User login
Gout flares linked to transient jump in MI, stroke risk
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.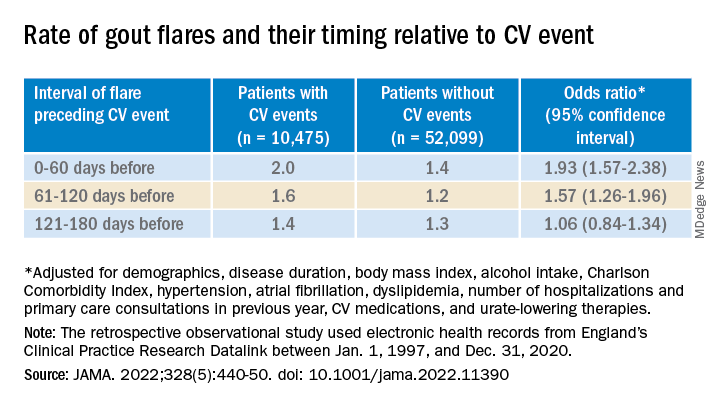
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
There is evidence that gout and heart disease are mechanistically linked by inflammation and patients with gout are at elevated risk for cardiovascular disease (CVD). But do gout flares, on their own, affect short-term risk for CV events? A new analysis based on records from British medical practices suggests that might be the case.
Risk for myocardial infarction or stroke climbed in the weeks after individual gout flare-ups in the study’s more than 60,000 patients with a recent gout diagnosis. The jump in risk, significant but small in absolute terms, held for about 4 months in the case-control study before going away.
A sensitivity analysis that excluded patients who already had CVD when their gout was diagnosed yielded similar results.
The observational study isn’t able to show that gout flares themselves transiently raise the risk for MI or stroke, but it’s enough to send a cautionary message to physicians who care for patients with gout, rheumatologist Abhishek Abhishek, PhD, Nottingham (England) City Hospital, said in an interview.
In such patients who also have conditions like hypertension, diabetes, or dyslipidemia, or a history of heart disease, he said, it’s important “to manage risk factors really aggressively, knowing that when these patients have a gout flare, there’s a temporary increase in risk of a cardiovascular event.”
Managing their absolute CV risk – whether with drug therapy, lifestyle changes, or other interventions – should help limit the transient jump in risk for MI or stroke following a gout flare, proposed Dr. Abhishek, who is senior author on the study published in JAMA, with lead author Edoardo Cipolletta, MD, also from Nottingham City Hospital.
First robust evidence
The case-control study, which involved more than 60,000 patients with a recent gout diagnosis, some who went on to have MI or stroke, looked at rates of such events at different time intervals after gout flares. Those who experienced such events showed a more than 90% increased likelihood of a gout flare-up in the preceding 60 days, a greater than 50% chance of a flare between 60 and 120 days before the event, but no increased likelihood prior to 120 days before the event.
Such a link between gout flares and CV events “has been suspected but never proven,” observed rheumatologist Hyon K. Choi, MD, Harvard Medical School, Boston, who was not associated with the analysis. “This is the first time it has actually been shown in a robust way,” he said in an interview.
The study suggests a “likely causative relationship” between gout flares and CV events, but – as the published report noted – has limitations like any observational study, said Dr. Choi, who also directs the Gout & Crystal Arthropathy Center at Massachusetts General Hospital, Boston. “Hopefully, this can be replicated in other cohorts.”
The analysis controlled for a number of relevant potential confounders, he noted, but couldn’t account for all issues that could argue against gout flares as a direct cause of the MIs and strokes.
Gout attacks are a complex experience with a range of potential indirect effects on CV risk, Dr. Choi observed. They can immobilize patients, possibly raising their risk for thrombotic events, for example. They can be exceptionally painful, which causes stress and can lead to frequent or chronic use of glucocorticoids or NSAIDs, all of which can exacerbate high blood pressure and possibly worsen CV risk.
A unique insight
The timing of gout flares relative to acute vascular events hasn’t been fully explored, observed an accompanying editorial. The current study’s “unique insight,” it stated, “is that disease activity from gout was associated with an incremental increase in risk for acute vascular events during the time period immediately following the gout flare.”
Although the study is observational, a “large body of evidence from animal and human research, mechanistic insights, and clinical interventions” support an association between flares and vascular events and “make a causal link eminently reasonable,” stated the editorialists, Jeffrey L. Anderson, MD, and Kirk U. Knowlton, MD, both with Intermountain Medical Center, Salt Lake City, Utah.
The findings, they wrote, “should alert clinicians and patients to the increased cardiovascular risk in the weeks beginning after a gout flare and should focus attention on optimizing preventive measures.” Those can include “lifestyle measures and standard risk-factor control including adherence to diet, statins, anti-inflammatory drugs (e.g., aspirin, colchicine), smoking cessation, diabetic and blood pressure control, and antithrombotic medications as indicated.”
Dr. Choi said the current results argue for more liberal use of colchicine, and for preferring colchicine over other anti-inflammatories, in patients with gout and traditional CV risk factors, given multiple randomized trials supporting the drug’s use in such cases. “If you use colchicine, you are covering their heart disease risk as well as their gout. It’s two birds with one stone.”
Nested case-control study
The investigators accessed electronic health records from 96,153 patients with recently diagnosed gout in England from 1997 to 2020; the cohort’s mean age was about 76 years, and 69% of participants were men. They matched 10,475 patients with at least one CV event to 52,099 others who didn’t have such an event by age, sex, and time from gout diagnosis. In each matched set of patients, those not experiencing a CV event were assigned a flare-to-event interval based on their matching with patients who did experience such an event.
Those with CV events, compared with patients without an event, had a greater than 90% increased likelihood of experiencing a gout flare-up in the 60 days preceding the event, a more than 50% greater chance of a flare-up 60-120 days before the CV event, but no increased likelihood more than 120 days before the event.
A self-controlled case series based on the same overall cohort with gout yielded similar results while sidestepping any potential for residual confounding, an inherent concern with any case–control analysis, the report notes. It involved 1,421 patients with one or more gout flare and at least one MI or stroke after the diagnosis of gout.
Among that cohort, the CV-event incidence rate ratio, adjusted for age and season of the year, by time interval after a gout flare, was 1.89 (95% confidence interval, 1.54-2.30) at 0-60 days, 1.64 (95% CI, 1.45-1.86) at 61-120 days, and1.29 (95% CI, 1.02-1.64) at 121-180 days.
Also similar, the report noted, were results of several sensitivity analyses, including one that excluded patients with confirmed CVD before their gout diagnosis; another that left out patients at low to moderate CV risk; and one that considered only gout flares treated with colchicine, corticosteroids, or NSAIDs.
The incremental CV event risks observed after flares in the study were small, which “has implications for both cost effectiveness and clinical relevance,” observed Dr. Anderson and Dr. Knowlton.
“An alternative to universal augmentation of cardiovascular risk prevention with therapies among patients with gout flares,” they wrote, would be “to further stratify risk by defining a group at highest near-term risk.” Such interventions could potentially be guided by markers of CV risk such as, for example, levels of high-sensitivity C-reactive protein or lipoprotein(a), or plaque burden on coronary-artery calcium scans.
Dr. Abhishek, Dr. Cipolletta, and the other authors reported no competing interests. Dr. Choi disclosed research support from Ironwood and Horizon; and consulting fees from Ironwood, Selecta, Horizon, Takeda, Kowa, and Vaxart. Dr. Anderson disclosed receiving grants to his institution from Novartis and Milestone.
A version of this article first appeared on Medscape.com.
FROM JAMA
‘Striking’ disparities in CVD deaths persist across COVID waves
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular disease (CVD) mortality rose significantly during the COVID-19 pandemic and persists more than 2 years on and, once again, Blacks and African Americans have been disproportionately affected, an analysis of death certificates shows.
The findings “suggest that the pandemic may reverse years or decades of work aimed at reducing gaps in cardiovascular outcomes,” Sadeer G. Al-Kindi, MD, Case Western Reserve University, Cleveland, said in an interview.
Although the disparities are in line with previous research, he said, “what was surprising is the persistence of excess cardiovascular mortality approximately 2 years after the pandemic started, even during a period of low COVID-19 mortality.”
“This suggests that the pandemic resulted in a disruption of health care access and, along with disparities in COVID-19 infection and its complications, he said, “may have a long-lasting effect on health care disparities, especially among vulnerable populations.”
The study was published online in Mayo Clinic Proceedings with lead author Scott E. Janus, MD, also of Case Western Reserve University.
Impact consistently greater for Blacks
Dr. Al-Kindi and colleagues used 3,598,352 U.S. death files to investigate trends in deaths caused specifically by CVD as well as its subtypes myocardial infarction, stroke, and heart failure (HF) in 2018 and 2019 (prepandemic) and the pandemic years 2020 and 2021. Baseline demographics showed a higher percentage of older, female, and Black individuals among the CVD subtypes of interest.
Overall, there was an excess CVD mortality of 6.7% during the pandemic, compared with prepandemic years, including a 2.5% rise in MI deaths and an 8.5% rise in stroke deaths. HF mortality remained relatively steady, rising only 0.1%.
Subgroup analyses revealed “striking differences” in excess mortality between Blacks and Whites, the authors noted. Blacks had an overall excess mortality of 13.8% versus 5.1% for Whites, compared with the prepandemic years. The differences were consistent across subtypes: MI (9.6% vs. 1.0%); stroke (14.5% vs. 6.9%); and HF (5.1% vs. –1.2%; P value for all < .001).
When the investigators looked at deaths on a yearly basis with 2018 as the baseline, they found CVD deaths increased by 1.5% in 2019, 15.8% in 2020, and 13.5% in 2021 among Black Americans, compared with 0.5%, 5.1%, and 5.7%, respectively, among White Americans.
Excess deaths from MI rose by 9.5% in 2020 and by 6.7% in 2021 among Blacks but fell by 1.2% in 2020 and by 1.0% in 2021 among Whites.
Disparities in excess HF mortality were similar, rising 9.1% and 4.1% in 2020 and 2021 among Blacks, while dipping 0.1% and 0.8% in 2020 and 2021 among Whites.
The “most striking difference” was in excess stroke mortality, which doubled among Blacks compared with whites in 2020 (14.9% vs. 6.7%) and in 2021 (17.5% vs. 8.1%), according to the authors.
Awareness urged
Although the disparities were expected, “there is clear value in documenting and quantifying the magnitude of these disparities,” Amil M. Shah, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital, both in Boston, said in an interview.
In addition to being observational, the main limitation of the study, he noted, is the quality and resolution of the death certificate data, which may limit the accuracy of the cause of death ascertainment and classification of race or ethnicity. “However, I think these potential inaccuracies are unlikely to materially impact the overall study findings.”
Dr. Shah, who was not involved in the study, said he would like to see additional research into the diversity and heterogeneity in risk among Black communities. “Understanding the environmental, social, and health care factors – both harmful and protective – that influence risk for CVD morbidity and mortality among Black individuals and communities offers the promise to provide actionable insights to mitigate these disparities.”
“Intervention studies testing approaches to mitigate disparities based on race/ethnicity” are also needed, he added. These may be at the policy, community, health system, or individual level, and community involvement in phases will be essential.”
Meanwhile, both Dr. Al-Kindi and Dr. Shah urged clinicians to be aware of the disparities and the need to improve access to care and address social determinants of health in vulnerable populations.
These disparities “are driven by structural factors, and are reinforced by individual behaviors. In this context, implicit bias training is important to help clinicians recognize and mitigate bias in their own practice,” Dr. Shah said. “Supporting diversity, equity, and inclusion efforts, and advocating for anti-racist policies and practices in their health systems” can also help.
Dr. Al-Kindi and Dr. Shah disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MAYO CLINIC PROCEEDINGS
‘Case closed’: Bridging thrombolysis remains ‘gold standard’ in stroke thrombectomy
Two new noninferiority trials address the controversial question of whether thrombolytic therapy can be omitted for acute ischemic stroke in patients undergoing endovascular thrombectomy for large-vessel occlusion.
Both trials show better outcomes when standard bridging thrombolytic therapy is used before thrombectomy, with comparable safety.
The results of SWIFT-DIRECT and DIRECT-SAFE were published online June 22 in The Lancet.
“The case appears closed. Bypass intravenous thrombolysis is highly unlikely to be noninferior to standard care by a clinically acceptable margin for most patients,” writes Pooja Khatri, MD, MSc, department of neurology, University of Cincinnati, in a linked comment.
SWIFT-DIRECT
SWIFT-DIRECT enrolled 408 patients (median age 72; 51% women) with acute stroke due to large vessel occlusion admitted to stroke centers in Europe and Canada. Half were randomly allocated to thrombectomy alone and half to intravenous alteplase and thrombectomy.
Successful reperfusion was less common in patients who had thrombectomy alone (91% vs. 96%; risk difference −5.1%; 95% confidence interval, −10.2 to 0.0, P = .047).
With combination therapy, more patients achieved functional independence with a modified Rankin scale score of 0-2 at 90 days (65% vs. 57%; adjusted risk difference −7.3%; 95% CI, −16·6 to 2·1, lower limit of one-sided 95% CI, −15·1%, crossing the noninferiority margin of −12%).
“Despite a very liberal noninferiority margin and strict inclusion and exclusion criteria aimed at studying a population most likely to benefit from thrombectomy alone, point estimates directionally favored intravenous thrombolysis plus thrombectomy,” Urs Fischer, MD, cochair of the Stroke Center, University Hospital Basel, Switzerland, told this news organization.
“Furthermore, we could demonstrate that overall reperfusion rates were extremely high and yet significantly better in patients receiving intravenous thrombolysis plus thrombectomy than in patients treated with thrombectomy alone, a finding which has not been shown before,” Dr. Fischer said.
There was no significant difference in the risk of symptomatic intracranial bleeding (3% with combination therapy and 2% with thrombectomy alone).
Based on the results, in patients suitable for thrombolysis, skipping it before thrombectomy “is not justified,” the study team concludes.
DIRECT-SAFE
DIRECT-SAFE enrolled 295 patients (median age 69; 43% women) with stroke and large vessel occlusion from Australia, New Zealand, China, and Vietnam, with half undergoing direct thrombectomy and half bridging therapy first.
Functional independence (modified Rankin Scale 0-2 or return to baseline at 90 days) was more common in the bridging group (61% vs. 55%).
Safety outcomes were similar between groups. Symptomatic intracerebral hemorrhage occurred in 2 (1%) patients in the direct group and 1 (1%) patient in the bridging group. There were 22 (15%) deaths in the direct group and 24 in the bridging group.
“There has been concern across the world regarding cost of treatment, together with fears of increasing bleeding risk or clot migration with intravenous thrombolytic,” lead investigator Peter Mitchell, MBBS, director, NeuroIntervention Service, The Royal Melbourne Hospital, Parkville, Victoria, Australia, told this news organization.
“We showed that patients in the bridging treatment arm had better outcomes across the entire study, especially in Asian region patients” and therefore remains “the gold standard,” Dr. Mitchell said.
To date, six published trials have addressed this question of endovascular therapy alone or with thrombolysis – SKIP, DIRECT-MT, MR CLEAN NO IV, SWIFT-DIRECT, and DIRECT-SAFE.
Dr. Fischer said the SWIFT-DIRECT study group plans to perform an individual participant data meta-analysis known as Improving Reperfusion Strategies in Ischemic Stroke (IRIS) of all six trials to see whether there are subgroups of patients in whom thrombectomy alone is as effective as thrombolysis plus thrombectomy.
Subgroups of interest, he said, include patients with early ischemic signs on imaging, those at increased risk for hemorrhagic complications, and patients with a high clot burden.
SWIFT-DIRECT was funding by Medtronic and University Hospital Bern. DIRECT-SAFE was funded by Australian National Health and Medical Research Council and Stryker USA. A complete list of author disclosures is available with the original articles.
A version of this article first appeared on Medscape.com.
Two new noninferiority trials address the controversial question of whether thrombolytic therapy can be omitted for acute ischemic stroke in patients undergoing endovascular thrombectomy for large-vessel occlusion.
Both trials show better outcomes when standard bridging thrombolytic therapy is used before thrombectomy, with comparable safety.
The results of SWIFT-DIRECT and DIRECT-SAFE were published online June 22 in The Lancet.
“The case appears closed. Bypass intravenous thrombolysis is highly unlikely to be noninferior to standard care by a clinically acceptable margin for most patients,” writes Pooja Khatri, MD, MSc, department of neurology, University of Cincinnati, in a linked comment.
SWIFT-DIRECT
SWIFT-DIRECT enrolled 408 patients (median age 72; 51% women) with acute stroke due to large vessel occlusion admitted to stroke centers in Europe and Canada. Half were randomly allocated to thrombectomy alone and half to intravenous alteplase and thrombectomy.
Successful reperfusion was less common in patients who had thrombectomy alone (91% vs. 96%; risk difference −5.1%; 95% confidence interval, −10.2 to 0.0, P = .047).
With combination therapy, more patients achieved functional independence with a modified Rankin scale score of 0-2 at 90 days (65% vs. 57%; adjusted risk difference −7.3%; 95% CI, −16·6 to 2·1, lower limit of one-sided 95% CI, −15·1%, crossing the noninferiority margin of −12%).
“Despite a very liberal noninferiority margin and strict inclusion and exclusion criteria aimed at studying a population most likely to benefit from thrombectomy alone, point estimates directionally favored intravenous thrombolysis plus thrombectomy,” Urs Fischer, MD, cochair of the Stroke Center, University Hospital Basel, Switzerland, told this news organization.
“Furthermore, we could demonstrate that overall reperfusion rates were extremely high and yet significantly better in patients receiving intravenous thrombolysis plus thrombectomy than in patients treated with thrombectomy alone, a finding which has not been shown before,” Dr. Fischer said.
There was no significant difference in the risk of symptomatic intracranial bleeding (3% with combination therapy and 2% with thrombectomy alone).
Based on the results, in patients suitable for thrombolysis, skipping it before thrombectomy “is not justified,” the study team concludes.
DIRECT-SAFE
DIRECT-SAFE enrolled 295 patients (median age 69; 43% women) with stroke and large vessel occlusion from Australia, New Zealand, China, and Vietnam, with half undergoing direct thrombectomy and half bridging therapy first.
Functional independence (modified Rankin Scale 0-2 or return to baseline at 90 days) was more common in the bridging group (61% vs. 55%).
Safety outcomes were similar between groups. Symptomatic intracerebral hemorrhage occurred in 2 (1%) patients in the direct group and 1 (1%) patient in the bridging group. There were 22 (15%) deaths in the direct group and 24 in the bridging group.
“There has been concern across the world regarding cost of treatment, together with fears of increasing bleeding risk or clot migration with intravenous thrombolytic,” lead investigator Peter Mitchell, MBBS, director, NeuroIntervention Service, The Royal Melbourne Hospital, Parkville, Victoria, Australia, told this news organization.
“We showed that patients in the bridging treatment arm had better outcomes across the entire study, especially in Asian region patients” and therefore remains “the gold standard,” Dr. Mitchell said.
To date, six published trials have addressed this question of endovascular therapy alone or with thrombolysis – SKIP, DIRECT-MT, MR CLEAN NO IV, SWIFT-DIRECT, and DIRECT-SAFE.
Dr. Fischer said the SWIFT-DIRECT study group plans to perform an individual participant data meta-analysis known as Improving Reperfusion Strategies in Ischemic Stroke (IRIS) of all six trials to see whether there are subgroups of patients in whom thrombectomy alone is as effective as thrombolysis plus thrombectomy.
Subgroups of interest, he said, include patients with early ischemic signs on imaging, those at increased risk for hemorrhagic complications, and patients with a high clot burden.
SWIFT-DIRECT was funding by Medtronic and University Hospital Bern. DIRECT-SAFE was funded by Australian National Health and Medical Research Council and Stryker USA. A complete list of author disclosures is available with the original articles.
A version of this article first appeared on Medscape.com.
Two new noninferiority trials address the controversial question of whether thrombolytic therapy can be omitted for acute ischemic stroke in patients undergoing endovascular thrombectomy for large-vessel occlusion.
Both trials show better outcomes when standard bridging thrombolytic therapy is used before thrombectomy, with comparable safety.
The results of SWIFT-DIRECT and DIRECT-SAFE were published online June 22 in The Lancet.
“The case appears closed. Bypass intravenous thrombolysis is highly unlikely to be noninferior to standard care by a clinically acceptable margin for most patients,” writes Pooja Khatri, MD, MSc, department of neurology, University of Cincinnati, in a linked comment.
SWIFT-DIRECT
SWIFT-DIRECT enrolled 408 patients (median age 72; 51% women) with acute stroke due to large vessel occlusion admitted to stroke centers in Europe and Canada. Half were randomly allocated to thrombectomy alone and half to intravenous alteplase and thrombectomy.
Successful reperfusion was less common in patients who had thrombectomy alone (91% vs. 96%; risk difference −5.1%; 95% confidence interval, −10.2 to 0.0, P = .047).
With combination therapy, more patients achieved functional independence with a modified Rankin scale score of 0-2 at 90 days (65% vs. 57%; adjusted risk difference −7.3%; 95% CI, −16·6 to 2·1, lower limit of one-sided 95% CI, −15·1%, crossing the noninferiority margin of −12%).
“Despite a very liberal noninferiority margin and strict inclusion and exclusion criteria aimed at studying a population most likely to benefit from thrombectomy alone, point estimates directionally favored intravenous thrombolysis plus thrombectomy,” Urs Fischer, MD, cochair of the Stroke Center, University Hospital Basel, Switzerland, told this news organization.
“Furthermore, we could demonstrate that overall reperfusion rates were extremely high and yet significantly better in patients receiving intravenous thrombolysis plus thrombectomy than in patients treated with thrombectomy alone, a finding which has not been shown before,” Dr. Fischer said.
There was no significant difference in the risk of symptomatic intracranial bleeding (3% with combination therapy and 2% with thrombectomy alone).
Based on the results, in patients suitable for thrombolysis, skipping it before thrombectomy “is not justified,” the study team concludes.
DIRECT-SAFE
DIRECT-SAFE enrolled 295 patients (median age 69; 43% women) with stroke and large vessel occlusion from Australia, New Zealand, China, and Vietnam, with half undergoing direct thrombectomy and half bridging therapy first.
Functional independence (modified Rankin Scale 0-2 or return to baseline at 90 days) was more common in the bridging group (61% vs. 55%).
Safety outcomes were similar between groups. Symptomatic intracerebral hemorrhage occurred in 2 (1%) patients in the direct group and 1 (1%) patient in the bridging group. There were 22 (15%) deaths in the direct group and 24 in the bridging group.
“There has been concern across the world regarding cost of treatment, together with fears of increasing bleeding risk or clot migration with intravenous thrombolytic,” lead investigator Peter Mitchell, MBBS, director, NeuroIntervention Service, The Royal Melbourne Hospital, Parkville, Victoria, Australia, told this news organization.
“We showed that patients in the bridging treatment arm had better outcomes across the entire study, especially in Asian region patients” and therefore remains “the gold standard,” Dr. Mitchell said.
To date, six published trials have addressed this question of endovascular therapy alone or with thrombolysis – SKIP, DIRECT-MT, MR CLEAN NO IV, SWIFT-DIRECT, and DIRECT-SAFE.
Dr. Fischer said the SWIFT-DIRECT study group plans to perform an individual participant data meta-analysis known as Improving Reperfusion Strategies in Ischemic Stroke (IRIS) of all six trials to see whether there are subgroups of patients in whom thrombectomy alone is as effective as thrombolysis plus thrombectomy.
Subgroups of interest, he said, include patients with early ischemic signs on imaging, those at increased risk for hemorrhagic complications, and patients with a high clot burden.
SWIFT-DIRECT was funding by Medtronic and University Hospital Bern. DIRECT-SAFE was funded by Australian National Health and Medical Research Council and Stryker USA. A complete list of author disclosures is available with the original articles.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
Nurses’ cohort study: Endometriosis elevates stroke risk
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
FROM STROKE
Moderate drinking shows more benefit for older vs. younger adults
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
FROM THE LANCET
Interventional imagers take on central role and more radiation
Interventional echocardiographers have become an increasingly critical part of the structural heart team but may be paying the price in terms of radiation exposure, a new study suggests.
Results showed that interventional echocardiographers receive threefold higher head-level radiation doses than interventional cardiologists during left atrial appendage occlusion (LAAO) closures and 11-fold higher doses during mitral valve transcatheter edge-to-edge repair (TEER).
“Over the last 5-10 years there’s been exponential growth in these two procedures, TEER and LAAO, and while that’s been very exciting, I think there hasn’t been as much research into how to protect these individuals,” lead author David A. McNamara, MD, MPH, Spectrum Health, Grand Rapids, Mich., told this news organization.
The study was published in JAMA Network Open.
Previous studies have focused largely on radiation exposure and mitigation efforts during coronary interventions, but the room set-up for LAAO and TEER and shielding techniques to mitigate radiation exposure are vastly different, he noted.
A 2017 study reported that radiation exposure was significantly higher for imaging specialists than structural heart specialists and varied by procedure type.
For the current study, Dr. McNamara, an echocardiographer by training, and colleagues collected data from 30 consecutive LAAO and 30 consecutive TEER procedures performed at their institution between July 2016 and January 2018.
Interventional imagers, interventional cardiologists, and sonographers all wore a lead skirt, apron, and thyroid collar, as well as a dosimeter to collect radiation data.
Interventional cardiologists stood immediately adjacent to the procedure table and used a ceiling-mounted, upper-body lead shield and a lower-body shield extending from the table to the floor. The echocardiographer stood at the patient’s head and used a mobile accessory shield raised to a height that allowed the imager to extend their arms over the shield to manipulate a transesophageal echocardiogram probe throughout the case.
The median fluoroscopy time was 9.2 minutes for LAAO and 20.9 minutes for TEER. The median air kerma was 164 mGy and 109 mGy, respectively.
Interventional echocardiographers received a median per case radiation dose of 10.6 µSv, compared with 2.1 µSv for interventional cardiologists. The result was similar for TEER (10.5 vs. 0.9 µSv) and LAAO (10.6 vs. 3.5 µSv; P < .001 for all).
The odds of interventional echocardiographers having a radiation dose greater than 20 µSV were 7.5 times greater than for interventional cardiologists (P < .001).
“It’s not the direction of the association, but really the magnitude is what surprised us,” observed Dr. McNamara.
The team was pleasantly surprised, he said, that sonographers, a “vastly understudied group,” received significantly lower median radiation doses than interventional imagers during LAAO (0.2 µSV) and TEER procedures (0.0 µSv; P < .001 for both).
The average distances from the radiation source were 26 cm (10.2 inches) for the echocardiographer, 36 cm (14.2 inches) for the interventional cardiologist, and 250 cm (8.2 feet) for the sonographer.
“These folks [sonographers] were much further away than both the physicians performing these cases, and that is what we hypothesize drove their very low rates, but that should also help inform our mitigation techniques for physicians and for all other cath lab members in the room,” Dr. McNamara said.
He noted that Spectrum Health has been at the forefront in terms of research into radiation exposure and mitigation, has good institutional radiation safety education, and used dose-lowering fluoroscopy systems (AlluraClarity, Philips) with real-time image noise reduction technology and a frame rate of 15 frames per second for the study. “So we’re hopeful that this actually represents a somewhat best-case scenario for what is being done at multiple institutions throughout the nation.”
Nevertheless, there is a huge amount of variability in radiation exposure, Dr. McNamara observed. “First and foremost, we really just have to identify our problem and highlight that this is something that needs some advocacy from our [professional] groups.”
Sunil Rao, MD, the newly minted president of the Society of Cardiovascular Angiography and Interventions (SCAI), said, “This is a really important study, because it expands the potential occupational hazards outside of what we traditionally think of as the team that does interventional procedures ... we have to recognize that the procedures we’re doing in the cath lab have changed.”
“Showing that our colleagues are getting 3-10 times radiation exposure is a really important piece of information to have out there. I think it’s really sort of a call to action,” Dr. Rao, professor of medicine at Duke University, Durham, N.C., told this news organization.
Nevertheless, he observed that practices have shifted somewhat since the study and that interventional cardiologists working with imaging physicians are more cognizant of radiation exposure issues.
“When I talk with our folks here that are doing structural heart procedures, they’re making sure that they’re not stepping on the fluoro pedal while the echocardiographer is manipulating the TE probe,” Dr. Rao said. “The echocardiographer is oftentimes using a much bigger shield than what was described in the study, and remember there’s an exponential decrease in the radiation exposure by distance, so they’re stepping back during the fluoroscopy time.”
Although the volume of TEER and LAAO procedures, as well as tricuspid interventions, will continue to climb, Dr. Rao said he expects radiation exposure to the imaging cardiologist will fall thanks to greater use of newer-generation imaging systems with dose-reduction features and better shielding strategies.
He noted that several of SCAI’s “best practices” documents call attention to radiation safety and that SCAI is creating a pathway where imaging cardiologists can become fellows of the society, which was traditionally reserved for interventionalists.
Still, imaging and cardiovascular societies have yet to endorse standardized safety procedures for interventional imagers, nor is information routinely collected on radiation exposure in national registries.
“We just don’t have the budgets or the interest nationally to do that kind of thing, so it has to be done locally,” Dr. Rao said. “And the person who I think is responsible for that is really the cath lab director and the cath lab nurse manager, who really should work hand-in-glove to make sure that radiation safety is at the top of the priority list.”
The study was funded by the Frederik Meijer Heart & Vascular Institute, Spectrum Health, and by Corindus. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication. Senior author Ryan Madder, MD, reports receiving research support, speaker honoraria, and grants, and serving on the advisory board of Corindus. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Interventional echocardiographers have become an increasingly critical part of the structural heart team but may be paying the price in terms of radiation exposure, a new study suggests.
Results showed that interventional echocardiographers receive threefold higher head-level radiation doses than interventional cardiologists during left atrial appendage occlusion (LAAO) closures and 11-fold higher doses during mitral valve transcatheter edge-to-edge repair (TEER).
“Over the last 5-10 years there’s been exponential growth in these two procedures, TEER and LAAO, and while that’s been very exciting, I think there hasn’t been as much research into how to protect these individuals,” lead author David A. McNamara, MD, MPH, Spectrum Health, Grand Rapids, Mich., told this news organization.
The study was published in JAMA Network Open.
Previous studies have focused largely on radiation exposure and mitigation efforts during coronary interventions, but the room set-up for LAAO and TEER and shielding techniques to mitigate radiation exposure are vastly different, he noted.
A 2017 study reported that radiation exposure was significantly higher for imaging specialists than structural heart specialists and varied by procedure type.
For the current study, Dr. McNamara, an echocardiographer by training, and colleagues collected data from 30 consecutive LAAO and 30 consecutive TEER procedures performed at their institution between July 2016 and January 2018.
Interventional imagers, interventional cardiologists, and sonographers all wore a lead skirt, apron, and thyroid collar, as well as a dosimeter to collect radiation data.
Interventional cardiologists stood immediately adjacent to the procedure table and used a ceiling-mounted, upper-body lead shield and a lower-body shield extending from the table to the floor. The echocardiographer stood at the patient’s head and used a mobile accessory shield raised to a height that allowed the imager to extend their arms over the shield to manipulate a transesophageal echocardiogram probe throughout the case.
The median fluoroscopy time was 9.2 minutes for LAAO and 20.9 minutes for TEER. The median air kerma was 164 mGy and 109 mGy, respectively.
Interventional echocardiographers received a median per case radiation dose of 10.6 µSv, compared with 2.1 µSv for interventional cardiologists. The result was similar for TEER (10.5 vs. 0.9 µSv) and LAAO (10.6 vs. 3.5 µSv; P < .001 for all).
The odds of interventional echocardiographers having a radiation dose greater than 20 µSV were 7.5 times greater than for interventional cardiologists (P < .001).
“It’s not the direction of the association, but really the magnitude is what surprised us,” observed Dr. McNamara.
The team was pleasantly surprised, he said, that sonographers, a “vastly understudied group,” received significantly lower median radiation doses than interventional imagers during LAAO (0.2 µSV) and TEER procedures (0.0 µSv; P < .001 for both).
The average distances from the radiation source were 26 cm (10.2 inches) for the echocardiographer, 36 cm (14.2 inches) for the interventional cardiologist, and 250 cm (8.2 feet) for the sonographer.
“These folks [sonographers] were much further away than both the physicians performing these cases, and that is what we hypothesize drove their very low rates, but that should also help inform our mitigation techniques for physicians and for all other cath lab members in the room,” Dr. McNamara said.
He noted that Spectrum Health has been at the forefront in terms of research into radiation exposure and mitigation, has good institutional radiation safety education, and used dose-lowering fluoroscopy systems (AlluraClarity, Philips) with real-time image noise reduction technology and a frame rate of 15 frames per second for the study. “So we’re hopeful that this actually represents a somewhat best-case scenario for what is being done at multiple institutions throughout the nation.”
Nevertheless, there is a huge amount of variability in radiation exposure, Dr. McNamara observed. “First and foremost, we really just have to identify our problem and highlight that this is something that needs some advocacy from our [professional] groups.”
Sunil Rao, MD, the newly minted president of the Society of Cardiovascular Angiography and Interventions (SCAI), said, “This is a really important study, because it expands the potential occupational hazards outside of what we traditionally think of as the team that does interventional procedures ... we have to recognize that the procedures we’re doing in the cath lab have changed.”
“Showing that our colleagues are getting 3-10 times radiation exposure is a really important piece of information to have out there. I think it’s really sort of a call to action,” Dr. Rao, professor of medicine at Duke University, Durham, N.C., told this news organization.
Nevertheless, he observed that practices have shifted somewhat since the study and that interventional cardiologists working with imaging physicians are more cognizant of radiation exposure issues.
“When I talk with our folks here that are doing structural heart procedures, they’re making sure that they’re not stepping on the fluoro pedal while the echocardiographer is manipulating the TE probe,” Dr. Rao said. “The echocardiographer is oftentimes using a much bigger shield than what was described in the study, and remember there’s an exponential decrease in the radiation exposure by distance, so they’re stepping back during the fluoroscopy time.”
Although the volume of TEER and LAAO procedures, as well as tricuspid interventions, will continue to climb, Dr. Rao said he expects radiation exposure to the imaging cardiologist will fall thanks to greater use of newer-generation imaging systems with dose-reduction features and better shielding strategies.
He noted that several of SCAI’s “best practices” documents call attention to radiation safety and that SCAI is creating a pathway where imaging cardiologists can become fellows of the society, which was traditionally reserved for interventionalists.
Still, imaging and cardiovascular societies have yet to endorse standardized safety procedures for interventional imagers, nor is information routinely collected on radiation exposure in national registries.
“We just don’t have the budgets or the interest nationally to do that kind of thing, so it has to be done locally,” Dr. Rao said. “And the person who I think is responsible for that is really the cath lab director and the cath lab nurse manager, who really should work hand-in-glove to make sure that radiation safety is at the top of the priority list.”
The study was funded by the Frederik Meijer Heart & Vascular Institute, Spectrum Health, and by Corindus. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication. Senior author Ryan Madder, MD, reports receiving research support, speaker honoraria, and grants, and serving on the advisory board of Corindus. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Interventional echocardiographers have become an increasingly critical part of the structural heart team but may be paying the price in terms of radiation exposure, a new study suggests.
Results showed that interventional echocardiographers receive threefold higher head-level radiation doses than interventional cardiologists during left atrial appendage occlusion (LAAO) closures and 11-fold higher doses during mitral valve transcatheter edge-to-edge repair (TEER).
“Over the last 5-10 years there’s been exponential growth in these two procedures, TEER and LAAO, and while that’s been very exciting, I think there hasn’t been as much research into how to protect these individuals,” lead author David A. McNamara, MD, MPH, Spectrum Health, Grand Rapids, Mich., told this news organization.
The study was published in JAMA Network Open.
Previous studies have focused largely on radiation exposure and mitigation efforts during coronary interventions, but the room set-up for LAAO and TEER and shielding techniques to mitigate radiation exposure are vastly different, he noted.
A 2017 study reported that radiation exposure was significantly higher for imaging specialists than structural heart specialists and varied by procedure type.
For the current study, Dr. McNamara, an echocardiographer by training, and colleagues collected data from 30 consecutive LAAO and 30 consecutive TEER procedures performed at their institution between July 2016 and January 2018.
Interventional imagers, interventional cardiologists, and sonographers all wore a lead skirt, apron, and thyroid collar, as well as a dosimeter to collect radiation data.
Interventional cardiologists stood immediately adjacent to the procedure table and used a ceiling-mounted, upper-body lead shield and a lower-body shield extending from the table to the floor. The echocardiographer stood at the patient’s head and used a mobile accessory shield raised to a height that allowed the imager to extend their arms over the shield to manipulate a transesophageal echocardiogram probe throughout the case.
The median fluoroscopy time was 9.2 minutes for LAAO and 20.9 minutes for TEER. The median air kerma was 164 mGy and 109 mGy, respectively.
Interventional echocardiographers received a median per case radiation dose of 10.6 µSv, compared with 2.1 µSv for interventional cardiologists. The result was similar for TEER (10.5 vs. 0.9 µSv) and LAAO (10.6 vs. 3.5 µSv; P < .001 for all).
The odds of interventional echocardiographers having a radiation dose greater than 20 µSV were 7.5 times greater than for interventional cardiologists (P < .001).
“It’s not the direction of the association, but really the magnitude is what surprised us,” observed Dr. McNamara.
The team was pleasantly surprised, he said, that sonographers, a “vastly understudied group,” received significantly lower median radiation doses than interventional imagers during LAAO (0.2 µSV) and TEER procedures (0.0 µSv; P < .001 for both).
The average distances from the radiation source were 26 cm (10.2 inches) for the echocardiographer, 36 cm (14.2 inches) for the interventional cardiologist, and 250 cm (8.2 feet) for the sonographer.
“These folks [sonographers] were much further away than both the physicians performing these cases, and that is what we hypothesize drove their very low rates, but that should also help inform our mitigation techniques for physicians and for all other cath lab members in the room,” Dr. McNamara said.
He noted that Spectrum Health has been at the forefront in terms of research into radiation exposure and mitigation, has good institutional radiation safety education, and used dose-lowering fluoroscopy systems (AlluraClarity, Philips) with real-time image noise reduction technology and a frame rate of 15 frames per second for the study. “So we’re hopeful that this actually represents a somewhat best-case scenario for what is being done at multiple institutions throughout the nation.”
Nevertheless, there is a huge amount of variability in radiation exposure, Dr. McNamara observed. “First and foremost, we really just have to identify our problem and highlight that this is something that needs some advocacy from our [professional] groups.”
Sunil Rao, MD, the newly minted president of the Society of Cardiovascular Angiography and Interventions (SCAI), said, “This is a really important study, because it expands the potential occupational hazards outside of what we traditionally think of as the team that does interventional procedures ... we have to recognize that the procedures we’re doing in the cath lab have changed.”
“Showing that our colleagues are getting 3-10 times radiation exposure is a really important piece of information to have out there. I think it’s really sort of a call to action,” Dr. Rao, professor of medicine at Duke University, Durham, N.C., told this news organization.
Nevertheless, he observed that practices have shifted somewhat since the study and that interventional cardiologists working with imaging physicians are more cognizant of radiation exposure issues.
“When I talk with our folks here that are doing structural heart procedures, they’re making sure that they’re not stepping on the fluoro pedal while the echocardiographer is manipulating the TE probe,” Dr. Rao said. “The echocardiographer is oftentimes using a much bigger shield than what was described in the study, and remember there’s an exponential decrease in the radiation exposure by distance, so they’re stepping back during the fluoroscopy time.”
Although the volume of TEER and LAAO procedures, as well as tricuspid interventions, will continue to climb, Dr. Rao said he expects radiation exposure to the imaging cardiologist will fall thanks to greater use of newer-generation imaging systems with dose-reduction features and better shielding strategies.
He noted that several of SCAI’s “best practices” documents call attention to radiation safety and that SCAI is creating a pathway where imaging cardiologists can become fellows of the society, which was traditionally reserved for interventionalists.
Still, imaging and cardiovascular societies have yet to endorse standardized safety procedures for interventional imagers, nor is information routinely collected on radiation exposure in national registries.
“We just don’t have the budgets or the interest nationally to do that kind of thing, so it has to be done locally,” Dr. Rao said. “And the person who I think is responsible for that is really the cath lab director and the cath lab nurse manager, who really should work hand-in-glove to make sure that radiation safety is at the top of the priority list.”
The study was funded by the Frederik Meijer Heart & Vascular Institute, Spectrum Health, and by Corindus. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication. Senior author Ryan Madder, MD, reports receiving research support, speaker honoraria, and grants, and serving on the advisory board of Corindus. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Shift schedule today could worsen that stroke tomorrow
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Body clocks and the shifting risks of stroke
Health care professionals, we’re sure, are no strangers to rotating shifts. And, as practitioners of the shiftly arts, you should know new research shows that working those kinds of hours can have lasting effects on your health. And it’s all based on your sleep-wake cycle.
In a study published in Neurobiology of Sleep and Circadian Rhythms, investigators at Texas A&M University looked at the effects of working these kinds of shifts for a long period of time and then returning to a regular 24-hour cycle later in life. The study piggybacks on a previous study, which showed that rats on shift schedules had more severe stroke outcomes than those who were on a 24-hour cycle.
The current study demonstrates that working rotating shifts does have a lasting effect, by way of messing with the sleep-wake cycle. Based on the research, the rats that performed those kinds of shifts never got back to a normal schedule. When strokes occurred, outcomes were much worse, and the females had a higher mortality rate and more severe functional deficits than the males.
Now for the “good” news: Even if you’re among those who haven’t worked a rotating shift, you may not be safe either.
People who have regular working hours have a tendency to take work home and stay up late, especially with so many moving to a remote-work model. And if you’re staying up late on the weekends you’re producing what lead author David J. Earnest, PhD, called “social jet lag,” which messes with your circadian rhythm to wind you down for sleep. All of these things can lead to the same kind of effects that working rotating shifts has on your health, he said in a written statement.
How do you combat this? Dr. Earnest recommended creating a sleep schedule and setting regular mealtimes. Also ease up on high-fat foods, drinking, and smoking. The connection between your brain and gut also could play a part in how severe a stroke can be.
So continue to work hard, but not too hard.
Got 3 minutes? You got time for culture
Much like a Krabby Patty, art is good for your soul. Seriously, staring at a 500-year-old painting may not seem like much, but research has proven time and again that going to a museum and looking at paintings by long-dead artists you probably know better as pizza-eating superhero turtles improves mood, stress, and well-being.
A couple of years ago, however, museums and art galleries ran into a big virus-shaped problem. You may have heard of it. All of a sudden it became a very bad idea for people to gather together in one building and huddle around the Mona Lisa, which, by the way, is a lot smaller in person than you might expect. But, rather than sit around with a bunch of priceless art for an indeterminate amount of time, museums brought their exhibits to the Internet so that people from all over the world could see great works from their couches.
This is absolutely a good thing for public access, but do these virtual art exhibits provide the same health benefits as going to a museum in person? That’s what a group of European researchers aimed to find out, and in a study published in Frontiers of Psychology, that’s exactly what they found.
Their directive to the 84 study participants was simple: Take a well-being survey, engage with either of a pair of online exhibits (a Monet painting and a display of Japanese culinary traditions) for just 3 minutes, then take another well-being assessment. The results were quite clear: Even just a couple of minutes of viewing art online improved all the well-being categories on the survey, such as lowering anxiety, negative mood, and loneliness, as well as increasing subjective well-being. Also, the more beautiful or meaningful a person found the art, the more their mood and well-being improved.
The researchers noted that these results could help access in places where access to art is limited, such as waiting rooms, hospitals, and rural areas. Let’s just hope it sticks to that, and that big businesses don’t take notice. Just imagine them plastering ads with classic Renaissance artworks. After all, art makes you feel good, and you know what else feels good on a hot summer day? An ice-cold Coca-Cola! By the way, we’re taking offers, advertising agencies. The LOTME staff can absolutely be bought.
Appetite for etymology
Today on “It’s a Thing,” we examine various states of hunger and what they should be called. Our first guest is that historically hungry royal person, King Henry VIII of England. Your majesty, have you ever been “hangry?”
KH8: First, let me thank you for inviting me on the show, Maurice. I’m a huge fan. A recent study done in the United Kingdom and Austria showed that “hunger is associated with greater levels of anger and irritability, as well as lower levels of pleasure,” according to a Eurekalert statement. So, yes, I have been “hangry.”
Maurice: Now to our next guest. Martha Stewart, can you add anything about that study?
Martha: Happy to, Maurice. The 64 participants used a smartphone app to record their hunger levels and emotional states five times a day for 21 days. It’s the first time that “hanger” was studied outside a lab, and it showed that hunger “was associated with 37% of the variance in irritability, 34% of the variance in anger, and 38% of the variance in pleasure recorded by the participants,” the investigators said in that statement.
Maurice: It’s official, then. Hangry is a thing, and we don’t need to put it in quotes anymore. Now let’s meet our third and final guest, Betty Crocker. Betty, I’m told you have a study to plug.
Betty: That’s right, Mo. Researchers at Tel Aviv University looked at survey data from almost 3,000 men and women and found that men ate 17% more food during the warmer months (March to September) than they did the rest of the year. Among women, however, caloric intake did not change.
KH8: I saw that study. Didn’t they put 27 people out in the sun and then take blood samples?
Betty: Indeed they did, Hank. After 25 minutes of sun exposure, the 13 men felt hungrier than before, but the 14 women did not. The men also had higher levels of ghrelin, an appetite-stimulating hormone, than the women.
Maurice: To sum all this up, then, we’ve got angry and hungry officially combining to make hangry, and now it looks like the sun is causing hunger in men, which makes them … sungry?
Martha: It’s a thing.
Chicken cutlets with a side of COVID
You stopped at the drive through at McDonald’s on the way home from work, and while you’re looking for something sweet in the refrigerator for dessert, you see that chicken breast that expires today.
Freezing meat that’s about to expire might be your go-to so it doesn’t go to waste, but it’s been found that SARS-CoV-2 can live in meat that’s been in the refrigerator or freezer for more than a month.
Researchers exposed chicken, beef, pork, and salmon to surrogate viruses that are similar to COVID but not as harmful and stored them in freezers at –4° F and in the refrigerator at 39.2° F. “We even found that the viruses could be cultured after [being frozen for] that length of time,” lead author Emily Bailey, PhD, of Campbell University in Buies Creek, N.C., said in Study Finds.
The team began its research after hearing of COVID-19 outbreaks where there were no reports of community transmission, such as in Southeast Asia. Tracing eventually led to packaged meats as the culprits in those cases. SARS-CoV-2 is able to replicate in the gut, as well as the respiratory tract, so it could affect the gut before respiratory symptoms start. It is crucial to ensure cross contamination doesn’t occur, and inadequate sanitation prior to packaging needs to be addressed, the investigators said.
Honestly, we didn’t think anything could survive in a freezer for that long, but SARS-CoV-2 is a fighter.
Adding salt to food linked to higher risk of premature death
in a new study.
In the study of more than 500,000 people, compared with those who never or rarely added salt, those who always added salt to their food had a 28% increased risk of dying prematurely (defined as death before the age of 75 years).
Results also showed that adding salt to food was linked to a lower life expectancy. At the age of 50 years, life expectancy was reduced by 1.5 years in women and by 2.28 years in men who always added salt to their food, compared with those who never or rarely did.
However, these increased risks appeared to be attenuated with increasing intakes of high-potassium foods (vegetables and fruits).
The study was published online in the European Heart Journal.
“As far as we are aware, this is the first study to analyze adding salt to meals as a unique measurement for dietary sodium intake. Such a measure is less likely affected by other dietary components, especially potassium intake,” senior author Lu Qi, MD, Tulane University, New Orleans, told this news organization.
“Our study provides supportive evidence from a novel perspective to show the adverse effects of high sodium intake on human health, which is still a controversial topic. Our findings support the advice that reduction of salt intake by reducing the salt added to meals may benefit health and improve life expectancy. Our results also suggest that high intakes of fruits and vegetables are beneficial regarding lowering the adverse effects of salt,” he added.
Link between dietary salt and health is subject of longstanding debate
The researchers explained that the relationship between dietary salt intake and health remains a subject of longstanding debate, with previous studies on the association between sodium intake and mortality having shown conflicting results.
They attributed the inconsistent results to the low accuracy of sodium measurement, noting that sodium intake varies widely from day to day, but the majority of previous studies have largely relied on a single day’s urine collection or dietary survey for estimating the sodium intake, which is inadequate to assess an individual’s usual consumption levels.
They also pointed out that it is difficult to separate the contributions of intakes of sodium and potassium to health based on current methods for measuring dietary sodium and potassium, and this may confound the association between sodium intake and health outcomes.
They noted that the hypothesis that a high-potassium intake may attenuate the adverse association of high-sodium intake with health outcomes has been proposed for many years, but studies assessing the interaction between sodium intake and potassium intake on the risk of mortality are scarce.
Adding salt to food at the table is a common eating behavior directly related to an individual’s long-term preference for salty tasting foods and habitual salt intake, the authors said, adding that commonly used table salt contains 97%-99% sodium chloride, minimizing the potential confounding effects of other dietary factors including potassium. “Therefore, adding salt to foods provides a unique assessment to evaluate the association between habitual sodium intake and mortality.”
UK Biobank study
For the current study Dr. Qi and colleagues analyzed data from 501,379 people taking part in the UK Biobank study. When joining the study between 2006 and 2010, the participants were asked whether they added salt to their foods never/rarely, sometimes, usually or always. Participants were then followed for a median of 9 years.
After adjustment for sex, age, race, smoking, moderate drinking, body mass index, physical activity, Townsend deprivation index, high cholesterol, chronic kidney disease, diabetes, cardiovascular disease, and cancer, results showed an increasing risk of all-cause premature mortality rose with increasing frequency of adding salt to foods.
The adjusted hazard ratios, compared with those who never or rarely added salt, were 1.02 (95% CI, 0.99-1.06) for those who added salt sometimes, 1.07 (95% CI, 1.02-1.11) for those who usually added salt, and 1.28 (95% CI, 1.20-1.35) for those who always added salt.
The researchers also estimated the lower survival time caused by the high frequency of adding salt to foods. At age 50, women who always added salt to foods had an average 1.50 fewer years of life expectancy, and men who always added salt had an average 2.28 fewer years of life expectancy, as compared with their counterparts who never/rarely added salt to foods.
For cause-specific premature mortality, results showed that higher frequency of adding salt to foods was significantly associated with a higher risk of cardiovascular mortality and cancer mortality, but not for dementia mortality or respiratory mortality. For the subtypes of cardiovascular mortality, adding salt to foods was significantly associated with higher risk of stroke mortality but not coronary heart disease mortality.
Other analyses suggested that the association of adding salt to foods with an increased risk of premature mortality appeared to be attenuated with increasing intake of food high in potassium (fruits and vegetables).
The authors point out that the amounts of discretionary sodium intake (the salt used at the table or in home cooking) have been largely overlooked in previous studies, even though adding salt to foods accounts for a considerable proportion of total sodium intake (6%-20%) in Western diets.
“Our findings also support the notion that even a modest reduction in sodium intake is likely to result in substantial health benefits, especially when it is achieved in the general population,” they conclude.
Conflicting information from different studies
But the current findings seem to directly contradict those from another recent study by Messerli and colleagues showing higher sodium intake correlates with improved life expectancy.
Addressing these contradictory results, Dr. Qi commented: “The study of Messerli et al. is based on an ecological design, in which the analysis is performed on country average sodium intake, rather than at the individual level. This type of ecological study has several major limitations, such as the lack of individuals’ sodium intake, uncontrolled confounding, and the cross-sectional nature. Typically, ecological studies are not considered useful for testing hypothesis in epidemiological studies.”
Dr. Qi noted that, in contrast, his current study analyzes individuals’ exposure, and has a prospective design. “Our findings are supported by previous large-scale observational studies and clinical trials which show the high intake of sodium may adversely affect chronic diseases such as cardiovascular disease and hypertension.” =
Lead author of the ecological study, Franz Messerli, MD, Bern (Switzerland) University Hospital, however, was not convinced by the findings from Dr. Qi’s study.
“The difference in 24-hour sodium intake between those who never/rarely added salt and those who always did is a minuscule 0.17 g. It is highly unlikely that such negligible quantity has any impact on blood pressure, not to mention cardiovascular mortality or life expectancy,” he commented in an interview.
He also pointed out that, in Dr. Qi’s study, people who added salt more frequently also consumed more red meat and processed meat, as well as less fish and less fruit and vegetables. “I would suggest that the bad habit of adding salt at the table is simply a powerful marker for an unhealthy diet.”
“There is no question that an excessive salt intake is harmful in hypertensive patients and increases the risk of stroke. But 0.17 g is not going to make any difference,” Dr. Messerli added.
What is the optimum level?
In an editorial accompanying the study by Dr. Qi and colleagues in the European Heart Journal, Annika Rosengren, MD, PhD, Sahlgrenska University Hospital, Gothenburg, Sweden, noted that guidelines recommend a salt intake below 5 g, or about a teaspoon, per day. But few individuals meet this recommendation.
Because several recent studies show a U- or J-shaped association between salt and atherosclerotic cardiovascular disease, reducing salt intake across the whole population may not be universally beneficial, Dr. Rosengren said.
“So far, what the collective evidence about salt seems to indicate is that healthy people consuming what constitutes normal levels of ordinary salt need not worry too much about their salt intake,” she wrote.
Instead, she advised a diet rich in fruit and vegetables should be a priority to counterbalance potentially harmful effects of salt, and for many other reasons.
And she added that people at high risk, such as those with hypertension who have a high salt intake, are probably well advised to cut down, and not adding extra salt to already prepared foods is one way of achieving this. However, at the individual level, the optimal salt consumption range, or the “sweet spot” remains to be determined.
“Not adding extra salt to food is unlikely to be harmful and could contribute to strategies to lower population blood pressure levels,” Dr. Rosengren concluded.
A version of this article first appeared on Medscape.com.
in a new study.
In the study of more than 500,000 people, compared with those who never or rarely added salt, those who always added salt to their food had a 28% increased risk of dying prematurely (defined as death before the age of 75 years).
Results also showed that adding salt to food was linked to a lower life expectancy. At the age of 50 years, life expectancy was reduced by 1.5 years in women and by 2.28 years in men who always added salt to their food, compared with those who never or rarely did.
However, these increased risks appeared to be attenuated with increasing intakes of high-potassium foods (vegetables and fruits).
The study was published online in the European Heart Journal.
“As far as we are aware, this is the first study to analyze adding salt to meals as a unique measurement for dietary sodium intake. Such a measure is less likely affected by other dietary components, especially potassium intake,” senior author Lu Qi, MD, Tulane University, New Orleans, told this news organization.
“Our study provides supportive evidence from a novel perspective to show the adverse effects of high sodium intake on human health, which is still a controversial topic. Our findings support the advice that reduction of salt intake by reducing the salt added to meals may benefit health and improve life expectancy. Our results also suggest that high intakes of fruits and vegetables are beneficial regarding lowering the adverse effects of salt,” he added.
Link between dietary salt and health is subject of longstanding debate
The researchers explained that the relationship between dietary salt intake and health remains a subject of longstanding debate, with previous studies on the association between sodium intake and mortality having shown conflicting results.
They attributed the inconsistent results to the low accuracy of sodium measurement, noting that sodium intake varies widely from day to day, but the majority of previous studies have largely relied on a single day’s urine collection or dietary survey for estimating the sodium intake, which is inadequate to assess an individual’s usual consumption levels.
They also pointed out that it is difficult to separate the contributions of intakes of sodium and potassium to health based on current methods for measuring dietary sodium and potassium, and this may confound the association between sodium intake and health outcomes.
They noted that the hypothesis that a high-potassium intake may attenuate the adverse association of high-sodium intake with health outcomes has been proposed for many years, but studies assessing the interaction between sodium intake and potassium intake on the risk of mortality are scarce.
Adding salt to food at the table is a common eating behavior directly related to an individual’s long-term preference for salty tasting foods and habitual salt intake, the authors said, adding that commonly used table salt contains 97%-99% sodium chloride, minimizing the potential confounding effects of other dietary factors including potassium. “Therefore, adding salt to foods provides a unique assessment to evaluate the association between habitual sodium intake and mortality.”
UK Biobank study
For the current study Dr. Qi and colleagues analyzed data from 501,379 people taking part in the UK Biobank study. When joining the study between 2006 and 2010, the participants were asked whether they added salt to their foods never/rarely, sometimes, usually or always. Participants were then followed for a median of 9 years.
After adjustment for sex, age, race, smoking, moderate drinking, body mass index, physical activity, Townsend deprivation index, high cholesterol, chronic kidney disease, diabetes, cardiovascular disease, and cancer, results showed an increasing risk of all-cause premature mortality rose with increasing frequency of adding salt to foods.
The adjusted hazard ratios, compared with those who never or rarely added salt, were 1.02 (95% CI, 0.99-1.06) for those who added salt sometimes, 1.07 (95% CI, 1.02-1.11) for those who usually added salt, and 1.28 (95% CI, 1.20-1.35) for those who always added salt.
The researchers also estimated the lower survival time caused by the high frequency of adding salt to foods. At age 50, women who always added salt to foods had an average 1.50 fewer years of life expectancy, and men who always added salt had an average 2.28 fewer years of life expectancy, as compared with their counterparts who never/rarely added salt to foods.
For cause-specific premature mortality, results showed that higher frequency of adding salt to foods was significantly associated with a higher risk of cardiovascular mortality and cancer mortality, but not for dementia mortality or respiratory mortality. For the subtypes of cardiovascular mortality, adding salt to foods was significantly associated with higher risk of stroke mortality but not coronary heart disease mortality.
Other analyses suggested that the association of adding salt to foods with an increased risk of premature mortality appeared to be attenuated with increasing intake of food high in potassium (fruits and vegetables).
The authors point out that the amounts of discretionary sodium intake (the salt used at the table or in home cooking) have been largely overlooked in previous studies, even though adding salt to foods accounts for a considerable proportion of total sodium intake (6%-20%) in Western diets.
“Our findings also support the notion that even a modest reduction in sodium intake is likely to result in substantial health benefits, especially when it is achieved in the general population,” they conclude.
Conflicting information from different studies
But the current findings seem to directly contradict those from another recent study by Messerli and colleagues showing higher sodium intake correlates with improved life expectancy.
Addressing these contradictory results, Dr. Qi commented: “The study of Messerli et al. is based on an ecological design, in which the analysis is performed on country average sodium intake, rather than at the individual level. This type of ecological study has several major limitations, such as the lack of individuals’ sodium intake, uncontrolled confounding, and the cross-sectional nature. Typically, ecological studies are not considered useful for testing hypothesis in epidemiological studies.”
Dr. Qi noted that, in contrast, his current study analyzes individuals’ exposure, and has a prospective design. “Our findings are supported by previous large-scale observational studies and clinical trials which show the high intake of sodium may adversely affect chronic diseases such as cardiovascular disease and hypertension.” =
Lead author of the ecological study, Franz Messerli, MD, Bern (Switzerland) University Hospital, however, was not convinced by the findings from Dr. Qi’s study.
“The difference in 24-hour sodium intake between those who never/rarely added salt and those who always did is a minuscule 0.17 g. It is highly unlikely that such negligible quantity has any impact on blood pressure, not to mention cardiovascular mortality or life expectancy,” he commented in an interview.
He also pointed out that, in Dr. Qi’s study, people who added salt more frequently also consumed more red meat and processed meat, as well as less fish and less fruit and vegetables. “I would suggest that the bad habit of adding salt at the table is simply a powerful marker for an unhealthy diet.”
“There is no question that an excessive salt intake is harmful in hypertensive patients and increases the risk of stroke. But 0.17 g is not going to make any difference,” Dr. Messerli added.
What is the optimum level?
In an editorial accompanying the study by Dr. Qi and colleagues in the European Heart Journal, Annika Rosengren, MD, PhD, Sahlgrenska University Hospital, Gothenburg, Sweden, noted that guidelines recommend a salt intake below 5 g, or about a teaspoon, per day. But few individuals meet this recommendation.
Because several recent studies show a U- or J-shaped association between salt and atherosclerotic cardiovascular disease, reducing salt intake across the whole population may not be universally beneficial, Dr. Rosengren said.
“So far, what the collective evidence about salt seems to indicate is that healthy people consuming what constitutes normal levels of ordinary salt need not worry too much about their salt intake,” she wrote.
Instead, she advised a diet rich in fruit and vegetables should be a priority to counterbalance potentially harmful effects of salt, and for many other reasons.
And she added that people at high risk, such as those with hypertension who have a high salt intake, are probably well advised to cut down, and not adding extra salt to already prepared foods is one way of achieving this. However, at the individual level, the optimal salt consumption range, or the “sweet spot” remains to be determined.
“Not adding extra salt to food is unlikely to be harmful and could contribute to strategies to lower population blood pressure levels,” Dr. Rosengren concluded.
A version of this article first appeared on Medscape.com.
in a new study.
In the study of more than 500,000 people, compared with those who never or rarely added salt, those who always added salt to their food had a 28% increased risk of dying prematurely (defined as death before the age of 75 years).
Results also showed that adding salt to food was linked to a lower life expectancy. At the age of 50 years, life expectancy was reduced by 1.5 years in women and by 2.28 years in men who always added salt to their food, compared with those who never or rarely did.
However, these increased risks appeared to be attenuated with increasing intakes of high-potassium foods (vegetables and fruits).
The study was published online in the European Heart Journal.
“As far as we are aware, this is the first study to analyze adding salt to meals as a unique measurement for dietary sodium intake. Such a measure is less likely affected by other dietary components, especially potassium intake,” senior author Lu Qi, MD, Tulane University, New Orleans, told this news organization.
“Our study provides supportive evidence from a novel perspective to show the adverse effects of high sodium intake on human health, which is still a controversial topic. Our findings support the advice that reduction of salt intake by reducing the salt added to meals may benefit health and improve life expectancy. Our results also suggest that high intakes of fruits and vegetables are beneficial regarding lowering the adverse effects of salt,” he added.
Link between dietary salt and health is subject of longstanding debate
The researchers explained that the relationship between dietary salt intake and health remains a subject of longstanding debate, with previous studies on the association between sodium intake and mortality having shown conflicting results.
They attributed the inconsistent results to the low accuracy of sodium measurement, noting that sodium intake varies widely from day to day, but the majority of previous studies have largely relied on a single day’s urine collection or dietary survey for estimating the sodium intake, which is inadequate to assess an individual’s usual consumption levels.
They also pointed out that it is difficult to separate the contributions of intakes of sodium and potassium to health based on current methods for measuring dietary sodium and potassium, and this may confound the association between sodium intake and health outcomes.
They noted that the hypothesis that a high-potassium intake may attenuate the adverse association of high-sodium intake with health outcomes has been proposed for many years, but studies assessing the interaction between sodium intake and potassium intake on the risk of mortality are scarce.
Adding salt to food at the table is a common eating behavior directly related to an individual’s long-term preference for salty tasting foods and habitual salt intake, the authors said, adding that commonly used table salt contains 97%-99% sodium chloride, minimizing the potential confounding effects of other dietary factors including potassium. “Therefore, adding salt to foods provides a unique assessment to evaluate the association between habitual sodium intake and mortality.”
UK Biobank study
For the current study Dr. Qi and colleagues analyzed data from 501,379 people taking part in the UK Biobank study. When joining the study between 2006 and 2010, the participants were asked whether they added salt to their foods never/rarely, sometimes, usually or always. Participants were then followed for a median of 9 years.
After adjustment for sex, age, race, smoking, moderate drinking, body mass index, physical activity, Townsend deprivation index, high cholesterol, chronic kidney disease, diabetes, cardiovascular disease, and cancer, results showed an increasing risk of all-cause premature mortality rose with increasing frequency of adding salt to foods.
The adjusted hazard ratios, compared with those who never or rarely added salt, were 1.02 (95% CI, 0.99-1.06) for those who added salt sometimes, 1.07 (95% CI, 1.02-1.11) for those who usually added salt, and 1.28 (95% CI, 1.20-1.35) for those who always added salt.
The researchers also estimated the lower survival time caused by the high frequency of adding salt to foods. At age 50, women who always added salt to foods had an average 1.50 fewer years of life expectancy, and men who always added salt had an average 2.28 fewer years of life expectancy, as compared with their counterparts who never/rarely added salt to foods.
For cause-specific premature mortality, results showed that higher frequency of adding salt to foods was significantly associated with a higher risk of cardiovascular mortality and cancer mortality, but not for dementia mortality or respiratory mortality. For the subtypes of cardiovascular mortality, adding salt to foods was significantly associated with higher risk of stroke mortality but not coronary heart disease mortality.
Other analyses suggested that the association of adding salt to foods with an increased risk of premature mortality appeared to be attenuated with increasing intake of food high in potassium (fruits and vegetables).
The authors point out that the amounts of discretionary sodium intake (the salt used at the table or in home cooking) have been largely overlooked in previous studies, even though adding salt to foods accounts for a considerable proportion of total sodium intake (6%-20%) in Western diets.
“Our findings also support the notion that even a modest reduction in sodium intake is likely to result in substantial health benefits, especially when it is achieved in the general population,” they conclude.
Conflicting information from different studies
But the current findings seem to directly contradict those from another recent study by Messerli and colleagues showing higher sodium intake correlates with improved life expectancy.
Addressing these contradictory results, Dr. Qi commented: “The study of Messerli et al. is based on an ecological design, in which the analysis is performed on country average sodium intake, rather than at the individual level. This type of ecological study has several major limitations, such as the lack of individuals’ sodium intake, uncontrolled confounding, and the cross-sectional nature. Typically, ecological studies are not considered useful for testing hypothesis in epidemiological studies.”
Dr. Qi noted that, in contrast, his current study analyzes individuals’ exposure, and has a prospective design. “Our findings are supported by previous large-scale observational studies and clinical trials which show the high intake of sodium may adversely affect chronic diseases such as cardiovascular disease and hypertension.” =
Lead author of the ecological study, Franz Messerli, MD, Bern (Switzerland) University Hospital, however, was not convinced by the findings from Dr. Qi’s study.
“The difference in 24-hour sodium intake between those who never/rarely added salt and those who always did is a minuscule 0.17 g. It is highly unlikely that such negligible quantity has any impact on blood pressure, not to mention cardiovascular mortality or life expectancy,” he commented in an interview.
He also pointed out that, in Dr. Qi’s study, people who added salt more frequently also consumed more red meat and processed meat, as well as less fish and less fruit and vegetables. “I would suggest that the bad habit of adding salt at the table is simply a powerful marker for an unhealthy diet.”
“There is no question that an excessive salt intake is harmful in hypertensive patients and increases the risk of stroke. But 0.17 g is not going to make any difference,” Dr. Messerli added.
What is the optimum level?
In an editorial accompanying the study by Dr. Qi and colleagues in the European Heart Journal, Annika Rosengren, MD, PhD, Sahlgrenska University Hospital, Gothenburg, Sweden, noted that guidelines recommend a salt intake below 5 g, or about a teaspoon, per day. But few individuals meet this recommendation.
Because several recent studies show a U- or J-shaped association between salt and atherosclerotic cardiovascular disease, reducing salt intake across the whole population may not be universally beneficial, Dr. Rosengren said.
“So far, what the collective evidence about salt seems to indicate is that healthy people consuming what constitutes normal levels of ordinary salt need not worry too much about their salt intake,” she wrote.
Instead, she advised a diet rich in fruit and vegetables should be a priority to counterbalance potentially harmful effects of salt, and for many other reasons.
And she added that people at high risk, such as those with hypertension who have a high salt intake, are probably well advised to cut down, and not adding extra salt to already prepared foods is one way of achieving this. However, at the individual level, the optimal salt consumption range, or the “sweet spot” remains to be determined.
“Not adding extra salt to food is unlikely to be harmful and could contribute to strategies to lower population blood pressure levels,” Dr. Rosengren concluded.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN HEART JOURNAL
Access to certified stroke centers divided by race, income
Hospitals in low-income and rural areas of the United States are much less likely to adopt stroke certification than hospitals in high-income and urban communities, a new study shows.
Further, other results showed that, after adjustment for population and hospital size, access to stroke-certified hospitals is significantly lower in Black, racially segregated communities.
The study was published online in JAMA Neurology.
Noting that stroke-certified hospitals provide higher-quality stroke care, the authors, led by Yu-Chu Shen, PhD, Naval Postgraduate School, Monterey, Calif., conclude that: “Our findings suggest that structural inequities in stroke care may be an important consideration in eliminating stroke disparities for vulnerable populations.”
In an audio interview on the JAMA Neurology website, senior author Renee Y. Hsia, MD, University of California, San Francisco, said: “Our findings show there are clear disparities in which communities are getting access to stroke certified hospitals.”
She called for more help for hospitals in underserved areas to obtain stroke certification.
Dr. Hsia explained that hospitals can seek certification at their own expense and that although stroke care is expensive, it is also lucrative in terms of reimbursement. So it tends to be the private for-profit hospitals that seek these certifications. “If you are a county hospital on a really tight budget, you’re not going to have the extra cash on hand to be applying for stroke certification,” she commented.
This can result in an increase in hospitals with stroke certification – but not in the areas that need it the most.
Dr. Hsia points out that this has happened in cardiac care. One study showed a 44% increase in hospitals providing percutaneous coronary intervention over a 10-year period, but the percentage of the population that had better access increased by less than 1%.
“In general, in the United States we have a mentality that ‘more is better,’ and because there is no government regulation in health care, any time a hospital applies for these specialized services we just generally think that’s a good thing. But this might not always be the case,” Dr. Hsia noted. “We have a very market-based approach, and this doesn’t lead to equity. It leads to profit maximization, and that is not synonymous with what’s good for patients or populations.”
She suggested that in future the process of certification should include some consideration of how it will affect population-based equity.
“Rather than rubber stamping an application just because hospitals have certain resources, we need to ask what the benefit is of providing this service,” Dr. Hsia said. “Does this community really need it? If not, maybe we should invest these resources into helping a hospital in a community that needs it more.”
Dr. Hsia explained that she and her colleagues conducted their study to investigate whether there were structural issues that might be contributing to disparities in stroke care.
“We like to think emergency stroke care is equitable. Anyone can call 911 or go the emergency room. But, actually, there is a big disparity on who receives what type of care,” she said. “We know Black patients are less likely to receive thrombolytics and mechanical thrombectomy compared to White patents. And wealthy patients are more likely to receive thrombectomy compared to patients from the poorest zip codes.”
She said there is a tendency to think this is a result of some sort of bias on the part of health care professionals. “We wanted to look deep down in the system and whether the built environment of health care supply and geographic distribution of services contributed to access and treatment inequities.”
The study combined a dataset of hospital stroke certification from all general acute nonfederal hospitals in the continental United States from January 2009 to December 2019. National, hospital, and census data were used to identify historically underserved communities by racial and ethnic composition, income distribution, and rurality.
A total of 4,984 hospitals were assessed. Results showed that over the 11-year study period, the number of hospitals with stroke certification grew from 961 (19%) to 1,763 (36%).
Without controlling for population and hospital size, hospitals in predominantly Black, racially segregated areas were 1.67-fold more likely to adopt stroke care of any level than those in predominantly non-Black, racially segregated areas (hazard ratio, 1.67; 95% confidence interval, 1.41-1.97).
However, after adjustment for population and hospital size, the likelihood of adopting stroke care among hospitals serving Black, racially segregated communities was significantly lower than among those serving non-Black, racially segregated communities (HR, 0.74; 95% CI, 0.62-0.89).
“In other words, on a per-capita basis, a hospital serving a predominantly Black, racially segregated community was 26% less likely to adopt stroke certification of any level than a hospital in a predominantly non-Black, racially segregated community,” the authors state.
In terms of socioeconomic factors, hospitals serving low-income, economically integrated (HR, 0.23) and low-income, economically segregated (HR, 0.29) areas were far less likely to adopt any level of stroke care certification than hospitals serving high-income areas, regardless of income segregation.
Rural hospitals were also much less likely to adopt any level of stroke care than urban hospitals (HR, 0.10).
“Our results suggest that it might be necessary to incentivize hospitals operating in underserved communities to seek stroke certification or to entice hospitals with higher propensity to adopt stroke care to operate in such communities so access at the per-patient level becomes more equitable,” the authors say.
This project was supported by the Pilot Project Award from the National Bureau of Economic Research Center for Aging and Health Research, funded by the National Institute on Aging and by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Shen and Dr. Hsia have received grants from the National Institute of Aging and the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
Hospitals in low-income and rural areas of the United States are much less likely to adopt stroke certification than hospitals in high-income and urban communities, a new study shows.
Further, other results showed that, after adjustment for population and hospital size, access to stroke-certified hospitals is significantly lower in Black, racially segregated communities.
The study was published online in JAMA Neurology.
Noting that stroke-certified hospitals provide higher-quality stroke care, the authors, led by Yu-Chu Shen, PhD, Naval Postgraduate School, Monterey, Calif., conclude that: “Our findings suggest that structural inequities in stroke care may be an important consideration in eliminating stroke disparities for vulnerable populations.”
In an audio interview on the JAMA Neurology website, senior author Renee Y. Hsia, MD, University of California, San Francisco, said: “Our findings show there are clear disparities in which communities are getting access to stroke certified hospitals.”
She called for more help for hospitals in underserved areas to obtain stroke certification.
Dr. Hsia explained that hospitals can seek certification at their own expense and that although stroke care is expensive, it is also lucrative in terms of reimbursement. So it tends to be the private for-profit hospitals that seek these certifications. “If you are a county hospital on a really tight budget, you’re not going to have the extra cash on hand to be applying for stroke certification,” she commented.
This can result in an increase in hospitals with stroke certification – but not in the areas that need it the most.
Dr. Hsia points out that this has happened in cardiac care. One study showed a 44% increase in hospitals providing percutaneous coronary intervention over a 10-year period, but the percentage of the population that had better access increased by less than 1%.
“In general, in the United States we have a mentality that ‘more is better,’ and because there is no government regulation in health care, any time a hospital applies for these specialized services we just generally think that’s a good thing. But this might not always be the case,” Dr. Hsia noted. “We have a very market-based approach, and this doesn’t lead to equity. It leads to profit maximization, and that is not synonymous with what’s good for patients or populations.”
She suggested that in future the process of certification should include some consideration of how it will affect population-based equity.
“Rather than rubber stamping an application just because hospitals have certain resources, we need to ask what the benefit is of providing this service,” Dr. Hsia said. “Does this community really need it? If not, maybe we should invest these resources into helping a hospital in a community that needs it more.”
Dr. Hsia explained that she and her colleagues conducted their study to investigate whether there were structural issues that might be contributing to disparities in stroke care.
“We like to think emergency stroke care is equitable. Anyone can call 911 or go the emergency room. But, actually, there is a big disparity on who receives what type of care,” she said. “We know Black patients are less likely to receive thrombolytics and mechanical thrombectomy compared to White patents. And wealthy patients are more likely to receive thrombectomy compared to patients from the poorest zip codes.”
She said there is a tendency to think this is a result of some sort of bias on the part of health care professionals. “We wanted to look deep down in the system and whether the built environment of health care supply and geographic distribution of services contributed to access and treatment inequities.”
The study combined a dataset of hospital stroke certification from all general acute nonfederal hospitals in the continental United States from January 2009 to December 2019. National, hospital, and census data were used to identify historically underserved communities by racial and ethnic composition, income distribution, and rurality.
A total of 4,984 hospitals were assessed. Results showed that over the 11-year study period, the number of hospitals with stroke certification grew from 961 (19%) to 1,763 (36%).
Without controlling for population and hospital size, hospitals in predominantly Black, racially segregated areas were 1.67-fold more likely to adopt stroke care of any level than those in predominantly non-Black, racially segregated areas (hazard ratio, 1.67; 95% confidence interval, 1.41-1.97).
However, after adjustment for population and hospital size, the likelihood of adopting stroke care among hospitals serving Black, racially segregated communities was significantly lower than among those serving non-Black, racially segregated communities (HR, 0.74; 95% CI, 0.62-0.89).
“In other words, on a per-capita basis, a hospital serving a predominantly Black, racially segregated community was 26% less likely to adopt stroke certification of any level than a hospital in a predominantly non-Black, racially segregated community,” the authors state.
In terms of socioeconomic factors, hospitals serving low-income, economically integrated (HR, 0.23) and low-income, economically segregated (HR, 0.29) areas were far less likely to adopt any level of stroke care certification than hospitals serving high-income areas, regardless of income segregation.
Rural hospitals were also much less likely to adopt any level of stroke care than urban hospitals (HR, 0.10).
“Our results suggest that it might be necessary to incentivize hospitals operating in underserved communities to seek stroke certification or to entice hospitals with higher propensity to adopt stroke care to operate in such communities so access at the per-patient level becomes more equitable,” the authors say.
This project was supported by the Pilot Project Award from the National Bureau of Economic Research Center for Aging and Health Research, funded by the National Institute on Aging and by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Shen and Dr. Hsia have received grants from the National Institute of Aging and the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
Hospitals in low-income and rural areas of the United States are much less likely to adopt stroke certification than hospitals in high-income and urban communities, a new study shows.
Further, other results showed that, after adjustment for population and hospital size, access to stroke-certified hospitals is significantly lower in Black, racially segregated communities.
The study was published online in JAMA Neurology.
Noting that stroke-certified hospitals provide higher-quality stroke care, the authors, led by Yu-Chu Shen, PhD, Naval Postgraduate School, Monterey, Calif., conclude that: “Our findings suggest that structural inequities in stroke care may be an important consideration in eliminating stroke disparities for vulnerable populations.”
In an audio interview on the JAMA Neurology website, senior author Renee Y. Hsia, MD, University of California, San Francisco, said: “Our findings show there are clear disparities in which communities are getting access to stroke certified hospitals.”
She called for more help for hospitals in underserved areas to obtain stroke certification.
Dr. Hsia explained that hospitals can seek certification at their own expense and that although stroke care is expensive, it is also lucrative in terms of reimbursement. So it tends to be the private for-profit hospitals that seek these certifications. “If you are a county hospital on a really tight budget, you’re not going to have the extra cash on hand to be applying for stroke certification,” she commented.
This can result in an increase in hospitals with stroke certification – but not in the areas that need it the most.
Dr. Hsia points out that this has happened in cardiac care. One study showed a 44% increase in hospitals providing percutaneous coronary intervention over a 10-year period, but the percentage of the population that had better access increased by less than 1%.
“In general, in the United States we have a mentality that ‘more is better,’ and because there is no government regulation in health care, any time a hospital applies for these specialized services we just generally think that’s a good thing. But this might not always be the case,” Dr. Hsia noted. “We have a very market-based approach, and this doesn’t lead to equity. It leads to profit maximization, and that is not synonymous with what’s good for patients or populations.”
She suggested that in future the process of certification should include some consideration of how it will affect population-based equity.
“Rather than rubber stamping an application just because hospitals have certain resources, we need to ask what the benefit is of providing this service,” Dr. Hsia said. “Does this community really need it? If not, maybe we should invest these resources into helping a hospital in a community that needs it more.”
Dr. Hsia explained that she and her colleagues conducted their study to investigate whether there were structural issues that might be contributing to disparities in stroke care.
“We like to think emergency stroke care is equitable. Anyone can call 911 or go the emergency room. But, actually, there is a big disparity on who receives what type of care,” she said. “We know Black patients are less likely to receive thrombolytics and mechanical thrombectomy compared to White patents. And wealthy patients are more likely to receive thrombectomy compared to patients from the poorest zip codes.”
She said there is a tendency to think this is a result of some sort of bias on the part of health care professionals. “We wanted to look deep down in the system and whether the built environment of health care supply and geographic distribution of services contributed to access and treatment inequities.”
The study combined a dataset of hospital stroke certification from all general acute nonfederal hospitals in the continental United States from January 2009 to December 2019. National, hospital, and census data were used to identify historically underserved communities by racial and ethnic composition, income distribution, and rurality.
A total of 4,984 hospitals were assessed. Results showed that over the 11-year study period, the number of hospitals with stroke certification grew from 961 (19%) to 1,763 (36%).
Without controlling for population and hospital size, hospitals in predominantly Black, racially segregated areas were 1.67-fold more likely to adopt stroke care of any level than those in predominantly non-Black, racially segregated areas (hazard ratio, 1.67; 95% confidence interval, 1.41-1.97).
However, after adjustment for population and hospital size, the likelihood of adopting stroke care among hospitals serving Black, racially segregated communities was significantly lower than among those serving non-Black, racially segregated communities (HR, 0.74; 95% CI, 0.62-0.89).
“In other words, on a per-capita basis, a hospital serving a predominantly Black, racially segregated community was 26% less likely to adopt stroke certification of any level than a hospital in a predominantly non-Black, racially segregated community,” the authors state.
In terms of socioeconomic factors, hospitals serving low-income, economically integrated (HR, 0.23) and low-income, economically segregated (HR, 0.29) areas were far less likely to adopt any level of stroke care certification than hospitals serving high-income areas, regardless of income segregation.
Rural hospitals were also much less likely to adopt any level of stroke care than urban hospitals (HR, 0.10).
“Our results suggest that it might be necessary to incentivize hospitals operating in underserved communities to seek stroke certification or to entice hospitals with higher propensity to adopt stroke care to operate in such communities so access at the per-patient level becomes more equitable,” the authors say.
This project was supported by the Pilot Project Award from the National Bureau of Economic Research Center for Aging and Health Research, funded by the National Institute on Aging and by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Shen and Dr. Hsia have received grants from the National Institute of Aging and the National Heart, Lung, and Blood Institute.
A version of this article first appeared on Medscape.com.
Cardiologists concerned for patient safety after abortion ruling
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.















