User login
Germline testing in advanced cancer can lead to targeted treatment
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
The study involved 11,974 patients with various tumor types. All the patients underwent germline genetic testing from 2015 to 2019 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York, using the next-generation sequencing panel MSK-IMPACT.
This testing showed that 17.1% of patients had variants in cancer predisposition genes, and 7.1%-8.6% had variants that could potentially be targeted.
“Of course, these numbers are not static,” commented lead author Zsofia K. Stadler, MD, a medical oncologist at MSKCC. “And with the emergence of novel targeted treatments with new FDA indications, the therapeutic actionability of germline variants is likely to increase over time.
“Our study demonstrates the first comprehensive assessment of the clinical utility of germline alterations for therapeutic actionability in a population of patients with advanced cancer,” she added.
Dr. Stadler presented the study results during a virtual scientific program of the American Society of Clinical Oncology 2020.
Testing for somatic mutations is evolving as the standard of care in many cancer types, and somatic genomic testing is rapidly becoming an integral part of the regimen for patients with advanced disease. Some studies suggest that 9%-11% of patients harbor actionable genetic alterations, as determined on the basis of tumor profiling.
“The take-home message from this is that now, more than ever before, germline testing is indicated for the selection of cancer treatment,” said Erin Wysong Hofstatter, MD, from Yale University, New Haven, Conn., in a Highlights of the Day session.
An emerging indication for germline testing is the selection of treatment in the advanced setting, she noted. “And it is important to know your test. Remember that tumor sequencing is not a substitute for comprehensive germline testing.”
Implications in cancer treatment
For their study, Dr. Stadler and colleagues reviewed the medical records of patients with likely pathogenic/pathogenic germline (LP/P) alterations in genes that had known therapeutic targets so as to identify germline-targeted treatment either in a clinical or research setting.
“Since 2015, patients undergoing MSK-IMPACT may also choose to provide additional consent for secondary germline genetic analysis, wherein up to 88 genes known to be associated with cancer predisposition are analyzed,” she said. “Likely pathogenic and pathogenic germline alterations identified are disclosed to the patient and treating physician via the Clinical Genetic Service.”
A total of 2043 (17.1%) patients who harbored LP/P variants in a cancer predisposition gene were identified. Of these, 11% of patients harbored pathogenic alterations in high or moderate penetrance cancer predisposition genes. When the analysis was limited to genes with targeted therapeutic actionability, or what the authors defined as tier 1 and tier 2 genes, 7.1% of patients (n = 849) harbored a targetable pathogenic germline alteration.
BRCA alterations accounted for half (52%) of the findings, and 20% were associated with Lynch syndrome.
The tier 2 genes, which included PALB2, ATM, RAD51C, and RAD51D, accounted for about a quarter of the findings. Dr. Hofstatter noted that, using strict criteria, 7.1% of patients (n = 849) were found to harbor a pathogenic alteration and a targetable gene. Using less stringent criteria, additional tier 3 genes and additional genes associated with DNA homologous recombination repair brought the number up to 8.6% (n = 1,003).
Therapeutic action
For determining therapeutic actionability, the strict criteria were used; 593 patients (4.95%) with recurrent or metastatic disease were identified. For these patients, consideration of a targeted therapy, either as part of standard care or as part of an investigation or research protocol, was important.
Of this group, 44% received therapy targeting the germline alteration. Regarding specific genes, 50% of BRCA1/2 carriers and 58% of Lynch syndrome patients received targeted treatment. With respect to tier 2 genes, 40% of patients with PALB2, 19% with ATM, and 37% with RAD51C or 51D received a poly (ADP-ribose) polymerase (PARP) inhibitor.
Among patients with a BRCA1/2 mutation who received a PARP inhibitor, 55.1% had breast or ovarian cancer, and 44.8% had other tumor types, including pancreas, prostate, bile duct, gastric cancers. These patients received the drug in a research setting.
For patients with PALB2 alterations who received PARP inhibitors, 53.3% had breast or pancreas cancer, and 46.7% had cancer of the prostate, ovary, or an unknown primary.
Looking ahead
The discussant for the paper, Funda Meric-Bernstam, MD, chair of the Department of Investigational Cancer Therapeutics at the University of Texas MD Anderson Cancer Center, Houston, pointed out that most of the BRCA-positive patients had cancers traditionally associated with the mutation. “There were no patients with PTEN mutations treated, and interestingly, no patients with NF1 were treated,” she said. “But actionability is evolving, as the MEK inhibitor selumitinib was recently approved for NF1.”
Some questions remain unanswered, she noted, such as: “What percentage of patients undergoing tumor-normal testing signed a germline protocol?” and “Does the population introduce a bias – such as younger patients, family history, and so on?”
It is also unknown what percentage of germline alterations were known in comparison with those identified through tumor/normal testing. Also of importance is the fact that in this study, the results of germline testing were delivered in an academic setting, she emphasized. “What if they were delivered elsewhere? What would be the impact of identifying these alterations in an environment with less access to trials?
“But to be fair, it is not easy to seek the germline mutations,” Dr. Meric-Bernstam continued. “These studies were done under institutional review board protocols, and it is important to note that most profiling is done as standard of care without consenting and soliciting patient preference on the return of germline results.”
An infrastructure is needed to return/counsel/offer cascade testing, and “analyses need to be facilitated to ensure that findings can be acted upon in a timely fashion,” she added.
The study was supported by MSKCC internal funding. Dr. Stadler reported relationships (institutional) with Adverum, Alimera Sciences, Allergan, Biomarin, Fortress Biotech, Genentech/Roche, Novartis, Optos, Regeneron, Regenxbio, and Spark Therapeutics. Dr. Meric-Bernstram reported relationships with numerous pharmaceutical companies.
This article first appeared on Medscape.com.
FROM ASCO 2020
Active cancer increases death risk in patients with COVID-19
Patients with COVID-19 and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer, according to data from the COVID-19 and Cancer Consortium (CCC19) registry.
Other independent risk factors for death in patients with COVID-19 and cancer were older age, male sex, former smoking, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and treatment with hydroxychloroquine plus azithromycin.
In fact, patients who received hydroxychloroquine and azithromycin had a nearly threefold higher risk of death than did patients who had not received the combination. However, this finding was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Warner presented these findings in an online press briefing. Additional findings from the CCC19 registry are set to be presented as part of the American Society of Clinical Oncology (ASCO) virtual scientific program. The findings were also published in The Lancet.
‘Severe impact’ in cancer patients
“For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment,” commented ASCO President Howard A. Burris III, MD, who moderated the press briefing but was not involved in the study of CCC19 registry data.
“ASCO members tell us that they have had to delay or modify treatment plans to reduce patients’ risk of infection, and we’re unclear what the impact of these changes will be. Delays in cancer screening and diagnosis are also a major concern,” Dr. Burris continued.
“This does confirm reports that have come out from other centers, including other parts of the world, where they have found that people who have cancer and COVID-19 have a worse outcome,” said Andrew T. Chan, MD, MPH, of Massachusetts General Hospital in Boston, who was not involved in the research.
Dr. Chan’s group has developed a COVID-19 symptom study app with the aim of defining whether people living with cancer are at increased risk for infections, in addition to whether cancer is an independent risk factor for COVID-19 severity or mortality.
“Using data from our app, we were able to show that people who reported living with cancer did have a higher risk of developing COVID and were more likely to be hospitalized related to COVID,” Dr. Chan said in an interview.
Study details
The CCC19 registry collects information from 104 participating institutions in the United States and Canada, as well as anonymous data from individuals in the United States, Argentina, Canada, the European Union, and the United Kingdom.
The sample of 928 patients Dr. Warner presented was evenly balanced by sex. The median age was 66 years, and 30% of patients were aged 75 years or older.
In all, 39% of patients were on active anticancer therapy, and 43% had measurable disease. Breast cancer was the most common diagnosis, followed by prostate cancer, gastrointestinal cancers, lymphomas, and thoracic cancers.
Two-thirds of the patients (68%) had an ECOG performance status of 0 or 1, 8% had a performance status of 2, and 5% a status of 3 or 4. The remaining patients had unknown performance status.
Slightly more than half of patients (52%) were never smokers, 37% were former smokers, and 5% were current smokers. The remaining 6% of patients had unknown smoking status.
At a median follow-up of 21 days, 121 patients (13%) had died. All deaths occurred within 30 days of COVID-19 diagnosis. Among patients who died, 78 were male, 64 were former smokers, 70 were aged 75 years or older, 41 had active stable or responding cancer, 25 had progressing cancer, and 42 had an ECOG performance status of 2 or higher.
In all, 466 patients were hospitalized, and 106 in this group (23%) died. Among the 132 patients admitted to an ICU, 50 (38%) died, including 27 patients aged 75 years or older, and 15 with an ECOG performance status of 2 or greater. Of the 116 patients who required intubation, 50 (43%) died, including 26 who were 75 years or older, and 11 who had a performance status of 2 or greater.
It’s early days yet, and a larger sample size with longer follow-up will be needed to get a more complete picture of how COVID-19 affects specific patient subsets over time, Dr. Warner said.
ASCO has established its own COVID-19 registry to collect both near-term and longitudinal data during the pandemic.
“We’ll be able to learn about both how the pandemic has impacted delivery of cancer care, as well as the longer-term effects of COVID-19 on cancer patients and understand what care approaches are working best,” said Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, during the briefing.
The study of CCC19 registry data was supported in part by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed stock/ownership in HemOnc.org, consulting for IBM and Westat, and travel expenses from IBM. Dr. Burris, Dr. Schilsky, and Dr. Chan reported no disclosures relevant to the study.
SOURCE: Warner J L et al. ASCO 2020, Abstract LBA110.
Patients with COVID-19 and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer, according to data from the COVID-19 and Cancer Consortium (CCC19) registry.
Other independent risk factors for death in patients with COVID-19 and cancer were older age, male sex, former smoking, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and treatment with hydroxychloroquine plus azithromycin.
In fact, patients who received hydroxychloroquine and azithromycin had a nearly threefold higher risk of death than did patients who had not received the combination. However, this finding was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Warner presented these findings in an online press briefing. Additional findings from the CCC19 registry are set to be presented as part of the American Society of Clinical Oncology (ASCO) virtual scientific program. The findings were also published in The Lancet.
‘Severe impact’ in cancer patients
“For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment,” commented ASCO President Howard A. Burris III, MD, who moderated the press briefing but was not involved in the study of CCC19 registry data.
“ASCO members tell us that they have had to delay or modify treatment plans to reduce patients’ risk of infection, and we’re unclear what the impact of these changes will be. Delays in cancer screening and diagnosis are also a major concern,” Dr. Burris continued.
“This does confirm reports that have come out from other centers, including other parts of the world, where they have found that people who have cancer and COVID-19 have a worse outcome,” said Andrew T. Chan, MD, MPH, of Massachusetts General Hospital in Boston, who was not involved in the research.
Dr. Chan’s group has developed a COVID-19 symptom study app with the aim of defining whether people living with cancer are at increased risk for infections, in addition to whether cancer is an independent risk factor for COVID-19 severity or mortality.
“Using data from our app, we were able to show that people who reported living with cancer did have a higher risk of developing COVID and were more likely to be hospitalized related to COVID,” Dr. Chan said in an interview.
Study details
The CCC19 registry collects information from 104 participating institutions in the United States and Canada, as well as anonymous data from individuals in the United States, Argentina, Canada, the European Union, and the United Kingdom.
The sample of 928 patients Dr. Warner presented was evenly balanced by sex. The median age was 66 years, and 30% of patients were aged 75 years or older.
In all, 39% of patients were on active anticancer therapy, and 43% had measurable disease. Breast cancer was the most common diagnosis, followed by prostate cancer, gastrointestinal cancers, lymphomas, and thoracic cancers.
Two-thirds of the patients (68%) had an ECOG performance status of 0 or 1, 8% had a performance status of 2, and 5% a status of 3 or 4. The remaining patients had unknown performance status.
Slightly more than half of patients (52%) were never smokers, 37% were former smokers, and 5% were current smokers. The remaining 6% of patients had unknown smoking status.
At a median follow-up of 21 days, 121 patients (13%) had died. All deaths occurred within 30 days of COVID-19 diagnosis. Among patients who died, 78 were male, 64 were former smokers, 70 were aged 75 years or older, 41 had active stable or responding cancer, 25 had progressing cancer, and 42 had an ECOG performance status of 2 or higher.
In all, 466 patients were hospitalized, and 106 in this group (23%) died. Among the 132 patients admitted to an ICU, 50 (38%) died, including 27 patients aged 75 years or older, and 15 with an ECOG performance status of 2 or greater. Of the 116 patients who required intubation, 50 (43%) died, including 26 who were 75 years or older, and 11 who had a performance status of 2 or greater.
It’s early days yet, and a larger sample size with longer follow-up will be needed to get a more complete picture of how COVID-19 affects specific patient subsets over time, Dr. Warner said.
ASCO has established its own COVID-19 registry to collect both near-term and longitudinal data during the pandemic.
“We’ll be able to learn about both how the pandemic has impacted delivery of cancer care, as well as the longer-term effects of COVID-19 on cancer patients and understand what care approaches are working best,” said Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, during the briefing.
The study of CCC19 registry data was supported in part by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed stock/ownership in HemOnc.org, consulting for IBM and Westat, and travel expenses from IBM. Dr. Burris, Dr. Schilsky, and Dr. Chan reported no disclosures relevant to the study.
SOURCE: Warner J L et al. ASCO 2020, Abstract LBA110.
Patients with COVID-19 and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer, according to data from the COVID-19 and Cancer Consortium (CCC19) registry.
Other independent risk factors for death in patients with COVID-19 and cancer were older age, male sex, former smoking, number of comorbidities, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and treatment with hydroxychloroquine plus azithromycin.
In fact, patients who received hydroxychloroquine and azithromycin had a nearly threefold higher risk of death than did patients who had not received the combination. However, this finding was of “uncertain validity due to a high risk of residual confounding; for example, patients receiving this combination were more likely to have severe disease or more likely to be hospitalized,” said Jeremy L. Warner, MD, of Vanderbilt University Medical Center in Nashville, Tennessee.
Dr. Warner presented these findings in an online press briefing. Additional findings from the CCC19 registry are set to be presented as part of the American Society of Clinical Oncology (ASCO) virtual scientific program. The findings were also published in The Lancet.
‘Severe impact’ in cancer patients
“For people with cancer, the impact of COVID-19 is especially severe, whether they have been exposed to the virus or not. Patients with cancer are typically older adults, often with other underlying conditions, and their immune systems may be suppressed by the cancer, or due to chemotherapy, radiation, or other treatment,” commented ASCO President Howard A. Burris III, MD, who moderated the press briefing but was not involved in the study of CCC19 registry data.
“ASCO members tell us that they have had to delay or modify treatment plans to reduce patients’ risk of infection, and we’re unclear what the impact of these changes will be. Delays in cancer screening and diagnosis are also a major concern,” Dr. Burris continued.
“This does confirm reports that have come out from other centers, including other parts of the world, where they have found that people who have cancer and COVID-19 have a worse outcome,” said Andrew T. Chan, MD, MPH, of Massachusetts General Hospital in Boston, who was not involved in the research.
Dr. Chan’s group has developed a COVID-19 symptom study app with the aim of defining whether people living with cancer are at increased risk for infections, in addition to whether cancer is an independent risk factor for COVID-19 severity or mortality.
“Using data from our app, we were able to show that people who reported living with cancer did have a higher risk of developing COVID and were more likely to be hospitalized related to COVID,” Dr. Chan said in an interview.
Study details
The CCC19 registry collects information from 104 participating institutions in the United States and Canada, as well as anonymous data from individuals in the United States, Argentina, Canada, the European Union, and the United Kingdom.
The sample of 928 patients Dr. Warner presented was evenly balanced by sex. The median age was 66 years, and 30% of patients were aged 75 years or older.
In all, 39% of patients were on active anticancer therapy, and 43% had measurable disease. Breast cancer was the most common diagnosis, followed by prostate cancer, gastrointestinal cancers, lymphomas, and thoracic cancers.
Two-thirds of the patients (68%) had an ECOG performance status of 0 or 1, 8% had a performance status of 2, and 5% a status of 3 or 4. The remaining patients had unknown performance status.
Slightly more than half of patients (52%) were never smokers, 37% were former smokers, and 5% were current smokers. The remaining 6% of patients had unknown smoking status.
At a median follow-up of 21 days, 121 patients (13%) had died. All deaths occurred within 30 days of COVID-19 diagnosis. Among patients who died, 78 were male, 64 were former smokers, 70 were aged 75 years or older, 41 had active stable or responding cancer, 25 had progressing cancer, and 42 had an ECOG performance status of 2 or higher.
In all, 466 patients were hospitalized, and 106 in this group (23%) died. Among the 132 patients admitted to an ICU, 50 (38%) died, including 27 patients aged 75 years or older, and 15 with an ECOG performance status of 2 or greater. Of the 116 patients who required intubation, 50 (43%) died, including 26 who were 75 years or older, and 11 who had a performance status of 2 or greater.
It’s early days yet, and a larger sample size with longer follow-up will be needed to get a more complete picture of how COVID-19 affects specific patient subsets over time, Dr. Warner said.
ASCO has established its own COVID-19 registry to collect both near-term and longitudinal data during the pandemic.
“We’ll be able to learn about both how the pandemic has impacted delivery of cancer care, as well as the longer-term effects of COVID-19 on cancer patients and understand what care approaches are working best,” said Richard L. Schilsky, MD, chief medical officer and executive vice president of ASCO, during the briefing.
The study of CCC19 registry data was supported in part by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed stock/ownership in HemOnc.org, consulting for IBM and Westat, and travel expenses from IBM. Dr. Burris, Dr. Schilsky, and Dr. Chan reported no disclosures relevant to the study.
SOURCE: Warner J L et al. ASCO 2020, Abstract LBA110.
FROM ASCO 2020
Key clinical point: Patients with progressing cancer and COVID-19 are at an especially high risk of 30-day mortality.
Major finding: Patients with COVID-19 whose cancers were progressing had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients in remission or with no evidence of cancer.
Study details: Analysis of data on 928 patients enrolled in the COVID-19 and Cancer Consortium (CCC19) registry.
Disclosures: The research was supported, in part, by the National Institutes of Health and the American Cancer Society. Dr. Warner disclosed relationships with HemOnc.org, IBM, and Westat.
Source: Warner J L et al. ASCO 2020, Abstract LBA110.
Tumor molecular profiling may help identify ‘exceptional responders’
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Virtually all oncologists, at one time or another, have treated a patient who defied the odds and achieved an unexpectedly long-lasting response. These “exceptional responders” are patients who experience a unique response to therapies that have largely failed to be effective for others with similar cancers.
Genetic and molecular mechanisms may partly account for these responses and may offer clues about why the treatment works for only a few and not for others. To delve more deeply into that area of research, the National Cancer Institute (NCI) began the Exceptional Responders Initiative (ERI) with the goal of identifying potential biological processes that may be responsible, at least in part, for these unusual responses.
NCI researchers have now successfully completed a pilot study that analyzed tumor specimens from more than 100 cases, and the study has affirmed the feasibility of this approach.
Of these cases, six were identified as involving potentially clinically actionable germline mutations.
The findings were published online ahead of print in the Journal of the National Cancer Institute.
“Clearly, the analysis and validation of these results will prove critical to determining the success of this approach,” write James M. Ford, MD, and Beverly S. Mitchell, MD, both of Stanford University School of Medicine, California, in an accompanying editorial. “Ultimately, prospective studies of tumors from exceptional responders, particularly to novel, genomically-targeted agents, may provide a powerful approach to cancer treatment discoveries.”
A special case
Molecular profiling technology, including next-generation sequencing, has significantly changed the landscape of the development of cancer therapies, and clinical trials in early drug development are increasingly selecting patients on the basis of molecular alterations.
The ERI grew out of several meetings held by the NCI in 2013 and 2014. It was built on the ability to profile archived tumor material, explained study author S. Percy Ivy, PhD, associate chief of the Investigational Drug Branch in the Division of Cancer Treatment and Diagnosis of the NCI. “This made it possible to collect cases from participating clinicians from all over the country.
“Published cases included patients treated with a targeted therapy but not treated with knowledge of their tumor’s genomics, who then later turned out to have genomic changes that made their tumor exquisitely sensitive to inhibition of a driving pathway,” she said. “There have been published cases as well as cases in the experience of practicing oncologists that seem to do much better than expected.
“We wondered if we could find molecular reasons why tumors respond not only to targeted therapies but also to standard chemotherapy,” said Percy. “If so, we could refine our choice of therapy to patients who are most likely to respond to it.”’
On its website, the NCI writes that there was a particular case that triggered the interest in going ahead with this initiative. Mutations in the TSC1 and NF2 genes, which result in a loss of gene function, were detected in a patient with metastatic bladder cancer. In a clinical trial, the patient was treated with everolimus (Afinitor, Novartis), an inhibitor of the mammalian target of rapamycin (mTORC1), and achieved a complete response with a duration of more than 2 years.
In a separate analysis, researchers sequenced tumors from 96 other individuals with high-grade bladder cancer and identified five TSC1 gene mutations. Tumors were sequenced from 13 patients with bladder cancer who had received everolimus. Results showed that 3 of 4 patients with TSC1 gene mutations experienced some degree of tumor shrinkage after treatment; 8 of 9 patients who did not have the mutation experienced disease progression.
The NCI notes that in “subsequent workshops and discussions, it became obvious that all clinicians have seen a few exceptional responders.”
Testing for feasibility
The aim of the current study was to assess the feasibility and potential usefulness of sequencing DNA and RNA from clinical tumor specimens from patients who had experienced unusually profound or durable responses to systemic therapy.
Its main feasibility goal was to identify at least 100 cases involving exceptional responders whose cases could be analyzed in less than 3 years.
An exceptional patient was defined as one who had experienced a complete response to one or more drugs in which complete responses were seen in fewer than 10% of patients who received similar treatment; or a partial response lasting at least 6 months in which such a response is seen in fewer than 10% of patients who receive similar treatment; or a complete or partial response of a duration that is three times the median response duration represented in the literature for the treatment.
Studying exceptional responders presents many challenges, the first being to define what an exceptional response is and what it is not, explained Ivy. “This definition relies on the existence of data that a particular therapy will produce particular responses in groups of patients with similar tumors, as defined by organ of origin,” she said.
Other challenges include obtaining tumor tissue and all the relevant clinical data, such as the number of prior treatments and the patient’s response, as well as any known molecular characteristics (eg, HER2/NEU amplification, estrogen-receptor expression, germline mutations). “We also do not have data on other exposures, such as smoking or chemical exposure,” she said. “In addition, when patients are not on clinical trial, the data are not uniformly obtained ― such as that scans may not be performed at particular intervals.”
Importantly, the molecular tools used to analyze tumors were not available in the past, so many trials did not collect tumor tissue for subsequent research. “Even now, we are learning that there are characteristics beyond DNA and RNA that are potentially important to the ability of a tumor to respond, such as the immune system or epigenetic changes,” she said.
From August 2014 to July 2017, a total of 520 cases were proposed by clinicians as possibly involving exceptional responders, and 222 cases met the criteria.
Analyzable tissue was available for 117 patients. Most of the responders (n = 80, 68.4%) had been treated with combination chemotherapy regimens; 34 patients (29.0%) had received one or more antiangiogenesis agents. In addition, six patients had an exceptional response following treatment with immune checkpoint inhibitors. The final analysis included 109 cases.
One exceptional responder was a woman with metastatic squamous lung cancer that was treated with paclitaxel and carboplatin. The patient achieved a 41-month complete response (expected rate, <10%). Another patient with esophageal adenocarcinoma who was treated with docetaxel and cisplatin experienced a partial response that lasted 128 months (reported median response duration, 24 months). After the patient’s tumor recurred, he experienced for the second time a response to concurrent chemoradiation with the same drug regimen.
Overall, potentially clinically relevant germline mutations were identified in six tumors. Pathogenic BRCA1 or BRCA2 mutations were found in two breast cancer patients, one patient with non–small cell lung cancer, and one patient with rectal cancer. A breast cancer patient had a pathogenic BRCA1 germline mutation, and another had a likely germline mutation in CHEK2. A patient with poorly differentiated lung cancer and a history of breast cancer had a PALB2 mutation.
Future steps
Molecular mechanisms are important, but other factors could also play a role in eliciting a response. One is the presence of comorbidities, which was not assessed in the study. Ivy noted that comorbidities could be very important to responses, along with medications that the patient is using for different types of ailments. In addition, the use of complementary and alternative medicines may also have an impact.
“As the field matures, we hope that others will collect these and other characteristics, so that all the data could be used to develop hypotheses about molecular and other factors that can better predict response or resistance,” she said.
The results from this pilot study demonstrated feasibility. Ivy noted that “additional collaboration in similar studies would be welcome, as would methods to use data from various sources to improve our ability to correlate patient characteristics, tumor characteristics and response.
“We envision a larger national and international effort to collect more exceptional responder cases, including more from patients treated with targeted therapies,” she added. “The NCI has been meeting with an interest group that focuses on ER cases in the UK, France, Italy, Canada, and Australia, and this collaborative effort is maturing, albeit slowly.”
The project has been funded in whole or in part with federal funds from the NCI and NIH. Ivy has disclosed no relevant financial relationships. Several coauthors report relationships with industry. The editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Video coaching may relieve anxiety and distress for long-distance cancer caregivers
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
FROM ASCO 2020
Mammography cuts risk for fatal breast cancers: New data
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Three experts who were approached by Medscape Medical News say this is further evidence that regular screening mammography significantly reduces the risk of dying from breast cancer, but one expert questioned the methodology used in the study.
The primary goal of cancer screening is to detect tumors at an early stage, when they are most treatable. The hope is that this will reduce the number of advanced cancers associated with poor prognosis and hence the risk of dying from that cancer.
So far, for mammography, the data have been somewhat conflicting. For example, some evidence suggests that widespread breast cancer screening may catch more small, slow-growing tumors that are unlikely to be fatal but will not curb the number of cancers that are diagnosed at a late stage.
The new study, published online in Cancer, refutes this view.
It followed a Swedish cohort of 549,091 women (covering approximately 30% of the Swedish screening-eligible population) who underwent regular mammography.
For the women in this cohort, there was a statistically significant 41% reduction in the risk of dying of breast cancer within 10 years and a 25% reduction in the incidence of advanced disease, compared to women who did not undergo screening. “Even in this age of effective treatments, early detection confers a substantial and significant additional reduction in risk of dying from breast cancer,” said lead author Stephen W. Duffy, MSc, from the Center for Cancer Prevention at Queen Mary University, London, United Kingdom.
The current study confirms the findings of a smaller earlier study (Cancer. 2019;125:515-23) from the same investigators. “It finds the same result with an extremely large evidence base, with more than half a million women, and it also adds further to the evidence that screening achieves this reduction in the context of routine healthcare, not only in the research context,” Duffy commented. “The results are generalizable to other populations, particularly in North America, Western Europe, and Australasia, where the epidemiology and demographics of breast cancer are similar,” said Duffy. “Clearly, more intensive screening is likely to achieve a greater benefit, but a trade-off between costs, both financial and human, and benefits always has to be made specific to each societal and healthcare environment.”
In Sweden, the policy regarding breast cancer screening is to screen women aged 40 to 54 years every 18 months. For those aged 55 to 69 years, screening is recommended every 24 months.
“The use of the incidence-based endpoints means that there is accurate classification of both the breast cancer cases and the whole study population in terms of exposure to screening and avoids a number of biases seen in other studies of service screening,” Duffy told Medscape Medical News.
“I have never seen persuasive evidence for the assertion that breast cancer screening does not reduce deaths from metastatic disease – indeed, the randomized trials seem to show the opposite,” said Duffy. “This may have arisen from a misunderstanding about the mechanism whereby screening works. It primarily works by diagnosing cancer early so that treatment is successful and recurrence with distant metastases, followed by death, does not occur some years later. I suspect some colleagues have confused this with distant metastases at initial diagnosis,” he added.
One expert questions methodology
One of the experts who was approached by Medscape Medical News to comment on the new study, Philippe Autier, MD, MPH, PhD, University of Strathclyde Institute of Global Public Health at the International Prevention Research Institute, Dardilly, France, questioned the methodology of the study. “This method is incorrect simply because women attending screening are different from women not attending screening,” he said. “The former are more health aware and have healthier behaviors than the latter, and this is a well-known fact and supported by the literature.”
Autier emphasized that it is practically impossible to control for that bias, which is known as confounding by indication.
“The statistical methods used for attenuating the so-called self-selection are very approximate and based on unverified assumptions,” he said. “For this reason, the Handbook on Breast Cancer Screening produced by the International Agency for Research on Cancer [IARC] clearly stated that ‘observational studies based on individual screening history, no matter how well designed and conducted, should not be regarded as providing evidence for an effect of screening,’ and the methodology in this paper has never been recommended by the IARC.”
A better way of conducting this type of study would have been to show the incidence trends of advanced-stage breast cancer in Sweden for the entire female population aged 40 years and older, he asserts. Autier used that methodology in his own study in the Netherlands, as previously reported by Medscape Medical News. That study found that in the Netherlands, screening mammography over a period of 24 years among women aged 50 to 74 years had little effect on reducing rates of advanced breast cancer or mortality from the disease.
Experts applaud the new findings
Three of the experts who were approached by Medscape Medical News to comment on the new findings applauded the efforts of Duffy and colleagues in providing evidence that mammography can reduce breast cancer–related mortality.
Marie Quinn, MD, director of diagnostic radiology at Roswell Park Comprehensive Cancer Center, Buffalo, New York, said this study adds to the growing body of scientific evidence that confirms that women who undergo regular screening mammography significantly reduce their risk of dying from breast cancer.
“Women who underwent regular screening also had a 25% reduction in the incidence of advanced-stage breast cancer,” she said. “This is important, because breast cancers are less fatal and often require less treatment when picked up at an earlier stage. We know the risk reduction benefit detected in this well-designed study can be attributed to screening mammography and not advances in cancer treatment, due to the long-term follow-up and outcome of cancer death within 10 years.”
The findings from this study support the guidelines recommending routine screening mammography in the United States, Quinn continued, but she pointed out that some aspects of screening (e.g., the age at which to begin screening and how often to screen) can vary. “This can be confusing for patients and providers,” she said. “Overall, research has shown us that women who undergo regular screening mammograms reduce their risk of dying from breast cancer. For women of average risk, the benefit of mammography is maximized with annual screening beginning at age 40,” she said.
Jay A. Baker, MD, FACR, FSBI, chief of the Division of Breast Imaging at Duke University Medical Center, Durham, North Carolina, emphasized that this is yet another study that confirms that the improvement in breast cancer mortality is not the result of improved treatments alone, as some have speculated. “Others have tried to model the benefit of screening vs treatment, but this study is a more direct measurement,” he said. “This conclusion is important for both patients and physicians to hear.”
Although the study strongly supports regular screening for all women, it does not specifically address which set of screening guidelines is optimal, Baker commented. “Fortunately, even though some organizations in the US curiously suggest a delayed start to screening, all organizations and professional societies agree that the most lives and the most years of life are saved by yearly screening beginning at age 40,” he added. “This new study tells us that new treatments alone aren’t enough and confirms that screening saves at least one-third more lives.”
Another expert, Bonnie N. Joe, MD, PhD, professor in residence and chief of breast imaging in the Department of Radiology and Biomedical Imaging at the University of California, San Francisco, agreed that the study shows the mortality benefits of regular screening mammography. “Notably, these benefits were related to participation in mammography screening and independent of any advances in treatment,” she said, “And these findings in this study support regular screening mammography to reduce advanced-stage breast cancers and to reduce a woman’s risk of dying from breast cancer.”
Joe noted that overall, this was a “well-done, large-scale screening study with long-term outcomes and should be applicable to other populations. In the US, we know that peak cancer incidence is in the 40s for minority women, and the results of this study support regular screening starting at 40.”
The study was supported by the American Cancer Society through a gift from the Longaberger Company’s Horizon of Hope Campaign. Additional financial support was provided by Brostcancerförbundet, Sweden. Duffy, Autier, Quinn, Joe, and Baker have disclosed no relevant financial relationships. One coauthor of the study has disclosed relationships with industry, as noted in the original article.
This article first appeared on Medscape.com.
Androgens may explain male vulnerability to COVID-19
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
As the COVID-19 pandemic has swept across the world, a striking difference has been seen between the sexes. But why are men so much more susceptible to severe outcomes from COVID-19 than women?
Suspicions naturally turn to the sex hormones, and there have been suggestions that estrogen may be protective against COVID-19 in females and/or that androgens worsen COVID-19 outcomes in males.
New data supporting the androgen theory come from a study in Italy.
These researchers found that patients with prostate cancer being treated with androgen deprivation therapy (ADT) were less likely to become infected with COVID-19 and die from the disease than other groups, including other patients with cancer.
The findings suggest that androgens somehow make the virus more virulent and that this exacerbates the severity of disease in men, they say. They also speculate that ADT may be protective against COVID-19.
The study was published online May 7 in Annals of Oncology.
The team analyzed data from 68 hospitals in the Veneto region, one of the areas in Italy most severely affected by the COVID-19 pandemic.
They found data on 9280 patients with laboratory-confirmed SARS-CoV-2 infection — of whom 4532 were males.
Women in the region were actually slightly more likely to be infected with COVID-19 than men, 56% vs 44%, the researchers point out.
However, men were more prone to develop more severe forms of the disease: 60% of men vs 40% of women required hospitalization, rising to 78% of men vs 22% of women who required intensive care. Also, more men died than women (62% vs 38%).
The team then turned their focus onto patients with cancer.
Of the entire male population of Veneto, those with cancer had an almost twofold higher risk of becoming infected with COVID-19 than men without cancer (P < .0001).
However, when the team looked specifically at men with prostate cancer in the region, they found “strikingly, only 4 out of 5273 patients receiving ADT developed SARS-CoV-2 infection and none of these patients died.”
This compared to 37,161 men with prostate cancer who were not receiving ADT, among whom 114 men developed COVID-19 and 18 died.
Among another 79,661 patients in the Veneto region with cancer other than prostate cancer, 312 developed COVID-19 and 57 died.
“This is the first paper to suggest a link between ADT and COVID-19,” commented lead author Andrea Alimonti, MD, PhD, Università della Svizzera Italiana in Lugano, Switzerland.
“Patients with prostate cancer receiving ADT had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT. An even greater difference (fivefold reduction in risk) was found when we compared prostate cancer patients receiving ADT to patients with any other type of cancer,” he said.
The finding raises “the hypothesis that androgen levels can facilitate coronavirus infections and increase the severity of symptoms, as has been seen in male patients,” he said.
“These data are very interesting and raise a fascinating hypothesis,” said Richard Martin, PhD, professor of clinical epidemiology at the University of Bristol, UK, commenting about the study. “But they do need independent validation in other large population-wide datasets...with appropriate statistical analysis including adjustment for important risk factors for SARS-CoV-2.”
He noted that the Italian study results were not adjusted for potential confounders, for example, age, body mass index, and cardiometabolic comorbidities, that are strong risk factors for SARS-CoV-2. In addition, men taking ADT may have been more likely to self-isolate and so be at reduced risk of getting the infection, he suggested.
How Do Androgens Interact With the Virus?
Alimonti and colleagues offer a mechanistic explanation of how androgens interact with the virus.
Coronavirus gains entry into the human cell by binding its viral spike (S) proteins to ACE2 and on S protein priming by TMPRSS2. TMPRSS2 is a member of a family of proteins called type II transmembrane serine proteases, which are involved in a number of processes including cancer and viral infections, they explain.
“Intriguingly, TMPRSS2 is an androgen-regulated gene that is upregulated in prostate cancer where it supports tumor progression,” they point out.
There is also evidence that the same androgen receptor regulates TMPRSS2 expression in nonprostatic tissues, including the lungs.
“[This] may explain the increased susceptibility of men to develop SARS-CoV-2 severe infections when compared to women,” the authors speculate.
Because ADT is known to decrease TMPRSS2 levels, they suggest that androgen receptor antagonists “could be used to block or decrease the severity of SARS-CoV-2 infection in male patients.”
They go even further and suggest that men without prostate cancer at high risk for COVID-19 could take ADT to prevent infection.
For men who do become infected with COVID-19, ADT might also help reduce symptom severity, they add.
Given that the effects of androgen receptor antagonists are reversible, “they could be used transiently (eg, 1 month) in patients affected by SARS-CoV-2, thereby reducing the risk of side effects due to long-term administration,” the authors suggest.
Another Theory: Is Estrogen Protective?
Another theory to explain the male/female difference for severe COVID-19 is that the female hormone estrogen may be protective.
“People have to stop putting estrogen in that ‘female hormone box’ because it’s a molecule that we all use as humans, it’s just not women,” Sharon Nachman, MD, told Medscape Medical News.
“Looking at estrogen as having potentially important immune effects is part of thinking outside the box,” she said.
Nachman is associate dean for research at the Renaissance School of Medicine, Stony Brook University in New York, and is working together with Antonios Gasparis, MD, professor of surgery at the same center.
They are exploring the use of a transdermal estrogen patch in patients with COVID-19 in a randomized trial with a placebo-controlled arm. They are recruiting patients who present to their emergency department with signs and symptoms of COVID-19, and enroll them into the trial if they are interested.
“We are testing everyone as well, but we are starting patients on the medication at the time of entry as opposed to waiting until we have a test result back,” Nachman explained.
The primary objective of the study is to evaluate whether the transdermal patch, applied to the skin for 7 days, might reduce the need for intubation in men and women infected with COVID-19 versus standard of care.
The product is the same single-use transdermal estradiol patch (Climara, 25 cm2, Bayer) prescribed for postmenopausal women and will be used at the same dose, which is known to be safe.
After the patch is removed, patients will be carefully tracked for symptoms over the next 45 days to see if the patch reduced symptom severity, and if so, in which patients.
Nachman would have preferred to enroll patients before they had overt symptoms, but this simply isn’t possible in a medical center where symptomatic patients present, she told Medscape Medical News.
However, she does know that even at their own medical center, the odds are stacked against male COVID-19 patients — and something is needed to mitigate its severity in this patient group.
As they were developing the protocol for the current study, the team decided to see who was in their ICU during a single study day.
The answer: mostly males. Intubation and death rates in men in their ICU for that single day was approximately 80% compared with only 20% among women.
“We have a new horrific pathogen that is pandemic and we’re all probably going to get it, it’s just a question of when and how sick we’ll be from it,” Nachman said.
Alimonti and coauthors have reported no relevant financial relationships, as did Goulder and Nachman.
This article first appeared on Medscape.com.
COVID-19 death rate was twice as high in cancer patients in NYC study
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
COVID-19 patients with cancer had double the fatality rate of COVID-19 patients without cancer treated in an urban New York hospital system, according to data from a retrospective study.
with COVID-19 treated during the same time period in the same hospital system.
Vikas Mehta, MD, of Montefiore Medical Center, New York, and colleagues reported these results in Cancer Discovery.
“As New York has emerged as the current epicenter of the pandemic, we sought to investigate the risk posed by COVID-19 to our cancer population,” the authors wrote.
They identified 218 cancer patients treated for COVID-19 in the Montefiore Health System between March 18 and April 8, 2020. Three-quarters of patients had solid tumors, and 25% had hematologic malignancies. Most patients were adults (98.6%), their median age was 69 years (range, 10-92 years), and 58% were men.
In all, 28% of the cancer patients (61/218) died from COVID-19, including 25% (41/164) of those with solid tumors and 37% (20/54) of those with hematologic malignancies.
Deaths by cancer type
Among the 164 patients with solid tumors, case fatality rates were as follows:
- Pancreatic – 67% (2/3)
- Lung – 55% (6/11)
- Colorectal – 38% (8/21)
- Upper gastrointestinal – 38% (3/8)
- Gynecologic – 38% (5/13)
- Skin – 33% (1/3)
- Hepatobiliary – 29% (2/7)
- Bone/soft tissue – 20% (1/5)
- Genitourinary – 15% (7/46)
- Breast – 14% (4/28)
- Neurologic – 13% (1/8)
- Head and neck – 13% (1/8).
None of the three patients with neuroendocrine tumors died.
Among the 54 patients with hematologic malignancies, case fatality rates were as follows:
- Chronic myeloid leukemia – 100% (1/1)
- Hodgkin lymphoma – 60% (3/5)
- Myelodysplastic syndromes – 60% (3/5)
- Multiple myeloma – 38% (5/13)
- Non-Hodgkin lymphoma – 33% (5/15)
- Chronic lymphocytic leukemia – 33% (1/3)
- Myeloproliferative neoplasms – 29% (2/7).
None of the four patients with acute lymphoblastic leukemia died, and there was one patient with acute myeloid leukemia who did not die.
Factors associated with increased mortality
The researchers compared the 218 cancer patients with COVID-19 with 1,090 age- and sex-matched noncancer patients with COVID-19 treated in the Montefiore Health System between March 18 and April 8, 2020.
Case fatality rates in cancer patients with COVID-19 were significantly increased in all age groups, but older age was associated with higher mortality.
“We observed case fatality rates were elevated in all age cohorts in cancer patients and achieved statistical significance in the age groups 45-64 and in patients older than 75 years of age,” the authors reported.
Other factors significantly associated with higher mortality in a multivariable analysis included the presence of multiple comorbidities; the need for ICU support; and increased levels of d-dimer, lactate, and lactate dehydrogenase.
Additional factors, such as socioeconomic and health disparities, may also be significant predictors of mortality, according to the authors. They noted that this cohort largely consisted of patients from a socioeconomically underprivileged community where mortality because of COVID-19 is reportedly higher.
Proactive strategies moving forward
“We have been addressing the significant burden of the COVID-19 pandemic on our vulnerable cancer patients through a variety of ways,” said study author Balazs Halmos, MD, of Montefiore Medical Center.
The center set up a separate infusion unit exclusively for COVID-positive patients and established separate inpatient areas. Dr. Halmos and colleagues are also providing telemedicine, virtual supportive care services, telephonic counseling, and bilingual peer-support programs.
“Many questions remain as we continue to establish new practices for our cancer patients,” Dr. Halmos said. “We will find answers to these questions as we continue to focus on adaptation and not acceptance in response to the COVID crisis. Our patients deserve nothing less.”
The Albert Einstein Cancer Center supported this study. The authors reported having no conflicts of interest.
SOURCE: Mehta V et al. Cancer Discov. 2020 May 1. doi: 10.1158/2159-8290.CD-20-0516.
FROM CANCER DISCOVERY
Three months of COVID-19 may mean 80,000 missed cancer diagnoses
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.
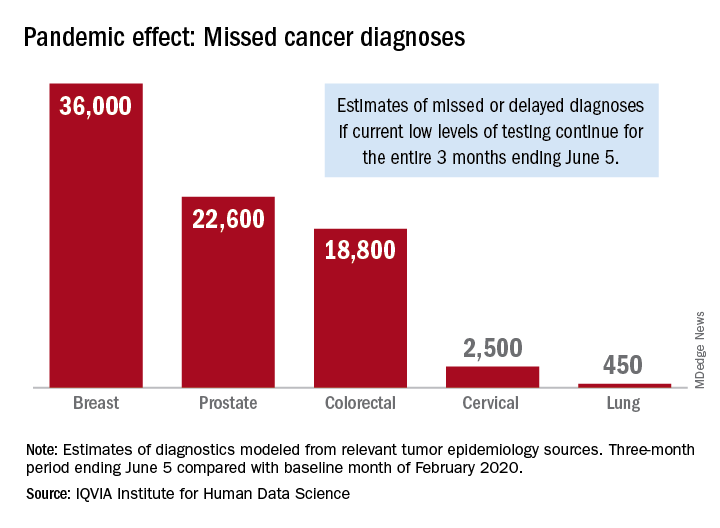
Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
, according to a report by the IQVIA Institute for Human Data Science looking at trends in the United States.

Screening and monitoring tests for breast, prostate, colorectal, cervical, and lung cancer were down 39%-90% in early April, compared with the baseline month of February, according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
These findings are based on data from IQVIA’s medical claims database, which includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data suggest that, at current positivity rates, there could be 36,000 missed or delayed diagnoses of breast cancer during the 3-month period from early March through early June. Estimates for missed diagnoses of the four other cancers analyzed include 450 for lung cancer, 2,500 for cervical cancer, 18,800 for colorectal cancer, and 22,600 for prostate cancer.
The authors project a total of 22 million canceled or delayed tests for the five cancers over the 3-month period ending June 5, based on a comparison of claims data for early April with the February baseline. Catching up on this backlog will be problematic, according to the authors.
“Current excess health care capacity ... would require providers to shift priorities to make time and space in schedules and facilities as well as the cooperation of patients to return to health care providers,” the authors wrote. “Both of these could be further disrupted by economic factors or reintroduction of social distancing in a reemergence of the outbreak.”
The report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Cancer screening, monitoring down during pandemic
according to a report by the IQVIA Institute for Human Data Science.
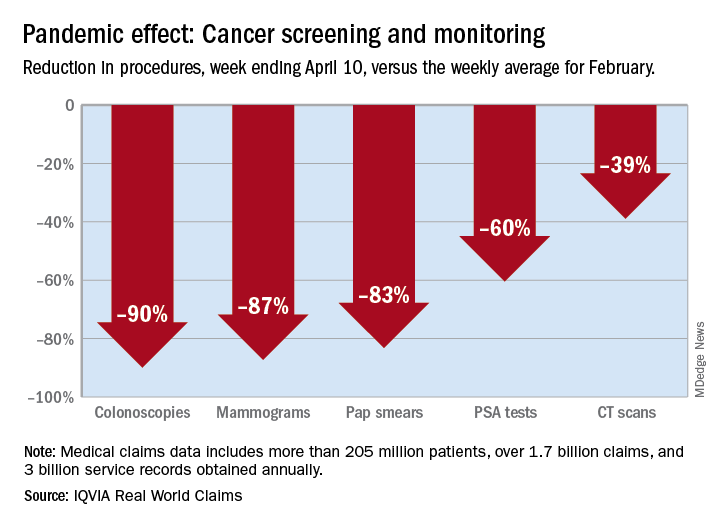
There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
according to a report by the IQVIA Institute for Human Data Science.

There were 90% fewer colonoscopies ordered during the week ending April 10, compared with the weekly average for Feb. 1-28, based on claims data analyzed by IQVIA.
IQVIA’s medical claims database includes more than 205 million patients, over 1.7 billion claims, and 3 billion service records obtained annually.
The data also showed an 87% reduction in mammograms and an 83% reduction in Pap smears during the week ending April 10. Prostate-specific antigen tests for prostate cancer decreased by 60%, and CT scans for lung cancer decreased by 39%.
The smaller decrease in CT scans for lung cancer “may reflect the generally more serious nature of those tumors or be due to concerns about ruling out COVID-related issues in some patients,” according to report authors Murray Aitken and Michael Kleinrock, both of IQVIA.
The report also showed that overall patient interactions with oncologists were down by 20% through April 3, based on medical and pharmacy claims processed since February, but there was variation by tumor type.
The authors noted “little or no disruption” in oncologist visits in March for patients with aggressive tumors or those diagnosed at advanced stages, compared with February. However, for patients with skin cancer or prostate cancer, visit rates were down by 20%-50% in March.
“This may reflect that oncologists who are providing care across multiple tumor types are prioritizing their time and efforts to those patients with more advanced or aggressive tumors,” the authors wrote.
This report was produced by the IQVIA Institute for Human Data Science without industry or government funding.
SOURCE: Murray A and Kleinrock M. Shifts in healthcare demand, delivery and care during the COVID-19 era. IQVIA Institute for Human Data Science. April 2020.
Adding a blood test to standard screening may improve early cancer detection
A minimally invasive multicancer blood test used with standard-of-care screening is safe, effective, and feasible for use in routine clinical care, according to interim findings from a large, prospective study.
The DETECT-A blood test, an early version of the CancerSEEK test currently in development, effectively guided patient management in real time, in some cases leading to diagnosis of early cancer and potentially curative surgery in asymptomatic women with no history of cancer.
Nickolas Papadopoulos, PhD, of Johns Hopkins Medicine in Baltimore, reported these findings at the AACR virtual meeting I. The findings were simultaneously published in Science.
The study enrolled 10,006 women, aged 65-75 years, with no prior cancer diagnosis. After exclusion and loss to follow-up, 9,911 women remained.
There were 26 patients who had cancer detected by the DETECT-A blood test, 15 of whom underwent follow-up PET-CT imaging and 9 of whom underwent surgical excision. An additional 24 cancers were detected by standard screening, and 46 were detected by other means.
The positive predictive value of the blood test was 19%. When the blood test was combined with imaging, the positive predictive value was 41%.
Improving upon standard screening
“Standard-of-care screening [was used] for three different organs: breast, lung, and colon. It was more sensitive for breast cancer,” Dr. Papadopoulos noted. “Blood testing, though, identified cancer in 10 different organs.”
In fact, the DETECT-A blood test detected 14 of 45 cancers in 7 organs for which no standard screening test is available.
In addition, 12 cancers in 3 organs (breast, lung, and colon) were first detected by DETECT-A rather than by standard screening. This increased the sensitivity of cancer detection from 47% with standard screening alone to 71% with standard screening plus blood testing.
“More important, 65% [of the cancers detected by blood test] were localized or regional, which have higher chance of successful treatment with intent to cure,” Dr. Papadopoulos said.
DETECT-A covers regions of 16 commonly mutated genes and 9 proteins known to be associated with cancer. In this study, 57% of cancers were detected by mutations.
Safety and additional screening
DETECT-A also proved safe, “without incurring a large number of futile invasive follow-up tests,” Dr. Papadopoulos said.
In fact, only 1% of patients without cancer underwent PET-CT imaging, and only 0.22% underwent a “futile” invasive follow-up procedure.
Three surgeries occurred in patients who were counted as false-positives, but the surgeries were determined to be indicated, Dr. Papadopoulos said. He explained that one was for large colonic polyps with high-grade dysplasia that could not be removed endoscopically, one was for an in situ carcinoma of the appendix, and one was for a 10-cm ovarian lesion that was found to be a mucinous cystadenoma.
The investigators also analyzed whether the availability of a “liquid biopsy” test like DETECT-A would inadvertently reduce patients’ use of standard screening and found that it did not. Mammography screening habits after receiving the baseline DETECT-A blood test did not differ significantly from those prior to study enrollment.
These findings are important because early detection is a key factor in reducing cancer-specific morbidity and mortality, and although minimally invasive screening tests, including liquid biopsies like DETECT-A, hold great promise, prospective clinical studies of these new methods are needed to ensure that the anticipated benefits outweigh the potential risks, Dr. Papadopoulos explained.
“The problem is that most cancers are detected at advanced stages when they are difficult to treat,” he said. “The earlier cancer is detected, the greater the chance of successful treatment.”
Unanswered questions and future studies
This study demonstrates that it is feasible for a minimally invasive blood test to safely detect multiple cancer types in patients without a history of cancer and to enable treatment with curative intent, at least in a subset of individuals, Dr. Papadopoulos said. He added that the findings also inform the design of future randomized trials “to establish clinical utility, cost-effectiveness, and benefit-to-risk ratio of future tests.”
Further studies will also be required to determine the clinical validity and utility of the strategy of using liquid biopsy as a complement to standard-of-care screening, Dr. Papadopoulos said.
Invited discussant David G. Huntsman, MD, of the University of British Columbia in Vancouver, applauded the investigators, saying this study serves to “move the field forward.” However, it still isn’t clear how sensitivity and negative predictive value will be determined and what the optimal testing schedule is.
“This is a prospective study that will provide the data on how this assay will be used [and] whether it should be used going forward,” Dr. Huntsman said, noting that the “much bigger and more important question” is whether it improves survival.
Cost-effectiveness will also be critical, he said.
This research was supported by The Marcus Foundation, Lustgarten Foundation for Pancreatic Cancer Research, The Virginia and D.K. Ludwig Fund for Cancer Research, The Sol Goldman Center for Pancreatic Cancer Research, Susan Wojcicki and Dennis Troper, the Rolfe Foundation, The Conrad R. Hilton Foundation, The John Templeton Foundation, Burroughs Wellcome Career Award For Medical Scientists, and grants/contracts from the National Institutes of Health.
Dr. Papadopoulos disclosed relationships with Thrive Earlier Detection Inc., PGDx Inc., NeoPhore, Cage Pharma, and other companies. Dr. Huntsman is a founder, shareholder, and chief medical officer for Contextual Genomics.
SOURCE: Papadopoulos N et al. AACR 2020, Abstract CT022; Lennon AM et al. Science. 2020 Apr 28. pii: eabb9601. doi: 10.1126/science.abb9601.
A minimally invasive multicancer blood test used with standard-of-care screening is safe, effective, and feasible for use in routine clinical care, according to interim findings from a large, prospective study.
The DETECT-A blood test, an early version of the CancerSEEK test currently in development, effectively guided patient management in real time, in some cases leading to diagnosis of early cancer and potentially curative surgery in asymptomatic women with no history of cancer.
Nickolas Papadopoulos, PhD, of Johns Hopkins Medicine in Baltimore, reported these findings at the AACR virtual meeting I. The findings were simultaneously published in Science.
The study enrolled 10,006 women, aged 65-75 years, with no prior cancer diagnosis. After exclusion and loss to follow-up, 9,911 women remained.
There were 26 patients who had cancer detected by the DETECT-A blood test, 15 of whom underwent follow-up PET-CT imaging and 9 of whom underwent surgical excision. An additional 24 cancers were detected by standard screening, and 46 were detected by other means.
The positive predictive value of the blood test was 19%. When the blood test was combined with imaging, the positive predictive value was 41%.
Improving upon standard screening
“Standard-of-care screening [was used] for three different organs: breast, lung, and colon. It was more sensitive for breast cancer,” Dr. Papadopoulos noted. “Blood testing, though, identified cancer in 10 different organs.”
In fact, the DETECT-A blood test detected 14 of 45 cancers in 7 organs for which no standard screening test is available.
In addition, 12 cancers in 3 organs (breast, lung, and colon) were first detected by DETECT-A rather than by standard screening. This increased the sensitivity of cancer detection from 47% with standard screening alone to 71% with standard screening plus blood testing.
“More important, 65% [of the cancers detected by blood test] were localized or regional, which have higher chance of successful treatment with intent to cure,” Dr. Papadopoulos said.
DETECT-A covers regions of 16 commonly mutated genes and 9 proteins known to be associated with cancer. In this study, 57% of cancers were detected by mutations.
Safety and additional screening
DETECT-A also proved safe, “without incurring a large number of futile invasive follow-up tests,” Dr. Papadopoulos said.
In fact, only 1% of patients without cancer underwent PET-CT imaging, and only 0.22% underwent a “futile” invasive follow-up procedure.
Three surgeries occurred in patients who were counted as false-positives, but the surgeries were determined to be indicated, Dr. Papadopoulos said. He explained that one was for large colonic polyps with high-grade dysplasia that could not be removed endoscopically, one was for an in situ carcinoma of the appendix, and one was for a 10-cm ovarian lesion that was found to be a mucinous cystadenoma.
The investigators also analyzed whether the availability of a “liquid biopsy” test like DETECT-A would inadvertently reduce patients’ use of standard screening and found that it did not. Mammography screening habits after receiving the baseline DETECT-A blood test did not differ significantly from those prior to study enrollment.
These findings are important because early detection is a key factor in reducing cancer-specific morbidity and mortality, and although minimally invasive screening tests, including liquid biopsies like DETECT-A, hold great promise, prospective clinical studies of these new methods are needed to ensure that the anticipated benefits outweigh the potential risks, Dr. Papadopoulos explained.
“The problem is that most cancers are detected at advanced stages when they are difficult to treat,” he said. “The earlier cancer is detected, the greater the chance of successful treatment.”
Unanswered questions and future studies
This study demonstrates that it is feasible for a minimally invasive blood test to safely detect multiple cancer types in patients without a history of cancer and to enable treatment with curative intent, at least in a subset of individuals, Dr. Papadopoulos said. He added that the findings also inform the design of future randomized trials “to establish clinical utility, cost-effectiveness, and benefit-to-risk ratio of future tests.”
Further studies will also be required to determine the clinical validity and utility of the strategy of using liquid biopsy as a complement to standard-of-care screening, Dr. Papadopoulos said.
Invited discussant David G. Huntsman, MD, of the University of British Columbia in Vancouver, applauded the investigators, saying this study serves to “move the field forward.” However, it still isn’t clear how sensitivity and negative predictive value will be determined and what the optimal testing schedule is.
“This is a prospective study that will provide the data on how this assay will be used [and] whether it should be used going forward,” Dr. Huntsman said, noting that the “much bigger and more important question” is whether it improves survival.
Cost-effectiveness will also be critical, he said.
This research was supported by The Marcus Foundation, Lustgarten Foundation for Pancreatic Cancer Research, The Virginia and D.K. Ludwig Fund for Cancer Research, The Sol Goldman Center for Pancreatic Cancer Research, Susan Wojcicki and Dennis Troper, the Rolfe Foundation, The Conrad R. Hilton Foundation, The John Templeton Foundation, Burroughs Wellcome Career Award For Medical Scientists, and grants/contracts from the National Institutes of Health.
Dr. Papadopoulos disclosed relationships with Thrive Earlier Detection Inc., PGDx Inc., NeoPhore, Cage Pharma, and other companies. Dr. Huntsman is a founder, shareholder, and chief medical officer for Contextual Genomics.
SOURCE: Papadopoulos N et al. AACR 2020, Abstract CT022; Lennon AM et al. Science. 2020 Apr 28. pii: eabb9601. doi: 10.1126/science.abb9601.
A minimally invasive multicancer blood test used with standard-of-care screening is safe, effective, and feasible for use in routine clinical care, according to interim findings from a large, prospective study.
The DETECT-A blood test, an early version of the CancerSEEK test currently in development, effectively guided patient management in real time, in some cases leading to diagnosis of early cancer and potentially curative surgery in asymptomatic women with no history of cancer.
Nickolas Papadopoulos, PhD, of Johns Hopkins Medicine in Baltimore, reported these findings at the AACR virtual meeting I. The findings were simultaneously published in Science.
The study enrolled 10,006 women, aged 65-75 years, with no prior cancer diagnosis. After exclusion and loss to follow-up, 9,911 women remained.
There were 26 patients who had cancer detected by the DETECT-A blood test, 15 of whom underwent follow-up PET-CT imaging and 9 of whom underwent surgical excision. An additional 24 cancers were detected by standard screening, and 46 were detected by other means.
The positive predictive value of the blood test was 19%. When the blood test was combined with imaging, the positive predictive value was 41%.
Improving upon standard screening
“Standard-of-care screening [was used] for three different organs: breast, lung, and colon. It was more sensitive for breast cancer,” Dr. Papadopoulos noted. “Blood testing, though, identified cancer in 10 different organs.”
In fact, the DETECT-A blood test detected 14 of 45 cancers in 7 organs for which no standard screening test is available.
In addition, 12 cancers in 3 organs (breast, lung, and colon) were first detected by DETECT-A rather than by standard screening. This increased the sensitivity of cancer detection from 47% with standard screening alone to 71% with standard screening plus blood testing.
“More important, 65% [of the cancers detected by blood test] were localized or regional, which have higher chance of successful treatment with intent to cure,” Dr. Papadopoulos said.
DETECT-A covers regions of 16 commonly mutated genes and 9 proteins known to be associated with cancer. In this study, 57% of cancers were detected by mutations.
Safety and additional screening
DETECT-A also proved safe, “without incurring a large number of futile invasive follow-up tests,” Dr. Papadopoulos said.
In fact, only 1% of patients without cancer underwent PET-CT imaging, and only 0.22% underwent a “futile” invasive follow-up procedure.
Three surgeries occurred in patients who were counted as false-positives, but the surgeries were determined to be indicated, Dr. Papadopoulos said. He explained that one was for large colonic polyps with high-grade dysplasia that could not be removed endoscopically, one was for an in situ carcinoma of the appendix, and one was for a 10-cm ovarian lesion that was found to be a mucinous cystadenoma.
The investigators also analyzed whether the availability of a “liquid biopsy” test like DETECT-A would inadvertently reduce patients’ use of standard screening and found that it did not. Mammography screening habits after receiving the baseline DETECT-A blood test did not differ significantly from those prior to study enrollment.
These findings are important because early detection is a key factor in reducing cancer-specific morbidity and mortality, and although minimally invasive screening tests, including liquid biopsies like DETECT-A, hold great promise, prospective clinical studies of these new methods are needed to ensure that the anticipated benefits outweigh the potential risks, Dr. Papadopoulos explained.
“The problem is that most cancers are detected at advanced stages when they are difficult to treat,” he said. “The earlier cancer is detected, the greater the chance of successful treatment.”
Unanswered questions and future studies
This study demonstrates that it is feasible for a minimally invasive blood test to safely detect multiple cancer types in patients without a history of cancer and to enable treatment with curative intent, at least in a subset of individuals, Dr. Papadopoulos said. He added that the findings also inform the design of future randomized trials “to establish clinical utility, cost-effectiveness, and benefit-to-risk ratio of future tests.”
Further studies will also be required to determine the clinical validity and utility of the strategy of using liquid biopsy as a complement to standard-of-care screening, Dr. Papadopoulos said.
Invited discussant David G. Huntsman, MD, of the University of British Columbia in Vancouver, applauded the investigators, saying this study serves to “move the field forward.” However, it still isn’t clear how sensitivity and negative predictive value will be determined and what the optimal testing schedule is.
“This is a prospective study that will provide the data on how this assay will be used [and] whether it should be used going forward,” Dr. Huntsman said, noting that the “much bigger and more important question” is whether it improves survival.
Cost-effectiveness will also be critical, he said.
This research was supported by The Marcus Foundation, Lustgarten Foundation for Pancreatic Cancer Research, The Virginia and D.K. Ludwig Fund for Cancer Research, The Sol Goldman Center for Pancreatic Cancer Research, Susan Wojcicki and Dennis Troper, the Rolfe Foundation, The Conrad R. Hilton Foundation, The John Templeton Foundation, Burroughs Wellcome Career Award For Medical Scientists, and grants/contracts from the National Institutes of Health.
Dr. Papadopoulos disclosed relationships with Thrive Earlier Detection Inc., PGDx Inc., NeoPhore, Cage Pharma, and other companies. Dr. Huntsman is a founder, shareholder, and chief medical officer for Contextual Genomics.
SOURCE: Papadopoulos N et al. AACR 2020, Abstract CT022; Lennon AM et al. Science. 2020 Apr 28. pii: eabb9601. doi: 10.1126/science.abb9601.
FROM AACR 2020



