User login
Clinical Guidelines Hub only
Guideline for overactive bladder adds new treatments
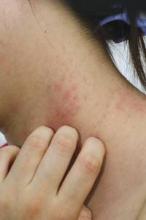
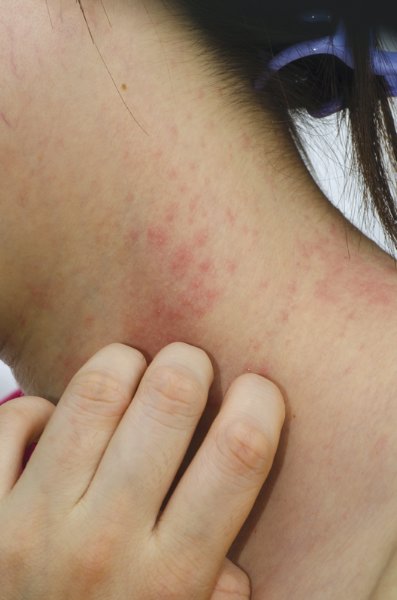
Updated recommendations for the diagnosis and treatment of non-neurogenic overactive bladder incorporate two new treatments approved since 2012 – oral mirabegron and intradetrusor injection of onabotulinumtoxinA.
The 2014 update from the American Urological Association and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (AUA/SUFU) also recognizes the growing number of therapeutic options by stressing the need to take a methodical approach and give an adequate trial of individual treatments before combining them.
"As we have more and more options on the market, you don’t want to stack one on top of another," Dr. Emily Cole said in an interview.
"All of these methodologies have some side effects. What you don’t want to do is have added side effects if something is not working," said Dr. Cole, a urologist specializing in female pelvic medicine and reconstructive surgery in a group practice in San Diego.
Compared with the 2012 AUA/SUFU recommendations, the 2014 guideline on "Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults" adds the beta3-adrenoceptor agonist mirabegron (Myrbetriq, Astellas Pharma) as a first- or second-line treatment option for some patients. OnabotulinumtoxinA (Botox, Allergan) has been upgraded to a "Standard Option" among third-line treatments instead of a nonapproved, off-label therapy.
The Food and Drug Administration approved mirabegron for adults with overactive bladder in June 2012. The FDA approved onabotulinumtoxinA (Botox, Allergan) in January 2013 for adults with overactive bladder who can’t use or don’t respond adequately to anticholinergics.
Clinicians always should start with first- and second-line therapies to reduce symptoms of overactive bladder, Dr. Cole said. By the time patients come to her, they typically have failed those options, but she offers them hope with third-line treatments.
"In most cases, we can really help patients. We may not make you completely dry," she said, but "we have enough tools now that we can make marked improvements."
That’s a big change in recent years, she added. "When I started out, we had two medications that had horrible side effects," she said. Dr. Cole was not involved in creation of the AUA/SUFU guideline.
The AUA/SUFU based the 2012 guideline on 151 articles on the treatment of overactive bladder and reviewed 72 more articles on treatment for the 2014 update.
The recommendations on diagnosis have not changed since 2012 and are based on expert opinion and clinical principles due to insufficient evidence for stronger recommendations.
First-line treatments are behavioral therapies such as bladder training and bladder control strategies or pelvic floor muscle training, which may be combined with pharmacologic management, the guideline states.
Second-line treatments include oral antimuscarinic drugs or mirabegron, preferably in an extended-release formulation if available to reduce the likelihood of dry mouth from immediate-release formulations. A transdermal patch that delivers the antimuscarinic drug oxybutynin became available to adult women over the counter (without a prescription) in 2013.
If a first antimuscarinic medication doesn’t work or causes unacceptable side effects, modify the dose or offer a different antimuscarinic or beta3-adrenoceptor agonist, the guideline states. If an antimuscarinic is effective but causes constipation or dry mouth, don’t give up on that drug class without trying to manage side effects through bowel management, fluid management, modifying the dose, or trying another antimuscarinic.
Be extremely cautious in using antimuscarinics in patients with impaired gastric emptying or a history of urinary retention, and don’t use antimuscarinics in patients with narrow-angle glaucoma without approval from a treating ophthalmologist. If a patient is on other medications with anticholinergic properties, be cautious about prescribing antimuscarinics.
Patients who fail first- and second-line therapies should be evaluated by a specialist if they still desire treatment, according to the guideline.
Among third-line treatment options, sacral neuromodulation may be offered to patients with severe refractory overactive bladder or patients who are not candidates for second-line treatments and who are willing to undergo a surgical procedure. Peripheral tibial nerve stimulation is another option in carefully selected patients.
Intradetrusor injections of Botox may be appropriate for carefully selected and "thoroughly counseled" patients who failed first- and second-line treatments if they are able and willing to return for frequent postvoid residual evaluations and to perform self-catheterization if necessary.
The guideline does not recommend indwelling catheters for management of overactive bladder except as a last resort in some patients, and says that augmentation cystoplasty or urinary diversion may be considered in rare cases of severe, refractory, complicated overactive bladder.
Dr. Cole has been a speaker for Allergan, which markets Botox.
On Twitter @sherryboschert
Updated recommendations for the diagnosis and treatment of non-neurogenic overactive bladder incorporate two new treatments approved since 2012 – oral mirabegron and intradetrusor injection of onabotulinumtoxinA.
The 2014 update from the American Urological Association and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (AUA/SUFU) also recognizes the growing number of therapeutic options by stressing the need to take a methodical approach and give an adequate trial of individual treatments before combining them.
"As we have more and more options on the market, you don’t want to stack one on top of another," Dr. Emily Cole said in an interview.
"All of these methodologies have some side effects. What you don’t want to do is have added side effects if something is not working," said Dr. Cole, a urologist specializing in female pelvic medicine and reconstructive surgery in a group practice in San Diego.
Compared with the 2012 AUA/SUFU recommendations, the 2014 guideline on "Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults" adds the beta3-adrenoceptor agonist mirabegron (Myrbetriq, Astellas Pharma) as a first- or second-line treatment option for some patients. OnabotulinumtoxinA (Botox, Allergan) has been upgraded to a "Standard Option" among third-line treatments instead of a nonapproved, off-label therapy.
The Food and Drug Administration approved mirabegron for adults with overactive bladder in June 2012. The FDA approved onabotulinumtoxinA (Botox, Allergan) in January 2013 for adults with overactive bladder who can’t use or don’t respond adequately to anticholinergics.
Clinicians always should start with first- and second-line therapies to reduce symptoms of overactive bladder, Dr. Cole said. By the time patients come to her, they typically have failed those options, but she offers them hope with third-line treatments.
"In most cases, we can really help patients. We may not make you completely dry," she said, but "we have enough tools now that we can make marked improvements."
That’s a big change in recent years, she added. "When I started out, we had two medications that had horrible side effects," she said. Dr. Cole was not involved in creation of the AUA/SUFU guideline.
The AUA/SUFU based the 2012 guideline on 151 articles on the treatment of overactive bladder and reviewed 72 more articles on treatment for the 2014 update.
The recommendations on diagnosis have not changed since 2012 and are based on expert opinion and clinical principles due to insufficient evidence for stronger recommendations.
First-line treatments are behavioral therapies such as bladder training and bladder control strategies or pelvic floor muscle training, which may be combined with pharmacologic management, the guideline states.
Second-line treatments include oral antimuscarinic drugs or mirabegron, preferably in an extended-release formulation if available to reduce the likelihood of dry mouth from immediate-release formulations. A transdermal patch that delivers the antimuscarinic drug oxybutynin became available to adult women over the counter (without a prescription) in 2013.
If a first antimuscarinic medication doesn’t work or causes unacceptable side effects, modify the dose or offer a different antimuscarinic or beta3-adrenoceptor agonist, the guideline states. If an antimuscarinic is effective but causes constipation or dry mouth, don’t give up on that drug class without trying to manage side effects through bowel management, fluid management, modifying the dose, or trying another antimuscarinic.
Be extremely cautious in using antimuscarinics in patients with impaired gastric emptying or a history of urinary retention, and don’t use antimuscarinics in patients with narrow-angle glaucoma without approval from a treating ophthalmologist. If a patient is on other medications with anticholinergic properties, be cautious about prescribing antimuscarinics.
Patients who fail first- and second-line therapies should be evaluated by a specialist if they still desire treatment, according to the guideline.
Among third-line treatment options, sacral neuromodulation may be offered to patients with severe refractory overactive bladder or patients who are not candidates for second-line treatments and who are willing to undergo a surgical procedure. Peripheral tibial nerve stimulation is another option in carefully selected patients.
Intradetrusor injections of Botox may be appropriate for carefully selected and "thoroughly counseled" patients who failed first- and second-line treatments if they are able and willing to return for frequent postvoid residual evaluations and to perform self-catheterization if necessary.
The guideline does not recommend indwelling catheters for management of overactive bladder except as a last resort in some patients, and says that augmentation cystoplasty or urinary diversion may be considered in rare cases of severe, refractory, complicated overactive bladder.
Dr. Cole has been a speaker for Allergan, which markets Botox.
On Twitter @sherryboschert
Updated recommendations for the diagnosis and treatment of non-neurogenic overactive bladder incorporate two new treatments approved since 2012 – oral mirabegron and intradetrusor injection of onabotulinumtoxinA.
The 2014 update from the American Urological Association and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction (AUA/SUFU) also recognizes the growing number of therapeutic options by stressing the need to take a methodical approach and give an adequate trial of individual treatments before combining them.
"As we have more and more options on the market, you don’t want to stack one on top of another," Dr. Emily Cole said in an interview.
"All of these methodologies have some side effects. What you don’t want to do is have added side effects if something is not working," said Dr. Cole, a urologist specializing in female pelvic medicine and reconstructive surgery in a group practice in San Diego.
Compared with the 2012 AUA/SUFU recommendations, the 2014 guideline on "Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults" adds the beta3-adrenoceptor agonist mirabegron (Myrbetriq, Astellas Pharma) as a first- or second-line treatment option for some patients. OnabotulinumtoxinA (Botox, Allergan) has been upgraded to a "Standard Option" among third-line treatments instead of a nonapproved, off-label therapy.
The Food and Drug Administration approved mirabegron for adults with overactive bladder in June 2012. The FDA approved onabotulinumtoxinA (Botox, Allergan) in January 2013 for adults with overactive bladder who can’t use or don’t respond adequately to anticholinergics.
Clinicians always should start with first- and second-line therapies to reduce symptoms of overactive bladder, Dr. Cole said. By the time patients come to her, they typically have failed those options, but she offers them hope with third-line treatments.
"In most cases, we can really help patients. We may not make you completely dry," she said, but "we have enough tools now that we can make marked improvements."
That’s a big change in recent years, she added. "When I started out, we had two medications that had horrible side effects," she said. Dr. Cole was not involved in creation of the AUA/SUFU guideline.
The AUA/SUFU based the 2012 guideline on 151 articles on the treatment of overactive bladder and reviewed 72 more articles on treatment for the 2014 update.
The recommendations on diagnosis have not changed since 2012 and are based on expert opinion and clinical principles due to insufficient evidence for stronger recommendations.
First-line treatments are behavioral therapies such as bladder training and bladder control strategies or pelvic floor muscle training, which may be combined with pharmacologic management, the guideline states.
Second-line treatments include oral antimuscarinic drugs or mirabegron, preferably in an extended-release formulation if available to reduce the likelihood of dry mouth from immediate-release formulations. A transdermal patch that delivers the antimuscarinic drug oxybutynin became available to adult women over the counter (without a prescription) in 2013.
If a first antimuscarinic medication doesn’t work or causes unacceptable side effects, modify the dose or offer a different antimuscarinic or beta3-adrenoceptor agonist, the guideline states. If an antimuscarinic is effective but causes constipation or dry mouth, don’t give up on that drug class without trying to manage side effects through bowel management, fluid management, modifying the dose, or trying another antimuscarinic.
Be extremely cautious in using antimuscarinics in patients with impaired gastric emptying or a history of urinary retention, and don’t use antimuscarinics in patients with narrow-angle glaucoma without approval from a treating ophthalmologist. If a patient is on other medications with anticholinergic properties, be cautious about prescribing antimuscarinics.
Patients who fail first- and second-line therapies should be evaluated by a specialist if they still desire treatment, according to the guideline.
Among third-line treatment options, sacral neuromodulation may be offered to patients with severe refractory overactive bladder or patients who are not candidates for second-line treatments and who are willing to undergo a surgical procedure. Peripheral tibial nerve stimulation is another option in carefully selected patients.
Intradetrusor injections of Botox may be appropriate for carefully selected and "thoroughly counseled" patients who failed first- and second-line treatments if they are able and willing to return for frequent postvoid residual evaluations and to perform self-catheterization if necessary.
The guideline does not recommend indwelling catheters for management of overactive bladder except as a last resort in some patients, and says that augmentation cystoplasty or urinary diversion may be considered in rare cases of severe, refractory, complicated overactive bladder.
Dr. Cole has been a speaker for Allergan, which markets Botox.
On Twitter @sherryboschert
Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures
Definitions for the strength of evidence (Class I-III) and strength of recommendations (Level A-C) are provided at the end of the "Major Recommendations" field.
- In patients with a first generalized convulsive seizure who have returned to their baseline clinical status, should antiepileptic therapy be initiated in the emergency department (ED) to prevent additional seizures?
Patient Management Recommendations
Level A recommendations. None specified.
Level B recommendations. None specified.
Level C recommendations.
- Emergency physicians need not initiate antiepileptic medication* in the ED for patients who have had a first provoked seizure. Precipitating medical conditions should be identified and treated.
- Emergency physicians need not initiate antiepileptic medication* in the ED for patients who have had a first unprovoked seizure without evidence of brain disease or injury.
- Emergency physicians may initiate antiepileptic medication* in the ED, or defer in coordination with other providers, for patients who experienced a first unprovoked seizure with a remote history of brain disease or injury.
*Antiepileptic medication in this document refers to medications prescribed for seizure prevention.
- In patients with a first unprovoked seizure who have returned to their baseline clinical status in the ED, should the patient be admitted to the hospital to prevent adverse events?
Patient Management Recommendations
Level A recommendations. None specified.
Level B recommendations. None specified.
Level C recommendations. Emergency physicians need not admit patients with a first unprovoked seizure who have returned to their clinical baseline in the ED.
- In patients with a known seizure disorder in which resuming their antiepileptic medication in the ED is deemed appropriate, does the route of administration impact recurrence of seizures?
Patient Management Recommendations
Level A recommendations. None specified.
Level B recommendations. None specified.
Level C recommendations. When resuming antiepileptic medication in the ED is deemed appropriate, the emergency physician may administer intravenous (IV) or oral medication at their discretion.
- In ED patients with generalized convulsive status epilepticus who continue to have seizures despite receiving optimal dosing of a benzodiazepine, which agent or agents should be administered next to terminate seizures?
Patient Management Recommendations
Level A recommendations. Emergency physicians should administer an additional antiepileptic medication in ED patients with refractory status epilepticus who have failed treatment with benzodiazepines.
Level B recommendations. Emergency physicians may administer IV phenytoin, fosphenytoin, or valproate in ED patients with refractory status epilepticus who have failed treatment with benzodiazepines.
Level C recommendations. Emergency physicians may administer IV levetiracetam, propofol, or barbiturates in ED patients with refractory status epilepticus who have failed treatment with benzodiazepines.
Definitions:
Strength of Evidence
Literature Classification Schema*
Design/Class Therapy† Diagnosis‡ Prognosis§ 1 Randomized, controlled trial or meta-analysis of randomized trials Prospective cohort using a criterion standard or meta-analysis of prospective studies Population prospective cohort or meta-analysis of prospective studies 2 Nonrandomized trial Retrospective observational Retrospective cohort
Case control3 Case series
Case report
Other (e.g., consensus, review)Case series
Case report
Other (e.g., consensus, review)Case series
Case report
Other (e.g., consensus, review)*Some designs (e.g., surveys) will not fit this schema and should be assessed individually.
†Objective is to measure therapeutic efficacy comparing interventions.
‡Objective is to determine the sensitivity and specificity of diagnostic tests.
§Objective is to predict outcome including mortality and morbidity.
Approach to Downgrading Strength of Evidence*
Design/Class Downgrading 1 2 3 None I II III 1 level II III X 2 levels III X X Fatally flawed X X X *See the "Description of Methods Used to Analyze the Evidence" field for more information.
Strength of recommendations regarding each critical question were made by subcommittee members using results from strength of evidence grading, expert opinion, and consensus among subcommittee members according to the following guidelines:
Strength of Recommendations
Level A recommendations. Generally accepted principles for patient care that reflect a high degree of clinical certainty (i.e., based on evidence from 1 or more Class of Evidence I or multiple Class of Evidence II studies).
Level B recommendations. Recommendations for patient care that may identify a particular strategy or range of strategies that reflect moderate clinical certainty (i.e., based on evidence from 1 or more Class of Evidence II studies or strong consensus of Class of Evidence III studies).
Level C recommendations. Recommendations for patient care that are based on evidence from Class of Evidence III studies or, in the absence of any adequate published literature, based on expert consensus. In instances where consensus recommendations are made, "consensus" is placed in parentheses at the end of the recommendation.
There are certain circumstances in which the recommendations stemming from a body of evidence should not be rated as highly as the individual studies on which they are based. Factors such as heterogeneity of results, uncertainty about effect magnitude and consequences, and publication bias, among others, might lead to such a downgrading of recommendations.
Definitions for the strength of evidence (Class I-III) and strength of recommendations (Level A-C) are provided at the end of the "Major Recommendations" field.
- In patients with a first generalized convulsive seizure who have returned to their baseline clinical status, should antiepileptic therapy be initiated in the emergency department (ED) to prevent additional seizures?
Patient Management Recommendations
Level A recommendations. None specified.
Level B recommendations. None specified.
Level C recommendations.
- Emergency physicians need not initiate antiepileptic medication* in the ED for patients who have had a first provoked seizure. Precipitating medical conditions should be identified and treated.
- Emergency physicians need not initiate antiepileptic medication* in the ED for patients who have had a first unprovoked seizure without evidence of brain disease or injury.
- Emergency physicians may initiate antiepileptic medication* in the ED, or defer in coordination with other providers, for patients who experienced a first unprovoked seizure with a remote history of brain disease or injury.
*Antiepileptic medication in this document refers to medications prescribed for seizure prevention.
- In patients with a first unprovoked seizure who have returned to their baseline clinical status in the ED, should the patient be admitted to the hospital to prevent adverse events?
Patient Management Recommendations
Level A recommendations. None specified.
Level B recommendations. None specified.
Level C recommendations. Emergency physicians need not admit patients with a first unprovoked seizure who have returned to their clinical baseline in the ED.
- In patients with a known seizure disorder in which resuming their antiepileptic medication in the ED is deemed appropriate, does the route of administration impact recurrence of seizures?
Patient Management Recommendations
Level A recommendations. None specified.
Level B recommendations. None specified.
Level C recommendations. When resuming antiepileptic medication in the ED is deemed appropriate, the emergency physician may administer intravenous (IV) or oral medication at their discretion.
- In ED patients with generalized convulsive status epilepticus who continue to have seizures despite receiving optimal dosing of a benzodiazepine, which agent or agents should be administered next to terminate seizures?
Patient Management Recommendations
Level A recommendations. Emergency physicians should administer an additional antiepileptic medication in ED patients with refractory status epilepticus who have failed treatment with benzodiazepines.
Level B recommendations. Emergency physicians may administer IV phenytoin, fosphenytoin, or valproate in ED patients with refractory status epilepticus who have failed treatment with benzodiazepines.
Level C recommendations. Emergency physicians may administer IV levetiracetam, propofol, or barbiturates in ED patients with refractory status epilepticus who have failed treatment with benzodiazepines.
Definitions:
Strength of Evidence
Literature Classification Schema*
Design/Class Therapy† Diagnosis‡ Prognosis§ 1 Randomized, controlled trial or meta-analysis of randomized trials Prospective cohort using a criterion standard or meta-analysis of prospective studies Population prospective cohort or meta-analysis of prospective studies 2 Nonrandomized trial Retrospective observational Retrospective cohort
Case control3 Case series
Case report
Other (e.g., consensus, review)Case series
Case report
Other (e.g., consensus, review)Case series
Case report
Other (e.g., consensus, review)*Some designs (e.g., surveys) will not fit this schema and should be assessed individually.
†Objective is to measure therapeutic efficacy comparing interventions.
‡Objective is to determine the sensitivity and specificity of diagnostic tests.
§Objective is to predict outcome including mortality and morbidity.
Approach to Downgrading Strength of Evidence*
Design/Class Downgrading 1 2 3 None I II III 1 level II III X 2 levels III X X Fatally flawed X X X *See the "Description of Methods Used to Analyze the Evidence" field for more information.
Strength of recommendations regarding each critical question were made by subcommittee members using results from strength of evidence grading, expert opinion, and consensus among subcommittee members according to the following guidelines:
Strength of Recommendations
Level A recommendations. Generally accepted principles for patient care that reflect a high degree of clinical certainty (i.e., based on evidence from 1 or more Class of Evidence I or multiple Class of Evidence II studies).
Level B recommendations. Recommendations for patient care that may identify a particular strategy or range of strategies that reflect moderate clinical certainty (i.e., based on evidence from 1 or more Class of Evidence II studies or strong consensus of Class of Evidence III studies).
Level C recommendations. Recommendations for patient care that are based on evidence from Class of Evidence III studies or, in the absence of any adequate published literature, based on expert consensus. In instances where consensus recommendations are made, "consensus" is placed in parentheses at the end of the recommendation.
There are certain circumstances in which the recommendations stemming from a body of evidence should not be rated as highly as the individual studies on which they are based. Factors such as heterogeneity of results, uncertainty about effect magnitude and consequences, and publication bias, among others, might lead to such a downgrading of recommendations.
Definitions for the strength of evidence (Class I-III) and strength of recommendations (Level A-C) are provided at the end of the "Major Recommendations" field.
- In patients with a first generalized convulsive seizure who have returned to their baseline clinical status, should antiepileptic therapy be initiated in the emergency department (ED) to prevent additional seizures?
Patient Management Recommendations
Level A recommendations. None specified.
Level B recommendations. None specified.
Level C recommendations.
- Emergency physicians need not initiate antiepileptic medication* in the ED for patients who have had a first provoked seizure. Precipitating medical conditions should be identified and treated.
- Emergency physicians need not initiate antiepileptic medication* in the ED for patients who have had a first unprovoked seizure without evidence of brain disease or injury.
- Emergency physicians may initiate antiepileptic medication* in the ED, or defer in coordination with other providers, for patients who experienced a first unprovoked seizure with a remote history of brain disease or injury.
*Antiepileptic medication in this document refers to medications prescribed for seizure prevention.
- In patients with a first unprovoked seizure who have returned to their baseline clinical status in the ED, should the patient be admitted to the hospital to prevent adverse events?
Patient Management Recommendations
Level A recommendations. None specified.
Level B recommendations. None specified.
Level C recommendations. Emergency physicians need not admit patients with a first unprovoked seizure who have returned to their clinical baseline in the ED.
- In patients with a known seizure disorder in which resuming their antiepileptic medication in the ED is deemed appropriate, does the route of administration impact recurrence of seizures?
Patient Management Recommendations
Level A recommendations. None specified.
Level B recommendations. None specified.
Level C recommendations. When resuming antiepileptic medication in the ED is deemed appropriate, the emergency physician may administer intravenous (IV) or oral medication at their discretion.
- In ED patients with generalized convulsive status epilepticus who continue to have seizures despite receiving optimal dosing of a benzodiazepine, which agent or agents should be administered next to terminate seizures?
Patient Management Recommendations
Level A recommendations. Emergency physicians should administer an additional antiepileptic medication in ED patients with refractory status epilepticus who have failed treatment with benzodiazepines.
Level B recommendations. Emergency physicians may administer IV phenytoin, fosphenytoin, or valproate in ED patients with refractory status epilepticus who have failed treatment with benzodiazepines.
Level C recommendations. Emergency physicians may administer IV levetiracetam, propofol, or barbiturates in ED patients with refractory status epilepticus who have failed treatment with benzodiazepines.
Definitions:
Strength of Evidence
Literature Classification Schema*
Design/Class Therapy† Diagnosis‡ Prognosis§ 1 Randomized, controlled trial or meta-analysis of randomized trials Prospective cohort using a criterion standard or meta-analysis of prospective studies Population prospective cohort or meta-analysis of prospective studies 2 Nonrandomized trial Retrospective observational Retrospective cohort
Case control3 Case series
Case report
Other (e.g., consensus, review)Case series
Case report
Other (e.g., consensus, review)Case series
Case report
Other (e.g., consensus, review)*Some designs (e.g., surveys) will not fit this schema and should be assessed individually.
†Objective is to measure therapeutic efficacy comparing interventions.
‡Objective is to determine the sensitivity and specificity of diagnostic tests.
§Objective is to predict outcome including mortality and morbidity.
Approach to Downgrading Strength of Evidence*
Design/Class Downgrading 1 2 3 None I II III 1 level II III X 2 levels III X X Fatally flawed X X X *See the "Description of Methods Used to Analyze the Evidence" field for more information.
Strength of recommendations regarding each critical question were made by subcommittee members using results from strength of evidence grading, expert opinion, and consensus among subcommittee members according to the following guidelines:
Strength of Recommendations
Level A recommendations. Generally accepted principles for patient care that reflect a high degree of clinical certainty (i.e., based on evidence from 1 or more Class of Evidence I or multiple Class of Evidence II studies).
Level B recommendations. Recommendations for patient care that may identify a particular strategy or range of strategies that reflect moderate clinical certainty (i.e., based on evidence from 1 or more Class of Evidence II studies or strong consensus of Class of Evidence III studies).
Level C recommendations. Recommendations for patient care that are based on evidence from Class of Evidence III studies or, in the absence of any adequate published literature, based on expert consensus. In instances where consensus recommendations are made, "consensus" is placed in parentheses at the end of the recommendation.
There are certain circumstances in which the recommendations stemming from a body of evidence should not be rated as highly as the individual studies on which they are based. Factors such as heterogeneity of results, uncertainty about effect magnitude and consequences, and publication bias, among others, might lead to such a downgrading of recommendations.
This clinical policy from the American College of Emergency Physicians is the revision of a 2004 policy on critical issues in the evaluation and management of adult patients with seizures in the emergency department. A writing subcommittee reviewed the literature to derive evidence-based recommendations to help clinicians answer four critical questions. A literature search was performed, the evidence was graded, and recommendations were given based on the strength of the available data in the medical literature.
Guidelines are copyright © 2014 American College of Emergency Physicians. All rights reserved. The summary is provided by the Agency for Healthcare Research and Quality.
Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis
Note from the National Guideline Clearinghouse (NGC): This document is the first section in a series of four and covers methods for diagnosis and assessment of atopic dermatitis (AD). The second guideline in the series will address the management and treatment of AD with pharmacologic and nonpharmacologic topical modalities; the third section will cover phototherapy and systemic treatment options; and the fourth section will address the minimization of disease flares, educational interventions, and use of adjunctive approaches.
Features to Be Considered in the Diagnosis of Patients with AD
| Essential Features—Must be present:
*Patterns Include:
Important Features—Seen in most cases, adding support to the diagnosis:
Associated Features—These clinical associations help to suggest the diagnosis of AD but are too nonspecific to be used for defining or detecting AD for research and epidemiologic studies:
Exclusionary Conditions—It should be noted that a diagnosis of AD depends on excluding conditions, such as:
Adapted from Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 2003;49:1088-95. Used with permission of the American Academy of Dermatology. |
Recommendation for the Diagnosis of AD
Patients with presumed AD should have their diagnosis based on the criteria summarized in the box above. On occasion, skin biopsy specimens or other tests (such as serum immunoglobulin E, potassium hydroxide preparation, patch testing, and/or genetic testing) may be helpful to rule out other or associated skin conditions.
Strength of Recommendations for the Diagnosis and Assessment of AD
| Recommendation | Strength of Recommendation | Level of Evidence | References |
|---|---|---|---|
| Diagnosis made using criteria in the box above | C | III | Mevorah et al., 1988; Gu et al., 2001; Lan et al., 2009; Diepgen, Sauerbrei, & Fartasch, 1996; De, Kanwar, & Handa, 2006; Loden, Andersson, & Lindberg, 1998; Samochocki & Dejewska, 2012; Samochocki, Paulochowska, & Zabielski, 2000; Chalmers et al., 2007; Firooz et al., 1999; Saeki et al., 2007; Firooz & Kashani, 2008; Hamada et al., 2005; Williams et al., 1994; Williams et al., 1996 |
| No specific biomarkers for diagnosis or severity assessment | B | II | Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Amon et al., 2000; Dhar et al., 2005; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al, 2006; Di Lorenzo et al., 2003; El Mongy et al., 2008; Ezzat, Hasan, & Shaheen, 2011; Jahnz-Rozyk et al., 2005; Nakazato et al., 2008; Belloni Fortina et al., 2006; Gutgesell et al., 2002; Hirai et al., 1996; Hon et al., 2007; Horikawa et al., 2002; Kakinuma et al., 2003; La Grutta et al., 2005; Leung et al., 2003; Mostafa et al., 2008; Oflazoglu et al., "CD30 expression," 2008; Oflazoglu et al., "CD40 expression," 2008; Ott et al., 2010; Raap et al., 2006; Song et al., 2006; Wolkerstorfer et al., 1998 |
| Immunoglobulin E levels not routinely recommended | A | I | Schneider et al., 2013; Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al., 2006; Vakirlis et al., 2011; Wu et al., 2011 |
| Available disease severity scales not for routine clinical use | C | II | Schmitt, Langan, & Williams, 2007; Schram et al., 2012; Sprikkelman et al., 1997; Angelova-Fischer et al., 2005; Wolkerstorfer et al., 1999; Linnet & Jemec, 1999; Hon et al., 2006; Barbier et al., 2004; Charman, Venn, & Williams, 2002; Charman, Venn, & Williams, 2004; Charman et al., 1999; Cosickic et al., 2010; Emerson, Charman, & Williams, 2000; Hanifin et al., 2001; Holm et al., 2007; Oranje et al., 1997; Rullo et al., 2008 |
| Available quality of life severity scales not for routine clinical use | C | II | Chamlin et al., 2007; Augustin et al., 2004; Hon et al, 2006; Misery et al., 2007 |
| Should query itch, sleep, impact on daily activity, and disease persistence | C | III | Chamlin et al., 2005; Hon et al., 2008; Dawn et al., 2009; Lewis-Jones, 2006; Weisshaar et al., 2008; Ricci et al., 2007; Bender et al., 2008; Ben-Gashir, Seed, & Hay, 2002 |
| Awareness and discussion of common associations | C | I and II | Chamlin et al., 2005; Hon et al., 2008; Batlles-Garrido et al., 2010; Chawes et al., 2010; Sultesz et al., 2010; Kyllonen et al., 2006; Hwang et al., 2010; Hyvarinen et al., 2005; Eller et al., 2009; Horwitz, Hossain, & Yousef, 2009; Bashir, Dar, & Rao, 2010; Schmitt et al., "Psychiatric comorbidity," 2009; Schmitt et al., "Atopic eczema," 2009; Yaghmaie, Koudelka, & Simpson, 2013; Harding et al., 2008; Synnerstad et al., 2008; Vajdic et al., 2009; Kajbaf, Asar, & Alipoor, 2011; Vlaski et al., 2006 |
| Integrated, multidisciplinary approach to care | C | III | Boguniewicz et al., 2008; Ricci et al., 2009 |
Recommendations for the Use of Biomarkers in the Assessment of AD
- For patients with presumed AD, there are no specific biomarkers that can be recommended for diagnosis and/or assessment of disease severity.
- Monitoring of immunoglobulin E levels is not recommended for the routine assessment of disease severity.
Recommendations for Disease Severity and Clinical Outcomes Assessment
- For the general management of patients with AD, available disease severity measurement scales are not recommended for routine clinical practice, because they were not usually designed for this purpose.
- For the general management of patients with AD, available patient quality of life measurement scales are not recommended for routine clinical practice.
- It is recommended that clinicians ask general questions about itch, sleep, impact on daily activity, and persistence of disease, and currently available scales be used mainly when practical.
Recommendations for the Assessment of Clinical Associations of AD
- Physicians should be aware of and assess for conditions associated with AD, such as rhinitis/rhinoconjunctivitis, asthma, food allergy, sleep disturbance, depression, and other neuropsychiatric conditions, and it is recommended that physicians discuss them with the patient as part of the treatment/management plan, when appropriate.
- An integrated, multidisciplinary approach to care may be valuable and is suggested for AD patients who present with common associations.
Definitions:
Levels of Evidence
- Good-quality patient-oriented evidence (i.e., evidence measuring outcomes that matter to patients: morbidity, mortality, symptom improvement, cost reduction, and quality of life)
- Limited-quality patient-oriented evidence
- Other evidence including consensus guidelines, opinion, case studies, or disease-oriented evidence (i.e., evidence measuring intermediate, physiologic, or surrogate end points that may or may not reflect improvements in patient outcomes)
Grades of Recommendation
- Recommendation based on consistent and good quality patient-oriented evidence
- Recommendation based on inconsistent or limited quality patient-oriented evidence
- Recommendation based on consensus, opinion, case studies, or disease-oriented evidence
Note from the National Guideline Clearinghouse (NGC): This document is the first section in a series of four and covers methods for diagnosis and assessment of atopic dermatitis (AD). The second guideline in the series will address the management and treatment of AD with pharmacologic and nonpharmacologic topical modalities; the third section will cover phototherapy and systemic treatment options; and the fourth section will address the minimization of disease flares, educational interventions, and use of adjunctive approaches.
Features to Be Considered in the Diagnosis of Patients with AD
| Essential Features—Must be present:
*Patterns Include:
Important Features—Seen in most cases, adding support to the diagnosis:
Associated Features—These clinical associations help to suggest the diagnosis of AD but are too nonspecific to be used for defining or detecting AD for research and epidemiologic studies:
Exclusionary Conditions—It should be noted that a diagnosis of AD depends on excluding conditions, such as:
Adapted from Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 2003;49:1088-95. Used with permission of the American Academy of Dermatology. |
Recommendation for the Diagnosis of AD
Patients with presumed AD should have their diagnosis based on the criteria summarized in the box above. On occasion, skin biopsy specimens or other tests (such as serum immunoglobulin E, potassium hydroxide preparation, patch testing, and/or genetic testing) may be helpful to rule out other or associated skin conditions.
Strength of Recommendations for the Diagnosis and Assessment of AD
| Recommendation | Strength of Recommendation | Level of Evidence | References |
|---|---|---|---|
| Diagnosis made using criteria in the box above | C | III | Mevorah et al., 1988; Gu et al., 2001; Lan et al., 2009; Diepgen, Sauerbrei, & Fartasch, 1996; De, Kanwar, & Handa, 2006; Loden, Andersson, & Lindberg, 1998; Samochocki & Dejewska, 2012; Samochocki, Paulochowska, & Zabielski, 2000; Chalmers et al., 2007; Firooz et al., 1999; Saeki et al., 2007; Firooz & Kashani, 2008; Hamada et al., 2005; Williams et al., 1994; Williams et al., 1996 |
| No specific biomarkers for diagnosis or severity assessment | B | II | Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Amon et al., 2000; Dhar et al., 2005; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al, 2006; Di Lorenzo et al., 2003; El Mongy et al., 2008; Ezzat, Hasan, & Shaheen, 2011; Jahnz-Rozyk et al., 2005; Nakazato et al., 2008; Belloni Fortina et al., 2006; Gutgesell et al., 2002; Hirai et al., 1996; Hon et al., 2007; Horikawa et al., 2002; Kakinuma et al., 2003; La Grutta et al., 2005; Leung et al., 2003; Mostafa et al., 2008; Oflazoglu et al., "CD30 expression," 2008; Oflazoglu et al., "CD40 expression," 2008; Ott et al., 2010; Raap et al., 2006; Song et al., 2006; Wolkerstorfer et al., 1998 |
| Immunoglobulin E levels not routinely recommended | A | I | Schneider et al., 2013; Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al., 2006; Vakirlis et al., 2011; Wu et al., 2011 |
| Available disease severity scales not for routine clinical use | C | II | Schmitt, Langan, & Williams, 2007; Schram et al., 2012; Sprikkelman et al., 1997; Angelova-Fischer et al., 2005; Wolkerstorfer et al., 1999; Linnet & Jemec, 1999; Hon et al., 2006; Barbier et al., 2004; Charman, Venn, & Williams, 2002; Charman, Venn, & Williams, 2004; Charman et al., 1999; Cosickic et al., 2010; Emerson, Charman, & Williams, 2000; Hanifin et al., 2001; Holm et al., 2007; Oranje et al., 1997; Rullo et al., 2008 |
| Available quality of life severity scales not for routine clinical use | C | II | Chamlin et al., 2007; Augustin et al., 2004; Hon et al, 2006; Misery et al., 2007 |
| Should query itch, sleep, impact on daily activity, and disease persistence | C | III | Chamlin et al., 2005; Hon et al., 2008; Dawn et al., 2009; Lewis-Jones, 2006; Weisshaar et al., 2008; Ricci et al., 2007; Bender et al., 2008; Ben-Gashir, Seed, & Hay, 2002 |
| Awareness and discussion of common associations | C | I and II | Chamlin et al., 2005; Hon et al., 2008; Batlles-Garrido et al., 2010; Chawes et al., 2010; Sultesz et al., 2010; Kyllonen et al., 2006; Hwang et al., 2010; Hyvarinen et al., 2005; Eller et al., 2009; Horwitz, Hossain, & Yousef, 2009; Bashir, Dar, & Rao, 2010; Schmitt et al., "Psychiatric comorbidity," 2009; Schmitt et al., "Atopic eczema," 2009; Yaghmaie, Koudelka, & Simpson, 2013; Harding et al., 2008; Synnerstad et al., 2008; Vajdic et al., 2009; Kajbaf, Asar, & Alipoor, 2011; Vlaski et al., 2006 |
| Integrated, multidisciplinary approach to care | C | III | Boguniewicz et al., 2008; Ricci et al., 2009 |
Recommendations for the Use of Biomarkers in the Assessment of AD
- For patients with presumed AD, there are no specific biomarkers that can be recommended for diagnosis and/or assessment of disease severity.
- Monitoring of immunoglobulin E levels is not recommended for the routine assessment of disease severity.
Recommendations for Disease Severity and Clinical Outcomes Assessment
- For the general management of patients with AD, available disease severity measurement scales are not recommended for routine clinical practice, because they were not usually designed for this purpose.
- For the general management of patients with AD, available patient quality of life measurement scales are not recommended for routine clinical practice.
- It is recommended that clinicians ask general questions about itch, sleep, impact on daily activity, and persistence of disease, and currently available scales be used mainly when practical.
Recommendations for the Assessment of Clinical Associations of AD
- Physicians should be aware of and assess for conditions associated with AD, such as rhinitis/rhinoconjunctivitis, asthma, food allergy, sleep disturbance, depression, and other neuropsychiatric conditions, and it is recommended that physicians discuss them with the patient as part of the treatment/management plan, when appropriate.
- An integrated, multidisciplinary approach to care may be valuable and is suggested for AD patients who present with common associations.
Definitions:
Levels of Evidence
- Good-quality patient-oriented evidence (i.e., evidence measuring outcomes that matter to patients: morbidity, mortality, symptom improvement, cost reduction, and quality of life)
- Limited-quality patient-oriented evidence
- Other evidence including consensus guidelines, opinion, case studies, or disease-oriented evidence (i.e., evidence measuring intermediate, physiologic, or surrogate end points that may or may not reflect improvements in patient outcomes)
Grades of Recommendation
- Recommendation based on consistent and good quality patient-oriented evidence
- Recommendation based on inconsistent or limited quality patient-oriented evidence
- Recommendation based on consensus, opinion, case studies, or disease-oriented evidence
Note from the National Guideline Clearinghouse (NGC): This document is the first section in a series of four and covers methods for diagnosis and assessment of atopic dermatitis (AD). The second guideline in the series will address the management and treatment of AD with pharmacologic and nonpharmacologic topical modalities; the third section will cover phototherapy and systemic treatment options; and the fourth section will address the minimization of disease flares, educational interventions, and use of adjunctive approaches.
Features to Be Considered in the Diagnosis of Patients with AD
| Essential Features—Must be present:
*Patterns Include:
Important Features—Seen in most cases, adding support to the diagnosis:
Associated Features—These clinical associations help to suggest the diagnosis of AD but are too nonspecific to be used for defining or detecting AD for research and epidemiologic studies:
Exclusionary Conditions—It should be noted that a diagnosis of AD depends on excluding conditions, such as:
Adapted from Eichenfield LF, Hanifin JM, Luger TA, Stevens SR, Pride HB. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol 2003;49:1088-95. Used with permission of the American Academy of Dermatology. |
Recommendation for the Diagnosis of AD
Patients with presumed AD should have their diagnosis based on the criteria summarized in the box above. On occasion, skin biopsy specimens or other tests (such as serum immunoglobulin E, potassium hydroxide preparation, patch testing, and/or genetic testing) may be helpful to rule out other or associated skin conditions.
Strength of Recommendations for the Diagnosis and Assessment of AD
| Recommendation | Strength of Recommendation | Level of Evidence | References |
|---|---|---|---|
| Diagnosis made using criteria in the box above | C | III | Mevorah et al., 1988; Gu et al., 2001; Lan et al., 2009; Diepgen, Sauerbrei, & Fartasch, 1996; De, Kanwar, & Handa, 2006; Loden, Andersson, & Lindberg, 1998; Samochocki & Dejewska, 2012; Samochocki, Paulochowska, & Zabielski, 2000; Chalmers et al., 2007; Firooz et al., 1999; Saeki et al., 2007; Firooz & Kashani, 2008; Hamada et al., 2005; Williams et al., 1994; Williams et al., 1996 |
| No specific biomarkers for diagnosis or severity assessment | B | II | Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Amon et al., 2000; Dhar et al., 2005; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al, 2006; Di Lorenzo et al., 2003; El Mongy et al., 2008; Ezzat, Hasan, & Shaheen, 2011; Jahnz-Rozyk et al., 2005; Nakazato et al., 2008; Belloni Fortina et al., 2006; Gutgesell et al., 2002; Hirai et al., 1996; Hon et al., 2007; Horikawa et al., 2002; Kakinuma et al., 2003; La Grutta et al., 2005; Leung et al., 2003; Mostafa et al., 2008; Oflazoglu et al., "CD30 expression," 2008; Oflazoglu et al., "CD40 expression," 2008; Ott et al., 2010; Raap et al., 2006; Song et al., 2006; Wolkerstorfer et al., 1998 |
| Immunoglobulin E levels not routinely recommended | A | I | Schneider et al., 2013; Murat-Susic et al., 2006; Schulte-Herbruggen et al., 2007; Gerdes, Kurrat, & Mrowietz, 2009; Aral et al., 2006; Vakirlis et al., 2011; Wu et al., 2011 |
| Available disease severity scales not for routine clinical use | C | II | Schmitt, Langan, & Williams, 2007; Schram et al., 2012; Sprikkelman et al., 1997; Angelova-Fischer et al., 2005; Wolkerstorfer et al., 1999; Linnet & Jemec, 1999; Hon et al., 2006; Barbier et al., 2004; Charman, Venn, & Williams, 2002; Charman, Venn, & Williams, 2004; Charman et al., 1999; Cosickic et al., 2010; Emerson, Charman, & Williams, 2000; Hanifin et al., 2001; Holm et al., 2007; Oranje et al., 1997; Rullo et al., 2008 |
| Available quality of life severity scales not for routine clinical use | C | II | Chamlin et al., 2007; Augustin et al., 2004; Hon et al, 2006; Misery et al., 2007 |
| Should query itch, sleep, impact on daily activity, and disease persistence | C | III | Chamlin et al., 2005; Hon et al., 2008; Dawn et al., 2009; Lewis-Jones, 2006; Weisshaar et al., 2008; Ricci et al., 2007; Bender et al., 2008; Ben-Gashir, Seed, & Hay, 2002 |
| Awareness and discussion of common associations | C | I and II | Chamlin et al., 2005; Hon et al., 2008; Batlles-Garrido et al., 2010; Chawes et al., 2010; Sultesz et al., 2010; Kyllonen et al., 2006; Hwang et al., 2010; Hyvarinen et al., 2005; Eller et al., 2009; Horwitz, Hossain, & Yousef, 2009; Bashir, Dar, & Rao, 2010; Schmitt et al., "Psychiatric comorbidity," 2009; Schmitt et al., "Atopic eczema," 2009; Yaghmaie, Koudelka, & Simpson, 2013; Harding et al., 2008; Synnerstad et al., 2008; Vajdic et al., 2009; Kajbaf, Asar, & Alipoor, 2011; Vlaski et al., 2006 |
| Integrated, multidisciplinary approach to care | C | III | Boguniewicz et al., 2008; Ricci et al., 2009 |
Recommendations for the Use of Biomarkers in the Assessment of AD
- For patients with presumed AD, there are no specific biomarkers that can be recommended for diagnosis and/or assessment of disease severity.
- Monitoring of immunoglobulin E levels is not recommended for the routine assessment of disease severity.
Recommendations for Disease Severity and Clinical Outcomes Assessment
- For the general management of patients with AD, available disease severity measurement scales are not recommended for routine clinical practice, because they were not usually designed for this purpose.
- For the general management of patients with AD, available patient quality of life measurement scales are not recommended for routine clinical practice.
- It is recommended that clinicians ask general questions about itch, sleep, impact on daily activity, and persistence of disease, and currently available scales be used mainly when practical.
Recommendations for the Assessment of Clinical Associations of AD
- Physicians should be aware of and assess for conditions associated with AD, such as rhinitis/rhinoconjunctivitis, asthma, food allergy, sleep disturbance, depression, and other neuropsychiatric conditions, and it is recommended that physicians discuss them with the patient as part of the treatment/management plan, when appropriate.
- An integrated, multidisciplinary approach to care may be valuable and is suggested for AD patients who present with common associations.
Definitions:
Levels of Evidence
- Good-quality patient-oriented evidence (i.e., evidence measuring outcomes that matter to patients: morbidity, mortality, symptom improvement, cost reduction, and quality of life)
- Limited-quality patient-oriented evidence
- Other evidence including consensus guidelines, opinion, case studies, or disease-oriented evidence (i.e., evidence measuring intermediate, physiologic, or surrogate end points that may or may not reflect improvements in patient outcomes)
Grades of Recommendation
- Recommendation based on consistent and good quality patient-oriented evidence
- Recommendation based on inconsistent or limited quality patient-oriented evidence
- Recommendation based on consensus, opinion, case studies, or disease-oriented evidence
Atopic dermatitis affects up to 25% of children and 2% to 3% of adults. This guideline addresses methods for the diagnosis and monitoring of disease, outcomes measures for assessment, and common clinical associations that affect patients with AD are discussed.
Guidelines are copyright © 2013 American Academy of Dermatology, Inc. Published by Mosby, Inc. All rights reserved. The summary is provided by the Agency for Healthcare Research and Quality
Guideline adjusts perioperative cardiac care in noncardiac surgery
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Professor of Anesthesiology and Critical Care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline pertinent to cardiologists, primary care physicians, or surgeons. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI), and the National Surgical Quality Improvement Project risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work of the committees. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Professor of Anesthesiology and Critical Care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline pertinent to cardiologists, primary care physicians, or surgeons. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI), and the National Surgical Quality Improvement Project risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work of the committees. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
A new clinical practice guideline on cardiovascular evaluation and management of patients undergoing noncardiac surgery adds some clarity around the controversial issue of beta-blocker therapy and updates other aspects of care.
If a patient on beta-blocker medication needs noncardiac surgery, continue the beta-blocker, because there is no evidence of harm from doing so; but you risk doing harm if the drug is stopped, according to the new guideline from the American College of Cardiology (ACC) and the American Heart Association (AHA).
Surgeons will be happy to hear that, said Dr. Lee A. Fleisher, the chair of the guideline-writing committee, because that conforms to one of the Surgical Care Improvement Project’s National Measures.
For patients at elevated risk of a cardiovascular event during noncardiac surgery who are not already on beta-blocker therapy, however, the new guideline steps back from the organization’s 2009 position that beta-blockers not be started, and says instead that it’s not unreasonable to start the drug, with a caveat. Be very cautious and start the drug early enough before surgery that you can titrate it to avoid causing hypotension or a low heart rate.
"Make sure that you’re giving the right amount and monitoring their blood pressure and heart rate," Dr. Fleisher, chair of the guideline writing committee, said in an interview. "Really think once, twice, and thrice about starting a protocol," added Dr. Fleisher, the Robert D. Dripps Professor of Anesthesiology and Critical Care at the University of Pennsylvania, Philadelphia.
The ACC and AHA commissioned a committee to review the evidence for and against beta-blockers in patients undergoing noncardiac surgery. A separate writing committee then considered the evidence review committee’s report, reviewed the literature on other aspects of perioperative care for noncardiac surgery, and compiled a 102-page guideline with a 59-page executive summary.
The "2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery" will be published online on the ACC and AHA websites.
Dr. Fleisher described other highlights of the new guideline pertinent to cardiologists, primary care physicians, or surgeons. For the first time, palliative care has been added as an option that may come out of the preoperative evaluation, he said. Patient categories of high risk and intermediate risk have been lumped together as having "elevated" risk for simplicity’s sake because recommendations for the two separate categories were so similar.
The guideline now endorses two tools to choose from for preoperative risk assessments: the Revised Cardiac Risk Index (RCRI), and the National Surgical Quality Improvement Project risk calculator. "There have been a lot of comments that [the NSQIP] is a very useful tool to have shared decision-making conversations with patients," he said.
Another change applies to patients who receive second- or third-generation coronary stents. Instead of a wait of a year after stent implantation to perform noncardiac surgery, a 6-month wait may be reasonable if the risks of delaying noncardiac surgery outweigh the risks of interrupting dual-antiplatelet therapy for the noncardiac surgery.
In addition, the guideline incorporates findings from the recent POISE-2 study to say that aspirin can be stopped and clonidine is not useful in patients without stents undergoing noncardiac surgery (N. Engl. J. Med. 2014;370:1494-503).
A new statement in the guideline about troponin says to check troponin in high-risk patients with signs or symptoms of trouble but not to include troponin in routine screening.
The recommendations on beta-blockers, however, address the most controversial topic in the guideline, Dr. Fleisher said. "There is a lot of confusing evidence" on the use of beta-blockers, "so we’ve tried to clarify as much as we can."
The ACC and AHA funded the work of the committees. Dr. Fleisher reported having no financial disclosures.
On Twitter @sherryboschert
Conventional DMARDs may be excluded from psoriatic arthritis enthesitis guidelines
NEW YORK – Conventional disease-modifying antirheumatic drugs will not be included among acceptable treatments for enthesitis related to psoriatic arthritis, according to a draft of guidelines being prepared for publication.
"The only controlled trial with a DMARD [disease-modifying antirheumatic drug] for enthesitis was conducted with sulfasalazine, and it was negative," said Dr. Evan L. Siegel, who is in group practice in rheumatology in Rockville, Md. Dr. Siegel led a group of experts preparing psoriatic arthritis enthesitis treatment recommendations to be issued by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In a preliminary report on the planned treatment recommendations, which were delivered at the joint meetings of GRAPPA and the Spondyloarthritis Research & Treatment Network (SPARTAN), Dr. Siegel reported that the new recommendations will recognize only two gradations of enthesitis: mild or moderate/severe.
"It is difficult to differentiate moderate from severe because these have not been treated as distinct entities in the clinical trials," Dr. Siegel said. Patients experience severity in regard to intensity of pain and limitation of function, either or both of which may represent severe enthesitis in any given individual patient, according to Dr. Siegel, who also noted that the number of sites of activity is also generally unhelpful.
"Significant activity at a single site may be sufficient to produce a major functional deficit in one individual, whereas the activity at multiple sites may not produce much symptomatology or functional loss in another," Dr. Siegel said.
Enthesitis, an inflammation at the site where tendons or ligaments attach to bone, has been reported in up to 70% of patients with psoriatic arthritis. In some patients, it is the dominant symptom. However, the number of treatment trials in which control of enthesitis is the primary outcome continues to be limited, according to Dr. Siegel. He acknowledged that many of the proposed treatment recommendations owed more to expert opinion than to data.
This is true of the proposed first-line recommendation, he said, which is the use of nonsteroidal anti-inflammatory drugs and physical therapy. For NSAIDs, the wording of the recommendation will emphasize the need to monitor side effects.
The expert opinion of the GRAPPA consensus group was nearly unanimous that NSAIDs and physical therapy are effective and should be tried initially in both mild and moderate/severe disease, even though Dr. Siegel said that the supportive data from controlled trials are limited.
For those with moderate/severe enthesitis not sufficiently controlled on NSAIDs, alternatives include tumor necrosis factor inhibitors, ustekinumab, and apremilast. Some supportive data are available for each of these options, even though the consensus group concluded that there is not enough comparative evidence "to recommend one over another," he said.
Rather, the consensus group is recommending that the choice of therapies beyond NSAIDs and physical therapy be individualized in relationship to comorbidities, personal preference, and other considerations.
Special wording is being developed in regard to the use of corticosteroid injections. This wording was partly inspired by a meta-analysis that associated corticosteroid injections with an adverse effect on the integrity of tendons. Although there were many criticisms of this report, there was sufficient concern among the consensus group to urge that this treatment be used with caution.
"We think that these injections should only be provided by experienced physicians," Dr. Siegel reported. The wording in the preliminary draft is that adjunctive corticosteroid injections "may be considered on an individual basis."
When published, the enthesitis guidelines will include grading for the quality of the evidence behind each recommendation as well as the relative strength of the recommendation conferred by the expert panel.
Dr. Siegel reported financial relationships with Amgen and AbbVie.
NEW YORK – Conventional disease-modifying antirheumatic drugs will not be included among acceptable treatments for enthesitis related to psoriatic arthritis, according to a draft of guidelines being prepared for publication.
"The only controlled trial with a DMARD [disease-modifying antirheumatic drug] for enthesitis was conducted with sulfasalazine, and it was negative," said Dr. Evan L. Siegel, who is in group practice in rheumatology in Rockville, Md. Dr. Siegel led a group of experts preparing psoriatic arthritis enthesitis treatment recommendations to be issued by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In a preliminary report on the planned treatment recommendations, which were delivered at the joint meetings of GRAPPA and the Spondyloarthritis Research & Treatment Network (SPARTAN), Dr. Siegel reported that the new recommendations will recognize only two gradations of enthesitis: mild or moderate/severe.
"It is difficult to differentiate moderate from severe because these have not been treated as distinct entities in the clinical trials," Dr. Siegel said. Patients experience severity in regard to intensity of pain and limitation of function, either or both of which may represent severe enthesitis in any given individual patient, according to Dr. Siegel, who also noted that the number of sites of activity is also generally unhelpful.
"Significant activity at a single site may be sufficient to produce a major functional deficit in one individual, whereas the activity at multiple sites may not produce much symptomatology or functional loss in another," Dr. Siegel said.
Enthesitis, an inflammation at the site where tendons or ligaments attach to bone, has been reported in up to 70% of patients with psoriatic arthritis. In some patients, it is the dominant symptom. However, the number of treatment trials in which control of enthesitis is the primary outcome continues to be limited, according to Dr. Siegel. He acknowledged that many of the proposed treatment recommendations owed more to expert opinion than to data.
This is true of the proposed first-line recommendation, he said, which is the use of nonsteroidal anti-inflammatory drugs and physical therapy. For NSAIDs, the wording of the recommendation will emphasize the need to monitor side effects.
The expert opinion of the GRAPPA consensus group was nearly unanimous that NSAIDs and physical therapy are effective and should be tried initially in both mild and moderate/severe disease, even though Dr. Siegel said that the supportive data from controlled trials are limited.
For those with moderate/severe enthesitis not sufficiently controlled on NSAIDs, alternatives include tumor necrosis factor inhibitors, ustekinumab, and apremilast. Some supportive data are available for each of these options, even though the consensus group concluded that there is not enough comparative evidence "to recommend one over another," he said.
Rather, the consensus group is recommending that the choice of therapies beyond NSAIDs and physical therapy be individualized in relationship to comorbidities, personal preference, and other considerations.
Special wording is being developed in regard to the use of corticosteroid injections. This wording was partly inspired by a meta-analysis that associated corticosteroid injections with an adverse effect on the integrity of tendons. Although there were many criticisms of this report, there was sufficient concern among the consensus group to urge that this treatment be used with caution.
"We think that these injections should only be provided by experienced physicians," Dr. Siegel reported. The wording in the preliminary draft is that adjunctive corticosteroid injections "may be considered on an individual basis."
When published, the enthesitis guidelines will include grading for the quality of the evidence behind each recommendation as well as the relative strength of the recommendation conferred by the expert panel.
Dr. Siegel reported financial relationships with Amgen and AbbVie.
NEW YORK – Conventional disease-modifying antirheumatic drugs will not be included among acceptable treatments for enthesitis related to psoriatic arthritis, according to a draft of guidelines being prepared for publication.
"The only controlled trial with a DMARD [disease-modifying antirheumatic drug] for enthesitis was conducted with sulfasalazine, and it was negative," said Dr. Evan L. Siegel, who is in group practice in rheumatology in Rockville, Md. Dr. Siegel led a group of experts preparing psoriatic arthritis enthesitis treatment recommendations to be issued by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In a preliminary report on the planned treatment recommendations, which were delivered at the joint meetings of GRAPPA and the Spondyloarthritis Research & Treatment Network (SPARTAN), Dr. Siegel reported that the new recommendations will recognize only two gradations of enthesitis: mild or moderate/severe.
"It is difficult to differentiate moderate from severe because these have not been treated as distinct entities in the clinical trials," Dr. Siegel said. Patients experience severity in regard to intensity of pain and limitation of function, either or both of which may represent severe enthesitis in any given individual patient, according to Dr. Siegel, who also noted that the number of sites of activity is also generally unhelpful.
"Significant activity at a single site may be sufficient to produce a major functional deficit in one individual, whereas the activity at multiple sites may not produce much symptomatology or functional loss in another," Dr. Siegel said.
Enthesitis, an inflammation at the site where tendons or ligaments attach to bone, has been reported in up to 70% of patients with psoriatic arthritis. In some patients, it is the dominant symptom. However, the number of treatment trials in which control of enthesitis is the primary outcome continues to be limited, according to Dr. Siegel. He acknowledged that many of the proposed treatment recommendations owed more to expert opinion than to data.
This is true of the proposed first-line recommendation, he said, which is the use of nonsteroidal anti-inflammatory drugs and physical therapy. For NSAIDs, the wording of the recommendation will emphasize the need to monitor side effects.
The expert opinion of the GRAPPA consensus group was nearly unanimous that NSAIDs and physical therapy are effective and should be tried initially in both mild and moderate/severe disease, even though Dr. Siegel said that the supportive data from controlled trials are limited.
For those with moderate/severe enthesitis not sufficiently controlled on NSAIDs, alternatives include tumor necrosis factor inhibitors, ustekinumab, and apremilast. Some supportive data are available for each of these options, even though the consensus group concluded that there is not enough comparative evidence "to recommend one over another," he said.
Rather, the consensus group is recommending that the choice of therapies beyond NSAIDs and physical therapy be individualized in relationship to comorbidities, personal preference, and other considerations.
Special wording is being developed in regard to the use of corticosteroid injections. This wording was partly inspired by a meta-analysis that associated corticosteroid injections with an adverse effect on the integrity of tendons. Although there were many criticisms of this report, there was sufficient concern among the consensus group to urge that this treatment be used with caution.
"We think that these injections should only be provided by experienced physicians," Dr. Siegel reported. The wording in the preliminary draft is that adjunctive corticosteroid injections "may be considered on an individual basis."
When published, the enthesitis guidelines will include grading for the quality of the evidence behind each recommendation as well as the relative strength of the recommendation conferred by the expert panel.
Dr. Siegel reported financial relationships with Amgen and AbbVie.
AT THE 2014 GRAPPA AND SPARTAN ANNUAL MEETINGS
An insider’s look at the 2014 atopic dermatitis guidelines
COEUR D’ALENE, IDAHO – The 2014 American Academy of Dermatology atopic dermatitis guidelines may already need an update, according to the cochair of the guidelines panel.
The guidelines were based upon studies published through 2012. Since then, new evidence has emerged that raises the level of uncertainty regarding several key questions the panel addressed, Dr. Robert Sidbury observed at the annual meeting of the Society for Pediatric Dermatology. Among these questions: To bathe or not to bathe? Will a child outgrow atopic dermatitis?
Serving as cochair of the guidelines committee was both a reassuring and daunting experience, according to Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital.
It was reassuring to note that the committee members, who included 17 atopic dermatitis experts from three countries, were free from financial conflicts as they sifted through the published data for evidence-based recommendations to inform practice. But it was daunting to learn how sketchy the supporting evidence is for some of the conventional wisdom regarding atopic dermatitis management, he explained.
By way of providing what he called "a peek behind the curtains" of the guidelines-development process, Dr. Sidbury highlighted the issue of daily bathing followed by application of emollients and moisturizers. This was among the topics the panel struggled with the most, which might come as a surprise to outsiders who consider this to be standard practice, he noted.
"It seems like a very straightforward thing. Almost everyone in this room, to a person, recommends a daily bath followed by moisturizers, yet when we examined the studies we realized that recommendation isn’t based upon much evidence," he said.
Thus, the panel concluded that bathing is "suggested" for atopic dermatitis patients, while adding that "there is no standard" for the duration or frequency of bathing. The panel rated the strength of their recommendation as C, and the level of evidence as III.
"That’s a fairly weak recommendation based upon fairly week evidence," Dr. Sidbury commented.
Moreover, since publication of the guidelines, two new studies have come forth that address the question of whether bathing plus moisturizers is beneficial in atopic dermatitis. The results conflict with each other, making recommendations even more difficult.
Data from a retrospective study of 75 patients with moderate or severe atopic dermatitis suggested that a daily 15- to 20-minute bath followed by a mid-potency topical steroid and moisturizer was indeed beneficial: 79% of subjects showed marked improvement based on Investigator’s Global Assessment at week 3, and 4% were clear (Dermatitis 2014;25:56-9).
By contrast, data from a prospective trial in which 28 children with atopic dermatitis were randomized to a daily vs. twice-weekly bath followed by appropriate care indicated that, while hydration with emollients was important, bathing frequency wasn’t (Clin. Pediatr. 2014;53:677-81).
"This paper makes me feel better about the guidelines not saying, ‘You should bathe every day,’ although that’s still my own recommendation to patients," Dr. Sidbury said.
This year also has brought two conflicting studies regarding the natural history of atopic dermatitis. A large national Taiwanese population-based cohort study of children diagnosed with atopic dermatitis within the first 2 years of life and followed from birth to age 10 years concluded that 70% of these early-onset patients eventually went into remission. A total of 19% of patients did so within the first year, and 49% in less than 4 years. The median disease duration was 4.2 years (Br. J. Dermatol. 2014;170:130-5).
On the other hand, a report from the 7,157-patient, cross-sectional, longitudinal Pediatric Eczema Elective Registry (PEER) found that by age 20, only 50% of the patients had experienced at least one symptom-free period lasting 6 months or more. The investigators concluded that atopic dermatitis is probably a lifelong disease (JAMA Dermatology 2014;150:593-600).
"That’s a provocative conclusion, and a tough thing to tell a parent," Dr. Sidbury observed. "I offer parents realistic but optimistic counsel. I tell them the tendency toward xerosis, irritancy, and infection will persist – the patient in front of you is never going to want to wear a wool sweater for the rest of their life. But the incessant itch, the need for treatment, the impact on quality of life – which is really the issue at hand – hopefully will not persist."
Since the release earlier this year of the first three of the four sections of the atopic dermatitis guidelines, Dr. Sidbury and the other panelists have received considerable feedback that the guidelines didn’t adequately address the topic of topical steroid addiction.
"Some say we missed the boat in not making coherent recommendations to parents about it. We got some very pointed comments," he conceded.
He noted that a systematic review presented at last year’s International Symposium on Atopic Dermatitis concluded that topical steroid withdrawal is a real phenomenon distinct from other topical steroid side effects. It comes in two rosacea-like variants: an erythroedematous form and a papulopustular form. An atopic dermatitis patient’s report of a burning sensation upon cessation of topical steroid therapy is a red flag.
Despite the occasional missed opportunity in drawing up the first AAD atopic guidelines in 10 years, the process was richly rewarding, Dr. Sidbury said. And although experts will continue to debate the unresolved controversies in atopic dermatitis, for him the most important lesson to emerge from the panel’s comprehensive review of the evidence was strikingly clear: "Time and time again, education trumps all. Education of patients and families leads to the best outcomes. I think that’s an important lesson to take home," he said.
Dr. Sidbury had no financial conflicts to disclose.
COEUR D’ALENE, IDAHO – The 2014 American Academy of Dermatology atopic dermatitis guidelines may already need an update, according to the cochair of the guidelines panel.
The guidelines were based upon studies published through 2012. Since then, new evidence has emerged that raises the level of uncertainty regarding several key questions the panel addressed, Dr. Robert Sidbury observed at the annual meeting of the Society for Pediatric Dermatology. Among these questions: To bathe or not to bathe? Will a child outgrow atopic dermatitis?
Serving as cochair of the guidelines committee was both a reassuring and daunting experience, according to Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital.
It was reassuring to note that the committee members, who included 17 atopic dermatitis experts from three countries, were free from financial conflicts as they sifted through the published data for evidence-based recommendations to inform practice. But it was daunting to learn how sketchy the supporting evidence is for some of the conventional wisdom regarding atopic dermatitis management, he explained.
By way of providing what he called "a peek behind the curtains" of the guidelines-development process, Dr. Sidbury highlighted the issue of daily bathing followed by application of emollients and moisturizers. This was among the topics the panel struggled with the most, which might come as a surprise to outsiders who consider this to be standard practice, he noted.
"It seems like a very straightforward thing. Almost everyone in this room, to a person, recommends a daily bath followed by moisturizers, yet when we examined the studies we realized that recommendation isn’t based upon much evidence," he said.
Thus, the panel concluded that bathing is "suggested" for atopic dermatitis patients, while adding that "there is no standard" for the duration or frequency of bathing. The panel rated the strength of their recommendation as C, and the level of evidence as III.
"That’s a fairly weak recommendation based upon fairly week evidence," Dr. Sidbury commented.
Moreover, since publication of the guidelines, two new studies have come forth that address the question of whether bathing plus moisturizers is beneficial in atopic dermatitis. The results conflict with each other, making recommendations even more difficult.
Data from a retrospective study of 75 patients with moderate or severe atopic dermatitis suggested that a daily 15- to 20-minute bath followed by a mid-potency topical steroid and moisturizer was indeed beneficial: 79% of subjects showed marked improvement based on Investigator’s Global Assessment at week 3, and 4% were clear (Dermatitis 2014;25:56-9).
By contrast, data from a prospective trial in which 28 children with atopic dermatitis were randomized to a daily vs. twice-weekly bath followed by appropriate care indicated that, while hydration with emollients was important, bathing frequency wasn’t (Clin. Pediatr. 2014;53:677-81).
"This paper makes me feel better about the guidelines not saying, ‘You should bathe every day,’ although that’s still my own recommendation to patients," Dr. Sidbury said.
This year also has brought two conflicting studies regarding the natural history of atopic dermatitis. A large national Taiwanese population-based cohort study of children diagnosed with atopic dermatitis within the first 2 years of life and followed from birth to age 10 years concluded that 70% of these early-onset patients eventually went into remission. A total of 19% of patients did so within the first year, and 49% in less than 4 years. The median disease duration was 4.2 years (Br. J. Dermatol. 2014;170:130-5).
On the other hand, a report from the 7,157-patient, cross-sectional, longitudinal Pediatric Eczema Elective Registry (PEER) found that by age 20, only 50% of the patients had experienced at least one symptom-free period lasting 6 months or more. The investigators concluded that atopic dermatitis is probably a lifelong disease (JAMA Dermatology 2014;150:593-600).
"That’s a provocative conclusion, and a tough thing to tell a parent," Dr. Sidbury observed. "I offer parents realistic but optimistic counsel. I tell them the tendency toward xerosis, irritancy, and infection will persist – the patient in front of you is never going to want to wear a wool sweater for the rest of their life. But the incessant itch, the need for treatment, the impact on quality of life – which is really the issue at hand – hopefully will not persist."
Since the release earlier this year of the first three of the four sections of the atopic dermatitis guidelines, Dr. Sidbury and the other panelists have received considerable feedback that the guidelines didn’t adequately address the topic of topical steroid addiction.
"Some say we missed the boat in not making coherent recommendations to parents about it. We got some very pointed comments," he conceded.
He noted that a systematic review presented at last year’s International Symposium on Atopic Dermatitis concluded that topical steroid withdrawal is a real phenomenon distinct from other topical steroid side effects. It comes in two rosacea-like variants: an erythroedematous form and a papulopustular form. An atopic dermatitis patient’s report of a burning sensation upon cessation of topical steroid therapy is a red flag.
Despite the occasional missed opportunity in drawing up the first AAD atopic guidelines in 10 years, the process was richly rewarding, Dr. Sidbury said. And although experts will continue to debate the unresolved controversies in atopic dermatitis, for him the most important lesson to emerge from the panel’s comprehensive review of the evidence was strikingly clear: "Time and time again, education trumps all. Education of patients and families leads to the best outcomes. I think that’s an important lesson to take home," he said.
Dr. Sidbury had no financial conflicts to disclose.
COEUR D’ALENE, IDAHO – The 2014 American Academy of Dermatology atopic dermatitis guidelines may already need an update, according to the cochair of the guidelines panel.
The guidelines were based upon studies published through 2012. Since then, new evidence has emerged that raises the level of uncertainty regarding several key questions the panel addressed, Dr. Robert Sidbury observed at the annual meeting of the Society for Pediatric Dermatology. Among these questions: To bathe or not to bathe? Will a child outgrow atopic dermatitis?
Serving as cochair of the guidelines committee was both a reassuring and daunting experience, according to Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital.
It was reassuring to note that the committee members, who included 17 atopic dermatitis experts from three countries, were free from financial conflicts as they sifted through the published data for evidence-based recommendations to inform practice. But it was daunting to learn how sketchy the supporting evidence is for some of the conventional wisdom regarding atopic dermatitis management, he explained.
By way of providing what he called "a peek behind the curtains" of the guidelines-development process, Dr. Sidbury highlighted the issue of daily bathing followed by application of emollients and moisturizers. This was among the topics the panel struggled with the most, which might come as a surprise to outsiders who consider this to be standard practice, he noted.
"It seems like a very straightforward thing. Almost everyone in this room, to a person, recommends a daily bath followed by moisturizers, yet when we examined the studies we realized that recommendation isn’t based upon much evidence," he said.
Thus, the panel concluded that bathing is "suggested" for atopic dermatitis patients, while adding that "there is no standard" for the duration or frequency of bathing. The panel rated the strength of their recommendation as C, and the level of evidence as III.
"That’s a fairly weak recommendation based upon fairly week evidence," Dr. Sidbury commented.
Moreover, since publication of the guidelines, two new studies have come forth that address the question of whether bathing plus moisturizers is beneficial in atopic dermatitis. The results conflict with each other, making recommendations even more difficult.
Data from a retrospective study of 75 patients with moderate or severe atopic dermatitis suggested that a daily 15- to 20-minute bath followed by a mid-potency topical steroid and moisturizer was indeed beneficial: 79% of subjects showed marked improvement based on Investigator’s Global Assessment at week 3, and 4% were clear (Dermatitis 2014;25:56-9).
By contrast, data from a prospective trial in which 28 children with atopic dermatitis were randomized to a daily vs. twice-weekly bath followed by appropriate care indicated that, while hydration with emollients was important, bathing frequency wasn’t (Clin. Pediatr. 2014;53:677-81).
"This paper makes me feel better about the guidelines not saying, ‘You should bathe every day,’ although that’s still my own recommendation to patients," Dr. Sidbury said.
This year also has brought two conflicting studies regarding the natural history of atopic dermatitis. A large national Taiwanese population-based cohort study of children diagnosed with atopic dermatitis within the first 2 years of life and followed from birth to age 10 years concluded that 70% of these early-onset patients eventually went into remission. A total of 19% of patients did so within the first year, and 49% in less than 4 years. The median disease duration was 4.2 years (Br. J. Dermatol. 2014;170:130-5).
On the other hand, a report from the 7,157-patient, cross-sectional, longitudinal Pediatric Eczema Elective Registry (PEER) found that by age 20, only 50% of the patients had experienced at least one symptom-free period lasting 6 months or more. The investigators concluded that atopic dermatitis is probably a lifelong disease (JAMA Dermatology 2014;150:593-600).
"That’s a provocative conclusion, and a tough thing to tell a parent," Dr. Sidbury observed. "I offer parents realistic but optimistic counsel. I tell them the tendency toward xerosis, irritancy, and infection will persist – the patient in front of you is never going to want to wear a wool sweater for the rest of their life. But the incessant itch, the need for treatment, the impact on quality of life – which is really the issue at hand – hopefully will not persist."
Since the release earlier this year of the first three of the four sections of the atopic dermatitis guidelines, Dr. Sidbury and the other panelists have received considerable feedback that the guidelines didn’t adequately address the topic of topical steroid addiction.
"Some say we missed the boat in not making coherent recommendations to parents about it. We got some very pointed comments," he conceded.
He noted that a systematic review presented at last year’s International Symposium on Atopic Dermatitis concluded that topical steroid withdrawal is a real phenomenon distinct from other topical steroid side effects. It comes in two rosacea-like variants: an erythroedematous form and a papulopustular form. An atopic dermatitis patient’s report of a burning sensation upon cessation of topical steroid therapy is a red flag.
Despite the occasional missed opportunity in drawing up the first AAD atopic guidelines in 10 years, the process was richly rewarding, Dr. Sidbury said. And although experts will continue to debate the unresolved controversies in atopic dermatitis, for him the most important lesson to emerge from the panel’s comprehensive review of the evidence was strikingly clear: "Time and time again, education trumps all. Education of patients and families leads to the best outcomes. I think that’s an important lesson to take home," he said.
Dr. Sidbury had no financial conflicts to disclose.
EXPERT OPINION FROM THE SPD ANNUAL MEETING
New guidelines proposed for nail involvement in psoriatic arthritis
NEW YORK – A consensus group has developed evidence-based treatment recommendations for patients with psoriatic arthritis and nail involvement and raised the possibility of issuing them independent of recommendations for the skin, according to a report on the deliberations.
"The suggestion has been made to separate out skin from nail because the assessment is quite different to the point where you can potentially have patients with severe nail disease but limited skin disease," reported Dr. April W. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
The proposal was made in the course of presenting the preliminary evidence-based recommendations on managing psoriatic arthritis (PsA) nail involvement to the full membership of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. GRAPPA is in the process of preparing a new set of PsA treatment recommendations.
The decision to address nails separately from skin was not a unanimous recommendation within the committee, and no conclusion was reached, but Dr. Armstrong brought the issue forward "to be clear about our ambiguity" regarding this specific aspect of how to best outline treatment options useful to clinicians.
Other recommendations, based on evidence, are more assured of making the final version, because they are evidence based. When presented at GRAPPA, which convened its most recent annual meeting jointly with the Spondyloarthritis Research & Treatment Network, each recommendation was graded for the quality of the evidence and strength of the consensus.
For example, the committee found little controlled evidence to support a significant benefit from topical therapies, including those commonly used, such as steroids and vitamin D analogs. Although the consensus committee concluded that the risk of adverse events is low and the costs are low to moderate, the efficacy is only modest. The strength for recommending topical therapies overall was characterized as "weak."
For procedural therapies, such as pulsed-dye laser or intralesional injections, no placebo-controlled trials could be identified by the committee, and the expert opinion of its members was that the efficacy is relatively low in general even if benefit is achieved in some individuals. The cost was characterized as low to moderate. The committee concluded that only a "weak" recommendation should be conferred to these types of interventions for nail involvement.
The proposed recommendation for oral therapies, such a methotrexate, cyclosporine, leflunomide, and acitretin, was considered "stronger than that for topical therapies" when nail involvement is moderate, but the committee also found supportive evidence of benefit to be of "low quality." Again, although recognizing that some PsA patients may benefit, the recommendation for oral therapies overall for nail involvement was characterized as weak.
For biologics, including both tumor necrosis factor (TNF) inhibitors and the interleukin 12/23 inhibitor ustekinumab, the consensus committee reported that there are good quality data showing efficacy in nails, including some studies showing superiority of TNF inhibitors to methotrexate and cyclosporine. For patients with moderate to severe nail involvement warranting the potential risks of these therapies, the preliminary recommendation for these agents was "strong" even if the costs of these therapies were characterized as "high to crazy."
One unresolved issue, however, is which strategy to recommend for placing nail involvement into "mild," "moderate," or "severe" categories. Objective scores, such as Nail Psoriasis Severity Index (NAPSI), were considered by the consensus committee to be helpful but insufficient.
"Perhaps in addition to objective scoring of nail disease, we should also take into account [the impact] on quality of life as well as function," Dr. Armstrong said.
The final GRAPPA treatment recommendations for nail involvement, as well as other aspects of PsA management, will be made after a discussion of the consensus group proposals over the next several months, followed by voting among the GRAPPA membership. As GRAPPA is an international organization, it is intended that final recommendations will be broadly applicable.
Dr. Armstrong reported financial relationships with AbbVie, Amgen, Janssen Biotech, Merck, Novartis, and Pfizer.
NEW YORK – A consensus group has developed evidence-based treatment recommendations for patients with psoriatic arthritis and nail involvement and raised the possibility of issuing them independent of recommendations for the skin, according to a report on the deliberations.
"The suggestion has been made to separate out skin from nail because the assessment is quite different to the point where you can potentially have patients with severe nail disease but limited skin disease," reported Dr. April W. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
The proposal was made in the course of presenting the preliminary evidence-based recommendations on managing psoriatic arthritis (PsA) nail involvement to the full membership of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. GRAPPA is in the process of preparing a new set of PsA treatment recommendations.
The decision to address nails separately from skin was not a unanimous recommendation within the committee, and no conclusion was reached, but Dr. Armstrong brought the issue forward "to be clear about our ambiguity" regarding this specific aspect of how to best outline treatment options useful to clinicians.
Other recommendations, based on evidence, are more assured of making the final version, because they are evidence based. When presented at GRAPPA, which convened its most recent annual meeting jointly with the Spondyloarthritis Research & Treatment Network, each recommendation was graded for the quality of the evidence and strength of the consensus.
For example, the committee found little controlled evidence to support a significant benefit from topical therapies, including those commonly used, such as steroids and vitamin D analogs. Although the consensus committee concluded that the risk of adverse events is low and the costs are low to moderate, the efficacy is only modest. The strength for recommending topical therapies overall was characterized as "weak."
For procedural therapies, such as pulsed-dye laser or intralesional injections, no placebo-controlled trials could be identified by the committee, and the expert opinion of its members was that the efficacy is relatively low in general even if benefit is achieved in some individuals. The cost was characterized as low to moderate. The committee concluded that only a "weak" recommendation should be conferred to these types of interventions for nail involvement.
The proposed recommendation for oral therapies, such a methotrexate, cyclosporine, leflunomide, and acitretin, was considered "stronger than that for topical therapies" when nail involvement is moderate, but the committee also found supportive evidence of benefit to be of "low quality." Again, although recognizing that some PsA patients may benefit, the recommendation for oral therapies overall for nail involvement was characterized as weak.
For biologics, including both tumor necrosis factor (TNF) inhibitors and the interleukin 12/23 inhibitor ustekinumab, the consensus committee reported that there are good quality data showing efficacy in nails, including some studies showing superiority of TNF inhibitors to methotrexate and cyclosporine. For patients with moderate to severe nail involvement warranting the potential risks of these therapies, the preliminary recommendation for these agents was "strong" even if the costs of these therapies were characterized as "high to crazy."
One unresolved issue, however, is which strategy to recommend for placing nail involvement into "mild," "moderate," or "severe" categories. Objective scores, such as Nail Psoriasis Severity Index (NAPSI), were considered by the consensus committee to be helpful but insufficient.
"Perhaps in addition to objective scoring of nail disease, we should also take into account [the impact] on quality of life as well as function," Dr. Armstrong said.
The final GRAPPA treatment recommendations for nail involvement, as well as other aspects of PsA management, will be made after a discussion of the consensus group proposals over the next several months, followed by voting among the GRAPPA membership. As GRAPPA is an international organization, it is intended that final recommendations will be broadly applicable.
Dr. Armstrong reported financial relationships with AbbVie, Amgen, Janssen Biotech, Merck, Novartis, and Pfizer.
NEW YORK – A consensus group has developed evidence-based treatment recommendations for patients with psoriatic arthritis and nail involvement and raised the possibility of issuing them independent of recommendations for the skin, according to a report on the deliberations.
"The suggestion has been made to separate out skin from nail because the assessment is quite different to the point where you can potentially have patients with severe nail disease but limited skin disease," reported Dr. April W. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
The proposal was made in the course of presenting the preliminary evidence-based recommendations on managing psoriatic arthritis (PsA) nail involvement to the full membership of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. GRAPPA is in the process of preparing a new set of PsA treatment recommendations.
The decision to address nails separately from skin was not a unanimous recommendation within the committee, and no conclusion was reached, but Dr. Armstrong brought the issue forward "to be clear about our ambiguity" regarding this specific aspect of how to best outline treatment options useful to clinicians.
Other recommendations, based on evidence, are more assured of making the final version, because they are evidence based. When presented at GRAPPA, which convened its most recent annual meeting jointly with the Spondyloarthritis Research & Treatment Network, each recommendation was graded for the quality of the evidence and strength of the consensus.
For example, the committee found little controlled evidence to support a significant benefit from topical therapies, including those commonly used, such as steroids and vitamin D analogs. Although the consensus committee concluded that the risk of adverse events is low and the costs are low to moderate, the efficacy is only modest. The strength for recommending topical therapies overall was characterized as "weak."
For procedural therapies, such as pulsed-dye laser or intralesional injections, no placebo-controlled trials could be identified by the committee, and the expert opinion of its members was that the efficacy is relatively low in general even if benefit is achieved in some individuals. The cost was characterized as low to moderate. The committee concluded that only a "weak" recommendation should be conferred to these types of interventions for nail involvement.
The proposed recommendation for oral therapies, such a methotrexate, cyclosporine, leflunomide, and acitretin, was considered "stronger than that for topical therapies" when nail involvement is moderate, but the committee also found supportive evidence of benefit to be of "low quality." Again, although recognizing that some PsA patients may benefit, the recommendation for oral therapies overall for nail involvement was characterized as weak.
For biologics, including both tumor necrosis factor (TNF) inhibitors and the interleukin 12/23 inhibitor ustekinumab, the consensus committee reported that there are good quality data showing efficacy in nails, including some studies showing superiority of TNF inhibitors to methotrexate and cyclosporine. For patients with moderate to severe nail involvement warranting the potential risks of these therapies, the preliminary recommendation for these agents was "strong" even if the costs of these therapies were characterized as "high to crazy."
One unresolved issue, however, is which strategy to recommend for placing nail involvement into "mild," "moderate," or "severe" categories. Objective scores, such as Nail Psoriasis Severity Index (NAPSI), were considered by the consensus committee to be helpful but insufficient.
"Perhaps in addition to objective scoring of nail disease, we should also take into account [the impact] on quality of life as well as function," Dr. Armstrong said.
The final GRAPPA treatment recommendations for nail involvement, as well as other aspects of PsA management, will be made after a discussion of the consensus group proposals over the next several months, followed by voting among the GRAPPA membership. As GRAPPA is an international organization, it is intended that final recommendations will be broadly applicable.
Dr. Armstrong reported financial relationships with AbbVie, Amgen, Janssen Biotech, Merck, Novartis, and Pfizer.
AT THE 2014 GRAPPA AND SPARTAN ANNUAL MEETINGS
WHO recommends HIV pre-exposure prophylaxis as a prevention option
MELBOURNE – Men who have sex with men should consider pre-exposure prophylaxis with antiretroviral medications as an additional option to prevent HIV infection, according to the latest World Health Organization guidelines on HIV prevention, diagnosis, treatment, and care in high-risk populations.
The guidelines, released at the 20th International AIDS Conference, also introduce a new recommendation on providing access to naloxone and instructions on its use for anyone likely to witness an opioid overdose in a friend or relative, as part of a broader harm-reduction effort.
These are the first WHO guidelines on HIV/AIDS that bring together advice on five key population groups: men who have sex with men, injection drug users, sex workers, transgender people, and people in prisons.
Dr. Rachel Baggaley, guidelines coordinator from the HIV department at WHO, said the latest UNAIDS estimates suggest up to 50% of new infections are occurring among these groups because they are not getting the services they need.
While the idea of pre-exposure prophylaxis (PrEP) among men who have sex with men was first raised 3 years ago, Dr. Baggaley said the evidence now justified a strengthening of the recommendation.
"We’re just opening the door to suggest that this can be considered as an additional prevention choice, given the high incidence rates we are continuing to see in this population," Dr. Baggaley said in an interview.
"At the moment PrEP is a daily dose, and so for gay men who would want to use it, it would be offered as a daily dose for a period of time and it would be reviewed ... in consultation with the health care provider," she said.
Other recommendations included voluntary medical male circumcision, particularly in areas with hyperendemic HIV and low prevalence of circumcision, for the prevention of heterosexually acquired HIV in men, and daily oral pre-exposure prophylaxis – tenofovir alone or tenofovir and emtricitabine – for uninfected partners of HIV-positive individuals if additional HIV prevention choices are needed.
Dr. Chris Beyrer, director of the Johns Hopkins Center for Public Health and Human Rights, Baltimore, said that the recommendation on PrEP was about providing more options for HIV prevention.
"PrEP is not being recommended as a lifetime approach – it is important for men to consider as an option for prevention when they are sexually active and at risk of HIV exposure," Dr. Beyrer told the conference.
The guidelines also recommended routine screening and management of mental health disorders such as depression and psychosocial stress among HIV-positive people from these key populations, to optimize health outcomes and improve antiretroviral adherence.
For injection drug users, the guidelines recommended all people should have access to sterile injection equipment through needle and syringe programs, as well as a recommendation for availability and training in naloxone use for opioid overdose.
Dr. Beyrer described the naloxone recommendation as a lifesaving intervention and a public health and human rights advance.
The guidelines were funded by UNAIDS, the U.S. President’s Emergency Plan for AIDS Relief, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. There were no relevant conflicts of interest declared.
MELBOURNE – Men who have sex with men should consider pre-exposure prophylaxis with antiretroviral medications as an additional option to prevent HIV infection, according to the latest World Health Organization guidelines on HIV prevention, diagnosis, treatment, and care in high-risk populations.
The guidelines, released at the 20th International AIDS Conference, also introduce a new recommendation on providing access to naloxone and instructions on its use for anyone likely to witness an opioid overdose in a friend or relative, as part of a broader harm-reduction effort.
These are the first WHO guidelines on HIV/AIDS that bring together advice on five key population groups: men who have sex with men, injection drug users, sex workers, transgender people, and people in prisons.
Dr. Rachel Baggaley, guidelines coordinator from the HIV department at WHO, said the latest UNAIDS estimates suggest up to 50% of new infections are occurring among these groups because they are not getting the services they need.
While the idea of pre-exposure prophylaxis (PrEP) among men who have sex with men was first raised 3 years ago, Dr. Baggaley said the evidence now justified a strengthening of the recommendation.
"We’re just opening the door to suggest that this can be considered as an additional prevention choice, given the high incidence rates we are continuing to see in this population," Dr. Baggaley said in an interview.
"At the moment PrEP is a daily dose, and so for gay men who would want to use it, it would be offered as a daily dose for a period of time and it would be reviewed ... in consultation with the health care provider," she said.
Other recommendations included voluntary medical male circumcision, particularly in areas with hyperendemic HIV and low prevalence of circumcision, for the prevention of heterosexually acquired HIV in men, and daily oral pre-exposure prophylaxis – tenofovir alone or tenofovir and emtricitabine – for uninfected partners of HIV-positive individuals if additional HIV prevention choices are needed.
Dr. Chris Beyrer, director of the Johns Hopkins Center for Public Health and Human Rights, Baltimore, said that the recommendation on PrEP was about providing more options for HIV prevention.
"PrEP is not being recommended as a lifetime approach – it is important for men to consider as an option for prevention when they are sexually active and at risk of HIV exposure," Dr. Beyrer told the conference.
The guidelines also recommended routine screening and management of mental health disorders such as depression and psychosocial stress among HIV-positive people from these key populations, to optimize health outcomes and improve antiretroviral adherence.
For injection drug users, the guidelines recommended all people should have access to sterile injection equipment through needle and syringe programs, as well as a recommendation for availability and training in naloxone use for opioid overdose.
Dr. Beyrer described the naloxone recommendation as a lifesaving intervention and a public health and human rights advance.
The guidelines were funded by UNAIDS, the U.S. President’s Emergency Plan for AIDS Relief, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. There were no relevant conflicts of interest declared.
MELBOURNE – Men who have sex with men should consider pre-exposure prophylaxis with antiretroviral medications as an additional option to prevent HIV infection, according to the latest World Health Organization guidelines on HIV prevention, diagnosis, treatment, and care in high-risk populations.
The guidelines, released at the 20th International AIDS Conference, also introduce a new recommendation on providing access to naloxone and instructions on its use for anyone likely to witness an opioid overdose in a friend or relative, as part of a broader harm-reduction effort.
These are the first WHO guidelines on HIV/AIDS that bring together advice on five key population groups: men who have sex with men, injection drug users, sex workers, transgender people, and people in prisons.
Dr. Rachel Baggaley, guidelines coordinator from the HIV department at WHO, said the latest UNAIDS estimates suggest up to 50% of new infections are occurring among these groups because they are not getting the services they need.
While the idea of pre-exposure prophylaxis (PrEP) among men who have sex with men was first raised 3 years ago, Dr. Baggaley said the evidence now justified a strengthening of the recommendation.
"We’re just opening the door to suggest that this can be considered as an additional prevention choice, given the high incidence rates we are continuing to see in this population," Dr. Baggaley said in an interview.
"At the moment PrEP is a daily dose, and so for gay men who would want to use it, it would be offered as a daily dose for a period of time and it would be reviewed ... in consultation with the health care provider," she said.
Other recommendations included voluntary medical male circumcision, particularly in areas with hyperendemic HIV and low prevalence of circumcision, for the prevention of heterosexually acquired HIV in men, and daily oral pre-exposure prophylaxis – tenofovir alone or tenofovir and emtricitabine – for uninfected partners of HIV-positive individuals if additional HIV prevention choices are needed.
Dr. Chris Beyrer, director of the Johns Hopkins Center for Public Health and Human Rights, Baltimore, said that the recommendation on PrEP was about providing more options for HIV prevention.
"PrEP is not being recommended as a lifetime approach – it is important for men to consider as an option for prevention when they are sexually active and at risk of HIV exposure," Dr. Beyrer told the conference.
The guidelines also recommended routine screening and management of mental health disorders such as depression and psychosocial stress among HIV-positive people from these key populations, to optimize health outcomes and improve antiretroviral adherence.
For injection drug users, the guidelines recommended all people should have access to sterile injection equipment through needle and syringe programs, as well as a recommendation for availability and training in naloxone use for opioid overdose.
Dr. Beyrer described the naloxone recommendation as a lifesaving intervention and a public health and human rights advance.
The guidelines were funded by UNAIDS, the U.S. President’s Emergency Plan for AIDS Relief, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. There were no relevant conflicts of interest declared.
AT AIDS 2014
Key clinical point: Men who have sex with men should consider pre-exposure prophylaxis with antiretroviral medications as an additional option to prevent HIV infection.
Major finding: WHO guidelines on HIV infection bring together advice on five key groups: men who have sex with men, injection drug users, sex workers, transgender people, and people in prisons. In addition, the guidelines recommend that naloxone be made available to anyone likely to witness an opioid overdose.
Data source: WHO’s Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations.
Disclosures: The guidelines were funded by UNAIDS, the U.S. President’s Emergency Plan for AIDS Relief, and the Global Fund to Fight AIDS, Tuberculosis and Malaria. There were no relevant conflicts of interest declared.
WHO recommends HIV pre-exposure prophylaxis as a prevention option
MELBOURNE – Men who have sex with men should consider pre-exposure prophylaxis with antiretroviral medications as an additional option to prevent HIV infection, according to the latest World Health Organization guidelines on HIV prevention, diagnosis, treatment, and care in high-risk populations.
The guidelines, released at the 20th International AIDS Conference, also introduce a new recommendation on providing access to naloxone and instructions on its use for anyone likely to witness an opioid overdose in a friend or relative, as part of a broader harm-reduction effort.
These are the first WHO guidelines on HIV/AIDS that bring together advice on five key population groups: men who have sex with men, injection drug users, sex workers, transgender people, and people in prisons.
Dr. Rachel Baggaley, guidelines coordinator from the HIV department at WHO, said the latest UNAIDS estimates suggest up to 50% of new infections are occurring among these groups because they are not getting the services they need.
While the idea of pre-exposure prophylaxis (PrEP) among men who have sex with men was first raised 3 years ago, Dr. Baggaley said the evidence now justified a strengthening of the recommendation.
"We’re just opening the door to suggest that this can be considered as an additional prevention choice, given the high incidence rates we are continuing to see in this population," Dr. Baggaley said in an interview.
"At the moment PrEP is a daily dose, and so for gay men who would want to use it, it would be offered as a daily dose for a period of time and it would be reviewed ... in consultation with the health care provider," she said.
Other recommendations included voluntary medical male circumcision, particularly in areas with hyperendemic HIV and low prevalence of circumcision, for the prevention of heterosexually acquired HIV in men, and daily oral pre-exposure prophylaxis – tenofovir alone or tenofovir and emtricitabine – for uninfected partners of HIV-positive individuals if additional HIV prevention choices are needed.
Dr. Chris Beyrer, director of the Johns Hopkins Center for Public Health and Human Rights, Baltimore, said that the recommendation on PrEP was about providing more options for HIV prevention.
"PrEP is not being recommended as a lifetime approach – it is important for men to consider as an option for prevention when they are sexually active and at risk of HIV exposure," Dr. Beyrer told the conference.
The guidelines also recommended routine screening and management of mental health disorders such as depression and psychosocial stress among HIV-positive people from these key populations, to optimize health outcomes and improve antiretroviral adherence.
For injection drug users, the guidelines recommended all people should have access to sterile injection equipment through needle and syringe programs, as well as a recommendation for availability and training in naloxone use for opioid overdose.
Dr. Beyrer described the naloxone recommendation as a lifesaving intervention and a public health and human rights advance.
The guidelines were funded by UNAIDS, the U.S. President’s Emergency Plan for AIDS Relief, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. There were no relevant conflicts of interest declared.
MELBOURNE – Men who have sex with men should consider pre-exposure prophylaxis with antiretroviral medications as an additional option to prevent HIV infection, according to the latest World Health Organization guidelines on HIV prevention, diagnosis, treatment, and care in high-risk populations.
The guidelines, released at the 20th International AIDS Conference, also introduce a new recommendation on providing access to naloxone and instructions on its use for anyone likely to witness an opioid overdose in a friend or relative, as part of a broader harm-reduction effort.
These are the first WHO guidelines on HIV/AIDS that bring together advice on five key population groups: men who have sex with men, injection drug users, sex workers, transgender people, and people in prisons.
Dr. Rachel Baggaley, guidelines coordinator from the HIV department at WHO, said the latest UNAIDS estimates suggest up to 50% of new infections are occurring among these groups because they are not getting the services they need.
While the idea of pre-exposure prophylaxis (PrEP) among men who have sex with men was first raised 3 years ago, Dr. Baggaley said the evidence now justified a strengthening of the recommendation.
"We’re just opening the door to suggest that this can be considered as an additional prevention choice, given the high incidence rates we are continuing to see in this population," Dr. Baggaley said in an interview.
"At the moment PrEP is a daily dose, and so for gay men who would want to use it, it would be offered as a daily dose for a period of time and it would be reviewed ... in consultation with the health care provider," she said.
Other recommendations included voluntary medical male circumcision, particularly in areas with hyperendemic HIV and low prevalence of circumcision, for the prevention of heterosexually acquired HIV in men, and daily oral pre-exposure prophylaxis – tenofovir alone or tenofovir and emtricitabine – for uninfected partners of HIV-positive individuals if additional HIV prevention choices are needed.
Dr. Chris Beyrer, director of the Johns Hopkins Center for Public Health and Human Rights, Baltimore, said that the recommendation on PrEP was about providing more options for HIV prevention.
"PrEP is not being recommended as a lifetime approach – it is important for men to consider as an option for prevention when they are sexually active and at risk of HIV exposure," Dr. Beyrer told the conference.
The guidelines also recommended routine screening and management of mental health disorders such as depression and psychosocial stress among HIV-positive people from these key populations, to optimize health outcomes and improve antiretroviral adherence.
For injection drug users, the guidelines recommended all people should have access to sterile injection equipment through needle and syringe programs, as well as a recommendation for availability and training in naloxone use for opioid overdose.
Dr. Beyrer described the naloxone recommendation as a lifesaving intervention and a public health and human rights advance.
The guidelines were funded by UNAIDS, the U.S. President’s Emergency Plan for AIDS Relief, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. There were no relevant conflicts of interest declared.
MELBOURNE – Men who have sex with men should consider pre-exposure prophylaxis with antiretroviral medications as an additional option to prevent HIV infection, according to the latest World Health Organization guidelines on HIV prevention, diagnosis, treatment, and care in high-risk populations.
The guidelines, released at the 20th International AIDS Conference, also introduce a new recommendation on providing access to naloxone and instructions on its use for anyone likely to witness an opioid overdose in a friend or relative, as part of a broader harm-reduction effort.
These are the first WHO guidelines on HIV/AIDS that bring together advice on five key population groups: men who have sex with men, injection drug users, sex workers, transgender people, and people in prisons.
Dr. Rachel Baggaley, guidelines coordinator from the HIV department at WHO, said the latest UNAIDS estimates suggest up to 50% of new infections are occurring among these groups because they are not getting the services they need.
While the idea of pre-exposure prophylaxis (PrEP) among men who have sex with men was first raised 3 years ago, Dr. Baggaley said the evidence now justified a strengthening of the recommendation.
"We’re just opening the door to suggest that this can be considered as an additional prevention choice, given the high incidence rates we are continuing to see in this population," Dr. Baggaley said in an interview.
"At the moment PrEP is a daily dose, and so for gay men who would want to use it, it would be offered as a daily dose for a period of time and it would be reviewed ... in consultation with the health care provider," she said.
Other recommendations included voluntary medical male circumcision, particularly in areas with hyperendemic HIV and low prevalence of circumcision, for the prevention of heterosexually acquired HIV in men, and daily oral pre-exposure prophylaxis – tenofovir alone or tenofovir and emtricitabine – for uninfected partners of HIV-positive individuals if additional HIV prevention choices are needed.
Dr. Chris Beyrer, director of the Johns Hopkins Center for Public Health and Human Rights, Baltimore, said that the recommendation on PrEP was about providing more options for HIV prevention.
"PrEP is not being recommended as a lifetime approach – it is important for men to consider as an option for prevention when they are sexually active and at risk of HIV exposure," Dr. Beyrer told the conference.
The guidelines also recommended routine screening and management of mental health disorders such as depression and psychosocial stress among HIV-positive people from these key populations, to optimize health outcomes and improve antiretroviral adherence.
For injection drug users, the guidelines recommended all people should have access to sterile injection equipment through needle and syringe programs, as well as a recommendation for availability and training in naloxone use for opioid overdose.
Dr. Beyrer described the naloxone recommendation as a lifesaving intervention and a public health and human rights advance.
The guidelines were funded by UNAIDS, the U.S. President’s Emergency Plan for AIDS Relief, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. There were no relevant conflicts of interest declared.
AT AIDS 2014
Key clinical point: Men who have sex with men should consider pre-exposure prophylaxis with antiretroviral medications as an additional option to prevent HIV infection.
Major finding: WHO guidelines on HIV infection bring together advice on five key groups: men who have sex with men, injection drug users, sex workers, transgender people, and people in prisons. In addition, the guidelines recommend that naloxone be made available to anyone likely to witness an opioid overdose.
Data source: WHO’s Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations.
Disclosures: The guidelines were funded by UNAIDS, the U.S. President’s Emergency Plan for AIDS Relief, and the Global Fund to Fight AIDS, Tuberculosis and Malaria. There were no relevant conflicts of interest declared.
First comorbidity guidelines drafted for psoriatic arthritis
NEW YORK – For the first time, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis is preparing evidence-based treatment recommendations for the diagnosis and management of the comorbidities associated with psoriatic arthritis.
"I am really excited that we are taking the initiative to treat and manage the psoriatic arthritis [PsA] patient as a whole," reported Dr. Elaine Husni, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic. The guidelines are meant to be a "platform on which to raise awareness" and foster education.
An initial set of guidelines on comorbidities was drafted at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Spondyloarthritis Research & Treatment Network (SPARTAN). Dr. Husni led the consensus group and fielded questions about key recommendations.
The first of these recommendations, which will undergo a process of discussion and review prior to formal adoption, states that all patients with PsA should be evaluated for cardiovascular (CV) disease. The consensus group labeled this recommendation "strong" even while conferring it with grade D evidence.
"The grade D was based on the fact that there are no outcomes data specifically in patients with psoriatic arthritis," observed Dr. Alexis R. Ogdie-Beatty, director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia. A member of the consensus group, Dr. Ogdie-Beatty suggested that benefit from CV screening is still a reasonable expectation "given the growing evidence that patients with PsA are at increased risk."
Similar "strong" recommendations but grade D evidence were given for screening for ophthalmic complications and inflammatory bowel disease. In these cases, there is good evidence for an association with PsA but limited evidence that screening will lead to improved outcome. The exception was obesity for which the group gave a B rating to the evidence for benefit from diagnosis and treatment.
Screening for diabetes was also included among recommendations, but it was given a "weak" rating and a grade D for supportive evidence.
Most of the other recommendations involved screening for various infections, such as hepatitis C virus (HCV), HIV, and tuberculosis, prior to initiating immunosuppressant therapies, particularly biologics. The level of evidence for these recommendations typically ranged between B and C even though all were given strong recommendations.
The consensus recommendations are not expected to include much detail about the specific management of comorbidities. The reason is concern about their applicability across various settings of care. It was thought that GRAPPA, as an international organization, should accommodate different types of practice. For example, the group cautioned against outlining steps of CV risk management, which may be managed by rheumatologists in some areas of the world but by specialists in others.
"We want to stay away from being minicardiologists," Dr. Husni explained. She indicated that the goal of the recommendations is to simply identify the specific types of comorbidity screening that should be considered "core recommendations" in the approach to PsA.
However, there is interest in creating a table regarding the use of specific medications for PsA treatment in the context of comorbidities. In a color-coded draft presented at the GRAPPA and SPARTAN meeting, some examples included caution in the use of NSAIDs in patients with CV disease, a need for monitoring when using cyclosporine in patients with chronic kidney disease, and a preference for etanercept over other tumor necrosis factor inhibitors in patients with HCV infection.
Overall, recommendations for comorbidities were identified as an important step in defining optimal care for PsA. According to Dr. Ogdie-Beatty, "the most important point may be to raise awareness." As these recommendations wend their way through an approval process at the organizational level, Dr. Ogdie-Beatty expressed hope that the final wording is specific enough to encourage attention to comorbidities without restricting a variety of valid approaches.
Dr. Husni reported a financial relationship with Celgene. Dr. Ogdie-Beatty had no potential conflicts of interest to report.
NEW YORK – For the first time, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis is preparing evidence-based treatment recommendations for the diagnosis and management of the comorbidities associated with psoriatic arthritis.
"I am really excited that we are taking the initiative to treat and manage the psoriatic arthritis [PsA] patient as a whole," reported Dr. Elaine Husni, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic. The guidelines are meant to be a "platform on which to raise awareness" and foster education.
An initial set of guidelines on comorbidities was drafted at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Spondyloarthritis Research & Treatment Network (SPARTAN). Dr. Husni led the consensus group and fielded questions about key recommendations.
The first of these recommendations, which will undergo a process of discussion and review prior to formal adoption, states that all patients with PsA should be evaluated for cardiovascular (CV) disease. The consensus group labeled this recommendation "strong" even while conferring it with grade D evidence.
"The grade D was based on the fact that there are no outcomes data specifically in patients with psoriatic arthritis," observed Dr. Alexis R. Ogdie-Beatty, director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia. A member of the consensus group, Dr. Ogdie-Beatty suggested that benefit from CV screening is still a reasonable expectation "given the growing evidence that patients with PsA are at increased risk."
Similar "strong" recommendations but grade D evidence were given for screening for ophthalmic complications and inflammatory bowel disease. In these cases, there is good evidence for an association with PsA but limited evidence that screening will lead to improved outcome. The exception was obesity for which the group gave a B rating to the evidence for benefit from diagnosis and treatment.
Screening for diabetes was also included among recommendations, but it was given a "weak" rating and a grade D for supportive evidence.
Most of the other recommendations involved screening for various infections, such as hepatitis C virus (HCV), HIV, and tuberculosis, prior to initiating immunosuppressant therapies, particularly biologics. The level of evidence for these recommendations typically ranged between B and C even though all were given strong recommendations.
The consensus recommendations are not expected to include much detail about the specific management of comorbidities. The reason is concern about their applicability across various settings of care. It was thought that GRAPPA, as an international organization, should accommodate different types of practice. For example, the group cautioned against outlining steps of CV risk management, which may be managed by rheumatologists in some areas of the world but by specialists in others.
"We want to stay away from being minicardiologists," Dr. Husni explained. She indicated that the goal of the recommendations is to simply identify the specific types of comorbidity screening that should be considered "core recommendations" in the approach to PsA.
However, there is interest in creating a table regarding the use of specific medications for PsA treatment in the context of comorbidities. In a color-coded draft presented at the GRAPPA and SPARTAN meeting, some examples included caution in the use of NSAIDs in patients with CV disease, a need for monitoring when using cyclosporine in patients with chronic kidney disease, and a preference for etanercept over other tumor necrosis factor inhibitors in patients with HCV infection.
Overall, recommendations for comorbidities were identified as an important step in defining optimal care for PsA. According to Dr. Ogdie-Beatty, "the most important point may be to raise awareness." As these recommendations wend their way through an approval process at the organizational level, Dr. Ogdie-Beatty expressed hope that the final wording is specific enough to encourage attention to comorbidities without restricting a variety of valid approaches.
Dr. Husni reported a financial relationship with Celgene. Dr. Ogdie-Beatty had no potential conflicts of interest to report.
NEW YORK – For the first time, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis is preparing evidence-based treatment recommendations for the diagnosis and management of the comorbidities associated with psoriatic arthritis.
"I am really excited that we are taking the initiative to treat and manage the psoriatic arthritis [PsA] patient as a whole," reported Dr. Elaine Husni, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic. The guidelines are meant to be a "platform on which to raise awareness" and foster education.
An initial set of guidelines on comorbidities was drafted at the joint meetings of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Spondyloarthritis Research & Treatment Network (SPARTAN). Dr. Husni led the consensus group and fielded questions about key recommendations.
The first of these recommendations, which will undergo a process of discussion and review prior to formal adoption, states that all patients with PsA should be evaluated for cardiovascular (CV) disease. The consensus group labeled this recommendation "strong" even while conferring it with grade D evidence.
"The grade D was based on the fact that there are no outcomes data specifically in patients with psoriatic arthritis," observed Dr. Alexis R. Ogdie-Beatty, director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia. A member of the consensus group, Dr. Ogdie-Beatty suggested that benefit from CV screening is still a reasonable expectation "given the growing evidence that patients with PsA are at increased risk."
Similar "strong" recommendations but grade D evidence were given for screening for ophthalmic complications and inflammatory bowel disease. In these cases, there is good evidence for an association with PsA but limited evidence that screening will lead to improved outcome. The exception was obesity for which the group gave a B rating to the evidence for benefit from diagnosis and treatment.
Screening for diabetes was also included among recommendations, but it was given a "weak" rating and a grade D for supportive evidence.
Most of the other recommendations involved screening for various infections, such as hepatitis C virus (HCV), HIV, and tuberculosis, prior to initiating immunosuppressant therapies, particularly biologics. The level of evidence for these recommendations typically ranged between B and C even though all were given strong recommendations.
The consensus recommendations are not expected to include much detail about the specific management of comorbidities. The reason is concern about their applicability across various settings of care. It was thought that GRAPPA, as an international organization, should accommodate different types of practice. For example, the group cautioned against outlining steps of CV risk management, which may be managed by rheumatologists in some areas of the world but by specialists in others.
"We want to stay away from being minicardiologists," Dr. Husni explained. She indicated that the goal of the recommendations is to simply identify the specific types of comorbidity screening that should be considered "core recommendations" in the approach to PsA.
However, there is interest in creating a table regarding the use of specific medications for PsA treatment in the context of comorbidities. In a color-coded draft presented at the GRAPPA and SPARTAN meeting, some examples included caution in the use of NSAIDs in patients with CV disease, a need for monitoring when using cyclosporine in patients with chronic kidney disease, and a preference for etanercept over other tumor necrosis factor inhibitors in patients with HCV infection.
Overall, recommendations for comorbidities were identified as an important step in defining optimal care for PsA. According to Dr. Ogdie-Beatty, "the most important point may be to raise awareness." As these recommendations wend their way through an approval process at the organizational level, Dr. Ogdie-Beatty expressed hope that the final wording is specific enough to encourage attention to comorbidities without restricting a variety of valid approaches.
Dr. Husni reported a financial relationship with Celgene. Dr. Ogdie-Beatty had no potential conflicts of interest to report.
AT THE 2014 GRAPPA AND SPARTAN ANNUAL MEETINGS