al. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002; 87:7–12.
84. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9.
85. Bruinstroop E, Klok FA, Van De Ree MA, et al. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost 2009;611–8.
86. Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149:481–90.
87. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25.
88. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna Prediction Model. Circulation2010; 121:1630–6.
89. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014; 3: e000467.
90. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31.
91. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
92. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577–85.
93. Marcucci M, Iorio A, Douketis J. Management of patients with unprovoked venous thromboembolism: an evidence-based and practical approach. Curr Treat Options Cardiovasc Med 2013;15:224–39.
94. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425–34.
95. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631–9.
96. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979–87.
97. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67.
98. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130:1062–71.
99. Makris M. Thrombophilia: grading the risk. Blood 2009;113:5038–9.
100. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;30:2472.
101. Ho WK, Hankey GH, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006;166:729–36.
102. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
103. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2009; 7:1737–40.
104. Brandt JT, Barna LK, Triplett DA. Laboratory identification of lupus anticoagulants: results of the Second International Workship for identification of lupus anticoagulants. On behalf of the subcommittee on lupus anticoagulants/antiphospholipid antibodies of the ISTH. Thromb Haemost 1995;74: 1597–603.
105. Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol 2001;106:25-32.
106. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23.
107. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (Suppl 1): I17–21.
108. Van Doormaal FF, Terpstra W, Van Der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost 2011;9:79–84.
109. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50.
110. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
111. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402-29.
112. Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: “clinical study.” J Surg Oncol 1973;5:189–93.
113. Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost 2007;5:729–37.
114. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153–68.
115. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
116. Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol 2012;24:235-40.
117. Marchiori A, Mosena L, Prandoni P. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost 2006;32:737–43.
118. Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med 2010;152:218–24.
119. Galanaud JP, Genty C, Sevestre MA, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost 2011;105:31–9.
120. Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg 2001;193:556–62.
121. Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg 1992;164:269–75.
122. Chengelis DL, Benedick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
123. Verlato F, Zuccetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the leg. J Vasc Surg 1999;30:1113–5.
124. Cosmi B, Filippini M, Tonti D, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost 2012;10:1026–35.
125. Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32.
126. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
127. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303.
128. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9.
129. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85.
130. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007;370:949–56.
131. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, non-inferiority trial. Thromb Haemost2011;105:721–9.
132. Friedman RJ, Dahl OE, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res 2010;126:175–82.
133. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009;101:77–85.
134. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;36:2342–52.
135. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18.
136. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75.
137. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomized controlled trial. Lancet 2008;372:31–9.
138. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86.
139. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2010;66:1099–108.
140. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23.
141. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510.
142. EINSTEIN-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97.
143. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808.
144. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708.
145. Hokusai-VTE investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15.
146. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596–605.
147. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
148. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete, and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at the American Society of Hematology 2014 Annual Meeting; San Francisco, CA.
149. Crowther MA, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Abstract #4269. Presented at the American Society of Hematology 2014 Annual Meeting, San Francisco, CA.
150. Crowther M, Levy GG, Lu G, et al. ANNEXATM-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT 064445), a universal antidote for factor Xa (fa) inhibitors. Presented at the American Heart Association 2014 Annual Meeting, Chicago, IL.
151. Ansell JE, Bakhru SH, Lauliche BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141–2.
152. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9.
153. Korber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost 2014;20:735–40.
154. Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013;80:443–51.
155. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014;123:1152–8.
1. White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(Suppl 1):I4-8.
2. Moster KM and Fedullo PF. The diagnosis of deep-vein thrombosis. N Engl J Med 1994;330:863–4.
3. Kearon C. Natural history of venous thromboembolism. Circulation 2003;(Suppl 1):122–30.
4. Heijboer H, Brandjes DP, Buller HR, et al. Deficiencies of coagulation-inhibiting and fibrinolytic proteins in outpatients with deep-vein thrombosis. N Engl J Med 1990;323:1512–6.
5. Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:441–51.
6. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334–49.
7. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
9. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102.
10. Mahmoodi BK, Gansevoort RT, Naess IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964–71.
11. Landolfi R, Marchioli R, Patrono C. Mechanisms of bleeding and thrombosis in myeloproliferative disorders. Thromb Haemost 1997;78:617–21.
12. Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:1253–8.
13.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63.
14. Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008;6:1262–6.
15. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an aymptomatic patient population. J Vasc Surg 2004;39:937–43.
16. Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878–80.
17. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298.
18. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–80.
19. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88.
20. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost 2014;20:244–9.
21. Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52.
22. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722–7.
23. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost 2013;11:116-23.
24. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (Suppl 1): I9–16.
25. Clemetson KJ. Platelet GPIb-V-IX complex. Thromb Haemost 1997;78:266–70.
26. Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood 1989;74:1181–95.
27. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
28. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost 2000;416–20.
29. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–71.
30. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004;140:589–602.
31. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res 2010;125:e123–27.
32. Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med 2005;31:48–55.
33. Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med 2009;122:1050–3.
34. Chan WS, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
35. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357–61.
36. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 2014; 146:1444–51.
37. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004; 109 (Suppl 1): I9–14.
38. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102–10.
39. Tapson VF, Carroll BA, Davidson BL, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999;160:1043–66.
40. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e351S–418S.
41. Pezzullo JA, Perkins AB, Cronan JJ. Symptomatic deep vein thrombosis: diagnosis with limited compression US. Radiology 1996;198:67–70.
42. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations Radiology 1996;199:45–7.
43. Remy-Jardin M, Remy J, Deschidre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996;200:699–706.
44. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27.
45. Goodacre S. In the clinic. Deep venous thrombosis. Ann Intern Med 2008;149:ITC3–1.
46. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266–74.
47. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9.
48. Constans J, Salmi LR, Sevestre-Pietri MA, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost 2008;99:202–7.
49. Meyer G, Vicault E, Danasys T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11.
50. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-bline, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459–68.
51. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):7S–47S.
52. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998;3:41–6.
53. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2010;9:CD001100.
54. Buller HR, Davidson BL, Decousus H, et al. Fondaprinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 2004;140: 867–73.
55. Buller HR, Davidson BL, Decousus H, et al. Subcutaenous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–702.
56. Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112:e53–60.
57. Pon TK, Dager WE, Roberts AJ, White RH. Subcutaneous enoxaparin for therapeutic anticoagulation in hemodialysis patients. Thromb Res 2014;133:1023–8.
58. Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A. Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 2013;28:3090–5.
59. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–6.
60. Jiminez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–9.
61. Schulman S. Advances in the management of venous thromboembolism. Best Pract Res Clin Haematol 2012;25:361–77.
62. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost 2010;8:2412–7.
63. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–72.
64. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9.
65. Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007;146:211–22.
66. Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553–62.
67. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study group. N Engl J Med 1995;332:1661–5.
68. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036.
69. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-07.
70. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19–25.
71. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal duration Italian trial investigators. N Engl J Med 2001;345:165–9.
72. Bauer KA. Long-term management of venous thromboembolism: a 61-year old woman with unprovoked venous thromboembolism. JAMA 2011;305:1336–45.
73. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:
1794–801.
74. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):257S–298S.
75. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993;95:315–28.
76. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost 2008;100:26–31.
77. Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e419S–94S.
78. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010;138:1093–1100.
79. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9.
80. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
81. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–84.
82. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110:515–9.
83. Palareti G, Legnani C, Cosmi B, et al. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002; 87:7–12.
84. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9.
85. Bruinstroop E, Klok FA, Van De Ree MA, et al. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost 2009;611–8.
86. Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149:481–90.
87. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25.
88. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna Prediction Model. Circulation2010; 121:1630–6.
89. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014; 3: e000467.
90. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31.
91. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
92. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577–85.
93. Marcucci M, Iorio A, Douketis J. Management of patients with unprovoked venous thromboembolism: an evidence-based and practical approach. Curr Treat Options Cardiovasc Med 2013;15:224–39.
94. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425–34.
95. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631–9.
96. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979–87.
97. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67.
98. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130:1062–71.
99. Makris M. Thrombophilia: grading the risk. Blood 2009;113:5038–9.
100. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;30:2472.
101. Ho WK, Hankey GH, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006;166:729–36.
102. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
103. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2009; 7:1737–40.
104. Brandt JT, Barna LK, Triplett DA. Laboratory identification of lupus anticoagulants: results of the Second International Workship for identification of lupus anticoagulants. On behalf of the subcommittee on lupus anticoagulants/antiphospholipid antibodies of the ISTH. Thromb Haemost 1995;74: 1597–603.
105. Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol 2001;106:25-32.
106. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23.
107. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (Suppl 1): I17–21.
108. Van Doormaal FF, Terpstra W, Van Der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost 2011;9:79–84.
109. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50.
110. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
111. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402-29.
112. Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: “clinical study.” J Surg Oncol 1973;5:189–93.
113. Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost 2007;5:729–37.
114. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153–68.
115. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
116. Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol 2012;24:235-40.
117. Marchiori A, Mosena L, Prandoni P. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost 2006;32:737–43.
118. Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med 2010;152:218–24.
119. Galanaud JP, Genty C, Sevestre MA, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost 2011;105:31–9.
120. Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg 2001;193:556–62.
121. Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg 1992;164:269–75.
122. Chengelis DL, Benedick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
123. Verlato F, Zuccetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the leg. J Vasc Surg 1999;30:1113–5.
124. Cosmi B, Filippini M, Tonti D, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost 2012;10:1026–35.
125. Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32.
126. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
127. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303.
128. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9.
129. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85.
130. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007;370:949–56.
131. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, non-inferiority trial. Thromb Haemost2011;105:721–9.
132. Friedman RJ, Dahl OE, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res 2010;126:175–82.
133. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009;101:77–85.
134. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;36:2342–52.
135. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18.
136. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75.
137. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomized controlled trial. Lancet 2008;372:31–9.
138. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86.
139. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2010;66:1099–108.
140. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23.
141. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510.
142. EINSTEIN-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97.
143. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808.
144. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708.
145. Hokusai-VTE investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15.
146. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596–605.
147. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
148. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete, and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at the American Society of Hematology 2014 Annual Meeting; San Francisco, CA.
149. Crowther MA, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Abstract #4269. Presented at the American Society of Hematology 2014 Annual Meeting, San Francisco, CA.
150. Crowther M, Levy GG, Lu G, et al. ANNEXATM-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT 064445), a universal antidote for factor Xa (fa) inhibitors. Presented at the American Heart Association 2014 Annual Meeting, Chicago, IL.
151. Ansell JE, Bakhru SH, Lauliche BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141–2.
152. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9.
153. Korber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost 2014;20:735–40.
154. Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013;80:443–51.
155. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014;123:1152–8.
A 79-year-old with acute portal vein thrombosis
A 79-year-old man presented with chills and fever. He had a history of polymyalgia rheumatica and had been tapered off corticosteroids 1 month before admission. One week before he presented, he had developed generalized myalgia, chills, and fatigue. A cortisol stimulation test at that time was normal, prednisone was restarted, and his symptoms had improved. But 1 day before he presented, the chills had returned, this time with fever. Laboratory testing at an outpatient clinic had revealed abnormal liver enzyme levels.
On the day he presented, he felt worse, with persistent chills, fever, and vague lower abdominal pain, but he denied nausea, vomiting, changes in bowel habits, melena, hematochezia, and hematemesis. He was admitted for additional evaluation.
His medical history also included coronary artery disease (for which he had undergone coronary artery bypass grafting), hypertension, stable liver cysts, and gout. He had no known inflammatory bowel disease and no recent abdominal surgery. His medications included prednisone, atorvastatin, atenolol, aspirin, niacin, and cholecalciferol. He had no history of smoking, significant drinking, or use of illicit drugs. He had no respiratory or cardiac symptoms or neurologic symptoms consistent with a transient ischemic attack or stroke. He denied any rashes.
On admission, he was febrile, with temperatures reaching 102˚F (38.9˚C). His blood pressure was 137/63 mm Hg, pulse 54 beats per minute, respiration rate 18 breaths per minute, and oxygen saturation 97% on room air. A harsh systolic murmur was noted on physical examination. His abdomen was nondistended, nontender, and without bruits.
Laboratory testing (Table 1) revealed leukocytosis, anemia, mildly abnormal aminotransferase levels, elevated alkaline phosphatase, and markedly elevated C-reactive protein.
A full workup for fever was performed, including blood and urine cultures; chest radiography; contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis; magnetic resonance imaging (MRI) of the abdomen; and colonoscopy. No source of infection—bacterial, viral, or fungal—was found. However, CT revealed new extensive thrombosis of the right portal vein and its branches (Figure 1).
CLINICAL PRESENTATION
1. Which of the following is least consistent with the clinical presentation of acute portal vein thrombosis?
- Abdominal pain
- Fever and chills
- Hematemesis
- Leukocytosis
- Absence of symptoms
Of these signs and symptoms, hematemesis is the least likely to be associated with acute portal vein thrombosis, although it can be associated with chronic cases.
