User login
ESC: Cancer itself may cause cardiotoxicity
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
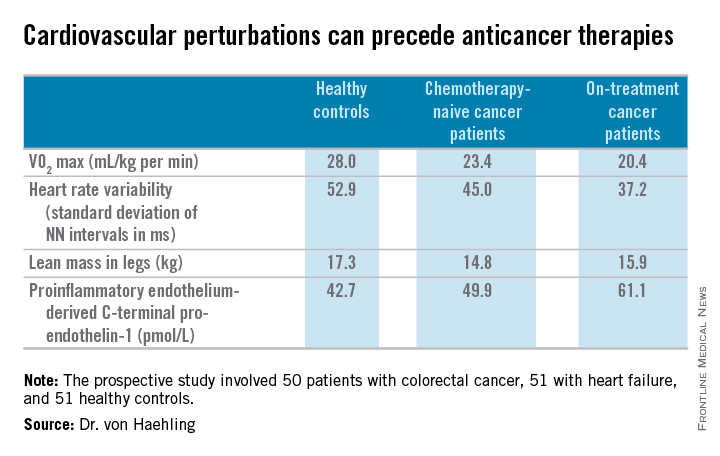
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.
For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.

For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.

For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
EXPERT ANALYSIS FROM THE ESC CONGRESS 2015
California governor signs physician-assisted suicide bill into law
California Gov. Jerry Brown (D) has signed into law a controversial measure that allows physicians to help terminally ill patients legally end their lives, making California the fourth state to permit doctor-assisted suicide through its legislature.
Gov. Brown, a former seminary student, approved the End of Life Option Act Oct. 5, after state lawmakers passed the bill Sept. 11.
In a signing message, Gov. Brown said that he had considered all sides of the issue and carefully weighed religious and theological perspectives that shortening a patient’s life is sinful.
“In the end, I was left to reflect on what I would want in the face of my own death,” Gov. Brown said in the message. “I do not know what I would do if I were dying in prolonged and excruciating pain. I am certain, however, that it would be a comfort to be able to consider the options afforded by this bill. And I wouldn’t deny that right to others.”
Modeled after Oregon’s statute, California’s law requires two doctors to determine that a patient has 6 months or less to live before doctors could prescribe life-ending medication. Patients must have the mental capacity to make medical decisions and would physically have to be able to swallow the drugs.
In addition, patients seeking physician aid in dying must submit two oral requests, a minimum of 15 days apart, and a written request to their physician. The attending physician must receive all three requests directly from the patient and not through a designee. Before prescribing end-of-life drugs, the attending physician must refer the patient to a consulting physician for confirmation of the diagnosis and prognosis and of the patient’s capacity to make the decision.
Oregon, Vermont, and Washington each have laws permitting physician-assisted death. Court rulings in New Mexico and Montana have allowed for the practice, but litigation in those states is ongoing and the decisions have yet to be enforced.
The signing ends nearly a year of passionate debate in California that divided physicians, religious groups, lawmakers, and community members. In May, the California Medical Association (CMA) became the first state medical society to change its stance against physician-assisted suicide to that of being neutral.
“The decision to participate in the End of Life Option Act is a very personal one between a doctor and their patient, which is why CMA has removed policy that outright objects to physicians aiding terminally ill patients in end of life options,” Dr. Luther F. Cobb, CMA president, said in a statement. “We believe it is up to the individual physician and their patient to decide voluntarily whether the End of Life Option Act is something in which they want to engage. Protecting that physician-patient relationship is essential.”
The California law will take effect 90 days after the state legislature adjourns its special session on health care, which may not be until early next year. The earliest likely enactment would be spring 2016.
On Twitter @legal_med
California Gov. Jerry Brown (D) has signed into law a controversial measure that allows physicians to help terminally ill patients legally end their lives, making California the fourth state to permit doctor-assisted suicide through its legislature.
Gov. Brown, a former seminary student, approved the End of Life Option Act Oct. 5, after state lawmakers passed the bill Sept. 11.
In a signing message, Gov. Brown said that he had considered all sides of the issue and carefully weighed religious and theological perspectives that shortening a patient’s life is sinful.
“In the end, I was left to reflect on what I would want in the face of my own death,” Gov. Brown said in the message. “I do not know what I would do if I were dying in prolonged and excruciating pain. I am certain, however, that it would be a comfort to be able to consider the options afforded by this bill. And I wouldn’t deny that right to others.”
Modeled after Oregon’s statute, California’s law requires two doctors to determine that a patient has 6 months or less to live before doctors could prescribe life-ending medication. Patients must have the mental capacity to make medical decisions and would physically have to be able to swallow the drugs.
In addition, patients seeking physician aid in dying must submit two oral requests, a minimum of 15 days apart, and a written request to their physician. The attending physician must receive all three requests directly from the patient and not through a designee. Before prescribing end-of-life drugs, the attending physician must refer the patient to a consulting physician for confirmation of the diagnosis and prognosis and of the patient’s capacity to make the decision.
Oregon, Vermont, and Washington each have laws permitting physician-assisted death. Court rulings in New Mexico and Montana have allowed for the practice, but litigation in those states is ongoing and the decisions have yet to be enforced.
The signing ends nearly a year of passionate debate in California that divided physicians, religious groups, lawmakers, and community members. In May, the California Medical Association (CMA) became the first state medical society to change its stance against physician-assisted suicide to that of being neutral.
“The decision to participate in the End of Life Option Act is a very personal one between a doctor and their patient, which is why CMA has removed policy that outright objects to physicians aiding terminally ill patients in end of life options,” Dr. Luther F. Cobb, CMA president, said in a statement. “We believe it is up to the individual physician and their patient to decide voluntarily whether the End of Life Option Act is something in which they want to engage. Protecting that physician-patient relationship is essential.”
The California law will take effect 90 days after the state legislature adjourns its special session on health care, which may not be until early next year. The earliest likely enactment would be spring 2016.
On Twitter @legal_med
California Gov. Jerry Brown (D) has signed into law a controversial measure that allows physicians to help terminally ill patients legally end their lives, making California the fourth state to permit doctor-assisted suicide through its legislature.
Gov. Brown, a former seminary student, approved the End of Life Option Act Oct. 5, after state lawmakers passed the bill Sept. 11.
In a signing message, Gov. Brown said that he had considered all sides of the issue and carefully weighed religious and theological perspectives that shortening a patient’s life is sinful.
“In the end, I was left to reflect on what I would want in the face of my own death,” Gov. Brown said in the message. “I do not know what I would do if I were dying in prolonged and excruciating pain. I am certain, however, that it would be a comfort to be able to consider the options afforded by this bill. And I wouldn’t deny that right to others.”
Modeled after Oregon’s statute, California’s law requires two doctors to determine that a patient has 6 months or less to live before doctors could prescribe life-ending medication. Patients must have the mental capacity to make medical decisions and would physically have to be able to swallow the drugs.
In addition, patients seeking physician aid in dying must submit two oral requests, a minimum of 15 days apart, and a written request to their physician. The attending physician must receive all three requests directly from the patient and not through a designee. Before prescribing end-of-life drugs, the attending physician must refer the patient to a consulting physician for confirmation of the diagnosis and prognosis and of the patient’s capacity to make the decision.
Oregon, Vermont, and Washington each have laws permitting physician-assisted death. Court rulings in New Mexico and Montana have allowed for the practice, but litigation in those states is ongoing and the decisions have yet to be enforced.
The signing ends nearly a year of passionate debate in California that divided physicians, religious groups, lawmakers, and community members. In May, the California Medical Association (CMA) became the first state medical society to change its stance against physician-assisted suicide to that of being neutral.
“The decision to participate in the End of Life Option Act is a very personal one between a doctor and their patient, which is why CMA has removed policy that outright objects to physicians aiding terminally ill patients in end of life options,” Dr. Luther F. Cobb, CMA president, said in a statement. “We believe it is up to the individual physician and their patient to decide voluntarily whether the End of Life Option Act is something in which they want to engage. Protecting that physician-patient relationship is essential.”
The California law will take effect 90 days after the state legislature adjourns its special session on health care, which may not be until early next year. The earliest likely enactment would be spring 2016.
On Twitter @legal_med
Chlorhexidine gel-pad dressing reduces bloodstream infections
SAN DIEGO – Chlorhexidine-containing intravenous catheter securement dressings significantly reduced the incidence of central venous catheter–related bloodstream infections in neutropenic patients, a multicenter randomized trial showed.
“Central venous catheters impose a risk of catheter-related bloodstream infections, especially when used in neutropenic patients, and the mortality risk has been reported to be up to 36%,” Dr. Lena M. Biehl said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
One way to prevent catheter-related bloodstream infections is to use chlorhexidine-containing intravenous catheter securement dressings in an effort to decrease skin colonization, said Dr. Biehl of the department of internal medicine at University Hospital of Cologne (Germany). “A few studies have looked at this, but most of them used chlorhexidine-containing sponges. There’s only one study using a more advanced chlorhexidine-containing gel pad, and this study was done in the ICU setting. The gel pad enables you to evaluate the insertion site, so you can see if there are signs of infection without removing the dressing.”
In a multicenter, randomized trial known as the COAT study that was conducted at 10 hematology departments in Germany from February 2012 to September 2014, Dr. Biehl and her associates set out to compare the incidence of central venous catheter–related bloodstream infections in two groups of neutropenic patients: those with chlorhexidine-containing IV catheter-securement dressings that included a gel pad (the chlorhexidine group) and those with conventional IV catheter-securement dressings that lacked a gel pad (the control group). They limited the analysis to patients undergoing intensive chemotherapy with expected neutropenia for at least 5 days and expected central venous catheter use of at least 10 days. The primary endpoint was the incidence of definite catheter-related bloodstream infections within 14 days of central venous catheter placement. The secondary endpoints were overall incidence of definite or probable central venous catheter–related bloodstream infections at 14 days and the overall incidence of definite or probable central venous catheter–related bloodstream infections.
Dr. Biehl presented results from 613 patients. Of these, 307 were in the chlorhexidine group and 306 were in the control group. The median age was 58 years and 59% were male, and the distribution of causative pathogens was similar between the two groups. The incidence of definite catheter-related bloodstream infections at 14 days was 2.6% in the chlorhexidine group, compared with 3.9% in the control group, a difference that did not reach statistical significance (P = .375). However, the overall incidence of definite and probable central venous catheter–related bloodstream infections together at 14 days was 6.5% in the chlorhexidine group, compared with 11.1% in the control group, a difference that reached statistical significance (P = .047). Finally, the overall incidence of definite and probable central venous catheter–related bloodstream infections was 10.4% in the chlorhexidine group, compared with 17% in the control group, a difference that also reached statistical significance (P = .019).
“The chlorhexidine dressings were very well tolerated, and in contrast to previous studies we saw no increase in skin and soft tissue abnormalities or contact dermatitis,” Dr. Biehl said. The researchers also observed no significant difference in mortality between the chlorhexidine and control groups (6.2 % vs. 5.6%, respectively).
The study was supported by a grant from 3M. Dr. Biehl disclosed that she is a member of the speakers bureau for Astellas Pharma and Merck/MSD. She has received travel grants from 3M and Gilead Sciences. Another study investigator, Dr. Maria J. G. T. Vehreschild, disclosed numerous financial ties to industry.
SAN DIEGO – Chlorhexidine-containing intravenous catheter securement dressings significantly reduced the incidence of central venous catheter–related bloodstream infections in neutropenic patients, a multicenter randomized trial showed.
“Central venous catheters impose a risk of catheter-related bloodstream infections, especially when used in neutropenic patients, and the mortality risk has been reported to be up to 36%,” Dr. Lena M. Biehl said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
One way to prevent catheter-related bloodstream infections is to use chlorhexidine-containing intravenous catheter securement dressings in an effort to decrease skin colonization, said Dr. Biehl of the department of internal medicine at University Hospital of Cologne (Germany). “A few studies have looked at this, but most of them used chlorhexidine-containing sponges. There’s only one study using a more advanced chlorhexidine-containing gel pad, and this study was done in the ICU setting. The gel pad enables you to evaluate the insertion site, so you can see if there are signs of infection without removing the dressing.”
In a multicenter, randomized trial known as the COAT study that was conducted at 10 hematology departments in Germany from February 2012 to September 2014, Dr. Biehl and her associates set out to compare the incidence of central venous catheter–related bloodstream infections in two groups of neutropenic patients: those with chlorhexidine-containing IV catheter-securement dressings that included a gel pad (the chlorhexidine group) and those with conventional IV catheter-securement dressings that lacked a gel pad (the control group). They limited the analysis to patients undergoing intensive chemotherapy with expected neutropenia for at least 5 days and expected central venous catheter use of at least 10 days. The primary endpoint was the incidence of definite catheter-related bloodstream infections within 14 days of central venous catheter placement. The secondary endpoints were overall incidence of definite or probable central venous catheter–related bloodstream infections at 14 days and the overall incidence of definite or probable central venous catheter–related bloodstream infections.
Dr. Biehl presented results from 613 patients. Of these, 307 were in the chlorhexidine group and 306 were in the control group. The median age was 58 years and 59% were male, and the distribution of causative pathogens was similar between the two groups. The incidence of definite catheter-related bloodstream infections at 14 days was 2.6% in the chlorhexidine group, compared with 3.9% in the control group, a difference that did not reach statistical significance (P = .375). However, the overall incidence of definite and probable central venous catheter–related bloodstream infections together at 14 days was 6.5% in the chlorhexidine group, compared with 11.1% in the control group, a difference that reached statistical significance (P = .047). Finally, the overall incidence of definite and probable central venous catheter–related bloodstream infections was 10.4% in the chlorhexidine group, compared with 17% in the control group, a difference that also reached statistical significance (P = .019).
“The chlorhexidine dressings were very well tolerated, and in contrast to previous studies we saw no increase in skin and soft tissue abnormalities or contact dermatitis,” Dr. Biehl said. The researchers also observed no significant difference in mortality between the chlorhexidine and control groups (6.2 % vs. 5.6%, respectively).
The study was supported by a grant from 3M. Dr. Biehl disclosed that she is a member of the speakers bureau for Astellas Pharma and Merck/MSD. She has received travel grants from 3M and Gilead Sciences. Another study investigator, Dr. Maria J. G. T. Vehreschild, disclosed numerous financial ties to industry.
SAN DIEGO – Chlorhexidine-containing intravenous catheter securement dressings significantly reduced the incidence of central venous catheter–related bloodstream infections in neutropenic patients, a multicenter randomized trial showed.
“Central venous catheters impose a risk of catheter-related bloodstream infections, especially when used in neutropenic patients, and the mortality risk has been reported to be up to 36%,” Dr. Lena M. Biehl said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
One way to prevent catheter-related bloodstream infections is to use chlorhexidine-containing intravenous catheter securement dressings in an effort to decrease skin colonization, said Dr. Biehl of the department of internal medicine at University Hospital of Cologne (Germany). “A few studies have looked at this, but most of them used chlorhexidine-containing sponges. There’s only one study using a more advanced chlorhexidine-containing gel pad, and this study was done in the ICU setting. The gel pad enables you to evaluate the insertion site, so you can see if there are signs of infection without removing the dressing.”
In a multicenter, randomized trial known as the COAT study that was conducted at 10 hematology departments in Germany from February 2012 to September 2014, Dr. Biehl and her associates set out to compare the incidence of central venous catheter–related bloodstream infections in two groups of neutropenic patients: those with chlorhexidine-containing IV catheter-securement dressings that included a gel pad (the chlorhexidine group) and those with conventional IV catheter-securement dressings that lacked a gel pad (the control group). They limited the analysis to patients undergoing intensive chemotherapy with expected neutropenia for at least 5 days and expected central venous catheter use of at least 10 days. The primary endpoint was the incidence of definite catheter-related bloodstream infections within 14 days of central venous catheter placement. The secondary endpoints were overall incidence of definite or probable central venous catheter–related bloodstream infections at 14 days and the overall incidence of definite or probable central venous catheter–related bloodstream infections.
Dr. Biehl presented results from 613 patients. Of these, 307 were in the chlorhexidine group and 306 were in the control group. The median age was 58 years and 59% were male, and the distribution of causative pathogens was similar between the two groups. The incidence of definite catheter-related bloodstream infections at 14 days was 2.6% in the chlorhexidine group, compared with 3.9% in the control group, a difference that did not reach statistical significance (P = .375). However, the overall incidence of definite and probable central venous catheter–related bloodstream infections together at 14 days was 6.5% in the chlorhexidine group, compared with 11.1% in the control group, a difference that reached statistical significance (P = .047). Finally, the overall incidence of definite and probable central venous catheter–related bloodstream infections was 10.4% in the chlorhexidine group, compared with 17% in the control group, a difference that also reached statistical significance (P = .019).
“The chlorhexidine dressings were very well tolerated, and in contrast to previous studies we saw no increase in skin and soft tissue abnormalities or contact dermatitis,” Dr. Biehl said. The researchers also observed no significant difference in mortality between the chlorhexidine and control groups (6.2 % vs. 5.6%, respectively).
The study was supported by a grant from 3M. Dr. Biehl disclosed that she is a member of the speakers bureau for Astellas Pharma and Merck/MSD. She has received travel grants from 3M and Gilead Sciences. Another study investigator, Dr. Maria J. G. T. Vehreschild, disclosed numerous financial ties to industry.
AT ICAAC 2015
Key clinical point: Using a chlorhexidine-containing securement dressing reduces the incidence of catheter-related bloodstream infections.
Major finding: The overall incidence of definite and probable central venous catheter–related bloodstream infections was 10.4% in the chlorhexidine group, compared with 17% in the control group, a difference that reached statistical significance (P = .019).
Data source: A randomized study of 613 neutropenic patients conducted at 10 hematology departments in Germany.
Disclosures: The study was supported by a grant from 3M. Dr. Biehl disclosed that she is a member of the speakers bureau for Astellas Pharma and Merck/MSD. She has received travel grants from 3M and Gilead Sciences. Another study investigator, Dr. Maria J. G. T. Vehreschild, disclosed numerous financial ties to industry.
VIDEO: Pediatric outcomes reassuring after cancer treatment during pregnancy
VIENNA – Children whose mothers had cancer while pregnant had similar cognitive, cardiac, and general development in early childhood as did those born to women without cancer, according to results presented at the European Cancer Congress and simultaneously published in the New England Journal of Medicine.
The case-control study found no significant differences in mental development among children exposed to chemotherapy, radiotherapy, surgery alone, or no treatment. In addition, the number of chemotherapy cycles during pregnancy, which ranged from 1 to 10, also had no impact on mental development when measured at 18 months and 3 years, Dr. Frédéric Amant, a gynecologic oncologist at University Hospitals Leuven, Belgium and at Antoni van Leeuwenhoek in Amsterdam, said during a press briefing.
Chemotherapy was given in only the second and third trimesters, he noted.
During pregnancy, 96 of the 129 children were exposed to chemotherapy alone or in combination with other treatments, 11 to radiotherapy alone or in combination with other treatments, 13 to surgery alone, 2 to other drugs therapies, and 14 to no treatment. The 129 controls were born to healthy mothers after uncomplicated pregnancies and deliveries (N Engl J Med. Sep 28, doi:10.1056/NEJMoa1508913.).
More than 60% of children with perinatal cancer exposure were born premature, but their development at a median age of 22 months was normal for their gestational age at birth.
The incidence of prematurity is high, but in most cases the children were born prematurely because of a medical decision to induce preterm so the mother could continue cancer therapy after delivery, Dr. Amant said in an interview.
Children with a birthweight below the 10th percentile were born more often to mothers with cancer during pregnancy than were children in the control group, but the difference was not significantly different (22% vs. 15.2%; P = .16).
“Overall, these data should be reassuring to women who are facing a new diagnosis of cancer during pregnancy and to their families,” Dr. Michael Greene, chief of obstetrics at Massachusetts General Hospital in Boston, and Dr. Dan Longo, an oncologist from the Dana Farber Cancer Institute, also in Boston, wrote in an accompanying editorial (N Engl J Med. Sep 28, doi:10.1056/NEJMe1512188.).
“Prudence continues to suggest avoiding cancer treatment in the first trimester, however, treatment in the second and third trimester is likely to be best for both mothers and their offspring,” they stated.
Dr. Peter Naredi, European CanCer Organization scientific cochair of the Congress, said that “while further follow-up of these children is required, the important message at this stage seems to be that doctors should not only start cancer treatment immediately, but should also try to maintain the pregnancy to as near full term as possible.”
The study was funded by the Belgian National Cancer Plan, Research Fund Flanders, Stichting tegen Kanker, Katholieke University Leuven, and Universitaire Ziekenhuizen Leuven. Dr. Amant reported having no financial disclosures. Dr. Greene and Dr. Longo reported that they are editors for the New England Journal of Medicine.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
VIENNA – Children whose mothers had cancer while pregnant had similar cognitive, cardiac, and general development in early childhood as did those born to women without cancer, according to results presented at the European Cancer Congress and simultaneously published in the New England Journal of Medicine.
The case-control study found no significant differences in mental development among children exposed to chemotherapy, radiotherapy, surgery alone, or no treatment. In addition, the number of chemotherapy cycles during pregnancy, which ranged from 1 to 10, also had no impact on mental development when measured at 18 months and 3 years, Dr. Frédéric Amant, a gynecologic oncologist at University Hospitals Leuven, Belgium and at Antoni van Leeuwenhoek in Amsterdam, said during a press briefing.
Chemotherapy was given in only the second and third trimesters, he noted.
During pregnancy, 96 of the 129 children were exposed to chemotherapy alone or in combination with other treatments, 11 to radiotherapy alone or in combination with other treatments, 13 to surgery alone, 2 to other drugs therapies, and 14 to no treatment. The 129 controls were born to healthy mothers after uncomplicated pregnancies and deliveries (N Engl J Med. Sep 28, doi:10.1056/NEJMoa1508913.).
More than 60% of children with perinatal cancer exposure were born premature, but their development at a median age of 22 months was normal for their gestational age at birth.
The incidence of prematurity is high, but in most cases the children were born prematurely because of a medical decision to induce preterm so the mother could continue cancer therapy after delivery, Dr. Amant said in an interview.
Children with a birthweight below the 10th percentile were born more often to mothers with cancer during pregnancy than were children in the control group, but the difference was not significantly different (22% vs. 15.2%; P = .16).
“Overall, these data should be reassuring to women who are facing a new diagnosis of cancer during pregnancy and to their families,” Dr. Michael Greene, chief of obstetrics at Massachusetts General Hospital in Boston, and Dr. Dan Longo, an oncologist from the Dana Farber Cancer Institute, also in Boston, wrote in an accompanying editorial (N Engl J Med. Sep 28, doi:10.1056/NEJMe1512188.).
“Prudence continues to suggest avoiding cancer treatment in the first trimester, however, treatment in the second and third trimester is likely to be best for both mothers and their offspring,” they stated.
Dr. Peter Naredi, European CanCer Organization scientific cochair of the Congress, said that “while further follow-up of these children is required, the important message at this stage seems to be that doctors should not only start cancer treatment immediately, but should also try to maintain the pregnancy to as near full term as possible.”
The study was funded by the Belgian National Cancer Plan, Research Fund Flanders, Stichting tegen Kanker, Katholieke University Leuven, and Universitaire Ziekenhuizen Leuven. Dr. Amant reported having no financial disclosures. Dr. Greene and Dr. Longo reported that they are editors for the New England Journal of Medicine.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
VIENNA – Children whose mothers had cancer while pregnant had similar cognitive, cardiac, and general development in early childhood as did those born to women without cancer, according to results presented at the European Cancer Congress and simultaneously published in the New England Journal of Medicine.
The case-control study found no significant differences in mental development among children exposed to chemotherapy, radiotherapy, surgery alone, or no treatment. In addition, the number of chemotherapy cycles during pregnancy, which ranged from 1 to 10, also had no impact on mental development when measured at 18 months and 3 years, Dr. Frédéric Amant, a gynecologic oncologist at University Hospitals Leuven, Belgium and at Antoni van Leeuwenhoek in Amsterdam, said during a press briefing.
Chemotherapy was given in only the second and third trimesters, he noted.
During pregnancy, 96 of the 129 children were exposed to chemotherapy alone or in combination with other treatments, 11 to radiotherapy alone or in combination with other treatments, 13 to surgery alone, 2 to other drugs therapies, and 14 to no treatment. The 129 controls were born to healthy mothers after uncomplicated pregnancies and deliveries (N Engl J Med. Sep 28, doi:10.1056/NEJMoa1508913.).
More than 60% of children with perinatal cancer exposure were born premature, but their development at a median age of 22 months was normal for their gestational age at birth.
The incidence of prematurity is high, but in most cases the children were born prematurely because of a medical decision to induce preterm so the mother could continue cancer therapy after delivery, Dr. Amant said in an interview.
Children with a birthweight below the 10th percentile were born more often to mothers with cancer during pregnancy than were children in the control group, but the difference was not significantly different (22% vs. 15.2%; P = .16).
“Overall, these data should be reassuring to women who are facing a new diagnosis of cancer during pregnancy and to their families,” Dr. Michael Greene, chief of obstetrics at Massachusetts General Hospital in Boston, and Dr. Dan Longo, an oncologist from the Dana Farber Cancer Institute, also in Boston, wrote in an accompanying editorial (N Engl J Med. Sep 28, doi:10.1056/NEJMe1512188.).
“Prudence continues to suggest avoiding cancer treatment in the first trimester, however, treatment in the second and third trimester is likely to be best for both mothers and their offspring,” they stated.
Dr. Peter Naredi, European CanCer Organization scientific cochair of the Congress, said that “while further follow-up of these children is required, the important message at this stage seems to be that doctors should not only start cancer treatment immediately, but should also try to maintain the pregnancy to as near full term as possible.”
The study was funded by the Belgian National Cancer Plan, Research Fund Flanders, Stichting tegen Kanker, Katholieke University Leuven, and Universitaire Ziekenhuizen Leuven. Dr. Amant reported having no financial disclosures. Dr. Greene and Dr. Longo reported that they are editors for the New England Journal of Medicine.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
AT THE EUROPEAN CANCER CONGRESS 2015
ASCO: APF530 superior to ondansetron in prevention of CINV
SAN FRANCISCO – APF530 is a long-acting 5-HT3 antagonist formulation in development for the prevention of chemotherapy-induced nausea and vomiting, and new data presented at the 2015 Breast Cancer Symposium suggest that it may have an edge over the standard regimen.
When administered with fosaprepitant and dexamethasone, it was superior to ondansetron, in combination with the same two agents, for preventing chemotherapy-induced nausea and vomiting (CINV) associated with delayed ( > 24-120 h) highly emetogenic chemotherapy (HEC).
Among the 450 patients who received the three-drug APF530 combination, 263 (58.4%) experienced a complete response, defined as having no emesis or need for rescue medication, as compared with 239/452 (53%) who received the ondansetron regimen (P= .092).
“APF530 is the first and only 5-HT3 receptor antagonist to demonstrate superiority in a phase III, three-drug versus three-drug regimen efficacy trial for the prevention of CINV in patients receiving HEC,” said Dr. Ian D. Schnadig, from Compass Oncology, The US Oncology Network, Tualatin, OR. “Managing delayed-phase CINV associated with HEC is an unmet need.”
APF530 is a novel, injectable, extended-release version of granisetron that provides sustained release over ≥ 5 days to prevent both acute (0-24 h) and delayed CINV. A large randomized double blind phase III trial has already demonstrated that this new agent is non-inferior to palonosetron in this setting.
In this double-blind, multicenter study known as the MAGIC trial, Dr. Schnadig randomized 902 patients to receive APF530 500 mg by subcutaneous injection (10 mg granisetron) or ondansetron 0.15 mg/kg IV, and the cohort was stratified by planned cisplatin therapy ( ≥ 50 mg/m2).
The primary end point was delayed-phase complete response, and secondary end points included complete response in acute and overall phases and complete control (complete response and no more than mild nausea) in acute, delayed, and overall phases.
A significantly higher percentage of patients in the APF530 cohort (65%) had delayed-phase complete response, as compared to those with ondansetron (57%) (P= .014). In addition, a significantly higher percentage (61% vs. 53%) of patients had delayed-phase complete control (P= .022).
The rates of complete response and complete control in acute and overall phases were numerically higher with APF530 compared with ondansetron, but this did not reach statistical significance.
In looking at exploratory efficacy end points, the authors observed that the proportion of patients who experienced an episode of nausea was generally higher with ondansetron than with APF530. Rates of no nausea were also numerically higher with APF530 compared with the ondansetron regimen in the delayed 49.7% vs. 44.25%; P= .099) and overall phases (45.3% vs. 44.2%; P= .138), but there were no statistically significant differences.
The regimen containing APR530 was generally well tolerated, without any new safety signals identified. The most common treatment related events were injection-site reactions, which were mostly mild or moderate. Injection site reactions were similar between the two groups, Dr. Schnadig noted.
In a discussion of the paper, Dr. Clifford Hudis reiterated that there was a higher complete response rate in late phase with APF530—“that’s an advantage and it was 8% higher in absolute terms, and it was 11% higher in the cisplatin group.”
If the drug is approved and it’s comparably priced to other agents and available, “why wouldn’t you use it?” he asked. “Its more convenient, it’s a little more effective, and its one less thing for all of us practicing to think very much about.”
SAN FRANCISCO – APF530 is a long-acting 5-HT3 antagonist formulation in development for the prevention of chemotherapy-induced nausea and vomiting, and new data presented at the 2015 Breast Cancer Symposium suggest that it may have an edge over the standard regimen.
When administered with fosaprepitant and dexamethasone, it was superior to ondansetron, in combination with the same two agents, for preventing chemotherapy-induced nausea and vomiting (CINV) associated with delayed ( > 24-120 h) highly emetogenic chemotherapy (HEC).
Among the 450 patients who received the three-drug APF530 combination, 263 (58.4%) experienced a complete response, defined as having no emesis or need for rescue medication, as compared with 239/452 (53%) who received the ondansetron regimen (P= .092).
“APF530 is the first and only 5-HT3 receptor antagonist to demonstrate superiority in a phase III, three-drug versus three-drug regimen efficacy trial for the prevention of CINV in patients receiving HEC,” said Dr. Ian D. Schnadig, from Compass Oncology, The US Oncology Network, Tualatin, OR. “Managing delayed-phase CINV associated with HEC is an unmet need.”
APF530 is a novel, injectable, extended-release version of granisetron that provides sustained release over ≥ 5 days to prevent both acute (0-24 h) and delayed CINV. A large randomized double blind phase III trial has already demonstrated that this new agent is non-inferior to palonosetron in this setting.
In this double-blind, multicenter study known as the MAGIC trial, Dr. Schnadig randomized 902 patients to receive APF530 500 mg by subcutaneous injection (10 mg granisetron) or ondansetron 0.15 mg/kg IV, and the cohort was stratified by planned cisplatin therapy ( ≥ 50 mg/m2).
The primary end point was delayed-phase complete response, and secondary end points included complete response in acute and overall phases and complete control (complete response and no more than mild nausea) in acute, delayed, and overall phases.
A significantly higher percentage of patients in the APF530 cohort (65%) had delayed-phase complete response, as compared to those with ondansetron (57%) (P= .014). In addition, a significantly higher percentage (61% vs. 53%) of patients had delayed-phase complete control (P= .022).
The rates of complete response and complete control in acute and overall phases were numerically higher with APF530 compared with ondansetron, but this did not reach statistical significance.
In looking at exploratory efficacy end points, the authors observed that the proportion of patients who experienced an episode of nausea was generally higher with ondansetron than with APF530. Rates of no nausea were also numerically higher with APF530 compared with the ondansetron regimen in the delayed 49.7% vs. 44.25%; P= .099) and overall phases (45.3% vs. 44.2%; P= .138), but there were no statistically significant differences.
The regimen containing APR530 was generally well tolerated, without any new safety signals identified. The most common treatment related events were injection-site reactions, which were mostly mild or moderate. Injection site reactions were similar between the two groups, Dr. Schnadig noted.
In a discussion of the paper, Dr. Clifford Hudis reiterated that there was a higher complete response rate in late phase with APF530—“that’s an advantage and it was 8% higher in absolute terms, and it was 11% higher in the cisplatin group.”
If the drug is approved and it’s comparably priced to other agents and available, “why wouldn’t you use it?” he asked. “Its more convenient, it’s a little more effective, and its one less thing for all of us practicing to think very much about.”
SAN FRANCISCO – APF530 is a long-acting 5-HT3 antagonist formulation in development for the prevention of chemotherapy-induced nausea and vomiting, and new data presented at the 2015 Breast Cancer Symposium suggest that it may have an edge over the standard regimen.
When administered with fosaprepitant and dexamethasone, it was superior to ondansetron, in combination with the same two agents, for preventing chemotherapy-induced nausea and vomiting (CINV) associated with delayed ( > 24-120 h) highly emetogenic chemotherapy (HEC).
Among the 450 patients who received the three-drug APF530 combination, 263 (58.4%) experienced a complete response, defined as having no emesis or need for rescue medication, as compared with 239/452 (53%) who received the ondansetron regimen (P= .092).
“APF530 is the first and only 5-HT3 receptor antagonist to demonstrate superiority in a phase III, three-drug versus three-drug regimen efficacy trial for the prevention of CINV in patients receiving HEC,” said Dr. Ian D. Schnadig, from Compass Oncology, The US Oncology Network, Tualatin, OR. “Managing delayed-phase CINV associated with HEC is an unmet need.”
APF530 is a novel, injectable, extended-release version of granisetron that provides sustained release over ≥ 5 days to prevent both acute (0-24 h) and delayed CINV. A large randomized double blind phase III trial has already demonstrated that this new agent is non-inferior to palonosetron in this setting.
In this double-blind, multicenter study known as the MAGIC trial, Dr. Schnadig randomized 902 patients to receive APF530 500 mg by subcutaneous injection (10 mg granisetron) or ondansetron 0.15 mg/kg IV, and the cohort was stratified by planned cisplatin therapy ( ≥ 50 mg/m2).
The primary end point was delayed-phase complete response, and secondary end points included complete response in acute and overall phases and complete control (complete response and no more than mild nausea) in acute, delayed, and overall phases.
A significantly higher percentage of patients in the APF530 cohort (65%) had delayed-phase complete response, as compared to those with ondansetron (57%) (P= .014). In addition, a significantly higher percentage (61% vs. 53%) of patients had delayed-phase complete control (P= .022).
The rates of complete response and complete control in acute and overall phases were numerically higher with APF530 compared with ondansetron, but this did not reach statistical significance.
In looking at exploratory efficacy end points, the authors observed that the proportion of patients who experienced an episode of nausea was generally higher with ondansetron than with APF530. Rates of no nausea were also numerically higher with APF530 compared with the ondansetron regimen in the delayed 49.7% vs. 44.25%; P= .099) and overall phases (45.3% vs. 44.2%; P= .138), but there were no statistically significant differences.
The regimen containing APR530 was generally well tolerated, without any new safety signals identified. The most common treatment related events were injection-site reactions, which were mostly mild or moderate. Injection site reactions were similar between the two groups, Dr. Schnadig noted.
In a discussion of the paper, Dr. Clifford Hudis reiterated that there was a higher complete response rate in late phase with APF530—“that’s an advantage and it was 8% higher in absolute terms, and it was 11% higher in the cisplatin group.”
If the drug is approved and it’s comparably priced to other agents and available, “why wouldn’t you use it?” he asked. “Its more convenient, it’s a little more effective, and its one less thing for all of us practicing to think very much about.”
FROM THE ASCO BREAST CANCER SYMPOSIUM 2015
Key clinical point:APF530 combined with fosaprepitant and dexamethasone was superior to ondansetron combined with the same agents in the prevention of chemotherapy induced vomiting.
Major finding: A significantly higher percentage of patients in the APF530 cohort (65%) had delayed-phase complete response, as compared to those with on-dansetron (57%) (P= .014), the primary endpoint of the study.
Data source: A phase III randomized trial that included 902 patients.
Disclosures: Research support for the study was provided by Heron Therapeutics. Dr. Schnadig is employed by Compass Oncology and reports relationships with HERON, Tesaro, and McKesson. Several of the co-authors also have disclosures with industry.
Generalizations of a generalist — common themes among systemic therapies for common cancers
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Effects of a self-care education program on quality of life of patients with gastric cancer after gastrectomy
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
A qualitative exploration of supports and unmet needs of diverse young women with breast cancer
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Changing oncology compliance standards: step 1 in re-valuing clinician workload for value-based cancer care
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
New assay may be a game changer in invasive candidiasis
SAN DIEGO – The T2 magnetic resonance assay for rapid diagnosis or rule-out of invasive candidiasis has the potential to significantly change the management and outcome of this common, deadly, and expensive disease, Dr. Peter G. Pappas asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This novel diagnostic instrument addresses a longstanding major unmet need in the field of infectious diseases: namely, the necessity for a substantially faster and more accurate test for invasive candidiasis than the decades-old current standard, which is automated blood cultures.
Blood cultures are notoriously insensitive. Indeed, they are negative in roughly 50% of patients with invasive candidiasis, mainly those with deep-seated, noncandidemic invasive candidiasis. And blood cultures are far too slow, taking 2-5 days to finalize results, explained Dr. Pappas, professor of medicine at the University of Alabama, Birmingham. “Management of invasive candidiasis involves time-critical decision making. The earlier we can approach the patient with specific therapy, the better the outcomes. That actually hasn’t been shown prospectively, but it’s a reasonable assumption based upon the available retrospective studies. We would like to be able to initiate effective treatment within 12-24 hours; that’s seldom possible with blood cultures,” he continued.
Dr. Pappas was principal investigator in the direct T2 pivotal clinical trial which led to Food and Drug Administration approval of the T2 magnetic resonance assay, known as the T2Candida platform. In this 1,801-patient multicenter study, the assay provided results in a mean of just over 4 hours with 91.4% sensitivity, 99.4% specificity, and a negative predictive value of 99.2%. In contrast, blood cultures, which were obtained in all participants, required an average of more than 120 hours to provide results (Clin Infect Dis. 2015 Mar 15;60[6]:892-9. doi: 10.1093/cid/ciu959).
At ICAAC 2015, the clinical trial was named one of the top 10 papers of the year in mycology.
Invasive candidiasis is a huge problem that’s seen little in the way of progress over the past 2 decades. Candida infections account for 6% of all hospital-acquired infections in the United States. More than 400,000 cases of invasive candidiasis occur annually worldwide. Attributable mortality rates of up to 49% have been reported. The disease is an important cause of prolonged hospitalization, with episodes adding an average of about $40,000 to the cost of a hospital stay.
The T2Candida test not only enables physicians to get effective antifungal agents started quickly, but a negative result will allow a drastic cutback in the now-routine use of empiric antifungal therapy prescribed during the lengthy wait for blood culture results. This will reduce needless exposure to drug side effects among uninfected patients, discourage the rise of resistant Candida strains, and substantially reduce health care costs.
Extrapolating from this trial’s data, and from other studies, Dr. Pappas said “the sweet spot” for the assay, where it has an impressively high 75%-85% positive predictive value, occurs when it is applied to patients with a pretest probability of invasive candidiasis in the 3%-10% range based upon well-known high-risk factors, including current cancer, neutropenia, organ or stem cell transplantation, having a central venous catheter, or being on steroid therapy.
The new assay bypasses blood cultures entirely, instead employing molecular diagnostics to directly analyze a whole blood sample. It can identify C. albicans and four other clinically relevant Candida species which collectively account for the vast majority of cases of invasive candidiasis. One of the reasons panelists at ICAAC 2015 named the T2Candida pivotal trial to their top-10 list of major papers in mycology is that the T2 magnetic resonance technology is a platform capable of also being applied to the diagnosis of other pathogens whose prompt diagnosis is critical.
Another advantage of the T2Candida platform is that the results are unaffected by antifungal therapy. In contrast, blood cultures become unreliable if a patient has empiric antifungal therapy onboard. In a separate presentation at ICAAC 2015, Dr. Pappas and coworkers presented interim results from an ongoing study that capitalizes on this advantage of the new technology.
To date, the study has enrolled 23 patients with culture-proven candidemia, all of whom underwent daily testing via both blood cultures and T2Candida during their first 7 days on antifungal therapy. Blood cultures remained positive for only two patients on-treatment, whereas T2Candida remained positive for nine patients on all 7 days and also detected one new case of intra-abdominal candidiasis missed by blood cultures.
Thus, the T2Candida platform may be an effective method not only for diagnosis of invasive candidiasis, Dr. Pappas observed, but for monitoring the response to therapy in the form of antifungal agents and/or removal of an offending contaminated catheter.
Dr. Pappas reported receiving research grants from and serving as an advisor to T2 Biosystems, which markets the assay. He has also received research support from Astellas, Gilead, and Merck.
SAN DIEGO – The T2 magnetic resonance assay for rapid diagnosis or rule-out of invasive candidiasis has the potential to significantly change the management and outcome of this common, deadly, and expensive disease, Dr. Peter G. Pappas asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This novel diagnostic instrument addresses a longstanding major unmet need in the field of infectious diseases: namely, the necessity for a substantially faster and more accurate test for invasive candidiasis than the decades-old current standard, which is automated blood cultures.
Blood cultures are notoriously insensitive. Indeed, they are negative in roughly 50% of patients with invasive candidiasis, mainly those with deep-seated, noncandidemic invasive candidiasis. And blood cultures are far too slow, taking 2-5 days to finalize results, explained Dr. Pappas, professor of medicine at the University of Alabama, Birmingham. “Management of invasive candidiasis involves time-critical decision making. The earlier we can approach the patient with specific therapy, the better the outcomes. That actually hasn’t been shown prospectively, but it’s a reasonable assumption based upon the available retrospective studies. We would like to be able to initiate effective treatment within 12-24 hours; that’s seldom possible with blood cultures,” he continued.
Dr. Pappas was principal investigator in the direct T2 pivotal clinical trial which led to Food and Drug Administration approval of the T2 magnetic resonance assay, known as the T2Candida platform. In this 1,801-patient multicenter study, the assay provided results in a mean of just over 4 hours with 91.4% sensitivity, 99.4% specificity, and a negative predictive value of 99.2%. In contrast, blood cultures, which were obtained in all participants, required an average of more than 120 hours to provide results (Clin Infect Dis. 2015 Mar 15;60[6]:892-9. doi: 10.1093/cid/ciu959).
At ICAAC 2015, the clinical trial was named one of the top 10 papers of the year in mycology.
Invasive candidiasis is a huge problem that’s seen little in the way of progress over the past 2 decades. Candida infections account for 6% of all hospital-acquired infections in the United States. More than 400,000 cases of invasive candidiasis occur annually worldwide. Attributable mortality rates of up to 49% have been reported. The disease is an important cause of prolonged hospitalization, with episodes adding an average of about $40,000 to the cost of a hospital stay.
The T2Candida test not only enables physicians to get effective antifungal agents started quickly, but a negative result will allow a drastic cutback in the now-routine use of empiric antifungal therapy prescribed during the lengthy wait for blood culture results. This will reduce needless exposure to drug side effects among uninfected patients, discourage the rise of resistant Candida strains, and substantially reduce health care costs.
Extrapolating from this trial’s data, and from other studies, Dr. Pappas said “the sweet spot” for the assay, where it has an impressively high 75%-85% positive predictive value, occurs when it is applied to patients with a pretest probability of invasive candidiasis in the 3%-10% range based upon well-known high-risk factors, including current cancer, neutropenia, organ or stem cell transplantation, having a central venous catheter, or being on steroid therapy.
The new assay bypasses blood cultures entirely, instead employing molecular diagnostics to directly analyze a whole blood sample. It can identify C. albicans and four other clinically relevant Candida species which collectively account for the vast majority of cases of invasive candidiasis. One of the reasons panelists at ICAAC 2015 named the T2Candida pivotal trial to their top-10 list of major papers in mycology is that the T2 magnetic resonance technology is a platform capable of also being applied to the diagnosis of other pathogens whose prompt diagnosis is critical.
Another advantage of the T2Candida platform is that the results are unaffected by antifungal therapy. In contrast, blood cultures become unreliable if a patient has empiric antifungal therapy onboard. In a separate presentation at ICAAC 2015, Dr. Pappas and coworkers presented interim results from an ongoing study that capitalizes on this advantage of the new technology.
To date, the study has enrolled 23 patients with culture-proven candidemia, all of whom underwent daily testing via both blood cultures and T2Candida during their first 7 days on antifungal therapy. Blood cultures remained positive for only two patients on-treatment, whereas T2Candida remained positive for nine patients on all 7 days and also detected one new case of intra-abdominal candidiasis missed by blood cultures.
Thus, the T2Candida platform may be an effective method not only for diagnosis of invasive candidiasis, Dr. Pappas observed, but for monitoring the response to therapy in the form of antifungal agents and/or removal of an offending contaminated catheter.
Dr. Pappas reported receiving research grants from and serving as an advisor to T2 Biosystems, which markets the assay. He has also received research support from Astellas, Gilead, and Merck.
SAN DIEGO – The T2 magnetic resonance assay for rapid diagnosis or rule-out of invasive candidiasis has the potential to significantly change the management and outcome of this common, deadly, and expensive disease, Dr. Peter G. Pappas asserted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
This novel diagnostic instrument addresses a longstanding major unmet need in the field of infectious diseases: namely, the necessity for a substantially faster and more accurate test for invasive candidiasis than the decades-old current standard, which is automated blood cultures.
Blood cultures are notoriously insensitive. Indeed, they are negative in roughly 50% of patients with invasive candidiasis, mainly those with deep-seated, noncandidemic invasive candidiasis. And blood cultures are far too slow, taking 2-5 days to finalize results, explained Dr. Pappas, professor of medicine at the University of Alabama, Birmingham. “Management of invasive candidiasis involves time-critical decision making. The earlier we can approach the patient with specific therapy, the better the outcomes. That actually hasn’t been shown prospectively, but it’s a reasonable assumption based upon the available retrospective studies. We would like to be able to initiate effective treatment within 12-24 hours; that’s seldom possible with blood cultures,” he continued.
Dr. Pappas was principal investigator in the direct T2 pivotal clinical trial which led to Food and Drug Administration approval of the T2 magnetic resonance assay, known as the T2Candida platform. In this 1,801-patient multicenter study, the assay provided results in a mean of just over 4 hours with 91.4% sensitivity, 99.4% specificity, and a negative predictive value of 99.2%. In contrast, blood cultures, which were obtained in all participants, required an average of more than 120 hours to provide results (Clin Infect Dis. 2015 Mar 15;60[6]:892-9. doi: 10.1093/cid/ciu959).
At ICAAC 2015, the clinical trial was named one of the top 10 papers of the year in mycology.
Invasive candidiasis is a huge problem that’s seen little in the way of progress over the past 2 decades. Candida infections account for 6% of all hospital-acquired infections in the United States. More than 400,000 cases of invasive candidiasis occur annually worldwide. Attributable mortality rates of up to 49% have been reported. The disease is an important cause of prolonged hospitalization, with episodes adding an average of about $40,000 to the cost of a hospital stay.
The T2Candida test not only enables physicians to get effective antifungal agents started quickly, but a negative result will allow a drastic cutback in the now-routine use of empiric antifungal therapy prescribed during the lengthy wait for blood culture results. This will reduce needless exposure to drug side effects among uninfected patients, discourage the rise of resistant Candida strains, and substantially reduce health care costs.
Extrapolating from this trial’s data, and from other studies, Dr. Pappas said “the sweet spot” for the assay, where it has an impressively high 75%-85% positive predictive value, occurs when it is applied to patients with a pretest probability of invasive candidiasis in the 3%-10% range based upon well-known high-risk factors, including current cancer, neutropenia, organ or stem cell transplantation, having a central venous catheter, or being on steroid therapy.
The new assay bypasses blood cultures entirely, instead employing molecular diagnostics to directly analyze a whole blood sample. It can identify C. albicans and four other clinically relevant Candida species which collectively account for the vast majority of cases of invasive candidiasis. One of the reasons panelists at ICAAC 2015 named the T2Candida pivotal trial to their top-10 list of major papers in mycology is that the T2 magnetic resonance technology is a platform capable of also being applied to the diagnosis of other pathogens whose prompt diagnosis is critical.
Another advantage of the T2Candida platform is that the results are unaffected by antifungal therapy. In contrast, blood cultures become unreliable if a patient has empiric antifungal therapy onboard. In a separate presentation at ICAAC 2015, Dr. Pappas and coworkers presented interim results from an ongoing study that capitalizes on this advantage of the new technology.
To date, the study has enrolled 23 patients with culture-proven candidemia, all of whom underwent daily testing via both blood cultures and T2Candida during their first 7 days on antifungal therapy. Blood cultures remained positive for only two patients on-treatment, whereas T2Candida remained positive for nine patients on all 7 days and also detected one new case of intra-abdominal candidiasis missed by blood cultures.
Thus, the T2Candida platform may be an effective method not only for diagnosis of invasive candidiasis, Dr. Pappas observed, but for monitoring the response to therapy in the form of antifungal agents and/or removal of an offending contaminated catheter.
Dr. Pappas reported receiving research grants from and serving as an advisor to T2 Biosystems, which markets the assay. He has also received research support from Astellas, Gilead, and Merck.
EXPERT ANALYSIS FROM ICAAC 2015










