User login
Building patient-centered care through values assessment integration with advance care planning
Oncologists frequently have to make diagnoses that portend bad outcomes and difficulties in management, among them, for stage IV lung or pancreatic cancer. Many recent studies have shown the importance of appropriate implementation of palliative care and the need for discussing with the patient the goals of treatment early in diagnosis.1-3
Click on the PDF icon at the top of this introduction to read the full article.
Oncologists frequently have to make diagnoses that portend bad outcomes and difficulties in management, among them, for stage IV lung or pancreatic cancer. Many recent studies have shown the importance of appropriate implementation of palliative care and the need for discussing with the patient the goals of treatment early in diagnosis.1-3
Click on the PDF icon at the top of this introduction to read the full article.
Oncologists frequently have to make diagnoses that portend bad outcomes and difficulties in management, among them, for stage IV lung or pancreatic cancer. Many recent studies have shown the importance of appropriate implementation of palliative care and the need for discussing with the patient the goals of treatment early in diagnosis.1-3
Click on the PDF icon at the top of this introduction to read the full article.
Guideline-recommended beta-blockers before noncardiac surgery shown to increase mortality by 27%
More than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by joint American College of Cardiology Foundation/American Heart Association and separate European Society of Cardiology guidelines.
These guidelines recommend perioperative beta-blockers in all patients undergoing vascular or intermediate-risk surgery with coronary artery disease, or with more than one risk factor for CAD, or with preexisting beta-blockade. These are all iatrogenic deaths, according to a meta-analysis of secure studies, which excluded data from the now discredited Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) family of trials.
"Refraining from this ESC [European Society of Cardiology] guideline would therefore be expected to prevent up to 10,000 iatrogenic deaths each year in the U.K.," according to Dr. Sonia Bouri and her coauthors at the National Heart and Lung Institute, Imperial College London.
The researchers analyzed nine secure randomized trials totaling 10,529 patients who met the guideline criteria, 291 of whom died. They found that initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in mortality.
In the secure trials, use of perioperative beta-blockers decreased nonfatal myocardial infarction significantly (RR, 0.73; P = .001), but increased stroke (RR, 1.73; P =.05) and hypotension (RR, 1.51; P less than .00001), according to the authors, who presented their data in Heart (2013 July 31 [doi: 10.1136/heartjnl-2013-304262]).
Of the 291 deaths recorded in the secure trials, 162 deaths (3.21%) occurred in 5,264 patients randomized to beta-blockers, and 129 deaths (2.45%) occurred in the 5,265 patients randomized to placebo.
Thus, the initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in all-cause mortality, Dr. Bouri and her coauthors stated. "Any remaining [perioperative beta-blocker] enthusiasts might best channel their energy into a further randomized trial, which should be designed carefully and honestly," they added.
The results from the DECREASE family of trials substantially contradicted the meta-analysis of the secure trials on the effect on mortality (P = .05 for divergence).
"All studies investigated in the DECREASE family for which data had not been lost were found to be insecure because of serious flaws. In one case, it was clear that the entire study database had been fabricated. DECREASE I, published in 1999, escaped investigation as the terms of the investigation only reached back 10 years," the researchers reported.
When the ESC and American College of Cardiology Foundation/American Heart Association guidelines were formulated, "the inclusion of insecure data caused them to reach the conclusion that beta-blockade had a neutral effect on mortality and allowed them to focus on the reduction of non-fatal MI as a surrogate endpoint," the authors explained.
The DECREASE family of studies was discredited almost 2 years ago and subsequently underwent lengthy internal investigation, the results of which have been public for some time, according to the authors. "Nevertheless, neither the European Society of Cardiology nor the AHA guidelines have been retracted," they said.
"Patient safety being paramount, guidelines for perioperative beta-blockers should be retracted without further delay. Future guidelines should be accompanied by a commitment from named individuals to retract them immediately if the advice given is later revealed to be harmful," the authors concluded.
The authors reported that they had no conflicts of interest.
For those of us in the "perioperative care business," the results of this meta-analysis are neither surprising nor informative. The increased all-cause mortality with perioperative beta blockade in the POISE trial is well documented in the literature and the findings of this most recent study by Bouri and colleagues is dominated by POISE data.

|
| Dr. Franklin Michota |
For those who might not recall POISE, it is the largest randomized clinical trial (RCT) to date that evaluated the safety and efficacy of perioperative beta-blockers in noncardiac surgery. A total of 8,351 patients aged older than 45 years who had or were at risk for atherosclerotic heart disease were randomized to beta-blockers or placebo.
In the treatment arm, patients received metoprolol CR (100 mg preop, 100 mg 6 hours postop, 200 mg 12 hours later, then daily for 30 days). This dose is significantly higher than what most clinicians are accustomed to using. And while it is true that we can no longer trust the conclusions from the DECREASE family of studies due to academic negligence, it does not equate to confirmatory evidence that titrated perioperative beta blockade at a lower dose than that used in POISE (thus avoiding sinus bradycardia and/or hypotension) is of no benefit or harmful. Unfortunately, we just won't know either way until further investigation is performed.

|
| Dr. Amir Jaffer |
To extrapolate that more than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by the ACC/AHA and separate ESC guidelines is pure sensationalism.
Following publication of the POISE trial, the ACC/AHA did publish a focused update on perioperative beta-blockers and specifically noted the possibility of harm from these medications and the importance of careful dose titration. Given the anecdotal experience by all of us who have titrated beta-blockers perioperatively, we find it difficult to believe that such an approach is causing harm. We certainly do question now whether such an approach is doing the patient any good until proven prospectively in a large RCT.
Dr. Franklin A. Michota is director of academic affairs in the department of hospital medicine at the Cleveland Clinic. Dr. Amir K. Jaffer is assistant chief medical officer and division chief of hospital medicine at Rush University Medical Center, Chicago. They are advisers to Hospitalist News.
For those of us in the "perioperative care business," the results of this meta-analysis are neither surprising nor informative. The increased all-cause mortality with perioperative beta blockade in the POISE trial is well documented in the literature and the findings of this most recent study by Bouri and colleagues is dominated by POISE data.

|
| Dr. Franklin Michota |
For those who might not recall POISE, it is the largest randomized clinical trial (RCT) to date that evaluated the safety and efficacy of perioperative beta-blockers in noncardiac surgery. A total of 8,351 patients aged older than 45 years who had or were at risk for atherosclerotic heart disease were randomized to beta-blockers or placebo.
In the treatment arm, patients received metoprolol CR (100 mg preop, 100 mg 6 hours postop, 200 mg 12 hours later, then daily for 30 days). This dose is significantly higher than what most clinicians are accustomed to using. And while it is true that we can no longer trust the conclusions from the DECREASE family of studies due to academic negligence, it does not equate to confirmatory evidence that titrated perioperative beta blockade at a lower dose than that used in POISE (thus avoiding sinus bradycardia and/or hypotension) is of no benefit or harmful. Unfortunately, we just won't know either way until further investigation is performed.

|
| Dr. Amir Jaffer |
To extrapolate that more than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by the ACC/AHA and separate ESC guidelines is pure sensationalism.
Following publication of the POISE trial, the ACC/AHA did publish a focused update on perioperative beta-blockers and specifically noted the possibility of harm from these medications and the importance of careful dose titration. Given the anecdotal experience by all of us who have titrated beta-blockers perioperatively, we find it difficult to believe that such an approach is causing harm. We certainly do question now whether such an approach is doing the patient any good until proven prospectively in a large RCT.
Dr. Franklin A. Michota is director of academic affairs in the department of hospital medicine at the Cleveland Clinic. Dr. Amir K. Jaffer is assistant chief medical officer and division chief of hospital medicine at Rush University Medical Center, Chicago. They are advisers to Hospitalist News.
For those of us in the "perioperative care business," the results of this meta-analysis are neither surprising nor informative. The increased all-cause mortality with perioperative beta blockade in the POISE trial is well documented in the literature and the findings of this most recent study by Bouri and colleagues is dominated by POISE data.

|
| Dr. Franklin Michota |
For those who might not recall POISE, it is the largest randomized clinical trial (RCT) to date that evaluated the safety and efficacy of perioperative beta-blockers in noncardiac surgery. A total of 8,351 patients aged older than 45 years who had or were at risk for atherosclerotic heart disease were randomized to beta-blockers or placebo.
In the treatment arm, patients received metoprolol CR (100 mg preop, 100 mg 6 hours postop, 200 mg 12 hours later, then daily for 30 days). This dose is significantly higher than what most clinicians are accustomed to using. And while it is true that we can no longer trust the conclusions from the DECREASE family of studies due to academic negligence, it does not equate to confirmatory evidence that titrated perioperative beta blockade at a lower dose than that used in POISE (thus avoiding sinus bradycardia and/or hypotension) is of no benefit or harmful. Unfortunately, we just won't know either way until further investigation is performed.

|
| Dr. Amir Jaffer |
To extrapolate that more than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by the ACC/AHA and separate ESC guidelines is pure sensationalism.
Following publication of the POISE trial, the ACC/AHA did publish a focused update on perioperative beta-blockers and specifically noted the possibility of harm from these medications and the importance of careful dose titration. Given the anecdotal experience by all of us who have titrated beta-blockers perioperatively, we find it difficult to believe that such an approach is causing harm. We certainly do question now whether such an approach is doing the patient any good until proven prospectively in a large RCT.
Dr. Franklin A. Michota is director of academic affairs in the department of hospital medicine at the Cleveland Clinic. Dr. Amir K. Jaffer is assistant chief medical officer and division chief of hospital medicine at Rush University Medical Center, Chicago. They are advisers to Hospitalist News.
More than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by joint American College of Cardiology Foundation/American Heart Association and separate European Society of Cardiology guidelines.
These guidelines recommend perioperative beta-blockers in all patients undergoing vascular or intermediate-risk surgery with coronary artery disease, or with more than one risk factor for CAD, or with preexisting beta-blockade. These are all iatrogenic deaths, according to a meta-analysis of secure studies, which excluded data from the now discredited Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) family of trials.
"Refraining from this ESC [European Society of Cardiology] guideline would therefore be expected to prevent up to 10,000 iatrogenic deaths each year in the U.K.," according to Dr. Sonia Bouri and her coauthors at the National Heart and Lung Institute, Imperial College London.
The researchers analyzed nine secure randomized trials totaling 10,529 patients who met the guideline criteria, 291 of whom died. They found that initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in mortality.
In the secure trials, use of perioperative beta-blockers decreased nonfatal myocardial infarction significantly (RR, 0.73; P = .001), but increased stroke (RR, 1.73; P =.05) and hypotension (RR, 1.51; P less than .00001), according to the authors, who presented their data in Heart (2013 July 31 [doi: 10.1136/heartjnl-2013-304262]).
Of the 291 deaths recorded in the secure trials, 162 deaths (3.21%) occurred in 5,264 patients randomized to beta-blockers, and 129 deaths (2.45%) occurred in the 5,265 patients randomized to placebo.
Thus, the initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in all-cause mortality, Dr. Bouri and her coauthors stated. "Any remaining [perioperative beta-blocker] enthusiasts might best channel their energy into a further randomized trial, which should be designed carefully and honestly," they added.
The results from the DECREASE family of trials substantially contradicted the meta-analysis of the secure trials on the effect on mortality (P = .05 for divergence).
"All studies investigated in the DECREASE family for which data had not been lost were found to be insecure because of serious flaws. In one case, it was clear that the entire study database had been fabricated. DECREASE I, published in 1999, escaped investigation as the terms of the investigation only reached back 10 years," the researchers reported.
When the ESC and American College of Cardiology Foundation/American Heart Association guidelines were formulated, "the inclusion of insecure data caused them to reach the conclusion that beta-blockade had a neutral effect on mortality and allowed them to focus on the reduction of non-fatal MI as a surrogate endpoint," the authors explained.
The DECREASE family of studies was discredited almost 2 years ago and subsequently underwent lengthy internal investigation, the results of which have been public for some time, according to the authors. "Nevertheless, neither the European Society of Cardiology nor the AHA guidelines have been retracted," they said.
"Patient safety being paramount, guidelines for perioperative beta-blockers should be retracted without further delay. Future guidelines should be accompanied by a commitment from named individuals to retract them immediately if the advice given is later revealed to be harmful," the authors concluded.
The authors reported that they had no conflicts of interest.
More than 1 in 4 patients who died from all causes after noncardiac surgery may have survived if they were not treated with perioperative beta-blockers as specified by joint American College of Cardiology Foundation/American Heart Association and separate European Society of Cardiology guidelines.
These guidelines recommend perioperative beta-blockers in all patients undergoing vascular or intermediate-risk surgery with coronary artery disease, or with more than one risk factor for CAD, or with preexisting beta-blockade. These are all iatrogenic deaths, according to a meta-analysis of secure studies, which excluded data from the now discredited Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE) family of trials.
"Refraining from this ESC [European Society of Cardiology] guideline would therefore be expected to prevent up to 10,000 iatrogenic deaths each year in the U.K.," according to Dr. Sonia Bouri and her coauthors at the National Heart and Lung Institute, Imperial College London.
The researchers analyzed nine secure randomized trials totaling 10,529 patients who met the guideline criteria, 291 of whom died. They found that initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in mortality.
In the secure trials, use of perioperative beta-blockers decreased nonfatal myocardial infarction significantly (RR, 0.73; P = .001), but increased stroke (RR, 1.73; P =.05) and hypotension (RR, 1.51; P less than .00001), according to the authors, who presented their data in Heart (2013 July 31 [doi: 10.1136/heartjnl-2013-304262]).
Of the 291 deaths recorded in the secure trials, 162 deaths (3.21%) occurred in 5,264 patients randomized to beta-blockers, and 129 deaths (2.45%) occurred in the 5,265 patients randomized to placebo.
Thus, the initiation of a course of beta-blockers as per guideline recommendations before surgery resulted in a 27% increase in all-cause mortality, Dr. Bouri and her coauthors stated. "Any remaining [perioperative beta-blocker] enthusiasts might best channel their energy into a further randomized trial, which should be designed carefully and honestly," they added.
The results from the DECREASE family of trials substantially contradicted the meta-analysis of the secure trials on the effect on mortality (P = .05 for divergence).
"All studies investigated in the DECREASE family for which data had not been lost were found to be insecure because of serious flaws. In one case, it was clear that the entire study database had been fabricated. DECREASE I, published in 1999, escaped investigation as the terms of the investigation only reached back 10 years," the researchers reported.
When the ESC and American College of Cardiology Foundation/American Heart Association guidelines were formulated, "the inclusion of insecure data caused them to reach the conclusion that beta-blockade had a neutral effect on mortality and allowed them to focus on the reduction of non-fatal MI as a surrogate endpoint," the authors explained.
The DECREASE family of studies was discredited almost 2 years ago and subsequently underwent lengthy internal investigation, the results of which have been public for some time, according to the authors. "Nevertheless, neither the European Society of Cardiology nor the AHA guidelines have been retracted," they said.
"Patient safety being paramount, guidelines for perioperative beta-blockers should be retracted without further delay. Future guidelines should be accompanied by a commitment from named individuals to retract them immediately if the advice given is later revealed to be harmful," the authors concluded.
The authors reported that they had no conflicts of interest.
FROM HEART
Nail complications of cancer therapies on the rise
NEW YORK – Nail complications are increasingly recognized as a problematic side effect of chemotherapies and biologic therapies for cancer patients.
In some cases these are cosmetic issues, but the side effects really do interfere with activities of daily living for many cancer patients, Dr. Patricia S. Myskowski, an attending dermatologist at Memorial Sloan Kettering Cancer Center, New York, reported at the American Academy of Dermatology summer meeting.
In a list of toxicities provided by the National Cancer Institute in 2006, only three categories of nail toxicities were listed, and these employed relatively vague descriptions, according to Dr. Myskowski. More recent summaries, including a literature review (J. Oncol. Pharm. Pract. 2009;15:143-55), have helped to classify and quantify nail complications as well as provide therapeutic guidance.
Taxanes, and specifically docetaxel, are "the worst offenders" of chemotherapies resulting in nails disorders, she said. More than 80% of patients treated with multiple cycles of docetaxel will develop some nail changes. Most are cosmetic reactions, such as depigmentation, but nearly one-third of patients have reactions that interfere with activities of daily living.
One of the most bothersome of these complications is subungual hematomas with hemopurulent discharge. In patients with nail infection, antibiotics may accelerate drainage and healing, but Dr. Myskowski suggested that this complication can be avoided by keeping the nails trimmed as short as possible.
Short nails are associated with a reduced risk of secondary infection, said Dr. Myskowski, who advised bacterial and fungal cultures when infection is suspected.
Biological therapies, particularly epidermal growth factor receptor (EGFR) inhibitors and tyrosine kinase inhibitors (TKIs), also are associated with a high rate of nail disorders, including paronychia, pyogenic granuloma, and infection. Although nail disorders are far less common than the characteristic rash associated with these agents, she cited one study suggesting a 12% incidence of symptomatic paronychia on EGFR inhibitors. Typically, nail complications emerge about 2 months after treatment is initiated.
To prevent paronychia associated with EGFR inhibitors, Dr. Myskowksi recommended following guidelines issued by the National Comprehensive Cancer Network (J. Natl. Compr. Canc. Netw. 2009;75:S5-S21).
Recommendations include avoiding frequent water immersion as well as contact with harsh chemicals. Applying petroleum jelly to the periungual soft tissue may be protective. In the event of nail infection, augmenting antibiotic therapy with topical therapies such as silver nitrate solution or white vinegar soaks may speed healing. In patients who have reinfections of toenails, disposing of shoes that may harbor bacteria sometimes resolves the problem.
Dr. Myskowski reported no financial disclosures relevant to her presentation.
NEW YORK – Nail complications are increasingly recognized as a problematic side effect of chemotherapies and biologic therapies for cancer patients.
In some cases these are cosmetic issues, but the side effects really do interfere with activities of daily living for many cancer patients, Dr. Patricia S. Myskowski, an attending dermatologist at Memorial Sloan Kettering Cancer Center, New York, reported at the American Academy of Dermatology summer meeting.
In a list of toxicities provided by the National Cancer Institute in 2006, only three categories of nail toxicities were listed, and these employed relatively vague descriptions, according to Dr. Myskowski. More recent summaries, including a literature review (J. Oncol. Pharm. Pract. 2009;15:143-55), have helped to classify and quantify nail complications as well as provide therapeutic guidance.
Taxanes, and specifically docetaxel, are "the worst offenders" of chemotherapies resulting in nails disorders, she said. More than 80% of patients treated with multiple cycles of docetaxel will develop some nail changes. Most are cosmetic reactions, such as depigmentation, but nearly one-third of patients have reactions that interfere with activities of daily living.
One of the most bothersome of these complications is subungual hematomas with hemopurulent discharge. In patients with nail infection, antibiotics may accelerate drainage and healing, but Dr. Myskowski suggested that this complication can be avoided by keeping the nails trimmed as short as possible.
Short nails are associated with a reduced risk of secondary infection, said Dr. Myskowski, who advised bacterial and fungal cultures when infection is suspected.
Biological therapies, particularly epidermal growth factor receptor (EGFR) inhibitors and tyrosine kinase inhibitors (TKIs), also are associated with a high rate of nail disorders, including paronychia, pyogenic granuloma, and infection. Although nail disorders are far less common than the characteristic rash associated with these agents, she cited one study suggesting a 12% incidence of symptomatic paronychia on EGFR inhibitors. Typically, nail complications emerge about 2 months after treatment is initiated.
To prevent paronychia associated with EGFR inhibitors, Dr. Myskowksi recommended following guidelines issued by the National Comprehensive Cancer Network (J. Natl. Compr. Canc. Netw. 2009;75:S5-S21).
Recommendations include avoiding frequent water immersion as well as contact with harsh chemicals. Applying petroleum jelly to the periungual soft tissue may be protective. In the event of nail infection, augmenting antibiotic therapy with topical therapies such as silver nitrate solution or white vinegar soaks may speed healing. In patients who have reinfections of toenails, disposing of shoes that may harbor bacteria sometimes resolves the problem.
Dr. Myskowski reported no financial disclosures relevant to her presentation.
NEW YORK – Nail complications are increasingly recognized as a problematic side effect of chemotherapies and biologic therapies for cancer patients.
In some cases these are cosmetic issues, but the side effects really do interfere with activities of daily living for many cancer patients, Dr. Patricia S. Myskowski, an attending dermatologist at Memorial Sloan Kettering Cancer Center, New York, reported at the American Academy of Dermatology summer meeting.
In a list of toxicities provided by the National Cancer Institute in 2006, only three categories of nail toxicities were listed, and these employed relatively vague descriptions, according to Dr. Myskowski. More recent summaries, including a literature review (J. Oncol. Pharm. Pract. 2009;15:143-55), have helped to classify and quantify nail complications as well as provide therapeutic guidance.
Taxanes, and specifically docetaxel, are "the worst offenders" of chemotherapies resulting in nails disorders, she said. More than 80% of patients treated with multiple cycles of docetaxel will develop some nail changes. Most are cosmetic reactions, such as depigmentation, but nearly one-third of patients have reactions that interfere with activities of daily living.
One of the most bothersome of these complications is subungual hematomas with hemopurulent discharge. In patients with nail infection, antibiotics may accelerate drainage and healing, but Dr. Myskowski suggested that this complication can be avoided by keeping the nails trimmed as short as possible.
Short nails are associated with a reduced risk of secondary infection, said Dr. Myskowski, who advised bacterial and fungal cultures when infection is suspected.
Biological therapies, particularly epidermal growth factor receptor (EGFR) inhibitors and tyrosine kinase inhibitors (TKIs), also are associated with a high rate of nail disorders, including paronychia, pyogenic granuloma, and infection. Although nail disorders are far less common than the characteristic rash associated with these agents, she cited one study suggesting a 12% incidence of symptomatic paronychia on EGFR inhibitors. Typically, nail complications emerge about 2 months after treatment is initiated.
To prevent paronychia associated with EGFR inhibitors, Dr. Myskowksi recommended following guidelines issued by the National Comprehensive Cancer Network (J. Natl. Compr. Canc. Netw. 2009;75:S5-S21).
Recommendations include avoiding frequent water immersion as well as contact with harsh chemicals. Applying petroleum jelly to the periungual soft tissue may be protective. In the event of nail infection, augmenting antibiotic therapy with topical therapies such as silver nitrate solution or white vinegar soaks may speed healing. In patients who have reinfections of toenails, disposing of shoes that may harbor bacteria sometimes resolves the problem.
Dr. Myskowski reported no financial disclosures relevant to her presentation.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2013
Dalteparin safely used for 12 months to prevent VTE recurrence in cancer patients
AMSTERDAM – Cancer patients who start dalteparin to prevent a recurrence of venous thromboembolism can continue on the drug for as long as a year without an increase in bleeding risk and with stable control against another venous thromboembolism.
But the high underlying mortality risk from cancer that many of these patients face makes it hard to judge which patients will benefit from continued prophylaxis with the low-molecular-weight heparin, Dr. Ajay K. Kakkar said at the congress of the International Society on Thrombosis and Haemostasis.
A third of the patients enrolled in the trial died before the yearlong study ended, and the vast majority of the deaths were due to cancer.
"One of the most important competing risks besides thrombosis that these cancer patients face is the risk of dying from their underlying malignancy. That’s an important determinant in trying to understand whether we should continue anticoagulant treatment," said Dr. Kakkar, a professor of surgery at University College London. "It requires clinical judgment to balance the risk of recurrent venous thromboembolism against the risk of bleeding [as an adverse effect of antithrombotic treatment] in patients with deteriorating health because of their progressive cancer," he said.
About 10 years ago, a 6-month course of treatment with a low-molecular-weight heparin became standard for cancer patients with a venous thromboembolism (VTE). Results from the CLOT (Randomized Comparison of Low Molecular Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer) trial showed that dalteparin (Fragmin) was more effective than warfarin or another oral anticoagulant for preventing recurrent VTE without increasing the risk of bleeding during 6 months of treatment (New Engl. J. Med. 2003;349:146-53).
To examine the impact of dalteparin treatment during months 7-12 following VTE, Dr. Kakkar and his associates conducted an open-label, phase IV study in 334 cancer patients who had a VTE. Trial participants received dalteparin for up to 12 months at 50 centers in the United States, Europe, and Canada. The patients averaged 64 years old, half were women, 92% had a solid tumor, and almost two-thirds had metastatic disease. The most common tumor type was lung cancer, followed by colorectal cancer, and breast and pancreatic cancer. Patients began the regimen by receiving 200 IU/kg dalteparin subcutaneously daily for the first 30 days, and then they received a reduced dosage of 150 IU/kg daily for the balance of their treatment.
The study’s primary endpoint was the rate of major bleeds during months 7-12 compared with months 2-6. Major bleeding occurred in 4.6% of the patients during months 2-6, and in 4.2% of the patients who received treatment during months 7-12. The highest rate of bleeding occurred during the first month of treatment, a 3.6% rate during the first 30 days on dalteparin, the time when the dosage was at its highest.
The incidence of all bleeding events was 13.2% during the first month on treatment, 17.3% during months 2-6, and 14.8% during months 7-12.
The incidence of new or recurrent VTEs was 5.7% during the first month on treatment, 3.4% during months 2-6, and 4.1% during months 7-12. The results suggest that dalteparin continued to suppress thrombosis during extended treatment. The overall VTE rate during the first 6 months was about 9%, which matched the 9% VTE rate on 6 months of dalteparin treatment reported in the CLOT study results in 2003. A logistic regression analysis failed to identify any patient factors that were significantly linked to the development of a new or recurrent VTE.
The study was sponsored by Eisai, the company that markets dalteparin (Fragmin). Dr. Kakkar said that he has been a consultant to and has received honoraria from Eisai as well as from several other drug companies.
[email protected]
On Twitter @mitchelzoler
AMSTERDAM – Cancer patients who start dalteparin to prevent a recurrence of venous thromboembolism can continue on the drug for as long as a year without an increase in bleeding risk and with stable control against another venous thromboembolism.
But the high underlying mortality risk from cancer that many of these patients face makes it hard to judge which patients will benefit from continued prophylaxis with the low-molecular-weight heparin, Dr. Ajay K. Kakkar said at the congress of the International Society on Thrombosis and Haemostasis.
A third of the patients enrolled in the trial died before the yearlong study ended, and the vast majority of the deaths were due to cancer.
"One of the most important competing risks besides thrombosis that these cancer patients face is the risk of dying from their underlying malignancy. That’s an important determinant in trying to understand whether we should continue anticoagulant treatment," said Dr. Kakkar, a professor of surgery at University College London. "It requires clinical judgment to balance the risk of recurrent venous thromboembolism against the risk of bleeding [as an adverse effect of antithrombotic treatment] in patients with deteriorating health because of their progressive cancer," he said.
About 10 years ago, a 6-month course of treatment with a low-molecular-weight heparin became standard for cancer patients with a venous thromboembolism (VTE). Results from the CLOT (Randomized Comparison of Low Molecular Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer) trial showed that dalteparin (Fragmin) was more effective than warfarin or another oral anticoagulant for preventing recurrent VTE without increasing the risk of bleeding during 6 months of treatment (New Engl. J. Med. 2003;349:146-53).
To examine the impact of dalteparin treatment during months 7-12 following VTE, Dr. Kakkar and his associates conducted an open-label, phase IV study in 334 cancer patients who had a VTE. Trial participants received dalteparin for up to 12 months at 50 centers in the United States, Europe, and Canada. The patients averaged 64 years old, half were women, 92% had a solid tumor, and almost two-thirds had metastatic disease. The most common tumor type was lung cancer, followed by colorectal cancer, and breast and pancreatic cancer. Patients began the regimen by receiving 200 IU/kg dalteparin subcutaneously daily for the first 30 days, and then they received a reduced dosage of 150 IU/kg daily for the balance of their treatment.
The study’s primary endpoint was the rate of major bleeds during months 7-12 compared with months 2-6. Major bleeding occurred in 4.6% of the patients during months 2-6, and in 4.2% of the patients who received treatment during months 7-12. The highest rate of bleeding occurred during the first month of treatment, a 3.6% rate during the first 30 days on dalteparin, the time when the dosage was at its highest.
The incidence of all bleeding events was 13.2% during the first month on treatment, 17.3% during months 2-6, and 14.8% during months 7-12.
The incidence of new or recurrent VTEs was 5.7% during the first month on treatment, 3.4% during months 2-6, and 4.1% during months 7-12. The results suggest that dalteparin continued to suppress thrombosis during extended treatment. The overall VTE rate during the first 6 months was about 9%, which matched the 9% VTE rate on 6 months of dalteparin treatment reported in the CLOT study results in 2003. A logistic regression analysis failed to identify any patient factors that were significantly linked to the development of a new or recurrent VTE.
The study was sponsored by Eisai, the company that markets dalteparin (Fragmin). Dr. Kakkar said that he has been a consultant to and has received honoraria from Eisai as well as from several other drug companies.
[email protected]
On Twitter @mitchelzoler
AMSTERDAM – Cancer patients who start dalteparin to prevent a recurrence of venous thromboembolism can continue on the drug for as long as a year without an increase in bleeding risk and with stable control against another venous thromboembolism.
But the high underlying mortality risk from cancer that many of these patients face makes it hard to judge which patients will benefit from continued prophylaxis with the low-molecular-weight heparin, Dr. Ajay K. Kakkar said at the congress of the International Society on Thrombosis and Haemostasis.
A third of the patients enrolled in the trial died before the yearlong study ended, and the vast majority of the deaths were due to cancer.
"One of the most important competing risks besides thrombosis that these cancer patients face is the risk of dying from their underlying malignancy. That’s an important determinant in trying to understand whether we should continue anticoagulant treatment," said Dr. Kakkar, a professor of surgery at University College London. "It requires clinical judgment to balance the risk of recurrent venous thromboembolism against the risk of bleeding [as an adverse effect of antithrombotic treatment] in patients with deteriorating health because of their progressive cancer," he said.
About 10 years ago, a 6-month course of treatment with a low-molecular-weight heparin became standard for cancer patients with a venous thromboembolism (VTE). Results from the CLOT (Randomized Comparison of Low Molecular Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer) trial showed that dalteparin (Fragmin) was more effective than warfarin or another oral anticoagulant for preventing recurrent VTE without increasing the risk of bleeding during 6 months of treatment (New Engl. J. Med. 2003;349:146-53).
To examine the impact of dalteparin treatment during months 7-12 following VTE, Dr. Kakkar and his associates conducted an open-label, phase IV study in 334 cancer patients who had a VTE. Trial participants received dalteparin for up to 12 months at 50 centers in the United States, Europe, and Canada. The patients averaged 64 years old, half were women, 92% had a solid tumor, and almost two-thirds had metastatic disease. The most common tumor type was lung cancer, followed by colorectal cancer, and breast and pancreatic cancer. Patients began the regimen by receiving 200 IU/kg dalteparin subcutaneously daily for the first 30 days, and then they received a reduced dosage of 150 IU/kg daily for the balance of their treatment.
The study’s primary endpoint was the rate of major bleeds during months 7-12 compared with months 2-6. Major bleeding occurred in 4.6% of the patients during months 2-6, and in 4.2% of the patients who received treatment during months 7-12. The highest rate of bleeding occurred during the first month of treatment, a 3.6% rate during the first 30 days on dalteparin, the time when the dosage was at its highest.
The incidence of all bleeding events was 13.2% during the first month on treatment, 17.3% during months 2-6, and 14.8% during months 7-12.
The incidence of new or recurrent VTEs was 5.7% during the first month on treatment, 3.4% during months 2-6, and 4.1% during months 7-12. The results suggest that dalteparin continued to suppress thrombosis during extended treatment. The overall VTE rate during the first 6 months was about 9%, which matched the 9% VTE rate on 6 months of dalteparin treatment reported in the CLOT study results in 2003. A logistic regression analysis failed to identify any patient factors that were significantly linked to the development of a new or recurrent VTE.
The study was sponsored by Eisai, the company that markets dalteparin (Fragmin). Dr. Kakkar said that he has been a consultant to and has received honoraria from Eisai as well as from several other drug companies.
[email protected]
On Twitter @mitchelzoler
AT THE 2013 ISTH CONGRESS
Major finding: Major bleeding occurred in 4.6% of the patients during months 2-6, and in 4.2% of the patients who received treatment during months 7-12.
Data source: Data came from a multicenter, open-label, phase IV study of dalteparin in 334 cancer patients following an index venous thromboembolism.
Disclosures: The study was sponsored by Eisai, the company that markets dalteparin (Fragmin). Dr. Kakkar said that he has been a consultant to and has received honoraria from Eisai as well as from several other drug companies.
Gender differences in the evolution of illness understanding among patients with advanced cancer
Background Patient understanding of advanced metastatic disease is central to decisions about care near death. Prior studies have focused on gender differences in communication style rather than on illness understanding.
Objectives To evaluate gender differences in terminal illness acknowledgement (TIA), understanding that the disease is incurable and the advanced stage of the disease. To evaluate gender differences in patients’ reports of discussions of life expectancy with oncology providers and its effect on differences in illness understanding.
Methods Coping with Cancer 2 patients (N 68) were interviewed before and after a visit with their oncology providers to discuss scan results.
Results At the prescan interview, there were no statistically significant gender differences in patient measures of illness understanding. At the postscan interview, women were more likely than men to recognize that their illness was incurable (Adjusted Odds Ratio, [AOR] 5.29; P .038), know that their cancer was at an advanced stage (AOR, 6.38; P, .013), and report having had discussions of life expectancy with their oncologist (AOR, 4.77; P, .021). Controlling discussions of life expectancy, women were more likely than men to report that their cancer was at an advanced stage (AOR, 9.53; P .050). Controlling for gender, discussions of life expectancy were associated with higher rates of TIA (AOR, 4.65; P, .036) and higher rates of understanding that the cancer was incurable (AOR, 4.09; P .085).
Conclusions Due largely to gender differences in communication, women over time have a better understanding of their illness than men. More frequent discussions of life expectancy should enhance illness understanding and reduce gender differences.
*For a PDF of the full article, click on the link to the left of this introduction.
Background Patient understanding of advanced metastatic disease is central to decisions about care near death. Prior studies have focused on gender differences in communication style rather than on illness understanding.
Objectives To evaluate gender differences in terminal illness acknowledgement (TIA), understanding that the disease is incurable and the advanced stage of the disease. To evaluate gender differences in patients’ reports of discussions of life expectancy with oncology providers and its effect on differences in illness understanding.
Methods Coping with Cancer 2 patients (N 68) were interviewed before and after a visit with their oncology providers to discuss scan results.
Results At the prescan interview, there were no statistically significant gender differences in patient measures of illness understanding. At the postscan interview, women were more likely than men to recognize that their illness was incurable (Adjusted Odds Ratio, [AOR] 5.29; P .038), know that their cancer was at an advanced stage (AOR, 6.38; P, .013), and report having had discussions of life expectancy with their oncologist (AOR, 4.77; P, .021). Controlling discussions of life expectancy, women were more likely than men to report that their cancer was at an advanced stage (AOR, 9.53; P .050). Controlling for gender, discussions of life expectancy were associated with higher rates of TIA (AOR, 4.65; P, .036) and higher rates of understanding that the cancer was incurable (AOR, 4.09; P .085).
Conclusions Due largely to gender differences in communication, women over time have a better understanding of their illness than men. More frequent discussions of life expectancy should enhance illness understanding and reduce gender differences.
*For a PDF of the full article, click on the link to the left of this introduction.
Background Patient understanding of advanced metastatic disease is central to decisions about care near death. Prior studies have focused on gender differences in communication style rather than on illness understanding.
Objectives To evaluate gender differences in terminal illness acknowledgement (TIA), understanding that the disease is incurable and the advanced stage of the disease. To evaluate gender differences in patients’ reports of discussions of life expectancy with oncology providers and its effect on differences in illness understanding.
Methods Coping with Cancer 2 patients (N 68) were interviewed before and after a visit with their oncology providers to discuss scan results.
Results At the prescan interview, there were no statistically significant gender differences in patient measures of illness understanding. At the postscan interview, women were more likely than men to recognize that their illness was incurable (Adjusted Odds Ratio, [AOR] 5.29; P .038), know that their cancer was at an advanced stage (AOR, 6.38; P, .013), and report having had discussions of life expectancy with their oncologist (AOR, 4.77; P, .021). Controlling discussions of life expectancy, women were more likely than men to report that their cancer was at an advanced stage (AOR, 9.53; P .050). Controlling for gender, discussions of life expectancy were associated with higher rates of TIA (AOR, 4.65; P, .036) and higher rates of understanding that the cancer was incurable (AOR, 4.09; P .085).
Conclusions Due largely to gender differences in communication, women over time have a better understanding of their illness than men. More frequent discussions of life expectancy should enhance illness understanding and reduce gender differences.
*For a PDF of the full article, click on the link to the left of this introduction.
Chlorpromazine bioavailability from a topical gel formulation in volunteers
Background Symptom management medications are often compounded into topical gel formulations providing an alternative route of administration for hospice and palliative care patients. Though commonly used, transdermal absorption and bioavailability studies of these gel products are lacking. Chlorpromazine was studied because it is FDA approved for treatment of nausea and vomiting and is used off-label for treatment of agitation and delirium.
Objective The objective of this study is to determine the transdermal absorption of chlorpromazine PLO gel in healthy adults.
Methods Twenty-five milligrams of chlorpromazine in PLO gel was applied to 10 subjects’ wrists and 100 mg was applied to 1 subject’s wrist. Blood draws were completed preapplication and 1, 2, and 4 hours postapplication. This single-center unblinded study recruited healthy adults between 18 and 70 years of age. Participants were not pregnant, did not have an allergy to any component of the study medication, and were not taking a phenothiazine medication.
Results Chlorpromazine was undetected in any of the 11 subjects’ blood samples.
Limitations There is an assumption of equivalent medication absorption in healthy patients and palliative care or hospice patients.
Conclusion Rapid relief of symptoms at end of life is essential. Chlorpromazine in PLO gel may not be an effective treatment option since blood levels were undetectable at 1, 2, and 4 hours after topical application.
*For a PDF of the full article, click on the link to the left of this introduction.
Background Symptom management medications are often compounded into topical gel formulations providing an alternative route of administration for hospice and palliative care patients. Though commonly used, transdermal absorption and bioavailability studies of these gel products are lacking. Chlorpromazine was studied because it is FDA approved for treatment of nausea and vomiting and is used off-label for treatment of agitation and delirium.
Objective The objective of this study is to determine the transdermal absorption of chlorpromazine PLO gel in healthy adults.
Methods Twenty-five milligrams of chlorpromazine in PLO gel was applied to 10 subjects’ wrists and 100 mg was applied to 1 subject’s wrist. Blood draws were completed preapplication and 1, 2, and 4 hours postapplication. This single-center unblinded study recruited healthy adults between 18 and 70 years of age. Participants were not pregnant, did not have an allergy to any component of the study medication, and were not taking a phenothiazine medication.
Results Chlorpromazine was undetected in any of the 11 subjects’ blood samples.
Limitations There is an assumption of equivalent medication absorption in healthy patients and palliative care or hospice patients.
Conclusion Rapid relief of symptoms at end of life is essential. Chlorpromazine in PLO gel may not be an effective treatment option since blood levels were undetectable at 1, 2, and 4 hours after topical application.
*For a PDF of the full article, click on the link to the left of this introduction.
Background Symptom management medications are often compounded into topical gel formulations providing an alternative route of administration for hospice and palliative care patients. Though commonly used, transdermal absorption and bioavailability studies of these gel products are lacking. Chlorpromazine was studied because it is FDA approved for treatment of nausea and vomiting and is used off-label for treatment of agitation and delirium.
Objective The objective of this study is to determine the transdermal absorption of chlorpromazine PLO gel in healthy adults.
Methods Twenty-five milligrams of chlorpromazine in PLO gel was applied to 10 subjects’ wrists and 100 mg was applied to 1 subject’s wrist. Blood draws were completed preapplication and 1, 2, and 4 hours postapplication. This single-center unblinded study recruited healthy adults between 18 and 70 years of age. Participants were not pregnant, did not have an allergy to any component of the study medication, and were not taking a phenothiazine medication.
Results Chlorpromazine was undetected in any of the 11 subjects’ blood samples.
Limitations There is an assumption of equivalent medication absorption in healthy patients and palliative care or hospice patients.
Conclusion Rapid relief of symptoms at end of life is essential. Chlorpromazine in PLO gel may not be an effective treatment option since blood levels were undetectable at 1, 2, and 4 hours after topical application.
*For a PDF of the full article, click on the link to the left of this introduction.
Complementary and alternative medicine (CAM) use in advanced cancer: a systematic review
This systematic review synthesizes knowledge about the use of complementary and alternative medicine (CAM) among advanced cancer patients. EBSCO and Ovid databases were searched using core concepts, including advanced cancer, CAM, integrative medicine, and decision-making. Articles included in the final review were analyzed using narrative synthesis methods, including thematic analysis, concept mapping, and critical reflection on the synthesis process. Results demonstrate that advanced cancer patients who are younger, female, more educated, have longer duration of disease, and have previously used CAM are more likely to use CAM during this stage of illness. Key themes identified include patterns of and reasons for use; and barriers and facilitators to informed CAM decision-making. Knowledge regarding the use of CAM in advanced cancer remains in its nascent stages. Findings suggest a need for more research on understanding the dynamic process of CAM decision-making in the advanced cancer population from the patients’ perspective.
*For a PDF of the full article, click on the link to the left of this introduction.
This systematic review synthesizes knowledge about the use of complementary and alternative medicine (CAM) among advanced cancer patients. EBSCO and Ovid databases were searched using core concepts, including advanced cancer, CAM, integrative medicine, and decision-making. Articles included in the final review were analyzed using narrative synthesis methods, including thematic analysis, concept mapping, and critical reflection on the synthesis process. Results demonstrate that advanced cancer patients who are younger, female, more educated, have longer duration of disease, and have previously used CAM are more likely to use CAM during this stage of illness. Key themes identified include patterns of and reasons for use; and barriers and facilitators to informed CAM decision-making. Knowledge regarding the use of CAM in advanced cancer remains in its nascent stages. Findings suggest a need for more research on understanding the dynamic process of CAM decision-making in the advanced cancer population from the patients’ perspective.
*For a PDF of the full article, click on the link to the left of this introduction.
This systematic review synthesizes knowledge about the use of complementary and alternative medicine (CAM) among advanced cancer patients. EBSCO and Ovid databases were searched using core concepts, including advanced cancer, CAM, integrative medicine, and decision-making. Articles included in the final review were analyzed using narrative synthesis methods, including thematic analysis, concept mapping, and critical reflection on the synthesis process. Results demonstrate that advanced cancer patients who are younger, female, more educated, have longer duration of disease, and have previously used CAM are more likely to use CAM during this stage of illness. Key themes identified include patterns of and reasons for use; and barriers and facilitators to informed CAM decision-making. Knowledge regarding the use of CAM in advanced cancer remains in its nascent stages. Findings suggest a need for more research on understanding the dynamic process of CAM decision-making in the advanced cancer population from the patients’ perspective.
*For a PDF of the full article, click on the link to the left of this introduction.
Best practices for pediatric palliative cancer care: a primer for clinical providers
Cancer is the leading cause of disease-related death in children and adolescents. Pediatric patients with cancer suffer greatly at the end of life. However, palliative care interventions can reduce suffering and significantly improve the care of these patients and their families. A large percentage of pediatric deaths occur outside of the hospital setting where pediatric palliative resources may not be readily available. This review focuses on the principles of best practice in the provision of palliative care for children and adolescents with cancer.
Click on the PDF icon at the top of this introduction to read the full article.
Cancer is the leading cause of disease-related death in children and adolescents. Pediatric patients with cancer suffer greatly at the end of life. However, palliative care interventions can reduce suffering and significantly improve the care of these patients and their families. A large percentage of pediatric deaths occur outside of the hospital setting where pediatric palliative resources may not be readily available. This review focuses on the principles of best practice in the provision of palliative care for children and adolescents with cancer.
Click on the PDF icon at the top of this introduction to read the full article.
Cancer is the leading cause of disease-related death in children and adolescents. Pediatric patients with cancer suffer greatly at the end of life. However, palliative care interventions can reduce suffering and significantly improve the care of these patients and their families. A large percentage of pediatric deaths occur outside of the hospital setting where pediatric palliative resources may not be readily available. This review focuses on the principles of best practice in the provision of palliative care for children and adolescents with cancer.
Click on the PDF icon at the top of this introduction to read the full article.
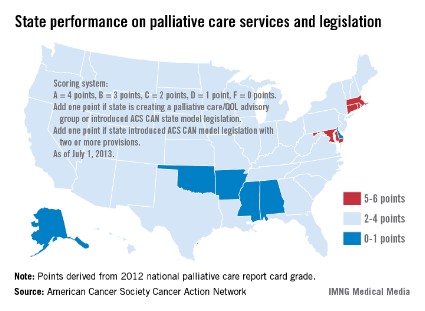
Few states meet palliative care benchmark
Only four states have effective strategies in place to improve access to and knowledge of palliative care services, the American Cancer Society Cancer Action Network reported.
The ACS CAN awarded top scores (5-6 points) to Connecticut, Maryland, Massachusetts, and Rhode Island using a scoring system that combines grades from the Center to Advance Palliative Care’s national palliative care report card with actions on model legislation.
The four states passed laws "this session that focus on improving patient quality of life through palliative care," the ACS CAN noted, with Maryland finally crossing "the finish line with a palliative care bill after a 3-year effort."
The six states on the low end of the scoring range (0-1 points) were Alabama, Alaska, Arkansas, Delaware, Mississippi, and Oklahoma, the ACS CAN said in its report.
Palliative care is 1 of 10 legislative priority areas – including comprehensive smoke-free laws, tobacco taxes, restrictions on tanning bed use by minors, improved access to Medicaid, balanced pain policies, and time requirements for physical education in schools – measured by the ACS CAN. The network said that 38 states have reached benchmarks in three or fewer of the 10 areas, and that no states met the benchmarks in more than six areas.
"Many state legislatures are missing opportunities to enact laws and policies that could not only generate new revenue and long-term health savings, but also save lives," the ACS CAN said in a statement.
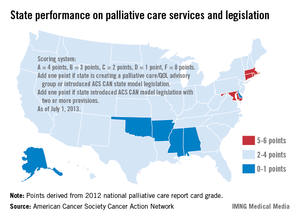
Only four states have effective strategies in place to improve access to and knowledge of palliative care services, the American Cancer Society Cancer Action Network reported.
The ACS CAN awarded top scores (5-6 points) to Connecticut, Maryland, Massachusetts, and Rhode Island using a scoring system that combines grades from the Center to Advance Palliative Care’s national palliative care report card with actions on model legislation.

The four states passed laws "this session that focus on improving patient quality of life through palliative care," the ACS CAN noted, with Maryland finally crossing "the finish line with a palliative care bill after a 3-year effort."
The six states on the low end of the scoring range (0-1 points) were Alabama, Alaska, Arkansas, Delaware, Mississippi, and Oklahoma, the ACS CAN said in its report.
Palliative care is 1 of 10 legislative priority areas – including comprehensive smoke-free laws, tobacco taxes, restrictions on tanning bed use by minors, improved access to Medicaid, balanced pain policies, and time requirements for physical education in schools – measured by the ACS CAN. The network said that 38 states have reached benchmarks in three or fewer of the 10 areas, and that no states met the benchmarks in more than six areas.
"Many state legislatures are missing opportunities to enact laws and policies that could not only generate new revenue and long-term health savings, but also save lives," the ACS CAN said in a statement.
Only four states have effective strategies in place to improve access to and knowledge of palliative care services, the American Cancer Society Cancer Action Network reported.
The ACS CAN awarded top scores (5-6 points) to Connecticut, Maryland, Massachusetts, and Rhode Island using a scoring system that combines grades from the Center to Advance Palliative Care’s national palliative care report card with actions on model legislation.

The four states passed laws "this session that focus on improving patient quality of life through palliative care," the ACS CAN noted, with Maryland finally crossing "the finish line with a palliative care bill after a 3-year effort."
The six states on the low end of the scoring range (0-1 points) were Alabama, Alaska, Arkansas, Delaware, Mississippi, and Oklahoma, the ACS CAN said in its report.
Palliative care is 1 of 10 legislative priority areas – including comprehensive smoke-free laws, tobacco taxes, restrictions on tanning bed use by minors, improved access to Medicaid, balanced pain policies, and time requirements for physical education in schools – measured by the ACS CAN. The network said that 38 states have reached benchmarks in three or fewer of the 10 areas, and that no states met the benchmarks in more than six areas.
"Many state legislatures are missing opportunities to enact laws and policies that could not only generate new revenue and long-term health savings, but also save lives," the ACS CAN said in a statement.
Oncologist compensation deserves evidence-based scrutiny and analysis
As community-based oncology practices have faced continued cutbacks in reimbursements under the Medicare Prescription Drug, Improvement, and Modernization Act and now through sequestration, many have had to close satellite sites, cut back on their supportive services, join networks – where possible – or hospitals or health systems, and scramble to engage payers in rethinking payment models. They have done so not only to cover their costs for the technological outlay, staffing, and other overheads necessary for them to provide quality oncology care, but also to ensure competitive compensation packages for their teams of highly trained, specialized physicians, midlevel practitioners, and nursing and administrative staff…
*Click on the link to the left for a PDF of the full article.
As community-based oncology practices have faced continued cutbacks in reimbursements under the Medicare Prescription Drug, Improvement, and Modernization Act and now through sequestration, many have had to close satellite sites, cut back on their supportive services, join networks – where possible – or hospitals or health systems, and scramble to engage payers in rethinking payment models. They have done so not only to cover their costs for the technological outlay, staffing, and other overheads necessary for them to provide quality oncology care, but also to ensure competitive compensation packages for their teams of highly trained, specialized physicians, midlevel practitioners, and nursing and administrative staff…
*Click on the link to the left for a PDF of the full article.
As community-based oncology practices have faced continued cutbacks in reimbursements under the Medicare Prescription Drug, Improvement, and Modernization Act and now through sequestration, many have had to close satellite sites, cut back on their supportive services, join networks – where possible – or hospitals or health systems, and scramble to engage payers in rethinking payment models. They have done so not only to cover their costs for the technological outlay, staffing, and other overheads necessary for them to provide quality oncology care, but also to ensure competitive compensation packages for their teams of highly trained, specialized physicians, midlevel practitioners, and nursing and administrative staff…
*Click on the link to the left for a PDF of the full article.