User login
20-year-old male college basketball prospect • wrist pain after falling on wrist • normal ROM • pain with active/passive wrist extension • Dx?
THE CASE
A 20-year-old man presented to our family medicine clinic with right wrist pain 4 days after falling on his wrist and hand while playing basketball. He denied any other previous injury or trauma. The pain was unchanged since the injury occurred.
Examination demonstrated mild edema over the palmar and ulnar aspect of the patient’s right wrist with no apparent ecchymosis. He had normal range of motion of his right wrist and hand. However, he experienced pain with active and passive wrist extension and ulnar deviation. There was significant tenderness in the palmar and ulnar aspects of his right wrist just distal to the ulnar styloid process.
THE DIAGNOSIS
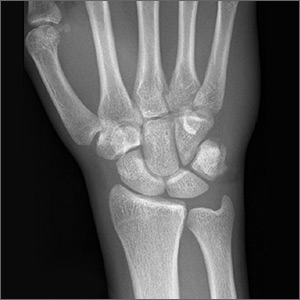
Standard plain x-rays of the right wrist revealed an isolated fracture of the body of the triquetrum (FIGURE 1). Since the patient refused to have a cast placed, his wrist was immobilized with a wrist brace. By Day 16 post injury, the pain and edema had improved significantly. After talking with the patient about the potential risks and benefits of continuing to play basketball—and despite our recommendation that he not play—he decided to continue playing since he was a college basketball prospect.
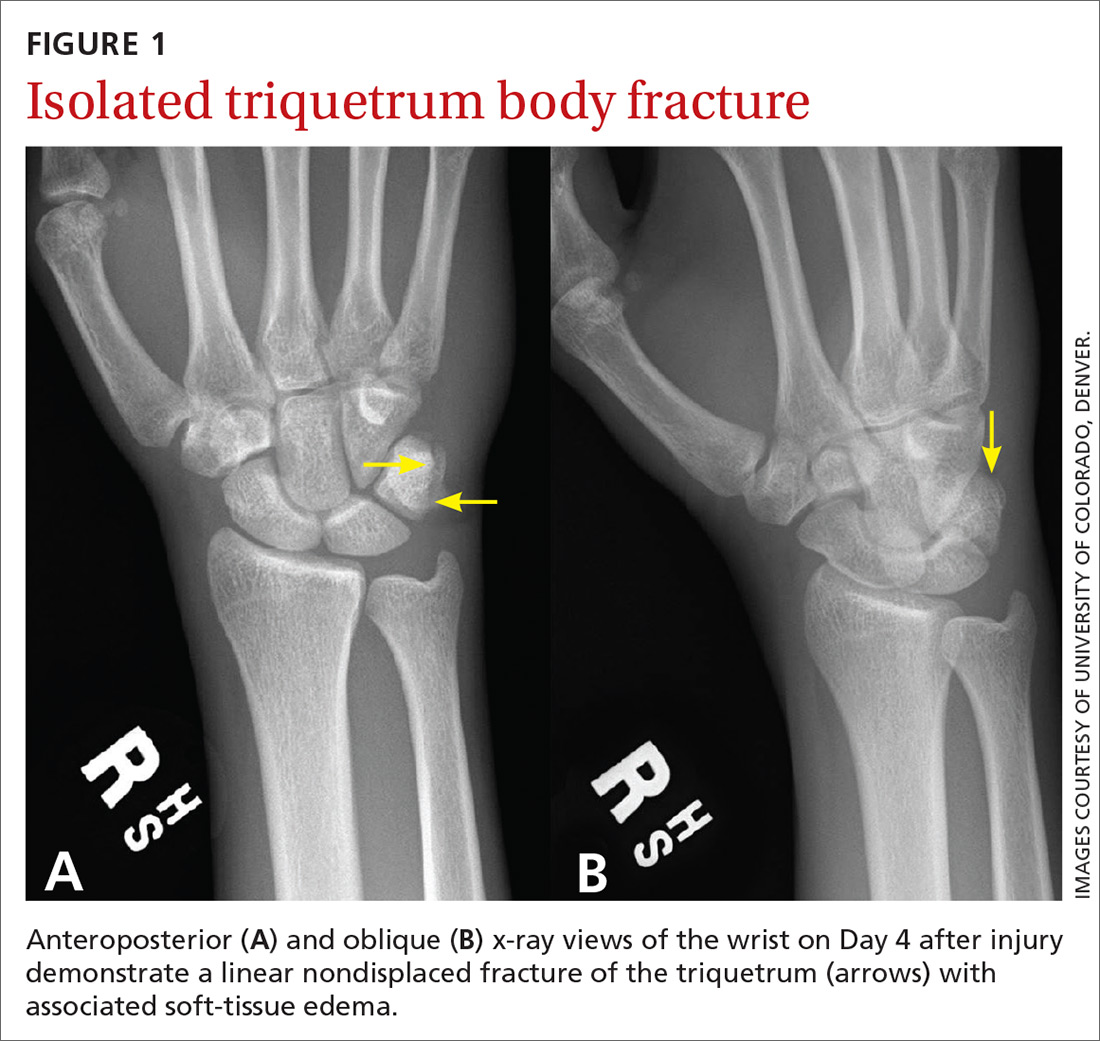
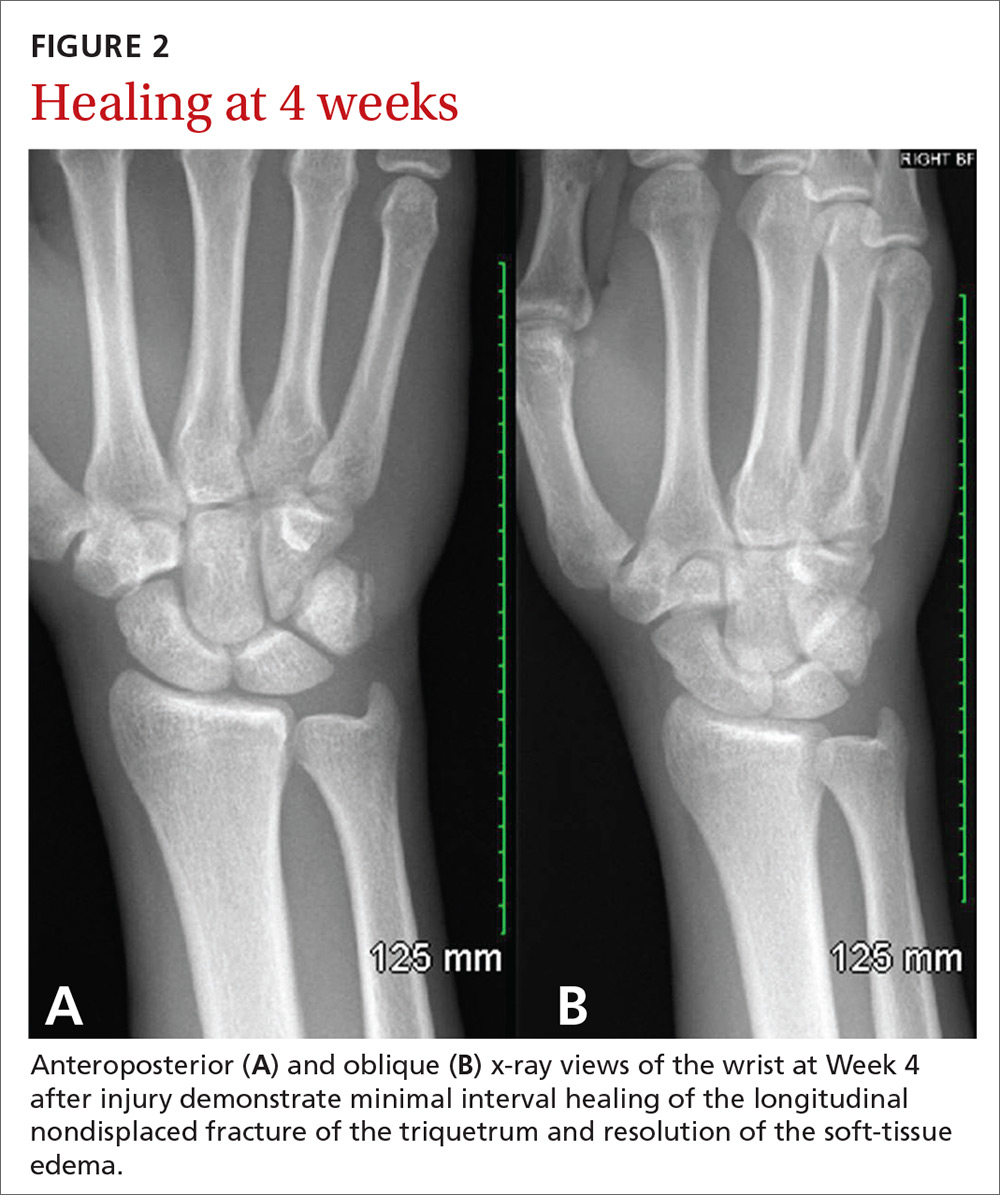
At 4 weeks post injury, x-rays demonstrated mild interval healing (FIGURE 2). At the 8-week visit, the patient had only very mild pain and tenderness, and x-ray images showed improvement (FIGURE 3). Within a few months, his symptoms resolved completely. No further imaging was performed.
DISCUSSION
In general, carpal fractures are uncommon.1 The triquetrum is the second most commonly injured carpal bone, involved in up to 18% of all carpal fractures.2,3 Triquetrum fractures most commonly occur as isolated injuries and are typically classified in 2 general categories: avulsion fractures (dorsal cortex or volar cortex) and fractures of the triquetrum body.4-8 Isolated avulsion fractures of the triquetral dorsal cortex are relatively common, occurring in about 95% of triquetrum injuries.4-9 Isolated fractures of the triquetrum body are less common, occurring in about 4% of triquetrum injuries, and can go unnoticed on conventional x-rays.4-9

Basketball presents a unique risk for hand or wrist fracture due to its high-impact nature, hard playing surfaces, and frequent use of the hands for dribbling, shooting, rebounding, and passing the ball.
In a retrospective study of sports-related fractures conducted at the Royal Infirmary of Edinburgh, basketball had the highest incidence of carpal injuries compared with other sports, including football, rugby, skiing, snowboarding, and ice-skating.4 Similarly, a retrospective study conducted at the University of California, Los Angeles, found that of all Division 1 collegiate athletes at the school, basketball players had the highest incidence of primary fractures, and the most common fracture location was the hand.10
Continue to: An injury that's easy to miss
An injury that’s easy to miss
Because the incidence of hand and wrist injuries is high among basketball players, it is imperative that triquetrum body fractures are not missed or misdiagnosed as more common hand and wrist injuries, such as triquetral dorsal avulsion fractures.
Our patient, who had an isolated triquetrum body fracture, presented with focal tenderness on the palmar and ulnar aspects of his wrist and pain with ulnar deviation. Since triquetral body fractures often have a clinical presentation quite similar to that of triquetral dorsal avulsion fractures, patients presenting with symptoms of wrist tenderness and pain should be treated with a high degree of clinical suspicion.
With our patient, anteroposterior and lateral x-rays were sufficient to demonstrate an isolated triquetrum body fracture; however, triquetral fractures can be missed in up to 20% of x-rays.4 Both magnetic resonance imaging and computerized tomography are useful in diagnosing occult triquetrum fractures and should be used to confirm clinical suspicion when traditional x-rays are inconclusive.11,12
Management varies
Management of isolated triquetrum body fractures varies depending on the fracture pattern and the status of bone consolidation. Triquetral body fractures typically heal well; it’s very rare that there is a nonunion. As our patient’s fracture was nondisplaced and stable, brace immobilization for 4 weeks was sufficient to facilitate healing and restore long-term hand and wrist functionality. This course of treatment is consistent with other cases of nondisplaced triquetrum body fractures reported in the literature.13
Long-term outcomes. The literature is sparse regarding the long-term functional outcome of nonsurgical treatment for nondisplaced triquetrum body fractures. Multiple carpal fractures, displaced triquetrum body fractures, and persistent pain for multiple months after nonsurgical management all indicate the need for referral to orthopedic surgery. In instances of fracture displacement or nonunion, management tends to be surgical, with open reduction and internal fixation (ORIF) used in multiple cases of nonunion for isolated triquetrum body fractures.3,14 Any diagnostic imaging that reveals displacement, malunion, or nonunion of the fracture is an indication for referral to an orthopedic surgeon.
Continue to: Return to play
Return to play. There is no evidence-based return-to-play recommendation for patients with a triquetrum fracture. However, our patient continued to play basketball through the early stages of injury management because he was a collegiate prospect. While medical, social, and economic factors should be considered when discussing treatment options with athletes, injuries should be managed so that there is no long-term loss of function or risk of injury exacerbation. When discussing early return from injury with athletes who have outside pressure to return to play, it’s important to make them aware of the associated long- and short-term risks.15
THE TAKEAWAY
Management of an isolated triquetrum body fracture is typically straightforward; however, if the fracture is displaced, refer the patient to an orthopedic surgeon as ORIF may be required. For this reason, it’s important to be able to promptly identify isolated triquetrum body fractures and to avoid confusing them with triquetrum dorsal avulsion fractures.
Depending on the sport played and the severity of the injury, athletes with conservatively managed nondisplaced triquetral body fractures may be candidates for early return to play. Nonetheless, athletes should understand both the short- and the long-term risks of playing with an injury, and they should never be advised to continue playing with an injury if it jeopardizes their well-being or the long-term functionality of the affected body part.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Suh N, Ek ET, Wolfe SW. Carpal fractures. J Hand Surg Am. 2014;39:785-791.
2. Hey HW, Chong AK, Murphy D. Prevalence of carpal fracture in Singapore. J Hand Surg Am. 2011;36:278-283.
3. Al Rashid M, Rasoli S, Khan WS. Non-union of isolated displaced triquetral body fracture—a case report. Ortop Traumatol Rehabil. 2012;14:71-74.
4. Becce F, Theumann N, Bollmann C, et al. Dorsal fractures of the triquetrum: MRI findings with an emphasis on dorsal carpal ligament injuries. AJR Am J Roentgenol. 2013;200:608-617.
5. Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365-1372.
6. Urch EY, Lee SK. Carpal fractures other than scaphoid. Clin Sports Med. 2015;34:51-67.
7. deWeber K. Triquetrum fractures. UpToDate. 2016. www.uptodate.com/contents/triquetrum-fractures. Accessed September 3, 2019.
8. Höcker K, Menschik A. Chip fractures of the triquetrum. Mechanism, classification and results. J Hand Surg Br. 1994;19:584-588.
9. Jarraya M, Hayashi D, Roemer FW, et al. Radiographically occult and subtle fractures: a pictorial review. Radiol Res Pract. 2013;2013:370169.
10. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446-451.
11. Hindman BW, Kulik WJ, Lee G, et al. Occult fractures of the carpals and metacarpals: demonstration by CT. AJR Am J Roentgenol. 1989;153:529-532.
12. Pierre-Jerome C, Moncayo V, Albastaki U, et al. Multiple occult wrist bone injuries and joint effusions: prevalence and distribution on MRI. Emerg Radiol. 2010;17:179-184.
13. Yildirim C, Akmaz I, Keklikçi K, et al. An unusual combined fracture pattern of the triquetrum. J Hand Surg Eur Vol. 2008;33:385-386.
14. Rasoli S, Ricks M, Packer G. Isolated displaced non-union of a triquetral body fracture: a case report. J Med Case Rep. 2012;6:54.
15. Strickland JW. Considerations for the treatment of the injured athlete. Clin Sports Med. 1998;17:397-400.
THE CASE
A 20-year-old man presented to our family medicine clinic with right wrist pain 4 days after falling on his wrist and hand while playing basketball. He denied any other previous injury or trauma. The pain was unchanged since the injury occurred.
Examination demonstrated mild edema over the palmar and ulnar aspect of the patient’s right wrist with no apparent ecchymosis. He had normal range of motion of his right wrist and hand. However, he experienced pain with active and passive wrist extension and ulnar deviation. There was significant tenderness in the palmar and ulnar aspects of his right wrist just distal to the ulnar styloid process.
THE DIAGNOSIS
Standard plain x-rays of the right wrist revealed an isolated fracture of the body of the triquetrum (FIGURE 1). Since the patient refused to have a cast placed, his wrist was immobilized with a wrist brace. By Day 16 post injury, the pain and edema had improved significantly. After talking with the patient about the potential risks and benefits of continuing to play basketball—and despite our recommendation that he not play—he decided to continue playing since he was a college basketball prospect.

At 4 weeks post injury, x-rays demonstrated mild interval healing (FIGURE 2). At the 8-week visit, the patient had only very mild pain and tenderness, and x-ray images showed improvement (FIGURE 3). Within a few months, his symptoms resolved completely. No further imaging was performed.

DISCUSSION
In general, carpal fractures are uncommon.1 The triquetrum is the second most commonly injured carpal bone, involved in up to 18% of all carpal fractures.2,3 Triquetrum fractures most commonly occur as isolated injuries and are typically classified in 2 general categories: avulsion fractures (dorsal cortex or volar cortex) and fractures of the triquetrum body.4-8 Isolated avulsion fractures of the triquetral dorsal cortex are relatively common, occurring in about 95% of triquetrum injuries.4-9 Isolated fractures of the triquetrum body are less common, occurring in about 4% of triquetrum injuries, and can go unnoticed on conventional x-rays.4-9

Basketball presents a unique risk for hand or wrist fracture due to its high-impact nature, hard playing surfaces, and frequent use of the hands for dribbling, shooting, rebounding, and passing the ball.
In a retrospective study of sports-related fractures conducted at the Royal Infirmary of Edinburgh, basketball had the highest incidence of carpal injuries compared with other sports, including football, rugby, skiing, snowboarding, and ice-skating.4 Similarly, a retrospective study conducted at the University of California, Los Angeles, found that of all Division 1 collegiate athletes at the school, basketball players had the highest incidence of primary fractures, and the most common fracture location was the hand.10
Continue to: An injury that's easy to miss
An injury that’s easy to miss
Because the incidence of hand and wrist injuries is high among basketball players, it is imperative that triquetrum body fractures are not missed or misdiagnosed as more common hand and wrist injuries, such as triquetral dorsal avulsion fractures.
Our patient, who had an isolated triquetrum body fracture, presented with focal tenderness on the palmar and ulnar aspects of his wrist and pain with ulnar deviation. Since triquetral body fractures often have a clinical presentation quite similar to that of triquetral dorsal avulsion fractures, patients presenting with symptoms of wrist tenderness and pain should be treated with a high degree of clinical suspicion.
With our patient, anteroposterior and lateral x-rays were sufficient to demonstrate an isolated triquetrum body fracture; however, triquetral fractures can be missed in up to 20% of x-rays.4 Both magnetic resonance imaging and computerized tomography are useful in diagnosing occult triquetrum fractures and should be used to confirm clinical suspicion when traditional x-rays are inconclusive.11,12
Management varies
Management of isolated triquetrum body fractures varies depending on the fracture pattern and the status of bone consolidation. Triquetral body fractures typically heal well; it’s very rare that there is a nonunion. As our patient’s fracture was nondisplaced and stable, brace immobilization for 4 weeks was sufficient to facilitate healing and restore long-term hand and wrist functionality. This course of treatment is consistent with other cases of nondisplaced triquetrum body fractures reported in the literature.13
Long-term outcomes. The literature is sparse regarding the long-term functional outcome of nonsurgical treatment for nondisplaced triquetrum body fractures. Multiple carpal fractures, displaced triquetrum body fractures, and persistent pain for multiple months after nonsurgical management all indicate the need for referral to orthopedic surgery. In instances of fracture displacement or nonunion, management tends to be surgical, with open reduction and internal fixation (ORIF) used in multiple cases of nonunion for isolated triquetrum body fractures.3,14 Any diagnostic imaging that reveals displacement, malunion, or nonunion of the fracture is an indication for referral to an orthopedic surgeon.
Continue to: Return to play
Return to play. There is no evidence-based return-to-play recommendation for patients with a triquetrum fracture. However, our patient continued to play basketball through the early stages of injury management because he was a collegiate prospect. While medical, social, and economic factors should be considered when discussing treatment options with athletes, injuries should be managed so that there is no long-term loss of function or risk of injury exacerbation. When discussing early return from injury with athletes who have outside pressure to return to play, it’s important to make them aware of the associated long- and short-term risks.15
THE TAKEAWAY
Management of an isolated triquetrum body fracture is typically straightforward; however, if the fracture is displaced, refer the patient to an orthopedic surgeon as ORIF may be required. For this reason, it’s important to be able to promptly identify isolated triquetrum body fractures and to avoid confusing them with triquetrum dorsal avulsion fractures.
Depending on the sport played and the severity of the injury, athletes with conservatively managed nondisplaced triquetral body fractures may be candidates for early return to play. Nonetheless, athletes should understand both the short- and the long-term risks of playing with an injury, and they should never be advised to continue playing with an injury if it jeopardizes their well-being or the long-term functionality of the affected body part.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
THE CASE
A 20-year-old man presented to our family medicine clinic with right wrist pain 4 days after falling on his wrist and hand while playing basketball. He denied any other previous injury or trauma. The pain was unchanged since the injury occurred.
Examination demonstrated mild edema over the palmar and ulnar aspect of the patient’s right wrist with no apparent ecchymosis. He had normal range of motion of his right wrist and hand. However, he experienced pain with active and passive wrist extension and ulnar deviation. There was significant tenderness in the palmar and ulnar aspects of his right wrist just distal to the ulnar styloid process.
THE DIAGNOSIS
Standard plain x-rays of the right wrist revealed an isolated fracture of the body of the triquetrum (FIGURE 1). Since the patient refused to have a cast placed, his wrist was immobilized with a wrist brace. By Day 16 post injury, the pain and edema had improved significantly. After talking with the patient about the potential risks and benefits of continuing to play basketball—and despite our recommendation that he not play—he decided to continue playing since he was a college basketball prospect.

At 4 weeks post injury, x-rays demonstrated mild interval healing (FIGURE 2). At the 8-week visit, the patient had only very mild pain and tenderness, and x-ray images showed improvement (FIGURE 3). Within a few months, his symptoms resolved completely. No further imaging was performed.

DISCUSSION
In general, carpal fractures are uncommon.1 The triquetrum is the second most commonly injured carpal bone, involved in up to 18% of all carpal fractures.2,3 Triquetrum fractures most commonly occur as isolated injuries and are typically classified in 2 general categories: avulsion fractures (dorsal cortex or volar cortex) and fractures of the triquetrum body.4-8 Isolated avulsion fractures of the triquetral dorsal cortex are relatively common, occurring in about 95% of triquetrum injuries.4-9 Isolated fractures of the triquetrum body are less common, occurring in about 4% of triquetrum injuries, and can go unnoticed on conventional x-rays.4-9

Basketball presents a unique risk for hand or wrist fracture due to its high-impact nature, hard playing surfaces, and frequent use of the hands for dribbling, shooting, rebounding, and passing the ball.
In a retrospective study of sports-related fractures conducted at the Royal Infirmary of Edinburgh, basketball had the highest incidence of carpal injuries compared with other sports, including football, rugby, skiing, snowboarding, and ice-skating.4 Similarly, a retrospective study conducted at the University of California, Los Angeles, found that of all Division 1 collegiate athletes at the school, basketball players had the highest incidence of primary fractures, and the most common fracture location was the hand.10
Continue to: An injury that's easy to miss
An injury that’s easy to miss
Because the incidence of hand and wrist injuries is high among basketball players, it is imperative that triquetrum body fractures are not missed or misdiagnosed as more common hand and wrist injuries, such as triquetral dorsal avulsion fractures.
Our patient, who had an isolated triquetrum body fracture, presented with focal tenderness on the palmar and ulnar aspects of his wrist and pain with ulnar deviation. Since triquetral body fractures often have a clinical presentation quite similar to that of triquetral dorsal avulsion fractures, patients presenting with symptoms of wrist tenderness and pain should be treated with a high degree of clinical suspicion.
With our patient, anteroposterior and lateral x-rays were sufficient to demonstrate an isolated triquetrum body fracture; however, triquetral fractures can be missed in up to 20% of x-rays.4 Both magnetic resonance imaging and computerized tomography are useful in diagnosing occult triquetrum fractures and should be used to confirm clinical suspicion when traditional x-rays are inconclusive.11,12
Management varies
Management of isolated triquetrum body fractures varies depending on the fracture pattern and the status of bone consolidation. Triquetral body fractures typically heal well; it’s very rare that there is a nonunion. As our patient’s fracture was nondisplaced and stable, brace immobilization for 4 weeks was sufficient to facilitate healing and restore long-term hand and wrist functionality. This course of treatment is consistent with other cases of nondisplaced triquetrum body fractures reported in the literature.13
Long-term outcomes. The literature is sparse regarding the long-term functional outcome of nonsurgical treatment for nondisplaced triquetrum body fractures. Multiple carpal fractures, displaced triquetrum body fractures, and persistent pain for multiple months after nonsurgical management all indicate the need for referral to orthopedic surgery. In instances of fracture displacement or nonunion, management tends to be surgical, with open reduction and internal fixation (ORIF) used in multiple cases of nonunion for isolated triquetrum body fractures.3,14 Any diagnostic imaging that reveals displacement, malunion, or nonunion of the fracture is an indication for referral to an orthopedic surgeon.
Continue to: Return to play
Return to play. There is no evidence-based return-to-play recommendation for patients with a triquetrum fracture. However, our patient continued to play basketball through the early stages of injury management because he was a collegiate prospect. While medical, social, and economic factors should be considered when discussing treatment options with athletes, injuries should be managed so that there is no long-term loss of function or risk of injury exacerbation. When discussing early return from injury with athletes who have outside pressure to return to play, it’s important to make them aware of the associated long- and short-term risks.15
THE TAKEAWAY
Management of an isolated triquetrum body fracture is typically straightforward; however, if the fracture is displaced, refer the patient to an orthopedic surgeon as ORIF may be required. For this reason, it’s important to be able to promptly identify isolated triquetrum body fractures and to avoid confusing them with triquetrum dorsal avulsion fractures.
Depending on the sport played and the severity of the injury, athletes with conservatively managed nondisplaced triquetral body fractures may be candidates for early return to play. Nonetheless, athletes should understand both the short- and the long-term risks of playing with an injury, and they should never be advised to continue playing with an injury if it jeopardizes their well-being or the long-term functionality of the affected body part.
CORRESPONDENCE
Morteza Khodaee, MD, MPH, University of Colorado School of Medicine, AFW Clinic, 3055 Roslyn Street, Denver, CO 80238; [email protected]
1. Suh N, Ek ET, Wolfe SW. Carpal fractures. J Hand Surg Am. 2014;39:785-791.
2. Hey HW, Chong AK, Murphy D. Prevalence of carpal fracture in Singapore. J Hand Surg Am. 2011;36:278-283.
3. Al Rashid M, Rasoli S, Khan WS. Non-union of isolated displaced triquetral body fracture—a case report. Ortop Traumatol Rehabil. 2012;14:71-74.
4. Becce F, Theumann N, Bollmann C, et al. Dorsal fractures of the triquetrum: MRI findings with an emphasis on dorsal carpal ligament injuries. AJR Am J Roentgenol. 2013;200:608-617.
5. Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365-1372.
6. Urch EY, Lee SK. Carpal fractures other than scaphoid. Clin Sports Med. 2015;34:51-67.
7. deWeber K. Triquetrum fractures. UpToDate. 2016. www.uptodate.com/contents/triquetrum-fractures. Accessed September 3, 2019.
8. Höcker K, Menschik A. Chip fractures of the triquetrum. Mechanism, classification and results. J Hand Surg Br. 1994;19:584-588.
9. Jarraya M, Hayashi D, Roemer FW, et al. Radiographically occult and subtle fractures: a pictorial review. Radiol Res Pract. 2013;2013:370169.
10. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446-451.
11. Hindman BW, Kulik WJ, Lee G, et al. Occult fractures of the carpals and metacarpals: demonstration by CT. AJR Am J Roentgenol. 1989;153:529-532.
12. Pierre-Jerome C, Moncayo V, Albastaki U, et al. Multiple occult wrist bone injuries and joint effusions: prevalence and distribution on MRI. Emerg Radiol. 2010;17:179-184.
13. Yildirim C, Akmaz I, Keklikçi K, et al. An unusual combined fracture pattern of the triquetrum. J Hand Surg Eur Vol. 2008;33:385-386.
14. Rasoli S, Ricks M, Packer G. Isolated displaced non-union of a triquetral body fracture: a case report. J Med Case Rep. 2012;6:54.
15. Strickland JW. Considerations for the treatment of the injured athlete. Clin Sports Med. 1998;17:397-400.
1. Suh N, Ek ET, Wolfe SW. Carpal fractures. J Hand Surg Am. 2014;39:785-791.
2. Hey HW, Chong AK, Murphy D. Prevalence of carpal fracture in Singapore. J Hand Surg Am. 2011;36:278-283.
3. Al Rashid M, Rasoli S, Khan WS. Non-union of isolated displaced triquetral body fracture—a case report. Ortop Traumatol Rehabil. 2012;14:71-74.
4. Becce F, Theumann N, Bollmann C, et al. Dorsal fractures of the triquetrum: MRI findings with an emphasis on dorsal carpal ligament injuries. AJR Am J Roentgenol. 2013;200:608-617.
5. Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365-1372.
6. Urch EY, Lee SK. Carpal fractures other than scaphoid. Clin Sports Med. 2015;34:51-67.
7. deWeber K. Triquetrum fractures. UpToDate. 2016. www.uptodate.com/contents/triquetrum-fractures. Accessed September 3, 2019.
8. Höcker K, Menschik A. Chip fractures of the triquetrum. Mechanism, classification and results. J Hand Surg Br. 1994;19:584-588.
9. Jarraya M, Hayashi D, Roemer FW, et al. Radiographically occult and subtle fractures: a pictorial review. Radiol Res Pract. 2013;2013:370169.
10. Hame SL, LaFemina JM, McAllister DR, et al. Fractures in the collegiate athlete. Am J Sports Med. 2004;32:446-451.
11. Hindman BW, Kulik WJ, Lee G, et al. Occult fractures of the carpals and metacarpals: demonstration by CT. AJR Am J Roentgenol. 1989;153:529-532.
12. Pierre-Jerome C, Moncayo V, Albastaki U, et al. Multiple occult wrist bone injuries and joint effusions: prevalence and distribution on MRI. Emerg Radiol. 2010;17:179-184.
13. Yildirim C, Akmaz I, Keklikçi K, et al. An unusual combined fracture pattern of the triquetrum. J Hand Surg Eur Vol. 2008;33:385-386.
14. Rasoli S, Ricks M, Packer G. Isolated displaced non-union of a triquetral body fracture: a case report. J Med Case Rep. 2012;6:54.
15. Strickland JW. Considerations for the treatment of the injured athlete. Clin Sports Med. 1998;17:397-400.
Novel research aims to improve ED care in sickle cell disease
Several initiatives are in the works to improve the management of patients with sickle cell disease in the ED, experts said at a recent webinar held by the National Heart, Lung, and Blood Institute.
In 2014, the NHLBI released evidence-based guidelines for the management of patients with sickle cell disease. The expert panel provided recommendations on the treatment of acute complications of sickle cell disease, many of which are common reasons for ED visits.
Optimizing the treatment of acute complications, namely vasoocclusive crisis, is essential to ensure improved long-term outcomes, explained Paula Tanabe, PhD, of Duke University, Durham, N.C.
Pain management
While the majority of pain-related ED visits in sickle cell are the result of vasoocclusive crisis, other causes, such as acute chest syndrome, abdominal catastrophes, and splenic sequestration, are also important.
The hallmark of pain management in this population is rapid and aggressive treatment with intravenous opioids. The use of individualized doses is also important, but if not available, an sickle cell disease–specific pain protocol can be used, she explained.
Recent evidence has confirmed the benefit of using an individualized (patient-specific) dosing protocol. Dr. Tanabe reported the results of a randomized pilot study that compared two pain protocols for patients undergoing a vasoocclusive episode in the ED.
“The reason we pursued this project is to generate additional evidence beyond the expert panel,” she said.
The primary outcome of the study was the difference in pain scores from arrival to discharge between patients receiving an individualized or weight-based dosing protocol. Secondary outcomes included safety, pain experience, and side effects, among others.
The researchers found that patients who received an individualized protocol had significantly lower pain scores, compared with a standard weight-based protocol (between-protocol pain score difference, 15.6 plus or minus 5.0; P = .002).
Additionally, patients in the individualized dosing arm were admitted less often than those in the weight-based arm (P = .03), Dr. Tanabe reported.
The findings from the previous study formed the basis for an ongoing study that is further examining the impact of patient-specific dosing in patients who present with a vasoocclusive episode. The COMPARE VOE study is currently enrolling patients and is being funded by NHLBI.
The NHLBI also provides funding to eight Sickle Cell Disease Implementation Consortium sites throughout the United States. The objective of this grant funding is to help implement NHLBI recommendations in the emergency setting.
Quality improvement
“One area [that] we want to improve is how quickly we administer [analgesic therapy] to patients when they are experiencing a vasoocclusive episode,” said Caroline Freiermuth, MD, of the University of Cincinnati.
Some common barriers to delivering rapid analgesia in this setting include difficulties in obtaining intravenous access, high patient volumes, lack of education, and provider biases, she explained.
With respect to high patient volumes, one strategy that may help overcome this barrier is to triage patients as Emergency Severity Index level 2, allowing for accelerated room placement.
Sickle cell patients undergoing vasoocclusive crisis meet the criteria for level 2 based on morbidity, degree of pain, and the level of resources often required.
Another important strategy is improving education related to sickle cell disease, particularly the high morbidity and mortality seen in these patients, Dr. Freiermuth said.
“The median lifespan for patients with HbSS disease is in the 40s, basically half of the lifespan of a typical American,” she said.
At present, acute chest syndrome is the principal cause of death in patients with sickle cell disease, and most frequently occurs during a vasoocclusive episode. As a result, screening for this complication is essential to reduce mortality in the emergency setting.
Dr. Freiermuth explained that one of the best ways to prevent acute chest syndrome is to encourage the use of incentive spirometry in patients undergoing a vasoocclusive episode.
In order to increase the likelihood of obtaining intravenous access, the use of ultrasound may help guide placement. Educating nurses on the proper use of ultrasound-guided placement of intravenous catheters is one practical approach, she said.
Alternatively, opioid analgesia can be administered subcutaneously. Benefits of subcutaneous delivery include comparable pharmacokinetics, less pain, and a reduced likelihood of sterile abscesses that are often seen with intramuscular administration.
Dr. Freiermuth outlined the quality-improvement initiative being tested at her institution, which involves the administration of parenteral opioid therapy during triage for sickle cell patients undergoing a suspected vasoocclusive crisis. The initiative was developed with input from both the emergency and hematology departments at the site.
Early results have shown no significant changes using this approach, but the data is still preliminary. Initial feedback has revealed that time to room placement has been the greatest barrier, she reported.
Dr. Tanabe reported grant/research support from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Freiermuth reported research support from Pfizer.
Several initiatives are in the works to improve the management of patients with sickle cell disease in the ED, experts said at a recent webinar held by the National Heart, Lung, and Blood Institute.
In 2014, the NHLBI released evidence-based guidelines for the management of patients with sickle cell disease. The expert panel provided recommendations on the treatment of acute complications of sickle cell disease, many of which are common reasons for ED visits.
Optimizing the treatment of acute complications, namely vasoocclusive crisis, is essential to ensure improved long-term outcomes, explained Paula Tanabe, PhD, of Duke University, Durham, N.C.
Pain management
While the majority of pain-related ED visits in sickle cell are the result of vasoocclusive crisis, other causes, such as acute chest syndrome, abdominal catastrophes, and splenic sequestration, are also important.
The hallmark of pain management in this population is rapid and aggressive treatment with intravenous opioids. The use of individualized doses is also important, but if not available, an sickle cell disease–specific pain protocol can be used, she explained.
Recent evidence has confirmed the benefit of using an individualized (patient-specific) dosing protocol. Dr. Tanabe reported the results of a randomized pilot study that compared two pain protocols for patients undergoing a vasoocclusive episode in the ED.
“The reason we pursued this project is to generate additional evidence beyond the expert panel,” she said.
The primary outcome of the study was the difference in pain scores from arrival to discharge between patients receiving an individualized or weight-based dosing protocol. Secondary outcomes included safety, pain experience, and side effects, among others.
The researchers found that patients who received an individualized protocol had significantly lower pain scores, compared with a standard weight-based protocol (between-protocol pain score difference, 15.6 plus or minus 5.0; P = .002).
Additionally, patients in the individualized dosing arm were admitted less often than those in the weight-based arm (P = .03), Dr. Tanabe reported.
The findings from the previous study formed the basis for an ongoing study that is further examining the impact of patient-specific dosing in patients who present with a vasoocclusive episode. The COMPARE VOE study is currently enrolling patients and is being funded by NHLBI.
The NHLBI also provides funding to eight Sickle Cell Disease Implementation Consortium sites throughout the United States. The objective of this grant funding is to help implement NHLBI recommendations in the emergency setting.
Quality improvement
“One area [that] we want to improve is how quickly we administer [analgesic therapy] to patients when they are experiencing a vasoocclusive episode,” said Caroline Freiermuth, MD, of the University of Cincinnati.
Some common barriers to delivering rapid analgesia in this setting include difficulties in obtaining intravenous access, high patient volumes, lack of education, and provider biases, she explained.
With respect to high patient volumes, one strategy that may help overcome this barrier is to triage patients as Emergency Severity Index level 2, allowing for accelerated room placement.
Sickle cell patients undergoing vasoocclusive crisis meet the criteria for level 2 based on morbidity, degree of pain, and the level of resources often required.
Another important strategy is improving education related to sickle cell disease, particularly the high morbidity and mortality seen in these patients, Dr. Freiermuth said.
“The median lifespan for patients with HbSS disease is in the 40s, basically half of the lifespan of a typical American,” she said.
At present, acute chest syndrome is the principal cause of death in patients with sickle cell disease, and most frequently occurs during a vasoocclusive episode. As a result, screening for this complication is essential to reduce mortality in the emergency setting.
Dr. Freiermuth explained that one of the best ways to prevent acute chest syndrome is to encourage the use of incentive spirometry in patients undergoing a vasoocclusive episode.
In order to increase the likelihood of obtaining intravenous access, the use of ultrasound may help guide placement. Educating nurses on the proper use of ultrasound-guided placement of intravenous catheters is one practical approach, she said.
Alternatively, opioid analgesia can be administered subcutaneously. Benefits of subcutaneous delivery include comparable pharmacokinetics, less pain, and a reduced likelihood of sterile abscesses that are often seen with intramuscular administration.
Dr. Freiermuth outlined the quality-improvement initiative being tested at her institution, which involves the administration of parenteral opioid therapy during triage for sickle cell patients undergoing a suspected vasoocclusive crisis. The initiative was developed with input from both the emergency and hematology departments at the site.
Early results have shown no significant changes using this approach, but the data is still preliminary. Initial feedback has revealed that time to room placement has been the greatest barrier, she reported.
Dr. Tanabe reported grant/research support from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Freiermuth reported research support from Pfizer.
Several initiatives are in the works to improve the management of patients with sickle cell disease in the ED, experts said at a recent webinar held by the National Heart, Lung, and Blood Institute.
In 2014, the NHLBI released evidence-based guidelines for the management of patients with sickle cell disease. The expert panel provided recommendations on the treatment of acute complications of sickle cell disease, many of which are common reasons for ED visits.
Optimizing the treatment of acute complications, namely vasoocclusive crisis, is essential to ensure improved long-term outcomes, explained Paula Tanabe, PhD, of Duke University, Durham, N.C.
Pain management
While the majority of pain-related ED visits in sickle cell are the result of vasoocclusive crisis, other causes, such as acute chest syndrome, abdominal catastrophes, and splenic sequestration, are also important.
The hallmark of pain management in this population is rapid and aggressive treatment with intravenous opioids. The use of individualized doses is also important, but if not available, an sickle cell disease–specific pain protocol can be used, she explained.
Recent evidence has confirmed the benefit of using an individualized (patient-specific) dosing protocol. Dr. Tanabe reported the results of a randomized pilot study that compared two pain protocols for patients undergoing a vasoocclusive episode in the ED.
“The reason we pursued this project is to generate additional evidence beyond the expert panel,” she said.
The primary outcome of the study was the difference in pain scores from arrival to discharge between patients receiving an individualized or weight-based dosing protocol. Secondary outcomes included safety, pain experience, and side effects, among others.
The researchers found that patients who received an individualized protocol had significantly lower pain scores, compared with a standard weight-based protocol (between-protocol pain score difference, 15.6 plus or minus 5.0; P = .002).
Additionally, patients in the individualized dosing arm were admitted less often than those in the weight-based arm (P = .03), Dr. Tanabe reported.
The findings from the previous study formed the basis for an ongoing study that is further examining the impact of patient-specific dosing in patients who present with a vasoocclusive episode. The COMPARE VOE study is currently enrolling patients and is being funded by NHLBI.
The NHLBI also provides funding to eight Sickle Cell Disease Implementation Consortium sites throughout the United States. The objective of this grant funding is to help implement NHLBI recommendations in the emergency setting.
Quality improvement
“One area [that] we want to improve is how quickly we administer [analgesic therapy] to patients when they are experiencing a vasoocclusive episode,” said Caroline Freiermuth, MD, of the University of Cincinnati.
Some common barriers to delivering rapid analgesia in this setting include difficulties in obtaining intravenous access, high patient volumes, lack of education, and provider biases, she explained.
With respect to high patient volumes, one strategy that may help overcome this barrier is to triage patients as Emergency Severity Index level 2, allowing for accelerated room placement.
Sickle cell patients undergoing vasoocclusive crisis meet the criteria for level 2 based on morbidity, degree of pain, and the level of resources often required.
Another important strategy is improving education related to sickle cell disease, particularly the high morbidity and mortality seen in these patients, Dr. Freiermuth said.
“The median lifespan for patients with HbSS disease is in the 40s, basically half of the lifespan of a typical American,” she said.
At present, acute chest syndrome is the principal cause of death in patients with sickle cell disease, and most frequently occurs during a vasoocclusive episode. As a result, screening for this complication is essential to reduce mortality in the emergency setting.
Dr. Freiermuth explained that one of the best ways to prevent acute chest syndrome is to encourage the use of incentive spirometry in patients undergoing a vasoocclusive episode.
In order to increase the likelihood of obtaining intravenous access, the use of ultrasound may help guide placement. Educating nurses on the proper use of ultrasound-guided placement of intravenous catheters is one practical approach, she said.
Alternatively, opioid analgesia can be administered subcutaneously. Benefits of subcutaneous delivery include comparable pharmacokinetics, less pain, and a reduced likelihood of sterile abscesses that are often seen with intramuscular administration.
Dr. Freiermuth outlined the quality-improvement initiative being tested at her institution, which involves the administration of parenteral opioid therapy during triage for sickle cell patients undergoing a suspected vasoocclusive crisis. The initiative was developed with input from both the emergency and hematology departments at the site.
Early results have shown no significant changes using this approach, but the data is still preliminary. Initial feedback has revealed that time to room placement has been the greatest barrier, she reported.
Dr. Tanabe reported grant/research support from the National Institutes of Health and the Agency for Healthcare Research and Quality. Dr. Freiermuth reported research support from Pfizer.
REPORTING FROM AN NIH WEBINAR
A few pearls can help prepare the mind
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
Myopathy for the general internist: Statins and much more
Myopathies can present with a wide variety of symptoms, so patients with muscle weakness are often seen initially by a general practitioner. Nonrheumatologists should be able to evaluate a patient presenting with muscle weakness or myalgia and be aware of red flags indicating potentially dangerous syndromes that require a prompt, thorough investigation.
This article reviews selected causes of muscle weakness, such as statin-induced and autoimmune disorders, and systemic features of inflammatory myopathies beyond myositis, such as dermatologic and pulmonary manifestations.
FOCUSING THE EVALUATION
The evaluation of a patient presenting with muscle weakness should include several assessments:
Temporal progression. Was the onset of symptoms rapid or insidious? Patterns of onset may give clues to etiology, including the possibility of an associated autoimmune condition.
Location of muscle weakness. Are symptoms global or localized? And if localized, are they proximal or distal? Proximal weakness can be manifested by difficulty rising from a chair (hip muscles) or combing one’s hair (shoulder muscles), whereas distal weakness can involve difficulty standing on toes (gastrocnemius and soleus muscles) or performing fine motor activities (intrinsic hand muscles).
Symmetry. A focal or asymmetric pattern often has a neurologic etiology, but this could also be consistent with inclusion body myositis.
Other symptoms. Arthritis, rash, and swallowing problems point to a possible underlying rheumatologic disease. Weight gain or loss may indicate a thyroid disorder.
Family history. Some patients report that others in their family have this pattern of weakness, indicating a likely genetic myopathy. If the patient reports a relative with multiple sclerosis, lupus erythematosus, rheumatoid arthritis, or another autoimmune disease, then an immune-mediated myopathy should be considered.
Medications should be reviewed, particularly statins.
CASE 1: SLOWLY PROGRESSIVE WEAKNESS
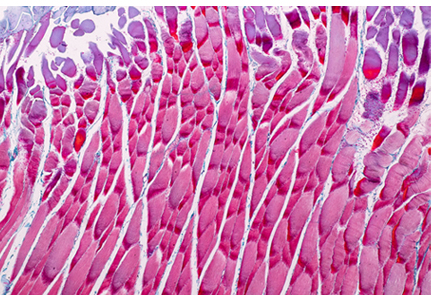
A 65-year-old man presented with the insidious onset of muscle weakness and episodes of falling. On review of his medical record, his serum creatine kinase (CK) levels were elevated at various periods at 2 to 4 times the upper limit of normal. Electromyography (EMG) previously showed a myopathic pattern, and a muscle biopsy was abnormal, consistent with endomysial inflammation (term is consistent with “polymyositis”). He was treated for polymyositis for several years with prednisone alone, with steroids plus methotrexate, and with combined immunosuppression including methotrexate and azathioprine, but with no improvement. Eventually, another muscle biopsy revealed inclusion bodies with rimmed vacuoles, consistent with inclusion body myositis.
Inclusion body myositis
Inclusion body myositis is the most common myopathy in middle-aged to elderly people, especially men. These patients are often told “You are just getting old,” but they have a defined condition. It should also be considered in patients failing to respond to treatment or with those with “refractory” polymyositis.
The onset of muscle weakness is insidious and painless, and the weakness progresses slowly. The pattern is distal and asymmetric (eg, foot drop), and muscle atrophy typically affects the forearm flexors, quadriceps, and intrinsic muscles of the hands.1
Magnetic resonance imaging may show marked muscle atrophy. Unfortunately, no treatment has shown efficacy, and most neuromuscular and rheumatology experts do not treat inclusion body myositis with immunosuppressive drugs.
CASE 2: MILD MYALGIA WITHOUT WEAKNESS
A black 52-year-old man was referred because of myalgia and a CK level of 862 U/L (reference range < 200). His physician wanted to start him on a statin but was hesitant to do so without first consulting a rheumatologist.
The patient had a long history of mild arthralgias and myalgias without muscle weakness. He had dyslipidemia and hypertension. He reported no family history of myopathy and no illicit drug use. He was formerly an athlete. Medications included a thiazide diuretic and a beta-blocker. On examination, his muscles were strong (rated 5 on a scale of 5) in the upper and lower extremities, without atrophy.
His records showed that his CK levels had risen and fallen repeatedly over the past few years, ranging from 600 to 1,100 U/L. On further questioning, he reported that when he had joined the army 30 years previously, a physician had recommended he undergo a liver biopsy in view of elevated liver function tests, but that he had refused because he felt fine.
Currently, his gamma-glutamyl transpeptidase levels were normal.
Idiopathic ‘hyperCKemia’
So-called idiopathic hyperCKemia is not a form of myositis but merely a laboratory result outside the “normal” range. Reference ranges are based predominantly on measurements in white people and on an assumption that the distribution is Gaussian (bell-shaped). A normal CK level is usually defined as less than 200 U/L. Using this standard, up to 20% of men and 5% of women have hyperCKemia.2
However, CK levels vary by sex and ethnicity, with mean levels highest in black men, followed by black women, white men, and white women. The mean level in black men is higher than the standard cutoff point for normal, and especially in this population, there is wide fluctuation around the mean, leading to hyperCKemia quite frequently in black men. Exercise and manual labor also drive up CK levels.3–5
Idiopathic hyperCKemia is benign. D’Adda et al6 followed 55 patients for a mean of 7.5 years. CK levels normalized in 12 patients or at least decreased in 24. Most remained symptom-free or had minimal symptoms.
Idiopathic hyperCKemia: Bottom line
Before prescribing a statin, determine the baseline CK level. If slightly elevated (ie, up to 3 to 5 times the upper limit of normal, or even higher) in the setting of normal muscle strength, there is no need for electromyography or muscle biopsy, and the patient can certainly receive a statin. Most of these patients do not need to see a rheumatologist but can simply have their CK and muscle strength monitored.
CLASSIFYING MYOSITIS
Myositis (idiopathic inflammatory myopathy) is a heterogeneous group of autoimmune syndromes of unknown cause characterized by chronic muscle weakness and inflammation of striated muscle. These syndromes likely arise as a result of genetic predisposition and an environmental or infectious “hit.”
Myositis is rare, with an incidence of 5 to 10 cases per million per year and an estimated prevalence of 50 to 90 cases per million. It has 2 incidence peaks: 1 in childhood (age 5–15) and another in adult midlife (age 30–50). Women are affected 2 to 3 times more often than men, with black women most commonly affected.
Myositis is traditionally classified as follows:
- Adult polymyositis
- Adult dermatomyositis
- Juvenile myositis (dermatomyositis much more frequent than polymyositis)
- Malignancy-associated myositis (usually dermatomyositis)
- Myositis overlapping with another autoimmune disease
- Inclusion body myositis.
However, polymyositis is less common than we originally thought, and the term necrotizing myopathy is now used in many patients, as noted in the case studies below. Further, myositis overlap syndromes are being increasingly diagnosed, likely related to the emergence of autoantibodies and clinical “syndromes” associated with these autoantibody subsets (discussed in cases below).
Dermatomyositis
Dermatomyositis is characterized by muscle weakness and a rash that can be obvious or subtle. Classic skin lesions are Gottron papules, which are raised, flat-topped red or purplish lesions over the knuckles, elbows, or knees.
Lesions may be confused with those of psoriasis. There can also be a V-neck rash over the anterior chest or upper back (“shawl sign”) or a rash over the lateral thigh (“holster sign”). A facial rash may occur, but unlike lupus, dermatomyositis does not spare the nasolabial area. However, the V-neck rash can be similar to that seen in lupus.
Dermatomyositis may cause muscle pain, perhaps related to muscle ischemia, whereas polymyositis and necrotizing myopathy are often painless. However, pain is also associated with fibromyalgia, which may be seen in many autoimmune conditions. It is important not to overtreat rheumatologic diseases with immunosuppression to try to control pain if the pain is actually caused by fibromyalgia.
Polymyositis mimics
Hypothyroid myopathy can present as classic polymyositis. The serum CK may be elevated, and there may be myalgias, muscle hypertrophy with stiffness, weakness, cramps, and even features of a proximal myopathy, and rhabdomyolysis. The electromyogram can be normal or myopathic. Results of muscle biopsy are often normal but may show focal necrosis and mild inflammatory infiltrates, thus mimicking that seen with inflammatory myopathy.7
Drug-induced or toxic myopathies can also mimic polymyositis. Statins are among the most commonly prescribed drugs in the United States, with more than 35 million people taking them. Statins are generally well tolerated but have a broad spectrum of toxicity, ranging from myalgias to life-threatening rhabdomyolysis. Myalgias lead to about 5% to 10% of patients refusing to take a statin or stopping it on their own.
Myalgias affect up to 20% of statin users in clinical practice.8,9 A small cross-sectional study10 of 1,000 patients in a primary care setting found that the risk of muscle complaints in statin users was 1.5 times higher than in nonstatin users, similar to findings in other studies.
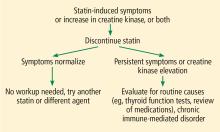
My strategy for managing a patient with possible statin-induced myopathy is illustrated in Figure 1.
CASE 3: WEAKNESS, VERY HIGH CK ON A STATIN
In March 2010, a 67-year-old woman presented with muscle weakness. She had a history of hypertension, hyperlipidemia, and, more than 10 years previously, uterine cancer. In 2004, she was given atorvastatin for dyslipidemia. Four years later, she developed lower-extremity weakness, which her doctor attributed to normal aging. A year after that, she found it difficult to walk up steps and lift her arms overhead. In June 2009, she stopped taking the atorvastatin on her own, but the weakness did not improve.
In September 2009, she returned to her doctor, who found her CK level was 6,473 U/L but believed it to be an error, so the test was repeated, with a result of 9,375 U/L. She had no rash or joint involvement.
She was admitted to the hospital and underwent muscle biopsy, which showed myonecrosis with no inflammation or vasculitis. She was treated with prednisone 60 mg/day, and her elevated CK level and weakness improved.
Immune-mediated necrotizing myopathy associated with statins
The hallmark of necrotizing myopathy is myonecrosis without significant inflammation.12 This pattern contrasts with that of polymyositis, which is characterized by lymphocytic inflammation.
Although statins became available in the United States in 1987, immune-mediated necrotizing myopathy associated with statins was first described only in 2010. In that report, Grable-Esposito et al13 described 25 patients from 2 neuromuscular centers seen between 2000 and 2008 who had elevated CK and proximal weakness during or after statin use, both of which persisted despite stopping the statin. Patients improved with immunosuppressive agents but had a relapse when steroids were stopped or tapered, a pattern typical in autoimmune disease.
Autoantibody defines subgroup of necrotizing myopathy
Also in 2010, Christopher-Stine et al14 reported an antibody associated with necrotizing myopathy. Of 38 patients with the condition, 16 were found to have an abnormal “doublet” autoantibody recognizing 200- and 100-kDa proteins. All patients had weakness and a high CK level, and 63% had statin exposure before the weakness (this percentage increased to 83% in patients older than 50). All responded to immunosuppressive therapy, and many had a relapse when it was withdrawn.
Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Co A reductase (HMGCR), and paradoxically, they also upregulate it. HMGCR has a molecular weight of 97 kDa. Mammen et al15 identified HMGCR as the 100-kDa target of the identified antibody and developed an enzyme-linked immunosorbent assay for it. Of 750 patients presenting to one center, only 45 (6%) had anti-HMGCR autoantibodies, but all 16 patients who had the abnormal doublet antibody tested positive for anti-HMGCR. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response after statins are discontinued.
Case 3 continued: Intravenous immunoglobulin brings improvement
In March 2010, when the 67-year-old patient presented to our myositis center, her CK level was 5,800 U/L, which increased as prednisone was tapered. She still felt weak. On examination, her muscle strength findings were deltoids 4+/5, neck flexors 4/5, and iliopsoas 3+/5. She was treated with methotrexate and azathioprine without benefit. She was next treated with intravenous immunoglobulin, and after 3 months, her strength normalized for the first time in years. Her CK level decreased but did not normalize. Testing showed that she was positive for anti-HMGCR autoantibody, as this test had become commercially available.
In 2015, Mammen and Tiniakou16 suggested using intravenous immunoglobulin as first-line therapy for statin-associated autoimmune necrotizing myopathy, based on experience at a single center with 3 patients who declined glucocorticoid treatment.
Necrotizing myopathy: Bottom line
Myositis overlap syndromes
Heterogeneity is the rule in myositis, and it can present with a wide variety of signs and symptoms as outlined in Table 2.
CASE 4: FEVER, NEW ‘RHEUMATOID ARTHRITIS,’ AND LUNG DISEASE
A 52-year-old woman with knee osteoarthritis saw her primary care physician in November 2013 for dyspnea and low-grade fever. The next month, she presented with polyarthritis, muscle weakness, and Raynaud phenomenon.
In January 2014, she developed acrocyanosis of her fingers. Examination revealed hyperkeratotic, cracked areas of her fingers. Her oxygen saturation by pulse oximetry was low. She was admitted to the hospital. Her doctor suspected new onset of rheumatoid arthritis, but blood tests revealed a negative antinuclear antibody, so an autoimmune condition was deemed unlikely. Her CK was mildly elevated at 350 U/L.
Because of her dyspnea, an open-lung biopsy was performed. High-resolution computed tomography (CT) revealed infiltrates and ground-glass opacities, leading to the diagnosis of nonspecific interstitial pneumonia. A rheumatologist was consulted and recommended pulse methylprednisolone, followed by prednisone 60 mg/day and mycophenolate mofetil. Testing for Jo-1 antibodies was positive.
Antisynthetase syndrome
The antisynthetase syndrome is a clinically heterogeneous condition that can occur with any or all of the following:
- Fever
- Myositis
- Arthritis (often misdiagnosed as rheumatoid arthritis)
- Raynaud phenomenon
- Mechanic’s hands (hyperkeratotic roughness with fissures on the lateral aspects of the fingers and finger pads)
- Interstitial lung disease.
The skin rashes and myositis may be subtle, making the presentation “lung-dominant,” and nonrheumatologists should be aware of this syndrome. Although in our patient the condition developed in a classic manner, with all of the aforementioned features of the antisynthetase syndrome, some patients will manifest one or a few of the features.
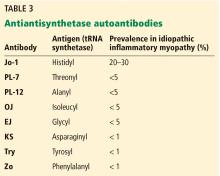
Clinically, patients with the Jo-1 antisynthetase syndrome often present differently than those with non-Jo-1 antisynthetase autoantibodies. When we compared 122 patients with Jo-1 vs 80 patients with a non-Jo-1 antisynthetase autoantibody, patients with Jo-1 antibodies were more likely to have initially received a diagnosis of myositis (83%), while myositis was the original diagnosis in only 17% of those possessing non-Jo-1 antisynthetase autoantibodies. In fact, many patients (approximately 50%) were diagnosed as having undifferentiated connective tissue disease or an overlap syndrome, and 13% had scleroderma as their first diagnosis.17
We also found that the survival rate was higher in patients with Jo-1 syndrome compared with patients with non-Jo-1 antisynthetase syndromes. We attributed the difference in survival rates to a delayed diagnosis in the non-Jo-1 group, perhaps due to their “nonclassic” presentations of the antisynthetase syndrome, delaying appropriate treatment. Patients received a diagnosis of Jo-1 antibody syndrome after a mean of 0.4 year (range 0.2–0.8), while those with a non-Jo-1 antisynthetase autoantibody had a delay in diagnosis of 1.0 year (range 0.4–5.1) (P < .01).17
In nearly half the cases in this cohort, pulmonary fibrosis was the cause of death, with primary pulmonary hypertension being the second leading cause (11%).
Antisynthetase syndrome: Bottom line
Antisynthetase syndrome is an often fatal disease that does not always present in a typical fashion with symptoms of myositis, as lung disease may be the predominant feature. A negative antinuclear antibody test result does not imply antibody negativity, as the autoantigen in these diseases is not located in the nucleus. Prompt diagnosis and appropriate immunosuppressive therapy are critical to improving outcomes.
CASE 5: FEVER, UNDIAGNOSED LUNG DISEASE, NO MYOSITIS
In January 2001, a 39-year-old woman was admitted to the hospital after 5 weeks of fever (temperatures 103°–104°F) and myalgias. An extensive workup was negative except for low-titer antinuclear antibody and for mild basilar fibrosis noted on chest radiography. She left the hospital against medical advice because of frustration with a lack of a specific diagnosis (“fever of unknown origin”).
Two months later, at a follow-up rheumatology consult, she reported more myalgias and arthralgias, as well as fever. Chest radiography now showed pleural effusions. Her fingers had color changes consistent with Raynaud phenomenon. At that time, I diagnosed an undifferentiated connective tissue disease and told her that I suspected an autoimmune condition that would need time to reveal itself. In the meantime, I treated her empirically with prednisone.
In April, she returned, much more short of breath and with more prominent diffuse pulmonary infiltrates. Physical examination revealed subtle Gottron changes. Testing revealed poor pulmonary function: forced vital capacity (FVC) 56%, forced expiratory volume in 1 second (FEV1) 52%, and diffusing capacity for carbon monoxide (Dlco) 40%. Blood testing was positive for anti-PL-12 antibody, one of the non-Jo-1 antisynthetase antibodies. At this time, we treated her with glucocorticoids and tacrolimus.
More than 15 years later, this patient is doing well. Her skin rash, joint symptoms, and fever have not returned, and interestingly, she never developed myositis. Her Raynaud symptoms are mild. Her most recent pulmonary function test results (January 2018) were FVC 75%, FEV1 87%, and Dlco 78%. Although these results are not normal, they are much improved and allow her to be completely functional without supplemental oxygen. Echocardiography showed normal pulmonary artery systolic pressure (25 mm Hg). She was still taking tacrolimus and prednisone. When we tried to stop tacrolimus after she had done well for many years, her condition flared.
Non-Jo-1 antisynthetase syndrome: Bottom line
Patients with a non-Jo-1 antisynthetase syndrome often present without myositis symptoms and may never manifest myositis symptoms. Likely because of this presentation, diagnosis of a specific connective tissue disorder is delayed, perhaps leading to increased mortality risk from pulmonary disease. Chronic immunosuppression is often required for these autoimmune conditions.
CASE 6: DERMATOMYOSITIS, RAPIDLY PROGRESSIVE INTERSTITIAL LUNG DISEASE
A 58-year-old woman presented in the summer of 2012 with a photosensitive rash. The following January, she returned with polyarthritis, mild muscle weakness, and a dermatomyositis-pattern rash. Her CK level was normal, and her antinuclear antibody and Sjögren syndrome antibody test results were negative. She improved on low-dose prednisone and methotrexate.
She was originally referred to me in May of that year for worsening rash and mild weakness. She denied pulmonary symptoms, but examination revealed faint basilar crackles. I increased her prednisone dosage to 20 mg/day and started mycophenolate mofetil mainly for the mild cutaneous and myositis features. I also recommended high-resolution CT of the lungs and pulmonary function tests, which she underwent in early June. High-resolution CT showed nonspecific mild infiltrates with minimal ground-glass opacities.
On July 1, she presented to her local emergency department with severe shortness of breath, requiring oxygen 12 L/min. She had a palmar rash. Repeat high-resolution CT showed dramatic worsening compared with the scan the previous month. Because of continued inadequate oxygenation, she was transferred to our center. A blood test later was positive for antimelanoma differentiation-associated gene 5 (MDA-5) autoantibody, previously known as anticlinically amyopathic dermatomyositis (anti-CADM)-140 antibody (based on immunoprecipitation results).
She died on the third day after transfer, just 2 months after I had originally seen her, at which time she had had no pulmonary symptoms.
Clinically amyopathic dermatomyositis
Anti-CADM-140, first reported from Asia,18–20 is an autoantibody-associated disease but not an antisynthetase. It is associated with dermatomyositis; patients often have a “vasculopathy” with cutaneous ulcerations and palmar papules.
MDA-5 is a cytoplasmic protein that “senses” viral RNA and induces production of type 1 interferon. It is involved in the innate immune defense against viruses.
Anti-MDA-5 positivity is associated with a poor pulmonary outcome.21 In our cohort from the University of Pittsburgh, many patients died within 3 years, compared with about a 40% survival rate in patients with dermatomyositis who tested negative for this antibody. That being said, many patients with anti-MDA-5 do not develop rapidly progressive interstitial lung disease.
Autoimmune interstitial lung disease: Bottom line
Autoimmune interstitial lung disease is easy to miss, especially in the case of a non-Jo-1 syndrome, for 3 important reasons:
- The autoimmune features may initially be subtle (eg, Raynaud phenomena, mild dermatomyositis rash, undifferentiated connective tissue disease)
- Autoantibody testing is not often ordered, is not standardized, or may be unavailable
- Providers are mistakenly reassured that a patient who tests negative for antinuclear antibody does not have an autoimmune condition.
To emphasize the last point, in a cohort of 202 patients who tested positive for an antisynthetase antibody, only half were antinuclear antibody-positive, but nearly three-quarters demonstrated anticytoplasmic staining on indirect immunofluorescence (due to the location of the autoantigen in the cytoplasm), making the latter a better screening test for an antisynthetase antibody. For scleroderma, 99% were antinculear antibody-positive, but for myositis, this test is much less sensitive.22
- Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001; 80(5):320–327. doi:10.1097/00005792-200109000-00006
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279(1-2):107–115. doi:10.1016/S0009-8981(98)00180-6
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21(7):494–500. doi:10.1016/j.nmd.2011.04.007
- Johnston JD, Lloyd M, Mathews JA, Hawthorne SW. Racial variation in serum creatine kinase levels. J R Soc Med 1996; 89(8):462-464. pmid:8795501
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249(3):305–311. pmid:11993531
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253(11):1399–1403. doi:10.1007/s00415-006-0223-y
- Madariaga MG. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid 2002; 12(4):331–336. doi:10.1089/10507250252949478
- de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ; Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90(2):181–184. doi:10.1016/s0002-9149(02)02449-9
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19(6):403–414. doi:10.1007/s10557-005-5686-z
- Mosshammer D, Lorenz G, Meznaric S, Schwarz J, Muche R, Mörike K. Statin use and its association with musculoskeletal symptoms—a cross-sectional study in primary care settings. Fam Pract 2009; 26(2):88–95. doi:10.1093/fampra/cmp006
- Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007; 29(8):1761–1770. doi:10.1016/j.clinthera.2007.08.022
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72(9):996–1003. doi:10.1001/jamaneurol.2015.1207
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41(2):185–190. doi:10.1002/mus.21486
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9):2757–2766. doi:10.1002/art.27572
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3):713–721. doi:10.1002/art.30156
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373(17):1680–1682. doi:10.1056/NEJMc1506163
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014; 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52(5):1571–1576. doi:10.1002/art.21023
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009; 60(7):2193–2200. doi:10.1002/art.24621
- Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32(12):3909–3915. doi:10.1007/s00296-011-2323-y
- Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016; 68(5):689–694. doi:10.1002/acr.22728
- Aggarwal R, Dhillon N, Fertig N, Koontz D, Qi Z, Oddis CV. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J Rheumatol 2017; 44(2):223–229. doi:10.3899/jrheum.160618
Myopathies can present with a wide variety of symptoms, so patients with muscle weakness are often seen initially by a general practitioner. Nonrheumatologists should be able to evaluate a patient presenting with muscle weakness or myalgia and be aware of red flags indicating potentially dangerous syndromes that require a prompt, thorough investigation.
This article reviews selected causes of muscle weakness, such as statin-induced and autoimmune disorders, and systemic features of inflammatory myopathies beyond myositis, such as dermatologic and pulmonary manifestations.
FOCUSING THE EVALUATION
The evaluation of a patient presenting with muscle weakness should include several assessments:
Temporal progression. Was the onset of symptoms rapid or insidious? Patterns of onset may give clues to etiology, including the possibility of an associated autoimmune condition.
Location of muscle weakness. Are symptoms global or localized? And if localized, are they proximal or distal? Proximal weakness can be manifested by difficulty rising from a chair (hip muscles) or combing one’s hair (shoulder muscles), whereas distal weakness can involve difficulty standing on toes (gastrocnemius and soleus muscles) or performing fine motor activities (intrinsic hand muscles).
Symmetry. A focal or asymmetric pattern often has a neurologic etiology, but this could also be consistent with inclusion body myositis.
Other symptoms. Arthritis, rash, and swallowing problems point to a possible underlying rheumatologic disease. Weight gain or loss may indicate a thyroid disorder.
Family history. Some patients report that others in their family have this pattern of weakness, indicating a likely genetic myopathy. If the patient reports a relative with multiple sclerosis, lupus erythematosus, rheumatoid arthritis, or another autoimmune disease, then an immune-mediated myopathy should be considered.
Medications should be reviewed, particularly statins.
CASE 1: SLOWLY PROGRESSIVE WEAKNESS
A 65-year-old man presented with the insidious onset of muscle weakness and episodes of falling. On review of his medical record, his serum creatine kinase (CK) levels were elevated at various periods at 2 to 4 times the upper limit of normal. Electromyography (EMG) previously showed a myopathic pattern, and a muscle biopsy was abnormal, consistent with endomysial inflammation (term is consistent with “polymyositis”). He was treated for polymyositis for several years with prednisone alone, with steroids plus methotrexate, and with combined immunosuppression including methotrexate and azathioprine, but with no improvement. Eventually, another muscle biopsy revealed inclusion bodies with rimmed vacuoles, consistent with inclusion body myositis.
Inclusion body myositis
Inclusion body myositis is the most common myopathy in middle-aged to elderly people, especially men. These patients are often told “You are just getting old,” but they have a defined condition. It should also be considered in patients failing to respond to treatment or with those with “refractory” polymyositis.
The onset of muscle weakness is insidious and painless, and the weakness progresses slowly. The pattern is distal and asymmetric (eg, foot drop), and muscle atrophy typically affects the forearm flexors, quadriceps, and intrinsic muscles of the hands.1
Magnetic resonance imaging may show marked muscle atrophy. Unfortunately, no treatment has shown efficacy, and most neuromuscular and rheumatology experts do not treat inclusion body myositis with immunosuppressive drugs.
CASE 2: MILD MYALGIA WITHOUT WEAKNESS
A black 52-year-old man was referred because of myalgia and a CK level of 862 U/L (reference range < 200). His physician wanted to start him on a statin but was hesitant to do so without first consulting a rheumatologist.
The patient had a long history of mild arthralgias and myalgias without muscle weakness. He had dyslipidemia and hypertension. He reported no family history of myopathy and no illicit drug use. He was formerly an athlete. Medications included a thiazide diuretic and a beta-blocker. On examination, his muscles were strong (rated 5 on a scale of 5) in the upper and lower extremities, without atrophy.
His records showed that his CK levels had risen and fallen repeatedly over the past few years, ranging from 600 to 1,100 U/L. On further questioning, he reported that when he had joined the army 30 years previously, a physician had recommended he undergo a liver biopsy in view of elevated liver function tests, but that he had refused because he felt fine.
Currently, his gamma-glutamyl transpeptidase levels were normal.
Idiopathic ‘hyperCKemia’
So-called idiopathic hyperCKemia is not a form of myositis but merely a laboratory result outside the “normal” range. Reference ranges are based predominantly on measurements in white people and on an assumption that the distribution is Gaussian (bell-shaped). A normal CK level is usually defined as less than 200 U/L. Using this standard, up to 20% of men and 5% of women have hyperCKemia.2
However, CK levels vary by sex and ethnicity, with mean levels highest in black men, followed by black women, white men, and white women. The mean level in black men is higher than the standard cutoff point for normal, and especially in this population, there is wide fluctuation around the mean, leading to hyperCKemia quite frequently in black men. Exercise and manual labor also drive up CK levels.3–5
Idiopathic hyperCKemia is benign. D’Adda et al6 followed 55 patients for a mean of 7.5 years. CK levels normalized in 12 patients or at least decreased in 24. Most remained symptom-free or had minimal symptoms.
Idiopathic hyperCKemia: Bottom line
Before prescribing a statin, determine the baseline CK level. If slightly elevated (ie, up to 3 to 5 times the upper limit of normal, or even higher) in the setting of normal muscle strength, there is no need for electromyography or muscle biopsy, and the patient can certainly receive a statin. Most of these patients do not need to see a rheumatologist but can simply have their CK and muscle strength monitored.
CLASSIFYING MYOSITIS
Myositis (idiopathic inflammatory myopathy) is a heterogeneous group of autoimmune syndromes of unknown cause characterized by chronic muscle weakness and inflammation of striated muscle. These syndromes likely arise as a result of genetic predisposition and an environmental or infectious “hit.”
Myositis is rare, with an incidence of 5 to 10 cases per million per year and an estimated prevalence of 50 to 90 cases per million. It has 2 incidence peaks: 1 in childhood (age 5–15) and another in adult midlife (age 30–50). Women are affected 2 to 3 times more often than men, with black women most commonly affected.
Myositis is traditionally classified as follows:
- Adult polymyositis
- Adult dermatomyositis
- Juvenile myositis (dermatomyositis much more frequent than polymyositis)
- Malignancy-associated myositis (usually dermatomyositis)
- Myositis overlapping with another autoimmune disease
- Inclusion body myositis.
However, polymyositis is less common than we originally thought, and the term necrotizing myopathy is now used in many patients, as noted in the case studies below. Further, myositis overlap syndromes are being increasingly diagnosed, likely related to the emergence of autoantibodies and clinical “syndromes” associated with these autoantibody subsets (discussed in cases below).
Dermatomyositis
Dermatomyositis is characterized by muscle weakness and a rash that can be obvious or subtle. Classic skin lesions are Gottron papules, which are raised, flat-topped red or purplish lesions over the knuckles, elbows, or knees.
Lesions may be confused with those of psoriasis. There can also be a V-neck rash over the anterior chest or upper back (“shawl sign”) or a rash over the lateral thigh (“holster sign”). A facial rash may occur, but unlike lupus, dermatomyositis does not spare the nasolabial area. However, the V-neck rash can be similar to that seen in lupus.
Dermatomyositis may cause muscle pain, perhaps related to muscle ischemia, whereas polymyositis and necrotizing myopathy are often painless. However, pain is also associated with fibromyalgia, which may be seen in many autoimmune conditions. It is important not to overtreat rheumatologic diseases with immunosuppression to try to control pain if the pain is actually caused by fibromyalgia.
Polymyositis mimics
Hypothyroid myopathy can present as classic polymyositis. The serum CK may be elevated, and there may be myalgias, muscle hypertrophy with stiffness, weakness, cramps, and even features of a proximal myopathy, and rhabdomyolysis. The electromyogram can be normal or myopathic. Results of muscle biopsy are often normal but may show focal necrosis and mild inflammatory infiltrates, thus mimicking that seen with inflammatory myopathy.7
Drug-induced or toxic myopathies can also mimic polymyositis. Statins are among the most commonly prescribed drugs in the United States, with more than 35 million people taking them. Statins are generally well tolerated but have a broad spectrum of toxicity, ranging from myalgias to life-threatening rhabdomyolysis. Myalgias lead to about 5% to 10% of patients refusing to take a statin or stopping it on their own.
Myalgias affect up to 20% of statin users in clinical practice.8,9 A small cross-sectional study10 of 1,000 patients in a primary care setting found that the risk of muscle complaints in statin users was 1.5 times higher than in nonstatin users, similar to findings in other studies.
My strategy for managing a patient with possible statin-induced myopathy is illustrated in Figure 1.
CASE 3: WEAKNESS, VERY HIGH CK ON A STATIN
In March 2010, a 67-year-old woman presented with muscle weakness. She had a history of hypertension, hyperlipidemia, and, more than 10 years previously, uterine cancer. In 2004, she was given atorvastatin for dyslipidemia. Four years later, she developed lower-extremity weakness, which her doctor attributed to normal aging. A year after that, she found it difficult to walk up steps and lift her arms overhead. In June 2009, she stopped taking the atorvastatin on her own, but the weakness did not improve.
In September 2009, she returned to her doctor, who found her CK level was 6,473 U/L but believed it to be an error, so the test was repeated, with a result of 9,375 U/L. She had no rash or joint involvement.
She was admitted to the hospital and underwent muscle biopsy, which showed myonecrosis with no inflammation or vasculitis. She was treated with prednisone 60 mg/day, and her elevated CK level and weakness improved.
Immune-mediated necrotizing myopathy associated with statins
The hallmark of necrotizing myopathy is myonecrosis without significant inflammation.12 This pattern contrasts with that of polymyositis, which is characterized by lymphocytic inflammation.
Although statins became available in the United States in 1987, immune-mediated necrotizing myopathy associated with statins was first described only in 2010. In that report, Grable-Esposito et al13 described 25 patients from 2 neuromuscular centers seen between 2000 and 2008 who had elevated CK and proximal weakness during or after statin use, both of which persisted despite stopping the statin. Patients improved with immunosuppressive agents but had a relapse when steroids were stopped or tapered, a pattern typical in autoimmune disease.
Autoantibody defines subgroup of necrotizing myopathy
Also in 2010, Christopher-Stine et al14 reported an antibody associated with necrotizing myopathy. Of 38 patients with the condition, 16 were found to have an abnormal “doublet” autoantibody recognizing 200- and 100-kDa proteins. All patients had weakness and a high CK level, and 63% had statin exposure before the weakness (this percentage increased to 83% in patients older than 50). All responded to immunosuppressive therapy, and many had a relapse when it was withdrawn.
Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Co A reductase (HMGCR), and paradoxically, they also upregulate it. HMGCR has a molecular weight of 97 kDa. Mammen et al15 identified HMGCR as the 100-kDa target of the identified antibody and developed an enzyme-linked immunosorbent assay for it. Of 750 patients presenting to one center, only 45 (6%) had anti-HMGCR autoantibodies, but all 16 patients who had the abnormal doublet antibody tested positive for anti-HMGCR. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response after statins are discontinued.
Case 3 continued: Intravenous immunoglobulin brings improvement
In March 2010, when the 67-year-old patient presented to our myositis center, her CK level was 5,800 U/L, which increased as prednisone was tapered. She still felt weak. On examination, her muscle strength findings were deltoids 4+/5, neck flexors 4/5, and iliopsoas 3+/5. She was treated with methotrexate and azathioprine without benefit. She was next treated with intravenous immunoglobulin, and after 3 months, her strength normalized for the first time in years. Her CK level decreased but did not normalize. Testing showed that she was positive for anti-HMGCR autoantibody, as this test had become commercially available.
In 2015, Mammen and Tiniakou16 suggested using intravenous immunoglobulin as first-line therapy for statin-associated autoimmune necrotizing myopathy, based on experience at a single center with 3 patients who declined glucocorticoid treatment.
Necrotizing myopathy: Bottom line
Myositis overlap syndromes
Heterogeneity is the rule in myositis, and it can present with a wide variety of signs and symptoms as outlined in Table 2.
CASE 4: FEVER, NEW ‘RHEUMATOID ARTHRITIS,’ AND LUNG DISEASE
A 52-year-old woman with knee osteoarthritis saw her primary care physician in November 2013 for dyspnea and low-grade fever. The next month, she presented with polyarthritis, muscle weakness, and Raynaud phenomenon.
In January 2014, she developed acrocyanosis of her fingers. Examination revealed hyperkeratotic, cracked areas of her fingers. Her oxygen saturation by pulse oximetry was low. She was admitted to the hospital. Her doctor suspected new onset of rheumatoid arthritis, but blood tests revealed a negative antinuclear antibody, so an autoimmune condition was deemed unlikely. Her CK was mildly elevated at 350 U/L.
Because of her dyspnea, an open-lung biopsy was performed. High-resolution computed tomography (CT) revealed infiltrates and ground-glass opacities, leading to the diagnosis of nonspecific interstitial pneumonia. A rheumatologist was consulted and recommended pulse methylprednisolone, followed by prednisone 60 mg/day and mycophenolate mofetil. Testing for Jo-1 antibodies was positive.
Antisynthetase syndrome
The antisynthetase syndrome is a clinically heterogeneous condition that can occur with any or all of the following:
- Fever
- Myositis
- Arthritis (often misdiagnosed as rheumatoid arthritis)
- Raynaud phenomenon
- Mechanic’s hands (hyperkeratotic roughness with fissures on the lateral aspects of the fingers and finger pads)
- Interstitial lung disease.
The skin rashes and myositis may be subtle, making the presentation “lung-dominant,” and nonrheumatologists should be aware of this syndrome. Although in our patient the condition developed in a classic manner, with all of the aforementioned features of the antisynthetase syndrome, some patients will manifest one or a few of the features.
Clinically, patients with the Jo-1 antisynthetase syndrome often present differently than those with non-Jo-1 antisynthetase autoantibodies. When we compared 122 patients with Jo-1 vs 80 patients with a non-Jo-1 antisynthetase autoantibody, patients with Jo-1 antibodies were more likely to have initially received a diagnosis of myositis (83%), while myositis was the original diagnosis in only 17% of those possessing non-Jo-1 antisynthetase autoantibodies. In fact, many patients (approximately 50%) were diagnosed as having undifferentiated connective tissue disease or an overlap syndrome, and 13% had scleroderma as their first diagnosis.17
We also found that the survival rate was higher in patients with Jo-1 syndrome compared with patients with non-Jo-1 antisynthetase syndromes. We attributed the difference in survival rates to a delayed diagnosis in the non-Jo-1 group, perhaps due to their “nonclassic” presentations of the antisynthetase syndrome, delaying appropriate treatment. Patients received a diagnosis of Jo-1 antibody syndrome after a mean of 0.4 year (range 0.2–0.8), while those with a non-Jo-1 antisynthetase autoantibody had a delay in diagnosis of 1.0 year (range 0.4–5.1) (P < .01).17
In nearly half the cases in this cohort, pulmonary fibrosis was the cause of death, with primary pulmonary hypertension being the second leading cause (11%).
Antisynthetase syndrome: Bottom line
Antisynthetase syndrome is an often fatal disease that does not always present in a typical fashion with symptoms of myositis, as lung disease may be the predominant feature. A negative antinuclear antibody test result does not imply antibody negativity, as the autoantigen in these diseases is not located in the nucleus. Prompt diagnosis and appropriate immunosuppressive therapy are critical to improving outcomes.
CASE 5: FEVER, UNDIAGNOSED LUNG DISEASE, NO MYOSITIS
In January 2001, a 39-year-old woman was admitted to the hospital after 5 weeks of fever (temperatures 103°–104°F) and myalgias. An extensive workup was negative except for low-titer antinuclear antibody and for mild basilar fibrosis noted on chest radiography. She left the hospital against medical advice because of frustration with a lack of a specific diagnosis (“fever of unknown origin”).
Two months later, at a follow-up rheumatology consult, she reported more myalgias and arthralgias, as well as fever. Chest radiography now showed pleural effusions. Her fingers had color changes consistent with Raynaud phenomenon. At that time, I diagnosed an undifferentiated connective tissue disease and told her that I suspected an autoimmune condition that would need time to reveal itself. In the meantime, I treated her empirically with prednisone.
In April, she returned, much more short of breath and with more prominent diffuse pulmonary infiltrates. Physical examination revealed subtle Gottron changes. Testing revealed poor pulmonary function: forced vital capacity (FVC) 56%, forced expiratory volume in 1 second (FEV1) 52%, and diffusing capacity for carbon monoxide (Dlco) 40%. Blood testing was positive for anti-PL-12 antibody, one of the non-Jo-1 antisynthetase antibodies. At this time, we treated her with glucocorticoids and tacrolimus.
More than 15 years later, this patient is doing well. Her skin rash, joint symptoms, and fever have not returned, and interestingly, she never developed myositis. Her Raynaud symptoms are mild. Her most recent pulmonary function test results (January 2018) were FVC 75%, FEV1 87%, and Dlco 78%. Although these results are not normal, they are much improved and allow her to be completely functional without supplemental oxygen. Echocardiography showed normal pulmonary artery systolic pressure (25 mm Hg). She was still taking tacrolimus and prednisone. When we tried to stop tacrolimus after she had done well for many years, her condition flared.
Non-Jo-1 antisynthetase syndrome: Bottom line
Patients with a non-Jo-1 antisynthetase syndrome often present without myositis symptoms and may never manifest myositis symptoms. Likely because of this presentation, diagnosis of a specific connective tissue disorder is delayed, perhaps leading to increased mortality risk from pulmonary disease. Chronic immunosuppression is often required for these autoimmune conditions.
CASE 6: DERMATOMYOSITIS, RAPIDLY PROGRESSIVE INTERSTITIAL LUNG DISEASE
A 58-year-old woman presented in the summer of 2012 with a photosensitive rash. The following January, she returned with polyarthritis, mild muscle weakness, and a dermatomyositis-pattern rash. Her CK level was normal, and her antinuclear antibody and Sjögren syndrome antibody test results were negative. She improved on low-dose prednisone and methotrexate.
She was originally referred to me in May of that year for worsening rash and mild weakness. She denied pulmonary symptoms, but examination revealed faint basilar crackles. I increased her prednisone dosage to 20 mg/day and started mycophenolate mofetil mainly for the mild cutaneous and myositis features. I also recommended high-resolution CT of the lungs and pulmonary function tests, which she underwent in early June. High-resolution CT showed nonspecific mild infiltrates with minimal ground-glass opacities.
On July 1, she presented to her local emergency department with severe shortness of breath, requiring oxygen 12 L/min. She had a palmar rash. Repeat high-resolution CT showed dramatic worsening compared with the scan the previous month. Because of continued inadequate oxygenation, she was transferred to our center. A blood test later was positive for antimelanoma differentiation-associated gene 5 (MDA-5) autoantibody, previously known as anticlinically amyopathic dermatomyositis (anti-CADM)-140 antibody (based on immunoprecipitation results).
She died on the third day after transfer, just 2 months after I had originally seen her, at which time she had had no pulmonary symptoms.
Clinically amyopathic dermatomyositis
Anti-CADM-140, first reported from Asia,18–20 is an autoantibody-associated disease but not an antisynthetase. It is associated with dermatomyositis; patients often have a “vasculopathy” with cutaneous ulcerations and palmar papules.
MDA-5 is a cytoplasmic protein that “senses” viral RNA and induces production of type 1 interferon. It is involved in the innate immune defense against viruses.
Anti-MDA-5 positivity is associated with a poor pulmonary outcome.21 In our cohort from the University of Pittsburgh, many patients died within 3 years, compared with about a 40% survival rate in patients with dermatomyositis who tested negative for this antibody. That being said, many patients with anti-MDA-5 do not develop rapidly progressive interstitial lung disease.
Autoimmune interstitial lung disease: Bottom line
Autoimmune interstitial lung disease is easy to miss, especially in the case of a non-Jo-1 syndrome, for 3 important reasons:
- The autoimmune features may initially be subtle (eg, Raynaud phenomena, mild dermatomyositis rash, undifferentiated connective tissue disease)
- Autoantibody testing is not often ordered, is not standardized, or may be unavailable
- Providers are mistakenly reassured that a patient who tests negative for antinuclear antibody does not have an autoimmune condition.
To emphasize the last point, in a cohort of 202 patients who tested positive for an antisynthetase antibody, only half were antinuclear antibody-positive, but nearly three-quarters demonstrated anticytoplasmic staining on indirect immunofluorescence (due to the location of the autoantigen in the cytoplasm), making the latter a better screening test for an antisynthetase antibody. For scleroderma, 99% were antinculear antibody-positive, but for myositis, this test is much less sensitive.22
Myopathies can present with a wide variety of symptoms, so patients with muscle weakness are often seen initially by a general practitioner. Nonrheumatologists should be able to evaluate a patient presenting with muscle weakness or myalgia and be aware of red flags indicating potentially dangerous syndromes that require a prompt, thorough investigation.
This article reviews selected causes of muscle weakness, such as statin-induced and autoimmune disorders, and systemic features of inflammatory myopathies beyond myositis, such as dermatologic and pulmonary manifestations.
FOCUSING THE EVALUATION
The evaluation of a patient presenting with muscle weakness should include several assessments:
Temporal progression. Was the onset of symptoms rapid or insidious? Patterns of onset may give clues to etiology, including the possibility of an associated autoimmune condition.
Location of muscle weakness. Are symptoms global or localized? And if localized, are they proximal or distal? Proximal weakness can be manifested by difficulty rising from a chair (hip muscles) or combing one’s hair (shoulder muscles), whereas distal weakness can involve difficulty standing on toes (gastrocnemius and soleus muscles) or performing fine motor activities (intrinsic hand muscles).
Symmetry. A focal or asymmetric pattern often has a neurologic etiology, but this could also be consistent with inclusion body myositis.
Other symptoms. Arthritis, rash, and swallowing problems point to a possible underlying rheumatologic disease. Weight gain or loss may indicate a thyroid disorder.
Family history. Some patients report that others in their family have this pattern of weakness, indicating a likely genetic myopathy. If the patient reports a relative with multiple sclerosis, lupus erythematosus, rheumatoid arthritis, or another autoimmune disease, then an immune-mediated myopathy should be considered.
Medications should be reviewed, particularly statins.
CASE 1: SLOWLY PROGRESSIVE WEAKNESS
A 65-year-old man presented with the insidious onset of muscle weakness and episodes of falling. On review of his medical record, his serum creatine kinase (CK) levels were elevated at various periods at 2 to 4 times the upper limit of normal. Electromyography (EMG) previously showed a myopathic pattern, and a muscle biopsy was abnormal, consistent with endomysial inflammation (term is consistent with “polymyositis”). He was treated for polymyositis for several years with prednisone alone, with steroids plus methotrexate, and with combined immunosuppression including methotrexate and azathioprine, but with no improvement. Eventually, another muscle biopsy revealed inclusion bodies with rimmed vacuoles, consistent with inclusion body myositis.
Inclusion body myositis
Inclusion body myositis is the most common myopathy in middle-aged to elderly people, especially men. These patients are often told “You are just getting old,” but they have a defined condition. It should also be considered in patients failing to respond to treatment or with those with “refractory” polymyositis.
The onset of muscle weakness is insidious and painless, and the weakness progresses slowly. The pattern is distal and asymmetric (eg, foot drop), and muscle atrophy typically affects the forearm flexors, quadriceps, and intrinsic muscles of the hands.1
Magnetic resonance imaging may show marked muscle atrophy. Unfortunately, no treatment has shown efficacy, and most neuromuscular and rheumatology experts do not treat inclusion body myositis with immunosuppressive drugs.
CASE 2: MILD MYALGIA WITHOUT WEAKNESS
A black 52-year-old man was referred because of myalgia and a CK level of 862 U/L (reference range < 200). His physician wanted to start him on a statin but was hesitant to do so without first consulting a rheumatologist.
The patient had a long history of mild arthralgias and myalgias without muscle weakness. He had dyslipidemia and hypertension. He reported no family history of myopathy and no illicit drug use. He was formerly an athlete. Medications included a thiazide diuretic and a beta-blocker. On examination, his muscles were strong (rated 5 on a scale of 5) in the upper and lower extremities, without atrophy.
His records showed that his CK levels had risen and fallen repeatedly over the past few years, ranging from 600 to 1,100 U/L. On further questioning, he reported that when he had joined the army 30 years previously, a physician had recommended he undergo a liver biopsy in view of elevated liver function tests, but that he had refused because he felt fine.
Currently, his gamma-glutamyl transpeptidase levels were normal.
Idiopathic ‘hyperCKemia’
So-called idiopathic hyperCKemia is not a form of myositis but merely a laboratory result outside the “normal” range. Reference ranges are based predominantly on measurements in white people and on an assumption that the distribution is Gaussian (bell-shaped). A normal CK level is usually defined as less than 200 U/L. Using this standard, up to 20% of men and 5% of women have hyperCKemia.2
However, CK levels vary by sex and ethnicity, with mean levels highest in black men, followed by black women, white men, and white women. The mean level in black men is higher than the standard cutoff point for normal, and especially in this population, there is wide fluctuation around the mean, leading to hyperCKemia quite frequently in black men. Exercise and manual labor also drive up CK levels.3–5
Idiopathic hyperCKemia is benign. D’Adda et al6 followed 55 patients for a mean of 7.5 years. CK levels normalized in 12 patients or at least decreased in 24. Most remained symptom-free or had minimal symptoms.
Idiopathic hyperCKemia: Bottom line
Before prescribing a statin, determine the baseline CK level. If slightly elevated (ie, up to 3 to 5 times the upper limit of normal, or even higher) in the setting of normal muscle strength, there is no need for electromyography or muscle biopsy, and the patient can certainly receive a statin. Most of these patients do not need to see a rheumatologist but can simply have their CK and muscle strength monitored.
CLASSIFYING MYOSITIS
Myositis (idiopathic inflammatory myopathy) is a heterogeneous group of autoimmune syndromes of unknown cause characterized by chronic muscle weakness and inflammation of striated muscle. These syndromes likely arise as a result of genetic predisposition and an environmental or infectious “hit.”
Myositis is rare, with an incidence of 5 to 10 cases per million per year and an estimated prevalence of 50 to 90 cases per million. It has 2 incidence peaks: 1 in childhood (age 5–15) and another in adult midlife (age 30–50). Women are affected 2 to 3 times more often than men, with black women most commonly affected.
Myositis is traditionally classified as follows:
- Adult polymyositis
- Adult dermatomyositis
- Juvenile myositis (dermatomyositis much more frequent than polymyositis)
- Malignancy-associated myositis (usually dermatomyositis)
- Myositis overlapping with another autoimmune disease
- Inclusion body myositis.
However, polymyositis is less common than we originally thought, and the term necrotizing myopathy is now used in many patients, as noted in the case studies below. Further, myositis overlap syndromes are being increasingly diagnosed, likely related to the emergence of autoantibodies and clinical “syndromes” associated with these autoantibody subsets (discussed in cases below).
Dermatomyositis
Dermatomyositis is characterized by muscle weakness and a rash that can be obvious or subtle. Classic skin lesions are Gottron papules, which are raised, flat-topped red or purplish lesions over the knuckles, elbows, or knees.
Lesions may be confused with those of psoriasis. There can also be a V-neck rash over the anterior chest or upper back (“shawl sign”) or a rash over the lateral thigh (“holster sign”). A facial rash may occur, but unlike lupus, dermatomyositis does not spare the nasolabial area. However, the V-neck rash can be similar to that seen in lupus.
Dermatomyositis may cause muscle pain, perhaps related to muscle ischemia, whereas polymyositis and necrotizing myopathy are often painless. However, pain is also associated with fibromyalgia, which may be seen in many autoimmune conditions. It is important not to overtreat rheumatologic diseases with immunosuppression to try to control pain if the pain is actually caused by fibromyalgia.
Polymyositis mimics
Hypothyroid myopathy can present as classic polymyositis. The serum CK may be elevated, and there may be myalgias, muscle hypertrophy with stiffness, weakness, cramps, and even features of a proximal myopathy, and rhabdomyolysis. The electromyogram can be normal or myopathic. Results of muscle biopsy are often normal but may show focal necrosis and mild inflammatory infiltrates, thus mimicking that seen with inflammatory myopathy.7
Drug-induced or toxic myopathies can also mimic polymyositis. Statins are among the most commonly prescribed drugs in the United States, with more than 35 million people taking them. Statins are generally well tolerated but have a broad spectrum of toxicity, ranging from myalgias to life-threatening rhabdomyolysis. Myalgias lead to about 5% to 10% of patients refusing to take a statin or stopping it on their own.
Myalgias affect up to 20% of statin users in clinical practice.8,9 A small cross-sectional study10 of 1,000 patients in a primary care setting found that the risk of muscle complaints in statin users was 1.5 times higher than in nonstatin users, similar to findings in other studies.
My strategy for managing a patient with possible statin-induced myopathy is illustrated in Figure 1.
CASE 3: WEAKNESS, VERY HIGH CK ON A STATIN
In March 2010, a 67-year-old woman presented with muscle weakness. She had a history of hypertension, hyperlipidemia, and, more than 10 years previously, uterine cancer. In 2004, she was given atorvastatin for dyslipidemia. Four years later, she developed lower-extremity weakness, which her doctor attributed to normal aging. A year after that, she found it difficult to walk up steps and lift her arms overhead. In June 2009, she stopped taking the atorvastatin on her own, but the weakness did not improve.
In September 2009, she returned to her doctor, who found her CK level was 6,473 U/L but believed it to be an error, so the test was repeated, with a result of 9,375 U/L. She had no rash or joint involvement.
She was admitted to the hospital and underwent muscle biopsy, which showed myonecrosis with no inflammation or vasculitis. She was treated with prednisone 60 mg/day, and her elevated CK level and weakness improved.
Immune-mediated necrotizing myopathy associated with statins
The hallmark of necrotizing myopathy is myonecrosis without significant inflammation.12 This pattern contrasts with that of polymyositis, which is characterized by lymphocytic inflammation.
Although statins became available in the United States in 1987, immune-mediated necrotizing myopathy associated with statins was first described only in 2010. In that report, Grable-Esposito et al13 described 25 patients from 2 neuromuscular centers seen between 2000 and 2008 who had elevated CK and proximal weakness during or after statin use, both of which persisted despite stopping the statin. Patients improved with immunosuppressive agents but had a relapse when steroids were stopped or tapered, a pattern typical in autoimmune disease.
Autoantibody defines subgroup of necrotizing myopathy
Also in 2010, Christopher-Stine et al14 reported an antibody associated with necrotizing myopathy. Of 38 patients with the condition, 16 were found to have an abnormal “doublet” autoantibody recognizing 200- and 100-kDa proteins. All patients had weakness and a high CK level, and 63% had statin exposure before the weakness (this percentage increased to 83% in patients older than 50). All responded to immunosuppressive therapy, and many had a relapse when it was withdrawn.
Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Co A reductase (HMGCR), and paradoxically, they also upregulate it. HMGCR has a molecular weight of 97 kDa. Mammen et al15 identified HMGCR as the 100-kDa target of the identified antibody and developed an enzyme-linked immunosorbent assay for it. Of 750 patients presenting to one center, only 45 (6%) had anti-HMGCR autoantibodies, but all 16 patients who had the abnormal doublet antibody tested positive for anti-HMGCR. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response after statins are discontinued.
Case 3 continued: Intravenous immunoglobulin brings improvement
In March 2010, when the 67-year-old patient presented to our myositis center, her CK level was 5,800 U/L, which increased as prednisone was tapered. She still felt weak. On examination, her muscle strength findings were deltoids 4+/5, neck flexors 4/5, and iliopsoas 3+/5. She was treated with methotrexate and azathioprine without benefit. She was next treated with intravenous immunoglobulin, and after 3 months, her strength normalized for the first time in years. Her CK level decreased but did not normalize. Testing showed that she was positive for anti-HMGCR autoantibody, as this test had become commercially available.
In 2015, Mammen and Tiniakou16 suggested using intravenous immunoglobulin as first-line therapy for statin-associated autoimmune necrotizing myopathy, based on experience at a single center with 3 patients who declined glucocorticoid treatment.
Necrotizing myopathy: Bottom line
Myositis overlap syndromes
Heterogeneity is the rule in myositis, and it can present with a wide variety of signs and symptoms as outlined in Table 2.
CASE 4: FEVER, NEW ‘RHEUMATOID ARTHRITIS,’ AND LUNG DISEASE
A 52-year-old woman with knee osteoarthritis saw her primary care physician in November 2013 for dyspnea and low-grade fever. The next month, she presented with polyarthritis, muscle weakness, and Raynaud phenomenon.
In January 2014, she developed acrocyanosis of her fingers. Examination revealed hyperkeratotic, cracked areas of her fingers. Her oxygen saturation by pulse oximetry was low. She was admitted to the hospital. Her doctor suspected new onset of rheumatoid arthritis, but blood tests revealed a negative antinuclear antibody, so an autoimmune condition was deemed unlikely. Her CK was mildly elevated at 350 U/L.
Because of her dyspnea, an open-lung biopsy was performed. High-resolution computed tomography (CT) revealed infiltrates and ground-glass opacities, leading to the diagnosis of nonspecific interstitial pneumonia. A rheumatologist was consulted and recommended pulse methylprednisolone, followed by prednisone 60 mg/day and mycophenolate mofetil. Testing for Jo-1 antibodies was positive.
Antisynthetase syndrome
The antisynthetase syndrome is a clinically heterogeneous condition that can occur with any or all of the following:
- Fever
- Myositis
- Arthritis (often misdiagnosed as rheumatoid arthritis)
- Raynaud phenomenon
- Mechanic’s hands (hyperkeratotic roughness with fissures on the lateral aspects of the fingers and finger pads)
- Interstitial lung disease.
The skin rashes and myositis may be subtle, making the presentation “lung-dominant,” and nonrheumatologists should be aware of this syndrome. Although in our patient the condition developed in a classic manner, with all of the aforementioned features of the antisynthetase syndrome, some patients will manifest one or a few of the features.
Clinically, patients with the Jo-1 antisynthetase syndrome often present differently than those with non-Jo-1 antisynthetase autoantibodies. When we compared 122 patients with Jo-1 vs 80 patients with a non-Jo-1 antisynthetase autoantibody, patients with Jo-1 antibodies were more likely to have initially received a diagnosis of myositis (83%), while myositis was the original diagnosis in only 17% of those possessing non-Jo-1 antisynthetase autoantibodies. In fact, many patients (approximately 50%) were diagnosed as having undifferentiated connective tissue disease or an overlap syndrome, and 13% had scleroderma as their first diagnosis.17
We also found that the survival rate was higher in patients with Jo-1 syndrome compared with patients with non-Jo-1 antisynthetase syndromes. We attributed the difference in survival rates to a delayed diagnosis in the non-Jo-1 group, perhaps due to their “nonclassic” presentations of the antisynthetase syndrome, delaying appropriate treatment. Patients received a diagnosis of Jo-1 antibody syndrome after a mean of 0.4 year (range 0.2–0.8), while those with a non-Jo-1 antisynthetase autoantibody had a delay in diagnosis of 1.0 year (range 0.4–5.1) (P < .01).17
In nearly half the cases in this cohort, pulmonary fibrosis was the cause of death, with primary pulmonary hypertension being the second leading cause (11%).
Antisynthetase syndrome: Bottom line
Antisynthetase syndrome is an often fatal disease that does not always present in a typical fashion with symptoms of myositis, as lung disease may be the predominant feature. A negative antinuclear antibody test result does not imply antibody negativity, as the autoantigen in these diseases is not located in the nucleus. Prompt diagnosis and appropriate immunosuppressive therapy are critical to improving outcomes.
CASE 5: FEVER, UNDIAGNOSED LUNG DISEASE, NO MYOSITIS
In January 2001, a 39-year-old woman was admitted to the hospital after 5 weeks of fever (temperatures 103°–104°F) and myalgias. An extensive workup was negative except for low-titer antinuclear antibody and for mild basilar fibrosis noted on chest radiography. She left the hospital against medical advice because of frustration with a lack of a specific diagnosis (“fever of unknown origin”).
Two months later, at a follow-up rheumatology consult, she reported more myalgias and arthralgias, as well as fever. Chest radiography now showed pleural effusions. Her fingers had color changes consistent with Raynaud phenomenon. At that time, I diagnosed an undifferentiated connective tissue disease and told her that I suspected an autoimmune condition that would need time to reveal itself. In the meantime, I treated her empirically with prednisone.
In April, she returned, much more short of breath and with more prominent diffuse pulmonary infiltrates. Physical examination revealed subtle Gottron changes. Testing revealed poor pulmonary function: forced vital capacity (FVC) 56%, forced expiratory volume in 1 second (FEV1) 52%, and diffusing capacity for carbon monoxide (Dlco) 40%. Blood testing was positive for anti-PL-12 antibody, one of the non-Jo-1 antisynthetase antibodies. At this time, we treated her with glucocorticoids and tacrolimus.
More than 15 years later, this patient is doing well. Her skin rash, joint symptoms, and fever have not returned, and interestingly, she never developed myositis. Her Raynaud symptoms are mild. Her most recent pulmonary function test results (January 2018) were FVC 75%, FEV1 87%, and Dlco 78%. Although these results are not normal, they are much improved and allow her to be completely functional without supplemental oxygen. Echocardiography showed normal pulmonary artery systolic pressure (25 mm Hg). She was still taking tacrolimus and prednisone. When we tried to stop tacrolimus after she had done well for many years, her condition flared.
Non-Jo-1 antisynthetase syndrome: Bottom line
Patients with a non-Jo-1 antisynthetase syndrome often present without myositis symptoms and may never manifest myositis symptoms. Likely because of this presentation, diagnosis of a specific connective tissue disorder is delayed, perhaps leading to increased mortality risk from pulmonary disease. Chronic immunosuppression is often required for these autoimmune conditions.
CASE 6: DERMATOMYOSITIS, RAPIDLY PROGRESSIVE INTERSTITIAL LUNG DISEASE
A 58-year-old woman presented in the summer of 2012 with a photosensitive rash. The following January, she returned with polyarthritis, mild muscle weakness, and a dermatomyositis-pattern rash. Her CK level was normal, and her antinuclear antibody and Sjögren syndrome antibody test results were negative. She improved on low-dose prednisone and methotrexate.
She was originally referred to me in May of that year for worsening rash and mild weakness. She denied pulmonary symptoms, but examination revealed faint basilar crackles. I increased her prednisone dosage to 20 mg/day and started mycophenolate mofetil mainly for the mild cutaneous and myositis features. I also recommended high-resolution CT of the lungs and pulmonary function tests, which she underwent in early June. High-resolution CT showed nonspecific mild infiltrates with minimal ground-glass opacities.
On July 1, she presented to her local emergency department with severe shortness of breath, requiring oxygen 12 L/min. She had a palmar rash. Repeat high-resolution CT showed dramatic worsening compared with the scan the previous month. Because of continued inadequate oxygenation, she was transferred to our center. A blood test later was positive for antimelanoma differentiation-associated gene 5 (MDA-5) autoantibody, previously known as anticlinically amyopathic dermatomyositis (anti-CADM)-140 antibody (based on immunoprecipitation results).
She died on the third day after transfer, just 2 months after I had originally seen her, at which time she had had no pulmonary symptoms.
Clinically amyopathic dermatomyositis
Anti-CADM-140, first reported from Asia,18–20 is an autoantibody-associated disease but not an antisynthetase. It is associated with dermatomyositis; patients often have a “vasculopathy” with cutaneous ulcerations and palmar papules.
MDA-5 is a cytoplasmic protein that “senses” viral RNA and induces production of type 1 interferon. It is involved in the innate immune defense against viruses.
Anti-MDA-5 positivity is associated with a poor pulmonary outcome.21 In our cohort from the University of Pittsburgh, many patients died within 3 years, compared with about a 40% survival rate in patients with dermatomyositis who tested negative for this antibody. That being said, many patients with anti-MDA-5 do not develop rapidly progressive interstitial lung disease.
Autoimmune interstitial lung disease: Bottom line
Autoimmune interstitial lung disease is easy to miss, especially in the case of a non-Jo-1 syndrome, for 3 important reasons:
- The autoimmune features may initially be subtle (eg, Raynaud phenomena, mild dermatomyositis rash, undifferentiated connective tissue disease)
- Autoantibody testing is not often ordered, is not standardized, or may be unavailable
- Providers are mistakenly reassured that a patient who tests negative for antinuclear antibody does not have an autoimmune condition.
To emphasize the last point, in a cohort of 202 patients who tested positive for an antisynthetase antibody, only half were antinuclear antibody-positive, but nearly three-quarters demonstrated anticytoplasmic staining on indirect immunofluorescence (due to the location of the autoantigen in the cytoplasm), making the latter a better screening test for an antisynthetase antibody. For scleroderma, 99% were antinculear antibody-positive, but for myositis, this test is much less sensitive.22
- Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001; 80(5):320–327. doi:10.1097/00005792-200109000-00006
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279(1-2):107–115. doi:10.1016/S0009-8981(98)00180-6
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21(7):494–500. doi:10.1016/j.nmd.2011.04.007
- Johnston JD, Lloyd M, Mathews JA, Hawthorne SW. Racial variation in serum creatine kinase levels. J R Soc Med 1996; 89(8):462-464. pmid:8795501
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249(3):305–311. pmid:11993531
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253(11):1399–1403. doi:10.1007/s00415-006-0223-y
- Madariaga MG. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid 2002; 12(4):331–336. doi:10.1089/10507250252949478
- de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ; Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90(2):181–184. doi:10.1016/s0002-9149(02)02449-9
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19(6):403–414. doi:10.1007/s10557-005-5686-z
- Mosshammer D, Lorenz G, Meznaric S, Schwarz J, Muche R, Mörike K. Statin use and its association with musculoskeletal symptoms—a cross-sectional study in primary care settings. Fam Pract 2009; 26(2):88–95. doi:10.1093/fampra/cmp006
- Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007; 29(8):1761–1770. doi:10.1016/j.clinthera.2007.08.022
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72(9):996–1003. doi:10.1001/jamaneurol.2015.1207
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41(2):185–190. doi:10.1002/mus.21486
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9):2757–2766. doi:10.1002/art.27572
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3):713–721. doi:10.1002/art.30156
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373(17):1680–1682. doi:10.1056/NEJMc1506163
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014; 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52(5):1571–1576. doi:10.1002/art.21023
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009; 60(7):2193–2200. doi:10.1002/art.24621
- Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32(12):3909–3915. doi:10.1007/s00296-011-2323-y
- Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016; 68(5):689–694. doi:10.1002/acr.22728
- Aggarwal R, Dhillon N, Fertig N, Koontz D, Qi Z, Oddis CV. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J Rheumatol 2017; 44(2):223–229. doi:10.3899/jrheum.160618
- Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001; 80(5):320–327. doi:10.1097/00005792-200109000-00006
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279(1-2):107–115. doi:10.1016/S0009-8981(98)00180-6
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21(7):494–500. doi:10.1016/j.nmd.2011.04.007
- Johnston JD, Lloyd M, Mathews JA, Hawthorne SW. Racial variation in serum creatine kinase levels. J R Soc Med 1996; 89(8):462-464. pmid:8795501
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249(3):305–311. pmid:11993531
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253(11):1399–1403. doi:10.1007/s00415-006-0223-y
- Madariaga MG. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid 2002; 12(4):331–336. doi:10.1089/10507250252949478
- de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ; Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90(2):181–184. doi:10.1016/s0002-9149(02)02449-9
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19(6):403–414. doi:10.1007/s10557-005-5686-z
- Mosshammer D, Lorenz G, Meznaric S, Schwarz J, Muche R, Mörike K. Statin use and its association with musculoskeletal symptoms—a cross-sectional study in primary care settings. Fam Pract 2009; 26(2):88–95. doi:10.1093/fampra/cmp006
- Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007; 29(8):1761–1770. doi:10.1016/j.clinthera.2007.08.022
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72(9):996–1003. doi:10.1001/jamaneurol.2015.1207
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41(2):185–190. doi:10.1002/mus.21486
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9):2757–2766. doi:10.1002/art.27572
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3):713–721. doi:10.1002/art.30156
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373(17):1680–1682. doi:10.1056/NEJMc1506163
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014; 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52(5):1571–1576. doi:10.1002/art.21023
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009; 60(7):2193–2200. doi:10.1002/art.24621
- Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32(12):3909–3915. doi:10.1007/s00296-011-2323-y
- Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016; 68(5):689–694. doi:10.1002/acr.22728
- Aggarwal R, Dhillon N, Fertig N, Koontz D, Qi Z, Oddis CV. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J Rheumatol 2017; 44(2):223–229. doi:10.3899/jrheum.160618
KEY POINTS
- Inclusion body myositis affects older men more than women and is characterized by slowly progressive, asymmetric, distal and proximal weakness and atrophy.
- Statin-associated muscle complaints are common, whereas necrotizing myopathy, characterized by a very high CK plus weakness, is rare but must be recognized.
- Elevated CK does not necessarily indicate myositis, especially in African Americans or after heavy exercise.
- Dermatomyositis is characterized by muscle weakness and raised red or purple Gottron papules over the knuckles, elbows, or knees.
- Autoimmune interstitial lung disease may be caused by a variety of antibodies, the most common being anti-Jo-1 (directed against histidyl tRNA synthetase).
- The rarer non-Jo-1 antisynthetase autoantibodies may be associated with rapidly progressive interstitial lung disease, which is a challenge to recognize because associated rheumatologic symptoms may be minimal.
Autism, pain, and the NMDA receptor
Ms. G, a 36-year-old woman, presented to the emergency department (ED) requesting a neurologic evaluation. She told clinicians she had “NMDA receptor encephalitis.”
Ms. G reported successful self-treatment of “life-long” body pain that was precipitated by multiple external stimuli (food, social encounters, interpersonal conflict, etc.). Through her own research, she had learned that both ketamine and magnesium could alter nociception in rats through N-methyl-
In the ED, Ms. G had a labile affect, pressured speech, and flight of ideas. She denied any history of psychiatric treatment, suicide attempts, or substance abuse. Ms. G’s family reported she had been unusually social, talkative, and impulsive. She was admitted to the inpatient psychiatric unit with a diagnosis of mania.
On psychiatric evaluation, Ms. G was grandiose, irritable, and perseverative about her aberrant symptoms. She felt she did not experience the world as other people did, but found relief from her chronic pain after taking Delsym. She was not taking other medications. Ms. G did not report a family history of bipolar disorder or psychosis. Her laboratory results, including a comprehensive metabolic panel, complete blood count, lipid panel, thyroid studies, urine drug screening, and urinalysis, were unremarkable. Her blood pressure was mildly elevated (141/82 mm Hg).
Ms. G’s eventual diagnosis was substance-induced mania (DXM). The DXM-containing cough syrup and magnesium were discontinued in the hospital. She was stabilized on lithium extended-release, 900 mg/d (blood level 0.8 mmol/L), and olanzapine, 10 mg/d at bedtime. However, after discharge, Ms. G resumed using Delsym, which resulted in 3 subsequent psychiatric hospitalizations for mania during the next year.
I first treated Ms. G as an outpatient after her second hospitalization. At that point, she was stable. Her mental status was calm and cooperative, and she had a linear thought process. At her baseline, in the absence of mania, she had a blunted affect. She understood that DXM caused her to have manic symptoms, but she continued to believe that Delsym and magnesium cured her physical suffering and social inhibition. I noticed Ms. G would use figurative language inappropriately. I later learned she had sensitivities to food textures and a specialized interest in electronics. Because of this, I suspected Ms. G was on the autism spectrum; she met several DSM-5 criteria for autism spectrum disorder (ASD), particularly deficits in social-emotional reciprocity, highly restricted interests, and hyperreactivity to sensory input.
Upon routine lab screening, Ms. G was found to have hypothyroidism, with a thyroid-stimulating hormone level of 6.67 mcIU/mL. This resolved after discontinuing lithium. Olanzapine caused adverse metabolic effects and also was discontinued. Ms. G remained euthymic without any mood-stabilizing medication, except during periods when she abused DXM, when she would again become manic. Eventually, her motivation to avoid hospitalization would promote her abstinence.
Continue to: Implications of NMDA receptor antagonism
Implications of NMDA receptor antagonism
The use of ketamine as an NMDA receptor antagonist for treating depression and other psychiatric illnesses has gained momentum. Esketamine, the S-enantiomer of racemic ketamine, is now available as an FDA-approved intranasal formulation for treatment-resistant depression. Ketamine stops afferent nociception to the brain and is used as an analgesic (at low concentrations) and anesthetic (at high concentrations).1
Dextromethorphan is abused as a recreational drug because at high doses it works similarly to both ketamine and phencyclidine. Individuals who abuse DXM can develop psychosis, motor/cognitive impairment, agitation, fevers, hypertension, tachycardia, and death.2 In patients with ASD, researchers have identified genetic variations of NMDA receptors that are linked to dysfunction of these receptors.3 In animal models, as well as in humans, researchers have found that suppression or excitation of the NMDA receptor can ameliorate ASD symptoms, including social withdrawal and repetitive behaviors.3
Many individuals with ASD suffer from sensory abnormalities, including a reduced sensitivity to pain or a crippling sensitivity to various stimuli. Patients with ASD may have difficulty describing these abnormalities, and as a result, they may be misdiagnosed. One case report described a 15-year-old girl diagnosed with social anxiety and chronic generalized pain when in social situations.4 Pediatric rheumatologists had diagnosed her with “amplified pain syndrome.” When she presented to a mental health clinic for a neurodevelopmental evaluation, she explained to clinicians how she simply “did not ‘get’ people; they are just empty shells” and subsequently was given a diagnosis of ASD.4
In psychiatric patients who have comorbid substance use disorders, it is vital for clinicians to not only detect the presence of substance misuse, but also to understand what drives the patient toward abuse. Ms. G’s case, with its combination of substance abuse and ASD, illustrates the importance of listening to our patients for more precise diagnostic formulations, which then shape our treatment recommendations.
1. Vadivelu N, Schermer E, Kodumudi V, et al. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298-306.
2. Martinak B, Bolis R, Black J, et al. Dextromethorphan in cough syrup: the poor man’s psychosis. Psychopharmacol Bull. 2017;47(4):59-63.
3. Lee E, Choi S, Kim E. NMDA receptor dysfunction in autism spectrum disorders. Curr Opin Pharmacol. 2015;20:8-13.
4. Clarke C. Autism spectrum disorder and amplified pain. Case Rep Psychiatry. 2015;2015:930874. doi: 10.1155/2015/930874.
Ms. G, a 36-year-old woman, presented to the emergency department (ED) requesting a neurologic evaluation. She told clinicians she had “NMDA receptor encephalitis.”
Ms. G reported successful self-treatment of “life-long” body pain that was precipitated by multiple external stimuli (food, social encounters, interpersonal conflict, etc.). Through her own research, she had learned that both ketamine and magnesium could alter nociception in rats through N-methyl-
In the ED, Ms. G had a labile affect, pressured speech, and flight of ideas. She denied any history of psychiatric treatment, suicide attempts, or substance abuse. Ms. G’s family reported she had been unusually social, talkative, and impulsive. She was admitted to the inpatient psychiatric unit with a diagnosis of mania.
On psychiatric evaluation, Ms. G was grandiose, irritable, and perseverative about her aberrant symptoms. She felt she did not experience the world as other people did, but found relief from her chronic pain after taking Delsym. She was not taking other medications. Ms. G did not report a family history of bipolar disorder or psychosis. Her laboratory results, including a comprehensive metabolic panel, complete blood count, lipid panel, thyroid studies, urine drug screening, and urinalysis, were unremarkable. Her blood pressure was mildly elevated (141/82 mm Hg).
Ms. G’s eventual diagnosis was substance-induced mania (DXM). The DXM-containing cough syrup and magnesium were discontinued in the hospital. She was stabilized on lithium extended-release, 900 mg/d (blood level 0.8 mmol/L), and olanzapine, 10 mg/d at bedtime. However, after discharge, Ms. G resumed using Delsym, which resulted in 3 subsequent psychiatric hospitalizations for mania during the next year.
I first treated Ms. G as an outpatient after her second hospitalization. At that point, she was stable. Her mental status was calm and cooperative, and she had a linear thought process. At her baseline, in the absence of mania, she had a blunted affect. She understood that DXM caused her to have manic symptoms, but she continued to believe that Delsym and magnesium cured her physical suffering and social inhibition. I noticed Ms. G would use figurative language inappropriately. I later learned she had sensitivities to food textures and a specialized interest in electronics. Because of this, I suspected Ms. G was on the autism spectrum; she met several DSM-5 criteria for autism spectrum disorder (ASD), particularly deficits in social-emotional reciprocity, highly restricted interests, and hyperreactivity to sensory input.
Upon routine lab screening, Ms. G was found to have hypothyroidism, with a thyroid-stimulating hormone level of 6.67 mcIU/mL. This resolved after discontinuing lithium. Olanzapine caused adverse metabolic effects and also was discontinued. Ms. G remained euthymic without any mood-stabilizing medication, except during periods when she abused DXM, when she would again become manic. Eventually, her motivation to avoid hospitalization would promote her abstinence.
Continue to: Implications of NMDA receptor antagonism
Implications of NMDA receptor antagonism
The use of ketamine as an NMDA receptor antagonist for treating depression and other psychiatric illnesses has gained momentum. Esketamine, the S-enantiomer of racemic ketamine, is now available as an FDA-approved intranasal formulation for treatment-resistant depression. Ketamine stops afferent nociception to the brain and is used as an analgesic (at low concentrations) and anesthetic (at high concentrations).1
Dextromethorphan is abused as a recreational drug because at high doses it works similarly to both ketamine and phencyclidine. Individuals who abuse DXM can develop psychosis, motor/cognitive impairment, agitation, fevers, hypertension, tachycardia, and death.2 In patients with ASD, researchers have identified genetic variations of NMDA receptors that are linked to dysfunction of these receptors.3 In animal models, as well as in humans, researchers have found that suppression or excitation of the NMDA receptor can ameliorate ASD symptoms, including social withdrawal and repetitive behaviors.3
Many individuals with ASD suffer from sensory abnormalities, including a reduced sensitivity to pain or a crippling sensitivity to various stimuli. Patients with ASD may have difficulty describing these abnormalities, and as a result, they may be misdiagnosed. One case report described a 15-year-old girl diagnosed with social anxiety and chronic generalized pain when in social situations.4 Pediatric rheumatologists had diagnosed her with “amplified pain syndrome.” When she presented to a mental health clinic for a neurodevelopmental evaluation, she explained to clinicians how she simply “did not ‘get’ people; they are just empty shells” and subsequently was given a diagnosis of ASD.4
In psychiatric patients who have comorbid substance use disorders, it is vital for clinicians to not only detect the presence of substance misuse, but also to understand what drives the patient toward abuse. Ms. G’s case, with its combination of substance abuse and ASD, illustrates the importance of listening to our patients for more precise diagnostic formulations, which then shape our treatment recommendations.
Ms. G, a 36-year-old woman, presented to the emergency department (ED) requesting a neurologic evaluation. She told clinicians she had “NMDA receptor encephalitis.”
Ms. G reported successful self-treatment of “life-long” body pain that was precipitated by multiple external stimuli (food, social encounters, interpersonal conflict, etc.). Through her own research, she had learned that both ketamine and magnesium could alter nociception in rats through N-methyl-
In the ED, Ms. G had a labile affect, pressured speech, and flight of ideas. She denied any history of psychiatric treatment, suicide attempts, or substance abuse. Ms. G’s family reported she had been unusually social, talkative, and impulsive. She was admitted to the inpatient psychiatric unit with a diagnosis of mania.
On psychiatric evaluation, Ms. G was grandiose, irritable, and perseverative about her aberrant symptoms. She felt she did not experience the world as other people did, but found relief from her chronic pain after taking Delsym. She was not taking other medications. Ms. G did not report a family history of bipolar disorder or psychosis. Her laboratory results, including a comprehensive metabolic panel, complete blood count, lipid panel, thyroid studies, urine drug screening, and urinalysis, were unremarkable. Her blood pressure was mildly elevated (141/82 mm Hg).
Ms. G’s eventual diagnosis was substance-induced mania (DXM). The DXM-containing cough syrup and magnesium were discontinued in the hospital. She was stabilized on lithium extended-release, 900 mg/d (blood level 0.8 mmol/L), and olanzapine, 10 mg/d at bedtime. However, after discharge, Ms. G resumed using Delsym, which resulted in 3 subsequent psychiatric hospitalizations for mania during the next year.
I first treated Ms. G as an outpatient after her second hospitalization. At that point, she was stable. Her mental status was calm and cooperative, and she had a linear thought process. At her baseline, in the absence of mania, she had a blunted affect. She understood that DXM caused her to have manic symptoms, but she continued to believe that Delsym and magnesium cured her physical suffering and social inhibition. I noticed Ms. G would use figurative language inappropriately. I later learned she had sensitivities to food textures and a specialized interest in electronics. Because of this, I suspected Ms. G was on the autism spectrum; she met several DSM-5 criteria for autism spectrum disorder (ASD), particularly deficits in social-emotional reciprocity, highly restricted interests, and hyperreactivity to sensory input.
Upon routine lab screening, Ms. G was found to have hypothyroidism, with a thyroid-stimulating hormone level of 6.67 mcIU/mL. This resolved after discontinuing lithium. Olanzapine caused adverse metabolic effects and also was discontinued. Ms. G remained euthymic without any mood-stabilizing medication, except during periods when she abused DXM, when she would again become manic. Eventually, her motivation to avoid hospitalization would promote her abstinence.
Continue to: Implications of NMDA receptor antagonism
Implications of NMDA receptor antagonism
The use of ketamine as an NMDA receptor antagonist for treating depression and other psychiatric illnesses has gained momentum. Esketamine, the S-enantiomer of racemic ketamine, is now available as an FDA-approved intranasal formulation for treatment-resistant depression. Ketamine stops afferent nociception to the brain and is used as an analgesic (at low concentrations) and anesthetic (at high concentrations).1
Dextromethorphan is abused as a recreational drug because at high doses it works similarly to both ketamine and phencyclidine. Individuals who abuse DXM can develop psychosis, motor/cognitive impairment, agitation, fevers, hypertension, tachycardia, and death.2 In patients with ASD, researchers have identified genetic variations of NMDA receptors that are linked to dysfunction of these receptors.3 In animal models, as well as in humans, researchers have found that suppression or excitation of the NMDA receptor can ameliorate ASD symptoms, including social withdrawal and repetitive behaviors.3
Many individuals with ASD suffer from sensory abnormalities, including a reduced sensitivity to pain or a crippling sensitivity to various stimuli. Patients with ASD may have difficulty describing these abnormalities, and as a result, they may be misdiagnosed. One case report described a 15-year-old girl diagnosed with social anxiety and chronic generalized pain when in social situations.4 Pediatric rheumatologists had diagnosed her with “amplified pain syndrome.” When she presented to a mental health clinic for a neurodevelopmental evaluation, she explained to clinicians how she simply “did not ‘get’ people; they are just empty shells” and subsequently was given a diagnosis of ASD.4
In psychiatric patients who have comorbid substance use disorders, it is vital for clinicians to not only detect the presence of substance misuse, but also to understand what drives the patient toward abuse. Ms. G’s case, with its combination of substance abuse and ASD, illustrates the importance of listening to our patients for more precise diagnostic formulations, which then shape our treatment recommendations.
1. Vadivelu N, Schermer E, Kodumudi V, et al. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298-306.
2. Martinak B, Bolis R, Black J, et al. Dextromethorphan in cough syrup: the poor man’s psychosis. Psychopharmacol Bull. 2017;47(4):59-63.
3. Lee E, Choi S, Kim E. NMDA receptor dysfunction in autism spectrum disorders. Curr Opin Pharmacol. 2015;20:8-13.
4. Clarke C. Autism spectrum disorder and amplified pain. Case Rep Psychiatry. 2015;2015:930874. doi: 10.1155/2015/930874.
1. Vadivelu N, Schermer E, Kodumudi V, et al. Role of ketamine for analgesia in adults and children. J Anaesthesiol Clin Pharmacol. 2016;32(3):298-306.
2. Martinak B, Bolis R, Black J, et al. Dextromethorphan in cough syrup: the poor man’s psychosis. Psychopharmacol Bull. 2017;47(4):59-63.
3. Lee E, Choi S, Kim E. NMDA receptor dysfunction in autism spectrum disorders. Curr Opin Pharmacol. 2015;20:8-13.
4. Clarke C. Autism spectrum disorder and amplified pain. Case Rep Psychiatry. 2015;2015:930874. doi: 10.1155/2015/930874.
Does this patient have bacterial conjunctivitis?
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
A 54-year-old pharmacist with a history of gout, hypertension, and conjunctivitis presents for evaluation of pink eye in the summer. The morning before coming into the office, he noticed that his right eye was red and inflamed. He self-treated with saline washes and eye drops, but upon awakening the next day, he found his right eye to be crusted shut with surrounding yellow discharge. He has not had any changes to his vision but endorses a somewhat uncomfortable, “gritty” sensation. He reports no recent cough, nasal congestion, or allergies, and he has not been around any sick contacts. His blood pressure is 102/58 mm Hg, pulse is 76 bpm, and body mass index is 27.3 kg/m2. His eye exam reveals unilateral conjunctival injections but no hyperemia of the conjunctiva adjacent to the cornea. Mucopurulent discharge was neither found on the undersurface of the eyelid nor emerging from the eye. Which of the following is the best treatment for this patient’s condition?
A) Erythromycin 5 mg/gram ophthalmic ointment.
B) Ofloxacin 0.3% ophthalmic drops.
C) Antihistamine drops.
D) Eye lubricant drops.
E) No treatment necessary.
This patient is an adult presenting with presumed conjunctivitis. Because he is presenting in the summer without observed purulent discharge, his condition is unlikely to be bacterial. This patient does not need treatment, although eye lubricant drops could reduce his discomfort.
After ruling out serious eye disease, clinicians need to determine which cases of suspected conjunctivitis are most likely to be bacterial to allow for judicious use of antibiotic eye drops. This is an important undertaking as most patients assume that antibiotics are needed.
How do we know which history and clinical exam findings to lean on when attempting to categorize conjunctivitis as bacterial or not? If a patient reports purulent discharge, doesn’t that mean it is bacterial? Surprisingly, a systematic review published in 2016 by Narayana and McGee found that a patient’s self-report of “purulent drainage” is diagnostically unhelpful, but if a clinician finds it on exam, the likelihood of a bacterial etiology increases.3
Narayana and McGee analyzed three studies that enrolled a total of 281 patients with presumed conjunctivitis who underwent bacterial cultures. They then determined which findings increased the probability of positive bacterial culture. From strongest to weakest, the best indicators of a bacterial cause were found to be: complete redness of the conjunctival membrane obscuring tarsal vessels (the vessels visible on the inside of everted upper or lower eyelids) (likelihood ratio, 4.6), observed purulent discharge (LR, 3.9), matting of both eyes in the morning (LR, 3.6), and presence during winter/spring months (LR, 1.9). On the other hand, failure to observe a red eye at 20 feet (LR, 0.2), absence of morning gluing of either eye (LR, 0.3), and presentation during summer months (LR, 0.4) all decreased the probability of a bacterial cause. This review and different study by Stenson et al. unfortunately have conflicting evidence regarding whether the following findings are diagnostically helpful: qualities of eye discomfort (such as burning or itching), preauricular adenopathy, conjunctival follicles, and conjunctival papillae.3,4 Rietveld and colleagues found that a history of conjunctivitis decreased the likelihood of bacterial conjunctivitis.5
Ultimately, if the former indicators are kept in mind, primary care clinicians should be able to decrease the prescribing of topical antimicrobials to patients with non-bacterial conjunctivitis.
Pearl: The best indicators of a bacterial cause in patients with presumed conjunctivitis are complete redness of the conjunctival membrane obscuring tarsal vessels, observed purulent discharge, and matting of both eyes in the morning. Presentation during the summer months and having a history of conjunctivitis decreases the likelihood of bacterial conjunctivitis.
Ms. Momany is a fourth-year medical student at University of Washington, Seattle. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Azari AA and Barney NP. JAMA. 2013 Oct 23; 310(16):1721-9.
2. Smith AF and Waycaster C. BMC Ophthalmol. 2009 Nov 25. doi: 10.1186/1471-2415-9-13.
3) Narayana S and McGee S. Am J Med. 2015;128(11):1220-4.e1.
4) Stenson S et al. Arch Ophthalmol. 1982;100(8):1275-7.
5) Rietveld RP et al. BMJ. 2004 Jul 24;329(7459):206-10.
Left ear pain
The FP suspected cutaneous vasculitis of the ear caused by levamisole-adulterated cocaine.
Levamisole is an antihelminthic drug approved for veterinary purposes. In the past, the drug had been used as an immune modulator in autoimmune disorders, but no longer is considered safe for human use, as it can cause agranulocytosis. Sellers around the world often lace cocaine with levamisole because it boosts the profits and potentiates the psychoactive effects of the cocaine. Cutaneous vasculitis secondary to levamisole-adulterated cocaine has been reported many times in the literature.
Levamisole-associated vasculitis presents with ear purpura, retiform (like a net) purpura of the trunk or extremities, and neutropenia. Patients will test positive for perinuclear antineutrophil cytoplasmic antibody (pANCA). This cutaneous vasculitis also may present on the nose or face. There are reports of cocaine/levamisole-associated autoimmune syndrome involving agranulocytosis and cutaneous vasculitis.
The patient tested positive for pANCA, as was expected. The FP told her to discontinue her cocaine use, as she ran the risk of worse manifestations. She refused any treatment for her drug use and stated she could stop it on her own. The FP referred the patient to Dermatology, but the vasculitis was barely visible by the time she was seen. Convincing the patient not to use cocaine again remained the only treatment.
Photo courtesy of Jon Karnes, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected cutaneous vasculitis of the ear caused by levamisole-adulterated cocaine.
Levamisole is an antihelminthic drug approved for veterinary purposes. In the past, the drug had been used as an immune modulator in autoimmune disorders, but no longer is considered safe for human use, as it can cause agranulocytosis. Sellers around the world often lace cocaine with levamisole because it boosts the profits and potentiates the psychoactive effects of the cocaine. Cutaneous vasculitis secondary to levamisole-adulterated cocaine has been reported many times in the literature.
Levamisole-associated vasculitis presents with ear purpura, retiform (like a net) purpura of the trunk or extremities, and neutropenia. Patients will test positive for perinuclear antineutrophil cytoplasmic antibody (pANCA). This cutaneous vasculitis also may present on the nose or face. There are reports of cocaine/levamisole-associated autoimmune syndrome involving agranulocytosis and cutaneous vasculitis.
The patient tested positive for pANCA, as was expected. The FP told her to discontinue her cocaine use, as she ran the risk of worse manifestations. She refused any treatment for her drug use and stated she could stop it on her own. The FP referred the patient to Dermatology, but the vasculitis was barely visible by the time she was seen. Convincing the patient not to use cocaine again remained the only treatment.
Photo courtesy of Jon Karnes, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected cutaneous vasculitis of the ear caused by levamisole-adulterated cocaine.
Levamisole is an antihelminthic drug approved for veterinary purposes. In the past, the drug had been used as an immune modulator in autoimmune disorders, but no longer is considered safe for human use, as it can cause agranulocytosis. Sellers around the world often lace cocaine with levamisole because it boosts the profits and potentiates the psychoactive effects of the cocaine. Cutaneous vasculitis secondary to levamisole-adulterated cocaine has been reported many times in the literature.
Levamisole-associated vasculitis presents with ear purpura, retiform (like a net) purpura of the trunk or extremities, and neutropenia. Patients will test positive for perinuclear antineutrophil cytoplasmic antibody (pANCA). This cutaneous vasculitis also may present on the nose or face. There are reports of cocaine/levamisole-associated autoimmune syndrome involving agranulocytosis and cutaneous vasculitis.
The patient tested positive for pANCA, as was expected. The FP told her to discontinue her cocaine use, as she ran the risk of worse manifestations. She refused any treatment for her drug use and stated she could stop it on her own. The FP referred the patient to Dermatology, but the vasculitis was barely visible by the time she was seen. Convincing the patient not to use cocaine again remained the only treatment.
Photo courtesy of Jon Karnes, MD, and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Sensory feedback may smooth walking with a prosthetic leg
A prosthetic leg that elicits the sensation of knee motion and the feeling of the sole of the foot touching the ground may improve walking performance and reduce phantom limb pain, according to a proof-of-concept study with two patients.
With the bionic leg system, the patients performed better during clinically important tests indoors and outdoors, study author Stanisa Raspopovic, PhD, explained during a press briefing about the research. The findings were published in Nature Medicine.
The results indicate that the use of sensory feedback “could be common practice” in prosthetic devices in the future, he said. Dr. Raspopovic is a researcher at Swiss Federal Institute of Technology Zürich and a founder of SensArs Neuroprosthetics, which is based in Lausanne, Switzerland.
Neural prosthetics allow the nervous system and external devices to interact. These brain-machine interfaces may improve quality of life for patients with brain or spinal cord injuries, degenerative disease, or loss of limbs.
“Conventional leg prostheses do not convey sensory information about motion or interaction with the ground to above-knee amputees, thereby reducing confidence and walking speed in the users,” the study authors wrote. Users may also have high levels of mental and physical fatigue, and the lack of physiologic feedback from the extremity to the brain may contribute to the generation of phantom limb pain.
To evaluate whether neural sensory feedback restoration could address these issues, investigators conducted a study with two patients who had undergone transfemoral amputations as a result of traumatic events. The patients were implanted with four intraneural stimulation electrodes in the remaining tibial nerve. The prosthetic leg device included sensors to represent foot touch and pressure and knee joint angle. The sensors transmitted sensory signals to the nervous system through the stimulation electrodes in the tibial nerve.
When the patients walked outdoors over a path traced in the sand, “participants’ speeds were significantly higher when sensory feedback was provided,” the authors wrote. One participant walked 3.56 m/min faster, and the other walked 5.68 m/min faster.
The participants also rated their confidence in the prosthesis on a scale from 0 to 10. For patient 1, self-rated confidence improved from 4.85 to 7.71 with the device. Patient 2 reported a confidence level that climbed from 2.7 to 5.55.
When tested indoors, both patients reached a 0.5 km/hour higher speed on the treadmill when stimulation was provided and both had a lower mean rate of oxygen uptake during the sensory feedback trials, the study authors reported.
Levels of phantom limb pain also decreased significantly after 10-minute stimulation sessions, but not during control sessions.
Longer studies with more patients are required, and fully implantable devices without transcutaneous cables need to be developed, the authors wrote.
Grants from the European Research Council, European Commission, and Swiss National Science Foundation funded the research. Dr. Raspopovic and two coauthors hold shares of SensArs Neuroprosthetics, a start-up company dealing with the commercialization of neurocontrolled artificial limbs.
SOURCE: Petrini FM et al. Nat Med. 2019 Sep 9. doi: 10.1038/s41591-019-0567-3.
A prosthetic leg that elicits the sensation of knee motion and the feeling of the sole of the foot touching the ground may improve walking performance and reduce phantom limb pain, according to a proof-of-concept study with two patients.
With the bionic leg system, the patients performed better during clinically important tests indoors and outdoors, study author Stanisa Raspopovic, PhD, explained during a press briefing about the research. The findings were published in Nature Medicine.
The results indicate that the use of sensory feedback “could be common practice” in prosthetic devices in the future, he said. Dr. Raspopovic is a researcher at Swiss Federal Institute of Technology Zürich and a founder of SensArs Neuroprosthetics, which is based in Lausanne, Switzerland.
Neural prosthetics allow the nervous system and external devices to interact. These brain-machine interfaces may improve quality of life for patients with brain or spinal cord injuries, degenerative disease, or loss of limbs.
“Conventional leg prostheses do not convey sensory information about motion or interaction with the ground to above-knee amputees, thereby reducing confidence and walking speed in the users,” the study authors wrote. Users may also have high levels of mental and physical fatigue, and the lack of physiologic feedback from the extremity to the brain may contribute to the generation of phantom limb pain.
To evaluate whether neural sensory feedback restoration could address these issues, investigators conducted a study with two patients who had undergone transfemoral amputations as a result of traumatic events. The patients were implanted with four intraneural stimulation electrodes in the remaining tibial nerve. The prosthetic leg device included sensors to represent foot touch and pressure and knee joint angle. The sensors transmitted sensory signals to the nervous system through the stimulation electrodes in the tibial nerve.
When the patients walked outdoors over a path traced in the sand, “participants’ speeds were significantly higher when sensory feedback was provided,” the authors wrote. One participant walked 3.56 m/min faster, and the other walked 5.68 m/min faster.
The participants also rated their confidence in the prosthesis on a scale from 0 to 10. For patient 1, self-rated confidence improved from 4.85 to 7.71 with the device. Patient 2 reported a confidence level that climbed from 2.7 to 5.55.
When tested indoors, both patients reached a 0.5 km/hour higher speed on the treadmill when stimulation was provided and both had a lower mean rate of oxygen uptake during the sensory feedback trials, the study authors reported.
Levels of phantom limb pain also decreased significantly after 10-minute stimulation sessions, but not during control sessions.
Longer studies with more patients are required, and fully implantable devices without transcutaneous cables need to be developed, the authors wrote.
Grants from the European Research Council, European Commission, and Swiss National Science Foundation funded the research. Dr. Raspopovic and two coauthors hold shares of SensArs Neuroprosthetics, a start-up company dealing with the commercialization of neurocontrolled artificial limbs.
SOURCE: Petrini FM et al. Nat Med. 2019 Sep 9. doi: 10.1038/s41591-019-0567-3.
A prosthetic leg that elicits the sensation of knee motion and the feeling of the sole of the foot touching the ground may improve walking performance and reduce phantom limb pain, according to a proof-of-concept study with two patients.
With the bionic leg system, the patients performed better during clinically important tests indoors and outdoors, study author Stanisa Raspopovic, PhD, explained during a press briefing about the research. The findings were published in Nature Medicine.
The results indicate that the use of sensory feedback “could be common practice” in prosthetic devices in the future, he said. Dr. Raspopovic is a researcher at Swiss Federal Institute of Technology Zürich and a founder of SensArs Neuroprosthetics, which is based in Lausanne, Switzerland.
Neural prosthetics allow the nervous system and external devices to interact. These brain-machine interfaces may improve quality of life for patients with brain or spinal cord injuries, degenerative disease, or loss of limbs.
“Conventional leg prostheses do not convey sensory information about motion or interaction with the ground to above-knee amputees, thereby reducing confidence and walking speed in the users,” the study authors wrote. Users may also have high levels of mental and physical fatigue, and the lack of physiologic feedback from the extremity to the brain may contribute to the generation of phantom limb pain.
To evaluate whether neural sensory feedback restoration could address these issues, investigators conducted a study with two patients who had undergone transfemoral amputations as a result of traumatic events. The patients were implanted with four intraneural stimulation electrodes in the remaining tibial nerve. The prosthetic leg device included sensors to represent foot touch and pressure and knee joint angle. The sensors transmitted sensory signals to the nervous system through the stimulation electrodes in the tibial nerve.
When the patients walked outdoors over a path traced in the sand, “participants’ speeds were significantly higher when sensory feedback was provided,” the authors wrote. One participant walked 3.56 m/min faster, and the other walked 5.68 m/min faster.
The participants also rated their confidence in the prosthesis on a scale from 0 to 10. For patient 1, self-rated confidence improved from 4.85 to 7.71 with the device. Patient 2 reported a confidence level that climbed from 2.7 to 5.55.
When tested indoors, both patients reached a 0.5 km/hour higher speed on the treadmill when stimulation was provided and both had a lower mean rate of oxygen uptake during the sensory feedback trials, the study authors reported.
Levels of phantom limb pain also decreased significantly after 10-minute stimulation sessions, but not during control sessions.
Longer studies with more patients are required, and fully implantable devices without transcutaneous cables need to be developed, the authors wrote.
Grants from the European Research Council, European Commission, and Swiss National Science Foundation funded the research. Dr. Raspopovic and two coauthors hold shares of SensArs Neuroprosthetics, a start-up company dealing with the commercialization of neurocontrolled artificial limbs.
SOURCE: Petrini FM et al. Nat Med. 2019 Sep 9. doi: 10.1038/s41591-019-0567-3.
FROM NATURE MEDICINE
Migraines linked to higher risk of dementia
, according to research published online Sept. 4 in the International Journal of Geriatric Psychiatry.
In the Manitoba Study of Health and Aging, a population-based, prospective cohort study, 679 community-dwelling adults with a mean age of 75.9 years were followed for 5 years. Participants screened as cognitively intact at baseline had complete data on migraine history and all covariates at baseline and were assessed for cognitive outcomes 5 years later.
The study showed that a history of migraines was associated with a 2.97-fold greater likelihood of dementia, after adjustment for age, education, and a history of stroke, compared with individuals without a history of migraine. Individuals with Alzheimer’s disease were more than four times more likely to have a history of migraines (odds ratio 4.22).
However, researchers found no significant association between vascular dementia and a history of migraines, either before or after adjusting for confounders but particularly after incorporating a history of stroke into the model.
Lead investigator Suzanne L. Tyas, PhD, associate professor in the School of Public Health and Health Systems at the University of Waterloo, Ont., and coauthors suggested that the association between migraine and dementia was largely driven by the strong association between migraines and Alzheimer’s disease.
“This interpretation is supported by the weaker association for dementia than for Alzheimer’s disease, reflecting a dilution of the association with migraines across all types of dementia including vascular dementia, where a significant association was not found,” the researchers wrote.
The study population was 61.9% female, and no men reporting a history of migraine were diagnosed with dementia. While the study reflected a strong association between migraine and dementia in women, the researchers said they were unable to assess potential gender differences in this association.
Commenting on possible mechanisms behind the association, the authors wrote that there were overlaps underlying the biological mechanisms of migraine and dementia. Vascular risk factors such as diabetes, hypertension, heart attack, and stroke are associated with the development of dementia, and a relationship of these risk factors and migraine also has been seen.
“Many of the mechanisms involved in migraine neurophysiology, such as inflammation and reduced cerebral blood flow, are also underlying causes of dementia,” they wrote. “Repeated activation of these pathways in chronic migraineurs has been shown to cause permanent neurological and vascular damage.”
They also observed that the association could be influenced by genetic factors, as individuals with presenilin-1 mutations, which predispose them to Alzheimer’s disease, are more likely to experience migraines or recurrent headaches.
They suggested their findings could inform preventive strategies and treatments for Alzheimer’s disease, as well as interventions such as earlier screening for cognitive decline in individuals who experience migraines.
The study was funded by Manitoba Health and the National Health Research and Development Program of Health Canada. No conflicts of interest were declared.
SOURCE: Morton R et al. Int J Geriatr Psychiatry, 2019 Sep 4. doi: 10.1002/gps.5180.
, according to research published online Sept. 4 in the International Journal of Geriatric Psychiatry.
In the Manitoba Study of Health and Aging, a population-based, prospective cohort study, 679 community-dwelling adults with a mean age of 75.9 years were followed for 5 years. Participants screened as cognitively intact at baseline had complete data on migraine history and all covariates at baseline and were assessed for cognitive outcomes 5 years later.
The study showed that a history of migraines was associated with a 2.97-fold greater likelihood of dementia, after adjustment for age, education, and a history of stroke, compared with individuals without a history of migraine. Individuals with Alzheimer’s disease were more than four times more likely to have a history of migraines (odds ratio 4.22).
However, researchers found no significant association between vascular dementia and a history of migraines, either before or after adjusting for confounders but particularly after incorporating a history of stroke into the model.
Lead investigator Suzanne L. Tyas, PhD, associate professor in the School of Public Health and Health Systems at the University of Waterloo, Ont., and coauthors suggested that the association between migraine and dementia was largely driven by the strong association between migraines and Alzheimer’s disease.
“This interpretation is supported by the weaker association for dementia than for Alzheimer’s disease, reflecting a dilution of the association with migraines across all types of dementia including vascular dementia, where a significant association was not found,” the researchers wrote.
The study population was 61.9% female, and no men reporting a history of migraine were diagnosed with dementia. While the study reflected a strong association between migraine and dementia in women, the researchers said they were unable to assess potential gender differences in this association.
Commenting on possible mechanisms behind the association, the authors wrote that there were overlaps underlying the biological mechanisms of migraine and dementia. Vascular risk factors such as diabetes, hypertension, heart attack, and stroke are associated with the development of dementia, and a relationship of these risk factors and migraine also has been seen.
“Many of the mechanisms involved in migraine neurophysiology, such as inflammation and reduced cerebral blood flow, are also underlying causes of dementia,” they wrote. “Repeated activation of these pathways in chronic migraineurs has been shown to cause permanent neurological and vascular damage.”
They also observed that the association could be influenced by genetic factors, as individuals with presenilin-1 mutations, which predispose them to Alzheimer’s disease, are more likely to experience migraines or recurrent headaches.
They suggested their findings could inform preventive strategies and treatments for Alzheimer’s disease, as well as interventions such as earlier screening for cognitive decline in individuals who experience migraines.
The study was funded by Manitoba Health and the National Health Research and Development Program of Health Canada. No conflicts of interest were declared.
SOURCE: Morton R et al. Int J Geriatr Psychiatry, 2019 Sep 4. doi: 10.1002/gps.5180.
, according to research published online Sept. 4 in the International Journal of Geriatric Psychiatry.
In the Manitoba Study of Health and Aging, a population-based, prospective cohort study, 679 community-dwelling adults with a mean age of 75.9 years were followed for 5 years. Participants screened as cognitively intact at baseline had complete data on migraine history and all covariates at baseline and were assessed for cognitive outcomes 5 years later.
The study showed that a history of migraines was associated with a 2.97-fold greater likelihood of dementia, after adjustment for age, education, and a history of stroke, compared with individuals without a history of migraine. Individuals with Alzheimer’s disease were more than four times more likely to have a history of migraines (odds ratio 4.22).
However, researchers found no significant association between vascular dementia and a history of migraines, either before or after adjusting for confounders but particularly after incorporating a history of stroke into the model.
Lead investigator Suzanne L. Tyas, PhD, associate professor in the School of Public Health and Health Systems at the University of Waterloo, Ont., and coauthors suggested that the association between migraine and dementia was largely driven by the strong association between migraines and Alzheimer’s disease.
“This interpretation is supported by the weaker association for dementia than for Alzheimer’s disease, reflecting a dilution of the association with migraines across all types of dementia including vascular dementia, where a significant association was not found,” the researchers wrote.
The study population was 61.9% female, and no men reporting a history of migraine were diagnosed with dementia. While the study reflected a strong association between migraine and dementia in women, the researchers said they were unable to assess potential gender differences in this association.
Commenting on possible mechanisms behind the association, the authors wrote that there were overlaps underlying the biological mechanisms of migraine and dementia. Vascular risk factors such as diabetes, hypertension, heart attack, and stroke are associated with the development of dementia, and a relationship of these risk factors and migraine also has been seen.
“Many of the mechanisms involved in migraine neurophysiology, such as inflammation and reduced cerebral blood flow, are also underlying causes of dementia,” they wrote. “Repeated activation of these pathways in chronic migraineurs has been shown to cause permanent neurological and vascular damage.”
They also observed that the association could be influenced by genetic factors, as individuals with presenilin-1 mutations, which predispose them to Alzheimer’s disease, are more likely to experience migraines or recurrent headaches.
They suggested their findings could inform preventive strategies and treatments for Alzheimer’s disease, as well as interventions such as earlier screening for cognitive decline in individuals who experience migraines.
The study was funded by Manitoba Health and the National Health Research and Development Program of Health Canada. No conflicts of interest were declared.
SOURCE: Morton R et al. Int J Geriatr Psychiatry, 2019 Sep 4. doi: 10.1002/gps.5180.
FROM THE INTERNATIONAL JOURNAL OF GERIATRIC PSYCHIATRY
Which oral nonopioid agents are most effective for OA pain?
EVIDENCE SUMMARY
All NSAIDs at maximum clinical doses reduced large joint OA pain more effectively than placebo and acetaminophen based on data from a network meta-analysis of 129 RCTs with 32,129 patients (TABLE 1).1 When various doses of NSAIDs are ranked for efficacy based on their effect size compared to placebo, diclofenac 150 mg/d had the greatest treatment effect, followed by ibuprofen 2400 mg/d.2 Lower doses of NSAIDs—including diclofenac 70 mg/d, naproxen 750 mg/d, and ibuprofen 1200 mg/d—were not statistically superior to placebo (TABLE 2).2
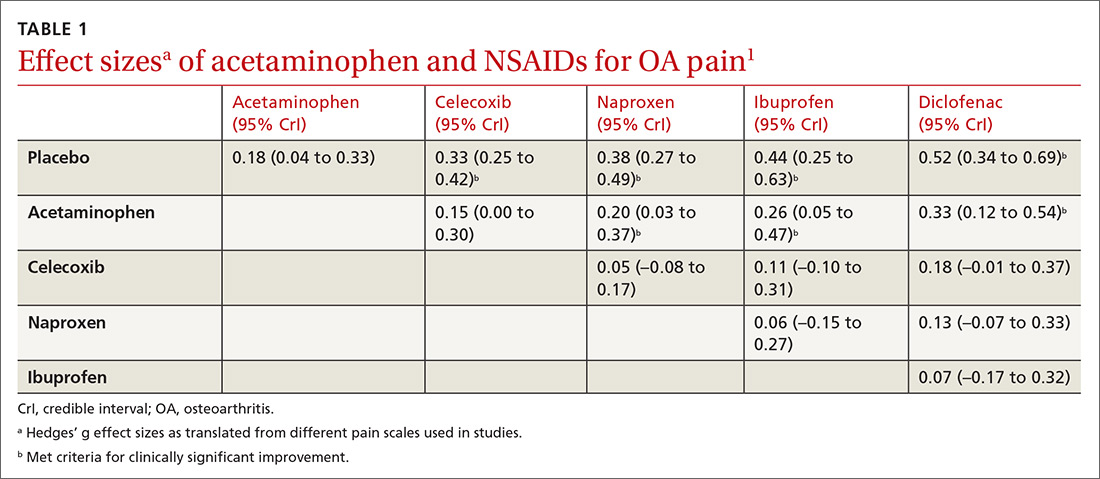
Selective vs nonselective. There was no statistical difference in pain relief between the selective COX-2 inhibitor celecoxib and the nonselective NSAIDs naproxen, diclofenac, and ibuprofen (TABLE 1).1
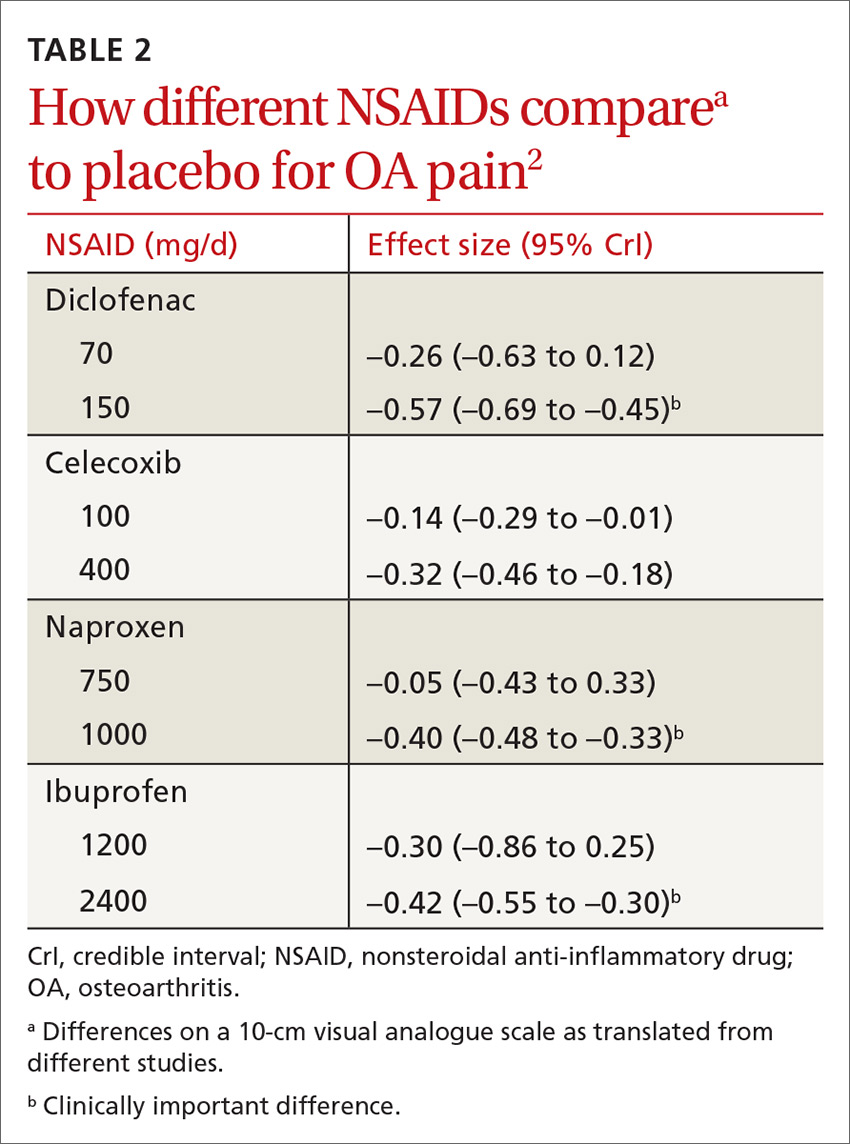
Meloxicam. A systematic review of 16 RCTs and 22,886 patients found that meloxicam reduced pain more effectively than placebo (10-point visual analogue scale [VAS] score pain difference of –6.8; 95% CI, –9.3 to –4.2) but was marginally less effective than other NSAIDs (VAS score pain difference of 1.7; 95% CI, 0.8 to 2.7).3
Acetaminophen. Data from 6 RCTs involving 2083 adults with knee OA indicate acetaminophen did not achieve clinical significance compared to placebo (TABLE 1).1 Another meta-analysis of 5 RCTs involving 1741 patients with hip or knee OA also demonstrated that acetaminophen failed to achieve a clinically significant effect on pain, defined as a reduction of 9 mm on a 0 to 100 mm VAS (–3.7; 95% CI, –5.5 to –1.9).4 Another network meta-analysis of 6 RCTs including 58,556 patients with knee or hip OA, with the primary outcome of pain (using a hierarchy of pain scores, with global pain score taking precedence) also found no clinically significant difference between acetaminophen at the highest dose (4000 mg/d) and placebo (–0.17; 95% credible interval [CrI], –0.27 to –0.6).2
RECOMMENDATIONS
In a systematic review of mixed evidence-based and expert opinion recommendations and guidelines on the management of OA, 10 of the 11 guidelines that included pharmacologic management recommended acetaminophen as a first-line agent, followed by topical NSAIDs, and then oral NSAIDs. The exception is the most recent American Academy of Orthopaedic Surgeons guideline, which continues to recommend NSAIDs but is now unable to recommend for or against acetaminophen.5
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
2. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e23-e33.
3. Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1-278, iii.
4. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
5. Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712.
EVIDENCE SUMMARY
All NSAIDs at maximum clinical doses reduced large joint OA pain more effectively than placebo and acetaminophen based on data from a network meta-analysis of 129 RCTs with 32,129 patients (TABLE 1).1 When various doses of NSAIDs are ranked for efficacy based on their effect size compared to placebo, diclofenac 150 mg/d had the greatest treatment effect, followed by ibuprofen 2400 mg/d.2 Lower doses of NSAIDs—including diclofenac 70 mg/d, naproxen 750 mg/d, and ibuprofen 1200 mg/d—were not statistically superior to placebo (TABLE 2).2

Selective vs nonselective. There was no statistical difference in pain relief between the selective COX-2 inhibitor celecoxib and the nonselective NSAIDs naproxen, diclofenac, and ibuprofen (TABLE 1).1

Meloxicam. A systematic review of 16 RCTs and 22,886 patients found that meloxicam reduced pain more effectively than placebo (10-point visual analogue scale [VAS] score pain difference of –6.8; 95% CI, –9.3 to –4.2) but was marginally less effective than other NSAIDs (VAS score pain difference of 1.7; 95% CI, 0.8 to 2.7).3
Acetaminophen. Data from 6 RCTs involving 2083 adults with knee OA indicate acetaminophen did not achieve clinical significance compared to placebo (TABLE 1).1 Another meta-analysis of 5 RCTs involving 1741 patients with hip or knee OA also demonstrated that acetaminophen failed to achieve a clinically significant effect on pain, defined as a reduction of 9 mm on a 0 to 100 mm VAS (–3.7; 95% CI, –5.5 to –1.9).4 Another network meta-analysis of 6 RCTs including 58,556 patients with knee or hip OA, with the primary outcome of pain (using a hierarchy of pain scores, with global pain score taking precedence) also found no clinically significant difference between acetaminophen at the highest dose (4000 mg/d) and placebo (–0.17; 95% credible interval [CrI], –0.27 to –0.6).2
RECOMMENDATIONS
In a systematic review of mixed evidence-based and expert opinion recommendations and guidelines on the management of OA, 10 of the 11 guidelines that included pharmacologic management recommended acetaminophen as a first-line agent, followed by topical NSAIDs, and then oral NSAIDs. The exception is the most recent American Academy of Orthopaedic Surgeons guideline, which continues to recommend NSAIDs but is now unable to recommend for or against acetaminophen.5
EVIDENCE SUMMARY
All NSAIDs at maximum clinical doses reduced large joint OA pain more effectively than placebo and acetaminophen based on data from a network meta-analysis of 129 RCTs with 32,129 patients (TABLE 1).1 When various doses of NSAIDs are ranked for efficacy based on their effect size compared to placebo, diclofenac 150 mg/d had the greatest treatment effect, followed by ibuprofen 2400 mg/d.2 Lower doses of NSAIDs—including diclofenac 70 mg/d, naproxen 750 mg/d, and ibuprofen 1200 mg/d—were not statistically superior to placebo (TABLE 2).2

Selective vs nonselective. There was no statistical difference in pain relief between the selective COX-2 inhibitor celecoxib and the nonselective NSAIDs naproxen, diclofenac, and ibuprofen (TABLE 1).1

Meloxicam. A systematic review of 16 RCTs and 22,886 patients found that meloxicam reduced pain more effectively than placebo (10-point visual analogue scale [VAS] score pain difference of –6.8; 95% CI, –9.3 to –4.2) but was marginally less effective than other NSAIDs (VAS score pain difference of 1.7; 95% CI, 0.8 to 2.7).3
Acetaminophen. Data from 6 RCTs involving 2083 adults with knee OA indicate acetaminophen did not achieve clinical significance compared to placebo (TABLE 1).1 Another meta-analysis of 5 RCTs involving 1741 patients with hip or knee OA also demonstrated that acetaminophen failed to achieve a clinically significant effect on pain, defined as a reduction of 9 mm on a 0 to 100 mm VAS (–3.7; 95% CI, –5.5 to –1.9).4 Another network meta-analysis of 6 RCTs including 58,556 patients with knee or hip OA, with the primary outcome of pain (using a hierarchy of pain scores, with global pain score taking precedence) also found no clinically significant difference between acetaminophen at the highest dose (4000 mg/d) and placebo (–0.17; 95% credible interval [CrI], –0.27 to –0.6).2
RECOMMENDATIONS
In a systematic review of mixed evidence-based and expert opinion recommendations and guidelines on the management of OA, 10 of the 11 guidelines that included pharmacologic management recommended acetaminophen as a first-line agent, followed by topical NSAIDs, and then oral NSAIDs. The exception is the most recent American Academy of Orthopaedic Surgeons guideline, which continues to recommend NSAIDs but is now unable to recommend for or against acetaminophen.5
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
2. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e23-e33.
3. Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1-278, iii.
4. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
5. Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712.
1. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54.
2. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e23-e33.
3. Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1-278, iii.
4. Machado GC, Maher CG, Ferreira PH, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225.
5. Nelson AE, Allen KD, Golightly YM, et al. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712.
EVIDENCE-BASED ANSWER:
Nonsteroidal anti-inflammatory drugs (NSAIDs), when used at the maximum clinically effective dose, reduce osteoarthritis (OA) pain in large joints more effectively than either placebo or acetaminophen (strength of recommendation [SOR]: A, network meta-analysis of randomized controlled trials [RCTs]).
When ranked for efficacy, diclofenac 150 mg/d was the most effective (SOR: A, network meta-analysis of RCTs). The selective COX-2 inhibitors, such as celecoxib, are not more effective at reducing pain than the nonselective NSAIDs (SOR: A, meta-analysis of RCTs). Meloxicam is superior to placebo but marginally inferior to other NSAIDs (SOR: A, systematic review of RCTs).
Acetaminophen is no more effective than placebo (SOR: A, meta-analysis of RCTs).