User login
Risky business: Most cancer drugs don’t reach the market
, a new analysis suggests.
The researchers also found that about 8% of approved agents were subsequently taken off the market.
“The 6% is not a big surprise to us, since a few other studies using different methodologies and foci have estimated similar percentages,” Alyson Haslam, PhD, University of California, San Francisco, told this news organization. “When you look at drug development, it makes sense that you have to test a lot of drugs to get one that works, but sometimes it is nice to quantify the actual percentage in order to fully appreciate the process.”
The fact that 8% were withdrawn, however, “elicits the question of how the approval process can be improved to avoid ineffective or harmful drugs from coming onto the market,” Dr. Haslam added.
The study was published online in the International Journal of Cancer.
More desirable features?
Monitoring trends over time helps oncologists assess whether more drugs are making it to market and if certain factors make some drugs more likely to get approved.
Prior published estimates put the likelihood of approval between 6.7% and 13.4%, but these estimates were for drugs tested more than a decade ago.
To provide updated estimates, the researchers searched the literature for all oncology drugs tested in phase 1 studies during 2015 and evaluated their fate in subsequent phase 2/3 studies through FDA clearance.
Overall, the team found 803 phase 1 studies that met initial inclusion criteria; 48 trials that included only Japanese participants were excluded because these studies often evaluated drugs already approved in the United States, leaving 755 studies for the analysis.
The most common tumor types were solid/multiple tumors (24.2%), leukemias (12.8%), and lung cancer (8.5%). Just under half (47%) of the trials tested a drug as monotherapy; 43% were combination trials with one dose-escalated drug; and about 10% were combination trials with both drugs dose-escalated.
The FDA approved 51 drugs during the study period. Four (7.8%) were subsequently withdrawn: nivolumab (Opdivo) and pembrolizumab (Keytruda) for small cell lung cancer, olaratumab (Lartruvo) for soft tissue sarcoma, and melflufen (Pepaxto) for multiple myeloma. These four were not counted in the overall number of approvals.
“We really wanted to look at the end fate of drugs (within a reasonable time frame), which is why we did not include the four drugs that were initially approved but later withdrawn, although this had little impact on the main finding,” Dr. Haslam explained.
The estimated probability of any drug or drug combination tested in a phase 1 trial published in 2015 and approved that year was 1.7% and reached 6.2% by the end of 2021, the researchers found.
Monoclonal antibodies had a higher probability of being approved (15.3%), compared with inhibitors (5.1%) and chemotherapy drugs (4.2%).
The FDA was also more apt to green-light drugs tested as monotherapy, compared with drug combinations (odds ratio, 0.22). Drugs tested in monotherapy had a 9.4% probability of approval versus those tested in combination, which had a 5.6% probability of being approved when pairing a novel drug with one or more established agents, as well as when combining two novel drugs. The probability of approval was less than 1% for trials testing two established drug combinations.
Other factors that boosted the odds of FDA approval include having a response rate over 40% in phase 1 testing, demonstrating an overall survival benefit in phase 3 testing, and having the trial sponsored by a top-20 drug company, compared with a non–top-20 drug company.
Dr. Haslam found the last finding rather surprising, given the recent trend for bigger companies to invest in smaller companies who are developing promising drugs, rather than doing all of the development themselves. “In fact, a recent analysis found that only 25% of new drugs are sponsored by larger companies,” she noted.
Reached for comment, Jeff Allen, PhD, who wasn’t involved in the study, noted that “these types of landscape analyses are quite helpful in understanding the current state of oncology science and drug development.”
When looking at a 6.2% success rate for phase 1–tested oncology drugs, “it can be difficult holistically to determine all factors for which development didn’t continue,” said Dr. Allen, president and CEO of the nonprofit Friends of Cancer Research.
For instance, lack of approval may not signal the drug was a failure “but rather an artifact of circumstances such as resource limitations or reprioritization,” Dr. Allen said.
Plus, he commented, “I don’t think that we should expect all these early studies to lead to eventual approvals, but it’s clear from the authors’ findings that continued efforts to improve the overall success rate in developing new cancer medicines are greatly needed.”
The study was funded by Arnold Ventures. Dr. Haslam and Dr. Allen have no relevant disclosures. Study author Vinay Prasad, MD, MPH, receives royalties from Arnold Ventures.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The researchers also found that about 8% of approved agents were subsequently taken off the market.
“The 6% is not a big surprise to us, since a few other studies using different methodologies and foci have estimated similar percentages,” Alyson Haslam, PhD, University of California, San Francisco, told this news organization. “When you look at drug development, it makes sense that you have to test a lot of drugs to get one that works, but sometimes it is nice to quantify the actual percentage in order to fully appreciate the process.”
The fact that 8% were withdrawn, however, “elicits the question of how the approval process can be improved to avoid ineffective or harmful drugs from coming onto the market,” Dr. Haslam added.
The study was published online in the International Journal of Cancer.
More desirable features?
Monitoring trends over time helps oncologists assess whether more drugs are making it to market and if certain factors make some drugs more likely to get approved.
Prior published estimates put the likelihood of approval between 6.7% and 13.4%, but these estimates were for drugs tested more than a decade ago.
To provide updated estimates, the researchers searched the literature for all oncology drugs tested in phase 1 studies during 2015 and evaluated their fate in subsequent phase 2/3 studies through FDA clearance.
Overall, the team found 803 phase 1 studies that met initial inclusion criteria; 48 trials that included only Japanese participants were excluded because these studies often evaluated drugs already approved in the United States, leaving 755 studies for the analysis.
The most common tumor types were solid/multiple tumors (24.2%), leukemias (12.8%), and lung cancer (8.5%). Just under half (47%) of the trials tested a drug as monotherapy; 43% were combination trials with one dose-escalated drug; and about 10% were combination trials with both drugs dose-escalated.
The FDA approved 51 drugs during the study period. Four (7.8%) were subsequently withdrawn: nivolumab (Opdivo) and pembrolizumab (Keytruda) for small cell lung cancer, olaratumab (Lartruvo) for soft tissue sarcoma, and melflufen (Pepaxto) for multiple myeloma. These four were not counted in the overall number of approvals.
“We really wanted to look at the end fate of drugs (within a reasonable time frame), which is why we did not include the four drugs that were initially approved but later withdrawn, although this had little impact on the main finding,” Dr. Haslam explained.
The estimated probability of any drug or drug combination tested in a phase 1 trial published in 2015 and approved that year was 1.7% and reached 6.2% by the end of 2021, the researchers found.
Monoclonal antibodies had a higher probability of being approved (15.3%), compared with inhibitors (5.1%) and chemotherapy drugs (4.2%).
The FDA was also more apt to green-light drugs tested as monotherapy, compared with drug combinations (odds ratio, 0.22). Drugs tested in monotherapy had a 9.4% probability of approval versus those tested in combination, which had a 5.6% probability of being approved when pairing a novel drug with one or more established agents, as well as when combining two novel drugs. The probability of approval was less than 1% for trials testing two established drug combinations.
Other factors that boosted the odds of FDA approval include having a response rate over 40% in phase 1 testing, demonstrating an overall survival benefit in phase 3 testing, and having the trial sponsored by a top-20 drug company, compared with a non–top-20 drug company.
Dr. Haslam found the last finding rather surprising, given the recent trend for bigger companies to invest in smaller companies who are developing promising drugs, rather than doing all of the development themselves. “In fact, a recent analysis found that only 25% of new drugs are sponsored by larger companies,” she noted.
Reached for comment, Jeff Allen, PhD, who wasn’t involved in the study, noted that “these types of landscape analyses are quite helpful in understanding the current state of oncology science and drug development.”
When looking at a 6.2% success rate for phase 1–tested oncology drugs, “it can be difficult holistically to determine all factors for which development didn’t continue,” said Dr. Allen, president and CEO of the nonprofit Friends of Cancer Research.
For instance, lack of approval may not signal the drug was a failure “but rather an artifact of circumstances such as resource limitations or reprioritization,” Dr. Allen said.
Plus, he commented, “I don’t think that we should expect all these early studies to lead to eventual approvals, but it’s clear from the authors’ findings that continued efforts to improve the overall success rate in developing new cancer medicines are greatly needed.”
The study was funded by Arnold Ventures. Dr. Haslam and Dr. Allen have no relevant disclosures. Study author Vinay Prasad, MD, MPH, receives royalties from Arnold Ventures.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The researchers also found that about 8% of approved agents were subsequently taken off the market.
“The 6% is not a big surprise to us, since a few other studies using different methodologies and foci have estimated similar percentages,” Alyson Haslam, PhD, University of California, San Francisco, told this news organization. “When you look at drug development, it makes sense that you have to test a lot of drugs to get one that works, but sometimes it is nice to quantify the actual percentage in order to fully appreciate the process.”
The fact that 8% were withdrawn, however, “elicits the question of how the approval process can be improved to avoid ineffective or harmful drugs from coming onto the market,” Dr. Haslam added.
The study was published online in the International Journal of Cancer.
More desirable features?
Monitoring trends over time helps oncologists assess whether more drugs are making it to market and if certain factors make some drugs more likely to get approved.
Prior published estimates put the likelihood of approval between 6.7% and 13.4%, but these estimates were for drugs tested more than a decade ago.
To provide updated estimates, the researchers searched the literature for all oncology drugs tested in phase 1 studies during 2015 and evaluated their fate in subsequent phase 2/3 studies through FDA clearance.
Overall, the team found 803 phase 1 studies that met initial inclusion criteria; 48 trials that included only Japanese participants were excluded because these studies often evaluated drugs already approved in the United States, leaving 755 studies for the analysis.
The most common tumor types were solid/multiple tumors (24.2%), leukemias (12.8%), and lung cancer (8.5%). Just under half (47%) of the trials tested a drug as monotherapy; 43% were combination trials with one dose-escalated drug; and about 10% were combination trials with both drugs dose-escalated.
The FDA approved 51 drugs during the study period. Four (7.8%) were subsequently withdrawn: nivolumab (Opdivo) and pembrolizumab (Keytruda) for small cell lung cancer, olaratumab (Lartruvo) for soft tissue sarcoma, and melflufen (Pepaxto) for multiple myeloma. These four were not counted in the overall number of approvals.
“We really wanted to look at the end fate of drugs (within a reasonable time frame), which is why we did not include the four drugs that were initially approved but later withdrawn, although this had little impact on the main finding,” Dr. Haslam explained.
The estimated probability of any drug or drug combination tested in a phase 1 trial published in 2015 and approved that year was 1.7% and reached 6.2% by the end of 2021, the researchers found.
Monoclonal antibodies had a higher probability of being approved (15.3%), compared with inhibitors (5.1%) and chemotherapy drugs (4.2%).
The FDA was also more apt to green-light drugs tested as monotherapy, compared with drug combinations (odds ratio, 0.22). Drugs tested in monotherapy had a 9.4% probability of approval versus those tested in combination, which had a 5.6% probability of being approved when pairing a novel drug with one or more established agents, as well as when combining two novel drugs. The probability of approval was less than 1% for trials testing two established drug combinations.
Other factors that boosted the odds of FDA approval include having a response rate over 40% in phase 1 testing, demonstrating an overall survival benefit in phase 3 testing, and having the trial sponsored by a top-20 drug company, compared with a non–top-20 drug company.
Dr. Haslam found the last finding rather surprising, given the recent trend for bigger companies to invest in smaller companies who are developing promising drugs, rather than doing all of the development themselves. “In fact, a recent analysis found that only 25% of new drugs are sponsored by larger companies,” she noted.
Reached for comment, Jeff Allen, PhD, who wasn’t involved in the study, noted that “these types of landscape analyses are quite helpful in understanding the current state of oncology science and drug development.”
When looking at a 6.2% success rate for phase 1–tested oncology drugs, “it can be difficult holistically to determine all factors for which development didn’t continue,” said Dr. Allen, president and CEO of the nonprofit Friends of Cancer Research.
For instance, lack of approval may not signal the drug was a failure “but rather an artifact of circumstances such as resource limitations or reprioritization,” Dr. Allen said.
Plus, he commented, “I don’t think that we should expect all these early studies to lead to eventual approvals, but it’s clear from the authors’ findings that continued efforts to improve the overall success rate in developing new cancer medicines are greatly needed.”
The study was funded by Arnold Ventures. Dr. Haslam and Dr. Allen have no relevant disclosures. Study author Vinay Prasad, MD, MPH, receives royalties from Arnold Ventures.
A version of this article first appeared on Medscape.com.
FROM INTERNATIONAL JOURNAL OF CANCER
If nuclear disaster strikes, U.S. hematologists stand ready
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.
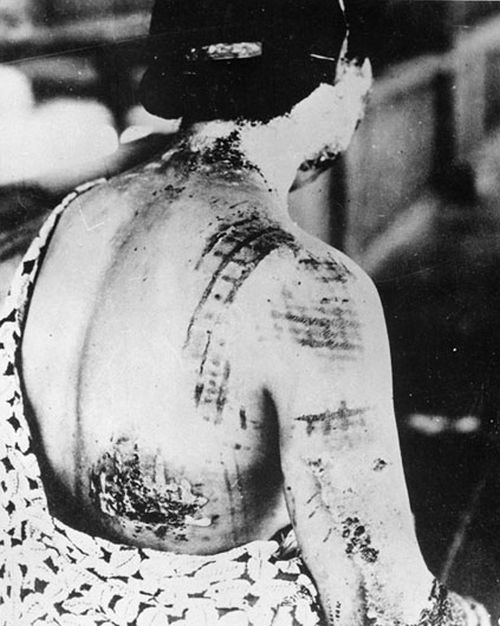
“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”
And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
For many Americans – especially those too young to know much about the Cold War or Hiroshima – Russia’s invasion of Ukraine might mark the first time they’ve truly considered the dangers of nuclear weapons. But dozens of hematologists in the United States already know the drill and have placed themselves on the front lines. These physicians stand prepared to treat patients exposed to radiation caused by nuclear accidents or attacks on U.S. soil.
They work nationwide at 74 medical centers that make up the Radiation Injury Treatment Network, ready to manage cases of acute radiation syndrome (ARS) during disasters. While RITN keeps a low profile, it’s been in the news lately amid anxieties about the Ukraine conflict, nuclear plant accidents, and the potential launching of nuclear weapons by foreign adversaries.
“The Radiation Injury Treatment Network helps plan responses for disaster scenarios where a person’s cells would be damaged after having been exposed to ionizing radiation,” program director Cullen Case Jr., MPA, said in an interview.
A U.S. Army veteran who took part in hurricane response early in his career, Mr. Case now oversees preparedness activities among all RITN hospitals, blood donor centers, and cord blood banks, in readiness for a mass casualty radiological incident. He also serves as a senior manager of the National Marrow Donor Program/Be a Match Marrow Registry.
Intense preparation for nuclear attacks or accidents is necessary, Mr. Case said, despite the doomsday scenarios disseminated on television shows and movies.
“The most frequent misconception we hear is that a nuclear disaster will encompass the whole world and be so complete that preparedness isn’t useful. However, many planning scenarios include smaller-scale incidents where survivors will need prompt and expert care,” he said.
In the wake of 9/11, the National Marrow Donor Program and the American Society for Blood and Marrow Transplantation established the RITN in 2006, with a mission to prepare for nuclear disaster and help manage the response if one occurs.

“The widespread availability of radioactive material has made future exposure events, accidental or intentional, nearly inevitable,” RITN leaders warned in a 2008 report. “Hematologists, oncologists, and HSCT [hematopoietic stem cell transplantation] physicians are uniquely suited to care for victims of radiation exposure, creating a collective responsibility to prepare for a variety of contingencies.”
RITN doesn’t just train physicians, Mr. Case noted. All medical centers within the RITN are required to conduct an annual tabletop exercise where a radiation disaster scenario and a set of discussion questions are presented to the team.
Hematologists specially equipped to treat radiation injuries
Why are hematologists involved in treating people exposed to dangerously high levels of radiation? The answer has to do with how radiation harms the body, said Dr. Ann A. Jakubowski, a hematologist/oncologist and transplant physician at Memorial Sloan Kettering Cancer Center, New York, who serves as a medical director for RITN.
“One of the most common toxicities from radiation exposure and a major player in acute radiation syndrome is hematologic toxicity– damage to the bone marrow by the radiation, with a resultant decrease in peripheral blood counts,” she said in an interview. “This is similar to what is often seen in the treatment of cancers with radiation and/or chemotherapy.”
In cases of severe and nonreversible radiation damage to the bone marrow, Dr. Jakubowski noted, “patients can be considered for a stem cell transplant to provide new healthy cells to repopulate the bone marrow, which provides recovery of peripheral blood counts. Hematologist/oncologists are the physicians who manage stem cell transplants.”
The crucial role of hematologists in radiation injuries is not new. In fact, these physicians have been closely intertwined with nuclear research since the dawn of the atomic age. The work of developing atomic bombs also led investigators to an understanding of the structure and processes of hematopoiesis and helped them to identify hematopoietic stem cells and prove their existence in humans.
Disaster response poses multiple challenges
As noted in a recent article in ASH Clinical News, the challenges of treating radiation injuries would be intense, especially in the event of a nuclear accident or attack that affects a wide area. For starters, how quickly can medical professionals be mobilized, and will there be enough physicians comfortable treating patients? Fortunately, irradiated patients should not pose a direct risk to medical professionals who treat them.
“The expectation is that the patients will all be decontaminated,” said Nelson Chao, MD, MBA, one of the founders of RITN and a hematologist/oncologist and transplant physician at Duke University, Durham, N.C.
Dr. Jakubowski questions whether there will be adequate resources to handle the influx of patients who need more intensive treatment, as well as outpatients who “received lower doses of radiation and may experience a period of low blood counts but are expected to eventually recover blood counts.”

And if many people are injured, Dr. Chao asks, how will physicians “adopt altered standards of care to treat large numbers of patients?”
There will also be a need for physicians who aren’t hematologists, Dr. Jakubowski said. “There may be many victims who have both radiation exposure and traumatic or burn injuries, which need to be addressed first, before the hematologist can start addressing the consequences of ARS. Traumatic and burn injuries will require surgical resources.”
In addition, ARS affects the gastrointestinal track and central nervous system/cardiovascular, and it has multiple stages, she noted.
“Although we have methods of supporting the hematopoietic system – transfusions and growth factors – and even replacing it with a stem cell transplant, this will not necessarily fix the badly damaged other organs, Dr. Jakubowski said. “Also, not all radioactive isotopes are equal in their effects, nor are the various types of radiation exposure.”
Training goes beyond transplants and drugs
RITN offers individual hematologists specialized education about treating radiation injuries through annual exercises, modules, and “just-in-time” training.
For example, the RITN webpage devoted to triage includes guidelines for transferring radiation injury patients, triage guidelines for cytokine administration in cases of ARS, an exposure and symptom triage tool, and more. The treatment page includes details about subjects such as when human leukocyte antigen typing of casualties is appropriate and how to keep yourself safe while treating patients.
Another focus is teaching hematologists to react quickly in disasters, Mr. Case said. “The vast majority of hematologists have little to no experience in responding to disasters and making decisions with imperfect or incomplete information, as emergency medicine practitioners must do regularly.”
“Some of the RITN tabletop exercises present physicians and advanced practitioners with an incomplete set of patient information and ask physicians to then determine and prioritize their care,” Mr. Case said. “The resulting discussions help to lay the groundwork for being able to shift to the crisis standards of care mindset that would be necessary during a radiological disaster.”
Here’s how hematologists can get involved
If you want to help improve the nation’s response to radiation injuries, Mr. Case suggests checking RITN’s list of participating hospitals. If your facility is already part of this network, he said, contact its bone marrow transplant unit for more information.
In such cases, Dr. Jakubowski suggests that you “consider periodically giving a presentation to staff on the basics of radiation injury and the center’s role in RITN.” And if you’re not part of RITN, she said, consider contacting the network about becoming a member.
Hematologists, Mr. Case said, can also take advantage of RITN’s free short overview courses, review the RITN Treatment Guidelines, or watch short videos on the RITN’s YouTube channel.
He highlighted the Radiation Emergency Medical Management website administered by the Department of Health & Human Services, the Center for Disease Control’s radiation emergencies webpage, and the Department of Energy’s Radiation Emergency Assistance Center/Training Site.
How to manage cancer pain when patients misuse opioids
Opioids remain a staple in pain management for cancer, but there is little guidance around how to treat patients who have a history of opioid misuse.
Recently,
“There is a tendency to ignore treatment of opioid use disorder in advanced cancer patients because people think: ‘Oh, this person has bigger fish to fry,’ but that’s not a very patient-centric way of looking at things,” senior author Jessica Merlin, MD, PhD, with the University of Pittsburgh, said in a news release.
“We know that opioid use disorder is a really important factor in quality of life, so addressing opioid addiction and prescription opioid misuse in people with advanced cancer is really critical,” Dr. Merlin added.
The study was published online in JAMA Oncology.
To improve care for people with advanced cancer and cancer-related pain, the researchers first assessed how clinicians currently treat patients with opioid complexity.
Using an online Delphi platform, the team invited 120 clinicians with expertise in palliative care, pain management, and addiction medicine to weigh in on three common clinical scenarios – a patient with a recent history of untreated opioid use disorder, a patient taking more opioids than prescribed, and a patient using nonprescribed benzodiazepines.
For a patient with cancer and a recent history of untreated opioid use disorder, regardless of prognosis, the panel deemed it appropriate to begin treatment with buprenorphine/naloxone for pain but inappropriate to refer the patient to a methadone clinic. The panel felt that going to a methadone clinic would be too burdensome for a patient with advanced cancer and not possible for those with limited prognoses.
“This underscores the importance of access to [opioid use disorder] treatment in cancer treatment settings, including non–addiction specialists waivered to prescribe buprenorphine/naloxone and addiction specialists for more complex cases,” the authors wrote.
For a patient with untreated opioid use disorder, the panel deemed split-dose methadone (two to three times daily) appropriate in those with limited prognosis of weeks to months but was uncertain about the suitability of this approach for patients with longer prognoses of a year or longer.
The appropriateness of initiating treatment with a full-agonist opioid was considered uncertain for a patient with limited prognosis and inappropriate for a patient with longer prognosis.
For a patient with cancer pain and no medical history of opioid use disorder but taking more opioids than prescribed, regardless of prognosis, the panel felt it was appropriate to increase monitoring and inappropriate to taper opioids. The panel was not certain about whether to increase opioids based on the patient’s account of what they need or transition to buprenorphine/naloxone.
For a patient with no history of opioid use disorder who was prescribed traditional opioids for pain and had a positive urine drug test for nonprescribed benzodiazepines, regardless of prognosis, the panel felt it was appropriate to continue opioids with close monitoring and inappropriate to taper opioids or transition to buprenorphine/naloxone.
The researchers said that improving education around buprenorphine and cancer pain management in the context of opioid use disorder or misuse is needed.
In a related editorial, two experts noted that the patients considered in this “important article” require considerable time and expertise from an interdisciplinary team.
“It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer,” wrote Joseph Arthur, MD, and Eduardo Bruera, MD, with the University of Texas MD Anderson Cancer Center, Houston.
In the wider context, Dr. Arthur and Dr. Bruera highlighted how treatments for patients with advanced cancer have evolved over the past 3 decades, yet patients have continued to be given opioids to address cancer-related pain. Developing more sophisticated drugs that relieve pain without significant side effects or addictive properties is imperative.
Dr. Arthur and Dr. Bruera said the study authors “appropriately emphasize the value of delivering compassionate and expert care for these particularly complex cases and the importance of conducting research on the best ways to alleviate the suffering in this rapidly growing patient population.”
This research was supported by Cambia Health Foundation and the National Institute of Nursing Research. Dr. Merlin, Dr. Arthur, and Dr. Bruera reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioids remain a staple in pain management for cancer, but there is little guidance around how to treat patients who have a history of opioid misuse.
Recently,
“There is a tendency to ignore treatment of opioid use disorder in advanced cancer patients because people think: ‘Oh, this person has bigger fish to fry,’ but that’s not a very patient-centric way of looking at things,” senior author Jessica Merlin, MD, PhD, with the University of Pittsburgh, said in a news release.
“We know that opioid use disorder is a really important factor in quality of life, so addressing opioid addiction and prescription opioid misuse in people with advanced cancer is really critical,” Dr. Merlin added.
The study was published online in JAMA Oncology.
To improve care for people with advanced cancer and cancer-related pain, the researchers first assessed how clinicians currently treat patients with opioid complexity.
Using an online Delphi platform, the team invited 120 clinicians with expertise in palliative care, pain management, and addiction medicine to weigh in on three common clinical scenarios – a patient with a recent history of untreated opioid use disorder, a patient taking more opioids than prescribed, and a patient using nonprescribed benzodiazepines.
For a patient with cancer and a recent history of untreated opioid use disorder, regardless of prognosis, the panel deemed it appropriate to begin treatment with buprenorphine/naloxone for pain but inappropriate to refer the patient to a methadone clinic. The panel felt that going to a methadone clinic would be too burdensome for a patient with advanced cancer and not possible for those with limited prognoses.
“This underscores the importance of access to [opioid use disorder] treatment in cancer treatment settings, including non–addiction specialists waivered to prescribe buprenorphine/naloxone and addiction specialists for more complex cases,” the authors wrote.
For a patient with untreated opioid use disorder, the panel deemed split-dose methadone (two to three times daily) appropriate in those with limited prognosis of weeks to months but was uncertain about the suitability of this approach for patients with longer prognoses of a year or longer.
The appropriateness of initiating treatment with a full-agonist opioid was considered uncertain for a patient with limited prognosis and inappropriate for a patient with longer prognosis.
For a patient with cancer pain and no medical history of opioid use disorder but taking more opioids than prescribed, regardless of prognosis, the panel felt it was appropriate to increase monitoring and inappropriate to taper opioids. The panel was not certain about whether to increase opioids based on the patient’s account of what they need or transition to buprenorphine/naloxone.
For a patient with no history of opioid use disorder who was prescribed traditional opioids for pain and had a positive urine drug test for nonprescribed benzodiazepines, regardless of prognosis, the panel felt it was appropriate to continue opioids with close monitoring and inappropriate to taper opioids or transition to buprenorphine/naloxone.
The researchers said that improving education around buprenorphine and cancer pain management in the context of opioid use disorder or misuse is needed.
In a related editorial, two experts noted that the patients considered in this “important article” require considerable time and expertise from an interdisciplinary team.
“It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer,” wrote Joseph Arthur, MD, and Eduardo Bruera, MD, with the University of Texas MD Anderson Cancer Center, Houston.
In the wider context, Dr. Arthur and Dr. Bruera highlighted how treatments for patients with advanced cancer have evolved over the past 3 decades, yet patients have continued to be given opioids to address cancer-related pain. Developing more sophisticated drugs that relieve pain without significant side effects or addictive properties is imperative.
Dr. Arthur and Dr. Bruera said the study authors “appropriately emphasize the value of delivering compassionate and expert care for these particularly complex cases and the importance of conducting research on the best ways to alleviate the suffering in this rapidly growing patient population.”
This research was supported by Cambia Health Foundation and the National Institute of Nursing Research. Dr. Merlin, Dr. Arthur, and Dr. Bruera reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Opioids remain a staple in pain management for cancer, but there is little guidance around how to treat patients who have a history of opioid misuse.
Recently,
“There is a tendency to ignore treatment of opioid use disorder in advanced cancer patients because people think: ‘Oh, this person has bigger fish to fry,’ but that’s not a very patient-centric way of looking at things,” senior author Jessica Merlin, MD, PhD, with the University of Pittsburgh, said in a news release.
“We know that opioid use disorder is a really important factor in quality of life, so addressing opioid addiction and prescription opioid misuse in people with advanced cancer is really critical,” Dr. Merlin added.
The study was published online in JAMA Oncology.
To improve care for people with advanced cancer and cancer-related pain, the researchers first assessed how clinicians currently treat patients with opioid complexity.
Using an online Delphi platform, the team invited 120 clinicians with expertise in palliative care, pain management, and addiction medicine to weigh in on three common clinical scenarios – a patient with a recent history of untreated opioid use disorder, a patient taking more opioids than prescribed, and a patient using nonprescribed benzodiazepines.
For a patient with cancer and a recent history of untreated opioid use disorder, regardless of prognosis, the panel deemed it appropriate to begin treatment with buprenorphine/naloxone for pain but inappropriate to refer the patient to a methadone clinic. The panel felt that going to a methadone clinic would be too burdensome for a patient with advanced cancer and not possible for those with limited prognoses.
“This underscores the importance of access to [opioid use disorder] treatment in cancer treatment settings, including non–addiction specialists waivered to prescribe buprenorphine/naloxone and addiction specialists for more complex cases,” the authors wrote.
For a patient with untreated opioid use disorder, the panel deemed split-dose methadone (two to three times daily) appropriate in those with limited prognosis of weeks to months but was uncertain about the suitability of this approach for patients with longer prognoses of a year or longer.
The appropriateness of initiating treatment with a full-agonist opioid was considered uncertain for a patient with limited prognosis and inappropriate for a patient with longer prognosis.
For a patient with cancer pain and no medical history of opioid use disorder but taking more opioids than prescribed, regardless of prognosis, the panel felt it was appropriate to increase monitoring and inappropriate to taper opioids. The panel was not certain about whether to increase opioids based on the patient’s account of what they need or transition to buprenorphine/naloxone.
For a patient with no history of opioid use disorder who was prescribed traditional opioids for pain and had a positive urine drug test for nonprescribed benzodiazepines, regardless of prognosis, the panel felt it was appropriate to continue opioids with close monitoring and inappropriate to taper opioids or transition to buprenorphine/naloxone.
The researchers said that improving education around buprenorphine and cancer pain management in the context of opioid use disorder or misuse is needed.
In a related editorial, two experts noted that the patients considered in this “important article” require considerable time and expertise from an interdisciplinary team.
“It is important that cancer centers establish and fund such teams mainly as a safety measure for these patients and also as a major contribution to the care of all patients with cancer,” wrote Joseph Arthur, MD, and Eduardo Bruera, MD, with the University of Texas MD Anderson Cancer Center, Houston.
In the wider context, Dr. Arthur and Dr. Bruera highlighted how treatments for patients with advanced cancer have evolved over the past 3 decades, yet patients have continued to be given opioids to address cancer-related pain. Developing more sophisticated drugs that relieve pain without significant side effects or addictive properties is imperative.
Dr. Arthur and Dr. Bruera said the study authors “appropriately emphasize the value of delivering compassionate and expert care for these particularly complex cases and the importance of conducting research on the best ways to alleviate the suffering in this rapidly growing patient population.”
This research was supported by Cambia Health Foundation and the National Institute of Nursing Research. Dr. Merlin, Dr. Arthur, and Dr. Bruera reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Patients with blood cancers underutilize palliative care
I used to attend the Supportive Care in Oncology Symposium every year, but to my dismay, the American Society for Clinical Oncology stopped hosting the symposium a few years ago. Instead, ASCO now incorporates palliative care research fully into its annual meeting which was held in early June in Chicago. Being integrated into the annual meeting means greater exposure to a broader audience that may not otherwise see this work. In this column, I highlight some presentations that stood out to me.
Palliative care studies for patients with hematologic malignancies
There continues to be low uptake of outpatient palliative care services among patients with hematologic malignancies. Fortunately, there are efforts underway to study the impact of integrating early palliative care into the routine care of hematology patients. In a study presented by Mazie Tsang, MD, a clinical fellow at the University of California, San Francisco, researchers embedded a palliative care nurse practitioner in a hematology clinic and studied the impact this single NP had over 4 years of integration. They found that patients were less likely to be hospitalized or visit the emergency department after integrating the NP. They also found that advance directives were more likely to be completed following NP integration. The results were limited by small sample size and lack of a true control group, but generally trended toward significance when compared with historical controls.
Other studies highlighted the relatively high symptom burden among patients with hematologic malignancies, such as myeloma, leukemia, and lymphoma. In a study presented by Sarah E. Monick, MD, of the University of Chicago, researchers found that, among adolescents and young adults with hematologic malignancies seen in a clinic where a palliative care provider was embedded, symptom burden was high across the board regardless of where patients were in their disease trajectory or their demographic characteristics. Due to the presence of high symptom burden among adolescents and young adults, the authors suggest that patients undergo screening at every visit and that supportive care be incorporated throughout the patient’s journey.
Kyle Fitzgibbon of the Princess Margaret Cancer Centre in Toronto shared details of an ongoing multicenter, randomized, controlled, phase 3 trial designed to evaluate the effect of a novel psychosocial/palliative care intervention for patients with acute leukemia hospitalized for induction chemotherapy. The intervention will consist of 8 weeks of psychological support as well as access to palliative care for physical symptoms. Participants will be randomized to receive either intervention or standard of care at the beginning of their hospitalization. Researchers plan to study the impact of the intervention on physical and psychological symptom severity, quality of life, and patient satisfaction at multiple time points. It will be exciting to see the results of this study given that there are very few research clinical trials examining early palliative care with patients who have hematologic malignancies.
Trends in palliative care integration with oncology care
One key trend that I am elated to see is the integration of palliative care throughout the entire patient journey. A secondary analysis of oncology practice data from the National Cancer Institute Community Oncology Research Program found that more than three-quarters of outpatient oncology practices surveyed in 2015 have integrated palliative care inpatient and outpatient services. 36% said they had an outpatient palliative care clinic. More availability of services typically translates to better access to care and improved outcomes for patients, so it is always nice to see these quality metrics continue to move in a positive direction. The analysis was presented by Tiffany M. Statler, PA, of Atrium Health Wake Forest Baptist, Winston Salem, N.C.
It turns out that patients are also advocating for integrated palliative care. A unique qualitative project brought together patient advocates from several countries to hold a moderated discussion about quality of life and treatment side effects. The advocates focused on the importance of maintaining independence with activities of daily living as a significant quality of life goal, particularly as treatments tend to cause cumulative mental and physical fatigue. They highlighted the importance of palliative care for helping achieve quality of life goals, especially in latter part of the disease trajectory. The project was presented by Paul Wheatley-Price, MD, of the Ottawa Hospital Cancer Centre, University of Ottawa.
In 2010, a study by Temel and colleagues was published, finding that patients with metastatic non–small cell lung cancer who received palliative care early had significant improvements in quality of life and mood as compared with patients who received standard care. It was a landmark study and is frequently cited. The Temel group reports on the planning process for a new randomized controlled trial of palliative care with metastatic lung cancer patients who have targetable mutations. With next generation sequencing of tumor tissue, many patients with metastatic lung cancer are identified at diagnosis as having a targetable mutation. As such, they may receive a targeted therapy as first-line treatment instead of traditional chemotherapy. This has lengthened survival considerably, but the disease remains incurable and ultimately fatal, and the trajectory can resemble a roller-coaster ride.
In this new randomized controlled trial, patients in the experimental arm will receive four monthly visits with a palliative care clinician who is specially trained to help patients manage the uncertainties of prolonged illness. The researchers plan to evaluate patients’ distress levels and prognostic awareness, as well as evidence of advance care planning in the chart.
And, a study presented by Roberto Enrique Ochoa Planchart, MD, of Chen Medical Centers, Miami, found that when primary care providers used declines in functional status as a trigger for referring advanced cancer patients to palliative care, those patients were less likely to be admitted to the hospital near the end of life, translating to an 86% cost savings. This study reiterated the importance of partnering with a patient’s nononcologic providers, that is, primary care and palliative care clinicians to improve outcomes at the end of life.
Use of technology in palliative care
Numerous studies were reported on innovative uses of technology for various functions relevant to palliative care. They included everything from capturing patient-reported outcomes through patient-facing smartphone apps, to using artificial intelligence and/or machine learning to build prognostication tools and to generate earlier referrals to palliative care. There were presentations on the use of online tools to assist with and document goals of care conversations.
As a clinician who is always looking for new ways to capture patient symptom information and motivate patients to engage in advance care planning, I am excited about the prospect of using some of these tools in real time.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I used to attend the Supportive Care in Oncology Symposium every year, but to my dismay, the American Society for Clinical Oncology stopped hosting the symposium a few years ago. Instead, ASCO now incorporates palliative care research fully into its annual meeting which was held in early June in Chicago. Being integrated into the annual meeting means greater exposure to a broader audience that may not otherwise see this work. In this column, I highlight some presentations that stood out to me.
Palliative care studies for patients with hematologic malignancies
There continues to be low uptake of outpatient palliative care services among patients with hematologic malignancies. Fortunately, there are efforts underway to study the impact of integrating early palliative care into the routine care of hematology patients. In a study presented by Mazie Tsang, MD, a clinical fellow at the University of California, San Francisco, researchers embedded a palliative care nurse practitioner in a hematology clinic and studied the impact this single NP had over 4 years of integration. They found that patients were less likely to be hospitalized or visit the emergency department after integrating the NP. They also found that advance directives were more likely to be completed following NP integration. The results were limited by small sample size and lack of a true control group, but generally trended toward significance when compared with historical controls.
Other studies highlighted the relatively high symptom burden among patients with hematologic malignancies, such as myeloma, leukemia, and lymphoma. In a study presented by Sarah E. Monick, MD, of the University of Chicago, researchers found that, among adolescents and young adults with hematologic malignancies seen in a clinic where a palliative care provider was embedded, symptom burden was high across the board regardless of where patients were in their disease trajectory or their demographic characteristics. Due to the presence of high symptom burden among adolescents and young adults, the authors suggest that patients undergo screening at every visit and that supportive care be incorporated throughout the patient’s journey.
Kyle Fitzgibbon of the Princess Margaret Cancer Centre in Toronto shared details of an ongoing multicenter, randomized, controlled, phase 3 trial designed to evaluate the effect of a novel psychosocial/palliative care intervention for patients with acute leukemia hospitalized for induction chemotherapy. The intervention will consist of 8 weeks of psychological support as well as access to palliative care for physical symptoms. Participants will be randomized to receive either intervention or standard of care at the beginning of their hospitalization. Researchers plan to study the impact of the intervention on physical and psychological symptom severity, quality of life, and patient satisfaction at multiple time points. It will be exciting to see the results of this study given that there are very few research clinical trials examining early palliative care with patients who have hematologic malignancies.
Trends in palliative care integration with oncology care
One key trend that I am elated to see is the integration of palliative care throughout the entire patient journey. A secondary analysis of oncology practice data from the National Cancer Institute Community Oncology Research Program found that more than three-quarters of outpatient oncology practices surveyed in 2015 have integrated palliative care inpatient and outpatient services. 36% said they had an outpatient palliative care clinic. More availability of services typically translates to better access to care and improved outcomes for patients, so it is always nice to see these quality metrics continue to move in a positive direction. The analysis was presented by Tiffany M. Statler, PA, of Atrium Health Wake Forest Baptist, Winston Salem, N.C.
It turns out that patients are also advocating for integrated palliative care. A unique qualitative project brought together patient advocates from several countries to hold a moderated discussion about quality of life and treatment side effects. The advocates focused on the importance of maintaining independence with activities of daily living as a significant quality of life goal, particularly as treatments tend to cause cumulative mental and physical fatigue. They highlighted the importance of palliative care for helping achieve quality of life goals, especially in latter part of the disease trajectory. The project was presented by Paul Wheatley-Price, MD, of the Ottawa Hospital Cancer Centre, University of Ottawa.
In 2010, a study by Temel and colleagues was published, finding that patients with metastatic non–small cell lung cancer who received palliative care early had significant improvements in quality of life and mood as compared with patients who received standard care. It was a landmark study and is frequently cited. The Temel group reports on the planning process for a new randomized controlled trial of palliative care with metastatic lung cancer patients who have targetable mutations. With next generation sequencing of tumor tissue, many patients with metastatic lung cancer are identified at diagnosis as having a targetable mutation. As such, they may receive a targeted therapy as first-line treatment instead of traditional chemotherapy. This has lengthened survival considerably, but the disease remains incurable and ultimately fatal, and the trajectory can resemble a roller-coaster ride.
In this new randomized controlled trial, patients in the experimental arm will receive four monthly visits with a palliative care clinician who is specially trained to help patients manage the uncertainties of prolonged illness. The researchers plan to evaluate patients’ distress levels and prognostic awareness, as well as evidence of advance care planning in the chart.
And, a study presented by Roberto Enrique Ochoa Planchart, MD, of Chen Medical Centers, Miami, found that when primary care providers used declines in functional status as a trigger for referring advanced cancer patients to palliative care, those patients were less likely to be admitted to the hospital near the end of life, translating to an 86% cost savings. This study reiterated the importance of partnering with a patient’s nononcologic providers, that is, primary care and palliative care clinicians to improve outcomes at the end of life.
Use of technology in palliative care
Numerous studies were reported on innovative uses of technology for various functions relevant to palliative care. They included everything from capturing patient-reported outcomes through patient-facing smartphone apps, to using artificial intelligence and/or machine learning to build prognostication tools and to generate earlier referrals to palliative care. There were presentations on the use of online tools to assist with and document goals of care conversations.
As a clinician who is always looking for new ways to capture patient symptom information and motivate patients to engage in advance care planning, I am excited about the prospect of using some of these tools in real time.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I used to attend the Supportive Care in Oncology Symposium every year, but to my dismay, the American Society for Clinical Oncology stopped hosting the symposium a few years ago. Instead, ASCO now incorporates palliative care research fully into its annual meeting which was held in early June in Chicago. Being integrated into the annual meeting means greater exposure to a broader audience that may not otherwise see this work. In this column, I highlight some presentations that stood out to me.
Palliative care studies for patients with hematologic malignancies
There continues to be low uptake of outpatient palliative care services among patients with hematologic malignancies. Fortunately, there are efforts underway to study the impact of integrating early palliative care into the routine care of hematology patients. In a study presented by Mazie Tsang, MD, a clinical fellow at the University of California, San Francisco, researchers embedded a palliative care nurse practitioner in a hematology clinic and studied the impact this single NP had over 4 years of integration. They found that patients were less likely to be hospitalized or visit the emergency department after integrating the NP. They also found that advance directives were more likely to be completed following NP integration. The results were limited by small sample size and lack of a true control group, but generally trended toward significance when compared with historical controls.
Other studies highlighted the relatively high symptom burden among patients with hematologic malignancies, such as myeloma, leukemia, and lymphoma. In a study presented by Sarah E. Monick, MD, of the University of Chicago, researchers found that, among adolescents and young adults with hematologic malignancies seen in a clinic where a palliative care provider was embedded, symptom burden was high across the board regardless of where patients were in their disease trajectory or their demographic characteristics. Due to the presence of high symptom burden among adolescents and young adults, the authors suggest that patients undergo screening at every visit and that supportive care be incorporated throughout the patient’s journey.
Kyle Fitzgibbon of the Princess Margaret Cancer Centre in Toronto shared details of an ongoing multicenter, randomized, controlled, phase 3 trial designed to evaluate the effect of a novel psychosocial/palliative care intervention for patients with acute leukemia hospitalized for induction chemotherapy. The intervention will consist of 8 weeks of psychological support as well as access to palliative care for physical symptoms. Participants will be randomized to receive either intervention or standard of care at the beginning of their hospitalization. Researchers plan to study the impact of the intervention on physical and psychological symptom severity, quality of life, and patient satisfaction at multiple time points. It will be exciting to see the results of this study given that there are very few research clinical trials examining early palliative care with patients who have hematologic malignancies.
Trends in palliative care integration with oncology care
One key trend that I am elated to see is the integration of palliative care throughout the entire patient journey. A secondary analysis of oncology practice data from the National Cancer Institute Community Oncology Research Program found that more than three-quarters of outpatient oncology practices surveyed in 2015 have integrated palliative care inpatient and outpatient services. 36% said they had an outpatient palliative care clinic. More availability of services typically translates to better access to care and improved outcomes for patients, so it is always nice to see these quality metrics continue to move in a positive direction. The analysis was presented by Tiffany M. Statler, PA, of Atrium Health Wake Forest Baptist, Winston Salem, N.C.
It turns out that patients are also advocating for integrated palliative care. A unique qualitative project brought together patient advocates from several countries to hold a moderated discussion about quality of life and treatment side effects. The advocates focused on the importance of maintaining independence with activities of daily living as a significant quality of life goal, particularly as treatments tend to cause cumulative mental and physical fatigue. They highlighted the importance of palliative care for helping achieve quality of life goals, especially in latter part of the disease trajectory. The project was presented by Paul Wheatley-Price, MD, of the Ottawa Hospital Cancer Centre, University of Ottawa.
In 2010, a study by Temel and colleagues was published, finding that patients with metastatic non–small cell lung cancer who received palliative care early had significant improvements in quality of life and mood as compared with patients who received standard care. It was a landmark study and is frequently cited. The Temel group reports on the planning process for a new randomized controlled trial of palliative care with metastatic lung cancer patients who have targetable mutations. With next generation sequencing of tumor tissue, many patients with metastatic lung cancer are identified at diagnosis as having a targetable mutation. As such, they may receive a targeted therapy as first-line treatment instead of traditional chemotherapy. This has lengthened survival considerably, but the disease remains incurable and ultimately fatal, and the trajectory can resemble a roller-coaster ride.
In this new randomized controlled trial, patients in the experimental arm will receive four monthly visits with a palliative care clinician who is specially trained to help patients manage the uncertainties of prolonged illness. The researchers plan to evaluate patients’ distress levels and prognostic awareness, as well as evidence of advance care planning in the chart.
And, a study presented by Roberto Enrique Ochoa Planchart, MD, of Chen Medical Centers, Miami, found that when primary care providers used declines in functional status as a trigger for referring advanced cancer patients to palliative care, those patients were less likely to be admitted to the hospital near the end of life, translating to an 86% cost savings. This study reiterated the importance of partnering with a patient’s nononcologic providers, that is, primary care and palliative care clinicians to improve outcomes at the end of life.
Use of technology in palliative care
Numerous studies were reported on innovative uses of technology for various functions relevant to palliative care. They included everything from capturing patient-reported outcomes through patient-facing smartphone apps, to using artificial intelligence and/or machine learning to build prognostication tools and to generate earlier referrals to palliative care. There were presentations on the use of online tools to assist with and document goals of care conversations.
As a clinician who is always looking for new ways to capture patient symptom information and motivate patients to engage in advance care planning, I am excited about the prospect of using some of these tools in real time.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
FROM ASCO 2022
Acetaminophen linked to diminished response to immunotherapy in cancer
The team found a strong association between the use of acetaminophen and a decreased response to immune checkpoint inhibitors in a study of three clinical cohorts involving more than 600 patients with advanced cancer.
Patients who took acetaminophen at the start of immunotherapy – with acetaminophen exposure confirmed by plasma testing – were found to have worse overall survival and progression-free survival than patients who did not take the analgesic. Multivariate analysis confirmed the association independent of other prognostic factors. “It is unlikely that our data are the result of bias or unmeasured confounding,” the authors comment.
The findings “present a compelling case for caution” in using acetaminophen in patients with cancer who are receiving immune checkpoint blockers, senior investigator Antoine Italiano, MD, PhD, a medical oncologist at the University of Bordeaux (France), and colleagues concluded.
The study was presented at the annual meeting of the American Society of Clinical Oncology and published simultaneously in Annals of Oncology.
“Patients with advanced cancer taking [acetaminophen] during immunotherapy experience worse clinical outcomes, which suggests that [acetaminophen] decreases T cell–mediated antitumor immunity,” the authors comment.
They also report bench research and blood studies in four healthy volunteers, which showed an up-regulation of immunosuppressive regulatory T cells (Tregs) with acetaminophen, and other findings that together suggest that acetaminophen undermines the antitumor immune processes by which checkpoint inhibitors work.
Reconsider acetaminophen pretreatment
After hearing Dr. Italiano present the results at the meeting, a Polish oncologist in the audience said he was concerned that his clinic premedicates with acetaminophen before immune checkpoint blockade and wanted to know if they should stop doing it.
“I don’t think inducing Tregs ... in cancer patients is a good approach. I do a lot of clinical trials,” and “I do not understand why in several cases sponsors required mandatory premedication with acetaminophen. I think ... we should reconsider this approach,” Dr. Italiano said.
There’s precedence for the findings. Acetaminophen – also known as paracetamol – has been shown in some studies to limit immune cell proliferation, T-cell–dependent antibody response, and viral clearance, among other things. After a randomized trial showing blunted responses to vaccines in individuals who were taking acetaminophen, the World Health Organization recommended in 2015 against concurrent use of acetaminophen with vaccines.
Steroids, antibiotics, and proton pump inhibitors have also recently been shown to worsen outcomes with pembrolizumab, noted invited discussant, Margaret Gatti-Mays, MD, a medical oncologist at Ohio State University, Columbus.
“We are starting to understand that ... commonly used medications may have a larger impact on the efficacy and toxicity of immune checkpoint blockade than historically seen with chemotherapy,” she said.
However, she expressed some uncertainty over the French findings, as she was concerned that even the multivariate analysis didn’t completely rule out that acetaminophen users had worse disease to begin with and so would be expected to have worse outcomes.
She was also unsure of how much acetaminophen is too much.
Acetaminophen has a half-life of around 3 hours or less, where the immune checkpoint inhibitors have a half-life of around 20 days or more.
Given that, Dr. Gatti-Mays wondered whether “a single dose of acetaminophen [is] enough to derail the benefit of checkpoint inhibition? Does exposure need to be continuous?”
She allowed that acetaminophen use may turn out to be one more of the many patient-level factors emerging lately – such as chronic stress, diet, body flora, and physiological age, among others – that might help explain why checkpoint inhibition works in only about 20% of eligible patients with cancer.
Study details
Dr. Italiano and his team analyzed plasma samples from 297 participants in the CheckMate 025 trial of nivolumab for renal cancer; 34 participants in the BIP study into actionable molecular alterations in cancer; and 297 participants in the PREMIS immune-related adverse events study. The patients in these last two studies had a variety of cancers and were taking various agents.
All 628 patients were on checkpoint inhibitors. The investigators divided them according to who had acetaminophen or its metabolite acetaminophen glucuronide in their plasma when they started checkpoint inhibition and those who did not.
In CheckMate 025, overall survival was significantly worse among participants who had detectable acetaminophen or its metabolite in plasma (hazard ratio, 0.67; P = .004).
None of the acetaminophen-positive participants in the BIP study responded to checkpoint blockade, compared with almost 30% of those who were negative. Acetaminophen-positive participants also trended toward worse progression-free survival (median, 1.87 vs. 4.72 months) and overall survival (median, 7.87 vs. 16.56 months).
In PREMIS, progression-free survival was a median of 2.63 months in the acetaminophen group versus 5.03 months in negative participants (P = .009); median overall survival was 8.43 months versus 14.93 months, respectively (P < .0001).
A multivariate analysis was performed in PREMIS. Acetaminophen exposure was associated with both progression-free survival (hazard ratio, 1.43; P =.015) and overall survival (HR, 1.78; P =.006) independently of performance status, liver metastases, bone metastases, number of metastases sites, tumor type, number of previous lines of treatment, steroid/antibiotic use, lactate dehydrogenase levels, and other factors.
There was no funding for the work. Dr. Italiano is a consultant for AstraZeneca, Bayer, Chugai, Deciphera, Merck, Parthenon, Roche, and Springworks, He also has grants from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, MSD, Novartis, Pharmamar, and Roche. Two authors work for Explicyte and one works for Amgen. Dr. Gatti-Mays is a consultant for Seattle Genetics.
A version of this article first appeared on Medscape.com.
The team found a strong association between the use of acetaminophen and a decreased response to immune checkpoint inhibitors in a study of three clinical cohorts involving more than 600 patients with advanced cancer.
Patients who took acetaminophen at the start of immunotherapy – with acetaminophen exposure confirmed by plasma testing – were found to have worse overall survival and progression-free survival than patients who did not take the analgesic. Multivariate analysis confirmed the association independent of other prognostic factors. “It is unlikely that our data are the result of bias or unmeasured confounding,” the authors comment.
The findings “present a compelling case for caution” in using acetaminophen in patients with cancer who are receiving immune checkpoint blockers, senior investigator Antoine Italiano, MD, PhD, a medical oncologist at the University of Bordeaux (France), and colleagues concluded.
The study was presented at the annual meeting of the American Society of Clinical Oncology and published simultaneously in Annals of Oncology.
“Patients with advanced cancer taking [acetaminophen] during immunotherapy experience worse clinical outcomes, which suggests that [acetaminophen] decreases T cell–mediated antitumor immunity,” the authors comment.
They also report bench research and blood studies in four healthy volunteers, which showed an up-regulation of immunosuppressive regulatory T cells (Tregs) with acetaminophen, and other findings that together suggest that acetaminophen undermines the antitumor immune processes by which checkpoint inhibitors work.
Reconsider acetaminophen pretreatment
After hearing Dr. Italiano present the results at the meeting, a Polish oncologist in the audience said he was concerned that his clinic premedicates with acetaminophen before immune checkpoint blockade and wanted to know if they should stop doing it.
“I don’t think inducing Tregs ... in cancer patients is a good approach. I do a lot of clinical trials,” and “I do not understand why in several cases sponsors required mandatory premedication with acetaminophen. I think ... we should reconsider this approach,” Dr. Italiano said.
There’s precedence for the findings. Acetaminophen – also known as paracetamol – has been shown in some studies to limit immune cell proliferation, T-cell–dependent antibody response, and viral clearance, among other things. After a randomized trial showing blunted responses to vaccines in individuals who were taking acetaminophen, the World Health Organization recommended in 2015 against concurrent use of acetaminophen with vaccines.
Steroids, antibiotics, and proton pump inhibitors have also recently been shown to worsen outcomes with pembrolizumab, noted invited discussant, Margaret Gatti-Mays, MD, a medical oncologist at Ohio State University, Columbus.
“We are starting to understand that ... commonly used medications may have a larger impact on the efficacy and toxicity of immune checkpoint blockade than historically seen with chemotherapy,” she said.
However, she expressed some uncertainty over the French findings, as she was concerned that even the multivariate analysis didn’t completely rule out that acetaminophen users had worse disease to begin with and so would be expected to have worse outcomes.
She was also unsure of how much acetaminophen is too much.
Acetaminophen has a half-life of around 3 hours or less, where the immune checkpoint inhibitors have a half-life of around 20 days or more.
Given that, Dr. Gatti-Mays wondered whether “a single dose of acetaminophen [is] enough to derail the benefit of checkpoint inhibition? Does exposure need to be continuous?”
She allowed that acetaminophen use may turn out to be one more of the many patient-level factors emerging lately – such as chronic stress, diet, body flora, and physiological age, among others – that might help explain why checkpoint inhibition works in only about 20% of eligible patients with cancer.
Study details
Dr. Italiano and his team analyzed plasma samples from 297 participants in the CheckMate 025 trial of nivolumab for renal cancer; 34 participants in the BIP study into actionable molecular alterations in cancer; and 297 participants in the PREMIS immune-related adverse events study. The patients in these last two studies had a variety of cancers and were taking various agents.
All 628 patients were on checkpoint inhibitors. The investigators divided them according to who had acetaminophen or its metabolite acetaminophen glucuronide in their plasma when they started checkpoint inhibition and those who did not.
In CheckMate 025, overall survival was significantly worse among participants who had detectable acetaminophen or its metabolite in plasma (hazard ratio, 0.67; P = .004).
None of the acetaminophen-positive participants in the BIP study responded to checkpoint blockade, compared with almost 30% of those who were negative. Acetaminophen-positive participants also trended toward worse progression-free survival (median, 1.87 vs. 4.72 months) and overall survival (median, 7.87 vs. 16.56 months).
In PREMIS, progression-free survival was a median of 2.63 months in the acetaminophen group versus 5.03 months in negative participants (P = .009); median overall survival was 8.43 months versus 14.93 months, respectively (P < .0001).
A multivariate analysis was performed in PREMIS. Acetaminophen exposure was associated with both progression-free survival (hazard ratio, 1.43; P =.015) and overall survival (HR, 1.78; P =.006) independently of performance status, liver metastases, bone metastases, number of metastases sites, tumor type, number of previous lines of treatment, steroid/antibiotic use, lactate dehydrogenase levels, and other factors.
There was no funding for the work. Dr. Italiano is a consultant for AstraZeneca, Bayer, Chugai, Deciphera, Merck, Parthenon, Roche, and Springworks, He also has grants from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, MSD, Novartis, Pharmamar, and Roche. Two authors work for Explicyte and one works for Amgen. Dr. Gatti-Mays is a consultant for Seattle Genetics.
A version of this article first appeared on Medscape.com.
The team found a strong association between the use of acetaminophen and a decreased response to immune checkpoint inhibitors in a study of three clinical cohorts involving more than 600 patients with advanced cancer.
Patients who took acetaminophen at the start of immunotherapy – with acetaminophen exposure confirmed by plasma testing – were found to have worse overall survival and progression-free survival than patients who did not take the analgesic. Multivariate analysis confirmed the association independent of other prognostic factors. “It is unlikely that our data are the result of bias or unmeasured confounding,” the authors comment.
The findings “present a compelling case for caution” in using acetaminophen in patients with cancer who are receiving immune checkpoint blockers, senior investigator Antoine Italiano, MD, PhD, a medical oncologist at the University of Bordeaux (France), and colleagues concluded.
The study was presented at the annual meeting of the American Society of Clinical Oncology and published simultaneously in Annals of Oncology.
“Patients with advanced cancer taking [acetaminophen] during immunotherapy experience worse clinical outcomes, which suggests that [acetaminophen] decreases T cell–mediated antitumor immunity,” the authors comment.
They also report bench research and blood studies in four healthy volunteers, which showed an up-regulation of immunosuppressive regulatory T cells (Tregs) with acetaminophen, and other findings that together suggest that acetaminophen undermines the antitumor immune processes by which checkpoint inhibitors work.
Reconsider acetaminophen pretreatment
After hearing Dr. Italiano present the results at the meeting, a Polish oncologist in the audience said he was concerned that his clinic premedicates with acetaminophen before immune checkpoint blockade and wanted to know if they should stop doing it.
“I don’t think inducing Tregs ... in cancer patients is a good approach. I do a lot of clinical trials,” and “I do not understand why in several cases sponsors required mandatory premedication with acetaminophen. I think ... we should reconsider this approach,” Dr. Italiano said.
There’s precedence for the findings. Acetaminophen – also known as paracetamol – has been shown in some studies to limit immune cell proliferation, T-cell–dependent antibody response, and viral clearance, among other things. After a randomized trial showing blunted responses to vaccines in individuals who were taking acetaminophen, the World Health Organization recommended in 2015 against concurrent use of acetaminophen with vaccines.
Steroids, antibiotics, and proton pump inhibitors have also recently been shown to worsen outcomes with pembrolizumab, noted invited discussant, Margaret Gatti-Mays, MD, a medical oncologist at Ohio State University, Columbus.
“We are starting to understand that ... commonly used medications may have a larger impact on the efficacy and toxicity of immune checkpoint blockade than historically seen with chemotherapy,” she said.
However, she expressed some uncertainty over the French findings, as she was concerned that even the multivariate analysis didn’t completely rule out that acetaminophen users had worse disease to begin with and so would be expected to have worse outcomes.
She was also unsure of how much acetaminophen is too much.
Acetaminophen has a half-life of around 3 hours or less, where the immune checkpoint inhibitors have a half-life of around 20 days or more.
Given that, Dr. Gatti-Mays wondered whether “a single dose of acetaminophen [is] enough to derail the benefit of checkpoint inhibition? Does exposure need to be continuous?”
She allowed that acetaminophen use may turn out to be one more of the many patient-level factors emerging lately – such as chronic stress, diet, body flora, and physiological age, among others – that might help explain why checkpoint inhibition works in only about 20% of eligible patients with cancer.
Study details
Dr. Italiano and his team analyzed plasma samples from 297 participants in the CheckMate 025 trial of nivolumab for renal cancer; 34 participants in the BIP study into actionable molecular alterations in cancer; and 297 participants in the PREMIS immune-related adverse events study. The patients in these last two studies had a variety of cancers and were taking various agents.
All 628 patients were on checkpoint inhibitors. The investigators divided them according to who had acetaminophen or its metabolite acetaminophen glucuronide in their plasma when they started checkpoint inhibition and those who did not.
In CheckMate 025, overall survival was significantly worse among participants who had detectable acetaminophen or its metabolite in plasma (hazard ratio, 0.67; P = .004).
None of the acetaminophen-positive participants in the BIP study responded to checkpoint blockade, compared with almost 30% of those who were negative. Acetaminophen-positive participants also trended toward worse progression-free survival (median, 1.87 vs. 4.72 months) and overall survival (median, 7.87 vs. 16.56 months).
In PREMIS, progression-free survival was a median of 2.63 months in the acetaminophen group versus 5.03 months in negative participants (P = .009); median overall survival was 8.43 months versus 14.93 months, respectively (P < .0001).
A multivariate analysis was performed in PREMIS. Acetaminophen exposure was associated with both progression-free survival (hazard ratio, 1.43; P =.015) and overall survival (HR, 1.78; P =.006) independently of performance status, liver metastases, bone metastases, number of metastases sites, tumor type, number of previous lines of treatment, steroid/antibiotic use, lactate dehydrogenase levels, and other factors.
There was no funding for the work. Dr. Italiano is a consultant for AstraZeneca, Bayer, Chugai, Deciphera, Merck, Parthenon, Roche, and Springworks, He also has grants from AstraZeneca, Bayer, Bristol-Myers Squibb, Merck, MSD, Novartis, Pharmamar, and Roche. Two authors work for Explicyte and one works for Amgen. Dr. Gatti-Mays is a consultant for Seattle Genetics.
A version of this article first appeared on Medscape.com.
FROM ASCO 2022
Time-restricted eating may reduce CVD risk after breast cancer
, a single-group feasibility study suggests.
The results show a 15% relative decline in cardiovascular risk, measured using the Framingham Risk Score, among at-risk breast cancer survivors (BCS) after only 8 weeks of following a time-restricted eating regimen, reported Amy A. Kirkham, PhD, assistant professor of kinesiology and physical education, University of Toronto, and colleagues.
“Time-restricted eating also significantly decreased visceral adipose tissue (VAT), which our team has previously found to accumulate rapidly with cardiotoxic treatment and predict later cardiac events among BCS,” the researchers add.
The findings were published online in the Journal of the American College of Cardiology: Cardiac Onco.
Physical activity is one of the main modalities for lowering cardiovascular risk, but it is not feasible for everyone because of physical limitations and other factors, noted Dr. Kirkham.
“I became interested in time-restricted eating when I came across the literature, which has really exploded in the last 5 years, showing that it can reduce the number of cardiovascular risk factors,” she said in an interview.
“However, most of these populations studied have had cardiometabolic conditions, like obesity, type 2 diabetes, prediabetes, and metabolic syndrome, and no one has looked at this” in either the population specifically at high risk for cardiovascular disease or in patients with overt cardiovascular disease, she said.
This approach is easy for patients to follow and is much simpler than many of the other dietary patterns, noted Dr. Kirkham. “It simply consists of having a start time or end time to your eating, so it is easy to prescribe,” she said. “You can see how that is much easier for a doctor to explain to a patient than trying to explain how to meet the physical activity guidelines each week.”
“This particular study definitely shows that time-restricted eating can decrease the calorie intake, and I think by decreasing the calorie intake you definitely would improve the body weight, which has numerous benefits irrespective of how we arrive at the end goal which is including the cardiovascular risk factors,” said Ajay Vallakati, MBBS, physician and clinical assistant professor of internal medicine, the Ohio State University, Columbus, commenting on the study.
“I think time-restricted eating is a tool we should look at, and a bigger study would help us to recommend this for our patients,” Dr. Vallakati told this news organization.
The study involved 22 participants. Mean age was 66 years. Mean body mass index was 31 ± 5 kg/m². In the cohort, 91% of participants were taking aromatase inhibitors and tamoxifen at the time of the study, and 50% underwent left-sided radiation.
The study group included breast cancer survivors who had risk factors for cardiovascular disease mortality, including completion of cardiotoxic therapy, like anthracyclines, within 1-6 years, obesity/overweight, and older age, defined as 60 years of age or older.
Participants were allowed to eat freely between 12 PM and 8 PM on weekdays and any time during weekends. Outside of the allotted hours, they could only drink black coffee, water, or black tea for the 8-week study period. They were not under any other physical activity or dietary restrictions.
All were provided with behavioral support, such as check-in phone calls with the research team at 1-, 3-, and 6-week follow-up and pre-interventional calls from a registered dietitian. During weekdays, they also received automated text messages twice a day asking what time they started and stopped eating.
Irritability and headaches were among the transient, minor symptoms reported, the researchers say. The study group responded to nearly all of the text messages that they received from the researchers. The participants also followed through with the fast for a median 98% of the prescribed days by fasting for 16 or more hours.
The results showed that after 8 weeks, median Framingham cardiovascular risk declined from 10.9% to 8.6%, a 15% relative reduction (P = .037). Modifiable aspects of Framingham, such as systolic blood pressure, total cholesterol, and high-density lipoprotein, remained relatively consistent overall, however, suggesting variation between individuals in the etiology of the risk decline.
Caloric intake fell by a median of 450 kcal, representing a relative reduction of about 22% (P < .001), they note.
The findings also showed a decline in median derived whole-body fat mass (–0.9 kg; P = .046), body mass (–1.0 kg; P = .025), and mean MRI-derived VAT (–5%; P = .009).
Other data showed that the average BMI remained the same (P = .10).
At the beginning of the study, 68% of the cohort was considered cardiometabolically unhealthy, given the benchmarks for pharmacologic preventive therapy of cardiovascular risk or metabolic syndrome based on Canadian Cardiovascular Society recommendations.
Notably, 53% of the cohort was no longer classified as meeting the criteria for metabolic syndrome or for the therapeutic treatment of cardiovascular risk after the intervention.
The study’s limitations include its short duration, selection bias, and that it did not involve a control group, the researchers acknowledge.
“Randomized controlled trials are needed to confirm these findings and to evaluate the health benefits, including potential health care cost savings and safety of longer-term time-restricted eating,” the researchers conclude.
Dr. Vallakati and Dr. Kirkham report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, a single-group feasibility study suggests.
The results show a 15% relative decline in cardiovascular risk, measured using the Framingham Risk Score, among at-risk breast cancer survivors (BCS) after only 8 weeks of following a time-restricted eating regimen, reported Amy A. Kirkham, PhD, assistant professor of kinesiology and physical education, University of Toronto, and colleagues.
“Time-restricted eating also significantly decreased visceral adipose tissue (VAT), which our team has previously found to accumulate rapidly with cardiotoxic treatment and predict later cardiac events among BCS,” the researchers add.
The findings were published online in the Journal of the American College of Cardiology: Cardiac Onco.
Physical activity is one of the main modalities for lowering cardiovascular risk, but it is not feasible for everyone because of physical limitations and other factors, noted Dr. Kirkham.
“I became interested in time-restricted eating when I came across the literature, which has really exploded in the last 5 years, showing that it can reduce the number of cardiovascular risk factors,” she said in an interview.
“However, most of these populations studied have had cardiometabolic conditions, like obesity, type 2 diabetes, prediabetes, and metabolic syndrome, and no one has looked at this” in either the population specifically at high risk for cardiovascular disease or in patients with overt cardiovascular disease, she said.
This approach is easy for patients to follow and is much simpler than many of the other dietary patterns, noted Dr. Kirkham. “It simply consists of having a start time or end time to your eating, so it is easy to prescribe,” she said. “You can see how that is much easier for a doctor to explain to a patient than trying to explain how to meet the physical activity guidelines each week.”
“This particular study definitely shows that time-restricted eating can decrease the calorie intake, and I think by decreasing the calorie intake you definitely would improve the body weight, which has numerous benefits irrespective of how we arrive at the end goal which is including the cardiovascular risk factors,” said Ajay Vallakati, MBBS, physician and clinical assistant professor of internal medicine, the Ohio State University, Columbus, commenting on the study.
“I think time-restricted eating is a tool we should look at, and a bigger study would help us to recommend this for our patients,” Dr. Vallakati told this news organization.
The study involved 22 participants. Mean age was 66 years. Mean body mass index was 31 ± 5 kg/m². In the cohort, 91% of participants were taking aromatase inhibitors and tamoxifen at the time of the study, and 50% underwent left-sided radiation.
The study group included breast cancer survivors who had risk factors for cardiovascular disease mortality, including completion of cardiotoxic therapy, like anthracyclines, within 1-6 years, obesity/overweight, and older age, defined as 60 years of age or older.
Participants were allowed to eat freely between 12 PM and 8 PM on weekdays and any time during weekends. Outside of the allotted hours, they could only drink black coffee, water, or black tea for the 8-week study period. They were not under any other physical activity or dietary restrictions.
All were provided with behavioral support, such as check-in phone calls with the research team at 1-, 3-, and 6-week follow-up and pre-interventional calls from a registered dietitian. During weekdays, they also received automated text messages twice a day asking what time they started and stopped eating.
Irritability and headaches were among the transient, minor symptoms reported, the researchers say. The study group responded to nearly all of the text messages that they received from the researchers. The participants also followed through with the fast for a median 98% of the prescribed days by fasting for 16 or more hours.
The results showed that after 8 weeks, median Framingham cardiovascular risk declined from 10.9% to 8.6%, a 15% relative reduction (P = .037). Modifiable aspects of Framingham, such as systolic blood pressure, total cholesterol, and high-density lipoprotein, remained relatively consistent overall, however, suggesting variation between individuals in the etiology of the risk decline.
Caloric intake fell by a median of 450 kcal, representing a relative reduction of about 22% (P < .001), they note.
The findings also showed a decline in median derived whole-body fat mass (–0.9 kg; P = .046), body mass (–1.0 kg; P = .025), and mean MRI-derived VAT (–5%; P = .009).
Other data showed that the average BMI remained the same (P = .10).
At the beginning of the study, 68% of the cohort was considered cardiometabolically unhealthy, given the benchmarks for pharmacologic preventive therapy of cardiovascular risk or metabolic syndrome based on Canadian Cardiovascular Society recommendations.
Notably, 53% of the cohort was no longer classified as meeting the criteria for metabolic syndrome or for the therapeutic treatment of cardiovascular risk after the intervention.
The study’s limitations include its short duration, selection bias, and that it did not involve a control group, the researchers acknowledge.
“Randomized controlled trials are needed to confirm these findings and to evaluate the health benefits, including potential health care cost savings and safety of longer-term time-restricted eating,” the researchers conclude.
Dr. Vallakati and Dr. Kirkham report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, a single-group feasibility study suggests.
The results show a 15% relative decline in cardiovascular risk, measured using the Framingham Risk Score, among at-risk breast cancer survivors (BCS) after only 8 weeks of following a time-restricted eating regimen, reported Amy A. Kirkham, PhD, assistant professor of kinesiology and physical education, University of Toronto, and colleagues.
“Time-restricted eating also significantly decreased visceral adipose tissue (VAT), which our team has previously found to accumulate rapidly with cardiotoxic treatment and predict later cardiac events among BCS,” the researchers add.
The findings were published online in the Journal of the American College of Cardiology: Cardiac Onco.
Physical activity is one of the main modalities for lowering cardiovascular risk, but it is not feasible for everyone because of physical limitations and other factors, noted Dr. Kirkham.
“I became interested in time-restricted eating when I came across the literature, which has really exploded in the last 5 years, showing that it can reduce the number of cardiovascular risk factors,” she said in an interview.
“However, most of these populations studied have had cardiometabolic conditions, like obesity, type 2 diabetes, prediabetes, and metabolic syndrome, and no one has looked at this” in either the population specifically at high risk for cardiovascular disease or in patients with overt cardiovascular disease, she said.
This approach is easy for patients to follow and is much simpler than many of the other dietary patterns, noted Dr. Kirkham. “It simply consists of having a start time or end time to your eating, so it is easy to prescribe,” she said. “You can see how that is much easier for a doctor to explain to a patient than trying to explain how to meet the physical activity guidelines each week.”
“This particular study definitely shows that time-restricted eating can decrease the calorie intake, and I think by decreasing the calorie intake you definitely would improve the body weight, which has numerous benefits irrespective of how we arrive at the end goal which is including the cardiovascular risk factors,” said Ajay Vallakati, MBBS, physician and clinical assistant professor of internal medicine, the Ohio State University, Columbus, commenting on the study.
“I think time-restricted eating is a tool we should look at, and a bigger study would help us to recommend this for our patients,” Dr. Vallakati told this news organization.
The study involved 22 participants. Mean age was 66 years. Mean body mass index was 31 ± 5 kg/m². In the cohort, 91% of participants were taking aromatase inhibitors and tamoxifen at the time of the study, and 50% underwent left-sided radiation.
The study group included breast cancer survivors who had risk factors for cardiovascular disease mortality, including completion of cardiotoxic therapy, like anthracyclines, within 1-6 years, obesity/overweight, and older age, defined as 60 years of age or older.
Participants were allowed to eat freely between 12 PM and 8 PM on weekdays and any time during weekends. Outside of the allotted hours, they could only drink black coffee, water, or black tea for the 8-week study period. They were not under any other physical activity or dietary restrictions.
All were provided with behavioral support, such as check-in phone calls with the research team at 1-, 3-, and 6-week follow-up and pre-interventional calls from a registered dietitian. During weekdays, they also received automated text messages twice a day asking what time they started and stopped eating.
Irritability and headaches were among the transient, minor symptoms reported, the researchers say. The study group responded to nearly all of the text messages that they received from the researchers. The participants also followed through with the fast for a median 98% of the prescribed days by fasting for 16 or more hours.
The results showed that after 8 weeks, median Framingham cardiovascular risk declined from 10.9% to 8.6%, a 15% relative reduction (P = .037). Modifiable aspects of Framingham, such as systolic blood pressure, total cholesterol, and high-density lipoprotein, remained relatively consistent overall, however, suggesting variation between individuals in the etiology of the risk decline.
Caloric intake fell by a median of 450 kcal, representing a relative reduction of about 22% (P < .001), they note.
The findings also showed a decline in median derived whole-body fat mass (–0.9 kg; P = .046), body mass (–1.0 kg; P = .025), and mean MRI-derived VAT (–5%; P = .009).
Other data showed that the average BMI remained the same (P = .10).
At the beginning of the study, 68% of the cohort was considered cardiometabolically unhealthy, given the benchmarks for pharmacologic preventive therapy of cardiovascular risk or metabolic syndrome based on Canadian Cardiovascular Society recommendations.
Notably, 53% of the cohort was no longer classified as meeting the criteria for metabolic syndrome or for the therapeutic treatment of cardiovascular risk after the intervention.
The study’s limitations include its short duration, selection bias, and that it did not involve a control group, the researchers acknowledge.
“Randomized controlled trials are needed to confirm these findings and to evaluate the health benefits, including potential health care cost savings and safety of longer-term time-restricted eating,” the researchers conclude.
Dr. Vallakati and Dr. Kirkham report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY: CARDIAC ONCO
How to manage drug interactions with Paxlovid for COVID-19
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cancer patients unaware of their increased thrombosis risk
It is up to their physician to discuss this with them.
This link is explained by the authors of an article in Cancer Treatment and Research Communications that reports results of a survey carried out by the European Cancer Patient Coalition (ECPC). “The aim of this pan-European patient survey was to assess patient awareness and knowledge about cancer-associated thrombosis (CAT), including risk factors, signs and symptoms, and interventions, to better prevent and treat CAT,” write the authors. “The idea was to create a sort of starting point for subsequent communication and information strategies and for comparing the results of any action taken in this area,” they add.
A roundtable discussion that included oncology healthcare professionals, policymakers, and patient advocates was convened to discuss and review the evidence regarding their ongoing concerns of excessive CAT-associated morbidity and mortality, as well as patients’ desire for greater CAT awareness.
“These discussions demonstrated that very little change had occurred over the years and that greater knowledge about CAT was still needed across the spectrum of healthcare practitioners and patients, particularly regarding primary and secondary prevention of thrombosis,” the authors write.
It was from this starting point that the idea for the pan-European survey was born. The ECPC, widely viewed as the “unified voice of cancer patients across Europe,” led the survey. This survey spanned six countries (France, Germany, Greece, Italy, United Kingdom, and Spain) and involved 1,365 patients and caregivers. The ECPC survey result was originally released at World Thrombosis Day in late 2018.
In an interview, Anna Falanga, MD, the main author of the article and professor of hematology at the University of Milan-Bicocca, Italy, reviewed the results and explained how to improve knowledge of CAT among patients with cancer.
“Data support that up to 20% of patients with cancer will experience venous thromboembolism (VTE), which is approximately 4–5 times higher than the general population,” said Dr. Falanga, who is also chief of the department of immunohematology and transfusion medicine and the Thrombosis and Hemostasis Center at the Hospital Papa Giovanni XXIII, in Bergamo, Italy.
“We have known about the link between thrombosis and cancer since the 19th century, but it has taken until midway through the last century for our level of understanding and awareness of the problem to reach its current level. Initially, this was limited to fundamental research, with large advances in our understanding of the mechanisms of the link between the two; it has only been more recently that we have had clinical studies that have piqued the interest of healthcare professionals, who were previously uninterested in the topic,” she said.
Poor understanding
One piece of data stands out from the European survey: Nearly three quarters of respondents (72%) said that before taking part in the survey, they were not aware that people with cancer have a higher-than-normal risk of developing thrombosis. “We asked participants to rate their overall understanding of CAT on a scale of 1 (low) to 10 (high), with the average (mean) score obtained being 4.1. Only 21% of patients gave a rating of 7 or above (high understanding). The average rating was very similar in the different countries surveyed,” write the authors. They note that the survey also assessed how much participants had learned about the topic from their physicians.
Approximately 35% of patients were made aware of CAT either immediately before or at the time of their cancer diagnosis. Of particular concern, one quarter (26%) of respondents (the largest proportion) noted that they first became aware of CAT when they suffered a blood clot. The average rating was very similar in the different countries surveyed. “Let us not forget that cancer and cancer treatments themselves cause a number of side effects, some of which can be very serious, so in some ways, a clot might be seen as a minor problem. Yet, in reality, it isn’t. It is a significant cause of death and disease in cancer patients,” said Dr. Falanga.
When discussing prevention, most respondents (87%) said they were aware that taking a walk could reduce their risk. Slightly fewer were aware that stopping smoking could reduce their risk (75%), and even fewer were aware that keeping hydrated (63%) and stretching their legs (55%) could reduce their risk.
Symptoms of CAT appeared to be relatively well known; 73% of survey participants indicated that they were aware that swelling in the foot, ankle, or leg could be a sign of DVT, and 71% indicated that shortness of breath could be a sign of pulmonary embolism (PE). “Other symptoms, however, were less well known, with just over half (57%) of participants being aware that pain, cramping, and tenderness could be a sign of DVT. About one third (33%) knew that irregular heartbeat could be a sign of PE. These results varied between countries,” according to the authors.
The survey highlighted that just over a third of respondents said that they were currently using anticoagulants, although almost all (96%) knew that anticoagulants could be used to effectively treat thrombosis. Only 41% of those using anticoagulants said they had been told about any possible side effects.
The Italian situation
The report containing the full results of the European survey goes even further, since, in addition to its overall results, it also gives information about individual countries.
The data from Italy, which are based on 246 persons, show that only 27% of patients and caregivers were aware of the increased risk of thrombosis after a cancer diagnosis. This figure is in line with the overall results of the survey, although the average score of the 10-point scale was lower for the Italy cohort (3.3/10 vs 4.1/10).
The results are more variable in terms of knowledge of risk factors. Most respondents (89%) said that they were aware of the risks related to inactivity. Just over half (52%), however, said that they were aware of the risks related to radiotherapy. Nevertheless, 75% of participants knew about the risks relating to cancer surgery and chemotherapy. “To all intents and purposes, all types of cancer drug can significantly affect the risk of developing a clot. And this is also the case for more modern types of treatment, such as immunotherapy,” said Dr. Falanga.
Most respondents reported that they got information about cancer-associated thrombosis verbally, usually from their hospital doctor (11%). Some respondents (6%) said that they found out about it from their own research, usually online. Almost 1 in 4 patients (24%) in Italy said that they first became aware of CAT when they suffered a blood clot. Answers to questions about knowledge of symptoms show that 58% of Italian patients and caregivers know that swelling of the lower limbs can be a symptom of DVT, and the same percentage knows that shortness of breath might indicate PE.
In terms of preventive action, the picture in Italy is somewhat variable: 74% of participants were aware of the importance of walking, but far fewer knew about the need to stop smoking (57%) and stretch the legs (35%). Of the 41% of Italians who were also taking an anticoagulant drug, 53% said that they knew about the possible side effects of such medication.
Which way forward?
“The high rate of CAT suggests that, despite the clinical evidence and clear guideline recommendations for patients with cancer, CAT prevention and recognition remain low among healthcare professionals,” the authors write.
The results of the ECPC survey further confirm those of previous studies, highlighting patients’ lack of knowledge about CAT and the need for more in-depth discussions between physician and patient.
So, what can be done? As highlighted by previous studies, “patients’ experiences are an education in themselves, particularly for the oncology care team,” the authors write. “Once the patient has a thrombosis, the opportunity for thrombosis prevention, which should be the most crucial focus of the care clinics (surgical, oncology, and palliative care), is gone,” they add.
“Oncology professionals, as well as other members of the patient’s care team (eg, internists, surgeons, nurses), need to perform better, at every stage of the patient’s cancer pathway, to ensure patients are aware of CAT and their individual risk to develop a blood clot,” said Dr. Falanga. She explained that in this group, it is the general practitioner who is the first contact. “These professionals are on the front line of the battle; they are among the first healthcare workers given the chance to suspect a clot and should, therefore, be fully aware of the increased risk in oncology patients,” she reiterated.
Experts agree on the fact that a multidisciplinary approach is of utmost importance in this context: the different roles in the team must be clear. “It is also fundamental to establish who does what in terms of educating and informing the patient,” said Dr. Falanga.
The researchers also put forward an example of a successful initiative: the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTE-PACC) program. The initiative was developed by experts from the University of Vermont and was described in a recent article in JCO Oncology Practice.
Numerous resources are available online to help physicians talk to their patients and explain the risks linked to CAT along the continuum of cancer care. Among them is a resource titled, “Cancer Associated Thrombosis (CAT): Be Clot Conscious,” which can be found on the ECPC’s website.
“We have a collective responsibility using the ECPC patient survey as a baseline to inform patients with cancer on how to identify signs and symptoms of CAT to enable faster diagnosis and treatment,” the authors conclude.
This article was translated from Univadis Italy.
It is up to their physician to discuss this with them.
This link is explained by the authors of an article in Cancer Treatment and Research Communications that reports results of a survey carried out by the European Cancer Patient Coalition (ECPC). “The aim of this pan-European patient survey was to assess patient awareness and knowledge about cancer-associated thrombosis (CAT), including risk factors, signs and symptoms, and interventions, to better prevent and treat CAT,” write the authors. “The idea was to create a sort of starting point for subsequent communication and information strategies and for comparing the results of any action taken in this area,” they add.
A roundtable discussion that included oncology healthcare professionals, policymakers, and patient advocates was convened to discuss and review the evidence regarding their ongoing concerns of excessive CAT-associated morbidity and mortality, as well as patients’ desire for greater CAT awareness.
“These discussions demonstrated that very little change had occurred over the years and that greater knowledge about CAT was still needed across the spectrum of healthcare practitioners and patients, particularly regarding primary and secondary prevention of thrombosis,” the authors write.
It was from this starting point that the idea for the pan-European survey was born. The ECPC, widely viewed as the “unified voice of cancer patients across Europe,” led the survey. This survey spanned six countries (France, Germany, Greece, Italy, United Kingdom, and Spain) and involved 1,365 patients and caregivers. The ECPC survey result was originally released at World Thrombosis Day in late 2018.
In an interview, Anna Falanga, MD, the main author of the article and professor of hematology at the University of Milan-Bicocca, Italy, reviewed the results and explained how to improve knowledge of CAT among patients with cancer.
“Data support that up to 20% of patients with cancer will experience venous thromboembolism (VTE), which is approximately 4–5 times higher than the general population,” said Dr. Falanga, who is also chief of the department of immunohematology and transfusion medicine and the Thrombosis and Hemostasis Center at the Hospital Papa Giovanni XXIII, in Bergamo, Italy.
“We have known about the link between thrombosis and cancer since the 19th century, but it has taken until midway through the last century for our level of understanding and awareness of the problem to reach its current level. Initially, this was limited to fundamental research, with large advances in our understanding of the mechanisms of the link between the two; it has only been more recently that we have had clinical studies that have piqued the interest of healthcare professionals, who were previously uninterested in the topic,” she said.
Poor understanding
One piece of data stands out from the European survey: Nearly three quarters of respondents (72%) said that before taking part in the survey, they were not aware that people with cancer have a higher-than-normal risk of developing thrombosis. “We asked participants to rate their overall understanding of CAT on a scale of 1 (low) to 10 (high), with the average (mean) score obtained being 4.1. Only 21% of patients gave a rating of 7 or above (high understanding). The average rating was very similar in the different countries surveyed,” write the authors. They note that the survey also assessed how much participants had learned about the topic from their physicians.
Approximately 35% of patients were made aware of CAT either immediately before or at the time of their cancer diagnosis. Of particular concern, one quarter (26%) of respondents (the largest proportion) noted that they first became aware of CAT when they suffered a blood clot. The average rating was very similar in the different countries surveyed. “Let us not forget that cancer and cancer treatments themselves cause a number of side effects, some of which can be very serious, so in some ways, a clot might be seen as a minor problem. Yet, in reality, it isn’t. It is a significant cause of death and disease in cancer patients,” said Dr. Falanga.
When discussing prevention, most respondents (87%) said they were aware that taking a walk could reduce their risk. Slightly fewer were aware that stopping smoking could reduce their risk (75%), and even fewer were aware that keeping hydrated (63%) and stretching their legs (55%) could reduce their risk.
Symptoms of CAT appeared to be relatively well known; 73% of survey participants indicated that they were aware that swelling in the foot, ankle, or leg could be a sign of DVT, and 71% indicated that shortness of breath could be a sign of pulmonary embolism (PE). “Other symptoms, however, were less well known, with just over half (57%) of participants being aware that pain, cramping, and tenderness could be a sign of DVT. About one third (33%) knew that irregular heartbeat could be a sign of PE. These results varied between countries,” according to the authors.
The survey highlighted that just over a third of respondents said that they were currently using anticoagulants, although almost all (96%) knew that anticoagulants could be used to effectively treat thrombosis. Only 41% of those using anticoagulants said they had been told about any possible side effects.
The Italian situation
The report containing the full results of the European survey goes even further, since, in addition to its overall results, it also gives information about individual countries.
The data from Italy, which are based on 246 persons, show that only 27% of patients and caregivers were aware of the increased risk of thrombosis after a cancer diagnosis. This figure is in line with the overall results of the survey, although the average score of the 10-point scale was lower for the Italy cohort (3.3/10 vs 4.1/10).
The results are more variable in terms of knowledge of risk factors. Most respondents (89%) said that they were aware of the risks related to inactivity. Just over half (52%), however, said that they were aware of the risks related to radiotherapy. Nevertheless, 75% of participants knew about the risks relating to cancer surgery and chemotherapy. “To all intents and purposes, all types of cancer drug can significantly affect the risk of developing a clot. And this is also the case for more modern types of treatment, such as immunotherapy,” said Dr. Falanga.
Most respondents reported that they got information about cancer-associated thrombosis verbally, usually from their hospital doctor (11%). Some respondents (6%) said that they found out about it from their own research, usually online. Almost 1 in 4 patients (24%) in Italy said that they first became aware of CAT when they suffered a blood clot. Answers to questions about knowledge of symptoms show that 58% of Italian patients and caregivers know that swelling of the lower limbs can be a symptom of DVT, and the same percentage knows that shortness of breath might indicate PE.
In terms of preventive action, the picture in Italy is somewhat variable: 74% of participants were aware of the importance of walking, but far fewer knew about the need to stop smoking (57%) and stretch the legs (35%). Of the 41% of Italians who were also taking an anticoagulant drug, 53% said that they knew about the possible side effects of such medication.
Which way forward?
“The high rate of CAT suggests that, despite the clinical evidence and clear guideline recommendations for patients with cancer, CAT prevention and recognition remain low among healthcare professionals,” the authors write.
The results of the ECPC survey further confirm those of previous studies, highlighting patients’ lack of knowledge about CAT and the need for more in-depth discussions between physician and patient.
So, what can be done? As highlighted by previous studies, “patients’ experiences are an education in themselves, particularly for the oncology care team,” the authors write. “Once the patient has a thrombosis, the opportunity for thrombosis prevention, which should be the most crucial focus of the care clinics (surgical, oncology, and palliative care), is gone,” they add.
“Oncology professionals, as well as other members of the patient’s care team (eg, internists, surgeons, nurses), need to perform better, at every stage of the patient’s cancer pathway, to ensure patients are aware of CAT and their individual risk to develop a blood clot,” said Dr. Falanga. She explained that in this group, it is the general practitioner who is the first contact. “These professionals are on the front line of the battle; they are among the first healthcare workers given the chance to suspect a clot and should, therefore, be fully aware of the increased risk in oncology patients,” she reiterated.
Experts agree on the fact that a multidisciplinary approach is of utmost importance in this context: the different roles in the team must be clear. “It is also fundamental to establish who does what in terms of educating and informing the patient,” said Dr. Falanga.
The researchers also put forward an example of a successful initiative: the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTE-PACC) program. The initiative was developed by experts from the University of Vermont and was described in a recent article in JCO Oncology Practice.
Numerous resources are available online to help physicians talk to their patients and explain the risks linked to CAT along the continuum of cancer care. Among them is a resource titled, “Cancer Associated Thrombosis (CAT): Be Clot Conscious,” which can be found on the ECPC’s website.
“We have a collective responsibility using the ECPC patient survey as a baseline to inform patients with cancer on how to identify signs and symptoms of CAT to enable faster diagnosis and treatment,” the authors conclude.
This article was translated from Univadis Italy.
It is up to their physician to discuss this with them.
This link is explained by the authors of an article in Cancer Treatment and Research Communications that reports results of a survey carried out by the European Cancer Patient Coalition (ECPC). “The aim of this pan-European patient survey was to assess patient awareness and knowledge about cancer-associated thrombosis (CAT), including risk factors, signs and symptoms, and interventions, to better prevent and treat CAT,” write the authors. “The idea was to create a sort of starting point for subsequent communication and information strategies and for comparing the results of any action taken in this area,” they add.
A roundtable discussion that included oncology healthcare professionals, policymakers, and patient advocates was convened to discuss and review the evidence regarding their ongoing concerns of excessive CAT-associated morbidity and mortality, as well as patients’ desire for greater CAT awareness.
“These discussions demonstrated that very little change had occurred over the years and that greater knowledge about CAT was still needed across the spectrum of healthcare practitioners and patients, particularly regarding primary and secondary prevention of thrombosis,” the authors write.
It was from this starting point that the idea for the pan-European survey was born. The ECPC, widely viewed as the “unified voice of cancer patients across Europe,” led the survey. This survey spanned six countries (France, Germany, Greece, Italy, United Kingdom, and Spain) and involved 1,365 patients and caregivers. The ECPC survey result was originally released at World Thrombosis Day in late 2018.
In an interview, Anna Falanga, MD, the main author of the article and professor of hematology at the University of Milan-Bicocca, Italy, reviewed the results and explained how to improve knowledge of CAT among patients with cancer.
“Data support that up to 20% of patients with cancer will experience venous thromboembolism (VTE), which is approximately 4–5 times higher than the general population,” said Dr. Falanga, who is also chief of the department of immunohematology and transfusion medicine and the Thrombosis and Hemostasis Center at the Hospital Papa Giovanni XXIII, in Bergamo, Italy.
“We have known about the link between thrombosis and cancer since the 19th century, but it has taken until midway through the last century for our level of understanding and awareness of the problem to reach its current level. Initially, this was limited to fundamental research, with large advances in our understanding of the mechanisms of the link between the two; it has only been more recently that we have had clinical studies that have piqued the interest of healthcare professionals, who were previously uninterested in the topic,” she said.
Poor understanding
One piece of data stands out from the European survey: Nearly three quarters of respondents (72%) said that before taking part in the survey, they were not aware that people with cancer have a higher-than-normal risk of developing thrombosis. “We asked participants to rate their overall understanding of CAT on a scale of 1 (low) to 10 (high), with the average (mean) score obtained being 4.1. Only 21% of patients gave a rating of 7 or above (high understanding). The average rating was very similar in the different countries surveyed,” write the authors. They note that the survey also assessed how much participants had learned about the topic from their physicians.
Approximately 35% of patients were made aware of CAT either immediately before or at the time of their cancer diagnosis. Of particular concern, one quarter (26%) of respondents (the largest proportion) noted that they first became aware of CAT when they suffered a blood clot. The average rating was very similar in the different countries surveyed. “Let us not forget that cancer and cancer treatments themselves cause a number of side effects, some of which can be very serious, so in some ways, a clot might be seen as a minor problem. Yet, in reality, it isn’t. It is a significant cause of death and disease in cancer patients,” said Dr. Falanga.
When discussing prevention, most respondents (87%) said they were aware that taking a walk could reduce their risk. Slightly fewer were aware that stopping smoking could reduce their risk (75%), and even fewer were aware that keeping hydrated (63%) and stretching their legs (55%) could reduce their risk.
Symptoms of CAT appeared to be relatively well known; 73% of survey participants indicated that they were aware that swelling in the foot, ankle, or leg could be a sign of DVT, and 71% indicated that shortness of breath could be a sign of pulmonary embolism (PE). “Other symptoms, however, were less well known, with just over half (57%) of participants being aware that pain, cramping, and tenderness could be a sign of DVT. About one third (33%) knew that irregular heartbeat could be a sign of PE. These results varied between countries,” according to the authors.
The survey highlighted that just over a third of respondents said that they were currently using anticoagulants, although almost all (96%) knew that anticoagulants could be used to effectively treat thrombosis. Only 41% of those using anticoagulants said they had been told about any possible side effects.
The Italian situation
The report containing the full results of the European survey goes even further, since, in addition to its overall results, it also gives information about individual countries.
The data from Italy, which are based on 246 persons, show that only 27% of patients and caregivers were aware of the increased risk of thrombosis after a cancer diagnosis. This figure is in line with the overall results of the survey, although the average score of the 10-point scale was lower for the Italy cohort (3.3/10 vs 4.1/10).
The results are more variable in terms of knowledge of risk factors. Most respondents (89%) said that they were aware of the risks related to inactivity. Just over half (52%), however, said that they were aware of the risks related to radiotherapy. Nevertheless, 75% of participants knew about the risks relating to cancer surgery and chemotherapy. “To all intents and purposes, all types of cancer drug can significantly affect the risk of developing a clot. And this is also the case for more modern types of treatment, such as immunotherapy,” said Dr. Falanga.
Most respondents reported that they got information about cancer-associated thrombosis verbally, usually from their hospital doctor (11%). Some respondents (6%) said that they found out about it from their own research, usually online. Almost 1 in 4 patients (24%) in Italy said that they first became aware of CAT when they suffered a blood clot. Answers to questions about knowledge of symptoms show that 58% of Italian patients and caregivers know that swelling of the lower limbs can be a symptom of DVT, and the same percentage knows that shortness of breath might indicate PE.
In terms of preventive action, the picture in Italy is somewhat variable: 74% of participants were aware of the importance of walking, but far fewer knew about the need to stop smoking (57%) and stretch the legs (35%). Of the 41% of Italians who were also taking an anticoagulant drug, 53% said that they knew about the possible side effects of such medication.
Which way forward?
“The high rate of CAT suggests that, despite the clinical evidence and clear guideline recommendations for patients with cancer, CAT prevention and recognition remain low among healthcare professionals,” the authors write.
The results of the ECPC survey further confirm those of previous studies, highlighting patients’ lack of knowledge about CAT and the need for more in-depth discussions between physician and patient.
So, what can be done? As highlighted by previous studies, “patients’ experiences are an education in themselves, particularly for the oncology care team,” the authors write. “Once the patient has a thrombosis, the opportunity for thrombosis prevention, which should be the most crucial focus of the care clinics (surgical, oncology, and palliative care), is gone,” they add.
“Oncology professionals, as well as other members of the patient’s care team (eg, internists, surgeons, nurses), need to perform better, at every stage of the patient’s cancer pathway, to ensure patients are aware of CAT and their individual risk to develop a blood clot,” said Dr. Falanga. She explained that in this group, it is the general practitioner who is the first contact. “These professionals are on the front line of the battle; they are among the first healthcare workers given the chance to suspect a clot and should, therefore, be fully aware of the increased risk in oncology patients,” she reiterated.
Experts agree on the fact that a multidisciplinary approach is of utmost importance in this context: the different roles in the team must be clear. “It is also fundamental to establish who does what in terms of educating and informing the patient,” said Dr. Falanga.
The researchers also put forward an example of a successful initiative: the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTE-PACC) program. The initiative was developed by experts from the University of Vermont and was described in a recent article in JCO Oncology Practice.
Numerous resources are available online to help physicians talk to their patients and explain the risks linked to CAT along the continuum of cancer care. Among them is a resource titled, “Cancer Associated Thrombosis (CAT): Be Clot Conscious,” which can be found on the ECPC’s website.
“We have a collective responsibility using the ECPC patient survey as a baseline to inform patients with cancer on how to identify signs and symptoms of CAT to enable faster diagnosis and treatment,” the authors conclude.
This article was translated from Univadis Italy.
FROM CANCER TREATMENT AND RESEARCH COMMUNICATIONS
COVID booster mounts ‘brisk’ response in patients with cancer
New data shed light on the durability of antibody responses to SARS-CoV-2 vaccines and the impact of booster doses for patients with cancer undergoing systemic therapy or who have received a stem cell transplant (SCT).
In a cross-sectional study of 453 such patients, anti–SARS-CoV-2 spike protein receptor binding domain (anti-RBD) antibodies peaked 1 month after the second dose of an mRNA vaccine and remained stable over the next 6 months.
Notably, compared with the primary vaccine course, patients experienced a 20-fold increase in anti-RBD antibodies after the third vaccine dose, “indicative of a brisk anamnestic response from memory B cells,” Qamar Khan, MD, a medical oncologist at the University of Kansas Medical Center, Kansas City, and colleagues report.
The study appeared online in JAMA Oncology.
Given the risk of poor outcomes among patients with cancer and recipients of SCTs who get COVID, Dr. Khan and colleagues wanted to understand the durability of the antibody response to COVID vaccines in this population.
Among the 453 patients enrolled in the study, 70% had solid tumors and 30% had hematologic malignancies. Just over 40% were receiving chemotherapy, 16% were receiving immunotherapy, 14% were receiving a targeted oral agent, 5% were receiving chemoimmunotherapy, and 25% had received an SCT.
Regarding vaccine type, 61% received the Pfizer-BioNTech mRNA vaccine, 36% received the Moderna mRNA vaccine, and 4% got the Janssen/Johnson & Johnson vaccine. The mean age of the cohort was 60.4 years; 56% were women.
Prior to vaccination, the geometric mean titer (GMT) of anti-RBD antibodies for all patients was 1.7; it increased to 18.65 2 weeks after the first dose.
At 1 month after the second mRNA dose (or 2 months after the Johnson & Johnson vaccine), GMTs of anti-RBD antibodies reached 470.38 and then decreased to 425.8 at 3 months after the second dose (or 4 months after the Johnson & Johnson vaccine). Patients who were male, older than 65 years, and who had been diagnosed with a hematologic malignant tumor were more likely to have lower anti-RBD GMT 3 months after the second vaccine dose.
GMTs subsequently increased to 447.23 6 months after the second dose (7 months for Johnson & Johnson).
One month after the third dose, GMTs of anti-RBD antibodies rose to 9,224.85 – more than 20 times the previous GMT value.
According to the investigators, roughly 80% of these patients remained above the threshold of an anti-RBD level of 100 U/mL or higher at 6 months.
“While still an arbitrary cutoff, an anti-RBD level of 100 U/mL or higher has been associated with protection and has been used to evaluate the effectiveness of a third dose of an mRNA vaccine in a randomized clinical trial of patients who received a solid organ transplant,” Dr. Khan and colleagues write.
“Although more data are needed to confirm this level as protective, if established, anti-RBD can potentially be used to prioritize additional vaccine doses, especially in regions of the world with limited vaccine resources,” the authors conclude.
The study was supported in part by the University of Kansas Cancer Center and the Investigator Initiated Steering Committee, by a grant from the National Institute of General Medical Sciences, and a University of Kansas Cancer Center Support Grant from the National Cancer Institute. Dr. Khan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data shed light on the durability of antibody responses to SARS-CoV-2 vaccines and the impact of booster doses for patients with cancer undergoing systemic therapy or who have received a stem cell transplant (SCT).
In a cross-sectional study of 453 such patients, anti–SARS-CoV-2 spike protein receptor binding domain (anti-RBD) antibodies peaked 1 month after the second dose of an mRNA vaccine and remained stable over the next 6 months.
Notably, compared with the primary vaccine course, patients experienced a 20-fold increase in anti-RBD antibodies after the third vaccine dose, “indicative of a brisk anamnestic response from memory B cells,” Qamar Khan, MD, a medical oncologist at the University of Kansas Medical Center, Kansas City, and colleagues report.
The study appeared online in JAMA Oncology.
Given the risk of poor outcomes among patients with cancer and recipients of SCTs who get COVID, Dr. Khan and colleagues wanted to understand the durability of the antibody response to COVID vaccines in this population.
Among the 453 patients enrolled in the study, 70% had solid tumors and 30% had hematologic malignancies. Just over 40% were receiving chemotherapy, 16% were receiving immunotherapy, 14% were receiving a targeted oral agent, 5% were receiving chemoimmunotherapy, and 25% had received an SCT.
Regarding vaccine type, 61% received the Pfizer-BioNTech mRNA vaccine, 36% received the Moderna mRNA vaccine, and 4% got the Janssen/Johnson & Johnson vaccine. The mean age of the cohort was 60.4 years; 56% were women.
Prior to vaccination, the geometric mean titer (GMT) of anti-RBD antibodies for all patients was 1.7; it increased to 18.65 2 weeks after the first dose.
At 1 month after the second mRNA dose (or 2 months after the Johnson & Johnson vaccine), GMTs of anti-RBD antibodies reached 470.38 and then decreased to 425.8 at 3 months after the second dose (or 4 months after the Johnson & Johnson vaccine). Patients who were male, older than 65 years, and who had been diagnosed with a hematologic malignant tumor were more likely to have lower anti-RBD GMT 3 months after the second vaccine dose.
GMTs subsequently increased to 447.23 6 months after the second dose (7 months for Johnson & Johnson).
One month after the third dose, GMTs of anti-RBD antibodies rose to 9,224.85 – more than 20 times the previous GMT value.
According to the investigators, roughly 80% of these patients remained above the threshold of an anti-RBD level of 100 U/mL or higher at 6 months.
“While still an arbitrary cutoff, an anti-RBD level of 100 U/mL or higher has been associated with protection and has been used to evaluate the effectiveness of a third dose of an mRNA vaccine in a randomized clinical trial of patients who received a solid organ transplant,” Dr. Khan and colleagues write.
“Although more data are needed to confirm this level as protective, if established, anti-RBD can potentially be used to prioritize additional vaccine doses, especially in regions of the world with limited vaccine resources,” the authors conclude.
The study was supported in part by the University of Kansas Cancer Center and the Investigator Initiated Steering Committee, by a grant from the National Institute of General Medical Sciences, and a University of Kansas Cancer Center Support Grant from the National Cancer Institute. Dr. Khan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data shed light on the durability of antibody responses to SARS-CoV-2 vaccines and the impact of booster doses for patients with cancer undergoing systemic therapy or who have received a stem cell transplant (SCT).
In a cross-sectional study of 453 such patients, anti–SARS-CoV-2 spike protein receptor binding domain (anti-RBD) antibodies peaked 1 month after the second dose of an mRNA vaccine and remained stable over the next 6 months.
Notably, compared with the primary vaccine course, patients experienced a 20-fold increase in anti-RBD antibodies after the third vaccine dose, “indicative of a brisk anamnestic response from memory B cells,” Qamar Khan, MD, a medical oncologist at the University of Kansas Medical Center, Kansas City, and colleagues report.
The study appeared online in JAMA Oncology.
Given the risk of poor outcomes among patients with cancer and recipients of SCTs who get COVID, Dr. Khan and colleagues wanted to understand the durability of the antibody response to COVID vaccines in this population.
Among the 453 patients enrolled in the study, 70% had solid tumors and 30% had hematologic malignancies. Just over 40% were receiving chemotherapy, 16% were receiving immunotherapy, 14% were receiving a targeted oral agent, 5% were receiving chemoimmunotherapy, and 25% had received an SCT.
Regarding vaccine type, 61% received the Pfizer-BioNTech mRNA vaccine, 36% received the Moderna mRNA vaccine, and 4% got the Janssen/Johnson & Johnson vaccine. The mean age of the cohort was 60.4 years; 56% were women.
Prior to vaccination, the geometric mean titer (GMT) of anti-RBD antibodies for all patients was 1.7; it increased to 18.65 2 weeks after the first dose.
At 1 month after the second mRNA dose (or 2 months after the Johnson & Johnson vaccine), GMTs of anti-RBD antibodies reached 470.38 and then decreased to 425.8 at 3 months after the second dose (or 4 months after the Johnson & Johnson vaccine). Patients who were male, older than 65 years, and who had been diagnosed with a hematologic malignant tumor were more likely to have lower anti-RBD GMT 3 months after the second vaccine dose.
GMTs subsequently increased to 447.23 6 months after the second dose (7 months for Johnson & Johnson).
One month after the third dose, GMTs of anti-RBD antibodies rose to 9,224.85 – more than 20 times the previous GMT value.
According to the investigators, roughly 80% of these patients remained above the threshold of an anti-RBD level of 100 U/mL or higher at 6 months.
“While still an arbitrary cutoff, an anti-RBD level of 100 U/mL or higher has been associated with protection and has been used to evaluate the effectiveness of a third dose of an mRNA vaccine in a randomized clinical trial of patients who received a solid organ transplant,” Dr. Khan and colleagues write.
“Although more data are needed to confirm this level as protective, if established, anti-RBD can potentially be used to prioritize additional vaccine doses, especially in regions of the world with limited vaccine resources,” the authors conclude.
The study was supported in part by the University of Kansas Cancer Center and the Investigator Initiated Steering Committee, by a grant from the National Institute of General Medical Sciences, and a University of Kansas Cancer Center Support Grant from the National Cancer Institute. Dr. Khan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA ONCOLOGY
Dodging potholes from cancer care to hospice transitions
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I’m often in the position of caring for patients after they’ve stopped active cancer treatments, but before they’ve made the decision to enroll in hospice. They remain under my care until they feel emotionally ready, or until their care needs have escalated to the point in which hospice is unavoidable.
Jenny, a mom in her 50s with metastatic pancreatic cancer, stopped coming to the clinic. She lived about 40 minutes away from the clinic and was no longer receiving treatment. The car rides were painful and difficult for her. I held weekly video visits with her for 2 months before she eventually went to hospice and passed away. Before she died, she shared with me her sadness that her oncologist – who had taken care of her for 3 years – had “washed his hands of [me].” She rarely heard from him after their final conversation in the clinic when he informed her that she was no longer a candidate for further therapy. The sense of abandonment Jenny described was visceral and devastating. With her permission, I let her oncology team know how she felt and they reached out to her just 1 week before her death. After she died, her husband told me how meaningful it had been for the whole family to hear from Jenny’s oncologist who told them that she had done everything possible to fight her cancer and that “no stone was left unturned.” Her husband felt this final conversation provided Jenny with the closure she needed to pass away peacefully.
Transitioning from active therapy to symptom management
Switching gears from an all-out pursuit of active therapy to focusing on cancer symptoms is often a scary transition for patients and their families. The transition is often viewed as a movement away from hope and optimism to “giving up the fight.” Whether you agree with the warrior language or not, many patients still describe their journey in these terms and thus, experience enrollment in hospice as a sense of having failed.
The sense of failure can be compounded by feelings of abandonment by oncology providers when they are referred without much guidance or continuity through the hospice enrollment process. Unfortunately, the consequences of suboptimal hospice transitions can be damaging, especially for the mental health and well-being of the patient and their surviving loved ones.
When managed poorly, hospice transitions can easily lead to patient and family harm, which is a claim supported by research. A qualitative study published in 2019 included 92 caregivers of patients with terminal cancer. The authors found three common pathways for end-of-life transitions – a frictionless transition in which the patient and family are well prepared in advance by their oncologist; a more turbulent transition in which patient and family had direct conversations with their oncologist about the incurability of the disease and the lack of efficacy of further treatments, but were given no guidance on prognosis; and a third type of transition marked by abrupt shifts toward end-of-life care occurring in extremis and typically in the hospital.
In the latter two groups, caregivers felt their loved ones died very quickly after stopping treatment, taking them by surprise and leaving them rushing to put end-of-life care plans in place without much support from their oncologists. In the last group, caregivers shared they received their first prognostic information from the hospital or ICU doctor caring for their actively dying loved one, leaving them with a sense of anger and betrayal toward their oncologist for allowing them to be so ill-prepared.
A Japanese survey published in 2018 in The Oncologist of families of cancer patients who had passed away under hospice care over a 2-year period (2012-2014), found that about one-quarter felt abandoned by oncologists. Several factors that were associated with feeling either more or less abandonment. Spouses of patients, patients aged less than 60 years, and patients whose oncologists informed them that there was “nothing more to do” felt more abandoned by oncologists; whereas families for whom the oncologist provided reassurance about the trajectory of care, recommended hospice, and engaged with a palliative care team felt less abandoned by oncologists. Families who felt more abandoned had higher levels of depression and grief when measured with standardized instruments.
‘Don’t just put in the hospice order and walk away’
Fortunately, there are a few low-resource interventions that can improve the quality of care-to-hospice transitions and prevent the sense of abandonment felt by many patients and families.
First, don’t just put in the hospice order and walk away. Designate a staffer in your office to contact hospice directly, ensure all medical records are faxed and received, and update the patient and family on this progress throughout the transition. Taking care of details like these ensures the patient enrolls in hospice in a timely manner and reduces the chance the patient, who is likely to be quite sick at this point, will end up in the hospital despite your best efforts to get hospice involved.
Make sure the patient and family understand that you are still their oncologist and still available to them. If they want to continue care with you, have them name you as the “non–hospice-attending physician” so that you can continue to bill for telemedicine and office visits using the terminal diagnosis (with a billing modifier). This does not mean that you will be expected to manage the patient’s hospice problem list or respond to hospice nurse calls at 2 a.m. – the hospice doctor will still do this. It just ensures that patients do not receive a bill if you continue to see them.
If ongoing office or video visits are too much for the patient and family, consider assigning a member of your team to call the patient and family on a weekly basis to check in and offer support. A small 2018 pilot study aimed at improving communication found that when caregivers of advanced cancer patients transitioning to hospice received weekly supportive phone calls by a member of their oncology team (typically a nurse or nurse practitioner), they felt emotionally supported, had good continuity of care throughout the hospice enrollment, and appreciated the ability to have closure with their oncology team. In other words, a sense of abandonment was prevented and the patient-provider relationship was actually deepened through the transition.
These suggestions are not rocket science – they are simple, obvious ways to try to restore patient-centeredness to a transition that for providers can seem routine, but for patients and families is often the first time they have confronted the reality that death is approaching. That reality is terrifying and overwhelming. Patients and caregivers need our support more during hospice transitions than at any other point during their cancer journey – except perhaps at diagnosis.
As with Jenny, my patient who felt abandoned, all it took was a single call by her oncology team to restore the trust and heal the sense of feeling forsaken by the people who cared for her for years. Sometimes, even just one more phone call can feel like a lot to a chronically overburdened provider – but what a difference a simple call can make.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.