Symptoms of portal vein thrombosis
Portal vein thrombosis causes extrahepatic obstruction of the portal venous system, which provides two-thirds of the total hepatic blood flow.
Acute. Often, thrombotic occlusion of the portal vein produces no acute symptoms because of immediate, compensatory vasodilation of the hepatic arterial system.1 Additionally, in the ensuing days, the thrombus becomes an organized collagenous plug, and collateral veins develop to bypass the blocked vein and maintain portal perfusion in a process called cavernous transformation.1,2 Thus, many patients have no symptoms.
If symptoms occur, portal vein thrombosis can initially present as transient abdominal pain with fever, as seen in this patient.3 Many patients with acute portal vein thrombosis experience abdominal pain due to intra-abdominal sepsis, also referred to as pylephlebitis.2,4 High, spiking fevers and chills also occur, caused by infected thrombi associated with intra-abdominal infections such as appendicitis, diverticulitis, and pancreatitis.5,6
Chronic. In contrast, symptomatic chronic portal vein thrombosis commonly presents with sequelae of portal hypertension, most notably gastrointestinal bleeding. Hematemesis from ruptured esophageal varices is the most frequent reason for seeking medical attention, though varices also develop in the stomach, duodenum, jejunum, gallbladder, and bile ducts.2,7 Abdominal pain is less common in chronic portal vein thrombosis unless the thrombus extends into the mesenteric veins and causes bowel ischemia or infarction. Long-standing portal vein thrombosis may also lead to dilated venous collaterals that compress large bile ducts, resulting in portal cholangiopathy.1,8
Portal vein thrombosis may present as acute intestinal ischemia and bowel infarction, though this is uncommon. This is generally seen with extensive occlusive portal vein thrombosis and concomitant mesenteric venous thrombosis.1,2
Other symptoms that are common but nonspecific are nausea, vomiting, diarrhea, weight loss, and anorexia.2
Signs of portal vein thrombosis
On examination, patients with acute portal vein thrombosis have minimal physical signs unless they have other contributing conditions. For example, acute portal vein thrombosis can result in abdominal distention secondary to ileus, or guarding and ascites secondary to intestinal infarction.3,9
Some patients with chronic portal vein thrombosis also have normal physical findings, but many have signs. Splenomegaly is seen in 75% to 100% of patients.2,7 Hepatomegaly, abdominal tenderness, and low-grade fever are common as well.2,10 Ascites is usually not present without underlying cirrhosis; however, mild and transient ascites can develop immediately after the thrombotic event before the patient develops collateral circulation.2
Laboratory testing for portal vein thrombosis
Laboratory test results are typically unremarkable. Liver function tests show preserved hepatic function but may reveal mild increases in aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and bilirubin.2,10
In acute cases, elevations of acute-phase reactant levels can occur.9 Leukocytosis and blood cultures growing Bacteroides species are seen in septic cases or pylephlebitis.11,12 There may be mild anemia, particularly after a recent bleeding episode, or mild leukopenia and thrombocytopenia due to hypersplenism. Suspicion of an underlying myeloproliferative disorder is high if thrombocytosis is present.2
DIAGNOSIS
2. All of the following would be appropriate initial diagnostic studies for portal vein thrombosis except which one?
- Doppler ultrasonography
- Contrast-enhanced CT
- Contrast-enhanced MRI
- Angiography
Portal vein thrombosis is most often diagnosed with noninvasive techniques, namely Doppler ultrasonography, CT, and MRI—not angiography.
Ultrasonography can reveal an echogenic thrombus in the vessel lumen with distention of the portal vein proximal to the occlusion and extensive collateral vessels. Plain ultrasonography fails to reveal the thrombus in up to one-third of patients. However, duplex ultrasonography with color flow Doppler imaging can confirm partial or complete absence of flow in the vein with 89% sensitivity and 92% specificity.13,14
On contrast-enhanced CT, the thrombus appears as a filling defect within the portal venous segment. Complete occlusion of the vein may produce a “train track” appearance due to contrast around the vessel.10 Without contrast, the clot will appear as hyperattenuating material in the portal vein, but contrast-enhanced imaging may be necessary to differentiate the thrombus from the vessel wall.15 Gas within the portal venous system is specific for pylephlebitis.4 Evidence of cavernous transformation is seen in chronic portal vein thrombosis.
Contrast-enhanced magnetic resonance angiography can also be used to evaluate patency and flow direction. In addition, it provides detailed anatomic information about the entire portal venous system, including the intrahepatic portal vessels, which is limited in CT imaging.2,10 CT and MRI can also help to identify predisposing conditions (eg, intra-abdominal infection, hepatocellular carcinoma) and complications (eg, intestinal infarction) associated with portal vein thrombosis.
Angiography can be considered if noninvasive techniques are inconclusive but is generally not necessary, given the increased use of CT and MRI.
In our patient, abdominal CT revealed occlusive thrombosis of the right portal vein and its branches (Figure 1). The left and main portal veins were patent. There was no evidence of intra-abdominal infection or infarction.
FINDING THE CAUSE
3. Which of the following is not a common cause
of portal vein thrombosis?
- A hypercoagulable state
- Immune deficiency
- Intra-abdominal infection
- Malignancy
- Portal hypertension
Once portal vein thrombosis has been diagnosed, the cause should be identified (Table 2). The differential diagnosis is broad, including both local factors (eg, injury to the portal vein, local inflammation, infection) and general factors (eg, inherited and acquired hypercoagulable conditions). Thrombophilias are identified in 60% of patients with portal vein thrombosis and local factors in 40%.7 Moreover, the etiology is often multifactorial. However, immune deficiency is not a common cause.
Hypercoagulability
Prothrombotic disorders can be either inherited or acquired.
Inherited deficiencies in the natural anticoagulants antithrombin, protein C, and protein S are associated with a high risk of thrombosis but have a low prevalence in the general population. In the setting of liver abnormalities, familial testing may be helpful to distinguish inherited causes of portal vein thrombosis from defective liver function as a consequence of portal vein thrombosis. The factor V Leiden mutation (G1691A) and the G20210A mutation in the prothrombin gene are more prevalent (> 2%) but generally confer a lower thrombosis risk.16 The prothrombin gene mutation G20210A is the most common risk factor for portal vein thrombosis, with prevalence of 2% to 22% in adults with nonmalignant, noncirrhotic portal vein thrombosis.3
Hyperhomocysteinemia due to a methylene tetrahydrofolate reductase (MTHFR) mutation (C677T) is another inherited associated risk factor for portal vein thrombosis, but hyperhomocysteinemia can also arise as a complication of portal vein thrombosis-related liver disease.3
Acquired prothrombotic disorders, particularly myeloproliferative diseases, are found in 22% to 48% of cases of portal vein thrombosis. Many young patients with myeloproliferative disorders present with portal vein thrombosis as the first symptom, and testing for the G1849T point mutation in JAK2 can make the diagnosis.17 Splenectomy with underlying myeloproliferative disorder confers a particularly high risk for portal vein thrombosis.18
Other thrombophilic disorders including antiphospholipid antibody syndrome, paroxysmal nocturnal hemoglobinuria, and malignancy can contribute to portal vein thrombosis.3 Pregnancy and oral contraceptive use have also been associated with hypercoagulability, and cessation of oral estrogen is recommended in such cases. The risk may be further increased in patients on oral contraceptives who have a previously unrecognized hypercoagulable state.3
Inflammation and infection
Inflammation and infection are local risk factors for portal vein thrombosis. Acute portal vein thrombosis has been associated with intra-abdominal infections (eg, appendicitis, cholecystitis) and with inflammatory conditions such as inflammatory bowel disease and pancreatitis.16,19 From 3% to 5% of all portal vein thrombosis cases result from pancreatitis, either from a single acute episode or from repeat inflammation of chronic pancreatitis.10 Portal vein thrombosis in the setting of inflammatory bowel disease can occur even when the disease is in remission, particularly in ulcerative colitis.20,21
Injury to the portal venous system
Abdominal surgery, particularly splenectomy, portosystemic shunting, colectomy, and blunt abdominal trauma can cause injury to the portal venous system, resulting in portal vein thrombosis. This is usually seen only in patients with portal hypertension, an underlying prothrombotic condition such as myeloproliferative disease, or inflammatory bowel disease.10,19,22
Impaired portal vein flow
Cirrhosis and malignancy are major risk factors for portal vein thrombosis. In case series, cirrhosis was found in 24% to 32% of patients with portal vein thrombosis.2,23 However, the overall prevalence of portal vein thrombosis in cirrhotic patients varies widely, from 0.6% to 28%, depending on the degree of cirrhosis.10
The pathogenesis of portal vein thrombosis in cirrhosis is unclear but may be multifactorial. Decreased portal blood flow (with subsequent stasis) and periportal lymphangitis and fibrosis are thought to stimulate thrombus formation.3,10 Additionally, patients with advanced cirrhosis are prothrombotic because of reduced hepatic synthesis of antithrombin, protein C, protein S, and coagulation factors.
Malignancy is associated with 21% to 24% of cases of portal vein thrombosis in adults, with pancreatic cancer and hepatocellular carcinoma being the most common.2,3 Others include cholangiocarcinoma and carcinomas of the stomach, lung, prostate, uterus, and kidney. Cancer causes portal vein thrombosis through a combination of tumor invasion into the portal vein, extrinsic compression by the tumor, periportal fibrosis following surgery or radiation, and hypercoagulability secondary to malignancy.9,16,24
Idiopathic portal vein thrombosis
Portal vein thrombosis is usually caused by one or more of the underlying factors mentioned above but is idiopathic in 8% to 15% of cases.10
Back to our patient
The cause of this patient’s portal vein thrombosis is unclear. He did not have a history of cirrhosis, inflammatory bowel disease, trauma, or abdominal surgery. His febrile illness could have precipitated the formation of a thrombus, but no definitive source of infection or inflammation was discovered. His workup was negative for pancreatitis, appendicitis, cholecystitis, diverticulitis, and prostatitis. No occult malignancy was found. It is also possible that his fever was the result of the thrombosis.
A full hypercoagulability panel revealed no striking abnormalities. He did have elevated fibrinogen and factor VIII levels that were consistent with an acute-phase reaction, along with an elevated erythrocyte sedimentation rate (> 90 mm/hr) and C-reactive protein level. Aside from the portal vein thrombosis, no potential source of inflammation could be identified.
Mildly reduced levels of antithrombin III activity were attributed to enoxaparin therapy and ultimately normalized on repeated testing. The patient had very minimally elevated titers of anticardiolipin immunoglobulin G (1:10 GPL) and anti-beta-2 glycoprotein immunoglobulin M (21 SMU), which were not thought to be significant. Tests for lupus anticoagulant, prothrombin gene mutation, activated protein C resistance, and JAK2 mutation were negative.
TREATMENT
4. Treatment of symptomatic portal vein thrombosis generally includes which two of the following?
- Anticoagulation
- Intravenous gamma globulin
- Broad-spectrum antibiotics
Anticoagulant therapy
Treatment of acute, symptomatic portal vein thrombosis involves anticoagulant therapy to prevent extension of the thrombus and, ultimately, to allow for recanalization of the obstructed veins. Anticoagulant therapy is initially intravenous unfractionated heparin or subcutaneous low-molecular-weight heparin, eventually bridged to an oral agent such as warfarin.3,9 Currently, there are inadequate data on the use of oral or parenteral factor Xa inhibitors or direct thrombin inhibitors in the treatment of this disease.
When started immediately, anticoagulation therapy is associated with complete recanalization in 38.3% and partial recanalization in 14% of patients presenting with complete thrombosis. Without anticoagulation, spontaneous recanalization is unusual.25
Although the optimal duration of anticoagulant therapy is unclear, a minimum of 3 months is generally recommended.9,26 If a hypercoagulable state is present or if the portal vein thrombosis is unprovoked (eg, by surgery, trauma, or an intra-abdominal infection), long-term treatment should be considered.26
Experience with thrombolytic therapy or mechanical recanalization has been limited, but the use of catheter-based techniques for pharmacomechanical thrombolysis has been reported.27–29 Transjugular intrahepatic portosystemic shunting is also an alternative to anticoagulation, but its role in treating portal vein thrombosis is complicated by technical difficulties of the procedure, postoperative complications, and recurrent occlusion of the shunt.25
Currently, there are no data comparing the risk-benefit ratio of early anticoagulation and that of invasive procedures. These more aggressive treatments are generally considered only when there is extensive thrombosis or ascites (which are both predictive factors of poor response to anticoagulation alone) and in patients for whom anticoagulation has failed.3 Surgical thrombectomy is rarely indicated, typically only in instances in which laparotomy is being performed for suspected bowel infarction.3
Antibiotics
In addition to anticoagulation, broad-spectrum antibiotics covering gram-negative and anaerobic bacteria are indicated for those cases of portal vein thrombosis associated with underlying infection.9
For chronic cases, the goals of management are to prevent and treat gastroesophageal variceal bleeding and to prevent recurrent thrombosis.9 Nonselective beta-blockers (eg, propranolol) and endoscopic band ligation have shown evidence of reducing the incidence of recurrent bleeding and prolonging survival in retrospective studies.9,30,31 Long-term anticoagulation is generally indicated to prevent further thrombosis and to increase the likelihood of recanalization only for patients with a permanent prothrombotic condition.9 In patients with clinically significant portal hypertension, the benefit of continued anticoagulation therapy must be weighed against the risk of esophageal and gastric variceal bleeding.
