User login
Over 20 Years, Pain Is on the Rise
Pain is becoming a fact of life for more and more people, and they are turning to opioids to treat it, according to a survey sponsored by the National Center for Complementary and Integrative Health.
Researchers looked at nearly 2 decades-worth of cumulative data from the Medical Expenditure Panel Survey (MEPS). They found that since 1997/1998, pain prevalence in US adults rose by 25%.
In 1997/1998, about 33% of American adults had at ≤ 1 painful health condition. In 2013/2014, that proportion was 41%. For about 68 million people, moderate-to-severe pain was interfering with normal work activities. And those people were turning more often to strong opioids—eg, fentanyl, morphine, oxycodone—for help. Use of opioids to manage pain more than doubled in just 10 years: from 4.1 million (11.5%) in 2001/2002 to 10.5 million (24.3%) in 2013/2014.
People with severe pain-related interference also were more likely to have had > 4 opioid prescriptions and to have visited a doctor’s office > 6 times for pain compared with those with minimal pain-related interference.
Opioid use peaked between 2005 and 2012, but since 2012, opioid use has slightly declined. The researchers say this ties to a reduction in use of weak opioids and in the number of patients reporting only 1 opioid prescription.
The survey also found some small downward shifts in health care visits. Ambulatory office visits plateaued between 2001/2002 and 2007/2008 and decreased through 2013/2014. The researchers also found small but statistically significant drops in pain-related emergency department visits and overnight hospital stays.
The researchers say their findings suggest more education about the risk/benefit ratio of opioids “appears warranted.”
Pain is becoming a fact of life for more and more people, and they are turning to opioids to treat it, according to a survey sponsored by the National Center for Complementary and Integrative Health.
Researchers looked at nearly 2 decades-worth of cumulative data from the Medical Expenditure Panel Survey (MEPS). They found that since 1997/1998, pain prevalence in US adults rose by 25%.
In 1997/1998, about 33% of American adults had at ≤ 1 painful health condition. In 2013/2014, that proportion was 41%. For about 68 million people, moderate-to-severe pain was interfering with normal work activities. And those people were turning more often to strong opioids—eg, fentanyl, morphine, oxycodone—for help. Use of opioids to manage pain more than doubled in just 10 years: from 4.1 million (11.5%) in 2001/2002 to 10.5 million (24.3%) in 2013/2014.
People with severe pain-related interference also were more likely to have had > 4 opioid prescriptions and to have visited a doctor’s office > 6 times for pain compared with those with minimal pain-related interference.
Opioid use peaked between 2005 and 2012, but since 2012, opioid use has slightly declined. The researchers say this ties to a reduction in use of weak opioids and in the number of patients reporting only 1 opioid prescription.
The survey also found some small downward shifts in health care visits. Ambulatory office visits plateaued between 2001/2002 and 2007/2008 and decreased through 2013/2014. The researchers also found small but statistically significant drops in pain-related emergency department visits and overnight hospital stays.
The researchers say their findings suggest more education about the risk/benefit ratio of opioids “appears warranted.”
Pain is becoming a fact of life for more and more people, and they are turning to opioids to treat it, according to a survey sponsored by the National Center for Complementary and Integrative Health.
Researchers looked at nearly 2 decades-worth of cumulative data from the Medical Expenditure Panel Survey (MEPS). They found that since 1997/1998, pain prevalence in US adults rose by 25%.
In 1997/1998, about 33% of American adults had at ≤ 1 painful health condition. In 2013/2014, that proportion was 41%. For about 68 million people, moderate-to-severe pain was interfering with normal work activities. And those people were turning more often to strong opioids—eg, fentanyl, morphine, oxycodone—for help. Use of opioids to manage pain more than doubled in just 10 years: from 4.1 million (11.5%) in 2001/2002 to 10.5 million (24.3%) in 2013/2014.
People with severe pain-related interference also were more likely to have had > 4 opioid prescriptions and to have visited a doctor’s office > 6 times for pain compared with those with minimal pain-related interference.
Opioid use peaked between 2005 and 2012, but since 2012, opioid use has slightly declined. The researchers say this ties to a reduction in use of weak opioids and in the number of patients reporting only 1 opioid prescription.
The survey also found some small downward shifts in health care visits. Ambulatory office visits plateaued between 2001/2002 and 2007/2008 and decreased through 2013/2014. The researchers also found small but statistically significant drops in pain-related emergency department visits and overnight hospital stays.
The researchers say their findings suggest more education about the risk/benefit ratio of opioids “appears warranted.”
Criteria-based fibromyalgia diagnosis and rheumatologists’ clinical diagnosis often disagree
particularly the most recent revision of criteria from 2011 that rely on patients’ self-report of symptoms.
Frederick Wolfe, MD, of the National Data Bank for Rheumatic Diseases and the University of Kansas, Wichita, and his colleagues reported in Arthritis Care & Research that clinicians failed to identify almost half of cases that met the 2011 modification of the American College of Rheumatology’s self-report criteria for fibromyalgia during a 3-month period at Rush Medical College, Chicago. This led them to conclude that the “overall agreement between clinicians’ diagnosis of fibromyalgia and diagnosis by fibromyalgia criteria is only fair.”
The findings call into question studies of fibromyalgia based on ICD-10 diagnosis, according to the authors.
Several widely accepted criteria sets have provided definitions and methods of diagnosis, but a number of studies have suggested that fibromyalgia is overdiagnosed, the authors wrote.
“In the current study, we consider underdiagnosis as well as the form of overdiagnosis that lead to diagnosis when fibromyalgia criteria are not satisfied and symptoms are insufficient for diagnosis,” they added.
The research included 497 consecutive patients attending the Rush Medical College rheumatology clinic who completed a questionnaire assessing fibromyalgia diagnostic variables used in the ACR 2010 preliminary diagnostic criteria for fibromyalgia and its 2011 modification for self-report immediately prior to being seen at the clinic. Clinicians were not given instructions on how any diseases were to be diagnosed, including no instructions on using 2011 fibromyalgia criteria.
The results showed that 121 (24.3%) of the patients satisfied the 2011 fibromyalgia criteria. The agreement between clinicians and criteria was 79.2%, but agreement beyond chance was considered “fair” based on a kappa score of 0.41 and a probabilistic benchmark interval of 0.21-0.40. The researchers wrote that this benchmark represents “the probability for each coefficient of falling into the selected benchmark interval along with the cumulative probability of exceeding the predetermined threshold associated with the interval.”
A total of 104 (20.9%) received a clinician ICD-10 diagnosis of fibromyalgia, but only 61 (58.7%) actually satisfied criteria. Physicians failed to identify 60 (49.6%) criteria-positive patients and incorrectly identified 43 (11.4%) criteria-negative patients.
In a subset of 88 patients with RA, agreement was 84.1%, and the kappa score was 0.32, indicating “slight to fair” agreement (probabilistic benchmark interval, 0.00-0.20). Among 13 RA patients with criteria-positive fibromyalgia, 5 were identified by clinicians; among those who were criteria negative, 6 were deemed positive by clinicians.
The authors noted than “even worse” results were obtained in patients with systemic lupus erythematosus, where the agreement between criteria and clinician ICD diagnosis yielded a kappa value of 0.08. In patients with osteoarthritis, the kappa score was 0.51 (probabilistic benchmark interval, 0.21-0.40).
Overall, the results showed that women and patients with more symptoms but fewer pain areas were more likely to receive a clinician’s diagnosis than to satisfy fibromyalgia criteria. “Clinicians gave greater weight in making a diagnosis to being a woman and having increased symptoms and were willing to diagnose patients with lower WPI [Widespread Pain Index] and PSD [polysymptomatic distress] scores,” the researchers noted.
Dr. Wolfe and his associates wrote that it is unclear why the physicians in the study who misclassified fibromyalgia and missed patients with the diagnosis did not do better. “They may have simply misdiagnosed the condition or not accepted the use of the formal diagnostic system as necessary or clinically useful for identifying fibromyalgia in routine care.”
The researchers noted that it is likely that diagnosis in the community by general physicians, for whom the criteria are not as well known, is even more inaccurate. “It is likely that misdiagnosis is a public health problem and one that can lead to overdiagnosis and overtreatment as well as to inappropriate treatment of individuals not recognized to have fibromyalgia symptoms.”
No disclosures or funding were reported.
SOURCE: Wolfe F et al. Arthritis Care Res. 2019 Feb 6. doi: 10.1002/acr.23731.
Despite voicing concern regarding the “self-report nature of fibromyalgia,” Wolfe et al. selected the 2011 self-report fibromyalgia criteria as their “gold standard” for fibromyalgia diagnosis. Their findings therefore should not be surprising.
The authors’ conclusion that expert physicians often misdiagnose fibromyalgia implies that published criteria are superior to expert clinical judgment for individual patient diagnosis.
However, this view fails to take into account the myriad variables seen by the clinician in the clinic. Until biomarkers or genetic testing allows for more objective disease markers, common conditions like fibromyalgia will continue to be symptom-based diagnoses. Therefore, the gold standard diagnostic criteria for fibromyalgia and other common illnesses is expert opinion.
Rheumatologists are the go-to experts for the diagnosis of fibromyalgia, whether or not we readily accept that role.
These comments are adapted from an accompanying editorial by Don Goldenberg, MD, of Oregon Health & Science University, Portland, and Tufts University, Boston (Arthritis Care Res. 2019 Feb 6. doi: 10.1002/acr.23727).
Despite voicing concern regarding the “self-report nature of fibromyalgia,” Wolfe et al. selected the 2011 self-report fibromyalgia criteria as their “gold standard” for fibromyalgia diagnosis. Their findings therefore should not be surprising.
The authors’ conclusion that expert physicians often misdiagnose fibromyalgia implies that published criteria are superior to expert clinical judgment for individual patient diagnosis.
However, this view fails to take into account the myriad variables seen by the clinician in the clinic. Until biomarkers or genetic testing allows for more objective disease markers, common conditions like fibromyalgia will continue to be symptom-based diagnoses. Therefore, the gold standard diagnostic criteria for fibromyalgia and other common illnesses is expert opinion.
Rheumatologists are the go-to experts for the diagnosis of fibromyalgia, whether or not we readily accept that role.
These comments are adapted from an accompanying editorial by Don Goldenberg, MD, of Oregon Health & Science University, Portland, and Tufts University, Boston (Arthritis Care Res. 2019 Feb 6. doi: 10.1002/acr.23727).
Despite voicing concern regarding the “self-report nature of fibromyalgia,” Wolfe et al. selected the 2011 self-report fibromyalgia criteria as their “gold standard” for fibromyalgia diagnosis. Their findings therefore should not be surprising.
The authors’ conclusion that expert physicians often misdiagnose fibromyalgia implies that published criteria are superior to expert clinical judgment for individual patient diagnosis.
However, this view fails to take into account the myriad variables seen by the clinician in the clinic. Until biomarkers or genetic testing allows for more objective disease markers, common conditions like fibromyalgia will continue to be symptom-based diagnoses. Therefore, the gold standard diagnostic criteria for fibromyalgia and other common illnesses is expert opinion.
Rheumatologists are the go-to experts for the diagnosis of fibromyalgia, whether or not we readily accept that role.
These comments are adapted from an accompanying editorial by Don Goldenberg, MD, of Oregon Health & Science University, Portland, and Tufts University, Boston (Arthritis Care Res. 2019 Feb 6. doi: 10.1002/acr.23727).
particularly the most recent revision of criteria from 2011 that rely on patients’ self-report of symptoms.
Frederick Wolfe, MD, of the National Data Bank for Rheumatic Diseases and the University of Kansas, Wichita, and his colleagues reported in Arthritis Care & Research that clinicians failed to identify almost half of cases that met the 2011 modification of the American College of Rheumatology’s self-report criteria for fibromyalgia during a 3-month period at Rush Medical College, Chicago. This led them to conclude that the “overall agreement between clinicians’ diagnosis of fibromyalgia and diagnosis by fibromyalgia criteria is only fair.”
The findings call into question studies of fibromyalgia based on ICD-10 diagnosis, according to the authors.
Several widely accepted criteria sets have provided definitions and methods of diagnosis, but a number of studies have suggested that fibromyalgia is overdiagnosed, the authors wrote.
“In the current study, we consider underdiagnosis as well as the form of overdiagnosis that lead to diagnosis when fibromyalgia criteria are not satisfied and symptoms are insufficient for diagnosis,” they added.
The research included 497 consecutive patients attending the Rush Medical College rheumatology clinic who completed a questionnaire assessing fibromyalgia diagnostic variables used in the ACR 2010 preliminary diagnostic criteria for fibromyalgia and its 2011 modification for self-report immediately prior to being seen at the clinic. Clinicians were not given instructions on how any diseases were to be diagnosed, including no instructions on using 2011 fibromyalgia criteria.
The results showed that 121 (24.3%) of the patients satisfied the 2011 fibromyalgia criteria. The agreement between clinicians and criteria was 79.2%, but agreement beyond chance was considered “fair” based on a kappa score of 0.41 and a probabilistic benchmark interval of 0.21-0.40. The researchers wrote that this benchmark represents “the probability for each coefficient of falling into the selected benchmark interval along with the cumulative probability of exceeding the predetermined threshold associated with the interval.”
A total of 104 (20.9%) received a clinician ICD-10 diagnosis of fibromyalgia, but only 61 (58.7%) actually satisfied criteria. Physicians failed to identify 60 (49.6%) criteria-positive patients and incorrectly identified 43 (11.4%) criteria-negative patients.
In a subset of 88 patients with RA, agreement was 84.1%, and the kappa score was 0.32, indicating “slight to fair” agreement (probabilistic benchmark interval, 0.00-0.20). Among 13 RA patients with criteria-positive fibromyalgia, 5 were identified by clinicians; among those who were criteria negative, 6 were deemed positive by clinicians.
The authors noted than “even worse” results were obtained in patients with systemic lupus erythematosus, where the agreement between criteria and clinician ICD diagnosis yielded a kappa value of 0.08. In patients with osteoarthritis, the kappa score was 0.51 (probabilistic benchmark interval, 0.21-0.40).
Overall, the results showed that women and patients with more symptoms but fewer pain areas were more likely to receive a clinician’s diagnosis than to satisfy fibromyalgia criteria. “Clinicians gave greater weight in making a diagnosis to being a woman and having increased symptoms and were willing to diagnose patients with lower WPI [Widespread Pain Index] and PSD [polysymptomatic distress] scores,” the researchers noted.
Dr. Wolfe and his associates wrote that it is unclear why the physicians in the study who misclassified fibromyalgia and missed patients with the diagnosis did not do better. “They may have simply misdiagnosed the condition or not accepted the use of the formal diagnostic system as necessary or clinically useful for identifying fibromyalgia in routine care.”
The researchers noted that it is likely that diagnosis in the community by general physicians, for whom the criteria are not as well known, is even more inaccurate. “It is likely that misdiagnosis is a public health problem and one that can lead to overdiagnosis and overtreatment as well as to inappropriate treatment of individuals not recognized to have fibromyalgia symptoms.”
No disclosures or funding were reported.
SOURCE: Wolfe F et al. Arthritis Care Res. 2019 Feb 6. doi: 10.1002/acr.23731.
particularly the most recent revision of criteria from 2011 that rely on patients’ self-report of symptoms.
Frederick Wolfe, MD, of the National Data Bank for Rheumatic Diseases and the University of Kansas, Wichita, and his colleagues reported in Arthritis Care & Research that clinicians failed to identify almost half of cases that met the 2011 modification of the American College of Rheumatology’s self-report criteria for fibromyalgia during a 3-month period at Rush Medical College, Chicago. This led them to conclude that the “overall agreement between clinicians’ diagnosis of fibromyalgia and diagnosis by fibromyalgia criteria is only fair.”
The findings call into question studies of fibromyalgia based on ICD-10 diagnosis, according to the authors.
Several widely accepted criteria sets have provided definitions and methods of diagnosis, but a number of studies have suggested that fibromyalgia is overdiagnosed, the authors wrote.
“In the current study, we consider underdiagnosis as well as the form of overdiagnosis that lead to diagnosis when fibromyalgia criteria are not satisfied and symptoms are insufficient for diagnosis,” they added.
The research included 497 consecutive patients attending the Rush Medical College rheumatology clinic who completed a questionnaire assessing fibromyalgia diagnostic variables used in the ACR 2010 preliminary diagnostic criteria for fibromyalgia and its 2011 modification for self-report immediately prior to being seen at the clinic. Clinicians were not given instructions on how any diseases were to be diagnosed, including no instructions on using 2011 fibromyalgia criteria.
The results showed that 121 (24.3%) of the patients satisfied the 2011 fibromyalgia criteria. The agreement between clinicians and criteria was 79.2%, but agreement beyond chance was considered “fair” based on a kappa score of 0.41 and a probabilistic benchmark interval of 0.21-0.40. The researchers wrote that this benchmark represents “the probability for each coefficient of falling into the selected benchmark interval along with the cumulative probability of exceeding the predetermined threshold associated with the interval.”
A total of 104 (20.9%) received a clinician ICD-10 diagnosis of fibromyalgia, but only 61 (58.7%) actually satisfied criteria. Physicians failed to identify 60 (49.6%) criteria-positive patients and incorrectly identified 43 (11.4%) criteria-negative patients.
In a subset of 88 patients with RA, agreement was 84.1%, and the kappa score was 0.32, indicating “slight to fair” agreement (probabilistic benchmark interval, 0.00-0.20). Among 13 RA patients with criteria-positive fibromyalgia, 5 were identified by clinicians; among those who were criteria negative, 6 were deemed positive by clinicians.
The authors noted than “even worse” results were obtained in patients with systemic lupus erythematosus, where the agreement between criteria and clinician ICD diagnosis yielded a kappa value of 0.08. In patients with osteoarthritis, the kappa score was 0.51 (probabilistic benchmark interval, 0.21-0.40).
Overall, the results showed that women and patients with more symptoms but fewer pain areas were more likely to receive a clinician’s diagnosis than to satisfy fibromyalgia criteria. “Clinicians gave greater weight in making a diagnosis to being a woman and having increased symptoms and were willing to diagnose patients with lower WPI [Widespread Pain Index] and PSD [polysymptomatic distress] scores,” the researchers noted.
Dr. Wolfe and his associates wrote that it is unclear why the physicians in the study who misclassified fibromyalgia and missed patients with the diagnosis did not do better. “They may have simply misdiagnosed the condition or not accepted the use of the formal diagnostic system as necessary or clinically useful for identifying fibromyalgia in routine care.”
The researchers noted that it is likely that diagnosis in the community by general physicians, for whom the criteria are not as well known, is even more inaccurate. “It is likely that misdiagnosis is a public health problem and one that can lead to overdiagnosis and overtreatment as well as to inappropriate treatment of individuals not recognized to have fibromyalgia symptoms.”
No disclosures or funding were reported.
SOURCE: Wolfe F et al. Arthritis Care Res. 2019 Feb 6. doi: 10.1002/acr.23731.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Clinicians failed to identify nearly 50% of criteria-positive cases
Major finding: Physicians failed to identify 60 (49.6%) criteria-positive patients and incorrectly identified 43 (11.4%) criteria-negative patients.
Study details: A group of 497 consecutive unselected rheumatology clinic attendees who completed a questionnaire assessing fibromyalgia diagnostic variables used in the American College of Rheumatology 2010 preliminary diagnostic criteria for fibromyalgia and its 2011 modification for self-report.
Disclosures: No disclosures or funding were reported.
Source: Wolfe F et al. Arthritis Care Res. 2019 Feb 6. doi: 10.1002/acr.23731.
International survey probes oxygen’s efficacy for cluster headache
According to the results, triptans also are highly effective, with some side effects. Newer medications deserve further study, the researchers said.
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, Stuart M. Pearson, a researcher in the department of psychology at the University of West Georgia in Carrollton, and his coauthors analyzed data from the Cluster Headache Questionnaire. Respondents from more than 50 countries completed the online survey; most were from the United States, the United Kingdom, and Canada. The survey included questions about cluster headache diagnostic criteria and medication effectiveness, complications, and access to medications.
In all, 3,251 subjects participated in the questionnaire, and 2,193 respondents met criteria for the study; 1,604 had cluster headache, and 589 had probable cluster headache. Among the respondents with cluster headache, 68.8% were male, 78.0% had episodic cluster headache, and the average age was 46 years. More than half of respondents reported complete or very effective treatment for triptans (54%) and oxygen (also 54%). The proportion of respondents who reported that ergot derivatives, caffeine or energy drinks, and intranasal ketamine were completely or very effective ranged from 14% to 25%. Patients were less likely to report high levels of efficacy for opioids (6%), intranasal capsaicin (5%), and intranasal lidocaine (2%).
Participants experienced few complications from oxygen, with 99% reporting no or minimal physical and medical complications, and 97% reporting no or minimal psychological and emotional complications. Patients also reported few complications from intranasal lidocaine, intranasal ketamine, intranasal capsaicin, and caffeine and energy drinks. For triptans, 74% of respondents reported no or minimal physical and medical complications, and 85% reported no or minimal psychological and emotional complications.
Among the 139 participants with cluster headache who were aged 65 years or older, responses were similar to those for the entire population. In addition, the 589 respondents with probable cluster headache reported similar efficacy data, compared with respondents with a full diagnosis of cluster headache.
“Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects,” the investigators said. “However, respondents reported that it was difficult to obtain.”
Limited insurance coverage of oxygen may affect access, even though the treatment has a Level A recommendation for the acute treatment of cluster headache in the American Headache Society guidelines, the authors said. Physicians also may pose a barrier. A prior study found that 12% of providers did not prescribe oxygen for cluster headache because they doubted its efficacy or did not know about it. In addition, there may be concerns that the treatment could be a fire hazard in a patient population that has high rates of smoking, the researchers said.
Limitations of the study include the survey’s use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of all triptans, rather than assessing individual triptan medications, such as sumatriptan subcutaneous, alone.
The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
This article was updated 3/7/2019.
SOURCE: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
According to the results, triptans also are highly effective, with some side effects. Newer medications deserve further study, the researchers said.
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, Stuart M. Pearson, a researcher in the department of psychology at the University of West Georgia in Carrollton, and his coauthors analyzed data from the Cluster Headache Questionnaire. Respondents from more than 50 countries completed the online survey; most were from the United States, the United Kingdom, and Canada. The survey included questions about cluster headache diagnostic criteria and medication effectiveness, complications, and access to medications.
In all, 3,251 subjects participated in the questionnaire, and 2,193 respondents met criteria for the study; 1,604 had cluster headache, and 589 had probable cluster headache. Among the respondents with cluster headache, 68.8% were male, 78.0% had episodic cluster headache, and the average age was 46 years. More than half of respondents reported complete or very effective treatment for triptans (54%) and oxygen (also 54%). The proportion of respondents who reported that ergot derivatives, caffeine or energy drinks, and intranasal ketamine were completely or very effective ranged from 14% to 25%. Patients were less likely to report high levels of efficacy for opioids (6%), intranasal capsaicin (5%), and intranasal lidocaine (2%).
Participants experienced few complications from oxygen, with 99% reporting no or minimal physical and medical complications, and 97% reporting no or minimal psychological and emotional complications. Patients also reported few complications from intranasal lidocaine, intranasal ketamine, intranasal capsaicin, and caffeine and energy drinks. For triptans, 74% of respondents reported no or minimal physical and medical complications, and 85% reported no or minimal psychological and emotional complications.
Among the 139 participants with cluster headache who were aged 65 years or older, responses were similar to those for the entire population. In addition, the 589 respondents with probable cluster headache reported similar efficacy data, compared with respondents with a full diagnosis of cluster headache.
“Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects,” the investigators said. “However, respondents reported that it was difficult to obtain.”
Limited insurance coverage of oxygen may affect access, even though the treatment has a Level A recommendation for the acute treatment of cluster headache in the American Headache Society guidelines, the authors said. Physicians also may pose a barrier. A prior study found that 12% of providers did not prescribe oxygen for cluster headache because they doubted its efficacy or did not know about it. In addition, there may be concerns that the treatment could be a fire hazard in a patient population that has high rates of smoking, the researchers said.
Limitations of the study include the survey’s use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of all triptans, rather than assessing individual triptan medications, such as sumatriptan subcutaneous, alone.
The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
This article was updated 3/7/2019.
SOURCE: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
According to the results, triptans also are highly effective, with some side effects. Newer medications deserve further study, the researchers said.
To assess the effectiveness and adverse effects of acute cluster headache medications in a large international sample, Stuart M. Pearson, a researcher in the department of psychology at the University of West Georgia in Carrollton, and his coauthors analyzed data from the Cluster Headache Questionnaire. Respondents from more than 50 countries completed the online survey; most were from the United States, the United Kingdom, and Canada. The survey included questions about cluster headache diagnostic criteria and medication effectiveness, complications, and access to medications.
In all, 3,251 subjects participated in the questionnaire, and 2,193 respondents met criteria for the study; 1,604 had cluster headache, and 589 had probable cluster headache. Among the respondents with cluster headache, 68.8% were male, 78.0% had episodic cluster headache, and the average age was 46 years. More than half of respondents reported complete or very effective treatment for triptans (54%) and oxygen (also 54%). The proportion of respondents who reported that ergot derivatives, caffeine or energy drinks, and intranasal ketamine were completely or very effective ranged from 14% to 25%. Patients were less likely to report high levels of efficacy for opioids (6%), intranasal capsaicin (5%), and intranasal lidocaine (2%).
Participants experienced few complications from oxygen, with 99% reporting no or minimal physical and medical complications, and 97% reporting no or minimal psychological and emotional complications. Patients also reported few complications from intranasal lidocaine, intranasal ketamine, intranasal capsaicin, and caffeine and energy drinks. For triptans, 74% of respondents reported no or minimal physical and medical complications, and 85% reported no or minimal psychological and emotional complications.
Among the 139 participants with cluster headache who were aged 65 years or older, responses were similar to those for the entire population. In addition, the 589 respondents with probable cluster headache reported similar efficacy data, compared with respondents with a full diagnosis of cluster headache.
“Oxygen in particular had a high rate of complete effectiveness, a low rate of ineffectiveness, and a low rate of physical, medical, emotional, and psychological side effects,” the investigators said. “However, respondents reported that it was difficult to obtain.”
Limited insurance coverage of oxygen may affect access, even though the treatment has a Level A recommendation for the acute treatment of cluster headache in the American Headache Society guidelines, the authors said. Physicians also may pose a barrier. A prior study found that 12% of providers did not prescribe oxygen for cluster headache because they doubted its efficacy or did not know about it. In addition, there may be concerns that the treatment could be a fire hazard in a patient population that has high rates of smoking, the researchers said.
Limitations of the study include the survey’s use of nonvalidated questions, the lack of a formal clinical diagnosis of cluster headache, and the grouping of all triptans, rather than assessing individual triptan medications, such as sumatriptan subcutaneous, alone.
The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
This article was updated 3/7/2019.
SOURCE: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
FROM HEADACHE
Key clinical point: Oxygen is a highly effective treatment for cluster headache with few complications.
Major finding: More than half of respondents (54%) reported that triptans and oxygen were completely or very effective.
Study details: Analysis of data from 1,604 people with cluster headache who completed the online Cluster Headache Questionnaire.
Disclosures: The study received funding from Autonomic Technologies and Clusterbusters. One of the authors has served as a paid consultant to Eli Lilly as a member of the data monitoring committee for clinical trials of galcanezumab for cluster headache and migraine.
Source: Pearson SM et al. Headache. 2019 Jan 11. doi: 10.1111/head.13473.
In California, opioids most often prescribed in low-income, mostly white areas
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The most common users of opioids according to prescription drug records are residents of mostly low-income, white neighborhoods.
Major finding: Compared with 23.6% of all Californians, 44.2% of individuals in zip codes containing mostly low-income, white residents had at least one opioid prescription each year, compared with 16.1% of individuals in high-income zip codes with the lowest population of white residents.
Study details: An analysis of 29.7 million opioid prescription drug records by race and income in California during 2011-2015.
Disclosures: Dr. Schriger reported support from the Korein Foundation for his time working on the study by Friedman et al. The other authors from Friedman et al. reported no conflicts of interest.
The Underrecognized Risk for Drug Overdose Deaths
The numbers are stunning: 1,643% increase in rates of deaths involving synthetic opioids. A 915% increase for heroin, 830% for benzodiazepines. Even more stunning: Those are the increases only in overdose death rates for women aged 30 to 64 years.
According to CDC data, between 1999 and 2010, the largest percentage change in the rates of overall drug overdose deaths was among women aged between 45 and 64 years. But that research did not account for trends in specific drugs or consider changes in age group distributions, say researchers from the CDC’s National Center for Injury Prevention and Control.
They examined overdose death rates among women aged 30 to 64 years between 1999 and 2017. The unadjusted death rate jumped 260%, from 4,314 deaths to 18,110 deaths. Among women aged 55 to 59 years, the number of deaths involving antidepressants increased approximately 300%; among women aged 60 to 64 years, nearly 400%. The crude rate of deaths involving prescription opioids skyrocketed > 1,000%.
The drug epidemic is “evolving,” the researchers note. In 1999, overdose death rates were highest among women aged 40 to 44 years. In 2017, they were highest among women aged 50 to 54 years. And as demographics shift, prevention programs need to shift as well. As women age, the researchers say, individual experiences can change the type of substance used or misused and in the experiences of pain that might result in an opioid prescription.
The researchers note that “substantial work” has focused on informing women of childbearing age about the risks and benefits of certain drugs. The current analysis demonstrates “the remaining need” to consider middle-aged women who are at risk.
Targeted efforts are needed, and the researchers suggest interventions: Medicaid and other health insurance programs can review records of controlled substance prescribing. States and local communities can expand capacity of drug use disorder treatments and links to care, particularly adding “gender-responsive” substance use disorder treatment centers.
A “multifaceted approach involving the full spectrum of care services is likely necessary,” the researchers say. Health care practitioners who treat women for pain, depression, or anxiety can discuss treatment options that consider the unique biopsychosocial needs of women.
Health care practitioners also can consider implementing the CDC Guideline for Prescribing Opioids for Chronic Pain, which says “Opioids are not first-line or routine therapy for chronic pain.” The guideline also says before starting and periodically during opioid therapy, clinicians should discuss with patients the “known risks and realistic benefits of opioid therapy.” In other words, listen to the women and prescribe carefully.
The numbers are stunning: 1,643% increase in rates of deaths involving synthetic opioids. A 915% increase for heroin, 830% for benzodiazepines. Even more stunning: Those are the increases only in overdose death rates for women aged 30 to 64 years.
According to CDC data, between 1999 and 2010, the largest percentage change in the rates of overall drug overdose deaths was among women aged between 45 and 64 years. But that research did not account for trends in specific drugs or consider changes in age group distributions, say researchers from the CDC’s National Center for Injury Prevention and Control.
They examined overdose death rates among women aged 30 to 64 years between 1999 and 2017. The unadjusted death rate jumped 260%, from 4,314 deaths to 18,110 deaths. Among women aged 55 to 59 years, the number of deaths involving antidepressants increased approximately 300%; among women aged 60 to 64 years, nearly 400%. The crude rate of deaths involving prescription opioids skyrocketed > 1,000%.
The drug epidemic is “evolving,” the researchers note. In 1999, overdose death rates were highest among women aged 40 to 44 years. In 2017, they were highest among women aged 50 to 54 years. And as demographics shift, prevention programs need to shift as well. As women age, the researchers say, individual experiences can change the type of substance used or misused and in the experiences of pain that might result in an opioid prescription.
The researchers note that “substantial work” has focused on informing women of childbearing age about the risks and benefits of certain drugs. The current analysis demonstrates “the remaining need” to consider middle-aged women who are at risk.
Targeted efforts are needed, and the researchers suggest interventions: Medicaid and other health insurance programs can review records of controlled substance prescribing. States and local communities can expand capacity of drug use disorder treatments and links to care, particularly adding “gender-responsive” substance use disorder treatment centers.
A “multifaceted approach involving the full spectrum of care services is likely necessary,” the researchers say. Health care practitioners who treat women for pain, depression, or anxiety can discuss treatment options that consider the unique biopsychosocial needs of women.
Health care practitioners also can consider implementing the CDC Guideline for Prescribing Opioids for Chronic Pain, which says “Opioids are not first-line or routine therapy for chronic pain.” The guideline also says before starting and periodically during opioid therapy, clinicians should discuss with patients the “known risks and realistic benefits of opioid therapy.” In other words, listen to the women and prescribe carefully.
The numbers are stunning: 1,643% increase in rates of deaths involving synthetic opioids. A 915% increase for heroin, 830% for benzodiazepines. Even more stunning: Those are the increases only in overdose death rates for women aged 30 to 64 years.
According to CDC data, between 1999 and 2010, the largest percentage change in the rates of overall drug overdose deaths was among women aged between 45 and 64 years. But that research did not account for trends in specific drugs or consider changes in age group distributions, say researchers from the CDC’s National Center for Injury Prevention and Control.
They examined overdose death rates among women aged 30 to 64 years between 1999 and 2017. The unadjusted death rate jumped 260%, from 4,314 deaths to 18,110 deaths. Among women aged 55 to 59 years, the number of deaths involving antidepressants increased approximately 300%; among women aged 60 to 64 years, nearly 400%. The crude rate of deaths involving prescription opioids skyrocketed > 1,000%.
The drug epidemic is “evolving,” the researchers note. In 1999, overdose death rates were highest among women aged 40 to 44 years. In 2017, they were highest among women aged 50 to 54 years. And as demographics shift, prevention programs need to shift as well. As women age, the researchers say, individual experiences can change the type of substance used or misused and in the experiences of pain that might result in an opioid prescription.
The researchers note that “substantial work” has focused on informing women of childbearing age about the risks and benefits of certain drugs. The current analysis demonstrates “the remaining need” to consider middle-aged women who are at risk.
Targeted efforts are needed, and the researchers suggest interventions: Medicaid and other health insurance programs can review records of controlled substance prescribing. States and local communities can expand capacity of drug use disorder treatments and links to care, particularly adding “gender-responsive” substance use disorder treatment centers.
A “multifaceted approach involving the full spectrum of care services is likely necessary,” the researchers say. Health care practitioners who treat women for pain, depression, or anxiety can discuss treatment options that consider the unique biopsychosocial needs of women.
Health care practitioners also can consider implementing the CDC Guideline for Prescribing Opioids for Chronic Pain, which says “Opioids are not first-line or routine therapy for chronic pain.” The guideline also says before starting and periodically during opioid therapy, clinicians should discuss with patients the “known risks and realistic benefits of opioid therapy.” In other words, listen to the women and prescribe carefully.
Cloud of inconsistency hangs over cannabis data
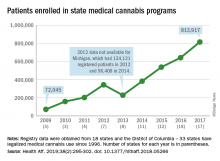
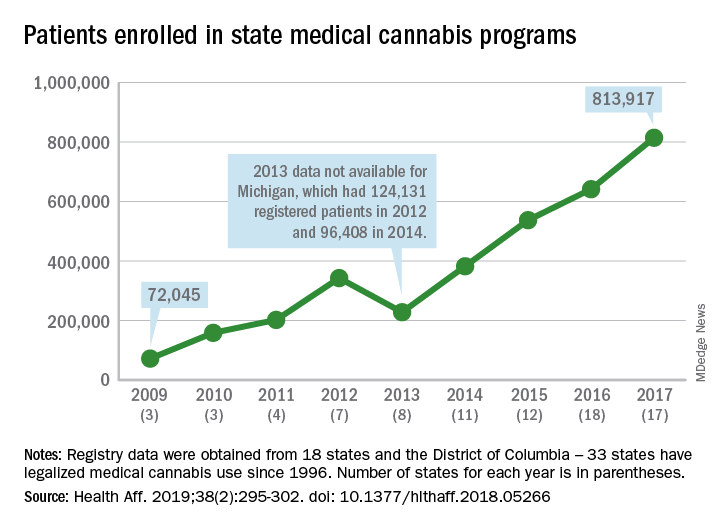
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
More people are using medical cannabis as it becomes legal in more states, but the lack of standardization in states’ data collection hindered investigators’ efforts to track that use.
Legalized medical cannabis is now available in 33 states and the District of Columbia, and the number of users has risen from just over 72,000 in 2009 to almost 814,000 in 2017. That 814,000, however, covers only 16 states and D.C., since 1 state (Connecticut) does not publish reports on medical cannabis use, 12 did not have statistics available, 2 (New York and Vermont) didn’t report data for 2017, and 2 (California and Maine) have voluntary registries that are unlikely to be accurate, according to Kevin F. Boehnke, PhD, of the University of Michigan, Ann Arbor, and his associates.
Michigan had the largest reported number of patients enrolled in its medical cannabis program in 2017, almost 270,000. California – the state with the oldest medical cannabis legislation (passed in 1996) and the largest overall population but a voluntary cannabis registry – reported its highest number of enrollees, 12,659, in 2009-2010, the investigators said. Colorado had more than 116,000 patients in its medical cannabis program in 2010 (Health Aff. 2019;38[2]:295-302).
The “many inconsistencies in data quality across states [suggest] the need for further standardization of data collection. Such standardization would add transparency to understanding how medical cannabis programs are used, which would help guide both research and policy needs,” Dr. Boehnke and his associates wrote.
More consistency was seen in the reasons for using medical cannabis. Chronic pain made up 62.2% of all qualifying conditions reported by patients during 1999-2016, with the annual average varying between 33.3% and 73%. Multiple sclerosis spasticity symptoms had the second-highest number of reports over the study period, followed by chemotherapy-induced nausea and vomiting, posttraumatic stress disorder, and cancer, they reported.
The investigators also looked at the appropriateness of cannabis and determined that its use in 85.5% of patient-reported conditions was “supported by conclusive or substantial evidence of therapeutic effectiveness, according to the 2017 National Academies report” on the health effects of cannabis.
“We believe not only that it is inappropriate for cannabis to remain a Schedule I substance, but also that state and federal policy makers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the health care system,” they concluded.
SOURCE: Boehnke KF et al. Health Aff. 2019;38(2):295-302.
FROM HEALTH AFFAIRS
Psoriatic arthritis eludes early diagnosis
Most patients with psoriatic arthritis first present with psoriasis only. Their skin disorder precedes any joint involvement, often by several years. That suggests targeting interventions to patients with psoriasis to prevent or slow their progression to psoriatic arthritis, as well as following psoriatic patients closely to diagnose psoriatic arthritis quickly when it first appears. It’s a simple and attractive management premise that’s been challenging to apply in practice.
It’s not that clinicians aren’t motivated to diagnose psoriatic arthritis (PsA) in patients early, hopefully as soon as it appears. The susceptibility of patients with psoriasis to develop PsA is well described, with an annual progression rate of about 3%, and adverse consequences result from even a 6-month delay in diagnosis.
“Some physicians still don’t ask psoriasis patients about joint pain, or their symptoms are misinterpreted as something else,” said Lihi Eder, MD, a rheumatologist at Women’s College Research Institute, Toronto, and the University of Toronto. “Although there is increased awareness about PsA, there are still delays in diagnosis,” she said in an interview.
“Often there is a massive delay in diagnosis, and we know from a number of studies that longer duration of symptoms before diagnosis is associated with poorer outcomes,” said Laura C. Coates, MBChB, PhD, a rheumatologist at the University of Oxford (England). The delay to PsA diagnosis is generally “longer than for equivalent rheumatoid arthritis patients. PsA patients take longer to ask a primary care physician for help, longer to get a referral to a rheumatologist, and longer to get a diagnosis” from a rheumatologist. “We need to educate patients with psoriasis about their risk so that they seek help, educate GPs about whom to refer, and educate rheumatologists about diagnosis,” Dr. Coates said.
“It is very important to diagnose PsA as early as possible. We know that a delay in diagnosis and treatment can lead to worse outcomes and joint damage,” said Soumya M. Reddy, MD, codirector of the Psoriasis and Psoriatic Arthritis Center at New York University Langone Health in New York. “The heterogeneity of clinical manifestations of PsA can make it difficult to diagnose, and in some cases this leads to delayed diagnosis.”
“We are increasingly interested in the concept of preventing PsA. Psoriasis is a unique disease state in which we have an at-risk population where 30% will develop an inflammatory and potentially damaging arthritis. This may become important as our skin treatments may also treat musculoskeletal components of the disease,” said Joseph F. Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital in Boston and double board certified in dermatology and rheumatology.
Focusing on patients with psoriasis
Treatment of psoriasis makes sense to address several quality-of-life issues, but controlling the severity of psoriatic skin manifestations gives patients no guarantees about their possible progression to PsA.
“So many people have mild psoriasis that, although they are less likely, proportionately, to get PsA, we still see many in the clinic,” said Dr. Coates. “Patients with severe skin involvement have a good reason to get treatment, which could help test whether a drug slows progression to arthritis. But it’s unethical to not treat severe psoriasis just to have a comparison group.” Aside from skin psoriasis, “we don’t have any other good markers,” Dr. Coates noted.
PsA can develop in patients who had psoriasis in the past but without currently active disease. “At the level of individual patients you can’t say someone is protected from developing PsA because their psoriasis is inactive,” Dr. Eder said. “No study has looked at whether treatment of psoriasis reduces the risk for progression to PsA. We don’t know whether any treatments that reduce inflammation in psoriasis also reduce progression to PsA.” Regardless, treating psoriasis is important because it improves quality of life and may have a beneficial, long-term effects on possible cardiovascular disease effects from psoriasis, Dr. Eder said.
Her research group is now studying the associations among different biological drugs taken by patients with psoriasis and their subsequent incidence of PsA with use of medical records from about 4 million Israeli residents. Other studies are also looking at this, but they are all observational and therefore are subject to unidentified confounders, she noted. A more definitive demonstration of PsA protection would need a randomized trial.
“The idea of preventing the transition from psoriasis to PsA is an exciting concept. But currently, there are no established treatments for preventing transition of psoriasis to PsA. It’s an area of active research, and the holy grail of psoriatic disease research. We do not yet know whether successful treatment of skin psoriasis delays the onset of arthritis,” Dr. Reddy said in an interview.
“The risk of developing PsA increases as the severity of skin psoriasis increases, measured by percentage of body surface area affected. However, there is only a weak correlation between the severity of skin disease and the severity of PsA,” said Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania in Philadelphia.
Widening the PsA diagnostic net
What’s elusive is some other parameter to identify the 3% of psoriasis patients who will develop PsA during the next year – or at least a better way to find patients as soon as they have what is identifiably PsA.
The diagnostic definition for PsA that many experts now accept is actually a set of classification criteria, CASPAR (Classification Criteria for Psoriatic Arthritis) (Arthritis Rheum. 2006 Aug;54[8]:2665-73). But in actual practice, diagnosing PsA relies heavily on clinical skill, case recognition, and ruling out mimickers.
“The CASPAR criteria were developed as classification criteria to use in studies, but they are also quite helpful in diagnosing PsA. It is the agreed on definition of PsA at the moment,” said Alexis Ogdie, MD, director of the psoriatic arthritis clinic at the University of Pennsylvania.
“CASPAR fills the role at present for clinical trials, but the diagnosis remains clinical, based on history, physical findings, and supported by lab and radiology findings,” Dr. Merola said. “A clinical prediction tool and a proper biomarker would be of great value.”
“We certainly use CASPAR criteria in the clinic, but they do not include all PsA patients; sometimes we diagnose PsA in patients who don’t meet the CASPAR criteria,” Dr. Coates said.
“In practice, rheumatologists mostly use their clinical judgment: a patient with history and findings typical of PsA after ruling out other causes. But distinguishing inflammatory from noninflammatory arthritis – like osteoarthritis or fibromyalgia – can be quite difficult; that’s the main challenge,” Dr. Eder said. “Rheumatologists rely on their clinical skill and experience. PsA can be difficult to diagnose. There is no biomarker for it. PsA is complex [to diagnose], and that’s why it’s diagnosed later. A primary care physician might get a negative result on a rheumatoid factor test and think that rules out PsA, but it doesn’t.”
Dr. Eder and others suggest that ultrasound may be a useful tool for earlier diagnosis of PsA. Ultrasound is more sensitive than a physical examination and can detect inflammation in joints and entheses, she noted. Another effective method may be more systematic use of screening questionnaires, Dr. Coates said, or simply more systematic questioning of patients.
“Questionnaires are not a high priority for a primary care physician who may have only a few patients with psoriasis. Even some dermatologists may not use the questionnaires because they take time to administer and assess. But just asking a psoriasis patient whether they have joint symptoms is enough,” Dr. Eder said. All clinicians who encounter patients with psoriasis should ask about musculoskeletal symptoms and refer when appropriate to a rheumatologist, Dr. Reddy said.
The earliest indicators of PsA
Evidence supporting ultrasound’s value for early PsA diagnosis came in a report at the most recent annual meeting of the American College of Rheumatology in Chicago in October 2018. Researchers from the University of Rochester (New York) used ultrasound to examine 78 patients with psoriasis but without PsA or musculoskeletal symptoms and 25 healthy controls. They found ultrasound abnormalities in almost half of the patients with psoriasis, significantly more than in the controls (Thiele R et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 2904).
Another report at the ACR annual meeting last October looked at the incidence of physician visits for nonspecific musculoskeletal symptoms during each of the 5 years preceding diagnosis of PsA. A prior report from Dr. Eder and her associates had documented in an observational cohort of 410 patients with psoriasis that, prior to development of PsA, patients often had nonspecific musculoskeletal symptoms of joint pain, fatigue, and stiffness that constituted a “preclinical” phase (Arthritis Rheumatol. 2017 March;69[3]:622-9).
In October, Dr. Eder reported how the appearance of musculoskeletal symptoms played out in terms of physician visits. She and her associates analyzed data from an Ontario health insurance database that included about 430,000 Ontario patients seen by 466 primary care physicians, which included 462 patients with a new diagnosis of PsA and 2,310 matched controls. The results showed that, in every year during the 5 years preceding diagnosis of PsA, the patients who would wind up getting diagnosed had roughly twice the number of visits to a primary care physician each year for nonspecific musculoskeletal issues. A similar pattern of doubled visits occurred for people prior to their PsA diagnosis going to physicians who specialize in musculoskeletal conditions, and when the analysis focused on visits to rheumatologists, patients who went on to get diagnosed with PsA had a nearly sevenfold increased rate of these visits, compared with controls, for each of the 5 years preceding their PsA diagnosis (Eder L et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 967).
These results “highlight that in many patients PsA is not an acute disease that starts suddenly. In many patients, there is a period when the patient experiences musculoskeletal symptoms and sees a primary care physician or rheumatologist and may be diagnosed with something that is not PsA. That means that the delay in diagnosis [of PsA] may have happened because the patients were misdiagnosed. It reinforces the need for better diagnostic tools,” Dr. Eder said. “We have focused on getting these patients to see a rheumatologist earlier, but that may not be enough. These patients may not receive routine follow-up; we need to do more follow-up on patients like these.” Diagnosing PsA early means earlier treatment, a better chance of reaching remission, less chance of permanent joint damage, and better quality of life.
The challenges of making an early diagnosis were also documented in a study reported by Dr. Ogdie during the June 2018 annual congress of the European League Against Rheumatism. Dr. Ogdie reported on the survey responses of 203 patients who said they had been diagnosed with PsA whose index diagnosis was a median of 6 years before they completed the survey. A total of 195 of these patients, or 96%, said that they had received at least one misdiagnosis prior to their PsA diagnosis (Odgie A et al. Ann Rheum Dis. 2018;77[Suppl 2]:163. Abstract THU0292). The most common misdiagnoses were psychosomatic disease, reported by 27% of the patients; osteoarthritis in 22%; anxiety or depression in 18%; and an orthopedic problem in 18%. (Patients could report more than one type of misdiagnosis.)
The results “showed that patients often had substantial delays and misdiagnoses before they received a PsA diagnosis,” Dr. Ogdie and her associates concluded. Although the CASPAR classification criteria may be the agreed on PsA definition, recent findings suggest a pre-PsA stage exists with musculoskeletal and other abnormalities. “How may we diagnose ‘pre-PsA’? How might we capture this transition phase from psoriasis to PsA before the CASPAR criteria are fulfilled,” she wondered in an interview. “If we could stop PsA before it is clinically relevant, that could dramatically change the course of the disease. This is a big need in the field right now.”
Weight loss and other interventions
Aside from treating psoriasis and perhaps putting a patient with psoriasis in a PsA-prevention trial, one of the best ways to prevent PsA may be weight loss.
Results from “some studies suggest that being overweight increases the risk for developing PsA. Obesity also exacerbates skin psoriasis, makes treatment less effective, and further increases the risk of cardiometabolic diseases associated with psoriasis,” Dr. Gelfand said. “All patients with psoriatic disease should be educated about the importance of maintaining a healthy body weight.”
“Several studies suggest that obesity is a risk factor for developing PsA. Obesity likely plays a role in driving or contributing to inflammation in psoriatic disease,” said Dr. Reddy, who noted that other PsA risk factors include nail psoriasis and first-degree relatives with PsA. Dr. Ogdie also cited uveitis and prior joint trauma as other risk factors.
“Strong observational data link obesity and PsA incidence. I talk to psoriasis patients about weight control, and selected patients could even consider bariatric surgery,” Dr. Eder said. Losing at least 5% of body mass index can make a difference, she added.
At the 2018 annual meeting of the American College of Rheumatology, researchers from the University of Bath (England) reported results from a retrospective, observational study of more than 90,000 people with recent-onset psoriasis; they found that people with an obese BMI had twice the rate of progression to PsA when compared with people with a normal BMI. Overweight people had a nearly 80% higher rate of incident PsA (Green A et al. Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2134).
Hints have also emerged that new approaches to treating psoriasis also could help to keep PsA precursors at bay. One recent example from researchers at the University of Leeds (England) was a phase 2 study of 73 patients with moderate to severe psoriasis but no PsA who underwent ultrasound screening of their entheses for signs of inflammatory changes. The 23 patients underwent 52 weeks of treatment with the drug ustekinumab (Stelara), an antagonist of interleukin-12 and -23 that is approved for U.S. marketing to treat both psoriasis and PsA. After 24 weeks on treatment, their mean inflammation scores had dropped by more than 40%, and the effect persisted through 52 weeks of treatment (Arthritis Rheum. 2018 Nov 22. doi: 10.1002/art.40778).
Despite this promise, the researchers “haven’t looked long enough or in enough people to see whether this actually stops patients from developing PsA,” Dr. Coates commented. It also remains unclear whether this or another ultrasound abnormality detectable in joints or entheses is a reliable predictor of PsA, she noted.
“We still have a lot to learn about how to classify patients as high risk” for PsA, Dr. Ogdie concluded.
Dr. Eder has received research and educational grants from AbbVie, Amgen, Celgene, Lilly, Novartis, and UCB. Dr. Coates has received honoraria, research funding, or both from more than a dozen companies. Dr. Reddy has been a consultant to AbbVie, Novartis, Pfizer, and UCB. Dr. Merola has been a consultant to AbbVie, Celgene, GlaxoSmithKline, Janssen, Lilly, Novartis, Samumed, Sanofi, and UCB and has received research grants from Aclaris, Biogen, Incyte, Novartis, Pfizer, and Sanofi. Dr. Gelfand has been a consultant, adviser, or both to more than a dozen companies. Dr. Ogdie has been a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Lilly, Novartis, Pfizer, and Takeda.
Most patients with psoriatic arthritis first present with psoriasis only. Their skin disorder precedes any joint involvement, often by several years. That suggests targeting interventions to patients with psoriasis to prevent or slow their progression to psoriatic arthritis, as well as following psoriatic patients closely to diagnose psoriatic arthritis quickly when it first appears. It’s a simple and attractive management premise that’s been challenging to apply in practice.
It’s not that clinicians aren’t motivated to diagnose psoriatic arthritis (PsA) in patients early, hopefully as soon as it appears. The susceptibility of patients with psoriasis to develop PsA is well described, with an annual progression rate of about 3%, and adverse consequences result from even a 6-month delay in diagnosis.
“Some physicians still don’t ask psoriasis patients about joint pain, or their symptoms are misinterpreted as something else,” said Lihi Eder, MD, a rheumatologist at Women’s College Research Institute, Toronto, and the University of Toronto. “Although there is increased awareness about PsA, there are still delays in diagnosis,” she said in an interview.
“Often there is a massive delay in diagnosis, and we know from a number of studies that longer duration of symptoms before diagnosis is associated with poorer outcomes,” said Laura C. Coates, MBChB, PhD, a rheumatologist at the University of Oxford (England). The delay to PsA diagnosis is generally “longer than for equivalent rheumatoid arthritis patients. PsA patients take longer to ask a primary care physician for help, longer to get a referral to a rheumatologist, and longer to get a diagnosis” from a rheumatologist. “We need to educate patients with psoriasis about their risk so that they seek help, educate GPs about whom to refer, and educate rheumatologists about diagnosis,” Dr. Coates said.
“It is very important to diagnose PsA as early as possible. We know that a delay in diagnosis and treatment can lead to worse outcomes and joint damage,” said Soumya M. Reddy, MD, codirector of the Psoriasis and Psoriatic Arthritis Center at New York University Langone Health in New York. “The heterogeneity of clinical manifestations of PsA can make it difficult to diagnose, and in some cases this leads to delayed diagnosis.”
“We are increasingly interested in the concept of preventing PsA. Psoriasis is a unique disease state in which we have an at-risk population where 30% will develop an inflammatory and potentially damaging arthritis. This may become important as our skin treatments may also treat musculoskeletal components of the disease,” said Joseph F. Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital in Boston and double board certified in dermatology and rheumatology.
Focusing on patients with psoriasis
Treatment of psoriasis makes sense to address several quality-of-life issues, but controlling the severity of psoriatic skin manifestations gives patients no guarantees about their possible progression to PsA.
“So many people have mild psoriasis that, although they are less likely, proportionately, to get PsA, we still see many in the clinic,” said Dr. Coates. “Patients with severe skin involvement have a good reason to get treatment, which could help test whether a drug slows progression to arthritis. But it’s unethical to not treat severe psoriasis just to have a comparison group.” Aside from skin psoriasis, “we don’t have any other good markers,” Dr. Coates noted.
PsA can develop in patients who had psoriasis in the past but without currently active disease. “At the level of individual patients you can’t say someone is protected from developing PsA because their psoriasis is inactive,” Dr. Eder said. “No study has looked at whether treatment of psoriasis reduces the risk for progression to PsA. We don’t know whether any treatments that reduce inflammation in psoriasis also reduce progression to PsA.” Regardless, treating psoriasis is important because it improves quality of life and may have a beneficial, long-term effects on possible cardiovascular disease effects from psoriasis, Dr. Eder said.
Her research group is now studying the associations among different biological drugs taken by patients with psoriasis and their subsequent incidence of PsA with use of medical records from about 4 million Israeli residents. Other studies are also looking at this, but they are all observational and therefore are subject to unidentified confounders, she noted. A more definitive demonstration of PsA protection would need a randomized trial.
“The idea of preventing the transition from psoriasis to PsA is an exciting concept. But currently, there are no established treatments for preventing transition of psoriasis to PsA. It’s an area of active research, and the holy grail of psoriatic disease research. We do not yet know whether successful treatment of skin psoriasis delays the onset of arthritis,” Dr. Reddy said in an interview.
“The risk of developing PsA increases as the severity of skin psoriasis increases, measured by percentage of body surface area affected. However, there is only a weak correlation between the severity of skin disease and the severity of PsA,” said Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania in Philadelphia.
Widening the PsA diagnostic net
What’s elusive is some other parameter to identify the 3% of psoriasis patients who will develop PsA during the next year – or at least a better way to find patients as soon as they have what is identifiably PsA.
The diagnostic definition for PsA that many experts now accept is actually a set of classification criteria, CASPAR (Classification Criteria for Psoriatic Arthritis) (Arthritis Rheum. 2006 Aug;54[8]:2665-73). But in actual practice, diagnosing PsA relies heavily on clinical skill, case recognition, and ruling out mimickers.
“The CASPAR criteria were developed as classification criteria to use in studies, but they are also quite helpful in diagnosing PsA. It is the agreed on definition of PsA at the moment,” said Alexis Ogdie, MD, director of the psoriatic arthritis clinic at the University of Pennsylvania.
“CASPAR fills the role at present for clinical trials, but the diagnosis remains clinical, based on history, physical findings, and supported by lab and radiology findings,” Dr. Merola said. “A clinical prediction tool and a proper biomarker would be of great value.”
“We certainly use CASPAR criteria in the clinic, but they do not include all PsA patients; sometimes we diagnose PsA in patients who don’t meet the CASPAR criteria,” Dr. Coates said.
“In practice, rheumatologists mostly use their clinical judgment: a patient with history and findings typical of PsA after ruling out other causes. But distinguishing inflammatory from noninflammatory arthritis – like osteoarthritis or fibromyalgia – can be quite difficult; that’s the main challenge,” Dr. Eder said. “Rheumatologists rely on their clinical skill and experience. PsA can be difficult to diagnose. There is no biomarker for it. PsA is complex [to diagnose], and that’s why it’s diagnosed later. A primary care physician might get a negative result on a rheumatoid factor test and think that rules out PsA, but it doesn’t.”
Dr. Eder and others suggest that ultrasound may be a useful tool for earlier diagnosis of PsA. Ultrasound is more sensitive than a physical examination and can detect inflammation in joints and entheses, she noted. Another effective method may be more systematic use of screening questionnaires, Dr. Coates said, or simply more systematic questioning of patients.
“Questionnaires are not a high priority for a primary care physician who may have only a few patients with psoriasis. Even some dermatologists may not use the questionnaires because they take time to administer and assess. But just asking a psoriasis patient whether they have joint symptoms is enough,” Dr. Eder said. All clinicians who encounter patients with psoriasis should ask about musculoskeletal symptoms and refer when appropriate to a rheumatologist, Dr. Reddy said.
The earliest indicators of PsA
Evidence supporting ultrasound’s value for early PsA diagnosis came in a report at the most recent annual meeting of the American College of Rheumatology in Chicago in October 2018. Researchers from the University of Rochester (New York) used ultrasound to examine 78 patients with psoriasis but without PsA or musculoskeletal symptoms and 25 healthy controls. They found ultrasound abnormalities in almost half of the patients with psoriasis, significantly more than in the controls (Thiele R et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 2904).
Another report at the ACR annual meeting last October looked at the incidence of physician visits for nonspecific musculoskeletal symptoms during each of the 5 years preceding diagnosis of PsA. A prior report from Dr. Eder and her associates had documented in an observational cohort of 410 patients with psoriasis that, prior to development of PsA, patients often had nonspecific musculoskeletal symptoms of joint pain, fatigue, and stiffness that constituted a “preclinical” phase (Arthritis Rheumatol. 2017 March;69[3]:622-9).
In October, Dr. Eder reported how the appearance of musculoskeletal symptoms played out in terms of physician visits. She and her associates analyzed data from an Ontario health insurance database that included about 430,000 Ontario patients seen by 466 primary care physicians, which included 462 patients with a new diagnosis of PsA and 2,310 matched controls. The results showed that, in every year during the 5 years preceding diagnosis of PsA, the patients who would wind up getting diagnosed had roughly twice the number of visits to a primary care physician each year for nonspecific musculoskeletal issues. A similar pattern of doubled visits occurred for people prior to their PsA diagnosis going to physicians who specialize in musculoskeletal conditions, and when the analysis focused on visits to rheumatologists, patients who went on to get diagnosed with PsA had a nearly sevenfold increased rate of these visits, compared with controls, for each of the 5 years preceding their PsA diagnosis (Eder L et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 967).
These results “highlight that in many patients PsA is not an acute disease that starts suddenly. In many patients, there is a period when the patient experiences musculoskeletal symptoms and sees a primary care physician or rheumatologist and may be diagnosed with something that is not PsA. That means that the delay in diagnosis [of PsA] may have happened because the patients were misdiagnosed. It reinforces the need for better diagnostic tools,” Dr. Eder said. “We have focused on getting these patients to see a rheumatologist earlier, but that may not be enough. These patients may not receive routine follow-up; we need to do more follow-up on patients like these.” Diagnosing PsA early means earlier treatment, a better chance of reaching remission, less chance of permanent joint damage, and better quality of life.
The challenges of making an early diagnosis were also documented in a study reported by Dr. Ogdie during the June 2018 annual congress of the European League Against Rheumatism. Dr. Ogdie reported on the survey responses of 203 patients who said they had been diagnosed with PsA whose index diagnosis was a median of 6 years before they completed the survey. A total of 195 of these patients, or 96%, said that they had received at least one misdiagnosis prior to their PsA diagnosis (Odgie A et al. Ann Rheum Dis. 2018;77[Suppl 2]:163. Abstract THU0292). The most common misdiagnoses were psychosomatic disease, reported by 27% of the patients; osteoarthritis in 22%; anxiety or depression in 18%; and an orthopedic problem in 18%. (Patients could report more than one type of misdiagnosis.)
The results “showed that patients often had substantial delays and misdiagnoses before they received a PsA diagnosis,” Dr. Ogdie and her associates concluded. Although the CASPAR classification criteria may be the agreed on PsA definition, recent findings suggest a pre-PsA stage exists with musculoskeletal and other abnormalities. “How may we diagnose ‘pre-PsA’? How might we capture this transition phase from psoriasis to PsA before the CASPAR criteria are fulfilled,” she wondered in an interview. “If we could stop PsA before it is clinically relevant, that could dramatically change the course of the disease. This is a big need in the field right now.”
Weight loss and other interventions
Aside from treating psoriasis and perhaps putting a patient with psoriasis in a PsA-prevention trial, one of the best ways to prevent PsA may be weight loss.
Results from “some studies suggest that being overweight increases the risk for developing PsA. Obesity also exacerbates skin psoriasis, makes treatment less effective, and further increases the risk of cardiometabolic diseases associated with psoriasis,” Dr. Gelfand said. “All patients with psoriatic disease should be educated about the importance of maintaining a healthy body weight.”
“Several studies suggest that obesity is a risk factor for developing PsA. Obesity likely plays a role in driving or contributing to inflammation in psoriatic disease,” said Dr. Reddy, who noted that other PsA risk factors include nail psoriasis and first-degree relatives with PsA. Dr. Ogdie also cited uveitis and prior joint trauma as other risk factors.
“Strong observational data link obesity and PsA incidence. I talk to psoriasis patients about weight control, and selected patients could even consider bariatric surgery,” Dr. Eder said. Losing at least 5% of body mass index can make a difference, she added.
At the 2018 annual meeting of the American College of Rheumatology, researchers from the University of Bath (England) reported results from a retrospective, observational study of more than 90,000 people with recent-onset psoriasis; they found that people with an obese BMI had twice the rate of progression to PsA when compared with people with a normal BMI. Overweight people had a nearly 80% higher rate of incident PsA (Green A et al. Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2134).
Hints have also emerged that new approaches to treating psoriasis also could help to keep PsA precursors at bay. One recent example from researchers at the University of Leeds (England) was a phase 2 study of 73 patients with moderate to severe psoriasis but no PsA who underwent ultrasound screening of their entheses for signs of inflammatory changes. The 23 patients underwent 52 weeks of treatment with the drug ustekinumab (Stelara), an antagonist of interleukin-12 and -23 that is approved for U.S. marketing to treat both psoriasis and PsA. After 24 weeks on treatment, their mean inflammation scores had dropped by more than 40%, and the effect persisted through 52 weeks of treatment (Arthritis Rheum. 2018 Nov 22. doi: 10.1002/art.40778).
Despite this promise, the researchers “haven’t looked long enough or in enough people to see whether this actually stops patients from developing PsA,” Dr. Coates commented. It also remains unclear whether this or another ultrasound abnormality detectable in joints or entheses is a reliable predictor of PsA, she noted.
“We still have a lot to learn about how to classify patients as high risk” for PsA, Dr. Ogdie concluded.
Dr. Eder has received research and educational grants from AbbVie, Amgen, Celgene, Lilly, Novartis, and UCB. Dr. Coates has received honoraria, research funding, or both from more than a dozen companies. Dr. Reddy has been a consultant to AbbVie, Novartis, Pfizer, and UCB. Dr. Merola has been a consultant to AbbVie, Celgene, GlaxoSmithKline, Janssen, Lilly, Novartis, Samumed, Sanofi, and UCB and has received research grants from Aclaris, Biogen, Incyte, Novartis, Pfizer, and Sanofi. Dr. Gelfand has been a consultant, adviser, or both to more than a dozen companies. Dr. Ogdie has been a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Lilly, Novartis, Pfizer, and Takeda.
Most patients with psoriatic arthritis first present with psoriasis only. Their skin disorder precedes any joint involvement, often by several years. That suggests targeting interventions to patients with psoriasis to prevent or slow their progression to psoriatic arthritis, as well as following psoriatic patients closely to diagnose psoriatic arthritis quickly when it first appears. It’s a simple and attractive management premise that’s been challenging to apply in practice.
It’s not that clinicians aren’t motivated to diagnose psoriatic arthritis (PsA) in patients early, hopefully as soon as it appears. The susceptibility of patients with psoriasis to develop PsA is well described, with an annual progression rate of about 3%, and adverse consequences result from even a 6-month delay in diagnosis.
“Some physicians still don’t ask psoriasis patients about joint pain, or their symptoms are misinterpreted as something else,” said Lihi Eder, MD, a rheumatologist at Women’s College Research Institute, Toronto, and the University of Toronto. “Although there is increased awareness about PsA, there are still delays in diagnosis,” she said in an interview.
“Often there is a massive delay in diagnosis, and we know from a number of studies that longer duration of symptoms before diagnosis is associated with poorer outcomes,” said Laura C. Coates, MBChB, PhD, a rheumatologist at the University of Oxford (England). The delay to PsA diagnosis is generally “longer than for equivalent rheumatoid arthritis patients. PsA patients take longer to ask a primary care physician for help, longer to get a referral to a rheumatologist, and longer to get a diagnosis” from a rheumatologist. “We need to educate patients with psoriasis about their risk so that they seek help, educate GPs about whom to refer, and educate rheumatologists about diagnosis,” Dr. Coates said.
“It is very important to diagnose PsA as early as possible. We know that a delay in diagnosis and treatment can lead to worse outcomes and joint damage,” said Soumya M. Reddy, MD, codirector of the Psoriasis and Psoriatic Arthritis Center at New York University Langone Health in New York. “The heterogeneity of clinical manifestations of PsA can make it difficult to diagnose, and in some cases this leads to delayed diagnosis.”
“We are increasingly interested in the concept of preventing PsA. Psoriasis is a unique disease state in which we have an at-risk population where 30% will develop an inflammatory and potentially damaging arthritis. This may become important as our skin treatments may also treat musculoskeletal components of the disease,” said Joseph F. Merola, MD, director of the Center for Skin and Related Musculoskeletal Diseases at Brigham and Women’s Hospital in Boston and double board certified in dermatology and rheumatology.
Focusing on patients with psoriasis
Treatment of psoriasis makes sense to address several quality-of-life issues, but controlling the severity of psoriatic skin manifestations gives patients no guarantees about their possible progression to PsA.
“So many people have mild psoriasis that, although they are less likely, proportionately, to get PsA, we still see many in the clinic,” said Dr. Coates. “Patients with severe skin involvement have a good reason to get treatment, which could help test whether a drug slows progression to arthritis. But it’s unethical to not treat severe psoriasis just to have a comparison group.” Aside from skin psoriasis, “we don’t have any other good markers,” Dr. Coates noted.
PsA can develop in patients who had psoriasis in the past but without currently active disease. “At the level of individual patients you can’t say someone is protected from developing PsA because their psoriasis is inactive,” Dr. Eder said. “No study has looked at whether treatment of psoriasis reduces the risk for progression to PsA. We don’t know whether any treatments that reduce inflammation in psoriasis also reduce progression to PsA.” Regardless, treating psoriasis is important because it improves quality of life and may have a beneficial, long-term effects on possible cardiovascular disease effects from psoriasis, Dr. Eder said.
Her research group is now studying the associations among different biological drugs taken by patients with psoriasis and their subsequent incidence of PsA with use of medical records from about 4 million Israeli residents. Other studies are also looking at this, but they are all observational and therefore are subject to unidentified confounders, she noted. A more definitive demonstration of PsA protection would need a randomized trial.
“The idea of preventing the transition from psoriasis to PsA is an exciting concept. But currently, there are no established treatments for preventing transition of psoriasis to PsA. It’s an area of active research, and the holy grail of psoriatic disease research. We do not yet know whether successful treatment of skin psoriasis delays the onset of arthritis,” Dr. Reddy said in an interview.
“The risk of developing PsA increases as the severity of skin psoriasis increases, measured by percentage of body surface area affected. However, there is only a weak correlation between the severity of skin disease and the severity of PsA,” said Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania in Philadelphia.
Widening the PsA diagnostic net
What’s elusive is some other parameter to identify the 3% of psoriasis patients who will develop PsA during the next year – or at least a better way to find patients as soon as they have what is identifiably PsA.
The diagnostic definition for PsA that many experts now accept is actually a set of classification criteria, CASPAR (Classification Criteria for Psoriatic Arthritis) (Arthritis Rheum. 2006 Aug;54[8]:2665-73). But in actual practice, diagnosing PsA relies heavily on clinical skill, case recognition, and ruling out mimickers.
“The CASPAR criteria were developed as classification criteria to use in studies, but they are also quite helpful in diagnosing PsA. It is the agreed on definition of PsA at the moment,” said Alexis Ogdie, MD, director of the psoriatic arthritis clinic at the University of Pennsylvania.
“CASPAR fills the role at present for clinical trials, but the diagnosis remains clinical, based on history, physical findings, and supported by lab and radiology findings,” Dr. Merola said. “A clinical prediction tool and a proper biomarker would be of great value.”
“We certainly use CASPAR criteria in the clinic, but they do not include all PsA patients; sometimes we diagnose PsA in patients who don’t meet the CASPAR criteria,” Dr. Coates said.
“In practice, rheumatologists mostly use their clinical judgment: a patient with history and findings typical of PsA after ruling out other causes. But distinguishing inflammatory from noninflammatory arthritis – like osteoarthritis or fibromyalgia – can be quite difficult; that’s the main challenge,” Dr. Eder said. “Rheumatologists rely on their clinical skill and experience. PsA can be difficult to diagnose. There is no biomarker for it. PsA is complex [to diagnose], and that’s why it’s diagnosed later. A primary care physician might get a negative result on a rheumatoid factor test and think that rules out PsA, but it doesn’t.”
Dr. Eder and others suggest that ultrasound may be a useful tool for earlier diagnosis of PsA. Ultrasound is more sensitive than a physical examination and can detect inflammation in joints and entheses, she noted. Another effective method may be more systematic use of screening questionnaires, Dr. Coates said, or simply more systematic questioning of patients.
“Questionnaires are not a high priority for a primary care physician who may have only a few patients with psoriasis. Even some dermatologists may not use the questionnaires because they take time to administer and assess. But just asking a psoriasis patient whether they have joint symptoms is enough,” Dr. Eder said. All clinicians who encounter patients with psoriasis should ask about musculoskeletal symptoms and refer when appropriate to a rheumatologist, Dr. Reddy said.
The earliest indicators of PsA
Evidence supporting ultrasound’s value for early PsA diagnosis came in a report at the most recent annual meeting of the American College of Rheumatology in Chicago in October 2018. Researchers from the University of Rochester (New York) used ultrasound to examine 78 patients with psoriasis but without PsA or musculoskeletal symptoms and 25 healthy controls. They found ultrasound abnormalities in almost half of the patients with psoriasis, significantly more than in the controls (Thiele R et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 2904).
Another report at the ACR annual meeting last October looked at the incidence of physician visits for nonspecific musculoskeletal symptoms during each of the 5 years preceding diagnosis of PsA. A prior report from Dr. Eder and her associates had documented in an observational cohort of 410 patients with psoriasis that, prior to development of PsA, patients often had nonspecific musculoskeletal symptoms of joint pain, fatigue, and stiffness that constituted a “preclinical” phase (Arthritis Rheumatol. 2017 March;69[3]:622-9).
In October, Dr. Eder reported how the appearance of musculoskeletal symptoms played out in terms of physician visits. She and her associates analyzed data from an Ontario health insurance database that included about 430,000 Ontario patients seen by 466 primary care physicians, which included 462 patients with a new diagnosis of PsA and 2,310 matched controls. The results showed that, in every year during the 5 years preceding diagnosis of PsA, the patients who would wind up getting diagnosed had roughly twice the number of visits to a primary care physician each year for nonspecific musculoskeletal issues. A similar pattern of doubled visits occurred for people prior to their PsA diagnosis going to physicians who specialize in musculoskeletal conditions, and when the analysis focused on visits to rheumatologists, patients who went on to get diagnosed with PsA had a nearly sevenfold increased rate of these visits, compared with controls, for each of the 5 years preceding their PsA diagnosis (Eder L et al. Arthritis Rheumatol. 2018;70[suppl 10]: Abstract 967).
These results “highlight that in many patients PsA is not an acute disease that starts suddenly. In many patients, there is a period when the patient experiences musculoskeletal symptoms and sees a primary care physician or rheumatologist and may be diagnosed with something that is not PsA. That means that the delay in diagnosis [of PsA] may have happened because the patients were misdiagnosed. It reinforces the need for better diagnostic tools,” Dr. Eder said. “We have focused on getting these patients to see a rheumatologist earlier, but that may not be enough. These patients may not receive routine follow-up; we need to do more follow-up on patients like these.” Diagnosing PsA early means earlier treatment, a better chance of reaching remission, less chance of permanent joint damage, and better quality of life.
The challenges of making an early diagnosis were also documented in a study reported by Dr. Ogdie during the June 2018 annual congress of the European League Against Rheumatism. Dr. Ogdie reported on the survey responses of 203 patients who said they had been diagnosed with PsA whose index diagnosis was a median of 6 years before they completed the survey. A total of 195 of these patients, or 96%, said that they had received at least one misdiagnosis prior to their PsA diagnosis (Odgie A et al. Ann Rheum Dis. 2018;77[Suppl 2]:163. Abstract THU0292). The most common misdiagnoses were psychosomatic disease, reported by 27% of the patients; osteoarthritis in 22%; anxiety or depression in 18%; and an orthopedic problem in 18%. (Patients could report more than one type of misdiagnosis.)
The results “showed that patients often had substantial delays and misdiagnoses before they received a PsA diagnosis,” Dr. Ogdie and her associates concluded. Although the CASPAR classification criteria may be the agreed on PsA definition, recent findings suggest a pre-PsA stage exists with musculoskeletal and other abnormalities. “How may we diagnose ‘pre-PsA’? How might we capture this transition phase from psoriasis to PsA before the CASPAR criteria are fulfilled,” she wondered in an interview. “If we could stop PsA before it is clinically relevant, that could dramatically change the course of the disease. This is a big need in the field right now.”
Weight loss and other interventions
Aside from treating psoriasis and perhaps putting a patient with psoriasis in a PsA-prevention trial, one of the best ways to prevent PsA may be weight loss.
Results from “some studies suggest that being overweight increases the risk for developing PsA. Obesity also exacerbates skin psoriasis, makes treatment less effective, and further increases the risk of cardiometabolic diseases associated with psoriasis,” Dr. Gelfand said. “All patients with psoriatic disease should be educated about the importance of maintaining a healthy body weight.”
“Several studies suggest that obesity is a risk factor for developing PsA. Obesity likely plays a role in driving or contributing to inflammation in psoriatic disease,” said Dr. Reddy, who noted that other PsA risk factors include nail psoriasis and first-degree relatives with PsA. Dr. Ogdie also cited uveitis and prior joint trauma as other risk factors.
“Strong observational data link obesity and PsA incidence. I talk to psoriasis patients about weight control, and selected patients could even consider bariatric surgery,” Dr. Eder said. Losing at least 5% of body mass index can make a difference, she added.
At the 2018 annual meeting of the American College of Rheumatology, researchers from the University of Bath (England) reported results from a retrospective, observational study of more than 90,000 people with recent-onset psoriasis; they found that people with an obese BMI had twice the rate of progression to PsA when compared with people with a normal BMI. Overweight people had a nearly 80% higher rate of incident PsA (Green A et al. Arthritis Rheumatol. 2018;70[Suppl 10]: Abstract 2134).
Hints have also emerged that new approaches to treating psoriasis also could help to keep PsA precursors at bay. One recent example from researchers at the University of Leeds (England) was a phase 2 study of 73 patients with moderate to severe psoriasis but no PsA who underwent ultrasound screening of their entheses for signs of inflammatory changes. The 23 patients underwent 52 weeks of treatment with the drug ustekinumab (Stelara), an antagonist of interleukin-12 and -23 that is approved for U.S. marketing to treat both psoriasis and PsA. After 24 weeks on treatment, their mean inflammation scores had dropped by more than 40%, and the effect persisted through 52 weeks of treatment (Arthritis Rheum. 2018 Nov 22. doi: 10.1002/art.40778).
Despite this promise, the researchers “haven’t looked long enough or in enough people to see whether this actually stops patients from developing PsA,” Dr. Coates commented. It also remains unclear whether this or another ultrasound abnormality detectable in joints or entheses is a reliable predictor of PsA, she noted.
“We still have a lot to learn about how to classify patients as high risk” for PsA, Dr. Ogdie concluded.
Dr. Eder has received research and educational grants from AbbVie, Amgen, Celgene, Lilly, Novartis, and UCB. Dr. Coates has received honoraria, research funding, or both from more than a dozen companies. Dr. Reddy has been a consultant to AbbVie, Novartis, Pfizer, and UCB. Dr. Merola has been a consultant to AbbVie, Celgene, GlaxoSmithKline, Janssen, Lilly, Novartis, Samumed, Sanofi, and UCB and has received research grants from Aclaris, Biogen, Incyte, Novartis, Pfizer, and Sanofi. Dr. Gelfand has been a consultant, adviser, or both to more than a dozen companies. Dr. Ogdie has been a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Lilly, Novartis, Pfizer, and Takeda.
Compounded pain creams no better than placebo creams in localized chronic pain
Specially formulated topical pain creams are no better than placebo creams for relieving localized chronic pain, according to results from a double-blind, randomized, placebo-controlled trial.
Study authors led by Robert E. Brutcher, PharmD, PhD, of Walter Reed National Military Medical Center in Bethesda, Md., said their findings published Feb. 4 in Annals of Internal Medicine suggest compounded pain creams should not be routinely used for chronic pain conditions.
The researchers noted that the use of compounded topical pain creams has increased dramatically despite “weak evidence” supporting their efficacy to treat localized pain, and this is particularly the case in military personnel, where the authors said treatments without central effect may be particularly beneficial because “opioid therapy may render a service member nondeployable and medications that affect the central nervous system may have a negative effect on judgment and motor skills.”
They noted a report from the U.S. Government Accountability Office that showed Tricare’s pharmacy benefits program paid $259 million for compounded medications in the 2013 fiscal year, a figure that increased to $746 million in 2014.
“The soaring costs, coupled with sparse efficacy data prompted the Defense Health Agency to evaluate this issue,” they wrote.
The objectives of the current study were to assess the efficacy of compounded pain creams for chronic pain conditions and determine whether efficacy differed for the various pain classifications.
“We hypothesized that, compared with placebo, compounded topical pain creams would provide greater pain relief and functional improvement,” they said.
The randomized trial involved 133 patients diagnosed with neuropathic pain, 133 with nociceptive pain, and 133 with mixed pain who had attended pain clinics at Walter Reed. Patients were aged between 18 and 90 years and had localized pain in the face, back or buttocks, neck, abdomen, chest, groin, or in up to two extremities. To be included in the study, they were also required to have an average pain score of 4 or greater on a 0- to 10-point numerical rating scale during the preceding week and have symptoms lasting longer than 6 weeks.
Patients in all three pain subgroups were randomized in a 1:1 ratio to receive either a compounded pain cream or a placebo cream. The authors noted that their “pain cream formulations were selected on the basis of accepted systemic indications for neuropathic and nociceptive pain.”
The formulation given to participants with neuropathic pain (n = 68) contained 10% ketamine, 6% gabapentin, 0.2% clonidine, and 2% lidocaine. The patients with nociceptive pain (n = 66) received a cream with 10% ketoprofen, 2% baclofen, 2% cyclobenzaprine, and 2% lidocaine. Those with mixed pain (n = 68) were given a cream containing 10% ketamine, 6% gabapentin, 3% diclofenac, 2% baclofen, 2% cyclobenzaprine, and 2% lidocaine. The authors said the concentrations of individual medications were based on previous trials that evaluated topical use.
The patients, who had a mean age across the groups that ranged from 48 to 57 years, applied cream to affected areas three times per day, with the amount dispensed determined by the size of the area experiencing pain. About half of the patients were women.
The primary outcome measures were an average pain score 1 month after treatment. A positive categorical response was a reduction in pain score of 2 or more points coupled with a score above 3 on a 5-point satisfaction scale. Secondary outcomes included Short Form-36 Health Survey scores, satisfaction, and categorical response. Participants with a positive outcome were followed to 3 months.
Change in the primary outcome of average pain score at 1 month did not differ between the active cream and placebo groups for any type of pain classification. The change was only –0.1 points (95% confidence interval, –0.8 to 0.5 points) for neuropathic pain, –0.3 points (95% CI, –0.9 to 0.2 points) for nociceptive pain, and –0.3 points (95% CI, –0.9 to 0.2 points) for mixed pain.
Among all patients combined, an overall change in average pain scores of –0.3 points (95% CI, –0.6 to 0.1 points) that favored the active-ingredient cream also did not differ between the treatment and placebo groups.
“The lower 95% confidence bounds for the 1-month between-group differences were all 0.9 points or less and excluded clinically meaningful benefits with the compounded topical pain cream,” the authors wrote.
Secondary outcomes also did not differ between the two study groups for any type of pain classification or for the entire cohort.
“Although participants in both treatment and control groups had improvement in their pain throughout the study, no significant differences were observed in pain scores, functional improvement, or satisfaction in the cohort or in any subgroup,” the authors concluded.
They noted that their findings were consistent with previous studies that also showed a lack of efficacy for most topical pain creams.
While some randomized trials have suggested positive findings for capsaicin, lidocaine, and NSAIDs, the authors noted that they did not find a similar benefit in their study population.
“Administered as stand-alone agents, lidocaine and NSAIDs may alleviate pain, although the effect size is small and the number needed to treat is large,” they wrote.
“Considering the increased costs of using a non–FDA-approved and regulated compounded cream rather than a single agent, we caution against routine use of compounded creams for chronic pain,” they wrote.
They noted that their study had several limitations, including that conventional treatments had failed in some of the participants before they enrolled in the study, increasing the likelihood that subsequent therapy would not be effective.
The authors suggested that future studies should aim to establish whether targeting specific types of pain or adding other agents like dimethyl sulfoxide would result in better outcomes.
The Centers for Rehabilitation Sciences Research in the U.S. Department of Defense’s Defense Health Agency funded the study. Two authors reported receiving grants and personal fees from several pharmaceutical companies.
SOURCE: Brutcher RE et al. Ann Intern Med. 2019 Feb 4. doi: 10.7326/M18-2736.
Specially formulated topical pain creams are no better than placebo creams for relieving localized chronic pain, according to results from a double-blind, randomized, placebo-controlled trial.
Study authors led by Robert E. Brutcher, PharmD, PhD, of Walter Reed National Military Medical Center in Bethesda, Md., said their findings published Feb. 4 in Annals of Internal Medicine suggest compounded pain creams should not be routinely used for chronic pain conditions.
The researchers noted that the use of compounded topical pain creams has increased dramatically despite “weak evidence” supporting their efficacy to treat localized pain, and this is particularly the case in military personnel, where the authors said treatments without central effect may be particularly beneficial because “opioid therapy may render a service member nondeployable and medications that affect the central nervous system may have a negative effect on judgment and motor skills.”
They noted a report from the U.S. Government Accountability Office that showed Tricare’s pharmacy benefits program paid $259 million for compounded medications in the 2013 fiscal year, a figure that increased to $746 million in 2014.
“The soaring costs, coupled with sparse efficacy data prompted the Defense Health Agency to evaluate this issue,” they wrote.
The objectives of the current study were to assess the efficacy of compounded pain creams for chronic pain conditions and determine whether efficacy differed for the various pain classifications.
“We hypothesized that, compared with placebo, compounded topical pain creams would provide greater pain relief and functional improvement,” they said.
The randomized trial involved 133 patients diagnosed with neuropathic pain, 133 with nociceptive pain, and 133 with mixed pain who had attended pain clinics at Walter Reed. Patients were aged between 18 and 90 years and had localized pain in the face, back or buttocks, neck, abdomen, chest, groin, or in up to two extremities. To be included in the study, they were also required to have an average pain score of 4 or greater on a 0- to 10-point numerical rating scale during the preceding week and have symptoms lasting longer than 6 weeks.
Patients in all three pain subgroups were randomized in a 1:1 ratio to receive either a compounded pain cream or a placebo cream. The authors noted that their “pain cream formulations were selected on the basis of accepted systemic indications for neuropathic and nociceptive pain.”
The formulation given to participants with neuropathic pain (n = 68) contained 10% ketamine, 6% gabapentin, 0.2% clonidine, and 2% lidocaine. The patients with nociceptive pain (n = 66) received a cream with 10% ketoprofen, 2% baclofen, 2% cyclobenzaprine, and 2% lidocaine. Those with mixed pain (n = 68) were given a cream containing 10% ketamine, 6% gabapentin, 3% diclofenac, 2% baclofen, 2% cyclobenzaprine, and 2% lidocaine. The authors said the concentrations of individual medications were based on previous trials that evaluated topical use.
The patients, who had a mean age across the groups that ranged from 48 to 57 years, applied cream to affected areas three times per day, with the amount dispensed determined by the size of the area experiencing pain. About half of the patients were women.
The primary outcome measures were an average pain score 1 month after treatment. A positive categorical response was a reduction in pain score of 2 or more points coupled with a score above 3 on a 5-point satisfaction scale. Secondary outcomes included Short Form-36 Health Survey scores, satisfaction, and categorical response. Participants with a positive outcome were followed to 3 months.
Change in the primary outcome of average pain score at 1 month did not differ between the active cream and placebo groups for any type of pain classification. The change was only –0.1 points (95% confidence interval, –0.8 to 0.5 points) for neuropathic pain, –0.3 points (95% CI, –0.9 to 0.2 points) for nociceptive pain, and –0.3 points (95% CI, –0.9 to 0.2 points) for mixed pain.
Among all patients combined, an overall change in average pain scores of –0.3 points (95% CI, –0.6 to 0.1 points) that favored the active-ingredient cream also did not differ between the treatment and placebo groups.
“The lower 95% confidence bounds for the 1-month between-group differences were all 0.9 points or less and excluded clinically meaningful benefits with the compounded topical pain cream,” the authors wrote.
Secondary outcomes also did not differ between the two study groups for any type of pain classification or for the entire cohort.
“Although participants in both treatment and control groups had improvement in their pain throughout the study, no significant differences were observed in pain scores, functional improvement, or satisfaction in the cohort or in any subgroup,” the authors concluded.
They noted that their findings were consistent with previous studies that also showed a lack of efficacy for most topical pain creams.
While some randomized trials have suggested positive findings for capsaicin, lidocaine, and NSAIDs, the authors noted that they did not find a similar benefit in their study population.
“Administered as stand-alone agents, lidocaine and NSAIDs may alleviate pain, although the effect size is small and the number needed to treat is large,” they wrote.
“Considering the increased costs of using a non–FDA-approved and regulated compounded cream rather than a single agent, we caution against routine use of compounded creams for chronic pain,” they wrote.
They noted that their study had several limitations, including that conventional treatments had failed in some of the participants before they enrolled in the study, increasing the likelihood that subsequent therapy would not be effective.
The authors suggested that future studies should aim to establish whether targeting specific types of pain or adding other agents like dimethyl sulfoxide would result in better outcomes.
The Centers for Rehabilitation Sciences Research in the U.S. Department of Defense’s Defense Health Agency funded the study. Two authors reported receiving grants and personal fees from several pharmaceutical companies.
SOURCE: Brutcher RE et al. Ann Intern Med. 2019 Feb 4. doi: 10.7326/M18-2736.
Specially formulated topical pain creams are no better than placebo creams for relieving localized chronic pain, according to results from a double-blind, randomized, placebo-controlled trial.
Study authors led by Robert E. Brutcher, PharmD, PhD, of Walter Reed National Military Medical Center in Bethesda, Md., said their findings published Feb. 4 in Annals of Internal Medicine suggest compounded pain creams should not be routinely used for chronic pain conditions.
The researchers noted that the use of compounded topical pain creams has increased dramatically despite “weak evidence” supporting their efficacy to treat localized pain, and this is particularly the case in military personnel, where the authors said treatments without central effect may be particularly beneficial because “opioid therapy may render a service member nondeployable and medications that affect the central nervous system may have a negative effect on judgment and motor skills.”
They noted a report from the U.S. Government Accountability Office that showed Tricare’s pharmacy benefits program paid $259 million for compounded medications in the 2013 fiscal year, a figure that increased to $746 million in 2014.
“The soaring costs, coupled with sparse efficacy data prompted the Defense Health Agency to evaluate this issue,” they wrote.
The objectives of the current study were to assess the efficacy of compounded pain creams for chronic pain conditions and determine whether efficacy differed for the various pain classifications.
“We hypothesized that, compared with placebo, compounded topical pain creams would provide greater pain relief and functional improvement,” they said.
The randomized trial involved 133 patients diagnosed with neuropathic pain, 133 with nociceptive pain, and 133 with mixed pain who had attended pain clinics at Walter Reed. Patients were aged between 18 and 90 years and had localized pain in the face, back or buttocks, neck, abdomen, chest, groin, or in up to two extremities. To be included in the study, they were also required to have an average pain score of 4 or greater on a 0- to 10-point numerical rating scale during the preceding week and have symptoms lasting longer than 6 weeks.
Patients in all three pain subgroups were randomized in a 1:1 ratio to receive either a compounded pain cream or a placebo cream. The authors noted that their “pain cream formulations were selected on the basis of accepted systemic indications for neuropathic and nociceptive pain.”
The formulation given to participants with neuropathic pain (n = 68) contained 10% ketamine, 6% gabapentin, 0.2% clonidine, and 2% lidocaine. The patients with nociceptive pain (n = 66) received a cream with 10% ketoprofen, 2% baclofen, 2% cyclobenzaprine, and 2% lidocaine. Those with mixed pain (n = 68) were given a cream containing 10% ketamine, 6% gabapentin, 3% diclofenac, 2% baclofen, 2% cyclobenzaprine, and 2% lidocaine. The authors said the concentrations of individual medications were based on previous trials that evaluated topical use.
The patients, who had a mean age across the groups that ranged from 48 to 57 years, applied cream to affected areas three times per day, with the amount dispensed determined by the size of the area experiencing pain. About half of the patients were women.
The primary outcome measures were an average pain score 1 month after treatment. A positive categorical response was a reduction in pain score of 2 or more points coupled with a score above 3 on a 5-point satisfaction scale. Secondary outcomes included Short Form-36 Health Survey scores, satisfaction, and categorical response. Participants with a positive outcome were followed to 3 months.
Change in the primary outcome of average pain score at 1 month did not differ between the active cream and placebo groups for any type of pain classification. The change was only –0.1 points (95% confidence interval, –0.8 to 0.5 points) for neuropathic pain, –0.3 points (95% CI, –0.9 to 0.2 points) for nociceptive pain, and –0.3 points (95% CI, –0.9 to 0.2 points) for mixed pain.
Among all patients combined, an overall change in average pain scores of –0.3 points (95% CI, –0.6 to 0.1 points) that favored the active-ingredient cream also did not differ between the treatment and placebo groups.
“The lower 95% confidence bounds for the 1-month between-group differences were all 0.9 points or less and excluded clinically meaningful benefits with the compounded topical pain cream,” the authors wrote.
Secondary outcomes also did not differ between the two study groups for any type of pain classification or for the entire cohort.
“Although participants in both treatment and control groups had improvement in their pain throughout the study, no significant differences were observed in pain scores, functional improvement, or satisfaction in the cohort or in any subgroup,” the authors concluded.
They noted that their findings were consistent with previous studies that also showed a lack of efficacy for most topical pain creams.
While some randomized trials have suggested positive findings for capsaicin, lidocaine, and NSAIDs, the authors noted that they did not find a similar benefit in their study population.
“Administered as stand-alone agents, lidocaine and NSAIDs may alleviate pain, although the effect size is small and the number needed to treat is large,” they wrote.
“Considering the increased costs of using a non–FDA-approved and regulated compounded cream rather than a single agent, we caution against routine use of compounded creams for chronic pain,” they wrote.
They noted that their study had several limitations, including that conventional treatments had failed in some of the participants before they enrolled in the study, increasing the likelihood that subsequent therapy would not be effective.
The authors suggested that future studies should aim to establish whether targeting specific types of pain or adding other agents like dimethyl sulfoxide would result in better outcomes.
The Centers for Rehabilitation Sciences Research in the U.S. Department of Defense’s Defense Health Agency funded the study. Two authors reported receiving grants and personal fees from several pharmaceutical companies.
SOURCE: Brutcher RE et al. Ann Intern Med. 2019 Feb 4. doi: 10.7326/M18-2736.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Compounded pain creams should not be routinely used to treat chronic localized pain.
Major finding: At 1 month, the researchers found no significant differences in pain scores, functional improvement, or satisfaction between study participants with localized pain who had received specifically formulated pain creams and those who had received a placebo cream.
Study details: This double-blind, randomized, controlled trial involved 399 patients with localized pain who received either a pain cream specifically compounded for their type of pain – neuropathic, nociceptive, or mixed – or a placebo cream.
Disclosures: The Centers for Rehabilitation Sciences Research in the U.S. Department of Defense’s Defense Health Agency funded the study. Two authors reported receiving grants and personal fees from several pharmaceutical companies.
Source: Brutcher RE et al. Ann Intern Med. 2019 Feb 4. doi: 10.7326/M18-2736.
Mapping the Pathway of Pain
What makes us pull a hand away from a hot stove or flinch at a pinprick? Researchers from the National Center for Complementary and Integrative Health say they have identified activity in the brain that governs these reactions.
Alexander Chesler, PhD, senior author of the study, says we already know a lot about local spinal cord circuits for simple reflexive responses, but “the mechanisms underlying more complex behaviors remain poorly understood.”
Using heat as the source of discomfort in their experiments, the researchers found a predictable sequence of behaviors—likened to the sequence of responding to walking cautiously on a hot beach, then hopping as the heat intensifies, then running to a water source. “This kind of ‘feed-forward’ circuitry is unique because it is an upward spiral,” says Arnab Barik, PhD, one of the study authors. “The more this pathway is activated by harmful activity, the more it reacts, leading to dramatic behavioral responses.”
The experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). Standing on a hot surface activated a group of nerve cells in the PBNI, triggering escape responses through connections to the MdD. Interestingly, the PBNI cells express a gene that codes for substances that also contribute to multiple disease processes.
“Our data provide evidence that the PBNI produces streams of information with distinct functional significance,” says Arnab Barik, PhD, one of the study authors. “The brainstem-spinal cord pathway identified in this study selectively controls pain response and elicits appropriate behaviors based on sensory input.”
Further investigation, the researchers say, can help us understand how pain is encoded in the brain. The study findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain.
What makes us pull a hand away from a hot stove or flinch at a pinprick? Researchers from the National Center for Complementary and Integrative Health say they have identified activity in the brain that governs these reactions.
Alexander Chesler, PhD, senior author of the study, says we already know a lot about local spinal cord circuits for simple reflexive responses, but “the mechanisms underlying more complex behaviors remain poorly understood.”
Using heat as the source of discomfort in their experiments, the researchers found a predictable sequence of behaviors—likened to the sequence of responding to walking cautiously on a hot beach, then hopping as the heat intensifies, then running to a water source. “This kind of ‘feed-forward’ circuitry is unique because it is an upward spiral,” says Arnab Barik, PhD, one of the study authors. “The more this pathway is activated by harmful activity, the more it reacts, leading to dramatic behavioral responses.”
The experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). Standing on a hot surface activated a group of nerve cells in the PBNI, triggering escape responses through connections to the MdD. Interestingly, the PBNI cells express a gene that codes for substances that also contribute to multiple disease processes.
“Our data provide evidence that the PBNI produces streams of information with distinct functional significance,” says Arnab Barik, PhD, one of the study authors. “The brainstem-spinal cord pathway identified in this study selectively controls pain response and elicits appropriate behaviors based on sensory input.”
Further investigation, the researchers say, can help us understand how pain is encoded in the brain. The study findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain.
What makes us pull a hand away from a hot stove or flinch at a pinprick? Researchers from the National Center for Complementary and Integrative Health say they have identified activity in the brain that governs these reactions.
Alexander Chesler, PhD, senior author of the study, says we already know a lot about local spinal cord circuits for simple reflexive responses, but “the mechanisms underlying more complex behaviors remain poorly understood.”
Using heat as the source of discomfort in their experiments, the researchers found a predictable sequence of behaviors—likened to the sequence of responding to walking cautiously on a hot beach, then hopping as the heat intensifies, then running to a water source. “This kind of ‘feed-forward’ circuitry is unique because it is an upward spiral,” says Arnab Barik, PhD, one of the study authors. “The more this pathway is activated by harmful activity, the more it reacts, leading to dramatic behavioral responses.”
The experiments showed that the parts of the brainstem involved in this circuit are the parabrachial nucleus (PBNI) and the dorsal reticular formation in the medulla (MdD). Standing on a hot surface activated a group of nerve cells in the PBNI, triggering escape responses through connections to the MdD. Interestingly, the PBNI cells express a gene that codes for substances that also contribute to multiple disease processes.
“Our data provide evidence that the PBNI produces streams of information with distinct functional significance,” says Arnab Barik, PhD, one of the study authors. “The brainstem-spinal cord pathway identified in this study selectively controls pain response and elicits appropriate behaviors based on sensory input.”
Further investigation, the researchers say, can help us understand how pain is encoded in the brain. The study findings may also offer opportunities to understand how the body becomes dysregulated during chronic pain.
Expert: There’s no single treatment for fibromyalgia
SAN DIEGO – There are many potential treatments for fibromyalgia, but a large number of them – NSAIDs, opioids, cannabis and more – come with caveats and nothing beats an old stand-by: physical rehabilitation.
With exercise, “we’re getting the muscles moving, and we’re getting [patients] used to stimulation that will hopefully deaden that pain response over time,” David E.J. Bazzo, MD, said at Pain Care for Primary Care. Still, “it’s going to take multiple things to best treat your patients.”
Fibromyalgia is unique, said Dr. Bazzo, professor of family medicine and public health at the University of California, San Diego. Diagnosis is based on self-reported symptoms since no laboratory tests are available. For diagnostic criteria, he recommends those released by the American College of Rheumatology in 2010 and 2011 and updated in 2016. The criteria, he said, recognize the importance of cognitive symptoms, unrefreshing sleep, fatigue, and certain somatic symptoms (Semin Arthritis Rheum. 2016;46[3]:319-29).
Poor sleep is an especially important problem in fibromyalgia, Dr. Bazzo said, although it’s “a bit of a chicken-and-egg discussion.” It’s not clear which comes first, but “we know that both happen hand-in-hand. We need to work on people’s sleep as one of the primary targets.”
When it comes to treatment, “you have to validate this person’s symptoms and say, ‘Yes, I believe you. I know that you are suffering, and that you’re having pain,’ ” Dr. Bazzo said at the meeting held by the American Pain Society and Global Academy for Medical Education. He advised clinicians to keep in mind conditions that can accompany fibromyalgia, such as depression, that may require other treatment options.
Dr. Bazzo offered advice about these approaches to treatment:
- Exercise. Research supports treadmill and cycle ergometry (BMJ 2002;325:185).
- Opioids. “There’s no convincing evidence that opioids have a role in treating fibromyalgia initially. If you’ve tried everything and patients have had problems, are just not responsive or had side effects, you could consider opioids. But that should be at the tail end of everything because the data is not there,” he said.
- Tramadol. “It’s like an opioid with potential for addiction,” he said. “Don’t just use it willy-nilly. Make sure you have a reason and a good plan. Would it be my first thing? No. Is it something that I keep in my back pocket when other things aren’t working? Perhaps. Would I use it before an opioid? For sure.”
- Second-line therapies. According to Dr. Bazzo, these include antiepileptics such as gabapentin and pregabalin, low-dose cyclobenzaprine, and dual reuptake inhibitors such as duloxetine. There are many other second-line options, he said, from behavioral approaches to yoga to guided physical therapy.
- NSAIDs. Not helpful.
- Cannabis. May interact with other medications.
- Pain clinics. Make sure you refer patients to a pain clinic that embraces a multidisciplinary approach, he said, not one that only offers “pain pills or shots.”
Dr. Bazzo reported no relevant conflicts of interest. The Global Academy for Medical Education and this news organization are owned by the same parent company.
SAN DIEGO – There are many potential treatments for fibromyalgia, but a large number of them – NSAIDs, opioids, cannabis and more – come with caveats and nothing beats an old stand-by: physical rehabilitation.
With exercise, “we’re getting the muscles moving, and we’re getting [patients] used to stimulation that will hopefully deaden that pain response over time,” David E.J. Bazzo, MD, said at Pain Care for Primary Care. Still, “it’s going to take multiple things to best treat your patients.”
Fibromyalgia is unique, said Dr. Bazzo, professor of family medicine and public health at the University of California, San Diego. Diagnosis is based on self-reported symptoms since no laboratory tests are available. For diagnostic criteria, he recommends those released by the American College of Rheumatology in 2010 and 2011 and updated in 2016. The criteria, he said, recognize the importance of cognitive symptoms, unrefreshing sleep, fatigue, and certain somatic symptoms (Semin Arthritis Rheum. 2016;46[3]:319-29).
Poor sleep is an especially important problem in fibromyalgia, Dr. Bazzo said, although it’s “a bit of a chicken-and-egg discussion.” It’s not clear which comes first, but “we know that both happen hand-in-hand. We need to work on people’s sleep as one of the primary targets.”
When it comes to treatment, “you have to validate this person’s symptoms and say, ‘Yes, I believe you. I know that you are suffering, and that you’re having pain,’ ” Dr. Bazzo said at the meeting held by the American Pain Society and Global Academy for Medical Education. He advised clinicians to keep in mind conditions that can accompany fibromyalgia, such as depression, that may require other treatment options.
Dr. Bazzo offered advice about these approaches to treatment:
- Exercise. Research supports treadmill and cycle ergometry (BMJ 2002;325:185).
- Opioids. “There’s no convincing evidence that opioids have a role in treating fibromyalgia initially. If you’ve tried everything and patients have had problems, are just not responsive or had side effects, you could consider opioids. But that should be at the tail end of everything because the data is not there,” he said.
- Tramadol. “It’s like an opioid with potential for addiction,” he said. “Don’t just use it willy-nilly. Make sure you have a reason and a good plan. Would it be my first thing? No. Is it something that I keep in my back pocket when other things aren’t working? Perhaps. Would I use it before an opioid? For sure.”
- Second-line therapies. According to Dr. Bazzo, these include antiepileptics such as gabapentin and pregabalin, low-dose cyclobenzaprine, and dual reuptake inhibitors such as duloxetine. There are many other second-line options, he said, from behavioral approaches to yoga to guided physical therapy.
- NSAIDs. Not helpful.
- Cannabis. May interact with other medications.
- Pain clinics. Make sure you refer patients to a pain clinic that embraces a multidisciplinary approach, he said, not one that only offers “pain pills or shots.”
Dr. Bazzo reported no relevant conflicts of interest. The Global Academy for Medical Education and this news organization are owned by the same parent company.
SAN DIEGO – There are many potential treatments for fibromyalgia, but a large number of them – NSAIDs, opioids, cannabis and more – come with caveats and nothing beats an old stand-by: physical rehabilitation.
With exercise, “we’re getting the muscles moving, and we’re getting [patients] used to stimulation that will hopefully deaden that pain response over time,” David E.J. Bazzo, MD, said at Pain Care for Primary Care. Still, “it’s going to take multiple things to best treat your patients.”
Fibromyalgia is unique, said Dr. Bazzo, professor of family medicine and public health at the University of California, San Diego. Diagnosis is based on self-reported symptoms since no laboratory tests are available. For diagnostic criteria, he recommends those released by the American College of Rheumatology in 2010 and 2011 and updated in 2016. The criteria, he said, recognize the importance of cognitive symptoms, unrefreshing sleep, fatigue, and certain somatic symptoms (Semin Arthritis Rheum. 2016;46[3]:319-29).
Poor sleep is an especially important problem in fibromyalgia, Dr. Bazzo said, although it’s “a bit of a chicken-and-egg discussion.” It’s not clear which comes first, but “we know that both happen hand-in-hand. We need to work on people’s sleep as one of the primary targets.”
When it comes to treatment, “you have to validate this person’s symptoms and say, ‘Yes, I believe you. I know that you are suffering, and that you’re having pain,’ ” Dr. Bazzo said at the meeting held by the American Pain Society and Global Academy for Medical Education. He advised clinicians to keep in mind conditions that can accompany fibromyalgia, such as depression, that may require other treatment options.
Dr. Bazzo offered advice about these approaches to treatment:
- Exercise. Research supports treadmill and cycle ergometry (BMJ 2002;325:185).
- Opioids. “There’s no convincing evidence that opioids have a role in treating fibromyalgia initially. If you’ve tried everything and patients have had problems, are just not responsive or had side effects, you could consider opioids. But that should be at the tail end of everything because the data is not there,” he said.
- Tramadol. “It’s like an opioid with potential for addiction,” he said. “Don’t just use it willy-nilly. Make sure you have a reason and a good plan. Would it be my first thing? No. Is it something that I keep in my back pocket when other things aren’t working? Perhaps. Would I use it before an opioid? For sure.”
- Second-line therapies. According to Dr. Bazzo, these include antiepileptics such as gabapentin and pregabalin, low-dose cyclobenzaprine, and dual reuptake inhibitors such as duloxetine. There are many other second-line options, he said, from behavioral approaches to yoga to guided physical therapy.
- NSAIDs. Not helpful.
- Cannabis. May interact with other medications.
- Pain clinics. Make sure you refer patients to a pain clinic that embraces a multidisciplinary approach, he said, not one that only offers “pain pills or shots.”
Dr. Bazzo reported no relevant conflicts of interest. The Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PAIN CARE FOR PRIMARY CARE