User login
Dr. Birds-n-Bees: How physicians are taking up the sex ed slack
An athletic coach stands in front of a packed gym full of high school students.
“Don’t have sex,” he instructs, “because you will get pregnant and die. Don’t have sex in the missionary position. Don’t have sex standing up. Just don’t do it, promise? Okay, everybody take some rubbers.”
Sad to say, this scene from the 2004 movie “Mean Girls” bears a striking resemblance to the actual sex education courses taught in schools across the United States today. In fact, things may have gotten measurably worse.
National data recently published by the Guttmacher Institute showed that adolescents were less likely to receive adequate sex education from 2015 to 2019 than they were in 1995. Only half of kids aged 15-19 received sex education that met minimum standards recommended by the Department of Health & Human Services, and fewer than half were given this information before having sex for the first time. With such a vast learning gap, it is no surprise that the United States has some of the highest rates of teenage pregnancy and sexually transmitted infections in the developed world.
Concerned and motivated by this need for sex education, physicians and other medical professionals are stepping in to fill the void, offering sexual health information through a range of methods to students of all ages (some a lot older than one may think). It is a calling that takes them outside their hospitals and exam rooms into workshops and through educational materials, video, and social media content created from scratch.
“The fact that we’re able to go in and provide factual, scientific, important information that can affect the trajectory of someone’s life is powerful,” said Julia Rossen, part of a contingent of med students at Brown University, Providence, R.I., who now teach sex ed as an elective.
Their goals are not just about protecting health. Many are also teaching about other topics commonly ignored in sex education classes, such as consent, pleasure, LGBTQ+ identities, and cultural competence. There is a mutually beneficial relationship, they say, between their sex education work and their medical practice.
Changing the status quo
A jumble of state laws govern how and when schools should offer sex education courses. Individual school districts often make the final decisions about their content, creating even more inconsistent standards. Only 29 states and the District of Columbia mandate sex education, and 13 of those do not require that it be medically accurate. Abstinence-only education, which has been shown to be ineffective, is exclusively taught in 16 states.
Without formal instruction, many young people must learn about sex from family members, who may be unwilling, or they may share knowledge between themselves, which is often incorrect, or navigate the limitless information and misinformation available on the internet.
The consequences of this were apparent to several medical students at Brown University in 2013. At the time, the rate of teenage pregnancy across Rhode Island was 1 in 100, but in the small city of Central Falls, it was 1 in 25. Aiming to improve this, the group created a comprehensive sex education program for a Central Falls middle school that was taught by medical student volunteers.
The Sex Ed by Brown Med program continues today. It consists of eight in-person sessions. Topics include anatomy, contraception, STIs, sexual decision-making, consent, sexual violence, and sexual and gender identity. Through this program, as well as other factors, the Central Falls teenage pregnancy rate declined to 1.6 in 100 from 2016 to 2020, according to the Rhode Island Department of Health.
“Historically, sexual education has been politicized,” said Ms. Rossen, one of the current program leaders. “It’s been at the discretion of a lot of different factors that aren’t under the control of the communities that are actually receiving the education.”
Among seventh graders, the teachers say they encounter different levels of maturity. But they feel that the kids are more receptive and open with younger adults who, like them, are still students. Some volunteers recall the flaws in their own sex education, particularly regarding topics such as consent and gender and sexual identity, and they believe middle school is the time to begin the sexual health conversation. “By the time you’re talking to college-age students, it’s pretty much too late,” said another group leader, Benjamin Stone.
Mr. Stone feels that practicing having these often-awkward discussions enhances their clinical skills as physicians. “Sex and sexual history are part of the comprehensive medical interview. People want to have these conversations, and they’re looking for someone to open the door. The kids are excited that we’re opening that door for them. And I think patients feel the same way.”
Conquering social media
Opening the door has been more like releasing a floodgate for Danielle Jones, MD, an ob.gyn. physician who is originally from Texas but who moved to New Zealand in 2021. Known on social media as “Mama Doctor Jones,” she has garnered more than 3 million followers across YouTube, TikTok, Twitter, Instagram, and Facebook. Dr. Jones produces short, friendly, entertaining videos on a range of reproductive health and sex education topics. They appeal to an adolescent audience hungry for a trustworthy voice on issues such as,: “5 ‘Strange’ Things Your Vagina Does That Are NORMAL” and “Condom Broke ... Now What?”
Dr. Jones uses her platform to debunk some of the misleading and inaccurate sexual health information being taught in classrooms, by other social media influencers, and that is found on the internet in general. Her no-nonsense-style videos call out such myths as being unable to pee with a tampon in, Plan B emergency contraception causing abortions, and COVID-19 vaccines damaging fertility.
“The way sex ed is done in the U.S. in most places is continuing the taboo by making it a one-time discussion or health class,” said Dr. Jones, “particularly if boys and girls are separated. That doesn’t further communication between people or foster an environment where it’s okay to discuss your body and puberty and changes in sexual health in general. And if you can’t talk about it in educational spaces, you’re certainly not going to be comfortable talking about that in a one-on-one situation with another 16-year-old.”
Taking on other taboos, Dr. Jones has been outspoken about abortion and the consequences of the recent Supreme Court decision, both as an ethical issue and a medical one that endangers lives. Raised in a deeply religious family, Dr. Jones said she was indoctrinated with antiabortion views, and it took time for her thinking to evolve “from a scientific and humanistic standpoint.” While working in a Texas private practice, Dr. Jones described being unable to mention abortion online because of fear of losing her patients and for her own safety.
Now free of those constraints, Dr. Jones feels that her videos can be important resources for teachers who may have little health training. And she is enthusiastic about the complementary relationship between her social media work and her clinical practice. “There are conversations I have all the time in the clinic where patients tell me: ‘Nobody’s ever really had this conversation in this way with me. Thank you for explaining that,’ ” said Dr. Jones. “And then I think: ‘Well, now I’ll have it with a hundred thousand other people too.’ ”
Promoting pleasure
While not an ob.gyn., discussing sexuality with patients has become a focus for Evelin Dacker, MD, a family physician in Salem, Ore. Dr. Dacker is certified in functional medicine, which takes a holistic and integrative approach. During her training she had a sudden realization: Sexuality had not been discussed at any point during her medical education.
“I recognized that this was a huge gap in how we deal with a person as a human,” Dr. Dacker explained. “Since sexuality plays a role in so many aspects of our humanness, not just having sex.”
Dr. Dacker believes in rethinking sexuality as a fundamental part of overall health, as vital as nutrition or blood pressure. Outside her medical practice, she teaches classes and workshops on sexual health and sex positivity for young adults and other physicians. She has also developed an educational framework for sexual health topics. Dr. Dacker said she frequently confronts the idea that sexuality is only about engaging with another person. She disagrees. Using food as a metaphor, she argues that just as the pleasure of eating something is purely for oneself, sexuality belongs to the individual.
Sexuality can also be a tool for pleasure, which Dr. Dacker believes plays an essential role in physical health. “Pleasure is a medicine,” Dr. Dacker said. “I actually prescribe self-pleasure practices to my patients, so they can start owning it within themselves. Make sure you get 7-8 hours of sleep, do some breathing exercises to help bring down your stress, and do self-pleasure so that you can integrate into your body better.”
She added that the impact of prioritizing one’s own desires, needs, and boundaries can transform how people view their sexuality. Her adult students frequently ask: “Why wasn’t I taught this as a teenager?”
Speaking of adult students – An older generation learns new tricks
While the teen cohort is usually the focus, the lack of sex education in previous decades – and the way sexual culture has evolved in that time – have an impact on older groups. Among U.S. adults aged 55 and older, the rate of STIs has more than doubled in the past 10 years, according to the Centers for Disease Control and Prevention. While the majority of STI cases still occur among teenagers and young adults, the consistent increase in STIs among older persons is cause for concern among physicians and researchers.
The issue worries Shannon Dowler, MD, a family physician in western North Carolina and chief medical officer for North Carolina Medicaid. Dr. Dowler, who has practiced in an STI clinic throughout her career, began seeing more and more older adults with chlamydia, herpes, and other STIs. Dowler cites several factors behind the rise, including the growing retirement community population, the availability of pharmaceuticals for sexual dysfunction, and the “hook-up culture” that is active on dating apps, which research shows are regularly used by more than a third of adults older than 55.
Dr. Dowler also sees a lack of communication about sexual health between physicians and their older patients. “Older adults are more likely to be in relationship with their physician outside the exam room, especially if they’re in a small community,” Dr. Dowler said. “Sometimes they aren’t as comfortable sharing what their risks are. But we are guilty in medicine all the time of not asking. We assume someone’s older so they’re not having sex anymore. But, in fact, they are, and we’re not taking the time to say: ‘Let’s talk about your sex life. Are you at risk for anything? Are you having any difficulties with sex?’ We tend to avoid it as a health care culture.”
In contrast, Dr. Dowler said she talks about sexual health with anyone who will listen. She teaches classes in private schools and universities and for church youth groups and other physicians. She often finds that public schools are not interested, which she attributes to fear of her discussing things “outside the rule book.”
Dr. Dowler takes creative approaches. In 2017, she released a hip-hop video, “STD’s Never Get Old,” in which she raps about safe sex for older adults. Her video went viral, was mentioned by several news outlets, and received over 50,000 views on YouTube. Dr. Dowler’s latest project is a book, “Never Too Late: Your Guide to Safer Sex after 60,” which is scheduled for publication on Valentine’s Day, 2023.
“It’s sex ed for seniors,” she explained. “It’s that gym class that some people got – I won’t say everyone got – in high school. This is the version for older adults who didn’t get that. There are new infections now that didn’t exist when they had sex education, if they had sex education.”
A big subject requires a big mission
For others in the sex education field, physicians are allies in their fight against agendas designed to obstruct or erode sex education. Alison Macklin, director of policy and advocacy at SIECUS: Sex Ed for Social Change, formerly the Sexuality Information and Education Council of the United States, sees this struggle playing out in school boards and state legislatures across the country. For every comprehensive sex education bill passed or school district victory, there is yet another blocked proposal or restrictive law somewhere else.
Ms. Macklin urged doctors to get more involved locally and to expand their knowledge of sexual health issues by reaching out to organizations such as Planned Parenthood and to be “hyper vigilant” in their own communities.
“Doctors are trusted. People really respect what they have to say,” Ms. Macklin said. “And this is an important time for them to speak up.”
A version of this article first appeared on Medscape.com.
An athletic coach stands in front of a packed gym full of high school students.
“Don’t have sex,” he instructs, “because you will get pregnant and die. Don’t have sex in the missionary position. Don’t have sex standing up. Just don’t do it, promise? Okay, everybody take some rubbers.”
Sad to say, this scene from the 2004 movie “Mean Girls” bears a striking resemblance to the actual sex education courses taught in schools across the United States today. In fact, things may have gotten measurably worse.
National data recently published by the Guttmacher Institute showed that adolescents were less likely to receive adequate sex education from 2015 to 2019 than they were in 1995. Only half of kids aged 15-19 received sex education that met minimum standards recommended by the Department of Health & Human Services, and fewer than half were given this information before having sex for the first time. With such a vast learning gap, it is no surprise that the United States has some of the highest rates of teenage pregnancy and sexually transmitted infections in the developed world.
Concerned and motivated by this need for sex education, physicians and other medical professionals are stepping in to fill the void, offering sexual health information through a range of methods to students of all ages (some a lot older than one may think). It is a calling that takes them outside their hospitals and exam rooms into workshops and through educational materials, video, and social media content created from scratch.
“The fact that we’re able to go in and provide factual, scientific, important information that can affect the trajectory of someone’s life is powerful,” said Julia Rossen, part of a contingent of med students at Brown University, Providence, R.I., who now teach sex ed as an elective.
Their goals are not just about protecting health. Many are also teaching about other topics commonly ignored in sex education classes, such as consent, pleasure, LGBTQ+ identities, and cultural competence. There is a mutually beneficial relationship, they say, between their sex education work and their medical practice.
Changing the status quo
A jumble of state laws govern how and when schools should offer sex education courses. Individual school districts often make the final decisions about their content, creating even more inconsistent standards. Only 29 states and the District of Columbia mandate sex education, and 13 of those do not require that it be medically accurate. Abstinence-only education, which has been shown to be ineffective, is exclusively taught in 16 states.
Without formal instruction, many young people must learn about sex from family members, who may be unwilling, or they may share knowledge between themselves, which is often incorrect, or navigate the limitless information and misinformation available on the internet.
The consequences of this were apparent to several medical students at Brown University in 2013. At the time, the rate of teenage pregnancy across Rhode Island was 1 in 100, but in the small city of Central Falls, it was 1 in 25. Aiming to improve this, the group created a comprehensive sex education program for a Central Falls middle school that was taught by medical student volunteers.
The Sex Ed by Brown Med program continues today. It consists of eight in-person sessions. Topics include anatomy, contraception, STIs, sexual decision-making, consent, sexual violence, and sexual and gender identity. Through this program, as well as other factors, the Central Falls teenage pregnancy rate declined to 1.6 in 100 from 2016 to 2020, according to the Rhode Island Department of Health.
“Historically, sexual education has been politicized,” said Ms. Rossen, one of the current program leaders. “It’s been at the discretion of a lot of different factors that aren’t under the control of the communities that are actually receiving the education.”
Among seventh graders, the teachers say they encounter different levels of maturity. But they feel that the kids are more receptive and open with younger adults who, like them, are still students. Some volunteers recall the flaws in their own sex education, particularly regarding topics such as consent and gender and sexual identity, and they believe middle school is the time to begin the sexual health conversation. “By the time you’re talking to college-age students, it’s pretty much too late,” said another group leader, Benjamin Stone.
Mr. Stone feels that practicing having these often-awkward discussions enhances their clinical skills as physicians. “Sex and sexual history are part of the comprehensive medical interview. People want to have these conversations, and they’re looking for someone to open the door. The kids are excited that we’re opening that door for them. And I think patients feel the same way.”
Conquering social media
Opening the door has been more like releasing a floodgate for Danielle Jones, MD, an ob.gyn. physician who is originally from Texas but who moved to New Zealand in 2021. Known on social media as “Mama Doctor Jones,” she has garnered more than 3 million followers across YouTube, TikTok, Twitter, Instagram, and Facebook. Dr. Jones produces short, friendly, entertaining videos on a range of reproductive health and sex education topics. They appeal to an adolescent audience hungry for a trustworthy voice on issues such as,: “5 ‘Strange’ Things Your Vagina Does That Are NORMAL” and “Condom Broke ... Now What?”
