User login
Two studies add to knowledge base of biosimilar use in psoriasis, HS
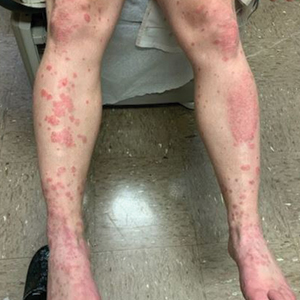
And another study, a small, single-center retrospective study of patients with hidradenitis suppurativa (HS), found that administration of infliximab and biosimilar infliximab were associated with similar and significant improvement in disease.
Both studies were published online in April in JAMA Dermatology and add to mounting evidence that biosimilars may be interchangeable in certain dermatologic conditions.
“Biosimilars are an exciting innovation in the field,” Joseph Zahn, MD, assistant professor of dermatology at George Washington University, Washington, said in an interview. “Their efficacy and price point will allow patients greater access to effective treatment.” To date, biosimilars approved in the United States that could be prescribed by dermatologists include those for rituximab, etanercept, adalimumab, and infliximab.
In the trial from Denmark, Nikolai Loft, MD, of the University of Copenhagen and colleagues evaluated outcomes following a mandatory medical switch from the brand name adalimumab, referred to as adalimumab originator, to adalimumab biosimilars among 726 individuals who were enrolled in a Danish nationwide registry of patients treated with biologics since 2007. The primary outcome was 1-year drug retention in patients switching to adalimumab biosimilars compared with patients treated with adalimumab originator.
The study population consisted of 348 patients with at least 2 years of exposure to adalimumab who had switched from originator to adalimumab biosimilars (a mean age of 52 and 72% male) and 378 patients who served as the adalimumab cohort (a mean age of 51 and 71% male). When the researchers compared the 1-year drug retention rates between the adalimumab biosimilar cohort and the adalimumab originator cohort, the rates were similar (92% vs. 92.1%, respectively).
The hazard ratios for other outcomes were similar as well. Specifically, the crude hazard ratios were 1.02 (P = .94) for all causes of drug discontinuation, 0.82 (P = .60) for insufficient effect, and 1.41 (P = .50) for adverse events (AEs) in the adalimumab biosimilar cohort, compared with the adalimumab originator cohort.
“Overall, results for any AEs were contradicting, but certain AEs were more prevalent in the adalimumab biosimilar cohort,” the authors wrote. Dermatologic AEs and AEs in the “other” category “were more prevalent, which could be attributable to more patients experiencing injection site reactions as a result of larger volumes and differences in excipients and syringes in the adalimumab biosimilars and the adalimumab originator.” Other potential explanations they offered were the nocebo effect and greater awareness of AEs among practitioners and patients.
“This study concludes that, when switched to a biosimilar medication, patients do not have worse control of their psoriasis nor do they switch to other medications,” Dr. Zahn, who was asked to comment about these results, said in the interview. “However, there was a trend toward a higher number of side effects in the biosimilar group. The main takeaway point from this study is that biosimilars of adalimumab seem to be relatively interchangeable in patients with psoriasis without loss of efficacy or significant increase in side effects that lead to a medication change for the patient.”
The researchers acknowledged certain limitations of their study, including the fact that it was limited to Danish patients and that individual AEs could not be examined. “Moreover, the surveillance of AEs is not as vigilant as in clinical trials, and AEs are most likely underreported,” they wrote. “Although no major differences were found when switching from adalimumab originator to adalimumab biosimilar versions, it was not possible to assess the performance of individual adalimumab biosimilar versions in this study.”
In the second study, Christopher Sayed, MD, associate professor of dermatology, University of North Carolina, Chapel Hill, and colleagues retrospectively evaluated the effectiveness of infliximab-abda versus infliximab administration in the treatment of 34 patients with HS who were cared for at the university’s dermatology clinic. Patients were treated with either agent for at least 10 weeks. The infliximab treatment group consisted of 20 patients with a mean age of 42 years who were mostly female (17; 85%), while the infliximab-abda treatment group included 14 patients with a mean age of 36 years who also were mostly female (13; 93%).
Both groups received loading doses of 10 mg/kg at weeks 0, 2, and 6, and treatment was continued with a maintenance dose administered every 4-8 weeks. The patients were followed between February 2016 and June 2020 and the primary outcome measure was Hidradenitis Suppurative Clinical Response (HiSCR), which was defined as at least a 50% decrease in inflammatory nodule count without any increase in the number of abscesses or draining sinuses.
The researchers found that 71% of patients in the infliximab-abda treatment group achieved a HiSCR, compared with 60% of their counterparts in the infliximab treatment group, a difference that did not reach statistical significance (P = .47). Three patients in the infliximab treatment group experienced AEs, compared with none in the infliximab-abda treatment group.
“The data are promising,” Dr. Zahn said. “Although this is a small study with a limited number of patients, it suggests that this particular biosimilar may be a reasonable or possibly even equivalent alternative to infliximab. A larger, prospective trial will be needed before we can be sure the results are equivalent.”
Dr. Sayed and colleagues noted certain limitations of their study, including the retrospective design and the use of concomitant medications by some participants. “There is also a risk of selection bias because copay and medication assistance programs are not available for infliximab-abda for patients with HS,” they wrote.
In an editorial accompanying the two studies, Mark Lebwohl, MD, professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, wrote that the introduction of biosimilars have been justified by “the hope that lower costs” will increase availability of treatments to patients with moderate to severe psoriasis. “Inroads in the U.S. market, however, have been limited,” he added, and there is concern that they “may be used to prevent access to newer interleukin-17 blockers and interleukin-23 blockers for which biosimilars are available and that do not carry the boxed warnings found on tumor necrosis factor blockers.”
Dr. Loft reported receiving personal fees from Eli Lilly and Janssen outside of the submitted work. Many of his coauthors reporting having numerous financial conflicts of interest with the pharmaceutical industry. The HS study was supported by a public health service research award from the National Institutes of Health. Dr. Sayed reported receiving personal fees or personal fees paid to the institution from AbbVie, Novartis, Chemocentryx, GlaxoSmithKline, Incyte, InflaRx, and UCB. No other disclosures were reported. Dr. Lebwohl disclosed receiving research funds from companies including AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, and Incyte; and receiving personal fees from multiple companies, outside of the submitted work. Dr. Zahn reported having no disclosures.
And another study, a small, single-center retrospective study of patients with hidradenitis suppurativa (HS), found that administration of infliximab and biosimilar infliximab were associated with similar and significant improvement in disease.
Both studies were published online in April in JAMA Dermatology and add to mounting evidence that biosimilars may be interchangeable in certain dermatologic conditions.
“Biosimilars are an exciting innovation in the field,” Joseph Zahn, MD, assistant professor of dermatology at George Washington University, Washington, said in an interview. “Their efficacy and price point will allow patients greater access to effective treatment.” To date, biosimilars approved in the United States that could be prescribed by dermatologists include those for rituximab, etanercept, adalimumab, and infliximab.
In the trial from Denmark, Nikolai Loft, MD, of the University of Copenhagen and colleagues evaluated outcomes following a mandatory medical switch from the brand name adalimumab, referred to as adalimumab originator, to adalimumab biosimilars among 726 individuals who were enrolled in a Danish nationwide registry of patients treated with biologics since 2007. The primary outcome was 1-year drug retention in patients switching to adalimumab biosimilars compared with patients treated with adalimumab originator.
The study population consisted of 348 patients with at least 2 years of exposure to adalimumab who had switched from originator to adalimumab biosimilars (a mean age of 52 and 72% male) and 378 patients who served as the adalimumab cohort (a mean age of 51 and 71% male). When the researchers compared the 1-year drug retention rates between the adalimumab biosimilar cohort and the adalimumab originator cohort, the rates were similar (92% vs. 92.1%, respectively).
The hazard ratios for other outcomes were similar as well. Specifically, the crude hazard ratios were 1.02 (P = .94) for all causes of drug discontinuation, 0.82 (P = .60) for insufficient effect, and 1.41 (P = .50) for adverse events (AEs) in the adalimumab biosimilar cohort, compared with the adalimumab originator cohort.
“Overall, results for any AEs were contradicting, but certain AEs were more prevalent in the adalimumab biosimilar cohort,” the authors wrote. Dermatologic AEs and AEs in the “other” category “were more prevalent, which could be attributable to more patients experiencing injection site reactions as a result of larger volumes and differences in excipients and syringes in the adalimumab biosimilars and the adalimumab originator.” Other potential explanations they offered were the nocebo effect and greater awareness of AEs among practitioners and patients.
“This study concludes that, when switched to a biosimilar medication, patients do not have worse control of their psoriasis nor do they switch to other medications,” Dr. Zahn, who was asked to comment about these results, said in the interview. “However, there was a trend toward a higher number of side effects in the biosimilar group. The main takeaway point from this study is that biosimilars of adalimumab seem to be relatively interchangeable in patients with psoriasis without loss of efficacy or significant increase in side effects that lead to a medication change for the patient.”
The researchers acknowledged certain limitations of their study, including the fact that it was limited to Danish patients and that individual AEs could not be examined. “Moreover, the surveillance of AEs is not as vigilant as in clinical trials, and AEs are most likely underreported,” they wrote. “Although no major differences were found when switching from adalimumab originator to adalimumab biosimilar versions, it was not possible to assess the performance of individual adalimumab biosimilar versions in this study.”
In the second study, Christopher Sayed, MD, associate professor of dermatology, University of North Carolina, Chapel Hill, and colleagues retrospectively evaluated the effectiveness of infliximab-abda versus infliximab administration in the treatment of 34 patients with HS who were cared for at the university’s dermatology clinic. Patients were treated with either agent for at least 10 weeks. The infliximab treatment group consisted of 20 patients with a mean age of 42 years who were mostly female (17; 85%), while the infliximab-abda treatment group included 14 patients with a mean age of 36 years who also were mostly female (13; 93%).
Both groups received loading doses of 10 mg/kg at weeks 0, 2, and 6, and treatment was continued with a maintenance dose administered every 4-8 weeks. The patients were followed between February 2016 and June 2020 and the primary outcome measure was Hidradenitis Suppurative Clinical Response (HiSCR), which was defined as at least a 50% decrease in inflammatory nodule count without any increase in the number of abscesses or draining sinuses.
The researchers found that 71% of patients in the infliximab-abda treatment group achieved a HiSCR, compared with 60% of their counterparts in the infliximab treatment group, a difference that did not reach statistical significance (P = .47). Three patients in the infliximab treatment group experienced AEs, compared with none in the infliximab-abda treatment group.
“The data are promising,” Dr. Zahn said. “Although this is a small study with a limited number of patients, it suggests that this particular biosimilar may be a reasonable or possibly even equivalent alternative to infliximab. A larger, prospective trial will be needed before we can be sure the results are equivalent.”
Dr. Sayed and colleagues noted certain limitations of their study, including the retrospective design and the use of concomitant medications by some participants. “There is also a risk of selection bias because copay and medication assistance programs are not available for infliximab-abda for patients with HS,” they wrote.
In an editorial accompanying the two studies, Mark Lebwohl, MD, professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, wrote that the introduction of biosimilars have been justified by “the hope that lower costs” will increase availability of treatments to patients with moderate to severe psoriasis. “Inroads in the U.S. market, however, have been limited,” he added, and there is concern that they “may be used to prevent access to newer interleukin-17 blockers and interleukin-23 blockers for which biosimilars are available and that do not carry the boxed warnings found on tumor necrosis factor blockers.”
Dr. Loft reported receiving personal fees from Eli Lilly and Janssen outside of the submitted work. Many of his coauthors reporting having numerous financial conflicts of interest with the pharmaceutical industry. The HS study was supported by a public health service research award from the National Institutes of Health. Dr. Sayed reported receiving personal fees or personal fees paid to the institution from AbbVie, Novartis, Chemocentryx, GlaxoSmithKline, Incyte, InflaRx, and UCB. No other disclosures were reported. Dr. Lebwohl disclosed receiving research funds from companies including AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, and Incyte; and receiving personal fees from multiple companies, outside of the submitted work. Dr. Zahn reported having no disclosures.
And another study, a small, single-center retrospective study of patients with hidradenitis suppurativa (HS), found that administration of infliximab and biosimilar infliximab were associated with similar and significant improvement in disease.
Both studies were published online in April in JAMA Dermatology and add to mounting evidence that biosimilars may be interchangeable in certain dermatologic conditions.
“Biosimilars are an exciting innovation in the field,” Joseph Zahn, MD, assistant professor of dermatology at George Washington University, Washington, said in an interview. “Their efficacy and price point will allow patients greater access to effective treatment.” To date, biosimilars approved in the United States that could be prescribed by dermatologists include those for rituximab, etanercept, adalimumab, and infliximab.
In the trial from Denmark, Nikolai Loft, MD, of the University of Copenhagen and colleagues evaluated outcomes following a mandatory medical switch from the brand name adalimumab, referred to as adalimumab originator, to adalimumab biosimilars among 726 individuals who were enrolled in a Danish nationwide registry of patients treated with biologics since 2007. The primary outcome was 1-year drug retention in patients switching to adalimumab biosimilars compared with patients treated with adalimumab originator.
The study population consisted of 348 patients with at least 2 years of exposure to adalimumab who had switched from originator to adalimumab biosimilars (a mean age of 52 and 72% male) and 378 patients who served as the adalimumab cohort (a mean age of 51 and 71% male). When the researchers compared the 1-year drug retention rates between the adalimumab biosimilar cohort and the adalimumab originator cohort, the rates were similar (92% vs. 92.1%, respectively).
The hazard ratios for other outcomes were similar as well. Specifically, the crude hazard ratios were 1.02 (P = .94) for all causes of drug discontinuation, 0.82 (P = .60) for insufficient effect, and 1.41 (P = .50) for adverse events (AEs) in the adalimumab biosimilar cohort, compared with the adalimumab originator cohort.
“Overall, results for any AEs were contradicting, but certain AEs were more prevalent in the adalimumab biosimilar cohort,” the authors wrote. Dermatologic AEs and AEs in the “other” category “were more prevalent, which could be attributable to more patients experiencing injection site reactions as a result of larger volumes and differences in excipients and syringes in the adalimumab biosimilars and the adalimumab originator.” Other potential explanations they offered were the nocebo effect and greater awareness of AEs among practitioners and patients.
“This study concludes that, when switched to a biosimilar medication, patients do not have worse control of their psoriasis nor do they switch to other medications,” Dr. Zahn, who was asked to comment about these results, said in the interview. “However, there was a trend toward a higher number of side effects in the biosimilar group. The main takeaway point from this study is that biosimilars of adalimumab seem to be relatively interchangeable in patients with psoriasis without loss of efficacy or significant increase in side effects that lead to a medication change for the patient.”
The researchers acknowledged certain limitations of their study, including the fact that it was limited to Danish patients and that individual AEs could not be examined. “Moreover, the surveillance of AEs is not as vigilant as in clinical trials, and AEs are most likely underreported,” they wrote. “Although no major differences were found when switching from adalimumab originator to adalimumab biosimilar versions, it was not possible to assess the performance of individual adalimumab biosimilar versions in this study.”
In the second study, Christopher Sayed, MD, associate professor of dermatology, University of North Carolina, Chapel Hill, and colleagues retrospectively evaluated the effectiveness of infliximab-abda versus infliximab administration in the treatment of 34 patients with HS who were cared for at the university’s dermatology clinic. Patients were treated with either agent for at least 10 weeks. The infliximab treatment group consisted of 20 patients with a mean age of 42 years who were mostly female (17; 85%), while the infliximab-abda treatment group included 14 patients with a mean age of 36 years who also were mostly female (13; 93%).
Both groups received loading doses of 10 mg/kg at weeks 0, 2, and 6, and treatment was continued with a maintenance dose administered every 4-8 weeks. The patients were followed between February 2016 and June 2020 and the primary outcome measure was Hidradenitis Suppurative Clinical Response (HiSCR), which was defined as at least a 50% decrease in inflammatory nodule count without any increase in the number of abscesses or draining sinuses.
The researchers found that 71% of patients in the infliximab-abda treatment group achieved a HiSCR, compared with 60% of their counterparts in the infliximab treatment group, a difference that did not reach statistical significance (P = .47). Three patients in the infliximab treatment group experienced AEs, compared with none in the infliximab-abda treatment group.
“The data are promising,” Dr. Zahn said. “Although this is a small study with a limited number of patients, it suggests that this particular biosimilar may be a reasonable or possibly even equivalent alternative to infliximab. A larger, prospective trial will be needed before we can be sure the results are equivalent.”
Dr. Sayed and colleagues noted certain limitations of their study, including the retrospective design and the use of concomitant medications by some participants. “There is also a risk of selection bias because copay and medication assistance programs are not available for infliximab-abda for patients with HS,” they wrote.
In an editorial accompanying the two studies, Mark Lebwohl, MD, professor of dermatology, Icahn School of Medicine at Mount Sinai, New York, wrote that the introduction of biosimilars have been justified by “the hope that lower costs” will increase availability of treatments to patients with moderate to severe psoriasis. “Inroads in the U.S. market, however, have been limited,” he added, and there is concern that they “may be used to prevent access to newer interleukin-17 blockers and interleukin-23 blockers for which biosimilars are available and that do not carry the boxed warnings found on tumor necrosis factor blockers.”
Dr. Loft reported receiving personal fees from Eli Lilly and Janssen outside of the submitted work. Many of his coauthors reporting having numerous financial conflicts of interest with the pharmaceutical industry. The HS study was supported by a public health service research award from the National Institutes of Health. Dr. Sayed reported receiving personal fees or personal fees paid to the institution from AbbVie, Novartis, Chemocentryx, GlaxoSmithKline, Incyte, InflaRx, and UCB. No other disclosures were reported. Dr. Lebwohl disclosed receiving research funds from companies including AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, and Incyte; and receiving personal fees from multiple companies, outside of the submitted work. Dr. Zahn reported having no disclosures.
FROM JAMA DERMATOLOGY
Debate: Should biologics be used for milder cases of psoriasis?
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
The issue was tackled in a debate at the American Academy of Dermatology Virtual Meeting Experience.
Taking the con side, Kenneth Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee, argued that, with the high cost of biologics, availability of many alternatives, and other issues, “we should just say no. ... There is no good reason that we need to expand the use of biologics in patients with limited disease.”
On the pro side, Richard Langley, MD, professor of dermatology at Dalhousie University Halifax, N.S, argued for a nuanced approach. He noted that patients with smaller patches of disease can be just as miserable as patients who hit traditional benchmarks of increased severity, such as high body surface area involvement – especially if those small areas are in sensitive locations like the scalp, palms, or genitals.
The decision to use a biologic should hinge on how badly patients and their quality of life are affected, not on “some artificial and limiting definition” of severity, Dr. Langley said.
Dr. Gordon didn’t disagree, noting that current use criteria include objective measures as well as disease in sensitive areas and failure of alternative treatments.
Rather, he was concerned about “expanding the definition of who is eligible beyond these criteria ... to chase every last bit of” disease. “I don’t think we have” a good rationale for that approach, he said.
Cost is the most important issue, Dr. Gordon said.
With more biologics on the way and prices continuing to go up, “there is going to a be a huge challenge to our use of these expensive medicines over the next few years” from payers. “It is important that we use them smartly in order to make sure we are able to use them for people with severe disease” who really need them. If “we start using biologics for all our patients with psoriasis,” it will be a “cost disaster,” Dr. Gordon said.
In addition, topicals and home phototherapy can be effective as long as patients adhere to them, as can alternative systemic agents, such as methotrexate and apremilast.
Often with biologics, “the issue is mainly convenience” rather than a fundamental problem with the alternatives, and despite the good safety record in trials, “chasing the last bit” of psoriasis with a biologic “is not necessarily” without risk for the patient, Dr. Gordon said.
Still, there can be a “pretty significant disconnect” between how patients perceive their psoriasis and “what physicians are thinking and prescribing” for it based on objective measures, Dr. Langley noted. Sometimes patients who have limited disease but are in significant distress aren’t even receiving treatment or are only given another cream to add to their collection of ones that haven’t worked.
One problem with traditional severity classifications is that they don’t generally take patients’ subjective experience into account, he added. There’s also been a lack of standardization to the point that dermatologists, researchers, and payers can sometimes disagree over severity in a given patient.
There’s movement toward better incorporation of patient experience into severity considerations, but for now at least, a designation of mild psoriasis can underestimate the true severity of disease, Dr. Langley said.
Dr. Gordon and Dr. Langley reported receiving honoraria and/or research support from many pharmaceutical companies, including AbbVie, Pfizer, and Lilly.
A version of this article first appeared on Medscape.com.
Clearance rates higher with bimekizumab vs. secukinumab in phase 3 psoriasis study
Secukinumab is the latest adult plaque psoriasis treatment to be bested by a newcomer, the interleukin 17A and 17F blocker bimekizumab.
Rates of complete clearance were substantially higher with bimekizumab in a phase 3 trial with 743 patients with moderate-to-severe plaque psoriasis, but oral candidiasis (oral thrush) again emerged as a particular issue with the agent.
Clinical improvements seen with bimekizumab have exceeded those with two standard options for adult plaque psoriasis — the tumor necrosis factor blocker adalimumab and the interleukin (IL) 12/23 inhibitor ustekinumab
— in phase 3 trials from manufacturer UCB Pharma, and it›s under review for the indication by the U.S. Food and Drug Administration and the European Medicines Agency.
The biologic is also being evaluated in phase 3 trials for treating psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa.
Results of the trial comparing bimekizumab to secukinumab, dubbed BE RADIANT, were presented at the American Academy of Dermatology Virtual Meeting Experience and published online concurrently April 23 in the New England Journal of Medicine.
The results “suggest that inhibition of both interleukin-17A and interleukin-17F with bimekizumab may provide greater clinical benefit for patients with moderate-to-severe plaque psoriasis than inhibition of interleukin-17A alone,” as with secukinumab, said the investigators, led by Kristian Reich, MD, professor of dermatology at the University Medical Center Hamburg-Eppendorf in Hamburg, Germany.
The trial randomly assigned 373 adults to bimekizumab 320 mg every 4 weeks to week 16, then rerandomized them to maintenance dosing either every 4 weeks or every 8 weeks to week 48; another 370 adults were randomly assigned to secukinumab 300 mg weekly for the first 4 weeks, then every 4 weeks to week 48. Baseline Psoriasis Area and Severity Index (PASI) scores were about 20 points in both treatment groups.
At the 1-month point, 71% in the bimekizumab group, vs 47.3% on secukinumab, had a 75% or greater reduction from their baseline PASI score. At 4 months, 61.7% of those on bimekizumab but 48.9% in the secukinumab group had complete clearance with a PASI score of 100.
At 48 weeks, 67% of those on bimekizumab had a PASI 100 response — which was numerically similar between the two bimekizumab dosing regimens after week 16 — vs 46.2% of the secukinumab group (P for all < .001).
The incidence of serious adverse events was just under 6% in both groups, with adverse events leading to discontinuation in 3.5% of bimekizumab and 2.7% of secukinumab subjects. The rate of serious infections was similar in both groups.
However, as in past trials, oral candidiasis was an issue, occurring in 19.3% of bimekizumab subjects vs 3% on secukinumab. Half of the 72 bimekizumab cases were classified as mild, and all but two of the rest as moderate. Over 40% of affected subjects reported more than one case, but none led to treatment discontinuation.
More than 85% of oral candidiasis cases in the study were treated with antifungal therapy and resolved during the trial. Inflammatory bowel disease is a concern with IL-17 blockade, but this hasn’t emerged as a particular issue with bimekizumab. There was one case each of ulcerative colitis in both the bimekizumab and secukinumab groups, and just one case of ulcerative colitis in three previous phase 3 bimekizumab trials, according to the investigators.
Among the trial limitations: Patients who had been on bimekizumab or secukinumab previously were excluded, as were patients who had no response to an IL-17 biologic or more than one biologic agent of any other class within the previous 12 weeks. The limitations could reduce generalizability, the investigators said.
Patients in the trial were about 45 years old, on average, and about two thirds were men; over 90% were White.
The study was funded by UCB Pharma. The investigators had numerous disclosures, including Reich who reported grants and personal fees from companies including UCB Pharma. The full list of disclosures can be found with the New England Journal of Medicine article.
A version of this article first appeared on Medscape.com .
Secukinumab is the latest adult plaque psoriasis treatment to be bested by a newcomer, the interleukin 17A and 17F blocker bimekizumab.
Rates of complete clearance were substantially higher with bimekizumab in a phase 3 trial with 743 patients with moderate-to-severe plaque psoriasis, but oral candidiasis (oral thrush) again emerged as a particular issue with the agent.
Clinical improvements seen with bimekizumab have exceeded those with two standard options for adult plaque psoriasis — the tumor necrosis factor blocker adalimumab and the interleukin (IL) 12/23 inhibitor ustekinumab
— in phase 3 trials from manufacturer UCB Pharma, and it›s under review for the indication by the U.S. Food and Drug Administration and the European Medicines Agency.
The biologic is also being evaluated in phase 3 trials for treating psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa.
Results of the trial comparing bimekizumab to secukinumab, dubbed BE RADIANT, were presented at the American Academy of Dermatology Virtual Meeting Experience and published online concurrently April 23 in the New England Journal of Medicine.
The results “suggest that inhibition of both interleukin-17A and interleukin-17F with bimekizumab may provide greater clinical benefit for patients with moderate-to-severe plaque psoriasis than inhibition of interleukin-17A alone,” as with secukinumab, said the investigators, led by Kristian Reich, MD, professor of dermatology at the University Medical Center Hamburg-Eppendorf in Hamburg, Germany.
The trial randomly assigned 373 adults to bimekizumab 320 mg every 4 weeks to week 16, then rerandomized them to maintenance dosing either every 4 weeks or every 8 weeks to week 48; another 370 adults were randomly assigned to secukinumab 300 mg weekly for the first 4 weeks, then every 4 weeks to week 48. Baseline Psoriasis Area and Severity Index (PASI) scores were about 20 points in both treatment groups.
At the 1-month point, 71% in the bimekizumab group, vs 47.3% on secukinumab, had a 75% or greater reduction from their baseline PASI score. At 4 months, 61.7% of those on bimekizumab but 48.9% in the secukinumab group had complete clearance with a PASI score of 100.
At 48 weeks, 67% of those on bimekizumab had a PASI 100 response — which was numerically similar between the two bimekizumab dosing regimens after week 16 — vs 46.2% of the secukinumab group (P for all < .001).
The incidence of serious adverse events was just under 6% in both groups, with adverse events leading to discontinuation in 3.5% of bimekizumab and 2.7% of secukinumab subjects. The rate of serious infections was similar in both groups.
However, as in past trials, oral candidiasis was an issue, occurring in 19.3% of bimekizumab subjects vs 3% on secukinumab. Half of the 72 bimekizumab cases were classified as mild, and all but two of the rest as moderate. Over 40% of affected subjects reported more than one case, but none led to treatment discontinuation.
More than 85% of oral candidiasis cases in the study were treated with antifungal therapy and resolved during the trial. Inflammatory bowel disease is a concern with IL-17 blockade, but this hasn’t emerged as a particular issue with bimekizumab. There was one case each of ulcerative colitis in both the bimekizumab and secukinumab groups, and just one case of ulcerative colitis in three previous phase 3 bimekizumab trials, according to the investigators.
Among the trial limitations: Patients who had been on bimekizumab or secukinumab previously were excluded, as were patients who had no response to an IL-17 biologic or more than one biologic agent of any other class within the previous 12 weeks. The limitations could reduce generalizability, the investigators said.
Patients in the trial were about 45 years old, on average, and about two thirds were men; over 90% were White.
The study was funded by UCB Pharma. The investigators had numerous disclosures, including Reich who reported grants and personal fees from companies including UCB Pharma. The full list of disclosures can be found with the New England Journal of Medicine article.
A version of this article first appeared on Medscape.com .
Secukinumab is the latest adult plaque psoriasis treatment to be bested by a newcomer, the interleukin 17A and 17F blocker bimekizumab.
Rates of complete clearance were substantially higher with bimekizumab in a phase 3 trial with 743 patients with moderate-to-severe plaque psoriasis, but oral candidiasis (oral thrush) again emerged as a particular issue with the agent.
Clinical improvements seen with bimekizumab have exceeded those with two standard options for adult plaque psoriasis — the tumor necrosis factor blocker adalimumab and the interleukin (IL) 12/23 inhibitor ustekinumab
— in phase 3 trials from manufacturer UCB Pharma, and it›s under review for the indication by the U.S. Food and Drug Administration and the European Medicines Agency.
The biologic is also being evaluated in phase 3 trials for treating psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa.
Results of the trial comparing bimekizumab to secukinumab, dubbed BE RADIANT, were presented at the American Academy of Dermatology Virtual Meeting Experience and published online concurrently April 23 in the New England Journal of Medicine.
The results “suggest that inhibition of both interleukin-17A and interleukin-17F with bimekizumab may provide greater clinical benefit for patients with moderate-to-severe plaque psoriasis than inhibition of interleukin-17A alone,” as with secukinumab, said the investigators, led by Kristian Reich, MD, professor of dermatology at the University Medical Center Hamburg-Eppendorf in Hamburg, Germany.
The trial randomly assigned 373 adults to bimekizumab 320 mg every 4 weeks to week 16, then rerandomized them to maintenance dosing either every 4 weeks or every 8 weeks to week 48; another 370 adults were randomly assigned to secukinumab 300 mg weekly for the first 4 weeks, then every 4 weeks to week 48. Baseline Psoriasis Area and Severity Index (PASI) scores were about 20 points in both treatment groups.
At the 1-month point, 71% in the bimekizumab group, vs 47.3% on secukinumab, had a 75% or greater reduction from their baseline PASI score. At 4 months, 61.7% of those on bimekizumab but 48.9% in the secukinumab group had complete clearance with a PASI score of 100.
At 48 weeks, 67% of those on bimekizumab had a PASI 100 response — which was numerically similar between the two bimekizumab dosing regimens after week 16 — vs 46.2% of the secukinumab group (P for all < .001).
The incidence of serious adverse events was just under 6% in both groups, with adverse events leading to discontinuation in 3.5% of bimekizumab and 2.7% of secukinumab subjects. The rate of serious infections was similar in both groups.
However, as in past trials, oral candidiasis was an issue, occurring in 19.3% of bimekizumab subjects vs 3% on secukinumab. Half of the 72 bimekizumab cases were classified as mild, and all but two of the rest as moderate. Over 40% of affected subjects reported more than one case, but none led to treatment discontinuation.
More than 85% of oral candidiasis cases in the study were treated with antifungal therapy and resolved during the trial. Inflammatory bowel disease is a concern with IL-17 blockade, but this hasn’t emerged as a particular issue with bimekizumab. There was one case each of ulcerative colitis in both the bimekizumab and secukinumab groups, and just one case of ulcerative colitis in three previous phase 3 bimekizumab trials, according to the investigators.
Among the trial limitations: Patients who had been on bimekizumab or secukinumab previously were excluded, as were patients who had no response to an IL-17 biologic or more than one biologic agent of any other class within the previous 12 weeks. The limitations could reduce generalizability, the investigators said.
Patients in the trial were about 45 years old, on average, and about two thirds were men; over 90% were White.
The study was funded by UCB Pharma. The investigators had numerous disclosures, including Reich who reported grants and personal fees from companies including UCB Pharma. The full list of disclosures can be found with the New England Journal of Medicine article.
A version of this article first appeared on Medscape.com .
Bimekizumab tops adalimumab for plaque psoriasis
The interleukin-17A and 17F blocker has also racked up significant wins against ustekinumab and secukinumab, other standard biologic options for adults with moderate to severe plaque psoriasis, and is currently under review for the indication by the U.S. Food and Drug Administration and European Medicines Agency.
In the adalimumab trial, dubbed BE SURE, bimekizumab had higher clinical response rates than the tumor necrosis factor (TNF) blocker over the 24-week head-to-head phase of the 478-patient trial, with substantial improvements in both Psoriasis Area and Severity Index (PASI) 90 response and Investigator’s Global Assessment (IGA) scores of 0 or 1, which signifies clear or almost clear skin.
The results were published in the New England Journal of Medicine and scheduled to be presented at the American Academy of Dermatology Virtual Meeting Experience on April 24.
“The data look good,” said psoriasis specialist Steven Feldman, MD, PhD, professor of dermatology at Wake Forest School of Medicine in Winston-Salem, N.C., when asked for comment.
Bimekizumab “appears more effective than current options. The big question is safety. The 10%-20% rate of oral candidiasis is much higher than other treatments but should be entirely manageable, as long as there are no unknown worse candida issues.” In addition, that there were no cases of inflammatory bowel disease in BE SURE “is very encouraging, as that is one of the limitations for existing IL-17 blockers,” he said.
The trial was launched after previous reports suggested that IL-17A inhibition may be better than TNF blockade in controlling psoriasis, said investigators led by Richard Warren, MBChB, PhD, a dermatology professor at the University of Manchester (England).
Patients were assigned evenly to one of three regimens: subcutaneous bimekizumab at a dose of 320 mg every 4 weeks for 56 weeks; bimekizumab at 320 mg every 4 weeks for 16 weeks, then every 8 weeks out to 56 weeks; or subcutaneous adalimumab at a dose of 40 mg every 2 weeks for 24 weeks, followed by bimekizumab at a dose of 320 mg every 4 weeks to week 56.
At week 16, 86.2% of those in the bimekizumab group but just 47.2% in the adalimumab group had a PASI 90 response (P < .001), and 85.3% of the bimekizumab versus 57.2% in the adalimumab group had an IGA score of 0 or 1 (P < .001).
About 52% of the adalimumab group had a PASI 90 response at week 24, when they were switched to bimekizumab. By week 56, their PASI 90 response rate rose to 81.8%. Skin clearance was maintained through week 56 whether subjects were dosed every 4 or every 8 weeks with the interleukin blocker.
The incidence of oral candidiasis (9.5%-17.4% vs. 0% with adalimumab alone) was similar to other trials and likely because of the short circuiting of interleukin-17, which plays a role protecting against candida. Most cases were mild to moderate.
The increased risk of oral thrush with bimekizumab “may not be particularly clinically meaningful, especially if” it can be managed by an occasional fluconazole pill. It’s “reassuring … if that’s the biggest problem with the drug, or we may wonder if, in real life use, more severe, perhaps esophageal or systemic fungal infection may be observed,” Dr. Feldman said in a recent editorial.
“Not knowing the future may make some physicians reticent about using the drug when other options are available, at least until data are available on much larger numbers of exposed patients treated for longer periods of time,” he and his colleague William Huang, MD, also a dermatologist at Wake Forest, said.
One of the limits of the trial was that the head-to-head portion was only 24 weeks, “which was too brief for a comparison of safety between bimekizumab and adalimumab in a lifelong disease,” the investigators noted.
The mean age of the patients was 44.9 years, and the mean baseline PASI score was 19.8.
Although the initial dose of adalimumab in the study was 40 mg, labeling recommends an initial dose of 80 mg for the TNF blocker.
Bimekizumab is also being evaluated in phase 3 trials for psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa, according to UCB Pharma.
The study was funded by UCB Pharma. The investigators had numerous disclosures, including Dr. Warren who reported grants and personal fees from the company. Dr. Feldman reported receiving research, speaking, and/or consulting support from UCB Pharma and other companies.
A version of this article first appeared on Medscape.com.
The interleukin-17A and 17F blocker has also racked up significant wins against ustekinumab and secukinumab, other standard biologic options for adults with moderate to severe plaque psoriasis, and is currently under review for the indication by the U.S. Food and Drug Administration and European Medicines Agency.
In the adalimumab trial, dubbed BE SURE, bimekizumab had higher clinical response rates than the tumor necrosis factor (TNF) blocker over the 24-week head-to-head phase of the 478-patient trial, with substantial improvements in both Psoriasis Area and Severity Index (PASI) 90 response and Investigator’s Global Assessment (IGA) scores of 0 or 1, which signifies clear or almost clear skin.
The results were published in the New England Journal of Medicine and scheduled to be presented at the American Academy of Dermatology Virtual Meeting Experience on April 24.
“The data look good,” said psoriasis specialist Steven Feldman, MD, PhD, professor of dermatology at Wake Forest School of Medicine in Winston-Salem, N.C., when asked for comment.
Bimekizumab “appears more effective than current options. The big question is safety. The 10%-20% rate of oral candidiasis is much higher than other treatments but should be entirely manageable, as long as there are no unknown worse candida issues.” In addition, that there were no cases of inflammatory bowel disease in BE SURE “is very encouraging, as that is one of the limitations for existing IL-17 blockers,” he said.
The trial was launched after previous reports suggested that IL-17A inhibition may be better than TNF blockade in controlling psoriasis, said investigators led by Richard Warren, MBChB, PhD, a dermatology professor at the University of Manchester (England).
Patients were assigned evenly to one of three regimens: subcutaneous bimekizumab at a dose of 320 mg every 4 weeks for 56 weeks; bimekizumab at 320 mg every 4 weeks for 16 weeks, then every 8 weeks out to 56 weeks; or subcutaneous adalimumab at a dose of 40 mg every 2 weeks for 24 weeks, followed by bimekizumab at a dose of 320 mg every 4 weeks to week 56.
At week 16, 86.2% of those in the bimekizumab group but just 47.2% in the adalimumab group had a PASI 90 response (P < .001), and 85.3% of the bimekizumab versus 57.2% in the adalimumab group had an IGA score of 0 or 1 (P < .001).
About 52% of the adalimumab group had a PASI 90 response at week 24, when they were switched to bimekizumab. By week 56, their PASI 90 response rate rose to 81.8%. Skin clearance was maintained through week 56 whether subjects were dosed every 4 or every 8 weeks with the interleukin blocker.
The incidence of oral candidiasis (9.5%-17.4% vs. 0% with adalimumab alone) was similar to other trials and likely because of the short circuiting of interleukin-17, which plays a role protecting against candida. Most cases were mild to moderate.
The increased risk of oral thrush with bimekizumab “may not be particularly clinically meaningful, especially if” it can be managed by an occasional fluconazole pill. It’s “reassuring … if that’s the biggest problem with the drug, or we may wonder if, in real life use, more severe, perhaps esophageal or systemic fungal infection may be observed,” Dr. Feldman said in a recent editorial.
“Not knowing the future may make some physicians reticent about using the drug when other options are available, at least until data are available on much larger numbers of exposed patients treated for longer periods of time,” he and his colleague William Huang, MD, also a dermatologist at Wake Forest, said.
One of the limits of the trial was that the head-to-head portion was only 24 weeks, “which was too brief for a comparison of safety between bimekizumab and adalimumab in a lifelong disease,” the investigators noted.
The mean age of the patients was 44.9 years, and the mean baseline PASI score was 19.8.
Although the initial dose of adalimumab in the study was 40 mg, labeling recommends an initial dose of 80 mg for the TNF blocker.
Bimekizumab is also being evaluated in phase 3 trials for psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa, according to UCB Pharma.
The study was funded by UCB Pharma. The investigators had numerous disclosures, including Dr. Warren who reported grants and personal fees from the company. Dr. Feldman reported receiving research, speaking, and/or consulting support from UCB Pharma and other companies.
A version of this article first appeared on Medscape.com.
The interleukin-17A and 17F blocker has also racked up significant wins against ustekinumab and secukinumab, other standard biologic options for adults with moderate to severe plaque psoriasis, and is currently under review for the indication by the U.S. Food and Drug Administration and European Medicines Agency.
In the adalimumab trial, dubbed BE SURE, bimekizumab had higher clinical response rates than the tumor necrosis factor (TNF) blocker over the 24-week head-to-head phase of the 478-patient trial, with substantial improvements in both Psoriasis Area and Severity Index (PASI) 90 response and Investigator’s Global Assessment (IGA) scores of 0 or 1, which signifies clear or almost clear skin.
The results were published in the New England Journal of Medicine and scheduled to be presented at the American Academy of Dermatology Virtual Meeting Experience on April 24.
“The data look good,” said psoriasis specialist Steven Feldman, MD, PhD, professor of dermatology at Wake Forest School of Medicine in Winston-Salem, N.C., when asked for comment.
Bimekizumab “appears more effective than current options. The big question is safety. The 10%-20% rate of oral candidiasis is much higher than other treatments but should be entirely manageable, as long as there are no unknown worse candida issues.” In addition, that there were no cases of inflammatory bowel disease in BE SURE “is very encouraging, as that is one of the limitations for existing IL-17 blockers,” he said.
The trial was launched after previous reports suggested that IL-17A inhibition may be better than TNF blockade in controlling psoriasis, said investigators led by Richard Warren, MBChB, PhD, a dermatology professor at the University of Manchester (England).
Patients were assigned evenly to one of three regimens: subcutaneous bimekizumab at a dose of 320 mg every 4 weeks for 56 weeks; bimekizumab at 320 mg every 4 weeks for 16 weeks, then every 8 weeks out to 56 weeks; or subcutaneous adalimumab at a dose of 40 mg every 2 weeks for 24 weeks, followed by bimekizumab at a dose of 320 mg every 4 weeks to week 56.
At week 16, 86.2% of those in the bimekizumab group but just 47.2% in the adalimumab group had a PASI 90 response (P < .001), and 85.3% of the bimekizumab versus 57.2% in the adalimumab group had an IGA score of 0 or 1 (P < .001).
About 52% of the adalimumab group had a PASI 90 response at week 24, when they were switched to bimekizumab. By week 56, their PASI 90 response rate rose to 81.8%. Skin clearance was maintained through week 56 whether subjects were dosed every 4 or every 8 weeks with the interleukin blocker.
The incidence of oral candidiasis (9.5%-17.4% vs. 0% with adalimumab alone) was similar to other trials and likely because of the short circuiting of interleukin-17, which plays a role protecting against candida. Most cases were mild to moderate.
The increased risk of oral thrush with bimekizumab “may not be particularly clinically meaningful, especially if” it can be managed by an occasional fluconazole pill. It’s “reassuring … if that’s the biggest problem with the drug, or we may wonder if, in real life use, more severe, perhaps esophageal or systemic fungal infection may be observed,” Dr. Feldman said in a recent editorial.
“Not knowing the future may make some physicians reticent about using the drug when other options are available, at least until data are available on much larger numbers of exposed patients treated for longer periods of time,” he and his colleague William Huang, MD, also a dermatologist at Wake Forest, said.
One of the limits of the trial was that the head-to-head portion was only 24 weeks, “which was too brief for a comparison of safety between bimekizumab and adalimumab in a lifelong disease,” the investigators noted.
The mean age of the patients was 44.9 years, and the mean baseline PASI score was 19.8.
Although the initial dose of adalimumab in the study was 40 mg, labeling recommends an initial dose of 80 mg for the TNF blocker.
Bimekizumab is also being evaluated in phase 3 trials for psoriatic arthritis, ankylosing spondylitis, nonradiographic axial spondyloarthritis, and hidradenitis suppurativa, according to UCB Pharma.
The study was funded by UCB Pharma. The investigators had numerous disclosures, including Dr. Warren who reported grants and personal fees from the company. Dr. Feldman reported receiving research, speaking, and/or consulting support from UCB Pharma and other companies.
A version of this article first appeared on Medscape.com.
Study aims to enhance understanding of ‘tremendously understudied’ prurigo nodularis
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
compared with age-matched controls, as well those with atopic dermatitis and psoriasis.
Those are key findings from a retrospective analysis of claims data that was published online April 3, 2021, in the Journal of Investigative Dermatology.
“Prurigo nodularis is a tremendously understudied inflammatory skin disease,” one of the study’s cosenior authors, Shawn G. Kwatra, MD, of the department of dermatology, Johns Hopkins University, Baltimore, said in an interview. “Prurigo nodularis patients have uncontrolled itch, which leads to reduced quality of life, and the association with many disease comorbidities. We focused on better understanding in this work the unique comorbidities of prurigo nodularis, compared to other inflammatory skin diseases.”
For the study, Dr. Kwatra, cosenior author Yevgeniy R. Semenov, MD, of the department of dermatology, Massachusetts General Hospital, Boston, and colleagues evaluated nationally representative, private insurance claims data from October 2015 to December 2019 to identify prurigo nodularis (PN) patients, who were defined as individuals with two or more medical claims for PN using ICD-10-CM codes. For comparison with patients with inflammatory skin diseases, they used the same claims data to identify patients with atopic dermatitis (AD) and psoriasis as well as to select controls who were age and gender matched to PN patients. Next, they quantified the overall comorbidity burden with the Charlson Comorbidity Index (CCI).
In 2016, the claims database included 2,658 patients with PN, 21,482 patients with AD, 21,073 patients with psoriasis, and 13,290 controls. The number of patients in each category rose each subsequent year, so that by the end of 2019 there were 9,426 patients with PN, 70,298 patients with AD, 59,509 patients with psoriasis, and 47,130 controls. Between 2016 and 2019 the mean age of PN patients increased from 57.5 to 59.8 years and the percent of male patients rose from 44.5% to 46.5%.
Between 2016 and 2019, the overall PN prevalence rates rose from 18 per 100,000 to 58 per 100,000, while the PN prevalence rates among adults increased from 22 per 100,000 to 70 per 100,000, and the rates among children rose grew from 2 per 100,000 to 7 per 100,000. “Our report shows an estimated disease prevalence of around 335,000 cases of PN in the United States,” said Dr. Kwatra, who was among a group of researchers to recently report on systemic Th22-polarized inflammation in PN patients.
The researchers also found that patients with PN had the highest mean CCI in both 2016 and 2019. In 2016, their mean CCI was 1.53, compared with 0.98 among controls, 0.53 among those with AD, and 1.16 among those with psoriasis. In 2019, the mean CCI had increased in all groups of patients, to 2.32 among those with PN, 1.57 among controls, 0.75 among those with AD patients, and 1.71 among those with psoriasis.
The top five medical specialties who cared for PN patients, defined as the estimated number of visits per year per patient, were internal medicine (2.01 visits), dermatology (1.87 visits), family practice (1.60 visits), cardiology or cardiovascular disease (0.85 visits), and orthopedics or orthopedic surgery (0.49 visits).
“If you encounter a patient with prurigo nodularis, it’s important to perform a screening for chronic kidney disease, diabetes, and liver disease,” Dr. Kwatra said. “These comorbidities along with emerging studies on circulating blood biomarkers suggest prurigo nodularis is a systemic inflammatory disorder; thus systemic agents are needed for most patients as part of multimodal therapy in prurigo nodularis.”
The researchers acknowledged certain limitations of the study, including its retrospective design and the identification of patients with PN with the ICD-10-CM code, which require further validation. “Furthermore, the increase in annual prevalence estimates for PN, AD, and psoriasis observed in the study could also be a result of increasing coding of these diagnoses in the claims data along with rising awareness by the medical profession,” they wrote.
Dr. Kwatra disclosed that he is an advisory board member/consultant for AbbVie, Galderma, Incyte, Pfizer, Regeneron, and Kiniksa Pharmaceuticals, and has received grant funding from Galderma, Pfizer, and Kiniksa. He has also received a Dermatology Foundation Medical Dermatology Career Development Award, a research grant from the Skin of Color Society, and is supported by the National Institutes of Health. One coauthor has been funded by NIH grants.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Secukinumab brings high PASI 75 results in 6- to 17-year-olds with psoriasis
at 24 weeks of follow-up in an ongoing 4-year phase 2 clinical trial, Adam Reich, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
Secukinumab (Cosentyx), a fully human monoclonal antibody that inhibits interleukin-17A, is widely approved for treatment of psoriasis in adults. In August 2020, the biologic received an expanded indication in Europe for treatment of 6- to 17-year-olds. Two phase 3 clinical trials are underway in an effort to gain a similar broadened indication in the United States to help address the high unmet need for new treatments for psoriasis in the pediatric population, said Dr. Reich, professor and head of the department of dermatology at the University of Rzeszow (Poland).
He reported on 84 pediatric patients participating in the open-label, phase 2, international study. They were randomized to one of two weight-based dosing regimens. Those in the low-dose arm received secukinumab dosed at 75 mg if they weighed less than 50 kg and 150 mg if they weighed more. In the high-dose arm, patients got secukinumab 75 mg if they weighed less than 25 kg, 150 mg if they weighed 25-50 kg, and 300 mg if they tipped the scales in excess of 50 kg.
The primary endpoint in the study was the week-12 rate of at least a 75% improvement from baseline in the Psoriasis Area and Severity Index score, or PASI 75. The rates were similar: 92.9% of patients in the high-dose arm achieved this endpoint, as did 90.5% in the low-dose arm. The PASI 90 rates were 83.3% and 78%, the PASI 100 rates were 61.9% and 54.8%, and clear or almost clear skin, as measured by the Investigator Global Assessment, was achieved in 88.7% of the high- and 85.7% of the low-dose groups. In addition,61.9% of those in the high-dose secukinumab group and 50% in the low-dose group had a score of 0 or 1 on the Children’s Dermatology Life Quality Index – indicating psoriasis has no impact on daily quality of life, he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
At week 24, roughly 95% of patients in both the low- and high-dose secukinumab groups had achieved PASI 75s, 88% reached a PASI 90 response, and 67% were at PASI 100. Nearly 60% of the low-dose and 70% of the high-dose groups had a score of 0 or 1 on the Children’s Dermatology Life Quality Index.
Treatment-emergent adverse event rates were similar in the two study arms. Of note, there was one case of new-onset inflammatory bowel disease in the high-dose group, and one case of vulvovaginal candidiasis as well.
Discussant Bruce E. Strober, MD, PhD, said that, if secukinumab gets a pediatric indication from the Food and Drug Administration, as seems likely, it won’t alter his biologic treatment hierarchy.
“I treat a lot of kids with psoriasis. We have three approved drugs now in etanercept [Enbrel], ustekinumab [Stelara], and ixekizumab [Taltz]. My bias is still towards ustekinumab because it’s infrequently dosed and that’s a huge issue for children. You want to expose them to as few injections as possible, for obvious reasons: It’s easier for parents and other caregivers,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn.
“The other issue is in IL-17 inhibition there has been a slight signal of inflammatory bowel disease popping up in children getting these drugs, and therefore you need to screen patients in this age group very carefully – not only the patients themselves, but their family – for IBD risk. If there is any sign of that I would move the IL-17 inhibitors to the back of the line, compared to ustekinumab and etanercept. Ustekinumab is still clearly the one that I think has to be used first line,” he said.
Dr. Strober offered a final word of advice for his colleagues: “You can’t be afraid to treat children with biologic therapies. In fact, preferentially I would use a biologic therapy over methotrexate or light therapy, which is really difficult for children.”
Dr. Reich and Dr. Strober reported receiving research grants from and serving as a consultant to numerous pharmaceutical companies, including Novartis, which markets secukinumab and funded the study.
MedscapeLIVE! and this news organization are owned by the same parent company.
at 24 weeks of follow-up in an ongoing 4-year phase 2 clinical trial, Adam Reich, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
Secukinumab (Cosentyx), a fully human monoclonal antibody that inhibits interleukin-17A, is widely approved for treatment of psoriasis in adults. In August 2020, the biologic received an expanded indication in Europe for treatment of 6- to 17-year-olds. Two phase 3 clinical trials are underway in an effort to gain a similar broadened indication in the United States to help address the high unmet need for new treatments for psoriasis in the pediatric population, said Dr. Reich, professor and head of the department of dermatology at the University of Rzeszow (Poland).
He reported on 84 pediatric patients participating in the open-label, phase 2, international study. They were randomized to one of two weight-based dosing regimens. Those in the low-dose arm received secukinumab dosed at 75 mg if they weighed less than 50 kg and 150 mg if they weighed more. In the high-dose arm, patients got secukinumab 75 mg if they weighed less than 25 kg, 150 mg if they weighed 25-50 kg, and 300 mg if they tipped the scales in excess of 50 kg.
The primary endpoint in the study was the week-12 rate of at least a 75% improvement from baseline in the Psoriasis Area and Severity Index score, or PASI 75. The rates were similar: 92.9% of patients in the high-dose arm achieved this endpoint, as did 90.5% in the low-dose arm. The PASI 90 rates were 83.3% and 78%, the PASI 100 rates were 61.9% and 54.8%, and clear or almost clear skin, as measured by the Investigator Global Assessment, was achieved in 88.7% of the high- and 85.7% of the low-dose groups. In addition,61.9% of those in the high-dose secukinumab group and 50% in the low-dose group had a score of 0 or 1 on the Children’s Dermatology Life Quality Index – indicating psoriasis has no impact on daily quality of life, he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
At week 24, roughly 95% of patients in both the low- and high-dose secukinumab groups had achieved PASI 75s, 88% reached a PASI 90 response, and 67% were at PASI 100. Nearly 60% of the low-dose and 70% of the high-dose groups had a score of 0 or 1 on the Children’s Dermatology Life Quality Index.
Treatment-emergent adverse event rates were similar in the two study arms. Of note, there was one case of new-onset inflammatory bowel disease in the high-dose group, and one case of vulvovaginal candidiasis as well.
Discussant Bruce E. Strober, MD, PhD, said that, if secukinumab gets a pediatric indication from the Food and Drug Administration, as seems likely, it won’t alter his biologic treatment hierarchy.
“I treat a lot of kids with psoriasis. We have three approved drugs now in etanercept [Enbrel], ustekinumab [Stelara], and ixekizumab [Taltz]. My bias is still towards ustekinumab because it’s infrequently dosed and that’s a huge issue for children. You want to expose them to as few injections as possible, for obvious reasons: It’s easier for parents and other caregivers,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn.
“The other issue is in IL-17 inhibition there has been a slight signal of inflammatory bowel disease popping up in children getting these drugs, and therefore you need to screen patients in this age group very carefully – not only the patients themselves, but their family – for IBD risk. If there is any sign of that I would move the IL-17 inhibitors to the back of the line, compared to ustekinumab and etanercept. Ustekinumab is still clearly the one that I think has to be used first line,” he said.
Dr. Strober offered a final word of advice for his colleagues: “You can’t be afraid to treat children with biologic therapies. In fact, preferentially I would use a biologic therapy over methotrexate or light therapy, which is really difficult for children.”
Dr. Reich and Dr. Strober reported receiving research grants from and serving as a consultant to numerous pharmaceutical companies, including Novartis, which markets secukinumab and funded the study.
MedscapeLIVE! and this news organization are owned by the same parent company.
at 24 weeks of follow-up in an ongoing 4-year phase 2 clinical trial, Adam Reich, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
Secukinumab (Cosentyx), a fully human monoclonal antibody that inhibits interleukin-17A, is widely approved for treatment of psoriasis in adults. In August 2020, the biologic received an expanded indication in Europe for treatment of 6- to 17-year-olds. Two phase 3 clinical trials are underway in an effort to gain a similar broadened indication in the United States to help address the high unmet need for new treatments for psoriasis in the pediatric population, said Dr. Reich, professor and head of the department of dermatology at the University of Rzeszow (Poland).
He reported on 84 pediatric patients participating in the open-label, phase 2, international study. They were randomized to one of two weight-based dosing regimens. Those in the low-dose arm received secukinumab dosed at 75 mg if they weighed less than 50 kg and 150 mg if they weighed more. In the high-dose arm, patients got secukinumab 75 mg if they weighed less than 25 kg, 150 mg if they weighed 25-50 kg, and 300 mg if they tipped the scales in excess of 50 kg.
The primary endpoint in the study was the week-12 rate of at least a 75% improvement from baseline in the Psoriasis Area and Severity Index score, or PASI 75. The rates were similar: 92.9% of patients in the high-dose arm achieved this endpoint, as did 90.5% in the low-dose arm. The PASI 90 rates were 83.3% and 78%, the PASI 100 rates were 61.9% and 54.8%, and clear or almost clear skin, as measured by the Investigator Global Assessment, was achieved in 88.7% of the high- and 85.7% of the low-dose groups. In addition,61.9% of those in the high-dose secukinumab group and 50% in the low-dose group had a score of 0 or 1 on the Children’s Dermatology Life Quality Index – indicating psoriasis has no impact on daily quality of life, he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
At week 24, roughly 95% of patients in both the low- and high-dose secukinumab groups had achieved PASI 75s, 88% reached a PASI 90 response, and 67% were at PASI 100. Nearly 60% of the low-dose and 70% of the high-dose groups had a score of 0 or 1 on the Children’s Dermatology Life Quality Index.
Treatment-emergent adverse event rates were similar in the two study arms. Of note, there was one case of new-onset inflammatory bowel disease in the high-dose group, and one case of vulvovaginal candidiasis as well.
Discussant Bruce E. Strober, MD, PhD, said that, if secukinumab gets a pediatric indication from the Food and Drug Administration, as seems likely, it won’t alter his biologic treatment hierarchy.
“I treat a lot of kids with psoriasis. We have three approved drugs now in etanercept [Enbrel], ustekinumab [Stelara], and ixekizumab [Taltz]. My bias is still towards ustekinumab because it’s infrequently dosed and that’s a huge issue for children. You want to expose them to as few injections as possible, for obvious reasons: It’s easier for parents and other caregivers,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn.
“The other issue is in IL-17 inhibition there has been a slight signal of inflammatory bowel disease popping up in children getting these drugs, and therefore you need to screen patients in this age group very carefully – not only the patients themselves, but their family – for IBD risk. If there is any sign of that I would move the IL-17 inhibitors to the back of the line, compared to ustekinumab and etanercept. Ustekinumab is still clearly the one that I think has to be used first line,” he said.
Dr. Strober offered a final word of advice for his colleagues: “You can’t be afraid to treat children with biologic therapies. In fact, preferentially I would use a biologic therapy over methotrexate or light therapy, which is really difficult for children.”
Dr. Reich and Dr. Strober reported receiving research grants from and serving as a consultant to numerous pharmaceutical companies, including Novartis, which markets secukinumab and funded the study.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Apremilast Uses and Relevance to the Military
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.
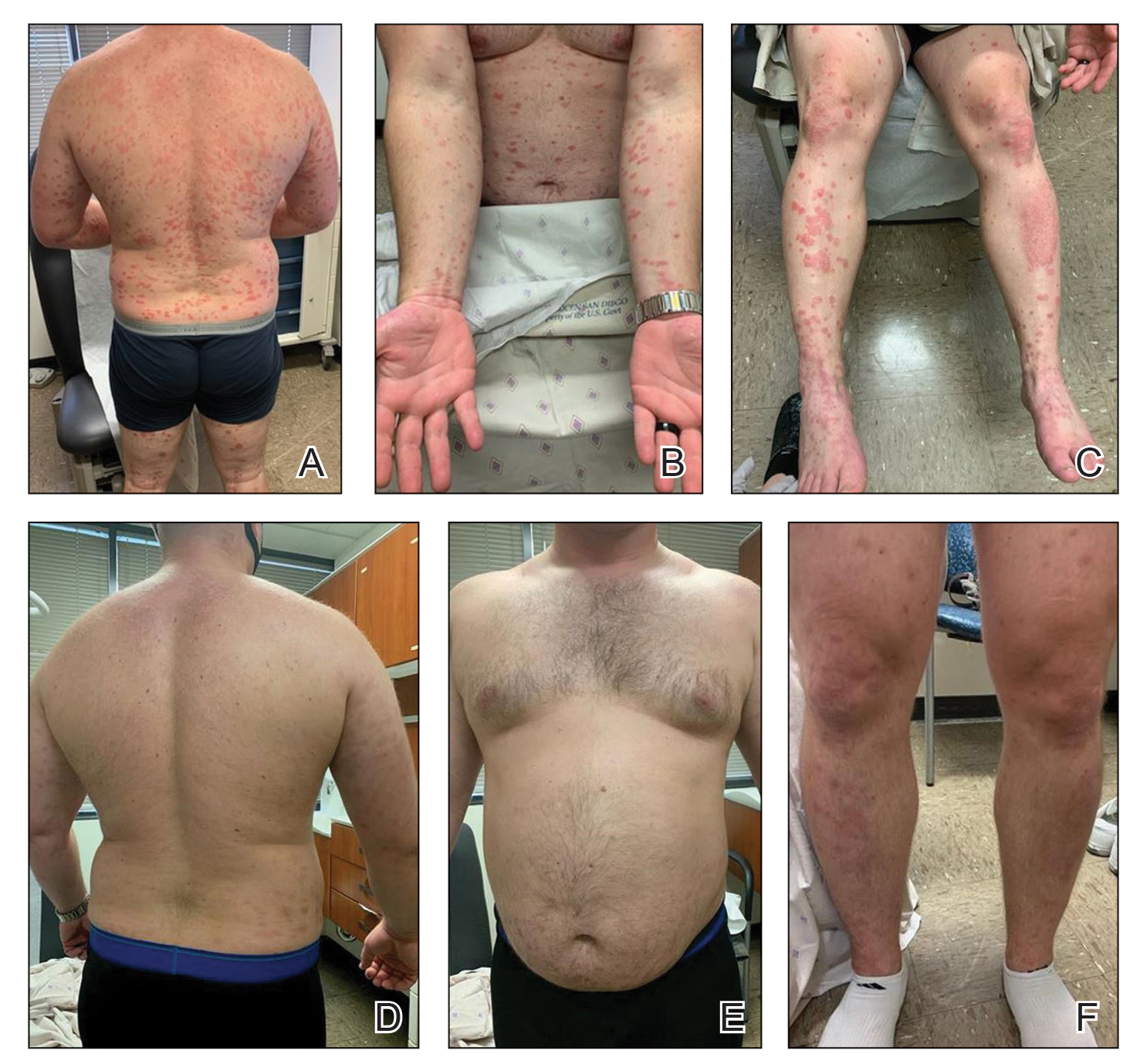
A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32
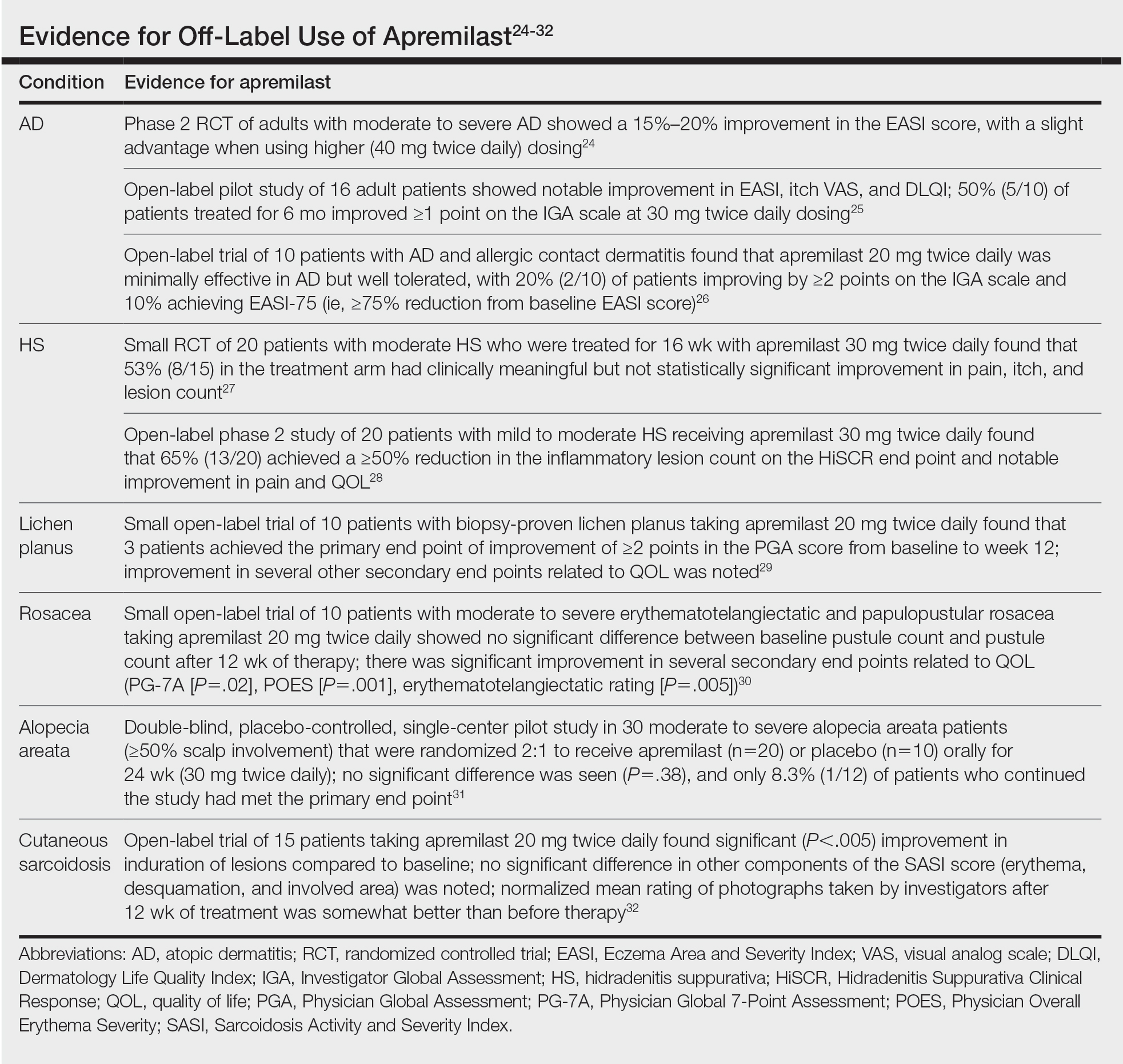
Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.

A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32

Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
Apremilast is a small-molecule biologic approved by the US Food and Drug Administration (FDA) for use in plaque psoriasis, psoriatic arthritis, and Behçet disease.1-6 Although apremilast is seemingly a less favorable choice for treating psoriasis in the era of injectable biologics, the drug is an important option for patients in the military. In recent months, apremilast also emerged as one of a few systemic medications recommended for the treatment of psoriasis and other dermatologic conditions during the COVID-19 pandemic.7
In this article, we review on-label indications and off-label uses for apremilast; highlight the importance of apremilast for managing psoriasis in the military population; and propose other patient populations in whom the use of apremilast is favorable. We also present a case report that highlights and embodies the benefit of apremilast for military service members.
CASE REPORT
A 28-year-old active-duty male US Navy service member developed extensive guttate psoriasis in a distribution too wide to manage with topical medication (Figure, A–C). His condition did not improve with a trial of oral antibiotics, and he reported itch that affected his sleep. He denied new joint pain, swelling, or deformity.

A review of the patient’s service history revealed that he was serving aboard a guided-missile cruiser ship for a tour extending an additional 2 years. Limited medical resources and lack of refrigeration made the use of injectable biologics, such as adalimumab, infeasible. Furthermore, the patient was too critical to the mission to be transported frequently off the ship to a higher level of care for injection of medication. He also had trouble returning for appointments and refills because of the high operational tempo of his command.
After discussion with the patient, oral apremilast was started at 30 mg/d and titrated up to the standard dosing of 30 mg twice daily, with excellent results by 3 months after he started therapy (Figure, D–F).
COMMENT
We reviewed the research on apremilast for its approved indications, including psoriasis; its off-label uses; and strategies for using the drug to treat psoriasis and other dermatologic conditions in military populations. The most recent evidence regarding the use of apremilast in dermatology, rheumatology, and other medical specialties was assessed using published English-language research data and review articles. We conducted a PubMed search of articles indexed for MEDLINE using the following terms: apremilast, Otezla, psoriasis, psoriatic arthritis, arthritis, off-label, Behçet’s, hidradenitis suppurativa, military, and armed forces. We also reviewed citations within relevant articles to identify additional relevant sources.
Off-label uses reviewed here are based on data from randomized controlled trials, large open-label trials, and large prospective case series. Articles with less evidence are not included in this review.
On-Label Usage Profile
Apremilast is an orally administered, small-molecule inhibitor of phosphodiesterase 4. Small-molecule inhibitors are a class of medications with low molecular weight, high stability, and short half-life. They act intracellularly to modulate proinflammatory states through regulation of the proinflammatory cytokine milieu.
Apremilast has been approved by the FDA for use in adult psoriasis and psoriatic arthritis since 2014 and for use in treating oral ulcers of Behçet disease since 2019.1-3,5,6 Recently, a phase 2, multicenter, open-label study on the use of apremilast in pediatric psoriasis patients (aged 12–17 years) demonstrated a similar safety profile with weight-based dosing8; phase 3 trials in this population are in the recruitment phase (ClinicalTrials.gov Identifier NCT03701763).
Because information regarding its use in pregnancy is limited, apremilast is not recommended in this population. It is unknown whether apremilast is present in breast milk; although the manufacturer does not make explicit recommendations regarding use during breastfeeding, an expert panel reviewing management of psoriasis in pregnant and breastfeeding women recommended avoiding its use while breastfeeding.9
Common Adverse Effects
Common adverse effects (AEs) include weight loss (>5% total body weight in 5% of patients; 5%–10% of total body weight in 10%–12% of patients; and ≥10% total body weight in 2% of patients), diarrhea and nausea, headache, and upper respiratory tract infection.10,11 Gastrointestinal AEs tend to be self-limited and improve or resolve after the first few weeks of therapy. Caution is advised in patients older than 65 years and in those at risk for hypotension or volume depletion. Although depressed mood is a rare AE (<1%), apremilast should be used cautiously in patients with a history of depression or suicidal ideation. Weight loss generally is self-limited; routine monitoring of weight is recommended.11
Apremilast in Psoriasis and Psoriatic Arthritis
Psoriasis
The ESTEEM trials established the safety and efficacy of apremilast for use in psoriasis.2,3 In a phase 3, multicenter, double-blind, placebo-controlled trial of 844 patients, apremilast demonstrated a statistically significant 75% or greater reduction from the baseline psoriasis area and severity index score (PASI-75) in 33.1% of patients receiving the medication compared to 5.3% of those receiving placebo.2 Data from real-world practice (outside constraints of clinical trials) suggest slightly greater efficacy than was demonstrated in the ESTEEM trials.
A recently published retrospective, cross-sectional study of 480 patients with psoriasis treated with apremilast reported that 48.6% of patients continuing therapy for a mean (SD) of 6 (1) months achieved PASI-75. Furthermore, the mean dermatology life quality index (DLQI) score of the surveyed population decreased from 13.4 at initiation of treatment to 5.7 at 6 (1) months of treatment—a marked improvement in quality of life.12 Other single-center and smaller study populations also have suggested increased real-world benefit.13,14
Nonetheless, the rate and degree of clearance of plaques with apremilast seem to lag behind what is observed with many of the biologics and traditional medications employed to treat psoriasis.15-19 Furthermore, indirect cost analysis comparisons suggest a much higher cost per level of PASI for apremilast compared to several biologics and to methotrexate.20,21 A study that used indirect methods of comparison to analyze the comparative cost and efficacy of apremilast and methotrexate found no evidence of greater efficacy for apremilast and that the incremental cost to achieve 1 additional PASI-75 responder by using apremilast is $187,888 annually.21
Psoriatic Arthritis
The PALACE clinical trials 1, 2, and 3 assessed the efficacy of apremilast in patients who had prior treatment with conventional disease-modifying antirheumatic drugs or biologics, or both. PALACE 4 evaluated efficacy in treatment-naïve patients; standard dosing of apremilast was found to produce improvement in psoriatic arthritis in treatment-naïve and non–treatment-naïve patients.4-6,22 In the 24-week placebo-controlled phase of the PALACE 1 trial, the American College of Rheumatology (ACR) baseline composite measurement of 20% disease improvement, or ACR20, was achieved in 40% of patients randomized to the standard dosing regimen compared to 19% of patients receiving placebo, a statistically significant result (P<.001).22
Evaluation of long-term study data is beyond the scope of this review, but those data suggest that disease outcomes continue to improve the longer therapy is utilized, with a greater percentage of patients achieving ACR20 as well as ACR50 (50% improvement) and ACR70 (70% improvement) responses. Indirect comparisons analyzing the cost and effectiveness for adalimumab, apremilast, and methotrexate in patients with psoriatic arthritis found that apremilast was less effective than adalimumab and as efficacious as methotrexate, though apremilast carries the highest price tag of these drugs.23
Off-Label Uses
Ease of oral administration and a favorable safety profile have prompted off-label study of apremilast in other inflammatory skin diseases, including atopic dermatitis, hidradenitis suppurativa, lichen planus, rosacea, alopecia areata, and cutaneous sarcoidosis. Publications with a minimum case series of 10 patients are included in the Table.24-32

Use in the Military and Beyond
Psoriasis and other inflammatory skin conditions are common in the military and can greatly hinder a service member’s ability to perform their duties and remain ready to deploy. A history of psoriasis is disqualifying for military recruits, but early entry into service, misdiagnosis, and low or no burden of disease at time of entry into the service all contribute to a substantial population of active-duty service members who require treatment of psoriasis.33 Necessity dictates that treatment of this condition extend to theater operations; from 2008 to 2015, more than 3600 soldiers sought care for psoriasis while deployed to a combat theater.34
In some cases, poorly controlled inflammatory skin conditions lead to medical separation.33 Although there are limited data on the use of apremilast in the military, its use during deployment for the treatment of psoriasis and psoriatic arthritis has been reported, with the great majority of service members retaining their deployable status even 1 year after the study period.35
The ideal medication for deployable military personnel should have low toxicity, simple storage, and minimal monitoring requirements, and it should not expose a service member to increased risk while in a combat theater. Worldwide deployability is a requirement for most military occupations. The risk for immunosuppression with targeted immune therapy must be fully weighed, as certain duty stations and deployments might increase the risk for exposure to Mycobacterium tuberculosis, endemic mycopathogens, hepatitis C virus, HIV, Leishmania, and Strongyloides.34
Furthermore, the tumor necrosis factor α inhibitors and IL-17 and IL-23 blockers used to treat psoriasis all require refrigeration; often, this requirement cannot be met in austere overseas settings. Additional requirements for laboratory monitoring, titration of medications, and frequent office visits might prohibit a service member from performing their duties, which, in turn, is detrimental to military readiness and the career of that service member.
Last, the Centers for Disease Control and Prevention recommend avoiding live virus vaccination while taking targeted immune therapy because of safety and effectiveness concerns during immunosuppression.36 This recommendation might disqualify military personnel from deployment to certain locations that require the protection that such vaccines afford. Therefore, apremilast is an ideal option for the military patient population, with many military-specific advantages.
Of course, the military is not the only population in whom ease of use and storage and simplified monitoring parameters are essential. Benefits of apremilast also may translate to patients who are placed in austere conditions or who participate in extended worldwide travel for work or leisure, such as government contractors who deploy in support of military operations, firefighters or national park employees who spend extended periods in resource-limited settings, and foreign-aid workers and diplomats who are engaged in frequent travel around the world. Furthermore, travel to certain regions might increase the risk for exposure to atypical pathogens as well as the desire for a therapeutic option that does not have potential to suppress the immune system. This subset of psoriasis patients might be better treated with novel agents such as apremilast than other drugs that would be the presumed standard of care in a domestic setting.
Final Thoughts
The benefits of apremilast translate to all patients in austere environments with limited resources and during times when immune function is of utmost concern. For military service members and many civilians in austere environments worldwide, apremilast could be considered a first-line systemic agent for psoriasis and psoriatic arthritis. In patients unable to use or tolerate other treatments, apremilast can be considered for off-label therapy (Table24-32). There are times when the approach to prescribing must look beyond primary efficacy, AE profile, and cost—to include occupation, environment, or duties—to select the optimal medication for a patient.
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
- Hatemi G, Melikoglu M, Tunc R, et al. Apremilast for Behçet’s syndrome—a phase 2, placebo-controlled study. N Engl J Med. 2015;372:1510-1518. doi:10.1056/NEJMoa1408684
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j.jaad.2015.03.049
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399. doi:10.1111/bjd.14164
- Cutolo M, Myerson GE, Fleischmann RM, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43:1724-1734. doi:10.3899/jrheum.151376
- Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75:1065-1073. doi:10.1136/annrheumdis-2015-207963
- Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57:1253-1263. doi:10.1093/rheumatology/key032
- Niaki OZ, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: a guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83:1150-1159. doi:10.1016/j.jaad.2020.06.051
- Paller AS, Hong Y, Becker EM, et al. Pharmacokinetics and safety of apremilast in pediatric patients with moderate to severe plaque psoriasis: results from a phase 2 open-label study. J Am Acad Dermatol. 2020;82:389-397. doi:10.1016/j.jaad.2019.08.019
- Rademaker M, Agnew K, Andrews M, et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas J Dermatol. 2018;59:86-100. doi:10.1111/ajd.12641
- Otezla. Prescribing information. Amgen Inc; June 2020. Accessed March 13, 2021. www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/otezla/otezla_pi_english.ashx
- Otezla. Product monograph. Amgen Canada Inc; Revised August 2020. Accessed March 13, 2021. www.amgen.ca/products/~/media/FB841218E06B4508B0E7213BC578E641.ashx
- Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: Results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2020;35:123-134. doi:10.1111/jdv.16431
- Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1173-1179. doi:10.1111/jdv.14832
- Wong TH, Sinclair S, Smith B, et al. Real‐world, single‐centre experience of apremilast for the treatment of moderate to severe psoriasis. Clin Exp Dermatol. 2017;42:675-676. doi:10.1111/ced.13150
- Saurat, J‐H, Stingl G, Dubertret L, et al; . Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158:558-566. doi:10.1111/j.1365-2133.2007.08315.x
- Kimball AB, Papp KA, Wasfi Y, et al; Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545. doi:10.1111/jdv.12046
- Langley, RG, Elewski BE, Lebwohl M, et al; ; Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338. doi:10.1056/NEJMoa1314258
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328. doi:10.1056/NEJMoa1503824
- Papp KA, Leonaridi CL, Blauvelt A, et al. Ixekizumab treatment for psoriasis: integrated efficacy analysis of three double‐blinded, controlled studies (UNCOVER‐1, UNCOVER‐2, UNCOVER‐3). Br J Dermatol. 2018;178:674-681. doi:10.1111/bjd.16050
- Kromer C, Celis D, Sonntag D, et al. Biologicals and small molecules in psoriasis: a systematic review of economic evaluations. PloS One. 2018;13:e0189765. doi:10.1371/journal.pone.0189765
- Armstrong AW, Betts KA, Sundaram M, et al. Comparative efficacy and incremental cost per responder of methotrexate versus apremilast for methotrexate-naïve patients with psoriasis. J Am Acad Dermatol. 2016;75:740-746. doi:10.1016/j.jaad.2016.05.040
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026. doi:10.1136/annrheumdis-2013-205056
- Betts KA, Griffith J, Friedman A, et al. An indirect comparison and cost per responder analysis of adalimumab, methotrexate and apremilast in the treatment of methotrexate-naïve patients with psoriatic arthritis. Curr Med Res Opin. 2016;32:721-729. doi:10.1185/03007995.2016.114002624. Simpson EL, Imafuku S, Poulin Y, et al. A phase 2 randomized trial of apremilast in patients with atopic dermatitis. J Invest Dermatol. 2019;139:1063-1072. doi:10.1016/j.jid.2018.10.043
- Samrao A, Berry TM, Goreshi R, et al. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148:890-897. doi:10.1001/archdermatol.2012.812
- Volf EM, Au S-C, Dumont N, et al. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11:341-346.
- Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol. 2019;80:80-88. doi:10.1016/j.jaad.2018.06.046
- Kerdel FR, Azevedo FA, Don CK, et al. Apremilast for the treatment of mild-to-moderate hidradenitis suppurativa in a prospective, open-label, phase 2 study. J Drugs Dermatol. 2019;18:170-176.
- Paul J, Foss CE, Hirano SA, et al. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68:255-261. doi:10.1016/j.jaad.2012.07.014
- Thompson BJ, Furniss M, Zhao W, et al. An oral phosphodiesterase inhibitor (apremilast) for inflammatory rosacea in adults: a pilot study. JAMA Dermatol. 2014;150:1013-1014. doi:10.1001/jamadermatol.2013.10526
- Mikhaylov D, Pavel A, Yao C, et al. A randomized placebo-controlled single-center pilot study of the safety and efficacy of apremilast in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res. 2019;311(1):29-36. doi:10.1007/s00403-018-1876-y
- Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262-264. doi:10.1001/archdermatol.2011.301
- Navy Medicine, US Navy. Manual of the Medical Department (MANMED), NAVMED P-117. Chapter 15. Updated October 20, 2020. Accessed March 13, 2021. https://www.med.navy.mil/directives/Pages/NAVMEDP-MANMED.aspx
- Rosenberg A, Meyerle J. The use of apremilast to treat psoriasis during deployment. Mil Med. 2017;182:1628-1631. doi:10.7205/MILMED-D-17-00047
- Price AD, Wagler VD, Donaldson C, et al. The effects of apremilast therapy on deployability in active duty US Army soldiers with plaque psoriasis and psoriatic arthritis [published online October 30, 2020]. J Clin Rheumatol. doi:10.1097/RHU.0000000000001601
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Hamborsky J, Kroger A, Wolfe S, eds. 13th ed. Washington D.C. Public Health Foundation, 2015. Accessed March 25,2021; https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/table-of-contents.pdf
Practice Points
- Apremilast is a versatile and easy-to-use therapeutic option for treatment of psoriasis and psoriatic arthritis.
- Ease of transport and storage as well as lack of necessary laboratory monitoring have made apremilast a compelling treatment option for psoriasis and psoriatic arthritis in military populations with high operational tempos.
- Dermatologists should consider apremilast for treatment in populations that work for prolonged periods in austere or resource-limited environments.
Quantifying Itch: Measurement on the Way to Management
Itch is one of the most protean manifestations of skin disease and can take a substantial physical and emotional toll on patients. For physicians, it is a frequent—if often dreaded—patient concern with a rising incidence. Lack of specific itch therapies as well as associations with multiple dermatologic conditions, including xerosis, psoriasis, atopic dermatitis, cutaneous lymphoma, contact dermatitis, and internal malignancies, make management of these itchy patients challenging and deserving of our attention. Studies evaluating patients with chronic pruritus identified a considerable impact on health-related quality of life, including development of depression, inability to perform activities of daily living, and sleep difficulties. 1
How to Classify Itching
Itch, or pruritus, originally was defined as an unpleasant sensation that provokes the desire to scratch,2 but this definition likely limits our ability to assess itch.
Pain is another complex subjective symptom but is one that has been better studied. A previous intensity theory postulated that itch is a form of pain: low-intensity noxious stimuli are perceived as itch, while high-intensity stimuli are perceived as pain. Over time, our understanding of itch evolved, and it became clear that a specific neuronal pathway for itch also exists.3 However, the pathophysiology of itch and pain remain intertwined. Scratching may elicit pain, providing a change in sensation that replaces the itch, whereas opioid analgesics suppress pain but may worsen the itch.
We are gaining a better understanding of the biology and classification of itch, which will hopefully enable the development of new measures to accurately assess itch. Four main categories of itch currently exist: neurogenic, psychogenic, neuropathic, and pruritoceptive.4 Patients may have one or multiple types of itch, which can be differentiated clinically and biochemically. Neurogenic (also known as systemic) itch is transmitted via the central nervous system with possible involvement of itch-specific neurons in the spinal cord and encompasses itch associated with pruritus from other organ systems. As the term implies, psychogenic itch is associated with psychiatric disorders. Neuropathic itch is generated from the inappropriate firing of peripheral or central sensory neurons in the absence of pruritogenic stimuli, which can be seen in notalgia paresthetica, brachioradial pruritus, and postherpetic neuralgia. Pruritoceptive itch most commonly is encountered in dermatology and is associated with skin inflammation or other dermatoses.4
How to Assess Itch Quantitatively
There currently are 2 major questions about quantitative assessments of itch. First, how do we measure itch in studies that are designed to relieve a different skin disease that is associated with itch? Most clinical trials investigating therapeutic options for atopic dermatitis and psoriasis now include itch assessment and improvement as a secondary outcome. Second, how do we measure itch in studies that are designed with relief of itch as the primary end point? Both of these scenarios require a fundamental set of decisions. Itch clearly is a subjective experience, but it also is one that can be local, regional, generalized, or transitory. Just as with pain, an individual can be distracted from their itch to some extent and consequently experience it more acutely when there are fewer stimuli in their environment. Classically, patients will report that itching is worse at night, preventing them from sleeping. Sleep disruption previously has been demonstrated.5 Of course, the environment also can exacerbate itch, as dry air and in some cases humidity can flare the sensation.
Fundamentally, therefore, the questions that are asked to assess itch are incredibly relevant, and there is a matrix of possible avenues of inquiry. Should you measure the peak itch in one area or the peak itch overall? Is the duration, the frequency, or the persistence of the itching most relevant? What is the correct time frame in which to do an assessment: the last 24 hours, the last 48 hours, or the last week? Because these parameters have been so challenging, most investigators have used a visual analog scale, similar to what is used to assess pain, at a 24-hour interval to decrease recall bias. The most commonly employed tool is the itch numeric rating scale (NRS), which asks patients to rate their symptoms on a scale of 0 (no itch) to 10 (worst imaginable itch). Although the psychometric properties of the itch NRS have been validated, debate still exists as to whether the itch NRS is best administered at a specific time of day or if it should be updated to evaluate peak pruritus scores explicitly. Regardless, implementing these scales often is time consuming and burdensome in the clinical trial setting, as participants are asked to complete daily diaries at the same time each day using either paper forms or electronic tablets.
Once scores are collected, we then need to quantitate a meaningful difference in itch. For pain, there has been some acceptance of a 30% difference, or a 2-point reduction, as being clinically meaningful; however, there was substantial debate at the time of the approval of ixekizumab as to whether that was a similarly appropriate threshold for itch. Using data from ixekizumab phase 2 and phase 3 trials, a 4-point reduction in itch NRS was found to be optimal for evaluating clinically significant changes in moderate to severe psoriasis.6 A more recent study of the validity of the itch NRS in prurigo nodularis suggested a 1-point change was correlated with minimal clinical improvement.7 Thus, the interesting question of how assessment of itch varies across clinical trials and disease states needs to be raised. Psoriasis classically has been thought of as not particularly itchy, and atopic dermatitis and prurigo nodularis have been regarded as extraordinarily itchy, yet one study comparing baseline itch scores in psoriasis and atopic dermatitis suggested that the experience actually is somewhat similar.8
Final Thoughts
The subjective nature of itch makes NRSs our best option at this time, but the best disease severity assessment tools are objective, sensitive, and generalizable. Unfortunately, we do not have such tools available to us yet, but technology—smart devices to monitor nocturnal scratching and machine learning algorithms that use electromagnetic impact to capture motion associated with itching and scratching9—may offer new objective measures for itch that can be used to further validate the current itch NRS. Even if these technology-based approaches become the standard of measurement, they will certainly help us understand what we are measuring. And even better, the focus on how to develop meaningful end points around the improvement of itch will likely lead us to measure it more and drive the development of therapeutics that address the effect and consequences of this pernicious problem.
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153-1156. doi:10.1001/archdermatol.2011.178
- Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 pt 1):268-269. doi:10.1016/s0190-9622(98)70087-8
- Ikoma A, Rukwied R, Ständer S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475-1478. doi:10.1001/archderm.139.11.1475
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84-91. doi:10.1111/dth.12025
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161. doi:10.1016/j.jaad.2016.07.034
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157-162. doi:10.1111/bjd.14464
- Kimel M, Zeidler C, Kwon P, et al. validation of psychometric properties of the itch numeric rating scale for pruritus associated with prurigo nodularis: a secondary analysis of a randomized clinical trial. JAMA Dermatol. 2020;156:1354-1358. doi:10.1001/jamadermatol.2020.3071
- Shahwan KT, Kimball AB. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: a meta-analysis. J Am Acad Dermatol. 2017;76:1198.el-1200.e1. doi:10.1016/j.jaad.2017.02.002
- Smith MP, Ly K, Thibodeaux Q, et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb). 2019;9:407-420. doi:10.1007/s13555-019-0312-3
Itch is one of the most protean manifestations of skin disease and can take a substantial physical and emotional toll on patients. For physicians, it is a frequent—if often dreaded—patient concern with a rising incidence. Lack of specific itch therapies as well as associations with multiple dermatologic conditions, including xerosis, psoriasis, atopic dermatitis, cutaneous lymphoma, contact dermatitis, and internal malignancies, make management of these itchy patients challenging and deserving of our attention. Studies evaluating patients with chronic pruritus identified a considerable impact on health-related quality of life, including development of depression, inability to perform activities of daily living, and sleep difficulties. 1
How to Classify Itching
Itch, or pruritus, originally was defined as an unpleasant sensation that provokes the desire to scratch,2 but this definition likely limits our ability to assess itch.
Pain is another complex subjective symptom but is one that has been better studied. A previous intensity theory postulated that itch is a form of pain: low-intensity noxious stimuli are perceived as itch, while high-intensity stimuli are perceived as pain. Over time, our understanding of itch evolved, and it became clear that a specific neuronal pathway for itch also exists.3 However, the pathophysiology of itch and pain remain intertwined. Scratching may elicit pain, providing a change in sensation that replaces the itch, whereas opioid analgesics suppress pain but may worsen the itch.
We are gaining a better understanding of the biology and classification of itch, which will hopefully enable the development of new measures to accurately assess itch. Four main categories of itch currently exist: neurogenic, psychogenic, neuropathic, and pruritoceptive.4 Patients may have one or multiple types of itch, which can be differentiated clinically and biochemically. Neurogenic (also known as systemic) itch is transmitted via the central nervous system with possible involvement of itch-specific neurons in the spinal cord and encompasses itch associated with pruritus from other organ systems. As the term implies, psychogenic itch is associated with psychiatric disorders. Neuropathic itch is generated from the inappropriate firing of peripheral or central sensory neurons in the absence of pruritogenic stimuli, which can be seen in notalgia paresthetica, brachioradial pruritus, and postherpetic neuralgia. Pruritoceptive itch most commonly is encountered in dermatology and is associated with skin inflammation or other dermatoses.4
How to Assess Itch Quantitatively
There currently are 2 major questions about quantitative assessments of itch. First, how do we measure itch in studies that are designed to relieve a different skin disease that is associated with itch? Most clinical trials investigating therapeutic options for atopic dermatitis and psoriasis now include itch assessment and improvement as a secondary outcome. Second, how do we measure itch in studies that are designed with relief of itch as the primary end point? Both of these scenarios require a fundamental set of decisions. Itch clearly is a subjective experience, but it also is one that can be local, regional, generalized, or transitory. Just as with pain, an individual can be distracted from their itch to some extent and consequently experience it more acutely when there are fewer stimuli in their environment. Classically, patients will report that itching is worse at night, preventing them from sleeping. Sleep disruption previously has been demonstrated.5 Of course, the environment also can exacerbate itch, as dry air and in some cases humidity can flare the sensation.
Fundamentally, therefore, the questions that are asked to assess itch are incredibly relevant, and there is a matrix of possible avenues of inquiry. Should you measure the peak itch in one area or the peak itch overall? Is the duration, the frequency, or the persistence of the itching most relevant? What is the correct time frame in which to do an assessment: the last 24 hours, the last 48 hours, or the last week? Because these parameters have been so challenging, most investigators have used a visual analog scale, similar to what is used to assess pain, at a 24-hour interval to decrease recall bias. The most commonly employed tool is the itch numeric rating scale (NRS), which asks patients to rate their symptoms on a scale of 0 (no itch) to 10 (worst imaginable itch). Although the psychometric properties of the itch NRS have been validated, debate still exists as to whether the itch NRS is best administered at a specific time of day or if it should be updated to evaluate peak pruritus scores explicitly. Regardless, implementing these scales often is time consuming and burdensome in the clinical trial setting, as participants are asked to complete daily diaries at the same time each day using either paper forms or electronic tablets.
Once scores are collected, we then need to quantitate a meaningful difference in itch. For pain, there has been some acceptance of a 30% difference, or a 2-point reduction, as being clinically meaningful; however, there was substantial debate at the time of the approval of ixekizumab as to whether that was a similarly appropriate threshold for itch. Using data from ixekizumab phase 2 and phase 3 trials, a 4-point reduction in itch NRS was found to be optimal for evaluating clinically significant changes in moderate to severe psoriasis.6 A more recent study of the validity of the itch NRS in prurigo nodularis suggested a 1-point change was correlated with minimal clinical improvement.7 Thus, the interesting question of how assessment of itch varies across clinical trials and disease states needs to be raised. Psoriasis classically has been thought of as not particularly itchy, and atopic dermatitis and prurigo nodularis have been regarded as extraordinarily itchy, yet one study comparing baseline itch scores in psoriasis and atopic dermatitis suggested that the experience actually is somewhat similar.8
Final Thoughts
The subjective nature of itch makes NRSs our best option at this time, but the best disease severity assessment tools are objective, sensitive, and generalizable. Unfortunately, we do not have such tools available to us yet, but technology—smart devices to monitor nocturnal scratching and machine learning algorithms that use electromagnetic impact to capture motion associated with itching and scratching9—may offer new objective measures for itch that can be used to further validate the current itch NRS. Even if these technology-based approaches become the standard of measurement, they will certainly help us understand what we are measuring. And even better, the focus on how to develop meaningful end points around the improvement of itch will likely lead us to measure it more and drive the development of therapeutics that address the effect and consequences of this pernicious problem.
Itch is one of the most protean manifestations of skin disease and can take a substantial physical and emotional toll on patients. For physicians, it is a frequent—if often dreaded—patient concern with a rising incidence. Lack of specific itch therapies as well as associations with multiple dermatologic conditions, including xerosis, psoriasis, atopic dermatitis, cutaneous lymphoma, contact dermatitis, and internal malignancies, make management of these itchy patients challenging and deserving of our attention. Studies evaluating patients with chronic pruritus identified a considerable impact on health-related quality of life, including development of depression, inability to perform activities of daily living, and sleep difficulties. 1
How to Classify Itching
Itch, or pruritus, originally was defined as an unpleasant sensation that provokes the desire to scratch,2 but this definition likely limits our ability to assess itch.
Pain is another complex subjective symptom but is one that has been better studied. A previous intensity theory postulated that itch is a form of pain: low-intensity noxious stimuli are perceived as itch, while high-intensity stimuli are perceived as pain. Over time, our understanding of itch evolved, and it became clear that a specific neuronal pathway for itch also exists.3 However, the pathophysiology of itch and pain remain intertwined. Scratching may elicit pain, providing a change in sensation that replaces the itch, whereas opioid analgesics suppress pain but may worsen the itch.
We are gaining a better understanding of the biology and classification of itch, which will hopefully enable the development of new measures to accurately assess itch. Four main categories of itch currently exist: neurogenic, psychogenic, neuropathic, and pruritoceptive.4 Patients may have one or multiple types of itch, which can be differentiated clinically and biochemically. Neurogenic (also known as systemic) itch is transmitted via the central nervous system with possible involvement of itch-specific neurons in the spinal cord and encompasses itch associated with pruritus from other organ systems. As the term implies, psychogenic itch is associated with psychiatric disorders. Neuropathic itch is generated from the inappropriate firing of peripheral or central sensory neurons in the absence of pruritogenic stimuli, which can be seen in notalgia paresthetica, brachioradial pruritus, and postherpetic neuralgia. Pruritoceptive itch most commonly is encountered in dermatology and is associated with skin inflammation or other dermatoses.4
How to Assess Itch Quantitatively
There currently are 2 major questions about quantitative assessments of itch. First, how do we measure itch in studies that are designed to relieve a different skin disease that is associated with itch? Most clinical trials investigating therapeutic options for atopic dermatitis and psoriasis now include itch assessment and improvement as a secondary outcome. Second, how do we measure itch in studies that are designed with relief of itch as the primary end point? Both of these scenarios require a fundamental set of decisions. Itch clearly is a subjective experience, but it also is one that can be local, regional, generalized, or transitory. Just as with pain, an individual can be distracted from their itch to some extent and consequently experience it more acutely when there are fewer stimuli in their environment. Classically, patients will report that itching is worse at night, preventing them from sleeping. Sleep disruption previously has been demonstrated.5 Of course, the environment also can exacerbate itch, as dry air and in some cases humidity can flare the sensation.
Fundamentally, therefore, the questions that are asked to assess itch are incredibly relevant, and there is a matrix of possible avenues of inquiry. Should you measure the peak itch in one area or the peak itch overall? Is the duration, the frequency, or the persistence of the itching most relevant? What is the correct time frame in which to do an assessment: the last 24 hours, the last 48 hours, or the last week? Because these parameters have been so challenging, most investigators have used a visual analog scale, similar to what is used to assess pain, at a 24-hour interval to decrease recall bias. The most commonly employed tool is the itch numeric rating scale (NRS), which asks patients to rate their symptoms on a scale of 0 (no itch) to 10 (worst imaginable itch). Although the psychometric properties of the itch NRS have been validated, debate still exists as to whether the itch NRS is best administered at a specific time of day or if it should be updated to evaluate peak pruritus scores explicitly. Regardless, implementing these scales often is time consuming and burdensome in the clinical trial setting, as participants are asked to complete daily diaries at the same time each day using either paper forms or electronic tablets.
Once scores are collected, we then need to quantitate a meaningful difference in itch. For pain, there has been some acceptance of a 30% difference, or a 2-point reduction, as being clinically meaningful; however, there was substantial debate at the time of the approval of ixekizumab as to whether that was a similarly appropriate threshold for itch. Using data from ixekizumab phase 2 and phase 3 trials, a 4-point reduction in itch NRS was found to be optimal for evaluating clinically significant changes in moderate to severe psoriasis.6 A more recent study of the validity of the itch NRS in prurigo nodularis suggested a 1-point change was correlated with minimal clinical improvement.7 Thus, the interesting question of how assessment of itch varies across clinical trials and disease states needs to be raised. Psoriasis classically has been thought of as not particularly itchy, and atopic dermatitis and prurigo nodularis have been regarded as extraordinarily itchy, yet one study comparing baseline itch scores in psoriasis and atopic dermatitis suggested that the experience actually is somewhat similar.8
Final Thoughts
The subjective nature of itch makes NRSs our best option at this time, but the best disease severity assessment tools are objective, sensitive, and generalizable. Unfortunately, we do not have such tools available to us yet, but technology—smart devices to monitor nocturnal scratching and machine learning algorithms that use electromagnetic impact to capture motion associated with itching and scratching9—may offer new objective measures for itch that can be used to further validate the current itch NRS. Even if these technology-based approaches become the standard of measurement, they will certainly help us understand what we are measuring. And even better, the focus on how to develop meaningful end points around the improvement of itch will likely lead us to measure it more and drive the development of therapeutics that address the effect and consequences of this pernicious problem.
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153-1156. doi:10.1001/archdermatol.2011.178
- Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 pt 1):268-269. doi:10.1016/s0190-9622(98)70087-8
- Ikoma A, Rukwied R, Ständer S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475-1478. doi:10.1001/archderm.139.11.1475
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84-91. doi:10.1111/dth.12025
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161. doi:10.1016/j.jaad.2016.07.034
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157-162. doi:10.1111/bjd.14464
- Kimel M, Zeidler C, Kwon P, et al. validation of psychometric properties of the itch numeric rating scale for pruritus associated with prurigo nodularis: a secondary analysis of a randomized clinical trial. JAMA Dermatol. 2020;156:1354-1358. doi:10.1001/jamadermatol.2020.3071
- Shahwan KT, Kimball AB. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: a meta-analysis. J Am Acad Dermatol. 2017;76:1198.el-1200.e1. doi:10.1016/j.jaad.2017.02.002
- Smith MP, Ly K, Thibodeaux Q, et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb). 2019;9:407-420. doi:10.1007/s13555-019-0312-3
- Kini SP, DeLong LK, Veledar E, et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147:1153-1156. doi:10.1001/archdermatol.2011.178
- Savin JA. How should we define itching? J Am Acad Dermatol. 1998;39(2 pt 1):268-269. doi:10.1016/s0190-9622(98)70087-8
- Ikoma A, Rukwied R, Ständer S, et al. Neurophysiology of pruritus: interaction of itch and pain. Arch Dermatol. 2003;139:1475-1478. doi:10.1001/archderm.139.11.1475
- Garibyan L, Rheingold CG, Lerner EA. Understanding the pathophysiology of itch. Dermatol Ther. 2013;26:84-91. doi:10.1111/dth.12025
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161. doi:10.1016/j.jaad.2016.07.034
- Kimball AB, Naegeli AN, Edson-Heredia E, et al. Psychometric properties of the Itch Numeric Rating Scale in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:157-162. doi:10.1111/bjd.14464
- Kimel M, Zeidler C, Kwon P, et al. validation of psychometric properties of the itch numeric rating scale for pruritus associated with prurigo nodularis: a secondary analysis of a randomized clinical trial. JAMA Dermatol. 2020;156:1354-1358. doi:10.1001/jamadermatol.2020.3071
- Shahwan KT, Kimball AB. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: a meta-analysis. J Am Acad Dermatol. 2017;76:1198.el-1200.e1. doi:10.1016/j.jaad.2017.02.002
- Smith MP, Ly K, Thibodeaux Q, et al. Emerging methods to objectively assess pruritus in atopic dermatitis. Dermatol Ther (Heidelb). 2019;9:407-420. doi:10.1007/s13555-019-0312-3
Researchers stress importance of second COVID-19 vaccine dose for infliximab users
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
FROM MEDRXIV
Bimekizumab superior to adalimumab in head-to-head psoriasis study
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY