User login
Pediatric emergencies associated with unnecessary testing: AAP
Children seen for these conditions in emergency settings and even in primary care offices could experience avoidable pain, exposure to harmful radiation, and other harms, according to the group.
“The emergency department has the ability to rapidly perform myriad diagnostic tests and receive results quickly,” said Paul Mullan, MD, MPH, chair of the AAP’s Section of Emergency Medicine’s Choosing Wisely task force. “However, this comes with the danger of diagnostic overtesting.”
The five recommendations are as follows:
- Radiographs should not be obtained for children with bronchiolitis, croup, asthma, or first-time wheezing.
- Laboratory tests for screening should not be undertaken in the medical clearance process of children who require inpatient psychiatric admission unless clinically indicated.
- Laboratory testing or a CT scan of the head should not be ordered for a child with an unprovoked, generalized seizure or a simple febrile seizure whose mental status has returned to baseline.
- Abdominal radiographs should not be obtained for suspected constipation.
- Comprehensive viral panel testing should not be undertaken for children who are suspected of having respiratory viral illnesses.
The AAP task force partnered with Choosing Wisely Canada to create the recommendations. The list is the first of its kind to be published jointly by two countries, according to the release.
“We hope this Choosing Wisely list will encourage clinicians to rely on their clinical skills and avoid unnecessary tests,” said Dr. Mullan, who is also a physician at Children’s Hospital of the King’s Daughters and professor of pediatrics at Eastern Virginia Medical School, Norfolk.
A version of this article first appeared on Medscape.com.
Children seen for these conditions in emergency settings and even in primary care offices could experience avoidable pain, exposure to harmful radiation, and other harms, according to the group.
“The emergency department has the ability to rapidly perform myriad diagnostic tests and receive results quickly,” said Paul Mullan, MD, MPH, chair of the AAP’s Section of Emergency Medicine’s Choosing Wisely task force. “However, this comes with the danger of diagnostic overtesting.”
The five recommendations are as follows:
- Radiographs should not be obtained for children with bronchiolitis, croup, asthma, or first-time wheezing.
- Laboratory tests for screening should not be undertaken in the medical clearance process of children who require inpatient psychiatric admission unless clinically indicated.
- Laboratory testing or a CT scan of the head should not be ordered for a child with an unprovoked, generalized seizure or a simple febrile seizure whose mental status has returned to baseline.
- Abdominal radiographs should not be obtained for suspected constipation.
- Comprehensive viral panel testing should not be undertaken for children who are suspected of having respiratory viral illnesses.
The AAP task force partnered with Choosing Wisely Canada to create the recommendations. The list is the first of its kind to be published jointly by two countries, according to the release.
“We hope this Choosing Wisely list will encourage clinicians to rely on their clinical skills and avoid unnecessary tests,” said Dr. Mullan, who is also a physician at Children’s Hospital of the King’s Daughters and professor of pediatrics at Eastern Virginia Medical School, Norfolk.
A version of this article first appeared on Medscape.com.
Children seen for these conditions in emergency settings and even in primary care offices could experience avoidable pain, exposure to harmful radiation, and other harms, according to the group.
“The emergency department has the ability to rapidly perform myriad diagnostic tests and receive results quickly,” said Paul Mullan, MD, MPH, chair of the AAP’s Section of Emergency Medicine’s Choosing Wisely task force. “However, this comes with the danger of diagnostic overtesting.”
The five recommendations are as follows:
- Radiographs should not be obtained for children with bronchiolitis, croup, asthma, or first-time wheezing.
- Laboratory tests for screening should not be undertaken in the medical clearance process of children who require inpatient psychiatric admission unless clinically indicated.
- Laboratory testing or a CT scan of the head should not be ordered for a child with an unprovoked, generalized seizure or a simple febrile seizure whose mental status has returned to baseline.
- Abdominal radiographs should not be obtained for suspected constipation.
- Comprehensive viral panel testing should not be undertaken for children who are suspected of having respiratory viral illnesses.
The AAP task force partnered with Choosing Wisely Canada to create the recommendations. The list is the first of its kind to be published jointly by two countries, according to the release.
“We hope this Choosing Wisely list will encourage clinicians to rely on their clinical skills and avoid unnecessary tests,” said Dr. Mullan, who is also a physician at Children’s Hospital of the King’s Daughters and professor of pediatrics at Eastern Virginia Medical School, Norfolk.
A version of this article first appeared on Medscape.com.
Women docs: How your next job contract can reflect your real goals
Rebecca Chester, MD, an Arizona-based interventional cardiologist, recently left her position in a private practice and started employment at a hospital system.
“When I was negotiating my previous contract with the private practice, I found that navigating contracts from the standpoint of a woman still in childbearing years was a little disappointing and challenging,” Dr. Chester told this news organization.
“I wanted to have more children and hired a lawyer recommended by a male colleague to help me not only understand the contract but also negotiate time off and maternity leave, but the lawyer discouraged me from advocating for maternity leave, feeling that it might stigmatize me and prevent me from getting a job,” she says.
He also didn’t explain very much. “He just said it falls under ‘disability leave’ and left it at that.”
Fortunately, Dr. Chester had a good experience with the group. “As things turned out, I did have a child later that year, and they treated me well – I actually got time off – and they didn’t make me take extra call. But it might have turned out very differently because I didn’t know what I was getting into. If I hadn’t worked for such a conscientious group, I might have been in a much tougher situation.”
Since then, Dr. Chester has spoken to female colleagues who received “more support from their legal advisors regarding maternity leave.” She suggests turning to female physicians for recommendations to a lawyer.
Although the central components of a contract (for example, noncompete covenants, malpractice “tail” coverage, bonus structure, vacation time, disability, and call) are relevant to physicians of all genders, the needs of women and men are often different.
Dennis Hursh, managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, told this news organization that women physicians have “several issues that need special attention when negotiating their physician employment agreements.”
It starts with the interview
“Women have to be sensitive to the interviewer’s casual ‘let’s-get-to-know-each-other’ types of questions that may seem natural but really are unlawful to bring into an employment interview,” said Mr. Hursh.
He warned women to beware of questions such as “Are you married? Do you have kids? Are you planning to start a family?” These may be friendly chit-chat for male interviewees but there may be other agendas when asked to a prospective female employee.
Many of Mr. Hursh’s female clients have been asked this type of question, which “should be regarded as a ‘red flag.’ Yes, it may be an innocent, well-intentioned ice-breaker, but it’s actually unlawful to bring that up in an employment setting and, according to the Equal Opportunity Commission, can be seen as a form of discrimination.” He advises female physicians not to engage with the question and simply to refocus the discussion.
Know your worth and go for it
Medscape’s Physician Compensation surveys have consistently found discrepancies in earnings between male and female physicians, both in primary care and in specialties. In 2022, male primary care physicians earned 23% more than their female counterparts, whereas male specialists earned 31% more.
One reason may be that women tend to be more timid about negotiating for better compensation packages. Amanda Hill of Hill Health Law, a health care practice based in Austin, Tex., told this news organization that in her experience one of the most “overarching” features of female physicians is “that they either don’t know what they’re worth or they undersell themselves.”
In contrast to men, “many women are afraid of coming across as greedy or crass, or even demanding or bossy. But it’s a misperception that if you ask for more money, your future employer will hate you or won’t hire you,” said Ms. Hill.
Ms. Hill and Mr. Hursh encourage physicians to find out what they’re worth, which varies by region and specialty, by consulting benchmarks provided by companies such as Medical Group Management Association.
Jon Appino, MBA, principal and founder of Contract Diagnostics, a Kansas City–based consulting company that specializes in physician employment contract reviews, told this news organization that it’s important to look beyond the salary at other aspects of the position. For example, some figures “don’t take into account how much call a physician is taking. You may know what the average ob.gyn. is making, but an ob.gyn. may be working 3 days a week, while another one is working 6 days a week, one may be on call 15 times per month and another may be on call 15 times a quarter.” Other components of compensation include relative value unit (RVU) thresholds and bonuses.
Once you have that information, “don’t be intimidated, even if you’re sitting in front of several executives who are savvy about negotiations, and don’t worry about coming across as ‘high-maintenance’ or ‘all about money,’ ” Mr. Appino says. Proceed with confidence, knowing your worth and pursuing it.
Part-time vs. full-time
Mr. Appino has seen “more female than male physicians who want to work less than full-time. So it’s important to clarify whether that’s a possibility now or in the future and to understand the implications of working part-time.”
He explained that a full-time employee typically puts in a 40-hour work week, which translates into 1.0 Full-Time Equivalent (FTE), or one unit of work. “For example, if a person wants to work 0.8 FTEs – 4 days a week – is vacation time pro-rated? At what point is there a medical insurance fall-off or a higher monthly premium?”
In medical settings, FTEs may be tied to different metrics rather than the number of weekly hours – for example, for a hospitalist, it might be a certain number of shifts and might also vary by specialty. And it affects the call schedule too. “Call is hard to pro-rate. Many hospital bylaws mandate that call be divided equally, but if one surgeon is working 1.0 FTEs and another is working 0.8 FTEs, how does that call schedule get divided?”
Maternity leave: A tricky question
Many attorneys counsel against raising the question out of fear of scaring away potential employers.
“On the one hand, it is and should be absolutely reasonable to ask about the maternity leave policy or even negotiate for paid leave or additional leave, but it also highlights that you’re planning to have a baby and be out for months,” said Ms. Hill.
“And as much as we want people to be fair and reasonable, on the side of the employer, bias still very much exists, especially in a situation where revenue is based on group numbers. So suddenly, the employer thinks up some ‘nondiscriminatory’ reason why that person isn’t a great fit for the organization.”
Andrew Knoll, MD, JD, a former hospitalist who is now a partner with Cohen Compagni Beckman Appler & Knoll PLLC in Syracuse, N.Y., said that maternity leave is “rare” in an employment agreement, except sometimes in small private practice groups, because it often falls under the purview of “disability leave,” and “from a legal perspective, it’s no different than any other type of disability leave.”
The Family and Medical Leave Act (FMLA), which applies if a group is large enough, allows employees 12 weeks of unpaid leave, during which time their job is protected and their benefits maintained. And some states require employers to offer paid family leave.
“During this time, the woman can take time off – albeit without pay sometimes – to bond with the baby,” Dr. Knoll says. “Since there are statutory laws that protect the employee’s job, offering specific paid maternity leave is very unlikely.”
Ms. Hill advises carefully examining the employer’s comprehensive benefits plan to ascertain if paid maternity leave is included in the benefits. “But unless you’re currently pregnant and want to start off the relationship with true transparency – ‘I’m due in April and curious how we can handle that if you hire me now’ – I would keep the family planning questions to yourself before you get the job.”
Mr. Hursh, author of “The Final Hurdle: A Physician’s Guide to Negotiating a Fair Employment Agreement” (Charleston, S.C.: Advantage, 2012), has a different perspective. “I think all women, no matter how old they are, should ask about maternity leave, whether or not they’re planning a family,” he said.
“The employer may say, ‘We treat maternity leave like any other disability; our policy is such-and-such.’ If they cite an unacceptable policy, it’s a red flag about how they treat women, and should give a woman pause before accepting a position at that organization. Even if your rights are protected under the law and the organization’s policy is violating the law, no one wants to go to battle with HR or to have to go to court.”
Do you want partnership?
Not all female physicians entering a private practice want to advance to becoming partners. Many who are balancing family and work commitments “would prefer to just go into the office, perform their clinical responsibilities, go home, and be done” without the extra headaches, tasks, and time involved with business leadership, says Mr. Appino.
Some private practices have different contracts for those on partnership vs. nonpartnership tracks, so “you should ascertain this information and make sure it’s not automatically assumed that you would like to be on a partnership track,” says Mr. Appino.
On the other hand, you may want to become a partner. “I always suggest asking about the possibility of obtaining a leadership position in an organization or becoming an owner in a private practice,” says Mr. Hursh. “You may be told, ‘We’ve never had a woman in leadership before.’ This might be innocent if you’re the first woman hired, but it might be a red flag as to how women are regarded.” Either way, it’s important to have the information and know what your options are.
The impact of shift schedule
Mr. Hursh advises drilling down into the specifics of the schedule if you’re considering becoming a hospitalist. “Does a ‘week-on/week-off’ shift schedule assume you’ll be taking your vacation time or completing your CME requirements during the week off? This is important for all physicians, but especially for women who might want to use their weeks off to attend to children and family.”
Moreover, “there should be limits on shifts. You shouldn’t have a full day shift followed by a night shift. And there should be a limit on the number of shifts you work without time off. Twelve would be a brutal schedule. Seven is a reasonable amount. Make sure this is in writing and that the contract protects you. Don’t allow the employer to say, ‘We expect you to do the work we assign’ and leave it vague.”
Often, hospitalists will receive an annual salary under the assumption that a certain minimum number of shifts will be completed, but there is no maximum. “It’s important that the salary includes the minimum number of shifts, but that a compensation structure is created so that additional shifts receive additional compensation,” Mr. Hursh said.
Removing the ‘golden handcuffs’
Ms. Hill observes that there are some “really terrible contracts out there, which physicians – especially women – often feel pressured into signing.” They’re told, “This is our standard contract. You won’t find anything better.” Or, “Don’t worry about the small print and legalese.” The physician “gets scared or is artificially reassured, signs an overwhelmingly unfair contract, and then feels stuck.”
Being stuck in a bad contract “is debilitating and adds to burnout, feeling of depression, and the sense that there is no recourse and nowhere to go, especially if your family depends on you,” Ms. Hill said.
“More women than men feel hamstrung or are resigned to being harassed – which is not uncommon in the medical setting, especially in surgical specialties – or just accept poor treatment,” Ms. Hill added. Yes, you can “fight the system and go to HR, but fighting the system is very hard.”
She urges women “not to feel stuck or imprisoned by the ‘golden handcuffs’ but to consult a good lawyer, even if you have to break the contract.” Be aware of the reasons for your unhappiness and bring them to your lawyer – perhaps the system has engaged in fraud, perhaps there has been sexual or racial harassment, perhaps the organization hasn’t followed its own compliance policies.
Dr. Chester consulted Ms. Hill before signing the contract for her current position. “I wanted someone who could give me personalized advice, not only generic advice, and who understood my needs as a woman.”
Ms. Hill helped her to understand “what was and wasn’t fair and reasonable, what changes I could request based on my goals and whether they were realistic, and how to pick my battles. For example, I tried to negotiate tail coverage up front in my previous job but was unsuccessful. The new employer paid tail for me, both from my previous employment and for my current employment.”
Dr. Chester advises other female physicians “never to sign anything without having a lawyer review it and to make sure that the lawyer is sensitive to their specific needs.”
It can be hard to be a female physician. Having the right knowledge and ammunition and knowing how to negotiate well paves the way for success and thriving in an often male-dominated market.
A version of this article first appeared on Medscape.com.
Rebecca Chester, MD, an Arizona-based interventional cardiologist, recently left her position in a private practice and started employment at a hospital system.
“When I was negotiating my previous contract with the private practice, I found that navigating contracts from the standpoint of a woman still in childbearing years was a little disappointing and challenging,” Dr. Chester told this news organization.
“I wanted to have more children and hired a lawyer recommended by a male colleague to help me not only understand the contract but also negotiate time off and maternity leave, but the lawyer discouraged me from advocating for maternity leave, feeling that it might stigmatize me and prevent me from getting a job,” she says.
He also didn’t explain very much. “He just said it falls under ‘disability leave’ and left it at that.”
Fortunately, Dr. Chester had a good experience with the group. “As things turned out, I did have a child later that year, and they treated me well – I actually got time off – and they didn’t make me take extra call. But it might have turned out very differently because I didn’t know what I was getting into. If I hadn’t worked for such a conscientious group, I might have been in a much tougher situation.”
Since then, Dr. Chester has spoken to female colleagues who received “more support from their legal advisors regarding maternity leave.” She suggests turning to female physicians for recommendations to a lawyer.
Although the central components of a contract (for example, noncompete covenants, malpractice “tail” coverage, bonus structure, vacation time, disability, and call) are relevant to physicians of all genders, the needs of women and men are often different.
Dennis Hursh, managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, told this news organization that women physicians have “several issues that need special attention when negotiating their physician employment agreements.”
It starts with the interview
“Women have to be sensitive to the interviewer’s casual ‘let’s-get-to-know-each-other’ types of questions that may seem natural but really are unlawful to bring into an employment interview,” said Mr. Hursh.
He warned women to beware of questions such as “Are you married? Do you have kids? Are you planning to start a family?” These may be friendly chit-chat for male interviewees but there may be other agendas when asked to a prospective female employee.
Many of Mr. Hursh’s female clients have been asked this type of question, which “should be regarded as a ‘red flag.’ Yes, it may be an innocent, well-intentioned ice-breaker, but it’s actually unlawful to bring that up in an employment setting and, according to the Equal Opportunity Commission, can be seen as a form of discrimination.” He advises female physicians not to engage with the question and simply to refocus the discussion.
Know your worth and go for it
Medscape’s Physician Compensation surveys have consistently found discrepancies in earnings between male and female physicians, both in primary care and in specialties. In 2022, male primary care physicians earned 23% more than their female counterparts, whereas male specialists earned 31% more.
One reason may be that women tend to be more timid about negotiating for better compensation packages. Amanda Hill of Hill Health Law, a health care practice based in Austin, Tex., told this news organization that in her experience one of the most “overarching” features of female physicians is “that they either don’t know what they’re worth or they undersell themselves.”
In contrast to men, “many women are afraid of coming across as greedy or crass, or even demanding or bossy. But it’s a misperception that if you ask for more money, your future employer will hate you or won’t hire you,” said Ms. Hill.
Ms. Hill and Mr. Hursh encourage physicians to find out what they’re worth, which varies by region and specialty, by consulting benchmarks provided by companies such as Medical Group Management Association.
Jon Appino, MBA, principal and founder of Contract Diagnostics, a Kansas City–based consulting company that specializes in physician employment contract reviews, told this news organization that it’s important to look beyond the salary at other aspects of the position. For example, some figures “don’t take into account how much call a physician is taking. You may know what the average ob.gyn. is making, but an ob.gyn. may be working 3 days a week, while another one is working 6 days a week, one may be on call 15 times per month and another may be on call 15 times a quarter.” Other components of compensation include relative value unit (RVU) thresholds and bonuses.
Once you have that information, “don’t be intimidated, even if you’re sitting in front of several executives who are savvy about negotiations, and don’t worry about coming across as ‘high-maintenance’ or ‘all about money,’ ” Mr. Appino says. Proceed with confidence, knowing your worth and pursuing it.
Part-time vs. full-time
Mr. Appino has seen “more female than male physicians who want to work less than full-time. So it’s important to clarify whether that’s a possibility now or in the future and to understand the implications of working part-time.”
He explained that a full-time employee typically puts in a 40-hour work week, which translates into 1.0 Full-Time Equivalent (FTE), or one unit of work. “For example, if a person wants to work 0.8 FTEs – 4 days a week – is vacation time pro-rated? At what point is there a medical insurance fall-off or a higher monthly premium?”
In medical settings, FTEs may be tied to different metrics rather than the number of weekly hours – for example, for a hospitalist, it might be a certain number of shifts and might also vary by specialty. And it affects the call schedule too. “Call is hard to pro-rate. Many hospital bylaws mandate that call be divided equally, but if one surgeon is working 1.0 FTEs and another is working 0.8 FTEs, how does that call schedule get divided?”
Maternity leave: A tricky question
Many attorneys counsel against raising the question out of fear of scaring away potential employers.
“On the one hand, it is and should be absolutely reasonable to ask about the maternity leave policy or even negotiate for paid leave or additional leave, but it also highlights that you’re planning to have a baby and be out for months,” said Ms. Hill.
“And as much as we want people to be fair and reasonable, on the side of the employer, bias still very much exists, especially in a situation where revenue is based on group numbers. So suddenly, the employer thinks up some ‘nondiscriminatory’ reason why that person isn’t a great fit for the organization.”
Andrew Knoll, MD, JD, a former hospitalist who is now a partner with Cohen Compagni Beckman Appler & Knoll PLLC in Syracuse, N.Y., said that maternity leave is “rare” in an employment agreement, except sometimes in small private practice groups, because it often falls under the purview of “disability leave,” and “from a legal perspective, it’s no different than any other type of disability leave.”
The Family and Medical Leave Act (FMLA), which applies if a group is large enough, allows employees 12 weeks of unpaid leave, during which time their job is protected and their benefits maintained. And some states require employers to offer paid family leave.
“During this time, the woman can take time off – albeit without pay sometimes – to bond with the baby,” Dr. Knoll says. “Since there are statutory laws that protect the employee’s job, offering specific paid maternity leave is very unlikely.”
Ms. Hill advises carefully examining the employer’s comprehensive benefits plan to ascertain if paid maternity leave is included in the benefits. “But unless you’re currently pregnant and want to start off the relationship with true transparency – ‘I’m due in April and curious how we can handle that if you hire me now’ – I would keep the family planning questions to yourself before you get the job.”
Mr. Hursh, author of “The Final Hurdle: A Physician’s Guide to Negotiating a Fair Employment Agreement” (Charleston, S.C.: Advantage, 2012), has a different perspective. “I think all women, no matter how old they are, should ask about maternity leave, whether or not they’re planning a family,” he said.
“The employer may say, ‘We treat maternity leave like any other disability; our policy is such-and-such.’ If they cite an unacceptable policy, it’s a red flag about how they treat women, and should give a woman pause before accepting a position at that organization. Even if your rights are protected under the law and the organization’s policy is violating the law, no one wants to go to battle with HR or to have to go to court.”
Do you want partnership?
Not all female physicians entering a private practice want to advance to becoming partners. Many who are balancing family and work commitments “would prefer to just go into the office, perform their clinical responsibilities, go home, and be done” without the extra headaches, tasks, and time involved with business leadership, says Mr. Appino.
Some private practices have different contracts for those on partnership vs. nonpartnership tracks, so “you should ascertain this information and make sure it’s not automatically assumed that you would like to be on a partnership track,” says Mr. Appino.
On the other hand, you may want to become a partner. “I always suggest asking about the possibility of obtaining a leadership position in an organization or becoming an owner in a private practice,” says Mr. Hursh. “You may be told, ‘We’ve never had a woman in leadership before.’ This might be innocent if you’re the first woman hired, but it might be a red flag as to how women are regarded.” Either way, it’s important to have the information and know what your options are.
The impact of shift schedule
Mr. Hursh advises drilling down into the specifics of the schedule if you’re considering becoming a hospitalist. “Does a ‘week-on/week-off’ shift schedule assume you’ll be taking your vacation time or completing your CME requirements during the week off? This is important for all physicians, but especially for women who might want to use their weeks off to attend to children and family.”
Moreover, “there should be limits on shifts. You shouldn’t have a full day shift followed by a night shift. And there should be a limit on the number of shifts you work without time off. Twelve would be a brutal schedule. Seven is a reasonable amount. Make sure this is in writing and that the contract protects you. Don’t allow the employer to say, ‘We expect you to do the work we assign’ and leave it vague.”
Often, hospitalists will receive an annual salary under the assumption that a certain minimum number of shifts will be completed, but there is no maximum. “It’s important that the salary includes the minimum number of shifts, but that a compensation structure is created so that additional shifts receive additional compensation,” Mr. Hursh said.
Removing the ‘golden handcuffs’
Ms. Hill observes that there are some “really terrible contracts out there, which physicians – especially women – often feel pressured into signing.” They’re told, “This is our standard contract. You won’t find anything better.” Or, “Don’t worry about the small print and legalese.” The physician “gets scared or is artificially reassured, signs an overwhelmingly unfair contract, and then feels stuck.”
Being stuck in a bad contract “is debilitating and adds to burnout, feeling of depression, and the sense that there is no recourse and nowhere to go, especially if your family depends on you,” Ms. Hill said.
“More women than men feel hamstrung or are resigned to being harassed – which is not uncommon in the medical setting, especially in surgical specialties – or just accept poor treatment,” Ms. Hill added. Yes, you can “fight the system and go to HR, but fighting the system is very hard.”
She urges women “not to feel stuck or imprisoned by the ‘golden handcuffs’ but to consult a good lawyer, even if you have to break the contract.” Be aware of the reasons for your unhappiness and bring them to your lawyer – perhaps the system has engaged in fraud, perhaps there has been sexual or racial harassment, perhaps the organization hasn’t followed its own compliance policies.
Dr. Chester consulted Ms. Hill before signing the contract for her current position. “I wanted someone who could give me personalized advice, not only generic advice, and who understood my needs as a woman.”
Ms. Hill helped her to understand “what was and wasn’t fair and reasonable, what changes I could request based on my goals and whether they were realistic, and how to pick my battles. For example, I tried to negotiate tail coverage up front in my previous job but was unsuccessful. The new employer paid tail for me, both from my previous employment and for my current employment.”
Dr. Chester advises other female physicians “never to sign anything without having a lawyer review it and to make sure that the lawyer is sensitive to their specific needs.”
It can be hard to be a female physician. Having the right knowledge and ammunition and knowing how to negotiate well paves the way for success and thriving in an often male-dominated market.
A version of this article first appeared on Medscape.com.
Rebecca Chester, MD, an Arizona-based interventional cardiologist, recently left her position in a private practice and started employment at a hospital system.
“When I was negotiating my previous contract with the private practice, I found that navigating contracts from the standpoint of a woman still in childbearing years was a little disappointing and challenging,” Dr. Chester told this news organization.
“I wanted to have more children and hired a lawyer recommended by a male colleague to help me not only understand the contract but also negotiate time off and maternity leave, but the lawyer discouraged me from advocating for maternity leave, feeling that it might stigmatize me and prevent me from getting a job,” she says.
He also didn’t explain very much. “He just said it falls under ‘disability leave’ and left it at that.”
Fortunately, Dr. Chester had a good experience with the group. “As things turned out, I did have a child later that year, and they treated me well – I actually got time off – and they didn’t make me take extra call. But it might have turned out very differently because I didn’t know what I was getting into. If I hadn’t worked for such a conscientious group, I might have been in a much tougher situation.”
Since then, Dr. Chester has spoken to female colleagues who received “more support from their legal advisors regarding maternity leave.” She suggests turning to female physicians for recommendations to a lawyer.
Although the central components of a contract (for example, noncompete covenants, malpractice “tail” coverage, bonus structure, vacation time, disability, and call) are relevant to physicians of all genders, the needs of women and men are often different.
Dennis Hursh, managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, told this news organization that women physicians have “several issues that need special attention when negotiating their physician employment agreements.”
It starts with the interview
“Women have to be sensitive to the interviewer’s casual ‘let’s-get-to-know-each-other’ types of questions that may seem natural but really are unlawful to bring into an employment interview,” said Mr. Hursh.
He warned women to beware of questions such as “Are you married? Do you have kids? Are you planning to start a family?” These may be friendly chit-chat for male interviewees but there may be other agendas when asked to a prospective female employee.
Many of Mr. Hursh’s female clients have been asked this type of question, which “should be regarded as a ‘red flag.’ Yes, it may be an innocent, well-intentioned ice-breaker, but it’s actually unlawful to bring that up in an employment setting and, according to the Equal Opportunity Commission, can be seen as a form of discrimination.” He advises female physicians not to engage with the question and simply to refocus the discussion.
Know your worth and go for it
Medscape’s Physician Compensation surveys have consistently found discrepancies in earnings between male and female physicians, both in primary care and in specialties. In 2022, male primary care physicians earned 23% more than their female counterparts, whereas male specialists earned 31% more.
One reason may be that women tend to be more timid about negotiating for better compensation packages. Amanda Hill of Hill Health Law, a health care practice based in Austin, Tex., told this news organization that in her experience one of the most “overarching” features of female physicians is “that they either don’t know what they’re worth or they undersell themselves.”
In contrast to men, “many women are afraid of coming across as greedy or crass, or even demanding or bossy. But it’s a misperception that if you ask for more money, your future employer will hate you or won’t hire you,” said Ms. Hill.
Ms. Hill and Mr. Hursh encourage physicians to find out what they’re worth, which varies by region and specialty, by consulting benchmarks provided by companies such as Medical Group Management Association.
Jon Appino, MBA, principal and founder of Contract Diagnostics, a Kansas City–based consulting company that specializes in physician employment contract reviews, told this news organization that it’s important to look beyond the salary at other aspects of the position. For example, some figures “don’t take into account how much call a physician is taking. You may know what the average ob.gyn. is making, but an ob.gyn. may be working 3 days a week, while another one is working 6 days a week, one may be on call 15 times per month and another may be on call 15 times a quarter.” Other components of compensation include relative value unit (RVU) thresholds and bonuses.
Once you have that information, “don’t be intimidated, even if you’re sitting in front of several executives who are savvy about negotiations, and don’t worry about coming across as ‘high-maintenance’ or ‘all about money,’ ” Mr. Appino says. Proceed with confidence, knowing your worth and pursuing it.
Part-time vs. full-time
Mr. Appino has seen “more female than male physicians who want to work less than full-time. So it’s important to clarify whether that’s a possibility now or in the future and to understand the implications of working part-time.”
He explained that a full-time employee typically puts in a 40-hour work week, which translates into 1.0 Full-Time Equivalent (FTE), or one unit of work. “For example, if a person wants to work 0.8 FTEs – 4 days a week – is vacation time pro-rated? At what point is there a medical insurance fall-off or a higher monthly premium?”
In medical settings, FTEs may be tied to different metrics rather than the number of weekly hours – for example, for a hospitalist, it might be a certain number of shifts and might also vary by specialty. And it affects the call schedule too. “Call is hard to pro-rate. Many hospital bylaws mandate that call be divided equally, but if one surgeon is working 1.0 FTEs and another is working 0.8 FTEs, how does that call schedule get divided?”
Maternity leave: A tricky question
Many attorneys counsel against raising the question out of fear of scaring away potential employers.
“On the one hand, it is and should be absolutely reasonable to ask about the maternity leave policy or even negotiate for paid leave or additional leave, but it also highlights that you’re planning to have a baby and be out for months,” said Ms. Hill.
“And as much as we want people to be fair and reasonable, on the side of the employer, bias still very much exists, especially in a situation where revenue is based on group numbers. So suddenly, the employer thinks up some ‘nondiscriminatory’ reason why that person isn’t a great fit for the organization.”
Andrew Knoll, MD, JD, a former hospitalist who is now a partner with Cohen Compagni Beckman Appler & Knoll PLLC in Syracuse, N.Y., said that maternity leave is “rare” in an employment agreement, except sometimes in small private practice groups, because it often falls under the purview of “disability leave,” and “from a legal perspective, it’s no different than any other type of disability leave.”
The Family and Medical Leave Act (FMLA), which applies if a group is large enough, allows employees 12 weeks of unpaid leave, during which time their job is protected and their benefits maintained. And some states require employers to offer paid family leave.
“During this time, the woman can take time off – albeit without pay sometimes – to bond with the baby,” Dr. Knoll says. “Since there are statutory laws that protect the employee’s job, offering specific paid maternity leave is very unlikely.”
Ms. Hill advises carefully examining the employer’s comprehensive benefits plan to ascertain if paid maternity leave is included in the benefits. “But unless you’re currently pregnant and want to start off the relationship with true transparency – ‘I’m due in April and curious how we can handle that if you hire me now’ – I would keep the family planning questions to yourself before you get the job.”
Mr. Hursh, author of “The Final Hurdle: A Physician’s Guide to Negotiating a Fair Employment Agreement” (Charleston, S.C.: Advantage, 2012), has a different perspective. “I think all women, no matter how old they are, should ask about maternity leave, whether or not they’re planning a family,” he said.
“The employer may say, ‘We treat maternity leave like any other disability; our policy is such-and-such.’ If they cite an unacceptable policy, it’s a red flag about how they treat women, and should give a woman pause before accepting a position at that organization. Even if your rights are protected under the law and the organization’s policy is violating the law, no one wants to go to battle with HR or to have to go to court.”
Do you want partnership?
Not all female physicians entering a private practice want to advance to becoming partners. Many who are balancing family and work commitments “would prefer to just go into the office, perform their clinical responsibilities, go home, and be done” without the extra headaches, tasks, and time involved with business leadership, says Mr. Appino.
Some private practices have different contracts for those on partnership vs. nonpartnership tracks, so “you should ascertain this information and make sure it’s not automatically assumed that you would like to be on a partnership track,” says Mr. Appino.
On the other hand, you may want to become a partner. “I always suggest asking about the possibility of obtaining a leadership position in an organization or becoming an owner in a private practice,” says Mr. Hursh. “You may be told, ‘We’ve never had a woman in leadership before.’ This might be innocent if you’re the first woman hired, but it might be a red flag as to how women are regarded.” Either way, it’s important to have the information and know what your options are.
The impact of shift schedule
Mr. Hursh advises drilling down into the specifics of the schedule if you’re considering becoming a hospitalist. “Does a ‘week-on/week-off’ shift schedule assume you’ll be taking your vacation time or completing your CME requirements during the week off? This is important for all physicians, but especially for women who might want to use their weeks off to attend to children and family.”
Moreover, “there should be limits on shifts. You shouldn’t have a full day shift followed by a night shift. And there should be a limit on the number of shifts you work without time off. Twelve would be a brutal schedule. Seven is a reasonable amount. Make sure this is in writing and that the contract protects you. Don’t allow the employer to say, ‘We expect you to do the work we assign’ and leave it vague.”
Often, hospitalists will receive an annual salary under the assumption that a certain minimum number of shifts will be completed, but there is no maximum. “It’s important that the salary includes the minimum number of shifts, but that a compensation structure is created so that additional shifts receive additional compensation,” Mr. Hursh said.
Removing the ‘golden handcuffs’
Ms. Hill observes that there are some “really terrible contracts out there, which physicians – especially women – often feel pressured into signing.” They’re told, “This is our standard contract. You won’t find anything better.” Or, “Don’t worry about the small print and legalese.” The physician “gets scared or is artificially reassured, signs an overwhelmingly unfair contract, and then feels stuck.”
Being stuck in a bad contract “is debilitating and adds to burnout, feeling of depression, and the sense that there is no recourse and nowhere to go, especially if your family depends on you,” Ms. Hill said.
“More women than men feel hamstrung or are resigned to being harassed – which is not uncommon in the medical setting, especially in surgical specialties – or just accept poor treatment,” Ms. Hill added. Yes, you can “fight the system and go to HR, but fighting the system is very hard.”
She urges women “not to feel stuck or imprisoned by the ‘golden handcuffs’ but to consult a good lawyer, even if you have to break the contract.” Be aware of the reasons for your unhappiness and bring them to your lawyer – perhaps the system has engaged in fraud, perhaps there has been sexual or racial harassment, perhaps the organization hasn’t followed its own compliance policies.
Dr. Chester consulted Ms. Hill before signing the contract for her current position. “I wanted someone who could give me personalized advice, not only generic advice, and who understood my needs as a woman.”
Ms. Hill helped her to understand “what was and wasn’t fair and reasonable, what changes I could request based on my goals and whether they were realistic, and how to pick my battles. For example, I tried to negotiate tail coverage up front in my previous job but was unsuccessful. The new employer paid tail for me, both from my previous employment and for my current employment.”
Dr. Chester advises other female physicians “never to sign anything without having a lawyer review it and to make sure that the lawyer is sensitive to their specific needs.”
It can be hard to be a female physician. Having the right knowledge and ammunition and knowing how to negotiate well paves the way for success and thriving in an often male-dominated market.
A version of this article first appeared on Medscape.com.
How blunt is too blunt for informed consent?
Sitting across from a patient explaining a complicated treatment proposal, protocol, or medication may be one of the most complex yet crucial tasks you have as a physician. Although informed consent is at the forefront of shared decisions between you and your patient, there’s a fine line between providing enough information on the risks and benefits of a particular treatment and knowing you’ve explained it well enough to fully educate your patient about their choices.
“It is a bit of a fine line because unless your patient happens to be a health care provider, medicine is complicated for patients to understand,” said David L. Feldman, MD, chief medical officer at The Doctors Company, the nation’s largest medical malpractice insurer in New York.
In addition, documenting the interaction is critical, said James Giordano, PhD, MPhil, professor in the departments of neurology and biochemistry and chief of the neuroethics studies program at the Pellegrino Center for Clinical Bioethics at Georgetown University Medical Center, Washington.
“As with anything in medicine, the key rule is that if it’s not documented, it’s not done,” he said. “This also means diligent documentation in all aspects of the medical record, including the electronic medical record and the written one.”
That said, it’s important to know what’s enough and what’s too granular when you discuss a procedure with your patients, said Erum N. Ilyas, MD, a board-certified dermatologist at Schweiger Dermatology and a bioethicist near Philadelphia.
“One of the most challenging aspects of informed consent, especially for young physicians, is how to discuss a procedure or a medication in a manner that is both relevant and concise,” Dr. llyas said. “I’ve had residents about to perform a skin biopsy spend several minutes covering every aspect of every potential outcome of a routine skin biopsy. The patient is left traumatized and confused as to whether they should proceed with the small procedure.”
Instead, the goal of informed consent is to ensure that the patient has a general overview of the procedure and is empowered, knowing that the decision to proceed is, indeed, part of their decision-making process.
How long an informed consent discussion takes depends on the procedure.
“When I was in practice as a plastic surgeon, the conversations varied from the straightforward ‘I’m taking this mole off your cheek, and there’s a risk of scarring and bleeding’ to talking about a mastectomy and breast reconstruction, which could take an hour or more to discuss,” Dr. Feldman said.
Ultimately, it’s as essential for doctors to explain the risks associated with a procedure as it is for patients to understand precisely what’s involved, Dr. Ilyas added.
She also recommends creating a flow to the conversation that places the discussion of risks within the context of why the procedure is being performed. This way, clarity about both the risks and the need for the treatment or procedure can be achieved.
When doing so, it’s critical to make sure you’re speaking your patient’s language – literally.
“Have a translator in the room if needed,” Dr. Feldman added. “If your patient is hearing or sight impaired, you need to have every contingency ready to ensure that everyone is in complete communication.”
Document, document, document!
To best protect yourself, the patient must consent to each procedure and intervention via active, informed consent, said Dr. Giordano.
“It’s not enough to hand a patient a piece of paper and say sign it,” he said. “There should be some documented evidence that the patient has not only read the document but that the key parts of the document have been explained and that the patient’s level of comprehension has been assessed and verified.”
It is vital if the patient has a disability, a neurological impairment, or a neurocognitive or psychiatric condition that might impede his or her ability to understand the consent that’s being sought.
In addition, it’s best if a ‘clinical proxy’ handles the consent (for example, a nurse, office worker, or case manager).
“This can be very helpful because it means you’ve had third-party documentation of informed consent,” Dr. Giordano said. “It should then be re-documented with you as the clinician and stated that the patient has affirmatively and actively agreed to treatment.”
What happens when things go wrong?
If you’re sued over informed consent, with the patient claiming that you didn’t fully explain the potential risks, the first thing to consider is why this happened.
“Very often, these situations occur if there was some difficulty or competency of communication,” Dr. Giordano said. “You may have done everything right, but somehow the patient hasn’t gained an understanding of the procedure required.”
Physicians must take a hard look at how they’re explaining risks and possible side effects. For doctors who perform these procedures regularly, the risks may seem small, and they may unconsciously minimize them to the patient. But when something goes wrong, the patient may then feel that they didn’t fully understand the frequency of poor outcomes, or the potential severity.
Next, it’s important to perform a ‘gap analysis’ to assess why something went awry. That means, look at all the potential factors involved to identify which one was the weak link.
“It might be that the patient was on a signing frenzy and signed away but didn’t receive active and informed content,” Dr. Giordano said. “The goal is to learn how to close the gap for this case and for future cases.”
To protect yourself, consider using technology to your advantage, especially since lawsuits over informed consent usually happen several years after the procedure. This is when a patient might argue that you didn’t tell them about possible complications and that they might have opted out of the procedure if they had known about those issues ahead of time.
“Even before the statute of limitations is up for a lawsuit, it could be five years from the time the procedure occurred due to the length of time a lawsuit can take,” Dr. Feldman said. “That’s why it’s important to take a video of your conversation or make a recording of the informed consent conversation. This way if there’s a question of what you said, there’s a video of it.”
For many physicians, this would be a big change – to video record and then store all their informed consent conversations. It could most likely help you if a lawsuit occurs, but some physicians may feel that process to be cumbersome and time-consuming, and they’d rather find another way to ensure that patients understand the risks.
Ultimately, however, if there’s a legal question involved with informed consent, the general thinking is that the effect on the patient must be harmful for it to stand up.
“The question becomes whether the outcome rendered that gap in the consenting process forgivable,” Dr. Giordano said. “The hope is that there was nothing harmful to the patient and that the benefit of the procedure was demonstrable despite any gaps in the informed consent process.”
In the end, informed consent should be a matter of good communication before, during, and after any treatment or procedure.
“When you form a relationship with a patient who needs any procedure, small or large, you’re going to be guiding them through a very scary thing,” Dr. Feldman said. “You want to make patients feel like you care about them and that, while neither you nor the system is perfect, you’ll take care of them. That’s the bottom line.”
A version of this article first appeared on Medscape.com.
Sitting across from a patient explaining a complicated treatment proposal, protocol, or medication may be one of the most complex yet crucial tasks you have as a physician. Although informed consent is at the forefront of shared decisions between you and your patient, there’s a fine line between providing enough information on the risks and benefits of a particular treatment and knowing you’ve explained it well enough to fully educate your patient about their choices.
“It is a bit of a fine line because unless your patient happens to be a health care provider, medicine is complicated for patients to understand,” said David L. Feldman, MD, chief medical officer at The Doctors Company, the nation’s largest medical malpractice insurer in New York.
In addition, documenting the interaction is critical, said James Giordano, PhD, MPhil, professor in the departments of neurology and biochemistry and chief of the neuroethics studies program at the Pellegrino Center for Clinical Bioethics at Georgetown University Medical Center, Washington.
“As with anything in medicine, the key rule is that if it’s not documented, it’s not done,” he said. “This also means diligent documentation in all aspects of the medical record, including the electronic medical record and the written one.”
That said, it’s important to know what’s enough and what’s too granular when you discuss a procedure with your patients, said Erum N. Ilyas, MD, a board-certified dermatologist at Schweiger Dermatology and a bioethicist near Philadelphia.
“One of the most challenging aspects of informed consent, especially for young physicians, is how to discuss a procedure or a medication in a manner that is both relevant and concise,” Dr. llyas said. “I’ve had residents about to perform a skin biopsy spend several minutes covering every aspect of every potential outcome of a routine skin biopsy. The patient is left traumatized and confused as to whether they should proceed with the small procedure.”
Instead, the goal of informed consent is to ensure that the patient has a general overview of the procedure and is empowered, knowing that the decision to proceed is, indeed, part of their decision-making process.
How long an informed consent discussion takes depends on the procedure.
“When I was in practice as a plastic surgeon, the conversations varied from the straightforward ‘I’m taking this mole off your cheek, and there’s a risk of scarring and bleeding’ to talking about a mastectomy and breast reconstruction, which could take an hour or more to discuss,” Dr. Feldman said.
Ultimately, it’s as essential for doctors to explain the risks associated with a procedure as it is for patients to understand precisely what’s involved, Dr. Ilyas added.
She also recommends creating a flow to the conversation that places the discussion of risks within the context of why the procedure is being performed. This way, clarity about both the risks and the need for the treatment or procedure can be achieved.
When doing so, it’s critical to make sure you’re speaking your patient’s language – literally.
“Have a translator in the room if needed,” Dr. Feldman added. “If your patient is hearing or sight impaired, you need to have every contingency ready to ensure that everyone is in complete communication.”
Document, document, document!
To best protect yourself, the patient must consent to each procedure and intervention via active, informed consent, said Dr. Giordano.
“It’s not enough to hand a patient a piece of paper and say sign it,” he said. “There should be some documented evidence that the patient has not only read the document but that the key parts of the document have been explained and that the patient’s level of comprehension has been assessed and verified.”
It is vital if the patient has a disability, a neurological impairment, or a neurocognitive or psychiatric condition that might impede his or her ability to understand the consent that’s being sought.
In addition, it’s best if a ‘clinical proxy’ handles the consent (for example, a nurse, office worker, or case manager).
“This can be very helpful because it means you’ve had third-party documentation of informed consent,” Dr. Giordano said. “It should then be re-documented with you as the clinician and stated that the patient has affirmatively and actively agreed to treatment.”
What happens when things go wrong?
If you’re sued over informed consent, with the patient claiming that you didn’t fully explain the potential risks, the first thing to consider is why this happened.
“Very often, these situations occur if there was some difficulty or competency of communication,” Dr. Giordano said. “You may have done everything right, but somehow the patient hasn’t gained an understanding of the procedure required.”
Physicians must take a hard look at how they’re explaining risks and possible side effects. For doctors who perform these procedures regularly, the risks may seem small, and they may unconsciously minimize them to the patient. But when something goes wrong, the patient may then feel that they didn’t fully understand the frequency of poor outcomes, or the potential severity.
Next, it’s important to perform a ‘gap analysis’ to assess why something went awry. That means, look at all the potential factors involved to identify which one was the weak link.
“It might be that the patient was on a signing frenzy and signed away but didn’t receive active and informed content,” Dr. Giordano said. “The goal is to learn how to close the gap for this case and for future cases.”
To protect yourself, consider using technology to your advantage, especially since lawsuits over informed consent usually happen several years after the procedure. This is when a patient might argue that you didn’t tell them about possible complications and that they might have opted out of the procedure if they had known about those issues ahead of time.
“Even before the statute of limitations is up for a lawsuit, it could be five years from the time the procedure occurred due to the length of time a lawsuit can take,” Dr. Feldman said. “That’s why it’s important to take a video of your conversation or make a recording of the informed consent conversation. This way if there’s a question of what you said, there’s a video of it.”
For many physicians, this would be a big change – to video record and then store all their informed consent conversations. It could most likely help you if a lawsuit occurs, but some physicians may feel that process to be cumbersome and time-consuming, and they’d rather find another way to ensure that patients understand the risks.
Ultimately, however, if there’s a legal question involved with informed consent, the general thinking is that the effect on the patient must be harmful for it to stand up.
“The question becomes whether the outcome rendered that gap in the consenting process forgivable,” Dr. Giordano said. “The hope is that there was nothing harmful to the patient and that the benefit of the procedure was demonstrable despite any gaps in the informed consent process.”