There is controversy regarding how to manage portal vein thrombosis that is incidentally identified and asymptomatic (eg, if it is discovered on an imaging study for another indication). Current guidelines recommend against anticoagulation in patients with incidentally discovered and asymptomatic splanchnic vein thrombosis, including portal vein thrombosis.26
Intravenous gamma globulin is not part of the treatment.
CASE CONTINUED
The patient’s presenting symptoms of fever, chills, and abdominal pain completely resolved after a course of antibiotic therapy. The erythrocyte sedimentation rate subsequently normalized and factor VIII activity improved. We believed that an underlying infectious or inflammatory process had contributed to the development of portal vein thrombosis, though the specific cause could not be identified. The patient was treated with enoxaparin 1 mg/kg twice a day and transitioned to warfarin.
Magnetic resonance venography done 3 months after diagnosis showed persistent right portal vein thrombosis that was largely unchanged. Anticoagulation was continued for 1 year with no change in his portal vein thrombosis on sequential imaging and was subsequently discontinued. To date, no malignancy or infectious process has been found, and the patient continues to do well 2 years later.
- Ponziani FR, Zocco MA, Campanale C, et al. Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment. World J Gastroenterol 2010; 16:143–155.
- Cohen J, Edelman RR, Chopra S. Portal vein thrombosis: a review. Am J Med 1992; 92:173–182.
- Primignani M. Portal vein thrombosis, revisited. Dig Liver Dis 2010; 42:163–170.
- Condat B, Valla D. Nonmalignant portal vein thrombosis in adults. Nat Clin Pract Gastroenterol Hepatol 2006; 3:505–515.
- Condat B, Pessione F, Helene Denninger M, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology 2000; 32:466–470.
- Sheen CL, Lamparelli H, Milne A, Green I, Ramage JK. Clinical features, diagnosis and outcome of acute portal vein thrombosis. QJM 2000; 93:531–534.
- Sogaard KK, Astrup LB, Vilstrup H, Gronbaek H. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol 2007; 7:34.
- Llop E, de Juan C, Seijo S, et al. Portal cholangiopathy: radiological classification and natural history. Gut 2011; 60:853–860.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Sobhonslidsuk A, Reddy KR. Portal vein thrombosis: a concise review. Am J Gastroenterol 2002; 97:535–541.
- Ni YH, Wang NC, Peng MY, Chou YY, Chang FY. Bacteroides fragilis bacteremia associated with portal vein and superior mesentery vein thrombosis secondary to antithrombin III and protein C deficiency: a case report. J Microbiol Immunol Infect 2002; 35:255–258.
- Trum J, Valla D, Cohen G, et al. Bacteroides bacteraemia of undetermined origin: strong association with portal vein thrombosis and cryptogenic pylephlebitis. Eur J Gastroenterol Hepatol 1993; 5:655–659.
- Ueno N, Sasaki A, Tomiyama T, Tano S, Kimura K. Color Doppler ultrasonography in the diagnosis of cavernous transformation of the portal vein. J Clin Ultrasound 1997; 25:227–233.
- Tessler FN, Gehring BJ, Gomes AS, et al. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol 1991; 157:293–296.
- Hidajat N, Stobbe H, Griesshaber V, Felix R, Schroder RJ. Imaging and radiological interventions of portal vein thrombosis. Acta Radiol 2005; 46:336–343.
- Valla DC, Condat B. Portal vein thrombosis in adults: pathophysiology, pathogenesis and management. J Hepatol 2000; 32:865–871.
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005; 352:1779–1790.
- Krauth MT, Lechner K, Neugebauer EA, Pabinger I. The postoperative splenic/portal vein thrombosis after splenectomy and its prevention—an unresolved issue. Haematologica 2008; 93:1227–1232.
- Sinagra E, Aragona E, Romano C, et al. The role of portal vein thrombosis in the clinical course of inflammatory bowel diseases: report on three cases and review of the literature. Gastroenterol Res Pract 2012; 2012:916428.
- Maconi G, Bolzacchini E, Dell’Era A, Russo U, Ardizzone S, de Franchis R. Portal vein thrombosis in inflammatory bowel diseases: a single-center case series. J Crohns Colitis 2012; 6:362–367.
- Jackson LM, O’Gorman PJ, O’Connell J, Cronin CC, Cotter KP, Shanahan F. Thrombosis in inflammatory bowel disease: clinical setting, procoagulant profile and factor V Leiden. QJM 1997; 90:183–188.
- Eguchi A, Hashizume M, Kitano S, Tanoue K, Wada H, Sugimachi K. High rate of portal thrombosis after splenectomy in patients with esophageal varices and idiopathic portal hypertension. Arch Surg 1991; 126:752–755.
- Ogren M, Bergqvist D, Björck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol 2006; 12:2115–2119.
- Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013; 11:223–233.
- Congly SE, Lee SS. Portal vein thrombosis: should anticoagulation be used? Curr Gastroenterol Rep 2013; 15:306.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Uflacker R. Applications of percutaneous mechanical thrombectomy in transjugular intrahepatic portosystemic shunt and portal vein thrombosis. Tech Vasc Interv Radiol 2003; 6:59–69.
- Takahashi N, Kuroki K, Yanaga K. Percutaneous transhepatic mechanical thrombectomy for acute mesenteric venous thrombosis. J Endovasc Ther 2005; 12:508–511.
- Lopera JE, Correa G, Brazzini A, et al. Percutaneous transhepatic treatment of symptomatic mesenteric venous thrombosis. J Vasc Surg 2002; 36:1058–1061.
- Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5:80–86.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001; 120:490–497.
A 79-year-old man presented with chills and fever. He had a history of polymyalgia rheumatica and had been tapered off corticosteroids 1 month before admission. One week before he presented, he had developed generalized myalgia, chills, and fatigue. A cortisol stimulation test at that time was normal, prednisone was restarted, and his symptoms had improved. But 1 day before he presented, the chills had returned, this time with fever. Laboratory testing at an outpatient clinic had revealed abnormal liver enzyme levels.
On the day he presented, he felt worse, with persistent chills, fever, and vague lower abdominal pain, but he denied nausea, vomiting, changes in bowel habits, melena, hematochezia, and hematemesis. He was admitted for additional evaluation.
His medical history also included coronary artery disease (for which he had undergone coronary artery bypass grafting), hypertension, stable liver cysts, and gout. He had no known inflammatory bowel disease and no recent abdominal surgery. His medications included prednisone, atorvastatin, atenolol, aspirin, niacin, and cholecalciferol. He had no history of smoking, significant drinking, or use of illicit drugs. He had no respiratory or cardiac symptoms or neurologic symptoms consistent with a transient ischemic attack or stroke. He denied any rashes.
On admission, he was febrile, with temperatures reaching 102˚F (38.9˚C). His blood pressure was 137/63 mm Hg, pulse 54 beats per minute, respiration rate 18 breaths per minute, and oxygen saturation 97% on room air. A harsh systolic murmur was noted on physical examination. His abdomen was nondistended, nontender, and without bruits.
Laboratory testing (Table 1) revealed leukocytosis, anemia, mildly abnormal aminotransferase levels, elevated alkaline phosphatase, and markedly elevated C-reactive protein.
A full workup for fever was performed, including blood and urine cultures; chest radiography; contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis; magnetic resonance imaging (MRI) of the abdomen; and colonoscopy. No source of infection—bacterial, viral, or fungal—was found. However, CT revealed new extensive thrombosis of the right portal vein and its branches (Figure 1).
CLINICAL PRESENTATION
1. Which of the following is least consistent with the clinical presentation of acute portal vein thrombosis?
- Abdominal pain
- Fever and chills
- Hematemesis
- Leukocytosis
- Absence of symptoms
Of these signs and symptoms, hematemesis is the least likely to be associated with acute portal vein thrombosis, although it can be associated with chronic cases.
Symptoms of portal vein thrombosis
Portal vein thrombosis causes extrahepatic obstruction of the portal venous system, which provides two-thirds of the total hepatic blood flow.
Acute. Often, thrombotic occlusion of the portal vein produces no acute symptoms because of immediate, compensatory vasodilation of the hepatic arterial system.1 Additionally, in the ensuing days, the thrombus becomes an organized collagenous plug, and collateral veins develop to bypass the blocked vein and maintain portal perfusion in a process called cavernous transformation.1,2 Thus, many patients have no symptoms.
If symptoms occur, portal vein thrombosis can initially present as transient abdominal pain with fever, as seen in this patient.3 Many patients with acute portal vein thrombosis experience abdominal pain due to intra-abdominal sepsis, also referred to as pylephlebitis.2,4 High, spiking fevers and chills also occur, caused by infected thrombi associated with intra-abdominal infections such as appendicitis, diverticulitis, and pancreatitis.5,6
Chronic. In contrast, symptomatic chronic portal vein thrombosis commonly presents with sequelae of portal hypertension, most notably gastrointestinal bleeding. Hematemesis from ruptured esophageal varices is the most frequent reason for seeking medical attention, though varices also develop in the stomach, duodenum, jejunum, gallbladder, and bile ducts.2,7 Abdominal pain is less common in chronic portal vein thrombosis unless the thrombus extends into the mesenteric veins and causes bowel ischemia or infarction. Long-standing portal vein thrombosis may also lead to dilated venous collaterals that compress large bile ducts, resulting in portal cholangiopathy.1,8
Portal vein thrombosis may present as acute intestinal ischemia and bowel infarction, though this is uncommon. This is generally seen with extensive occlusive portal vein thrombosis and concomitant mesenteric venous thrombosis.1,2
Other symptoms that are common but nonspecific are nausea, vomiting, diarrhea, weight loss, and anorexia.2
Signs of portal vein thrombosis
On examination, patients with acute portal vein thrombosis have minimal physical signs unless they have other contributing conditions. For example, acute portal vein thrombosis can result in abdominal distention secondary to ileus, or guarding and ascites secondary to intestinal infarction.3,9
Some patients with chronic portal vein thrombosis also have normal physical findings, but many have signs. Splenomegaly is seen in 75% to 100% of patients.2,7 Hepatomegaly, abdominal tenderness, and low-grade fever are common as well.2,10 Ascites is usually not present without underlying cirrhosis; however, mild and transient ascites can develop immediately after the thrombotic event before the patient develops collateral circulation.2
Laboratory testing for portal vein thrombosis
Laboratory test results are typically unremarkable. Liver function tests show preserved hepatic function but may reveal mild increases in aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and bilirubin.2,10
In acute cases, elevations of acute-phase reactant levels can occur.9 Leukocytosis and blood cultures growing Bacteroides species are seen in septic cases or pylephlebitis.11,12 There may be mild anemia, particularly after a recent bleeding episode, or mild leukopenia and thrombocytopenia due to hypersplenism. Suspicion of an underlying myeloproliferative disorder is high if thrombocytosis is present.2
DIAGNOSIS
2. All of the following would be appropriate initial diagnostic studies for portal vein thrombosis except which one?
- Doppler ultrasonography
- Contrast-enhanced CT
- Contrast-enhanced MRI
- Angiography
Portal vein thrombosis is most often diagnosed with noninvasive techniques, namely Doppler ultrasonography, CT, and MRI—not angiography.
Ultrasonography can reveal an echogenic thrombus in the vessel lumen with distention of the portal vein proximal to the occlusion and extensive collateral vessels. Plain ultrasonography fails to reveal the thrombus in up to one-third of patients. However, duplex ultrasonography with color flow Doppler imaging can confirm partial or complete absence of flow in the vein with 89% sensitivity and 92% specificity.13,14
On contrast-enhanced CT, the thrombus appears as a filling defect within the portal venous segment. Complete occlusion of the vein may produce a “train track” appearance due to contrast around the vessel.10 Without contrast, the clot will appear as hyperattenuating material in the portal vein, but contrast-enhanced imaging may be necessary to differentiate the thrombus from the vessel wall.15 Gas within the portal venous system is specific for pylephlebitis.4 Evidence of cavernous transformation is seen in chronic portal vein thrombosis.
Contrast-enhanced magnetic resonance angiography can also be used to evaluate patency and flow direction. In addition, it provides detailed anatomic information about the entire portal venous system, including the intrahepatic portal vessels, which is limited in CT imaging.2,10 CT and MRI can also help to identify predisposing conditions (eg, intra-abdominal infection, hepatocellular carcinoma) and complications (eg, intestinal infarction) associated with portal vein thrombosis.
Angiography can be considered if noninvasive techniques are inconclusive but is generally not necessary, given the increased use of CT and MRI.
