User login
Tofacitinib shows mortality benefit in patients with COVID-19 pneumonia
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
The Janus kinase inhibitor tofacitinib reduces the risk of both death and respiratory failure in hospitalized adults with COVID-19 pneumonia, a new Brazilian study has found.
“Whether the use of JAK inhibitors is superior or additive to other specific immunomodulatory therapies in patients hospitalized with COVID-19 remains to be determined,” Patrícia O. Guimarães, MD, PhD, of the Hospital Israelita Albert Einstein in São Paulo, and coauthors wrote. The study was published in the New England Journal of Medicine.
The results of previous trials that tested JAK inhibitors as therapies for COVID-19 have been mixed. The second iteration of the Adaptive COVID-19 Treatment Trial (ACTT-2) found that a combination treatment of baricitinib and the Food and Drug Administration–authorized remdesivir was superior to remdesivir alone, but ACTT-4 – which compared baricitinib plus remdesivir with dexamethasone plus remdesivir – was stopped for futility in April 2021.
To assess the efficacy and safety of tofacitinib as a potential treatment for COVID-19, the researchers launched a randomized, double-blind trial made up of 289 patients from 15 sites in Brazil. The Study of Tofacitinib in Hospitalized Patients with COVID-19 Pneumonia (STOP-COVID) split its participants into two groups: one (n = 144) received 10 mg of oral tofacitinib twice daily and the other (n = 145) received placebo. Treatment was to be administered for up to 14 days or until hospital discharge. The participants’ mean age was 56 years, and 34.9% were women.
Over 89% of participants received glucocorticoids during hospitalization, a significant increase, compared with ACTT-2’s 12%. Through 28 days, death or respiratory failure occurred in 18.1% of the tofacitinib group and in 29.0% of the placebo group (risk ratio, 0.63; 95% confidence interval, 0.41-0.97; P = .04). Death from any cause occurred in 2.8% of the tofacitinib group and 5.5% of the placebo group (hazard ratio, 0.49; 95% CI, 0.15-1.63). The median number of days that treatment was administered was 5 in the tofacitinib group and 6 in the placebo group, and the median duration of hospital and ICU stays were similar across groups.
On the eight-level National Institute of Allergy and Infectious Diseases ordinal scale of disease severity, the proportional odds of having a worse score with tofacitinib, compared with placebo, was 0.6 (95% CI, 0.36-1.00) at day 14 and 0.54 (95% CI, 0.27-1.06) at day 28. Adverse events occurred in 26.1% of the tofacitinib group and 22.5% of the placebo group, with serious adverse events occurring in 20 patients (14.1%) on tofacitinib and 17 patients (12%) on placebo. Patients on tofacitinib suffered from events like deep vein thrombosis, acute myocardial infarction, ventricular tachycardia, and myocarditis, each of which affected one person, while one placebo patient each suffered from hemorrhagic stroke and cardiogenic shock. The incidence of serious infection was 3.5% in the tofacitinib group and 4.2% in the placebo group.
Timing may be everything
“There is a lot of interest in repurposing a variety of disease-modifying antirheumatic drugs for the treatment of COVID-19, which includes JAK inhibitors,” Zachary S. Wallace, MD, of the rheumatology unit at Massachusetts General Hospital, Boston, said in an interview. “The ACTT-2 data was compelling; it did suggest perhaps a benefit associated with baricitinib for COVID. This study certainly is more compelling.”
“For many people, there is this hyperinflammatory response in COVID-19 that seems to drive a lot of the morbidity and mortality that we see,” he added. “I think we all hypothesize that some of our treatments may be beneficial there. The challenge that we face is figuring out when the best time is to administer these medicines, and whether they need to be administered as part of a cocktail of therapy.”
Along those lines, Dr. Wallace cited a recent study he coauthored in which rheumatoid arthritis patients who were on JAK inhibitors at baseline had worse COVID-19 severity. But he emphasized that, despite their differing findings, the two studies are not irreconcilable.
“What this might speak to is, the timing of your exposure may be really important,” he said. “At the time of your initial infection, you may need certain aspects of your immune system that a JAK inhibitor may interfere with. But when you initiate a JAK inhibitor, once that phase is complete and you’re in this hyperinflammatory phase, you may have more benefit to target and treat the intense inflammation that we observe in patients who have COVID.”
He also offered up another variable potentially in play: different JAK inhibitors having different targets among the JAK receptors. “It may be that targeting specific JAKs is more beneficial when it comes to treating the hyperinflammatory response of COVID-19.”
The trial was sponsored by Pfizer. Several authors acknowledged potential conflicts of interest, including receiving grants and personal fees from Pfizer and various other pharmaceutical companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Clean indoor air is vital for infection control
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Health workers already know that indoor air quality can be as important to human health as clean water and uncontaminated food. But before the COVID-19 pandemic, its importance in the prevention of respiratory illnesses outside of health circles was only whispered about.
Now, a team of nearly 40 scientists from 14 countries is calling for “a paradigm shift,” so that improvements in indoor air quality are viewed as essential to curb respiratory infections.
Most countries do not have indoor air-quality standards, the scientists point out in their recent report, and those that do often fall short in scope and enforcement.
“We expect everywhere in the world to have clean water flowing from our taps. In most parts of the developed world, it is happening and we take it completely for granted,” said lead investigator Lidia Morawska, PhD, of the International Laboratory for Air Quality and Health at the Queensland University of Technology in Brisbane, Australia.
But bacteria and viruses can circulate freely in the air, and “no one thinks about this, whatsoever, apart from health care facilities,” she said.
A first step is to recognize the risk posed by airborne pathogens, something not yet universally acknowledged. The investigators also want the World Health Organization to extend its guidelines to cover airborne pathogens, and for ventilation standards to include higher airflow and filtration rates.
Germany has been at the forefront of air-quality measures, Dr. Morawska said. Years ago, she observed a monitor showing the carbon dioxide level and relative humidity in the room where she was attending a meeting. The screen was accompanied by red, yellow, and green signals to communicate risk. Such indicators are also commonly displayed in German schools so teachers know when to open the windows or adjust the ventilation.
Monitors show carbon dioxide levels
But this is not yet being done in most other countries, Dr. Morawska said. Levels of carbon dioxide are one measure of indoor air quality, but they serve as a proxy for ventilation, she pointed out. Although the technology is available, sensors that can test a variety of components in a building in real time are not yet affordable.
Dr. Morawska envisions a future where the air quality numbers of the places people frequent are displayed so they know the risk for airborne transmission of respiratory illnesses. And people can begin to expect clean indoor air when they enter a business, office, or entertainment space and request changes when the air quality dips and improvement is needed, she said.
It is a daunting challenge to clean indoor air for several reasons. Air is not containable in the same way water is, which makes it difficult to trace contaminants. And infections transmitted through dirty water and food are usually evident immediately, whereas infections transmitted through airborne pathogens can take days to develop. Plus, the necessary infrastructure changes will be expensive.
However, the initial cost required to change the flow and quality of indoor air might be less than the cost of infections, the scientists pointed out. It is estimated that the global harm caused by COVID-19 alone costs $1 trillion each month.
“In the United States, the yearly cost – direct and indirect – of influenza has been calculated at $11.2 billion. For respiratory infections other than influenza, the yearly cost stood at $40 billion,” the team noted.
“If even half of this was caused by inhalation, we are still talking about massive costs,” said Dr. Morawska.
Bigger is not always better
It is tempting to see the solution as increased ventilation, said Ehsan Mousavi, PhD, assistant professor of construction science and management at Clemson (S.C.) University, who studies indoor air quality and ventilation in hospitals.
“We are ventilating the heck out of hospitals,” he said in an interview. But there is much debate about how much ventilation is the right amount. Too much and “you can blow pathogens into an open wound,” he explained. “Bigger is not always better.”
And there is still debate about the best mix of outside and recirculated air. An increase in the intake of outdoor air can refresh indoor air if it is clean, but that depends on where you live, he pointed out.
The mix used in most standard office buildings is 15% outside air and 85% recirculated air, Dr. Mousavi said. Boosting the percentage of outside air increases costs and energy use.
In fact, it can take five times more energy to ventilate hospital spaces than office spaces, he reported.
Engineers searching for clean-air solutions need to know what particulates are in the air and whether they are harmful to humans, but the sensors currently available can’t identify whether a virus is present in real time.
Samples have to be taken to a lab and, “by the time you know a virus was in the space, the moment is gone,” Dr. Mousavi explained.
More research is needed. “We need a reasonable answer that looks at the problem holistically, not just from the infectious disease perspective,” he said.
Hydrating indoor air
Research is making it clear that health care environments can play a significant role in patient recovery, according to Stephanie Taylor, MD. Dr. Taylor is president of Building4Health, which she founded to help businesses assess the quality of air in their buildings and find solutions. The company uses an algorithm to arrive at a health assessment score.
Air hydration is the most important aspect to target, she said.
Since the 1980s, research has shown that a relative humidity of 40%-60% is healthy for humans, she said. Currently, in an office building in a winter climate, the humidity level is more like 20%.
Canada is the first country to officially recommend the 40%-60% range for senior citizen centers and residential homes.
“Properly hydrated air supports our immune system and prevents skin problems and respiratory problems. It also inactivates many bacteria and viruses,” Dr. Taylor explained. Inhaling dry air compromises the ability of the body to restrict influenza virus infection, researchers showed in a 2019 study.
In the case of COVID-19, as virus particles attach to water molecules, they get bigger and heavier and eventually drop out of the breathing zone and onto surfaces where they can be wiped away, she explained.
But when the particles “are very small – like 5 microns in diameter – and you inhale them, they can lodge deep in the lungs,” she said.
In properly hydrated air, particles will be larger – about 10-20 microns when they attach to the water vapor – so they will get stuck in the nose or the back of the throat, where they can be washed away by mucous and not travel to the lungs.
“Indoor air metrics” can support our health or contribute to disease, “not just over time, but quickly, within minutes or hours,” she said.
No one expects the world’s building stock to suddenly upgrade to the ideal air quality. “But that doesn’t mean we shouldn’t move in that direction,” Dr. Taylor said. Changes can start small and gradually increase.
New research targets indoor air
Humidity is one of the key areas for current research, said Karl Rockne, PhD, director of the environmental engineering program at the National Science Foundation.
“When a virus comes out, it’s not just a naked virus, which is exceptionally small. It’s a virus encapsulated in liquid. And that’s why the humidity is so key. The degree of humidity can determine how fast the water evaporates from the particle,” he said in an interview.
In the wake of COVID-19, his institution is funding more cross-disciplinary research in biology, building science, architecture, and physics, he pointed out.
One such effort involved the development of a sensor that can capture live COVID-19 virus. This so-called “smoking gun,” which proved that the virus can spread through the air, took the combined expertise of professionals in medicine, engineering, and several other disciplines.
Currently, investigators are examining indoor air quality and water supplies in offices that have been left empty during the pandemic, and the effect they will have on human health. And others are looking at the way outside air quality affects indoor air quality, particularly where outdoor air quality is poor, such as in areas experiencing wildfires.
So will COVID-19 be the catalyst that finally drives changes to building design, regulation, and public expectations of air quality in the spaces where we spend close to 90% of our time?
“If not COVID, what else? It affected every country, every sector,” Dr. Morawska said. “There’s enough momentum now to do something about this. And enough realization there is a problem.”
A version of this article first appeared on Medscape.com.
Pediatric bronchiolitis: Less is more
A common cause of infant morbidity and hospitalization in developed countries, infant viral bronchiolitis, has long been bedeviled by treatment uncertainty beyond supportive care.
Rationales for most pharmacologic treatments continue to be debated, and clinical practice guidelines generally advise respiratory and hydration support, discouraging the use of chest radiography, albuterol, glucocorticoids, antibiotics, and epinephrine.
Despite evidence that the latter interventions are ineffective, they are still too often applied, according to two recent studies, one in Pediatrics, the other in JAMA Pediatrics.
“The pull of the therapeutic vacuum surrounding this disease has been noted in the pages of this journal for at least 50 years, with Wright and Beem writing in 1965 that ‘energies should not be frittered away by the annoyance of unnecessary or futile medications and procedures’ for the child with bronchiolitis,” said emergency physicians Matthew J. Lipshaw, MD, MS, of the Cincinnati Children’s Hospital Medical Center, and Todd A. Florin, MD, MSCE, of Ann and Robert H. Lurie Children’s Hospital of Chicago.
These remarks came in their editorial in Pediatrics wryly titled: “Don’t Just Do Something, Stand There” and published online to accompany a recent study of three network meta-analyses.
Led by Sarah A. Elliott, PhD, of the Alberta Research Centre for Health Evidence at the University of Alberta in Edmonton, this analysis amalgamated 150 randomized, controlled trials comparing a placebo or active comparator with any bronchodilator, glucocorticoid steroid, hypertonic saline solution, antibiotic, helium-oxygen therapy, or high-flow oxygen therapy. It then looked at the following outcomes in children aged 2 years and younger: hospital admission rate on day 1, hospital admission rate within 7 days, and total hospital length of stay.
Few treatments seemed more effective than nebulized placebo (0.9% saline) for short-term outcomes, the authors found. While nebulized epinephrine and nebulized hypertonic saline plus salbutamol appeared to reduce admission rates during the index ED presentation, and hypertonic saline, alone or in combination with epinephrine, seemed to reduce hospital stays, such treatment had no effect on admissions within 7 days of initial presentation. Furthermore, most benefits disappeared in higher-quality studies.
Concluding, albeit with weak evidence and low confidence, that some benefit might accrue with hypertonic saline with salbutamol to reduce admission rates on initial presentation to the ED, the authors called for well-designed studies on treatments in inpatients and outpatients.
According to Dr. Lipshaw, assistant professor of clinical pediatrics, the lack of benefit observed in superior studies limits the applicability of Dr. Elliott and colleagues’ results to immediate clinical practice. “These findings could be used, however, to target future high-quality studies toward the medications that they found might be useful,” he said in an interview.
For the present, other recent research augurs well for strategically reducing unnecessary care. In a paper published online in JAMA Pediatrics, Libby Haskell, MN, of the ED at Starship Children’s Hospital in Auckland, New Zealand, and associates reported on a cluster-randomized, controlled trial of targeted interventions.
Conducted in 2017 at 26 hospitals and with 3,727 babies in New Zealand and Australia, the study addressed drivers of non–evidence-based approaches with behavior-modifying approaches such as on-site clinical leads, stakeholder meetings, a train-the-trainer workshop, education, and audit and feedback.
The authors reported a 14.1% difference in rates of compliance during the first 24 hours of hospitalization favoring the intervention group for all five bronchiolitis guideline recommendations. The greatest change was seen in albuterol and chest radiography use, with other improvements in ED visits, inpatient consultations, and throughout hospitalization.
“These results provide clinicians and hospitals with clear implementation strategies to address unnecessary treatment of infants with bronchiolitis,” Dr. Haskell’s group wrote. Dr. Lipshaw agreed that multifaceted deimplementation packages including clinician and family education, audit and feedback, and clinical decision support have been successful. “Haskell et al. demonstrated that it is possible to successfully deimplement non–evidence-based practices for bronchiolitis with targeted inventions,” he said. “It would be wonderful to see their success replicated in the U.S.”
Why the slow adoption of guidelines?
The American Academy of Pediatrics issued bronchiolitis guidelines for babies to 23 months in 2014 and updated them in 2018. Why, then, has care in some centers been seemingly all over the map and counter to guidelines? “Both parents and clinicians are acting in what they believe to be the best interests of the child, and in the absence of high-value interventions, can feel the need to do something, even if that something is not supported by evidence,” Dr. Lipshaw said.
Furthermore, with children in obvious distress, breathing fast and with difficulty, and sometimes unable to eat or drink, “we feel like we should have some way to make them feel better quicker. Unfortunately, none of the medications we have tried seem to be useful for most children, and we are left with supportive care measures such as suctioning their noses, giving them oxygen if their oxygen is low, and giving them fluids if they are dehydrated.”
Other physicians agree that taking a less-is-more approach can be challenging and even counterintuitive. “To families, seeing their child’s doctor ‘doing less’ can be frustrating,” admitted Diana S. Lee, MD, assistant professor of pediatrics at Icahn School of Medicine at Mount Sinai, New York.
Beyond that, altering practice behavior will need more than guidelines, Dr. Lee said in an interview. “Haskell et al. showed targeted behavior-change interventions improved compliance with bronchiolitis guidelines, but such change requires motivation and resources, and the sustainability of this effect over time remains to be seen.”
At Dr. Lipshaw’s institution, treatment depends on the attending physician, “but we have an emergency department care algorithm, which does not recommend any inhaled medications or steroids in accordance with the 2014 AAP guidelines,” he said.
Similarly at Mount Sinai, practitioners strive to follow the AAP guidelines, although their implementation has not been immediate, Dr. Lee said. “This is a situation where we must make the effort to choose not to do more, given current evidence.”
But Michelle Dunn, MD, an attending physician in the division of general pediatrics at the Children’s Hospital of Philadelphia, said the American practice norm already tends more to the observance than the breach of the guidelines, noting that since 2014 quality improvement efforts have been made throughout the country. “At our institution, we have effectively reduced the use of albuterol in patients with bronchiolitis and we use evidence-based therapy as much as possible, which in the case of bronchiolitis generally involves supportive management alone,” she said in an interview.
Still, Dr. Dunn added, many patients receive unnecessary diagnostic testing and ineffective therapies, with some providers facing psychological barriers to doing less. “However, with more and more evidence to support this, hopefully, physicians will become more comfortable with this.”
To that end, Dr. Lipshaw’s editorial urges physicians to “curb the rampant use of therapies repeatedly revealed to be ineffective,” citing team engagement, clear practice guidelines, and information technology as key factors in deimplementation. In the meantime, his mantra remains: “Don’t just do something, stand there.”
The study by Dr. Elliot and colleagues was supported by the Canadian Institutes of Health Research Knowledge Synthesis grant program. One coauthor is supported by a University of Ottawa Tier I Research Chair in Pediatric Emergency Medicine. Another is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and the Stollery Science Laboratory. Dr. Lipshaw and Dr. Florin disclosed no financial relationships relevant to their commentary. Dr. Haskell and colleagues were supported, variously, by the National Health and Medical Research Council of New Zealand, the Center of Research Excellence for Pediatric Emergency Medicine, the Victorian Government’s Operational Infrastructure Support Program, Cure Kids New Zealand, the Royal Children’s Hospital Foundation, and the Starship Foundation. Dr. Lee and Dr. Dunn had no competing interests to disclose with regard to their comments.
A common cause of infant morbidity and hospitalization in developed countries, infant viral bronchiolitis, has long been bedeviled by treatment uncertainty beyond supportive care.
Rationales for most pharmacologic treatments continue to be debated, and clinical practice guidelines generally advise respiratory and hydration support, discouraging the use of chest radiography, albuterol, glucocorticoids, antibiotics, and epinephrine.
Despite evidence that the latter interventions are ineffective, they are still too often applied, according to two recent studies, one in Pediatrics, the other in JAMA Pediatrics.
“The pull of the therapeutic vacuum surrounding this disease has been noted in the pages of this journal for at least 50 years, with Wright and Beem writing in 1965 that ‘energies should not be frittered away by the annoyance of unnecessary or futile medications and procedures’ for the child with bronchiolitis,” said emergency physicians Matthew J. Lipshaw, MD, MS, of the Cincinnati Children’s Hospital Medical Center, and Todd A. Florin, MD, MSCE, of Ann and Robert H. Lurie Children’s Hospital of Chicago.
These remarks came in their editorial in Pediatrics wryly titled: “Don’t Just Do Something, Stand There” and published online to accompany a recent study of three network meta-analyses.
Led by Sarah A. Elliott, PhD, of the Alberta Research Centre for Health Evidence at the University of Alberta in Edmonton, this analysis amalgamated 150 randomized, controlled trials comparing a placebo or active comparator with any bronchodilator, glucocorticoid steroid, hypertonic saline solution, antibiotic, helium-oxygen therapy, or high-flow oxygen therapy. It then looked at the following outcomes in children aged 2 years and younger: hospital admission rate on day 1, hospital admission rate within 7 days, and total hospital length of stay.
Few treatments seemed more effective than nebulized placebo (0.9% saline) for short-term outcomes, the authors found. While nebulized epinephrine and nebulized hypertonic saline plus salbutamol appeared to reduce admission rates during the index ED presentation, and hypertonic saline, alone or in combination with epinephrine, seemed to reduce hospital stays, such treatment had no effect on admissions within 7 days of initial presentation. Furthermore, most benefits disappeared in higher-quality studies.
Concluding, albeit with weak evidence and low confidence, that some benefit might accrue with hypertonic saline with salbutamol to reduce admission rates on initial presentation to the ED, the authors called for well-designed studies on treatments in inpatients and outpatients.
According to Dr. Lipshaw, assistant professor of clinical pediatrics, the lack of benefit observed in superior studies limits the applicability of Dr. Elliott and colleagues’ results to immediate clinical practice. “These findings could be used, however, to target future high-quality studies toward the medications that they found might be useful,” he said in an interview.
For the present, other recent research augurs well for strategically reducing unnecessary care. In a paper published online in JAMA Pediatrics, Libby Haskell, MN, of the ED at Starship Children’s Hospital in Auckland, New Zealand, and associates reported on a cluster-randomized, controlled trial of targeted interventions.
Conducted in 2017 at 26 hospitals and with 3,727 babies in New Zealand and Australia, the study addressed drivers of non–evidence-based approaches with behavior-modifying approaches such as on-site clinical leads, stakeholder meetings, a train-the-trainer workshop, education, and audit and feedback.
The authors reported a 14.1% difference in rates of compliance during the first 24 hours of hospitalization favoring the intervention group for all five bronchiolitis guideline recommendations. The greatest change was seen in albuterol and chest radiography use, with other improvements in ED visits, inpatient consultations, and throughout hospitalization.
“These results provide clinicians and hospitals with clear implementation strategies to address unnecessary treatment of infants with bronchiolitis,” Dr. Haskell’s group wrote. Dr. Lipshaw agreed that multifaceted deimplementation packages including clinician and family education, audit and feedback, and clinical decision support have been successful. “Haskell et al. demonstrated that it is possible to successfully deimplement non–evidence-based practices for bronchiolitis with targeted inventions,” he said. “It would be wonderful to see their success replicated in the U.S.”
Why the slow adoption of guidelines?
The American Academy of Pediatrics issued bronchiolitis guidelines for babies to 23 months in 2014 and updated them in 2018. Why, then, has care in some centers been seemingly all over the map and counter to guidelines? “Both parents and clinicians are acting in what they believe to be the best interests of the child, and in the absence of high-value interventions, can feel the need to do something, even if that something is not supported by evidence,” Dr. Lipshaw said.
Furthermore, with children in obvious distress, breathing fast and with difficulty, and sometimes unable to eat or drink, “we feel like we should have some way to make them feel better quicker. Unfortunately, none of the medications we have tried seem to be useful for most children, and we are left with supportive care measures such as suctioning their noses, giving them oxygen if their oxygen is low, and giving them fluids if they are dehydrated.”
Other physicians agree that taking a less-is-more approach can be challenging and even counterintuitive. “To families, seeing their child’s doctor ‘doing less’ can be frustrating,” admitted Diana S. Lee, MD, assistant professor of pediatrics at Icahn School of Medicine at Mount Sinai, New York.
Beyond that, altering practice behavior will need more than guidelines, Dr. Lee said in an interview. “Haskell et al. showed targeted behavior-change interventions improved compliance with bronchiolitis guidelines, but such change requires motivation and resources, and the sustainability of this effect over time remains to be seen.”
At Dr. Lipshaw’s institution, treatment depends on the attending physician, “but we have an emergency department care algorithm, which does not recommend any inhaled medications or steroids in accordance with the 2014 AAP guidelines,” he said.
Similarly at Mount Sinai, practitioners strive to follow the AAP guidelines, although their implementation has not been immediate, Dr. Lee said. “This is a situation where we must make the effort to choose not to do more, given current evidence.”
But Michelle Dunn, MD, an attending physician in the division of general pediatrics at the Children’s Hospital of Philadelphia, said the American practice norm already tends more to the observance than the breach of the guidelines, noting that since 2014 quality improvement efforts have been made throughout the country. “At our institution, we have effectively reduced the use of albuterol in patients with bronchiolitis and we use evidence-based therapy as much as possible, which in the case of bronchiolitis generally involves supportive management alone,” she said in an interview.
Still, Dr. Dunn added, many patients receive unnecessary diagnostic testing and ineffective therapies, with some providers facing psychological barriers to doing less. “However, with more and more evidence to support this, hopefully, physicians will become more comfortable with this.”
To that end, Dr. Lipshaw’s editorial urges physicians to “curb the rampant use of therapies repeatedly revealed to be ineffective,” citing team engagement, clear practice guidelines, and information technology as key factors in deimplementation. In the meantime, his mantra remains: “Don’t just do something, stand there.”
The study by Dr. Elliot and colleagues was supported by the Canadian Institutes of Health Research Knowledge Synthesis grant program. One coauthor is supported by a University of Ottawa Tier I Research Chair in Pediatric Emergency Medicine. Another is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and the Stollery Science Laboratory. Dr. Lipshaw and Dr. Florin disclosed no financial relationships relevant to their commentary. Dr. Haskell and colleagues were supported, variously, by the National Health and Medical Research Council of New Zealand, the Center of Research Excellence for Pediatric Emergency Medicine, the Victorian Government’s Operational Infrastructure Support Program, Cure Kids New Zealand, the Royal Children’s Hospital Foundation, and the Starship Foundation. Dr. Lee and Dr. Dunn had no competing interests to disclose with regard to their comments.
A common cause of infant morbidity and hospitalization in developed countries, infant viral bronchiolitis, has long been bedeviled by treatment uncertainty beyond supportive care.
Rationales for most pharmacologic treatments continue to be debated, and clinical practice guidelines generally advise respiratory and hydration support, discouraging the use of chest radiography, albuterol, glucocorticoids, antibiotics, and epinephrine.
Despite evidence that the latter interventions are ineffective, they are still too often applied, according to two recent studies, one in Pediatrics, the other in JAMA Pediatrics.
“The pull of the therapeutic vacuum surrounding this disease has been noted in the pages of this journal for at least 50 years, with Wright and Beem writing in 1965 that ‘energies should not be frittered away by the annoyance of unnecessary or futile medications and procedures’ for the child with bronchiolitis,” said emergency physicians Matthew J. Lipshaw, MD, MS, of the Cincinnati Children’s Hospital Medical Center, and Todd A. Florin, MD, MSCE, of Ann and Robert H. Lurie Children’s Hospital of Chicago.
These remarks came in their editorial in Pediatrics wryly titled: “Don’t Just Do Something, Stand There” and published online to accompany a recent study of three network meta-analyses.
Led by Sarah A. Elliott, PhD, of the Alberta Research Centre for Health Evidence at the University of Alberta in Edmonton, this analysis amalgamated 150 randomized, controlled trials comparing a placebo or active comparator with any bronchodilator, glucocorticoid steroid, hypertonic saline solution, antibiotic, helium-oxygen therapy, or high-flow oxygen therapy. It then looked at the following outcomes in children aged 2 years and younger: hospital admission rate on day 1, hospital admission rate within 7 days, and total hospital length of stay.
Few treatments seemed more effective than nebulized placebo (0.9% saline) for short-term outcomes, the authors found. While nebulized epinephrine and nebulized hypertonic saline plus salbutamol appeared to reduce admission rates during the index ED presentation, and hypertonic saline, alone or in combination with epinephrine, seemed to reduce hospital stays, such treatment had no effect on admissions within 7 days of initial presentation. Furthermore, most benefits disappeared in higher-quality studies.
Concluding, albeit with weak evidence and low confidence, that some benefit might accrue with hypertonic saline with salbutamol to reduce admission rates on initial presentation to the ED, the authors called for well-designed studies on treatments in inpatients and outpatients.
According to Dr. Lipshaw, assistant professor of clinical pediatrics, the lack of benefit observed in superior studies limits the applicability of Dr. Elliott and colleagues’ results to immediate clinical practice. “These findings could be used, however, to target future high-quality studies toward the medications that they found might be useful,” he said in an interview.
For the present, other recent research augurs well for strategically reducing unnecessary care. In a paper published online in JAMA Pediatrics, Libby Haskell, MN, of the ED at Starship Children’s Hospital in Auckland, New Zealand, and associates reported on a cluster-randomized, controlled trial of targeted interventions.
Conducted in 2017 at 26 hospitals and with 3,727 babies in New Zealand and Australia, the study addressed drivers of non–evidence-based approaches with behavior-modifying approaches such as on-site clinical leads, stakeholder meetings, a train-the-trainer workshop, education, and audit and feedback.
The authors reported a 14.1% difference in rates of compliance during the first 24 hours of hospitalization favoring the intervention group for all five bronchiolitis guideline recommendations. The greatest change was seen in albuterol and chest radiography use, with other improvements in ED visits, inpatient consultations, and throughout hospitalization.
“These results provide clinicians and hospitals with clear implementation strategies to address unnecessary treatment of infants with bronchiolitis,” Dr. Haskell’s group wrote. Dr. Lipshaw agreed that multifaceted deimplementation packages including clinician and family education, audit and feedback, and clinical decision support have been successful. “Haskell et al. demonstrated that it is possible to successfully deimplement non–evidence-based practices for bronchiolitis with targeted inventions,” he said. “It would be wonderful to see their success replicated in the U.S.”
Why the slow adoption of guidelines?
The American Academy of Pediatrics issued bronchiolitis guidelines for babies to 23 months in 2014 and updated them in 2018. Why, then, has care in some centers been seemingly all over the map and counter to guidelines? “Both parents and clinicians are acting in what they believe to be the best interests of the child, and in the absence of high-value interventions, can feel the need to do something, even if that something is not supported by evidence,” Dr. Lipshaw said.
Furthermore, with children in obvious distress, breathing fast and with difficulty, and sometimes unable to eat or drink, “we feel like we should have some way to make them feel better quicker. Unfortunately, none of the medications we have tried seem to be useful for most children, and we are left with supportive care measures such as suctioning their noses, giving them oxygen if their oxygen is low, and giving them fluids if they are dehydrated.”
Other physicians agree that taking a less-is-more approach can be challenging and even counterintuitive. “To families, seeing their child’s doctor ‘doing less’ can be frustrating,” admitted Diana S. Lee, MD, assistant professor of pediatrics at Icahn School of Medicine at Mount Sinai, New York.
Beyond that, altering practice behavior will need more than guidelines, Dr. Lee said in an interview. “Haskell et al. showed targeted behavior-change interventions improved compliance with bronchiolitis guidelines, but such change requires motivation and resources, and the sustainability of this effect over time remains to be seen.”
At Dr. Lipshaw’s institution, treatment depends on the attending physician, “but we have an emergency department care algorithm, which does not recommend any inhaled medications or steroids in accordance with the 2014 AAP guidelines,” he said.
Similarly at Mount Sinai, practitioners strive to follow the AAP guidelines, although their implementation has not been immediate, Dr. Lee said. “This is a situation where we must make the effort to choose not to do more, given current evidence.”
But Michelle Dunn, MD, an attending physician in the division of general pediatrics at the Children’s Hospital of Philadelphia, said the American practice norm already tends more to the observance than the breach of the guidelines, noting that since 2014 quality improvement efforts have been made throughout the country. “At our institution, we have effectively reduced the use of albuterol in patients with bronchiolitis and we use evidence-based therapy as much as possible, which in the case of bronchiolitis generally involves supportive management alone,” she said in an interview.
Still, Dr. Dunn added, many patients receive unnecessary diagnostic testing and ineffective therapies, with some providers facing psychological barriers to doing less. “However, with more and more evidence to support this, hopefully, physicians will become more comfortable with this.”
To that end, Dr. Lipshaw’s editorial urges physicians to “curb the rampant use of therapies repeatedly revealed to be ineffective,” citing team engagement, clear practice guidelines, and information technology as key factors in deimplementation. In the meantime, his mantra remains: “Don’t just do something, stand there.”
The study by Dr. Elliot and colleagues was supported by the Canadian Institutes of Health Research Knowledge Synthesis grant program. One coauthor is supported by a University of Ottawa Tier I Research Chair in Pediatric Emergency Medicine. Another is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and the Stollery Science Laboratory. Dr. Lipshaw and Dr. Florin disclosed no financial relationships relevant to their commentary. Dr. Haskell and colleagues were supported, variously, by the National Health and Medical Research Council of New Zealand, the Center of Research Excellence for Pediatric Emergency Medicine, the Victorian Government’s Operational Infrastructure Support Program, Cure Kids New Zealand, the Royal Children’s Hospital Foundation, and the Starship Foundation. Dr. Lee and Dr. Dunn had no competing interests to disclose with regard to their comments.
Hospitalization not rare for children with COVID, study says
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Nearly a third of those had severe disease that required mechanical ventilation or admission to an intensive care unit, according to a new study published in JAMA Network Open on April 9.*
That means about 1 in 9 kids with COVID-19 in this cohort needed hospitalization, and about 1 in 28 had severe COVID-19.
“Although most children with COVID-19 experience mild illness, some children develop serious illness that leads to hospitalization, use of invasive mechanical ventilation, and death,” the researchers wrote.
The research team analyzed discharge data from 869 medical facilities in the Premier Healthcare Database Special COVID-19 Release. They looked for COVID-19 patients ages 18 and under who had an in-patient or emergency department visit between March and October 2020.
More than 20,700 children with COVID-19 had an in-patient or an emergency department visit, and 2,430 were hospitalized with COVID-19. Among those, 756 children had severe COVID-19 and were admitted to an intensive care unit or needed mechanical ventilation.
About 53% of the COVID-19 patients were girls, and about 54% were between ages 12-18. In addition, about 29% had at least one chronic condition.
Similar to COVID-19 studies in adults, Hispanic, Latino and Black patients were overrepresented. About 39% of the children were Hispanic or Latino, and 24% were Black. However, the researchers didn’t find an association between severe COVID-19 and race or ethnicity.
The likelihood of severe COVID-19 increased if the patient had at least one chronic condition, was male, or was between ages 2-11.
“Understanding factors associated with severe COVID-19 disease among children could help inform prevention and control strategies,” they added. “Reducing infection risk through community mitigation strategies is critical for protecting children from COVID-19 and preventing poor outcomes.”
As of April 8, more than 3.54 million U.S. children have tested positive for COVID-19, according to the latest report from the American Academy of Pediatrics and Children’s Hospital Association. Cases among children are increasing slightly, with about 73,000 new cases reported during the first week of April.
Children represent about 13.5% of the COVID-19 cases in the country, according to the report. Among the 24 states that provide data, children represented 1% to 3% of all COVID-19 hospitalizations, and less than 2% of all child COVID-19 cases resulted in hospitalization.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the two groups wrote.
“However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects,” they added.
A version of this article first appeared on WebMD.com.
*CORRECTION, 6/7/21 – This story has been corrected to clarify that the patient sample study reflects only those children who presented to an emergency department or received inpatient care for COVID-19 in a hospital network and were included in the Premier Healthcare Database Special COVID-19 Release. A previous version of the story incorrectly implied that 12% of all U.S. children with COVID-19 had required inpatient care.
Prospective data support delaying antibiotics for pediatric respiratory infections
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
For pediatric patients with respiratory tract infections (RTIs), immediately prescribing antibiotics may do more harm than good, based on prospective data from 436 children treated by primary care pediatricians in Spain.
In the largest trial of its kind to date, children who were immediately prescribed antibiotics showed no significant difference in symptom severity or duration from those who received a delayed prescription for antibiotics, or no prescription at all; yet those in the immediate-prescription group had a higher rate of gastrointestinal adverse events, reported lead author Gemma Mas-Dalmau, MD, of the Sant Pau Institute for Biomedical Research, Barcelona, and colleagues.
“Most RTIs are self-limiting, and antibiotics hardly alter the course of the condition, yet antibiotics are frequently prescribed for these conditions,” the investigators wrote in Pediatrics. “Antibiotic prescription for RTIs in children is especially considered to be inappropriately high.”
This clinical behavior is driven by several factors, according to Dr. Mas-Dalmau and colleagues, including limited diagnostics in primary care, pressure to meet parental expectations, and concern for possible complications if antibiotics are withheld or delayed.
In an accompanying editorial, Jeffrey S. Gerber, MD, PhD and Bonnie F. Offit, MD, of Children’s Hospital of Philadelphia, noted that “children in the United States receive more than one antibiotic prescription per year, driven largely by acute RTIs.”
Dr. Gerber and Dr. Offit noted that some RTIs are indeed caused by bacteria, and therefore benefit from antibiotics, but it’s “not always easy” to identify these cases.
“Primary care, urgent care, and emergency medicine clinicians have a hard job,” they wrote.
According to the Centers for Disease Control and Prevention, delayed prescription of antibiotics, in which a prescription is filled upon persistence or worsening of symptoms, can balance clinical caution and antibiotic stewardship.
“An example of this approach is acute otitis media, in which delayed prescribing has been shown to safely reduce antibiotic exposure,” wrote Dr. Gerber and Dr. Offit.
In a 2017 Cochrane systematic review of both adults and children with RTIs, antibiotic prescriptions, whether immediate, delayed, or not given at all, had no significant effect on most symptoms or complications. Although several randomized trials have evaluated delayed antibiotic prescriptions in children, Dr. Mas-Dalmau and colleagues described the current body of evidence as “scant.”
The present study built upon this knowledge base by prospectively following 436 children treated at 39 primary care centers in Spain from 2012 to 2016. Patients were between 2 and 14 years of age and presented for rhinosinusitis, pharyngitis, acute otitis media, or acute bronchitis. Inclusion in the study required the pediatrician to have “reasonable doubts about the need to prescribe an antibiotic.” Clinics with access to rapid streptococcal testing did not enroll patients with pharyngitis.
Patients were randomized in approximately equal groups to receive either immediate prescription of antibiotics, delayed prescription, or no prescription. In the delayed group, caregivers were advised to fill prescriptions if any of following three events occurred:
- No symptom improvement after a certain amount of days, depending on presenting complaint (acute otitis media, 4 days; pharyngitis, 7 days; acute rhinosinusitis, 15 days; acute bronchitis, 20 days).
- Temperature of at least 39° C after 24 hours, or at least 38° C but less than 39° C after 48 hours.
- Patient feeling “much worse.”
Primary outcomes were severity and duration of symptoms over 30 days, while secondary outcomes included antibiotic use over 30 days, additional unscheduled visits to primary care over 30 days, and parental satisfaction and beliefs regarding antibiotic efficacy.
In the final dataset, 148 patients received immediate antibiotic prescriptions, while 146 received delayed prescriptions, and 142 received no prescription. Rate of antibiotic use was highest in the immediate prescription group, at 96%, versus 25.3% in the delayed group and 12% among those who received no prescription upon first presentation (P < .001).
Although the mean duration of severe symptoms was longest in the delayed-prescription group, at 12.4 days, versus 10.9 days in the no-prescription group and 10.1 days in the immediate-prescription group, these differences were not statistically significant (P = .539). Median score for greatest severity of any symptom was also similar across groups. Secondary outcomes echoed this pattern, in which reconsultation rates and caregiver satisfaction were statistically similar regardless of treatment type.
In contrast, patients who received immediate antibiotic prescriptions had a significantly higher rate of gastrointestinal adverse events (8.8%) than those who received a delayed prescription (3.4%) or no prescription (2.8%; P = .037).
“Delayed antibiotic prescription is an efficacious and safe strategy for reducing inappropriate antibiotic treatment of uncomplicated RTIs in children when the doctor has reasonable doubts regarding the indication,” the investigators concluded. “[It] is therefore a useful tool for addressing the public health issue of bacterial resistance. However, no antibiotic prescription remains the recommended strategy when it is clear that antibiotics are not indicated, like in most cases of acute bronchitis.”
“These data are reassuring,” wrote Dr. Gerber and Dr. Offit; however, they went on to suggest that the data “might not substantially move the needle.”
“With rare exceptions, children with acute pharyngitis should first receive a group A streptococcal test,” they wrote. “If results are positive, all patients should get antibiotics; if results are negative, no one gets them. Acute bronchitis (whatever that is in children) is viral. Acute sinusitis with persistent symptoms (the most commonly diagnosed variety) already has a delayed option, and the current study ... was not powered for this outcome. We are left with acute otitis media, which dominated enrollment but already has an evidence-based guideline.”
Still, Dr. Gerber and Dr. Offit suggested that the findings should further encourage pediatricians to prescribe antibiotics judiciously, and when elected, to choose the shortest duration and narrowest spectrum possible.
In a joint comment, Rana El Feghaly, MD, MSCI, director of outpatient antibiotic stewardship at Children’s Mercy, Kansas City, and her colleague, Mary Anne Jackson, MD, noted that the findings are “in accordance” with the 2017 Cochrane review.
Dr. Feghaly and Dr. Jackson said that these new data provide greater support for conservative use of antibiotics, which is badly needed, considering approximately 50% of outpatient prescriptions are unnecessary or inappropriate .
Delayed antibiotic prescription is part of a multifaceted approach to the issue, they said, joining “communication skills training, antibiotic justification documentation, audit and feedback reporting with peer comparison, diagnostic stewardship, [and] the use of clinician education on practice-based guidelines.”
“Leveraging delayed antibiotic prescription may be an excellent way to combat antibiotic overuse in the outpatient setting, while avoiding provider and parental fear of the ‘no antibiotic’ approach,” Dr. Feghaly and Dr. Jackson said.
Karlyn Kinsella, MD, of Pediatric Associates of Cheshire, Conn., suggested that clinicians discuss these findings with parents who request antibiotics for “otitis, pharyngitis, bronchitis, or sinusitis.”
“We can cite this study that antibiotics have no effect on symptom duration or severity for these illnesses,” Dr. Kinsella said. “Of course, our clinical opinion in each case takes precedent.”
According to Dr. Kinsella, conversations with parents also need to cover reasonable expectations, as the study did, with clear time frames for each condition in which children should start to get better.
“I think this is really key in our anticipatory guidance so that patients know what to expect,” she said.
The study was funded by Instituto de Salud Carlos III, the European Union, and the Spanish Ministry of Health, Social Services, and Equality. The investigators and interviewees reported no conflicts of interest.
FROM PEDIATRICS
Arthritis drugs ‘impressive’ for severe COVID but not ‘magic cure’
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
COVID-19 symptoms persist months after acute infection
, according to a follow-up study involving 1,733 patients.
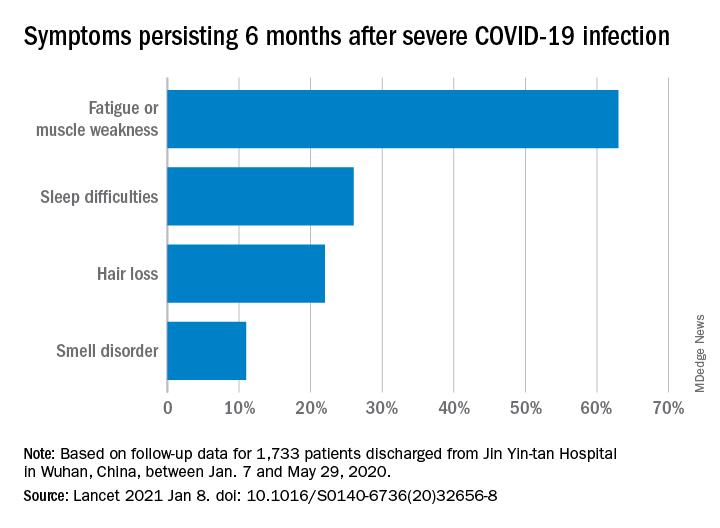
“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
, according to a follow-up study involving 1,733 patients.

“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
, according to a follow-up study involving 1,733 patients.

“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
FROM THE LANCET
Seeking new vaccines against whooping cough: The PERISCOPE project
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
FROM ESPID 2020
Metapneumovirus infections clinically indistinguishable from flu, RSV
The all-consuming news about SARS-CoV-2 and COVID-19 has overshadowed other viral pathogens that are the cause of severe or fatal lower respiratory infections (LRI) including human metapneumovirus (HMPV).
“MPV is really a leading cause of LRI not just in children but in adults, with high mortality rates in the frail elderly, long-term care facilities, and cancer patients with pneumonia, “ said John Williams, MD, from the department of pediatric infectious diseases at the University of Pittsburgh Medical Center.
“Right now we have no effective antivirals. There are monoclonal antibodies in development that my group and others have discovered. In fact, some of these treat MPV and RSV [respiratory syncytial virus], so we may have good options,” he said in an online presentation during an annual scientific meeting on infectious diseases.
The virus preys, wolf-like, on the most vulnerable patients, including children and frail elderly adults, as well as other adults with predisposing conditions, he said.
HMPV causes acute respiratory illnesses in approximately 2%-11% of hospitalized adults, 3%-25% of organ transplant recipients or cancer patients, 4%-12% of chronic obstructive pulmonary disease exacerbations, 5%-20% of asthma exacerbations, and it has been identified in multiple outbreaks at long-term care facilities.
Relative newcomer
Metapneumovirus was isolated and discovered from children with respiratory tract disease in the early 2000s. Once included in the family of paramyxoviruses (including measles, mumps, Nipah virus, and parainfluenza virus 1-4), HMPV and RSV are now classified as pneumoviruses, based on gene order and other characteristics, Dr. Williams explained.
Various studies have consistently placed the prevalence of HMPV ranging from 5%-14% in young children with LRI, children hospitalized for wheezing, adults with cancer and LRI, adults with asthma admissions, children with upper respiratory infections, and children hospitalized in the United States and Jordan for LRI, as well as children hospitalized in the United States and Peru with acute respiratory infections.
A study tracking respiratory infections in a Rochester, N.Y., cohort from 1999 through 2003 showed that healthy elderly patients had and annual incidence of HMPV infections of 5.9%, compared with 9.1% for high-risk patients, 13.1% for young patients, and 8.5% among hospitalized adult patients.
“These percentages are virtually identical to what has been seen in the same cohort for respiratory syncytial virus, so in this multiyear prospective cohort, metapneumovirus was as common as RSV,” Dr. Williams said.
Although the incidences of both HMPV and RSV were lower among hospitalized adults “clinically, we can’t tell these respiratory viruses apart. If we know it’s circulating we can make a guess, but we really can’t discriminate them,” he added.
In the Rochester cohort the frequency of clinical symptoms – including congestion, sore throat, cough, sputum production, dyspnea, and fever – were similar among patients infected with HMPV, RSV, or influenza A, with the exception of a slightly higher incidence of wheezing (80%) with HMPV, compared with influenza.
“I can tell you as a pediatrician, this is absolutely true in children, that metapneumovirus is indistinguishable from other respiratory viruses in kids,” he said.
Fatalities among older adults
As noted before, HMPV can cause severe and fatal illness in adults. For example, during an outbreak in North Dakota in 2016, 3 of 27 hospitalized adults with HMPV (median age, 69 years) died, and 10 required mechanical or noninvasive ventilation.
In a study from Korea comparing outcomes of severe HMPV-associated community-acquired pneumonia (CAP) with those of severe influenza-associated CAP, the investigators found that 30- and 60-day mortality rates were similar between the groups, at 24% of patients with HMPV-associated CAP and 32.1% for influenza-associated CAP, and 32% versus 38.5%, respectively.
Patients at high risk for severe disease or death from HMPV infection include those over 65 years, especially frail elderly, patients with chronic obstructive pulmonary disease, immunocompromised patients, and those with cardiopulmonary diseases such as congestive heart failure.
Supportive care only
“Do we have anything for treatment? The short answer is, No,” Dr. Williams.
Supportive care is currently the only effective approach for patients with severe HMPV infection.
Ribavirin, used to treat patients with acute RSV infection, has poor in vitro activity against HMPV and poor oral bioavailability and hemolysis, and there are no randomized controlled trials to support its use in this situation.
“It really can’t be recommended, and I don’t recommend it,” he said.
Virology may still help
Mark J. Siedner, MD, an infectious diseases physician at Mass General and associate professor of medicine at Harvard Medical School, both in Boston, who was not involved in the study, said that, despite the inability to clinically distinguish HMPV from RSV or influenza A, there is still clinical value to identifying HMPV infections.
“We spend millions of dollar each year treating people for upper respiratory tract infections, often with antibacterials, sometimes with antivirals, but those have costs to the health care system, and they also have costs in terms of drug resistance,” he said in an interview seeking objective commentary.
“Diagnostic tests that determine the actual source or the cause of these upper respiratory tract infections and encourage both patients and physicians not to be using antibiotics have value,” he said.
Identifying the pathogen can also help clinicians take appropriate infection-control precautions to prevent patient-to-clinician or patient-to-patient transmission of viral infections, he added.
Dr. Williams’ research is supported by the National Institutes of Health, Henry L. Hillman Foundation, and Asher Krop Memorial Fund of Children’s Hospital of Pittsburgh. Dr. Williams and Dr. Siedner reported no relevant conflict of interest disclosures.
The all-consuming news about SARS-CoV-2 and COVID-19 has overshadowed other viral pathogens that are the cause of severe or fatal lower respiratory infections (LRI) including human metapneumovirus (HMPV).
“MPV is really a leading cause of LRI not just in children but in adults, with high mortality rates in the frail elderly, long-term care facilities, and cancer patients with pneumonia, “ said John Williams, MD, from the department of pediatric infectious diseases at the University of Pittsburgh Medical Center.
“Right now we have no effective antivirals. There are monoclonal antibodies in development that my group and others have discovered. In fact, some of these treat MPV and RSV [respiratory syncytial virus], so we may have good options,” he said in an online presentation during an annual scientific meeting on infectious diseases.
The virus preys, wolf-like, on the most vulnerable patients, including children and frail elderly adults, as well as other adults with predisposing conditions, he said.
HMPV causes acute respiratory illnesses in approximately 2%-11% of hospitalized adults, 3%-25% of organ transplant recipients or cancer patients, 4%-12% of chronic obstructive pulmonary disease exacerbations, 5%-20% of asthma exacerbations, and it has been identified in multiple outbreaks at long-term care facilities.
Relative newcomer
Metapneumovirus was isolated and discovered from children with respiratory tract disease in the early 2000s. Once included in the family of paramyxoviruses (including measles, mumps, Nipah virus, and parainfluenza virus 1-4), HMPV and RSV are now classified as pneumoviruses, based on gene order and other characteristics, Dr. Williams explained.
Various studies have consistently placed the prevalence of HMPV ranging from 5%-14% in young children with LRI, children hospitalized for wheezing, adults with cancer and LRI, adults with asthma admissions, children with upper respiratory infections, and children hospitalized in the United States and Jordan for LRI, as well as children hospitalized in the United States and Peru with acute respiratory infections.
A study tracking respiratory infections in a Rochester, N.Y., cohort from 1999 through 2003 showed that healthy elderly patients had and annual incidence of HMPV infections of 5.9%, compared with 9.1% for high-risk patients, 13.1% for young patients, and 8.5% among hospitalized adult patients.
“These percentages are virtually identical to what has been seen in the same cohort for respiratory syncytial virus, so in this multiyear prospective cohort, metapneumovirus was as common as RSV,” Dr. Williams said.
Although the incidences of both HMPV and RSV were lower among hospitalized adults “clinically, we can’t tell these respiratory viruses apart. If we know it’s circulating we can make a guess, but we really can’t discriminate them,” he added.
In the Rochester cohort the frequency of clinical symptoms – including congestion, sore throat, cough, sputum production, dyspnea, and fever – were similar among patients infected with HMPV, RSV, or influenza A, with the exception of a slightly higher incidence of wheezing (80%) with HMPV, compared with influenza.
“I can tell you as a pediatrician, this is absolutely true in children, that metapneumovirus is indistinguishable from other respiratory viruses in kids,” he said.
Fatalities among older adults
As noted before, HMPV can cause severe and fatal illness in adults. For example, during an outbreak in North Dakota in 2016, 3 of 27 hospitalized adults with HMPV (median age, 69 years) died, and 10 required mechanical or noninvasive ventilation.
In a study from Korea comparing outcomes of severe HMPV-associated community-acquired pneumonia (CAP) with those of severe influenza-associated CAP, the investigators found that 30- and 60-day mortality rates were similar between the groups, at 24% of patients with HMPV-associated CAP and 32.1% for influenza-associated CAP, and 32% versus 38.5%, respectively.
Patients at high risk for severe disease or death from HMPV infection include those over 65 years, especially frail elderly, patients with chronic obstructive pulmonary disease, immunocompromised patients, and those with cardiopulmonary diseases such as congestive heart failure.
Supportive care only
“Do we have anything for treatment? The short answer is, No,” Dr. Williams.
Supportive care is currently the only effective approach for patients with severe HMPV infection.
Ribavirin, used to treat patients with acute RSV infection, has poor in vitro activity against HMPV and poor oral bioavailability and hemolysis, and there are no randomized controlled trials to support its use in this situation.
“It really can’t be recommended, and I don’t recommend it,” he said.
Virology may still help
Mark J. Siedner, MD, an infectious diseases physician at Mass General and associate professor of medicine at Harvard Medical School, both in Boston, who was not involved in the study, said that, despite the inability to clinically distinguish HMPV from RSV or influenza A, there is still clinical value to identifying HMPV infections.
“We spend millions of dollar each year treating people for upper respiratory tract infections, often with antibacterials, sometimes with antivirals, but those have costs to the health care system, and they also have costs in terms of drug resistance,” he said in an interview seeking objective commentary.
“Diagnostic tests that determine the actual source or the cause of these upper respiratory tract infections and encourage both patients and physicians not to be using antibiotics have value,” he said.
Identifying the pathogen can also help clinicians take appropriate infection-control precautions to prevent patient-to-clinician or patient-to-patient transmission of viral infections, he added.
Dr. Williams’ research is supported by the National Institutes of Health, Henry L. Hillman Foundation, and Asher Krop Memorial Fund of Children’s Hospital of Pittsburgh. Dr. Williams and Dr. Siedner reported no relevant conflict of interest disclosures.
The all-consuming news about SARS-CoV-2 and COVID-19 has overshadowed other viral pathogens that are the cause of severe or fatal lower respiratory infections (LRI) including human metapneumovirus (HMPV).
“MPV is really a leading cause of LRI not just in children but in adults, with high mortality rates in the frail elderly, long-term care facilities, and cancer patients with pneumonia, “ said John Williams, MD, from the department of pediatric infectious diseases at the University of Pittsburgh Medical Center.
“Right now we have no effective antivirals. There are monoclonal antibodies in development that my group and others have discovered. In fact, some of these treat MPV and RSV [respiratory syncytial virus], so we may have good options,” he said in an online presentation during an annual scientific meeting on infectious diseases.
The virus preys, wolf-like, on the most vulnerable patients, including children and frail elderly adults, as well as other adults with predisposing conditions, he said.
HMPV causes acute respiratory illnesses in approximately 2%-11% of hospitalized adults, 3%-25% of organ transplant recipients or cancer patients, 4%-12% of chronic obstructive pulmonary disease exacerbations, 5%-20% of asthma exacerbations, and it has been identified in multiple outbreaks at long-term care facilities.
Relative newcomer
Metapneumovirus was isolated and discovered from children with respiratory tract disease in the early 2000s. Once included in the family of paramyxoviruses (including measles, mumps, Nipah virus, and parainfluenza virus 1-4), HMPV and RSV are now classified as pneumoviruses, based on gene order and other characteristics, Dr. Williams explained.
Various studies have consistently placed the prevalence of HMPV ranging from 5%-14% in young children with LRI, children hospitalized for wheezing, adults with cancer and LRI, adults with asthma admissions, children with upper respiratory infections, and children hospitalized in the United States and Jordan for LRI, as well as children hospitalized in the United States and Peru with acute respiratory infections.
A study tracking respiratory infections in a Rochester, N.Y., cohort from 1999 through 2003 showed that healthy elderly patients had and annual incidence of HMPV infections of 5.9%, compared with 9.1% for high-risk patients, 13.1% for young patients, and 8.5% among hospitalized adult patients.
“These percentages are virtually identical to what has been seen in the same cohort for respiratory syncytial virus, so in this multiyear prospective cohort, metapneumovirus was as common as RSV,” Dr. Williams said.
Although the incidences of both HMPV and RSV were lower among hospitalized adults “clinically, we can’t tell these respiratory viruses apart. If we know it’s circulating we can make a guess, but we really can’t discriminate them,” he added.
In the Rochester cohort the frequency of clinical symptoms – including congestion, sore throat, cough, sputum production, dyspnea, and fever – were similar among patients infected with HMPV, RSV, or influenza A, with the exception of a slightly higher incidence of wheezing (80%) with HMPV, compared with influenza.
“I can tell you as a pediatrician, this is absolutely true in children, that metapneumovirus is indistinguishable from other respiratory viruses in kids,” he said.
Fatalities among older adults
As noted before, HMPV can cause severe and fatal illness in adults. For example, during an outbreak in North Dakota in 2016, 3 of 27 hospitalized adults with HMPV (median age, 69 years) died, and 10 required mechanical or noninvasive ventilation.
In a study from Korea comparing outcomes of severe HMPV-associated community-acquired pneumonia (CAP) with those of severe influenza-associated CAP, the investigators found that 30- and 60-day mortality rates were similar between the groups, at 24% of patients with HMPV-associated CAP and 32.1% for influenza-associated CAP, and 32% versus 38.5%, respectively.
Patients at high risk for severe disease or death from HMPV infection include those over 65 years, especially frail elderly, patients with chronic obstructive pulmonary disease, immunocompromised patients, and those with cardiopulmonary diseases such as congestive heart failure.
Supportive care only
“Do we have anything for treatment? The short answer is, No,” Dr. Williams.
Supportive care is currently the only effective approach for patients with severe HMPV infection.
Ribavirin, used to treat patients with acute RSV infection, has poor in vitro activity against HMPV and poor oral bioavailability and hemolysis, and there are no randomized controlled trials to support its use in this situation.
“It really can’t be recommended, and I don’t recommend it,” he said.
Virology may still help
Mark J. Siedner, MD, an infectious diseases physician at Mass General and associate professor of medicine at Harvard Medical School, both in Boston, who was not involved in the study, said that, despite the inability to clinically distinguish HMPV from RSV or influenza A, there is still clinical value to identifying HMPV infections.
“We spend millions of dollar each year treating people for upper respiratory tract infections, often with antibacterials, sometimes with antivirals, but those have costs to the health care system, and they also have costs in terms of drug resistance,” he said in an interview seeking objective commentary.
“Diagnostic tests that determine the actual source or the cause of these upper respiratory tract infections and encourage both patients and physicians not to be using antibiotics have value,” he said.
Identifying the pathogen can also help clinicians take appropriate infection-control precautions to prevent patient-to-clinician or patient-to-patient transmission of viral infections, he added.
Dr. Williams’ research is supported by the National Institutes of Health, Henry L. Hillman Foundation, and Asher Krop Memorial Fund of Children’s Hospital of Pittsburgh. Dr. Williams and Dr. Siedner reported no relevant conflict of interest disclosures.
FROM IDWEEK 2020
Potentially practice-changing bacterial therapy trials analyzed
A new formulation of an existing antibacterial agent and a potential therapeutic approach to a challenging clinical problem were the focus of a session on potentially practice-changing clinical trials in antimicrobial therapy presented during IDWeek 2020, an annual scientific meeting on infectious diseases.
“I know it has been a big year for viral disease of course, with COVID, but there has been some really good work that has gone on in the bacterial space, and of course as those of you who are on service know, you may have your fair share of COVID patients, but these are infections that we still deal with on a daily basis,” said Michael Satlin, MD, an infectious disease specialist at Weill Cornell Medicine in New York.
He combed through studies published during the previous 12 months in leading medical journals, including the New England Journal of Medicine, JAMA network publications, Lancet Infectious Diseases, Lancet Respiratory Medicine, Clinical Infectious Diseases, and Clinical Microbiology and Infection, looking for randomized trials of interventions to treat bacterial infections, and selecting those most likely to change practice of U.S. infectious diseases practitioners.
He excluded meta-analyses, post hoc analyses, evaluations of diagnostic tests, stewardship, or any studies presented previously at IDWeek.
Two of the trials he highlighted are described here.
Fosfomycin for injection
In the United States, fosfomycin, the only antibiotic in its class, is currently available only in an oral sachet formulation (Monurol), “and typically we’ve only given this for patients with cystitis because we know that we don’t achieve significant levels [of drug] in the kidney or in the bloodstream for other types of infections,” Dr. Satlin said.
In Europe, however fosfomycin for injection (ZTI-01) has been available for several years.
“There’s been a lot of interest in fosfomycin because it has a different mechanism of action from other agents. It’s an epoxide antibiotic that inhibits early peptidoglycan synthesis by binding to MurA,” he explained.
The phase 2/3 randomized ZEUS trial compared ZTI-01 with piperacillin/tazobactam (pip/taz) for treatment of complicated urinary tract infection (UTI) including acute pyelonephritis.
A total of 465 hospitalized adults with suspected or microbiologically confirmed complicated UTI or acute pyelonephritis were randomized to 6 g of ZTI-01 every 8 hours or 4.5 g of intravenous pip/taz every 8 hours for a fixed 7-day course with no oral switch; patients with concomitant bacteremia (about 9% of the study population) could receive the assigned therapy for up to 14 days.
The primary endpoint of noninferiority of ZTI-01 was met and clinical cure rates were high and similar between the treatments, at approximately 91% each. Treatment-emergent adverse events, including hypokalemia and elevated serum aminotransferases, were mostly mild and transient.
The hypokalemia seen in the trial may be attributable to the high salt load of fosfomycin relative to pip/taz, Dr. Satlin said.
“How might this change your practice? Well, if IV fosfomycin is ever FDA [Food and Drug Administration] approved – and my understanding is that the delays have been more related to manufacturing than scientific quality of data – it could potentially be an alternative to beta-lactams and fluoroquinolones” and has activity against most extend spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae, he said.
Fosfomycin susceptibility testing is challenging, however, with no Clinical & Laboratory Standards Institute (CLSI) or FDA breakpoints for Enterobacterales other than Escherichia coli, and there are questions about the step-down therapy.
“Do you just give a 3-gram sachet chaser when they walk out the door? Do you switch to another agent? I think that needs to be worked out,” he said.
Inhaled amikacin
“We know that some IV antibiotics, particularly for resistant organisms, may not achieve sufficient concentrations in the lung to treat pneumonia. We know that inhaled antibiotics can give a lot of concentration of that drug right at the at the site of infection, but we don’t really have [randomized controlled trial] data to see whether it really helps,” Dr. Satlin said.
The INHALE trial was a double-blind, placebo-controlled superiority trial to see whether adding inhaled amikacin to IV standard-of-care antibiotics could improve outcomes for mechanically ventilated patients with gram-negative pneumonia.
The investigators enrolled 725 adults who were receiving mechanical ventilation for pneumonia, 45% of who had ventilator-associated pneumonia (VAP). Of the total cohort, 508 patients analyzed for efficacy had gram-negative pathogens, including 32% with Pseudomonas aeurginosa, 29% with Acinetobacter baumannii, 30% with E. coli, and the remainder with Klebsiella pneumoniae.
Patients were randomized to standard-of-care intravenous antibiotics plus either inhaled amikacin 400 mg twice daily for 10 days or inhaled saline placebo.
“Of note, the median standard-of-care antibiotics in this study was 18 days, which is certainly longer than what our guidelines recommend.”
There was no significant difference between study arms in the primary endpoint of survival at days 28-32 for all patients who had received at least one dose of study drug, were infected with a gram-negative pathogen, and an Acute Physiology and Chronic Health Evaluation (APACHE) II score of at 10 or higher at diagnosis. The respective survival rates for the inhaled amikacin and placebo groups were 75% and 77%. The incidence of treatment-emergent adverse events or serious treatment-emergent adverse events were similar between the two treatment arms.
“No matter how you sliced and diced it – days of mechanical ventilation, duration of ICU stay – essentially they looked the same. Even for [extensively drug resistant] pathogens where you might expect that you’d see the benefit of inhaled amikacin, they didn’t really see a mortality benefit in this study,” Dr. Satlin said.
The study is practice changing, he said “because I think inhaled aminoglycosides should not be routinely added to the standard of care IV antibiotics for pneumonia in ventilated patients,” he said.
It’s still unclear whether inhaled aminoglycosides might play a role in the treatment of select patients infected with organisms resistant to all beta-lactams and fluoroquinolones, he added.
Tempting strategy
“Adding inhaled antibiotics is a tempting strategy for treatment of ventilated pneumonia, which often has poor outcomes,” commented Thomas Holland, MD, a hospitalist and infectious disease specialist at Duke University Hospital in Durham, N.C. “This is valuable and practical information as clinicians choose antibiotics regimens for this difficult-to-treat syndrome,” he said in an interview.
Dr. Holland comoderated the session in which Dr. Satlin presented the study findings and opinions.
No funding source for the presentation was reported. Dr. Satlin reported consulting for Shionogi and Achaogen and research grants from Allergan, Merck, and BioFire Diagnostics. Dr. Holland disclosed consulting fees and other material support from Basilea Pharmaceutica, Genetech, Karius and Theravance.
A new formulation of an existing antibacterial agent and a potential therapeutic approach to a challenging clinical problem were the focus of a session on potentially practice-changing clinical trials in antimicrobial therapy presented during IDWeek 2020, an annual scientific meeting on infectious diseases.
“I know it has been a big year for viral disease of course, with COVID, but there has been some really good work that has gone on in the bacterial space, and of course as those of you who are on service know, you may have your fair share of COVID patients, but these are infections that we still deal with on a daily basis,” said Michael Satlin, MD, an infectious disease specialist at Weill Cornell Medicine in New York.
He combed through studies published during the previous 12 months in leading medical journals, including the New England Journal of Medicine, JAMA network publications, Lancet Infectious Diseases, Lancet Respiratory Medicine, Clinical Infectious Diseases, and Clinical Microbiology and Infection, looking for randomized trials of interventions to treat bacterial infections, and selecting those most likely to change practice of U.S. infectious diseases practitioners.
He excluded meta-analyses, post hoc analyses, evaluations of diagnostic tests, stewardship, or any studies presented previously at IDWeek.
Two of the trials he highlighted are described here.
Fosfomycin for injection
In the United States, fosfomycin, the only antibiotic in its class, is currently available only in an oral sachet formulation (Monurol), “and typically we’ve only given this for patients with cystitis because we know that we don’t achieve significant levels [of drug] in the kidney or in the bloodstream for other types of infections,” Dr. Satlin said.
In Europe, however fosfomycin for injection (ZTI-01) has been available for several years.
“There’s been a lot of interest in fosfomycin because it has a different mechanism of action from other agents. It’s an epoxide antibiotic that inhibits early peptidoglycan synthesis by binding to MurA,” he explained.
The phase 2/3 randomized ZEUS trial compared ZTI-01 with piperacillin/tazobactam (pip/taz) for treatment of complicated urinary tract infection (UTI) including acute pyelonephritis.
A total of 465 hospitalized adults with suspected or microbiologically confirmed complicated UTI or acute pyelonephritis were randomized to 6 g of ZTI-01 every 8 hours or 4.5 g of intravenous pip/taz every 8 hours for a fixed 7-day course with no oral switch; patients with concomitant bacteremia (about 9% of the study population) could receive the assigned therapy for up to 14 days.
The primary endpoint of noninferiority of ZTI-01 was met and clinical cure rates were high and similar between the treatments, at approximately 91% each. Treatment-emergent adverse events, including hypokalemia and elevated serum aminotransferases, were mostly mild and transient.
The hypokalemia seen in the trial may be attributable to the high salt load of fosfomycin relative to pip/taz, Dr. Satlin said.
“How might this change your practice? Well, if IV fosfomycin is ever FDA [Food and Drug Administration] approved – and my understanding is that the delays have been more related to manufacturing than scientific quality of data – it could potentially be an alternative to beta-lactams and fluoroquinolones” and has activity against most extend spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae, he said.
Fosfomycin susceptibility testing is challenging, however, with no Clinical & Laboratory Standards Institute (CLSI) or FDA breakpoints for Enterobacterales other than Escherichia coli, and there are questions about the step-down therapy.
“Do you just give a 3-gram sachet chaser when they walk out the door? Do you switch to another agent? I think that needs to be worked out,” he said.
Inhaled amikacin
“We know that some IV antibiotics, particularly for resistant organisms, may not achieve sufficient concentrations in the lung to treat pneumonia. We know that inhaled antibiotics can give a lot of concentration of that drug right at the at the site of infection, but we don’t really have [randomized controlled trial] data to see whether it really helps,” Dr. Satlin said.
The INHALE trial was a double-blind, placebo-controlled superiority trial to see whether adding inhaled amikacin to IV standard-of-care antibiotics could improve outcomes for mechanically ventilated patients with gram-negative pneumonia.
The investigators enrolled 725 adults who were receiving mechanical ventilation for pneumonia, 45% of who had ventilator-associated pneumonia (VAP). Of the total cohort, 508 patients analyzed for efficacy had gram-negative pathogens, including 32% with Pseudomonas aeurginosa, 29% with Acinetobacter baumannii, 30% with E. coli, and the remainder with Klebsiella pneumoniae.
Patients were randomized to standard-of-care intravenous antibiotics plus either inhaled amikacin 400 mg twice daily for 10 days or inhaled saline placebo.
“Of note, the median standard-of-care antibiotics in this study was 18 days, which is certainly longer than what our guidelines recommend.”
There was no significant difference between study arms in the primary endpoint of survival at days 28-32 for all patients who had received at least one dose of study drug, were infected with a gram-negative pathogen, and an Acute Physiology and Chronic Health Evaluation (APACHE) II score of at 10 or higher at diagnosis. The respective survival rates for the inhaled amikacin and placebo groups were 75% and 77%. The incidence of treatment-emergent adverse events or serious treatment-emergent adverse events were similar between the two treatment arms.
“No matter how you sliced and diced it – days of mechanical ventilation, duration of ICU stay – essentially they looked the same. Even for [extensively drug resistant] pathogens where you might expect that you’d see the benefit of inhaled amikacin, they didn’t really see a mortality benefit in this study,” Dr. Satlin said.
The study is practice changing, he said “because I think inhaled aminoglycosides should not be routinely added to the standard of care IV antibiotics for pneumonia in ventilated patients,” he said.
It’s still unclear whether inhaled aminoglycosides might play a role in the treatment of select patients infected with organisms resistant to all beta-lactams and fluoroquinolones, he added.
Tempting strategy
“Adding inhaled antibiotics is a tempting strategy for treatment of ventilated pneumonia, which often has poor outcomes,” commented Thomas Holland, MD, a hospitalist and infectious disease specialist at Duke University Hospital in Durham, N.C. “This is valuable and practical information as clinicians choose antibiotics regimens for this difficult-to-treat syndrome,” he said in an interview.
Dr. Holland comoderated the session in which Dr. Satlin presented the study findings and opinions.
No funding source for the presentation was reported. Dr. Satlin reported consulting for Shionogi and Achaogen and research grants from Allergan, Merck, and BioFire Diagnostics. Dr. Holland disclosed consulting fees and other material support from Basilea Pharmaceutica, Genetech, Karius and Theravance.
A new formulation of an existing antibacterial agent and a potential therapeutic approach to a challenging clinical problem were the focus of a session on potentially practice-changing clinical trials in antimicrobial therapy presented during IDWeek 2020, an annual scientific meeting on infectious diseases.
“I know it has been a big year for viral disease of course, with COVID, but there has been some really good work that has gone on in the bacterial space, and of course as those of you who are on service know, you may have your fair share of COVID patients, but these are infections that we still deal with on a daily basis,” said Michael Satlin, MD, an infectious disease specialist at Weill Cornell Medicine in New York.
He combed through studies published during the previous 12 months in leading medical journals, including the New England Journal of Medicine, JAMA network publications, Lancet Infectious Diseases, Lancet Respiratory Medicine, Clinical Infectious Diseases, and Clinical Microbiology and Infection, looking for randomized trials of interventions to treat bacterial infections, and selecting those most likely to change practice of U.S. infectious diseases practitioners.
He excluded meta-analyses, post hoc analyses, evaluations of diagnostic tests, stewardship, or any studies presented previously at IDWeek.
Two of the trials he highlighted are described here.
Fosfomycin for injection
In the United States, fosfomycin, the only antibiotic in its class, is currently available only in an oral sachet formulation (Monurol), “and typically we’ve only given this for patients with cystitis because we know that we don’t achieve significant levels [of drug] in the kidney or in the bloodstream for other types of infections,” Dr. Satlin said.
In Europe, however fosfomycin for injection (ZTI-01) has been available for several years.
“There’s been a lot of interest in fosfomycin because it has a different mechanism of action from other agents. It’s an epoxide antibiotic that inhibits early peptidoglycan synthesis by binding to MurA,” he explained.
The phase 2/3 randomized ZEUS trial compared ZTI-01 with piperacillin/tazobactam (pip/taz) for treatment of complicated urinary tract infection (UTI) including acute pyelonephritis.
A total of 465 hospitalized adults with suspected or microbiologically confirmed complicated UTI or acute pyelonephritis were randomized to 6 g of ZTI-01 every 8 hours or 4.5 g of intravenous pip/taz every 8 hours for a fixed 7-day course with no oral switch; patients with concomitant bacteremia (about 9% of the study population) could receive the assigned therapy for up to 14 days.
The primary endpoint of noninferiority of ZTI-01 was met and clinical cure rates were high and similar between the treatments, at approximately 91% each. Treatment-emergent adverse events, including hypokalemia and elevated serum aminotransferases, were mostly mild and transient.
The hypokalemia seen in the trial may be attributable to the high salt load of fosfomycin relative to pip/taz, Dr. Satlin said.
“How might this change your practice? Well, if IV fosfomycin is ever FDA [Food and Drug Administration] approved – and my understanding is that the delays have been more related to manufacturing than scientific quality of data – it could potentially be an alternative to beta-lactams and fluoroquinolones” and has activity against most extend spectrum beta-lactamase (ESBL)–producing Enterobacteriaceae, he said.
Fosfomycin susceptibility testing is challenging, however, with no Clinical & Laboratory Standards Institute (CLSI) or FDA breakpoints for Enterobacterales other than Escherichia coli, and there are questions about the step-down therapy.
“Do you just give a 3-gram sachet chaser when they walk out the door? Do you switch to another agent? I think that needs to be worked out,” he said.
Inhaled amikacin
“We know that some IV antibiotics, particularly for resistant organisms, may not achieve sufficient concentrations in the lung to treat pneumonia. We know that inhaled antibiotics can give a lot of concentration of that drug right at the at the site of infection, but we don’t really have [randomized controlled trial] data to see whether it really helps,” Dr. Satlin said.
The INHALE trial was a double-blind, placebo-controlled superiority trial to see whether adding inhaled amikacin to IV standard-of-care antibiotics could improve outcomes for mechanically ventilated patients with gram-negative pneumonia.
The investigators enrolled 725 adults who were receiving mechanical ventilation for pneumonia, 45% of who had ventilator-associated pneumonia (VAP). Of the total cohort, 508 patients analyzed for efficacy had gram-negative pathogens, including 32% with Pseudomonas aeurginosa, 29% with Acinetobacter baumannii, 30% with E. coli, and the remainder with Klebsiella pneumoniae.
Patients were randomized to standard-of-care intravenous antibiotics plus either inhaled amikacin 400 mg twice daily for 10 days or inhaled saline placebo.
“Of note, the median standard-of-care antibiotics in this study was 18 days, which is certainly longer than what our guidelines recommend.”
There was no significant difference between study arms in the primary endpoint of survival at days 28-32 for all patients who had received at least one dose of study drug, were infected with a gram-negative pathogen, and an Acute Physiology and Chronic Health Evaluation (APACHE) II score of at 10 or higher at diagnosis. The respective survival rates for the inhaled amikacin and placebo groups were 75% and 77%. The incidence of treatment-emergent adverse events or serious treatment-emergent adverse events were similar between the two treatment arms.
“No matter how you sliced and diced it – days of mechanical ventilation, duration of ICU stay – essentially they looked the same. Even for [extensively drug resistant] pathogens where you might expect that you’d see the benefit of inhaled amikacin, they didn’t really see a mortality benefit in this study,” Dr. Satlin said.
The study is practice changing, he said “because I think inhaled aminoglycosides should not be routinely added to the standard of care IV antibiotics for pneumonia in ventilated patients,” he said.
It’s still unclear whether inhaled aminoglycosides might play a role in the treatment of select patients infected with organisms resistant to all beta-lactams and fluoroquinolones, he added.
Tempting strategy
“Adding inhaled antibiotics is a tempting strategy for treatment of ventilated pneumonia, which often has poor outcomes,” commented Thomas Holland, MD, a hospitalist and infectious disease specialist at Duke University Hospital in Durham, N.C. “This is valuable and practical information as clinicians choose antibiotics regimens for this difficult-to-treat syndrome,” he said in an interview.
Dr. Holland comoderated the session in which Dr. Satlin presented the study findings and opinions.
No funding source for the presentation was reported. Dr. Satlin reported consulting for Shionogi and Achaogen and research grants from Allergan, Merck, and BioFire Diagnostics. Dr. Holland disclosed consulting fees and other material support from Basilea Pharmaceutica, Genetech, Karius and Theravance.
FROM IDWEEK 2020