User login
Expert makes the case for not subtyping patients with rosacea
. At least they should be, according to Julie C. Harper, MD.
“How many people with papules and pustules don’t also have redness?” Dr. Harper, who practices in Birmingham, Ala., said at Medscape Live’s annual Coastal Dermatology Symposium. “If we’re not careful, and we try to classify a person into a subtype of rosacea, we end up treating only part of their rosacea; we don’t treat all of it. We have seen this in the literature,” she added.
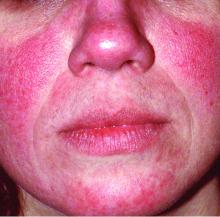
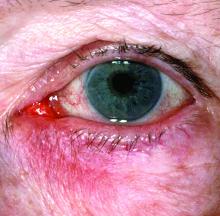
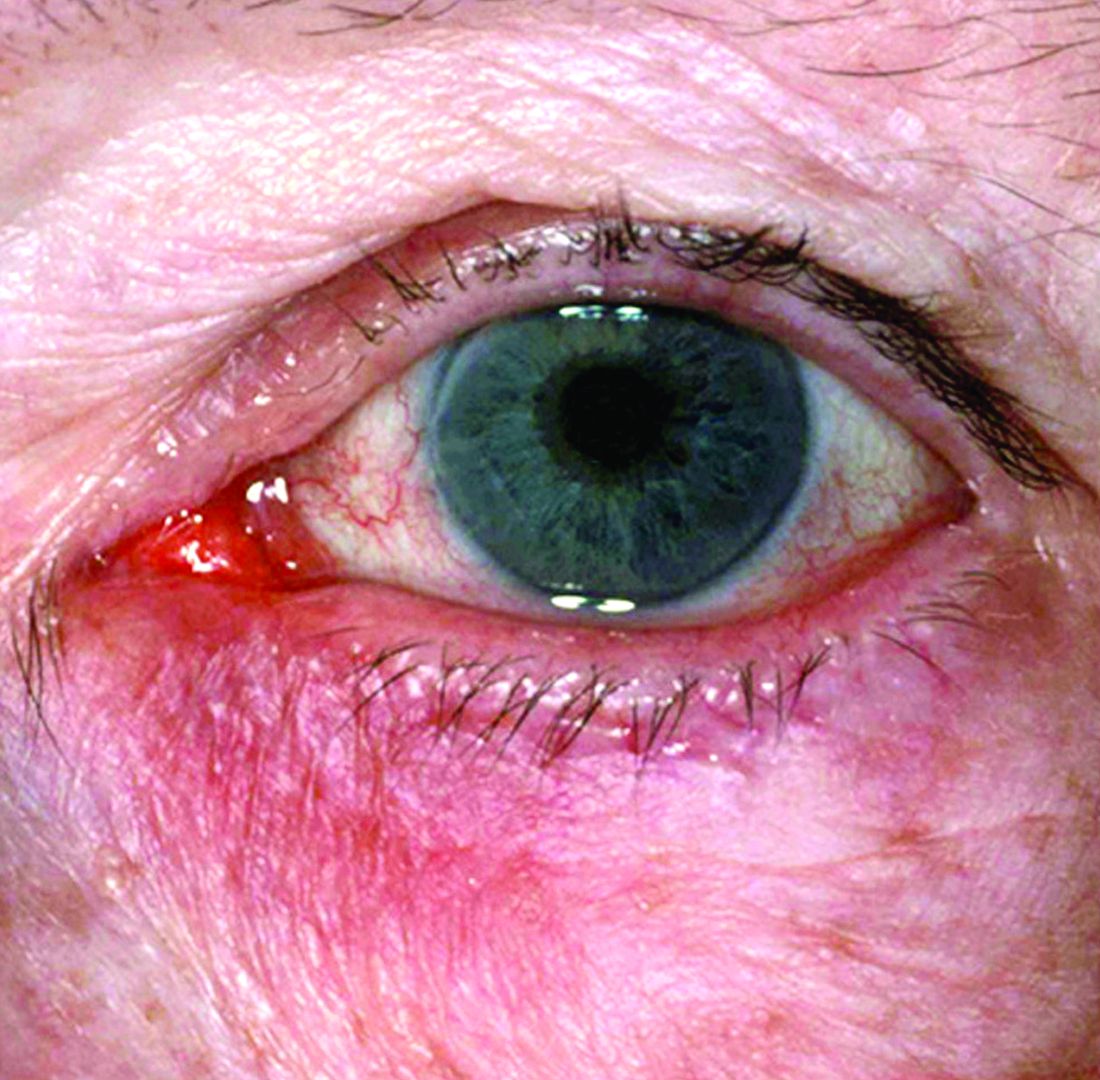
“The idea now is to take a phenotypic approach to rosacea. What we mean by that is that you look at the patient, you document every part of rosacea that you see, and you treat according to that,” she continued. “That person with papules and pustules may also have phyma and ocular disease. They may have telangiectasia and persistent background erythema. They may also have flushing.”
Dr. Harper incorporates the mnemonic “STOP” to her visits with rosacea patients.
S stands for: Identify signs and symptoms of the condition. “Listen to the patient for symptoms,” she advised. “We’ve learned to listen to darker skinned patients for what they tell us about erythema, for example, because we may not be able to see it, yet they are experiencing it. They may also have symptomatic burning, itching, and stinging.”
T stands for: Discuss triggers. “Ask patients, ‘what is it that makes your rosacea worse?’ That’s different for everyone,” she said.
O stands for: Agree on a treatment outcome. “Ask, ‘what is it that really bothers you? Are you bothered by the bumps? The redness?’ ” she said.
“The P stands for: Develop a plan that addresses all of that,” she said.
Different treatments for different rosacea symptoms
No one-size-fits-all treatment exists for rosacea. Options that work well for papules and pustules aren’t effective for redness. Similarly, products that work for redness don’t work for telangiectasia.
“Different lesions and signs of rosacea will likely require multiple modes of treatment,” Dr. Harper said. “So, when you evaluate your rosacea patients, if they’re doing great, don’t change their regimen. But if you see somebody who is not well controlled, is there an opportunity for you to come in and add something to that regimen that may make them better? Maybe so.”
Treatment options indicated for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and encapsulated benzoyl peroxide.
Options indicated for persistent background erythema include brimonidine and oxymetazoline, while device-based treatments include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Anti-inflammatory action for pustules and papules
A relatively new product indicated for pustules and papules is minocycline 1.5% foam, the only minocycline that is FDA approved to treat rosacea.
“There is no oral minocycline product approved for rosacea yet,” Dr. Harper said. “There is not a known bacterial pathogen in rosacea. Tetracyclines likely work in rosacea by inhibiting neutrophil chemotaxis, inhibiting MMP and thus KLK-5 and LL-37, inhibiting pro-inflammatory cytokines, downregulating reactive oxygen species, and inhibiting angiogenesis.”
In two 12-week, phase 3 randomized studies of 1,522 patients with moderate to severe rosacea, participants were assigned to receive minocycline 5% foam or a vehicle that contained mineral oil and coconut oil.
At week 12, about 50% of patients who received minocycline 5% foam were clear, compared with about 40% of those in the vehicle arm. Also, the reduction of lesion count was about 63% for patients in the treatment group, compared with a reduction of about 54% in the vehicle arm.
Dr. Harper characterized the 63% reduction as “pretty good, but is it good enough or fast enough? I don’t think so, so even with a great drug like this, I would use something else. You can use two medications sometimes to get people better faster. There’s room to bring in something for that background erythema.”
Minocycline 1.5% foam is colored yellow and may stain fabric. “It contains coconut oil, soybean oil, and light mineral oil,” she said. “Most people prefer to use this at bedtime, but you don’t have to.”
Another treatment option is 5% microencapsulated benzoyl peroxide cream, which is FDA approved for inflammatory lesions of rosacea.
“What’s the mechanism of action? Probably not being antimicrobial,” Dr. Harper said. “I think it’s probably at least in part anti-inflammatory, because we have some data to show that it’s killing Demodex [mites]. If Demodex [are] a trigger of inflammation, and we can lessen Demodex, then we could lessen the inflammatory response after that.”
The drug’s approval was based on data from two positive, identical phase 3 randomized, double-blind, multicenter, 12-week clinical trials that evaluated its safety compared with vehicle in 733 people with inflammatory lesions of rosacea (NCT03564119 and NCT03448939).
At week 12, inflammatory lesions of rosacea were reduced by nearly 70% in both trials among those who received 5% microencapsulated benzoyl peroxide cream, compared with 38%-46% among those who received the vehicle. Also, nearly 50% of subjects in the treatment groups were clear or almost clear at 12 weeks, compared with 38%-46% of those who received the vehicle.
Dr. Harper added that about one-quarter of patients in the treatment group of the trials were clear or almost clear by week 4. “That’s pretty fast,” she said, noting that the product’s microencapsulated shell acts as a fenestrated barrier. “It has little openings, which means that it takes a while for the drug to work itself out,” she said. “I think of it as being like a speed bump for benzoyl peroxide delivery. It has to get through this little maze before it lands on the skin. We think that is what has helped with tolerability.”
Oral sarecycline, a narrow spectrum tetracycline that was FDA approved for acne in 2018, may also benefit rosacea patients. In a 12-week, investigator-blinded pilot study, 72 patients with papulopustular rosacea were assigned to receive sarecycline, while 25 received a multivitamin.
By week 12, 75% of patients in the sarecycline group were clear, compared with 16% of those in the multivitamin group, while the inflammatory lesion counts dropped from baseline by 80% and 60%, respectively. Studies of sarecycline for acne have demonstrated similar rates of vertigo, dizziness, and sunburn to those of placebo.
“There were also low rates of gastrointestinal disturbances,” Dr. Harper said. “That’s important in rosacea, because there is no bacterial pathogen.”
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speakers bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
. At least they should be, according to Julie C. Harper, MD.
“How many people with papules and pustules don’t also have redness?” Dr. Harper, who practices in Birmingham, Ala., said at Medscape Live’s annual Coastal Dermatology Symposium. “If we’re not careful, and we try to classify a person into a subtype of rosacea, we end up treating only part of their rosacea; we don’t treat all of it. We have seen this in the literature,” she added.
“The idea now is to take a phenotypic approach to rosacea. What we mean by that is that you look at the patient, you document every part of rosacea that you see, and you treat according to that,” she continued. “That person with papules and pustules may also have phyma and ocular disease. They may have telangiectasia and persistent background erythema. They may also have flushing.”
Dr. Harper incorporates the mnemonic “STOP” to her visits with rosacea patients.
S stands for: Identify signs and symptoms of the condition. “Listen to the patient for symptoms,” she advised. “We’ve learned to listen to darker skinned patients for what they tell us about erythema, for example, because we may not be able to see it, yet they are experiencing it. They may also have symptomatic burning, itching, and stinging.”
T stands for: Discuss triggers. “Ask patients, ‘what is it that makes your rosacea worse?’ That’s different for everyone,” she said.
O stands for: Agree on a treatment outcome. “Ask, ‘what is it that really bothers you? Are you bothered by the bumps? The redness?’ ” she said.
“The P stands for: Develop a plan that addresses all of that,” she said.
Different treatments for different rosacea symptoms
No one-size-fits-all treatment exists for rosacea. Options that work well for papules and pustules aren’t effective for redness. Similarly, products that work for redness don’t work for telangiectasia.
“Different lesions and signs of rosacea will likely require multiple modes of treatment,” Dr. Harper said. “So, when you evaluate your rosacea patients, if they’re doing great, don’t change their regimen. But if you see somebody who is not well controlled, is there an opportunity for you to come in and add something to that regimen that may make them better? Maybe so.”
Treatment options indicated for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and encapsulated benzoyl peroxide.
Options indicated for persistent background erythema include brimonidine and oxymetazoline, while device-based treatments include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Anti-inflammatory action for pustules and papules
A relatively new product indicated for pustules and papules is minocycline 1.5% foam, the only minocycline that is FDA approved to treat rosacea.
“There is no oral minocycline product approved for rosacea yet,” Dr. Harper said. “There is not a known bacterial pathogen in rosacea. Tetracyclines likely work in rosacea by inhibiting neutrophil chemotaxis, inhibiting MMP and thus KLK-5 and LL-37, inhibiting pro-inflammatory cytokines, downregulating reactive oxygen species, and inhibiting angiogenesis.”
In two 12-week, phase 3 randomized studies of 1,522 patients with moderate to severe rosacea, participants were assigned to receive minocycline 5% foam or a vehicle that contained mineral oil and coconut oil.
At week 12, about 50% of patients who received minocycline 5% foam were clear, compared with about 40% of those in the vehicle arm. Also, the reduction of lesion count was about 63% for patients in the treatment group, compared with a reduction of about 54% in the vehicle arm.
Dr. Harper characterized the 63% reduction as “pretty good, but is it good enough or fast enough? I don’t think so, so even with a great drug like this, I would use something else. You can use two medications sometimes to get people better faster. There’s room to bring in something for that background erythema.”
Minocycline 1.5% foam is colored yellow and may stain fabric. “It contains coconut oil, soybean oil, and light mineral oil,” she said. “Most people prefer to use this at bedtime, but you don’t have to.”
Another treatment option is 5% microencapsulated benzoyl peroxide cream, which is FDA approved for inflammatory lesions of rosacea.
“What’s the mechanism of action? Probably not being antimicrobial,” Dr. Harper said. “I think it’s probably at least in part anti-inflammatory, because we have some data to show that it’s killing Demodex [mites]. If Demodex [are] a trigger of inflammation, and we can lessen Demodex, then we could lessen the inflammatory response after that.”
The drug’s approval was based on data from two positive, identical phase 3 randomized, double-blind, multicenter, 12-week clinical trials that evaluated its safety compared with vehicle in 733 people with inflammatory lesions of rosacea (NCT03564119 and NCT03448939).
At week 12, inflammatory lesions of rosacea were reduced by nearly 70% in both trials among those who received 5% microencapsulated benzoyl peroxide cream, compared with 38%-46% among those who received the vehicle. Also, nearly 50% of subjects in the treatment groups were clear or almost clear at 12 weeks, compared with 38%-46% of those who received the vehicle.
Dr. Harper added that about one-quarter of patients in the treatment group of the trials were clear or almost clear by week 4. “That’s pretty fast,” she said, noting that the product’s microencapsulated shell acts as a fenestrated barrier. “It has little openings, which means that it takes a while for the drug to work itself out,” she said. “I think of it as being like a speed bump for benzoyl peroxide delivery. It has to get through this little maze before it lands on the skin. We think that is what has helped with tolerability.”
Oral sarecycline, a narrow spectrum tetracycline that was FDA approved for acne in 2018, may also benefit rosacea patients. In a 12-week, investigator-blinded pilot study, 72 patients with papulopustular rosacea were assigned to receive sarecycline, while 25 received a multivitamin.
By week 12, 75% of patients in the sarecycline group were clear, compared with 16% of those in the multivitamin group, while the inflammatory lesion counts dropped from baseline by 80% and 60%, respectively. Studies of sarecycline for acne have demonstrated similar rates of vertigo, dizziness, and sunburn to those of placebo.
“There were also low rates of gastrointestinal disturbances,” Dr. Harper said. “That’s important in rosacea, because there is no bacterial pathogen.”
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speakers bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
. At least they should be, according to Julie C. Harper, MD.
“How many people with papules and pustules don’t also have redness?” Dr. Harper, who practices in Birmingham, Ala., said at Medscape Live’s annual Coastal Dermatology Symposium. “If we’re not careful, and we try to classify a person into a subtype of rosacea, we end up treating only part of their rosacea; we don’t treat all of it. We have seen this in the literature,” she added.
“The idea now is to take a phenotypic approach to rosacea. What we mean by that is that you look at the patient, you document every part of rosacea that you see, and you treat according to that,” she continued. “That person with papules and pustules may also have phyma and ocular disease. They may have telangiectasia and persistent background erythema. They may also have flushing.”
Dr. Harper incorporates the mnemonic “STOP” to her visits with rosacea patients.
S stands for: Identify signs and symptoms of the condition. “Listen to the patient for symptoms,” she advised. “We’ve learned to listen to darker skinned patients for what they tell us about erythema, for example, because we may not be able to see it, yet they are experiencing it. They may also have symptomatic burning, itching, and stinging.”
T stands for: Discuss triggers. “Ask patients, ‘what is it that makes your rosacea worse?’ That’s different for everyone,” she said.
O stands for: Agree on a treatment outcome. “Ask, ‘what is it that really bothers you? Are you bothered by the bumps? The redness?’ ” she said.
“The P stands for: Develop a plan that addresses all of that,” she said.
Different treatments for different rosacea symptoms
No one-size-fits-all treatment exists for rosacea. Options that work well for papules and pustules aren’t effective for redness. Similarly, products that work for redness don’t work for telangiectasia.
“Different lesions and signs of rosacea will likely require multiple modes of treatment,” Dr. Harper said. “So, when you evaluate your rosacea patients, if they’re doing great, don’t change their regimen. But if you see somebody who is not well controlled, is there an opportunity for you to come in and add something to that regimen that may make them better? Maybe so.”
Treatment options indicated for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and encapsulated benzoyl peroxide.
Options indicated for persistent background erythema include brimonidine and oxymetazoline, while device-based treatments include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Anti-inflammatory action for pustules and papules
A relatively new product indicated for pustules and papules is minocycline 1.5% foam, the only minocycline that is FDA approved to treat rosacea.
