User login
Guidelines issued on radiation-induced heart disease
Cancer patients undergoing radiation therapy need to have baseline studies of cardiac function and routine screening for heart disease, according to recommendations from the European Society of Cardiology and the American Society of Echocardiography published July 16 in the European Heart Journal–Cardiovascular Imaging.
The groups recommend baseline preradiation echocardiography along with a cardiac exam as well as screening for risk factors. An annual cardiac history and physical should be performed to check for new-onset heart problems.
Within 10 years of treatment, 10%-30% of patients who undergo radiation therapy develop radiation-induced heart diseases (RIHD), including chronic pericarditis, myocardial fibrosis, coronary artery disease, aortic calcification, and valve regurgitation or stenosis. The hope of screening is to catch early RIHD, but screening is not currently routine.
"We wrote the expert consensus to raise the alarm that the risks of radiation-induced heart disease should not be ignored. The prevalence ... is increasing because the rate of cancer survival has improved," said Dr. Patrizio Lancellotti, who is a professor of cardiology at the University Hospital of Liège, Belgium, and led the recommendations task force.
Radiotherapy is given in more targeted form and at lower doses than it once was, but "patients are still at increased risk of RIHD, particularly when the heart is in the radiation field. This applies to patients treated for lymphoma, breast cancer, and esophageal cancer. Patients who receive radiotherapy for neck cancer are also at risk because lesions can develop on the carotid artery and increase the risk of stroke," Dr. Lancellotti said in a statement.
Using targeted radiation and alternate radiation fields, with avoidance and shielding of the heart, remain "the most important interventions to prevent" cardiac complications, the authors noted.
The task force advises that high-risk patients without evidence of heart disease on history and physical should have screening echocardiography every 5 years and noninvasive stress testing every 5-10 years; low-risk patients should have screening echocardiography every 10 years. If heart disorders are detected, routine monitoring should include echocardiography, cardiac magnetic resonance imaging, or carotid ultrasound as appropriate.
High-risk patients include those who received radiotherapy at younger ages; those who have cardiovascular risk factors or preexisting heart disease; and those who receive high-dose radiation (greater than 30 Gy), concomitant chemotherapy, radiation without shielding, or anterior or left chest radiation (Eur. Heart J. Cardiovasc. Imaging 2013;14:721-40).
The recommendations are based on an extensive literature review and analysis by Dr. Lancellotti and other specialists.
The authors reported no financial conflicts or outside funding for their work.
Cancer patients undergoing radiation therapy need to have baseline studies of cardiac function and routine screening for heart disease, according to recommendations from the European Society of Cardiology and the American Society of Echocardiography published July 16 in the European Heart Journal–Cardiovascular Imaging.
The groups recommend baseline preradiation echocardiography along with a cardiac exam as well as screening for risk factors. An annual cardiac history and physical should be performed to check for new-onset heart problems.
Within 10 years of treatment, 10%-30% of patients who undergo radiation therapy develop radiation-induced heart diseases (RIHD), including chronic pericarditis, myocardial fibrosis, coronary artery disease, aortic calcification, and valve regurgitation or stenosis. The hope of screening is to catch early RIHD, but screening is not currently routine.
"We wrote the expert consensus to raise the alarm that the risks of radiation-induced heart disease should not be ignored. The prevalence ... is increasing because the rate of cancer survival has improved," said Dr. Patrizio Lancellotti, who is a professor of cardiology at the University Hospital of Liège, Belgium, and led the recommendations task force.
Radiotherapy is given in more targeted form and at lower doses than it once was, but "patients are still at increased risk of RIHD, particularly when the heart is in the radiation field. This applies to patients treated for lymphoma, breast cancer, and esophageal cancer. Patients who receive radiotherapy for neck cancer are also at risk because lesions can develop on the carotid artery and increase the risk of stroke," Dr. Lancellotti said in a statement.
Using targeted radiation and alternate radiation fields, with avoidance and shielding of the heart, remain "the most important interventions to prevent" cardiac complications, the authors noted.
The task force advises that high-risk patients without evidence of heart disease on history and physical should have screening echocardiography every 5 years and noninvasive stress testing every 5-10 years; low-risk patients should have screening echocardiography every 10 years. If heart disorders are detected, routine monitoring should include echocardiography, cardiac magnetic resonance imaging, or carotid ultrasound as appropriate.
High-risk patients include those who received radiotherapy at younger ages; those who have cardiovascular risk factors or preexisting heart disease; and those who receive high-dose radiation (greater than 30 Gy), concomitant chemotherapy, radiation without shielding, or anterior or left chest radiation (Eur. Heart J. Cardiovasc. Imaging 2013;14:721-40).
The recommendations are based on an extensive literature review and analysis by Dr. Lancellotti and other specialists.
The authors reported no financial conflicts or outside funding for their work.
Cancer patients undergoing radiation therapy need to have baseline studies of cardiac function and routine screening for heart disease, according to recommendations from the European Society of Cardiology and the American Society of Echocardiography published July 16 in the European Heart Journal–Cardiovascular Imaging.
The groups recommend baseline preradiation echocardiography along with a cardiac exam as well as screening for risk factors. An annual cardiac history and physical should be performed to check for new-onset heart problems.
Within 10 years of treatment, 10%-30% of patients who undergo radiation therapy develop radiation-induced heart diseases (RIHD), including chronic pericarditis, myocardial fibrosis, coronary artery disease, aortic calcification, and valve regurgitation or stenosis. The hope of screening is to catch early RIHD, but screening is not currently routine.
"We wrote the expert consensus to raise the alarm that the risks of radiation-induced heart disease should not be ignored. The prevalence ... is increasing because the rate of cancer survival has improved," said Dr. Patrizio Lancellotti, who is a professor of cardiology at the University Hospital of Liège, Belgium, and led the recommendations task force.
Radiotherapy is given in more targeted form and at lower doses than it once was, but "patients are still at increased risk of RIHD, particularly when the heart is in the radiation field. This applies to patients treated for lymphoma, breast cancer, and esophageal cancer. Patients who receive radiotherapy for neck cancer are also at risk because lesions can develop on the carotid artery and increase the risk of stroke," Dr. Lancellotti said in a statement.
Using targeted radiation and alternate radiation fields, with avoidance and shielding of the heart, remain "the most important interventions to prevent" cardiac complications, the authors noted.
The task force advises that high-risk patients without evidence of heart disease on history and physical should have screening echocardiography every 5 years and noninvasive stress testing every 5-10 years; low-risk patients should have screening echocardiography every 10 years. If heart disorders are detected, routine monitoring should include echocardiography, cardiac magnetic resonance imaging, or carotid ultrasound as appropriate.
High-risk patients include those who received radiotherapy at younger ages; those who have cardiovascular risk factors or preexisting heart disease; and those who receive high-dose radiation (greater than 30 Gy), concomitant chemotherapy, radiation without shielding, or anterior or left chest radiation (Eur. Heart J. Cardiovasc. Imaging 2013;14:721-40).
The recommendations are based on an extensive literature review and analysis by Dr. Lancellotti and other specialists.
The authors reported no financial conflicts or outside funding for their work.
FROM THE EUROPEAN HEART JOURNAL – CARDIOVASCULAR IMAGING
Khorana score tracks risk of cancer death
AMSTERDAM – A score routinely used to quantify cancer patients’ risk for developing venous thromboembolism also can be used to gauge their risk of death, according to an analysis of data from more than 1,500 patients.
The new findings are only exploratory, but they suggest that the well-established risk calculator known as the Khorana score "may support clinical decision making not only for VTE prophylaxis but also for anticancer treatment strategies," Dr. Cihan Ay said at the Congress of the International Society on Thrombosis and Haemostasis.
The link between higher Khorana scores and an increased rate of death over the following 2 years was independent of VTE occurrence, suggesting that the Khorana score identifies susceptibilities in addition to VTE than can cause death, said Dr. Ay, a hematologist-oncologist at the Medical University of Vienna.
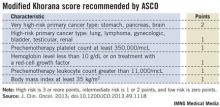
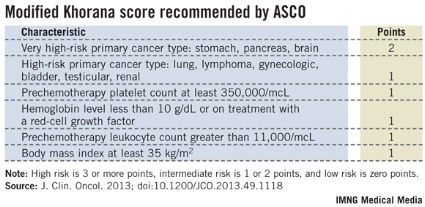
Following the Khorana score’s introduction in 2008 by Dr. Alok Khorana and his associates (Blood 2008;111:4902-7) to assess a patient’s risk for VTE and need for anticoagulant prophylaxis, the score underwent several validations. Earlier this year, the score was adopted in a slightly modified form as part of the VTE management guidelines of the American Society of Clinical Oncology (J. Clin. Onc. 2013 [doi: 10.1200/JCO.2013.49.1118]).
The ASCO guidelines say that "the Panel recommends that patients with cancer be assessed for VTE risk at the time of chemotherapy initiation and periodically thereafter" using the slightly modified form of the Khorana score (see box). The 2013 formula from the ASCO Panel revised the original 2008 version of the Khorana score by adding primary brain tumors to the 2-point category and renal tumors to the 1-point category.
"The Khorana score is used to identify patients for primary thromboprophylaxis. My idea is to also use it to get information on general prognosis," Dr. Ay said in an interview. "There is often a discussion of the patient’s prognosis and how far to go with treatment, how intensive treatment should be. If patients have a poor prognosis, some treatments may not be worth trying."
Dr. Ay and his associates applied their own slight modification of the Khorana score to 1,544 patients with a variety of cancers enrolled in the Vienna Cancer and Thrombosis Study. The patients averaged 62 years old, about 45% were women, and they were followed for a mean of about 19 months, during which overall, all-cause mortality was 42%. The most common tumor type was lung cancer, in about 250 patients, followed by lymphoma, breast cancer, and brain tumors, which each affected more than 200 patients. The researchers modified the Khorana score by adding melanoma to the 1-point group of cancers and removing gynecologic, bladder, and testicular cancer from being assigned any points.
The analysis showed a strong link between patients’ Khorana scores at baseline, before treatment began, and their survival over the next 2 years of follow-up. The 466 patients (30%) with a score of 0 had a 27% mortality rate; the 489 patients (32%) with a score of 1 had a 38% mortality rate (adjusted hazard ratio, 61% compared with patients with a score of 0). The 405 patients (26%) with a score of 2 had a 51% mortality rate, a 2.8-fold increased mortality rate after adjustment, and the 184 patients (12%) with a score of 3 or more had a 63% mortality rate, a fourfold higher mortality rate after adjustment compared with patients with a score of 0. Between-group differences in mortality were all statistically significant. The hazard model adjusted for age, sex, and incident VTE.
Overall in the adjusted model, every 1-point increase in modified Khorana score linked with a statistically significant 56% increase in mortality, Dr. Ay said.
Dr. Ay said that he had no relevant disclosures.
On Twitter @mitchelzoler
AMSTERDAM – A score routinely used to quantify cancer patients’ risk for developing venous thromboembolism also can be used to gauge their risk of death, according to an analysis of data from more than 1,500 patients.
The new findings are only exploratory, but they suggest that the well-established risk calculator known as the Khorana score "may support clinical decision making not only for VTE prophylaxis but also for anticancer treatment strategies," Dr. Cihan Ay said at the Congress of the International Society on Thrombosis and Haemostasis.
The link between higher Khorana scores and an increased rate of death over the following 2 years was independent of VTE occurrence, suggesting that the Khorana score identifies susceptibilities in addition to VTE than can cause death, said Dr. Ay, a hematologist-oncologist at the Medical University of Vienna.
Following the Khorana score’s introduction in 2008 by Dr. Alok Khorana and his associates (Blood 2008;111:4902-7) to assess a patient’s risk for VTE and need for anticoagulant prophylaxis, the score underwent several validations. Earlier this year, the score was adopted in a slightly modified form as part of the VTE management guidelines of the American Society of Clinical Oncology (J. Clin. Onc. 2013 [doi: 10.1200/JCO.2013.49.1118]).
The ASCO guidelines say that "the Panel recommends that patients with cancer be assessed for VTE risk at the time of chemotherapy initiation and periodically thereafter" using the slightly modified form of the Khorana score (see box). The 2013 formula from the ASCO Panel revised the original 2008 version of the Khorana score by adding primary brain tumors to the 2-point category and renal tumors to the 1-point category.
"The Khorana score is used to identify patients for primary thromboprophylaxis. My idea is to also use it to get information on general prognosis," Dr. Ay said in an interview. "There is often a discussion of the patient’s prognosis and how far to go with treatment, how intensive treatment should be. If patients have a poor prognosis, some treatments may not be worth trying."
Dr. Ay and his associates applied their own slight modification of the Khorana score to 1,544 patients with a variety of cancers enrolled in the Vienna Cancer and Thrombosis Study. The patients averaged 62 years old, about 45% were women, and they were followed for a mean of about 19 months, during which overall, all-cause mortality was 42%. The most common tumor type was lung cancer, in about 250 patients, followed by lymphoma, breast cancer, and brain tumors, which each affected more than 200 patients. The researchers modified the Khorana score by adding melanoma to the 1-point group of cancers and removing gynecologic, bladder, and testicular cancer from being assigned any points.
The analysis showed a strong link between patients’ Khorana scores at baseline, before treatment began, and their survival over the next 2 years of follow-up. The 466 patients (30%) with a score of 0 had a 27% mortality rate; the 489 patients (32%) with a score of 1 had a 38% mortality rate (adjusted hazard ratio, 61% compared with patients with a score of 0). The 405 patients (26%) with a score of 2 had a 51% mortality rate, a 2.8-fold increased mortality rate after adjustment, and the 184 patients (12%) with a score of 3 or more had a 63% mortality rate, a fourfold higher mortality rate after adjustment compared with patients with a score of 0. Between-group differences in mortality were all statistically significant. The hazard model adjusted for age, sex, and incident VTE.
Overall in the adjusted model, every 1-point increase in modified Khorana score linked with a statistically significant 56% increase in mortality, Dr. Ay said.
Dr. Ay said that he had no relevant disclosures.
On Twitter @mitchelzoler
AMSTERDAM – A score routinely used to quantify cancer patients’ risk for developing venous thromboembolism also can be used to gauge their risk of death, according to an analysis of data from more than 1,500 patients.
The new findings are only exploratory, but they suggest that the well-established risk calculator known as the Khorana score "may support clinical decision making not only for VTE prophylaxis but also for anticancer treatment strategies," Dr. Cihan Ay said at the Congress of the International Society on Thrombosis and Haemostasis.
The link between higher Khorana scores and an increased rate of death over the following 2 years was independent of VTE occurrence, suggesting that the Khorana score identifies susceptibilities in addition to VTE than can cause death, said Dr. Ay, a hematologist-oncologist at the Medical University of Vienna.
Following the Khorana score’s introduction in 2008 by Dr. Alok Khorana and his associates (Blood 2008;111:4902-7) to assess a patient’s risk for VTE and need for anticoagulant prophylaxis, the score underwent several validations. Earlier this year, the score was adopted in a slightly modified form as part of the VTE management guidelines of the American Society of Clinical Oncology (J. Clin. Onc. 2013 [doi: 10.1200/JCO.2013.49.1118]).
The ASCO guidelines say that "the Panel recommends that patients with cancer be assessed for VTE risk at the time of chemotherapy initiation and periodically thereafter" using the slightly modified form of the Khorana score (see box). The 2013 formula from the ASCO Panel revised the original 2008 version of the Khorana score by adding primary brain tumors to the 2-point category and renal tumors to the 1-point category.
"The Khorana score is used to identify patients for primary thromboprophylaxis. My idea is to also use it to get information on general prognosis," Dr. Ay said in an interview. "There is often a discussion of the patient’s prognosis and how far to go with treatment, how intensive treatment should be. If patients have a poor prognosis, some treatments may not be worth trying."
Dr. Ay and his associates applied their own slight modification of the Khorana score to 1,544 patients with a variety of cancers enrolled in the Vienna Cancer and Thrombosis Study. The patients averaged 62 years old, about 45% were women, and they were followed for a mean of about 19 months, during which overall, all-cause mortality was 42%. The most common tumor type was lung cancer, in about 250 patients, followed by lymphoma, breast cancer, and brain tumors, which each affected more than 200 patients. The researchers modified the Khorana score by adding melanoma to the 1-point group of cancers and removing gynecologic, bladder, and testicular cancer from being assigned any points.
The analysis showed a strong link between patients’ Khorana scores at baseline, before treatment began, and their survival over the next 2 years of follow-up. The 466 patients (30%) with a score of 0 had a 27% mortality rate; the 489 patients (32%) with a score of 1 had a 38% mortality rate (adjusted hazard ratio, 61% compared with patients with a score of 0). The 405 patients (26%) with a score of 2 had a 51% mortality rate, a 2.8-fold increased mortality rate after adjustment, and the 184 patients (12%) with a score of 3 or more had a 63% mortality rate, a fourfold higher mortality rate after adjustment compared with patients with a score of 0. Between-group differences in mortality were all statistically significant. The hazard model adjusted for age, sex, and incident VTE.
Overall in the adjusted model, every 1-point increase in modified Khorana score linked with a statistically significant 56% increase in mortality, Dr. Ay said.
Dr. Ay said that he had no relevant disclosures.
On Twitter @mitchelzoler
AT THE 2013 ISTH CONGRESS
Major finding: In the adjusted model, every 1-point increase in modified Khorana score linked with a statistically significant 56% increase in mortality.
Data source: An analysis of 1,544 cancer patients followed prospectively at an Austrian center.
Disclosures: Dr. Ay said that he had no relevant disclosures.
Cancer survivors may have lower Alzheimer’s risk
BOSTON – It’s a trade-off that few people would be likely to make, but having cancer – particularly a type treated by chemotherapy – is associated with a significantly decreased risk for developing Alzheimer’s disease, a retrospective study has shown.
An inverse relationship was found between the incidence of the majority of different types of cancer and Alzheimer’s disease (AD) risk, in a review of the records of nearly 3.5 million U.S. veterans.
Liver cancer survivors had the lowest risk for AD (hazard ratio = 0.49), followed by survivors of pancreatic cancer (HR = 0.56), esophageal cancer (0.67), and multiple myeloma (0.74), reported Dr. Laura Frain of the geriatric research education and clinical center at Boston VA Medical Center.
However, survivors of prostate cancer had a small but significantly higher risk for AD (HR = 1.11), and other screening-detected cancers (colorectal cancer, melanoma) were not associated with a reduced risk, Dr. Frain and her associates found.
"There may be something different about prostate cancer survivors, but whether that’s biologic or related to the way that they’re screened is unclear," Dr. Frain said in an interview.
Many types of cancer were associated with an increased risk for non-AD dementias, with increased risks ranging from an HR of 1.11 for head and neck cancer to 2.64 for brain cancer.
The investigators attempted to control for the possibility that people with cancer may not survive long enough to develop frank dementia by looking at other age-related conditions, including stroke, osteoarthritis, cataracts, and macular degeneration. They found that all cancers were associated with an increased risk for other age-related conditions, suggesting that their findings of reduced AD risk were valid, Dr. Frain said at the Alzheimer’s Association International Conference 2013.
Findings corroborated
The findings parallel those of a recently published study of more than 1 million residents of Northern Italy (Neurology 2013 July 10 [doi:10.1212/WNL.0b013e31829c5ec1]). The investigators in that study found that "[t]he risk of cancer in patients with AD dementia was halved, and the risk of AD dementia in patients with cancer was 35% reduced. This relationship was observed in almost all subgroup analyses, suggesting that some anticipated potential confounding factors did not significantly influence the results."
Dr. Frain noted that three previous prospective cohort studies had found that older adults with cancer had a reduction in risk for incident AD, ranging from a 33% to a 60% decline. Those studies, however, were limited by small numbers of patients with cancer, limiting the ability to look for associations with specific cancer or treatment types.
The current study drew on data from the massive U.S. National Veterans Affairs Healthcare System to assemble a cohort of 3,499,378 veterans who received outpatient care within the system from 1997 to 2011. The investigators used regression analysis models adjusted for cancer treatment, multiple comorbidities, follow-up time, and number of visits per year before baseline. In addition, the researchers performed subanalyses of patients with diagnoses of prostate, lung, colorectal, and bladder cancers and lymphoma, with the models adjusted for cancer stage and grade.
The median age of the cohort was 71 years, 98% were males, and 66% were white. A total of 771,285 veterans (22%) had a cancer diagnosis. Of the 82,028 veterans who developed AD (2.3% of the entire cohort), 24% were cancer survivors, and the remainder had no cancer history.
The investigators also found that "treatment with chemotherapy conferred additional protection against AD in nearly all cancer types, suggesting that some forms of chemotherapy may have neuroprotective action."
Chemotherapy lowered the risk of AD associated with all cancers except prostate cancer by 20%-45%.
The investigators plan to look at individual categories of anticancer agents, and to see whether they may be common factors between cancer and AD in regard to proteins, genes, and biochemical pathways.
One intriguing target for exploration, Dr. Frain said, is peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN1), an enzyme that when upregulated has been associated with the pathogenesis of some forms of cancer, and when downregulated may be associated with the development of AD.
The study was supported by grants from the Department of Veterans Affairs. Dr. Frain is supported by a VA Special Fellowship in geriatrics.
BOSTON – It’s a trade-off that few people would be likely to make, but having cancer – particularly a type treated by chemotherapy – is associated with a significantly decreased risk for developing Alzheimer’s disease, a retrospective study has shown.
An inverse relationship was found between the incidence of the majority of different types of cancer and Alzheimer’s disease (AD) risk, in a review of the records of nearly 3.5 million U.S. veterans.
Liver cancer survivors had the lowest risk for AD (hazard ratio = 0.49), followed by survivors of pancreatic cancer (HR = 0.56), esophageal cancer (0.67), and multiple myeloma (0.74), reported Dr. Laura Frain of the geriatric research education and clinical center at Boston VA Medical Center.
However, survivors of prostate cancer had a small but significantly higher risk for AD (HR = 1.11), and other screening-detected cancers (colorectal cancer, melanoma) were not associated with a reduced risk, Dr. Frain and her associates found.
"There may be something different about prostate cancer survivors, but whether that’s biologic or related to the way that they’re screened is unclear," Dr. Frain said in an interview.
Many types of cancer were associated with an increased risk for non-AD dementias, with increased risks ranging from an HR of 1.11 for head and neck cancer to 2.64 for brain cancer.
The investigators attempted to control for the possibility that people with cancer may not survive long enough to develop frank dementia by looking at other age-related conditions, including stroke, osteoarthritis, cataracts, and macular degeneration. They found that all cancers were associated with an increased risk for other age-related conditions, suggesting that their findings of reduced AD risk were valid, Dr. Frain said at the Alzheimer’s Association International Conference 2013.
Findings corroborated
The findings parallel those of a recently published study of more than 1 million residents of Northern Italy (Neurology 2013 July 10 [doi:10.1212/WNL.0b013e31829c5ec1]). The investigators in that study found that "[t]he risk of cancer in patients with AD dementia was halved, and the risk of AD dementia in patients with cancer was 35% reduced. This relationship was observed in almost all subgroup analyses, suggesting that some anticipated potential confounding factors did not significantly influence the results."
Dr. Frain noted that three previous prospective cohort studies had found that older adults with cancer had a reduction in risk for incident AD, ranging from a 33% to a 60% decline. Those studies, however, were limited by small numbers of patients with cancer, limiting the ability to look for associations with specific cancer or treatment types.
The current study drew on data from the massive U.S. National Veterans Affairs Healthcare System to assemble a cohort of 3,499,378 veterans who received outpatient care within the system from 1997 to 2011. The investigators used regression analysis models adjusted for cancer treatment, multiple comorbidities, follow-up time, and number of visits per year before baseline. In addition, the researchers performed subanalyses of patients with diagnoses of prostate, lung, colorectal, and bladder cancers and lymphoma, with the models adjusted for cancer stage and grade.
The median age of the cohort was 71 years, 98% were males, and 66% were white. A total of 771,285 veterans (22%) had a cancer diagnosis. Of the 82,028 veterans who developed AD (2.3% of the entire cohort), 24% were cancer survivors, and the remainder had no cancer history.
The investigators also found that "treatment with chemotherapy conferred additional protection against AD in nearly all cancer types, suggesting that some forms of chemotherapy may have neuroprotective action."
Chemotherapy lowered the risk of AD associated with all cancers except prostate cancer by 20%-45%.
The investigators plan to look at individual categories of anticancer agents, and to see whether they may be common factors between cancer and AD in regard to proteins, genes, and biochemical pathways.
One intriguing target for exploration, Dr. Frain said, is peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN1), an enzyme that when upregulated has been associated with the pathogenesis of some forms of cancer, and when downregulated may be associated with the development of AD.
The study was supported by grants from the Department of Veterans Affairs. Dr. Frain is supported by a VA Special Fellowship in geriatrics.
BOSTON – It’s a trade-off that few people would be likely to make, but having cancer – particularly a type treated by chemotherapy – is associated with a significantly decreased risk for developing Alzheimer’s disease, a retrospective study has shown.
An inverse relationship was found between the incidence of the majority of different types of cancer and Alzheimer’s disease (AD) risk, in a review of the records of nearly 3.5 million U.S. veterans.
Liver cancer survivors had the lowest risk for AD (hazard ratio = 0.49), followed by survivors of pancreatic cancer (HR = 0.56), esophageal cancer (0.67), and multiple myeloma (0.74), reported Dr. Laura Frain of the geriatric research education and clinical center at Boston VA Medical Center.
However, survivors of prostate cancer had a small but significantly higher risk for AD (HR = 1.11), and other screening-detected cancers (colorectal cancer, melanoma) were not associated with a reduced risk, Dr. Frain and her associates found.
"There may be something different about prostate cancer survivors, but whether that’s biologic or related to the way that they’re screened is unclear," Dr. Frain said in an interview.
Many types of cancer were associated with an increased risk for non-AD dementias, with increased risks ranging from an HR of 1.11 for head and neck cancer to 2.64 for brain cancer.
The investigators attempted to control for the possibility that people with cancer may not survive long enough to develop frank dementia by looking at other age-related conditions, including stroke, osteoarthritis, cataracts, and macular degeneration. They found that all cancers were associated with an increased risk for other age-related conditions, suggesting that their findings of reduced AD risk were valid, Dr. Frain said at the Alzheimer’s Association International Conference 2013.
Findings corroborated
The findings parallel those of a recently published study of more than 1 million residents of Northern Italy (Neurology 2013 July 10 [doi:10.1212/WNL.0b013e31829c5ec1]). The investigators in that study found that "[t]he risk of cancer in patients with AD dementia was halved, and the risk of AD dementia in patients with cancer was 35% reduced. This relationship was observed in almost all subgroup analyses, suggesting that some anticipated potential confounding factors did not significantly influence the results."
Dr. Frain noted that three previous prospective cohort studies had found that older adults with cancer had a reduction in risk for incident AD, ranging from a 33% to a 60% decline. Those studies, however, were limited by small numbers of patients with cancer, limiting the ability to look for associations with specific cancer or treatment types.
The current study drew on data from the massive U.S. National Veterans Affairs Healthcare System to assemble a cohort of 3,499,378 veterans who received outpatient care within the system from 1997 to 2011. The investigators used regression analysis models adjusted for cancer treatment, multiple comorbidities, follow-up time, and number of visits per year before baseline. In addition, the researchers performed subanalyses of patients with diagnoses of prostate, lung, colorectal, and bladder cancers and lymphoma, with the models adjusted for cancer stage and grade.
The median age of the cohort was 71 years, 98% were males, and 66% were white. A total of 771,285 veterans (22%) had a cancer diagnosis. Of the 82,028 veterans who developed AD (2.3% of the entire cohort), 24% were cancer survivors, and the remainder had no cancer history.
The investigators also found that "treatment with chemotherapy conferred additional protection against AD in nearly all cancer types, suggesting that some forms of chemotherapy may have neuroprotective action."
Chemotherapy lowered the risk of AD associated with all cancers except prostate cancer by 20%-45%.
The investigators plan to look at individual categories of anticancer agents, and to see whether they may be common factors between cancer and AD in regard to proteins, genes, and biochemical pathways.
One intriguing target for exploration, Dr. Frain said, is peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN1), an enzyme that when upregulated has been associated with the pathogenesis of some forms of cancer, and when downregulated may be associated with the development of AD.
The study was supported by grants from the Department of Veterans Affairs. Dr. Frain is supported by a VA Special Fellowship in geriatrics.
AT AAIC 2013
Major finding: The risk for incident Alzheimer’s disease was reduced by 51% in liver cancer survivors, 44% in survivors of pancreatic cancer, and 23% in survivors of esophageal cancer.
Data source: A retrospective cohort study of 3,499,378 U.S. veterans.
Disclosures: The study was supported by grants from the Department of Veterans Affairs. Dr. Frain is supported by a VA Special Fellowship in geriatrics.
VTE rate triples in pediatric cancer survivors
AMSTERDAM – Children, adolescents, and young adults who survived a diagnosis and treatment of cancer had a greater than threefold higher rate of acute venous thromboembolism during roughly 10 years of follow-up, compared with matched controls from the general population, in a study that included more than 30,000 Canadians.
The increased rate of VTE appeared to be linked to the chemotherapy and radiation treatments that patients received, because patients who were managed only by surgery had a substantially reduced rate of VTE during follow-up.
"Our working hypothesis is that VTE that develops during [initial] treatment of childhood cancers then places these patients at an increased risk" for a second VTE later in their life, Dr. Ketan P. Kulkarni said at the congress of the International Society on Thrombosis and Haemostasis.
If a first episode of VTE during or soon after the initial therapy that young cancer patients receive can be clearly established as a major risk factor for a subsequent episode of VTE, the next step would be to test whether improved prophylaxis during initial therapy can prevent a first episode and thereby also cut patients’ risk for a second VTE several months or years later. Clinicians who manage children, adolescents, and young adults with cancer need increased awareness that VTE "is a major problem" during both initial treatment and follow-up, Dr. Kulkarni said in an interview. "We think the VTEs during follow-up are recurrences of clots that first formed during treatment." He and his associates have begun to review the medical records of each survivor to better determine how many of the VTEs seen during follow-up were recurrences.
Another major finding from this analysis of people who survived at least 5 years following cancer diagnosis at age 0-24 years was that the entire range of cancers posed a VTE risk to patients, not just leukemia as some had previously though. The VTE rate during follow-up of the survivors was roughly the same regardless of whether patients had leukemia, lymphoma, carcinoma, or some other type of cancer.
"We have clearly dispelled the myth that it’s only leukemias. It’s all cancers," said Dr. Kulkarni, a pediatric hematologist-oncologist at the University of Alberta in Edmonton.
The researchers used provincial health insurance records from British Columbia during 1981-1999 to identify 2,857 patients who were aged 0-24 years at the time of their initial cancer diagnosis and then lived for at least another 5 years. The survivors averaged about 14 years old at the time of their initial cancer diagnosis. The investigators also assembled a control group matched by age and sex from the general British Columbia population, taking 10 controls for each case for a total of 28,570 controls.
During follow-up that ranged from 5 to 21 years and averaged nearly 10 years, they found that 43 survivors had an episode of VTE, a 1.5% incidence rate, compared with a 0.5% rate among the controls. In a multivariate analysis that controlled for sex, socioeconomic status, and region of residence, patients who were cancer survivors had a statistically significant, 3.4-fold increased rate of VTE compared with the controls, Dr. Kulkarni reported. Among the survivors the incidence of deep vein thrombosis was roughly 0.8%, the incidence of pulmonary embolism was roughly 0.5%, and VTE in other locations occurred in about 0.3% of the survivors (the total is 1.6% because of rounding). The incidence of VTEs was highest during the first 6 months following cancer diagnosis.
Cancer survivors who had been treated by surgery alone, without chemotherapy or radiation, had a statistically significant, 81% lower rate of developing a VTE compared with the patients treated by chemotherapy alone, radiation alone, or both.
"This supports the hypothesis that treatment by radiation or by chemotherapy increases the VTE risk," Dr. Kulkarni said.
The VTE rate was also substantially higher in survivors who had a relapse of their cancer during follow-up. Patients with relapses had a 2.5-fold higher rate of VTE compared with survivors who did not have a relapse.
Dr. Kulkarni said that he had no disclosures.
[email protected]
On Twitter @mitchelzoler
AMSTERDAM – Children, adolescents, and young adults who survived a diagnosis and treatment of cancer had a greater than threefold higher rate of acute venous thromboembolism during roughly 10 years of follow-up, compared with matched controls from the general population, in a study that included more than 30,000 Canadians.
The increased rate of VTE appeared to be linked to the chemotherapy and radiation treatments that patients received, because patients who were managed only by surgery had a substantially reduced rate of VTE during follow-up.
"Our working hypothesis is that VTE that develops during [initial] treatment of childhood cancers then places these patients at an increased risk" for a second VTE later in their life, Dr. Ketan P. Kulkarni said at the congress of the International Society on Thrombosis and Haemostasis.
If a first episode of VTE during or soon after the initial therapy that young cancer patients receive can be clearly established as a major risk factor for a subsequent episode of VTE, the next step would be to test whether improved prophylaxis during initial therapy can prevent a first episode and thereby also cut patients’ risk for a second VTE several months or years later. Clinicians who manage children, adolescents, and young adults with cancer need increased awareness that VTE "is a major problem" during both initial treatment and follow-up, Dr. Kulkarni said in an interview. "We think the VTEs during follow-up are recurrences of clots that first formed during treatment." He and his associates have begun to review the medical records of each survivor to better determine how many of the VTEs seen during follow-up were recurrences.
Another major finding from this analysis of people who survived at least 5 years following cancer diagnosis at age 0-24 years was that the entire range of cancers posed a VTE risk to patients, not just leukemia as some had previously though. The VTE rate during follow-up of the survivors was roughly the same regardless of whether patients had leukemia, lymphoma, carcinoma, or some other type of cancer.
"We have clearly dispelled the myth that it’s only leukemias. It’s all cancers," said Dr. Kulkarni, a pediatric hematologist-oncologist at the University of Alberta in Edmonton.
The researchers used provincial health insurance records from British Columbia during 1981-1999 to identify 2,857 patients who were aged 0-24 years at the time of their initial cancer diagnosis and then lived for at least another 5 years. The survivors averaged about 14 years old at the time of their initial cancer diagnosis. The investigators also assembled a control group matched by age and sex from the general British Columbia population, taking 10 controls for each case for a total of 28,570 controls.
During follow-up that ranged from 5 to 21 years and averaged nearly 10 years, they found that 43 survivors had an episode of VTE, a 1.5% incidence rate, compared with a 0.5% rate among the controls. In a multivariate analysis that controlled for sex, socioeconomic status, and region of residence, patients who were cancer survivors had a statistically significant, 3.4-fold increased rate of VTE compared with the controls, Dr. Kulkarni reported. Among the survivors the incidence of deep vein thrombosis was roughly 0.8%, the incidence of pulmonary embolism was roughly 0.5%, and VTE in other locations occurred in about 0.3% of the survivors (the total is 1.6% because of rounding). The incidence of VTEs was highest during the first 6 months following cancer diagnosis.
Cancer survivors who had been treated by surgery alone, without chemotherapy or radiation, had a statistically significant, 81% lower rate of developing a VTE compared with the patients treated by chemotherapy alone, radiation alone, or both.
"This supports the hypothesis that treatment by radiation or by chemotherapy increases the VTE risk," Dr. Kulkarni said.
The VTE rate was also substantially higher in survivors who had a relapse of their cancer during follow-up. Patients with relapses had a 2.5-fold higher rate of VTE compared with survivors who did not have a relapse.
Dr. Kulkarni said that he had no disclosures.
[email protected]
On Twitter @mitchelzoler
AMSTERDAM – Children, adolescents, and young adults who survived a diagnosis and treatment of cancer had a greater than threefold higher rate of acute venous thromboembolism during roughly 10 years of follow-up, compared with matched controls from the general population, in a study that included more than 30,000 Canadians.
The increased rate of VTE appeared to be linked to the chemotherapy and radiation treatments that patients received, because patients who were managed only by surgery had a substantially reduced rate of VTE during follow-up.
"Our working hypothesis is that VTE that develops during [initial] treatment of childhood cancers then places these patients at an increased risk" for a second VTE later in their life, Dr. Ketan P. Kulkarni said at the congress of the International Society on Thrombosis and Haemostasis.
If a first episode of VTE during or soon after the initial therapy that young cancer patients receive can be clearly established as a major risk factor for a subsequent episode of VTE, the next step would be to test whether improved prophylaxis during initial therapy can prevent a first episode and thereby also cut patients’ risk for a second VTE several months or years later. Clinicians who manage children, adolescents, and young adults with cancer need increased awareness that VTE "is a major problem" during both initial treatment and follow-up, Dr. Kulkarni said in an interview. "We think the VTEs during follow-up are recurrences of clots that first formed during treatment." He and his associates have begun to review the medical records of each survivor to better determine how many of the VTEs seen during follow-up were recurrences.
Another major finding from this analysis of people who survived at least 5 years following cancer diagnosis at age 0-24 years was that the entire range of cancers posed a VTE risk to patients, not just leukemia as some had previously though. The VTE rate during follow-up of the survivors was roughly the same regardless of whether patients had leukemia, lymphoma, carcinoma, or some other type of cancer.
"We have clearly dispelled the myth that it’s only leukemias. It’s all cancers," said Dr. Kulkarni, a pediatric hematologist-oncologist at the University of Alberta in Edmonton.
The researchers used provincial health insurance records from British Columbia during 1981-1999 to identify 2,857 patients who were aged 0-24 years at the time of their initial cancer diagnosis and then lived for at least another 5 years. The survivors averaged about 14 years old at the time of their initial cancer diagnosis. The investigators also assembled a control group matched by age and sex from the general British Columbia population, taking 10 controls for each case for a total of 28,570 controls.
During follow-up that ranged from 5 to 21 years and averaged nearly 10 years, they found that 43 survivors had an episode of VTE, a 1.5% incidence rate, compared with a 0.5% rate among the controls. In a multivariate analysis that controlled for sex, socioeconomic status, and region of residence, patients who were cancer survivors had a statistically significant, 3.4-fold increased rate of VTE compared with the controls, Dr. Kulkarni reported. Among the survivors the incidence of deep vein thrombosis was roughly 0.8%, the incidence of pulmonary embolism was roughly 0.5%, and VTE in other locations occurred in about 0.3% of the survivors (the total is 1.6% because of rounding). The incidence of VTEs was highest during the first 6 months following cancer diagnosis.
Cancer survivors who had been treated by surgery alone, without chemotherapy or radiation, had a statistically significant, 81% lower rate of developing a VTE compared with the patients treated by chemotherapy alone, radiation alone, or both.
"This supports the hypothesis that treatment by radiation or by chemotherapy increases the VTE risk," Dr. Kulkarni said.
The VTE rate was also substantially higher in survivors who had a relapse of their cancer during follow-up. Patients with relapses had a 2.5-fold higher rate of VTE compared with survivors who did not have a relapse.
Dr. Kulkarni said that he had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE 2013 ISTH CONGRESS
Major finding: Pediatric cancer survivors had a 3.4-fold increased rate of venous thromboembolism during follow-up, compared with controls.
Data source: Data came from a case-control study of 2,857 pediatric and young adult Canadian cancer survivors and 28,570 age-matched Canadian residents who did not have cancer, during an average follow-up of about 10 years.
Disclosures: Dr. Kulkarni said that he had no disclosures.
Practical considerations in the delivery of genetic counseling and testing services for inherited cancer predisposition
Many professional entities endorse the need to deliver cancer genetics risk assessment (CGRA) services through a multidisciplinary team that includes trained genetics professionals. However, market forces, a lack of regulation of genetic testing, patent laws, cost barriers, and a limited workforce in genetics have resulted in an increasing number of community practitioners who order and interpret genetic testing. In addition, varying state-level laws and licensure requirements for genetic counselors may contribute to the nonuniform delivery of CGRA services across the United States. Those who perform genetic testing without having adequate training or expertise may incur liability risks. Moreover, the patient might not enjoy the maximum benefit of testing at the hands of an inadequately trained individual. In the setting of a limited number of professional who are trained in CGRA and a dearth of education and training resources, it is a challenge to integrate genetic testing services into clinical care. With advances in genomics and the implementation of personalized medicine, the problem will only be magnified, and it is critical that there are more opportunities for high quality education and training in clinical cancer genetics free of commercial bias. Successful strategies for delivering comprehensive CGRA services include academic-community partnerships that focus on collaboration with nongenetics providers or the inclusion of a genetics professional in the community setting as part of multidisciplinary patient care. These approaches can leverage the expertise of genetics professionals while allowing patients to remain in their community and enjoy better access to resources for long-term follow-up care.
*Click on the link to the left for a PDF of the full article.
Many professional entities endorse the need to deliver cancer genetics risk assessment (CGRA) services through a multidisciplinary team that includes trained genetics professionals. However, market forces, a lack of regulation of genetic testing, patent laws, cost barriers, and a limited workforce in genetics have resulted in an increasing number of community practitioners who order and interpret genetic testing. In addition, varying state-level laws and licensure requirements for genetic counselors may contribute to the nonuniform delivery of CGRA services across the United States. Those who perform genetic testing without having adequate training or expertise may incur liability risks. Moreover, the patient might not enjoy the maximum benefit of testing at the hands of an inadequately trained individual. In the setting of a limited number of professional who are trained in CGRA and a dearth of education and training resources, it is a challenge to integrate genetic testing services into clinical care. With advances in genomics and the implementation of personalized medicine, the problem will only be magnified, and it is critical that there are more opportunities for high quality education and training in clinical cancer genetics free of commercial bias. Successful strategies for delivering comprehensive CGRA services include academic-community partnerships that focus on collaboration with nongenetics providers or the inclusion of a genetics professional in the community setting as part of multidisciplinary patient care. These approaches can leverage the expertise of genetics professionals while allowing patients to remain in their community and enjoy better access to resources for long-term follow-up care.
*Click on the link to the left for a PDF of the full article.
Many professional entities endorse the need to deliver cancer genetics risk assessment (CGRA) services through a multidisciplinary team that includes trained genetics professionals. However, market forces, a lack of regulation of genetic testing, patent laws, cost barriers, and a limited workforce in genetics have resulted in an increasing number of community practitioners who order and interpret genetic testing. In addition, varying state-level laws and licensure requirements for genetic counselors may contribute to the nonuniform delivery of CGRA services across the United States. Those who perform genetic testing without having adequate training or expertise may incur liability risks. Moreover, the patient might not enjoy the maximum benefit of testing at the hands of an inadequately trained individual. In the setting of a limited number of professional who are trained in CGRA and a dearth of education and training resources, it is a challenge to integrate genetic testing services into clinical care. With advances in genomics and the implementation of personalized medicine, the problem will only be magnified, and it is critical that there are more opportunities for high quality education and training in clinical cancer genetics free of commercial bias. Successful strategies for delivering comprehensive CGRA services include academic-community partnerships that focus on collaboration with nongenetics providers or the inclusion of a genetics professional in the community setting as part of multidisciplinary patient care. These approaches can leverage the expertise of genetics professionals while allowing patients to remain in their community and enjoy better access to resources for long-term follow-up care.
*Click on the link to the left for a PDF of the full article.
Opioid overdose deaths skyrocket in women
American women are dying from prescription drug overdose at historically high rates, the Centers for Disease Control and Prevention announced July 2.
Between 1999 and 2010, the percentage increase in deaths from prescription opioid pain relievers increased more than 415% among women, compared with 265% among men, according to an analysis of national data sets.
In addition, for every woman who died of a prescription painkiller overdose, 30 went to the emergency department for painkiller misuse or abuse.
"Mothers, wives, sisters, and daughters are dying from overdoses at rates that we have never seen before," Dr. Tom Frieden, CDC director, said during a media teleconference. "The increase in opiate overdoses and opiate overdose deaths is directly proportional to the increase in prescribing of painkillers."
Prescriptions for opioid pain relievers such as hydrocodone, oxycodone, and oxymorphone "are increasing to an extent that we would not have anticipated and that could not possibly be clinically indicated," he said.
The findings underscore the importance of reserving prescriptions of opioid pain relievers for situations such as severe cancer pain, "where they can provide important and essential palliation," Dr. Frieden said. "But in many other situations, the risks far outweigh the benefits. Prescribing an opiate may condemn a patient to lifelong addiction and life-threatening complications."
For the analysis, CDC researchers used data from the 1999-2010 National Vital Statistics System and the 2004-2010 Drug Abuse Warning Network to analyze rates of fatal overdoses and ED visits related to drug use or misuse among women (MMWR 2013;62:1-6).
In 2010, 15,323 deaths among women were linked to drug overdose, for a rate of 9.8 per 100,000 population. Between 1999 and 2010, 47,935 women died of opioid pain reliever overdoses. Over that time period, the percentage increase in deaths related to opioid pain relievers was 415% for women and 265% for men. Rates for all drug overdose deaths were highest among women aged 45-54 years (a rate of 21.8 per 100,000 population).
The researchers also reported that in 2010, women made 943,365 ED visits for drug misuse or abuse, a rate of 601 per 100,000 population. The highest ED visit rates were for cocaine or heroin (147.2 per 100,000), benzodiazepines (134.6 per 100,000) and opioid pain relievers (129.6 per 100,000). ED visit rates among women for all drugs tended to be highest among those aged 25-34 years.
Compared with men, Dr. Frieden said that women "are more likely to have chronic pain, to be prescribed painkillers and other medications, to be given higher doses, and to use them for longer time periods. It may be that some of the most common forms of pain are more prevalent among women than men [such as] abdominal pain, migraines, and musculoskeletal pain."
Dr. Frieden advised prescribing clinicians to talk with patients about the risks and benefits of taking opioid pain relievers and to follow guidelines for responsible prescribing "such as screening and monitoring patients for substance abuse and for mental health problems, and [using] prescription drug monitoring programs to identify patients who may be improperly using prescription painkillers."
He also called on states to "improve and implement prescription drug monitoring programs. These programs are just getting up and running in many states."
States "need to do more to ensure that the programs are real-time, complete, and actively managed so that we identify patients who need drug treatment and doctors who need [prescribing] information and education," Dr. Frieden said.
As an example, Dr. Frieden highlighted efforts made in recent years in the state of Washington. Officials there worked with clinicians, health care insurers, and worker compensation programs to develop a consensus on how and when prescription opioids should be used, what some of the alternative treatments are, and resources for patients who are addicted.
"They enforced those guidelines through regulation and saw a more than 20% reduction in opioid deaths in about 3 years," he said.
In the MMWR article, researchers acknowledged certain limitations of the study, including the fact that vital statistics "underestimate the rates of drug involvement in deaths because the type of drug is not specified on many death certificates" and that injury mortality data "might underestimate by up to 35% the actual numbers of deaths for American Indian/Alaska natives and certain other racial/ethnic populations (e.g., Hispanics) because of the misclassification of race/ethnicity of decedents on death certificates."
The researchers had no relevant financial conflicts to disclose.
American women are dying from prescription drug overdose at historically high rates, the Centers for Disease Control and Prevention announced July 2.
Between 1999 and 2010, the percentage increase in deaths from prescription opioid pain relievers increased more than 415% among women, compared with 265% among men, according to an analysis of national data sets.
In addition, for every woman who died of a prescription painkiller overdose, 30 went to the emergency department for painkiller misuse or abuse.
"Mothers, wives, sisters, and daughters are dying from overdoses at rates that we have never seen before," Dr. Tom Frieden, CDC director, said during a media teleconference. "The increase in opiate overdoses and opiate overdose deaths is directly proportional to the increase in prescribing of painkillers."
Prescriptions for opioid pain relievers such as hydrocodone, oxycodone, and oxymorphone "are increasing to an extent that we would not have anticipated and that could not possibly be clinically indicated," he said.
The findings underscore the importance of reserving prescriptions of opioid pain relievers for situations such as severe cancer pain, "where they can provide important and essential palliation," Dr. Frieden said. "But in many other situations, the risks far outweigh the benefits. Prescribing an opiate may condemn a patient to lifelong addiction and life-threatening complications."
For the analysis, CDC researchers used data from the 1999-2010 National Vital Statistics System and the 2004-2010 Drug Abuse Warning Network to analyze rates of fatal overdoses and ED visits related to drug use or misuse among women (MMWR 2013;62:1-6).
In 2010, 15,323 deaths among women were linked to drug overdose, for a rate of 9.8 per 100,000 population. Between 1999 and 2010, 47,935 women died of opioid pain reliever overdoses. Over that time period, the percentage increase in deaths related to opioid pain relievers was 415% for women and 265% for men. Rates for all drug overdose deaths were highest among women aged 45-54 years (a rate of 21.8 per 100,000 population).
The researchers also reported that in 2010, women made 943,365 ED visits for drug misuse or abuse, a rate of 601 per 100,000 population. The highest ED visit rates were for cocaine or heroin (147.2 per 100,000), benzodiazepines (134.6 per 100,000) and opioid pain relievers (129.6 per 100,000). ED visit rates among women for all drugs tended to be highest among those aged 25-34 years.
Compared with men, Dr. Frieden said that women "are more likely to have chronic pain, to be prescribed painkillers and other medications, to be given higher doses, and to use them for longer time periods. It may be that some of the most common forms of pain are more prevalent among women than men [such as] abdominal pain, migraines, and musculoskeletal pain."
Dr. Frieden advised prescribing clinicians to talk with patients about the risks and benefits of taking opioid pain relievers and to follow guidelines for responsible prescribing "such as screening and monitoring patients for substance abuse and for mental health problems, and [using] prescription drug monitoring programs to identify patients who may be improperly using prescription painkillers."
He also called on states to "improve and implement prescription drug monitoring programs. These programs are just getting up and running in many states."
States "need to do more to ensure that the programs are real-time, complete, and actively managed so that we identify patients who need drug treatment and doctors who need [prescribing] information and education," Dr. Frieden said.
As an example, Dr. Frieden highlighted efforts made in recent years in the state of Washington. Officials there worked with clinicians, health care insurers, and worker compensation programs to develop a consensus on how and when prescription opioids should be used, what some of the alternative treatments are, and resources for patients who are addicted.
"They enforced those guidelines through regulation and saw a more than 20% reduction in opioid deaths in about 3 years," he said.
In the MMWR article, researchers acknowledged certain limitations of the study, including the fact that vital statistics "underestimate the rates of drug involvement in deaths because the type of drug is not specified on many death certificates" and that injury mortality data "might underestimate by up to 35% the actual numbers of deaths for American Indian/Alaska natives and certain other racial/ethnic populations (e.g., Hispanics) because of the misclassification of race/ethnicity of decedents on death certificates."
The researchers had no relevant financial conflicts to disclose.
American women are dying from prescription drug overdose at historically high rates, the Centers for Disease Control and Prevention announced July 2.
Between 1999 and 2010, the percentage increase in deaths from prescription opioid pain relievers increased more than 415% among women, compared with 265% among men, according to an analysis of national data sets.
In addition, for every woman who died of a prescription painkiller overdose, 30 went to the emergency department for painkiller misuse or abuse.
"Mothers, wives, sisters, and daughters are dying from overdoses at rates that we have never seen before," Dr. Tom Frieden, CDC director, said during a media teleconference. "The increase in opiate overdoses and opiate overdose deaths is directly proportional to the increase in prescribing of painkillers."
Prescriptions for opioid pain relievers such as hydrocodone, oxycodone, and oxymorphone "are increasing to an extent that we would not have anticipated and that could not possibly be clinically indicated," he said.
The findings underscore the importance of reserving prescriptions of opioid pain relievers for situations such as severe cancer pain, "where they can provide important and essential palliation," Dr. Frieden said. "But in many other situations, the risks far outweigh the benefits. Prescribing an opiate may condemn a patient to lifelong addiction and life-threatening complications."
For the analysis, CDC researchers used data from the 1999-2010 National Vital Statistics System and the 2004-2010 Drug Abuse Warning Network to analyze rates of fatal overdoses and ED visits related to drug use or misuse among women (MMWR 2013;62:1-6).
In 2010, 15,323 deaths among women were linked to drug overdose, for a rate of 9.8 per 100,000 population. Between 1999 and 2010, 47,935 women died of opioid pain reliever overdoses. Over that time period, the percentage increase in deaths related to opioid pain relievers was 415% for women and 265% for men. Rates for all drug overdose deaths were highest among women aged 45-54 years (a rate of 21.8 per 100,000 population).
The researchers also reported that in 2010, women made 943,365 ED visits for drug misuse or abuse, a rate of 601 per 100,000 population. The highest ED visit rates were for cocaine or heroin (147.2 per 100,000), benzodiazepines (134.6 per 100,000) and opioid pain relievers (129.6 per 100,000). ED visit rates among women for all drugs tended to be highest among those aged 25-34 years.
Compared with men, Dr. Frieden said that women "are more likely to have chronic pain, to be prescribed painkillers and other medications, to be given higher doses, and to use them for longer time periods. It may be that some of the most common forms of pain are more prevalent among women than men [such as] abdominal pain, migraines, and musculoskeletal pain."
Dr. Frieden advised prescribing clinicians to talk with patients about the risks and benefits of taking opioid pain relievers and to follow guidelines for responsible prescribing "such as screening and monitoring patients for substance abuse and for mental health problems, and [using] prescription drug monitoring programs to identify patients who may be improperly using prescription painkillers."
He also called on states to "improve and implement prescription drug monitoring programs. These programs are just getting up and running in many states."
States "need to do more to ensure that the programs are real-time, complete, and actively managed so that we identify patients who need drug treatment and doctors who need [prescribing] information and education," Dr. Frieden said.
As an example, Dr. Frieden highlighted efforts made in recent years in the state of Washington. Officials there worked with clinicians, health care insurers, and worker compensation programs to develop a consensus on how and when prescription opioids should be used, what some of the alternative treatments are, and resources for patients who are addicted.
"They enforced those guidelines through regulation and saw a more than 20% reduction in opioid deaths in about 3 years," he said.
In the MMWR article, researchers acknowledged certain limitations of the study, including the fact that vital statistics "underestimate the rates of drug involvement in deaths because the type of drug is not specified on many death certificates" and that injury mortality data "might underestimate by up to 35% the actual numbers of deaths for American Indian/Alaska natives and certain other racial/ethnic populations (e.g., Hispanics) because of the misclassification of race/ethnicity of decedents on death certificates."
The researchers had no relevant financial conflicts to disclose.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Major finding: Between 1999 and 2010, the percentage increase in deaths from overdose of opioid pain relievers rose 415% among women, compared with 265% among men.
Data source: Analysis of data from the 1999-2010 National Vital Statistics System and the 2004-2010 Drug Abuse Warning Network.
Disclosures: The researchers disclosed no relevant financial conflicts of interest.
Cancer health disparities and risk factors: lessons from a woman with a 20-cm chest wall mass, growing for 2 years
The National Cancer Institute (NCI) has defined cancer health disparities as adverse differences in incidence, prevalence, mortality, survivorship, and burden of cancer or related health conditions that exist among specific populations in the United States.1 African Americans are more likely than members of any other racial or ethnic population to develop and die from cancer.2 African American women are more likely than are white women to die of breast cancer, although African American women have a lower incidence rate of this disease than white women.3,4 The most conspicuous factors that contribute to the observed disparities are associated with a lack of health care coverage, low socioeconomic status, and race/ethnicity. We recently provided care to a woman who presented to the emergency room with 20-cm chest wall mass. She was found to have inoperable stage IV triple-negative breast cancer with significantly poor prognosis. We describe her presentation, diagnosis, and treatment, identify the factors that contributed to her current condition, discuss the cancer health disparities and the associated risk factors, and reiterate what physicians should know to prevent similar unfortunate and unnecessary scenarios.
*Click on the link to the left for a PDF of the full article.
The National Cancer Institute (NCI) has defined cancer health disparities as adverse differences in incidence, prevalence, mortality, survivorship, and burden of cancer or related health conditions that exist among specific populations in the United States.1 African Americans are more likely than members of any other racial or ethnic population to develop and die from cancer.2 African American women are more likely than are white women to die of breast cancer, although African American women have a lower incidence rate of this disease than white women.3,4 The most conspicuous factors that contribute to the observed disparities are associated with a lack of health care coverage, low socioeconomic status, and race/ethnicity. We recently provided care to a woman who presented to the emergency room with 20-cm chest wall mass. She was found to have inoperable stage IV triple-negative breast cancer with significantly poor prognosis. We describe her presentation, diagnosis, and treatment, identify the factors that contributed to her current condition, discuss the cancer health disparities and the associated risk factors, and reiterate what physicians should know to prevent similar unfortunate and unnecessary scenarios.
*Click on the link to the left for a PDF of the full article.
The National Cancer Institute (NCI) has defined cancer health disparities as adverse differences in incidence, prevalence, mortality, survivorship, and burden of cancer or related health conditions that exist among specific populations in the United States.1 African Americans are more likely than members of any other racial or ethnic population to develop and die from cancer.2 African American women are more likely than are white women to die of breast cancer, although African American women have a lower incidence rate of this disease than white women.3,4 The most conspicuous factors that contribute to the observed disparities are associated with a lack of health care coverage, low socioeconomic status, and race/ethnicity. We recently provided care to a woman who presented to the emergency room with 20-cm chest wall mass. She was found to have inoperable stage IV triple-negative breast cancer with significantly poor prognosis. We describe her presentation, diagnosis, and treatment, identify the factors that contributed to her current condition, discuss the cancer health disparities and the associated risk factors, and reiterate what physicians should know to prevent similar unfortunate and unnecessary scenarios.
*Click on the link to the left for a PDF of the full article.
How will sequestration affect American cancer care?
In an interview at the ASCO 2013 meeting, ASCO's chief medical officer, Dr. Richard Schilsky, discusses the negative impact federal spending cuts are having on physicians' care of cancer patients.
In an interview at the ASCO 2013 meeting, ASCO's chief medical officer, Dr. Richard Schilsky, discusses the negative impact federal spending cuts are having on physicians' care of cancer patients.
In an interview at the ASCO 2013 meeting, ASCO's chief medical officer, Dr. Richard Schilsky, discusses the negative impact federal spending cuts are having on physicians' care of cancer patients.
Oral HPV-related cancer risk not transmitted to sex partners
CHICAGO – Partners of patients with human papillomavirus-positive oropharyngeal cancer do not appear to be at an increased risk for HPV-related cancers, investigators reported at the annual meeting of the American Society of Clinical Oncology.
Of 88 female partners of persons with HPV-positive oropharyngeal cancers, 4 were found to be positive for HPV infection, and 2 of the 4 were positive for the oncogenic HPV-16 subtype. The prevalence of HPV infection in this sample was 5%, similar to that of women in the general population (3.6%), reported Gypsyamber D’Souza, Ph.D., an epidemiologist at the Johns Hopkins Bloomberg School of Public Health in Baltimore, Md.
None of the male partners in this small pilot study tested positive for oral HPV 16, but the male sample size was too small to draw significant conclusions about the risks of HPV transmission in men, Dr. D’Souza said.
"This is the first study to examine oral HPV prevalence among spouses of HPV-related oropharyngeal cancer patients, and it’s very reassuring that the oral HPV prevalence was similar to that observed in the general population. This suggests that risk of HPV-related cancer remains low among these spouses," she said in a media briefing prior to her presentation.
The study also supports epidemiologic data showing that although HPV infections are common in the general population, the overwhelming majority of people who are infected will spontaneously clear the virus and not get cancer.
"We certainly will need longer follow-up on how HPV infection and co-infection in the partners will affect patients and the development of cancer, but we recognize this as an important breakthrough in the understanding of the biology of oropharyngeal cancer," commented Dr. Gregory Masters, a medical oncologist at the Helen F. Graham Cancer Center in Newark, Del., who moderated the briefing.
Dr. Marcia S. Brose, a head and neck surgeon at the Hospital of the University of Pennsylvania in Philadelphia, said that despite the handful of male partners, the study sends an important message to patients and their partners.
"As oncologists, we’re not just in the business of the disease itself; we also have to treat the family members and their concerns. If you’re the spouse of someone who has a communicable disease-related cancer, you’re going to have to make decisions that affect your private life, and it means a lot that someone is doing the research that says you don’t have to worry," she said in an interview.
The study was prompted by concerns frequently expressed by patients with oropharyngeal cancer and their spouses/sexual partners about oral transmission of HPV and increased cancer risk, Dr. D’Souza said.
The investigators enrolled 147 men and 19 women (median age 56) with oropharyngeal cancers, and 94 of their spouses or long-term partners.
Oral rinse and gargle samples were collected from all cases and partners in the study at baseline and 1 year later. The samples were tested for the presence of HPV DNA. Imperfect as it is in sensitivity and specificity for HPV, the test is nonetheless the gold standard for detecting HPV in oral cancers, Dr. D’Souza said.
At the time of diagnosis, 65% of patients tested positive for HPV, and 54% of all patients had HPV 16.
At 1 year, however, following completion of therapy, only 5.6% of patients still tested positive for HPV 16.
Overall, the prevalence of oral HPV among partners was 6.5%, comparable to HPV prevalence among the general population in National Health and Nutrition Examination Survery (NHANES) data from 2009-2010. Among male partners, the prevalence of HPV also was similar to that seen among men in the general population (10.1%), but the small number made it difficult if not impossible to draw meaningful conclusions.
No oral or oropharyngeal cancers were detected in partners in an oral cancer screen, but three patients reported having previous partners with oropharyngeal cancer. In addition, five male patients reported having a partner who developed cervical cancer or a precancerous condition (one current partner and two previous partners had cervical cancers, and two current partners had cervical dysplasia).
The study was supported by the Johns Hopkins Innovation Fund. Dr. D’Souza reported receiving research support from Merck, maker of Gardasil.
CHICAGO – Partners of patients with human papillomavirus-positive oropharyngeal cancer do not appear to be at an increased risk for HPV-related cancers, investigators reported at the annual meeting of the American Society of Clinical Oncology.
Of 88 female partners of persons with HPV-positive oropharyngeal cancers, 4 were found to be positive for HPV infection, and 2 of the 4 were positive for the oncogenic HPV-16 subtype. The prevalence of HPV infection in this sample was 5%, similar to that of women in the general population (3.6%), reported Gypsyamber D’Souza, Ph.D., an epidemiologist at the Johns Hopkins Bloomberg School of Public Health in Baltimore, Md.
None of the male partners in this small pilot study tested positive for oral HPV 16, but the male sample size was too small to draw significant conclusions about the risks of HPV transmission in men, Dr. D’Souza said.
"This is the first study to examine oral HPV prevalence among spouses of HPV-related oropharyngeal cancer patients, and it’s very reassuring that the oral HPV prevalence was similar to that observed in the general population. This suggests that risk of HPV-related cancer remains low among these spouses," she said in a media briefing prior to her presentation.
The study also supports epidemiologic data showing that although HPV infections are common in the general population, the overwhelming majority of people who are infected will spontaneously clear the virus and not get cancer.
"We certainly will need longer follow-up on how HPV infection and co-infection in the partners will affect patients and the development of cancer, but we recognize this as an important breakthrough in the understanding of the biology of oropharyngeal cancer," commented Dr. Gregory Masters, a medical oncologist at the Helen F. Graham Cancer Center in Newark, Del., who moderated the briefing.
Dr. Marcia S. Brose, a head and neck surgeon at the Hospital of the University of Pennsylvania in Philadelphia, said that despite the handful of male partners, the study sends an important message to patients and their partners.
"As oncologists, we’re not just in the business of the disease itself; we also have to treat the family members and their concerns. If you’re the spouse of someone who has a communicable disease-related cancer, you’re going to have to make decisions that affect your private life, and it means a lot that someone is doing the research that says you don’t have to worry," she said in an interview.
The study was prompted by concerns frequently expressed by patients with oropharyngeal cancer and their spouses/sexual partners about oral transmission of HPV and increased cancer risk, Dr. D’Souza said.
The investigators enrolled 147 men and 19 women (median age 56) with oropharyngeal cancers, and 94 of their spouses or long-term partners.
Oral rinse and gargle samples were collected from all cases and partners in the study at baseline and 1 year later. The samples were tested for the presence of HPV DNA. Imperfect as it is in sensitivity and specificity for HPV, the test is nonetheless the gold standard for detecting HPV in oral cancers, Dr. D’Souza said.
At the time of diagnosis, 65% of patients tested positive for HPV, and 54% of all patients had HPV 16.
At 1 year, however, following completion of therapy, only 5.6% of patients still tested positive for HPV 16.
Overall, the prevalence of oral HPV among partners was 6.5%, comparable to HPV prevalence among the general population in National Health and Nutrition Examination Survery (NHANES) data from 2009-2010. Among male partners, the prevalence of HPV also was similar to that seen among men in the general population (10.1%), but the small number made it difficult if not impossible to draw meaningful conclusions.
No oral or oropharyngeal cancers were detected in partners in an oral cancer screen, but three patients reported having previous partners with oropharyngeal cancer. In addition, five male patients reported having a partner who developed cervical cancer or a precancerous condition (one current partner and two previous partners had cervical cancers, and two current partners had cervical dysplasia).
The study was supported by the Johns Hopkins Innovation Fund. Dr. D’Souza reported receiving research support from Merck, maker of Gardasil.
CHICAGO – Partners of patients with human papillomavirus-positive oropharyngeal cancer do not appear to be at an increased risk for HPV-related cancers, investigators reported at the annual meeting of the American Society of Clinical Oncology.
Of 88 female partners of persons with HPV-positive oropharyngeal cancers, 4 were found to be positive for HPV infection, and 2 of the 4 were positive for the oncogenic HPV-16 subtype. The prevalence of HPV infection in this sample was 5%, similar to that of women in the general population (3.6%), reported Gypsyamber D’Souza, Ph.D., an epidemiologist at the Johns Hopkins Bloomberg School of Public Health in Baltimore, Md.
None of the male partners in this small pilot study tested positive for oral HPV 16, but the male sample size was too small to draw significant conclusions about the risks of HPV transmission in men, Dr. D’Souza said.
"This is the first study to examine oral HPV prevalence among spouses of HPV-related oropharyngeal cancer patients, and it’s very reassuring that the oral HPV prevalence was similar to that observed in the general population. This suggests that risk of HPV-related cancer remains low among these spouses," she said in a media briefing prior to her presentation.
The study also supports epidemiologic data showing that although HPV infections are common in the general population, the overwhelming majority of people who are infected will spontaneously clear the virus and not get cancer.
"We certainly will need longer follow-up on how HPV infection and co-infection in the partners will affect patients and the development of cancer, but we recognize this as an important breakthrough in the understanding of the biology of oropharyngeal cancer," commented Dr. Gregory Masters, a medical oncologist at the Helen F. Graham Cancer Center in Newark, Del., who moderated the briefing.
Dr. Marcia S. Brose, a head and neck surgeon at the Hospital of the University of Pennsylvania in Philadelphia, said that despite the handful of male partners, the study sends an important message to patients and their partners.
"As oncologists, we’re not just in the business of the disease itself; we also have to treat the family members and their concerns. If you’re the spouse of someone who has a communicable disease-related cancer, you’re going to have to make decisions that affect your private life, and it means a lot that someone is doing the research that says you don’t have to worry," she said in an interview.
The study was prompted by concerns frequently expressed by patients with oropharyngeal cancer and their spouses/sexual partners about oral transmission of HPV and increased cancer risk, Dr. D’Souza said.
The investigators enrolled 147 men and 19 women (median age 56) with oropharyngeal cancers, and 94 of their spouses or long-term partners.
Oral rinse and gargle samples were collected from all cases and partners in the study at baseline and 1 year later. The samples were tested for the presence of HPV DNA. Imperfect as it is in sensitivity and specificity for HPV, the test is nonetheless the gold standard for detecting HPV in oral cancers, Dr. D’Souza said.
At the time of diagnosis, 65% of patients tested positive for HPV, and 54% of all patients had HPV 16.
At 1 year, however, following completion of therapy, only 5.6% of patients still tested positive for HPV 16.
Overall, the prevalence of oral HPV among partners was 6.5%, comparable to HPV prevalence among the general population in National Health and Nutrition Examination Survery (NHANES) data from 2009-2010. Among male partners, the prevalence of HPV also was similar to that seen among men in the general population (10.1%), but the small number made it difficult if not impossible to draw meaningful conclusions.
No oral or oropharyngeal cancers were detected in partners in an oral cancer screen, but three patients reported having previous partners with oropharyngeal cancer. In addition, five male patients reported having a partner who developed cervical cancer or a precancerous condition (one current partner and two previous partners had cervical cancers, and two current partners had cervical dysplasia).
The study was supported by the Johns Hopkins Innovation Fund. Dr. D’Souza reported receiving research support from Merck, maker of Gardasil.
AT THE ASCO ANNUAL MEETING 2013
Major finding: The prevalence of human papillomavirus infections is 5% among female partners of patients with HPV-related oropharyngeal cancers and 3.6% in the general population of women.
Data source: Pilot surveillance study of 166 people with oropharyngeal cancer and 94 of their spouses/long-term partners.
Disclosures: The study was supported by the Johns Hopkins Innovation Fund. Dr. D'Souza reported receiving research support from Merck, maker of Gardasil.
ASCO guidelines for fertility preservation affirm oocyte preservation
Guidelines for fertility preservation in newly diagnosed cancer patients have been updated to include oocyte preservation as a standard practice rather than an experimental option, according to the American Society of Clinical Oncology.
With the exception of the recommendation on oocyte preservation, however, the update is essentially comparable to the one that ASCO issued in 2006. The latest guidelines also include literature updates used to create a framework for physician-patient discussions of fertility preservation. The guidelines appear in the May 28 online edition of the Journal of Clinical Oncology (doi: 10.1200/JCO.2013.49.2678).
The discussion of fertility preservation needs to occur early, as part of education and informed consent before treatment. "Current evidence suggests that discussions about fertility and fertility preservation are of great importance to patients with cancer," wrote Dr. Alison Loren, cochair of the guidelines update panel, and her colleagues. "Discussing infertility and introducing the possibility of fertility preservation lead to improved quality of life and diminished distress in all patient populations."
"It may be difficult for physicians to know how important fertility preservation is to their patients unless they ask, because many patients may not bring up the topic," wrote Dr. Loren of the University of Pennsylvania, Philadelphia, and her colleagues. "They may be overwhelmed by and focused exclusively on the cancer diagnosis, they may be unaware that potential fertility loss may occur, or they may be concerned that pursuing fertility preservation will delay their treatment, leading to increased morbidity or mortality."
"There is evidence to suggest that, at least among women, patients may make cancer treatment decisions based on fertility concerns. ... [In a recent study,] 29% of women with breast cancer reported that infertility concerns influenced their treatment decisions," the panel wrote.
The panel examined 18 recent randomized controlled trials; 6 systematic reviews, meta-analyses, and previous guidelines; and dozens of narrative reviews, case series, case studies, and editorials. From this review, they produced guidelines for fertility preservation in men, women, and post- and prepubertal children.
Such discussions should occur with all cancer patients of reproductive age (and with parents or guardians of children and adolescents) before treatment starts when infertility is a potential risk of therapy. Discussions should address whether fertility preservation might have an impact on successful cancer treatment. Those interested in fertility preservation, including ambivalent patients, should be referred to reproductive specialists and the discussion documented in the medical record. Distress about potential infertility warrants a referral to a psychosocial provider. Those who opt for fertility preservation should be encouraged to participate in registries and clinical studies.
For men
Men who choose to move ahead with sperm cryopreservation can usually do so expeditiously with no cancer treatment delay.
Sperm cryopreservation is the only established fertility preservation method; sperm is usually easy to obtain, and samples can be recovered every 24 hours. Testicular hormone suppression and testicular tissue or spermatogonial freezing for later transplanting – including testis xenografting – are experimental and unproven in humans. Men should be advised of a potentially higher risk of genetic damage in sperm collected after initiation of chemotherapy.
For women
"Fertility preservation options in females depend on patient age, diagnosis, type of treatment, presence or participation of a male partner, and/or patient preferences regarding the use of banked donor sperm, time available, and likelihood that cancer has metastasized to her ovaries," the panel wrote. Timing is an issue: Oocyte and embryo cryopreservation are proven methods, but the ovarian stimulation needed to accomplish these generally takes at least 2-4 weeks. Prior worries about hormonal stimulation and its possible effect on hormone-sensitive cancers have been somewhat allayed.
Letrozole combined with standard fertility drugs can induce ovulation without spiking estrogen; the number of eggs and embryos from these inductions appears no different from those obtained with traditional drugs.
Ovarian transposition (oophoropexy) is an option when pelvic radiation therapy is performed as cancer treatment.
Ovarian suppression with gonadotropin-releasing hormone agonists doesn’t seem to have any significant benefit in fertility preservation, according to the panel. No differences have been noted in post-treatment resumption of menstruation or in hormonal or imaging markers of fertility.
Ovarian tissue cryopreservation for the purpose of future transplantation is still experimental. At least 19 live births have been reported using frozen ovarian tissue transplanted after treatment. "There is a theoretic concern with re-implanting ovarian tissue and the potential for reintroducing cancer cells depending on the type and stage of cancer, although so far there have been no reports of cancer recurrence in humans."
For teens and children
Postpubertal adolescents can choose to freeze eggs or sperm, a process that requires consent from both the teen and the parents.
There are no standard options for preserving the fertility of prepubertal children; all approaches are experimental. There are some reports of oocyte cryopreservation in girls aged 13 years and older, but no reports of live births associated with later re-implantation.
Testicular tissue has also been frozen, but again, there are no reports of re-implantation.
"Efforts to preserve the fertility of children using experimental methods should be attempted only under institutional review board–approved protocols," the panel cautioned.
Data are lacking on the fertility impact of new biologic and targeted treatments, with the exceptions of bevacizumab and tyrosine kinase inhibitors.
In 2011, the Food and Drug Administration reported a 34% rate of ovarian failure in women who got bevacizumab as part of a colorectal cancer treatment regimen; 1 in 5 women regained ovarian function. This possible complication should be addressed with women.
Young patients taking tyrosine kinase inhibitors for chronic myeloid leukemia may also face later fertility problems, the panel noted, including an increased risk of complications in pregnancy or congenital anomalies. Data on these findings are incomplete, but patients should understand the possible risks, the panel wrote.
Supplements and clinical tools and resources can be found at www.asco.org/guidelines/fertility. Patient information is also available at www.cancer.net.
Neither Dr. Loren nor any of the other panel members had any financial disclosures.
Guidelines for fertility preservation in newly diagnosed cancer patients have been updated to include oocyte preservation as a standard practice rather than an experimental option, according to the American Society of Clinical Oncology.
With the exception of the recommendation on oocyte preservation, however, the update is essentially comparable to the one that ASCO issued in 2006. The latest guidelines also include literature updates used to create a framework for physician-patient discussions of fertility preservation. The guidelines appear in the May 28 online edition of the Journal of Clinical Oncology (doi: 10.1200/JCO.2013.49.2678).
The discussion of fertility preservation needs to occur early, as part of education and informed consent before treatment. "Current evidence suggests that discussions about fertility and fertility preservation are of great importance to patients with cancer," wrote Dr. Alison Loren, cochair of the guidelines update panel, and her colleagues. "Discussing infertility and introducing the possibility of fertility preservation lead to improved quality of life and diminished distress in all patient populations."
"It may be difficult for physicians to know how important fertility preservation is to their patients unless they ask, because many patients may not bring up the topic," wrote Dr. Loren of the University of Pennsylvania, Philadelphia, and her colleagues. "They may be overwhelmed by and focused exclusively on the cancer diagnosis, they may be unaware that potential fertility loss may occur, or they may be concerned that pursuing fertility preservation will delay their treatment, leading to increased morbidity or mortality."
"There is evidence to suggest that, at least among women, patients may make cancer treatment decisions based on fertility concerns. ... [In a recent study,] 29% of women with breast cancer reported that infertility concerns influenced their treatment decisions," the panel wrote.
The panel examined 18 recent randomized controlled trials; 6 systematic reviews, meta-analyses, and previous guidelines; and dozens of narrative reviews, case series, case studies, and editorials. From this review, they produced guidelines for fertility preservation in men, women, and post- and prepubertal children.
Such discussions should occur with all cancer patients of reproductive age (and with parents or guardians of children and adolescents) before treatment starts when infertility is a potential risk of therapy. Discussions should address whether fertility preservation might have an impact on successful cancer treatment. Those interested in fertility preservation, including ambivalent patients, should be referred to reproductive specialists and the discussion documented in the medical record. Distress about potential infertility warrants a referral to a psychosocial provider. Those who opt for fertility preservation should be encouraged to participate in registries and clinical studies.
For men
Men who choose to move ahead with sperm cryopreservation can usually do so expeditiously with no cancer treatment delay.
Sperm cryopreservation is the only established fertility preservation method; sperm is usually easy to obtain, and samples can be recovered every 24 hours. Testicular hormone suppression and testicular tissue or spermatogonial freezing for later transplanting – including testis xenografting – are experimental and unproven in humans. Men should be advised of a potentially higher risk of genetic damage in sperm collected after initiation of chemotherapy.
For women
"Fertility preservation options in females depend on patient age, diagnosis, type of treatment, presence or participation of a male partner, and/or patient preferences regarding the use of banked donor sperm, time available, and likelihood that cancer has metastasized to her ovaries," the panel wrote. Timing is an issue: Oocyte and embryo cryopreservation are proven methods, but the ovarian stimulation needed to accomplish these generally takes at least 2-4 weeks. Prior worries about hormonal stimulation and its possible effect on hormone-sensitive cancers have been somewhat allayed.
Letrozole combined with standard fertility drugs can induce ovulation without spiking estrogen; the number of eggs and embryos from these inductions appears no different from those obtained with traditional drugs.
Ovarian transposition (oophoropexy) is an option when pelvic radiation therapy is performed as cancer treatment.
Ovarian suppression with gonadotropin-releasing hormone agonists doesn’t seem to have any significant benefit in fertility preservation, according to the panel. No differences have been noted in post-treatment resumption of menstruation or in hormonal or imaging markers of fertility.
Ovarian tissue cryopreservation for the purpose of future transplantation is still experimental. At least 19 live births have been reported using frozen ovarian tissue transplanted after treatment. "There is a theoretic concern with re-implanting ovarian tissue and the potential for reintroducing cancer cells depending on the type and stage of cancer, although so far there have been no reports of cancer recurrence in humans."
For teens and children
Postpubertal adolescents can choose to freeze eggs or sperm, a process that requires consent from both the teen and the parents.
There are no standard options for preserving the fertility of prepubertal children; all approaches are experimental. There are some reports of oocyte cryopreservation in girls aged 13 years and older, but no reports of live births associated with later re-implantation.
Testicular tissue has also been frozen, but again, there are no reports of re-implantation.
"Efforts to preserve the fertility of children using experimental methods should be attempted only under institutional review board–approved protocols," the panel cautioned.
Data are lacking on the fertility impact of new biologic and targeted treatments, with the exceptions of bevacizumab and tyrosine kinase inhibitors.
In 2011, the Food and Drug Administration reported a 34% rate of ovarian failure in women who got bevacizumab as part of a colorectal cancer treatment regimen; 1 in 5 women regained ovarian function. This possible complication should be addressed with women.
Young patients taking tyrosine kinase inhibitors for chronic myeloid leukemia may also face later fertility problems, the panel noted, including an increased risk of complications in pregnancy or congenital anomalies. Data on these findings are incomplete, but patients should understand the possible risks, the panel wrote.
Supplements and clinical tools and resources can be found at www.asco.org/guidelines/fertility. Patient information is also available at www.cancer.net.
Neither Dr. Loren nor any of the other panel members had any financial disclosures.
Guidelines for fertility preservation in newly diagnosed cancer patients have been updated to include oocyte preservation as a standard practice rather than an experimental option, according to the American Society of Clinical Oncology.
With the exception of the recommendation on oocyte preservation, however, the update is essentially comparable to the one that ASCO issued in 2006. The latest guidelines also include literature updates used to create a framework for physician-patient discussions of fertility preservation. The guidelines appear in the May 28 online edition of the Journal of Clinical Oncology (doi: 10.1200/JCO.2013.49.2678).
The discussion of fertility preservation needs to occur early, as part of education and informed consent before treatment. "Current evidence suggests that discussions about fertility and fertility preservation are of great importance to patients with cancer," wrote Dr. Alison Loren, cochair of the guidelines update panel, and her colleagues. "Discussing infertility and introducing the possibility of fertility preservation lead to improved quality of life and diminished distress in all patient populations."
"It may be difficult for physicians to know how important fertility preservation is to their patients unless they ask, because many patients may not bring up the topic," wrote Dr. Loren of the University of Pennsylvania, Philadelphia, and her colleagues. "They may be overwhelmed by and focused exclusively on the cancer diagnosis, they may be unaware that potential fertility loss may occur, or they may be concerned that pursuing fertility preservation will delay their treatment, leading to increased morbidity or mortality."
"There is evidence to suggest that, at least among women, patients may make cancer treatment decisions based on fertility concerns. ... [In a recent study,] 29% of women with breast cancer reported that infertility concerns influenced their treatment decisions," the panel wrote.
The panel examined 18 recent randomized controlled trials; 6 systematic reviews, meta-analyses, and previous guidelines; and dozens of narrative reviews, case series, case studies, and editorials. From this review, they produced guidelines for fertility preservation in men, women, and post- and prepubertal children.
Such discussions should occur with all cancer patients of reproductive age (and with parents or guardians of children and adolescents) before treatment starts when infertility is a potential risk of therapy. Discussions should address whether fertility preservation might have an impact on successful cancer treatment. Those interested in fertility preservation, including ambivalent patients, should be referred to reproductive specialists and the discussion documented in the medical record. Distress about potential infertility warrants a referral to a psychosocial provider. Those who opt for fertility preservation should be encouraged to participate in registries and clinical studies.
For men
Men who choose to move ahead with sperm cryopreservation can usually do so expeditiously with no cancer treatment delay.
Sperm cryopreservation is the only established fertility preservation method; sperm is usually easy to obtain, and samples can be recovered every 24 hours. Testicular hormone suppression and testicular tissue or spermatogonial freezing for later transplanting – including testis xenografting – are experimental and unproven in humans. Men should be advised of a potentially higher risk of genetic damage in sperm collected after initiation of chemotherapy.
For women
"Fertility preservation options in females depend on patient age, diagnosis, type of treatment, presence or participation of a male partner, and/or patient preferences regarding the use of banked donor sperm, time available, and likelihood that cancer has metastasized to her ovaries," the panel wrote. Timing is an issue: Oocyte and embryo cryopreservation are proven methods, but the ovarian stimulation needed to accomplish these generally takes at least 2-4 weeks. Prior worries about hormonal stimulation and its possible effect on hormone-sensitive cancers have been somewhat allayed.
Letrozole combined with standard fertility drugs can induce ovulation without spiking estrogen; the number of eggs and embryos from these inductions appears no different from those obtained with traditional drugs.
Ovarian transposition (oophoropexy) is an option when pelvic radiation therapy is performed as cancer treatment.
Ovarian suppression with gonadotropin-releasing hormone agonists doesn’t seem to have any significant benefit in fertility preservation, according to the panel. No differences have been noted in post-treatment resumption of menstruation or in hormonal or imaging markers of fertility.
Ovarian tissue cryopreservation for the purpose of future transplantation is still experimental. At least 19 live births have been reported using frozen ovarian tissue transplanted after treatment. "There is a theoretic concern with re-implanting ovarian tissue and the potential for reintroducing cancer cells depending on the type and stage of cancer, although so far there have been no reports of cancer recurrence in humans."
For teens and children
Postpubertal adolescents can choose to freeze eggs or sperm, a process that requires consent from both the teen and the parents.
There are no standard options for preserving the fertility of prepubertal children; all approaches are experimental. There are some reports of oocyte cryopreservation in girls aged 13 years and older, but no reports of live births associated with later re-implantation.
Testicular tissue has also been frozen, but again, there are no reports of re-implantation.
"Efforts to preserve the fertility of children using experimental methods should be attempted only under institutional review board–approved protocols," the panel cautioned.
Data are lacking on the fertility impact of new biologic and targeted treatments, with the exceptions of bevacizumab and tyrosine kinase inhibitors.
In 2011, the Food and Drug Administration reported a 34% rate of ovarian failure in women who got bevacizumab as part of a colorectal cancer treatment regimen; 1 in 5 women regained ovarian function. This possible complication should be addressed with women.
Young patients taking tyrosine kinase inhibitors for chronic myeloid leukemia may also face later fertility problems, the panel noted, including an increased risk of complications in pregnancy or congenital anomalies. Data on these findings are incomplete, but patients should understand the possible risks, the panel wrote.
Supplements and clinical tools and resources can be found at www.asco.org/guidelines/fertility. Patient information is also available at www.cancer.net.
Neither Dr. Loren nor any of the other panel members had any financial disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY