User login
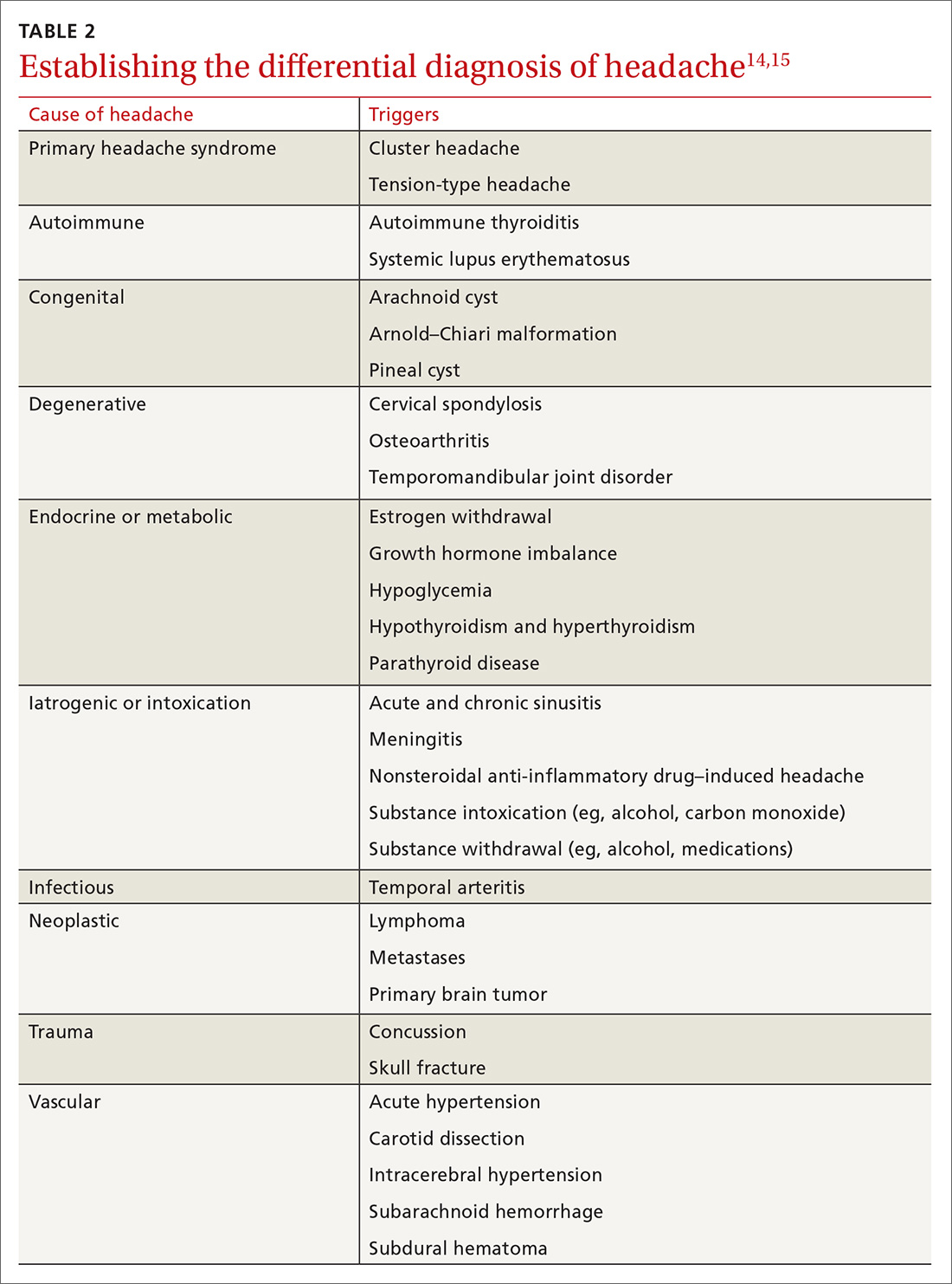
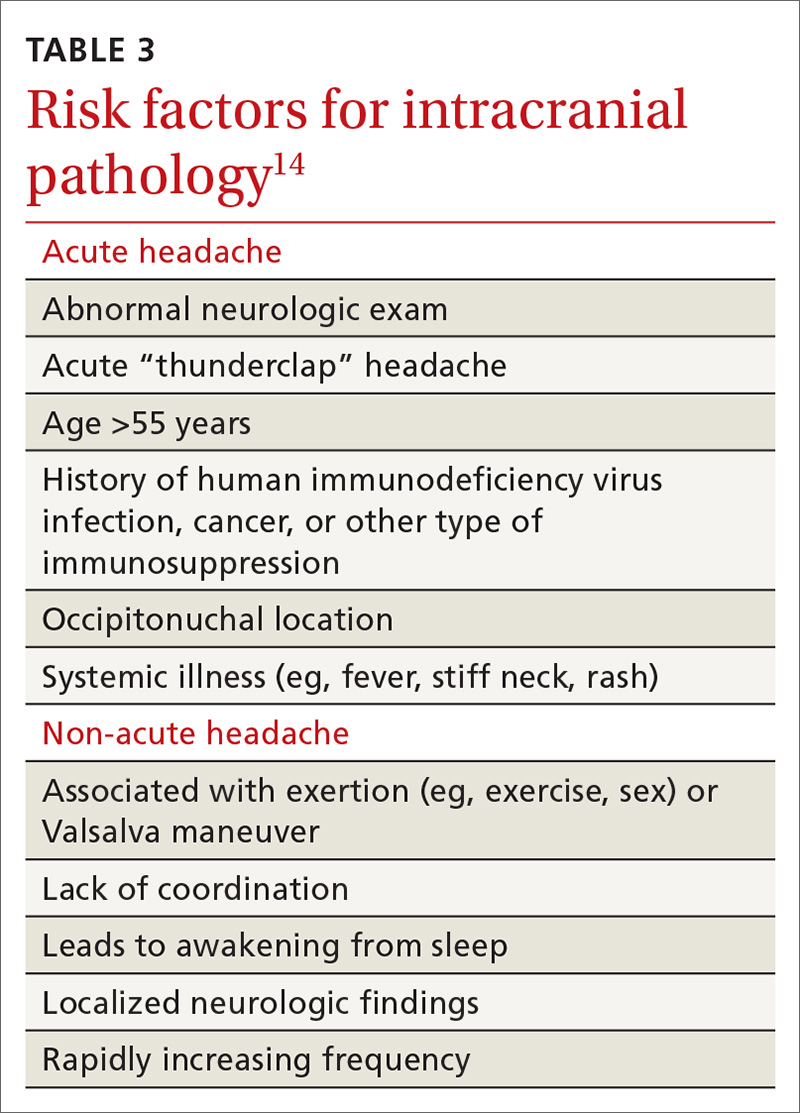
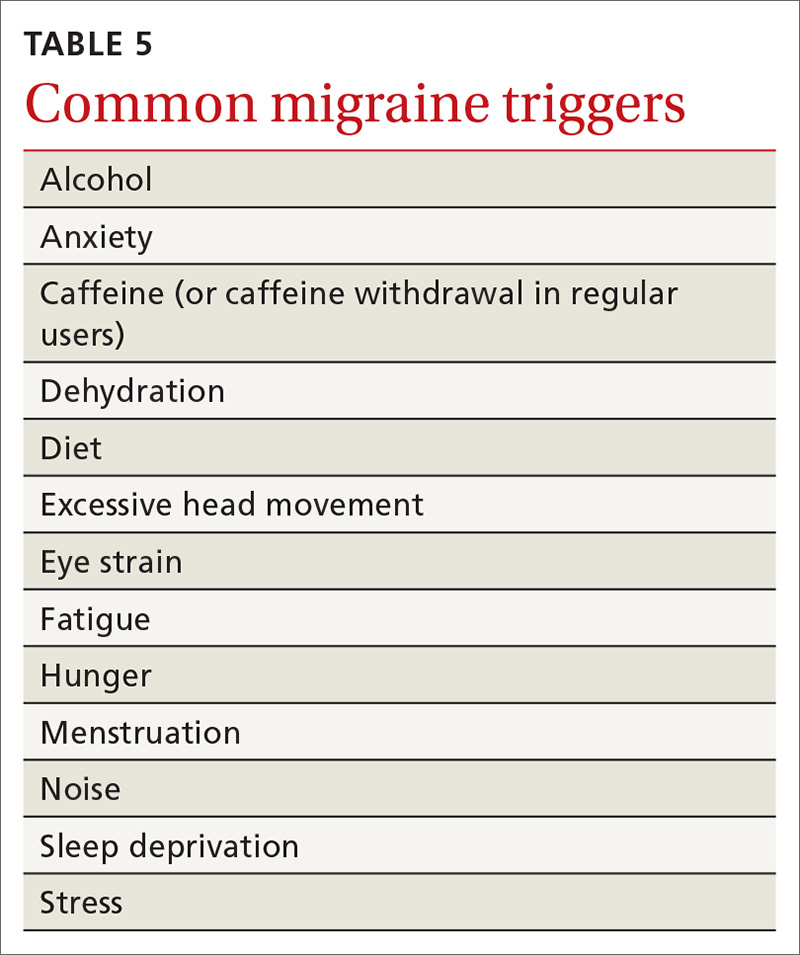
History of melanoma in situ • dyspnea • rib pain • Dx?
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.
A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
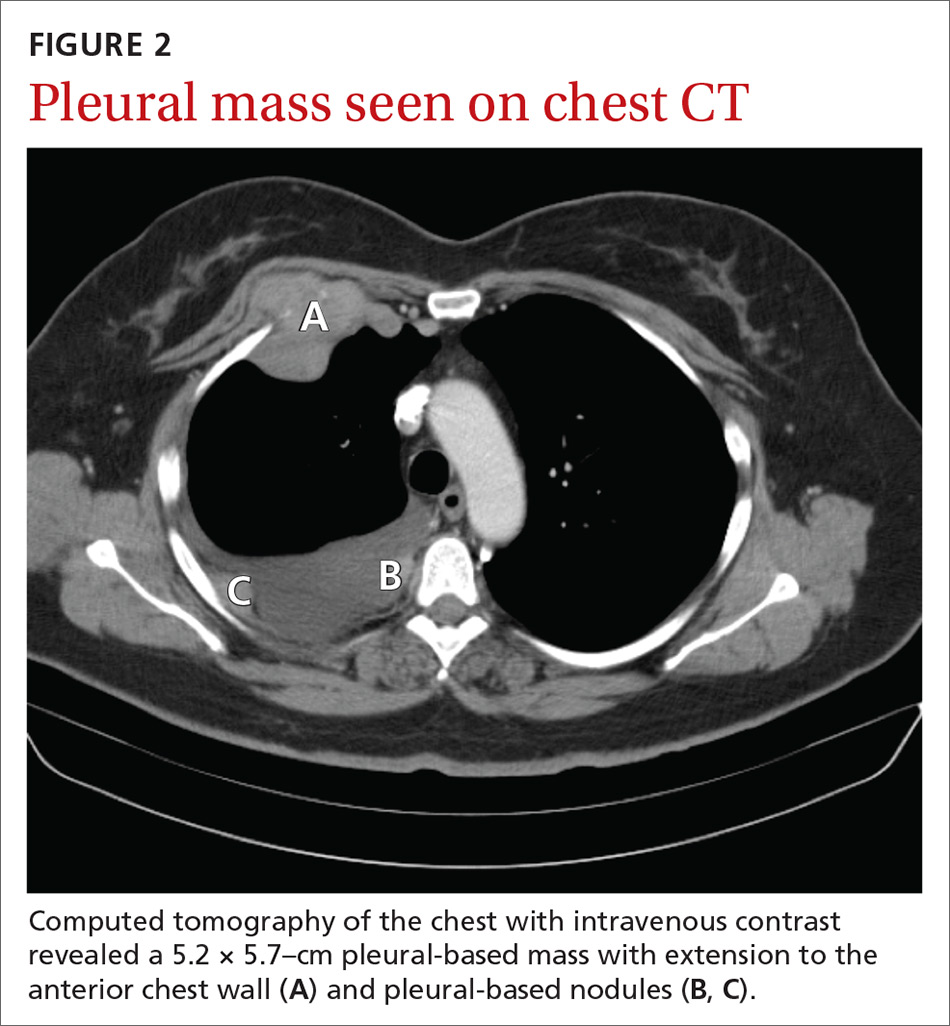
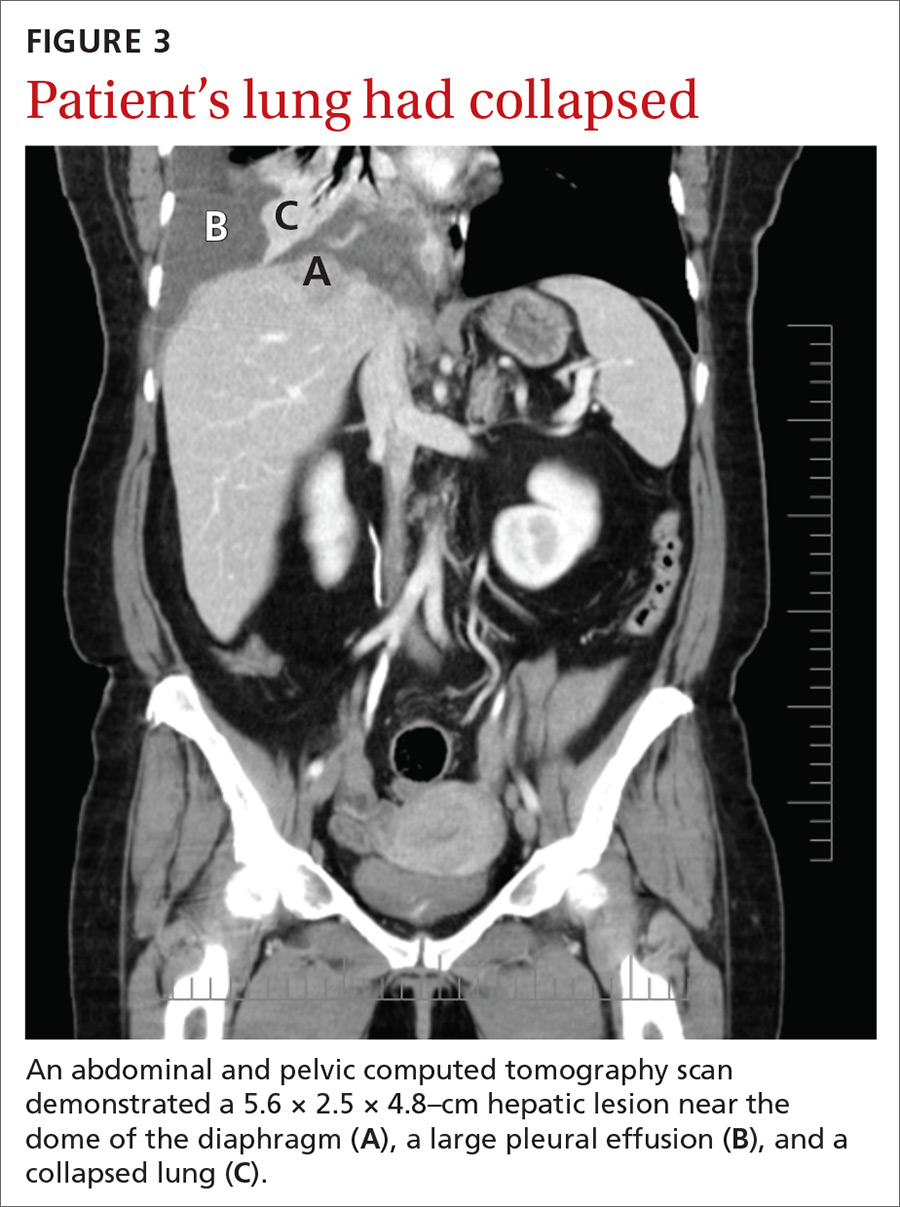
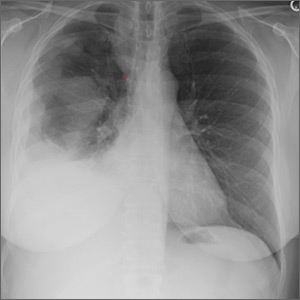
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
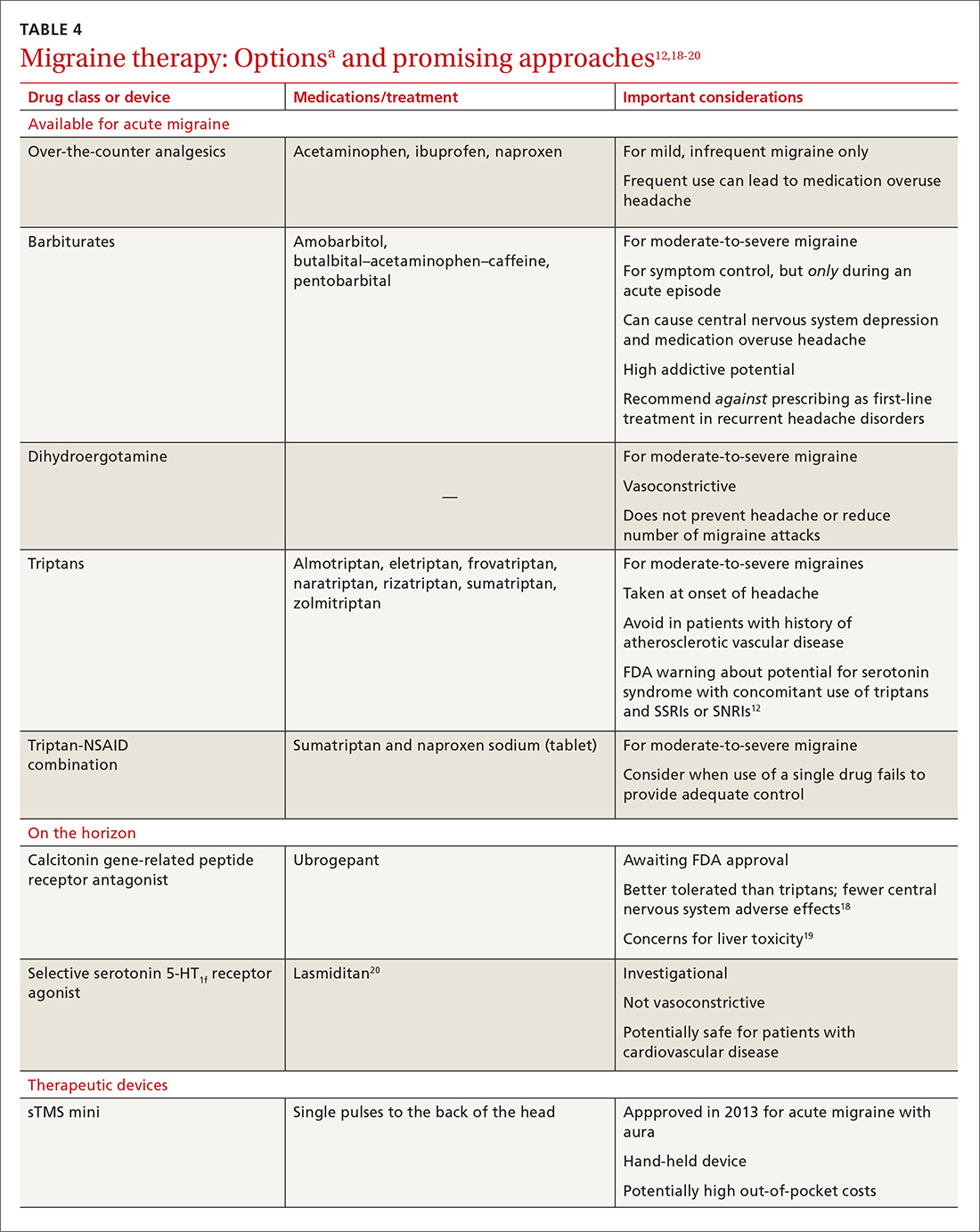
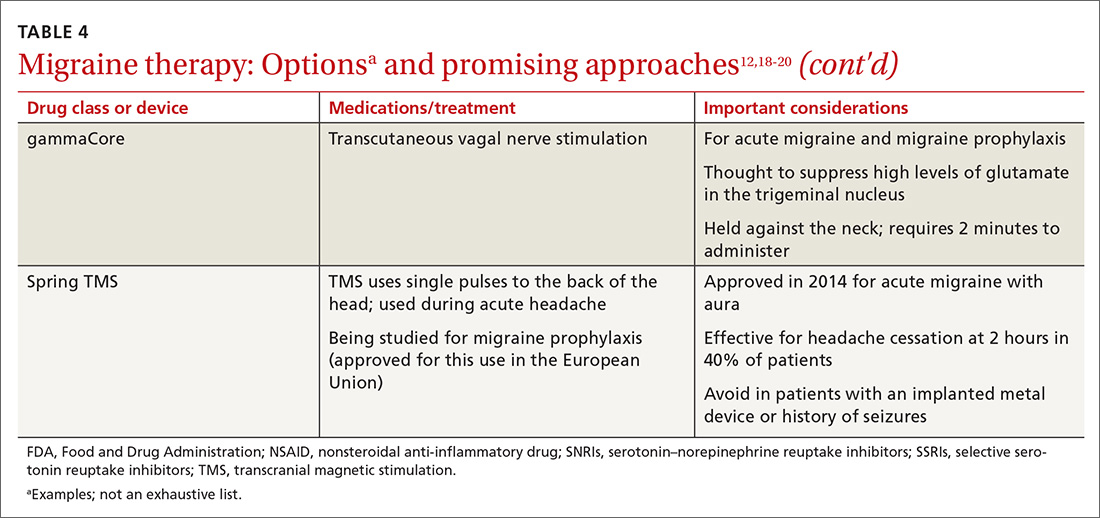
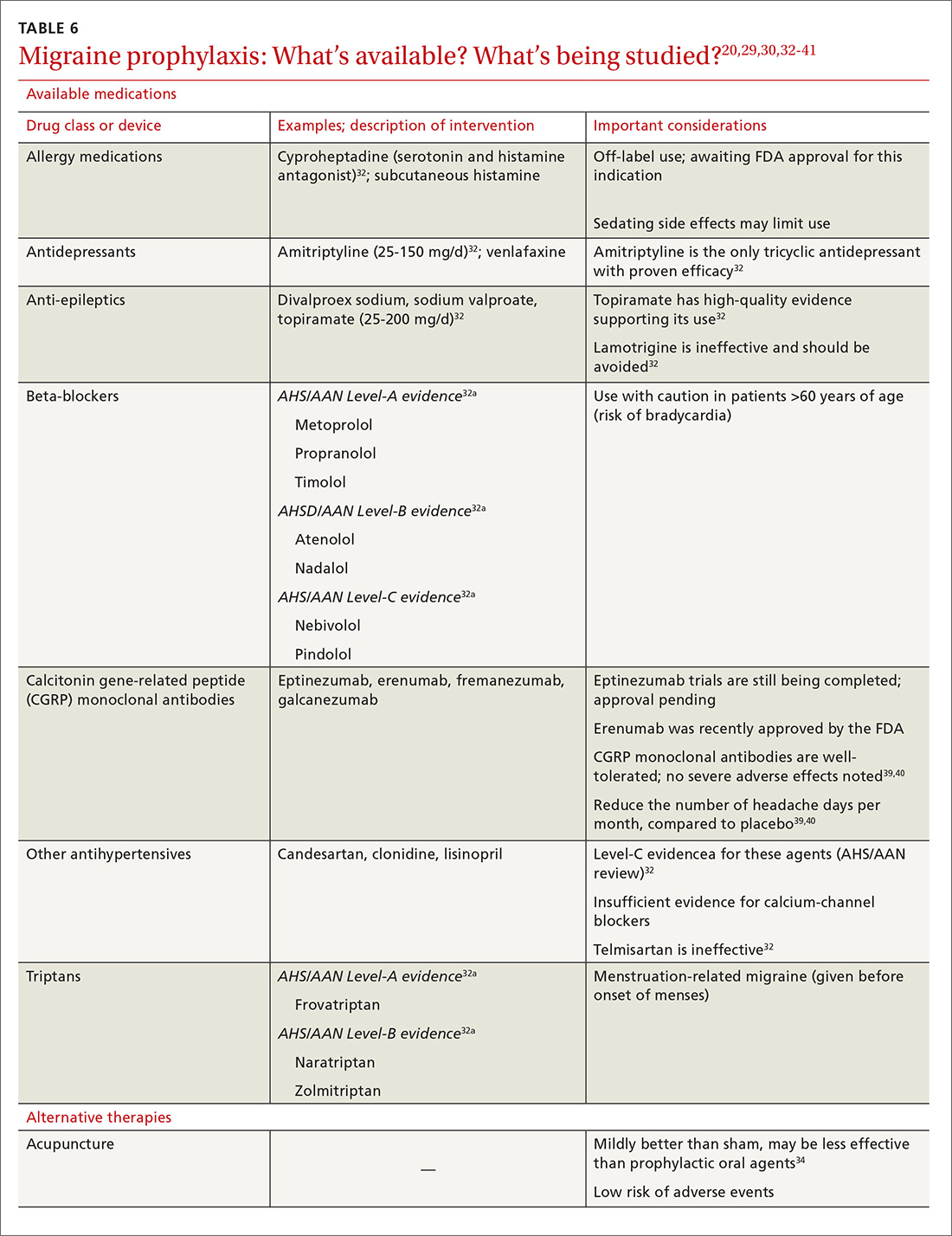
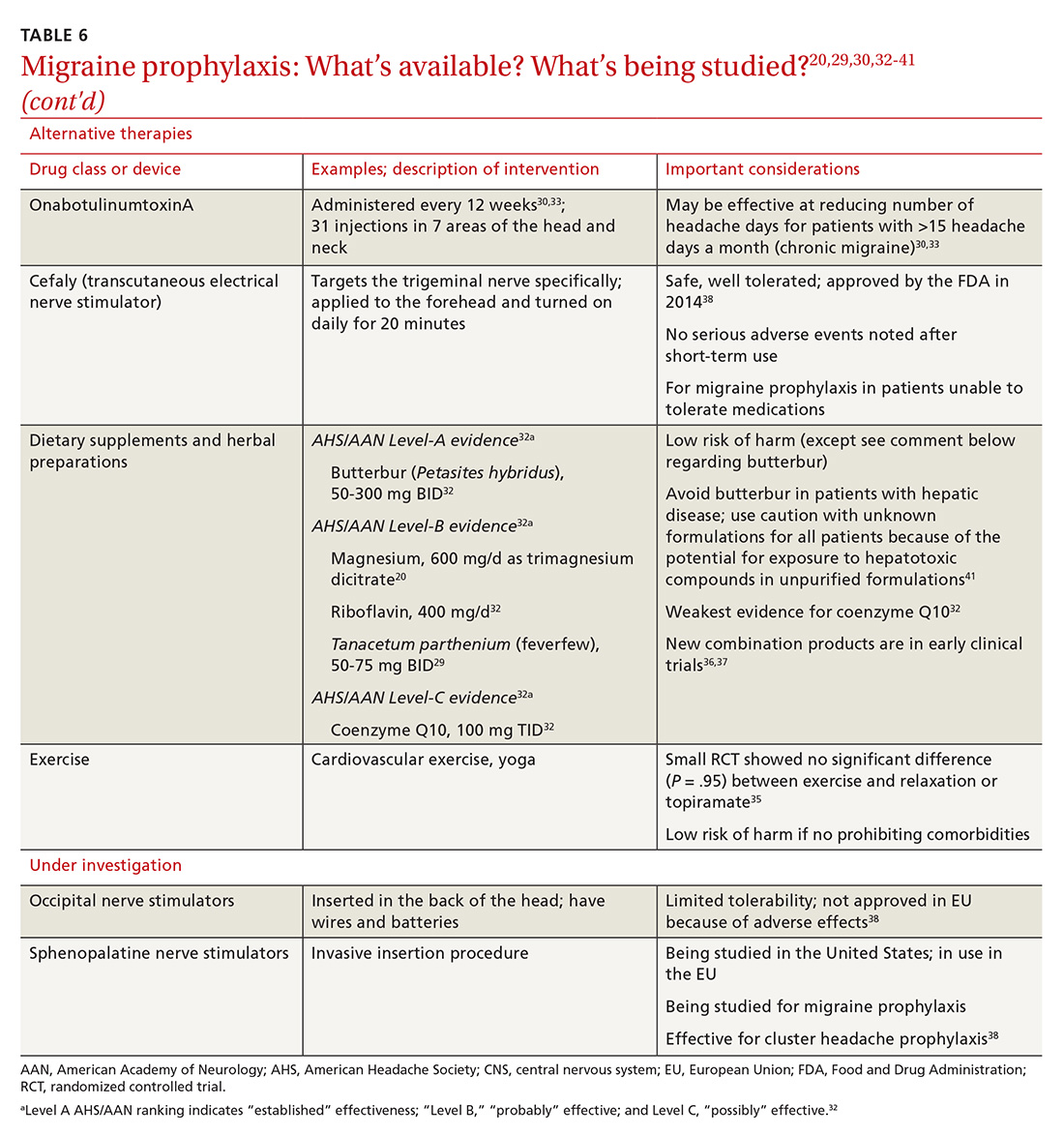
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; [email protected]
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.
A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; [email protected]
THE CASE
A 56-year-old woman with a history of melanoma in situ presented with progressive dyspnea on exertion, cough productive of clear sputum, and right-sided rib pain radiating to the upper back of 5 weeks’ duration.
Twenty-four years earlier, the patient had undergone excision of a skin lesion from the right side of her back. Pathology revealed melanoma in situ with no evidence of invasion of the underlying dermis. Because of close margins, she underwent wider excision 2 weeks later and no residual tumor was found. The patient subsequently underwent routine biannual dermatologic follow-up and transitioned (within the past few years) to annual dermatologic follow-up. At a recent dermatologic visit (9 months earlier), there were no suspicious skin lesions.
At current presentation, she denied fever, chills, night sweats, or unintentional weight loss. On examination, her vital signs were normal. Her pulse oximetry on room air was 95% at rest and 94% with ambulation. She had decreased breath sounds at the right lung base and a fixed 2 × 2-cm nontender, indurated mass in the right upper anterior chest wall, superior to the right breast. A skin examination was not performed at this time.
A complete blood count revealed a white blood cell count of 8220/mcL (reference range, 4500–11,000/mcL), hemoglobin of 13.6 g/dL (reference range, 14–17.5 g/dL), and a platelet count of 162 × 103/mcL (reference range, 150–350 × 103/mcL). The patient’s electrolytes were within normal limits, with a creatinine level of 0.67 mg/dL (reference range, 0.1–1.2 mg/dL) and a calcium level of 9.4 mg/dL (reference range, 8.2–10.2 mg/dL). Lactate dehydrogenase was elevated at 308 U/L (reference range, 100–200 U/L).
A chest radiograph revealed a right upper lobe mass, right lower lobe consolidation, and a large right-sided pleural effusion (FIGURE 1). Chest computed tomography (CT) with intravenous contrast revealed a 5.2 × 5.7–cm right pleural-based mass with extension to the anterior chest wall, 3 left-sided pulmonary nodules, numerous right-sided pleural-based nodules (FIGURE 2), and multiple low-density liver lesions. An abdominal and pelvic CT scan revealed a 5.6 × 2.5 × 4.8–cm hepatic lesion (FIGURE 3) with scattered hepatic cysts.
THE DIAGNOSIS
A diagnostic thoracentesis was performed, and pleural fluid cytology results revealed metastatic melanoma. Magnetic resonance imaging (MRI) of the brain showed no evidence of metastases.
After the patient’s initial presentation, her dermatologist performed a biopsy on a pre-existing skin lesion on the patient’s left abdomen, which initially was thought to be a cherry angioma. This left abdominal skin lesion was in a location different from her previous melanoma in situ, which was located on the right side of the back. Biopsy results of the presumed cherry angioma revealed a nodular malignant melanoma (which was partially removed), adjacent to a cherry angioma (which was completely excised).
Continue to: Two primary melanomas
Two primary melanomas. Our patient had 2 different primary melanomas: a melanoma in situ on the right back diagnosed 24 years prior to the current presentation and the more recently identified melanoma on the left abdomen with metastases to the lung and liver.
We referred the patient to Oncology and she was enrolled in a clinical study with ipilimumab and nivolumab, monoclonal antibodies directed against negative regulators of T-cell activation.
DISCUSSION
In the United States, melanoma is the fifth leading cancer in men and the sixth leading cancer in women.1 A prior history of melanoma or melanoma in situ increases the risk for a second melanoma,2-4 and the risk remains elevated for more than 20 years after the initial diagnosis.2 One- and 2-year survival rates for metastatic melanoma are 32% to 65% and 18% to 40%, respectively5; the 5-year survival rate of metastatic melanoma to the lung is approximately 16%.6
Recommendations regarding the appropriate follow-up of patients with a history of melanoma in situ and melanoma vary widely.7 For patients with a history of melanoma in situ, the American Academy of Dermatology and the National Comprehensive Cancer Network recommend annual skin examination indefinitely and self-examination of the skin and lymph nodes monthly.4,7,8
Novel therapies are powerful allies in fight against melanoma
Previous standard treatment of metastatic melanoma included surgery, radiation, and cytotoxic chemotherapy. Resection rarely is curative in distant metastatic melanoma, and cytotoxic chemotherapy has low response rates, has a response duration of 4 to 6 months, and does not improve overall survival in advanced melanoma.9-12
Continue to: Novel therapies...
Novel therapies, such as immunotherapy and molecular-targeted therapies, are dramatically increasing survival rates in metastatic melanoma. Melanoma frequently is associated with somatic mutations, and each patient may have a unique collection of mutations resulting in the expression of antigens that bind to certain T-cell receptors, which serve as targets for inhibitor immunotherapy.
Ipilimumab and nivolumab are monoclonal antibodies directed against negative regulators of T-cell activation. When ipilimumab and nivolumab bind to their receptors, feedback inhibition is prevented, which results in an immune response against the tumor. In a trial of 53 patients with advanced melanoma treated with both drugs, the overall survival rate at 1 and 2 years was 94% and 88%, respectively.13
Dabrafenib and trametinib. Mutations that activate the serine/threonine kinase gene, BRAF, are present in approximately 40% to 60% of advanced melanomas and lead to clonal expansion and tumor progression.14,15 Inhibition of BRAF produces rapid tumor regression—even in extensive disease. Treatment with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase inhibitor, has been shown to be superior to a BRAF inhibitor alone and is associated with a survival rate of 72% at 1 year.16
Our patient. Seven months after enrolling in the clinical trial with ipilimumab and nivolumab, our patient developed brain metastases and was withdrawn from the trial. A resection of her brain metastases and radiation therapy followed. The patient was then started on molecular-targeted therapy with dabrafenib and trametinib. Twelve weeks later, a repeat CT scan of the chest, abdomen, and pelvis demonstrated an interval decrease in the size of the majority of the metastatic lesions, and a repeat brain MRI showed no additional metastases.
More than 4 years after her diagnosis, our patient remains on dabrafenib and trametinib therapy and her metastatic lesions to the lung and liver remain stable.
Continue to: THE TAKEAWAY
THE TAKEAWAY
Patients with a prior melanoma in situ or invasive melanoma have a higher risk for a subsequent invasive melanoma, and this risk remains elevated for more than 20 years. While patients with a history of melanoma in situ do not require specific oncologic follow-up, they do require annual dermatologic follow-up indefinitely and should perform monthly self-examination of their skin and lymph nodes.
Heightened awareness of the risk for a second primary melanoma should prompt primary care physicians to conduct ongoing patient surveillance. Family physicians should also keep in mind that novel therapies for metastatic melanoma, such as molecular-targeted therapies and immunotherapy, are associated with a much higher survival rate than previous standard therapy.
CORRESPONDENCE
Iris Tong, MD, Associate Professor, Division of General Internal Medicine, Department of Medicine, Alpert Medical School of Brown University, 146 W River St, Providence, RI 02904; [email protected]
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018 [published online January 4, 2018]. Cancer J Clin. 2018;68:7-30.
2. Bradford PT, Freedman DM, Goldstein AM, et al. Increased risk of second primary cancers after diagnosis of melanoma. Arch Dermatol. 2010;146:265-272.
3. Balamurugan A, Rees JR, Kosary C, et al. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J Am Acad Dermatol. 2011;65(5) (suppl 1):S69-S77.
4. Pomerantz H, Huang D, Weinstock MA. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: a population-based study from 1973 to 2011. J Am Acad Dermatol. 2015;72:794-800.
5. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
6. American Cancer Society. Cancer facts & figures 2015. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. Accessed November 26, 2018.
7. Coit DG, Andtbacka R, Anker CJ, et al. Melanoma: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;3:366-400.
8. Bichakjian CK, Halpern AC, Johnson TM. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;5:1032-1047.
9. Atkins MB. The role of cytotoxic chemotherapeutic agents either alone or in combination with biological response modifiers. In: Kirkwood JK, ed. Molecular Diagnosis, Prevention & Therapy of Melanoma. New York, NY: Marcel Dekker; 1997:219.
10. Patel PM, Suciu S, Mortier L, et al; EORTC Melanoma Group. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032) [published online May 18, 2011]. Eur J Cancer. 2011;47:1476-1483.
11. Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma [published online December 17, 2012]. J Clin Oncol. 2013;31:373-379.
12. Sosman JA, Moon J, Tuthill RJ, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430 [published online March 31, 2011]. Cancer. 2011;117:4740-4746.
13. Sznol M, Kluger HM, Callahan MK, et al. Abstract LBA9003. Presented at: 2014 American Society of Clinical Oncology (ASCO) Annual Meeting; May 30–June 3, 2014; Chicago, IL.
14. Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res. 2003;9:6483-6488.
15. Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
16. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
Pain in right shoulder • recent influenza vaccination • history of hypertension and myocardial infarction • Dx?
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
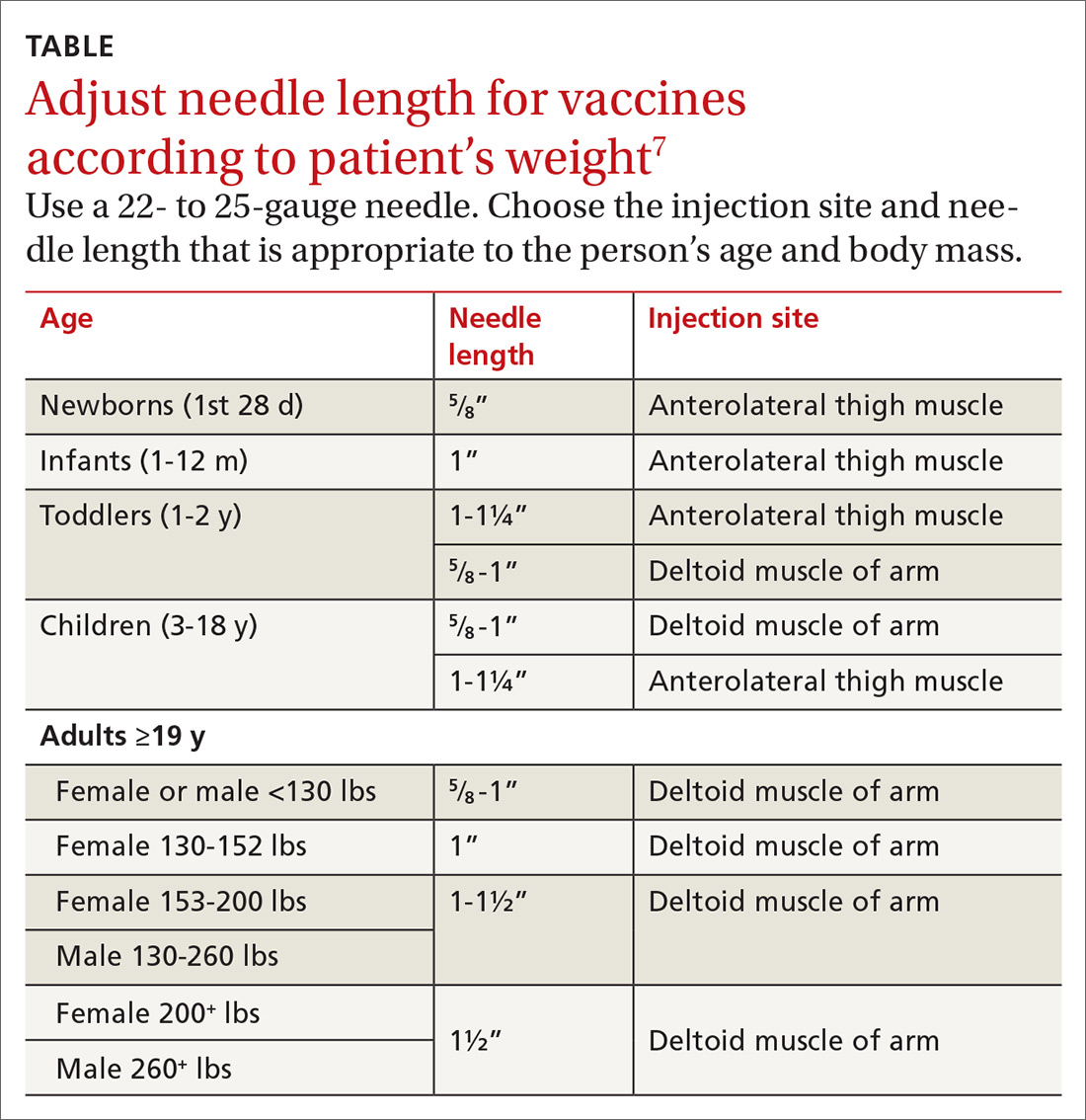
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; [email protected]
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; [email protected]
THE CASE
A 61-year-old Caucasian woman presented with acute right shoulder pain that began after she received an influenza vaccination at a local pharmacy 2 weeks earlier. She pointed to the proximal-most aspect of her lateral right upper arm as the vaccination site. Her pain intensified with shoulder abduction, forward flexion, and reaching movements. She denied recent and past injury to her shoulder, fever, chills, rash, or skin changes at the injection site. She said her left shoulder did not bother her.
The patient had continued to participate in her aerobics class, despite the discomfort. Her medical history included hypertension and myocardial infarction, and the medications she was taking included lisinopril 20 mg/d, atenolol 50 mg/d, and aspirin 81 mg/d.
The physical exam revealed a thin female with no visible rashes or erythema on her right shoulder. While there was no deltoid atrophy in comparison to her unaffected shoulder, she generally had low muscle mass in both arms. A painful arc of abduction was present, as was pain with palpation of the supraspinatus insertion. No pain was appreciated over the short or long head of the biceps tendon or the sternoclavicular or acromioclavicular joints. Strength was 5/5 for all movements of the rotator cuff, but pain was reproduced with resisted shoulder abduction. A Hawkin’s test was positive, while Speed’s, Yergason’s, cross-arm abduction, and O’Brien’s tests were all negative.
THE DIAGNOSIS
Anteroposterior, Grashey, Y-view, and axillary view radiographs of the right shoulder were normal without any calcific tendinopathy, degenerative changes, or acute fractures. The patient’s history and physical exam were consistent with a rotator cuff tendinitis secondary to an immune response to an influenza vaccination that infiltrated the supraspinatus tendon.
DISCUSSION
Soreness, redness and swelling at the injection site, fever, body aches, and headache are common adverse effects of the influenza vaccine.1Although rare, acute brachial neuritis, infection, rotator cuff injuries, and contusions of the humeral head have also been reported. 2-5 Collectively, these conditions are referred to as shoulder injuries related to vaccination administration (SIRVA). There have been multiple SIRVA cases reported in the United States, and the US Court of Federal Claims has compensated >100 patients for SIRVA since 2011.6 There is currently no listing of SIRVA as a potential adverse reaction to the influenza vaccine on the package inserts or on the Centers for Disease Control and Prevention (CDC) Web site.
Shoulder soreness lasting <72 hours without functional impairment is likely due to soreness at the injection site. If symptoms do not resolve within 72 to 96 hours, consider a more thorough workup, with SIRVA being a possible diagnosis.1,7 The etiology of SIRVA remains uncertain, but an inflammatory reaction from a vaccine mistakenly administered into the subacromial/subdeltoid bursa has been suggested. Whether this reaction is dependent on the nonantigenic or antigenic components of the vaccine has yet to be determined.
Symptoms of SIRVA include pain with arm movement, pain that is worse at night or awakens the patient from sleep, restricted range of motion, or arm weakness. Examination will reveal pain when resisting rotator cuff movements, particularly shoulder abduction. Advanced imaging can be considered when the diagnosis is in question. In previous cases of vaccine-associated rotator cuff tendinopathy in the authors’ practice, T2 magnetic resonance imaging (MRI) has shown focal inflammatory signal within the supraspinatus tendon and subacromial bursa.
Continue to: With support from the CDC...
With support from the CDC, the Immunization Action Coalition (IAC), a source of immunization information for health care professionals, recommends that vaccines be administered into the deltoid or vastus lateralis for individuals between the ages of 3 and 18 years and recommends the deltoid as the preferred location in adults ≥19 years. The IAC suggests increasing the needle length for intramuscular (IM) immunizations (depending on the weight of the patient), although in the authors’ experience, the adjustment of needle length may often be overlooked (TABLE7).
The majority of reported SIRVA cases caused by overpenetration have occurred in individuals weighing <140 lb or those who had little deltoid muscle bulk. An MRI study to evaluate optimal intramuscular needle length in pediatric patients found that the IAC-recommended needle lengths still allowed penetration of the subdeltoid space in a substantial number of patients.8 Classic teaching of IM deltoid injection landmarks is 3 fingerbreadths distal to the acromion, and a more proximal administration of a vaccine would allow penetration of the rotator cuff structures below.
How to manage the patient
Patients who develop SIRVA should be managed similarly to patients with tendinopathy from other causes. Treatment options include: physical therapy, anti-inflammatory medications, and subacromial corticosteroid injections. Given the significant discomfort and nighttime pain associated with rotator cuff tendinopathy, corticosteroid injections can offer rapid relief.
Limited data exist on the effect of corticosteroids on the suppression of the immune response in immunocompetent patients. Vaccinations are generally thought to stimulate an adequate immune response 14 days following administration, so our suggestion would be to re-vaccinate patients if a corticosteroid injection to treat SIRVA is completed prior to this.9
Our patient’s outcome
We talked to the patient about treatment options, which included physical therapy and nonsteroidal anti-inflammatory drugs (NSAIDs), but the patient elected to go forward with a corticosteroid injection. We administered 2 cc of Depo-Medrol 40 mg/mL with 2 cc of 1% lidocaine without epinephrine and 2 cc of 0.5% ropivacaine into her right shoulder subacromial space using a posterior approach. The patient noticed a 70% improvement in her pain immediately following the injection.
Continue to: Considering her influenza vaccine...
Considering her influenza vaccine was administered more than 14 days prior to her corticosteroid injection, we felt that she had mounted enough of an immune response for the vaccination to have been adequate for protection.9 Therefore, we told her that she didn’t need to be revaccinated for influenza this season. The case was reported to the Vaccine Adverse Event Reporting System (VAERS).
At the patient’s 2-month follow up, she reported an overall 80% improvement in pain. She continued to have occasional discomfort with certain movements, although the pain was relieved with over-the-counter anti-inflammatory medication. On physical exam she had an intact arc of abduction of the right shoulder to 150° without pain. Forward flexion and external and internal rotation were normal and pain free. She had mild pain with resisted abduction and a positive Hawkin’s test. The patient agreed to go to physical therapy to work on rotator cuff strengthening. She denied any known influenza infection up to that time.
THE TAKEAWAY
It’s important to consider rotator cuff injuries or SIRVA as a potential adverse effect of influenza vaccination administration. Thin patients and those with low deltoid muscle mass are at risk of vaccine over-penetration, and proximally placed deltoid vaccines may reach the rotator cuff structures below. Staff should be trained on appropriate techniques for administering influenza vaccinations to avoid causing SIRVA. Specifically:
- Intramuscular vaccines injected into the deltoid muscle should be 3 fingerbreadths distal to the acromion. A more proximal approach could potentially contact the rotator cuff muscles.
- Vaccine administration should mirror the position of the patient (eg, if the patient is sitting, the administrator should be sitting; if the patient is standing, the administrator should be standing).
- Needle length for vaccine administration should be adjusted according to the patient’s weight (TABLE7).
Following vaccination, it is important to keep 2 other points in mind. First, if a subacromial corticosteroid injection is used for treatment of SIRVA within the first 2 weeks of vaccine administration, consider revaccination. Second, be sure to use the VAERS to report any clinically significant medical event that occurs after vaccination. VAERS is a national vaccine safety surveillance program that is supported by the CDC and the US Food and Drug Administration. The VAERS reporting system can be accessed through www.vaers.hhhs.gov.
CORRESPONDENCE
Dusty Marie Narducci, MD, 5290 Big Island Drive, Unit 1303, Jacksonville, FL 32246; [email protected]
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
1. Centers for Disease Control and Prevention. Flu vaccine safety information. https://www.cdc.gov/flu/protect/vaccine/general.htm. Updated October 23, 2018. Accessed January 2, 2019.
2. Barnes MG, Ledford C, Hogan K. A “needling” problem: shoulder injury related to vaccine administration. J Am Board Fam Med. 2012;25:919-922.
3. Shaikh MF, Baqai TJ, Tahir H. Acute brachial neuritis following influenza vaccine. BMJ Case Rep. 2012. doi:10.1136/bcr-2012-007673.
4. Miller JD, Pruitt S, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
5. Atanasoff S, Ryan T, Lightfoot R, et al. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28:8049-8052.
6. Dugan IJ. Vaccine injury payouts rise. The Wall Street Journal. August 24, 2015. https://www.wsj.com/articles/vaccine-injury-payouts-rise-1440430702. Accessed December 3, 2018.
7. Immunization Action Coalition. Administering vaccines: dose, route, site, and needle size. www.immunize.org/catg.d/p3085.pdf. Accessed January 3, 2019.
8. Lippert WC, Wall EJ. Optimal intramuscular needle-penetration depth. Pediatrics. 2008;122:e556-e563.
9. Kroger AT, Sumaya CV, Pickering LK, et al. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers of Disease Control and Prevention Web site. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6002a1.htm. Published January 28, 2011. Accessed December 3, 2018.
Less is more when it comes to ketorolac for pain
ILLUSTRATIVE CASE
A 46-year-old man with no significant past medical history presents to the emergency department (ED) with right flank pain and nausea. A computed tomography scan reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning on starting him on intravenous (IV) ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective nonsteroidal anti-inflammatory drug (NSAID). As a non-opiate analgesic, it is often the optimal first choice for the treatment of acute conditions such as flank, abdominal, musculoskeletal, and headache pains.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries a US Food and Drug Administration black box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose,” a dose at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the analgesic ceiling dose of ketorolac as 10 mg across dosage forms.4,5 Yet, the majority of research and the majority of health care providers in current practice use higher doses of 20 to 60 mg. The US Food and Drug Administration label provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose of 10 mg in atleast 97% of patients who received IV doses and in at least 96% of patients receiving intramuscular (IM) doses in a US emergency department.6 If ketorolac 10 mg is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
Though often used at higher doses, 10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of 3 different doses of IV ketorolac for acute pain in 240 adult patients, ages 18 to 65 years, presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset ≤30 days.
Patients were randomized to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, and the syringes were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0 to 10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine 0.1 mg/kg was offered. The primary outcome was numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue medication.
The groups were similar in terms of demographics and baseline vital signs. The mean age of the participants was 39 to 42 years. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores were similar...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, but there was no difference between the groups; for the 10- and 15-mg groups, the mean pain scores post-intervention were 5.1 (95% confidence interval [CI] 4.5-5.7 and 4.5-5.6, respectively); and for the 30-mg group, the mean pain score was 4.8 (95% CI, 4.2-5.5). No P values were provided. There was no difference between the groups at any other time intervals. There was also no difference in the number of patients who needed rescue medication (morphine) at 30 minutes between the groups (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group; no P values were provided). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose (10 mg) of IV ketorolac is just as effective for acute pain control as higher 15- and 30-mg doses.
CAVEATS
A 2-hour time limit and no look at long-term effects
The ketorolac dose of 10 mg IV was specially prepared by the study pharmacist; it is unlikely this will be readily available in clinical settings. However, the 15-mg IV dose is also as effective as the higher 30-mg dose based on study results and is readily available.
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects like bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
A 10-mg single-dose vial is not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared. However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
2. Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Labratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
ILLUSTRATIVE CASE
A 46-year-old man with no significant past medical history presents to the emergency department (ED) with right flank pain and nausea. A computed tomography scan reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning on starting him on intravenous (IV) ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective nonsteroidal anti-inflammatory drug (NSAID). As a non-opiate analgesic, it is often the optimal first choice for the treatment of acute conditions such as flank, abdominal, musculoskeletal, and headache pains.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries a US Food and Drug Administration black box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose,” a dose at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the analgesic ceiling dose of ketorolac as 10 mg across dosage forms.4,5 Yet, the majority of research and the majority of health care providers in current practice use higher doses of 20 to 60 mg. The US Food and Drug Administration label provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose of 10 mg in atleast 97% of patients who received IV doses and in at least 96% of patients receiving intramuscular (IM) doses in a US emergency department.6 If ketorolac 10 mg is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
Though often used at higher doses, 10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of 3 different doses of IV ketorolac for acute pain in 240 adult patients, ages 18 to 65 years, presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset ≤30 days.
Patients were randomized to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, and the syringes were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0 to 10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine 0.1 mg/kg was offered. The primary outcome was numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue medication.
The groups were similar in terms of demographics and baseline vital signs. The mean age of the participants was 39 to 42 years. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores were similar...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, but there was no difference between the groups; for the 10- and 15-mg groups, the mean pain scores post-intervention were 5.1 (95% confidence interval [CI] 4.5-5.7 and 4.5-5.6, respectively); and for the 30-mg group, the mean pain score was 4.8 (95% CI, 4.2-5.5). No P values were provided. There was no difference between the groups at any other time intervals. There was also no difference in the number of patients who needed rescue medication (morphine) at 30 minutes between the groups (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group; no P values were provided). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose (10 mg) of IV ketorolac is just as effective for acute pain control as higher 15- and 30-mg doses.
CAVEATS
A 2-hour time limit and no look at long-term effects
The ketorolac dose of 10 mg IV was specially prepared by the study pharmacist; it is unlikely this will be readily available in clinical settings. However, the 15-mg IV dose is also as effective as the higher 30-mg dose based on study results and is readily available.
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects like bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
A 10-mg single-dose vial is not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared. However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 46-year-old man with no significant past medical history presents to the emergency department (ED) with right flank pain and nausea. A computed tomography scan reveals a 5-mm ureteral stone with no obstruction or hydronephrosis. You are planning on starting him on intravenous (IV) ketorolac for pain. What is the most appropriate dose?
Ketorolac tromethamine is a highly effective nonsteroidal anti-inflammatory drug (NSAID). As a non-opiate analgesic, it is often the optimal first choice for the treatment of acute conditions such as flank, abdominal, musculoskeletal, and headache pains.2 While it is not associated with euphoria, withdrawal effects, or respiratory depression (like its opiate analgesic counterparts), ketorolac carries a US Food and Drug Administration black box warning for gastrointestinal, cardiovascular, renal, and bleeding risks.3
NSAIDs are known to have a “ceiling dose,” a dose at which maximum analgesic benefit is achieved; higher doses will not provide further pain relief. Higher doses of ketorolac may be used when anti-inflammatory effects of NSAIDs are desired, but they are likely to cause more adverse effects.4 Available data describe the analgesic ceiling dose of ketorolac as 10 mg across dosage forms.4,5 Yet, the majority of research and the majority of health care providers in current practice use higher doses of 20 to 60 mg. The US Food and Drug Administration label provides for a maximum dose of 60 mg/d.3
In one recent study, ketorolac was prescribed above its ceiling dose of 10 mg in atleast 97% of patients who received IV doses and in at least 96% of patients receiving intramuscular (IM) doses in a US emergency department.6 If ketorolac 10 mg is an effective analgesic dose, current practice exceeds the label recommendation to use the lowest effective dose. This study sought to determine the comparative efficacy of 3 different doses of IV ketorolac for acute pain management in an ED.
STUDY SUMMARY
Though often used at higher doses, 10 mg of ketorolac is enough for pain
This randomized double-blind trial evaluated the effectiveness of 3 different doses of IV ketorolac for acute pain in 240 adult patients, ages 18 to 65 years, presenting to an ED with acute flank, abdominal, musculoskeletal, or headache pain.1 Acute pain was defined as onset ≤30 days.
Patients were randomized to receive either 10, 15, or 30 mg of IV ketorolac in 10 mL of normal saline. A pharmacist prepared the medication in identical syringes, and the syringes were delivered in a blinded manner to the nurses caring for the patients. Pain (measured using a 0 to 10 scale), vital signs, and adverse effects were assessed at baseline and at 15, 30, 60, 90, and 120 minutes. If patients were still in pain at 30 minutes, IV morphine 0.1 mg/kg was offered. The primary outcome was numerical pain score at 30 minutes after ketorolac administration; secondary outcomes included the occurrence of adverse events and the use of rescue medication.
The groups were similar in terms of demographics and baseline vital signs. The mean age of the participants was 39 to 42 years. Across the 3 groups, 36% to 40% of patients had abdominal pain, 26% to 39% had flank pain, 20% to 26% had musculoskeletal pain, and 1% to 11% had headache pain. Patients had pain for an average of 1.5 to 3.5 days.
Continue to: Baseline pain scores were similar...
Baseline pain scores were similar for all 3 groups (7.5-7.8 on a 10-point scale). In the intention-to-treat analysis, all 3 doses of ketorolac decreased pain significantly at 30 minutes, but there was no difference between the groups; for the 10- and 15-mg groups, the mean pain scores post-intervention were 5.1 (95% confidence interval [CI] 4.5-5.7 and 4.5-5.6, respectively); and for the 30-mg group, the mean pain score was 4.8 (95% CI, 4.2-5.5). No P values were provided. There was no difference between the groups at any other time intervals. There was also no difference in the number of patients who needed rescue medication (morphine) at 30 minutes between the groups (4 patients in the 10-mg group, 3 patients in the 15-mg group, and 4 patients in the 30-mg group; no P values were provided). In addition, adverse events (eg, dizziness, nausea, headache, itching, flushing) did not differ between the groups.
WHAT’S NEW
10 mg is just as effective as 30 mg
This trial confirms that a low dose (10 mg) of IV ketorolac is just as effective for acute pain control as higher 15- and 30-mg doses.
CAVEATS
A 2-hour time limit and no look at long-term effects
The ketorolac dose of 10 mg IV was specially prepared by the study pharmacist; it is unlikely this will be readily available in clinical settings. However, the 15-mg IV dose is also as effective as the higher 30-mg dose based on study results and is readily available.
It isn’t known whether the higher dose would have provided greater pain relief beyond the 120 minutes evaluated in this trial, or if alternative dosage forms (oral or IM) would result in different outcomes. This study was not designed to compare serious long-term adverse effects like bleeding, renal impairment, or cardiovascular events. Additionally, this study was not powered to look at specific therapeutic indications or anti-inflammatory response.
CHALLENGES TO IMPLEMENTATION
A 10-mg single-dose vial is not readily available
Ketorolac tromethamine for injection is available in the United States in 15-, 30-, and 60-mg single-dose vials. Because a 10-mg dose is not available as a single-dose vial, it would need to be specially prepared. However, this study should reassure providers that using the lowest available dose (eg, 15 mg IV if that is what is available) will relieve acute pain as well as higher doses.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
2. Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Labratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
1. Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
2. Buckley MM, Brogden RN. Ketorolac. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential. Drugs. 1990;39:86-109.
3. Ketorolac tromethamine [package insert]. Bedford, OH: Bedford Labratories; 2009.
4. Catapano MS. The analgesic efficacy of ketorolac for acute pain. J Emerg Med. 1996;14:67-75.
5. García Rodríguez LA, Cattaruzzi C, Troncon MG, et al. Risk of hospitalization for upper gastrointestinal tract bleeding associated with ketorolac, other nonsteroidal anti-inflammatory drugs, calcium antagonists, and other antihypertensive drugs. Arch Intern Med. 1998;158:33-39.
6. Soleyman-Zomalan E, Motov S, Likourezos A, et al. Patterns of ketorolac dosing by emergency physicians. World J Emerg Med. 2017;8:43-46.
PRACTICE CHANGER
Use a low dose (10 mg) of intravenous ketorolac for moderate to severe acute pain in adults because it is as effective as higher doses (15-30 mg).1
STRENGTH OF RECOMMENDATION
B: Based on a single, good-quality randomized controlled trial.
Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70:177-184.
Migraine: Expanding our Tx arsenal
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
Migraine is a highly disabling primary headache disorder that affects more than 44 million Americans annually.1 The disorder causes pain, photophobia, phonophobia, and nausea that can last for hours, even days. Migraine headaches are 2 times more common in women than in men; although migraine is most common in people 30 to 39 years of age, all ages are affected.2,3 Frequency of migraine headache is variable; chronic migraineurs experience more than 15 headache days a month.
Recent estimates indicate that the cost of acute and chronic migraine headaches reaches approximately $78 million a year in the United States. 4 This high burden of disease has made effective migraine treatment options absolutely essential. Recent advances in our understanding of migraine pathophysiology have led to new therapeutic targets; there are now many novel treatment approaches on the horizon.
In this article, we review the diagnosis and management of migraine in detail. Our emphasis is on evidence-based approaches to acute and prophylactic treatment, including tried-and-true options and newly emerging therapies.
Neuronal dysfunction and a genetic predisposition
Although migraine was once thought to be caused by abnormalities of vasodilation, current research suggests that the disorder has its origins in primary neuronal dysfunction. There appears to be a genetic predisposition toward widespread neuronal hyperexcitability in migraineurs.5 In addition, hypothalamic neurons are thought to initiate migraine by responding to changes in brain homeostasis. Increased parasympathetic tone might activate meningeal pain receptors or lower the threshold for transmitting pain signals from the thalamus to the cortex.6
Prodromal symptoms and aura appear to originate from multiple areas across the brain, including the hypothalamus, cortex, limbic system, and brainstem. This widespread brain involvement might explain why some headache sufferers concurrently experience a variety of symptoms, including fatigue, depression, muscle pain, and an abnormal sensitivity to light, sound, and smell.6,7
Although the exact mechanisms behind each of these symptoms have yet to be defined precisely, waves of neuronal depolarization—known as cortical spreading depression—are suspected to cause migraine aura.8-10 Cortical spreading depression activates the trigeminal pain pathway and leads to the release of pro-inflammatory markers such as calcitonin gene-related protein (CGRP).6 A better understanding of these complex signaling pathways has helped provide potential therapeutic targets for new migraine drugs.
Diagnosis: Close patient inquiry is most helpful
The International Headache Society (IHS) criteria for primary headache disorders serve as the basis for the diagnosis of migraine and its subtypes, which include migraine without aura and migraine with aura. Due to variability of presentation, migraine with aura is further subdivided into migraine with typical aura (with and without headache), migraine with brainstem aura, hemiplegic migraine, and retinal migraine.11
Continue to: How is migraine defined?
How is migraine defined? Simply, migraine is classically defined as a unilateral, pulsating headache of moderate to severe intensity lasting 4 to 72 hours, associated with photophobia and phonophobia or nausea and vomiting, or both.11 Often visual in nature, aura is a set of neurologic symptoms that lasts for minutes and precedes the onset of the headache. The visual aura is often described as a scintillating scotoma that begins near the point of visual fixation and then spreads left or right. Other aura symptoms include tingling or numbness (second most common), speech disturbance (aphasia), motor changes and, in rare cases, a combination of these in succession. By definition, all of these symptoms fully resolve between attacks.11
Validated valuable questionnaires. To help with accurate and timely diagnosis, researchers have developed and validated simplified questionnaires that can be completed independently by patients presenting to primary care (TABLE 112,13):
- ID Migraine is a set of 3 questions that scores positive when a patient endorses at least 2 of the 3 symptoms. 12
- MS-Q is similar to the ID Migraine but includes 5 items. A score of ≥4 is a positive screen. 13
The sensitivity and specificity of MS-Q (0.93 and 0.81, respectively) are slightly higher than those of ID Migraine (0.81 and 0.75).13
Remember POUND. This mnemonic device can also be used during history-taking to aid in diagnostic accuracy. Migraine is highly likely (92%) in patients who endorse 4 of the following 5 symptoms and unlikely (17%) in those who endorse ≤2 symptoms14: Pulsatile quality of headache 4 to 72 hOurs in duration, Unilateral location, Nausea or vomiting, and Disabling intensity.
Differential Dx. Although the differential diagnosis of headache is broad (TABLE 214,15), the history alone can often guide clinicians towards the correct assessment. After taking the initial history (headache onset, location, duration, and associated symptoms), focus your attention on assessing the risk of intracranial pathology. This is best accomplished by assessing specific details of the history (TABLE 314) and findings on physical examination15:
- blood pressure measurement (seated, legs uncrossed, feet flat on the floor; having rested for 5 minutes; arm well supported)
- cranial nerve exam
- extremity strength testing
- eye exam (vision, extra-ocular muscles, visual fields, pupillary reactivity, and funduscopic exam)
- gait (tandem walk)
- reflexes.
Continue to: Further testing needed?
Further testing needed? Neuroimaging should be considered only in patients with an abnormal neurologic exam, atypical headache features, or certain risk factors, such as an immune deficiency. There is no role for electroencephalography or other diagnostic testing in migraine.16
Take a multipronged approach to treatment
As with other complex, chronic conditions, the treatment of migraine should take a multifaceted approach, including management of acute symptoms as well as prevention of future headaches. In 2015, the American Headache Society published a systematic review that specified particular treatment goals for migraine sufferers. 17 These goals include:
- headache reduction
- headache relief
- decreased disability from headache
- elimination of nausea and vomiting
- elimination of photophobia and phonophobia.
Our review, which follows, of therapeutic options focuses on the management of migraine in adults. Approaches in special populations (older adults, pregnant women, and children) are discussed afterward.
Pharmacotherapy for acute migraine
Acute migraine should be treated with an abortive medication at the onset of headache. The immediate goal is to relieve pain within 2 hours and prevent its recurrence within the subsequent 48 hours (TABLE 412,18-20).
In the general population, mild, infrequent migraines can be managed with acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs).21
Continue to: For moderate-to-severe migraine...
For moderate-to-severe migraine, triptans, which target serotonin receptors, are the drug of choice for most patients.21 Triptans are superior to placebo in achieving a pain-free state at 2 and 24 hours after administration; eletriptan has the most desirable outcome, with 68% of patients pain free at 2 hours and 54% pain free at 24 hours.22 Triptans are available as sublingual tablets and nasal sprays, as well as subcutaneous injections for patients with significant associated nausea and vomiting. Avoid prescribing triptans for patients with known vascular disease (eg, history of stroke, myocardial infarction, peripheral vascular disease, uncontrolled hypertension, or signs and symptoms of these conditions), as well as for patients with severe hepatic impairment.
Importantly, although triptans all have a similar mechanism of action, patients might respond differently to different drugs within the class. If a patient does not get adequate headache relief from an appropriate dosage of a given triptan during a particular migraine episode, a different triptan can be tried during the next migraine.22 Additionally, if a patient experiences an adverse effect from one triptan, this does not necessarily mean that a trial of another triptan at a later time is contraindicated.
For patients who have an incomplete response to migraine treatment or for those with frequent recurrence, the combination formulation of sumatriptan, 85 mg, and naproxen, 500 mg, showed the highest rate of resolution of headache within 2 hours compared with either drug alone.23 A similar result might be found by combining a triptan known to be effective for a patient and an NSAID other than naproxen. If migraine persists despite initial treatment of an attack, a different class of medication should be tried during the course of that attack to attain relief of symptoms of that migraine.21
When a patient is seen in an acute care setting (eg, emergency department, urgent care center) while suffering a migraine, additional treatment options are available. Intravenous (IV) anti-emetics are useful for relieving the pain of migraine and nausea, and can be used in combination with an IV NSAID (eg, ketorolac).21 The most effective anti-emetics are dopamine receptor type-2 blockers, including chlorpromazine, droperidol, metoclopramide, and prochlorperazine, which has the highest level of efficacy.24 Note that these medications do present the risk of a dystonic reaction; diphenhydramine is therefore often used in tandem to mitigate such a response.
Looking ahead. Although triptans are the current first-line therapy for acute migraine, their effectiveness is limited. Only 20% of patients report sustained relief of pain in the 2 to 24 hours after treatment, and the response can vary from episode to episode.25
Continue to: With better understading of the pathophysiology of migraine...
With better understanding of the pathophysiology of migraine, a host of novel anti-migraine drugs are on the horizon.
CGRP receptor antagonists. The neuropeptide CGRP, which mediates central and peripheral nervous system pain signaling, has been noted to be elevated during acute migraine attacks26; clinical trials are therefore underway to evaluate the safety and efficacy of CGRP receptor antagonists.18 These agents appear to be better tolerated than triptans, have fewer vascular and central nervous system adverse effects, and present less of a risk of medication overuse headache.18 Liver toxicity has been seen with some medications in this class and remains an important concern in their development.19
Phase 3 clinical trials for 1 drug in this class, ubrogepant, were completed in late 2017; full analysis of the data is not yet available. Primary outcomes being evaluated include relief of pain at 2 hours and relief from the most bothersome symptoms again at 2 hours.27
Selective serotonin-HT1f receptor agonists, such as lasmiditan, offer another potential approach. Although the exact mechanism of action of these agents is not entirely clear, clinical trials have supported their efficacy and safety.20 Importantly, ongoing trials are specifically targeting patients with known cardiovascular risk factors because they are most likely to benefit from the nonvasoconstrictive mechanism of action.28,29 Adverse effects reported primarily include dizziness, fatigue, and vertigo.
Strategies for managing recurrent episodic migraine
Because of the risk of medication overuse headache with acute treatment, daily preventive therapy for migraine is indicated for any patient with 30 :
- ≥6 headache days a month
- ≥4 headache days a month with some impairment
- ≥3 headache days a month with severe impairment.
Continue to: Treatment begins by having patients identify...
Treatment begins by having patients identify, and then avoid, migraine triggers (TABLE 5). This can be accomplished by having patients keep a headache diary, in which they can enter notations about personal and environmental situations that precede a headache.
For the individual patient, some triggers are modifiable; others are not. Helping a patient develop strategies for coping with triggers, rather than aiming for complete avoidance, might help her (him) manage those that are inescapable (eg stress, menstruation, etc).31 For many patients, however, this is not an adequate intervention and other approaches must be explored. When considering which therapy might be best for a given patient, evaluate her (his) comorbidities and assess that particular treatment for potential secondary benefits and the possibility of adverse effects. Pay attention to the choice of preventive therapy in women who are considering pregnancy because many available treatments are potentially teratogenic.
Oral medications. Oral agents from several classes of drugs can be used for migraine prophylaxis, including anti-epileptics,antidepressants, and antihypertensives (TABLE 620,29,30,32-41). Selected anti-epileptics (divalproex sodium, sodium valproate, topiramate) and beta-blockers (metoprolol, propranolol, and timolol) have the strongest evidence to support their use.32 Overall, regular use of prophylactic medications can reduce headache frequency by 50% for approximately 40% to 45% of patients who take them.29 However, adherence may be limited by adverse effects or perceived lack of efficacy, thus reducing their potential for benefit.42
OnabotulinumtoxinA. In patients with chronic migraine (≥15 headache days a month for at least 3 months) who have failed oral medications, the American Academy of Neurology (AAN) recommends the use of onabotulinumtoxinA.30 The treatment regimen comprises 31 injections at various sites on the head, neck, and shoulders every 3 months.33
A 2010 large randomized controlled trial showed a decrease in the frequency of headache days for patients receiving onabotulinumtoxinA compared to placebo after a 24-week treatment period (7.8 fewer headache days a month, compared to 6.4 fewer in the placebo group).33 A recent systematic review also noted a reduction of 2 headache days a month compared with placebo; the authors cautioned, however, that data with which to evaluate onabotulinumtoxinA in comparison to other prophylactic agents are limited.43
Continue to: In both studies...
In both studies, the risk of adverse drug events due to onabotulinumtoxinA was high and led to a significant rate of discontinuation.33,43 Despite this, onabotulinumtoxinA remains the only Food and Drug Administration (FDA)–approved treatment for chronic migraine, making it reasonable to consider for appropriate patients.
Acupuncture. A 2016 Cochrane review found benefit for patients using acupuncture compared with sham acupuncture.34 When acupuncture was compared with prophylactic agents such as beta-blockers, calcium-channel blockers, and anti-epileptics, however, there was no significant difference between the procedure and pharmacotherapy. Patients willing and able to try acupuncture might see a reduction in the overall number of headaches. Acupuncture has few adverse effects; however, long-term data are lacking.34
Exercise is not supported by robust data for its role as a prophylactic treatment. It is generally considered safe in most populations, however, and can be pursued with little out-of-pocket cost.35
Cognitive behavioral therapy (CBT). The AAN recommends CBT, relaxation therapy, and biofeedback therapy. Accessibility of these services remains limited for many patients, and cost can be prohibitive.16
Supplements used to help prevent migraine include the root of Petasites hybridus (butterbur), magnesium, vitamin B2 (riboflavin), Tanacetum parthenium (feverfew), and coenzyme Q10.16 Although the strength of evidence for these therapies is limited by small trials, their overall risk of adverse effects is low, and they might be easier for patients to obtain than acupuncture or CBT.
Continue to: Butterbur, in particular...
Butterbur, in particular, has been found to be beneficial for migraine prevention in 2 small placebo-controlled trials. In a randomized controlled study of 245 patients P hybridus, (specifically, the German formulation, Petadolex), 75 mg BID, reduced the frequency of migraine attack by 48% at 4 months, compared to placebo (number needed to treat, 5.3).44 No difference was found at lower dosages. The most common reported adverse effect was burping.
Regrettably, unpurified butterbur extract contains pyrrolizidine alkaloids, potentially hepatotoxic and carcinogenic compounds. Because of variations in purification in production facilities in the United States, butterbur supplements might not have all of these compounds removed—and so should be used with caution.41
Magnesium. Studies evaluating the use of magnesium have demonstrated varied results; differences in methods and dosing have limited broad application of findings. As with most supplements considered for prophylactic treatment, magnesium dosing is poorly understood, and bioavailability varies in its different forms. Oral supplementation can be given as magnesium dicitrate, 600 mg/d.45
Recently, products containing various combinations of feverfew, coenzyme Q10, riboflavin, magnesium, and other supplements have shown benefit in early clinical trials.36,37
Neural stimulation. Over the past few years, a variety of transcutaneous nerve stimulator devices have gained FDA approval for use in migraine prophylaxis. The long-term safety and efficacy of these devices is not yet well understood, but they appear to provide headache relief in the short term and decrease the frequency of headache.38 Use of the noninvasive stimulators is limited today by high cost and poor coverage by US health care insurers.
Continue to: Newly available medical therapy
Newly available medical therapy. The FDA recently approved erenumab, a fully human monoclonal antibody for prevention of migraine in adults. This is the first drug in the CGRP antagonist class to be approved for this indication. Trials of this once-monthly, self-injectable drug show promising results for patients whose migraines have been refractory to other therapies.
A recent large trial evaluated 955 adults with migraine, randomizing them to receive erenumab, 70 mg; erenumab, 140 mg; or placebo over 28 weeks.39 The groups receiving erenumab had a nearly 2-fold higher odds of having their migraine reduced by 50%, compared with placebo (number needed to treat with the 140-mg dose, 4.27). Similar numbers of participants from all groups discontinued the study.39 Phase 3 trials that are not yet formally published have produced similarly beneficial results.40,46 The FDA has listed injection site reaction and constipation as the most reported adverse effects.40
Three other anti-CGRP antibodies are likely to be approved in the near future: fremanezumab, galcanezumab, and eptinezumab.
The approach to migraine in special populations
Management of acute and chronic migraine in children, pregnant women, and older adults requires special attention: Treatment approaches are different than they are for adults 19 to 65 years of age.
Pediatric patients. Migraine is the most common acute and recurrent headache syndrome in children. Headaches differ from those of adult migraine as a result of variations in brain maturation, plasticity, and cognitive development.47 Migraine attacks are often of shorter duration in children, lasting 1 to 2 hours, but can still be preceded by visual aura.48 Just as with adults, imaging, electroencephalography, lumbar puncture, and routine labs should be considered only if a child has an abnormal neurological exam or other concerning features (TABLE 214,15).
Continue to: The general approach to migraine treatment...
The general approach to migraine treatment in the pediatric population includes education of the child and family about symptom management. Acetaminophen, NSAIDs, and triptans are approved for abortive therapy in children and should be used for acute headache relief in the same way that they are used in adults. Oral rizatriptan, the most well studied triptan in the pediatric population, is approved for use in children as young as 6 years49; the pediatric dosage is 5 mg/d for patients weighing 20 to 39 kg and 10 mg/d for patients weighing more than 40 kg (same as the adult dosage).
Oral almotriptan and zolmitriptan are also approved for use in children 12 to 17 years of age. Usual dosages are: almotriptan, 12.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 25 mg/d); and zolmitriptan, 2.5 mg at onset, can repeat in 2 hours as needed (maximum dosage, 10 mg/d).50
For children who are unable to swallow pills or who are vomiting, a non-oral route of administration is preferable. Rizatriptan is available as an orally disintegrating tablet. Zolmitriptan is available in a nasal spray at a dose of 5 mg for children 12 years and older. Sumatriptan is not approved for use in patients younger than 18 years; however, recent studies have shown that it might have good efficacy and tolerability.50
Daily prophylactic treatment for recurrent migraine in the pediatric population is an evolving subject; published guidelines do not exist. It is reasonable to consider treatment using the same guidelines as those in place for adults.51 Topiramate, 1 to 2 mg/kg/d, is the only therapy approved by the FDA for episodic migraine preventive therapy in adolescents.50
Notably, a nonpharmacotherapeutic approach may be more effective for pediatric prevention. In 2017, a large double-blind, placebo-controlled trial investigated the use of amitriptyline, topiramate, and placebo for the treatment of recurrent migraine in children 8 to 17 years of age. An interim analysis of the 328 children enrolled found no significant differences in reduction of headache frequency with treatment compared with placebo over a 24-week period; the trial was stopped early due to futility.52
Continue to: The study did show...
The study did show, however, that reducing migraine triggers provided a high level of benefit to study participants. Stress is one of the most common migraine triggers in children; lack of sleep, exposure to a warm climate, and exposure to video games are also notable triggers.53 CBT may augment the efficacy of standard migraine medications in the pediatric population and may help prevent recurrence of episodes.54
Pregnancy. The treatment of migraine is different in pregnant women than it is in nonpregnant adults because of a concern over adverse effects on fetal development. For acute headache treatment, first-line therapies include trigger avoidance and acetaminophen, 1000 mg (maximum dosage, 4000 mg/d).55 If this is ineffective, a 10-mg dose of metoclopramide, as often as every 6 hours (not an FDA-approved indication), can be considered. During the second trimester, NSAIDs can be considered second-line therapy.
Triptans—specifically, sumatriptan and rizatriptan—can also be considered if first-line therapies fail.56 Triptan-exposed pregnant women with migraine have a rate of congenital malformations, spontaneous abortions, and prematurity that is similar to what is seen in pregnant women with migraine who have not been exposed to triptans. However, when triptan-exposed women are compared with healthy, non-migraine-suffering women, the rate of spontaneous abortion appears to be increased in the triptan-exposed population.57
Ergotamine is contraindicated during pregnancy because of its potential to induce uterine contractions and vasospasm, which can be detrimental to the fetus.56Nonpharmacotherapeutic interventions such as heat, ice, massage, rest, and avoidance of triggers are as successful in the pregnant population as in the nonpregnant population. For migraine prevention, coenzyme Q10, vitamins B2 and B6 (pyridoxine), and oral magnesium can be considered. Feverfew and butterbur should be avoided because of concerns about fetal malformation and preterm labor.58
Older adults. Choosing appropriate migraine therapy for older adults requires special consideration because of changes in drug metabolism and risks associated with drug adverse effects. Additionally, few studies of migraine drugs have included large populations of adults older than 65 years; medications should therefore be prescribed cautiously in this population, with particular attention to drug–drug interactions.
Continue to: Just as for younger adults...
Just as for younger adults, mild symptoms can be managed effectively with acetaminophen. NSAIDs may be used as well, but carry increased risks of gastric bleeding and elevation in blood pressure.59 The use of triptans is acceptable for the appropriate patient, but should be avoided in patients with known vascular disease.60 Antiemetics present an increased risk of extrapyramidal adverse effects in the elderly and should be used with caution at the lowest effective dosage.59 Novel mechanisms of action make some of the newer agents potentially safer for use in older adults when treating acute migraine.
For migraine prevention in older adults, particular attention should be paid to reducing triggers and minimizing polypharmacy.
More and more, successful treatment is within reach
With many clinical trials evaluating novel drugs underway, and additional studies contributing to our understanding of nonpharmacotherapeutic approaches to migraine treatment, improved headache control may become increasingly common over the next few years.
CORRESPONDENCE
Kathryn McGrath, MD, Department of Family and Community Medicine, Thomas Jefferson University, 1015 Walnut St, Philadelphia PA 19107; [email protected].
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
1. Stokes M, Becker WJ, Lipton RB, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51:1058-1077.
2. Smitherman TA, Burch R, Sheikh H, et al. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427-436.
3. Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
4. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479-484.
5. Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65-80.
6. Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosc. 2015;35:6619-6629.
7. Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2013;137(Pt 1):232-241.
8. Cutrer FM, Sorensen AG, Weisskoff RM, et al. Perfusion‐weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25-31.
9. Hadjikhani N, Sanchez Del Rio MS, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692.
10. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Ann Rev Physiol. 2013;75:365-391.
11. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, (beta version). Cephalalgia. 2013;33:629-808.
12. Lipton RB, Dodick D, Sadovsky RE, et al; ID Migraine validation study. A self-administered screener for migraine in primary care: The ID Migraine™ validation study. Neurology. 2003;61:375-382.
13. Láinez MJ, Domínguez M, Rejas J, et al. Development and validation of the Migraine Screen Questionnaire (MS‐Q). Headache. 2005;45:1328-1338.
14. Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
15. Becker WJ, Findlay T, Moga C, et al. Guideline for primary care management of headache in adults. Can Fam Physician. 2015;61:670-679.
16. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
17. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3-20.
18. Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36:887-898.
19. Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
20. Färkkilä M, Diener HC, Géraud G, et al; COL MIG-202 study group. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11:405-413.
21. Pringsheim T, Davenport WJ, Marmura MJ, et al. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56:1194-1200.
22. Thorlund K, Mills EJ, Wu P, et al. Comparative efficacy of triptans for the abortive treatment of migraine: a multiple treatment comparison meta-analysis. Cephalalgia. 2014;34:258-267.
23. Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database Syst Rev. 2013;(10):CD008541.
24. Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia. 2015;35:271-284.
25. Ferrari MD, Goadsby PJ, Roon KI, et al. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633-658.
26. Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48-56.
27. A phase 3, multicenter, randomized, double-blind, placebo-controlled single attack study to evaluate the efficacy, safety, and tolerability of oral ubrogepant in the acute treatment of migraine. https://clinicaltrials.gov/ct2/show/study/NCT02828020. Accessed November 16, 2018.
28. Rubio-Beltrán E, Labastida-Ramírez A, Villalón CM, et al. Is selective 5-HT1F receptor agonism an entity apart from that of the triptans in antimigraine therapy? Pharmacol Ther. 2018;186:88-97.
29. Diener HC, Charles A, Goadsby PJ, et al. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-1022.
30. Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122.
31. Martin PR. Behavioral management of migraine headache triggers: learning to cope with triggers. Curr Pain Headache Rep. 2010;14:221-227.
32. Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache. 2012;52:930-945.
33. Dodick DW, Turkel CC, DeGryse RE, et al; PREEMPT Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921-936.
34. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016(6):CD001218.
35. Varkey E, Cider Å, Carlsson J, et al. Exercise as migraine prophylaxis: a randomized study using relaxation and topiramate as controls. Cephalalgia. 2011;31:1428-1438.
36. Guilbot A, Bangratz M, Abdellah SA, et al. A combination of coenzyme Q10, feverfew and magnesium for migraine prophylaxis: a prospective observational study. BMC Complement Altern Med. 2017;17:433.
37. Dalla Volta G, Zavarize P, Ngonga G, et al. Combination of Tanacethum partenium, 5-hydrossitriptophan (5-Http) and magnesium in the prophylaxis of episodic migraine without aura (AURASTOP®) an observational study. Int J Neuro Brain Dis. 2017;4:1-4.
38. Puledda F, Goadsby PJ. An update on non‐pharmacological neuromodulation for the acute and preventive treatment of migraine. Headache. 2017;57:685-691.
39. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123-2132.
40. Reuter U. Efficacy and safety of erenumab in episodic migraine patients with 2-4 prior preventive treatment failures: Results from the Phase 3b LIBERTY study. Abstract 009, AAN 2018 Annual Meeting; April 24, 2018.
41. Diener HC, Freitag FG, Danesch U. Safety profile of a special butterbur extract from Petasites hybridus in migraine prevention with emphasis on the liver. Cephalalgia Reports. https://journals.sagepub.com/doi/10.1177/2515816318759304. 2018 May 2. Accessed December 15, 2018.
42. Kingston WS, Halker R. Determinants of suboptimal migraine diagnosis and treatment in the primary care setting. J Clin Outcomes Manag. 2017;24:319-324.
43. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database of Syst Rev. 2018;6:CD011616.
44. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
45. Von Luckner A, Riederer F. Magnesium in migraine prophylaxis—is there an evidence‐based rationale? A systematic review. Headache. 2018;58:199-209.
46. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16:425-434.
47. Sonal Sekhar M, Sasidharan S, Joseph S, et al. Migraine management: How do the adult and paediatric migraines differ? Saudi Pharm J. 2012;20:1-7.
48. Lewis DW. Pediatric migraine. In: Lewis DW. Clinician’s Manual on Treatment of Pediatric Migraine. London, UK: Springer Healthcare Ltd; 2010:15-26.
49. Ho TW, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan in pediatric migraineurs: results from a randomized double-blind, placebo controlled trial using a novel adaptive enrichment design. Cephalagia. 2012;32:750-765.
50. Khrizman M, Pakalnis A. Management of pediatric migraine: current therapies. Pediatr Ann. 2018;47:e55-e60.
51. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
52. Powers SW, Coffey CS, Chamberlin LA, et al; CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
53. Neut D, Fily A, Cuvellier JC, et al. The prevalence of triggers in paediatric migraine: a questionnaire study in 102 children and adolescents. J Headache Pain. 2012;13:61-65.
54. Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta‐analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. s2017;57:349-362.
55. Lipton RB, Baggish JS, Stewart WF, et al. Efficacy and safety of acetaminophen in the treatment of migraine: results of a randomized, double-blind, placebo-controlled, population-based study. Arch Intern Med. 2000;160:3486-3492.
56. Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392-398.
57. Marchenko A, Etwel F, Olutunfesse O, et al. Pregnancy outcome following prenatal exposure to triptan medications: a meta-analysis. Headache. 2015:55:490-501.
58. Wells RE, Turner DP, Lee M, et al. Managing migraine during pregnancy and lactation. Curr Neurol Neurosci Rep. 2016;16:40.
59. Haan J, Hollander J, Ferrari MD. Migraine in the elderly: a review. Cephalalgia. 2007;27:97-106.
60. Gladstone JP, Eross EJ, Dodick DW. Migraine in special populations. Treatment strategies for children and adolescents, pregnant women, and the elderly. Postgrad Med. 2004;115:39-44,47-50.
PRACTICE RECOMMENDATIONS
› Offer treatment with a triptan to adult patients with moderate-to-severe episodic migraine. A
› Consider prescribing topiramate, divalproex sodium, metoprolol, propranolol, or the herbal, Petasites hybridum, for the prevention of recurrent episodic migraine that has not responded to a reduction in headache triggers. A
› Add onabotulinumtoxinA injection to your therapeutic toolbox as an effective preventive treatment for chronic migraine (≥15 headache days a month for 3 months). B
› Recommend magnesium and feverfew as adjunctive preventive treatments for migraine. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Age of migraine onset may affect stroke risk
The age at which a patient develops migraine with aura may be an important factor in assessing stroke risk, according to a prospective cohort study published in Headache.
Patients who had onset of migraine with visual aura after age 50 years had an increased risk of ischemic stroke, compared with patients with no headache, the researchers found. Patients with longer exposure to migraine with visual aura – that is, onset before age 50 years – did not have significantly increased ischemic stroke risk, said X. Michelle Androulakis, MD, of the department of neurology at the University of South Carolina in Columbia, and her colleagues.
“Migraine, especially migraine with aura, is associated with increased risk of ischemic stroke,” but whether age of migraine onset affects the risk of cardiovascular disease has been unclear, the researchers said.
To examine the risk of ischemic stroke in migraineurs with and without aura with onset before and after age 50 years, the investigators conducted a post hoc analysis of data from the ongoing Atherosclerosis Risk in Communities (ARIC) study. The researchers adjusted for potential confounders, including diabetes, body mass index, hypertension, and hyperlipidemia.
In ARIC, participants completed a questionnaire about their migraine history at their third study visit (1993-1995) and were followed for ischemic stroke incidence over 20 years.
Of the 11,592 ARIC participants included in the analysis (mean age, 61 years; 76.5% white; and 55.3% female), 447 had migraine with aura, and 1,128 had migraine without aura. Onset of migraine with aura at age 50 years or older (average duration, 4.75 years) was associated with more than twofold greater risk of ischemic stroke, compared with no headache (multivariable adjusted hazard ratio = 2.17). Onset of migraine with aura before age 50 years (average duration, 28.17 years) was not significantly associated with stroke. A logistic regression model yielded consistent results.
In addition, patients with migraine without aura did not have an increased risk of stroke, regardless of the age of onset. The absolute risk for stroke in migraine with aura was 8.27%, and the absolute risk in migraine without aura was 4.25%.
“We found unexpected results suggesting that the onset of migraine with aura before age 50 is not associated with ischemic stroke. ... These results are specific to first-time ischemic stroke incidents that occurred in mid- to late life; therefore, it cannot be generalized to stroke in younger patients,” the authors wrote.
It could be that migraine with aura symptoms that start at a later age are a red flag for paradoxical emboli from a patent foramen ovale or microemboli, Dr. Androulakis and her colleagues noted. It also is possible that the degree of cortical spreading depression required to induce migraine with aura symptoms is different later in life versus earlier in life.
“This study underscores the importance of MA symptoms onset in evaluation of ischemic stroke risk in late life,” the researchers concluded.
The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
SOURCE: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
The age at which a patient develops migraine with aura may be an important factor in assessing stroke risk, according to a prospective cohort study published in Headache.
Patients who had onset of migraine with visual aura after age 50 years had an increased risk of ischemic stroke, compared with patients with no headache, the researchers found. Patients with longer exposure to migraine with visual aura – that is, onset before age 50 years – did not have significantly increased ischemic stroke risk, said X. Michelle Androulakis, MD, of the department of neurology at the University of South Carolina in Columbia, and her colleagues.
“Migraine, especially migraine with aura, is associated with increased risk of ischemic stroke,” but whether age of migraine onset affects the risk of cardiovascular disease has been unclear, the researchers said.
To examine the risk of ischemic stroke in migraineurs with and without aura with onset before and after age 50 years, the investigators conducted a post hoc analysis of data from the ongoing Atherosclerosis Risk in Communities (ARIC) study. The researchers adjusted for potential confounders, including diabetes, body mass index, hypertension, and hyperlipidemia.
In ARIC, participants completed a questionnaire about their migraine history at their third study visit (1993-1995) and were followed for ischemic stroke incidence over 20 years.
Of the 11,592 ARIC participants included in the analysis (mean age, 61 years; 76.5% white; and 55.3% female), 447 had migraine with aura, and 1,128 had migraine without aura. Onset of migraine with aura at age 50 years or older (average duration, 4.75 years) was associated with more than twofold greater risk of ischemic stroke, compared with no headache (multivariable adjusted hazard ratio = 2.17). Onset of migraine with aura before age 50 years (average duration, 28.17 years) was not significantly associated with stroke. A logistic regression model yielded consistent results.
In addition, patients with migraine without aura did not have an increased risk of stroke, regardless of the age of onset. The absolute risk for stroke in migraine with aura was 8.27%, and the absolute risk in migraine without aura was 4.25%.
“We found unexpected results suggesting that the onset of migraine with aura before age 50 is not associated with ischemic stroke. ... These results are specific to first-time ischemic stroke incidents that occurred in mid- to late life; therefore, it cannot be generalized to stroke in younger patients,” the authors wrote.
It could be that migraine with aura symptoms that start at a later age are a red flag for paradoxical emboli from a patent foramen ovale or microemboli, Dr. Androulakis and her colleagues noted. It also is possible that the degree of cortical spreading depression required to induce migraine with aura symptoms is different later in life versus earlier in life.
“This study underscores the importance of MA symptoms onset in evaluation of ischemic stroke risk in late life,” the researchers concluded.
The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
SOURCE: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
The age at which a patient develops migraine with aura may be an important factor in assessing stroke risk, according to a prospective cohort study published in Headache.
Patients who had onset of migraine with visual aura after age 50 years had an increased risk of ischemic stroke, compared with patients with no headache, the researchers found. Patients with longer exposure to migraine with visual aura – that is, onset before age 50 years – did not have significantly increased ischemic stroke risk, said X. Michelle Androulakis, MD, of the department of neurology at the University of South Carolina in Columbia, and her colleagues.
“Migraine, especially migraine with aura, is associated with increased risk of ischemic stroke,” but whether age of migraine onset affects the risk of cardiovascular disease has been unclear, the researchers said.
To examine the risk of ischemic stroke in migraineurs with and without aura with onset before and after age 50 years, the investigators conducted a post hoc analysis of data from the ongoing Atherosclerosis Risk in Communities (ARIC) study. The researchers adjusted for potential confounders, including diabetes, body mass index, hypertension, and hyperlipidemia.
In ARIC, participants completed a questionnaire about their migraine history at their third study visit (1993-1995) and were followed for ischemic stroke incidence over 20 years.
Of the 11,592 ARIC participants included in the analysis (mean age, 61 years; 76.5% white; and 55.3% female), 447 had migraine with aura, and 1,128 had migraine without aura. Onset of migraine with aura at age 50 years or older (average duration, 4.75 years) was associated with more than twofold greater risk of ischemic stroke, compared with no headache (multivariable adjusted hazard ratio = 2.17). Onset of migraine with aura before age 50 years (average duration, 28.17 years) was not significantly associated with stroke. A logistic regression model yielded consistent results.
In addition, patients with migraine without aura did not have an increased risk of stroke, regardless of the age of onset. The absolute risk for stroke in migraine with aura was 8.27%, and the absolute risk in migraine without aura was 4.25%.
“We found unexpected results suggesting that the onset of migraine with aura before age 50 is not associated with ischemic stroke. ... These results are specific to first-time ischemic stroke incidents that occurred in mid- to late life; therefore, it cannot be generalized to stroke in younger patients,” the authors wrote.
It could be that migraine with aura symptoms that start at a later age are a red flag for paradoxical emboli from a patent foramen ovale or microemboli, Dr. Androulakis and her colleagues noted. It also is possible that the degree of cortical spreading depression required to induce migraine with aura symptoms is different later in life versus earlier in life.
“This study underscores the importance of MA symptoms onset in evaluation of ischemic stroke risk in late life,” the researchers concluded.
The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
SOURCE: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
FROM HEADACHE
Key clinical point: Age of migraine onset may be an important factor in assessing stroke risk.
Major finding: (multivariable adjusted hazard ratio = 2.17).
Study details: A post hoc analysis of data from more than 11,500 participants in the Atherosclerosis Risk in Communities (ARIC) study.
Disclosures: The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
Source: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
Study hints at lacosamide’s efficacy for small fiber neuropathy
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
As a treatment for small fiber neuropathy (SFN), lacosamide decreased pain and had a positive effect on sleep quality with minimal adverse events in patients with mutations in the gene SCN9A that encodes the voltage-gated sodium channel Nav1.7, according to a randomized, placebo-controlled, double-blind, crossover-design study published in Brain.
“This is the first study that investigated the efficacy of lacosamide [Vimpat] in patients with SFN,” wrote lead author Bianca T.A. de Greef, MD, of Maastricht University Medical Center, the Netherlands, and her coauthors. “Compared with placebo, lacosamide appeared to be safe to use and well tolerated in this cohort of patients.”
Lacosamide, which is approved in the United States to treat partial-onset seizures in people aged 4 years and older, has been shown to bind to and inhibit Nav1.7.
The investigators randomized 25 Dutch patients with Nav1.7-related SFN into the Lacosamide-Efficacy-’N’-Safety in SFN (LENSS) study to receive lacosamide followed by placebo, or vice versa. The patients were recruited between November 2014 and July 2016; 1 patient dropped out before treatment and another after the first treatment period, leaving 24 patients who received lacosamide and 23 patients who received placebo. They went through a 3-week titration period, an 8-week treatment period, a 2-week tapering period, and a washout period of at least 2 weeks, after which they switched to the other treatment arm and repeated the same schedule.
Through the daily pain intensity numerical rating scale and the daily sleep interference scale (DSIS), among other questionnaires, the investigators sought to determine if lacosamide reduced pain and thereby improved sleep quality. Lacosamide treatment led to a decrease in mean average pain by at least 1 point in 50.0% of patients, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213). In addition, 25.0% of the lacosamide group reported at least a 2-point decrease in mean average pain versus 8.7% in the placebo group. There was also a notable difference in pain’s impact on sleep quality between the two, with the lacosamide period seeing a DSIS median value of 5.3, compared with 5.7 for the placebo period.
According to the patients’ global impression of change questionnaire, 33.3% felt better while using lacosamide versus 4.3% who felt better while using placebo (P = .0156). Six serious adverse events occurred during the study, though only two occurred during the lacosamide period. The most common adverse events for patients taking lacosamide included dizziness, headache, and nausea, all of which were comparable with adverse events in patients taking placebo.
Dr. de Greef and her colleagues noted the study’s potential limitations, including a carryover effect that could have confounded direct treatment effects (which they attempted to mitigate via a lengthier washout period) and a small cohort that was limited to very specific patients. However, the authors chose this particular cohort because “our aim was to demonstrate proof of-concept, which can be used for future studies involving larger groups of patients diagnosed with SFN.” They observed that their response rates were slightly lower than expected, but they noted that “lacosamide appears to be as effective as currently available neuropathic pain treatment.”
The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
SOURCE: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
FROM BRAIN
Key clinical point:
Major finding: In the lacosamide group, 50.0% of patients reported mean average pain decreasing by at least 1 point, compared with 21.7% in the placebo group (odds ratio, 4.45; 95% confidence interval, 1.38-14.36; P = .0213).
Study details: A randomized, placebo-controlled, double-blind, crossover-design study of 25 patients with Nav1.7-related small fiber neuropathy who received lacosamide followed by placebo, or vice versa.
Disclosures: The study was funded by the Prinses Beatrix Spierfonds. Some of the authors reported receiving grants, personal fees, funding for research, and/or honoraria from foundations, pharmaceutical companies, life sciences companies, and the European Commission.
Source: de Greef BTA et al. Brain. 2019 Jan 14. doi: 10.1093/brain/awy329.
TAPs top epidurals in ventral hernia repair
, according to a review of 246 cases at the Greenville Health System, in South Carolina.
“Regional anesthesia using TAP block[s] provides an effective alternative to epidural analgesia or opioid use alone for perioperative pain control ... In light of these findings, use of TAP block should be strongly considered as an adjunct to abdominal surgery,” wrote investigators led by general surgeon Jeremy Warren, MD, of the University of South Carolina School of Medicine, Greenville, in the Journal of the American College of Surgeons.
Prompted by favorable reports in the literature, the team switched from epidural analgesia to TAP blocks in early 2017. To see how it’s worked out, they reviewed all patients who had ventral hernia repairs at the Greenville Health System from Feb. 2015 to March 2018. They were all mesh cases, without parastomal hernias or enterostomy reversal.
Seventy-four patients had TAP blocks, which were placed in the OR after anesthesia induction and consisted of 200 mg ropivacaine, 100 mcg epinephrine, and 100 mcg clonidine in 60 ml saline, with 30 ml injected on each side under ultrasound guidance.
Their outcomes were compared with 172 patients who received epidurals, which were placed preoperatively and consisted of 0.125% bupivacaine initiated shortly before patients came out of anesthesia, at a rate of 8-12 ml/hr.
Hospital lengths of stay were significantly shorter in the TAP group, a median of 2.4 versus 4.5 days (P less than .001), and TAP patients received fewer opioids, a mean of 40 versus 54.1 morphine milligram equivalents (MME) on postop day 1, and 36.1 versus 52.5 MME on postop day 2 (P = .018).
There were no differences in the rates of surgical site infections or other wound complications. The mean duration of epidural infusion was 49.5 hours.
The shorter length of stay with TAP block was probably related to side effects of epidurals, which can include leg paresthesias, hypotension, and urinary retention, all of which get in the way of early ambulation. “Additionally, the decision of when to discontinue epidural analgesia in our series was left to the judgment of the pain management and surgical team based on reporting of patient pain, rather than duration determined by a protocol,” which may have also played a role, the study team said.
Overall, the results mirror outcomes from previous TAP block studies, but there were caveats. Epidural patients had wider hernias (median 10.8 cm versus 8.8 cm); required more myofascial releases; and had longer operative times, “indicating a higher degree of complexity that may influence the need for longer hospitalization and greater opioid use,” the investigators said.
Also, a greater number of TAP block patients received non-opioid pain killers, including ketorolac and acetaminophen.
The study was conducted within the health system’s enhanced recovery after surgery protocol, which includes a preoperative cocktail of pregabalin 75 mg, celecoxib 400 mg, and acetaminophen 1,000 mg, given within 1-2 hours of surgery. Post-operative management includes intravenous ketamine infusions at sub-anesthetic doses, NSAIDs, and acetaminophen, among other measures. The approach has pretty much eliminated patient-controlled analgesia.
There were slightly more men than women in the review. Study participants, on average, were in their late 50s. There were no significant differences in comorbidities.
No funding was reported, and the investigators didn’t have any relevant disclosures.
SOURCE: Warren JA et al., J Am Coll Surg. 2019 Jan 7. pii: S1072-7515(19)30014-6. doi: 10.1016/j.jamcollsurg.2018.12.017
, according to a review of 246 cases at the Greenville Health System, in South Carolina.
“Regional anesthesia using TAP block[s] provides an effective alternative to epidural analgesia or opioid use alone for perioperative pain control ... In light of these findings, use of TAP block should be strongly considered as an adjunct to abdominal surgery,” wrote investigators led by general surgeon Jeremy Warren, MD, of the University of South Carolina School of Medicine, Greenville, in the Journal of the American College of Surgeons.
Prompted by favorable reports in the literature, the team switched from epidural analgesia to TAP blocks in early 2017. To see how it’s worked out, they reviewed all patients who had ventral hernia repairs at the Greenville Health System from Feb. 2015 to March 2018. They were all mesh cases, without parastomal hernias or enterostomy reversal.
Seventy-four patients had TAP blocks, which were placed in the OR after anesthesia induction and consisted of 200 mg ropivacaine, 100 mcg epinephrine, and 100 mcg clonidine in 60 ml saline, with 30 ml injected on each side under ultrasound guidance.
Their outcomes were compared with 172 patients who received epidurals, which were placed preoperatively and consisted of 0.125% bupivacaine initiated shortly before patients came out of anesthesia, at a rate of 8-12 ml/hr.
Hospital lengths of stay were significantly shorter in the TAP group, a median of 2.4 versus 4.5 days (P less than .001), and TAP patients received fewer opioids, a mean of 40 versus 54.1 morphine milligram equivalents (MME) on postop day 1, and 36.1 versus 52.5 MME on postop day 2 (P = .018).
There were no differences in the rates of surgical site infections or other wound complications. The mean duration of epidural infusion was 49.5 hours.
The shorter length of stay with TAP block was probably related to side effects of epidurals, which can include leg paresthesias, hypotension, and urinary retention, all of which get in the way of early ambulation. “Additionally, the decision of when to discontinue epidural analgesia in our series was left to the judgment of the pain management and surgical team based on reporting of patient pain, rather than duration determined by a protocol,” which may have also played a role, the study team said.
Overall, the results mirror outcomes from previous TAP block studies, but there were caveats. Epidural patients had wider hernias (median 10.8 cm versus 8.8 cm); required more myofascial releases; and had longer operative times, “indicating a higher degree of complexity that may influence the need for longer hospitalization and greater opioid use,” the investigators said.
Also, a greater number of TAP block patients received non-opioid pain killers, including ketorolac and acetaminophen.
The study was conducted within the health system’s enhanced recovery after surgery protocol, which includes a preoperative cocktail of pregabalin 75 mg, celecoxib 400 mg, and acetaminophen 1,000 mg, given within 1-2 hours of surgery. Post-operative management includes intravenous ketamine infusions at sub-anesthetic doses, NSAIDs, and acetaminophen, among other measures. The approach has pretty much eliminated patient-controlled analgesia.
There were slightly more men than women in the review. Study participants, on average, were in their late 50s. There were no significant differences in comorbidities.
No funding was reported, and the investigators didn’t have any relevant disclosures.
SOURCE: Warren JA et al., J Am Coll Surg. 2019 Jan 7. pii: S1072-7515(19)30014-6. doi: 10.1016/j.jamcollsurg.2018.12.017
, according to a review of 246 cases at the Greenville Health System, in South Carolina.
“Regional anesthesia using TAP block[s] provides an effective alternative to epidural analgesia or opioid use alone for perioperative pain control ... In light of these findings, use of TAP block should be strongly considered as an adjunct to abdominal surgery,” wrote investigators led by general surgeon Jeremy Warren, MD, of the University of South Carolina School of Medicine, Greenville, in the Journal of the American College of Surgeons.
Prompted by favorable reports in the literature, the team switched from epidural analgesia to TAP blocks in early 2017. To see how it’s worked out, they reviewed all patients who had ventral hernia repairs at the Greenville Health System from Feb. 2015 to March 2018. They were all mesh cases, without parastomal hernias or enterostomy reversal.
Seventy-four patients had TAP blocks, which were placed in the OR after anesthesia induction and consisted of 200 mg ropivacaine, 100 mcg epinephrine, and 100 mcg clonidine in 60 ml saline, with 30 ml injected on each side under ultrasound guidance.
Their outcomes were compared with 172 patients who received epidurals, which were placed preoperatively and consisted of 0.125% bupivacaine initiated shortly before patients came out of anesthesia, at a rate of 8-12 ml/hr.
Hospital lengths of stay were significantly shorter in the TAP group, a median of 2.4 versus 4.5 days (P less than .001), and TAP patients received fewer opioids, a mean of 40 versus 54.1 morphine milligram equivalents (MME) on postop day 1, and 36.1 versus 52.5 MME on postop day 2 (P = .018).
There were no differences in the rates of surgical site infections or other wound complications. The mean duration of epidural infusion was 49.5 hours.
The shorter length of stay with TAP block was probably related to side effects of epidurals, which can include leg paresthesias, hypotension, and urinary retention, all of which get in the way of early ambulation. “Additionally, the decision of when to discontinue epidural analgesia in our series was left to the judgment of the pain management and surgical team based on reporting of patient pain, rather than duration determined by a protocol,” which may have also played a role, the study team said.
Overall, the results mirror outcomes from previous TAP block studies, but there were caveats. Epidural patients had wider hernias (median 10.8 cm versus 8.8 cm); required more myofascial releases; and had longer operative times, “indicating a higher degree of complexity that may influence the need for longer hospitalization and greater opioid use,” the investigators said.
Also, a greater number of TAP block patients received non-opioid pain killers, including ketorolac and acetaminophen.
The study was conducted within the health system’s enhanced recovery after surgery protocol, which includes a preoperative cocktail of pregabalin 75 mg, celecoxib 400 mg, and acetaminophen 1,000 mg, given within 1-2 hours of surgery. Post-operative management includes intravenous ketamine infusions at sub-anesthetic doses, NSAIDs, and acetaminophen, among other measures. The approach has pretty much eliminated patient-controlled analgesia.
There were slightly more men than women in the review. Study participants, on average, were in their late 50s. There were no significant differences in comorbidities.
No funding was reported, and the investigators didn’t have any relevant disclosures.
SOURCE: Warren JA et al., J Am Coll Surg. 2019 Jan 7. pii: S1072-7515(19)30014-6. doi: 10.1016/j.jamcollsurg.2018.12.017
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: TAP blocks are better than epidurals for pain control after ventral hernia repair.
Major finding: Hospital lengths of stay were significantly shorter in the TAP group, a median of 2.4 versus 4.5 days (P less than .001), and TAP patients received fewer opioids, a mean of 40 versus 54.1 morphine milligram equivalents (MME) on postop day 1.
Study details: Review of 246 repairs
Disclosures: No funding was reported, and the investigators didn’t have any relevant disclosures.
Source: Warren JA et al., J Am Coll Surg. 2019 Jan 7. pii: S1072-7515(19)30014-6. doi: 10.1016/j.jamcollsurg.2018.12.017
FDA labeling templates smooth way for OTC naloxone
Drug facts labels (DFLs) are required for all OTC drugs, and it’s usually up to manufacturers to develop and test their own to ensure that consumers understand how to use their products.
“Some stakeholders have identified the requirement ... as a barrier to development of OTC naloxone products,” so the agency developed two DFLs on its own – one for nasal spray naloxone, the other for auto-injectors – and completed the necessary label comprehension testing, according to an announcement from FDA Commissioner Scott Gottlieb, MD.
There’s not much else manufactures have to do, except deal with the details of their own products. They “can now focus their efforts on ... how well consumers understand the product-specific information that hasn’t been already tested in the model” DFLs, according to the announcement.
As deaths from opioid abuse continue to climb, the FDA is committed to increasing access to naloxone, which currently requires a prescription. The new DFLs “should jump-start the development of OTC naloxone products ... I personally urge companies to take notice of this pathway that the FDA has opened for them and come to the Agency with applications as soon as possible,” Dr. Gottlieb said.
Comprehension was assessed in more than 700 people, including heroin and prescription opioid users, their friends and families, and adolescents. “Overall, the study demonstrated that” the DFLs are “well-understood by consumers” and acceptable “for use by manufacturers in support of their ... development programs,” according to the announcement.
In a press statement, the American Medical Association applauded the agency’s move “to provide labeling that would allow for over-the-counter availability of naloxone, a move that will save people from opioid-related overdose ... The action should spur efforts by naloxone manufacturers to submit applications for their products to receive over-the-counter status.”
Drug facts labels (DFLs) are required for all OTC drugs, and it’s usually up to manufacturers to develop and test their own to ensure that consumers understand how to use their products.
“Some stakeholders have identified the requirement ... as a barrier to development of OTC naloxone products,” so the agency developed two DFLs on its own – one for nasal spray naloxone, the other for auto-injectors – and completed the necessary label comprehension testing, according to an announcement from FDA Commissioner Scott Gottlieb, MD.
There’s not much else manufactures have to do, except deal with the details of their own products. They “can now focus their efforts on ... how well consumers understand the product-specific information that hasn’t been already tested in the model” DFLs, according to the announcement.
As deaths from opioid abuse continue to climb, the FDA is committed to increasing access to naloxone, which currently requires a prescription. The new DFLs “should jump-start the development of OTC naloxone products ... I personally urge companies to take notice of this pathway that the FDA has opened for them and come to the Agency with applications as soon as possible,” Dr. Gottlieb said.
Comprehension was assessed in more than 700 people, including heroin and prescription opioid users, their friends and families, and adolescents. “Overall, the study demonstrated that” the DFLs are “well-understood by consumers” and acceptable “for use by manufacturers in support of their ... development programs,” according to the announcement.
In a press statement, the American Medical Association applauded the agency’s move “to provide labeling that would allow for over-the-counter availability of naloxone, a move that will save people from opioid-related overdose ... The action should spur efforts by naloxone manufacturers to submit applications for their products to receive over-the-counter status.”
Drug facts labels (DFLs) are required for all OTC drugs, and it’s usually up to manufacturers to develop and test their own to ensure that consumers understand how to use their products.
“Some stakeholders have identified the requirement ... as a barrier to development of OTC naloxone products,” so the agency developed two DFLs on its own – one for nasal spray naloxone, the other for auto-injectors – and completed the necessary label comprehension testing, according to an announcement from FDA Commissioner Scott Gottlieb, MD.
There’s not much else manufactures have to do, except deal with the details of their own products. They “can now focus their efforts on ... how well consumers understand the product-specific information that hasn’t been already tested in the model” DFLs, according to the announcement.
As deaths from opioid abuse continue to climb, the FDA is committed to increasing access to naloxone, which currently requires a prescription. The new DFLs “should jump-start the development of OTC naloxone products ... I personally urge companies to take notice of this pathway that the FDA has opened for them and come to the Agency with applications as soon as possible,” Dr. Gottlieb said.
Comprehension was assessed in more than 700 people, including heroin and prescription opioid users, their friends and families, and adolescents. “Overall, the study demonstrated that” the DFLs are “well-understood by consumers” and acceptable “for use by manufacturers in support of their ... development programs,” according to the announcement.
In a press statement, the American Medical Association applauded the agency’s move “to provide labeling that would allow for over-the-counter availability of naloxone, a move that will save people from opioid-related overdose ... The action should spur efforts by naloxone manufacturers to submit applications for their products to receive over-the-counter status.”
Courts stop contraceptive mandate
Too much sleep and too little sleep are linked to atherosclerosis, there is no drop in gout prevalence, but there isn’t an increase either, and back pain persists in one in five patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Too much sleep and too little sleep are linked to atherosclerosis, there is no drop in gout prevalence, but there isn’t an increase either, and back pain persists in one in five patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Too much sleep and too little sleep are linked to atherosclerosis, there is no drop in gout prevalence, but there isn’t an increase either, and back pain persists in one in five patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Back pain persists in one in five patients
according to data from a population-based study of more than 12,000 adults in Canada.
“Given that back pain [BP] is often recurrent, it is important to understand the course of back pain over time as this can provide additional insights on risk factors for nonfavorable outcomes,” wrote Mayilee Canizares, PhD, and her colleagues at the University Health Network’s Krembil Research Institute in Toronto.
In a longitudinal study published in Arthritis Care & Research, the investigators followed 12,782 adults from 1994 to 2011. The study population was a representative sample of the Canadian population via the National Population Health Survey, which collected data every 2 years for a total of nine cycles of data. They included people aged 15 years or older in 1994-1995 who had at least three cycles of data from baseline onward.
Over the 16-year study period, 46% of the participants reported at least one episode of back pain. Of these, 18% were identified as persistent, 28% as developing, 21% as recovering, and 33% as occasional.
“A major finding from this study is the negative impact of persistent BP on a range of health-related outcomes, including health care use, after adjustments for sociodemographic, behavior-related factors, and comorbidities,” the researchers wrote.
They examined several sociodemographic variables, including age, gender, educational level, and household income, as well as behavior-related variables including physical activity, work activity, smoking status, and obesity. The average age of the participants at baseline was 39 years; 51% were female.
Individuals who reported any back pain were more likely than those with no back pain to be overweight or obese, to smoke, to engage in moderate to heavy physical activity each day, and to have chronic conditions, including arthritis, depression, high blood pressure, and migraine.
Overall, individuals with persistent or developing BP had more pain, disability, health care visits, and medication use, compared with those in the recovery and occasional BP groups. However, individuals in the recovery group showed increased use of opioids and antidepressants over time as well, suggesting a need for long-term monitoring of back pain patients.
The trend in general disability was greatest for individuals in the persistent group followed by the developing group, recovery group, and occasional BP group.
The study findings were limited by several factors, including the use of self-reports, potential selection bias, and the inability to differentiate the specific types of back pain, the researchers noted. However, the results support and extend data from previous studies and provide clinical implications for understanding back pain.
The researchers concluded that “the different trajectory patterns potentially represent subgroups in the population that may require different interventions. In light of the trend of marked worsening outcomes, particularly for the persistent and developing groups, studies are needed to determine the nature of these groups.”
The authors reported no relevant financial conflicts.
SOURCE: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
according to data from a population-based study of more than 12,000 adults in Canada.
“Given that back pain [BP] is often recurrent, it is important to understand the course of back pain over time as this can provide additional insights on risk factors for nonfavorable outcomes,” wrote Mayilee Canizares, PhD, and her colleagues at the University Health Network’s Krembil Research Institute in Toronto.
In a longitudinal study published in Arthritis Care & Research, the investigators followed 12,782 adults from 1994 to 2011. The study population was a representative sample of the Canadian population via the National Population Health Survey, which collected data every 2 years for a total of nine cycles of data. They included people aged 15 years or older in 1994-1995 who had at least three cycles of data from baseline onward.
Over the 16-year study period, 46% of the participants reported at least one episode of back pain. Of these, 18% were identified as persistent, 28% as developing, 21% as recovering, and 33% as occasional.
“A major finding from this study is the negative impact of persistent BP on a range of health-related outcomes, including health care use, after adjustments for sociodemographic, behavior-related factors, and comorbidities,” the researchers wrote.
They examined several sociodemographic variables, including age, gender, educational level, and household income, as well as behavior-related variables including physical activity, work activity, smoking status, and obesity. The average age of the participants at baseline was 39 years; 51% were female.
Individuals who reported any back pain were more likely than those with no back pain to be overweight or obese, to smoke, to engage in moderate to heavy physical activity each day, and to have chronic conditions, including arthritis, depression, high blood pressure, and migraine.
Overall, individuals with persistent or developing BP had more pain, disability, health care visits, and medication use, compared with those in the recovery and occasional BP groups. However, individuals in the recovery group showed increased use of opioids and antidepressants over time as well, suggesting a need for long-term monitoring of back pain patients.
The trend in general disability was greatest for individuals in the persistent group followed by the developing group, recovery group, and occasional BP group.
The study findings were limited by several factors, including the use of self-reports, potential selection bias, and the inability to differentiate the specific types of back pain, the researchers noted. However, the results support and extend data from previous studies and provide clinical implications for understanding back pain.
The researchers concluded that “the different trajectory patterns potentially represent subgroups in the population that may require different interventions. In light of the trend of marked worsening outcomes, particularly for the persistent and developing groups, studies are needed to determine the nature of these groups.”
The authors reported no relevant financial conflicts.
SOURCE: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
according to data from a population-based study of more than 12,000 adults in Canada.
“Given that back pain [BP] is often recurrent, it is important to understand the course of back pain over time as this can provide additional insights on risk factors for nonfavorable outcomes,” wrote Mayilee Canizares, PhD, and her colleagues at the University Health Network’s Krembil Research Institute in Toronto.
In a longitudinal study published in Arthritis Care & Research, the investigators followed 12,782 adults from 1994 to 2011. The study population was a representative sample of the Canadian population via the National Population Health Survey, which collected data every 2 years for a total of nine cycles of data. They included people aged 15 years or older in 1994-1995 who had at least three cycles of data from baseline onward.
Over the 16-year study period, 46% of the participants reported at least one episode of back pain. Of these, 18% were identified as persistent, 28% as developing, 21% as recovering, and 33% as occasional.
“A major finding from this study is the negative impact of persistent BP on a range of health-related outcomes, including health care use, after adjustments for sociodemographic, behavior-related factors, and comorbidities,” the researchers wrote.
They examined several sociodemographic variables, including age, gender, educational level, and household income, as well as behavior-related variables including physical activity, work activity, smoking status, and obesity. The average age of the participants at baseline was 39 years; 51% were female.
Individuals who reported any back pain were more likely than those with no back pain to be overweight or obese, to smoke, to engage in moderate to heavy physical activity each day, and to have chronic conditions, including arthritis, depression, high blood pressure, and migraine.
Overall, individuals with persistent or developing BP had more pain, disability, health care visits, and medication use, compared with those in the recovery and occasional BP groups. However, individuals in the recovery group showed increased use of opioids and antidepressants over time as well, suggesting a need for long-term monitoring of back pain patients.
The trend in general disability was greatest for individuals in the persistent group followed by the developing group, recovery group, and occasional BP group.
The study findings were limited by several factors, including the use of self-reports, potential selection bias, and the inability to differentiate the specific types of back pain, the researchers noted. However, the results support and extend data from previous studies and provide clinical implications for understanding back pain.
The researchers concluded that “the different trajectory patterns potentially represent subgroups in the population that may require different interventions. In light of the trend of marked worsening outcomes, particularly for the persistent and developing groups, studies are needed to determine the nature of these groups.”
The authors reported no relevant financial conflicts.
SOURCE: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Back pain is a common health problem, and one in five Canadian adults reported persistent back pain.
Major finding: Approximately half (46%) of Canadian adults reported some type of back pain over a 16-year period.
Study details: The data come from a population-based study of 12,782 adults followed from 1994 to 2011.
Disclosures: The authors reported no relevant financial conflicts.
Source: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.