User login
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
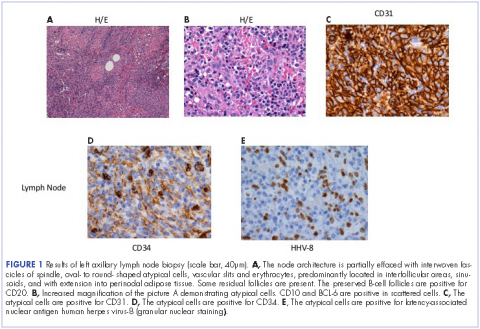
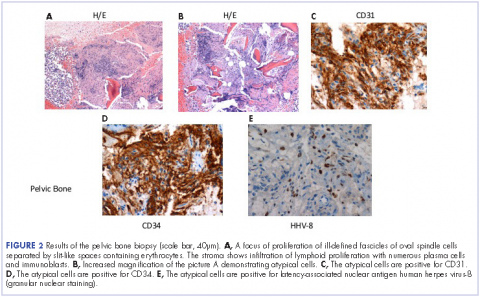
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
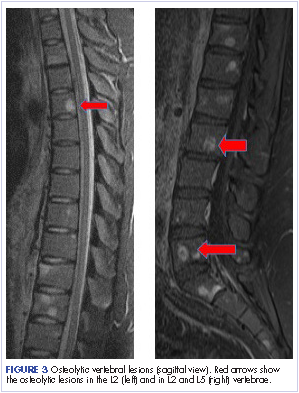
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.