User login
Presence of autoantibodies most predictive of long COVID in study
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
FROM CELL
Is it time to approach spontaneous pneumothorax more conservatively?
ILLUSTRATIVE CASE
A 26-year-old man presents to the emergency department complaining of sudden-onset left-side chest pain and mild dyspnea that started while he was playing basketball. He denies any medical problems and takes no medications. He is able to speak in complete sentences as he answers your questions. His O2 saturation is 95% and a chest x-ray reveals a left-side, moderate-to-large pneumothorax.
A primary spontaneous pneumothorax is one that occurs in the absence of underlying clinical lung disease and is not associated with an inciting cause, such as a rib fracture.2 In the United States, the estimated incidence of primary spontaneous pneumothorax is 7.4 cases per 100,000 men and 1.2 cases per 100,000 women.3 The etiology is often unknown, but it is associated with several risk factors, including male sex, smoking, and a tall, thin body habitus.2
The management strategy for stable patients with a primary spontaneous pneumothorax largely depends on pneumothorax size and institutional practice. Multiple methods define pneumothorax size; the US standard cutoff for a small or large pneumothorax is 3 cm, between the pleural line and chest wall at the level of the apex,4 compared with 2 cm in Europe, when evaluating the distance at the hilum in an upright chest radiograph.5 The Collins method uses a formula to calculate the percentage of lung area affected based on 3 distinct measurements on a posterior/anterior upright chest radiograph.6
Management options include observation, supplemental oxygen, simple aspiration, and thoracostomy or chest tube placement. British Thoracic Society guidelines published in 2010 state that only a small pneumothorax can be managed conservatively with observation alone; for a large pneumothorax, the guidelines recommend needle aspiration to achieve lung reinflation, followed by chest tube placement if unsuccessful.5
In practice, management of a large primary spontaneous pneumothorax varies, but the most common treatment is chest tube placement.7 This procedure can be painful and may result in complications such as bleeding, infection, injury to internal structures, or the need for surgical intervention.7 In addition, once a chest tube is placed, hospital admission ensues, lasting an average of 4 days.8 Given these consequences, there is a need for safe and feasible treatment options for a large primary spontaneous pneumothorax.
STUDY SUMMARY
Observational management judged noninferior, with multiple advantages
The Primary Spontaneous Pneumothorax (PSP) trial was a prospective noninferiority trial conducted at 39 hospitals in Australia and New Zealand. This randomized controlled trial compared observational (“watch and wait”) vs interventional (chest tube placement) management of uncomplicated, unilateral, primary spontaneous pneumothorax. Patients ages 14 to 50 years with a moderate-to-large pneumothorax—32% or greater, as defined by the Collins method4—were randomly assigned to a study group to examine the primary outcome of lung reexpansion at 8 weeks.
The intervention included chest tube insertion attached to an underwater seal without suction for 1 hour, followed by an x-ray and clamping for 4 hours if there was no air leak, followed by a repeat chest x-ray. If there was no evidence of radiographic resolution, or if during observation the pneumothorax recurred, the underwater seal was recommenced and the patient was admitted to the hospital, with further intervention at the discretion of the inpatient clinicians. If radiographic improvement was seen, the tube was removed and the patient discharged.
Continue to: In contrast...
In contrast, conservative management entailed patient observation for at least 4 hours followed by a repeat chest x-ray. If after the observation period, patients were walking comfortably and without supplemental oxygen, they were discharged. Patients in the observation group underwent an intervention if they met a variety of criteria, including unstable vitals or an enlarging pneumothorax. All patients received standard care with analgesia and supplemental oxygen as needed.
A total of 316 patients were randomized, with 154 assigned to the intervention group and 162 to the observation group. The mean age for all participants was 26. Most patients were male (84.4% in the intervention group and 87.7% in the observation group) and almost half were current smokers (49.3% in the intervention and 42.5% in the observation group). The mean body mass index of participants was 21.4 in the intervention and 21.3 in the observation group. Twenty-five patients (15%) in the observation group underwent interventions for reasons specified in the research protocol (eg, “significant symptoms” such as abnormal physiologic observations and intolerable symptoms, or patient unwillingness to continue in the assigned group), and 10 patients assigned to the intervention group declined treatment.
Using a complete-case analysis, 129 of 131 patients (98.5%) in the intervention group and 118 of 125 patients (94.4%) in the observation group met the primary outcome of radiographic resolution within 8 weeks (risk difference [RD] = –4.1%; 95% CI, –8.6 to 0.5), thereby falling within the prespecified margin for noninferiority of less than 9%.
Per-protocol analysis at 8 weeks also proved observational management noninferior, with 124 of 126 patients (98.4%) in the intervention group and 123 of 130 patients (94.6%) in the observation group achieving lung reexpansion within 8 weeks (RD = –3.8%; 95% CI, –8.3 to 0.7). The time to symptom resolution was similar between groups, with a median time of 15.5 days in the intervention group compared with 14 days in the observation group (hazard ratio = 1.11; 95% CI, 0.88-1.4). A lower risk of serious adverse events (relative risk [RR] = 3.3; 95% CI, 1.37-8.1) and pneumothorax recurrence (absolute RD = 8%; 95% CI, 0.5-15.4) occurred in the observation group vs the intervention group. The average length of hospital stay for patients in the intervention group was 6.1 days, vs 1.6 days in the observation group (RR = 2.8; 95% CI, 1.8-3.6).
Two additional sensitivity analyses were performed because multiple study participants were lost to follow-up or had data collected after 8 weeks. Noninferiority was maintained when data collected after the 8-week visit were included and extended to 63 days (RD = –3.7%: 95% CI, –7.9 to 0.6). However, noninferiority was lost when missing data after 8 weeks were deemed “treatment failure” (RD = –11%; 95% CI, –18.4 to –3.5).
Continue to: WHAT'S NEW
WHAT’S NEW
Conservative management enabled most patients to avoid invasive Tx risks
In this specific patient population, conservative management of primary spontaneous pneumothorax was noninferior to interventional management and had a lower risk of serious adverse events. This management practice spared 85% of the patients from invasive intervention. As a result, they experienced a shortened hospital stay, fewer days missed from school or work, less exposure to radiation from repeat chest x-rays, and a lower rate of adverse events. Additionally, fewer of these patients had early pneumothorax recurrence.
CAVEATS
There were limitations in the trial’s original statistical design
This study had a specific follow-up timetable, and some of the participants were not examined until after the 8-week checkpoint or were lost to follow-up entirely. The authors attempted to address these limitations (and show transparency) by providing additional sensitivity analyses as well as providing the intention-to-treat and per-protocol analyses for the primary outcome at 8 weeks. Noninferiority was maintained in all analyses except for the sensitivity analysis that treated missing data as treatment failure. Therefore, the authors note these approaches result in “statistical fragility” and are exploratory.
CHALLENGES TO IMPLEMENTATION
Pneumothorax is not commonly seen in outpatient settings
Family physicians working in outpatient settings generally do not encounter pneumothorax and, using current guidelines, would refer for emergency or inpatient care. This study opens the possibility of managing selected patients in an outpatient setting; however, this would require at least a 4-hour period of observation, which may be impractical for many outpatient-based physicians. Additionally, the study uses the Collins method to define moderate-to-large pneumothorax, which is likely an uncommon practice and thus not applicable in most primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Brown SGA, Ball EL, Perrin K, et al; PSP Investigators. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382:405-415. doi: 10.1056/NEJMoa1910775
2. Hallifax RJ, Goldacre R, Landray MJ, et al. Trends in the incidence and recurrence of inpatient-treated spontaneous pneumothorax, 1968-2016. JAMA. 2018;320:1471-1480. doi: 10.1001/jama.2018.14299
3. Melton LJ III, Hepper NGG, Offord KP. Incidence of spontaneous pneumothorax in Olmstead County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379-1382. doi: 10.1164/arrd.1979.120.6.1379
4. Baumann MH, Strange C, Heffner JE, et al; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602. doi: 10.1378/chest.119.2.590
5. MacDuff A, Arnold A, Harvey J; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl):ii18-ii31. doi: 10.1136/thx.2010.136986
6. Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. Am J Roentgenol. 1995;165:1127-1130. doi: 10.2214/ajr.165.5.7572489
7. Kwiatt M, Tarbox A, Seamon MJ, et al. Thoracostomy tubes: a comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:143-155. doi: 10.4103/2229-5151.134182
8. Maskell NA, Medford A, Gleeson FV. Seldinger chest drain insertion: simpler but not necessarily safer. Thorax. 2010;65:5-6. doi: 10.1136/thx.2009.117200
ILLUSTRATIVE CASE
A 26-year-old man presents to the emergency department complaining of sudden-onset left-side chest pain and mild dyspnea that started while he was playing basketball. He denies any medical problems and takes no medications. He is able to speak in complete sentences as he answers your questions. His O2 saturation is 95% and a chest x-ray reveals a left-side, moderate-to-large pneumothorax.
A primary spontaneous pneumothorax is one that occurs in the absence of underlying clinical lung disease and is not associated with an inciting cause, such as a rib fracture.2 In the United States, the estimated incidence of primary spontaneous pneumothorax is 7.4 cases per 100,000 men and 1.2 cases per 100,000 women.3 The etiology is often unknown, but it is associated with several risk factors, including male sex, smoking, and a tall, thin body habitus.2
The management strategy for stable patients with a primary spontaneous pneumothorax largely depends on pneumothorax size and institutional practice. Multiple methods define pneumothorax size; the US standard cutoff for a small or large pneumothorax is 3 cm, between the pleural line and chest wall at the level of the apex,4 compared with 2 cm in Europe, when evaluating the distance at the hilum in an upright chest radiograph.5 The Collins method uses a formula to calculate the percentage of lung area affected based on 3 distinct measurements on a posterior/anterior upright chest radiograph.6
Management options include observation, supplemental oxygen, simple aspiration, and thoracostomy or chest tube placement. British Thoracic Society guidelines published in 2010 state that only a small pneumothorax can be managed conservatively with observation alone; for a large pneumothorax, the guidelines recommend needle aspiration to achieve lung reinflation, followed by chest tube placement if unsuccessful.5
In practice, management of a large primary spontaneous pneumothorax varies, but the most common treatment is chest tube placement.7 This procedure can be painful and may result in complications such as bleeding, infection, injury to internal structures, or the need for surgical intervention.7 In addition, once a chest tube is placed, hospital admission ensues, lasting an average of 4 days.8 Given these consequences, there is a need for safe and feasible treatment options for a large primary spontaneous pneumothorax.
STUDY SUMMARY
Observational management judged noninferior, with multiple advantages
The Primary Spontaneous Pneumothorax (PSP) trial was a prospective noninferiority trial conducted at 39 hospitals in Australia and New Zealand. This randomized controlled trial compared observational (“watch and wait”) vs interventional (chest tube placement) management of uncomplicated, unilateral, primary spontaneous pneumothorax. Patients ages 14 to 50 years with a moderate-to-large pneumothorax—32% or greater, as defined by the Collins method4—were randomly assigned to a study group to examine the primary outcome of lung reexpansion at 8 weeks.
The intervention included chest tube insertion attached to an underwater seal without suction for 1 hour, followed by an x-ray and clamping for 4 hours if there was no air leak, followed by a repeat chest x-ray. If there was no evidence of radiographic resolution, or if during observation the pneumothorax recurred, the underwater seal was recommenced and the patient was admitted to the hospital, with further intervention at the discretion of the inpatient clinicians. If radiographic improvement was seen, the tube was removed and the patient discharged.
Continue to: In contrast...
In contrast, conservative management entailed patient observation for at least 4 hours followed by a repeat chest x-ray. If after the observation period, patients were walking comfortably and without supplemental oxygen, they were discharged. Patients in the observation group underwent an intervention if they met a variety of criteria, including unstable vitals or an enlarging pneumothorax. All patients received standard care with analgesia and supplemental oxygen as needed.
A total of 316 patients were randomized, with 154 assigned to the intervention group and 162 to the observation group. The mean age for all participants was 26. Most patients were male (84.4% in the intervention group and 87.7% in the observation group) and almost half were current smokers (49.3% in the intervention and 42.5% in the observation group). The mean body mass index of participants was 21.4 in the intervention and 21.3 in the observation group. Twenty-five patients (15%) in the observation group underwent interventions for reasons specified in the research protocol (eg, “significant symptoms” such as abnormal physiologic observations and intolerable symptoms, or patient unwillingness to continue in the assigned group), and 10 patients assigned to the intervention group declined treatment.
Using a complete-case analysis, 129 of 131 patients (98.5%) in the intervention group and 118 of 125 patients (94.4%) in the observation group met the primary outcome of radiographic resolution within 8 weeks (risk difference [RD] = –4.1%; 95% CI, –8.6 to 0.5), thereby falling within the prespecified margin for noninferiority of less than 9%.
Per-protocol analysis at 8 weeks also proved observational management noninferior, with 124 of 126 patients (98.4%) in the intervention group and 123 of 130 patients (94.6%) in the observation group achieving lung reexpansion within 8 weeks (RD = –3.8%; 95% CI, –8.3 to 0.7). The time to symptom resolution was similar between groups, with a median time of 15.5 days in the intervention group compared with 14 days in the observation group (hazard ratio = 1.11; 95% CI, 0.88-1.4). A lower risk of serious adverse events (relative risk [RR] = 3.3; 95% CI, 1.37-8.1) and pneumothorax recurrence (absolute RD = 8%; 95% CI, 0.5-15.4) occurred in the observation group vs the intervention group. The average length of hospital stay for patients in the intervention group was 6.1 days, vs 1.6 days in the observation group (RR = 2.8; 95% CI, 1.8-3.6).
Two additional sensitivity analyses were performed because multiple study participants were lost to follow-up or had data collected after 8 weeks. Noninferiority was maintained when data collected after the 8-week visit were included and extended to 63 days (RD = –3.7%: 95% CI, –7.9 to 0.6). However, noninferiority was lost when missing data after 8 weeks were deemed “treatment failure” (RD = –11%; 95% CI, –18.4 to –3.5).
Continue to: WHAT'S NEW
WHAT’S NEW
Conservative management enabled most patients to avoid invasive Tx risks
In this specific patient population, conservative management of primary spontaneous pneumothorax was noninferior to interventional management and had a lower risk of serious adverse events. This management practice spared 85% of the patients from invasive intervention. As a result, they experienced a shortened hospital stay, fewer days missed from school or work, less exposure to radiation from repeat chest x-rays, and a lower rate of adverse events. Additionally, fewer of these patients had early pneumothorax recurrence.
CAVEATS
There were limitations in the trial’s original statistical design
This study had a specific follow-up timetable, and some of the participants were not examined until after the 8-week checkpoint or were lost to follow-up entirely. The authors attempted to address these limitations (and show transparency) by providing additional sensitivity analyses as well as providing the intention-to-treat and per-protocol analyses for the primary outcome at 8 weeks. Noninferiority was maintained in all analyses except for the sensitivity analysis that treated missing data as treatment failure. Therefore, the authors note these approaches result in “statistical fragility” and are exploratory.
CHALLENGES TO IMPLEMENTATION
Pneumothorax is not commonly seen in outpatient settings
Family physicians working in outpatient settings generally do not encounter pneumothorax and, using current guidelines, would refer for emergency or inpatient care. This study opens the possibility of managing selected patients in an outpatient setting; however, this would require at least a 4-hour period of observation, which may be impractical for many outpatient-based physicians. Additionally, the study uses the Collins method to define moderate-to-large pneumothorax, which is likely an uncommon practice and thus not applicable in most primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 26-year-old man presents to the emergency department complaining of sudden-onset left-side chest pain and mild dyspnea that started while he was playing basketball. He denies any medical problems and takes no medications. He is able to speak in complete sentences as he answers your questions. His O2 saturation is 95% and a chest x-ray reveals a left-side, moderate-to-large pneumothorax.
A primary spontaneous pneumothorax is one that occurs in the absence of underlying clinical lung disease and is not associated with an inciting cause, such as a rib fracture.2 In the United States, the estimated incidence of primary spontaneous pneumothorax is 7.4 cases per 100,000 men and 1.2 cases per 100,000 women.3 The etiology is often unknown, but it is associated with several risk factors, including male sex, smoking, and a tall, thin body habitus.2
The management strategy for stable patients with a primary spontaneous pneumothorax largely depends on pneumothorax size and institutional practice. Multiple methods define pneumothorax size; the US standard cutoff for a small or large pneumothorax is 3 cm, between the pleural line and chest wall at the level of the apex,4 compared with 2 cm in Europe, when evaluating the distance at the hilum in an upright chest radiograph.5 The Collins method uses a formula to calculate the percentage of lung area affected based on 3 distinct measurements on a posterior/anterior upright chest radiograph.6
Management options include observation, supplemental oxygen, simple aspiration, and thoracostomy or chest tube placement. British Thoracic Society guidelines published in 2010 state that only a small pneumothorax can be managed conservatively with observation alone; for a large pneumothorax, the guidelines recommend needle aspiration to achieve lung reinflation, followed by chest tube placement if unsuccessful.5
In practice, management of a large primary spontaneous pneumothorax varies, but the most common treatment is chest tube placement.7 This procedure can be painful and may result in complications such as bleeding, infection, injury to internal structures, or the need for surgical intervention.7 In addition, once a chest tube is placed, hospital admission ensues, lasting an average of 4 days.8 Given these consequences, there is a need for safe and feasible treatment options for a large primary spontaneous pneumothorax.
STUDY SUMMARY
Observational management judged noninferior, with multiple advantages
The Primary Spontaneous Pneumothorax (PSP) trial was a prospective noninferiority trial conducted at 39 hospitals in Australia and New Zealand. This randomized controlled trial compared observational (“watch and wait”) vs interventional (chest tube placement) management of uncomplicated, unilateral, primary spontaneous pneumothorax. Patients ages 14 to 50 years with a moderate-to-large pneumothorax—32% or greater, as defined by the Collins method4—were randomly assigned to a study group to examine the primary outcome of lung reexpansion at 8 weeks.
The intervention included chest tube insertion attached to an underwater seal without suction for 1 hour, followed by an x-ray and clamping for 4 hours if there was no air leak, followed by a repeat chest x-ray. If there was no evidence of radiographic resolution, or if during observation the pneumothorax recurred, the underwater seal was recommenced and the patient was admitted to the hospital, with further intervention at the discretion of the inpatient clinicians. If radiographic improvement was seen, the tube was removed and the patient discharged.
Continue to: In contrast...
In contrast, conservative management entailed patient observation for at least 4 hours followed by a repeat chest x-ray. If after the observation period, patients were walking comfortably and without supplemental oxygen, they were discharged. Patients in the observation group underwent an intervention if they met a variety of criteria, including unstable vitals or an enlarging pneumothorax. All patients received standard care with analgesia and supplemental oxygen as needed.
A total of 316 patients were randomized, with 154 assigned to the intervention group and 162 to the observation group. The mean age for all participants was 26. Most patients were male (84.4% in the intervention group and 87.7% in the observation group) and almost half were current smokers (49.3% in the intervention and 42.5% in the observation group). The mean body mass index of participants was 21.4 in the intervention and 21.3 in the observation group. Twenty-five patients (15%) in the observation group underwent interventions for reasons specified in the research protocol (eg, “significant symptoms” such as abnormal physiologic observations and intolerable symptoms, or patient unwillingness to continue in the assigned group), and 10 patients assigned to the intervention group declined treatment.
Using a complete-case analysis, 129 of 131 patients (98.5%) in the intervention group and 118 of 125 patients (94.4%) in the observation group met the primary outcome of radiographic resolution within 8 weeks (risk difference [RD] = –4.1%; 95% CI, –8.6 to 0.5), thereby falling within the prespecified margin for noninferiority of less than 9%.
Per-protocol analysis at 8 weeks also proved observational management noninferior, with 124 of 126 patients (98.4%) in the intervention group and 123 of 130 patients (94.6%) in the observation group achieving lung reexpansion within 8 weeks (RD = –3.8%; 95% CI, –8.3 to 0.7). The time to symptom resolution was similar between groups, with a median time of 15.5 days in the intervention group compared with 14 days in the observation group (hazard ratio = 1.11; 95% CI, 0.88-1.4). A lower risk of serious adverse events (relative risk [RR] = 3.3; 95% CI, 1.37-8.1) and pneumothorax recurrence (absolute RD = 8%; 95% CI, 0.5-15.4) occurred in the observation group vs the intervention group. The average length of hospital stay for patients in the intervention group was 6.1 days, vs 1.6 days in the observation group (RR = 2.8; 95% CI, 1.8-3.6).
Two additional sensitivity analyses were performed because multiple study participants were lost to follow-up or had data collected after 8 weeks. Noninferiority was maintained when data collected after the 8-week visit were included and extended to 63 days (RD = –3.7%: 95% CI, –7.9 to 0.6). However, noninferiority was lost when missing data after 8 weeks were deemed “treatment failure” (RD = –11%; 95% CI, –18.4 to –3.5).
Continue to: WHAT'S NEW
WHAT’S NEW
Conservative management enabled most patients to avoid invasive Tx risks
In this specific patient population, conservative management of primary spontaneous pneumothorax was noninferior to interventional management and had a lower risk of serious adverse events. This management practice spared 85% of the patients from invasive intervention. As a result, they experienced a shortened hospital stay, fewer days missed from school or work, less exposure to radiation from repeat chest x-rays, and a lower rate of adverse events. Additionally, fewer of these patients had early pneumothorax recurrence.
CAVEATS
There were limitations in the trial’s original statistical design
This study had a specific follow-up timetable, and some of the participants were not examined until after the 8-week checkpoint or were lost to follow-up entirely. The authors attempted to address these limitations (and show transparency) by providing additional sensitivity analyses as well as providing the intention-to-treat and per-protocol analyses for the primary outcome at 8 weeks. Noninferiority was maintained in all analyses except for the sensitivity analysis that treated missing data as treatment failure. Therefore, the authors note these approaches result in “statistical fragility” and are exploratory.
CHALLENGES TO IMPLEMENTATION
Pneumothorax is not commonly seen in outpatient settings
Family physicians working in outpatient settings generally do not encounter pneumothorax and, using current guidelines, would refer for emergency or inpatient care. This study opens the possibility of managing selected patients in an outpatient setting; however, this would require at least a 4-hour period of observation, which may be impractical for many outpatient-based physicians. Additionally, the study uses the Collins method to define moderate-to-large pneumothorax, which is likely an uncommon practice and thus not applicable in most primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Brown SGA, Ball EL, Perrin K, et al; PSP Investigators. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382:405-415. doi: 10.1056/NEJMoa1910775
2. Hallifax RJ, Goldacre R, Landray MJ, et al. Trends in the incidence and recurrence of inpatient-treated spontaneous pneumothorax, 1968-2016. JAMA. 2018;320:1471-1480. doi: 10.1001/jama.2018.14299
3. Melton LJ III, Hepper NGG, Offord KP. Incidence of spontaneous pneumothorax in Olmstead County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379-1382. doi: 10.1164/arrd.1979.120.6.1379
4. Baumann MH, Strange C, Heffner JE, et al; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602. doi: 10.1378/chest.119.2.590
5. MacDuff A, Arnold A, Harvey J; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl):ii18-ii31. doi: 10.1136/thx.2010.136986
6. Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. Am J Roentgenol. 1995;165:1127-1130. doi: 10.2214/ajr.165.5.7572489
7. Kwiatt M, Tarbox A, Seamon MJ, et al. Thoracostomy tubes: a comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:143-155. doi: 10.4103/2229-5151.134182
8. Maskell NA, Medford A, Gleeson FV. Seldinger chest drain insertion: simpler but not necessarily safer. Thorax. 2010;65:5-6. doi: 10.1136/thx.2009.117200
1. Brown SGA, Ball EL, Perrin K, et al; PSP Investigators. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382:405-415. doi: 10.1056/NEJMoa1910775
2. Hallifax RJ, Goldacre R, Landray MJ, et al. Trends in the incidence and recurrence of inpatient-treated spontaneous pneumothorax, 1968-2016. JAMA. 2018;320:1471-1480. doi: 10.1001/jama.2018.14299
3. Melton LJ III, Hepper NGG, Offord KP. Incidence of spontaneous pneumothorax in Olmstead County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379-1382. doi: 10.1164/arrd.1979.120.6.1379
4. Baumann MH, Strange C, Heffner JE, et al; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602. doi: 10.1378/chest.119.2.590
5. MacDuff A, Arnold A, Harvey J; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl):ii18-ii31. doi: 10.1136/thx.2010.136986
6. Collins CD, Lopez A, Mathie A, et al. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. Am J Roentgenol. 1995;165:1127-1130. doi: 10.2214/ajr.165.5.7572489
7. Kwiatt M, Tarbox A, Seamon MJ, et al. Thoracostomy tubes: a comprehensive review of complications and related topics. Int J Crit Illn Inj Sci. 2014;4:143-155. doi: 10.4103/2229-5151.134182
8. Maskell NA, Medford A, Gleeson FV. Seldinger chest drain insertion: simpler but not necessarily safer. Thorax. 2010;65:5-6. doi: 10.1136/thx.2009.117200
PRACTICE CHANGER
Consider observation rather than chest tube placement for primary, uncomplicated, unilateral moderate-to-large spontaneous pneumothorax in patients ages 14 to 50.
STRENGTH OF RECOMMENDATION
B: Based on a single, lower-quality randomized controlled trial1
Brown SGA, Ball EL, Perrin K, et al; PSP Investigators. Conservative versus interventional treatment for spontaneous pneumothorax. N Engl J Med. 2020;382:405-415. doi: 10.1056/NEJMoa1910775
58-year-old man • bilateral shoulder pain • history of prostate cancer • limited shoulder range of motion • Dx?
THE CASE
A 58-year-old African American man with a past medical history of prostate cancer, hypertension, hyperlipidemia, osteoarthritis, and gastroesophageal reflux disease presented to our office to establish care with a new provider. He complained of bilateral shoulder pain, that was worse on the right side, for the past year. He denied any previous falls, trauma, or injury. He reported that lifting his grandkids was becoming increasingly difficult due to the pain but denied any weakness or neurologic symptoms. He had been using over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs), which provided minimal relief.
On physical examination, the overlying skin was normal and there was no tenderness to palpation. His shoulder range of motion was limited with complete flexion, but otherwise intact. Muscle strength was 5 out of 5 bilaterally, and neurovascular and sensory examinations were normal. On the right side, the Empty Can Test was positive, but the Neer and Apley tests were negative. All testing was negative on the left side.
The patient was referred for 10 sessions of physical therapy, which he completed. His pain persisted, and an x-ray of his right shoulder was performed. The x-ray indicated a high-riding humeral head, and magnetic resonance imaging (MRI) of the right shoulder was recommended due to possible rotator cuff tendinopathy.
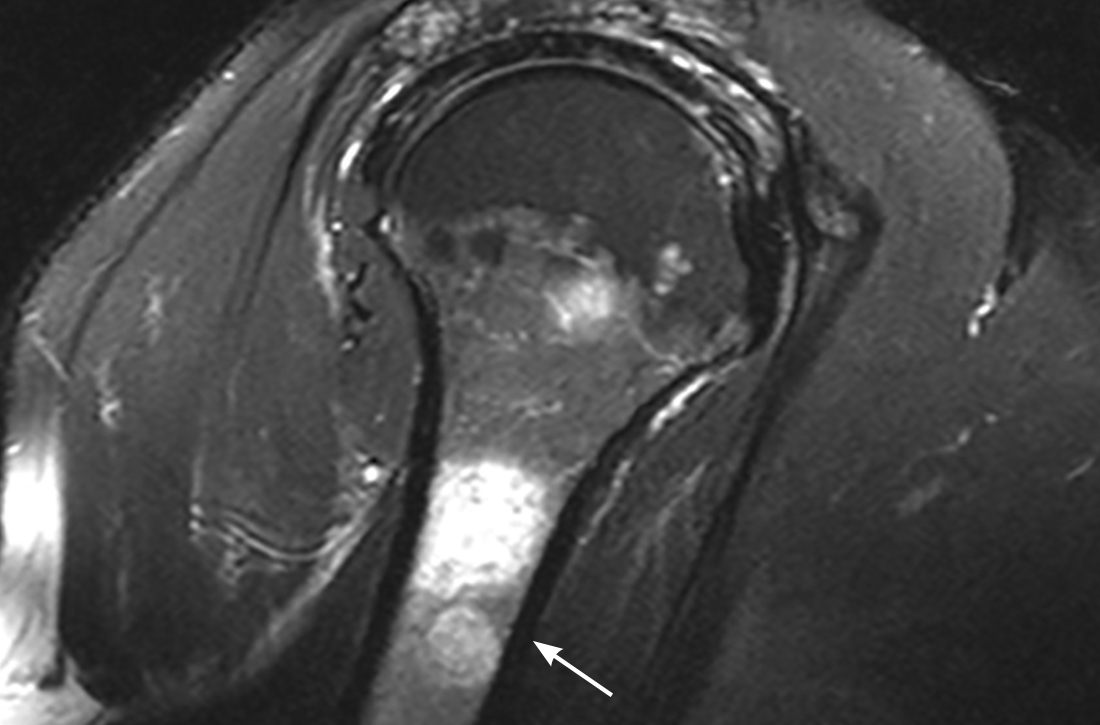
The MRI demonstrated a full-thickness tear of the distal supraspinatus tendon along with “metastatic lesions” (FIGURE). As a result, a bone scan was obtained and revealed activity in the proximal right humerus; however, it was nonconclusive for osteoblastic metastasis. A positron emission tomography (PET) scan was ordered, which revealed findings suggestive of bony metastasis in the proximal left tibia, distal shaft of the right tibia, and the right and left humeral heads. The patient was then scheduled for a bone biopsy; a chest, abdomen, and pelvis computed tomography (CT) scan with IV and oral contrast was also ordered.
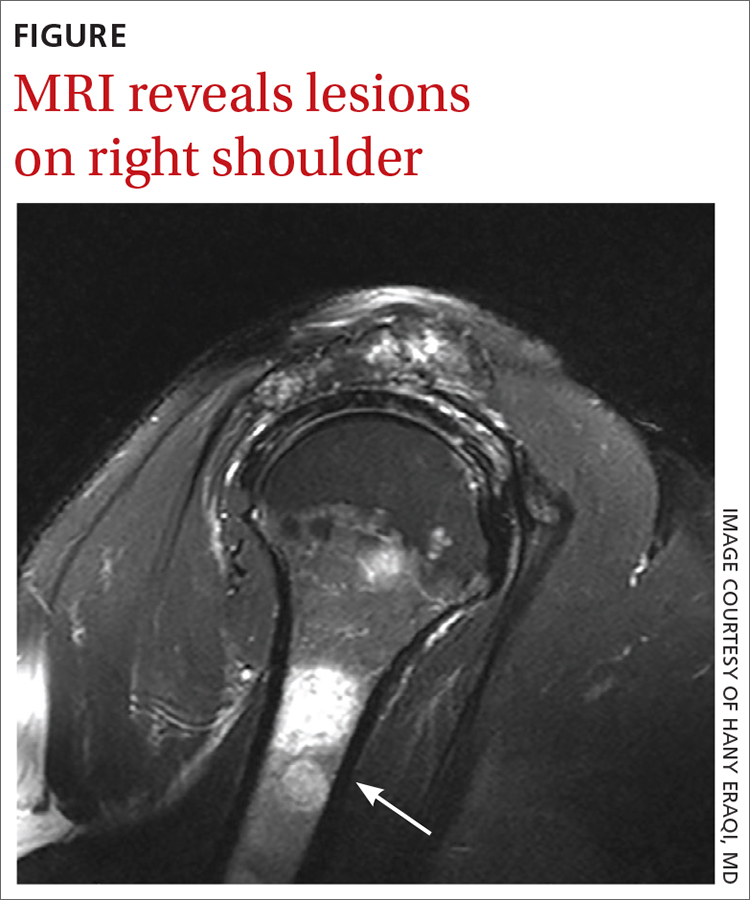
THE DIAGNOSIS
A bone biopsy of the left tibia indicated prominent non-necrotizing granulomatous inflammation and stains were negative for microorganisms. The CT scan demonstrated peribronchial vascular reticulonodular opacities in the upper lung zones compatible with sarcoidosis; no metastatic lesions were identified. Laboratory studies were obtained and demonstrated an elevated angiotensin-converting enzyme (ACE) level consistent with sarcoidosis. The cumulative test results pointed to a diagnosis of osseous sarcoidosis.
DISCUSSION
Osseous sarcoidosis is a rare manifestation of larger systemic disease. It is estimated that bony lesions occur in only 3% to 13% of patients with sarcoidosis.1 Bone involvement is most common in African Americans and occurs primarily in the hands and feet.1-3
Osseous lesions are comprised of noncaseating granulomatous inflammation.4,5 They are often asymptomatic but can be painful and associated with overlying skin disease and soft-tissue swelling.1,4 Although it’s not typical, patients may present with symptoms such as pain, stiffness, or fractures. On CT imaging and MRI (as in this case), osseous lesions can be confused with metastatic bone disease, and biopsy may be required for diagnosis.4
Continue to: There are multiple patterns of bone involvement
There are multiple patterns of bone involvement in osseous sarcoidosis, ranging from large cystic lesions that can lead to stress fractures to “tunnels” or “lace-like” reticulated patterns found in the bones of the hands and feet. 2,3,5,6 Long bone involvement is typically limited to the proximal and distal thirds of the bone.6 Sarcoidosis is also known to involve the axial skeleton, and less commonly, the cranial vault.6 Although multiple variations may manifest over time, skin changes usually precede bone lesions3,6; however, that was not the case with this patient.
Treatment entails pain management
Up to 50% of patients with bone lesions are symptomatic and may require treatment.3,5 Treatment is reserved for these symptomatic patients, with the goal of pain reduction.2,3,7
Low- to moderate-dose corticosteroids have been shown to relieve soft-tissue swelling and decrease pain.2,3,7 A prolonged course of steroids is not recommended, due to the risk of osteoporosis and fractures, and does not normalize bone structure.3,7
Other options. NSAIDs, such as colchicine and indomethacin, have also been found to be effective in pain management.7 Treatments such as methotrexate and hydroxychloroquine may be considered for those cases that are refractory to steroids.2
Given the extent of our patient’s disease, he was referred to multiple specialists to rule out further organ involvement. He was found to have neurosarcoidosis on brain imaging and was subsequently treated with prednisone 10 mg/d. The patient is being routinely monitored for active disease at various intervals or as symptoms arise.
THE TAKEAWAY
Consideration for systemic diseases (eg, sarcoidosis) should be given to patients presenting with musculoskeletal complaints without a significant history of trauma or injury. In those with risk factors associated with a higher incidence of sarcoidosis, such as age and race, a work-up should include imaging and biopsy. Treatment (eg, corticosteroids, NSAIDs) is provided to those patients who are symptomatic, with the goal of symptom relief.3
1. Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013;39:277-297. doi: 10.1016/j.rdc.2013.02.007
2. Kobak S. Sarcoidosis: a rheumatologist’s perspective. Ther Adv Musculoskelet Dis. 2015;7:196-205. doi: 10.1177/1759720X15591310
3. Bechman K, Christidis D, Walsh S, et al. A review of the musculoskeletal manifestations of sarcoidosis. Rheumatology (Oxford). 2018;57:777-783. doi: 10.1093/rheumatology/kex317
4. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165. doi: 10.1056/NEJMra071714
5. Yachoui R, Parker BJ, Nguyen TT. Bone and bone marrow involvement in sarcoidosis. Rheumatol Int. 2015;35:1917-1924. doi: 10.1007/s00296-015-3341-y
6. Aptel S, Lecocq-Teixeira S, Olivier P, et al. Multimodality evaluation of musculoskeletal sarcoidosis: Imaging findings and literature review. Diagn Interv Imaging. 2016;97:5-18. doi: 10.1016/j.diii.2014.11.038
7. Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol. 2000;12:321-330. doi: 10.1097/00002281-200007000-00016
THE CASE
A 58-year-old African American man with a past medical history of prostate cancer, hypertension, hyperlipidemia, osteoarthritis, and gastroesophageal reflux disease presented to our office to establish care with a new provider. He complained of bilateral shoulder pain, that was worse on the right side, for the past year. He denied any previous falls, trauma, or injury. He reported that lifting his grandkids was becoming increasingly difficult due to the pain but denied any weakness or neurologic symptoms. He had been using over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs), which provided minimal relief.
On physical examination, the overlying skin was normal and there was no tenderness to palpation. His shoulder range of motion was limited with complete flexion, but otherwise intact. Muscle strength was 5 out of 5 bilaterally, and neurovascular and sensory examinations were normal. On the right side, the Empty Can Test was positive, but the Neer and Apley tests were negative. All testing was negative on the left side.
The patient was referred for 10 sessions of physical therapy, which he completed. His pain persisted, and an x-ray of his right shoulder was performed. The x-ray indicated a high-riding humeral head, and magnetic resonance imaging (MRI) of the right shoulder was recommended due to possible rotator cuff tendinopathy.
The MRI demonstrated a full-thickness tear of the distal supraspinatus tendon along with “metastatic lesions” (FIGURE). As a result, a bone scan was obtained and revealed activity in the proximal right humerus; however, it was nonconclusive for osteoblastic metastasis. A positron emission tomography (PET) scan was ordered, which revealed findings suggestive of bony metastasis in the proximal left tibia, distal shaft of the right tibia, and the right and left humeral heads. The patient was then scheduled for a bone biopsy; a chest, abdomen, and pelvis computed tomography (CT) scan with IV and oral contrast was also ordered.

THE DIAGNOSIS
A bone biopsy of the left tibia indicated prominent non-necrotizing granulomatous inflammation and stains were negative for microorganisms. The CT scan demonstrated peribronchial vascular reticulonodular opacities in the upper lung zones compatible with sarcoidosis; no metastatic lesions were identified. Laboratory studies were obtained and demonstrated an elevated angiotensin-converting enzyme (ACE) level consistent with sarcoidosis. The cumulative test results pointed to a diagnosis of osseous sarcoidosis.
DISCUSSION
Osseous sarcoidosis is a rare manifestation of larger systemic disease. It is estimated that bony lesions occur in only 3% to 13% of patients with sarcoidosis.1 Bone involvement is most common in African Americans and occurs primarily in the hands and feet.1-3
Osseous lesions are comprised of noncaseating granulomatous inflammation.4,5 They are often asymptomatic but can be painful and associated with overlying skin disease and soft-tissue swelling.1,4 Although it’s not typical, patients may present with symptoms such as pain, stiffness, or fractures. On CT imaging and MRI (as in this case), osseous lesions can be confused with metastatic bone disease, and biopsy may be required for diagnosis.4
Continue to: There are multiple patterns of bone involvement
There are multiple patterns of bone involvement in osseous sarcoidosis, ranging from large cystic lesions that can lead to stress fractures to “tunnels” or “lace-like” reticulated patterns found in the bones of the hands and feet. 2,3,5,6 Long bone involvement is typically limited to the proximal and distal thirds of the bone.6 Sarcoidosis is also known to involve the axial skeleton, and less commonly, the cranial vault.6 Although multiple variations may manifest over time, skin changes usually precede bone lesions3,6; however, that was not the case with this patient.
Treatment entails pain management
Up to 50% of patients with bone lesions are symptomatic and may require treatment.3,5 Treatment is reserved for these symptomatic patients, with the goal of pain reduction.2,3,7
Low- to moderate-dose corticosteroids have been shown to relieve soft-tissue swelling and decrease pain.2,3,7 A prolonged course of steroids is not recommended, due to the risk of osteoporosis and fractures, and does not normalize bone structure.3,7
Other options. NSAIDs, such as colchicine and indomethacin, have also been found to be effective in pain management.7 Treatments such as methotrexate and hydroxychloroquine may be considered for those cases that are refractory to steroids.2
Given the extent of our patient’s disease, he was referred to multiple specialists to rule out further organ involvement. He was found to have neurosarcoidosis on brain imaging and was subsequently treated with prednisone 10 mg/d. The patient is being routinely monitored for active disease at various intervals or as symptoms arise.
THE TAKEAWAY
Consideration for systemic diseases (eg, sarcoidosis) should be given to patients presenting with musculoskeletal complaints without a significant history of trauma or injury. In those with risk factors associated with a higher incidence of sarcoidosis, such as age and race, a work-up should include imaging and biopsy. Treatment (eg, corticosteroids, NSAIDs) is provided to those patients who are symptomatic, with the goal of symptom relief.3
THE CASE
A 58-year-old African American man with a past medical history of prostate cancer, hypertension, hyperlipidemia, osteoarthritis, and gastroesophageal reflux disease presented to our office to establish care with a new provider. He complained of bilateral shoulder pain, that was worse on the right side, for the past year. He denied any previous falls, trauma, or injury. He reported that lifting his grandkids was becoming increasingly difficult due to the pain but denied any weakness or neurologic symptoms. He had been using over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs), which provided minimal relief.
On physical examination, the overlying skin was normal and there was no tenderness to palpation. His shoulder range of motion was limited with complete flexion, but otherwise intact. Muscle strength was 5 out of 5 bilaterally, and neurovascular and sensory examinations were normal. On the right side, the Empty Can Test was positive, but the Neer and Apley tests were negative. All testing was negative on the left side.
The patient was referred for 10 sessions of physical therapy, which he completed. His pain persisted, and an x-ray of his right shoulder was performed. The x-ray indicated a high-riding humeral head, and magnetic resonance imaging (MRI) of the right shoulder was recommended due to possible rotator cuff tendinopathy.
The MRI demonstrated a full-thickness tear of the distal supraspinatus tendon along with “metastatic lesions” (FIGURE). As a result, a bone scan was obtained and revealed activity in the proximal right humerus; however, it was nonconclusive for osteoblastic metastasis. A positron emission tomography (PET) scan was ordered, which revealed findings suggestive of bony metastasis in the proximal left tibia, distal shaft of the right tibia, and the right and left humeral heads. The patient was then scheduled for a bone biopsy; a chest, abdomen, and pelvis computed tomography (CT) scan with IV and oral contrast was also ordered.

THE DIAGNOSIS
A bone biopsy of the left tibia indicated prominent non-necrotizing granulomatous inflammation and stains were negative for microorganisms. The CT scan demonstrated peribronchial vascular reticulonodular opacities in the upper lung zones compatible with sarcoidosis; no metastatic lesions were identified. Laboratory studies were obtained and demonstrated an elevated angiotensin-converting enzyme (ACE) level consistent with sarcoidosis. The cumulative test results pointed to a diagnosis of osseous sarcoidosis.
DISCUSSION
Osseous sarcoidosis is a rare manifestation of larger systemic disease. It is estimated that bony lesions occur in only 3% to 13% of patients with sarcoidosis.1 Bone involvement is most common in African Americans and occurs primarily in the hands and feet.1-3
Osseous lesions are comprised of noncaseating granulomatous inflammation.4,5 They are often asymptomatic but can be painful and associated with overlying skin disease and soft-tissue swelling.1,4 Although it’s not typical, patients may present with symptoms such as pain, stiffness, or fractures. On CT imaging and MRI (as in this case), osseous lesions can be confused with metastatic bone disease, and biopsy may be required for diagnosis.4
Continue to: There are multiple patterns of bone involvement
There are multiple patterns of bone involvement in osseous sarcoidosis, ranging from large cystic lesions that can lead to stress fractures to “tunnels” or “lace-like” reticulated patterns found in the bones of the hands and feet. 2,3,5,6 Long bone involvement is typically limited to the proximal and distal thirds of the bone.6 Sarcoidosis is also known to involve the axial skeleton, and less commonly, the cranial vault.6 Although multiple variations may manifest over time, skin changes usually precede bone lesions3,6; however, that was not the case with this patient.
Treatment entails pain management
Up to 50% of patients with bone lesions are symptomatic and may require treatment.3,5 Treatment is reserved for these symptomatic patients, with the goal of pain reduction.2,3,7
Low- to moderate-dose corticosteroids have been shown to relieve soft-tissue swelling and decrease pain.2,3,7 A prolonged course of steroids is not recommended, due to the risk of osteoporosis and fractures, and does not normalize bone structure.3,7
Other options. NSAIDs, such as colchicine and indomethacin, have also been found to be effective in pain management.7 Treatments such as methotrexate and hydroxychloroquine may be considered for those cases that are refractory to steroids.2
Given the extent of our patient’s disease, he was referred to multiple specialists to rule out further organ involvement. He was found to have neurosarcoidosis on brain imaging and was subsequently treated with prednisone 10 mg/d. The patient is being routinely monitored for active disease at various intervals or as symptoms arise.
THE TAKEAWAY
Consideration for systemic diseases (eg, sarcoidosis) should be given to patients presenting with musculoskeletal complaints without a significant history of trauma or injury. In those with risk factors associated with a higher incidence of sarcoidosis, such as age and race, a work-up should include imaging and biopsy. Treatment (eg, corticosteroids, NSAIDs) is provided to those patients who are symptomatic, with the goal of symptom relief.3
1. Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013;39:277-297. doi: 10.1016/j.rdc.2013.02.007
2. Kobak S. Sarcoidosis: a rheumatologist’s perspective. Ther Adv Musculoskelet Dis. 2015;7:196-205. doi: 10.1177/1759720X15591310
3. Bechman K, Christidis D, Walsh S, et al. A review of the musculoskeletal manifestations of sarcoidosis. Rheumatology (Oxford). 2018;57:777-783. doi: 10.1093/rheumatology/kex317
4. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165. doi: 10.1056/NEJMra071714
5. Yachoui R, Parker BJ, Nguyen TT. Bone and bone marrow involvement in sarcoidosis. Rheumatol Int. 2015;35:1917-1924. doi: 10.1007/s00296-015-3341-y
6. Aptel S, Lecocq-Teixeira S, Olivier P, et al. Multimodality evaluation of musculoskeletal sarcoidosis: Imaging findings and literature review. Diagn Interv Imaging. 2016;97:5-18. doi: 10.1016/j.diii.2014.11.038
7. Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol. 2000;12:321-330. doi: 10.1097/00002281-200007000-00016
1. Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013;39:277-297. doi: 10.1016/j.rdc.2013.02.007
2. Kobak S. Sarcoidosis: a rheumatologist’s perspective. Ther Adv Musculoskelet Dis. 2015;7:196-205. doi: 10.1177/1759720X15591310
3. Bechman K, Christidis D, Walsh S, et al. A review of the musculoskeletal manifestations of sarcoidosis. Rheumatology (Oxford). 2018;57:777-783. doi: 10.1093/rheumatology/kex317
4. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165. doi: 10.1056/NEJMra071714
5. Yachoui R, Parker BJ, Nguyen TT. Bone and bone marrow involvement in sarcoidosis. Rheumatol Int. 2015;35:1917-1924. doi: 10.1007/s00296-015-3341-y
6. Aptel S, Lecocq-Teixeira S, Olivier P, et al. Multimodality evaluation of musculoskeletal sarcoidosis: Imaging findings and literature review. Diagn Interv Imaging. 2016;97:5-18. doi: 10.1016/j.diii.2014.11.038
7. Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol. 2000;12:321-330. doi: 10.1097/00002281-200007000-00016
Severe outcomes increased in youth hospitalized after positive COVID-19 test
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM JAMA NETWORK OPEN
Pediatric community-acquired pneumonia: 5 days of antibiotics better than 10 days
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
Pediatric antibiotic prescriptions plummeted in pandemic
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
Antibiotic prescribing in pediatric primary care decreased dramatically when the COVID-19 pandemic hit, and new research indicates that drop was sustained through June of 2021.
Lauren Dutcher, MD, with the division of infectious diseases at Hospital of the University of Pennsylvania in Philadelphia, led a study of 27 pediatric primary care practices in the United States. Encounters from Jan. 1, 2018, through June 30, 2021, were included.
Researchers found a 72.7% drop in antibiotic prescriptions when they compared prepandemic April 2019 through December 2019 with the same period in 2020.
Prescriptions remained at the lower levels, primarily driven by reductions in respiratory tract infection (RTI) encounters, and began to rise only in April of 2021, the authors write.
Findings were published online Jan. 11 in Pediatrics.
Researchers report there were 69,327 antibiotic prescriptions from April through December in 2019 and 18,935 antibiotic prescriptions during the same months in 2020.
“The reduction in prescriptions at visits for respiratory tract infection (RTI) accounted for 87.3% of this decrease,” the authors write.
Both prescribing and acute non–COVID-19 respiratory tract infection diagnoses decreased.
Researchers conclude reductions in viral RTI transmission likely played a large role in reduced RTI pediatric visits and antibiotic prescriptions.
Dr. Dutcher told this publication the reduction was likely caused by a combination of less viral transmission of respiratory infections, helped in part by masking and distancing, but also avoidance of health care in the pandemic.
She said the data reinforce the need for appropriate prescribing.
“Antibiotic prescribing is really heavily driven by respiratory infections so this should continue to clue providers in on how frequently that can be unnecessary,“ she said.
Dr. Dutcher said there was probably a reduction in secondary bacterial infections as well as the viral infections.
The research is more comprehensive than some other previous studies, the authors write.
“Although other studies demonstrated early reductions in RTIs and antibiotic prescribing during the COVID-19 pandemic, to our knowledge, this is the first study to demonstrate a sustained decrease in antibiotic prescribing in pediatric primary care throughout 2020 and early 2021,” they write.
The findings also suggest benefits of preventive measures during the pandemic, the authors say.
“Our data suggest that reducing community viral RTI transmission through social distancing and masking corresponds with a reduction in antibiotic prescribing,” they write.
Kao-Ping Chua, MD, a pediatrician and an assistant professor of pediatrics at the University of Michigan in Ann Arbor, said the reductions indicate one of two things is happening: either children aren’t getting sick as often during the pandemic or they are getting sick, but not coming in.
But if they were sick and not coming in, the expectation would be that they would show up in large numbers in emergency departments from untreated infections, he said.
“We just haven’t seen that,” he said.
He said one of the main points the authors make is that masks, distancing, and hand washing may be keeping kids from diseases beyond COVID-19.
He said longer-term data will be needed to show if the trend highlighted in this paper lasts, given children have now returned to school and pediatricians started to see lots of respiratory syncytial virus (RSV) cases this summer.
Anecdotally, he said, he has been prescribing more antibiotics of late for presentations such as ear infections.
Dr. Dutcher said that, though her team doesn’t have data yet since the end of the study period, she agreed that anecdotally it is likely that the prescriptions have been on the rise since June.
Dr. Chua said the reduction in visits also reduces the chance that a physician will be tempted to give in to families’ demands to prescribe an antibiotic.
“Every visit for a sick child represents an opportunity to inappropriately prescribe antibiotics,” Dr. Chua said. Dr. Chua’s own research has found that up to one-quarter of pediatric and adult antibiotic prescriptions are unnecessary.
This work was supported by a Centers for Disease Control and Prevention cooperative agreement, Epicenters for the Prevention of Healthcare Associated Infections. Dr. Dutcher and Dr. Chua had no relevant financial disclosures.
This article was updated 1/11/22.
FROM PEDIATRICS
COVID-vaccine myocarditis: Rare, mild, and usually in young men
The risk of myocarditis after immunization with mRNA-based vaccines against SARS-CoV-2 raised concerns when it came to light in early 2021. But as report after report showed such cases to be rare and usually mild and self-limited, focus has turned to the “how and why.”
The mechanism linking the BNT162b2 (Pfizer-BioNTech) and especially mRNA-1273 (Moderna) vaccines to the occurrence of myocarditis is unclear for now, but one potential driver may be tied to a peculiarity that became apparent early: It occurs overwhelmingly in younger males, from 16 to perhaps 40 or 50 years of age. Excess risk has not been consistently seen among women, girls, and older men.
That observation has led to speculation that higher testosterone levels in adolescent boys and young men may somehow promote the adverse vaccine effect, whereas greater levels of estrogen among girls and women in the same age range may be cardioprotective.
Unlikely, brief, and ‘benign’
“Most of the myocarditis is benign, by which I mean that maybe the patients are admitted due to chest pain, but without reduction in ventricular function,” Enrico Ammirati, MD, PhD, a myocarditis expert at De Gasperis Cardio Center and Transplant Center, Niguarda Hospital, Milan, said in an interview.
In a Nov. 14 address on this topic at the annual scientific sessions of the American Heart Association, Dror Mevorach, MD, described the typical case presentation as “mild” and one that clears in fairly short order based on resolution of “clinical symptoms, inflammatory markers and troponin decline, EKG normalization, echo normalization, and a relatively short length of hospital stay.”
Dr. Mevorach, of Hadassah Hebrew University Medical Center, Jerusalem, subsequently published the findings in a report in the New England Journal of Medicine that described 136 confirmed myocarditis cases among more than 5 million people in Israel immunized with the Pfizer-BioNTech vaccine. Myocarditis was considered “mild” in 129 cases, or 95%.
And the risk is tiny, compared with myocarditis from infection by SARS-CoV-2, not to mention the possibility of nasty clinical COVID-19 complications such as pneumonia and pulmonary embolism, Dr. Mevorach observed.
Many other reports agree that the incidence is minimal, especially given the rewards of vaccination. In a separate NEJM publication in September 2021 – from Noam Barda, MD, Clalit (Israel) Research Institute, and colleagues on 1.7 million people in that country, about half unvaccinated and half given the Pfizer-BioNTech vaccine – there were an estimated 2.7 cases of myocarditis per 100,000 vaccinated persons. There were also 11 cases of myocarditis per 100,000 persons who were positive for SARS-CoV-2 infection.
And in a recent case series of vaccinated people aged 16 or older, the myocarditis rate after a first or second Pfizer-BioNTech or Moderna injection was estimated at 1 or fewer per 100,000. The corresponding estimate was 4 such cases per 100,000 after a positive SARS-CoV-2 test among the same population, notes a report published Dec.14, 2021, in Nature Medicine.
In general, “the risk of any kind of cardiac injury is vastly lower with a vaccine than it is with the actual viral infection,” Leslie T. Cooper Jr., MD, a myocarditis expert and clinical trialist at the Mayo Clinic, Jacksonville, Fla., said in an interview. With the mRNA-based vaccines, “we do not have any conceivable danger signal that would outweigh the benefit of vaccination.”
Males of a certain age
Evidence that such myocarditis predominates in young adult men and adolescent boys, especially following a second vaccine dose, is remarkably consistent.
The risk was elevated only among mRNA-based vaccine recipients who were younger than 40 in the recent Nature Medicine analysis. Among that group, estimates after a second dose numbered fewer than 1 case per 100,000 for Pfizer-BioNTech and 1.5 per 100,000 for Moderna.
In a third analysis from Israel – also in NEJM, from Guy Witberg, MD, Rabin Medical Center, Petah Tikva, and colleagues, based on 2.5 million people aged 16 and older with at least one Pfizer-BioNTech injection – 2.1 cases per 100,000 were estimated overall, but the number rose to 10.7 per 100,000 among those aged 16-29 years.
In Dr. Mevorach’s NEJM report, estimates after a second Pfizer-BioNTech vaccine dose were 1 per 26,000 males versus 1 in 218,000 females, compared with 1 myocarditis case in 10,857 persons among “the general unvaccinated population.”
Most recipients of a first vaccine dose were younger than 50, and 16- to 29-year-olds accounted for most who completed two doses, noted Dr. Mevorach. Younger males bore the brunt of any myocarditis: the estimated prevalence after a second dose among males aged 16-19 was 1 per 6,637, compared with 1 per 99,853 females in the same age range, the group reported.
In the BMJ report, based on about 5 million people 12 years of age or older in Denmark, the estimated rates of myocarditis or pericarditis associated with Moderna immunization were 2 per 100,000 among women but 6.3 per 100,000 for men. The incidence and sex difference was much lower among those getting the Pfizer-BioNTech vaccine: 1.3 per 100,000 and 1.5 per 100,000 in women and men, respectively.
Sex hormones may be key
The predominance of vaccine-associated myocarditis among adolescent and young adult males is probably more about the myocarditis itself than the vaccines, observed Biykem Bozkurt, MD, PhD, who has been studying COVID-related myocarditis at Baylor College of Medicine, Houston.
Male sex historically is associated in both epidemiologic studies and experimental models with a greater propensity for most any form of myocarditis, Dr. Bozkurt said in an interview. Given that males aged 16-19 or so appear to be at highest risk of myocarditis as a complication of SARS-CoV-2 vaccination, the mechanism may well be related to sex hormones.
“Therefore, testosterone is implicated as a player in their higher risk of inflammation and injury and lack of adaptive response in terms of healing, and in terms of prevention of injury,” Dr. Bozkurt said. For its part, estrogen inhibits proinflammatory processes and, in particular, “blunts cell-mediated immune responses.”
“We don’t know the mechanism, but a theory that attributes a protective role to estrogen, or a risk associated with testosterone, is reasonable. It makes sense, at least based on epidemiological data,” Dr. Ammirati agreed. Still, “we do not have any direct evidence in human beings.”
Sex-associated differences in experimental myocarditis have been reported in the journals for at least 70 years, but “the testosterone literature and the estrogen literature have not been evaluated in detail in vaccine-associated myocarditis,” Dr. Cooper said.
Most myocarditis in the laboratory is viral, Dr. Cooper observed, and “the links between testosterone, viruses, and inflammation have been pretty well worked out, I would say, if you’re a mouse. If you’re a human, I think it’s still a bit uncertain.”
Were it to apply in humans, greater testosterone levels might independently promote myocarditis, “and if estrogen is cardioprotective, it would be another mechanism,” Dr. Cooper said. “That would translate to slight male predominance in most kinds of myocarditis.”
In males, compared with females, “the heart can be more vulnerable to events such as arrhythmias or to immune-mediated phenomena. So, probably there is also higher vulnerability to myocarditis in men,” Dr. Ammirati noted.
Male predominance in vaccine-related myocarditis is provocative, so it’s worth considering whether testosterone is part of the mechanism as well as the possibility of estrogen cardioprotection, Dr. Ammirati said. But given limitations of the animal models, “we don’t really have robust data to support any part of that.”
Although myocarditis is in some way immune mediated, “and hormones can modulate the response,” the mechanism has to be more than just sex hormones, he said. “They probably cannot explain the specificity for the heart. It’s not a systemic response, it’s an organ-specific response.”
Modulation of immune responses
Details about the immune processes underlying mRNA-vaccine myocarditis, hormone modulated or not, have been elusive. The complication doesn’t resemble serum sickness, nor does it seem to be a reaction to infection by other cardiotropic viruses, such as coxsackie virus B, a cause of viral myocarditis, Dr. Bozkurt said. The latter had been a compelling possibility because such hypersensitivity to smallpox vaccination is well recognized.
“We don’t know the mechanism, that’s the short answer. But there are many hypotheses,” she said. One candidate widely proposed in the literature: autoantibodies driven by molecular mimicry between the SARS-CoV-2 spike protein targeted by the mRNA vaccines and a structurally similar myocardial protein, possibly alpha-myosin, noted Dr. Bozkurt and colleagues in a recent publication.
But elevations in specific “antiheart antibodies” have not been documented in recipients of the two mRNA-based vaccines, said Dr. Cooper. “So, I would say that – although molecular mimicry is a well-established mechanism of, for example, rheumatic carditis after a streptococcal A infection – that has not been demonstrated yet for COVID-19 mRNA vaccination–related myocarditis.”
“We probably won’t know, ever, with a huge level of certainty, the exact mechanisms,” Dr. Cooper added. There is no animal model for vaccine-induced myocarditis, and “We’re still talking very, very small numbers of patients. The vast majority of them recover,” and so don’t generally provide mechanistic clues.
Prospects for younger children
Vaccination against SARS-CoV-2 has now been authorized by the Centers for Disease Control and Prevention for kids as young as 5-11 years, using the Pfizer-BioNTech vaccine. Experience so far suggests the immunization is safe in that age group with negligible risk of myocarditis or other complications. But with prospects of possible authorization in children younger than 5, should myocarditis be a concern for them?
Probably not, if the complication is driven primarily by sex hormones, Dr. Cooper proposed. “One would predict that before puberty you would have a lower – much, much lower – rate of myocarditis in males than you would in the 16- to 19-year-old range, and that it would be roughly equal to females.” Dr. Ammirati and Dr. Bozkurt largely agreed.
It remains to be seen whether the vaccine-related myocarditis risk applies to children younger than 12, “but I doubt it. I think it’s going to be puberty-related,” Dr. Bozkurt said. Still, “I don’t want to hypothesize without data.”
A version of this article first appeared on Medscape.com.
The risk of myocarditis after immunization with mRNA-based vaccines against SARS-CoV-2 raised concerns when it came to light in early 2021. But as report after report showed such cases to be rare and usually mild and self-limited, focus has turned to the “how and why.”
The mechanism linking the BNT162b2 (Pfizer-BioNTech) and especially mRNA-1273 (Moderna) vaccines to the occurrence of myocarditis is unclear for now, but one potential driver may be tied to a peculiarity that became apparent early: It occurs overwhelmingly in younger males, from 16 to perhaps 40 or 50 years of age. Excess risk has not been consistently seen among women, girls, and older men.
That observation has led to speculation that higher testosterone levels in adolescent boys and young men may somehow promote the adverse vaccine effect, whereas greater levels of estrogen among girls and women in the same age range may be cardioprotective.
Unlikely, brief, and ‘benign’
“Most of the myocarditis is benign, by which I mean that maybe the patients are admitted due to chest pain, but without reduction in ventricular function,” Enrico Ammirati, MD, PhD, a myocarditis expert at De Gasperis Cardio Center and Transplant Center, Niguarda Hospital, Milan, said in an interview.
In a Nov. 14 address on this topic at the annual scientific sessions of the American Heart Association, Dror Mevorach, MD, described the typical case presentation as “mild” and one that clears in fairly short order based on resolution of “clinical symptoms, inflammatory markers and troponin decline, EKG normalization, echo normalization, and a relatively short length of hospital stay.”
Dr. Mevorach, of Hadassah Hebrew University Medical Center, Jerusalem, subsequently published the findings in a report in the New England Journal of Medicine that described 136 confirmed myocarditis cases among more than 5 million people in Israel immunized with the Pfizer-BioNTech vaccine. Myocarditis was considered “mild” in 129 cases, or 95%.
And the risk is tiny, compared with myocarditis from infection by SARS-CoV-2, not to mention the possibility of nasty clinical COVID-19 complications such as pneumonia and pulmonary embolism, Dr. Mevorach observed.
Many other reports agree that the incidence is minimal, especially given the rewards of vaccination. In a separate NEJM publication in September 2021 – from Noam Barda, MD, Clalit (Israel) Research Institute, and colleagues on 1.7 million people in that country, about half unvaccinated and half given the Pfizer-BioNTech vaccine – there were an estimated 2.7 cases of myocarditis per 100,000 vaccinated persons. There were also 11 cases of myocarditis per 100,000 persons who were positive for SARS-CoV-2 infection.
And in a recent case series of vaccinated people aged 16 or older, the myocarditis rate after a first or second Pfizer-BioNTech or Moderna injection was estimated at 1 or fewer per 100,000. The corresponding estimate was 4 such cases per 100,000 after a positive SARS-CoV-2 test among the same population, notes a report published Dec.14, 2021, in Nature Medicine.
In general, “the risk of any kind of cardiac injury is vastly lower with a vaccine than it is with the actual viral infection,” Leslie T. Cooper Jr., MD, a myocarditis expert and clinical trialist at the Mayo Clinic, Jacksonville, Fla., said in an interview. With the mRNA-based vaccines, “we do not have any conceivable danger signal that would outweigh the benefit of vaccination.”
Males of a certain age
Evidence that such myocarditis predominates in young adult men and adolescent boys, especially following a second vaccine dose, is remarkably consistent.
The risk was elevated only among mRNA-based vaccine recipients who were younger than 40 in the recent Nature Medicine analysis. Among that group, estimates after a second dose numbered fewer than 1 case per 100,000 for Pfizer-BioNTech and 1.5 per 100,000 for Moderna.
In a third analysis from Israel – also in NEJM, from Guy Witberg, MD, Rabin Medical Center, Petah Tikva, and colleagues, based on 2.5 million people aged 16 and older with at least one Pfizer-BioNTech injection – 2.1 cases per 100,000 were estimated overall, but the number rose to 10.7 per 100,000 among those aged 16-29 years.
In Dr. Mevorach’s NEJM report, estimates after a second Pfizer-BioNTech vaccine dose were 1 per 26,000 males versus 1 in 218,000 females, compared with 1 myocarditis case in 10,857 persons among “the general unvaccinated population.”
Most recipients of a first vaccine dose were younger than 50, and 16- to 29-year-olds accounted for most who completed two doses, noted Dr. Mevorach. Younger males bore the brunt of any myocarditis: the estimated prevalence after a second dose among males aged 16-19 was 1 per 6,637, compared with 1 per 99,853 females in the same age range, the group reported.
In the BMJ report, based on about 5 million people 12 years of age or older in Denmark, the estimated rates of myocarditis or pericarditis associated with Moderna immunization were 2 per 100,000 among women but 6.3 per 100,000 for men. The incidence and sex difference was much lower among those getting the Pfizer-BioNTech vaccine: 1.3 per 100,000 and 1.5 per 100,000 in women and men, respectively.
Sex hormones may be key
The predominance of vaccine-associated myocarditis among adolescent and young adult males is probably more about the myocarditis itself than the vaccines, observed Biykem Bozkurt, MD, PhD, who has been studying COVID-related myocarditis at Baylor College of Medicine, Houston.
Male sex historically is associated in both epidemiologic studies and experimental models with a greater propensity for most any form of myocarditis, Dr. Bozkurt said in an interview. Given that males aged 16-19 or so appear to be at highest risk of myocarditis as a complication of SARS-CoV-2 vaccination, the mechanism may well be related to sex hormones.
“Therefore, testosterone is implicated as a player in their higher risk of inflammation and injury and lack of adaptive response in terms of healing, and in terms of prevention of injury,” Dr. Bozkurt said. For its part, estrogen inhibits proinflammatory processes and, in particular, “blunts cell-mediated immune responses.”
“We don’t know the mechanism, but a theory that attributes a protective role to estrogen, or a risk associated with testosterone, is reasonable. It makes sense, at least based on epidemiological data,” Dr. Ammirati agreed. Still, “we do not have any direct evidence in human beings.”
Sex-associated differences in experimental myocarditis have been reported in the journals for at least 70 years, but “the testosterone literature and the estrogen literature have not been evaluated in detail in vaccine-associated myocarditis,” Dr. Cooper said.
Most myocarditis in the laboratory is viral, Dr. Cooper observed, and “the links between testosterone, viruses, and inflammation have been pretty well worked out, I would say, if you’re a mouse. If you’re a human, I think it’s still a bit uncertain.”
Were it to apply in humans, greater testosterone levels might independently promote myocarditis, “and if estrogen is cardioprotective, it would be another mechanism,” Dr. Cooper said. “That would translate to slight male predominance in most kinds of myocarditis.”
In males, compared with females, “the heart can be more vulnerable to events such as arrhythmias or to immune-mediated phenomena. So, probably there is also higher vulnerability to myocarditis in men,” Dr. Ammirati noted.
Male predominance in vaccine-related myocarditis is provocative, so it’s worth considering whether testosterone is part of the mechanism as well as the possibility of estrogen cardioprotection, Dr. Ammirati said. But given limitations of the animal models, “we don’t really have robust data to support any part of that.”
Although myocarditis is in some way immune mediated, “and hormones can modulate the response,” the mechanism has to be more than just sex hormones, he said. “They probably cannot explain the specificity for the heart. It’s not a systemic response, it’s an organ-specific response.”
Modulation of immune responses
Details about the immune processes underlying mRNA-vaccine myocarditis, hormone modulated or not, have been elusive. The complication doesn’t resemble serum sickness, nor does it seem to be a reaction to infection by other cardiotropic viruses, such as coxsackie virus B, a cause of viral myocarditis, Dr. Bozkurt said. The latter had been a compelling possibility because such hypersensitivity to smallpox vaccination is well recognized.
“We don’t know the mechanism, that’s the short answer. But there are many hypotheses,” she said. One candidate widely proposed in the literature: autoantibodies driven by molecular mimicry between the SARS-CoV-2 spike protein targeted by the mRNA vaccines and a structurally similar myocardial protein, possibly alpha-myosin, noted Dr. Bozkurt and colleagues in a recent publication.
But elevations in specific “antiheart antibodies” have not been documented in recipients of the two mRNA-based vaccines, said Dr. Cooper. “So, I would say that – although molecular mimicry is a well-established mechanism of, for example, rheumatic carditis after a streptococcal A infection – that has not been demonstrated yet for COVID-19 mRNA vaccination–related myocarditis.”
“We probably won’t know, ever, with a huge level of certainty, the exact mechanisms,” Dr. Cooper added. There is no animal model for vaccine-induced myocarditis, and “We’re still talking very, very small numbers of patients. The vast majority of them recover,” and so don’t generally provide mechanistic clues.
Prospects for younger children
Vaccination against SARS-CoV-2 has now been authorized by the Centers for Disease Control and Prevention for kids as young as 5-11 years, using the Pfizer-BioNTech vaccine. Experience so far suggests the immunization is safe in that age group with negligible risk of myocarditis or other complications. But with prospects of possible authorization in children younger than 5, should myocarditis be a concern for them?
Probably not, if the complication is driven primarily by sex hormones, Dr. Cooper proposed. “One would predict that before puberty you would have a lower – much, much lower – rate of myocarditis in males than you would in the 16- to 19-year-old range, and that it would be roughly equal to females.” Dr. Ammirati and Dr. Bozkurt largely agreed.
It remains to be seen whether the vaccine-related myocarditis risk applies to children younger than 12, “but I doubt it. I think it’s going to be puberty-related,” Dr. Bozkurt said. Still, “I don’t want to hypothesize without data.”
A version of this article first appeared on Medscape.com.
The risk of myocarditis after immunization with mRNA-based vaccines against SARS-CoV-2 raised concerns when it came to light in early 2021. But as report after report showed such cases to be rare and usually mild and self-limited, focus has turned to the “how and why.”
The mechanism linking the BNT162b2 (Pfizer-BioNTech) and especially mRNA-1273 (Moderna) vaccines to the occurrence of myocarditis is unclear for now, but one potential driver may be tied to a peculiarity that became apparent early: It occurs overwhelmingly in younger males, from 16 to perhaps 40 or 50 years of age. Excess risk has not been consistently seen among women, girls, and older men.
That observation has led to speculation that higher testosterone levels in adolescent boys and young men may somehow promote the adverse vaccine effect, whereas greater levels of estrogen among girls and women in the same age range may be cardioprotective.
Unlikely, brief, and ‘benign’
“Most of the myocarditis is benign, by which I mean that maybe the patients are admitted due to chest pain, but without reduction in ventricular function,” Enrico Ammirati, MD, PhD, a myocarditis expert at De Gasperis Cardio Center and Transplant Center, Niguarda Hospital, Milan, said in an interview.
In a Nov. 14 address on this topic at the annual scientific sessions of the American Heart Association, Dror Mevorach, MD, described the typical case presentation as “mild” and one that clears in fairly short order based on resolution of “clinical symptoms, inflammatory markers and troponin decline, EKG normalization, echo normalization, and a relatively short length of hospital stay.”
Dr. Mevorach, of Hadassah Hebrew University Medical Center, Jerusalem, subsequently published the findings in a report in the New England Journal of Medicine that described 136 confirmed myocarditis cases among more than 5 million people in Israel immunized with the Pfizer-BioNTech vaccine. Myocarditis was considered “mild” in 129 cases, or 95%.
And the risk is tiny, compared with myocarditis from infection by SARS-CoV-2, not to mention the possibility of nasty clinical COVID-19 complications such as pneumonia and pulmonary embolism, Dr. Mevorach observed.
Many other reports agree that the incidence is minimal, especially given the rewards of vaccination. In a separate NEJM publication in September 2021 – from Noam Barda, MD, Clalit (Israel) Research Institute, and colleagues on 1.7 million people in that country, about half unvaccinated and half given the Pfizer-BioNTech vaccine – there were an estimated 2.7 cases of myocarditis per 100,000 vaccinated persons. There were also 11 cases of myocarditis per 100,000 persons who were positive for SARS-CoV-2 infection.
And in a recent case series of vaccinated people aged 16 or older, the myocarditis rate after a first or second Pfizer-BioNTech or Moderna injection was estimated at 1 or fewer per 100,000. The corresponding estimate was 4 such cases per 100,000 after a positive SARS-CoV-2 test among the same population, notes a report published Dec.14, 2021, in Nature Medicine.
In general, “the risk of any kind of cardiac injury is vastly lower with a vaccine than it is with the actual viral infection,” Leslie T. Cooper Jr., MD, a myocarditis expert and clinical trialist at the Mayo Clinic, Jacksonville, Fla., said in an interview. With the mRNA-based vaccines, “we do not have any conceivable danger signal that would outweigh the benefit of vaccination.”
Males of a certain age
Evidence that such myocarditis predominates in young adult men and adolescent boys, especially following a second vaccine dose, is remarkably consistent.
The risk was elevated only among mRNA-based vaccine recipients who were younger than 40 in the recent Nature Medicine analysis. Among that group, estimates after a second dose numbered fewer than 1 case per 100,000 for Pfizer-BioNTech and 1.5 per 100,000 for Moderna.
In a third analysis from Israel – also in NEJM, from Guy Witberg, MD, Rabin Medical Center, Petah Tikva, and colleagues, based on 2.5 million people aged 16 and older with at least one Pfizer-BioNTech injection – 2.1 cases per 100,000 were estimated overall, but the number rose to 10.7 per 100,000 among those aged 16-29 years.
In Dr. Mevorach’s NEJM report, estimates after a second Pfizer-BioNTech vaccine dose were 1 per 26,000 males versus 1 in 218,000 females, compared with 1 myocarditis case in 10,857 persons among “the general unvaccinated population.”
Most recipients of a first vaccine dose were younger than 50, and 16- to 29-year-olds accounted for most who completed two doses, noted Dr. Mevorach. Younger males bore the brunt of any myocarditis: the estimated prevalence after a second dose among males aged 16-19 was 1 per 6,637, compared with 1 per 99,853 females in the same age range, the group reported.
In the BMJ report, based on about 5 million people 12 years of age or older in Denmark, the estimated rates of myocarditis or pericarditis associated with Moderna immunization were 2 per 100,000 among women but 6.3 per 100,000 for men. The incidence and sex difference was much lower among those getting the Pfizer-BioNTech vaccine: 1.3 per 100,000 and 1.5 per 100,000 in women and men, respectively.
Sex hormones may be key
The predominance of vaccine-associated myocarditis among adolescent and young adult males is probably more about the myocarditis itself than the vaccines, observed Biykem Bozkurt, MD, PhD, who has been studying COVID-related myocarditis at Baylor College of Medicine, Houston.
Male sex historically is associated in both epidemiologic studies and experimental models with a greater propensity for most any form of myocarditis, Dr. Bozkurt said in an interview. Given that males aged 16-19 or so appear to be at highest risk of myocarditis as a complication of SARS-CoV-2 vaccination, the mechanism may well be related to sex hormones.
“Therefore, testosterone is implicated as a player in their higher risk of inflammation and injury and lack of adaptive response in terms of healing, and in terms of prevention of injury,” Dr. Bozkurt said. For its part, estrogen inhibits proinflammatory processes and, in particular, “blunts cell-mediated immune responses.”
“We don’t know the mechanism, but a theory that attributes a protective role to estrogen, or a risk associated with testosterone, is reasonable. It makes sense, at least based on epidemiological data,” Dr. Ammirati agreed. Still, “we do not have any direct evidence in human beings.”
Sex-associated differences in experimental myocarditis have been reported in the journals for at least 70 years, but “the testosterone literature and the estrogen literature have not been evaluated in detail in vaccine-associated myocarditis,” Dr. Cooper said.
Most myocarditis in the laboratory is viral, Dr. Cooper observed, and “the links between testosterone, viruses, and inflammation have been pretty well worked out, I would say, if you’re a mouse. If you’re a human, I think it’s still a bit uncertain.”
Were it to apply in humans, greater testosterone levels might independently promote myocarditis, “and if estrogen is cardioprotective, it would be another mechanism,” Dr. Cooper said. “That would translate to slight male predominance in most kinds of myocarditis.”
In males, compared with females, “the heart can be more vulnerable to events such as arrhythmias or to immune-mediated phenomena. So, probably there is also higher vulnerability to myocarditis in men,” Dr. Ammirati noted.
Male predominance in vaccine-related myocarditis is provocative, so it’s worth considering whether testosterone is part of the mechanism as well as the possibility of estrogen cardioprotection, Dr. Ammirati said. But given limitations of the animal models, “we don’t really have robust data to support any part of that.”
Although myocarditis is in some way immune mediated, “and hormones can modulate the response,” the mechanism has to be more than just sex hormones, he said. “They probably cannot explain the specificity for the heart. It’s not a systemic response, it’s an organ-specific response.”
Modulation of immune responses
Details about the immune processes underlying mRNA-vaccine myocarditis, hormone modulated or not, have been elusive. The complication doesn’t resemble serum sickness, nor does it seem to be a reaction to infection by other cardiotropic viruses, such as coxsackie virus B, a cause of viral myocarditis, Dr. Bozkurt said. The latter had been a compelling possibility because such hypersensitivity to smallpox vaccination is well recognized.
“We don’t know the mechanism, that’s the short answer. But there are many hypotheses,” she said. One candidate widely proposed in the literature: autoantibodies driven by molecular mimicry between the SARS-CoV-2 spike protein targeted by the mRNA vaccines and a structurally similar myocardial protein, possibly alpha-myosin, noted Dr. Bozkurt and colleagues in a recent publication.
But elevations in specific “antiheart antibodies” have not been documented in recipients of the two mRNA-based vaccines, said Dr. Cooper. “So, I would say that – although molecular mimicry is a well-established mechanism of, for example, rheumatic carditis after a streptococcal A infection – that has not been demonstrated yet for COVID-19 mRNA vaccination–related myocarditis.”
“We probably won’t know, ever, with a huge level of certainty, the exact mechanisms,” Dr. Cooper added. There is no animal model for vaccine-induced myocarditis, and “We’re still talking very, very small numbers of patients. The vast majority of them recover,” and so don’t generally provide mechanistic clues.
Prospects for younger children
Vaccination against SARS-CoV-2 has now been authorized by the Centers for Disease Control and Prevention for kids as young as 5-11 years, using the Pfizer-BioNTech vaccine. Experience so far suggests the immunization is safe in that age group with negligible risk of myocarditis or other complications. But with prospects of possible authorization in children younger than 5, should myocarditis be a concern for them?
Probably not, if the complication is driven primarily by sex hormones, Dr. Cooper proposed. “One would predict that before puberty you would have a lower – much, much lower – rate of myocarditis in males than you would in the 16- to 19-year-old range, and that it would be roughly equal to females.” Dr. Ammirati and Dr. Bozkurt largely agreed.
It remains to be seen whether the vaccine-related myocarditis risk applies to children younger than 12, “but I doubt it. I think it’s going to be puberty-related,” Dr. Bozkurt said. Still, “I don’t want to hypothesize without data.”
A version of this article first appeared on Medscape.com.
COVID-19 linked to increased diabetes risk in youth
SARS-CoV-2 infection was associated with an increased risk for diabetes among youth, whereas other acute respiratory infections were not, new data from the U.S. Centers for Disease Control and Prevention indicate.
The results from two large U.S. health claims databases were published in an early release in the CDC’s Morbidity and Mortality Weekly Report by Catherine E. Barrett, PhD, and colleagues of the CDC’s COVID-19 Emergency Response Team and Division of Diabetes Translation.
Clinicians should monitor individuals younger than 18 years in the months following a SARS-CoV-2 infection for new diabetes onset, they advise.
The findings, which are supported by independent studies in adults, “underscore the importance of COVID-19 prevention among all age groups, including vaccination for all eligible children and adolescents, and chronic disease prevention and treatment,” Dr. Barrett and colleagues say.
Diabetes type couldn’t be reliably distinguished from the databases, which is noted as an important study limitation.
“SARS-CoV-2 infection might lead to type 1 or type 2 diabetes through complex and differing mechanisms,” they say.
Emerging evidence began to suggest, in mid-2020, that COVID-19 may trigger the onset of diabetes in healthy people. A new global registry was subsequently established to collect data on patients with COVID-19–related diabetes, called the CoviDiab registry.
Not clear if diabetes after COVID-19 is transient or permanent
From one of the databases used in the new study, known as IQVIA, 80,893 individuals aged younger than 18 years diagnosed with COVID-19 during March 2020 to February 26, 2021, were compared with age- and sex-matched people during that period who did not have COVID-19 and to prepandemic groups with and without a diagnosis of acute respiratory illness during March 1, 2017, to February 26, 2018.
From the second database, HealthVerity, 439,439 youth diagnosed with COVID-19 during March 1, 2020, to June 28, 2021, were compared with age- and sex-matched youth without COVID-19. Here, there was no prepandemic comparison group.
Diabetes diagnoses were coded in 0.08% with COVID-19 vs. 0.03% without COVID-19 in IQVIA and in 0.25% vs. 0.19% in HealthVerity.
Thus, new diabetes diagnoses were 166% and 31% more likely to occur in those with COVID-19 in IQVIA and HealthVerity, respectively. And in IQVIA, those with COVID-19 were 116% more likely to develop diabetes than were those with prepandemic acute respiratory illnesses. Those differences were all significant, whereas non–SARS-CoV-2 respiratory infections were not associated with diabetes, Dr. Barrett and colleagues say.
In both databases, diabetic ketoacidosis (DKA) was more common at diabetes onset among those with, vs. without, COVID-19: 48.5% vs. 13.6% in IQVIA and 40.2% vs. 29.7% in HealthVerity. In IQVIA, 22.0% with prepandemic acute respiratory illness presented with DKA.
Dr. Barrett and colleagues offer several potential explanations for the observed association between COVID-19 and diabetes, including a direct attack on pancreatic beta cells expressing angiotensin-converting enzyme 2 receptors, or via stress hyperglycemia resulting from cytokine storm and alterations in glucose metabolism.
Another possibility is the precipitation to diabetes from prediabetes; the latter is a condition present in one in five U.S. adolescents.
Steroid treatment during hospitalization might have led to transient hyperglycemia, but only 1.5% to 2.2% of diabetes codes were for drug- or chemical-induced diabetes. The majority were for type 1 or 2.
Alternatively, pandemic-associated weight gain might have also contributed to risks for both severe COVID-19 and type 2 diabetes.
“Although this study can provide information on the risk for diabetes following SARS-CoV-2 infection, additional data are needed to understand underlying pathogenic mechanisms, either those caused by SARS-CoV-2 infection itself or resulting from treatments, and whether a COVID-19–associated diabetes diagnosis is transient or leads to a chronic condition,” Dr. Barrett and colleagues conclude.
A version of this article first appeared on Medscape.com.
SARS-CoV-2 infection was associated with an increased risk for diabetes among youth, whereas other acute respiratory infections were not, new data from the U.S. Centers for Disease Control and Prevention indicate.
The results from two large U.S. health claims databases were published in an early release in the CDC’s Morbidity and Mortality Weekly Report by Catherine E. Barrett, PhD, and colleagues of the CDC’s COVID-19 Emergency Response Team and Division of Diabetes Translation.
Clinicians should monitor individuals younger than 18 years in the months following a SARS-CoV-2 infection for new diabetes onset, they advise.
The findings, which are supported by independent studies in adults, “underscore the importance of COVID-19 prevention among all age groups, including vaccination for all eligible children and adolescents, and chronic disease prevention and treatment,” Dr. Barrett and colleagues say.
Diabetes type couldn’t be reliably distinguished from the databases, which is noted as an important study limitation.
“SARS-CoV-2 infection might lead to type 1 or type 2 diabetes through complex and differing mechanisms,” they say.
Emerging evidence began to suggest, in mid-2020, that COVID-19 may trigger the onset of diabetes in healthy people. A new global registry was subsequently established to collect data on patients with COVID-19–related diabetes, called the CoviDiab registry.
Not clear if diabetes after COVID-19 is transient or permanent
From one of the databases used in the new study, known as IQVIA, 80,893 individuals aged younger than 18 years diagnosed with COVID-19 during March 2020 to February 26, 2021, were compared with age- and sex-matched people during that period who did not have COVID-19 and to prepandemic groups with and without a diagnosis of acute respiratory illness during March 1, 2017, to February 26, 2018.
From the second database, HealthVerity, 439,439 youth diagnosed with COVID-19 during March 1, 2020, to June 28, 2021, were compared with age- and sex-matched youth without COVID-19. Here, there was no prepandemic comparison group.
Diabetes diagnoses were coded in 0.08% with COVID-19 vs. 0.03% without COVID-19 in IQVIA and in 0.25% vs. 0.19% in HealthVerity.
Thus, new diabetes diagnoses were 166% and 31% more likely to occur in those with COVID-19 in IQVIA and HealthVerity, respectively. And in IQVIA, those with COVID-19 were 116% more likely to develop diabetes than were those with prepandemic acute respiratory illnesses. Those differences were all significant, whereas non–SARS-CoV-2 respiratory infections were not associated with diabetes, Dr. Barrett and colleagues say.
In both databases, diabetic ketoacidosis (DKA) was more common at diabetes onset among those with, vs. without, COVID-19: 48.5% vs. 13.6% in IQVIA and 40.2% vs. 29.7% in HealthVerity. In IQVIA, 22.0% with prepandemic acute respiratory illness presented with DKA.
Dr. Barrett and colleagues offer several potential explanations for the observed association between COVID-19 and diabetes, including a direct attack on pancreatic beta cells expressing angiotensin-converting enzyme 2 receptors, or via stress hyperglycemia resulting from cytokine storm and alterations in glucose metabolism.
Another possibility is the precipitation to diabetes from prediabetes; the latter is a condition present in one in five U.S. adolescents.
Steroid treatment during hospitalization might have led to transient hyperglycemia, but only 1.5% to 2.2% of diabetes codes were for drug- or chemical-induced diabetes. The majority were for type 1 or 2.
Alternatively, pandemic-associated weight gain might have also contributed to risks for both severe COVID-19 and type 2 diabetes.
“Although this study can provide information on the risk for diabetes following SARS-CoV-2 infection, additional data are needed to understand underlying pathogenic mechanisms, either those caused by SARS-CoV-2 infection itself or resulting from treatments, and whether a COVID-19–associated diabetes diagnosis is transient or leads to a chronic condition,” Dr. Barrett and colleagues conclude.
A version of this article first appeared on Medscape.com.
SARS-CoV-2 infection was associated with an increased risk for diabetes among youth, whereas other acute respiratory infections were not, new data from the U.S. Centers for Disease Control and Prevention indicate.
The results from two large U.S. health claims databases were published in an early release in the CDC’s Morbidity and Mortality Weekly Report by Catherine E. Barrett, PhD, and colleagues of the CDC’s COVID-19 Emergency Response Team and Division of Diabetes Translation.
Clinicians should monitor individuals younger than 18 years in the months following a SARS-CoV-2 infection for new diabetes onset, they advise.
The findings, which are supported by independent studies in adults, “underscore the importance of COVID-19 prevention among all age groups, including vaccination for all eligible children and adolescents, and chronic disease prevention and treatment,” Dr. Barrett and colleagues say.
Diabetes type couldn’t be reliably distinguished from the databases, which is noted as an important study limitation.
“SARS-CoV-2 infection might lead to type 1 or type 2 diabetes through complex and differing mechanisms,” they say.
Emerging evidence began to suggest, in mid-2020, that COVID-19 may trigger the onset of diabetes in healthy people. A new global registry was subsequently established to collect data on patients with COVID-19–related diabetes, called the CoviDiab registry.
Not clear if diabetes after COVID-19 is transient or permanent
From one of the databases used in the new study, known as IQVIA, 80,893 individuals aged younger than 18 years diagnosed with COVID-19 during March 2020 to February 26, 2021, were compared with age- and sex-matched people during that period who did not have COVID-19 and to prepandemic groups with and without a diagnosis of acute respiratory illness during March 1, 2017, to February 26, 2018.
From the second database, HealthVerity, 439,439 youth diagnosed with COVID-19 during March 1, 2020, to June 28, 2021, were compared with age- and sex-matched youth without COVID-19. Here, there was no prepandemic comparison group.
Diabetes diagnoses were coded in 0.08% with COVID-19 vs. 0.03% without COVID-19 in IQVIA and in 0.25% vs. 0.19% in HealthVerity.
Thus, new diabetes diagnoses were 166% and 31% more likely to occur in those with COVID-19 in IQVIA and HealthVerity, respectively. And in IQVIA, those with COVID-19 were 116% more likely to develop diabetes than were those with prepandemic acute respiratory illnesses. Those differences were all significant, whereas non–SARS-CoV-2 respiratory infections were not associated with diabetes, Dr. Barrett and colleagues say.
In both databases, diabetic ketoacidosis (DKA) was more common at diabetes onset among those with, vs. without, COVID-19: 48.5% vs. 13.6% in IQVIA and 40.2% vs. 29.7% in HealthVerity. In IQVIA, 22.0% with prepandemic acute respiratory illness presented with DKA.
Dr. Barrett and colleagues offer several potential explanations for the observed association between COVID-19 and diabetes, including a direct attack on pancreatic beta cells expressing angiotensin-converting enzyme 2 receptors, or via stress hyperglycemia resulting from cytokine storm and alterations in glucose metabolism.
Another possibility is the precipitation to diabetes from prediabetes; the latter is a condition present in one in five U.S. adolescents.
Steroid treatment during hospitalization might have led to transient hyperglycemia, but only 1.5% to 2.2% of diabetes codes were for drug- or chemical-induced diabetes. The majority were for type 1 or 2.
Alternatively, pandemic-associated weight gain might have also contributed to risks for both severe COVID-19 and type 2 diabetes.
“Although this study can provide information on the risk for diabetes following SARS-CoV-2 infection, additional data are needed to understand underlying pathogenic mechanisms, either those caused by SARS-CoV-2 infection itself or resulting from treatments, and whether a COVID-19–associated diabetes diagnosis is transient or leads to a chronic condition,” Dr. Barrett and colleagues conclude.
A version of this article first appeared on Medscape.com.
FROM MMWR
Experimental plasma exchange shows promise for IPF flares in preliminary study
Acute flares of idiopathic pulmonary fibrosis have a mortality rate as high as 90% or more, depending on their severity. But an experimental regimen that includes autoantibody reduction was found to improve survival significantly, as well as oxygen levels and walk distances, according to a small preliminary study published in PLOS ONE.
“It’s a preliminary study, but it’s very exciting,” Amit Gaggar, MD, PhD, an endowed professor of medicine at the University of Alabama at Birmingham (UAB), said in an interview. “We don’t really have a treatment for acute exacerbations of pulmonary fibrosis, and the mortality is extremely high, so it’s really critical that we start thinking outside the box a little bit for therapeutics.” Dr. Gaggar isn’t affiliated with the study.
Study leader Steven R. Duncan, MD, also of UAB, acknowledged that the experimental therapy has its detractors. “There’s been a tremendous bias against the role of immunologic therapy in idiopathic fibrosis, although it seems to be lessening,” he said.
The preliminary study treated 24 patients who had acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF) with a 19-day regimen called triple-modality autoantibody reduction. The three contributing modalities are therapeutic plasma exchange (TPE), rituximab, and intravenous immunoglobulin treatments. The standard treatment for AE-IPF consists of antibiotics and corticosteroids.
Dr. Duncan led the only other study of autoantibody reduction for AE-IPF, published in PLOS ONE in 2015. The latest preliminary study is a precursor to a National Heart, Lung, and Blood Institute–funded phase 2 randomized clinical trial, called STRIVE-IPF, currently enrolling AE-IPF patients at six sites.
Overall survival rates at 1, 3, and 6 months were 67%, 63%, and 46%. The study couldn’t identify characteristics of survivors versus nonsurvivors, although the latter had a trend toward greater initial oxygen requirements. Among the 10 patients who needed less than 25 L/min supplemental O2, the survival rate was 57%. In patients who needed more than 25 L/min, the survival rate was 20% (P = .07). Only 1 of 5 patients who needed greater than 40 L/min survived a year (P = .36).
After the 19-day regimen, 15 patients, or 63%, had significant drops in supplemental O2 requirements, from an average of 15 L/min to 3 L/min (P = .0007). Thirteen (87%) of the patients who were taking an antifibrotic medication (either pirfenidone or nintedanib) at baseline needed less O2 and/or had increased walking distances, compared with five who weren’t prescribed either of the agents (P = .15), although 1-year survival didn’t vary significantly with antifibrotic use.
The mechanism of antibody reduction is to filter out B-cells, infiltrates of which are typically found in lungs of AE-IPF patients, Dr. Duncan said. The regimen involves nine TPEs over 15 days, two IV rituximab 1-gm treatments over that course, and IV Ig 0.5-gm/kg treatments daily on days 16 through 19.
“Plasma exchange rapidly gets rid of the antibodies,” Dr. Duncan said in an interview. “It’s the basis for a number of autoantibody-mediated diseases, such as myasthenia gravis.”
While the TPE removes the B-cells, they have a proclivity to re-emerge, hence the rituximab treatment, he said. IV Ig further inhibits B-cell activity. “The IV Ig probably works in large part by feedback inhibition of the B-cells that have survived the rituximab,” Dr. Duncan said.
He added that with the TPE and rituximab patients had “sometimes amazing response” but then would relapse. “Since we added IV Ig, we see far fewer relapses,” he said. “And interestingly, if they do relapse, we can salvage them by giving them this treatment again.”
The preliminary study doesn’t make clear what patients would benefit most from the triple-modality therapy, but it did provide some clues. “We found that patients who have higher levels of antibodies against epithelial cells tend to do the best, and patients who had less severe disease – that is, less disturbance of gas exchange requiring less O2 – tend to do better,” Dr. Duncan said. The STRIVE trial should serve to identify specific biomarkers, he said.
Dr. Gaggar, the UAB professor who’s not affiliated with the study, concurred that it’s “too early to tell” which patients would benefit. “Certainly, these patients that undergo exacerbations would be of high interest,” he said, “but the potential is there that the other chronic lung diseases that have exacerbations may also benefit from this kind of therapy.”
He noted that the preliminary study focused on one type of autoantibody generating from epithelial cells. “In many of these studies where we limit ourselves to a single autoantibody population, we might be at the tip of iceberg,” Dr. Gaggar said. “There might be autoantibodies generated from other cells in the lung or the body that might be also pathogenic. This is really powerful because this is a subgroup of autoantibodies, but they still had that kind of impact in this small study.”
The STRIVE study is scheduled for completion in September 2022.
Dr. Duncan disclosed relationships with Novartis and Tyr Pharma outside the study subject. Dr. Gaggar has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Acute flares of idiopathic pulmonary fibrosis have a mortality rate as high as 90% or more, depending on their severity. But an experimental regimen that includes autoantibody reduction was found to improve survival significantly, as well as oxygen levels and walk distances, according to a small preliminary study published in PLOS ONE.
“It’s a preliminary study, but it’s very exciting,” Amit Gaggar, MD, PhD, an endowed professor of medicine at the University of Alabama at Birmingham (UAB), said in an interview. “We don’t really have a treatment for acute exacerbations of pulmonary fibrosis, and the mortality is extremely high, so it’s really critical that we start thinking outside the box a little bit for therapeutics.” Dr. Gaggar isn’t affiliated with the study.
Study leader Steven R. Duncan, MD, also of UAB, acknowledged that the experimental therapy has its detractors. “There’s been a tremendous bias against the role of immunologic therapy in idiopathic fibrosis, although it seems to be lessening,” he said.
The preliminary study treated 24 patients who had acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF) with a 19-day regimen called triple-modality autoantibody reduction. The three contributing modalities are therapeutic plasma exchange (TPE), rituximab, and intravenous immunoglobulin treatments. The standard treatment for AE-IPF consists of antibiotics and corticosteroids.
Dr. Duncan led the only other study of autoantibody reduction for AE-IPF, published in PLOS ONE in 2015. The latest preliminary study is a precursor to a National Heart, Lung, and Blood Institute–funded phase 2 randomized clinical trial, called STRIVE-IPF, currently enrolling AE-IPF patients at six sites.
Overall survival rates at 1, 3, and 6 months were 67%, 63%, and 46%. The study couldn’t identify characteristics of survivors versus nonsurvivors, although the latter had a trend toward greater initial oxygen requirements. Among the 10 patients who needed less than 25 L/min supplemental O2, the survival rate was 57%. In patients who needed more than 25 L/min, the survival rate was 20% (P = .07). Only 1 of 5 patients who needed greater than 40 L/min survived a year (P = .36).
After the 19-day regimen, 15 patients, or 63%, had significant drops in supplemental O2 requirements, from an average of 15 L/min to 3 L/min (P = .0007). Thirteen (87%) of the patients who were taking an antifibrotic medication (either pirfenidone or nintedanib) at baseline needed less O2 and/or had increased walking distances, compared with five who weren’t prescribed either of the agents (P = .15), although 1-year survival didn’t vary significantly with antifibrotic use.
The mechanism of antibody reduction is to filter out B-cells, infiltrates of which are typically found in lungs of AE-IPF patients, Dr. Duncan said. The regimen involves nine TPEs over 15 days, two IV rituximab 1-gm treatments over that course, and IV Ig 0.5-gm/kg treatments daily on days 16 through 19.
“Plasma exchange rapidly gets rid of the antibodies,” Dr. Duncan said in an interview. “It’s the basis for a number of autoantibody-mediated diseases, such as myasthenia gravis.”
While the TPE removes the B-cells, they have a proclivity to re-emerge, hence the rituximab treatment, he said. IV Ig further inhibits B-cell activity. “The IV Ig probably works in large part by feedback inhibition of the B-cells that have survived the rituximab,” Dr. Duncan said.
He added that with the TPE and rituximab patients had “sometimes amazing response” but then would relapse. “Since we added IV Ig, we see far fewer relapses,” he said. “And interestingly, if they do relapse, we can salvage them by giving them this treatment again.”
The preliminary study doesn’t make clear what patients would benefit most from the triple-modality therapy, but it did provide some clues. “We found that patients who have higher levels of antibodies against epithelial cells tend to do the best, and patients who had less severe disease – that is, less disturbance of gas exchange requiring less O2 – tend to do better,” Dr. Duncan said. The STRIVE trial should serve to identify specific biomarkers, he said.
Dr. Gaggar, the UAB professor who’s not affiliated with the study, concurred that it’s “too early to tell” which patients would benefit. “Certainly, these patients that undergo exacerbations would be of high interest,” he said, “but the potential is there that the other chronic lung diseases that have exacerbations may also benefit from this kind of therapy.”
He noted that the preliminary study focused on one type of autoantibody generating from epithelial cells. “In many of these studies where we limit ourselves to a single autoantibody population, we might be at the tip of iceberg,” Dr. Gaggar said. “There might be autoantibodies generated from other cells in the lung or the body that might be also pathogenic. This is really powerful because this is a subgroup of autoantibodies, but they still had that kind of impact in this small study.”
The STRIVE study is scheduled for completion in September 2022.
Dr. Duncan disclosed relationships with Novartis and Tyr Pharma outside the study subject. Dr. Gaggar has no relevant disclosures.
A version of this article first appeared on Medscape.com.
Acute flares of idiopathic pulmonary fibrosis have a mortality rate as high as 90% or more, depending on their severity. But an experimental regimen that includes autoantibody reduction was found to improve survival significantly, as well as oxygen levels and walk distances, according to a small preliminary study published in PLOS ONE.
“It’s a preliminary study, but it’s very exciting,” Amit Gaggar, MD, PhD, an endowed professor of medicine at the University of Alabama at Birmingham (UAB), said in an interview. “We don’t really have a treatment for acute exacerbations of pulmonary fibrosis, and the mortality is extremely high, so it’s really critical that we start thinking outside the box a little bit for therapeutics.” Dr. Gaggar isn’t affiliated with the study.
Study leader Steven R. Duncan, MD, also of UAB, acknowledged that the experimental therapy has its detractors. “There’s been a tremendous bias against the role of immunologic therapy in idiopathic fibrosis, although it seems to be lessening,” he said.
The preliminary study treated 24 patients who had acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF) with a 19-day regimen called triple-modality autoantibody reduction. The three contributing modalities are therapeutic plasma exchange (TPE), rituximab, and intravenous immunoglobulin treatments. The standard treatment for AE-IPF consists of antibiotics and corticosteroids.
Dr. Duncan led the only other study of autoantibody reduction for AE-IPF, published in PLOS ONE in 2015. The latest preliminary study is a precursor to a National Heart, Lung, and Blood Institute–funded phase 2 randomized clinical trial, called STRIVE-IPF, currently enrolling AE-IPF patients at six sites.
Overall survival rates at 1, 3, and 6 months were 67%, 63%, and 46%. The study couldn’t identify characteristics of survivors versus nonsurvivors, although the latter had a trend toward greater initial oxygen requirements. Among the 10 patients who needed less than 25 L/min supplemental O2, the survival rate was 57%. In patients who needed more than 25 L/min, the survival rate was 20% (P = .07). Only 1 of 5 patients who needed greater than 40 L/min survived a year (P = .36).
After the 19-day regimen, 15 patients, or 63%, had significant drops in supplemental O2 requirements, from an average of 15 L/min to 3 L/min (P = .0007). Thirteen (87%) of the patients who were taking an antifibrotic medication (either pirfenidone or nintedanib) at baseline needed less O2 and/or had increased walking distances, compared with five who weren’t prescribed either of the agents (P = .15), although 1-year survival didn’t vary significantly with antifibrotic use.
The mechanism of antibody reduction is to filter out B-cells, infiltrates of which are typically found in lungs of AE-IPF patients, Dr. Duncan said. The regimen involves nine TPEs over 15 days, two IV rituximab 1-gm treatments over that course, and IV Ig 0.5-gm/kg treatments daily on days 16 through 19.
“Plasma exchange rapidly gets rid of the antibodies,” Dr. Duncan said in an interview. “It’s the basis for a number of autoantibody-mediated diseases, such as myasthenia gravis.”
While the TPE removes the B-cells, they have a proclivity to re-emerge, hence the rituximab treatment, he said. IV Ig further inhibits B-cell activity. “The IV Ig probably works in large part by feedback inhibition of the B-cells that have survived the rituximab,” Dr. Duncan said.
He added that with the TPE and rituximab patients had “sometimes amazing response” but then would relapse. “Since we added IV Ig, we see far fewer relapses,” he said. “And interestingly, if they do relapse, we can salvage them by giving them this treatment again.”
The preliminary study doesn’t make clear what patients would benefit most from the triple-modality therapy, but it did provide some clues. “We found that patients who have higher levels of antibodies against epithelial cells tend to do the best, and patients who had less severe disease – that is, less disturbance of gas exchange requiring less O2 – tend to do better,” Dr. Duncan said. The STRIVE trial should serve to identify specific biomarkers, he said.
Dr. Gaggar, the UAB professor who’s not affiliated with the study, concurred that it’s “too early to tell” which patients would benefit. “Certainly, these patients that undergo exacerbations would be of high interest,” he said, “but the potential is there that the other chronic lung diseases that have exacerbations may also benefit from this kind of therapy.”
He noted that the preliminary study focused on one type of autoantibody generating from epithelial cells. “In many of these studies where we limit ourselves to a single autoantibody population, we might be at the tip of iceberg,” Dr. Gaggar said. “There might be autoantibodies generated from other cells in the lung or the body that might be also pathogenic. This is really powerful because this is a subgroup of autoantibodies, but they still had that kind of impact in this small study.”
The STRIVE study is scheduled for completion in September 2022.
Dr. Duncan disclosed relationships with Novartis and Tyr Pharma outside the study subject. Dr. Gaggar has no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
Nicotine and Nicotine Replacement Therapy Use During Myocardial Perfusion Imaging
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
Chest pain is one of the most common concerns in patients presenting to the emergency department in the United States, accounting for approximately 7.6 million visits annually.1 Given the high mortality rate associated with acute coronary syndromes, prompt evaluation of chest pain is essential.2 Even in mild cases, recognition of newly onset or worsening coronary artery disease (CAD) is crucial to ensure that patients receive optimal medication therapy.
In symptomatic patients with risk factors for CAD, such as advanced age, hypertension, hyperlipidemia, obesity, and diabetes mellitus, myocardial perfusion imaging (MPI) is frequently used as a modality to assess the presence, location, and severity of ischemic or infarcted myocardium.2 MPI requires administration of a radiopharmaceutical before and after the patient undergoes a form of stress.2 This radiopharmaceutical is then detected in the myocardium with a nuclear camera, and images are obtained of the heart to assess myocardial blood flow.2
MPI can be performed using exercise-induced stress via a treadmill, or medication-induced stress (Table 1). In both strategies, healthy coronary arteries dilate to provide the myocardium with more blood flow to meet the increasing myocardial oxygen demand during this period of stress. While healthy vessels are able to dilate appropriately, coronary arteries with flow-limiting stenoses are unable to dilate to the same extent in response to stress.2 Because radioactive isotope uptake by the myocardium is directly related to arterial blood flow, MPI is able to demonstrate a mismatch in coronary blood flow between healthy and diseased coronary arteries indicated by differences in radioisotope uptake.2 The presence of such a mismatch, in conjunction with clinical history, potentially suggests the presence of CAD.
Prior to conducting MPI with a medication, certain substances should be avoided. For instance, methylxanthines, such as caffeine, aminophylline, and theophylline, antagonize adenosine receptors and can have major drug interactions with regadenoson, adenosine, and dipyridamole. Therefore, it is advised that these substances be stopped for at least 12 hours before testing.3 In some cases, other medications that can affect coronary blood flow, such as long-acting nitrates, β-blockers, and calcium channel blockers, are recommended to be avoided for 12 to 48 hours in order to obtain the most accurate depiction of underlying coronary disease.4
Because nicotine and nicotine replacement therapy (NRT) may have substantial effects on coronary circulation, a current area of controversy is whether these should be stopped prior to the use of a stress-inducing medication during MPI. To date, no formal drug interaction studies have been conducted between nicotine and regadenoson.5 Similarly, the ADVANCE MPI 2 Trial, which led to the US Food and Drug Administration approval of regadenoson, did not specify restrictions on the use of nicotine prior to stress testing in the protocol.6 However, as this trial was multicenter, investigators admit that individual study sites could have had their own restrictions on the use of nicotine prior to stress testing with regadenoson, but this information was not collected.6 The current review focuses on how the simultaneous use of nicotine or NRT during MPI with pharmacologic agents, such as regadenoson, may affect the accuracy of imaging results and the clinical impact of this interaction.
Nicotine Coronary Artery Effect
It is well documented that long-term cigarette smoking is a major risk factor for CAD.7 Compared with nonsmokers, cigarette smokers experience 2 times greater risk of morbidity and mortality from ischemic heart disease.7 There are several mechanisms by which nicotine induces damage to the myocardium (Figure). Nicotine has direct effects on both the sympathetic nervous system (SNS) and myocardial endothelium.8 Together, these factors result in reduced coronary blood flow, leading to less oxygen supply to meet an increased oxygen demand, resulting in myocardial ischemia.
Nicotine’s effect on coronary vasomotor tone occurs primarily through noradrenergic stimulation of α and β receptors associated with coronary vasoconstriction or vasodilation, respectively.9,10 These competing influences on coronary blood flow appear to manifest differently based on whether patients are at rest or in a stressed state. A study by Czerin and colleagues demonstrated that in healthy patients with relatively short smoking histories and in a healthy nonsmoker control group, coronary blood flow increased by 25% and 40%, respectively, with nicotine use at rest.9 However, when these patients were stressed with dipyramidole and while smoking during the examination, myocardial blood flow was reduced by 11% in the study group and 14% in the control group.9 This is likely because the patients studied had relatively healthy coronary arteries that were able to maximally dilate when stressed. In this scenario, nicotine’s dilatory effects are offset by nicotine’s α-receptor–mediated vasoconstriction effects.9 Of note, patients in the study group experienced a somewhat diminished increase in coronary blood flow at rest with nicotine use, suggesting that even a short smoking history may damage the myocardial endothelium, rendering it less responsive to nicotine’s vasodilatory effects.9
These principles similarly apply to patients with underlying moderate-to-severe cardiovascular disease (CVD). With nicotine use at rest, patients with significant CAD do not experience as dramatic of an increase in coronary blood flow, which typically decreases or remains the same despite increased myocardial work.10 This may be because patients with moderate-to-severe CAD often have flow-limiting stenoses and damaged endothelium that do not allow vessels to respond as efficiently to increased myocardial demand or to nicotine’s β-receptor–mediated vasodilatory effects.10,11 Moreover, when stressed, diseased coronary arteries are not able to further dilate and nicotine’s α-receptor–mediated vasoconstriction effects dominate.10,11
In a study by Quillen and colleagues of patients with moderate-to-severe CAD, the mean diameter of proximal coronary artery segments decreased by 5%, the distal coronary diameter decreased 8%, and the coronary vascular resistance increased by 21% while smoking at rest.12 The investigators did not analyze how parameters changed when these diseased coronary arteries were stressed using a medication during MPI. However, it can be predicted that coronary arteries would have constricted to a similar or greater degree than observed in Czerin and colleagues’ study, given that the underlying myocardium was diseased and more susceptible to nicotine’s vasoconstriction effects.9 Importantly, these studies have several limitations, most notably that they are older and have small sample sizes. Additionally, while statistically significant differences were found in the degree of changes in coronary circulation with nicotine use at rest and during stress, it is unclear whether this translates to a clinically significant and impactful finding.9-12
Nicotine Replacement Therapy and Stress Testing
Given the association between cigarette smoking and CAD, medical practitioners strongly encourage patients to quit smoking to reduce their risk of adverse cardiovascular outcomes. Various smoking cessation treatments are available for patients. Common, readily accessible forms of therapy include nicotine replacement products (Table 2).
Early studies of NRT in patients with underlying CVD found an increased risk of cardiovascular events, such as myocardial infarction, presumably due to the nicotine content of these products.13,14 However, the concentration of nicotine in NRT is substantially lower than that found in cigarettes and in some formulations, such as transdermal patches, nicotine is delivered over a prolonged period of time.15 For this reason, NRT is thought to be safe in patients with underlying CVD and stable ischemic heart disease. A recent systematic review and meta-analysis found that while NRT may be associated with tachycardia, it did not increase the risk of more serious cardiovascular adverse effects (AEs).16,17
Given the lower nicotine concentration in NRT products, the associated hemodynamic effect of nicotine also is thought to be less pronounced. In a study conducted by Tzivoni and colleagues in patients with CAD using transdermal nicotine patches, no differences in blood pressure, heart rate, ischemia, or arrhythmias were found from baseline to 2 weeks.18 These findings were further confirmed in a small study by Lucini and colleagues, which found that nicotine patches produced slight hemodynamic effects, but to a lesser extent than cigarette smoking.19 For the NRT gum formulation, while a small study found that 4 mg produced coronary vasoconstriction in patients with underlying CAD, a study by Nitenberg and Antony demonstrated that healthy and diseased coronary arteries did not significantly constrict while patients were using nicotine gum both before and after a cold pressor test, suggesting a lesser degree of coronary vasoconstriction than nicotine from cigarette smoking.20,21 Similar findings have been described with the nicotine intranasal spray in a study by Keeley and colleagues, which showed no additional AEs on myocardial demand or vasoconstriction when an intranasal nicotine spray was added to cigarette smoking.22 Importantly, a review of the transdermal and gum formulations found that these less pronounced hemodynamic effects were observed across different doses of NRT; however, further studies are needed to clarify the relationship between NRT dose and cardiovascular effects.23
Overall, NRT does not seem to activate the SNS to the same degree as nicotine obtained via cigarette smoking and likely does not increase the myocardial oxygen demand as much. Additionally, by containing a lower concentration of nicotine, NRT may not impair the myocardium’s ability to supply oxygen to coronary arteries to the same extent as nicotine from cigarette smoking. Therefore, the effects of NRT on MPI using a stress-inducing medication may not be as pronounced. However, due to study limitations, results should be interpreted cautiously.18-23
Conclusions
Because of the close relationship between cigarette smoking and CAD, many patients with underlying CVD are either current smokers or may be using NRT for smoking cessation. Therefore, the question of whether to refrain from nicotine use prior to MPI is clinically relevant. Currently, there is a lack of high-quality studies demonstrating the effects of nicotine and NRT on coronary perfusion. Because of this, the impact of nicotine and NRT use on the accuracy of MPI using stress-inducing medications remains uncertain. Nevertheless, given that nicotine and NRT may largely affect the accuracy of imaging results, several institutions have adopted protocols that prohibit patients from using these drugs on the day of nuclear stress testing.
There are currently no data specifying the number of hours to hold nicotine products prior to cardiac stress testing. It is generally recommended that other medications that affect coronary blood flow be held for 5 half-lives before conducting MPI.4 Following the same guidance for nicotine and NRT may present a reasonable approach to ensure accurate imaging results. Based on the discussed literature, patients should be instructed to refrain from cigarette smoking for at least 5 to 10 hours prior to MPI, given nicotine’s half-life of about 1 to 2 hours.24
The data for NRT are less clear. While use of NRT may not be an absolute contraindication to conducting MPI, it is important to consider that this may affect the accuracy of results. Given this uncertainty, it is likely ideal to hold NRT prior to MPI, based on the specific formulation of NRT and that product's half-life. Further robust studies are needed to analyze the impact of nicotine and NRT on the accuracy of nuclear stress testing using a medication.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.
1. Rui P, Kang K, Ashman JJ. National Hospital Ambulatory Medical Care Survey: 2016 emergency department summary tables. Published 2016. Accessed March 30, 2020. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2016_ed_web_tables.pdf
2. Lange RA. Cardiovascular testing. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10th ed. McGraw Hill; 2017.
3. Mace S. Observation Medicine: Principles and Protocols. Cambridge University Press; 2017.
4. Currie GM. Pharmacology, part 4: nuclear cardiology. J Nucl Med Technol. 2019;47(2):97-110. doi:10.2967/jnmt.118.219675
5. Regadenoson; Package insert. Astellas Pharma US Inc; 2008.
6. Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645-658. doi:10.1016/j.nuclcard.2007.06.114
7. Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18(3):109-114. doi:10.4103/HEARTVIEWS.HEARTVIEWS_106_17
8. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515-523. doi:10.1016/j.tcm.2016.03.001
9. Czernin J, Sun K, Brunken R, Böttcher M, Phelps M, Schelbert H. Effect of acute and long-term smoking on myocardial blood flow and flow reserve. Circulation. 1995;91:2891-2897. doi:10.1161/01.CIR.91.12.2891
10. Winniford MD, Wheelan KR, Kremers MS, et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662-667. doi:10.1161/01.cir.73.4.662
11. Klein LW, Ambrose J, Pichard A, Holt J, Gorlin R, Teichholz LE. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J Am Coll Cardiol. 1984;3(4):879-886. doi:10.1016/s0735-1097(84)80344-7
12. Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22(3):642-647. doi:10.1016/0735-1097(93)90170-6
13. Dacosta A, Guy JM, Tardy B, et al. Myocardial infarction and nicotine patch: a contributing or causative factor?. Eur Heart J. 1993;14(12):1709-1711. doi:10.1093/eurheartj/14.12.1709
14. Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107(6):1765-1766. doi:10.1378/chest.107.6.1765
15. Dollerup J, Vestbo J, Murray-Thomas T, et al. Cardiovascular risks in smokers treated with nicotine replacement therapy: a historical cohort study. Clin Epidemiol. 2017;9:231-243. Published 2017 Apr 26. doi:10.2147/CLEP.S127775
16. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation. A systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8(1):8. Published 2010 Jul 13. doi:10.1186/1617-9625-8-8
17. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28-41. doi:10.1161/CIRCULATIONAHA.113.003961
18. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12(3):239-244. doi:10.1023/a:1007757530765
19. Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol. 1998;31(5):714-720. doi:10.1097/00005344-199805000-00010
20. Kaijser L, Berglund B. Effect of nicotine on coronary blood-flow in man. Clin Physiol. 1985;5(6):541-552. doi:10.1111/j.1475-097x.1985.tb00767.x
21. Nitenberg A, Antony I. Effects of nicotine gum on coronary vasomotor responses during sympathetic stimulation in patients with coronary artery stenosis. J Cardiovasc Pharmacol. 1999;34(5):694-699. doi:10.1097/00005344-199911000-00011
22. Keeley EC, Pirwitz MJ, Landau C, et al. Intranasal nicotine spray does not augment the adverse effects of cigarette smoking on myocardial oxygen demand or coronary arterial dimensions. Am J Med. 1996;101(4):357-363. doi:10.1016/s0002-9343(96)00237-9
23. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29(7):1422-1431. doi:10.1016/s0735-1097(97)00079-x
24. Flowers L. Nicotine replacement therapy. Amer J Psych. 2017;11(6):4-7.
25. Adenosine; Package insert. Astellas Pharma US Inc; 1989.
26. Dipyridamole; Package insert. Boehringer Ingelheim Pharmaceuticals Inc; 2019.
27. Dobutamine; Package insert. Baxter Healthcare Corporation; 2012.