In our patient, abdominal CT revealed occlusive thrombosis of the right portal vein and its branches (Figure 1). The left and main portal veins were patent. There was no evidence of intra-abdominal infection or infarction.
FINDING THE CAUSE
3. Which of the following is not a common cause
of portal vein thrombosis?
- A hypercoagulable state
- Immune deficiency
- Intra-abdominal infection
- Malignancy
- Portal hypertension
Once portal vein thrombosis has been diagnosed, the cause should be identified (Table 2). The differential diagnosis is broad, including both local factors (eg, injury to the portal vein, local inflammation, infection) and general factors (eg, inherited and acquired hypercoagulable conditions). Thrombophilias are identified in 60% of patients with portal vein thrombosis and local factors in 40%.7 Moreover, the etiology is often multifactorial. However, immune deficiency is not a common cause.
Hypercoagulability
Prothrombotic disorders can be either inherited or acquired.
Inherited deficiencies in the natural anticoagulants antithrombin, protein C, and protein S are associated with a high risk of thrombosis but have a low prevalence in the general population. In the setting of liver abnormalities, familial testing may be helpful to distinguish inherited causes of portal vein thrombosis from defective liver function as a consequence of portal vein thrombosis. The factor V Leiden mutation (G1691A) and the G20210A mutation in the prothrombin gene are more prevalent (> 2%) but generally confer a lower thrombosis risk.16 The prothrombin gene mutation G20210A is the most common risk factor for portal vein thrombosis, with prevalence of 2% to 22% in adults with nonmalignant, noncirrhotic portal vein thrombosis.3
Hyperhomocysteinemia due to a methylene tetrahydrofolate reductase (MTHFR) mutation (C677T) is another inherited associated risk factor for portal vein thrombosis, but hyperhomocysteinemia can also arise as a complication of portal vein thrombosis-related liver disease.3
Acquired prothrombotic disorders, particularly myeloproliferative diseases, are found in 22% to 48% of cases of portal vein thrombosis. Many young patients with myeloproliferative disorders present with portal vein thrombosis as the first symptom, and testing for the G1849T point mutation in JAK2 can make the diagnosis.17 Splenectomy with underlying myeloproliferative disorder confers a particularly high risk for portal vein thrombosis.18
Other thrombophilic disorders including antiphospholipid antibody syndrome, paroxysmal nocturnal hemoglobinuria, and malignancy can contribute to portal vein thrombosis.3 Pregnancy and oral contraceptive use have also been associated with hypercoagulability, and cessation of oral estrogen is recommended in such cases. The risk may be further increased in patients on oral contraceptives who have a previously unrecognized hypercoagulable state.3
Inflammation and infection
Inflammation and infection are local risk factors for portal vein thrombosis. Acute portal vein thrombosis has been associated with intra-abdominal infections (eg, appendicitis, cholecystitis) and with inflammatory conditions such as inflammatory bowel disease and pancreatitis.16,19 From 3% to 5% of all portal vein thrombosis cases result from pancreatitis, either from a single acute episode or from repeat inflammation of chronic pancreatitis.10 Portal vein thrombosis in the setting of inflammatory bowel disease can occur even when the disease is in remission, particularly in ulcerative colitis.20,21
Injury to the portal venous system
Abdominal surgery, particularly splenectomy, portosystemic shunting, colectomy, and blunt abdominal trauma can cause injury to the portal venous system, resulting in portal vein thrombosis. This is usually seen only in patients with portal hypertension, an underlying prothrombotic condition such as myeloproliferative disease, or inflammatory bowel disease.10,19,22
Impaired portal vein flow
Cirrhosis and malignancy are major risk factors for portal vein thrombosis. In case series, cirrhosis was found in 24% to 32% of patients with portal vein thrombosis.2,23 However, the overall prevalence of portal vein thrombosis in cirrhotic patients varies widely, from 0.6% to 28%, depending on the degree of cirrhosis.10
The pathogenesis of portal vein thrombosis in cirrhosis is unclear but may be multifactorial. Decreased portal blood flow (with subsequent stasis) and periportal lymphangitis and fibrosis are thought to stimulate thrombus formation.3,10 Additionally, patients with advanced cirrhosis are prothrombotic because of reduced hepatic synthesis of antithrombin, protein C, protein S, and coagulation factors.
Malignancy is associated with 21% to 24% of cases of portal vein thrombosis in adults, with pancreatic cancer and hepatocellular carcinoma being the most common.2,3 Others include cholangiocarcinoma and carcinomas of the stomach, lung, prostate, uterus, and kidney. Cancer causes portal vein thrombosis through a combination of tumor invasion into the portal vein, extrinsic compression by the tumor, periportal fibrosis following surgery or radiation, and hypercoagulability secondary to malignancy.9,16,24
Idiopathic portal vein thrombosis
Portal vein thrombosis is usually caused by one or more of the underlying factors mentioned above but is idiopathic in 8% to 15% of cases.10
Back to our patient
The cause of this patient’s portal vein thrombosis is unclear. He did not have a history of cirrhosis, inflammatory bowel disease, trauma, or abdominal surgery. His febrile illness could have precipitated the formation of a thrombus, but no definitive source of infection or inflammation was discovered. His workup was negative for pancreatitis, appendicitis, cholecystitis, diverticulitis, and prostatitis. No occult malignancy was found. It is also possible that his fever was the result of the thrombosis.
A full hypercoagulability panel revealed no striking abnormalities. He did have elevated fibrinogen and factor VIII levels that were consistent with an acute-phase reaction, along with an elevated erythrocyte sedimentation rate (> 90 mm/hr) and C-reactive protein level. Aside from the portal vein thrombosis, no potential source of inflammation could be identified.
Mildly reduced levels of antithrombin III activity were attributed to enoxaparin therapy and ultimately normalized on repeated testing. The patient had very minimally elevated titers of anticardiolipin immunoglobulin G (1:10 GPL) and anti-beta-2 glycoprotein immunoglobulin M (21 SMU), which were not thought to be significant. Tests for lupus anticoagulant, prothrombin gene mutation, activated protein C resistance, and JAK2 mutation were negative.
TREATMENT
4. Treatment of symptomatic portal vein thrombosis generally includes which two of the following?
- Anticoagulation
- Intravenous gamma globulin
- Broad-spectrum antibiotics
Anticoagulant therapy
Treatment of acute, symptomatic portal vein thrombosis involves anticoagulant therapy to prevent extension of the thrombus and, ultimately, to allow for recanalization of the obstructed veins. Anticoagulant therapy is initially intravenous unfractionated heparin or subcutaneous low-molecular-weight heparin, eventually bridged to an oral agent such as warfarin.3,9 Currently, there are inadequate data on the use of oral or parenteral factor Xa inhibitors or direct thrombin inhibitors in the treatment of this disease.
When started immediately, anticoagulation therapy is associated with complete recanalization in 38.3% and partial recanalization in 14% of patients presenting with complete thrombosis. Without anticoagulation, spontaneous recanalization is unusual.25
Although the optimal duration of anticoagulant therapy is unclear, a minimum of 3 months is generally recommended.9,26 If a hypercoagulable state is present or if the portal vein thrombosis is unprovoked (eg, by surgery, trauma, or an intra-abdominal infection), long-term treatment should be considered.26
Experience with thrombolytic therapy or mechanical recanalization has been limited, but the use of catheter-based techniques for pharmacomechanical thrombolysis has been reported.27–29 Transjugular intrahepatic portosystemic shunting is also an alternative to anticoagulation, but its role in treating portal vein thrombosis is complicated by technical difficulties of the procedure, postoperative complications, and recurrent occlusion of the shunt.25
Currently, there are no data comparing the risk-benefit ratio of early anticoagulation and that of invasive procedures. These more aggressive treatments are generally considered only when there is extensive thrombosis or ascites (which are both predictive factors of poor response to anticoagulation alone) and in patients for whom anticoagulation has failed.3 Surgical thrombectomy is rarely indicated, typically only in instances in which laparotomy is being performed for suspected bowel infarction.3
Antibiotics
In addition to anticoagulation, broad-spectrum antibiotics covering gram-negative and anaerobic bacteria are indicated for those cases of portal vein thrombosis associated with underlying infection.9
For chronic cases, the goals of management are to prevent and treat gastroesophageal variceal bleeding and to prevent recurrent thrombosis.9 Nonselective beta-blockers (eg, propranolol) and endoscopic band ligation have shown evidence of reducing the incidence of recurrent bleeding and prolonging survival in retrospective studies.9,30,31 Long-term anticoagulation is generally indicated to prevent further thrombosis and to increase the likelihood of recanalization only for patients with a permanent prothrombotic condition.9 In patients with clinically significant portal hypertension, the benefit of continued anticoagulation therapy must be weighed against the risk of esophageal and gastric variceal bleeding.
There is controversy regarding how to manage portal vein thrombosis that is incidentally identified and asymptomatic (eg, if it is discovered on an imaging study for another indication). Current guidelines recommend against anticoagulation in patients with incidentally discovered and asymptomatic splanchnic vein thrombosis, including portal vein thrombosis.26
Intravenous gamma globulin is not part of the treatment.
CASE CONTINUED
The patient’s presenting symptoms of fever, chills, and abdominal pain completely resolved after a course of antibiotic therapy. The erythrocyte sedimentation rate subsequently normalized and factor VIII activity improved. We believed that an underlying infectious or inflammatory process had contributed to the development of portal vein thrombosis, though the specific cause could not be identified. The patient was treated with enoxaparin 1 mg/kg twice a day and transitioned to warfarin.
Magnetic resonance venography done 3 months after diagnosis showed persistent right portal vein thrombosis that was largely unchanged. Anticoagulation was continued for 1 year with no change in his portal vein thrombosis on sequential imaging and was subsequently discontinued. To date, no malignancy or infectious process has been found, and the patient continues to do well 2 years later.
A 79-year-old man presented with chills and fever. He had a history of polymyalgia rheumatica and had been tapered off corticosteroids 1 month before admission. One week before he presented, he had developed generalized myalgia, chills, and fatigue. A cortisol stimulation test at that time was normal, prednisone was restarted, and his symptoms had improved. But 1 day before he presented, the chills had returned, this time with fever. Laboratory testing at an outpatient clinic had revealed abnormal liver enzyme levels.
On the day he presented, he felt worse, with persistent chills, fever, and vague lower abdominal pain, but he denied nausea, vomiting, changes in bowel habits, melena, hematochezia, and hematemesis. He was admitted for additional evaluation.
His medical history also included coronary artery disease (for which he had undergone coronary artery bypass grafting), hypertension, stable liver cysts, and gout. He had no known inflammatory bowel disease and no recent abdominal surgery. His medications included prednisone, atorvastatin, atenolol, aspirin, niacin, and cholecalciferol. He had no history of smoking, significant drinking, or use of illicit drugs. He had no respiratory or cardiac symptoms or neurologic symptoms consistent with a transient ischemic attack or stroke. He denied any rashes.
On admission, he was febrile, with temperatures reaching 102˚F (38.9˚C). His blood pressure was 137/63 mm Hg, pulse 54 beats per minute, respiration rate 18 breaths per minute, and oxygen saturation 97% on room air. A harsh systolic murmur was noted on physical examination. His abdomen was nondistended, nontender, and without bruits.
Laboratory testing (Table 1) revealed leukocytosis, anemia, mildly abnormal aminotransferase levels, elevated alkaline phosphatase, and markedly elevated C-reactive protein.
A full workup for fever was performed, including blood and urine cultures; chest radiography; contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis; magnetic resonance imaging (MRI) of the abdomen; and colonoscopy. No source of infection—bacterial, viral, or fungal—was found. However, CT revealed new extensive thrombosis of the right portal vein and its branches (Figure 1).
CLINICAL PRESENTATION
1. Which of the following is least consistent with the clinical presentation of acute portal vein thrombosis?
- Abdominal pain
- Fever and chills
- Hematemesis
- Leukocytosis
- Absence of symptoms
Of these signs and symptoms, hematemesis is the least likely to be associated with acute portal vein thrombosis, although it can be associated with chronic cases.
Symptoms of portal vein thrombosis
Portal vein thrombosis causes extrahepatic obstruction of the portal venous system, which provides two-thirds of the total hepatic blood flow.
Acute. Often, thrombotic occlusion of the portal vein produces no acute symptoms because of immediate, compensatory vasodilation of the hepatic arterial system.1 Additionally, in the ensuing days, the thrombus becomes an organized collagenous plug, and collateral veins develop to bypass the blocked vein and maintain portal perfusion in a process called cavernous transformation.1,2 Thus, many patients have no symptoms.
If symptoms occur, portal vein thrombosis can initially present as transient abdominal pain with fever, as seen in this patient.3 Many patients with acute portal vein thrombosis experience abdominal pain due to intra-abdominal sepsis, also referred to as pylephlebitis.2,4 High, spiking fevers and chills also occur, caused by infected thrombi associated with intra-abdominal infections such as appendicitis, diverticulitis, and pancreatitis.5,6
Chronic. In contrast, symptomatic chronic portal vein thrombosis commonly presents with sequelae of portal hypertension, most notably gastrointestinal bleeding. Hematemesis from ruptured esophageal varices is the most frequent reason for seeking medical attention, though varices also develop in the stomach, duodenum, jejunum, gallbladder, and bile ducts.2,7 Abdominal pain is less common in chronic portal vein thrombosis unless the thrombus extends into the mesenteric veins and causes bowel ischemia or infarction. Long-standing portal vein thrombosis may also lead to dilated venous collaterals that compress large bile ducts, resulting in portal cholangiopathy.1,8
Portal vein thrombosis may present as acute intestinal ischemia and bowel infarction, though this is uncommon. This is generally seen with extensive occlusive portal vein thrombosis and concomitant mesenteric venous thrombosis.1,2
Other symptoms that are common but nonspecific are nausea, vomiting, diarrhea, weight loss, and anorexia.2
Signs of portal vein thrombosis
On examination, patients with acute portal vein thrombosis have minimal physical signs unless they have other contributing conditions. For example, acute portal vein thrombosis can result in abdominal distention secondary to ileus, or guarding and ascites secondary to intestinal infarction.3,9
Some patients with chronic portal vein thrombosis also have normal physical findings, but many have signs. Splenomegaly is seen in 75% to 100% of patients.2,7 Hepatomegaly, abdominal tenderness, and low-grade fever are common as well.2,10 Ascites is usually not present without underlying cirrhosis; however, mild and transient ascites can develop immediately after the thrombotic event before the patient develops collateral circulation.2
Laboratory testing for portal vein thrombosis
Laboratory test results are typically unremarkable. Liver function tests show preserved hepatic function but may reveal mild increases in aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and bilirubin.2,10
In acute cases, elevations of acute-phase reactant levels can occur.9 Leukocytosis and blood cultures growing Bacteroides species are seen in septic cases or pylephlebitis.11,12 There may be mild anemia, particularly after a recent bleeding episode, or mild leukopenia and thrombocytopenia due to hypersplenism. Suspicion of an underlying myeloproliferative disorder is high if thrombocytosis is present.2
DIAGNOSIS
2. All of the following would be appropriate initial diagnostic studies for portal vein thrombosis except which one?
- Doppler ultrasonography
- Contrast-enhanced CT
- Contrast-enhanced MRI
- Angiography
Portal vein thrombosis is most often diagnosed with noninvasive techniques, namely Doppler ultrasonography, CT, and MRI—not angiography.
Ultrasonography can reveal an echogenic thrombus in the vessel lumen with distention of the portal vein proximal to the occlusion and extensive collateral vessels. Plain ultrasonography fails to reveal the thrombus in up to one-third of patients. However, duplex ultrasonography with color flow Doppler imaging can confirm partial or complete absence of flow in the vein with 89% sensitivity and 92% specificity.13,14
On contrast-enhanced CT, the thrombus appears as a filling defect within the portal venous segment. Complete occlusion of the vein may produce a “train track” appearance due to contrast around the vessel.10 Without contrast, the clot will appear as hyperattenuating material in the portal vein, but contrast-enhanced imaging may be necessary to differentiate the thrombus from the vessel wall.15 Gas within the portal venous system is specific for pylephlebitis.4 Evidence of cavernous transformation is seen in chronic portal vein thrombosis.
Contrast-enhanced magnetic resonance angiography can also be used to evaluate patency and flow direction. In addition, it provides detailed anatomic information about the entire portal venous system, including the intrahepatic portal vessels, which is limited in CT imaging.2,10 CT and MRI can also help to identify predisposing conditions (eg, intra-abdominal infection, hepatocellular carcinoma) and complications (eg, intestinal infarction) associated with portal vein thrombosis.
Angiography can be considered if noninvasive techniques are inconclusive but is generally not necessary, given the increased use of CT and MRI.
In our patient, abdominal CT revealed occlusive thrombosis of the right portal vein and its branches (Figure 1). The left and main portal veins were patent. There was no evidence of intra-abdominal infection or infarction.
FINDING THE CAUSE
3. Which of the following is not a common cause
of portal vein thrombosis?
- A hypercoagulable state
- Immune deficiency
- Intra-abdominal infection
- Malignancy
- Portal hypertension
Once portal vein thrombosis has been diagnosed, the cause should be identified (Table 2). The differential diagnosis is broad, including both local factors (eg, injury to the portal vein, local inflammation, infection) and general factors (eg, inherited and acquired hypercoagulable conditions). Thrombophilias are identified in 60% of patients with portal vein thrombosis and local factors in 40%.7 Moreover, the etiology is often multifactorial. However, immune deficiency is not a common cause.
Hypercoagulability
Prothrombotic disorders can be either inherited or acquired.
Inherited deficiencies in the natural anticoagulants antithrombin, protein C, and protein S are associated with a high risk of thrombosis but have a low prevalence in the general population. In the setting of liver abnormalities, familial testing may be helpful to distinguish inherited causes of portal vein thrombosis from defective liver function as a consequence of portal vein thrombosis. The factor V Leiden mutation (G1691A) and the G20210A mutation in the prothrombin gene are more prevalent (> 2%) but generally confer a lower thrombosis risk.16 The prothrombin gene mutation G20210A is the most common risk factor for portal vein thrombosis, with prevalence of 2% to 22% in adults with nonmalignant, noncirrhotic portal vein thrombosis.3
Hyperhomocysteinemia due to a methylene tetrahydrofolate reductase (MTHFR) mutation (C677T) is another inherited associated risk factor for portal vein thrombosis, but hyperhomocysteinemia can also arise as a complication of portal vein thrombosis-related liver disease.3
Acquired prothrombotic disorders, particularly myeloproliferative diseases, are found in 22% to 48% of cases of portal vein thrombosis. Many young patients with myeloproliferative disorders present with portal vein thrombosis as the first symptom, and testing for the G1849T point mutation in JAK2 can make the diagnosis.17 Splenectomy with underlying myeloproliferative disorder confers a particularly high risk for portal vein thrombosis.18
Other thrombophilic disorders including antiphospholipid antibody syndrome, paroxysmal nocturnal hemoglobinuria, and malignancy can contribute to portal vein thrombosis.3 Pregnancy and oral contraceptive use have also been associated with hypercoagulability, and cessation of oral estrogen is recommended in such cases. The risk may be further increased in patients on oral contraceptives who have a previously unrecognized hypercoagulable state.3
Inflammation and infection
Inflammation and infection are local risk factors for portal vein thrombosis. Acute portal vein thrombosis has been associated with intra-abdominal infections (eg, appendicitis, cholecystitis) and with inflammatory conditions such as inflammatory bowel disease and pancreatitis.16,19 From 3% to 5% of all portal vein thrombosis cases result from pancreatitis, either from a single acute episode or from repeat inflammation of chronic pancreatitis.10 Portal vein thrombosis in the setting of inflammatory bowel disease can occur even when the disease is in remission, particularly in ulcerative colitis.20,21
Injury to the portal venous system
Abdominal surgery, particularly splenectomy, portosystemic shunting, colectomy, and blunt abdominal trauma can cause injury to the portal venous system, resulting in portal vein thrombosis. This is usually seen only in patients with portal hypertension, an underlying prothrombotic condition such as myeloproliferative disease, or inflammatory bowel disease.10,19,22
Impaired portal vein flow
Cirrhosis and malignancy are major risk factors for portal vein thrombosis. In case series, cirrhosis was found in 24% to 32% of patients with portal vein thrombosis.2,23 However, the overall prevalence of portal vein thrombosis in cirrhotic patients varies widely, from 0.6% to 28%, depending on the degree of cirrhosis.10
The pathogenesis of portal vein thrombosis in cirrhosis is unclear but may be multifactorial. Decreased portal blood flow (with subsequent stasis) and periportal lymphangitis and fibrosis are thought to stimulate thrombus formation.3,10 Additionally, patients with advanced cirrhosis are prothrombotic because of reduced hepatic synthesis of antithrombin, protein C, protein S, and coagulation factors.
Malignancy is associated with 21% to 24% of cases of portal vein thrombosis in adults, with pancreatic cancer and hepatocellular carcinoma being the most common.2,3 Others include cholangiocarcinoma and carcinomas of the stomach, lung, prostate, uterus, and kidney. Cancer causes portal vein thrombosis through a combination of tumor invasion into the portal vein, extrinsic compression by the tumor, periportal fibrosis following surgery or radiation, and hypercoagulability secondary to malignancy.9,16,24
Idiopathic portal vein thrombosis
Portal vein thrombosis is usually caused by one or more of the underlying factors mentioned above but is idiopathic in 8% to 15% of cases.10
Back to our patient
The cause of this patient’s portal vein thrombosis is unclear. He did not have a history of cirrhosis, inflammatory bowel disease, trauma, or abdominal surgery. His febrile illness could have precipitated the formation of a thrombus, but no definitive source of infection or inflammation was discovered. His workup was negative for pancreatitis, appendicitis, cholecystitis, diverticulitis, and prostatitis. No occult malignancy was found. It is also possible that his fever was the result of the thrombosis.
A full hypercoagulability panel revealed no striking abnormalities. He did have elevated fibrinogen and factor VIII levels that were consistent with an acute-phase reaction, along with an elevated erythrocyte sedimentation rate (> 90 mm/hr) and C-reactive protein level. Aside from the portal vein thrombosis, no potential source of inflammation could be identified.
Mildly reduced levels of antithrombin III activity were attributed to enoxaparin therapy and ultimately normalized on repeated testing. The patient had very minimally elevated titers of anticardiolipin immunoglobulin G (1:10 GPL) and anti-beta-2 glycoprotein immunoglobulin M (21 SMU), which were not thought to be significant. Tests for lupus anticoagulant, prothrombin gene mutation, activated protein C resistance, and JAK2 mutation were negative.
TREATMENT
4. Treatment of symptomatic portal vein thrombosis generally includes which two of the following?
- Anticoagulation
- Intravenous gamma globulin
- Broad-spectrum antibiotics
Anticoagulant therapy
Treatment of acute, symptomatic portal vein thrombosis involves anticoagulant therapy to prevent extension of the thrombus and, ultimately, to allow for recanalization of the obstructed veins. Anticoagulant therapy is initially intravenous unfractionated heparin or subcutaneous low-molecular-weight heparin, eventually bridged to an oral agent such as warfarin.3,9 Currently, there are inadequate data on the use of oral or parenteral factor Xa inhibitors or direct thrombin inhibitors in the treatment of this disease.
When started immediately, anticoagulation therapy is associated with complete recanalization in 38.3% and partial recanalization in 14% of patients presenting with complete thrombosis. Without anticoagulation, spontaneous recanalization is unusual.25
Although the optimal duration of anticoagulant therapy is unclear, a minimum of 3 months is generally recommended.9,26 If a hypercoagulable state is present or if the portal vein thrombosis is unprovoked (eg, by surgery, trauma, or an intra-abdominal infection), long-term treatment should be considered.26
Experience with thrombolytic therapy or mechanical recanalization has been limited, but the use of catheter-based techniques for pharmacomechanical thrombolysis has been reported.27–29 Transjugular intrahepatic portosystemic shunting is also an alternative to anticoagulation, but its role in treating portal vein thrombosis is complicated by technical difficulties of the procedure, postoperative complications, and recurrent occlusion of the shunt.25
Currently, there are no data comparing the risk-benefit ratio of early anticoagulation and that of invasive procedures. These more aggressive treatments are generally considered only when there is extensive thrombosis or ascites (which are both predictive factors of poor response to anticoagulation alone) and in patients for whom anticoagulation has failed.3 Surgical thrombectomy is rarely indicated, typically only in instances in which laparotomy is being performed for suspected bowel infarction.3
Antibiotics
In addition to anticoagulation, broad-spectrum antibiotics covering gram-negative and anaerobic bacteria are indicated for those cases of portal vein thrombosis associated with underlying infection.9
For chronic cases, the goals of management are to prevent and treat gastroesophageal variceal bleeding and to prevent recurrent thrombosis.9 Nonselective beta-blockers (eg, propranolol) and endoscopic band ligation have shown evidence of reducing the incidence of recurrent bleeding and prolonging survival in retrospective studies.9,30,31 Long-term anticoagulation is generally indicated to prevent further thrombosis and to increase the likelihood of recanalization only for patients with a permanent prothrombotic condition.9 In patients with clinically significant portal hypertension, the benefit of continued anticoagulation therapy must be weighed against the risk of esophageal and gastric variceal bleeding.
There is controversy regarding how to manage portal vein thrombosis that is incidentally identified and asymptomatic (eg, if it is discovered on an imaging study for another indication). Current guidelines recommend against anticoagulation in patients with incidentally discovered and asymptomatic splanchnic vein thrombosis, including portal vein thrombosis.26
Intravenous gamma globulin is not part of the treatment.
CASE CONTINUED
The patient’s presenting symptoms of fever, chills, and abdominal pain completely resolved after a course of antibiotic therapy. The erythrocyte sedimentation rate subsequently normalized and factor VIII activity improved. We believed that an underlying infectious or inflammatory process had contributed to the development of portal vein thrombosis, though the specific cause could not be identified. The patient was treated with enoxaparin 1 mg/kg twice a day and transitioned to warfarin.
