User login
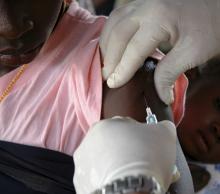
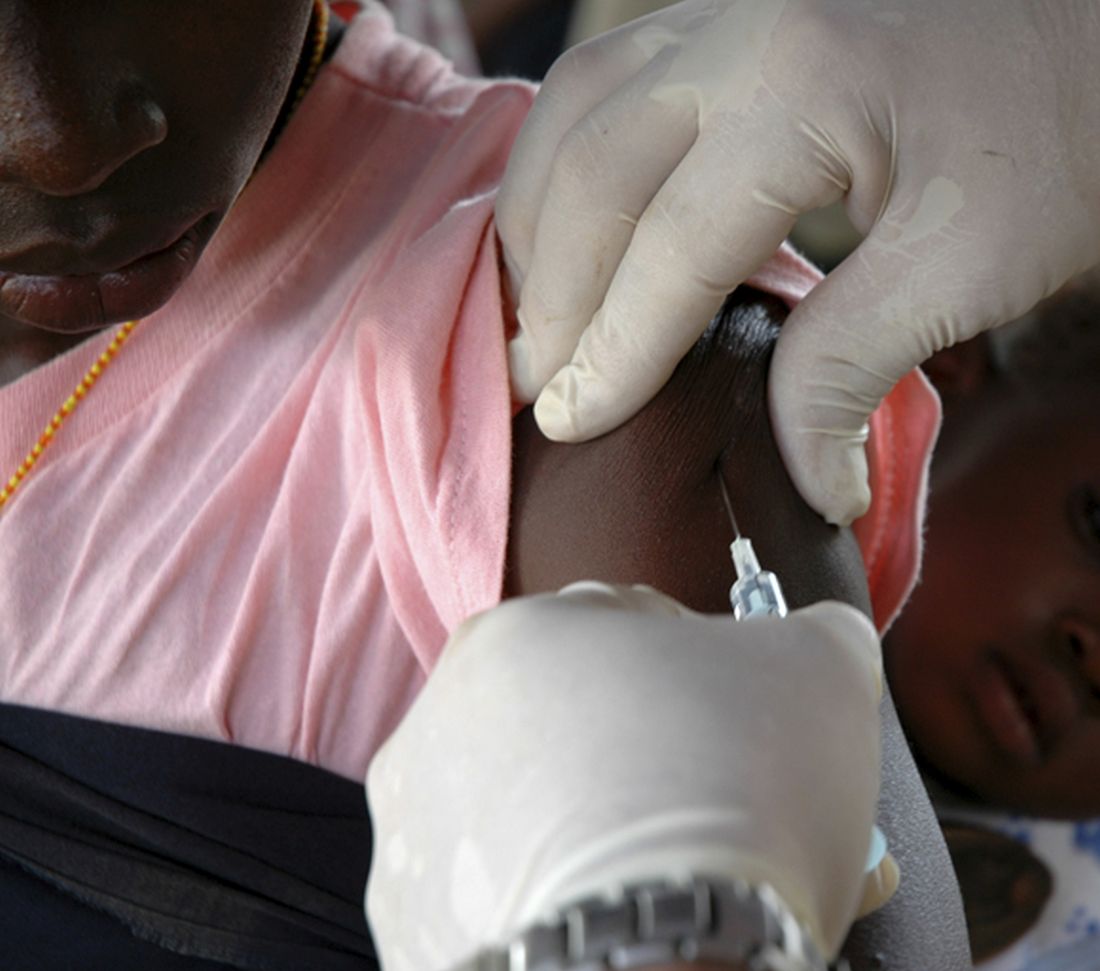
Cannabis vaping triggers respiratory symptoms in teens
, according to findings of a study based on a national sample of teens.
Most studies of electronic nicotine delivery systems (ENDS) use in teens have not addressed cannabis vaping, although e-cigarette– or vaping product use–associated lung injury (EVALI) has been predominately associated with cannabis products, wrote Carol J. Boyd, PhD, of the University of Michigan School of Nursing, Ann Arbor, and colleagues.
“At this time, relatively little is known about the population-level health consequences of adolescents’ use of ENDS, including use with cannabis and controlling for a history of asthma,” they said.
In a study published in the Journal of Adolescent Health, the researchers identified 14,798 adolescents aged 12-17 years using Wave 4 data from the Population Assessment of Tobacco and Health Study. Of these, 17.6% had a baseline asthma diagnosis, 8.9% reported ever using cannabis in ENDS, and 4.7% reported any cannabis use. In addition, 4.2% reported current e-cigarette use, 3.1% reported current cigarette use, 51% were male, and 69.2% were white.
Any cannabis vaping makes impact
In a fully-adjusted model, teens who had ever vaped cannabis had higher odds of five respiratory symptoms in the past year, compared with those with no history of cannabis vaping: wheezing or whistling in the chest (adjusted odds ratio, 1.81); sleep disturbed by wheezing or whistling (AOR, 1.71); speech limited because of wheezing (AOR, 1.96); wheezy during and after exercise (AOR, 1.33), and a dry cough at night independent of a cold or chest infection (AOR, 1.26).
Neither e-cigarettes nor cigarettes were significantly associated with any of these five respiratory symptoms in the fully adjusted models. In addition, “past 30-day use of cigarettes, e-cigarettes and cannabis use were associated with some respiratory symptoms in bivariate analyses but not in the adjusted models,” the researchers noted. In addition, the associations of an asthma diagnosis and respiratory symptoms had greater magnitudes than either cigarette, e-cigarette, and cannabis use or vaping cannabis with ENDS.
The study findings were limited by several factors including the inherent limitations of secondary database analysis, the researchers noted. “Another limitation is that co-use of cannabis and tobacco/nicotine was not assessed and, in the future, should be examined: Researchers have found that co-use is related to EVALI symptoms among young adults,” they said.
However, the study is the first known to include ENDS product use and respiratory symptoms while accounting for baseline asthma, and an asthma diagnosis was even more strongly associated with all five respiratory symptoms, the researchers said.
The results suggest that “the inhalation of cannabis via vaping is associated with some pulmonary irritation and symptoms of lung diseases (both known and unknown),” that may be predictive of later EVALI, they concluded.
Product details aid in diagnosis
“As we continue to see patients presenting with EVALI in pediatric hospitals, it is important for us to identify if there are specific products (or categories) that are more likely to cause it,” said Brandon Seay, MD, FCCP, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, in an interview. “When we are trying to diagnose EVALI, we should be asking appropriate questions about exposures to specific products to get the best answers. If we simply ask ‘Are you smoking e-cigarettes?’ the patient may not [equate] e-cigarette smoking to vaping cannabis products,” he said.
Dr. Seay said he was not surprised by the study findings. “A lot of the patients I see with EVALI have reported vaping THC products, and most of them also report that the products were mixed by a friend or an individual instead of being a commercially produced product,” he noted. “This is not surprising, as THC is still illegal in most states and there would not be any commercially available products,” he said. “The mixing of these products by individuals increases the risk of ingredients being more toxic or irritating to the lungs,” Dr. Seay added. “This does highlight the need for more regulation of vaping products. As more states legalize marijuana, more of these products will become available, which will provide an opportunity for increased regulation, he said.
The take-home message for clinicians is to seek specific details from their young patients, Dr. Seay emphasized. “When we are educating our patients on the dangers of vaping/e-cigarettes, we need to make sure we are asking specifically which products they are using and know the terminology,” he said. “The use of THC-containing products will be increasing across the country with more legalization, so we need to keep ourselves apprised of the different risks between THC- and nicotine-containing devices,” he added.
As for additional research, it would be interesting to know whether patients were asked where they had gotten their products (commercially available products vs. those mixed by individuals) and explore any difference between the two, said Dr. Seay. “Also, as these products are relatively new to the market, compared to cigarettes, data on the longitudinal effects of vaping (nicotine and THC) over a long period of time, compared to traditional combustible cigarettes, will be needed,” he said.
The study was funded by grants from the National Institutes of Health, National Institute on Drug Abuse, and National Cancer Institute. The researchers had no financial conflicts to disclose.
Dr. Seay had no financial disclosures, but serves as a member of the CHEST Physician editorial board.
, according to findings of a study based on a national sample of teens.
Most studies of electronic nicotine delivery systems (ENDS) use in teens have not addressed cannabis vaping, although e-cigarette– or vaping product use–associated lung injury (EVALI) has been predominately associated with cannabis products, wrote Carol J. Boyd, PhD, of the University of Michigan School of Nursing, Ann Arbor, and colleagues.
“At this time, relatively little is known about the population-level health consequences of adolescents’ use of ENDS, including use with cannabis and controlling for a history of asthma,” they said.
In a study published in the Journal of Adolescent Health, the researchers identified 14,798 adolescents aged 12-17 years using Wave 4 data from the Population Assessment of Tobacco and Health Study. Of these, 17.6% had a baseline asthma diagnosis, 8.9% reported ever using cannabis in ENDS, and 4.7% reported any cannabis use. In addition, 4.2% reported current e-cigarette use, 3.1% reported current cigarette use, 51% were male, and 69.2% were white.
Any cannabis vaping makes impact
In a fully-adjusted model, teens who had ever vaped cannabis had higher odds of five respiratory symptoms in the past year, compared with those with no history of cannabis vaping: wheezing or whistling in the chest (adjusted odds ratio, 1.81); sleep disturbed by wheezing or whistling (AOR, 1.71); speech limited because of wheezing (AOR, 1.96); wheezy during and after exercise (AOR, 1.33), and a dry cough at night independent of a cold or chest infection (AOR, 1.26).
Neither e-cigarettes nor cigarettes were significantly associated with any of these five respiratory symptoms in the fully adjusted models. In addition, “past 30-day use of cigarettes, e-cigarettes and cannabis use were associated with some respiratory symptoms in bivariate analyses but not in the adjusted models,” the researchers noted. In addition, the associations of an asthma diagnosis and respiratory symptoms had greater magnitudes than either cigarette, e-cigarette, and cannabis use or vaping cannabis with ENDS.
The study findings were limited by several factors including the inherent limitations of secondary database analysis, the researchers noted. “Another limitation is that co-use of cannabis and tobacco/nicotine was not assessed and, in the future, should be examined: Researchers have found that co-use is related to EVALI symptoms among young adults,” they said.
However, the study is the first known to include ENDS product use and respiratory symptoms while accounting for baseline asthma, and an asthma diagnosis was even more strongly associated with all five respiratory symptoms, the researchers said.
The results suggest that “the inhalation of cannabis via vaping is associated with some pulmonary irritation and symptoms of lung diseases (both known and unknown),” that may be predictive of later EVALI, they concluded.
Product details aid in diagnosis
“As we continue to see patients presenting with EVALI in pediatric hospitals, it is important for us to identify if there are specific products (or categories) that are more likely to cause it,” said Brandon Seay, MD, FCCP, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, in an interview. “When we are trying to diagnose EVALI, we should be asking appropriate questions about exposures to specific products to get the best answers. If we simply ask ‘Are you smoking e-cigarettes?’ the patient may not [equate] e-cigarette smoking to vaping cannabis products,” he said.
Dr. Seay said he was not surprised by the study findings. “A lot of the patients I see with EVALI have reported vaping THC products, and most of them also report that the products were mixed by a friend or an individual instead of being a commercially produced product,” he noted. “This is not surprising, as THC is still illegal in most states and there would not be any commercially available products,” he said. “The mixing of these products by individuals increases the risk of ingredients being more toxic or irritating to the lungs,” Dr. Seay added. “This does highlight the need for more regulation of vaping products. As more states legalize marijuana, more of these products will become available, which will provide an opportunity for increased regulation, he said.
The take-home message for clinicians is to seek specific details from their young patients, Dr. Seay emphasized. “When we are educating our patients on the dangers of vaping/e-cigarettes, we need to make sure we are asking specifically which products they are using and know the terminology,” he said. “The use of THC-containing products will be increasing across the country with more legalization, so we need to keep ourselves apprised of the different risks between THC- and nicotine-containing devices,” he added.
As for additional research, it would be interesting to know whether patients were asked where they had gotten their products (commercially available products vs. those mixed by individuals) and explore any difference between the two, said Dr. Seay. “Also, as these products are relatively new to the market, compared to cigarettes, data on the longitudinal effects of vaping (nicotine and THC) over a long period of time, compared to traditional combustible cigarettes, will be needed,” he said.
The study was funded by grants from the National Institutes of Health, National Institute on Drug Abuse, and National Cancer Institute. The researchers had no financial conflicts to disclose.
Dr. Seay had no financial disclosures, but serves as a member of the CHEST Physician editorial board.
, according to findings of a study based on a national sample of teens.
Most studies of electronic nicotine delivery systems (ENDS) use in teens have not addressed cannabis vaping, although e-cigarette– or vaping product use–associated lung injury (EVALI) has been predominately associated with cannabis products, wrote Carol J. Boyd, PhD, of the University of Michigan School of Nursing, Ann Arbor, and colleagues.
“At this time, relatively little is known about the population-level health consequences of adolescents’ use of ENDS, including use with cannabis and controlling for a history of asthma,” they said.
In a study published in the Journal of Adolescent Health, the researchers identified 14,798 adolescents aged 12-17 years using Wave 4 data from the Population Assessment of Tobacco and Health Study. Of these, 17.6% had a baseline asthma diagnosis, 8.9% reported ever using cannabis in ENDS, and 4.7% reported any cannabis use. In addition, 4.2% reported current e-cigarette use, 3.1% reported current cigarette use, 51% were male, and 69.2% were white.
Any cannabis vaping makes impact
In a fully-adjusted model, teens who had ever vaped cannabis had higher odds of five respiratory symptoms in the past year, compared with those with no history of cannabis vaping: wheezing or whistling in the chest (adjusted odds ratio, 1.81); sleep disturbed by wheezing or whistling (AOR, 1.71); speech limited because of wheezing (AOR, 1.96); wheezy during and after exercise (AOR, 1.33), and a dry cough at night independent of a cold or chest infection (AOR, 1.26).
Neither e-cigarettes nor cigarettes were significantly associated with any of these five respiratory symptoms in the fully adjusted models. In addition, “past 30-day use of cigarettes, e-cigarettes and cannabis use were associated with some respiratory symptoms in bivariate analyses but not in the adjusted models,” the researchers noted. In addition, the associations of an asthma diagnosis and respiratory symptoms had greater magnitudes than either cigarette, e-cigarette, and cannabis use or vaping cannabis with ENDS.
The study findings were limited by several factors including the inherent limitations of secondary database analysis, the researchers noted. “Another limitation is that co-use of cannabis and tobacco/nicotine was not assessed and, in the future, should be examined: Researchers have found that co-use is related to EVALI symptoms among young adults,” they said.
However, the study is the first known to include ENDS product use and respiratory symptoms while accounting for baseline asthma, and an asthma diagnosis was even more strongly associated with all five respiratory symptoms, the researchers said.
The results suggest that “the inhalation of cannabis via vaping is associated with some pulmonary irritation and symptoms of lung diseases (both known and unknown),” that may be predictive of later EVALI, they concluded.
Product details aid in diagnosis
“As we continue to see patients presenting with EVALI in pediatric hospitals, it is important for us to identify if there are specific products (or categories) that are more likely to cause it,” said Brandon Seay, MD, FCCP, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta, in an interview. “When we are trying to diagnose EVALI, we should be asking appropriate questions about exposures to specific products to get the best answers. If we simply ask ‘Are you smoking e-cigarettes?’ the patient may not [equate] e-cigarette smoking to vaping cannabis products,” he said.
Dr. Seay said he was not surprised by the study findings. “A lot of the patients I see with EVALI have reported vaping THC products, and most of them also report that the products were mixed by a friend or an individual instead of being a commercially produced product,” he noted. “This is not surprising, as THC is still illegal in most states and there would not be any commercially available products,” he said. “The mixing of these products by individuals increases the risk of ingredients being more toxic or irritating to the lungs,” Dr. Seay added. “This does highlight the need for more regulation of vaping products. As more states legalize marijuana, more of these products will become available, which will provide an opportunity for increased regulation, he said.
The take-home message for clinicians is to seek specific details from their young patients, Dr. Seay emphasized. “When we are educating our patients on the dangers of vaping/e-cigarettes, we need to make sure we are asking specifically which products they are using and know the terminology,” he said. “The use of THC-containing products will be increasing across the country with more legalization, so we need to keep ourselves apprised of the different risks between THC- and nicotine-containing devices,” he added.
As for additional research, it would be interesting to know whether patients were asked where they had gotten their products (commercially available products vs. those mixed by individuals) and explore any difference between the two, said Dr. Seay. “Also, as these products are relatively new to the market, compared to cigarettes, data on the longitudinal effects of vaping (nicotine and THC) over a long period of time, compared to traditional combustible cigarettes, will be needed,” he said.
The study was funded by grants from the National Institutes of Health, National Institute on Drug Abuse, and National Cancer Institute. The researchers had no financial conflicts to disclose.
Dr. Seay had no financial disclosures, but serves as a member of the CHEST Physician editorial board.
FROM THE JOURNAL OF ADOLESCENT HEALTH
Tocilizumab (Actemra) scores FDA approval for systemic sclerosis–associated interstitial lung disease
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
The Food and Drug Administration has approved subcutaneously-injected tocilizumab (Actemra) to reduce the rate of pulmonary function decline in systemic sclerosis–associated interstitial lung disease (SSc-ILD) patients, according to a press release from manufacturer Genentech.
Tocilizumab is the first biologic to be approved by the agency for adults with SSc-ILD, a rare and potentially life-threatening condition that may affect up to 80% of SSc patients and lead to lung inflammation and scarring.
The approval was based primarily on data from a phase 3 randomized, double-blind, placebo-controlled clinical trial (the focuSSced trial) that included 212 adults with SSc. Although that study failed to meet its primary endpoint of change from baseline to 48 weeks in the modified Rodnan Skin Score, the researchers observed a significantly reduced lung function decline as measured by forced vital capacity (FVC) and percent predicted forced vital capacity (ppFVC) among tocilizumab-treated patients, compared with those who received placebo. A total of 68 patients (65%) in the tocilizumab group and 68 patients (64%) in the placebo group had SSc-ILD at baseline.
In a subgroup analysis, patients taking tocilizumab had a smaller decline in mean ppFVC, compared with placebo patients (0.07% vs. –6.4%; mean difference, 6.47%), and a smaller decline in FVC (mean change –14 mL vs. –255 mL with placebo; mean difference, 241 mL).
The mean change from baseline to week 48 in modified Rodnan Skin Score was –5.88 for patients on tocilizumab and –3.77 with placebo.
Safety data were similar between tocilizumab and placebo groups through 48 weeks, and similar for patients with and without SSc-ILD. In general, tocilizumab side effects include increased susceptibility to infections, and serious side effects may include stomach tears, hepatotoxicity, and increased risk of cancer and hepatitis B, according to the prescribing information. However, the most common side effects are upper respiratory tract infections, headache, hypertension, and injection-site reactions.
Tocilizumab, an interleukin-6 receptor antagonist, is already approved for the treatment of adult patients with moderately to severely active rheumatoid arthritis, as well as for adult patients with giant cell arteritis; patients aged 2 years and older with active polyarticular juvenile idiopathic arthritis or active systemic juvenile idiopathic arthritis; and adults and pediatric patients 2 years of age and older with chimeric antigen receptor T-cell–induced severe or life-threatening cytokine release syndrome.
Prescribing information is available here.
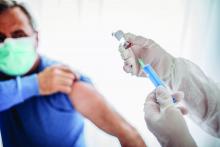
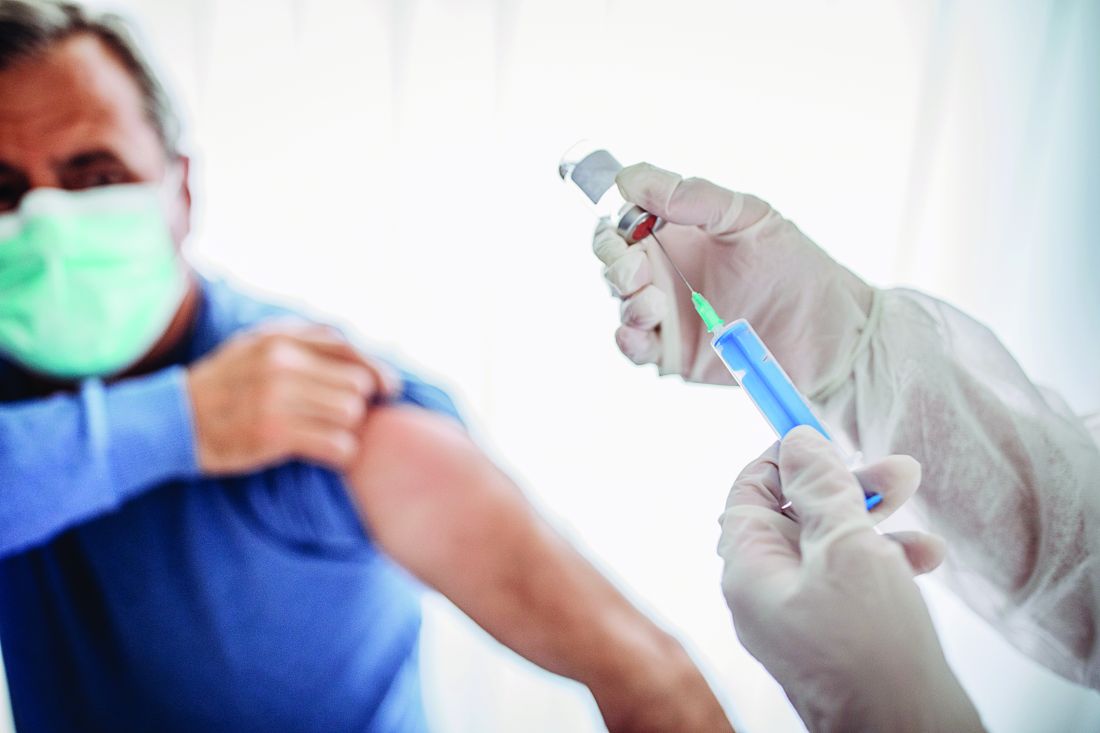
Routine vaccinations missed by older adults during pandemic
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
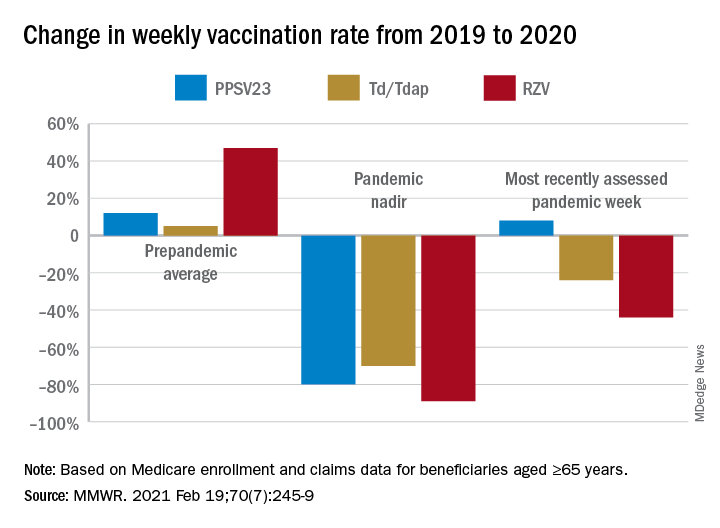
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.
In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
Physicians are going to have to play catch-up when it comes to getting older patients their routine, but important, vaccinations missed during the pandemic.
and have recovered only partially and gradually, according to a report by Kai Hong, PhD, and colleagues at the Centers for Disease Control and Prevention, published in the Morbidity and Mortality Weekly Report. “As the pandemic continues,” the investigators stated, “vaccination providers should continue efforts to resolve disruptions in routine adult vaccination.”
The CDC issued guidance recommending postponement of routine adult vaccination in response to the March 13, 2020, COVID-19 national emergency declaration by the U.S. government and also to state and local shelter-in-place orders. Health care facility operations were restricted because of safety concerns around exposure to the SARS-CoV-2 virus. The result was a significant drop in routine medical care including adult vaccinations.
The investigators examined Medicare enrollment and claims data to assess the change in weekly receipt of four routine adult vaccines by Medicare beneficiaries aged ≥65 during the pandemic: (13-valent pneumococcal conjugate vaccine [PCV13], 23-valent pneumococcal polysaccharide vaccine [PPSV23], tetanus-diphtheria or tetanus-diphtheria-acellular pertussis vaccine [Td/Tdap], and recombinant zoster vaccine [RZV]). The comparison periods were Jan. 6–July 20, 2019, and Jan. 5–July 18, 2020.
Of the Medicare enrollees in the study sample, 85% were White, 7% Black, 2% Asian, 2% Hispanic, and 4% other racial and ethnic groups. For each of the four vaccines overall, weekly rates of vaccination declined sharply after the emergency declaration, compared with corresponding weeks in 2019. In the period prior to the emergency declaration (Jan. 5–March 14, 2020), weekly percentages of Medicare beneficiaries vaccinated with PPSV23, Td/Tdap, and RZV were consistently higher than rates during the same period in 2019.
After the March 13 declaration, while weekly vaccination rates plummeted 25% for PPSV23 and 62% for RZV in the first week, the greatest weekly declines were during April 5-11, 2020, for PCV13, PPSV23, and Td/Tdap, and during April 12-18, 2020, for RZV. The pandemic weekly vaccination rate nadirs revealed declines of 88% for PCV13, 80% for PPSV23, 70% for Td/Tdap, and 89% for RZV.
Routine vaccinations increased midyear
Vaccination rates recovered gradually. For the most recently assessed pandemic week (July 12-18, 2020), the rate for PPSV23 was 8% higher than in the corresponding period in 2019. Weekly corresponding rates for other examined vaccines, however, remained much lower than in 2019: 44% lower for RZV, 24% lower for Td/Tdap and 43% lower for PCV13. The CDC Advisory Committee on Immunization Practices voted in June 2019 to stop recommending PCV13 for adults aged ≥65 years and so vaccination with PCV13 among this population declined in 2020, compared with that in 2019.
Another significant drop in the rates of adult vaccinations may have occurred because of the surge in COVID-19 infections in the fall of 2020 and subsequent closures and renewal of lockdown in many localities.
Disparities in routine vaccination trends
Dr. Hong and colleagues noted that their findings are consistent with prior reports of declines in pediatric vaccine ordering, administration, and coverage during the pandemic. While the reductions were similar across all racial and ethnic groups, the magnitudes of recovery varied, with vaccination rates lower among racial and ethnic minority adults than among White adults.

In view of the disproportionate COVID-19 pandemic effects among some racial and ethnic minorities, the investigators recommended monitoring and subsequent early intervention to mitigate similar indirect pandemic effects, such as reduced utilization of other preventive services. “Many members of racial and ethnic minority groups face barriers to routine medical care, which means they have fewer opportunities to receive preventive interventions such as vaccination,” Dr. Hong said in an interview. “When clinicians are following up with patients who have missed vaccinations, it is important for them to remember that patients may face new barriers to vaccination such as loss of income or health insurance, and to work with them to remove those barriers,” he added.
“If vaccination is deferred, older adults and adults with underlying medical conditions who subsequently become infected with a vaccine-preventable disease are at increased risk for complications,” Dr. Hong said. “The most important thing clinicians can do is identify patients who are due for or who have missed vaccinations, and contact them to schedule visits. Immunization Information Systems and electronic health records may be able to support this work. In addition, the vaccination status of all patients should be assessed at every health care visit to reduce missed opportunities for vaccination.”
FROM MMWR
BMI, age, and sex affect COVID-19 vaccine antibody response
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
Novel oral agent effective in teens with atopic dermatitis
Abrocitinib, an investigational drug proven to be a safe and effective treatment for moderate to severe atopic dermatitis (AD) in adults 18 years and older, is also safe and effective in patients aged 12-17 years, according to a randomized trial of the oral, once-daily Janus kinase (JAK) 1 selective inhibitor, used in combination with medicated topical therapy.
The results, from the phase 3 JADE TEEN study, were presented during an oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“We’re very excited about the introduction of oral JAKs into our armamentarium for atopic dermatitis,” lead author Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and chief of pediatric and adolescent dermatology, Rady Children’s Hospital, also in San Diego, said in an interview.
AD ranges in severity, and there is a great deal of moderate to severe AD that has a tremendous negative impact on the individual, Dr. Eichenfield said. “Traditionally we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control.”
JAK inhibitors are effective mediators of the inflammation response that occurs in moderate to severe AD. They inhibit the stimulation of the JAK pathway and allow anti-inflammatory effects and therefore have potential, especially in more severe disease, Dr. Eichenfield said.
In the current study, which is a spin-off of the original study that looked at abrocitinib in adults, he and his team randomly assigned 285 teens (mean age, 14.9 years; 50.9% male; 56.1% White) with moderate to severe AD to receive one of the following treatments for 12 weeks: abrocitinib 200 mg plus topical therapy (95); abrocitinib 100 mg plus topical therapy (95); or placebo, which consisted of topical therapy alone (95).
The primary endpoints were an Investigator’s Global Assessment response of clear or almost clear (scores of 0 and 1, respectively), with an improvement of at least 2 points, and an improvement in Eczema Area and Severity Index score of at least 75% at week 12.
Secondary endpoints included an improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) response of at least 4 points at week 12.
The teens who received abrocitinib along with medicated topical therapy showed significant improvement in the severity of their AD at the end of the 12-week period, compared with those in the placebo group.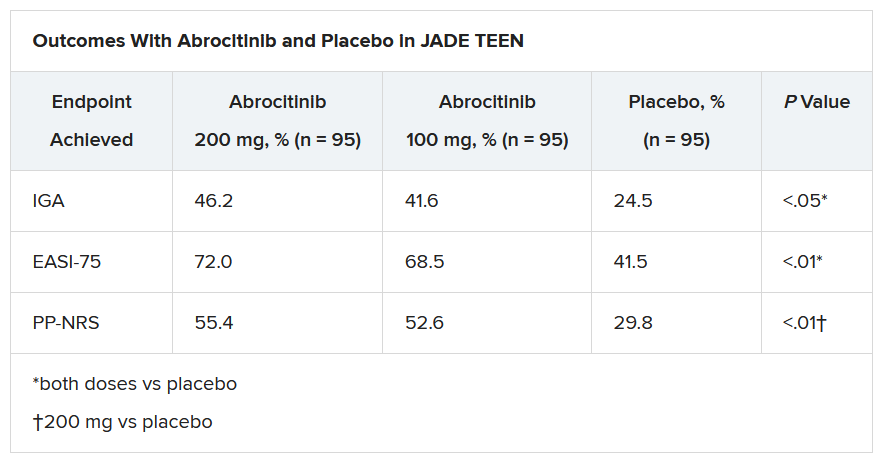
“The percentage of patients achieving essentially no itch, as captured in the fact that more than half of those on the higher dose of abrocitinib made it to no itch, is a new data point and is important to note,” Dr. Eichenfield said. “A lot of the other medicines don’t really get a significant percentage of the population to an itch score of 0 to 1. This drug brought about a rapid and profound itch relief.”
He added: “The results from JADE TEEN extend the drug’s utility in this younger population and show that abrocitinib performs the same with regard to efficacy and safety in the teenagers. Having atopic dermatitis that does not respond to treatment is especially hard for adolescents, but now we know that abrocitinib will be safe and effective and so we now have something to offer these kids.”
“Abrocitinib achieved a good response in this study that was statistically significant, compared to standard treatment,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, commented in an interview.
“JAK inhibitors are very promising, and this study adds to that promise. They play an important role in atopic dermatitis, so obviously, teenagers with AD represent an important population,” said Dr. Bernstein, who was not part of the study. “These results are very encouraging, and I think that we will probably see some of these JAK inhibitors approved by the FDA, if not this year, probably next.”
The study was sponsored by Pfizer. Dr. Eichenfield serves as an investigator, speaker, and consultant for Pfizer; and as an investigator, speaker, consultant, and/or is on a data safety monitoring board for AbbVie, Almirall, Amgen, Arcutis, Asana, Dermavant, Dermira, Forte, Galderma, Ichnos/Glenmark, Incyte, LEO, Lilly, L’Oreal, Novartis, Regeneron, Sanofi-Genzyme, and Verrica. Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Abrocitinib, an investigational drug proven to be a safe and effective treatment for moderate to severe atopic dermatitis (AD) in adults 18 years and older, is also safe and effective in patients aged 12-17 years, according to a randomized trial of the oral, once-daily Janus kinase (JAK) 1 selective inhibitor, used in combination with medicated topical therapy.
The results, from the phase 3 JADE TEEN study, were presented during an oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“We’re very excited about the introduction of oral JAKs into our armamentarium for atopic dermatitis,” lead author Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and chief of pediatric and adolescent dermatology, Rady Children’s Hospital, also in San Diego, said in an interview.
AD ranges in severity, and there is a great deal of moderate to severe AD that has a tremendous negative impact on the individual, Dr. Eichenfield said. “Traditionally we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control.”
JAK inhibitors are effective mediators of the inflammation response that occurs in moderate to severe AD. They inhibit the stimulation of the JAK pathway and allow anti-inflammatory effects and therefore have potential, especially in more severe disease, Dr. Eichenfield said.
In the current study, which is a spin-off of the original study that looked at abrocitinib in adults, he and his team randomly assigned 285 teens (mean age, 14.9 years; 50.9% male; 56.1% White) with moderate to severe AD to receive one of the following treatments for 12 weeks: abrocitinib 200 mg plus topical therapy (95); abrocitinib 100 mg plus topical therapy (95); or placebo, which consisted of topical therapy alone (95).
The primary endpoints were an Investigator’s Global Assessment response of clear or almost clear (scores of 0 and 1, respectively), with an improvement of at least 2 points, and an improvement in Eczema Area and Severity Index score of at least 75% at week 12.
Secondary endpoints included an improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) response of at least 4 points at week 12.
The teens who received abrocitinib along with medicated topical therapy showed significant improvement in the severity of their AD at the end of the 12-week period, compared with those in the placebo group.
“The percentage of patients achieving essentially no itch, as captured in the fact that more than half of those on the higher dose of abrocitinib made it to no itch, is a new data point and is important to note,” Dr. Eichenfield said. “A lot of the other medicines don’t really get a significant percentage of the population to an itch score of 0 to 1. This drug brought about a rapid and profound itch relief.”
He added: “The results from JADE TEEN extend the drug’s utility in this younger population and show that abrocitinib performs the same with regard to efficacy and safety in the teenagers. Having atopic dermatitis that does not respond to treatment is especially hard for adolescents, but now we know that abrocitinib will be safe and effective and so we now have something to offer these kids.”
“Abrocitinib achieved a good response in this study that was statistically significant, compared to standard treatment,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, commented in an interview.
“JAK inhibitors are very promising, and this study adds to that promise. They play an important role in atopic dermatitis, so obviously, teenagers with AD represent an important population,” said Dr. Bernstein, who was not part of the study. “These results are very encouraging, and I think that we will probably see some of these JAK inhibitors approved by the FDA, if not this year, probably next.”
The study was sponsored by Pfizer. Dr. Eichenfield serves as an investigator, speaker, and consultant for Pfizer; and as an investigator, speaker, consultant, and/or is on a data safety monitoring board for AbbVie, Almirall, Amgen, Arcutis, Asana, Dermavant, Dermira, Forte, Galderma, Ichnos/Glenmark, Incyte, LEO, Lilly, L’Oreal, Novartis, Regeneron, Sanofi-Genzyme, and Verrica. Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Abrocitinib, an investigational drug proven to be a safe and effective treatment for moderate to severe atopic dermatitis (AD) in adults 18 years and older, is also safe and effective in patients aged 12-17 years, according to a randomized trial of the oral, once-daily Janus kinase (JAK) 1 selective inhibitor, used in combination with medicated topical therapy.
The results, from the phase 3 JADE TEEN study, were presented during an oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“We’re very excited about the introduction of oral JAKs into our armamentarium for atopic dermatitis,” lead author Lawrence Eichenfield, MD, professor of dermatology and pediatrics, University of California, San Diego, and chief of pediatric and adolescent dermatology, Rady Children’s Hospital, also in San Diego, said in an interview.
AD ranges in severity, and there is a great deal of moderate to severe AD that has a tremendous negative impact on the individual, Dr. Eichenfield said. “Traditionally we have treated it with intermittent topical corticosteroids, but this has left a significant percentage of patients without long-term disease control.”
JAK inhibitors are effective mediators of the inflammation response that occurs in moderate to severe AD. They inhibit the stimulation of the JAK pathway and allow anti-inflammatory effects and therefore have potential, especially in more severe disease, Dr. Eichenfield said.
In the current study, which is a spin-off of the original study that looked at abrocitinib in adults, he and his team randomly assigned 285 teens (mean age, 14.9 years; 50.9% male; 56.1% White) with moderate to severe AD to receive one of the following treatments for 12 weeks: abrocitinib 200 mg plus topical therapy (95); abrocitinib 100 mg plus topical therapy (95); or placebo, which consisted of topical therapy alone (95).
The primary endpoints were an Investigator’s Global Assessment response of clear or almost clear (scores of 0 and 1, respectively), with an improvement of at least 2 points, and an improvement in Eczema Area and Severity Index score of at least 75% at week 12.
Secondary endpoints included an improvement in Peak Pruritus Numerical Rating Scale (PP-NRS) response of at least 4 points at week 12.
The teens who received abrocitinib along with medicated topical therapy showed significant improvement in the severity of their AD at the end of the 12-week period, compared with those in the placebo group.
“The percentage of patients achieving essentially no itch, as captured in the fact that more than half of those on the higher dose of abrocitinib made it to no itch, is a new data point and is important to note,” Dr. Eichenfield said. “A lot of the other medicines don’t really get a significant percentage of the population to an itch score of 0 to 1. This drug brought about a rapid and profound itch relief.”
He added: “The results from JADE TEEN extend the drug’s utility in this younger population and show that abrocitinib performs the same with regard to efficacy and safety in the teenagers. Having atopic dermatitis that does not respond to treatment is especially hard for adolescents, but now we know that abrocitinib will be safe and effective and so we now have something to offer these kids.”
“Abrocitinib achieved a good response in this study that was statistically significant, compared to standard treatment,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, commented in an interview.
“JAK inhibitors are very promising, and this study adds to that promise. They play an important role in atopic dermatitis, so obviously, teenagers with AD represent an important population,” said Dr. Bernstein, who was not part of the study. “These results are very encouraging, and I think that we will probably see some of these JAK inhibitors approved by the FDA, if not this year, probably next.”
The study was sponsored by Pfizer. Dr. Eichenfield serves as an investigator, speaker, and consultant for Pfizer; and as an investigator, speaker, consultant, and/or is on a data safety monitoring board for AbbVie, Almirall, Amgen, Arcutis, Asana, Dermavant, Dermira, Forte, Galderma, Ichnos/Glenmark, Incyte, LEO, Lilly, L’Oreal, Novartis, Regeneron, Sanofi-Genzyme, and Verrica. Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study: Shared decision-making in lung cancer screening needs work
Shared decision-making is an integral step in lung cancer screening with low-dose CT (LDCT) in high-risk patients, but a cross-sectional study at two academic medical centers in Texas has found wide variability in the quality of shared decision-making encounters and that nearly a third of patients reported being conflicted about their decisions to pursue screening.
Lead author Shawn P.E. Nishi, MD, associate professor in the division of pulmonary critical care and sleep medicine, department of internal medicine, of the University of Texas Medical Branch, Galveston, noted two striking findings of the study, published in Chest: that physicians rarely used decision aids according to Centers for Medicare & Medicaid Services direction, and that a “considerable imbalance” exists in the way physicians present management choices to patients. “As physicians, we want to focus on the positive,” she said, “but in shared decision-making (SDM) there needs to be a better balance between presentation and understanding of the risks and the benefits of lung cancer screening (LCS).”
Since 2015, CMS has reimbursed for LCS counseling and an shared decision-making visit before a patient has the screening.
The study analyzed self-reported survey results of 266 patients who had been through SDM at UTMB Galveston and MD Anderson Cancer Center in Houston in 2017. They completed patient surveys the following year. The study population was 87% White, 38% had a family history of lung cancer, and 39% were current smokers. The mean pack-year history was 40.4 years.
A high percentage – 86.6% – said they were satisfied with the level in which they were involved in their screening decision. Patients reported that their doctors talked to them about the benefits of LCS far more frequently than the potential harms, 68.3% to 20.8%. And 12.5% said they understood that an abnormal scan was likely to result in a negative finding. Only 30.7% said they’d received educational materials about LCS during the screening process.
A year after completing the SDM process, their knowledge of LCS was variable at best; on average, they answered 41.4% of the questions correctly, and almost one-third (31%) indicated that screening, rather than quitting smoking, was the best way reduce their lung cancer risk.
The study noted that, for patients who derive a small benefit from LCS, the absolute risk reduction is only 0.3%, which may not be enough to offset the potential harms of LDCT.
“The LCS exam itself is a simple noninvasive procedure; you get a scan and go about your day once it’s read,” Dr. Nishi said. “However there is a high false-positive rate, and the question really becomes that, as you start to work up those false positives and even true positives, however small, there is a risk associated with every procedure or evaluation thereafter. So the shared decision-making process is really there to ensure that patients value finding their lung cancer early if they do have it versus the potential harms down the line.”
However, as this study points out, there aren’t many parameters for what SDM entails. “It’s more than just an information exchange back and forth,” Dr. Nishi said. “It’s about having good-quality communication between the provider and patients so that the right decision can ultimately be made for each patient. It takes a very dedicated person that can commit the time and expertise to it. I don’t think that it should be taken lightly.”
As Dr. Nishi and colleagues pointed out in their study, SDM incorporates three essential elements: recognizing and acknowledging that a decision has to be made, knowing and understanding the best available evidence, and incorporating the patient’s own values and preferences in the decision.
CMS outlines specific components of SDM. It includes, beyond a discussion of the potential benefits and harms and use of a decision aid, education on the need for adherence to annual screening, and counseling on either stopping smoking or continued abstinence.
For physicians, dedicating the time and energy SDM needs can be a challenge, Dr. Nishi noted, “Health care doesn’t have a lot of support to perform shared decision-making,” she said. “In a very busy practice it’s very hard to make sure you have a good process where you can sit down and take all the time you need with a patient to open up a dialog about the risks and benefits.”
After they completed the screening process, 33.6% of patients said they had some conflicting feelings about their decision. Non-White patients were about four times more likely than White patients to feel conflicted about their choices (odds ratio, 4.31; 95% confidence interval, 1.36-13.70), as were former smokers, compared with current smokers (OR, 1.93; 95% CI, 1.04-3.55).
Future studies of SDM in LCS should focus on outcomes, said Dr. Nishi. “Hopefully then we can focus on those things that benefit patients the most.”
Abbie Begnaud, MD, FCCP, a pulmonologist at the University of Minnesota, Minneapolis, said this study confirmed what other studies found about shortcomings of SDM, with one difference. “We already knew we were not doing a great job at shared decision-making,” she said. “To me, the difference in this study is that most of the patients were pretty satisfied with their degree of involvement.”
She noted the low percentage of patients who understood that abnormal scans may be noncancerous. “This is one area that I think is an important place for us to improve,” Dr. Begnaud said.
The findings about non-White patients and former smokers are also telling, Dr. Begnaud said. “This highlights that we need to pay close attention to these two groups – people who have traditionally, historically been marginalized in medical care – and provide them the support they need to make a decision.”
Dr. Nishi and colleagues have no relevant disclosures. The study was supported by the Cancer Prevention and Research Institute of Texas and received grants from the National Cancer Institute and the University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment. Dr. Begnaud has no relevant relationships to disclose.
Shared decision-making is an integral step in lung cancer screening with low-dose CT (LDCT) in high-risk patients, but a cross-sectional study at two academic medical centers in Texas has found wide variability in the quality of shared decision-making encounters and that nearly a third of patients reported being conflicted about their decisions to pursue screening.
Lead author Shawn P.E. Nishi, MD, associate professor in the division of pulmonary critical care and sleep medicine, department of internal medicine, of the University of Texas Medical Branch, Galveston, noted two striking findings of the study, published in Chest: that physicians rarely used decision aids according to Centers for Medicare & Medicaid Services direction, and that a “considerable imbalance” exists in the way physicians present management choices to patients. “As physicians, we want to focus on the positive,” she said, “but in shared decision-making (SDM) there needs to be a better balance between presentation and understanding of the risks and the benefits of lung cancer screening (LCS).”
Since 2015, CMS has reimbursed for LCS counseling and an shared decision-making visit before a patient has the screening.
The study analyzed self-reported survey results of 266 patients who had been through SDM at UTMB Galveston and MD Anderson Cancer Center in Houston in 2017. They completed patient surveys the following year. The study population was 87% White, 38% had a family history of lung cancer, and 39% were current smokers. The mean pack-year history was 40.4 years.
A high percentage – 86.6% – said they were satisfied with the level in which they were involved in their screening decision. Patients reported that their doctors talked to them about the benefits of LCS far more frequently than the potential harms, 68.3% to 20.8%. And 12.5% said they understood that an abnormal scan was likely to result in a negative finding. Only 30.7% said they’d received educational materials about LCS during the screening process.
A year after completing the SDM process, their knowledge of LCS was variable at best; on average, they answered 41.4% of the questions correctly, and almost one-third (31%) indicated that screening, rather than quitting smoking, was the best way reduce their lung cancer risk.
The study noted that, for patients who derive a small benefit from LCS, the absolute risk reduction is only 0.3%, which may not be enough to offset the potential harms of LDCT.
“The LCS exam itself is a simple noninvasive procedure; you get a scan and go about your day once it’s read,” Dr. Nishi said. “However there is a high false-positive rate, and the question really becomes that, as you start to work up those false positives and even true positives, however small, there is a risk associated with every procedure or evaluation thereafter. So the shared decision-making process is really there to ensure that patients value finding their lung cancer early if they do have it versus the potential harms down the line.”
However, as this study points out, there aren’t many parameters for what SDM entails. “It’s more than just an information exchange back and forth,” Dr. Nishi said. “It’s about having good-quality communication between the provider and patients so that the right decision can ultimately be made for each patient. It takes a very dedicated person that can commit the time and expertise to it. I don’t think that it should be taken lightly.”
As Dr. Nishi and colleagues pointed out in their study, SDM incorporates three essential elements: recognizing and acknowledging that a decision has to be made, knowing and understanding the best available evidence, and incorporating the patient’s own values and preferences in the decision.
CMS outlines specific components of SDM. It includes, beyond a discussion of the potential benefits and harms and use of a decision aid, education on the need for adherence to annual screening, and counseling on either stopping smoking or continued abstinence.
For physicians, dedicating the time and energy SDM needs can be a challenge, Dr. Nishi noted, “Health care doesn’t have a lot of support to perform shared decision-making,” she said. “In a very busy practice it’s very hard to make sure you have a good process where you can sit down and take all the time you need with a patient to open up a dialog about the risks and benefits.”
After they completed the screening process, 33.6% of patients said they had some conflicting feelings about their decision. Non-White patients were about four times more likely than White patients to feel conflicted about their choices (odds ratio, 4.31; 95% confidence interval, 1.36-13.70), as were former smokers, compared with current smokers (OR, 1.93; 95% CI, 1.04-3.55).
Future studies of SDM in LCS should focus on outcomes, said Dr. Nishi. “Hopefully then we can focus on those things that benefit patients the most.”
Abbie Begnaud, MD, FCCP, a pulmonologist at the University of Minnesota, Minneapolis, said this study confirmed what other studies found about shortcomings of SDM, with one difference. “We already knew we were not doing a great job at shared decision-making,” she said. “To me, the difference in this study is that most of the patients were pretty satisfied with their degree of involvement.”
She noted the low percentage of patients who understood that abnormal scans may be noncancerous. “This is one area that I think is an important place for us to improve,” Dr. Begnaud said.
The findings about non-White patients and former smokers are also telling, Dr. Begnaud said. “This highlights that we need to pay close attention to these two groups – people who have traditionally, historically been marginalized in medical care – and provide them the support they need to make a decision.”
Dr. Nishi and colleagues have no relevant disclosures. The study was supported by the Cancer Prevention and Research Institute of Texas and received grants from the National Cancer Institute and the University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment. Dr. Begnaud has no relevant relationships to disclose.
Shared decision-making is an integral step in lung cancer screening with low-dose CT (LDCT) in high-risk patients, but a cross-sectional study at two academic medical centers in Texas has found wide variability in the quality of shared decision-making encounters and that nearly a third of patients reported being conflicted about their decisions to pursue screening.
Lead author Shawn P.E. Nishi, MD, associate professor in the division of pulmonary critical care and sleep medicine, department of internal medicine, of the University of Texas Medical Branch, Galveston, noted two striking findings of the study, published in Chest: that physicians rarely used decision aids according to Centers for Medicare & Medicaid Services direction, and that a “considerable imbalance” exists in the way physicians present management choices to patients. “As physicians, we want to focus on the positive,” she said, “but in shared decision-making (SDM) there needs to be a better balance between presentation and understanding of the risks and the benefits of lung cancer screening (LCS).”
Since 2015, CMS has reimbursed for LCS counseling and an shared decision-making visit before a patient has the screening.
The study analyzed self-reported survey results of 266 patients who had been through SDM at UTMB Galveston and MD Anderson Cancer Center in Houston in 2017. They completed patient surveys the following year. The study population was 87% White, 38% had a family history of lung cancer, and 39% were current smokers. The mean pack-year history was 40.4 years.
A high percentage – 86.6% – said they were satisfied with the level in which they were involved in their screening decision. Patients reported that their doctors talked to them about the benefits of LCS far more frequently than the potential harms, 68.3% to 20.8%. And 12.5% said they understood that an abnormal scan was likely to result in a negative finding. Only 30.7% said they’d received educational materials about LCS during the screening process.
A year after completing the SDM process, their knowledge of LCS was variable at best; on average, they answered 41.4% of the questions correctly, and almost one-third (31%) indicated that screening, rather than quitting smoking, was the best way reduce their lung cancer risk.
The study noted that, for patients who derive a small benefit from LCS, the absolute risk reduction is only 0.3%, which may not be enough to offset the potential harms of LDCT.
“The LCS exam itself is a simple noninvasive procedure; you get a scan and go about your day once it’s read,” Dr. Nishi said. “However there is a high false-positive rate, and the question really becomes that, as you start to work up those false positives and even true positives, however small, there is a risk associated with every procedure or evaluation thereafter. So the shared decision-making process is really there to ensure that patients value finding their lung cancer early if they do have it versus the potential harms down the line.”
However, as this study points out, there aren’t many parameters for what SDM entails. “It’s more than just an information exchange back and forth,” Dr. Nishi said. “It’s about having good-quality communication between the provider and patients so that the right decision can ultimately be made for each patient. It takes a very dedicated person that can commit the time and expertise to it. I don’t think that it should be taken lightly.”
As Dr. Nishi and colleagues pointed out in their study, SDM incorporates three essential elements: recognizing and acknowledging that a decision has to be made, knowing and understanding the best available evidence, and incorporating the patient’s own values and preferences in the decision.
CMS outlines specific components of SDM. It includes, beyond a discussion of the potential benefits and harms and use of a decision aid, education on the need for adherence to annual screening, and counseling on either stopping smoking or continued abstinence.
For physicians, dedicating the time and energy SDM needs can be a challenge, Dr. Nishi noted, “Health care doesn’t have a lot of support to perform shared decision-making,” she said. “In a very busy practice it’s very hard to make sure you have a good process where you can sit down and take all the time you need with a patient to open up a dialog about the risks and benefits.”
After they completed the screening process, 33.6% of patients said they had some conflicting feelings about their decision. Non-White patients were about four times more likely than White patients to feel conflicted about their choices (odds ratio, 4.31; 95% confidence interval, 1.36-13.70), as were former smokers, compared with current smokers (OR, 1.93; 95% CI, 1.04-3.55).
Future studies of SDM in LCS should focus on outcomes, said Dr. Nishi. “Hopefully then we can focus on those things that benefit patients the most.”
Abbie Begnaud, MD, FCCP, a pulmonologist at the University of Minnesota, Minneapolis, said this study confirmed what other studies found about shortcomings of SDM, with one difference. “We already knew we were not doing a great job at shared decision-making,” she said. “To me, the difference in this study is that most of the patients were pretty satisfied with their degree of involvement.”
She noted the low percentage of patients who understood that abnormal scans may be noncancerous. “This is one area that I think is an important place for us to improve,” Dr. Begnaud said.
The findings about non-White patients and former smokers are also telling, Dr. Begnaud said. “This highlights that we need to pay close attention to these two groups – people who have traditionally, historically been marginalized in medical care – and provide them the support they need to make a decision.”
Dr. Nishi and colleagues have no relevant disclosures. The study was supported by the Cancer Prevention and Research Institute of Texas and received grants from the National Cancer Institute and the University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment. Dr. Begnaud has no relevant relationships to disclose.
FROM CHEST
Study clarifies who gets post–COVID-19 interstitial lung disease
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
A study of post–COVID-19 patients in the United Kingdom who developed severe lung inflammation after they left the hospital may provide greater clarity on which patients are most likely to have persistent lung dysfunction.
In addition to pinpointing those most at risk, the findings showed that conventional corticosteroid treatment is highly effective in improving lung function and reducing symptoms.
Researchers from Guy’s and St. Thomas’ National Health Foundation Trust in London reported that a small percentage of patients – 4.8%, or 35 of 837 patients in the study – had severe persistent interstitial lung disease (ILD), mostly organizing pneumonia, 4 weeks after discharge. Of these patients, 30 received steroid treatment, all of whom showed improvement in lung function.
Lead author Katherine Jane Myall, MRCP, and colleagues wrote that the most common radiologic finding in acute COVID-19 is bilateral ground-glass opacification, and findings of organizing pneumonia are common. However, no reports exist of the role of inflammatory infiltrates during recovery from COVID-19 or of the effectiveness of treatments for persistent ILD. “The long-term respiratory morbidity remains unclear,” Dr. Myall and colleagues wrote.
The study findings are significant because they quantify the degree of lung disease that patients have after COVID-19, said Sachin Gupta, MD, FCCP, a pulmonologist and critical care specialist at Alameda Health System in Oakland, Calif. He added that the disease course and presentation followed the pattern of organizing pneumonia in some patients, and traditional corticosteroid therapy seemed to resolve symptoms and improve lung function.
“This is a really important piece to get out there because it describes what a lot of us are worried about in patients with post-COVID lung disease and about what type of lung disease they have. It offers a potential treatment,” he said.
Dr. Myall and colleagues noted that even a “relatively small proportion” of patients with persistent, severe ILD – as reported in this study – pose “a significant disease burden.” They added: “Prompt therapy may avoid potentially permanent fibrosis and functional impairment.”
The single-center, prospective, observational study followed discharged patients with telephone calls 4 weeks after discharge to determine their status. At that point, 39% of the study cohort (n = 325) reported ongoing symptoms.
The patients had outpatient examinations at 6 weeks post discharge, at which time 42.9% (n = 138) had no signs or symptoms of persistent disease; 33.8% (n = 110) had symptoms but no radiologic findings and received referrals to other departments; and 24% (n = 77) were referred to the post-COVID lung disease multidisciplinary team. A total of 59 were diagnosed with persistent post-COVID interstitial change, 35 of whom had organizing pneumonia, hence the rationale for using steroids in this group, Dr. Myall and colleagues stated.
The 30 patients treated with corticosteroids received a maximum initial dose of 0.5 mg/kg prednisolone, which was rapidly weaned over 3 weeks. Some patients received lower doses depending on their comorbidities.
Treatment resulted in an average relative increase in transfer factor of 31.6% (P < .001) and forced vital capacity of 9.6% (P = .014), along with significant improvement in symptoms and x-ray signs.
The study identified some key characteristics of the patients who had persistent post–COVID-19 inflammatory ILD. They were mostly male (71.5%) and overweight with an average body mass index of 28.3, but only 26% were obese. Most had at least one comorbidity, with the most common being diabetes and asthma (22.9%). Their average hospital stay was 16.9 days, 82.9% required oxygen, 55% were in the ICU, and 46% needed invasive mechanical ventilation.
The patients most vulnerable to ILD and organizing pneumonia were the “sicker” of the whole cohort, Dr. Gupta said. “In one sense, it’s reassuring that this is not just happening in anyone; this is happening in patients who had the worst course and were hospitalized in the ICU for the most part.”
The study shows that identifying these patients early on and initiating steroid therapy could avoid persistent lung injury and scarring, Dr. Gupta said.
The London researchers noted that theirs wasn’t a radiologic study, so CT scans weren’t formally scored before and after treatment. They also acknowledged vagueness about imaging and clinical findings representing “nothing other than slow ongoing recovery.”
Patients with post–COVID-19 ILD will require ongoing follow-up to better understand the disease course, Dr. Myall and colleagues stated, although they predicted organizing pneumonia is unlikely to recur once it resolves.
Dr. Myall and coauthors had no relevant relationships to disclose. Dr. Gupta disclosed he is also an employee and shareholder at Genentech.
FROM ANNALS OF THE AMERICAN THORACIC SOCIETY
Novel agent shows promise against cat allergy
One dose of the novel agent, REGN1908-1909 (Regeneron Pharmaceuticals) resulted in a rapid and durable reduction in cat-allergen-induced bronchoconstriction in cat-allergic subjects with mild asthma.
The finding, from a phase 2 randomized placebo-controlled study, is good news for the millions of people who are plagued by cat allergies, the investigators say.
The study, which was sponsored by Regeneron, was presented in a late breaking oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“REGN1908-1909 contains antibodies against Fel d 1, the major cat allergen, and here we show that it quickly and lastingly reduces acute bronchoconstriction in people with cat allergy,” lead author Frederic J. de Blay, MD, Strasbourg University Hospital, France, said in an interview.
Dr. de Blay admitted he is “quite excited” about the results.
“This study was performed in an environmental exposure chamber, and we clearly demonstrate that these antibodies decrease the asthmatic response to cat allergen within 8 days, and that these effects last 3 months. I never saw that in my life. I was a little bit skeptical at first, so to obtain such robust results after just 8 days, after just one injection, I was very surprised,” he said.
Dr. de Blay and his team screened potential participants to make sure they were cat allergic by exposing them to cat allergen for up to 2 hours while they were in the environmental exposure chamber. To be eligible for the study, participants had to show an early asthmatic response (EAR), defined as a reduction in forced expiratory volume in 1 second (FEV1) of at least 20% from baseline.
The participants were then randomized to receive either single-dose REGN1908-1909, 600 mg, subcutaneously (n = 29 patients) or placebo (n = 27 patients) prior to cat-allergen exposure in the controlled environmental chamber.
Dr. de Blay developed the chamber used in the study: the ALYATEC environmental exposure chamber.
“The chamber is 60 meters square, or 150 cubic meters, and can accommodate 20 patients. We are able to nebulize cat allergen, mice allergen, or whatever we wish to study so we can standardize the exposure. We can control the particle size and the amount so we know the exact amount of allergen that the patient has been exposed to,” he explained.
To test the efficacy of REGN1908-1909 in reducing acute bronchoconstriction, or EAR, the researchers measured FEV1 at baseline, and on days 8, 29, 57, and 85 in both groups. During each exposure, measurements were taken every 10 minutes for periods that lasted up to 4 hours.
They found that the probability of remaining in the chamber with no asthmatic response was substantially elevated in the group treated with REGN1908-1909.
Compared with placebo, REGN1908-1909 significantly increased the median time to EAR, from 51 minutes at baseline to more than 4 hours on day 8, (hazard ratio [HR], 0.36; P < .0083), day 29 (HR, 0.24; P < .0001), day 57 (HR, 0.45; P = .0222), and day 85 (HR, 0.27; P = .0003).
The FEV1 area under the curve (AUC) was also better with REGN1908-1909 than with placebo at day 8 (15.2% vs. 1.6%; P < .001). And a single dose reduced skin-test reactivity to cat allergen at 1 week, which persisted for up to 4 months.
In addition, participants who received REGN1908-1909 were able to tolerate a threefold higher amount of the cat allergen than those who received placebo (P = .003).
“We initially gave 40 nanograms of cat allergen, and then 8 days later they were able to stay longer in the chamber and inhale more of the allergen, to almost triple the amount they had originally been given. That 40 nanograms is very close to real world exposure,” Dr. de Blay noted.
Regeneron plans to start a phase 3 trial soon, he reported.
Promising results
“The study is well designed and shows a reduction in drop of FEV1 in response to cat allergen provocation and a decreased AUC in cat SPT response over 4 months,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, said in an interview.
“These are very promising results, which show that REGN1908-1909 can be a novel treatment for cat-induced asthma, which is often the only sensitization patients have. And they love their cats – one-third of the U.S. population has a cat and one-third has a dog, and 50% have both,” noted Dr. Bernstein, who was not involved with the study.
“This novel study used our scientific knowledge of the cat allergen itself to design a targeted antibody-based treatment that demonstrates significant benefit even after the first shot,” added Edwin H. Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill.
“This strategy has the potential to revolutionize not only our treatment of common environmental allergies but also other allergic diseases with well-described triggers, such as food and drug allergy,” Dr. Kim, who was not part of the study, said in an interview.
Dr. de Blay reported a financial relationship with Regeneron Pharmaceuticals, which sponsored the study. Dr. Bernstein and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One dose of the novel agent, REGN1908-1909 (Regeneron Pharmaceuticals) resulted in a rapid and durable reduction in cat-allergen-induced bronchoconstriction in cat-allergic subjects with mild asthma.
The finding, from a phase 2 randomized placebo-controlled study, is good news for the millions of people who are plagued by cat allergies, the investigators say.
The study, which was sponsored by Regeneron, was presented in a late breaking oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“REGN1908-1909 contains antibodies against Fel d 1, the major cat allergen, and here we show that it quickly and lastingly reduces acute bronchoconstriction in people with cat allergy,” lead author Frederic J. de Blay, MD, Strasbourg University Hospital, France, said in an interview.
Dr. de Blay admitted he is “quite excited” about the results.
“This study was performed in an environmental exposure chamber, and we clearly demonstrate that these antibodies decrease the asthmatic response to cat allergen within 8 days, and that these effects last 3 months. I never saw that in my life. I was a little bit skeptical at first, so to obtain such robust results after just 8 days, after just one injection, I was very surprised,” he said.
Dr. de Blay and his team screened potential participants to make sure they were cat allergic by exposing them to cat allergen for up to 2 hours while they were in the environmental exposure chamber. To be eligible for the study, participants had to show an early asthmatic response (EAR), defined as a reduction in forced expiratory volume in 1 second (FEV1) of at least 20% from baseline.
The participants were then randomized to receive either single-dose REGN1908-1909, 600 mg, subcutaneously (n = 29 patients) or placebo (n = 27 patients) prior to cat-allergen exposure in the controlled environmental chamber.
Dr. de Blay developed the chamber used in the study: the ALYATEC environmental exposure chamber.
“The chamber is 60 meters square, or 150 cubic meters, and can accommodate 20 patients. We are able to nebulize cat allergen, mice allergen, or whatever we wish to study so we can standardize the exposure. We can control the particle size and the amount so we know the exact amount of allergen that the patient has been exposed to,” he explained.
To test the efficacy of REGN1908-1909 in reducing acute bronchoconstriction, or EAR, the researchers measured FEV1 at baseline, and on days 8, 29, 57, and 85 in both groups. During each exposure, measurements were taken every 10 minutes for periods that lasted up to 4 hours.
They found that the probability of remaining in the chamber with no asthmatic response was substantially elevated in the group treated with REGN1908-1909.
Compared with placebo, REGN1908-1909 significantly increased the median time to EAR, from 51 minutes at baseline to more than 4 hours on day 8, (hazard ratio [HR], 0.36; P < .0083), day 29 (HR, 0.24; P < .0001), day 57 (HR, 0.45; P = .0222), and day 85 (HR, 0.27; P = .0003).
The FEV1 area under the curve (AUC) was also better with REGN1908-1909 than with placebo at day 8 (15.2% vs. 1.6%; P < .001). And a single dose reduced skin-test reactivity to cat allergen at 1 week, which persisted for up to 4 months.
In addition, participants who received REGN1908-1909 were able to tolerate a threefold higher amount of the cat allergen than those who received placebo (P = .003).
“We initially gave 40 nanograms of cat allergen, and then 8 days later they were able to stay longer in the chamber and inhale more of the allergen, to almost triple the amount they had originally been given. That 40 nanograms is very close to real world exposure,” Dr. de Blay noted.
Regeneron plans to start a phase 3 trial soon, he reported.
Promising results
“The study is well designed and shows a reduction in drop of FEV1 in response to cat allergen provocation and a decreased AUC in cat SPT response over 4 months,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, said in an interview.
“These are very promising results, which show that REGN1908-1909 can be a novel treatment for cat-induced asthma, which is often the only sensitization patients have. And they love their cats – one-third of the U.S. population has a cat and one-third has a dog, and 50% have both,” noted Dr. Bernstein, who was not involved with the study.
“This novel study used our scientific knowledge of the cat allergen itself to design a targeted antibody-based treatment that demonstrates significant benefit even after the first shot,” added Edwin H. Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill.
“This strategy has the potential to revolutionize not only our treatment of common environmental allergies but also other allergic diseases with well-described triggers, such as food and drug allergy,” Dr. Kim, who was not part of the study, said in an interview.
Dr. de Blay reported a financial relationship with Regeneron Pharmaceuticals, which sponsored the study. Dr. Bernstein and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One dose of the novel agent, REGN1908-1909 (Regeneron Pharmaceuticals) resulted in a rapid and durable reduction in cat-allergen-induced bronchoconstriction in cat-allergic subjects with mild asthma.
The finding, from a phase 2 randomized placebo-controlled study, is good news for the millions of people who are plagued by cat allergies, the investigators say.
The study, which was sponsored by Regeneron, was presented in a late breaking oral abstract session at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
“REGN1908-1909 contains antibodies against Fel d 1, the major cat allergen, and here we show that it quickly and lastingly reduces acute bronchoconstriction in people with cat allergy,” lead author Frederic J. de Blay, MD, Strasbourg University Hospital, France, said in an interview.
Dr. de Blay admitted he is “quite excited” about the results.
“This study was performed in an environmental exposure chamber, and we clearly demonstrate that these antibodies decrease the asthmatic response to cat allergen within 8 days, and that these effects last 3 months. I never saw that in my life. I was a little bit skeptical at first, so to obtain such robust results after just 8 days, after just one injection, I was very surprised,” he said.
Dr. de Blay and his team screened potential participants to make sure they were cat allergic by exposing them to cat allergen for up to 2 hours while they were in the environmental exposure chamber. To be eligible for the study, participants had to show an early asthmatic response (EAR), defined as a reduction in forced expiratory volume in 1 second (FEV1) of at least 20% from baseline.
The participants were then randomized to receive either single-dose REGN1908-1909, 600 mg, subcutaneously (n = 29 patients) or placebo (n = 27 patients) prior to cat-allergen exposure in the controlled environmental chamber.
Dr. de Blay developed the chamber used in the study: the ALYATEC environmental exposure chamber.
“The chamber is 60 meters square, or 150 cubic meters, and can accommodate 20 patients. We are able to nebulize cat allergen, mice allergen, or whatever we wish to study so we can standardize the exposure. We can control the particle size and the amount so we know the exact amount of allergen that the patient has been exposed to,” he explained.
To test the efficacy of REGN1908-1909 in reducing acute bronchoconstriction, or EAR, the researchers measured FEV1 at baseline, and on days 8, 29, 57, and 85 in both groups. During each exposure, measurements were taken every 10 minutes for periods that lasted up to 4 hours.
They found that the probability of remaining in the chamber with no asthmatic response was substantially elevated in the group treated with REGN1908-1909.
Compared with placebo, REGN1908-1909 significantly increased the median time to EAR, from 51 minutes at baseline to more than 4 hours on day 8, (hazard ratio [HR], 0.36; P < .0083), day 29 (HR, 0.24; P < .0001), day 57 (HR, 0.45; P = .0222), and day 85 (HR, 0.27; P = .0003).
The FEV1 area under the curve (AUC) was also better with REGN1908-1909 than with placebo at day 8 (15.2% vs. 1.6%; P < .001). And a single dose reduced skin-test reactivity to cat allergen at 1 week, which persisted for up to 4 months.
In addition, participants who received REGN1908-1909 were able to tolerate a threefold higher amount of the cat allergen than those who received placebo (P = .003).
“We initially gave 40 nanograms of cat allergen, and then 8 days later they were able to stay longer in the chamber and inhale more of the allergen, to almost triple the amount they had originally been given. That 40 nanograms is very close to real world exposure,” Dr. de Blay noted.
Regeneron plans to start a phase 3 trial soon, he reported.
Promising results
“The study is well designed and shows a reduction in drop of FEV1 in response to cat allergen provocation and a decreased AUC in cat SPT response over 4 months,” Jonathan A. Bernstein, MD, professor of medicine at the University of Cincinnati, said in an interview.
“These are very promising results, which show that REGN1908-1909 can be a novel treatment for cat-induced asthma, which is often the only sensitization patients have. And they love their cats – one-third of the U.S. population has a cat and one-third has a dog, and 50% have both,” noted Dr. Bernstein, who was not involved with the study.
“This novel study used our scientific knowledge of the cat allergen itself to design a targeted antibody-based treatment that demonstrates significant benefit even after the first shot,” added Edwin H. Kim, MD, director of the UNC Food Allergy Initiative at the University of North Carolina at Chapel Hill.
“This strategy has the potential to revolutionize not only our treatment of common environmental allergies but also other allergic diseases with well-described triggers, such as food and drug allergy,” Dr. Kim, who was not part of the study, said in an interview.
Dr. de Blay reported a financial relationship with Regeneron Pharmaceuticals, which sponsored the study. Dr. Bernstein and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAAAI
Patients with asthma say most doctors don’t ask about cannabis use
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
Among individuals with asthma and allergies who use cannabis, more than half said they aren’t willing to discuss their use of cannabis with their doctor and their doctor doesn’t ask, according to recent research at the annual meeting of the American Academy of Allergy, Asthma, and Immunology, held virtually this year.
In an online survey of respondents with asthma and allergies in the Allergy & Asthma Network, 88 of 489 (18.0%) reported cannabis use. Of these respondents, 37.5% said they wanted to discuss their cannabis use with their doctor, 51.1% said they would not want to, and 11.4% reported they were unsure. In addition, 40.9% of respondents said their doctor inquired about cannabis use, while 51.1% said their doctor did not bring up cannabis use at all, either through a verbal discussion or on an intake form.
To date, there has not been much research on use of cannabis among patients with allergies and asthma, Joanna S. Zeiger, MS, PhD, of the Canna Research Foundation in Boulder, Colo., said in her presentation. “This is a group with whom route of administration could have broad adverse effects. Smoking or vaping cannabis in this population could lead to increased symptoms of cough and wheeze, as well as increased use of asthma medications and exacerbations of their disease.”
Dr. Zeiger and colleagues recruited 489 respondents for the AAN Pain, Exercise, and Cannabis Experience Survey study through social media channels between May 2020 and September 2020. In the survey, the researchers asked questions about the nature of the respondent’s cannabis use (medical, recreational, or both), the types of cannabinoids used (tetrahydrocannabinol [THC], cannabidiol [CBD], or both), the route of administration (capsule, edible, oil/tincture, smoke, spray, topical, or vaporizer), and subjective effects. Most of the respondents reported using both THC and CBD, with smoking, edibles, and vaping being the most comment route of administration.
Of the 88 respondents who said they currently used cannabis, 60.2% were aged less than 50 years, 72.4% were women, and 71.6% were White. A majority of respondents had been using cannabis for 3 or more years (54.5%) , used it less than one time per day (60.2%), and used it for pain (68.2%). Current asthma was reported in 51 respondents (58.0%), and 39.2% had uncontrolled asthma. Half of those respondents with uncontrolled asthma reported smoking cannabis, and 25.0% reported coughing because of cannabis. Both THC and CBD were used by 47.7% of respondents; 33% reported THC use alone, while 19.3% used CBD alone.
Reported effects of cannabis use
The most common positive effects of using cannabis reported among respondents were that it helped with sleep (66 respondents), calmed them down (60 respondents), reduced pain (60 respondents), or decreased anxiety (59 respondents). Many respondents who reported positive effects were using both THC and CBD. For example, respondents who reported using cannabinoids for calming, 46.7% reported using both, compared with 36.7% who used THC only and 16.7% who used CBD only. Among respondents who reported that cannabis helped them sleep, 51.5% used both THC and CBD.
Regarding adverse effects, there were no significant differences based on use of THC or CBD, but 31.9% of respondents who said they smoked cannabis and 4.9% of respondents who used cannabis through a route of administration that wasn’t smoking reported they coughed with their cannabis use (P < .001). No respondents reported anaphyalaxis, although, among individuals who did not use cannabis, 2.5% reported a cannabis allergy.
‘Cannabis allergy is real’
Commenting on the research, Gordon L. Sussman MD, allergist, clinical immunologist, and clinical professor of medicine at the University of Toronto, said the survey is a thorough questionnaire that is likely representative of attitudes about cannabis in the United States and countries where cannabis is not broadly legalized.
Cannabis allergy, however, is not uncommon, and “is something that people should be aware of,” he said. “Cannabis IgE allergy is real, is probably fairly common, and is something that [clinicians] should be asking about routinely.”
One limitation of the research was not knowing the number of people who declined to answer the survey, as there may be a bias in the results toward people who want to answer the questions, compared with those who did not want to answer. “When you do a survey, only a certain number of people are going to answer, and [you also want input from] people that don’t answer,” Dr. Sussman said.
Dr. Sussman acknowledged it can be difficult to get patients to admit cannabis use, even in countries like Canada where it is legal. Surveys like the one administered by Dr. Zeiger and colleagues are “the first step” to getting updated assessments of cannabis attitudes and recommendations. “The next step is doing an international survey, so you get different countries’ viewpoints and perspectives,” he said.
This study was supported by the Allergy & Asthma Network and the Canna Research Foundation. Three authors are affiliated with the Canna Research Foundation. Dr. Sussman reported no financial conflicts of interest. Dr. Sussman participates in the International Cannabis Allergy KAP Collaboration, a group founded by one of the coauthors, William Silvers, MD, but Dr. Sussman was not involved with this study.
FROM AAAAI 2021
COVID-19 vaccination linked to less mechanical ventilation
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.
new evidence reveals.
Compared with residents younger than 50 – so far vaccinated at lower rates than those of the higher-risk older people – Israelis 70 and older were 67% less likely to require mechanical ventilation for SARS-CoV-2 infection in February 2021 compared with October-December 2020.
“This study provides preliminary evidence at the population level for the reduction in risk for severe COVID-19, as manifested by need for mechanical ventilation, after vaccination with the Pfizer-BioNTech COVID-19 vaccine,” wrote lead author Ehud Rinott, department of public health, faculty of health sciences, Ben-Gurion University of the Negev in Beer-Sheva, Israel, and colleagues.
The study was published online Feb. 26, 2021, in Morbidity and Mortality Weekly Report.
The progress of COVID-19 vaccination across Israel presents researchers with a unique opportunity to study effectiveness on a population level. In this study, 84% of residents 70 and older received two-dose vaccinations. In contrast, only 10% of people in Israel younger than 50 received the same vaccine coverage.
Along with senior author Yair Lewis, MD, PhD, and coauthor Ilan Youngster, MD, Mr. Rinott compared mechanical ventilation rates between Oct. 2, 2020, and Feb. 9, 2021. They found that the ratio of people 70 and older compared with those younger than 50 requiring mechanical ventilation changed from 5.8:1 to 1.9:1 between these periods. This translates to the 67% decrease.
The study offers a “real-world” look at vaccination effectiveness, adding to more controlled evidence from clinical trials. “Achieving high vaccination coverage through intensive vaccination campaigns has the potential to substantially reduce COVID-19-associated morbidity and mortality,” the researchers wrote.
Israel started a national vaccination program on Dec. 20, 2020, targeting high-risk residents including people 60 and older, health care workers, and those with relevant comorbidities. At the same time, in addition to immunization, Israel has used strategies like stay-at-home orders, school closures, mask mandates, and more.
Potential limitations include a limited ability to account for the effect of the stay-at-home orders, spread of virus variants, and other concomitant factors; a potential for a delayed reporting of cases; and variability in mitigation measures by age group.
Dr. Youngster reported receipt of consulting fees from MyBiotix Ltd.
A version of this article first appeared on Medscape.com.