User login
Through the eyes of migraine: Ocular considerations
STOWE, VT. – said Kathleen Digre, MD, at the annual meeting of the Headache Cooperative of New England. Specifically, she said, dry eye and photophobia are two symptoms that have biologic underpinnings, can be diagnosed, and can be treated. Dr. Digre is a professor of neurology and ophthalmology at the University of Utah, Salt Lake City, and is the current president of the American Headache Society.

Dr. Digre explained that dry eyes and migraine could have a cyclical relationship where dry eyes provoke the migraine, and the migraine may provoke the feeling of dry eye, regardless of whether it can be objectively measured.
Regarding photophobia, Dr. Digre stressed the importance of an accurate diagnosis that rules out eye disorders and other causes of photophobia. She discussed the problem of patient overreliance on dark glasses and encourages a return to light to break the cycle of dark adapting the retina.
Finally, Dr. Digre discussed how proper treatment of migraine and any associated anxiety or depression can help resolve eye issues that may be contributing to migraine.
STOWE, VT. – said Kathleen Digre, MD, at the annual meeting of the Headache Cooperative of New England. Specifically, she said, dry eye and photophobia are two symptoms that have biologic underpinnings, can be diagnosed, and can be treated. Dr. Digre is a professor of neurology and ophthalmology at the University of Utah, Salt Lake City, and is the current president of the American Headache Society.

Dr. Digre explained that dry eyes and migraine could have a cyclical relationship where dry eyes provoke the migraine, and the migraine may provoke the feeling of dry eye, regardless of whether it can be objectively measured.
Regarding photophobia, Dr. Digre stressed the importance of an accurate diagnosis that rules out eye disorders and other causes of photophobia. She discussed the problem of patient overreliance on dark glasses and encourages a return to light to break the cycle of dark adapting the retina.
Finally, Dr. Digre discussed how proper treatment of migraine and any associated anxiety or depression can help resolve eye issues that may be contributing to migraine.
STOWE, VT. – said Kathleen Digre, MD, at the annual meeting of the Headache Cooperative of New England. Specifically, she said, dry eye and photophobia are two symptoms that have biologic underpinnings, can be diagnosed, and can be treated. Dr. Digre is a professor of neurology and ophthalmology at the University of Utah, Salt Lake City, and is the current president of the American Headache Society.

Dr. Digre explained that dry eyes and migraine could have a cyclical relationship where dry eyes provoke the migraine, and the migraine may provoke the feeling of dry eye, regardless of whether it can be objectively measured.
Regarding photophobia, Dr. Digre stressed the importance of an accurate diagnosis that rules out eye disorders and other causes of photophobia. She discussed the problem of patient overreliance on dark glasses and encourages a return to light to break the cycle of dark adapting the retina.
Finally, Dr. Digre discussed how proper treatment of migraine and any associated anxiety or depression can help resolve eye issues that may be contributing to migraine.
REPORTING FROM HCNE STOWE 2019
CGRP drugs: How is it going?
STOWE, VT. – These are the early days of the “CGRP monoclonal antibody era,”

In an interview at the annual meeting of the Headache Cooperative of New England, Dr. McAllister said, “We are comforted that we have now 1-year, 3-year, and 5-year data” from clinical trials, but the sample size is small.
In the time since the first three drugs were approved, “we have probably in the ballpark of over 200,000 patients who have received a monoclonal antibody, and so far there has been nothing that makes us stop cold in our tracks and say there’s something wrong here. That is very comforting,” he said. Dr. McAllister is the medical director of the New England Institute for Neurology and Headache in Stamford, Conn.
What is still unknown, however, is the long-term safety and efficacy; what happens in a larger pool of patients taking these drugs; what happens in pregnancy and effects on the fetus; how and when to safely switch from one monoclonal antibody to another; the systemic effects of these drugs; and other concerns that may arise in postmarketing studies.
STOWE, VT. – These are the early days of the “CGRP monoclonal antibody era,”

In an interview at the annual meeting of the Headache Cooperative of New England, Dr. McAllister said, “We are comforted that we have now 1-year, 3-year, and 5-year data” from clinical trials, but the sample size is small.
In the time since the first three drugs were approved, “we have probably in the ballpark of over 200,000 patients who have received a monoclonal antibody, and so far there has been nothing that makes us stop cold in our tracks and say there’s something wrong here. That is very comforting,” he said. Dr. McAllister is the medical director of the New England Institute for Neurology and Headache in Stamford, Conn.
What is still unknown, however, is the long-term safety and efficacy; what happens in a larger pool of patients taking these drugs; what happens in pregnancy and effects on the fetus; how and when to safely switch from one monoclonal antibody to another; the systemic effects of these drugs; and other concerns that may arise in postmarketing studies.
STOWE, VT. – These are the early days of the “CGRP monoclonal antibody era,”

In an interview at the annual meeting of the Headache Cooperative of New England, Dr. McAllister said, “We are comforted that we have now 1-year, 3-year, and 5-year data” from clinical trials, but the sample size is small.
In the time since the first three drugs were approved, “we have probably in the ballpark of over 200,000 patients who have received a monoclonal antibody, and so far there has been nothing that makes us stop cold in our tracks and say there’s something wrong here. That is very comforting,” he said. Dr. McAllister is the medical director of the New England Institute for Neurology and Headache in Stamford, Conn.
What is still unknown, however, is the long-term safety and efficacy; what happens in a larger pool of patients taking these drugs; what happens in pregnancy and effects on the fetus; how and when to safely switch from one monoclonal antibody to another; the systemic effects of these drugs; and other concerns that may arise in postmarketing studies.
REPORTING FROM HCNE STOWE 2019
Opioid overdose risk greater among HIV patients
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
SEATTLE – People with HIV are more likely to die from an opioid overdose than the general public, according to investigators from the Centers for Disease Control and Prevention.
“We looked into this because we know persons with HIV are more likely to have chronic pain and more likely to receive opioid analgesic treatments, and receive higher doses. In addition, they are more likely to have substance use disorders and mental illness than the U.S. general populations,” CDC epidemiologist Karin A. Bosh, PhD, said at the Conference on Retroviruses and Opportunistic Infections.
To see how that played out in terms of unintentional opioid overdose deaths, they turned to the National HIV Surveillance System and focused on overdose deaths during 2011-2015, the latest data available at the time of the work.
There were 1,363 overdose deaths among persons with HIV during that period, with the rate increasing 42.7% – from 23.2/100,000 HIV patients in 2011 to 33.1/100,000 in 2015.
Although the rate of increase was comparable to the general population, the crude rate was “actually substantially higher among persons with HIV,” Dr. Bosh said. Deaths were highest among persons aged 50-59 years (41.9/100,000), whites (49.1/100,000), injection drug users (137.4/100,000), and people who live in the Northeast (60.6/100,000).
Surprisingly, there was no increase in the rate of overdose deaths among HIV patients on the West Coast, possibly because heroin there was less likely to be cut with fentanyl.
Also, the rate of opioid overdose deaths was higher among women with HIV (35.2/100,000) than among men, perhaps because women are more likely to contract HIV by injection drug use, so they are more likely to be injection drug users at baseline, while the vast majority of men are infected through male-male sex, the investigators said.
The findings underscore the importance of intensifying overdose prevention in the HIV community, and better integrating HIV and substance use disorder treatment, they concluded.
That comes down to screening people for problems, especially in the subgroups identified in the study, and connecting them to drug treatment services. If HIV and substance disorder services were in the same clinic it would help, as would an increase in the number of buprenorphine providers, according to Sheryl B. Lyss, PhD, a coinvestigator and CDC epidemiologist.
“Obviously, when substance use is addressed, people can be much more adherent with their [HIV] medications,” she noted.
The work was funded by the Centers for Disease Control and Prevention. The investigators had no relevant disclosures.
SOURCE: Bosh KA et al. CROI 2019, Abstract 147.
REPORTING FROM CROI 2019
Possible mortality risk seen with tramadol in osteoarthritis
Tramadol appears to be associated with higher mortality risk among older patients with osteoarthritis when compared against common NSAIDs, according to findings from a study published online March 12 in JAMA.
The findings from the retrospective cohort study are worth noting despite their susceptibility to confounding by indication because “tramadol is a weak opioid agonist and has been considered a potential alternative to NSAIDs and traditional opioids because of its assumed relatively lower risk of serious cardiovascular and gastrointestinal adverse effects than NSAIDs, as well as a lower risk of addiction and respiratory depression compared with other opioids,” wrote Chao Zeng, MD, PhD, of Xiangya Hospital of Central South University, Changsha, China, and his coauthors.
The investigators analyzed data from a combined total of 88,902 individuals aged 50 years and older with knee, hip, or hand osteoarthritis who were seen during 2000-2015 and had visits recorded in the United Kingdom’s The Health Improvement Network (THIN) electronic medical records database. Participants were matched on sociodemographic and lifestyle factors, as well as osteoarthritis duration, comorbidities, other prescriptions, and health care utilization prior to the index date of the study.
Over 1 year of follow-up, researchers saw a 71% higher risk of all-cause mortality in patients taking tramadol than that in seen in those taking naproxen, 88% higher than in those taking diclofenac, 70% higher than in those taking celecoxib, and about twice as high as in patients taking etoricoxib.
However, there was no significant difference in risk of all-cause mortality between tramadol and codeine, the researchers found.
The authors suggested that tramadol may have adverse effects on the neurologic system by inhibiting central serotonin and norepinephrine uptake, which could potentially lead to serotonin syndrome. They also speculated that it could increase the risk of postoperative delirium, cause fatal poisoning or respiratory depression if taken in conjunction with alcohol or other drugs, or increase the risk of hypoglycemia, hyponatremia, fractures, or falls.
The numbers of deaths from cardiovascular, gastrointestinal, infection, cancer, and respiratory diseases were all higher in the tramadol group, compared with patients taking NSAIDs, but the differences were not statistically significant because of the relatively small number of deaths, the authors said.
Overall, 44,451 patients were taking tramadol, 12,397 were taking naproxen, 6,512 were taking diclofenac, 5,674 were taking celecoxib, 2,946 were taking etoricoxib, and 16,922 were taking codeine.
Patients in the tramadol cohort were generally older, with higher body mass index, a longer duration of osteoarthritis, and had a higher prevalence of comorbidities, higher health care utilization, and more prescriptions of other medications.
The authors noted that, while the patients from each medication cohort were matched on propensity score, the results were still susceptible to confounding by indication and should be interpreted with caution.
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Natural Science Foundation of China. One author declared funding from the National Institute on Drug Abuse during the conduct of the study and grants from Optum Labs outside the study. No other conflicts of interest were declared.
SOURCE: Zeng C et al. JAMA. 2019;321:969-82.
Tramadol appears to be associated with higher mortality risk among older patients with osteoarthritis when compared against common NSAIDs, according to findings from a study published online March 12 in JAMA.
The findings from the retrospective cohort study are worth noting despite their susceptibility to confounding by indication because “tramadol is a weak opioid agonist and has been considered a potential alternative to NSAIDs and traditional opioids because of its assumed relatively lower risk of serious cardiovascular and gastrointestinal adverse effects than NSAIDs, as well as a lower risk of addiction and respiratory depression compared with other opioids,” wrote Chao Zeng, MD, PhD, of Xiangya Hospital of Central South University, Changsha, China, and his coauthors.
The investigators analyzed data from a combined total of 88,902 individuals aged 50 years and older with knee, hip, or hand osteoarthritis who were seen during 2000-2015 and had visits recorded in the United Kingdom’s The Health Improvement Network (THIN) electronic medical records database. Participants were matched on sociodemographic and lifestyle factors, as well as osteoarthritis duration, comorbidities, other prescriptions, and health care utilization prior to the index date of the study.
Over 1 year of follow-up, researchers saw a 71% higher risk of all-cause mortality in patients taking tramadol than that in seen in those taking naproxen, 88% higher than in those taking diclofenac, 70% higher than in those taking celecoxib, and about twice as high as in patients taking etoricoxib.
However, there was no significant difference in risk of all-cause mortality between tramadol and codeine, the researchers found.
The authors suggested that tramadol may have adverse effects on the neurologic system by inhibiting central serotonin and norepinephrine uptake, which could potentially lead to serotonin syndrome. They also speculated that it could increase the risk of postoperative delirium, cause fatal poisoning or respiratory depression if taken in conjunction with alcohol or other drugs, or increase the risk of hypoglycemia, hyponatremia, fractures, or falls.
The numbers of deaths from cardiovascular, gastrointestinal, infection, cancer, and respiratory diseases were all higher in the tramadol group, compared with patients taking NSAIDs, but the differences were not statistically significant because of the relatively small number of deaths, the authors said.
Overall, 44,451 patients were taking tramadol, 12,397 were taking naproxen, 6,512 were taking diclofenac, 5,674 were taking celecoxib, 2,946 were taking etoricoxib, and 16,922 were taking codeine.
Patients in the tramadol cohort were generally older, with higher body mass index, a longer duration of osteoarthritis, and had a higher prevalence of comorbidities, higher health care utilization, and more prescriptions of other medications.
The authors noted that, while the patients from each medication cohort were matched on propensity score, the results were still susceptible to confounding by indication and should be interpreted with caution.
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Natural Science Foundation of China. One author declared funding from the National Institute on Drug Abuse during the conduct of the study and grants from Optum Labs outside the study. No other conflicts of interest were declared.
SOURCE: Zeng C et al. JAMA. 2019;321:969-82.
Tramadol appears to be associated with higher mortality risk among older patients with osteoarthritis when compared against common NSAIDs, according to findings from a study published online March 12 in JAMA.
The findings from the retrospective cohort study are worth noting despite their susceptibility to confounding by indication because “tramadol is a weak opioid agonist and has been considered a potential alternative to NSAIDs and traditional opioids because of its assumed relatively lower risk of serious cardiovascular and gastrointestinal adverse effects than NSAIDs, as well as a lower risk of addiction and respiratory depression compared with other opioids,” wrote Chao Zeng, MD, PhD, of Xiangya Hospital of Central South University, Changsha, China, and his coauthors.
The investigators analyzed data from a combined total of 88,902 individuals aged 50 years and older with knee, hip, or hand osteoarthritis who were seen during 2000-2015 and had visits recorded in the United Kingdom’s The Health Improvement Network (THIN) electronic medical records database. Participants were matched on sociodemographic and lifestyle factors, as well as osteoarthritis duration, comorbidities, other prescriptions, and health care utilization prior to the index date of the study.
Over 1 year of follow-up, researchers saw a 71% higher risk of all-cause mortality in patients taking tramadol than that in seen in those taking naproxen, 88% higher than in those taking diclofenac, 70% higher than in those taking celecoxib, and about twice as high as in patients taking etoricoxib.
However, there was no significant difference in risk of all-cause mortality between tramadol and codeine, the researchers found.
The authors suggested that tramadol may have adverse effects on the neurologic system by inhibiting central serotonin and norepinephrine uptake, which could potentially lead to serotonin syndrome. They also speculated that it could increase the risk of postoperative delirium, cause fatal poisoning or respiratory depression if taken in conjunction with alcohol or other drugs, or increase the risk of hypoglycemia, hyponatremia, fractures, or falls.
The numbers of deaths from cardiovascular, gastrointestinal, infection, cancer, and respiratory diseases were all higher in the tramadol group, compared with patients taking NSAIDs, but the differences were not statistically significant because of the relatively small number of deaths, the authors said.
Overall, 44,451 patients were taking tramadol, 12,397 were taking naproxen, 6,512 were taking diclofenac, 5,674 were taking celecoxib, 2,946 were taking etoricoxib, and 16,922 were taking codeine.
Patients in the tramadol cohort were generally older, with higher body mass index, a longer duration of osteoarthritis, and had a higher prevalence of comorbidities, higher health care utilization, and more prescriptions of other medications.
The authors noted that, while the patients from each medication cohort were matched on propensity score, the results were still susceptible to confounding by indication and should be interpreted with caution.
The study was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Natural Science Foundation of China. One author declared funding from the National Institute on Drug Abuse during the conduct of the study and grants from Optum Labs outside the study. No other conflicts of interest were declared.
SOURCE: Zeng C et al. JAMA. 2019;321:969-82.
FROM JAMA
Juvenile idiopathic arthritis: Old disease, new tactics
Juvenile idiopathic arthritis (JIA) is a clinically heterogeneous group of arthritides that are characterized by onset before 16 years of age and defined in part as lasting ≥6 weeks.1 Significantly, the etiology of JIA is unknown, making it a diagnosis of exclusion.2
The most common autoimmune condition of childhood, JIA has a prevalence of 3.8 to 400 affected children for every 100,000 people.3,4 As the leading cause of musculoskeletal disability in children,5 and comprising 7 categories of disease, JIA must be managed with appropriate initial and ongoing intervention.
The amalgam of care that a JIA patient requires—medical, social, physical, psychological—calls for a primary care physician’s expert ability to collaborate and coordinate with medical specialists and subspecialists, including rheumatology, ophthalmology, social work, physical and occupational therapy, and psychology. The goal? As this article describes, the goal is to provide prompt diagnosis, suitable and effective intervention, and continuity of care. (JIA is a lifelong disease, in many cases.)
How JIA is classifiedfor diagnosis and treatment
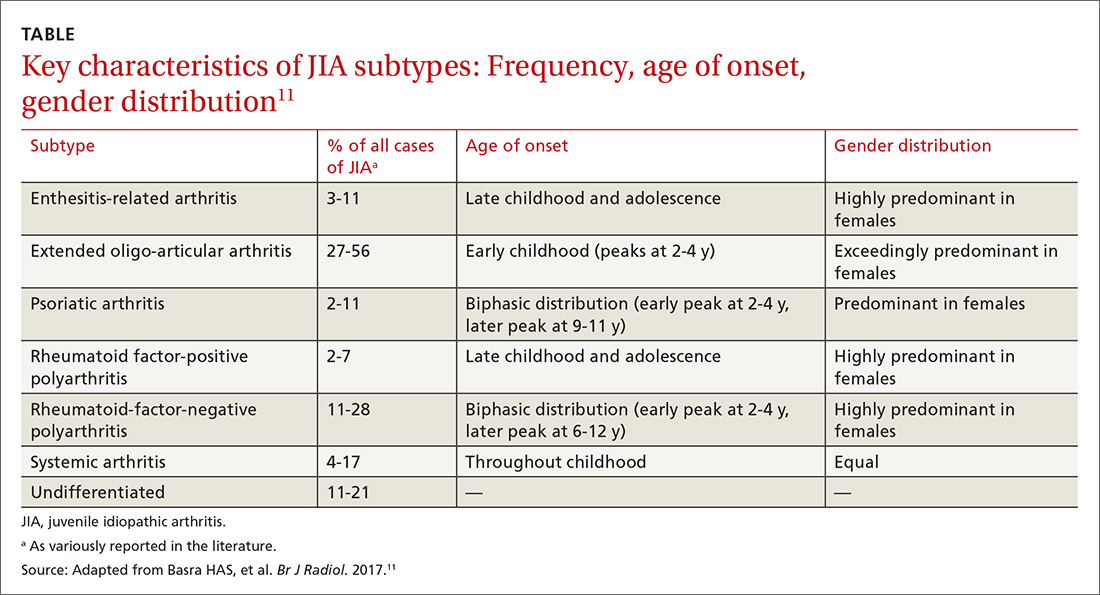
JIA comprises 7 categories, or classes.6 The scheme devised by the International League of Associations for Rheumatology (ILAR), now widely accepted, classifies JIA on the basis of clinical and biochemical markers that aid detection and treatment of the disorder, as well as research. (See “How efforts to classify JIA have caused confusion.”7-10) The ILAR classes (TABLE11) are:
- enthesitis-related arthritis (ERA)
- extended oligo-articular JIA (eoJIA), which involves ≤4 joints
- juvenile psoriatic arthritis (jPsA)
- rheumatoid factor (RF)-positive polyarticular JIA (RF+ pJIA)
- RF-negative polyarticular JIA (RF– pJIA)
- systemic-onset JIA (sJIA)
- undifferentiated JIA, which, generally, involves ≥4 joints.
SIDEBAR
How efforts to classiy JIA have caused confusion7-10
Various classifications of juvenile arthritis have been proposed and used over the past 3 decades. First was the American College of Rheumatology’s 1972 criteria for juvenile rheumatoid arthritis7; next came the European League against Rheumatism (EULAR) criteria for juvenile chronic arthritis, developed in 1977.8 Being contemporaneous, the 2 classifications led to a complicated, dichotomous definition of JIA among clinicians and researchers.
As a result of this disarray, the 1997 Durban, South Africa, meeting of the Pediatric Standing Committee of the International League of Associations for Rheumatology (ILAR)9 proposed that juvenile idiopathic arthritis be adopted as the umbrella term for the misunderstood terms juvenile rheumatoid arthritis and juvenile chronic arthritis. The intent of including “idiopathic” in the term was to acknowledge that the cause of these diseases was (and is still) unknown.
The novel classification proposed by the Pediatric Standing Committee was followed, in 2001, by an ILAR task force meeting in Edmonton, Alberta, Canada, on the classification of childhood arthritis. The outcome was a recommendation to add exclusion and inclusion criteria, to make all classes of JIA mutually exclusive.10 Most recently, as discussed in the body of this article, updated ILAR guidelines on JIA classification emphasize 1) heterogeneity among the 7 disease subtypes and 2) the fact that overlapping and exclusive features exist from class to class.
Updated guidelines regarding the 7 ILAR classes of JIA emphasize heterogeneity among disease subtypes, with overlapping and exclusive features noted from class to class.11
Extended oligo-articular JIA (27%-56%), pJIA (13%-35%), sJIA (4%-17%), and ERA,(3%-11%) are the most common JIA subtypes,12 with age of onset and sex predilection differing according to JIA class.11 The disease occurs more often in girls than in boys,11 and the predisposition is higher among Whites and Asians. The incidence of JIA (all classes taken together, for every 100,000 people) is: in Japan, 10 to 15 cases13; in Turkey, 64 cases14; in Norway, 65 cases15; and in the United States and Canada, taken together, 10 to 15 cases.16
What causes JIA?
The etiology of JIA remains unclear. It is known that the disease involves inflammation of the synovium and destruction of hard and soft tissues in joints.17 It has been postulated, therefore, that a combination of genetic, environmental, and immunogenic mechanisms might be responsible for JIA.
Continue to: For example, there is an increased...
For example, there is an increased frequency of autoimmune diseases among JIA patients.18 There are also reports documenting an increased rate of infection, including with enteric pathogens, parvovirus B,19 rubella, mumps, hepatitis B, Epstein-Barr virus, mycoplasma, and chlamydia.19 Stress and trauma have also been implicated.12
The T-lymphocyte percentage is increased in the synovial fluid of JIA patients, although that percentage varies from subtype to subtype.20 This elevation results in an increase in the number of macrophages, which are induced by secreted cytokines to produce interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-a). This activity of cellular immunity leads to joint destruction.21
Clinical features
The most common signs and symptoms of JIA are arthralgias (39%), arthritis (25%), fever (18%), limping (9%), rash (8%), abdominal pain (1.3%), and uveitis (1.3%).15 Forty percent of JIA patients are reported to have temporomandibular joint involvement at some point in their life; mandibular asymmetry secondary to condylar resorption and remodeling17 is the most common presenting complaint—not arthralgia or pain, as would be expected.
Most JIA patients (52%) first present to the emergency department; another 42% present to the office of a general medical practitioner.15 On average, 3 visits to a physician, over the course of approximately 3 months, are made before a definitive diagnosis (usually by a pediatric rheumatologist) is made.15
Pertinent questions to ask a patient who has a confirmed diagnosis of JIA include the nature, severity, and duration of morning stiffness and pain, as well as any encumbering factors to regular functioning at home or school.22 Different scoring charts can be used to determine the extent of pain and disability, including the Juvenile Arthritis Disease Activity Score (JADAS)23 and the clinical JADAS (cJADAS),24 which measure minimal disease activity25 and clinically inactive disease26 cutoffs.
Continue to: Macrophage-activating syndrome increases risk of morbidity, mortality
Macrophage-activating syndrome increases risk of morbidity, mortality
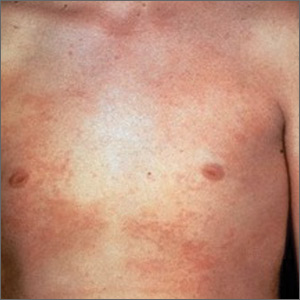
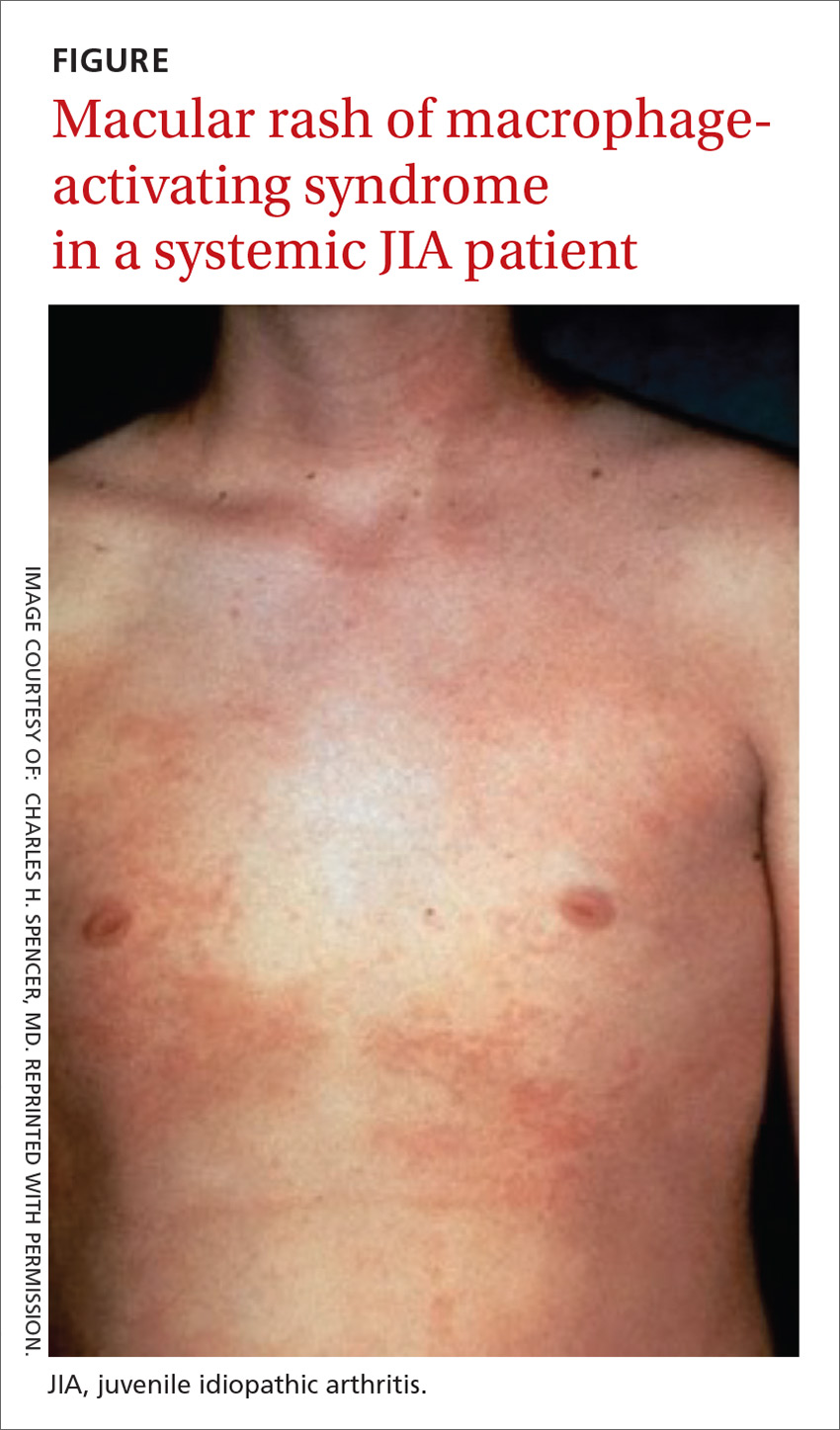
An overactivation and expansion of T lymphocytes and macrophagic histiocytes with hemophagocytic activity, macrophage-activating syndrome (MAS) occurs in approximately 10% of JIA patients,27 increasing their risk of morbidity and mortality. The syndrome, which typically presents as fever, seizures, hypotension, purpura, hepatitis, splenomegaly, and occasionally, multisystem organ failure, is seen in 30% to 40% of sJIA patients; approximately 11% of them experience sudden death as a consequence.28
The clinical setting of MAS includes presenting symptoms of fever and a salmon-pink macular rash (FIGURE). For many sJIA patients with MAS, the diagnosis is made when laboratory results show hyperferritinemia, thrombocytopenia, anemia, leukopenia, coagulopathy, and elevated levels of C-reactive protein and D-dimer.27
Different classes, different features
The following clinical profiles have been documented in different classes of JIA:
Systemic JIA presents with intermittent fever of at least 2 weeks’ duration, arthritis, and occasionally, a rash.
Extended oligo-articular JIA involves pain, in a mono-articular lower-extremity joint, that can develop suddenly or insidiously, and is characterized by early-morning stiffness and uveitis (especially in early-onset, antinuclear antibody-positive JIA patients).
Continue to: Poly-articular JIA
Poly-articular JIA patients present with mild fever, weight loss, and anemia.
Enthesis-related arthritis patients have findings of enthesopathy; asymmetric arthritis of the lower extremities, particularly the Achilles tendon29; and recurrent acute, symptomatic iridocyclitis.30
Juvenile psoriatic arthritis can involve any joint but is readily differentiated from pJIA by involvement of distal interphalangeal joints and psoriatic skin and nail changes.29
Investigations
Imaging
Radiography is still the most widely used imaging tool for making the diagnosis of JIA. Plain films demonstrate structural joint damage and disturbances of growth and maturation in bones. Radiography has poor sensitivity for detecting acute synovitis and limited utility in visualizing erosion changes early in the course of disease, however, which has led to increased use of ultrasonography (US) and contrast-enhanced magnetic resonance imaging (MRI) to diagnose JIA.30
Contrast-enhanced MRI is superior to US for detecting early inflammation and monitoring subsequent joint disease. Of course, MRI is more expensive than US, and less widely available. Other imaging options are computed tomography and positron emission tomography, but these scans are not as sensitive as contrast-enhanced MRI and have the disadvantage of radiation exposure (in the former) and cost (in the latter).
Continue to: Laboratory testing
Laboratory testing
No diagnostic tests for JIA exist. Assays of acute-phase reactants, including C-reactive protein, the erythrocyte sedimentation rate, and serum amyloid-A proteins, can be utilized to demonstrate inflammation but not to confirm the diagnosis. For some classes of JIA, various tests, including rheumatoid factor, antinuclear antibody, human leukocyte antigen B-27, and cyclic citrullated peptide antibodies, can be used to confirm a specific class but, again, are not recommended for confirming JIA.6
The complete blood count, blood cultures, and tests of uric acid and lactate dehydrogenase can be ordered during treatment to monitor for complications, such as malignancy, infection, MAS, and sepsis.
Treatment is based on disease class
Nonsteroidal anti-inflammatory drugs (NSAIDs) and intra-articular steroids are used in all JIA classes, as an adjunct to class-specific treatment, or as induction agents.31 These therapies, although they alleviate acute signs and symptoms, such as pain, inflammation, swelling and joint contractures, are not useful for long-term treatment of JIA because they do not halt disease progression.
Systemic steroids can be utilized in exceptional cases, including chronic uveitis with arthritis or in patients with destructive arthritis and poor prognostic features, including cyclic citrullated peptide antibodies, positive RF, erosions, and joint-space narrowing.32
Other drugs. Options include traditional disease-modifying anti-rheumatic drugs (csDMARDs), such as methotrexate and leflunomide; biologic agents, such as TNF-a inhibitors (eg, etanercept, adalimumab, and infliximab); and anti-IL monoclonal antibody drugs (eg, the IL-6 inhibitor tocilizumab and IL-1 inhibitors anakinra, and canakinumab).31 Indications by class include:
- csDMARDs as first-line therapy in persistent eoJIA and pJIA;
- TNF-Symbolα inhibitors for refractory eoJIA and for pJIA episodes31;
- tocilizumab, recommended for sJIA patients who have persistent systemic signs; and
- anakinra and canakinumab for refractory SJIA patients.32
Continue to: Failure
Failure
When treatment of JIA fails with a given drug, options include increasing the dosage; switching to another agent in the same drug class; switching to a different class; and combining an NSAID with a csDMARD or a biologic agent.32 In class-specific JIA cases, a change in a drug regimen is warranted on the basis of the evidence-based historical clinical response rate.32
What is the prognosis?
Treatment of JIA with novel agents, such as biologics, has opened up the possibility that JIA patients can live not just with suppressed symptoms but immunologically inactive disease. This is the result of better understanding of the pathogenesis of JIA and the mechanism of action of targeted drugs, and identification of biomarkers that are helpful in predicting prognosis, adverse effects, and response to treatment.
JIA is often a lifelong disease; one-third of patients continue to exhibit symptoms into adulthood.4 If their disease is properly managed, however, these patients do not develop typical features of rheumatoid arthritis, including hand, limb, and spine deformities. Last, patients with JIA who have only intermittent disease tend to do better over the long term than those whose disease is continual.32
The mortality rate of JIA has dropped: from 1% to 4% in the mid-1970s to 0.3% to 1% today4—an improvement in life expectancy that is echoed in enhanced quality of life for patients. According to the 4-level Steinbrocker functional classification scale33 (used to rate the extent of physical disability), 15% of JIA patients were Class III (limited to few or no activities of the patient’s usual occupation) or Class IV (bedridden with little or no self-care) in the period from 1976 to 1994—a percentage that had declined to 5% by 2002.34
The family physician plays pivotal role in JIA care
For the family physician, appropriate initial intervention in the management of JIA is imperative. This includes ordering imaging (whether plain films or MRI), laboratory tests as described earlier (although not to make the diagnosis), and the use of NSAIDs, intra-articular steroids, and other induction agents. Once the diagnosis is made, and a drug regimen is put in place, you will need to monitor for adverse effects. This monitoring will need to occur when a patient is escalated to csDMARDs, biological agents, or systemic steroids; is maintained on an NSAID; or is placed on a combination regimen.
Continue to: Before beginning therapy with a biologic agent...
Before beginning therapy with a biologic agent, it’s important to screen for hepatitis B, hepatitis C, human immunodeficiency virus infection, tuberculosis, and fungal infection (eg, Histoplasma capsulatum, Coccidioides immitis32). Be sure to make a timely referral to the ophthalmology service for a bi-annual eye exam and, in the event that surgery is necessary, conduct a preoperative evaluation, with the knowledge of how long before surgery a biologic agent must be withheld (duration varies by drug).32
CORRESPONDENCE
Tobe Momah, MD, Department of Family Medicine, Clinical Science Building, 4th Floor, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
1. Adriano LS, de França Fonteles MM, de Fátima Menezes Azevedo M, et al. Medication adherence in patients with juvenile idiopathic arthritis. Rev Bras Reumatol Engl Ed. 2017;57:23-29.
2. Akioka S. A better understanding of juvenile idiopathic arthritis with classification criteria. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39:513-521.
3. Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81:112-117.
4. Petty RE, Laxer RM, Lindsley CB, et al. Pediatric Rheumatology. Philadelphia, PA: Elsevier; 2016:188-201.e6.
5. Scott C, Brice N. Juvenile idiopathic arthritis–an update on its diagnosis and management. S Afr Med J. 2015;105:1077.
6. Giancane G, Consolaro A, Lanni S, et al. Juvenile idiopathic arthritis: diagnosis and treatment. Rheumatol Ther. 2016;3:187-207.
7. Criteria for the classification of juvenile rheumatoid arthritis. Bull Rheum Dis. 1972;23:712-719.
8. Wood PHN: Special meeting on nomenclature and classification of arthritis in children. In: Munthe E, ed. The Care of Rheumatic Children. Basel, Switzerland: EULAR Publishers; 1978:47-50.
9. Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991-1994.
10. Petty RE, Southwood TR, Manners P, et al; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392.
11. Basra HAS, Humphries PD. Juvenile idiopathic arthritis: what is the utility of ultrasound? Br J Radiol. 2017;90:20160920.
12. Weiss J, Ilowite NT. Juvenile idiopathic arthritis. Pediatr Clin North Am. 2005;52:413-442, vi.
13. Fujikawa S, Okuni M. A nationwide surveillance study of rheumatic diseases among Japanese children. Acta Pediatric Jpn. 1997:39:242-244.
14. Ozen S, Karaaslan Y, Ozdemir O, et al. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: a field study. J Rheumatol. 1998;25:2445-2449.
15. Aoust L, Rossi-Semerano L, Koné-PauL I, et al. Time to diagnosis in juvenile idiopathic arthritis: a French perspective. Orphanet J Rare Dis. 2017;12:43.
16. Moe N, Rygg M. Epidemiology of juvenile chronic arthritis in northern Norway; a ten-year retrospective study. Clin Exp Rheumatol. 1998;16:99-101.
17. Abramowicz S, Kim S, Prahalad S, et al. Juvenile arthritis: current concepts in terminology, etiopathogenesis, diagnosis, and management. Int J Oral Maxillofac Surg. 2016;45:801-812.
18. Prahalad S, Shear ES, Thompson SD, et al. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1851-1856.
19. Gonzalez B, Larrañaga C, León O, et al. Parvovirus B19 may have a role in the pathogenesis of juvenile idiopathic arthritis. J Rheumatol. 2007;34:1336-1340.
20. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138-2149.
21. Zhou J, Ding Y, Zhang Y, et al. CD3+CD56+ natural killer T cell activity in children with different forms of juvenile idiopathic arthritis and the influence of etanercept treatment on polyarticular subgroup. Clin Immunol. 2016;176:1-11.
22. Shoop-Worrall SJW, Verstappen SMM, Baildam E, et al. How common is clinically inactive disease in a prospective cohort of patients with juvenile idiopathic arthritis? The importance of definition. Ann Rheum Dis. 2017;0:1-8.
23. Nordal EB, Zak M, Berntson L, et al. Juvenile Arthritis Disease Activity Score (JADAS) based on CRP; validity and predictive ability in a Nordic population-based setting. Pediatr Rheumatol Online J. 2011;9(suppl 1):155.
24. Swart JF, Dijkhuizen EHP, Wulffraat NM, et al. Clinical Juvenile Arthritis Disease Activity Score proves to be a useful tool in treat-to-target therapy in juvenile idiopathic arthritis. Ann Rheum Dis. 2018;77:336-342.
25. Horneff G, Klein A, Ganser G, et al. Protocols on classification, monitoring and therapy in children’s rheumatology (PRO-KIND): results of the working group polyarticular juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2017;15:78.
26. Shoop-Worrall SJW, Verstappen SMM, McDonagh JE, et al. Long‐term outcomes following achievement of clinically inactive disease in juvenile idiopathic arthritis. Arthritis Rheumatol. 2018;70:1519-1529.
27. Ahn SS, Yoo BW, Jung SM, et al. In-hospital mortality in febrile lupus patients based on 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome. Sem Arthritis Rheum. 2017;.47:216-221.
28. Yokota S, Mori M, Imagawa T, et al. Proposal for juvenile idiopathic arthritis guidance on diagnosis and treatment for primary care pediatricians and nonpediatric rheumatologists (2007). Mod Rheumatol. 2007;17:353-363.
29. Barut K, Adrovic A, Şahin S, et al. Juvenile idiopathic arthritis. Balkan Med J. 2017;34:90-101.
30. Colebatch-Bourn AN, Edwards CJ, et al. EULAR-PReS points to consider for the use of imaging in the diagnosis and management of juvenile idiopathic arthritis in clinical practice. Ann Rheum Dis. 2015;74:1946-1957.
31. Blazina Š, Markelj G, AvramoviČ MZ, et al. Management of juvenile idiopathic arthritis: a clinical guide. Pediatr Drugs. 2016;18:397-412.
32. Santos MJ, Conde M, Mourão AF, et al. 2016 update of the Portuguese recommendations for the use of biologic therapies in children and adolescents with juvenile idiopathic arthritis. Acta Rheumatol Port. 2016;41:194-212.
33. Steinbrocker 0, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659-662.
34. Oen K, Malleson PN, Cabral D, et al. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29:1989-1999.
Juvenile idiopathic arthritis (JIA) is a clinically heterogeneous group of arthritides that are characterized by onset before 16 years of age and defined in part as lasting ≥6 weeks.1 Significantly, the etiology of JIA is unknown, making it a diagnosis of exclusion.2
The most common autoimmune condition of childhood, JIA has a prevalence of 3.8 to 400 affected children for every 100,000 people.3,4 As the leading cause of musculoskeletal disability in children,5 and comprising 7 categories of disease, JIA must be managed with appropriate initial and ongoing intervention.
The amalgam of care that a JIA patient requires—medical, social, physical, psychological—calls for a primary care physician’s expert ability to collaborate and coordinate with medical specialists and subspecialists, including rheumatology, ophthalmology, social work, physical and occupational therapy, and psychology. The goal? As this article describes, the goal is to provide prompt diagnosis, suitable and effective intervention, and continuity of care. (JIA is a lifelong disease, in many cases.)
How JIA is classifiedfor diagnosis and treatment
JIA comprises 7 categories, or classes.6 The scheme devised by the International League of Associations for Rheumatology (ILAR), now widely accepted, classifies JIA on the basis of clinical and biochemical markers that aid detection and treatment of the disorder, as well as research. (See “How efforts to classify JIA have caused confusion.”7-10) The ILAR classes (TABLE11) are:
- enthesitis-related arthritis (ERA)
- extended oligo-articular JIA (eoJIA), which involves ≤4 joints
- juvenile psoriatic arthritis (jPsA)
- rheumatoid factor (RF)-positive polyarticular JIA (RF+ pJIA)
- RF-negative polyarticular JIA (RF– pJIA)
- systemic-onset JIA (sJIA)
- undifferentiated JIA, which, generally, involves ≥4 joints.

SIDEBAR
How efforts to classiy JIA have caused confusion7-10
Various classifications of juvenile arthritis have been proposed and used over the past 3 decades. First was the American College of Rheumatology’s 1972 criteria for juvenile rheumatoid arthritis7; next came the European League against Rheumatism (EULAR) criteria for juvenile chronic arthritis, developed in 1977.8 Being contemporaneous, the 2 classifications led to a complicated, dichotomous definition of JIA among clinicians and researchers.
As a result of this disarray, the 1997 Durban, South Africa, meeting of the Pediatric Standing Committee of the International League of Associations for Rheumatology (ILAR)9 proposed that juvenile idiopathic arthritis be adopted as the umbrella term for the misunderstood terms juvenile rheumatoid arthritis and juvenile chronic arthritis. The intent of including “idiopathic” in the term was to acknowledge that the cause of these diseases was (and is still) unknown.
The novel classification proposed by the Pediatric Standing Committee was followed, in 2001, by an ILAR task force meeting in Edmonton, Alberta, Canada, on the classification of childhood arthritis. The outcome was a recommendation to add exclusion and inclusion criteria, to make all classes of JIA mutually exclusive.10 Most recently, as discussed in the body of this article, updated ILAR guidelines on JIA classification emphasize 1) heterogeneity among the 7 disease subtypes and 2) the fact that overlapping and exclusive features exist from class to class.
Updated guidelines regarding the 7 ILAR classes of JIA emphasize heterogeneity among disease subtypes, with overlapping and exclusive features noted from class to class.11
Extended oligo-articular JIA (27%-56%), pJIA (13%-35%), sJIA (4%-17%), and ERA,(3%-11%) are the most common JIA subtypes,12 with age of onset and sex predilection differing according to JIA class.11 The disease occurs more often in girls than in boys,11 and the predisposition is higher among Whites and Asians. The incidence of JIA (all classes taken together, for every 100,000 people) is: in Japan, 10 to 15 cases13; in Turkey, 64 cases14; in Norway, 65 cases15; and in the United States and Canada, taken together, 10 to 15 cases.16
What causes JIA?
The etiology of JIA remains unclear. It is known that the disease involves inflammation of the synovium and destruction of hard and soft tissues in joints.17 It has been postulated, therefore, that a combination of genetic, environmental, and immunogenic mechanisms might be responsible for JIA.
Continue to: For example, there is an increased...
For example, there is an increased frequency of autoimmune diseases among JIA patients.18 There are also reports documenting an increased rate of infection, including with enteric pathogens, parvovirus B,19 rubella, mumps, hepatitis B, Epstein-Barr virus, mycoplasma, and chlamydia.19 Stress and trauma have also been implicated.12
The T-lymphocyte percentage is increased in the synovial fluid of JIA patients, although that percentage varies from subtype to subtype.20 This elevation results in an increase in the number of macrophages, which are induced by secreted cytokines to produce interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-a). This activity of cellular immunity leads to joint destruction.21
Clinical features
The most common signs and symptoms of JIA are arthralgias (39%), arthritis (25%), fever (18%), limping (9%), rash (8%), abdominal pain (1.3%), and uveitis (1.3%).15 Forty percent of JIA patients are reported to have temporomandibular joint involvement at some point in their life; mandibular asymmetry secondary to condylar resorption and remodeling17 is the most common presenting complaint—not arthralgia or pain, as would be expected.
Most JIA patients (52%) first present to the emergency department; another 42% present to the office of a general medical practitioner.15 On average, 3 visits to a physician, over the course of approximately 3 months, are made before a definitive diagnosis (usually by a pediatric rheumatologist) is made.15
Pertinent questions to ask a patient who has a confirmed diagnosis of JIA include the nature, severity, and duration of morning stiffness and pain, as well as any encumbering factors to regular functioning at home or school.22 Different scoring charts can be used to determine the extent of pain and disability, including the Juvenile Arthritis Disease Activity Score (JADAS)23 and the clinical JADAS (cJADAS),24 which measure minimal disease activity25 and clinically inactive disease26 cutoffs.
Continue to: Macrophage-activating syndrome increases risk of morbidity, mortality
Macrophage-activating syndrome increases risk of morbidity, mortality
An overactivation and expansion of T lymphocytes and macrophagic histiocytes with hemophagocytic activity, macrophage-activating syndrome (MAS) occurs in approximately 10% of JIA patients,27 increasing their risk of morbidity and mortality. The syndrome, which typically presents as fever, seizures, hypotension, purpura, hepatitis, splenomegaly, and occasionally, multisystem organ failure, is seen in 30% to 40% of sJIA patients; approximately 11% of them experience sudden death as a consequence.28
The clinical setting of MAS includes presenting symptoms of fever and a salmon-pink macular rash (FIGURE). For many sJIA patients with MAS, the diagnosis is made when laboratory results show hyperferritinemia, thrombocytopenia, anemia, leukopenia, coagulopathy, and elevated levels of C-reactive protein and D-dimer.27

Different classes, different features
The following clinical profiles have been documented in different classes of JIA:
Systemic JIA presents with intermittent fever of at least 2 weeks’ duration, arthritis, and occasionally, a rash.
Extended oligo-articular JIA involves pain, in a mono-articular lower-extremity joint, that can develop suddenly or insidiously, and is characterized by early-morning stiffness and uveitis (especially in early-onset, antinuclear antibody-positive JIA patients).
Continue to: Poly-articular JIA
Poly-articular JIA patients present with mild fever, weight loss, and anemia.
Enthesis-related arthritis patients have findings of enthesopathy; asymmetric arthritis of the lower extremities, particularly the Achilles tendon29; and recurrent acute, symptomatic iridocyclitis.30
Juvenile psoriatic arthritis can involve any joint but is readily differentiated from pJIA by involvement of distal interphalangeal joints and psoriatic skin and nail changes.29
Investigations
Imaging
Radiography is still the most widely used imaging tool for making the diagnosis of JIA. Plain films demonstrate structural joint damage and disturbances of growth and maturation in bones. Radiography has poor sensitivity for detecting acute synovitis and limited utility in visualizing erosion changes early in the course of disease, however, which has led to increased use of ultrasonography (US) and contrast-enhanced magnetic resonance imaging (MRI) to diagnose JIA.30
Contrast-enhanced MRI is superior to US for detecting early inflammation and monitoring subsequent joint disease. Of course, MRI is more expensive than US, and less widely available. Other imaging options are computed tomography and positron emission tomography, but these scans are not as sensitive as contrast-enhanced MRI and have the disadvantage of radiation exposure (in the former) and cost (in the latter).
Continue to: Laboratory testing
Laboratory testing
No diagnostic tests for JIA exist. Assays of acute-phase reactants, including C-reactive protein, the erythrocyte sedimentation rate, and serum amyloid-A proteins, can be utilized to demonstrate inflammation but not to confirm the diagnosis. For some classes of JIA, various tests, including rheumatoid factor, antinuclear antibody, human leukocyte antigen B-27, and cyclic citrullated peptide antibodies, can be used to confirm a specific class but, again, are not recommended for confirming JIA.6
The complete blood count, blood cultures, and tests of uric acid and lactate dehydrogenase can be ordered during treatment to monitor for complications, such as malignancy, infection, MAS, and sepsis.
Treatment is based on disease class
Nonsteroidal anti-inflammatory drugs (NSAIDs) and intra-articular steroids are used in all JIA classes, as an adjunct to class-specific treatment, or as induction agents.31 These therapies, although they alleviate acute signs and symptoms, such as pain, inflammation, swelling and joint contractures, are not useful for long-term treatment of JIA because they do not halt disease progression.
Systemic steroids can be utilized in exceptional cases, including chronic uveitis with arthritis or in patients with destructive arthritis and poor prognostic features, including cyclic citrullated peptide antibodies, positive RF, erosions, and joint-space narrowing.32
Other drugs. Options include traditional disease-modifying anti-rheumatic drugs (csDMARDs), such as methotrexate and leflunomide; biologic agents, such as TNF-a inhibitors (eg, etanercept, adalimumab, and infliximab); and anti-IL monoclonal antibody drugs (eg, the IL-6 inhibitor tocilizumab and IL-1 inhibitors anakinra, and canakinumab).31 Indications by class include:
- csDMARDs as first-line therapy in persistent eoJIA and pJIA;
- TNF-Symbolα inhibitors for refractory eoJIA and for pJIA episodes31;
- tocilizumab, recommended for sJIA patients who have persistent systemic signs; and
- anakinra and canakinumab for refractory SJIA patients.32
Continue to: Failure
Failure
When treatment of JIA fails with a given drug, options include increasing the dosage; switching to another agent in the same drug class; switching to a different class; and combining an NSAID with a csDMARD or a biologic agent.32 In class-specific JIA cases, a change in a drug regimen is warranted on the basis of the evidence-based historical clinical response rate.32
What is the prognosis?
Treatment of JIA with novel agents, such as biologics, has opened up the possibility that JIA patients can live not just with suppressed symptoms but immunologically inactive disease. This is the result of better understanding of the pathogenesis of JIA and the mechanism of action of targeted drugs, and identification of biomarkers that are helpful in predicting prognosis, adverse effects, and response to treatment.
JIA is often a lifelong disease; one-third of patients continue to exhibit symptoms into adulthood.4 If their disease is properly managed, however, these patients do not develop typical features of rheumatoid arthritis, including hand, limb, and spine deformities. Last, patients with JIA who have only intermittent disease tend to do better over the long term than those whose disease is continual.32
The mortality rate of JIA has dropped: from 1% to 4% in the mid-1970s to 0.3% to 1% today4—an improvement in life expectancy that is echoed in enhanced quality of life for patients. According to the 4-level Steinbrocker functional classification scale33 (used to rate the extent of physical disability), 15% of JIA patients were Class III (limited to few or no activities of the patient’s usual occupation) or Class IV (bedridden with little or no self-care) in the period from 1976 to 1994—a percentage that had declined to 5% by 2002.34
The family physician plays pivotal role in JIA care
For the family physician, appropriate initial intervention in the management of JIA is imperative. This includes ordering imaging (whether plain films or MRI), laboratory tests as described earlier (although not to make the diagnosis), and the use of NSAIDs, intra-articular steroids, and other induction agents. Once the diagnosis is made, and a drug regimen is put in place, you will need to monitor for adverse effects. This monitoring will need to occur when a patient is escalated to csDMARDs, biological agents, or systemic steroids; is maintained on an NSAID; or is placed on a combination regimen.
Continue to: Before beginning therapy with a biologic agent...
Before beginning therapy with a biologic agent, it’s important to screen for hepatitis B, hepatitis C, human immunodeficiency virus infection, tuberculosis, and fungal infection (eg, Histoplasma capsulatum, Coccidioides immitis32). Be sure to make a timely referral to the ophthalmology service for a bi-annual eye exam and, in the event that surgery is necessary, conduct a preoperative evaluation, with the knowledge of how long before surgery a biologic agent must be withheld (duration varies by drug).32
CORRESPONDENCE
Tobe Momah, MD, Department of Family Medicine, Clinical Science Building, 4th Floor, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
Juvenile idiopathic arthritis (JIA) is a clinically heterogeneous group of arthritides that are characterized by onset before 16 years of age and defined in part as lasting ≥6 weeks.1 Significantly, the etiology of JIA is unknown, making it a diagnosis of exclusion.2
The most common autoimmune condition of childhood, JIA has a prevalence of 3.8 to 400 affected children for every 100,000 people.3,4 As the leading cause of musculoskeletal disability in children,5 and comprising 7 categories of disease, JIA must be managed with appropriate initial and ongoing intervention.
The amalgam of care that a JIA patient requires—medical, social, physical, psychological—calls for a primary care physician’s expert ability to collaborate and coordinate with medical specialists and subspecialists, including rheumatology, ophthalmology, social work, physical and occupational therapy, and psychology. The goal? As this article describes, the goal is to provide prompt diagnosis, suitable and effective intervention, and continuity of care. (JIA is a lifelong disease, in many cases.)
How JIA is classifiedfor diagnosis and treatment
JIA comprises 7 categories, or classes.6 The scheme devised by the International League of Associations for Rheumatology (ILAR), now widely accepted, classifies JIA on the basis of clinical and biochemical markers that aid detection and treatment of the disorder, as well as research. (See “How efforts to classify JIA have caused confusion.”7-10) The ILAR classes (TABLE11) are:
- enthesitis-related arthritis (ERA)
- extended oligo-articular JIA (eoJIA), which involves ≤4 joints
- juvenile psoriatic arthritis (jPsA)
- rheumatoid factor (RF)-positive polyarticular JIA (RF+ pJIA)
- RF-negative polyarticular JIA (RF– pJIA)
- systemic-onset JIA (sJIA)
- undifferentiated JIA, which, generally, involves ≥4 joints.

SIDEBAR
How efforts to classiy JIA have caused confusion7-10
Various classifications of juvenile arthritis have been proposed and used over the past 3 decades. First was the American College of Rheumatology’s 1972 criteria for juvenile rheumatoid arthritis7; next came the European League against Rheumatism (EULAR) criteria for juvenile chronic arthritis, developed in 1977.8 Being contemporaneous, the 2 classifications led to a complicated, dichotomous definition of JIA among clinicians and researchers.
As a result of this disarray, the 1997 Durban, South Africa, meeting of the Pediatric Standing Committee of the International League of Associations for Rheumatology (ILAR)9 proposed that juvenile idiopathic arthritis be adopted as the umbrella term for the misunderstood terms juvenile rheumatoid arthritis and juvenile chronic arthritis. The intent of including “idiopathic” in the term was to acknowledge that the cause of these diseases was (and is still) unknown.
The novel classification proposed by the Pediatric Standing Committee was followed, in 2001, by an ILAR task force meeting in Edmonton, Alberta, Canada, on the classification of childhood arthritis. The outcome was a recommendation to add exclusion and inclusion criteria, to make all classes of JIA mutually exclusive.10 Most recently, as discussed in the body of this article, updated ILAR guidelines on JIA classification emphasize 1) heterogeneity among the 7 disease subtypes and 2) the fact that overlapping and exclusive features exist from class to class.
Updated guidelines regarding the 7 ILAR classes of JIA emphasize heterogeneity among disease subtypes, with overlapping and exclusive features noted from class to class.11
Extended oligo-articular JIA (27%-56%), pJIA (13%-35%), sJIA (4%-17%), and ERA,(3%-11%) are the most common JIA subtypes,12 with age of onset and sex predilection differing according to JIA class.11 The disease occurs more often in girls than in boys,11 and the predisposition is higher among Whites and Asians. The incidence of JIA (all classes taken together, for every 100,000 people) is: in Japan, 10 to 15 cases13; in Turkey, 64 cases14; in Norway, 65 cases15; and in the United States and Canada, taken together, 10 to 15 cases.16
What causes JIA?
The etiology of JIA remains unclear. It is known that the disease involves inflammation of the synovium and destruction of hard and soft tissues in joints.17 It has been postulated, therefore, that a combination of genetic, environmental, and immunogenic mechanisms might be responsible for JIA.
Continue to: For example, there is an increased...
For example, there is an increased frequency of autoimmune diseases among JIA patients.18 There are also reports documenting an increased rate of infection, including with enteric pathogens, parvovirus B,19 rubella, mumps, hepatitis B, Epstein-Barr virus, mycoplasma, and chlamydia.19 Stress and trauma have also been implicated.12
The T-lymphocyte percentage is increased in the synovial fluid of JIA patients, although that percentage varies from subtype to subtype.20 This elevation results in an increase in the number of macrophages, which are induced by secreted cytokines to produce interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-a). This activity of cellular immunity leads to joint destruction.21
Clinical features
The most common signs and symptoms of JIA are arthralgias (39%), arthritis (25%), fever (18%), limping (9%), rash (8%), abdominal pain (1.3%), and uveitis (1.3%).15 Forty percent of JIA patients are reported to have temporomandibular joint involvement at some point in their life; mandibular asymmetry secondary to condylar resorption and remodeling17 is the most common presenting complaint—not arthralgia or pain, as would be expected.
Most JIA patients (52%) first present to the emergency department; another 42% present to the office of a general medical practitioner.15 On average, 3 visits to a physician, over the course of approximately 3 months, are made before a definitive diagnosis (usually by a pediatric rheumatologist) is made.15
Pertinent questions to ask a patient who has a confirmed diagnosis of JIA include the nature, severity, and duration of morning stiffness and pain, as well as any encumbering factors to regular functioning at home or school.22 Different scoring charts can be used to determine the extent of pain and disability, including the Juvenile Arthritis Disease Activity Score (JADAS)23 and the clinical JADAS (cJADAS),24 which measure minimal disease activity25 and clinically inactive disease26 cutoffs.
Continue to: Macrophage-activating syndrome increases risk of morbidity, mortality
Macrophage-activating syndrome increases risk of morbidity, mortality
An overactivation and expansion of T lymphocytes and macrophagic histiocytes with hemophagocytic activity, macrophage-activating syndrome (MAS) occurs in approximately 10% of JIA patients,27 increasing their risk of morbidity and mortality. The syndrome, which typically presents as fever, seizures, hypotension, purpura, hepatitis, splenomegaly, and occasionally, multisystem organ failure, is seen in 30% to 40% of sJIA patients; approximately 11% of them experience sudden death as a consequence.28
The clinical setting of MAS includes presenting symptoms of fever and a salmon-pink macular rash (FIGURE). For many sJIA patients with MAS, the diagnosis is made when laboratory results show hyperferritinemia, thrombocytopenia, anemia, leukopenia, coagulopathy, and elevated levels of C-reactive protein and D-dimer.27

Different classes, different features
The following clinical profiles have been documented in different classes of JIA:
Systemic JIA presents with intermittent fever of at least 2 weeks’ duration, arthritis, and occasionally, a rash.
Extended oligo-articular JIA involves pain, in a mono-articular lower-extremity joint, that can develop suddenly or insidiously, and is characterized by early-morning stiffness and uveitis (especially in early-onset, antinuclear antibody-positive JIA patients).
Continue to: Poly-articular JIA
Poly-articular JIA patients present with mild fever, weight loss, and anemia.
Enthesis-related arthritis patients have findings of enthesopathy; asymmetric arthritis of the lower extremities, particularly the Achilles tendon29; and recurrent acute, symptomatic iridocyclitis.30
Juvenile psoriatic arthritis can involve any joint but is readily differentiated from pJIA by involvement of distal interphalangeal joints and psoriatic skin and nail changes.29
Investigations
Imaging
Radiography is still the most widely used imaging tool for making the diagnosis of JIA. Plain films demonstrate structural joint damage and disturbances of growth and maturation in bones. Radiography has poor sensitivity for detecting acute synovitis and limited utility in visualizing erosion changes early in the course of disease, however, which has led to increased use of ultrasonography (US) and contrast-enhanced magnetic resonance imaging (MRI) to diagnose JIA.30
Contrast-enhanced MRI is superior to US for detecting early inflammation and monitoring subsequent joint disease. Of course, MRI is more expensive than US, and less widely available. Other imaging options are computed tomography and positron emission tomography, but these scans are not as sensitive as contrast-enhanced MRI and have the disadvantage of radiation exposure (in the former) and cost (in the latter).
Continue to: Laboratory testing
Laboratory testing
No diagnostic tests for JIA exist. Assays of acute-phase reactants, including C-reactive protein, the erythrocyte sedimentation rate, and serum amyloid-A proteins, can be utilized to demonstrate inflammation but not to confirm the diagnosis. For some classes of JIA, various tests, including rheumatoid factor, antinuclear antibody, human leukocyte antigen B-27, and cyclic citrullated peptide antibodies, can be used to confirm a specific class but, again, are not recommended for confirming JIA.6
The complete blood count, blood cultures, and tests of uric acid and lactate dehydrogenase can be ordered during treatment to monitor for complications, such as malignancy, infection, MAS, and sepsis.
Treatment is based on disease class
Nonsteroidal anti-inflammatory drugs (NSAIDs) and intra-articular steroids are used in all JIA classes, as an adjunct to class-specific treatment, or as induction agents.31 These therapies, although they alleviate acute signs and symptoms, such as pain, inflammation, swelling and joint contractures, are not useful for long-term treatment of JIA because they do not halt disease progression.
Systemic steroids can be utilized in exceptional cases, including chronic uveitis with arthritis or in patients with destructive arthritis and poor prognostic features, including cyclic citrullated peptide antibodies, positive RF, erosions, and joint-space narrowing.32
Other drugs. Options include traditional disease-modifying anti-rheumatic drugs (csDMARDs), such as methotrexate and leflunomide; biologic agents, such as TNF-a inhibitors (eg, etanercept, adalimumab, and infliximab); and anti-IL monoclonal antibody drugs (eg, the IL-6 inhibitor tocilizumab and IL-1 inhibitors anakinra, and canakinumab).31 Indications by class include:
- csDMARDs as first-line therapy in persistent eoJIA and pJIA;
- TNF-Symbolα inhibitors for refractory eoJIA and for pJIA episodes31;
- tocilizumab, recommended for sJIA patients who have persistent systemic signs; and
- anakinra and canakinumab for refractory SJIA patients.32
Continue to: Failure
Failure
When treatment of JIA fails with a given drug, options include increasing the dosage; switching to another agent in the same drug class; switching to a different class; and combining an NSAID with a csDMARD or a biologic agent.32 In class-specific JIA cases, a change in a drug regimen is warranted on the basis of the evidence-based historical clinical response rate.32
What is the prognosis?
Treatment of JIA with novel agents, such as biologics, has opened up the possibility that JIA patients can live not just with suppressed symptoms but immunologically inactive disease. This is the result of better understanding of the pathogenesis of JIA and the mechanism of action of targeted drugs, and identification of biomarkers that are helpful in predicting prognosis, adverse effects, and response to treatment.
JIA is often a lifelong disease; one-third of patients continue to exhibit symptoms into adulthood.4 If their disease is properly managed, however, these patients do not develop typical features of rheumatoid arthritis, including hand, limb, and spine deformities. Last, patients with JIA who have only intermittent disease tend to do better over the long term than those whose disease is continual.32
The mortality rate of JIA has dropped: from 1% to 4% in the mid-1970s to 0.3% to 1% today4—an improvement in life expectancy that is echoed in enhanced quality of life for patients. According to the 4-level Steinbrocker functional classification scale33 (used to rate the extent of physical disability), 15% of JIA patients were Class III (limited to few or no activities of the patient’s usual occupation) or Class IV (bedridden with little or no self-care) in the period from 1976 to 1994—a percentage that had declined to 5% by 2002.34
The family physician plays pivotal role in JIA care
For the family physician, appropriate initial intervention in the management of JIA is imperative. This includes ordering imaging (whether plain films or MRI), laboratory tests as described earlier (although not to make the diagnosis), and the use of NSAIDs, intra-articular steroids, and other induction agents. Once the diagnosis is made, and a drug regimen is put in place, you will need to monitor for adverse effects. This monitoring will need to occur when a patient is escalated to csDMARDs, biological agents, or systemic steroids; is maintained on an NSAID; or is placed on a combination regimen.
Continue to: Before beginning therapy with a biologic agent...
Before beginning therapy with a biologic agent, it’s important to screen for hepatitis B, hepatitis C, human immunodeficiency virus infection, tuberculosis, and fungal infection (eg, Histoplasma capsulatum, Coccidioides immitis32). Be sure to make a timely referral to the ophthalmology service for a bi-annual eye exam and, in the event that surgery is necessary, conduct a preoperative evaluation, with the knowledge of how long before surgery a biologic agent must be withheld (duration varies by drug).32
CORRESPONDENCE
Tobe Momah, MD, Department of Family Medicine, Clinical Science Building, 4th Floor, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; [email protected].
1. Adriano LS, de França Fonteles MM, de Fátima Menezes Azevedo M, et al. Medication adherence in patients with juvenile idiopathic arthritis. Rev Bras Reumatol Engl Ed. 2017;57:23-29.
2. Akioka S. A better understanding of juvenile idiopathic arthritis with classification criteria. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39:513-521.
3. Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81:112-117.
4. Petty RE, Laxer RM, Lindsley CB, et al. Pediatric Rheumatology. Philadelphia, PA: Elsevier; 2016:188-201.e6.
5. Scott C, Brice N. Juvenile idiopathic arthritis–an update on its diagnosis and management. S Afr Med J. 2015;105:1077.
6. Giancane G, Consolaro A, Lanni S, et al. Juvenile idiopathic arthritis: diagnosis and treatment. Rheumatol Ther. 2016;3:187-207.
7. Criteria for the classification of juvenile rheumatoid arthritis. Bull Rheum Dis. 1972;23:712-719.
8. Wood PHN: Special meeting on nomenclature and classification of arthritis in children. In: Munthe E, ed. The Care of Rheumatic Children. Basel, Switzerland: EULAR Publishers; 1978:47-50.
9. Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991-1994.
10. Petty RE, Southwood TR, Manners P, et al; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392.
11. Basra HAS, Humphries PD. Juvenile idiopathic arthritis: what is the utility of ultrasound? Br J Radiol. 2017;90:20160920.
12. Weiss J, Ilowite NT. Juvenile idiopathic arthritis. Pediatr Clin North Am. 2005;52:413-442, vi.
13. Fujikawa S, Okuni M. A nationwide surveillance study of rheumatic diseases among Japanese children. Acta Pediatric Jpn. 1997:39:242-244.
14. Ozen S, Karaaslan Y, Ozdemir O, et al. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: a field study. J Rheumatol. 1998;25:2445-2449.
15. Aoust L, Rossi-Semerano L, Koné-PauL I, et al. Time to diagnosis in juvenile idiopathic arthritis: a French perspective. Orphanet J Rare Dis. 2017;12:43.
16. Moe N, Rygg M. Epidemiology of juvenile chronic arthritis in northern Norway; a ten-year retrospective study. Clin Exp Rheumatol. 1998;16:99-101.
17. Abramowicz S, Kim S, Prahalad S, et al. Juvenile arthritis: current concepts in terminology, etiopathogenesis, diagnosis, and management. Int J Oral Maxillofac Surg. 2016;45:801-812.
18. Prahalad S, Shear ES, Thompson SD, et al. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1851-1856.
19. Gonzalez B, Larrañaga C, León O, et al. Parvovirus B19 may have a role in the pathogenesis of juvenile idiopathic arthritis. J Rheumatol. 2007;34:1336-1340.
20. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138-2149.
21. Zhou J, Ding Y, Zhang Y, et al. CD3+CD56+ natural killer T cell activity in children with different forms of juvenile idiopathic arthritis and the influence of etanercept treatment on polyarticular subgroup. Clin Immunol. 2016;176:1-11.
22. Shoop-Worrall SJW, Verstappen SMM, Baildam E, et al. How common is clinically inactive disease in a prospective cohort of patients with juvenile idiopathic arthritis? The importance of definition. Ann Rheum Dis. 2017;0:1-8.
23. Nordal EB, Zak M, Berntson L, et al. Juvenile Arthritis Disease Activity Score (JADAS) based on CRP; validity and predictive ability in a Nordic population-based setting. Pediatr Rheumatol Online J. 2011;9(suppl 1):155.
24. Swart JF, Dijkhuizen EHP, Wulffraat NM, et al. Clinical Juvenile Arthritis Disease Activity Score proves to be a useful tool in treat-to-target therapy in juvenile idiopathic arthritis. Ann Rheum Dis. 2018;77:336-342.
25. Horneff G, Klein A, Ganser G, et al. Protocols on classification, monitoring and therapy in children’s rheumatology (PRO-KIND): results of the working group polyarticular juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2017;15:78.
26. Shoop-Worrall SJW, Verstappen SMM, McDonagh JE, et al. Long‐term outcomes following achievement of clinically inactive disease in juvenile idiopathic arthritis. Arthritis Rheumatol. 2018;70:1519-1529.
27. Ahn SS, Yoo BW, Jung SM, et al. In-hospital mortality in febrile lupus patients based on 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome. Sem Arthritis Rheum. 2017;.47:216-221.
28. Yokota S, Mori M, Imagawa T, et al. Proposal for juvenile idiopathic arthritis guidance on diagnosis and treatment for primary care pediatricians and nonpediatric rheumatologists (2007). Mod Rheumatol. 2007;17:353-363.
29. Barut K, Adrovic A, Şahin S, et al. Juvenile idiopathic arthritis. Balkan Med J. 2017;34:90-101.
30. Colebatch-Bourn AN, Edwards CJ, et al. EULAR-PReS points to consider for the use of imaging in the diagnosis and management of juvenile idiopathic arthritis in clinical practice. Ann Rheum Dis. 2015;74:1946-1957.
31. Blazina Š, Markelj G, AvramoviČ MZ, et al. Management of juvenile idiopathic arthritis: a clinical guide. Pediatr Drugs. 2016;18:397-412.
32. Santos MJ, Conde M, Mourão AF, et al. 2016 update of the Portuguese recommendations for the use of biologic therapies in children and adolescents with juvenile idiopathic arthritis. Acta Rheumatol Port. 2016;41:194-212.
33. Steinbrocker 0, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659-662.
34. Oen K, Malleson PN, Cabral D, et al. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29:1989-1999.
1. Adriano LS, de França Fonteles MM, de Fátima Menezes Azevedo M, et al. Medication adherence in patients with juvenile idiopathic arthritis. Rev Bras Reumatol Engl Ed. 2017;57:23-29.
2. Akioka S. A better understanding of juvenile idiopathic arthritis with classification criteria. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39:513-521.
3. Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint Bone Spine. 2014;81:112-117.
4. Petty RE, Laxer RM, Lindsley CB, et al. Pediatric Rheumatology. Philadelphia, PA: Elsevier; 2016:188-201.e6.
5. Scott C, Brice N. Juvenile idiopathic arthritis–an update on its diagnosis and management. S Afr Med J. 2015;105:1077.
6. Giancane G, Consolaro A, Lanni S, et al. Juvenile idiopathic arthritis: diagnosis and treatment. Rheumatol Ther. 2016;3:187-207.
7. Criteria for the classification of juvenile rheumatoid arthritis. Bull Rheum Dis. 1972;23:712-719.
8. Wood PHN: Special meeting on nomenclature and classification of arthritis in children. In: Munthe E, ed. The Care of Rheumatic Children. Basel, Switzerland: EULAR Publishers; 1978:47-50.
9. Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol. 1998;25:1991-1994.
10. Petty RE, Southwood TR, Manners P, et al; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392.
11. Basra HAS, Humphries PD. Juvenile idiopathic arthritis: what is the utility of ultrasound? Br J Radiol. 2017;90:20160920.
12. Weiss J, Ilowite NT. Juvenile idiopathic arthritis. Pediatr Clin North Am. 2005;52:413-442, vi.
13. Fujikawa S, Okuni M. A nationwide surveillance study of rheumatic diseases among Japanese children. Acta Pediatric Jpn. 1997:39:242-244.
14. Ozen S, Karaaslan Y, Ozdemir O, et al. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey: a field study. J Rheumatol. 1998;25:2445-2449.
15. Aoust L, Rossi-Semerano L, Koné-PauL I, et al. Time to diagnosis in juvenile idiopathic arthritis: a French perspective. Orphanet J Rare Dis. 2017;12:43.
16. Moe N, Rygg M. Epidemiology of juvenile chronic arthritis in northern Norway; a ten-year retrospective study. Clin Exp Rheumatol. 1998;16:99-101.
17. Abramowicz S, Kim S, Prahalad S, et al. Juvenile arthritis: current concepts in terminology, etiopathogenesis, diagnosis, and management. Int J Oral Maxillofac Surg. 2016;45:801-812.
18. Prahalad S, Shear ES, Thompson SD, et al. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46:1851-1856.
19. Gonzalez B, Larrañaga C, León O, et al. Parvovirus B19 may have a role in the pathogenesis of juvenile idiopathic arthritis. J Rheumatol. 2007;34:1336-1340.
20. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138-2149.
21. Zhou J, Ding Y, Zhang Y, et al. CD3+CD56+ natural killer T cell activity in children with different forms of juvenile idiopathic arthritis and the influence of etanercept treatment on polyarticular subgroup. Clin Immunol. 2016;176:1-11.
22. Shoop-Worrall SJW, Verstappen SMM, Baildam E, et al. How common is clinically inactive disease in a prospective cohort of patients with juvenile idiopathic arthritis? The importance of definition. Ann Rheum Dis. 2017;0:1-8.
23. Nordal EB, Zak M, Berntson L, et al. Juvenile Arthritis Disease Activity Score (JADAS) based on CRP; validity and predictive ability in a Nordic population-based setting. Pediatr Rheumatol Online J. 2011;9(suppl 1):155.
24. Swart JF, Dijkhuizen EHP, Wulffraat NM, et al. Clinical Juvenile Arthritis Disease Activity Score proves to be a useful tool in treat-to-target therapy in juvenile idiopathic arthritis. Ann Rheum Dis. 2018;77:336-342.
25. Horneff G, Klein A, Ganser G, et al. Protocols on classification, monitoring and therapy in children’s rheumatology (PRO-KIND): results of the working group polyarticular juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2017;15:78.
26. Shoop-Worrall SJW, Verstappen SMM, McDonagh JE, et al. Long‐term outcomes following achievement of clinically inactive disease in juvenile idiopathic arthritis. Arthritis Rheumatol. 2018;70:1519-1529.
27. Ahn SS, Yoo BW, Jung SM, et al. In-hospital mortality in febrile lupus patients based on 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome. Sem Arthritis Rheum. 2017;.47:216-221.
28. Yokota S, Mori M, Imagawa T, et al. Proposal for juvenile idiopathic arthritis guidance on diagnosis and treatment for primary care pediatricians and nonpediatric rheumatologists (2007). Mod Rheumatol. 2007;17:353-363.
29. Barut K, Adrovic A, Şahin S, et al. Juvenile idiopathic arthritis. Balkan Med J. 2017;34:90-101.
30. Colebatch-Bourn AN, Edwards CJ, et al. EULAR-PReS points to consider for the use of imaging in the diagnosis and management of juvenile idiopathic arthritis in clinical practice. Ann Rheum Dis. 2015;74:1946-1957.
31. Blazina Š, Markelj G, AvramoviČ MZ, et al. Management of juvenile idiopathic arthritis: a clinical guide. Pediatr Drugs. 2016;18:397-412.
32. Santos MJ, Conde M, Mourão AF, et al. 2016 update of the Portuguese recommendations for the use of biologic therapies in children and adolescents with juvenile idiopathic arthritis. Acta Rheumatol Port. 2016;41:194-212.
33. Steinbrocker 0, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659-662.
34. Oen K, Malleson PN, Cabral D, et al. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002;29:1989-1999.
PRACTICE RECOMMENDATIONS
› Pair the findings of your clinical exam with the results of imaging and laboratory testing to make the diagnosis of juvenile idiopathic arthritis (JIA), as it is a diagnosis of exclusion. B
› Individualize treatment based on where the patient falls in the JIA disease spectrum to increase the likelihood that medical therapy will be effective. A
› Consider treating diagnosed JIA with an available biologic agent, which can provide a long asymptomatic period. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Is intra-articular platelet-rich plasma injection an effective treatment for knee OA?
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).
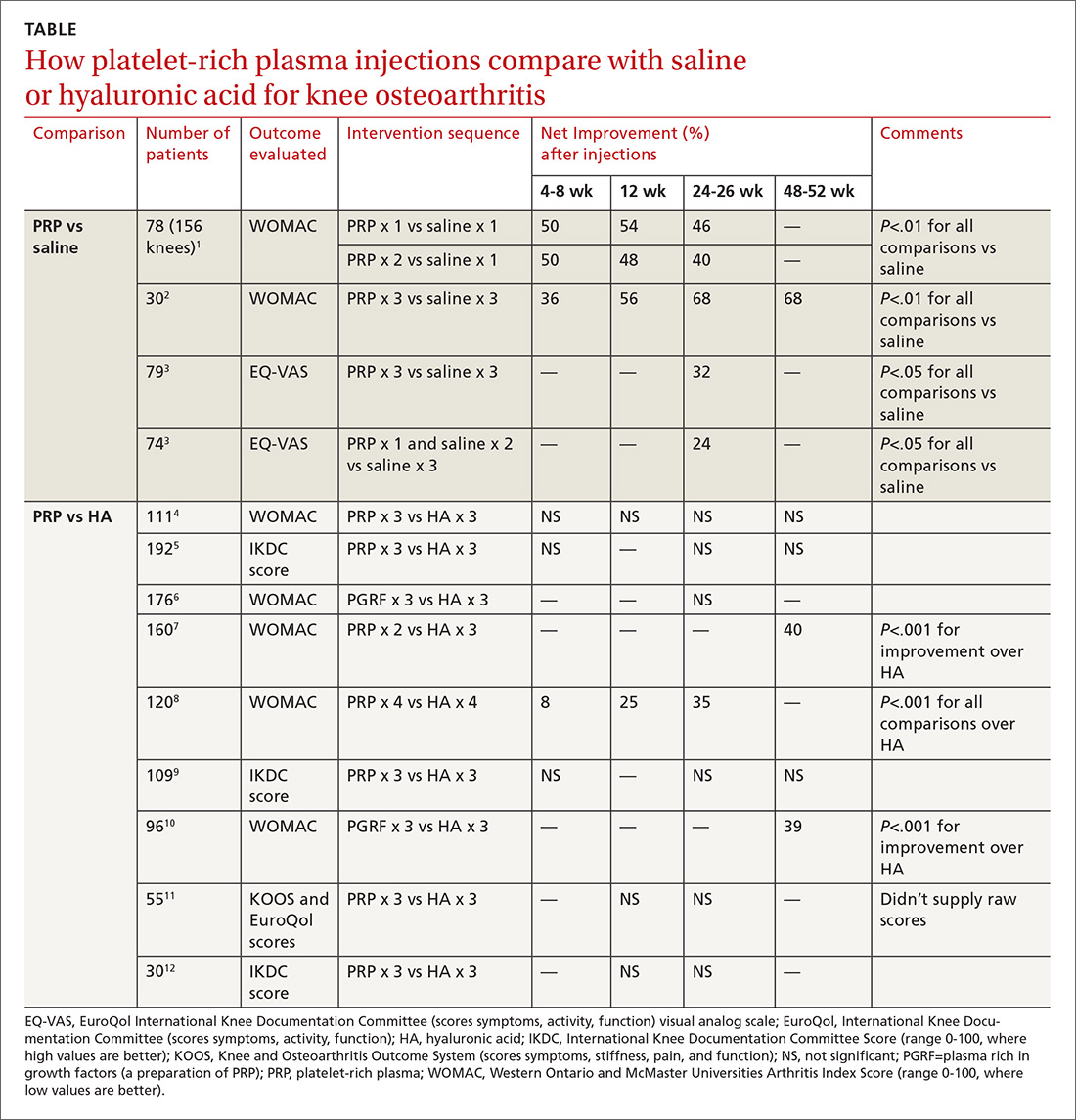
The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).

The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).

The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
EVIDENCE-BASED ANSWER:
Probably not, based on the balance of evidence. While low-quality evidence may suggest potential benefit, the balance of evidence suggests it is no better than placebo.
Compared with saline placebo, platelet-rich plasma (PRP) injections may improve standardized scores for knee osteoarthritis (OA) pain, function, and stiffness by 24% to 70% for periods of 6 to 52 weeks in patients with early to moderate OA (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with methodologic flaws).
Compared with hyaluronic acid (HA), PRP probably improves scores by a similar amount for periods of 8 to 52 weeks (SOR: B, multiple RCTs with conflicting results favoring no difference). However, since HA alone likely doesn’t improve scores more than placebo (SOR: B, RCTs of moderate quality), if both HA and PRP are about the same, then both are not better than placebo.
A systematic approach to chronic abnormal uterine bleeding
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.
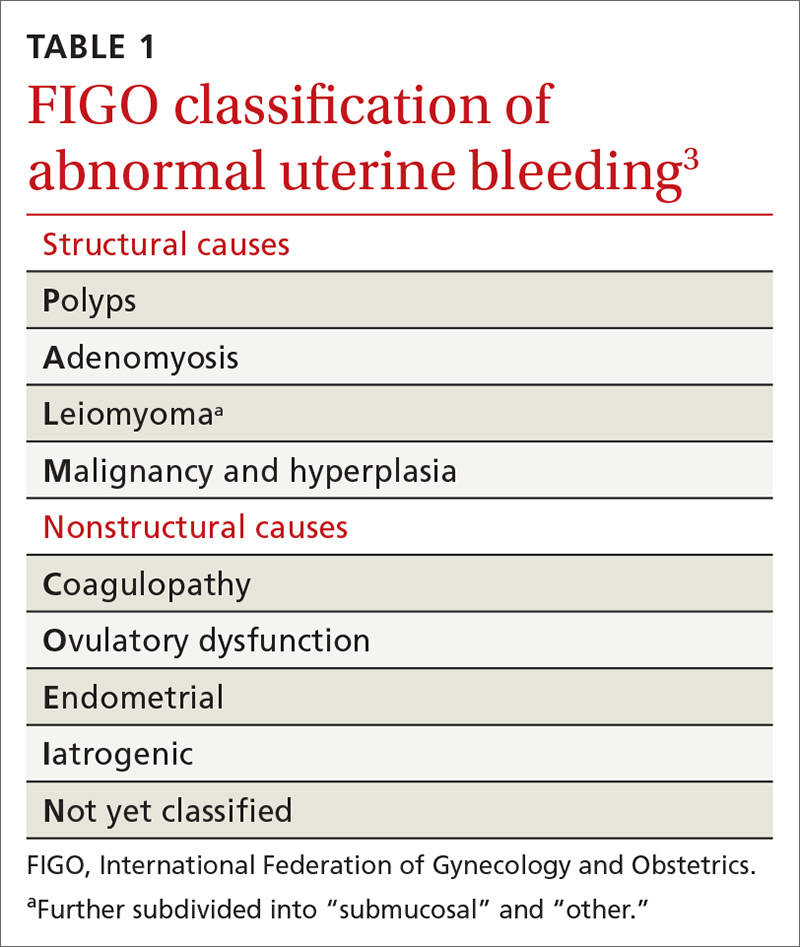
Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).
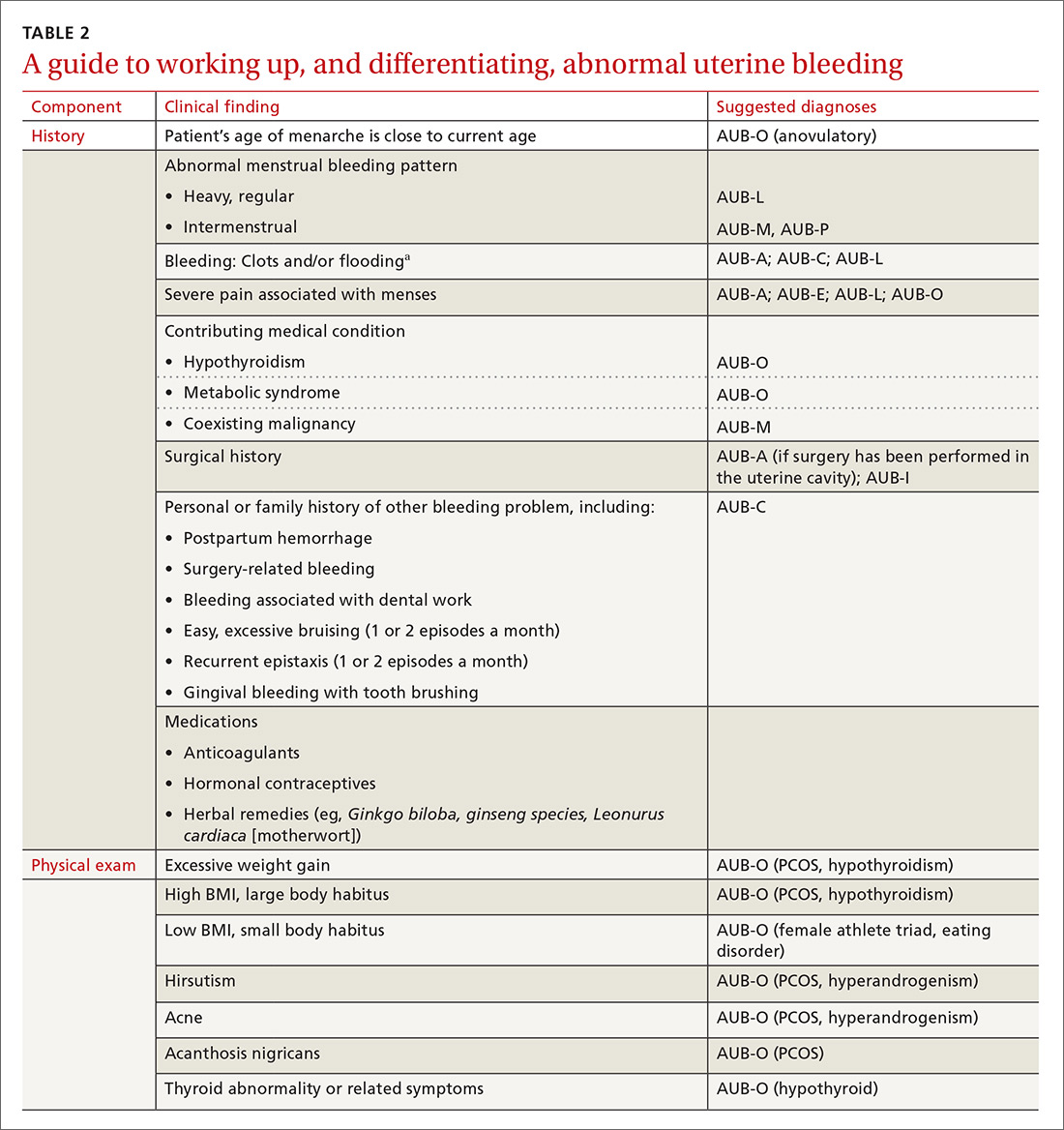
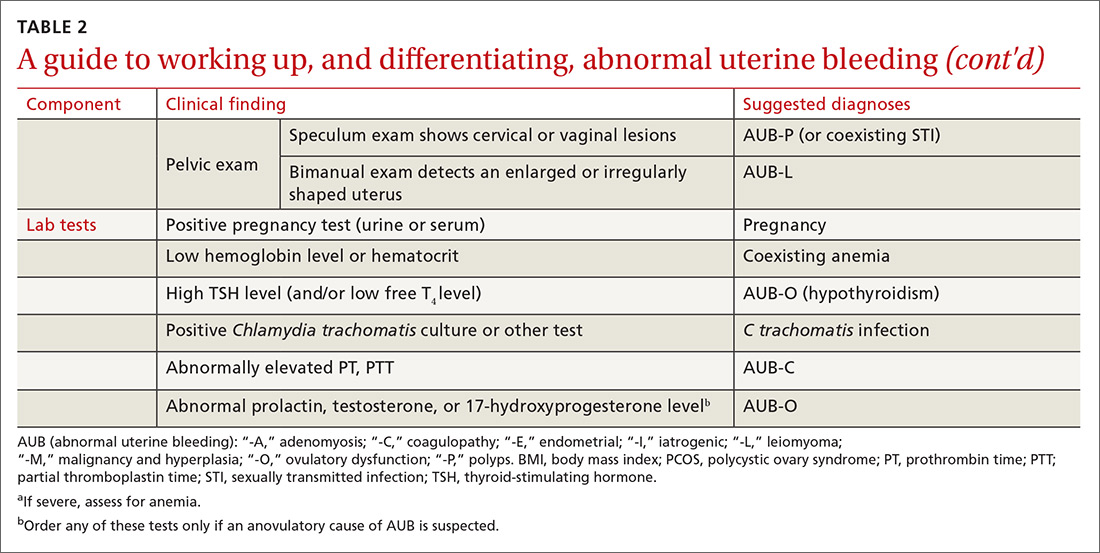
The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1
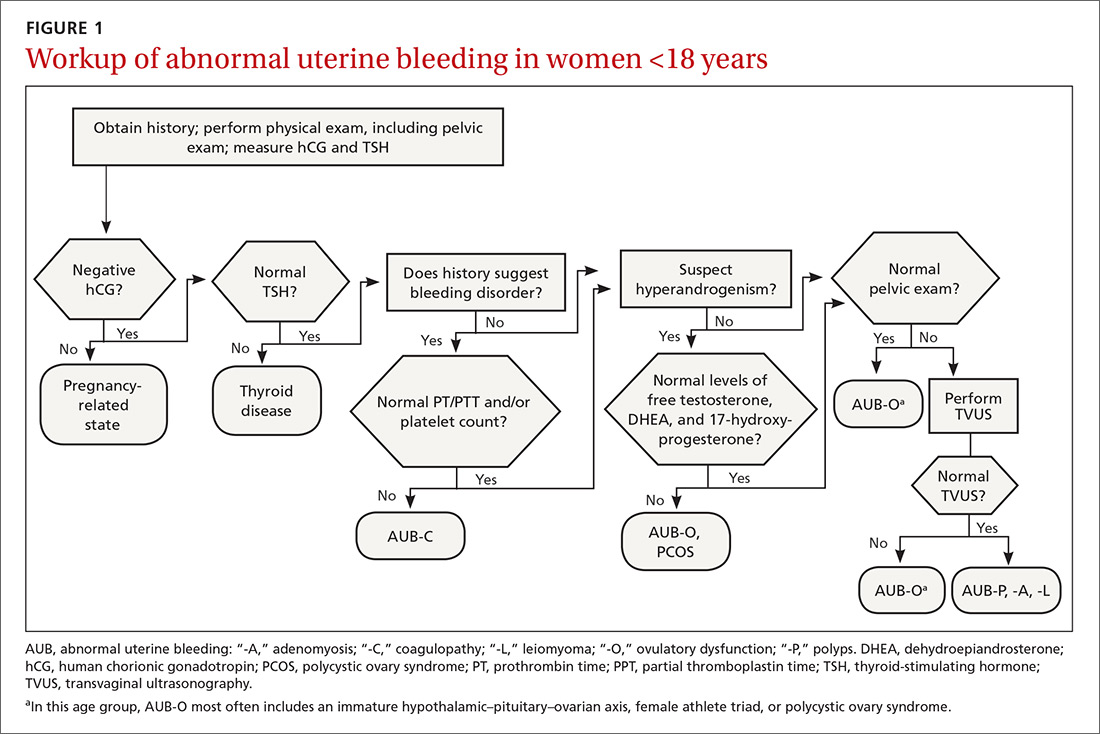
The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).
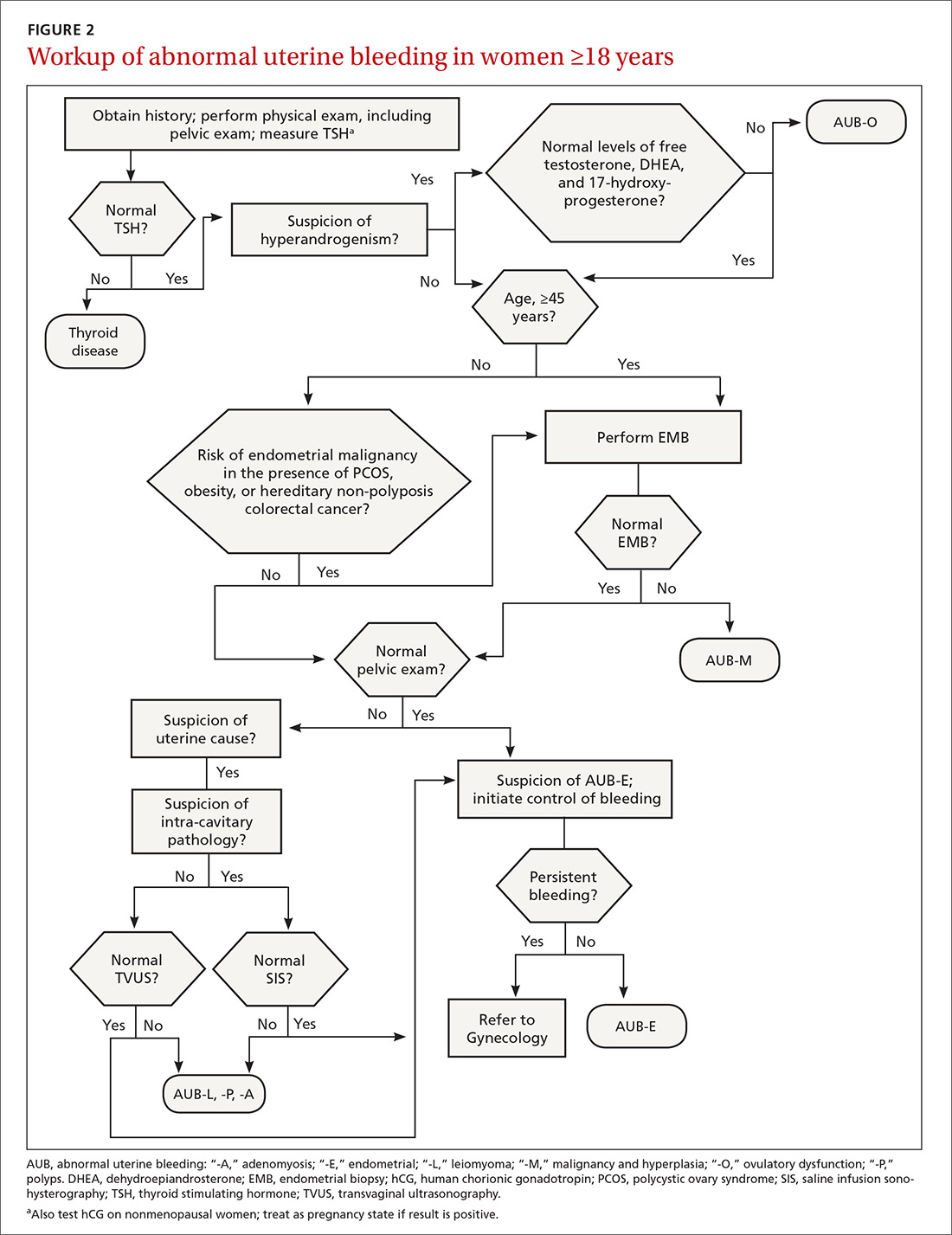
We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).

CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; [email protected].
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.

Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).


The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1

The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).

We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).

CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; [email protected].
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.

Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).


The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1

The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).

We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).

CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; [email protected].
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
PRACTICE RECOMMENDATIONS
› Perform endometrial biopsy on all women who have abnormal uterine bleeding and risk factors for endometrial cancer and on all women ≥45 years, regardless of risk. C
› Initiate a workup for a coagulation disorder in women who are close to the onset of menarche and have a history of heavy menstrual bleeding. C
› Promote lifestyle changes and weight loss as primary treatments for polycystic ovary syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Click for Credit: Endometriosis surgery benefits; diabetes & aging; more
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
MS research: “Our patients can’t wait” for conventional techniques
DALLAS – The time is right to bring big data and high-horsepower computation to the thorniest problems in multiple sclerosis (MS) research, said Jennifer Graves, MD, who cochaired the closing session at the meeting held by the Americas Committee for Research and Treatment in Multiple Sclerosis. The session focused on harnessing machine learning, deep learning, and the newest noninvasive observational techniques to move research and clinical care forward.
“We’ve reached a point in MS research where we’re hitting some stumbling blocks. And a lot of those stumbling blocks are related to how well and how precisely we can measure phenotype in MS. The reason that’s important is that our next frontier is treating progressive MS – and what that requires is finding things that let us know what’s happening at the biological level, so that we can screen drugs faster. We can’t afford to have 3- to 5-year clinical trials. ... Because our patients can’t wait,” said Dr. Graves, an associate professor of neuroscience at the University of California, San Diego.
“We can use all sorts of big data sources, whether it’s the rich imaging data we get on patients when they go into the MRI scanner, whether it’s wearable sensors,” or even newer technology, Dr. Graves said. “We can use technology to give us the sensitivity that we’ve been missing.”
Wearable technology, including accelerometers, can track physical activity that tracks with outcomes in MS, she added. As the tech armament increases, so will data available for analysis and correlation.
However, the key to progress will be to focus on technology that measures change over time. “This is the key: sensitivity to change over time. A lot of things can be associated with disability,” said Dr. Graves, but the key is tracking what changes in an individual patient with disease progression, “so that we can detect treatment effects or side effects.”
DALLAS – The time is right to bring big data and high-horsepower computation to the thorniest problems in multiple sclerosis (MS) research, said Jennifer Graves, MD, who cochaired the closing session at the meeting held by the Americas Committee for Research and Treatment in Multiple Sclerosis. The session focused on harnessing machine learning, deep learning, and the newest noninvasive observational techniques to move research and clinical care forward.
“We’ve reached a point in MS research where we’re hitting some stumbling blocks. And a lot of those stumbling blocks are related to how well and how precisely we can measure phenotype in MS. The reason that’s important is that our next frontier is treating progressive MS – and what that requires is finding things that let us know what’s happening at the biological level, so that we can screen drugs faster. We can’t afford to have 3- to 5-year clinical trials. ... Because our patients can’t wait,” said Dr. Graves, an associate professor of neuroscience at the University of California, San Diego.
“We can use all sorts of big data sources, whether it’s the rich imaging data we get on patients when they go into the MRI scanner, whether it’s wearable sensors,” or even newer technology, Dr. Graves said. “We can use technology to give us the sensitivity that we’ve been missing.”
Wearable technology, including accelerometers, can track physical activity that tracks with outcomes in MS, she added. As the tech armament increases, so will data available for analysis and correlation.
However, the key to progress will be to focus on technology that measures change over time. “This is the key: sensitivity to change over time. A lot of things can be associated with disability,” said Dr. Graves, but the key is tracking what changes in an individual patient with disease progression, “so that we can detect treatment effects or side effects.”
DALLAS – The time is right to bring big data and high-horsepower computation to the thorniest problems in multiple sclerosis (MS) research, said Jennifer Graves, MD, who cochaired the closing session at the meeting held by the Americas Committee for Research and Treatment in Multiple Sclerosis. The session focused on harnessing machine learning, deep learning, and the newest noninvasive observational techniques to move research and clinical care forward.
“We’ve reached a point in MS research where we’re hitting some stumbling blocks. And a lot of those stumbling blocks are related to how well and how precisely we can measure phenotype in MS. The reason that’s important is that our next frontier is treating progressive MS – and what that requires is finding things that let us know what’s happening at the biological level, so that we can screen drugs faster. We can’t afford to have 3- to 5-year clinical trials. ... Because our patients can’t wait,” said Dr. Graves, an associate professor of neuroscience at the University of California, San Diego.
“We can use all sorts of big data sources, whether it’s the rich imaging data we get on patients when they go into the MRI scanner, whether it’s wearable sensors,” or even newer technology, Dr. Graves said. “We can use technology to give us the sensitivity that we’ve been missing.”
Wearable technology, including accelerometers, can track physical activity that tracks with outcomes in MS, she added. As the tech armament increases, so will data available for analysis and correlation.
However, the key to progress will be to focus on technology that measures change over time. “This is the key: sensitivity to change over time. A lot of things can be associated with disability,” said Dr. Graves, but the key is tracking what changes in an individual patient with disease progression, “so that we can detect treatment effects or side effects.”
REPORTING FROM ACTRIMS FORUM 2019
Migraine associated with more severe disability in patients with MS
DALLAS – researchers reported at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Traditional migraine risk factors such as obesity, anxiety, and depression were also overrepresented in our cohort” of patients with multiple sclerosis (MS) and migraine, said Anne M. Damian, MD, of Johns Hopkins University, Baltimore, and her research colleagues.
Migraine is common in patients with MS, but whether migraine plays a role in MS disease course or MS symptom severity is unknown. Dr. Damian and her colleagues conducted an observational study to examine the associations between migraine history, disability, and neurologic function in patients with MS and whether migraine tends to occur with other comorbid conditions in MS.
They analyzed data from 289 patients (79% female; mean age, 49.2 years) patients with MS who completed the Multiple Sclerosis Performance Test (MSPT), an iPad version of the MS Functional Composite. MS outcome measures included disability (such as the Patient Determined Disease Steps) and objective neurologic outcomes (such as walking speed, manual dexterity, and processing speed). Patients also completed a questionnaire about comorbidities, including history of physician-diagnosed migraine, diabetes, hypertension, hypercholesterolemia, heart disease, sleep apnea, depression, and anxiety.
The researchers used generalized linear models adjusted for age, sex, MS subtype, MS duration, years of education, and body mass index to evaluate the association between history of migraine and MS outcomes.
Compared with patients with MS without migraine, migraineurs (n = 65) tended to be younger (mean age, 44.3 years vs. 50.4 years) and were more likely to be overweight or obese (73.9% vs. 51.6%). In addition, patients with MS and migraine were more likely to have a history of depression (46.2% vs. 24.2%), anxiety (30.8% vs. 18.8%), and severe rather than mild disability (odds ratio, 3.08; 95% confidence, 1.04-9.20). Migraine also was associated with significantly slower walking speeds (9.08% slower; 95% CI, 0.82%-18.77%). Migraine was not associated with processing speed or manual dexterity, however.
If an association between migraine history and worse MS disability is confirmed, migraine history may be a factor that neurologists could consider when making MS treatment decisions, Dr. Damian said. The researchers noted that migraine was reported by patients and not detected using a validated questionnaire. Future studies should investigate whether MS lesions on MRI differ in migraineurs and whether migraine predicts future neurologic disability in patients with MS.
Collection of the MSPT outcomes was sponsored by Biogen.
SOURCE: Damian AM et al. ACTRIMS Forum 2019, Abstract 78.
DALLAS – researchers reported at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Traditional migraine risk factors such as obesity, anxiety, and depression were also overrepresented in our cohort” of patients with multiple sclerosis (MS) and migraine, said Anne M. Damian, MD, of Johns Hopkins University, Baltimore, and her research colleagues.
Migraine is common in patients with MS, but whether migraine plays a role in MS disease course or MS symptom severity is unknown. Dr. Damian and her colleagues conducted an observational study to examine the associations between migraine history, disability, and neurologic function in patients with MS and whether migraine tends to occur with other comorbid conditions in MS.
They analyzed data from 289 patients (79% female; mean age, 49.2 years) patients with MS who completed the Multiple Sclerosis Performance Test (MSPT), an iPad version of the MS Functional Composite. MS outcome measures included disability (such as the Patient Determined Disease Steps) and objective neurologic outcomes (such as walking speed, manual dexterity, and processing speed). Patients also completed a questionnaire about comorbidities, including history of physician-diagnosed migraine, diabetes, hypertension, hypercholesterolemia, heart disease, sleep apnea, depression, and anxiety.
The researchers used generalized linear models adjusted for age, sex, MS subtype, MS duration, years of education, and body mass index to evaluate the association between history of migraine and MS outcomes.
Compared with patients with MS without migraine, migraineurs (n = 65) tended to be younger (mean age, 44.3 years vs. 50.4 years) and were more likely to be overweight or obese (73.9% vs. 51.6%). In addition, patients with MS and migraine were more likely to have a history of depression (46.2% vs. 24.2%), anxiety (30.8% vs. 18.8%), and severe rather than mild disability (odds ratio, 3.08; 95% confidence, 1.04-9.20). Migraine also was associated with significantly slower walking speeds (9.08% slower; 95% CI, 0.82%-18.77%). Migraine was not associated with processing speed or manual dexterity, however.
If an association between migraine history and worse MS disability is confirmed, migraine history may be a factor that neurologists could consider when making MS treatment decisions, Dr. Damian said. The researchers noted that migraine was reported by patients and not detected using a validated questionnaire. Future studies should investigate whether MS lesions on MRI differ in migraineurs and whether migraine predicts future neurologic disability in patients with MS.
Collection of the MSPT outcomes was sponsored by Biogen.
SOURCE: Damian AM et al. ACTRIMS Forum 2019, Abstract 78.
DALLAS – researchers reported at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
“Traditional migraine risk factors such as obesity, anxiety, and depression were also overrepresented in our cohort” of patients with multiple sclerosis (MS) and migraine, said Anne M. Damian, MD, of Johns Hopkins University, Baltimore, and her research colleagues.
Migraine is common in patients with MS, but whether migraine plays a role in MS disease course or MS symptom severity is unknown. Dr. Damian and her colleagues conducted an observational study to examine the associations between migraine history, disability, and neurologic function in patients with MS and whether migraine tends to occur with other comorbid conditions in MS.
They analyzed data from 289 patients (79% female; mean age, 49.2 years) patients with MS who completed the Multiple Sclerosis Performance Test (MSPT), an iPad version of the MS Functional Composite. MS outcome measures included disability (such as the Patient Determined Disease Steps) and objective neurologic outcomes (such as walking speed, manual dexterity, and processing speed). Patients also completed a questionnaire about comorbidities, including history of physician-diagnosed migraine, diabetes, hypertension, hypercholesterolemia, heart disease, sleep apnea, depression, and anxiety.
The researchers used generalized linear models adjusted for age, sex, MS subtype, MS duration, years of education, and body mass index to evaluate the association between history of migraine and MS outcomes.
Compared with patients with MS without migraine, migraineurs (n = 65) tended to be younger (mean age, 44.3 years vs. 50.4 years) and were more likely to be overweight or obese (73.9% vs. 51.6%). In addition, patients with MS and migraine were more likely to have a history of depression (46.2% vs. 24.2%), anxiety (30.8% vs. 18.8%), and severe rather than mild disability (odds ratio, 3.08; 95% confidence, 1.04-9.20). Migraine also was associated with significantly slower walking speeds (9.08% slower; 95% CI, 0.82%-18.77%). Migraine was not associated with processing speed or manual dexterity, however.
If an association between migraine history and worse MS disability is confirmed, migraine history may be a factor that neurologists could consider when making MS treatment decisions, Dr. Damian said. The researchers noted that migraine was reported by patients and not detected using a validated questionnaire. Future studies should investigate whether MS lesions on MRI differ in migraineurs and whether migraine predicts future neurologic disability in patients with MS.
Collection of the MSPT outcomes was sponsored by Biogen.
SOURCE: Damian AM et al. ACTRIMS Forum 2019, Abstract 78.
REPORTING FROM ACTRIMS FORUM 2019