Magnetic resonance venography done 3 months after diagnosis showed persistent right portal vein thrombosis that was largely unchanged. Anticoagulation was continued for 1 year with no change in his portal vein thrombosis on sequential imaging and was subsequently discontinued. To date, no malignancy or infectious process has been found, and the patient continues to do well 2 years later.
- Ponziani FR, Zocco MA, Campanale C, et al. Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment. World J Gastroenterol 2010; 16:143–155.
- Cohen J, Edelman RR, Chopra S. Portal vein thrombosis: a review. Am J Med 1992; 92:173–182.
- Primignani M. Portal vein thrombosis, revisited. Dig Liver Dis 2010; 42:163–170.
- Condat B, Valla D. Nonmalignant portal vein thrombosis in adults. Nat Clin Pract Gastroenterol Hepatol 2006; 3:505–515.
- Condat B, Pessione F, Helene Denninger M, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology 2000; 32:466–470.
- Sheen CL, Lamparelli H, Milne A, Green I, Ramage JK. Clinical features, diagnosis and outcome of acute portal vein thrombosis. QJM 2000; 93:531–534.
- Sogaard KK, Astrup LB, Vilstrup H, Gronbaek H. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol 2007; 7:34.
- Llop E, de Juan C, Seijo S, et al. Portal cholangiopathy: radiological classification and natural history. Gut 2011; 60:853–860.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Sobhonslidsuk A, Reddy KR. Portal vein thrombosis: a concise review. Am J Gastroenterol 2002; 97:535–541.
- Ni YH, Wang NC, Peng MY, Chou YY, Chang FY. Bacteroides fragilis bacteremia associated with portal vein and superior mesentery vein thrombosis secondary to antithrombin III and protein C deficiency: a case report. J Microbiol Immunol Infect 2002; 35:255–258.
- Trum J, Valla D, Cohen G, et al. Bacteroides bacteraemia of undetermined origin: strong association with portal vein thrombosis and cryptogenic pylephlebitis. Eur J Gastroenterol Hepatol 1993; 5:655–659.
- Ueno N, Sasaki A, Tomiyama T, Tano S, Kimura K. Color Doppler ultrasonography in the diagnosis of cavernous transformation of the portal vein. J Clin Ultrasound 1997; 25:227–233.
- Tessler FN, Gehring BJ, Gomes AS, et al. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol 1991; 157:293–296.
- Hidajat N, Stobbe H, Griesshaber V, Felix R, Schroder RJ. Imaging and radiological interventions of portal vein thrombosis. Acta Radiol 2005; 46:336–343.
- Valla DC, Condat B. Portal vein thrombosis in adults: pathophysiology, pathogenesis and management. J Hepatol 2000; 32:865–871.
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005; 352:1779–1790.
- Krauth MT, Lechner K, Neugebauer EA, Pabinger I. The postoperative splenic/portal vein thrombosis after splenectomy and its prevention—an unresolved issue. Haematologica 2008; 93:1227–1232.
- Sinagra E, Aragona E, Romano C, et al. The role of portal vein thrombosis in the clinical course of inflammatory bowel diseases: report on three cases and review of the literature. Gastroenterol Res Pract 2012; 2012:916428.
- Maconi G, Bolzacchini E, Dell’Era A, Russo U, Ardizzone S, de Franchis R. Portal vein thrombosis in inflammatory bowel diseases: a single-center case series. J Crohns Colitis 2012; 6:362–367.
- Jackson LM, O’Gorman PJ, O’Connell J, Cronin CC, Cotter KP, Shanahan F. Thrombosis in inflammatory bowel disease: clinical setting, procoagulant profile and factor V Leiden. QJM 1997; 90:183–188.
- Eguchi A, Hashizume M, Kitano S, Tanoue K, Wada H, Sugimachi K. High rate of portal thrombosis after splenectomy in patients with esophageal varices and idiopathic portal hypertension. Arch Surg 1991; 126:752–755.
- Ogren M, Bergqvist D, Björck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol 2006; 12:2115–2119.
- Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013; 11:223–233.
- Congly SE, Lee SS. Portal vein thrombosis: should anticoagulation be used? Curr Gastroenterol Rep 2013; 15:306.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Uflacker R. Applications of percutaneous mechanical thrombectomy in transjugular intrahepatic portosystemic shunt and portal vein thrombosis. Tech Vasc Interv Radiol 2003; 6:59–69.
- Takahashi N, Kuroki K, Yanaga K. Percutaneous transhepatic mechanical thrombectomy for acute mesenteric venous thrombosis. J Endovasc Ther 2005; 12:508–511.
- Lopera JE, Correa G, Brazzini A, et al. Percutaneous transhepatic treatment of symptomatic mesenteric venous thrombosis. J Vasc Surg 2002; 36:1058–1061.
- Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5:80–86.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001; 120:490–497.
- Ponziani FR, Zocco MA, Campanale C, et al. Portal vein thrombosis: insight into physiopathology, diagnosis, and treatment. World J Gastroenterol 2010; 16:143–155.
- Cohen J, Edelman RR, Chopra S. Portal vein thrombosis: a review. Am J Med 1992; 92:173–182.
- Primignani M. Portal vein thrombosis, revisited. Dig Liver Dis 2010; 42:163–170.
- Condat B, Valla D. Nonmalignant portal vein thrombosis in adults. Nat Clin Pract Gastroenterol Hepatol 2006; 3:505–515.
- Condat B, Pessione F, Helene Denninger M, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology 2000; 32:466–470.
- Sheen CL, Lamparelli H, Milne A, Green I, Ramage JK. Clinical features, diagnosis and outcome of acute portal vein thrombosis. QJM 2000; 93:531–534.
- Sogaard KK, Astrup LB, Vilstrup H, Gronbaek H. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol 2007; 7:34.
- Llop E, de Juan C, Seijo S, et al. Portal cholangiopathy: radiological classification and natural history. Gut 2011; 60:853–860.
- DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study of Liver Diseases. Vascular disorders of the liver. Hepatology 2009; 49:1729–1764.
- Sobhonslidsuk A, Reddy KR. Portal vein thrombosis: a concise review. Am J Gastroenterol 2002; 97:535–541.
- Ni YH, Wang NC, Peng MY, Chou YY, Chang FY. Bacteroides fragilis bacteremia associated with portal vein and superior mesentery vein thrombosis secondary to antithrombin III and protein C deficiency: a case report. J Microbiol Immunol Infect 2002; 35:255–258.
- Trum J, Valla D, Cohen G, et al. Bacteroides bacteraemia of undetermined origin: strong association with portal vein thrombosis and cryptogenic pylephlebitis. Eur J Gastroenterol Hepatol 1993; 5:655–659.
- Ueno N, Sasaki A, Tomiyama T, Tano S, Kimura K. Color Doppler ultrasonography in the diagnosis of cavernous transformation of the portal vein. J Clin Ultrasound 1997; 25:227–233.
- Tessler FN, Gehring BJ, Gomes AS, et al. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol 1991; 157:293–296.
- Hidajat N, Stobbe H, Griesshaber V, Felix R, Schroder RJ. Imaging and radiological interventions of portal vein thrombosis. Acta Radiol 2005; 46:336–343.
- Valla DC, Condat B. Portal vein thrombosis in adults: pathophysiology, pathogenesis and management. J Hepatol 2000; 32:865–871.
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005; 352:1779–1790.
- Krauth MT, Lechner K, Neugebauer EA, Pabinger I. The postoperative splenic/portal vein thrombosis after splenectomy and its prevention—an unresolved issue. Haematologica 2008; 93:1227–1232.
- Sinagra E, Aragona E, Romano C, et al. The role of portal vein thrombosis in the clinical course of inflammatory bowel diseases: report on three cases and review of the literature. Gastroenterol Res Pract 2012; 2012:916428.
- Maconi G, Bolzacchini E, Dell’Era A, Russo U, Ardizzone S, de Franchis R. Portal vein thrombosis in inflammatory bowel diseases: a single-center case series. J Crohns Colitis 2012; 6:362–367.
- Jackson LM, O’Gorman PJ, O’Connell J, Cronin CC, Cotter KP, Shanahan F. Thrombosis in inflammatory bowel disease: clinical setting, procoagulant profile and factor V Leiden. QJM 1997; 90:183–188.
- Eguchi A, Hashizume M, Kitano S, Tanoue K, Wada H, Sugimachi K. High rate of portal thrombosis after splenectomy in patients with esophageal varices and idiopathic portal hypertension. Arch Surg 1991; 126:752–755.
- Ogren M, Bergqvist D, Björck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol 2006; 12:2115–2119.
- Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013; 11:223–233.
- Congly SE, Lee SS. Portal vein thrombosis: should anticoagulation be used? Curr Gastroenterol Rep 2013; 15:306.
- Kearon C, Akl EA, Comerota AJ, et al; American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e419S–e494S.
- Uflacker R. Applications of percutaneous mechanical thrombectomy in transjugular intrahepatic portosystemic shunt and portal vein thrombosis. Tech Vasc Interv Radiol 2003; 6:59–69.
- Takahashi N, Kuroki K, Yanaga K. Percutaneous transhepatic mechanical thrombectomy for acute mesenteric venous thrombosis. J Endovasc Ther 2005; 12:508–511.
- Lopera JE, Correa G, Brazzini A, et al. Percutaneous transhepatic treatment of symptomatic mesenteric venous thrombosis. J Vasc Surg 2002; 36:1058–1061.
- Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5:80–86.
- Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology 2001; 120:490–497.
Implantable filter doesn’t cut rate of recurrent PE
Implanting a retrievable filter in the inferior vena cava did not reduce the rate of recurrent pulmonary embolism or mortality in high-risk patients, according to a report published online April 28 in JAMA.
In recent years, there has been a sharp increase in the use of these devices as an add-on to anticoagulant therapy among patients hospitalized for acute PE associated with lower-limb deep or superficial vein thrombosis. Several clinical guidelines advocate this strategy, though others do not, citing the paucity of reliable data concerning both risks and benefits.
The findings in this study “do not support the use of this type of filter in patients who can be treated with anticoagulation alone,” and clinical guidelines recommending this approach should be reexamined, Dr. Patrick Mismetti of the University Hospital of Saint-Etienne, France, and his associates said.
They performed a randomized, open-label clinical study at 17 French medical centers to compare anticoagulation alone against anticoagulation plus implanting a filter to be retrieved 3 months later.
The study participants were 399 adults enrolled during a 6-year period who were deemed at high risk for recurrent PE because of advanced age, active cancer, chronic cardiac or respiratory insufficiency, ischemic stroke with leg paralysis, DVT that was bilateral or affected the iliocaval segment, or signs of right ventricular dysfunction or myocardial injury.
The primary efficacy outcome, recurrent PE within 3 months of hospitalization, developed in 6 of 200 patients assigned to receive an implantable filter (3%) and 3 of the 199 assigned to the control group (1.5%). All but one of these episodes of recurrent PE were fatal. One additional PE developed in each study group between 3 and 6 months. There were no differences between patients who received an inferior vena cava filter and those who did not in the incidence of DVT, major bleeding, or death from any cause at 3 or 6 months, the investigators said (JAMA 2015 April 28 [doi:10.1001/jama.2015.3780]).
Besides failing to prevent recurrent PE, the filter implantation caused access site hematomas in five patients, and the filter itself caused thrombosis formation in three. One patient developed cardiac arrest during the procedure. In addition, retrieval of the device failed in 11 patients because of mechanical problems.
Implanting a retrievable filter in the inferior vena cava did not reduce the rate of recurrent pulmonary embolism or mortality in high-risk patients, according to a report published online April 28 in JAMA.
In recent years, there has been a sharp increase in the use of these devices as an add-on to anticoagulant therapy among patients hospitalized for acute PE associated with lower-limb deep or superficial vein thrombosis. Several clinical guidelines advocate this strategy, though others do not, citing the paucity of reliable data concerning both risks and benefits.
The findings in this study “do not support the use of this type of filter in patients who can be treated with anticoagulation alone,” and clinical guidelines recommending this approach should be reexamined, Dr. Patrick Mismetti of the University Hospital of Saint-Etienne, France, and his associates said.
They performed a randomized, open-label clinical study at 17 French medical centers to compare anticoagulation alone against anticoagulation plus implanting a filter to be retrieved 3 months later.
The study participants were 399 adults enrolled during a 6-year period who were deemed at high risk for recurrent PE because of advanced age, active cancer, chronic cardiac or respiratory insufficiency, ischemic stroke with leg paralysis, DVT that was bilateral or affected the iliocaval segment, or signs of right ventricular dysfunction or myocardial injury.
The primary efficacy outcome, recurrent PE within 3 months of hospitalization, developed in 6 of 200 patients assigned to receive an implantable filter (3%) and 3 of the 199 assigned to the control group (1.5%). All but one of these episodes of recurrent PE were fatal. One additional PE developed in each study group between 3 and 6 months. There were no differences between patients who received an inferior vena cava filter and those who did not in the incidence of DVT, major bleeding, or death from any cause at 3 or 6 months, the investigators said (JAMA 2015 April 28 [doi:10.1001/jama.2015.3780]).
Besides failing to prevent recurrent PE, the filter implantation caused access site hematomas in five patients, and the filter itself caused thrombosis formation in three. One patient developed cardiac arrest during the procedure. In addition, retrieval of the device failed in 11 patients because of mechanical problems.
Implanting a retrievable filter in the inferior vena cava did not reduce the rate of recurrent pulmonary embolism or mortality in high-risk patients, according to a report published online April 28 in JAMA.
In recent years, there has been a sharp increase in the use of these devices as an add-on to anticoagulant therapy among patients hospitalized for acute PE associated with lower-limb deep or superficial vein thrombosis. Several clinical guidelines advocate this strategy, though others do not, citing the paucity of reliable data concerning both risks and benefits.
The findings in this study “do not support the use of this type of filter in patients who can be treated with anticoagulation alone,” and clinical guidelines recommending this approach should be reexamined, Dr. Patrick Mismetti of the University Hospital of Saint-Etienne, France, and his associates said.
They performed a randomized, open-label clinical study at 17 French medical centers to compare anticoagulation alone against anticoagulation plus implanting a filter to be retrieved 3 months later.
The study participants were 399 adults enrolled during a 6-year period who were deemed at high risk for recurrent PE because of advanced age, active cancer, chronic cardiac or respiratory insufficiency, ischemic stroke with leg paralysis, DVT that was bilateral or affected the iliocaval segment, or signs of right ventricular dysfunction or myocardial injury.
The primary efficacy outcome, recurrent PE within 3 months of hospitalization, developed in 6 of 200 patients assigned to receive an implantable filter (3%) and 3 of the 199 assigned to the control group (1.5%). All but one of these episodes of recurrent PE were fatal. One additional PE developed in each study group between 3 and 6 months. There were no differences between patients who received an inferior vena cava filter and those who did not in the incidence of DVT, major bleeding, or death from any cause at 3 or 6 months, the investigators said (JAMA 2015 April 28 [doi:10.1001/jama.2015.3780]).
Besides failing to prevent recurrent PE, the filter implantation caused access site hematomas in five patients, and the filter itself caused thrombosis formation in three. One patient developed cardiac arrest during the procedure. In addition, retrieval of the device failed in 11 patients because of mechanical problems.
FROM JAMA
Key clinical point: Use of a retrievable filter implanted in the inferior vena cava did not reduce the rate of recurrent pulmonary embolism.
Major finding: The primary efficacy outcome, recurrent PE within 3 months of hospitalization, developed in 6 of 200 patients assigned to receive an implantable filter (3%) and 3 of 199 assigned to the control group (1.5%).
Data source: An open-label randomized trial involving 399 adults hospitalized in France for acute PE.
Disclosures: This study was sponsored by the University Hospital of Saint-Etienne and supported by the French Department of Health, Fondation de l’Avenir, and Fondation de France. Vena cava filters were provided free of charge by ALN Implants Chirurgicaux. Dr. Mismetti and his associates reported ties to numerous industry sources.
Angiojet system found safe, effective in lower-extremity deep venous thrombosis
More than 80% of patients with lower-extremity deep venous thrombosis who underwent endovascular treatment with the Angiojet rheolytic thrombectomy system were free of rethrombosis a year later, based on final results from the PEARL registry study.
Almost 4% of patients had bleeding events after treatment, but none of these events was tied to use of the Angiojet system, reported Dr. Mark Garcia of Mount Sinai Medical Center in New York and his associates.
“PEARL registry data demonstrate that rheolytic pharmacomechanical catheter-directed thrombolysis treatment of deep venous thrombosis is safe and effective, and can potentially reduce the need for concomitant catheter-directed thrombolysis and intensive care,” the researchers wrote.
The rates of venous thromboembolism are rising, and the number of affected adults is expected to double in the next 40 years as the population ages and experiences recurrent episodes. Lower-extremity deep venous thrombosis (DVT) is especially likely to recur or to develop complications such as pulmonary embolism and post-thrombotic syndrome. For this reason, practice guidelines now advocate early removal of iliofemoral clots if patients are functional, have a good life expectancy, are within 14 days of symptom onset, and are unlikely to develop bleeding complications. Options for clot removal include catheter-directed thrombolysis (CDT) or pharmacomechanical CDT, which combines catheterization with intervention to break up or aspirate the clot while infusing it with a thrombolytic drug, said the investigators (J. Vasc. Interv. Radiol. 2015 Mar. 27 [doi:10.1016/j.jvir.2015.01.036]).
The PEARL registry study prospectively followed patients who underwent PCDT for arterial or venous thrombosis with the AngioJet thrombectomy catheter system. Researchers analyzed data from 329 patients with severe lower-extremity DVT who were treated at 32 sites in the United States and Europe between 2007 and 2013. Two-thirds of the patients underwent Angiojet thrombectomy within 2 weeks of symptom onset, while 19% were treated within 15 to 30 days and 14% were treated for chronic lesions. A total of 57% of patients were men, and the average age at onset was 52 years. The cohort’s most prevalent risk factors for thrombosis included a history of DVT, preexisting caval filters, past or current tobacco use, and prior pulmonary embolism, the investigators reported.
Grade III (100%) clot removal was possible without needing to use CDT in 39% of patients. Most of these patients underwent PCDT alone (lasting a median of 2 hours), while the rest underwent rheolytic thrombectomy without a lytic agent (median, 1.4 hours). However, just over half of patients underwent PCDT and catheter-directed thrombolysis, lasting a median of 22 hours, and 9% underwent rheolytic thrombectomy with CDT (median, 41 hours). About three-quarters of patients had procedures lasting under 24 hours, and about one in three were done within 6 hours. Also, 86% of procedures required no more than two catheter laboratory sessions.
Three months after treatment, 94% of patients were free from rethrombosis, and 87% and 83% of the cohort remained so at 6 and 12 months, respectively, the researchers added. Even patients with chronic thrombi improved so much on the 12-item Short-Form Health Survey that their scores approximated population norms with a year of treatment, they said.
A total of nine patients (2.7%) had adverse events possibly related to treatment, including one case of acute renal failure, said the investigators. Clinicians should follow recommendations for hydration and limit run time to four minutes in a free-flowing vessel to prevent that outcome, they added.
Dr. Garcia and his associates reported being paid consultants for Boston Scientific, which makes the Angiojet thrombectomy catheter system and funded the study. Dr. Garcia also reported grant funds and consulting fees from BTG/EKOS and Cook. Four coauthors reported receiving consulting fees from Cordis, Cook, Medtronic, AstraZeneca, and Covidien.
More than 80% of patients with lower-extremity deep venous thrombosis who underwent endovascular treatment with the Angiojet rheolytic thrombectomy system were free of rethrombosis a year later, based on final results from the PEARL registry study.
Almost 4% of patients had bleeding events after treatment, but none of these events was tied to use of the Angiojet system, reported Dr. Mark Garcia of Mount Sinai Medical Center in New York and his associates.
“PEARL registry data demonstrate that rheolytic pharmacomechanical catheter-directed thrombolysis treatment of deep venous thrombosis is safe and effective, and can potentially reduce the need for concomitant catheter-directed thrombolysis and intensive care,” the researchers wrote.
The rates of venous thromboembolism are rising, and the number of affected adults is expected to double in the next 40 years as the population ages and experiences recurrent episodes. Lower-extremity deep venous thrombosis (DVT) is especially likely to recur or to develop complications such as pulmonary embolism and post-thrombotic syndrome. For this reason, practice guidelines now advocate early removal of iliofemoral clots if patients are functional, have a good life expectancy, are within 14 days of symptom onset, and are unlikely to develop bleeding complications. Options for clot removal include catheter-directed thrombolysis (CDT) or pharmacomechanical CDT, which combines catheterization with intervention to break up or aspirate the clot while infusing it with a thrombolytic drug, said the investigators (J. Vasc. Interv. Radiol. 2015 Mar. 27 [doi:10.1016/j.jvir.2015.01.036]).
The PEARL registry study prospectively followed patients who underwent PCDT for arterial or venous thrombosis with the AngioJet thrombectomy catheter system. Researchers analyzed data from 329 patients with severe lower-extremity DVT who were treated at 32 sites in the United States and Europe between 2007 and 2013. Two-thirds of the patients underwent Angiojet thrombectomy within 2 weeks of symptom onset, while 19% were treated within 15 to 30 days and 14% were treated for chronic lesions. A total of 57% of patients were men, and the average age at onset was 52 years. The cohort’s most prevalent risk factors for thrombosis included a history of DVT, preexisting caval filters, past or current tobacco use, and prior pulmonary embolism, the investigators reported.
Grade III (100%) clot removal was possible without needing to use CDT in 39% of patients. Most of these patients underwent PCDT alone (lasting a median of 2 hours), while the rest underwent rheolytic thrombectomy without a lytic agent (median, 1.4 hours). However, just over half of patients underwent PCDT and catheter-directed thrombolysis, lasting a median of 22 hours, and 9% underwent rheolytic thrombectomy with CDT (median, 41 hours). About three-quarters of patients had procedures lasting under 24 hours, and about one in three were done within 6 hours. Also, 86% of procedures required no more than two catheter laboratory sessions.
Three months after treatment, 94% of patients were free from rethrombosis, and 87% and 83% of the cohort remained so at 6 and 12 months, respectively, the researchers added. Even patients with chronic thrombi improved so much on the 12-item Short-Form Health Survey that their scores approximated population norms with a year of treatment, they said.
A total of nine patients (2.7%) had adverse events possibly related to treatment, including one case of acute renal failure, said the investigators. Clinicians should follow recommendations for hydration and limit run time to four minutes in a free-flowing vessel to prevent that outcome, they added.
Dr. Garcia and his associates reported being paid consultants for Boston Scientific, which makes the Angiojet thrombectomy catheter system and funded the study. Dr. Garcia also reported grant funds and consulting fees from BTG/EKOS and Cook. Four coauthors reported receiving consulting fees from Cordis, Cook, Medtronic, AstraZeneca, and Covidien.
More than 80% of patients with lower-extremity deep venous thrombosis who underwent endovascular treatment with the Angiojet rheolytic thrombectomy system were free of rethrombosis a year later, based on final results from the PEARL registry study.
Almost 4% of patients had bleeding events after treatment, but none of these events was tied to use of the Angiojet system, reported Dr. Mark Garcia of Mount Sinai Medical Center in New York and his associates.
“PEARL registry data demonstrate that rheolytic pharmacomechanical catheter-directed thrombolysis treatment of deep venous thrombosis is safe and effective, and can potentially reduce the need for concomitant catheter-directed thrombolysis and intensive care,” the researchers wrote.
The rates of venous thromboembolism are rising, and the number of affected adults is expected to double in the next 40 years as the population ages and experiences recurrent episodes. Lower-extremity deep venous thrombosis (DVT) is especially likely to recur or to develop complications such as pulmonary embolism and post-thrombotic syndrome. For this reason, practice guidelines now advocate early removal of iliofemoral clots if patients are functional, have a good life expectancy, are within 14 days of symptom onset, and are unlikely to develop bleeding complications. Options for clot removal include catheter-directed thrombolysis (CDT) or pharmacomechanical CDT, which combines catheterization with intervention to break up or aspirate the clot while infusing it with a thrombolytic drug, said the investigators (J. Vasc. Interv. Radiol. 2015 Mar. 27 [doi:10.1016/j.jvir.2015.01.036]).
The PEARL registry study prospectively followed patients who underwent PCDT for arterial or venous thrombosis with the AngioJet thrombectomy catheter system. Researchers analyzed data from 329 patients with severe lower-extremity DVT who were treated at 32 sites in the United States and Europe between 2007 and 2013. Two-thirds of the patients underwent Angiojet thrombectomy within 2 weeks of symptom onset, while 19% were treated within 15 to 30 days and 14% were treated for chronic lesions. A total of 57% of patients were men, and the average age at onset was 52 years. The cohort’s most prevalent risk factors for thrombosis included a history of DVT, preexisting caval filters, past or current tobacco use, and prior pulmonary embolism, the investigators reported.
Grade III (100%) clot removal was possible without needing to use CDT in 39% of patients. Most of these patients underwent PCDT alone (lasting a median of 2 hours), while the rest underwent rheolytic thrombectomy without a lytic agent (median, 1.4 hours). However, just over half of patients underwent PCDT and catheter-directed thrombolysis, lasting a median of 22 hours, and 9% underwent rheolytic thrombectomy with CDT (median, 41 hours). About three-quarters of patients had procedures lasting under 24 hours, and about one in three were done within 6 hours. Also, 86% of procedures required no more than two catheter laboratory sessions.
Three months after treatment, 94% of patients were free from rethrombosis, and 87% and 83% of the cohort remained so at 6 and 12 months, respectively, the researchers added. Even patients with chronic thrombi improved so much on the 12-item Short-Form Health Survey that their scores approximated population norms with a year of treatment, they said.
A total of nine patients (2.7%) had adverse events possibly related to treatment, including one case of acute renal failure, said the investigators. Clinicians should follow recommendations for hydration and limit run time to four minutes in a free-flowing vessel to prevent that outcome, they added.
Dr. Garcia and his associates reported being paid consultants for Boston Scientific, which makes the Angiojet thrombectomy catheter system and funded the study. Dr. Garcia also reported grant funds and consulting fees from BTG/EKOS and Cook. Four coauthors reported receiving consulting fees from Cordis, Cook, Medtronic, AstraZeneca, and Covidien.
FROM THE JOURNAL OF VASCULAR INTERVENTIONAL RADIOLOGY
Key clinical point: Endovascular treatment with the Angiojet rheolytic thrombectomy systemwas found safe and effective for lower-extremity deep venous thrombosis.
Major finding: 83% of patients were free of rethrombosis a year later, and only one suffered a serious adverse event (acute renal failure).
Data source: A multicenter prospective registry study of 329 patients who underwent Angiojet thrombectomy with or without catheter-directed thrombolysis.
Disclosures: Dr. Garcia and his associates reported being paid consultants for Boston Scientific, which makes the Angiojet thrombectomy catheter system and funded the study. Dr. Garcia also reported grant funds and consulting fees from BTG/EKOS and Cook. Four coauthors reported receiving consulting fees from Cordis, Cook, Medtronic, AstraZeneca, and Covidien.
Study: More DVTs than expected in patients who had varicose vein surgeries
A retrospective study of patients who underwent varicose vein surgeries with a tourniquet found a greater incidence of deep vein thromboses (DVTs) than previous studies.
Within the first 3 postoperative days, 113 (7.7%) of the 1,461 patients had DVTs. The researchers also found that DVTs occurred significantly more often in patients with gastrocnemius vein dilation (GVD). A total of 410 (28%) of the study’s participants had GVTs, and the incidence of DVTs was significantly greater in individuals with GVD compared to those without such a symptom. GVD had a higher predictive power for postoperative DVT than did all of the other risk factors examined in univariate and multivariate analyses.
The vast majority of the DVTs diagnosed were isolated distal. While 94 patients suffered from this kind of DVT, the remaining 19 DVTs were proximal. According to Dr. Chen Kai of Wenzhou (China) Medical University, and colleagues, proximal DVTs were nearly always asymptomatic and a larger percentage of them took more time to disappear than did the distal DVTs. Within 6 months following anticoagulant therapy, 94.3% of the distal DVTs exhibited thrombus resolution and 55.6% of the proximal DVTs were thrombus free. None of the study’s participants had died because of DVT or pulmonary embolus during the 6 months following their surgeries.
This study’s “present data reflect a higher incidence of postoperative DVT than previous studies, and we also identify GVD as a significant risk factor. Larger prospective studies will be needed to evaluate this issue precisely and to understand the clinical relevance of these results,” wrote the researchers.Find the full study in Thombosis Research (doi: 10.1016/j.thromres.2015.03.008).
A retrospective study of patients who underwent varicose vein surgeries with a tourniquet found a greater incidence of deep vein thromboses (DVTs) than previous studies.
Within the first 3 postoperative days, 113 (7.7%) of the 1,461 patients had DVTs. The researchers also found that DVTs occurred significantly more often in patients with gastrocnemius vein dilation (GVD). A total of 410 (28%) of the study’s participants had GVTs, and the incidence of DVTs was significantly greater in individuals with GVD compared to those without such a symptom. GVD had a higher predictive power for postoperative DVT than did all of the other risk factors examined in univariate and multivariate analyses.
The vast majority of the DVTs diagnosed were isolated distal. While 94 patients suffered from this kind of DVT, the remaining 19 DVTs were proximal. According to Dr. Chen Kai of Wenzhou (China) Medical University, and colleagues, proximal DVTs were nearly always asymptomatic and a larger percentage of them took more time to disappear than did the distal DVTs. Within 6 months following anticoagulant therapy, 94.3% of the distal DVTs exhibited thrombus resolution and 55.6% of the proximal DVTs were thrombus free. None of the study’s participants had died because of DVT or pulmonary embolus during the 6 months following their surgeries.
This study’s “present data reflect a higher incidence of postoperative DVT than previous studies, and we also identify GVD as a significant risk factor. Larger prospective studies will be needed to evaluate this issue precisely and to understand the clinical relevance of these results,” wrote the researchers.Find the full study in Thombosis Research (doi: 10.1016/j.thromres.2015.03.008).
A retrospective study of patients who underwent varicose vein surgeries with a tourniquet found a greater incidence of deep vein thromboses (DVTs) than previous studies.
Within the first 3 postoperative days, 113 (7.7%) of the 1,461 patients had DVTs. The researchers also found that DVTs occurred significantly more often in patients with gastrocnemius vein dilation (GVD). A total of 410 (28%) of the study’s participants had GVTs, and the incidence of DVTs was significantly greater in individuals with GVD compared to those without such a symptom. GVD had a higher predictive power for postoperative DVT than did all of the other risk factors examined in univariate and multivariate analyses.
The vast majority of the DVTs diagnosed were isolated distal. While 94 patients suffered from this kind of DVT, the remaining 19 DVTs were proximal. According to Dr. Chen Kai of Wenzhou (China) Medical University, and colleagues, proximal DVTs were nearly always asymptomatic and a larger percentage of them took more time to disappear than did the distal DVTs. Within 6 months following anticoagulant therapy, 94.3% of the distal DVTs exhibited thrombus resolution and 55.6% of the proximal DVTs were thrombus free. None of the study’s participants had died because of DVT or pulmonary embolus during the 6 months following their surgeries.
This study’s “present data reflect a higher incidence of postoperative DVT than previous studies, and we also identify GVD as a significant risk factor. Larger prospective studies will be needed to evaluate this issue precisely and to understand the clinical relevance of these results,” wrote the researchers.Find the full study in Thombosis Research (doi: 10.1016/j.thromres.2015.03.008).
Few PE patients treated with catheter-directed interventions had complications
A review of research suggests that catheter-directed interventions (CDIs) have fewer complications but are not necessarily better at preventing mortality than are standard treatments for pulmonary embolisms, according to Dr. Efthymios D. Avgerinos and Dr. Rabih A. Chaer of the University of Pittsburgh.
Of 594 patients with massive pulmonary embolisms (PEs) who received various forms of CDI, 86.5% survived (range, 40%-100%), according to a systematic review of 35 noncontrolled studies.
“In 95% of these patients, CDIs were initiated without prior intravenous thrombolysis,” while 60%-67% of the patients also received a thrombolytic agent during the procedure, they wrote. The patient survival rate was 91.2% in studies that provided at least 80% of their patients with local thrombolytic therapy during a CDI, compared with 82.8% in studies in which less than 80% of participants received thrombolytic therapy.
Not all findings, however, suggested that it was more favorable for the patients to receive the thrombolytic therapy, Overall, the pooled rates of major and minor complications were 7.9% and 2.4%, respectively. The 25 major complications reported included bleeding complications requiring transfusion, renal failure requiring hemodialysis, cardiopulmonary events, cerebrovascular events, and death.
Other research on CDIs found that right ventricle dilation was reversed in patients with submassive PEs who received fixed-dose, ultrasound-assisted, catheter-directed thrombosis combined with anticoagulation. According to the recently published randomized controlled trial, which compared the effects of fixed-dose, ultrasound-assisted, catheter-directed thrombosis and anticoagulation to anticoagulation alone, the mean right-to-left-ventricle ratio was reduced for patients in the CDI group after 1 day. Such a change did not occur in the control group, but at 90 days, the average ratio “became comparable between the two groups … with a trend in favor of the [CDI],” according to Dr. Avgerinos and Dr. Chaer. None of this study’s participants suffered from major bleeding complications.
“There is increasing evidence that percutaneous CDIs are an essential, effective and safe alternative to systemic thrombolysis or anticoagulation in the contemporary management of massive and submassive PE,” the reviewers noted. More research is needed to confirm the differences in the outcomes between using systemic thrombolysis and catheter-based techniques for treating PEs, as no clinical trial comparing CDIs with systemic thrombolysis for PE has been done, they added.
Read the full review of research in the Journal of Vascular Surgery (doi:10.1016/j.jvs.2014.10.036).
A review of research suggests that catheter-directed interventions (CDIs) have fewer complications but are not necessarily better at preventing mortality than are standard treatments for pulmonary embolisms, according to Dr. Efthymios D. Avgerinos and Dr. Rabih A. Chaer of the University of Pittsburgh.
Of 594 patients with massive pulmonary embolisms (PEs) who received various forms of CDI, 86.5% survived (range, 40%-100%), according to a systematic review of 35 noncontrolled studies.
“In 95% of these patients, CDIs were initiated without prior intravenous thrombolysis,” while 60%-67% of the patients also received a thrombolytic agent during the procedure, they wrote. The patient survival rate was 91.2% in studies that provided at least 80% of their patients with local thrombolytic therapy during a CDI, compared with 82.8% in studies in which less than 80% of participants received thrombolytic therapy.
Not all findings, however, suggested that it was more favorable for the patients to receive the thrombolytic therapy, Overall, the pooled rates of major and minor complications were 7.9% and 2.4%, respectively. The 25 major complications reported included bleeding complications requiring transfusion, renal failure requiring hemodialysis, cardiopulmonary events, cerebrovascular events, and death.
Other research on CDIs found that right ventricle dilation was reversed in patients with submassive PEs who received fixed-dose, ultrasound-assisted, catheter-directed thrombosis combined with anticoagulation. According to the recently published randomized controlled trial, which compared the effects of fixed-dose, ultrasound-assisted, catheter-directed thrombosis and anticoagulation to anticoagulation alone, the mean right-to-left-ventricle ratio was reduced for patients in the CDI group after 1 day. Such a change did not occur in the control group, but at 90 days, the average ratio “became comparable between the two groups … with a trend in favor of the [CDI],” according to Dr. Avgerinos and Dr. Chaer. None of this study’s participants suffered from major bleeding complications.
“There is increasing evidence that percutaneous CDIs are an essential, effective and safe alternative to systemic thrombolysis or anticoagulation in the contemporary management of massive and submassive PE,” the reviewers noted. More research is needed to confirm the differences in the outcomes between using systemic thrombolysis and catheter-based techniques for treating PEs, as no clinical trial comparing CDIs with systemic thrombolysis for PE has been done, they added.
Read the full review of research in the Journal of Vascular Surgery (doi:10.1016/j.jvs.2014.10.036).
A review of research suggests that catheter-directed interventions (CDIs) have fewer complications but are not necessarily better at preventing mortality than are standard treatments for pulmonary embolisms, according to Dr. Efthymios D. Avgerinos and Dr. Rabih A. Chaer of the University of Pittsburgh.
Of 594 patients with massive pulmonary embolisms (PEs) who received various forms of CDI, 86.5% survived (range, 40%-100%), according to a systematic review of 35 noncontrolled studies.
“In 95% of these patients, CDIs were initiated without prior intravenous thrombolysis,” while 60%-67% of the patients also received a thrombolytic agent during the procedure, they wrote. The patient survival rate was 91.2% in studies that provided at least 80% of their patients with local thrombolytic therapy during a CDI, compared with 82.8% in studies in which less than 80% of participants received thrombolytic therapy.
Not all findings, however, suggested that it was more favorable for the patients to receive the thrombolytic therapy, Overall, the pooled rates of major and minor complications were 7.9% and 2.4%, respectively. The 25 major complications reported included bleeding complications requiring transfusion, renal failure requiring hemodialysis, cardiopulmonary events, cerebrovascular events, and death.
Other research on CDIs found that right ventricle dilation was reversed in patients with submassive PEs who received fixed-dose, ultrasound-assisted, catheter-directed thrombosis combined with anticoagulation. According to the recently published randomized controlled trial, which compared the effects of fixed-dose, ultrasound-assisted, catheter-directed thrombosis and anticoagulation to anticoagulation alone, the mean right-to-left-ventricle ratio was reduced for patients in the CDI group after 1 day. Such a change did not occur in the control group, but at 90 days, the average ratio “became comparable between the two groups … with a trend in favor of the [CDI],” according to Dr. Avgerinos and Dr. Chaer. None of this study’s participants suffered from major bleeding complications.
“There is increasing evidence that percutaneous CDIs are an essential, effective and safe alternative to systemic thrombolysis or anticoagulation in the contemporary management of massive and submassive PE,” the reviewers noted. More research is needed to confirm the differences in the outcomes between using systemic thrombolysis and catheter-based techniques for treating PEs, as no clinical trial comparing CDIs with systemic thrombolysis for PE has been done, they added.
Read the full review of research in the Journal of Vascular Surgery (doi:10.1016/j.jvs.2014.10.036).