User login
Minorities bear brunt of pediatric COVID-19 cases
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
Black and Hispanic children comprised significantly more cases of COVID-19, compared with White children, based on data from a large, cross-sectional study of 1,000 cases.
“Data regarding disparities in SARS-CoV-2 infection and outcomes have been, thus far, mostly limited to adults,” wrote Monika K. Goyal, MD, of Children’s National Hospital, Washington, and colleagues. “Additional data further suggest that low socioeconomic status may further exacerbate health outcomes for racial and ethnic minorities.”
In a study published in Pediatrics, the researchers conducted a cross-sectional analysis of 1,000 children from a registry of non–acutely ill pediatric patients seen at a drive-through and walk-up COVID-19 test site.
Minority, socioeconomic status affect pediatric outcomes too
The median age of the study population was 8 years, and approximately half were male.
The researchers also examined the association of median family income (MFI) using census block group estimates data from the American Community Survey (2014–2018) to represent socioeconomic status.
Infection rates were significantly higher among children in the lowest three quartiles of MFI (24%, 27%, and 38% for quartiles 3, 2, and 1, respectively), compared with the highest quartile of MFI (9%).
After adjusting for age, sex, and MFI, Hispanic children were six times more likely and non-Hispanic Black children were twice as likely to test positive for COVID-19 than non-Hispanic White children (adjusted odds ratios, 6.3 and 2.3, respectively).
The study findings were limited by several factors including the use of clinician-reported ethnicity and thus potential for misclassification, the researchers noted. In addition, the socioeconomic and racial disparities may be underestimated because these groups have less access to primary care, and the study did not allow for confounding variables including housing conditions or occupancy.
“Although it was beyond the scope of this study to understand the causes for these differential rates of infection, the causes may be multifactorial, including, but not limited to, structural factors, poorer access to health care, limited resources, and bias and discrimination,” the researchers noted. In addition, the high infection rate among minority children may be impacted by parents who are less able to telework, find child care, or avoid public transportation, Dr. Goyal and associates wrote.
Future research should address “the modifiable reasons for these observed disparities as well as their differential impact in terms of SARS-CoV-2–related morbidity and mortality outcomes to mitigate the spread of infection and its health effects,” they concluded.
How to help
“This study is important because we need to understand which groups of children are at highest risk for SARS-CoV-2 infection in order to maximize efforts for screening, allocating resources, and prioritizing vaccine administration,” Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
Dr. Kinsella said she was not surprised at the higher infection rates in general in minorities and low socioeconomic groups. “We already knew that adult COVID-19 rates were higher for people in certain racial/ethnic groups and those with socioeconomic disadvantages; however, I was shocked by the percentages. That is a huge burden for a population that already has disparities in health outcomes.”
“As the authors cite, this was not a research study of why these groups were more likely to be COVID-19 positive, but they speculated that crowded living conditions, multigenerational families living together, and many minorities being essential workers unable to work from home,” said Dr. Kinsella. Additional factors contributing to higher infection rates may include limited access to care, transportation issues, insurance coverage, schedule challenges, and fear of deportation. Some of these problems might be addressed by coming into communities in mobile vans, visiting community health centers and schools with free educational materials, using masks and hand sanitizer, and offering free access to testing.
“Future studies could confirm the cause of this discrepancy, as well as study community-based interventions and their outcomes,” Dr. Kinsella said. In the meantime, a take-home message for clinicians is the need to prioritize screening, resources, and vaccines to reflect the higher rates of SARS-CoV-2 infections in children from disadvantaged racial and socioeconomic backgrounds.
The study received no outside funding. The researchers had no financial conflicts to disclose, but lead author Dr. Goyal is a member of the Pediatrics editorial board. Dr. Kinsella had no financial conflicts to disclose, but serves on the Pediatric News editorial advisory board.
SOURCE: Goyal MK et al. Pediatrics. 2020 Sep 24. doi: 10.1542/peds.2020-009951.
FROM PEDIATRICS
Use of e-cigarettes may be linked to sleep deprivation
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.
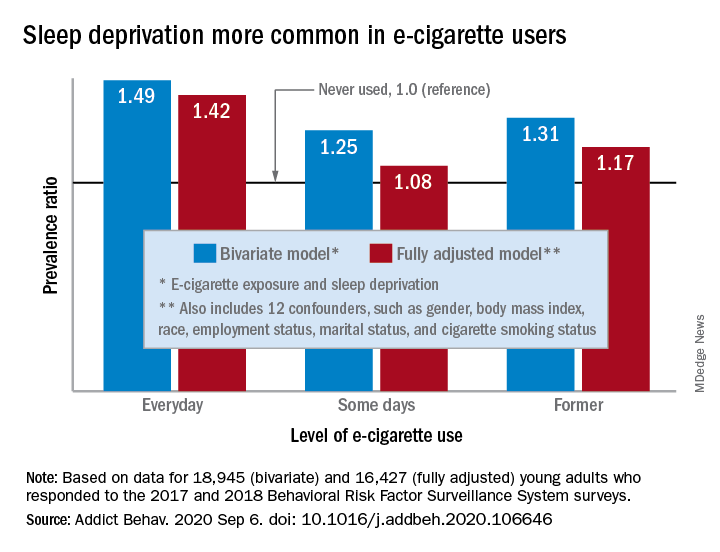
“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
compared with those who have never used e-cigarettes, according to the first study to evaluate the association in a large, nationally representative population of young adults.

“The e-cigarette use and sleep deprivation association seems to have a dose-response nature as the point estimate of the association increased with increased exposure to e-cigarette,” Sina Kianersi, DVM, and associates at Indiana University, Bloomington, said in Addictive Behaviors.
Sleep deprivation was 49% more prevalent among everyday users of e-cigarettes, compared with nonusers. Prevalence ratios for former users (1.31) and occasional users (1.25) also showed significantly higher sleep deprivation, compared with nonusers, they reported based on a bivariate analysis of data from young adults aged 18-24 years who participated in the 2017 and 2018 Behavioral Risk Factor Surveillance System surveys.
After adjustment for multiple confounders, young adults who currently used e-cigarettes every day were 42% more likely to report sleep deprivation than those who never used e-cigarettes, a difference that was statistically significant. The prevalence of sleep deprivation among those who used e-cigarettes on some days was not significantly higher (prevalence ratio, 1.08), but the ratio between former users and never users was a significant 1.17, the investigators said.
“The nicotine in the inhaled e-cigarette aerosols may have negative effects on sleep architecture and disturb the neurotransmitters that regulate sleep cycle,” they suggested, and since higher doses of nicotine produce greater reductions in sleep duration, “those who use e-cigarette on a daily basis might consume higher doses of nicotine, compared to some days, former, and never users, and therefore get fewer hours of sleep.”
Nicotine withdrawal, on the other hand, has been found to increase sleep duration in a dose-dependent manner, which “could explain the smaller [prevalence ratios] observed for the association between e-cigarette use and sleep deprivation among former and some days e-cigarette users,” Dr. Kianersi and associates added.
The bivariate analysis involved 18,945 survey respondents, of whom 16,427 were included in the fully adjusted model using 12 confounding factors.
SOURCE: Kianersi S et al. Addict Behav. 2020 Sep 6. doi: 10.1016/j.addbeh.2020.106646.
FROM ADDICTIVE BEHAVIORS
COVID-19 airway management: Expert tips on infection control
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
As continue to evolve, practicing vigilant transmission-based infection control precautions remains essential.
This starts with observing droplet precautions to prevent exposure to droplets larger than 5 microns in size, Charles Griffis, PhD, CRNA, said at a Society for Critical Care Medicine virtual meeting: COVID-19: What’s Next. “These are particles exhaled from infected persons and which fall within around 6 feet and involve an exposure time of 15 or more minutes of contact,” said Dr. Griffis, of the department of anesthesiology at the University of Southern California, Los Angeles. “We will always observe standard precautions, which include hand hygiene, gloves, hair and eye cover, medical mask, and face shield. We will observe these at all times for all patients and layer our transmission-based precautions on top.”
During aerosol-producing procedures such as airway management maneuvers, tracheostomies, and bronchoscopies, very fine microscopic particles less than 5 microns in size are produced, which remain airborne for potentially many hours and travel long distances. “We will add an N95 mask or a powered air-purifying respirator (PAPR) device to filter out tiny particles in addition to our ever-present standard precautions,” he said. “Contact precautions are indicated for direct contact with patient saliva, blood, urine, and stool. In addition to standard precautions, we’re going to add an impermeable gown and we’ll continue with gloves, eye protection, and shoe covers. The message is to all of us. We have to observe all of the infection precautions that all of us have learned and trained in to avoid exposure.”
In terms of airway management for infected patients for elective procedures and surgery, recommendations based on current and previous coronavirus outbreaks suggest that all patients get polymerase chain reaction (PCR) tested within 24-48 hours of elective procedures or surgeries. If positive, they should be quarantined for 10-14 days and then, if asymptomatic, these patients may be retested or they can be regarded as negative. “Patients who are PCR positive with active infection and active symptoms receive only urgent or emergent care in most settings,” said Dr. Griffis, a member of the American Association of Nurse Anesthetists Infection Control Advisory Panel. “The care provided to our patients, whether they’re positive or not, is individualized per patient needs and institutional policy. Some folks have made the decision to treat all patients as infected and to use airborne precautions for all aerosol-producing procedures for all patients all the time.”
When a COVID-19 patient requires emergent or urgent airway management because of respiratory failure or some other surgical or procedural intervention necessitating airway management, preprocedural planning is key, he continued. This means establishing the steps in airway management scenarios for infected patients and rehearsing those steps in each ICU setting with key personnel such as nurses, respiratory therapists, and medical staff. “You want to make sure that the PPE is readily available and determine and limit the number of personnel that are going to enter the patient’s room or area for airway management,” Dr. Griffis said. “Have all the airway equipment and drugs immediately available. Perhaps you could organize them in a cart which is decontaminated after every use.”
He also recommends forming an intubation team for ICUs and perhaps even for ORs, where the most experienced clinicians perform airway management. “This helps to avoid unnecessary airway manipulation and minimizes personnel exposure and time to airway establishment,” he said.
Always attempt to house the infected patient in an airborne isolation, negative-pressure room, with a minimum of 12 exchanges per hour and which will take 35 minutes for 99.99% removal of airborne contaminants after airway management. “These numbers are important to remember for room turnover safety,” he said.
Patient factors to review during airway management include assessing the past medical history, inspecting the airway and considering the patient’s current physiological status as time permits. Previously in the pandemic, intubation was used earlier in the disease course, but now data suggest that patients do better without intubation if possible (Am J Trop Med Hyg. 2020;102[6]. doi: 10.4269/aitmh.20-0283). “This is because the pathophysiology of COVID-19 is such that the lung tissue is predisposed to iatrogenic barotrauma damage from positive-pressure ventilation,” Dr. Griffis said. “In addition, COVID patients appear to tolerate significant hypoxemia without distress in many cases. Therefore, many clinicians now hold off on intubation until the hypoxemic patient begins exhibiting signs and symptoms of respiratory distress.”
Options for delivering noninvasive airway support for COVID-19 patients include high-flow nasal cannula and noninvasive positive-pressure ventilation via CPAP or BiPAP. To mitigate the associated aerosol production, consider applying a surgical mask, helmet, or face mask over the airway device/patient’s face. “Another measure that has proven helpful in general respiratory support is to actually put the patient in a prone position to help redistribute ventilation throughout the lungs,” Dr. Griffis said (see Resp Care. 2015;60[11]:1660-87).
To prepare for the actual intubation procedure, gather two expert intubators who are going to be entering the patient’s room. The team should perform hand hygiene and don full PPE prior to entry. “It’s recommended that you consider wearing double gloves for the intubation,” he said. “Have the airway equipment easily accessible in a central location on a cart or in a kit, and use disposable, single-use equipment if possible. All of the usual intubation equipment to maintain a clear airway and give positive pressure ventilation should be arranged for easy access. A video laryngoscope should be used, if possible, for greater accuracy and reduced procedure time. Ready access to sedation and muscle relaxant drugs must be assured at all times.”
For the intubation procedure itself, Dr. Griffis recommends ensuring that an oxygen source, positive-pressure ventilation, and suction and resuscitation drugs and equipment are available per institutional protocol. Assign one person outside the room to coordinate supplies and assistance. “Preoxygenate the patient as permitted by clinical status,” he said. “A nonrebreathing oxygen mask can be used if sufficient spontaneous ventilation is present. Assess the airway, check and arrange equipment for easy access, and develop the safest airway management plan. Consider a rapid sequence induction and intubation as the first option.” Avoid positive-pressure ventilation or awake fiber optic intubation unless absolutely necessary, thus avoiding aerosol production. “Only ventilate the patient after the endotracheal tube cuff is inflated, to avoid aerosol release,” he said.
For intubation, administer airway procedural drugs and insert the laryngoscope – ideally a video laryngoscope if available. Intubate the trachea under direct vision, inflate the cuff, and remove outer gloves. Then attach the Ambu bag with a 99% filtration efficiency, heat-and-moisture exchange filter; and proceed to ventilate the patient, checking for chest rise, breath sounds, and CO2 production. “Discard contaminated equipment in designated bins and secure the tube,” Dr. Griffis advised. “Attach the ventilator with an HMEF filter to protect the ventilator circuit and inner parts of the machine. Recheck your breath sounds, CO2 production, and oxygen saturation, and adjust your vent settings as indicated.”
For post intubation, Dr. Griffis recommends securing contaminated discardable equipment in biohazard-labeled bins or bags, safely doffing your PPE, and retaining your N95 mask in the room. Remove your inner gloves, perform hand hygiene with soap and water if available, with alcohol-based hand rub if not, then don clean gloves. Exit the room, safely transporting any contaminated equipment that will be reused such as a cart or video laryngoscope to decontamination areas for processing. “Once clear of the room, order your chest x-ray to confirm your tube position per institutional protocol, understanding that radiology techs are all going to be following infection control procedures and wearing their PPE,” he said.
For extubation, Dr. Griffis recommends excusing all nonessential personnel from the patient room and assigning an assistant outside the room for necessary help. An experienced airway management expert should evaluate the patient wearing full PPE and be double-gloved. “If the extubation criteria are met, suction the pharynx and extubate,” he said. “Remove outer gloves and apply desired oxygen delivery equipment to the patient and assess respiratory status and vital signs for stability.” Next, discard all contaminated equipment in designated bins, doff contaminated PPE, and retain your N95 mask. Doff inner gloves, perform hand hygiene, and don clean gloves. “Exit the room, hand off contaminated equipment that is reusable, doff your gloves outside, do hand hygiene, then proceed to change your scrubs and complete your own personal hygiene measures,” he said.
Dr. Griffis reported having no financial disclosures.
“While the PPE used for intubation of a coronavirus patient is certainly more than the typical droplet precautions observed when intubating any other patient, the process and best practices aren’t terribly different from usual standard of care: Ensuring all necessary equipment is readily available with backup plans should the airway be difficult,” said Megan Conroy, MD, assistant professor of clinical medicine at The Ohio State University.
“We’ve been streamlining the team that’s present in the room for intubations of COVID patients, but I’m always amazed at the team members that stand at the ready to lend additional assistance just from the other side of the door. So while fewer personnel may be exposed, I wouldn’t consider the team needed for intubation to actually be much smaller, we’re just functioning differently.
In my practice the decision of when to intubate, clinically, doesn’t vary too much from any other form of severe ARDS. We may tolerate higher FiO2 requirements on heated high-flow nasal cannula if the patient exhibits acceptable work of breathing, but I wouldn’t advise allowing a patient to remain hypoxemic with oxygen needs unmet by noninvasive methods out of fear of intubation or ventilator management. In my opinion, this simply delays a necessary therapy and only makes for a higher risk intubation. Certainly, the decision to intubate is never based on only one single data point, but takes an expert assessment of the whole clinical picture.
I’d assert that it’s true in every disease that patients do better if it’s possible to avoid intubation – but I would argue that the ability to avoid intubation is determined primarily by the disease course and clinical scenario, and not by whether the physician wishes to avoid intubation or not. If I can safely manage a patient off of a ventilator, I will always do so, COVID or otherwise. I think in this phase of the pandemic, patients ‘do better without intubation’ because those who didn’t require intubation were inherently doing better!”
FROM AN SCCM VIRTUAL MEETING
What to do when a patient is not ready to stop smoking
Recommendations from the American Thoracic Society
Below is a case involving a patient who is not yet ready to quit smoking. We later provide treatment recommendations for this patient based on a new guideline from the American Thoracic Society.
Case
A 58-year-old female comes into the office for a physical exam. She has been smoking two packs a day since she was 23 years of age. You have tried at previous visits to get her to quit, but she hasn’t been interested. The patient says she has a lot of stress, and that it is still not the right time for her to stop smoking. You tell her she needs to quit and, though the patient understands that quitting would be beneficial for her health, she just isn’t ready to try to kick the habit. How do you proceed?
The Guideline in context
Even though this patient stated that she is not ready to stop smoking, she is still a candidate for pharmacological treatment for her tobacco dependence and can be offered varenicline, according to the ATS guideline.1
In a previously published column, we have discussed the ATS’ recommended approaches for treating patients who are ready to stop smoking cigarettes. The reality is that many patients, if not most, are not ready to quit when we speak to them during any given office visit. The ATS guideline addresses this critical issue by recommending treatment with varenicline in patients who are not ready to stop smoking. It also states that this is a better strategy than waiting to start treatment until patients say they are ready for it.
This recommendation – to prescribe varenicline to smokers even when they are not ready to quit smoking – is based on solid clinical trial evidence. Research has shown that behavior change is dynamic and that the decision to stop smoking is not always a planned one.1 Patients often make quit attempts between office visits, and are often successful in those attempts. Because the decision to try to stop smoking is influenced by the satisfaction and physical addiction that comes from smoking, a medication such as varenicline that is a partial agonist/antagonist at the alpha4-beta2 nicotinic receptor might increase the likelihood that a patient would decide to try to stop smoking. This is because taking this type of a drug would lead the patient to no longer experience the reinforcing effects of nicotine.2 This hypothesis was examined in five randomized trials.1
In these studies, regular smokers who were not ready to make a quit attempt were randomized to varenicline versus placebo. Twice as many individuals who took varenicline stopped smoking 6 months after starting treatment.1
Suggested treatment
This patient should be offered varenicline. This individual meets the criteria for this treatment according to the ATS guideline in that the patient is a regular smoker who doesn’t think she is ready to stop smoking but understands she needs to stop and is open to taking medication to assist her with quitting.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Sprogell is a third-year resident in the family medicine residency program at Abington Jefferson Health. They have no conflicts related to the content of this piece. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Leone F T et al. Initiating pharmacologic treatment in tobacco-dependent adults: An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020 Jul 15;202(2):e5–e31.
2. Ebbert JO et al. Varenicline for smoking cessation: Efficacy, safety, and treatment recommendations. Patient Prefer Adherence. 2010;4:355-62.
Recommendations from the American Thoracic Society
Recommendations from the American Thoracic Society
Below is a case involving a patient who is not yet ready to quit smoking. We later provide treatment recommendations for this patient based on a new guideline from the American Thoracic Society.
Case
A 58-year-old female comes into the office for a physical exam. She has been smoking two packs a day since she was 23 years of age. You have tried at previous visits to get her to quit, but she hasn’t been interested. The patient says she has a lot of stress, and that it is still not the right time for her to stop smoking. You tell her she needs to quit and, though the patient understands that quitting would be beneficial for her health, she just isn’t ready to try to kick the habit. How do you proceed?
The Guideline in context
Even though this patient stated that she is not ready to stop smoking, she is still a candidate for pharmacological treatment for her tobacco dependence and can be offered varenicline, according to the ATS guideline.1
In a previously published column, we have discussed the ATS’ recommended approaches for treating patients who are ready to stop smoking cigarettes. The reality is that many patients, if not most, are not ready to quit when we speak to them during any given office visit. The ATS guideline addresses this critical issue by recommending treatment with varenicline in patients who are not ready to stop smoking. It also states that this is a better strategy than waiting to start treatment until patients say they are ready for it.
This recommendation – to prescribe varenicline to smokers even when they are not ready to quit smoking – is based on solid clinical trial evidence. Research has shown that behavior change is dynamic and that the decision to stop smoking is not always a planned one.1 Patients often make quit attempts between office visits, and are often successful in those attempts. Because the decision to try to stop smoking is influenced by the satisfaction and physical addiction that comes from smoking, a medication such as varenicline that is a partial agonist/antagonist at the alpha4-beta2 nicotinic receptor might increase the likelihood that a patient would decide to try to stop smoking. This is because taking this type of a drug would lead the patient to no longer experience the reinforcing effects of nicotine.2 This hypothesis was examined in five randomized trials.1
In these studies, regular smokers who were not ready to make a quit attempt were randomized to varenicline versus placebo. Twice as many individuals who took varenicline stopped smoking 6 months after starting treatment.1
Suggested treatment
This patient should be offered varenicline. This individual meets the criteria for this treatment according to the ATS guideline in that the patient is a regular smoker who doesn’t think she is ready to stop smoking but understands she needs to stop and is open to taking medication to assist her with quitting.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Sprogell is a third-year resident in the family medicine residency program at Abington Jefferson Health. They have no conflicts related to the content of this piece. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Leone F T et al. Initiating pharmacologic treatment in tobacco-dependent adults: An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020 Jul 15;202(2):e5–e31.
2. Ebbert JO et al. Varenicline for smoking cessation: Efficacy, safety, and treatment recommendations. Patient Prefer Adherence. 2010;4:355-62.
Below is a case involving a patient who is not yet ready to quit smoking. We later provide treatment recommendations for this patient based on a new guideline from the American Thoracic Society.
Case
A 58-year-old female comes into the office for a physical exam. She has been smoking two packs a day since she was 23 years of age. You have tried at previous visits to get her to quit, but she hasn’t been interested. The patient says she has a lot of stress, and that it is still not the right time for her to stop smoking. You tell her she needs to quit and, though the patient understands that quitting would be beneficial for her health, she just isn’t ready to try to kick the habit. How do you proceed?
The Guideline in context
Even though this patient stated that she is not ready to stop smoking, she is still a candidate for pharmacological treatment for her tobacco dependence and can be offered varenicline, according to the ATS guideline.1
In a previously published column, we have discussed the ATS’ recommended approaches for treating patients who are ready to stop smoking cigarettes. The reality is that many patients, if not most, are not ready to quit when we speak to them during any given office visit. The ATS guideline addresses this critical issue by recommending treatment with varenicline in patients who are not ready to stop smoking. It also states that this is a better strategy than waiting to start treatment until patients say they are ready for it.
This recommendation – to prescribe varenicline to smokers even when they are not ready to quit smoking – is based on solid clinical trial evidence. Research has shown that behavior change is dynamic and that the decision to stop smoking is not always a planned one.1 Patients often make quit attempts between office visits, and are often successful in those attempts. Because the decision to try to stop smoking is influenced by the satisfaction and physical addiction that comes from smoking, a medication such as varenicline that is a partial agonist/antagonist at the alpha4-beta2 nicotinic receptor might increase the likelihood that a patient would decide to try to stop smoking. This is because taking this type of a drug would lead the patient to no longer experience the reinforcing effects of nicotine.2 This hypothesis was examined in five randomized trials.1
In these studies, regular smokers who were not ready to make a quit attempt were randomized to varenicline versus placebo. Twice as many individuals who took varenicline stopped smoking 6 months after starting treatment.1
Suggested treatment
This patient should be offered varenicline. This individual meets the criteria for this treatment according to the ATS guideline in that the patient is a regular smoker who doesn’t think she is ready to stop smoking but understands she needs to stop and is open to taking medication to assist her with quitting.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Hospital–Jefferson Health. Dr. Sprogell is a third-year resident in the family medicine residency program at Abington Jefferson Health. They have no conflicts related to the content of this piece. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Leone F T et al. Initiating pharmacologic treatment in tobacco-dependent adults: An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020 Jul 15;202(2):e5–e31.
2. Ebbert JO et al. Varenicline for smoking cessation: Efficacy, safety, and treatment recommendations. Patient Prefer Adherence. 2010;4:355-62.
Smart health devices – promises and pitfalls
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
What needs to be done before the data deluge hits the office
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Clinical Utility of Methicillin-Resistant Staphylococcus aureus Polymerase Chain Reaction Nasal Swab Testing in Lower Respiratory Tract Infections
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).
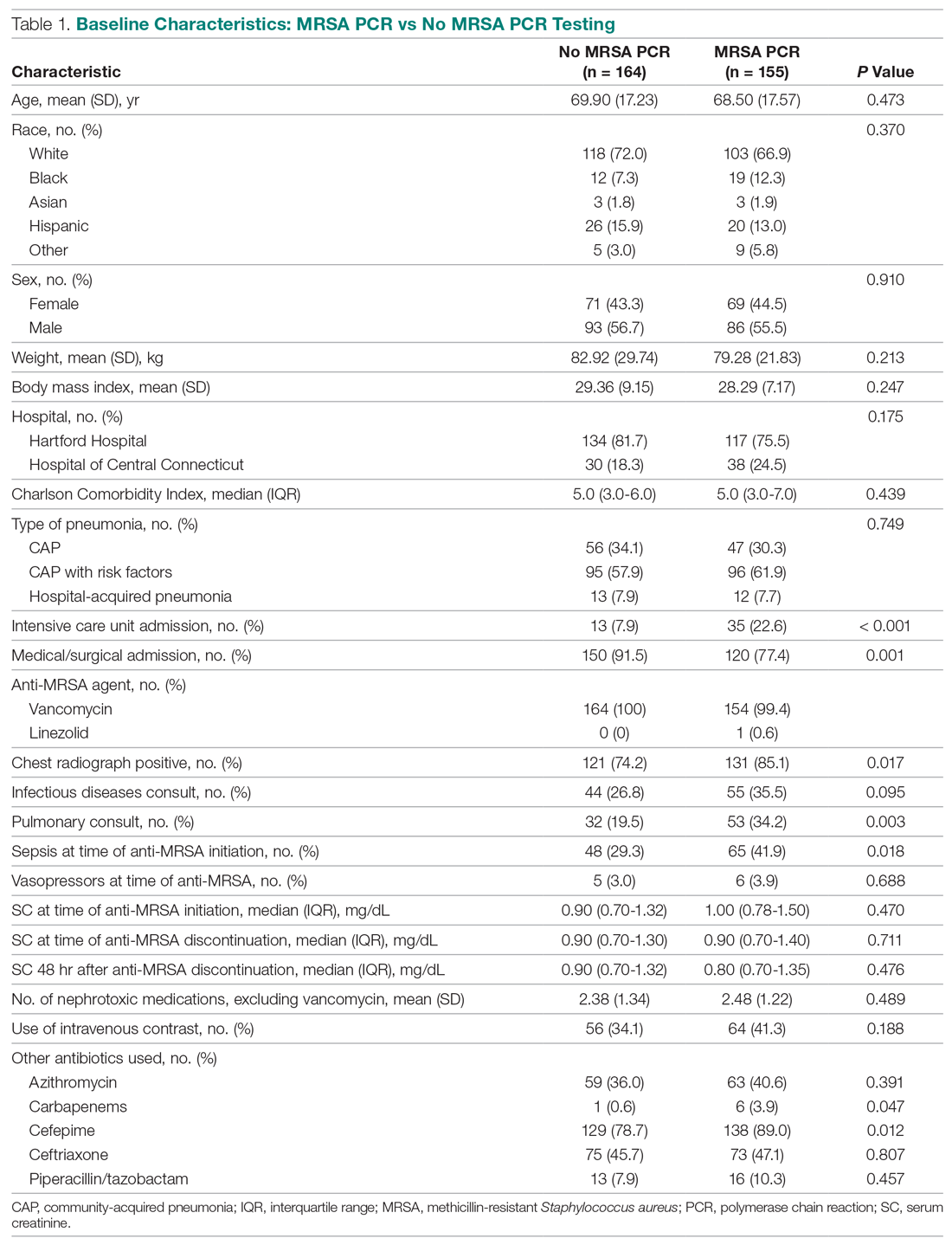
In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.
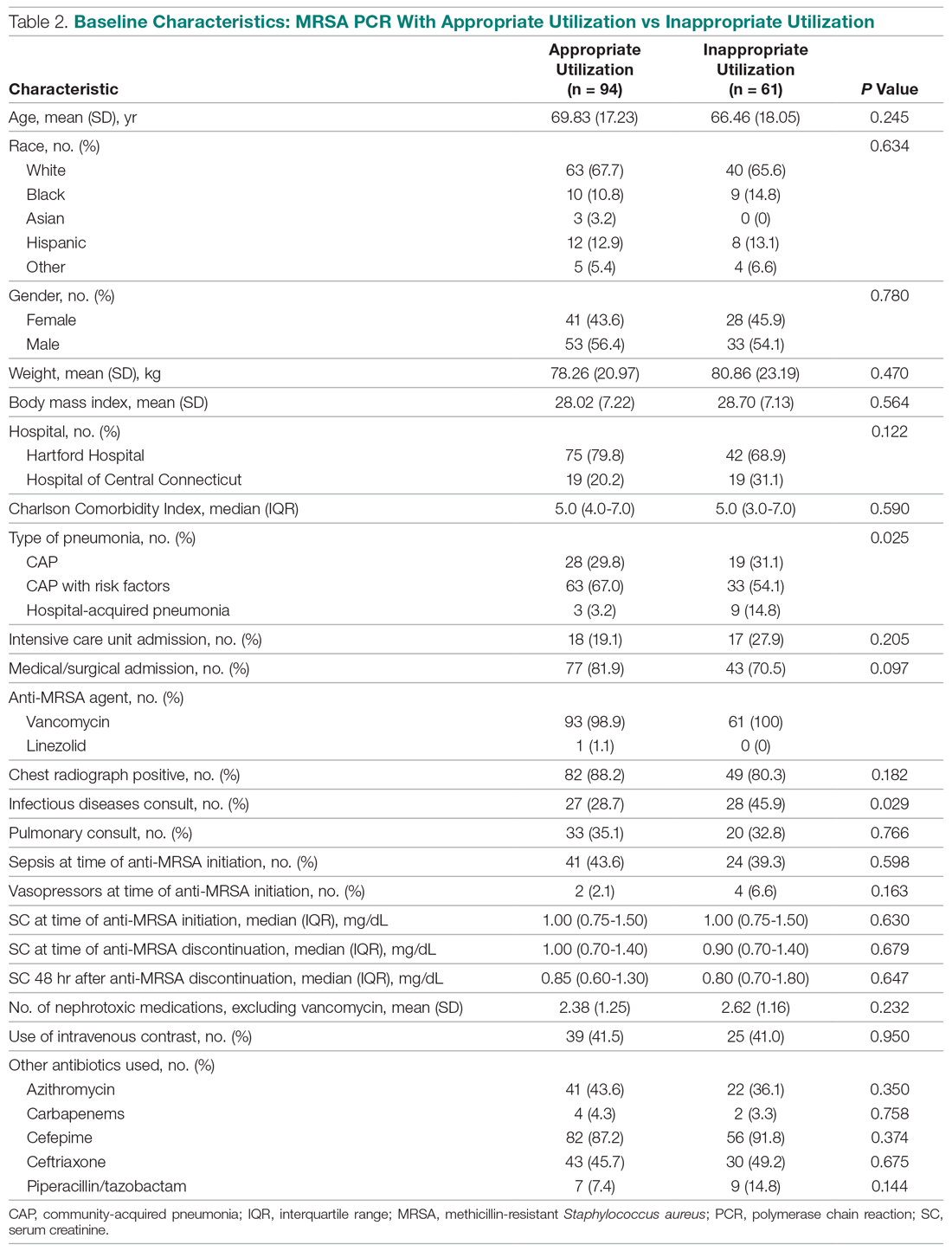
Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.
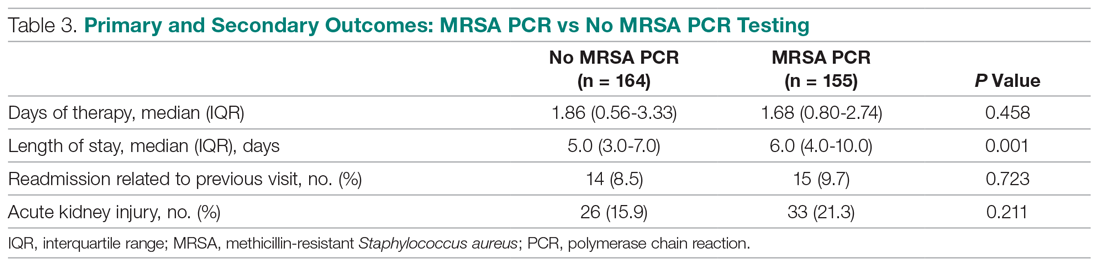
In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.
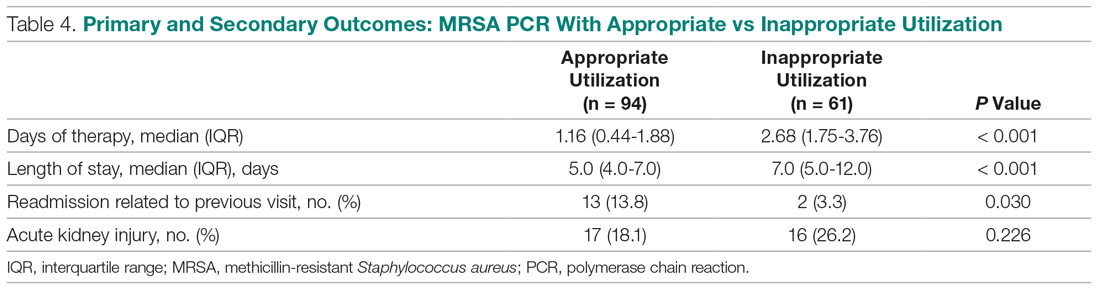
A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.
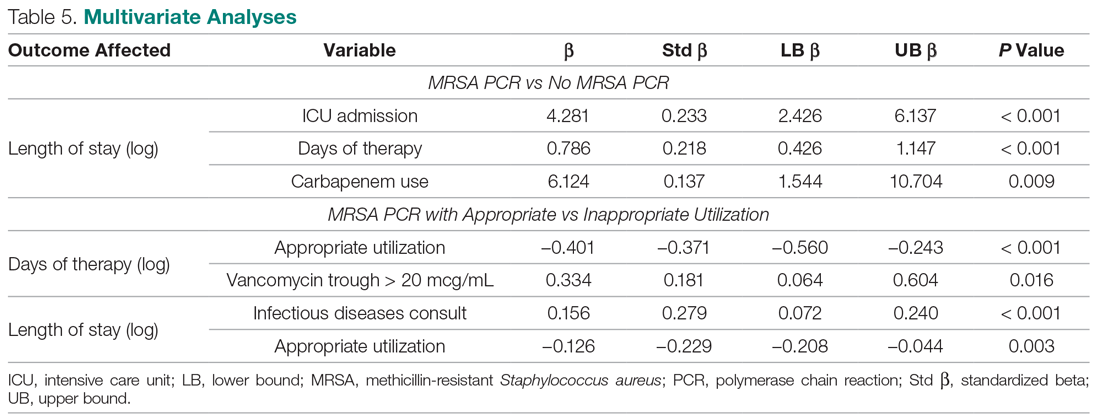
Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
From the Hospital of Central Connecticut, New Britain, CT (Dr. Caulfield and Dr. Shepard); Hartford Hospital, Hartford, CT (Dr. Linder and Dr. Dempsey); and the Hartford HealthCare Research Program, Hartford, CT (Dr. O’Sullivan).
Abstract
- Objective: To assess the utility of methicillin-resistant Staphylococcus aureus (MRSA) polymerase chain reaction (PCR) nasal swab testing in patients with lower respiratory tract infections (LRTI).
- Design and setting: Multicenter, retrospective, electronic chart review conducted within the Hartford HealthCare system.
- Participants: Patients who were treated for LRTIs at the Hospital of Central Connecticut or Hartford Hospital between July 1, 2018, and June 30, 2019.
- Measurements: The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing versus those who did not. In a subgroup analysis, we compared anti-MRSA DOT among patients with appropriate versus inappropriate utilization of the MRSA PCR test.
- Results: Of the 319 patients treated for LRTIs, 155 (48.6%) had a MRSA PCR ordered, and appropriate utilization occurred in 94 (60.6%) of these patients. Anti-MRSA DOT in the MRSA PCR group (n = 155) was shorter than in the group that did not undergo MRSA PCR testing (n = 164), but this difference did not reach statistical significance (1.68 days [interquartile range {IQR}, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458). In the subgroup analysis, anti-MRSA DOT was significantly shorter in the MRSA PCR group with appropriate utilization compared to the inappropriate utilization group (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76], P < 0.001)
- Conclusion: Appropriate utilization of MRSA PCR nasal swab testing can reduce DOT in patients with LRTI. Further education is necessary to expand the appropriate use of the MRSA PCR test across our health system.
Keywords: MRSA; LRTI; pneumonia; antimicrobial stewardship; antibiotic resistance.
More than 300,000 patients were hospitalized with methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States in 2017, and at least 10,000 of these cases resulted in mortality.1 While MRSA infections overall are decreasing, it is crucial to continue to employ antimicrobial stewardship tactics to keep these infections at bay. Recently, strains of S. aureus have become resistant to vancomycin, making this bacterium even more difficult to treat.2
A novel tactic in antimicrobial stewardship involves the use of MRSA polymerase chain reaction (PCR) nasal swab testing to rule out the presence of MRSA in patients with lower respiratory tract infections (LRTI). If used appropriately, this approach may decrease the number of days patients are treated with anti-MRSA antimicrobials. Waiting for cultures to speciate can take up to 72 hours,3 meaning that patients may be exposed to 3 days of unnecessary broad-spectrum antibiotics. Results of MRSA PCR assay of nasal swab specimens can be available in 1 to 2 hours,4 allowing for more rapid de-escalation of therapy. Numerous studies have shown that this test has a negative predictive value (NPV) greater than 95%, indicating that a negative nasal swab result may be useful to guide de-escalation of antibiotic therapy.5-8 The purpose of this study was to assess the utility of MRSA PCR nasal swab testing in patients with LRTI throughout the Hartford HealthCare system.
Methods
Design
This study was a multicenter, retrospective, electronic chart review. It was approved by the Hartford HealthCare Institutional Review Board (HHC-2019-0169).
Selection of Participants
Patients were identified through electronic medical record reports based on ICD-10 codes. Records were categorized into 2 groups: patients who received a MRSA PCR nasal swab testing and patients who did not. Patients who received the MRSA PCR were further categorized by appropriate or inappropriate utilization. Appropriate utilization of the MRSA PCR was defined as MRSA PCR ordered within 48 hours of a new vancomycin or linezolid order, and anti-MRSA therapy discontinued within 24 hours of a negative result. Inappropriate utilization of the MRSA PCR was defined as MRSA PCR ordered more than 48 hours after a new vancomycin or linezolid order, or continuation of anti-MRSA therapy despite a negative MRSA PCR and no other evidence of a MRSA infection.
Patients were included if they met all of the following criteria: age 18 years or older, with no upper age limit; treated for a LRTI, identified by ICD-10 codes (J13-22, J44, J85); treated with empiric antibiotics active against MRSA, specifically vancomycin or linezolid; and treated at the Hospital of Central Connecticut (HOCC) or Hartford Hospital (HH) between July 1, 2018, and June 30, 2019, inclusive. Patients were excluded if they met 1 or more of the following criteria: age less than 18 years old; admitted for 48 hours or fewer or discharged from the emergency department; not treated at either facility; treated before July 1, 2018, or after June 30, 2019; treated for ventilator-associated pneumonia; received anti-MRSA therapy within 30 days prior to admission; or treated for a concurrent bacterial infection requiring anti-MRSA therapy.
Outcome Measures
The primary outcome was anti-MRSA days of therapy (DOT) in patients who underwent MRSA PCR testing compared to patients who did not undergo MRSA PCR testing. A subgroup analysis was completed to compare anti-MRSA DOT within patients in the MRSA PCR group. Patients in the subgroup were categorized by appropriate or inappropriate utilization, and anti-MRSA DOT were compared between these groups. Secondary outcomes that were evaluated included length of stay (LOS), 30-day readmission rate, and incidence of acute kidney injury (AKI). Thirty-day readmission was defined as admission to HOCC, HH, or any institution within Hartford HealthCare within 30 days of discharge. AKI was defined as an increase in serum creatinine by ≥ 0.3 mg/dL in 48 hours, increase ≥ 1.5 times baseline, or a urine volume < 0.5 mL/kg/hr for 6 hours.
Statistical Analyses
The study was powered for the primary outcome, anti-MRSA DOT, with a clinically meaningful difference of 1 day. Group sample sizes of 240 in the MRSA PCR group and 160 in the no MRSA PCR group would have afforded 92% power to detect that difference, if the null hypothesis was that both group means were 4 days and the alternative hypothesis was that the mean of the MRSA PCR group was 3 days, with estimated group standard deviations of 80% of each mean. This estimate used an alpha level of 0.05 with a 2-sided t-test. Among those who received a MRSA PCR test, a clinically meaningful difference between appropriate and inappropriate utilization was 5%.
Descriptive statistics were provided for all variables as a function of the individual hospital and for the combined data set. Continuous data were summarized with means and standard deviations (SD), or with median and interquartile ranges (IQR), depending on distribution. Categorical variables were reported as frequencies, using percentages. All data were evaluated for normality of distribution. Inferential statistics comprised a Student’s t-test to compare normally distributed, continuous data between groups. Nonparametric distributions were compared using a Mann-Whitney U test. Categorical comparisons were made using a Fisher’s exact test for 2×2 tables and a Pearson chi-square test for comparisons involving more than 2 groups.
Since anti-MRSA DOT (primary outcome) and LOS (secondary outcome) are often non-normally distributed, they have been transformed (eg, log or square root, again depending on distribution). Whichever native variable or transformation variable was appropriate was used as the outcome measure in a linear regression model to account for the influence of factors (covariates) that show significant univariate differences. Given the relatively small sample size, a maximum of 10 variables were included in the model. All factors were iterated in a forward regression model (most influential first) until no significant changes were observed.
All calculations were performed with SPSS v. 21 (IBM; Armonk, NY) using an a priori alpha level of 0.05, such that all results yielding P < 0.05 were deemed statistically significant.
Results
Of the 561 patient records reviewed, 319 patients were included and 242 patients were excluded. Reasons for exclusion included 65 patients admitted for a duration of 48 hours or less or discharged from the emergency department; 61 patients having another infection requiring anti-MRSA therapy; 60 patients not having a diagnosis of a LRTI or not receiving anti-MRSA therapy; 52 patients having received anti-MRSA therapy within 30 days prior to admission; and 4 patients treated outside of the specified date range.
Of the 319 patients included, 155 (48.6%) were in the MRSA PCR group and 164 (51.4%) were in the group that did not undergo MRSA PCR (Table 1). Of the 155 patients with a MRSA PCR ordered, the test was utilized appropriately in 94 (60.6%) patients and inappropriately in 61 (39.4%) patients (Table 2). In the MRSA PCR group, 135 patients had a negative result on PCR assay, with 133 of those patients having negative respiratory cultures, resulting in a NPV of 98.5%. Differences in baseline characteristics between the MRSA PCR and no MRSA PCR groups were observed. The patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, as indicated by statistically significant differences in intensive care unit (ICU) admissions (P = 0.001), positive chest radiographs (P = 0.034), sepsis at time of anti-MRSA initiation (P = 0.013), pulmonary consults placed (P = 0.003), and carbapenem usage (P = 0.047).

In the subgroup analysis comparing appropriate and inappropriate utilization within the MRSA PCR group, the inappropriate utilization group had significantly higher numbers of infectious diseases consults placed, patients with hospital-acquired pneumonia, and patients with community-acquired pneumonia with risk factors.

Outcomes
Median anti-MRSA DOT in the MRSA PCR group was shorter than DOT in the no MRSA PCR group, but this difference did not reach statistical significance (1.68 [IQR, 0.80-2.74] vs 1.86 days [IQR, 0.56-3.33], P = 0.458; Table 3). LOS in the MRSA PCR group was longer than in the no MRSA PCR group (6.0 [IQR, 4.0-10.0] vs 5.0 [IQR, 3.0-7.0] days, P = 0.001). There was no difference in 30-day readmissions that were related to the previous visit or incidence of AKI between groups.

In the subgroup analysis, anti-MRSA DOT in the MRSA PCR group with appropriate utilization was shorter than DOT in the MRSA PCR group with inappropriate utilization (1.16 [IQR, 0.44-1.88] vs 2.68 [IQR, 1.75-3.76] days, P < 0.001; Table 4). LOS in the MRSA PCR group with appropriate utilization was shorter than LOS in the inappropriate utilization group (5.0 [IQR, 4.0-7.0] vs 7.0 [IQR, 5.0-12.0] days, P < 0.001). Thirty-day readmissions that were related to the previous visit were significantly higher in patients in the MRSA PCR group with appropriate utilization (13 vs 2, P = 0.030). There was no difference in incidence of AKI between the groups.

A multivariate analysis was completed to determine whether the sicker MRSA PCR population was confounding outcomes, particularly the secondary outcome of LOS, which was noted to be longer in the MRSA PCR group (Table 5). When comparing LOS in the MRSA PCR and the no MRSA PCR patients, the multivariate analysis showed that admission to the ICU and carbapenem use were associated with a longer LOS (P < 0.001 and P = 0.009, respectively). The incidence of admission to the ICU and carbapenem use were higher in the MRSA PCR group (P = 0.001 and P = 0.047). Therefore, longer LOS in the MRSA PCR patients could be a result of the higher prevalence of ICU admissions and infections requiring carbapenem therapy rather than the result of the MRSA PCR itself.

Discussion
A MRSA PCR nasal swab protocol can be used to minimize a patient’s exposure to unnecessary broad-spectrum antibiotics, thereby preventing antimicrobial resistance. Thus, it is important to assess how our health system is utilizing this antimicrobial stewardship tactic. With the MRSA PCR’s high NPV, providers can be confident that MRSA pneumonia is unlikely in the absence of MRSA colonization. Our study established a NPV of 98.5%, which is similar to other studies, all of which have shown NPVs greater than 95%.5-8 Despite the high NPV, this study demonstrated that only 51.4% of patients with LRTI had orders for a MRSA PCR. Of the 155 patients with a MRSA PCR, the test was utilized appropriately only 60.6% of the time. A majority of the inappropriately utilized tests were due to MRSA PCR orders placed more than 48 hours after anti-MRSA therapy initiation. To our knowledge, no other studies have assessed the clinical utility of MRSA PCR nasal swabs as an antimicrobial stewardship tool in a diverse health system; therefore, these results are useful to guide future practices at our institution. There is a clear need for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing for patients with LRTI being treated with anti-MRSA therapy. Additionally, clinician education regarding the initial timing of the MRSA PCR order and the proper utilization of the results of the MRSA PCR likely will benefit patient outcomes at our institution.
When evaluating anti-MRSA DOT, this study demonstrated a reduction of only 0.18 days (about 4 hours) of anti-MRSA therapy in the patients who received MRSA PCR testing compared to the patients without a MRSA PCR ordered. Our anti-MRSA DOT reduction was lower than what has been reported in similar studies. For example, Baby et al found that the use of the MRSA PCR was associated with 46.6 fewer hours of unnecessary antimicrobial treatment. Willis et al evaluated a pharmacist-driven protocol that resulted in a reduction of 1.8 days of anti-MRSA therapy, despite a protocol compliance rate of only 55%.9,10 In our study, the patients in the MRSA PCR group appeared to be significantly more ill than those in the no MRSA PCR group, which may be the reason for the incongruences in our results compared to the current literature. Characteristics such as ICU admissions, positive chest radiographs, sepsis cases, pulmonary consults, and carbapenem usage—all of which are indicative of a sicker population—were more prevalent in the MRSA PCR group. This sicker population could have underestimated the reduction of DOT in the MRSA PCR group compared to the no MRSA PCR group.
After isolating the MRSA PCR patients in the subgroup analysis, anti-MRSA DOT was 1.5 days shorter when the test was appropriately utilized, which is more comparable to what has been reported in the literature.9,10 Only 60.6% of the MRSA PCR patients had their anti-MRSA therapy appropriately managed based on the MRSA PCR. Interestingly, a majority of patients in the inappropriate utilization group had MRSA PCR tests ordered more than 48 hours after beginning anti-MRSA therapy. More prompt and efficient ordering of the MRSA PCR may have resulted in more opportunities for earlier de-escalation of therapy. Due to these factors, the patients in the inappropriate utilization group could have further contributed to the underestimated difference in anti-MRSA DOT between the MRSA PCR and no MRSA PCR patients in the primary outcome. Additionally, there were no notable differences between the appropriate and inappropriate utilization groups, unlike in the MRSA PCR and no MRSA PCR groups, which is why we were able to draw more robust conclusions in the subgroup analysis. Therefore, the subgroup analysis confirmed that if the results of the MRSA PCR are used appropriately to guide anti-MRSA therapy, patients can potentially avoid 36 hours of broad-spectrum antibiotics.
Data on how the utilization of the MRSA PCR nasal swab can affect LOS are limited; however, one study did report a 2.8-day reduction in LOS after implementation of a pharmacist-driven MRSA PCR nasal swab protocol.11 Our study demonstrated that LOS was significantly longer in the MRSA PCR group than in the no MRSA PCR group. This result was likely affected by the aforementioned sicker MRSA PCR population. Our multivariate analysis further confirmed that ICU admissions were associated with a longer LOS, and, given that the MRSA PCR group had a significantly higher ICU population, this likely confounded these results. If our 2 groups had had more evenly distributed characteristics, it is possible that we could have found a shorter LOS in the MRSA PCR group, similar to what is reported in the literature. In the subgroup analysis, LOS was 2 days shorter in the appropriate utilization group compared to the inappropriate utilization group. This further affirms that the results of the MRSA PCR must be used appropriately in order for patient outcomes, like LOS, to benefit.
The effects of the MRSA PCR nasal swab on 30-day readmission rates and incidence of AKI are not well-documented in the literature. One study did report 30-day readmission rates as an outcome, but did not cite any difference after the implementation of a protocol that utilized MRSA PCR nasal swab testing.12 The outcome of AKI is slightly better represented in the literature, but the results are conflicting. Some studies report no difference after the implementation of a MRSA PCR-based protocol,11 and others report a significant decrease in AKI with the use of the MRSA PCR.9 Our study detected no difference in 30-day readmission rates related to the previous admission or in AKI between the MRSA PCR and no MRSA PCR populations. In the subgroup analysis, 30-day readmission rates were significantly higher in the MRSA PCR group with appropriate utilization than in the group with inappropriate utilization; however, our study was not powered to detect a difference in this secondary outcome.
This study had some limitations that may have affected our results. First, this study was a retrospective chart review. Additionally, the baseline characteristics were not well balanced across the different groups. There were sicker patients in the MRSA PCR group, which may have led to an underestimate of the reduction in DOT and LOS in these patients. Finally, we did not include enough patient records to reach power in the MRSA PCR group due to a higher than expected number of patients meeting exclusion criteria. Had we attained sufficient power, there may have been more profound reductions in DOT and LOS.
Conclusion
MRSA infections are a common cause for hospitalization, and there is a growing need for antimicrobial stewardship efforts to limit unnecessary antibiotic usage in order to prevent resistance. As illustrated in our study, appropriate utilization of the MRSA PCR can reduce DOT up to 1.5 days. However, our results suggest that there is room for provider and pharmacist education to increase the use of MRSA PCR nasal swab testing in patients with LRTI receiving anti-MRSA therapy. Further emphasis on the appropriate utilization of the MRSA PCR within our health care system is essential.
Corresponding author: Casey Dempsey, PharmD, BCIDP, 80 Seymour St., Hartford, CT 06106; [email protected].
Financial disclosures: None.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
1. Antimicrobial resistance threats. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest-threats.html. Accessed September 9, 2020.
2. Biggest threats and data. Centers for Disease Control and Prevention web site. www.cdc.gov/drugresistance/biggest_threats.html#mrsa. Accessed September 9, 2020.
3. Smith MN, Erdman MJ, Ferreira JA, et al. Clinical utility of methicillin-resistant Staphylococcus aureus nasal polymerase chain reaction assay in critically ill patients with nosocomial pneumonia. J Crit Care. 2017;38:168-171.
4. Giancola SE, Nguyen AT, Le B, et al. Clinical utility of a nasal swab methicillin-resistant Staphylococcus aureus polymerase chain reaction test in intensive and intermediate care unit patients with pneumonia. Diagn Microbiol Infect Dis. 2016;86:307-310.
5. Dangerfield B, Chung A, Webb B, Seville MT. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother. 2014;58:859-864.
6. Johnson JA, Wright ME, Sheperd LA, et al. Nasal methicillin-resistant Staphylococcus aureus polymerase chain reaction: a potential use in guiding antibiotic therapy for pneumonia. Perm J. 2015;19: 34-36.
7. Dureau AF, Duclos G, Antonini F, et al. Rapid diagnostic test and use of antibiotic against methicillin-resistant Staphylococcus aureus in adult intensive care unit. Eur J Clin Microbiol Infect Dis. 2017;36:267-272.
8. Tilahun B, Faust AC, McCorstin P, Ortegon A. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care. 2015;24:8-12.
9. Baby N, Faust AC, Smith T, et al. Nasal methicillin-resistant Staphylococcus aureus (MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother. 2017;61:e02432-16.
10. Willis C, Allen B, Tucker C, et al. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus surveillance protocol. Am J Health-Syst Pharm. 2017;74:1765-1773.
11. Dadzie P, Dietrich T, Ashurst J. Impact of a pharmacist-driven methicillin-resistant Staphylococcus aureus polymerase chain reaction nasal swab protocol on the de-escalation of empiric vancomycin in patients with pneumonia in a rural healthcare setting. Cureus. 2019;11:e6378
12. Dunaway S, Orwig KW, Arbogast ZQ, et al. Evaluation of a pharmacy-driven methicillin-resistant Staphylococcus aureus surveillance protocol in pneumonia. Int J Clin Pharm. 2018;40;526-532.
Wildfires’ toxic air leaves damage long after the smoke clears
When researchers arrived in Seeley Lake, Mont., a town tucked in the northern Rockies, 3 years ago, they could still smell the smoke a day after it cleared from devastating wildfires. Their plan was to chart how long it took for people to recover from living for 7 weeks surrounded by relentless smoke.
They still don’t know, because most residents haven’t recovered. In fact, they’ve gotten worse.
Forest fires had funneled hazardous air into Seeley Lake, a town of fewer than 2,000 people, for 49 days. The air quality was so bad that on some days the monitoring stations couldn’t measure the extent of the pollution. The intensity of the smoke and the length of time residents had been trapped in it were unprecedented, prompting county officials to issue their first evacuation orders because of smoke, not fire risk.
Many people stayed. That made Seeley Lake an ideal place to track the long-term health of people inundated by wildfire pollution.
So far, researchers have found that people’s lung capacity declined in the first 2 years after the smoke cleared. Chris Migliaccio, PhD, an immunologist with the University of Montana, Missoula, and associates found the percentage of residents whose lung function sank below normal thresholds more than doubled in the first year after the fire and remained low a year after that.
“There’s something wrong there,” Dr. Migliaccio said.
While it’s long been known that smoke can be dangerous when in the thick of it – triggering asthma attacks, cardiac arrests, hospitalizations and more – the Seeley Lake research confirmed what public health experts feared: Wildfire haze can have consequences long after it’s gone.
That doesn’t bode well for the 78 million people in the western United States now confronting historic wildfires.
Toxic air from fires has blanketed California and the Pacific Northwest for weeks now, causing some of the world’s worst air quality. California fires have burned roughly 2.3 million acres so far this year, and the wildfire season isn’t over yet. Oregon estimates 500,000 people in the state have been under a notice to either prepare to evacuate or leave. Smoke from the West Coast blazes has drifted as far away as Europe.
Extreme wildfires are predicted to become a regular occurrence because of climate change. And, as more people increasingly settle in fire-prone places, the risks increase. That’s shifted wildfires from being a perennial reality for rural mountain towns to becoming an annual threat for areas across the West.
Perry Hystad, PhD, an associate professor at Oregon State University, Corvallis, said the Seeley Lake research offers unique insights into wildfire smoke’s impact, which until recently had largely been unexplored. He said similar studies are likely to follow because of this fire season.
“This is the question that everybody is asking,” Dr. Hystad said. “‘I’ve been sitting in smoke for 2 weeks, how concerned should I be?’”
Dr. Migliaccio wants to know whether the lung damage he saw in Seeley Lake is reversible – or even treatable. (Think of an inhaler for asthma or other medication that prevents swollen airways.)
But those discoveries will have to wait. The team hasn’t been able to return to Seeley Lake this year because of the coronavirus pandemic.
Dr. Migliaccio said more research is needed on whether wildfire smoke damages organs besides the lungs, and whether routine exposure makes people more susceptible to diseases.
The combination of the fire season and the pandemic has spurred other questions as well, like whether heavy smoke exposure could lead to more COVID-19 deaths. A recent study showed a spike in influenza cases following major fire seasons.
“Now you have the combination of flu season and COVID and the wildfires,” Dr. Migliaccio said. “How are all these things going to interact come late fall or winter?”
A case study
Seeley Lake has long known smoke. It sits in a narrow valley between vast stretches of thick forests.
On a recent September day, Boyd Gossard stood on his back porch and pointed toward the mountains that were ablaze in 2017.
Mr. Gossard, 80, expects to have some summer days veiled in haze. But that year, he said, he could hardly see his neighbor’s house a few hundred feet away.
“I’ve seen a lot of smoke in my career,” said Mr. Gossard, who worked in timber management and served as a wildland firefighter. “But having to just live in it like this was very different. It got to you after a while.”
When Missoula County health officials urged people to leave town and flee the hazardous smoke, many residents stayed close to home. Some said their jobs wouldn’t let them leave. Others didn’t have a place to go – or the money to get there.
Health officials warned those who stayed to avoid exercising and breathing too hard, to remain inside, and to follow steps to make their homes as smoke free as possible. The health department also worked to get air filters to those who needed them most.
But when flames got too close, some people had to sleep outside in campsites on the other side of town.
Understanding the science of smoke
One of the known dangers of smoke is particulate matter. Smaller than the width of a human hair, it can bypass a body’s defenses, lodging deep into lungs. Lu Hu, PhD, an atmospheric chemist with the University of Montana, said air quality reports are based on how much of that pollution is in the air.
“It’s like lead; there’s no safe level, but still we have a safety measure for what’s allowable,” Dr. Hu said. “Some things kill you fast and some things kill you slowly.”
While air quality measurements can gauge the overall amount of pollution, they can’t assess which specific toxins people are inhaling. Dr. Hu is collaborating with other scientists to better predict how smoke travels and what pollutants people actually breathe.
He said smoke’s chemistry changes based on how far it travels and what’s burning, among other factors.
Over the past few years, teams of researchers drove trucks along fire lines to collect smoke samples. Other scientists boarded cargo planes and flew into smoke plumes to take samples right from a fire’s source. Still others stationed at a mountain lookout captured smoke drifting in from nearby fires. And ground-level machines at a Missoula site logged data over 2 summers.
Bob Yokelson, PhD, a longtime smoke researcher with the University of Montana, said scientists are getting closer to understanding its contents. And, he said, “it’s not all bad news.”
Temperature and sunlight can change some pollutants over time. Some dangerous particles seem to disappear. But others, such as ozone, can increase as smoke ages.
Dr. Yokelson said scientists are still a long way from determining a safe level of exposure to the hundred-odd pollutants in smoke.
“We can complete the circle by measuring not only what’s in smoke, but measuring what’s happening to the people who breathe it,” Dr. Yokelson said. “That’s where the future of health research on smoke is going to go.”
Coping with nowhere to flee
In the meantime, those studying wildland smoke hope what they’ve learned so far can better prepare people to live in the haze when evacuation isn’t an option.
Joan Wollan, 82, was one of the Seeley Lake study participants. She stayed put during the 2017 fire because her house at the time sat on a border of the evacuation zone. The air made her eyes burn and her husband cough. She ordered air filters to create cleaner air inside her home, which helped.
On a recent day, the air in Mrs. Wollan’s new neighborhood in Missoula turned that familiar gray-orange as traces of fires from elsewhere appeared. Local health officials warned that western Montana could get hit by some of the worst air quality the state had seen since those 2017 fires.
If it got bad enough, Mrs. Wollan said, she’d get the filters out of storage or look for a way to get to cleaner air – “if there is someplace in Montana that isn’t smoky.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
When researchers arrived in Seeley Lake, Mont., a town tucked in the northern Rockies, 3 years ago, they could still smell the smoke a day after it cleared from devastating wildfires. Their plan was to chart how long it took for people to recover from living for 7 weeks surrounded by relentless smoke.
They still don’t know, because most residents haven’t recovered. In fact, they’ve gotten worse.
Forest fires had funneled hazardous air into Seeley Lake, a town of fewer than 2,000 people, for 49 days. The air quality was so bad that on some days the monitoring stations couldn’t measure the extent of the pollution. The intensity of the smoke and the length of time residents had been trapped in it were unprecedented, prompting county officials to issue their first evacuation orders because of smoke, not fire risk.
Many people stayed. That made Seeley Lake an ideal place to track the long-term health of people inundated by wildfire pollution.
So far, researchers have found that people’s lung capacity declined in the first 2 years after the smoke cleared. Chris Migliaccio, PhD, an immunologist with the University of Montana, Missoula, and associates found the percentage of residents whose lung function sank below normal thresholds more than doubled in the first year after the fire and remained low a year after that.
“There’s something wrong there,” Dr. Migliaccio said.
While it’s long been known that smoke can be dangerous when in the thick of it – triggering asthma attacks, cardiac arrests, hospitalizations and more – the Seeley Lake research confirmed what public health experts feared: Wildfire haze can have consequences long after it’s gone.
That doesn’t bode well for the 78 million people in the western United States now confronting historic wildfires.
Toxic air from fires has blanketed California and the Pacific Northwest for weeks now, causing some of the world’s worst air quality. California fires have burned roughly 2.3 million acres so far this year, and the wildfire season isn’t over yet. Oregon estimates 500,000 people in the state have been under a notice to either prepare to evacuate or leave. Smoke from the West Coast blazes has drifted as far away as Europe.
Extreme wildfires are predicted to become a regular occurrence because of climate change. And, as more people increasingly settle in fire-prone places, the risks increase. That’s shifted wildfires from being a perennial reality for rural mountain towns to becoming an annual threat for areas across the West.
Perry Hystad, PhD, an associate professor at Oregon State University, Corvallis, said the Seeley Lake research offers unique insights into wildfire smoke’s impact, which until recently had largely been unexplored. He said similar studies are likely to follow because of this fire season.
“This is the question that everybody is asking,” Dr. Hystad said. “‘I’ve been sitting in smoke for 2 weeks, how concerned should I be?’”
Dr. Migliaccio wants to know whether the lung damage he saw in Seeley Lake is reversible – or even treatable. (Think of an inhaler for asthma or other medication that prevents swollen airways.)
But those discoveries will have to wait. The team hasn’t been able to return to Seeley Lake this year because of the coronavirus pandemic.
Dr. Migliaccio said more research is needed on whether wildfire smoke damages organs besides the lungs, and whether routine exposure makes people more susceptible to diseases.
The combination of the fire season and the pandemic has spurred other questions as well, like whether heavy smoke exposure could lead to more COVID-19 deaths. A recent study showed a spike in influenza cases following major fire seasons.
“Now you have the combination of flu season and COVID and the wildfires,” Dr. Migliaccio said. “How are all these things going to interact come late fall or winter?”
A case study
Seeley Lake has long known smoke. It sits in a narrow valley between vast stretches of thick forests.
On a recent September day, Boyd Gossard stood on his back porch and pointed toward the mountains that were ablaze in 2017.
Mr. Gossard, 80, expects to have some summer days veiled in haze. But that year, he said, he could hardly see his neighbor’s house a few hundred feet away.
“I’ve seen a lot of smoke in my career,” said Mr. Gossard, who worked in timber management and served as a wildland firefighter. “But having to just live in it like this was very different. It got to you after a while.”
When Missoula County health officials urged people to leave town and flee the hazardous smoke, many residents stayed close to home. Some said their jobs wouldn’t let them leave. Others didn’t have a place to go – or the money to get there.
Health officials warned those who stayed to avoid exercising and breathing too hard, to remain inside, and to follow steps to make their homes as smoke free as possible. The health department also worked to get air filters to those who needed them most.
But when flames got too close, some people had to sleep outside in campsites on the other side of town.
Understanding the science of smoke
One of the known dangers of smoke is particulate matter. Smaller than the width of a human hair, it can bypass a body’s defenses, lodging deep into lungs. Lu Hu, PhD, an atmospheric chemist with the University of Montana, said air quality reports are based on how much of that pollution is in the air.
“It’s like lead; there’s no safe level, but still we have a safety measure for what’s allowable,” Dr. Hu said. “Some things kill you fast and some things kill you slowly.”
While air quality measurements can gauge the overall amount of pollution, they can’t assess which specific toxins people are inhaling. Dr. Hu is collaborating with other scientists to better predict how smoke travels and what pollutants people actually breathe.
He said smoke’s chemistry changes based on how far it travels and what’s burning, among other factors.
Over the past few years, teams of researchers drove trucks along fire lines to collect smoke samples. Other scientists boarded cargo planes and flew into smoke plumes to take samples right from a fire’s source. Still others stationed at a mountain lookout captured smoke drifting in from nearby fires. And ground-level machines at a Missoula site logged data over 2 summers.
Bob Yokelson, PhD, a longtime smoke researcher with the University of Montana, said scientists are getting closer to understanding its contents. And, he said, “it’s not all bad news.”
Temperature and sunlight can change some pollutants over time. Some dangerous particles seem to disappear. But others, such as ozone, can increase as smoke ages.
Dr. Yokelson said scientists are still a long way from determining a safe level of exposure to the hundred-odd pollutants in smoke.
“We can complete the circle by measuring not only what’s in smoke, but measuring what’s happening to the people who breathe it,” Dr. Yokelson said. “That’s where the future of health research on smoke is going to go.”
Coping with nowhere to flee
In the meantime, those studying wildland smoke hope what they’ve learned so far can better prepare people to live in the haze when evacuation isn’t an option.
Joan Wollan, 82, was one of the Seeley Lake study participants. She stayed put during the 2017 fire because her house at the time sat on a border of the evacuation zone. The air made her eyes burn and her husband cough. She ordered air filters to create cleaner air inside her home, which helped.
On a recent day, the air in Mrs. Wollan’s new neighborhood in Missoula turned that familiar gray-orange as traces of fires from elsewhere appeared. Local health officials warned that western Montana could get hit by some of the worst air quality the state had seen since those 2017 fires.
If it got bad enough, Mrs. Wollan said, she’d get the filters out of storage or look for a way to get to cleaner air – “if there is someplace in Montana that isn’t smoky.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
When researchers arrived in Seeley Lake, Mont., a town tucked in the northern Rockies, 3 years ago, they could still smell the smoke a day after it cleared from devastating wildfires. Their plan was to chart how long it took for people to recover from living for 7 weeks surrounded by relentless smoke.
They still don’t know, because most residents haven’t recovered. In fact, they’ve gotten worse.
Forest fires had funneled hazardous air into Seeley Lake, a town of fewer than 2,000 people, for 49 days. The air quality was so bad that on some days the monitoring stations couldn’t measure the extent of the pollution. The intensity of the smoke and the length of time residents had been trapped in it were unprecedented, prompting county officials to issue their first evacuation orders because of smoke, not fire risk.
Many people stayed. That made Seeley Lake an ideal place to track the long-term health of people inundated by wildfire pollution.
So far, researchers have found that people’s lung capacity declined in the first 2 years after the smoke cleared. Chris Migliaccio, PhD, an immunologist with the University of Montana, Missoula, and associates found the percentage of residents whose lung function sank below normal thresholds more than doubled in the first year after the fire and remained low a year after that.
“There’s something wrong there,” Dr. Migliaccio said.
While it’s long been known that smoke can be dangerous when in the thick of it – triggering asthma attacks, cardiac arrests, hospitalizations and more – the Seeley Lake research confirmed what public health experts feared: Wildfire haze can have consequences long after it’s gone.
That doesn’t bode well for the 78 million people in the western United States now confronting historic wildfires.
Toxic air from fires has blanketed California and the Pacific Northwest for weeks now, causing some of the world’s worst air quality. California fires have burned roughly 2.3 million acres so far this year, and the wildfire season isn’t over yet. Oregon estimates 500,000 people in the state have been under a notice to either prepare to evacuate or leave. Smoke from the West Coast blazes has drifted as far away as Europe.
Extreme wildfires are predicted to become a regular occurrence because of climate change. And, as more people increasingly settle in fire-prone places, the risks increase. That’s shifted wildfires from being a perennial reality for rural mountain towns to becoming an annual threat for areas across the West.
Perry Hystad, PhD, an associate professor at Oregon State University, Corvallis, said the Seeley Lake research offers unique insights into wildfire smoke’s impact, which until recently had largely been unexplored. He said similar studies are likely to follow because of this fire season.
“This is the question that everybody is asking,” Dr. Hystad said. “‘I’ve been sitting in smoke for 2 weeks, how concerned should I be?’”
Dr. Migliaccio wants to know whether the lung damage he saw in Seeley Lake is reversible – or even treatable. (Think of an inhaler for asthma or other medication that prevents swollen airways.)
But those discoveries will have to wait. The team hasn’t been able to return to Seeley Lake this year because of the coronavirus pandemic.
Dr. Migliaccio said more research is needed on whether wildfire smoke damages organs besides the lungs, and whether routine exposure makes people more susceptible to diseases.
The combination of the fire season and the pandemic has spurred other questions as well, like whether heavy smoke exposure could lead to more COVID-19 deaths. A recent study showed a spike in influenza cases following major fire seasons.
“Now you have the combination of flu season and COVID and the wildfires,” Dr. Migliaccio said. “How are all these things going to interact come late fall or winter?”
A case study
Seeley Lake has long known smoke. It sits in a narrow valley between vast stretches of thick forests.
On a recent September day, Boyd Gossard stood on his back porch and pointed toward the mountains that were ablaze in 2017.
Mr. Gossard, 80, expects to have some summer days veiled in haze. But that year, he said, he could hardly see his neighbor’s house a few hundred feet away.
“I’ve seen a lot of smoke in my career,” said Mr. Gossard, who worked in timber management and served as a wildland firefighter. “But having to just live in it like this was very different. It got to you after a while.”
When Missoula County health officials urged people to leave town and flee the hazardous smoke, many residents stayed close to home. Some said their jobs wouldn’t let them leave. Others didn’t have a place to go – or the money to get there.
Health officials warned those who stayed to avoid exercising and breathing too hard, to remain inside, and to follow steps to make their homes as smoke free as possible. The health department also worked to get air filters to those who needed them most.
But when flames got too close, some people had to sleep outside in campsites on the other side of town.
Understanding the science of smoke
One of the known dangers of smoke is particulate matter. Smaller than the width of a human hair, it can bypass a body’s defenses, lodging deep into lungs. Lu Hu, PhD, an atmospheric chemist with the University of Montana, said air quality reports are based on how much of that pollution is in the air.
“It’s like lead; there’s no safe level, but still we have a safety measure for what’s allowable,” Dr. Hu said. “Some things kill you fast and some things kill you slowly.”
While air quality measurements can gauge the overall amount of pollution, they can’t assess which specific toxins people are inhaling. Dr. Hu is collaborating with other scientists to better predict how smoke travels and what pollutants people actually breathe.
He said smoke’s chemistry changes based on how far it travels and what’s burning, among other factors.
Over the past few years, teams of researchers drove trucks along fire lines to collect smoke samples. Other scientists boarded cargo planes and flew into smoke plumes to take samples right from a fire’s source. Still others stationed at a mountain lookout captured smoke drifting in from nearby fires. And ground-level machines at a Missoula site logged data over 2 summers.
Bob Yokelson, PhD, a longtime smoke researcher with the University of Montana, said scientists are getting closer to understanding its contents. And, he said, “it’s not all bad news.”
Temperature and sunlight can change some pollutants over time. Some dangerous particles seem to disappear. But others, such as ozone, can increase as smoke ages.
Dr. Yokelson said scientists are still a long way from determining a safe level of exposure to the hundred-odd pollutants in smoke.
“We can complete the circle by measuring not only what’s in smoke, but measuring what’s happening to the people who breathe it,” Dr. Yokelson said. “That’s where the future of health research on smoke is going to go.”
Coping with nowhere to flee
In the meantime, those studying wildland smoke hope what they’ve learned so far can better prepare people to live in the haze when evacuation isn’t an option.
Joan Wollan, 82, was one of the Seeley Lake study participants. She stayed put during the 2017 fire because her house at the time sat on a border of the evacuation zone. The air made her eyes burn and her husband cough. She ordered air filters to create cleaner air inside her home, which helped.
On a recent day, the air in Mrs. Wollan’s new neighborhood in Missoula turned that familiar gray-orange as traces of fires from elsewhere appeared. Local health officials warned that western Montana could get hit by some of the worst air quality the state had seen since those 2017 fires.
If it got bad enough, Mrs. Wollan said, she’d get the filters out of storage or look for a way to get to cleaner air – “if there is someplace in Montana that isn’t smoky.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Nocturnal oxygen no help for isolated desaturation in COPD
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Nocturnal oxygen therapy for patients with COPD and isolated nocturnal oxygen desaturation does not improve survival or delay disease progression, according to findings published Sept. 17 in The New England Journal of Medicine. The new report adds to evidence that the widely implemented and costly practice may be unnecessary.
Patients with COPD who do not qualify for long-term oxygen therapy (LTOT) are commonly prescribed nocturnal oxygen in the belief that it can delay disease progression, possibly by decreasing alveolar hypoventilation and ventilation-perfusion mismatch.
But investigations so far and the new study from the International Nocturnal Oxygen (INOX) Trial have not borne this out.
“There is no indication that nocturnal oxygen has a positive or negative effect on survival or progression to long-term oxygen therapy in patients with nocturnal hypoxemia in COPD. Consequently, there is no reason for physicians to screen for nocturnal hypoxemia in COPD,” study leader Yves Lacasse, MD, told Medscape Medical News.
Lacasse is from the Institut Universitaire de Cardiologie et de Pneumologie de Québec–Université Laval, Quebec, Canada.
The idea that the therapy helps is firmly entrenched.
In the early 1980s, two trials indicated that patients who had COPD and severe chronic daytime hypoxemia benefit from LTOT (15-18 hours a day or longer).
A decade later, two landmark trials (the Nocturnal Oxygen Therapy Trial and the British Medical Research Council Trial) added to evidence that LTOT may prolong life for patients with COPD and severe daytime hypoxemia.
“The good news from both trials was that oxygen saves lives. From this moment, oxygen therapy became a standard of care, and confirmatory trials would be considered unethical,” Lacasse explained.
“Oxygen therapy gained widespread acceptance by official organizations for treatment of most chronic cardiorespiratory conditions complicated by severe hypoxemia, even if proof of efficacy is lacking. New indications emerged, such as isolated nocturnal oxygen desaturation. Even in COPD, inappropriate prescriptions of home oxygen therapy are not unusual. Oxygen is everywhere,” Lacasse continued.
A meta-analysis from 2005 identified two trials that evaluated home oxygen therapy specifically for isolated nocturnal desaturation. Both found no survival benefit from nocturnal oxygen.
The study by Lacasse and colleagues assessed effects on mortality or worsening of disease (progression to LTOT) with 3-4 years of nocturnal oxygen supplementation.
Participants, whose oxygen saturation was less than 90% for at least 30% of the recording time on nocturnal oximetry, received oxygen or ambient air from a sham device as a placebo for at least 4 hours per session. The goal of treatment was nocturnal oxygen saturation exceeding 90% for at least 90% of the recorded time.
The trial protocol excluded patients with severe obesity, apnea, lung cancer, left heart failure, interstitial lung disease, or bronchiectasis.
The study was initially powered in 2010 to include 600 participants, with half to receive placebo. The study assumed mortality of 20% among control patients over 3 years; 20% of patients progressed to LTOT.
When recruiting lagged, the data safety monitoring board and steering committee extended follow-up to 4 years. In 2014, they requested an interim analysis, and recruitment ceased. Overall, 243 patients participated.
Lacasse cited several reasons for the difficulty with recruitment as well as retention: unwillingness to take the risk of receiving placebo instead of a readily available treatment, fading interest over time, and frailty that affects compliance.
Patients in the study came from 28 community or university-affiliated hospitals in Canada, Portugal, Spain, and France. At the 3-year mark, 39% of patients (48 of 123) who were assigned to nocturnal oxygen therapy and 42% (50 of 119) of those taking placebo had met criteria for LTOT or had died (difference, −3.0 percentage points; P = .64). The groups did not differ appreciably in rates of exacerbation and hospitalization.
The researchers could not analyze subgroups because the patients were very similar with regard to the severity of nocturnal oxygen desaturation, Lacasse said.
Economics enters into the picture – home oxygen therapy is second only to hospitalization as the most expensive healthcare expenditure associated with clinical care for COPD in developed countries. “The math is simple. There is enormous potential for saving money if the results of our clinical trial are applied appropriately,” said Lacasse.
William Bailey, MD, professor emeritus of pulmonary, allergy, and critical care medicine at the University of Alabama at Birmingham, agrees that the practice is overused.
“There is a built-in bias in the medical community. Most believe that anyone with lung disease benefits from oxygen. Even some of our investigators had a hard time believing the results. The study was well designed, carefully carried out, and I feel confident that the results are reliable,” he said.
Shawn P. E. Nishi, MD, director of bronchoscopy and advanced pulmonary procedures, division of pulmonary and critical care medicine, the University of Texas Medical Branch, Galveston, Texas, mentioned the study’s main limitation, which the authors readily acknowledge.
“Unfortunately, the trial had difficulty recruiting subjects, with less than half of expected enrollment achieved, and was underpowered to make any conclusions. Other studies have examined nocturnal oxygen use and have not shown a mortality benefit,” Nishi explained.
She added that the study did not evaluate use of LTOT for improving outcomes other than mortality, including quality of life, cardiovascular morbidity, depression, cognitive function, exercise capacity, and frequency of COPD exacerbations or hospitalization.
Other limitations of the study include suboptimal adherence to the therapy and interpretation of the clinical significance on the basis of a survey of Canadian pulmonologists.
This article first appeared on Medscape.com.
Pharmacologic Management of COPD
A Discussion of the new American Thoracic Society Clinical Practice Guideline
Chronic obstructive pulmonary disease (COPD) is caused by airway and alveolar abnormalities and is the third most common cause of death worldwide. COPD results in airflow obstruction that is not fully reversible. The diagnosis of COPD should be considered in patients over 40 years who have chronic cough and/or dyspnea, particularly if they have a history of tobacco use. The diagnosis is confirmed by a diminished forced expiratory volume in 1 second (FEV1) that is not fully reversible with the use of a bronchodilator and an FEV1/forced vital capacity ratio of less than or equal to 0.7.1
Recommendation 1
Patients with COPD who report dyspnea or exercise intolerance should be treated with both a long-acting muscarinic antagonist (LAMA) and a long-acting beta agonist (LABA) (dual LAMA/LABA therapy) instead of monotherapy, the guideline says.
This recommendation represents a critical change in care and is based on strong evidence. For years practitioners have been using single bronchodilator therapy, often a LAMA as the entrance to treatment for patients with symptomatic COPD. The recommendation to begin treatment with dual bronchodilator therapy is an important one. This is the only recommendation that received a “strong” grade.
The evidence comes from the compilation of 24 randomized controlled trials that altogether included 45,441 patients. Dual therapy versus monotherapy was evaluated by examining differences in dyspnea, health-related quality of life, exacerbations (which were defined as requiring antibiotics, oral steroids, or hospitalizations), and hospitalizations independently. Marked improvements were observed for exacerbations and hospitalizations in the dual LAMA/LABA group, compared with treatment with use of a single bronchodilator. In 22,733 patients across 15 RCTs, there were 88 fewer exacerbations per 1,000 patients with a rate ratio (RR) of 0.80 (P < .002), the guideline states.
The decrease in exacerbations is a critical factor in treating patients with COPD because each exacerbation can lead to a sustained decrease in airflow and increases the risk of future exacerbations.
Recommendation 2
In COPD patients who report dyspnea or exercise intolerance, with an exacerbation in the last year, the guideline recommends triple therapy with an inhaled corticosteroid (ICS) instead of just dual LAMA/LABA therapy.
In the past many clinicians have relegated triple therapy to a “last ditch resort.” This recommendation makes it clear that triple therapy is appropriate for a broad range of patients with moderate to severe COPD.
Recommendation 3
In patients with COPD who are on triple therapy, the inhaled corticosteroid component can be withdrawn if patients have not had an exacerbation within the last year, according to the guideline.
It should be noted that the committee said that the ICS can be withdrawn, not that it necessarily needs to be withdrawn. The data showed that it would be safe to withdraw the ICS, but the data is limited in time to 1 year’s follow-up.
Recommendation 4
ATS was not able to make a recommendation for or against ICS as an additive therapy to LAMA/LABA in those without an exacerbation and elevated blood eosinophilia (defined as ≥2% blood eosinophils or >149 cell/mcL). In those with at least one exacerbation and increased blood eosinophilia, the society does recommend addition of ICS to dual LAMA/LABA therapy.
An area of ongoing discussion is at what point in disease severity, before exacerbations occur, might ICS be useful in preventing a first exacerbation. This awaits further studies and evidence.
Recommendation 5
In COPD patients with frequent and severe exacerbations who are otherwise medically optimized, the ATS advises against the use of maintenance oral corticosteroid therapy.
It has been known and accepted for years that oral steroids should be avoided if at all possible because they have little benefit and can cause significant harm. The guideline reinforces this.
The Bottom Line
Dual LAMA/LABA therapy in symptomatic patients is the standard of care. If a patient has had an exacerbation within the last year, add an ICS to the LAMA/LABA, most conveniently given in the form of triple therapy in one inhaler. Finally, even in refractory COPD, maintenance oral corticosteroids bring more harm than benefit.
Dr. Skolnik is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Matthews is a second-year resident in the family medicine residency program at Abington Jefferson Health.
References
1. Wells C, Joo MJ. COPD and asthma: Diagnostic accuracy requires spirometry. J Fam Pract. 2019;68(2):76-81.
2. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-69.
A Discussion of the new American Thoracic Society Clinical Practice Guideline
A Discussion of the new American Thoracic Society Clinical Practice Guideline
Chronic obstructive pulmonary disease (COPD) is caused by airway and alveolar abnormalities and is the third most common cause of death worldwide. COPD results in airflow obstruction that is not fully reversible. The diagnosis of COPD should be considered in patients over 40 years who have chronic cough and/or dyspnea, particularly if they have a history of tobacco use. The diagnosis is confirmed by a diminished forced expiratory volume in 1 second (FEV1) that is not fully reversible with the use of a bronchodilator and an FEV1/forced vital capacity ratio of less than or equal to 0.7.1
Recommendation 1
Patients with COPD who report dyspnea or exercise intolerance should be treated with both a long-acting muscarinic antagonist (LAMA) and a long-acting beta agonist (LABA) (dual LAMA/LABA therapy) instead of monotherapy, the guideline says.
This recommendation represents a critical change in care and is based on strong evidence. For years practitioners have been using single bronchodilator therapy, often a LAMA as the entrance to treatment for patients with symptomatic COPD. The recommendation to begin treatment with dual bronchodilator therapy is an important one. This is the only recommendation that received a “strong” grade.
The evidence comes from the compilation of 24 randomized controlled trials that altogether included 45,441 patients. Dual therapy versus monotherapy was evaluated by examining differences in dyspnea, health-related quality of life, exacerbations (which were defined as requiring antibiotics, oral steroids, or hospitalizations), and hospitalizations independently. Marked improvements were observed for exacerbations and hospitalizations in the dual LAMA/LABA group, compared with treatment with use of a single bronchodilator. In 22,733 patients across 15 RCTs, there were 88 fewer exacerbations per 1,000 patients with a rate ratio (RR) of 0.80 (P < .002), the guideline states.
The decrease in exacerbations is a critical factor in treating patients with COPD because each exacerbation can lead to a sustained decrease in airflow and increases the risk of future exacerbations.
Recommendation 2
In COPD patients who report dyspnea or exercise intolerance, with an exacerbation in the last year, the guideline recommends triple therapy with an inhaled corticosteroid (ICS) instead of just dual LAMA/LABA therapy.
In the past many clinicians have relegated triple therapy to a “last ditch resort.” This recommendation makes it clear that triple therapy is appropriate for a broad range of patients with moderate to severe COPD.
Recommendation 3
In patients with COPD who are on triple therapy, the inhaled corticosteroid component can be withdrawn if patients have not had an exacerbation within the last year, according to the guideline.
It should be noted that the committee said that the ICS can be withdrawn, not that it necessarily needs to be withdrawn. The data showed that it would be safe to withdraw the ICS, but the data is limited in time to 1 year’s follow-up.
Recommendation 4
ATS was not able to make a recommendation for or against ICS as an additive therapy to LAMA/LABA in those without an exacerbation and elevated blood eosinophilia (defined as ≥2% blood eosinophils or >149 cell/mcL). In those with at least one exacerbation and increased blood eosinophilia, the society does recommend addition of ICS to dual LAMA/LABA therapy.
An area of ongoing discussion is at what point in disease severity, before exacerbations occur, might ICS be useful in preventing a first exacerbation. This awaits further studies and evidence.
Recommendation 5
In COPD patients with frequent and severe exacerbations who are otherwise medically optimized, the ATS advises against the use of maintenance oral corticosteroid therapy.
It has been known and accepted for years that oral steroids should be avoided if at all possible because they have little benefit and can cause significant harm. The guideline reinforces this.
The Bottom Line
Dual LAMA/LABA therapy in symptomatic patients is the standard of care. If a patient has had an exacerbation within the last year, add an ICS to the LAMA/LABA, most conveniently given in the form of triple therapy in one inhaler. Finally, even in refractory COPD, maintenance oral corticosteroids bring more harm than benefit.
Dr. Skolnik is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Matthews is a second-year resident in the family medicine residency program at Abington Jefferson Health.
References
1. Wells C, Joo MJ. COPD and asthma: Diagnostic accuracy requires spirometry. J Fam Pract. 2019;68(2):76-81.
2. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-69.
Chronic obstructive pulmonary disease (COPD) is caused by airway and alveolar abnormalities and is the third most common cause of death worldwide. COPD results in airflow obstruction that is not fully reversible. The diagnosis of COPD should be considered in patients over 40 years who have chronic cough and/or dyspnea, particularly if they have a history of tobacco use. The diagnosis is confirmed by a diminished forced expiratory volume in 1 second (FEV1) that is not fully reversible with the use of a bronchodilator and an FEV1/forced vital capacity ratio of less than or equal to 0.7.1
Recommendation 1
Patients with COPD who report dyspnea or exercise intolerance should be treated with both a long-acting muscarinic antagonist (LAMA) and a long-acting beta agonist (LABA) (dual LAMA/LABA therapy) instead of monotherapy, the guideline says.
This recommendation represents a critical change in care and is based on strong evidence. For years practitioners have been using single bronchodilator therapy, often a LAMA as the entrance to treatment for patients with symptomatic COPD. The recommendation to begin treatment with dual bronchodilator therapy is an important one. This is the only recommendation that received a “strong” grade.
The evidence comes from the compilation of 24 randomized controlled trials that altogether included 45,441 patients. Dual therapy versus monotherapy was evaluated by examining differences in dyspnea, health-related quality of life, exacerbations (which were defined as requiring antibiotics, oral steroids, or hospitalizations), and hospitalizations independently. Marked improvements were observed for exacerbations and hospitalizations in the dual LAMA/LABA group, compared with treatment with use of a single bronchodilator. In 22,733 patients across 15 RCTs, there were 88 fewer exacerbations per 1,000 patients with a rate ratio (RR) of 0.80 (P < .002), the guideline states.
The decrease in exacerbations is a critical factor in treating patients with COPD because each exacerbation can lead to a sustained decrease in airflow and increases the risk of future exacerbations.
Recommendation 2
In COPD patients who report dyspnea or exercise intolerance, with an exacerbation in the last year, the guideline recommends triple therapy with an inhaled corticosteroid (ICS) instead of just dual LAMA/LABA therapy.
In the past many clinicians have relegated triple therapy to a “last ditch resort.” This recommendation makes it clear that triple therapy is appropriate for a broad range of patients with moderate to severe COPD.
Recommendation 3
In patients with COPD who are on triple therapy, the inhaled corticosteroid component can be withdrawn if patients have not had an exacerbation within the last year, according to the guideline.
It should be noted that the committee said that the ICS can be withdrawn, not that it necessarily needs to be withdrawn. The data showed that it would be safe to withdraw the ICS, but the data is limited in time to 1 year’s follow-up.
Recommendation 4
ATS was not able to make a recommendation for or against ICS as an additive therapy to LAMA/LABA in those without an exacerbation and elevated blood eosinophilia (defined as ≥2% blood eosinophils or >149 cell/mcL). In those with at least one exacerbation and increased blood eosinophilia, the society does recommend addition of ICS to dual LAMA/LABA therapy.
An area of ongoing discussion is at what point in disease severity, before exacerbations occur, might ICS be useful in preventing a first exacerbation. This awaits further studies and evidence.
Recommendation 5
In COPD patients with frequent and severe exacerbations who are otherwise medically optimized, the ATS advises against the use of maintenance oral corticosteroid therapy.
It has been known and accepted for years that oral steroids should be avoided if at all possible because they have little benefit and can cause significant harm. The guideline reinforces this.
The Bottom Line
Dual LAMA/LABA therapy in symptomatic patients is the standard of care. If a patient has had an exacerbation within the last year, add an ICS to the LAMA/LABA, most conveniently given in the form of triple therapy in one inhaler. Finally, even in refractory COPD, maintenance oral corticosteroids bring more harm than benefit.
Dr. Skolnik is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Dr. Matthews is a second-year resident in the family medicine residency program at Abington Jefferson Health.
References
1. Wells C, Joo MJ. COPD and asthma: Diagnostic accuracy requires spirometry. J Fam Pract. 2019;68(2):76-81.
2. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56-69.
Many Americans still concerned about access to health care
according to the results of a survey conducted Aug. 7-26.
Nationally, 23.8% of respondents said that they were very concerned about being able to receive care during the pandemic, and another 27.4% said that they were somewhat concerned. Just under a quarter, 24.3%, said they were not very concerned, while 20.4% were not at all concerned, the COVID-19 Consortium for Understanding the Public’s Policy Preferences Across States reported after surveying 21,196 adults.
At the state level, Mississippi had the most adults (35.5%) who were very concerned about their access to care, followed by Texas (32.7%) and Nevada (32.4%). The residents of Montana were least likely (10.5%) to be very concerned, with Vermont next at 11.6% and Wyoming slightly higher at 13.8%. Montana also had the highest proportion of adults, 30.2%, who were not at all concerned, the consortium’s data show.
When asked about getting the coronavirus themselves, 67.8% of U.S. adults came down on the concerned side (33.3% somewhat and 34.5% very concerned) versus 30.8% who were not concerned (18.6% were not very concerned; 12.2% were not concerned at all.). Respondents’ concern was higher for their family members’ risk of getting coronavirus: 30.2% were somewhat concerned and 47.6% were very concerned, the consortium said.
Among many other topics, respondents were asked how closely they had followed recommended health guidelines in the last week, with the two extremes shown here:
- Avoiding contact with other people: 49.3% very closely, 4.8% not at all closely.
- Frequently washing hands: 74.7% very, 1.6% not at all.
- Disinfecting often-touched surfaces: 54.4% very, 4.3% not at all.
- Wearing a face mask in public: 75.7% very, 3.5% not at all.
The consortium is a joint project of the Network Science Institute of Northeastern University; the Shorenstein Center on Media, Politics, and Public Policy of Harvard University; Harvard Medical School; the School of Communication and Information at Rutgers University; and the department of political science at Northwestern University. The project is supported by grants from the National Science Foundation.
according to the results of a survey conducted Aug. 7-26.
Nationally, 23.8% of respondents said that they were very concerned about being able to receive care during the pandemic, and another 27.4% said that they were somewhat concerned. Just under a quarter, 24.3%, said they were not very concerned, while 20.4% were not at all concerned, the COVID-19 Consortium for Understanding the Public’s Policy Preferences Across States reported after surveying 21,196 adults.
At the state level, Mississippi had the most adults (35.5%) who were very concerned about their access to care, followed by Texas (32.7%) and Nevada (32.4%). The residents of Montana were least likely (10.5%) to be very concerned, with Vermont next at 11.6% and Wyoming slightly higher at 13.8%. Montana also had the highest proportion of adults, 30.2%, who were not at all concerned, the consortium’s data show.
When asked about getting the coronavirus themselves, 67.8% of U.S. adults came down on the concerned side (33.3% somewhat and 34.5% very concerned) versus 30.8% who were not concerned (18.6% were not very concerned; 12.2% were not concerned at all.). Respondents’ concern was higher for their family members’ risk of getting coronavirus: 30.2% were somewhat concerned and 47.6% were very concerned, the consortium said.
Among many other topics, respondents were asked how closely they had followed recommended health guidelines in the last week, with the two extremes shown here:
- Avoiding contact with other people: 49.3% very closely, 4.8% not at all closely.
- Frequently washing hands: 74.7% very, 1.6% not at all.
- Disinfecting often-touched surfaces: 54.4% very, 4.3% not at all.
- Wearing a face mask in public: 75.7% very, 3.5% not at all.
The consortium is a joint project of the Network Science Institute of Northeastern University; the Shorenstein Center on Media, Politics, and Public Policy of Harvard University; Harvard Medical School; the School of Communication and Information at Rutgers University; and the department of political science at Northwestern University. The project is supported by grants from the National Science Foundation.
according to the results of a survey conducted Aug. 7-26.
Nationally, 23.8% of respondents said that they were very concerned about being able to receive care during the pandemic, and another 27.4% said that they were somewhat concerned. Just under a quarter, 24.3%, said they were not very concerned, while 20.4% were not at all concerned, the COVID-19 Consortium for Understanding the Public’s Policy Preferences Across States reported after surveying 21,196 adults.
At the state level, Mississippi had the most adults (35.5%) who were very concerned about their access to care, followed by Texas (32.7%) and Nevada (32.4%). The residents of Montana were least likely (10.5%) to be very concerned, with Vermont next at 11.6% and Wyoming slightly higher at 13.8%. Montana also had the highest proportion of adults, 30.2%, who were not at all concerned, the consortium’s data show.
When asked about getting the coronavirus themselves, 67.8% of U.S. adults came down on the concerned side (33.3% somewhat and 34.5% very concerned) versus 30.8% who were not concerned (18.6% were not very concerned; 12.2% were not concerned at all.). Respondents’ concern was higher for their family members’ risk of getting coronavirus: 30.2% were somewhat concerned and 47.6% were very concerned, the consortium said.
Among many other topics, respondents were asked how closely they had followed recommended health guidelines in the last week, with the two extremes shown here:
- Avoiding contact with other people: 49.3% very closely, 4.8% not at all closely.
- Frequently washing hands: 74.7% very, 1.6% not at all.
- Disinfecting often-touched surfaces: 54.4% very, 4.3% not at all.
- Wearing a face mask in public: 75.7% very, 3.5% not at all.
The consortium is a joint project of the Network Science Institute of Northeastern University; the Shorenstein Center on Media, Politics, and Public Policy of Harvard University; Harvard Medical School; the School of Communication and Information at Rutgers University; and the department of political science at Northwestern University. The project is supported by grants from the National Science Foundation.