In the end, informed consent should be a matter of good communication before, during, and after any treatment or procedure.
“When you form a relationship with a patient who needs any procedure, small or large, you’re going to be guiding them through a very scary thing,” Dr. Feldman said. “You want to make patients feel like you care about them and that, while neither you nor the system is perfect, you’ll take care of them. That’s the bottom line.”
A version of this article first appeared on Medscape.com.
Sitting across from a patient explaining a complicated treatment proposal, protocol, or medication may be one of the most complex yet crucial tasks you have as a physician. Although informed consent is at the forefront of shared decisions between you and your patient, there’s a fine line between providing enough information on the risks and benefits of a particular treatment and knowing you’ve explained it well enough to fully educate your patient about their choices.
“It is a bit of a fine line because unless your patient happens to be a health care provider, medicine is complicated for patients to understand,” said David L. Feldman, MD, chief medical officer at The Doctors Company, the nation’s largest medical malpractice insurer in New York.
In addition, documenting the interaction is critical, said James Giordano, PhD, MPhil, professor in the departments of neurology and biochemistry and chief of the neuroethics studies program at the Pellegrino Center for Clinical Bioethics at Georgetown University Medical Center, Washington.
“As with anything in medicine, the key rule is that if it’s not documented, it’s not done,” he said. “This also means diligent documentation in all aspects of the medical record, including the electronic medical record and the written one.”
That said, it’s important to know what’s enough and what’s too granular when you discuss a procedure with your patients, said Erum N. Ilyas, MD, a board-certified dermatologist at Schweiger Dermatology and a bioethicist near Philadelphia.
“One of the most challenging aspects of informed consent, especially for young physicians, is how to discuss a procedure or a medication in a manner that is both relevant and concise,” Dr. llyas said. “I’ve had residents about to perform a skin biopsy spend several minutes covering every aspect of every potential outcome of a routine skin biopsy. The patient is left traumatized and confused as to whether they should proceed with the small procedure.”
Instead, the goal of informed consent is to ensure that the patient has a general overview of the procedure and is empowered, knowing that the decision to proceed is, indeed, part of their decision-making process.
How long an informed consent discussion takes depends on the procedure.
“When I was in practice as a plastic surgeon, the conversations varied from the straightforward ‘I’m taking this mole off your cheek, and there’s a risk of scarring and bleeding’ to talking about a mastectomy and breast reconstruction, which could take an hour or more to discuss,” Dr. Feldman said.
Ultimately, it’s as essential for doctors to explain the risks associated with a procedure as it is for patients to understand precisely what’s involved, Dr. Ilyas added.
She also recommends creating a flow to the conversation that places the discussion of risks within the context of why the procedure is being performed. This way, clarity about both the risks and the need for the treatment or procedure can be achieved.
When doing so, it’s critical to make sure you’re speaking your patient’s language – literally.
“Have a translator in the room if needed,” Dr. Feldman added. “If your patient is hearing or sight impaired, you need to have every contingency ready to ensure that everyone is in complete communication.”
Document, document, document!
To best protect yourself, the patient must consent to each procedure and intervention via active, informed consent, said Dr. Giordano.
“It’s not enough to hand a patient a piece of paper and say sign it,” he said. “There should be some documented evidence that the patient has not only read the document but that the key parts of the document have been explained and that the patient’s level of comprehension has been assessed and verified.”
It is vital if the patient has a disability, a neurological impairment, or a neurocognitive or psychiatric condition that might impede his or her ability to understand the consent that’s being sought.
In addition, it’s best if a ‘clinical proxy’ handles the consent (for example, a nurse, office worker, or case manager).
“This can be very helpful because it means you’ve had third-party documentation of informed consent,” Dr. Giordano said. “It should then be re-documented with you as the clinician and stated that the patient has affirmatively and actively agreed to treatment.”
What happens when things go wrong?
If you’re sued over informed consent, with the patient claiming that you didn’t fully explain the potential risks, the first thing to consider is why this happened.
“Very often, these situations occur if there was some difficulty or competency of communication,” Dr. Giordano said. “You may have done everything right, but somehow the patient hasn’t gained an understanding of the procedure required.”
Physicians must take a hard look at how they’re explaining risks and possible side effects. For doctors who perform these procedures regularly, the risks may seem small, and they may unconsciously minimize them to the patient. But when something goes wrong, the patient may then feel that they didn’t fully understand the frequency of poor outcomes, or the potential severity.
Next, it’s important to perform a ‘gap analysis’ to assess why something went awry. That means, look at all the potential factors involved to identify which one was the weak link.
“It might be that the patient was on a signing frenzy and signed away but didn’t receive active and informed content,” Dr. Giordano said. “The goal is to learn how to close the gap for this case and for future cases.”
To protect yourself, consider using technology to your advantage, especially since lawsuits over informed consent usually happen several years after the procedure. This is when a patient might argue that you didn’t tell them about possible complications and that they might have opted out of the procedure if they had known about those issues ahead of time.
“Even before the statute of limitations is up for a lawsuit, it could be five years from the time the procedure occurred due to the length of time a lawsuit can take,” Dr. Feldman said. “That’s why it’s important to take a video of your conversation or make a recording of the informed consent conversation. This way if there’s a question of what you said, there’s a video of it.”
For many physicians, this would be a big change – to video record and then store all their informed consent conversations. It could most likely help you if a lawsuit occurs, but some physicians may feel that process to be cumbersome and time-consuming, and they’d rather find another way to ensure that patients understand the risks.
Ultimately, however, if there’s a legal question involved with informed consent, the general thinking is that the effect on the patient must be harmful for it to stand up.
“The question becomes whether the outcome rendered that gap in the consenting process forgivable,” Dr. Giordano said. “The hope is that there was nothing harmful to the patient and that the benefit of the procedure was demonstrable despite any gaps in the informed consent process.”
In the end, informed consent should be a matter of good communication before, during, and after any treatment or procedure.
“When you form a relationship with a patient who needs any procedure, small or large, you’re going to be guiding them through a very scary thing,” Dr. Feldman said. “You want to make patients feel like you care about them and that, while neither you nor the system is perfect, you’ll take care of them. That’s the bottom line.”
A version of this article first appeared on Medscape.com.
Analysis of doctors’ EHR email finds infrequent but notable hostility
Among the emails, 43% were from patients; the remainder were mostly from other physicians or clinicians, or automated. The content of the messages wasn’t associated with doctor burnout, as the researchers had hypothesized. And only about 5% of the messages had negative sentiment.
But the researchers were struck by the hostility of that sentiment, displayed in messages like these that surely would be distressing for physicians to read:
“I hope and expect that you will spend eternity in he**. You are an abusive, nasty, cheap person.”
“Your office is full of liars, hypocrites and I will do everything in my power to prevent anyone from going to your bullsh** office again.”
About 5% of emails had an overall negative sentiment, with high-frequency words like “cancel,” “pain,” or “problem.” Among patient messages, 3% were negative and contained words and expletives suggesting hatred, hostility, or violence.
“F***” was the most common expletive used by patients.
Researchers provided examples of profanity-laced messages, including one patient who said, “I am so upset that I was told the blood work would include the gender of the baby. I have been waiting 5 [days] to find it, and it wasn’t even fu**ing tested!!!! What a disappointment in your office and the bullsh** I was told. I will be switching plans because this is sh**!”
Researchers also noted some high-frequency words associated with violence, such as “shoot,” “fight,” and “kill.”
“This is concerning, especially given documentation of patient-inflicted violence against physicians. Health systems should be proactive in ensuring that the in-basket does not become a venue for physician abuse and cyberbullying,” the researchers wrote in JAMA Network Open.
“Posting reminders in EHR patient portals to use kind language when sending messages, applying filters for expletives or threatening words, and creating frameworks for identifying patients who frequently send negative messages are potential strategies for mitigating this risk.”
Using a form of artificial intelligence technology called natural language processing (NLP), researchers at the University of California, San Diego, analyzed the characteristics of more than 1.4 million emails received by the university’s physicians, 43% of them from patients. They specifically looked at the volume of messages, word count, and overall sentiment.
Whereas other studies have examined the growing burden of EHR messaging for doctors, this type of email sentiment analysis could help in creating solutions. Researchers say that one such solution could involve applying filters for expletives or threatening words. It also could help identify fixable health system issues that make patients so angry, the researchers say.
Among the emails from physicians to physicians, just over half reported burnout, which correlated to the following phrases: “I am beginning to burn out and have one or more symptoms of burnout” and “I feel completely burned out [and] am at the point where I may need to seek help.”
On average, physicians who reported burnout received a greater volume of patient messages. The odds of burnout were significantly higher among Hispanic/Latinx physicians and females. Physicians with more than 15 years of clinical practice had markedly lower burnout.
Despite physicians now spending more time on EHR in-basket tasks than they did before the pandemic, the study found no significant associations between message characteristics and burnout.
Data for the cross-sectional study were collected from multiple specialties from April to September 2020. Physicians then completed a survey and assessed their burnout on a 5-point scale. Of the 609 physician responses, approximately 49% of participants were women, 56% were White, and 64% worked in outpatient settings. About 70% of the doctors had been in practice for 15 years or less.
The sentiment score was based on word content as well as the use of negation, punctuation, degree modifiers, all caps, emoticons, emojis, and acronyms. Positive patient messages were more likely to convey gratitude and thanks, along with casual expressions, such as “fyi” and “lol.”
A version of this article first appeared on Medscape.com.
Among the emails, 43% were from patients; the remainder were mostly from other physicians or clinicians, or automated. The content of the messages wasn’t associated with doctor burnout, as the researchers had hypothesized. And only about 5% of the messages had negative sentiment.
But the researchers were struck by the hostility of that sentiment, displayed in messages like these that surely would be distressing for physicians to read:
“I hope and expect that you will spend eternity in he**. You are an abusive, nasty, cheap person.”
“Your office is full of liars, hypocrites and I will do everything in my power to prevent anyone from going to your bullsh** office again.”
About 5% of emails had an overall negative sentiment, with high-frequency words like “cancel,” “pain,” or “problem.” Among patient messages, 3% were negative and contained words and expletives suggesting hatred, hostility, or violence.
“F***” was the most common expletive used by patients.
Researchers provided examples of profanity-laced messages, including one patient who said, “I am so upset that I was told the blood work would include the gender of the baby. I have been waiting 5 [days] to find it, and it wasn’t even fu**ing tested!!!! What a disappointment in your office and the bullsh** I was told. I will be switching plans because this is sh**!”
Researchers also noted some high-frequency words associated with violence, such as “shoot,” “fight,” and “kill.”
“This is concerning, especially given documentation of patient-inflicted violence against physicians. Health systems should be proactive in ensuring that the in-basket does not become a venue for physician abuse and cyberbullying,” the researchers wrote in JAMA Network Open.
“Posting reminders in EHR patient portals to use kind language when sending messages, applying filters for expletives or threatening words, and creating frameworks for identifying patients who frequently send negative messages are potential strategies for mitigating this risk.”
Using a form of artificial intelligence technology called natural language processing (NLP), researchers at the University of California, San Diego, analyzed the characteristics of more than 1.4 million emails received by the university’s physicians, 43% of them from patients. They specifically looked at the volume of messages, word count, and overall sentiment.
Whereas other studies have examined the growing burden of EHR messaging for doctors, this type of email sentiment analysis could help in creating solutions. Researchers say that one such solution could involve applying filters for expletives or threatening words. It also could help identify fixable health system issues that make patients so angry, the researchers say.
Among the emails from physicians to physicians, just over half reported burnout, which correlated to the following phrases: “I am beginning to burn out and have one or more symptoms of burnout” and “I feel completely burned out [and] am at the point where I may need to seek help.”
On average, physicians who reported burnout received a greater volume of patient messages. The odds of burnout were significantly higher among Hispanic/Latinx physicians and females. Physicians with more than 15 years of clinical practice had markedly lower burnout.
Despite physicians now spending more time on EHR in-basket tasks than they did before the pandemic, the study found no significant associations between message characteristics and burnout.
Data for the cross-sectional study were collected from multiple specialties from April to September 2020. Physicians then completed a survey and assessed their burnout on a 5-point scale. Of the 609 physician responses, approximately 49% of participants were women, 56% were White, and 64% worked in outpatient settings. About 70% of the doctors had been in practice for 15 years or less.
The sentiment score was based on word content as well as the use of negation, punctuation, degree modifiers, all caps, emoticons, emojis, and acronyms. Positive patient messages were more likely to convey gratitude and thanks, along with casual expressions, such as “fyi” and “lol.”
A version of this article first appeared on Medscape.com.
Among the emails, 43% were from patients; the remainder were mostly from other physicians or clinicians, or automated. The content of the messages wasn’t associated with doctor burnout, as the researchers had hypothesized. And only about 5% of the messages had negative sentiment.
But the researchers were struck by the hostility of that sentiment, displayed in messages like these that surely would be distressing for physicians to read:
“I hope and expect that you will spend eternity in he**. You are an abusive, nasty, cheap person.”
“Your office is full of liars, hypocrites and I will do everything in my power to prevent anyone from going to your bullsh** office again.”
About 5% of emails had an overall negative sentiment, with high-frequency words like “cancel,” “pain,” or “problem.” Among patient messages, 3% were negative and contained words and expletives suggesting hatred, hostility, or violence.
“F***” was the most common expletive used by patients.
Researchers provided examples of profanity-laced messages, including one patient who said, “I am so upset that I was told the blood work would include the gender of the baby. I have been waiting 5 [days] to find it, and it wasn’t even fu**ing tested!!!! What a disappointment in your office and the bullsh** I was told. I will be switching plans because this is sh**!”
Researchers also noted some high-frequency words associated with violence, such as “shoot,” “fight,” and “kill.”
“This is concerning, especially given documentation of patient-inflicted violence against physicians. Health systems should be proactive in ensuring that the in-basket does not become a venue for physician abuse and cyberbullying,” the researchers wrote in JAMA Network Open.
“Posting reminders in EHR patient portals to use kind language when sending messages, applying filters for expletives or threatening words, and creating frameworks for identifying patients who frequently send negative messages are potential strategies for mitigating this risk.”
Using a form of artificial intelligence technology called natural language processing (NLP), researchers at the University of California, San Diego, analyzed the characteristics of more than 1.4 million emails received by the university’s physicians, 43% of them from patients. They specifically looked at the volume of messages, word count, and overall sentiment.
Whereas other studies have examined the growing burden of EHR messaging for doctors, this type of email sentiment analysis could help in creating solutions. Researchers say that one such solution could involve applying filters for expletives or threatening words. It also could help identify fixable health system issues that make patients so angry, the researchers say.
Among the emails from physicians to physicians, just over half reported burnout, which correlated to the following phrases: “I am beginning to burn out and have one or more symptoms of burnout” and “I feel completely burned out [and] am at the point where I may need to seek help.”
On average, physicians who reported burnout received a greater volume of patient messages. The odds of burnout were significantly higher among Hispanic/Latinx physicians and females. Physicians with more than 15 years of clinical practice had markedly lower burnout.
Despite physicians now spending more time on EHR in-basket tasks than they did before the pandemic, the study found no significant associations between message characteristics and burnout.
Data for the cross-sectional study were collected from multiple specialties from April to September 2020. Physicians then completed a survey and assessed their burnout on a 5-point scale. Of the 609 physician responses, approximately 49% of participants were women, 56% were White, and 64% worked in outpatient settings. About 70% of the doctors had been in practice for 15 years or less.
The sentiment score was based on word content as well as the use of negation, punctuation, degree modifiers, all caps, emoticons, emojis, and acronyms. Positive patient messages were more likely to convey gratitude and thanks, along with casual expressions, such as “fyi” and “lol.”
