User login
Ocular Manifestations of Patients With Cutaneous Rosacea With and Without Demodex Infection
Acne rosacea is a chronic inflammatory disease that may affect the facial skin, eyes, and eyelids.1 It is characterized by transient or persistent flushing, facial erythema, and telangiectases, generally located on the central portion of the face, and may progress to papules and pustules.2,3 At the late stage of the disease, dermal edema or fibroplasia and sebaceous gland hypertrophy may cause phymatous alterations in the skin. In 2004, the National Rosacea Society Expert Committee developed a classification system for rosacea to standardize subtypes and variants that has since been widely accepted and continues to aid in research and epidemiologic studies.4 The committee defined 4 subtypes based on clinical characteristics: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea.2,3
Ocular rosacea may accompany mild, moderate, and severe dermatologic disease or may occur in the absence of diagnostic skin disease.5 Ocular signs include eyelid margin telangiectasia, spade-shaped infiltrates in the cornea, scleritis, and sclerokeratitis. Common symptoms include burning, stinging, light sensitivity, and foreign-body sensation. Ocular signs commonly seen in rosacea are meibomian gland dysfunction characterized by inspissation and inflammation of the meibomian glands (chalazia), conjunctivitis, honey crust and cylindrical collarette accumulation at the base of the eyelashes, irregularity of the eyelid margin architecture, and evaporative tear dysfunction.5,6
The physiopathology of rosacea is still unknown. Potential factors include genetic predisposition, abnormal inflammation, vascular dysfunction, and involvement of several microbial agents, such as commensal Demodex mites. The number of Demodex mites on normal skin flora is less than 5/cm2; however, the increased vascular dilation and capillary permeability associated with rosacea that result from sunlight and heat exposure increase the density of Demodex folliculorum.7 Elevated Demodex mite density has been observed in the lumens of the sebaceous follicles in patients with rosacea. However, because the severity of the clinical manifestations of the disease is not directly associated with the density of D folliculorum, it generally is accepted that D folliculorum is not a pathogenetic but rather an exacerbating factor.8 It has been reported that this species of mite is mostly found on the face and around the eyelashes and scalp of patients and that it can cause ocular surface inflammation.8
Most studies have researched ocular manifestations of rosacea but not ocular involvement in rosacea patients with and without Demodex mite infestation. In our study, we sought to compare the ocular surface, meibomian gland characteristics, and tear film abnormalities among patients with cutaneous rosacea with and without Demodex infestation.
Materials and Methods
We conducted a retrospective study of 60 patients with cutaneous rosacea. This study was approved by the ethics committee of the local hospital (2018/002-003), and all patients provided verbal and written informed consent before participating in the study. The study was carried out according to the guidelines of the Declaration of Helsinki.
Patient Selection and Evaluation
Patients diagnosed with rosacea by a dermatologist within 6 months were included in the study. Diagnosis of the disease was made after a detailed anamnesis and dermatologic examination. Rosacea was diagnosed if patients had an itching sensation, erythema and/or erythema attacks, and papules and pustules, and fulfilled the diagnostic criteria according to the National Rosacea Society. The skin disease was classified according to the subtypes as ETR, PPR, phymatous rosacea, or ocular rosacea.
The standard skin surface biopsy method was used in 60 patients for detecting Demodex density. When more than 5 mites were detected per square centimeter, the result was recorded as positive. Thirty consecutive, newly diagnosed patients with cutaneous acne rosacea with Demodex infestation and 30 consecutive, newly diagnosed sex- and age-matched patients with acne rosacea without Demodex infestation admitted to the dermatology outpatient clinic were included to this study. The patients who did not have any known dermatologic, systemic, or ocular diseases were included in the study. Patients who met any of the following criteria were excluded from the study: prior anti-inflammatory topical and/or systemic treatment for rosacea during the last 3 months, contact lens wear, eyelid surgery, or autoimmune disease requiring treatment.
Microscopic Demodex Examination
Demodex count was determined using a standardized skin surface biopsy, which is a noninvasive method. Every patient gave samples from the cheeks. This biopsy was repeated from the same site. A drop of cyanoacrylate was placed on a clean slide, pressed against a skin lesion, held in place for 1 minute, and removed. The obtained samples were evaluated under a light microscope (Nikon E200) with oil immersion. When more than 5 mites were detected per square centimeter, the result was recorded as positive.
Ophthalmologic Examination
A complete ophthalmologic examination including visual acuity assessment, standardized slit lamp examination, and fundus examination was done for all patients. Ocular rosacea was diagnosed on detection of 1 or more of the following: watery or bloodshot appearance, foreign-body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectases of the conjunctiva and eyelid margin, eyelid lid and periocular erythema, anterior blepharitis, meibomian gland dysfunction, or irregularity of eyelid margins. All patients were screened for the signs and symptoms of ocular rosacea and underwent other ophthalmologic examinations, including tear function tests. Tear functions were evaluated with Schirmer tests without anesthesia and fluorescein tear breakup time (TBUT). Tear film breakup time was assessed after instillation of 2% fluorescein staining under a cobalt blue filter. The time interval between the last complete blink and the appearance of the first dry spot was recorded. The mean of 3 consecutive measurements was obtained. The Schirmer test was performed without topical anesthesia using a standardized filter strip (Bio-Tech Vision Care). The amount of wetting was measured after 5 minutes. Meibomian gland expressibility was assessed by applying digital pressure to the eyelid margin.
Statistical Analysis
Statistical analysis of the study was performed with SPSS Statistics Version 22.0 (SPSS Inc). Continuous variables were reported as mean (SD), and categorical variables were reported as percentages and counts. Descriptive statistics for numerical variables were created. An independent sample t test was used for normally distributed continuous variables. The Kolmogorov-Smirnov test was used to determine normality. The Schirmer test without anesthesia and TBUT values among groups were compared using one-way analysis of variance. The differences were calculated using the multiple comparison Tukey test. P<.05 was considered statistically significant.
Results
Demographic Characteristics of Rosacea Patients
Sixty eyes of 30 newly diagnosed patients with acne rosacea with Demodex infestation and 60 eyes of 30 newly diagnosed patients with acne rosacea without Demodex infestation were enrolled in this study. The mean age (SD) of the 60 patients was 37.63 (10.01) years. The mean TBUT (SD) of the 120 eyes was 6.65 (3.44) seconds, and the mean Schirmer score (SD) was 12.59 (6.71) mm (Table 1).
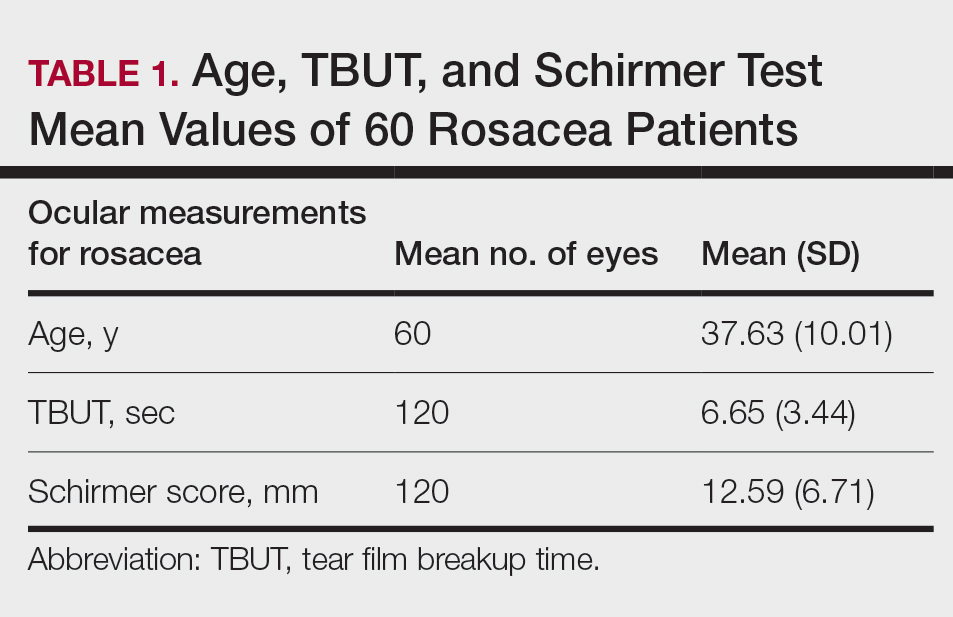
Meibomian Gland Dysfunction vs Subgroup of Rosacea Patients
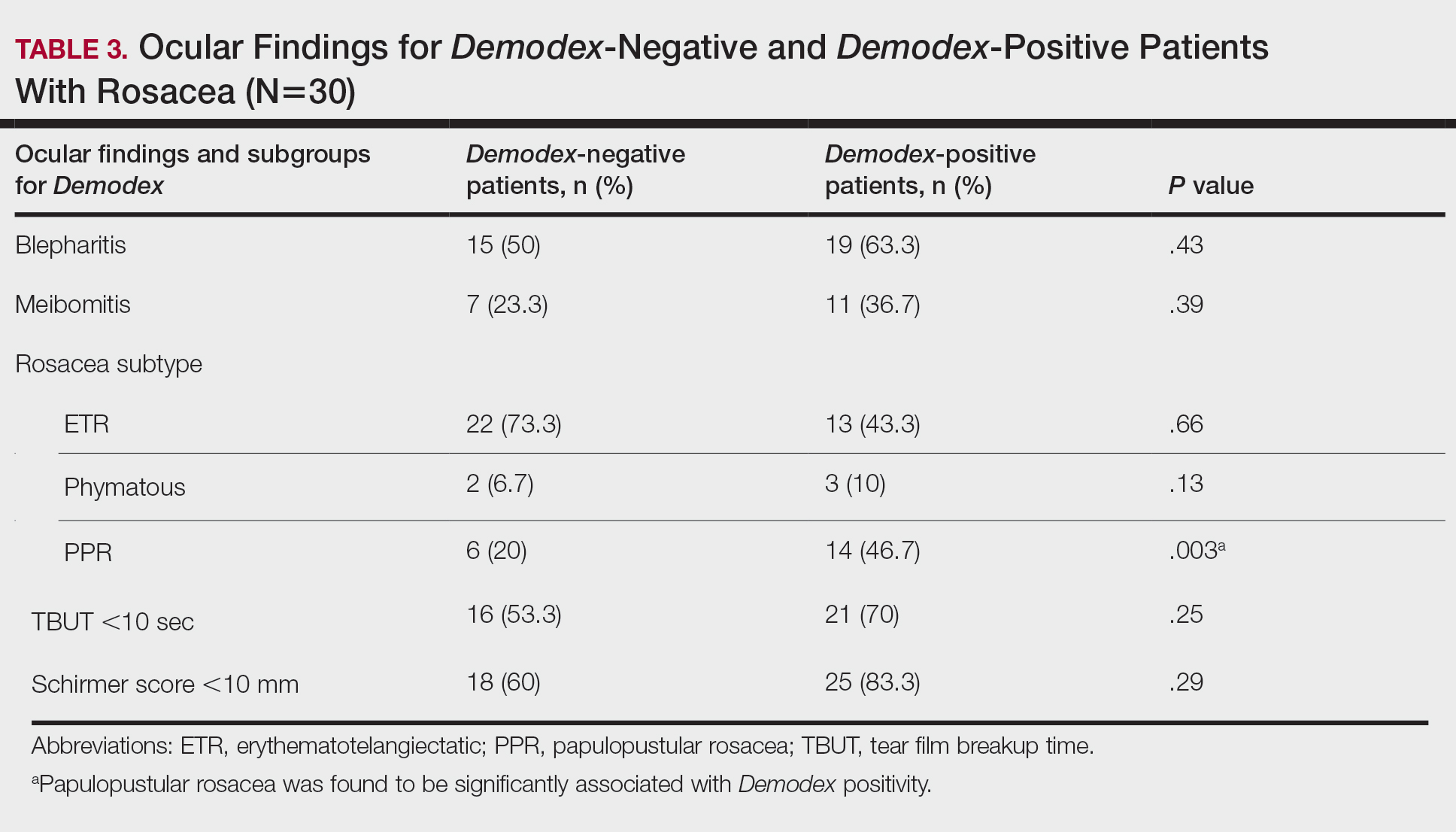
Thirty-four (57%) patients had blepharitis, and 18 (30%) patients had meibomitis. Thirty-five (58.3%) patients had ETR, 5 (8.3%) patients had phymatous rosacea, and 20 (33.4%) patients had PPR (Table 2). Of the Demodex-negative patients, 73.3% (22/30) had ETR, 20% (6/30) had PPR, and 6.7% (2/30) had phymatous rosacea. Of the Demodex-positive patients, 43.3% (13/30) had ETR, 46.7% (14/30) had PPR, and 10% (3/30) had phymatous rosacea (Table 3). Papulopustular rosacea was found to be significantly associated with Demodex positivity (P=.003); neither ETR nor phymatous rosacea was found to be significantly associated with Demodex infestation (P=.66 and P=.13, respectively)(Table 3).
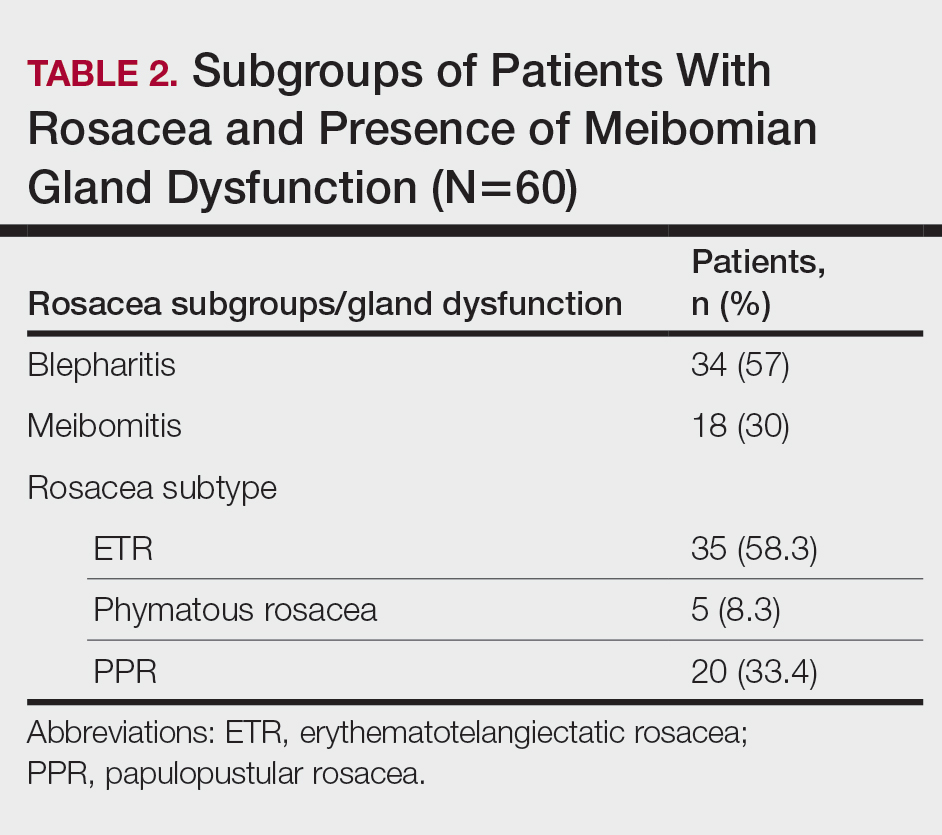
There was no statistically significant difference between the Demodex-negative and Demodex-positive groups for mean age (SD)(37.4 [11.54] years vs 37.87 [8.41] years; P=.85), mean TBUT (SD)(6.73 [3.62] seconds vs 6.57 [3.33] seconds; P=.85), and mean Schirmer score (SD)(13.68 [7.23] mm vs 11.5 [6.08] mm; P=.21)(Table 4).
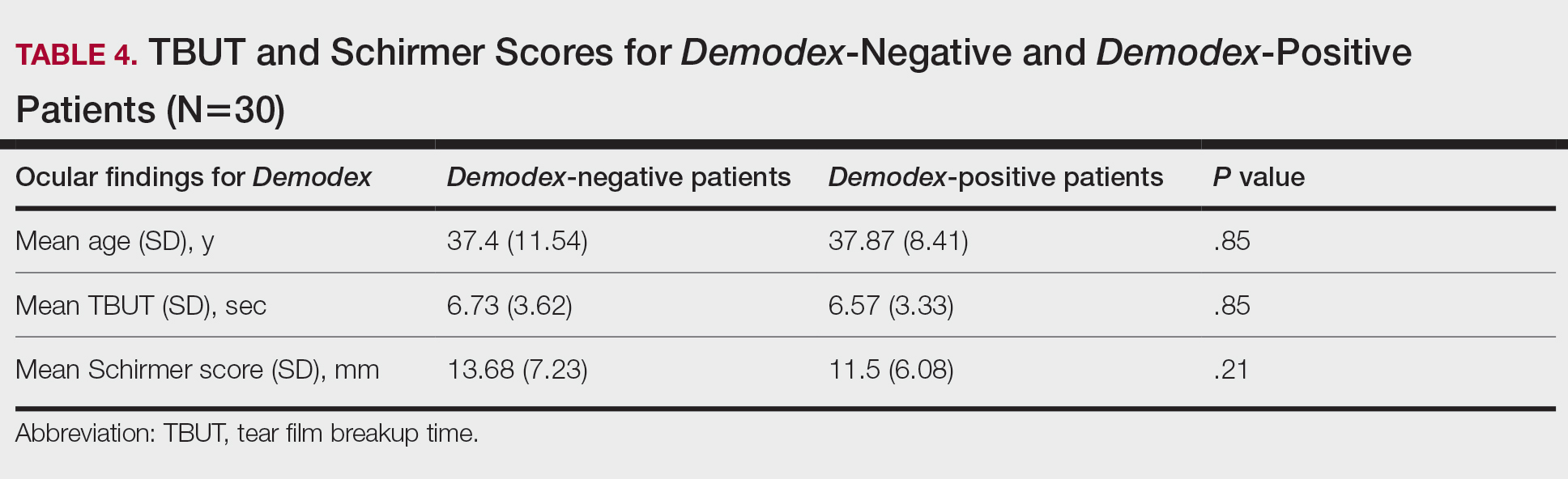
Fifteen (50%) patients (30 eyes) in the Demodex-negative group and 19 (63.3%) patients (38 eyes) in the Demodex-positive group had blepharitis, with no statistically significant difference between the groups (P=.43). Seven (23.3%) patients (14 eyes) in the Demodex-negative group and 11 (36.7%) patients (22 eyes) in the Demodex-positive group had meibomitis, with no statistically significant difference between the groups (P=.39)(Table 3).
Sixteen (53.3%) patients (32 eyes) in the Demodex-negative group and 21 (70%) patients (42 eyes) in the Demodex-positive group had TBUT values less than 10 seconds. Eighteen (60%) patients (36 eyes) in the Demodex-negative group and 25 (83.3%) patients (50 eyes) in the Demodex-positive group had Schirmer scores less than 10 mm (Table 3). The 2 groups were not significantly different in dry eye findings (P=.25 and P=.29, respectively).
Comment
Inflammation in Rosacea
It is known that the density of nonfloral bacteria as well as D folliculorum and Demodex brevis increases in skin affected by rosacea compared to normal skin. Vascular dilation associated with rosacea that results from sunlight and heat causes increased capillary permeability and creates the ideal environment for the proliferation of D folliculorum. Demodex is thought to act as a vector for the activity of certain other microorganisms, particularly Bacillus oleronius, and thus initiates the inflammatory response associated with rosacea.9
One study reported that the inflammation associated with rosacea that was caused by Demodex and other environmental stimuli occurred through toll-like receptor 2 and various cytokines.10 It has been reported that the abnormal function of toll-like receptor 2 in the epidermis leads to the increased production of cathelicidin. Cathelicidin is an antimicrobial peptide with both vasoactive and proinflammatory activity and has been used as a basis to explain the pathogenesis of facial erythema, flushing, and telangiectasia in the context of rosacea.11,12 In addition, it has been reported that the increased secretion of proinflammatory cytokines such as IL-1 and gelatinase B in ocular rosacea leads to tearing film abnormalities that result from increased bacterial flora in the eyelids, which subsequently leads to decreased tear drainage and dry eyes.13 In addition, B oleronius isolated from a D folliculorum mite from patients with PPR produced proteins that induced an inflammatory immune response in 73% (16/22) of patients with rosacea.14
Ocular Findings in Rosacea Patients
In our study, PPR was found to be significantly associated with Demodex positivity compared to ETR and phymatous rosacea (P=.003). However, ocular inflammation findings such as blepharitis and meibomitis were not significantly different between Demodex-positive and Demodex-negative patients. Although the mean Schirmer score of Demodex-positive patients was lower than Demodex-negative patients, this difference was not statistically significant. We evaluated a TBUT of less than 10 seconds and a Schirmer score less than 10 mm as dry eye. Accordingly, the number of patients with dry eye was higher in the Demodex-positive group, but this difference was not statistically significant.
Chronic blepharitis, conjunctival inflammation, and meibomian gland dysfunction are among the most common findings of ocular rosacea.15,16 Patients with ocular rosacea commonly have dry eye and abnormal TBUT and Schirmer scores.17 In our study, we found that the fluorescein TBUT and Schirmer scores were more likely to be abnormal in the Demodex-positive group, but the difference between the 2 groups was not statistically significant.
It has been reported that proinflammatory cytokines due to a weakened immune system in rosacea patients were increased. The weakened immune system was further supported by the increased concentrations of proinflammatory cytokines such as IL-1 and matrix metalloproteinase 9 in these patients’ tears and the improvement of symptoms after the inhibition of these cytokines.11 Luo et al18 reported that Demodex inflammation causes dry eye, particularly with D brevis. Ayyildiz and Sezgin19 reported that Schirmer scores were significantly lower and that the Ocular Surface Disease Index had significantly increased in the Demodex-positive group compared to the Demodex-negative group (P=.001 for both). A Korean study reported that Demodex density was correlated with age, sex, and TBUT results, but there was no significant relationship between Demodex density and Schirmer scores.16
Sobolewska et al20 administered ivermectin cream 1% to 10 patients with cutaneous and ocular rosacea, but only to the forehead, chin, nose, cheeks, and regions close to the eyelids, and observed a significant improvement in blepharitis (P=.004). They stated that ivermectin, as applied only to the face, suppressed the proinflammatory cytokines associated with rosacea and showed anti-inflammatory effects by reducing Demodex mites.20Li et al21 demonstrated a strong correlation between ocular Demodex inflammation and serum reactivity to these bacterial proteins in patients with ocular rosacea, and they found that eyelid margin inflammation and facial rosacea correlated with reactivity to these proteins. These studies suggest a possible role for Demodex infestation and bacterial proteins in the etiology of rosacea.
Gonzalez-Hinojosa et al22 demonstrated that even though eyelash blepharitis was more common in PPR than ETR, there was no statistically significant association between rosacea and Demodex blepharitis. In our study, we found a significant correlation between PPR and Demodex positivity. Also, meibomian gland dysfunction was more common in the Demodex-positive group; however, this result was not statistically significant. One study compared patients with primary demodicosis and patients with rosacea with Demodex-induced blepharitis to healthy controls and found that patients with primary demodicosis and patients with rosacea did not have significantly different ocular findings.23 In contrast, Forton and De Maertelaer24 reported that patients with PPR had significantly more severe ocular manifestations compared with patients with demodicosis (P=.004).
Mizuno et al25 compared the normal (nonrosacea) population with and without Demodex-infested eyelashes and found that the 2 groups were not significantly different for meibomian gland dysfunction, fluorescein TBUT, or ocular surface discomfort.
Varying results have been reported regarding the association between Demodex and blepharitis or ocular surface discomfort with or without rosacea. In our study, we found that Demodex did not affect tear function tests or meibomian gland function in patients with rosacea. We believe this study is important because it demonstrates the effects of Demodex on ocular findings in patients with cutaneous rosacea.
Limitations
Our study has some limitations. The number of patients was relatively small, resulting in few significant differences between the comparison groups. A larger prospective research study is required to assess the prevalence of Demodex mites in the ocular rosacea population along with associated symptoms and findings.
Conclusion
Rosacea is a chronic disease associated with skin and ocular manifestations that range from mild to severe, that progresses in the form of attacks, and that requires long-term follow-up and treatment. Rosacea most often presents as a disease that causes ocular surface inflammation of varying degrees. Demodex infestation may increase cutaneous or ocular inflammation in rosacea. Therefore, every patient diagnosed with rosacea should be given a dermatologic examination to determine Demodex positivity and an ophthalmologic examination to determine ocular manifestations.
- O’Reilly N, Gallagher C, Reddy Katikireddy K, et al. Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci. 2012;53:3250-3259.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 suppl 1):S42-S43.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094.
- Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17:1-4.
- Erbagcı Z, Ozgoztası O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36:81‐86.
- Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97:242‐248.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Gold LM, Draelos ZD. New and emerging treatments for rosacea. Am J Clin Dermatol. 2015;16:457-461.
- Two AM, Del Rosso JQ. Kallikrein 5-mediated inflammation in rosacea: clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J Clin Aesthet Dermatol. 2014;7:20-25.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474-481.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74-87.
- Lee SH, Chun YS, Kim JH, et al. The relationship between Demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906-2911.
- Awais M, Anwar MI, Ilfikhar R, et al. Rosacea—the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34:161-166.
- Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9-S14.
- Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(suppl 1):S39-S41.
- Sobolewska B, Doycheva D, Deuter CM, et al. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea [published online April 7, 2020]. Ocul Immunol Inflamm. doi:10.1080/09273948.2020.1727531
- Li J, O‘Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. 2010;117:870-877.
- Gonzalez‐Hinojosa D, Jaime‐Villalonga A, Aguilar‐Montes G, et al. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66:36‐38.
- Sarac G, Cankaya C, Ozcan KN, et al. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J Cosmet Dermatol. 2020;19:1260-1265.
- Forton FMN, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99:47-52.
- Mizuno M, Kawashima M, Uchino M, et al. Demodex-mite infestation in cilia and its association with ocular surface parameters in Japanese volunteers. Eye Contact Lens. 2020;46:291-296.
Acne rosacea is a chronic inflammatory disease that may affect the facial skin, eyes, and eyelids.1 It is characterized by transient or persistent flushing, facial erythema, and telangiectases, generally located on the central portion of the face, and may progress to papules and pustules.2,3 At the late stage of the disease, dermal edema or fibroplasia and sebaceous gland hypertrophy may cause phymatous alterations in the skin. In 2004, the National Rosacea Society Expert Committee developed a classification system for rosacea to standardize subtypes and variants that has since been widely accepted and continues to aid in research and epidemiologic studies.4 The committee defined 4 subtypes based on clinical characteristics: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea.2,3
Ocular rosacea may accompany mild, moderate, and severe dermatologic disease or may occur in the absence of diagnostic skin disease.5 Ocular signs include eyelid margin telangiectasia, spade-shaped infiltrates in the cornea, scleritis, and sclerokeratitis. Common symptoms include burning, stinging, light sensitivity, and foreign-body sensation. Ocular signs commonly seen in rosacea are meibomian gland dysfunction characterized by inspissation and inflammation of the meibomian glands (chalazia), conjunctivitis, honey crust and cylindrical collarette accumulation at the base of the eyelashes, irregularity of the eyelid margin architecture, and evaporative tear dysfunction.5,6
The physiopathology of rosacea is still unknown. Potential factors include genetic predisposition, abnormal inflammation, vascular dysfunction, and involvement of several microbial agents, such as commensal Demodex mites. The number of Demodex mites on normal skin flora is less than 5/cm2; however, the increased vascular dilation and capillary permeability associated with rosacea that result from sunlight and heat exposure increase the density of Demodex folliculorum.7 Elevated Demodex mite density has been observed in the lumens of the sebaceous follicles in patients with rosacea. However, because the severity of the clinical manifestations of the disease is not directly associated with the density of D folliculorum, it generally is accepted that D folliculorum is not a pathogenetic but rather an exacerbating factor.8 It has been reported that this species of mite is mostly found on the face and around the eyelashes and scalp of patients and that it can cause ocular surface inflammation.8
Most studies have researched ocular manifestations of rosacea but not ocular involvement in rosacea patients with and without Demodex mite infestation. In our study, we sought to compare the ocular surface, meibomian gland characteristics, and tear film abnormalities among patients with cutaneous rosacea with and without Demodex infestation.
Materials and Methods
We conducted a retrospective study of 60 patients with cutaneous rosacea. This study was approved by the ethics committee of the local hospital (2018/002-003), and all patients provided verbal and written informed consent before participating in the study. The study was carried out according to the guidelines of the Declaration of Helsinki.
Patient Selection and Evaluation
Patients diagnosed with rosacea by a dermatologist within 6 months were included in the study. Diagnosis of the disease was made after a detailed anamnesis and dermatologic examination. Rosacea was diagnosed if patients had an itching sensation, erythema and/or erythema attacks, and papules and pustules, and fulfilled the diagnostic criteria according to the National Rosacea Society. The skin disease was classified according to the subtypes as ETR, PPR, phymatous rosacea, or ocular rosacea.
The standard skin surface biopsy method was used in 60 patients for detecting Demodex density. When more than 5 mites were detected per square centimeter, the result was recorded as positive. Thirty consecutive, newly diagnosed patients with cutaneous acne rosacea with Demodex infestation and 30 consecutive, newly diagnosed sex- and age-matched patients with acne rosacea without Demodex infestation admitted to the dermatology outpatient clinic were included to this study. The patients who did not have any known dermatologic, systemic, or ocular diseases were included in the study. Patients who met any of the following criteria were excluded from the study: prior anti-inflammatory topical and/or systemic treatment for rosacea during the last 3 months, contact lens wear, eyelid surgery, or autoimmune disease requiring treatment.
Microscopic Demodex Examination
Demodex count was determined using a standardized skin surface biopsy, which is a noninvasive method. Every patient gave samples from the cheeks. This biopsy was repeated from the same site. A drop of cyanoacrylate was placed on a clean slide, pressed against a skin lesion, held in place for 1 minute, and removed. The obtained samples were evaluated under a light microscope (Nikon E200) with oil immersion. When more than 5 mites were detected per square centimeter, the result was recorded as positive.
Ophthalmologic Examination
A complete ophthalmologic examination including visual acuity assessment, standardized slit lamp examination, and fundus examination was done for all patients. Ocular rosacea was diagnosed on detection of 1 or more of the following: watery or bloodshot appearance, foreign-body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectases of the conjunctiva and eyelid margin, eyelid lid and periocular erythema, anterior blepharitis, meibomian gland dysfunction, or irregularity of eyelid margins. All patients were screened for the signs and symptoms of ocular rosacea and underwent other ophthalmologic examinations, including tear function tests. Tear functions were evaluated with Schirmer tests without anesthesia and fluorescein tear breakup time (TBUT). Tear film breakup time was assessed after instillation of 2% fluorescein staining under a cobalt blue filter. The time interval between the last complete blink and the appearance of the first dry spot was recorded. The mean of 3 consecutive measurements was obtained. The Schirmer test was performed without topical anesthesia using a standardized filter strip (Bio-Tech Vision Care). The amount of wetting was measured after 5 minutes. Meibomian gland expressibility was assessed by applying digital pressure to the eyelid margin.
Statistical Analysis
Statistical analysis of the study was performed with SPSS Statistics Version 22.0 (SPSS Inc). Continuous variables were reported as mean (SD), and categorical variables were reported as percentages and counts. Descriptive statistics for numerical variables were created. An independent sample t test was used for normally distributed continuous variables. The Kolmogorov-Smirnov test was used to determine normality. The Schirmer test without anesthesia and TBUT values among groups were compared using one-way analysis of variance. The differences were calculated using the multiple comparison Tukey test. P<.05 was considered statistically significant.
Results
Demographic Characteristics of Rosacea Patients
Sixty eyes of 30 newly diagnosed patients with acne rosacea with Demodex infestation and 60 eyes of 30 newly diagnosed patients with acne rosacea without Demodex infestation were enrolled in this study. The mean age (SD) of the 60 patients was 37.63 (10.01) years. The mean TBUT (SD) of the 120 eyes was 6.65 (3.44) seconds, and the mean Schirmer score (SD) was 12.59 (6.71) mm (Table 1).

Meibomian Gland Dysfunction vs Subgroup of Rosacea Patients
Thirty-four (57%) patients had blepharitis, and 18 (30%) patients had meibomitis. Thirty-five (58.3%) patients had ETR, 5 (8.3%) patients had phymatous rosacea, and 20 (33.4%) patients had PPR (Table 2). Of the Demodex-negative patients, 73.3% (22/30) had ETR, 20% (6/30) had PPR, and 6.7% (2/30) had phymatous rosacea. Of the Demodex-positive patients, 43.3% (13/30) had ETR, 46.7% (14/30) had PPR, and 10% (3/30) had phymatous rosacea (Table 3). Papulopustular rosacea was found to be significantly associated with Demodex positivity (P=.003); neither ETR nor phymatous rosacea was found to be significantly associated with Demodex infestation (P=.66 and P=.13, respectively)(Table 3).


There was no statistically significant difference between the Demodex-negative and Demodex-positive groups for mean age (SD)(37.4 [11.54] years vs 37.87 [8.41] years; P=.85), mean TBUT (SD)(6.73 [3.62] seconds vs 6.57 [3.33] seconds; P=.85), and mean Schirmer score (SD)(13.68 [7.23] mm vs 11.5 [6.08] mm; P=.21)(Table 4).

Fifteen (50%) patients (30 eyes) in the Demodex-negative group and 19 (63.3%) patients (38 eyes) in the Demodex-positive group had blepharitis, with no statistically significant difference between the groups (P=.43). Seven (23.3%) patients (14 eyes) in the Demodex-negative group and 11 (36.7%) patients (22 eyes) in the Demodex-positive group had meibomitis, with no statistically significant difference between the groups (P=.39)(Table 3).
Sixteen (53.3%) patients (32 eyes) in the Demodex-negative group and 21 (70%) patients (42 eyes) in the Demodex-positive group had TBUT values less than 10 seconds. Eighteen (60%) patients (36 eyes) in the Demodex-negative group and 25 (83.3%) patients (50 eyes) in the Demodex-positive group had Schirmer scores less than 10 mm (Table 3). The 2 groups were not significantly different in dry eye findings (P=.25 and P=.29, respectively).
Comment
Inflammation in Rosacea
It is known that the density of nonfloral bacteria as well as D folliculorum and Demodex brevis increases in skin affected by rosacea compared to normal skin. Vascular dilation associated with rosacea that results from sunlight and heat causes increased capillary permeability and creates the ideal environment for the proliferation of D folliculorum. Demodex is thought to act as a vector for the activity of certain other microorganisms, particularly Bacillus oleronius, and thus initiates the inflammatory response associated with rosacea.9
One study reported that the inflammation associated with rosacea that was caused by Demodex and other environmental stimuli occurred through toll-like receptor 2 and various cytokines.10 It has been reported that the abnormal function of toll-like receptor 2 in the epidermis leads to the increased production of cathelicidin. Cathelicidin is an antimicrobial peptide with both vasoactive and proinflammatory activity and has been used as a basis to explain the pathogenesis of facial erythema, flushing, and telangiectasia in the context of rosacea.11,12 In addition, it has been reported that the increased secretion of proinflammatory cytokines such as IL-1 and gelatinase B in ocular rosacea leads to tearing film abnormalities that result from increased bacterial flora in the eyelids, which subsequently leads to decreased tear drainage and dry eyes.13 In addition, B oleronius isolated from a D folliculorum mite from patients with PPR produced proteins that induced an inflammatory immune response in 73% (16/22) of patients with rosacea.14
Ocular Findings in Rosacea Patients
In our study, PPR was found to be significantly associated with Demodex positivity compared to ETR and phymatous rosacea (P=.003). However, ocular inflammation findings such as blepharitis and meibomitis were not significantly different between Demodex-positive and Demodex-negative patients. Although the mean Schirmer score of Demodex-positive patients was lower than Demodex-negative patients, this difference was not statistically significant. We evaluated a TBUT of less than 10 seconds and a Schirmer score less than 10 mm as dry eye. Accordingly, the number of patients with dry eye was higher in the Demodex-positive group, but this difference was not statistically significant.
Chronic blepharitis, conjunctival inflammation, and meibomian gland dysfunction are among the most common findings of ocular rosacea.15,16 Patients with ocular rosacea commonly have dry eye and abnormal TBUT and Schirmer scores.17 In our study, we found that the fluorescein TBUT and Schirmer scores were more likely to be abnormal in the Demodex-positive group, but the difference between the 2 groups was not statistically significant.
It has been reported that proinflammatory cytokines due to a weakened immune system in rosacea patients were increased. The weakened immune system was further supported by the increased concentrations of proinflammatory cytokines such as IL-1 and matrix metalloproteinase 9 in these patients’ tears and the improvement of symptoms after the inhibition of these cytokines.11 Luo et al18 reported that Demodex inflammation causes dry eye, particularly with D brevis. Ayyildiz and Sezgin19 reported that Schirmer scores were significantly lower and that the Ocular Surface Disease Index had significantly increased in the Demodex-positive group compared to the Demodex-negative group (P=.001 for both). A Korean study reported that Demodex density was correlated with age, sex, and TBUT results, but there was no significant relationship between Demodex density and Schirmer scores.16
Sobolewska et al20 administered ivermectin cream 1% to 10 patients with cutaneous and ocular rosacea, but only to the forehead, chin, nose, cheeks, and regions close to the eyelids, and observed a significant improvement in blepharitis (P=.004). They stated that ivermectin, as applied only to the face, suppressed the proinflammatory cytokines associated with rosacea and showed anti-inflammatory effects by reducing Demodex mites.20Li et al21 demonstrated a strong correlation between ocular Demodex inflammation and serum reactivity to these bacterial proteins in patients with ocular rosacea, and they found that eyelid margin inflammation and facial rosacea correlated with reactivity to these proteins. These studies suggest a possible role for Demodex infestation and bacterial proteins in the etiology of rosacea.
Gonzalez-Hinojosa et al22 demonstrated that even though eyelash blepharitis was more common in PPR than ETR, there was no statistically significant association between rosacea and Demodex blepharitis. In our study, we found a significant correlation between PPR and Demodex positivity. Also, meibomian gland dysfunction was more common in the Demodex-positive group; however, this result was not statistically significant. One study compared patients with primary demodicosis and patients with rosacea with Demodex-induced blepharitis to healthy controls and found that patients with primary demodicosis and patients with rosacea did not have significantly different ocular findings.23 In contrast, Forton and De Maertelaer24 reported that patients with PPR had significantly more severe ocular manifestations compared with patients with demodicosis (P=.004).
Mizuno et al25 compared the normal (nonrosacea) population with and without Demodex-infested eyelashes and found that the 2 groups were not significantly different for meibomian gland dysfunction, fluorescein TBUT, or ocular surface discomfort.
Varying results have been reported regarding the association between Demodex and blepharitis or ocular surface discomfort with or without rosacea. In our study, we found that Demodex did not affect tear function tests or meibomian gland function in patients with rosacea. We believe this study is important because it demonstrates the effects of Demodex on ocular findings in patients with cutaneous rosacea.
Limitations
Our study has some limitations. The number of patients was relatively small, resulting in few significant differences between the comparison groups. A larger prospective research study is required to assess the prevalence of Demodex mites in the ocular rosacea population along with associated symptoms and findings.
Conclusion
Rosacea is a chronic disease associated with skin and ocular manifestations that range from mild to severe, that progresses in the form of attacks, and that requires long-term follow-up and treatment. Rosacea most often presents as a disease that causes ocular surface inflammation of varying degrees. Demodex infestation may increase cutaneous or ocular inflammation in rosacea. Therefore, every patient diagnosed with rosacea should be given a dermatologic examination to determine Demodex positivity and an ophthalmologic examination to determine ocular manifestations.
Acne rosacea is a chronic inflammatory disease that may affect the facial skin, eyes, and eyelids.1 It is characterized by transient or persistent flushing, facial erythema, and telangiectases, generally located on the central portion of the face, and may progress to papules and pustules.2,3 At the late stage of the disease, dermal edema or fibroplasia and sebaceous gland hypertrophy may cause phymatous alterations in the skin. In 2004, the National Rosacea Society Expert Committee developed a classification system for rosacea to standardize subtypes and variants that has since been widely accepted and continues to aid in research and epidemiologic studies.4 The committee defined 4 subtypes based on clinical characteristics: erythematotelangiectatic (ETR), papulopustular (PPR), phymatous, and ocular rosacea.2,3
Ocular rosacea may accompany mild, moderate, and severe dermatologic disease or may occur in the absence of diagnostic skin disease.5 Ocular signs include eyelid margin telangiectasia, spade-shaped infiltrates in the cornea, scleritis, and sclerokeratitis. Common symptoms include burning, stinging, light sensitivity, and foreign-body sensation. Ocular signs commonly seen in rosacea are meibomian gland dysfunction characterized by inspissation and inflammation of the meibomian glands (chalazia), conjunctivitis, honey crust and cylindrical collarette accumulation at the base of the eyelashes, irregularity of the eyelid margin architecture, and evaporative tear dysfunction.5,6
The physiopathology of rosacea is still unknown. Potential factors include genetic predisposition, abnormal inflammation, vascular dysfunction, and involvement of several microbial agents, such as commensal Demodex mites. The number of Demodex mites on normal skin flora is less than 5/cm2; however, the increased vascular dilation and capillary permeability associated with rosacea that result from sunlight and heat exposure increase the density of Demodex folliculorum.7 Elevated Demodex mite density has been observed in the lumens of the sebaceous follicles in patients with rosacea. However, because the severity of the clinical manifestations of the disease is not directly associated with the density of D folliculorum, it generally is accepted that D folliculorum is not a pathogenetic but rather an exacerbating factor.8 It has been reported that this species of mite is mostly found on the face and around the eyelashes and scalp of patients and that it can cause ocular surface inflammation.8
Most studies have researched ocular manifestations of rosacea but not ocular involvement in rosacea patients with and without Demodex mite infestation. In our study, we sought to compare the ocular surface, meibomian gland characteristics, and tear film abnormalities among patients with cutaneous rosacea with and without Demodex infestation.