“There is no oral minocycline product approved for rosacea yet,” Dr. Harper said. “There is not a known bacterial pathogen in rosacea. Tetracyclines likely work in rosacea by inhibiting neutrophil chemotaxis, inhibiting MMP and thus KLK-5 and LL-37, inhibiting pro-inflammatory cytokines, downregulating reactive oxygen species, and inhibiting angiogenesis.”
In two 12-week, phase 3 randomized studies of 1,522 patients with moderate to severe rosacea, participants were assigned to receive minocycline 5% foam or a vehicle that contained mineral oil and coconut oil.
At week 12, about 50% of patients who received minocycline 5% foam were clear, compared with about 40% of those in the vehicle arm. Also, the reduction of lesion count was about 63% for patients in the treatment group, compared with a reduction of about 54% in the vehicle arm.
Dr. Harper characterized the 63% reduction as “pretty good, but is it good enough or fast enough? I don’t think so, so even with a great drug like this, I would use something else. You can use two medications sometimes to get people better faster. There’s room to bring in something for that background erythema.”
Minocycline 1.5% foam is colored yellow and may stain fabric. “It contains coconut oil, soybean oil, and light mineral oil,” she said. “Most people prefer to use this at bedtime, but you don’t have to.”
Another treatment option is 5% microencapsulated benzoyl peroxide cream, which is FDA approved for inflammatory lesions of rosacea.
“What’s the mechanism of action? Probably not being antimicrobial,” Dr. Harper said. “I think it’s probably at least in part anti-inflammatory, because we have some data to show that it’s killing Demodex [mites]. If Demodex [are] a trigger of inflammation, and we can lessen Demodex, then we could lessen the inflammatory response after that.”
The drug’s approval was based on data from two positive, identical phase 3 randomized, double-blind, multicenter, 12-week clinical trials that evaluated its safety compared with vehicle in 733 people with inflammatory lesions of rosacea (NCT03564119 and NCT03448939).
At week 12, inflammatory lesions of rosacea were reduced by nearly 70% in both trials among those who received 5% microencapsulated benzoyl peroxide cream, compared with 38%-46% among those who received the vehicle. Also, nearly 50% of subjects in the treatment groups were clear or almost clear at 12 weeks, compared with 38%-46% of those who received the vehicle.
Dr. Harper added that about one-quarter of patients in the treatment group of the trials were clear or almost clear by week 4. “That’s pretty fast,” she said, noting that the product’s microencapsulated shell acts as a fenestrated barrier. “It has little openings, which means that it takes a while for the drug to work itself out,” she said. “I think of it as being like a speed bump for benzoyl peroxide delivery. It has to get through this little maze before it lands on the skin. We think that is what has helped with tolerability.”
Oral sarecycline, a narrow spectrum tetracycline that was FDA approved for acne in 2018, may also benefit rosacea patients. In a 12-week, investigator-blinded pilot study, 72 patients with papulopustular rosacea were assigned to receive sarecycline, while 25 received a multivitamin.
By week 12, 75% of patients in the sarecycline group were clear, compared with 16% of those in the multivitamin group, while the inflammatory lesion counts dropped from baseline by 80% and 60%, respectively. Studies of sarecycline for acne have demonstrated similar rates of vertigo, dizziness, and sunburn to those of placebo.
“There were also low rates of gastrointestinal disturbances,” Dr. Harper said. “That’s important in rosacea, because there is no bacterial pathogen.”
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speakers bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
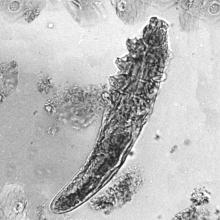
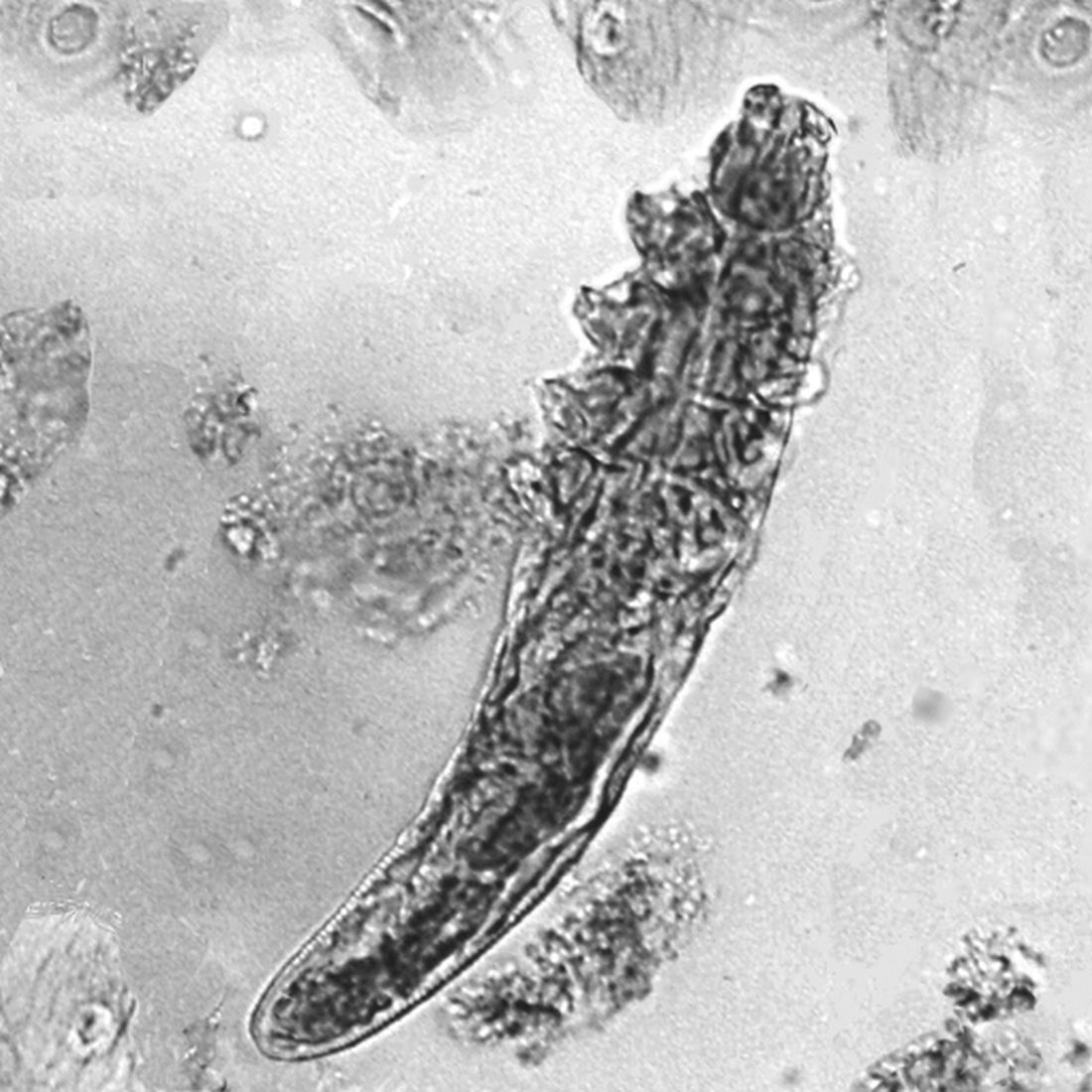
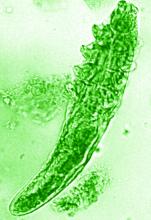
What’s the true role of Demodex mites in the development of papulopustular rosacea?
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
, a narrative review proposes.
According to the author, Fabienne Forton, MD, PhD, a dermatologist based in Brussels, recent studies suggest that Demodex induces two opposite actions on host immunity: A defensive immune response aimed at eliminating the mite and an immunosuppressive action aimed at favoring its own proliferation. “Moreover, the initial defensive immune response is likely diverted towards benefit for the mite, via T-cell exhaustion induced by the immunosuppressive properties of vascular endothelial growth factor (VEGF), which may also explain the favorable influence that the altered vascular background of rosacea seems to exert on Demodex proliferation,” she wrote in the review, which was published in JEADV, the Journal of the European Academy of Dermatology and Venereology.
She presented several arguments for and against a causal role of Demodex in rosacea. Three on the “for” side are:
High Demodex densities (Dds) are observed in almost all cases of rosacea with papulopustules (PPR). Dr. Forton pointed out that Demodex proliferation presents in as many as 98.6% of cases of PPR when two consecutive standardized skin surface biopsies (SSSBs) are performed (Acta Derm Venereol. 2017;97:242-8). “Dds in patients with PPR are as high as those in patients with demodicosis, much higher than in healthy skin and other facial dermatoses (except when these are associated with demodicosis [as is often the case with seborrheic dermatitis and acne vulgaris]),” she wrote.
The Demodex mite has the elements necessary to stimulate the host’s innate and adaptative immune system. Dr. Forton characterized Demodex as “the only microorganism found in abundance in almost all subjects with PPR, which can, in addition, alter the skin barrier. To feed and move around, Demodex mites attack the epidermal wall of the pilosebaceous follicles mechanically (via their stylets, mouth palps and motor palps) and chemically (through enzymes secreted from salivary glands for pre-oral digestion).”
The Demodex mite stimulates the immune system (which ultimately results in phymatous changes). A healthy immune system, including T helper 17 cells, seems necessary to adequately control mite proliferation. Dr. Forton noted that researchers have observed a perivascular and perifollicular infiltrate in people with rosacea, “which invades the epidermis and is often associated with the presence of Demodex. The lympho-histiocytic perifollicular infiltrate is correlated with the presence and the numbers of mites inside the follicles, and giant cell granulomas can be seen around intradermal Demodex mites, which attempt to phagocytize the mites.”
The three arguments that she presented against a causal role of Demodex in rosacea are the following:
No relationship with the mite was observed in two early histological studies. Rosacea biopsies conducted in these two analyses, published in 1969 and 1988, showed only mild infiltrate, with few parasites and no inflammation around the infested follicles.
However, she countered, “these data are now obsolete, because it has since been clearly demonstrated that the perifollicular infiltrate is a characteristic of rosacea, that this infiltrate is statistically related to the presence and the number of Demodex mites, and that high Dds are observed in almost all subjects with PPR.”
Demodex is not always associated with inflammatory symptoms. This argument holds that Demodex is present in all individuals and can be observed in very high densities without causing significant symptoms. Studies that support this viewpoint include the following: J Eur Acad Dermatol Venereol. 2001;15:441–4 and J Zhejiang Univ Sci B. 2011;12:998-1007.
However, Dr. Forton pointed out that the normal, low-density presence of Demodex in the skin “does not contradict a pathogenic effect when it proliferates excessively or penetrates into the dermis. The absence of intense inflammatory symptoms when the Dd is very high does not negate its potential pathogenicity.”
Demodex proliferation could be a consequence rather than a cause. Dr. Forton cited a study, suggesting that inflammation could be responsible for alteration of the skin barrier, “which, secondarily, would favor proliferation of the parasites, as with skin affected by atopic dermatitis that becomes superinfected by Staphylococcus aureus”. On the other hand, she argued, “unlike S. aureus, Demodex does not require alteration of the skin barrier to implant or proliferate. It also does not require an inflammatory background.” She added that if mite proliferation was a consequence of clinical lesions, “the Demodex mite should logically proliferate in other inflammatory facial skin conditions, which is not the case.”
A Sept. 14 National Rosacea Society (NRS) press release featured the paper by Dr. Forton, titled, “Which Comes First, The Rosacea Blemish or The Mite?” In the release, Richard Gallo, MD, PhD, who chaired the NRS Expert Committee that updated the standard classification of rosacea in 2018, said that “growing knowledge of rosacea’s pathophysiology has established that a consistent multivariate disease process underlies its potential manifestations, and the clinical significance of each of these elements is increasing as more is understood.”
While the potential role of Demodex in rosacea has been controversial in the past, “these new insights suggest where it may play a role as a meaningful cofactor in the development of the disorder,” added Dr. Gallo, chair of the department of dermatology at the University of California, San Diego.
Dr. Forton reported having no financial disclosures.
FROM JEADV
Can dietary tweaks improve some skin diseases?
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
Since 1950, the terms “diet and skin” in the medical literature have markedly increased, said Vivian Shi, MD associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock, who talked about nutritional approaches for select skin diseases at MedscapeLive’s Women’s and Pediatric Dermatology Seminar.
Myths abound, but some associations of diet with skin diseases hold water, and she said.
Acne
What’s known, Dr. Shi said, is that the prevalence of acne is substantially lower in non-Westernized countries, and that diets in those countries generally have a low glycemic load, which decreases IGF-1 insulinlike growth factor 1 (IGF-1) concentrations, an accepted risk factor for acne. The Western diet also includes the hormonal effects of cow’s milk products.
Whey protein, which is popular as a supplement, isn’t good for acne, Dr. Shi said. It takes a couple of hours to digest, while casein protein digests more slowly, over 5-7 hours. If casein protein isn’t acceptable, good alternatives to whey protein are hemp seed, plant protein blends (peas, seeds, berries), egg white, brown rice isolate, and soy isolate protein.
Dairy products increase IGF-1 levels, hormonal mediators that can make acne worse. In addition, industrial cow’s milk can contain anabolic steroids and growth factor, leading to sebogenesis, Dr. Shi said. As for the type of milk, skim milk tends to be the most acnegenic and associated with the highest blood levels of IGF-1.
Supplementing with omega-3 fatty acids and gamma-linolenic acid improved mild to moderate acne in a double-blind, controlled study. Researchers randomized 45 patients with mild to moderate acne to an omega-3 fatty acid group (2,000 mg of eicosapentaenoic acid and docosahexaenoic acid), a gamma-linolenic acid group (borage oil with 400 mg gamma-linolenic acid) or a control group. After 10 weeks in both treatment groups, there was a significant reduction in inflammatory and noninflammatory lesions.
Those with acne are more likely to be deficient in Vitamin D, research suggests. Researchers also found that among those who had vitamin D deficiency, supplementing with 1,000 IU daily for 2 months reduced inflammatory lesions by 35% after 8 weeks, compared with a 6% reduction in the control group.
Other research has found that those with a low serum zinc level had more severe acne and that 30-200 mg of zinc orally for 2-4 months reduced inflammatory acne. However, Dr. Shi cautioned that those taking zinc for more than 2 months also need a copper supplement, as zinc reduces the amount of copper absorbed by the body.
Dr. Shi’s “do’s” diet list for acne patients is a follows: Paleolithic and Mediterranean diets, omega-3 fatty acids, gamma-linolenic acids, Vitamin D, zinc, tubers, legumes, vegetables, fruits, and fish.
Unknowns, she said, include chocolate, caffeine, green tea, and high salt.
Hidradenitis suppurativa
Patents with HS who follow a Mediterranean diet most closely have less severe disease, research has found. In this study, those patients with HS with the lowest adherence had a Sartorius HS score of 59.38, while those who followed it the most closely had a score of 39 (of 80).
In another study, patients with HS reported the following foods as exacerbating HS: sweets, bread/pasta/rice, dairy, and high-fat foods. Alleviating foods included vegetables, fruit, chicken, and fish.
Dr. Shi’s dietary recommendations for patients with HS: Follow a Mediterranean diet, avoid high fat foods and highly processed foods, and focus on eating more vegetables, fresh fruit, corn-based cereal, white meat, and fish.
A retrospective study of patients with Hurley stage 1 and 2 found that oral zinc gluconate, 90 mg a day, combined with 2% topical triclosan twice a day, resulted in significantly decreased HS scores and nodules and improved quality of life after 3 months. Expect vitamin D deficiency, she added.
Lastly, Dr. Shi recommended, if necessary, “weight loss to reduce the inflammatory burden.”
Rosacea
Dietary triggers for rosacea are thought to include high-fat foods, dairy foods, spicy foods, hot drinks, cinnamon, and vanilla.
A population-based case-control study in China, which evaluated 1,347 rosacea patients and 1,290 healthy controls, found that a high intake of fatty foods positively correlated with erythematotelangiectatic rosacea (ETR) and phymatous rosacea. High-frequency dairy intake negatively correlated with ETR and papulopustular rosacea, which was a surprise, she said. And in this study, no significant correlations were found between sweets, coffee, and spicy foods. That goes against the traditional thinking, she said, but this was a Chinese cohort and their diet is probably vastly different than those in the United States.
Other rosacea triggers, Dr. Shi said, are niacin-containing foods such as turkey, chicken breast, crustaceans, dried Shiitake mushrooms, peanuts, tuna, and liver, as well as cold drinks, and formalin-containing foods (fish, squid, tofu, wet noodles).
As the field of nutrigenics – how genes affect how the body responds to food – evolves, more answers about the impact of diet on these diseases will be forthcoming, Dr. Shi said.
In an interactive panel discussion, she was asked if she talks about diet with all her patients with acne, rosacea, and HS, or just those not responding to traditional therapy.
“I think it’s an important conversation to have,” Dr. Shi responded. “When I’m done with the medication [instructions], I say: ‘There is something else you can do to augment what I just told you.’ ” That’s when she explains the dietary information. She also has a handout on diet and routinely refers patients for dietary counseling.
MedscapeLive and this news organization are owned by the same parent company. Dr. Shi disclosed consulting, investigative and research funding from several sources, but not directly related to the content of her talk.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Cutaneous Body Image: How the Mental Health Benefits of Treating Dermatologic Disease Support Military Readiness in Service Members
According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
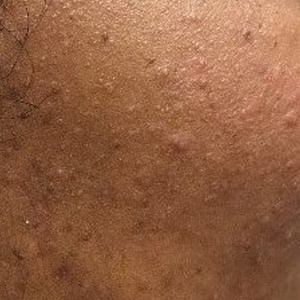
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
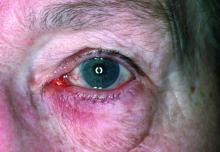
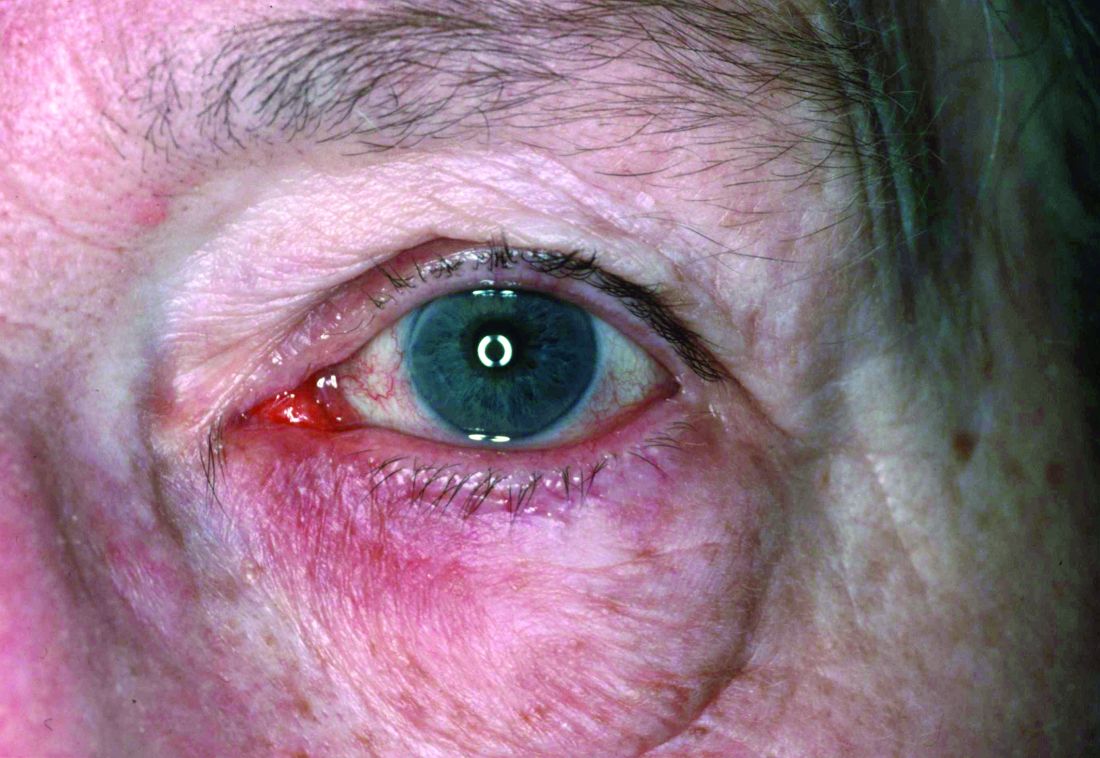
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
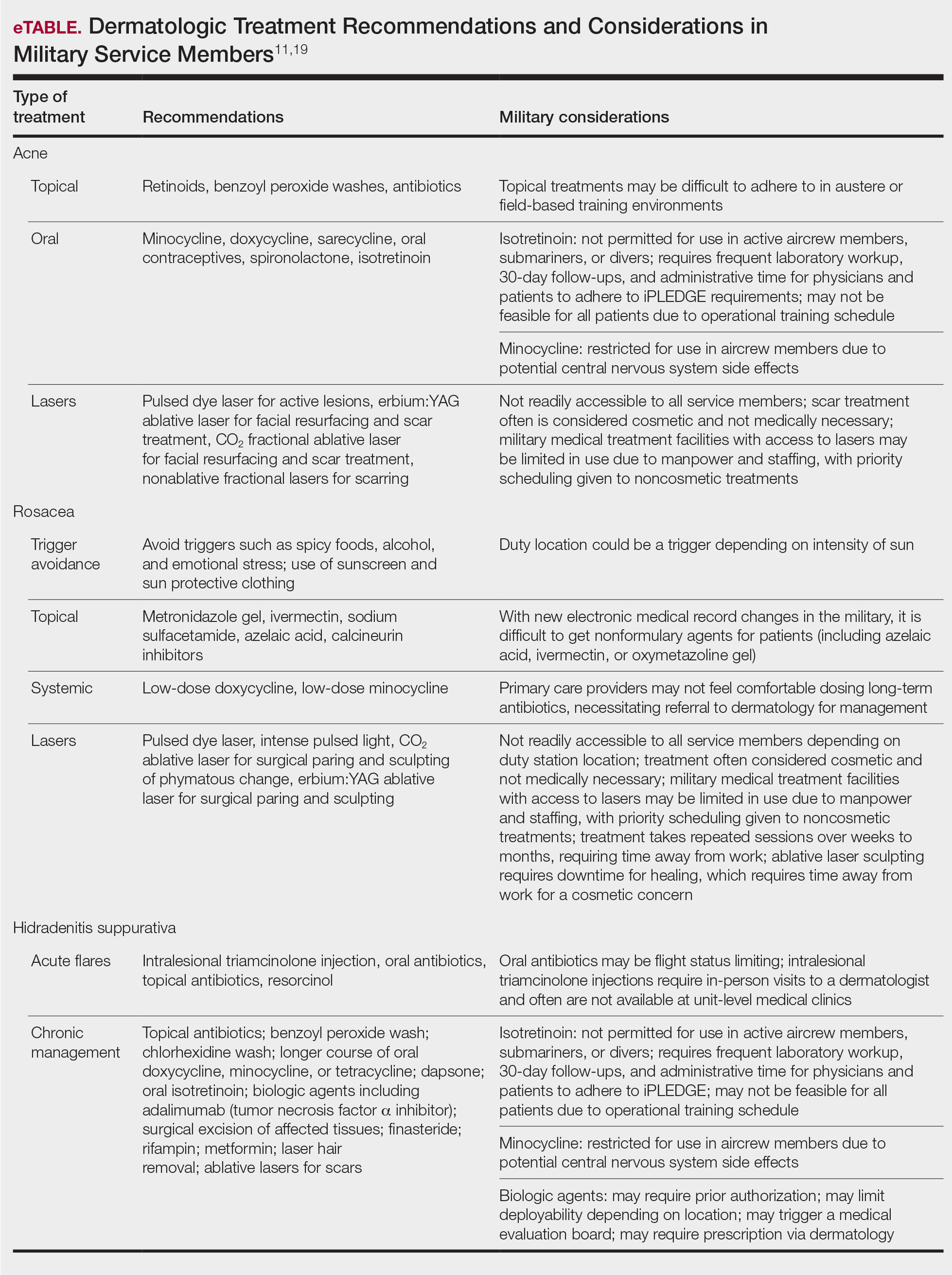
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.
Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.

- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.

Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.

According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.

Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.

- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
Practice Points
- The term readiness refers to the ability to recruit, train, deploy, and sustain military forces that are ready to “fight tonight” and succeed in combat.
- Maintaining readiness requires a holistic approach, as it is directly affected by physical and mental health outcomes.
- Cutaneous body image (CBI) refers to an individual’s mental perception of the condition of their hair, nails, and skin. Positive CBI is related to increased quality of life, while negative CBI, which often is associated with dermatologic disease, is associated with poorer health outcomes and even self-injury.
- Treatment of dermatologic disease in the context of active-duty military members can positively influence CBI, which may in turn increase service members’ quality of life and overall military readiness.
Dryness, conjunctival telangiectasia among ocular symptoms common in rosacea
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study recently published in International Ophthalmology.
In the study, investigators compared the right eyes of 76 patients with acne rosacea and 113 age-matched and gender-matched patients without rosacea. The mean age of the patients was 47-48 years, and about 63% were females. Ophthalmologic examinations that included tear breakup time and optical CT-assisted infrared meibography were conducted, and participants were asked to complete the Ocular Surface Disease Index (OSDI) questionnaire, which the authors say is widely used to assess aspects of ocular surface diseases.
Compared with controls, significantly more patients with rosacea had itching (35.5% vs. 17.7%), dryness (46.1% vs. 10.6%), hyperemia (10.5% vs. 2.7%), conjunctival telangiectasia (26.3% vs. 1.8%), and meibomitis (52.6% vs. 31%) (P ≤ .05 for all), according to the investigators, from the departments of ophthalmology and dermatology, Dokuz Eylul University, Izmir, Turkey. The most common ocular symptom among those with rosacea was having a foreign body sensation (53.9% vs. 24.8%, P < .001).
Ocular surface problems were also more common among those with rosacea, and OSDI scores were significantly higher among those with rosacea, compared with controls.
Estee Williams, MD, a dermatologist in private practice in New York and assistant clinical professor of dermatology at Mount Sinai Hospital, also in New York, who was not involved with the study, said the results reinforce the need to keep ocular rosacea in mind when examining a patient.
“The study is a reminder that ocular rosacea is, like its facial counterpart, an inflammatory disease that can manifest in many ways; for this reason, it’s often misdiagnosed or missed altogether,” Dr. Williams told this news organization. “This is unfortunate because it is usually easily managed.”
She added that there is a need for more randomized, controlled studies to determine optimal treatments for ocular rosacea, which is underdiagnosed. Part of the reason she believes it is underdiagnosed is that often “ophthalmologists don’t think about ocular rosacea specifically, unless they are given the information that the patient suffers from rosacea. The patient may not be aware that their skin and eye problems are connected.”
The take-home message of the study, Dr. Williams added, is that dermatologists who treat rosacea should be ready to screen their patients with rosacea for ocular symptoms, as well as have a basic understanding of ocular rosacea and know when to refer patients to an ophthalmologist.
“Preservative-free eye drops are usually well tolerated and a good starting point for those cases that are limited to symptoms only,” she said. “However, once a patient has signs of overt inflammation on exam, such as arcades of blood vessels on the eyelid margin or on the white of the eye, prescription medication is usually needed.”
A limitation of the study is that both eyes of patients were not included, said Dr. Williams, noting that ocular rosacea is usually bilateral.
Also asked to comment on the results, Marc Lupin, MD, a dermatologist in Victoria, B.C., and clinical instructor in the department of dermatology and skin science, University of British Columbia, Vancouver, noted that one of the shortcomings of the study is that it did not account for any effect of treatment.
“Were they on treatment for their rosacea either during the study or before the study?” asked Dr. Lupin. “That would affect the ocular findings.” Still, he agreed that the study underlines the need for dermatologists to be aware of the high incidence of ocular rosacea in patients and to appreciate that it can present subtly.
The study authors, Dr. Williams, and Dr. Lupin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNATIONAL OPTHALMOLOGY
Rosacea is in the eye of the beholder, expert says
In the clinical experience of Emmy Graber, MD, MBA, rosacea is in the eye of the beholder.
“It’s not really up to us as the providers as to what’s important to the patient or how bad their rosacea is,” she said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It really is up to the patient,” added Dr. Graber, president of The Dermatology Institute of Boston, who recommends asking patients about how severe they consider their rosacea to be, and what about rosacea bothers them most. Their responses may be surprising.
A study published in 2017 showed that complete resolution of even mild rosacea prolongs remission of rosacea, and most importantly, improves the quality of life for patients. “So, don’t discount what you consider to be mild rosacea in patients,” she said.
Skin care recommendations
“And don’t forget about basic skin care,” she advised. A recently published Chinese study of 999 rosacea patients and 1,010 controls with healthy skin found that a high frequency of cleansing and expansive use of cleansers were positively correlated with rosacea occurrence, suggesting that overcleansing can be a risk factor for rosacea. “Ask your patient, ‘how often are you cleaning your face?’ ” Dr. Graber suggested. “You might find that they’re overdoing it by washing three or four times a day. Several studies have shown that basic skin care alone improves rosacea.”
Skin care recommendations for patients with rosacea include avoiding chemical or physical exfoliants and alcohol-based topical products, and moisturizing and washing their faces with mild, synthetic detergent-based products rather than traditional soaps, which may further alkalinize and irritate the skin. “Patients should also be counseled to use physical-based sunscreens rather than chemical-based sunscreens,” she said.
Treating erythema
For treating erythema with topicals, a systematic review published in 2019 found the most evidence for brimonidine 0.33% gel, an alpha2-adrenergic agonist, and oxymetazoline 1% cream, an alpha1-adrenergic agonist. “Both of these products functionally constrict facial blood vessels,” and are Food and Drug Administration approved for treating persistent erythema, Dr. Graber said. “These products improve erythema within 3 hours of and up to 12 hours after application and overall, they are well tolerated.”
Based on clinical trial results, about 15% of patients on brimonidine report adverse reactions such as dermatitis, burning, pruritus, and erythema, compared with 8% of patients on oxymetazoline. At the same time, up to 20% of individuals on brimonidine report rebound erythema, compared with fewer than 1% of those using oxymetazoline. Laser and light therapies such as pulse-dye lasers, potassium-titanyl-phosphate lasers, and intense-pulse light devices are also effective in treating persistent erythema but are less effective for transient flushing.
Treatment of papules and pustules
For treating papules and pustules, the 2019 systemic review also found high-certainty evidence for using azelaic acid and topical ivermectin, and moderate-certainty evidence for using topical metronidazole and topical minocycline. “Topical ivermectin was demonstrated to be the most effective topical treatment for papulopustular rosacea and to provide the greatest psychological benefit to these patients,” Dr. Graber said.
In a double-blind, multicenter 15-week trial comparing azelaic acid 15% gel with metronidazole 0.75% gel in patients with papulopustular rosacea, both agents were found to be effective. But those treated with azelaic acid 15% gel had a greater reduction in lesion counts and erythema, and improvement in global assessments, compared with metronidazole 0.75% gel. However, the azelaic acid 15% gel was associated with more stinging compared with metronidazole 0.75% gel, although it was usually transient.
Another study, a double-blind, single-center, 15-week trial, compared the efficacy of azelaic acid 20% cream with metronidazole 0.75% cream. Both agents were found to be effective and had similar levels of reductions in papules and pustules. However, patients in the azelaic acid 20% cream arm had significantly higher physician ratings of global improvement, as well as overall higher patient satisfaction.
More recently, a phase 3 study of 962 patients found that ivermectin 1% cream once daily improved quality of life slightly more than metronidazole 0.75% cream twice daily. No difference in adverse events were noted between the two agents.
Other options for treating papules and pustules include topical minocycline 1.5% foam, which is FDA approved for rosacea, as well as second-line agents topical sodium sulfacetamide with sulfur cleanser (cream or lotion), and permethrin, Dr. Graber said.
As for treating papules and pustules with oral agents, the strongest evidence favors oral tetracyclines and isotretinoin, she noted.
Doxycycline, minocycline, tetracycline, and sarecycline can be used as monotherapy or coadministered with topical agents. “The addition of topical agents may also help to shorten the duration of antibiotic use, which is very important,” Dr. Graber said.
She noted that oral beta-blockers might be useful to treat persistent erythema and flushing because they antagonize the effects of sympathetic nerve stimulation and circulating catecholamines at b-adrenoceptors. Carvedilol and propranolol have been the most studied. The most common potential side effects are hypotension and bradycardia.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
In the clinical experience of Emmy Graber, MD, MBA, rosacea is in the eye of the beholder.
“It’s not really up to us as the providers as to what’s important to the patient or how bad their rosacea is,” she said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It really is up to the patient,” added Dr. Graber, president of The Dermatology Institute of Boston, who recommends asking patients about how severe they consider their rosacea to be, and what about rosacea bothers them most. Their responses may be surprising.
A study published in 2017 showed that complete resolution of even mild rosacea prolongs remission of rosacea, and most importantly, improves the quality of life for patients. “So, don’t discount what you consider to be mild rosacea in patients,” she said.
Skin care recommendations
“And don’t forget about basic skin care,” she advised. A recently published Chinese study of 999 rosacea patients and 1,010 controls with healthy skin found that a high frequency of cleansing and expansive use of cleansers were positively correlated with rosacea occurrence, suggesting that overcleansing can be a risk factor for rosacea. “Ask your patient, ‘how often are you cleaning your face?’ ” Dr. Graber suggested. “You might find that they’re overdoing it by washing three or four times a day. Several studies have shown that basic skin care alone improves rosacea.”
Skin care recommendations for patients with rosacea include avoiding chemical or physical exfoliants and alcohol-based topical products, and moisturizing and washing their faces with mild, synthetic detergent-based products rather than traditional soaps, which may further alkalinize and irritate the skin. “Patients should also be counseled to use physical-based sunscreens rather than chemical-based sunscreens,” she said.
Treating erythema
For treating erythema with topicals, a systematic review published in 2019 found the most evidence for brimonidine 0.33% gel, an alpha2-adrenergic agonist, and oxymetazoline 1% cream, an alpha1-adrenergic agonist. “Both of these products functionally constrict facial blood vessels,” and are Food and Drug Administration approved for treating persistent erythema, Dr. Graber said. “These products improve erythema within 3 hours of and up to 12 hours after application and overall, they are well tolerated.”
Based on clinical trial results, about 15% of patients on brimonidine report adverse reactions such as dermatitis, burning, pruritus, and erythema, compared with 8% of patients on oxymetazoline. At the same time, up to 20% of individuals on brimonidine report rebound erythema, compared with fewer than 1% of those using oxymetazoline. Laser and light therapies such as pulse-dye lasers, potassium-titanyl-phosphate lasers, and intense-pulse light devices are also effective in treating persistent erythema but are less effective for transient flushing.
Treatment of papules and pustules
For treating papules and pustules, the 2019 systemic review also found high-certainty evidence for using azelaic acid and topical ivermectin, and moderate-certainty evidence for using topical metronidazole and topical minocycline. “Topical ivermectin was demonstrated to be the most effective topical treatment for papulopustular rosacea and to provide the greatest psychological benefit to these patients,” Dr. Graber said.
In a double-blind, multicenter 15-week trial comparing azelaic acid 15% gel with metronidazole 0.75% gel in patients with papulopustular rosacea, both agents were found to be effective. But those treated with azelaic acid 15% gel had a greater reduction in lesion counts and erythema, and improvement in global assessments, compared with metronidazole 0.75% gel. However, the azelaic acid 15% gel was associated with more stinging compared with metronidazole 0.75% gel, although it was usually transient.
Another study, a double-blind, single-center, 15-week trial, compared the efficacy of azelaic acid 20% cream with metronidazole 0.75% cream. Both agents were found to be effective and had similar levels of reductions in papules and pustules. However, patients in the azelaic acid 20% cream arm had significantly higher physician ratings of global improvement, as well as overall higher patient satisfaction.
More recently, a phase 3 study of 962 patients found that ivermectin 1% cream once daily improved quality of life slightly more than metronidazole 0.75% cream twice daily. No difference in adverse events were noted between the two agents.
Other options for treating papules and pustules include topical minocycline 1.5% foam, which is FDA approved for rosacea, as well as second-line agents topical sodium sulfacetamide with sulfur cleanser (cream or lotion), and permethrin, Dr. Graber said.
As for treating papules and pustules with oral agents, the strongest evidence favors oral tetracyclines and isotretinoin, she noted.
Doxycycline, minocycline, tetracycline, and sarecycline can be used as monotherapy or coadministered with topical agents. “The addition of topical agents may also help to shorten the duration of antibiotic use, which is very important,” Dr. Graber said.
She noted that oral beta-blockers might be useful to treat persistent erythema and flushing because they antagonize the effects of sympathetic nerve stimulation and circulating catecholamines at b-adrenoceptors. Carvedilol and propranolol have been the most studied. The most common potential side effects are hypotension and bradycardia.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
In the clinical experience of Emmy Graber, MD, MBA, rosacea is in the eye of the beholder.
“It’s not really up to us as the providers as to what’s important to the patient or how bad their rosacea is,” she said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It really is up to the patient,” added Dr. Graber, president of The Dermatology Institute of Boston, who recommends asking patients about how severe they consider their rosacea to be, and what about rosacea bothers them most. Their responses may be surprising.
A study published in 2017 showed that complete resolution of even mild rosacea prolongs remission of rosacea, and most importantly, improves the quality of life for patients. “So, don’t discount what you consider to be mild rosacea in patients,” she said.
Skin care recommendations
“And don’t forget about basic skin care,” she advised. A recently published Chinese study of 999 rosacea patients and 1,010 controls with healthy skin found that a high frequency of cleansing and expansive use of cleansers were positively correlated with rosacea occurrence, suggesting that overcleansing can be a risk factor for rosacea. “Ask your patient, ‘how often are you cleaning your face?’ ” Dr. Graber suggested. “You might find that they’re overdoing it by washing three or four times a day. Several studies have shown that basic skin care alone improves rosacea.”
Skin care recommendations for patients with rosacea include avoiding chemical or physical exfoliants and alcohol-based topical products, and moisturizing and washing their faces with mild, synthetic detergent-based products rather than traditional soaps, which may further alkalinize and irritate the skin. “Patients should also be counseled to use physical-based sunscreens rather than chemical-based sunscreens,” she said.
Treating erythema
For treating erythema with topicals, a systematic review published in 2019 found the most evidence for brimonidine 0.33% gel, an alpha2-adrenergic agonist, and oxymetazoline 1% cream, an alpha1-adrenergic agonist. “Both of these products functionally constrict facial blood vessels,” and are Food and Drug Administration approved for treating persistent erythema, Dr. Graber said. “These products improve erythema within 3 hours of and up to 12 hours after application and overall, they are well tolerated.”
Based on clinical trial results, about 15% of patients on brimonidine report adverse reactions such as dermatitis, burning, pruritus, and erythema, compared with 8% of patients on oxymetazoline. At the same time, up to 20% of individuals on brimonidine report rebound erythema, compared with fewer than 1% of those using oxymetazoline. Laser and light therapies such as pulse-dye lasers, potassium-titanyl-phosphate lasers, and intense-pulse light devices are also effective in treating persistent erythema but are less effective for transient flushing.
Treatment of papules and pustules
For treating papules and pustules, the 2019 systemic review also found high-certainty evidence for using azelaic acid and topical ivermectin, and moderate-certainty evidence for using topical metronidazole and topical minocycline. “Topical ivermectin was demonstrated to be the most effective topical treatment for papulopustular rosacea and to provide the greatest psychological benefit to these patients,” Dr. Graber said.
In a double-blind, multicenter 15-week trial comparing azelaic acid 15% gel with metronidazole 0.75% gel in patients with papulopustular rosacea, both agents were found to be effective. But those treated with azelaic acid 15% gel had a greater reduction in lesion counts and erythema, and improvement in global assessments, compared with metronidazole 0.75% gel. However, the azelaic acid 15% gel was associated with more stinging compared with metronidazole 0.75% gel, although it was usually transient.
Another study, a double-blind, single-center, 15-week trial, compared the efficacy of azelaic acid 20% cream with metronidazole 0.75% cream. Both agents were found to be effective and had similar levels of reductions in papules and pustules. However, patients in the azelaic acid 20% cream arm had significantly higher physician ratings of global improvement, as well as overall higher patient satisfaction.
More recently, a phase 3 study of 962 patients found that ivermectin 1% cream once daily improved quality of life slightly more than metronidazole 0.75% cream twice daily. No difference in adverse events were noted between the two agents.
Other options for treating papules and pustules include topical minocycline 1.5% foam, which is FDA approved for rosacea, as well as second-line agents topical sodium sulfacetamide with sulfur cleanser (cream or lotion), and permethrin, Dr. Graber said.
As for treating papules and pustules with oral agents, the strongest evidence favors oral tetracyclines and isotretinoin, she noted.
Doxycycline, minocycline, tetracycline, and sarecycline can be used as monotherapy or coadministered with topical agents. “The addition of topical agents may also help to shorten the duration of antibiotic use, which is very important,” Dr. Graber said.
She noted that oral beta-blockers might be useful to treat persistent erythema and flushing because they antagonize the effects of sympathetic nerve stimulation and circulating catecholamines at b-adrenoceptors. Carvedilol and propranolol have been the most studied. The most common potential side effects are hypotension and bradycardia.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Microbiome studies among those awarded National Rosacea Society grants
A study on this year, as part of the organization’s research grants program.
The NRS research grants program was created to increase knowledge and understanding of not only the potential causes of rosacea, but other aspects of the disease that may inform prevention, treatment, or a potential cure, according to the press release announcing the recipients.
New research grant recipient Sezen Karakus, MD, of the Johns Hopkins Wilmer Eye Institute, Baltimore, received $15,000 for a study on the contribution of the ocular surface microbiome to the development of rosacea. Ocular rosacea can result in corneal complications severe enough to affect vision, and identifying the microorganisms on the ocular surface may lead to new treatment strategies, Dr. Karakus said in the release. He will collaborate on this research with dermatologist Noori Kim, MD, of Johns Hopkins University, Baltimore.
A second new research grant went to Emmanuel Contassot, MD, project leader in the dermatology department at of the University Hospital of Basel, Switzerland, who received $5,000 to investigate whether certain elevated intracellular signals in rosacea lesions may promote the skin inflammation that may be a root cause of the condition.
The NRS also renewed its support of a pair of ongoing studies. Michelle Trautwein, MD, of the Institute for Biodiversity Science and Sustainability at the California Academy of Sciences, continues her work on the first study to sequence the genome of Demodex mites; the study also identifies associated bacteria that may play a role in rosacea.
A second ongoing study by Tissa Hata, MD, of the University of California, San Diego, focuses on the normalization of the microbiome in people with rosacea. Dr. Hata’s work identifies types of bacteria associated with rosacea, as well as bacteria that may be associated with healthy skin after successful treatment of rosacea, including Cutibacterium acnes and Staphylococcus epidermidis.
The deadline to submit research proposals for next year’s grants is June 17, 2022. Researchers can find forms and instructions at the research grants section of the NRS website or by contacting the National Rosacea Society at 111 Lions Dr., Suite 216, Barrington, Ill., 60010, by telephone at 1-888-662-5874, or by email at [email protected].
A study on this year, as part of the organization’s research grants program.
The NRS research grants program was created to increase knowledge and understanding of not only the potential causes of rosacea, but other aspects of the disease that may inform prevention, treatment, or a potential cure, according to the press release announcing the recipients.
New research grant recipient Sezen Karakus, MD, of the Johns Hopkins Wilmer Eye Institute, Baltimore, received $15,000 for a study on the contribution of the ocular surface microbiome to the development of rosacea. Ocular rosacea can result in corneal complications severe enough to affect vision, and identifying the microorganisms on the ocular surface may lead to new treatment strategies, Dr. Karakus said in the release. He will collaborate on this research with dermatologist Noori Kim, MD, of Johns Hopkins University, Baltimore.
A second new research grant went to Emmanuel Contassot, MD, project leader in the dermatology department at of the University Hospital of Basel, Switzerland, who received $5,000 to investigate whether certain elevated intracellular signals in rosacea lesions may promote the skin inflammation that may be a root cause of the condition.
The NRS also renewed its support of a pair of ongoing studies. Michelle Trautwein, MD, of the Institute for Biodiversity Science and Sustainability at the California Academy of Sciences, continues her work on the first study to sequence the genome of Demodex mites; the study also identifies associated bacteria that may play a role in rosacea.
A second ongoing study by Tissa Hata, MD, of the University of California, San Diego, focuses on the normalization of the microbiome in people with rosacea. Dr. Hata’s work identifies types of bacteria associated with rosacea, as well as bacteria that may be associated with healthy skin after successful treatment of rosacea, including Cutibacterium acnes and Staphylococcus epidermidis.
The deadline to submit research proposals for next year’s grants is June 17, 2022. Researchers can find forms and instructions at the research grants section of the NRS website or by contacting the National Rosacea Society at 111 Lions Dr., Suite 216, Barrington, Ill., 60010, by telephone at 1-888-662-5874, or by email at [email protected].
A study on this year, as part of the organization’s research grants program.
The NRS research grants program was created to increase knowledge and understanding of not only the potential causes of rosacea, but other aspects of the disease that may inform prevention, treatment, or a potential cure, according to the press release announcing the recipients.
New research grant recipient Sezen Karakus, MD, of the Johns Hopkins Wilmer Eye Institute, Baltimore, received $15,000 for a study on the contribution of the ocular surface microbiome to the development of rosacea. Ocular rosacea can result in corneal complications severe enough to affect vision, and identifying the microorganisms on the ocular surface may lead to new treatment strategies, Dr. Karakus said in the release. He will collaborate on this research with dermatologist Noori Kim, MD, of Johns Hopkins University, Baltimore.
A second new research grant went to Emmanuel Contassot, MD, project leader in the dermatology department at of the University Hospital of Basel, Switzerland, who received $5,000 to investigate whether certain elevated intracellular signals in rosacea lesions may promote the skin inflammation that may be a root cause of the condition.
The NRS also renewed its support of a pair of ongoing studies. Michelle Trautwein, MD, of the Institute for Biodiversity Science and Sustainability at the California Academy of Sciences, continues her work on the first study to sequence the genome of Demodex mites; the study also identifies associated bacteria that may play a role in rosacea.
A second ongoing study by Tissa Hata, MD, of the University of California, San Diego, focuses on the normalization of the microbiome in people with rosacea. Dr. Hata’s work identifies types of bacteria associated with rosacea, as well as bacteria that may be associated with healthy skin after successful treatment of rosacea, including Cutibacterium acnes and Staphylococcus epidermidis.
The deadline to submit research proposals for next year’s grants is June 17, 2022. Researchers can find forms and instructions at the research grants section of the NRS website or by contacting the National Rosacea Society at 111 Lions Dr., Suite 216, Barrington, Ill., 60010, by telephone at 1-888-662-5874, or by email at [email protected].
Contact Allergy to Topical Medicaments, Part 1: A Double-edged Sword
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.
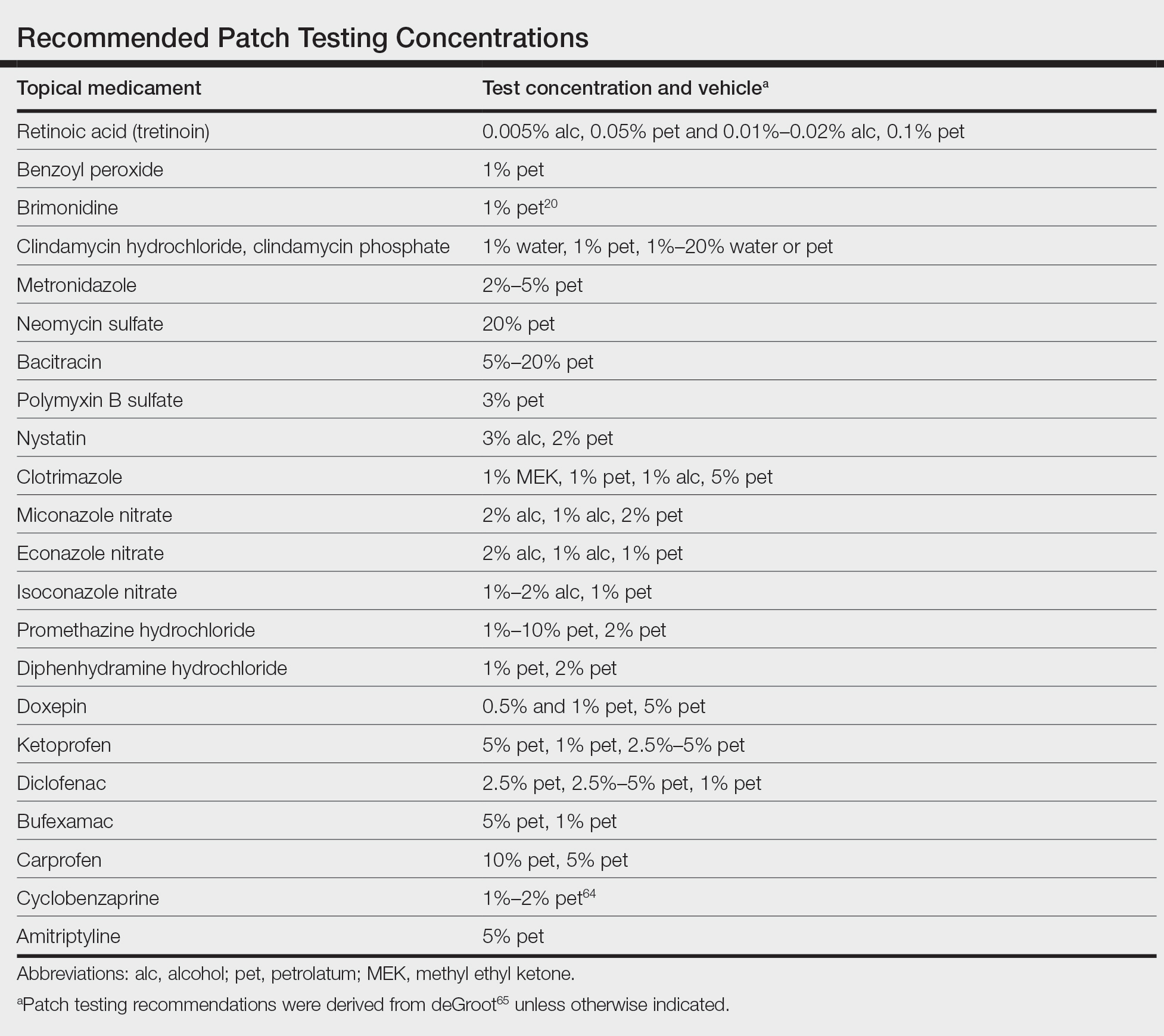
Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.

Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
Topical medications frequently are prescribed in dermatology and provide the advantages of direct skin penetration and targeted application while typically sparing patients from systemic effects. Adverse cutaneous effects include allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), photosensitivity, urticaria, hyperpigmentation or hypopigmentation, atrophy, periorificial dermatitis, and acneform eruptions. Allergic contact dermatitis can develop from the active drug or vehicle components.
Patients with medicament ACD often present with symptoms of pruritus and dermatitis at the site of topical application. They may express concern that the medication is no longer working or seems to be making things worse. Certain sites are more prone to developing medicament dermatitis, including the face, groin, and lower legs. Older adults may be more at risk. Other risk factors include pre-existing skin diseases such as stasis dermatitis, acne, psoriasis, atopic dermatitis, and genital dermatoses.1 A review of 14,911 patch-tested patients from a single referral clinic revealed that 17.4% had iatrogenic contact dermatitis, with the most common culprits being topical antibiotics, antiseptics, and steroids.2
In this 2-part series, we will focus on the active drug as a source of ACD. Part 1 explores ACD associated with acne and rosacea medications, antimicrobials, antihistamines, and topical pain preparations.
Acne and Rosacea Medications
Retinoids—Topical retinoids are first-line acne treatments that help normalize skin keratinization. Irritant contact dermatitis from retinoids is a well-known and common side effect. Although far less common than ICD, ACD from topical retinoid use has been reported.3,4 Reactions to tretinoin are most frequently reported in the literature compared to adapalene gel5 and tazarotene foam, which have lower potential for sensitization.6 Allergic contact dermatitis also has been reported from retinyl palmitate7,8 in cosmetic creams and from occupational exposure in settings of industrial vitamin A production.9 Both ICD and ACD from topical retinoids can present with pruritus, erythema, and scaling. Given this clinical overlap between ACD and ICD, patch testing is crucial in differentiating the underlying etiology of the dermatitis.
Benzoyl Peroxide—Benzoyl peroxide (BP) is another popular topical acne treatment that targets Cutibacterium acnes, a bacterium often implicated in the pathogenesis of acne vulgaris. Similar to retinoids, ICD is more common than ACD. Several cases of ACD to BP have been reported.10-14 Occasionally, honey-colored crusting associated with ACD to BP can mimic impetigo.10 Aside from use of BP as an acne treatment, other potential exposures to BP include bleached flour13 and orthopedic bone cement. Occupations at risk for potential BP exposure include dental technicians15 and those working in plastic manufacturing.
Brimonidine—Brimonidine tartrate is a selective α2-adrenergic agonist initially used to treat open-angle glaucoma and also is used as a topical treatment for rosacea. Allergic reactions to brimonidine eye drops may present with periorbital hyperpigmentation and pruritic bullous lesions.16 Case reports of topical brimonidine ACD have demonstrated mixed patch test results, with positive patch tests to Mirvaso (Galderma) as is but negative patch tests to pure brimonidine tartrate 0.33%.17,18 Ringuet and Houle19 reported the first known positive patch test reaction to pure topical brimonidine, testing with brimonidine tartrate 1% in petrolatum.20,21 Clinicians should be attuned to ACD to topical brimonidine in patients previously treated for glaucoma, as prior use of ophthalmic preparations may result in sensitization.18,20
Antimicrobials
Clindamycin—Clindamycin targets bacterial protein synthesis and is an effective adjunct in the treatment of acne. Despite its widespread and often long-term use, topical clindamycin is a weak sensitizer.22 To date, limited case reports on ACD to topical clindamycin exist.23-28 Rare clinical patterns of ACD to clindamycin include mimickers of irritant retinoid dermatitis, erythema multiforme, or pustular rosacea.25,26,29
Metronidazole—Metronidazole is a bactericidal agent that disrupts nucleic acid synthesis with additional anti-inflammatory properties used in the treatment of rosacea. Allergic contact dermatitis to topical metronidazole has been reported.30-34 In 2006, Beutner at al35 patch tested 215 patients using metronidazole gel 1%, which revealed no positive reactions to indicate contact sensitization. Similarly, Jappe et al36 found no positive reactions to metronidazole 2% in petrolatum in their prospective analysis of 78 rosacea patients, further highlighting the exceptionally low incidence of ACD. Cross-reaction with isothiazolinone, which shares structurally similar properties to metronidazole, has been speculated.31,34 One patient developed an acute reaction to metronidazole gel 0.75% within 24 hours of application, suggesting that isothiazolinone may act as a sensitizer, though this relationship has not been proven.31
Neomycin—Neomycin blocks bacterial protein synthesis and is available in both prescription and over-the-counter (OTC) formulations. It commonly is used to treat and prevent superficial wound infections as an OTC antibiotic and also has otic, ophthalmologic, gastroenterologic, urologic, and peritoneal formulations. It also can be used in the dental and veterinary fields and is present in some animal feeds and in trace amounts in some vaccines for humans. Neomycin is a common antibiotic contact allergen, and the most recently reported 2017-2018 North American Contact Dermatitis Group data cycle placed it at number 12 with 5.4% positivity.37 Co-reactions with bacitracin can occur, substantially limiting OTC topical antibiotic options for allergic patients. A safe alternative for patients with neomycin (and bacitracin and polymyxin) contact allergy is prescription mupirocin.
Bacitracin—Bacitracin interferes with peptidoglycan and cell-wall synthesis to treat superficial cutaneous infections. Similar to neomycin, it also can be found in OTC antibiotic ointments as well as in antibacterial bandages. There are several case reports of patients with both type IV delayed hypersensitivity (contact dermatitis) and type I anaphylactic reactions to bacitracin38-40; patch testers should be aware of this rare association. Bacitracin was positive in 5.5% of patch tested patients in the 2017-2018 North American Contact Dermatitis Group data cycle,37 and as with neomycin, bacitracin also is commonly patch tested in most screening patch test series.
Polymyxin—Polymyxin is a polypeptide topical antibiotic that is used to treat superficial wound infections and can be used in combination with neomycin and/or bacitracin. Historically, it is a less common antibiotic allergen; however, it is now frequently included in comprehensive patch test series, as the frequency of positive reactions seems to be increasing, probably due to polysensitization with neomycin and bacitracin.
Nystatin—Nystatin is an antifungal that binds to ergosterol and disrupts the cell wall. Cases exist of ACD to topical nystatin as well as systemic ACD from oral exposure, though both are quite rare. Authors have surmised that the overall low rates of ACD may be due to poor skin absorption of nystatin, which also can confound patch testing.41,42 For patients with suspected ACD to nystatin, repeat open application testing also can be performed to confirm allergy.
Imidazole Antifungals—Similar to nystatins, imidazole antifungals also work by disrupting the fungal cell wall. Imidazole antifungal preparations that have been reported to cause ACD include clotrimazole, miconazole, econazole, and isoconazole, and although cross-reactivity patterns have been described, they are not always reproducible with patch testing.43 In one reported case, tioconazole found in an antifungal nail lacquer triggered ACD involving not only the fingers and toes but also the trunk.44 Erythema multiforme–like reactions also have been described from topical use.45 Commercial patch test preparations of the most common imidazole allergens do exist. Nonimidazole antifungals remain a safe option for allergic patients.
Antihistamines
Antihistamines, or H1-receptor antagonists, are marketed to be applied topically for relief of pruritus associated with allergic cutaneous reactions. Ironically, they are known to be potent sensitizers themselves. There are 6 main chemical classes of antihistamines: phenothiazines, ethylenediamines, ethanolamines, alkylamines, piperazines, and piperidines. Goossens and Linsen46 patch tested 12,460 patients from 1978 to 1997 and found the most positive reactions to promethazine (phenothiazine)(n=12), followed by diphenhydramine (ethanolamine)(n=8) and clemizole (benzimidazole)(n=6). The authors also noted cross-reactions between diphenhydramine derivatives and between promethazine and chlorpromazine.46
Doxepin is a tricyclic antidepressant with antihistamine activity and is a well-documented sensitizer.47-52 Taylor et al47 evaluated 97 patients with chronic dermatoses, and patch testing revealed 17 (17.5%) positive reactions to doxepin cream, 13 (76.5%) of which were positive reactions to both the commercial cream and the active ingredient. Patch testing using doxepin dilution as low as 0.5% in petrolatum is sufficient to provoke a strong (++) allergic reaction.50,51 Early-onset ACD following the use of doxepin cream suggests the possibility of prior sensitization, perhaps with a structurally similar phenothiazine drug.51 A keen suspicion for ACD in patients using doxepin cream for longer than the recommended duration can help make the diagnosis.49,52
Topical Analgesics
Nonsteroidal Anti-inflammatory Drugs—Ketoprofen is one of the most frequent culprits of photoallergic contact dermatitis. Pruritic, papulovesicular, and bullous lesions typically develop acutely weeks after exposure. Prolonged photosensitivity is common and can last years after discontinuation of the nonsteroidal anti-inflammatory drug.53 Cases of cross-reactions and co-sensitization to structurally similar substances have been reported, including to benzophenone-related chemicals in sunscreen and aldehyde groups in fragrance mix.53,54
Diclofenac gel generally is well tolerated in the topical treatment of joint pain and inflammation. In the setting of ACD, patients typically present with dermatitis localized to the area of application.55 Immediate cessation and avoidance of topical diclofenac are crucial components of management. Although systemic contact dermatitis has been reported with oral diclofenac use,56 a recent report suggested that oral diclofenac may be well tolerated for some patients with topical ACD.57
Publications on bufexamac-induced ACD mainly consist of international reports, as this medication has been discontinued in the United States. Bufexamac is a highly sensitizing agent that can lead to severe polymorphic eruptions requiring treatment with prednisolone and even hospitalization.58 In one Australian case report, a mother developed an edematous, erythematous, papulovesicular eruption on the breast while breastfeeding her baby, who was being treated with bufexamac cream 5% for infantile eczema.59 Carprofen-induced photoallergic contact dermatitis is associated with occupational exposure in pharmaceutical workers.60,61 A few case reports on other nonsteroidal anti-inflammatory drugs, including etofenamate and aceclofenac, have been published.62,63
Compounded Medications—Compounded topical analgesics, which help to control pain via multiple combined effects, have gained increasing popularity in the management of chronic neuropathic pain disorders. Only a few recent retrospective studies assessing the efficacy and safety of these medications have mentioned suspected allergic cutaneous reactions.62,63 In 2015, Turrentine et al64 reported a case of ACD to cyclobenzaprine in a compound containing ketamine 10%, diclofenac 5%, baclofen 2%, bupivacaine 1%, cyclobenzaprine 2%, gabapentin 6%, ibuprofen 3%, and pentoxifylline 3% in a proprietary cream base. When patients present with suspected ACD to a compounded pain medication, obtaining individual components for patch testing is key to determining the allergic ingredient(s). We suspect that we will see a rise in reports of ACD as these topical compounds become readily adopted in clinical practices.
Patch Testing for Diagnosis
When patients present with symptoms concerning for ACD to medicaments, the astute clinician should promptly stop the suspected topical medication and consider patch testing. For common allergens such as neomycin, bacitracin, or ethylenediamine, commercial patch test preparations exist and should be used; however, for drugs that do not have a commercial patch test preparation, the patient’s product can be applied as is, keeping in mind that certain preparations (such as retinoids) can cause irritant patch test reactions, which may confound the reading. Alternatively, individual ingredients in the medication’s formulation can be requested from the manufacturer or a compounding pharmacy for targeted testing. Suggested concentrations for patch testing based on the literature and expert reference are listed in the Table. The authors (M.R., A.R.A.) frequently rely on an expert reference66 to determine ideal concentrations for patch testing. Referral to a specialized patch test clinic may be appropriate.

Final Interpretation
Although their intent is to heal, topical medicaments also can be a source of ACD. The astute clinician should consider ACD when topicals either no longer seem to help the patient or trigger new-onset dermatitis. Patch testing directly with the culprit medicament, or individual medication ingredients when needed, can lead to the diagnosis, though caution is advised. Stay tuned for part 2 of this series in which we will discuss ACD to topical steroids, immunomodulators, and anesthetic medications.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
- Davis MD. Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009;27:289-297, vi. doi:10.1016/j.det.2009.05.003
- Gilissen L, Goossens A. Frequency and trends of contact allergy to and iatrogenic contact dermatitis caused by topical drugs over a 25-year period. Contact Dermatitis. 2016;75:290-302. doi:10.1111/cod.12621
- Balato N, Patruno C, Lembo G, et al. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32:51. doi:10.1111/j.1600-0536.1995.tb00846.x
- Berg JE, Bowman JP, Saenz AB. Cumulative irritation potential and contact sensitization potential of tazarotene foam 0.1% in 2 phase 1 patch studies. Cutis. 2012;90:206-211.
- Numata T, Jo R, Kobayashi Y, et al. Allergic contact dermatitis caused by adapalene. Contact Dermatitis. 2015;73:187-188. doi:10.1111/cod.12410
- Anderson A, Gebauer K. Periorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55:152-153. doi:10.1111/ajd.12041
- Blondeel A. Contact allergy to vitamin A. Contact Dermatitis. 1984;11:191-192. doi:10.1111/j.1600-0536.1984.tb00976.x
- Manzano D, Aguirre A, Gardeazabal J, et al. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31:324. doi:10.1111/j.1600-0536.1994.tb02030.x
- Heidenheim M, Jemec GB. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33:439. doi:10.1111/j.1600-0536.1995.tb02091.x
- Kim C, Craiglow BG, Watsky KL, et al. Allergic contact dermatitis to benzoyl peroxide resembling impetigo. Pediatr Dermatol. 2015;32:E161-E162. doi:10.1111/pde.12585
- Sandre M, Skotnicki-Grant S. A case of a paediatric patient with allergic contact dermatitis to benzoyl peroxide. J Cutan Med Surg. 2018;22:226-228. doi:10.1177/1203475417733462
- Corazza M, Amendolagine G, Musmeci D, et al. Sometimes even Dr Google is wrong: an unusual contact dermatitis caused by benzoyl peroxide. Contact Dermatitis. 2018;79:380-381. doi:10.1111/cod.13086
- Adelman M, Mohammad T, Kerr H. Allergic contact dermatitis due to benzoyl peroxide from an unlikely source. Dermatitis. 2019;30:230-231. doi:10.1097/DER.0000000000000470
- Gatica-Ortega ME, Pastor-Nieto MA. Allergic contact dermatitis to Glycyrrhiza inflata root extract in an anti-acne cosmetic product [published online April 28, 2021]. Contact Dermatitis. doi:10.1111/cod.13872
- Ockenfels HM, Uter W, Lessmann H, et al. Patch testing with benzoyl peroxide: reaction profile and interpretation of positive patch test reactions. Contact Dermatitis. 2009;61:209-216. doi:10.1111/j.1600-0536.2009.01603.x
- Sodhi PK, Verma L, Ratan J. Dermatological side effects of brimonidine: a report of three cases. J Dermatol. 2003;30:697-700. doi:10.1111/j.1346-8138.2003.tb00461.x
- Swanson LA, Warshaw EM. Allergic contact dermatitis to topical brimonidine tartrate gel 0.33% for treatment of rosacea. J Am Acad Dermatol. 2014;71:832-833. doi:10.1016/j.jaad.2014.05.073
- Bangsgaard N, Fischer LA, Zachariae C. Sensitization to and allergic contact dermatitis caused by Mirvaso(®)(brimonidine tartrate) for treatment of rosacea—2 cases. Contact Dermatitis. 2016;74:378-379. doi:10.1111/cod.12547
- Ringuet J, Houle MC. Case report: allergic contact dermatitis to topical brimonidine demonstrated with patch testing: insights on evaluation of brimonidine sensitization. J Cutan Med Surg. 2018;22:636-638. doi:10.1177/1203475418789020
- Cookson H, McFadden J, White J, et al. Allergic contact dermatitis caused by Mirvaso®, brimonidine tartrate gel 0.33%, a new topical treatment for rosaceal erythema. Contact Dermatitis. 2015;73:366-367. doi:10.1111/cod.12476
- Rajagopalan A, Rajagopalan B. Allergic contact dermatitis to topical brimonidine. Australas J Dermatol. 2015;56:235. doi:10.1111/ajd.12299
- Veraldi S, Brena M, Barbareschi M. Allergic contact dermatitis caused by topical antiacne drugs. Expert Rev Clin Pharmacol. 2015;8:377-381. doi:10.1586/17512433.2015.1046839
- Vejlstrup E, Menné T. Contact dermatitis from clindamycin. Contact Dermatitis. 1995;32:110. doi:10.1111/j.1600-0536.1995.tb00759.x
- García R, Galindo PA, Feo F, et al. Delayed allergic reactions to amoxycillin and clindamycin. Contact Dermatitis. 1996;35:116-117. doi:10.1111/j.1600-0536.1996.tb02312.x
- Muñoz D, Del Pozo MD, Audicana M, et al. Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermatitis. 1996;34:227-228. doi:10.1111/j.1600-0536.1996.tb02187.x
- Romita P, Ettorre G, Corazza M, et al. Allergic contact dermatitis caused by clindamycin mimicking ‘retinoid flare.’ Contact Dermatitis. 2017;77:181-182. doi:10.1111/cod.12784
- Veraldi S, Guanziroli E, Ferrucci S, et al. Allergic contact dermatitis caused by clindamycin. Contact Dermatitis. 2019;80:68-69. doi:10.1111/cod.13133
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314. doi:10.1111/cod.13465
- de Kort WJ, de Groot AC. Clindamycin allergy presenting as rosacea. Contact Dermatitis. 1989;20:72-73. doi:10.1111/j.1600-0536.1989.tb03108.x
- Vincenzi C, Lucente P, Ricci C, et al. Facial contact dermatitis due to metronidazole. Contact Dermatitis. 1997;36:116-117. doi:10.1111/j.1600-0536.1997.tb00434.x
- Wolf R, Orion E, Matz H. Co-existing sensitivity to metronidazole and isothiazolinone. Clin Exp Dermatol. 2003;28:506-507. doi:10.1046/j.1365-2230.2003.01364.x
- Madsen JT, Thormann J, Kerre S, et al. Allergic contact dermatitis to topical metronidazole—3 cases. Contact Dermatitis. 2007;56:364-366. doi:10.1111/j.1600-0536.2006.01064.x
- Fernández-Jorge B, Goday Buján J, Fernández-Torres R, et al. Concomitant allergic contact dermatitis from diphenhydramine and metronidazole. Contact Dermatitis. 2008;59:115-116. doi:10.1111/j.1600-0536.2008.01332.x
- Madsen JT, Lorentzen HF, Paulsen E. Contact sensitization to metronidazole from possible occupational exposure. Contact Dermatitis. 2009;60:117-118. doi:10.1111/j.1600-0536.2008.01490.x
- Beutner KR, Lemke S, Calvarese B. A look at the safety of metronidazole 1% gel: cumulative irritation, contact sensitization, phototoxicity, and photoallergy potential. Cutis. 2006;77(4 suppl):12-17.
- Jappe U, Schäfer T, Schnuch A, et al. Contact allergy in patients with rosacea: a clinic-based, prospective epidemiological study. J Eur Acad Dermatol Venereol. 2008;22:1208-1214. doi:10.1111/j.1468-3083.2008.02778.x
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123. doi:10.1097/DER.0000000000000729
- Comaish JS, Cunliffe WJ. Absorption of drugs from varicose ulcers: a cause of anaphylaxis. Br J Clin Pract. 1967;21:97-98.
- Roupe G, Strannegård O. Anaphylactic shock elicited by topical administration of bacitracin. Arch Dermatol. 1969;100:450-452.
- Farley M, Pak H, Carregal V, et al. Anaphylaxis to topically applied bacitracin. Am J Contact Dermat. 1995;6:28-31.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60. doi:10.1034/j.1600-0536.2001.045001060.x
- Cooper SM, Shaw S. Contact allergy to nystatin: an unusual allergen. Contact Dermatitis. 1999;41:120. doi:10.1111/j.1600-0536.1999.tb06254.x
- Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77. doi:10.1111/j.1600-0536.1995.tb00504.x
- Pérez-Mesonero R, Schneller-Pavelescu L, Ochando-Ibernón G, et al. Is tioconazole contact dermatitis still a concern? bringing allergic contact dermatitis caused by topical tioconazole back into the spotlight. Contact Dermatitis. 2019;80:168-169.
- Tang MM, Corti MA, Stirnimann R, et al. Severe cutaneous allergic reactions following topical antifungal therapy. Contact Dermatitis. 2013;68:56-57.
- Goossens A, Linsen G. Contact allergy to antihistamines is not common. Contact Dermatitis. 1998;39:38. doi:10.1111/j.1600-0536.1998.tb05817.x
- Taylor JS, Praditsuwan P, Handel D, et al. Allergic contact dermatitis from doxepin cream. one-year patch test clinic experience. Arch Dermatol. 1996;132:515-518.
- Bilbao I, Aguirre A, Vicente JM, et al. Allergic contact dermatitis due to 5% doxepin cream. Contact Dermatitis. 1996;35:254-255. doi:10.1111/j.1600-0536.1996.tb02374.x
- Shelley WB, Shelley ED, Talanin NY. Self-potentiating allergic contact dermatitis caused by doxepin hydrochloride cream. J Am Acad Dermatol. 1996;34:143-144. doi:10.1016/s0190-9622(96)90864-6
- Wakelin SH, Rycroft RJ. Allergic contact dermatitis from doxepin. Contact Dermatitis. 1999;40:214. doi:10.1111/j.1600-0536.1999.tb06037.x
- Horn HM, Tidman MJ, Aldridge RD. Allergic contact dermatitis due to doxepin cream in a patient with dystrophic epidermolysis bullosa. Contact Dermatitis. 2001;45:115. doi:10.1034/j.1600-0536.2001.045002115.x
- Bonnel RA, La Grenade L, Karwoski CB, et al. Allergic contact dermatitis from topical doxepin: Food and Drug Administration’s postmarketing surveillance experience. J Am Acad Dermatol. 2003;48:294-296. doi:10.1067/mjd.2003.46
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166. doi:10.1111/j.1600-0536.2007.01296.x
- Foti C, Bonamonte D, Conserva A, et al. Allergic and photoallergic contact dermatitis from ketoprofen: evaluation of cross-reactivities by a combination of photopatch testing and computerized conformational analysis. Curr Pharm Des. 2008;14:2833-2839. doi:10.2174/138161208786369696
- Gulin SJ, Chiriac A. Diclofenac-induced allergic contact dermatitis: a series of four patients. Drug Saf Case Rep. 2016;3:15. doi:10.1007/s40800-016-0039-3
- Lakshmi C, Srinivas CR. Systemic (allergic) contact dermatitis to diclofenac. Indian J Dermatol Venereol Leprol. 2011;77:536. doi:10.4103/0378-6323.82424
- Beutner C, Forkel S, Kreipe K, et al. Contact allergy to topical diclofenac with systemic tolerance [published online August 22, 2021]. Contact Dermatitis. doi:10.1111/cod.13961
- Pan Y, Nixon R. Allergic contact dermatitis to topical preparations of bufexamac. Australas J Dermatol. 2012;53:207-210. doi:10.1111/j.1440-0960.2012.00876.x
- Nakada T, Matsuzawa Y. Allergic contact dermatitis syndrome from bufexamac for nursing infant. Dermatitis. 2012;23:185-186. doi:10.1097/DER.0b013e318260d774
- Kerr AC, Muller F, Ferguson J, et al. Occupational carprofen photoallergic contact dermatitis. Br J Dermatol. 2008;159:1303-1308. doi:10.1111/j.1365-2133.2008.08847.x
- Kiely C, Murphy G. Photoallergic contact dermatitis caused by occupational exposure to the canine non-steroidal anti-inflammatory drug carprofen. Contact Dermatitis. 2010;63:364-365. doi:10.1111/j.1600-0536.2010.01820.x
- Somberg J, Molnar J. Retrospective evaluation on the analgesic activities of 2 compounded topical creams and voltaren gel in chronic noncancer pain. Am J Ther. 2015;22:342-349. doi:10.1097/MJT.0000000000000275
- Lee HG, Grossman SK, Valdes-Rodriguez R, et al. Topical ketamine-amitriptyline-lidocaine for chronic pruritus: a retrospective study assessing efficacy and tolerability. J Am Acad Dermatol. 2017;76:760-761. doi:10.1016/j.jaad.2016.10.030
- Turrentine JE, Marrazzo G, Cruz PD Jr. Novel use of patch testing in the first report of allergic contact dermatitis to cyclobenzaprine. Dermatitis. 2015;26:60-61. doi:10.1097/DER.0000000000000099
- de Groot A. Patch Testing. 3rd ed. acdegroot publishing; 2008.
- de Groot A. Patch Testing. 4th ed. acdegroot publishing; 2018.
Practice Points
- Allergic contact dermatitis should be suspected in patients with persistent or worsening dermatitis after use of topical medications.
- Prior sensitization is not always apparent, and cross-reactions may occur between structurally similar compounds.
- Although most medicaments can be patch tested as is, patch testing to the individual components may be necessary to identify the causative allergen.
Granulomatous Facial Dermatoses
Cutaneous granulomatous diseases encompass many entities that are skin-limited or systemic. The prototypical cutaneous granuloma is a painless, rounded, well-defined, red-pink or flesh-colored papule1 and is smooth, owing to minimal epidermal involvement. Examples of conditions that present with such lesions include granulomatous periorificial dermatitis (GPD), granulomatous rosacea (GR), lupus miliaris disseminatus faciei (LMDF), and papular sarcoidosis. These entities commonly are seen on the face and can be a source of distress to patients when they are extensive. Several reports have raised the possibility that these conditions lie on a spectrum.2-4 We present 2 cases of patients with facial papular granulomas, discuss potential causes of the lesions, review historical aspects from the literature, and highlight the challenges that these lesions can pose to the clinician.
Case Reports
Patient 1—A 10-year-old Ethiopian girl with a history of atopic dermatitis presented with a facial rash of 4 months’ duration. Her pediatrician initially treated the rash as pityriasis alba and prescribed hydrocortisone cream. Two months into treatment, the patient developed an otherwise asymptomatic, unilateral, papular dermatosis on the right cheek. She subsequently was switched to treatment with benzoyl peroxide and topical clindamycin, which she had been using for 2 months with no improvement at the time of the current presentation. The lesions then spread bilaterally and periorally.
At the current presentation, physical examination demonstrated fine, diffuse, follicular-based, flesh-colored papules over both cheeks, the right side of the nose, and the perioral region (Figure 1). A biopsy of a papular lesion from the right cheek revealed well-formed, noncaseating granulomas in the superficial and mid dermis with an associated lymphocytic infiltrate (Figure 2). No organisms were identified on acid-fast, Fite, or periodic acid–Schiff staining. A tuberculin skin test was negative. A chest radiograph showed small calcified hilar lymph nodes bilaterally. Pulmonary function tests were unremarkable. Calcium and angiotensin-converting enzyme levels were normal.
The patient denied any fever, chills, hemoptysis, cough, dyspnea, lymphadenopathy, scleral or conjunctival pain or erythema, visual disturbances, or arthralgias. Hydroxychloroquine 200 mg twice daily was started with minimal improvement after 5 months. Methotrexate 20 mg once weekly was then added. Topical fluocinonide 0.05% also was started at this time, as the patient had required several prednisone tapers over the past 3 months for symptomatic relief. The lesions improved minimally after 5 more months of treatment, at which time she had developed inflammatory papules, pustules, and open comedones in the same areas as well as the glabella.
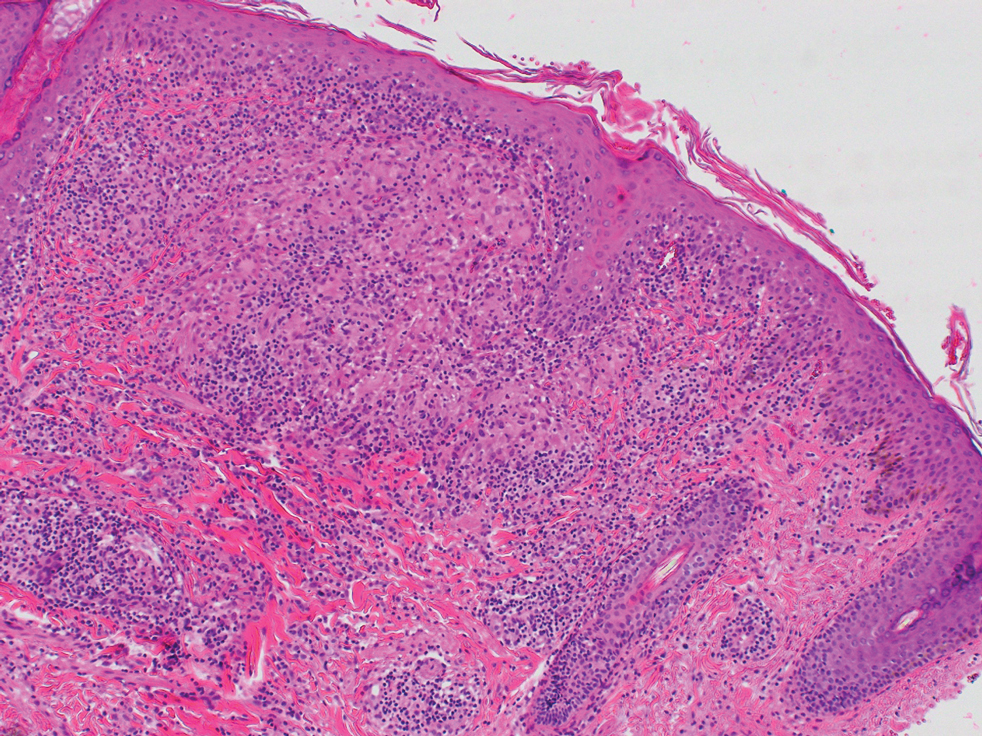
Repeat biopsy of a papular lesion demonstrated noncaseating granulomas and an associated chronic lymphocytic infiltrate in a follicular and perifollicular distribution (Figure 3). Biopsy of a pustule demonstrated acute Demodex folliculitis. Fluocinonide was stopped, and anti-mite therapy with ivermectin, permethrin cream 5%, and selenium sulfide lotion 2.5% was started, with good response from the pustular lesions.
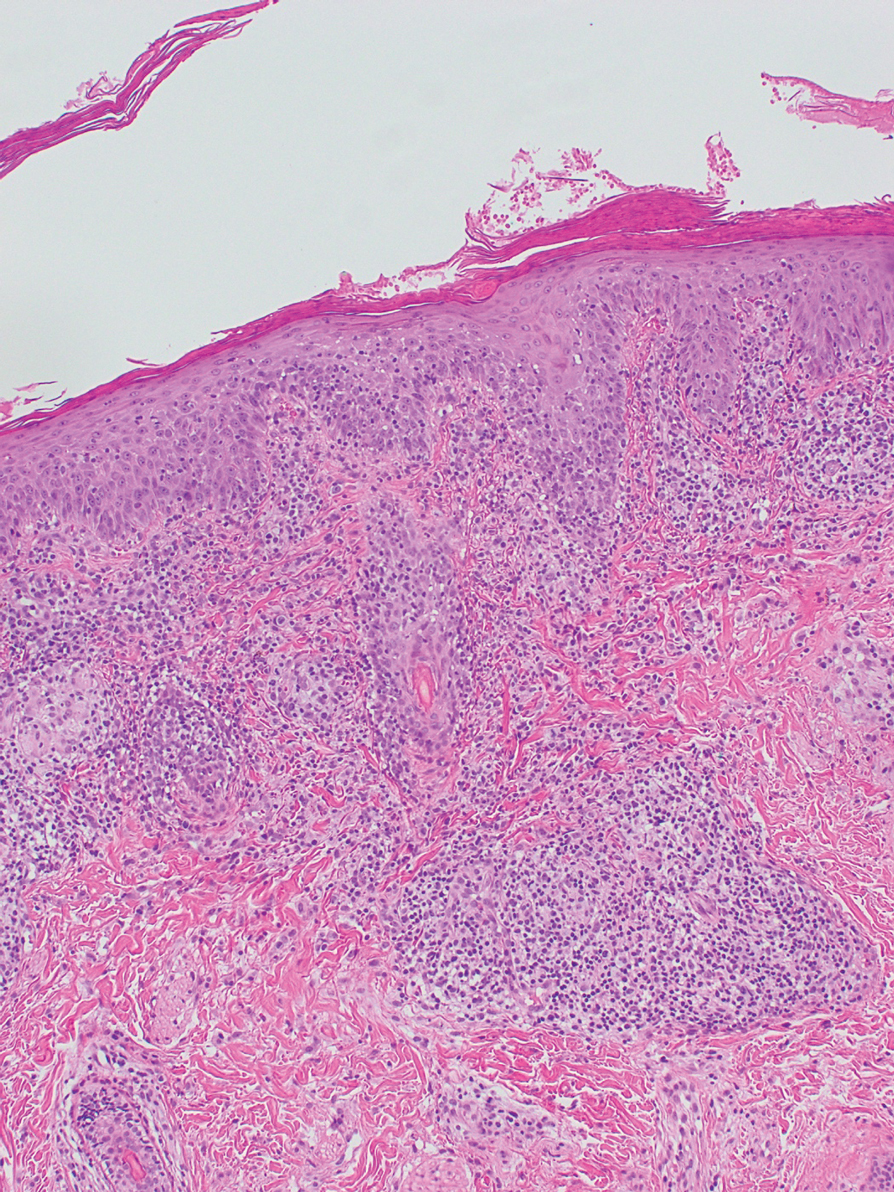
The patient continued taking methotrexate 20 mg once weekly during this time, with improvement in the papular lesions. She discontinued methotrexate after 12 months with complete resolution. At follow-up 12 months after stopping the methotrexate (roughly 2 years after initial presentation), she showed sustained resolution, with small pitted scars on both cheeks and the nasal tip.
Patient 2—A 33-year-old Ethiopian woman presented with a facial rash of 15 years’ duration. The lesions had been accumulating slowly and were asymptomatic. Physical examination revealed multiple follicular-based, flesh-colored, and erythematous papules on the cheeks, chin, perioral area, and forehead (Figure 4). There were no pustules or telangiectasias. Treatment with tretinoin cream 0.05% for 6 months offered minimal relief.
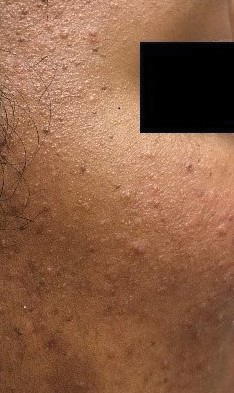
Biopsy of a papule from the left mandible showed superficial vascular telangiectasias, noncaseating granulomas comprising epithelioid histiocytes and lymphocytes in the superficial dermis, and a perifollicular lymphocytic infiltrate (Figure 5). No organisms were identified on Fite or Gomori methenamine silver staining.
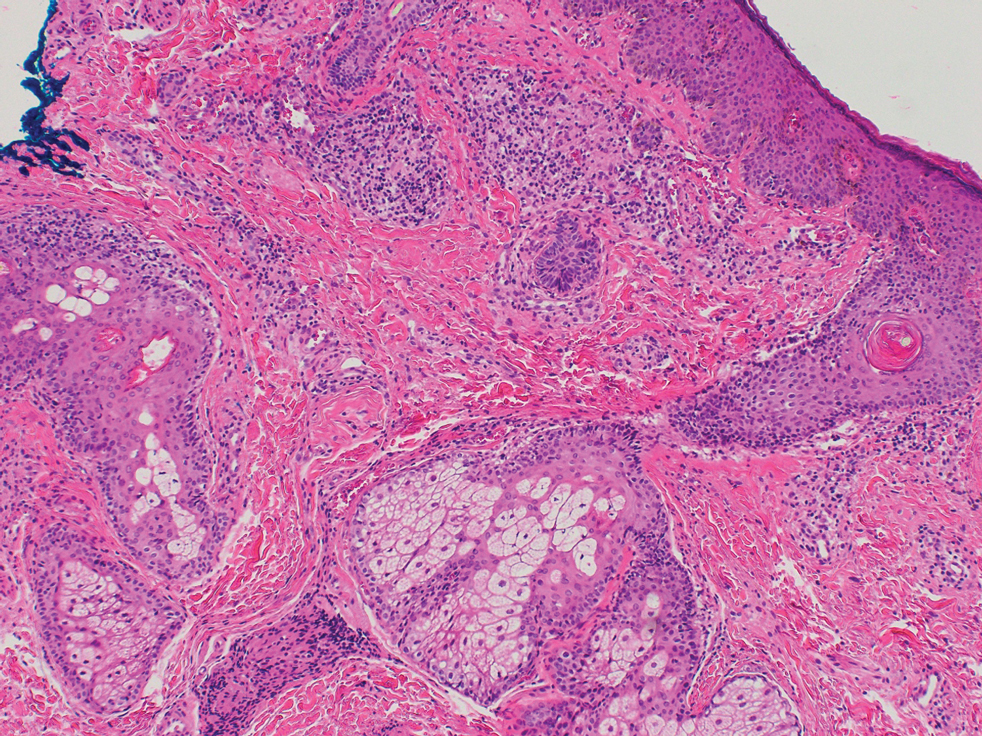
Comment
The first step in differentiating cutaneous granulomatous lesions should be to distinguish infectious from noninfectious causes.1 Noninfectious cutaneous granulomas can appear nearly anywhere; however, certain processes have a predilection for the face, including GPD, GR, LMDF, and papular sarcoidosis.5-7 These conditions generally present with papular granulomas with features as described above.
Granulomatous Periorificial Dermatitis—In 1970, Gianotti and colleagues8 briefly described the first possible cases of GPD in 5 children. The eruption comprised numerous yellow, dome-shaped papules in a mostly perioral distribution. Tuberculin and the Kveim tests were nonreactive; histopathology was described as sarcoid-type and not necessarily follicular or perifollicular.8 In 1974, Marten et al9 described 22 Afro-Caribbean children with flesh-colored, papular eruptions on the face that did not show histologic granulomatous changes but were morphologically similar to the reports by Gianotti et al.8 By 1989, Frieden and colleagues10 described this facial eruption as “granulomatous perioral dermatitis in children”. Additionally, the investigators observed granulomatous infiltrates in a perifollicular distribution and suggested follicular disruption as a possible cause. It was clear from the case discussions that these eruptions were not uncommonly diagnosed as papular sarcoidosis.10 The following year, Williams et al11 reported 5 cases of similar papular eruptions in 5 Afro-Caribbean children, coining the term facial Afro-Caribbean eruption.11 Knautz and Lesher12 referred to this entity as “childhood GPD” in 1996 to avoid limiting the diagnosis to Afro-Caribbean patients and to a perioral distribution; this is the most popular current terminology.12 Since then, reports of extrafacial involvement and disease in adults have been published.13,14
Granulomatous periorificial dermatitis often is seen in the perinasal, periocular, and perioral regions of the face.2 It is associated with topical steroid exposure.5 Histologically, noncaseating granulomas around the upper half of undisrupted hair follicles with a lymphocytic infiltrate are typical.13 Treatment should begin with cessation of any topical steroids; first-line agents are oral tetracycline or macrolide antibiotics.5 These agents can be used alone or in combination with topical erythromycin, metronidazole, or sulfur-based lotions.13 Rarely, GPD presents extrafacially.13 Even so, it usually resolves within 2 weeks to 6 months, especially with therapy; scarring is unusual.5,13,15
Granulomatous Rosacea—A report in the early 20th century described patients with tuberculoid granulomas resembling papular rosacea; the initial belief was that this finding represented a rosacealike tuberculid eruption.5 However, this belief was questioned by Snapp,16 among others, who demonstrated near universal lack of reactivity to tuberculin among 20 of these patients in 1949; more recent evidence has substantiated these findings.17 Still, Snapp16 postulated that these rosacealike granulomatous lesions were distinct from classic rosacea because they lacked vascular symptoms and pustules and were recalcitrant to rosacea treatment modalities.
In 1970, Mullanax and colleagues18 introduced the term granulomatous rosacea, reiterating that this entity was not tuberculous. They documented papulopustular lesions as well as telangiectasias, raising the possibility that GR does overlap with acne rosacea. More recent studies have established the current theory that GR is a histologic variant of acne rosacea because, in addition to typical granulomatous papules, its microscopic features can be seen across subtypes of acne rosacea.19,20
Various causes have been proposed for GR. Demodex mites have been reported in association with GR for nearly 30 years.19,20 In the past 10 years, molecular studies have started to define the role of metalloproteinases, UV radiation, and cutaneous peptides in the pathogenesis of acne rosacea and GR.21,22
Granulomatous rosacea typically is seen in middle-aged women.20,23 Hallmarks of rosacea, such as facial erythema, flushing, telangiectasias, pustules, and rhinophyma, are not always present in GR.5,20,23 Lesions usually are distributed around the central face, although extension to the cheeks, total facial involvement, and extrafacial lesions are possible.5,20 Histologically, perifollicular and follicular-based noncaseating granulomas with dilatation of the dermal papillary vasculature are seen.17,23 As a whole, rosacea is comparatively uncommon in dark-skinned patients; when it does occur, GR is a frequent presentation.24
First-line treatment for GR is tetracycline antibiotics.5 Unresponsive cases have been treated—largely anecdotally—with topical modalities (eg, metronidazole, steroids, immunomodulators), systemic agents (eg, dapsone, erythromycin, isotretinoin), and other therapies.5 Granulomatous rosacea tends to have a chronic course.5,23
Lupus Miliaris Disseminatus Faciei—Classic LMDF demonstrates caseating perifollicular granulomas histologically.6,17,25 Lesions tend to appear on the central face, particularly the eyelids, and can be seen extrafacially.3,6,25,26 Although LMDF originally was categorized as a tuberculid eruption, this no longer is thought to be the case.27 It is now regarded by some as a variant of GR25; however, LMDF responds poorly to tetracyclines, is more common in males, and lacks rosacealike vascular abnormalities, leading some to question this association.3,6,17 In the past 20 years, some have proposed renaming LMDF to better reflect its clinical course and to consider it independent of tuberculosis and GR.28 It usually resolves spontaneously after 1 to 3 years, leaving pitted scars.3,6
Papular Sarcoidosis—The first potential documented case of sarcoidosis was by Hutchinson29 in 1869 in a patient seen in London. The author labeled purple plaques on the index patient’s legs and hands as “livid papillary psoriasis.” In 1889, Besnier30 described a patient with violaceous swellings on the nose, ears, and fingers, which he called “lupus pernio”; his contemporary, Tenneson,31 published a case of lupus pernio and described its histologic profile as comprising epithelioid cells and giant cells. It was not until 1899 that the term sarkoid was used to describe these cutaneous lesions by Boeck,32 who thought they were reminiscent of sarcoma. In 1915, Kuznitsky and Bittorf33 described a patient with cutaneous lesions histologically consistent with Boeck’s sarkoid but additionally with hilar lymphadenopathy and pulmonary infiltrates. Around 1916 or 1917, Schaumann34 described patients with cutaneous lesions and additionally with involvement of pulmonary, osseous, hepatosplenic, and tonsillar tissue. These reports are among the first to recognize the multisystemic nature of sarcoidosis. The first possible case of childhood sarcoidosis might have been reported by Osler35 in the United States in 1898.
In the past century or so, an ongoing effort by researchers has focused on identifying etiologic triggers for sarcoidosis. Microbial agents have been considered in this role, with Mycobacterium and Propionibacterium organisms the most intensively studied; the possibility that foreign material contributes to the formation of granulomas also has been raised.36 Current models of the pathogenesis of sarcoidosis involve an interplay between the immune system in genetically predisposed patients and an infection that leads to a hyperimmune type 1 T–helper cell response that clears the infection but not antigens generated by the microbes and the acute host response, including proteins such as serum amyloid A and vimentin.36,37 These antigens aggregate and serve as a nidus for granuloma formation and maintenance long after infection has resolved.
Cutaneous lesions of sarcoidosis include macules, papules, plaques, and lupus pernio, as well as lesions arising within scars or tattoos, with many less common presentations.7,38 Papular sarcoidosis is common on the face but also can involve the extremities.4,7 Strictly, at least 2 organ systems must be involved to diagnose sarcoidosis, but this is debatable.4,7 Among 41 patients with cutaneous sarcoidosis, 24 (58.5%) had systemic disease; cutaneous lesions were the presenting sign in 87.5% (21/24) of patients.38 Histologic analysis, regardless of the lesion, usually shows noncaseating so-called “naked” granulomas, which have minimal lymphocytic infiltrate associated with the epithelioid histiocytes.38,39 Perifollicular granulomas are possible but unusual.40
Treatment depends on the extent of cutaneous and systemic involvement. Pharmacotherapeutic modalities include topical steroids, immunomodulators, and retinoids; systemic immunomodulators and immunosuppressants; and biologic agents.7 Isolated cutaneous sarcoidosis, particularly the papular variant, usually is associated with acute disease lasting less than 2 years, with resolution of skin lesions.7,38 That said, a recent report suggested that cutaneous sarcoidosis can progress to multisystemic disease as long as 7 years after the initial diagnosis.41
Clinical and Histologic Overlap—Despite this categorization of noninfectious facial granulomatous conditions, each has some clinical and histologic overlap with the others, which must be considered when encountering a granulomatous facial dermatosis. Both GPD and GR tend to present with lesions near the eyes, mouth, and nose, although GR can extend to lateral aspects of the face, below the mandible, and the forehead and has different demographic features.15,20,23 Granulomas in both GPD and GR generally are noncaseating and form in a follicular or perifollicular distribution within the dermis.2,15,23 Lupus miliaris disseminatus faciei and GR share a similar facial distribution in some cases.17,20 Even papular cutaneous sarcoidosis has masqueraded as GR clinically and histologically.4
Diagnostic and Treatment Difficulty—Our cases illustrate the range of difficulty in evaluating and managing patients with facial papular granulomas. On one hand, our adult patient’s clinical and histologic findings were highly consistent with GR; on the other hand, our younger patient had clinicopathologic features of both sarcoidosis and GPD at varying times. Both conditions are more common in dark-skinned patients.11,42
Juvenile sarcoidosis is comparatively rare, with a reported annual incidence of 0.22 to 0.27 for every 100,000 children younger than 15 years; however, juvenile sarcoidosis commonly presents around 8 to 15 years of age.43
It is unusual for sarcoid granulomas to be isolated to the skin, much less to the face.4,7,43,44 Patient 1 initially presented in this manner and lacked convincing laboratory or radiographic evidence of systemic sarcoidosis. Bilateral hilar calcifications in sarcoidosis are more typical among adults after 5 to 20 years; there were no signs or symptoms of active infection that could account for the pulmonary and cutaneous lesions.45
The presence of perifollicular granulomas with associated lymphocytic infiltrates on repeat biopsy, coupled with the use of topical steroids, made it difficult to rule out a contribution by GPD to her clinical course. That her lesions resolved with pitted scarring while she was taking methotrexate and after topical steroids had been stopped could be the result of successful management or spontaneous resolution of her dermatosis; both papular sarcoidosis and GPD tend to have a self-limited course.7,13
Conclusion
We present 2 cases of papular facial granulomas in patients with similar skin types who had different clinical courses. Evaluation of such lesions remains challenging given the similarity between specific entities that present in this manner. Certainly, it is reasonable to consider a spectrum upon which all of these conditions fall, in light of the findings of these cases and those reported previously.
- Beretta-Piccoli BT, Mainetti C, Peeters M-A, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146. doi:10.1007/s12016-017-8666-8
- Lucas CR, Korman NJ, Gilliam AC. Granulomatous periorificial dermatitis: a variant of granulomatous rosacea in children? J Cutan Med Surg. 2009;13:115-118. doi:10.2310/7750.2008.07088
- van de Scheur MR, van der Waal RIF, Starink TM. Lupus miliaris disseminatus faciei: a distinctive rosacea-like syndrome and not a granulomatous form of rosacea. Dermatology. 2003;206:120-123. doi:10.1159/000068457
- Simonart T, Lowy M, Rasquin F, et al. Overlap of sarcoidosis and rosacea. Dermatology. 1997;194:416-418. doi:10.1159/000246165
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management. Dermatol Clin. 2015;33:447-455. doi:10.1016/j.det.2015.03.009
- Michaels JD, Cook-Norris RH, Lehman JS, et al. Adult with papular eruption of the central aspect of the face. J Am Acad Dermatol. 2014;71:410-412. doi:10.1016/j.jaad.2012.06.039
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;38:685-702. doi:10.1016/j.ccm.2015.08.010
- Gianotti F, Ermacora E, Benelli MG, et al. Particulière dermatite peri-orale infantile. observations sur 5 cas. Bull Soc Fr Dermatol Syphiligr. 1970;77:341.
- Marten RH, Presbury DG, Adamson JE, et al. An unusual papular and acneiform facial eruption in the negro child. Br J Dermatol. 1974;91:435-438. doi:10.1111/j.1365-2133.1974.tb13083.x
- Frieden IJ, Prose NS, Fletcher V, et al. Granulomatous perioral dermatitis in children. Arch Dermatol. 1989;125:369-373.
- Williams HC, Ashworth J, Pembroke AC, et al. FACE—facial Afro-Caribbean childhood eruption. Clin Exp Dermatol. 1990;15:163-166. doi:10.1111/j.1365-2230.1990.tb02063.x
- Knautz MA, Lesher JL Jr. Childhood granulomatous periorificial dermatitis. Pediatr Dermatol. 1996;13:131-134. doi:10.1111/j.1525-1470.1996.tb01419.x
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358. doi:10.1001/archderm.138.10.1354
- Vincenzi C, Parente G, Tosti A. Perioral granulomatous dermatitis: two cases treated with clarithromycin. J Dermatol Treat. 2000;11:57-61.
- Kim YJ, Shin JW, Lee JS, et al. Childhood granulomatous periorificial dermatitis. Ann Dermatol. 2011;23:386-388. doi:10.5021/ad.2011.23.3.386
- Snapp RH. Lewandowsky’s rosacea-like eruption; a clinical study. J Invest Dermatol. 1949;13:175-190. doi:10.1038/jid.1949.86
- Chougule A, Chatterjee D, Sethi S, et al. Granulomatous rosacea versus lupus miliaris disseminatus faciei—2 faces of facial granulomatous disorder: a clinicohistological and molecular study. Am J Dermatopathol. 2018;40:819-823. doi:10.1097/DAD.0000000000001243
- Mullanax MG, Kierland RR. Granulomatous rosacea. Arch Dermatol. 1970;101:206-211.
- Sánchez JL, Berlingeri-Ramos AC, Dueño DV. Granulomatous rosacea. Am J Dermatopathol. 2008;30:6-9. doi:10.1097/DAD.0b013e31815bc191
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25:1038-1043. doi:10.1016/0190-9622(91)70304-k
- Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Invest Dermatol. 2012;132:1435-1442. doi:10.1038/jid.2012.14
- Jang YH, Sim JH, Kang HY, et al. Immunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the non-granulomatous rosacea. J Eur Acad Dermatol Venereol. 2011;25:544-548. doi:10.1111/j.1468-3083.2010.03825.x
- Khokhar O, Khachemoune A. A case of granulomatous rosacea: sorting granulomatous rosacea from other granulomatous diseases that affect the face. Dermatol Online J. 2004;10:6.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73. doi:10.1016/s0190-9622(87)70173-x
- Adams AK, Davis JL, Davis MDP, et al. What is your diagnosis? granulomatous rosacea (lupus miliaris disseminatus faciei, acne agminata). Cutis. 2008;82:103-112.
- Shitara A. Lupus miliaris disseminatus faciei. Int J Dermatol. 1984;23:542-544. doi:10.1111/j.1365-4362.1984.tb04206.x
- Hodak E, Trattner A, Feuerman H, et al. Lupus miliaris disseminatus faciei—the DNA of Mycobacterium tuberculosis is not detectable in active lesions by polymerase chain reaction. Br J Dermatol. 1997;137:614-619. doi: 10.1111/j.1365-2133.1997.tb03797.x
- Skowron F, Causeret AS, Pabion C, et al. F.I.GU.R.E.: facial idiopathic granulomas with regressive evolution. Dermatology. 2000;201:287-289. doi:10.1159/000051539
- Hutchinson J. Case of livid papillary psoriasis. In: London J, Churchill A, eds. Illustrations of Clinical Surgery. J&A Churchill; 1877:42-43.
- Besnier E. Lupus pernio of the face [in French]. Ann Dermatol Syphiligr (Paris). 1889;10:33-36.
- Tenneson H. Lupus pernio. Ann Dermatol Syphiligr (Paris). 1889;10:333-336.
- Boeck C. Multiple benign sarkoid of the skin [in Norwegian]. Norsk Mag Laegevidensk. 1899;14:1321-1334.
- Kuznitsky E, Bittorf A. Sarkoid mit beteiligung innerer organe. Münch Med Wochenschr. 1915;62:1349-1353.
- Schaumann J. Etude sur le lupus pernio et ses rapports avec les sarcoides et la tuberculose. Ann Dermatol Syphiligr. 1916-1917;6:357-373.
- Osler W. On chronic symmetrical enlargement of the salivary and lacrimal glands. Am J Med Sci. 1898;115:27-30.
- Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:6-18. doi:10.1007/s12016-015-8481-z
- Eberhardt C, Thillai M, Parker R, et al. Proteomic analysis of Kveim reagent identifies targets of cellular immunity in sarcoidosis. PLoS One. 2017;12:e0170285. doi:10.1371/journal.pone.0170285
- Esteves TC, Aparicio G, Ferrer B, et al. Prognostic value of skin lesions in sarcoidosis: clinical and histopathological clues. Eur J Dermatol. 2015;25:556-562. doi:10.1684/ejd.2015.2666
- Cardoso JC, Cravo M, Reis JP, et al. Cutaneous sarcoidosis: a histopathological study. J Eur Acad Dermatol Venereol. 2009;23:678-682. doi:10.1111/j.1468-3083.2009.03153.x
- Mangas C, Fernández-Figueras M-T, Fité E, et al. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol. 2006;33:772-777. doi:10.1111/j.1600-0560.2006.00563.x
- García-Colmenero L, Sánchez-Schmidt JM, Barranco C, et al. The natural history of cutaneous sarcoidosis. clinical spectrum and histological analysis of 40 cases. Int J Dermatol. 2019;58:178-184. doi: 10.1111/ijd.14218
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16. doi:10.1186/1546-0096-6-16
- Milman N, Hoffmann AL, Byg KE. Sarcoidosis in children. epidemiology in Danes, clinical features, diagnosis, treatment and prognosis. Acta Paediatr. 1998;87:871-878. doi:10.1080/08035259875001366244. A, H, Yapıcı I. Isolated cutaneous sarcoidosis. Arch Bronconeumol. 2016;52:220.
- Scadding JG. The late stages of pulmonary sarcoidosis. Postgrad Med J. 1970;46:530-536. doi:10.1136/pgmj.46.538.530
Cutaneous granulomatous diseases encompass many entities that are skin-limited or systemic. The prototypical cutaneous granuloma is a painless, rounded, well-defined, red-pink or flesh-colored papule1 and is smooth, owing to minimal epidermal involvement. Examples of conditions that present with such lesions include granulomatous periorificial dermatitis (GPD), granulomatous rosacea (GR), lupus miliaris disseminatus faciei (LMDF), and papular sarcoidosis. These entities commonly are seen on the face and can be a source of distress to patients when they are extensive. Several reports have raised the possibility that these conditions lie on a spectrum.2-4 We present 2 cases of patients with facial papular granulomas, discuss potential causes of the lesions, review historical aspects from the literature, and highlight the challenges that these lesions can pose to the clinician.
Case Reports
Patient 1—A 10-year-old Ethiopian girl with a history of atopic dermatitis presented with a facial rash of 4 months’ duration. Her pediatrician initially treated the rash as pityriasis alba and prescribed hydrocortisone cream. Two months into treatment, the patient developed an otherwise asymptomatic, unilateral, papular dermatosis on the right cheek. She subsequently was switched to treatment with benzoyl peroxide and topical clindamycin, which she had been using for 2 months with no improvement at the time of the current presentation. The lesions then spread bilaterally and periorally.
At the current presentation, physical examination demonstrated fine, diffuse, follicular-based, flesh-colored papules over both cheeks, the right side of the nose, and the perioral region (Figure 1). A biopsy of a papular lesion from the right cheek revealed well-formed, noncaseating granulomas in the superficial and mid dermis with an associated lymphocytic infiltrate (Figure 2). No organisms were identified on acid-fast, Fite, or periodic acid–Schiff staining. A tuberculin skin test was negative. A chest radiograph showed small calcified hilar lymph nodes bilaterally. Pulmonary function tests were unremarkable. Calcium and angiotensin-converting enzyme levels were normal.

The patient denied any fever, chills, hemoptysis, cough, dyspnea, lymphadenopathy, scleral or conjunctival pain or erythema, visual disturbances, or arthralgias. Hydroxychloroquine 200 mg twice daily was started with minimal improvement after 5 months. Methotrexate 20 mg once weekly was then added. Topical fluocinonide 0.05% also was started at this time, as the patient had required several prednisone tapers over the past 3 months for symptomatic relief. The lesions improved minimally after 5 more months of treatment, at which time she had developed inflammatory papules, pustules, and open comedones in the same areas as well as the glabella.

Repeat biopsy of a papular lesion demonstrated noncaseating granulomas and an associated chronic lymphocytic infiltrate in a follicular and perifollicular distribution (Figure 3). Biopsy of a pustule demonstrated acute Demodex folliculitis. Fluocinonide was stopped, and anti-mite therapy with ivermectin, permethrin cream 5%, and selenium sulfide lotion 2.5% was started, with good response from the pustular lesions.

The patient continued taking methotrexate 20 mg once weekly during this time, with improvement in the papular lesions. She discontinued methotrexate after 12 months with complete resolution. At follow-up 12 months after stopping the methotrexate (roughly 2 years after initial presentation), she showed sustained resolution, with small pitted scars on both cheeks and the nasal tip.
Patient 2—A 33-year-old Ethiopian woman presented with a facial rash of 15 years’ duration. The lesions had been accumulating slowly and were asymptomatic. Physical examination revealed multiple follicular-based, flesh-colored, and erythematous papules on the cheeks, chin, perioral area, and forehead (Figure 4). There were no pustules or telangiectasias. Treatment with tretinoin cream 0.05% for 6 months offered minimal relief.

Biopsy of a papule from the left mandible showed superficial vascular telangiectasias, noncaseating granulomas comprising epithelioid histiocytes and lymphocytes in the superficial dermis, and a perifollicular lymphocytic infiltrate (Figure 5). No organisms were identified on Fite or Gomori methenamine silver staining.

Comment
The first step in differentiating cutaneous granulomatous lesions should be to distinguish infectious from noninfectious causes.1 Noninfectious cutaneous granulomas can appear nearly anywhere; however, certain processes have a predilection for the face, including GPD, GR, LMDF, and papular sarcoidosis.5-7 These conditions generally present with papular granulomas with features as described above.
Granulomatous Periorificial Dermatitis—In 1970, Gianotti and colleagues8 briefly described the first possible cases of GPD in 5 children. The eruption comprised numerous yellow, dome-shaped papules in a mostly perioral distribution. Tuberculin and the Kveim tests were nonreactive; histopathology was described as sarcoid-type and not necessarily follicular or perifollicular.8 In 1974, Marten et al9 described 22 Afro-Caribbean children with flesh-colored, papular eruptions on the face that did not show histologic granulomatous changes but were morphologically similar to the reports by Gianotti et al.8 By 1989, Frieden and colleagues10 described this facial eruption as “granulomatous perioral dermatitis in children”. Additionally, the investigators observed granulomatous infiltrates in a perifollicular distribution and suggested follicular disruption as a possible cause. It was clear from the case discussions that these eruptions were not uncommonly diagnosed as papular sarcoidosis.10 The following year, Williams et al11 reported 5 cases of similar papular eruptions in 5 Afro-Caribbean children, coining the term facial Afro-Caribbean eruption.11 Knautz and Lesher12 referred to this entity as “childhood GPD” in 1996 to avoid limiting the diagnosis to Afro-Caribbean patients and to a perioral distribution; this is the most popular current terminology.12 Since then, reports of extrafacial involvement and disease in adults have been published.13,14
Granulomatous periorificial dermatitis often is seen in the perinasal, periocular, and perioral regions of the face.2 It is associated with topical steroid exposure.5 Histologically, noncaseating granulomas around the upper half of undisrupted hair follicles with a lymphocytic infiltrate are typical.13 Treatment should begin with cessation of any topical steroids; first-line agents are oral tetracycline or macrolide antibiotics.5 These agents can be used alone or in combination with topical erythromycin, metronidazole, or sulfur-based lotions.13 Rarely, GPD presents extrafacially.13 Even so, it usually resolves within 2 weeks to 6 months, especially with therapy; scarring is unusual.5,13,15
Granulomatous Rosacea—A report in the early 20th century described patients with tuberculoid granulomas resembling papular rosacea; the initial belief was that this finding represented a rosacealike tuberculid eruption.5 However, this belief was questioned by Snapp,16 among others, who demonstrated near universal lack of reactivity to tuberculin among 20 of these patients in 1949; more recent evidence has substantiated these findings.17 Still, Snapp16 postulated that these rosacealike granulomatous lesions were distinct from classic rosacea because they lacked vascular symptoms and pustules and were recalcitrant to rosacea treatment modalities.
In 1970, Mullanax and colleagues18 introduced the term granulomatous rosacea, reiterating that this entity was not tuberculous. They documented papulopustular lesions as well as telangiectasias, raising the possibility that GR does overlap with acne rosacea. More recent studies have established the current theory that GR is a histologic variant of acne rosacea because, in addition to typical granulomatous papules, its microscopic features can be seen across subtypes of acne rosacea.19,20
Various causes have been proposed for GR. Demodex mites have been reported in association with GR for nearly 30 years.19,20 In the past 10 years, molecular studies have started to define the role of metalloproteinases, UV radiation, and cutaneous peptides in the pathogenesis of acne rosacea and GR.21,22
Granulomatous rosacea typically is seen in middle-aged women.20,23 Hallmarks of rosacea, such as facial erythema, flushing, telangiectasias, pustules, and rhinophyma, are not always present in GR.5,20,23 Lesions usually are distributed around the central face, although extension to the cheeks, total facial involvement, and extrafacial lesions are possible.5,20 Histologically, perifollicular and follicular-based noncaseating granulomas with dilatation of the dermal papillary vasculature are seen.17,23 As a whole, rosacea is comparatively uncommon in dark-skinned patients; when it does occur, GR is a frequent presentation.24
First-line treatment for GR is tetracycline antibiotics.5 Unresponsive cases have been treated—largely anecdotally—with topical modalities (eg, metronidazole, steroids, immunomodulators), systemic agents (eg, dapsone, erythromycin, isotretinoin), and other therapies.5 Granulomatous rosacea tends to have a chronic course.5,23
Lupus Miliaris Disseminatus Faciei—Classic LMDF demonstrates caseating perifollicular granulomas histologically.6,17,25 Lesions tend to appear on the central face, particularly the eyelids, and can be seen extrafacially.3,6,25,26 Although LMDF originally was categorized as a tuberculid eruption, this no longer is thought to be the case.27 It is now regarded by some as a variant of GR25; however, LMDF responds poorly to tetracyclines, is more common in males, and lacks rosacealike vascular abnormalities, leading some to question this association.3,6,17 In the past 20 years, some have proposed renaming LMDF to better reflect its clinical course and to consider it independent of tuberculosis and GR.28 It usually resolves spontaneously after 1 to 3 years, leaving pitted scars.3,6
Papular Sarcoidosis—The first potential documented case of sarcoidosis was by Hutchinson29 in 1869 in a patient seen in London. The author labeled purple plaques on the index patient’s legs and hands as “livid papillary psoriasis.” In 1889, Besnier30 described a patient with violaceous swellings on the nose, ears, and fingers, which he called “lupus pernio”; his contemporary, Tenneson,31 published a case of lupus pernio and described its histologic profile as comprising epithelioid cells and giant cells. It was not until 1899 that the term sarkoid was used to describe these cutaneous lesions by Boeck,32 who thought they were reminiscent of sarcoma. In 1915, Kuznitsky and Bittorf33 described a patient with cutaneous lesions histologically consistent with Boeck’s sarkoid but additionally with hilar lymphadenopathy and pulmonary infiltrates. Around 1916 or 1917, Schaumann34 described patients with cutaneous lesions and additionally with involvement of pulmonary, osseous, hepatosplenic, and tonsillar tissue. These reports are among the first to recognize the multisystemic nature of sarcoidosis. The first possible case of childhood sarcoidosis might have been reported by Osler35 in the United States in 1898.
In the past century or so, an ongoing effort by researchers has focused on identifying etiologic triggers for sarcoidosis. Microbial agents have been considered in this role, with Mycobacterium and Propionibacterium organisms the most intensively studied; the possibility that foreign material contributes to the formation of granulomas also has been raised.36 Current models of the pathogenesis of sarcoidosis involve an interplay between the immune system in genetically predisposed patients and an infection that leads to a hyperimmune type 1 T–helper cell response that clears the infection but not antigens generated by the microbes and the acute host response, including proteins such as serum amyloid A and vimentin.36,37 These antigens aggregate and serve as a nidus for granuloma formation and maintenance long after infection has resolved.
Cutaneous lesions of sarcoidosis include macules, papules, plaques, and lupus pernio, as well as lesions arising within scars or tattoos, with many less common presentations.7,38 Papular sarcoidosis is common on the face but also can involve the extremities.4,7 Strictly, at least 2 organ systems must be involved to diagnose sarcoidosis, but this is debatable.4,7 Among 41 patients with cutaneous sarcoidosis, 24 (58.5%) had systemic disease; cutaneous lesions were the presenting sign in 87.5% (21/24) of patients.38 Histologic analysis, regardless of the lesion, usually shows noncaseating so-called “naked” granulomas, which have minimal lymphocytic infiltrate associated with the epithelioid histiocytes.38,39 Perifollicular granulomas are possible but unusual.40
Treatment depends on the extent of cutaneous and systemic involvement. Pharmacotherapeutic modalities include topical steroids, immunomodulators, and retinoids; systemic immunomodulators and immunosuppressants; and biologic agents.7 Isolated cutaneous sarcoidosis, particularly the papular variant, usually is associated with acute disease lasting less than 2 years, with resolution of skin lesions.7,38 That said, a recent report suggested that cutaneous sarcoidosis can progress to multisystemic disease as long as 7 years after the initial diagnosis.41
Clinical and Histologic Overlap—Despite this categorization of noninfectious facial granulomatous conditions, each has some clinical and histologic overlap with the others, which must be considered when encountering a granulomatous facial dermatosis. Both GPD and GR tend to present with lesions near the eyes, mouth, and nose, although GR can extend to lateral aspects of the face, below the mandible, and the forehead and has different demographic features.15,20,23 Granulomas in both GPD and GR generally are noncaseating and form in a follicular or perifollicular distribution within the dermis.2,15,23 Lupus miliaris disseminatus faciei and GR share a similar facial distribution in some cases.17,20 Even papular cutaneous sarcoidosis has masqueraded as GR clinically and histologically.4
Diagnostic and Treatment Difficulty—Our cases illustrate the range of difficulty in evaluating and managing patients with facial papular granulomas. On one hand, our adult patient’s clinical and histologic findings were highly consistent with GR; on the other hand, our younger patient had clinicopathologic features of both sarcoidosis and GPD at varying times. Both conditions are more common in dark-skinned patients.11,42
Juvenile sarcoidosis is comparatively rare, with a reported annual incidence of 0.22 to 0.27 for every 100,000 children younger than 15 years; however, juvenile sarcoidosis commonly presents around 8 to 15 years of age.43
It is unusual for sarcoid granulomas to be isolated to the skin, much less to the face.4,7,43,44 Patient 1 initially presented in this manner and lacked convincing laboratory or radiographic evidence of systemic sarcoidosis. Bilateral hilar calcifications in sarcoidosis are more typical among adults after 5 to 20 years; there were no signs or symptoms of active infection that could account for the pulmonary and cutaneous lesions.45
The presence of perifollicular granulomas with associated lymphocytic infiltrates on repeat biopsy, coupled with the use of topical steroids, made it difficult to rule out a contribution by GPD to her clinical course. That her lesions resolved with pitted scarring while she was taking methotrexate and after topical steroids had been stopped could be the result of successful management or spontaneous resolution of her dermatosis; both papular sarcoidosis and GPD tend to have a self-limited course.7,13
Conclusion
We present 2 cases of papular facial granulomas in patients with similar skin types who had different clinical courses. Evaluation of such lesions remains challenging given the similarity between specific entities that present in this manner. Certainly, it is reasonable to consider a spectrum upon which all of these conditions fall, in light of the findings of these cases and those reported previously.
Cutaneous granulomatous diseases encompass many entities that are skin-limited or systemic. The prototypical cutaneous granuloma is a painless, rounded, well-defined, red-pink or flesh-colored papule1 and is smooth, owing to minimal epidermal involvement. Examples of conditions that present with such lesions include granulomatous periorificial dermatitis (GPD), granulomatous rosacea (GR), lupus miliaris disseminatus faciei (LMDF), and papular sarcoidosis. These entities commonly are seen on the face and can be a source of distress to patients when they are extensive. Several reports have raised the possibility that these conditions lie on a spectrum.2-4 We present 2 cases of patients with facial papular granulomas, discuss potential causes of the lesions, review historical aspects from the literature, and highlight the challenges that these lesions can pose to the clinician.
Case Reports
Patient 1—A 10-year-old Ethiopian girl with a history of atopic dermatitis presented with a facial rash of 4 months’ duration. Her pediatrician initially treated the rash as pityriasis alba and prescribed hydrocortisone cream. Two months into treatment, the patient developed an otherwise asymptomatic, unilateral, papular dermatosis on the right cheek. She subsequently was switched to treatment with benzoyl peroxide and topical clindamycin, which she had been using for 2 months with no improvement at the time of the current presentation. The lesions then spread bilaterally and periorally.
At the current presentation, physical examination demonstrated fine, diffuse, follicular-based, flesh-colored papules over both cheeks, the right side of the nose, and the perioral region (Figure 1). A biopsy of a papular lesion from the right cheek revealed well-formed, noncaseating granulomas in the superficial and mid dermis with an associated lymphocytic infiltrate (Figure 2). No organisms were identified on acid-fast, Fite, or periodic acid–Schiff staining. A tuberculin skin test was negative. A chest radiograph showed small calcified hilar lymph nodes bilaterally. Pulmonary function tests were unremarkable. Calcium and angiotensin-converting enzyme levels were normal.

The patient denied any fever, chills, hemoptysis, cough, dyspnea, lymphadenopathy, scleral or conjunctival pain or erythema, visual disturbances, or arthralgias. Hydroxychloroquine 200 mg twice daily was started with minimal improvement after 5 months. Methotrexate 20 mg once weekly was then added. Topical fluocinonide 0.05% also was started at this time, as the patient had required several prednisone tapers over the past 3 months for symptomatic relief. The lesions improved minimally after 5 more months of treatment, at which time she had developed inflammatory papules, pustules, and open comedones in the same areas as well as the glabella.

Repeat biopsy of a papular lesion demonstrated noncaseating granulomas and an associated chronic lymphocytic infiltrate in a follicular and perifollicular distribution (Figure 3). Biopsy of a pustule demonstrated acute Demodex folliculitis. Fluocinonide was stopped, and anti-mite therapy with ivermectin, permethrin cream 5%, and selenium sulfide lotion 2.5% was started, with good response from the pustular lesions.

The patient continued taking methotrexate 20 mg once weekly during this time, with improvement in the papular lesions. She discontinued methotrexate after 12 months with complete resolution. At follow-up 12 months after stopping the methotrexate (roughly 2 years after initial presentation), she showed sustained resolution, with small pitted scars on both cheeks and the nasal tip.
Patient 2—A 33-year-old Ethiopian woman presented with a facial rash of 15 years’ duration. The lesions had been accumulating slowly and were asymptomatic. Physical examination revealed multiple follicular-based, flesh-colored, and erythematous papules on the cheeks, chin, perioral area, and forehead (Figure 4). There were no pustules or telangiectasias. Treatment with tretinoin cream 0.05% for 6 months offered minimal relief.

Biopsy of a papule from the left mandible showed superficial vascular telangiectasias, noncaseating granulomas comprising epithelioid histiocytes and lymphocytes in the superficial dermis, and a perifollicular lymphocytic infiltrate (Figure 5). No organisms were identified on Fite or Gomori methenamine silver staining.

Comment
The first step in differentiating cutaneous granulomatous lesions should be to distinguish infectious from noninfectious causes.1 Noninfectious cutaneous granulomas can appear nearly anywhere; however, certain processes have a predilection for the face, including GPD, GR, LMDF, and papular sarcoidosis.5-7 These conditions generally present with papular granulomas with features as described above.
Granulomatous Periorificial Dermatitis—In 1970, Gianotti and colleagues8 briefly described the first possible cases of GPD in 5 children. The eruption comprised numerous yellow, dome-shaped papules in a mostly perioral distribution. Tuberculin and the Kveim tests were nonreactive; histopathology was described as sarcoid-type and not necessarily follicular or perifollicular.8 In 1974, Marten et al9 described 22 Afro-Caribbean children with flesh-colored, papular eruptions on the face that did not show histologic granulomatous changes but were morphologically similar to the reports by Gianotti et al.8 By 1989, Frieden and colleagues10 described this facial eruption as “granulomatous perioral dermatitis in children”. Additionally, the investigators observed granulomatous infiltrates in a perifollicular distribution and suggested follicular disruption as a possible cause. It was clear from the case discussions that these eruptions were not uncommonly diagnosed as papular sarcoidosis.10 The following year, Williams et al11 reported 5 cases of similar papular eruptions in 5 Afro-Caribbean children, coining the term facial Afro-Caribbean eruption.11 Knautz and Lesher12 referred to this entity as “childhood GPD” in 1996 to avoid limiting the diagnosis to Afro-Caribbean patients and to a perioral distribution; this is the most popular current terminology.12 Since then, reports of extrafacial involvement and disease in adults have been published.13,14
Granulomatous periorificial dermatitis often is seen in the perinasal, periocular, and perioral regions of the face.2 It is associated with topical steroid exposure.5 Histologically, noncaseating granulomas around the upper half of undisrupted hair follicles with a lymphocytic infiltrate are typical.13 Treatment should begin with cessation of any topical steroids; first-line agents are oral tetracycline or macrolide antibiotics.5 These agents can be used alone or in combination with topical erythromycin, metronidazole, or sulfur-based lotions.13 Rarely, GPD presents extrafacially.13 Even so, it usually resolves within 2 weeks to 6 months, especially with therapy; scarring is unusual.5,13,15
Granulomatous Rosacea—A report in the early 20th century described patients with tuberculoid granulomas resembling papular rosacea; the initial belief was that this finding represented a rosacealike tuberculid eruption.5 However, this belief was questioned by Snapp,16 among others, who demonstrated near universal lack of reactivity to tuberculin among 20 of these patients in 1949; more recent evidence has substantiated these findings.17 Still, Snapp16 postulated that these rosacealike granulomatous lesions were distinct from classic rosacea because they lacked vascular symptoms and pustules and were recalcitrant to rosacea treatment modalities.
In 1970, Mullanax and colleagues18 introduced the term granulomatous rosacea, reiterating that this entity was not tuberculous. They documented papulopustular lesions as well as telangiectasias, raising the possibility that GR does overlap with acne rosacea. More recent studies have established the current theory that GR is a histologic variant of acne rosacea because, in addition to typical granulomatous papules, its microscopic features can be seen across subtypes of acne rosacea.19,20
Various causes have been proposed for GR. Demodex mites have been reported in association with GR for nearly 30 years.19,20 In the past 10 years, molecular studies have started to define the role of metalloproteinases, UV radiation, and cutaneous peptides in the pathogenesis of acne rosacea and GR.21,22
Granulomatous rosacea typically is seen in middle-aged women.20,23 Hallmarks of rosacea, such as facial erythema, flushing, telangiectasias, pustules, and rhinophyma, are not always present in GR.5,20,23 Lesions usually are distributed around the central face, although extension to the cheeks, total facial involvement, and extrafacial lesions are possible.5,20 Histologically, perifollicular and follicular-based noncaseating granulomas with dilatation of the dermal papillary vasculature are seen.17,23 As a whole, rosacea is comparatively uncommon in dark-skinned patients; when it does occur, GR is a frequent presentation.24
First-line treatment for GR is tetracycline antibiotics.5 Unresponsive cases have been treated—largely anecdotally—with topical modalities (eg, metronidazole, steroids, immunomodulators), systemic agents (eg, dapsone, erythromycin, isotretinoin), and other therapies.5 Granulomatous rosacea tends to have a chronic course.5,23
Lupus Miliaris Disseminatus Faciei—Classic LMDF demonstrates caseating perifollicular granulomas histologically.6,17,25 Lesions tend to appear on the central face, particularly the eyelids, and can be seen extrafacially.3,6,25,26 Although LMDF originally was categorized as a tuberculid eruption, this no longer is thought to be the case.27 It is now regarded by some as a variant of GR25; however, LMDF responds poorly to tetracyclines, is more common in males, and lacks rosacealike vascular abnormalities, leading some to question this association.3,6,17 In the past 20 years, some have proposed renaming LMDF to better reflect its clinical course and to consider it independent of tuberculosis and GR.28 It usually resolves spontaneously after 1 to 3 years, leaving pitted scars.3,6
Papular Sarcoidosis—The first potential documented case of sarcoidosis was by Hutchinson29 in 1869 in a patient seen in London. The author labeled purple plaques on the index patient’s legs and hands as “livid papillary psoriasis.” In 1889, Besnier30 described a patient with violaceous swellings on the nose, ears, and fingers, which he called “lupus pernio”; his contemporary, Tenneson,31 published a case of lupus pernio and described its histologic profile as comprising epithelioid cells and giant cells. It was not until 1899 that the term sarkoid was used to describe these cutaneous lesions by Boeck,32 who thought they were reminiscent of sarcoma. In 1915, Kuznitsky and Bittorf33 described a patient with cutaneous lesions histologically consistent with Boeck’s sarkoid but additionally with hilar lymphadenopathy and pulmonary infiltrates. Around 1916 or 1917, Schaumann34 described patients with cutaneous lesions and additionally with involvement of pulmonary, osseous, hepatosplenic, and tonsillar tissue. These reports are among the first to recognize the multisystemic nature of sarcoidosis. The first possible case of childhood sarcoidosis might have been reported by Osler35 in the United States in 1898.
In the past century or so, an ongoing effort by researchers has focused on identifying etiologic triggers for sarcoidosis. Microbial agents have been considered in this role, with Mycobacterium and Propionibacterium organisms the most intensively studied; the possibility that foreign material contributes to the formation of granulomas also has been raised.36 Current models of the pathogenesis of sarcoidosis involve an interplay between the immune system in genetically predisposed patients and an infection that leads to a hyperimmune type 1 T–helper cell response that clears the infection but not antigens generated by the microbes and the acute host response, including proteins such as serum amyloid A and vimentin.36,37 These antigens aggregate and serve as a nidus for granuloma formation and maintenance long after infection has resolved.
Cutaneous lesions of sarcoidosis include macules, papules, plaques, and lupus pernio, as well as lesions arising within scars or tattoos, with many less common presentations.7,38 Papular sarcoidosis is common on the face but also can involve the extremities.4,7 Strictly, at least 2 organ systems must be involved to diagnose sarcoidosis, but this is debatable.4,7 Among 41 patients with cutaneous sarcoidosis, 24 (58.5%) had systemic disease; cutaneous lesions were the presenting sign in 87.5% (21/24) of patients.38 Histologic analysis, regardless of the lesion, usually shows noncaseating so-called “naked” granulomas, which have minimal lymphocytic infiltrate associated with the epithelioid histiocytes.38,39 Perifollicular granulomas are possible but unusual.40
Treatment depends on the extent of cutaneous and systemic involvement. Pharmacotherapeutic modalities include topical steroids, immunomodulators, and retinoids; systemic immunomodulators and immunosuppressants; and biologic agents.7 Isolated cutaneous sarcoidosis, particularly the papular variant, usually is associated with acute disease lasting less than 2 years, with resolution of skin lesions.7,38 That said, a recent report suggested that cutaneous sarcoidosis can progress to multisystemic disease as long as 7 years after the initial diagnosis.41
Clinical and Histologic Overlap—Despite this categorization of noninfectious facial granulomatous conditions, each has some clinical and histologic overlap with the others, which must be considered when encountering a granulomatous facial dermatosis. Both GPD and GR tend to present with lesions near the eyes, mouth, and nose, although GR can extend to lateral aspects of the face, below the mandible, and the forehead and has different demographic features.15,20,23 Granulomas in both GPD and GR generally are noncaseating and form in a follicular or perifollicular distribution within the dermis.2,15,23 Lupus miliaris disseminatus faciei and GR share a similar facial distribution in some cases.17,20 Even papular cutaneous sarcoidosis has masqueraded as GR clinically and histologically.4
Diagnostic and Treatment Difficulty—Our cases illustrate the range of difficulty in evaluating and managing patients with facial papular granulomas. On one hand, our adult patient’s clinical and histologic findings were highly consistent with GR; on the other hand, our younger patient had clinicopathologic features of both sarcoidosis and GPD at varying times. Both conditions are more common in dark-skinned patients.11,42
Juvenile sarcoidosis is comparatively rare, with a reported annual incidence of 0.22 to 0.27 for every 100,000 children younger than 15 years; however, juvenile sarcoidosis commonly presents around 8 to 15 years of age.43
It is unusual for sarcoid granulomas to be isolated to the skin, much less to the face.4,7,43,44 Patient 1 initially presented in this manner and lacked convincing laboratory or radiographic evidence of systemic sarcoidosis. Bilateral hilar calcifications in sarcoidosis are more typical among adults after 5 to 20 years; there were no signs or symptoms of active infection that could account for the pulmonary and cutaneous lesions.45
The presence of perifollicular granulomas with associated lymphocytic infiltrates on repeat biopsy, coupled with the use of topical steroids, made it difficult to rule out a contribution by GPD to her clinical course. That her lesions resolved with pitted scarring while she was taking methotrexate and after topical steroids had been stopped could be the result of successful management or spontaneous resolution of her dermatosis; both papular sarcoidosis and GPD tend to have a self-limited course.7,13
Conclusion
We present 2 cases of papular facial granulomas in patients with similar skin types who had different clinical courses. Evaluation of such lesions remains challenging given the similarity between specific entities that present in this manner. Certainly, it is reasonable to consider a spectrum upon which all of these conditions fall, in light of the findings of these cases and those reported previously.
- Beretta-Piccoli BT, Mainetti C, Peeters M-A, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146. doi:10.1007/s12016-017-8666-8
- Lucas CR, Korman NJ, Gilliam AC. Granulomatous periorificial dermatitis: a variant of granulomatous rosacea in children? J Cutan Med Surg. 2009;13:115-118. doi:10.2310/7750.2008.07088
- van de Scheur MR, van der Waal RIF, Starink TM. Lupus miliaris disseminatus faciei: a distinctive rosacea-like syndrome and not a granulomatous form of rosacea. Dermatology. 2003;206:120-123. doi:10.1159/000068457
- Simonart T, Lowy M, Rasquin F, et al. Overlap of sarcoidosis and rosacea. Dermatology. 1997;194:416-418. doi:10.1159/000246165
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management. Dermatol Clin. 2015;33:447-455. doi:10.1016/j.det.2015.03.009
- Michaels JD, Cook-Norris RH, Lehman JS, et al. Adult with papular eruption of the central aspect of the face. J Am Acad Dermatol. 2014;71:410-412. doi:10.1016/j.jaad.2012.06.039
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;38:685-702. doi:10.1016/j.ccm.2015.08.010
- Gianotti F, Ermacora E, Benelli MG, et al. Particulière dermatite peri-orale infantile. observations sur 5 cas. Bull Soc Fr Dermatol Syphiligr. 1970;77:341.
- Marten RH, Presbury DG, Adamson JE, et al. An unusual papular and acneiform facial eruption in the negro child. Br J Dermatol. 1974;91:435-438. doi:10.1111/j.1365-2133.1974.tb13083.x
- Frieden IJ, Prose NS, Fletcher V, et al. Granulomatous perioral dermatitis in children. Arch Dermatol. 1989;125:369-373.
- Williams HC, Ashworth J, Pembroke AC, et al. FACE—facial Afro-Caribbean childhood eruption. Clin Exp Dermatol. 1990;15:163-166. doi:10.1111/j.1365-2230.1990.tb02063.x
- Knautz MA, Lesher JL Jr. Childhood granulomatous periorificial dermatitis. Pediatr Dermatol. 1996;13:131-134. doi:10.1111/j.1525-1470.1996.tb01419.x
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358. doi:10.1001/archderm.138.10.1354
- Vincenzi C, Parente G, Tosti A. Perioral granulomatous dermatitis: two cases treated with clarithromycin. J Dermatol Treat. 2000;11:57-61.
- Kim YJ, Shin JW, Lee JS, et al. Childhood granulomatous periorificial dermatitis. Ann Dermatol. 2011;23:386-388. doi:10.5021/ad.2011.23.3.386
- Snapp RH. Lewandowsky’s rosacea-like eruption; a clinical study. J Invest Dermatol. 1949;13:175-190. doi:10.1038/jid.1949.86
- Chougule A, Chatterjee D, Sethi S, et al. Granulomatous rosacea versus lupus miliaris disseminatus faciei—2 faces of facial granulomatous disorder: a clinicohistological and molecular study. Am J Dermatopathol. 2018;40:819-823. doi:10.1097/DAD.0000000000001243
- Mullanax MG, Kierland RR. Granulomatous rosacea. Arch Dermatol. 1970;101:206-211.
- Sánchez JL, Berlingeri-Ramos AC, Dueño DV. Granulomatous rosacea. Am J Dermatopathol. 2008;30:6-9. doi:10.1097/DAD.0b013e31815bc191
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25:1038-1043. doi:10.1016/0190-9622(91)70304-k
- Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Invest Dermatol. 2012;132:1435-1442. doi:10.1038/jid.2012.14
- Jang YH, Sim JH, Kang HY, et al. Immunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the non-granulomatous rosacea. J Eur Acad Dermatol Venereol. 2011;25:544-548. doi:10.1111/j.1468-3083.2010.03825.x
- Khokhar O, Khachemoune A. A case of granulomatous rosacea: sorting granulomatous rosacea from other granulomatous diseases that affect the face. Dermatol Online J. 2004;10:6.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73. doi:10.1016/s0190-9622(87)70173-x
- Adams AK, Davis JL, Davis MDP, et al. What is your diagnosis? granulomatous rosacea (lupus miliaris disseminatus faciei, acne agminata). Cutis. 2008;82:103-112.
- Shitara A. Lupus miliaris disseminatus faciei. Int J Dermatol. 1984;23:542-544. doi:10.1111/j.1365-4362.1984.tb04206.x
- Hodak E, Trattner A, Feuerman H, et al. Lupus miliaris disseminatus faciei—the DNA of Mycobacterium tuberculosis is not detectable in active lesions by polymerase chain reaction. Br J Dermatol. 1997;137:614-619. doi: 10.1111/j.1365-2133.1997.tb03797.x
- Skowron F, Causeret AS, Pabion C, et al. F.I.GU.R.E.: facial idiopathic granulomas with regressive evolution. Dermatology. 2000;201:287-289. doi:10.1159/000051539
- Hutchinson J. Case of livid papillary psoriasis. In: London J, Churchill A, eds. Illustrations of Clinical Surgery. J&A Churchill; 1877:42-43.
- Besnier E. Lupus pernio of the face [in French]. Ann Dermatol Syphiligr (Paris). 1889;10:33-36.
- Tenneson H. Lupus pernio. Ann Dermatol Syphiligr (Paris). 1889;10:333-336.
- Boeck C. Multiple benign sarkoid of the skin [in Norwegian]. Norsk Mag Laegevidensk. 1899;14:1321-1334.
- Kuznitsky E, Bittorf A. Sarkoid mit beteiligung innerer organe. Münch Med Wochenschr. 1915;62:1349-1353.
- Schaumann J. Etude sur le lupus pernio et ses rapports avec les sarcoides et la tuberculose. Ann Dermatol Syphiligr. 1916-1917;6:357-373.
- Osler W. On chronic symmetrical enlargement of the salivary and lacrimal glands. Am J Med Sci. 1898;115:27-30.
- Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:6-18. doi:10.1007/s12016-015-8481-z
- Eberhardt C, Thillai M, Parker R, et al. Proteomic analysis of Kveim reagent identifies targets of cellular immunity in sarcoidosis. PLoS One. 2017;12:e0170285. doi:10.1371/journal.pone.0170285
- Esteves TC, Aparicio G, Ferrer B, et al. Prognostic value of skin lesions in sarcoidosis: clinical and histopathological clues. Eur J Dermatol. 2015;25:556-562. doi:10.1684/ejd.2015.2666
- Cardoso JC, Cravo M, Reis JP, et al. Cutaneous sarcoidosis: a histopathological study. J Eur Acad Dermatol Venereol. 2009;23:678-682. doi:10.1111/j.1468-3083.2009.03153.x
- Mangas C, Fernández-Figueras M-T, Fité E, et al. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol. 2006;33:772-777. doi:10.1111/j.1600-0560.2006.00563.x
- García-Colmenero L, Sánchez-Schmidt JM, Barranco C, et al. The natural history of cutaneous sarcoidosis. clinical spectrum and histological analysis of 40 cases. Int J Dermatol. 2019;58:178-184. doi: 10.1111/ijd.14218
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16. doi:10.1186/1546-0096-6-16
- Milman N, Hoffmann AL, Byg KE. Sarcoidosis in children. epidemiology in Danes, clinical features, diagnosis, treatment and prognosis. Acta Paediatr. 1998;87:871-878. doi:10.1080/08035259875001366244. A, H, Yapıcı I. Isolated cutaneous sarcoidosis. Arch Bronconeumol. 2016;52:220.
- Scadding JG. The late stages of pulmonary sarcoidosis. Postgrad Med J. 1970;46:530-536. doi:10.1136/pgmj.46.538.530
- Beretta-Piccoli BT, Mainetti C, Peeters M-A, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146. doi:10.1007/s12016-017-8666-8
- Lucas CR, Korman NJ, Gilliam AC. Granulomatous periorificial dermatitis: a variant of granulomatous rosacea in children? J Cutan Med Surg. 2009;13:115-118. doi:10.2310/7750.2008.07088
- van de Scheur MR, van der Waal RIF, Starink TM. Lupus miliaris disseminatus faciei: a distinctive rosacea-like syndrome and not a granulomatous form of rosacea. Dermatology. 2003;206:120-123. doi:10.1159/000068457
- Simonart T, Lowy M, Rasquin F, et al. Overlap of sarcoidosis and rosacea. Dermatology. 1997;194:416-418. doi:10.1159/000246165
- Lee GL, Zirwas MJ. Granulomatous rosacea and periorificial dermatitis: controversies and review of management. Dermatol Clin. 2015;33:447-455. doi:10.1016/j.det.2015.03.009
- Michaels JD, Cook-Norris RH, Lehman JS, et al. Adult with papular eruption of the central aspect of the face. J Am Acad Dermatol. 2014;71:410-412. doi:10.1016/j.jaad.2012.06.039
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;38:685-702. doi:10.1016/j.ccm.2015.08.010
- Gianotti F, Ermacora E, Benelli MG, et al. Particulière dermatite peri-orale infantile. observations sur 5 cas. Bull Soc Fr Dermatol Syphiligr. 1970;77:341.
- Marten RH, Presbury DG, Adamson JE, et al. An unusual papular and acneiform facial eruption in the negro child. Br J Dermatol. 1974;91:435-438. doi:10.1111/j.1365-2133.1974.tb13083.x
- Frieden IJ, Prose NS, Fletcher V, et al. Granulomatous perioral dermatitis in children. Arch Dermatol. 1989;125:369-373.
- Williams HC, Ashworth J, Pembroke AC, et al. FACE—facial Afro-Caribbean childhood eruption. Clin Exp Dermatol. 1990;15:163-166. doi:10.1111/j.1365-2230.1990.tb02063.x
- Knautz MA, Lesher JL Jr. Childhood granulomatous periorificial dermatitis. Pediatr Dermatol. 1996;13:131-134. doi:10.1111/j.1525-1470.1996.tb01419.x
- Urbatsch AJ, Frieden I, Williams ML, et al. Extrafacial and generalized granulomatous periorificial dermatitis. Arch Dermatol. 2002;138:1354-1358. doi:10.1001/archderm.138.10.1354
- Vincenzi C, Parente G, Tosti A. Perioral granulomatous dermatitis: two cases treated with clarithromycin. J Dermatol Treat. 2000;11:57-61.
- Kim YJ, Shin JW, Lee JS, et al. Childhood granulomatous periorificial dermatitis. Ann Dermatol. 2011;23:386-388. doi:10.5021/ad.2011.23.3.386
- Snapp RH. Lewandowsky’s rosacea-like eruption; a clinical study. J Invest Dermatol. 1949;13:175-190. doi:10.1038/jid.1949.86
- Chougule A, Chatterjee D, Sethi S, et al. Granulomatous rosacea versus lupus miliaris disseminatus faciei—2 faces of facial granulomatous disorder: a clinicohistological and molecular study. Am J Dermatopathol. 2018;40:819-823. doi:10.1097/DAD.0000000000001243
- Mullanax MG, Kierland RR. Granulomatous rosacea. Arch Dermatol. 1970;101:206-211.
- Sánchez JL, Berlingeri-Ramos AC, Dueño DV. Granulomatous rosacea. Am J Dermatopathol. 2008;30:6-9. doi:10.1097/DAD.0b013e31815bc191
- Helm KF, Menz J, Gibson LE, et al. A clinical and histopathologic study of granulomatous rosacea. J Am Acad Dermatol. 1991;25:1038-1043. doi:10.1016/0190-9622(91)70304-k
- Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Invest Dermatol. 2012;132:1435-1442. doi:10.1038/jid.2012.14
- Jang YH, Sim JH, Kang HY, et al. Immunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the non-granulomatous rosacea. J Eur Acad Dermatol Venereol. 2011;25:544-548. doi:10.1111/j.1468-3083.2010.03825.x
- Khokhar O, Khachemoune A. A case of granulomatous rosacea: sorting granulomatous rosacea from other granulomatous diseases that affect the face. Dermatol Online J. 2004;10:6.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73. doi:10.1016/s0190-9622(87)70173-x
- Adams AK, Davis JL, Davis MDP, et al. What is your diagnosis? granulomatous rosacea (lupus miliaris disseminatus faciei, acne agminata). Cutis. 2008;82:103-112.
- Shitara A. Lupus miliaris disseminatus faciei. Int J Dermatol. 1984;23:542-544. doi:10.1111/j.1365-4362.1984.tb04206.x
- Hodak E, Trattner A, Feuerman H, et al. Lupus miliaris disseminatus faciei—the DNA of Mycobacterium tuberculosis is not detectable in active lesions by polymerase chain reaction. Br J Dermatol. 1997;137:614-619. doi: 10.1111/j.1365-2133.1997.tb03797.x
- Skowron F, Causeret AS, Pabion C, et al. F.I.GU.R.E.: facial idiopathic granulomas with regressive evolution. Dermatology. 2000;201:287-289. doi:10.1159/000051539
- Hutchinson J. Case of livid papillary psoriasis. In: London J, Churchill A, eds. Illustrations of Clinical Surgery. J&A Churchill; 1877:42-43.
- Besnier E. Lupus pernio of the face [in French]. Ann Dermatol Syphiligr (Paris). 1889;10:33-36.
- Tenneson H. Lupus pernio. Ann Dermatol Syphiligr (Paris). 1889;10:333-336.
- Boeck C. Multiple benign sarkoid of the skin [in Norwegian]. Norsk Mag Laegevidensk. 1899;14:1321-1334.
- Kuznitsky E, Bittorf A. Sarkoid mit beteiligung innerer organe. Münch Med Wochenschr. 1915;62:1349-1353.
- Schaumann J. Etude sur le lupus pernio et ses rapports avec les sarcoides et la tuberculose. Ann Dermatol Syphiligr. 1916-1917;6:357-373.
- Osler W. On chronic symmetrical enlargement of the salivary and lacrimal glands. Am J Med Sci. 1898;115:27-30.
- Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:6-18. doi:10.1007/s12016-015-8481-z
- Eberhardt C, Thillai M, Parker R, et al. Proteomic analysis of Kveim reagent identifies targets of cellular immunity in sarcoidosis. PLoS One. 2017;12:e0170285. doi:10.1371/journal.pone.0170285
- Esteves TC, Aparicio G, Ferrer B, et al. Prognostic value of skin lesions in sarcoidosis: clinical and histopathological clues. Eur J Dermatol. 2015;25:556-562. doi:10.1684/ejd.2015.2666
- Cardoso JC, Cravo M, Reis JP, et al. Cutaneous sarcoidosis: a histopathological study. J Eur Acad Dermatol Venereol. 2009;23:678-682. doi:10.1111/j.1468-3083.2009.03153.x
- Mangas C, Fernández-Figueras M-T, Fité E, et al. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol. 2006;33:772-777. doi:10.1111/j.1600-0560.2006.00563.x
- García-Colmenero L, Sánchez-Schmidt JM, Barranco C, et al. The natural history of cutaneous sarcoidosis. clinical spectrum and histological analysis of 40 cases. Int J Dermatol. 2019;58:178-184. doi: 10.1111/ijd.14218
- Shetty AK, Gedalia A. Childhood sarcoidosis: a rare but fascinating disorder. Pediatr Rheumatol Online J. 2008;6:16. doi:10.1186/1546-0096-6-16
- Milman N, Hoffmann AL, Byg KE. Sarcoidosis in children. epidemiology in Danes, clinical features, diagnosis, treatment and prognosis. Acta Paediatr. 1998;87:871-878. doi:10.1080/08035259875001366244. A, H, Yapıcı I. Isolated cutaneous sarcoidosis. Arch Bronconeumol. 2016;52:220.
- Scadding JG. The late stages of pulmonary sarcoidosis. Postgrad Med J. 1970;46:530-536. doi:10.1136/pgmj.46.538.530
Practice Points
- Dermatologists should be aware that noninfectious granulomatous dermatosis of the face can be caused by granulomatous periorificial dermatitis, granulomatous rosacea, lupus miliaris disseminatus faciei, and papular sarcoidosis.
- These conditions lie on a spectrum, suggested by their historical description and clinical and histological features.
- Because their clinical courses can vary considerably from patient to patient, a thorough effort should be made to differentiate these conditions.
Unexpected Complications: A Case of Rosacea Fulminans in Pregnancy
Rosacea fulminans (RF) is a rare facial dermatosis characterized by its fulminating course. 1 It presents with superficial and deep-seated papules, pustules, and nodules combined with an intense reddish or cyanotic erythema localized to the face. Furthermore, there is an absence of comedones and involvement of the chest or back. 2 Rosacea fulminans primarily affects women and often is, but not always, proceeded by seborrhea, chronic acne vulgaris, or rosacea. Although the etiology of RF remains unknown, immunologic, hormonal, and vascular factors have been implicated. 3 We report a case of RF in a pregnant patient with a history of mild acne as a teenager that was long ago resolved.
Case Report
A 32-year-old pregnant woman (10 weeks’ gestation) presented with a rapidly progressing inflammatory disorder of the face of 1 month’s duration. The lesions developed 3 weeks after beginning progesterone therapy (200 mg vaginal suppository) for infertility due to polycystic ovary syndrome. Despite discontinuing progesterone for the last month, the patient’s lesions had dramatically worsened (Figure 1). Empiric cephalosporin treatment prescribed by her primary care physician yielded no improvement. Physical examination at the current presentation revealed erythematous nodules and pustules all over the face, coalescing into large thick plaques on the patient’s right cheek and chin. Submental nodes were palpable and tender. Based on the initial clinical findings, acne conglobata secondary to progesterone therapy was considered. The patient was given intralesional triamcinolone (2.5 mg/cc) injections to all larger nodules and several blue light treatments.
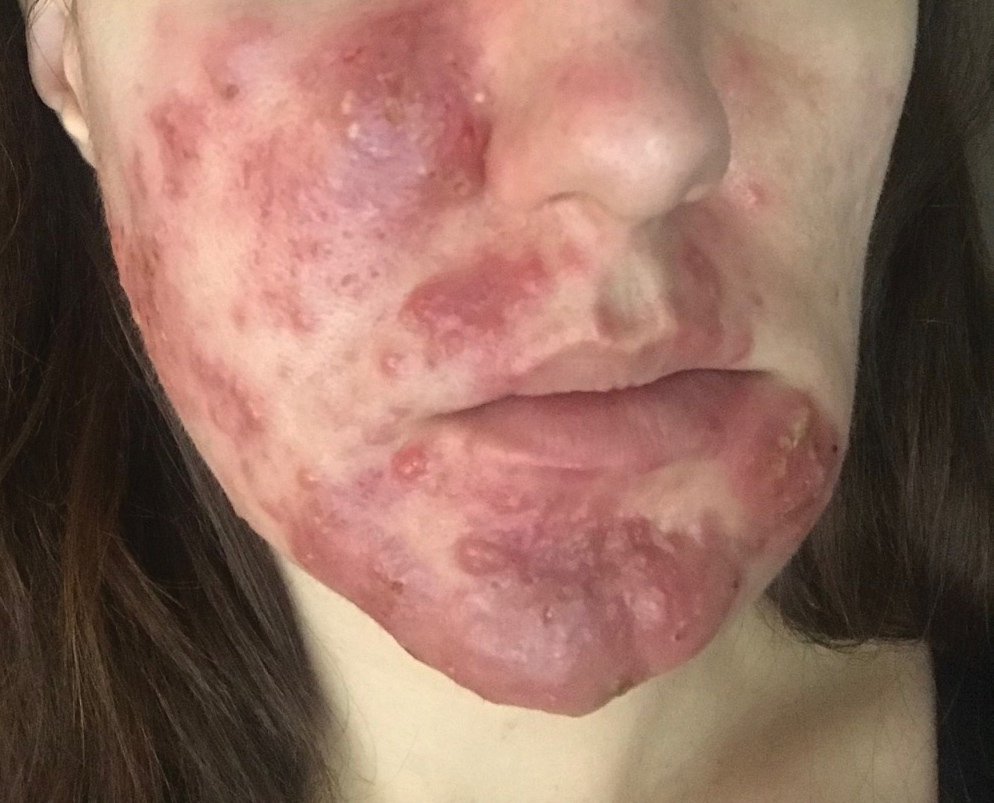
The injected areas had improved 5 days after the initial visit; however, the chin and right paranasal cheek developed even more nodules and papules coalescing into large plaques. After consulting the patient’s obstetrician, prednisone (20 mg once daily) was initiated. Three weeks later, the patient’s nodular lesions had improved, but there was a showering of more than 100 pustules and increased general erythema of the entire face (Figure 2). Crotamiton cream 10% (every day before noon), ivermectin cream 1% (every night at bedtime), and sodium sulfacetamide cleanser 10% once daily were added to the treatment plan.

At 16 weeks’ gestation, there was slight improvement; however, there was still erythema on the entire face with scattered pustules and multiple papules and nodules. Many small ice-pick scars were seen on the cheeks and forehead. No comedones were observed. A punch biopsy of an intact papule showed a prominent inflammatory infiltrate with granulomatous reaction and numerous neutrophils predominantly affecting hair follicles. Based on the clinical presentation and histopathology, a diagnosis of RF was made. Azithromycin (250 mg once daily) and metronidazole cream 0.75% twice daily were added. Two weeks later there were fewer nodules but many papules, edema, and intense erythema. The prednisone dosage was increased to 40 mg once daily. Two weeks later, the patient showed improvement with fewer lesions, less edema, and less erythema. The patient was instructed to finish the azithromycin course and discontinue use. At 28 weeks’ gestation, a prednisone taper was started with the intention to reduce the daily dose by delivery.
The patient delivered a healthy girl (birth weight, 1.985 kg) prematurely at 34 weeks’ gestation. At 2 months postpartum, the patient’s existing lesions continued to spontaneously improve; however, she still had numerous nodules and papules and continued to develop new lesions and form additional scars. Isotretinoin was instituted at 3 months postpartum upon cessation of nursing. Three months later (40 mg/d isotretinoin), the patient was nearly clear. At 8 months postpartum, isotretinoin was discontinued after a course of 150 mg/kg.
Comment
Rosacea fulminans initially was called pyoderma faciale but was later regarded as a severe form of rosacea and was renamed rosacea fulminans.2 According to a PubMed search of articles indexed for MEDLINE using the terms pregnancy and rosacea fulminans or pyoderma faciale, we identified 12 publications reporting 20 cases of RF associated with pregnancy (Table). Although there is no substantial evidence regarding the exact mechanism, these cases indicate that pregnancy can be an exacerbating or causative factor in the pathogenesis of RF.
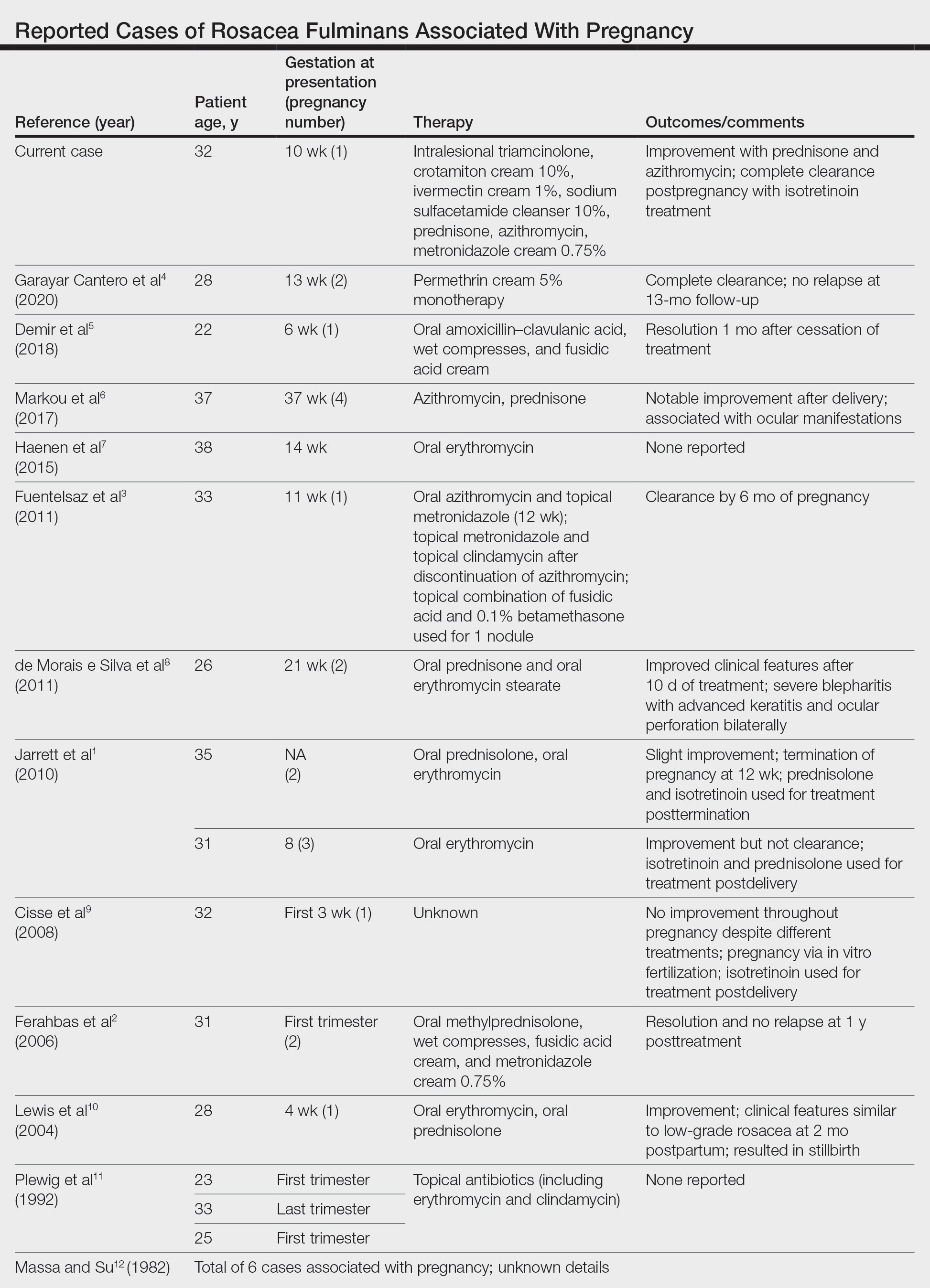
In addition to pregnancy, RF has been associated with inflammatory bowel disease, thyroid and liver disease, erythema nodosum, and severe emotional trauma. However, no organism has been consistently isolated, and no evidence of family history has been reported.1 Histopathologic findings are dependent on the stage of disease. Massive infiltrates of neutrophils may be observed in early stages. In older lesions, infiltrates take the form of epithelioid cell granulomas.2
Treatment of RF during pregnancy is challenging. Early and aggressive treatment with retinoids, tetracycline antibiotics, antiandrogenic contraceptives, and dapsone is recommended in patients who are not pregnant; these therapies are all contraindicated in pregnancy. Topical steroids can be safely used; however, systemic steroids usually are required to control RF. The use of systemic steroids can only be justified if the risks for intrauterine growth retardation, maternal diabetes mellitus, and hypertension outweigh the benefits of treating this severe disfiguring skin condition.10 A study by Bakar et al13 indicated that azithromycin is an effective and safe alternative in the treatment of RF. It has a superior pharmacokinetic profile compared to other macrolides and does not pose increased risks for congenital malformation or miscarriage. Because of the concomitant use of both azithromycin and prednisone, it is not possible to determine which had the larger role in the patient’s improvement.
Isotretinoin therapy in our patient led to substantial improvement of RF. Time will tell if the response will be durable. Also unknown is the risk for recurrence with subsequent pregnancies, which has not been reported in the literature. Although it is difficult to confidently say that pregnancy was the inciting factor in this patient’s RF, this case certainly provides more evidence for a link between pregnancy and RF.
- Jarrett R, Gonsalves R, Anstey AV. Differing obstetric outcomes of rosacea fulminans in pregnancy: report of three cases with review of pathogenesis and management. Clin Exp Dermatol. 2010;35:888-891. doi:10.1111/j.1365-2230.2010.03846.x
- Ferahbas A, Utas S, Mistik S, et al. Rosacea fulminans in pregnancy: case report and review of the literature. Am J Clin Dermatol. 2006;7:141-144. doi:10.2165/00128071-200607020-00007
- Fuentelsaz V, Ara M, Corredera C, et al. Rosacea fulminans in pregnancy: successful treatment with azithromycin. Clin Exp Dermatol. 2011;36:674-676. doi:10.1111/j.1365-2230.2011.04042.x
- Garayar Cantero M, Garabito Solovera E, Aguado García Á, et al. Use of permethrin in the treatment of rosacea fulminans during pregnancy: one case report. Dermatol Ther. 2020;33:E13436. doi:10.1111/dth.13436
- Demir O, Tas IS, Gunay B, et al. A rare dermatologic disease in pregnancy: rosacea fulminans—case report and review of the literature. Open Access Maced J Med Sci. 2018;6:1438-1441. doi:10.3889/oamjms.2018.267
- Markou AG, Alessandrini V, Muray JM, et al. Rosacea fulminans during pregnancy. Clin Exp Obstet Gynecol. 2017;44:157-159.
- Haenen CCP, Kouwenhoven STP, van Doorn R. Rosacea fulminans in pregnancy [in Dutch]. Ned Tijdschr Geneeskd. 2015;159:A8334.
- de Morais e Silva FA, Bonassi M, Steiner D, et al. Rosacea fulminans in pregnancy with ocular perforation. J Dtsch Dermatol Ges. 2011;9:542-543. doi:10.1111/j.1610-0387.2011.07616.x
- Cisse M, Maruani A, Bré C. Rosacea fulminans in the early course of a pregnancy by in vitro fertilization with embryo transfer [in French]. Ann Dermatol Venereol. 2008;135:675-678. doi:10.1016/j.annder.2008.04.015
- Lewis VJ, Holme SA, Wright A, et al. Rosacea fulminans in pregnancy. Br J Dermatol. 2004;151:917-919. doi:10.1111/j.1365-2133.2004.06190.x
- Plewig G, Jansen T, Kligman AM. Pyoderma faciale. a review and report of 20 additional cases: is it rosacea? Arch Dermatol. 1992;128:1611-1617. doi:10.1001/archderm.128.12.1611
- Massa MC, Su WP. Pyoderma faciale: a clinical study of twenty-nine patients. J Am Acad Dermatol. 1982;6:84-91. doi:10.1016/s0190-9622(82)70008-8
- Bakar O, Demirçay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154. doi:10.1111/j.1365-4632.2004.01958.x
Rosacea fulminans (RF) is a rare facial dermatosis characterized by its fulminating course. 1 It presents with superficial and deep-seated papules, pustules, and nodules combined with an intense reddish or cyanotic erythema localized to the face. Furthermore, there is an absence of comedones and involvement of the chest or back. 2 Rosacea fulminans primarily affects women and often is, but not always, proceeded by seborrhea, chronic acne vulgaris, or rosacea. Although the etiology of RF remains unknown, immunologic, hormonal, and vascular factors have been implicated. 3 We report a case of RF in a pregnant patient with a history of mild acne as a teenager that was long ago resolved.
Case Report
A 32-year-old pregnant woman (10 weeks’ gestation) presented with a rapidly progressing inflammatory disorder of the face of 1 month’s duration. The lesions developed 3 weeks after beginning progesterone therapy (200 mg vaginal suppository) for infertility due to polycystic ovary syndrome. Despite discontinuing progesterone for the last month, the patient’s lesions had dramatically worsened (Figure 1). Empiric cephalosporin treatment prescribed by her primary care physician yielded no improvement. Physical examination at the current presentation revealed erythematous nodules and pustules all over the face, coalescing into large thick plaques on the patient’s right cheek and chin. Submental nodes were palpable and tender. Based on the initial clinical findings, acne conglobata secondary to progesterone therapy was considered. The patient was given intralesional triamcinolone (2.5 mg/cc) injections to all larger nodules and several blue light treatments.

The injected areas had improved 5 days after the initial visit; however, the chin and right paranasal cheek developed even more nodules and papules coalescing into large plaques. After consulting the patient’s obstetrician, prednisone (20 mg once daily) was initiated. Three weeks later, the patient’s nodular lesions had improved, but there was a showering of more than 100 pustules and increased general erythema of the entire face (Figure 2). Crotamiton cream 10% (every day before noon), ivermectin cream 1% (every night at bedtime), and sodium sulfacetamide cleanser 10% once daily were added to the treatment plan.

At 16 weeks’ gestation, there was slight improvement; however, there was still erythema on the entire face with scattered pustules and multiple papules and nodules. Many small ice-pick scars were seen on the cheeks and forehead. No comedones were observed. A punch biopsy of an intact papule showed a prominent inflammatory infiltrate with granulomatous reaction and numerous neutrophils predominantly affecting hair follicles. Based on the clinical presentation and histopathology, a diagnosis of RF was made. Azithromycin (250 mg once daily) and metronidazole cream 0.75% twice daily were added. Two weeks later there were fewer nodules but many papules, edema, and intense erythema. The prednisone dosage was increased to 40 mg once daily. Two weeks later, the patient showed improvement with fewer lesions, less edema, and less erythema. The patient was instructed to finish the azithromycin course and discontinue use. At 28 weeks’ gestation, a prednisone taper was started with the intention to reduce the daily dose by delivery.
The patient delivered a healthy girl (birth weight, 1.985 kg) prematurely at 34 weeks’ gestation. At 2 months postpartum, the patient’s existing lesions continued to spontaneously improve; however, she still had numerous nodules and papules and continued to develop new lesions and form additional scars. Isotretinoin was instituted at 3 months postpartum upon cessation of nursing. Three months later (40 mg/d isotretinoin), the patient was nearly clear. At 8 months postpartum, isotretinoin was discontinued after a course of 150 mg/kg.
Comment
Rosacea fulminans initially was called pyoderma faciale but was later regarded as a severe form of rosacea and was renamed rosacea fulminans.2 According to a PubMed search of articles indexed for MEDLINE using the terms pregnancy and rosacea fulminans or pyoderma faciale, we identified 12 publications reporting 20 cases of RF associated with pregnancy (Table). Although there is no substantial evidence regarding the exact mechanism, these cases indicate that pregnancy can be an exacerbating or causative factor in the pathogenesis of RF.

In addition to pregnancy, RF has been associated with inflammatory bowel disease, thyroid and liver disease, erythema nodosum, and severe emotional trauma. However, no organism has been consistently isolated, and no evidence of family history has been reported.1 Histopathologic findings are dependent on the stage of disease. Massive infiltrates of neutrophils may be observed in early stages. In older lesions, infiltrates take the form of epithelioid cell granulomas.2
Treatment of RF during pregnancy is challenging. Early and aggressive treatment with retinoids, tetracycline antibiotics, antiandrogenic contraceptives, and dapsone is recommended in patients who are not pregnant; these therapies are all contraindicated in pregnancy. Topical steroids can be safely used; however, systemic steroids usually are required to control RF. The use of systemic steroids can only be justified if the risks for intrauterine growth retardation, maternal diabetes mellitus, and hypertension outweigh the benefits of treating this severe disfiguring skin condition.10 A study by Bakar et al13 indicated that azithromycin is an effective and safe alternative in the treatment of RF. It has a superior pharmacokinetic profile compared to other macrolides and does not pose increased risks for congenital malformation or miscarriage. Because of the concomitant use of both azithromycin and prednisone, it is not possible to determine which had the larger role in the patient’s improvement.
Isotretinoin therapy in our patient led to substantial improvement of RF. Time will tell if the response will be durable. Also unknown is the risk for recurrence with subsequent pregnancies, which has not been reported in the literature. Although it is difficult to confidently say that pregnancy was the inciting factor in this patient’s RF, this case certainly provides more evidence for a link between pregnancy and RF.
Rosacea fulminans (RF) is a rare facial dermatosis characterized by its fulminating course. 1 It presents with superficial and deep-seated papules, pustules, and nodules combined with an intense reddish or cyanotic erythema localized to the face. Furthermore, there is an absence of comedones and involvement of the chest or back. 2 Rosacea fulminans primarily affects women and often is, but not always, proceeded by seborrhea, chronic acne vulgaris, or rosacea. Although the etiology of RF remains unknown, immunologic, hormonal, and vascular factors have been implicated. 3 We report a case of RF in a pregnant patient with a history of mild acne as a teenager that was long ago resolved.
Case Report
A 32-year-old pregnant woman (10 weeks’ gestation) presented with a rapidly progressing inflammatory disorder of the face of 1 month’s duration. The lesions developed 3 weeks after beginning progesterone therapy (200 mg vaginal suppository) for infertility due to polycystic ovary syndrome. Despite discontinuing progesterone for the last month, the patient’s lesions had dramatically worsened (Figure 1). Empiric cephalosporin treatment prescribed by her primary care physician yielded no improvement. Physical examination at the current presentation revealed erythematous nodules and pustules all over the face, coalescing into large thick plaques on the patient’s right cheek and chin. Submental nodes were palpable and tender. Based on the initial clinical findings, acne conglobata secondary to progesterone therapy was considered. The patient was given intralesional triamcinolone (2.5 mg/cc) injections to all larger nodules and several blue light treatments.

The injected areas had improved 5 days after the initial visit; however, the chin and right paranasal cheek developed even more nodules and papules coalescing into large plaques. After consulting the patient’s obstetrician, prednisone (20 mg once daily) was initiated. Three weeks later, the patient’s nodular lesions had improved, but there was a showering of more than 100 pustules and increased general erythema of the entire face (Figure 2). Crotamiton cream 10% (every day before noon), ivermectin cream 1% (every night at bedtime), and sodium sulfacetamide cleanser 10% once daily were added to the treatment plan.

At 16 weeks’ gestation, there was slight improvement; however, there was still erythema on the entire face with scattered pustules and multiple papules and nodules. Many small ice-pick scars were seen on the cheeks and forehead. No comedones were observed. A punch biopsy of an intact papule showed a prominent inflammatory infiltrate with granulomatous reaction and numerous neutrophils predominantly affecting hair follicles. Based on the clinical presentation and histopathology, a diagnosis of RF was made. Azithromycin (250 mg once daily) and metronidazole cream 0.75% twice daily were added. Two weeks later there were fewer nodules but many papules, edema, and intense erythema. The prednisone dosage was increased to 40 mg once daily. Two weeks later, the patient showed improvement with fewer lesions, less edema, and less erythema. The patient was instructed to finish the azithromycin course and discontinue use. At 28 weeks’ gestation, a prednisone taper was started with the intention to reduce the daily dose by delivery.
The patient delivered a healthy girl (birth weight, 1.985 kg) prematurely at 34 weeks’ gestation. At 2 months postpartum, the patient’s existing lesions continued to spontaneously improve; however, she still had numerous nodules and papules and continued to develop new lesions and form additional scars. Isotretinoin was instituted at 3 months postpartum upon cessation of nursing. Three months later (40 mg/d isotretinoin), the patient was nearly clear. At 8 months postpartum, isotretinoin was discontinued after a course of 150 mg/kg.
Comment
Rosacea fulminans initially was called pyoderma faciale but was later regarded as a severe form of rosacea and was renamed rosacea fulminans.2 According to a PubMed search of articles indexed for MEDLINE using the terms pregnancy and rosacea fulminans or pyoderma faciale, we identified 12 publications reporting 20 cases of RF associated with pregnancy (Table). Although there is no substantial evidence regarding the exact mechanism, these cases indicate that pregnancy can be an exacerbating or causative factor in the pathogenesis of RF.

In addition to pregnancy, RF has been associated with inflammatory bowel disease, thyroid and liver disease, erythema nodosum, and severe emotional trauma. However, no organism has been consistently isolated, and no evidence of family history has been reported.1 Histopathologic findings are dependent on the stage of disease. Massive infiltrates of neutrophils may be observed in early stages. In older lesions, infiltrates take the form of epithelioid cell granulomas.2
Treatment of RF during pregnancy is challenging. Early and aggressive treatment with retinoids, tetracycline antibiotics, antiandrogenic contraceptives, and dapsone is recommended in patients who are not pregnant; these therapies are all contraindicated in pregnancy. Topical steroids can be safely used; however, systemic steroids usually are required to control RF. The use of systemic steroids can only be justified if the risks for intrauterine growth retardation, maternal diabetes mellitus, and hypertension outweigh the benefits of treating this severe disfiguring skin condition.10 A study by Bakar et al13 indicated that azithromycin is an effective and safe alternative in the treatment of RF. It has a superior pharmacokinetic profile compared to other macrolides and does not pose increased risks for congenital malformation or miscarriage. Because of the concomitant use of both azithromycin and prednisone, it is not possible to determine which had the larger role in the patient’s improvement.
Isotretinoin therapy in our patient led to substantial improvement of RF. Time will tell if the response will be durable. Also unknown is the risk for recurrence with subsequent pregnancies, which has not been reported in the literature. Although it is difficult to confidently say that pregnancy was the inciting factor in this patient’s RF, this case certainly provides more evidence for a link between pregnancy and RF.
- Jarrett R, Gonsalves R, Anstey AV. Differing obstetric outcomes of rosacea fulminans in pregnancy: report of three cases with review of pathogenesis and management. Clin Exp Dermatol. 2010;35:888-891. doi:10.1111/j.1365-2230.2010.03846.x
- Ferahbas A, Utas S, Mistik S, et al. Rosacea fulminans in pregnancy: case report and review of the literature. Am J Clin Dermatol. 2006;7:141-144. doi:10.2165/00128071-200607020-00007
- Fuentelsaz V, Ara M, Corredera C, et al. Rosacea fulminans in pregnancy: successful treatment with azithromycin. Clin Exp Dermatol. 2011;36:674-676. doi:10.1111/j.1365-2230.2011.04042.x
- Garayar Cantero M, Garabito Solovera E, Aguado García Á, et al. Use of permethrin in the treatment of rosacea fulminans during pregnancy: one case report. Dermatol Ther. 2020;33:E13436. doi:10.1111/dth.13436
- Demir O, Tas IS, Gunay B, et al. A rare dermatologic disease in pregnancy: rosacea fulminans—case report and review of the literature. Open Access Maced J Med Sci. 2018;6:1438-1441. doi:10.3889/oamjms.2018.267
- Markou AG, Alessandrini V, Muray JM, et al. Rosacea fulminans during pregnancy. Clin Exp Obstet Gynecol. 2017;44:157-159.
- Haenen CCP, Kouwenhoven STP, van Doorn R. Rosacea fulminans in pregnancy [in Dutch]. Ned Tijdschr Geneeskd. 2015;159:A8334.
- de Morais e Silva FA, Bonassi M, Steiner D, et al. Rosacea fulminans in pregnancy with ocular perforation. J Dtsch Dermatol Ges. 2011;9:542-543. doi:10.1111/j.1610-0387.2011.07616.x
- Cisse M, Maruani A, Bré C. Rosacea fulminans in the early course of a pregnancy by in vitro fertilization with embryo transfer [in French]. Ann Dermatol Venereol. 2008;135:675-678. doi:10.1016/j.annder.2008.04.015
- Lewis VJ, Holme SA, Wright A, et al. Rosacea fulminans in pregnancy. Br J Dermatol. 2004;151:917-919. doi:10.1111/j.1365-2133.2004.06190.x
- Plewig G, Jansen T, Kligman AM. Pyoderma faciale. a review and report of 20 additional cases: is it rosacea? Arch Dermatol. 1992;128:1611-1617. doi:10.1001/archderm.128.12.1611
- Massa MC, Su WP. Pyoderma faciale: a clinical study of twenty-nine patients. J Am Acad Dermatol. 1982;6:84-91. doi:10.1016/s0190-9622(82)70008-8
- Bakar O, Demirçay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154. doi:10.1111/j.1365-4632.2004.01958.x
- Jarrett R, Gonsalves R, Anstey AV. Differing obstetric outcomes of rosacea fulminans in pregnancy: report of three cases with review of pathogenesis and management. Clin Exp Dermatol. 2010;35:888-891. doi:10.1111/j.1365-2230.2010.03846.x
- Ferahbas A, Utas S, Mistik S, et al. Rosacea fulminans in pregnancy: case report and review of the literature. Am J Clin Dermatol. 2006;7:141-144. doi:10.2165/00128071-200607020-00007
- Fuentelsaz V, Ara M, Corredera C, et al. Rosacea fulminans in pregnancy: successful treatment with azithromycin. Clin Exp Dermatol. 2011;36:674-676. doi:10.1111/j.1365-2230.2011.04042.x
- Garayar Cantero M, Garabito Solovera E, Aguado García Á, et al. Use of permethrin in the treatment of rosacea fulminans during pregnancy: one case report. Dermatol Ther. 2020;33:E13436. doi:10.1111/dth.13436
- Demir O, Tas IS, Gunay B, et al. A rare dermatologic disease in pregnancy: rosacea fulminans—case report and review of the literature. Open Access Maced J Med Sci. 2018;6:1438-1441. doi:10.3889/oamjms.2018.267
- Markou AG, Alessandrini V, Muray JM, et al. Rosacea fulminans during pregnancy. Clin Exp Obstet Gynecol. 2017;44:157-159.
- Haenen CCP, Kouwenhoven STP, van Doorn R. Rosacea fulminans in pregnancy [in Dutch]. Ned Tijdschr Geneeskd. 2015;159:A8334.
- de Morais e Silva FA, Bonassi M, Steiner D, et al. Rosacea fulminans in pregnancy with ocular perforation. J Dtsch Dermatol Ges. 2011;9:542-543. doi:10.1111/j.1610-0387.2011.07616.x
- Cisse M, Maruani A, Bré C. Rosacea fulminans in the early course of a pregnancy by in vitro fertilization with embryo transfer [in French]. Ann Dermatol Venereol. 2008;135:675-678. doi:10.1016/j.annder.2008.04.015
- Lewis VJ, Holme SA, Wright A, et al. Rosacea fulminans in pregnancy. Br J Dermatol. 2004;151:917-919. doi:10.1111/j.1365-2133.2004.06190.x
- Plewig G, Jansen T, Kligman AM. Pyoderma faciale. a review and report of 20 additional cases: is it rosacea? Arch Dermatol. 1992;128:1611-1617. doi:10.1001/archderm.128.12.1611
- Massa MC, Su WP. Pyoderma faciale: a clinical study of twenty-nine patients. J Am Acad Dermatol. 1982;6:84-91. doi:10.1016/s0190-9622(82)70008-8
- Bakar O, Demirçay Z, Gürbüz O. Therapeutic potential of azithromycin in rosacea. Int J Dermatol. 2004;43:151-154. doi:10.1111/j.1365-4632.2004.01958.x
Practice Points
- Rosacea fulminans (RF) is a rare facial dermatosis that can present in pregnant patients.
- Treatment of RF in a pregnant patient requires special considerations because typical therapies are contraindicated in pregnancy.