User login
Palliative care at time of cancer diagnosis improves survival
CHICAGO – Early palliative care delivered almost exclusively by telephone improved survival among patients with advanced cancer in the ENABLE III study.
After a median follow-up of a little more than 1 year, 46% of patients receiving palliative care from the time of cancer diagnosis and 54% of those with delayed palliative care had died.
Overall median survival was 18.3 months for the immediate group and 11.9 months for the delayed group (P = .17).
In preplanned analyses, the risk of death at 1 year was significantly lower in the immediate group (hazard ratio 0.72; P = .003), with a catch-up effect thereafter, Marie Bakitas, DNSc, reported at the annual meeting of the American Society of Clinical Oncology.
"Enhanced medical care, reduced aggressive care and chemotherapy use, longer access to hospice, and biologic impacts of improved quality of life have all been proposed as mechanisms to explain this survival advantage," Dr. Bakitas said. "However, at the present time, we do not have the data to support a particular mechanism and we are actively exploring this question through secondary analyses."
ENABLE (Educate, Nurture, Advise, Before Life Ends) III is the first study to examine the timing of early palliative care, but not the first to identify a survival advantage.
A recent study (N. Engl. J. Med. 2010:363:733-42) found that patients with metastatic non–small cell lung cancer (NSCLC) who received palliative care at the time of randomization lived a significant 2.7 months longer than did those receiving standard oncologic care, despite receiving significantly less aggressive end-of-life care (33% vs. 54%).
In ENABLE III, 207 patients with advanced cancer, and their caregivers, were randomized as a dyad to begin usual cancer care plus the intervention at the time of diagnosis (immediate group) or usual care alone for 3 months followed by the intervention (delayed group).
The intervention consisted of a traditional outpatient palliative care consult and six weekly structured telephone calls with a nurse coach using a guidebook that covers such topics as problem solving, symptom management, communication, and advanced care planning, explained Dr. Bakitas, the Marie O’Koren Endowed Chair and Professor, School of Nursing, and associate director of the Center for Palliative and Supportive Care, University of Alabama, Birmingham.
Usual care included the clinical consult, but not the telephone intervention.
The participants’ mean age was 64 years, half were male, 60% lived in a rural area, and 65% were married or living with a partner. Lung cancer was the most common diagnosis at 42%.
At baseline, 75% of patients were receiving chemotherapy, 19% were undergoing radiation, and 43% had an advanced directive completed at diagnosis.
Unlike the group’s prior trial comparing palliative care to usual care at 3 months, immediate versus delayed palliative care did not lead to significant improvements in quality of life on the Functional Assessment of Chronic Illness Therapy-Palliative care scale (129.9 vs. 127.2; P = .34), mood on the Center for Epidemiologic Studies Depression scale (11.2 vs. 10.8; P = .33), or symptom impact on the Quality of Life at the End of Life symptom impact subscale (11.4 vs. 12.2; P = .09).
One plausible reason for the findings is that there may not have been enough care differences between the two groups, with 40% of the delayed group receiving their first palliative care contact an average of 30 days before they were scheduled to do so on day 84, Dr. Bakitas said.
Second, difficulties in accrual and decreased study power may have made it difficult to pick up between-group differences on the subjective instruments, resulting in a type 2 error.
"A 3-month delay is still very early," Dr. Bakitas said.
She noted that early intervention allowed the palliative care team to have contact with patients for 1 year on average (range 240-493 days), compared with a median of 41-90 days from referral to death reported for outpatient clinics in a national survey of 142 National Cancer Institute and non-NCI cancer centers (JAMA 2010;303:1054-61).
Resource and chemotherapy use in ENABLE III was also comparable in both groups. Decedents in the immediate and delayed groups spent a median of 5 and 6 days, respectively, in hospital in the 7-9 months preceding death, while 8% and 5% received chemotherapy in the last 2 weeks of life.
This compares favorably with a national average of more than 8 hospital days in the last 6 months of life observed in the 2014 Dartmouth Atlas of Health Care, and a chemotherapy rate of 17.5% reported in the previously noted NSCLC study, Dr. Bakitas said.
She called for more studies of early palliative care to determine the optimal timing, personnel, essential elements, and mechanisms of improved survival.
"While the benefits of these approaches have been demonstrated when provided early after a cancer diagnosis, in practice these potentially beneficial palliative care services are often provided very late, sometimes hours or weeks before death," Dr. Bakitas said. "This trend is likely to continue in the absence of clear direction on the very pragmatic questions of who, what, and when."
The study was funded by National Institute for Nursing Research. Dr. Bakitas reported having no relevant disclosures.
Palliative care support buoys caregivers of advanced cancer patients
Providing early palliative care support to caregivers of advanced cancer patients improves their quality of life, depression, and stress burden, the ENABLE III study found.
"Similar to patients, waiting to provide these caregiver services until patients are in their last weeks to days of life may not adequately address the distress that they experience," Nick Dionne-Odom, Ph.D., RN, said at the meeting.
Caregivers for the 13 million cancer patients in the United States living with advanced disease can spend up to 8 hours per day providing assistance in activities that include symptom management, emotional and spiritual support, meal preparation, arranging medical appointments, and transportation.
The combination of this burden and witnessing someone close to you struggle with illness can cause psychological distress equal to or sometimes greater than that experienced by the patient, said Dr. Dionne-Odom, a postdoctoral fellow at the University of Alabama at Birmingham.
In ENABLE III, 122 caregivers were randomized at the time of the patient’s cancer diagnosis or 12 weeks later to a palliative care intervention that consisted of three weekly structured educational telephone calls from an advanced practice nurse coach, monthly check-in calls to address new or ongoing issues, and a bereavement call for caregivers whose loved ones died.
Caregivers were not restricted to family members, but could include close friends and even neighbors. Their mean age was 60 years, 79% were female, 75% were spouses, and all had at least a high school education.
At 12 weeks from the start of the intervention, caregivers in the immediate versus delayed group had significantly better quality of life on the Caregiver Quality of Life Index–Cancer scale (mean 50.2 vs. 56.1; P = .02) and less depressive symptoms on the Center for Epidemiologic Studies Depression (CESD) scale (10.2 vs. 16.6; P = .0006), Dr. Dionne-Odom said. Notably, the delayed group surpassed the clinical cutoff for depression of 16 on the CESD scale, he added.
The intervention did not appear to change the perception among caregivers of what was demanded of them by the patient or their objective burden, though there was a trend among the immediate group for improved caregiver stress burden on the Montgomery Borgatta Caregiver Burden Scale (13.2 vs. 13.8; P = .10).
There was no significant difference between groups in depression or grief scores for caregivers of decedents. A difference may have been detected with a larger sample size, he said, adding that prior studies have shown that reducing caregiver stress before patients’ death is associated with better bereavement adjustment.
As for why caregivers appear to benefit more than the patients from the parallel palliative care interventions, Dr. Bakitas said in an interview it may be the timing of the assessments, adding that other studies have shown an impact of palliative care at 4 months, but not at 3 months.
CHICAGO – Early palliative care delivered almost exclusively by telephone improved survival among patients with advanced cancer in the ENABLE III study.
After a median follow-up of a little more than 1 year, 46% of patients receiving palliative care from the time of cancer diagnosis and 54% of those with delayed palliative care had died.
Overall median survival was 18.3 months for the immediate group and 11.9 months for the delayed group (P = .17).
In preplanned analyses, the risk of death at 1 year was significantly lower in the immediate group (hazard ratio 0.72; P = .003), with a catch-up effect thereafter, Marie Bakitas, DNSc, reported at the annual meeting of the American Society of Clinical Oncology.
"Enhanced medical care, reduced aggressive care and chemotherapy use, longer access to hospice, and biologic impacts of improved quality of life have all been proposed as mechanisms to explain this survival advantage," Dr. Bakitas said. "However, at the present time, we do not have the data to support a particular mechanism and we are actively exploring this question through secondary analyses."
ENABLE (Educate, Nurture, Advise, Before Life Ends) III is the first study to examine the timing of early palliative care, but not the first to identify a survival advantage.
A recent study (N. Engl. J. Med. 2010:363:733-42) found that patients with metastatic non–small cell lung cancer (NSCLC) who received palliative care at the time of randomization lived a significant 2.7 months longer than did those receiving standard oncologic care, despite receiving significantly less aggressive end-of-life care (33% vs. 54%).
In ENABLE III, 207 patients with advanced cancer, and their caregivers, were randomized as a dyad to begin usual cancer care plus the intervention at the time of diagnosis (immediate group) or usual care alone for 3 months followed by the intervention (delayed group).
The intervention consisted of a traditional outpatient palliative care consult and six weekly structured telephone calls with a nurse coach using a guidebook that covers such topics as problem solving, symptom management, communication, and advanced care planning, explained Dr. Bakitas, the Marie O’Koren Endowed Chair and Professor, School of Nursing, and associate director of the Center for Palliative and Supportive Care, University of Alabama, Birmingham.
Usual care included the clinical consult, but not the telephone intervention.
The participants’ mean age was 64 years, half were male, 60% lived in a rural area, and 65% were married or living with a partner. Lung cancer was the most common diagnosis at 42%.
At baseline, 75% of patients were receiving chemotherapy, 19% were undergoing radiation, and 43% had an advanced directive completed at diagnosis.
Unlike the group’s prior trial comparing palliative care to usual care at 3 months, immediate versus delayed palliative care did not lead to significant improvements in quality of life on the Functional Assessment of Chronic Illness Therapy-Palliative care scale (129.9 vs. 127.2; P = .34), mood on the Center for Epidemiologic Studies Depression scale (11.2 vs. 10.8; P = .33), or symptom impact on the Quality of Life at the End of Life symptom impact subscale (11.4 vs. 12.2; P = .09).
One plausible reason for the findings is that there may not have been enough care differences between the two groups, with 40% of the delayed group receiving their first palliative care contact an average of 30 days before they were scheduled to do so on day 84, Dr. Bakitas said.
Second, difficulties in accrual and decreased study power may have made it difficult to pick up between-group differences on the subjective instruments, resulting in a type 2 error.
"A 3-month delay is still very early," Dr. Bakitas said.
She noted that early intervention allowed the palliative care team to have contact with patients for 1 year on average (range 240-493 days), compared with a median of 41-90 days from referral to death reported for outpatient clinics in a national survey of 142 National Cancer Institute and non-NCI cancer centers (JAMA 2010;303:1054-61).
Resource and chemotherapy use in ENABLE III was also comparable in both groups. Decedents in the immediate and delayed groups spent a median of 5 and 6 days, respectively, in hospital in the 7-9 months preceding death, while 8% and 5% received chemotherapy in the last 2 weeks of life.
This compares favorably with a national average of more than 8 hospital days in the last 6 months of life observed in the 2014 Dartmouth Atlas of Health Care, and a chemotherapy rate of 17.5% reported in the previously noted NSCLC study, Dr. Bakitas said.
She called for more studies of early palliative care to determine the optimal timing, personnel, essential elements, and mechanisms of improved survival.
"While the benefits of these approaches have been demonstrated when provided early after a cancer diagnosis, in practice these potentially beneficial palliative care services are often provided very late, sometimes hours or weeks before death," Dr. Bakitas said. "This trend is likely to continue in the absence of clear direction on the very pragmatic questions of who, what, and when."
The study was funded by National Institute for Nursing Research. Dr. Bakitas reported having no relevant disclosures.
Palliative care support buoys caregivers of advanced cancer patients
Providing early palliative care support to caregivers of advanced cancer patients improves their quality of life, depression, and stress burden, the ENABLE III study found.
"Similar to patients, waiting to provide these caregiver services until patients are in their last weeks to days of life may not adequately address the distress that they experience," Nick Dionne-Odom, Ph.D., RN, said at the meeting.
Caregivers for the 13 million cancer patients in the United States living with advanced disease can spend up to 8 hours per day providing assistance in activities that include symptom management, emotional and spiritual support, meal preparation, arranging medical appointments, and transportation.
The combination of this burden and witnessing someone close to you struggle with illness can cause psychological distress equal to or sometimes greater than that experienced by the patient, said Dr. Dionne-Odom, a postdoctoral fellow at the University of Alabama at Birmingham.
In ENABLE III, 122 caregivers were randomized at the time of the patient’s cancer diagnosis or 12 weeks later to a palliative care intervention that consisted of three weekly structured educational telephone calls from an advanced practice nurse coach, monthly check-in calls to address new or ongoing issues, and a bereavement call for caregivers whose loved ones died.
Caregivers were not restricted to family members, but could include close friends and even neighbors. Their mean age was 60 years, 79% were female, 75% were spouses, and all had at least a high school education.
At 12 weeks from the start of the intervention, caregivers in the immediate versus delayed group had significantly better quality of life on the Caregiver Quality of Life Index–Cancer scale (mean 50.2 vs. 56.1; P = .02) and less depressive symptoms on the Center for Epidemiologic Studies Depression (CESD) scale (10.2 vs. 16.6; P = .0006), Dr. Dionne-Odom said. Notably, the delayed group surpassed the clinical cutoff for depression of 16 on the CESD scale, he added.
The intervention did not appear to change the perception among caregivers of what was demanded of them by the patient or their objective burden, though there was a trend among the immediate group for improved caregiver stress burden on the Montgomery Borgatta Caregiver Burden Scale (13.2 vs. 13.8; P = .10).
There was no significant difference between groups in depression or grief scores for caregivers of decedents. A difference may have been detected with a larger sample size, he said, adding that prior studies have shown that reducing caregiver stress before patients’ death is associated with better bereavement adjustment.
As for why caregivers appear to benefit more than the patients from the parallel palliative care interventions, Dr. Bakitas said in an interview it may be the timing of the assessments, adding that other studies have shown an impact of palliative care at 4 months, but not at 3 months.
CHICAGO – Early palliative care delivered almost exclusively by telephone improved survival among patients with advanced cancer in the ENABLE III study.
After a median follow-up of a little more than 1 year, 46% of patients receiving palliative care from the time of cancer diagnosis and 54% of those with delayed palliative care had died.
Overall median survival was 18.3 months for the immediate group and 11.9 months for the delayed group (P = .17).
In preplanned analyses, the risk of death at 1 year was significantly lower in the immediate group (hazard ratio 0.72; P = .003), with a catch-up effect thereafter, Marie Bakitas, DNSc, reported at the annual meeting of the American Society of Clinical Oncology.
"Enhanced medical care, reduced aggressive care and chemotherapy use, longer access to hospice, and biologic impacts of improved quality of life have all been proposed as mechanisms to explain this survival advantage," Dr. Bakitas said. "However, at the present time, we do not have the data to support a particular mechanism and we are actively exploring this question through secondary analyses."
ENABLE (Educate, Nurture, Advise, Before Life Ends) III is the first study to examine the timing of early palliative care, but not the first to identify a survival advantage.
A recent study (N. Engl. J. Med. 2010:363:733-42) found that patients with metastatic non–small cell lung cancer (NSCLC) who received palliative care at the time of randomization lived a significant 2.7 months longer than did those receiving standard oncologic care, despite receiving significantly less aggressive end-of-life care (33% vs. 54%).
In ENABLE III, 207 patients with advanced cancer, and their caregivers, were randomized as a dyad to begin usual cancer care plus the intervention at the time of diagnosis (immediate group) or usual care alone for 3 months followed by the intervention (delayed group).
The intervention consisted of a traditional outpatient palliative care consult and six weekly structured telephone calls with a nurse coach using a guidebook that covers such topics as problem solving, symptom management, communication, and advanced care planning, explained Dr. Bakitas, the Marie O’Koren Endowed Chair and Professor, School of Nursing, and associate director of the Center for Palliative and Supportive Care, University of Alabama, Birmingham.
Usual care included the clinical consult, but not the telephone intervention.
The participants’ mean age was 64 years, half were male, 60% lived in a rural area, and 65% were married or living with a partner. Lung cancer was the most common diagnosis at 42%.
At baseline, 75% of patients were receiving chemotherapy, 19% were undergoing radiation, and 43% had an advanced directive completed at diagnosis.
Unlike the group’s prior trial comparing palliative care to usual care at 3 months, immediate versus delayed palliative care did not lead to significant improvements in quality of life on the Functional Assessment of Chronic Illness Therapy-Palliative care scale (129.9 vs. 127.2; P = .34), mood on the Center for Epidemiologic Studies Depression scale (11.2 vs. 10.8; P = .33), or symptom impact on the Quality of Life at the End of Life symptom impact subscale (11.4 vs. 12.2; P = .09).
One plausible reason for the findings is that there may not have been enough care differences between the two groups, with 40% of the delayed group receiving their first palliative care contact an average of 30 days before they were scheduled to do so on day 84, Dr. Bakitas said.
Second, difficulties in accrual and decreased study power may have made it difficult to pick up between-group differences on the subjective instruments, resulting in a type 2 error.
"A 3-month delay is still very early," Dr. Bakitas said.
She noted that early intervention allowed the palliative care team to have contact with patients for 1 year on average (range 240-493 days), compared with a median of 41-90 days from referral to death reported for outpatient clinics in a national survey of 142 National Cancer Institute and non-NCI cancer centers (JAMA 2010;303:1054-61).
Resource and chemotherapy use in ENABLE III was also comparable in both groups. Decedents in the immediate and delayed groups spent a median of 5 and 6 days, respectively, in hospital in the 7-9 months preceding death, while 8% and 5% received chemotherapy in the last 2 weeks of life.
This compares favorably with a national average of more than 8 hospital days in the last 6 months of life observed in the 2014 Dartmouth Atlas of Health Care, and a chemotherapy rate of 17.5% reported in the previously noted NSCLC study, Dr. Bakitas said.
She called for more studies of early palliative care to determine the optimal timing, personnel, essential elements, and mechanisms of improved survival.
"While the benefits of these approaches have been demonstrated when provided early after a cancer diagnosis, in practice these potentially beneficial palliative care services are often provided very late, sometimes hours or weeks before death," Dr. Bakitas said. "This trend is likely to continue in the absence of clear direction on the very pragmatic questions of who, what, and when."
The study was funded by National Institute for Nursing Research. Dr. Bakitas reported having no relevant disclosures.
Palliative care support buoys caregivers of advanced cancer patients
Providing early palliative care support to caregivers of advanced cancer patients improves their quality of life, depression, and stress burden, the ENABLE III study found.
"Similar to patients, waiting to provide these caregiver services until patients are in their last weeks to days of life may not adequately address the distress that they experience," Nick Dionne-Odom, Ph.D., RN, said at the meeting.
Caregivers for the 13 million cancer patients in the United States living with advanced disease can spend up to 8 hours per day providing assistance in activities that include symptom management, emotional and spiritual support, meal preparation, arranging medical appointments, and transportation.
The combination of this burden and witnessing someone close to you struggle with illness can cause psychological distress equal to or sometimes greater than that experienced by the patient, said Dr. Dionne-Odom, a postdoctoral fellow at the University of Alabama at Birmingham.
In ENABLE III, 122 caregivers were randomized at the time of the patient’s cancer diagnosis or 12 weeks later to a palliative care intervention that consisted of three weekly structured educational telephone calls from an advanced practice nurse coach, monthly check-in calls to address new or ongoing issues, and a bereavement call for caregivers whose loved ones died.
Caregivers were not restricted to family members, but could include close friends and even neighbors. Their mean age was 60 years, 79% were female, 75% were spouses, and all had at least a high school education.
At 12 weeks from the start of the intervention, caregivers in the immediate versus delayed group had significantly better quality of life on the Caregiver Quality of Life Index–Cancer scale (mean 50.2 vs. 56.1; P = .02) and less depressive symptoms on the Center for Epidemiologic Studies Depression (CESD) scale (10.2 vs. 16.6; P = .0006), Dr. Dionne-Odom said. Notably, the delayed group surpassed the clinical cutoff for depression of 16 on the CESD scale, he added.
The intervention did not appear to change the perception among caregivers of what was demanded of them by the patient or their objective burden, though there was a trend among the immediate group for improved caregiver stress burden on the Montgomery Borgatta Caregiver Burden Scale (13.2 vs. 13.8; P = .10).
There was no significant difference between groups in depression or grief scores for caregivers of decedents. A difference may have been detected with a larger sample size, he said, adding that prior studies have shown that reducing caregiver stress before patients’ death is associated with better bereavement adjustment.
As for why caregivers appear to benefit more than the patients from the parallel palliative care interventions, Dr. Bakitas said in an interview it may be the timing of the assessments, adding that other studies have shown an impact of palliative care at 4 months, but not at 3 months.
AT THE ASCO ANNUAL MEETING 2014
Key clinical point: Palliative care delivered at the time of cancer diagnosis improves survival.
Major finding: The risk of death at 1 year was significantly lower in the immediate palliative care group versus the delayed palliative care group (hazard ratio 0.72; P = .003).
Data source: A randomized trial of palliative oncology care in 207 patients with advanced cancer and their caregivers.
Disclosures: The National Institute for Nursing Research funded the study. Dr. Bakitas reported having no relevant disclosures.
New tool measures ‘financial toxicity’ of cancer treatment
The financial distress of cancer patients can be measured using a newly developed 11-item, patient-reported outcome measure, investigators said June 20 online in Cancer.
The content for a comprehensive score for financial toxicity (COST) was developed with a stepwise approach involving 155 patients with advanced cancer. A literature review and semistructured, qualitative interviews with patients were followed by patients’ assessment of the items for importance to their quality of life, pilot testing, and finally an exploratory factor analysis, reported Dr. Jonas A. de Souza of the University of Chicago Medicine and his associates.
The final content included 11 questions: 1 financial item, 2 resource items, and 8 affect items. In the factor analysis, no sociodemographic factor was found significantly associated with the COST score, including household income (Cancer 2014 June 20 [doi:10.1002/cncr.28814]).
COST is a "first and major step" towards measuring how financial distress impacts the lives of patients with cancer, the authors said.
"A thoughtful, concise tool that could help predict a patient’s risk for financial toxicity might open the lines of communication. This gives us a way to launch that discussion," Dr. de Souza and his associates wrote.
The researchers are now conducting a further study to validate and correlate the COST scale with quality of life and anxiety in cancer patients.
All study participants had advanced cancer, were privately insured, were on chemotherapy, and had received treatment for at least 3 months and had therefore received cancer care bills.
One of the study coauthors disclosed receiving grants and personal fees from several pharmaceutical companies and owning stock options in Biscayne Pharmaceuticals.
The financial distress of cancer patients can be measured using a newly developed 11-item, patient-reported outcome measure, investigators said June 20 online in Cancer.
The content for a comprehensive score for financial toxicity (COST) was developed with a stepwise approach involving 155 patients with advanced cancer. A literature review and semistructured, qualitative interviews with patients were followed by patients’ assessment of the items for importance to their quality of life, pilot testing, and finally an exploratory factor analysis, reported Dr. Jonas A. de Souza of the University of Chicago Medicine and his associates.
The final content included 11 questions: 1 financial item, 2 resource items, and 8 affect items. In the factor analysis, no sociodemographic factor was found significantly associated with the COST score, including household income (Cancer 2014 June 20 [doi:10.1002/cncr.28814]).
COST is a "first and major step" towards measuring how financial distress impacts the lives of patients with cancer, the authors said.
"A thoughtful, concise tool that could help predict a patient’s risk for financial toxicity might open the lines of communication. This gives us a way to launch that discussion," Dr. de Souza and his associates wrote.
The researchers are now conducting a further study to validate and correlate the COST scale with quality of life and anxiety in cancer patients.
All study participants had advanced cancer, were privately insured, were on chemotherapy, and had received treatment for at least 3 months and had therefore received cancer care bills.
One of the study coauthors disclosed receiving grants and personal fees from several pharmaceutical companies and owning stock options in Biscayne Pharmaceuticals.
The financial distress of cancer patients can be measured using a newly developed 11-item, patient-reported outcome measure, investigators said June 20 online in Cancer.
The content for a comprehensive score for financial toxicity (COST) was developed with a stepwise approach involving 155 patients with advanced cancer. A literature review and semistructured, qualitative interviews with patients were followed by patients’ assessment of the items for importance to their quality of life, pilot testing, and finally an exploratory factor analysis, reported Dr. Jonas A. de Souza of the University of Chicago Medicine and his associates.
The final content included 11 questions: 1 financial item, 2 resource items, and 8 affect items. In the factor analysis, no sociodemographic factor was found significantly associated with the COST score, including household income (Cancer 2014 June 20 [doi:10.1002/cncr.28814]).
COST is a "first and major step" towards measuring how financial distress impacts the lives of patients with cancer, the authors said.
"A thoughtful, concise tool that could help predict a patient’s risk for financial toxicity might open the lines of communication. This gives us a way to launch that discussion," Dr. de Souza and his associates wrote.
The researchers are now conducting a further study to validate and correlate the COST scale with quality of life and anxiety in cancer patients.
All study participants had advanced cancer, were privately insured, were on chemotherapy, and had received treatment for at least 3 months and had therefore received cancer care bills.
One of the study coauthors disclosed receiving grants and personal fees from several pharmaceutical companies and owning stock options in Biscayne Pharmaceuticals.
FROM CANCER
Cancer survivors have higher medical costs
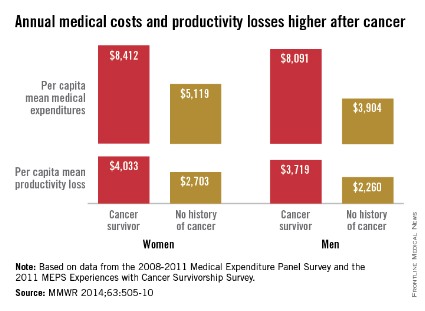
Average annual medical expenses for male cancer survivors were more than double those of men with no history of cancer in 2008-2011, while expenditures for female cancer survivors were 64% higher than those of women without a history of cancer, the Centers for Disease Control and Prevention reported.
A male cancer survivor had a mean annual medical expenditure of $8,091 from 2008 to 2011, along with $3,719 in lost productivity from employment disability, missed work days, and lost household productivity. A man without a history of cancer, by comparison, had an annual medical expenditure of $3,904 and a total productivity loss of $2,260, according to the CDC investigators (MMWR 2014;63:505-10).
For a female cancer survivor, annual medical expenditures totaled $8,412, with $4,033 in lost productivity in 2008-2011. In that same time period, a woman with no history of cancer had medical costs of $5,119 and productivity losses of $2,703, the CDC said.
The report was based on data from the 2008-2011 Medical Expenditure Panel Survey (6,722 cancer survivors and 86,865 with no history) and the 2011 MEPS Experiences with Cancer Survivorship Survey (1,202 cancer survivors).
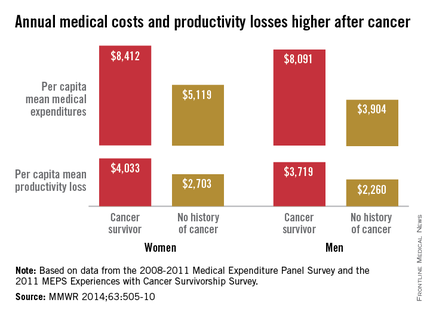
Average annual medical expenses for male cancer survivors were more than double those of men with no history of cancer in 2008-2011, while expenditures for female cancer survivors were 64% higher than those of women without a history of cancer, the Centers for Disease Control and Prevention reported.
A male cancer survivor had a mean annual medical expenditure of $8,091 from 2008 to 2011, along with $3,719 in lost productivity from employment disability, missed work days, and lost household productivity. A man without a history of cancer, by comparison, had an annual medical expenditure of $3,904 and a total productivity loss of $2,260, according to the CDC investigators (MMWR 2014;63:505-10).
For a female cancer survivor, annual medical expenditures totaled $8,412, with $4,033 in lost productivity in 2008-2011. In that same time period, a woman with no history of cancer had medical costs of $5,119 and productivity losses of $2,703, the CDC said.
The report was based on data from the 2008-2011 Medical Expenditure Panel Survey (6,722 cancer survivors and 86,865 with no history) and the 2011 MEPS Experiences with Cancer Survivorship Survey (1,202 cancer survivors).

Average annual medical expenses for male cancer survivors were more than double those of men with no history of cancer in 2008-2011, while expenditures for female cancer survivors were 64% higher than those of women without a history of cancer, the Centers for Disease Control and Prevention reported.
A male cancer survivor had a mean annual medical expenditure of $8,091 from 2008 to 2011, along with $3,719 in lost productivity from employment disability, missed work days, and lost household productivity. A man without a history of cancer, by comparison, had an annual medical expenditure of $3,904 and a total productivity loss of $2,260, according to the CDC investigators (MMWR 2014;63:505-10).
For a female cancer survivor, annual medical expenditures totaled $8,412, with $4,033 in lost productivity in 2008-2011. In that same time period, a woman with no history of cancer had medical costs of $5,119 and productivity losses of $2,703, the CDC said.
The report was based on data from the 2008-2011 Medical Expenditure Panel Survey (6,722 cancer survivors and 86,865 with no history) and the 2011 MEPS Experiences with Cancer Survivorship Survey (1,202 cancer survivors).

FROM MORBIDITY AND MORTALITY WEEKLY REPORT
VIDEO: Less frequent zoledronic acid is safe, retains efficacy
CHICAGO – Women with breast cancer and bone metastasis can safely scale back the frequency of their zoledronic acid infusions from every 4 weeks to every 12 weeks without a loss in efficacy*, according to results of the phase III OPTIMIZE 2 trial.
Notably, the dreaded bisphosphonate side effect of osteonecrosis of the jaw was seen in two patients in the monthly arm, but none of those in the every-3-month treatment arm.
The findings apply only to breast cancer patients who’ve completed at least 1 year of monthly zoledronic acid therapy, according to study author Dr. Gabriel N. Hortobagyi, a professor of medicine at the University of Texas M.D. Anderson Cancer Center, Houston.
In an interview with us at the 50th anniversary of the American Society of Clinical Oncology, this past ASCO president said that the findings from this late-breaking abstract study will have implications for the costs of cancer care and possibly for patients with other cancers.
Dr. Hortobagyi reported consultant or advisory roles and research funding with Novartis, the study sponsor. Several coauthors are employees of or have leadership positions with Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Correction, 5/31/2014: An earlier version of this article misstated the duration of their zoledronic acid infusion treatments.
CHICAGO – Women with breast cancer and bone metastasis can safely scale back the frequency of their zoledronic acid infusions from every 4 weeks to every 12 weeks without a loss in efficacy*, according to results of the phase III OPTIMIZE 2 trial.
Notably, the dreaded bisphosphonate side effect of osteonecrosis of the jaw was seen in two patients in the monthly arm, but none of those in the every-3-month treatment arm.
The findings apply only to breast cancer patients who’ve completed at least 1 year of monthly zoledronic acid therapy, according to study author Dr. Gabriel N. Hortobagyi, a professor of medicine at the University of Texas M.D. Anderson Cancer Center, Houston.
In an interview with us at the 50th anniversary of the American Society of Clinical Oncology, this past ASCO president said that the findings from this late-breaking abstract study will have implications for the costs of cancer care and possibly for patients with other cancers.
Dr. Hortobagyi reported consultant or advisory roles and research funding with Novartis, the study sponsor. Several coauthors are employees of or have leadership positions with Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Correction, 5/31/2014: An earlier version of this article misstated the duration of their zoledronic acid infusion treatments.
CHICAGO – Women with breast cancer and bone metastasis can safely scale back the frequency of their zoledronic acid infusions from every 4 weeks to every 12 weeks without a loss in efficacy*, according to results of the phase III OPTIMIZE 2 trial.
Notably, the dreaded bisphosphonate side effect of osteonecrosis of the jaw was seen in two patients in the monthly arm, but none of those in the every-3-month treatment arm.
The findings apply only to breast cancer patients who’ve completed at least 1 year of monthly zoledronic acid therapy, according to study author Dr. Gabriel N. Hortobagyi, a professor of medicine at the University of Texas M.D. Anderson Cancer Center, Houston.
In an interview with us at the 50th anniversary of the American Society of Clinical Oncology, this past ASCO president said that the findings from this late-breaking abstract study will have implications for the costs of cancer care and possibly for patients with other cancers.
Dr. Hortobagyi reported consultant or advisory roles and research funding with Novartis, the study sponsor. Several coauthors are employees of or have leadership positions with Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Correction, 5/31/2014: An earlier version of this article misstated the duration of their zoledronic acid infusion treatments.
AT THE ASCO ANNUAL MEETING 2014
The importance of hematologic, cytogenetic, and molecular testing and mutational analysis in chronic myeloid leukemia
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) for treatment of chronic myeloid leukemia (CML) has made it possible for this cancer to be controlled in many patients for long periods with chronic medication and regular monitoring of disease status. Hematologic and cytogenetic testing, molecular monitoring, and BCR-ABL1 mutational analysis have become integral to the routine management of CML. The information that each type of test provides is essential to confirm a diagnosis, determine the disease stage, assess response to treatment, and monitor for signals of disease progression – all of which can be used to identify patients who might require further evaluation, closer follow-up, and additional intervention, and to guide clinical decisions.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) for treatment of chronic myeloid leukemia (CML) has made it possible for this cancer to be controlled in many patients for long periods with chronic medication and regular monitoring of disease status. Hematologic and cytogenetic testing, molecular monitoring, and BCR-ABL1 mutational analysis have become integral to the routine management of CML. The information that each type of test provides is essential to confirm a diagnosis, determine the disease stage, assess response to treatment, and monitor for signals of disease progression – all of which can be used to identify patients who might require further evaluation, closer follow-up, and additional intervention, and to guide clinical decisions.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) for treatment of chronic myeloid leukemia (CML) has made it possible for this cancer to be controlled in many patients for long periods with chronic medication and regular monitoring of disease status. Hematologic and cytogenetic testing, molecular monitoring, and BCR-ABL1 mutational analysis have become integral to the routine management of CML. The information that each type of test provides is essential to confirm a diagnosis, determine the disease stage, assess response to treatment, and monitor for signals of disease progression – all of which can be used to identify patients who might require further evaluation, closer follow-up, and additional intervention, and to guide clinical decisions.
Click on the PDF icon at the top of this introduction to read the full article.
Molecular monitoring and minimal residual disease in the management of chronic myelogenous leukemia
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in 2001 for treatment of chronic myelogenous leukemia (CML) marked a paradigm shift in management of the disease. With that advance, CML has been largely managed as a chronic condition, with daily medication and frequent monitoring. Optimizing monitoring methods and identifying factors associated with response and long-term outcomes has thus been a major clinical research focus. Given the improved understanding of surveillance techniques in CML and the advent of several recently approved second- and third-generation TKIs, there have been recent updates to clinical practice guidelines.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in 2001 for treatment of chronic myelogenous leukemia (CML) marked a paradigm shift in management of the disease. With that advance, CML has been largely managed as a chronic condition, with daily medication and frequent monitoring. Optimizing monitoring methods and identifying factors associated with response and long-term outcomes has thus been a major clinical research focus. Given the improved understanding of surveillance techniques in CML and the advent of several recently approved second- and third-generation TKIs, there have been recent updates to clinical practice guidelines.
Click on the PDF icon at the top of this introduction to read the full article.
The introduction of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in 2001 for treatment of chronic myelogenous leukemia (CML) marked a paradigm shift in management of the disease. With that advance, CML has been largely managed as a chronic condition, with daily medication and frequent monitoring. Optimizing monitoring methods and identifying factors associated with response and long-term outcomes has thus been a major clinical research focus. Given the improved understanding of surveillance techniques in CML and the advent of several recently approved second- and third-generation TKIs, there have been recent updates to clinical practice guidelines.
Click on the PDF icon at the top of this introduction to read the full article.
Performance status of real-world oncology patients before and after first course of chemotherapy
Background Eastern Cooperative Oncology Group Performance Status (ECOG-PS) scores are used to quantify overall disease status and are widely used to stratify participants at clinical trial entry. Longitudinal ECOG-PS measurement between 2 tumor types may provide important data for patient management in community settings.
Objective To describe oncology patients’ performance status before and after their first course of chemotherapy.
Methods ECOG-PS scores from electronic medical records (EMRs) of 47 oncology clinics across the United States were retrieved. The included patients had breast, lymphoma, prostate, colorectal, or lung cancers and ECOG-PS scores within ± 14 days of initiation and completion of the first chemotherapy course. Descriptive statistics of ECOG-PS were analyzed and compared within tumor types (via the Wilcoxon signed-rank test) and between tumor types (via the Kruskal-Wallis test).
Results In all, 7,912 cancer patients were identified as having breast cancer, lymphoma, prostate cancer, colorectal cancer, or lung cancer. At baseline, patients’ mean (SD) ECOG-PS scores were breast cancer, 0.51 (0.01); lymphoma, 0.82 (0.02); prostate cancer, 1.04 (0.05); colorectal cancer, 0.72 (0.02); and lung cancer, 0.97 (0.02). The percentages of patients with ECOG-PS < 2 at chemotherapy start were 94%, 86%, 78%, 89%, and 81% for each tumor, respectively; percentages at the end of the first course were 88%, 80%, 68%, 84%, and 66%, respectively. All pre- and postchemotherapy comparisons of scores between tumor types were statistically significantly different (P < .001), with the exceptions of lung and prostate cancer before chemotherapy, and lung, prostate, lymphoma, and colorectal cancers after chemotherapy. Changes of ECOG-PS scores from baseline to postchemotherapy assessments were statistically significant in all tumor types (P < .01).
Limitations The lack of a standardized method for collecting ECOG-PS scores in routine oncology practice led to the unavailability of scores for many patients.
Conclusions This study describes a national sample of community oncology patients’ performance status. Even though there was a significant drop in ECOG-PS scores from pre- to postchemotherapy, good ECOG-PS scores were maintained in a majority of patients. These findings demonstrate that ECOG-PS scores can be routinely assessed and can aid in decisions throughout chemo- therapy and in the planning for future treatments.
Funding Amgen Inc funded the study.
Click on the PDF icon at the top of this introduction to read the full article.
Background Eastern Cooperative Oncology Group Performance Status (ECOG-PS) scores are used to quantify overall disease status and are widely used to stratify participants at clinical trial entry. Longitudinal ECOG-PS measurement between 2 tumor types may provide important data for patient management in community settings.
Objective To describe oncology patients’ performance status before and after their first course of chemotherapy.
Methods ECOG-PS scores from electronic medical records (EMRs) of 47 oncology clinics across the United States were retrieved. The included patients had breast, lymphoma, prostate, colorectal, or lung cancers and ECOG-PS scores within ± 14 days of initiation and completion of the first chemotherapy course. Descriptive statistics of ECOG-PS were analyzed and compared within tumor types (via the Wilcoxon signed-rank test) and between tumor types (via the Kruskal-Wallis test).
Results In all, 7,912 cancer patients were identified as having breast cancer, lymphoma, prostate cancer, colorectal cancer, or lung cancer. At baseline, patients’ mean (SD) ECOG-PS scores were breast cancer, 0.51 (0.01); lymphoma, 0.82 (0.02); prostate cancer, 1.04 (0.05); colorectal cancer, 0.72 (0.02); and lung cancer, 0.97 (0.02). The percentages of patients with ECOG-PS < 2 at chemotherapy start were 94%, 86%, 78%, 89%, and 81% for each tumor, respectively; percentages at the end of the first course were 88%, 80%, 68%, 84%, and 66%, respectively. All pre- and postchemotherapy comparisons of scores between tumor types were statistically significantly different (P < .001), with the exceptions of lung and prostate cancer before chemotherapy, and lung, prostate, lymphoma, and colorectal cancers after chemotherapy. Changes of ECOG-PS scores from baseline to postchemotherapy assessments were statistically significant in all tumor types (P < .01).
Limitations The lack of a standardized method for collecting ECOG-PS scores in routine oncology practice led to the unavailability of scores for many patients.
Conclusions This study describes a national sample of community oncology patients’ performance status. Even though there was a significant drop in ECOG-PS scores from pre- to postchemotherapy, good ECOG-PS scores were maintained in a majority of patients. These findings demonstrate that ECOG-PS scores can be routinely assessed and can aid in decisions throughout chemo- therapy and in the planning for future treatments.
Funding Amgen Inc funded the study.
Click on the PDF icon at the top of this introduction to read the full article.
Background Eastern Cooperative Oncology Group Performance Status (ECOG-PS) scores are used to quantify overall disease status and are widely used to stratify participants at clinical trial entry. Longitudinal ECOG-PS measurement between 2 tumor types may provide important data for patient management in community settings.
Objective To describe oncology patients’ performance status before and after their first course of chemotherapy.
Methods ECOG-PS scores from electronic medical records (EMRs) of 47 oncology clinics across the United States were retrieved. The included patients had breast, lymphoma, prostate, colorectal, or lung cancers and ECOG-PS scores within ± 14 days of initiation and completion of the first chemotherapy course. Descriptive statistics of ECOG-PS were analyzed and compared within tumor types (via the Wilcoxon signed-rank test) and between tumor types (via the Kruskal-Wallis test).
Results In all, 7,912 cancer patients were identified as having breast cancer, lymphoma, prostate cancer, colorectal cancer, or lung cancer. At baseline, patients’ mean (SD) ECOG-PS scores were breast cancer, 0.51 (0.01); lymphoma, 0.82 (0.02); prostate cancer, 1.04 (0.05); colorectal cancer, 0.72 (0.02); and lung cancer, 0.97 (0.02). The percentages of patients with ECOG-PS < 2 at chemotherapy start were 94%, 86%, 78%, 89%, and 81% for each tumor, respectively; percentages at the end of the first course were 88%, 80%, 68%, 84%, and 66%, respectively. All pre- and postchemotherapy comparisons of scores between tumor types were statistically significantly different (P < .001), with the exceptions of lung and prostate cancer before chemotherapy, and lung, prostate, lymphoma, and colorectal cancers after chemotherapy. Changes of ECOG-PS scores from baseline to postchemotherapy assessments were statistically significant in all tumor types (P < .01).
Limitations The lack of a standardized method for collecting ECOG-PS scores in routine oncology practice led to the unavailability of scores for many patients.
Conclusions This study describes a national sample of community oncology patients’ performance status. Even though there was a significant drop in ECOG-PS scores from pre- to postchemotherapy, good ECOG-PS scores were maintained in a majority of patients. These findings demonstrate that ECOG-PS scores can be routinely assessed and can aid in decisions throughout chemo- therapy and in the planning for future treatments.
Funding Amgen Inc funded the study.
Click on the PDF icon at the top of this introduction to read the full article.
Barriers to palliative care research for emergency department patients with advanced cancer
Background Patients with advanced cancer often visit the emergency department (ED). Little is known about their willingness or ability to engage in palliative care research, although enrollment in clinical trials of other seriously ill ED patients -- those with stroke, for example -- has been shown to be feasible.
Objective To identify barriers to the enrollment of ED patients with advanced cancer in palliative care research.
Methods We prospectively tracked factors that affected patient accrual into a trial of palliative care for adults with metastatic solid tumors at an urban, academic ED. Research staff screened the electronic medical records for patients admitted to the hospital with metastatic solid tumors 8-12 hours a day, Monday through Friday. The ED attending of record and the patient’s medical oncologist had to agree before research staff invited the patient to participate. Informed consent was obtained at the bedside in the ED, and patients were offered a $20 incentive to participate.
Results Attempts were made to enroll 150 eligible patients in the study, and 73 were enrolled (49% enrollment rate). Barriers to enrollment for the 77 patients who did not participate were deduced from the field notes and placed into the following categories: patient refusal (n = 38, 49%), diagnostic uncertainty regarding cancer stage (n = 11, 14%), symptom burden (n = 9, 12%), family refusal (n = 7, 9%), physician refusal (n = 7, 9%), and/or patient unaware of illness or stage (n = 5, 7%).
Limitations The findings are descriptive and do not test predetermined hypotheses.
Conclusion Patient refusal, symptom burden, and diagnostic disparities are common barriers encountered when recruiting ED patients with advanced cancer. Despite the barriers, recruitment was feasible for such ED patients.
Funding/sponsor This study was funded by a Mentored Research Scholar Grant from the American Cancer Society (Dr Grudzen), a Medical Student Training in Aging Research Grant from the American Federation on Aging (Mr Kandarian), and by a Mid- Career Investigator Award in Patient Oriented Research (K24 AG022345) from the National Institute on Aging (Dr Morrison).
Click on the PDF icon at the top of this introduction to read the full article.
Background Patients with advanced cancer often visit the emergency department (ED). Little is known about their willingness or ability to engage in palliative care research, although enrollment in clinical trials of other seriously ill ED patients -- those with stroke, for example -- has been shown to be feasible.
Objective To identify barriers to the enrollment of ED patients with advanced cancer in palliative care research.
Methods We prospectively tracked factors that affected patient accrual into a trial of palliative care for adults with metastatic solid tumors at an urban, academic ED. Research staff screened the electronic medical records for patients admitted to the hospital with metastatic solid tumors 8-12 hours a day, Monday through Friday. The ED attending of record and the patient’s medical oncologist had to agree before research staff invited the patient to participate. Informed consent was obtained at the bedside in the ED, and patients were offered a $20 incentive to participate.
Results Attempts were made to enroll 150 eligible patients in the study, and 73 were enrolled (49% enrollment rate). Barriers to enrollment for the 77 patients who did not participate were deduced from the field notes and placed into the following categories: patient refusal (n = 38, 49%), diagnostic uncertainty regarding cancer stage (n = 11, 14%), symptom burden (n = 9, 12%), family refusal (n = 7, 9%), physician refusal (n = 7, 9%), and/or patient unaware of illness or stage (n = 5, 7%).
Limitations The findings are descriptive and do not test predetermined hypotheses.
Conclusion Patient refusal, symptom burden, and diagnostic disparities are common barriers encountered when recruiting ED patients with advanced cancer. Despite the barriers, recruitment was feasible for such ED patients.
Funding/sponsor This study was funded by a Mentored Research Scholar Grant from the American Cancer Society (Dr Grudzen), a Medical Student Training in Aging Research Grant from the American Federation on Aging (Mr Kandarian), and by a Mid- Career Investigator Award in Patient Oriented Research (K24 AG022345) from the National Institute on Aging (Dr Morrison).
Click on the PDF icon at the top of this introduction to read the full article.
Background Patients with advanced cancer often visit the emergency department (ED). Little is known about their willingness or ability to engage in palliative care research, although enrollment in clinical trials of other seriously ill ED patients -- those with stroke, for example -- has been shown to be feasible.
Objective To identify barriers to the enrollment of ED patients with advanced cancer in palliative care research.
Methods We prospectively tracked factors that affected patient accrual into a trial of palliative care for adults with metastatic solid tumors at an urban, academic ED. Research staff screened the electronic medical records for patients admitted to the hospital with metastatic solid tumors 8-12 hours a day, Monday through Friday. The ED attending of record and the patient’s medical oncologist had to agree before research staff invited the patient to participate. Informed consent was obtained at the bedside in the ED, and patients were offered a $20 incentive to participate.
Results Attempts were made to enroll 150 eligible patients in the study, and 73 were enrolled (49% enrollment rate). Barriers to enrollment for the 77 patients who did not participate were deduced from the field notes and placed into the following categories: patient refusal (n = 38, 49%), diagnostic uncertainty regarding cancer stage (n = 11, 14%), symptom burden (n = 9, 12%), family refusal (n = 7, 9%), physician refusal (n = 7, 9%), and/or patient unaware of illness or stage (n = 5, 7%).
Limitations The findings are descriptive and do not test predetermined hypotheses.
Conclusion Patient refusal, symptom burden, and diagnostic disparities are common barriers encountered when recruiting ED patients with advanced cancer. Despite the barriers, recruitment was feasible for such ED patients.
Funding/sponsor This study was funded by a Mentored Research Scholar Grant from the American Cancer Society (Dr Grudzen), a Medical Student Training in Aging Research Grant from the American Federation on Aging (Mr Kandarian), and by a Mid- Career Investigator Award in Patient Oriented Research (K24 AG022345) from the National Institute on Aging (Dr Morrison).
Click on the PDF icon at the top of this introduction to read the full article.
Taking the data and findings into the real-world setting
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
No link seen between ondansetron and tachyarrhythmias in healthy children
Vancouver, B.C. – The antinausea and antiemetic agent ondansetron does not trigger malignant tachyarrhythmias in pediatric patients who have a healthy, native heart, suggests a retrospective cohort study presented at the annual meeting of the Pediatric Academic Societies.
Findings of the study, conducted at a large tertiary care children’s hospital and its affiliated clinics, and spanning a 6-year period, showed that the incidence of tachyarrhythmia within 24 hours after ondansetron (Zofran) administration was just 0.02%, or 1 in 6,299 patients.
All of the patients developing tachyarrhythmias had a preexisting cardiac diagnosis, including cardiac transplantation in some cases, and most also had other risk factors, reported lead researcher Dr. Breanne K.P. Shah, a fellow at the Medical College of Wisconsin and Children’s Hospital of Wisconsin in Madison.
The median dose of ondansetron administered was consistent with recommendations, although it ranged widely, she noted.
"Only children with cardiac diagnoses were found to have arrhythmia after ondansetron administration," Dr. Shah commented. "In subgroup analyses of patient populations who either received frequent doses or doses higher than average, such as patients in the ED or those with oncologic diagnoses, there were no documented tachyarrhythmias in the 24 hours after receiving ondansetron. It appears that these patients are at low risk."
"The findings of our study support consideration of cardiac monitoring for children with cardiac diagnoses who are receiving ondansetron. They do not support ECG screening or continuous monitoring of other pediatric populations receiving ondansetron," she concluded.
Session comoderator Dr. Julie Brown, an emergency medicine attending physician at Seattle Children’s Hospital who is with the department of pediatrics at the University of Washington, commented, "This is a really important study that I think probably illustrates the ‘creep’ of therapy from what is well studied into applications to a wider population, which is a little frightening."
"Sometimes, we come across something striking in our clinical work that prompts us to investigate a problem that could also maybe stack the deck with adverse events. So I’m wondering if you were aware of any of the identified cases before you proceeded with the retrospective study," she said.
"We were not," Dr. Shah replied, noting that the research was prompted by a 2011 Food and Drug Administration warning about potentially fatal abnormal heart rhythms in patients administered ondansetron.
In their warning about the drug, FDA "recommended avoiding use in patients with congenital long QT. Additionally, they recommended for ECG monitoring in patients with heart failure or bradyarrhythmias, patients taking other medications that can lead to QT prolongation, or patients with electrolyte abnormalities," Dr. Shah noted.
However, "to our knowledge, no pediatric studies have been done to evaluate adverse clinical outcomes with the use of ondansetron."
The researchers retrospectively studied 58,009 visits by patients aged 0-18 years in which ondansetron was administered. A total of 199,773 doses were given to 37,794 patients.
For single doses, the median dose was 4 mg (range, 0.11 to 36 mg) and the median dosage was 0.1 mg/kg per dose (range, 0.005 to 0.86 mg/kg per dose).
Overall, six patients developed a tachyarrhythmia within 24 hours of receiving ondansetron, for an incidence of 0.02%. They ranged in age from 9 weeks to 17 years, and two-thirds were male.
None died at the time of the event, according to Dr. Shah.
The average ondansetron dosage in these patients was 0.1 mg/kg per dose, and the time between dosing and onset of tachyarrhythmia ranged from 2 to 20 hours.
All six patients had underlying cardiac diagnoses (congenital conduction abnormality, congenital heart defect, cardiac tumor, or heart transplantation). And five had concomitant risk factors (use of medications known to prolong the QT interval, electrolyte abnormalities, or prolonged QTc interval).
"The retrospective nature of this study limits our ability to make conclusions," Dr. Shah acknowledged. "We used a conservative approach, assuming that any documentation of a tachyarrhythmia in the medical record within 24 hours of ondansetron constituted an ondansetron-related event, but we cannot prove cause."
Dr. Shah disclosed no relevant conflicts of interest.
Vancouver, B.C. – The antinausea and antiemetic agent ondansetron does not trigger malignant tachyarrhythmias in pediatric patients who have a healthy, native heart, suggests a retrospective cohort study presented at the annual meeting of the Pediatric Academic Societies.
Findings of the study, conducted at a large tertiary care children’s hospital and its affiliated clinics, and spanning a 6-year period, showed that the incidence of tachyarrhythmia within 24 hours after ondansetron (Zofran) administration was just 0.02%, or 1 in 6,299 patients.
All of the patients developing tachyarrhythmias had a preexisting cardiac diagnosis, including cardiac transplantation in some cases, and most also had other risk factors, reported lead researcher Dr. Breanne K.P. Shah, a fellow at the Medical College of Wisconsin and Children’s Hospital of Wisconsin in Madison.
The median dose of ondansetron administered was consistent with recommendations, although it ranged widely, she noted.
"Only children with cardiac diagnoses were found to have arrhythmia after ondansetron administration," Dr. Shah commented. "In subgroup analyses of patient populations who either received frequent doses or doses higher than average, such as patients in the ED or those with oncologic diagnoses, there were no documented tachyarrhythmias in the 24 hours after receiving ondansetron. It appears that these patients are at low risk."
"The findings of our study support consideration of cardiac monitoring for children with cardiac diagnoses who are receiving ondansetron. They do not support ECG screening or continuous monitoring of other pediatric populations receiving ondansetron," she concluded.
Session comoderator Dr. Julie Brown, an emergency medicine attending physician at Seattle Children’s Hospital who is with the department of pediatrics at the University of Washington, commented, "This is a really important study that I think probably illustrates the ‘creep’ of therapy from what is well studied into applications to a wider population, which is a little frightening."
"Sometimes, we come across something striking in our clinical work that prompts us to investigate a problem that could also maybe stack the deck with adverse events. So I’m wondering if you were aware of any of the identified cases before you proceeded with the retrospective study," she said.
"We were not," Dr. Shah replied, noting that the research was prompted by a 2011 Food and Drug Administration warning about potentially fatal abnormal heart rhythms in patients administered ondansetron.
In their warning about the drug, FDA "recommended avoiding use in patients with congenital long QT. Additionally, they recommended for ECG monitoring in patients with heart failure or bradyarrhythmias, patients taking other medications that can lead to QT prolongation, or patients with electrolyte abnormalities," Dr. Shah noted.
However, "to our knowledge, no pediatric studies have been done to evaluate adverse clinical outcomes with the use of ondansetron."
The researchers retrospectively studied 58,009 visits by patients aged 0-18 years in which ondansetron was administered. A total of 199,773 doses were given to 37,794 patients.
For single doses, the median dose was 4 mg (range, 0.11 to 36 mg) and the median dosage was 0.1 mg/kg per dose (range, 0.005 to 0.86 mg/kg per dose).
Overall, six patients developed a tachyarrhythmia within 24 hours of receiving ondansetron, for an incidence of 0.02%. They ranged in age from 9 weeks to 17 years, and two-thirds were male.
None died at the time of the event, according to Dr. Shah.
The average ondansetron dosage in these patients was 0.1 mg/kg per dose, and the time between dosing and onset of tachyarrhythmia ranged from 2 to 20 hours.
All six patients had underlying cardiac diagnoses (congenital conduction abnormality, congenital heart defect, cardiac tumor, or heart transplantation). And five had concomitant risk factors (use of medications known to prolong the QT interval, electrolyte abnormalities, or prolonged QTc interval).
"The retrospective nature of this study limits our ability to make conclusions," Dr. Shah acknowledged. "We used a conservative approach, assuming that any documentation of a tachyarrhythmia in the medical record within 24 hours of ondansetron constituted an ondansetron-related event, but we cannot prove cause."
Dr. Shah disclosed no relevant conflicts of interest.
Vancouver, B.C. – The antinausea and antiemetic agent ondansetron does not trigger malignant tachyarrhythmias in pediatric patients who have a healthy, native heart, suggests a retrospective cohort study presented at the annual meeting of the Pediatric Academic Societies.
Findings of the study, conducted at a large tertiary care children’s hospital and its affiliated clinics, and spanning a 6-year period, showed that the incidence of tachyarrhythmia within 24 hours after ondansetron (Zofran) administration was just 0.02%, or 1 in 6,299 patients.
All of the patients developing tachyarrhythmias had a preexisting cardiac diagnosis, including cardiac transplantation in some cases, and most also had other risk factors, reported lead researcher Dr. Breanne K.P. Shah, a fellow at the Medical College of Wisconsin and Children’s Hospital of Wisconsin in Madison.
The median dose of ondansetron administered was consistent with recommendations, although it ranged widely, she noted.
"Only children with cardiac diagnoses were found to have arrhythmia after ondansetron administration," Dr. Shah commented. "In subgroup analyses of patient populations who either received frequent doses or doses higher than average, such as patients in the ED or those with oncologic diagnoses, there were no documented tachyarrhythmias in the 24 hours after receiving ondansetron. It appears that these patients are at low risk."
"The findings of our study support consideration of cardiac monitoring for children with cardiac diagnoses who are receiving ondansetron. They do not support ECG screening or continuous monitoring of other pediatric populations receiving ondansetron," she concluded.
Session comoderator Dr. Julie Brown, an emergency medicine attending physician at Seattle Children’s Hospital who is with the department of pediatrics at the University of Washington, commented, "This is a really important study that I think probably illustrates the ‘creep’ of therapy from what is well studied into applications to a wider population, which is a little frightening."
"Sometimes, we come across something striking in our clinical work that prompts us to investigate a problem that could also maybe stack the deck with adverse events. So I’m wondering if you were aware of any of the identified cases before you proceeded with the retrospective study," she said.
"We were not," Dr. Shah replied, noting that the research was prompted by a 2011 Food and Drug Administration warning about potentially fatal abnormal heart rhythms in patients administered ondansetron.
In their warning about the drug, FDA "recommended avoiding use in patients with congenital long QT. Additionally, they recommended for ECG monitoring in patients with heart failure or bradyarrhythmias, patients taking other medications that can lead to QT prolongation, or patients with electrolyte abnormalities," Dr. Shah noted.
However, "to our knowledge, no pediatric studies have been done to evaluate adverse clinical outcomes with the use of ondansetron."
The researchers retrospectively studied 58,009 visits by patients aged 0-18 years in which ondansetron was administered. A total of 199,773 doses were given to 37,794 patients.
For single doses, the median dose was 4 mg (range, 0.11 to 36 mg) and the median dosage was 0.1 mg/kg per dose (range, 0.005 to 0.86 mg/kg per dose).
Overall, six patients developed a tachyarrhythmia within 24 hours of receiving ondansetron, for an incidence of 0.02%. They ranged in age from 9 weeks to 17 years, and two-thirds were male.
None died at the time of the event, according to Dr. Shah.
The average ondansetron dosage in these patients was 0.1 mg/kg per dose, and the time between dosing and onset of tachyarrhythmia ranged from 2 to 20 hours.
All six patients had underlying cardiac diagnoses (congenital conduction abnormality, congenital heart defect, cardiac tumor, or heart transplantation). And five had concomitant risk factors (use of medications known to prolong the QT interval, electrolyte abnormalities, or prolonged QTc interval).
"The retrospective nature of this study limits our ability to make conclusions," Dr. Shah acknowledged. "We used a conservative approach, assuming that any documentation of a tachyarrhythmia in the medical record within 24 hours of ondansetron constituted an ondansetron-related event, but we cannot prove cause."
Dr. Shah disclosed no relevant conflicts of interest.
AT THE PAS ANNUAL MEETING
Top clinical point: The study findings support cardiac monitoring for children with cardiac diagnoses who are receiving ondansetron. They do not support ECG screening or continuous monitoring of other pediatric populations receiving ondansetron
Major finding: The incidence of tachyarrhythmia after ondansetron administration was 0.02%; all affected patients had preexisting cardiac diagnoses.
Data source: A retrospective cohort study of 58,009 visits to a children’s hospital in which ondansetron was given.
Disclosures: Dr. Shah disclosed no relevant conflicts of interest.