User login
Endocrine Society raises concerns about FDA’s “safe” classification of bisphenol A in food containers
An initial report from the Endocrine Society has raised concerns about bisphenol A use in products such as food and drink containers, toys, and medical devices, citing recent data that show the synthetic compound is linked to reproductive, behavioral, and metabolic disorders.
Although the Food and Drug Administration classifies bisphenol A (BPA) as safe to use in food containers, there have been hundreds of studies tying BPA to health problems such as “neurological outcomes, abnormal metabolism, reproductive effects as well as growth and development effects,” according to Laura N. Vandenberg, PhD, an Endocrine Society spokesperson, who spoke at a press briefing held on Oct. 23. Dr. Vandenberg explained the FDA’s 2014 position on BPA safety comes from a small subset of publicly available data, but these are not all the data on BPA, as some academic data are still under review.
The Endocrine Society recently held the news conference because they are concerned the FDA has “jumped the gun” before all the research has been published. “Even considering the fact that the data that have been presented by FDA have been interpreted by FDA as suggesting that BPA is safe, scientists still disagree,” Dr. Vandenberg said.
However, the Endocrine Society noted there is an issue with the current literature, which can be used to interpret and report different results. Heather Patisaul, PhD, cited a joint report from the Food and Agriculture Organization of the United Nations and World Health Organization, as well as a report from the National Toxicology Program (NTP), to illustrate this problem. Both reports expressed concern about BPA safety but took different approaches and a wider viewpoint, and came to different conclusions, she said.
“These two documents both concluded that there was some concern about bisphenol A and behavior, but they identified there was a big problem with trying to pool all this literature together because the experimental protocols were different, the animals were different, the dosing was different,” she said. “It was not a very harmonious literature.”
To combat this issue, the National Institute of Environmental Health Sciences and the FDA have funded the CLARITY-BPA (Consortium Linking Academic and Regulatory Insights on BPA Toxicity) study. Dr. Patisaul said CLARITY-BPA is “the most ambitious project that’s ever been done” to study the health effects of a chemical, bringing together scientists from academic institutions, the NTP, and the FDA to help create data for risk assessment.
“The goal was to create this culture of partnership and communication between the agencies that have to make these decisions about safety and the scientists who are producing the data that’s trying to inform those assessments,” Dr. Patisaul said.
Dr. Vandenberg and Dr. Patisaul presented results from the CLARITY-BPA Core Study, which studied the effects of continuous doses of BPA in rats starting from 6 days of pregnancy; after birth, the rat offspring were fed doses of BPA for 1 year and 2 years. A second group of rats in a stop-dose group were fed BPA from early development, where the mothers were fed BPA at day 6 of pregnancy and the offspring fed BPA from birth until puberty (21 days) and followed for 1 year or 2 years. The researchers also examined 2.5 mcg/kg, 25 mcg/kg, 250 mcg/kg, 2,500 mcg/kg, and 25,000 mcg/kg doses of BPA exposure as well as continuous ethinyl estradiol exposure as a positive control.
In the FDA Core Study, there was a significantly increased incidence of mammary adenocarcinoma in the stop dose group and inflammation of the dorsal and lateral lobes of the prostate in the continuous dose group at a dose of 2.5 mcg/kg. In addition, kidney nephropathy and increased body weight in female rats in the continuous group were also seen at the 2.5 mcg/kg dose, Dr. Vandenberg noted.
“I think one of the reasons why FDA is dismissing those low-dose effects is that there’s an expectation with increasing dose, there should be an increase in an effect,” Dr. Vandenberg said.
In the low-dose range, BPA could be acting as a hormone such as estrogen, but also could be acting through other hormone receptors or as a toxicant at the high-dose range, she explained.
Dr. Patisaul also presented results of BPA-related effects on the brain and behavior in the existing literature from the TEDX Low-dose Bisphenol A project, which is a comparison of 391 in vivo and in vitro studies of BPA prior to 2009. The results showed brain and behavior was “heavily impacted” by BPA, as were organ systems such as the heart, which supports the results from the CLARITY-BPA study, Dr. Patisaul noted.
“When you think about reproducibility in the broadest sense, and you look at the effects that the FDA found at low dose, you look at the effects the CLARITY investigators found at low dose, and you go back and look at the existing literature, you see a very clear picture of BPA-produced effects on brain and behavior, female reproductive systems, and the cardiovascular system,” she said.
Dr. Patisaul is a study investigator for CLARITY-BPA.
An initial report from the Endocrine Society has raised concerns about bisphenol A use in products such as food and drink containers, toys, and medical devices, citing recent data that show the synthetic compound is linked to reproductive, behavioral, and metabolic disorders.
Although the Food and Drug Administration classifies bisphenol A (BPA) as safe to use in food containers, there have been hundreds of studies tying BPA to health problems such as “neurological outcomes, abnormal metabolism, reproductive effects as well as growth and development effects,” according to Laura N. Vandenberg, PhD, an Endocrine Society spokesperson, who spoke at a press briefing held on Oct. 23. Dr. Vandenberg explained the FDA’s 2014 position on BPA safety comes from a small subset of publicly available data, but these are not all the data on BPA, as some academic data are still under review.
The Endocrine Society recently held the news conference because they are concerned the FDA has “jumped the gun” before all the research has been published. “Even considering the fact that the data that have been presented by FDA have been interpreted by FDA as suggesting that BPA is safe, scientists still disagree,” Dr. Vandenberg said.
However, the Endocrine Society noted there is an issue with the current literature, which can be used to interpret and report different results. Heather Patisaul, PhD, cited a joint report from the Food and Agriculture Organization of the United Nations and World Health Organization, as well as a report from the National Toxicology Program (NTP), to illustrate this problem. Both reports expressed concern about BPA safety but took different approaches and a wider viewpoint, and came to different conclusions, she said.
“These two documents both concluded that there was some concern about bisphenol A and behavior, but they identified there was a big problem with trying to pool all this literature together because the experimental protocols were different, the animals were different, the dosing was different,” she said. “It was not a very harmonious literature.”
To combat this issue, the National Institute of Environmental Health Sciences and the FDA have funded the CLARITY-BPA (Consortium Linking Academic and Regulatory Insights on BPA Toxicity) study. Dr. Patisaul said CLARITY-BPA is “the most ambitious project that’s ever been done” to study the health effects of a chemical, bringing together scientists from academic institutions, the NTP, and the FDA to help create data for risk assessment.
“The goal was to create this culture of partnership and communication between the agencies that have to make these decisions about safety and the scientists who are producing the data that’s trying to inform those assessments,” Dr. Patisaul said.
Dr. Vandenberg and Dr. Patisaul presented results from the CLARITY-BPA Core Study, which studied the effects of continuous doses of BPA in rats starting from 6 days of pregnancy; after birth, the rat offspring were fed doses of BPA for 1 year and 2 years. A second group of rats in a stop-dose group were fed BPA from early development, where the mothers were fed BPA at day 6 of pregnancy and the offspring fed BPA from birth until puberty (21 days) and followed for 1 year or 2 years. The researchers also examined 2.5 mcg/kg, 25 mcg/kg, 250 mcg/kg, 2,500 mcg/kg, and 25,000 mcg/kg doses of BPA exposure as well as continuous ethinyl estradiol exposure as a positive control.
In the FDA Core Study, there was a significantly increased incidence of mammary adenocarcinoma in the stop dose group and inflammation of the dorsal and lateral lobes of the prostate in the continuous dose group at a dose of 2.5 mcg/kg. In addition, kidney nephropathy and increased body weight in female rats in the continuous group were also seen at the 2.5 mcg/kg dose, Dr. Vandenberg noted.
“I think one of the reasons why FDA is dismissing those low-dose effects is that there’s an expectation with increasing dose, there should be an increase in an effect,” Dr. Vandenberg said.
In the low-dose range, BPA could be acting as a hormone such as estrogen, but also could be acting through other hormone receptors or as a toxicant at the high-dose range, she explained.
Dr. Patisaul also presented results of BPA-related effects on the brain and behavior in the existing literature from the TEDX Low-dose Bisphenol A project, which is a comparison of 391 in vivo and in vitro studies of BPA prior to 2009. The results showed brain and behavior was “heavily impacted” by BPA, as were organ systems such as the heart, which supports the results from the CLARITY-BPA study, Dr. Patisaul noted.
“When you think about reproducibility in the broadest sense, and you look at the effects that the FDA found at low dose, you look at the effects the CLARITY investigators found at low dose, and you go back and look at the existing literature, you see a very clear picture of BPA-produced effects on brain and behavior, female reproductive systems, and the cardiovascular system,” she said.
Dr. Patisaul is a study investigator for CLARITY-BPA.
An initial report from the Endocrine Society has raised concerns about bisphenol A use in products such as food and drink containers, toys, and medical devices, citing recent data that show the synthetic compound is linked to reproductive, behavioral, and metabolic disorders.
Although the Food and Drug Administration classifies bisphenol A (BPA) as safe to use in food containers, there have been hundreds of studies tying BPA to health problems such as “neurological outcomes, abnormal metabolism, reproductive effects as well as growth and development effects,” according to Laura N. Vandenberg, PhD, an Endocrine Society spokesperson, who spoke at a press briefing held on Oct. 23. Dr. Vandenberg explained the FDA’s 2014 position on BPA safety comes from a small subset of publicly available data, but these are not all the data on BPA, as some academic data are still under review.
The Endocrine Society recently held the news conference because they are concerned the FDA has “jumped the gun” before all the research has been published. “Even considering the fact that the data that have been presented by FDA have been interpreted by FDA as suggesting that BPA is safe, scientists still disagree,” Dr. Vandenberg said.
However, the Endocrine Society noted there is an issue with the current literature, which can be used to interpret and report different results. Heather Patisaul, PhD, cited a joint report from the Food and Agriculture Organization of the United Nations and World Health Organization, as well as a report from the National Toxicology Program (NTP), to illustrate this problem. Both reports expressed concern about BPA safety but took different approaches and a wider viewpoint, and came to different conclusions, she said.
“These two documents both concluded that there was some concern about bisphenol A and behavior, but they identified there was a big problem with trying to pool all this literature together because the experimental protocols were different, the animals were different, the dosing was different,” she said. “It was not a very harmonious literature.”
To combat this issue, the National Institute of Environmental Health Sciences and the FDA have funded the CLARITY-BPA (Consortium Linking Academic and Regulatory Insights on BPA Toxicity) study. Dr. Patisaul said CLARITY-BPA is “the most ambitious project that’s ever been done” to study the health effects of a chemical, bringing together scientists from academic institutions, the NTP, and the FDA to help create data for risk assessment.
“The goal was to create this culture of partnership and communication between the agencies that have to make these decisions about safety and the scientists who are producing the data that’s trying to inform those assessments,” Dr. Patisaul said.
Dr. Vandenberg and Dr. Patisaul presented results from the CLARITY-BPA Core Study, which studied the effects of continuous doses of BPA in rats starting from 6 days of pregnancy; after birth, the rat offspring were fed doses of BPA for 1 year and 2 years. A second group of rats in a stop-dose group were fed BPA from early development, where the mothers were fed BPA at day 6 of pregnancy and the offspring fed BPA from birth until puberty (21 days) and followed for 1 year or 2 years. The researchers also examined 2.5 mcg/kg, 25 mcg/kg, 250 mcg/kg, 2,500 mcg/kg, and 25,000 mcg/kg doses of BPA exposure as well as continuous ethinyl estradiol exposure as a positive control.
In the FDA Core Study, there was a significantly increased incidence of mammary adenocarcinoma in the stop dose group and inflammation of the dorsal and lateral lobes of the prostate in the continuous dose group at a dose of 2.5 mcg/kg. In addition, kidney nephropathy and increased body weight in female rats in the continuous group were also seen at the 2.5 mcg/kg dose, Dr. Vandenberg noted.
“I think one of the reasons why FDA is dismissing those low-dose effects is that there’s an expectation with increasing dose, there should be an increase in an effect,” Dr. Vandenberg said.
In the low-dose range, BPA could be acting as a hormone such as estrogen, but also could be acting through other hormone receptors or as a toxicant at the high-dose range, she explained.
Dr. Patisaul also presented results of BPA-related effects on the brain and behavior in the existing literature from the TEDX Low-dose Bisphenol A project, which is a comparison of 391 in vivo and in vitro studies of BPA prior to 2009. The results showed brain and behavior was “heavily impacted” by BPA, as were organ systems such as the heart, which supports the results from the CLARITY-BPA study, Dr. Patisaul noted.
“When you think about reproducibility in the broadest sense, and you look at the effects that the FDA found at low dose, you look at the effects the CLARITY investigators found at low dose, and you go back and look at the existing literature, you see a very clear picture of BPA-produced effects on brain and behavior, female reproductive systems, and the cardiovascular system,” she said.
Dr. Patisaul is a study investigator for CLARITY-BPA.
Key clinical point: Despite claims from the Food and Drug Administration, results from the CLARITY-BPA (Consortium Linking Academic and Regulatory Insights on BPA Toxicity) Core Study show serious effects in humans of bisphenol A at low doses.
Major finding: Research from CLARITY-BPA has shown brain and behavior, female reproduction, and organ systems such as the heart can be adversely affected by bisphenol A even at low doses.
Study details: An initial report from the CLARITY-BPA Core Study.
Disclosures: Dr. Patisaul is a study investigator for CLARITY-BPA.
Study: Problems persist with APMs
Physicians continue to support advanced alternative payment models despite the fact that operational issues have not improved over the last 4 years and new ones have cropped up, according to a follow-up survey conducted by the RAND Corporation for the American Medical Association.
“All the things we heard in 2014 were still present in 2018. Both the challenges that practices had experienced back in 2014 having to do with data timeliness, data completeness and accuracy, payment model execution, all those challenges persisted,” Mark W. Friedberg, MD, senior physician policy researcher at RAND, said in an interview.
RAND surveyed 31 practices of varying practice size and specialty across six geographic regions, some of which participated in the 2014 survey. Supplemental information was provided by interviews with 32 market observers, 8 health plan leaders, 10 hospital and hospital system leaders, 10 state and local medical society leaders, and 4 chapter leaders with MGMA (formerly the Medical Group Management Association).
“We had thought we would hear that the problem had gotten a little bit better since there has been some investment in trying to tamp down the wide range of measures that are involved in these alternative payment models,” Dr. Friedberg said. “We did not see any evidence of that having any effect on the practices that participated in this study this time around.”
Indeed, concerns reported in 2014 were again reported in 2018, along with a new set of concerns, including the perceived pace of change in alternative payment models (APMs), the complexity of APMs, and physician concerns over two-sided risk models.
“Practices, especially those that participated both times, said in 2014 we had these challenges [of rapid changes in APM models] and since then, things have just gotten a lot faster,” he said, noting that doctors are complaining of models that are going through changes, sometimes without much warning. “They are changing quite rapidly from year to year. If you look at the MACRA QPP [Quality Payment Program] for example, that model changes every year to some extent and those things are hard for them to keep up with.”
Running hand in hand with the change is the complexity of the changes, a result of expanding performance measures and uncertainty with thresholds for penalties and rewards and in some ways has had little impact on improving care.
Dr. Friedberg noted that some practices are hiring people to examine APMs to devise strategic ways to choose and report data for maximum return.
“In a practice, for example, if their quality of care was already very good, what these folks ended up doing was help them choose measures and work the attribution algorithms in a strategic way to either guarantee a bonus or minimize the risk of incurring a penalty,” he said.
He also noted that practices appear to becoming more risk averse.
“We heard a lot more of the following thing, which is that if [practices] were in a two-sided risk model, several of them reported trying and succeeding in some cases offloading the downside risk to partners,” Dr. Friedberg reported. “And what this resulted in was that the practice, even though from the payer’s perspective they are in a two-sided model, the practice was actually in a one-sided model with a partner who is taking all of the downside risk and a portion of the upside risk, leaving a small upside risk proposition that remained for the practice.”
He said the range of partners that were absorbing the downside risk included hospitals, device manufacturers, consulting companies, or private equity firms.
Despite the concerns surrounding APMs, Dr. Friedberg said that “we did not hear practices broadly saying that they just weren’t interested in alternative payment models. In general, practices still remained pretty enthusiastic about these alternative payment models in theory. If they could be made simpler, if the pace of change weren’t quite so fast, that they would have a chance to really do some important care improvements in alternative payment models.”
He noted some of the surveyed practices were able to make investments in care as a direct result of participating in APMs, such as in behavioral health capabilities in primary care, for example, leading to quality of care improvements.
However, these issues could reveal a future unwillingness to participate in APMs, especially two-sided risk models, something at least the Centers for Medicare & Medicaid Services are pushing for as a stated goal of the QPP is to get practices to participate in APMs and take on more risk.
The growing aversion to taking on downside risk could lead practices to simply stay in fee for service and simply take the payment penalty because it is a fixed amount that can be planned for, as opposed to the fluctuations of bonuses and penalties that comes with a rapidly changing APM environment, Dr. Friedberg said.
Going forward, the report makes a number of recommendations to help create an environment that would potentially make APMs more successful, including simplifying the models; creating stable, predictable, and moderately paced pathways to APM participation; making data available in a more timely fashion; minimizing downside risk or helping practices better manage it; and designing APMs that will encourage clinical changes to help improve the effectiveness of care delivered.
Physicians continue to support advanced alternative payment models despite the fact that operational issues have not improved over the last 4 years and new ones have cropped up, according to a follow-up survey conducted by the RAND Corporation for the American Medical Association.
“All the things we heard in 2014 were still present in 2018. Both the challenges that practices had experienced back in 2014 having to do with data timeliness, data completeness and accuracy, payment model execution, all those challenges persisted,” Mark W. Friedberg, MD, senior physician policy researcher at RAND, said in an interview.
RAND surveyed 31 practices of varying practice size and specialty across six geographic regions, some of which participated in the 2014 survey. Supplemental information was provided by interviews with 32 market observers, 8 health plan leaders, 10 hospital and hospital system leaders, 10 state and local medical society leaders, and 4 chapter leaders with MGMA (formerly the Medical Group Management Association).
“We had thought we would hear that the problem had gotten a little bit better since there has been some investment in trying to tamp down the wide range of measures that are involved in these alternative payment models,” Dr. Friedberg said. “We did not see any evidence of that having any effect on the practices that participated in this study this time around.”
Indeed, concerns reported in 2014 were again reported in 2018, along with a new set of concerns, including the perceived pace of change in alternative payment models (APMs), the complexity of APMs, and physician concerns over two-sided risk models.
“Practices, especially those that participated both times, said in 2014 we had these challenges [of rapid changes in APM models] and since then, things have just gotten a lot faster,” he said, noting that doctors are complaining of models that are going through changes, sometimes without much warning. “They are changing quite rapidly from year to year. If you look at the MACRA QPP [Quality Payment Program] for example, that model changes every year to some extent and those things are hard for them to keep up with.”
Running hand in hand with the change is the complexity of the changes, a result of expanding performance measures and uncertainty with thresholds for penalties and rewards and in some ways has had little impact on improving care.
Dr. Friedberg noted that some practices are hiring people to examine APMs to devise strategic ways to choose and report data for maximum return.
“In a practice, for example, if their quality of care was already very good, what these folks ended up doing was help them choose measures and work the attribution algorithms in a strategic way to either guarantee a bonus or minimize the risk of incurring a penalty,” he said.
He also noted that practices appear to becoming more risk averse.
“We heard a lot more of the following thing, which is that if [practices] were in a two-sided risk model, several of them reported trying and succeeding in some cases offloading the downside risk to partners,” Dr. Friedberg reported. “And what this resulted in was that the practice, even though from the payer’s perspective they are in a two-sided model, the practice was actually in a one-sided model with a partner who is taking all of the downside risk and a portion of the upside risk, leaving a small upside risk proposition that remained for the practice.”
He said the range of partners that were absorbing the downside risk included hospitals, device manufacturers, consulting companies, or private equity firms.
Despite the concerns surrounding APMs, Dr. Friedberg said that “we did not hear practices broadly saying that they just weren’t interested in alternative payment models. In general, practices still remained pretty enthusiastic about these alternative payment models in theory. If they could be made simpler, if the pace of change weren’t quite so fast, that they would have a chance to really do some important care improvements in alternative payment models.”
He noted some of the surveyed practices were able to make investments in care as a direct result of participating in APMs, such as in behavioral health capabilities in primary care, for example, leading to quality of care improvements.
However, these issues could reveal a future unwillingness to participate in APMs, especially two-sided risk models, something at least the Centers for Medicare & Medicaid Services are pushing for as a stated goal of the QPP is to get practices to participate in APMs and take on more risk.
The growing aversion to taking on downside risk could lead practices to simply stay in fee for service and simply take the payment penalty because it is a fixed amount that can be planned for, as opposed to the fluctuations of bonuses and penalties that comes with a rapidly changing APM environment, Dr. Friedberg said.
Going forward, the report makes a number of recommendations to help create an environment that would potentially make APMs more successful, including simplifying the models; creating stable, predictable, and moderately paced pathways to APM participation; making data available in a more timely fashion; minimizing downside risk or helping practices better manage it; and designing APMs that will encourage clinical changes to help improve the effectiveness of care delivered.
Physicians continue to support advanced alternative payment models despite the fact that operational issues have not improved over the last 4 years and new ones have cropped up, according to a follow-up survey conducted by the RAND Corporation for the American Medical Association.
“All the things we heard in 2014 were still present in 2018. Both the challenges that practices had experienced back in 2014 having to do with data timeliness, data completeness and accuracy, payment model execution, all those challenges persisted,” Mark W. Friedberg, MD, senior physician policy researcher at RAND, said in an interview.
RAND surveyed 31 practices of varying practice size and specialty across six geographic regions, some of which participated in the 2014 survey. Supplemental information was provided by interviews with 32 market observers, 8 health plan leaders, 10 hospital and hospital system leaders, 10 state and local medical society leaders, and 4 chapter leaders with MGMA (formerly the Medical Group Management Association).
“We had thought we would hear that the problem had gotten a little bit better since there has been some investment in trying to tamp down the wide range of measures that are involved in these alternative payment models,” Dr. Friedberg said. “We did not see any evidence of that having any effect on the practices that participated in this study this time around.”
Indeed, concerns reported in 2014 were again reported in 2018, along with a new set of concerns, including the perceived pace of change in alternative payment models (APMs), the complexity of APMs, and physician concerns over two-sided risk models.
“Practices, especially those that participated both times, said in 2014 we had these challenges [of rapid changes in APM models] and since then, things have just gotten a lot faster,” he said, noting that doctors are complaining of models that are going through changes, sometimes without much warning. “They are changing quite rapidly from year to year. If you look at the MACRA QPP [Quality Payment Program] for example, that model changes every year to some extent and those things are hard for them to keep up with.”
Running hand in hand with the change is the complexity of the changes, a result of expanding performance measures and uncertainty with thresholds for penalties and rewards and in some ways has had little impact on improving care.
Dr. Friedberg noted that some practices are hiring people to examine APMs to devise strategic ways to choose and report data for maximum return.
“In a practice, for example, if their quality of care was already very good, what these folks ended up doing was help them choose measures and work the attribution algorithms in a strategic way to either guarantee a bonus or minimize the risk of incurring a penalty,” he said.
He also noted that practices appear to becoming more risk averse.
“We heard a lot more of the following thing, which is that if [practices] were in a two-sided risk model, several of them reported trying and succeeding in some cases offloading the downside risk to partners,” Dr. Friedberg reported. “And what this resulted in was that the practice, even though from the payer’s perspective they are in a two-sided model, the practice was actually in a one-sided model with a partner who is taking all of the downside risk and a portion of the upside risk, leaving a small upside risk proposition that remained for the practice.”
He said the range of partners that were absorbing the downside risk included hospitals, device manufacturers, consulting companies, or private equity firms.
Despite the concerns surrounding APMs, Dr. Friedberg said that “we did not hear practices broadly saying that they just weren’t interested in alternative payment models. In general, practices still remained pretty enthusiastic about these alternative payment models in theory. If they could be made simpler, if the pace of change weren’t quite so fast, that they would have a chance to really do some important care improvements in alternative payment models.”
He noted some of the surveyed practices were able to make investments in care as a direct result of participating in APMs, such as in behavioral health capabilities in primary care, for example, leading to quality of care improvements.
However, these issues could reveal a future unwillingness to participate in APMs, especially two-sided risk models, something at least the Centers for Medicare & Medicaid Services are pushing for as a stated goal of the QPP is to get practices to participate in APMs and take on more risk.
The growing aversion to taking on downside risk could lead practices to simply stay in fee for service and simply take the payment penalty because it is a fixed amount that can be planned for, as opposed to the fluctuations of bonuses and penalties that comes with a rapidly changing APM environment, Dr. Friedberg said.
Going forward, the report makes a number of recommendations to help create an environment that would potentially make APMs more successful, including simplifying the models; creating stable, predictable, and moderately paced pathways to APM participation; making data available in a more timely fashion; minimizing downside risk or helping practices better manage it; and designing APMs that will encourage clinical changes to help improve the effectiveness of care delivered.
Does the preterm birth racial disparity persist among black and white IVF users?
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1
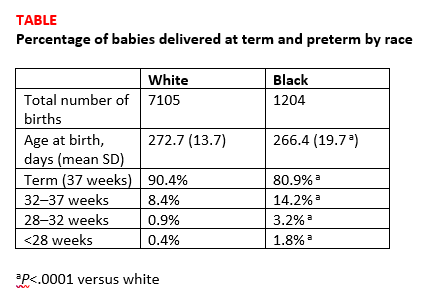
Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1

Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Investigators from the National Institutes of Health and Shady Grove Fertility found that among women having a singleton live birth resulting from in vitro fertilization (IVF) that black women are at higher risk for lower gestational age and preterm delivery than white women.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Kate Devine, MD, coinvestigator of the retrospective cohort study said in an interview with OBG Management that “It’s been well documented that African Americans have a higher preterm birth rate in the United States compared to Caucasians and the overall population. While the exact mechanism of preterm birth is unknown and likely varied, and while the mechanism for the preterm birth rate being higher in African Americans is not well understood, it has been hypothesized that socioeconomic factors are responsible at least in part.”2 She added that the investigators used a population of women receiving IVF for the study because “access to reproductive care and IVF is in some way a leveling factor in terms of socioeconomics.”
Details of the study. The investigators reviewed all singleton IVF pregnancies ending in live birth among women self-identifying as white, black, Asian, or Hispanic from 2004 to 2016 at a private IVF practice (N=10,371). The primary outcome was gestational age at birth, calculated as the number of days from oocyte retrieval to birth, plus 14, among white, black, Asian, and Hispanic women receiving IVF.
Births among black women occurred more than 6 days earlier than births among white women. The researchers noted that some of the shorter gestations among the black women could be explained by the higher average body mass index of the group (P<.0001). Dr. Devine explained that another contributing factor was the higher incidence of fibroid uterus among the black women (P<.0001). But after adjusting for these and other demographic variables, the black women still delivered 5.5 days earlier than the white women, and they were more than 3 times as likely to have either very preterm or extremely preterm deliveries (TABLE).1

Research implications. Dr. Devine said that black pregnant patients “perhaps should be monitored more closely” for signs or symptoms suggestive of preterm labor and would like to see more research into understanding the mechanisms of preterm birth that are resulting in greater rates of preterm birth among black women. She mentioned that research into how fibroids impact obstetric outcomes is also important.
Share your thoughts! Send your Letters to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
- Bishop LA, Devine K, Sasson I, et al. Lower gestational age and increased risk of preterm birth associated with singleton live birth resulting from in vitro fertilization (IVF) among African American versus comparable Caucasian women. Fertil Steril. 2018;110(45 suppl):e7.
Prior fertility treatment is associated with higher maternal morbidity during delivery
Investigators from the Stanford Hospital and Clinics in California found that while absolute risk is low, women who have received an infertility diagnosis or who have received fertility treatment are at higher risk of several markers of severe maternal morbidity than women who never received an infertility diagnosis or fertility treatment.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Gaya Murugappan, MD, lead investigator on the study, explained in an interview with OBG Management, “We know that in the last decade or so the rate of maternal morbidity has been rising gradually in the US, and we know that the utilization of fertility technology and the incidence of infertility are also rising.” The retrospective analysis set out to determine if a connection exists.
Methods. The investigators used a large insurance claims database to look at data from 2003 to 2016. They identified a group of infertile women who later conceived without fertility treatment (n=1822 deliveries) and a group of women who received fertility treatment (n=782 deliveries) and compared them with a control group of women who never received an infertility diagnosis or fertility treatment (n=37,944 deliveries). Women who currently or previously had cancer were excluded from the study.
The primary outcome was the number of indicators of severe maternal morbidity that occurred during the 6 months prior to or following delivery.
Findings. Compared with the control group, the women diagnosed with infertility were almost 4 times as likely to experience severe anesthesia complications (0.38% vs 0.11%; odds ratio [OR], 3.83; 95% confidence interval [CI], 1.69–8.70), about twice as likely to experience intraoperative heart failure (0.71% vs 0.31%; OR, 1.88; 95% CI, 1.05–3.34), and more than 3 times as likely to receive a hysterectomy (1.04% vs 0.28%; OR, 3.30; 95% CI, 2.02–5.40).
Similarly, compared with controls, women who had received fertility treatment had an OR of 2.66 for disseminated intravascular coagulation (2.81% vs 0.91%; 95% CI, 1.66–4.24), an OR of 5.17 for shock (0.90% vs 0.15%; 95% CI, 2.21–12.06), an OR of 1.61 for blood transfusions (3.71% vs 1.64%; 95% CI, 1.07–2.42), and an OR of 1.43 for cardiac monitoring (13.17% vs 8.14%; 95% CI, 1.14–1.79).
More research is needed. Dr. Murugappan noted, “I hope that these data help us identify high-risk populations of women so that we can minimize the occurrence of these potentially devastating health outcomes. Women need to be telling their ObGyns that they have a history of infertility and/or fertility treatment. Some women may not want to say that they conceived with donor egg, for example, but that could be a critical element of a patient’s history that an ObGyn should be aware of.”
More study is necessary, she added. For instance, “a study in the future looking at risk of maternal morbidity in patients who are infertile but then who go on to conceive spontaneously. Then we can tease out what is the effect of infertility versus the effect of fertility treatment.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of maternal morbidity in infertile women: analysis of US claims data. Fertil Steril. 2018;110(45 suppl):e9.
Investigators from the Stanford Hospital and Clinics in California found that while absolute risk is low, women who have received an infertility diagnosis or who have received fertility treatment are at higher risk of several markers of severe maternal morbidity than women who never received an infertility diagnosis or fertility treatment.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Gaya Murugappan, MD, lead investigator on the study, explained in an interview with OBG Management, “We know that in the last decade or so the rate of maternal morbidity has been rising gradually in the US, and we know that the utilization of fertility technology and the incidence of infertility are also rising.” The retrospective analysis set out to determine if a connection exists.
Methods. The investigators used a large insurance claims database to look at data from 2003 to 2016. They identified a group of infertile women who later conceived without fertility treatment (n=1822 deliveries) and a group of women who received fertility treatment (n=782 deliveries) and compared them with a control group of women who never received an infertility diagnosis or fertility treatment (n=37,944 deliveries). Women who currently or previously had cancer were excluded from the study.
The primary outcome was the number of indicators of severe maternal morbidity that occurred during the 6 months prior to or following delivery.
Findings. Compared with the control group, the women diagnosed with infertility were almost 4 times as likely to experience severe anesthesia complications (0.38% vs 0.11%; odds ratio [OR], 3.83; 95% confidence interval [CI], 1.69–8.70), about twice as likely to experience intraoperative heart failure (0.71% vs 0.31%; OR, 1.88; 95% CI, 1.05–3.34), and more than 3 times as likely to receive a hysterectomy (1.04% vs 0.28%; OR, 3.30; 95% CI, 2.02–5.40).
Similarly, compared with controls, women who had received fertility treatment had an OR of 2.66 for disseminated intravascular coagulation (2.81% vs 0.91%; 95% CI, 1.66–4.24), an OR of 5.17 for shock (0.90% vs 0.15%; 95% CI, 2.21–12.06), an OR of 1.61 for blood transfusions (3.71% vs 1.64%; 95% CI, 1.07–2.42), and an OR of 1.43 for cardiac monitoring (13.17% vs 8.14%; 95% CI, 1.14–1.79).
More research is needed. Dr. Murugappan noted, “I hope that these data help us identify high-risk populations of women so that we can minimize the occurrence of these potentially devastating health outcomes. Women need to be telling their ObGyns that they have a history of infertility and/or fertility treatment. Some women may not want to say that they conceived with donor egg, for example, but that could be a critical element of a patient’s history that an ObGyn should be aware of.”
More study is necessary, she added. For instance, “a study in the future looking at risk of maternal morbidity in patients who are infertile but then who go on to conceive spontaneously. Then we can tease out what is the effect of infertility versus the effect of fertility treatment.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Investigators from the Stanford Hospital and Clinics in California found that while absolute risk is low, women who have received an infertility diagnosis or who have received fertility treatment are at higher risk of several markers of severe maternal morbidity than women who never received an infertility diagnosis or fertility treatment.1 The study results were presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6 to 10, Denver, Colorado).
Gaya Murugappan, MD, lead investigator on the study, explained in an interview with OBG Management, “We know that in the last decade or so the rate of maternal morbidity has been rising gradually in the US, and we know that the utilization of fertility technology and the incidence of infertility are also rising.” The retrospective analysis set out to determine if a connection exists.
