User login
Upadacitinib (Rinvoq) gains psoriatic arthritis as second FDA-approved indication
upadacitinib (Rinvoq) for adults with psoriatic arthritis who had an inadequate response or intolerance to one or more anti-tumor necrosis factor drugs, manufacturer AbbVie announced December 14.
The approval is the second indication given by the agency for the selective Janus kinase (JAK) inhibitor upadacitinib, which was previously approved for rheumatoid arthritis (RA) in 2019.
Upadacitinib 15 mg is also approved by the European Commission for adults with RA, psoriatic arthritis, and ankylosing spondylitis. The European Commission also approved the drug for moderate to severe atopic dermatitis at both 15- and 30-mg doses for adults and at 15 mg for adolescents.
The approval is based on two phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, which together randomized more than 2,300 patients with psoriatic arthritis. In the trials, significantly more patients who took upadacitinib 15 mg met their primary endpoint of 20% improvement in American College of Rheumatology response criteria (ACR20) at week 12 (71% in SELECT-PsA 1 and 57% in SELECT-PsA 2) vs placebo (36% and 24%, respectively). Both trials also included treatment arms for upadacitinib at 30 mg, but the FDA approved only the 15-mg dose.
In the announcement, AbbVie noted that significantly higher percentages of patients treated with upadacitinib 15 mg in the SELECT-PSA 1 and 2 trials, respectively, met ACR50 (38% and 32%) and ACR70 (16% and 9%) criteria than did patients on placebo (13% and 5% for ACR50 and 2% and 1% for ACR70). Symptoms of dactylitis and enthesitis improved with upadacitinib for patients who had them at baseline.
The trials’ 12-week results also indicated that upadacitinib significantly improved physical function relative to placebo at baseline, based on the Health Assessment Questionnaire-Disability Index, as well as fatigue, according to Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scores. Skin manifestations also improved during the trial, but upadacitinib has not been studied for treating plaque psoriasis.
AbbVie reported that the safety results of upadacitinib in the trials were consistent with the results seen in patients with rheumatoid arthritis, and during the trials’ 24-week placebo-controlled period, the most common adverse events reported with upadacitinib were upper respiratory tract infection and blood creatine phosphokinase elevations.
Upadacitinib comes with a boxed warning that was formally placed on the drug’s label this month after data from a postmarketing trial of the JAK inhibitor tofacitinib (Xeljanz and Xeljanz XR) in patients with RA aged 50 years and older with at least one cardiovascular risk factor showed numerically higher risks for all-cause mortality; lymphoma and other malignancies; major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke); and thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis.
Upadacitinib also carries a boxed warning for an elevated risk of serious infection leading to hospitalization or death. In the SELECT-PsA 1 and 2 trials overall, rates of herpes zoster and herpes simplex were 1.1% and 1.4% with upadacitinib, compared with 0.8% and 1.3% with placebo.
Phase 3 trials of upadacitinib in RA, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, ulcerative colitis, giant cell arteritis, and Takayasu arteritis are ongoing, according to AbbVie.
A version of this article first appeared on Medscape.com.
upadacitinib (Rinvoq) for adults with psoriatic arthritis who had an inadequate response or intolerance to one or more anti-tumor necrosis factor drugs, manufacturer AbbVie announced December 14.
The approval is the second indication given by the agency for the selective Janus kinase (JAK) inhibitor upadacitinib, which was previously approved for rheumatoid arthritis (RA) in 2019.
Upadacitinib 15 mg is also approved by the European Commission for adults with RA, psoriatic arthritis, and ankylosing spondylitis. The European Commission also approved the drug for moderate to severe atopic dermatitis at both 15- and 30-mg doses for adults and at 15 mg for adolescents.
The approval is based on two phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, which together randomized more than 2,300 patients with psoriatic arthritis. In the trials, significantly more patients who took upadacitinib 15 mg met their primary endpoint of 20% improvement in American College of Rheumatology response criteria (ACR20) at week 12 (71% in SELECT-PsA 1 and 57% in SELECT-PsA 2) vs placebo (36% and 24%, respectively). Both trials also included treatment arms for upadacitinib at 30 mg, but the FDA approved only the 15-mg dose.
In the announcement, AbbVie noted that significantly higher percentages of patients treated with upadacitinib 15 mg in the SELECT-PSA 1 and 2 trials, respectively, met ACR50 (38% and 32%) and ACR70 (16% and 9%) criteria than did patients on placebo (13% and 5% for ACR50 and 2% and 1% for ACR70). Symptoms of dactylitis and enthesitis improved with upadacitinib for patients who had them at baseline.
The trials’ 12-week results also indicated that upadacitinib significantly improved physical function relative to placebo at baseline, based on the Health Assessment Questionnaire-Disability Index, as well as fatigue, according to Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scores. Skin manifestations also improved during the trial, but upadacitinib has not been studied for treating plaque psoriasis.
AbbVie reported that the safety results of upadacitinib in the trials were consistent with the results seen in patients with rheumatoid arthritis, and during the trials’ 24-week placebo-controlled period, the most common adverse events reported with upadacitinib were upper respiratory tract infection and blood creatine phosphokinase elevations.
Upadacitinib comes with a boxed warning that was formally placed on the drug’s label this month after data from a postmarketing trial of the JAK inhibitor tofacitinib (Xeljanz and Xeljanz XR) in patients with RA aged 50 years and older with at least one cardiovascular risk factor showed numerically higher risks for all-cause mortality; lymphoma and other malignancies; major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke); and thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis.
Upadacitinib also carries a boxed warning for an elevated risk of serious infection leading to hospitalization or death. In the SELECT-PsA 1 and 2 trials overall, rates of herpes zoster and herpes simplex were 1.1% and 1.4% with upadacitinib, compared with 0.8% and 1.3% with placebo.
Phase 3 trials of upadacitinib in RA, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, ulcerative colitis, giant cell arteritis, and Takayasu arteritis are ongoing, according to AbbVie.
A version of this article first appeared on Medscape.com.
upadacitinib (Rinvoq) for adults with psoriatic arthritis who had an inadequate response or intolerance to one or more anti-tumor necrosis factor drugs, manufacturer AbbVie announced December 14.
The approval is the second indication given by the agency for the selective Janus kinase (JAK) inhibitor upadacitinib, which was previously approved for rheumatoid arthritis (RA) in 2019.
Upadacitinib 15 mg is also approved by the European Commission for adults with RA, psoriatic arthritis, and ankylosing spondylitis. The European Commission also approved the drug for moderate to severe atopic dermatitis at both 15- and 30-mg doses for adults and at 15 mg for adolescents.
The approval is based on two phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, which together randomized more than 2,300 patients with psoriatic arthritis. In the trials, significantly more patients who took upadacitinib 15 mg met their primary endpoint of 20% improvement in American College of Rheumatology response criteria (ACR20) at week 12 (71% in SELECT-PsA 1 and 57% in SELECT-PsA 2) vs placebo (36% and 24%, respectively). Both trials also included treatment arms for upadacitinib at 30 mg, but the FDA approved only the 15-mg dose.
In the announcement, AbbVie noted that significantly higher percentages of patients treated with upadacitinib 15 mg in the SELECT-PSA 1 and 2 trials, respectively, met ACR50 (38% and 32%) and ACR70 (16% and 9%) criteria than did patients on placebo (13% and 5% for ACR50 and 2% and 1% for ACR70). Symptoms of dactylitis and enthesitis improved with upadacitinib for patients who had them at baseline.
The trials’ 12-week results also indicated that upadacitinib significantly improved physical function relative to placebo at baseline, based on the Health Assessment Questionnaire-Disability Index, as well as fatigue, according to Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scores. Skin manifestations also improved during the trial, but upadacitinib has not been studied for treating plaque psoriasis.
AbbVie reported that the safety results of upadacitinib in the trials were consistent with the results seen in patients with rheumatoid arthritis, and during the trials’ 24-week placebo-controlled period, the most common adverse events reported with upadacitinib were upper respiratory tract infection and blood creatine phosphokinase elevations.
Upadacitinib comes with a boxed warning that was formally placed on the drug’s label this month after data from a postmarketing trial of the JAK inhibitor tofacitinib (Xeljanz and Xeljanz XR) in patients with RA aged 50 years and older with at least one cardiovascular risk factor showed numerically higher risks for all-cause mortality; lymphoma and other malignancies; major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke); and thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis.
Upadacitinib also carries a boxed warning for an elevated risk of serious infection leading to hospitalization or death. In the SELECT-PsA 1 and 2 trials overall, rates of herpes zoster and herpes simplex were 1.1% and 1.4% with upadacitinib, compared with 0.8% and 1.3% with placebo.
Phase 3 trials of upadacitinib in RA, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, ulcerative colitis, giant cell arteritis, and Takayasu arteritis are ongoing, according to AbbVie.
A version of this article first appeared on Medscape.com.
Alternative rheumatology practice models aim to avoid traditional limitations
Elizabeth Ortiz, MD, knew she needed a change. Working at an academic county clinic, she was often worn down and pulled in different directions. “When I thought about what I really liked about my job, it was patient care and spending time with my patients, which I wasn’t able to do,” Dr. Ortiz said during the annual meeting of the American College of Rheumatology.
She’d heard of direct or concierge care but wasn’t sure if it was a good fit for her. COVID-19 offered a catalyst of sorts for a move to a new care model.
Ten weeks after she moved to Dallas, the pandemic hit full force. Seeing how telehealth was taking off, Dr. Ortiz began crafting a new model of care, a hybrid of telemedicine and house calls that offered multiple venues to connect with patients. The practice is just a year old, and “it’s working and it’s a constant experiment,” said Dr. Ortiz, who offers membership plans and prepaid appointments. She also does “a la carte” visits where established patients can see her at a one-off price. Her goal is to achieve 100% membership.
Although she operates through a direct pay and cash-only model, only recently has she become comfortable with the word “concierge.” There’s a preconceived notion of what that word means, she said.
Direct care: A definition
Following the trend of some primary care practices, more rheumatologists who are dissatisfied with the status quo are embracing these models of care.
Direct and concierge care are often mentioned in tandem, but there are nuanced differences. Direct specialty care removes third-party payers to protect the best interests of patients, according to Diana Girnita, MD, founder and CEO of Rheumatologist OnCall, a direct care practice. Her patient base hails from rural and urban areas in least 10 states. She also created a Facebook group for specialists in direct care and is the cofounder of the Direct Specialty Care Alliance.
Direct care offers a membership fee and additional fees for “as needed” services. “As the physician, I do not have to be contracted to an insurance company to see patients. I contract directly with patients. It is the patient’s choice to contract with an insurance and use the insurance for ancillary services and medication,” Dr. Girnita said. Patients with out-of-network benefits can claim the insurance to cover part of the consultation cost, she added.
In concierge or retainer medicine, a patient pays an annual or monthly fee or retainer to get access to the physician practice. In addition to this fee, the practice can bill the patient’s insurance for consultations or other services. “The concierge model does not eliminate the sub payer. You still contract with the patient’s insurance,” explained Dr. Girnita.
Physicians who establish these models sometimes do a hybrid of cash only and insurance. Micah Yu, MD, who practices rheumatology in Newport Beach, Calif., only takes Medicare. “Otherwise, patients are private pay. I am mainly fee for service, so patients are paying me for my time,” he said.
By tailoring their patient base and services, adopters find they have more time to spend with patients. “In my model, I spend 30 minutes for follow-up and 1 hour for new patients,” Dr. Yu said.
Limitations of traditional care
Carrying insurance doesn’t guarantee you the right care, Dr. Girnita said. Wait times to see a rheumatologist range from 4 to 6 months. For physicians who contract with insurance companies, reimbursement for services isn’t always paid promptly and decreases every year. A new cut in reimbursement is expected for rheumatology services in 2022.
Patients in direct care “pay a small amount for memberships that cover the cost of their visits and the time physicians spend in coordinating their additional care between the visits. The cost of the visits is always transparent,” Dr. Girnita said.
Irene Kazmers, MD, a solo private rheumatology practitioner in northern Michigan, was seeing 20-plus patients a day before she made the leap to a concierge model. “The paperwork and administrative burdens of practicing rheumatology as a solo [physician] have mushroomed in the last 10 years,” she said during the ACR meeting. She and staff were spending an inordinate amount of time on prior authorizations, step therapy requirements, electronic health record documentation, and other administrative burdens.
Reimbursements from payers have progressively declined as administrative challenges have necessitated more staff. “I was struggling to maintain an ample financial margin,” she said.
Improved communication, unlimited visits
Dr. Kazmers attests that the transition to the concierge model has enabled and fostered a higher level of communication and specialty care for her patients.
Patients who enroll in the practice pay an annual membership fee and get access to her personal cell phone number and email address. “If they need an urgent appointment, it is typically arranged the same or next day,” she said in an interview. “Visits are not as rushed as in the traditional model, conducive to incorporating beneficial integrative medicine modalities such as dietary, exercise, and mind-body approaches as appropriate, in addition to state-of-the-art treatment.”
She also has more time to coordinate care with her patients’ primary care providers and other care team professionals and to give patients feedback on lab and study results.
Dr. Girnita has ramped down from 28 to 15 patients a day. She’s able to spend 60 minutes for new patients and 30 minutes for follow-ups. Like Dr. Kazmers, she feels she has more time to address patient needs and listen to their concerns.
She’s kept her hospital affiliations but finds that she doesn’t have to go to the hospital as much as she used to. Direct care “reduces hospital visits because physicians significantly have much more time to spend with the patient and address the needs of the patient.” A patient with a gout flare, for example, may end up in the hospital under traditional care because there’s no room in the physician’s schedule to address the patient’s needs.
Dr. Girnita recalled when she assisted a patient who had developed inflammatory arthritis and was desperate to see a doctor. The patient had good insurance, but appointments in her area weren’t available for at least 6 months. “Her primary care physician called me. I saw her and provided her with the appropriate care. A couple of months later she is doing great.”
What insurance does and doesn’t cover
Many patients who seek out direct or concierge models retain their insurance. At least 90% of Dr. Girnita’s patients have insurance with high deductibles. The other 10% have other types of insurance or no insurance.
Ellen McKnight, MD, who has a hybrid rheumatology practice in Pensacola, Fla., still accepts commercial insurance, but has opted out of Medicare. Her patients mostly come from rural areas in Florida, and their insurance situations vary widely. “In my practice, I estimate that 65% have insurance and 35% do not. Most of my patients have commercial insurance, and a substantial portion, about 40%, are just paying cash. My cash pay patients have Medicare, HMOs, and others are uninsured,” she said in an interview.
Direct care practices may continue to bill traditional insurance for items like visits, injections, and ultrasound.
Dr. Girnita’s patients have the option of submitting a “superbill” or invoice to insurance companies for patients to be reimbursed by their insurance for the cost of the visit. It contains the CPT code for the visit along with the ICD-10 codes for diagnoses. “I use a company called Reimbursify to help patients submit their invoice to their insurance company,” Dr. Girnita said.
Dr. Ortiz takes a different approach, offering superbills for consults and individual appointments, but not for patients enrolled in her membership program.
Some in the payer industry contend that direct care arrangements increase costs and distort risk pools. If most direct care patients already have a comprehensive health insurance policy, it’s likely they’re being billed twice for services, said David Allen, spokesperson for America’s Health Insurance Plans.
“Duplicative payments inflate the cost of care at a convenience to the providers and increase the cost of insurance premiums when insurers receive bills for those same services from providers. In other words, patients are being double billed,” Mr. Allen said.
These providers are assuming risk without state insurance oversight or regulations to ensure patient protections and safeguards are in place, he continued. “If utilization of services outpaces capacity, the provider may ultimately be unable to provide the amount of care expected by the patient because their practice agreed to unlimited visits and services with little or no restrictions.”
Eliminating ‘surprise’ bills
Adopters of direct care/concierge services counter that it’s the insurance and pharmaceutical companies driving up costs. Patients – especially those who have high-deductible plans – save money through these models. “In the direct care model, doctors have worked out advocacy for patients that are unsurpassed. Insurance companies don’t do that,” Dr. McKnight said.
Consumers know up front what the price is for other services. When you go to a restaurant, you always look on the menu to see what the price is for a bottle of wine or steak, Dr. Girnita said. “Only in the medical field you don’t know anything. And you’re shocked about the price you must pay.” Not many practices list their prices on their website, although federal rules seek to further increase price transparency in hospitals and among insurers.
Patients will sometimes get a “surprise” bill for their visit, laboratory, or imaging tests. According to Dr. Girnita, “that doesn’t happen in my practice. I discuss all prices with them before they get to the lab or MRI. I don’t charge copayments or anything extra.” Without a copayment – usually $50-$75 for specialist services – or a surprise bill, patients are always paying less, she said.
Costs through insurance are oftentimes higher, she continued. For routine lab work, a patient in a direct care practice pays about $30-$40. If they request this work through a lab, they’re likely to pay $150. “Think about an MRI. Through a direct care practice like mine, you pay $450-$700. In a hospital setting, you pay at least $5,000.”
Patients with high-deductible insurance plans often pay thousands of dollars before meeting their deductible, Dr. Girnita and others noted. A patient with this type of plan may pay $250 for a vitamin D lab if they haven’t met their deductible, Dr. McKnight explained. “With direct care, you’ll be paying $12.50.”
Dr. Girnita said her members get excellent discounts for labs and imaging. In the direct care models, physicians can help with this by contracting directly with labs, imaging centers, and independent pharmacies, giving patients access to affordable and transparent prices for their medical care.
What patients pay for services
In direct and concierge care membership models, coverage for services and fees vary widely from practice to practice.
Dr. Girnita offers several membership options. One package, which is $199 a month, is for patients with stable symptoms that guarantee continuity of care. It includes four visits a year and immediate access to the practice in case of emergency (including two additional urgent visits). “This works for a lot of patients. They consider that affordable, and they have all the benefits of a concierge practice. They can have direct communication with me, and they have guaranteed continuity of care,” Dr. Girnita said.
The other model, which is $299 per month, is for patients who need monthly contact with the rheumatologist for visits, telephone and email communications, urgent appointments, integrative medicine consultations, and many other benefits. For 1-hour consultations, Dr. Girnita charges $399.
Dr. Ortiz, who offers a direct pay model, charges $899 for an initial consult, which covers 3.5 hours of her time. “We do an hour of telemedicine, and we do a house call, which is 1.5-2 hours.” She follows up with a telehealth visit. Labs and x-rays are not included and go through the patient’s insurance.
Once the consult takes place, she assesses what a patient needs and offers them either a 6- or 12-month membership, which includes unlimited visits.
Patients can also buy a prepaid, six-appointment package with a 12% discount. Dr. Ortiz prices her telehealth visits at $350 and house calls at $550.
Dr. McKnight’s cash-only model for established patients offers four visits a year, reducing the fee for each visit. For example, a patient will pay $95 for the first visit, then $90, $85, and $80 for subsequent visits.
Accessing medications through direct care
One challenge with this model is finding affordable medications for patients outside of insurance.
Insurance dictates what’s covered, leaving fewer options for patients, Dr. McKnight said. “You have to jump through hoops, and there’s prior authorizations.” For a condition like severe osteoporosis, treatment should start sequentially with the true bone builders first, then move on to a medication like alendronate (Fosamax).
“Insurers will make you go to Fosamax first and then fail it,” she said. This results in the patient potentially developing worsening bone loss or possibly even sustaining a fracture.
Prior authorization requirements demand excessive staff time and effort, Dr. Kazmers said. This can translate to more than $90,000 a year in human resource costs for rheumatologists, who often deal with many specialty drug authorizations. “Every practice needs to hire staff to handle prior authorizations. We receive no compensation for this from the pharmaceutical companies and middlemen who ultimately profit from this cumbersome process,” she added.
Among the two big classes for rheumatology patients, conventional synthetic disease-modifying antirheumatic drugs (DMARDs) are the most widely available. Pharmacies can offer DMARDs for cash, although some are limited in terms of where they can ship, Dr. Girnita said.
The other class, biologic DMARDs, are the most expensive medications rheumatologists use for conditions such as rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis.
With biologics, it’s more difficult, as they’re very expensive, typically $6,000 a month or more, sources told this news organization.
“Unfortunately, we can’t partner at this time with pharmaceutical companies that produce biologics or independent pharmacies,” Dr. Girnita said. Physicians can’t control biologic prices either. “Insurance companies and pharmacy benefit managers have the control on these prices.”
Physicians can direct patients to multiple resources where they can find assistance.
Biologics companies that offer patient assistance programs can sometimes offer medications for free, while others offer savings cards or copay cards, “which helps a lot,” Dr. Girnita said. She assists her patients by filing some of the paperwork necessary to qualify for these programs, and the patients submit the rest.
“For these companies to help the patient, they need the patient’s financial information,” she said. “But I do most of that work; I complete the forms and send to the company and justify need for the medication.”
What’s ahead for direct specialty care
While some patients have benefited, others have had to seek alternatives as their doctors transition to alternative models.
Not everyone can afford the concierge retainer fee, said Dr. Kazmers, who practices in a rural area of Michigan, where rheumatologists are scarce. Enrollment in her concierge practice filled months before the switchover from her traditional practice took place. There are 70 patients on a waiting list.
Patients who elect not to enroll in the concierge practice need to find another source of rheumatology care. This is a downside to the practice transition, she acknowledged. “The closest rheumatologist taking new patients is a 3- to 4-hour drive away, which simply reflects the shortage of medical school graduates choosing to go into rheumatology in the United States,” Dr. Kazmers added.
One physician caring for thousands of chronically ill, complicated patients within systems that don’t allow them the time to really care for their patients threatens to make the access problem worse, Dr. Ortiz said. The direct care/concierge model offers an alternative for the provider “and is a way to keep providers in the workforce, who may otherwise consider leaving.”
Direct care/concierge medicine isn’t for all doctors. But for Dr. Kazmers, it’s the best option for her at this point in her career. “I’ve been practicing for 45 years in various models, including academic positions and private practice employment. I have worked for years in settings accepting Medicaid. I understand that if every rheumatologist went concierge tomorrow, this would constrict access to needed specialty care. But in my case, it provided a viable alternative to closing the practice’s doors altogether.”
Ultimately, the U.S. medical system needs more rheumatologists and other specialists. “If you really want to increase the service, then Medicare or other sources should support opening more residency and fellowship spots for medical graduates to pursue,” Dr. Girnita said.
Other solutions call for more systemic and institutional changes, such as expanding rheumatology divisions and faculties at institutions that train fellows and addressing medical school debt, Dr. Ortiz said.
Some practices see themselves branching out from individual patient care and partnering with local businesses to provide care for employees. That’s the future for direct specialty care, said Dr. Girnita, who’s been in discussions with a few employers to make such arrangements.
The direct primary care community has already started to contract with employers. “Their employees get care they need for just a fraction of the cost. These discussions are arising more and more,” she said.
A version of this article first appeared on Medscape.com.
Elizabeth Ortiz, MD, knew she needed a change. Working at an academic county clinic, she was often worn down and pulled in different directions. “When I thought about what I really liked about my job, it was patient care and spending time with my patients, which I wasn’t able to do,” Dr. Ortiz said during the annual meeting of the American College of Rheumatology.
She’d heard of direct or concierge care but wasn’t sure if it was a good fit for her. COVID-19 offered a catalyst of sorts for a move to a new care model.
Ten weeks after she moved to Dallas, the pandemic hit full force. Seeing how telehealth was taking off, Dr. Ortiz began crafting a new model of care, a hybrid of telemedicine and house calls that offered multiple venues to connect with patients. The practice is just a year old, and “it’s working and it’s a constant experiment,” said Dr. Ortiz, who offers membership plans and prepaid appointments. She also does “a la carte” visits where established patients can see her at a one-off price. Her goal is to achieve 100% membership.
Although she operates through a direct pay and cash-only model, only recently has she become comfortable with the word “concierge.” There’s a preconceived notion of what that word means, she said.
Direct care: A definition
Following the trend of some primary care practices, more rheumatologists who are dissatisfied with the status quo are embracing these models of care.
Direct and concierge care are often mentioned in tandem, but there are nuanced differences. Direct specialty care removes third-party payers to protect the best interests of patients, according to Diana Girnita, MD, founder and CEO of Rheumatologist OnCall, a direct care practice. Her patient base hails from rural and urban areas in least 10 states. She also created a Facebook group for specialists in direct care and is the cofounder of the Direct Specialty Care Alliance.
Direct care offers a membership fee and additional fees for “as needed” services. “As the physician, I do not have to be contracted to an insurance company to see patients. I contract directly with patients. It is the patient’s choice to contract with an insurance and use the insurance for ancillary services and medication,” Dr. Girnita said. Patients with out-of-network benefits can claim the insurance to cover part of the consultation cost, she added.
In concierge or retainer medicine, a patient pays an annual or monthly fee or retainer to get access to the physician practice. In addition to this fee, the practice can bill the patient’s insurance for consultations or other services. “The concierge model does not eliminate the sub payer. You still contract with the patient’s insurance,” explained Dr. Girnita.
Physicians who establish these models sometimes do a hybrid of cash only and insurance. Micah Yu, MD, who practices rheumatology in Newport Beach, Calif., only takes Medicare. “Otherwise, patients are private pay. I am mainly fee for service, so patients are paying me for my time,” he said.
By tailoring their patient base and services, adopters find they have more time to spend with patients. “In my model, I spend 30 minutes for follow-up and 1 hour for new patients,” Dr. Yu said.
Limitations of traditional care
Carrying insurance doesn’t guarantee you the right care, Dr. Girnita said. Wait times to see a rheumatologist range from 4 to 6 months. For physicians who contract with insurance companies, reimbursement for services isn’t always paid promptly and decreases every year. A new cut in reimbursement is expected for rheumatology services in 2022.
Patients in direct care “pay a small amount for memberships that cover the cost of their visits and the time physicians spend in coordinating their additional care between the visits. The cost of the visits is always transparent,” Dr. Girnita said.
Irene Kazmers, MD, a solo private rheumatology practitioner in northern Michigan, was seeing 20-plus patients a day before she made the leap to a concierge model. “The paperwork and administrative burdens of practicing rheumatology as a solo [physician] have mushroomed in the last 10 years,” she said during the ACR meeting. She and staff were spending an inordinate amount of time on prior authorizations, step therapy requirements, electronic health record documentation, and other administrative burdens.
Reimbursements from payers have progressively declined as administrative challenges have necessitated more staff. “I was struggling to maintain an ample financial margin,” she said.
Improved communication, unlimited visits
Dr. Kazmers attests that the transition to the concierge model has enabled and fostered a higher level of communication and specialty care for her patients.
Patients who enroll in the practice pay an annual membership fee and get access to her personal cell phone number and email address. “If they need an urgent appointment, it is typically arranged the same or next day,” she said in an interview. “Visits are not as rushed as in the traditional model, conducive to incorporating beneficial integrative medicine modalities such as dietary, exercise, and mind-body approaches as appropriate, in addition to state-of-the-art treatment.”
She also has more time to coordinate care with her patients’ primary care providers and other care team professionals and to give patients feedback on lab and study results.
Dr. Girnita has ramped down from 28 to 15 patients a day. She’s able to spend 60 minutes for new patients and 30 minutes for follow-ups. Like Dr. Kazmers, she feels she has more time to address patient needs and listen to their concerns.
She’s kept her hospital affiliations but finds that she doesn’t have to go to the hospital as much as she used to. Direct care “reduces hospital visits because physicians significantly have much more time to spend with the patient and address the needs of the patient.” A patient with a gout flare, for example, may end up in the hospital under traditional care because there’s no room in the physician’s schedule to address the patient’s needs.
Dr. Girnita recalled when she assisted a patient who had developed inflammatory arthritis and was desperate to see a doctor. The patient had good insurance, but appointments in her area weren’t available for at least 6 months. “Her primary care physician called me. I saw her and provided her with the appropriate care. A couple of months later she is doing great.”
What insurance does and doesn’t cover
Many patients who seek out direct or concierge models retain their insurance. At least 90% of Dr. Girnita’s patients have insurance with high deductibles. The other 10% have other types of insurance or no insurance.
Ellen McKnight, MD, who has a hybrid rheumatology practice in Pensacola, Fla., still accepts commercial insurance, but has opted out of Medicare. Her patients mostly come from rural areas in Florida, and their insurance situations vary widely. “In my practice, I estimate that 65% have insurance and 35% do not. Most of my patients have commercial insurance, and a substantial portion, about 40%, are just paying cash. My cash pay patients have Medicare, HMOs, and others are uninsured,” she said in an interview.
Direct care practices may continue to bill traditional insurance for items like visits, injections, and ultrasound.
Dr. Girnita’s patients have the option of submitting a “superbill” or invoice to insurance companies for patients to be reimbursed by their insurance for the cost of the visit. It contains the CPT code for the visit along with the ICD-10 codes for diagnoses. “I use a company called Reimbursify to help patients submit their invoice to their insurance company,” Dr. Girnita said.
Dr. Ortiz takes a different approach, offering superbills for consults and individual appointments, but not for patients enrolled in her membership program.
Some in the payer industry contend that direct care arrangements increase costs and distort risk pools. If most direct care patients already have a comprehensive health insurance policy, it’s likely they’re being billed twice for services, said David Allen, spokesperson for America’s Health Insurance Plans.
“Duplicative payments inflate the cost of care at a convenience to the providers and increase the cost of insurance premiums when insurers receive bills for those same services from providers. In other words, patients are being double billed,” Mr. Allen said.
These providers are assuming risk without state insurance oversight or regulations to ensure patient protections and safeguards are in place, he continued. “If utilization of services outpaces capacity, the provider may ultimately be unable to provide the amount of care expected by the patient because their practice agreed to unlimited visits and services with little or no restrictions.”
Eliminating ‘surprise’ bills
Adopters of direct care/concierge services counter that it’s the insurance and pharmaceutical companies driving up costs. Patients – especially those who have high-deductible plans – save money through these models. “In the direct care model, doctors have worked out advocacy for patients that are unsurpassed. Insurance companies don’t do that,” Dr. McKnight said.
Consumers know up front what the price is for other services. When you go to a restaurant, you always look on the menu to see what the price is for a bottle of wine or steak, Dr. Girnita said. “Only in the medical field you don’t know anything. And you’re shocked about the price you must pay.” Not many practices list their prices on their website, although federal rules seek to further increase price transparency in hospitals and among insurers.
Patients will sometimes get a “surprise” bill for their visit, laboratory, or imaging tests. According to Dr. Girnita, “that doesn’t happen in my practice. I discuss all prices with them before they get to the lab or MRI. I don’t charge copayments or anything extra.” Without a copayment – usually $50-$75 for specialist services – or a surprise bill, patients are always paying less, she said.
Costs through insurance are oftentimes higher, she continued. For routine lab work, a patient in a direct care practice pays about $30-$40. If they request this work through a lab, they’re likely to pay $150. “Think about an MRI. Through a direct care practice like mine, you pay $450-$700. In a hospital setting, you pay at least $5,000.”
Patients with high-deductible insurance plans often pay thousands of dollars before meeting their deductible, Dr. Girnita and others noted. A patient with this type of plan may pay $250 for a vitamin D lab if they haven’t met their deductible, Dr. McKnight explained. “With direct care, you’ll be paying $12.50.”
Dr. Girnita said her members get excellent discounts for labs and imaging. In the direct care models, physicians can help with this by contracting directly with labs, imaging centers, and independent pharmacies, giving patients access to affordable and transparent prices for their medical care.
What patients pay for services
In direct and concierge care membership models, coverage for services and fees vary widely from practice to practice.
Dr. Girnita offers several membership options. One package, which is $199 a month, is for patients with stable symptoms that guarantee continuity of care. It includes four visits a year and immediate access to the practice in case of emergency (including two additional urgent visits). “This works for a lot of patients. They consider that affordable, and they have all the benefits of a concierge practice. They can have direct communication with me, and they have guaranteed continuity of care,” Dr. Girnita said.
The other model, which is $299 per month, is for patients who need monthly contact with the rheumatologist for visits, telephone and email communications, urgent appointments, integrative medicine consultations, and many other benefits. For 1-hour consultations, Dr. Girnita charges $399.
Dr. Ortiz, who offers a direct pay model, charges $899 for an initial consult, which covers 3.5 hours of her time. “We do an hour of telemedicine, and we do a house call, which is 1.5-2 hours.” She follows up with a telehealth visit. Labs and x-rays are not included and go through the patient’s insurance.
Once the consult takes place, she assesses what a patient needs and offers them either a 6- or 12-month membership, which includes unlimited visits.
Patients can also buy a prepaid, six-appointment package with a 12% discount. Dr. Ortiz prices her telehealth visits at $350 and house calls at $550.
Dr. McKnight’s cash-only model for established patients offers four visits a year, reducing the fee for each visit. For example, a patient will pay $95 for the first visit, then $90, $85, and $80 for subsequent visits.
Accessing medications through direct care
One challenge with this model is finding affordable medications for patients outside of insurance.
Insurance dictates what’s covered, leaving fewer options for patients, Dr. McKnight said. “You have to jump through hoops, and there’s prior authorizations.” For a condition like severe osteoporosis, treatment should start sequentially with the true bone builders first, then move on to a medication like alendronate (Fosamax).
“Insurers will make you go to Fosamax first and then fail it,” she said. This results in the patient potentially developing worsening bone loss or possibly even sustaining a fracture.
Prior authorization requirements demand excessive staff time and effort, Dr. Kazmers said. This can translate to more than $90,000 a year in human resource costs for rheumatologists, who often deal with many specialty drug authorizations. “Every practice needs to hire staff to handle prior authorizations. We receive no compensation for this from the pharmaceutical companies and middlemen who ultimately profit from this cumbersome process,” she added.
Among the two big classes for rheumatology patients, conventional synthetic disease-modifying antirheumatic drugs (DMARDs) are the most widely available. Pharmacies can offer DMARDs for cash, although some are limited in terms of where they can ship, Dr. Girnita said.
The other class, biologic DMARDs, are the most expensive medications rheumatologists use for conditions such as rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis.
With biologics, it’s more difficult, as they’re very expensive, typically $6,000 a month or more, sources told this news organization.
“Unfortunately, we can’t partner at this time with pharmaceutical companies that produce biologics or independent pharmacies,” Dr. Girnita said. Physicians can’t control biologic prices either. “Insurance companies and pharmacy benefit managers have the control on these prices.”
Physicians can direct patients to multiple resources where they can find assistance.
Biologics companies that offer patient assistance programs can sometimes offer medications for free, while others offer savings cards or copay cards, “which helps a lot,” Dr. Girnita said. She assists her patients by filing some of the paperwork necessary to qualify for these programs, and the patients submit the rest.
“For these companies to help the patient, they need the patient’s financial information,” she said. “But I do most of that work; I complete the forms and send to the company and justify need for the medication.”
What’s ahead for direct specialty care
While some patients have benefited, others have had to seek alternatives as their doctors transition to alternative models.
Not everyone can afford the concierge retainer fee, said Dr. Kazmers, who practices in a rural area of Michigan, where rheumatologists are scarce. Enrollment in her concierge practice filled months before the switchover from her traditional practice took place. There are 70 patients on a waiting list.
Patients who elect not to enroll in the concierge practice need to find another source of rheumatology care. This is a downside to the practice transition, she acknowledged. “The closest rheumatologist taking new patients is a 3- to 4-hour drive away, which simply reflects the shortage of medical school graduates choosing to go into rheumatology in the United States,” Dr. Kazmers added.
One physician caring for thousands of chronically ill, complicated patients within systems that don’t allow them the time to really care for their patients threatens to make the access problem worse, Dr. Ortiz said. The direct care/concierge model offers an alternative for the provider “and is a way to keep providers in the workforce, who may otherwise consider leaving.”
Direct care/concierge medicine isn’t for all doctors. But for Dr. Kazmers, it’s the best option for her at this point in her career. “I’ve been practicing for 45 years in various models, including academic positions and private practice employment. I have worked for years in settings accepting Medicaid. I understand that if every rheumatologist went concierge tomorrow, this would constrict access to needed specialty care. But in my case, it provided a viable alternative to closing the practice’s doors altogether.”
Ultimately, the U.S. medical system needs more rheumatologists and other specialists. “If you really want to increase the service, then Medicare or other sources should support opening more residency and fellowship spots for medical graduates to pursue,” Dr. Girnita said.
Other solutions call for more systemic and institutional changes, such as expanding rheumatology divisions and faculties at institutions that train fellows and addressing medical school debt, Dr. Ortiz said.
Some practices see themselves branching out from individual patient care and partnering with local businesses to provide care for employees. That’s the future for direct specialty care, said Dr. Girnita, who’s been in discussions with a few employers to make such arrangements.
The direct primary care community has already started to contract with employers. “Their employees get care they need for just a fraction of the cost. These discussions are arising more and more,” she said.
A version of this article first appeared on Medscape.com.
Elizabeth Ortiz, MD, knew she needed a change. Working at an academic county clinic, she was often worn down and pulled in different directions. “When I thought about what I really liked about my job, it was patient care and spending time with my patients, which I wasn’t able to do,” Dr. Ortiz said during the annual meeting of the American College of Rheumatology.
She’d heard of direct or concierge care but wasn’t sure if it was a good fit for her. COVID-19 offered a catalyst of sorts for a move to a new care model.
Ten weeks after she moved to Dallas, the pandemic hit full force. Seeing how telehealth was taking off, Dr. Ortiz began crafting a new model of care, a hybrid of telemedicine and house calls that offered multiple venues to connect with patients. The practice is just a year old, and “it’s working and it’s a constant experiment,” said Dr. Ortiz, who offers membership plans and prepaid appointments. She also does “a la carte” visits where established patients can see her at a one-off price. Her goal is to achieve 100% membership.
Although she operates through a direct pay and cash-only model, only recently has she become comfortable with the word “concierge.” There’s a preconceived notion of what that word means, she said.
Direct care: A definition
Following the trend of some primary care practices, more rheumatologists who are dissatisfied with the status quo are embracing these models of care.
Direct and concierge care are often mentioned in tandem, but there are nuanced differences. Direct specialty care removes third-party payers to protect the best interests of patients, according to Diana Girnita, MD, founder and CEO of Rheumatologist OnCall, a direct care practice. Her patient base hails from rural and urban areas in least 10 states. She also created a Facebook group for specialists in direct care and is the cofounder of the Direct Specialty Care Alliance.
Direct care offers a membership fee and additional fees for “as needed” services. “As the physician, I do not have to be contracted to an insurance company to see patients. I contract directly with patients. It is the patient’s choice to contract with an insurance and use the insurance for ancillary services and medication,” Dr. Girnita said. Patients with out-of-network benefits can claim the insurance to cover part of the consultation cost, she added.
In concierge or retainer medicine, a patient pays an annual or monthly fee or retainer to get access to the physician practice. In addition to this fee, the practice can bill the patient’s insurance for consultations or other services. “The concierge model does not eliminate the sub payer. You still contract with the patient’s insurance,” explained Dr. Girnita.
Physicians who establish these models sometimes do a hybrid of cash only and insurance. Micah Yu, MD, who practices rheumatology in Newport Beach, Calif., only takes Medicare. “Otherwise, patients are private pay. I am mainly fee for service, so patients are paying me for my time,” he said.
By tailoring their patient base and services, adopters find they have more time to spend with patients. “In my model, I spend 30 minutes for follow-up and 1 hour for new patients,” Dr. Yu said.
Limitations of traditional care
Carrying insurance doesn’t guarantee you the right care, Dr. Girnita said. Wait times to see a rheumatologist range from 4 to 6 months. For physicians who contract with insurance companies, reimbursement for services isn’t always paid promptly and decreases every year. A new cut in reimbursement is expected for rheumatology services in 2022.
Patients in direct care “pay a small amount for memberships that cover the cost of their visits and the time physicians spend in coordinating their additional care between the visits. The cost of the visits is always transparent,” Dr. Girnita said.
Irene Kazmers, MD, a solo private rheumatology practitioner in northern Michigan, was seeing 20-plus patients a day before she made the leap to a concierge model. “The paperwork and administrative burdens of practicing rheumatology as a solo [physician] have mushroomed in the last 10 years,” she said during the ACR meeting. She and staff were spending an inordinate amount of time on prior authorizations, step therapy requirements, electronic health record documentation, and other administrative burdens.
Reimbursements from payers have progressively declined as administrative challenges have necessitated more staff. “I was struggling to maintain an ample financial margin,” she said.
Improved communication, unlimited visits
Dr. Kazmers attests that the transition to the concierge model has enabled and fostered a higher level of communication and specialty care for her patients.
Patients who enroll in the practice pay an annual membership fee and get access to her personal cell phone number and email address. “If they need an urgent appointment, it is typically arranged the same or next day,” she said in an interview. “Visits are not as rushed as in the traditional model, conducive to incorporating beneficial integrative medicine modalities such as dietary, exercise, and mind-body approaches as appropriate, in addition to state-of-the-art treatment.”
She also has more time to coordinate care with her patients’ primary care providers and other care team professionals and to give patients feedback on lab and study results.
Dr. Girnita has ramped down from 28 to 15 patients a day. She’s able to spend 60 minutes for new patients and 30 minutes for follow-ups. Like Dr. Kazmers, she feels she has more time to address patient needs and listen to their concerns.
She’s kept her hospital affiliations but finds that she doesn’t have to go to the hospital as much as she used to. Direct care “reduces hospital visits because physicians significantly have much more time to spend with the patient and address the needs of the patient.” A patient with a gout flare, for example, may end up in the hospital under traditional care because there’s no room in the physician’s schedule to address the patient’s needs.
Dr. Girnita recalled when she assisted a patient who had developed inflammatory arthritis and was desperate to see a doctor. The patient had good insurance, but appointments in her area weren’t available for at least 6 months. “Her primary care physician called me. I saw her and provided her with the appropriate care. A couple of months later she is doing great.”
What insurance does and doesn’t cover
Many patients who seek out direct or concierge models retain their insurance. At least 90% of Dr. Girnita’s patients have insurance with high deductibles. The other 10% have other types of insurance or no insurance.
Ellen McKnight, MD, who has a hybrid rheumatology practice in Pensacola, Fla., still accepts commercial insurance, but has opted out of Medicare. Her patients mostly come from rural areas in Florida, and their insurance situations vary widely. “In my practice, I estimate that 65% have insurance and 35% do not. Most of my patients have commercial insurance, and a substantial portion, about 40%, are just paying cash. My cash pay patients have Medicare, HMOs, and others are uninsured,” she said in an interview.
Direct care practices may continue to bill traditional insurance for items like visits, injections, and ultrasound.
Dr. Girnita’s patients have the option of submitting a “superbill” or invoice to insurance companies for patients to be reimbursed by their insurance for the cost of the visit. It contains the CPT code for the visit along with the ICD-10 codes for diagnoses. “I use a company called Reimbursify to help patients submit their invoice to their insurance company,” Dr. Girnita said.
Dr. Ortiz takes a different approach, offering superbills for consults and individual appointments, but not for patients enrolled in her membership program.
Some in the payer industry contend that direct care arrangements increase costs and distort risk pools. If most direct care patients already have a comprehensive health insurance policy, it’s likely they’re being billed twice for services, said David Allen, spokesperson for America’s Health Insurance Plans.
“Duplicative payments inflate the cost of care at a convenience to the providers and increase the cost of insurance premiums when insurers receive bills for those same services from providers. In other words, patients are being double billed,” Mr. Allen said.
These providers are assuming risk without state insurance oversight or regulations to ensure patient protections and safeguards are in place, he continued. “If utilization of services outpaces capacity, the provider may ultimately be unable to provide the amount of care expected by the patient because their practice agreed to unlimited visits and services with little or no restrictions.”
Eliminating ‘surprise’ bills
Adopters of direct care/concierge services counter that it’s the insurance and pharmaceutical companies driving up costs. Patients – especially those who have high-deductible plans – save money through these models. “In the direct care model, doctors have worked out advocacy for patients that are unsurpassed. Insurance companies don’t do that,” Dr. McKnight said.
Consumers know up front what the price is for other services. When you go to a restaurant, you always look on the menu to see what the price is for a bottle of wine or steak, Dr. Girnita said. “Only in the medical field you don’t know anything. And you’re shocked about the price you must pay.” Not many practices list their prices on their website, although federal rules seek to further increase price transparency in hospitals and among insurers.
Patients will sometimes get a “surprise” bill for their visit, laboratory, or imaging tests. According to Dr. Girnita, “that doesn’t happen in my practice. I discuss all prices with them before they get to the lab or MRI. I don’t charge copayments or anything extra.” Without a copayment – usually $50-$75 for specialist services – or a surprise bill, patients are always paying less, she said.
Costs through insurance are oftentimes higher, she continued. For routine lab work, a patient in a direct care practice pays about $30-$40. If they request this work through a lab, they’re likely to pay $150. “Think about an MRI. Through a direct care practice like mine, you pay $450-$700. In a hospital setting, you pay at least $5,000.”
Patients with high-deductible insurance plans often pay thousands of dollars before meeting their deductible, Dr. Girnita and others noted. A patient with this type of plan may pay $250 for a vitamin D lab if they haven’t met their deductible, Dr. McKnight explained. “With direct care, you’ll be paying $12.50.”
Dr. Girnita said her members get excellent discounts for labs and imaging. In the direct care models, physicians can help with this by contracting directly with labs, imaging centers, and independent pharmacies, giving patients access to affordable and transparent prices for their medical care.
What patients pay for services
In direct and concierge care membership models, coverage for services and fees vary widely from practice to practice.
Dr. Girnita offers several membership options. One package, which is $199 a month, is for patients with stable symptoms that guarantee continuity of care. It includes four visits a year and immediate access to the practice in case of emergency (including two additional urgent visits). “This works for a lot of patients. They consider that affordable, and they have all the benefits of a concierge practice. They can have direct communication with me, and they have guaranteed continuity of care,” Dr. Girnita said.
The other model, which is $299 per month, is for patients who need monthly contact with the rheumatologist for visits, telephone and email communications, urgent appointments, integrative medicine consultations, and many other benefits. For 1-hour consultations, Dr. Girnita charges $399.
Dr. Ortiz, who offers a direct pay model, charges $899 for an initial consult, which covers 3.5 hours of her time. “We do an hour of telemedicine, and we do a house call, which is 1.5-2 hours.” She follows up with a telehealth visit. Labs and x-rays are not included and go through the patient’s insurance.
Once the consult takes place, she assesses what a patient needs and offers them either a 6- or 12-month membership, which includes unlimited visits.
Patients can also buy a prepaid, six-appointment package with a 12% discount. Dr. Ortiz prices her telehealth visits at $350 and house calls at $550.
Dr. McKnight’s cash-only model for established patients offers four visits a year, reducing the fee for each visit. For example, a patient will pay $95 for the first visit, then $90, $85, and $80 for subsequent visits.
Accessing medications through direct care
One challenge with this model is finding affordable medications for patients outside of insurance.
Insurance dictates what’s covered, leaving fewer options for patients, Dr. McKnight said. “You have to jump through hoops, and there’s prior authorizations.” For a condition like severe osteoporosis, treatment should start sequentially with the true bone builders first, then move on to a medication like alendronate (Fosamax).
“Insurers will make you go to Fosamax first and then fail it,” she said. This results in the patient potentially developing worsening bone loss or possibly even sustaining a fracture.
Prior authorization requirements demand excessive staff time and effort, Dr. Kazmers said. This can translate to more than $90,000 a year in human resource costs for rheumatologists, who often deal with many specialty drug authorizations. “Every practice needs to hire staff to handle prior authorizations. We receive no compensation for this from the pharmaceutical companies and middlemen who ultimately profit from this cumbersome process,” she added.
Among the two big classes for rheumatology patients, conventional synthetic disease-modifying antirheumatic drugs (DMARDs) are the most widely available. Pharmacies can offer DMARDs for cash, although some are limited in terms of where they can ship, Dr. Girnita said.
The other class, biologic DMARDs, are the most expensive medications rheumatologists use for conditions such as rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis.
With biologics, it’s more difficult, as they’re very expensive, typically $6,000 a month or more, sources told this news organization.
“Unfortunately, we can’t partner at this time with pharmaceutical companies that produce biologics or independent pharmacies,” Dr. Girnita said. Physicians can’t control biologic prices either. “Insurance companies and pharmacy benefit managers have the control on these prices.”
Physicians can direct patients to multiple resources where they can find assistance.
Biologics companies that offer patient assistance programs can sometimes offer medications for free, while others offer savings cards or copay cards, “which helps a lot,” Dr. Girnita said. She assists her patients by filing some of the paperwork necessary to qualify for these programs, and the patients submit the rest.
“For these companies to help the patient, they need the patient’s financial information,” she said. “But I do most of that work; I complete the forms and send to the company and justify need for the medication.”
What’s ahead for direct specialty care
While some patients have benefited, others have had to seek alternatives as their doctors transition to alternative models.
Not everyone can afford the concierge retainer fee, said Dr. Kazmers, who practices in a rural area of Michigan, where rheumatologists are scarce. Enrollment in her concierge practice filled months before the switchover from her traditional practice took place. There are 70 patients on a waiting list.
Patients who elect not to enroll in the concierge practice need to find another source of rheumatology care. This is a downside to the practice transition, she acknowledged. “The closest rheumatologist taking new patients is a 3- to 4-hour drive away, which simply reflects the shortage of medical school graduates choosing to go into rheumatology in the United States,” Dr. Kazmers added.
One physician caring for thousands of chronically ill, complicated patients within systems that don’t allow them the time to really care for their patients threatens to make the access problem worse, Dr. Ortiz said. The direct care/concierge model offers an alternative for the provider “and is a way to keep providers in the workforce, who may otherwise consider leaving.”
Direct care/concierge medicine isn’t for all doctors. But for Dr. Kazmers, it’s the best option for her at this point in her career. “I’ve been practicing for 45 years in various models, including academic positions and private practice employment. I have worked for years in settings accepting Medicaid. I understand that if every rheumatologist went concierge tomorrow, this would constrict access to needed specialty care. But in my case, it provided a viable alternative to closing the practice’s doors altogether.”
Ultimately, the U.S. medical system needs more rheumatologists and other specialists. “If you really want to increase the service, then Medicare or other sources should support opening more residency and fellowship spots for medical graduates to pursue,” Dr. Girnita said.
Other solutions call for more systemic and institutional changes, such as expanding rheumatology divisions and faculties at institutions that train fellows and addressing medical school debt, Dr. Ortiz said.
Some practices see themselves branching out from individual patient care and partnering with local businesses to provide care for employees. That’s the future for direct specialty care, said Dr. Girnita, who’s been in discussions with a few employers to make such arrangements.
The direct primary care community has already started to contract with employers. “Their employees get care they need for just a fraction of the cost. These discussions are arising more and more,” she said.
A version of this article first appeared on Medscape.com.
Giant cell arteritis fast-track clinics pave way for greater ultrasound use in U.S.
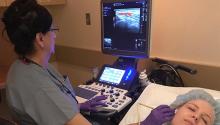
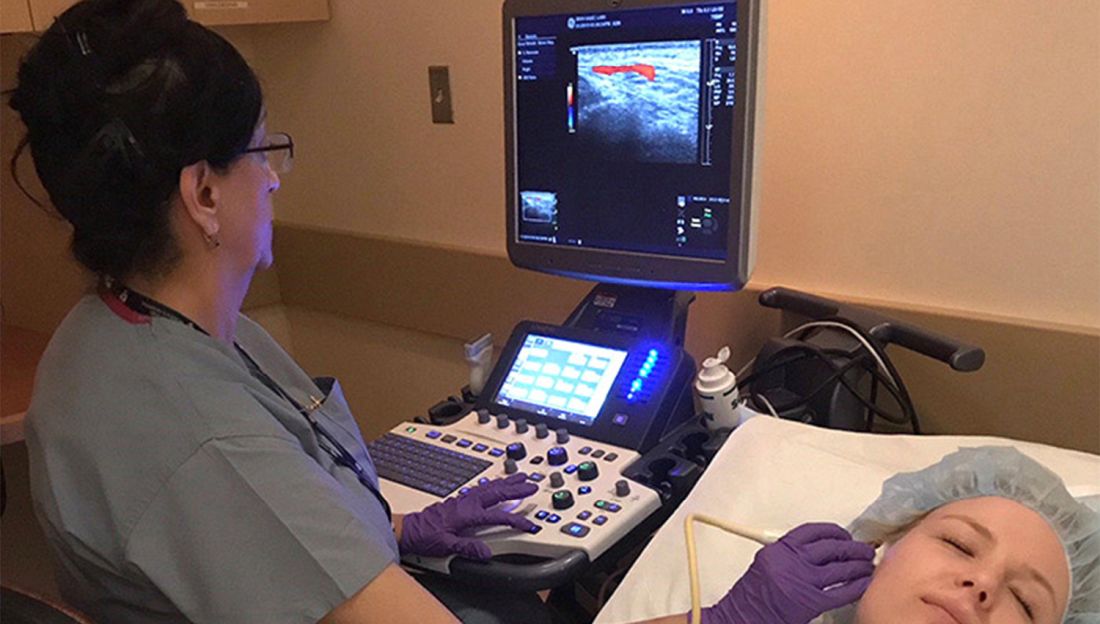
Temporal artery biopsy has been the standard for diagnosing giant cell arteritis (GCA), but vascular ultrasound, a procedure that’s less invasive, less time-intensive, less expensive, and more convenient, has gained widespread use in Europe, and now clinics in the United States are adopting this approach and moving toward having rheumatologists take on the role of ultrasonographer.
However, directors at these clinics – known as GCA fast-track clinics – caution that the bar can be high for adopting vascular ultrasound (VUS) as a tool to diagnose GCA. Ultrasonographers need specialized training to perform the task and an adequate caseload to maintain their skills. Clinics also need to be outfitted with high-definition ultrasound machines.
“It definitely takes adequate training and learning of how to adjust the settings on the ultrasound machine to be able to visualize the findings appropriately,” said Minna Kohler, MD, director of the rheumatology and musculoskeletal ultrasound program at Massachusetts General Hospital in Boston, which has its own GCA fast-track clinic.
“And the clinical context is very important,” she said. “If you have a high suspicion for someone with temporal arteritis, or GCA, and the patient has been on steroids for weeks before you see them, the ultrasound findings may not show signs of the disease. In those cases in which the imaging is equivocal, we would still pursue a biopsy.”
The idea of the fast-track clinic is as the name implies: to quickly confirm the presence of GCA in a matter of hours, or days at the most, in an outpatient setting without the hassles of a biopsy. Temporal artery biopsy (TAB), by comparison, “is more costly because it requires operating room time, a surgical consultation, and surgery time, whereas ultrasound is a very inexpensive exam since it’s done in the clinic by the rheumatologist,” Dr. Kohler said.
European experience
Use of VUS to diagnose GCA is supplanting TAB in Europe and other countries. In Denmark alone – with a population of 6 million – three outpatient fast-track clinics are operating. The United States, with a population more than 50 times larger than Denmark’s, has six.
Stavros Chrysidis, MD, PhD, chief of rheumatology at the Hospital South West Jutland, one of the fast-track clinic sites in Denmark, led a recent multicenter study, known as EUREKA, of VUS in patients with suspected GCA. He and his colleagues reported in The Lancet Rheumatology that the sensitivity and specificity of VUS was superior to TAB in confirming a diagnosis of GCA. Dr. Chrysidis has instructed U.S. rheumatologists and ultrasonographers in performing and interpreting VUS for GCA.
The study emphasizes the importance of training for ultrasonographers, said Dr. Chrysidis, who regularly performs VUS at his institution. “The most important finding is that, when we apply VUS by systematically trained ultrasonographers using appropriate equipment in appropriate settings, it has excellent diagnostic accuracy on GCA,” he told this news organization.
He noted that The Lancet Rheumatology report is the first multicenter study of VUS for diagnosing GCA in which all the ultrasonographers participated in a standardized training program, which his group developed. “Ultrasound is very operator dependent,” he said. “That’s why the training is very important.”
The training occurred over a year and included two workshops consisting of 5 days of theoretical training on VUS; supervised hands-on evaluation of healthy individuals and patients with known GCA; and evaluation of ultrasound images. Over the course of the year, trainees performed at least 50 VUS evaluations, half of which were in patients with confirmed GCA. During the training period, an external rheumatologist with broad experience in VUS made the final diagnosis.
“The equipment and settings are very important because ultrasound can be very time consuming if you are not educated well and if your equipment is not adjusted well,” Dr. Chrysidis said. The equipment must be calibrated beforehand “so you don’t spend time on adjustments.”
For diagnosing temporal artery anomalies, the ultrasound equipment must have a resolution of 0.3-0.4 mm, he said. “When you have a transducer of 10 MHz, you cannot visualize changes smaller than 5 mm.”
The EUREKA study stated that VUS could replace TAB as a first-line diagnostic tool for GCA – provided the ultrasonographers are systematically trained and the equipment and settings are appropriate. In the Jutland clinic, VUS already has replaced TAB, Dr. Chrysidis said.
“In my department since 2017, when we started the fast-track clinic after the EUREKA study was terminated, we have performed three temporal artery biopsies in the last 4 years, and we screen 60-70 patients per year because we use ultrasound as the primary imaging,” he said. In cases when the results are inconclusive, they order a PET scan. “We don’t perform biopsies anymore,” he said.
U.S. fast-track clinic models
The fast-track clinic models in the United States vary. Results of a survey of the U.S. clinics were presented as an abstract at the 2021 American College of Rheumatology annual meeting. Some centers have a vasculitis specialist obtain and interpret the imaging. At others, a vasculitis specialist refers patients to a VUS-trained rheumatologist to perform and interpret the test. Another approach is to have vasculitis specialists refer patients to ultrasound technicians trained in VUS, with a vascular surgeon interpreting the images and either a VUS-trained rheumatologist or vascular medicine specialist verifying the images.
The take-home message of that survey is that “ultrasound evaluation should be considered in the hands of experts, realizing that not everyone has that skill set, but if it is available, it’s a way to expedite diagnosis and it can be helpful in managing the GCA patient in an appropriate way, quicker than trying to schedule cross-sectional imaging,” said Massachusetts General’s Dr. Kohler, who is a coauthor of the abstract. “Certainly, cross-sectional imaging also plays an important role, but when it comes to confirming whether to continue with treatment or not for a very serious condition, ultrasound is a quick way to get the answer.”
In addition to the fast-track clinic at Massachusetts General, the survey included fast-track clinics at the University of Washington, Seattle; Brigham and Women’s Hospital, Boston; Loma Linda (Calif.) University; University of California, Los Angeles; and at a private practice, Arthritis and Rheumatism Associates in the Washington area.
Advantages of VUS vs. TAB
At Massachusetts General, some of the rheumatologists are trained to perform VUS. The rheumatologists also perform the clinical evaluation of suspected cases of GCA. The advantage of VUS, Dr. Kohler said, is that the answer is “right there”; that is, the imaging yields a diagnosis almost instantaneously whereas a biopsy must be sent to a lab for analysis.
“Since a lot of patients with suspected vasculitis may already come to us on steroid therapy, and if there’s a low probability or low suspicion for vasculitis, [VUS] actually confirms that, and we’re able to taper prednisone or steroids quickly rather than keep them on a prolonged course.”
Alison Bays, MD, MPH, of the department of rheumatology at the University of Washington, said that the advantages of avoiding biopsy aren’t to be overlooked. “Temporal artery biopsies are invasive and carry surgical risks, especially as many of our patients are elderly,” she told this news organization.
“These patients occasionally refuse biopsy, but the acceptance of ultrasound is high,” Dr. Bays said. “Scheduling surgery can be more complicated, resulting in delays to biopsy and potentially higher rates of false negatives. Additionally, ultrasound has resulted in a higher rate of diagnosis with GCA as TAB misses large-vessel involvement.” The fast-track clinic at the university has evaluated 250 patients since it opened in 2017.
Dr. Bays and colleagues published the first United States study of a fast-track protocol using vascular sonographers. “Our group has demonstrated that fast-track clinics can rapidly and effectively evaluate patients, and we demonstrated a different method of evaluation using vascular sonographers rather than rheumatologists to do the vascular ultrasound,” she said. “It utilizes the familiarity vascular sonographers already have with imaging blood vessels.”
She added that the TABUL study in the United Kingdom in 2016 demonstrated that VUS yielded a savings of $686 (£484 as reported in the study), compared with TAB. “Further studies need to be done in the United States,” she said. “Beyond direct comparison of costs, reduction in steroid burden due to quick evaluation and diagnosis may carry additional benefits.”
At Brigham and Women’s Hospital, the division of rheumatology and the vascular section of the cardiology division collaborate on the GCA evaluation, said Sara Tedeschi, MD, MPH, codirector of the fast-track clinic there. Trained vascular technologists credentialed in the procedure and specifically trained in using VUS for evaluating arteritis perform the VUS. Cardiologists with a subspecialty in vascular medicine interpret the studies.
VUS patients have a rheumatology evaluation just before they have the ultrasound. “The rheumatology evaluation is then able to incorporate information from the VUS together with laboratory data, the patient’s history, and physical examination,” Dr. Tedeschi said.
“If the rheumatologist recommends a temporal artery biopsy as a next step, we arrange this with vascular surgery,” she said. “If the rheumatologist recommends other imaging such as MRA [magnetic resonance angiography] or PET-CT, we frequently review the images together with our colleagues in cardiovascular radiology and/or nuclear medicine.”
But in the United States, it will take some time for GCA fast-track clinics to become the standard, Dr. Tedeschi said. “Temporal artery biopsy may be faster to arrange in certain practice settings if VUS is not already being employed for giant cell arteritis evaluation,” she said.
Dr. Bays recognized this limitation, saying, “We are hoping that in the future, the American College of Rheumatology will consider vascular ultrasound as a first-line diagnostic test in diagnosis as rheumatologists and vascular sonographers gain familiarity over time.”
But that would mean that centers performing VUS for GCA would have to meet rigorous standards for the procedure. “With that, standardization of a protocol, high-quality equipment, and adequate training are necessary to ensure quality and reduce the chance of false-positive or false-negative results,” she said.
Dr. Chrysidis, Dr. Bays, and Dr. Tedeschi have disclosed no relevant financial relationships. Dr. Kohler is a board member of Ultrasound School of North American Rheumatologists.
A version of this article first appeared on Medscape.com.
Temporal artery biopsy has been the standard for diagnosing giant cell arteritis (GCA), but vascular ultrasound, a procedure that’s less invasive, less time-intensive, less expensive, and more convenient, has gained widespread use in Europe, and now clinics in the United States are adopting this approach and moving toward having rheumatologists take on the role of ultrasonographer.
However, directors at these clinics – known as GCA fast-track clinics – caution that the bar can be high for adopting vascular ultrasound (VUS) as a tool to diagnose GCA. Ultrasonographers need specialized training to perform the task and an adequate caseload to maintain their skills. Clinics also need to be outfitted with high-definition ultrasound machines.
“It definitely takes adequate training and learning of how to adjust the settings on the ultrasound machine to be able to visualize the findings appropriately,” said Minna Kohler, MD, director of the rheumatology and musculoskeletal ultrasound program at Massachusetts General Hospital in Boston, which has its own GCA fast-track clinic.
“And the clinical context is very important,” she said. “If you have a high suspicion for someone with temporal arteritis, or GCA, and the patient has been on steroids for weeks before you see them, the ultrasound findings may not show signs of the disease. In those cases in which the imaging is equivocal, we would still pursue a biopsy.”
The idea of the fast-track clinic is as the name implies: to quickly confirm the presence of GCA in a matter of hours, or days at the most, in an outpatient setting without the hassles of a biopsy. Temporal artery biopsy (TAB), by comparison, “is more costly because it requires operating room time, a surgical consultation, and surgery time, whereas ultrasound is a very inexpensive exam since it’s done in the clinic by the rheumatologist,” Dr. Kohler said.
European experience
Use of VUS to diagnose GCA is supplanting TAB in Europe and other countries. In Denmark alone – with a population of 6 million – three outpatient fast-track clinics are operating. The United States, with a population more than 50 times larger than Denmark’s, has six.
Stavros Chrysidis, MD, PhD, chief of rheumatology at the Hospital South West Jutland, one of the fast-track clinic sites in Denmark, led a recent multicenter study, known as EUREKA, of VUS in patients with suspected GCA. He and his colleagues reported in The Lancet Rheumatology that the sensitivity and specificity of VUS was superior to TAB in confirming a diagnosis of GCA. Dr. Chrysidis has instructed U.S. rheumatologists and ultrasonographers in performing and interpreting VUS for GCA.
The study emphasizes the importance of training for ultrasonographers, said Dr. Chrysidis, who regularly performs VUS at his institution. “The most important finding is that, when we apply VUS by systematically trained ultrasonographers using appropriate equipment in appropriate settings, it has excellent diagnostic accuracy on GCA,” he told this news organization.
He noted that The Lancet Rheumatology report is the first multicenter study of VUS for diagnosing GCA in which all the ultrasonographers participated in a standardized training program, which his group developed. “Ultrasound is very operator dependent,” he said. “That’s why the training is very important.”
The training occurred over a year and included two workshops consisting of 5 days of theoretical training on VUS; supervised hands-on evaluation of healthy individuals and patients with known GCA; and evaluation of ultrasound images. Over the course of the year, trainees performed at least 50 VUS evaluations, half of which were in patients with confirmed GCA. During the training period, an external rheumatologist with broad experience in VUS made the final diagnosis.
“The equipment and settings are very important because ultrasound can be very time consuming if you are not educated well and if your equipment is not adjusted well,” Dr. Chrysidis said. The equipment must be calibrated beforehand “so you don’t spend time on adjustments.”
For diagnosing temporal artery anomalies, the ultrasound equipment must have a resolution of 0.3-0.4 mm, he said. “When you have a transducer of 10 MHz, you cannot visualize changes smaller than 5 mm.”
The EUREKA study stated that VUS could replace TAB as a first-line diagnostic tool for GCA – provided the ultrasonographers are systematically trained and the equipment and settings are appropriate. In the Jutland clinic, VUS already has replaced TAB, Dr. Chrysidis said.
“In my department since 2017, when we started the fast-track clinic after the EUREKA study was terminated, we have performed three temporal artery biopsies in the last 4 years, and we screen 60-70 patients per year because we use ultrasound as the primary imaging,” he said. In cases when the results are inconclusive, they order a PET scan. “We don’t perform biopsies anymore,” he said.
U.S. fast-track clinic models
The fast-track clinic models in the United States vary. Results of a survey of the U.S. clinics were presented as an abstract at the 2021 American College of Rheumatology annual meeting. Some centers have a vasculitis specialist obtain and interpret the imaging. At others, a vasculitis specialist refers patients to a VUS-trained rheumatologist to perform and interpret the test. Another approach is to have vasculitis specialists refer patients to ultrasound technicians trained in VUS, with a vascular surgeon interpreting the images and either a VUS-trained rheumatologist or vascular medicine specialist verifying the images.
The take-home message of that survey is that “ultrasound evaluation should be considered in the hands of experts, realizing that not everyone has that skill set, but if it is available, it’s a way to expedite diagnosis and it can be helpful in managing the GCA patient in an appropriate way, quicker than trying to schedule cross-sectional imaging,” said Massachusetts General’s Dr. Kohler, who is a coauthor of the abstract. “Certainly, cross-sectional imaging also plays an important role, but when it comes to confirming whether to continue with treatment or not for a very serious condition, ultrasound is a quick way to get the answer.”
In addition to the fast-track clinic at Massachusetts General, the survey included fast-track clinics at the University of Washington, Seattle; Brigham and Women’s Hospital, Boston; Loma Linda (Calif.) University; University of California, Los Angeles; and at a private practice, Arthritis and Rheumatism Associates in the Washington area.
Advantages of VUS vs. TAB
At Massachusetts General, some of the rheumatologists are trained to perform VUS. The rheumatologists also perform the clinical evaluation of suspected cases of GCA. The advantage of VUS, Dr. Kohler said, is that the answer is “right there”; that is, the imaging yields a diagnosis almost instantaneously whereas a biopsy must be sent to a lab for analysis.
“Since a lot of patients with suspected vasculitis may already come to us on steroid therapy, and if there’s a low probability or low suspicion for vasculitis, [VUS] actually confirms that, and we’re able to taper prednisone or steroids quickly rather than keep them on a prolonged course.”
Alison Bays, MD, MPH, of the department of rheumatology at the University of Washington, said that the advantages of avoiding biopsy aren’t to be overlooked. “Temporal artery biopsies are invasive and carry surgical risks, especially as many of our patients are elderly,” she told this news organization.
“These patients occasionally refuse biopsy, but the acceptance of ultrasound is high,” Dr. Bays said. “Scheduling surgery can be more complicated, resulting in delays to biopsy and potentially higher rates of false negatives. Additionally, ultrasound has resulted in a higher rate of diagnosis with GCA as TAB misses large-vessel involvement.” The fast-track clinic at the university has evaluated 250 patients since it opened in 2017.
Dr. Bays and colleagues published the first United States study of a fast-track protocol using vascular sonographers. “Our group has demonstrated that fast-track clinics can rapidly and effectively evaluate patients, and we demonstrated a different method of evaluation using vascular sonographers rather than rheumatologists to do the vascular ultrasound,” she said. “It utilizes the familiarity vascular sonographers already have with imaging blood vessels.”
She added that the TABUL study in the United Kingdom in 2016 demonstrated that VUS yielded a savings of $686 (£484 as reported in the study), compared with TAB. “Further studies need to be done in the United States,” she said. “Beyond direct comparison of costs, reduction in steroid burden due to quick evaluation and diagnosis may carry additional benefits.”
At Brigham and Women’s Hospital, the division of rheumatology and the vascular section of the cardiology division collaborate on the GCA evaluation, said Sara Tedeschi, MD, MPH, codirector of the fast-track clinic there. Trained vascular technologists credentialed in the procedure and specifically trained in using VUS for evaluating arteritis perform the VUS. Cardiologists with a subspecialty in vascular medicine interpret the studies.
VUS patients have a rheumatology evaluation just before they have the ultrasound. “The rheumatology evaluation is then able to incorporate information from the VUS together with laboratory data, the patient’s history, and physical examination,” Dr. Tedeschi said.
“If the rheumatologist recommends a temporal artery biopsy as a next step, we arrange this with vascular surgery,” she said. “If the rheumatologist recommends other imaging such as MRA [magnetic resonance angiography] or PET-CT, we frequently review the images together with our colleagues in cardiovascular radiology and/or nuclear medicine.”
But in the United States, it will take some time for GCA fast-track clinics to become the standard, Dr. Tedeschi said. “Temporal artery biopsy may be faster to arrange in certain practice settings if VUS is not already being employed for giant cell arteritis evaluation,” she said.
Dr. Bays recognized this limitation, saying, “We are hoping that in the future, the American College of Rheumatology will consider vascular ultrasound as a first-line diagnostic test in diagnosis as rheumatologists and vascular sonographers gain familiarity over time.”
But that would mean that centers performing VUS for GCA would have to meet rigorous standards for the procedure. “With that, standardization of a protocol, high-quality equipment, and adequate training are necessary to ensure quality and reduce the chance of false-positive or false-negative results,” she said.
Dr. Chrysidis, Dr. Bays, and Dr. Tedeschi have disclosed no relevant financial relationships. Dr. Kohler is a board member of Ultrasound School of North American Rheumatologists.
A version of this article first appeared on Medscape.com.
Temporal artery biopsy has been the standard for diagnosing giant cell arteritis (GCA), but vascular ultrasound, a procedure that’s less invasive, less time-intensive, less expensive, and more convenient, has gained widespread use in Europe, and now clinics in the United States are adopting this approach and moving toward having rheumatologists take on the role of ultrasonographer.
However, directors at these clinics – known as GCA fast-track clinics – caution that the bar can be high for adopting vascular ultrasound (VUS) as a tool to diagnose GCA. Ultrasonographers need specialized training to perform the task and an adequate caseload to maintain their skills. Clinics also need to be outfitted with high-definition ultrasound machines.
“It definitely takes adequate training and learning of how to adjust the settings on the ultrasound machine to be able to visualize the findings appropriately,” said Minna Kohler, MD, director of the rheumatology and musculoskeletal ultrasound program at Massachusetts General Hospital in Boston, which has its own GCA fast-track clinic.
“And the clinical context is very important,” she said. “If you have a high suspicion for someone with temporal arteritis, or GCA, and the patient has been on steroids for weeks before you see them, the ultrasound findings may not show signs of the disease. In those cases in which the imaging is equivocal, we would still pursue a biopsy.”
The idea of the fast-track clinic is as the name implies: to quickly confirm the presence of GCA in a matter of hours, or days at the most, in an outpatient setting without the hassles of a biopsy. Temporal artery biopsy (TAB), by comparison, “is more costly because it requires operating room time, a surgical consultation, and surgery time, whereas ultrasound is a very inexpensive exam since it’s done in the clinic by the rheumatologist,” Dr. Kohler said.
European experience
Use of VUS to diagnose GCA is supplanting TAB in Europe and other countries. In Denmark alone – with a population of 6 million – three outpatient fast-track clinics are operating. The United States, with a population more than 50 times larger than Denmark’s, has six.
Stavros Chrysidis, MD, PhD, chief of rheumatology at the Hospital South West Jutland, one of the fast-track clinic sites in Denmark, led a recent multicenter study, known as EUREKA, of VUS in patients with suspected GCA. He and his colleagues reported in The Lancet Rheumatology that the sensitivity and specificity of VUS was superior to TAB in confirming a diagnosis of GCA. Dr. Chrysidis has instructed U.S. rheumatologists and ultrasonographers in performing and interpreting VUS for GCA.
The study emphasizes the importance of training for ultrasonographers, said Dr. Chrysidis, who regularly performs VUS at his institution. “The most important finding is that, when we apply VUS by systematically trained ultrasonographers using appropriate equipment in appropriate settings, it has excellent diagnostic accuracy on GCA,” he told this news organization.
He noted that The Lancet Rheumatology report is the first multicenter study of VUS for diagnosing GCA in which all the ultrasonographers participated in a standardized training program, which his group developed. “Ultrasound is very operator dependent,” he said. “That’s why the training is very important.”
The training occurred over a year and included two workshops consisting of 5 days of theoretical training on VUS; supervised hands-on evaluation of healthy individuals and patients with known GCA; and evaluation of ultrasound images. Over the course of the year, trainees performed at least 50 VUS evaluations, half of which were in patients with confirmed GCA. During the training period, an external rheumatologist with broad experience in VUS made the final diagnosis.
“The equipment and settings are very important because ultrasound can be very time consuming if you are not educated well and if your equipment is not adjusted well,” Dr. Chrysidis said. The equipment must be calibrated beforehand “so you don’t spend time on adjustments.”
For diagnosing temporal artery anomalies, the ultrasound equipment must have a resolution of 0.3-0.4 mm, he said. “When you have a transducer of 10 MHz, you cannot visualize changes smaller than 5 mm.”
The EUREKA study stated that VUS could replace TAB as a first-line diagnostic tool for GCA – provided the ultrasonographers are systematically trained and the equipment and settings are appropriate. In the Jutland clinic, VUS already has replaced TAB, Dr. Chrysidis said.
“In my department since 2017, when we started the fast-track clinic after the EUREKA study was terminated, we have performed three temporal artery biopsies in the last 4 years, and we screen 60-70 patients per year because we use ultrasound as the primary imaging,” he said. In cases when the results are inconclusive, they order a PET scan. “We don’t perform biopsies anymore,” he said.
U.S. fast-track clinic models
The fast-track clinic models in the United States vary. Results of a survey of the U.S. clinics were presented as an abstract at the 2021 American College of Rheumatology annual meeting. Some centers have a vasculitis specialist obtain and interpret the imaging. At others, a vasculitis specialist refers patients to a VUS-trained rheumatologist to perform and interpret the test. Another approach is to have vasculitis specialists refer patients to ultrasound technicians trained in VUS, with a vascular surgeon interpreting the images and either a VUS-trained rheumatologist or vascular medicine specialist verifying the images.
The take-home message of that survey is that “ultrasound evaluation should be considered in the hands of experts, realizing that not everyone has that skill set, but if it is available, it’s a way to expedite diagnosis and it can be helpful in managing the GCA patient in an appropriate way, quicker than trying to schedule cross-sectional imaging,” said Massachusetts General’s Dr. Kohler, who is a coauthor of the abstract. “Certainly, cross-sectional imaging also plays an important role, but when it comes to confirming whether to continue with treatment or not for a very serious condition, ultrasound is a quick way to get the answer.”
In addition to the fast-track clinic at Massachusetts General, the survey included fast-track clinics at the University of Washington, Seattle; Brigham and Women’s Hospital, Boston; Loma Linda (Calif.) University; University of California, Los Angeles; and at a private practice, Arthritis and Rheumatism Associates in the Washington area.
Advantages of VUS vs. TAB
At Massachusetts General, some of the rheumatologists are trained to perform VUS. The rheumatologists also perform the clinical evaluation of suspected cases of GCA. The advantage of VUS, Dr. Kohler said, is that the answer is “right there”; that is, the imaging yields a diagnosis almost instantaneously whereas a biopsy must be sent to a lab for analysis.
“Since a lot of patients with suspected vasculitis may already come to us on steroid therapy, and if there’s a low probability or low suspicion for vasculitis, [VUS] actually confirms that, and we’re able to taper prednisone or steroids quickly rather than keep them on a prolonged course.”
Alison Bays, MD, MPH, of the department of rheumatology at the University of Washington, said that the advantages of avoiding biopsy aren’t to be overlooked. “Temporal artery biopsies are invasive and carry surgical risks, especially as many of our patients are elderly,” she told this news organization.
“These patients occasionally refuse biopsy, but the acceptance of ultrasound is high,” Dr. Bays said. “Scheduling surgery can be more complicated, resulting in delays to biopsy and potentially higher rates of false negatives. Additionally, ultrasound has resulted in a higher rate of diagnosis with GCA as TAB misses large-vessel involvement.” The fast-track clinic at the university has evaluated 250 patients since it opened in 2017.
Dr. Bays and colleagues published the first United States study of a fast-track protocol using vascular sonographers. “Our group has demonstrated that fast-track clinics can rapidly and effectively evaluate patients, and we demonstrated a different method of evaluation using vascular sonographers rather than rheumatologists to do the vascular ultrasound,” she said. “It utilizes the familiarity vascular sonographers already have with imaging blood vessels.”
She added that the TABUL study in the United Kingdom in 2016 demonstrated that VUS yielded a savings of $686 (£484 as reported in the study), compared with TAB. “Further studies need to be done in the United States,” she said. “Beyond direct comparison of costs, reduction in steroid burden due to quick evaluation and diagnosis may carry additional benefits.”
At Brigham and Women’s Hospital, the division of rheumatology and the vascular section of the cardiology division collaborate on the GCA evaluation, said Sara Tedeschi, MD, MPH, codirector of the fast-track clinic there. Trained vascular technologists credentialed in the procedure and specifically trained in using VUS for evaluating arteritis perform the VUS. Cardiologists with a subspecialty in vascular medicine interpret the studies.
VUS patients have a rheumatology evaluation just before they have the ultrasound. “The rheumatology evaluation is then able to incorporate information from the VUS together with laboratory data, the patient’s history, and physical examination,” Dr. Tedeschi said.
“If the rheumatologist recommends a temporal artery biopsy as a next step, we arrange this with vascular surgery,” she said. “If the rheumatologist recommends other imaging such as MRA [magnetic resonance angiography] or PET-CT, we frequently review the images together with our colleagues in cardiovascular radiology and/or nuclear medicine.”
But in the United States, it will take some time for GCA fast-track clinics to become the standard, Dr. Tedeschi said. “Temporal artery biopsy may be faster to arrange in certain practice settings if VUS is not already being employed for giant cell arteritis evaluation,” she said.
Dr. Bays recognized this limitation, saying, “We are hoping that in the future, the American College of Rheumatology will consider vascular ultrasound as a first-line diagnostic test in diagnosis as rheumatologists and vascular sonographers gain familiarity over time.”
But that would mean that centers performing VUS for GCA would have to meet rigorous standards for the procedure. “With that, standardization of a protocol, high-quality equipment, and adequate training are necessary to ensure quality and reduce the chance of false-positive or false-negative results,” she said.
Dr. Chrysidis, Dr. Bays, and Dr. Tedeschi have disclosed no relevant financial relationships. Dr. Kohler is a board member of Ultrasound School of North American Rheumatologists.
A version of this article first appeared on Medscape.com.
Guselkumab’s efficacy, safety confirmed in patients with psoriatic arthritis and prior TNFi exposure
A new study has established guselkumab (Tremfya) as both a safe and effective treatment option for psoriatic arthritis (PsA) in patients who had previously responded poorly to tumor necrosis factor inhibitors (TNFis).
“While the positive guselkumab benefit-risk profile observed through week 24 was maintained through 1 year, real-world evidence will further inform long-term guselkumab persistence in TNFi-inadequate response patients,” writes Laura C. Coates, MBChB, PhD, of the University of Oxford (England), and her coauthors. The study was published in the Annals of the Rheumatic Diseases.
Previous studies indicated that the anti–interleukin-23p19 monoclonal antibody improved outcomes in patients with PsA, even after 1 year, but some uncertainty remained regarding the surprisingly similar level of effectiveness in biologic-naive and TNFi-treated patients. Guselkumab is approved for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy and adults with active psoriatic arthritis.
Clarity on guselkumab’s effectiveness in certain patients
“In previous studies that cemented guselkumab as a treatment option for PsA, what was odd was that the results were pretty comparable,” Eric M. Ruderman, MD, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago, Illinois, said in an interview. “We didn’t really have a sense of how well it worked in patients who had failed other biologics, which is where you might expect a drug with a new mechanism to be used when it comes into a particular disease category.
“Not surprisingly, in this study, the overall response rate was a little less than the response rate in the other two trials,” said Dr. Ruderman, who was not involved in the study. “You can’t really compare across studies, but it does fit with what we might expect: People who’ve previously failed a TNF inhibitor might be a little less likely to respond to guselkumab, compared to someone who hasn’t seen a TNF inhibitor.”
When asked about potential follow-up studies, Dr. Ruderman noted that “the missing piece of the puzzle is that we still really have no way to compare this to other biologics. The next step would be to ask, in a single trial, what happens if you give some people TNF inhibitors and some people guselkumab? Just to try to give us context. Is this equivalent? Is it less effective? More effective? Where does it fit? Without that information, rheumatologists may struggle to figure out who is the right person for this drug and how often should they use it.”
Study details
To assess the efficacy and safety of guselkumab in patients who had previously taken TNFis but stopped because of inefficacy or intolerance, the researchers launched a randomized, double-blind study called COSMOS at 84 European sites from March 2019 to November 2020. The study’s 285 patients – 52% of whom were women, with an average overall age of 49 – were assigned to two groups: guselkumab (n = 189) or placebo (n = 96). A total of 88% of all patients had used one TNFi prior; 12% had used two.
The guselkumab group received 100-mg injections at week 0, week 4, and then every 8 weeks through week 44; the placebo group received injections at weeks 0, 4, 12, and 20, followed by 100 mg of guselkumab at weeks 24, 28, 36, and 44. Patients with less than 5% improvement from baseline in both tender and swollen joint counts at week 16 qualified for early escape to “initiate or increase the dose of one permitted concomitant medication up to the maximum allowed dose at the physician’s discretion.” Ultimately, 88% of patients in the guselkumab arm and 83% of the placebo arm completed the study.
At 24 weeks, more than 44% of the guselkumab group achieved a 20% or greater improvement in American College of Rheumatology criteria (ACR20), compared with just under 20% of the placebo group, a difference of nearly 25% (95% confidence interval, 14.1%-35.2%; multiplicity-adjusted P < .001). At 48 weeks, nearly 58% of the guselkumab group had achieved ACR20; of the 51 patients in the placebo arm who started taking guselkumab at week 24, 55% achieved ACR20 by week 48.
Through 24 weeks, 80 patients in the guselkumab group (42%) and 46 patients in the placebo group (48%) experienced adverse events; only 3.7% and 3.1% developed serious adverse events, respectively. The most common adverse events in the guselkumab group at that point included nasopharyngitis (5%) and upper respiratory tract infection (4%), which occurred at a similar frequency (5% and 3%) in the placebo group.
The authors acknowledge their study’s limitations, including imbalances in baseline characteristics such as gender and weight, as well as the COSMOS study being restricted to European patients and thus potentially limiting diversity. In addition, while the COVID-19 pandemic may have increased major protocol deviations near the end of the study, the authors note that “most were related to timing of study visits and did not impact efficacy.”
The study was funded by Janssen, and six authors reported being employees of the company. The authors also acknowledge numerous potential conflicts of interest, including receiving consulting fees and research grants from various pharmaceutical companies, including Janssen. Dr. Ruderman is a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, and Janssen and served on the data safety monitoring committee for two other phase 3 guselkumab trials.
A version of this article first appeared on Medscape.com.
A new study has established guselkumab (Tremfya) as both a safe and effective treatment option for psoriatic arthritis (PsA) in patients who had previously responded poorly to tumor necrosis factor inhibitors (TNFis).
“While the positive guselkumab benefit-risk profile observed through week 24 was maintained through 1 year, real-world evidence will further inform long-term guselkumab persistence in TNFi-inadequate response patients,” writes Laura C. Coates, MBChB, PhD, of the University of Oxford (England), and her coauthors. The study was published in the Annals of the Rheumatic Diseases.
Previous studies indicated that the anti–interleukin-23p19 monoclonal antibody improved outcomes in patients with PsA, even after 1 year, but some uncertainty remained regarding the surprisingly similar level of effectiveness in biologic-naive and TNFi-treated patients. Guselkumab is approved for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy and adults with active psoriatic arthritis.
Clarity on guselkumab’s effectiveness in certain patients
“In previous studies that cemented guselkumab as a treatment option for PsA, what was odd was that the results were pretty comparable,” Eric M. Ruderman, MD, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago, Illinois, said in an interview. “We didn’t really have a sense of how well it worked in patients who had failed other biologics, which is where you might expect a drug with a new mechanism to be used when it comes into a particular disease category.
“Not surprisingly, in this study, the overall response rate was a little less than the response rate in the other two trials,” said Dr. Ruderman, who was not involved in the study. “You can’t really compare across studies, but it does fit with what we might expect: People who’ve previously failed a TNF inhibitor might be a little less likely to respond to guselkumab, compared to someone who hasn’t seen a TNF inhibitor.”
When asked about potential follow-up studies, Dr. Ruderman noted that “the missing piece of the puzzle is that we still really have no way to compare this to other biologics. The next step would be to ask, in a single trial, what happens if you give some people TNF inhibitors and some people guselkumab? Just to try to give us context. Is this equivalent? Is it less effective? More effective? Where does it fit? Without that information, rheumatologists may struggle to figure out who is the right person for this drug and how often should they use it.”
Study details
To assess the efficacy and safety of guselkumab in patients who had previously taken TNFis but stopped because of inefficacy or intolerance, the researchers launched a randomized, double-blind study called COSMOS at 84 European sites from March 2019 to November 2020. The study’s 285 patients – 52% of whom were women, with an average overall age of 49 – were assigned to two groups: guselkumab (n = 189) or placebo (n = 96). A total of 88% of all patients had used one TNFi prior; 12% had used two.
The guselkumab group received 100-mg injections at week 0, week 4, and then every 8 weeks through week 44; the placebo group received injections at weeks 0, 4, 12, and 20, followed by 100 mg of guselkumab at weeks 24, 28, 36, and 44. Patients with less than 5% improvement from baseline in both tender and swollen joint counts at week 16 qualified for early escape to “initiate or increase the dose of one permitted concomitant medication up to the maximum allowed dose at the physician’s discretion.” Ultimately, 88% of patients in the guselkumab arm and 83% of the placebo arm completed the study.
At 24 weeks, more than 44% of the guselkumab group achieved a 20% or greater improvement in American College of Rheumatology criteria (ACR20), compared with just under 20% of the placebo group, a difference of nearly 25% (95% confidence interval, 14.1%-35.2%; multiplicity-adjusted P < .001). At 48 weeks, nearly 58% of the guselkumab group had achieved ACR20; of the 51 patients in the placebo arm who started taking guselkumab at week 24, 55% achieved ACR20 by week 48.
Through 24 weeks, 80 patients in the guselkumab group (42%) and 46 patients in the placebo group (48%) experienced adverse events; only 3.7% and 3.1% developed serious adverse events, respectively. The most common adverse events in the guselkumab group at that point included nasopharyngitis (5%) and upper respiratory tract infection (4%), which occurred at a similar frequency (5% and 3%) in the placebo group.
The authors acknowledge their study’s limitations, including imbalances in baseline characteristics such as gender and weight, as well as the COSMOS study being restricted to European patients and thus potentially limiting diversity. In addition, while the COVID-19 pandemic may have increased major protocol deviations near the end of the study, the authors note that “most were related to timing of study visits and did not impact efficacy.”
The study was funded by Janssen, and six authors reported being employees of the company. The authors also acknowledge numerous potential conflicts of interest, including receiving consulting fees and research grants from various pharmaceutical companies, including Janssen. Dr. Ruderman is a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, and Janssen and served on the data safety monitoring committee for two other phase 3 guselkumab trials.
A version of this article first appeared on Medscape.com.
A new study has established guselkumab (Tremfya) as both a safe and effective treatment option for psoriatic arthritis (PsA) in patients who had previously responded poorly to tumor necrosis factor inhibitors (TNFis).
“While the positive guselkumab benefit-risk profile observed through week 24 was maintained through 1 year, real-world evidence will further inform long-term guselkumab persistence in TNFi-inadequate response patients,” writes Laura C. Coates, MBChB, PhD, of the University of Oxford (England), and her coauthors. The study was published in the Annals of the Rheumatic Diseases.
Previous studies indicated that the anti–interleukin-23p19 monoclonal antibody improved outcomes in patients with PsA, even after 1 year, but some uncertainty remained regarding the surprisingly similar level of effectiveness in biologic-naive and TNFi-treated patients. Guselkumab is approved for treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy and adults with active psoriatic arthritis.
Clarity on guselkumab’s effectiveness in certain patients
“In previous studies that cemented guselkumab as a treatment option for PsA, what was odd was that the results were pretty comparable,” Eric M. Ruderman, MD, professor of medicine and associate chief for clinical affairs in the division of rheumatology at Northwestern University in Chicago, Illinois, said in an interview. “We didn’t really have a sense of how well it worked in patients who had failed other biologics, which is where you might expect a drug with a new mechanism to be used when it comes into a particular disease category.
“Not surprisingly, in this study, the overall response rate was a little less than the response rate in the other two trials,” said Dr. Ruderman, who was not involved in the study. “You can’t really compare across studies, but it does fit with what we might expect: People who’ve previously failed a TNF inhibitor might be a little less likely to respond to guselkumab, compared to someone who hasn’t seen a TNF inhibitor.”
When asked about potential follow-up studies, Dr. Ruderman noted that “the missing piece of the puzzle is that we still really have no way to compare this to other biologics. The next step would be to ask, in a single trial, what happens if you give some people TNF inhibitors and some people guselkumab? Just to try to give us context. Is this equivalent? Is it less effective? More effective? Where does it fit? Without that information, rheumatologists may struggle to figure out who is the right person for this drug and how often should they use it.”
Study details
To assess the efficacy and safety of guselkumab in patients who had previously taken TNFis but stopped because of inefficacy or intolerance, the researchers launched a randomized, double-blind study called COSMOS at 84 European sites from March 2019 to November 2020. The study’s 285 patients – 52% of whom were women, with an average overall age of 49 – were assigned to two groups: guselkumab (n = 189) or placebo (n = 96). A total of 88% of all patients had used one TNFi prior; 12% had used two.
The guselkumab group received 100-mg injections at week 0, week 4, and then every 8 weeks through week 44; the placebo group received injections at weeks 0, 4, 12, and 20, followed by 100 mg of guselkumab at weeks 24, 28, 36, and 44. Patients with less than 5% improvement from baseline in both tender and swollen joint counts at week 16 qualified for early escape to “initiate or increase the dose of one permitted concomitant medication up to the maximum allowed dose at the physician’s discretion.” Ultimately, 88% of patients in the guselkumab arm and 83% of the placebo arm completed the study.
At 24 weeks, more than 44% of the guselkumab group achieved a 20% or greater improvement in American College of Rheumatology criteria (ACR20), compared with just under 20% of the placebo group, a difference of nearly 25% (95% confidence interval, 14.1%-35.2%; multiplicity-adjusted P < .001). At 48 weeks, nearly 58% of the guselkumab group had achieved ACR20; of the 51 patients in the placebo arm who started taking guselkumab at week 24, 55% achieved ACR20 by week 48.
Through 24 weeks, 80 patients in the guselkumab group (42%) and 46 patients in the placebo group (48%) experienced adverse events; only 3.7% and 3.1% developed serious adverse events, respectively. The most common adverse events in the guselkumab group at that point included nasopharyngitis (5%) and upper respiratory tract infection (4%), which occurred at a similar frequency (5% and 3%) in the placebo group.
The authors acknowledge their study’s limitations, including imbalances in baseline characteristics such as gender and weight, as well as the COSMOS study being restricted to European patients and thus potentially limiting diversity. In addition, while the COVID-19 pandemic may have increased major protocol deviations near the end of the study, the authors note that “most were related to timing of study visits and did not impact efficacy.”
The study was funded by Janssen, and six authors reported being employees of the company. The authors also acknowledge numerous potential conflicts of interest, including receiving consulting fees and research grants from various pharmaceutical companies, including Janssen. Dr. Ruderman is a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, and Janssen and served on the data safety monitoring committee for two other phase 3 guselkumab trials.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Validity of commercial serologic tests for dermatomyositis still questionable
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Platelet-rich plasma injections show no benefit in knee OA in placebo-controlled trial
A large randomized, placebo-controlled trial of platelet-rich plasma injections for knee osteoarthritis has found almost no symptomatic or structural benefit from the treatment, giving some clarity to an evidence base that has seen both positive and negative trials for the treatment modality.
Given the need for better disease-modifying treatments for osteoarthritis, there has been a lot of interest in biological therapies such as platelet-rich plasma and stem cells, the lead author of the study, Kim Bennell, PhD, told this news organization. “People have started to use it to treat osteoarthritis, but the evidence to support it was limited in terms of its quality, and there’s been very little work looking at effects on structure,” said Dr. Bennell, a research physiotherapist and chair of physiotherapy at the University of Melbourne.
Platelet-rich plasma contains a range of growth factors and cytokines that are thought to be beneficial in building cartilage and reducing inflammation. There have been several clinical trials of the treatment in knee osteoarthritis, but the current study’s authors said these were limited by factors such as a lack of blinding and were at high risk of bias. “That was the impetus to do a large, high-quality study and to look at joint structure,” Dr. Bennell said.
Study details
For the study, which was published Nov. 23 in JAMA, the researchers enrolled 288 adults older than 50 with knee osteoarthritis who had experienced knee pain on most days of the past month and had radiographic evidence of mild to moderate osteoarthritis of the tibiofemoral joint.
After having stopped all nonsteroidal anti-inflammatory and pain-relief drugs 2 weeks prior – except acetaminophen – participants were randomly assigned to receive three weekly intra-articular knee injections of either a commercially available leukocyte-poor platelet-rich plasma or saline placebo. They were then followed for 12 months.
Among the 288 participants in the study, researchers saw no statistically significant difference in the change in pain scores between the treatment and placebo groups at 12 months, although there was a nonsignificantly greater reduction in pain scores among those given platelet-rich plasma. The study also found no statistically significant difference between the two groups in the change in medial tibial cartilage volume.
The researchers also looked at a large number of secondary outcomes, including the effects of treatment on pain and function at 2 months, change in Knee Injury and Osteoarthritis Outcome (KOOS) scores, and change in quality of life scores. There were no indications of any benefits from the treatment at the 2-month follow-up, and at 12 months, the study showed no significant improvements in knee pain while walking or in pain scores, KOOS scores, or quality of life measures.
However, significantly more participants in the treatment group than in the placebo group reported overall improvement at the 2-month point – 48.2% of those in the treatment arm, compared with 36.2% of the placebo group (risk ratio, 1.37; 95% confidence interval, 1.05-1.80; P = .02). At 12 months, 42.8% of those who received platelet-rich plasma reported improved function, compared with 32.1% of those in the placebo group (risk ratio, 1.36; 95% CI, 1.00-1.86, P = .05).
The study also found that significantly more people in the platelet-rich plasma group had three or more areas of cartilage thinning at 12 months (17.1% vs. 6.8%; risk ratio, 2.71; 95% CI, 1.16-6.34; P = .02).
Even when researchers looked for treatment effects in subgroups – for example, based on disease severity, body mass index, or knee alignment – they found no significant differences from placebo.
Dr. Bennell said the results were disappointing but not surprising. “Anecdotally, people do report that they get better, but we know that there is a very large placebo effect with treatment of pain,” she said.
Results emphasize importance of placebo controls
In an accompanying editorial by Jeffrey N. Katz, MD, director of the Orthopaedic and Arthritis Center for Outcomes Research at Brigham and Women’s Hospital, professor of medicine and orthopedic surgery at Harvard Medical School, and professor of epidemiology and environmental health at the Harvard T.H. Chan School of Public Health, all in Boston, draws parallels between this study and two earlier studies of platelet-rich plasma for ankle osteoarthritis and Achilles tendinopathy, both published in JAMA in 2021. None of the three studies showed any significant improvements over and above placebo.
“These findings emphasize the importance of comparing interventions with placebos in trials of injection therapies,” Dr. Katz writes. However, he notes that these studies do suggest possible benefits in secondary outcomes, such as self-reported pain and function, and that earlier studies of the treatment had had more positive outcomes.
Dr. Katz said it was premature to dismiss platelet-rich plasma as a treatment for knee osteoarthritis, but “until a new generation of trials using standardized approaches to PRP [platelet-rich plasma] therapy provides evidence of efficacy, it would be prudent to pause the use of PRP for OA and Achilles tendinitis.”
Not ready to stop using platelet-rich plasma?
When asked for comment, sports medicine physician Maarten Moen, MD, from the Bergman Clinics Naarden (the Netherlands) said the study was the largest yet of the use of platelet-rich plasma for knee osteoarthritis and that it was a well-designed, double-blind, placebo-controlled trial.
However, he also pointed out that at least six earlier randomized, placebo-controlled studies of this treatment approach have been conducted, and of those six, all but two found positive benefits for patients. “It’s a very well-performed study, but for me, it would be a bridge too far to say, ‘Now we have this study, let’s stop doing it,’ ” Dr. Moen said.
Dr. Moen said he would like to see what effect this study had on meta-analyses and systematic reviews of the treatment, as that would give the clearest indication of the overall picture of its effectiveness.
Dr. Moen’s own experience of treating patients with platelet-rich plasma also suggested that, among those who do benefit from the treatment, that benefit would most likely show between 2 and 12 months afterward. He said it would have been useful to see outcomes at 3- and 6-month intervals.
“What I tell people is that, on average, around 9 months’ effect is to be expected,” he said.
Dr. Bennell said the research group chose the 12-month follow-up because they wanted to see if there were long-term improvements in joint structure which they hoped for, given the cost of treatment.
The study was funded by the Australian National Health and Medical Research Council, and Regen Lab SA provided platelet-rich plasma kits free of charge. Two authors reported using platelet-rich plasma injections in clinical practice, one reported scientific advisory board fees from Biobone, Novartis, Tissuegene, Pfizer, and Lilly; two reported fees for contributing to UpToDate clinical guidelines, and two reported grants from the National Health and Medical Research Council outside the submitted work. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
A large randomized, placebo-controlled trial of platelet-rich plasma injections for knee osteoarthritis has found almost no symptomatic or structural benefit from the treatment, giving some clarity to an evidence base that has seen both positive and negative trials for the treatment modality.
Given the need for better disease-modifying treatments for osteoarthritis, there has been a lot of interest in biological therapies such as platelet-rich plasma and stem cells, the lead author of the study, Kim Bennell, PhD, told this news organization. “People have started to use it to treat osteoarthritis, but the evidence to support it was limited in terms of its quality, and there’s been very little work looking at effects on structure,” said Dr. Bennell, a research physiotherapist and chair of physiotherapy at the University of Melbourne.
Platelet-rich plasma contains a range of growth factors and cytokines that are thought to be beneficial in building cartilage and reducing inflammation. There have been several clinical trials of the treatment in knee osteoarthritis, but the current study’s authors said these were limited by factors such as a lack of blinding and were at high risk of bias. “That was the impetus to do a large, high-quality study and to look at joint structure,” Dr. Bennell said.
Study details
For the study, which was published Nov. 23 in JAMA, the researchers enrolled 288 adults older than 50 with knee osteoarthritis who had experienced knee pain on most days of the past month and had radiographic evidence of mild to moderate osteoarthritis of the tibiofemoral joint.
After having stopped all nonsteroidal anti-inflammatory and pain-relief drugs 2 weeks prior – except acetaminophen – participants were randomly assigned to receive three weekly intra-articular knee injections of either a commercially available leukocyte-poor platelet-rich plasma or saline placebo. They were then followed for 12 months.
Among the 288 participants in the study, researchers saw no statistically significant difference in the change in pain scores between the treatment and placebo groups at 12 months, although there was a nonsignificantly greater reduction in pain scores among those given platelet-rich plasma. The study also found no statistically significant difference between the two groups in the change in medial tibial cartilage volume.
The researchers also looked at a large number of secondary outcomes, including the effects of treatment on pain and function at 2 months, change in Knee Injury and Osteoarthritis Outcome (KOOS) scores, and change in quality of life scores. There were no indications of any benefits from the treatment at the 2-month follow-up, and at 12 months, the study showed no significant improvements in knee pain while walking or in pain scores, KOOS scores, or quality of life measures.
However, significantly more participants in the treatment group than in the placebo group reported overall improvement at the 2-month point – 48.2% of those in the treatment arm, compared with 36.2% of the placebo group (risk ratio, 1.37; 95% confidence interval, 1.05-1.80; P = .02). At 12 months, 42.8% of those who received platelet-rich plasma reported improved function, compared with 32.1% of those in the placebo group (risk ratio, 1.36; 95% CI, 1.00-1.86, P = .05).
The study also found that significantly more people in the platelet-rich plasma group had three or more areas of cartilage thinning at 12 months (17.1% vs. 6.8%; risk ratio, 2.71; 95% CI, 1.16-6.34; P = .02).
Even when researchers looked for treatment effects in subgroups – for example, based on disease severity, body mass index, or knee alignment – they found no significant differences from placebo.
Dr. Bennell said the results were disappointing but not surprising. “Anecdotally, people do report that they get better, but we know that there is a very large placebo effect with treatment of pain,” she said.
Results emphasize importance of placebo controls
In an accompanying editorial by Jeffrey N. Katz, MD, director of the Orthopaedic and Arthritis Center for Outcomes Research at Brigham and Women’s Hospital, professor of medicine and orthopedic surgery at Harvard Medical School, and professor of epidemiology and environmental health at the Harvard T.H. Chan School of Public Health, all in Boston, draws parallels between this study and two earlier studies of platelet-rich plasma for ankle osteoarthritis and Achilles tendinopathy, both published in JAMA in 2021. None of the three studies showed any significant improvements over and above placebo.
“These findings emphasize the importance of comparing interventions with placebos in trials of injection therapies,” Dr. Katz writes. However, he notes that these studies do suggest possible benefits in secondary outcomes, such as self-reported pain and function, and that earlier studies of the treatment had had more positive outcomes.
Dr. Katz said it was premature to dismiss platelet-rich plasma as a treatment for knee osteoarthritis, but “until a new generation of trials using standardized approaches to PRP [platelet-rich plasma] therapy provides evidence of efficacy, it would be prudent to pause the use of PRP for OA and Achilles tendinitis.”
Not ready to stop using platelet-rich plasma?
When asked for comment, sports medicine physician Maarten Moen, MD, from the Bergman Clinics Naarden (the Netherlands) said the study was the largest yet of the use of platelet-rich plasma for knee osteoarthritis and that it was a well-designed, double-blind, placebo-controlled trial.
However, he also pointed out that at least six earlier randomized, placebo-controlled studies of this treatment approach have been conducted, and of those six, all but two found positive benefits for patients. “It’s a very well-performed study, but for me, it would be a bridge too far to say, ‘Now we have this study, let’s stop doing it,’ ” Dr. Moen said.
Dr. Moen said he would like to see what effect this study had on meta-analyses and systematic reviews of the treatment, as that would give the clearest indication of the overall picture of its effectiveness.
Dr. Moen’s own experience of treating patients with platelet-rich plasma also suggested that, among those who do benefit from the treatment, that benefit would most likely show between 2 and 12 months afterward. He said it would have been useful to see outcomes at 3- and 6-month intervals.
“What I tell people is that, on average, around 9 months’ effect is to be expected,” he said.
Dr. Bennell said the research group chose the 12-month follow-up because they wanted to see if there were long-term improvements in joint structure which they hoped for, given the cost of treatment.
The study was funded by the Australian National Health and Medical Research Council, and Regen Lab SA provided platelet-rich plasma kits free of charge. Two authors reported using platelet-rich plasma injections in clinical practice, one reported scientific advisory board fees from Biobone, Novartis, Tissuegene, Pfizer, and Lilly; two reported fees for contributing to UpToDate clinical guidelines, and two reported grants from the National Health and Medical Research Council outside the submitted work. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
A large randomized, placebo-controlled trial of platelet-rich plasma injections for knee osteoarthritis has found almost no symptomatic or structural benefit from the treatment, giving some clarity to an evidence base that has seen both positive and negative trials for the treatment modality.
Given the need for better disease-modifying treatments for osteoarthritis, there has been a lot of interest in biological therapies such as platelet-rich plasma and stem cells, the lead author of the study, Kim Bennell, PhD, told this news organization. “People have started to use it to treat osteoarthritis, but the evidence to support it was limited in terms of its quality, and there’s been very little work looking at effects on structure,” said Dr. Bennell, a research physiotherapist and chair of physiotherapy at the University of Melbourne.
Platelet-rich plasma contains a range of growth factors and cytokines that are thought to be beneficial in building cartilage and reducing inflammation. There have been several clinical trials of the treatment in knee osteoarthritis, but the current study’s authors said these were limited by factors such as a lack of blinding and were at high risk of bias. “That was the impetus to do a large, high-quality study and to look at joint structure,” Dr. Bennell said.
Study details
For the study, which was published Nov. 23 in JAMA, the researchers enrolled 288 adults older than 50 with knee osteoarthritis who had experienced knee pain on most days of the past month and had radiographic evidence of mild to moderate osteoarthritis of the tibiofemoral joint.
After having stopped all nonsteroidal anti-inflammatory and pain-relief drugs 2 weeks prior – except acetaminophen – participants were randomly assigned to receive three weekly intra-articular knee injections of either a commercially available leukocyte-poor platelet-rich plasma or saline placebo. They were then followed for 12 months.
Among the 288 participants in the study, researchers saw no statistically significant difference in the change in pain scores between the treatment and placebo groups at 12 months, although there was a nonsignificantly greater reduction in pain scores among those given platelet-rich plasma. The study also found no statistically significant difference between the two groups in the change in medial tibial cartilage volume.
The researchers also looked at a large number of secondary outcomes, including the effects of treatment on pain and function at 2 months, change in Knee Injury and Osteoarthritis Outcome (KOOS) scores, and change in quality of life scores. There were no indications of any benefits from the treatment at the 2-month follow-up, and at 12 months, the study showed no significant improvements in knee pain while walking or in pain scores, KOOS scores, or quality of life measures.
However, significantly more participants in the treatment group than in the placebo group reported overall improvement at the 2-month point – 48.2% of those in the treatment arm, compared with 36.2% of the placebo group (risk ratio, 1.37; 95% confidence interval, 1.05-1.80; P = .02). At 12 months, 42.8% of those who received platelet-rich plasma reported improved function, compared with 32.1% of those in the placebo group (risk ratio, 1.36; 95% CI, 1.00-1.86, P = .05).
The study also found that significantly more people in the platelet-rich plasma group had three or more areas of cartilage thinning at 12 months (17.1% vs. 6.8%; risk ratio, 2.71; 95% CI, 1.16-6.34; P = .02).
Even when researchers looked for treatment effects in subgroups – for example, based on disease severity, body mass index, or knee alignment – they found no significant differences from placebo.
Dr. Bennell said the results were disappointing but not surprising. “Anecdotally, people do report that they get better, but we know that there is a very large placebo effect with treatment of pain,” she said.
Results emphasize importance of placebo controls
In an accompanying editorial by Jeffrey N. Katz, MD, director of the Orthopaedic and Arthritis Center for Outcomes Research at Brigham and Women’s Hospital, professor of medicine and orthopedic surgery at Harvard Medical School, and professor of epidemiology and environmental health at the Harvard T.H. Chan School of Public Health, all in Boston, draws parallels between this study and two earlier studies of platelet-rich plasma for ankle osteoarthritis and Achilles tendinopathy, both published in JAMA in 2021. None of the three studies showed any significant improvements over and above placebo.
“These findings emphasize the importance of comparing interventions with placebos in trials of injection therapies,” Dr. Katz writes. However, he notes that these studies do suggest possible benefits in secondary outcomes, such as self-reported pain and function, and that earlier studies of the treatment had had more positive outcomes.
Dr. Katz said it was premature to dismiss platelet-rich plasma as a treatment for knee osteoarthritis, but “until a new generation of trials using standardized approaches to PRP [platelet-rich plasma] therapy provides evidence of efficacy, it would be prudent to pause the use of PRP for OA and Achilles tendinitis.”
Not ready to stop using platelet-rich plasma?
When asked for comment, sports medicine physician Maarten Moen, MD, from the Bergman Clinics Naarden (the Netherlands) said the study was the largest yet of the use of platelet-rich plasma for knee osteoarthritis and that it was a well-designed, double-blind, placebo-controlled trial.
However, he also pointed out that at least six earlier randomized, placebo-controlled studies of this treatment approach have been conducted, and of those six, all but two found positive benefits for patients. “It’s a very well-performed study, but for me, it would be a bridge too far to say, ‘Now we have this study, let’s stop doing it,’ ” Dr. Moen said.
Dr. Moen said he would like to see what effect this study had on meta-analyses and systematic reviews of the treatment, as that would give the clearest indication of the overall picture of its effectiveness.
Dr. Moen’s own experience of treating patients with platelet-rich plasma also suggested that, among those who do benefit from the treatment, that benefit would most likely show between 2 and 12 months afterward. He said it would have been useful to see outcomes at 3- and 6-month intervals.
“What I tell people is that, on average, around 9 months’ effect is to be expected,” he said.
Dr. Bennell said the research group chose the 12-month follow-up because they wanted to see if there were long-term improvements in joint structure which they hoped for, given the cost of treatment.
The study was funded by the Australian National Health and Medical Research Council, and Regen Lab SA provided platelet-rich plasma kits free of charge. Two authors reported using platelet-rich plasma injections in clinical practice, one reported scientific advisory board fees from Biobone, Novartis, Tissuegene, Pfizer, and Lilly; two reported fees for contributing to UpToDate clinical guidelines, and two reported grants from the National Health and Medical Research Council outside the submitted work. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
FROM JAMA
Secukinumab beat placebo for sustained remission of giant cell arteritis after steroid taper
Patients with giant cell arteritis (GCA) remained in remission longer when they took secukinumab (Cosentyx) during a 6-month–long taper of glucocorticoids, a monoclonal antibody drug that inhibits interleukin-17A, compared with placebo, according to phase 2 trial results presented at the virtual annual meeting of the American College of Rheumatology.
The mainstay of GCA treatment is glucocorticoids, although IL-6 inhibition with tocilizumab (Actemra) has recently become another option, Jens Thiel, MD, vice director of the Clinic for Rheumatology and Clinical Immunology at University Hospital Freiburg (Germany), told attendees.
“Secukinumab has shown significant improvements in the signs and symptoms of IL-17A-driven medical conditions such as psoriasis and psoriatic arthritis, and it has a very favorable long-term safety profile,” Dr. Thiel said. “There is experimental and preclinical data that points toward the role of IL-17A in the pathogenesis of giant cell arteritis, and therefore IL-17A inhibition, blocking vascular inflammation, is potentially a new therapeutic target for GCA.”
Christopher R. Palma, MD, ScM, assistant professor in the division of allergy, immunology, and rheumatology at the University of Rochester (N.Y.), said in an interview that the trial’s preliminary findings were exciting because they suggest that IL-17A is likely to be an effective strategy for treating GCA. ”This aligns with known pathophysiology of GCA, where IL-17A is an important part of pathology of disease,” Dr. Palma said.
In the randomized, controlled, double-blind trial, researchers enrolled 52 patients, all at least 50 years old, who had never taken a biologic for GCA. Most of the participants (80.8%) had new-onset GCA, diagnosed within the previous 6 weeks, and 19.2% had relapsing GCA. The participants received either 300 mg of secukinumab or placebo every week for 5 weeks, and then every 4 weeks for 48 total weeks. At baseline, all participants also began a 26-week taper of prednisolone from a dose of 25-60 mg/day at baseline to 0 at week 27. The primary endpoint was the proportion of participants in sustained remission through week 28.
Among the 27 participants taking secukinumab and the 25 taking placebo, 37 completed the study treatment (71%). At week 28, those still in remission included 70.1% of participants taking secukinumab and 20.3% of those taking placebo (odds ratio [OR], 9.3). Through week 52, the proportion of participants in remission included 59.3% of the secukinumab group and 8% of the placebo group.
Determination of flare was based on signs and symptoms along with a C-reactive protein level of more than 10 mg/L or an increased erythrocyte sedimentation rate. Dr. Thiel did not provide more details on these parameters or on how flares were determined, but reported that participants taking secukinumab did not reach a median time to first flare, compared to a median 197 days in the placebo group.
All the participants taking secukinumab and 96% of those taking placebo experienced treatment-emergent adverse events, with serious adverse events occurring in 22.2% of those taking secukinumab and 44% of those taking placebo. Two patients in each group discontinued the treatment because of adverse events, and one participant in each group died from causes determined to be unrelated to the treatment.
The trial’s effect size was large, but Dr. Palma noted that the study’s generalizability is limited by prednisolone tapering in the placebo arm because that’s not reflective of most clinical practice for treatment of GCA.
“We would be unlikely to mimic this trial design as we know rates of disease flare and recurrence are high without long-term therapy of some kind,” Dr. Palma said. ”The real challenge will be in assessing relative benefit and risk among many possible therapies and how IL-17A–directed therapies fit in. Obviously, a head-to-head trial design would help answer many of these questions.”
Dr. Palma said it’s too early to recommend widespread off-label use of secukinumab for GCA, but he would encourage his patients with GCA to consider participating in a phase 3 trial of the drug.
Novartis, which markets secukinumab, funded the research. Dr. Thiel has received speaking and/or advising fees from AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, and Novartis, and research grants from Bristol-Myers Squibb and Novartis. His coauthors had disclosures for a wide range of pharmaceutical companies. Dr. Palma has received research funding from AbbVie, Incyte, and Regeneron.
Patients with giant cell arteritis (GCA) remained in remission longer when they took secukinumab (Cosentyx) during a 6-month–long taper of glucocorticoids, a monoclonal antibody drug that inhibits interleukin-17A, compared with placebo, according to phase 2 trial results presented at the virtual annual meeting of the American College of Rheumatology.
The mainstay of GCA treatment is glucocorticoids, although IL-6 inhibition with tocilizumab (Actemra) has recently become another option, Jens Thiel, MD, vice director of the Clinic for Rheumatology and Clinical Immunology at University Hospital Freiburg (Germany), told attendees.
“Secukinumab has shown significant improvements in the signs and symptoms of IL-17A-driven medical conditions such as psoriasis and psoriatic arthritis, and it has a very favorable long-term safety profile,” Dr. Thiel said. “There is experimental and preclinical data that points toward the role of IL-17A in the pathogenesis of giant cell arteritis, and therefore IL-17A inhibition, blocking vascular inflammation, is potentially a new therapeutic target for GCA.”
Christopher R. Palma, MD, ScM, assistant professor in the division of allergy, immunology, and rheumatology at the University of Rochester (N.Y.), said in an interview that the trial’s preliminary findings were exciting because they suggest that IL-17A is likely to be an effective strategy for treating GCA. ”This aligns with known pathophysiology of GCA, where IL-17A is an important part of pathology of disease,” Dr. Palma said.
In the randomized, controlled, double-blind trial, researchers enrolled 52 patients, all at least 50 years old, who had never taken a biologic for GCA. Most of the participants (80.8%) had new-onset GCA, diagnosed within the previous 6 weeks, and 19.2% had relapsing GCA. The participants received either 300 mg of secukinumab or placebo every week for 5 weeks, and then every 4 weeks for 48 total weeks. At baseline, all participants also began a 26-week taper of prednisolone from a dose of 25-60 mg/day at baseline to 0 at week 27. The primary endpoint was the proportion of participants in sustained remission through week 28.
Among the 27 participants taking secukinumab and the 25 taking placebo, 37 completed the study treatment (71%). At week 28, those still in remission included 70.1% of participants taking secukinumab and 20.3% of those taking placebo (odds ratio [OR], 9.3). Through week 52, the proportion of participants in remission included 59.3% of the secukinumab group and 8% of the placebo group.
Determination of flare was based on signs and symptoms along with a C-reactive protein level of more than 10 mg/L or an increased erythrocyte sedimentation rate. Dr. Thiel did not provide more details on these parameters or on how flares were determined, but reported that participants taking secukinumab did not reach a median time to first flare, compared to a median 197 days in the placebo group.
All the participants taking secukinumab and 96% of those taking placebo experienced treatment-emergent adverse events, with serious adverse events occurring in 22.2% of those taking secukinumab and 44% of those taking placebo. Two patients in each group discontinued the treatment because of adverse events, and one participant in each group died from causes determined to be unrelated to the treatment.
The trial’s effect size was large, but Dr. Palma noted that the study’s generalizability is limited by prednisolone tapering in the placebo arm because that’s not reflective of most clinical practice for treatment of GCA.
“We would be unlikely to mimic this trial design as we know rates of disease flare and recurrence are high without long-term therapy of some kind,” Dr. Palma said. ”The real challenge will be in assessing relative benefit and risk among many possible therapies and how IL-17A–directed therapies fit in. Obviously, a head-to-head trial design would help answer many of these questions.”
Dr. Palma said it’s too early to recommend widespread off-label use of secukinumab for GCA, but he would encourage his patients with GCA to consider participating in a phase 3 trial of the drug.
Novartis, which markets secukinumab, funded the research. Dr. Thiel has received speaking and/or advising fees from AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, and Novartis, and research grants from Bristol-Myers Squibb and Novartis. His coauthors had disclosures for a wide range of pharmaceutical companies. Dr. Palma has received research funding from AbbVie, Incyte, and Regeneron.
Patients with giant cell arteritis (GCA) remained in remission longer when they took secukinumab (Cosentyx) during a 6-month–long taper of glucocorticoids, a monoclonal antibody drug that inhibits interleukin-17A, compared with placebo, according to phase 2 trial results presented at the virtual annual meeting of the American College of Rheumatology.
The mainstay of GCA treatment is glucocorticoids, although IL-6 inhibition with tocilizumab (Actemra) has recently become another option, Jens Thiel, MD, vice director of the Clinic for Rheumatology and Clinical Immunology at University Hospital Freiburg (Germany), told attendees.
“Secukinumab has shown significant improvements in the signs and symptoms of IL-17A-driven medical conditions such as psoriasis and psoriatic arthritis, and it has a very favorable long-term safety profile,” Dr. Thiel said. “There is experimental and preclinical data that points toward the role of IL-17A in the pathogenesis of giant cell arteritis, and therefore IL-17A inhibition, blocking vascular inflammation, is potentially a new therapeutic target for GCA.”
Christopher R. Palma, MD, ScM, assistant professor in the division of allergy, immunology, and rheumatology at the University of Rochester (N.Y.), said in an interview that the trial’s preliminary findings were exciting because they suggest that IL-17A is likely to be an effective strategy for treating GCA. ”This aligns with known pathophysiology of GCA, where IL-17A is an important part of pathology of disease,” Dr. Palma said.
In the randomized, controlled, double-blind trial, researchers enrolled 52 patients, all at least 50 years old, who had never taken a biologic for GCA. Most of the participants (80.8%) had new-onset GCA, diagnosed within the previous 6 weeks, and 19.2% had relapsing GCA. The participants received either 300 mg of secukinumab or placebo every week for 5 weeks, and then every 4 weeks for 48 total weeks. At baseline, all participants also began a 26-week taper of prednisolone from a dose of 25-60 mg/day at baseline to 0 at week 27. The primary endpoint was the proportion of participants in sustained remission through week 28.
Among the 27 participants taking secukinumab and the 25 taking placebo, 37 completed the study treatment (71%). At week 28, those still in remission included 70.1% of participants taking secukinumab and 20.3% of those taking placebo (odds ratio [OR], 9.3). Through week 52, the proportion of participants in remission included 59.3% of the secukinumab group and 8% of the placebo group.
Determination of flare was based on signs and symptoms along with a C-reactive protein level of more than 10 mg/L or an increased erythrocyte sedimentation rate. Dr. Thiel did not provide more details on these parameters or on how flares were determined, but reported that participants taking secukinumab did not reach a median time to first flare, compared to a median 197 days in the placebo group.
All the participants taking secukinumab and 96% of those taking placebo experienced treatment-emergent adverse events, with serious adverse events occurring in 22.2% of those taking secukinumab and 44% of those taking placebo. Two patients in each group discontinued the treatment because of adverse events, and one participant in each group died from causes determined to be unrelated to the treatment.
The trial’s effect size was large, but Dr. Palma noted that the study’s generalizability is limited by prednisolone tapering in the placebo arm because that’s not reflective of most clinical practice for treatment of GCA.
“We would be unlikely to mimic this trial design as we know rates of disease flare and recurrence are high without long-term therapy of some kind,” Dr. Palma said. ”The real challenge will be in assessing relative benefit and risk among many possible therapies and how IL-17A–directed therapies fit in. Obviously, a head-to-head trial design would help answer many of these questions.”
Dr. Palma said it’s too early to recommend widespread off-label use of secukinumab for GCA, but he would encourage his patients with GCA to consider participating in a phase 3 trial of the drug.
Novartis, which markets secukinumab, funded the research. Dr. Thiel has received speaking and/or advising fees from AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, and Novartis, and research grants from Bristol-Myers Squibb and Novartis. His coauthors had disclosures for a wide range of pharmaceutical companies. Dr. Palma has received research funding from AbbVie, Incyte, and Regeneron.
FROM ACR 2021
Serious infection hospitalizations have declined in patients with PsA
The rate of U.S. hospitalizations for three types of serious infections in patients with psoriatic arthritis (PsA) appears to have declined from 2012 to 2017, according to research presented at the virtual annual meeting of the American College of Rheumatology.
Several of the standard treatments for PsA have an increased risk of infections, but the rates vary amongst conventional disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, biologics, and other therapies.
“Given the uptake of biological therapies has increased over recent years, we sought to investigate the national trends in serious infections in patients with psoriatic arthritis from the years 2012 to 2017,” Vagishwari Murugesan, MBBS, a psoriatic arthritis clinical fellow at the University of Toronto, told attendees in a prerecorded poster presentation. Dr. Murugesan was a fellow at Boston University when she conducted the research.
The researchers analyzed data from 2012 to 2017 in the U.S. National Inpatient Sample (NIS), which includes approximately 20% of all discharges from U.S. community hospitals except rehabilitation and long-term acute care institutions. Using ICD-9 and ICD-10 codes, the researchers identified all discharge records containing a diagnosis of PsA as well as pneumonia, sepsis, urinary tract infection (UTI), and skin and soft-tissue infections. After making adjustments to match U.S. population age distributions over the years, they examined trends in serious infections among patients with PsA for that 6-year period.
Demographics over those years changed little: The average age of discharged patients was 59.5 in 2012 and 60.8 in 2017. Similarly, the patient population was 56% women and 88.5% Whites in 2012 and 57.7% women and 88.4% Whites in 2017. The average length of stay was also similar: 4.7 days in 2012, compared with 4.9 days in 2017.
Among 50,700 discharges of patients with PsA in 2012, the researchers identified 125 with pneumonia, 230 with sepsis, 312 with skin and soft-tissue infections, and 174 with a UTI. Among the 179,400 discharges in 2017 of patients with PsA, 344 had pneumonia, 374 had sepsis, 681 had skin and soft-tissue infections, and 348 had a UTI. After statistical analysis, the researchers found no significant differences in pneumonia diagnoses during the years studied, but they did find a statistically significant decline in sepsis, skin and soft tissue infections, and UTI discharges (P < .001).
A notable limitation of the study is the NIS database’s lack of data on treatments or outpatient data, making it impossible to determine if more infections were occurring but simply being treated in outpatient settings, although it’s not clear why such a substantial shift would occur in just 5 years. It’s also possible that coding practices differ across hospital, but, presumably, the ways they might differ in 2012 would be similar to any differences in 2017.
Arthur Kavanaugh, MD, a professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, found the results interesting for what he considers an important topic.
“What makes these data interesting is the same thing that limits their reliability: The authors note that infections decreased ‘despite the increase in use of biologics over this time,’ ” Dr. Kavanaugh said in an interview. “These are claims data, so there is no way to support any association between those serious infections and biologic use. Indeed, multiple factors could have also impacted these data. It is not possible to tell from claims data.”
Dr. Kavanaugh said the question is worth investigating further with data from other sources.
The research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One study coauthor reported ties to UCB; Dr. Murugesan and her other coauthors reported no disclosures. Dr. Kavanaugh had no disclosures.
The rate of U.S. hospitalizations for three types of serious infections in patients with psoriatic arthritis (PsA) appears to have declined from 2012 to 2017, according to research presented at the virtual annual meeting of the American College of Rheumatology.
Several of the standard treatments for PsA have an increased risk of infections, but the rates vary amongst conventional disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, biologics, and other therapies.
“Given the uptake of biological therapies has increased over recent years, we sought to investigate the national trends in serious infections in patients with psoriatic arthritis from the years 2012 to 2017,” Vagishwari Murugesan, MBBS, a psoriatic arthritis clinical fellow at the University of Toronto, told attendees in a prerecorded poster presentation. Dr. Murugesan was a fellow at Boston University when she conducted the research.
The researchers analyzed data from 2012 to 2017 in the U.S. National Inpatient Sample (NIS), which includes approximately 20% of all discharges from U.S. community hospitals except rehabilitation and long-term acute care institutions. Using ICD-9 and ICD-10 codes, the researchers identified all discharge records containing a diagnosis of PsA as well as pneumonia, sepsis, urinary tract infection (UTI), and skin and soft-tissue infections. After making adjustments to match U.S. population age distributions over the years, they examined trends in serious infections among patients with PsA for that 6-year period.
Demographics over those years changed little: The average age of discharged patients was 59.5 in 2012 and 60.8 in 2017. Similarly, the patient population was 56% women and 88.5% Whites in 2012 and 57.7% women and 88.4% Whites in 2017. The average length of stay was also similar: 4.7 days in 2012, compared with 4.9 days in 2017.
Among 50,700 discharges of patients with PsA in 2012, the researchers identified 125 with pneumonia, 230 with sepsis, 312 with skin and soft-tissue infections, and 174 with a UTI. Among the 179,400 discharges in 2017 of patients with PsA, 344 had pneumonia, 374 had sepsis, 681 had skin and soft-tissue infections, and 348 had a UTI. After statistical analysis, the researchers found no significant differences in pneumonia diagnoses during the years studied, but they did find a statistically significant decline in sepsis, skin and soft tissue infections, and UTI discharges (P < .001).
A notable limitation of the study is the NIS database’s lack of data on treatments or outpatient data, making it impossible to determine if more infections were occurring but simply being treated in outpatient settings, although it’s not clear why such a substantial shift would occur in just 5 years. It’s also possible that coding practices differ across hospital, but, presumably, the ways they might differ in 2012 would be similar to any differences in 2017.
Arthur Kavanaugh, MD, a professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, found the results interesting for what he considers an important topic.
“What makes these data interesting is the same thing that limits their reliability: The authors note that infections decreased ‘despite the increase in use of biologics over this time,’ ” Dr. Kavanaugh said in an interview. “These are claims data, so there is no way to support any association between those serious infections and biologic use. Indeed, multiple factors could have also impacted these data. It is not possible to tell from claims data.”
Dr. Kavanaugh said the question is worth investigating further with data from other sources.
The research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One study coauthor reported ties to UCB; Dr. Murugesan and her other coauthors reported no disclosures. Dr. Kavanaugh had no disclosures.
The rate of U.S. hospitalizations for three types of serious infections in patients with psoriatic arthritis (PsA) appears to have declined from 2012 to 2017, according to research presented at the virtual annual meeting of the American College of Rheumatology.
Several of the standard treatments for PsA have an increased risk of infections, but the rates vary amongst conventional disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, biologics, and other therapies.
“Given the uptake of biological therapies has increased over recent years, we sought to investigate the national trends in serious infections in patients with psoriatic arthritis from the years 2012 to 2017,” Vagishwari Murugesan, MBBS, a psoriatic arthritis clinical fellow at the University of Toronto, told attendees in a prerecorded poster presentation. Dr. Murugesan was a fellow at Boston University when she conducted the research.
The researchers analyzed data from 2012 to 2017 in the U.S. National Inpatient Sample (NIS), which includes approximately 20% of all discharges from U.S. community hospitals except rehabilitation and long-term acute care institutions. Using ICD-9 and ICD-10 codes, the researchers identified all discharge records containing a diagnosis of PsA as well as pneumonia, sepsis, urinary tract infection (UTI), and skin and soft-tissue infections. After making adjustments to match U.S. population age distributions over the years, they examined trends in serious infections among patients with PsA for that 6-year period.
Demographics over those years changed little: The average age of discharged patients was 59.5 in 2012 and 60.8 in 2017. Similarly, the patient population was 56% women and 88.5% Whites in 2012 and 57.7% women and 88.4% Whites in 2017. The average length of stay was also similar: 4.7 days in 2012, compared with 4.9 days in 2017.
Among 50,700 discharges of patients with PsA in 2012, the researchers identified 125 with pneumonia, 230 with sepsis, 312 with skin and soft-tissue infections, and 174 with a UTI. Among the 179,400 discharges in 2017 of patients with PsA, 344 had pneumonia, 374 had sepsis, 681 had skin and soft-tissue infections, and 348 had a UTI. After statistical analysis, the researchers found no significant differences in pneumonia diagnoses during the years studied, but they did find a statistically significant decline in sepsis, skin and soft tissue infections, and UTI discharges (P < .001).
A notable limitation of the study is the NIS database’s lack of data on treatments or outpatient data, making it impossible to determine if more infections were occurring but simply being treated in outpatient settings, although it’s not clear why such a substantial shift would occur in just 5 years. It’s also possible that coding practices differ across hospital, but, presumably, the ways they might differ in 2012 would be similar to any differences in 2017.
Arthur Kavanaugh, MD, a professor of medicine and director of the Center for Innovative Therapy at the University of California, San Diego, found the results interesting for what he considers an important topic.
“What makes these data interesting is the same thing that limits their reliability: The authors note that infections decreased ‘despite the increase in use of biologics over this time,’ ” Dr. Kavanaugh said in an interview. “These are claims data, so there is no way to support any association between those serious infections and biologic use. Indeed, multiple factors could have also impacted these data. It is not possible to tell from claims data.”
Dr. Kavanaugh said the question is worth investigating further with data from other sources.
The research was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. One study coauthor reported ties to UCB; Dr. Murugesan and her other coauthors reported no disclosures. Dr. Kavanaugh had no disclosures.
FROM ACR 2021
Low-dose rituximab may keep RA disease activity low in responders
Rituximab doses as low as 200 mg reduced disease activity in patients with rheumatoid arthritis to an extent that’s similar to the standard 1,000-mg dose during more than 3 years of follow-up, according to results from an extension study of a clinical trial in the Netherlands.
“We could not formally statistically show that the lower doses were less effective than the higher dose,” study leader Nathan den Broeder, MSc, a PhD candidate at St. Maarten Clinic and the Radboud Institute for Health Sciences in Nijmegen, the Netherlands, said in a presentation at the virtual annual meeting of the American College of Rheumatology. “We concluded at this time that only 6% of patients needed to switch to another biologic or targeted disease-modifying antirheumatic drug and that mean disease activity remained very low,” he said of the patients treated with 200- and 500-mg doses of rituximab.
The extension study included 118 of 142 patients in the REDO trial, following them from the start of the trial in 2017-2018 through April 2021. They were randomized to three treatment arms: the standard 1,000-mg dose (24 patients), a 500-mg dose (n = 48), and the 200-mg dose (n = 46). The mean follow-up was 3.2 years.
The study evaluated disease activity by mean Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP), which during follow-up were 2.2-2.3 in the groups. Seven patients in the study cohort switched to a different DMARD, he said.
“On average, we saw a DAS28-CRP 0.15 points lower per 1,000 mg rituximab used in the past year,” Mr. den Broeder said in an interview. “For context, this is compared to a measurement error of about 0.6. A good response to a drug would be a reduction in DAS28-CRP of 1.2. This means we can just about get an effect that is bigger than the measurement error if we compare the highest dose in our study to the lowest one.”
After a year, the median yearly rituximab dose was 978 mg, with an interquartile range of 704-1,425 mg. At the end of the study, 31% of patients took 200 mg every 6 months, 40% took 500 mg every 6.2 months, and 29% took 1,000 mg every 6.4 months.
“It’s important to note, though, this is in a situation where patients are given a dose based on disease activity,” Mr. den Broeder said. “That is, we try one dose; if the patient does well, we try a lower one; if not, we might go back up to a higher dose. We could expect somewhat larger differences if all patients were to be switched to a lower dose, regardless of whether that works well for them.”
The results were achieved without a high reliance on glucocorticoids (GCs), he said. Use of comedication in the extension study population was 0.38 GC injections per patient-year and starting or increasing an oral GC occurred at a rate of only 0.05 per patient-year.
“As a result of this study, we are now implementing a strategy of rituximab dose reduction in clinical practice at our center,” Mr. den Broeder said. Patients with RA start on a 1,000-mg dose for 6 months, and if they respond well they’re put on a 500-mg dose. If they respond well after 6 months on the 500-mg dose, they’re then moved to the 200-mg dose. “With this, we hope to gain that patients have fewer side effects,” he said. “We hope to reduce the cost of treatment, and also, what we instantly gain is that the infusion time for patients is also reduced.”
Future research considerations include evaluating the 200-mg dose as a subcutaneous injection. “Another thing you might think of as well: Are even lower doses possible?” he said.
Session moderator Maya Buch, MD, professor of rheumatology and director of Experimental Medicine at the Centre for Musculoskeletal Research at the University of Manchester (England), asked if the investigators used CD19 testing to measure B-cell levels or immunoglobulin G levels to determine dose escalation.
Mr. den Broeder said that CD19 wasn’t used in clinical practice but was used in the original trial. However, it wasn’t found to have any predictive ability, while immunoglobulin G levels were measured in patients who had multiple infections. “Sporadically, a lower dose might have been initiated because of that, but not systematically,” he said.
Mr. den Broeder had no relevant relationships to disclose. Dr. Buch reported financial relationships with AbbVie, Eli Lilly, Gilead Sciences, Merck-Serono, Pfizer, Roche, Sanofi, and UCB.
Rituximab doses as low as 200 mg reduced disease activity in patients with rheumatoid arthritis to an extent that’s similar to the standard 1,000-mg dose during more than 3 years of follow-up, according to results from an extension study of a clinical trial in the Netherlands.
“We could not formally statistically show that the lower doses were less effective than the higher dose,” study leader Nathan den Broeder, MSc, a PhD candidate at St. Maarten Clinic and the Radboud Institute for Health Sciences in Nijmegen, the Netherlands, said in a presentation at the virtual annual meeting of the American College of Rheumatology. “We concluded at this time that only 6% of patients needed to switch to another biologic or targeted disease-modifying antirheumatic drug and that mean disease activity remained very low,” he said of the patients treated with 200- and 500-mg doses of rituximab.
The extension study included 118 of 142 patients in the REDO trial, following them from the start of the trial in 2017-2018 through April 2021. They were randomized to three treatment arms: the standard 1,000-mg dose (24 patients), a 500-mg dose (n = 48), and the 200-mg dose (n = 46). The mean follow-up was 3.2 years.
The study evaluated disease activity by mean Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP), which during follow-up were 2.2-2.3 in the groups. Seven patients in the study cohort switched to a different DMARD, he said.
“On average, we saw a DAS28-CRP 0.15 points lower per 1,000 mg rituximab used in the past year,” Mr. den Broeder said in an interview. “For context, this is compared to a measurement error of about 0.6. A good response to a drug would be a reduction in DAS28-CRP of 1.2. This means we can just about get an effect that is bigger than the measurement error if we compare the highest dose in our study to the lowest one.”
After a year, the median yearly rituximab dose was 978 mg, with an interquartile range of 704-1,425 mg. At the end of the study, 31% of patients took 200 mg every 6 months, 40% took 500 mg every 6.2 months, and 29% took 1,000 mg every 6.4 months.
“It’s important to note, though, this is in a situation where patients are given a dose based on disease activity,” Mr. den Broeder said. “That is, we try one dose; if the patient does well, we try a lower one; if not, we might go back up to a higher dose. We could expect somewhat larger differences if all patients were to be switched to a lower dose, regardless of whether that works well for them.”
The results were achieved without a high reliance on glucocorticoids (GCs), he said. Use of comedication in the extension study population was 0.38 GC injections per patient-year and starting or increasing an oral GC occurred at a rate of only 0.05 per patient-year.
“As a result of this study, we are now implementing a strategy of rituximab dose reduction in clinical practice at our center,” Mr. den Broeder said. Patients with RA start on a 1,000-mg dose for 6 months, and if they respond well they’re put on a 500-mg dose. If they respond well after 6 months on the 500-mg dose, they’re then moved to the 200-mg dose. “With this, we hope to gain that patients have fewer side effects,” he said. “We hope to reduce the cost of treatment, and also, what we instantly gain is that the infusion time for patients is also reduced.”
Future research considerations include evaluating the 200-mg dose as a subcutaneous injection. “Another thing you might think of as well: Are even lower doses possible?” he said.
Session moderator Maya Buch, MD, professor of rheumatology and director of Experimental Medicine at the Centre for Musculoskeletal Research at the University of Manchester (England), asked if the investigators used CD19 testing to measure B-cell levels or immunoglobulin G levels to determine dose escalation.
Mr. den Broeder said that CD19 wasn’t used in clinical practice but was used in the original trial. However, it wasn’t found to have any predictive ability, while immunoglobulin G levels were measured in patients who had multiple infections. “Sporadically, a lower dose might have been initiated because of that, but not systematically,” he said.
Mr. den Broeder had no relevant relationships to disclose. Dr. Buch reported financial relationships with AbbVie, Eli Lilly, Gilead Sciences, Merck-Serono, Pfizer, Roche, Sanofi, and UCB.
Rituximab doses as low as 200 mg reduced disease activity in patients with rheumatoid arthritis to an extent that’s similar to the standard 1,000-mg dose during more than 3 years of follow-up, according to results from an extension study of a clinical trial in the Netherlands.
“We could not formally statistically show that the lower doses were less effective than the higher dose,” study leader Nathan den Broeder, MSc, a PhD candidate at St. Maarten Clinic and the Radboud Institute for Health Sciences in Nijmegen, the Netherlands, said in a presentation at the virtual annual meeting of the American College of Rheumatology. “We concluded at this time that only 6% of patients needed to switch to another biologic or targeted disease-modifying antirheumatic drug and that mean disease activity remained very low,” he said of the patients treated with 200- and 500-mg doses of rituximab.
The extension study included 118 of 142 patients in the REDO trial, following them from the start of the trial in 2017-2018 through April 2021. They were randomized to three treatment arms: the standard 1,000-mg dose (24 patients), a 500-mg dose (n = 48), and the 200-mg dose (n = 46). The mean follow-up was 3.2 years.
The study evaluated disease activity by mean Disease Activity Score in 28 joints with C-reactive protein (DAS28-CRP), which during follow-up were 2.2-2.3 in the groups. Seven patients in the study cohort switched to a different DMARD, he said.
“On average, we saw a DAS28-CRP 0.15 points lower per 1,000 mg rituximab used in the past year,” Mr. den Broeder said in an interview. “For context, this is compared to a measurement error of about 0.6. A good response to a drug would be a reduction in DAS28-CRP of 1.2. This means we can just about get an effect that is bigger than the measurement error if we compare the highest dose in our study to the lowest one.”
After a year, the median yearly rituximab dose was 978 mg, with an interquartile range of 704-1,425 mg. At the end of the study, 31% of patients took 200 mg every 6 months, 40% took 500 mg every 6.2 months, and 29% took 1,000 mg every 6.4 months.
“It’s important to note, though, this is in a situation where patients are given a dose based on disease activity,” Mr. den Broeder said. “That is, we try one dose; if the patient does well, we try a lower one; if not, we might go back up to a higher dose. We could expect somewhat larger differences if all patients were to be switched to a lower dose, regardless of whether that works well for them.”
The results were achieved without a high reliance on glucocorticoids (GCs), he said. Use of comedication in the extension study population was 0.38 GC injections per patient-year and starting or increasing an oral GC occurred at a rate of only 0.05 per patient-year.
“As a result of this study, we are now implementing a strategy of rituximab dose reduction in clinical practice at our center,” Mr. den Broeder said. Patients with RA start on a 1,000-mg dose for 6 months, and if they respond well they’re put on a 500-mg dose. If they respond well after 6 months on the 500-mg dose, they’re then moved to the 200-mg dose. “With this, we hope to gain that patients have fewer side effects,” he said. “We hope to reduce the cost of treatment, and also, what we instantly gain is that the infusion time for patients is also reduced.”
Future research considerations include evaluating the 200-mg dose as a subcutaneous injection. “Another thing you might think of as well: Are even lower doses possible?” he said.
Session moderator Maya Buch, MD, professor of rheumatology and director of Experimental Medicine at the Centre for Musculoskeletal Research at the University of Manchester (England), asked if the investigators used CD19 testing to measure B-cell levels or immunoglobulin G levels to determine dose escalation.
Mr. den Broeder said that CD19 wasn’t used in clinical practice but was used in the original trial. However, it wasn’t found to have any predictive ability, while immunoglobulin G levels were measured in patients who had multiple infections. “Sporadically, a lower dose might have been initiated because of that, but not systematically,” he said.
Mr. den Broeder had no relevant relationships to disclose. Dr. Buch reported financial relationships with AbbVie, Eli Lilly, Gilead Sciences, Merck-Serono, Pfizer, Roche, Sanofi, and UCB.
FROM ACR 2021
ADVOCATE: Avacopan shows renal benefits in ANCA vasculitis
Treatment of antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis and renal disease with the oral C5a receptor inhibitor avacopan (Tavneos, ChemoCentryx) provides significant recovery of kidney function, compared with prednisone, particularly in patients with severe kidney disease, novel research indicates.
The new analysis underscores that “the real value of avacopan is that we can now expect to get our patients steroid free,” said first author David R.W. Jayne, MD, a professor of clinical autoimmunity at the University of Cambridge (England), when presenting the findings at the American Society of Nephrology’s Kidney Week 2021.
“Whether or not we’re brave enough to initiate treatment without steroids, I think that will perhaps come with some patient experience,” he added.
The findings are from a subanalysis of renal effects in the phase 3 ADVOCATE trial, which was published in February 2021 in the New England Journal of Medicine and included 330 patients with ANCA-associated vasculitis.
The trial in large part led to the U.S. approval of avacopan by the Food and Drug Administration in October as an adjunctive treatment for adults with severe active ANCA-associated vasculitis in combination with standard therapy including glucocorticoids.
The approval was greeted with enthusiasm as suggesting a much-needed option to help reduce, or even potentially eliminate, the need for glucocorticoids and their side effects. Other agents included in treatment regimens for ANCA-associated vasculitis include cyclophosphamide and rituximab.
Dr. Jayne emphasized that, before avacopan, treatment options had been limited.
“There is nothing else new in the clinic apart from rituximab, which we have now been using for almost 20 years,” he said in an interview. “Avacopan is new, the mode of action is different from any drugs in use at the moment, and the speed of action is very quick.”
The need to more closely investigate the trial’s renal outcomes in this new analysis was important because the high mortality rates in ANCA-associated vasculitis – a rare systemic autoimmune disease causing overactivation of complement resulting in inflammation of small blood vessels – is largely driven by those with MPO and PR3 autoantibody renal vasculitis, Dr. Jayne explained.
Commenting on the study, J. Charles Jennette, MD, a professor of pathology and laboratory medicine and professor of medicine at the University of North Carolina at Chapel Hill, said the new findings on renal outcomes, such as proteinuria, may offer key insights on avacopan’s efficacy.
“To me, the most impressive outcome of the ADVOCATE Phase 3 trial was the more rapid reduction in hematuria and proteinuria with avacopan compared to conventional prednisone therapy,” he said in an interview.
Recovery of eGFR with avacopan best in those with severe renal disease
In the trial, patients with ANCA-associated vasculitis were randomized 1:1 to treatment with oral avacopan 30 mg twice daily or oral prednisone on a tapering schedule.
All patients also received background immunosuppression – about two-thirds received rituximab and a third received cyclophosphamide – followed by azathioprine.
The main study results showed similar rates of remission in both groups at week 26 and a superior remission rate with avacopan, in terms of sustained remission, at week 52 (65.7% vs. 54.9%; P < .001).
Approximately 80% of patients in the trial had renal involvement of ANCA vasculitis, the focus of the new analysis, and they had a baseline mean estimated glomerular filtration rate (eGFR) of 45 mL/min per 1.73 m2.
Among those with renal involvement, patients treated with avacopan had a significantly greater eGFR recovery, compared with the prednisone group at week 26 (P = .046) and week 52 (P < .029).
The strongest improvements were observed among patients with moderate to severe kidney damage, who had a mean eGFR of 21 mL/min per 1.73 m2 at baseline. Among those patients, the mean increase in eGFR was 13.7 mL/min per 1.73 m2 in the avacopan-treated group (n = 52) versus 8.2 mL/min per 1.73 m2 in the prednisone group (n = 48; P < .01) by week 52.
Improvements in urinary albumin:creatinine ratios (UACR) of as much as 40% were also observed in the avacopan group within the first 4 weeks of treatment, while no changes were observed in the same period in the prednisone group.
In other findings, the study also showed more rapid declines in proteinuria within 4 weeks in the avacopan group, and fewer patients had hematuria and there were greater reductions in MCP-1 in avacopan-treated patients at week 52, Dr. Jayne reported.
In terms of safety, there were no differences between the groups, with trends of fewer deaths and severe adverse events in the avacopan group.
“We found that the improved recovery of eGFR with avacopan was accentuated among those with more severe renal disease,” Dr. Jayne said.
He noted that, while the study’s aim was for the avacopan group to be steroid free, the patients received brief, reduced doses of about a third of the normal oral steroid dose early in the trial. However, using a Glucocorticoid Toxicity Index, the authors found those in the avacopan group did have fewer glucocorticoid-related adverse events.
Future issues to be examined include what happens when avacopan is discontinued and whether there will be a high relapse rate, Dr. Jayne noted.
Overall, however, “we anticipate that with longer-term follow-up, this better eGFR recovery will have a [favorable] effect on kidney failure and potentially mortality risk in these patients,” he concluded.
Targeted therapy is good for patients and doctors
Expanding upon his comments regarding the new drug, Dr. Jennette said it implies “that the C5a receptor inhibitor was targeting an event that blocks injury more quickly and effectively than prednisone.”
“This may be because prednisone has more complex pharmacodynamics and less targeted effects than a C5a receptor inhibitor,” he said.
Overall, the findings bode well for a potentially beneficial therapy, he added. “We have entered a new era of more targeted therapies, for example, targeted B-cell therapy using an anti-CD20 antibody, and targeted complement-mediated injury therapy using C5a receptor inhibitor.”
“The validation of this targeted therapy to block complement-mediated autoimmune inflammatory injury is another advance toward targeted precision therapy versus empirical therapy. This will be good for the doctors and good for the patients,” Dr. Jennette concluded.
The study was funded by ChemoCentryx. Dr. Jayne has reported receiving grants and/or consulting for AstraZeneca, ChemoCentryx, GlaxoSmithKline, MiroBio, Vifor, and Roche/Genentech. Dr. Jennette has received funding from ChemoCentryx for preclinical validation studies of avacopan in a mouse model of ANCA glomerulonephritis.
A version of this article first appeared on Medscape.com.
Treatment of antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis and renal disease with the oral C5a receptor inhibitor avacopan (Tavneos, ChemoCentryx) provides significant recovery of kidney function, compared with prednisone, particularly in patients with severe kidney disease, novel research indicates.
The new analysis underscores that “the real value of avacopan is that we can now expect to get our patients steroid free,” said first author David R.W. Jayne, MD, a professor of clinical autoimmunity at the University of Cambridge (England), when presenting the findings at the American Society of Nephrology’s Kidney Week 2021.
“Whether or not we’re brave enough to initiate treatment without steroids, I think that will perhaps come with some patient experience,” he added.
The findings are from a subanalysis of renal effects in the phase 3 ADVOCATE trial, which was published in February 2021 in the New England Journal of Medicine and included 330 patients with ANCA-associated vasculitis.
The trial in large part led to the U.S. approval of avacopan by the Food and Drug Administration in October as an adjunctive treatment for adults with severe active ANCA-associated vasculitis in combination with standard therapy including glucocorticoids.
The approval was greeted with enthusiasm as suggesting a much-needed option to help reduce, or even potentially eliminate, the need for glucocorticoids and their side effects. Other agents included in treatment regimens for ANCA-associated vasculitis include cyclophosphamide and rituximab.
Dr. Jayne emphasized that, before avacopan, treatment options had been limited.
“There is nothing else new in the clinic apart from rituximab, which we have now been using for almost 20 years,” he said in an interview. “Avacopan is new, the mode of action is different from any drugs in use at the moment, and the speed of action is very quick.”
The need to more closely investigate the trial’s renal outcomes in this new analysis was important because the high mortality rates in ANCA-associated vasculitis – a rare systemic autoimmune disease causing overactivation of complement resulting in inflammation of small blood vessels – is largely driven by those with MPO and PR3 autoantibody renal vasculitis, Dr. Jayne explained.
Commenting on the study, J. Charles Jennette, MD, a professor of pathology and laboratory medicine and professor of medicine at the University of North Carolina at Chapel Hill, said the new findings on renal outcomes, such as proteinuria, may offer key insights on avacopan’s efficacy.
“To me, the most impressive outcome of the ADVOCATE Phase 3 trial was the more rapid reduction in hematuria and proteinuria with avacopan compared to conventional prednisone therapy,” he said in an interview.
Recovery of eGFR with avacopan best in those with severe renal disease
In the trial, patients with ANCA-associated vasculitis were randomized 1:1 to treatment with oral avacopan 30 mg twice daily or oral prednisone on a tapering schedule.
All patients also received background immunosuppression – about two-thirds received rituximab and a third received cyclophosphamide – followed by azathioprine.
The main study results showed similar rates of remission in both groups at week 26 and a superior remission rate with avacopan, in terms of sustained remission, at week 52 (65.7% vs. 54.9%; P < .001).
Approximately 80% of patients in the trial had renal involvement of ANCA vasculitis, the focus of the new analysis, and they had a baseline mean estimated glomerular filtration rate (eGFR) of 45 mL/min per 1.73 m2.
Among those with renal involvement, patients treated with avacopan had a significantly greater eGFR recovery, compared with the prednisone group at week 26 (P = .046) and week 52 (P < .029).
The strongest improvements were observed among patients with moderate to severe kidney damage, who had a mean eGFR of 21 mL/min per 1.73 m2 at baseline. Among those patients, the mean increase in eGFR was 13.7 mL/min per 1.73 m2 in the avacopan-treated group (n = 52) versus 8.2 mL/min per 1.73 m2 in the prednisone group (n = 48; P < .01) by week 52.
Improvements in urinary albumin:creatinine ratios (UACR) of as much as 40% were also observed in the avacopan group within the first 4 weeks of treatment, while no changes were observed in the same period in the prednisone group.
In other findings, the study also showed more rapid declines in proteinuria within 4 weeks in the avacopan group, and fewer patients had hematuria and there were greater reductions in MCP-1 in avacopan-treated patients at week 52, Dr. Jayne reported.
In terms of safety, there were no differences between the groups, with trends of fewer deaths and severe adverse events in the avacopan group.
“We found that the improved recovery of eGFR with avacopan was accentuated among those with more severe renal disease,” Dr. Jayne said.
He noted that, while the study’s aim was for the avacopan group to be steroid free, the patients received brief, reduced doses of about a third of the normal oral steroid dose early in the trial. However, using a Glucocorticoid Toxicity Index, the authors found those in the avacopan group did have fewer glucocorticoid-related adverse events.
Future issues to be examined include what happens when avacopan is discontinued and whether there will be a high relapse rate, Dr. Jayne noted.
Overall, however, “we anticipate that with longer-term follow-up, this better eGFR recovery will have a [favorable] effect on kidney failure and potentially mortality risk in these patients,” he concluded.
Targeted therapy is good for patients and doctors
Expanding upon his comments regarding the new drug, Dr. Jennette said it implies “that the C5a receptor inhibitor was targeting an event that blocks injury more quickly and effectively than prednisone.”
“This may be because prednisone has more complex pharmacodynamics and less targeted effects than a C5a receptor inhibitor,” he said.
Overall, the findings bode well for a potentially beneficial therapy, he added. “We have entered a new era of more targeted therapies, for example, targeted B-cell therapy using an anti-CD20 antibody, and targeted complement-mediated injury therapy using C5a receptor inhibitor.”
“The validation of this targeted therapy to block complement-mediated autoimmune inflammatory injury is another advance toward targeted precision therapy versus empirical therapy. This will be good for the doctors and good for the patients,” Dr. Jennette concluded.
The study was funded by ChemoCentryx. Dr. Jayne has reported receiving grants and/or consulting for AstraZeneca, ChemoCentryx, GlaxoSmithKline, MiroBio, Vifor, and Roche/Genentech. Dr. Jennette has received funding from ChemoCentryx for preclinical validation studies of avacopan in a mouse model of ANCA glomerulonephritis.
A version of this article first appeared on Medscape.com.
Treatment of antineutrophil cytoplasmic autoantibody (ANCA)–associated vasculitis and renal disease with the oral C5a receptor inhibitor avacopan (Tavneos, ChemoCentryx) provides significant recovery of kidney function, compared with prednisone, particularly in patients with severe kidney disease, novel research indicates.
The new analysis underscores that “the real value of avacopan is that we can now expect to get our patients steroid free,” said first author David R.W. Jayne, MD, a professor of clinical autoimmunity at the University of Cambridge (England), when presenting the findings at the American Society of Nephrology’s Kidney Week 2021.
“Whether or not we’re brave enough to initiate treatment without steroids, I think that will perhaps come with some patient experience,” he added.
The findings are from a subanalysis of renal effects in the phase 3 ADVOCATE trial, which was published in February 2021 in the New England Journal of Medicine and included 330 patients with ANCA-associated vasculitis.
The trial in large part led to the U.S. approval of avacopan by the Food and Drug Administration in October as an adjunctive treatment for adults with severe active ANCA-associated vasculitis in combination with standard therapy including glucocorticoids.
The approval was greeted with enthusiasm as suggesting a much-needed option to help reduce, or even potentially eliminate, the need for glucocorticoids and their side effects. Other agents included in treatment regimens for ANCA-associated vasculitis include cyclophosphamide and rituximab.
Dr. Jayne emphasized that, before avacopan, treatment options had been limited.
“There is nothing else new in the clinic apart from rituximab, which we have now been using for almost 20 years,” he said in an interview. “Avacopan is new, the mode of action is different from any drugs in use at the moment, and the speed of action is very quick.”
The need to more closely investigate the trial’s renal outcomes in this new analysis was important because the high mortality rates in ANCA-associated vasculitis – a rare systemic autoimmune disease causing overactivation of complement resulting in inflammation of small blood vessels – is largely driven by those with MPO and PR3 autoantibody renal vasculitis, Dr. Jayne explained.
Commenting on the study, J. Charles Jennette, MD, a professor of pathology and laboratory medicine and professor of medicine at the University of North Carolina at Chapel Hill, said the new findings on renal outcomes, such as proteinuria, may offer key insights on avacopan’s efficacy.
“To me, the most impressive outcome of the ADVOCATE Phase 3 trial was the more rapid reduction in hematuria and proteinuria with avacopan compared to conventional prednisone therapy,” he said in an interview.
Recovery of eGFR with avacopan best in those with severe renal disease
In the trial, patients with ANCA-associated vasculitis were randomized 1:1 to treatment with oral avacopan 30 mg twice daily or oral prednisone on a tapering schedule.
All patients also received background immunosuppression – about two-thirds received rituximab and a third received cyclophosphamide – followed by azathioprine.
The main study results showed similar rates of remission in both groups at week 26 and a superior remission rate with avacopan, in terms of sustained remission, at week 52 (65.7% vs. 54.9%; P < .001).
Approximately 80% of patients in the trial had renal involvement of ANCA vasculitis, the focus of the new analysis, and they had a baseline mean estimated glomerular filtration rate (eGFR) of 45 mL/min per 1.73 m2.
Among those with renal involvement, patients treated with avacopan had a significantly greater eGFR recovery, compared with the prednisone group at week 26 (P = .046) and week 52 (P < .029).
The strongest improvements were observed among patients with moderate to severe kidney damage, who had a mean eGFR of 21 mL/min per 1.73 m2 at baseline. Among those patients, the mean increase in eGFR was 13.7 mL/min per 1.73 m2 in the avacopan-treated group (n = 52) versus 8.2 mL/min per 1.73 m2 in the prednisone group (n = 48; P < .01) by week 52.
Improvements in urinary albumin:creatinine ratios (UACR) of as much as 40% were also observed in the avacopan group within the first 4 weeks of treatment, while no changes were observed in the same period in the prednisone group.
In other findings, the study also showed more rapid declines in proteinuria within 4 weeks in the avacopan group, and fewer patients had hematuria and there were greater reductions in MCP-1 in avacopan-treated patients at week 52, Dr. Jayne reported.
In terms of safety, there were no differences between the groups, with trends of fewer deaths and severe adverse events in the avacopan group.
“We found that the improved recovery of eGFR with avacopan was accentuated among those with more severe renal disease,” Dr. Jayne said.
He noted that, while the study’s aim was for the avacopan group to be steroid free, the patients received brief, reduced doses of about a third of the normal oral steroid dose early in the trial. However, using a Glucocorticoid Toxicity Index, the authors found those in the avacopan group did have fewer glucocorticoid-related adverse events.
Future issues to be examined include what happens when avacopan is discontinued and whether there will be a high relapse rate, Dr. Jayne noted.
Overall, however, “we anticipate that with longer-term follow-up, this better eGFR recovery will have a [favorable] effect on kidney failure and potentially mortality risk in these patients,” he concluded.
Targeted therapy is good for patients and doctors
Expanding upon his comments regarding the new drug, Dr. Jennette said it implies “that the C5a receptor inhibitor was targeting an event that blocks injury more quickly and effectively than prednisone.”
“This may be because prednisone has more complex pharmacodynamics and less targeted effects than a C5a receptor inhibitor,” he said.
Overall, the findings bode well for a potentially beneficial therapy, he added. “We have entered a new era of more targeted therapies, for example, targeted B-cell therapy using an anti-CD20 antibody, and targeted complement-mediated injury therapy using C5a receptor inhibitor.”
“The validation of this targeted therapy to block complement-mediated autoimmune inflammatory injury is another advance toward targeted precision therapy versus empirical therapy. This will be good for the doctors and good for the patients,” Dr. Jennette concluded.
The study was funded by ChemoCentryx. Dr. Jayne has reported receiving grants and/or consulting for AstraZeneca, ChemoCentryx, GlaxoSmithKline, MiroBio, Vifor, and Roche/Genentech. Dr. Jennette has received funding from ChemoCentryx for preclinical validation studies of avacopan in a mouse model of ANCA glomerulonephritis.
A version of this article first appeared on Medscape.com.
FROM KIDNEY WEEK 2021