Materials and Methods
We conducted a retrospective study of 60 patients with cutaneous rosacea. This study was approved by the ethics committee of the local hospital (2018/002-003), and all patients provided verbal and written informed consent before participating in the study. The study was carried out according to the guidelines of the Declaration of Helsinki.
Patient Selection and Evaluation
Patients diagnosed with rosacea by a dermatologist within 6 months were included in the study. Diagnosis of the disease was made after a detailed anamnesis and dermatologic examination. Rosacea was diagnosed if patients had an itching sensation, erythema and/or erythema attacks, and papules and pustules, and fulfilled the diagnostic criteria according to the National Rosacea Society. The skin disease was classified according to the subtypes as ETR, PPR, phymatous rosacea, or ocular rosacea.
The standard skin surface biopsy method was used in 60 patients for detecting Demodex density. When more than 5 mites were detected per square centimeter, the result was recorded as positive. Thirty consecutive, newly diagnosed patients with cutaneous acne rosacea with Demodex infestation and 30 consecutive, newly diagnosed sex- and age-matched patients with acne rosacea without Demodex infestation admitted to the dermatology outpatient clinic were included to this study. The patients who did not have any known dermatologic, systemic, or ocular diseases were included in the study. Patients who met any of the following criteria were excluded from the study: prior anti-inflammatory topical and/or systemic treatment for rosacea during the last 3 months, contact lens wear, eyelid surgery, or autoimmune disease requiring treatment.
Microscopic Demodex Examination
Demodex count was determined using a standardized skin surface biopsy, which is a noninvasive method. Every patient gave samples from the cheeks. This biopsy was repeated from the same site. A drop of cyanoacrylate was placed on a clean slide, pressed against a skin lesion, held in place for 1 minute, and removed. The obtained samples were evaluated under a light microscope (Nikon E200) with oil immersion. When more than 5 mites were detected per square centimeter, the result was recorded as positive.
Ophthalmologic Examination
A complete ophthalmologic examination including visual acuity assessment, standardized slit lamp examination, and fundus examination was done for all patients. Ocular rosacea was diagnosed on detection of 1 or more of the following: watery or bloodshot appearance, foreign-body sensation, burning or stinging, dryness, itching, light sensitivity, blurred vision, telangiectases of the conjunctiva and eyelid margin, eyelid lid and periocular erythema, anterior blepharitis, meibomian gland dysfunction, or irregularity of eyelid margins. All patients were screened for the signs and symptoms of ocular rosacea and underwent other ophthalmologic examinations, including tear function tests. Tear functions were evaluated with Schirmer tests without anesthesia and fluorescein tear breakup time (TBUT). Tear film breakup time was assessed after instillation of 2% fluorescein staining under a cobalt blue filter. The time interval between the last complete blink and the appearance of the first dry spot was recorded. The mean of 3 consecutive measurements was obtained. The Schirmer test was performed without topical anesthesia using a standardized filter strip (Bio-Tech Vision Care). The amount of wetting was measured after 5 minutes. Meibomian gland expressibility was assessed by applying digital pressure to the eyelid margin.
Statistical Analysis
Statistical analysis of the study was performed with SPSS Statistics Version 22.0 (SPSS Inc). Continuous variables were reported as mean (SD), and categorical variables were reported as percentages and counts. Descriptive statistics for numerical variables were created. An independent sample t test was used for normally distributed continuous variables. The Kolmogorov-Smirnov test was used to determine normality. The Schirmer test without anesthesia and TBUT values among groups were compared using one-way analysis of variance. The differences were calculated using the multiple comparison Tukey test. P<.05 was considered statistically significant.
Results
Demographic Characteristics of Rosacea Patients
Sixty eyes of 30 newly diagnosed patients with acne rosacea with Demodex infestation and 60 eyes of 30 newly diagnosed patients with acne rosacea without Demodex infestation were enrolled in this study. The mean age (SD) of the 60 patients was 37.63 (10.01) years. The mean TBUT (SD) of the 120 eyes was 6.65 (3.44) seconds, and the mean Schirmer score (SD) was 12.59 (6.71) mm (Table 1).

Meibomian Gland Dysfunction vs Subgroup of Rosacea Patients
Thirty-four (57%) patients had blepharitis, and 18 (30%) patients had meibomitis. Thirty-five (58.3%) patients had ETR, 5 (8.3%) patients had phymatous rosacea, and 20 (33.4%) patients had PPR (Table 2). Of the Demodex-negative patients, 73.3% (22/30) had ETR, 20% (6/30) had PPR, and 6.7% (2/30) had phymatous rosacea. Of the Demodex-positive patients, 43.3% (13/30) had ETR, 46.7% (14/30) had PPR, and 10% (3/30) had phymatous rosacea (Table 3). Papulopustular rosacea was found to be significantly associated with Demodex positivity (P=.003); neither ETR nor phymatous rosacea was found to be significantly associated with Demodex infestation (P=.66 and P=.13, respectively)(Table 3).


There was no statistically significant difference between the Demodex-negative and Demodex-positive groups for mean age (SD)(37.4 [11.54] years vs 37.87 [8.41] years; P=.85), mean TBUT (SD)(6.73 [3.62] seconds vs 6.57 [3.33] seconds; P=.85), and mean Schirmer score (SD)(13.68 [7.23] mm vs 11.5 [6.08] mm; P=.21)(Table 4).

Fifteen (50%) patients (30 eyes) in the Demodex-negative group and 19 (63.3%) patients (38 eyes) in the Demodex-positive group had blepharitis, with no statistically significant difference between the groups (P=.43). Seven (23.3%) patients (14 eyes) in the Demodex-negative group and 11 (36.7%) patients (22 eyes) in the Demodex-positive group had meibomitis, with no statistically significant difference between the groups (P=.39)(Table 3).
Sixteen (53.3%) patients (32 eyes) in the Demodex-negative group and 21 (70%) patients (42 eyes) in the Demodex-positive group had TBUT values less than 10 seconds. Eighteen (60%) patients (36 eyes) in the Demodex-negative group and 25 (83.3%) patients (50 eyes) in the Demodex-positive group had Schirmer scores less than 10 mm (Table 3). The 2 groups were not significantly different in dry eye findings (P=.25 and P=.29, respectively).
Comment
Inflammation in Rosacea
It is known that the density of nonfloral bacteria as well as D folliculorum and Demodex brevis increases in skin affected by rosacea compared to normal skin. Vascular dilation associated with rosacea that results from sunlight and heat causes increased capillary permeability and creates the ideal environment for the proliferation of D folliculorum. Demodex is thought to act as a vector for the activity of certain other microorganisms, particularly Bacillus oleronius, and thus initiates the inflammatory response associated with rosacea.9
One study reported that the inflammation associated with rosacea that was caused by Demodex and other environmental stimuli occurred through toll-like receptor 2 and various cytokines.10 It has been reported that the abnormal function of toll-like receptor 2 in the epidermis leads to the increased production of cathelicidin. Cathelicidin is an antimicrobial peptide with both vasoactive and proinflammatory activity and has been used as a basis to explain the pathogenesis of facial erythema, flushing, and telangiectasia in the context of rosacea.11,12 In addition, it has been reported that the increased secretion of proinflammatory cytokines such as IL-1 and gelatinase B in ocular rosacea leads to tearing film abnormalities that result from increased bacterial flora in the eyelids, which subsequently leads to decreased tear drainage and dry eyes.13 In addition, B oleronius isolated from a D folliculorum mite from patients with PPR produced proteins that induced an inflammatory immune response in 73% (16/22) of patients with rosacea.14
Ocular Findings in Rosacea Patients
In our study, PPR was found to be significantly associated with Demodex positivity compared to ETR and phymatous rosacea (P=.003). However, ocular inflammation findings such as blepharitis and meibomitis were not significantly different between Demodex-positive and Demodex-negative patients. Although the mean Schirmer score of Demodex-positive patients was lower than Demodex-negative patients, this difference was not statistically significant. We evaluated a TBUT of less than 10 seconds and a Schirmer score less than 10 mm as dry eye. Accordingly, the number of patients with dry eye was higher in the Demodex-positive group, but this difference was not statistically significant.
Chronic blepharitis, conjunctival inflammation, and meibomian gland dysfunction are among the most common findings of ocular rosacea.15,16 Patients with ocular rosacea commonly have dry eye and abnormal TBUT and Schirmer scores.17 In our study, we found that the fluorescein TBUT and Schirmer scores were more likely to be abnormal in the Demodex-positive group, but the difference between the 2 groups was not statistically significant.
It has been reported that proinflammatory cytokines due to a weakened immune system in rosacea patients were increased. The weakened immune system was further supported by the increased concentrations of proinflammatory cytokines such as IL-1 and matrix metalloproteinase 9 in these patients’ tears and the improvement of symptoms after the inhibition of these cytokines.11 Luo et al18 reported that Demodex inflammation causes dry eye, particularly with D brevis. Ayyildiz and Sezgin19 reported that Schirmer scores were significantly lower and that the Ocular Surface Disease Index had significantly increased in the Demodex-positive group compared to the Demodex-negative group (P=.001 for both). A Korean study reported that Demodex density was correlated with age, sex, and TBUT results, but there was no significant relationship between Demodex density and Schirmer scores.16
Sobolewska et al20 administered ivermectin cream 1% to 10 patients with cutaneous and ocular rosacea, but only to the forehead, chin, nose, cheeks, and regions close to the eyelids, and observed a significant improvement in blepharitis (P=.004). They stated that ivermectin, as applied only to the face, suppressed the proinflammatory cytokines associated with rosacea and showed anti-inflammatory effects by reducing Demodex mites.20Li et al21 demonstrated a strong correlation between ocular Demodex inflammation and serum reactivity to these bacterial proteins in patients with ocular rosacea, and they found that eyelid margin inflammation and facial rosacea correlated with reactivity to these proteins. These studies suggest a possible role for Demodex infestation and bacterial proteins in the etiology of rosacea.
Gonzalez-Hinojosa et al22 demonstrated that even though eyelash blepharitis was more common in PPR than ETR, there was no statistically significant association between rosacea and Demodex blepharitis. In our study, we found a significant correlation between PPR and Demodex positivity. Also, meibomian gland dysfunction was more common in the Demodex-positive group; however, this result was not statistically significant. One study compared patients with primary demodicosis and patients with rosacea with Demodex-induced blepharitis to healthy controls and found that patients with primary demodicosis and patients with rosacea did not have significantly different ocular findings.23 In contrast, Forton and De Maertelaer24 reported that patients with PPR had significantly more severe ocular manifestations compared with patients with demodicosis (P=.004).
Mizuno et al25 compared the normal (nonrosacea) population with and without Demodex-infested eyelashes and found that the 2 groups were not significantly different for meibomian gland dysfunction, fluorescein TBUT, or ocular surface discomfort.
Varying results have been reported regarding the association between Demodex and blepharitis or ocular surface discomfort with or without rosacea. In our study, we found that Demodex did not affect tear function tests or meibomian gland function in patients with rosacea. We believe this study is important because it demonstrates the effects of Demodex on ocular findings in patients with cutaneous rosacea.
Limitations
Our study has some limitations. The number of patients was relatively small, resulting in few significant differences between the comparison groups. A larger prospective research study is required to assess the prevalence of Demodex mites in the ocular rosacea population along with associated symptoms and findings.
Conclusion
Rosacea is a chronic disease associated with skin and ocular manifestations that range from mild to severe, that progresses in the form of attacks, and that requires long-term follow-up and treatment. Rosacea most often presents as a disease that causes ocular surface inflammation of varying degrees. Demodex infestation may increase cutaneous or ocular inflammation in rosacea. Therefore, every patient diagnosed with rosacea should be given a dermatologic examination to determine Demodex positivity and an ophthalmologic examination to determine ocular manifestations.
- O’Reilly N, Gallagher C, Reddy Katikireddy K, et al. Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci. 2012;53:3250-3259.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 suppl 1):S42-S43.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094.
- Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17:1-4.
- Erbagcı Z, Ozgoztası O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36:81‐86.
- Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97:242‐248.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Gold LM, Draelos ZD. New and emerging treatments for rosacea. Am J Clin Dermatol. 2015;16:457-461.
- Two AM, Del Rosso JQ. Kallikrein 5-mediated inflammation in rosacea: clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J Clin Aesthet Dermatol. 2014;7:20-25.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474-481.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74-87.
- Lee SH, Chun YS, Kim JH, et al. The relationship between Demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906-2911.
- Awais M, Anwar MI, Ilfikhar R, et al. Rosacea—the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34:161-166.
- Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9-S14.
- Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(suppl 1):S39-S41.
- Sobolewska B, Doycheva D, Deuter CM, et al. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea [published online April 7, 2020]. Ocul Immunol Inflamm. doi:10.1080/09273948.2020.1727531
- Li J, O‘Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. 2010;117:870-877.
- Gonzalez‐Hinojosa D, Jaime‐Villalonga A, Aguilar‐Montes G, et al. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66:36‐38.
- Sarac G, Cankaya C, Ozcan KN, et al. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J Cosmet Dermatol. 2020;19:1260-1265.
- Forton FMN, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99:47-52.
- Mizuno M, Kawashima M, Uchino M, et al. Demodex-mite infestation in cilia and its association with ocular surface parameters in Japanese volunteers. Eye Contact Lens. 2020;46:291-296.
- O’Reilly N, Gallagher C, Reddy Katikireddy K, et al. Demodex-associated Bacillus proteins induce an aberrant wound healing response in a corneal epithelial cell line: possible implications for corneal ulcer formation in ocular rosacea. Invest Ophthalmol Vis Sci. 2012;53:3250-3259.
- Webster G, Schaller M. Ocular rosacea: a dermatologic perspective. J Am Acad Dermatol. 2013;69(6 suppl 1):S42-S43.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341.
- Wilkin J, Dahl M, Detmar M, et al. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50:907-912.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Gao YY, Di Pascuale MA, Li W, et al. High prevalence of Demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci. 2005;46:3089-3094.
- Fallen RS, Gooderham M. Rosacea: update on management and emerging therapies. Skin Therapy Lett. 2012;17:1-4.
- Erbagcı Z, Ozgoztası O. The significance of Demodex folliculorum density in rosacea. Int J Dermatol. 1998;37:421-425.
- Ahn CS, Huang WW. Rosacea pathogenesis. Dermatol Clin. 2018;36:81‐86.
- Forton FMN, De Maertelaer V. Two consecutive standardized skin surface biopsies: an improved sampling method to evaluate Demodex density as a diagnostic tool for rosacea and demodicosis. Acta Derm Venereol. 2017;97:242‐248.
- Yamasaki K, Kanada K, Macleod DT, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011;131:688-697.
- Gold LM, Draelos ZD. New and emerging treatments for rosacea. Am J Clin Dermatol. 2015;16:457-461.
- Two AM, Del Rosso JQ. Kallikrein 5-mediated inflammation in rosacea: clinically relevant correlations with acute and chronic manifestations in rosacea and how individual treatments may provide therapeutic benefit. J Clin Aesthet Dermatol. 2014;7:20-25.
- Lacey N, Delaney S, Kavanagh K, et al. Mite-related bacterial antigens stimulate inflammatory cells in rosacea. Br J Dermatol. 2007;157:474-481.
- Forton F, Germaux MA, Brasseur T, et al. Demodicosis and rosacea: epidemiology and significance in daily dermatologic practice. J Am Acad Dermatol. 2005;52:74-87.
- Lee SH, Chun YS, Kim JH, et al. The relationship between Demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51:2906-2911.
- Awais M, Anwar MI, Ilfikhar R, et al. Rosacea—the ophthalmic perspective. Cutan Ocul Toxicol. 2015;34:161-166.
- Luo X, Li J, Chen C, et al. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. 2017;36(suppl 1):S9-S14.
- Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on Schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. 2020;46(suppl 1):S39-S41.
- Sobolewska B, Doycheva D, Deuter CM, et al. Efficacy of topical ivermectin for the treatment of cutaneous and ocular rosacea [published online April 7, 2020]. Ocul Immunol Inflamm. doi:10.1080/09273948.2020.1727531
- Li J, O‘Reilly N, Sheha H, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. 2010;117:870-877.
- Gonzalez‐Hinojosa D, Jaime‐Villalonga A, Aguilar‐Montes G, et al. Demodex and rosacea: is there a relationship? Indian J Ophthalmol. 2018;66:36‐38.
- Sarac G, Cankaya C, Ozcan KN, et al. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J Cosmet Dermatol. 2020;19:1260-1265.
- Forton FMN, De Maertelaer V. Rosacea and demodicosis: little-known diagnostic signs and symptoms. Acta Derm Venereol. 2019;99:47-52.
- Mizuno M, Kawashima M, Uchino M, et al. Demodex-mite infestation in cilia and its association with ocular surface parameters in Japanese volunteers. Eye Contact Lens. 2020;46:291-296.
Practice Points
- Rosacea is a common chronic inflammatory skin disease of the central facial skin and is of unknown origin. Patients with ocular rosacea may report dryness, itching, and photophobia.
- Demodex infestation may increase cutaneous or ocular inflammation in rosacea.
Reexamining the Role of Diet in Dermatology
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
Within the last decade, almost 3000 articles have been published on the role of diet in the prevention and management of dermatologic conditions. Patients are increasingly interested in—and employing—dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.1 It is essential that dermatologists are familiar with existing evidence on the role of diet in dermatology to counsel patients appropriately. Herein, we discuss the compositions of several popular diets and their proposed utility for dermatologic purposes. We highlight the limited literature that exists surrounding this topic and emphasize the need for future, well-designed clinical trials that study the impact of diet on skin disease.
Ketogenic Diet
The ketogenic diet has a macronutrient profile composed of high fat, low to moderate protein, and very low carbohydrates. Nutritional ketosis occurs as the body begins to use free fatty acids (via beta oxidation) as the primary metabolite driving cellular metabolism. It has been suggested that the ketogenic diet may impart beneficial effects on skin disease; however, limited literature exists on the role of nutritional ketosis in the treatment of dermatologic conditions.
Mechanistically, the ketogenic diet decreases the secretion of insulin and insulinlike growth factor 1, resulting in a reduction of circulating androgens and increased activity of the retinoid X receptor.2 In acne vulgaris, it has been suggested that the ketogenic diet may be beneficial in decreasing androgen-induced sebum production and the overproliferation of keratinocytes.2-7 The ketogenic diet is one of the most rapidly effective dietary strategies for normalizing both insulin and androgens, thus it may theoretically be useful for other metabolic and hormone-dependent skin diseases, such as hidradenitis suppurativa.8,9
The cutaneous manifestations associated with chronic hyperinsulinemia and hyperglycemia are numerous and include acanthosis nigricans, acrochordons, diabetic dermopathy, scleredema diabeticorum, bullosis diabeticorum, keratosis pilaris, and generalized granuloma annulare. There also is an increased risk for bacterial and fungal skin infections associated with hyperglycemic states.10 The ketogenic diet is an effective nonpharmacologic tool for normalizing serum insulin and glucose levels in most patients and may have utility in the aforementioned conditions.11,12 In addition to improving insulin sensitivity, it has been used as a dietary strategy for weight loss.11-15 Because obesity and metabolic syndrome are highly correlated with common skin conditions such as psoriasis, hidradenitis suppurativa, and androgenetic alopecia, there may be a role for employing the ketogenic diet in these patient populations.16,17
Although robust clinical studies on ketogenic diets in skin disease are lacking, a recent single-arm, open-label clinical trial observed benefit in all 37 drug-naïve, overweight patients with chronic plaque psoriasis who underwent a ketogenic weight loss protocol. Significant reductions in psoriasis area and severity index (PASI) score and dermatology life quality index score were reported (P<.001).18 Another study of 30 patients with psoriasis found that a 4-week, low-calorie, ketogenic diet resulted in 50% improvement of PASI scores, 10% weight loss, and a reduction in the proinflammatory cytokines IL-1β and IL-2.19 Despite these results, it is a challenge to tease out if the specific dietary intervention or its associated weight loss was the main driver in these reported improvements in skin disease.
There is mixed evidence on the anti-inflammatory nature of the ketogenic diet, likely due to wide variation in the composition of foods included in individual diets. In many instances, the ketogenic diet is thought to possess considerable antioxidant and anti-inflammatory capabilities. Ketones are known activators of the nuclear factor erythroid 2–related factor 2 pathway, which upregulates the production of glutathione, a major endogenous intracellular antioxidant.20 Additionally, dietary compounds from foods that are encouraged while on the ketogenic diet, such as sulforaphane from broccoli, also are independent activators of nuclear factor erythroid 2–related factor 2.21 Ketones are efficiently utilized by mitochondria, which also may result in the decreased production of reactive oxygen species and lower oxidative stress.22 Moreover, the ketone body β-hydroxybutyrate has demonstrated the ability to reduce proinflammatory IL-1β levels via suppression of nucleotide-binding domain-like receptor protein 3 inflammasome activity.23,24 The activity of IL-1β is known to be elevated in many dermatologic conditions, including juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, hidradenitis suppurativa, Behçet disease, and other autoinflammatory syndromes.25 Ketones also have been shown to inhibit the nuclear factor–κB proinflammatory signaling pathway.22,26,27 Overexpression of IL-1β and aberrant activation of nuclear factor–κB are implicated in a variety of inflammatory, autoimmune, and oncologic cutaneous pathologies. The ketogenic diet may prove to be an effective adjunctive treatment for dermatologists to consider in select patient populations.23,24,28-30
For patients with keratinocyte carcinomas, the ketogenic diet may offer the aforementioned anti-inflammatory and antioxidant effects, as well as suppression of the mechanistic target of rapamycin, a major regulator of cell metabolism and proliferation.31,32 Inhibition of mechanistic target of rapamycin activity has been shown to slow tumor growth and reduce the development of squamous cell carcinoma.25,33,34 The ketogenic diet also may exploit the preferential utilization of glucose exhibited by many types of cancer cells, thereby “starving” the tumor of its primary fuel source.35,36 In vitro and animal studies in a variety of cancer types have demonstrated that a ketogenic metabolic state—achieved through the ketogenic diet or fasting—can sensitize tumor cells to chemotherapy and radiation while conferring a protective effect to normal cells.37-40 This recently described phenomenon is known as differential stress resistance, but it has not been studied in keratinocyte malignancies or melanoma to date. Importantly, some basal cell carcinomas and BRAF V600E–mutated melanomas have worsened while on the ketogenic diet, suggesting more data is needed before it can be recommended for all cancer patients.41,42 Furthermore, other skin conditions such as prurigo pigmentosa have been associated with initiation of the ketogenic diet.43
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) are short-chain carbohydrates that are poorly absorbed, osmotically active, and rapidly fermented by intestinal bacteria.44 The low FODMAP diet has been shown to be efficacious for treatment of irritable bowel syndrome, small intestinal bacterial overgrowth (SIBO), and some cases of inflammatory bowel disease (IBD).44-49 A low FODMAP diet may have potential implications for several dermatologic conditions.
Rosacea has been associated with various gastrointestinal tract disorders including irritable bowel syndrome, SIBO, and IBD.50-54 A single study found that patients with rosacea had a 13-fold increased risk for SIBO.55,56 Treatment of 40 patients with SIBO using rifaximin resulted in complete resolution of rosacea in all patients, with no relapse after a 3-year follow-up period.55 Psoriasis also has been associated with SIBO and IBD.57,58 One small study found that eradication of SIBO in psoriatic patients resulted in improved PASI scores and colorimetric values.59
Although the long-term health consequences of the low FODMAP diet are unknown, further research on such dietary interventions for inflammatory skin conditions is warranted given the mounting evidence of a gut-skin connection and the role of the intestinal microbiome in skin health.50,51
Gluten-Free Diet
Gluten is a protein found in a variety of grains. Although the role of gluten in the pathogenesis of celiac disease and dermatitis herpetiformis is indisputable, the deleterious effects of gluten outside of the context of these diseases remain controversial. There may be a compelling case for eliminating gluten in psoriasis patients with seropositivity for celiac disease. A recent systematic review found a 2.2-fold increased risk for celiac disease in psoriasis patients.60 Antigliadin antibody titers also were found to be positively correlated with psoriatic disease severity.61 In addition, one open-label study found a reduction in PASI scores in 73% of patients with antigliadin antibodies after 3 months on a gluten-free diet compared to those without antibodies; however, the study only included 22 patients.62 Several other small studies have yielded similar results63,64; however, antigliadin antibodies are neither the most sensitive nor specific markers of celiac disease, and additional testing should be completed in any patient who may carry this diagnosis. A survey study by the National Psoriasis Foundation found that the dietary change associated with the greatest skin improvement was removal of gluten and nightshade vegetables in approximately 50% of the 1200 psoriasis patients that responded.65 Case reports of various dermatologic conditions including sarcoidosis, vitiligo, alopecia areata, lichen planus, dermatomyositis, pyoderma gangrenosum, erythema nodosum, leukocytoclastic vasculitis, linear IgA bullous dermatosis, and aphthous ulcerations have reportedly improved with a gluten-free diet; however, this should not be used as primary therapy in patients without celiac disease.66-71 Because gluten-free diets can be expensive and challenging to follow, a formal assessment for celiac disease should be considered before recommendation of this dietary intervention.
Low Histamine Diet
Histamine is a biogenic amine produced by the decarboxylation of the amino acid histidine.72 It is found in several foods in varying amounts. Because bacteria can convert histidine into histamine, many fermented and aged foods such as kimchi, sauerkraut, cheese, and red wine contain high levels of histamine. Individuals who have decreased activity of diamine oxidase (DAO), an enzyme that degrades histamine, may be more susceptible to histamine intolerance.72 The symptoms of histamine intolerance are numerous and include gastrointestinal tract distress, rhinorrhea and nasal congestion, headache, urticaria, flushing, and pruritus. Histamine intolerance can mimic an IgE-mediated food allergy; however, allergy testing is negative in these patients. Unfortunately, there is no laboratory test for histamine intolerance; a double-blind, placebo-controlled food challenge is considered the gold-standard test.72
As it pertains to dermatology, a low histamine diet may play a role in the treatment of certain patients with atopic dermatitis and chronic spontaneous urticaria. One study reported that 17 of 54 (31.5%) atopic patients had higher basal levels of serum histamine compared to controls.73 Another study found that a histamine-free diet led to improvement in both histamine intolerance symptoms and atopic dermatitis disease severity (SCORing atopic dermatitis) in patients with low DAO activity.74 In chronic spontaneous urticaria, a recent systematic review found that in 223 patients placed on a low histamine diet for 3 to 4 weeks, 12% and 44% achieved complete and partial remission, respectively.75 Although treatment response based on a patient’s DAO activity level has not been correlated, a diet low in histamine may prove useful for patients with persistent atopic dermatitis and chronic spontaneous urticaria who have negative food allergy tests and report exacerbation of symptoms after ingestion of histamine-rich foods.76,77
Mediterranean Diet
The Mediterranean diet has been touted as one of the healthiest diets to date, and large randomized clinical trials have demonstrated its effectiveness in weight loss, improving insulin sensitivity, and reducing inflammatory cytokine profiles.78,79 A major criticism of the Mediterranean diet is that it has considerable ambiguity and lacks a precise definition due to the variability of what is consumed in different Mediterranean regions. Generally, the diet emphasizes high consumption of colorful fruits and vegetables, aromatic herbs and spices, olive oil, nuts, and seafood, as well as modest amounts of dairy, eggs, and red meat.80 The anti-inflammatory effects of this diet largely have been attributed to its abundance of polyphenols, carotenoids, monounsaturated fatty acids, and omega-3 polyunsaturated fatty acids (PUFAs).80,81 Examples of polyphenols include resveratrol in red grapes, quercetin in apples and red onions, and curcumin in turmeric, while examples of carotenoids include lycopene in tomatoes and zeaxanthin in dark leafy greens. Oleic acid is a monounsaturated fatty acid present in high concentrations in olive oil, while eicosapentaenoic acid and docosahexaenoic acid are omega-3 PUFAs predominantly found in fish.82
Unfortunately, rigorous clinical trials regarding the Mediterranean diet as it pertains to dermatology have not been undertaken. Numerous observational studies in patients with psoriasis have suggested that close adherence to the Mediterranean diet was associated with improvement in PASI scores.83-86 The National Psoriasis Foundation now recommends a trial of the Mediterranean diet in some patients with psoriasis, emphasizing increased dietary intake of olive oil, fish, and vegetables.87 Adherence to a Mediterranean diet also has been inversely correlated to the severity of acne vulgaris and hidradenitis suppurativa88,89; however, these studies failed to account for the multifactorial risk factors associated with these conditions. Mediterranean diets also may impart a chemopreventive effect, supported by a number of in vivo and in vitro studies demonstrating the inhibition and/or reversal of cutaneous DNA damage induced by UV radiation through supplementation with various phytonutrients and omega-3 PUFAs.81,90-92 Although small case-control studies have found a decreased risk of basal cell carcinoma in those who closely adhered to a Mediterranean diet, more rigorous clinical research is needed.93
Whole-Food, Plant-Based Diet
A whole-food, plant-based (WFPB) diet is another popular dietary approach that consists of eating fruits, vegetables, legumes, nuts, seeds, and grains in their whole natural form.94 This diet discourages all animal products, including red meat, seafood, dairy, and eggs. It is similar to a vegan diet except that it eliminates all highly refined carbohydrates, vegetable oils, and other processed foods.94 Randomized clinical studies have demonstrated the WFPB diet to be effective in the treatment of obesity and metabolic syndrome.95,96
A WFPB diet has been shown to increase the antioxidant capacity of cells, lengthen telomeres, and reduce formation of advanced glycation end products.94,97,98 These benefits may help combat accelerated skin aging, including increased skin permeability, reduced elasticity and hydration, decreased angiogenesis, impaired immune function, and decreased vitamin D synthesis. Accelerated skin aging can result in delayed wound healing and susceptibility to skin tears and ecchymoses and also may promote the development of cutaneous malignancies.99 There remains a lack of clinical data studying a properly formulated WFPB diet in the dermatologic setting.
Paleolithic Diet
The paleolithic (Paleo) diet is an increasingly popular way of eating that attempts to mirror what our ancestors may have consumed between 10,000 and 2.5 million years ago.100 It is similar to the Mediterranean diet but excludes grains, dairy, legumes, and nightshade vegetables. It also calls for elimination of highly processed sugars and oils as well as chemical food additives and preservatives. There is a strict variation of the diet for individuals with autoimmune disease that also excludes eggs, nuts, and seeds, as these can be inflammatory or immunogenic in some patients.100-106 Other variations of the diet exist, including the ketogenic Paleo diet, pegan (Paleo vegan) diet, and lacto-Paleo diet.100 An often cited criticism of the Paleo diet is the low intake of calcium and risk for osteoporosis; however, consumption of calcium-rich foods or a calcium supplement can address this concern.107
Although small clinical studies have found the Paleo diet to be beneficial for various autoimmune diseases, clinical data evaluating the utility of the diet for cutaneous disease is lacking.108,109 Numerous randomized trials have demonstrated the Paleo diet to be effective for weight loss and improving insulin sensitivity and lipid levels.110-116 Thus, the Paleo diet may theoretically serve as a viable adjunct dietary approach to the treatment of cutaneous diseases associated with obesity and metabolic derangement.117
Carnivore Diet
Arguably the most controversial and radical diet is the carnivore diet. As the name implies, the carnivore diet is based on consuming solely animal products. A properly structured carnivore diet emphasizes a “nose-to-tail” eating approach where all parts of the animal including the muscle meats, organs, and fat are consumed. Proponents of the diet cite anthropologic evidence from fossil-stable carbon-13/carbon-12 isotope analyses, craniodental features, and numerous other adaptations that indicate increased consumption of meat during human evolution.118-122 Notably, many early humans ate a carnivore diet, but life span was very short at this time, suggesting the diet may not be as beneficial as has been suggested.
Despite the abundance of anecdotal evidence supporting its use for a variety of chronic conditions, including cutaneous autoimmune disease, there is a virtual absence of high-quality research on the carnivore diet.123-125
The purported benefits of the carnivore diet may be attributed to the consumption of organ meats that contain highly bioavailable essential vitamins and minerals, such as iron, zinc, copper, selenium, thiamine, niacin, folate, vitamin B6, vitamin B12, vitamin A, vitamin D, vitamin K, and choline.126-128 Other dietary compounds that have demonstrated benefit for skin health and are predominantly found in animal foods include carnosine, carnitine, creatine, taurine, coenzyme Q10, and collagen.129-134 Nevertheless, there is no data to recommend the elimination of antioxidant- and micronutrient-dense plant-based foods. Rigorous clinical research evaluating the efficacy and safety of the carnivore diet in dermatologic patients is needed. A carnivore diet should not be undertaken without the assistance of a dietician who can ensure adequate micronutrient and macronutrient support.
Final Thoughts
The adjunctive role of diet in the treatment of skin disease is expanding and becoming more widely accepted among dermatologists. Unfortunately, there remains a lack of randomized controlled trials confirming the efficacy of various dietary interventions in the dermatologic setting. Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
Ultimately, dietary recommendations must be personalized, considering a patient’s comorbidities, personal beliefs and preferences, and nutrigenetics. The emerging field of dermatonutrigenomics—the study of how dietary compounds interact with one’s genes to influence skin health—may allow for precise dietary recommendations to be made in dermatologic practice. Direct-to-consumer genetic tests targeted toward dermatology patients are already on the market, but their clinical utility awaits validation.1 Because nutritional science is a constantly evolving field, becoming familiar with these popular diets will serve both dermatologists and their patients well.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
- Jaros J, Katta R, Shi VY. Dermatonutrigenomics: past, present, and future. Dermatology. 2019;235:164-166.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Melnik BC, Schmitz G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp Dermatol. 2009;18:833-841.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Smith R, Mann N, Mäkeläinen H, et al. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: a nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52:718-726.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Khandalavala BN, Do MV. Finasteride in hidradenitis suppurativa: a "male" therapy for a predominantly "female" disease. J Clin Aesthet Dermatol. 2016;9:44.
- Nikolakis G, Karagiannidis I, Vaiopoulos AG, et al. Endocrinological mechanisms in the pathophysiology of hidradenitis suppurativa [in German]. Hautarzt. 2020;71:762-771.
- Karadag AS, Ozlu E, Lavery MJ. Cutaneous manifestations of diabetes mellitus and the metabolic syndrome. Clin Dermatology. 2018;36:89-93.
- Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969-977.
- Anton SD, Hida A, Heekin K, et al. Effects of popular diets without specific calorie targets on weight loss outcomes: systematic review of findings from clinical trials. Nutrients. 2017;9:822.
- Castellana M, Conte E, Cignarelli A, et al. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:5-16.
- Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. 2020;18:104.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Lian N, Chen M. Metabolic syndrome and skin disease: potential connection and risk. Int J Dermatol Venereol. 2019;2:89-93.
- Hu Y, Zhu Y, Lian N, et al. Metabolic syndrome and skin diseases. Front Endocrinol (Lausanne). 2019;10:788.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Milder J, Liang L-P, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40:238-244.
- Kubo E, Chhunchha B, Singh P, et al. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7:14130.
- Pinto A, Bonucci A, Maggi E, et al. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants (Basel). 2018;7:63.
- Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Rahman M, Muhammad S, Khan MA, et al. The β-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:1-11.
- Lu Y, Yang YY, Zhou MW, et al. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13-18.
- Hamarsheh S, Zeiser R. NLRP3 inflammasome activation in cancer: a double-edged sword. Front Immunol. 2020;11:1444.
- Bell S, Degitz K, Quirling M, et al. Involvement of NF-κB signalling in skin physiology and disease. Cell Signal. 2003;15:1-7.
- Goldminz AM, Au SC, Kim N, et al. NF-κB: an essential transcription factor in psoriasis. J Dermatol Sci. 2013;69:89-94.
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589.
- McDaniel S, Rensing N, Yamada K, et al. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:E7-E11.
- Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR-inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480-488.
- Feldmeyer L, Hofbauer GF, Böni T, et al. Mammalian target of rapamycin (mTOR) inhibitors slow skin carcinogenesis, but impair wound healing. Br J Dermatol. 2012;166:422-424.
- Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41:211-218.
- Li W. "Warburg effect" and mitochondrial metabolism in skin cancer.J Carcinogene Mutagene. 2012:S4.
- Naveed S, Aslam M, Ahmad A. Starvation based differential chemotherapy: a novel approach for cancer treatment. Oman Med J. 2014;29:391-398.
- Raffaghello L, Lee C, Safdie FM, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215-8220.
- Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271-280.
- de Groot S, Pijl H, van der Hoeven JJM, et al. Effects of short-term fasting on cancer treatment. J Exp Clin Cancer Res. 2019;38:209.
- Hosseini M, Kasraian Z, Rezvani HR. Energy metabolism in skin cancers: a therapeutic perspective. Biochim Biophys Acta Bioenerg. 2017;1858:712-722.
- Feichtinger RG, Lang R, Geilberger R, et al. Melanoma tumors exhibit a variable but distinct metabolic signature. Exp Dermatol. 2018;27:204-207.
- Alshaya MA, Turkmani MG, Alissa AM. Prurigo pigmentosa following ketogenic diet and bariatric surgery: a growing association. JAAD Case Rep. 2019;5:504-507.
- Bellini M, Tonarelli S, Nagy AG, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12:148.
- Kwiatkowski L, Rice E, Langland J. Integrative treatment of chronic abdominal bloating and pain associated with overgrowth of small intestinal bacteria: a case report. Altern Ther Health Med. 2017;23:56-61.
- Hubkova T. No more pain in the gut: lifestyle medicine approach to irritable bowel syndrome. Am J Lifestyle Med. 2017;11:223-226.
- Schumann D, Klose P, Lauche R, et al. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24-31.
- Cox SR, Prince AC, Myers CE, et al. Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11:1420-1429.
- Damas OM, Garces L, Abreu MT. Diet as adjunctive treatment for inflammatory bowel disease: review and update of the latest literature. Curr Treat Options Gastroenterol. 2019;17:313-325.
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424.
- Daou H, Paradiso M, Hennessy K, et al. Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 2021;11:1-12.
- Weinstock LB, Steinhoff M. Rosacea and small intestinal bacterial overgrowth: prevalence and response to rifaximin. J Am Acad Dermatol. 2013;68:875-876.
- Wu CY, Chang YT, Juan CK, et al. Risk of inflammatory bowel disease in patients with rosacea: results from a nationwide cohort study in Taiwan. J Am Acad Dermatol. 2017;76:911-917.
- Egeberg A, Weinstock LB, Thyssen EP, et al. Rosacea and gastrointestinal disorders: a population-based cohort study. Br J Dermatol. 2017;176:100-106.
- Drago F, De Col E, Agnoletti AF, et al. The role of small intestinal bacterial overgrowth in rosacea: a 3-year follow-up. J Am Acad Dermatol. 2016;75:E113-E115.
- Parodi A, Paolino S, Greco A, et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764.
- Ojetti V, De Simone C, Aguilar Sanchez J, et al. Malabsorption in psoriatic patients: cause or consequence? Scand J Gastroenterol. 2006;41:1267-1271.
- Kim M, Choi KH, Hwang SW, et al. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017;76:40-48.
- Drago F, Ciccarese G, Indemini E, et al. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113.
- Acharya P, Mathur M. Association between psoriasis and celiac disease: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1376-1385.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Michaëlsson G, Gerdén B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142:44-51.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2018;11:13-19.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Loche F, Bazex J. Celiac disease associated with cutaneous sarcoidosic granuloma [in French]. Rev Med Interne. 1997;18:975-978.
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210.
- Wijarnpreecha K, Panjawatanan P, Corral JE, et al. Celiac disease and risk of sarcoidosis: a systematic review and meta-analysis. J Evid Based Med. 2019;12:194-199.
- Rodrigo L, Beteta-Gorriti V, Alvarez N, et al. Cutaneous and mucosal manifestations associated with celiac disease. Nutrients. 2018;10:800.
- Song MS, Farber D, Bitton A, et al. Dermatomyositis associated with celiac disease: response to a gluten-free diet. Can J Gastroenterol. 2006;20:433-435.
- Egan CA, Smith EP, Taylor TB, et al. Linear IgA bullous dermatosis responsive to a gluten-free diet. Am J Gastroenterol. 2001;96:1927-1929.
- Comas-Basté O, Sánchez-Pérez S, Veciana-Nogués MT, et al. Histamine intolerance: the current state of the art. Biomolecules. 2020;10:1181.
- Ring J. Plasma histamine concentrations in atopic eczema. Clin Allergy. 1983;13:545-552.
- Maintz L, Benfadal S, Allam JP, et al. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J Allergy Clin Immunol. 2006;117:1106-1112.
- Cornillier H, Giraudeau B, Samimi M, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99:127-132.
- Son JH, Chung BY, Kim HO, et al. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30:164-172.
- Wagner N, Dirk D, Peveling-Oberhag A, et al. A popular myth - low-histamine diet improves chronic spontaneous urticaria - fact or fiction? J Eur Acad Dermatol Venereol. 2017;31:650-655.
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446.
- Steffen LM, Van Horn L, Daviglus ML, et al. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (coronary artery risk development in young adults) study. Br J Nutr. 2014;112:1654-1661.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional Mediterranean diet. Crit Rev Food Sci Nutr. 2016;56:2728-2746.
- Bosch R, Philips N, Suárez-Pérez JA, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants (Basel). 2015;4:248-268.
- Katsimbri P, Korakas E, Kountouri A, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants. 2021;10:157.
- Molina-Leyva A, Cuenca-Barrales C, Vega-Castillo JJ, et al. Adherence to Mediterranean diet in Spanish patients with psoriasis: cardiovascular benefits? Dermatol Ther. 2019;32:E12810.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:1-10.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Korovesi A, Dalamaga M, Kotopouli M, et al. Adherence to the Mediterranean diet is independently associated with psoriasis risk, severity, and quality of life: a cross-sectional observational study. Int J Dermatol. 2019;58:E164-E165.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934-950.
- Skroza N, Tolino E, Semyonov L, et al. Mediterranean diet and familial dysmetabolism as factors influencing the development of acne. Scand J Public Health. 2012;40:466-474.
- Barrea L, Fabbrocini G, Annunziata G, et al. Role of nutrition and adherence to the Mediterranean diet in the multidisciplinary approach of hidradenitis suppurativa: evaluation of nutritional status and its association with severity of disease. Nutrients. 2018;11:57.
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71-83.
- Huang T-H, Wang P-W, Yang S-C, et al. Cosmetic and therapeutic applications of fish oil's fatty acids on the skin. Mar Drugs. 2018;16:256.
- Rizwan M, Rodriguez-Blanco I, Harbottle A, et al. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol. 2011;164:154-162.
- Leone A, Martínez-González M, Martin-Gorgojo A, et al. Mediterranean diet, dietary approaches to stop hypertension, and pro-vegetarian dietary pattern in relation to the risk of basal cell carcinoma: a nested case-control study within the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2020;112:364-372.
- Solway J, McBride M, Haq F, et al. Diet and dermatology: the role of a whole-food, plant-based diet in preventing and reversing skin aging--a review. J Clin Aesthet Dermatol. 2020;13:38-43.
- Greger M. A whole food plant-based diet is effective for weight loss: the evidence. Am J Lifestyle Med. 2020;14:500-510.
- Wright N, Wilson L, Smith M, et al. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:E256.
- Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14:1112-1120.
- Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048-1057.
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3-14.
- Gupta L, Khandelwal D, Lal PR, et al. Palaeolithic diet in diabesity and endocrinopathies--a vegan's perspective. Eur Endocrinol. 2019;15:77-82.
- Chassaing B, Van de Wiele T, De Bodt J, et al. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017;66:1414-1427.
- Thorburn Alison N, Macia L, Mackay Charles R. Diet, metabolites, and "Western lifestyle" inflammatory diseases. Immunity. 2014;40:833-842.
- Katta R, Schlichte M. Diet and dermatitis: food triggers. J Clin Aesthet Dermatol. 2014;7:30-36.
- Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645-648.
- Birmingham N, Thanesvorakul S, Gangur V. Relative immunogenicity of commonly allergenic foods versus rarely allergenic and nonallergenic foods in mice. J Food Prot. 2002;65:1988-1991.
- Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16:751-765.
- Kowalski LM, Bujko J. Evaluation of biological and clinical potential of paleolithic diet [in Polish]. Rocz Panstw Zakl Hig. 2012;63:9-15.
- Lee JE, Titcomb TJ, Bisht B, et al. A modified MCT-based ketogenic diet increases plasma β-hydroxybutyrate but has less effect on fatigue and quality of life in people with multiple sclerosis compared to a modified paleolithic diet: a waitlist-controlled, randomized pilot study. J Am Coll Nutr. 2021;40:13-25.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:E4556.
- Lindeberg S, Jönsson T, Granfeldt Y, et al. A palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia. 2007;50:1795-1807.
- Jönsson T, Granfeldt Y, Ahrén B, et al. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35.
- Boers I, Muskiet FAJ, Berkelaar E, et al. Favourable effects of consuming a palaeolithic-type diet on characteristics of the metabolic syndrome: a randomized controlled pilot-study. Lipids Health Dis. 2014;13:160.
- Ghaedi E, Mohammadi M, Mohammadi H, et al. Effects of a paleolithic diet on cardiovascular disease risk factors: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10:634-646.
- Mellberg C, Sandberg S, Ryberg M, et al. Long-term effects of a palaeolithic-type diet in obese postmenopausal women: a 2-year randomized trial. Eur J Clin Nutr. 2014;68:350-357.
- Pastore RL, Brooks JT, Carbone JW. Paleolithic nutrition improves plasma lipid concentrations of hypercholesterolemic adults to a greater extent than traditional heart-healthy dietary recommendations. Nutr Res. 2015;35:474-479.
- Otten J, Stomby A, Waling M, et al. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:E2828.
- Stefanadi EC, Dimitrakakis G, Antoniou C-K, et al. Metabolic syndrome and the skin: a more than superficial association. reviewing the association between skin diseases and metabolic syndrome and a clinical decision algorithm for high risk patients. Diabetol Metab Syndr. 2018;10:9.
- Mann N. Meat in the human diet: an anthropological perspective. Nutr Dietetics. 2007;64(suppl 4):S102-S107.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345-352.
- Kuhn JE. Throwing, the shoulder, and human evolution. Am J Orthop (Belle Mead NJ). 2016;45:110-114.
- Kobayashi H, Kohshima S. Unique morphology of the human eye and its adaptive meaning: comparative studies on external morphology of the primate eye. J Hum Evol. 2001;40:419-435.
- Cordain L, Eaton SB, Miller JB, et al. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(suppl 1):S42-S52.
- McClellan WS, Du Bois EF. Clinical calorimetry: XLV. prolonged meat diets with a study of kidney function and ketosis. J Biol Chem. 1930;87:651-668.
- O'Hearn A. Can a carnivore diet provide all essential nutrients? Curr Opin Endocrinol Diabetes Obes. 2020;27:312-316.
- O'Hearn LA. A survey of improvements experienced on a carnivore diet compared to only carbohydrate restriction. Open Science Forum website. Published February 12, 2019. Accessed May 17, 2021. doi:10.17605/OSF.IO/5FU4D
- Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(suppl 4):S113-S119.
- Biel W, Czerniawska-Piątkowska E, Kowalczyk A. Offal chemical composition from veal, beef, and lamb maintained in organic production systems. Animals (Basel). 2019;9:489.
- Elmadfa I, Meyer AL. The role of the status of selected micronutrients in shaping the immune function. Endocr Metab Immune Disord Drug Targets. 2019;19:1100-1115.
- Babizhayev M. Treatment of skin aging and photoaging with innovative oral dosage forms of nonhydrolized carnosine and carcinine. Int J Clin Derm Res. 2017;5:116-143.
- Danby FW. Nutrition and aging skin: sugar and glycation. Clin Dermatol. 2010;28:409-411.
- Siefken W, Carstensen S, Springmann G, et al. Role of taurine accumulation in keratinocyte hydration. J Invest Dermatol. 2003;121:354-361.
- Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19:3059.
- Fischer F, Achterberg V, März A, et al. Folic acid and creatineimprove the firmness of human skin in vivo. J Cosmet Dermatol. 2011;10:15-23.
- Blatt T, Lenz H, Weber T. Topical application of creatine is multibeneficial for human skin. J Am Acad Dermatol. 2005;52:P32.
Practice Points
- Patients are increasingly interested in dietary modifications that may influence skin appearance and aid in the treatment of cutaneous disease.
- Although evidence-based dietary recommendations currently are limited, it is important for dermatologists to be aware of the varied and nuanced dietary interventions employed by patients.
- There remains a lack of randomized controlled trials assessing the efficacy of various dietary interventions in the dermatologic setting.
Review finds diverse outcomes in clinical trials of rosacea
according to authors of a new systematic review of rosacea treatment studies.
“Rosacea is a chronic dermatologic condition that affects 16 million Americans,” one of the study authors, Sarah A. Ibrahim, told this news organization after the annual conference of the American Society for Laser Medicine and Surgery. “The features of rosacea, such as inflammatory lesions, redness, burning sensations, and swelling, can have a negative impact on the quality of life for many patients. Additionally, patients with rosacea are at an increased risk for other conditions such as autoimmune diseases, like inflammatory bowel disease.”
In an effort led by principal investigator Murad Alam, MD, vice chair of the department of dermatology at Northwestern University, Chicago, Ms. Ibrahim conducted a systematic review to identify all outcomes that have previously been reported in clinical trials of rosacea, as part of the development of the core outcome set established by the Measurement of Priority Outcome Variables in Dermatologic Surgery (IMPROVED) group. “This has not been done before and is an important first step in understanding what outcomes should be measured in every future clinical study of rosacea,” said Ms. Ibrahim, a medical student at Northwestern University, and predoctoral research fellow in Northwestern’s department of dermatology.
The researchers limited their analysis to randomized, controlled trials of rosacea interventions published between 2010 and 2020 and categorized outcomes into domains based on similar themes.
A total of 58 studies were included in the systematic review, of which 7 (12%) evaluated laser-based interventions. The researchers identified 55 unique outcomes that encompassed eight domains: Quality of life, treatment effects, patient perception of health, clinical assessment, acceptance of care, laboratory assessment, physiological skin assessment, and patient satisfaction. Of the eight domains, clinical assessment-related outcomes were measured in all studies. Nontransient erythema was the most commonly reported outcome (43 studies, 78%), followed by inflammatory lesions (36 studies, 65%) and telangiectasia (22 studies, 40%).
Outcomes pertaining to treatment effects such as adverse events were measured in 49 of the 55 studies (89%), while patient-reported outcomes were measured in 21 (38%). Quality of life and patient satisfaction were reported in 18 (33%) and 13 (24%) studies, respectively.
“There were two main take-home messages of our study,” said Ms. Ibrahim, who presented the results at the meeting. “The first is that there is a wide range of outcomes that are reported in clinical trials of rosacea therapies. Second, that there is a need to standardize the outcomes that are reported in clinical trials of rosacea, in order to be able to combine the results from different studies to better understand which interventions for rosacea are most effective.”
She acknowledged certain limitations of the review, including that other trials related to the topic were not included. “Because of the date range and types of studies that we used to narrow down our search, it is possible that additional outcomes were reported in studies that were not included here,” she said.
“This is a very important study because rosacea is a very common condition and one that I have seen more frequently in clinic since the pandemic started,” said Omar Ibrahimi, PhD, MD, a dermatologist with the Connecticut Skin Institute in Stamford, who was asked to comment on the work. “One of the limitations with rosacea studies is that the studies done are often fairly small and the outcome measures are heterogenous. The current study by Ibrahim and coworkers does a wonderful job of highlighting the various outcomes measures used to measure the success of rosacea treatments with energy-based devices.”
This information, he added, “will be very useful for further research studies because it forms the basis for formulating a set of core outcome measures to judge treatment interventions with consensus input from a variety of key opinion leaders. This will prove to be valuable because if we can have a uniform set of outcome measures to judge rosacea treatments with then we will be able to compare the results from different studies better.”
Ms. Ibrahim and colleagues reported having no relevant financial disclosures. Dr. Ibrahimi disclosed that he has been a speaker for both Candela and Cutera and he is currently on the medical advisory board for Cutera.
according to authors of a new systematic review of rosacea treatment studies.
“Rosacea is a chronic dermatologic condition that affects 16 million Americans,” one of the study authors, Sarah A. Ibrahim, told this news organization after the annual conference of the American Society for Laser Medicine and Surgery. “The features of rosacea, such as inflammatory lesions, redness, burning sensations, and swelling, can have a negative impact on the quality of life for many patients. Additionally, patients with rosacea are at an increased risk for other conditions such as autoimmune diseases, like inflammatory bowel disease.”
In an effort led by principal investigator Murad Alam, MD, vice chair of the department of dermatology at Northwestern University, Chicago, Ms. Ibrahim conducted a systematic review to identify all outcomes that have previously been reported in clinical trials of rosacea, as part of the development of the core outcome set established by the Measurement of Priority Outcome Variables in Dermatologic Surgery (IMPROVED) group. “This has not been done before and is an important first step in understanding what outcomes should be measured in every future clinical study of rosacea,” said Ms. Ibrahim, a medical student at Northwestern University, and predoctoral research fellow in Northwestern’s department of dermatology.
The researchers limited their analysis to randomized, controlled trials of rosacea interventions published between 2010 and 2020 and categorized outcomes into domains based on similar themes.
A total of 58 studies were included in the systematic review, of which 7 (12%) evaluated laser-based interventions. The researchers identified 55 unique outcomes that encompassed eight domains: Quality of life, treatment effects, patient perception of health, clinical assessment, acceptance of care, laboratory assessment, physiological skin assessment, and patient satisfaction. Of the eight domains, clinical assessment-related outcomes were measured in all studies. Nontransient erythema was the most commonly reported outcome (43 studies, 78%), followed by inflammatory lesions (36 studies, 65%) and telangiectasia (22 studies, 40%).
Outcomes pertaining to treatment effects such as adverse events were measured in 49 of the 55 studies (89%), while patient-reported outcomes were measured in 21 (38%). Quality of life and patient satisfaction were reported in 18 (33%) and 13 (24%) studies, respectively.
“There were two main take-home messages of our study,” said Ms. Ibrahim, who presented the results at the meeting. “The first is that there is a wide range of outcomes that are reported in clinical trials of rosacea therapies. Second, that there is a need to standardize the outcomes that are reported in clinical trials of rosacea, in order to be able to combine the results from different studies to better understand which interventions for rosacea are most effective.”
She acknowledged certain limitations of the review, including that other trials related to the topic were not included. “Because of the date range and types of studies that we used to narrow down our search, it is possible that additional outcomes were reported in studies that were not included here,” she said.
“This is a very important study because rosacea is a very common condition and one that I have seen more frequently in clinic since the pandemic started,” said Omar Ibrahimi, PhD, MD, a dermatologist with the Connecticut Skin Institute in Stamford, who was asked to comment on the work. “One of the limitations with rosacea studies is that the studies done are often fairly small and the outcome measures are heterogenous. The current study by Ibrahim and coworkers does a wonderful job of highlighting the various outcomes measures used to measure the success of rosacea treatments with energy-based devices.”
This information, he added, “will be very useful for further research studies because it forms the basis for formulating a set of core outcome measures to judge treatment interventions with consensus input from a variety of key opinion leaders. This will prove to be valuable because if we can have a uniform set of outcome measures to judge rosacea treatments with then we will be able to compare the results from different studies better.”
Ms. Ibrahim and colleagues reported having no relevant financial disclosures. Dr. Ibrahimi disclosed that he has been a speaker for both Candela and Cutera and he is currently on the medical advisory board for Cutera.
according to authors of a new systematic review of rosacea treatment studies.
“Rosacea is a chronic dermatologic condition that affects 16 million Americans,” one of the study authors, Sarah A. Ibrahim, told this news organization after the annual conference of the American Society for Laser Medicine and Surgery. “The features of rosacea, such as inflammatory lesions, redness, burning sensations, and swelling, can have a negative impact on the quality of life for many patients. Additionally, patients with rosacea are at an increased risk for other conditions such as autoimmune diseases, like inflammatory bowel disease.”
In an effort led by principal investigator Murad Alam, MD, vice chair of the department of dermatology at Northwestern University, Chicago, Ms. Ibrahim conducted a systematic review to identify all outcomes that have previously been reported in clinical trials of rosacea, as part of the development of the core outcome set established by the Measurement of Priority Outcome Variables in Dermatologic Surgery (IMPROVED) group. “This has not been done before and is an important first step in understanding what outcomes should be measured in every future clinical study of rosacea,” said Ms. Ibrahim, a medical student at Northwestern University, and predoctoral research fellow in Northwestern’s department of dermatology.
The researchers limited their analysis to randomized, controlled trials of rosacea interventions published between 2010 and 2020 and categorized outcomes into domains based on similar themes.
A total of 58 studies were included in the systematic review, of which 7 (12%) evaluated laser-based interventions. The researchers identified 55 unique outcomes that encompassed eight domains: Quality of life, treatment effects, patient perception of health, clinical assessment, acceptance of care, laboratory assessment, physiological skin assessment, and patient satisfaction. Of the eight domains, clinical assessment-related outcomes were measured in all studies. Nontransient erythema was the most commonly reported outcome (43 studies, 78%), followed by inflammatory lesions (36 studies, 65%) and telangiectasia (22 studies, 40%).
Outcomes pertaining to treatment effects such as adverse events were measured in 49 of the 55 studies (89%), while patient-reported outcomes were measured in 21 (38%). Quality of life and patient satisfaction were reported in 18 (33%) and 13 (24%) studies, respectively.
“There were two main take-home messages of our study,” said Ms. Ibrahim, who presented the results at the meeting. “The first is that there is a wide range of outcomes that are reported in clinical trials of rosacea therapies. Second, that there is a need to standardize the outcomes that are reported in clinical trials of rosacea, in order to be able to combine the results from different studies to better understand which interventions for rosacea are most effective.”
She acknowledged certain limitations of the review, including that other trials related to the topic were not included. “Because of the date range and types of studies that we used to narrow down our search, it is possible that additional outcomes were reported in studies that were not included here,” she said.
“This is a very important study because rosacea is a very common condition and one that I have seen more frequently in clinic since the pandemic started,” said Omar Ibrahimi, PhD, MD, a dermatologist with the Connecticut Skin Institute in Stamford, who was asked to comment on the work. “One of the limitations with rosacea studies is that the studies done are often fairly small and the outcome measures are heterogenous. The current study by Ibrahim and coworkers does a wonderful job of highlighting the various outcomes measures used to measure the success of rosacea treatments with energy-based devices.”
This information, he added, “will be very useful for further research studies because it forms the basis for formulating a set of core outcome measures to judge treatment interventions with consensus input from a variety of key opinion leaders. This will prove to be valuable because if we can have a uniform set of outcome measures to judge rosacea treatments with then we will be able to compare the results from different studies better.”
Ms. Ibrahim and colleagues reported having no relevant financial disclosures. Dr. Ibrahimi disclosed that he has been a speaker for both Candela and Cutera and he is currently on the medical advisory board for Cutera.
FROM ASLMS 2021
Oral sarecycline promising for papulopustular rosacea
Linda Stein Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The oral broad-spectrum second-generation tetracyclines doxycycline and minocycline have long been considered first-line therapy for papulopustular rosacea that isn’t cleared using topical agents. But the widespread use of these oral tetracyclines has encouraged the development of antimicrobial resistance. In contrast, sarecycline (Seysara) is a third-generation, narrow-spectrum tetracycline designed to minimize antibiotic resistance. The Food and Drug Administration approved the drug for treatment of moderate to severe acne vulgaris in 2018.
At the meeting, Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit, highlighted a recent pilot study of oral sarecycline for papulopustular rosacea carried out by James Q. Del Rosso, DO, of Las Vegas and coinvestigators. Although she wasn’t involved in the study, she is a veteran clinical trialist with vast experience leading studies of new therapies for rosacea, acne, and other major dermatologic disorders.
The 12-week, prospective, investigator-blinded study included 97 adults with moderate to severe papulopustular rosacea; 72 were randomized to weight-based dosing of once-daily sarecycline, while the 25 controls took a daily oral vitamin.
One coprimary endpoint was achievement of an Investigator Global Assessment score of 0 or 1, meaning clear or almost clear skin, at week 12. The rates were 75% in the sarecycline group and 16% in controls. The other coprimary endpoint was the percent reduction from baseline to week 12 in inflammatory lesion count. Here again, there was a statistically significant difference in favor of the third-generation tetracycline derivative, which achieved an 80% reduction, compared with 50% in the control group.
Of note, the difference was already significant at the first evaluation at week 4, with a 58% reduction in inflammatory lesions in the sarecycline group versus 31% decrease in controls, Dr. Stein Gold observed at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also at week 12, 96% of patients on sarecycline reported having no or only trace symptoms of facial burning, 63% had no or only trace facial erythema, and 94% had no or trace facial itch, compared with 76%, 12%, and 76% of controls, respectively. The sarecycline group was also significantly more likely to report no or trace skin dryness and oiliness.
The side-effect profile was favorable and the same as encountered with the use of sarecycline for acne: no major photosensitivity issues, no serious adverse events, and only 2 of the original 75 patients in the active-treatment arm discontinued sarecycline for treatment-emergent headache or gastroenteritis considered “probably” related to the study drug. The investigators deemed further studies of sarecycline for rosacea to be warranted as a potential expanded indication.
Aiming for clear skin rather than ‘almost clear’
Dr. Stein Gold shared her mantra for rosacea therapy: “Always aim for clear skin.”
She cited a study led by Guy Webster, MD, professor of dermatology, Thomas Jefferson University, Philadelphia, in which he and his coinvestigators looked at the durability of treatment response in a pooled analysis of 1,366 rosacea patients in four clinical trials. If patients improved to “almost clear” after treatment, their median time to relapse was 3 months; if they reached “clear,” it was more than 8 months. Also, more clear patients rated their outcomes as excellent and reported that their skin disease no longer had any effect on their quality of life.
“That’s more than a 5-month difference,” Dr. Stein Gold noted. “It shows the importance of really striving to get that skin completely clear.”
The sarecycline study was funded by Almirall, which markets the antibiotic. Dr. Stein Gold, who has no financial relationship with Almirall, has received research funding from and/or served as a consultant to roughly a dozen other pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Linda Stein Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The oral broad-spectrum second-generation tetracyclines doxycycline and minocycline have long been considered first-line therapy for papulopustular rosacea that isn’t cleared using topical agents. But the widespread use of these oral tetracyclines has encouraged the development of antimicrobial resistance. In contrast, sarecycline (Seysara) is a third-generation, narrow-spectrum tetracycline designed to minimize antibiotic resistance. The Food and Drug Administration approved the drug for treatment of moderate to severe acne vulgaris in 2018.
At the meeting, Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit, highlighted a recent pilot study of oral sarecycline for papulopustular rosacea carried out by James Q. Del Rosso, DO, of Las Vegas and coinvestigators. Although she wasn’t involved in the study, she is a veteran clinical trialist with vast experience leading studies of new therapies for rosacea, acne, and other major dermatologic disorders.
The 12-week, prospective, investigator-blinded study included 97 adults with moderate to severe papulopustular rosacea; 72 were randomized to weight-based dosing of once-daily sarecycline, while the 25 controls took a daily oral vitamin.