Methods. The investigators used a large insurance claims database to look at data from 2003 to 2016. They identified a group of infertile women who later conceived without fertility treatment (n=1822 deliveries) and a group of women who received fertility treatment (n=782 deliveries) and compared them with a control group of women who never received an infertility diagnosis or fertility treatment (n=37,944 deliveries). Women who currently or previously had cancer were excluded from the study.
The primary outcome was the number of indicators of severe maternal morbidity that occurred during the 6 months prior to or following delivery.
Findings. Compared with the control group, the women diagnosed with infertility were almost 4 times as likely to experience severe anesthesia complications (0.38% vs 0.11%; odds ratio [OR], 3.83; 95% confidence interval [CI], 1.69–8.70), about twice as likely to experience intraoperative heart failure (0.71% vs 0.31%; OR, 1.88; 95% CI, 1.05–3.34), and more than 3 times as likely to receive a hysterectomy (1.04% vs 0.28%; OR, 3.30; 95% CI, 2.02–5.40).
Similarly, compared with controls, women who had received fertility treatment had an OR of 2.66 for disseminated intravascular coagulation (2.81% vs 0.91%; 95% CI, 1.66–4.24), an OR of 5.17 for shock (0.90% vs 0.15%; 95% CI, 2.21–12.06), an OR of 1.61 for blood transfusions (3.71% vs 1.64%; 95% CI, 1.07–2.42), and an OR of 1.43 for cardiac monitoring (13.17% vs 8.14%; 95% CI, 1.14–1.79).
More research is needed. Dr. Murugappan noted, “I hope that these data help us identify high-risk populations of women so that we can minimize the occurrence of these potentially devastating health outcomes. Women need to be telling their ObGyns that they have a history of infertility and/or fertility treatment. Some women may not want to say that they conceived with donor egg, for example, but that could be a critical element of a patient’s history that an ObGyn should be aware of.”
More study is necessary, she added. For instance, “a study in the future looking at risk of maternal morbidity in patients who are infertile but then who go on to conceive spontaneously. Then we can tease out what is the effect of infertility versus the effect of fertility treatment.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of maternal morbidity in infertile women: analysis of US claims data. Fertil Steril. 2018;110(45 suppl):e9.
- Murugappan G, Li S, Lathi RB, Baker VL, Eisenberg ML. Increased risk of maternal morbidity in infertile women: analysis of US claims data. Fertil Steril. 2018;110(45 suppl):e9.
Should return to fertility be a concern for nulliparous patients using an IUD?
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1
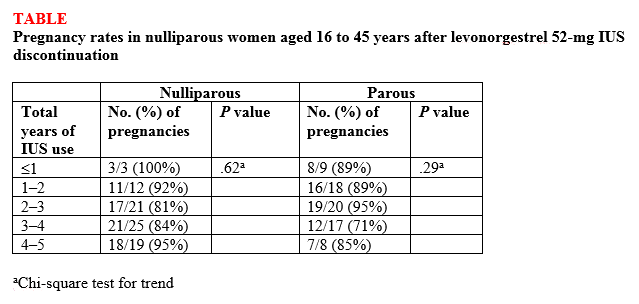
“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
Investigators from the University of Texas Southwestern are dispelling the myth that you shouldn’t recommend intrauterine devices (IUDs) for nulliparous women because the devices might make it more difficult for them to become pregnant after discontinuation. They found that nulliparous women can just as easily get pregnant after using a progestin intrauterine system (IUS) as parous women,1 according to results of a study presented at the American Society for Reproductive Medicine (ASRM) 2018 annual meeting (October 6–10, Denver, Colorado).
Bruce R. Carr, MD, lead investigator of the study, explained in an interview with OBG Management, “There have been a number of studies—maybe 10 to 15 years ago—that looked at pregnancy rates when patients stopped using IUDs, but most of these studies were done in women who were multiparous. There is almost no data on patients who are nulliparous stopping an IUD and trying to get pregnant.”
Participants and methods. This prospective, multicenter, clinical trial, which is still ongoing, is evaluating the efficacy and safety for up to 10 years of the Liletta levonorgestrel 52-mg IUS in nulliparous and parous women ages 16 to 45 years. Every 3 months for up to 1 year, the investigators contacted the women who discontinued the IUS during the first 5 years of use and who were trying to become pregnant to determine pregnancy status.
Outcomes. The primary outcome was time to pregnancy among nulliparous vs parous women after discontinuation of a progestin IUS.
Findings. Overall, 132 (87%) of 152 women ages 16 to 35 years at the beginning of the study who attempted to become pregnant did so within 1 year of discontinuing the IUS, and there was no difference in pregnancy rates between nulliparous and parous women (87.5% vs 86.1%, respectively; P<.82) or between nulligravid and gravid women (88.2% vs 85.7%, respectively; P<.81). High percentages of women became pregnant by the end of 3 months (43.4%) and 6 months (69.7%), with a median time to conception of 91.5 days. The women used the IUS for a median of 34 months before discontinuation. Length of IUS use and age of the women at IUS discontinuation did not affect pregnancy rates at 12 months postdiscontinuation in either nulliparous or parous women (TABLE).1

“The bottom line,” according to Dr. Carr, is that the “pregnancy rates were the same in women who had never been pregnant compared with women who had previously been pregnant.” He continued, “People worried that if a patient who had never been pregnant used an IUD that maybe she was going to have a harder time getting pregnant after discontinuing, and now we know that is not true. It [the study] reinforces the option of using progestin IUDs and not having to worry about future pregnancy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
This article was updated October 15, 2018.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
- Carr BR, Thomas MA, Gangestad A, Eisenberg DL, Olariu AI, Creinin MD. Return of fertility in nulliparous and parous women after levonorgestrel 52 mg intrauterine system discontinuation [ASRM abstract O-104]. Fertil Steril. 2018;110(45 suppl):e46.
Consider ART for younger endometriosis patients
but non-ART infertility treatment is less likely to succeed in the endometriosis population, according to data from approximately 1,800 women with infertility.
Moderate to severe endometriosis had a negative impact on the outcome of ART, but “the efficacy of non-ART treatment in patients with endometriosis remains elusive” wrote Wataru Isono of the University of Tokyo, and colleagues.
In a study published in the Journal of Obstetrics & Gynaecology Research, the investigators sought to determine the impact of endometriosis severity on the effectiveness of non-ART treatment. They calculated the cumulative live birth rates for women treated with ART and non-ART.
Overall, 49% of the 894 ART patients and 22% of the 1,358 non-ART patients gave birth after treatment. The birth rate remained more than twice as high among ART patients across all age groups, but declined among ART patients starting at 35 years of age and declined sharply as patients reached 40 years of age.
“The most important aspect of this study was that we determined the limitations of non-ART and sought to identify optimal management methods, as non-ART can be a more cost-effective treatment than ART in certain circumstances,” the researchers noted.
They then focused on 288 patients with advanced endometriosis, defined as stage III or IV according to the revised American Society for Reproductive Medicine score. Notably, the presence of moderate to severe endometriosis had a significant effect on the outcomes of non-ART patients in their 30s, but ART was effective in this age group in a multivariate analysis. The cumulative live birth rate in advanced endometriosis patients was significantly lower than in those without the condition who underwent non-ART treatment (10% vs. 25%). However, cumulative live birth rates after ART were not significantly different among advanced endometriosis patients, compared with patients without advanced endometriosis (45% vs. 51%).
The study was limited by several factors including the inability to analyze the varied types of infertility treatment, Dr. Isono and associates noted. However, “our results may provide infertility patients with accurate information regarding their expected probabilities of achieving a live birth and may help them select the optimal treatment based on their classification according to various risk factors,” they said.
The researchers had no financial conflicts to disclose. The study was supported by the Japan Society for the Promotion of Sciences; Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture; the Japan Agency for Medical Research and Development; and the Ministry of Health, Labor and Welfare.
SOURCE: Isono W et al. J Obstet Gynaecol Res. 2018. doi: 10.1111/jog.13826.
but non-ART infertility treatment is less likely to succeed in the endometriosis population, according to data from approximately 1,800 women with infertility.
Moderate to severe endometriosis had a negative impact on the outcome of ART, but “the efficacy of non-ART treatment in patients with endometriosis remains elusive” wrote Wataru Isono of the University of Tokyo, and colleagues.
In a study published in the Journal of Obstetrics & Gynaecology Research, the investigators sought to determine the impact of endometriosis severity on the effectiveness of non-ART treatment. They calculated the cumulative live birth rates for women treated with ART and non-ART.
Overall, 49% of the 894 ART patients and 22% of the 1,358 non-ART patients gave birth after treatment. The birth rate remained more than twice as high among ART patients across all age groups, but declined among ART patients starting at 35 years of age and declined sharply as patients reached 40 years of age.
“The most important aspect of this study was that we determined the limitations of non-ART and sought to identify optimal management methods, as non-ART can be a more cost-effective treatment than ART in certain circumstances,” the researchers noted.
They then focused on 288 patients with advanced endometriosis, defined as stage III or IV according to the revised American Society for Reproductive Medicine score. Notably, the presence of moderate to severe endometriosis had a significant effect on the outcomes of non-ART patients in their 30s, but ART was effective in this age group in a multivariate analysis. The cumulative live birth rate in advanced endometriosis patients was significantly lower than in those without the condition who underwent non-ART treatment (10% vs. 25%). However, cumulative live birth rates after ART were not significantly different among advanced endometriosis patients, compared with patients without advanced endometriosis (45% vs. 51%).
The study was limited by several factors including the inability to analyze the varied types of infertility treatment, Dr. Isono and associates noted. However, “our results may provide infertility patients with accurate information regarding their expected probabilities of achieving a live birth and may help them select the optimal treatment based on their classification according to various risk factors,” they said.
The researchers had no financial conflicts to disclose. The study was supported by the Japan Society for the Promotion of Sciences; Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture; the Japan Agency for Medical Research and Development; and the Ministry of Health, Labor and Welfare.
SOURCE: Isono W et al. J Obstet Gynaecol Res. 2018. doi: 10.1111/jog.13826.
but non-ART infertility treatment is less likely to succeed in the endometriosis population, according to data from approximately 1,800 women with infertility.
Moderate to severe endometriosis had a negative impact on the outcome of ART, but “the efficacy of non-ART treatment in patients with endometriosis remains elusive” wrote Wataru Isono of the University of Tokyo, and colleagues.
In a study published in the Journal of Obstetrics & Gynaecology Research, the investigators sought to determine the impact of endometriosis severity on the effectiveness of non-ART treatment. They calculated the cumulative live birth rates for women treated with ART and non-ART.
Overall, 49% of the 894 ART patients and 22% of the 1,358 non-ART patients gave birth after treatment. The birth rate remained more than twice as high among ART patients across all age groups, but declined among ART patients starting at 35 years of age and declined sharply as patients reached 40 years of age.
“The most important aspect of this study was that we determined the limitations of non-ART and sought to identify optimal management methods, as non-ART can be a more cost-effective treatment than ART in certain circumstances,” the researchers noted.
They then focused on 288 patients with advanced endometriosis, defined as stage III or IV according to the revised American Society for Reproductive Medicine score. Notably, the presence of moderate to severe endometriosis had a significant effect on the outcomes of non-ART patients in their 30s, but ART was effective in this age group in a multivariate analysis. The cumulative live birth rate in advanced endometriosis patients was significantly lower than in those without the condition who underwent non-ART treatment (10% vs. 25%). However, cumulative live birth rates after ART were not significantly different among advanced endometriosis patients, compared with patients without advanced endometriosis (45% vs. 51%).
The study was limited by several factors including the inability to analyze the varied types of infertility treatment, Dr. Isono and associates noted. However, “our results may provide infertility patients with accurate information regarding their expected probabilities of achieving a live birth and may help them select the optimal treatment based on their classification according to various risk factors,” they said.
The researchers had no financial conflicts to disclose. The study was supported by the Japan Society for the Promotion of Sciences; Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture; the Japan Agency for Medical Research and Development; and the Ministry of Health, Labor and Welfare.
SOURCE: Isono W et al. J Obstet Gynaecol Res. 2018. doi: 10.1111/jog.13826.
FROM THE JOURNAL OF OBSTETRICS AND GYNAECOLOGY RESEARCH
Key clinical point: ART was more effective than was non-ART against infertility in women with endometriosis, especially among younger women.
Major finding: The cumulative live birth rate in advanced endometriosis patients was significantly lower than in those without the condition who underwent non-ART treatment (10% vs. 25%).
Study details: A retrospective study of 1,864 infertile women in Japan, including 288 with advanced endometriosis.
Disclosures: The researchers had no financial conflicts to disclose. The study was supported by the Japan Society for the Promotion of Sciences; Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture; the Japan Agency for Medical Research and Development; and the Ministry of Health, Labor and Welfare.
Source: Isono W et al. J Obstet Gynaecol Res. 2018. doi: 10.1111/jog.13826.
All children deserve support for their gender identities
The Atlantic published the article “When Children Say They’re Trans” by Jesse Singal in its July/August edition not too long ago. In this article, the author wrote about the increasing availability of treatments for affirming one’s gender identity and the rising concerns about the risks surrounding those treatments.
A key issue in the article is the concept of desistance. Desistance is a phenomenon in which individuals no longer feel that their gender identities are incongruent with their physical appearance. Highly related to desistance is detransitioning, a phenomenon in which transgender individuals no longer take the steps (e.g., hormone therapy) to affirm their gender identity. Singal highlights the concern surrounding starting medical treatments to affirm an individual’s gender identity, considering that the changes are irreversible and that it is possible for children to change their minds. Implied in the article is a call for a cautious approach for treating children who identify as transgender because it will be difficult to predict what one’s final gender identity is; however, I believe that a better approach is to support the child in the journey in affirming the gender identity.
The evidence on the rate of desistance may not be accurate
One argument for the cautious approach is the often cited statistic that 80% of children with gender nonconforming behaviors do not identify as transgender when they are adults. This is derived from four published studies that track the gender identity of individuals with gender nonconforming behaviors in childhood.1-4 These estimates may not be accurate, mainly due to these studies’ methodological shortcomings. For example, those who were lost to follow-up were assumed to be cisgender as adults and no efforts were made to verify these individuals’ gender identity.2-4 I do not intend to thoroughly critique these studies in this column. This is best left to peer-reviewed commentaries (a good example is one written by Newhook et al. 2018).5 I worry, however, that some clinicians may dismiss a child’s gender identity based on these studies and recommend to the parents to delay supporting a transition until the child “knows for sure.” The problem with this approach is that it may worsen the health and well-being of transgender youth, as there is growing evidence that transgender children who are supported by their parents are less likely to have mental health problems.6,7
The reasons for desistance are far more complicated
The common narrative of desistance is that the individuals simply change their minds because they were “confused” during adolescence. However, the truth is more complicated. Children can identify their own gender as early as 2 years old;8 however, when a child’s gender identity matches the assigned sex at birth, this is often reinforced. In contrast, if a child’s gender identity does not match the assigned sex at birth, it often is challenged by peers and adults. This challenge by peers, their families, and medical providers may be one of the reasons why transitioning is so difficult for many transgender youth – and many do give up.3,9 In these cases, some people wait for years, if not decades, to come out again and start transitioning when they finally feel supported and safe – even in their 90s! Other transgender people realize that their gender identity is not on the binary (neither male nor female), so they no longer need cross-sex hormones or surgeries to affirm their gender identity. Finally, others are concerned about the side effects, such as infertility, and feel that the risks for those side effects are not worth it, so they find other, nonmedical or nonsurgical ways to affirm their gender identity or manage their gender dysphoria.
Positive outcomes are more common
Reports of youth detransitioning highlight many physicians’ fears of making a mistake; however, these reports obscure the more common – and positive – outcomes for transgender individuals who took steps to affirm their gender identity. The Report of the 2011 Transition Survey shows that 97% were satisfied with being on hormone therapy and 90% were satisfied with obtaining bottom surgery.10 Furthermore, there is growing evidence showing that such treatments are associated with better health.11 A study by de Vries et al. found that transgender youth who transitioned in adolescence had less depression and better adjustment as adults.12 Finally, there is a lack of evidence supporting the concept that someone whose gender identity is fluid over time is any less healthy than those whose gender identity is static over time. Rare outcomes should never be dismissed; however, providers should not use rare events as the primary driver for discouraging evidence-based treatment.
The key is support
I believe that every child’s gender identity should be supported and affirmed. Clinicians can provide this support and affirmation through the following actions:
- At the first visit, clinicians should ask what the child’s hopes and expectations are for pursuing gender-affirming medical treatments.
- Clinicians should allow the child the opportunity to describe and process their gender identity instead of assuming that they are on the binary.
- Clinicians must recognize the varied reasons for desistance – stigma, discrimination, shame, or need to fit within a gender binary – and find ways to address those factors.
- Clinicians should have a thorough discussion with patients and their families about the risks of not supporting the child’s gender identity versus the risk of medical or surgical treatments used to affirm one’s gender identity and process with the child and the family where the values and wishes are within the context of those risks.
- Most importantly, clinicians should emphasize support for whatever decisions the child makes to affirm their gender identity. Providing support is essential in promoting the health and well-being of any child.
Dr. Montano is assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Dev Psychol. 2008;44(1):34-45.
2. J Am Acad Child Adolesc Psychiatry. 2013 Jun;52(6):582-90.
3. Clin Child Psychol Psychiatry. 2011 Oct;16(4):499-516.
4. J Am Acad Child Adolesc Psychiatry. 2008 Dec;47(12):1413-23.
5. International Journal of Transgenderism. 2018;19(2):212-24.
6. Pediatrics. 2016 Mar;137(3):e20153223.
7. J Sex Marital Ther. 2010;36(1):6-23.
8. “Adolescence,” 11th ed. (New York: McGraw-Hill Education; 2016).
9. Graduate Journal of Social Science. 2010;7(2):26-43.
10. “Affirming Gender, Affirming Lives: A Report of the 2011 Transition Survey,” Gender Advocacy Training & Education, 2012.
11. Transgend Health. 2016 Jan;1(1):21-31.
12. Pediatrics. 2014 Oct;134(4):696-704.
The Atlantic published the article “When Children Say They’re Trans” by Jesse Singal in its July/August edition not too long ago. In this article, the author wrote about the increasing availability of treatments for affirming one’s gender identity and the rising concerns about the risks surrounding those treatments.
A key issue in the article is the concept of desistance. Desistance is a phenomenon in which individuals no longer feel that their gender identities are incongruent with their physical appearance. Highly related to desistance is detransitioning, a phenomenon in which transgender individuals no longer take the steps (e.g., hormone therapy) to affirm their gender identity. Singal highlights the concern surrounding starting medical treatments to affirm an individual’s gender identity, considering that the changes are irreversible and that it is possible for children to change their minds. Implied in the article is a call for a cautious approach for treating children who identify as transgender because it will be difficult to predict what one’s final gender identity is; however, I believe that a better approach is to support the child in the journey in affirming the gender identity.
The evidence on the rate of desistance may not be accurate
One argument for the cautious approach is the often cited statistic that 80% of children with gender nonconforming behaviors do not identify as transgender when they are adults. This is derived from four published studies that track the gender identity of individuals with gender nonconforming behaviors in childhood.1-4 These estimates may not be accurate, mainly due to these studies’ methodological shortcomings. For example, those who were lost to follow-up were assumed to be cisgender as adults and no efforts were made to verify these individuals’ gender identity.2-4 I do not intend to thoroughly critique these studies in this column. This is best left to peer-reviewed commentaries (a good example is one written by Newhook et al. 2018).5 I worry, however, that some clinicians may dismiss a child’s gender identity based on these studies and recommend to the parents to delay supporting a transition until the child “knows for sure.” The problem with this approach is that it may worsen the health and well-being of transgender youth, as there is growing evidence that transgender children who are supported by their parents are less likely to have mental health problems.6,7
The reasons for desistance are far more complicated
The common narrative of desistance is that the individuals simply change their minds because they were “confused” during adolescence. However, the truth is more complicated. Children can identify their own gender as early as 2 years old;8 however, when a child’s gender identity matches the assigned sex at birth, this is often reinforced. In contrast, if a child’s gender identity does not match the assigned sex at birth, it often is challenged by peers and adults. This challenge by peers, their families, and medical providers may be one of the reasons why transitioning is so difficult for many transgender youth – and many do give up.3,9 In these cases, some people wait for years, if not decades, to come out again and start transitioning when they finally feel supported and safe – even in their 90s! Other transgender people realize that their gender identity is not on the binary (neither male nor female), so they no longer need cross-sex hormones or surgeries to affirm their gender identity. Finally, others are concerned about the side effects, such as infertility, and feel that the risks for those side effects are not worth it, so they find other, nonmedical or nonsurgical ways to affirm their gender identity or manage their gender dysphoria.
Positive outcomes are more common
Reports of youth detransitioning highlight many physicians’ fears of making a mistake; however, these reports obscure the more common – and positive – outcomes for transgender individuals who took steps to affirm their gender identity. The Report of the 2011 Transition Survey shows that 97% were satisfied with being on hormone therapy and 90% were satisfied with obtaining bottom surgery.10 Furthermore, there is growing evidence showing that such treatments are associated with better health.11 A study by de Vries et al. found that transgender youth who transitioned in adolescence had less depression and better adjustment as adults.12 Finally, there is a lack of evidence supporting the concept that someone whose gender identity is fluid over time is any less healthy than those whose gender identity is static over time. Rare outcomes should never be dismissed; however, providers should not use rare events as the primary driver for discouraging evidence-based treatment.
The key is support
I believe that every child’s gender identity should be supported and affirmed. Clinicians can provide this support and affirmation through the following actions:
- At the first visit, clinicians should ask what the child’s hopes and expectations are for pursuing gender-affirming medical treatments.
- Clinicians should allow the child the opportunity to describe and process their gender identity instead of assuming that they are on the binary.
- Clinicians must recognize the varied reasons for desistance – stigma, discrimination, shame, or need to fit within a gender binary – and find ways to address those factors.
- Clinicians should have a thorough discussion with patients and their families about the risks of not supporting the child’s gender identity versus the risk of medical or surgical treatments used to affirm one’s gender identity and process with the child and the family where the values and wishes are within the context of those risks.
- Most importantly, clinicians should emphasize support for whatever decisions the child makes to affirm their gender identity. Providing support is essential in promoting the health and well-being of any child.
Dr. Montano is assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Dev Psychol. 2008;44(1):34-45.
2. J Am Acad Child Adolesc Psychiatry. 2013 Jun;52(6):582-90.
3. Clin Child Psychol Psychiatry. 2011 Oct;16(4):499-516.
4. J Am Acad Child Adolesc Psychiatry. 2008 Dec;47(12):1413-23.
5. International Journal of Transgenderism. 2018;19(2):212-24.
6. Pediatrics. 2016 Mar;137(3):e20153223.
7. J Sex Marital Ther. 2010;36(1):6-23.
8. “Adolescence,” 11th ed. (New York: McGraw-Hill Education; 2016).
9. Graduate Journal of Social Science. 2010;7(2):26-43.
10. “Affirming Gender, Affirming Lives: A Report of the 2011 Transition Survey,” Gender Advocacy Training & Education, 2012.
11. Transgend Health. 2016 Jan;1(1):21-31.
12. Pediatrics. 2014 Oct;134(4):696-704.
The Atlantic published the article “When Children Say They’re Trans” by Jesse Singal in its July/August edition not too long ago. In this article, the author wrote about the increasing availability of treatments for affirming one’s gender identity and the rising concerns about the risks surrounding those treatments.
A key issue in the article is the concept of desistance. Desistance is a phenomenon in which individuals no longer feel that their gender identities are incongruent with their physical appearance. Highly related to desistance is detransitioning, a phenomenon in which transgender individuals no longer take the steps (e.g., hormone therapy) to affirm their gender identity. Singal highlights the concern surrounding starting medical treatments to affirm an individual’s gender identity, considering that the changes are irreversible and that it is possible for children to change their minds. Implied in the article is a call for a cautious approach for treating children who identify as transgender because it will be difficult to predict what one’s final gender identity is; however, I believe that a better approach is to support the child in the journey in affirming the gender identity.
The evidence on the rate of desistance may not be accurate
One argument for the cautious approach is the often cited statistic that 80% of children with gender nonconforming behaviors do not identify as transgender when they are adults. This is derived from four published studies that track the gender identity of individuals with gender nonconforming behaviors in childhood.1-4 These estimates may not be accurate, mainly due to these studies’ methodological shortcomings. For example, those who were lost to follow-up were assumed to be cisgender as adults and no efforts were made to verify these individuals’ gender identity.2-4 I do not intend to thoroughly critique these studies in this column. This is best left to peer-reviewed commentaries (a good example is one written by Newhook et al. 2018).5 I worry, however, that some clinicians may dismiss a child’s gender identity based on these studies and recommend to the parents to delay supporting a transition until the child “knows for sure.” The problem with this approach is that it may worsen the health and well-being of transgender youth, as there is growing evidence that transgender children who are supported by their parents are less likely to have mental health problems.6,7
The reasons for desistance are far more complicated
The common narrative of desistance is that the individuals simply change their minds because they were “confused” during adolescence. However, the truth is more complicated. Children can identify their own gender as early as 2 years old;8 however, when a child’s gender identity matches the assigned sex at birth, this is often reinforced. In contrast, if a child’s gender identity does not match the assigned sex at birth, it often is challenged by peers and adults. This challenge by peers, their families, and medical providers may be one of the reasons why transitioning is so difficult for many transgender youth – and many do give up.3,9 In these cases, some people wait for years, if not decades, to come out again and start transitioning when they finally feel supported and safe – even in their 90s! Other transgender people realize that their gender identity is not on the binary (neither male nor female), so they no longer need cross-sex hormones or surgeries to affirm their gender identity. Finally, others are concerned about the side effects, such as infertility, and feel that the risks for those side effects are not worth it, so they find other, nonmedical or nonsurgical ways to affirm their gender identity or manage their gender dysphoria.
Positive outcomes are more common
Reports of youth detransitioning highlight many physicians’ fears of making a mistake; however, these reports obscure the more common – and positive – outcomes for transgender individuals who took steps to affirm their gender identity. The Report of the 2011 Transition Survey shows that 97% were satisfied with being on hormone therapy and 90% were satisfied with obtaining bottom surgery.10 Furthermore, there is growing evidence showing that such treatments are associated with better health.11 A study by de Vries et al. found that transgender youth who transitioned in adolescence had less depression and better adjustment as adults.12 Finally, there is a lack of evidence supporting the concept that someone whose gender identity is fluid over time is any less healthy than those whose gender identity is static over time. Rare outcomes should never be dismissed; however, providers should not use rare events as the primary driver for discouraging evidence-based treatment.
The key is support
I believe that every child’s gender identity should be supported and affirmed. Clinicians can provide this support and affirmation through the following actions:
- At the first visit, clinicians should ask what the child’s hopes and expectations are for pursuing gender-affirming medical treatments.
- Clinicians should allow the child the opportunity to describe and process their gender identity instead of assuming that they are on the binary.
- Clinicians must recognize the varied reasons for desistance – stigma, discrimination, shame, or need to fit within a gender binary – and find ways to address those factors.
- Clinicians should have a thorough discussion with patients and their families about the risks of not supporting the child’s gender identity versus the risk of medical or surgical treatments used to affirm one’s gender identity and process with the child and the family where the values and wishes are within the context of those risks.
- Most importantly, clinicians should emphasize support for whatever decisions the child makes to affirm their gender identity. Providing support is essential in promoting the health and well-being of any child.
Dr. Montano is assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. Dev Psychol. 2008;44(1):34-45.
2. J Am Acad Child Adolesc Psychiatry. 2013 Jun;52(6):582-90.
3. Clin Child Psychol Psychiatry. 2011 Oct;16(4):499-516.
4. J Am Acad Child Adolesc Psychiatry. 2008 Dec;47(12):1413-23.
5. International Journal of Transgenderism. 2018;19(2):212-24.
6. Pediatrics. 2016 Mar;137(3):e20153223.
7. J Sex Marital Ther. 2010;36(1):6-23.
8. “Adolescence,” 11th ed. (New York: McGraw-Hill Education; 2016).
9. Graduate Journal of Social Science. 2010;7(2):26-43.
10. “Affirming Gender, Affirming Lives: A Report of the 2011 Transition Survey,” Gender Advocacy Training & Education, 2012.
11. Transgend Health. 2016 Jan;1(1):21-31.
12. Pediatrics. 2014 Oct;134(4):696-704.
Ulipristal reduces bleeding with contraceptive implant
Ulipristal acetate reduces breakthrough bleeding in women with etonogestrel implants, according to new findings.
After 30 days, patients treated with ulipristal were more satisfied with their bleeding profiles than were those given placebo, reported Rachel E. Zigler, MD, of the department of obstetrics and gynecology at Washington University in St. Louis and her colleagues.
About half of women experience unscheduled bleeding within the first 6 months of etonogestrel implantation, causing many to discontinue treatment. The etiology of this phenomenon is poorly understood.
“One leading theory is that sustained exposure to a progestin can lead to endometrial angiogenesis disruption, resulting in the development of a dense venous network that is fragile and prone to bleeding,” the researchers wrote in Obstetrics & Gynecology.
Ulipristal acetate is a selective progesterone receptor modulator approved for emergency contraception in the United States. Outside the United States, it is used to treat abnormal uterine bleeding in cases of uterine leiomyoma. Ulipristal acts directly upon myometrial and endometrial tissue and “may also displace local progestin within the uterus to counteract bleeding secondary to … a dense, fragile venous network.”
The double-blind, placebo-controlled study included 65 women aged 18-45 years with etonogestrel implants. Eligibility required that implants be in place for more than 90 days and less than 3 years and that participants experienced more than one bleeding episode over a 24-day time frame.
Patients received either 15 mg ulipristal (n = 32) or placebo (n = 33) daily for 7 days. From the first day of treatment until 30 days, patients self-reported bleeding events. Weekly phone questionnaires also were conducted to determine satisfaction with medication and side effects.
Ten days after starting treatment, bleeding resolved in 34% of patients treated with ulipristal versus 10% of patients given placebo (P = .03).
The ulipristal group reported a median of 5 fewer bleeding days, compared with the placebo group, over the month-long evaluation (7 vs. 12; P = .002). Treatment satisfaction rates were also better in the ulipristal group, with nearly three-quarters (72%) “very happy” with results versus about one-quarter (27%) of women who received placebo.
Consequently, more ulipristal patients desired to keep their implants, compared with placebo patients. All patients receiving ulipristal would consider ulipristal for breakthrough bleeding in the future, compared with two-thirds of patients in the placebo group.
Side effects were uncommon for both groups; the most common side effect was headache, reported in 9% in the ulipristal group and 19% in the placebo group.
“Increased satisfaction with the etonogestrel implant may lead to a decrease in discontinuation rates and potentially a decrease in unintended pregnancy rates in this population,” the researchers wrote.
Study funding was provided by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences. The authors reported financial disclosures related to Bayer and Merck.
SOURCE: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
The recent trial by Zigler et al. showed that ulipristal acetate may reduce bleeding days in contraceptive implant users, but larger studies are needed, and concerns about logistics, dose, and toxicity must be addressed before clinical roll out, according to Eve Espey, MD.
The FDA halted the present trial after another ulipristal study overseas detected liver toxicity. The overseas study (for uterine leiomyoma) involved daily administration of ulipristal (5 mg) for 3 months, which is significantly longer than the present study for breakthrough bleeding. The European Medicines Agency has since determined that women with liver issues should not receive ulipristal and that others should have close liver monitoring before, during, and after ulipristal therapy.
Despite the above concerns, ulipristal still holds promise for a common clinical problem.
“This study contributes to the literature on management of bothersome bleeding with the contraceptive implant,” Dr. Espey said. “It is an important area because bothersome bleeding leads both to dissatisfaction and method discontinuation. As a recent Cochrane review pointed out, although several different medications have been used, studies are small and not yet conclusive. Despite these caveats, the findings were promising, similar to findings of prior work with a similar compound, mifepristone. Future directions would include clinical trials utilizing ulipristal acetate in a larger population powered for discontinuation.”
Dr. Espey is a professor in and chair of the department of obstetrics and gynecology and the family planning fellowship director at the University of New Mexico, Albuquerque. These comments are adapted from an interview. Dr. Espey said she had no relevant financial disclosures.
The recent trial by Zigler et al. showed that ulipristal acetate may reduce bleeding days in contraceptive implant users, but larger studies are needed, and concerns about logistics, dose, and toxicity must be addressed before clinical roll out, according to Eve Espey, MD.
The FDA halted the present trial after another ulipristal study overseas detected liver toxicity. The overseas study (for uterine leiomyoma) involved daily administration of ulipristal (5 mg) for 3 months, which is significantly longer than the present study for breakthrough bleeding. The European Medicines Agency has since determined that women with liver issues should not receive ulipristal and that others should have close liver monitoring before, during, and after ulipristal therapy.
Despite the above concerns, ulipristal still holds promise for a common clinical problem.
“This study contributes to the literature on management of bothersome bleeding with the contraceptive implant,” Dr. Espey said. “It is an important area because bothersome bleeding leads both to dissatisfaction and method discontinuation. As a recent Cochrane review pointed out, although several different medications have been used, studies are small and not yet conclusive. Despite these caveats, the findings were promising, similar to findings of prior work with a similar compound, mifepristone. Future directions would include clinical trials utilizing ulipristal acetate in a larger population powered for discontinuation.”
Dr. Espey is a professor in and chair of the department of obstetrics and gynecology and the family planning fellowship director at the University of New Mexico, Albuquerque. These comments are adapted from an interview. Dr. Espey said she had no relevant financial disclosures.
The recent trial by Zigler et al. showed that ulipristal acetate may reduce bleeding days in contraceptive implant users, but larger studies are needed, and concerns about logistics, dose, and toxicity must be addressed before clinical roll out, according to Eve Espey, MD.
The FDA halted the present trial after another ulipristal study overseas detected liver toxicity. The overseas study (for uterine leiomyoma) involved daily administration of ulipristal (5 mg) for 3 months, which is significantly longer than the present study for breakthrough bleeding. The European Medicines Agency has since determined that women with liver issues should not receive ulipristal and that others should have close liver monitoring before, during, and after ulipristal therapy.
Despite the above concerns, ulipristal still holds promise for a common clinical problem.
“This study contributes to the literature on management of bothersome bleeding with the contraceptive implant,” Dr. Espey said. “It is an important area because bothersome bleeding leads both to dissatisfaction and method discontinuation. As a recent Cochrane review pointed out, although several different medications have been used, studies are small and not yet conclusive. Despite these caveats, the findings were promising, similar to findings of prior work with a similar compound, mifepristone. Future directions would include clinical trials utilizing ulipristal acetate in a larger population powered for discontinuation.”
Dr. Espey is a professor in and chair of the department of obstetrics and gynecology and the family planning fellowship director at the University of New Mexico, Albuquerque. These comments are adapted from an interview. Dr. Espey said she had no relevant financial disclosures.
Ulipristal acetate reduces breakthrough bleeding in women with etonogestrel implants, according to new findings.
After 30 days, patients treated with ulipristal were more satisfied with their bleeding profiles than were those given placebo, reported Rachel E. Zigler, MD, of the department of obstetrics and gynecology at Washington University in St. Louis and her colleagues.
About half of women experience unscheduled bleeding within the first 6 months of etonogestrel implantation, causing many to discontinue treatment. The etiology of this phenomenon is poorly understood.
“One leading theory is that sustained exposure to a progestin can lead to endometrial angiogenesis disruption, resulting in the development of a dense venous network that is fragile and prone to bleeding,” the researchers wrote in Obstetrics & Gynecology.
Ulipristal acetate is a selective progesterone receptor modulator approved for emergency contraception in the United States. Outside the United States, it is used to treat abnormal uterine bleeding in cases of uterine leiomyoma. Ulipristal acts directly upon myometrial and endometrial tissue and “may also displace local progestin within the uterus to counteract bleeding secondary to … a dense, fragile venous network.”
The double-blind, placebo-controlled study included 65 women aged 18-45 years with etonogestrel implants. Eligibility required that implants be in place for more than 90 days and less than 3 years and that participants experienced more than one bleeding episode over a 24-day time frame.
Patients received either 15 mg ulipristal (n = 32) or placebo (n = 33) daily for 7 days. From the first day of treatment until 30 days, patients self-reported bleeding events. Weekly phone questionnaires also were conducted to determine satisfaction with medication and side effects.
Ten days after starting treatment, bleeding resolved in 34% of patients treated with ulipristal versus 10% of patients given placebo (P = .03).
The ulipristal group reported a median of 5 fewer bleeding days, compared with the placebo group, over the month-long evaluation (7 vs. 12; P = .002). Treatment satisfaction rates were also better in the ulipristal group, with nearly three-quarters (72%) “very happy” with results versus about one-quarter (27%) of women who received placebo.
Consequently, more ulipristal patients desired to keep their implants, compared with placebo patients. All patients receiving ulipristal would consider ulipristal for breakthrough bleeding in the future, compared with two-thirds of patients in the placebo group.
Side effects were uncommon for both groups; the most common side effect was headache, reported in 9% in the ulipristal group and 19% in the placebo group.
“Increased satisfaction with the etonogestrel implant may lead to a decrease in discontinuation rates and potentially a decrease in unintended pregnancy rates in this population,” the researchers wrote.
Study funding was provided by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences. The authors reported financial disclosures related to Bayer and Merck.
SOURCE: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
Ulipristal acetate reduces breakthrough bleeding in women with etonogestrel implants, according to new findings.
After 30 days, patients treated with ulipristal were more satisfied with their bleeding profiles than were those given placebo, reported Rachel E. Zigler, MD, of the department of obstetrics and gynecology at Washington University in St. Louis and her colleagues.
About half of women experience unscheduled bleeding within the first 6 months of etonogestrel implantation, causing many to discontinue treatment. The etiology of this phenomenon is poorly understood.
“One leading theory is that sustained exposure to a progestin can lead to endometrial angiogenesis disruption, resulting in the development of a dense venous network that is fragile and prone to bleeding,” the researchers wrote in Obstetrics & Gynecology.
Ulipristal acetate is a selective progesterone receptor modulator approved for emergency contraception in the United States. Outside the United States, it is used to treat abnormal uterine bleeding in cases of uterine leiomyoma. Ulipristal acts directly upon myometrial and endometrial tissue and “may also displace local progestin within the uterus to counteract bleeding secondary to … a dense, fragile venous network.”
The double-blind, placebo-controlled study included 65 women aged 18-45 years with etonogestrel implants. Eligibility required that implants be in place for more than 90 days and less than 3 years and that participants experienced more than one bleeding episode over a 24-day time frame.
Patients received either 15 mg ulipristal (n = 32) or placebo (n = 33) daily for 7 days. From the first day of treatment until 30 days, patients self-reported bleeding events. Weekly phone questionnaires also were conducted to determine satisfaction with medication and side effects.
Ten days after starting treatment, bleeding resolved in 34% of patients treated with ulipristal versus 10% of patients given placebo (P = .03).
The ulipristal group reported a median of 5 fewer bleeding days, compared with the placebo group, over the month-long evaluation (7 vs. 12; P = .002). Treatment satisfaction rates were also better in the ulipristal group, with nearly three-quarters (72%) “very happy” with results versus about one-quarter (27%) of women who received placebo.
Consequently, more ulipristal patients desired to keep their implants, compared with placebo patients. All patients receiving ulipristal would consider ulipristal for breakthrough bleeding in the future, compared with two-thirds of patients in the placebo group.
Side effects were uncommon for both groups; the most common side effect was headache, reported in 9% in the ulipristal group and 19% in the placebo group.
“Increased satisfaction with the etonogestrel implant may lead to a decrease in discontinuation rates and potentially a decrease in unintended pregnancy rates in this population,” the researchers wrote.
Study funding was provided by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences. The authors reported financial disclosures related to Bayer and Merck.
SOURCE: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Treatment with ulipristal was associated with 5 fewer bleeding days per month, compared with placebo (P = .002).
Study details: The double-blind, placebo-controlled trial involved 65 women with etonogestrel implants who reported more than one bleeding episode in a 24-day time frame.
Disclosures: The study was funded by the Society of Family Planning Research Fund, the Washington University Institute of Clinical and Translational Sciences, and the National Center for Advancing Translational Sciences (NCATS). The authors reported financial disclosures related to Bayer and Merck.
Source: Zigler RE et al. Obstet Gynecol. 2018;132:888-94.
One-step gestational diabetes screening doesn’t improve outcomes
according to data from a before-and-after cohort study of women in the state of Washington.
The one-step test, a 75-g 2-hour oral glucose tolerance test (OGTT), was recommended for all pregnant women in 2010, although the traditional two-step test – a 50-g screening glucose challenge test followed by a 100-g 3-hour OGTT – remains widely used, wrote Gaia Pocobelli, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues. “No randomized trial has been published comparing outcomes of the two approaches.”
In a study published in Obstetrics & Gynecology, the researchers compared data from 23,257 women who received prenatal care in Washington State between January 2009 and December 2014, including 8,363 women who received care before the guideline change, 4,103 who received care during a transition period, and 10,791 after the guideline change. Approximately 60% of the women received care from clinicians internal to Kaiser Permanente; 40% received care from external providers. Most (87%) of the internal clinicians switched to the one-step approach, the researchers said. Only 5% of external providers did so.
Overall, adopting the one-step approach was associated with a 41% increase in the diagnosis of GDM without improved maternal or neonatal outcomes, the researchers noted.
The incidence of GDM increased from 7% before the guideline change to 11% afterward for women seen by internal providers. For women seen by external providers, gestational diabetes incidence increased from 10% to 11%.
For women seen by internal providers, the use of insulin increased from 1% before the guideline change to 4% afterward; for women seen by external providers, use of insulin increased from 1.3% to 1.4% (change between the groups P less than .001).
In addition, women seen by internal providers were more likely to undergo induction of labor after the guideline change (25% to 29%), while labor induction decreased for women seen by external providers (31% to 29%) for a relative risk of 1.2.
Neonatal hypoglycemia increased from 1% to 2% among women seen by internal providers, but decreased slightly from 2.4% to 2.1% for women seen by external providers, for a relative risk of 1.77.
There were no significant differences between the women seen by internal and external providers in risk of primary cesarean section, large for gestational age, small for gestational age, or neonatal ICU admission.
The main limitation of the study was the potential confounding variables including maternal diet and exercise, and possible underreporting of risk factors such as smoking, the researchers noted. However, the results were strengthened by the large study population, and the results “do not suggest a benefit of adopting the one-step over the two-step approach.
“Kaiser Permanente Washington has revised [its] guidelines to return to a two-step process. We recommend that any health care system considering switching to the one-step approach incorporate a rigorous evaluation of changes in maternal and neonatal outcomes,” Dr. Pocobelli and her associates added.
Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
Diabetes is a significant global public health concern, but is especially problematic for women of reproductive age because diabetes in pregnancy can cause significant health complications for the mother and baby. Gestational diabetes mellitus (GDM) affects up to 10% of pregnancies in the United States annually, and is associated with perinatal loss, operative delivery, macrosomia, hypoglycemia, respiratory distress syndrome, and metabolic derangements for the offspring. For the mother, GDM is associated with hypertensive disorders, infections, hydramnios, and increased risk for developing type 2 diabetes later in life. As the incidence of GDM continues to rise, studies examining how to reduce, manage or prevent this condition become increasingly important.
The authors’ conclusions, that adopting the one-step approach increased the number of women with diagnosed GDM but did not significantly improve maternal or neonatal outcomes, are not surprising. Since the initial publication of the Hyperglycemia and Adverse Pregnancy Outcome Study, upon which the International Association of the Diabetes in Pregnancy Study Groups based its recommendations to go to a one-step approach, much debate has ensued about the best method to diagnose GDM. Indeed, the National Institutes of Health convened a consensus panel to review the literature and determine whether the one-step approach should be universally adopted (the panel concluded that more information was needed, and that the current two-step approach should continue to be used).
As the authors concede, studies have shown conflicting results, and no large-scale randomized controlled trial has been conducted to date. However, the literature does not bear out the idea that the one-step approach is truly better. The current study, although including a significant number of women and a reasonable control group, only serves as yet another study to reinforce what has previously been published.
I would agree with the researchers’ conclusions that the one-step approach is not necessarily beneficial. Although the one-step approach may identify a subset of patients who might not otherwise be diagnosed with GDM, it still remains unclear whether the outcomes for these patients will be improved. Furthermore, additional testing, need for insulin or other oral antidiabetic medications, etc., would result in additional stress to the patient and the health care system. Based on the authors’ findings, and results of other studies, it remains to be determined if the effort (diagnosing additional patients with GDM) is justified medically, economically, or otherwise.
As ob.gyns., we must continually ask ourselves: “By not doing something, are we causing harm to our patients?” If we change the diagnostic criteria for GDM, thereby increasing the number of women with the condition who would then require additional care, medications, and, potentially, more complex decisions around timing and mode of delivery, we need to be certain that we are not doing harm. This, and other studies examining the use of the one- versus two-step approach have yet to demonstrate, unequivocally, that changing the criteria reduces harm, and, perhaps, might – unintentionally – cause more.
As the study authors and the NIH consensus panel concluded, more rigorous evaluation is needed; that is, a large, multicenter randomized controlled trial that examines not only the benefits during pregnancy but also the long-term benefits to women and their children.
E. Albert Reece, MD, PhD, MBA, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He provided commentary on the study by Pocobelli et al. Dr. Reece said he had no relevant financial disclosures.
Diabetes is a significant global public health concern, but is especially problematic for women of reproductive age because diabetes in pregnancy can cause significant health complications for the mother and baby. Gestational diabetes mellitus (GDM) affects up to 10% of pregnancies in the United States annually, and is associated with perinatal loss, operative delivery, macrosomia, hypoglycemia, respiratory distress syndrome, and metabolic derangements for the offspring. For the mother, GDM is associated with hypertensive disorders, infections, hydramnios, and increased risk for developing type 2 diabetes later in life. As the incidence of GDM continues to rise, studies examining how to reduce, manage or prevent this condition become increasingly important.
The authors’ conclusions, that adopting the one-step approach increased the number of women with diagnosed GDM but did not significantly improve maternal or neonatal outcomes, are not surprising. Since the initial publication of the Hyperglycemia and Adverse Pregnancy Outcome Study, upon which the International Association of the Diabetes in Pregnancy Study Groups based its recommendations to go to a one-step approach, much debate has ensued about the best method to diagnose GDM. Indeed, the National Institutes of Health convened a consensus panel to review the literature and determine whether the one-step approach should be universally adopted (the panel concluded that more information was needed, and that the current two-step approach should continue to be used).
As the authors concede, studies have shown conflicting results, and no large-scale randomized controlled trial has been conducted to date. However, the literature does not bear out the idea that the one-step approach is truly better. The current study, although including a significant number of women and a reasonable control group, only serves as yet another study to reinforce what has previously been published.
I would agree with the researchers’ conclusions that the one-step approach is not necessarily beneficial. Although the one-step approach may identify a subset of patients who might not otherwise be diagnosed with GDM, it still remains unclear whether the outcomes for these patients will be improved. Furthermore, additional testing, need for insulin or other oral antidiabetic medications, etc., would result in additional stress to the patient and the health care system. Based on the authors’ findings, and results of other studies, it remains to be determined if the effort (diagnosing additional patients with GDM) is justified medically, economically, or otherwise.
As ob.gyns., we must continually ask ourselves: “By not doing something, are we causing harm to our patients?” If we change the diagnostic criteria for GDM, thereby increasing the number of women with the condition who would then require additional care, medications, and, potentially, more complex decisions around timing and mode of delivery, we need to be certain that we are not doing harm. This, and other studies examining the use of the one- versus two-step approach have yet to demonstrate, unequivocally, that changing the criteria reduces harm, and, perhaps, might – unintentionally – cause more.
As the study authors and the NIH consensus panel concluded, more rigorous evaluation is needed; that is, a large, multicenter randomized controlled trial that examines not only the benefits during pregnancy but also the long-term benefits to women and their children.
E. Albert Reece, MD, PhD, MBA, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He provided commentary on the study by Pocobelli et al. Dr. Reece said he had no relevant financial disclosures.
Diabetes is a significant global public health concern, but is especially problematic for women of reproductive age because diabetes in pregnancy can cause significant health complications for the mother and baby. Gestational diabetes mellitus (GDM) affects up to 10% of pregnancies in the United States annually, and is associated with perinatal loss, operative delivery, macrosomia, hypoglycemia, respiratory distress syndrome, and metabolic derangements for the offspring. For the mother, GDM is associated with hypertensive disorders, infections, hydramnios, and increased risk for developing type 2 diabetes later in life. As the incidence of GDM continues to rise, studies examining how to reduce, manage or prevent this condition become increasingly important.
The authors’ conclusions, that adopting the one-step approach increased the number of women with diagnosed GDM but did not significantly improve maternal or neonatal outcomes, are not surprising. Since the initial publication of the Hyperglycemia and Adverse Pregnancy Outcome Study, upon which the International Association of the Diabetes in Pregnancy Study Groups based its recommendations to go to a one-step approach, much debate has ensued about the best method to diagnose GDM. Indeed, the National Institutes of Health convened a consensus panel to review the literature and determine whether the one-step approach should be universally adopted (the panel concluded that more information was needed, and that the current two-step approach should continue to be used).
As the authors concede, studies have shown conflicting results, and no large-scale randomized controlled trial has been conducted to date. However, the literature does not bear out the idea that the one-step approach is truly better. The current study, although including a significant number of women and a reasonable control group, only serves as yet another study to reinforce what has previously been published.
I would agree with the researchers’ conclusions that the one-step approach is not necessarily beneficial. Although the one-step approach may identify a subset of patients who might not otherwise be diagnosed with GDM, it still remains unclear whether the outcomes for these patients will be improved. Furthermore, additional testing, need for insulin or other oral antidiabetic medications, etc., would result in additional stress to the patient and the health care system. Based on the authors’ findings, and results of other studies, it remains to be determined if the effort (diagnosing additional patients with GDM) is justified medically, economically, or otherwise.
As ob.gyns., we must continually ask ourselves: “By not doing something, are we causing harm to our patients?” If we change the diagnostic criteria for GDM, thereby increasing the number of women with the condition who would then require additional care, medications, and, potentially, more complex decisions around timing and mode of delivery, we need to be certain that we are not doing harm. This, and other studies examining the use of the one- versus two-step approach have yet to demonstrate, unequivocally, that changing the criteria reduces harm, and, perhaps, might – unintentionally – cause more.
As the study authors and the NIH consensus panel concluded, more rigorous evaluation is needed; that is, a large, multicenter randomized controlled trial that examines not only the benefits during pregnancy but also the long-term benefits to women and their children.
E. Albert Reece, MD, PhD, MBA, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He provided commentary on the study by Pocobelli et al. Dr. Reece said he had no relevant financial disclosures.
according to data from a before-and-after cohort study of women in the state of Washington.
The one-step test, a 75-g 2-hour oral glucose tolerance test (OGTT), was recommended for all pregnant women in 2010, although the traditional two-step test – a 50-g screening glucose challenge test followed by a 100-g 3-hour OGTT – remains widely used, wrote Gaia Pocobelli, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues. “No randomized trial has been published comparing outcomes of the two approaches.”
In a study published in Obstetrics & Gynecology, the researchers compared data from 23,257 women who received prenatal care in Washington State between January 2009 and December 2014, including 8,363 women who received care before the guideline change, 4,103 who received care during a transition period, and 10,791 after the guideline change. Approximately 60% of the women received care from clinicians internal to Kaiser Permanente; 40% received care from external providers. Most (87%) of the internal clinicians switched to the one-step approach, the researchers said. Only 5% of external providers did so.
Overall, adopting the one-step approach was associated with a 41% increase in the diagnosis of GDM without improved maternal or neonatal outcomes, the researchers noted.
The incidence of GDM increased from 7% before the guideline change to 11% afterward for women seen by internal providers. For women seen by external providers, gestational diabetes incidence increased from 10% to 11%.
For women seen by internal providers, the use of insulin increased from 1% before the guideline change to 4% afterward; for women seen by external providers, use of insulin increased from 1.3% to 1.4% (change between the groups P less than .001).
In addition, women seen by internal providers were more likely to undergo induction of labor after the guideline change (25% to 29%), while labor induction decreased for women seen by external providers (31% to 29%) for a relative risk of 1.2.
Neonatal hypoglycemia increased from 1% to 2% among women seen by internal providers, but decreased slightly from 2.4% to 2.1% for women seen by external providers, for a relative risk of 1.77.
There were no significant differences between the women seen by internal and external providers in risk of primary cesarean section, large for gestational age, small for gestational age, or neonatal ICU admission.
The main limitation of the study was the potential confounding variables including maternal diet and exercise, and possible underreporting of risk factors such as smoking, the researchers noted. However, the results were strengthened by the large study population, and the results “do not suggest a benefit of adopting the one-step over the two-step approach.
“Kaiser Permanente Washington has revised [its] guidelines to return to a two-step process. We recommend that any health care system considering switching to the one-step approach incorporate a rigorous evaluation of changes in maternal and neonatal outcomes,” Dr. Pocobelli and her associates added.
Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
according to data from a before-and-after cohort study of women in the state of Washington.
The one-step test, a 75-g 2-hour oral glucose tolerance test (OGTT), was recommended for all pregnant women in 2010, although the traditional two-step test – a 50-g screening glucose challenge test followed by a 100-g 3-hour OGTT – remains widely used, wrote Gaia Pocobelli, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues. “No randomized trial has been published comparing outcomes of the two approaches.”
In a study published in Obstetrics & Gynecology, the researchers compared data from 23,257 women who received prenatal care in Washington State between January 2009 and December 2014, including 8,363 women who received care before the guideline change, 4,103 who received care during a transition period, and 10,791 after the guideline change. Approximately 60% of the women received care from clinicians internal to Kaiser Permanente; 40% received care from external providers. Most (87%) of the internal clinicians switched to the one-step approach, the researchers said. Only 5% of external providers did so.
Overall, adopting the one-step approach was associated with a 41% increase in the diagnosis of GDM without improved maternal or neonatal outcomes, the researchers noted.
The incidence of GDM increased from 7% before the guideline change to 11% afterward for women seen by internal providers. For women seen by external providers, gestational diabetes incidence increased from 10% to 11%.
For women seen by internal providers, the use of insulin increased from 1% before the guideline change to 4% afterward; for women seen by external providers, use of insulin increased from 1.3% to 1.4% (change between the groups P less than .001).
In addition, women seen by internal providers were more likely to undergo induction of labor after the guideline change (25% to 29%), while labor induction decreased for women seen by external providers (31% to 29%) for a relative risk of 1.2.
Neonatal hypoglycemia increased from 1% to 2% among women seen by internal providers, but decreased slightly from 2.4% to 2.1% for women seen by external providers, for a relative risk of 1.77.
There were no significant differences between the women seen by internal and external providers in risk of primary cesarean section, large for gestational age, small for gestational age, or neonatal ICU admission.
The main limitation of the study was the potential confounding variables including maternal diet and exercise, and possible underreporting of risk factors such as smoking, the researchers noted. However, the results were strengthened by the large study population, and the results “do not suggest a benefit of adopting the one-step over the two-step approach.
“Kaiser Permanente Washington has revised [its] guidelines to return to a two-step process. We recommend that any health care system considering switching to the one-step approach incorporate a rigorous evaluation of changes in maternal and neonatal outcomes,” Dr. Pocobelli and her associates added.
Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Increased diagnoses of gestational diabetes did not significantly improve maternal or fetal outcomes.
Major finding: Adoption of a one-step screening process for gestational diabetes increased diagnoses by 41%.
Study details: The data come from a before-and-after cohort study with a population of 23,257 women.
Disclosures: Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
Source: Pocobelli G et al. Obstet Gynecol. 2018;132:859-67.
Hormonal contraceptive use linked to leukemia risk in offspring
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: Recent maternal hormonal contraceptive use was linked to one additional case of leukemia per 47,170 children.
Study details: Danish nationwide cohort study in 1,185,157 children.
Disclosures: The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
Source: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.