Dr. Jones uses her platform to debunk some of the misleading and inaccurate sexual health information being taught in classrooms, by other social media influencers, and that is found on the internet in general. Her no-nonsense-style videos call out such myths as being unable to pee with a tampon in, Plan B emergency contraception causing abortions, and COVID-19 vaccines damaging fertility.
“The way sex ed is done in the U.S. in most places is continuing the taboo by making it a one-time discussion or health class,” said Dr. Jones, “particularly if boys and girls are separated. That doesn’t further communication between people or foster an environment where it’s okay to discuss your body and puberty and changes in sexual health in general. And if you can’t talk about it in educational spaces, you’re certainly not going to be comfortable talking about that in a one-on-one situation with another 16-year-old.”
Taking on other taboos, Dr. Jones has been outspoken about abortion and the consequences of the recent Supreme Court decision, both as an ethical issue and a medical one that endangers lives. Raised in a deeply religious family, Dr. Jones said she was indoctrinated with antiabortion views, and it took time for her thinking to evolve “from a scientific and humanistic standpoint.” While working in a Texas private practice, Dr. Jones described being unable to mention abortion online because of fear of losing her patients and for her own safety.
Now free of those constraints, Dr. Jones feels that her videos can be important resources for teachers who may have little health training. And she is enthusiastic about the complementary relationship between her social media work and her clinical practice. “There are conversations I have all the time in the clinic where patients tell me: ‘Nobody’s ever really had this conversation in this way with me. Thank you for explaining that,’ ” said Dr. Jones. “And then I think: ‘Well, now I’ll have it with a hundred thousand other people too.’ ”
Promoting pleasure
While not an ob.gyn., discussing sexuality with patients has become a focus for Evelin Dacker, MD, a family physician in Salem, Ore. Dr. Dacker is certified in functional medicine, which takes a holistic and integrative approach. During her training she had a sudden realization: Sexuality had not been discussed at any point during her medical education.
“I recognized that this was a huge gap in how we deal with a person as a human,” Dr. Dacker explained. “Since sexuality plays a role in so many aspects of our humanness, not just having sex.”
Dr. Dacker believes in rethinking sexuality as a fundamental part of overall health, as vital as nutrition or blood pressure. Outside her medical practice, she teaches classes and workshops on sexual health and sex positivity for young adults and other physicians. She has also developed an educational framework for sexual health topics. Dr. Dacker said she frequently confronts the idea that sexuality is only about engaging with another person. She disagrees. Using food as a metaphor, she argues that just as the pleasure of eating something is purely for oneself, sexuality belongs to the individual.
Sexuality can also be a tool for pleasure, which Dr. Dacker believes plays an essential role in physical health. “Pleasure is a medicine,” Dr. Dacker said. “I actually prescribe self-pleasure practices to my patients, so they can start owning it within themselves. Make sure you get 7-8 hours of sleep, do some breathing exercises to help bring down your stress, and do self-pleasure so that you can integrate into your body better.”
She added that the impact of prioritizing one’s own desires, needs, and boundaries can transform how people view their sexuality. Her adult students frequently ask: “Why wasn’t I taught this as a teenager?”
Speaking of adult students – An older generation learns new tricks
While the teen cohort is usually the focus, the lack of sex education in previous decades – and the way sexual culture has evolved in that time – have an impact on older groups. Among U.S. adults aged 55 and older, the rate of STIs has more than doubled in the past 10 years, according to the Centers for Disease Control and Prevention. While the majority of STI cases still occur among teenagers and young adults, the consistent increase in STIs among older persons is cause for concern among physicians and researchers.
The issue worries Shannon Dowler, MD, a family physician in western North Carolina and chief medical officer for North Carolina Medicaid. Dr. Dowler, who has practiced in an STI clinic throughout her career, began seeing more and more older adults with chlamydia, herpes, and other STIs. Dowler cites several factors behind the rise, including the growing retirement community population, the availability of pharmaceuticals for sexual dysfunction, and the “hook-up culture” that is active on dating apps, which research shows are regularly used by more than a third of adults older than 55.
Dr. Dowler also sees a lack of communication about sexual health between physicians and their older patients. “Older adults are more likely to be in relationship with their physician outside the exam room, especially if they’re in a small community,” Dr. Dowler said. “Sometimes they aren’t as comfortable sharing what their risks are. But we are guilty in medicine all the time of not asking. We assume someone’s older so they’re not having sex anymore. But, in fact, they are, and we’re not taking the time to say: ‘Let’s talk about your sex life. Are you at risk for anything? Are you having any difficulties with sex?’ We tend to avoid it as a health care culture.”
In contrast, Dr. Dowler said she talks about sexual health with anyone who will listen. She teaches classes in private schools and universities and for church youth groups and other physicians. She often finds that public schools are not interested, which she attributes to fear of her discussing things “outside the rule book.”
Dr. Dowler takes creative approaches. In 2017, she released a hip-hop video, “STD’s Never Get Old,” in which she raps about safe sex for older adults. Her video went viral, was mentioned by several news outlets, and received over 50,000 views on YouTube. Dr. Dowler’s latest project is a book, “Never Too Late: Your Guide to Safer Sex after 60,” which is scheduled for publication on Valentine’s Day, 2023.
“It’s sex ed for seniors,” she explained. “It’s that gym class that some people got – I won’t say everyone got – in high school. This is the version for older adults who didn’t get that. There are new infections now that didn’t exist when they had sex education, if they had sex education.”
A big subject requires a big mission
For others in the sex education field, physicians are allies in their fight against agendas designed to obstruct or erode sex education. Alison Macklin, director of policy and advocacy at SIECUS: Sex Ed for Social Change, formerly the Sexuality Information and Education Council of the United States, sees this struggle playing out in school boards and state legislatures across the country. For every comprehensive sex education bill passed or school district victory, there is yet another blocked proposal or restrictive law somewhere else.
Ms. Macklin urged doctors to get more involved locally and to expand their knowledge of sexual health issues by reaching out to organizations such as Planned Parenthood and to be “hyper vigilant” in their own communities.
“Doctors are trusted. People really respect what they have to say,” Ms. Macklin said. “And this is an important time for them to speak up.”
A version of this article first appeared on Medscape.com.
An athletic coach stands in front of a packed gym full of high school students.
“Don’t have sex,” he instructs, “because you will get pregnant and die. Don’t have sex in the missionary position. Don’t have sex standing up. Just don’t do it, promise? Okay, everybody take some rubbers.”
Sad to say, this scene from the 2004 movie “Mean Girls” bears a striking resemblance to the actual sex education courses taught in schools across the United States today. In fact, things may have gotten measurably worse.
National data recently published by the Guttmacher Institute showed that adolescents were less likely to receive adequate sex education from 2015 to 2019 than they were in 1995. Only half of kids aged 15-19 received sex education that met minimum standards recommended by the Department of Health & Human Services, and fewer than half were given this information before having sex for the first time. With such a vast learning gap, it is no surprise that the United States has some of the highest rates of teenage pregnancy and sexually transmitted infections in the developed world.
Concerned and motivated by this need for sex education, physicians and other medical professionals are stepping in to fill the void, offering sexual health information through a range of methods to students of all ages (some a lot older than one may think). It is a calling that takes them outside their hospitals and exam rooms into workshops and through educational materials, video, and social media content created from scratch.
“The fact that we’re able to go in and provide factual, scientific, important information that can affect the trajectory of someone’s life is powerful,” said Julia Rossen, part of a contingent of med students at Brown University, Providence, R.I., who now teach sex ed as an elective.
Their goals are not just about protecting health. Many are also teaching about other topics commonly ignored in sex education classes, such as consent, pleasure, LGBTQ+ identities, and cultural competence. There is a mutually beneficial relationship, they say, between their sex education work and their medical practice.
Changing the status quo
A jumble of state laws govern how and when schools should offer sex education courses. Individual school districts often make the final decisions about their content, creating even more inconsistent standards. Only 29 states and the District of Columbia mandate sex education, and 13 of those do not require that it be medically accurate. Abstinence-only education, which has been shown to be ineffective, is exclusively taught in 16 states.
Without formal instruction, many young people must learn about sex from family members, who may be unwilling, or they may share knowledge between themselves, which is often incorrect, or navigate the limitless information and misinformation available on the internet.
The consequences of this were apparent to several medical students at Brown University in 2013. At the time, the rate of teenage pregnancy across Rhode Island was 1 in 100, but in the small city of Central Falls, it was 1 in 25. Aiming to improve this, the group created a comprehensive sex education program for a Central Falls middle school that was taught by medical student volunteers.
The Sex Ed by Brown Med program continues today. It consists of eight in-person sessions. Topics include anatomy, contraception, STIs, sexual decision-making, consent, sexual violence, and sexual and gender identity. Through this program, as well as other factors, the Central Falls teenage pregnancy rate declined to 1.6 in 100 from 2016 to 2020, according to the Rhode Island Department of Health.
“Historically, sexual education has been politicized,” said Ms. Rossen, one of the current program leaders. “It’s been at the discretion of a lot of different factors that aren’t under the control of the communities that are actually receiving the education.”
Among seventh graders, the teachers say they encounter different levels of maturity. But they feel that the kids are more receptive and open with younger adults who, like them, are still students. Some volunteers recall the flaws in their own sex education, particularly regarding topics such as consent and gender and sexual identity, and they believe middle school is the time to begin the sexual health conversation. “By the time you’re talking to college-age students, it’s pretty much too late,” said another group leader, Benjamin Stone.
Mr. Stone feels that practicing having these often-awkward discussions enhances their clinical skills as physicians. “Sex and sexual history are part of the comprehensive medical interview. People want to have these conversations, and they’re looking for someone to open the door. The kids are excited that we’re opening that door for them. And I think patients feel the same way.”
Conquering social media
Opening the door has been more like releasing a floodgate for Danielle Jones, MD, an ob.gyn. physician who is originally from Texas but who moved to New Zealand in 2021. Known on social media as “Mama Doctor Jones,” she has garnered more than 3 million followers across YouTube, TikTok, Twitter, Instagram, and Facebook. Dr. Jones produces short, friendly, entertaining videos on a range of reproductive health and sex education topics. They appeal to an adolescent audience hungry for a trustworthy voice on issues such as,: “5 ‘Strange’ Things Your Vagina Does That Are NORMAL” and “Condom Broke ... Now What?”
Dr. Jones uses her platform to debunk some of the misleading and inaccurate sexual health information being taught in classrooms, by other social media influencers, and that is found on the internet in general. Her no-nonsense-style videos call out such myths as being unable to pee with a tampon in, Plan B emergency contraception causing abortions, and COVID-19 vaccines damaging fertility.
“The way sex ed is done in the U.S. in most places is continuing the taboo by making it a one-time discussion or health class,” said Dr. Jones, “particularly if boys and girls are separated. That doesn’t further communication between people or foster an environment where it’s okay to discuss your body and puberty and changes in sexual health in general. And if you can’t talk about it in educational spaces, you’re certainly not going to be comfortable talking about that in a one-on-one situation with another 16-year-old.”
Taking on other taboos, Dr. Jones has been outspoken about abortion and the consequences of the recent Supreme Court decision, both as an ethical issue and a medical one that endangers lives. Raised in a deeply religious family, Dr. Jones said she was indoctrinated with antiabortion views, and it took time for her thinking to evolve “from a scientific and humanistic standpoint.” While working in a Texas private practice, Dr. Jones described being unable to mention abortion online because of fear of losing her patients and for her own safety.
Now free of those constraints, Dr. Jones feels that her videos can be important resources for teachers who may have little health training. And she is enthusiastic about the complementary relationship between her social media work and her clinical practice. “There are conversations I have all the time in the clinic where patients tell me: ‘Nobody’s ever really had this conversation in this way with me. Thank you for explaining that,’ ” said Dr. Jones. “And then I think: ‘Well, now I’ll have it with a hundred thousand other people too.’ ”
Promoting pleasure
While not an ob.gyn., discussing sexuality with patients has become a focus for Evelin Dacker, MD, a family physician in Salem, Ore. Dr. Dacker is certified in functional medicine, which takes a holistic and integrative approach. During her training she had a sudden realization: Sexuality had not been discussed at any point during her medical education.
“I recognized that this was a huge gap in how we deal with a person as a human,” Dr. Dacker explained. “Since sexuality plays a role in so many aspects of our humanness, not just having sex.”
Dr. Dacker believes in rethinking sexuality as a fundamental part of overall health, as vital as nutrition or blood pressure. Outside her medical practice, she teaches classes and workshops on sexual health and sex positivity for young adults and other physicians. She has also developed an educational framework for sexual health topics. Dr. Dacker said she frequently confronts the idea that sexuality is only about engaging with another person. She disagrees. Using food as a metaphor, she argues that just as the pleasure of eating something is purely for oneself, sexuality belongs to the individual.
Sexuality can also be a tool for pleasure, which Dr. Dacker believes plays an essential role in physical health. “Pleasure is a medicine,” Dr. Dacker said. “I actually prescribe self-pleasure practices to my patients, so they can start owning it within themselves. Make sure you get 7-8 hours of sleep, do some breathing exercises to help bring down your stress, and do self-pleasure so that you can integrate into your body better.”
She added that the impact of prioritizing one’s own desires, needs, and boundaries can transform how people view their sexuality. Her adult students frequently ask: “Why wasn’t I taught this as a teenager?”
Speaking of adult students – An older generation learns new tricks
While the teen cohort is usually the focus, the lack of sex education in previous decades – and the way sexual culture has evolved in that time – have an impact on older groups. Among U.S. adults aged 55 and older, the rate of STIs has more than doubled in the past 10 years, according to the Centers for Disease Control and Prevention. While the majority of STI cases still occur among teenagers and young adults, the consistent increase in STIs among older persons is cause for concern among physicians and researchers.
The issue worries Shannon Dowler, MD, a family physician in western North Carolina and chief medical officer for North Carolina Medicaid. Dr. Dowler, who has practiced in an STI clinic throughout her career, began seeing more and more older adults with chlamydia, herpes, and other STIs. Dowler cites several factors behind the rise, including the growing retirement community population, the availability of pharmaceuticals for sexual dysfunction, and the “hook-up culture” that is active on dating apps, which research shows are regularly used by more than a third of adults older than 55.
Dr. Dowler also sees a lack of communication about sexual health between physicians and their older patients. “Older adults are more likely to be in relationship with their physician outside the exam room, especially if they’re in a small community,” Dr. Dowler said. “Sometimes they aren’t as comfortable sharing what their risks are. But we are guilty in medicine all the time of not asking. We assume someone’s older so they’re not having sex anymore. But, in fact, they are, and we’re not taking the time to say: ‘Let’s talk about your sex life. Are you at risk for anything? Are you having any difficulties with sex?’ We tend to avoid it as a health care culture.”
In contrast, Dr. Dowler said she talks about sexual health with anyone who will listen. She teaches classes in private schools and universities and for church youth groups and other physicians. She often finds that public schools are not interested, which she attributes to fear of her discussing things “outside the rule book.”
Dr. Dowler takes creative approaches. In 2017, she released a hip-hop video, “STD’s Never Get Old,” in which she raps about safe sex for older adults. Her video went viral, was mentioned by several news outlets, and received over 50,000 views on YouTube. Dr. Dowler’s latest project is a book, “Never Too Late: Your Guide to Safer Sex after 60,” which is scheduled for publication on Valentine’s Day, 2023.
“It’s sex ed for seniors,” she explained. “It’s that gym class that some people got – I won’t say everyone got – in high school. This is the version for older adults who didn’t get that. There are new infections now that didn’t exist when they had sex education, if they had sex education.”
A big subject requires a big mission
For others in the sex education field, physicians are allies in their fight against agendas designed to obstruct or erode sex education. Alison Macklin, director of policy and advocacy at SIECUS: Sex Ed for Social Change, formerly the Sexuality Information and Education Council of the United States, sees this struggle playing out in school boards and state legislatures across the country. For every comprehensive sex education bill passed or school district victory, there is yet another blocked proposal or restrictive law somewhere else.
Ms. Macklin urged doctors to get more involved locally and to expand their knowledge of sexual health issues by reaching out to organizations such as Planned Parenthood and to be “hyper vigilant” in their own communities.
“Doctors are trusted. People really respect what they have to say,” Ms. Macklin said. “And this is an important time for them to speak up.”
A version of this article first appeared on Medscape.com.
Texas district court allows employers to deny HIV PrEP coverage
Fort Worth, Tex. – A case decision made by Texas U.S. District Judge Reed Charles O’Connor that will allow employers to deny health care insurance coverage for HIV preexposure prophylaxis (PrEP) is already provoking HIV activists, medical associations, nonprofits, and patients.
As this news organization first reported in August, the class action suit (Kelley v. Azar) has a broader goal – to dismantle the Affordable Care Act using the argument that many of the preventive services it covers, including PrEP, violate the Religious Freedom Restoration Act.
“Judge O’Connor has a long history of issuing rulings against the Affordable Care Act and LGBT individuals, and we expect the case to be successfully appealed as has been the case with his previous discriminatory decisions,” said Carl Schmid, executive director of the HIV+Hepatitis Policy Institute in Washington, in a prepared statement issued shortly after the ruling.
“To single out PrEP, which are FDA approved drugs that effectively prevent HIV, and conclude that its coverage violates the religious freedom of certain individuals, is plain wrong, highly discriminatory, and impedes the public health of our nation,” he said.
PrEP is not just for men who have sex with men. According to the Centers for Disease Control and Prevention, more than 1 million Americans could benefit from PrEP, and roughly 20% are heterosexual women – a fact both Mr. Schmid and the HIV Medicine Association pointed out in response to Judge O’Connor’s ruling.
“Denying access to PrEP threatens the health of more than 1.2 million Americans who could benefit from this potentially life saving intervention,” stated Marwan Haddad, MD, MPH, chair of the HIV Medicine Association, in a press release issued by the organization.
“This ruling is yet one more instance of unacceptable interference in scientific, evidence-based health care practices that must remain within the sanctity of the provider-patient relationship,” she said.
The ruling is also outside what is normally considered religious “conscientious objection.”
While the American Medical Association supports the rights of physicians to act in accordance with conscience, medical ethicists like Abram Brummett, PhD, assistant professor, department of foundational medical studies, Oakland University, Rochester, Mich., previously told this news organization that this ruling actually reflects a phenomenon known as “conscience creep” – that is, the way conscientious objection creeps outside traditional contexts like abortion, sterilization, and organ transplantation.
Incidentally, the case is not yet completed; Judge O’Connor still has to decide on challenges to contraceptives and HPV mandates. He has requested that defendants and plaintiffs file a supplemental briefing before he makes a final decision.
Regardless of how it plays out, it is unclear whether the U.S. Department of Health and Human Services will appeal.
A version of this article first appeared on Medscape.com.
Fort Worth, Tex. – A case decision made by Texas U.S. District Judge Reed Charles O’Connor that will allow employers to deny health care insurance coverage for HIV preexposure prophylaxis (PrEP) is already provoking HIV activists, medical associations, nonprofits, and patients.
As this news organization first reported in August, the class action suit (Kelley v. Azar) has a broader goal – to dismantle the Affordable Care Act using the argument that many of the preventive services it covers, including PrEP, violate the Religious Freedom Restoration Act.
“Judge O’Connor has a long history of issuing rulings against the Affordable Care Act and LGBT individuals, and we expect the case to be successfully appealed as has been the case with his previous discriminatory decisions,” said Carl Schmid, executive director of the HIV+Hepatitis Policy Institute in Washington, in a prepared statement issued shortly after the ruling.
“To single out PrEP, which are FDA approved drugs that effectively prevent HIV, and conclude that its coverage violates the religious freedom of certain individuals, is plain wrong, highly discriminatory, and impedes the public health of our nation,” he said.
PrEP is not just for men who have sex with men. According to the Centers for Disease Control and Prevention, more than 1 million Americans could benefit from PrEP, and roughly 20% are heterosexual women – a fact both Mr. Schmid and the HIV Medicine Association pointed out in response to Judge O’Connor’s ruling.
“Denying access to PrEP threatens the health of more than 1.2 million Americans who could benefit from this potentially life saving intervention,” stated Marwan Haddad, MD, MPH, chair of the HIV Medicine Association, in a press release issued by the organization.
“This ruling is yet one more instance of unacceptable interference in scientific, evidence-based health care practices that must remain within the sanctity of the provider-patient relationship,” she said.
The ruling is also outside what is normally considered religious “conscientious objection.”
While the American Medical Association supports the rights of physicians to act in accordance with conscience, medical ethicists like Abram Brummett, PhD, assistant professor, department of foundational medical studies, Oakland University, Rochester, Mich., previously told this news organization that this ruling actually reflects a phenomenon known as “conscience creep” – that is, the way conscientious objection creeps outside traditional contexts like abortion, sterilization, and organ transplantation.
Incidentally, the case is not yet completed; Judge O’Connor still has to decide on challenges to contraceptives and HPV mandates. He has requested that defendants and plaintiffs file a supplemental briefing before he makes a final decision.
Regardless of how it plays out, it is unclear whether the U.S. Department of Health and Human Services will appeal.
A version of this article first appeared on Medscape.com.
Fort Worth, Tex. – A case decision made by Texas U.S. District Judge Reed Charles O’Connor that will allow employers to deny health care insurance coverage for HIV preexposure prophylaxis (PrEP) is already provoking HIV activists, medical associations, nonprofits, and patients.
As this news organization first reported in August, the class action suit (Kelley v. Azar) has a broader goal – to dismantle the Affordable Care Act using the argument that many of the preventive services it covers, including PrEP, violate the Religious Freedom Restoration Act.
“Judge O’Connor has a long history of issuing rulings against the Affordable Care Act and LGBT individuals, and we expect the case to be successfully appealed as has been the case with his previous discriminatory decisions,” said Carl Schmid, executive director of the HIV+Hepatitis Policy Institute in Washington, in a prepared statement issued shortly after the ruling.
“To single out PrEP, which are FDA approved drugs that effectively prevent HIV, and conclude that its coverage violates the religious freedom of certain individuals, is plain wrong, highly discriminatory, and impedes the public health of our nation,” he said.
PrEP is not just for men who have sex with men. According to the Centers for Disease Control and Prevention, more than 1 million Americans could benefit from PrEP, and roughly 20% are heterosexual women – a fact both Mr. Schmid and the HIV Medicine Association pointed out in response to Judge O’Connor’s ruling.
“Denying access to PrEP threatens the health of more than 1.2 million Americans who could benefit from this potentially life saving intervention,” stated Marwan Haddad, MD, MPH, chair of the HIV Medicine Association, in a press release issued by the organization.
“This ruling is yet one more instance of unacceptable interference in scientific, evidence-based health care practices that must remain within the sanctity of the provider-patient relationship,” she said.
The ruling is also outside what is normally considered religious “conscientious objection.”
While the American Medical Association supports the rights of physicians to act in accordance with conscience, medical ethicists like Abram Brummett, PhD, assistant professor, department of foundational medical studies, Oakland University, Rochester, Mich., previously told this news organization that this ruling actually reflects a phenomenon known as “conscience creep” – that is, the way conscientious objection creeps outside traditional contexts like abortion, sterilization, and organ transplantation.
Incidentally, the case is not yet completed; Judge O’Connor still has to decide on challenges to contraceptives and HPV mandates. He has requested that defendants and plaintiffs file a supplemental briefing before he makes a final decision.
Regardless of how it plays out, it is unclear whether the U.S. Department of Health and Human Services will appeal.
A version of this article first appeared on Medscape.com.
Monkeypox in children and women remains rare, CDC data show
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox virus found in asymptomatic people
The findings, published in Annals of Internal Medicine, follow a similar, non–peer-reviewed report from Belgium. Researchers in both studies tested swabs for monkeypox in men who have sex with men. These swabs had been collected for routine STI screening.
It’s unclear whether asymptomatic individuals who test positive for monkeypox can spread the virus, the French team wrote. But if so, public health strategies to vaccinate those with known exposure “may not be sufficient to contain spread.”
In an editorial accompanying their paper, Stuart Isaacs, MD, associate professor at the University of Pennsylvania, Philadelphia, said it “raises the question of whether asymptomatic or subclinical infections are contributing to the current worldwide outbreak.”
Historically, transmission of monkeypox and its close relative, smallpox, was thought to be greatest when a rash was present, Dr. Isaacs wrote. “Long chains of human-to-human transmission were rare” with monkeypox.
That’s changed with the current outbreak, which was first detected in May. On Aug. 17, the World Health Organization reported more than 35,000 cases in 92 countries, with 12 deaths.
Research methods
For the French study, researchers conducted polymerase chain reaction tests on 200 anorectal swabs from asymptomatic individuals that had been collected from June 5 to July 11 in order to screen for gonorrhea and chlamydia. Of those, 13 (6.5%) were positive for monkeypox.
During the study period, STI testing had been suspended in individuals with monkeypox symptoms because of safety concerns, the researchers reported.
The research team contacted the 13 monkeypox-positive patients and advised them to limit sexual activity for 21 days following their test and notify recent sexual partners. None reported having developed symptoms, but two subsequently returned to the clinic with symptoms – one had an anal rash and the other a sore throat.
In the Belgian report, posted publicly on June 21 as a preprint, 3 of 224 anal samples collected for STI screening in May tested positive for monkeypox. All three of the men who tested positive said they did not have any symptoms in the weeks before and after the sample was taken.
At follow-up testing, 21-37 days after the initial samples were taken, all patients who had previously tested positive were negative. This was “likely as a consequence of spontaneous clearance of the infection,” the authors of that paper wrote.
Clinical implications of findings are uncertain
Monica Gandhi, MD, MPH, a professor of medicine at the University of California, San Francisco, said in an interview that the clinical implications of the findings are uncertain because it’s not known how much viral transmission results from asymptomatic individuals.
Nevertheless, Dr. Gandhi said that “vaccinating all gay men for monkeypox who will accept the vaccine is prudent,” compared with a less aggressive strategy of only vaccinating those with known exposure, which is called ring vaccination. That way, “we can be assured to provide immunity to large swaths of the at-risk population.”
Dr. Gandhi said that movement toward mass vaccination of gay men is occurring in the United States, Canada, Europe, and Australia, despite limited vaccine supply.
She added that, although monkeypox has been concentrated in communities of men who have sex with men, “anyone with multiple sexual partners should be vaccinated given the data.”
However, a WHO official recently cautioned that reports of breakthrough infections in individuals who were vaccinated against monkeypox constitute a reminder that “vaccine is not a silver bullet.”
Non-vaccine interventions are also needed
Other experts stressed the need for nonvaccine interventions.
In his editorial, Dr. Isaacs said an “expanded” ring vaccination strategy in communities of high risk is likely needed, but ultimately the outbreak will only be controlled if vaccination is accompanied by other measures such as identifying and isolating cases, making treatment available, and educating individuals about how to reduce their risk.
Aileen Marty, MD, a professor of infectious diseases at Florida International University, Miami, said in an interview that the new evidence makes it “incredibly important” to inform people that they might be infected by a sex partner even if that person does not have telltale lesions.
Dr. Marty said she has been advising men who have sex with men to “reduce or eliminate situations in which they find themselves with multiple anonymous individuals.”
Although most individuals recover from monkeypox, the disease can lead to hospitalization, disfigurement, blindness, and even death, Dr. Marty noted, adding that monkeypox is “absolutely a disease to avoid.”
Authors of the French study reported financial relationships with Gilead Sciences, Viiv Healthcare, MSD, AstraZeneca, Theratechnologies, Janssen Pharmaceuticals, Pfizer, GlaxoSmithKline, and bioMérieux. Dr. Isaacs reported grants from the Department of Veterans Affairs and the National Institutes of Health and royalties from UpToDate. Dr. Gandhi and Dr. Marty reported no relevant financial interests.
The findings, published in Annals of Internal Medicine, follow a similar, non–peer-reviewed report from Belgium. Researchers in both studies tested swabs for monkeypox in men who have sex with men. These swabs had been collected for routine STI screening.
It’s unclear whether asymptomatic individuals who test positive for monkeypox can spread the virus, the French team wrote. But if so, public health strategies to vaccinate those with known exposure “may not be sufficient to contain spread.”
In an editorial accompanying their paper, Stuart Isaacs, MD, associate professor at the University of Pennsylvania, Philadelphia, said it “raises the question of whether asymptomatic or subclinical infections are contributing to the current worldwide outbreak.”
Historically, transmission of monkeypox and its close relative, smallpox, was thought to be greatest when a rash was present, Dr. Isaacs wrote. “Long chains of human-to-human transmission were rare” with monkeypox.
That’s changed with the current outbreak, which was first detected in May. On Aug. 17, the World Health Organization reported more than 35,000 cases in 92 countries, with 12 deaths.
Research methods
For the French study, researchers conducted polymerase chain reaction tests on 200 anorectal swabs from asymptomatic individuals that had been collected from June 5 to July 11 in order to screen for gonorrhea and chlamydia. Of those, 13 (6.5%) were positive for monkeypox.
During the study period, STI testing had been suspended in individuals with monkeypox symptoms because of safety concerns, the researchers reported.
The research team contacted the 13 monkeypox-positive patients and advised them to limit sexual activity for 21 days following their test and notify recent sexual partners. None reported having developed symptoms, but two subsequently returned to the clinic with symptoms – one had an anal rash and the other a sore throat.
In the Belgian report, posted publicly on June 21 as a preprint, 3 of 224 anal samples collected for STI screening in May tested positive for monkeypox. All three of the men who tested positive said they did not have any symptoms in the weeks before and after the sample was taken.
At follow-up testing, 21-37 days after the initial samples were taken, all patients who had previously tested positive were negative. This was “likely as a consequence of spontaneous clearance of the infection,” the authors of that paper wrote.
Clinical implications of findings are uncertain
Monica Gandhi, MD, MPH, a professor of medicine at the University of California, San Francisco, said in an interview that the clinical implications of the findings are uncertain because it’s not known how much viral transmission results from asymptomatic individuals.
Nevertheless, Dr. Gandhi said that “vaccinating all gay men for monkeypox who will accept the vaccine is prudent,” compared with a less aggressive strategy of only vaccinating those with known exposure, which is called ring vaccination. That way, “we can be assured to provide immunity to large swaths of the at-risk population.”
Dr. Gandhi said that movement toward mass vaccination of gay men is occurring in the United States, Canada, Europe, and Australia, despite limited vaccine supply.
She added that, although monkeypox has been concentrated in communities of men who have sex with men, “anyone with multiple sexual partners should be vaccinated given the data.”
However, a WHO official recently cautioned that reports of breakthrough infections in individuals who were vaccinated against monkeypox constitute a reminder that “vaccine is not a silver bullet.”
Non-vaccine interventions are also needed
Other experts stressed the need for nonvaccine interventions.
In his editorial, Dr. Isaacs said an “expanded” ring vaccination strategy in communities of high risk is likely needed, but ultimately the outbreak will only be controlled if vaccination is accompanied by other measures such as identifying and isolating cases, making treatment available, and educating individuals about how to reduce their risk.
Aileen Marty, MD, a professor of infectious diseases at Florida International University, Miami, said in an interview that the new evidence makes it “incredibly important” to inform people that they might be infected by a sex partner even if that person does not have telltale lesions.
Dr. Marty said she has been advising men who have sex with men to “reduce or eliminate situations in which they find themselves with multiple anonymous individuals.”
Although most individuals recover from monkeypox, the disease can lead to hospitalization, disfigurement, blindness, and even death, Dr. Marty noted, adding that monkeypox is “absolutely a disease to avoid.”
Authors of the French study reported financial relationships with Gilead Sciences, Viiv Healthcare, MSD, AstraZeneca, Theratechnologies, Janssen Pharmaceuticals, Pfizer, GlaxoSmithKline, and bioMérieux. Dr. Isaacs reported grants from the Department of Veterans Affairs and the National Institutes of Health and royalties from UpToDate. Dr. Gandhi and Dr. Marty reported no relevant financial interests.
The findings, published in Annals of Internal Medicine, follow a similar, non–peer-reviewed report from Belgium. Researchers in both studies tested swabs for monkeypox in men who have sex with men. These swabs had been collected for routine STI screening.
It’s unclear whether asymptomatic individuals who test positive for monkeypox can spread the virus, the French team wrote. But if so, public health strategies to vaccinate those with known exposure “may not be sufficient to contain spread.”
In an editorial accompanying their paper, Stuart Isaacs, MD, associate professor at the University of Pennsylvania, Philadelphia, said it “raises the question of whether asymptomatic or subclinical infections are contributing to the current worldwide outbreak.”
Historically, transmission of monkeypox and its close relative, smallpox, was thought to be greatest when a rash was present, Dr. Isaacs wrote. “Long chains of human-to-human transmission were rare” with monkeypox.
That’s changed with the current outbreak, which was first detected in May. On Aug. 17, the World Health Organization reported more than 35,000 cases in 92 countries, with 12 deaths.
Research methods
For the French study, researchers conducted polymerase chain reaction tests on 200 anorectal swabs from asymptomatic individuals that had been collected from June 5 to July 11 in order to screen for gonorrhea and chlamydia. Of those, 13 (6.5%) were positive for monkeypox.
During the study period, STI testing had been suspended in individuals with monkeypox symptoms because of safety concerns, the researchers reported.
The research team contacted the 13 monkeypox-positive patients and advised them to limit sexual activity for 21 days following their test and notify recent sexual partners. None reported having developed symptoms, but two subsequently returned to the clinic with symptoms – one had an anal rash and the other a sore throat.
In the Belgian report, posted publicly on June 21 as a preprint, 3 of 224 anal samples collected for STI screening in May tested positive for monkeypox. All three of the men who tested positive said they did not have any symptoms in the weeks before and after the sample was taken.
At follow-up testing, 21-37 days after the initial samples were taken, all patients who had previously tested positive were negative. This was “likely as a consequence of spontaneous clearance of the infection,” the authors of that paper wrote.
Clinical implications of findings are uncertain
Monica Gandhi, MD, MPH, a professor of medicine at the University of California, San Francisco, said in an interview that the clinical implications of the findings are uncertain because it’s not known how much viral transmission results from asymptomatic individuals.
Nevertheless, Dr. Gandhi said that “vaccinating all gay men for monkeypox who will accept the vaccine is prudent,” compared with a less aggressive strategy of only vaccinating those with known exposure, which is called ring vaccination. That way, “we can be assured to provide immunity to large swaths of the at-risk population.”
Dr. Gandhi said that movement toward mass vaccination of gay men is occurring in the United States, Canada, Europe, and Australia, despite limited vaccine supply.
She added that, although monkeypox has been concentrated in communities of men who have sex with men, “anyone with multiple sexual partners should be vaccinated given the data.”
However, a WHO official recently cautioned that reports of breakthrough infections in individuals who were vaccinated against monkeypox constitute a reminder that “vaccine is not a silver bullet.”
Non-vaccine interventions are also needed
Other experts stressed the need for nonvaccine interventions.
In his editorial, Dr. Isaacs said an “expanded” ring vaccination strategy in communities of high risk is likely needed, but ultimately the outbreak will only be controlled if vaccination is accompanied by other measures such as identifying and isolating cases, making treatment available, and educating individuals about how to reduce their risk.
Aileen Marty, MD, a professor of infectious diseases at Florida International University, Miami, said in an interview that the new evidence makes it “incredibly important” to inform people that they might be infected by a sex partner even if that person does not have telltale lesions.
Dr. Marty said she has been advising men who have sex with men to “reduce or eliminate situations in which they find themselves with multiple anonymous individuals.”
Although most individuals recover from monkeypox, the disease can lead to hospitalization, disfigurement, blindness, and even death, Dr. Marty noted, adding that monkeypox is “absolutely a disease to avoid.”
Authors of the French study reported financial relationships with Gilead Sciences, Viiv Healthcare, MSD, AstraZeneca, Theratechnologies, Janssen Pharmaceuticals, Pfizer, GlaxoSmithKline, and bioMérieux. Dr. Isaacs reported grants from the Department of Veterans Affairs and the National Institutes of Health and royalties from UpToDate. Dr. Gandhi and Dr. Marty reported no relevant financial interests.
FROM ANNALS OF INTERNAL MEDICINE
Docs not talking about anal sex may put women at risk
Clinicians’ reluctance to discuss possible harms of anal sex may be letting down a generation of young women who are unaware of the risks, two researchers from the United Kingdom write in an opinion article published in The BMJ.
Failure to discuss the subject “exposes women to missed diagnoses, futile treatments, and further harm arising from a lack of medical advice,” write Tabitha Gana, MD, and Lesley Hunt, MD, with Sheffield Teaching Hospitals NHS Foundation Trust and Northern General Hospital, both in Sheffield, United Kingdom.
In their opinion, health care professionals, particularly those in general practice, gastroenterology, and colorectal surgery, “have a duty to acknowledge changes in society around anal sex in young women and to meet these changes with open, neutral, and non-judgmental conversations to ensure that all women have the information they need to make informed choices about sex.”
Asking about anal sex is standard practice in genitourinary medicine clinics, but it’s less common in general practice and colorectal clinics, they point out.
No longer taboo
Anal intercourse is becoming more common among young heterosexual couples. In the United Kingdom, participation in heterosexual anal intercourse among people aged 16-24 years rose from about 13% to 29% over the last few decades, according to national survey data.
The same thing is happening in the United States, where research suggests 30%-44% of men and women report having anal sex.
Individual motivation for anal sex varies. Young women cite pleasure, curiosity, pleasing the male partners, and coercion as factors. Up to 25% of women with experience of anal sex report they have been pressured into it at least once, Dr. Gana and Dr. Hunt say.
However, because of its association with alcohol, drug use, and multiple sex partners, anal intercourse is considered a risky sexual behavior.
It’s also associated with specific health concerns, Dr. Gana and Dr. Hunt point out. These include fecal incontinence and anal sphincter injury, which have been reported in women who engage in anal intercourse. When it comes to incontinence, women are at higher risk than men because of their different anatomy and the effects of hormones, pregnancy, and childbirth on the pelvic floor.
“Women have less robust anal sphincters and lower anal canal pressures than men, and damage caused by anal penetration is therefore more consequential,” Dr. Gana and Dr. Hunt point out.
“The pain and bleeding women report after anal sex is indicative of trauma, and risks may be increased if anal sex is coerced,” they add.
Knowledge of the underlying risk factors and taking a good history are key to effective management of anorectal disorders, they say.
Dr. Gana and Dr. Hunt worry that clinicians may shy away from talking about anal sex, influenced by society’s taboos.
Currently, NHS patient information on anal sex considers only sexually transmitted infections, making no mention of anal trauma, incontinence, or the psychological aftermath of being coerced into anal sex.
“It may not be just avoidance or stigma that prevents health professionals [from] talking to young women about the risks of anal sex. There is genuine concern that the message may be seen as judgmental or even misconstrued as homophobic,” Dr. Gana and Dr. Hunt write.
“However, by avoiding these discussions, we may be failing a generation of young women who are unaware of the risks,” they add.
“With better information, women who want anal sex would be able to protect themselves more effectively from possible harm, and those who agree to anal sex reluctantly to meet society’s expectations or please partners may feel better empowered to say no,” Dr. Gana and Dr. Hunt say.
This research had no specific funding. Dr. Gana and Dr. Hunt report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians’ reluctance to discuss possible harms of anal sex may be letting down a generation of young women who are unaware of the risks, two researchers from the United Kingdom write in an opinion article published in The BMJ.
Failure to discuss the subject “exposes women to missed diagnoses, futile treatments, and further harm arising from a lack of medical advice,” write Tabitha Gana, MD, and Lesley Hunt, MD, with Sheffield Teaching Hospitals NHS Foundation Trust and Northern General Hospital, both in Sheffield, United Kingdom.
In their opinion, health care professionals, particularly those in general practice, gastroenterology, and colorectal surgery, “have a duty to acknowledge changes in society around anal sex in young women and to meet these changes with open, neutral, and non-judgmental conversations to ensure that all women have the information they need to make informed choices about sex.”
Asking about anal sex is standard practice in genitourinary medicine clinics, but it’s less common in general practice and colorectal clinics, they point out.
No longer taboo
Anal intercourse is becoming more common among young heterosexual couples. In the United Kingdom, participation in heterosexual anal intercourse among people aged 16-24 years rose from about 13% to 29% over the last few decades, according to national survey data.
The same thing is happening in the United States, where research suggests 30%-44% of men and women report having anal sex.
Individual motivation for anal sex varies. Young women cite pleasure, curiosity, pleasing the male partners, and coercion as factors. Up to 25% of women with experience of anal sex report they have been pressured into it at least once, Dr. Gana and Dr. Hunt say.
However, because of its association with alcohol, drug use, and multiple sex partners, anal intercourse is considered a risky sexual behavior.
It’s also associated with specific health concerns, Dr. Gana and Dr. Hunt point out. These include fecal incontinence and anal sphincter injury, which have been reported in women who engage in anal intercourse. When it comes to incontinence, women are at higher risk than men because of their different anatomy and the effects of hormones, pregnancy, and childbirth on the pelvic floor.
“Women have less robust anal sphincters and lower anal canal pressures than men, and damage caused by anal penetration is therefore more consequential,” Dr. Gana and Dr. Hunt point out.
“The pain and bleeding women report after anal sex is indicative of trauma, and risks may be increased if anal sex is coerced,” they add.
Knowledge of the underlying risk factors and taking a good history are key to effective management of anorectal disorders, they say.
Dr. Gana and Dr. Hunt worry that clinicians may shy away from talking about anal sex, influenced by society’s taboos.
Currently, NHS patient information on anal sex considers only sexually transmitted infections, making no mention of anal trauma, incontinence, or the psychological aftermath of being coerced into anal sex.
“It may not be just avoidance or stigma that prevents health professionals [from] talking to young women about the risks of anal sex. There is genuine concern that the message may be seen as judgmental or even misconstrued as homophobic,” Dr. Gana and Dr. Hunt write.
“However, by avoiding these discussions, we may be failing a generation of young women who are unaware of the risks,” they add.
“With better information, women who want anal sex would be able to protect themselves more effectively from possible harm, and those who agree to anal sex reluctantly to meet society’s expectations or please partners may feel better empowered to say no,” Dr. Gana and Dr. Hunt say.
This research had no specific funding. Dr. Gana and Dr. Hunt report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians’ reluctance to discuss possible harms of anal sex may be letting down a generation of young women who are unaware of the risks, two researchers from the United Kingdom write in an opinion article published in The BMJ.
Failure to discuss the subject “exposes women to missed diagnoses, futile treatments, and further harm arising from a lack of medical advice,” write Tabitha Gana, MD, and Lesley Hunt, MD, with Sheffield Teaching Hospitals NHS Foundation Trust and Northern General Hospital, both in Sheffield, United Kingdom.
In their opinion, health care professionals, particularly those in general practice, gastroenterology, and colorectal surgery, “have a duty to acknowledge changes in society around anal sex in young women and to meet these changes with open, neutral, and non-judgmental conversations to ensure that all women have the information they need to make informed choices about sex.”
Asking about anal sex is standard practice in genitourinary medicine clinics, but it’s less common in general practice and colorectal clinics, they point out.
No longer taboo
Anal intercourse is becoming more common among young heterosexual couples. In the United Kingdom, participation in heterosexual anal intercourse among people aged 16-24 years rose from about 13% to 29% over the last few decades, according to national survey data.
The same thing is happening in the United States, where research suggests 30%-44% of men and women report having anal sex.
Individual motivation for anal sex varies. Young women cite pleasure, curiosity, pleasing the male partners, and coercion as factors. Up to 25% of women with experience of anal sex report they have been pressured into it at least once, Dr. Gana and Dr. Hunt say.
However, because of its association with alcohol, drug use, and multiple sex partners, anal intercourse is considered a risky sexual behavior.
It’s also associated with specific health concerns, Dr. Gana and Dr. Hunt point out. These include fecal incontinence and anal sphincter injury, which have been reported in women who engage in anal intercourse. When it comes to incontinence, women are at higher risk than men because of their different anatomy and the effects of hormones, pregnancy, and childbirth on the pelvic floor.
“Women have less robust anal sphincters and lower anal canal pressures than men, and damage caused by anal penetration is therefore more consequential,” Dr. Gana and Dr. Hunt point out.
“The pain and bleeding women report after anal sex is indicative of trauma, and risks may be increased if anal sex is coerced,” they add.
Knowledge of the underlying risk factors and taking a good history are key to effective management of anorectal disorders, they say.
Dr. Gana and Dr. Hunt worry that clinicians may shy away from talking about anal sex, influenced by society’s taboos.
Currently, NHS patient information on anal sex considers only sexually transmitted infections, making no mention of anal trauma, incontinence, or the psychological aftermath of being coerced into anal sex.
“It may not be just avoidance or stigma that prevents health professionals [from] talking to young women about the risks of anal sex. There is genuine concern that the message may be seen as judgmental or even misconstrued as homophobic,” Dr. Gana and Dr. Hunt write.
“However, by avoiding these discussions, we may be failing a generation of young women who are unaware of the risks,” they add.
“With better information, women who want anal sex would be able to protect themselves more effectively from possible harm, and those who agree to anal sex reluctantly to meet society’s expectations or please partners may feel better empowered to say no,” Dr. Gana and Dr. Hunt say.
This research had no specific funding. Dr. Gana and Dr. Hunt report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
2022 Update on female sexual health
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
Monkeypox: Another emerging threat?
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16
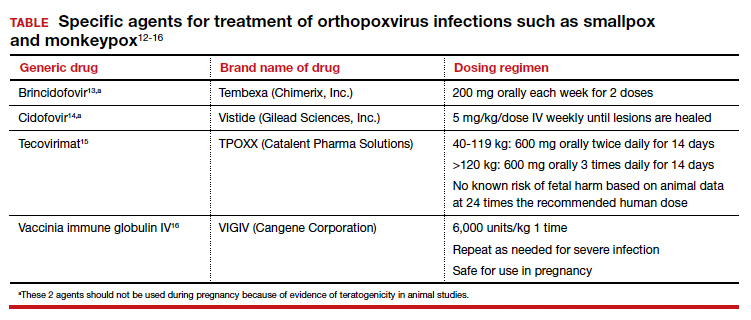
Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

CASE Pregnant woman’s husband is ill after traveling
A 29-year-old primigravid woman at 18 weeks’ gestation just returned from a 10-day trip to Nigeria with her husband. While in Nigeria, the couple went on safari. On several occasions during the safari, they consumed bushmeat prepared by their guides. Her husband now has severe malaise, fever, chills, myalgias, cough, and prominent submandibular, cervical, and inguinal adenopathy. In addition, he has developed a diffuse papular-vesicular rash on his trunk and extremities.
- What is the most likely diagnosis?
- Does this condition pose a danger to his wife?
- What treatment is indicated for his wife?
What we know
In recent weeks, the specter of another poorly understood biological threat has emerged in the medical literature and lay press: monkeypox. This article will first review the epidemiology, clinical manifestations, and diagnosis of this infection, followed by a discussion of how to prevent and treat the condition, with special emphasis on the risks that this infection poses in pregnant women.
Virology
The monkeypox virus is a member of the orthopoxvirus genus. The variola (smallpox) virus and vaccinia virus are included in this genus. It is one of the largest of all viruses, measuring 200-250 nm. It is enveloped and contains double-stranded DNA. Its natural reservoir is probably African rodents. Two distinct strains of monkeypox exist in different geographical regions of Africa: the Central African clade and the West African clade. The Central African clade is significantly more virulent than the latter, with a mortality rate approaching 10%, versus 1% in the West African clade. The incubation period of the virus ranges from 4-20 days and averages 12 days.1,2
Epidemiology
Monkeypox was first discovered in 1958 by Preben von Magnus in a colony of research monkeys in Copenhagen, Denmark. The first case of monkeypox in humans occurred in the Democratic Republic of Congo in 1970 in a 9-year-old boy. Subsequently, cases were reported in the Ivory Coast, Liberia, Nigeria, and Sierra Leone. The infection was limited to the rain forests of central and western Africa until 2003. At that time, the first cases in the United States were reported. The US cases occurred in the Midwest and were traced to exposure to pet prairie dogs. These animals all came from a single distributor, and they apparently were infected when they were housed in the same space with Gambian rats, which are well recognized reservoirs of monkeypox in their native habitat in Africa.1-3
A limited outbreak of monkeypox occurred in the United Kingdom in 2018. Seventy-one cases, with no fatalities, were reported. In 2021 another US case of monkeypox was reported in Dallas, Texas, in an individual who had recently traveled to the United States from Nigeria. A second US case was reported in November 2021 from a patient in Maryland who had returned from a visit to Nigeria. Those were the only 2 reported cases of monkeypox in the United States in 2021.1-3
Then in early May 2022, the United Kingdom reported 9 cases of monkeypox. The first infected patient had recently traveled to Nigeria and, subsequently, infected 2 members of his family.4 On May 18, the Massachusetts Department of Public Health confirmed a case of monkeypox in an adult man who had recently traveled to Canada. As of July 7, 6,027 cases have been reported from at least 39 countries.
The current outbreak is unusual in that, previously, almost all cases occurred in western and central Africa in remote tropical rain forests. Infection usually resulted from close exposure to rats, rabbits, squirrels, monkeys, porcupines, and gazelles. Exposure occurred when persons captured, slaughtered, prepared, and then ate these animals for food without properly cooking the flesh.
The leading theory is that the present outbreak originated among men who had sex with men at 2 raves held in Spain and Belgium. The virus appears to have been spread by skin-to-skin contact, by respiratory droplets, by contact with contaminated bedding, and probably by sperm.2,4,6
Continue to: Clinical manifestations...
Clinical manifestations
Monkeypox evolves through 2 stages: a pre-eruptive stage and an eruptive stage. Prodromal symptoms include malaise, severe headache, myalgias, fever, drenching sweats, backache, fatigue, sore throat, dyspnea, and cough. Within 2-3 days, the characteristic skin eruption develops. The lesions usually begin on the face and then spread in a centrifugal manner to the trunk and extremities, including the palms of the hands and soles of the feet. The lesions typically progress from macules to papules to vesicles to pustules. They then crust and scab over. An interesting additional finding is the presence of prominent lymphadenopathy behind the ear, beneath the mandible, in the neck, and in the groin.1
Several different illnesses must be considered in the differential diagnosis of monkeypox infection. They include measles, scabies, secondary syphilis, and medication-associated allergic reactions. However, the 2 conditions most likely to be confused with monkeypox are chickenpox (varicella) and smallpox. Lymphadenopathy is much more prominent in monkeypox compared with chickenpox. Moreover, with monkeypox, all lesions tend to be at the same stage of evolution as opposed to appearing in crops as they do in chickenpox. Smallpox would be extremely unlikely in the absence of a recognized laboratory accident or a bioterrorism incident.7
Diagnosis
The presumptive diagnosis of monkeypox infection is made primarily based on clinical examination. However, laboratory testing is indicated to definitively differentiate monkeypox from other orthopoxvirus infections such as varicella and smallpox.
In specialized laboratories that employ highly trained personnel and maintain strict safety precautions, the virus can be isolated in mammalian cell cultures. Electron microscopy is a valuable tool for identifying the characteristic brick-shaped poxvirus virions. Routine histologic examination of a lesion will show ballooning degeneration of keratinocytes, prominent spongiosis, dermal edema, and acute inflammation, although these findings are not unique to monkeypox.1
The Centers for Disease Control and Prevention (CDC) has developed serologic tests that detect immunoglobulin (Ig) M- and IgG-specific antibody. However, the most useful and practical diagnostic test is assessment of a skin scraping by polymerase chain reaction (PCR). This test is more sensitive than assessment of serum PCR.1
When the diagnosis of monkeypox is being considered, the clinician should coordinate testing through the local and state public health departments and through the CDC. Effective communication with all agencies will ensure that laboratory specimens are processed in a timely and efficient manner. The CDC website presents information on specimen collection.8
How do we manage monkeypox?
Prevention
The first step in prevention of infection is to isolate infected individuals until all lesions have dried and crusted over. Susceptible people should avoid close contact with skin lesions, respiratory and genital secretions, and bedding of patients who are infected.
The ultimate preventive measure, however, is vaccination of susceptible people either immediately before exposure (eg, military personnel, first responders, infection control investigators, health care workers) or immediately after exposure (general population). Older individuals who received the original smallpox vaccine likely have immunity to monkeypox infection. Unfortunately, very few women who currently are of reproductive age received this vaccine because its use was discontinued in the United States in the early 1970s. Therefore, the vast majority of our patients are uniquely susceptible to this infection and should be vaccinated if there is an outbreak of monkeypox in their locality.7,9
The current preferred vaccine for prevention of both smallpox and monkeypox is the Jynneos (Bavarian Nordic A/S) vaccine.10 This agent incorporates a replication-deficient live virus and does not pose the same risk for adverse events as the original versions of the smallpox vaccine. Jynneos is administered subcutaneously rather than by scarification. Two 0.5-mL doses, delivered 28 days apart, are required for optimal effect. The vaccine must be obtained from local and state health departments, in consultation with the CDC.7,9
There is very little published information on the safety of the Jynneos vaccine in pregnant or lactating women, although animal data are reassuring. Moreover, the dangers of monkeypox infection are significant, and in the event of an outbreak, vaccination of susceptible individuals, including pregnant women, is indicated.
- Monkeypox is a member of the orthopoxvirus genus and is closely related to the smallpox virus. It is a large, double-stranded, enveloped DNA virus.
- The virus is transmitted primarily by close contact with infected animals or other humans or by consumption of contaminated bushmeat.
- The infection evolves in 2 phases. The pre-eruptive phase is characterized by severe flu-like symptoms and signs. The eruptive phase is distinguished by a diffuse papular-vesicular rash.
- The most valuable test for confirming the diagnosis is a polymerase chain reaction test of a fresh skin lesion.
- In women who are pregnant, monkeypox has been associated with spontaneous abortion and fetal death.
- Three antiviral agents may be of value in treating infected patients: cidofovir, brincidofovir, and tecovirimat. Only the latter has an acceptable safety profile for women who are pregnant or lactating.
- The new nonreplicating smallpox vaccine Jynneos (Bavarian Nordic A/S) is of great value for pre- and post-exposure prophylaxis.
Continue to: Treatment...
Treatment
Infected pregnant women should receive acetaminophen 1,000 mg orally every 8 hours, to control fever and provide analgesia. An antihistamine such as diphenhydramine 25 mg orally every 6-8 hours, may be used to control pruritus and provide mild sedation. Adequate fluid intake and optimal nutrition should be encouraged. Skin lesions should be inspected regularly to detect signs of superimposed bacterial infections. Small, localized bacterial skin infections can be treated with topical application of mupirocin ointment 2%, 3 times daily for 7-14 days. For diffuse and more severe bacterial skin infections, a systemic antibiotic may be necessary. Reasonable choices include amoxicillin-clavulanate 875 mg/125 mg orally every 12 hours, or trimethoprim-sulfamethoxazole double strength 800 mg/160 mg orally every 12 hours.11 The latter agent should be avoided in the first trimester of pregnancy because of potential teratogenic effects.
Several specific agents are available through the CDC for treatment of orthopoxvirus infections such as smallpox and monkeypox. Information about these agents is summarized in the TABLE.12-16

Unique considerations in pregnancy
Because monkeypox is so rare, there is very little information about the effects of this infection in pregnant women. The report most commonly cited in the literature is that by Mbala et al, which was published in 2017.17 These authors described 4 pregnant patients in the Democratic Republic of Congo who contracted monkeypox infection over a 4-year period. All 4 women were hospitalized and treated with systemic antibiotics, antiparasitic medications, and analgesics. One patient delivered a healthy infant. Two women had spontaneous abortions in the first trimester. The fourth patient experienced a stillbirth at 22 weeks’ gestation. At postmortem examination, the fetus had diffuse cutaneous lesions, prominent hepatomegaly, and hydrops. No structural malformations were noted. The placenta demonstrated numerous punctate hemorrhages, and high concentrations of virus were recovered from the placenta and from fetal tissue.
Although the information on pregnancy outcome is quite limited, it seems clear that the virus can cross the placenta and cause adverse effects such as spontaneous abortion and fetal death. Accordingly, I think the following guidelines are a reasonable approach to a pregnant patient who has been exposed to monkeypox or who has developed manifestations of infection.3,7,9
- In the event of a community outbreak, bioterrorism event, or exposure to a person with suspected or confirmed monkeypox infection, the pregnant patient should receive the Jynneos vaccine.
- The pregnant patient should be isolated from any individual with suspected or confirmed monkeypox.
- If infection develops despite these measures, the patient should be treated with either tecovirimat or vaccinia immune globulin IV. Hospitalization may be necessary for seriously ill individuals.
- Within 2 weeks of infection, a comprehensive ultrasound examination should be performed to assess for structural abnormalities in the fetus.
- Subsequently, serial ultrasound examinations should be performed at intervals of 4-6 weeks to assess fetal growth and re-evaluate fetal anatomy.
- Following delivery, a detailed neonatal examination should be performed to assess for evidence of viral injury. Neonatal skin lesions and neonatal serum can be assessed by PCR for monkeypox virus. The newborn should be isolated from the mother until all the mother’s lesions have dried and crusted over.
CASE Resolved
Given the husband’s recent travel to Nigeria and consumption of bushmeat, he most likely has monkeypox. The infection can be spread from person to person by close contact; thus, his wife is at risk. The couple should isolate until all of his lesions have dried and crusted over. The woman also should receive the Jynneos vaccine. If she becomes symptomatic, she should be treated with tecovirimat or vaccinia immune globulin IV. ●

- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
- Isaacs SN, Shenoy ES. Monkeypox. UpToDate. Updated June 28,2022. Accessed July 1, 2022. https://www.uptodate.com /contents/monkeypox?topicRef=8349&source=see_link
- Graham MB. Monkeypox. Medscape. Updated June 29, 2022. Accessed July 1, 2022. https://emedicine.medscape.com /article/1134714-overview.
- Khalil A, Samara A, O’Brien P, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022;60:22-27. doi:10.1002/uog.24968.
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. May 18, 2022. Accessed July 1, 2022. https://www.who.int/emergencies/diseaseoutbreak-news/item/2022-DON383.
- WHO reports two new monkeypox deaths, cases in new areas. Reuters. July 7, 2022. https://www.reuters.com/world /who-reports-two-new-monkeypox-deaths-2022-07-07/. Accessed July 19, 2022.
- World Health Organization. Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. Accessed July 1, 2022. https://www.who.int /emergencies/disease-outbreak-news/item/2022 -DON388#:~:text=Multi%2Dcountry%20monkeypox%20 outbreak%20in%20non%2Dendemic%20countries%3A%20 Update,-29%20May%202022&text=Since%2013%20 May%202022%2C%20monkeypox,Epidemiological%20 investigations%20are%20ongoing.
- Cono J, Cragan JD, Jamieson DJ, Rasmussen SA. Prophylaxis and treatment of pregnant women for emerging infections andbioterrorism emergencies. Emerg Infect Dis. 2006;12:16311637. doi:10.3201/eid1211.060618.
- Centers for Disease Control and Prevention. Preparation and collection of specimens. Reviewed June 29, 2022. Accessed July 6, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/prep-collection-specimens.html.
- Rao AK, Petersen BW, Whitehill F, et al. Monkeypox vaccination. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi:10.15585/mmwr.mm7122e1.
- Smallpox and monkeypox vaccine, live, nonreplicating. Package insert. Bavarian Nordic A/S; 2021. Accessed July 1, 2022. https://www.fda.gov/media/131078/download.
- Duff P. Commonly used antibiotics in ObGyn practice. OBG Manag. 2022;34:29, 36-40. doi:10.12788/obgm.0191.
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals: interim clinical guidance for the treatment of monkeypox. Reviewed June 17, 2022. Accessed July 1, 2022. https://www.cdc.gov/poxvirus /monkeypox/clinicians/treatment.html.
- Brincidofovir. Prescribing information. Chimerix, Inc.; 2021. Accessed July 1, 2022. https://www.accessdata.fda.gov /drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf.
- Cidofovir. Package insert. Gilead Sciences, Inc.; 2010. Accessed July 1, 2022. https://www.gilead.com/~/media /Files/pdfs/medicines/other/vistide/vistide.pdf.
- Tecovirimat. Prescribing information. Catalent Pharma Solutions; 2022. Accessed July 1, 2022. https://www.accessdata.fda.gov/drugsatfda_docs /label/2022/214518s000lbl.pdf.
- Vaccinia immune globulin IV. Prescribing information. Cangene Corporation; 2010. Accessed July 1, 2022. https: //www.fda.gov/media/77004/download.
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260.
Single dose of HPV vaccine is ‘game changer,’ says WHO
The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) has changed the recommendation for vaccines against human papillomavirus (HPV).
From the available evidence, SAGE has concluded that a single dose of vaccine offers solid protection against HPV, comparable to that achieved with two-dose schedules.
This could be a “game-changer for the prevention of the disease,” as it would allow “more doses of the life-saving jab reach more girls,” the WHO declared in a press release.
SAGE recommends updating HPV dose schedules as follows:
- One- or two-dose schedule for the primary target of girls aged 9-14 years.
- One- or two-dose schedule for young women aged 15-20.
- Two doses with a 6-month interval for women older than 21.
The HPV vaccine is highly effective for the prevention of HPV serotypes 16 and 18, which cause 70% of cases of cervical cancer, said Alejandro Cravioto, MD, PhD, SAGE chair, in a statement.
“SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch up of missed and older cohorts of girls. These recommendations will enable more girls and women to be vaccinated and thus preventing them from having cervical cancer and all its consequences over the course of their lifetimes,” he added.
For individuals who are immunocompromised, including those with HIV, three doses of the vaccine should be given if feasible, and if not, then at least two doses. There is limited evidence regarding the efficacy of a single dose in this group, the advisory group noted.
Policy makers need to make changes
Now that the WHO has deemed that one dose of HPV vaccine is sufficient, policy makers should make changes, say experts in a recent editorial comment published in The Lancet Oncology.
“Policy makers should consider modifying their HPV immunization schedules for girls aged 9-14 years from a two-dose regimen to a one-dose regimen,” wrote Jeff D’Souza, PhD, Institute for Better Health, Trillium Health Partners, Mississauga, Ont., and David Nderitu, PhD, Egerton University, Nakuru County, Kenya.
Policy makers also need to consider reorienting their efforts on cervical cancer screening and treatment, and they should ensure that all girls globally have access to an effective HPV vaccination schedule, they add.
The editorialists also make a radical proposal.
Existing supply constraints of the HPV vaccine at the country level are expected to continue for the next 3 years, and the vast majority of new cervical cancer cases and related deaths occur in low- and middle-income countries (LMICs).
To overcome these problems, they suggest that “high-income countries that currently offer two-dose regimens to girls aged 9-14 years should consider opting for a one-dose vaccination schedule, and give any excess of vaccines to countries in greater need of them.”
Two doses in high-income countries
But it is unclear whether high-income countries are ready to move to a one-dose schedule.
Approached for comment, Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, Philadelphia, told this news organization that while he can’t say for certain, he suspects that the United States will be slower to accept this recommendation for a single dose of HPV vaccine “as a component of a ‘standard-of-care’ approach.”
However, it “might formally acknowledge that if an individual/parent will only accept a single vaccine dose (or ultimately refuses to return for a recommended second dose), this will be considered a favorable outcome, both for the individual and society.
“I do not know if regulatory bodies in the United States will accept the existing studies performed to address the one-dose vaccination strategy to rather dramatically change the approach in our country,” he said. “The issue would be that if a single dose was stated to be a clinically acceptable option in the United States, it would rapidly become the standard approach, and the regulators would want to be as certain as possible that this would not have a negative effect on what is now recognized to be a remarkably safe and effective cancer prevention effort.”
Another expert who was approached for comment, Stephanie V. Blank, MD, professor of gynecologic oncology at the Icahn School of Medicine at Mount Sinai, New York, said: “In higher-resourced countries, two doses are still preferred, as they are more effective than one.
“The modeling on which the SAGE recommendation is based is all from studies in LMICs and other modeling studies,” she added.
At present, the Centers for Disease Control and Prevention recommends a two-dose schedule of HPV vaccines for individuals who receive the first dose before their 15th birthday. The three-dose schedule is recommended for those who receive the first dose on or after their 15th birthday and for people with certain immunocompromising conditions.
Studies have shown that two doses of HPV vaccine given to children aged 9-14 years provide as good or better protection than three doses given to older adolescents or young adults.
But even with a two-dose schedule, the WHO reports that uptake of the vaccine has been slow, and coverage is much lower than their 90% target. In 2020, global coverage with two doses was only 13%.
Factors that have influenced the slow uptake and low coverage of HPV vaccines include supply challenges, programmatic challenges, and costs related to delivering a two-dose regimen to older girls who are not typically included in childhood vaccination programs. The relatively high cost of HPV vaccines has also been problematic, particularly for middle-income countries.
Trials of one-dose schedules
The one-dose vaccine schedule has garnered a lot of interest, with several studies showing efficacy.
The KEN SHE trial, based in Kenya, showed that a single dose of the HPV vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens. Vaccine efficacy was 97.5% (P < .001) against HPV 16/18 for both the bivalent and monovalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers noted.
A study in India found that efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% for the single dose, 93.1% for the two-dose schedule, and 93.3% for the three-dose series.
Commenting on this trial in India in a recent interview with this news organization, Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, said the findings from India would need “to be confirmed by other studies.” The results were nonetheless “excellent news for developing countries where there are challenges when it comes to access to vaccination.”
Speaking at the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases, he emphasized that at this stage, the findings “cannot be extrapolated” to France. HPV vaccination coverage is low in France (it is estimated that the rate is 23.7%, placing the country 28th of 31 countries in Europe), and he recommended continuing with the two- or three-dose schedule for the time being.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
Ethics of the vaccine
In their editorial, Dr. D’Souza and Dr. Nderitu note that there are ethical considerations with the HPV vaccine that can “help guide deliberations, covering nonmaleficence, beneficence, health equity, stewardship, and solidarity.”
It would be inequitable and unjustifiable, they write, to offer a two-dose regimen to girls aged 9-14 years without also introducing multi-age cohort catch-up campaigns or programs for women who do not have access. “When it comes to an effective HPV vaccination schedule, no woman or girl should be left behind,” they say.
To achieve the goal of eliminating cervical cancer, “countries must ensure that 90% of girls are vaccinated, 70% of women are screened, and 90% of women with precancerous lesions receive treatment and care,” they write. “Given resource constraints, particularly in low-middle income countries, policy makers have a responsibility to ensure that resources are used in an optimal manner that promotes the right to health of all individuals.”
Thus, countries that are lagging far behind in cervical cancer education, screening, and treatment should consider opting for a one-dose regimen for girls aged 9-14 years, as well as using additional resources to close the gap in these other areas.
Dr. Markman has relationships with Genentech, AstraZeneca, Celgene, Clovis, and Amgen; he is also a regular contributor to Medscape Oncology with the Markamn on Oncology video column. Dr. D’Souza and Dr. Nderitu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) has changed the recommendation for vaccines against human papillomavirus (HPV).
From the available evidence, SAGE has concluded that a single dose of vaccine offers solid protection against HPV, comparable to that achieved with two-dose schedules.
This could be a “game-changer for the prevention of the disease,” as it would allow “more doses of the life-saving jab reach more girls,” the WHO declared in a press release.
SAGE recommends updating HPV dose schedules as follows:
- One- or two-dose schedule for the primary target of girls aged 9-14 years.
- One- or two-dose schedule for young women aged 15-20.
- Two doses with a 6-month interval for women older than 21.
The HPV vaccine is highly effective for the prevention of HPV serotypes 16 and 18, which cause 70% of cases of cervical cancer, said Alejandro Cravioto, MD, PhD, SAGE chair, in a statement.
“SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch up of missed and older cohorts of girls. These recommendations will enable more girls and women to be vaccinated and thus preventing them from having cervical cancer and all its consequences over the course of their lifetimes,” he added.
For individuals who are immunocompromised, including those with HIV, three doses of the vaccine should be given if feasible, and if not, then at least two doses. There is limited evidence regarding the efficacy of a single dose in this group, the advisory group noted.
Policy makers need to make changes
Now that the WHO has deemed that one dose of HPV vaccine is sufficient, policy makers should make changes, say experts in a recent editorial comment published in The Lancet Oncology.
“Policy makers should consider modifying their HPV immunization schedules for girls aged 9-14 years from a two-dose regimen to a one-dose regimen,” wrote Jeff D’Souza, PhD, Institute for Better Health, Trillium Health Partners, Mississauga, Ont., and David Nderitu, PhD, Egerton University, Nakuru County, Kenya.
Policy makers also need to consider reorienting their efforts on cervical cancer screening and treatment, and they should ensure that all girls globally have access to an effective HPV vaccination schedule, they add.
The editorialists also make a radical proposal.
Existing supply constraints of the HPV vaccine at the country level are expected to continue for the next 3 years, and the vast majority of new cervical cancer cases and related deaths occur in low- and middle-income countries (LMICs).
To overcome these problems, they suggest that “high-income countries that currently offer two-dose regimens to girls aged 9-14 years should consider opting for a one-dose vaccination schedule, and give any excess of vaccines to countries in greater need of them.”
Two doses in high-income countries
But it is unclear whether high-income countries are ready to move to a one-dose schedule.
Approached for comment, Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, Philadelphia, told this news organization that while he can’t say for certain, he suspects that the United States will be slower to accept this recommendation for a single dose of HPV vaccine “as a component of a ‘standard-of-care’ approach.”
However, it “might formally acknowledge that if an individual/parent will only accept a single vaccine dose (or ultimately refuses to return for a recommended second dose), this will be considered a favorable outcome, both for the individual and society.
“I do not know if regulatory bodies in the United States will accept the existing studies performed to address the one-dose vaccination strategy to rather dramatically change the approach in our country,” he said. “The issue would be that if a single dose was stated to be a clinically acceptable option in the United States, it would rapidly become the standard approach, and the regulators would want to be as certain as possible that this would not have a negative effect on what is now recognized to be a remarkably safe and effective cancer prevention effort.”
Another expert who was approached for comment, Stephanie V. Blank, MD, professor of gynecologic oncology at the Icahn School of Medicine at Mount Sinai, New York, said: “In higher-resourced countries, two doses are still preferred, as they are more effective than one.
“The modeling on which the SAGE recommendation is based is all from studies in LMICs and other modeling studies,” she added.
At present, the Centers for Disease Control and Prevention recommends a two-dose schedule of HPV vaccines for individuals who receive the first dose before their 15th birthday. The three-dose schedule is recommended for those who receive the first dose on or after their 15th birthday and for people with certain immunocompromising conditions.
Studies have shown that two doses of HPV vaccine given to children aged 9-14 years provide as good or better protection than three doses given to older adolescents or young adults.
But even with a two-dose schedule, the WHO reports that uptake of the vaccine has been slow, and coverage is much lower than their 90% target. In 2020, global coverage with two doses was only 13%.
Factors that have influenced the slow uptake and low coverage of HPV vaccines include supply challenges, programmatic challenges, and costs related to delivering a two-dose regimen to older girls who are not typically included in childhood vaccination programs. The relatively high cost of HPV vaccines has also been problematic, particularly for middle-income countries.
Trials of one-dose schedules
The one-dose vaccine schedule has garnered a lot of interest, with several studies showing efficacy.
The KEN SHE trial, based in Kenya, showed that a single dose of the HPV vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens. Vaccine efficacy was 97.5% (P < .001) against HPV 16/18 for both the bivalent and monovalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers noted.
A study in India found that efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% for the single dose, 93.1% for the two-dose schedule, and 93.3% for the three-dose series.
Commenting on this trial in India in a recent interview with this news organization, Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, said the findings from India would need “to be confirmed by other studies.” The results were nonetheless “excellent news for developing countries where there are challenges when it comes to access to vaccination.”
Speaking at the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases, he emphasized that at this stage, the findings “cannot be extrapolated” to France. HPV vaccination coverage is low in France (it is estimated that the rate is 23.7%, placing the country 28th of 31 countries in Europe), and he recommended continuing with the two- or three-dose schedule for the time being.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
Ethics of the vaccine
In their editorial, Dr. D’Souza and Dr. Nderitu note that there are ethical considerations with the HPV vaccine that can “help guide deliberations, covering nonmaleficence, beneficence, health equity, stewardship, and solidarity.”
It would be inequitable and unjustifiable, they write, to offer a two-dose regimen to girls aged 9-14 years without also introducing multi-age cohort catch-up campaigns or programs for women who do not have access. “When it comes to an effective HPV vaccination schedule, no woman or girl should be left behind,” they say.
To achieve the goal of eliminating cervical cancer, “countries must ensure that 90% of girls are vaccinated, 70% of women are screened, and 90% of women with precancerous lesions receive treatment and care,” they write. “Given resource constraints, particularly in low-middle income countries, policy makers have a responsibility to ensure that resources are used in an optimal manner that promotes the right to health of all individuals.”
Thus, countries that are lagging far behind in cervical cancer education, screening, and treatment should consider opting for a one-dose regimen for girls aged 9-14 years, as well as using additional resources to close the gap in these other areas.
Dr. Markman has relationships with Genentech, AstraZeneca, Celgene, Clovis, and Amgen; he is also a regular contributor to Medscape Oncology with the Markamn on Oncology video column. Dr. D’Souza and Dr. Nderitu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The World Health Organization’s Strategic Advisory Group of Experts on Immunization (SAGE) has changed the recommendation for vaccines against human papillomavirus (HPV).
From the available evidence, SAGE has concluded that a single dose of vaccine offers solid protection against HPV, comparable to that achieved with two-dose schedules.
This could be a “game-changer for the prevention of the disease,” as it would allow “more doses of the life-saving jab reach more girls,” the WHO declared in a press release.
SAGE recommends updating HPV dose schedules as follows:
- One- or two-dose schedule for the primary target of girls aged 9-14 years.
- One- or two-dose schedule for young women aged 15-20.
- Two doses with a 6-month interval for women older than 21.
The HPV vaccine is highly effective for the prevention of HPV serotypes 16 and 18, which cause 70% of cases of cervical cancer, said Alejandro Cravioto, MD, PhD, SAGE chair, in a statement.
“SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch up of missed and older cohorts of girls. These recommendations will enable more girls and women to be vaccinated and thus preventing them from having cervical cancer and all its consequences over the course of their lifetimes,” he added.
For individuals who are immunocompromised, including those with HIV, three doses of the vaccine should be given if feasible, and if not, then at least two doses. There is limited evidence regarding the efficacy of a single dose in this group, the advisory group noted.
Policy makers need to make changes
Now that the WHO has deemed that one dose of HPV vaccine is sufficient, policy makers should make changes, say experts in a recent editorial comment published in The Lancet Oncology.
“Policy makers should consider modifying their HPV immunization schedules for girls aged 9-14 years from a two-dose regimen to a one-dose regimen,” wrote Jeff D’Souza, PhD, Institute for Better Health, Trillium Health Partners, Mississauga, Ont., and David Nderitu, PhD, Egerton University, Nakuru County, Kenya.
Policy makers also need to consider reorienting their efforts on cervical cancer screening and treatment, and they should ensure that all girls globally have access to an effective HPV vaccination schedule, they add.
The editorialists also make a radical proposal.
Existing supply constraints of the HPV vaccine at the country level are expected to continue for the next 3 years, and the vast majority of new cervical cancer cases and related deaths occur in low- and middle-income countries (LMICs).
To overcome these problems, they suggest that “high-income countries that currently offer two-dose regimens to girls aged 9-14 years should consider opting for a one-dose vaccination schedule, and give any excess of vaccines to countries in greater need of them.”
Two doses in high-income countries
But it is unclear whether high-income countries are ready to move to a one-dose schedule.
Approached for comment, Maurie Markman, MD, president of medicine and science at Cancer Treatment Centers of America, Philadelphia, told this news organization that while he can’t say for certain, he suspects that the United States will be slower to accept this recommendation for a single dose of HPV vaccine “as a component of a ‘standard-of-care’ approach.”
However, it “might formally acknowledge that if an individual/parent will only accept a single vaccine dose (or ultimately refuses to return for a recommended second dose), this will be considered a favorable outcome, both for the individual and society.
“I do not know if regulatory bodies in the United States will accept the existing studies performed to address the one-dose vaccination strategy to rather dramatically change the approach in our country,” he said. “The issue would be that if a single dose was stated to be a clinically acceptable option in the United States, it would rapidly become the standard approach, and the regulators would want to be as certain as possible that this would not have a negative effect on what is now recognized to be a remarkably safe and effective cancer prevention effort.”
Another expert who was approached for comment, Stephanie V. Blank, MD, professor of gynecologic oncology at the Icahn School of Medicine at Mount Sinai, New York, said: “In higher-resourced countries, two doses are still preferred, as they are more effective than one.
“The modeling on which the SAGE recommendation is based is all from studies in LMICs and other modeling studies,” she added.
At present, the Centers for Disease Control and Prevention recommends a two-dose schedule of HPV vaccines for individuals who receive the first dose before their 15th birthday. The three-dose schedule is recommended for those who receive the first dose on or after their 15th birthday and for people with certain immunocompromising conditions.
Studies have shown that two doses of HPV vaccine given to children aged 9-14 years provide as good or better protection than three doses given to older adolescents or young adults.
But even with a two-dose schedule, the WHO reports that uptake of the vaccine has been slow, and coverage is much lower than their 90% target. In 2020, global coverage with two doses was only 13%.
Factors that have influenced the slow uptake and low coverage of HPV vaccines include supply challenges, programmatic challenges, and costs related to delivering a two-dose regimen to older girls who are not typically included in childhood vaccination programs. The relatively high cost of HPV vaccines has also been problematic, particularly for middle-income countries.
Trials of one-dose schedules
The one-dose vaccine schedule has garnered a lot of interest, with several studies showing efficacy.
The KEN SHE trial, based in Kenya, showed that a single dose of the HPV vaccine was highly effective at preventing oncogenic infection, rivaling the protection offered by multidose regimens. Vaccine efficacy was 97.5% (P < .001) against HPV 16/18 for both the bivalent and monovalent vaccines, which is “comparable to that seen in multidose vaccine trials,” the researchers noted.
A study in India found that efficacy against persistent HPV 16 and 18 infection among participants evaluable for the endpoint was 95.4% for the single dose, 93.1% for the two-dose schedule, and 93.3% for the three-dose series.
Commenting on this trial in India in a recent interview with this news organization, Geoffroy Canlorbe, MD, PhD, of the department of gynecologic and breast surgery and oncology, Pitié-Salpêtrière Hospital, Paris, said the findings from India would need “to be confirmed by other studies.” The results were nonetheless “excellent news for developing countries where there are challenges when it comes to access to vaccination.”
Speaking at the 45th Congress of the French Society for Colposcopy and Cervical and Vaginal Diseases, he emphasized that at this stage, the findings “cannot be extrapolated” to France. HPV vaccination coverage is low in France (it is estimated that the rate is 23.7%, placing the country 28th of 31 countries in Europe), and he recommended continuing with the two- or three-dose schedule for the time being.
“This poor coverage has nothing to do with health care–related logistical or organizational issues; instead, it has to do with people’s mistrust when it comes to vaccination. Here, people who get the first dose get the subsequent ones,” said Dr. Canlorbe. “The very fact of getting two to three doses allows the person’s body to increase the production of antibodies and get a longer-lasting response to the vaccine.”
Ethics of the vaccine
In their editorial, Dr. D’Souza and Dr. Nderitu note that there are ethical considerations with the HPV vaccine that can “help guide deliberations, covering nonmaleficence, beneficence, health equity, stewardship, and solidarity.”
It would be inequitable and unjustifiable, they write, to offer a two-dose regimen to girls aged 9-14 years without also introducing multi-age cohort catch-up campaigns or programs for women who do not have access. “When it comes to an effective HPV vaccination schedule, no woman or girl should be left behind,” they say.
To achieve the goal of eliminating cervical cancer, “countries must ensure that 90% of girls are vaccinated, 70% of women are screened, and 90% of women with precancerous lesions receive treatment and care,” they write. “Given resource constraints, particularly in low-middle income countries, policy makers have a responsibility to ensure that resources are used in an optimal manner that promotes the right to health of all individuals.”
Thus, countries that are lagging far behind in cervical cancer education, screening, and treatment should consider opting for a one-dose regimen for girls aged 9-14 years, as well as using additional resources to close the gap in these other areas.
Dr. Markman has relationships with Genentech, AstraZeneca, Celgene, Clovis, and Amgen; he is also a regular contributor to Medscape Oncology with the Markamn on Oncology video column. Dr. D’Souza and Dr. Nderitu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
To gauge monkeypox spread, researchers eye cases in women
As cases of monkeypox continue to mount in the United States and abroad, infectious disease experts are closely monitoring one group of people in particular: women.
So far, the overwhelming majority of cases of the viral disease have been reported in men who have sex with men. But in recent days, officials have learned of a handful of cases in women – possibly indicating that the outbreak may be widening.
Researchers are keeping close tabs on the proportion of cases in women to “assess whether the outbreak is moving away” from networks of men who have sex with men, where most of the initial cases have been identified, according to a briefing from the UK Health Security Agency (UKHSA).
“There is insufficient evidence to support a change in the transmission dynamics,” the agency said. “However, over the last few weeks the proportion of female cases has been increasing, so this trend needs to be monitored closely.”
A global collaboration of researchers and clinicians recently described 528 cases of monkeypox in 16 countries – but none were in women.
Since data collection for that study ended in June, the research group has confirmed cases in women, said study coauthor John P. Thornhill, MD, PhD, consultant physician in sexual health and HIV and clinical senior lecturer at Barts Health NHS Trust and Queen Mary University of London.
“Cases in women have certainly been reported but are currently far less common,” Dr. Thornhill told this news organization.
Although infections in women have been outliers during the current outbreak, they can be severe when they do occur. Several women in England have been hospitalized with severe symptoms.
A similar pattern has been seen in New York City, where just one woman is among the 639 total cases, according to a July 21 report from the city’s health agency.
Researchers have recently published guidance on monkeypox for ob.gyns., maternal-fetal medicine subspecialists, and people who are pregnant or breastfeeding in anticipation of the possibility of more cases in women.
The Centers for Disease Control and Prevention advises that “pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment” of monkeypox if needed.
One monkeypox vaccine, Jynneos, can be offered to people who are pregnant or breastfeeding and are otherwise eligible for vaccination on the basis of confirmed or likely contact with cases, ideally within 4 days of exposure. Some people at high risk for exposure, such as laboratory workers, may receive the vaccine preemptively.
Another vaccine, ACAM2000, is contraindicated in people who are pregnant or breastfeeding, according to the CDC.
Transmission dynamics
Investigators have not yet identified substantial spread of monkeypox beyond men who have sex with men, although transmission among household contacts, including women and children, has been reported.
Most initial infections during the current outbreak occurred during sexual activity. But monkeypox can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC. It also may spread from mother to child in utero.
Infected pets have been known to spread the disease as well. A multistate monkeypox outbreak in the United States in 2003 was linked to pet prairie dogs, including in childcare and school settings. That year, 55% of the 71 cases occurred in female patients.
More testing, higher positivity rates in men
Since May, more men than women in the United Kingdom have undergone testing for monkeypox, with 3,467 tests in men versus 447 tests in women. Among those tested, the positivity rate has been far higher in men than in women, 54% versus 2.2%, respectively.
As of July 20, about 0.65% of U.K. cases with known gender were in women. Two weeks prior, about 0.4% were in women.
In all, 13 monkeypox cases in England have been in women, and four had severe manifestations that required hospitalization, according to the UKHSA.
Globally, more than 16,000 monkeypox cases have been reported, according to the World Health Organization. The agency said that it plans to rename the disease to reduce stigma.
Monkeypox and pregnancy
Ob.gyns. are often on the “front line in terms of identifying people with infectious diseases,” said Denise J. Jamieson, MD, MPH, Emory University, Atlanta. Dr. Jamieson coauthored “A Primer on Monkeypox Virus for Obstetrician-Gynecologists,” published in Obstetrics & Gynecology.
“Obstetricians need to be aware of what infectious diseases are circulating and be aware of what is going on in the community,” she said.
With monkeypox, “it is anybody’s guess as to how widespread this is going to be,” Dr. Jamieson said.
“The initial monkeypox cases in the current outbreak have been predominately but not exclusively among men who have sex with men; enhanced transmission in this group may be facilitated by sexual activity and spread through complex sexual networks,” Dr. Thornhill said. “As the outbreak continues, we will likely see more monkeypox infections” outside that group.
“Those working in sexual health should have a high index of suspicion in all individuals presenting with genital and oral ulcers and those with proctitis,” he added.
During previous monkeypox outbreaks, the chain of household transmissions has been short, typically two or three people, said Chloe M. Orkin, MD, professor of HIV medicine at Queen Mary University of London. Dr. Orkin directs the Sexual Health and HIV All East Research (SHARE) Collaborative, which has worked to compile the international case series.
Though monkeypox has mainly been transmitted among men who have sex with men, not all identify as gay and some may also have female and nonbinary partners, Dr. Orkin said.
“Clinicians should bear this in mind when examining any person,” she said.
A version of this article first appeared on Medscape.com.
As cases of monkeypox continue to mount in the United States and abroad, infectious disease experts are closely monitoring one group of people in particular: women.
So far, the overwhelming majority of cases of the viral disease have been reported in men who have sex with men. But in recent days, officials have learned of a handful of cases in women – possibly indicating that the outbreak may be widening.
Researchers are keeping close tabs on the proportion of cases in women to “assess whether the outbreak is moving away” from networks of men who have sex with men, where most of the initial cases have been identified, according to a briefing from the UK Health Security Agency (UKHSA).
“There is insufficient evidence to support a change in the transmission dynamics,” the agency said. “However, over the last few weeks the proportion of female cases has been increasing, so this trend needs to be monitored closely.”
A global collaboration of researchers and clinicians recently described 528 cases of monkeypox in 16 countries – but none were in women.
Since data collection for that study ended in June, the research group has confirmed cases in women, said study coauthor John P. Thornhill, MD, PhD, consultant physician in sexual health and HIV and clinical senior lecturer at Barts Health NHS Trust and Queen Mary University of London.
“Cases in women have certainly been reported but are currently far less common,” Dr. Thornhill told this news organization.
Although infections in women have been outliers during the current outbreak, they can be severe when they do occur. Several women in England have been hospitalized with severe symptoms.
A similar pattern has been seen in New York City, where just one woman is among the 639 total cases, according to a July 21 report from the city’s health agency.
Researchers have recently published guidance on monkeypox for ob.gyns., maternal-fetal medicine subspecialists, and people who are pregnant or breastfeeding in anticipation of the possibility of more cases in women.
The Centers for Disease Control and Prevention advises that “pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment” of monkeypox if needed.
One monkeypox vaccine, Jynneos, can be offered to people who are pregnant or breastfeeding and are otherwise eligible for vaccination on the basis of confirmed or likely contact with cases, ideally within 4 days of exposure. Some people at high risk for exposure, such as laboratory workers, may receive the vaccine preemptively.
Another vaccine, ACAM2000, is contraindicated in people who are pregnant or breastfeeding, according to the CDC.
Transmission dynamics
Investigators have not yet identified substantial spread of monkeypox beyond men who have sex with men, although transmission among household contacts, including women and children, has been reported.
Most initial infections during the current outbreak occurred during sexual activity. But monkeypox can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC. It also may spread from mother to child in utero.
Infected pets have been known to spread the disease as well. A multistate monkeypox outbreak in the United States in 2003 was linked to pet prairie dogs, including in childcare and school settings. That year, 55% of the 71 cases occurred in female patients.
More testing, higher positivity rates in men
Since May, more men than women in the United Kingdom have undergone testing for monkeypox, with 3,467 tests in men versus 447 tests in women. Among those tested, the positivity rate has been far higher in men than in women, 54% versus 2.2%, respectively.
As of July 20, about 0.65% of U.K. cases with known gender were in women. Two weeks prior, about 0.4% were in women.
In all, 13 monkeypox cases in England have been in women, and four had severe manifestations that required hospitalization, according to the UKHSA.
Globally, more than 16,000 monkeypox cases have been reported, according to the World Health Organization. The agency said that it plans to rename the disease to reduce stigma.
Monkeypox and pregnancy
Ob.gyns. are often on the “front line in terms of identifying people with infectious diseases,” said Denise J. Jamieson, MD, MPH, Emory University, Atlanta. Dr. Jamieson coauthored “A Primer on Monkeypox Virus for Obstetrician-Gynecologists,” published in Obstetrics & Gynecology.
“Obstetricians need to be aware of what infectious diseases are circulating and be aware of what is going on in the community,” she said.
With monkeypox, “it is anybody’s guess as to how widespread this is going to be,” Dr. Jamieson said.
“The initial monkeypox cases in the current outbreak have been predominately but not exclusively among men who have sex with men; enhanced transmission in this group may be facilitated by sexual activity and spread through complex sexual networks,” Dr. Thornhill said. “As the outbreak continues, we will likely see more monkeypox infections” outside that group.
“Those working in sexual health should have a high index of suspicion in all individuals presenting with genital and oral ulcers and those with proctitis,” he added.
During previous monkeypox outbreaks, the chain of household transmissions has been short, typically two or three people, said Chloe M. Orkin, MD, professor of HIV medicine at Queen Mary University of London. Dr. Orkin directs the Sexual Health and HIV All East Research (SHARE) Collaborative, which has worked to compile the international case series.
Though monkeypox has mainly been transmitted among men who have sex with men, not all identify as gay and some may also have female and nonbinary partners, Dr. Orkin said.
“Clinicians should bear this in mind when examining any person,” she said.
A version of this article first appeared on Medscape.com.
As cases of monkeypox continue to mount in the United States and abroad, infectious disease experts are closely monitoring one group of people in particular: women.
So far, the overwhelming majority of cases of the viral disease have been reported in men who have sex with men. But in recent days, officials have learned of a handful of cases in women – possibly indicating that the outbreak may be widening.
Researchers are keeping close tabs on the proportion of cases in women to “assess whether the outbreak is moving away” from networks of men who have sex with men, where most of the initial cases have been identified, according to a briefing from the UK Health Security Agency (UKHSA).
“There is insufficient evidence to support a change in the transmission dynamics,” the agency said. “However, over the last few weeks the proportion of female cases has been increasing, so this trend needs to be monitored closely.”
A global collaboration of researchers and clinicians recently described 528 cases of monkeypox in 16 countries – but none were in women.
Since data collection for that study ended in June, the research group has confirmed cases in women, said study coauthor John P. Thornhill, MD, PhD, consultant physician in sexual health and HIV and clinical senior lecturer at Barts Health NHS Trust and Queen Mary University of London.
“Cases in women have certainly been reported but are currently far less common,” Dr. Thornhill told this news organization.
Although infections in women have been outliers during the current outbreak, they can be severe when they do occur. Several women in England have been hospitalized with severe symptoms.
A similar pattern has been seen in New York City, where just one woman is among the 639 total cases, according to a July 21 report from the city’s health agency.
Researchers have recently published guidance on monkeypox for ob.gyns., maternal-fetal medicine subspecialists, and people who are pregnant or breastfeeding in anticipation of the possibility of more cases in women.
The Centers for Disease Control and Prevention advises that “pregnant, recently pregnant, and breastfeeding people should be prioritized for medical treatment” of monkeypox if needed.
One monkeypox vaccine, Jynneos, can be offered to people who are pregnant or breastfeeding and are otherwise eligible for vaccination on the basis of confirmed or likely contact with cases, ideally within 4 days of exposure. Some people at high risk for exposure, such as laboratory workers, may receive the vaccine preemptively.
Another vaccine, ACAM2000, is contraindicated in people who are pregnant or breastfeeding, according to the CDC.
Transmission dynamics
Investigators have not yet identified substantial spread of monkeypox beyond men who have sex with men, although transmission among household contacts, including women and children, has been reported.
Most initial infections during the current outbreak occurred during sexual activity. But monkeypox can spread through any close contact with skin lesions or body fluids and possibly through touching contaminated items like clothing or linens, according to the CDC. It also may spread from mother to child in utero.
Infected pets have been known to spread the disease as well. A multistate monkeypox outbreak in the United States in 2003 was linked to pet prairie dogs, including in childcare and school settings. That year, 55% of the 71 cases occurred in female patients.
More testing, higher positivity rates in men
Since May, more men than women in the United Kingdom have undergone testing for monkeypox, with 3,467 tests in men versus 447 tests in women. Among those tested, the positivity rate has been far higher in men than in women, 54% versus 2.2%, respectively.
As of July 20, about 0.65% of U.K. cases with known gender were in women. Two weeks prior, about 0.4% were in women.
In all, 13 monkeypox cases in England have been in women, and four had severe manifestations that required hospitalization, according to the UKHSA.
Globally, more than 16,000 monkeypox cases have been reported, according to the World Health Organization. The agency said that it plans to rename the disease to reduce stigma.
Monkeypox and pregnancy
Ob.gyns. are often on the “front line in terms of identifying people with infectious diseases,” said Denise J. Jamieson, MD, MPH, Emory University, Atlanta. Dr. Jamieson coauthored “A Primer on Monkeypox Virus for Obstetrician-Gynecologists,” published in Obstetrics & Gynecology.
“Obstetricians need to be aware of what infectious diseases are circulating and be aware of what is going on in the community,” she said.
With monkeypox, “it is anybody’s guess as to how widespread this is going to be,” Dr. Jamieson said.
“The initial monkeypox cases in the current outbreak have been predominately but not exclusively among men who have sex with men; enhanced transmission in this group may be facilitated by sexual activity and spread through complex sexual networks,” Dr. Thornhill said. “As the outbreak continues, we will likely see more monkeypox infections” outside that group.
“Those working in sexual health should have a high index of suspicion in all individuals presenting with genital and oral ulcers and those with proctitis,” he added.
During previous monkeypox outbreaks, the chain of household transmissions has been short, typically two or three people, said Chloe M. Orkin, MD, professor of HIV medicine at Queen Mary University of London. Dr. Orkin directs the Sexual Health and HIV All East Research (SHARE) Collaborative, which has worked to compile the international case series.
Though monkeypox has mainly been transmitted among men who have sex with men, not all identify as gay and some may also have female and nonbinary partners, Dr. Orkin said.
“Clinicians should bear this in mind when examining any person,” she said.
A version of this article first appeared on Medscape.com.
Hormone therapy didn’t increase recurrence or mortality in women treated for breast cancer
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
Hormone therapy did not increase mortality in postmenopausal women treated for early-stage estrogen receptor–positive breast cancer, but, in longitudinal data from Denmark, there was a recurrence risk with vaginal estrogen therapy among those treated with aromatase inhibitors.
Genitourinary syndrome of menopause (GSM) – including vaginal dryness, burning, and urinary incontinence – is common in women treated for breast cancer. Adjuvant endocrine therapy, particularly aromatase inhibitors, can aggravate these symptoms. Both local and systemic estrogen therapy are recommended for alleviating GSM symptoms in healthy women, but concerns have been raised about their use in women with breast cancer. Previous studies examining this have suggested possible risks for breast cancer recurrence, but those studies have had several limitations including small samples and short follow-up, particularly for vaginal estrogen therapy.
In the new study, from a national Danish cohort of 8,461 postmenopausal women diagnosed between 1997 and 2004 and treated for early-stage invasive estrogen receptor–positive nonmetastatic breast cancer, neither systemic menopausal hormone therapy (MHT) nor local vaginal estrogen therapy (VET) were associated with an overall increased risk for either breast cancer recurrence or mortality. However, in the subset who had received an aromatase inhibitor – with or without tamoxifen – there was a statistically significant increased risk for breast cancer recurrence, but not mortality.
The results were published in the Journal of the National Cancer Institute.
“The data are reassuring for the majority of women with no adjuvant therapy or tamoxifen. But for those using adjuvant aromatase inhibitors, there might be a small risk,” study lead author Søren Cold, MD, PhD, senior oncologist in the department of oncology at Odense (Denmark) University Hospital, Odense, said in an interview.
Moreover, Dr. Cold noted, while this study didn’t find an increased recurrence risk with MHT for women taking aromatase inhibitors, other studies have. One in particular was stopped because of harm. The reason for the difference here is likely that the previous sample was small – just 133 women.
“Our study is mainly focusing on the use of vaginal estrogen. We had so few patients using systemic menopausal hormone therapy, those data don’t mean much. ... The risk with systemic therapy has been established. The vaginal use hasn’t been thoroughly studied before,” he noted.
Breast cancer recurrence elevated with VET and aromatase inhibitors
The study pool was 9,710 women who underwent complete resection for estrogen-positive breast cancer and were all allocated to 5 years of adjuvant endocrine treatment or no adjuvant treatment, according to guidelines. Overall, 3,112 received no adjuvant endocrine treatment, 2,007 were treated with tamoxifen only, 403 with an aromatase inhibitor, and 2,939 with a sequence of tamoxifen and an aromatase inhibitor.
After exclusion of 1,249 who had received VET or MHT prior to breast cancer diagnosis, there were 6,391 not prescribed any estrogen hormonal treatment, 1,957 prescribed VET, and 133 prescribed MHT with or without VET.
During an estimated median 9.8 years’ follow-up, 1,333 women (16%) had a breast cancer recurrence. Of those, 111 had received VET, 16 MHT, and 1,206 neither. Compared with those receiving no hormonal treatment, the adjusted risk of recurrence was similar for the VET users (hazard ratio, 1.08; 95% confidence interval, 0.89-1.32).
However, there was an increased risk for recurrence associated with initiating VET during aromatase inhibitor treatment (HR, 1.39, 95% CI, 1.04-1.85). For women receiving MHT, the adjusted relative risk of recurrence with aromatase inhibitors wasn’t significant (HR, 1.05; 95% CI, 0.62-1.78).
Overall, compared with women who never used hormonal treatment, the absolute 10-year breast cancer recurrence risk was 19.2% for never-users of VET or MHT, 15.4% in VET users, and 17.1% in MHT users.
No differences found for mortality
Of the 8,461 women in the study, 40% (3,370) died during an estimated median follow-up of 15.2 years. Of those, 497 had received VET, 47 MHT, and 2,826 neither. Compared with the never-users of estrogen therapy, the adjusted HR for overall survival in VET users was 0.78 (95% CI, 0.71-0.87). The analysis stratified by adjuvant endocrine therapy didn’t show an increase in VET users by use of aromatase inhibitors (aHR, 0.94, 95% CI, 0.70-1.26). The same was found for women prescribed MHT, compared with never-users (aHR, 0.94; 95% CI, 0.70-1.26).
Never-users of VET or MHT had an absolute 10-year overall survival of 73.8% versus 79.5% and 80.5% among the women who used VET or MHT, respectively.
Asked to comment, Nanette Santoro, MD, professor and E. Stewart Taylor Chair of Obstetrics & Gynecology at the University of Colorado at Denver, Aurora, said in an interview: “It is important to look at this issue. These findings raise but don’t answer the question that vaginal estradiol may not be as safe as we hope it is for women with breast cancer using an aromatase inhibitor.”
However, she also pointed out that “the overall increase in risk is not enormous; mortality risk was not increased. Women need to consider that there may be some risk associated with this option in their decision making about taking it. Having a satisfying sex life is also important for many women! It is really compassionate use for quality of life, so there is always that unknown element of risk in the discussion. That unknown risk has to be balanced against the benefit that the estrogen provides.”
And, Dr. Santoro also noted that the use of prescription data poses limitations. “It cannot tell us what was going on in the minds of the patient and the prescriber. There may be differences in the prescriber’s impression of the patient’s risk of recurrence that influenced the decision to provide a prescription. ... Women using AIs [aromatase inhibitors] often get pretty severe vaginal dryness symptoms and may need more estrogen to be comfortable with intercourse, but we really cannot tell this from what is in this paper.”
Indeed, Dr. Cold said: “We admit it’s not a randomized study, but we’ve done what was possible to take [confounding] factors into account, including age, tumor size, nodal status, histology, and comorbidities.”
He suggested that a potential therapeutic approach to reducing the recurrence risk might be to switch VET-treated women to tamoxifen after 2-3 years of aromatase inhibitors.
This work was supported by Breast Friends, a part of the Danish Cancer Society. Dr. Cold received support from Breast Friends for the current study. Some of the other coauthors have pharmaceutical company disclosures. Dr. Santoro is a member of the scientific advisory boards for Astellas, Menogenix, Que Oncology, and Amazon Ember, and is a consultant for Ansh Labs.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE