User login
DEA moves hydrocodone combination products to schedule II
The Drug Enforcement Administration is making it harder to prescribe hydrocodone combination products.
The move was expected, as the agency proposed in February to move hydrocodone combinations from schedule III to schedule II in response to requests from both the U.S. Department of Health & Human Services and the Food and Drug Administration.
Some physician groups have opposed the move, saying that it will lead to more administrative burdens, do nothing to curb abuse and diversion, and potentially decrease access to medications.
The DEA will publish the final rule on the rescheduling in the Federal Register on Aug. 22. Manufacturers, distributors, and prescribers will have to comply by Oct. 13.
The agency said it is time to rein in opioid prescribing and that rescheduling will help accomplish that goal.
"Almost 7 million Americans abuse controlled-substance prescription medications, including opioid painkillers, resulting in more deaths from prescription drug overdoses than auto accidents," DEA administrator Michele Leonhart said in a statement. "Today’s action recognizes that these products are some of the most addictive and potentially dangerous prescription medications available."
Hydrocodone combination products were placed on schedule III by Congress in 1970 when it created the Controlled Substances Act, in part because it was believed that adding acetaminophen or other non-narcotics might lessen the abuse potential. Hydrocodone itself was placed on schedule II.
Now, "the scientific, medical, and epidemiological data are robust and support rescheduling [of hydrocodone combination products] into schedule II," according to the final rule.
Data show that the products are widely diverted and abused at rates similar to that of oxycodone products, which are schedule II, said the agency, which added that abuse is associated with severe psychological or physical dependence, and many are being admitted to addiction treatment.
The hydrocodone combinations are also associated with large numbers of deaths, said the agency. More than 16,000 deaths in 2010 were due to abuse of opioids, including hydrocodone combinations, according to the DEA.
About 137 million prescriptions for hydrocodone combinations were dispensed in 2013, the agency said. The most frequently prescribed combination is hydrocodone/acetaminophen.
In comments to the proposed rule in April, the American Medical Association, along with a group of organizations and companies in the long-term care field, asked the agency to delay the final rule until an exception was made for nursing homes and other long-term care facilities. The AMA’s House of Delegates had also voted in 2013 to oppose rescheduling.
The American College of Emergency Physicians also urged against rescheduling, telling the DEA in April that it would not likely solve the abuse and diversion problems, but would lead to a greater administrative hassle for physicians.
The DEA said it received 573 comments after it proposed rescheduling, with 52% in favor, 41% opposed, and 7% taking no position. The agency received the most comments from the general public (44%; 250 comments) and pharmacists and pharmacy students (21%; 122 comments), physicians (13%; 73 comments), patients (6%; 35 comments), and midlevel practitioners (5%; 31 comments). Just over half of the physician comments supported, or supported with qualification, rescheduling.
Most of the commenters who were opposed to rescheduling were pharmacists, pharmacy students, and patients. Those opposed were concerned about how it would affect prescribing practices and patient access to medicine, and how it might impact long-term care facilities. Commenters also said that it would not prevent abuse or diversion.
The DEA said that although moving to schedule II does, for instance, prohibit refills, it would not necessarily block physicians from writing prescriptions for supplies of longer than 30 days, or from writing multiple prescriptions at once. State laws might have limits, however, and those will take precedence over the DEA rule.
The DEA rescheduling follows an FDA advisory committee recommendation in Jan. 2013 to do so, and the FDA’s backing of the proposal in Oct. 2013. HHS followed with its own recommendation to the DEA.
In comments on the proposed rule, manufacturers and pharmacies asked for more time to implement the rescheduling, but the DEA said no, citing high rates of abuse, overdose, and deaths relating to hydrocodone combination products.
The rescheduling goes into effect on Oct. 13, 45 days from the date of the rule’s publication in the Federal Register.
On Twitter @aliciaault
The Drug Enforcement Administration is making it harder to prescribe hydrocodone combination products.
The move was expected, as the agency proposed in February to move hydrocodone combinations from schedule III to schedule II in response to requests from both the U.S. Department of Health & Human Services and the Food and Drug Administration.
Some physician groups have opposed the move, saying that it will lead to more administrative burdens, do nothing to curb abuse and diversion, and potentially decrease access to medications.
The DEA will publish the final rule on the rescheduling in the Federal Register on Aug. 22. Manufacturers, distributors, and prescribers will have to comply by Oct. 13.
The agency said it is time to rein in opioid prescribing and that rescheduling will help accomplish that goal.
"Almost 7 million Americans abuse controlled-substance prescription medications, including opioid painkillers, resulting in more deaths from prescription drug overdoses than auto accidents," DEA administrator Michele Leonhart said in a statement. "Today’s action recognizes that these products are some of the most addictive and potentially dangerous prescription medications available."
Hydrocodone combination products were placed on schedule III by Congress in 1970 when it created the Controlled Substances Act, in part because it was believed that adding acetaminophen or other non-narcotics might lessen the abuse potential. Hydrocodone itself was placed on schedule II.
Now, "the scientific, medical, and epidemiological data are robust and support rescheduling [of hydrocodone combination products] into schedule II," according to the final rule.
Data show that the products are widely diverted and abused at rates similar to that of oxycodone products, which are schedule II, said the agency, which added that abuse is associated with severe psychological or physical dependence, and many are being admitted to addiction treatment.
The hydrocodone combinations are also associated with large numbers of deaths, said the agency. More than 16,000 deaths in 2010 were due to abuse of opioids, including hydrocodone combinations, according to the DEA.
About 137 million prescriptions for hydrocodone combinations were dispensed in 2013, the agency said. The most frequently prescribed combination is hydrocodone/acetaminophen.
In comments to the proposed rule in April, the American Medical Association, along with a group of organizations and companies in the long-term care field, asked the agency to delay the final rule until an exception was made for nursing homes and other long-term care facilities. The AMA’s House of Delegates had also voted in 2013 to oppose rescheduling.
The American College of Emergency Physicians also urged against rescheduling, telling the DEA in April that it would not likely solve the abuse and diversion problems, but would lead to a greater administrative hassle for physicians.
The DEA said it received 573 comments after it proposed rescheduling, with 52% in favor, 41% opposed, and 7% taking no position. The agency received the most comments from the general public (44%; 250 comments) and pharmacists and pharmacy students (21%; 122 comments), physicians (13%; 73 comments), patients (6%; 35 comments), and midlevel practitioners (5%; 31 comments). Just over half of the physician comments supported, or supported with qualification, rescheduling.
Most of the commenters who were opposed to rescheduling were pharmacists, pharmacy students, and patients. Those opposed were concerned about how it would affect prescribing practices and patient access to medicine, and how it might impact long-term care facilities. Commenters also said that it would not prevent abuse or diversion.
The DEA said that although moving to schedule II does, for instance, prohibit refills, it would not necessarily block physicians from writing prescriptions for supplies of longer than 30 days, or from writing multiple prescriptions at once. State laws might have limits, however, and those will take precedence over the DEA rule.
The DEA rescheduling follows an FDA advisory committee recommendation in Jan. 2013 to do so, and the FDA’s backing of the proposal in Oct. 2013. HHS followed with its own recommendation to the DEA.
In comments on the proposed rule, manufacturers and pharmacies asked for more time to implement the rescheduling, but the DEA said no, citing high rates of abuse, overdose, and deaths relating to hydrocodone combination products.
The rescheduling goes into effect on Oct. 13, 45 days from the date of the rule’s publication in the Federal Register.
On Twitter @aliciaault
The Drug Enforcement Administration is making it harder to prescribe hydrocodone combination products.
The move was expected, as the agency proposed in February to move hydrocodone combinations from schedule III to schedule II in response to requests from both the U.S. Department of Health & Human Services and the Food and Drug Administration.
Some physician groups have opposed the move, saying that it will lead to more administrative burdens, do nothing to curb abuse and diversion, and potentially decrease access to medications.
The DEA will publish the final rule on the rescheduling in the Federal Register on Aug. 22. Manufacturers, distributors, and prescribers will have to comply by Oct. 13.
The agency said it is time to rein in opioid prescribing and that rescheduling will help accomplish that goal.
"Almost 7 million Americans abuse controlled-substance prescription medications, including opioid painkillers, resulting in more deaths from prescription drug overdoses than auto accidents," DEA administrator Michele Leonhart said in a statement. "Today’s action recognizes that these products are some of the most addictive and potentially dangerous prescription medications available."
Hydrocodone combination products were placed on schedule III by Congress in 1970 when it created the Controlled Substances Act, in part because it was believed that adding acetaminophen or other non-narcotics might lessen the abuse potential. Hydrocodone itself was placed on schedule II.
Now, "the scientific, medical, and epidemiological data are robust and support rescheduling [of hydrocodone combination products] into schedule II," according to the final rule.
Data show that the products are widely diverted and abused at rates similar to that of oxycodone products, which are schedule II, said the agency, which added that abuse is associated with severe psychological or physical dependence, and many are being admitted to addiction treatment.
The hydrocodone combinations are also associated with large numbers of deaths, said the agency. More than 16,000 deaths in 2010 were due to abuse of opioids, including hydrocodone combinations, according to the DEA.
About 137 million prescriptions for hydrocodone combinations were dispensed in 2013, the agency said. The most frequently prescribed combination is hydrocodone/acetaminophen.
In comments to the proposed rule in April, the American Medical Association, along with a group of organizations and companies in the long-term care field, asked the agency to delay the final rule until an exception was made for nursing homes and other long-term care facilities. The AMA’s House of Delegates had also voted in 2013 to oppose rescheduling.
The American College of Emergency Physicians also urged against rescheduling, telling the DEA in April that it would not likely solve the abuse and diversion problems, but would lead to a greater administrative hassle for physicians.
The DEA said it received 573 comments after it proposed rescheduling, with 52% in favor, 41% opposed, and 7% taking no position. The agency received the most comments from the general public (44%; 250 comments) and pharmacists and pharmacy students (21%; 122 comments), physicians (13%; 73 comments), patients (6%; 35 comments), and midlevel practitioners (5%; 31 comments). Just over half of the physician comments supported, or supported with qualification, rescheduling.
Most of the commenters who were opposed to rescheduling were pharmacists, pharmacy students, and patients. Those opposed were concerned about how it would affect prescribing practices and patient access to medicine, and how it might impact long-term care facilities. Commenters also said that it would not prevent abuse or diversion.
The DEA said that although moving to schedule II does, for instance, prohibit refills, it would not necessarily block physicians from writing prescriptions for supplies of longer than 30 days, or from writing multiple prescriptions at once. State laws might have limits, however, and those will take precedence over the DEA rule.
The DEA rescheduling follows an FDA advisory committee recommendation in Jan. 2013 to do so, and the FDA’s backing of the proposal in Oct. 2013. HHS followed with its own recommendation to the DEA.
In comments on the proposed rule, manufacturers and pharmacies asked for more time to implement the rescheduling, but the DEA said no, citing high rates of abuse, overdose, and deaths relating to hydrocodone combination products.
The rescheduling goes into effect on Oct. 13, 45 days from the date of the rule’s publication in the Federal Register.
On Twitter @aliciaault
Radiotherapy plus chemotherapy drive risk of pancreatic cancer in HL patients
Hodgkin’s lymphoma survivors who undergo both radiotherapy and chemotherapy face an increased risk of subsequent pancreatic cancer, an intervention case-control study demonstrated. In fact, the risk was 18-fold among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent.
"Several studies have reported significantly increased risks of pancreatic cancer among long-term HL [Hodgkin’s Lymphoma] survivors, but no prior study of HL survivors has assessed the risk of pancreatic cancer in relation to radiation dose or specific chemotherapeutic agents," researchers led by Dr. Graca M. Dores wrote in the July 25, 2014 issue of the Annals of Oncology. "In the general U.S. population, pancreatic cancer is the fourth most common cause of cancer death, with an overall 5-year relative survival of 5.8%," they noted.
In what the investigators characterized as the first analysis of its kind, Dr. Dores of the division of cancer epidemiology and genetics at the National Cancer Institute and associates drew from six population-based registries and data from main hospitals in the Netherlands to locate HL survivors who received a diagnosis of HL as their primary cancer between 1953 and 2003 and who had survived at least 5 years beyond the initial diagnosis. The cohort was comprised of 19,882 HL survivors, including 36 cases of pancreatic cancer and 70 matched controls. The researchers used logistic regression to estimate odds ratios for pancreatic cancer by comparing the histories of case patients to those of matched controls (Ann. Oncol. July 25 [doi:10.1093/annonc/mdu287]).
The median age at HL diagnosis was 47 years, and 73% had stage I or II disease. Among the 36 patients who developed pancreatic cancer, the median age of pancreatic cancer onset among cases was 61 years, a median of 19 years following the initial HL diagnosis. Dr. Dores and associates found that the risk of pancreatic cancer increased with increasing radiation dose to the location of the pancreatic tumor (P = .005) and increasing number of chemotherapy cycles that contained alkylating agents (P = .008). The risk of prostate cancer was 18-fold higher among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent. "This risk was significantly greater than the OR of 3.8 predicted by an additive model (P = .041) and nonsignificantly greater than the OR of 5.4 predicted by a multiplicative model (P = 0.29)," the researchers wrote. "Analyses based on continuous variables yielded similar results."
In discussing the implications of the findings, Dr. Dores and associates acknowledged that treatment approaches for HL "have changed considerably over the past several decades in an effort to maximize efficacy and minimize toxicity. Although radiotherapy remains an important therapeutic modality, radiation volumes and doses have decreased considerably over time, and subdiaphragmatic radiotherapy is infrequently indicated. While the first-line therapy for many HL patients today includes doxorubicin and dacarbazine, procarbazine and cyclophosphamide continue to be used, although often with lower cumulative doses than used in the past. Our findings for topoisomerase II inhibitors are equivocal, but warrant further investigation."
They went on to note that the study extends the range of solid cancers associated with chemotherapy "and adds to the evidence that the combination of chemotherapy and radiotherapy can increase risks beyond those predicted by a multiplicative model. For HL patients, radiation dose-response relationships have now been demonstrated for second cancers of the lung, female breast, stomach, and pancreas and, with the exception of breast cancer, increased risks of these cancers have been observed after receipt of AA [alkylating agent]-containing therapy. Changes in HL therapy over time should reduce second cancer risks compared to those observed with past treatments. In the interim, health care providers caring for long-term HL survivors should be alert to this treatment sequela and encourage a healthy lifestyle to minimize additional cancer risk factors."
The study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute contracts to Cancer Care Ontario, Toronto; Danish Cancer Society, Copenhagen; Finnish Cancer Registry, Helsinki; Information Management Services, Inc., Silver Spring, Md.; Karolinska Institute, Stockholm; University of Iowa; The University of Texas MD Anderson Cancer Center; and Westat, Inc., Rockville, Md. The Dutch study also was supported by the Lance Armstrong Foundation and the Dutch Cancer Society.
On Twitter @dougbrunk
Hodgkin’s lymphoma survivors who undergo both radiotherapy and chemotherapy face an increased risk of subsequent pancreatic cancer, an intervention case-control study demonstrated. In fact, the risk was 18-fold among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent.
"Several studies have reported significantly increased risks of pancreatic cancer among long-term HL [Hodgkin’s Lymphoma] survivors, but no prior study of HL survivors has assessed the risk of pancreatic cancer in relation to radiation dose or specific chemotherapeutic agents," researchers led by Dr. Graca M. Dores wrote in the July 25, 2014 issue of the Annals of Oncology. "In the general U.S. population, pancreatic cancer is the fourth most common cause of cancer death, with an overall 5-year relative survival of 5.8%," they noted.
In what the investigators characterized as the first analysis of its kind, Dr. Dores of the division of cancer epidemiology and genetics at the National Cancer Institute and associates drew from six population-based registries and data from main hospitals in the Netherlands to locate HL survivors who received a diagnosis of HL as their primary cancer between 1953 and 2003 and who had survived at least 5 years beyond the initial diagnosis. The cohort was comprised of 19,882 HL survivors, including 36 cases of pancreatic cancer and 70 matched controls. The researchers used logistic regression to estimate odds ratios for pancreatic cancer by comparing the histories of case patients to those of matched controls (Ann. Oncol. July 25 [doi:10.1093/annonc/mdu287]).
The median age at HL diagnosis was 47 years, and 73% had stage I or II disease. Among the 36 patients who developed pancreatic cancer, the median age of pancreatic cancer onset among cases was 61 years, a median of 19 years following the initial HL diagnosis. Dr. Dores and associates found that the risk of pancreatic cancer increased with increasing radiation dose to the location of the pancreatic tumor (P = .005) and increasing number of chemotherapy cycles that contained alkylating agents (P = .008). The risk of prostate cancer was 18-fold higher among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent. "This risk was significantly greater than the OR of 3.8 predicted by an additive model (P = .041) and nonsignificantly greater than the OR of 5.4 predicted by a multiplicative model (P = 0.29)," the researchers wrote. "Analyses based on continuous variables yielded similar results."
In discussing the implications of the findings, Dr. Dores and associates acknowledged that treatment approaches for HL "have changed considerably over the past several decades in an effort to maximize efficacy and minimize toxicity. Although radiotherapy remains an important therapeutic modality, radiation volumes and doses have decreased considerably over time, and subdiaphragmatic radiotherapy is infrequently indicated. While the first-line therapy for many HL patients today includes doxorubicin and dacarbazine, procarbazine and cyclophosphamide continue to be used, although often with lower cumulative doses than used in the past. Our findings for topoisomerase II inhibitors are equivocal, but warrant further investigation."
They went on to note that the study extends the range of solid cancers associated with chemotherapy "and adds to the evidence that the combination of chemotherapy and radiotherapy can increase risks beyond those predicted by a multiplicative model. For HL patients, radiation dose-response relationships have now been demonstrated for second cancers of the lung, female breast, stomach, and pancreas and, with the exception of breast cancer, increased risks of these cancers have been observed after receipt of AA [alkylating agent]-containing therapy. Changes in HL therapy over time should reduce second cancer risks compared to those observed with past treatments. In the interim, health care providers caring for long-term HL survivors should be alert to this treatment sequela and encourage a healthy lifestyle to minimize additional cancer risk factors."
The study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute contracts to Cancer Care Ontario, Toronto; Danish Cancer Society, Copenhagen; Finnish Cancer Registry, Helsinki; Information Management Services, Inc., Silver Spring, Md.; Karolinska Institute, Stockholm; University of Iowa; The University of Texas MD Anderson Cancer Center; and Westat, Inc., Rockville, Md. The Dutch study also was supported by the Lance Armstrong Foundation and the Dutch Cancer Society.
On Twitter @dougbrunk
Hodgkin’s lymphoma survivors who undergo both radiotherapy and chemotherapy face an increased risk of subsequent pancreatic cancer, an intervention case-control study demonstrated. In fact, the risk was 18-fold among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent.
"Several studies have reported significantly increased risks of pancreatic cancer among long-term HL [Hodgkin’s Lymphoma] survivors, but no prior study of HL survivors has assessed the risk of pancreatic cancer in relation to radiation dose or specific chemotherapeutic agents," researchers led by Dr. Graca M. Dores wrote in the July 25, 2014 issue of the Annals of Oncology. "In the general U.S. population, pancreatic cancer is the fourth most common cause of cancer death, with an overall 5-year relative survival of 5.8%," they noted.
In what the investigators characterized as the first analysis of its kind, Dr. Dores of the division of cancer epidemiology and genetics at the National Cancer Institute and associates drew from six population-based registries and data from main hospitals in the Netherlands to locate HL survivors who received a diagnosis of HL as their primary cancer between 1953 and 2003 and who had survived at least 5 years beyond the initial diagnosis. The cohort was comprised of 19,882 HL survivors, including 36 cases of pancreatic cancer and 70 matched controls. The researchers used logistic regression to estimate odds ratios for pancreatic cancer by comparing the histories of case patients to those of matched controls (Ann. Oncol. July 25 [doi:10.1093/annonc/mdu287]).
The median age at HL diagnosis was 47 years, and 73% had stage I or II disease. Among the 36 patients who developed pancreatic cancer, the median age of pancreatic cancer onset among cases was 61 years, a median of 19 years following the initial HL diagnosis. Dr. Dores and associates found that the risk of pancreatic cancer increased with increasing radiation dose to the location of the pancreatic tumor (P = .005) and increasing number of chemotherapy cycles that contained alkylating agents (P = .008). The risk of prostate cancer was 18-fold higher among those who received subdiaphragmatic radiation delivered at 10 Gy or higher in addition to six or more cycles of chemotherapy that contained an alkylating agent. "This risk was significantly greater than the OR of 3.8 predicted by an additive model (P = .041) and nonsignificantly greater than the OR of 5.4 predicted by a multiplicative model (P = 0.29)," the researchers wrote. "Analyses based on continuous variables yielded similar results."
In discussing the implications of the findings, Dr. Dores and associates acknowledged that treatment approaches for HL "have changed considerably over the past several decades in an effort to maximize efficacy and minimize toxicity. Although radiotherapy remains an important therapeutic modality, radiation volumes and doses have decreased considerably over time, and subdiaphragmatic radiotherapy is infrequently indicated. While the first-line therapy for many HL patients today includes doxorubicin and dacarbazine, procarbazine and cyclophosphamide continue to be used, although often with lower cumulative doses than used in the past. Our findings for topoisomerase II inhibitors are equivocal, but warrant further investigation."
They went on to note that the study extends the range of solid cancers associated with chemotherapy "and adds to the evidence that the combination of chemotherapy and radiotherapy can increase risks beyond those predicted by a multiplicative model. For HL patients, radiation dose-response relationships have now been demonstrated for second cancers of the lung, female breast, stomach, and pancreas and, with the exception of breast cancer, increased risks of these cancers have been observed after receipt of AA [alkylating agent]-containing therapy. Changes in HL therapy over time should reduce second cancer risks compared to those observed with past treatments. In the interim, health care providers caring for long-term HL survivors should be alert to this treatment sequela and encourage a healthy lifestyle to minimize additional cancer risk factors."
The study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute contracts to Cancer Care Ontario, Toronto; Danish Cancer Society, Copenhagen; Finnish Cancer Registry, Helsinki; Information Management Services, Inc., Silver Spring, Md.; Karolinska Institute, Stockholm; University of Iowa; The University of Texas MD Anderson Cancer Center; and Westat, Inc., Rockville, Md. The Dutch study also was supported by the Lance Armstrong Foundation and the Dutch Cancer Society.
On Twitter @dougbrunk
FROM ANNALS OF ONCOLOGY
Key clinical point: Both radiotherapy and chemotherapy increase pancreatic cancer risk among Hodgkin’s lymphoma survivors.
Major finding: Survivors of Hodgkin’s lymphoma treated with both subdiaphragmatic radiation and six or more cycles of alkylating agent-containing therapy were 18 times more likely to develop pancreatic cancer, compared with patients who received no such treatment.
Data source: An international case-control study within a cohort of 19,882 HL survivors diagnosed from 1953 to 2003, including 36 cases with pancreatic cancer and 70 matched controls.
Disclosures: The study was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and National Cancer Institute contracts to Cancer Care Ontario, Toronto; Danish Cancer Society, Copenhagen; Finnish Cancer Registry, Helsinki; Information Management Services, Inc., Silver Spring, Md.; Karolinska Institute, Stockholm; University of Iowa; The University of Texas MD Anderson Cancer Center; and Westat, Inc., Rockville, Md. The Dutch study also was supported by the Lance Armstrong Foundation and the Dutch Cancer Society.
House bill would allow corrective action plan for DEA violators
A new bill aims to clarify the rules of the Controlled Substances Act to ensure that legitimate operators stay in business and patients get needed medication, according to congressional backers of the bill, which was approved by the House of Representatives on July 29.
The bill still has to be taken up by the Senate, where there is no companion legislation.
The Ensuring Patient Access and Effective Drug Enforcement Act of 2014 (H.R. 4709) would ensure that restrictions on distribution of controlled substances are not so onerous as to inhibit access for patients, would require the U.S. Attorney General to give DEA registrant pharmacies and physicians who violate the rules an opportunity to submit a corrective action plan that might defer suspension of their registration, and would establish a working group to make recommendations to Congress on federal policies to reduce prescription drug diversion and abuse.
These measures are among the major policy goals of the Alliance to Prevent the Abuse of Medicines. The Washington, D.C.–based group includes among its members the American Medical Association, Cardinal Health, CVS Caremark, the Health Industry Distributors Association, and Teva.
The National Association of Chain Drug Stores "and chain pharmacy are committed to partnering with federal and state agencies, law enforcement personnel, policymakers, and other stakeholders to work on viable strategies to simultaneously advance patient health and prevent prescription drug abuse," NACDS President and CEO Steven C. Anderson, said in a statement regarding the bill.
Rep. Marsha Blackburn (R-Tenn.), a cosponsor of the bill, said in a statement that simply acknowledging the epidemic of prescription drug abuse isn’t enough. "Congress has a responsibility to make sure the law is crystal clear for both the DEA and legitimate businesses who want to understand what the rules are so they can do the right thing."
In House testimony last April, DEA Deputy Assistant Administrator Joseph Rannazzisi said the agency’s job is getting tougher. The number of registrants that the DEA regulates has mushroomed from 480,000 in 1973 to 1.5 million today, he said. At the same time, diversion and abuse have risen steeply, with opioids selling on the black market for 5-10 times their retail value.
In the last 3 years, the DEA’s Tactical Diversion Squads have increased from 37 to 66, and the agency has been applying its stiffest penalty – an immediate suspension order – in a judicious manner, according to Mr. Rannazzisi. From October 2013 through March 2014, 20 suspensions were ordered.
On Twitter @aliciaault
A new bill aims to clarify the rules of the Controlled Substances Act to ensure that legitimate operators stay in business and patients get needed medication, according to congressional backers of the bill, which was approved by the House of Representatives on July 29.
The bill still has to be taken up by the Senate, where there is no companion legislation.
The Ensuring Patient Access and Effective Drug Enforcement Act of 2014 (H.R. 4709) would ensure that restrictions on distribution of controlled substances are not so onerous as to inhibit access for patients, would require the U.S. Attorney General to give DEA registrant pharmacies and physicians who violate the rules an opportunity to submit a corrective action plan that might defer suspension of their registration, and would establish a working group to make recommendations to Congress on federal policies to reduce prescription drug diversion and abuse.
These measures are among the major policy goals of the Alliance to Prevent the Abuse of Medicines. The Washington, D.C.–based group includes among its members the American Medical Association, Cardinal Health, CVS Caremark, the Health Industry Distributors Association, and Teva.
The National Association of Chain Drug Stores "and chain pharmacy are committed to partnering with federal and state agencies, law enforcement personnel, policymakers, and other stakeholders to work on viable strategies to simultaneously advance patient health and prevent prescription drug abuse," NACDS President and CEO Steven C. Anderson, said in a statement regarding the bill.
Rep. Marsha Blackburn (R-Tenn.), a cosponsor of the bill, said in a statement that simply acknowledging the epidemic of prescription drug abuse isn’t enough. "Congress has a responsibility to make sure the law is crystal clear for both the DEA and legitimate businesses who want to understand what the rules are so they can do the right thing."
In House testimony last April, DEA Deputy Assistant Administrator Joseph Rannazzisi said the agency’s job is getting tougher. The number of registrants that the DEA regulates has mushroomed from 480,000 in 1973 to 1.5 million today, he said. At the same time, diversion and abuse have risen steeply, with opioids selling on the black market for 5-10 times their retail value.
In the last 3 years, the DEA’s Tactical Diversion Squads have increased from 37 to 66, and the agency has been applying its stiffest penalty – an immediate suspension order – in a judicious manner, according to Mr. Rannazzisi. From October 2013 through March 2014, 20 suspensions were ordered.
On Twitter @aliciaault
A new bill aims to clarify the rules of the Controlled Substances Act to ensure that legitimate operators stay in business and patients get needed medication, according to congressional backers of the bill, which was approved by the House of Representatives on July 29.
The bill still has to be taken up by the Senate, where there is no companion legislation.
The Ensuring Patient Access and Effective Drug Enforcement Act of 2014 (H.R. 4709) would ensure that restrictions on distribution of controlled substances are not so onerous as to inhibit access for patients, would require the U.S. Attorney General to give DEA registrant pharmacies and physicians who violate the rules an opportunity to submit a corrective action plan that might defer suspension of their registration, and would establish a working group to make recommendations to Congress on federal policies to reduce prescription drug diversion and abuse.
These measures are among the major policy goals of the Alliance to Prevent the Abuse of Medicines. The Washington, D.C.–based group includes among its members the American Medical Association, Cardinal Health, CVS Caremark, the Health Industry Distributors Association, and Teva.
The National Association of Chain Drug Stores "and chain pharmacy are committed to partnering with federal and state agencies, law enforcement personnel, policymakers, and other stakeholders to work on viable strategies to simultaneously advance patient health and prevent prescription drug abuse," NACDS President and CEO Steven C. Anderson, said in a statement regarding the bill.
Rep. Marsha Blackburn (R-Tenn.), a cosponsor of the bill, said in a statement that simply acknowledging the epidemic of prescription drug abuse isn’t enough. "Congress has a responsibility to make sure the law is crystal clear for both the DEA and legitimate businesses who want to understand what the rules are so they can do the right thing."
In House testimony last April, DEA Deputy Assistant Administrator Joseph Rannazzisi said the agency’s job is getting tougher. The number of registrants that the DEA regulates has mushroomed from 480,000 in 1973 to 1.5 million today, he said. At the same time, diversion and abuse have risen steeply, with opioids selling on the black market for 5-10 times their retail value.
In the last 3 years, the DEA’s Tactical Diversion Squads have increased from 37 to 66, and the agency has been applying its stiffest penalty – an immediate suspension order – in a judicious manner, according to Mr. Rannazzisi. From October 2013 through March 2014, 20 suspensions were ordered.
On Twitter @aliciaault
Look for nephrotoxicity in adult survivors of childhood cancer
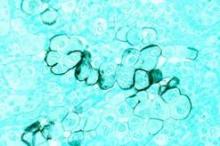
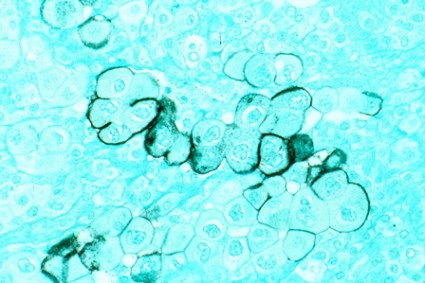
LAS VEGAS – Adult survivors of childhood cancer treated with high-dose cisplatin or high-dose ifosfamide are at markedly increased risk for chronic renal impairment, according to a large Dutch study with a median 18.3-year follow-up.
Long-term treatment-related nephrotoxicity was also seen in the adult survivors of childhood cancer who underwent unilateral nephrectomy combined with abdominal radiation therapy.
"This study is perhaps a warning to us when we’re seeing these adult survivors of childhood cancer in clinic, particularly if they have a history of ifosfamide or cisplatin use, to pay closer attention to the development of chronic kidney disease so they can be managed better in the future," Dr. Anushree C. Shirali said at a meeting sponsored by the National Kidney Foundation.
Drug-induced acute kidney injury is a common event during cancer therapy. Its mechanisms and treatments are well studied. In contrast, the long-term nephrotoxicity of the powerful therapies used in treating childhood cancers has received much less scrutiny. But it’s an increasingly relevant issue because childhood cancer survival rates have improved substantially. Indeed, as the Dutch investigators observed, today 1 in 570 young adults is a childhood cancer survivor (Clin. J. Am. Soc. Nephrol. 2013;8:922-9).
Dr. Shirali, a nephrologist at Yale University in New Haven, Conn., highlighted the Dutch study of 763 adult survivors of childhood cancer because of its unusually long and complete follow-up. The investigators included nearly 90% of all adult survivors treated at Erasmus University in Rotterdam, the Netherlands, during 1964-2005.
High-dose ifosfamide was associated with a 6.2-fold greater likelihood of an increased urinary beta2-microglobulin/creatinine ratio indicative of persisting tubular dysfunction, compared with cancer survivors not receiving that therapy. High-dose cisplatin was associated with a 5.2-fold increased risk of albuminuria. The estimated glomerular filtration rate in adult survivors who had received high-dose ifosfamide was 88 mL/min per 1.73 m2, significantly lower than the 98 mL/min per 1.73 m2 in others. Similarly, patients who had received high-dose cisplatin had an average estimated glomerular filtration rate of 83 mL/min per 1.73 m2, compared with 101 mL/min per 1.73 m2 in survivors not treated with high-dose cisplatin.
In contrast to ifosfamide, its isomer cyclophosphamide was not associated with long-term nephrotoxicity; neither was carboplatin, a cisplatin analogue, or methotrexate. Although methotrexate is known to cause acute nephrotoxicity, this phenomenon appears to be completely reversible, since methotrexate-treated, long-term cancer survivors didn’t develop tubular or glomerular dysfunction.
This long-term study was supported by the Dutch Kidney Foundation. Dr. Shirali, who was not involved in the study, reported having no financial conflicts.
LAS VEGAS – Adult survivors of childhood cancer treated with high-dose cisplatin or high-dose ifosfamide are at markedly increased risk for chronic renal impairment, according to a large Dutch study with a median 18.3-year follow-up.
Long-term treatment-related nephrotoxicity was also seen in the adult survivors of childhood cancer who underwent unilateral nephrectomy combined with abdominal radiation therapy.
"This study is perhaps a warning to us when we’re seeing these adult survivors of childhood cancer in clinic, particularly if they have a history of ifosfamide or cisplatin use, to pay closer attention to the development of chronic kidney disease so they can be managed better in the future," Dr. Anushree C. Shirali said at a meeting sponsored by the National Kidney Foundation.
Drug-induced acute kidney injury is a common event during cancer therapy. Its mechanisms and treatments are well studied. In contrast, the long-term nephrotoxicity of the powerful therapies used in treating childhood cancers has received much less scrutiny. But it’s an increasingly relevant issue because childhood cancer survival rates have improved substantially. Indeed, as the Dutch investigators observed, today 1 in 570 young adults is a childhood cancer survivor (Clin. J. Am. Soc. Nephrol. 2013;8:922-9).
Dr. Shirali, a nephrologist at Yale University in New Haven, Conn., highlighted the Dutch study of 763 adult survivors of childhood cancer because of its unusually long and complete follow-up. The investigators included nearly 90% of all adult survivors treated at Erasmus University in Rotterdam, the Netherlands, during 1964-2005.
High-dose ifosfamide was associated with a 6.2-fold greater likelihood of an increased urinary beta2-microglobulin/creatinine ratio indicative of persisting tubular dysfunction, compared with cancer survivors not receiving that therapy. High-dose cisplatin was associated with a 5.2-fold increased risk of albuminuria. The estimated glomerular filtration rate in adult survivors who had received high-dose ifosfamide was 88 mL/min per 1.73 m2, significantly lower than the 98 mL/min per 1.73 m2 in others. Similarly, patients who had received high-dose cisplatin had an average estimated glomerular filtration rate of 83 mL/min per 1.73 m2, compared with 101 mL/min per 1.73 m2 in survivors not treated with high-dose cisplatin.
In contrast to ifosfamide, its isomer cyclophosphamide was not associated with long-term nephrotoxicity; neither was carboplatin, a cisplatin analogue, or methotrexate. Although methotrexate is known to cause acute nephrotoxicity, this phenomenon appears to be completely reversible, since methotrexate-treated, long-term cancer survivors didn’t develop tubular or glomerular dysfunction.
This long-term study was supported by the Dutch Kidney Foundation. Dr. Shirali, who was not involved in the study, reported having no financial conflicts.
LAS VEGAS – Adult survivors of childhood cancer treated with high-dose cisplatin or high-dose ifosfamide are at markedly increased risk for chronic renal impairment, according to a large Dutch study with a median 18.3-year follow-up.
Long-term treatment-related nephrotoxicity was also seen in the adult survivors of childhood cancer who underwent unilateral nephrectomy combined with abdominal radiation therapy.
"This study is perhaps a warning to us when we’re seeing these adult survivors of childhood cancer in clinic, particularly if they have a history of ifosfamide or cisplatin use, to pay closer attention to the development of chronic kidney disease so they can be managed better in the future," Dr. Anushree C. Shirali said at a meeting sponsored by the National Kidney Foundation.
Drug-induced acute kidney injury is a common event during cancer therapy. Its mechanisms and treatments are well studied. In contrast, the long-term nephrotoxicity of the powerful therapies used in treating childhood cancers has received much less scrutiny. But it’s an increasingly relevant issue because childhood cancer survival rates have improved substantially. Indeed, as the Dutch investigators observed, today 1 in 570 young adults is a childhood cancer survivor (Clin. J. Am. Soc. Nephrol. 2013;8:922-9).
Dr. Shirali, a nephrologist at Yale University in New Haven, Conn., highlighted the Dutch study of 763 adult survivors of childhood cancer because of its unusually long and complete follow-up. The investigators included nearly 90% of all adult survivors treated at Erasmus University in Rotterdam, the Netherlands, during 1964-2005.
High-dose ifosfamide was associated with a 6.2-fold greater likelihood of an increased urinary beta2-microglobulin/creatinine ratio indicative of persisting tubular dysfunction, compared with cancer survivors not receiving that therapy. High-dose cisplatin was associated with a 5.2-fold increased risk of albuminuria. The estimated glomerular filtration rate in adult survivors who had received high-dose ifosfamide was 88 mL/min per 1.73 m2, significantly lower than the 98 mL/min per 1.73 m2 in others. Similarly, patients who had received high-dose cisplatin had an average estimated glomerular filtration rate of 83 mL/min per 1.73 m2, compared with 101 mL/min per 1.73 m2 in survivors not treated with high-dose cisplatin.
In contrast to ifosfamide, its isomer cyclophosphamide was not associated with long-term nephrotoxicity; neither was carboplatin, a cisplatin analogue, or methotrexate. Although methotrexate is known to cause acute nephrotoxicity, this phenomenon appears to be completely reversible, since methotrexate-treated, long-term cancer survivors didn’t develop tubular or glomerular dysfunction.
This long-term study was supported by the Dutch Kidney Foundation. Dr. Shirali, who was not involved in the study, reported having no financial conflicts.
AT SCM 14
Key clinical point: Today 1 in 570 young adults is a childhood cancer survivor, and many are on the road to chronic kidney disease.
Major finding: Adult survivors of childhood cancer treated with high-dose cisplatin or high-dose ifosfamide had a significantly lower estimated glomerular filtration rate than those who were not.
Data source: This was a retrospective, single-center study involving 763 adult survivors of childhood cancer with a median 18.3 years of follow-up.
Disclosures: This long-term study was supported by the Dutch Kidney Foundation. Dr. Shirali, who was not involved in the study, reported having no financial conflicts.
Latest evidence clarifies management of prostate cancer in elderly
A review of the existing evidence to date on the management of prostate cancer in the older population was published online July 28 as part of a special issue of the Journal of Clinical Oncology devoted to geriatric oncology.
Underlying health status and age-related changes can have an effect on tolerance of hormonal therapy and chemotherapy in men with advanced disease, said Dr. Chunkit Fung of the University of Rochester (N.Y.) and his associates.
They recommend using common geriatric assessment tools to categorize men into "stages of age" to assist with decision making. They used this framework – categorizing men as fit, vulnerable, and frail – to provide an evidence-based guide to the management of older men with prostate cancer, with a focus on systemic disease. The review is available on the journal’s website here.
On Twitter @nikolaideslaura
A review of the existing evidence to date on the management of prostate cancer in the older population was published online July 28 as part of a special issue of the Journal of Clinical Oncology devoted to geriatric oncology.
Underlying health status and age-related changes can have an effect on tolerance of hormonal therapy and chemotherapy in men with advanced disease, said Dr. Chunkit Fung of the University of Rochester (N.Y.) and his associates.
They recommend using common geriatric assessment tools to categorize men into "stages of age" to assist with decision making. They used this framework – categorizing men as fit, vulnerable, and frail – to provide an evidence-based guide to the management of older men with prostate cancer, with a focus on systemic disease. The review is available on the journal’s website here.
On Twitter @nikolaideslaura
A review of the existing evidence to date on the management of prostate cancer in the older population was published online July 28 as part of a special issue of the Journal of Clinical Oncology devoted to geriatric oncology.
Underlying health status and age-related changes can have an effect on tolerance of hormonal therapy and chemotherapy in men with advanced disease, said Dr. Chunkit Fung of the University of Rochester (N.Y.) and his associates.
They recommend using common geriatric assessment tools to categorize men into "stages of age" to assist with decision making. They used this framework – categorizing men as fit, vulnerable, and frail – to provide an evidence-based guide to the management of older men with prostate cancer, with a focus on systemic disease. The review is available on the journal’s website here.
On Twitter @nikolaideslaura
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Insurers play growing role in palliative care expansion
Health plans are starting to expand their coverage of palliative care, offering higher payments to physicians who meet certain standards or paying for home care for a wider range of seriously ill patients.
The offerings vary greatly by region and by plan, but the common theme is that these types of programs are on the rise.
Cambia Health Solutions, which operates six health plans in Oregon, Washington, Idaho, and Utah, recently garnered attention with the launch of a wide-ranging palliative care program.
Starting this year, Cambia plans will pay for services previously not covered, such as home health aides and advance care planning counseling. They are also partnering with the University of Washington to offer palliative care training to physicians, nurses, and social workers. Palliative care physicians will be able to earn certification through a 1-year program. There’s also a shorter training option in the works to teach primary care physicians how to start palliative care conversations and make referrals.
The program also aims to address the social needs of seriously ill patients by forming case management units with specially trained nurses and social workers who can assist members with issues such as home food delivery, according to Dr. Csaba Mera, chief medical officer at Cambia.
The new program’s goal is to build a "best in class" model that could be used nationally, Dr. Mera said. At the core of the program, he noted, is the goal of honoring the wishes of seriously ill patients.
"That has not been happening in health care," he said. "It’s amazing how often somebody decides they don’t want certain kinds of treatment, and they still get it."
While Cambia’s program is wide reaching, the health plan is not the only payer active in palliative care. The insurance giant Aetna has a decade of experience with specialized case management services designed to help patients and caregivers coping with advanced illness.
Through Aetna’s Compassionate Care Program, nurses and social workers work with physicians’ offices and patients and their families to provide education, assistance with pain medications, and other psychosocial support. They also work to ensure that advance directives are in place and followed. In most cases, the case managers are located in call centers. But increasingly, the health plan has embedded them in the offices of participating medical groups.
The case managers are "almost a lifeline" to patients and their families, explained Dr. Randall Krakauer, Aetna’s vice president and national medical director for medical strategy.
It’s also been a no-brainer for Aetna financially. The specialized case managers, who are used in both commercial and Medicare Advantage plans, have significantly reduced costly hospital stays and the use of the emergency department. There’s typically a $12,000 decrease in costs for each person enrolled in the case management program, according to Aetna.
Among Aetna Medicare Advantage members, there has been an 82% drop in days spent in the hospital for acute care, an 86% drop in days spent in the intensive care unit, and a 78% drop in use of the emergency department.
"If there is an opportunity for favorable impact at the intersection of quality and cost, particularly in Medicare, this is in fact the mother lode," Dr. Krakauer said.
Some regional payers have also begun to partner with physicians and other providers in the community to offer more than just traditional hospice coverage. For instance, Excellus BlueCross BlueShield, which operates in upstate New York, worked with the University of Rochester Medical Center to develop courses for physicians that focus on advance care planning, how to use the Medical Orders for Life-Sustaining Treatment (MOLST) protocol, and myths about CPR and life-sustaining treatment. Physicians who complete the online training and a testing module about it can qualify for bonus payments.
Highmark, which operates in Pennsylvania, Delaware, and West Virginia, set up a palliative care indicator to measure the performance of its contracted hospitals. The insurer measures the percentage of patients who have a palliative care consult, and the percentage of adult intensive care unit patients who have a documented resuscitation status, designated health care proxy, and documented interdisciplinary family meeting.
What’s driving the expansion into palliative care? Part of the reason is demographics, noted J. Donald Schumacher, Psy.D., president and CEO of the National Hospice and Palliative Care Organization. Baby boomers are aging and will demand health care on their own terms, including having help with both their medical and psychosocial needs when dealing with serious illness.
"I think it’s great the health plans are doing it," he said. "People are asking for good pain and symptom management now more than ever before."
The growth of accountable care organizations (ACOs) is also driving the expansion of palliative care programs. Because ACOs assume risk for the total cost of their members’ health care, expanding palliative care services in the community offers the potential to lower costly hospital admissions and readmissions, Dr. Schumacher said. Even though ACOs are developing very differently depending on the local community, they all seem interested in hospice and palliative care programs, he said.
Dr. Diane E. Meier, director of the Center to Advance Palliative Care, recently created a toolkit for payers outlining the rationale for establishing a palliative program and elements with a proven track record of improving quality and decreasing costs. For instance, a meaningful 24/7 clinical response is an essential element, she said, because it keeps patients from calling 911 frequently and ending up in the emergency department.
"Payers are recognizing that the reason their members don’t get access to palliative care in the community is because no one will pay for it – and they can fix that," said Dr. Meier, who is also a professor of geriatrics and palliative medicine at Mount Sinai Hospital in New York.
But expanding access to palliative care is likely to take more than just financial incentives, said Dr. Krakauer. The difficulty in making changes to health care delivery is one obstacle, he said. Another issue is that palliative care still runs contrary to the medical culture.
"A physician might feel that this represents personal failure as a professional, and he may feel that this is not what his patients would expect of him," Dr. Krakauer said. "We know this isn’t true, but I think that this type of feeling is persistent."
On Twitter @maryellenny
Health plans are starting to expand their coverage of palliative care, offering higher payments to physicians who meet certain standards or paying for home care for a wider range of seriously ill patients.
The offerings vary greatly by region and by plan, but the common theme is that these types of programs are on the rise.
Cambia Health Solutions, which operates six health plans in Oregon, Washington, Idaho, and Utah, recently garnered attention with the launch of a wide-ranging palliative care program.
Starting this year, Cambia plans will pay for services previously not covered, such as home health aides and advance care planning counseling. They are also partnering with the University of Washington to offer palliative care training to physicians, nurses, and social workers. Palliative care physicians will be able to earn certification through a 1-year program. There’s also a shorter training option in the works to teach primary care physicians how to start palliative care conversations and make referrals.
The program also aims to address the social needs of seriously ill patients by forming case management units with specially trained nurses and social workers who can assist members with issues such as home food delivery, according to Dr. Csaba Mera, chief medical officer at Cambia.
The new program’s goal is to build a "best in class" model that could be used nationally, Dr. Mera said. At the core of the program, he noted, is the goal of honoring the wishes of seriously ill patients.
"That has not been happening in health care," he said. "It’s amazing how often somebody decides they don’t want certain kinds of treatment, and they still get it."
While Cambia’s program is wide reaching, the health plan is not the only payer active in palliative care. The insurance giant Aetna has a decade of experience with specialized case management services designed to help patients and caregivers coping with advanced illness.
Through Aetna’s Compassionate Care Program, nurses and social workers work with physicians’ offices and patients and their families to provide education, assistance with pain medications, and other psychosocial support. They also work to ensure that advance directives are in place and followed. In most cases, the case managers are located in call centers. But increasingly, the health plan has embedded them in the offices of participating medical groups.
The case managers are "almost a lifeline" to patients and their families, explained Dr. Randall Krakauer, Aetna’s vice president and national medical director for medical strategy.
It’s also been a no-brainer for Aetna financially. The specialized case managers, who are used in both commercial and Medicare Advantage plans, have significantly reduced costly hospital stays and the use of the emergency department. There’s typically a $12,000 decrease in costs for each person enrolled in the case management program, according to Aetna.
Among Aetna Medicare Advantage members, there has been an 82% drop in days spent in the hospital for acute care, an 86% drop in days spent in the intensive care unit, and a 78% drop in use of the emergency department.
"If there is an opportunity for favorable impact at the intersection of quality and cost, particularly in Medicare, this is in fact the mother lode," Dr. Krakauer said.
Some regional payers have also begun to partner with physicians and other providers in the community to offer more than just traditional hospice coverage. For instance, Excellus BlueCross BlueShield, which operates in upstate New York, worked with the University of Rochester Medical Center to develop courses for physicians that focus on advance care planning, how to use the Medical Orders for Life-Sustaining Treatment (MOLST) protocol, and myths about CPR and life-sustaining treatment. Physicians who complete the online training and a testing module about it can qualify for bonus payments.
Highmark, which operates in Pennsylvania, Delaware, and West Virginia, set up a palliative care indicator to measure the performance of its contracted hospitals. The insurer measures the percentage of patients who have a palliative care consult, and the percentage of adult intensive care unit patients who have a documented resuscitation status, designated health care proxy, and documented interdisciplinary family meeting.
What’s driving the expansion into palliative care? Part of the reason is demographics, noted J. Donald Schumacher, Psy.D., president and CEO of the National Hospice and Palliative Care Organization. Baby boomers are aging and will demand health care on their own terms, including having help with both their medical and psychosocial needs when dealing with serious illness.
"I think it’s great the health plans are doing it," he said. "People are asking for good pain and symptom management now more than ever before."
The growth of accountable care organizations (ACOs) is also driving the expansion of palliative care programs. Because ACOs assume risk for the total cost of their members’ health care, expanding palliative care services in the community offers the potential to lower costly hospital admissions and readmissions, Dr. Schumacher said. Even though ACOs are developing very differently depending on the local community, they all seem interested in hospice and palliative care programs, he said.
Dr. Diane E. Meier, director of the Center to Advance Palliative Care, recently created a toolkit for payers outlining the rationale for establishing a palliative program and elements with a proven track record of improving quality and decreasing costs. For instance, a meaningful 24/7 clinical response is an essential element, she said, because it keeps patients from calling 911 frequently and ending up in the emergency department.
"Payers are recognizing that the reason their members don’t get access to palliative care in the community is because no one will pay for it – and they can fix that," said Dr. Meier, who is also a professor of geriatrics and palliative medicine at Mount Sinai Hospital in New York.
But expanding access to palliative care is likely to take more than just financial incentives, said Dr. Krakauer. The difficulty in making changes to health care delivery is one obstacle, he said. Another issue is that palliative care still runs contrary to the medical culture.
"A physician might feel that this represents personal failure as a professional, and he may feel that this is not what his patients would expect of him," Dr. Krakauer said. "We know this isn’t true, but I think that this type of feeling is persistent."
On Twitter @maryellenny
Health plans are starting to expand their coverage of palliative care, offering higher payments to physicians who meet certain standards or paying for home care for a wider range of seriously ill patients.
The offerings vary greatly by region and by plan, but the common theme is that these types of programs are on the rise.
Cambia Health Solutions, which operates six health plans in Oregon, Washington, Idaho, and Utah, recently garnered attention with the launch of a wide-ranging palliative care program.
Starting this year, Cambia plans will pay for services previously not covered, such as home health aides and advance care planning counseling. They are also partnering with the University of Washington to offer palliative care training to physicians, nurses, and social workers. Palliative care physicians will be able to earn certification through a 1-year program. There’s also a shorter training option in the works to teach primary care physicians how to start palliative care conversations and make referrals.
The program also aims to address the social needs of seriously ill patients by forming case management units with specially trained nurses and social workers who can assist members with issues such as home food delivery, according to Dr. Csaba Mera, chief medical officer at Cambia.
The new program’s goal is to build a "best in class" model that could be used nationally, Dr. Mera said. At the core of the program, he noted, is the goal of honoring the wishes of seriously ill patients.
"That has not been happening in health care," he said. "It’s amazing how often somebody decides they don’t want certain kinds of treatment, and they still get it."
While Cambia’s program is wide reaching, the health plan is not the only payer active in palliative care. The insurance giant Aetna has a decade of experience with specialized case management services designed to help patients and caregivers coping with advanced illness.
Through Aetna’s Compassionate Care Program, nurses and social workers work with physicians’ offices and patients and their families to provide education, assistance with pain medications, and other psychosocial support. They also work to ensure that advance directives are in place and followed. In most cases, the case managers are located in call centers. But increasingly, the health plan has embedded them in the offices of participating medical groups.
The case managers are "almost a lifeline" to patients and their families, explained Dr. Randall Krakauer, Aetna’s vice president and national medical director for medical strategy.
It’s also been a no-brainer for Aetna financially. The specialized case managers, who are used in both commercial and Medicare Advantage plans, have significantly reduced costly hospital stays and the use of the emergency department. There’s typically a $12,000 decrease in costs for each person enrolled in the case management program, according to Aetna.
Among Aetna Medicare Advantage members, there has been an 82% drop in days spent in the hospital for acute care, an 86% drop in days spent in the intensive care unit, and a 78% drop in use of the emergency department.
"If there is an opportunity for favorable impact at the intersection of quality and cost, particularly in Medicare, this is in fact the mother lode," Dr. Krakauer said.
Some regional payers have also begun to partner with physicians and other providers in the community to offer more than just traditional hospice coverage. For instance, Excellus BlueCross BlueShield, which operates in upstate New York, worked with the University of Rochester Medical Center to develop courses for physicians that focus on advance care planning, how to use the Medical Orders for Life-Sustaining Treatment (MOLST) protocol, and myths about CPR and life-sustaining treatment. Physicians who complete the online training and a testing module about it can qualify for bonus payments.
Highmark, which operates in Pennsylvania, Delaware, and West Virginia, set up a palliative care indicator to measure the performance of its contracted hospitals. The insurer measures the percentage of patients who have a palliative care consult, and the percentage of adult intensive care unit patients who have a documented resuscitation status, designated health care proxy, and documented interdisciplinary family meeting.
What’s driving the expansion into palliative care? Part of the reason is demographics, noted J. Donald Schumacher, Psy.D., president and CEO of the National Hospice and Palliative Care Organization. Baby boomers are aging and will demand health care on their own terms, including having help with both their medical and psychosocial needs when dealing with serious illness.
"I think it’s great the health plans are doing it," he said. "People are asking for good pain and symptom management now more than ever before."
The growth of accountable care organizations (ACOs) is also driving the expansion of palliative care programs. Because ACOs assume risk for the total cost of their members’ health care, expanding palliative care services in the community offers the potential to lower costly hospital admissions and readmissions, Dr. Schumacher said. Even though ACOs are developing very differently depending on the local community, they all seem interested in hospice and palliative care programs, he said.
Dr. Diane E. Meier, director of the Center to Advance Palliative Care, recently created a toolkit for payers outlining the rationale for establishing a palliative program and elements with a proven track record of improving quality and decreasing costs. For instance, a meaningful 24/7 clinical response is an essential element, she said, because it keeps patients from calling 911 frequently and ending up in the emergency department.
"Payers are recognizing that the reason their members don’t get access to palliative care in the community is because no one will pay for it – and they can fix that," said Dr. Meier, who is also a professor of geriatrics and palliative medicine at Mount Sinai Hospital in New York.
But expanding access to palliative care is likely to take more than just financial incentives, said Dr. Krakauer. The difficulty in making changes to health care delivery is one obstacle, he said. Another issue is that palliative care still runs contrary to the medical culture.
"A physician might feel that this represents personal failure as a professional, and he may feel that this is not what his patients would expect of him," Dr. Krakauer said. "We know this isn’t true, but I think that this type of feeling is persistent."
On Twitter @maryellenny
Palonosetron versus older 5-HT3 receptor antagonists for nausea prevention in patients receiving chemotherapy: a multistudy analysis
Background No clinical standard currently exists for the optimal management of nausea induced by emetogenic chemotherapy, particularly delayed nausea.
Objective To compare the efficacy and safety of palonosetron with older 5-HT3 receptor antagonists (RAs) in preventing chemotherapy-induced nausea.
Methods Data were pooled from 4 similarly designed multicenter, randomized, double-blind, clinical trials that compared single intravenous doses of palonosetron 0.25 mg or 0.75 mg with ondansetron 32 mg, dolasetron 100 mg, or granisetron 40 μg/kg, administered 30 minutes before moderately emetogenic chemotherapy (MEC ) or highly emetogenic chemotherapy (HEC). Pooled data within each chemotherapy category (MEC: n = 1,132; HEC: n = 1,781) were analyzed by a logistic regression model. Nausea endpoints were complete control rates (ie, no more than mild nausea, no vomiting, and no rescue medication), nausea-free rates, nausea severity, and requirement for rescue antiemetic/antinausea medication over 5 days following chemotherapy. Pooled safety data were summarized descriptively.
Results Numerically more palonosetron-treated patients were nausea-free on each day, and fewer had moderate-severe nausea. Similarly, usage of rescue medication was less frequent among palonosetron-treated patients. Complete control rates for palonosetron and older 5-HT3 RAs in the acute phase were 66% vs 63%, 52% vs 42% in the delayed phase (24-120 hours), and 46% vs 37% in the overall phase. The incidence of adverse events was similar for palonosetron and older 5-HT3 RAs.
Limitations This post hoc analysis summarized data for palonosetron and several other 5-HT3 RAs but was not powered for statistical comparisons between individual agents. Because nausea is inherently subjective, the reliability of assessments of some aspects (eg, severity) may be influenced by interindividual variability.
Conclusion Palonosetron may be more effective than older 5-HT3 RAs in preventing nausea, with comparable tolerability.
Disclosures and funding Dr Schwartzberg is a consultant to and Dr Cox an employee at Esai. Mr Ballinari is a member of staff at and Dr Thorn consults for Helsinn Healthcare SA. Funding to support this study and the preparation of this manuscript was provided by Eisai Inc.
Click on the PDF icon at the top of this introduction to read the full article.
Background No clinical standard currently exists for the optimal management of nausea induced by emetogenic chemotherapy, particularly delayed nausea.
Objective To compare the efficacy and safety of palonosetron with older 5-HT3 receptor antagonists (RAs) in preventing chemotherapy-induced nausea.
Methods Data were pooled from 4 similarly designed multicenter, randomized, double-blind, clinical trials that compared single intravenous doses of palonosetron 0.25 mg or 0.75 mg with ondansetron 32 mg, dolasetron 100 mg, or granisetron 40 μg/kg, administered 30 minutes before moderately emetogenic chemotherapy (MEC ) or highly emetogenic chemotherapy (HEC). Pooled data within each chemotherapy category (MEC: n = 1,132; HEC: n = 1,781) were analyzed by a logistic regression model. Nausea endpoints were complete control rates (ie, no more than mild nausea, no vomiting, and no rescue medication), nausea-free rates, nausea severity, and requirement for rescue antiemetic/antinausea medication over 5 days following chemotherapy. Pooled safety data were summarized descriptively.
Results Numerically more palonosetron-treated patients were nausea-free on each day, and fewer had moderate-severe nausea. Similarly, usage of rescue medication was less frequent among palonosetron-treated patients. Complete control rates for palonosetron and older 5-HT3 RAs in the acute phase were 66% vs 63%, 52% vs 42% in the delayed phase (24-120 hours), and 46% vs 37% in the overall phase. The incidence of adverse events was similar for palonosetron and older 5-HT3 RAs.
Limitations This post hoc analysis summarized data for palonosetron and several other 5-HT3 RAs but was not powered for statistical comparisons between individual agents. Because nausea is inherently subjective, the reliability of assessments of some aspects (eg, severity) may be influenced by interindividual variability.
Conclusion Palonosetron may be more effective than older 5-HT3 RAs in preventing nausea, with comparable tolerability.
Disclosures and funding Dr Schwartzberg is a consultant to and Dr Cox an employee at Esai. Mr Ballinari is a member of staff at and Dr Thorn consults for Helsinn Healthcare SA. Funding to support this study and the preparation of this manuscript was provided by Eisai Inc.
Click on the PDF icon at the top of this introduction to read the full article.
Background No clinical standard currently exists for the optimal management of nausea induced by emetogenic chemotherapy, particularly delayed nausea.
Objective To compare the efficacy and safety of palonosetron with older 5-HT3 receptor antagonists (RAs) in preventing chemotherapy-induced nausea.
Methods Data were pooled from 4 similarly designed multicenter, randomized, double-blind, clinical trials that compared single intravenous doses of palonosetron 0.25 mg or 0.75 mg with ondansetron 32 mg, dolasetron 100 mg, or granisetron 40 μg/kg, administered 30 minutes before moderately emetogenic chemotherapy (MEC ) or highly emetogenic chemotherapy (HEC). Pooled data within each chemotherapy category (MEC: n = 1,132; HEC: n = 1,781) were analyzed by a logistic regression model. Nausea endpoints were complete control rates (ie, no more than mild nausea, no vomiting, and no rescue medication), nausea-free rates, nausea severity, and requirement for rescue antiemetic/antinausea medication over 5 days following chemotherapy. Pooled safety data were summarized descriptively.
Results Numerically more palonosetron-treated patients were nausea-free on each day, and fewer had moderate-severe nausea. Similarly, usage of rescue medication was less frequent among palonosetron-treated patients. Complete control rates for palonosetron and older 5-HT3 RAs in the acute phase were 66% vs 63%, 52% vs 42% in the delayed phase (24-120 hours), and 46% vs 37% in the overall phase. The incidence of adverse events was similar for palonosetron and older 5-HT3 RAs.
Limitations This post hoc analysis summarized data for palonosetron and several other 5-HT3 RAs but was not powered for statistical comparisons between individual agents. Because nausea is inherently subjective, the reliability of assessments of some aspects (eg, severity) may be influenced by interindividual variability.
Conclusion Palonosetron may be more effective than older 5-HT3 RAs in preventing nausea, with comparable tolerability.
Disclosures and funding Dr Schwartzberg is a consultant to and Dr Cox an employee at Esai. Mr Ballinari is a member of staff at and Dr Thorn consults for Helsinn Healthcare SA. Funding to support this study and the preparation of this manuscript was provided by Eisai Inc.
Click on the PDF icon at the top of this introduction to read the full article.
Oncology hospitalist field is small, but growing
Have you met an oncology hospitalist yet? If you haven’t, you probably will soon.
The latest offshoot of hospital medicine aims to take all the strengths of the hospitalist movement – increased efficiency and improved quality and safety – and apply them to inpatient cancer care.
While there is no typical oncology-hospitalist program, most manage the complications of a patient’s cancer and treatment, as well as providing some type of end-of-life services. Oncology hospitalists may be oncologists with an interest in taking care of hospitalized patients. Or they could be hospitalists trained in internal or family medicine, who have an interest in caring for cancer patients.
"People are very interested in this, and we really want to grow it," said Dr. Maria-Claudia Campagna, an oncology-hospitalist at the University of Texas MD Anderson Cancer Center in Houston.
Dr. Campagna is part of a nine-physician oncology-hospitalist program at MD Anderson. The program was launched in 2006 with just one hospitalist, but has grown to nine hospitalists over the last several years. And the program is in the process of recruiting three more physicians.
They are also piloting an observation unit geared toward oncology patients, she said.
Much like in the early days of hospital medicine, Dr. Campagna said the program initially got pushback from oncologists who didn’t want to give up care of their patients in the hospital. But over time, the hospitalists have proven their competence and oncologists have gotten even busier with their outpatient practices.
"They know we take good care of their patients, so ultimately they trust our criteria. And when we don’t know, we tell them," Dr. Campagna said. "So we have a very symbiotic relationship."
The experience at MD Anderson is being replicated at cancer centers and academic medical centers around the country. Even some community hospitals are exploring the idea.
The reason is simple, said Dr. Eddy Chen, an oncology hospitalist at Dana-Farber/Brigham and Women’s Cancer Center in Boston. The combination of a coming shortage of oncologists combined with an expected surge in cancer patients among aging baby boomers means that virtually every hospital will see a marked increase in cancer patients over the next several years.
"In the future, there are going to be a lot more patients with cancer who are going to be coming into the hospital," Dr. Chen said. "And who is going to take care of these patients?"
But I’m not an oncologist ...
At Dana-Farber/Brigham and Women’s, they have three oncology hospitalists, including Dr. Chen. Along with the regular cadre of oncologists, they manage all of the hospitalized cancer patients. What makes hospitalist management different from that of the oncologists, who are treating both inpatients and outpatients, is the focus on quality improvement, patient safety, and research, said Dr. Chen.
Dr. Chen, who is trained as an oncologist, said hospitalists don’t need to be oncologists to do this job. But as the field develops, there are likely to be some training or prerequisites that will develop. For now, Dr. Chen said hospitalists need to have an interest in treating complex patients and be willing to develop a deeper understanding of the principles of cancer medicine.
"We are now at a point in the road of this endeavor where best practices, and understanding these issues, can be further defined," Dr. Chen said.
As Dr. Chen proves, oncology hospitalists can be oncologists or traditional hospitalists trained in internal medicine or family medicine. But they must all be prepared to handle complex patients and take on end-of-life discussions.
At Memorial Sloan-Kettering Cancer Center in New York, where the oncology-hospitalist team works mainly with GI oncology and lymphoma patients, they treat patients with very advanced disease, many of whom are in the last 6 months of life.
"We’ve attained a lot of experience and expertise in end-of-life care, but it’s all been on-the-job training," said Dr. Barbara C. Egan, chief of the hospital medicine service at Sloan-Kettering.
Because of the heavy focus on end-of-life care, Dr. Egan and some of the other hospitalists in her group were recently board certified in hospice and palliative medicine based in part on their clinical experience working with cancer patients.
Emotional days
The work is very different from a general medicine hospitalist service. For oncology hospitalists, all of the patients have multisystem organ disease and also typically have complicated psychosocial dynamics end-of-life care. The result is a time-consuming, emotionally charged day that isn’t accurately measured by RVUs (relative value units) or the number of encounters per day, Dr. Egan said.
"It’s very different to round on a 25-year-old who’s dying of colon cancer, than in a general medicine hospital where you might have several patients on the service who are there for single issue, uncomplicated soft rule-out MI." she said. "It’s definitely very emotionally draining on the physicians."
To try to prevent burnout among their physicians, Sloan-Kettering’s program consists of seven daytime hospitalists who work a typical 2-week on/2-week off schedule. The other 10 hospitalists are dedicated nocturnalists. The model has resulted in virtually no turnover among the daytime hospitalists. The nocturnal group has high turnover, which is typical of most night-shift work. At this point, there’s no definitive count of the number of oncology hospitalists working in the United States today. But what is clear, is that the small niche is growing.
Dr. A. Charlotta Weaver, medical director of oncology hospitalists at Northwestern Memorial Hospital in Chicago, epitomizes the appeal for some young physicians.
Dr. Weaver graduated from residency in 2008, a year after Northwestern launched its oncology-hospitalist program. She had been considering a fellowship in hematology/oncology when she heard about the new program. "This little light went off in my head that that’s what I wanted to do," she said.
Initially, she thought about working as an oncology hospitalist for a few years as a bridge to fellowship, but ultimately decided to stay on with the program. The appeals, she said, was a combination of the hospitalist schedule and taking care of hematology/oncology patients.
"I think of it as real medicine. They are really sick," Dr. Weaver said. "There is something legitimately wrong with them and I can help; whereas in general medicine, you don’t always have that sense."
On Twitter @maryellenny
Have you met an oncology hospitalist yet? If you haven’t, you probably will soon.
The latest offshoot of hospital medicine aims to take all the strengths of the hospitalist movement – increased efficiency and improved quality and safety – and apply them to inpatient cancer care.
While there is no typical oncology-hospitalist program, most manage the complications of a patient’s cancer and treatment, as well as providing some type of end-of-life services. Oncology hospitalists may be oncologists with an interest in taking care of hospitalized patients. Or they could be hospitalists trained in internal or family medicine, who have an interest in caring for cancer patients.
"People are very interested in this, and we really want to grow it," said Dr. Maria-Claudia Campagna, an oncology-hospitalist at the University of Texas MD Anderson Cancer Center in Houston.
Dr. Campagna is part of a nine-physician oncology-hospitalist program at MD Anderson. The program was launched in 2006 with just one hospitalist, but has grown to nine hospitalists over the last several years. And the program is in the process of recruiting three more physicians.
They are also piloting an observation unit geared toward oncology patients, she said.
Much like in the early days of hospital medicine, Dr. Campagna said the program initially got pushback from oncologists who didn’t want to give up care of their patients in the hospital. But over time, the hospitalists have proven their competence and oncologists have gotten even busier with their outpatient practices.
"They know we take good care of their patients, so ultimately they trust our criteria. And when we don’t know, we tell them," Dr. Campagna said. "So we have a very symbiotic relationship."
The experience at MD Anderson is being replicated at cancer centers and academic medical centers around the country. Even some community hospitals are exploring the idea.
The reason is simple, said Dr. Eddy Chen, an oncology hospitalist at Dana-Farber/Brigham and Women’s Cancer Center in Boston. The combination of a coming shortage of oncologists combined with an expected surge in cancer patients among aging baby boomers means that virtually every hospital will see a marked increase in cancer patients over the next several years.
"In the future, there are going to be a lot more patients with cancer who are going to be coming into the hospital," Dr. Chen said. "And who is going to take care of these patients?"
But I’m not an oncologist ...
At Dana-Farber/Brigham and Women’s, they have three oncology hospitalists, including Dr. Chen. Along with the regular cadre of oncologists, they manage all of the hospitalized cancer patients. What makes hospitalist management different from that of the oncologists, who are treating both inpatients and outpatients, is the focus on quality improvement, patient safety, and research, said Dr. Chen.
Dr. Chen, who is trained as an oncologist, said hospitalists don’t need to be oncologists to do this job. But as the field develops, there are likely to be some training or prerequisites that will develop. For now, Dr. Chen said hospitalists need to have an interest in treating complex patients and be willing to develop a deeper understanding of the principles of cancer medicine.
"We are now at a point in the road of this endeavor where best practices, and understanding these issues, can be further defined," Dr. Chen said.
As Dr. Chen proves, oncology hospitalists can be oncologists or traditional hospitalists trained in internal medicine or family medicine. But they must all be prepared to handle complex patients and take on end-of-life discussions.
At Memorial Sloan-Kettering Cancer Center in New York, where the oncology-hospitalist team works mainly with GI oncology and lymphoma patients, they treat patients with very advanced disease, many of whom are in the last 6 months of life.
"We’ve attained a lot of experience and expertise in end-of-life care, but it’s all been on-the-job training," said Dr. Barbara C. Egan, chief of the hospital medicine service at Sloan-Kettering.
Because of the heavy focus on end-of-life care, Dr. Egan and some of the other hospitalists in her group were recently board certified in hospice and palliative medicine based in part on their clinical experience working with cancer patients.
Emotional days
The work is very different from a general medicine hospitalist service. For oncology hospitalists, all of the patients have multisystem organ disease and also typically have complicated psychosocial dynamics end-of-life care. The result is a time-consuming, emotionally charged day that isn’t accurately measured by RVUs (relative value units) or the number of encounters per day, Dr. Egan said.
"It’s very different to round on a 25-year-old who’s dying of colon cancer, than in a general medicine hospital where you might have several patients on the service who are there for single issue, uncomplicated soft rule-out MI." she said. "It’s definitely very emotionally draining on the physicians."
To try to prevent burnout among their physicians, Sloan-Kettering’s program consists of seven daytime hospitalists who work a typical 2-week on/2-week off schedule. The other 10 hospitalists are dedicated nocturnalists. The model has resulted in virtually no turnover among the daytime hospitalists. The nocturnal group has high turnover, which is typical of most night-shift work. At this point, there’s no definitive count of the number of oncology hospitalists working in the United States today. But what is clear, is that the small niche is growing.
Dr. A. Charlotta Weaver, medical director of oncology hospitalists at Northwestern Memorial Hospital in Chicago, epitomizes the appeal for some young physicians.
Dr. Weaver graduated from residency in 2008, a year after Northwestern launched its oncology-hospitalist program. She had been considering a fellowship in hematology/oncology when she heard about the new program. "This little light went off in my head that that’s what I wanted to do," she said.
Initially, she thought about working as an oncology hospitalist for a few years as a bridge to fellowship, but ultimately decided to stay on with the program. The appeals, she said, was a combination of the hospitalist schedule and taking care of hematology/oncology patients.
"I think of it as real medicine. They are really sick," Dr. Weaver said. "There is something legitimately wrong with them and I can help; whereas in general medicine, you don’t always have that sense."
On Twitter @maryellenny
Have you met an oncology hospitalist yet? If you haven’t, you probably will soon.
The latest offshoot of hospital medicine aims to take all the strengths of the hospitalist movement – increased efficiency and improved quality and safety – and apply them to inpatient cancer care.
While there is no typical oncology-hospitalist program, most manage the complications of a patient’s cancer and treatment, as well as providing some type of end-of-life services. Oncology hospitalists may be oncologists with an interest in taking care of hospitalized patients. Or they could be hospitalists trained in internal or family medicine, who have an interest in caring for cancer patients.
"People are very interested in this, and we really want to grow it," said Dr. Maria-Claudia Campagna, an oncology-hospitalist at the University of Texas MD Anderson Cancer Center in Houston.
Dr. Campagna is part of a nine-physician oncology-hospitalist program at MD Anderson. The program was launched in 2006 with just one hospitalist, but has grown to nine hospitalists over the last several years. And the program is in the process of recruiting three more physicians.
They are also piloting an observation unit geared toward oncology patients, she said.
Much like in the early days of hospital medicine, Dr. Campagna said the program initially got pushback from oncologists who didn’t want to give up care of their patients in the hospital. But over time, the hospitalists have proven their competence and oncologists have gotten even busier with their outpatient practices.
"They know we take good care of their patients, so ultimately they trust our criteria. And when we don’t know, we tell them," Dr. Campagna said. "So we have a very symbiotic relationship."
The experience at MD Anderson is being replicated at cancer centers and academic medical centers around the country. Even some community hospitals are exploring the idea.
The reason is simple, said Dr. Eddy Chen, an oncology hospitalist at Dana-Farber/Brigham and Women’s Cancer Center in Boston. The combination of a coming shortage of oncologists combined with an expected surge in cancer patients among aging baby boomers means that virtually every hospital will see a marked increase in cancer patients over the next several years.
"In the future, there are going to be a lot more patients with cancer who are going to be coming into the hospital," Dr. Chen said. "And who is going to take care of these patients?"
But I’m not an oncologist ...
At Dana-Farber/Brigham and Women’s, they have three oncology hospitalists, including Dr. Chen. Along with the regular cadre of oncologists, they manage all of the hospitalized cancer patients. What makes hospitalist management different from that of the oncologists, who are treating both inpatients and outpatients, is the focus on quality improvement, patient safety, and research, said Dr. Chen.
Dr. Chen, who is trained as an oncologist, said hospitalists don’t need to be oncologists to do this job. But as the field develops, there are likely to be some training or prerequisites that will develop. For now, Dr. Chen said hospitalists need to have an interest in treating complex patients and be willing to develop a deeper understanding of the principles of cancer medicine.
"We are now at a point in the road of this endeavor where best practices, and understanding these issues, can be further defined," Dr. Chen said.
As Dr. Chen proves, oncology hospitalists can be oncologists or traditional hospitalists trained in internal medicine or family medicine. But they must all be prepared to handle complex patients and take on end-of-life discussions.
At Memorial Sloan-Kettering Cancer Center in New York, where the oncology-hospitalist team works mainly with GI oncology and lymphoma patients, they treat patients with very advanced disease, many of whom are in the last 6 months of life.
"We’ve attained a lot of experience and expertise in end-of-life care, but it’s all been on-the-job training," said Dr. Barbara C. Egan, chief of the hospital medicine service at Sloan-Kettering.
Because of the heavy focus on end-of-life care, Dr. Egan and some of the other hospitalists in her group were recently board certified in hospice and palliative medicine based in part on their clinical experience working with cancer patients.
Emotional days
The work is very different from a general medicine hospitalist service. For oncology hospitalists, all of the patients have multisystem organ disease and also typically have complicated psychosocial dynamics end-of-life care. The result is a time-consuming, emotionally charged day that isn’t accurately measured by RVUs (relative value units) or the number of encounters per day, Dr. Egan said.
"It’s very different to round on a 25-year-old who’s dying of colon cancer, than in a general medicine hospital where you might have several patients on the service who are there for single issue, uncomplicated soft rule-out MI." she said. "It’s definitely very emotionally draining on the physicians."
To try to prevent burnout among their physicians, Sloan-Kettering’s program consists of seven daytime hospitalists who work a typical 2-week on/2-week off schedule. The other 10 hospitalists are dedicated nocturnalists. The model has resulted in virtually no turnover among the daytime hospitalists. The nocturnal group has high turnover, which is typical of most night-shift work. At this point, there’s no definitive count of the number of oncology hospitalists working in the United States today. But what is clear, is that the small niche is growing.
Dr. A. Charlotta Weaver, medical director of oncology hospitalists at Northwestern Memorial Hospital in Chicago, epitomizes the appeal for some young physicians.
Dr. Weaver graduated from residency in 2008, a year after Northwestern launched its oncology-hospitalist program. She had been considering a fellowship in hematology/oncology when she heard about the new program. "This little light went off in my head that that’s what I wanted to do," she said.
Initially, she thought about working as an oncology hospitalist for a few years as a bridge to fellowship, but ultimately decided to stay on with the program. The appeals, she said, was a combination of the hospitalist schedule and taking care of hematology/oncology patients.
"I think of it as real medicine. They are really sick," Dr. Weaver said. "There is something legitimately wrong with them and I can help; whereas in general medicine, you don’t always have that sense."
On Twitter @maryellenny
A supportive care clinic for cancer patients embedded within an oncology practice
Background Most cancer patients have symptoms from their disease or treatment. Symptoms are not ideally managed in the context of busy clinics, resulting in potentially avoidable emergency department (ED) visits and hospitalizations. Adjunct supportive care clinics (SCCs) may more effectively address patient needs, but they contribute to fractionation of care if different personnel are involved.
Objective We describe an SCC embedded within a physician practice in which an employed nurse practitioner delivered most of the care. We measured the disposition of patients from the SCC to the ED, and the effect on ED visits and admissions for symptom management.
Methods We conducted a retrospective review of the patients attending the SCC over a period of 11 months. Demographics and disposition outcomes were tracked and compared with pre-intervention controls.
Results In all, 340 visits were recorded from 330 unique patients. Same-day and next-day appointments with a nurse practitioner were arranged for 62% and 25% of patients, respectively. The most common complaints related to pain and gastrointestinal issues. Most of the patients were discharged home. A few needed hospitalization or ED-level care. Admissions for symptom-related care fell by 31%. An estimated 66 ED visits were avoided by patients accessing the SCC.
Limitations The study was retrospective. It did not include detailed financial data. Results may not be generalizable because of the high level of central planning and use of a shared electronic medical record system, which may be lacking in some practices.
Conclusions An embedded supportive care clinic allowed rapid access to experienced oncology care under supervision by the patient’s own oncologists. The clinic was associated with less use of the ED and need for hospitalization. New methods of reimbursing medical care will increasingly require oncology practices to improve patient access to symptom-related care to avoid unnecessary admissions. An embedded SCC can accomplish these goals while avoiding further fractionation of care.
Click on the PDF icon below for the full article.
Background Most cancer patients have symptoms from their disease or treatment. Symptoms are not ideally managed in the context of busy clinics, resulting in potentially avoidable emergency department (ED) visits and hospitalizations. Adjunct supportive care clinics (SCCs) may more effectively address patient needs, but they contribute to fractionation of care if different personnel are involved.
Objective We describe an SCC embedded within a physician practice in which an employed nurse practitioner delivered most of the care. We measured the disposition of patients from the SCC to the ED, and the effect on ED visits and admissions for symptom management.
Methods We conducted a retrospective review of the patients attending the SCC over a period of 11 months. Demographics and disposition outcomes were tracked and compared with pre-intervention controls.
Results In all, 340 visits were recorded from 330 unique patients. Same-day and next-day appointments with a nurse practitioner were arranged for 62% and 25% of patients, respectively. The most common complaints related to pain and gastrointestinal issues. Most of the patients were discharged home. A few needed hospitalization or ED-level care. Admissions for symptom-related care fell by 31%. An estimated 66 ED visits were avoided by patients accessing the SCC.
Limitations The study was retrospective. It did not include detailed financial data. Results may not be generalizable because of the high level of central planning and use of a shared electronic medical record system, which may be lacking in some practices.
Conclusions An embedded supportive care clinic allowed rapid access to experienced oncology care under supervision by the patient’s own oncologists. The clinic was associated with less use of the ED and need for hospitalization. New methods of reimbursing medical care will increasingly require oncology practices to improve patient access to symptom-related care to avoid unnecessary admissions. An embedded SCC can accomplish these goals while avoiding further fractionation of care.
Click on the PDF icon below for the full article.
Background Most cancer patients have symptoms from their disease or treatment. Symptoms are not ideally managed in the context of busy clinics, resulting in potentially avoidable emergency department (ED) visits and hospitalizations. Adjunct supportive care clinics (SCCs) may more effectively address patient needs, but they contribute to fractionation of care if different personnel are involved.
Objective We describe an SCC embedded within a physician practice in which an employed nurse practitioner delivered most of the care. We measured the disposition of patients from the SCC to the ED, and the effect on ED visits and admissions for symptom management.
Methods We conducted a retrospective review of the patients attending the SCC over a period of 11 months. Demographics and disposition outcomes were tracked and compared with pre-intervention controls.
Results In all, 340 visits were recorded from 330 unique patients. Same-day and next-day appointments with a nurse practitioner were arranged for 62% and 25% of patients, respectively. The most common complaints related to pain and gastrointestinal issues. Most of the patients were discharged home. A few needed hospitalization or ED-level care. Admissions for symptom-related care fell by 31%. An estimated 66 ED visits were avoided by patients accessing the SCC.
Limitations The study was retrospective. It did not include detailed financial data. Results may not be generalizable because of the high level of central planning and use of a shared electronic medical record system, which may be lacking in some practices.
Conclusions An embedded supportive care clinic allowed rapid access to experienced oncology care under supervision by the patient’s own oncologists. The clinic was associated with less use of the ED and need for hospitalization. New methods of reimbursing medical care will increasingly require oncology practices to improve patient access to symptom-related care to avoid unnecessary admissions. An embedded SCC can accomplish these goals while avoiding further fractionation of care.
Click on the PDF icon below for the full article.
Frail women less likely to initiate hormonal therapy for breast cancer
Older women with breast cancer are less likely to initiate adjuvant hormonal therapy if they are frail, according to researchers.
In a prospective cohort study of women aged 65 and older diagnosed with invasive, nonmetastatic estrogen receptor–positive breast cancers (n = 1,062), about a quarter of the women were frail or prefrail (4.9% and 18.7%, respectively) based on a validated frailty score determined at baseline, reported Vanessa Sheppard, Ph.D., of Georgetown University Medical Center, Washington (J. Clin. Oncol. 2014 June 16 [doi:10.1200/JCO.2013.51.7367]).
Overall, 14% of the women in the study did not initiate hormonal therapy. Women considered prefrail or frail were significantly less likely to initiate therapy than were those considered "robust" or nonfrail (odds ratio, 1.63; 95% CI, 1.11-2.40; P =.013). Nonwhite race was also significantly associated with noninitiation of therapy (OR, 1.71; CI, 1.04-2.80; P = 0.33).
Baseline frailty was not predictive of discontinuation of therapy after a median follow-up of 3 years, Dr. Sheppard and colleagues reported.
The findings suggest "women and/or their providers are making informed judgments about the risks and benefits," the researchers wrote. "An alternative explanation is that women with greater frailty may have been concerned about adverse effects based on interactions of hormonal therapy and specific comorbidities, such as cardio- and/or cerebrovascular disease and risk of thromboembolic events," they added.
Limitations of the study include a potential for bias associated with self-reporting (discontinuation of therapy was measured only through self-report) and measurement of frailty only at baseline, the investigators said.
The National Institutes of Health, Amgen, and the Cancer and Leukemia Group B (CALGB) Foundation funded the study. Four of Dr. Sheppard’s coauthors, Dr. Gretchen Kimmick, Dr. Eric Winer, Dr. Arti Hurria, and Dr. Claudine Isaacs reported ties with pharmaceutical manufacturers.
Older women with breast cancer are less likely to initiate adjuvant hormonal therapy if they are frail, according to researchers.
In a prospective cohort study of women aged 65 and older diagnosed with invasive, nonmetastatic estrogen receptor–positive breast cancers (n = 1,062), about a quarter of the women were frail or prefrail (4.9% and 18.7%, respectively) based on a validated frailty score determined at baseline, reported Vanessa Sheppard, Ph.D., of Georgetown University Medical Center, Washington (J. Clin. Oncol. 2014 June 16 [doi:10.1200/JCO.2013.51.7367]).
Overall, 14% of the women in the study did not initiate hormonal therapy. Women considered prefrail or frail were significantly less likely to initiate therapy than were those considered "robust" or nonfrail (odds ratio, 1.63; 95% CI, 1.11-2.40; P =.013). Nonwhite race was also significantly associated with noninitiation of therapy (OR, 1.71; CI, 1.04-2.80; P = 0.33).
Baseline frailty was not predictive of discontinuation of therapy after a median follow-up of 3 years, Dr. Sheppard and colleagues reported.
The findings suggest "women and/or their providers are making informed judgments about the risks and benefits," the researchers wrote. "An alternative explanation is that women with greater frailty may have been concerned about adverse effects based on interactions of hormonal therapy and specific comorbidities, such as cardio- and/or cerebrovascular disease and risk of thromboembolic events," they added.
Limitations of the study include a potential for bias associated with self-reporting (discontinuation of therapy was measured only through self-report) and measurement of frailty only at baseline, the investigators said.
The National Institutes of Health, Amgen, and the Cancer and Leukemia Group B (CALGB) Foundation funded the study. Four of Dr. Sheppard’s coauthors, Dr. Gretchen Kimmick, Dr. Eric Winer, Dr. Arti Hurria, and Dr. Claudine Isaacs reported ties with pharmaceutical manufacturers.
Older women with breast cancer are less likely to initiate adjuvant hormonal therapy if they are frail, according to researchers.
In a prospective cohort study of women aged 65 and older diagnosed with invasive, nonmetastatic estrogen receptor–positive breast cancers (n = 1,062), about a quarter of the women were frail or prefrail (4.9% and 18.7%, respectively) based on a validated frailty score determined at baseline, reported Vanessa Sheppard, Ph.D., of Georgetown University Medical Center, Washington (J. Clin. Oncol. 2014 June 16 [doi:10.1200/JCO.2013.51.7367]).
Overall, 14% of the women in the study did not initiate hormonal therapy. Women considered prefrail or frail were significantly less likely to initiate therapy than were those considered "robust" or nonfrail (odds ratio, 1.63; 95% CI, 1.11-2.40; P =.013). Nonwhite race was also significantly associated with noninitiation of therapy (OR, 1.71; CI, 1.04-2.80; P = 0.33).
Baseline frailty was not predictive of discontinuation of therapy after a median follow-up of 3 years, Dr. Sheppard and colleagues reported.
The findings suggest "women and/or their providers are making informed judgments about the risks and benefits," the researchers wrote. "An alternative explanation is that women with greater frailty may have been concerned about adverse effects based on interactions of hormonal therapy and specific comorbidities, such as cardio- and/or cerebrovascular disease and risk of thromboembolic events," they added.
Limitations of the study include a potential for bias associated with self-reporting (discontinuation of therapy was measured only through self-report) and measurement of frailty only at baseline, the investigators said.
The National Institutes of Health, Amgen, and the Cancer and Leukemia Group B (CALGB) Foundation funded the study. Four of Dr. Sheppard’s coauthors, Dr. Gretchen Kimmick, Dr. Eric Winer, Dr. Arti Hurria, and Dr. Claudine Isaacs reported ties with pharmaceutical manufacturers.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Older women with breast cancer are less likely to initiate adjuvant hormonal therapy if they are frail.
Major finding: Odds ratio for noninitiation of hormonal therapy was 1.63 for frail women compared with nonfrail women (95% CI, 1.11 to 2.40; P = .013) after covariate adjustment.
Data source: A prospective cohort of 1,288 older women diagnosed with invasive, nonmetastatic breast cancer recruited from 78 sites from 2004 to 2011, of which 1,062 had estrogen receptor–positive tumors.
Disclosures: The National Institutes of Health, Amgen, and the Cancer and Leukemia Group B (CALGB) Foundation funded the study. Four of Dr. Sheppard’s coauthors, Dr. Gretchen Kimmick, Dr. Eric Winer, Dr. Arti Hurria, and Dr. Claudine Isaacs reported ties with pharmaceutical manufacturers.