One coprimary endpoint was achievement of an Investigator Global Assessment score of 0 or 1, meaning clear or almost clear skin, at week 12. The rates were 75% in the sarecycline group and 16% in controls. The other coprimary endpoint was the percent reduction from baseline to week 12 in inflammatory lesion count. Here again, there was a statistically significant difference in favor of the third-generation tetracycline derivative, which achieved an 80% reduction, compared with 50% in the control group.
Of note, the difference was already significant at the first evaluation at week 4, with a 58% reduction in inflammatory lesions in the sarecycline group versus 31% decrease in controls, Dr. Stein Gold observed at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also at week 12, 96% of patients on sarecycline reported having no or only trace symptoms of facial burning, 63% had no or only trace facial erythema, and 94% had no or trace facial itch, compared with 76%, 12%, and 76% of controls, respectively. The sarecycline group was also significantly more likely to report no or trace skin dryness and oiliness.
The side-effect profile was favorable and the same as encountered with the use of sarecycline for acne: no major photosensitivity issues, no serious adverse events, and only 2 of the original 75 patients in the active-treatment arm discontinued sarecycline for treatment-emergent headache or gastroenteritis considered “probably” related to the study drug. The investigators deemed further studies of sarecycline for rosacea to be warranted as a potential expanded indication.
Aiming for clear skin rather than ‘almost clear’
Dr. Stein Gold shared her mantra for rosacea therapy: “Always aim for clear skin.”
She cited a study led by Guy Webster, MD, professor of dermatology, Thomas Jefferson University, Philadelphia, in which he and his coinvestigators looked at the durability of treatment response in a pooled analysis of 1,366 rosacea patients in four clinical trials. If patients improved to “almost clear” after treatment, their median time to relapse was 3 months; if they reached “clear,” it was more than 8 months. Also, more clear patients rated their outcomes as excellent and reported that their skin disease no longer had any effect on their quality of life.
“That’s more than a 5-month difference,” Dr. Stein Gold noted. “It shows the importance of really striving to get that skin completely clear.”
The sarecycline study was funded by Almirall, which markets the antibiotic. Dr. Stein Gold, who has no financial relationship with Almirall, has received research funding from and/or served as a consultant to roughly a dozen other pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Linda Stein Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The oral broad-spectrum second-generation tetracyclines doxycycline and minocycline have long been considered first-line therapy for papulopustular rosacea that isn’t cleared using topical agents. But the widespread use of these oral tetracyclines has encouraged the development of antimicrobial resistance. In contrast, sarecycline (Seysara) is a third-generation, narrow-spectrum tetracycline designed to minimize antibiotic resistance. The Food and Drug Administration approved the drug for treatment of moderate to severe acne vulgaris in 2018.
At the meeting, Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit, highlighted a recent pilot study of oral sarecycline for papulopustular rosacea carried out by James Q. Del Rosso, DO, of Las Vegas and coinvestigators. Although she wasn’t involved in the study, she is a veteran clinical trialist with vast experience leading studies of new therapies for rosacea, acne, and other major dermatologic disorders.
The 12-week, prospective, investigator-blinded study included 97 adults with moderate to severe papulopustular rosacea; 72 were randomized to weight-based dosing of once-daily sarecycline, while the 25 controls took a daily oral vitamin.
One coprimary endpoint was achievement of an Investigator Global Assessment score of 0 or 1, meaning clear or almost clear skin, at week 12. The rates were 75% in the sarecycline group and 16% in controls. The other coprimary endpoint was the percent reduction from baseline to week 12 in inflammatory lesion count. Here again, there was a statistically significant difference in favor of the third-generation tetracycline derivative, which achieved an 80% reduction, compared with 50% in the control group.
Of note, the difference was already significant at the first evaluation at week 4, with a 58% reduction in inflammatory lesions in the sarecycline group versus 31% decrease in controls, Dr. Stein Gold observed at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also at week 12, 96% of patients on sarecycline reported having no or only trace symptoms of facial burning, 63% had no or only trace facial erythema, and 94% had no or trace facial itch, compared with 76%, 12%, and 76% of controls, respectively. The sarecycline group was also significantly more likely to report no or trace skin dryness and oiliness.
The side-effect profile was favorable and the same as encountered with the use of sarecycline for acne: no major photosensitivity issues, no serious adverse events, and only 2 of the original 75 patients in the active-treatment arm discontinued sarecycline for treatment-emergent headache or gastroenteritis considered “probably” related to the study drug. The investigators deemed further studies of sarecycline for rosacea to be warranted as a potential expanded indication.
Aiming for clear skin rather than ‘almost clear’
Dr. Stein Gold shared her mantra for rosacea therapy: “Always aim for clear skin.”
She cited a study led by Guy Webster, MD, professor of dermatology, Thomas Jefferson University, Philadelphia, in which he and his coinvestigators looked at the durability of treatment response in a pooled analysis of 1,366 rosacea patients in four clinical trials. If patients improved to “almost clear” after treatment, their median time to relapse was 3 months; if they reached “clear,” it was more than 8 months. Also, more clear patients rated their outcomes as excellent and reported that their skin disease no longer had any effect on their quality of life.
“That’s more than a 5-month difference,” Dr. Stein Gold noted. “It shows the importance of really striving to get that skin completely clear.”
The sarecycline study was funded by Almirall, which markets the antibiotic. Dr. Stein Gold, who has no financial relationship with Almirall, has received research funding from and/or served as a consultant to roughly a dozen other pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Vasodilatory medications found protective against rosacea
.
“Our initial hypothesis was that perhaps antihypertensive agents might be associated with worsening rosacea,” one of the study authors, Jennifer G. Powers, MD, associate professor of dermatology at the University of Iowa, Iowa City, said in an interview. “What we found was exactly the opposite – that in fact their presence in a medical chart correlated with lower rates of rosacea diagnoses, as defined by ICD 9/10 codes.”
According to the researchers, who published their findings in the Journal of the American Academy of Dermatology, cases of acute vasodilator-induced rosacea have been reported, but no long-term association has been established. “In fact, many widely used antihypertensive medications modulate peripheral vascular tone,” they wrote. “Therefore, chronic use in patients with hypertension may reduce damage to peripheral vessels, and thus decrease risk of rosacea.”
To determine the correlates between vasodilator use and risk of rosacea, Dr. Powers and colleagues identified 680 hypertensive patients being treated with vasodilators or a thiazide diuretic in whom rosacea developed within 5 years of initiating therapy between June 1, 2006, and April 31, 2019. Vasodilator therapies included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs). Patients on thiazide diuretics served as the control group. The researchers stratified the patients by age, gender, race, diabetes, chronic kidney disease, and coronary artery disease and calculated relative risk estimates comparing vasodilators with thiazides between strata.
Of the 680 patients, all but 40 were White; 127 were on thiazides, and the remaining 553 were on vasodilators. Overall, the researchers observed that use of vasodilators had a protective effect on the development of rosacea within 5 years, compared with thiazides (relative risk [RR], 0.56; P less than .0001). Specifically, the relative risk was 0.50 for ACE-inhibitors (P less than .0001); 0.69 for ARBs (P = .041); 0.55 for beta-blockers (P less than .0001); and 0.39 for CCBs (P less than .0001).
Dr. Powers and colleagues also observed significant inverse correlations in ACE-inhibitors, beta-blockers, and CCBs among White women aged 50 and older, but no significance was observed in non-White subgroups. The cohorts of patients with chronic kidney disease and coronary artery disease were too small for analysis.
“We were very surprised to find that many of the agents we think of as vasodilators might actually be beneficial for rosacea,” Dr. Powers said. “We would like to see these results reproduced in larger population studies. There are also potential questions about the mechanism at play. However, should these findings hold true, [it’s] all the more reason for our rosacea patients with hypertension to be managed well. They need not fear that those medications are worsening disease. Also, there might be new therapeutic options based on this data.”
The study received funding support from the National Center for Advancing Translational Sciences. The researchers reported having no financial disclosures.
One of Dr. Powers’ coauthors is her husband, Edward M. Powers, MD, a cardiology fellow at the University of Iowa. “We sometimes bounce ideas off one another and will talk about how systemic effects on the vasculature may impact skin disease,” she said, noting that they also published a report on statins and atopic dermatitis.
.
“Our initial hypothesis was that perhaps antihypertensive agents might be associated with worsening rosacea,” one of the study authors, Jennifer G. Powers, MD, associate professor of dermatology at the University of Iowa, Iowa City, said in an interview. “What we found was exactly the opposite – that in fact their presence in a medical chart correlated with lower rates of rosacea diagnoses, as defined by ICD 9/10 codes.”
According to the researchers, who published their findings in the Journal of the American Academy of Dermatology, cases of acute vasodilator-induced rosacea have been reported, but no long-term association has been established. “In fact, many widely used antihypertensive medications modulate peripheral vascular tone,” they wrote. “Therefore, chronic use in patients with hypertension may reduce damage to peripheral vessels, and thus decrease risk of rosacea.”
To determine the correlates between vasodilator use and risk of rosacea, Dr. Powers and colleagues identified 680 hypertensive patients being treated with vasodilators or a thiazide diuretic in whom rosacea developed within 5 years of initiating therapy between June 1, 2006, and April 31, 2019. Vasodilator therapies included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs). Patients on thiazide diuretics served as the control group. The researchers stratified the patients by age, gender, race, diabetes, chronic kidney disease, and coronary artery disease and calculated relative risk estimates comparing vasodilators with thiazides between strata.
Of the 680 patients, all but 40 were White; 127 were on thiazides, and the remaining 553 were on vasodilators. Overall, the researchers observed that use of vasodilators had a protective effect on the development of rosacea within 5 years, compared with thiazides (relative risk [RR], 0.56; P less than .0001). Specifically, the relative risk was 0.50 for ACE-inhibitors (P less than .0001); 0.69 for ARBs (P = .041); 0.55 for beta-blockers (P less than .0001); and 0.39 for CCBs (P less than .0001).
Dr. Powers and colleagues also observed significant inverse correlations in ACE-inhibitors, beta-blockers, and CCBs among White women aged 50 and older, but no significance was observed in non-White subgroups. The cohorts of patients with chronic kidney disease and coronary artery disease were too small for analysis.
“We were very surprised to find that many of the agents we think of as vasodilators might actually be beneficial for rosacea,” Dr. Powers said. “We would like to see these results reproduced in larger population studies. There are also potential questions about the mechanism at play. However, should these findings hold true, [it’s] all the more reason for our rosacea patients with hypertension to be managed well. They need not fear that those medications are worsening disease. Also, there might be new therapeutic options based on this data.”
The study received funding support from the National Center for Advancing Translational Sciences. The researchers reported having no financial disclosures.
One of Dr. Powers’ coauthors is her husband, Edward M. Powers, MD, a cardiology fellow at the University of Iowa. “We sometimes bounce ideas off one another and will talk about how systemic effects on the vasculature may impact skin disease,” she said, noting that they also published a report on statins and atopic dermatitis.
.
“Our initial hypothesis was that perhaps antihypertensive agents might be associated with worsening rosacea,” one of the study authors, Jennifer G. Powers, MD, associate professor of dermatology at the University of Iowa, Iowa City, said in an interview. “What we found was exactly the opposite – that in fact their presence in a medical chart correlated with lower rates of rosacea diagnoses, as defined by ICD 9/10 codes.”
According to the researchers, who published their findings in the Journal of the American Academy of Dermatology, cases of acute vasodilator-induced rosacea have been reported, but no long-term association has been established. “In fact, many widely used antihypertensive medications modulate peripheral vascular tone,” they wrote. “Therefore, chronic use in patients with hypertension may reduce damage to peripheral vessels, and thus decrease risk of rosacea.”
To determine the correlates between vasodilator use and risk of rosacea, Dr. Powers and colleagues identified 680 hypertensive patients being treated with vasodilators or a thiazide diuretic in whom rosacea developed within 5 years of initiating therapy between June 1, 2006, and April 31, 2019. Vasodilator therapies included angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, and calcium channel blockers (CCBs). Patients on thiazide diuretics served as the control group. The researchers stratified the patients by age, gender, race, diabetes, chronic kidney disease, and coronary artery disease and calculated relative risk estimates comparing vasodilators with thiazides between strata.
Of the 680 patients, all but 40 were White; 127 were on thiazides, and the remaining 553 were on vasodilators. Overall, the researchers observed that use of vasodilators had a protective effect on the development of rosacea within 5 years, compared with thiazides (relative risk [RR], 0.56; P less than .0001). Specifically, the relative risk was 0.50 for ACE-inhibitors (P less than .0001); 0.69 for ARBs (P = .041); 0.55 for beta-blockers (P less than .0001); and 0.39 for CCBs (P less than .0001).
Dr. Powers and colleagues also observed significant inverse correlations in ACE-inhibitors, beta-blockers, and CCBs among White women aged 50 and older, but no significance was observed in non-White subgroups. The cohorts of patients with chronic kidney disease and coronary artery disease were too small for analysis.
“We were very surprised to find that many of the agents we think of as vasodilators might actually be beneficial for rosacea,” Dr. Powers said. “We would like to see these results reproduced in larger population studies. There are also potential questions about the mechanism at play. However, should these findings hold true, [it’s] all the more reason for our rosacea patients with hypertension to be managed well. They need not fear that those medications are worsening disease. Also, there might be new therapeutic options based on this data.”
The study received funding support from the National Center for Advancing Translational Sciences. The researchers reported having no financial disclosures.
One of Dr. Powers’ coauthors is her husband, Edward M. Powers, MD, a cardiology fellow at the University of Iowa. “We sometimes bounce ideas off one another and will talk about how systemic effects on the vasculature may impact skin disease,” she said, noting that they also published a report on statins and atopic dermatitis.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Energy-based devices: Expert shares treatment tips for rosacea, scars
, according to a 2020 international consensus publication that Jeremy B. Green, MD, reviewed during a virtual course on laser and aesthetic skin therapy.
During his presentation, he also reviewed laser treatment of scars. “Erythema is an indicator of scar activity,” said Dr. Green, a dermatologist in Coral Gables, Fla. “So, with flat, red scars, vascular devices are the first choice. If you’re going to treat with multiple lasers in a single session, use the vascular laser first, followed by a resurfacing laser if needed. If you treat with a resurfacing laser first, you’ll cause erythema and edema and you’ll obscure that blood vessel target.”
The manuscript, which was created by a panel of 26 dermatologists and plastic and reconstructive surgeons from 13 different countries, also calls for using scar treatment settings that are lower than those used for port wine stains, with mild purpura as the clinical endpoint to strive for.
Vascular lasers are also the expert panel’s first choice when a scar is painful or pruritic, while the second choice is an ablative fractional laser with intralesional triamcinolone and/or 5-fluorouracil (5-FU). “If the scar is hypertrophic, I will combine a vascular laser, then a nonablative or an ablative fractional laser, then intralesional triamcinolone mixed with 5-FU,” said Dr. Green, who was not involved in drafting the recommendations.
As for the first treatment of choice, 80% of the experts chose a pulsed dye laser, while others chose the KTP laser, intense pulsed light (IPL) and the neodymium yttrium aluminum garnet (Nd:YAG) laser. With regard to settings, when using a PDL and a 10-mm spot size, 41% of experts recommend a fluence of 5-6 J/cm2, 27% recommend a fluence of 4-5 J/cm2, and 27% recommend a fluence of 6-7 J/cm2. Pertaining to pulse duration, 50% favor 1.5 milliseconds, 18% use 3 milliseconds, and 18% use .45 milliseconds.
As for timing post surgery, 70% report treating less than 1 week after surgery and 90% report treating within 1 month post surgery. “I prefer to treat about 1 week after sutures are removed so the skin is re-epithelialized,” Dr. Green said. “The bottom line is, with postsurgical, posttraumatic scars, once the skin is healed, the sooner you get at it, the better.”
Rosacea
He also discussed the microvascular effects of PDL in combination with oxymetazoline 1% cream, an alpha1A adrenoceptor agonist, which is approved by the Food and Drug Administration for treatment of persistent facial erythema associated with rosacea. “This has been a hot topic lately,” Dr. Green said. “When the studies were done for FDA approval, there was an observation that vasodilation occurs 5 minutes after application of oxymetazoline, so the venule diameter increases. Sixty minutes after application, vasoconstriction happens, which is the desired clinical effect for patients with facial erythema.”
In a mouse study, researchers led by Bernard Choi, PhD, and Kristin M. Kelly, MD, of the Beckman Laser Institute and Medical Clinic, University of California, Irvine, found that the combination protocol of oxymetazoline application, followed 5 minutes later by PDL, induced persistent vascular shutdown 7 days after irradiation. Vascular shutdown occurred in 67% of vessels treated with oxymetazoline plus PDL at day 7 vs. 17% in those treated with saline plus PDL.
“This is fascinating,” Dr. Green said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “There is no publication I’m aware of in humans that has explored this timing, but I have used oxymetazoline in my clinic in patients with stubborn erythema and treated them with the vascular laser 5 minutes later.”
In a separate open-label study of 46 patients with moderate to severe facial erythema associated with rosacea, researchers found that oxymetazoline 1% as adjunctive therapy with energy-based therapy was safe and well tolerated, and reduced facial erythema in patients with moderate to severe persistent facial erythema associated with rosacea. Energy sources used were the PDL, KTP, or IPL.
In a study presented during the 2020 American Society for Laser Medicine & Surgery meeting, researchers led by Pooja Sodha, MD, of George Washington University, Washington, conducted a pilot trial of PDL plus oxymetazoline 1% cream for erythematotelangiectatic rosacea. Between baseline and 6 months’ follow-up the Clinician’s Erythema Assessment score fell from 4 to 2.
“Of note, I would also throw the kitchen sink at these patients medically, meaning I love topical ivermectin 1% cream,” Dr. Green said. “In some cases I’ll even use oral ivermectin and an oral tetracycline class antibiotic.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
, according to a 2020 international consensus publication that Jeremy B. Green, MD, reviewed during a virtual course on laser and aesthetic skin therapy.
During his presentation, he also reviewed laser treatment of scars. “Erythema is an indicator of scar activity,” said Dr. Green, a dermatologist in Coral Gables, Fla. “So, with flat, red scars, vascular devices are the first choice. If you’re going to treat with multiple lasers in a single session, use the vascular laser first, followed by a resurfacing laser if needed. If you treat with a resurfacing laser first, you’ll cause erythema and edema and you’ll obscure that blood vessel target.”
The manuscript, which was created by a panel of 26 dermatologists and plastic and reconstructive surgeons from 13 different countries, also calls for using scar treatment settings that are lower than those used for port wine stains, with mild purpura as the clinical endpoint to strive for.
Vascular lasers are also the expert panel’s first choice when a scar is painful or pruritic, while the second choice is an ablative fractional laser with intralesional triamcinolone and/or 5-fluorouracil (5-FU). “If the scar is hypertrophic, I will combine a vascular laser, then a nonablative or an ablative fractional laser, then intralesional triamcinolone mixed with 5-FU,” said Dr. Green, who was not involved in drafting the recommendations.
As for the first treatment of choice, 80% of the experts chose a pulsed dye laser, while others chose the KTP laser, intense pulsed light (IPL) and the neodymium yttrium aluminum garnet (Nd:YAG) laser. With regard to settings, when using a PDL and a 10-mm spot size, 41% of experts recommend a fluence of 5-6 J/cm2, 27% recommend a fluence of 4-5 J/cm2, and 27% recommend a fluence of 6-7 J/cm2. Pertaining to pulse duration, 50% favor 1.5 milliseconds, 18% use 3 milliseconds, and 18% use .45 milliseconds.
As for timing post surgery, 70% report treating less than 1 week after surgery and 90% report treating within 1 month post surgery. “I prefer to treat about 1 week after sutures are removed so the skin is re-epithelialized,” Dr. Green said. “The bottom line is, with postsurgical, posttraumatic scars, once the skin is healed, the sooner you get at it, the better.”
Rosacea
He also discussed the microvascular effects of PDL in combination with oxymetazoline 1% cream, an alpha1A adrenoceptor agonist, which is approved by the Food and Drug Administration for treatment of persistent facial erythema associated with rosacea. “This has been a hot topic lately,” Dr. Green said. “When the studies were done for FDA approval, there was an observation that vasodilation occurs 5 minutes after application of oxymetazoline, so the venule diameter increases. Sixty minutes after application, vasoconstriction happens, which is the desired clinical effect for patients with facial erythema.”
In a mouse study, researchers led by Bernard Choi, PhD, and Kristin M. Kelly, MD, of the Beckman Laser Institute and Medical Clinic, University of California, Irvine, found that the combination protocol of oxymetazoline application, followed 5 minutes later by PDL, induced persistent vascular shutdown 7 days after irradiation. Vascular shutdown occurred in 67% of vessels treated with oxymetazoline plus PDL at day 7 vs. 17% in those treated with saline plus PDL.
“This is fascinating,” Dr. Green said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “There is no publication I’m aware of in humans that has explored this timing, but I have used oxymetazoline in my clinic in patients with stubborn erythema and treated them with the vascular laser 5 minutes later.”
In a separate open-label study of 46 patients with moderate to severe facial erythema associated with rosacea, researchers found that oxymetazoline 1% as adjunctive therapy with energy-based therapy was safe and well tolerated, and reduced facial erythema in patients with moderate to severe persistent facial erythema associated with rosacea. Energy sources used were the PDL, KTP, or IPL.
In a study presented during the 2020 American Society for Laser Medicine & Surgery meeting, researchers led by Pooja Sodha, MD, of George Washington University, Washington, conducted a pilot trial of PDL plus oxymetazoline 1% cream for erythematotelangiectatic rosacea. Between baseline and 6 months’ follow-up the Clinician’s Erythema Assessment score fell from 4 to 2.
“Of note, I would also throw the kitchen sink at these patients medically, meaning I love topical ivermectin 1% cream,” Dr. Green said. “In some cases I’ll even use oral ivermectin and an oral tetracycline class antibiotic.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
, according to a 2020 international consensus publication that Jeremy B. Green, MD, reviewed during a virtual course on laser and aesthetic skin therapy.
During his presentation, he also reviewed laser treatment of scars. “Erythema is an indicator of scar activity,” said Dr. Green, a dermatologist in Coral Gables, Fla. “So, with flat, red scars, vascular devices are the first choice. If you’re going to treat with multiple lasers in a single session, use the vascular laser first, followed by a resurfacing laser if needed. If you treat with a resurfacing laser first, you’ll cause erythema and edema and you’ll obscure that blood vessel target.”
The manuscript, which was created by a panel of 26 dermatologists and plastic and reconstructive surgeons from 13 different countries, also calls for using scar treatment settings that are lower than those used for port wine stains, with mild purpura as the clinical endpoint to strive for.
Vascular lasers are also the expert panel’s first choice when a scar is painful or pruritic, while the second choice is an ablative fractional laser with intralesional triamcinolone and/or 5-fluorouracil (5-FU). “If the scar is hypertrophic, I will combine a vascular laser, then a nonablative or an ablative fractional laser, then intralesional triamcinolone mixed with 5-FU,” said Dr. Green, who was not involved in drafting the recommendations.
As for the first treatment of choice, 80% of the experts chose a pulsed dye laser, while others chose the KTP laser, intense pulsed light (IPL) and the neodymium yttrium aluminum garnet (Nd:YAG) laser. With regard to settings, when using a PDL and a 10-mm spot size, 41% of experts recommend a fluence of 5-6 J/cm2, 27% recommend a fluence of 4-5 J/cm2, and 27% recommend a fluence of 6-7 J/cm2. Pertaining to pulse duration, 50% favor 1.5 milliseconds, 18% use 3 milliseconds, and 18% use .45 milliseconds.
As for timing post surgery, 70% report treating less than 1 week after surgery and 90% report treating within 1 month post surgery. “I prefer to treat about 1 week after sutures are removed so the skin is re-epithelialized,” Dr. Green said. “The bottom line is, with postsurgical, posttraumatic scars, once the skin is healed, the sooner you get at it, the better.”
Rosacea
He also discussed the microvascular effects of PDL in combination with oxymetazoline 1% cream, an alpha1A adrenoceptor agonist, which is approved by the Food and Drug Administration for treatment of persistent facial erythema associated with rosacea. “This has been a hot topic lately,” Dr. Green said. “When the studies were done for FDA approval, there was an observation that vasodilation occurs 5 minutes after application of oxymetazoline, so the venule diameter increases. Sixty minutes after application, vasoconstriction happens, which is the desired clinical effect for patients with facial erythema.”
In a mouse study, researchers led by Bernard Choi, PhD, and Kristin M. Kelly, MD, of the Beckman Laser Institute and Medical Clinic, University of California, Irvine, found that the combination protocol of oxymetazoline application, followed 5 minutes later by PDL, induced persistent vascular shutdown 7 days after irradiation. Vascular shutdown occurred in 67% of vessels treated with oxymetazoline plus PDL at day 7 vs. 17% in those treated with saline plus PDL.
“This is fascinating,” Dr. Green said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “There is no publication I’m aware of in humans that has explored this timing, but I have used oxymetazoline in my clinic in patients with stubborn erythema and treated them with the vascular laser 5 minutes later.”
In a separate open-label study of 46 patients with moderate to severe facial erythema associated with rosacea, researchers found that oxymetazoline 1% as adjunctive therapy with energy-based therapy was safe and well tolerated, and reduced facial erythema in patients with moderate to severe persistent facial erythema associated with rosacea. Energy sources used were the PDL, KTP, or IPL.
In a study presented during the 2020 American Society for Laser Medicine & Surgery meeting, researchers led by Pooja Sodha, MD, of George Washington University, Washington, conducted a pilot trial of PDL plus oxymetazoline 1% cream for erythematotelangiectatic rosacea. Between baseline and 6 months’ follow-up the Clinician’s Erythema Assessment score fell from 4 to 2.
“Of note, I would also throw the kitchen sink at these patients medically, meaning I love topical ivermectin 1% cream,” Dr. Green said. “In some cases I’ll even use oral ivermectin and an oral tetracycline class antibiotic.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
EXPERT ANALYSIS FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Face masks can aggravate rosacea
The “maskne” phenomenon – that is, new onset or exacerbation of preexisting acne due to prolonged wearing of protective face masks – has become commonplace during the COVID-19 pandemic. Less well appreciated is that rosacea often markedly worsens, too, Giovanni Damiani, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This is particularly interesting because two inflammatory dermatoses with different pathogenesis are both mechanically and microbiologically triggered by mask use,” observed Dr. Damiani, a dermatologist at the University of Milan.
He presented . These patients – 23 with papulopustular and 13 with erythematotelangiectatic rosacea – were wearing face masks for at least 6 hours per day during quarantine. Most were using what Dr. Damiani termed “community masks,” meaning they weren’t approved by the European regulatory agency as personal protective equipment.
Every yardstick Dr. Damiani and coinvestigators employed to characterize the patients’ rosacea demonstrated that the dermatosis was significantly worse during the prolonged mask-wearing period. For example, the average prequarantine score on the Global Flushing Severity Scale was 2.56, jumping to 3.97 after a month of masked quarantine. The flushing score climbed from 1.83 to 2.78 in the subgroup with papulopustular rosacea, and from 3.85 to 6.08 in patients with erythematotelangiectatic rosacea. Scores on the Clinician’s Erythema Assessment rose from 1.09 to 1.7 in the papulopustular rosacea patients, and from 2.46 to 3.54 in those with erythematotelangiectatic rosacea.
Scores on the Dermatology Life Quality Index climbed from 7.35 prequarantine to 10.65 in the subgroup with papulopustular rosacea and from 5.15 to 8.69 in patients with erythematotelangiectatic rosacea. Investigator Global Assessment and Patient’s Self-Assessment scores also deteriorated significantly after a month in masked quarantine.
Clinically, the mask-aggravated rosacea, or “maskacea,” was mainly localized to the dorsal lower third of the nose as well as the cheeks. The ocular and perioral areas and the chin were least affected.
Dr. Damiani advised his colleagues to intensify therapy promptly when patients report any worsening of their preexisting rosacea in connection with use of face masks. He has found this condition is often relatively treatment resistant so long as affected patients continue to wear face masks as an essential tool in preventing transmission of COVID-19.
The dermatologist noted that not all face masks are equal offenders when it comes to aggravating common facial dermatoses. During the spring 2020 pandemic quarantine in Milan, 11.6% of 318 mask wearers, none health care professionals, presented to Dr. Damiani and coinvestigators for treatment of facial dermatoses. The facial dermatosis rate was 5.4% among 168 users of masks bearing the European Union CE mark signifying the devices met relevant safety and performance standards, compared with 18.7% in 150 users of community masks with no CE mark. The rate of irritant contact dermatitis was zero with the CE mark masks and 4.7% with the community masks.
During quarantine, however, these patients wore their protective face masks for only a limited time, since for the most part they were restricted to home. In contrast, during the first week after the quarantine was lifted in early May and the daily hours of mask use increased, facial dermatoses were diagnosed in 8.7% of 23 users of CE-approved masks, compared with 45% of 71 wearers of community masks. Dr. Damiani and colleagues diagnosed irritant contact dermatitis in 16% of the community mask wearers post quarantine, but in not a single user of a mask bearing the CE mark.
The National Rosacea Society has issued patient guidance on avoiding rosacea flare-ups during the Covid-19 pandemic.
Dr. Damiani reported having no financial conflicts regarding his study.
The “maskne” phenomenon – that is, new onset or exacerbation of preexisting acne due to prolonged wearing of protective face masks – has become commonplace during the COVID-19 pandemic. Less well appreciated is that rosacea often markedly worsens, too, Giovanni Damiani, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This is particularly interesting because two inflammatory dermatoses with different pathogenesis are both mechanically and microbiologically triggered by mask use,” observed Dr. Damiani, a dermatologist at the University of Milan.
He presented . These patients – 23 with papulopustular and 13 with erythematotelangiectatic rosacea – were wearing face masks for at least 6 hours per day during quarantine. Most were using what Dr. Damiani termed “community masks,” meaning they weren’t approved by the European regulatory agency as personal protective equipment.
Every yardstick Dr. Damiani and coinvestigators employed to characterize the patients’ rosacea demonstrated that the dermatosis was significantly worse during the prolonged mask-wearing period. For example, the average prequarantine score on the Global Flushing Severity Scale was 2.56, jumping to 3.97 after a month of masked quarantine. The flushing score climbed from 1.83 to 2.78 in the subgroup with papulopustular rosacea, and from 3.85 to 6.08 in patients with erythematotelangiectatic rosacea. Scores on the Clinician’s Erythema Assessment rose from 1.09 to 1.7 in the papulopustular rosacea patients, and from 2.46 to 3.54 in those with erythematotelangiectatic rosacea.
Scores on the Dermatology Life Quality Index climbed from 7.35 prequarantine to 10.65 in the subgroup with papulopustular rosacea and from 5.15 to 8.69 in patients with erythematotelangiectatic rosacea. Investigator Global Assessment and Patient’s Self-Assessment scores also deteriorated significantly after a month in masked quarantine.
Clinically, the mask-aggravated rosacea, or “maskacea,” was mainly localized to the dorsal lower third of the nose as well as the cheeks. The ocular and perioral areas and the chin were least affected.
Dr. Damiani advised his colleagues to intensify therapy promptly when patients report any worsening of their preexisting rosacea in connection with use of face masks. He has found this condition is often relatively treatment resistant so long as affected patients continue to wear face masks as an essential tool in preventing transmission of COVID-19.
The dermatologist noted that not all face masks are equal offenders when it comes to aggravating common facial dermatoses. During the spring 2020 pandemic quarantine in Milan, 11.6% of 318 mask wearers, none health care professionals, presented to Dr. Damiani and coinvestigators for treatment of facial dermatoses. The facial dermatosis rate was 5.4% among 168 users of masks bearing the European Union CE mark signifying the devices met relevant safety and performance standards, compared with 18.7% in 150 users of community masks with no CE mark. The rate of irritant contact dermatitis was zero with the CE mark masks and 4.7% with the community masks.
During quarantine, however, these patients wore their protective face masks for only a limited time, since for the most part they were restricted to home. In contrast, during the first week after the quarantine was lifted in early May and the daily hours of mask use increased, facial dermatoses were diagnosed in 8.7% of 23 users of CE-approved masks, compared with 45% of 71 wearers of community masks. Dr. Damiani and colleagues diagnosed irritant contact dermatitis in 16% of the community mask wearers post quarantine, but in not a single user of a mask bearing the CE mark.
The National Rosacea Society has issued patient guidance on avoiding rosacea flare-ups during the Covid-19 pandemic.
Dr. Damiani reported having no financial conflicts regarding his study.
The “maskne” phenomenon – that is, new onset or exacerbation of preexisting acne due to prolonged wearing of protective face masks – has become commonplace during the COVID-19 pandemic. Less well appreciated is that rosacea often markedly worsens, too, Giovanni Damiani, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
“This is particularly interesting because two inflammatory dermatoses with different pathogenesis are both mechanically and microbiologically triggered by mask use,” observed Dr. Damiani, a dermatologist at the University of Milan.
He presented . These patients – 23 with papulopustular and 13 with erythematotelangiectatic rosacea – were wearing face masks for at least 6 hours per day during quarantine. Most were using what Dr. Damiani termed “community masks,” meaning they weren’t approved by the European regulatory agency as personal protective equipment.
Every yardstick Dr. Damiani and coinvestigators employed to characterize the patients’ rosacea demonstrated that the dermatosis was significantly worse during the prolonged mask-wearing period. For example, the average prequarantine score on the Global Flushing Severity Scale was 2.56, jumping to 3.97 after a month of masked quarantine. The flushing score climbed from 1.83 to 2.78 in the subgroup with papulopustular rosacea, and from 3.85 to 6.08 in patients with erythematotelangiectatic rosacea. Scores on the Clinician’s Erythema Assessment rose from 1.09 to 1.7 in the papulopustular rosacea patients, and from 2.46 to 3.54 in those with erythematotelangiectatic rosacea.
Scores on the Dermatology Life Quality Index climbed from 7.35 prequarantine to 10.65 in the subgroup with papulopustular rosacea and from 5.15 to 8.69 in patients with erythematotelangiectatic rosacea. Investigator Global Assessment and Patient’s Self-Assessment scores also deteriorated significantly after a month in masked quarantine.
Clinically, the mask-aggravated rosacea, or “maskacea,” was mainly localized to the dorsal lower third of the nose as well as the cheeks. The ocular and perioral areas and the chin were least affected.
Dr. Damiani advised his colleagues to intensify therapy promptly when patients report any worsening of their preexisting rosacea in connection with use of face masks. He has found this condition is often relatively treatment resistant so long as affected patients continue to wear face masks as an essential tool in preventing transmission of COVID-19.
The dermatologist noted that not all face masks are equal offenders when it comes to aggravating common facial dermatoses. During the spring 2020 pandemic quarantine in Milan, 11.6% of 318 mask wearers, none health care professionals, presented to Dr. Damiani and coinvestigators for treatment of facial dermatoses. The facial dermatosis rate was 5.4% among 168 users of masks bearing the European Union CE mark signifying the devices met relevant safety and performance standards, compared with 18.7% in 150 users of community masks with no CE mark. The rate of irritant contact dermatitis was zero with the CE mark masks and 4.7% with the community masks.
During quarantine, however, these patients wore their protective face masks for only a limited time, since for the most part they were restricted to home. In contrast, during the first week after the quarantine was lifted in early May and the daily hours of mask use increased, facial dermatoses were diagnosed in 8.7% of 23 users of CE-approved masks, compared with 45% of 71 wearers of community masks. Dr. Damiani and colleagues diagnosed irritant contact dermatitis in 16% of the community mask wearers post quarantine, but in not a single user of a mask bearing the CE mark.
The National Rosacea Society has issued patient guidance on avoiding rosacea flare-ups during the Covid-19 pandemic.
Dr. Damiani reported having no financial conflicts regarding his study.
FROM THE EADV CONGRESS
Study results support screening rosacea patients for cardiometabolic disease
according to the results of a meta-analysis of more than 50,000 patients.
To date, “mounting comorbidities of rosacea have been identified, suggesting that rosacea is not simply a skin disease but has links to multiple systemic illnesses,” wrote Qi Chen, MD, of Central South University, Changsha, China, and colleagues. The association with rosacea and cardiometabolic disease has been controversial, they added.
In a study published in the Journal of the American Academy of Dermatology, they identified 13 studies including 50,442 rosacea patients and 1,525,864 controls. Approximately 71% of the rosacea patients were women.
Overall, patients with rosacea showed a statistically significant association for hypertension (risk ratio, 1.20; 95% confidence interval, 1.08-1.34; P = .001) and dyslipidemia (RR, 1.32; 95% CI, 1.10-1.58; P = .002). Specifically, rosacea patients averaged higher standard mean differences of systolic and diastolic blood pressure, total cholesterol, HDL cholesterol and LDL cholesterol, and triglycerides, compared with controls.
Rosacea was not significantly associated with an increased risk for ischemic heart disease, stroke, or diabetes, although the rosacea patients showed significantly increased risk of higher fasting blood glucose, compared with controls.
Findings don’t show causality
The study findings were limited by several factors, including the observational nature of some of the studies and the inability to perform subgroup analyses based on subtype and disease severity, the researchers noted. In addition, most of the rosacea patients were outpatients. “Further investigations are warranted to identify the relationship between rosacea and [cardiometabolic disease] in general populations to further validate the significance of our findings.”
However, the results support the value of screening for cardiometabolic disease in rosacea patients to facilitate diagnosis and treatment of disease at an early stage, they concluded.
“Rosacea has been linked statistically to many comorbidities including depression, anxiety, hypertension, and diabetes mellitus,” Julie Harper, MD, of the Dermatology and Skin Care Center of Birmingham (Alabama), said in an interview.
“This study looked more specifically at cardiometabolic disease and found a statistically significant correlation between rosacea and hypertension, higher total cholesterol, higher triglycerides and higher fasting blood glucose,” she said. However, “while there is an association present in this meta-analysis, we cannot assume a cause-and-effect relationship.”
Although the analysis does not prove causality, the key message for clinicians is that cardiometabolic disease is quite common in rosacea patients, and risk factors should be identified and treated early, said Dr. Harper. “Our patients with and without rosacea will benefit from age-appropriate screening, physical examination, and laboratory evaluation with a primary care physician. For rosacea patients in particular, we can advise them that early research suggests that individuals with rosacea might have an increased risk of hypertension and/or high cholesterol and triglycerides. It never hurts to make an appointment with primary care and to be checked.”
“We need more confirmatory studies that minimize the influence of confounding,” Dr. Harper added. Rosacea also has also been linked to obesity, which is another risk factor for cardiometabolic disease.
The study was supported by multiple grants from the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Harper had no relevant financial conflicts to disclose.
SOURCE: Chen Q et al. J Am Acad Dermatol. 2020 Nov;83(5):1331-40.
according to the results of a meta-analysis of more than 50,000 patients.
To date, “mounting comorbidities of rosacea have been identified, suggesting that rosacea is not simply a skin disease but has links to multiple systemic illnesses,” wrote Qi Chen, MD, of Central South University, Changsha, China, and colleagues. The association with rosacea and cardiometabolic disease has been controversial, they added.
In a study published in the Journal of the American Academy of Dermatology, they identified 13 studies including 50,442 rosacea patients and 1,525,864 controls. Approximately 71% of the rosacea patients were women.
Overall, patients with rosacea showed a statistically significant association for hypertension (risk ratio, 1.20; 95% confidence interval, 1.08-1.34; P = .001) and dyslipidemia (RR, 1.32; 95% CI, 1.10-1.58; P = .002). Specifically, rosacea patients averaged higher standard mean differences of systolic and diastolic blood pressure, total cholesterol, HDL cholesterol and LDL cholesterol, and triglycerides, compared with controls.
Rosacea was not significantly associated with an increased risk for ischemic heart disease, stroke, or diabetes, although the rosacea patients showed significantly increased risk of higher fasting blood glucose, compared with controls.
Findings don’t show causality
The study findings were limited by several factors, including the observational nature of some of the studies and the inability to perform subgroup analyses based on subtype and disease severity, the researchers noted. In addition, most of the rosacea patients were outpatients. “Further investigations are warranted to identify the relationship between rosacea and [cardiometabolic disease] in general populations to further validate the significance of our findings.”
However, the results support the value of screening for cardiometabolic disease in rosacea patients to facilitate diagnosis and treatment of disease at an early stage, they concluded.
“Rosacea has been linked statistically to many comorbidities including depression, anxiety, hypertension, and diabetes mellitus,” Julie Harper, MD, of the Dermatology and Skin Care Center of Birmingham (Alabama), said in an interview.
“This study looked more specifically at cardiometabolic disease and found a statistically significant correlation between rosacea and hypertension, higher total cholesterol, higher triglycerides and higher fasting blood glucose,” she said. However, “while there is an association present in this meta-analysis, we cannot assume a cause-and-effect relationship.”
Although the analysis does not prove causality, the key message for clinicians is that cardiometabolic disease is quite common in rosacea patients, and risk factors should be identified and treated early, said Dr. Harper. “Our patients with and without rosacea will benefit from age-appropriate screening, physical examination, and laboratory evaluation with a primary care physician. For rosacea patients in particular, we can advise them that early research suggests that individuals with rosacea might have an increased risk of hypertension and/or high cholesterol and triglycerides. It never hurts to make an appointment with primary care and to be checked.”
“We need more confirmatory studies that minimize the influence of confounding,” Dr. Harper added. Rosacea also has also been linked to obesity, which is another risk factor for cardiometabolic disease.
The study was supported by multiple grants from the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Harper had no relevant financial conflicts to disclose.
SOURCE: Chen Q et al. J Am Acad Dermatol. 2020 Nov;83(5):1331-40.
according to the results of a meta-analysis of more than 50,000 patients.
To date, “mounting comorbidities of rosacea have been identified, suggesting that rosacea is not simply a skin disease but has links to multiple systemic illnesses,” wrote Qi Chen, MD, of Central South University, Changsha, China, and colleagues. The association with rosacea and cardiometabolic disease has been controversial, they added.
In a study published in the Journal of the American Academy of Dermatology, they identified 13 studies including 50,442 rosacea patients and 1,525,864 controls. Approximately 71% of the rosacea patients were women.
Overall, patients with rosacea showed a statistically significant association for hypertension (risk ratio, 1.20; 95% confidence interval, 1.08-1.34; P = .001) and dyslipidemia (RR, 1.32; 95% CI, 1.10-1.58; P = .002). Specifically, rosacea patients averaged higher standard mean differences of systolic and diastolic blood pressure, total cholesterol, HDL cholesterol and LDL cholesterol, and triglycerides, compared with controls.
Rosacea was not significantly associated with an increased risk for ischemic heart disease, stroke, or diabetes, although the rosacea patients showed significantly increased risk of higher fasting blood glucose, compared with controls.
Findings don’t show causality
The study findings were limited by several factors, including the observational nature of some of the studies and the inability to perform subgroup analyses based on subtype and disease severity, the researchers noted. In addition, most of the rosacea patients were outpatients. “Further investigations are warranted to identify the relationship between rosacea and [cardiometabolic disease] in general populations to further validate the significance of our findings.”
However, the results support the value of screening for cardiometabolic disease in rosacea patients to facilitate diagnosis and treatment of disease at an early stage, they concluded.
“Rosacea has been linked statistically to many comorbidities including depression, anxiety, hypertension, and diabetes mellitus,” Julie Harper, MD, of the Dermatology and Skin Care Center of Birmingham (Alabama), said in an interview.
“This study looked more specifically at cardiometabolic disease and found a statistically significant correlation between rosacea and hypertension, higher total cholesterol, higher triglycerides and higher fasting blood glucose,” she said. However, “while there is an association present in this meta-analysis, we cannot assume a cause-and-effect relationship.”
Although the analysis does not prove causality, the key message for clinicians is that cardiometabolic disease is quite common in rosacea patients, and risk factors should be identified and treated early, said Dr. Harper. “Our patients with and without rosacea will benefit from age-appropriate screening, physical examination, and laboratory evaluation with a primary care physician. For rosacea patients in particular, we can advise them that early research suggests that individuals with rosacea might have an increased risk of hypertension and/or high cholesterol and triglycerides. It never hurts to make an appointment with primary care and to be checked.”
“We need more confirmatory studies that minimize the influence of confounding,” Dr. Harper added. Rosacea also has also been linked to obesity, which is another risk factor for cardiometabolic disease.
The study was supported by multiple grants from the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Harper had no relevant financial conflicts to disclose.
SOURCE: Chen Q et al. J Am Acad Dermatol. 2020 Nov;83(5):1331-40.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Moving from subtypes to phenotypes is simplifying management of rosacea
When a new phenotype approach to the diagnosis of rosacea was proposed 2 years ago, this simpler and more accurate method was accompanied by several corollary advantages, including a more rational approach to treatment and better methods of measuring treatment efficacy, according to an expert speaking at the annual Coastal Dermatology Symposium, held virtually.
“By looking at rosacea in a more simple way – but a more accurate way – we are able to track what happens [to key features] over time,” explained Jerry Tan, MD, of the University of Western Ontario, London.
With the previous method of subtyping, many rosacea patients failed to fit neatly into any of the four categories, producing confusion and diverting attention from troublesome symptoms.
“Rosacea patients often present with a range of features that span multiple subtypes or progress between them,” Dr. Tan explained. The risk is not just a delay in diagnosis but a failure to focus on symptoms patients find most bothersome.
The previous diagnostic criteria for rosacea, published in 2002, identified primary and secondary symptoms within its four subtypes. The new diagnostic criteria, endorsed by the National Rosacea Society and published in 2018, rely on phenotypes defined by diagnostic, major, and minor symptoms. Rather than the four previous subtypes, which were erythematotelangiectatic, papulopustular, phymatous, and ocular, the phenotypes facilitate diagnosis in patients with mixed features.
By replacing “the old thought process of subtyping” with a newer focus on phenotypes, the updated criteria were “aimed toward accuracy, simplicity and practicality,” Dr. Tan said.
Moreover, without squeezing patients into subgroups where they do not neatly fit, the new criteria draw attention to the specific symptoms that bring patients to the clinician.
The phenotype approach to treatment strategies was reflected in a systematic review of treatments based on phenotypes that was published in 2019, not long after the new classification system became available. In this review, coauthored by Dr. Tan, the GRADE certainty-of-evidence approach was employed to identify effective therapies, matching specific symptoms with specific therapies such as low-dose isotretinoin for papules or omega-3 fatty acids for dry eyes.
Based on a patient-centric approach that emphasizes control of key symptoms, Dr. Tan also described a method of documenting the severity of major and minor symptoms at each visit. With this method, called a rosacea patient tracker, patients and physicians can determine whether therapies are effective against the signs and symptoms of disease that they find most burdensome, according to Dr. Tan, who was the first author of an article he cited as a reference to this phenotype-based methodology.
Overall, the phenotype approach to rosacea “rationalizes treatment,” he said.
Specifically, the heterogeneity of symptoms in rosacea is mirrored in the heterogeneity of underlying pathophysiology. According to Dr. Tan, the upregulation of cytokines for inflammation, of angiogenic pathways for vascular symptoms, and of matrix metalloproteinases for tissue remodeling are all implicated in rosacea but drive different types of symptoms. While appropriate skin care and efforts to identify and minimize symptom triggers is appropriate for all patients, phenotypes provide a guide to the most appropriate therapies.
He said he hopes that the focus on phenotypes will draw attention to differences in these pathophysiological mechanisms. According to Dr. Tan, evaluating rosacea from the perspective of phenotypes has represented an important paradigm shift that extends beyond diagnosis.
“The move to the phenotype approach is hopefully simpler, more accurate, and more relevant,” Dr. Tan said.
This same approach has been advocated by others, including Esther J. van Zurren, MD, professor of dermatology at Leiden University Medical Centre in the Netherlands, the lead author of the 2018 systematic review article discussed by Dr. Tan. In this review article on the phenotype approach, specific strategies were recommended for specific symptoms on the basis of grading by an international group of experts that included Dr. Tan, a coauthor.
“These strategies should be directed toward achieving improvements in general well-being by targeting those aspects most bothersome to the patient,” the article advises. Like Dr. Tan, she considers this phenotype-based approach to diagnosis and treatment to be a meaningful clinical advance over the guidelines published in 2002.
“Management strategies for people with rosacea should include phenotype-based treatments,” she agreed, adding that specific choices should be made on the basis of these phenotypes “instead of the previous subtype classification.”
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
When a new phenotype approach to the diagnosis of rosacea was proposed 2 years ago, this simpler and more accurate method was accompanied by several corollary advantages, including a more rational approach to treatment and better methods of measuring treatment efficacy, according to an expert speaking at the annual Coastal Dermatology Symposium, held virtually.
“By looking at rosacea in a more simple way – but a more accurate way – we are able to track what happens [to key features] over time,” explained Jerry Tan, MD, of the University of Western Ontario, London.
With the previous method of subtyping, many rosacea patients failed to fit neatly into any of the four categories, producing confusion and diverting attention from troublesome symptoms.
“Rosacea patients often present with a range of features that span multiple subtypes or progress between them,” Dr. Tan explained. The risk is not just a delay in diagnosis but a failure to focus on symptoms patients find most bothersome.
The previous diagnostic criteria for rosacea, published in 2002, identified primary and secondary symptoms within its four subtypes. The new diagnostic criteria, endorsed by the National Rosacea Society and published in 2018, rely on phenotypes defined by diagnostic, major, and minor symptoms. Rather than the four previous subtypes, which were erythematotelangiectatic, papulopustular, phymatous, and ocular, the phenotypes facilitate diagnosis in patients with mixed features.
By replacing “the old thought process of subtyping” with a newer focus on phenotypes, the updated criteria were “aimed toward accuracy, simplicity and practicality,” Dr. Tan said.
Moreover, without squeezing patients into subgroups where they do not neatly fit, the new criteria draw attention to the specific symptoms that bring patients to the clinician.
The phenotype approach to treatment strategies was reflected in a systematic review of treatments based on phenotypes that was published in 2019, not long after the new classification system became available. In this review, coauthored by Dr. Tan, the GRADE certainty-of-evidence approach was employed to identify effective therapies, matching specific symptoms with specific therapies such as low-dose isotretinoin for papules or omega-3 fatty acids for dry eyes.
Based on a patient-centric approach that emphasizes control of key symptoms, Dr. Tan also described a method of documenting the severity of major and minor symptoms at each visit. With this method, called a rosacea patient tracker, patients and physicians can determine whether therapies are effective against the signs and symptoms of disease that they find most burdensome, according to Dr. Tan, who was the first author of an article he cited as a reference to this phenotype-based methodology.
Overall, the phenotype approach to rosacea “rationalizes treatment,” he said.
Specifically, the heterogeneity of symptoms in rosacea is mirrored in the heterogeneity of underlying pathophysiology. According to Dr. Tan, the upregulation of cytokines for inflammation, of angiogenic pathways for vascular symptoms, and of matrix metalloproteinases for tissue remodeling are all implicated in rosacea but drive different types of symptoms. While appropriate skin care and efforts to identify and minimize symptom triggers is appropriate for all patients, phenotypes provide a guide to the most appropriate therapies.
He said he hopes that the focus on phenotypes will draw attention to differences in these pathophysiological mechanisms. According to Dr. Tan, evaluating rosacea from the perspective of phenotypes has represented an important paradigm shift that extends beyond diagnosis.
“The move to the phenotype approach is hopefully simpler, more accurate, and more relevant,” Dr. Tan said.
This same approach has been advocated by others, including Esther J. van Zurren, MD, professor of dermatology at Leiden University Medical Centre in the Netherlands, the lead author of the 2018 systematic review article discussed by Dr. Tan. In this review article on the phenotype approach, specific strategies were recommended for specific symptoms on the basis of grading by an international group of experts that included Dr. Tan, a coauthor.
“These strategies should be directed toward achieving improvements in general well-being by targeting those aspects most bothersome to the patient,” the article advises. Like Dr. Tan, she considers this phenotype-based approach to diagnosis and treatment to be a meaningful clinical advance over the guidelines published in 2002.
“Management strategies for people with rosacea should include phenotype-based treatments,” she agreed, adding that specific choices should be made on the basis of these phenotypes “instead of the previous subtype classification.”
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
When a new phenotype approach to the diagnosis of rosacea was proposed 2 years ago, this simpler and more accurate method was accompanied by several corollary advantages, including a more rational approach to treatment and better methods of measuring treatment efficacy, according to an expert speaking at the annual Coastal Dermatology Symposium, held virtually.
“By looking at rosacea in a more simple way – but a more accurate way – we are able to track what happens [to key features] over time,” explained Jerry Tan, MD, of the University of Western Ontario, London.
With the previous method of subtyping, many rosacea patients failed to fit neatly into any of the four categories, producing confusion and diverting attention from troublesome symptoms.
“Rosacea patients often present with a range of features that span multiple subtypes or progress between them,” Dr. Tan explained. The risk is not just a delay in diagnosis but a failure to focus on symptoms patients find most bothersome.
The previous diagnostic criteria for rosacea, published in 2002, identified primary and secondary symptoms within its four subtypes. The new diagnostic criteria, endorsed by the National Rosacea Society and published in 2018, rely on phenotypes defined by diagnostic, major, and minor symptoms. Rather than the four previous subtypes, which were erythematotelangiectatic, papulopustular, phymatous, and ocular, the phenotypes facilitate diagnosis in patients with mixed features.
By replacing “the old thought process of subtyping” with a newer focus on phenotypes, the updated criteria were “aimed toward accuracy, simplicity and practicality,” Dr. Tan said.
Moreover, without squeezing patients into subgroups where they do not neatly fit, the new criteria draw attention to the specific symptoms that bring patients to the clinician.
The phenotype approach to treatment strategies was reflected in a systematic review of treatments based on phenotypes that was published in 2019, not long after the new classification system became available. In this review, coauthored by Dr. Tan, the GRADE certainty-of-evidence approach was employed to identify effective therapies, matching specific symptoms with specific therapies such as low-dose isotretinoin for papules or omega-3 fatty acids for dry eyes.
Based on a patient-centric approach that emphasizes control of key symptoms, Dr. Tan also described a method of documenting the severity of major and minor symptoms at each visit. With this method, called a rosacea patient tracker, patients and physicians can determine whether therapies are effective against the signs and symptoms of disease that they find most burdensome, according to Dr. Tan, who was the first author of an article he cited as a reference to this phenotype-based methodology.
Overall, the phenotype approach to rosacea “rationalizes treatment,” he said.
Specifically, the heterogeneity of symptoms in rosacea is mirrored in the heterogeneity of underlying pathophysiology. According to Dr. Tan, the upregulation of cytokines for inflammation, of angiogenic pathways for vascular symptoms, and of matrix metalloproteinases for tissue remodeling are all implicated in rosacea but drive different types of symptoms. While appropriate skin care and efforts to identify and minimize symptom triggers is appropriate for all patients, phenotypes provide a guide to the most appropriate therapies.
He said he hopes that the focus on phenotypes will draw attention to differences in these pathophysiological mechanisms. According to Dr. Tan, evaluating rosacea from the perspective of phenotypes has represented an important paradigm shift that extends beyond diagnosis.
“The move to the phenotype approach is hopefully simpler, more accurate, and more relevant,” Dr. Tan said.
This same approach has been advocated by others, including Esther J. van Zurren, MD, professor of dermatology at Leiden University Medical Centre in the Netherlands, the lead author of the 2018 systematic review article discussed by Dr. Tan. In this review article on the phenotype approach, specific strategies were recommended for specific symptoms on the basis of grading by an international group of experts that included Dr. Tan, a coauthor.
“These strategies should be directed toward achieving improvements in general well-being by targeting those aspects most bothersome to the patient,” the article advises. Like Dr. Tan, she considers this phenotype-based approach to diagnosis and treatment to be a meaningful clinical advance over the guidelines published in 2002.
“Management strategies for people with rosacea should include phenotype-based treatments,” she agreed, adding that specific choices should be made on the basis of these phenotypes “instead of the previous subtype classification.”
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are owned by the same parent company.
FROM COASTAL DERM
Role of lasers and light sources in medicine continue to expand
suggests R. Rox Anderson, MD.
“I’ve been doing this in my practice for a number of years and it’s quite gratifying,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “You treat the periorbital skin, mostly under the eye, just as if you were treating telangiectasia rosacea. The meibomian glands under the upper eyelid that cause this disease are sebaceous glands, and most of the people with dry eye have rosacea.”
In a retrospective noncomparative, interventional case series, 78 patients with severe dry eye syndrome were treated with intense pulsed-light therapy and gland expression at a single outpatient clinic over 30 months. Physician-judged improvement in dry eye tear breakup time was found for 87% of patients with an average of seven treatment visits and four maintenance visits, while 93% of patients reported posttreatment satisfaction with the degree of dry eye syndrome symptoms. More information about the approach were published in Investigative Ophthalmology & Visual Science and Current Opinion in Ophthalmology.
“What’s gratifying here is that most patients will get about 2 months of relief after a single treatment,” Dr. Anderson said. “They are very happy – some of the happiest patients in my practice. Many ophthalmologists don’t have the technology, so I think you can do this depending on your local referral system.”
Light-based approaches are also making promising inroads in cancer treatment. A recent study led by Martin Purschke, PhD, at the Wellman Center evaluated the use of a novel radio-phototherapy approach for killing cancer cells. The center of solid tissue tumors that are treated with radiotherapy is hypoxic, Dr. Anderson explained, “and oxygen is typically located around the perimeter of the tumor. After a radiation therapy treatment, you kill only the outer portion of it, and then the remaining cells grow back, and you end up with the same tumor. This is why you have to do radiation therapy over and over again. In contrast, if you add scintillating nanoparticles, which are particles with a very high C number atoms in them that pick up the x-ray photon and then emit many UV photons from one x-ray photon, they are very efficient at converting x-ray energy to UV energy.” The x-ray, he added, “generates UV light, and the UV light kills the tumor. We’re hoping that we can make a dent in radiotherapy this way.”
Dr. Anderson predicted that fiber lasers, which are highly advanced for industrial applications, will play an increasing role in dermatology and in other areas of medicine. “There are not a new kid on the block anymore but fiber lasers are relatively new to medicine,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “We are seeing incredible capabilities with fiber lasers: essentially any wavelength, any power, any pulse duration you want. The lasers are efficient, small, rugged, and their lifetime exceeds your lifetime. They are likely to displace many of our old lasers in dermatology. I don’t know when, but I know it will happen.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
suggests R. Rox Anderson, MD.
“I’ve been doing this in my practice for a number of years and it’s quite gratifying,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “You treat the periorbital skin, mostly under the eye, just as if you were treating telangiectasia rosacea. The meibomian glands under the upper eyelid that cause this disease are sebaceous glands, and most of the people with dry eye have rosacea.”
In a retrospective noncomparative, interventional case series, 78 patients with severe dry eye syndrome were treated with intense pulsed-light therapy and gland expression at a single outpatient clinic over 30 months. Physician-judged improvement in dry eye tear breakup time was found for 87% of patients with an average of seven treatment visits and four maintenance visits, while 93% of patients reported posttreatment satisfaction with the degree of dry eye syndrome symptoms. More information about the approach were published in Investigative Ophthalmology & Visual Science and Current Opinion in Ophthalmology.
“What’s gratifying here is that most patients will get about 2 months of relief after a single treatment,” Dr. Anderson said. “They are very happy – some of the happiest patients in my practice. Many ophthalmologists don’t have the technology, so I think you can do this depending on your local referral system.”
Light-based approaches are also making promising inroads in cancer treatment. A recent study led by Martin Purschke, PhD, at the Wellman Center evaluated the use of a novel radio-phototherapy approach for killing cancer cells. The center of solid tissue tumors that are treated with radiotherapy is hypoxic, Dr. Anderson explained, “and oxygen is typically located around the perimeter of the tumor. After a radiation therapy treatment, you kill only the outer portion of it, and then the remaining cells grow back, and you end up with the same tumor. This is why you have to do radiation therapy over and over again. In contrast, if you add scintillating nanoparticles, which are particles with a very high C number atoms in them that pick up the x-ray photon and then emit many UV photons from one x-ray photon, they are very efficient at converting x-ray energy to UV energy.” The x-ray, he added, “generates UV light, and the UV light kills the tumor. We’re hoping that we can make a dent in radiotherapy this way.”
Dr. Anderson predicted that fiber lasers, which are highly advanced for industrial applications, will play an increasing role in dermatology and in other areas of medicine. “There are not a new kid on the block anymore but fiber lasers are relatively new to medicine,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “We are seeing incredible capabilities with fiber lasers: essentially any wavelength, any power, any pulse duration you want. The lasers are efficient, small, rugged, and their lifetime exceeds your lifetime. They are likely to displace many of our old lasers in dermatology. I don’t know when, but I know it will happen.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
suggests R. Rox Anderson, MD.
“I’ve been doing this in my practice for a number of years and it’s quite gratifying,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “You treat the periorbital skin, mostly under the eye, just as if you were treating telangiectasia rosacea. The meibomian glands under the upper eyelid that cause this disease are sebaceous glands, and most of the people with dry eye have rosacea.”
In a retrospective noncomparative, interventional case series, 78 patients with severe dry eye syndrome were treated with intense pulsed-light therapy and gland expression at a single outpatient clinic over 30 months. Physician-judged improvement in dry eye tear breakup time was found for 87% of patients with an average of seven treatment visits and four maintenance visits, while 93% of patients reported posttreatment satisfaction with the degree of dry eye syndrome symptoms. More information about the approach were published in Investigative Ophthalmology & Visual Science and Current Opinion in Ophthalmology.
“What’s gratifying here is that most patients will get about 2 months of relief after a single treatment,” Dr. Anderson said. “They are very happy – some of the happiest patients in my practice. Many ophthalmologists don’t have the technology, so I think you can do this depending on your local referral system.”
Light-based approaches are also making promising inroads in cancer treatment. A recent study led by Martin Purschke, PhD, at the Wellman Center evaluated the use of a novel radio-phototherapy approach for killing cancer cells. The center of solid tissue tumors that are treated with radiotherapy is hypoxic, Dr. Anderson explained, “and oxygen is typically located around the perimeter of the tumor. After a radiation therapy treatment, you kill only the outer portion of it, and then the remaining cells grow back, and you end up with the same tumor. This is why you have to do radiation therapy over and over again. In contrast, if you add scintillating nanoparticles, which are particles with a very high C number atoms in them that pick up the x-ray photon and then emit many UV photons from one x-ray photon, they are very efficient at converting x-ray energy to UV energy.” The x-ray, he added, “generates UV light, and the UV light kills the tumor. We’re hoping that we can make a dent in radiotherapy this way.”
Dr. Anderson predicted that fiber lasers, which are highly advanced for industrial applications, will play an increasing role in dermatology and in other areas of medicine. “There are not a new kid on the block anymore but fiber lasers are relatively new to medicine,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “We are seeing incredible capabilities with fiber lasers: essentially any wavelength, any power, any pulse duration you want. The lasers are efficient, small, rugged, and their lifetime exceeds your lifetime. They are likely to displace many of our old lasers in dermatology. I don’t know when, but I know it will happen.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE