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
U.S. biosimilar competition, use, and availability still lags behind European countries
The uptake and treatment costs of biosimilar drugs in the United States from 2011 to 2020 were significantly higher than in both Germany and Switzerland, based on data from a cohort study of publicly available commercial databases.
Biologics remain the fastest growing segment of drug research and development, but their costs remain high, David L. Carl, MSc, of the University of Zurich, and colleagues wrote in their study, published online in JAMA Network Open.
As patents and regulatory exclusivity periods expire, biologics face competition from biosimilars, which may drive competition and lower prices, they said.
“However, studies have shown that there are varying policies and biosimilar uptake in European countries and that the observed levels of competition and uptake have not reached the expected levels in the U.S.,” the researchers said.
To assist the discussions of policy makers in the United States and Europe as they consider legislative and regulatory reforms that are intended to promote the competition of biosimilars, the researchers reviewed data from 15 biosimilars and 6 biologics in the United States, 52 biosimilars and 15 biologics in Germany, and 28 biosimilars and 13 biologics in Switzerland.
They analyzed temporal trends in the uptake of biosimilars and their relative prices, compared with the prices of biologics in each country, by obtaining wholesale acquisition costs from online drug pricing databases. They extracted quarterly sales volume data for 2011-2020 from the IQVIA database. In the case of confidential rebates in Switzerland, the researchers obtained list prices.
Overall, the uptake of biosimilars increased in all three countries during the study period. However, the prices of biosimilars and the reference products were significantly higher in the United States, compared with Germany and Switzerland, both of which have national mechanisms for drug price negotiation. The monthly treatment cost of biosimilars was a median of 1.94 and 2.74 times higher in the United States than in Germany and Switzerland, respectively.
On average, the biosimilar market share at launch was highest in Germany; however, it increased at the fastest rate in the United States.
The findings were limited by several factors, including the sample size and the inclusion only of sales data provided by IQVIA, and by the use of list prices only without accounting for drug rebates, the researchers noted. Other limitations were the inability to compare conclusions from the United States and European Union directly because the drugs entered markets at different times, and not all the same drugs have been approved or designated as biosimilars, they said.
However, the results illustrate a difference in uptake of biosimilars in the United States with a reduced impact on drug costs, they said.
Looking ahead, “Policies for drug pricing negotiations in the U.S. against anticompetitive practices of exclusionary contracts could allow biosimilars to enter the market sooner and at lower costs, which could result in lower health care costs and improved patient access,” they concluded.
The study was partially funded by the Swiss National Science Foundation. Lead author Mr. Carl had no financial conflicts to disclose; several coauthors disclosed funding from organizations including The Health Foundation, the U.K. National Institute for Health Research, and the Pharmaceutical Group of the European Union; all were unrelated to the current study.
The uptake and treatment costs of biosimilar drugs in the United States from 2011 to 2020 were significantly higher than in both Germany and Switzerland, based on data from a cohort study of publicly available commercial databases.
Biologics remain the fastest growing segment of drug research and development, but their costs remain high, David L. Carl, MSc, of the University of Zurich, and colleagues wrote in their study, published online in JAMA Network Open.
As patents and regulatory exclusivity periods expire, biologics face competition from biosimilars, which may drive competition and lower prices, they said.
“However, studies have shown that there are varying policies and biosimilar uptake in European countries and that the observed levels of competition and uptake have not reached the expected levels in the U.S.,” the researchers said.
To assist the discussions of policy makers in the United States and Europe as they consider legislative and regulatory reforms that are intended to promote the competition of biosimilars, the researchers reviewed data from 15 biosimilars and 6 biologics in the United States, 52 biosimilars and 15 biologics in Germany, and 28 biosimilars and 13 biologics in Switzerland.
They analyzed temporal trends in the uptake of biosimilars and their relative prices, compared with the prices of biologics in each country, by obtaining wholesale acquisition costs from online drug pricing databases. They extracted quarterly sales volume data for 2011-2020 from the IQVIA database. In the case of confidential rebates in Switzerland, the researchers obtained list prices.
Overall, the uptake of biosimilars increased in all three countries during the study period. However, the prices of biosimilars and the reference products were significantly higher in the United States, compared with Germany and Switzerland, both of which have national mechanisms for drug price negotiation. The monthly treatment cost of biosimilars was a median of 1.94 and 2.74 times higher in the United States than in Germany and Switzerland, respectively.
On average, the biosimilar market share at launch was highest in Germany; however, it increased at the fastest rate in the United States.
The findings were limited by several factors, including the sample size and the inclusion only of sales data provided by IQVIA, and by the use of list prices only without accounting for drug rebates, the researchers noted. Other limitations were the inability to compare conclusions from the United States and European Union directly because the drugs entered markets at different times, and not all the same drugs have been approved or designated as biosimilars, they said.
However, the results illustrate a difference in uptake of biosimilars in the United States with a reduced impact on drug costs, they said.
Looking ahead, “Policies for drug pricing negotiations in the U.S. against anticompetitive practices of exclusionary contracts could allow biosimilars to enter the market sooner and at lower costs, which could result in lower health care costs and improved patient access,” they concluded.
The study was partially funded by the Swiss National Science Foundation. Lead author Mr. Carl had no financial conflicts to disclose; several coauthors disclosed funding from organizations including The Health Foundation, the U.K. National Institute for Health Research, and the Pharmaceutical Group of the European Union; all were unrelated to the current study.
The uptake and treatment costs of biosimilar drugs in the United States from 2011 to 2020 were significantly higher than in both Germany and Switzerland, based on data from a cohort study of publicly available commercial databases.
Biologics remain the fastest growing segment of drug research and development, but their costs remain high, David L. Carl, MSc, of the University of Zurich, and colleagues wrote in their study, published online in JAMA Network Open.
As patents and regulatory exclusivity periods expire, biologics face competition from biosimilars, which may drive competition and lower prices, they said.
“However, studies have shown that there are varying policies and biosimilar uptake in European countries and that the observed levels of competition and uptake have not reached the expected levels in the U.S.,” the researchers said.
To assist the discussions of policy makers in the United States and Europe as they consider legislative and regulatory reforms that are intended to promote the competition of biosimilars, the researchers reviewed data from 15 biosimilars and 6 biologics in the United States, 52 biosimilars and 15 biologics in Germany, and 28 biosimilars and 13 biologics in Switzerland.
They analyzed temporal trends in the uptake of biosimilars and their relative prices, compared with the prices of biologics in each country, by obtaining wholesale acquisition costs from online drug pricing databases. They extracted quarterly sales volume data for 2011-2020 from the IQVIA database. In the case of confidential rebates in Switzerland, the researchers obtained list prices.
Overall, the uptake of biosimilars increased in all three countries during the study period. However, the prices of biosimilars and the reference products were significantly higher in the United States, compared with Germany and Switzerland, both of which have national mechanisms for drug price negotiation. The monthly treatment cost of biosimilars was a median of 1.94 and 2.74 times higher in the United States than in Germany and Switzerland, respectively.
On average, the biosimilar market share at launch was highest in Germany; however, it increased at the fastest rate in the United States.
The findings were limited by several factors, including the sample size and the inclusion only of sales data provided by IQVIA, and by the use of list prices only without accounting for drug rebates, the researchers noted. Other limitations were the inability to compare conclusions from the United States and European Union directly because the drugs entered markets at different times, and not all the same drugs have been approved or designated as biosimilars, they said.
However, the results illustrate a difference in uptake of biosimilars in the United States with a reduced impact on drug costs, they said.
Looking ahead, “Policies for drug pricing negotiations in the U.S. against anticompetitive practices of exclusionary contracts could allow biosimilars to enter the market sooner and at lower costs, which could result in lower health care costs and improved patient access,” they concluded.
The study was partially funded by the Swiss National Science Foundation. Lead author Mr. Carl had no financial conflicts to disclose; several coauthors disclosed funding from organizations including The Health Foundation, the U.K. National Institute for Health Research, and the Pharmaceutical Group of the European Union; all were unrelated to the current study.
FROM JAMA NETWORK OPEN
How to Foster Camaraderie in Dermatology Residency
Change is inevitable in residency as well as in life. Every year on July 1, the atmosphere and social structure of residencies change with the new postgraduate year 2 class. Each class brings a unique perspective and energy. Residents come together from different backgrounds and life situations. Some residents are single, some are engaged or married, and some are starting or expanding their families. Some residents will have prior careers, others will have graduate degrees or expertise in various fields. They will have different ethnic backgrounds, religious and/or spiritual beliefs, familial upbringings, personalities, and methods of communicating. These differences all are important to consider when developing a mindset of inclusion and camaraderie. As residents start their journey together, it is important to remember that residency is a team endeavor. The principles of teamwork apply directly to residents and are founded on creating a climate of trust and building strong relationships with one another.1 Trust is the foundation of good relationships in the workplace; it allows people to communicate freely and foster the belief that everyone is working for each other’s best interests. Being open and sharing knowledge about networking opportunities, scholarships, and research projects is one way to foster collaboration and trust in residency.
Diversity, equity, and inclusion in dermatology is a work in progress. In the 2020-2021 dermatology application cycle, only 4.8% of applicants identified as Hispanic or Latino, and 7.8% identified as Black or African American.2 The American Academy of Dermatology took an active role in promoting diversity by creating a task force in 2018 to increase the exposure and recruitment into dermatology of medical students who are underrepresented in medicine.2 As standards for diversity are met in dermatology, we will have the wonderful opportunity to welcome even more diversity into our lives.
Listening, showing curiosity about your co-residents’ lives outside of work, and asking questions can help build respect, friendships, and camaraderie. Ask your co-residents what makes them happy and what their goals are in residency. Finding common goals and cultivating the mindset that you all work together to achieve your goals is key to the success of a residency class. Now that we discussed accepting and welcoming differences, how do you foster camaraderie in a social setting?
Establish a Social Committee
As a class, consider 1 or 2 residents who are always excited to try new activities such as attend restaurant openings, exercise classes, concerts, or movie nights. Consider nominating these co-residents along with one attending to be social chairs of your residency. The social chairs should meet and establish at least 1 social event per season, with 4 total for the academic year. There are only 2 rules with social events: (1) they must be held outside of clinic, and (2) everyone should try their best to attend.
Social chairs should try to prioritize a location-specific event that allows the residents who are not from the area to experience something local, which can be anything from apple picking at an orchard in the fall to beach volleyball in the summer. Planning these parties gives everyone an event to look forward to and a chance to spend time together and grow closer. The memories and inside jokes that arise from these outings are invaluable and increase joy inside and outside of clinic.
Utilize Social Media
Another project can be developing a social media account for your program with the approval of your faculty. @unmcdermatology, @uwderm, and @gwdermres can help foster social relationships by establishing a lighthearted space to celebrate the residency’s achievements, new publications, volunteer events, or social gatherings.
Encourage Local and National Conference Attendance
All residents should be encouraged to submit abstracts to local and national conferences and attend with their co-residents. Conferences are peak opportunities to foster camaraderie within residency classes, as they involve a sense of togetherness in the specialty along with the excitement of traveling to a new city and meeting other like-minded individuals. Conferences allow collaboration within the specialty on a national level and foster relationships between residency programs.
In addition, national groups such as the Women’s Dermatologic Society, the Skin of Color Society, and the American Academy of Dermatology Diversity, Equity, and Inclusion task force meet at the national conferences and discuss their next initiatives and projects. Joining a society of your interest can lead to many new networks and relationships you may not have had before. Even if you are not interested in specializing after general dermatology, consider attending a surgery, dermatopathology, or pediatric or cosmetic dermatology conference to learn more about the field from the experts.
Repair Conflicts and Build a Climate of Collaboration
Conflicts and disagreements unfortunately are inevitable during residency. Whether they involve planning vacation times or coordinating call schedules, everyone will not agree on every decision. Learning how to handle and approach conflict with co-residents is of utmost importance to maintaining the hard work you have put in to create trust, camaraderie, and a good social atmosphere. If you are having an issue with a circumstance involving a co-resident, holding a grudge will only sour your experience and the experience of others. Talking to your co-resident directly about your concerns before escalating the issue to a chief resident or faculty member is a great start. Consider asking them about their thought process and show concern for their point of view. Listen to them openly before going into your preferences. It is important to remember that working as a team requires sacrifices, and sometimes you will not be satisfied with the outcome of a conflict.
It also is important to remember that feelings change, and an issue you feel you must address immediately can wait to be addressed at a better time when you have calmed down. You may even find that you decide not to address it at all. At the end of the day, if a conflict cannot be worked out between those involved, consider confiding in a chief resident or a faculty mentor for advice on the next steps to take to resolve the problem. Ultimately, having a good foundation of respect and strong bonds with your residents will help tremendously when conflicts arise.
Final Thoughts
Fostering camaraderie in residency will improve the overall experience and lives of the residents, as well as the experience of the faculty, staff, and patients by the trickle-down effect. Creating a cheerful and fun atmosphere filled with inside jokes and excitement regarding upcoming social events or conferences will certainly result in a time you will cherish for the rest of your life.
- Kouzes JM, Posner BZ. Foster collaboration. In: Kouzes JM, Posner BZ, eds. The Leadership Challenge. 6th ed. John Wiley & Sons, Inc; 2017:195-217.
- Cooper J, Shao K, Feng H. Racial/ethnic health disparities in dermatology in the United States, part 1: overview of contributing factors and management strategies [published online February 7, 2022]. J Am Acad Dermatol. 2022;87:723-730. doi:10.1016/j.jaad.2021.12.061
Change is inevitable in residency as well as in life. Every year on July 1, the atmosphere and social structure of residencies change with the new postgraduate year 2 class. Each class brings a unique perspective and energy. Residents come together from different backgrounds and life situations. Some residents are single, some are engaged or married, and some are starting or expanding their families. Some residents will have prior careers, others will have graduate degrees or expertise in various fields. They will have different ethnic backgrounds, religious and/or spiritual beliefs, familial upbringings, personalities, and methods of communicating. These differences all are important to consider when developing a mindset of inclusion and camaraderie. As residents start their journey together, it is important to remember that residency is a team endeavor. The principles of teamwork apply directly to residents and are founded on creating a climate of trust and building strong relationships with one another.1 Trust is the foundation of good relationships in the workplace; it allows people to communicate freely and foster the belief that everyone is working for each other’s best interests. Being open and sharing knowledge about networking opportunities, scholarships, and research projects is one way to foster collaboration and trust in residency.
Diversity, equity, and inclusion in dermatology is a work in progress. In the 2020-2021 dermatology application cycle, only 4.8% of applicants identified as Hispanic or Latino, and 7.8% identified as Black or African American.2 The American Academy of Dermatology took an active role in promoting diversity by creating a task force in 2018 to increase the exposure and recruitment into dermatology of medical students who are underrepresented in medicine.2 As standards for diversity are met in dermatology, we will have the wonderful opportunity to welcome even more diversity into our lives.
Listening, showing curiosity about your co-residents’ lives outside of work, and asking questions can help build respect, friendships, and camaraderie. Ask your co-residents what makes them happy and what their goals are in residency. Finding common goals and cultivating the mindset that you all work together to achieve your goals is key to the success of a residency class. Now that we discussed accepting and welcoming differences, how do you foster camaraderie in a social setting?
Establish a Social Committee
As a class, consider 1 or 2 residents who are always excited to try new activities such as attend restaurant openings, exercise classes, concerts, or movie nights. Consider nominating these co-residents along with one attending to be social chairs of your residency. The social chairs should meet and establish at least 1 social event per season, with 4 total for the academic year. There are only 2 rules with social events: (1) they must be held outside of clinic, and (2) everyone should try their best to attend.
Social chairs should try to prioritize a location-specific event that allows the residents who are not from the area to experience something local, which can be anything from apple picking at an orchard in the fall to beach volleyball in the summer. Planning these parties gives everyone an event to look forward to and a chance to spend time together and grow closer. The memories and inside jokes that arise from these outings are invaluable and increase joy inside and outside of clinic.
Utilize Social Media
Another project can be developing a social media account for your program with the approval of your faculty. @unmcdermatology, @uwderm, and @gwdermres can help foster social relationships by establishing a lighthearted space to celebrate the residency’s achievements, new publications, volunteer events, or social gatherings.
Encourage Local and National Conference Attendance
All residents should be encouraged to submit abstracts to local and national conferences and attend with their co-residents. Conferences are peak opportunities to foster camaraderie within residency classes, as they involve a sense of togetherness in the specialty along with the excitement of traveling to a new city and meeting other like-minded individuals. Conferences allow collaboration within the specialty on a national level and foster relationships between residency programs.
In addition, national groups such as the Women’s Dermatologic Society, the Skin of Color Society, and the American Academy of Dermatology Diversity, Equity, and Inclusion task force meet at the national conferences and discuss their next initiatives and projects. Joining a society of your interest can lead to many new networks and relationships you may not have had before. Even if you are not interested in specializing after general dermatology, consider attending a surgery, dermatopathology, or pediatric or cosmetic dermatology conference to learn more about the field from the experts.
Repair Conflicts and Build a Climate of Collaboration
Conflicts and disagreements unfortunately are inevitable during residency. Whether they involve planning vacation times or coordinating call schedules, everyone will not agree on every decision. Learning how to handle and approach conflict with co-residents is of utmost importance to maintaining the hard work you have put in to create trust, camaraderie, and a good social atmosphere. If you are having an issue with a circumstance involving a co-resident, holding a grudge will only sour your experience and the experience of others. Talking to your co-resident directly about your concerns before escalating the issue to a chief resident or faculty member is a great start. Consider asking them about their thought process and show concern for their point of view. Listen to them openly before going into your preferences. It is important to remember that working as a team requires sacrifices, and sometimes you will not be satisfied with the outcome of a conflict.
It also is important to remember that feelings change, and an issue you feel you must address immediately can wait to be addressed at a better time when you have calmed down. You may even find that you decide not to address it at all. At the end of the day, if a conflict cannot be worked out between those involved, consider confiding in a chief resident or a faculty mentor for advice on the next steps to take to resolve the problem. Ultimately, having a good foundation of respect and strong bonds with your residents will help tremendously when conflicts arise.
Final Thoughts
Fostering camaraderie in residency will improve the overall experience and lives of the residents, as well as the experience of the faculty, staff, and patients by the trickle-down effect. Creating a cheerful and fun atmosphere filled with inside jokes and excitement regarding upcoming social events or conferences will certainly result in a time you will cherish for the rest of your life.
Change is inevitable in residency as well as in life. Every year on July 1, the atmosphere and social structure of residencies change with the new postgraduate year 2 class. Each class brings a unique perspective and energy. Residents come together from different backgrounds and life situations. Some residents are single, some are engaged or married, and some are starting or expanding their families. Some residents will have prior careers, others will have graduate degrees or expertise in various fields. They will have different ethnic backgrounds, religious and/or spiritual beliefs, familial upbringings, personalities, and methods of communicating. These differences all are important to consider when developing a mindset of inclusion and camaraderie. As residents start their journey together, it is important to remember that residency is a team endeavor. The principles of teamwork apply directly to residents and are founded on creating a climate of trust and building strong relationships with one another.1 Trust is the foundation of good relationships in the workplace; it allows people to communicate freely and foster the belief that everyone is working for each other’s best interests. Being open and sharing knowledge about networking opportunities, scholarships, and research projects is one way to foster collaboration and trust in residency.
Diversity, equity, and inclusion in dermatology is a work in progress. In the 2020-2021 dermatology application cycle, only 4.8% of applicants identified as Hispanic or Latino, and 7.8% identified as Black or African American.2 The American Academy of Dermatology took an active role in promoting diversity by creating a task force in 2018 to increase the exposure and recruitment into dermatology of medical students who are underrepresented in medicine.2 As standards for diversity are met in dermatology, we will have the wonderful opportunity to welcome even more diversity into our lives.
Listening, showing curiosity about your co-residents’ lives outside of work, and asking questions can help build respect, friendships, and camaraderie. Ask your co-residents what makes them happy and what their goals are in residency. Finding common goals and cultivating the mindset that you all work together to achieve your goals is key to the success of a residency class. Now that we discussed accepting and welcoming differences, how do you foster camaraderie in a social setting?
Establish a Social Committee
As a class, consider 1 or 2 residents who are always excited to try new activities such as attend restaurant openings, exercise classes, concerts, or movie nights. Consider nominating these co-residents along with one attending to be social chairs of your residency. The social chairs should meet and establish at least 1 social event per season, with 4 total for the academic year. There are only 2 rules with social events: (1) they must be held outside of clinic, and (2) everyone should try their best to attend.
Social chairs should try to prioritize a location-specific event that allows the residents who are not from the area to experience something local, which can be anything from apple picking at an orchard in the fall to beach volleyball in the summer. Planning these parties gives everyone an event to look forward to and a chance to spend time together and grow closer. The memories and inside jokes that arise from these outings are invaluable and increase joy inside and outside of clinic.
Utilize Social Media
Another project can be developing a social media account for your program with the approval of your faculty. @unmcdermatology, @uwderm, and @gwdermres can help foster social relationships by establishing a lighthearted space to celebrate the residency’s achievements, new publications, volunteer events, or social gatherings.
Encourage Local and National Conference Attendance
All residents should be encouraged to submit abstracts to local and national conferences and attend with their co-residents. Conferences are peak opportunities to foster camaraderie within residency classes, as they involve a sense of togetherness in the specialty along with the excitement of traveling to a new city and meeting other like-minded individuals. Conferences allow collaboration within the specialty on a national level and foster relationships between residency programs.
In addition, national groups such as the Women’s Dermatologic Society, the Skin of Color Society, and the American Academy of Dermatology Diversity, Equity, and Inclusion task force meet at the national conferences and discuss their next initiatives and projects. Joining a society of your interest can lead to many new networks and relationships you may not have had before. Even if you are not interested in specializing after general dermatology, consider attending a surgery, dermatopathology, or pediatric or cosmetic dermatology conference to learn more about the field from the experts.
Repair Conflicts and Build a Climate of Collaboration
Conflicts and disagreements unfortunately are inevitable during residency. Whether they involve planning vacation times or coordinating call schedules, everyone will not agree on every decision. Learning how to handle and approach conflict with co-residents is of utmost importance to maintaining the hard work you have put in to create trust, camaraderie, and a good social atmosphere. If you are having an issue with a circumstance involving a co-resident, holding a grudge will only sour your experience and the experience of others. Talking to your co-resident directly about your concerns before escalating the issue to a chief resident or faculty member is a great start. Consider asking them about their thought process and show concern for their point of view. Listen to them openly before going into your preferences. It is important to remember that working as a team requires sacrifices, and sometimes you will not be satisfied with the outcome of a conflict.
It also is important to remember that feelings change, and an issue you feel you must address immediately can wait to be addressed at a better time when you have calmed down. You may even find that you decide not to address it at all. At the end of the day, if a conflict cannot be worked out between those involved, consider confiding in a chief resident or a faculty mentor for advice on the next steps to take to resolve the problem. Ultimately, having a good foundation of respect and strong bonds with your residents will help tremendously when conflicts arise.
Final Thoughts
Fostering camaraderie in residency will improve the overall experience and lives of the residents, as well as the experience of the faculty, staff, and patients by the trickle-down effect. Creating a cheerful and fun atmosphere filled with inside jokes and excitement regarding upcoming social events or conferences will certainly result in a time you will cherish for the rest of your life.
- Kouzes JM, Posner BZ. Foster collaboration. In: Kouzes JM, Posner BZ, eds. The Leadership Challenge. 6th ed. John Wiley & Sons, Inc; 2017:195-217.
- Cooper J, Shao K, Feng H. Racial/ethnic health disparities in dermatology in the United States, part 1: overview of contributing factors and management strategies [published online February 7, 2022]. J Am Acad Dermatol. 2022;87:723-730. doi:10.1016/j.jaad.2021.12.061
- Kouzes JM, Posner BZ. Foster collaboration. In: Kouzes JM, Posner BZ, eds. The Leadership Challenge. 6th ed. John Wiley & Sons, Inc; 2017:195-217.
- Cooper J, Shao K, Feng H. Racial/ethnic health disparities in dermatology in the United States, part 1: overview of contributing factors and management strategies [published online February 7, 2022]. J Am Acad Dermatol. 2022;87:723-730. doi:10.1016/j.jaad.2021.12.061
Resident Pearls
- Camaraderie in residency is a special dynamic that can be enhanced and fostered in many different ways.
- The relationships among residents should be treated with importance, as some of the friends you make will last a career and/or a lifetime.
- Conflicts inevitably will arise and learning how to handle them effectively can improve the residency experience.
More on social entropy
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
As leaders of the American Psychiatric Association, we received dozens of communications from members who were shocked by the discriminatory and transphobic commentary in the recent editorial “The accelerating societal entropy undermines mental health” (
Specifically, citing “lack of certainty about gender identity in children and adults” as an indicator of societal turmoil that undermines mental health is contrary to the scientific understanding of gender identity. Physicians have professional obligations to advance patients’ well-being and do no harm.
The medical profession, including psychiatry, is at a critical juncture in coming to terms with and dismantling its longstanding history of systemic racism and discrimination. Authors and editors must be aware that harmful and divisive language negatively affects mental health, especially for people who have been subject to discrimination individually and/or as members of historically excluded and/or minoritized groups.
In publishing this editorial,
Rebecca W. Brendel, MD, JD, DFAPA
President
American Psychiatric Association
Saul Levin, MD, MPA, FRCP-E, FRCPsych
CEO and Medical Director
American Psychiatric Association
Disclosures
The authors report no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
Dr. Nasrallah responds
I regret that the sentence about gender identity in my October editorial was regarded as transphobic and harmful. While the phrasing reflected my patients’ comments to me, I realize my unfortunate choice of words deeply offended individuals who are transgender, who have been subjected to ongoing discrimination and prejudice.
I apologize to our readers; to my American Psychiatric Association LGBTQAI+ friends, colleagues, and relatives; and to the LGBTQAI+ community at large. The sentence has been deleted from the online version of my editorial. This has been a teachable moment for me.
Henry A. Nasrallah, MD
Editor-In-Chief
Continue to: More on psychiatric documentation
More on psychiatric documentation
Dr. Joshi’s helpful discussion of clinical documentation strategies (“Medical record documentation: What to do, and what to avoid,”
The mental health record may not always be as confidential as psychiatrists think (or hope) it is. The Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule, for example, generally does not distinguish between medical and mental health information, nor does it provide special rules for the latter (although certain state laws may do so). HIPAA provides added protections for “psychotherapy notes,” but this category explicitly excludes progress notes that discuss treatment modalities, diagnosis, and clinical milestones. To retain their protected status, psychotherapists’ private, “desk-drawer memory joggers” must never be comingled with the patient chart.1 For mental health professionals, this distinction underscores the importance of keeping personal details broad in the progress note; scandalous or embarrassing narratives recounted in the medical record itself are routinely accessible to the patient and may be lawfully disclosed to others under specified circumstances.
In addition to avoiding speculation and including patient quotes when appropriate, documenting objectively and nonjudgmentally means annotating facts and observations that helped the clinician arrive at their conclusion. For example, “patient appears intoxicated” is less helpful than noting the patient’s slurred speech, impaired gait and/or coordination, and alcohol odor.
Clinical care and its associated documentation are so intertwined that they can become virtually indistinguishable. In a medical malpractice case, the burden is on the plaintiff to prove their injury resulted from substandard care. Some courts, however, have held that missing or incomplete records can effectively shift the burden from the recipient to the provider of care to show that the treatment at issue was rendered non-negligently.2 Statutes of limitations restricting the amount of time in which a patient can sue after an adverse event are sometimes triggered by the date on which they knew or should have known of the alleged malpractice.3 One of the best ways of ascertaining this date, and starting the statute of limitations clock, can be a clear annotation in the medical record that the patient was apprised of an unanticipated outcome or iatrogenic harm. In this way, a timely and thorough note can be critical not just to defending the physician’s quality of care, but potentially to precluding a cognizable lawsuit altogether.
Charles G. Kels, JD
Defense Health Agency
San Antonio, Texas
Disclosures
The views expressed are those of the author and do not necessarily reflect those of any government agency, nor do they constitute individualized legal advice. The author reports no financial relationships with any companies whose products are mentioned in this letter, or with manufacturers of competing products.
References
1. 45 CFR Parts 160 and 164, Subparts A and E.
2. Valcin v Public Health Trust, 473 So. 2d 1297 (1984).
3. US v Kubrick, 444 US 111 (1979).
An overlooked cause of catatonia
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.
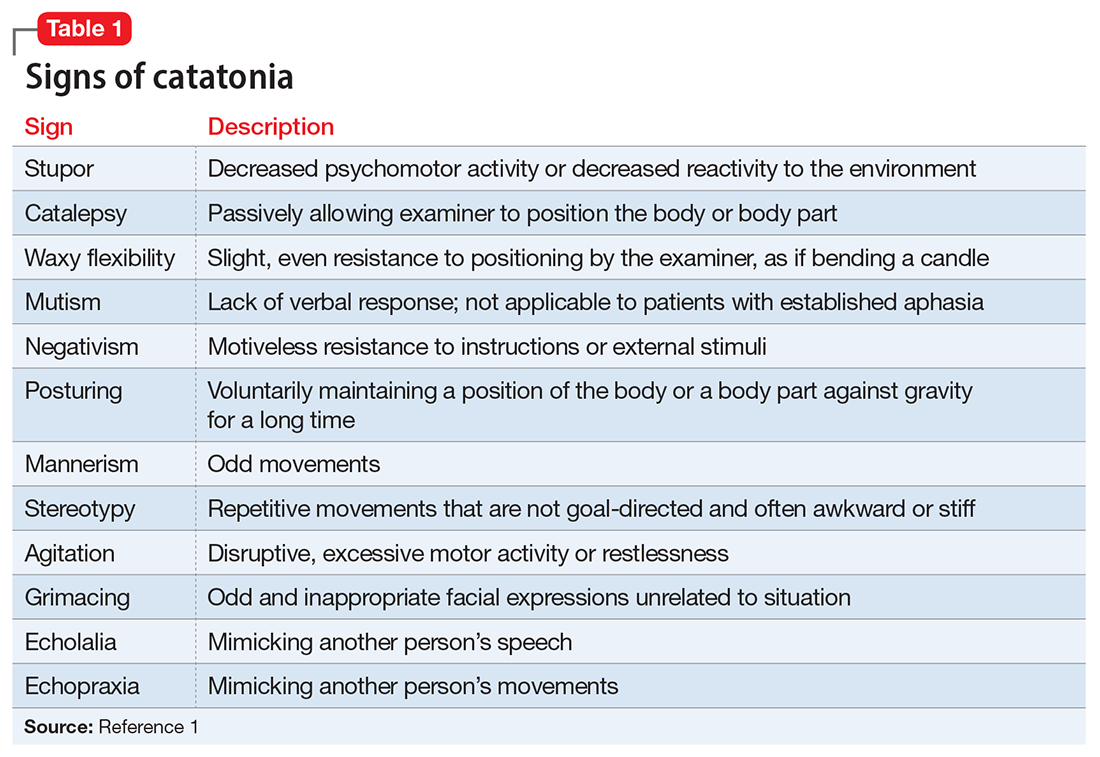
EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).
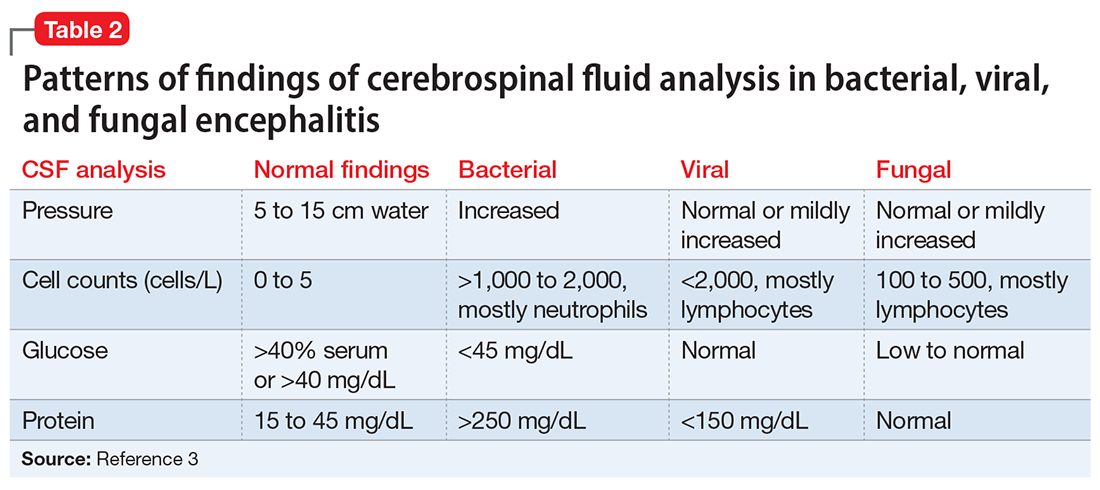
[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.
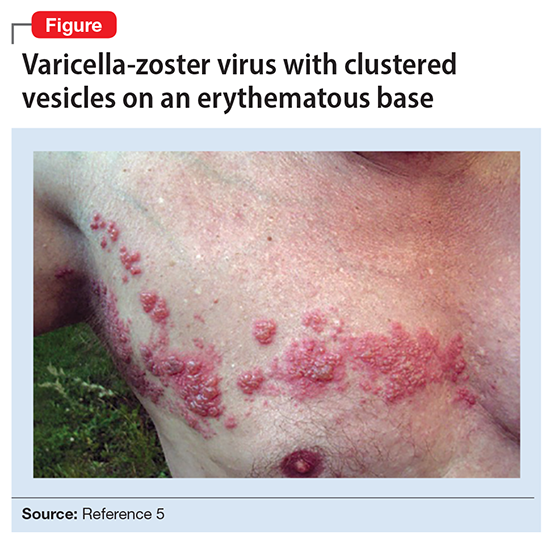
Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16
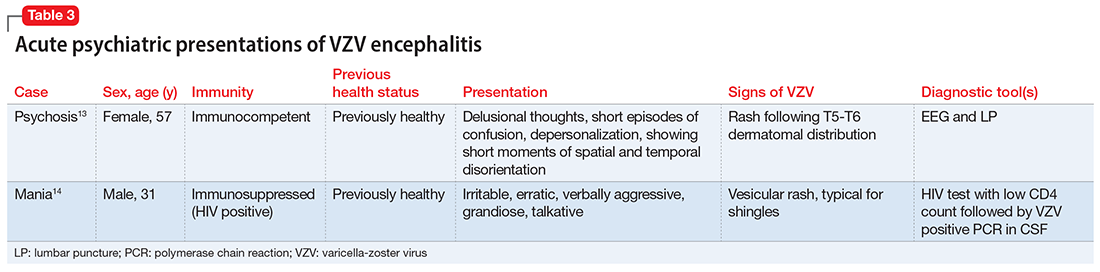
To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-
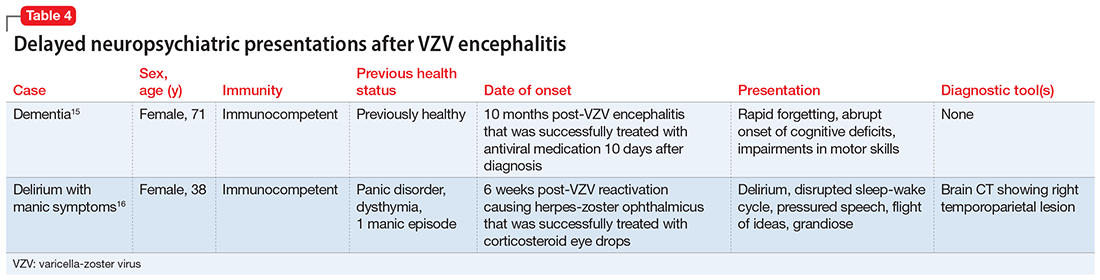
Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
CASE Agitation and bizarre behavior
Ms. L, age 40, presents to the emergency department (ED) for altered mental status and bizarre behavior. Before arriving at the ED, she had experienced a severe headache and an episode of vomiting. At home she had been irritable and agitated, repetitively dressing and undressing, urinating outside the toilet, and opening and closing water faucets in the house. She also had stopped eating and drinking. Ms. L’s home medications consist of levothyroxine 100 mcg/d for hypothyroidism.
In the ED, Ms. L has severe psychomotor agitation. She is restless and displays purposeless repetitive movements with her hands. She is mostly mute, but does groan at times.
HISTORY Multiple trips to the ED
In addition to hypothyroidism, Ms. L has a history of migraines and asthma. Four days before presenting to the ED, she complained of a severe headache and generalized fatigue, with vomiting and nausea. Two days later, she presented to the ED at a different hospital and underwent a brain CT scan; the results were unremarkable. At that facility, a laboratory work-up—including complete blood count, urea, creatinine, C-reactive protein, electrolytes, magnesium, phosphorus, calcium, full liver function tests, amylase, lipase, bilirubin, thyroid function test, and beta-human chorionic gonadotropin—was normal except for low thyroid-stimulating hormone levels (0.016 mIU/L). Ms. L was diagnosed with a severe migraine attack and discharged home with instructions to follow up with her endocrinologist.
Ms. L has no previous psychiatric history. Her family’s psychiatric history includes depression with psychotic features (mother), depression (maternal aunt), and generalized anxiety disorder (mother’s maternal aunt).
[polldaddy:11252938]
The authors’ observations
Catatonia is a behavioral syndrome with heterogeneous signs and symptoms. According to DSM-5, the diagnosis is considered when a patient presents with ≥3 of the 12 signs outlined in Table 1.1 It usually occurs in the context of an underlying psychiatric disorder such as schizophrenia or depression, or a medical disorder such as CNS infection or encephalopathy due to metabolic causes.1 Ms. L exhibited mutism, negativism, mannerism, stereotypy, and agitation and thus met the criteria for a catatonia diagnosis.

EVALUATION Unexpected finding on physical exam
In the ED, Ms. L is hemodynamically stable. Her blood pressure is 140/80 mm Hg; heart rate is 103 beats per minute; oxygen saturation is 98%; respiratory rate is 14 breaths per minute; and temperature is 37.5° C. Results from a brain MRI and total body scan performed prior to admission are unremarkable.
Ms. L is admitted to the psychiatric ward under the care of neurology for a psychiatry consultation. For approximately 24 hours, she receives IV diazepam 5 mg every 8 hours (due to the unavailability of lorazepam) for management of her catatonic symptoms, and olanzapine 10 mg every 8 hours orally as needed for agitation. Collateral history rules out a current mood episode or onset of psychosis in the weeks before she came to the ED. Diazepam improves Ms. L’s psychomotor agitation, which allows the primary team an opportunity to examine her.
Continue to: A physical exam reveals...
A physical exam reveals small vesicular lesions (1 to 2 cm in diameter) on an erythematous base on the left breast associated with an erythematous plaque with no evident vesicles on the left inner arm. The vesicular lesions display in a segmented pattern of dermatomal distribution.
[polldaddy:11252941]
The authors’ observations
Catatonic symptoms, coupled with psychomotor agitation in an immunocompetent middle-aged adult with a history of migraine headaches, strong family history of severe mental illness, and noncontributory findings on brain imaging, prompted a Psychiatry consultation and administration of psychotropic medications. A thorough physical exam revealing the small area of shingles and acute altered mental status prompted more aggressive investigations to explore the possibility of encephalitis.
Physicians should have a low index of suspicion for encephalitis (viral, bacterial, autoimmune, etc) and perform a lumbar puncture (LP) when necessary, despite the invasiveness of this test. A direct physical examination is often underutilized, notably in psychiatric patients, which can lead to the omission of important clinical information.2 Normal vital signs, blood workup, and MRI before admission are not sufficient to correctly guide diagnosis.
EVALUATION Additional lab results establish the diagnosis
An LP reveals Ms. L’s protein levels are 44 mg/dL, her glucose levels are 85 mg/dL, red blood cell count is 4/µL, and white blood cell count is 200/µL with 92% lymphocytes and 1% neutrophils. Ms. L’s CSF analysis profile indicates a viral CNS infection (Table 23).

[polldaddy:11252943]
The authors’ observations
Varicella-zoster virus (VZV) and herpes simplex virus (HSV) are human neurotropic alphaherpesviruses that cause lifelong infections in ganglia, and their reactivation can come in the form of encephalitis.4
Continue to: Ms. L's clinical presentation...
Ms. L’s clinical presentation most likely implicated VZV. Skin lesions of VZV may look exactly like HSV, with clustered vesicles on an erythematous base (Figure5). However, VZV rash tends to follow a dermatomal distribution (as in Ms. L’s case), which can help distinguish it from herpetic lesions.

Cases of VZV infection have been increasing worldwide. It is usually seen in older adults or those with compromised immunity.6 Significantly higher rates of VZV complications have been reported in such patients. A serious complication is VZV encephalitis, which is rare but possible, even in healthy individuals.6 VZV encephalitis can present with atypical psychiatric features. Ms. L exhibited several symptoms of VZV encephalitis, which include headache, fever, vomiting, altered level of consciousness, and seizures. An EEG also showed intermittent generalized slow waves in the range of theta commonly seen in encephalitis.
Ms. L’s case shows the importance of early recognition of VZV infection. The diagnosis is confirmed through CSF analysis. There is an urgency to promptly conduct the LP to confirm the diagnosis and quickly initiate antiviral treatment to stop the progression of the infection and its life-threatening sequelae.
In the absence of underlying medical cause, typical treatment of catatonia involves the sublingual or IM administration of 1 to 2 mg lorazepam that can be repeated twice at 3-hour intervals if the patient’s symptoms do not resolve. ECT is indicated if the patient experiences minimal or no response to lorazepam.
The use of antipsychotics for catatonia is controversial. High-potency antipsychotics such as haloperidol and risperidone are not recommended due to increased risk of the progression of catatonia into neuroleptic malignant syndrome.7
Continue to: OUTCOME Prompt recovery with an antiviral
OUTCOME Prompt recovery with an antiviral
Ms. L receives IV acyclovir 1,200 mg every 8 hours for 14 days. Just 48 hours after starting this antiviral medication, her bizarre behavior and catatonic features cease, and she returns to her baseline mental functioning. Olanzapine is discontinued, and lorazepam is progressively decreased. The CSF polymerase chain reaction assay indicates Ms. L is positive for VZV, which confirms the diagnosis of VZV encephalitis. A spine MRI is also performed and rules out myelitis as a sequela of the infection.
The authors’ observations
Chickenpox is caused by a primary encounter with VZV. Inside the ganglions of neurons, a dormant form of VZV resides. Its reactivation leads to the spread of the infection to the skin innervated by these neurons, causing shingles. Reactivation occurs in approximately 1 million people in the United States each year. The annual incidence is 5 to 6.5 cases per 1,000 people at age 60, and 8 to 11 cases per 1,000 people at age 70.8
In 2006, the FDA approved the first zoster vaccine (Zostavax) for use in nonimmunocompromised, VZV-seropositive adults age >60 (later lowered to age 50). This vaccine reduces the incidence of shingles by 51%, the incidence of postherpetic neuralgia by 66%, and the burden of illness by 61%. In 2017, the FDA approved a second VZV vaccine (Shingrix, recombinant nonlive vaccine). In 2021, Shingrix was approved for use in immunosuppressed patients.9
Reactivation of VZV starts with a prodromal phase, characterized by pain, itching, numbness, and dysesthesias in 1 to 3 dermatomes. A maculopapular rash appears on the affected area a few days later, evolving into vesicles that scab over in 10 days.10
Dissemination of the virus leading specifically to VZV encephalitis typically occurs in immunosuppressed individuals and older patients. According to the World Health Organization, encephalitis is a life-threatening complication of VZV and occurs in 1 of 33,000 to 50,000 cases.11
Continue to: Delay in the diagnosis...
Delay in the diagnosis and treatment of VZV encephalitis can be detrimental or even fatal. Kodadhala et al12 found that the mortality rate for VZV encephalitis is 5% to 10% and ≤80% in immunosuppressed individuals.
Sometimes, VZV encephalitis can masquerade as a psychiatric presentation. Few cases presenting with acute or delayed neuropsychiatric symptoms related to VZV encephalitis have been previously reported in the literature. Some are summarized in Table 313,14 and Table 4.15,16

To our knowledge, this is the first case report of catatonia as a presentation of VZV encephalitis. The catatonic presentation has been previously described in autoimmune encephalitis such as N-methyl-

Bottom Line
In the setting of a patient with an abrupt change in mental status/behavior, physicians must be aware of the importance of a thorough physical examination to better ascertain a diagnosis and to rule out an underlying medical disorder. Reactivation of varicella-zoster virus (VZV) can result in encephalitis that might masquerade as a psychiatric presentation, including symptoms of catatonia.
Related Resources
- Baum ML, Johnson MC, Lizano P. Is it psychosis, or an autoimmune encephalitis? Current Psychiatry. 2022;21(8): 31-38,44. doi:10.12788/cp.0273
- Reinfold S. Are we failing to diagnose and treat the many faces of catatonia? Current Psychiatry. 2022;21(1):e3-e5. doi:10.12788/cp.0208
Drug Brand Names
Acyclovir • Sitavig
Diazepam • Valium
Haloperidol • Haldol
Lorazepam • Ativan
Levothyroxine • Levoxyl
Olanzapine • Zyprexa
Risperidone • Risperdal
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
2. Sanders RD, Keshavan MS. Physical and neurologic examinations in neuropsychiatry. Semin Clin Neuropsychiatry. 2002;7(1):18-29.
3. Howes DS, Lazoff M. Encephalitis workup. Medscape. Updated August 7, 2018. Accessed August 9, 2022. https://emedicine.medscape.com/article/791896-workup#c11
4. Kennedy PG, Rovnak J, Badani H, et al. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(Pt 7):1581-1602.
5. Fisle, CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0). Wikimedia Commons. https://upload.wikimedia.org/wikipedia/commons/1/19/Herpes_zoster_chest.png
6. John AR, Canaday DH. Herpes zoster in the older adult. Infect Dis Clin North Am. 2017;31(4):811-826.
7. Rosebush PI, Mazurek MF. Catatonia and its treatment. Schizophr Bull. 2010;36(2):239-242.
8. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016.
9. Raedler LA. Shingrix (zoster vaccine recombinant) a new vaccine approved for herpes zoster prevention in older adults. American Health & Drug Benefits, Ninth Annual Payers’ Guide. March 2018. Updated August 30, 2021. Accessed August 9, 2022. https://www.ahdbonline.com/issues/2018/april-2018-vol-11-ninth-annual-payers-guide/2567-shingrix-zoster-vaccine-recombinant-a-new-vaccine-approved-for-herpes-zoster-prevention-in-older-adults
10. Nair PA, Patel BC. Herpes zoster. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK441824/
11. Lizzi J, Hill T, Jakubowski J. Varicella zoster virus encephalitis. Clin Pract Cases Emerg Med. 2019;3(4):380-382.
12. Kodadhala V, Dessalegn M, Barned S, et al. 578: Varicella encephalitis: a rare complication of herpes zoster in an elderly patient. Crit Care Med. 2019;47(1):269.
13. Tremolizzo L, Tremolizzo S, Beghi M, et al. Mood disorder with psychotic symptoms and overlooked skin lesions: the strange case of Mrs. O. Riv Psichiatr. 2012;47(5):447-450.
14. George O, Daniel J, Forsyth S, et al. Mania presenting as a VZV encephalitis in the context of HIV. BMJ Case Rep. 2020;13(9):e230512.
15. Bangen KJ, Delano-Wood L, Wierenga CE, et al. Dementia following herpes zoster encephalitis. Clin Neuropsychol. 2010;24(7):1193-1203.
16. McKenna KF, Warneke LB. Encephalitis associated with herpes zoster: a case report and review. Can J Psychiatry. 1992;37(4):271-273.
17. Rogers JP, Pollak TA, Blackman G, et al. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6(7):620-630.
Good news, bad news for GI in 2023 CMS physician fee schedule
Medicare expanded coverage of colorectal cancer (CRC) testing through the 2023 physician payment rule while also finalizing certain mandated budget cuts.
The 2023 Medicare Physician Fee Schedule (MPFS) lowers the minimum age for CRC screening to 45 from 50 years, in keeping with the recommendation from the U.S. Preventive Services Task Force. The physician payment rule, which was unveiled on November 1, also ends the copay for colonoscopies that follow a positive stool-based colon cancer test. However, it is important to note that colonoscopies that involve polyp removal are still subject to Medicare coinsurance requirements, although the financial responsibility eventually diminishes to zero by 2030: From 2023 to 2026, patient responsibility is 15% of the cost; from 2027 to 2029 it falls to 10%; and by 2030 it will be covered 100% by Medicare.
These changes come after a year of intense advocacy led by AGA, including multiple meetings with senior officials at the Centers for Medicare and Medicaid Services and legislative pressure by members across the country. In the 2023 MPFS proposed rule, CMS attributed its decision to expand Medicare benefits to colonoscopy following a positive stool test to involvement from AGA, saying, “We consulted with and reviewed recommendations from a number of professional societies in developing this proposal, including supportive letters and communications with representatives from American Gastroenterological Association, American Cancer Society, and Fight Colorectal Cancer.”
“This is a win for all patients and should elevate our nation’s screening rates while lowering the overall cancer burden, saving lives. Importantly, the changes will lessen colorectal cancer disparities, eliminating a financial burden for many patients,” says AGA President John Carethers, MD, AGAF, who met with CMS earlier this year to advocate for the coverage of colonoscopy following a positive noninvasive colorectal cancer screening test.
David Lieberman, MD, AGAF, who met with CMS officials multiple times, offered, “Cost-sharing is a well-recognized barrier to screening and has resulted in disparities. Patients can now engage in a CRC screening program and be confident that they will not face unexpected cost-sharing for colonoscopy after a positive noninvasive screening test.”
‘Déjà vu all over again’
CMS uses its annual updates of the Physician Fee Schedule to make myriad policy decisions, with the 2023 version of the rule running close to 3,000 pages. AGA’s summary of the 2023 MPFS final rule highlights changes that impact gastroenterologists.
But the most controversial provisions in the rule involve federal mandates meant to control spending that CMS has no control over. These include a reduction in one of the variables used in determining payment, known as the conversion factor. This will fall by $1.55 from the current level of $34.61 to $33.06 in 2023.
There’s widespread agreement that Congress needs to reconsider its approach to setting Medicare payment for clinicians.
Between 2003 and April 2014, Congress passed 17 laws overriding the cuts to physician pay that were required under the old sustainable growth rate (SGR) formula.
The Medicare Access and CHIP Reauthorization Act of 2015 was supposed to end the annual battles over reimbursement cuts resulting from the SGR formula by changing the way physician payment is updated each year.
However, physicians face a 4.42% Medicare payment cut under the new payment system, as reflected in 2023 payment rule.
Two physicians serving in Congress, Rep. Ami Bera, MD (D-CA), and Rep. Larry Bucshon, MD (R-IN), have introduced legislation that would block next year’s cuts.
The current fight to stave off 2023 cuts seems like “déjà vu all over again,” said Kathleen Teixeira, AGA’s vice president of government affairs, in an interview with this news organization. Congress needs to shift away from the “Band-Aid approach” and concentrate on longer-term issues with physician payment, she said.
Rep. Bera and Rep. Buchson in September issued a letter seeking feedback on ways to “stabilize the Medicare payment system” without dramatically increasing the cost to taxpayers.
Louis Wilson, MD, chair of the American College of Gastroenterology’s legislative and public policy council, told this news organization that Congress needs to revisit Medicare’s physician payment system, especially in terms of addressing inflation.
Lawmakers’ attempts to restrain growth in Medicare physician payments have had the unintended consequence of fueling the acquisition of practices by hospitals, said Dr. Wilson, the managing partner of a physician-owned single-specialty private practice in Wichita Falls, Tex. Once doctors are employed by hospitals, Medicare often pays higher rates for their services than it would pay to physicians for providing the same care in a private practice.
Indeed, the Federal Trade Commission has said the U.S. physician workplace is “undergoing a dramatic restructuring,” with traditional solo practices and small single-specialty group practices rapidly being replaced by large multispecialty physician group practices, or practices that are owned or employed by hospital systems. The FTC is in the midst of a major series of studies on the effects of this consolidation.
“There’s been so much market distortion, so much limitation in innovation by failing to adequately pay in the Physician Fee Schedule, that the consequence is the widespread consolidation,” said Dr. Wilson. “That’s recognized on both sides of the aisle as being essentially expensive and inefficient and not in patients’ best interest.”
Medicare expanded coverage of colorectal cancer (CRC) testing through the 2023 physician payment rule while also finalizing certain mandated budget cuts.
The 2023 Medicare Physician Fee Schedule (MPFS) lowers the minimum age for CRC screening to 45 from 50 years, in keeping with the recommendation from the U.S. Preventive Services Task Force. The physician payment rule, which was unveiled on November 1, also ends the copay for colonoscopies that follow a positive stool-based colon cancer test. However, it is important to note that colonoscopies that involve polyp removal are still subject to Medicare coinsurance requirements, although the financial responsibility eventually diminishes to zero by 2030: From 2023 to 2026, patient responsibility is 15% of the cost; from 2027 to 2029 it falls to 10%; and by 2030 it will be covered 100% by Medicare.
These changes come after a year of intense advocacy led by AGA, including multiple meetings with senior officials at the Centers for Medicare and Medicaid Services and legislative pressure by members across the country. In the 2023 MPFS proposed rule, CMS attributed its decision to expand Medicare benefits to colonoscopy following a positive stool test to involvement from AGA, saying, “We consulted with and reviewed recommendations from a number of professional societies in developing this proposal, including supportive letters and communications with representatives from American Gastroenterological Association, American Cancer Society, and Fight Colorectal Cancer.”
“This is a win for all patients and should elevate our nation’s screening rates while lowering the overall cancer burden, saving lives. Importantly, the changes will lessen colorectal cancer disparities, eliminating a financial burden for many patients,” says AGA President John Carethers, MD, AGAF, who met with CMS earlier this year to advocate for the coverage of colonoscopy following a positive noninvasive colorectal cancer screening test.
David Lieberman, MD, AGAF, who met with CMS officials multiple times, offered, “Cost-sharing is a well-recognized barrier to screening and has resulted in disparities. Patients can now engage in a CRC screening program and be confident that they will not face unexpected cost-sharing for colonoscopy after a positive noninvasive screening test.”
‘Déjà vu all over again’
CMS uses its annual updates of the Physician Fee Schedule to make myriad policy decisions, with the 2023 version of the rule running close to 3,000 pages. AGA’s summary of the 2023 MPFS final rule highlights changes that impact gastroenterologists.
But the most controversial provisions in the rule involve federal mandates meant to control spending that CMS has no control over. These include a reduction in one of the variables used in determining payment, known as the conversion factor. This will fall by $1.55 from the current level of $34.61 to $33.06 in 2023.
There’s widespread agreement that Congress needs to reconsider its approach to setting Medicare payment for clinicians.
Between 2003 and April 2014, Congress passed 17 laws overriding the cuts to physician pay that were required under the old sustainable growth rate (SGR) formula.
The Medicare Access and CHIP Reauthorization Act of 2015 was supposed to end the annual battles over reimbursement cuts resulting from the SGR formula by changing the way physician payment is updated each year.
However, physicians face a 4.42% Medicare payment cut under the new payment system, as reflected in 2023 payment rule.
Two physicians serving in Congress, Rep. Ami Bera, MD (D-CA), and Rep. Larry Bucshon, MD (R-IN), have introduced legislation that would block next year’s cuts.
The current fight to stave off 2023 cuts seems like “déjà vu all over again,” said Kathleen Teixeira, AGA’s vice president of government affairs, in an interview with this news organization. Congress needs to shift away from the “Band-Aid approach” and concentrate on longer-term issues with physician payment, she said.
Rep. Bera and Rep. Buchson in September issued a letter seeking feedback on ways to “stabilize the Medicare payment system” without dramatically increasing the cost to taxpayers.
Louis Wilson, MD, chair of the American College of Gastroenterology’s legislative and public policy council, told this news organization that Congress needs to revisit Medicare’s physician payment system, especially in terms of addressing inflation.
Lawmakers’ attempts to restrain growth in Medicare physician payments have had the unintended consequence of fueling the acquisition of practices by hospitals, said Dr. Wilson, the managing partner of a physician-owned single-specialty private practice in Wichita Falls, Tex. Once doctors are employed by hospitals, Medicare often pays higher rates for their services than it would pay to physicians for providing the same care in a private practice.
Indeed, the Federal Trade Commission has said the U.S. physician workplace is “undergoing a dramatic restructuring,” with traditional solo practices and small single-specialty group practices rapidly being replaced by large multispecialty physician group practices, or practices that are owned or employed by hospital systems. The FTC is in the midst of a major series of studies on the effects of this consolidation.
“There’s been so much market distortion, so much limitation in innovation by failing to adequately pay in the Physician Fee Schedule, that the consequence is the widespread consolidation,” said Dr. Wilson. “That’s recognized on both sides of the aisle as being essentially expensive and inefficient and not in patients’ best interest.”
Medicare expanded coverage of colorectal cancer (CRC) testing through the 2023 physician payment rule while also finalizing certain mandated budget cuts.
The 2023 Medicare Physician Fee Schedule (MPFS) lowers the minimum age for CRC screening to 45 from 50 years, in keeping with the recommendation from the U.S. Preventive Services Task Force. The physician payment rule, which was unveiled on November 1, also ends the copay for colonoscopies that follow a positive stool-based colon cancer test. However, it is important to note that colonoscopies that involve polyp removal are still subject to Medicare coinsurance requirements, although the financial responsibility eventually diminishes to zero by 2030: From 2023 to 2026, patient responsibility is 15% of the cost; from 2027 to 2029 it falls to 10%; and by 2030 it will be covered 100% by Medicare.
These changes come after a year of intense advocacy led by AGA, including multiple meetings with senior officials at the Centers for Medicare and Medicaid Services and legislative pressure by members across the country. In the 2023 MPFS proposed rule, CMS attributed its decision to expand Medicare benefits to colonoscopy following a positive stool test to involvement from AGA, saying, “We consulted with and reviewed recommendations from a number of professional societies in developing this proposal, including supportive letters and communications with representatives from American Gastroenterological Association, American Cancer Society, and Fight Colorectal Cancer.”
“This is a win for all patients and should elevate our nation’s screening rates while lowering the overall cancer burden, saving lives. Importantly, the changes will lessen colorectal cancer disparities, eliminating a financial burden for many patients,” says AGA President John Carethers, MD, AGAF, who met with CMS earlier this year to advocate for the coverage of colonoscopy following a positive noninvasive colorectal cancer screening test.
David Lieberman, MD, AGAF, who met with CMS officials multiple times, offered, “Cost-sharing is a well-recognized barrier to screening and has resulted in disparities. Patients can now engage in a CRC screening program and be confident that they will not face unexpected cost-sharing for colonoscopy after a positive noninvasive screening test.”
‘Déjà vu all over again’
CMS uses its annual updates of the Physician Fee Schedule to make myriad policy decisions, with the 2023 version of the rule running close to 3,000 pages. AGA’s summary of the 2023 MPFS final rule highlights changes that impact gastroenterologists.
But the most controversial provisions in the rule involve federal mandates meant to control spending that CMS has no control over. These include a reduction in one of the variables used in determining payment, known as the conversion factor. This will fall by $1.55 from the current level of $34.61 to $33.06 in 2023.
There’s widespread agreement that Congress needs to reconsider its approach to setting Medicare payment for clinicians.
Between 2003 and April 2014, Congress passed 17 laws overriding the cuts to physician pay that were required under the old sustainable growth rate (SGR) formula.
The Medicare Access and CHIP Reauthorization Act of 2015 was supposed to end the annual battles over reimbursement cuts resulting from the SGR formula by changing the way physician payment is updated each year.
However, physicians face a 4.42% Medicare payment cut under the new payment system, as reflected in 2023 payment rule.
Two physicians serving in Congress, Rep. Ami Bera, MD (D-CA), and Rep. Larry Bucshon, MD (R-IN), have introduced legislation that would block next year’s cuts.
The current fight to stave off 2023 cuts seems like “déjà vu all over again,” said Kathleen Teixeira, AGA’s vice president of government affairs, in an interview with this news organization. Congress needs to shift away from the “Band-Aid approach” and concentrate on longer-term issues with physician payment, she said.
Rep. Bera and Rep. Buchson in September issued a letter seeking feedback on ways to “stabilize the Medicare payment system” without dramatically increasing the cost to taxpayers.
Louis Wilson, MD, chair of the American College of Gastroenterology’s legislative and public policy council, told this news organization that Congress needs to revisit Medicare’s physician payment system, especially in terms of addressing inflation.
Lawmakers’ attempts to restrain growth in Medicare physician payments have had the unintended consequence of fueling the acquisition of practices by hospitals, said Dr. Wilson, the managing partner of a physician-owned single-specialty private practice in Wichita Falls, Tex. Once doctors are employed by hospitals, Medicare often pays higher rates for their services than it would pay to physicians for providing the same care in a private practice.
Indeed, the Federal Trade Commission has said the U.S. physician workplace is “undergoing a dramatic restructuring,” with traditional solo practices and small single-specialty group practices rapidly being replaced by large multispecialty physician group practices, or practices that are owned or employed by hospital systems. The FTC is in the midst of a major series of studies on the effects of this consolidation.
“There’s been so much market distortion, so much limitation in innovation by failing to adequately pay in the Physician Fee Schedule, that the consequence is the widespread consolidation,” said Dr. Wilson. “That’s recognized on both sides of the aisle as being essentially expensive and inefficient and not in patients’ best interest.”
Don’t call me ‘Dr.,’ say some physicians – but most prefer the title
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN