User login
Ibrutinib becomes first FDA-approved treatment for chronic GVHD
Ibrutinib (Imbruvica) added another notch on its indications belt with its Aug. 2 approval by the U.S. Food and Drug Administration for the treatment of adult patients with chronic graft versus host disease (cGVHD) after failure of one or more lines of systemic therapy.
The new indication makes ibrutinib the first FDA-approved therapy for the treatment of cGVHD, according to an FDA press release.
Ibrutinib’s other approved indications include chronic lymphocytic leukemia/small lymphocytic lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma with 17p deletion, Waldenström’s macroglobulinemia, marginal zone lymphoma, and mantle cell lymphoma, according to a press release from the FDA.
The recommended dose of ibrutinib for cGVHD is 420 mg (three 140 mg capsules once daily). Prescribing information is available on the FDA website.
Imbruvica is manufactured by Pharmacyclics.
[email protected]
On Twitter @maryjodales
Ibrutinib (Imbruvica) added another notch on its indications belt with its Aug. 2 approval by the U.S. Food and Drug Administration for the treatment of adult patients with chronic graft versus host disease (cGVHD) after failure of one or more lines of systemic therapy.
The new indication makes ibrutinib the first FDA-approved therapy for the treatment of cGVHD, according to an FDA press release.
Ibrutinib’s other approved indications include chronic lymphocytic leukemia/small lymphocytic lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma with 17p deletion, Waldenström’s macroglobulinemia, marginal zone lymphoma, and mantle cell lymphoma, according to a press release from the FDA.
The recommended dose of ibrutinib for cGVHD is 420 mg (three 140 mg capsules once daily). Prescribing information is available on the FDA website.
Imbruvica is manufactured by Pharmacyclics.
[email protected]
On Twitter @maryjodales
Ibrutinib (Imbruvica) added another notch on its indications belt with its Aug. 2 approval by the U.S. Food and Drug Administration for the treatment of adult patients with chronic graft versus host disease (cGVHD) after failure of one or more lines of systemic therapy.
The new indication makes ibrutinib the first FDA-approved therapy for the treatment of cGVHD, according to an FDA press release.
Ibrutinib’s other approved indications include chronic lymphocytic leukemia/small lymphocytic lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma with 17p deletion, Waldenström’s macroglobulinemia, marginal zone lymphoma, and mantle cell lymphoma, according to a press release from the FDA.
The recommended dose of ibrutinib for cGVHD is 420 mg (three 140 mg capsules once daily). Prescribing information is available on the FDA website.
Imbruvica is manufactured by Pharmacyclics.
[email protected]
On Twitter @maryjodales
Pancreatitis associated with newer classes of antineoplastic therapies
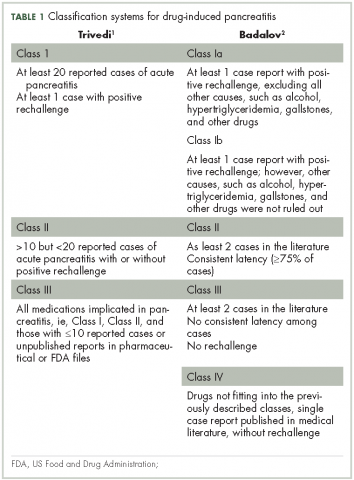
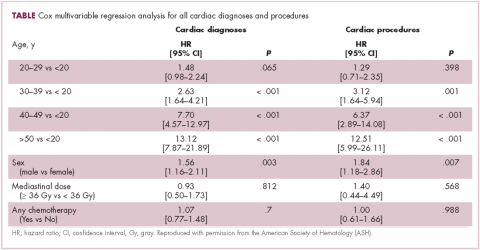
Patients with advanced malignancies may develop pancreatitis during therapy for their cancer. Acute pancreatitis is inflammation of the pancreas. Common symptoms include abdominal pain, nausea, vomiting, shortness of breath, dehydration. Laboratory evidence of acute pancreatitis includes elevations of the amylase and lipase. Mild pancreatitis occurs when there is no organ dysfunction, moderate pancreatitis is associated with one organ dysfunction, and severe pancreatitis is complicated by multiple organ dysfunction. Hypotension, hypocalcemia, or anemia suggest a more severe course of the pancreatitis. In some instances, the pancreatitis may be an adverse reaction to the therapy being given. However, other causes such as hypercalcemia, hypertriglyceridemia, cholelithiasis, and underlying malignancy must be ruled out before ascribing pancreatitis to a specific drug. To date, two classifications systems have been proposed by Trivedi1 and Badalov2 to evaluate the degree to which a drug is responsible for pancreatitis (Table 1). Furthermore, Naranjo and colleagues have proposed a more general method of assessing the causal relationship between drugs and adverse events.3 The Naranjo algorithm is not specific for pancreatitis. Jones and colleagues4 reported that 0.1%-2% of acute pancreatitis cases were owing to drugs. In 2015, they listed the older chemotherapy agents associated with pancreatitis. However, more recently, many new agents have been approved for the management of cancers. The newer classes of antineoplastic agents including proteasome inhibitors, immune-modulating agents, tyrosine kinase inhibitors, monoclonal antibodies against programmed cell death-1 (PD-1) and its ligand PD-L1 and antibody-toxin conjugates are now associated with acute pancreatitis.
Methods
We conducted a search in PubMed, Google Scholar, and Micromedex for pancreatitis related to antineoplastic agents, including proteasome inhibitors, immune checkpoint inhibitors, monoclonal antibodies, immune-modulating agents, drug-induced pancreatitis. Terms used for the searches included each specific agent and pancreatitis, immunotherapy and pancreatitis, tyrosine kinase inhibitors and pancreatitis, auto immune pancreatitis, and toxicities of molecular target therapies. Reference lists from the identified manuscripts were reviewed for further studies of pancreatitis as a result of antineoplastic therapy. The most recent search date was February 15, 2017.
The degree to which each agent was associated with inducing pancreatitis was evaluated using the Badalov classification system2 in addition to the Naranjo Adverse Drug Reaction (ADR) Probability Scale.3 The Naranjo scale consists of 10 questions with values assigned to each answer. Total scores range from -4 to 13, where 13-9 indicates the reaction is considered definitely attributable to the drug; 8-5, probably attributable; 4-1, possibly attributable; and ≤0, doubtful if attributable.
A total of 67 manuscripts and abstracts were identified. Four manuscripts and 3 abstracts were excluded because they had insufficient information about possible pancreatitis or there was a presence of multiple other agents or conditions that might have caused pancreatitis. In total, 60 publications met inclusion criteria and were evaluated.
Results
Immune checkpoint inhibitors
In a review of toxicities of anti-programmed cell death-1 (PD-1) therapy, pancreatitis was reported to occur in about 1.8% of patients who received nivolumab or pembrolizumab.5 The 9 patients with pancreatitis attributed to an immune etiology were treated with corticosteroids. Pancreatitis was grade 2 in 3 patients (1.5-2 times upper limit of normal [ULN]), grade 3 in 4 patients (>2-5 ULN), and grade 4 ( >5 ULN) in 2 patients.
In asymptomatic individuals, pancreatitis has been detected on a positron-emission tomography–computed tomography (CT) scan after anti-PD-1 therapy.5 By contrast, there was a case report of a patient treated with nivolumab for lung cancer who developed anorexia, vomiting, and back pain on day 18 of therapy with an elevation of the amylase and lipase levels, but a negative CT.6 Later the patient developed a swollen pancreas on CT. Autoimmune pancreatitis comes in two forms. The most common relates to elevated levels of immunoglobulin G4 (IgG4; normal, 135 mg/dL ULN)7 The mechanism of immune pancreatitis associated with anti-PD-1 therapy is unknown.
Ipilimumab (an anti-CTLA4 antibody) has been approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. Pancreatitis occurred in 1 patient in a phase 1 trial in pediatric patients.9 In a summary of 14 phase 1-3 trials of ipilimumab in advanced melanoma, pancreatitis was reported in fewer than 1% of the patients.10 In management guidelines for therapy with ipilimumab, pancreatitis may present as an asymptomatic increase in the levels of amylase and lipase, or with fevers, malaise, or abdominal pain. Oral prednisone or dexamethasone were given for the immune pancreatitis, but the decline in enzymes was slow, often taking months.11 In a preclinical model of autoimmune pancreatitis due to the blocking of CTLA4, there was suppression of regulatory T-cell function. The autoimmune pancreatitis responded to cyclosporin or rapamycin but there are no clinical data for these agents.12 The anti-PD-L1 agent atezolizumab has been associated with acute pancreatitis in 2 of 1,978 patients (0.1%).13 A review by Champiat and colleagues on dysimmune toxcities related to immune checkpoint inhibitors includes pancreatitis as an autoimmune complication of such therapies.14
Blinatumomab is an anti-CD19–directed CD3 T-cell engager that has been approved by the FDA for refractory B-cell acute lymphoblastic leukemia. In August 2016, the maker of the drug, Amgen, advised hematologists and oncologists that since February 2016, 10 patients out of more than 2,000 treated with blinatumomab had developed pancreatitis.15 Other medications the patients were receiving such as high-dose steroids might have caused or contributed to the pancreatitis. In one case, the pancreatitis improved with stopping blinatumomab but worsened with re-challenge. It is possible that the mechanism of the associated pancreatitis relates to a change in immune checkpoint inhibition. CD19-positive, CD24-high, CD27-positive regulatory B cells are decreased in autoimmune pancreatitis.16 Treatment with blinatumomab may decrease the CD19-positive cells.
Molecularly targeted agents, including TKIs
Molecularly targeted agents such as tyrosine kinase inhibitors (TKIs) or other kinase inhibitors have been associated with pancreatitis.17, 18 In a retrospective study by Tiruman and colleagues,19 the investigators found 91 patients with pancreatitis on imaging, of whom 15 were receiving molecularly target drugs. The pancreatitis was asymptomatic in 2 patients, but 13 had abdominal pain, many with nausea. Four of the patients also had gallstones, but the drug was deemed to be the cause of the pancreatitis. In 4 of the 9 patients in whom a rechallenge was done with the TKI, the pancreatitis relapsed. The pancreatitis resolved in 14 of the 15 patients; 1 patient died because of progressive cancer before the pancreatitis resolved. The pancreatitis was mild, 7 of the 15 patients had normal pancreatic enzymes and the pancreatitis was diagnosed by radiology.
Ghatlia and colleagues17 performed a meta-analysis of trials of TKI. They found 9 cases of pancreatitis in patients on sunitinib therapy. Of those, 4 patients were on sunitinib alone, and 5 were on other chemotherapy agents in combination with sunitinib. Eight cases of pancreatitis due to sorafenib were found. Three of the patients were on sorafenib alone, and 5 were on other chemotherapy including 1 on transcatheter embolization (TACE). Three cases of pancreatitis were associated with vandetanib; 2 of those patients had other concurrent chemotherapy. One case of axitinib induced pancreatitis was described.
Pancreatitis was reported in the phase 1 trials of sorafenib and sunitinib. In all, 3 of 69 patients treated with sorafenib had grade 3 pancreatitis and asymptomatic elevations of amylase and lipase levels were present in about 5% of patients receiving sunitinib.18,19
Other TKIs associated with pancreatitis include pazopanib,20,21 axitinib,22 and nilotinib.23 Pezzilli and coleagues24 described 5 patients with pancreatitis on sorafenib, 3 on sunitinib, 6 on nilotinib. It is possible that some of these cases appeared in other reviews. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, caused a single case of pancreatitis in 9 patients.25
Vemurafenib, a BRAF kinase inhibitor, was associated with pancreatitis in one case. In this case, the pancreatitis resolved on stopping the medication but recurred when vemurafenib rechallenge was attempted.26 There is a report of dabrafenib being associated with pancreatitis in 1 patient.27
Agents that inhibit the TKIs associated with BCR-ABL in chronic myelogenous leukemia are associated with acute pancreatitis. Imatinib-induced pancreatitis was reported in a small number of cases.28 Nilotinib has caused amylase/lipase elevations with and without symptomatic pancreatitis.29,30 Ponatinib, an inhibitor of BCR-ABL tyrosine kinase, is associated with pancreatitis.31 Pancreatitis occurred in 11 of 81 patients treated with ponatinib, and in 8 patients it was described as serious. Further elevation of amylase or lipase levels without clinical pancreatitis was noted in 7 other patients.
Proteosome inhibitors
In 2010, Elouni and colleagues32 reported a case of IV bortezomib-induced pancreatitis, which recurred on rechallenge with bortezomib. This same patient was also reported in an abstract in 2009.33 In 2009, there was an editorial comment which was added to the end of the abstract that the World Health Organization Adverse Drug Reaction database had 11 reports of bortezomib associated pancreatitis. Talamo and colleagues34 reported a case of bortezomib-induced pancreatitis due to bortezomib that had been administered subcutaneously. At that time, they also summarized 7 previous reports of bortezomib-associated pancreatitis. The mechanism of bortezomib-induced pancreatitis is not known.35-37
Fotoh and colleagues reported a patient with myeloma who had elevated triglyceride levels after bortezomib therapy.38 In one case of bortezomib-associated pancreatitis, the patient had an elevated triglyceride level, but it was not extremely high.39 Multiple myeloma itself may be associated with hyperlipidemia but only rarely.40 Gozetti and colleagues reported a patient who developed hyperlipidemia after two courses of bortezomib;41 stopping bisphosphonates may be associated with a rise in triglycerides. There was one case of carfilzomib causing pancreatitis during a phase 1 trial.42
Older chemotherapy agents
Reviews of drug-induced pancreatitis have listed many chemotherapy agents which may cause pancreatitis.1,43 The agent most frequently associated with acute pancreatitis has been asparaginase,44 with 2%-16% of patients undergoing asparaginase therapy developing pancreatitis. Asparaginase-related pancreatitis is grade 3 or 4 in 5%-10% of patients, and recurs in 63% of patients on rechallenge. Other chemotherapy agents associated with pancreatitis include: mercaptopurine, cytosine arabinoside, cisplatin, interferon alfa-2b, doxorubicin, tamoxifen, gefitinib, vinorelbine, oxaliplatin, levamisole, methotrexate, azathioprine, 5-fluorouracil, capecitabine, ifosfamide, paclitaxel, and all-trans retinoic acid.
Oxaliplatin carries a 0.1%-2% incidence of drug-induced pancreatitis. In one series of 6 patients, cessation of the agent allowed for resolution of symptoms and decrease in serum lipase and amylase levels.45 With capecitabine there are 2 case reports of pancreatitis.46 Cases of pancreatitis associated with trifluridine or tipiracil were not present in the literature.
Thalidomide caused severe pancreatitis in a patient when it was used to treat chronic graft-versus-host disease.47 This patient suffered recurrent pancreatitis on retreatment with the thalidomide. The authors further referenced two other suspected cases of thalidomide-induced, acute pancreatitis. However, in view of the extensive use of thalidomide for multiple myeloma before the development of lenalidomide, thalidomide-associated pancreatitis would be <1% of patients.
Agents that cause hypertriglyceridemia may cause pancreatitis. This mechanism has been reported as the cause of pancreatitis for everolimus48 and tamoxifen.49,50-52 Everolimus causes elevated triglycerides in 30%-50% of patients. There are case reports and a review of tamoxifen-associated pancreatitis caused by elevated triglycerides.52 There has also been a case of temsirolimus-associated pancreatitis,53 another agent that elevates triglycerides.
Pancreatitis associated with hepatic embolization or HIPEC
TACE leads to symptomatic acute pancreatitis in 0.4%-2% of patients, but nonselective TACE (into the hepatic artery and not just feeder vessels), may lead to elevated amylase levels in 15%-40% of patients.54-56 The risk of pancreatitis would depend on which chemotherapy drug is being infused into the liver. It would also be greater if the chemotherapy has to be infused into a larger part of the liver than into a small portion of the liver. In one patient, severe pancreatitis secondary to TACE occurred after two previous embolizations; prior embolization may have led to occlusion of the previously infused vessels.57 Radioembolization with 90Y microspheres was associated with one case of pancreatitis in 112 consecutive patients.58 The postembolization syndrome in the first 24 hours after the procedure may involve fever, abdominal pain, nausea, and vomiting due to acute pancreatitis in some instances.
Acute pancreatitis has also been described as a complication of hyperthermic intraperitoneal chemotherapy (HIPEC).59,60 Two of 13 patients receiving HIPEC for gastric cancer developed pancreatitis.59 In 25 patients with colon cancer who were treated with HIPEC, 1 patient had pancreatitis.60
Antibody-drug conjugates
Muzaffar and colleagues reported a patient with acute pancreatitis 3 days after starting therapy with ado-trastuzumab emtansine.61 Urru and colleagues62 reported a patient who developed acute pancreatitis after brentuximab vedotin therapy. Ghandi and colleagues63 identified 2 cases of fatal acute pancreatitis with brentuximab vedotin and 6 cases of nonfatal pancreatitis. Two of the nonfatal patients were rechallenged, and 1 developed recurrent pancreatitis. Because abdominal pain may occur in up to 18% of patients receiving brentuximab vedotin, the incidence of pancreatitis may be underestimated with this agent.64
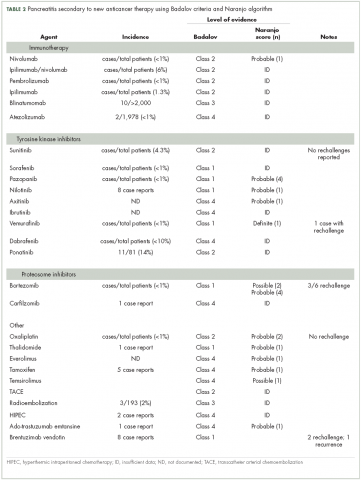
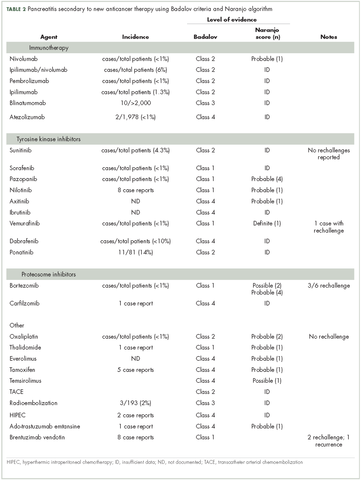
In Table 2, ado-trastuzumab emtansine and brentuximab vedotin are listed with incidence and level of association given by the Baldavo2 and Naranjo.3 With greater awareness, the incidence of pancreatitis associated with these agents may rise or fall as more data is accumulated. In many instances, there are insufficient numbers of reported cases or insufficient information in single-case reports to complete the entire table.
Discussion
Acute pancreatitis is an uncommon complication of tyrosine kinase inhibitors, other kinase inhibitors, proteasome inhibitors, monoclonal antibody-drug conjugates and anti-PD-1 immunotherapies. As nausea, abdominal pain, emesis are common in patients with cancer on antineoplastic therapy, some patients may have acute pancreatitis which is undiagnosed. It is not clear whether a patient with pancreatitis secondary to a TKI can be safely switched to a different TKI. As more molecularly targeted agents and more monoclonal antibodies targeting PD-L1 and PD-1 are under development, screening for amylase and lipase levels during phase 1/2 testing may prove helpful.
The natural history of cancer-drug–associated pancreatitis may depend on which agent is the cause. Although there are descriptions of the course of autoimmune pancreatitis, these studies have not included pancreatitis associated with anti-PD-L1 or -PD-1 therapies.65 It is possible that once an autoimmune pancreatitis has developed, simply stopping the inciting anti-PD-L1 or -PD-1 antibody may not lead to immediate resolution. Therapy with combined immune checkpoint blockade agents (eg, nivolumab and ipilimumab) may cause a higher incidence of pancreatitis than therapy with a single agent.66
In a report of 119 patients with melanoma who were treated with nivolumab and ipilimumab, there were 2 cases of acute pancreatitis, though 20% of patients had a grade 3 or higher amylase level, and just over 6% had a grade 3 or higher lipase.67 Stopping this type of immunotherapy early for grade 1,2, or 3 rises in pancreatic enzymes might prevent symptomatic pancreatitis from developing, but would stop potentially curative therapy for many patients who would have never developed clinically serious pancreatitis. Patients who suffer immune toxicities with anti-PD-1 therapies may be more apt to obtain some clinical benefit. The development of immune-related toxicities in patients treated with ipilimumab ( an anti CTLA4 antibody) seemed to correlate the tumor regression.68 This has also been suggested by the fact that the development of vitiligo correlates with clinical response in melanoma patients treated with nivolumab.69 Although clinically significant pancreatitis might be averted by stopping immune therapies earlier, stopping before it is deemed necessary might prevent cancer patients from receiving life-prolonging therapy.
Acute pancreatitis in general is severe in about 25% of cases and is associated with a significant risk of death. Scoring systems such as Ranson criteria and Apache 2 are used to assess the severity of pancreatitis although their utility is debated.70 Asparaginase is the chemotherapy agent most frequently associated with pancreatitis. It has been used to treat acute lymphoblastic leukemia for more than 30 years. This allowed for a study of 5,185 children and young adults who received asparaginase to determine what clinical factors and genomic factors were associated with the development of acute pancreatitis in 117 individuals.71 Further information gathered from programs such as the FDA and the adverse drug reaction program at Northwestern University in Chicago, coupled with the publication of other cases of pancreatitis associated with newer cancer agents may provide more insight into the mechanism causing pancreatitis due to a specific agent. With more cases being published, it may also become possible to determine if there are specific predisposing factors based on the clearance or metabolism of the offending agent or any genetic predisposition for drug-related pancreatitis.
1. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;29:709-716.
2. Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroeneterol Hepatol. 2007;5:648-661.
3. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
4. Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Oschner J. 2015;15:45-51.
5. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
6. Alabed YZ, Aghayev A, Sakellis C, et al. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-529.
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer. 2016;90:148-150.
8. Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532-539.
9. Merchant MS, Baird K, Wexler L, et al. Ipilimumab: first results of a phase I trial in pediatric patients with advanced solid tumors. J Clin Oncol. 2012;30:abstract 9545.
10. Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:abstract 8583.
11. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
12. Mayerle J, van den Brandt C, Schwaiger T, et al. Blockage of CTLA-4 suggests that autoimmune pancreatitis is a T-cell mediated disease responsive to ciclosporin A and rapamycin . Pancreatology. 2012;12:579(abstract S8-3).
13. Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2016.
14. Champiat S, Lambotte E, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27:559-574.
15. Amgen. New safety information for Blincyto (blinatumomab) Risk of pancreatitis. August 2016 and update to Micromedex 2016.
16. Sumimoto K, Uchida K, KusudaT, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high, CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis . Pancreatology. 2014;14:193-200.
17. Ghatalia P, Morgan CJ, Choueiri TK, et al. Pancreatitis with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:136-145.
18. Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis . J Oncol Pharm Pract. 2012;19:257-260.
19. Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology . 2013;13:461-467.
20. Russano M, Vincenzi B, Benditti O, et al. Pazopanib and pancreatic toxicity: a case report. BMC Res notes. 2015;8:196-198.
21. Kawakubo K, Hata H, Kawakami H, et al. Pazopanib induced severe acute pancreatitis. Case Rep Oncol. 2015;8:356-358.
22. Peron J, Khenifer S, Potier V, et al. Axitinib induced acute pancreatitis: a case report . Anticancer Drugs. 2014;25:478-479.
23. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis . Ann Pharmaco. 2013;37:33.
24. Pezzilli R, Corinaldesi R, Morselli-LabateAM. Tyrosine kinase inhibitors and acute pancreatitis. http://www.serena.unina.it/index.php/jop/article/view/3836/4278. Published May 5, 2010. Accessed May 22 , 2017.
25. Blum KA, Christian B, Flynn JM, et al. A phase I trial of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab and bendamustine in patients with relapsed/refractory non Hodgkin’s lymphoma. Blood. 2012;120:abstract 1643.
26. Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: a case report. Pharmacotherapy. 2013;33:e43-e44.
27. Dabrafenib. In Life-Sciences-Europe.com from Tafinlar. EU Summary of Product Characteristics. 30 August 2013.
28. Varma MR, Mathew S, Krishnadas D, et al. Imatinib-induced pancreatitis. Indian J Pharmacol. 2010;42:50-52.
29. Palandri F, Castagnetti F, Soverinie S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758-1761.
30. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis. Ann of Pharmacother. 2013;47:e.3
31. Cortesk JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadephia chromosome-positive leukemias. New Engl J Med. 2012;367:2075-2088.
32. Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis [Letter]. J Pancreas. 2010;119:275-276.
33. Elouni B. Acute pancreatitis induced by Velcade ( bortezomib) with positive rechallenge. 9th Annual meeting of the International Society of Pharmacovigilance. Oct 2009 abstract 74.
34. Talamo G, Sikik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis. Case report and review of the literature . J Oncol Pharm Prac. 2016;22:332-334.
35. SolakogluT, Akyol P, Guney T, et al. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189-190.
36. Mihaila RG. A possible rare complication of bortezomib treatment, acute pancreatitis. Acta Medica Transilvanica. 2013;2:269-171
37. Gupta H, Bansal R, Khanna S, et al. An unusual complication of bortezomib therapy: acute pancreatitis. Indian J Nephr. 2014;24:135-136.
38. Fotoh M, KitaharaT, Sakuta J, et al. Multiple lipoma with hyperlipidemia in a multiple myeloma patient treated with bortezomib/dexamethasone. Leuk Res. 2010;34:e120-121.
39. Wang HH, Tsui J, Wang XY, et al. Bortezomib induced acute pancreatitis in a patient with multiple myeloma. Leuk Lymphoma. 2014;55:1404-1405.
40. Misselwitz B, Goede JS, Pestalozzi BC, et al. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol. 2010;89:569-577.
41. Gozzetti A, Fabbri A, Defina M, et al. Hyperlipidemia in a myeloma patient after bortezomib treatment. Leuk Research. 2010;34:e250.
42. Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893-897.
43. Runzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109.
44. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757.
45. Butt W, Saadati H, Wasif- Saif M. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res. 2010;30:5113-5115.
46. Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Onc Pharm Pract. 2010;16:133-134.
47. Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87:421-424.
48. Subramaniam S, Zell JA, Kunz PL. Everolimus causing severe hypertriglyceridemia and acute pancreatitis. J Natl Compr Canc Netw. 2013;11:5-9.
49. Wadood A, Chesner R, Mirza M, et al. Tamoxifen precipitation of familial hypertriglyceridaemia: a rare cause of acute pancreatitis. BMJ Case Rep. Published August 3, 2016. doi: 10.1136/bcr-2016-214837.
50. Sakhri J, BenSalem C, Fathallah H, et al. Severe pancreatitis due to tamoxifen induced hypertriglyceridemia with positive rechallenge. J Pancreas. 2010;11:382-384.
51. Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067-1069.
52. Artac M, Sari R, Altunbas J, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14:309-311.
53. [Author name not available]. Acute pancreatitis: 15 case reports. React Wkly. 2015;1546:29.
54. Ozcinar B, Guven K, Poylani A, et al. Necrotizing pancreatitis after transcatheter embolization for hepatocellular carcinoma. Diagn In
56. She WH, Chan ACY, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93-98.
57. Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:321-325.
58. Peterson JL, Vallow LA, Johnson DW, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy. 2013;12:573-579.
59. Piso P, Glockzin G, Schlitt HJ. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from gastric cancer. J Clin Oncol. 2011;29(suppl 4):abstract 132.
60. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3356888/?report=reader. Published 2012. Accessed May 23, 2017.
61. Muzaffar M, Jia J, Liles D, et al. Acute pancreatitis associated with ado-trastuzumab emtansine. Am J Ther. 2016;23:e572-574.
62. Urru SA, Mariotti E, Carta P, et al. Acute pancreatitis following brentuzimab vedotin therapy for refractory Hodgkin lymphoma: a case report. Drugs R D. 2014;14:9-11.
63. Gandhi MD, Evens AM, Fenske TS, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895-2897.
64. Brentuximab vedotin in Micromedex solutions, Truven Health Analytics. 2016.
65. Okazaki K, Uchida K. Autoimmune pancreatitis: the past, present and future. Pancreas. 2015;44:1006-1016.
66. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122-133.
67. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab and ipilimumab. J Natl Cancer Inst. 2017;109:[page numbers not available].
68. Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571-584.
69. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
70 . Di MY, Liu H, Zu-Yao Y, et al. Prediction models of mortality in acute pancreatitis in adults. A systematic review. Ann Int Med. 2016;165:482-490.
71. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;18:2133-2140.
Patients with advanced malignancies may develop pancreatitis during therapy for their cancer. Acute pancreatitis is inflammation of the pancreas. Common symptoms include abdominal pain, nausea, vomiting, shortness of breath, dehydration. Laboratory evidence of acute pancreatitis includes elevations of the amylase and lipase. Mild pancreatitis occurs when there is no organ dysfunction, moderate pancreatitis is associated with one organ dysfunction, and severe pancreatitis is complicated by multiple organ dysfunction. Hypotension, hypocalcemia, or anemia suggest a more severe course of the pancreatitis. In some instances, the pancreatitis may be an adverse reaction to the therapy being given. However, other causes such as hypercalcemia, hypertriglyceridemia, cholelithiasis, and underlying malignancy must be ruled out before ascribing pancreatitis to a specific drug. To date, two classifications systems have been proposed by Trivedi1 and Badalov2 to evaluate the degree to which a drug is responsible for pancreatitis (Table 1). Furthermore, Naranjo and colleagues have proposed a more general method of assessing the causal relationship between drugs and adverse events.3 The Naranjo algorithm is not specific for pancreatitis. Jones and colleagues4 reported that 0.1%-2% of acute pancreatitis cases were owing to drugs. In 2015, they listed the older chemotherapy agents associated with pancreatitis. However, more recently, many new agents have been approved for the management of cancers. The newer classes of antineoplastic agents including proteasome inhibitors, immune-modulating agents, tyrosine kinase inhibitors, monoclonal antibodies against programmed cell death-1 (PD-1) and its ligand PD-L1 and antibody-toxin conjugates are now associated with acute pancreatitis.
Methods
We conducted a search in PubMed, Google Scholar, and Micromedex for pancreatitis related to antineoplastic agents, including proteasome inhibitors, immune checkpoint inhibitors, monoclonal antibodies, immune-modulating agents, drug-induced pancreatitis. Terms used for the searches included each specific agent and pancreatitis, immunotherapy and pancreatitis, tyrosine kinase inhibitors and pancreatitis, auto immune pancreatitis, and toxicities of molecular target therapies. Reference lists from the identified manuscripts were reviewed for further studies of pancreatitis as a result of antineoplastic therapy. The most recent search date was February 15, 2017.
The degree to which each agent was associated with inducing pancreatitis was evaluated using the Badalov classification system2 in addition to the Naranjo Adverse Drug Reaction (ADR) Probability Scale.3 The Naranjo scale consists of 10 questions with values assigned to each answer. Total scores range from -4 to 13, where 13-9 indicates the reaction is considered definitely attributable to the drug; 8-5, probably attributable; 4-1, possibly attributable; and ≤0, doubtful if attributable.
A total of 67 manuscripts and abstracts were identified. Four manuscripts and 3 abstracts were excluded because they had insufficient information about possible pancreatitis or there was a presence of multiple other agents or conditions that might have caused pancreatitis. In total, 60 publications met inclusion criteria and were evaluated.
Results
Immune checkpoint inhibitors
In a review of toxicities of anti-programmed cell death-1 (PD-1) therapy, pancreatitis was reported to occur in about 1.8% of patients who received nivolumab or pembrolizumab.5 The 9 patients with pancreatitis attributed to an immune etiology were treated with corticosteroids. Pancreatitis was grade 2 in 3 patients (1.5-2 times upper limit of normal [ULN]), grade 3 in 4 patients (>2-5 ULN), and grade 4 ( >5 ULN) in 2 patients.
In asymptomatic individuals, pancreatitis has been detected on a positron-emission tomography–computed tomography (CT) scan after anti-PD-1 therapy.5 By contrast, there was a case report of a patient treated with nivolumab for lung cancer who developed anorexia, vomiting, and back pain on day 18 of therapy with an elevation of the amylase and lipase levels, but a negative CT.6 Later the patient developed a swollen pancreas on CT. Autoimmune pancreatitis comes in two forms. The most common relates to elevated levels of immunoglobulin G4 (IgG4; normal, 135 mg/dL ULN)7 The mechanism of immune pancreatitis associated with anti-PD-1 therapy is unknown.
Ipilimumab (an anti-CTLA4 antibody) has been approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. Pancreatitis occurred in 1 patient in a phase 1 trial in pediatric patients.9 In a summary of 14 phase 1-3 trials of ipilimumab in advanced melanoma, pancreatitis was reported in fewer than 1% of the patients.10 In management guidelines for therapy with ipilimumab, pancreatitis may present as an asymptomatic increase in the levels of amylase and lipase, or with fevers, malaise, or abdominal pain. Oral prednisone or dexamethasone were given for the immune pancreatitis, but the decline in enzymes was slow, often taking months.11 In a preclinical model of autoimmune pancreatitis due to the blocking of CTLA4, there was suppression of regulatory T-cell function. The autoimmune pancreatitis responded to cyclosporin or rapamycin but there are no clinical data for these agents.12 The anti-PD-L1 agent atezolizumab has been associated with acute pancreatitis in 2 of 1,978 patients (0.1%).13 A review by Champiat and colleagues on dysimmune toxcities related to immune checkpoint inhibitors includes pancreatitis as an autoimmune complication of such therapies.14
Blinatumomab is an anti-CD19–directed CD3 T-cell engager that has been approved by the FDA for refractory B-cell acute lymphoblastic leukemia. In August 2016, the maker of the drug, Amgen, advised hematologists and oncologists that since February 2016, 10 patients out of more than 2,000 treated with blinatumomab had developed pancreatitis.15 Other medications the patients were receiving such as high-dose steroids might have caused or contributed to the pancreatitis. In one case, the pancreatitis improved with stopping blinatumomab but worsened with re-challenge. It is possible that the mechanism of the associated pancreatitis relates to a change in immune checkpoint inhibition. CD19-positive, CD24-high, CD27-positive regulatory B cells are decreased in autoimmune pancreatitis.16 Treatment with blinatumomab may decrease the CD19-positive cells.
Molecularly targeted agents, including TKIs
Molecularly targeted agents such as tyrosine kinase inhibitors (TKIs) or other kinase inhibitors have been associated with pancreatitis.17, 18 In a retrospective study by Tiruman and colleagues,19 the investigators found 91 patients with pancreatitis on imaging, of whom 15 were receiving molecularly target drugs. The pancreatitis was asymptomatic in 2 patients, but 13 had abdominal pain, many with nausea. Four of the patients also had gallstones, but the drug was deemed to be the cause of the pancreatitis. In 4 of the 9 patients in whom a rechallenge was done with the TKI, the pancreatitis relapsed. The pancreatitis resolved in 14 of the 15 patients; 1 patient died because of progressive cancer before the pancreatitis resolved. The pancreatitis was mild, 7 of the 15 patients had normal pancreatic enzymes and the pancreatitis was diagnosed by radiology.
Ghatlia and colleagues17 performed a meta-analysis of trials of TKI. They found 9 cases of pancreatitis in patients on sunitinib therapy. Of those, 4 patients were on sunitinib alone, and 5 were on other chemotherapy agents in combination with sunitinib. Eight cases of pancreatitis due to sorafenib were found. Three of the patients were on sorafenib alone, and 5 were on other chemotherapy including 1 on transcatheter embolization (TACE). Three cases of pancreatitis were associated with vandetanib; 2 of those patients had other concurrent chemotherapy. One case of axitinib induced pancreatitis was described.
Pancreatitis was reported in the phase 1 trials of sorafenib and sunitinib. In all, 3 of 69 patients treated with sorafenib had grade 3 pancreatitis and asymptomatic elevations of amylase and lipase levels were present in about 5% of patients receiving sunitinib.18,19
Other TKIs associated with pancreatitis include pazopanib,20,21 axitinib,22 and nilotinib.23 Pezzilli and coleagues24 described 5 patients with pancreatitis on sorafenib, 3 on sunitinib, 6 on nilotinib. It is possible that some of these cases appeared in other reviews. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, caused a single case of pancreatitis in 9 patients.25
Vemurafenib, a BRAF kinase inhibitor, was associated with pancreatitis in one case. In this case, the pancreatitis resolved on stopping the medication but recurred when vemurafenib rechallenge was attempted.26 There is a report of dabrafenib being associated with pancreatitis in 1 patient.27
Agents that inhibit the TKIs associated with BCR-ABL in chronic myelogenous leukemia are associated with acute pancreatitis. Imatinib-induced pancreatitis was reported in a small number of cases.28 Nilotinib has caused amylase/lipase elevations with and without symptomatic pancreatitis.29,30 Ponatinib, an inhibitor of BCR-ABL tyrosine kinase, is associated with pancreatitis.31 Pancreatitis occurred in 11 of 81 patients treated with ponatinib, and in 8 patients it was described as serious. Further elevation of amylase or lipase levels without clinical pancreatitis was noted in 7 other patients.
Proteosome inhibitors
In 2010, Elouni and colleagues32 reported a case of IV bortezomib-induced pancreatitis, which recurred on rechallenge with bortezomib. This same patient was also reported in an abstract in 2009.33 In 2009, there was an editorial comment which was added to the end of the abstract that the World Health Organization Adverse Drug Reaction database had 11 reports of bortezomib associated pancreatitis. Talamo and colleagues34 reported a case of bortezomib-induced pancreatitis due to bortezomib that had been administered subcutaneously. At that time, they also summarized 7 previous reports of bortezomib-associated pancreatitis. The mechanism of bortezomib-induced pancreatitis is not known.35-37
Fotoh and colleagues reported a patient with myeloma who had elevated triglyceride levels after bortezomib therapy.38 In one case of bortezomib-associated pancreatitis, the patient had an elevated triglyceride level, but it was not extremely high.39 Multiple myeloma itself may be associated with hyperlipidemia but only rarely.40 Gozetti and colleagues reported a patient who developed hyperlipidemia after two courses of bortezomib;41 stopping bisphosphonates may be associated with a rise in triglycerides. There was one case of carfilzomib causing pancreatitis during a phase 1 trial.42
Older chemotherapy agents
Reviews of drug-induced pancreatitis have listed many chemotherapy agents which may cause pancreatitis.1,43 The agent most frequently associated with acute pancreatitis has been asparaginase,44 with 2%-16% of patients undergoing asparaginase therapy developing pancreatitis. Asparaginase-related pancreatitis is grade 3 or 4 in 5%-10% of patients, and recurs in 63% of patients on rechallenge. Other chemotherapy agents associated with pancreatitis include: mercaptopurine, cytosine arabinoside, cisplatin, interferon alfa-2b, doxorubicin, tamoxifen, gefitinib, vinorelbine, oxaliplatin, levamisole, methotrexate, azathioprine, 5-fluorouracil, capecitabine, ifosfamide, paclitaxel, and all-trans retinoic acid.
Oxaliplatin carries a 0.1%-2% incidence of drug-induced pancreatitis. In one series of 6 patients, cessation of the agent allowed for resolution of symptoms and decrease in serum lipase and amylase levels.45 With capecitabine there are 2 case reports of pancreatitis.46 Cases of pancreatitis associated with trifluridine or tipiracil were not present in the literature.
Thalidomide caused severe pancreatitis in a patient when it was used to treat chronic graft-versus-host disease.47 This patient suffered recurrent pancreatitis on retreatment with the thalidomide. The authors further referenced two other suspected cases of thalidomide-induced, acute pancreatitis. However, in view of the extensive use of thalidomide for multiple myeloma before the development of lenalidomide, thalidomide-associated pancreatitis would be <1% of patients.
Agents that cause hypertriglyceridemia may cause pancreatitis. This mechanism has been reported as the cause of pancreatitis for everolimus48 and tamoxifen.49,50-52 Everolimus causes elevated triglycerides in 30%-50% of patients. There are case reports and a review of tamoxifen-associated pancreatitis caused by elevated triglycerides.52 There has also been a case of temsirolimus-associated pancreatitis,53 another agent that elevates triglycerides.
Pancreatitis associated with hepatic embolization or HIPEC
TACE leads to symptomatic acute pancreatitis in 0.4%-2% of patients, but nonselective TACE (into the hepatic artery and not just feeder vessels), may lead to elevated amylase levels in 15%-40% of patients.54-56 The risk of pancreatitis would depend on which chemotherapy drug is being infused into the liver. It would also be greater if the chemotherapy has to be infused into a larger part of the liver than into a small portion of the liver. In one patient, severe pancreatitis secondary to TACE occurred after two previous embolizations; prior embolization may have led to occlusion of the previously infused vessels.57 Radioembolization with 90Y microspheres was associated with one case of pancreatitis in 112 consecutive patients.58 The postembolization syndrome in the first 24 hours after the procedure may involve fever, abdominal pain, nausea, and vomiting due to acute pancreatitis in some instances.
Acute pancreatitis has also been described as a complication of hyperthermic intraperitoneal chemotherapy (HIPEC).59,60 Two of 13 patients receiving HIPEC for gastric cancer developed pancreatitis.59 In 25 patients with colon cancer who were treated with HIPEC, 1 patient had pancreatitis.60
Antibody-drug conjugates
Muzaffar and colleagues reported a patient with acute pancreatitis 3 days after starting therapy with ado-trastuzumab emtansine.61 Urru and colleagues62 reported a patient who developed acute pancreatitis after brentuximab vedotin therapy. Ghandi and colleagues63 identified 2 cases of fatal acute pancreatitis with brentuximab vedotin and 6 cases of nonfatal pancreatitis. Two of the nonfatal patients were rechallenged, and 1 developed recurrent pancreatitis. Because abdominal pain may occur in up to 18% of patients receiving brentuximab vedotin, the incidence of pancreatitis may be underestimated with this agent.64
In Table 2, ado-trastuzumab emtansine and brentuximab vedotin are listed with incidence and level of association given by the Baldavo2 and Naranjo.3 With greater awareness, the incidence of pancreatitis associated with these agents may rise or fall as more data is accumulated. In many instances, there are insufficient numbers of reported cases or insufficient information in single-case reports to complete the entire table.
Discussion
Acute pancreatitis is an uncommon complication of tyrosine kinase inhibitors, other kinase inhibitors, proteasome inhibitors, monoclonal antibody-drug conjugates and anti-PD-1 immunotherapies. As nausea, abdominal pain, emesis are common in patients with cancer on antineoplastic therapy, some patients may have acute pancreatitis which is undiagnosed. It is not clear whether a patient with pancreatitis secondary to a TKI can be safely switched to a different TKI. As more molecularly targeted agents and more monoclonal antibodies targeting PD-L1 and PD-1 are under development, screening for amylase and lipase levels during phase 1/2 testing may prove helpful.
The natural history of cancer-drug–associated pancreatitis may depend on which agent is the cause. Although there are descriptions of the course of autoimmune pancreatitis, these studies have not included pancreatitis associated with anti-PD-L1 or -PD-1 therapies.65 It is possible that once an autoimmune pancreatitis has developed, simply stopping the inciting anti-PD-L1 or -PD-1 antibody may not lead to immediate resolution. Therapy with combined immune checkpoint blockade agents (eg, nivolumab and ipilimumab) may cause a higher incidence of pancreatitis than therapy with a single agent.66
In a report of 119 patients with melanoma who were treated with nivolumab and ipilimumab, there were 2 cases of acute pancreatitis, though 20% of patients had a grade 3 or higher amylase level, and just over 6% had a grade 3 or higher lipase.67 Stopping this type of immunotherapy early for grade 1,2, or 3 rises in pancreatic enzymes might prevent symptomatic pancreatitis from developing, but would stop potentially curative therapy for many patients who would have never developed clinically serious pancreatitis. Patients who suffer immune toxicities with anti-PD-1 therapies may be more apt to obtain some clinical benefit. The development of immune-related toxicities in patients treated with ipilimumab ( an anti CTLA4 antibody) seemed to correlate the tumor regression.68 This has also been suggested by the fact that the development of vitiligo correlates with clinical response in melanoma patients treated with nivolumab.69 Although clinically significant pancreatitis might be averted by stopping immune therapies earlier, stopping before it is deemed necessary might prevent cancer patients from receiving life-prolonging therapy.
Acute pancreatitis in general is severe in about 25% of cases and is associated with a significant risk of death. Scoring systems such as Ranson criteria and Apache 2 are used to assess the severity of pancreatitis although their utility is debated.70 Asparaginase is the chemotherapy agent most frequently associated with pancreatitis. It has been used to treat acute lymphoblastic leukemia for more than 30 years. This allowed for a study of 5,185 children and young adults who received asparaginase to determine what clinical factors and genomic factors were associated with the development of acute pancreatitis in 117 individuals.71 Further information gathered from programs such as the FDA and the adverse drug reaction program at Northwestern University in Chicago, coupled with the publication of other cases of pancreatitis associated with newer cancer agents may provide more insight into the mechanism causing pancreatitis due to a specific agent. With more cases being published, it may also become possible to determine if there are specific predisposing factors based on the clearance or metabolism of the offending agent or any genetic predisposition for drug-related pancreatitis.
Patients with advanced malignancies may develop pancreatitis during therapy for their cancer. Acute pancreatitis is inflammation of the pancreas. Common symptoms include abdominal pain, nausea, vomiting, shortness of breath, dehydration. Laboratory evidence of acute pancreatitis includes elevations of the amylase and lipase. Mild pancreatitis occurs when there is no organ dysfunction, moderate pancreatitis is associated with one organ dysfunction, and severe pancreatitis is complicated by multiple organ dysfunction. Hypotension, hypocalcemia, or anemia suggest a more severe course of the pancreatitis. In some instances, the pancreatitis may be an adverse reaction to the therapy being given. However, other causes such as hypercalcemia, hypertriglyceridemia, cholelithiasis, and underlying malignancy must be ruled out before ascribing pancreatitis to a specific drug. To date, two classifications systems have been proposed by Trivedi1 and Badalov2 to evaluate the degree to which a drug is responsible for pancreatitis (Table 1). Furthermore, Naranjo and colleagues have proposed a more general method of assessing the causal relationship between drugs and adverse events.3 The Naranjo algorithm is not specific for pancreatitis. Jones and colleagues4 reported that 0.1%-2% of acute pancreatitis cases were owing to drugs. In 2015, they listed the older chemotherapy agents associated with pancreatitis. However, more recently, many new agents have been approved for the management of cancers. The newer classes of antineoplastic agents including proteasome inhibitors, immune-modulating agents, tyrosine kinase inhibitors, monoclonal antibodies against programmed cell death-1 (PD-1) and its ligand PD-L1 and antibody-toxin conjugates are now associated with acute pancreatitis.
Methods
We conducted a search in PubMed, Google Scholar, and Micromedex for pancreatitis related to antineoplastic agents, including proteasome inhibitors, immune checkpoint inhibitors, monoclonal antibodies, immune-modulating agents, drug-induced pancreatitis. Terms used for the searches included each specific agent and pancreatitis, immunotherapy and pancreatitis, tyrosine kinase inhibitors and pancreatitis, auto immune pancreatitis, and toxicities of molecular target therapies. Reference lists from the identified manuscripts were reviewed for further studies of pancreatitis as a result of antineoplastic therapy. The most recent search date was February 15, 2017.
The degree to which each agent was associated with inducing pancreatitis was evaluated using the Badalov classification system2 in addition to the Naranjo Adverse Drug Reaction (ADR) Probability Scale.3 The Naranjo scale consists of 10 questions with values assigned to each answer. Total scores range from -4 to 13, where 13-9 indicates the reaction is considered definitely attributable to the drug; 8-5, probably attributable; 4-1, possibly attributable; and ≤0, doubtful if attributable.
A total of 67 manuscripts and abstracts were identified. Four manuscripts and 3 abstracts were excluded because they had insufficient information about possible pancreatitis or there was a presence of multiple other agents or conditions that might have caused pancreatitis. In total, 60 publications met inclusion criteria and were evaluated.
Results
Immune checkpoint inhibitors
In a review of toxicities of anti-programmed cell death-1 (PD-1) therapy, pancreatitis was reported to occur in about 1.8% of patients who received nivolumab or pembrolizumab.5 The 9 patients with pancreatitis attributed to an immune etiology were treated with corticosteroids. Pancreatitis was grade 2 in 3 patients (1.5-2 times upper limit of normal [ULN]), grade 3 in 4 patients (>2-5 ULN), and grade 4 ( >5 ULN) in 2 patients.
In asymptomatic individuals, pancreatitis has been detected on a positron-emission tomography–computed tomography (CT) scan after anti-PD-1 therapy.5 By contrast, there was a case report of a patient treated with nivolumab for lung cancer who developed anorexia, vomiting, and back pain on day 18 of therapy with an elevation of the amylase and lipase levels, but a negative CT.6 Later the patient developed a swollen pancreas on CT. Autoimmune pancreatitis comes in two forms. The most common relates to elevated levels of immunoglobulin G4 (IgG4; normal, 135 mg/dL ULN)7 The mechanism of immune pancreatitis associated with anti-PD-1 therapy is unknown.
Ipilimumab (an anti-CTLA4 antibody) has been approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. Pancreatitis occurred in 1 patient in a phase 1 trial in pediatric patients.9 In a summary of 14 phase 1-3 trials of ipilimumab in advanced melanoma, pancreatitis was reported in fewer than 1% of the patients.10 In management guidelines for therapy with ipilimumab, pancreatitis may present as an asymptomatic increase in the levels of amylase and lipase, or with fevers, malaise, or abdominal pain. Oral prednisone or dexamethasone were given for the immune pancreatitis, but the decline in enzymes was slow, often taking months.11 In a preclinical model of autoimmune pancreatitis due to the blocking of CTLA4, there was suppression of regulatory T-cell function. The autoimmune pancreatitis responded to cyclosporin or rapamycin but there are no clinical data for these agents.12 The anti-PD-L1 agent atezolizumab has been associated with acute pancreatitis in 2 of 1,978 patients (0.1%).13 A review by Champiat and colleagues on dysimmune toxcities related to immune checkpoint inhibitors includes pancreatitis as an autoimmune complication of such therapies.14
Blinatumomab is an anti-CD19–directed CD3 T-cell engager that has been approved by the FDA for refractory B-cell acute lymphoblastic leukemia. In August 2016, the maker of the drug, Amgen, advised hematologists and oncologists that since February 2016, 10 patients out of more than 2,000 treated with blinatumomab had developed pancreatitis.15 Other medications the patients were receiving such as high-dose steroids might have caused or contributed to the pancreatitis. In one case, the pancreatitis improved with stopping blinatumomab but worsened with re-challenge. It is possible that the mechanism of the associated pancreatitis relates to a change in immune checkpoint inhibition. CD19-positive, CD24-high, CD27-positive regulatory B cells are decreased in autoimmune pancreatitis.16 Treatment with blinatumomab may decrease the CD19-positive cells.
Molecularly targeted agents, including TKIs
Molecularly targeted agents such as tyrosine kinase inhibitors (TKIs) or other kinase inhibitors have been associated with pancreatitis.17, 18 In a retrospective study by Tiruman and colleagues,19 the investigators found 91 patients with pancreatitis on imaging, of whom 15 were receiving molecularly target drugs. The pancreatitis was asymptomatic in 2 patients, but 13 had abdominal pain, many with nausea. Four of the patients also had gallstones, but the drug was deemed to be the cause of the pancreatitis. In 4 of the 9 patients in whom a rechallenge was done with the TKI, the pancreatitis relapsed. The pancreatitis resolved in 14 of the 15 patients; 1 patient died because of progressive cancer before the pancreatitis resolved. The pancreatitis was mild, 7 of the 15 patients had normal pancreatic enzymes and the pancreatitis was diagnosed by radiology.
Ghatlia and colleagues17 performed a meta-analysis of trials of TKI. They found 9 cases of pancreatitis in patients on sunitinib therapy. Of those, 4 patients were on sunitinib alone, and 5 were on other chemotherapy agents in combination with sunitinib. Eight cases of pancreatitis due to sorafenib were found. Three of the patients were on sorafenib alone, and 5 were on other chemotherapy including 1 on transcatheter embolization (TACE). Three cases of pancreatitis were associated with vandetanib; 2 of those patients had other concurrent chemotherapy. One case of axitinib induced pancreatitis was described.
Pancreatitis was reported in the phase 1 trials of sorafenib and sunitinib. In all, 3 of 69 patients treated with sorafenib had grade 3 pancreatitis and asymptomatic elevations of amylase and lipase levels were present in about 5% of patients receiving sunitinib.18,19
Other TKIs associated with pancreatitis include pazopanib,20,21 axitinib,22 and nilotinib.23 Pezzilli and coleagues24 described 5 patients with pancreatitis on sorafenib, 3 on sunitinib, 6 on nilotinib. It is possible that some of these cases appeared in other reviews. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, caused a single case of pancreatitis in 9 patients.25
Vemurafenib, a BRAF kinase inhibitor, was associated with pancreatitis in one case. In this case, the pancreatitis resolved on stopping the medication but recurred when vemurafenib rechallenge was attempted.26 There is a report of dabrafenib being associated with pancreatitis in 1 patient.27
Agents that inhibit the TKIs associated with BCR-ABL in chronic myelogenous leukemia are associated with acute pancreatitis. Imatinib-induced pancreatitis was reported in a small number of cases.28 Nilotinib has caused amylase/lipase elevations with and without symptomatic pancreatitis.29,30 Ponatinib, an inhibitor of BCR-ABL tyrosine kinase, is associated with pancreatitis.31 Pancreatitis occurred in 11 of 81 patients treated with ponatinib, and in 8 patients it was described as serious. Further elevation of amylase or lipase levels without clinical pancreatitis was noted in 7 other patients.
Proteosome inhibitors
In 2010, Elouni and colleagues32 reported a case of IV bortezomib-induced pancreatitis, which recurred on rechallenge with bortezomib. This same patient was also reported in an abstract in 2009.33 In 2009, there was an editorial comment which was added to the end of the abstract that the World Health Organization Adverse Drug Reaction database had 11 reports of bortezomib associated pancreatitis. Talamo and colleagues34 reported a case of bortezomib-induced pancreatitis due to bortezomib that had been administered subcutaneously. At that time, they also summarized 7 previous reports of bortezomib-associated pancreatitis. The mechanism of bortezomib-induced pancreatitis is not known.35-37
Fotoh and colleagues reported a patient with myeloma who had elevated triglyceride levels after bortezomib therapy.38 In one case of bortezomib-associated pancreatitis, the patient had an elevated triglyceride level, but it was not extremely high.39 Multiple myeloma itself may be associated with hyperlipidemia but only rarely.40 Gozetti and colleagues reported a patient who developed hyperlipidemia after two courses of bortezomib;41 stopping bisphosphonates may be associated with a rise in triglycerides. There was one case of carfilzomib causing pancreatitis during a phase 1 trial.42
Older chemotherapy agents
Reviews of drug-induced pancreatitis have listed many chemotherapy agents which may cause pancreatitis.1,43 The agent most frequently associated with acute pancreatitis has been asparaginase,44 with 2%-16% of patients undergoing asparaginase therapy developing pancreatitis. Asparaginase-related pancreatitis is grade 3 or 4 in 5%-10% of patients, and recurs in 63% of patients on rechallenge. Other chemotherapy agents associated with pancreatitis include: mercaptopurine, cytosine arabinoside, cisplatin, interferon alfa-2b, doxorubicin, tamoxifen, gefitinib, vinorelbine, oxaliplatin, levamisole, methotrexate, azathioprine, 5-fluorouracil, capecitabine, ifosfamide, paclitaxel, and all-trans retinoic acid.
Oxaliplatin carries a 0.1%-2% incidence of drug-induced pancreatitis. In one series of 6 patients, cessation of the agent allowed for resolution of symptoms and decrease in serum lipase and amylase levels.45 With capecitabine there are 2 case reports of pancreatitis.46 Cases of pancreatitis associated with trifluridine or tipiracil were not present in the literature.
Thalidomide caused severe pancreatitis in a patient when it was used to treat chronic graft-versus-host disease.47 This patient suffered recurrent pancreatitis on retreatment with the thalidomide. The authors further referenced two other suspected cases of thalidomide-induced, acute pancreatitis. However, in view of the extensive use of thalidomide for multiple myeloma before the development of lenalidomide, thalidomide-associated pancreatitis would be <1% of patients.
Agents that cause hypertriglyceridemia may cause pancreatitis. This mechanism has been reported as the cause of pancreatitis for everolimus48 and tamoxifen.49,50-52 Everolimus causes elevated triglycerides in 30%-50% of patients. There are case reports and a review of tamoxifen-associated pancreatitis caused by elevated triglycerides.52 There has also been a case of temsirolimus-associated pancreatitis,53 another agent that elevates triglycerides.
Pancreatitis associated with hepatic embolization or HIPEC
TACE leads to symptomatic acute pancreatitis in 0.4%-2% of patients, but nonselective TACE (into the hepatic artery and not just feeder vessels), may lead to elevated amylase levels in 15%-40% of patients.54-56 The risk of pancreatitis would depend on which chemotherapy drug is being infused into the liver. It would also be greater if the chemotherapy has to be infused into a larger part of the liver than into a small portion of the liver. In one patient, severe pancreatitis secondary to TACE occurred after two previous embolizations; prior embolization may have led to occlusion of the previously infused vessels.57 Radioembolization with 90Y microspheres was associated with one case of pancreatitis in 112 consecutive patients.58 The postembolization syndrome in the first 24 hours after the procedure may involve fever, abdominal pain, nausea, and vomiting due to acute pancreatitis in some instances.
Acute pancreatitis has also been described as a complication of hyperthermic intraperitoneal chemotherapy (HIPEC).59,60 Two of 13 patients receiving HIPEC for gastric cancer developed pancreatitis.59 In 25 patients with colon cancer who were treated with HIPEC, 1 patient had pancreatitis.60
Antibody-drug conjugates
Muzaffar and colleagues reported a patient with acute pancreatitis 3 days after starting therapy with ado-trastuzumab emtansine.61 Urru and colleagues62 reported a patient who developed acute pancreatitis after brentuximab vedotin therapy. Ghandi and colleagues63 identified 2 cases of fatal acute pancreatitis with brentuximab vedotin and 6 cases of nonfatal pancreatitis. Two of the nonfatal patients were rechallenged, and 1 developed recurrent pancreatitis. Because abdominal pain may occur in up to 18% of patients receiving brentuximab vedotin, the incidence of pancreatitis may be underestimated with this agent.64
In Table 2, ado-trastuzumab emtansine and brentuximab vedotin are listed with incidence and level of association given by the Baldavo2 and Naranjo.3 With greater awareness, the incidence of pancreatitis associated with these agents may rise or fall as more data is accumulated. In many instances, there are insufficient numbers of reported cases or insufficient information in single-case reports to complete the entire table.
Discussion
Acute pancreatitis is an uncommon complication of tyrosine kinase inhibitors, other kinase inhibitors, proteasome inhibitors, monoclonal antibody-drug conjugates and anti-PD-1 immunotherapies. As nausea, abdominal pain, emesis are common in patients with cancer on antineoplastic therapy, some patients may have acute pancreatitis which is undiagnosed. It is not clear whether a patient with pancreatitis secondary to a TKI can be safely switched to a different TKI. As more molecularly targeted agents and more monoclonal antibodies targeting PD-L1 and PD-1 are under development, screening for amylase and lipase levels during phase 1/2 testing may prove helpful.
The natural history of cancer-drug–associated pancreatitis may depend on which agent is the cause. Although there are descriptions of the course of autoimmune pancreatitis, these studies have not included pancreatitis associated with anti-PD-L1 or -PD-1 therapies.65 It is possible that once an autoimmune pancreatitis has developed, simply stopping the inciting anti-PD-L1 or -PD-1 antibody may not lead to immediate resolution. Therapy with combined immune checkpoint blockade agents (eg, nivolumab and ipilimumab) may cause a higher incidence of pancreatitis than therapy with a single agent.66
In a report of 119 patients with melanoma who were treated with nivolumab and ipilimumab, there were 2 cases of acute pancreatitis, though 20% of patients had a grade 3 or higher amylase level, and just over 6% had a grade 3 or higher lipase.67 Stopping this type of immunotherapy early for grade 1,2, or 3 rises in pancreatic enzymes might prevent symptomatic pancreatitis from developing, but would stop potentially curative therapy for many patients who would have never developed clinically serious pancreatitis. Patients who suffer immune toxicities with anti-PD-1 therapies may be more apt to obtain some clinical benefit. The development of immune-related toxicities in patients treated with ipilimumab ( an anti CTLA4 antibody) seemed to correlate the tumor regression.68 This has also been suggested by the fact that the development of vitiligo correlates with clinical response in melanoma patients treated with nivolumab.69 Although clinically significant pancreatitis might be averted by stopping immune therapies earlier, stopping before it is deemed necessary might prevent cancer patients from receiving life-prolonging therapy.
Acute pancreatitis in general is severe in about 25% of cases and is associated with a significant risk of death. Scoring systems such as Ranson criteria and Apache 2 are used to assess the severity of pancreatitis although their utility is debated.70 Asparaginase is the chemotherapy agent most frequently associated with pancreatitis. It has been used to treat acute lymphoblastic leukemia for more than 30 years. This allowed for a study of 5,185 children and young adults who received asparaginase to determine what clinical factors and genomic factors were associated with the development of acute pancreatitis in 117 individuals.71 Further information gathered from programs such as the FDA and the adverse drug reaction program at Northwestern University in Chicago, coupled with the publication of other cases of pancreatitis associated with newer cancer agents may provide more insight into the mechanism causing pancreatitis due to a specific agent. With more cases being published, it may also become possible to determine if there are specific predisposing factors based on the clearance or metabolism of the offending agent or any genetic predisposition for drug-related pancreatitis.
1. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;29:709-716.
2. Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroeneterol Hepatol. 2007;5:648-661.
3. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
4. Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Oschner J. 2015;15:45-51.
5. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
6. Alabed YZ, Aghayev A, Sakellis C, et al. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-529.
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer. 2016;90:148-150.
8. Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532-539.
9. Merchant MS, Baird K, Wexler L, et al. Ipilimumab: first results of a phase I trial in pediatric patients with advanced solid tumors. J Clin Oncol. 2012;30:abstract 9545.
10. Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:abstract 8583.
11. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
12. Mayerle J, van den Brandt C, Schwaiger T, et al. Blockage of CTLA-4 suggests that autoimmune pancreatitis is a T-cell mediated disease responsive to ciclosporin A and rapamycin . Pancreatology. 2012;12:579(abstract S8-3).
13. Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2016.
14. Champiat S, Lambotte E, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27:559-574.
15. Amgen. New safety information for Blincyto (blinatumomab) Risk of pancreatitis. August 2016 and update to Micromedex 2016.
16. Sumimoto K, Uchida K, KusudaT, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high, CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis . Pancreatology. 2014;14:193-200.
17. Ghatalia P, Morgan CJ, Choueiri TK, et al. Pancreatitis with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:136-145.
18. Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis . J Oncol Pharm Pract. 2012;19:257-260.
19. Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology . 2013;13:461-467.
20. Russano M, Vincenzi B, Benditti O, et al. Pazopanib and pancreatic toxicity: a case report. BMC Res notes. 2015;8:196-198.
21. Kawakubo K, Hata H, Kawakami H, et al. Pazopanib induced severe acute pancreatitis. Case Rep Oncol. 2015;8:356-358.
22. Peron J, Khenifer S, Potier V, et al. Axitinib induced acute pancreatitis: a case report . Anticancer Drugs. 2014;25:478-479.
23. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis . Ann Pharmaco. 2013;37:33.
24. Pezzilli R, Corinaldesi R, Morselli-LabateAM. Tyrosine kinase inhibitors and acute pancreatitis. http://www.serena.unina.it/index.php/jop/article/view/3836/4278. Published May 5, 2010. Accessed May 22 , 2017.
25. Blum KA, Christian B, Flynn JM, et al. A phase I trial of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab and bendamustine in patients with relapsed/refractory non Hodgkin’s lymphoma. Blood. 2012;120:abstract 1643.
26. Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: a case report. Pharmacotherapy. 2013;33:e43-e44.
27. Dabrafenib. In Life-Sciences-Europe.com from Tafinlar. EU Summary of Product Characteristics. 30 August 2013.
28. Varma MR, Mathew S, Krishnadas D, et al. Imatinib-induced pancreatitis. Indian J Pharmacol. 2010;42:50-52.
29. Palandri F, Castagnetti F, Soverinie S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758-1761.
30. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis. Ann of Pharmacother. 2013;47:e.3
31. Cortesk JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadephia chromosome-positive leukemias. New Engl J Med. 2012;367:2075-2088.
32. Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis [Letter]. J Pancreas. 2010;119:275-276.
33. Elouni B. Acute pancreatitis induced by Velcade ( bortezomib) with positive rechallenge. 9th Annual meeting of the International Society of Pharmacovigilance. Oct 2009 abstract 74.
34. Talamo G, Sikik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis. Case report and review of the literature . J Oncol Pharm Prac. 2016;22:332-334.
35. SolakogluT, Akyol P, Guney T, et al. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189-190.
36. Mihaila RG. A possible rare complication of bortezomib treatment, acute pancreatitis. Acta Medica Transilvanica. 2013;2:269-171
37. Gupta H, Bansal R, Khanna S, et al. An unusual complication of bortezomib therapy: acute pancreatitis. Indian J Nephr. 2014;24:135-136.
38. Fotoh M, KitaharaT, Sakuta J, et al. Multiple lipoma with hyperlipidemia in a multiple myeloma patient treated with bortezomib/dexamethasone. Leuk Res. 2010;34:e120-121.
39. Wang HH, Tsui J, Wang XY, et al. Bortezomib induced acute pancreatitis in a patient with multiple myeloma. Leuk Lymphoma. 2014;55:1404-1405.
40. Misselwitz B, Goede JS, Pestalozzi BC, et al. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol. 2010;89:569-577.
41. Gozzetti A, Fabbri A, Defina M, et al. Hyperlipidemia in a myeloma patient after bortezomib treatment. Leuk Research. 2010;34:e250.
42. Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893-897.
43. Runzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109.
44. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757.
45. Butt W, Saadati H, Wasif- Saif M. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res. 2010;30:5113-5115.
46. Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Onc Pharm Pract. 2010;16:133-134.
47. Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87:421-424.
48. Subramaniam S, Zell JA, Kunz PL. Everolimus causing severe hypertriglyceridemia and acute pancreatitis. J Natl Compr Canc Netw. 2013;11:5-9.
49. Wadood A, Chesner R, Mirza M, et al. Tamoxifen precipitation of familial hypertriglyceridaemia: a rare cause of acute pancreatitis. BMJ Case Rep. Published August 3, 2016. doi: 10.1136/bcr-2016-214837.
50. Sakhri J, BenSalem C, Fathallah H, et al. Severe pancreatitis due to tamoxifen induced hypertriglyceridemia with positive rechallenge. J Pancreas. 2010;11:382-384.
51. Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067-1069.
52. Artac M, Sari R, Altunbas J, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14:309-311.
53. [Author name not available]. Acute pancreatitis: 15 case reports. React Wkly. 2015;1546:29.
54. Ozcinar B, Guven K, Poylani A, et al. Necrotizing pancreatitis after transcatheter embolization for hepatocellular carcinoma. Diagn In
56. She WH, Chan ACY, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93-98.
57. Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:321-325.
58. Peterson JL, Vallow LA, Johnson DW, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy. 2013;12:573-579.
59. Piso P, Glockzin G, Schlitt HJ. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from gastric cancer. J Clin Oncol. 2011;29(suppl 4):abstract 132.
60. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3356888/?report=reader. Published 2012. Accessed May 23, 2017.
61. Muzaffar M, Jia J, Liles D, et al. Acute pancreatitis associated with ado-trastuzumab emtansine. Am J Ther. 2016;23:e572-574.
62. Urru SA, Mariotti E, Carta P, et al. Acute pancreatitis following brentuzimab vedotin therapy for refractory Hodgkin lymphoma: a case report. Drugs R D. 2014;14:9-11.
63. Gandhi MD, Evens AM, Fenske TS, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895-2897.
64. Brentuximab vedotin in Micromedex solutions, Truven Health Analytics. 2016.
65. Okazaki K, Uchida K. Autoimmune pancreatitis: the past, present and future. Pancreas. 2015;44:1006-1016.
66. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122-133.
67. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab and ipilimumab. J Natl Cancer Inst. 2017;109:[page numbers not available].
68. Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571-584.
69. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
70 . Di MY, Liu H, Zu-Yao Y, et al. Prediction models of mortality in acute pancreatitis in adults. A systematic review. Ann Int Med. 2016;165:482-490.
71. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;18:2133-2140.
1. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;29:709-716.
2. Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroeneterol Hepatol. 2007;5:648-661.
3. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
4. Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Oschner J. 2015;15:45-51.
5. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
6. Alabed YZ, Aghayev A, Sakellis C, et al. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-529.
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer. 2016;90:148-150.
8. Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532-539.
9. Merchant MS, Baird K, Wexler L, et al. Ipilimumab: first results of a phase I trial in pediatric patients with advanced solid tumors. J Clin Oncol. 2012;30:abstract 9545.
10. Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:abstract 8583.
11. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
12. Mayerle J, van den Brandt C, Schwaiger T, et al. Blockage of CTLA-4 suggests that autoimmune pancreatitis is a T-cell mediated disease responsive to ciclosporin A and rapamycin . Pancreatology. 2012;12:579(abstract S8-3).
13. Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2016.
14. Champiat S, Lambotte E, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27:559-574.
15. Amgen. New safety information for Blincyto (blinatumomab) Risk of pancreatitis. August 2016 and update to Micromedex 2016.
16. Sumimoto K, Uchida K, KusudaT, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high, CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis . Pancreatology. 2014;14:193-200.
17. Ghatalia P, Morgan CJ, Choueiri TK, et al. Pancreatitis with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:136-145.
18. Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis . J Oncol Pharm Pract. 2012;19:257-260.
19. Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology . 2013;13:461-467.
20. Russano M, Vincenzi B, Benditti O, et al. Pazopanib and pancreatic toxicity: a case report. BMC Res notes. 2015;8:196-198.
21. Kawakubo K, Hata H, Kawakami H, et al. Pazopanib induced severe acute pancreatitis. Case Rep Oncol. 2015;8:356-358.
22. Peron J, Khenifer S, Potier V, et al. Axitinib induced acute pancreatitis: a case report . Anticancer Drugs. 2014;25:478-479.
23. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis . Ann Pharmaco. 2013;37:33.
24. Pezzilli R, Corinaldesi R, Morselli-LabateAM. Tyrosine kinase inhibitors and acute pancreatitis. http://www.serena.unina.it/index.php/jop/article/view/3836/4278. Published May 5, 2010. Accessed May 22 , 2017.
25. Blum KA, Christian B, Flynn JM, et al. A phase I trial of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab and bendamustine in patients with relapsed/refractory non Hodgkin’s lymphoma. Blood. 2012;120:abstract 1643.
26. Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: a case report. Pharmacotherapy. 2013;33:e43-e44.
27. Dabrafenib. In Life-Sciences-Europe.com from Tafinlar. EU Summary of Product Characteristics. 30 August 2013.
28. Varma MR, Mathew S, Krishnadas D, et al. Imatinib-induced pancreatitis. Indian J Pharmacol. 2010;42:50-52.
29. Palandri F, Castagnetti F, Soverinie S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758-1761.
30. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis. Ann of Pharmacother. 2013;47:e.3
31. Cortesk JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadephia chromosome-positive leukemias. New Engl J Med. 2012;367:2075-2088.
32. Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis [Letter]. J Pancreas. 2010;119:275-276.
33. Elouni B. Acute pancreatitis induced by Velcade ( bortezomib) with positive rechallenge. 9th Annual meeting of the International Society of Pharmacovigilance. Oct 2009 abstract 74.
34. Talamo G, Sikik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis. Case report and review of the literature . J Oncol Pharm Prac. 2016;22:332-334.
35. SolakogluT, Akyol P, Guney T, et al. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189-190.
36. Mihaila RG. A possible rare complication of bortezomib treatment, acute pancreatitis. Acta Medica Transilvanica. 2013;2:269-171
37. Gupta H, Bansal R, Khanna S, et al. An unusual complication of bortezomib therapy: acute pancreatitis. Indian J Nephr. 2014;24:135-136.
38. Fotoh M, KitaharaT, Sakuta J, et al. Multiple lipoma with hyperlipidemia in a multiple myeloma patient treated with bortezomib/dexamethasone. Leuk Res. 2010;34:e120-121.
39. Wang HH, Tsui J, Wang XY, et al. Bortezomib induced acute pancreatitis in a patient with multiple myeloma. Leuk Lymphoma. 2014;55:1404-1405.
40. Misselwitz B, Goede JS, Pestalozzi BC, et al. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol. 2010;89:569-577.
41. Gozzetti A, Fabbri A, Defina M, et al. Hyperlipidemia in a myeloma patient after bortezomib treatment. Leuk Research. 2010;34:e250.
42. Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893-897.
43. Runzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109.
44. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757.
45. Butt W, Saadati H, Wasif- Saif M. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res. 2010;30:5113-5115.
46. Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Onc Pharm Pract. 2010;16:133-134.
47. Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87:421-424.
48. Subramaniam S, Zell JA, Kunz PL. Everolimus causing severe hypertriglyceridemia and acute pancreatitis. J Natl Compr Canc Netw. 2013;11:5-9.
49. Wadood A, Chesner R, Mirza M, et al. Tamoxifen precipitation of familial hypertriglyceridaemia: a rare cause of acute pancreatitis. BMJ Case Rep. Published August 3, 2016. doi: 10.1136/bcr-2016-214837.
50. Sakhri J, BenSalem C, Fathallah H, et al. Severe pancreatitis due to tamoxifen induced hypertriglyceridemia with positive rechallenge. J Pancreas. 2010;11:382-384.
51. Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067-1069.
52. Artac M, Sari R, Altunbas J, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14:309-311.
53. [Author name not available]. Acute pancreatitis: 15 case reports. React Wkly. 2015;1546:29.
54. Ozcinar B, Guven K, Poylani A, et al. Necrotizing pancreatitis after transcatheter embolization for hepatocellular carcinoma. Diagn In
56. She WH, Chan ACY, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93-98.
57. Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:321-325.
58. Peterson JL, Vallow LA, Johnson DW, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy. 2013;12:573-579.
59. Piso P, Glockzin G, Schlitt HJ. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from gastric cancer. J Clin Oncol. 2011;29(suppl 4):abstract 132.
60. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3356888/?report=reader. Published 2012. Accessed May 23, 2017.
61. Muzaffar M, Jia J, Liles D, et al. Acute pancreatitis associated with ado-trastuzumab emtansine. Am J Ther. 2016;23:e572-574.
62. Urru SA, Mariotti E, Carta P, et al. Acute pancreatitis following brentuzimab vedotin therapy for refractory Hodgkin lymphoma: a case report. Drugs R D. 2014;14:9-11.
63. Gandhi MD, Evens AM, Fenske TS, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895-2897.
64. Brentuximab vedotin in Micromedex solutions, Truven Health Analytics. 2016.
65. Okazaki K, Uchida K. Autoimmune pancreatitis: the past, present and future. Pancreas. 2015;44:1006-1016.
66. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122-133.
67. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab and ipilimumab. J Natl Cancer Inst. 2017;109:[page numbers not available].
68. Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571-584.
69. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
70 . Di MY, Liu H, Zu-Yao Y, et al. Prediction models of mortality in acute pancreatitis in adults. A systematic review. Ann Int Med. 2016;165:482-490.
71. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;18:2133-2140.
Physician liability in opioid deaths
Question: Regarding opioid deaths, which of the following is incorrect?
A. The term refers to accidental or intentional deaths caused mostly by heroin.
B. They are reaching epidemic proportions.
C. May form the basis for a wrongful death lawsuit.
D. May lead to loss of medical license.
E. The physician may face prosecution for homicide.
Answer: A. Opioids are a class of drugs that include the illegal drug heroin, as well as prescription drugs such as fentanyl, oxycodone, hydrocodone, codeine, and morphine. To be sure, opioid deaths occur in addicts from the deliberate or accidental use of heroin; but other opioids, especially painkillers, are also widely implicated. In addition, deaths have resulted from the careless, negligent, reckless, or wanton conduct of doctors who prescribe them without the proper indications or in inappropriate amounts, and then fail to provide careful follow-up.
Physicians may face both civil and criminal liabilities in such a situation. One remedy sought in wrongful death is a civil action, i.e., a malpractice lawsuit against the negligent doctor. The plaintiff is asserting that by violating community professional standards, the physician’s substandard conduct breached his duty of due care and was a proximate cause of the patient’s death. The evidentiary proof that is required to sustain such an allegation is “more probable than not” or “preponderance of evidence,” and expert medical testimony is typically necessary to establish the requisite standard of care and causation. Where there is gross negligence, i.e., egregious conduct that was reckless, the jury may award punitive damages.
Not infrequently, the wayward doctor faces triple liability: a civil lawsuit, state medical board action, and criminal prosecution for homicide. Given the publicity over soaring opioid death rates, one can expect aggressive prosecution of dealers and doctors alike.
This was not the first such case in Oklahoma. In 2014, a 71-year-old pain management doctor pleaded guilty to eight counts of second-degree murder in connection with several drug overdose deaths and will serve 8 years in prison. The doctor had reportedly prescribed more controlled drugs than any other physician in the state of Oklahoma. These drugs included hydrocodone, oxycodone, alprazolam, diazepam (Valium), and carisoprodol (Soma) – as many as 600 pills at a time. He allegedly accepted only cash payment for the office visits, and a review of his patient files revealed inadequate assessment of patient complaints or physical findings to justify the prescriptions.
Other states have been equally aggressive in prosecuting doctors over opioid deaths from reckless prescribing habits.
For the first time, New York in 2014 convicted a doctor of manslaughter in the overdose deaths of patients from oxycodone and alprazolam (Xanax). Some of the patients were prescribed as many as 500-800 pills over a 5- to 6-week period. The defendant, an anesthesiologist and pain management specialist, allegedly saw upward of 90 patients a day in his Queens weekend storefront clinic, charging them on a per-prescription basis. In his defense, he claimed that he was simply trying to help suffering people who misused medications and who misled him (“tough patients and good liars”).
Likewise, a Los Angeles–area doctor was recently convicted of second-degree murder for prescribing painkillers that killed three patients, and he was sentenced to 30 years to life in prison.
According to the Centers for Disease Control and Prevention, both drug overdose and opioid-involved deaths continue to increase in the United States.2 The majority of drug overdose deaths (more than 6 out of 10) involve an opioid, and the number has quadrupled since 1999.2 It has been estimated that more than 18,000 overdose deaths in 2014 involved prescription painkillers, while an additional 10,000 fatalities were attributed to heroin and 5,000 to fentanyl and other synthetic opioids. Overdose deaths exceed motor vehicle accidents as the leading cause of injury-related deaths. About 90 Americans die every day from an opioid overdose, and opioids have been forecast to kill 500,000 Americans over the next decade.
The CDC acknowledges that prescription opioids are a driving factor, noting that since 1999, the amount sold in the United States has nearly quadrupled, yet there has not been an overall change in the amount of pain that Americans report.
States such as Missouri, faced with the skyrocketing cost of treating the opioid epidemic, have sued the drug manufacturers, blaming them for their “campaign of fraud and deception.” At the same time, doctors have been deemed the “biggest culprit” for the opioid addiction epidemic, and one author has pointedly asserted that “by refusing to accept their inability to separate pain relief from addiction, physicians have long suffered the sin of hubris – and their patients have paid the price.”3
The U.S. Surgeon General recently took the historic step of writing to all American doctors asking for their help. And the American Medical Association has developed an educational module explaining the epidemic and how opioid misuse is linked to heroin addiction. The module also outlines risk-reducing steps when using opioids for pain relief.4
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. Some of the materials here have appeared in previous columns by the author. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Available at www.foxnews.om/health/2017/06/26/oklahoma-doctor-charged-in-opioid-deaths-5-patients.html. Accessed June 28, 2017.
2. Available at www.cdc.gov/drugoverdose/epidemic/index.html. Accessed June 28, 2017.
3. Available at www.thedailybeast.com/the-doctors-who-started-the-opioid-epidemic. Accessed June 27, 2017.
4. https://www.end-opioid-epidemic.org. Accessed July 5, 2017.
Question: Regarding opioid deaths, which of the following is incorrect?
A. The term refers to accidental or intentional deaths caused mostly by heroin.
B. They are reaching epidemic proportions.
C. May form the basis for a wrongful death lawsuit.
D. May lead to loss of medical license.
E. The physician may face prosecution for homicide.
Answer: A. Opioids are a class of drugs that include the illegal drug heroin, as well as prescription drugs such as fentanyl, oxycodone, hydrocodone, codeine, and morphine. To be sure, opioid deaths occur in addicts from the deliberate or accidental use of heroin; but other opioids, especially painkillers, are also widely implicated. In addition, deaths have resulted from the careless, negligent, reckless, or wanton conduct of doctors who prescribe them without the proper indications or in inappropriate amounts, and then fail to provide careful follow-up.
Physicians may face both civil and criminal liabilities in such a situation. One remedy sought in wrongful death is a civil action, i.e., a malpractice lawsuit against the negligent doctor. The plaintiff is asserting that by violating community professional standards, the physician’s substandard conduct breached his duty of due care and was a proximate cause of the patient’s death. The evidentiary proof that is required to sustain such an allegation is “more probable than not” or “preponderance of evidence,” and expert medical testimony is typically necessary to establish the requisite standard of care and causation. Where there is gross negligence, i.e., egregious conduct that was reckless, the jury may award punitive damages.
Not infrequently, the wayward doctor faces triple liability: a civil lawsuit, state medical board action, and criminal prosecution for homicide. Given the publicity over soaring opioid death rates, one can expect aggressive prosecution of dealers and doctors alike.
This was not the first such case in Oklahoma. In 2014, a 71-year-old pain management doctor pleaded guilty to eight counts of second-degree murder in connection with several drug overdose deaths and will serve 8 years in prison. The doctor had reportedly prescribed more controlled drugs than any other physician in the state of Oklahoma. These drugs included hydrocodone, oxycodone, alprazolam, diazepam (Valium), and carisoprodol (Soma) – as many as 600 pills at a time. He allegedly accepted only cash payment for the office visits, and a review of his patient files revealed inadequate assessment of patient complaints or physical findings to justify the prescriptions.
Other states have been equally aggressive in prosecuting doctors over opioid deaths from reckless prescribing habits.
For the first time, New York in 2014 convicted a doctor of manslaughter in the overdose deaths of patients from oxycodone and alprazolam (Xanax). Some of the patients were prescribed as many as 500-800 pills over a 5- to 6-week period. The defendant, an anesthesiologist and pain management specialist, allegedly saw upward of 90 patients a day in his Queens weekend storefront clinic, charging them on a per-prescription basis. In his defense, he claimed that he was simply trying to help suffering people who misused medications and who misled him (“tough patients and good liars”).
Likewise, a Los Angeles–area doctor was recently convicted of second-degree murder for prescribing painkillers that killed three patients, and he was sentenced to 30 years to life in prison.
According to the Centers for Disease Control and Prevention, both drug overdose and opioid-involved deaths continue to increase in the United States.2 The majority of drug overdose deaths (more than 6 out of 10) involve an opioid, and the number has quadrupled since 1999.2 It has been estimated that more than 18,000 overdose deaths in 2014 involved prescription painkillers, while an additional 10,000 fatalities were attributed to heroin and 5,000 to fentanyl and other synthetic opioids. Overdose deaths exceed motor vehicle accidents as the leading cause of injury-related deaths. About 90 Americans die every day from an opioid overdose, and opioids have been forecast to kill 500,000 Americans over the next decade.
The CDC acknowledges that prescription opioids are a driving factor, noting that since 1999, the amount sold in the United States has nearly quadrupled, yet there has not been an overall change in the amount of pain that Americans report.
States such as Missouri, faced with the skyrocketing cost of treating the opioid epidemic, have sued the drug manufacturers, blaming them for their “campaign of fraud and deception.” At the same time, doctors have been deemed the “biggest culprit” for the opioid addiction epidemic, and one author has pointedly asserted that “by refusing to accept their inability to separate pain relief from addiction, physicians have long suffered the sin of hubris – and their patients have paid the price.”3
The U.S. Surgeon General recently took the historic step of writing to all American doctors asking for their help. And the American Medical Association has developed an educational module explaining the epidemic and how opioid misuse is linked to heroin addiction. The module also outlines risk-reducing steps when using opioids for pain relief.4
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. Some of the materials here have appeared in previous columns by the author. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Available at www.foxnews.om/health/2017/06/26/oklahoma-doctor-charged-in-opioid-deaths-5-patients.html. Accessed June 28, 2017.
2. Available at www.cdc.gov/drugoverdose/epidemic/index.html. Accessed June 28, 2017.
3. Available at www.thedailybeast.com/the-doctors-who-started-the-opioid-epidemic. Accessed June 27, 2017.
4. https://www.end-opioid-epidemic.org. Accessed July 5, 2017.
Question: Regarding opioid deaths, which of the following is incorrect?
A. The term refers to accidental or intentional deaths caused mostly by heroin.
B. They are reaching epidemic proportions.
C. May form the basis for a wrongful death lawsuit.
D. May lead to loss of medical license.
E. The physician may face prosecution for homicide.
Answer: A. Opioids are a class of drugs that include the illegal drug heroin, as well as prescription drugs such as fentanyl, oxycodone, hydrocodone, codeine, and morphine. To be sure, opioid deaths occur in addicts from the deliberate or accidental use of heroin; but other opioids, especially painkillers, are also widely implicated. In addition, deaths have resulted from the careless, negligent, reckless, or wanton conduct of doctors who prescribe them without the proper indications or in inappropriate amounts, and then fail to provide careful follow-up.
Physicians may face both civil and criminal liabilities in such a situation. One remedy sought in wrongful death is a civil action, i.e., a malpractice lawsuit against the negligent doctor. The plaintiff is asserting that by violating community professional standards, the physician’s substandard conduct breached his duty of due care and was a proximate cause of the patient’s death. The evidentiary proof that is required to sustain such an allegation is “more probable than not” or “preponderance of evidence,” and expert medical testimony is typically necessary to establish the requisite standard of care and causation. Where there is gross negligence, i.e., egregious conduct that was reckless, the jury may award punitive damages.
Not infrequently, the wayward doctor faces triple liability: a civil lawsuit, state medical board action, and criminal prosecution for homicide. Given the publicity over soaring opioid death rates, one can expect aggressive prosecution of dealers and doctors alike.
This was not the first such case in Oklahoma. In 2014, a 71-year-old pain management doctor pleaded guilty to eight counts of second-degree murder in connection with several drug overdose deaths and will serve 8 years in prison. The doctor had reportedly prescribed more controlled drugs than any other physician in the state of Oklahoma. These drugs included hydrocodone, oxycodone, alprazolam, diazepam (Valium), and carisoprodol (Soma) – as many as 600 pills at a time. He allegedly accepted only cash payment for the office visits, and a review of his patient files revealed inadequate assessment of patient complaints or physical findings to justify the prescriptions.
Other states have been equally aggressive in prosecuting doctors over opioid deaths from reckless prescribing habits.
For the first time, New York in 2014 convicted a doctor of manslaughter in the overdose deaths of patients from oxycodone and alprazolam (Xanax). Some of the patients were prescribed as many as 500-800 pills over a 5- to 6-week period. The defendant, an anesthesiologist and pain management specialist, allegedly saw upward of 90 patients a day in his Queens weekend storefront clinic, charging them on a per-prescription basis. In his defense, he claimed that he was simply trying to help suffering people who misused medications and who misled him (“tough patients and good liars”).
Likewise, a Los Angeles–area doctor was recently convicted of second-degree murder for prescribing painkillers that killed three patients, and he was sentenced to 30 years to life in prison.
According to the Centers for Disease Control and Prevention, both drug overdose and opioid-involved deaths continue to increase in the United States.2 The majority of drug overdose deaths (more than 6 out of 10) involve an opioid, and the number has quadrupled since 1999.2 It has been estimated that more than 18,000 overdose deaths in 2014 involved prescription painkillers, while an additional 10,000 fatalities were attributed to heroin and 5,000 to fentanyl and other synthetic opioids. Overdose deaths exceed motor vehicle accidents as the leading cause of injury-related deaths. About 90 Americans die every day from an opioid overdose, and opioids have been forecast to kill 500,000 Americans over the next decade.
The CDC acknowledges that prescription opioids are a driving factor, noting that since 1999, the amount sold in the United States has nearly quadrupled, yet there has not been an overall change in the amount of pain that Americans report.
States such as Missouri, faced with the skyrocketing cost of treating the opioid epidemic, have sued the drug manufacturers, blaming them for their “campaign of fraud and deception.” At the same time, doctors have been deemed the “biggest culprit” for the opioid addiction epidemic, and one author has pointedly asserted that “by refusing to accept their inability to separate pain relief from addiction, physicians have long suffered the sin of hubris – and their patients have paid the price.”3
The U.S. Surgeon General recently took the historic step of writing to all American doctors asking for their help. And the American Medical Association has developed an educational module explaining the epidemic and how opioid misuse is linked to heroin addiction. The module also outlines risk-reducing steps when using opioids for pain relief.4
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. Some of the materials here have appeared in previous columns by the author. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Available at www.foxnews.om/health/2017/06/26/oklahoma-doctor-charged-in-opioid-deaths-5-patients.html. Accessed June 28, 2017.
2. Available at www.cdc.gov/drugoverdose/epidemic/index.html. Accessed June 28, 2017.
3. Available at www.thedailybeast.com/the-doctors-who-started-the-opioid-epidemic. Accessed June 27, 2017.
4. https://www.end-opioid-epidemic.org. Accessed July 5, 2017.
Idarucizumab reversed dabigatran completely and rapidly in study
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
One IV 5-g dose of idarucizumab completely, rapidly, and safely reversed the anticoagulant effect of dabigatran, according to final results for 503 patients in the multicenter, prospective, open-label, uncontrolled RE-VERSE AD study.
Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group of 202 patients, 197 were able to undergo urgent procedures after a median of 1.6 hours, Charles V. Pollack Jr., MD, and his associates reported at the International Society on Thrombosis and Haemostasis congress. The report was simultaneously published in the New England Journal of Medicine.
Idarucizumab was specifically developed to reverse the anticoagulant effect of dabigatran. Many countries have already licensed the humanized monoclonal antibody fragment based on interim results for the first 90 patients enrolled in the Reversal Effects of Idarucizumab on Active Dabigatran (RE-VERSE AD) study (NCT02104947), noted Dr. Pollack, of Thomas Jefferson University, Philadelphia.
The final RE-VERSE AD cohort included 301 patients with uncontrolled gastrointestinal, intracranial, or trauma-related bleeding and 202 patients who needed urgent procedures. Participants from both groups typically were white, in their late 70s (age range, 21-96 years), and receiving 110 mg (75-150 mg) dabigatran twice daily. The primary endpoint was maximum percentage reversal within 4 hours after patients received idarucizumab, based on diluted thrombin time and ecarin clotting time.
The median maximum percentage reversal of dabigatran was 100% (95% confidence interval, 100% to 100%) in more than 98% of patients, and the effect usually lasted 24 hours. Among patients who underwent procedures, intraprocedural hemostasis was considered normal in 93% of cases, mildly abnormal in 5% of cases, and moderately abnormal in 2% of cases, the researchers noted. Seven patients received another dose of idarucizumab after developing recurrent or postoperative bleeding.
A total of 24 patients had an adjudicated thrombotic event within 30 days after receiving idarucizumab. These events included pulmonary embolism, systemic embolism, ischemic stroke, deep vein thrombosis, and myocardial infarction. The fact that many patients did not restart anticoagulation could have contributed to these thrombotic events, the researchers asserted. They noted that idarucizumab had no procoagulant activity in studies of animals and healthy human volunteers.
About 19% of patients in both groups died within 90 days. “Patients enrolled in this study were elderly, had numerous coexisting conditions, and presented with serious index events, such as intracranial hemorrhage, multiple trauma, sepsis, acute abdomen, or open fracture,” the investigators wrote. “Most of the deaths that occurred within 5 days after enrollment appeared to be related to the severity of the index event or to coexisting conditions, such as respiratory failure or multiple organ failure, whereas deaths that occurred after 30 days were more likely to be independent events or related to coexisting conditions.”
Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, Bristol-Myers Squibb/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators also disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
FROM 2017 ISTH CONGRESS
Key clinical point:
Major finding: Uncontrolled bleeding stopped a median of 2.5 hours after 134 patients received idarucizumab. In a separate group, 197 patients were able to undergo urgent procedures after a median of 1.6 hours.
Data source: A multicenter, prospective, open-label study of 503 patients (RE-VERSE AD).
Disclosures: Boehringer Ingelheim Pharmaceuticals provided funding. Dr. Pollack disclosed grant support from Boehringer Ingelheim during the course of the study and ties to Daiichi Sankyo, Portola, CSL Behring, BMS/Pfizer, Janssen Pharma, and AstraZeneca. Eighteen coinvestigators disclosed ties to Boehringer Ingelheim and a number of other pharmaceutical companies. Two coinvestigators had no relevant financial disclosures.
Tool indicates fracture risk after HSCT
MADRID – The risk of osteoporotic fracture associated with hematopoietic stem cell transplantation (HSCT) could be assessed using the Fracture Risk Assessment Tool (FRAX), researchers from the University of Texas MD Anderson Cancer Center have found.
In a retrospective cohort study, Huifang Lu, MD, and her collaborators found that FRAX could predict the risk of fracture with reasonable accuracy. The area under the receiver operating characteristic curve was 0.66 for predicting a fracture 10 years after HSCT.
“Current guidelines recommend the evaluation of bone health at 1 year following the transplant, but we recommend that this needs to happen at a much earlier time,” Dr. Lu said at the European Congress of Rheumatology.
Determining how to assess risk earlier and prevent bone loss remains a challenge, however. FRAX is an easy and quick tool to use, but its predictive ability is modest, she said.
As the finding comes from a retrospective study, prospective evaluation of FRAX is needed in HSCT patients. If shown predictive in this setting, bone health could be assessed earlier using FRAX, ideally at or before the time of the transplant, to allow appropriate action to be taken, such as prescribing bisphosphonates to those identified to be at high risk.
There is no consensus on preventing and treating bone loss following HSCT, said Dr. Lu. In a meta-analysis performed by Dr. Lu and her associates (Bone Marrow Transplant. 2017;52[5]:663-70), less bone loss was seen in patients who received a bisphosphonate.
As the use of HSCT has expanded over the past two decades, there is an expanding population of survivors with potential long-term effects such as bone loss and a higher risk of fractures, compared with the general population, Dr. Lu explained.
The FRAX tool takes into account pre-HSCT factors such as age, smoking status, alcohol use, prior fracture, body mass index, and corticosteroid use. This can be considered in association with the fracture risk related to the various conditioning and supporting regimens that patients receive around the time of their transplants.
The study included 5,170 adult patients who had undergone HSCT at the University of Texas MD Anderson Cancer Center over a 10-year period. Patients were considered to have entered the cohort at the time of their transplants, Dr. Lu said. Their history of osteoporotic fractures up to 3.3 years later was obtained and verified by radiology and physician assessment. FRAX probabilities were then derived from baseline information.
The mean age of patients included was 52 years, 57% were male and 75% were white. One-quarter had experienced a prior fracture. Of note, 26% of the cohort underwent HSCT for multiple myeloma, 70% of whom had already had a fracture, compared with 9% of those who underwent HSCT for another reason such as leukemia or lymphoma.
Multivariate analyses were performed with and without considering death as a competing risk, and similar results were obtained. Higher FRAX scores (20 or greater) were more likely to be recorded in individuals who sustained a fracture than in those who did not. Patients who had an allogeneic HSCT were 15% more likely to have a fracture as those who received an autologous transplant. Perhaps not surprisingly, patients with multiple myeloma were more likely than those who had HSCT for other reasons to sustain a fracture by 10 years based on FRAX results (hazard ratio, 3.16).
Future research needs to look at the optimal cut offs for FRAX scores predictive of events and see if there is any association between the loss of bone and fracture risk. There also needs to be an evaluation of the use of concomitant medications and health economic analyses performed.
Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
MADRID – The risk of osteoporotic fracture associated with hematopoietic stem cell transplantation (HSCT) could be assessed using the Fracture Risk Assessment Tool (FRAX), researchers from the University of Texas MD Anderson Cancer Center have found.
In a retrospective cohort study, Huifang Lu, MD, and her collaborators found that FRAX could predict the risk of fracture with reasonable accuracy. The area under the receiver operating characteristic curve was 0.66 for predicting a fracture 10 years after HSCT.
“Current guidelines recommend the evaluation of bone health at 1 year following the transplant, but we recommend that this needs to happen at a much earlier time,” Dr. Lu said at the European Congress of Rheumatology.
Determining how to assess risk earlier and prevent bone loss remains a challenge, however. FRAX is an easy and quick tool to use, but its predictive ability is modest, she said.
As the finding comes from a retrospective study, prospective evaluation of FRAX is needed in HSCT patients. If shown predictive in this setting, bone health could be assessed earlier using FRAX, ideally at or before the time of the transplant, to allow appropriate action to be taken, such as prescribing bisphosphonates to those identified to be at high risk.
There is no consensus on preventing and treating bone loss following HSCT, said Dr. Lu. In a meta-analysis performed by Dr. Lu and her associates (Bone Marrow Transplant. 2017;52[5]:663-70), less bone loss was seen in patients who received a bisphosphonate.
As the use of HSCT has expanded over the past two decades, there is an expanding population of survivors with potential long-term effects such as bone loss and a higher risk of fractures, compared with the general population, Dr. Lu explained.
The FRAX tool takes into account pre-HSCT factors such as age, smoking status, alcohol use, prior fracture, body mass index, and corticosteroid use. This can be considered in association with the fracture risk related to the various conditioning and supporting regimens that patients receive around the time of their transplants.
The study included 5,170 adult patients who had undergone HSCT at the University of Texas MD Anderson Cancer Center over a 10-year period. Patients were considered to have entered the cohort at the time of their transplants, Dr. Lu said. Their history of osteoporotic fractures up to 3.3 years later was obtained and verified by radiology and physician assessment. FRAX probabilities were then derived from baseline information.
The mean age of patients included was 52 years, 57% were male and 75% were white. One-quarter had experienced a prior fracture. Of note, 26% of the cohort underwent HSCT for multiple myeloma, 70% of whom had already had a fracture, compared with 9% of those who underwent HSCT for another reason such as leukemia or lymphoma.
Multivariate analyses were performed with and without considering death as a competing risk, and similar results were obtained. Higher FRAX scores (20 or greater) were more likely to be recorded in individuals who sustained a fracture than in those who did not. Patients who had an allogeneic HSCT were 15% more likely to have a fracture as those who received an autologous transplant. Perhaps not surprisingly, patients with multiple myeloma were more likely than those who had HSCT for other reasons to sustain a fracture by 10 years based on FRAX results (hazard ratio, 3.16).
Future research needs to look at the optimal cut offs for FRAX scores predictive of events and see if there is any association between the loss of bone and fracture risk. There also needs to be an evaluation of the use of concomitant medications and health economic analyses performed.
Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
MADRID – The risk of osteoporotic fracture associated with hematopoietic stem cell transplantation (HSCT) could be assessed using the Fracture Risk Assessment Tool (FRAX), researchers from the University of Texas MD Anderson Cancer Center have found.
In a retrospective cohort study, Huifang Lu, MD, and her collaborators found that FRAX could predict the risk of fracture with reasonable accuracy. The area under the receiver operating characteristic curve was 0.66 for predicting a fracture 10 years after HSCT.
“Current guidelines recommend the evaluation of bone health at 1 year following the transplant, but we recommend that this needs to happen at a much earlier time,” Dr. Lu said at the European Congress of Rheumatology.
Determining how to assess risk earlier and prevent bone loss remains a challenge, however. FRAX is an easy and quick tool to use, but its predictive ability is modest, she said.
As the finding comes from a retrospective study, prospective evaluation of FRAX is needed in HSCT patients. If shown predictive in this setting, bone health could be assessed earlier using FRAX, ideally at or before the time of the transplant, to allow appropriate action to be taken, such as prescribing bisphosphonates to those identified to be at high risk.
There is no consensus on preventing and treating bone loss following HSCT, said Dr. Lu. In a meta-analysis performed by Dr. Lu and her associates (Bone Marrow Transplant. 2017;52[5]:663-70), less bone loss was seen in patients who received a bisphosphonate.
As the use of HSCT has expanded over the past two decades, there is an expanding population of survivors with potential long-term effects such as bone loss and a higher risk of fractures, compared with the general population, Dr. Lu explained.
The FRAX tool takes into account pre-HSCT factors such as age, smoking status, alcohol use, prior fracture, body mass index, and corticosteroid use. This can be considered in association with the fracture risk related to the various conditioning and supporting regimens that patients receive around the time of their transplants.
The study included 5,170 adult patients who had undergone HSCT at the University of Texas MD Anderson Cancer Center over a 10-year period. Patients were considered to have entered the cohort at the time of their transplants, Dr. Lu said. Their history of osteoporotic fractures up to 3.3 years later was obtained and verified by radiology and physician assessment. FRAX probabilities were then derived from baseline information.
The mean age of patients included was 52 years, 57% were male and 75% were white. One-quarter had experienced a prior fracture. Of note, 26% of the cohort underwent HSCT for multiple myeloma, 70% of whom had already had a fracture, compared with 9% of those who underwent HSCT for another reason such as leukemia or lymphoma.
Multivariate analyses were performed with and without considering death as a competing risk, and similar results were obtained. Higher FRAX scores (20 or greater) were more likely to be recorded in individuals who sustained a fracture than in those who did not. Patients who had an allogeneic HSCT were 15% more likely to have a fracture as those who received an autologous transplant. Perhaps not surprisingly, patients with multiple myeloma were more likely than those who had HSCT for other reasons to sustain a fracture by 10 years based on FRAX results (hazard ratio, 3.16).
Future research needs to look at the optimal cut offs for FRAX scores predictive of events and see if there is any association between the loss of bone and fracture risk. There also needs to be an evaluation of the use of concomitant medications and health economic analyses performed.
Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
AT THE EULAR 2017 CONGRESS
Key clinical point: The Fracture Risk Assessment Tool (FRAX) helped in predicting osteoporotic fracture risk after hematopoietic stem cell transplantation (HSCT).
Major finding: The area under the receiver operating characteristic curve was 0.66, indicating modest predictive ability,10 years after HSCT.
Data source: A retrospective cohort study of 5,170 adult patients who received HSCT at the University of Texas MD Anderson Cancer Center between 2001 and 2010.
Disclosures: Dr. Lu had no conflicts of interest. The study was funded by the Rolanette and Berdon Lawrence Bone Disease Program of Texas and via Cancer Survivorship Research Seed Monday Grants from the University Cancer Foundation and the Duncan Family Institute for Cancer Prevention and Risk Assessment to the University of Texas MD Anderson Cancer Center.
Prehabilitation for lymphedema in head and neck cancer patients at a community cancer center
Lymphedema is the swelling of tissue caused by the accumulation of interstitial fluid in any area of the body where lymphatic flow has been compromised.1 Secondary lymphedema is an acquired abnormality in lymph drainage1,2 and is the type commonly seen in cancer patients. Secondary lymphedema can be described as external or internal. Internal lymphedema, swelling of deep structures and tissues, is very difficult to quantify.
Lymphedema in patients with head and neck cancers
Lymphedema is a complicating morbidity frequently seen in head and neck cancer patients who have undergone treatment with surgery, radiation, and chemotherapy. However, although it is one of the most prevalent side effects of treatment, it is both under-recognized and under-treated.3
In head and neck cancer patients, internal swelling may develop in the soft tissues of the upper aero-digestive tract,4 affecting articulation and swallowing. Currently, there does not seem to be an effective practical and reliable tool with which to measure internal lymphedema. In addition, it is generally accepted that there is no effective way to treat internal lymphedema. By contrast, external lymphedema is more readily observed, but both subjective and objective assessments are difficult. External swelling may occur in the face, jaw, and neck. However, the subjective scales currently available are insufficient to capture very important characteristics of external lymphedema.5 The Edge Task Force on Head and Neck Cancer in 2015 was not able to recommend any outcome measures for objectively quantifying external edema.6 Furthermore, objective measurements of head and neck lymphedema can be expensive and time consuming.
Extent and risk
A combination of both internal and external swelling is seen in more than 50% of patients.7 Risk factors include “throat” tumors, multicancer treatment approaches, higher total radiation dose, a greater number of radiation procedures, and radiation at the surgical site.5 More than 500,000 survivors of head and neck cancer in the United States are at risk of lymphedema.5 Although recent advances in treatment have reduced the incidence of other morbidities, 50% of patients who are treated for head and neck cancer may still develop lymphedema.1,8 The reported incidence in some centers may be much higher, with up to 75% of patients developing lymphedema following treatment.9
Measurement modalities for clinical evaluation
There is little current research into lymphedema of the head and neck, despite the high prevalence of the condition.8 According to Deng and colleagues, measurement of head and neck lymphedema is a challenge, which has an impact on clinical assessment, diagnosis, and treatment of this under-recognized, under-reported and under-addressed problem in head and neck cancer patients.10 In a review of the literature, Deng and colleagues identified three measurement modalities available for clinical evaluation: patient-reported outcomes, clinician-reported outcomes, and technology.10 One major factor, though, in detecting lymphedema, is physician awareness: physicians, health care professionals, and even some lymphedema therapists are not well educated about this problem.8
Treatment
The effectiveness of traditional lymphedema treatment is not well defined.8 Currently, complete decongestive therapy (CDT), is considered the standard of care for lymphedema. The National Lymphedema Network has stated that modifications of CDT, especially manual lymphatic drainage and modified compressive garments for external lymphedema, have been shown to be beneficial for the treatment of lymphedema in head and neck cancer patients.11 Most findings in lymphedema research, mainly in breast cancer patients, have shown that early intervention is the best management and yields the best outcomes. As with other chronic conditions, early identification and timely, appropriate treatment of lymphedema is critical to improve clinical outcomes, to decrease symptom burden and functional impairment, and to improve overall quality of life in head and neck cancer patients.10
Improving recognition and treatment
Head and neck oncologic treatment is increasingly offered outside the network of specialist academic hospitals, at hospitals serving more localized communities where the neediest, sickest patient groups may be receiving less than optimal care.3 This challenges community hospitals to provide optimal treatment, similar to that being offered at nationally recognized institutions. In January 2012, we implemented a prehabilitation program in our community hospital cancer center to provide early intervention for our patients based on the understanding that proper and prompt treatment for patients with early signs of lymphedema should be a priority.12 In this article, we outline how we implemented the program and the describe improvements we observed before and after the implementation of the program.
The prehabilitation program
The role of the nurse navigator
Before the introduction of the prehabilitation program, our pattern of practice was to refer patients to oncology rehabilitation for lymphedema management after they had completed their medical treatment with surgery, radiation, and chemotherapy. In 2012, that was changed to a prehabilitation model of care that was overseen by a head and neck nurse navigator. This focus on prehabilitation begins with patients being referred to oncology rehabilitation at the time of cancer diagnosis for baseline assessment of head and neck swelling. In addition, there is assessment of the many possible other side effects associated with head and neck cancer and its treatment, namely loss of range of motion of the neck, jaw (trismus), and/or shoulders, postural deficits, functional loss, pain, balance dysfunction with fall risk, weakness, and fatigue. Therapeutic interventions are initiated as needed and appropriate. This process also raises awareness of a condition that has been described as under-recognized and under-treated.3
The nurse navigator sits in on each radiation oncology consultation and aids in “navigating” patients through their treatment. The nurse ensures that each patient is referred to different ancillary services from the outset, such as seeing a dietician, social worker, physical/occupational therapist and certified lymphedema therapist, speech pathologist, and financial assistance advisor, if necessary (Table 1).
Assessment of lymphedema
Measurement of head and neck lymphedema is a challenge.10 In our program, the physical therapy assessment also includes the evaluation of several other morbidities associated with head and neck cancer and its treatment, such as range of motion, weakness, fatigue, radiation fibrosis, balance dysfunction, and risk of falling (Table 2).
Patient-reported outcomes are essential to fully capture observable and unobservable symptoms (eg, sensations) as well as the functional impacts of lymphedema.10 In addition to lymphedema, there are many other morbidities that may be assessed on the basis of patient-reported outcome tools, such as upper extremity function with QuickDASH.13 At our clinic for head and neck cancer patients we use the Neck Disability Index (NDI)14 and Care Connections (CC)15 survey for the patient-reported outcomes. The Quick DASH, NDI, and CC tools all assess standard functional outcomes that are not specific to lymphedema, but are useful in documenting changes related to lymphedema. We initially used the CC survey and later transitioned to using the NDI. Neck pain is common with lymphedema in the head and neck region, and the NDI is a valid, reliable, responsive and internally consistent clinical tool to measure self-reported disability in patients with neck pain.16 These questionnaires were completed by the patients at their initial assessment, at reassessment, and at time of discharge.
Although objective criteria for external lymphedema have not been established, simple measurements such as using a tape measure to record neck circumference, allow a useful longitudinal assessment. Digital photography may be effective in the documentation and subjective evaluation of changes of external lymphedema.10,17 However, there are some limitations with photography because although external photographs (including digital photography and three-dimensional imaging) can capture some features, such as changes in contours, symmetry, and changes in skin quality and color, they do not detect changes in skin and soft tissue texture and compliance (Table 3).10
Impact on clinical outcomes
We retrospectively reviewed the medical records of 230 head and neck cancer patients who had been treated at our center between June 2008 and June 2015. Complete clinical data were available for 190 patients. The following information was extracted from each patient’s chart: whether they developed lymphedema, tumor stage, had surgery, radiation dose, type of chemotherapy given, their smoking history, if they had had a neck dissection and the primary site of the tumor (Table 3).
Incidence in different time periods. Of the 190 patients with complete records 78 (41%) were found to have lymphedema. These were all patients undergoing treatment for head and neck cancer during June 2008-June 2015. The prehabilitation program was initiated with the hiring of a nurse navigator for head and neck cancer, starting in January 2012. It is interesting to note that the incidence of lymphedema was 27% before the program was started, but after nurse navigator joined the team, the incidence increased significantly to 48% (P = .0002), in line with published expectations. This increase in recorded incidence may be attributable to the greater awareness of lymphedema intentionally fostered by the prehabilitation program.
Smoking history. Patients’ lifetime smoking history was retrieved from their medical records, based on their verbal admission of tobacco use. Most of the patients (n = 110) self-reported a history of smoking. Of those with a history of smoking, 36 (33%) developed external lymphedema after treatment for head and neck cancer, and 74 (67%) did not. However, this difference was not statistically significant. Hence, although smoking is a risk factor for head and neck cancer, it was not associated with the development of external lymphedema in our cohort of patients.
Type of tumor
Most of the patients (n = 156, 82%) had squamous cell carcinomas (SCC). Of those, 45% developed external lymphedema and 55% did not. Therefore, having SCC did not predispose to lymphedema. The other cancers were mixed type, mainly adenocaricoma, but their numbers were too small to draw statistical conclusions.
Stage of the tumor
About two thirds of the patients (n = 121, 64%) had stage 3 or 4 cancer. However, treatment of more advanced cancers was not associated with lymphedema development.
Site of the tumor
The literature suggests that patients with a primary tumor in the throat are at increased risk for lymphedema.5 The American Cancer Society has defined cancers of the oropharynx (throat) as including the base of the tongue (back third of the tongue), the soft palate, the tonsils, and the side and back walls of the throat.18 In our head and neck cancer cohort, patients with primary tumors of the oropharnyx were, perhaps, more susceptible to lymphedema (P = .044, Table 3). By contrast, in our cohort of patients, those with nasopharyngeal, hypopharyngeal, and parotid gland tumors were significantly less likely to develop lymphedema (Ps = .017, .04, .012, respectively).
No surgery
Half of our patients (n = 95) were not treated with surgery. In the patients who did not have surgery, 25 (26%) developed lymphedema, whereas 70 (74%) did not. Hence, although the incidence of lymphedema was significantly lower in patients who did not have surgery (P = .015), lymphedema did develop in patients who did not have a surgical procedure.
Resection of primary tumor without neck dissection
Of the 64 patients who had surgery, but without neck dissection, 35 (55%) developed external lymphedema. Compared with the no-surgery patients, the doubling of the incidence (from 26% to 55%) was highly significant (P = .0004). These findings are compatible with the literature reports that surgery increases the incidence of lymphedema, which is not surprising because surgery and subsequent scarring is known to compromise the lymphatic system.
Resection of primary tumor with neck dissection
The incidence of external lymphedema was increased to 69% when patients were subjected to both surgery and neck dissection. Compared with the June 2008-June 2015 cohort, there was a significant increase in the incidence of lymphedema in the neck dissection group (P = .007). Neck dissection involves the removal of lymph nodes and disruption of the lymphatic vessels, so it is not surprising that there is a higher incidence of external lymphedema. In our practice, neck dissections increased in frequency every year from June 2008 until December 2011, when 8 patients underwent neck dissections, 6 (75%) of whom developed lymphedema. Since January 2012, when the prehabilitation program was implemented, the number of neck dissections have declined, with more patients receiving chemoradiation and surgery being reserved for surgery. Hamoir and colleagues have reported that neck dissection is no longer justified unless there is clinically residual disease in the neck.19
Radiation
Lymphedema occurred in patients regardless of the dose of radiation received. Although the incidence of lymphedema seemed to be higher in patients who received more than 60 cGy, that difference was not statistically significant (Table 3). We had expected a relationship between radiation damage and greater lymphedema, but that was not evident in our patients.
Chemotherapy
The majority of patients (n = 131, 69%) received chemotherapy. The exposure to chemotherapy was not correlated with the risk of external lymphedema in our cohort of patients, with 58 of the 131 treated patients (44%) developing lymphedema, compared with 73 (56%) of treated patients who did not (Table 3).
Complete decongestive therapy
All patients with documented lymphedema were evaluated for complete decongestive therapy (CDT). Contraindications to CDT included congestive heart failure, renal failure, acute infection, peripheral artery disease, upper-quadrant deep vein thrombosis, and carotid artery stenosis. Eligible patients were referred to a certified lymphedema therapist for CDT. As the program evolved, patients at risk for lymphedema were referred for CDT early on, usually at the time of diagnosis, to improve early identification and surveillance of lymphedema.
CDT included manual lymph drainage,
Patients’ responses to CDT were documented with digital photographs that were taken at each visit and, more recently, use of the NDI.
Communication and education
The head and neck cancer nurse navigator attends the cancer center’s multidisciplinary head and neck tumor board, which has representation from otolaryngology, diagnostic radiology, pathology, radiation oncology, medical oncology, reconstructive surgery, oncology rehabilitation (physical/occupational therapist), dietary services, speech pathology, social services and clinical research. This regular contact allows for earlier awareness about which patients are at greater risk for developing lymphedema, thus enabling early intervention (and patient education) in a timely manner.
Education of the patient, before cancer therapy, of the risks of lymphedema is very important. Before the implementation of the prehabilitation program, some patients did not fully comprehend what a painful and debilitating consequence of cancer treatment lymphedema could be.
Discussion
We introduced a prehabilitation program to detect and treat lymphedema in head and neck cancer patients in January 2012 part way through following an observation cohort from June2008 through June2015. Central to this, in our center, was the appointment of a nurse navigator whose primary focus was on head and neck cancer patients. We placed a high priority on the early detection and treatment of lymphedema because do so has been associated with better outcomes in other centers.
One immediate consequence of the inception of our program was the identification of more patients with external lymphedema. Our detected incidence rose significantly (P = .0002), from 27% in the period June 2008-December 20112010, before the program, to 48% during the January 2012-June 2015 period, after the inception of the program. This later incidence rate is in line with published incidence rates in most centers. However, it is still somewhat short of the 75% suggested in one center,9 which suggests we are either we are underdetecting lymphedema or there are differences in definition criteria or sensitivity levels for defining lymphedema.
There are currently no specific objective measures of lymphedema, so there is bound to be some variation in diagnosis rates. In our program, we rely heavily on the patient-reported outcome measures, the NDI instrument, and digital photography to detect and monitor lymphedema, starting with the pretreatment baseline values that are established for each patient.
The use of digital photography in our community hospital setting, which includes taking photographs before and after treatment and at each visit, motivates and encourages patients and provides a tool for clinical lymphedema therapists to visually document benefits of treatment. Patients’ motivation and compliance with their established home program for head and neck lymphedema self-management are essential. The elements of the home program may include self-manual lymph drainage, home-modified compression bandaging and garment wear, therapeutic exercises, and skin care. Patients with lymphedema who adhered closely with their therapy program were more than 8 times more likely to improve compared with noncompliant patients.17
Some groups of patients have a greater risk of developing lymphedema than others,5 so the development of an algorithm to predict lymphedema seemed possible. However, in our cohort of patients, only neck dissection, with its disruption of the lymphatic system of the neck, was strongly associated with external lymphedema (Table 3). It is important to note that some patients who did not undergo surgery developed lymphedema. In our patients, high doses of radiation alone did not seem to predispose to lymphedema. That suggests that no group of head and neck cancer patients should be ignored, which is why we did routine screening of all patients before, during, and after treatment.
Our protocol falls short in the detection of internal lymphedema. For example, information on swallowing gathered by our speech pathologists (in a different department) has not, so far, been included in our assessment. This is one opportunity to improve on our approach, especially because speech difficulties may be associated with internal lymphedema. In addition, we are not equipped for the requisite internal examinations. Unfortunately, there are no practical and successful treatments for patients suffering from internal swelling. This represents a challenge for the medical community to better meet this need. Therefore, although we are missing some assessments of internal lymphedema, this is of little therapeutic consequence at this time.
The increase in the detected incidence of external lymphedema points to a practice gap that has been resolved by the appointment of a dedicated nurse navigator who attends oncology reviews to share knowledge and information. Another educational effort has been made with the patients themselves to increase compliance and improve continuous care at home.
There is always room for improvement, however, either by feedback acquired from other institutions and hospitals or through the future introduction of more objective assessment techniques.
Conclusions
The introduction of the prehabilitation program at our center has coincided with a significantly improved detection rate for external lymphedema in head and neck cancer patients. It may be because the program emphasizes education about lymphedema that awareness of the condition has increased throughout the center. It is now widely recognized that all patients are at risk of lymphedema regardless of whether they fall into an acknowledged high-risk group. Our experience shows that there is no significant difference between treatment modalities apart from neck dissection. In our population, the use of this procedure is decreasing. External lymphedema can develop even in patients who do not have surgery. Therefore, there is no sound way to predict which patients are most likely to suffer from the accumulation of fluid in their head and neck after treatment for head and neck cancer. Thus, an assessment as described here, during and after treatment for all patients, is warranted. Patients are now being seen earlier as a part of the prehabilitation program, which facilitates access to complete decongestive treatment at an earlier stage, improves patient outcomes, and increases patient satisfaction with their treatment. Our prehabilitation program could serve as a model for other community hospital centers in achieving outcomes that are as good as those in academic centers.
Acknowledgments
The authors thank Irene Kadota and Heather Peters, from the Department of Radiation Oncology, and Julianne Courtenay, from the Department of Physical Therapy at the Disney Family Cancer Center, Burbank, California, for providing the original clinical data for analysis.
1. The National Lymphedema Medical Advisory Committee. The diagnosis and treatment of lymphedema. National Lymphedema Network. http://www.lymphnet.org/pdfDocs/nlntreatment.pdf. Updated February 2011. Accessed April 26, 2017.
2. McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care (Engl). 2014;23(3):317-327.
3. Bhattacharyya N, Abemayor E. Patterns of hospital utilization for head and neck cancer care: changing demographics. JAMA Otolaryngol Head Neck Surg. 2015;141(4):307-312.
4. Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43(2):244-252.
5. Deng J, Ridner SH, Dietrich MS, et al. Factors associated with external and internal lymphedema in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;84(3):e319-328.
6. Flores AM, Spinelli BA, Eden MM, Galantino ML. EDGE task force on head and neck cancer outcomes: a systematic review of outcomes measures for quantifying external lymphedema. Rehabil Oncol. 2015;33(2):15-23.
7. Ridner SH, Doersam J, Galford E. An update on lymphedema of the head and neck. http://www.lymphnet.org/pdfDocs/Vol_28-N2_Update_HN.pdf. Published April-June 2015. Accessed April 26, 2017.
8. Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152(2);284-291.
9. Naqvi SHS, Karni RJ, Tan IC, et al. Int J Rad Oncol Biol Phys. 2016;4:927-928.
10. Deng J, Ridner SH, Aulino JM, Murphy BA. Assessment and measurement of head and neck lymphedema: state-of-the-science and future directions. Oral Oncol. 2015; 51(5):431-437.
11. Purcell A. Head and neck lymphedema management practices. J Lymphedema. 2013;8(2):8-15.
12. Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis treatment and impact: a review. J Clinl Oncol. 2012;30(30):3726-3733.
13. Quick DASH questionnaire. http://www.dash.iwh.on.ca/about-quickdash. [Last update not stated.] Accessed May 18, 2017.
14. Neck Disability Index questionnaire. www.aaos.org/uploadedFiles/NDI.pdf Accessed May 18, 2017.
15. Care Connections questionnaire. http://www.careconnections.com/. Accessed May 18, 2017.
16. Galantino ML, Eden MM, Spinelli BA, Flores AM. EDGE task force on head and neck cancer outcomes a systematic review of outcome measures for temporomandibular-related dysfunction. Rehabil Oncol. 2015;33(1):6-14.
17. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38(1):e1-e10.
18. What are oral cavity and oropharyngeal cancers? American Cancer Society. http://www.cancer.org/cancer/oralcavityandoropharyngealcancer/detailedguide/oral-cavity-and-oropharyngeal-cancer-what-is-oral-cavity-cancer. Last revised August 8, 2016. Accessed April 26, 2017.
19. Hamoir M, Schmitz S, Gregoire V. The role of neck dissection in squamous cell carcinoma of the head and neck. Curr Treat Options Oncol. 2014;15:611-624.
Lymphedema is the swelling of tissue caused by the accumulation of interstitial fluid in any area of the body where lymphatic flow has been compromised.1 Secondary lymphedema is an acquired abnormality in lymph drainage1,2 and is the type commonly seen in cancer patients. Secondary lymphedema can be described as external or internal. Internal lymphedema, swelling of deep structures and tissues, is very difficult to quantify.
Lymphedema in patients with head and neck cancers
Lymphedema is a complicating morbidity frequently seen in head and neck cancer patients who have undergone treatment with surgery, radiation, and chemotherapy. However, although it is one of the most prevalent side effects of treatment, it is both under-recognized and under-treated.3
In head and neck cancer patients, internal swelling may develop in the soft tissues of the upper aero-digestive tract,4 affecting articulation and swallowing. Currently, there does not seem to be an effective practical and reliable tool with which to measure internal lymphedema. In addition, it is generally accepted that there is no effective way to treat internal lymphedema. By contrast, external lymphedema is more readily observed, but both subjective and objective assessments are difficult. External swelling may occur in the face, jaw, and neck. However, the subjective scales currently available are insufficient to capture very important characteristics of external lymphedema.5 The Edge Task Force on Head and Neck Cancer in 2015 was not able to recommend any outcome measures for objectively quantifying external edema.6 Furthermore, objective measurements of head and neck lymphedema can be expensive and time consuming.
Extent and risk
A combination of both internal and external swelling is seen in more than 50% of patients.7 Risk factors include “throat” tumors, multicancer treatment approaches, higher total radiation dose, a greater number of radiation procedures, and radiation at the surgical site.5 More than 500,000 survivors of head and neck cancer in the United States are at risk of lymphedema.5 Although recent advances in treatment have reduced the incidence of other morbidities, 50% of patients who are treated for head and neck cancer may still develop lymphedema.1,8 The reported incidence in some centers may be much higher, with up to 75% of patients developing lymphedema following treatment.9
Measurement modalities for clinical evaluation
There is little current research into lymphedema of the head and neck, despite the high prevalence of the condition.8 According to Deng and colleagues, measurement of head and neck lymphedema is a challenge, which has an impact on clinical assessment, diagnosis, and treatment of this under-recognized, under-reported and under-addressed problem in head and neck cancer patients.10 In a review of the literature, Deng and colleagues identified three measurement modalities available for clinical evaluation: patient-reported outcomes, clinician-reported outcomes, and technology.10 One major factor, though, in detecting lymphedema, is physician awareness: physicians, health care professionals, and even some lymphedema therapists are not well educated about this problem.8
Treatment
The effectiveness of traditional lymphedema treatment is not well defined.8 Currently, complete decongestive therapy (CDT), is considered the standard of care for lymphedema. The National Lymphedema Network has stated that modifications of CDT, especially manual lymphatic drainage and modified compressive garments for external lymphedema, have been shown to be beneficial for the treatment of lymphedema in head and neck cancer patients.11 Most findings in lymphedema research, mainly in breast cancer patients, have shown that early intervention is the best management and yields the best outcomes. As with other chronic conditions, early identification and timely, appropriate treatment of lymphedema is critical to improve clinical outcomes, to decrease symptom burden and functional impairment, and to improve overall quality of life in head and neck cancer patients.10
Improving recognition and treatment
Head and neck oncologic treatment is increasingly offered outside the network of specialist academic hospitals, at hospitals serving more localized communities where the neediest, sickest patient groups may be receiving less than optimal care.3 This challenges community hospitals to provide optimal treatment, similar to that being offered at nationally recognized institutions. In January 2012, we implemented a prehabilitation program in our community hospital cancer center to provide early intervention for our patients based on the understanding that proper and prompt treatment for patients with early signs of lymphedema should be a priority.12 In this article, we outline how we implemented the program and the describe improvements we observed before and after the implementation of the program.
The prehabilitation program
The role of the nurse navigator
Before the introduction of the prehabilitation program, our pattern of practice was to refer patients to oncology rehabilitation for lymphedema management after they had completed their medical treatment with surgery, radiation, and chemotherapy. In 2012, that was changed to a prehabilitation model of care that was overseen by a head and neck nurse navigator. This focus on prehabilitation begins with patients being referred to oncology rehabilitation at the time of cancer diagnosis for baseline assessment of head and neck swelling. In addition, there is assessment of the many possible other side effects associated with head and neck cancer and its treatment, namely loss of range of motion of the neck, jaw (trismus), and/or shoulders, postural deficits, functional loss, pain, balance dysfunction with fall risk, weakness, and fatigue. Therapeutic interventions are initiated as needed and appropriate. This process also raises awareness of a condition that has been described as under-recognized and under-treated.3
The nurse navigator sits in on each radiation oncology consultation and aids in “navigating” patients through their treatment. The nurse ensures that each patient is referred to different ancillary services from the outset, such as seeing a dietician, social worker, physical/occupational therapist and certified lymphedema therapist, speech pathologist, and financial assistance advisor, if necessary (Table 1).
Assessment of lymphedema
Measurement of head and neck lymphedema is a challenge.10 In our program, the physical therapy assessment also includes the evaluation of several other morbidities associated with head and neck cancer and its treatment, such as range of motion, weakness, fatigue, radiation fibrosis, balance dysfunction, and risk of falling (Table 2).
Patient-reported outcomes are essential to fully capture observable and unobservable symptoms (eg, sensations) as well as the functional impacts of lymphedema.10 In addition to lymphedema, there are many other morbidities that may be assessed on the basis of patient-reported outcome tools, such as upper extremity function with QuickDASH.13 At our clinic for head and neck cancer patients we use the Neck Disability Index (NDI)14 and Care Connections (CC)15 survey for the patient-reported outcomes. The Quick DASH, NDI, and CC tools all assess standard functional outcomes that are not specific to lymphedema, but are useful in documenting changes related to lymphedema. We initially used the CC survey and later transitioned to using the NDI. Neck pain is common with lymphedema in the head and neck region, and the NDI is a valid, reliable, responsive and internally consistent clinical tool to measure self-reported disability in patients with neck pain.16 These questionnaires were completed by the patients at their initial assessment, at reassessment, and at time of discharge.
Although objective criteria for external lymphedema have not been established, simple measurements such as using a tape measure to record neck circumference, allow a useful longitudinal assessment. Digital photography may be effective in the documentation and subjective evaluation of changes of external lymphedema.10,17 However, there are some limitations with photography because although external photographs (including digital photography and three-dimensional imaging) can capture some features, such as changes in contours, symmetry, and changes in skin quality and color, they do not detect changes in skin and soft tissue texture and compliance (Table 3).10
Impact on clinical outcomes
We retrospectively reviewed the medical records of 230 head and neck cancer patients who had been treated at our center between June 2008 and June 2015. Complete clinical data were available for 190 patients. The following information was extracted from each patient’s chart: whether they developed lymphedema, tumor stage, had surgery, radiation dose, type of chemotherapy given, their smoking history, if they had had a neck dissection and the primary site of the tumor (Table 3).
Incidence in different time periods. Of the 190 patients with complete records 78 (41%) were found to have lymphedema. These were all patients undergoing treatment for head and neck cancer during June 2008-June 2015. The prehabilitation program was initiated with the hiring of a nurse navigator for head and neck cancer, starting in January 2012. It is interesting to note that the incidence of lymphedema was 27% before the program was started, but after nurse navigator joined the team, the incidence increased significantly to 48% (P = .0002), in line with published expectations. This increase in recorded incidence may be attributable to the greater awareness of lymphedema intentionally fostered by the prehabilitation program.
Smoking history. Patients’ lifetime smoking history was retrieved from their medical records, based on their verbal admission of tobacco use. Most of the patients (n = 110) self-reported a history of smoking. Of those with a history of smoking, 36 (33%) developed external lymphedema after treatment for head and neck cancer, and 74 (67%) did not. However, this difference was not statistically significant. Hence, although smoking is a risk factor for head and neck cancer, it was not associated with the development of external lymphedema in our cohort of patients.
Type of tumor
Most of the patients (n = 156, 82%) had squamous cell carcinomas (SCC). Of those, 45% developed external lymphedema and 55% did not. Therefore, having SCC did not predispose to lymphedema. The other cancers were mixed type, mainly adenocaricoma, but their numbers were too small to draw statistical conclusions.
Stage of the tumor
About two thirds of the patients (n = 121, 64%) had stage 3 or 4 cancer. However, treatment of more advanced cancers was not associated with lymphedema development.
Site of the tumor
The literature suggests that patients with a primary tumor in the throat are at increased risk for lymphedema.5 The American Cancer Society has defined cancers of the oropharynx (throat) as including the base of the tongue (back third of the tongue), the soft palate, the tonsils, and the side and back walls of the throat.18 In our head and neck cancer cohort, patients with primary tumors of the oropharnyx were, perhaps, more susceptible to lymphedema (P = .044, Table 3). By contrast, in our cohort of patients, those with nasopharyngeal, hypopharyngeal, and parotid gland tumors were significantly less likely to develop lymphedema (Ps = .017, .04, .012, respectively).
No surgery
Half of our patients (n = 95) were not treated with surgery. In the patients who did not have surgery, 25 (26%) developed lymphedema, whereas 70 (74%) did not. Hence, although the incidence of lymphedema was significantly lower in patients who did not have surgery (P = .015), lymphedema did develop in patients who did not have a surgical procedure.
Resection of primary tumor without neck dissection
Of the 64 patients who had surgery, but without neck dissection, 35 (55%) developed external lymphedema. Compared with the no-surgery patients, the doubling of the incidence (from 26% to 55%) was highly significant (P = .0004). These findings are compatible with the literature reports that surgery increases the incidence of lymphedema, which is not surprising because surgery and subsequent scarring is known to compromise the lymphatic system.
Resection of primary tumor with neck dissection
The incidence of external lymphedema was increased to 69% when patients were subjected to both surgery and neck dissection. Compared with the June 2008-June 2015 cohort, there was a significant increase in the incidence of lymphedema in the neck dissection group (P = .007). Neck dissection involves the removal of lymph nodes and disruption of the lymphatic vessels, so it is not surprising that there is a higher incidence of external lymphedema. In our practice, neck dissections increased in frequency every year from June 2008 until December 2011, when 8 patients underwent neck dissections, 6 (75%) of whom developed lymphedema. Since January 2012, when the prehabilitation program was implemented, the number of neck dissections have declined, with more patients receiving chemoradiation and surgery being reserved for surgery. Hamoir and colleagues have reported that neck dissection is no longer justified unless there is clinically residual disease in the neck.19
Radiation
Lymphedema occurred in patients regardless of the dose of radiation received. Although the incidence of lymphedema seemed to be higher in patients who received more than 60 cGy, that difference was not statistically significant (Table 3). We had expected a relationship between radiation damage and greater lymphedema, but that was not evident in our patients.
Chemotherapy
The majority of patients (n = 131, 69%) received chemotherapy. The exposure to chemotherapy was not correlated with the risk of external lymphedema in our cohort of patients, with 58 of the 131 treated patients (44%) developing lymphedema, compared with 73 (56%) of treated patients who did not (Table 3).
Complete decongestive therapy
All patients with documented lymphedema were evaluated for complete decongestive therapy (CDT). Contraindications to CDT included congestive heart failure, renal failure, acute infection, peripheral artery disease, upper-quadrant deep vein thrombosis, and carotid artery stenosis. Eligible patients were referred to a certified lymphedema therapist for CDT. As the program evolved, patients at risk for lymphedema were referred for CDT early on, usually at the time of diagnosis, to improve early identification and surveillance of lymphedema.
CDT included manual lymph drainage,
Patients’ responses to CDT were documented with digital photographs that were taken at each visit and, more recently, use of the NDI.
Communication and education
The head and neck cancer nurse navigator attends the cancer center’s multidisciplinary head and neck tumor board, which has representation from otolaryngology, diagnostic radiology, pathology, radiation oncology, medical oncology, reconstructive surgery, oncology rehabilitation (physical/occupational therapist), dietary services, speech pathology, social services and clinical research. This regular contact allows for earlier awareness about which patients are at greater risk for developing lymphedema, thus enabling early intervention (and patient education) in a timely manner.
Education of the patient, before cancer therapy, of the risks of lymphedema is very important. Before the implementation of the prehabilitation program, some patients did not fully comprehend what a painful and debilitating consequence of cancer treatment lymphedema could be.
Discussion
We introduced a prehabilitation program to detect and treat lymphedema in head and neck cancer patients in January 2012 part way through following an observation cohort from June2008 through June2015. Central to this, in our center, was the appointment of a nurse navigator whose primary focus was on head and neck cancer patients. We placed a high priority on the early detection and treatment of lymphedema because do so has been associated with better outcomes in other centers.
One immediate consequence of the inception of our program was the identification of more patients with external lymphedema. Our detected incidence rose significantly (P = .0002), from 27% in the period June 2008-December 20112010, before the program, to 48% during the January 2012-June 2015 period, after the inception of the program. This later incidence rate is in line with published incidence rates in most centers. However, it is still somewhat short of the 75% suggested in one center,9 which suggests we are either we are underdetecting lymphedema or there are differences in definition criteria or sensitivity levels for defining lymphedema.
There are currently no specific objective measures of lymphedema, so there is bound to be some variation in diagnosis rates. In our program, we rely heavily on the patient-reported outcome measures, the NDI instrument, and digital photography to detect and monitor lymphedema, starting with the pretreatment baseline values that are established for each patient.
The use of digital photography in our community hospital setting, which includes taking photographs before and after treatment and at each visit, motivates and encourages patients and provides a tool for clinical lymphedema therapists to visually document benefits of treatment. Patients’ motivation and compliance with their established home program for head and neck lymphedema self-management are essential. The elements of the home program may include self-manual lymph drainage, home-modified compression bandaging and garment wear, therapeutic exercises, and skin care. Patients with lymphedema who adhered closely with their therapy program were more than 8 times more likely to improve compared with noncompliant patients.17
Some groups of patients have a greater risk of developing lymphedema than others,5 so the development of an algorithm to predict lymphedema seemed possible. However, in our cohort of patients, only neck dissection, with its disruption of the lymphatic system of the neck, was strongly associated with external lymphedema (Table 3). It is important to note that some patients who did not undergo surgery developed lymphedema. In our patients, high doses of radiation alone did not seem to predispose to lymphedema. That suggests that no group of head and neck cancer patients should be ignored, which is why we did routine screening of all patients before, during, and after treatment.
Our protocol falls short in the detection of internal lymphedema. For example, information on swallowing gathered by our speech pathologists (in a different department) has not, so far, been included in our assessment. This is one opportunity to improve on our approach, especially because speech difficulties may be associated with internal lymphedema. In addition, we are not equipped for the requisite internal examinations. Unfortunately, there are no practical and successful treatments for patients suffering from internal swelling. This represents a challenge for the medical community to better meet this need. Therefore, although we are missing some assessments of internal lymphedema, this is of little therapeutic consequence at this time.
The increase in the detected incidence of external lymphedema points to a practice gap that has been resolved by the appointment of a dedicated nurse navigator who attends oncology reviews to share knowledge and information. Another educational effort has been made with the patients themselves to increase compliance and improve continuous care at home.
There is always room for improvement, however, either by feedback acquired from other institutions and hospitals or through the future introduction of more objective assessment techniques.
Conclusions
The introduction of the prehabilitation program at our center has coincided with a significantly improved detection rate for external lymphedema in head and neck cancer patients. It may be because the program emphasizes education about lymphedema that awareness of the condition has increased throughout the center. It is now widely recognized that all patients are at risk of lymphedema regardless of whether they fall into an acknowledged high-risk group. Our experience shows that there is no significant difference between treatment modalities apart from neck dissection. In our population, the use of this procedure is decreasing. External lymphedema can develop even in patients who do not have surgery. Therefore, there is no sound way to predict which patients are most likely to suffer from the accumulation of fluid in their head and neck after treatment for head and neck cancer. Thus, an assessment as described here, during and after treatment for all patients, is warranted. Patients are now being seen earlier as a part of the prehabilitation program, which facilitates access to complete decongestive treatment at an earlier stage, improves patient outcomes, and increases patient satisfaction with their treatment. Our prehabilitation program could serve as a model for other community hospital centers in achieving outcomes that are as good as those in academic centers.
Acknowledgments
The authors thank Irene Kadota and Heather Peters, from the Department of Radiation Oncology, and Julianne Courtenay, from the Department of Physical Therapy at the Disney Family Cancer Center, Burbank, California, for providing the original clinical data for analysis.
Lymphedema is the swelling of tissue caused by the accumulation of interstitial fluid in any area of the body where lymphatic flow has been compromised.1 Secondary lymphedema is an acquired abnormality in lymph drainage1,2 and is the type commonly seen in cancer patients. Secondary lymphedema can be described as external or internal. Internal lymphedema, swelling of deep structures and tissues, is very difficult to quantify.
Lymphedema in patients with head and neck cancers
Lymphedema is a complicating morbidity frequently seen in head and neck cancer patients who have undergone treatment with surgery, radiation, and chemotherapy. However, although it is one of the most prevalent side effects of treatment, it is both under-recognized and under-treated.3
In head and neck cancer patients, internal swelling may develop in the soft tissues of the upper aero-digestive tract,4 affecting articulation and swallowing. Currently, there does not seem to be an effective practical and reliable tool with which to measure internal lymphedema. In addition, it is generally accepted that there is no effective way to treat internal lymphedema. By contrast, external lymphedema is more readily observed, but both subjective and objective assessments are difficult. External swelling may occur in the face, jaw, and neck. However, the subjective scales currently available are insufficient to capture very important characteristics of external lymphedema.5 The Edge Task Force on Head and Neck Cancer in 2015 was not able to recommend any outcome measures for objectively quantifying external edema.6 Furthermore, objective measurements of head and neck lymphedema can be expensive and time consuming.
Extent and risk
A combination of both internal and external swelling is seen in more than 50% of patients.7 Risk factors include “throat” tumors, multicancer treatment approaches, higher total radiation dose, a greater number of radiation procedures, and radiation at the surgical site.5 More than 500,000 survivors of head and neck cancer in the United States are at risk of lymphedema.5 Although recent advances in treatment have reduced the incidence of other morbidities, 50% of patients who are treated for head and neck cancer may still develop lymphedema.1,8 The reported incidence in some centers may be much higher, with up to 75% of patients developing lymphedema following treatment.9
Measurement modalities for clinical evaluation
There is little current research into lymphedema of the head and neck, despite the high prevalence of the condition.8 According to Deng and colleagues, measurement of head and neck lymphedema is a challenge, which has an impact on clinical assessment, diagnosis, and treatment of this under-recognized, under-reported and under-addressed problem in head and neck cancer patients.10 In a review of the literature, Deng and colleagues identified three measurement modalities available for clinical evaluation: patient-reported outcomes, clinician-reported outcomes, and technology.10 One major factor, though, in detecting lymphedema, is physician awareness: physicians, health care professionals, and even some lymphedema therapists are not well educated about this problem.8
Treatment
The effectiveness of traditional lymphedema treatment is not well defined.8 Currently, complete decongestive therapy (CDT), is considered the standard of care for lymphedema. The National Lymphedema Network has stated that modifications of CDT, especially manual lymphatic drainage and modified compressive garments for external lymphedema, have been shown to be beneficial for the treatment of lymphedema in head and neck cancer patients.11 Most findings in lymphedema research, mainly in breast cancer patients, have shown that early intervention is the best management and yields the best outcomes. As with other chronic conditions, early identification and timely, appropriate treatment of lymphedema is critical to improve clinical outcomes, to decrease symptom burden and functional impairment, and to improve overall quality of life in head and neck cancer patients.10
Improving recognition and treatment
Head and neck oncologic treatment is increasingly offered outside the network of specialist academic hospitals, at hospitals serving more localized communities where the neediest, sickest patient groups may be receiving less than optimal care.3 This challenges community hospitals to provide optimal treatment, similar to that being offered at nationally recognized institutions. In January 2012, we implemented a prehabilitation program in our community hospital cancer center to provide early intervention for our patients based on the understanding that proper and prompt treatment for patients with early signs of lymphedema should be a priority.12 In this article, we outline how we implemented the program and the describe improvements we observed before and after the implementation of the program.
The prehabilitation program
The role of the nurse navigator
Before the introduction of the prehabilitation program, our pattern of practice was to refer patients to oncology rehabilitation for lymphedema management after they had completed their medical treatment with surgery, radiation, and chemotherapy. In 2012, that was changed to a prehabilitation model of care that was overseen by a head and neck nurse navigator. This focus on prehabilitation begins with patients being referred to oncology rehabilitation at the time of cancer diagnosis for baseline assessment of head and neck swelling. In addition, there is assessment of the many possible other side effects associated with head and neck cancer and its treatment, namely loss of range of motion of the neck, jaw (trismus), and/or shoulders, postural deficits, functional loss, pain, balance dysfunction with fall risk, weakness, and fatigue. Therapeutic interventions are initiated as needed and appropriate. This process also raises awareness of a condition that has been described as under-recognized and under-treated.3
The nurse navigator sits in on each radiation oncology consultation and aids in “navigating” patients through their treatment. The nurse ensures that each patient is referred to different ancillary services from the outset, such as seeing a dietician, social worker, physical/occupational therapist and certified lymphedema therapist, speech pathologist, and financial assistance advisor, if necessary (Table 1).
Assessment of lymphedema
Measurement of head and neck lymphedema is a challenge.10 In our program, the physical therapy assessment also includes the evaluation of several other morbidities associated with head and neck cancer and its treatment, such as range of motion, weakness, fatigue, radiation fibrosis, balance dysfunction, and risk of falling (Table 2).
Patient-reported outcomes are essential to fully capture observable and unobservable symptoms (eg, sensations) as well as the functional impacts of lymphedema.10 In addition to lymphedema, there are many other morbidities that may be assessed on the basis of patient-reported outcome tools, such as upper extremity function with QuickDASH.13 At our clinic for head and neck cancer patients we use the Neck Disability Index (NDI)14 and Care Connections (CC)15 survey for the patient-reported outcomes. The Quick DASH, NDI, and CC tools all assess standard functional outcomes that are not specific to lymphedema, but are useful in documenting changes related to lymphedema. We initially used the CC survey and later transitioned to using the NDI. Neck pain is common with lymphedema in the head and neck region, and the NDI is a valid, reliable, responsive and internally consistent clinical tool to measure self-reported disability in patients with neck pain.16 These questionnaires were completed by the patients at their initial assessment, at reassessment, and at time of discharge.
Although objective criteria for external lymphedema have not been established, simple measurements such as using a tape measure to record neck circumference, allow a useful longitudinal assessment. Digital photography may be effective in the documentation and subjective evaluation of changes of external lymphedema.10,17 However, there are some limitations with photography because although external photographs (including digital photography and three-dimensional imaging) can capture some features, such as changes in contours, symmetry, and changes in skin quality and color, they do not detect changes in skin and soft tissue texture and compliance (Table 3).10
Impact on clinical outcomes
We retrospectively reviewed the medical records of 230 head and neck cancer patients who had been treated at our center between June 2008 and June 2015. Complete clinical data were available for 190 patients. The following information was extracted from each patient’s chart: whether they developed lymphedema, tumor stage, had surgery, radiation dose, type of chemotherapy given, their smoking history, if they had had a neck dissection and the primary site of the tumor (Table 3).
Incidence in different time periods. Of the 190 patients with complete records 78 (41%) were found to have lymphedema. These were all patients undergoing treatment for head and neck cancer during June 2008-June 2015. The prehabilitation program was initiated with the hiring of a nurse navigator for head and neck cancer, starting in January 2012. It is interesting to note that the incidence of lymphedema was 27% before the program was started, but after nurse navigator joined the team, the incidence increased significantly to 48% (P = .0002), in line with published expectations. This increase in recorded incidence may be attributable to the greater awareness of lymphedema intentionally fostered by the prehabilitation program.
Smoking history. Patients’ lifetime smoking history was retrieved from their medical records, based on their verbal admission of tobacco use. Most of the patients (n = 110) self-reported a history of smoking. Of those with a history of smoking, 36 (33%) developed external lymphedema after treatment for head and neck cancer, and 74 (67%) did not. However, this difference was not statistically significant. Hence, although smoking is a risk factor for head and neck cancer, it was not associated with the development of external lymphedema in our cohort of patients.
Type of tumor
Most of the patients (n = 156, 82%) had squamous cell carcinomas (SCC). Of those, 45% developed external lymphedema and 55% did not. Therefore, having SCC did not predispose to lymphedema. The other cancers were mixed type, mainly adenocaricoma, but their numbers were too small to draw statistical conclusions.
Stage of the tumor
About two thirds of the patients (n = 121, 64%) had stage 3 or 4 cancer. However, treatment of more advanced cancers was not associated with lymphedema development.
Site of the tumor
The literature suggests that patients with a primary tumor in the throat are at increased risk for lymphedema.5 The American Cancer Society has defined cancers of the oropharynx (throat) as including the base of the tongue (back third of the tongue), the soft palate, the tonsils, and the side and back walls of the throat.18 In our head and neck cancer cohort, patients with primary tumors of the oropharnyx were, perhaps, more susceptible to lymphedema (P = .044, Table 3). By contrast, in our cohort of patients, those with nasopharyngeal, hypopharyngeal, and parotid gland tumors were significantly less likely to develop lymphedema (Ps = .017, .04, .012, respectively).
No surgery
Half of our patients (n = 95) were not treated with surgery. In the patients who did not have surgery, 25 (26%) developed lymphedema, whereas 70 (74%) did not. Hence, although the incidence of lymphedema was significantly lower in patients who did not have surgery (P = .015), lymphedema did develop in patients who did not have a surgical procedure.
Resection of primary tumor without neck dissection
Of the 64 patients who had surgery, but without neck dissection, 35 (55%) developed external lymphedema. Compared with the no-surgery patients, the doubling of the incidence (from 26% to 55%) was highly significant (P = .0004). These findings are compatible with the literature reports that surgery increases the incidence of lymphedema, which is not surprising because surgery and subsequent scarring is known to compromise the lymphatic system.
Resection of primary tumor with neck dissection
The incidence of external lymphedema was increased to 69% when patients were subjected to both surgery and neck dissection. Compared with the June 2008-June 2015 cohort, there was a significant increase in the incidence of lymphedema in the neck dissection group (P = .007). Neck dissection involves the removal of lymph nodes and disruption of the lymphatic vessels, so it is not surprising that there is a higher incidence of external lymphedema. In our practice, neck dissections increased in frequency every year from June 2008 until December 2011, when 8 patients underwent neck dissections, 6 (75%) of whom developed lymphedema. Since January 2012, when the prehabilitation program was implemented, the number of neck dissections have declined, with more patients receiving chemoradiation and surgery being reserved for surgery. Hamoir and colleagues have reported that neck dissection is no longer justified unless there is clinically residual disease in the neck.19
Radiation
Lymphedema occurred in patients regardless of the dose of radiation received. Although the incidence of lymphedema seemed to be higher in patients who received more than 60 cGy, that difference was not statistically significant (Table 3). We had expected a relationship between radiation damage and greater lymphedema, but that was not evident in our patients.
Chemotherapy
The majority of patients (n = 131, 69%) received chemotherapy. The exposure to chemotherapy was not correlated with the risk of external lymphedema in our cohort of patients, with 58 of the 131 treated patients (44%) developing lymphedema, compared with 73 (56%) of treated patients who did not (Table 3).
Complete decongestive therapy
All patients with documented lymphedema were evaluated for complete decongestive therapy (CDT). Contraindications to CDT included congestive heart failure, renal failure, acute infection, peripheral artery disease, upper-quadrant deep vein thrombosis, and carotid artery stenosis. Eligible patients were referred to a certified lymphedema therapist for CDT. As the program evolved, patients at risk for lymphedema were referred for CDT early on, usually at the time of diagnosis, to improve early identification and surveillance of lymphedema.
CDT included manual lymph drainage,
Patients’ responses to CDT were documented with digital photographs that were taken at each visit and, more recently, use of the NDI.
Communication and education
The head and neck cancer nurse navigator attends the cancer center’s multidisciplinary head and neck tumor board, which has representation from otolaryngology, diagnostic radiology, pathology, radiation oncology, medical oncology, reconstructive surgery, oncology rehabilitation (physical/occupational therapist), dietary services, speech pathology, social services and clinical research. This regular contact allows for earlier awareness about which patients are at greater risk for developing lymphedema, thus enabling early intervention (and patient education) in a timely manner.
Education of the patient, before cancer therapy, of the risks of lymphedema is very important. Before the implementation of the prehabilitation program, some patients did not fully comprehend what a painful and debilitating consequence of cancer treatment lymphedema could be.
Discussion
We introduced a prehabilitation program to detect and treat lymphedema in head and neck cancer patients in January 2012 part way through following an observation cohort from June2008 through June2015. Central to this, in our center, was the appointment of a nurse navigator whose primary focus was on head and neck cancer patients. We placed a high priority on the early detection and treatment of lymphedema because do so has been associated with better outcomes in other centers.
One immediate consequence of the inception of our program was the identification of more patients with external lymphedema. Our detected incidence rose significantly (P = .0002), from 27% in the period June 2008-December 20112010, before the program, to 48% during the January 2012-June 2015 period, after the inception of the program. This later incidence rate is in line with published incidence rates in most centers. However, it is still somewhat short of the 75% suggested in one center,9 which suggests we are either we are underdetecting lymphedema or there are differences in definition criteria or sensitivity levels for defining lymphedema.
There are currently no specific objective measures of lymphedema, so there is bound to be some variation in diagnosis rates. In our program, we rely heavily on the patient-reported outcome measures, the NDI instrument, and digital photography to detect and monitor lymphedema, starting with the pretreatment baseline values that are established for each patient.
The use of digital photography in our community hospital setting, which includes taking photographs before and after treatment and at each visit, motivates and encourages patients and provides a tool for clinical lymphedema therapists to visually document benefits of treatment. Patients’ motivation and compliance with their established home program for head and neck lymphedema self-management are essential. The elements of the home program may include self-manual lymph drainage, home-modified compression bandaging and garment wear, therapeutic exercises, and skin care. Patients with lymphedema who adhered closely with their therapy program were more than 8 times more likely to improve compared with noncompliant patients.17
Some groups of patients have a greater risk of developing lymphedema than others,5 so the development of an algorithm to predict lymphedema seemed possible. However, in our cohort of patients, only neck dissection, with its disruption of the lymphatic system of the neck, was strongly associated with external lymphedema (Table 3). It is important to note that some patients who did not undergo surgery developed lymphedema. In our patients, high doses of radiation alone did not seem to predispose to lymphedema. That suggests that no group of head and neck cancer patients should be ignored, which is why we did routine screening of all patients before, during, and after treatment.
Our protocol falls short in the detection of internal lymphedema. For example, information on swallowing gathered by our speech pathologists (in a different department) has not, so far, been included in our assessment. This is one opportunity to improve on our approach, especially because speech difficulties may be associated with internal lymphedema. In addition, we are not equipped for the requisite internal examinations. Unfortunately, there are no practical and successful treatments for patients suffering from internal swelling. This represents a challenge for the medical community to better meet this need. Therefore, although we are missing some assessments of internal lymphedema, this is of little therapeutic consequence at this time.
The increase in the detected incidence of external lymphedema points to a practice gap that has been resolved by the appointment of a dedicated nurse navigator who attends oncology reviews to share knowledge and information. Another educational effort has been made with the patients themselves to increase compliance and improve continuous care at home.
There is always room for improvement, however, either by feedback acquired from other institutions and hospitals or through the future introduction of more objective assessment techniques.
Conclusions
The introduction of the prehabilitation program at our center has coincided with a significantly improved detection rate for external lymphedema in head and neck cancer patients. It may be because the program emphasizes education about lymphedema that awareness of the condition has increased throughout the center. It is now widely recognized that all patients are at risk of lymphedema regardless of whether they fall into an acknowledged high-risk group. Our experience shows that there is no significant difference between treatment modalities apart from neck dissection. In our population, the use of this procedure is decreasing. External lymphedema can develop even in patients who do not have surgery. Therefore, there is no sound way to predict which patients are most likely to suffer from the accumulation of fluid in their head and neck after treatment for head and neck cancer. Thus, an assessment as described here, during and after treatment for all patients, is warranted. Patients are now being seen earlier as a part of the prehabilitation program, which facilitates access to complete decongestive treatment at an earlier stage, improves patient outcomes, and increases patient satisfaction with their treatment. Our prehabilitation program could serve as a model for other community hospital centers in achieving outcomes that are as good as those in academic centers.
Acknowledgments
The authors thank Irene Kadota and Heather Peters, from the Department of Radiation Oncology, and Julianne Courtenay, from the Department of Physical Therapy at the Disney Family Cancer Center, Burbank, California, for providing the original clinical data for analysis.
1. The National Lymphedema Medical Advisory Committee. The diagnosis and treatment of lymphedema. National Lymphedema Network. http://www.lymphnet.org/pdfDocs/nlntreatment.pdf. Updated February 2011. Accessed April 26, 2017.
2. McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care (Engl). 2014;23(3):317-327.
3. Bhattacharyya N, Abemayor E. Patterns of hospital utilization for head and neck cancer care: changing demographics. JAMA Otolaryngol Head Neck Surg. 2015;141(4):307-312.
4. Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43(2):244-252.
5. Deng J, Ridner SH, Dietrich MS, et al. Factors associated with external and internal lymphedema in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;84(3):e319-328.
6. Flores AM, Spinelli BA, Eden MM, Galantino ML. EDGE task force on head and neck cancer outcomes: a systematic review of outcomes measures for quantifying external lymphedema. Rehabil Oncol. 2015;33(2):15-23.
7. Ridner SH, Doersam J, Galford E. An update on lymphedema of the head and neck. http://www.lymphnet.org/pdfDocs/Vol_28-N2_Update_HN.pdf. Published April-June 2015. Accessed April 26, 2017.
8. Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152(2);284-291.
9. Naqvi SHS, Karni RJ, Tan IC, et al. Int J Rad Oncol Biol Phys. 2016;4:927-928.
10. Deng J, Ridner SH, Aulino JM, Murphy BA. Assessment and measurement of head and neck lymphedema: state-of-the-science and future directions. Oral Oncol. 2015; 51(5):431-437.
11. Purcell A. Head and neck lymphedema management practices. J Lymphedema. 2013;8(2):8-15.
12. Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis treatment and impact: a review. J Clinl Oncol. 2012;30(30):3726-3733.
13. Quick DASH questionnaire. http://www.dash.iwh.on.ca/about-quickdash. [Last update not stated.] Accessed May 18, 2017.
14. Neck Disability Index questionnaire. www.aaos.org/uploadedFiles/NDI.pdf Accessed May 18, 2017.
15. Care Connections questionnaire. http://www.careconnections.com/. Accessed May 18, 2017.
16. Galantino ML, Eden MM, Spinelli BA, Flores AM. EDGE task force on head and neck cancer outcomes a systematic review of outcome measures for temporomandibular-related dysfunction. Rehabil Oncol. 2015;33(1):6-14.
17. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38(1):e1-e10.
18. What are oral cavity and oropharyngeal cancers? American Cancer Society. http://www.cancer.org/cancer/oralcavityandoropharyngealcancer/detailedguide/oral-cavity-and-oropharyngeal-cancer-what-is-oral-cavity-cancer. Last revised August 8, 2016. Accessed April 26, 2017.
19. Hamoir M, Schmitz S, Gregoire V. The role of neck dissection in squamous cell carcinoma of the head and neck. Curr Treat Options Oncol. 2014;15:611-624.
1. The National Lymphedema Medical Advisory Committee. The diagnosis and treatment of lymphedema. National Lymphedema Network. http://www.lymphnet.org/pdfDocs/nlntreatment.pdf. Updated February 2011. Accessed April 26, 2017.
2. McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care (Engl). 2014;23(3):317-327.
3. Bhattacharyya N, Abemayor E. Patterns of hospital utilization for head and neck cancer care: changing demographics. JAMA Otolaryngol Head Neck Surg. 2015;141(4):307-312.
4. Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43(2):244-252.
5. Deng J, Ridner SH, Dietrich MS, et al. Factors associated with external and internal lymphedema in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;84(3):e319-328.
6. Flores AM, Spinelli BA, Eden MM, Galantino ML. EDGE task force on head and neck cancer outcomes: a systematic review of outcomes measures for quantifying external lymphedema. Rehabil Oncol. 2015;33(2):15-23.
7. Ridner SH, Doersam J, Galford E. An update on lymphedema of the head and neck. http://www.lymphnet.org/pdfDocs/Vol_28-N2_Update_HN.pdf. Published April-June 2015. Accessed April 26, 2017.
8. Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152(2);284-291.
9. Naqvi SHS, Karni RJ, Tan IC, et al. Int J Rad Oncol Biol Phys. 2016;4:927-928.
10. Deng J, Ridner SH, Aulino JM, Murphy BA. Assessment and measurement of head and neck lymphedema: state-of-the-science and future directions. Oral Oncol. 2015; 51(5):431-437.
11. Purcell A. Head and neck lymphedema management practices. J Lymphedema. 2013;8(2):8-15.
12. Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis treatment and impact: a review. J Clinl Oncol. 2012;30(30):3726-3733.
13. Quick DASH questionnaire. http://www.dash.iwh.on.ca/about-quickdash. [Last update not stated.] Accessed May 18, 2017.
14. Neck Disability Index questionnaire. www.aaos.org/uploadedFiles/NDI.pdf Accessed May 18, 2017.
15. Care Connections questionnaire. http://www.careconnections.com/. Accessed May 18, 2017.
16. Galantino ML, Eden MM, Spinelli BA, Flores AM. EDGE task force on head and neck cancer outcomes a systematic review of outcome measures for temporomandibular-related dysfunction. Rehabil Oncol. 2015;33(1):6-14.
17. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38(1):e1-e10.
18. What are oral cavity and oropharyngeal cancers? American Cancer Society. http://www.cancer.org/cancer/oralcavityandoropharyngealcancer/detailedguide/oral-cavity-and-oropharyngeal-cancer-what-is-oral-cavity-cancer. Last revised August 8, 2016. Accessed April 26, 2017.
19. Hamoir M, Schmitz S, Gregoire V. The role of neck dissection in squamous cell carcinoma of the head and neck. Curr Treat Options Oncol. 2014;15:611-624.
Oncology and the heart
A typical example is a patient who had Hodgkin disease in his teens and received mediastinal mantle radiation. Fifteen to 25 years later, the patient has a pacemaker for heart block, coronary artery disease that requires a stent, and most recently has two valves replaced—so aortic and mitral valve replacement because of late radiation effects. This scenario is typical for the “old” days. The 20-year cumulative incidence of radiation-induced cardiac toxicity is 15%-20% (Table, Figure).1 Sitting with a patient about to begin chest radiation, the absolute risks are unknown but presumed to be less as treatment is delivered according to the modern techniques that you described in the question.
DH They’re so much better now, so this is less common.
JC With the shielding and breath-holding techniques and position changes, doing upright radiation rather than supine, and because the technology has improved both in the delivery of radiation and the technology in understanding where all the radiation is going, in today’s world, we can calculate pretty precisely how much radiation the heart actually receives. Ultimately, with the protective mechanisms that are in place going forward, the risks that I described for that survivor are probably exponentially less than what’s reported in the literature and what we see clinically. Radiation has become much, much safer. There is still probably some small risk of development of late changes, but I don’t think we know what that risk is today because the shielding and things we do to protect the heart have not yet been studied in the long term.
DH Of course, the patient is breathing and there’ll be some movement of the target. Some of the radiation techniques can follow the target despite the breathing?
JC Yes, definitely true. Radiation delivery is much more precise today. Not only has the delivery changed, but so has what we know about the location of potential arterial disease. For example, if you read any textbook, it says that for the coronaries, that it’s ostial and proximal disease of the left main, or the left anterior descending, or the right coronary artery. Today, somebody who gets chest/mediastinal radiation, for either breast cancer, lymphoma, or for a mediastinal tumor, the location of potential disease is more likely to mimic the location of classic coronary disease in the mid-portion of the left anterior descending artery rather than at the ostium. It’s going to be a different disease going forward.2,3
DH Let’s switch from radiation to chemotherapy. Of course, all of us worry about and are very familiar with the toxicity potential of doxorubicin and trastuzumab. I remember an American Society of Clinical Oncology meeting a few years ago, one of the speakers was a cardiologist and was advising us that perhaps the ejection fraction, albeit readily available and reproducible, was probably too simple and we should watch more closely with other techniques. My final question and then I’ll let you comment – I thought I recalled 5-fluorouracil (5-FU) infusions, which we do in some of our colorectal cancers, for example, can cause a vasospasm, Prinzmetal-type angina from time to time, and is that true in capecitabine? What are your thoughts on how to follow the doxorubicin, trastuzumab analogs, and anything about 5-FU and its analogs?
JC Okay, this is a giant question. I’ll take them in order. First, doxorubicin. Cumulative dose-related cardiotoxicity was first described by Von Hoff in 1979.4 That is, the more you get, the higher likelihood of developing cardiotoxicity. Up to a total of 400 mg/m2, the risk is <1%, with a sharp rise as the dose increases beyond this level.4 That being said, there is a clear large and individual variation: I’ve seen sarcoma patients who’ve gotten close to 1,000 mg/m2 without cardiac dysfunction, and some people with minimal exposure have full-blown cardiomyopathy. One of the protective strategies that we developed over the years is to give less of the drug, and with that get the same cancer treatment efficacy. There is definitely a risk for anthracyclines. Full-blown heart failure is probably in the 4%-8% range – and that’s cumulative lifetime – it’s not as high as we once thought it was. That doesn’t mean that it isn’t there, but, relatively speaking, from the standpoint of benefit of anthracyclines, the benefit certainly clearly outweighs the cardiac risk.
With administration of the anthracyclines, we try to do whatever protective things we can do. There are some people who believe that continuous infusion is safer for the heart than bolus injection. It’s pretty controversial. Dexrazoxane, which is a chelating agent, has been shown to reduce cardiotoxicity, and using a lipophilic anthracycline preparation may also have less cardiac toxicity.
DH I have a population in which a lot of liposomal doxorubicin is used and I’ve given a lot and rarely if ever get cardiac toxicity. You see that as well?
JC Yes. There’s a significant financial difference between doxorubicin and liposomal doxorubicin; the latter is more expensive. From the standpoint of safety, and from the standpoint of if I ever needed doxorubicin, I would probably jump on that and ask for the liposomal preparation and/or dexrazoxane.
DH For trastuzumab, we are getting echo- cardiograms every 9 weeks. That seems awfully simple, but there’s a whole algorithm we follow for particular change in ejection fraction and watch the drug or stop the drug. Are we doing that correctly?
JC The first statement I would make about that is that there are too many women who need trastuzumab whose therapy has been prematurely stopped because of just looking at the ejection fractions. So, there has to be more to decision-making other than just the number of the ejection fraction. We’re pretty aggressive and tend to try to get women to get the full dose and whatever dose-effective dose they need, especially with curative intent in the adjuvant setting that we make decisions based not only on the ejection fraction.
We also have, I would say, a handful of our medical breast oncologists who do not follow the package insert. We don’t get ejection fractions every 3 cycles. We have substituted a little bit by following biomarkers so that we use N-terminal pro b-type natriuretic peptide (NT-proBNP) to monitor people, either with each cycle or every third cycle. The benefit of BNP is its negative predictive value. If it’s normal, it’s hard to have any clinically significant myocardial dysfunction.
What we’re going to see over – I would hope – the next year or two is that the recommendations about getting echocardiograms frequently will go away.
DH That would be welcome because in our electronic medical records, it’s 9 weeks, stop, do this, etc. How about a comment on infusional 5-FU and possibly its cousins, such as capecitabine, and any coronary issues?
JC Let me come back, just one more thing about trastuzumab. For metastatic disease, we do whatever is necessary to continue effective cancer therapy and in the absence of any cardiac symptoms or abnormal physical findings, we continue cancer treatment without any serial echocardiographic monitoring.
DH You think the NT-proBNP might be useful? I know that’s excreted by the kidneys, so that might rise in renal failure, but we can adjust for that.
JC The negative predictive value of having a normal BNP is helpful. I think what I wanted to say was that screening echocardiograms and looking at ejection fraction in low-risk populations probably is clearly not cost-effective. It probably never alters decision making. If you have a 30-year-old person with no cardiac risk factors and no past cardiac history who develops B-cell lymphoma and is going to get anthracycline-based chemotherapy, the likelihood of finding a reason not to give that therapy based on an echocardiogram is quite small. I would even go further and say close to zero. We’ve begun to look at this. There is literature that supports the concept. Also, that in low-risk people – if you can define the low-risk population in an accurate way – for lymphoma patients or women with breast cancer getting either anthracyclines, trastuzumab, or the other human epidermal growth factor receptor-2 (HER2)-directed therapies, there’s probably little yield to even getting a baseline study.
DH Very interesting. I would agree with you.
JC We’re going to talk about 5-FU, of course. The 5-FU thing has become a passion of mine. Over the last two to two-and-a- half years we have gotten very aggressive with treating coronary spasm that’s induced by the fluoropyrimidines. That’s 5-FU and capecitabine, the oral version.
There is an incidence that the literature says is less than 1%. It probably is somewhere between 3% and 5%. It’s a little bit more common than has been reported. The reason is the way that it presents has classically been described in the literature as different than what occurs in real life. It is a phenomenon. It’s the most common cardiac side effect. Sometimes it is large epicardial coronary artery spasm. Sometimes it’s small vessel spasm. You can have chest pain with no electrocardiographic changes or ECG changes without chest pain (so-called silent ischemia). The description doesn’t always sound like classic angina but symptoms are temporally related to getting the drug.
So, we’ve developed a protocol to treat documented spasm as an outpatient to be able to continue those drugs to their logical conclusion from an oncologic standpoint. In fact, we just submitted a manuscript to the
DH Finally, it occurred to me that we cause problems with radiation. We cause problems with chemotherapy and other infusions. Are there particular cancers that you think of or you’re called in to see that you worry about cardiac involvement by their location? What comes to mind are cases I’ve had in which there is pericardial involvement and tamponade or restrictive pericarditis.
JC We see metastatic disease to the pericardium with breast cancer, lung cancer, and lymphoma. Renal cell has an interesting predilection to go to the pericardium. We’ve seen in the last probably 6 months 2 cases of bladder cancer with pericardial metastases. When we reviewed the literature, we were only able to find 9 or 10 case reports. It’s rare, but it occurs.
Fluid in the pericardium with and without tamponade is increasingly common, and because we do a better job in treating complicated cancer, people successively can receive cycles of sequential chemotherapeutic regimens – they are living longer, their cancer can get more complicated and/or resistant and with it, there’s more time for metastatic disease to occur. Tamponade is a common phenomenon. We always say that at 4 o’clock on Friday we always see somebody who has tamponade. We see a lot of pericardial disease.
Then, another area of a concern is the tyrosine kinase inhibitors that can cause hypertension, which is very common. We’ve become pretty aggressive. The oncologists recognize the importance of being able to follow and treat blood pressures to allow patients to get these treatments. I guess we couldn’t end without talking about checkpoint inhibitors and the recent lay press flurry about reporting myocarditis.
DH I haven’t personally experienced that. How common is that, and how do we watch for it?
JC Personally, I’ve seen probably four or five people who were referred because of heart failure on checkpoint inhibitors. For each of them, there was historically something as a preexisting problem before the checkpoint inhibitor. It was coincident that with either fluid changes or blood pressure changes associated with the treatment that they had a flare-up of heart failure.
We have not seen, fortunately, the dynamics that were reported in the
DH Well, certainly with the proliferation of the checkpoint inhibitors, and so many different tumors, and so much widespread use, it looks like there is a small safety signal there but still yet to be defined. How common is that, and what should we watch for?
JC Actually, it’s serendipitous that yesterday I was walking to the parking lot with one of the nurse practitioners who takes care of the melanoma population. She said to me, “Now, do you think that we should be getting BNP levels on everybody who is getting a checkpoint inhibitor?”
I don’t think that we’re there. Just the awareness to ask the right questions when you see a patient and before starting ask, is this somebody who, in the absence of a checkpoint inhibitor, could be at risk for myocardial disease? Recognize that and use the cardiology and oncology community to work together and try to make sure that you do whatever cardioprotective things you can do and to monitor them a little bit more closely. I’m not sure that everybody who is going to start a checkpoint inhibitor needs a cardiac evaluation, doesn’t need an echocardiogram, and doesn’t need baseline biomarkers to decide if there’s a potential cardiotoxicity problem.
DH Well certainly, you’ve raised my awareness. It was not something that I had been thinking of with checkpoint inhibitors. Now, I certainly would if the patient has some comorbid illness that involves the heart, maybe think about it, wait to see how these reports develop, and what you and the registry do.
JC You’ve seen people who get this sort of immunologic reaction that they require steroids for fluid accumulation, rash, or other things that are in this constellation. I wouldn’t be surprised if that group might have some subclinical myocarditis that just gets better when they get treated for the other things.
We have actually been trying to get a quick look at the left ventricle when patients on checkpoint inhibitors present with systemic, noncardiac symptoms to see if there is a cardiac signal we are missing. We have a handheld portable echocardiogram device called a Vscan (General Electric Company, Fairfield, CT). It’s not much bigger than the larger cellphones that are available. We’ve been going to the bedside when people have the reaction and sticking the transducer on to get a feeling of what the ventricle looks like. There’s a lot that we don’t know. It’s a fertile ground for investigation.
DH Well, I couldn’t ask you to end on a higher note than covering the checkpoint inhibitors, which are so popular and so interesting and used everywhere. We’re still managing that whole concept. I want to thank you very much.
JC It was a great pleasure. Thank you.
1. Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412-418.
2. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
3. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854-862.
4. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710-717.
5. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755.
A typical example is a patient who had Hodgkin disease in his teens and received mediastinal mantle radiation. Fifteen to 25 years later, the patient has a pacemaker for heart block, coronary artery disease that requires a stent, and most recently has two valves replaced—so aortic and mitral valve replacement because of late radiation effects. This scenario is typical for the “old” days. The 20-year cumulative incidence of radiation-induced cardiac toxicity is 15%-20% (Table, Figure).1 Sitting with a patient about to begin chest radiation, the absolute risks are unknown but presumed to be less as treatment is delivered according to the modern techniques that you described in the question.
DH They’re so much better now, so this is less common.
JC With the shielding and breath-holding techniques and position changes, doing upright radiation rather than supine, and because the technology has improved both in the delivery of radiation and the technology in understanding where all the radiation is going, in today’s world, we can calculate pretty precisely how much radiation the heart actually receives. Ultimately, with the protective mechanisms that are in place going forward, the risks that I described for that survivor are probably exponentially less than what’s reported in the literature and what we see clinically. Radiation has become much, much safer. There is still probably some small risk of development of late changes, but I don’t think we know what that risk is today because the shielding and things we do to protect the heart have not yet been studied in the long term.
DH Of course, the patient is breathing and there’ll be some movement of the target. Some of the radiation techniques can follow the target despite the breathing?
JC Yes, definitely true. Radiation delivery is much more precise today. Not only has the delivery changed, but so has what we know about the location of potential arterial disease. For example, if you read any textbook, it says that for the coronaries, that it’s ostial and proximal disease of the left main, or the left anterior descending, or the right coronary artery. Today, somebody who gets chest/mediastinal radiation, for either breast cancer, lymphoma, or for a mediastinal tumor, the location of potential disease is more likely to mimic the location of classic coronary disease in the mid-portion of the left anterior descending artery rather than at the ostium. It’s going to be a different disease going forward.2,3
DH Let’s switch from radiation to chemotherapy. Of course, all of us worry about and are very familiar with the toxicity potential of doxorubicin and trastuzumab. I remember an American Society of Clinical Oncology meeting a few years ago, one of the speakers was a cardiologist and was advising us that perhaps the ejection fraction, albeit readily available and reproducible, was probably too simple and we should watch more closely with other techniques. My final question and then I’ll let you comment – I thought I recalled 5-fluorouracil (5-FU) infusions, which we do in some of our colorectal cancers, for example, can cause a vasospasm, Prinzmetal-type angina from time to time, and is that true in capecitabine? What are your thoughts on how to follow the doxorubicin, trastuzumab analogs, and anything about 5-FU and its analogs?
JC Okay, this is a giant question. I’ll take them in order. First, doxorubicin. Cumulative dose-related cardiotoxicity was first described by Von Hoff in 1979.4 That is, the more you get, the higher likelihood of developing cardiotoxicity. Up to a total of 400 mg/m2, the risk is <1%, with a sharp rise as the dose increases beyond this level.4 That being said, there is a clear large and individual variation: I’ve seen sarcoma patients who’ve gotten close to 1,000 mg/m2 without cardiac dysfunction, and some people with minimal exposure have full-blown cardiomyopathy. One of the protective strategies that we developed over the years is to give less of the drug, and with that get the same cancer treatment efficacy. There is definitely a risk for anthracyclines. Full-blown heart failure is probably in the 4%-8% range – and that’s cumulative lifetime – it’s not as high as we once thought it was. That doesn’t mean that it isn’t there, but, relatively speaking, from the standpoint of benefit of anthracyclines, the benefit certainly clearly outweighs the cardiac risk.
With administration of the anthracyclines, we try to do whatever protective things we can do. There are some people who believe that continuous infusion is safer for the heart than bolus injection. It’s pretty controversial. Dexrazoxane, which is a chelating agent, has been shown to reduce cardiotoxicity, and using a lipophilic anthracycline preparation may also have less cardiac toxicity.
DH I have a population in which a lot of liposomal doxorubicin is used and I’ve given a lot and rarely if ever get cardiac toxicity. You see that as well?
JC Yes. There’s a significant financial difference between doxorubicin and liposomal doxorubicin; the latter is more expensive. From the standpoint of safety, and from the standpoint of if I ever needed doxorubicin, I would probably jump on that and ask for the liposomal preparation and/or dexrazoxane.
DH For trastuzumab, we are getting echo- cardiograms every 9 weeks. That seems awfully simple, but there’s a whole algorithm we follow for particular change in ejection fraction and watch the drug or stop the drug. Are we doing that correctly?
JC The first statement I would make about that is that there are too many women who need trastuzumab whose therapy has been prematurely stopped because of just looking at the ejection fractions. So, there has to be more to decision-making other than just the number of the ejection fraction. We’re pretty aggressive and tend to try to get women to get the full dose and whatever dose-effective dose they need, especially with curative intent in the adjuvant setting that we make decisions based not only on the ejection fraction.
We also have, I would say, a handful of our medical breast oncologists who do not follow the package insert. We don’t get ejection fractions every 3 cycles. We have substituted a little bit by following biomarkers so that we use N-terminal pro b-type natriuretic peptide (NT-proBNP) to monitor people, either with each cycle or every third cycle. The benefit of BNP is its negative predictive value. If it’s normal, it’s hard to have any clinically significant myocardial dysfunction.
What we’re going to see over – I would hope – the next year or two is that the recommendations about getting echocardiograms frequently will go away.
DH That would be welcome because in our electronic medical records, it’s 9 weeks, stop, do this, etc. How about a comment on infusional 5-FU and possibly its cousins, such as capecitabine, and any coronary issues?
JC Let me come back, just one more thing about trastuzumab. For metastatic disease, we do whatever is necessary to continue effective cancer therapy and in the absence of any cardiac symptoms or abnormal physical findings, we continue cancer treatment without any serial echocardiographic monitoring.
DH You think the NT-proBNP might be useful? I know that’s excreted by the kidneys, so that might rise in renal failure, but we can adjust for that.
JC The negative predictive value of having a normal BNP is helpful. I think what I wanted to say was that screening echocardiograms and looking at ejection fraction in low-risk populations probably is clearly not cost-effective. It probably never alters decision making. If you have a 30-year-old person with no cardiac risk factors and no past cardiac history who develops B-cell lymphoma and is going to get anthracycline-based chemotherapy, the likelihood of finding a reason not to give that therapy based on an echocardiogram is quite small. I would even go further and say close to zero. We’ve begun to look at this. There is literature that supports the concept. Also, that in low-risk people – if you can define the low-risk population in an accurate way – for lymphoma patients or women with breast cancer getting either anthracyclines, trastuzumab, or the other human epidermal growth factor receptor-2 (HER2)-directed therapies, there’s probably little yield to even getting a baseline study.
DH Very interesting. I would agree with you.
JC We’re going to talk about 5-FU, of course. The 5-FU thing has become a passion of mine. Over the last two to two-and-a- half years we have gotten very aggressive with treating coronary spasm that’s induced by the fluoropyrimidines. That’s 5-FU and capecitabine, the oral version.
There is an incidence that the literature says is less than 1%. It probably is somewhere between 3% and 5%. It’s a little bit more common than has been reported. The reason is the way that it presents has classically been described in the literature as different than what occurs in real life. It is a phenomenon. It’s the most common cardiac side effect. Sometimes it is large epicardial coronary artery spasm. Sometimes it’s small vessel spasm. You can have chest pain with no electrocardiographic changes or ECG changes without chest pain (so-called silent ischemia). The description doesn’t always sound like classic angina but symptoms are temporally related to getting the drug.
So, we’ve developed a protocol to treat documented spasm as an outpatient to be able to continue those drugs to their logical conclusion from an oncologic standpoint. In fact, we just submitted a manuscript to the
DH Finally, it occurred to me that we cause problems with radiation. We cause problems with chemotherapy and other infusions. Are there particular cancers that you think of or you’re called in to see that you worry about cardiac involvement by their location? What comes to mind are cases I’ve had in which there is pericardial involvement and tamponade or restrictive pericarditis.
JC We see metastatic disease to the pericardium with breast cancer, lung cancer, and lymphoma. Renal cell has an interesting predilection to go to the pericardium. We’ve seen in the last probably 6 months 2 cases of bladder cancer with pericardial metastases. When we reviewed the literature, we were only able to find 9 or 10 case reports. It’s rare, but it occurs.
Fluid in the pericardium with and without tamponade is increasingly common, and because we do a better job in treating complicated cancer, people successively can receive cycles of sequential chemotherapeutic regimens – they are living longer, their cancer can get more complicated and/or resistant and with it, there’s more time for metastatic disease to occur. Tamponade is a common phenomenon. We always say that at 4 o’clock on Friday we always see somebody who has tamponade. We see a lot of pericardial disease.
Then, another area of a concern is the tyrosine kinase inhibitors that can cause hypertension, which is very common. We’ve become pretty aggressive. The oncologists recognize the importance of being able to follow and treat blood pressures to allow patients to get these treatments. I guess we couldn’t end without talking about checkpoint inhibitors and the recent lay press flurry about reporting myocarditis.
DH I haven’t personally experienced that. How common is that, and how do we watch for it?
JC Personally, I’ve seen probably four or five people who were referred because of heart failure on checkpoint inhibitors. For each of them, there was historically something as a preexisting problem before the checkpoint inhibitor. It was coincident that with either fluid changes or blood pressure changes associated with the treatment that they had a flare-up of heart failure.
We have not seen, fortunately, the dynamics that were reported in the
DH Well, certainly with the proliferation of the checkpoint inhibitors, and so many different tumors, and so much widespread use, it looks like there is a small safety signal there but still yet to be defined. How common is that, and what should we watch for?
JC Actually, it’s serendipitous that yesterday I was walking to the parking lot with one of the nurse practitioners who takes care of the melanoma population. She said to me, “Now, do you think that we should be getting BNP levels on everybody who is getting a checkpoint inhibitor?”
I don’t think that we’re there. Just the awareness to ask the right questions when you see a patient and before starting ask, is this somebody who, in the absence of a checkpoint inhibitor, could be at risk for myocardial disease? Recognize that and use the cardiology and oncology community to work together and try to make sure that you do whatever cardioprotective things you can do and to monitor them a little bit more closely. I’m not sure that everybody who is going to start a checkpoint inhibitor needs a cardiac evaluation, doesn’t need an echocardiogram, and doesn’t need baseline biomarkers to decide if there’s a potential cardiotoxicity problem.
DH Well certainly, you’ve raised my awareness. It was not something that I had been thinking of with checkpoint inhibitors. Now, I certainly would if the patient has some comorbid illness that involves the heart, maybe think about it, wait to see how these reports develop, and what you and the registry do.
JC You’ve seen people who get this sort of immunologic reaction that they require steroids for fluid accumulation, rash, or other things that are in this constellation. I wouldn’t be surprised if that group might have some subclinical myocarditis that just gets better when they get treated for the other things.
We have actually been trying to get a quick look at the left ventricle when patients on checkpoint inhibitors present with systemic, noncardiac symptoms to see if there is a cardiac signal we are missing. We have a handheld portable echocardiogram device called a Vscan (General Electric Company, Fairfield, CT). It’s not much bigger than the larger cellphones that are available. We’ve been going to the bedside when people have the reaction and sticking the transducer on to get a feeling of what the ventricle looks like. There’s a lot that we don’t know. It’s a fertile ground for investigation.
DH Well, I couldn’t ask you to end on a higher note than covering the checkpoint inhibitors, which are so popular and so interesting and used everywhere. We’re still managing that whole concept. I want to thank you very much.
JC It was a great pleasure. Thank you.
A typical example is a patient who had Hodgkin disease in his teens and received mediastinal mantle radiation. Fifteen to 25 years later, the patient has a pacemaker for heart block, coronary artery disease that requires a stent, and most recently has two valves replaced—so aortic and mitral valve replacement because of late radiation effects. This scenario is typical for the “old” days. The 20-year cumulative incidence of radiation-induced cardiac toxicity is 15%-20% (Table, Figure).1 Sitting with a patient about to begin chest radiation, the absolute risks are unknown but presumed to be less as treatment is delivered according to the modern techniques that you described in the question.
DH They’re so much better now, so this is less common.
JC With the shielding and breath-holding techniques and position changes, doing upright radiation rather than supine, and because the technology has improved both in the delivery of radiation and the technology in understanding where all the radiation is going, in today’s world, we can calculate pretty precisely how much radiation the heart actually receives. Ultimately, with the protective mechanisms that are in place going forward, the risks that I described for that survivor are probably exponentially less than what’s reported in the literature and what we see clinically. Radiation has become much, much safer. There is still probably some small risk of development of late changes, but I don’t think we know what that risk is today because the shielding and things we do to protect the heart have not yet been studied in the long term.
DH Of course, the patient is breathing and there’ll be some movement of the target. Some of the radiation techniques can follow the target despite the breathing?
JC Yes, definitely true. Radiation delivery is much more precise today. Not only has the delivery changed, but so has what we know about the location of potential arterial disease. For example, if you read any textbook, it says that for the coronaries, that it’s ostial and proximal disease of the left main, or the left anterior descending, or the right coronary artery. Today, somebody who gets chest/mediastinal radiation, for either breast cancer, lymphoma, or for a mediastinal tumor, the location of potential disease is more likely to mimic the location of classic coronary disease in the mid-portion of the left anterior descending artery rather than at the ostium. It’s going to be a different disease going forward.2,3
DH Let’s switch from radiation to chemotherapy. Of course, all of us worry about and are very familiar with the toxicity potential of doxorubicin and trastuzumab. I remember an American Society of Clinical Oncology meeting a few years ago, one of the speakers was a cardiologist and was advising us that perhaps the ejection fraction, albeit readily available and reproducible, was probably too simple and we should watch more closely with other techniques. My final question and then I’ll let you comment – I thought I recalled 5-fluorouracil (5-FU) infusions, which we do in some of our colorectal cancers, for example, can cause a vasospasm, Prinzmetal-type angina from time to time, and is that true in capecitabine? What are your thoughts on how to follow the doxorubicin, trastuzumab analogs, and anything about 5-FU and its analogs?
JC Okay, this is a giant question. I’ll take them in order. First, doxorubicin. Cumulative dose-related cardiotoxicity was first described by Von Hoff in 1979.4 That is, the more you get, the higher likelihood of developing cardiotoxicity. Up to a total of 400 mg/m2, the risk is <1%, with a sharp rise as the dose increases beyond this level.4 That being said, there is a clear large and individual variation: I’ve seen sarcoma patients who’ve gotten close to 1,000 mg/m2 without cardiac dysfunction, and some people with minimal exposure have full-blown cardiomyopathy. One of the protective strategies that we developed over the years is to give less of the drug, and with that get the same cancer treatment efficacy. There is definitely a risk for anthracyclines. Full-blown heart failure is probably in the 4%-8% range – and that’s cumulative lifetime – it’s not as high as we once thought it was. That doesn’t mean that it isn’t there, but, relatively speaking, from the standpoint of benefit of anthracyclines, the benefit certainly clearly outweighs the cardiac risk.
With administration of the anthracyclines, we try to do whatever protective things we can do. There are some people who believe that continuous infusion is safer for the heart than bolus injection. It’s pretty controversial. Dexrazoxane, which is a chelating agent, has been shown to reduce cardiotoxicity, and using a lipophilic anthracycline preparation may also have less cardiac toxicity.
DH I have a population in which a lot of liposomal doxorubicin is used and I’ve given a lot and rarely if ever get cardiac toxicity. You see that as well?
JC Yes. There’s a significant financial difference between doxorubicin and liposomal doxorubicin; the latter is more expensive. From the standpoint of safety, and from the standpoint of if I ever needed doxorubicin, I would probably jump on that and ask for the liposomal preparation and/or dexrazoxane.
DH For trastuzumab, we are getting echo- cardiograms every 9 weeks. That seems awfully simple, but there’s a whole algorithm we follow for particular change in ejection fraction and watch the drug or stop the drug. Are we doing that correctly?
JC The first statement I would make about that is that there are too many women who need trastuzumab whose therapy has been prematurely stopped because of just looking at the ejection fractions. So, there has to be more to decision-making other than just the number of the ejection fraction. We’re pretty aggressive and tend to try to get women to get the full dose and whatever dose-effective dose they need, especially with curative intent in the adjuvant setting that we make decisions based not only on the ejection fraction.
We also have, I would say, a handful of our medical breast oncologists who do not follow the package insert. We don’t get ejection fractions every 3 cycles. We have substituted a little bit by following biomarkers so that we use N-terminal pro b-type natriuretic peptide (NT-proBNP) to monitor people, either with each cycle or every third cycle. The benefit of BNP is its negative predictive value. If it’s normal, it’s hard to have any clinically significant myocardial dysfunction.
What we’re going to see over – I would hope – the next year or two is that the recommendations about getting echocardiograms frequently will go away.
DH That would be welcome because in our electronic medical records, it’s 9 weeks, stop, do this, etc. How about a comment on infusional 5-FU and possibly its cousins, such as capecitabine, and any coronary issues?
JC Let me come back, just one more thing about trastuzumab. For metastatic disease, we do whatever is necessary to continue effective cancer therapy and in the absence of any cardiac symptoms or abnormal physical findings, we continue cancer treatment without any serial echocardiographic monitoring.
DH You think the NT-proBNP might be useful? I know that’s excreted by the kidneys, so that might rise in renal failure, but we can adjust for that.
JC The negative predictive value of having a normal BNP is helpful. I think what I wanted to say was that screening echocardiograms and looking at ejection fraction in low-risk populations probably is clearly not cost-effective. It probably never alters decision making. If you have a 30-year-old person with no cardiac risk factors and no past cardiac history who develops B-cell lymphoma and is going to get anthracycline-based chemotherapy, the likelihood of finding a reason not to give that therapy based on an echocardiogram is quite small. I would even go further and say close to zero. We’ve begun to look at this. There is literature that supports the concept. Also, that in low-risk people – if you can define the low-risk population in an accurate way – for lymphoma patients or women with breast cancer getting either anthracyclines, trastuzumab, or the other human epidermal growth factor receptor-2 (HER2)-directed therapies, there’s probably little yield to even getting a baseline study.
DH Very interesting. I would agree with you.
JC We’re going to talk about 5-FU, of course. The 5-FU thing has become a passion of mine. Over the last two to two-and-a- half years we have gotten very aggressive with treating coronary spasm that’s induced by the fluoropyrimidines. That’s 5-FU and capecitabine, the oral version.
There is an incidence that the literature says is less than 1%. It probably is somewhere between 3% and 5%. It’s a little bit more common than has been reported. The reason is the way that it presents has classically been described in the literature as different than what occurs in real life. It is a phenomenon. It’s the most common cardiac side effect. Sometimes it is large epicardial coronary artery spasm. Sometimes it’s small vessel spasm. You can have chest pain with no electrocardiographic changes or ECG changes without chest pain (so-called silent ischemia). The description doesn’t always sound like classic angina but symptoms are temporally related to getting the drug.
So, we’ve developed a protocol to treat documented spasm as an outpatient to be able to continue those drugs to their logical conclusion from an oncologic standpoint. In fact, we just submitted a manuscript to the
DH Finally, it occurred to me that we cause problems with radiation. We cause problems with chemotherapy and other infusions. Are there particular cancers that you think of or you’re called in to see that you worry about cardiac involvement by their location? What comes to mind are cases I’ve had in which there is pericardial involvement and tamponade or restrictive pericarditis.
JC We see metastatic disease to the pericardium with breast cancer, lung cancer, and lymphoma. Renal cell has an interesting predilection to go to the pericardium. We’ve seen in the last probably 6 months 2 cases of bladder cancer with pericardial metastases. When we reviewed the literature, we were only able to find 9 or 10 case reports. It’s rare, but it occurs.
Fluid in the pericardium with and without tamponade is increasingly common, and because we do a better job in treating complicated cancer, people successively can receive cycles of sequential chemotherapeutic regimens – they are living longer, their cancer can get more complicated and/or resistant and with it, there’s more time for metastatic disease to occur. Tamponade is a common phenomenon. We always say that at 4 o’clock on Friday we always see somebody who has tamponade. We see a lot of pericardial disease.
Then, another area of a concern is the tyrosine kinase inhibitors that can cause hypertension, which is very common. We’ve become pretty aggressive. The oncologists recognize the importance of being able to follow and treat blood pressures to allow patients to get these treatments. I guess we couldn’t end without talking about checkpoint inhibitors and the recent lay press flurry about reporting myocarditis.
DH I haven’t personally experienced that. How common is that, and how do we watch for it?
JC Personally, I’ve seen probably four or five people who were referred because of heart failure on checkpoint inhibitors. For each of them, there was historically something as a preexisting problem before the checkpoint inhibitor. It was coincident that with either fluid changes or blood pressure changes associated with the treatment that they had a flare-up of heart failure.
We have not seen, fortunately, the dynamics that were reported in the
DH Well, certainly with the proliferation of the checkpoint inhibitors, and so many different tumors, and so much widespread use, it looks like there is a small safety signal there but still yet to be defined. How common is that, and what should we watch for?
JC Actually, it’s serendipitous that yesterday I was walking to the parking lot with one of the nurse practitioners who takes care of the melanoma population. She said to me, “Now, do you think that we should be getting BNP levels on everybody who is getting a checkpoint inhibitor?”
I don’t think that we’re there. Just the awareness to ask the right questions when you see a patient and before starting ask, is this somebody who, in the absence of a checkpoint inhibitor, could be at risk for myocardial disease? Recognize that and use the cardiology and oncology community to work together and try to make sure that you do whatever cardioprotective things you can do and to monitor them a little bit more closely. I’m not sure that everybody who is going to start a checkpoint inhibitor needs a cardiac evaluation, doesn’t need an echocardiogram, and doesn’t need baseline biomarkers to decide if there’s a potential cardiotoxicity problem.
DH Well certainly, you’ve raised my awareness. It was not something that I had been thinking of with checkpoint inhibitors. Now, I certainly would if the patient has some comorbid illness that involves the heart, maybe think about it, wait to see how these reports develop, and what you and the registry do.
JC You’ve seen people who get this sort of immunologic reaction that they require steroids for fluid accumulation, rash, or other things that are in this constellation. I wouldn’t be surprised if that group might have some subclinical myocarditis that just gets better when they get treated for the other things.
We have actually been trying to get a quick look at the left ventricle when patients on checkpoint inhibitors present with systemic, noncardiac symptoms to see if there is a cardiac signal we are missing. We have a handheld portable echocardiogram device called a Vscan (General Electric Company, Fairfield, CT). It’s not much bigger than the larger cellphones that are available. We’ve been going to the bedside when people have the reaction and sticking the transducer on to get a feeling of what the ventricle looks like. There’s a lot that we don’t know. It’s a fertile ground for investigation.
DH Well, I couldn’t ask you to end on a higher note than covering the checkpoint inhibitors, which are so popular and so interesting and used everywhere. We’re still managing that whole concept. I want to thank you very much.
JC It was a great pleasure. Thank you.
1. Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412-418.
2. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
3. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854-862.
4. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710-717.
5. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755.
1. Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117(2):412-418.
2. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
3. Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854-862.
4. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710-717.
5. Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749-1755.
Prescriber adherence to antiemetic guidelines with the new agent trifluridine-tipiracil
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
Cancer drugs are becoming available at an unprecedented rate. In 2015 alone, the US Food and Drug Administration (FDA) approved 18 new agents.1 Although many of those agents have adverse event profiles that are more favorable than those seen with conventional chemotherapy, nausea and vomiting still occur. In fact, nausea and vomiting continue to be ranked as among the most common and distressing of cancer symptoms.2,3 In a 2004 study, Grunberg and colleagues reported that as many as 75% of health care providers misjudge the risk for chemotherapy-induced nausea and vomiting (CINV), even when prescribing cancer drugs that have been available for years,4 thus amplifying concerns that such risk assessment might be even worse when new cancer agents are prescribed for the first time.
In this study, we hypothesized that patients prescribed a new cancer drug, trifluridine-tipiracil, would be at risk for CINV because of poor guideline adherence on the part of health care providers. The correct matching of antiemetics to chemotherapy is important. Inadequate antiemetic prophylaxis predisposes to nausea and vomiting with dehydration and metabolic and electrolyte derangements – complications that can occur in up to one-third of patients who receive moderately or highly emetogenic chemotherapy and who have been reported to achieve poor symptom control.4 Over-prophylaxis also has drawbacks. For example, antiemetics are expensive and, at times, they can induce their own adverse events, such as lethargy, dyskinesia, constipation, headaches, hiccups, fatigue, and even cardiac arrhythmias.5 The best approach is to appropriately match the antiemetic to the chemotherapy. Indeed, adherence to evidence-based guidelines has yielded success in symptom control, but the guidelines work on the assumption that the emetogenic potential of new chemotherapy agents has been accurately determined and then disseminated to and acted upon by health care providers.6,7 To our knowledge, no previous studies have tested that assumption, as we do in the present study.
Trifluridine-tipiracil was selected as the focus of this project and as illustrative of other newly approved chemotherapy agents for two reasons. First, it became available for routine prescribing in pretreated patients with metastatic colorectal cancer in the United States in September 2015.1 That timing allowed us to analyze much of the early prescribing period, both during the 9 months before approval, when the drug was available on a compassionate-use basis at our institution, and the 3 months after approval. Second, trifluridine-tipiracil has classifiably low emetogenic potential, and mismatching of antiemetics tends to occur more often with low emetogenic chemotherapy.9 Trifluridine-tipiracil and placebo patients manifest rates of nausea at 48% and 24%, respectively, and rates of vomiting at 28% and 14%, respectively.8
Hence, the goal of this study was to explore whether a guideline-based prophylactic antiemetic regimen was appropriately matched to the new chemotherapy agent, trifluridine-tipiracil, to report whether such symptoms of nausea and vomiting are kept at bay, and to identify a potentially vulnerable interval – immediately after drug approval – when cancer patients may be at risk for CINV because of poor adherence to antiemetic guideline prescribing practices by health care providers.
Methods
Overview
The Mayo Clinic Institutional Review Board approved this study. We obtained the identifying information of all patients treated with trifluridine-tipiracil at our institution from the Mayo Clinic Specialty Pharmacy, which uses an electronic prescribing system that contributed to the comprehensiveness of the data set. Patients included those who had participated in a colorectal cancer compassionate-use program before the September 2015 approval of the drug and those who received the drug shortly after its approval. In essence, this retrospective, single-institution study included every patient who received trifluridine-tipiracil for metastatic colorectal cancer in 2015 (January through December); this approach enabled us to systematically report on early first-cycle prescribing practices 9 months before and 3 months after the drug’s approval in September of 2015.
Determination of guideline adherence
This project relied on the National Comprehensive Cancer Network (NCCN) Guidelines (v1.2015, behind paywall) because they had been updated in 2015 (and hence coincided with this project’s study dates) to incorporate recommendations specific to oral chemotherapy and because they seemed concordant with other guidelines.10,11
Antiemetic prophylaxis for a specific patient was deemed guideline adherent if a version of the recommended NCCN antiemetic regimen had been prescribed during the first cycle of chemotherapy. This regimen consisted of metoclopramide, prochlorperazine, haloperidol, or a 5-hydroxytryptamine receptor antagonist. In contrast, if a patient had been prescribed a more aggressive or less aggressive regimen, such prescribing practices were deemed non–guideline adherent/aggressive (received more prophylaxis than called for) or non–guideline adherent/less aggressive (including no antiemetics), respectively. Again, medical record prescribing determined adherence.
Data reporting
The primary goal of this study was to report the percentage of patients who had been prescribed a first-cycle antiemetic prophylaxis regimen concordant with NCCN guidelines. Secondary goals included reporting the incidence of nausea and vomiting, the use of rescue antiemetics other than those prescribed up front, the need for an unplanned medical encounter to address nausea and vomiting, and change in antiemetic prescribing before the second chemotherapy cycle. Confidence intervals were calculated with JMP Pro 10.0.0. This study was too limited in sample size to assess sex-based differences in outcomes.
Results
Demographics
This report focuses on 44 patients who received first-cycle trifluridine-tipiracil during the first calendar year of the drug’s FDA approval. All patients had metastatic colorectal cancer and had previous exposures to other chemotherapy agents (Table 1). Of note, 28 patients (64%) had experienced CINV before starting trifluridine-tipiracil and all these patients had been heavily pretreated with multiple lines of chemotherapy.
Guideline adherence
Patients were most commonly prescribed prochlorperazine and ondansetron prophylaxis for CINV before the first chemotherapy cycle of trifluridine-tipiracil (Table 2): 15 patients were prescribed combination antiemetic therapy, typically two of the most commonly prescribed single agents with different mechanisms of action. Twenty-five patients (57%; 95% confidence interval (CI): 42%, 70%) were prescribed antiemetics in a manner consistent with guidelines; 15 (34%; 95% CI: 22%, 49%) were prescribed antiemetics in a non–guideline-adherent/more aggressive manner (received more prophylaxis than called for); and 4 (9%; 95% CI: 4%, 21%) were prescribed them in a non–guideline-adherent/less aggressive manner.
Clinical outcomes based on guideline adherence
In guideline-adherent patients, first-cycle nausea and vomiting occurred in 13 patients (52%) and 6 patients (24%), respectively, with 1 patient requiring an unscheduled clinic visit and another an emergency department visit and hospital admission – all for nausea and vomiting (Table 3). In non–guideline-adherent/more aggressive patients, those symptoms occurred in 5 patients (33%, nausea) and 4 patients (27%, vomiting), with 1 patient requiring a clinic visit and emergency department visit and another an emergency department visit – again, all for nausea and vomiting. In non–guideline-adherent/less aggressive patients, no nausea or vomiting was reported.
Discussion
This study examined adherence to antiemetic guidelines in the setting of a soon-to-be-approved or newly approved antineoplastic agent. As hypothesized, a substantial proportion of patients (43% in this study) were prescribed antiemetics in a nonadherent manner with respect to guidelines, thus identifying the period shortly before and after FDA approval as a particularly vulnerable interval with respect to antiemetic guideline adherence. It is possible that our institution’s practice of testing novel chemotherapy agents for the treatment of colorectal cancer prompted a heightened awareness of potential adverse events, leading to greater guideline adherence than might have occurred in other settings and resulting in judicious straying from guideline adherence only when appropriate.12-14 Thus, these high rates of poor adherence may in fact represent an underestimate of what one might see in other clinical practices; and, similarly, these rates of symptom control might also be more favorable than those one might see in other clinical practices. To our knowledge, antiemetic prescribing practices with newer chemotherapy agents have not been explored before now, and our data underscore a clear need to do so – particularly during this limited interval when health care providers begin to prescribe new chemotherapy agents for the first time.
It is worth noting that despite the high rates of guideline nonadherence, rates of nausea and vomiting seemed to be comparable in patients prescribed antiemetics in a guideline-adherent manner and those prescribed antiemetics in a non–guideline-adherent/aggressive manner.A small number of patients in both the guideline-adherent and non–guideline-adherent/aggressive groups required rescue medications, unscheduled medical visits for nausea and vomiting, and additional antiemetics during the second cycle of chemotherapy. Of note,none of those interventions occurred in patients who were prescribed antiemetics in a non–guideline-adherent/less aggressive manner. These findings might reflect the fact that the patients had proven themselves to be at risk for nausea and vomiting with previous chemotherapy. Before they became candidates for trifluridine-tipiracil, patients had been heavily pretreated with other chemotherapy agents, most had experienced CINV, and many were therefore highly predisposed to nausea and vomiting. These observations underscore the fact that guidelines – even those that are well accepted and widely used – should be implemented in concert with good clinical judgment.10,11 This study has shortcomings, most notably its small sample size. However, had we extended our study beyond 3 months of the FDA approval to include more patients, our findings would have reflected more experienced prescribing practices and we thereby would have deviated from our primary goal of assessing antiemetic prescribing practices with only recently-approved and available chemotherapy agents. In this context, this limited sample size aptly serves a primary role of capturing outcomes within a fleeting but critical interval of new drug availability.In summary, this study found a notable rate of poor guideline adherence when prescribing antiemetics for trifluridine-tipiracil, a new chemotherapy agent of low emetogenic potential. Although the resultant rates of nausea and vomiting suggest that good clinical judgment might have influenced whether or not guidelines were adhered to, these findings nonetheless underscore the need to assess adherence to antiemetic guidelines when new chemotherapy drugs become available and potentially to put in place institutional infrastructure rapidly to promote improved adherence. Such an assessment should be deliberate, formalized, and prompt within individual oncology clinics and cancer centers after a new cancer drug becomes available. In conjunction with clinical judgment, such measures might lead to improved symptom control.
Acknowledgment
This paper is based on a poster that was presented at the 2016 Palliative Care in Oncology Symposium, on September 10, 2016: Adherence to antiemetic guidelines with a newly approved chemotherapy agent, trifluridine-tipiracil (TAS-102): a single-institution study. Daniel Childs and Aminah Jatoi, Mayo Clinic, Rochester, MN. http://meetinglibrary.asco.org/record/136444/abstract. J Clin Oncol. 2016;34(suppl 26S):abstract 221.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
1. CenterWatch. FDA website. FDA approved drugs for oncology: drugs approved for 2015. https://www.centerwatch.com/drug-information/fda-approved-drugs/therapeutic-area/12/oncology. Last updated April 2017. Accessed June 4, 2016.
2. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356-1367.
3. Kottschade L, Novotny P, Lyss A, et al. Chemotherapy-induced nausea and vomiting: incidence and characteristics of persistent symptoms and future directions NCCTG N08C3. Support Care Cancer. 2016;24:2661-2667.
4. Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261-2268.
5. Navari RM. The safety of antiemetic medications for the prevention of chemotherapy-induced nausea and vomiting. Expert Opin Drug Saf. 2016; 15:343-356.
6. Gilmore JW, Peacock NW, Gu A, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE study. J Oncol Pract. 2014;10:68-74.
7. Mertens WC, Higby DJ, Brown D, et al. Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol. 2003;21:1373-1378.
8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919.
9. Schwartzberg L, Morrow G, Balu S, et al. Chemotherapy-induced nausea and vomiting and antiemetic prophylaxis with palonosetron versus other 5-HT3 receptor antagonists in patients with cancer treated with low emetogenic chemotherapy in a hospital outpatient setting in the United States. Curr Med Res Opin. 2011;27:1613-1622.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Antiemesis, Version1,2015 [behind paywall]. https://www.nccn.org. Last update not known. Accessed June 4, 2016.
11. Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21:v232-v243.
12. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomized, placebo-controlled, phase 3 study. Lancet. 2013;381:303-312.
13. Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393.
14. Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347-3353.
Comprehensive assessment of cancer survivors’ concerns to inform program development
Complex cancer treatments, limited personnel resources, and a growing number of cancer survivors are challenging cancer health care professionals’ abilities to provide comprehensive care. Cancer survivors have a range of needs that extend over the cancer care trajectory and that represent physical, psychological, social, and spiritual domains. Numerous studies have explored supportive care needs and recent systematic reviews have highlighted the supportive care needs related to cancer1 and to specific cancer types, including prostate cancer,2 breast cancer,3 gynecologic cancer,4 hematological cancer,5 and lung cancer.6 However, reviews are limited in that they do not always assess needs across the cancer trajectory or identify demographic or clinical variables that are associated with needs. These data are needed to focus survivorship program development in cancer centers in order to target populations most likely at risk for unmet needs, identify what salient concerns to address, and to appropriately schedule supportive care programs.
The importance of assessing the patient’s subjective view of his/her needs or concerns is well acknowledged as being fundamental to patient-centered care.7 Clinicians routinely assess needs in practice using a variety of screening tools. However, there needs to be a broader assessment of concerns and needs in a population of survivors with mixed cancer diagnoses, along with their appraisal of how well their needs were addressed by their health care team, to provide an overall identification of gaps in supportive care. The primary purpose of the present study was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs; ascertain survivors’ perceived importance of those needs and the extent to which our institution, the University Hospitals Seidman Cancer Center, was attentive to those needs; and to identify who might be at risk for having greater concerns. The overall goal was to use the data to inform survivorship and supportive care program development.
Methods
Design, sample and setting
We used a cross-sectional design. Surveys were mailed once to a convenience sample of 2,750 adult patients who had been seen in follow-up during the previous 2 years (2010-2011) at all clinical sites of University Hospitals Seidman Cancer Center, a Midwestern National Cancer Institute-designated Comprehensive Cancer Center. Patients who had a noncancer diagnosis were excluded. The distribution list was screened for deceased individuals and those patients who had multiple visits during the time period. The project was reviewed and approved as nonresearch by the Case Western Reserve University Cancer Institutional Review Board.
Survey
An interdisciplinary team of clinicians, administrators, and researchers adapted the Mayo Clinic Cancer Center’s Cancer Survivors Survey of Needs8 to create a comprehensive survey for the cancer center. Input regarding the scope of the survey was sought from the Patient and Family Advisory Council of the cancer center. The survey, which was formatted for scanning purposes, consisted of 33 questions that were compiled into 4 sections. Sections 1 and 2 focused on demographic and treatment-related information, including use of community and hospital support services and preferences for follow-up care. In section 3, a quality-of-life framework was used to assess physical, social, emotional, and spiritual needs. Respondents were asked to rate their current level of concern for 19 physical effects, 10 social effects, 10 emotional effects, and 5 spiritual effects on a scale ranging from 0 (no concern) to 5 (extreme concern). In section 4, respondents were asked to indicate the importance of the cancer team addressing their physical, social, emotional, and spiritual needs. This was followed by their rating of the cancer team’s attention to their needs as Poor, Fair, Good, Excellent, or They did not ask about my needs. Respondents were asked about preferences for learning about physical, social, emotional, and spiritual effects. In addition to the 33 questions, there were 6 open-ended questions in which respondents were encouraged to share additional information about their needs, sources of support, and other concerns.
Procedures
Eligible respondents were mailed a cover letter explaining the survey from both the director and president of the cancer center, a survey, and a postage-paid return envelope. The option to respond to the survey by a telephone call to the director of the Office of Cancer Survivorship was offered in the cover letter.
Data analysis
Returned surveys were scanned into a Teleform database, verified, and exported into an SPSS data file. Data quality was checked by running frequency analyses and summarizing variables. Time-since-treatment responses were collapsed into 4 categories: on treatment, up to 2 years posttreatment, 2-5 years posttreatment, and more than 5 years posttreatment. Descriptive statistics were used to summarize demographic and medical characteristics of the respondents and to calculate the mean score for each concern for the total sample and then for each category of time since treatment. Because of the large number of respondents with breast cancer, the respondents were stratified into two groups, one of breast cancer the other of nonbreast cancer respondents. Then, the Mann-Whitney test was performed for each concern to examine differences between respondents with and without breast cancer.
To identify the most prevalent concerns, ratings for concerns were recoded into no concern (rated as 0), low concern (1 or 2), and moderate/high concern (3, 4, or 5). Since our interest was in the moderate and high concerns, the responses were dichotomized into moderate/high concerns and all other levels. Logistic regression models were then used to identify associations between a set of survivor characteristics or covariates (age, sex, living status, marital status, employment status, cancer type, and time since treatment) with the 12 most highly rated moderate/high concerns. All the analyses were performed using statistical software SPSS 20 and Stata 13.0
Results
Respondents
A total of 1,005 surveys were returned for a 37% response rate. Forty-two patients responded by telephone. The mean age of respondents was 64.9 years (range, 22-98; SD, 12.8). The typical respondent was female, white, and married (Table 1). Twenty-four percent of the respondents (n = 240) reported living alone. Although about 47% of respondents (n = 473) reported a breast cancer diagnosis, more than 17 cancers were identified, and 14% of respondents (n = 145) listed multiple diagnoses. About a third of respondents were receiving treatment when they completed the survey.
Just under half of the respondents (n = 498) reported using community resources for support and information about cancer, and 29.5% (n = 296) sought information on the internet during their cancer experience. The most commonly used community resources were The Gathering Place, a local organization offering free supportive programs and services to individuals with cancer and their families (n = 167), and the American Cancer Society (n = 138). Of the 496 respondents who reported accessing hospital resources, most (n = 322) said they used information that their health care team recommended. Other supportive options were used to a lesser degree: support groups (n = 92), chemotherapy and radiation therapy classes (n = 129), and supportive/educational programs offered by the cancer center (n = 27). Most of the respondents (n = 822, 88.6%) preferred to have their follow-up care remain with their cancer care team 1 year after treatments are completed. Almost two-thirds of respondents (n = 601, 64%) cited being seen at the cancer center for follow-up care as the most important factor in considering follow-up care.
Concerns In determining whether the large proportion of respondents with breast cancer skewed the study results, it was determined that median scores differed significantly in only four concerns. Compared with respondents without breast cancer, respondents with breast cancer were more likely to have significantly lower scores for concerns related to fatigue (P <.001) and sexual issues/intimacy (P = .001). Respondents with breast cancer were more likely to have significantly higher scores than respondents without breast cancer for concerns related to genetic counseling (P = .001) and fear of developing a new cancer (P = .010).
Fears of the cancer returning and developing a new cancer were the two most prevalent concerns, identified by 51% (n = 486) and 47.5% (n = 459), respectively (Table 2). Physical concerns, rated as moderate/high concerns by at least 25% of the sample, were fatigue (n = 336, 34.8%), changes in [the] body after cancer (n = 323, 33.7%), trouble sleeping (n = 302, 31.0%), sexual issues/intimacy (n = 263, 28.0%), memory and concentration (n = 261, 26.7%), and weight changes (n = 248, 25.5%). The most prevalent moderate/high social concerns were related to finances (n = 265, 27.5%) and debt from medical bills (n = 232, 25.1%). Managing stress (n = 279, 29.2%) and difficult emotions (n = 244, 25.1%) were prevalent moderate/high emotional concerns. Spiritual concerns were less often rated as moderate/high concerns. Having a breast cancer diagnosis was not significantly related to the number of reported moderate to high concerns (P = 1.00).
Variables associated with the 12 most frequent moderate/high concerns are shown in Tables 3 and 4. Age was associated with the most moderate/high concerns. With every decade of age, the odds of having the following moderate/high concerns decreased: bodily changes after cancer (odds ratio [OR], 0.75), sexual intimacy (OR, 0.81), memory and concentration (OR, 0.83), weight changes (OR, 0.77), financial (OR, 0.75), debt (OR, 0.71), cancer returning (OR, 0.66), developing a new cancer (OR, 0.67), managing stress (OR, 0.67), and managing difficult emotions (OR, 0.67).
Female sex was associated with lower odds of having a concern about sexual intimacy (OR, 0.30) and increased odds of having concerns related to memory and concentration (OR, 1.78), managing stress (OR, 2.35), and managing difficult emotions (OR, 1.77). Race was another demographic characteristic statistically associated with numerous moderate/high concerns. Survivors who identified white, were more likely than other people of other races to have fewer moderate/high concerns regarding bodily changes after cancer (OR, 0.46), weight change (OR, 0.46), finances (OR, 0.46), debt (OR, 0.40), managing stress (OR, 0.55), and managing difficult emotions (OR, 0.49). The odds of having a moderate/high concern regarding debt was 2.25 times higher given widowed marital status compared with those survivors who were single. Unemployment status, when compared with full-time employment, was significantly associated with increased odds of having moderate/high concerns related to fatigue (OR, 2.08), bodily changes after cancer (OR, 1.72), memory and concentration (OR, 2.45), weight changes (OR, 2.17), finances (OR, 1.93), developing a new cancer (OR, 1.91), and managing difficult emotions (OR, 1.80).
As expected, respondents who had completed treatment were less likely to have many of the moderate/high concerns as those still undergoing treatment. Survivors who were up to 2 years posttreatment were significantly more likely than those survivors receiving treatment to have fewer moderate/high concerns regarding fatigue (OR, 0.56), sexual intimacy (OR, 0.54), weight change (OR, 0.55), fears of the cancer returning (OR, 0.48), developing a new cancer (OR, 0.35), managing stress (OR, 0.43), and managing difficult emotions (OR, 0.49).
However, those improved odds were not sustained over the cancer trajectory. Compared with survivors who were receiving treatment, survivors who were between 2-5 years posttreatment did not have significantly reduced odds for moderate/high concerns related to fatigue, sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress, and managing difficult emotions. They did have significantly reduced odds for having concerns only related to finances (OR, 0.61) and debt (OR, 0.52).
Long-term survivors, who were beyond 5 years posttreatment, had significantly reduced odds for having moderate/high concerns related to fatigue (OR, 0.45), finances (OR, 0.52), debt (OR, 0.47), and managing difficult emotions (OR, 0.54), compared with survivors receiving treatment. Moderate/high concerns related to sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress did not have improved odds for these long-term survivors.
Attention to needs
The health care teams were rated highly for their attention to the patients’ physical needs. Most respondents (n = 845, 92.4%) viewed the health care team’s attention their physical needs as important and 763 (77.6%) survivors rated the team’s attention to these needs as excellent. The importance of addressing emotional needs was affirmed by 723 (78.5%) respondents, and although 454 (46.8%) viewed the team’s attention to these needs as excellent, 119 (12.3%) reported that the health care team did not ask about emotional needs. In addition, 566 respondents (60%) viewed having the health care team address their social needs as important, and most (n = 715, 74.2%) rated the team’s attention to social needs as good or excellent. Yet, 162 (16.8%) respondents reported that team did not ask about their social needs. The health care team’s addressing of spiritual needs was viewed as important by 346 (37.5%) respondents and ratings for how well the team attended to spiritual needs were: 148 (15.6%) poor or fair, 204 (21.5%) good, and 150 (15.8%) excellent. However, 448 (47.2%) respondents reported that the health care team did not ask about their spiritual needs.
Discussion
The primary purpose of this project was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs and to assess the perceived importance of these needs and the extent to which the cancer center staff were attentive to those needs. The overall goal of this assessment was to inform the development of survivorship and supportive care programs by highlighting common concerns, demographic and medical factors associated with specific concerns, and timing of moderate/high level concerns along the cancer trajectory. There were 3 main findings.
First, the results support the need for enhancing supportive care services to meet emotional concerns of survivors beyond the treatment phase. Similar to other studies,8,9 emotional concerns ranked higher than all other concerns in this study with about 50% of the sample rating “fear the cancer will return” and “fear of developing a new cancer” as moderate/high concern. Although the odds of not having these emotional concerns improved up to 2 years posttreatment, these concerns are likely to resurface, as odds for survivors beyond 2 years were not significantly different from those receiving treatment. A recent systematic review reported that fear of cancer recurrence is experienced by about 73% of cancer survivors, with 49% reporting a moderate to high degree.10 It can have a chronic, stable trajectory for some survivors and is strongly associated with higher levels of anxiety, distress, and depression, and less global, emotional/mental, physical, role, social, and cognitive quality of life.10 In this sample, managing stress and difficult emotions were also rated as moderate/high concerns by at least 25% of the sample.
Second, the findings identified patients at risk for cancer-related concerns throughout the cancer trajectory. As demonstrated in other studies, younger age was associated with greater odds of having multiple greater moderate/high concerns.11-13 Unemployment was the second most common demographic factor associated with multiple moderate/high concerns related to physical symptoms, finances and emotions. Similarly, identifying as black, Asian, American Indian/Alaskan Native, or other was also associated with greater odds of having numerous physical, financial, and emotional concerns. Women had greater concerns related to memory, sexual intimacy, coping with difficult emotions, and stress.
Third, the results helped to identify gaps in supportive care at our cancer center. Although spiritual concerns were not prevalent as being moderate/high, they were still viewed by about a third of survivors as being an important area for the health care team to address. Yet, consistent with other need assessments, spiritual concerns in this study were least often addressed by staff.1 Assessment of spiritual care needs, screening for spiritual distress, and providing spiritual care are essential components of a clinician-patient relationship that supports healing.14 The importance of attending to spiritual care needs was underscored by a recent systematic review that found a positive association between overall spiritual well-being and quality of life in patients with cancer, with the meaning/peace factor consistently and positively associated with physical and mental health.15 Another identified gap was the health care team’s lack of attention to the patient’s social needs, which included concerns related to finances and debt from medical bills. In all, 46% of the respondents reported having financial concerns, with the odds of having moderate/high financial concerns being greatest during treatment to 2 years posttreatment. Attention to the financial burden of cancer patients is critical because the magnitude of cancer-related financial concerns is a significant, strong predictor of quality of life and adverse psychological issues such as depression, anxiety, and distress.16,17
There were several program implications based on the results. A periodic audit of the concerns of survivors and their views on how well their needs were being met was a relatively low cost endeavor. Although the findings were consistent with the literature, the results, when shared with administrators and clinicians, were instrumental in effecting change because they represented the concerns of survivors at the cancer center. Another program directive, based on the results, was to extend the routine screening of patients’ needs during treatment to posttreatment survivorship. Patients who are young, unemployed, do not identify as white, and female warrant more thorough assessment of needs and concerns along the cancer trajectory. Integral to these screenings is the need for patient-centered communication, with discussion of how cancer is affecting the different domains of quality of life within the context of the patient’s life. Lastly, the results clearly indicated the need for additional training of health care providers on how to assess and address spiritual well-being in cancer survivors.
There were limitations to this study, including use of a nonvalidated survey and cross-sectional approach that limited our ability to explore how concerns might change over the trajectory. Also, it was not possible to clarify medical information of the respondents, such as cancer stage. Although the response rate of this study was not high, we are confident in the results because of the large sample size and the finding that the large proportion of respondents with breast cancer was not influential. Despite these limitations, this needs assessment of cancer survivors over the trajectory of care provided insight into the scope of their concerns, identified vulnerable groups of survivors, and highlighted gaps in addressing those concerns. A quality- of-life framework for assessing needs assured a comprehensive focus and generated practice changes to strengthen holistic, comprehensive oncology care.
1. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
2. Paterson C, Robertson A, Smith A, Nabi G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur J Oncol Nurs. 2015;19:405-418.
3. Fiszer C, Dolbeault S, Sultan S, Bredart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology. 2014;23:361-374.
4. Maguire R, Kotronoulas G, Simpson M, Paterson C. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol. 2015;136:478-490.
5. Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: A critical review of the literature. Crit Rev Oncol Hematol. 2013;88:102-116.
6. Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449-464.
7. Adler NE, Page EK. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; Institute of Medicine, 2008.
8. Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40:35-42.
9. Swash B, Hulbert-Williams N, Bramwell R. Unmet psychosocial needs in haematological cancer: A systematic review. Support Care Cancer. 2014;22:1131-1141.
10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322.
11. Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: A multi-center, cross-sectional study in Korea. Psychooncology. 2013;22:1073-1080.
12. Pauwels EE, Charlier C, De Bourdeaudhuij I, Lechner L, Van Hoof E. Care needs after primary breast cancer treatment. Survivors’ associated sociodemographic and medical characteristics. Psychooncology. 2013;22:125-132.
13. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
14. Puchalski CM, Blatt B, Kogan M, Butler A. Spirituality and health: The development of a field. Academic Medicine. 2014;89:10-16.
15. Bai M, Lazenby M. A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med. 2015;18:286-298.
16. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332-338.
17. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological wellbeing among individuals living with cancer. Psychooncology. 2013;22:745-755.
Complex cancer treatments, limited personnel resources, and a growing number of cancer survivors are challenging cancer health care professionals’ abilities to provide comprehensive care. Cancer survivors have a range of needs that extend over the cancer care trajectory and that represent physical, psychological, social, and spiritual domains. Numerous studies have explored supportive care needs and recent systematic reviews have highlighted the supportive care needs related to cancer1 and to specific cancer types, including prostate cancer,2 breast cancer,3 gynecologic cancer,4 hematological cancer,5 and lung cancer.6 However, reviews are limited in that they do not always assess needs across the cancer trajectory or identify demographic or clinical variables that are associated with needs. These data are needed to focus survivorship program development in cancer centers in order to target populations most likely at risk for unmet needs, identify what salient concerns to address, and to appropriately schedule supportive care programs.
The importance of assessing the patient’s subjective view of his/her needs or concerns is well acknowledged as being fundamental to patient-centered care.7 Clinicians routinely assess needs in practice using a variety of screening tools. However, there needs to be a broader assessment of concerns and needs in a population of survivors with mixed cancer diagnoses, along with their appraisal of how well their needs were addressed by their health care team, to provide an overall identification of gaps in supportive care. The primary purpose of the present study was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs; ascertain survivors’ perceived importance of those needs and the extent to which our institution, the University Hospitals Seidman Cancer Center, was attentive to those needs; and to identify who might be at risk for having greater concerns. The overall goal was to use the data to inform survivorship and supportive care program development.
Methods
Design, sample and setting
We used a cross-sectional design. Surveys were mailed once to a convenience sample of 2,750 adult patients who had been seen in follow-up during the previous 2 years (2010-2011) at all clinical sites of University Hospitals Seidman Cancer Center, a Midwestern National Cancer Institute-designated Comprehensive Cancer Center. Patients who had a noncancer diagnosis were excluded. The distribution list was screened for deceased individuals and those patients who had multiple visits during the time period. The project was reviewed and approved as nonresearch by the Case Western Reserve University Cancer Institutional Review Board.
Survey
An interdisciplinary team of clinicians, administrators, and researchers adapted the Mayo Clinic Cancer Center’s Cancer Survivors Survey of Needs8 to create a comprehensive survey for the cancer center. Input regarding the scope of the survey was sought from the Patient and Family Advisory Council of the cancer center. The survey, which was formatted for scanning purposes, consisted of 33 questions that were compiled into 4 sections. Sections 1 and 2 focused on demographic and treatment-related information, including use of community and hospital support services and preferences for follow-up care. In section 3, a quality-of-life framework was used to assess physical, social, emotional, and spiritual needs. Respondents were asked to rate their current level of concern for 19 physical effects, 10 social effects, 10 emotional effects, and 5 spiritual effects on a scale ranging from 0 (no concern) to 5 (extreme concern). In section 4, respondents were asked to indicate the importance of the cancer team addressing their physical, social, emotional, and spiritual needs. This was followed by their rating of the cancer team’s attention to their needs as Poor, Fair, Good, Excellent, or They did not ask about my needs. Respondents were asked about preferences for learning about physical, social, emotional, and spiritual effects. In addition to the 33 questions, there were 6 open-ended questions in which respondents were encouraged to share additional information about their needs, sources of support, and other concerns.
Procedures
Eligible respondents were mailed a cover letter explaining the survey from both the director and president of the cancer center, a survey, and a postage-paid return envelope. The option to respond to the survey by a telephone call to the director of the Office of Cancer Survivorship was offered in the cover letter.
Data analysis
Returned surveys were scanned into a Teleform database, verified, and exported into an SPSS data file. Data quality was checked by running frequency analyses and summarizing variables. Time-since-treatment responses were collapsed into 4 categories: on treatment, up to 2 years posttreatment, 2-5 years posttreatment, and more than 5 years posttreatment. Descriptive statistics were used to summarize demographic and medical characteristics of the respondents and to calculate the mean score for each concern for the total sample and then for each category of time since treatment. Because of the large number of respondents with breast cancer, the respondents were stratified into two groups, one of breast cancer the other of nonbreast cancer respondents. Then, the Mann-Whitney test was performed for each concern to examine differences between respondents with and without breast cancer.
To identify the most prevalent concerns, ratings for concerns were recoded into no concern (rated as 0), low concern (1 or 2), and moderate/high concern (3, 4, or 5). Since our interest was in the moderate and high concerns, the responses were dichotomized into moderate/high concerns and all other levels. Logistic regression models were then used to identify associations between a set of survivor characteristics or covariates (age, sex, living status, marital status, employment status, cancer type, and time since treatment) with the 12 most highly rated moderate/high concerns. All the analyses were performed using statistical software SPSS 20 and Stata 13.0
Results
Respondents
A total of 1,005 surveys were returned for a 37% response rate. Forty-two patients responded by telephone. The mean age of respondents was 64.9 years (range, 22-98; SD, 12.8). The typical respondent was female, white, and married (Table 1). Twenty-four percent of the respondents (n = 240) reported living alone. Although about 47% of respondents (n = 473) reported a breast cancer diagnosis, more than 17 cancers were identified, and 14% of respondents (n = 145) listed multiple diagnoses. About a third of respondents were receiving treatment when they completed the survey.
Just under half of the respondents (n = 498) reported using community resources for support and information about cancer, and 29.5% (n = 296) sought information on the internet during their cancer experience. The most commonly used community resources were The Gathering Place, a local organization offering free supportive programs and services to individuals with cancer and their families (n = 167), and the American Cancer Society (n = 138). Of the 496 respondents who reported accessing hospital resources, most (n = 322) said they used information that their health care team recommended. Other supportive options were used to a lesser degree: support groups (n = 92), chemotherapy and radiation therapy classes (n = 129), and supportive/educational programs offered by the cancer center (n = 27). Most of the respondents (n = 822, 88.6%) preferred to have their follow-up care remain with their cancer care team 1 year after treatments are completed. Almost two-thirds of respondents (n = 601, 64%) cited being seen at the cancer center for follow-up care as the most important factor in considering follow-up care.
Concerns In determining whether the large proportion of respondents with breast cancer skewed the study results, it was determined that median scores differed significantly in only four concerns. Compared with respondents without breast cancer, respondents with breast cancer were more likely to have significantly lower scores for concerns related to fatigue (P <.001) and sexual issues/intimacy (P = .001). Respondents with breast cancer were more likely to have significantly higher scores than respondents without breast cancer for concerns related to genetic counseling (P = .001) and fear of developing a new cancer (P = .010).
Fears of the cancer returning and developing a new cancer were the two most prevalent concerns, identified by 51% (n = 486) and 47.5% (n = 459), respectively (Table 2). Physical concerns, rated as moderate/high concerns by at least 25% of the sample, were fatigue (n = 336, 34.8%), changes in [the] body after cancer (n = 323, 33.7%), trouble sleeping (n = 302, 31.0%), sexual issues/intimacy (n = 263, 28.0%), memory and concentration (n = 261, 26.7%), and weight changes (n = 248, 25.5%). The most prevalent moderate/high social concerns were related to finances (n = 265, 27.5%) and debt from medical bills (n = 232, 25.1%). Managing stress (n = 279, 29.2%) and difficult emotions (n = 244, 25.1%) were prevalent moderate/high emotional concerns. Spiritual concerns were less often rated as moderate/high concerns. Having a breast cancer diagnosis was not significantly related to the number of reported moderate to high concerns (P = 1.00).
Variables associated with the 12 most frequent moderate/high concerns are shown in Tables 3 and 4. Age was associated with the most moderate/high concerns. With every decade of age, the odds of having the following moderate/high concerns decreased: bodily changes after cancer (odds ratio [OR], 0.75), sexual intimacy (OR, 0.81), memory and concentration (OR, 0.83), weight changes (OR, 0.77), financial (OR, 0.75), debt (OR, 0.71), cancer returning (OR, 0.66), developing a new cancer (OR, 0.67), managing stress (OR, 0.67), and managing difficult emotions (OR, 0.67).
Female sex was associated with lower odds of having a concern about sexual intimacy (OR, 0.30) and increased odds of having concerns related to memory and concentration (OR, 1.78), managing stress (OR, 2.35), and managing difficult emotions (OR, 1.77). Race was another demographic characteristic statistically associated with numerous moderate/high concerns. Survivors who identified white, were more likely than other people of other races to have fewer moderate/high concerns regarding bodily changes after cancer (OR, 0.46), weight change (OR, 0.46), finances (OR, 0.46), debt (OR, 0.40), managing stress (OR, 0.55), and managing difficult emotions (OR, 0.49). The odds of having a moderate/high concern regarding debt was 2.25 times higher given widowed marital status compared with those survivors who were single. Unemployment status, when compared with full-time employment, was significantly associated with increased odds of having moderate/high concerns related to fatigue (OR, 2.08), bodily changes after cancer (OR, 1.72), memory and concentration (OR, 2.45), weight changes (OR, 2.17), finances (OR, 1.93), developing a new cancer (OR, 1.91), and managing difficult emotions (OR, 1.80).
As expected, respondents who had completed treatment were less likely to have many of the moderate/high concerns as those still undergoing treatment. Survivors who were up to 2 years posttreatment were significantly more likely than those survivors receiving treatment to have fewer moderate/high concerns regarding fatigue (OR, 0.56), sexual intimacy (OR, 0.54), weight change (OR, 0.55), fears of the cancer returning (OR, 0.48), developing a new cancer (OR, 0.35), managing stress (OR, 0.43), and managing difficult emotions (OR, 0.49).
However, those improved odds were not sustained over the cancer trajectory. Compared with survivors who were receiving treatment, survivors who were between 2-5 years posttreatment did not have significantly reduced odds for moderate/high concerns related to fatigue, sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress, and managing difficult emotions. They did have significantly reduced odds for having concerns only related to finances (OR, 0.61) and debt (OR, 0.52).
Long-term survivors, who were beyond 5 years posttreatment, had significantly reduced odds for having moderate/high concerns related to fatigue (OR, 0.45), finances (OR, 0.52), debt (OR, 0.47), and managing difficult emotions (OR, 0.54), compared with survivors receiving treatment. Moderate/high concerns related to sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress did not have improved odds for these long-term survivors.
Attention to needs
The health care teams were rated highly for their attention to the patients’ physical needs. Most respondents (n = 845, 92.4%) viewed the health care team’s attention their physical needs as important and 763 (77.6%) survivors rated the team’s attention to these needs as excellent. The importance of addressing emotional needs was affirmed by 723 (78.5%) respondents, and although 454 (46.8%) viewed the team’s attention to these needs as excellent, 119 (12.3%) reported that the health care team did not ask about emotional needs. In addition, 566 respondents (60%) viewed having the health care team address their social needs as important, and most (n = 715, 74.2%) rated the team’s attention to social needs as good or excellent. Yet, 162 (16.8%) respondents reported that team did not ask about their social needs. The health care team’s addressing of spiritual needs was viewed as important by 346 (37.5%) respondents and ratings for how well the team attended to spiritual needs were: 148 (15.6%) poor or fair, 204 (21.5%) good, and 150 (15.8%) excellent. However, 448 (47.2%) respondents reported that the health care team did not ask about their spiritual needs.
Discussion
The primary purpose of this project was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs and to assess the perceived importance of these needs and the extent to which the cancer center staff were attentive to those needs. The overall goal of this assessment was to inform the development of survivorship and supportive care programs by highlighting common concerns, demographic and medical factors associated with specific concerns, and timing of moderate/high level concerns along the cancer trajectory. There were 3 main findings.
First, the results support the need for enhancing supportive care services to meet emotional concerns of survivors beyond the treatment phase. Similar to other studies,8,9 emotional concerns ranked higher than all other concerns in this study with about 50% of the sample rating “fear the cancer will return” and “fear of developing a new cancer” as moderate/high concern. Although the odds of not having these emotional concerns improved up to 2 years posttreatment, these concerns are likely to resurface, as odds for survivors beyond 2 years were not significantly different from those receiving treatment. A recent systematic review reported that fear of cancer recurrence is experienced by about 73% of cancer survivors, with 49% reporting a moderate to high degree.10 It can have a chronic, stable trajectory for some survivors and is strongly associated with higher levels of anxiety, distress, and depression, and less global, emotional/mental, physical, role, social, and cognitive quality of life.10 In this sample, managing stress and difficult emotions were also rated as moderate/high concerns by at least 25% of the sample.
Second, the findings identified patients at risk for cancer-related concerns throughout the cancer trajectory. As demonstrated in other studies, younger age was associated with greater odds of having multiple greater moderate/high concerns.11-13 Unemployment was the second most common demographic factor associated with multiple moderate/high concerns related to physical symptoms, finances and emotions. Similarly, identifying as black, Asian, American Indian/Alaskan Native, or other was also associated with greater odds of having numerous physical, financial, and emotional concerns. Women had greater concerns related to memory, sexual intimacy, coping with difficult emotions, and stress.
Third, the results helped to identify gaps in supportive care at our cancer center. Although spiritual concerns were not prevalent as being moderate/high, they were still viewed by about a third of survivors as being an important area for the health care team to address. Yet, consistent with other need assessments, spiritual concerns in this study were least often addressed by staff.1 Assessment of spiritual care needs, screening for spiritual distress, and providing spiritual care are essential components of a clinician-patient relationship that supports healing.14 The importance of attending to spiritual care needs was underscored by a recent systematic review that found a positive association between overall spiritual well-being and quality of life in patients with cancer, with the meaning/peace factor consistently and positively associated with physical and mental health.15 Another identified gap was the health care team’s lack of attention to the patient’s social needs, which included concerns related to finances and debt from medical bills. In all, 46% of the respondents reported having financial concerns, with the odds of having moderate/high financial concerns being greatest during treatment to 2 years posttreatment. Attention to the financial burden of cancer patients is critical because the magnitude of cancer-related financial concerns is a significant, strong predictor of quality of life and adverse psychological issues such as depression, anxiety, and distress.16,17
There were several program implications based on the results. A periodic audit of the concerns of survivors and their views on how well their needs were being met was a relatively low cost endeavor. Although the findings were consistent with the literature, the results, when shared with administrators and clinicians, were instrumental in effecting change because they represented the concerns of survivors at the cancer center. Another program directive, based on the results, was to extend the routine screening of patients’ needs during treatment to posttreatment survivorship. Patients who are young, unemployed, do not identify as white, and female warrant more thorough assessment of needs and concerns along the cancer trajectory. Integral to these screenings is the need for patient-centered communication, with discussion of how cancer is affecting the different domains of quality of life within the context of the patient’s life. Lastly, the results clearly indicated the need for additional training of health care providers on how to assess and address spiritual well-being in cancer survivors.
There were limitations to this study, including use of a nonvalidated survey and cross-sectional approach that limited our ability to explore how concerns might change over the trajectory. Also, it was not possible to clarify medical information of the respondents, such as cancer stage. Although the response rate of this study was not high, we are confident in the results because of the large sample size and the finding that the large proportion of respondents with breast cancer was not influential. Despite these limitations, this needs assessment of cancer survivors over the trajectory of care provided insight into the scope of their concerns, identified vulnerable groups of survivors, and highlighted gaps in addressing those concerns. A quality- of-life framework for assessing needs assured a comprehensive focus and generated practice changes to strengthen holistic, comprehensive oncology care.
Complex cancer treatments, limited personnel resources, and a growing number of cancer survivors are challenging cancer health care professionals’ abilities to provide comprehensive care. Cancer survivors have a range of needs that extend over the cancer care trajectory and that represent physical, psychological, social, and spiritual domains. Numerous studies have explored supportive care needs and recent systematic reviews have highlighted the supportive care needs related to cancer1 and to specific cancer types, including prostate cancer,2 breast cancer,3 gynecologic cancer,4 hematological cancer,5 and lung cancer.6 However, reviews are limited in that they do not always assess needs across the cancer trajectory or identify demographic or clinical variables that are associated with needs. These data are needed to focus survivorship program development in cancer centers in order to target populations most likely at risk for unmet needs, identify what salient concerns to address, and to appropriately schedule supportive care programs.
The importance of assessing the patient’s subjective view of his/her needs or concerns is well acknowledged as being fundamental to patient-centered care.7 Clinicians routinely assess needs in practice using a variety of screening tools. However, there needs to be a broader assessment of concerns and needs in a population of survivors with mixed cancer diagnoses, along with their appraisal of how well their needs were addressed by their health care team, to provide an overall identification of gaps in supportive care. The primary purpose of the present study was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs; ascertain survivors’ perceived importance of those needs and the extent to which our institution, the University Hospitals Seidman Cancer Center, was attentive to those needs; and to identify who might be at risk for having greater concerns. The overall goal was to use the data to inform survivorship and supportive care program development.
Methods
Design, sample and setting
We used a cross-sectional design. Surveys were mailed once to a convenience sample of 2,750 adult patients who had been seen in follow-up during the previous 2 years (2010-2011) at all clinical sites of University Hospitals Seidman Cancer Center, a Midwestern National Cancer Institute-designated Comprehensive Cancer Center. Patients who had a noncancer diagnosis were excluded. The distribution list was screened for deceased individuals and those patients who had multiple visits during the time period. The project was reviewed and approved as nonresearch by the Case Western Reserve University Cancer Institutional Review Board.
Survey
An interdisciplinary team of clinicians, administrators, and researchers adapted the Mayo Clinic Cancer Center’s Cancer Survivors Survey of Needs8 to create a comprehensive survey for the cancer center. Input regarding the scope of the survey was sought from the Patient and Family Advisory Council of the cancer center. The survey, which was formatted for scanning purposes, consisted of 33 questions that were compiled into 4 sections. Sections 1 and 2 focused on demographic and treatment-related information, including use of community and hospital support services and preferences for follow-up care. In section 3, a quality-of-life framework was used to assess physical, social, emotional, and spiritual needs. Respondents were asked to rate their current level of concern for 19 physical effects, 10 social effects, 10 emotional effects, and 5 spiritual effects on a scale ranging from 0 (no concern) to 5 (extreme concern). In section 4, respondents were asked to indicate the importance of the cancer team addressing their physical, social, emotional, and spiritual needs. This was followed by their rating of the cancer team’s attention to their needs as Poor, Fair, Good, Excellent, or They did not ask about my needs. Respondents were asked about preferences for learning about physical, social, emotional, and spiritual effects. In addition to the 33 questions, there were 6 open-ended questions in which respondents were encouraged to share additional information about their needs, sources of support, and other concerns.
Procedures
Eligible respondents were mailed a cover letter explaining the survey from both the director and president of the cancer center, a survey, and a postage-paid return envelope. The option to respond to the survey by a telephone call to the director of the Office of Cancer Survivorship was offered in the cover letter.
Data analysis
Returned surveys were scanned into a Teleform database, verified, and exported into an SPSS data file. Data quality was checked by running frequency analyses and summarizing variables. Time-since-treatment responses were collapsed into 4 categories: on treatment, up to 2 years posttreatment, 2-5 years posttreatment, and more than 5 years posttreatment. Descriptive statistics were used to summarize demographic and medical characteristics of the respondents and to calculate the mean score for each concern for the total sample and then for each category of time since treatment. Because of the large number of respondents with breast cancer, the respondents were stratified into two groups, one of breast cancer the other of nonbreast cancer respondents. Then, the Mann-Whitney test was performed for each concern to examine differences between respondents with and without breast cancer.
To identify the most prevalent concerns, ratings for concerns were recoded into no concern (rated as 0), low concern (1 or 2), and moderate/high concern (3, 4, or 5). Since our interest was in the moderate and high concerns, the responses were dichotomized into moderate/high concerns and all other levels. Logistic regression models were then used to identify associations between a set of survivor characteristics or covariates (age, sex, living status, marital status, employment status, cancer type, and time since treatment) with the 12 most highly rated moderate/high concerns. All the analyses were performed using statistical software SPSS 20 and Stata 13.0
Results
Respondents
A total of 1,005 surveys were returned for a 37% response rate. Forty-two patients responded by telephone. The mean age of respondents was 64.9 years (range, 22-98; SD, 12.8). The typical respondent was female, white, and married (Table 1). Twenty-four percent of the respondents (n = 240) reported living alone. Although about 47% of respondents (n = 473) reported a breast cancer diagnosis, more than 17 cancers were identified, and 14% of respondents (n = 145) listed multiple diagnoses. About a third of respondents were receiving treatment when they completed the survey.
Just under half of the respondents (n = 498) reported using community resources for support and information about cancer, and 29.5% (n = 296) sought information on the internet during their cancer experience. The most commonly used community resources were The Gathering Place, a local organization offering free supportive programs and services to individuals with cancer and their families (n = 167), and the American Cancer Society (n = 138). Of the 496 respondents who reported accessing hospital resources, most (n = 322) said they used information that their health care team recommended. Other supportive options were used to a lesser degree: support groups (n = 92), chemotherapy and radiation therapy classes (n = 129), and supportive/educational programs offered by the cancer center (n = 27). Most of the respondents (n = 822, 88.6%) preferred to have their follow-up care remain with their cancer care team 1 year after treatments are completed. Almost two-thirds of respondents (n = 601, 64%) cited being seen at the cancer center for follow-up care as the most important factor in considering follow-up care.
Concerns In determining whether the large proportion of respondents with breast cancer skewed the study results, it was determined that median scores differed significantly in only four concerns. Compared with respondents without breast cancer, respondents with breast cancer were more likely to have significantly lower scores for concerns related to fatigue (P <.001) and sexual issues/intimacy (P = .001). Respondents with breast cancer were more likely to have significantly higher scores than respondents without breast cancer for concerns related to genetic counseling (P = .001) and fear of developing a new cancer (P = .010).
Fears of the cancer returning and developing a new cancer were the two most prevalent concerns, identified by 51% (n = 486) and 47.5% (n = 459), respectively (Table 2). Physical concerns, rated as moderate/high concerns by at least 25% of the sample, were fatigue (n = 336, 34.8%), changes in [the] body after cancer (n = 323, 33.7%), trouble sleeping (n = 302, 31.0%), sexual issues/intimacy (n = 263, 28.0%), memory and concentration (n = 261, 26.7%), and weight changes (n = 248, 25.5%). The most prevalent moderate/high social concerns were related to finances (n = 265, 27.5%) and debt from medical bills (n = 232, 25.1%). Managing stress (n = 279, 29.2%) and difficult emotions (n = 244, 25.1%) were prevalent moderate/high emotional concerns. Spiritual concerns were less often rated as moderate/high concerns. Having a breast cancer diagnosis was not significantly related to the number of reported moderate to high concerns (P = 1.00).
Variables associated with the 12 most frequent moderate/high concerns are shown in Tables 3 and 4. Age was associated with the most moderate/high concerns. With every decade of age, the odds of having the following moderate/high concerns decreased: bodily changes after cancer (odds ratio [OR], 0.75), sexual intimacy (OR, 0.81), memory and concentration (OR, 0.83), weight changes (OR, 0.77), financial (OR, 0.75), debt (OR, 0.71), cancer returning (OR, 0.66), developing a new cancer (OR, 0.67), managing stress (OR, 0.67), and managing difficult emotions (OR, 0.67).
Female sex was associated with lower odds of having a concern about sexual intimacy (OR, 0.30) and increased odds of having concerns related to memory and concentration (OR, 1.78), managing stress (OR, 2.35), and managing difficult emotions (OR, 1.77). Race was another demographic characteristic statistically associated with numerous moderate/high concerns. Survivors who identified white, were more likely than other people of other races to have fewer moderate/high concerns regarding bodily changes after cancer (OR, 0.46), weight change (OR, 0.46), finances (OR, 0.46), debt (OR, 0.40), managing stress (OR, 0.55), and managing difficult emotions (OR, 0.49). The odds of having a moderate/high concern regarding debt was 2.25 times higher given widowed marital status compared with those survivors who were single. Unemployment status, when compared with full-time employment, was significantly associated with increased odds of having moderate/high concerns related to fatigue (OR, 2.08), bodily changes after cancer (OR, 1.72), memory and concentration (OR, 2.45), weight changes (OR, 2.17), finances (OR, 1.93), developing a new cancer (OR, 1.91), and managing difficult emotions (OR, 1.80).
As expected, respondents who had completed treatment were less likely to have many of the moderate/high concerns as those still undergoing treatment. Survivors who were up to 2 years posttreatment were significantly more likely than those survivors receiving treatment to have fewer moderate/high concerns regarding fatigue (OR, 0.56), sexual intimacy (OR, 0.54), weight change (OR, 0.55), fears of the cancer returning (OR, 0.48), developing a new cancer (OR, 0.35), managing stress (OR, 0.43), and managing difficult emotions (OR, 0.49).
However, those improved odds were not sustained over the cancer trajectory. Compared with survivors who were receiving treatment, survivors who were between 2-5 years posttreatment did not have significantly reduced odds for moderate/high concerns related to fatigue, sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress, and managing difficult emotions. They did have significantly reduced odds for having concerns only related to finances (OR, 0.61) and debt (OR, 0.52).
Long-term survivors, who were beyond 5 years posttreatment, had significantly reduced odds for having moderate/high concerns related to fatigue (OR, 0.45), finances (OR, 0.52), debt (OR, 0.47), and managing difficult emotions (OR, 0.54), compared with survivors receiving treatment. Moderate/high concerns related to sleep, sexual intimacy, body changes, weight changes, memory, fears of the cancer returning, developing a new cancer, managing stress did not have improved odds for these long-term survivors.
Attention to needs
The health care teams were rated highly for their attention to the patients’ physical needs. Most respondents (n = 845, 92.4%) viewed the health care team’s attention their physical needs as important and 763 (77.6%) survivors rated the team’s attention to these needs as excellent. The importance of addressing emotional needs was affirmed by 723 (78.5%) respondents, and although 454 (46.8%) viewed the team’s attention to these needs as excellent, 119 (12.3%) reported that the health care team did not ask about emotional needs. In addition, 566 respondents (60%) viewed having the health care team address their social needs as important, and most (n = 715, 74.2%) rated the team’s attention to social needs as good or excellent. Yet, 162 (16.8%) respondents reported that team did not ask about their social needs. The health care team’s addressing of spiritual needs was viewed as important by 346 (37.5%) respondents and ratings for how well the team attended to spiritual needs were: 148 (15.6%) poor or fair, 204 (21.5%) good, and 150 (15.8%) excellent. However, 448 (47.2%) respondents reported that the health care team did not ask about their spiritual needs.
Discussion
The primary purpose of this project was to prioritize survivors’ most salient physical, social, emotional, and spiritual concerns or needs and to assess the perceived importance of these needs and the extent to which the cancer center staff were attentive to those needs. The overall goal of this assessment was to inform the development of survivorship and supportive care programs by highlighting common concerns, demographic and medical factors associated with specific concerns, and timing of moderate/high level concerns along the cancer trajectory. There were 3 main findings.
First, the results support the need for enhancing supportive care services to meet emotional concerns of survivors beyond the treatment phase. Similar to other studies,8,9 emotional concerns ranked higher than all other concerns in this study with about 50% of the sample rating “fear the cancer will return” and “fear of developing a new cancer” as moderate/high concern. Although the odds of not having these emotional concerns improved up to 2 years posttreatment, these concerns are likely to resurface, as odds for survivors beyond 2 years were not significantly different from those receiving treatment. A recent systematic review reported that fear of cancer recurrence is experienced by about 73% of cancer survivors, with 49% reporting a moderate to high degree.10 It can have a chronic, stable trajectory for some survivors and is strongly associated with higher levels of anxiety, distress, and depression, and less global, emotional/mental, physical, role, social, and cognitive quality of life.10 In this sample, managing stress and difficult emotions were also rated as moderate/high concerns by at least 25% of the sample.
Second, the findings identified patients at risk for cancer-related concerns throughout the cancer trajectory. As demonstrated in other studies, younger age was associated with greater odds of having multiple greater moderate/high concerns.11-13 Unemployment was the second most common demographic factor associated with multiple moderate/high concerns related to physical symptoms, finances and emotions. Similarly, identifying as black, Asian, American Indian/Alaskan Native, or other was also associated with greater odds of having numerous physical, financial, and emotional concerns. Women had greater concerns related to memory, sexual intimacy, coping with difficult emotions, and stress.
Third, the results helped to identify gaps in supportive care at our cancer center. Although spiritual concerns were not prevalent as being moderate/high, they were still viewed by about a third of survivors as being an important area for the health care team to address. Yet, consistent with other need assessments, spiritual concerns in this study were least often addressed by staff.1 Assessment of spiritual care needs, screening for spiritual distress, and providing spiritual care are essential components of a clinician-patient relationship that supports healing.14 The importance of attending to spiritual care needs was underscored by a recent systematic review that found a positive association between overall spiritual well-being and quality of life in patients with cancer, with the meaning/peace factor consistently and positively associated with physical and mental health.15 Another identified gap was the health care team’s lack of attention to the patient’s social needs, which included concerns related to finances and debt from medical bills. In all, 46% of the respondents reported having financial concerns, with the odds of having moderate/high financial concerns being greatest during treatment to 2 years posttreatment. Attention to the financial burden of cancer patients is critical because the magnitude of cancer-related financial concerns is a significant, strong predictor of quality of life and adverse psychological issues such as depression, anxiety, and distress.16,17
There were several program implications based on the results. A periodic audit of the concerns of survivors and their views on how well their needs were being met was a relatively low cost endeavor. Although the findings were consistent with the literature, the results, when shared with administrators and clinicians, were instrumental in effecting change because they represented the concerns of survivors at the cancer center. Another program directive, based on the results, was to extend the routine screening of patients’ needs during treatment to posttreatment survivorship. Patients who are young, unemployed, do not identify as white, and female warrant more thorough assessment of needs and concerns along the cancer trajectory. Integral to these screenings is the need for patient-centered communication, with discussion of how cancer is affecting the different domains of quality of life within the context of the patient’s life. Lastly, the results clearly indicated the need for additional training of health care providers on how to assess and address spiritual well-being in cancer survivors.
There were limitations to this study, including use of a nonvalidated survey and cross-sectional approach that limited our ability to explore how concerns might change over the trajectory. Also, it was not possible to clarify medical information of the respondents, such as cancer stage. Although the response rate of this study was not high, we are confident in the results because of the large sample size and the finding that the large proportion of respondents with breast cancer was not influential. Despite these limitations, this needs assessment of cancer survivors over the trajectory of care provided insight into the scope of their concerns, identified vulnerable groups of survivors, and highlighted gaps in addressing those concerns. A quality- of-life framework for assessing needs assured a comprehensive focus and generated practice changes to strengthen holistic, comprehensive oncology care.
1. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
2. Paterson C, Robertson A, Smith A, Nabi G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur J Oncol Nurs. 2015;19:405-418.
3. Fiszer C, Dolbeault S, Sultan S, Bredart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology. 2014;23:361-374.
4. Maguire R, Kotronoulas G, Simpson M, Paterson C. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol. 2015;136:478-490.
5. Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: A critical review of the literature. Crit Rev Oncol Hematol. 2013;88:102-116.
6. Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449-464.
7. Adler NE, Page EK. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; Institute of Medicine, 2008.
8. Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40:35-42.
9. Swash B, Hulbert-Williams N, Bramwell R. Unmet psychosocial needs in haematological cancer: A systematic review. Support Care Cancer. 2014;22:1131-1141.
10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322.
11. Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: A multi-center, cross-sectional study in Korea. Psychooncology. 2013;22:1073-1080.
12. Pauwels EE, Charlier C, De Bourdeaudhuij I, Lechner L, Van Hoof E. Care needs after primary breast cancer treatment. Survivors’ associated sociodemographic and medical characteristics. Psychooncology. 2013;22:125-132.
13. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
14. Puchalski CM, Blatt B, Kogan M, Butler A. Spirituality and health: The development of a field. Academic Medicine. 2014;89:10-16.
15. Bai M, Lazenby M. A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med. 2015;18:286-298.
16. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332-338.
17. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological wellbeing among individuals living with cancer. Psychooncology. 2013;22:745-755.
1. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
2. Paterson C, Robertson A, Smith A, Nabi G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur J Oncol Nurs. 2015;19:405-418.
3. Fiszer C, Dolbeault S, Sultan S, Bredart A. Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology. 2014;23:361-374.
4. Maguire R, Kotronoulas G, Simpson M, Paterson C. A systematic review of the supportive care needs of women living with and beyond cervical cancer. Gynecol Oncol. 2015;136:478-490.
5. Hall A, Lynagh M, Bryant J, Sanson-Fisher R. Supportive care needs of hematological cancer survivors: A critical review of the literature. Crit Rev Oncol Hematol. 2013;88:102-116.
6. Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449-464.
7. Adler NE, Page EK. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press; Institute of Medicine, 2008.
8. Ness S, Kokal J, Fee-Schroeder K, Novotny P, Satele D, Barton D. Concerns across the survivorship trajectory: results from a survey of cancer survivors. Oncol Nurs Forum. 2013;40:35-42.
9. Swash B, Hulbert-Williams N, Bramwell R. Unmet psychosocial needs in haematological cancer: A systematic review. Support Care Cancer. 2014;22:1131-1141.
10. Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: A systematic review of quantitative studies. J Cancer Surviv. 2013;7:300-322.
11. Choi KH, Park JH, Park JH, Park JS. Psychosocial needs of cancer patients and related factors: A multi-center, cross-sectional study in Korea. Psychooncology. 2013;22:1073-1080.
12. Pauwels EE, Charlier C, De Bourdeaudhuij I, Lechner L, Van Hoof E. Care needs after primary breast cancer treatment. Survivors’ associated sociodemographic and medical characteristics. Psychooncology. 2013;22:125-132.
13. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117-1128.
14. Puchalski CM, Blatt B, Kogan M, Butler A. Spirituality and health: The development of a field. Academic Medicine. 2014;89:10-16.
15. Bai M, Lazenby M. A systematic review of associations between spiritual well-being and quality of life at the scale and factor levels in studies among patients with cancer. J Palliat Med. 2015;18:286-298.
16. Fenn KM, Evans SB, McCorkle R, et al. Impact of financial burden of cancer on survivors’ quality of life. J Oncol Pract. 2014;10:332-338.
17. Sharp L, Carsin AE, Timmons A. Associations between cancer-related financial stress and strain and psychological wellbeing among individuals living with cancer. Psychooncology. 2013;22:745-755.
Bilateral chylothorax in an AIDS patient with newly diagnosed Kaposi sarcoma
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-8 (HHV-8). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremities, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia. The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma.
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDS-related Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-8 (HHV-8). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremities, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia. The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma.
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-8 (HHV-8). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremities, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia. The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma.
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDS-related Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDS-related Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.