User login
Improving cancer care through modern portfolio theory
We struggle daily to improve cancer care – to improve our therapeutic outcomes in cancer – as individual physicians and as researchers. We work collectively to disseminate information and collaborate, and there are welcome calls for open data sharing to accelerate progress.1 We enroll patients on clinical trials, or we work in a basic science lab to discover mechanisms of carcinogenesis and potential therapeutic targets. We discuss “n of 1” trials and the “paradigm shift of precision oncology,” and we are optimistic about the future of cancer care.
Leaving the world of biology and clinical trials for a minute, we also can apply economic theory in our never-ending quest to improve cancer outcomes. One area of interest may be modern portfolio theory (MPT), which the economist Harry Markowitz introduced in an essay in 1952 and later won the Nobel Prize for his work.
At least 71 billionaires live in the San Francisco Bay Area, where I live, but 14,000 children (13%) in the area live below the poverty line.3 When there is a range of asset allocations in health care, results can vary not on the basis of the underlying disease state or the quality of the provider, but on access to care. As an example, most pediatric cancers are curable, yet a recent retrospective analysis of data in the SEER-Medicare registry showed that mortality within 1 month of diagnosis of childhood cancer related in part to socioeconomic factors – those patients with a lower socioeconomic status (which correlates with being an ethnic minority in the United States) were more likely to die within a month of diagnosis of their cancer than were patients with a higher socioeconomic status.3 Here is where MPT can transform the cancer outcomes landscape at no additional investment in basic science or costly precision medicine5: by triaging these patients according to their disease state rather than their ability to pay, they could be administered curative chemotherapy, placed on the appropriate clinical trial, and be cured of their cancer like other children of higher socioeconomic status.
My colleagues and I observed a similar trend when we looked at treatment of diffuse large-cell non-Hodgkin lymphoma in Medicare recipients.6 Although the cure rate is as high as 60%-80% with the use of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or R (rituxin)-CHOP chemotherapy, we found that many patients had received suboptimal chemotherapy. Upon closer examination, we found that there were variations in care by socioeconomic status even in a single-payer system. Thus aspects of cultural literacy and additional efforts for triage need to be developed, but again, application of MPT could be instrumental in improving cancer cure rates by reducing disparities in care by allocating assets to solve access-to-care issues, and curing these patients of their non-Hodgkin lymphoma.
A physician at a Bay Area health care system notes that the open slots in his schedule are triaged by his employer by the patient’s ability to pay – well-insured patients are seen within a few days, but there are very few slots for Medicaid patients, who have to wait weeks or longer to be seen. During this time, their malignancies have time to grow, and potentially metastasize. This may provide suboptimal outcomes for some patients in his community.
We solved this problem at a local hospital where all patients were on Medicaid or uninsured. We triaged patients according to severity of illness, with patients with rapidly growing cancers, particularly curable ones, were brought in as soon as possible and patients with stable benign hematologic conditions seen on a less urgent basis. A social worker and I saw patients together. She would find them resources such as transportation, food, copay assistance to help them through their treatment, and I would optimize their cancer care clinically. On a small scale, this application of MPT (or asset allocation) worked quite well. Perhaps it can be reproduced on a much larger scale. Return on investment relates largely to how you allocate your assets. What’s nice about these applications of MPT is that the return on investment – increasing the cure rate of cancer - is quite large for just a minimal change in asset allocation.
1. Bertagnolli M, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. 2017;376(12):1178-1181.
2. Baum M. Justice. In: The scepticaemic surgeon: how not to win friends and influence people. New York: Nova Science Pubkishers; November 30, 2014.
3. Glaeser E. Gentfrification and its discontents. Wall Street Journal. May 5, 2017.
4. Green AL, Furutani E, Riberio KB, Galindo CR. Death within 1 month of diagnosis in childhood cancer : analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320-1327.
5. McCartney M. Are we too captivated by precision medicine? http://www.bmj.com/content/356/bmj.j1168.long. Published March 9, 2017. Accessed May 12, 2017.
6. Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995801/. Published November 12, 2010. Accessed May 12, 2017.
We struggle daily to improve cancer care – to improve our therapeutic outcomes in cancer – as individual physicians and as researchers. We work collectively to disseminate information and collaborate, and there are welcome calls for open data sharing to accelerate progress.1 We enroll patients on clinical trials, or we work in a basic science lab to discover mechanisms of carcinogenesis and potential therapeutic targets. We discuss “n of 1” trials and the “paradigm shift of precision oncology,” and we are optimistic about the future of cancer care.
Leaving the world of biology and clinical trials for a minute, we also can apply economic theory in our never-ending quest to improve cancer outcomes. One area of interest may be modern portfolio theory (MPT), which the economist Harry Markowitz introduced in an essay in 1952 and later won the Nobel Prize for his work.
At least 71 billionaires live in the San Francisco Bay Area, where I live, but 14,000 children (13%) in the area live below the poverty line.3 When there is a range of asset allocations in health care, results can vary not on the basis of the underlying disease state or the quality of the provider, but on access to care. As an example, most pediatric cancers are curable, yet a recent retrospective analysis of data in the SEER-Medicare registry showed that mortality within 1 month of diagnosis of childhood cancer related in part to socioeconomic factors – those patients with a lower socioeconomic status (which correlates with being an ethnic minority in the United States) were more likely to die within a month of diagnosis of their cancer than were patients with a higher socioeconomic status.3 Here is where MPT can transform the cancer outcomes landscape at no additional investment in basic science or costly precision medicine5: by triaging these patients according to their disease state rather than their ability to pay, they could be administered curative chemotherapy, placed on the appropriate clinical trial, and be cured of their cancer like other children of higher socioeconomic status.
My colleagues and I observed a similar trend when we looked at treatment of diffuse large-cell non-Hodgkin lymphoma in Medicare recipients.6 Although the cure rate is as high as 60%-80% with the use of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or R (rituxin)-CHOP chemotherapy, we found that many patients had received suboptimal chemotherapy. Upon closer examination, we found that there were variations in care by socioeconomic status even in a single-payer system. Thus aspects of cultural literacy and additional efforts for triage need to be developed, but again, application of MPT could be instrumental in improving cancer cure rates by reducing disparities in care by allocating assets to solve access-to-care issues, and curing these patients of their non-Hodgkin lymphoma.
A physician at a Bay Area health care system notes that the open slots in his schedule are triaged by his employer by the patient’s ability to pay – well-insured patients are seen within a few days, but there are very few slots for Medicaid patients, who have to wait weeks or longer to be seen. During this time, their malignancies have time to grow, and potentially metastasize. This may provide suboptimal outcomes for some patients in his community.
We solved this problem at a local hospital where all patients were on Medicaid or uninsured. We triaged patients according to severity of illness, with patients with rapidly growing cancers, particularly curable ones, were brought in as soon as possible and patients with stable benign hematologic conditions seen on a less urgent basis. A social worker and I saw patients together. She would find them resources such as transportation, food, copay assistance to help them through their treatment, and I would optimize their cancer care clinically. On a small scale, this application of MPT (or asset allocation) worked quite well. Perhaps it can be reproduced on a much larger scale. Return on investment relates largely to how you allocate your assets. What’s nice about these applications of MPT is that the return on investment – increasing the cure rate of cancer - is quite large for just a minimal change in asset allocation.
We struggle daily to improve cancer care – to improve our therapeutic outcomes in cancer – as individual physicians and as researchers. We work collectively to disseminate information and collaborate, and there are welcome calls for open data sharing to accelerate progress.1 We enroll patients on clinical trials, or we work in a basic science lab to discover mechanisms of carcinogenesis and potential therapeutic targets. We discuss “n of 1” trials and the “paradigm shift of precision oncology,” and we are optimistic about the future of cancer care.
Leaving the world of biology and clinical trials for a minute, we also can apply economic theory in our never-ending quest to improve cancer outcomes. One area of interest may be modern portfolio theory (MPT), which the economist Harry Markowitz introduced in an essay in 1952 and later won the Nobel Prize for his work.
At least 71 billionaires live in the San Francisco Bay Area, where I live, but 14,000 children (13%) in the area live below the poverty line.3 When there is a range of asset allocations in health care, results can vary not on the basis of the underlying disease state or the quality of the provider, but on access to care. As an example, most pediatric cancers are curable, yet a recent retrospective analysis of data in the SEER-Medicare registry showed that mortality within 1 month of diagnosis of childhood cancer related in part to socioeconomic factors – those patients with a lower socioeconomic status (which correlates with being an ethnic minority in the United States) were more likely to die within a month of diagnosis of their cancer than were patients with a higher socioeconomic status.3 Here is where MPT can transform the cancer outcomes landscape at no additional investment in basic science or costly precision medicine5: by triaging these patients according to their disease state rather than their ability to pay, they could be administered curative chemotherapy, placed on the appropriate clinical trial, and be cured of their cancer like other children of higher socioeconomic status.
My colleagues and I observed a similar trend when we looked at treatment of diffuse large-cell non-Hodgkin lymphoma in Medicare recipients.6 Although the cure rate is as high as 60%-80% with the use of CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or R (rituxin)-CHOP chemotherapy, we found that many patients had received suboptimal chemotherapy. Upon closer examination, we found that there were variations in care by socioeconomic status even in a single-payer system. Thus aspects of cultural literacy and additional efforts for triage need to be developed, but again, application of MPT could be instrumental in improving cancer cure rates by reducing disparities in care by allocating assets to solve access-to-care issues, and curing these patients of their non-Hodgkin lymphoma.
A physician at a Bay Area health care system notes that the open slots in his schedule are triaged by his employer by the patient’s ability to pay – well-insured patients are seen within a few days, but there are very few slots for Medicaid patients, who have to wait weeks or longer to be seen. During this time, their malignancies have time to grow, and potentially metastasize. This may provide suboptimal outcomes for some patients in his community.
We solved this problem at a local hospital where all patients were on Medicaid or uninsured. We triaged patients according to severity of illness, with patients with rapidly growing cancers, particularly curable ones, were brought in as soon as possible and patients with stable benign hematologic conditions seen on a less urgent basis. A social worker and I saw patients together. She would find them resources such as transportation, food, copay assistance to help them through their treatment, and I would optimize their cancer care clinically. On a small scale, this application of MPT (or asset allocation) worked quite well. Perhaps it can be reproduced on a much larger scale. Return on investment relates largely to how you allocate your assets. What’s nice about these applications of MPT is that the return on investment – increasing the cure rate of cancer - is quite large for just a minimal change in asset allocation.
1. Bertagnolli M, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. 2017;376(12):1178-1181.
2. Baum M. Justice. In: The scepticaemic surgeon: how not to win friends and influence people. New York: Nova Science Pubkishers; November 30, 2014.
3. Glaeser E. Gentfrification and its discontents. Wall Street Journal. May 5, 2017.
4. Green AL, Furutani E, Riberio KB, Galindo CR. Death within 1 month of diagnosis in childhood cancer : analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320-1327.
5. McCartney M. Are we too captivated by precision medicine? http://www.bmj.com/content/356/bmj.j1168.long. Published March 9, 2017. Accessed May 12, 2017.
6. Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995801/. Published November 12, 2010. Accessed May 12, 2017.
1. Bertagnolli M, Sartor O, Chabner BA, et al. Advantages of a truly open-access data-sharing model. N Engl J Med. 2017;376(12):1178-1181.
2. Baum M. Justice. In: The scepticaemic surgeon: how not to win friends and influence people. New York: Nova Science Pubkishers; November 30, 2014.
3. Glaeser E. Gentfrification and its discontents. Wall Street Journal. May 5, 2017.
4. Green AL, Furutani E, Riberio KB, Galindo CR. Death within 1 month of diagnosis in childhood cancer : analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320-1327.
5. McCartney M. Are we too captivated by precision medicine? http://www.bmj.com/content/356/bmj.j1168.long. Published March 9, 2017. Accessed May 12, 2017.
6. Griffiths R, Gleeson M, Knopf K, Danese M. Racial differences in treatment and survival in older patients with diffuse large B-cell lymphoma. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2995801/. Published November 12, 2010. Accessed May 12, 2017.
Metastatic Kaposi sarcoma with osseous involvement in a patient with AIDS
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
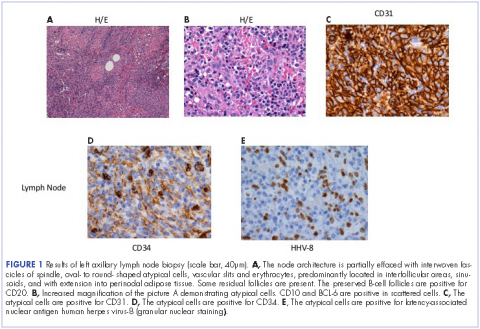
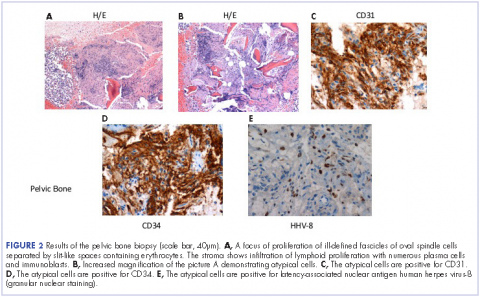
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
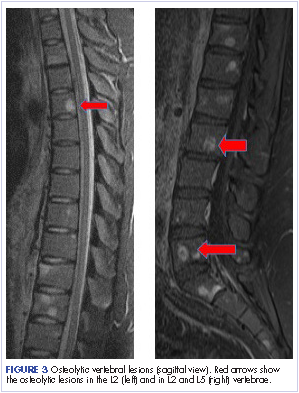
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
A rare case of hypoglycemia induced by a classic gastrointestinal stromal tumor
Hypoglycemia, a frequently encountered medical emergency, is usually seen in patients with diabetes, most commonly as a result of iatrogenesis. However, it can also be encountered in nondiabetic patients. Various causes, such as pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma and hypothyroidism, have been found to contribute to the condition in the nondiabetic population.1 In rare cases, an excessive production of insulin-like growth factor (IGF-2) – a condition known as nonislet cell tumor-induced hypoglycemia (NICTH) – has also been found to cause hypoglycemia. Hypoinsulinemic hypoglycemia, with low IGF-1 levels and an IGF-2-IgF1 ratio of greater than 10, is found to be suggestive of NICTH.
Case presentation and summary
An 81-year-old man with a history of diabetes mellitus, systolic heart failure, chronic kidney disease, and metastatic classical gastrointestinal spindle cell sarcoma presented to the emergency department with an acute change in mental status resulting from a new onset hypoglycemia. He was admitted, and during his hospital stay, he experienced severe hypoglycemic episodes with symptomatic presentations of diaphoresis on multiple occasions. A detailed history revealed that for diabetes, the patient had been on insulin for the first 12 years after his diagnosis, after which he was switched to metformin 500 mg twice daily for about 2 years, and as a satisfactory glycemic control was attained, eventually metformin had also been stopped 3 years prior to the current presentation.
The patient’s past medical records were obtained from the hospital at which he had been diagnosed gastrointestinal spindle cell sarcoma. Patient had not received treatment for the cancer as the disease was too widespread to be treated. The gastrointestinal spindle cell sarcoma, which had initially been surgically resected 7 years before the current presentation, had a recurrence 3 years later with abdominal and pulmonary metastasis, but no liver metastasis. No further intervention was carried out because the widely metastasized disease would not have benefited from any more surgical intervention and chemotherapy was not initiated because of the patient’s comorbid illnesses.
A blood sample drawn from the patient at the time of one hypoglycemic event, revealed low serum insulin <0.1 U/ml (normal, 2-19.6 U/ml); low C-peptide level, 0.59 ng/ml (0.8-3.85 ng/ml); low IGF-1, 16 ng/ml (5-4 ng/ml); and IGF-3, 0.9 ng/ml (2.2-4.5 ng/ml). IGF-2 levels were found to be markedly elevated at 945 ng/ml (47-350 ng/ml). The calculated IGF-2-IGF-1 ratio was 59.06 (normal, <10), suggesting NICTH as the etiology for the patient’s hypoglycemia.
The hypoglycemic episodes were initially treated with a continuous dextrose infusion followed by diazoxide treatment. However, diazoxide did not prevent his hypoglycemic episodes, so dexamethasone was considered as an alternative for his condition. The dexamethasone treatment resulted in the normalization of the patient’s serum glucose levels and resolution of his symptoms. The patient was discharged in a satisfactory state few days later and followed up thereafter. No recurrence of hypoglycemic episodes was found, and he was continued on dexamethasone therapy.
Discussion
Hypoglycemia due to NICTH is rare, with a prevalence of four times less than that of insulinoma.3 In most cases, NICTH occurs in patients with solid tumors of mesenchymal and epithelial origins such as hepatocellular carcinoma, gastric carcinoma or mesothelioma.4 In NICTH, the serum levels of insulin, C-peptide, and IGF-1 are usually decreased or undetectable. However, the circulating levels of total IGF2 may be increased, decreased, or normal. Concurrent normal to high morning cortisol and normal response to cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH. An IGF-2: IGF-1 ratio of >10 is considered to be clinically significant and highly suggestive of NICTH.5 Hypoglycemia in NICTH can be managed by administration of oral glucose, intravenous dextrose or glucagon. In some cases, diazoxide, a potent inhibitor of insulin secretion, has been found to be useful.6 Diazoxide directly inhibits the release of insulin through stimulation of adrenergic receptors and also has an extra pancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis.
Glucocorticoid therapy has been shown to suppress IGF-2 in a dose dependent manner and also by increasing gluconeogenesis.7 Surgical resection of the tumor whenever possible is the treatment of choice followed by radiotherapy and chemotherapy for inoperable disease and if successful, usually results in resolution of hypoglycemia. Imatinib, is the chemotherapeutic drug of choice for metastatic GIST, but many case reports have suggested worsening of hypoglycemia in advanced GIST with the use of the drug.8 The patient described in our report was not on any chemotherapy, hence hypoglycemia could not be attributed to it. On the basis of findings among 24 patients with GIST, Rikhof and colleagues have recommended monitoring plasma levels of pro-IGF-IIE to identify patients at high risk for developing hypoglycemia, especially those with progressive disease.9 Furthermore, over expression of IGF-2 as a predictor of potential relapse may be an area for potential research and further study.10
1. Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91.
2. Dutta P, Aggarwal A, Gogate Y, Nahar U, Shah VN, Singla M. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130046
3. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-93.
4. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor II producing non-islet-cell tumor hypoglycemia
5. Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998;5:111-129.
6. Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758.
7. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol .1998;49:491-498.
8. Hamberg P, De Jong FA, Boonstra JG, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30-e31.
9. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582-1588.
10. Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298.
Hypoglycemia, a frequently encountered medical emergency, is usually seen in patients with diabetes, most commonly as a result of iatrogenesis. However, it can also be encountered in nondiabetic patients. Various causes, such as pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma and hypothyroidism, have been found to contribute to the condition in the nondiabetic population.1 In rare cases, an excessive production of insulin-like growth factor (IGF-2) – a condition known as nonislet cell tumor-induced hypoglycemia (NICTH) – has also been found to cause hypoglycemia. Hypoinsulinemic hypoglycemia, with low IGF-1 levels and an IGF-2-IgF1 ratio of greater than 10, is found to be suggestive of NICTH.
Case presentation and summary
An 81-year-old man with a history of diabetes mellitus, systolic heart failure, chronic kidney disease, and metastatic classical gastrointestinal spindle cell sarcoma presented to the emergency department with an acute change in mental status resulting from a new onset hypoglycemia. He was admitted, and during his hospital stay, he experienced severe hypoglycemic episodes with symptomatic presentations of diaphoresis on multiple occasions. A detailed history revealed that for diabetes, the patient had been on insulin for the first 12 years after his diagnosis, after which he was switched to metformin 500 mg twice daily for about 2 years, and as a satisfactory glycemic control was attained, eventually metformin had also been stopped 3 years prior to the current presentation.
The patient’s past medical records were obtained from the hospital at which he had been diagnosed gastrointestinal spindle cell sarcoma. Patient had not received treatment for the cancer as the disease was too widespread to be treated. The gastrointestinal spindle cell sarcoma, which had initially been surgically resected 7 years before the current presentation, had a recurrence 3 years later with abdominal and pulmonary metastasis, but no liver metastasis. No further intervention was carried out because the widely metastasized disease would not have benefited from any more surgical intervention and chemotherapy was not initiated because of the patient’s comorbid illnesses.
A blood sample drawn from the patient at the time of one hypoglycemic event, revealed low serum insulin <0.1 U/ml (normal, 2-19.6 U/ml); low C-peptide level, 0.59 ng/ml (0.8-3.85 ng/ml); low IGF-1, 16 ng/ml (5-4 ng/ml); and IGF-3, 0.9 ng/ml (2.2-4.5 ng/ml). IGF-2 levels were found to be markedly elevated at 945 ng/ml (47-350 ng/ml). The calculated IGF-2-IGF-1 ratio was 59.06 (normal, <10), suggesting NICTH as the etiology for the patient’s hypoglycemia.
The hypoglycemic episodes were initially treated with a continuous dextrose infusion followed by diazoxide treatment. However, diazoxide did not prevent his hypoglycemic episodes, so dexamethasone was considered as an alternative for his condition. The dexamethasone treatment resulted in the normalization of the patient’s serum glucose levels and resolution of his symptoms. The patient was discharged in a satisfactory state few days later and followed up thereafter. No recurrence of hypoglycemic episodes was found, and he was continued on dexamethasone therapy.
Discussion
Hypoglycemia due to NICTH is rare, with a prevalence of four times less than that of insulinoma.3 In most cases, NICTH occurs in patients with solid tumors of mesenchymal and epithelial origins such as hepatocellular carcinoma, gastric carcinoma or mesothelioma.4 In NICTH, the serum levels of insulin, C-peptide, and IGF-1 are usually decreased or undetectable. However, the circulating levels of total IGF2 may be increased, decreased, or normal. Concurrent normal to high morning cortisol and normal response to cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH. An IGF-2: IGF-1 ratio of >10 is considered to be clinically significant and highly suggestive of NICTH.5 Hypoglycemia in NICTH can be managed by administration of oral glucose, intravenous dextrose or glucagon. In some cases, diazoxide, a potent inhibitor of insulin secretion, has been found to be useful.6 Diazoxide directly inhibits the release of insulin through stimulation of adrenergic receptors and also has an extra pancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis.
Glucocorticoid therapy has been shown to suppress IGF-2 in a dose dependent manner and also by increasing gluconeogenesis.7 Surgical resection of the tumor whenever possible is the treatment of choice followed by radiotherapy and chemotherapy for inoperable disease and if successful, usually results in resolution of hypoglycemia. Imatinib, is the chemotherapeutic drug of choice for metastatic GIST, but many case reports have suggested worsening of hypoglycemia in advanced GIST with the use of the drug.8 The patient described in our report was not on any chemotherapy, hence hypoglycemia could not be attributed to it. On the basis of findings among 24 patients with GIST, Rikhof and colleagues have recommended monitoring plasma levels of pro-IGF-IIE to identify patients at high risk for developing hypoglycemia, especially those with progressive disease.9 Furthermore, over expression of IGF-2 as a predictor of potential relapse may be an area for potential research and further study.10
Hypoglycemia, a frequently encountered medical emergency, is usually seen in patients with diabetes, most commonly as a result of iatrogenesis. However, it can also be encountered in nondiabetic patients. Various causes, such as pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma and hypothyroidism, have been found to contribute to the condition in the nondiabetic population.1 In rare cases, an excessive production of insulin-like growth factor (IGF-2) – a condition known as nonislet cell tumor-induced hypoglycemia (NICTH) – has also been found to cause hypoglycemia. Hypoinsulinemic hypoglycemia, with low IGF-1 levels and an IGF-2-IgF1 ratio of greater than 10, is found to be suggestive of NICTH.
Case presentation and summary
An 81-year-old man with a history of diabetes mellitus, systolic heart failure, chronic kidney disease, and metastatic classical gastrointestinal spindle cell sarcoma presented to the emergency department with an acute change in mental status resulting from a new onset hypoglycemia. He was admitted, and during his hospital stay, he experienced severe hypoglycemic episodes with symptomatic presentations of diaphoresis on multiple occasions. A detailed history revealed that for diabetes, the patient had been on insulin for the first 12 years after his diagnosis, after which he was switched to metformin 500 mg twice daily for about 2 years, and as a satisfactory glycemic control was attained, eventually metformin had also been stopped 3 years prior to the current presentation.
The patient’s past medical records were obtained from the hospital at which he had been diagnosed gastrointestinal spindle cell sarcoma. Patient had not received treatment for the cancer as the disease was too widespread to be treated. The gastrointestinal spindle cell sarcoma, which had initially been surgically resected 7 years before the current presentation, had a recurrence 3 years later with abdominal and pulmonary metastasis, but no liver metastasis. No further intervention was carried out because the widely metastasized disease would not have benefited from any more surgical intervention and chemotherapy was not initiated because of the patient’s comorbid illnesses.
A blood sample drawn from the patient at the time of one hypoglycemic event, revealed low serum insulin <0.1 U/ml (normal, 2-19.6 U/ml); low C-peptide level, 0.59 ng/ml (0.8-3.85 ng/ml); low IGF-1, 16 ng/ml (5-4 ng/ml); and IGF-3, 0.9 ng/ml (2.2-4.5 ng/ml). IGF-2 levels were found to be markedly elevated at 945 ng/ml (47-350 ng/ml). The calculated IGF-2-IGF-1 ratio was 59.06 (normal, <10), suggesting NICTH as the etiology for the patient’s hypoglycemia.
The hypoglycemic episodes were initially treated with a continuous dextrose infusion followed by diazoxide treatment. However, diazoxide did not prevent his hypoglycemic episodes, so dexamethasone was considered as an alternative for his condition. The dexamethasone treatment resulted in the normalization of the patient’s serum glucose levels and resolution of his symptoms. The patient was discharged in a satisfactory state few days later and followed up thereafter. No recurrence of hypoglycemic episodes was found, and he was continued on dexamethasone therapy.
Discussion
Hypoglycemia due to NICTH is rare, with a prevalence of four times less than that of insulinoma.3 In most cases, NICTH occurs in patients with solid tumors of mesenchymal and epithelial origins such as hepatocellular carcinoma, gastric carcinoma or mesothelioma.4 In NICTH, the serum levels of insulin, C-peptide, and IGF-1 are usually decreased or undetectable. However, the circulating levels of total IGF2 may be increased, decreased, or normal. Concurrent normal to high morning cortisol and normal response to cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH. An IGF-2: IGF-1 ratio of >10 is considered to be clinically significant and highly suggestive of NICTH.5 Hypoglycemia in NICTH can be managed by administration of oral glucose, intravenous dextrose or glucagon. In some cases, diazoxide, a potent inhibitor of insulin secretion, has been found to be useful.6 Diazoxide directly inhibits the release of insulin through stimulation of adrenergic receptors and also has an extra pancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis.
Glucocorticoid therapy has been shown to suppress IGF-2 in a dose dependent manner and also by increasing gluconeogenesis.7 Surgical resection of the tumor whenever possible is the treatment of choice followed by radiotherapy and chemotherapy for inoperable disease and if successful, usually results in resolution of hypoglycemia. Imatinib, is the chemotherapeutic drug of choice for metastatic GIST, but many case reports have suggested worsening of hypoglycemia in advanced GIST with the use of the drug.8 The patient described in our report was not on any chemotherapy, hence hypoglycemia could not be attributed to it. On the basis of findings among 24 patients with GIST, Rikhof and colleagues have recommended monitoring plasma levels of pro-IGF-IIE to identify patients at high risk for developing hypoglycemia, especially those with progressive disease.9 Furthermore, over expression of IGF-2 as a predictor of potential relapse may be an area for potential research and further study.10
1. Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91.
2. Dutta P, Aggarwal A, Gogate Y, Nahar U, Shah VN, Singla M. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130046
3. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-93.
4. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor II producing non-islet-cell tumor hypoglycemia
5. Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998;5:111-129.
6. Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758.
7. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol .1998;49:491-498.
8. Hamberg P, De Jong FA, Boonstra JG, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30-e31.
9. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582-1588.
10. Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298.
1. Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91.
2. Dutta P, Aggarwal A, Gogate Y, Nahar U, Shah VN, Singla M. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130046
3. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-93.
4. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor II producing non-islet-cell tumor hypoglycemia
5. Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998;5:111-129.
6. Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758.
7. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol .1998;49:491-498.
8. Hamberg P, De Jong FA, Boonstra JG, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30-e31.
9. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582-1588.
10. Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298.
NSAIDs remain a concern in colorectal ERAS protocols
SEATTLE – Nonselective NSAIDs increase the risk of anastomotic leaks after colorectal surgery, according to a meta-analysis from the University of Sydney, Australia.
After combing results from six randomized, controlled trials and seven retrospective studies involving a total of 23,508 patients, investigators found that postop nonselective NSAIDs (odds ratio, 0.54; 95% CI, 0.43-0.67; P less than .00001), and especially diclofenac (OR, 0.39; 95% CI, 0.28-0.55; P less than .00001), were both associated with an increased risk of leakage.
There was an increased risk with all NSAIDs compared to patients who did not receive them after surgery, but the risk was statistically significant only for nonselective options like diclofenac on subgroup analysis. There was a trend for increased leakage with the nonselective agent ketorolac, as well, but it was not significant (OR, 0.71; 95% CI, 0.35-1.43; P = .34).
“I’m not going to say we need to wait for more studies; there’s something here. We have to be aware there could be a high risk of leakage with nonsteroidals, and we have to be mindful of that with our ERAS [Enhanced Recovery after Surgery] protocols. I don’t think you should be using nonsteroidals unless you are using them in a trial” and collecting data, “because of the uncertainty,” lead investigator and colorectal surgeon Christopher Young, MD, a clinical associate professor of surgery at the University of Sydney, said at the American Society of Colon and Rectal Surgeons annual meeting.
NSAIDS are a routine part of colorectal ERAS protocols in some places to limit opioid use and hasten recovery and hospital discharge, but there’s been concern for some time that they might also increase the risk of anastomotic leakage. The new Australian findings fit in with previous investigations that raised concerns.
A 2016 review, for instance, found that among 856 patients undergoing an elective colon or rectal resection for cancer, the anastomotic leakage rate was significantly higher in the group that received nonsteroidal anti-inflammatory drugs compared to patients who did not (9.2% versus 5.3%). The higher rate was only seen in patients receiving diclofenac. “The use of diclofenac in colorectal surgery can no longer be recommended. Alternatives for postoperative analgesia need to be explored within an enhanced recovery program,” the investigators concluded (J Gastrointest Surg. 2016 Apr;20[4]:776-82. doi: 10.1007/s11605-015-3010-1).
A review of 13,082 bariatric and colorectal surgery patients in Washington State found that NSAIDs beginning within 24 hours after surgery were associated with a 70% increased risk of anastomotic leaks in nonelective colorectal surgery, with a leak rate of 12.3% in the NSAID group and 8.3% in the non-NSAID group (OR, 1.70; 95% CI, 1.11–2.68; P = .01). Although it was unclear which nonsteroidals patients received, intravenous ketorolac or ibuprofen were likely the most common (JAMA Surg. 2015 Mar 1;150[3]: 223–8).
It’s unknown why, exactly, NSAIDs impair healing and anastomotic strength, but it’s thought to be related to effects on prostaglandin synthesis, Dr. Young noted.
Dr. Young had no disclosures.
SEATTLE – Nonselective NSAIDs increase the risk of anastomotic leaks after colorectal surgery, according to a meta-analysis from the University of Sydney, Australia.
After combing results from six randomized, controlled trials and seven retrospective studies involving a total of 23,508 patients, investigators found that postop nonselective NSAIDs (odds ratio, 0.54; 95% CI, 0.43-0.67; P less than .00001), and especially diclofenac (OR, 0.39; 95% CI, 0.28-0.55; P less than .00001), were both associated with an increased risk of leakage.
There was an increased risk with all NSAIDs compared to patients who did not receive them after surgery, but the risk was statistically significant only for nonselective options like diclofenac on subgroup analysis. There was a trend for increased leakage with the nonselective agent ketorolac, as well, but it was not significant (OR, 0.71; 95% CI, 0.35-1.43; P = .34).
“I’m not going to say we need to wait for more studies; there’s something here. We have to be aware there could be a high risk of leakage with nonsteroidals, and we have to be mindful of that with our ERAS [Enhanced Recovery after Surgery] protocols. I don’t think you should be using nonsteroidals unless you are using them in a trial” and collecting data, “because of the uncertainty,” lead investigator and colorectal surgeon Christopher Young, MD, a clinical associate professor of surgery at the University of Sydney, said at the American Society of Colon and Rectal Surgeons annual meeting.
NSAIDS are a routine part of colorectal ERAS protocols in some places to limit opioid use and hasten recovery and hospital discharge, but there’s been concern for some time that they might also increase the risk of anastomotic leakage. The new Australian findings fit in with previous investigations that raised concerns.
A 2016 review, for instance, found that among 856 patients undergoing an elective colon or rectal resection for cancer, the anastomotic leakage rate was significantly higher in the group that received nonsteroidal anti-inflammatory drugs compared to patients who did not (9.2% versus 5.3%). The higher rate was only seen in patients receiving diclofenac. “The use of diclofenac in colorectal surgery can no longer be recommended. Alternatives for postoperative analgesia need to be explored within an enhanced recovery program,” the investigators concluded (J Gastrointest Surg. 2016 Apr;20[4]:776-82. doi: 10.1007/s11605-015-3010-1).
A review of 13,082 bariatric and colorectal surgery patients in Washington State found that NSAIDs beginning within 24 hours after surgery were associated with a 70% increased risk of anastomotic leaks in nonelective colorectal surgery, with a leak rate of 12.3% in the NSAID group and 8.3% in the non-NSAID group (OR, 1.70; 95% CI, 1.11–2.68; P = .01). Although it was unclear which nonsteroidals patients received, intravenous ketorolac or ibuprofen were likely the most common (JAMA Surg. 2015 Mar 1;150[3]: 223–8).
It’s unknown why, exactly, NSAIDs impair healing and anastomotic strength, but it’s thought to be related to effects on prostaglandin synthesis, Dr. Young noted.
Dr. Young had no disclosures.
SEATTLE – Nonselective NSAIDs increase the risk of anastomotic leaks after colorectal surgery, according to a meta-analysis from the University of Sydney, Australia.
After combing results from six randomized, controlled trials and seven retrospective studies involving a total of 23,508 patients, investigators found that postop nonselective NSAIDs (odds ratio, 0.54; 95% CI, 0.43-0.67; P less than .00001), and especially diclofenac (OR, 0.39; 95% CI, 0.28-0.55; P less than .00001), were both associated with an increased risk of leakage.
There was an increased risk with all NSAIDs compared to patients who did not receive them after surgery, but the risk was statistically significant only for nonselective options like diclofenac on subgroup analysis. There was a trend for increased leakage with the nonselective agent ketorolac, as well, but it was not significant (OR, 0.71; 95% CI, 0.35-1.43; P = .34).
“I’m not going to say we need to wait for more studies; there’s something here. We have to be aware there could be a high risk of leakage with nonsteroidals, and we have to be mindful of that with our ERAS [Enhanced Recovery after Surgery] protocols. I don’t think you should be using nonsteroidals unless you are using them in a trial” and collecting data, “because of the uncertainty,” lead investigator and colorectal surgeon Christopher Young, MD, a clinical associate professor of surgery at the University of Sydney, said at the American Society of Colon and Rectal Surgeons annual meeting.
NSAIDS are a routine part of colorectal ERAS protocols in some places to limit opioid use and hasten recovery and hospital discharge, but there’s been concern for some time that they might also increase the risk of anastomotic leakage. The new Australian findings fit in with previous investigations that raised concerns.
A 2016 review, for instance, found that among 856 patients undergoing an elective colon or rectal resection for cancer, the anastomotic leakage rate was significantly higher in the group that received nonsteroidal anti-inflammatory drugs compared to patients who did not (9.2% versus 5.3%). The higher rate was only seen in patients receiving diclofenac. “The use of diclofenac in colorectal surgery can no longer be recommended. Alternatives for postoperative analgesia need to be explored within an enhanced recovery program,” the investigators concluded (J Gastrointest Surg. 2016 Apr;20[4]:776-82. doi: 10.1007/s11605-015-3010-1).
A review of 13,082 bariatric and colorectal surgery patients in Washington State found that NSAIDs beginning within 24 hours after surgery were associated with a 70% increased risk of anastomotic leaks in nonelective colorectal surgery, with a leak rate of 12.3% in the NSAID group and 8.3% in the non-NSAID group (OR, 1.70; 95% CI, 1.11–2.68; P = .01). Although it was unclear which nonsteroidals patients received, intravenous ketorolac or ibuprofen were likely the most common (JAMA Surg. 2015 Mar 1;150[3]: 223–8).
It’s unknown why, exactly, NSAIDs impair healing and anastomotic strength, but it’s thought to be related to effects on prostaglandin synthesis, Dr. Young noted.
Dr. Young had no disclosures.
AT ASCRS 2017
Key clinical point:
Major finding: Postop nonselective NSAIDs (OR, 0.54; 95% CI, 0.43-0.67; P less than .00001), and especially diclofenac (OR, 0.39; 95% CI, 0.28-0.55; P less than .00001), were both associated with an increased risk of leakage.
Data source: Meta-analysis involving 23,508 patients
Disclosures: The presenter had no disclosures.
Ibrutinib dons new anti-GVHD hat
MADRID – Talk about versatility: Ibrutinib (Imbruvica), a drug with marked activity against B-cell malignancies, also appears to be a safe and acceptable option for the treatment of patients with chronic graft vs. host disease (cGVHD) for whom frontline therapies have failed.
Among 42 patients in a phase II study with steroid-refractory cGVHD, the overall response rate with ibrutinib was 67%, with one-third of responders having a complete response, reported Iskra Pusic, MD, from Washington University School of Medicine in St. Louis.
Corticosteroids are the most commonly used therapy for cGVHD in the United States, but for those patients for whom corticosteroids are a bust, there is no established second-line therapy, and patients with refractory cGVHD are usually recommended for clinical trials, Dr. Pusic said.
The therapeutic rationale underpinning the use of ibrutinib in cGVHD, a condition marked by extensive immune dysregulation, is that the agent is an irreversible inhibitor of Bruton’s tyrosine kinase and interleukin-2 inducible T-cell kinase, and thus has wide-ranging immune-dampening activity, Dr. Pusic said.
She and colleagues in a multicenter study enrolled 42 patients with cGVHD that corticosteroids had failed to treat adequately, and treated them with oral ibrutinib 420 mg daily until cGVHD progression or unacceptable toxicity.
At a median follow-up of 13.9 months, a total of 28 patients (67%) had a response according to 2005 National Institutes of Health (NIH) criteria, including nine with a complete response, and 19 with partial responses.
Of the patients with responses, 79% had a response at the time of the first assessment for response, and 71% of responders had responses lasting at least 5 months.
Among patients with multiorgan involvement, responses were seen in two or more organs.
Grade 3 or greater adverse events included fatigue, diarrhea, muscles spasms, pneumonia, pyrexia, and headache. Two patients died on study, one from multilobular pneumonia and one from bronchopulmonary aspergillosis.
In general, the safety profile of ibrutinib was similar to that seen in studies of the drug in B-cell malignancies and to that seen with corticosteroid therapy for patients with cGVHD, Dr. Pusic said.
Investigators are currently enrolling patients in a double-blind clinical trial comparing ibrutinib or placebo in combination with corticosteroids in patients with newly diagnosed cGVHD, she noted.
The study was supported by Pharmacyclics. Dr. Pusic did not report disclosures.
MADRID – Talk about versatility: Ibrutinib (Imbruvica), a drug with marked activity against B-cell malignancies, also appears to be a safe and acceptable option for the treatment of patients with chronic graft vs. host disease (cGVHD) for whom frontline therapies have failed.
Among 42 patients in a phase II study with steroid-refractory cGVHD, the overall response rate with ibrutinib was 67%, with one-third of responders having a complete response, reported Iskra Pusic, MD, from Washington University School of Medicine in St. Louis.
Corticosteroids are the most commonly used therapy for cGVHD in the United States, but for those patients for whom corticosteroids are a bust, there is no established second-line therapy, and patients with refractory cGVHD are usually recommended for clinical trials, Dr. Pusic said.
The therapeutic rationale underpinning the use of ibrutinib in cGVHD, a condition marked by extensive immune dysregulation, is that the agent is an irreversible inhibitor of Bruton’s tyrosine kinase and interleukin-2 inducible T-cell kinase, and thus has wide-ranging immune-dampening activity, Dr. Pusic said.
She and colleagues in a multicenter study enrolled 42 patients with cGVHD that corticosteroids had failed to treat adequately, and treated them with oral ibrutinib 420 mg daily until cGVHD progression or unacceptable toxicity.
At a median follow-up of 13.9 months, a total of 28 patients (67%) had a response according to 2005 National Institutes of Health (NIH) criteria, including nine with a complete response, and 19 with partial responses.
Of the patients with responses, 79% had a response at the time of the first assessment for response, and 71% of responders had responses lasting at least 5 months.
Among patients with multiorgan involvement, responses were seen in two or more organs.
Grade 3 or greater adverse events included fatigue, diarrhea, muscles spasms, pneumonia, pyrexia, and headache. Two patients died on study, one from multilobular pneumonia and one from bronchopulmonary aspergillosis.
In general, the safety profile of ibrutinib was similar to that seen in studies of the drug in B-cell malignancies and to that seen with corticosteroid therapy for patients with cGVHD, Dr. Pusic said.
Investigators are currently enrolling patients in a double-blind clinical trial comparing ibrutinib or placebo in combination with corticosteroids in patients with newly diagnosed cGVHD, she noted.
The study was supported by Pharmacyclics. Dr. Pusic did not report disclosures.
MADRID – Talk about versatility: Ibrutinib (Imbruvica), a drug with marked activity against B-cell malignancies, also appears to be a safe and acceptable option for the treatment of patients with chronic graft vs. host disease (cGVHD) for whom frontline therapies have failed.
Among 42 patients in a phase II study with steroid-refractory cGVHD, the overall response rate with ibrutinib was 67%, with one-third of responders having a complete response, reported Iskra Pusic, MD, from Washington University School of Medicine in St. Louis.
Corticosteroids are the most commonly used therapy for cGVHD in the United States, but for those patients for whom corticosteroids are a bust, there is no established second-line therapy, and patients with refractory cGVHD are usually recommended for clinical trials, Dr. Pusic said.
The therapeutic rationale underpinning the use of ibrutinib in cGVHD, a condition marked by extensive immune dysregulation, is that the agent is an irreversible inhibitor of Bruton’s tyrosine kinase and interleukin-2 inducible T-cell kinase, and thus has wide-ranging immune-dampening activity, Dr. Pusic said.
She and colleagues in a multicenter study enrolled 42 patients with cGVHD that corticosteroids had failed to treat adequately, and treated them with oral ibrutinib 420 mg daily until cGVHD progression or unacceptable toxicity.
At a median follow-up of 13.9 months, a total of 28 patients (67%) had a response according to 2005 National Institutes of Health (NIH) criteria, including nine with a complete response, and 19 with partial responses.
Of the patients with responses, 79% had a response at the time of the first assessment for response, and 71% of responders had responses lasting at least 5 months.
Among patients with multiorgan involvement, responses were seen in two or more organs.
Grade 3 or greater adverse events included fatigue, diarrhea, muscles spasms, pneumonia, pyrexia, and headache. Two patients died on study, one from multilobular pneumonia and one from bronchopulmonary aspergillosis.
In general, the safety profile of ibrutinib was similar to that seen in studies of the drug in B-cell malignancies and to that seen with corticosteroid therapy for patients with cGVHD, Dr. Pusic said.
Investigators are currently enrolling patients in a double-blind clinical trial comparing ibrutinib or placebo in combination with corticosteroids in patients with newly diagnosed cGVHD, she noted.
The study was supported by Pharmacyclics. Dr. Pusic did not report disclosures.
AT EHA 2017
Key clinical point: The tyrosine kinase inhibitor ibrutinib was associated with complete and partial responses in two-thirds of patients with steroid-refractory chronic graft vs. host disease (cGVHD).
Major finding: A total of 28 patients (67%) had responses, including 9 complete responses.
Data source: Phase II clinical trial in 42 patients with cGVHD for whom corticosteroids had failed.
Disclosures: The study was supported by Pharmacyclics. Dr. Pusic did not report disclosures.
Major bleeding deaths may outweigh VTE risk in older cancer patients
MADRID – Look before you leap into anticoagulation therapy for older cancer patients, results from a Canadian cohort study suggest.
Among patients 65 and older with cancer and a venous thromboembolic event (VTE) within 6 months of the cancer diagnosis, the 7-day mortality rate from VTE was 0.5%, compared with an 11% rate of death from a major bleeding episode, reported Alejandro Lazo-Langner MD, MSc from Western University in London, Canada.
If their findings are confirmed in further studies, “it would actually change what we do in terms of the treatment of thrombosis,” he said.
Risks for both VTE and for bleeding are known to be higher among patients with cancer than in the general population. Although a previously published systematic review suggested that mortality rates from recurrent VTE and major bleeding events were similar in the first 6 months of anticoagulation therapy, those results were limited by the heterogeneity of designs in the various studies included in the review, and by differences in outcome measures and the types of populations included, Dr. Lazo-Langner said.
To get a better idea of the case fatality rates of VTE recurrence and major bleeding and the case fatality rate ratio for each, the authors conducted a retrospective population-based cohort study in the Province of Ontario using de-identified linked administrative health care databases.
They assembled a cohort of patients 65 years of age and older who had a VTE event within 6 months of an initial cancer diagnosis. Recurrent VTE and major bleeding events were assessed within 180 days of the index date.
They found that from 2004 through 2014 there were 6,967 VTEs in cancer patients over 65 years of age (mean age 75) that were treated with an anticoagulant, either low-molecular-weight heparin (LMWH), LMWH plus warfarin, warfarin alone, or rivaroxaban (Xarelto).
Six months after the index VTE events, 235 patients (3%) had experienced a major bleeding event, and 1,184 (17%) had a recurrent VTE.
Within 7 days of the outcome event the mortality rate due to major bleeding was 11%, compared with 0.5% for recurrent VTEs. This translated into a mortality rate ratio for major bleeding vs. VTE of 21.8
Elizabeth Macintyre, MD, from the Hôpital Necker-Enfants Malades and University of Paris, France, who was not involved in the study, said in an interview that the results indicate that clinicians should not automatically assume that anticoagulation is a good idea for every older cancer patient.
“We now need to consider whether we should be reducing the time of anticoagulation, but that obviously depends on all sorts of other risk factors, so it has to be an individual patient decision. But the message is clearly don’t just anticoagulate to avoid the risk of thrombosis, and do in bear in mind that the other side of the coin could be just as serious,” she said.
The study was supported by the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Lazo-Langner and Dr. Macintyre reported having no conflicts of interest to disclose.
MADRID – Look before you leap into anticoagulation therapy for older cancer patients, results from a Canadian cohort study suggest.
Among patients 65 and older with cancer and a venous thromboembolic event (VTE) within 6 months of the cancer diagnosis, the 7-day mortality rate from VTE was 0.5%, compared with an 11% rate of death from a major bleeding episode, reported Alejandro Lazo-Langner MD, MSc from Western University in London, Canada.
If their findings are confirmed in further studies, “it would actually change what we do in terms of the treatment of thrombosis,” he said.
Risks for both VTE and for bleeding are known to be higher among patients with cancer than in the general population. Although a previously published systematic review suggested that mortality rates from recurrent VTE and major bleeding events were similar in the first 6 months of anticoagulation therapy, those results were limited by the heterogeneity of designs in the various studies included in the review, and by differences in outcome measures and the types of populations included, Dr. Lazo-Langner said.
To get a better idea of the case fatality rates of VTE recurrence and major bleeding and the case fatality rate ratio for each, the authors conducted a retrospective population-based cohort study in the Province of Ontario using de-identified linked administrative health care databases.
They assembled a cohort of patients 65 years of age and older who had a VTE event within 6 months of an initial cancer diagnosis. Recurrent VTE and major bleeding events were assessed within 180 days of the index date.
They found that from 2004 through 2014 there were 6,967 VTEs in cancer patients over 65 years of age (mean age 75) that were treated with an anticoagulant, either low-molecular-weight heparin (LMWH), LMWH plus warfarin, warfarin alone, or rivaroxaban (Xarelto).
Six months after the index VTE events, 235 patients (3%) had experienced a major bleeding event, and 1,184 (17%) had a recurrent VTE.
Within 7 days of the outcome event the mortality rate due to major bleeding was 11%, compared with 0.5% for recurrent VTEs. This translated into a mortality rate ratio for major bleeding vs. VTE of 21.8
Elizabeth Macintyre, MD, from the Hôpital Necker-Enfants Malades and University of Paris, France, who was not involved in the study, said in an interview that the results indicate that clinicians should not automatically assume that anticoagulation is a good idea for every older cancer patient.
“We now need to consider whether we should be reducing the time of anticoagulation, but that obviously depends on all sorts of other risk factors, so it has to be an individual patient decision. But the message is clearly don’t just anticoagulate to avoid the risk of thrombosis, and do in bear in mind that the other side of the coin could be just as serious,” she said.
The study was supported by the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Lazo-Langner and Dr. Macintyre reported having no conflicts of interest to disclose.
MADRID – Look before you leap into anticoagulation therapy for older cancer patients, results from a Canadian cohort study suggest.
Among patients 65 and older with cancer and a venous thromboembolic event (VTE) within 6 months of the cancer diagnosis, the 7-day mortality rate from VTE was 0.5%, compared with an 11% rate of death from a major bleeding episode, reported Alejandro Lazo-Langner MD, MSc from Western University in London, Canada.
If their findings are confirmed in further studies, “it would actually change what we do in terms of the treatment of thrombosis,” he said.
Risks for both VTE and for bleeding are known to be higher among patients with cancer than in the general population. Although a previously published systematic review suggested that mortality rates from recurrent VTE and major bleeding events were similar in the first 6 months of anticoagulation therapy, those results were limited by the heterogeneity of designs in the various studies included in the review, and by differences in outcome measures and the types of populations included, Dr. Lazo-Langner said.
To get a better idea of the case fatality rates of VTE recurrence and major bleeding and the case fatality rate ratio for each, the authors conducted a retrospective population-based cohort study in the Province of Ontario using de-identified linked administrative health care databases.
They assembled a cohort of patients 65 years of age and older who had a VTE event within 6 months of an initial cancer diagnosis. Recurrent VTE and major bleeding events were assessed within 180 days of the index date.
They found that from 2004 through 2014 there were 6,967 VTEs in cancer patients over 65 years of age (mean age 75) that were treated with an anticoagulant, either low-molecular-weight heparin (LMWH), LMWH plus warfarin, warfarin alone, or rivaroxaban (Xarelto).
Six months after the index VTE events, 235 patients (3%) had experienced a major bleeding event, and 1,184 (17%) had a recurrent VTE.
Within 7 days of the outcome event the mortality rate due to major bleeding was 11%, compared with 0.5% for recurrent VTEs. This translated into a mortality rate ratio for major bleeding vs. VTE of 21.8
Elizabeth Macintyre, MD, from the Hôpital Necker-Enfants Malades and University of Paris, France, who was not involved in the study, said in an interview that the results indicate that clinicians should not automatically assume that anticoagulation is a good idea for every older cancer patient.
“We now need to consider whether we should be reducing the time of anticoagulation, but that obviously depends on all sorts of other risk factors, so it has to be an individual patient decision. But the message is clearly don’t just anticoagulate to avoid the risk of thrombosis, and do in bear in mind that the other side of the coin could be just as serious,” she said.
The study was supported by the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Lazo-Langner and Dr. Macintyre reported having no conflicts of interest to disclose.
AT EHA 2017
Key clinical point: In cancer patients older than 65, the risk of major bleeding from anticoagulants may outweigh the risk of venous thromboembolism.
Major finding: 7-day mortality rates were 11% for major bleeding vs 0.5% for recurrent VTE.
Data source: Retrospective cohort study of 6,967 cancer patients age 65 and older from Ontario, Canada.
Disclosures: The study was supported by the Institute for Clinical Evaluative Sciences, funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. Dr. Lazo-Langner and Dr. Macintyre reported having no conflicts of interest to disclose.
VIDEO: No cancer risk found from biological DMARDs
MADRID – Additional real-world evidence confirmed that biological disease modifying drugs used to treat rheumatoid arthritis produced no spikes in new cancers or in cancer recurrences in registry data from tens of thousands of Swedish patients.
Among rheumatoid arthritis patients with a history of cancer, patients treated with a tumor necrosis factor inhibitor (TNFi) were not at an increased risk for cancer recurrence, Johan Askling, MD, said at the European Congress of Rheumatology. In a second study, patients with rheumatoid arthritis (RA) treated with a non-TNFi, biological, disease-modifying drug, specifically abatacept, rituximab, or tocilizumab, had no significantly different rate of new cancer onset when compared with RA patients who never received a biological disease modifying drug nor when compared with the general Swedish adult population, said Dr. Askling, a professor of clinical epidemiology at the Karolinska Institute in Stockholm.
“Five-year data are a good start, but we need data on 30-year risk,” Dr. Askling said in an interview.
The cancer-recurrence risk study with TNFi treatment used data collected by the Swedish national outpatient care registry on nearly 62,000 people, the Swedish cancer registry, and a rheumatology treatment registry called ARTIS. It also included patients treated during 2001-2014. From these sources, the researchers identified 446 RA patients with a history of at least one cancer who then began treatment with any type of TNFi and matched these cases with 1,278 similar RA patients with a cancer history who had never received a biologic drug. On average, the patients were nearly 10 years removed from their initial cancer diagnoses, and the average duration on TNFi treatment was nearly 5 years.
The adjusted hazard ratio for cancer recurrence among the TNFi recipients was reduced by a nominal 30%, compared with that of the controls, a difference that was not statistically significant, Dr. Askling reported.
The second study used data from similar sources for patients treated during 2006-2014 and included nearly 100,000 Swedes from the general population, more than 42,000 RA patients who did not receive a biological drug, more than 14,000 treated with either a first or second TNFi drug, and 1,693 patients treated with tocilizumab (Actemra), 1,894 on abatacept (Orencia), and 3,119 on rituximab (Rituxan).
The rates of new onset cancer in any of these treatment groups, including the patients on tocilizumab, abatacept, or rituximab, was not significantly different from the rate among RA patients who never received a biologic drug, nor from the general Swedish population rate, Dr. Askling said.
This is “one of the first large-scale assessments” of the cancer risk posed by non-TNFi biological drugs, aside from what was reported from the pivotal trials for these drugs, Dr. Askling said.
Dr. Askling has received research support from AbbVie, Lilly, MSD, Pfizer, Roche, and UCB.
[email protected]
On Twitter @mitchelzoler
Rheumatologists began having concerns about the possible impact of biological drugs on cancer when these types of drugs first became available 20 or more years ago. Registries have allowed us to follow these patients, and, so far, we have consistently seen that the risk for cancer is very low. The major adverse effect from treatment with biological drugs is infection.
The most confirmed finding has been that biologic drugs do not cause new cancers. We have known less about the risk patients with a history of cancer face for recurrence by taking a biological drug. The data on this have so far been scarce. Most guidelines advise that, when patients have had cancer, the possible use of a biologic drug should be the subject of a shared-decision discussion with the patient. The new data reported by Dr. Askling add to the risk information we have available to discuss with patients.
The risk that biologic drugs poses for infections is more complex. The infection risk also depends on a patient’s use of glucocorticoids, their age, and their comorbidities. The infection risk faced by a patient from treatment with a biological drug requires an individualized discussion that takes into account the severity of all the relevant risk factors.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
João E. Fonseca, MD is a professor of rheumatology at the University of Lisbon. He has been a speaker for or has received research funding from Abbvie, MSD, Pfizer, Roche, and UCB. He made these comments in a video interview.
Rheumatologists began having concerns about the possible impact of biological drugs on cancer when these types of drugs first became available 20 or more years ago. Registries have allowed us to follow these patients, and, so far, we have consistently seen that the risk for cancer is very low. The major adverse effect from treatment with biological drugs is infection.
The most confirmed finding has been that biologic drugs do not cause new cancers. We have known less about the risk patients with a history of cancer face for recurrence by taking a biological drug. The data on this have so far been scarce. Most guidelines advise that, when patients have had cancer, the possible use of a biologic drug should be the subject of a shared-decision discussion with the patient. The new data reported by Dr. Askling add to the risk information we have available to discuss with patients.
The risk that biologic drugs poses for infections is more complex. The infection risk also depends on a patient’s use of glucocorticoids, their age, and their comorbidities. The infection risk faced by a patient from treatment with a biological drug requires an individualized discussion that takes into account the severity of all the relevant risk factors.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
João E. Fonseca, MD is a professor of rheumatology at the University of Lisbon. He has been a speaker for or has received research funding from Abbvie, MSD, Pfizer, Roche, and UCB. He made these comments in a video interview.
Rheumatologists began having concerns about the possible impact of biological drugs on cancer when these types of drugs first became available 20 or more years ago. Registries have allowed us to follow these patients, and, so far, we have consistently seen that the risk for cancer is very low. The major adverse effect from treatment with biological drugs is infection.
The most confirmed finding has been that biologic drugs do not cause new cancers. We have known less about the risk patients with a history of cancer face for recurrence by taking a biological drug. The data on this have so far been scarce. Most guidelines advise that, when patients have had cancer, the possible use of a biologic drug should be the subject of a shared-decision discussion with the patient. The new data reported by Dr. Askling add to the risk information we have available to discuss with patients.
The risk that biologic drugs poses for infections is more complex. The infection risk also depends on a patient’s use of glucocorticoids, their age, and their comorbidities. The infection risk faced by a patient from treatment with a biological drug requires an individualized discussion that takes into account the severity of all the relevant risk factors.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
João E. Fonseca, MD is a professor of rheumatology at the University of Lisbon. He has been a speaker for or has received research funding from Abbvie, MSD, Pfizer, Roche, and UCB. He made these comments in a video interview.
MADRID – Additional real-world evidence confirmed that biological disease modifying drugs used to treat rheumatoid arthritis produced no spikes in new cancers or in cancer recurrences in registry data from tens of thousands of Swedish patients.
Among rheumatoid arthritis patients with a history of cancer, patients treated with a tumor necrosis factor inhibitor (TNFi) were not at an increased risk for cancer recurrence, Johan Askling, MD, said at the European Congress of Rheumatology. In a second study, patients with rheumatoid arthritis (RA) treated with a non-TNFi, biological, disease-modifying drug, specifically abatacept, rituximab, or tocilizumab, had no significantly different rate of new cancer onset when compared with RA patients who never received a biological disease modifying drug nor when compared with the general Swedish adult population, said Dr. Askling, a professor of clinical epidemiology at the Karolinska Institute in Stockholm.
“Five-year data are a good start, but we need data on 30-year risk,” Dr. Askling said in an interview.
The cancer-recurrence risk study with TNFi treatment used data collected by the Swedish national outpatient care registry on nearly 62,000 people, the Swedish cancer registry, and a rheumatology treatment registry called ARTIS. It also included patients treated during 2001-2014. From these sources, the researchers identified 446 RA patients with a history of at least one cancer who then began treatment with any type of TNFi and matched these cases with 1,278 similar RA patients with a cancer history who had never received a biologic drug. On average, the patients were nearly 10 years removed from their initial cancer diagnoses, and the average duration on TNFi treatment was nearly 5 years.
The adjusted hazard ratio for cancer recurrence among the TNFi recipients was reduced by a nominal 30%, compared with that of the controls, a difference that was not statistically significant, Dr. Askling reported.
The second study used data from similar sources for patients treated during 2006-2014 and included nearly 100,000 Swedes from the general population, more than 42,000 RA patients who did not receive a biological drug, more than 14,000 treated with either a first or second TNFi drug, and 1,693 patients treated with tocilizumab (Actemra), 1,894 on abatacept (Orencia), and 3,119 on rituximab (Rituxan).
The rates of new onset cancer in any of these treatment groups, including the patients on tocilizumab, abatacept, or rituximab, was not significantly different from the rate among RA patients who never received a biologic drug, nor from the general Swedish population rate, Dr. Askling said.
This is “one of the first large-scale assessments” of the cancer risk posed by non-TNFi biological drugs, aside from what was reported from the pivotal trials for these drugs, Dr. Askling said.
Dr. Askling has received research support from AbbVie, Lilly, MSD, Pfizer, Roche, and UCB.
[email protected]
On Twitter @mitchelzoler
MADRID – Additional real-world evidence confirmed that biological disease modifying drugs used to treat rheumatoid arthritis produced no spikes in new cancers or in cancer recurrences in registry data from tens of thousands of Swedish patients.
Among rheumatoid arthritis patients with a history of cancer, patients treated with a tumor necrosis factor inhibitor (TNFi) were not at an increased risk for cancer recurrence, Johan Askling, MD, said at the European Congress of Rheumatology. In a second study, patients with rheumatoid arthritis (RA) treated with a non-TNFi, biological, disease-modifying drug, specifically abatacept, rituximab, or tocilizumab, had no significantly different rate of new cancer onset when compared with RA patients who never received a biological disease modifying drug nor when compared with the general Swedish adult population, said Dr. Askling, a professor of clinical epidemiology at the Karolinska Institute in Stockholm.
“Five-year data are a good start, but we need data on 30-year risk,” Dr. Askling said in an interview.
The cancer-recurrence risk study with TNFi treatment used data collected by the Swedish national outpatient care registry on nearly 62,000 people, the Swedish cancer registry, and a rheumatology treatment registry called ARTIS. It also included patients treated during 2001-2014. From these sources, the researchers identified 446 RA patients with a history of at least one cancer who then began treatment with any type of TNFi and matched these cases with 1,278 similar RA patients with a cancer history who had never received a biologic drug. On average, the patients were nearly 10 years removed from their initial cancer diagnoses, and the average duration on TNFi treatment was nearly 5 years.
The adjusted hazard ratio for cancer recurrence among the TNFi recipients was reduced by a nominal 30%, compared with that of the controls, a difference that was not statistically significant, Dr. Askling reported.
The second study used data from similar sources for patients treated during 2006-2014 and included nearly 100,000 Swedes from the general population, more than 42,000 RA patients who did not receive a biological drug, more than 14,000 treated with either a first or second TNFi drug, and 1,693 patients treated with tocilizumab (Actemra), 1,894 on abatacept (Orencia), and 3,119 on rituximab (Rituxan).
The rates of new onset cancer in any of these treatment groups, including the patients on tocilizumab, abatacept, or rituximab, was not significantly different from the rate among RA patients who never received a biologic drug, nor from the general Swedish population rate, Dr. Askling said.
This is “one of the first large-scale assessments” of the cancer risk posed by non-TNFi biological drugs, aside from what was reported from the pivotal trials for these drugs, Dr. Askling said.
Dr. Askling has received research support from AbbVie, Lilly, MSD, Pfizer, Roche, and UCB.
[email protected]
On Twitter @mitchelzoler
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Former cancer patients on a TNFi had no increased cancer recurrences, compared with patients on other rheumatic treatments.
Data source: Data from the Swedish national registries.
Disclosures: Dr. Askling has received research support from AbbVie, Lilly, MSD, Pfizer, Roche, and UCB.
Hypogonadism after testicular cancer treatment can have lifelong impact
CHICAGO – Hypogonadism may compromise the long-term health outlook for many younger men who have been successfully treated for testicular cancer, according to an analysis conducted by the Platinum Study Group.
“Today, 95% of all testicular cancer patients are cured of their disease thanks to cisplatin-based chemotherapy. Nowadays, testicular cancer survivors can expect to live for over 40 years from the time of their diagnosis,” lead investigator Mohammad Issam Abu Zaid, MBBS, said in a press briefing at the annual meeting of the American Society of Clinical Oncology. “However, they are at risk of other health problems that may be related to their cancer treatment, including late complications from chemotherapy.”
“Testicular cancer survivors, especially those treated with chemotherapy, are at increased risk for hypogonadism, a problem that can be associated with predisposing factors for heart disease,” summarized Dr. Abu Zaid, of Indiana University in Indianapolis. “Mitigating approaches are the usual weight control, exercise, and monitoring of blood pressure and cholesterol levels.”
Expert perspective
“This is an important study, and it sends a loud message to those of us who take care of testis cancer patients, my area of expertise. ... We need to watch for hypogonadism, and we need to ask survivors about it. We need to examine them thinking about it, and, in patients who we are worried [they] might have hypogonadism, we need to do blood tests for testosterone and other hormone levels,” commented ASCO Expert Timothy D. Gilligan, MD, MSc, of the Cleveland Clinic in Ohio. “These are young patients, they have many years of life, so it’s many decades of suffering from consequences of this if it’s undetected.”
The findings were not surprising based on his personal experience and on evidence from the prostate cancer field showing the adverse metabolic effects of withdrawing testosterone, he said. Although the prevalence of hypogonadism found in the study was higher than that found in other studies, given the large size of the cohort, it should be taken seriously. Additionally, even if the true prevalence is somewhat lower, the absolute number of survivors affected would be substantial.
“We need to be cautious though and make sure people don’t misunderstand and think that this means we should test testosterone levels in all patients, which is a risky thing to do because the definition of normal testosterone is very fuzzy,” Dr. Gilligan stressed. “There is a wide range of normal, and what’s normal for me may not be the same as what’s normal for another man. So, looking for symptoms is really what guides this work, and, when there are symptoms, then testing is important.”
Study details
The Platinum Study is a large, ongoing, multicenter North American–based cohort study of testicular cancer survivors who received cisplatin as part of their chemotherapy.
Dr. Abu Zaid and his colleagues analyzed data from 491 participants who were aged 50 years or younger at diagnosis. All completed questionnaires addressing comorbidities, medications, and health behaviors; underwent physical examination; had measurement of testosterone levels; and had a genetic analysis.
The men were by and large young at the time of evaluation, with a mean age of just 38 years, he reported in the press briefing and a poster session at the meeting.
Overall, 38.5% had hypogonadism, defined in the study as a serum testosterone level of 3 ng/mL or lower or as use of testosterone replacement therapy.
In a multivariate analysis, survivors’ odds of hypogonadism increased with age (odds ratio, 1.41 per 10-year increment; P = .007) and were higher for those having a body mass index of 25 kg/m2 or greater, vs. lower (OR, 2.22; P = .003). On the other hand, there was a trend whereby survivors who engaged in any vigorous-intensity physical activity, vs. none, were less likely to have hypogonadism (OR, 0.64; P = .06).
The odds rose with number of risk alleles in the sex hormone–binding globulin gene, but findings were not significant, Dr. Abu Zaid said. They were also statistically indistinguishable across groups differing with respect to the chemotherapy regimen received and socioeconomic factors.
Compared with counterparts having normal testosterone levels, survivors having hypogonadism were up to four times more likely to be taking medication to treat dyslipidemia (20.1% vs. 6.0%; P less than .001), hypertension (18.5% vs. 10.6%; P = .013), erectile dysfunction (19.6% vs. 11.9%; P = .018), diabetes (5.8% vs. 2.6%; P = .067), and anxiety or depression (14.8% vs. 9.3%; P = .060).
Testicular cancer survivors should not universally have testosterone testing, agreed Dr. Abu Zaid. Such a practice might lead to overtreatment, and, in older men at least, use of testosterone itself has been linked to elevated cardiovascular risk.
“You really want to treat somebody who has symptoms related to hypogonadism: constant fatigue, night sweats, and depressed mood and some other symptoms that are well known to the clinician,” he maintained. “At the moment, we have no studies done looking at testosterone replacement in young men. I would hypothesize that we actually help them by giving them testosterone, and that’s what I tell my patients.”
CHICAGO – Hypogonadism may compromise the long-term health outlook for many younger men who have been successfully treated for testicular cancer, according to an analysis conducted by the Platinum Study Group.
“Today, 95% of all testicular cancer patients are cured of their disease thanks to cisplatin-based chemotherapy. Nowadays, testicular cancer survivors can expect to live for over 40 years from the time of their diagnosis,” lead investigator Mohammad Issam Abu Zaid, MBBS, said in a press briefing at the annual meeting of the American Society of Clinical Oncology. “However, they are at risk of other health problems that may be related to their cancer treatment, including late complications from chemotherapy.”
“Testicular cancer survivors, especially those treated with chemotherapy, are at increased risk for hypogonadism, a problem that can be associated with predisposing factors for heart disease,” summarized Dr. Abu Zaid, of Indiana University in Indianapolis. “Mitigating approaches are the usual weight control, exercise, and monitoring of blood pressure and cholesterol levels.”
Expert perspective
“This is an important study, and it sends a loud message to those of us who take care of testis cancer patients, my area of expertise. ... We need to watch for hypogonadism, and we need to ask survivors about it. We need to examine them thinking about it, and, in patients who we are worried [they] might have hypogonadism, we need to do blood tests for testosterone and other hormone levels,” commented ASCO Expert Timothy D. Gilligan, MD, MSc, of the Cleveland Clinic in Ohio. “These are young patients, they have many years of life, so it’s many decades of suffering from consequences of this if it’s undetected.”
The findings were not surprising based on his personal experience and on evidence from the prostate cancer field showing the adverse metabolic effects of withdrawing testosterone, he said. Although the prevalence of hypogonadism found in the study was higher than that found in other studies, given the large size of the cohort, it should be taken seriously. Additionally, even if the true prevalence is somewhat lower, the absolute number of survivors affected would be substantial.
“We need to be cautious though and make sure people don’t misunderstand and think that this means we should test testosterone levels in all patients, which is a risky thing to do because the definition of normal testosterone is very fuzzy,” Dr. Gilligan stressed. “There is a wide range of normal, and what’s normal for me may not be the same as what’s normal for another man. So, looking for symptoms is really what guides this work, and, when there are symptoms, then testing is important.”
Study details
The Platinum Study is a large, ongoing, multicenter North American–based cohort study of testicular cancer survivors who received cisplatin as part of their chemotherapy.
Dr. Abu Zaid and his colleagues analyzed data from 491 participants who were aged 50 years or younger at diagnosis. All completed questionnaires addressing comorbidities, medications, and health behaviors; underwent physical examination; had measurement of testosterone levels; and had a genetic analysis.
The men were by and large young at the time of evaluation, with a mean age of just 38 years, he reported in the press briefing and a poster session at the meeting.
Overall, 38.5% had hypogonadism, defined in the study as a serum testosterone level of 3 ng/mL or lower or as use of testosterone replacement therapy.
In a multivariate analysis, survivors’ odds of hypogonadism increased with age (odds ratio, 1.41 per 10-year increment; P = .007) and were higher for those having a body mass index of 25 kg/m2 or greater, vs. lower (OR, 2.22; P = .003). On the other hand, there was a trend whereby survivors who engaged in any vigorous-intensity physical activity, vs. none, were less likely to have hypogonadism (OR, 0.64; P = .06).
The odds rose with number of risk alleles in the sex hormone–binding globulin gene, but findings were not significant, Dr. Abu Zaid said. They were also statistically indistinguishable across groups differing with respect to the chemotherapy regimen received and socioeconomic factors.
Compared with counterparts having normal testosterone levels, survivors having hypogonadism were up to four times more likely to be taking medication to treat dyslipidemia (20.1% vs. 6.0%; P less than .001), hypertension (18.5% vs. 10.6%; P = .013), erectile dysfunction (19.6% vs. 11.9%; P = .018), diabetes (5.8% vs. 2.6%; P = .067), and anxiety or depression (14.8% vs. 9.3%; P = .060).
Testicular cancer survivors should not universally have testosterone testing, agreed Dr. Abu Zaid. Such a practice might lead to overtreatment, and, in older men at least, use of testosterone itself has been linked to elevated cardiovascular risk.
“You really want to treat somebody who has symptoms related to hypogonadism: constant fatigue, night sweats, and depressed mood and some other symptoms that are well known to the clinician,” he maintained. “At the moment, we have no studies done looking at testosterone replacement in young men. I would hypothesize that we actually help them by giving them testosterone, and that’s what I tell my patients.”
CHICAGO – Hypogonadism may compromise the long-term health outlook for many younger men who have been successfully treated for testicular cancer, according to an analysis conducted by the Platinum Study Group.
“Today, 95% of all testicular cancer patients are cured of their disease thanks to cisplatin-based chemotherapy. Nowadays, testicular cancer survivors can expect to live for over 40 years from the time of their diagnosis,” lead investigator Mohammad Issam Abu Zaid, MBBS, said in a press briefing at the annual meeting of the American Society of Clinical Oncology. “However, they are at risk of other health problems that may be related to their cancer treatment, including late complications from chemotherapy.”
“Testicular cancer survivors, especially those treated with chemotherapy, are at increased risk for hypogonadism, a problem that can be associated with predisposing factors for heart disease,” summarized Dr. Abu Zaid, of Indiana University in Indianapolis. “Mitigating approaches are the usual weight control, exercise, and monitoring of blood pressure and cholesterol levels.”
Expert perspective
“This is an important study, and it sends a loud message to those of us who take care of testis cancer patients, my area of expertise. ... We need to watch for hypogonadism, and we need to ask survivors about it. We need to examine them thinking about it, and, in patients who we are worried [they] might have hypogonadism, we need to do blood tests for testosterone and other hormone levels,” commented ASCO Expert Timothy D. Gilligan, MD, MSc, of the Cleveland Clinic in Ohio. “These are young patients, they have many years of life, so it’s many decades of suffering from consequences of this if it’s undetected.”
The findings were not surprising based on his personal experience and on evidence from the prostate cancer field showing the adverse metabolic effects of withdrawing testosterone, he said. Although the prevalence of hypogonadism found in the study was higher than that found in other studies, given the large size of the cohort, it should be taken seriously. Additionally, even if the true prevalence is somewhat lower, the absolute number of survivors affected would be substantial.
“We need to be cautious though and make sure people don’t misunderstand and think that this means we should test testosterone levels in all patients, which is a risky thing to do because the definition of normal testosterone is very fuzzy,” Dr. Gilligan stressed. “There is a wide range of normal, and what’s normal for me may not be the same as what’s normal for another man. So, looking for symptoms is really what guides this work, and, when there are symptoms, then testing is important.”
Study details
The Platinum Study is a large, ongoing, multicenter North American–based cohort study of testicular cancer survivors who received cisplatin as part of their chemotherapy.
Dr. Abu Zaid and his colleagues analyzed data from 491 participants who were aged 50 years or younger at diagnosis. All completed questionnaires addressing comorbidities, medications, and health behaviors; underwent physical examination; had measurement of testosterone levels; and had a genetic analysis.
The men were by and large young at the time of evaluation, with a mean age of just 38 years, he reported in the press briefing and a poster session at the meeting.
Overall, 38.5% had hypogonadism, defined in the study as a serum testosterone level of 3 ng/mL or lower or as use of testosterone replacement therapy.
In a multivariate analysis, survivors’ odds of hypogonadism increased with age (odds ratio, 1.41 per 10-year increment; P = .007) and were higher for those having a body mass index of 25 kg/m2 or greater, vs. lower (OR, 2.22; P = .003). On the other hand, there was a trend whereby survivors who engaged in any vigorous-intensity physical activity, vs. none, were less likely to have hypogonadism (OR, 0.64; P = .06).
The odds rose with number of risk alleles in the sex hormone–binding globulin gene, but findings were not significant, Dr. Abu Zaid said. They were also statistically indistinguishable across groups differing with respect to the chemotherapy regimen received and socioeconomic factors.
Compared with counterparts having normal testosterone levels, survivors having hypogonadism were up to four times more likely to be taking medication to treat dyslipidemia (20.1% vs. 6.0%; P less than .001), hypertension (18.5% vs. 10.6%; P = .013), erectile dysfunction (19.6% vs. 11.9%; P = .018), diabetes (5.8% vs. 2.6%; P = .067), and anxiety or depression (14.8% vs. 9.3%; P = .060).
Testicular cancer survivors should not universally have testosterone testing, agreed Dr. Abu Zaid. Such a practice might lead to overtreatment, and, in older men at least, use of testosterone itself has been linked to elevated cardiovascular risk.
“You really want to treat somebody who has symptoms related to hypogonadism: constant fatigue, night sweats, and depressed mood and some other symptoms that are well known to the clinician,” he maintained. “At the moment, we have no studies done looking at testosterone replacement in young men. I would hypothesize that we actually help them by giving them testosterone, and that’s what I tell my patients.”
AT ASCO 2017
Key clinical point:
Major finding: Fully 38.5% of survivors had low testosterone levels, and men in this group were more likely to be taking medications for dyslipidemia, hypertension, diabetes, erectile dysfunction, and anxiety or depression.
Data source: A cohort study of 491 testicular cancer survivors who had been treated with cisplatin-based chemotherapy (Platinum Study).
Disclosures: Dr. Abu Zaid reported that he had no relevant disclosures.
VIDEO: Survival improves when cancer patients self-report symptoms
CHICAGO – Patients with metastatic cancer who self-reported symptoms during routine cancer treatment experienced a number of benefits, including a statistically significant improvement in overall survival, according to findings from a randomized, controlled clinical trial.
The median overall survival among 441 patients receiving treatment for metastatic breast, lung, genitourinary, or gynecologic cancer who were randomized to the intervention arm was more than 5 months longer – a nearly 20% increase – than in 325 patients who received standard care (31.2 vs. 26 months), Ethan Basch, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Additionally, 31% of patients in the intervention arm had better quality of life/physical functioning, compared with those in the control arm, and 7% fewer patients in the intervention arm visited an emergency room during the course of the study. The duration of potentially life-prolonging chemotherapy was increased by an average of 2 months in the intervention arm, he said.
The findings were simultaneously published online in a research letter in JAMA (2017 Jun 4. doi: 10.1001/jama.2017.7156).
Symptoms such as nausea, pain, and fatigue are common among patients with metastatic cancer, Dr. Basch said. “Unfortunately, they often go undetected by doctors and nurses until they become severe and physically debilitating,” he added, explaining that patients are often hesitant to call the office to report symptoms between visits.
Even at office visits, competing topics can interfere with communication about symptoms, he noted.
He and his colleagues hypothesized that self-reporting of patient symptoms between visits or prior to a visit while in the clinic waiting area would prompt earlier intervention and improve symptom control and outcomes.
Study subjects were patients at Memorial Sloan Kettering Cancer Center who had advanced solid genitourinary, gynecologic, breast, or lung tumors and who were receiving outpatient chemotherapy. Those assigned to the intervention group used tablet computers and an online web survey system to report on 12 symptoms commonly experienced during chemotherapy. The system triggers an alert to a nurse when a severe or worsening symptom is reported. Patients in the usual care group discussed symptoms during office visits and were encouraged to call the office between visits if they experienced concerning symptoms.
Patients remained on the study until discontinuation of all cancer treatment, hospice, or death.
One possible explanation for the findings is that this self-reporting approach prompts clinicians to manage symptoms before they cause serious downstream complications, Dr. Basch said.
The approach may also keep patients more physically functional, which is known from prior studies to have a strong association with better survival, and the approach may also improve management of chemotherapy side effects, enabling longer duration of beneficial cancer treatment, he said, explaining that, “in oncology, we often are limited in our ability to give life-prolonging treatment because people don’t tolerate it well.”
“This approach should be considered for inclusion in standard symptoms management as a component of high quality cancer care,” he concluded, noting that efforts are underway to test the next generation of systems to improve communication between patients and care teams and to figure out how best to integrate these tools into oncology practice.
The system used in the this study was designed for research, but a number of companies have tools currently available for patient-reported outcomes, and others are being developed, Dr. Basch said, noting that a National Cancer Institute questionnaire – the PRO-CTCAE – is publicly available and could be loaded into patients’ electronic health records for this purpose as well.
ASCO’s chief medical officer, Richard L. Schilsky, MD, said the findings demonstrate that “these frequent touches between the patient and their health care providers obviously can make a huge difference in their outcomes.”
Additionally, ASCO expert Harold J. Burstein, MD, of Dana-Farber Cancer Institute, Boston, said this “exciting and compelling study” validates the feeling among many clinicians that patient-focused, team-based care can improve outcomes in a meaningful way for patients. In a video interview, he further discusses the challenges with implementing a system like this and particularly with obtaining funding to support implementation.
“If this was a drug, if it was iPad-olizumab, it would be worth tens, if not hundreds of thousands, of dollars per year to have something that improved overall survival. We don’t have those same kinds of dollars to help implement these into our electronic health records or our systems. We need to find ways to support that and make it happen,” he said.
This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr. Basch and Dr. Burstein each reported having no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Patients with metastatic cancer who self-reported symptoms during routine cancer treatment experienced a number of benefits, including a statistically significant improvement in overall survival, according to findings from a randomized, controlled clinical trial.
The median overall survival among 441 patients receiving treatment for metastatic breast, lung, genitourinary, or gynecologic cancer who were randomized to the intervention arm was more than 5 months longer – a nearly 20% increase – than in 325 patients who received standard care (31.2 vs. 26 months), Ethan Basch, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Additionally, 31% of patients in the intervention arm had better quality of life/physical functioning, compared with those in the control arm, and 7% fewer patients in the intervention arm visited an emergency room during the course of the study. The duration of potentially life-prolonging chemotherapy was increased by an average of 2 months in the intervention arm, he said.
The findings were simultaneously published online in a research letter in JAMA (2017 Jun 4. doi: 10.1001/jama.2017.7156).
Symptoms such as nausea, pain, and fatigue are common among patients with metastatic cancer, Dr. Basch said. “Unfortunately, they often go undetected by doctors and nurses until they become severe and physically debilitating,” he added, explaining that patients are often hesitant to call the office to report symptoms between visits.
Even at office visits, competing topics can interfere with communication about symptoms, he noted.
He and his colleagues hypothesized that self-reporting of patient symptoms between visits or prior to a visit while in the clinic waiting area would prompt earlier intervention and improve symptom control and outcomes.
Study subjects were patients at Memorial Sloan Kettering Cancer Center who had advanced solid genitourinary, gynecologic, breast, or lung tumors and who were receiving outpatient chemotherapy. Those assigned to the intervention group used tablet computers and an online web survey system to report on 12 symptoms commonly experienced during chemotherapy. The system triggers an alert to a nurse when a severe or worsening symptom is reported. Patients in the usual care group discussed symptoms during office visits and were encouraged to call the office between visits if they experienced concerning symptoms.
Patients remained on the study until discontinuation of all cancer treatment, hospice, or death.
One possible explanation for the findings is that this self-reporting approach prompts clinicians to manage symptoms before they cause serious downstream complications, Dr. Basch said.
The approach may also keep patients more physically functional, which is known from prior studies to have a strong association with better survival, and the approach may also improve management of chemotherapy side effects, enabling longer duration of beneficial cancer treatment, he said, explaining that, “in oncology, we often are limited in our ability to give life-prolonging treatment because people don’t tolerate it well.”
“This approach should be considered for inclusion in standard symptoms management as a component of high quality cancer care,” he concluded, noting that efforts are underway to test the next generation of systems to improve communication between patients and care teams and to figure out how best to integrate these tools into oncology practice.
The system used in the this study was designed for research, but a number of companies have tools currently available for patient-reported outcomes, and others are being developed, Dr. Basch said, noting that a National Cancer Institute questionnaire – the PRO-CTCAE – is publicly available and could be loaded into patients’ electronic health records for this purpose as well.
ASCO’s chief medical officer, Richard L. Schilsky, MD, said the findings demonstrate that “these frequent touches between the patient and their health care providers obviously can make a huge difference in their outcomes.”
Additionally, ASCO expert Harold J. Burstein, MD, of Dana-Farber Cancer Institute, Boston, said this “exciting and compelling study” validates the feeling among many clinicians that patient-focused, team-based care can improve outcomes in a meaningful way for patients. In a video interview, he further discusses the challenges with implementing a system like this and particularly with obtaining funding to support implementation.
“If this was a drug, if it was iPad-olizumab, it would be worth tens, if not hundreds of thousands, of dollars per year to have something that improved overall survival. We don’t have those same kinds of dollars to help implement these into our electronic health records or our systems. We need to find ways to support that and make it happen,” he said.
This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr. Basch and Dr. Burstein each reported having no disclosures.
CHICAGO – Patients with metastatic cancer who self-reported symptoms during routine cancer treatment experienced a number of benefits, including a statistically significant improvement in overall survival, according to findings from a randomized, controlled clinical trial.
The median overall survival among 441 patients receiving treatment for metastatic breast, lung, genitourinary, or gynecologic cancer who were randomized to the intervention arm was more than 5 months longer – a nearly 20% increase – than in 325 patients who received standard care (31.2 vs. 26 months), Ethan Basch, MD, reported at the annual meeting of the American Society of Clinical Oncology.
Additionally, 31% of patients in the intervention arm had better quality of life/physical functioning, compared with those in the control arm, and 7% fewer patients in the intervention arm visited an emergency room during the course of the study. The duration of potentially life-prolonging chemotherapy was increased by an average of 2 months in the intervention arm, he said.
The findings were simultaneously published online in a research letter in JAMA (2017 Jun 4. doi: 10.1001/jama.2017.7156).
Symptoms such as nausea, pain, and fatigue are common among patients with metastatic cancer, Dr. Basch said. “Unfortunately, they often go undetected by doctors and nurses until they become severe and physically debilitating,” he added, explaining that patients are often hesitant to call the office to report symptoms between visits.
Even at office visits, competing topics can interfere with communication about symptoms, he noted.
He and his colleagues hypothesized that self-reporting of patient symptoms between visits or prior to a visit while in the clinic waiting area would prompt earlier intervention and improve symptom control and outcomes.
Study subjects were patients at Memorial Sloan Kettering Cancer Center who had advanced solid genitourinary, gynecologic, breast, or lung tumors and who were receiving outpatient chemotherapy. Those assigned to the intervention group used tablet computers and an online web survey system to report on 12 symptoms commonly experienced during chemotherapy. The system triggers an alert to a nurse when a severe or worsening symptom is reported. Patients in the usual care group discussed symptoms during office visits and were encouraged to call the office between visits if they experienced concerning symptoms.
Patients remained on the study until discontinuation of all cancer treatment, hospice, or death.
One possible explanation for the findings is that this self-reporting approach prompts clinicians to manage symptoms before they cause serious downstream complications, Dr. Basch said.
The approach may also keep patients more physically functional, which is known from prior studies to have a strong association with better survival, and the approach may also improve management of chemotherapy side effects, enabling longer duration of beneficial cancer treatment, he said, explaining that, “in oncology, we often are limited in our ability to give life-prolonging treatment because people don’t tolerate it well.”
“This approach should be considered for inclusion in standard symptoms management as a component of high quality cancer care,” he concluded, noting that efforts are underway to test the next generation of systems to improve communication between patients and care teams and to figure out how best to integrate these tools into oncology practice.
The system used in the this study was designed for research, but a number of companies have tools currently available for patient-reported outcomes, and others are being developed, Dr. Basch said, noting that a National Cancer Institute questionnaire – the PRO-CTCAE – is publicly available and could be loaded into patients’ electronic health records for this purpose as well.
ASCO’s chief medical officer, Richard L. Schilsky, MD, said the findings demonstrate that “these frequent touches between the patient and their health care providers obviously can make a huge difference in their outcomes.”
Additionally, ASCO expert Harold J. Burstein, MD, of Dana-Farber Cancer Institute, Boston, said this “exciting and compelling study” validates the feeling among many clinicians that patient-focused, team-based care can improve outcomes in a meaningful way for patients. In a video interview, he further discusses the challenges with implementing a system like this and particularly with obtaining funding to support implementation.
“If this was a drug, if it was iPad-olizumab, it would be worth tens, if not hundreds of thousands, of dollars per year to have something that improved overall survival. We don’t have those same kinds of dollars to help implement these into our electronic health records or our systems. We need to find ways to support that and make it happen,” he said.
This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr. Basch and Dr. Burstein each reported having no disclosures.
AT THE 2017 ASCO ANNUAL MEETING
Key clinical point:
Major finding: Median overall survival was 31.2, vs. 26 months, with self-reporting of symptoms, vs. usual care.
Data source: A randomized controlled clinical trial of 766 patients.
Disclosures: This study was supported by the National Institutes of Health and the Conquer Cancer Foundation of the American Society of Clinical Oncology. Dr. Basch and Dr. Burstein each reported having no disclosures.
Test goes wide and deep to detect free tumor DNA in blood
CHICAGO – An experimental liquid biopsy method plumbs the depths of the genome to detect a broad array of mutations in blood samples that closely correspond to variants in tumors.
The high-intensity method for detecting circulating free DNA (cfDNA) in plasma detected at least one significant genetic variant in samples from 89% of patients with advanced breast, lung, and prostate cancers.
If validated in further studies, the technique could inform more accurate drug selection in patients with refractory disease and eventually may form the basis for plasma-based assays to detect early-stage cancers, said Pedram Razavi, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“This novel, high-intensity sequencing assay incorporates an unprecedented combination of depth and breadth of coverage compared to previous assays. High levels of concordance for variants between plasma and tissue provide strong evidence for high rates of tumor DNA detection in plasma,” he said at a press briefing at the annual meeting of the American Society of Clinical Oncology.
The technique also provides important insights into tumor biology, “including first exploration of mutational signatures in the plasma,” he added.
Deep scanning
The high-intensity sequencing method scans for 508 genes in a 2 million base-pair swath of genome, performing 60,000 repeat reads on each genome region to improve the assay’s sensitivity, and deep sequencing allows for detection of rare “needle-in-a-haystack” variants.
Dr. Razavi and his colleagues examined prospectively collected blood and tissues from 161 patients, 124 of whom had samples sufficient for concordance studies.
The samples were collected within 6 weeks of a de novo cancer diagnosis or evidence of disease progression, before the initiation of therapy.
Both cfDNA and genomic data from white blood cells (WBCs) of each patient were sequenced with the aforementioned 508-gene panel covering a broad range of known cancer variants and mutations.
Tumor tissues were sequenced with MSK-IMPACT, a 410 gene assay, with blinding of results in regard to plasma and WBC sequencing. Variants were classified as clonal or subclonal based on tumor sequencing in breast cancer and non–small cell lung cancer (NSCLC).
As noted before, 89% of patients had at least one genetic variant detected in both the tumor and in plasma, including 97% of patients with metastatic breast cancer, 85% of those with NSCLC, and 84% of patients with metastatic prostate cancer.
The investigators identified 864 clonal or subclonal variants in tissue samples from all three of these cancers, and 627 of the variants also were found in plasma.
In addition, 76% of clinically actionable somatic mutations identified in tumors also were found in plasma, which suggests that the high-intensity sequencing technique may be able to identify tumor heterogeneity that is not always evident in single tumor biopsies, Dr. Razavi said.
‘A clear advance’
“The work by Dr. Razavi and colleagues is a clear advance in the field because it surveys for the first time a much broader panel of genes – 508 genes in this case – and it does it with much deeper sequencing, which means it can detect much rarer alterations,” commented ASCO expert John Heymach, MD, PhD, from the University of Texas MD Anderson Cancer Center in Houston.
The study “helps illuminate a path toward a day when we will be using circulating tumor DNA assays for early detection of cancer, and not just for selecting certain therapies,” he added.
“We’re a long way from utilizing liquid biopsy for detecting cancers; already though, we’re seeing some utility of circulating tumor DNA in the domain of identifying novel alterations as a means of segmentation clinical trials,” commented ASCO expert Sumanta Kumar Pal, MD, from City of Hope in Duarte, Calif.
Dr. Heymach and Dr. Pal were not involved in the study, but were invited discussants at the briefing.
The study was funded in part by GRAIL. Dr. Razavi reported institutional research funding from the company. Multiple coauthors are employees of the company.
CHICAGO – An experimental liquid biopsy method plumbs the depths of the genome to detect a broad array of mutations in blood samples that closely correspond to variants in tumors.
The high-intensity method for detecting circulating free DNA (cfDNA) in plasma detected at least one significant genetic variant in samples from 89% of patients with advanced breast, lung, and prostate cancers.
If validated in further studies, the technique could inform more accurate drug selection in patients with refractory disease and eventually may form the basis for plasma-based assays to detect early-stage cancers, said Pedram Razavi, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“This novel, high-intensity sequencing assay incorporates an unprecedented combination of depth and breadth of coverage compared to previous assays. High levels of concordance for variants between plasma and tissue provide strong evidence for high rates of tumor DNA detection in plasma,” he said at a press briefing at the annual meeting of the American Society of Clinical Oncology.
The technique also provides important insights into tumor biology, “including first exploration of mutational signatures in the plasma,” he added.
Deep scanning
The high-intensity sequencing method scans for 508 genes in a 2 million base-pair swath of genome, performing 60,000 repeat reads on each genome region to improve the assay’s sensitivity, and deep sequencing allows for detection of rare “needle-in-a-haystack” variants.
Dr. Razavi and his colleagues examined prospectively collected blood and tissues from 161 patients, 124 of whom had samples sufficient for concordance studies.
The samples were collected within 6 weeks of a de novo cancer diagnosis or evidence of disease progression, before the initiation of therapy.
Both cfDNA and genomic data from white blood cells (WBCs) of each patient were sequenced with the aforementioned 508-gene panel covering a broad range of known cancer variants and mutations.
Tumor tissues were sequenced with MSK-IMPACT, a 410 gene assay, with blinding of results in regard to plasma and WBC sequencing. Variants were classified as clonal or subclonal based on tumor sequencing in breast cancer and non–small cell lung cancer (NSCLC).
As noted before, 89% of patients had at least one genetic variant detected in both the tumor and in plasma, including 97% of patients with metastatic breast cancer, 85% of those with NSCLC, and 84% of patients with metastatic prostate cancer.
The investigators identified 864 clonal or subclonal variants in tissue samples from all three of these cancers, and 627 of the variants also were found in plasma.
In addition, 76% of clinically actionable somatic mutations identified in tumors also were found in plasma, which suggests that the high-intensity sequencing technique may be able to identify tumor heterogeneity that is not always evident in single tumor biopsies, Dr. Razavi said.
‘A clear advance’
“The work by Dr. Razavi and colleagues is a clear advance in the field because it surveys for the first time a much broader panel of genes – 508 genes in this case – and it does it with much deeper sequencing, which means it can detect much rarer alterations,” commented ASCO expert John Heymach, MD, PhD, from the University of Texas MD Anderson Cancer Center in Houston.
The study “helps illuminate a path toward a day when we will be using circulating tumor DNA assays for early detection of cancer, and not just for selecting certain therapies,” he added.
“We’re a long way from utilizing liquid biopsy for detecting cancers; already though, we’re seeing some utility of circulating tumor DNA in the domain of identifying novel alterations as a means of segmentation clinical trials,” commented ASCO expert Sumanta Kumar Pal, MD, from City of Hope in Duarte, Calif.
Dr. Heymach and Dr. Pal were not involved in the study, but were invited discussants at the briefing.
The study was funded in part by GRAIL. Dr. Razavi reported institutional research funding from the company. Multiple coauthors are employees of the company.
CHICAGO – An experimental liquid biopsy method plumbs the depths of the genome to detect a broad array of mutations in blood samples that closely correspond to variants in tumors.
The high-intensity method for detecting circulating free DNA (cfDNA) in plasma detected at least one significant genetic variant in samples from 89% of patients with advanced breast, lung, and prostate cancers.
If validated in further studies, the technique could inform more accurate drug selection in patients with refractory disease and eventually may form the basis for plasma-based assays to detect early-stage cancers, said Pedram Razavi, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“This novel, high-intensity sequencing assay incorporates an unprecedented combination of depth and breadth of coverage compared to previous assays. High levels of concordance for variants between plasma and tissue provide strong evidence for high rates of tumor DNA detection in plasma,” he said at a press briefing at the annual meeting of the American Society of Clinical Oncology.
The technique also provides important insights into tumor biology, “including first exploration of mutational signatures in the plasma,” he added.
Deep scanning
The high-intensity sequencing method scans for 508 genes in a 2 million base-pair swath of genome, performing 60,000 repeat reads on each genome region to improve the assay’s sensitivity, and deep sequencing allows for detection of rare “needle-in-a-haystack” variants.
Dr. Razavi and his colleagues examined prospectively collected blood and tissues from 161 patients, 124 of whom had samples sufficient for concordance studies.
The samples were collected within 6 weeks of a de novo cancer diagnosis or evidence of disease progression, before the initiation of therapy.
Both cfDNA and genomic data from white blood cells (WBCs) of each patient were sequenced with the aforementioned 508-gene panel covering a broad range of known cancer variants and mutations.
Tumor tissues were sequenced with MSK-IMPACT, a 410 gene assay, with blinding of results in regard to plasma and WBC sequencing. Variants were classified as clonal or subclonal based on tumor sequencing in breast cancer and non–small cell lung cancer (NSCLC).
As noted before, 89% of patients had at least one genetic variant detected in both the tumor and in plasma, including 97% of patients with metastatic breast cancer, 85% of those with NSCLC, and 84% of patients with metastatic prostate cancer.
The investigators identified 864 clonal or subclonal variants in tissue samples from all three of these cancers, and 627 of the variants also were found in plasma.
In addition, 76% of clinically actionable somatic mutations identified in tumors also were found in plasma, which suggests that the high-intensity sequencing technique may be able to identify tumor heterogeneity that is not always evident in single tumor biopsies, Dr. Razavi said.
‘A clear advance’
“The work by Dr. Razavi and colleagues is a clear advance in the field because it surveys for the first time a much broader panel of genes – 508 genes in this case – and it does it with much deeper sequencing, which means it can detect much rarer alterations,” commented ASCO expert John Heymach, MD, PhD, from the University of Texas MD Anderson Cancer Center in Houston.
The study “helps illuminate a path toward a day when we will be using circulating tumor DNA assays for early detection of cancer, and not just for selecting certain therapies,” he added.
“We’re a long way from utilizing liquid biopsy for detecting cancers; already though, we’re seeing some utility of circulating tumor DNA in the domain of identifying novel alterations as a means of segmentation clinical trials,” commented ASCO expert Sumanta Kumar Pal, MD, from City of Hope in Duarte, Calif.
Dr. Heymach and Dr. Pal were not involved in the study, but were invited discussants at the briefing.
The study was funded in part by GRAIL. Dr. Razavi reported institutional research funding from the company. Multiple coauthors are employees of the company.
AT ASCO 2017
Key clinical point: High-intensity sequencing of plasma samples appears capable of detecting actionable tumor mutations in a large proportion of samples.
Major finding: In 89% of patients with advanced cancers, genetic variants were identified in both tumor samples and circulating free DNA testing.
Data source: Prospective study of tissue and plasma samples from 124 patients with non–small cell lung cancer or metastatic breast and prostate cancers.
Disclosures: The study was funded in part by GRAIL. Dr. Razavi reported institutional research funding from the company. Multiple coauthors are employees of the company.