User login
How best to address breast pain in nonbreastfeeding women
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
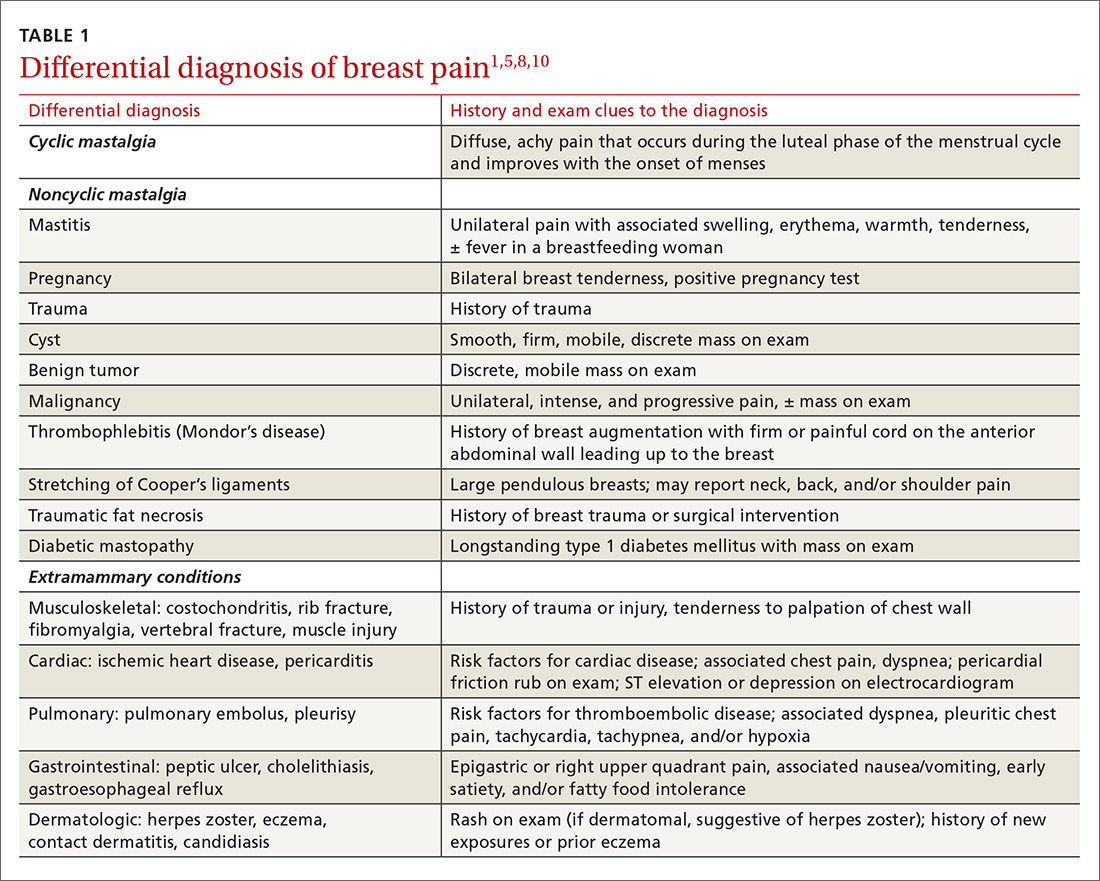
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).
Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
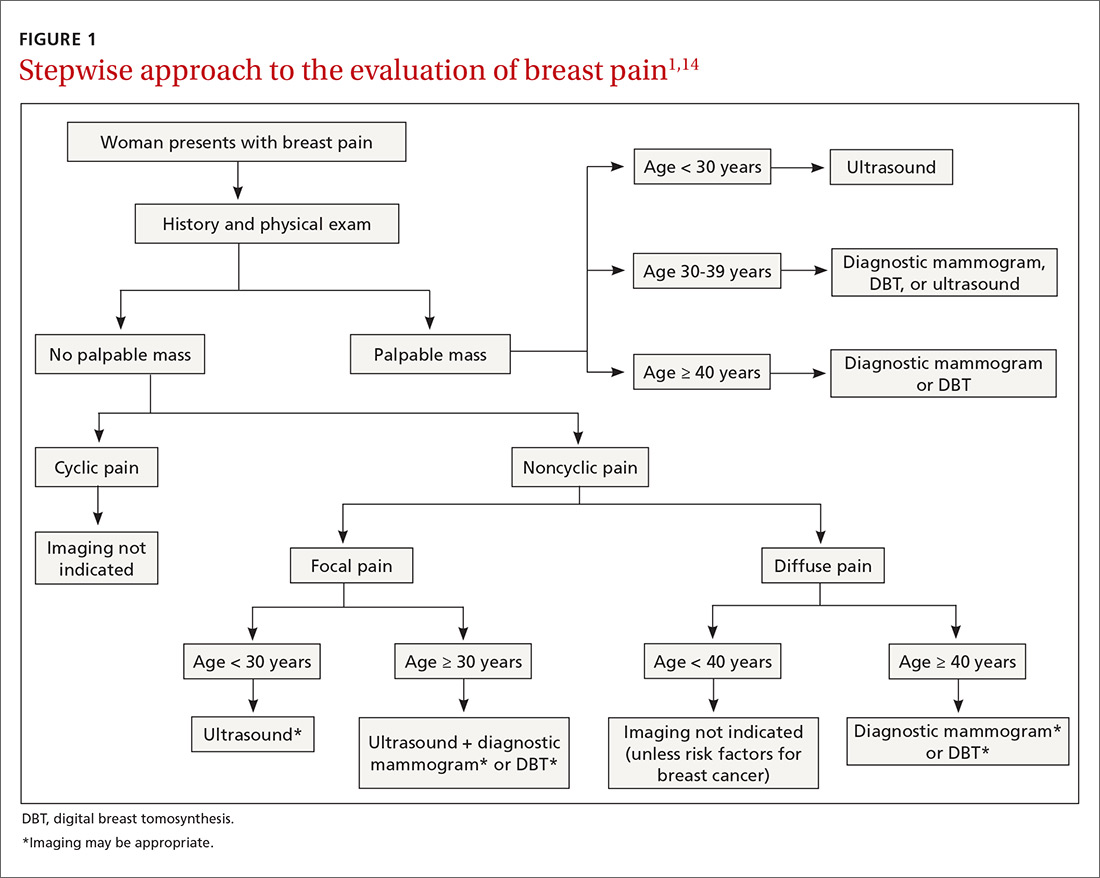
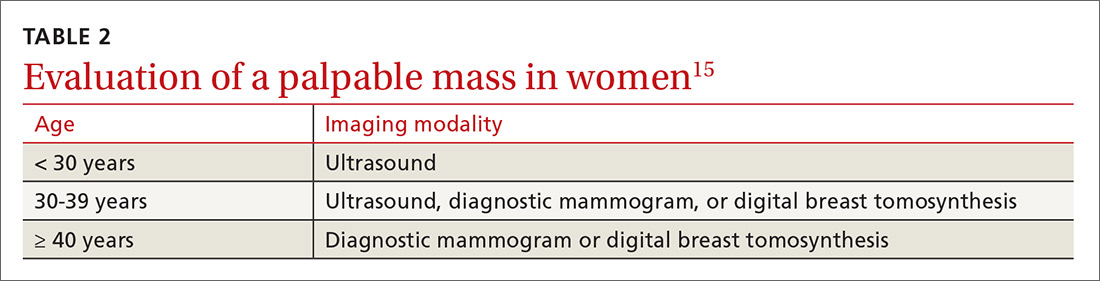
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15
Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15
Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
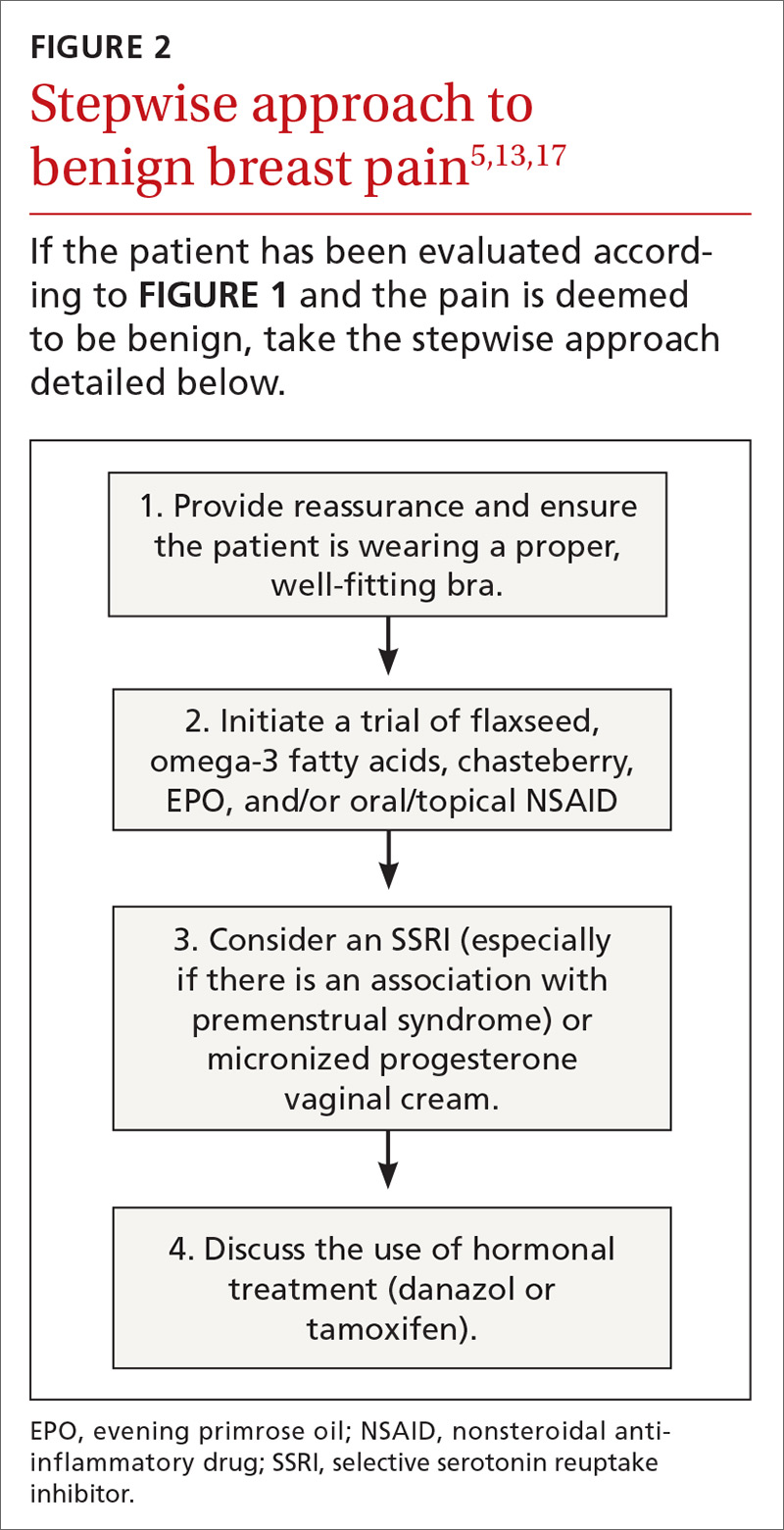
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).
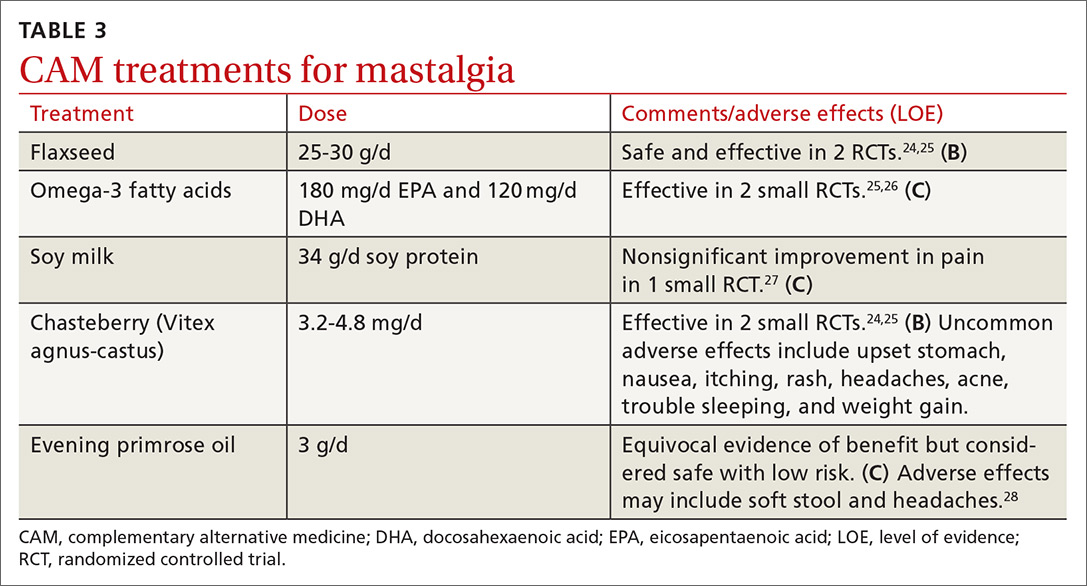
Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38
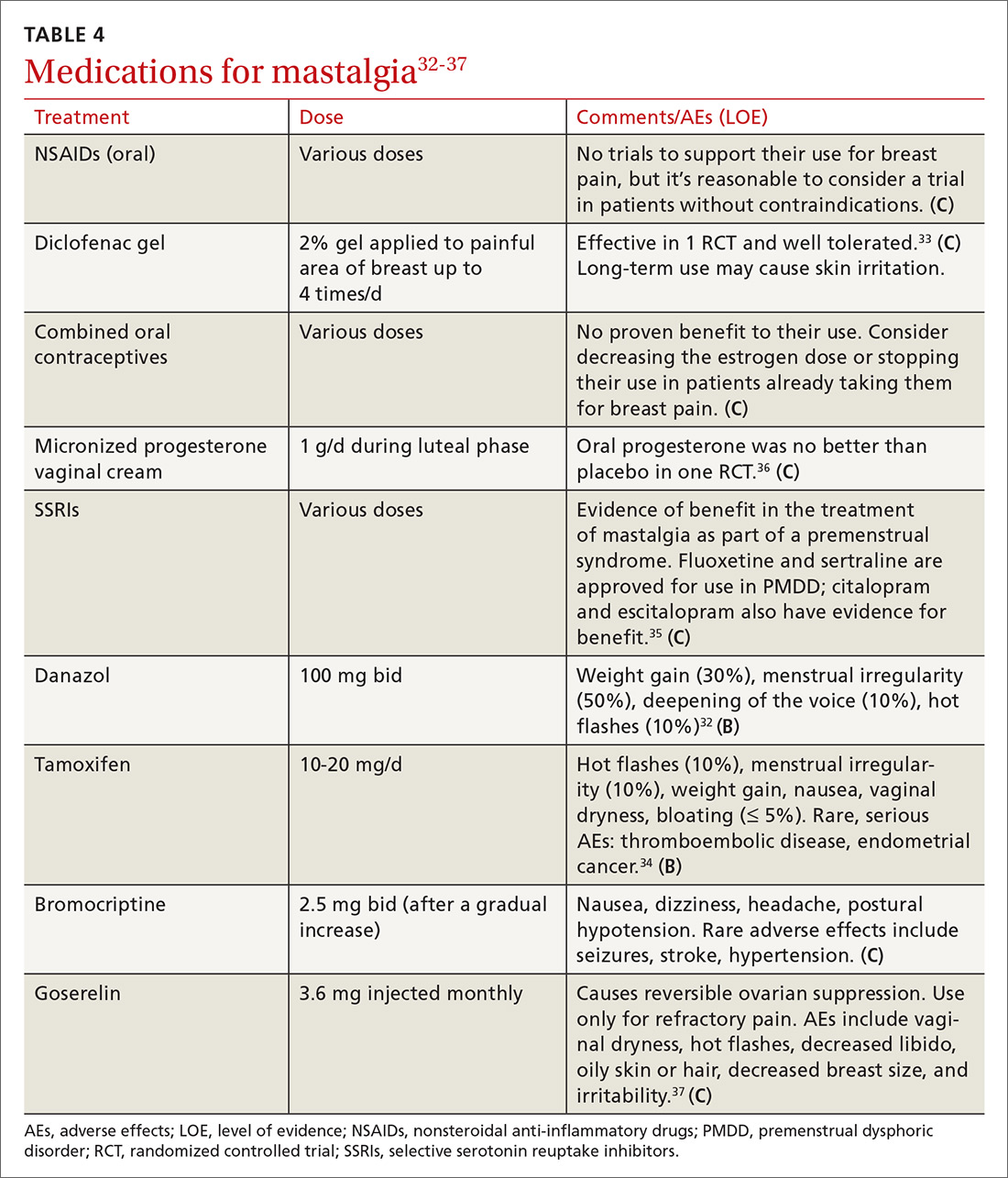
SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35
Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; [email protected].
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).

Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15

Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15

Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).

Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38

SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35

Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; [email protected].
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).

Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15

Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15

Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).

Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38

SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35

Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; [email protected].
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
PRACTICE RECOMMENDATIONS
› Instruct patients to maintain a pain diary, which, along with a careful history and physical examination, helps to determine the cause of breast pain and the type of evaluation needed. C
› Treat cyclic, bilateral breast pain with chasteberry and flaxseed. B
› Consider short-term treatment with danazol or tamoxifen for women with severe pain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CDC, SAMHSA commit $1.8 billion to combat opioid crisis
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
More financial reinforcements are arriving in the battle against the opioid crisis, with the Trump administration promising more than $1.8 billion in new funds to help states address the crisis.
Speaking at a Sept. 4 press conference announcing the funding, President Donald Trump said the money will be used “to increase access to medication and medication-assisted treatment and mental health resources, which are critical for ending homelessness and getting people the help they deserve.” The president added that the grants also will help state and local governments obtain high-quality, comprehensive data.
The Centers for Disease Control and Prevention will provide more than $900 million in new funding over the next 3 years to “advance the understanding of the opioid overdose epidemic and to scale-up prevention and response activities,” the Department of Health & Human Services said in a statement announcing the funding.
“This money will help states and local communities track overdose data and develop strategies that save lives,” HHS Secretary Alex Azar said during the press conference.
He noted that, when the Trump administration began, overdose data were published with a 12-month lag. That lag has since shortened to 6 months. One of the goals with the new funding is to bring data publishing as close to real time as possible.
Separately, the Substance Abuse and Mental Health Services Administration awarded $932 million to all 50 states as part of its State Opioid Response grants, which “provide flexible funding to state governments to support prevention, treatment, and recovery services in the ways that meet the needs of their state,” according to the HHS statement.
That flexibility “can mean everything from expanding the use of medication-assisted treatment in criminal justice settings or in rural areas via telemedicine, to youth-focused community-based prevention efforts,” Secretary Azar explained. The funds can also support employment coaching and naloxone distribution, he added.
Multispecialty Opioid Risk Reduction Program Targeting Chronic Pain and Addiction Management in Veterans
Chronic pain significantly affects 100 million Americans.1,2 Pain accounts for $560 to $635 billion in annual financial costs to society, including health care costs and loss of productivity (ie, days missed from work, hours of work lost, and lower wages).2,3 Although pain prevalence exceeds other chronic diseases, such as diabetes mellitus, cancer, and heart disease, it lacks a sufficient body of evidence-based research and guidelines on the underlying mechanisms, valid methods of assessment, and comparative effectiveness of treatments to effectively implement into clinical practice.2,4 Prevention and treatment of pain are often delayed, inaccessible, or inadequate.2 Primary care providers (PCPs) are most often sought for pain management and treat about 52% of chronic pain patients.2,3,5 Veterans are especially vulnerable to chronic pain and are at risk for inadequate treatment.2
Background
There is an epidemic of drug abuse and mortality from opioid prescription medication.6 In the US, rates of overdose deaths from prescription opioids were 6.1 per 100,000 for men and 4.2 per 100,000 for women in 2017. Opioids were involved in 47,600 overdose deaths in 2017, accounting for 67.8% of all drug overdose deaths.7
A large number of patients on long-term opioids have preexisting substance use disorders and/or psychiatric disease, further complicating chronic pain management.8-10 Prescription opioid use has been the precursor for about 80% of people who are now heroin addicts.11 Iatrogenic addiction from prescription medications isn’t easily captured by standard addiction criteria. Consequently, in patients who are on opioid therapy for prolonged periods, separating complex opioid dependence from addiction is difficult.12 Improved addiction screening and risk mitigation strategies are needed along with aggressive treatment monitoring to curb the opioid epidemic.
Opioid Management in Primary Care
The majority of opioid medications are prescribed by PCPs, which is magnified in the US Department of Veterans Affairs (VA) health care system due to the high prevalence of service-related injuries.3,13 The VA is at the forefront of addressing the complexities of opioid addiction through several initiatives.14 The ability to offer the frequent visits needed to safely manage patients prescribed opioids and the integration of mental health and addiction treatment are often lacking in non-VA primary care clinics. Therefore, a key to solving the opioid crisis is developing these capabilities so they can be delivered within the primary care setting. There is substantial evidence in support of nonopioid alternatives to chronic pain management, including other pharmacologic approaches, exercise, physical therapy, acupuncture, weight loss, smoking cessation, chiropractic care, cognitive behavioral therapy (CBT), and other integrative health modalities.
A 2009 VA directive mandated the development of a comprehensive, integrated, systemwide approach to pain management.15 The VA Stepped-Care Biopsychosocial Model for Pain Management is dependent on timely access to secondary consultation from pain medicine, behavioral health, physical medicine, and other specialty consultation.15
History of VHA SCAN-ECHO Model
The Specialty Care Access Network–Extension for Community Health Outcomes (SCAN-ECHO) is a Veterans Health Administration (VHA) adaptation of a program that originated at the University of Mexico.16,17 The SCAN-ECHO model uses a multisite videoconferencing network to provide specialty care consultations to PCPs and patient aligned care teams (PACTs). During the 60- to 90-minute weekly sessions, case presentations are analyzed in real time so that over time, the PCPs gain knowledge, competency, and confidence in learning how to handle complex chronic conditions.
Since its implementation, the SCAN-ECHO program has been adopted across the VHA in a variety of specialties. One program, the SCAN-ECHO for Pain Management (SCAN-ECHO-PM) was implemented in 7 VHA networks in 31 states, spanning 47 medical centers and 148 community-based outpatient clinics (CBOCs).18 The SCAN-ECHO-PM program successfully conducted 257 multidisciplinary pain consultations between 2011 and 2013, resulting in increased initiation of nonopioid medications.18
The aim of this article is to describe the implementation of a multicomponent primary care-based pain clinic with a fully integrated mental health service and addiction service at the North Florida/South Georgia Veterans Health System (NF/SGVHS). A practiced-based intervention of the biopsychosocial model with robust patient engagement has guided the development of the NF/SGVHS pain clinic (Figure 1).4,19
Pain CLinic
NF/SGVHS comprises the Malcom Randall and Lake City VA medical centers (VAMCs) hospitals, 3 satellite outpatient clinics, and 8 CBOCs. Spanning 33 counties in North Florida and 19 counties in South Georgia, the NF/SGVHS serves more than 140,000 patients. In 2010, the Malcom Randall VAMC established a multidisciplinary primary care pain clinic to manage veterans at high-risk for noncancer chronic pain and addiction. The noncancer pain policy was revised after garnering support from stakeholders who treat chronic pain, including the chiefs of psychiatry, rehabilitation medicine, neurosurgery, psychology, interventional pain, pharmacy, nursing, addiction medicine, and primary care. The clinic is staffed by primary care physicians trained in internal medicine and family medicine and is structured with 1-hour first visits, and 30-minute follow-up visits to allow enough time for comprehensive evaluation while meeting the needs for close follow-up support.
All physicians in the clinic have buprenorphine prescribing credentials to aid in the management of opioid addiction, as some patients feel more comfortable receiving addiction treatment in a primary care setting. The multimodal care model consists of several services that include addiction psychiatrists, interventional pain specialists, pain psychologists, and pain pharmacologists who coordinate the care to the veterans. The addiction psychiatrists offer a full range of services with inpatient residential and outpatient programs. Through recurring meetings with primary care pain clinic staff, the addiction psychiatrists are available to discuss use of buprenorphine and arrange follow-up for patients with complex pain addiction. There is ongoing collaboration to develop the best care plan that meets the patient’s needs for chronic pain, addiction, and/or mental health issues. The interventional pain service has 3 fellowship-trained pain care providers who deliver comprehensive evaluation, pharmacologic recommendations, and a full range of interventional and complementary therapies with an emphasis on objective functional improvement. Pain care providers offer alternatives to patients who are being weaned from opioids and support the multidisciplinary patient engagement model.
The pain psychology program, established in 2011, delivers CBT to 5 onsite locations and 5 telehealth locations. The service includes an advanced CBT program and a couples CBT program. The pharmacy pain fellowship program provides staff for an outpatient e-consult pain management service and an inpatient pharmacy consult service. Harnessing pain specialty pharmacists, the pharmacy service addresses pharmacokinetic issues, urine drug screen (UDS) results, opioid tapering and discharge planning for pain, addiction and mental health needs. The NF/SGVHS Primary Care Pain Clinic was established to support PCPs who did not feel comfortable managing chronic pain patients. These patients were typically on high-dose opioid therapy (> 100-mg morphine equivalent daily doses [MEDDs]); patients with a history of opioid addiction; patients with an addiction to opioids combined with benzodiazepines; and patients with comorbid medical issues (eg, sleep apnea), which complicated their management. The process of addressing opioid safety in these complex pain patients can be labor intensive and generally cannot be accomplished in a brief visit in a primary care setting where many other medical problems often need to be addressed.
Most patients on high-dose opioids are fearful of any changes in their medications. The difficult conversation regarding opioid safety is a lengthy one and frequently will occur over multiple visits. In addition, safely tapering opioids requires frequent follow-up to provide psychological support and to address withdrawal and mental health issues that may arise. As opioids are tapered, the clinic reinforces improved pain care through a multimodal biopsychosocial model. All veterans receiving pain care outside the VA are monitored annually to assure they are receiving evidence-based pain care as defined by the biopsychosocial model.
Education
Since 2011, the NF/SGVHS SCAN-ECHO pain and addiction educational forum has created > 50 hours of approved annual continuing medical education (CME) on pain management and addiction for PCPs. Initially, the 1-hour weekly educational audioconferences presented a pain management case along with related topics and involved specialists from interventional pain, physical therapy, psychiatry, nursing, neurology, and psychology departments. In 2013, in conjunction with the VA SCAN-ECHO program of Hunter Holmes McGuire VAMC in Richmond, Virginia, and Walter Reed National Military Medical Center in Bethesda, Maryland, the audioconference was expanded to 2 days each week with additional topics on addiction management. Residency and fellowship rotations were developed that specifically targeted fellows from psychiatry, pharmacology, and interventional pain departments.
Currently, an 8-session pain school is delivered onsite and at 7 telehealth locations. The school is a collaborative effort involving interventional pain, psychology, pharmacy, nutrition, and the primary care pain clinic staff. As the cornerstone of the program, the pain school stresses the biopsychosocial patient engagement model.
Program Evaluation
The VA is equipped with multiple telehealth service networks that allow for the delivery of programs, such as the pain school, a pain psychology program, and a yoga program, onsite or offsite. The VA Computerized Patient Record System (CPRS) manages electronic health records, allowing for rapid chart review and e-consults. The NF/SGVHS Pain Management Program provides about 1500 e-consults yearly. The CPRS includes templates with pain metrics to help PCPs deliver pain care more efficiently and evaluate performance measures. This system also allows for the capture of data to track improvements in the care of the veterans served.
From 2012 to 2017, more than 5000 NF/SGVHS patients were weaned from opioids. Overall, there was an 87% reduction in patients receiving opioids ( ≥ 100-mg MEDDs) within the NF/SGVHS, which is significantly more than the 49% seen nationally across the VHA (Figure 2). Percent reduction was calculated by taking the difference in number of patients receiving opioids in 2012 and 2017, dividing by the number of patients receiving opioids in 2012 and multiplying by 100. The largest proportion of opioid dose reductions for NF/SGVHS and VHA patients, respectively, were seen in 300-mg to 399-mg MEDDs (95% vs 67%, respectively); followed by ≥ 400-mg MEDDs (94% vs 71%, respectively); 200-mg to 299-mg MEDDs (91% vs 58%, respectively); and 100-mg to 199-mg MEDDs (84% vs 40%, respectively). When examining NF/SGVHS trends over time, there has been a consistent decline in patients prescribed opioids (18 223 in 2012 compared with 12 877 in 2017) with similar trends in benzodiazepine-opioid combination therapy (2694 in 2012 compared with 833 in 2017) (Figure 3).
Similar declines are seen when patients are stratified by the MEDD (Figure 4). From 2012 to 2017, 92% of the patients were successfully tapered off doses ≥ 400-mg MEDD (2012, n = 72; 2017, n = 6), and tapered off 300-mg to 399-mg MEDD (2012, n = 107; 2017, n = 5); 95% were tapered off 200-mg to 299-mg MEDD (2012, n = 262; 2017, n = 22); and 86% were tapered off 100-mg to 199-mg MEDD (2012, n = 876; 2017; n = 127).
Conclusion
Successful integration of primary care with mental health and addiction services is paramount to aggressively taper patients with chronic pain from opioids. There is evidence that drug dependence and chronic pain should be treated like other chronic illness.20 Both chronic pain and addiction can be treated with a multidimensional self-management approach. In view of the high incidence of mental health and addiction associated with opioid use, it makes sense that an integrated, 1-stop pain and addiction clinic that understands and addresses both issues is more likely to improve patient outcomes.
Acknowledgments
This material is the result of work supported by the resources and facilities at the North Florida/South Georgia Veterans Health System, Geriatric Research Education Clinical Center in Gainesville, Florida.
1. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457-467.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: Institute of Medicine; 2011.
3. Breuer B, Cruciani R, Portenoy RK. Pain management by primary care physicians, pain physicians, chiropractors, and acupuncturists: a national survey. South Med J. 2010;103(8):738-747.
4. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130.
5. Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: directions for health policy, education, practice, and research. Pain Med. 2012;13(1):5-28.
6. McHugh RK, Nielsen S, Weiss RD. Prescription drug abuse: from epidemiology to public policy. J Subst Abuse Treat. 2015;48(1):1-7.
7. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths-United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427.
8. Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8.
9. Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain. 2007;11(5):490-518.
10. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
11. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559-574.
12. Ballantyne JC, Sullivan MD, Kolodny A. Opioid dependence vs addiction: a distinction without a difference? Arch Intern Med. 2012;172(17):1342-1343.
13. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413.
14. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612.
15. US Department of Veterans Affairs. Veteran Health Administration Directive 2009-053, Pain Management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed August 19, 2019.
16. Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82(2):154-160.
17. Kirsh S, Su GL, Sales A, Jain R. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30(1):88-90.
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100.
19. Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. 2017;158 (suppl 1):S11-S18.
20. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
Chronic pain significantly affects 100 million Americans.1,2 Pain accounts for $560 to $635 billion in annual financial costs to society, including health care costs and loss of productivity (ie, days missed from work, hours of work lost, and lower wages).2,3 Although pain prevalence exceeds other chronic diseases, such as diabetes mellitus, cancer, and heart disease, it lacks a sufficient body of evidence-based research and guidelines on the underlying mechanisms, valid methods of assessment, and comparative effectiveness of treatments to effectively implement into clinical practice.2,4 Prevention and treatment of pain are often delayed, inaccessible, or inadequate.2 Primary care providers (PCPs) are most often sought for pain management and treat about 52% of chronic pain patients.2,3,5 Veterans are especially vulnerable to chronic pain and are at risk for inadequate treatment.2
Background
There is an epidemic of drug abuse and mortality from opioid prescription medication.6 In the US, rates of overdose deaths from prescription opioids were 6.1 per 100,000 for men and 4.2 per 100,000 for women in 2017. Opioids were involved in 47,600 overdose deaths in 2017, accounting for 67.8% of all drug overdose deaths.7
A large number of patients on long-term opioids have preexisting substance use disorders and/or psychiatric disease, further complicating chronic pain management.8-10 Prescription opioid use has been the precursor for about 80% of people who are now heroin addicts.11 Iatrogenic addiction from prescription medications isn’t easily captured by standard addiction criteria. Consequently, in patients who are on opioid therapy for prolonged periods, separating complex opioid dependence from addiction is difficult.12 Improved addiction screening and risk mitigation strategies are needed along with aggressive treatment monitoring to curb the opioid epidemic.
Opioid Management in Primary Care
The majority of opioid medications are prescribed by PCPs, which is magnified in the US Department of Veterans Affairs (VA) health care system due to the high prevalence of service-related injuries.3,13 The VA is at the forefront of addressing the complexities of opioid addiction through several initiatives.14 The ability to offer the frequent visits needed to safely manage patients prescribed opioids and the integration of mental health and addiction treatment are often lacking in non-VA primary care clinics. Therefore, a key to solving the opioid crisis is developing these capabilities so they can be delivered within the primary care setting. There is substantial evidence in support of nonopioid alternatives to chronic pain management, including other pharmacologic approaches, exercise, physical therapy, acupuncture, weight loss, smoking cessation, chiropractic care, cognitive behavioral therapy (CBT), and other integrative health modalities.
A 2009 VA directive mandated the development of a comprehensive, integrated, systemwide approach to pain management.15 The VA Stepped-Care Biopsychosocial Model for Pain Management is dependent on timely access to secondary consultation from pain medicine, behavioral health, physical medicine, and other specialty consultation.15
History of VHA SCAN-ECHO Model
The Specialty Care Access Network–Extension for Community Health Outcomes (SCAN-ECHO) is a Veterans Health Administration (VHA) adaptation of a program that originated at the University of Mexico.16,17 The SCAN-ECHO model uses a multisite videoconferencing network to provide specialty care consultations to PCPs and patient aligned care teams (PACTs). During the 60- to 90-minute weekly sessions, case presentations are analyzed in real time so that over time, the PCPs gain knowledge, competency, and confidence in learning how to handle complex chronic conditions.
Since its implementation, the SCAN-ECHO program has been adopted across the VHA in a variety of specialties. One program, the SCAN-ECHO for Pain Management (SCAN-ECHO-PM) was implemented in 7 VHA networks in 31 states, spanning 47 medical centers and 148 community-based outpatient clinics (CBOCs).18 The SCAN-ECHO-PM program successfully conducted 257 multidisciplinary pain consultations between 2011 and 2013, resulting in increased initiation of nonopioid medications.18
The aim of this article is to describe the implementation of a multicomponent primary care-based pain clinic with a fully integrated mental health service and addiction service at the North Florida/South Georgia Veterans Health System (NF/SGVHS). A practiced-based intervention of the biopsychosocial model with robust patient engagement has guided the development of the NF/SGVHS pain clinic (Figure 1).4,19
Pain CLinic
NF/SGVHS comprises the Malcom Randall and Lake City VA medical centers (VAMCs) hospitals, 3 satellite outpatient clinics, and 8 CBOCs. Spanning 33 counties in North Florida and 19 counties in South Georgia, the NF/SGVHS serves more than 140,000 patients. In 2010, the Malcom Randall VAMC established a multidisciplinary primary care pain clinic to manage veterans at high-risk for noncancer chronic pain and addiction. The noncancer pain policy was revised after garnering support from stakeholders who treat chronic pain, including the chiefs of psychiatry, rehabilitation medicine, neurosurgery, psychology, interventional pain, pharmacy, nursing, addiction medicine, and primary care. The clinic is staffed by primary care physicians trained in internal medicine and family medicine and is structured with 1-hour first visits, and 30-minute follow-up visits to allow enough time for comprehensive evaluation while meeting the needs for close follow-up support.
All physicians in the clinic have buprenorphine prescribing credentials to aid in the management of opioid addiction, as some patients feel more comfortable receiving addiction treatment in a primary care setting. The multimodal care model consists of several services that include addiction psychiatrists, interventional pain specialists, pain psychologists, and pain pharmacologists who coordinate the care to the veterans. The addiction psychiatrists offer a full range of services with inpatient residential and outpatient programs. Through recurring meetings with primary care pain clinic staff, the addiction psychiatrists are available to discuss use of buprenorphine and arrange follow-up for patients with complex pain addiction. There is ongoing collaboration to develop the best care plan that meets the patient’s needs for chronic pain, addiction, and/or mental health issues. The interventional pain service has 3 fellowship-trained pain care providers who deliver comprehensive evaluation, pharmacologic recommendations, and a full range of interventional and complementary therapies with an emphasis on objective functional improvement. Pain care providers offer alternatives to patients who are being weaned from opioids and support the multidisciplinary patient engagement model.
The pain psychology program, established in 2011, delivers CBT to 5 onsite locations and 5 telehealth locations. The service includes an advanced CBT program and a couples CBT program. The pharmacy pain fellowship program provides staff for an outpatient e-consult pain management service and an inpatient pharmacy consult service. Harnessing pain specialty pharmacists, the pharmacy service addresses pharmacokinetic issues, urine drug screen (UDS) results, opioid tapering and discharge planning for pain, addiction and mental health needs. The NF/SGVHS Primary Care Pain Clinic was established to support PCPs who did not feel comfortable managing chronic pain patients. These patients were typically on high-dose opioid therapy (> 100-mg morphine equivalent daily doses [MEDDs]); patients with a history of opioid addiction; patients with an addiction to opioids combined with benzodiazepines; and patients with comorbid medical issues (eg, sleep apnea), which complicated their management. The process of addressing opioid safety in these complex pain patients can be labor intensive and generally cannot be accomplished in a brief visit in a primary care setting where many other medical problems often need to be addressed.
Most patients on high-dose opioids are fearful of any changes in their medications. The difficult conversation regarding opioid safety is a lengthy one and frequently will occur over multiple visits. In addition, safely tapering opioids requires frequent follow-up to provide psychological support and to address withdrawal and mental health issues that may arise. As opioids are tapered, the clinic reinforces improved pain care through a multimodal biopsychosocial model. All veterans receiving pain care outside the VA are monitored annually to assure they are receiving evidence-based pain care as defined by the biopsychosocial model.
Education
Since 2011, the NF/SGVHS SCAN-ECHO pain and addiction educational forum has created > 50 hours of approved annual continuing medical education (CME) on pain management and addiction for PCPs. Initially, the 1-hour weekly educational audioconferences presented a pain management case along with related topics and involved specialists from interventional pain, physical therapy, psychiatry, nursing, neurology, and psychology departments. In 2013, in conjunction with the VA SCAN-ECHO program of Hunter Holmes McGuire VAMC in Richmond, Virginia, and Walter Reed National Military Medical Center in Bethesda, Maryland, the audioconference was expanded to 2 days each week with additional topics on addiction management. Residency and fellowship rotations were developed that specifically targeted fellows from psychiatry, pharmacology, and interventional pain departments.
Currently, an 8-session pain school is delivered onsite and at 7 telehealth locations. The school is a collaborative effort involving interventional pain, psychology, pharmacy, nutrition, and the primary care pain clinic staff. As the cornerstone of the program, the pain school stresses the biopsychosocial patient engagement model.
Program Evaluation
The VA is equipped with multiple telehealth service networks that allow for the delivery of programs, such as the pain school, a pain psychology program, and a yoga program, onsite or offsite. The VA Computerized Patient Record System (CPRS) manages electronic health records, allowing for rapid chart review and e-consults. The NF/SGVHS Pain Management Program provides about 1500 e-consults yearly. The CPRS includes templates with pain metrics to help PCPs deliver pain care more efficiently and evaluate performance measures. This system also allows for the capture of data to track improvements in the care of the veterans served.
From 2012 to 2017, more than 5000 NF/SGVHS patients were weaned from opioids. Overall, there was an 87% reduction in patients receiving opioids ( ≥ 100-mg MEDDs) within the NF/SGVHS, which is significantly more than the 49% seen nationally across the VHA (Figure 2). Percent reduction was calculated by taking the difference in number of patients receiving opioids in 2012 and 2017, dividing by the number of patients receiving opioids in 2012 and multiplying by 100. The largest proportion of opioid dose reductions for NF/SGVHS and VHA patients, respectively, were seen in 300-mg to 399-mg MEDDs (95% vs 67%, respectively); followed by ≥ 400-mg MEDDs (94% vs 71%, respectively); 200-mg to 299-mg MEDDs (91% vs 58%, respectively); and 100-mg to 199-mg MEDDs (84% vs 40%, respectively). When examining NF/SGVHS trends over time, there has been a consistent decline in patients prescribed opioids (18 223 in 2012 compared with 12 877 in 2017) with similar trends in benzodiazepine-opioid combination therapy (2694 in 2012 compared with 833 in 2017) (Figure 3).
Similar declines are seen when patients are stratified by the MEDD (Figure 4). From 2012 to 2017, 92% of the patients were successfully tapered off doses ≥ 400-mg MEDD (2012, n = 72; 2017, n = 6), and tapered off 300-mg to 399-mg MEDD (2012, n = 107; 2017, n = 5); 95% were tapered off 200-mg to 299-mg MEDD (2012, n = 262; 2017, n = 22); and 86% were tapered off 100-mg to 199-mg MEDD (2012, n = 876; 2017; n = 127).
Conclusion
Successful integration of primary care with mental health and addiction services is paramount to aggressively taper patients with chronic pain from opioids. There is evidence that drug dependence and chronic pain should be treated like other chronic illness.20 Both chronic pain and addiction can be treated with a multidimensional self-management approach. In view of the high incidence of mental health and addiction associated with opioid use, it makes sense that an integrated, 1-stop pain and addiction clinic that understands and addresses both issues is more likely to improve patient outcomes.
Acknowledgments
This material is the result of work supported by the resources and facilities at the North Florida/South Georgia Veterans Health System, Geriatric Research Education Clinical Center in Gainesville, Florida.
Chronic pain significantly affects 100 million Americans.1,2 Pain accounts for $560 to $635 billion in annual financial costs to society, including health care costs and loss of productivity (ie, days missed from work, hours of work lost, and lower wages).2,3 Although pain prevalence exceeds other chronic diseases, such as diabetes mellitus, cancer, and heart disease, it lacks a sufficient body of evidence-based research and guidelines on the underlying mechanisms, valid methods of assessment, and comparative effectiveness of treatments to effectively implement into clinical practice.2,4 Prevention and treatment of pain are often delayed, inaccessible, or inadequate.2 Primary care providers (PCPs) are most often sought for pain management and treat about 52% of chronic pain patients.2,3,5 Veterans are especially vulnerable to chronic pain and are at risk for inadequate treatment.2
Background
There is an epidemic of drug abuse and mortality from opioid prescription medication.6 In the US, rates of overdose deaths from prescription opioids were 6.1 per 100,000 for men and 4.2 per 100,000 for women in 2017. Opioids were involved in 47,600 overdose deaths in 2017, accounting for 67.8% of all drug overdose deaths.7
A large number of patients on long-term opioids have preexisting substance use disorders and/or psychiatric disease, further complicating chronic pain management.8-10 Prescription opioid use has been the precursor for about 80% of people who are now heroin addicts.11 Iatrogenic addiction from prescription medications isn’t easily captured by standard addiction criteria. Consequently, in patients who are on opioid therapy for prolonged periods, separating complex opioid dependence from addiction is difficult.12 Improved addiction screening and risk mitigation strategies are needed along with aggressive treatment monitoring to curb the opioid epidemic.
Opioid Management in Primary Care
The majority of opioid medications are prescribed by PCPs, which is magnified in the US Department of Veterans Affairs (VA) health care system due to the high prevalence of service-related injuries.3,13 The VA is at the forefront of addressing the complexities of opioid addiction through several initiatives.14 The ability to offer the frequent visits needed to safely manage patients prescribed opioids and the integration of mental health and addiction treatment are often lacking in non-VA primary care clinics. Therefore, a key to solving the opioid crisis is developing these capabilities so they can be delivered within the primary care setting. There is substantial evidence in support of nonopioid alternatives to chronic pain management, including other pharmacologic approaches, exercise, physical therapy, acupuncture, weight loss, smoking cessation, chiropractic care, cognitive behavioral therapy (CBT), and other integrative health modalities.
A 2009 VA directive mandated the development of a comprehensive, integrated, systemwide approach to pain management.15 The VA Stepped-Care Biopsychosocial Model for Pain Management is dependent on timely access to secondary consultation from pain medicine, behavioral health, physical medicine, and other specialty consultation.15
History of VHA SCAN-ECHO Model
The Specialty Care Access Network–Extension for Community Health Outcomes (SCAN-ECHO) is a Veterans Health Administration (VHA) adaptation of a program that originated at the University of Mexico.16,17 The SCAN-ECHO model uses a multisite videoconferencing network to provide specialty care consultations to PCPs and patient aligned care teams (PACTs). During the 60- to 90-minute weekly sessions, case presentations are analyzed in real time so that over time, the PCPs gain knowledge, competency, and confidence in learning how to handle complex chronic conditions.
Since its implementation, the SCAN-ECHO program has been adopted across the VHA in a variety of specialties. One program, the SCAN-ECHO for Pain Management (SCAN-ECHO-PM) was implemented in 7 VHA networks in 31 states, spanning 47 medical centers and 148 community-based outpatient clinics (CBOCs).18 The SCAN-ECHO-PM program successfully conducted 257 multidisciplinary pain consultations between 2011 and 2013, resulting in increased initiation of nonopioid medications.18
The aim of this article is to describe the implementation of a multicomponent primary care-based pain clinic with a fully integrated mental health service and addiction service at the North Florida/South Georgia Veterans Health System (NF/SGVHS). A practiced-based intervention of the biopsychosocial model with robust patient engagement has guided the development of the NF/SGVHS pain clinic (Figure 1).4,19
Pain CLinic
NF/SGVHS comprises the Malcom Randall and Lake City VA medical centers (VAMCs) hospitals, 3 satellite outpatient clinics, and 8 CBOCs. Spanning 33 counties in North Florida and 19 counties in South Georgia, the NF/SGVHS serves more than 140,000 patients. In 2010, the Malcom Randall VAMC established a multidisciplinary primary care pain clinic to manage veterans at high-risk for noncancer chronic pain and addiction. The noncancer pain policy was revised after garnering support from stakeholders who treat chronic pain, including the chiefs of psychiatry, rehabilitation medicine, neurosurgery, psychology, interventional pain, pharmacy, nursing, addiction medicine, and primary care. The clinic is staffed by primary care physicians trained in internal medicine and family medicine and is structured with 1-hour first visits, and 30-minute follow-up visits to allow enough time for comprehensive evaluation while meeting the needs for close follow-up support.
All physicians in the clinic have buprenorphine prescribing credentials to aid in the management of opioid addiction, as some patients feel more comfortable receiving addiction treatment in a primary care setting. The multimodal care model consists of several services that include addiction psychiatrists, interventional pain specialists, pain psychologists, and pain pharmacologists who coordinate the care to the veterans. The addiction psychiatrists offer a full range of services with inpatient residential and outpatient programs. Through recurring meetings with primary care pain clinic staff, the addiction psychiatrists are available to discuss use of buprenorphine and arrange follow-up for patients with complex pain addiction. There is ongoing collaboration to develop the best care plan that meets the patient’s needs for chronic pain, addiction, and/or mental health issues. The interventional pain service has 3 fellowship-trained pain care providers who deliver comprehensive evaluation, pharmacologic recommendations, and a full range of interventional and complementary therapies with an emphasis on objective functional improvement. Pain care providers offer alternatives to patients who are being weaned from opioids and support the multidisciplinary patient engagement model.
The pain psychology program, established in 2011, delivers CBT to 5 onsite locations and 5 telehealth locations. The service includes an advanced CBT program and a couples CBT program. The pharmacy pain fellowship program provides staff for an outpatient e-consult pain management service and an inpatient pharmacy consult service. Harnessing pain specialty pharmacists, the pharmacy service addresses pharmacokinetic issues, urine drug screen (UDS) results, opioid tapering and discharge planning for pain, addiction and mental health needs. The NF/SGVHS Primary Care Pain Clinic was established to support PCPs who did not feel comfortable managing chronic pain patients. These patients were typically on high-dose opioid therapy (> 100-mg morphine equivalent daily doses [MEDDs]); patients with a history of opioid addiction; patients with an addiction to opioids combined with benzodiazepines; and patients with comorbid medical issues (eg, sleep apnea), which complicated their management. The process of addressing opioid safety in these complex pain patients can be labor intensive and generally cannot be accomplished in a brief visit in a primary care setting where many other medical problems often need to be addressed.
Most patients on high-dose opioids are fearful of any changes in their medications. The difficult conversation regarding opioid safety is a lengthy one and frequently will occur over multiple visits. In addition, safely tapering opioids requires frequent follow-up to provide psychological support and to address withdrawal and mental health issues that may arise. As opioids are tapered, the clinic reinforces improved pain care through a multimodal biopsychosocial model. All veterans receiving pain care outside the VA are monitored annually to assure they are receiving evidence-based pain care as defined by the biopsychosocial model.
Education
Since 2011, the NF/SGVHS SCAN-ECHO pain and addiction educational forum has created > 50 hours of approved annual continuing medical education (CME) on pain management and addiction for PCPs. Initially, the 1-hour weekly educational audioconferences presented a pain management case along with related topics and involved specialists from interventional pain, physical therapy, psychiatry, nursing, neurology, and psychology departments. In 2013, in conjunction with the VA SCAN-ECHO program of Hunter Holmes McGuire VAMC in Richmond, Virginia, and Walter Reed National Military Medical Center in Bethesda, Maryland, the audioconference was expanded to 2 days each week with additional topics on addiction management. Residency and fellowship rotations were developed that specifically targeted fellows from psychiatry, pharmacology, and interventional pain departments.
Currently, an 8-session pain school is delivered onsite and at 7 telehealth locations. The school is a collaborative effort involving interventional pain, psychology, pharmacy, nutrition, and the primary care pain clinic staff. As the cornerstone of the program, the pain school stresses the biopsychosocial patient engagement model.
Program Evaluation
The VA is equipped with multiple telehealth service networks that allow for the delivery of programs, such as the pain school, a pain psychology program, and a yoga program, onsite or offsite. The VA Computerized Patient Record System (CPRS) manages electronic health records, allowing for rapid chart review and e-consults. The NF/SGVHS Pain Management Program provides about 1500 e-consults yearly. The CPRS includes templates with pain metrics to help PCPs deliver pain care more efficiently and evaluate performance measures. This system also allows for the capture of data to track improvements in the care of the veterans served.
From 2012 to 2017, more than 5000 NF/SGVHS patients were weaned from opioids. Overall, there was an 87% reduction in patients receiving opioids ( ≥ 100-mg MEDDs) within the NF/SGVHS, which is significantly more than the 49% seen nationally across the VHA (Figure 2). Percent reduction was calculated by taking the difference in number of patients receiving opioids in 2012 and 2017, dividing by the number of patients receiving opioids in 2012 and multiplying by 100. The largest proportion of opioid dose reductions for NF/SGVHS and VHA patients, respectively, were seen in 300-mg to 399-mg MEDDs (95% vs 67%, respectively); followed by ≥ 400-mg MEDDs (94% vs 71%, respectively); 200-mg to 299-mg MEDDs (91% vs 58%, respectively); and 100-mg to 199-mg MEDDs (84% vs 40%, respectively). When examining NF/SGVHS trends over time, there has been a consistent decline in patients prescribed opioids (18 223 in 2012 compared with 12 877 in 2017) with similar trends in benzodiazepine-opioid combination therapy (2694 in 2012 compared with 833 in 2017) (Figure 3).
Similar declines are seen when patients are stratified by the MEDD (Figure 4). From 2012 to 2017, 92% of the patients were successfully tapered off doses ≥ 400-mg MEDD (2012, n = 72; 2017, n = 6), and tapered off 300-mg to 399-mg MEDD (2012, n = 107; 2017, n = 5); 95% were tapered off 200-mg to 299-mg MEDD (2012, n = 262; 2017, n = 22); and 86% were tapered off 100-mg to 199-mg MEDD (2012, n = 876; 2017; n = 127).
Conclusion
Successful integration of primary care with mental health and addiction services is paramount to aggressively taper patients with chronic pain from opioids. There is evidence that drug dependence and chronic pain should be treated like other chronic illness.20 Both chronic pain and addiction can be treated with a multidimensional self-management approach. In view of the high incidence of mental health and addiction associated with opioid use, it makes sense that an integrated, 1-stop pain and addiction clinic that understands and addresses both issues is more likely to improve patient outcomes.
Acknowledgments
This material is the result of work supported by the resources and facilities at the North Florida/South Georgia Veterans Health System, Geriatric Research Education Clinical Center in Gainesville, Florida.
1. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457-467.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: Institute of Medicine; 2011.
3. Breuer B, Cruciani R, Portenoy RK. Pain management by primary care physicians, pain physicians, chiropractors, and acupuncturists: a national survey. South Med J. 2010;103(8):738-747.
4. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130.
5. Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: directions for health policy, education, practice, and research. Pain Med. 2012;13(1):5-28.
6. McHugh RK, Nielsen S, Weiss RD. Prescription drug abuse: from epidemiology to public policy. J Subst Abuse Treat. 2015;48(1):1-7.
7. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths-United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427.
8. Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8.
9. Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain. 2007;11(5):490-518.
10. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
11. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559-574.
12. Ballantyne JC, Sullivan MD, Kolodny A. Opioid dependence vs addiction: a distinction without a difference? Arch Intern Med. 2012;172(17):1342-1343.
13. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413.
14. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612.
15. US Department of Veterans Affairs. Veteran Health Administration Directive 2009-053, Pain Management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed August 19, 2019.
16. Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82(2):154-160.
17. Kirsh S, Su GL, Sales A, Jain R. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30(1):88-90.
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100.
19. Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. 2017;158 (suppl 1):S11-S18.
20. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
1. Dueñas M, Ojeda B, Salazar A, Mico JA, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. 2016;9:457-467.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: Institute of Medicine; 2011.
3. Breuer B, Cruciani R, Portenoy RK. Pain management by primary care physicians, pain physicians, chiropractors, and acupuncturists: a national survey. South Med J. 2010;103(8):738-747.
4. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130.
5. Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a national agenda to eliminate disparities in pain care: directions for health policy, education, practice, and research. Pain Med. 2012;13(1):5-28.
6. McHugh RK, Nielsen S, Weiss RD. Prescription drug abuse: from epidemiology to public policy. J Subst Abuse Treat. 2015;48(1):1-7.
7. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths-United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-1427.
8. Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8.
9. Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain. 2007;11(5):490-518.
10. Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
11. Kolodny A, Courtwright DT, Hwang CS, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559-574.
12. Ballantyne JC, Sullivan MD, Kolodny A. Opioid dependence vs addiction: a distinction without a difference? Arch Intern Med. 2012;172(17):1342-1343.
13. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007-2012. Am J Prev Med. 2015;49(3):409-413.
14. Gellad WF, Good CB, Shulkin DJ. Addressing the opioid epidemic in the United States: lessons from the Department of Veterans Affairs. JAMA Intern Med. 2017;177(5):611-612.
15. US Department of Veterans Affairs. Veteran Health Administration Directive 2009-053, Pain Management. https://www.va.gov/painmanagement/docs/vha09paindirective.pdf. Published October 28, 2009. Accessed August 19, 2019.
16. Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82(2):154-160.
17. Kirsh S, Su GL, Sales A, Jain R. Access to outpatient specialty care: solutions from an integrated health care system. Am J Med Qual. 2015;30(1):88-90.
18. Frank JW, Carey EP, Fagan KM, et al. Evaluation of a telementoring intervention for pain management in the Veterans Health Administration. Pain Med. 2015;16(6):1090-1100.
19. Fillingim RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. 2017;158 (suppl 1):S11-S18.
20. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689-1695.
Cannabidiol may interact with rheumatologic drugs
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.

“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.
FROM RHEUMATOLOGY
MSK Clinic: Evaluating shoulder pain using IPASS



Opioid Epidemic
Galcanezumab benefits patients with migraine and medication overuse
PHILADELPHIA –
“When you have targeted preventive treatment and you reduce the burden of illness, medication overuse seems to be reduced as well,” said Sheena Aurora, MD, adjunct clinical associate professor of anesthesiology and perioperative and pain medicine at Stanford (Calif.) Health Care. Dr. Aurora also is a medical fellow and global launch leader for galcanezumab at Eli Lilly, which has developed the treatment.
Galcanezumab is a humanized monoclonal antibody that selectively binds to the calcitonin gene-related peptide. The phase 3 EVOLVE-1, EVOLVE-2, and REGAIN studies indicated galcanezumab’s superiority to placebo in preventing episodic and chronic migraine.
A post hoc analysis of phase 3 data
Dr. Aurora and colleagues conducted a post hoc analysis of data from the three phase 3 studies to examine galcanezumab’s effect in patients with medication overuse. EVOLVE-1 and EVOLVE-2 included patients with episodic migraine, and REGAIN included patients with chronic migraine. All participants were randomized to monthly subcutaneous injections of placebo or galcanezumab (120 mg/month or 240 mg/month) for 3-6 months. Based on information obtained through electronic patient-reported outcome diaries, investigators determined headache medication overuse using criteria adapted from the International Classification of Headache Disorders, third edition. They estimated mean changes in monthly headache days and the proportion of patients with medication overuse after randomization using mixed modeling.
The demographic characteristics of the three study populations were similar to those reported in epidemiologic studies of migraine, said Dr. Aurora. Most participants were women, and most patients were between ages 40 and 49 years. At baseline, the mean number of monthly migraine headache days was 20 among patients with chronic migraine and 9 among patients with episodic migraine.
The rate of medication overuse was higher in the combined study population than in the literature. Patients with medication overuse had greater disability and greater health care resource utilization, compared with patients without medication overuse. Among patients with chronic migraine, participants who overused medication were not significantly different from those who did not. But among patients with episodic migraine, participants who overused medication had higher headache frequency than those who did not.
In the EVOLVE trials, the proportion of patients with baseline medication overuse was 19.3% in the placebo arm, 17.0% in the galcanezumab 120-mg arm, and 19.2% in the galcanezumab 240-mg arm. In REGAIN, the proportion of patients with baseline medication overuse was 63.4% in the placebo arm, 64.3% in the galcanezumab 120-mg arm, and 64.1% in the galcanezumab 240-mg arm.
Galcanezumab reduced medication overuse
Compared with placebo, both doses of galcanezumab significantly decreased mean monthly migraine headache days in patients with baseline medication overuse. In the EVOLVE studies, this endpoint decreased by 2.71 in the placebo group, 6.26 in the galcanezumab 120-mg group, and 5.77 in the galcanezumab 240-mg group. In REGAIN, the reductions were 2.25 in the placebo group, 4.78 in the galcanezumab 120-mg group, and 4.51 in the galcanezumab 240-mg group. The effect size was higher in patients who were overusing medications, compared with those who were not, said Dr. Aurora. “This is clinically relevant, because most of us ... had this belief that patients who were overusing medications may be more treatment-resistant to prevention.”
In addition, galcanezumab was associated with significantly lower rates of average monthly medication overuse, compared with placebo. In the EVOLVE studies, the average rate of monthly medication overuse was 15.9% for the placebo group, 6.2% for the galcanezumab 120-mg group, and 7.9% for the galcanezumab 240-mg group. In REGAIN, the average rate of monthly medication overuse was 40.6% in the placebo group, 24.3% in the galcanezumab 120-mg group, and 23.1% in the galcanezumab 240-mg group. About 85% of patients with episodic migraine and medication overuse had a reduction in medication overuse, and approximately 50% of patients with chronic migraine and medication overuse had a reduction in medication overuse, said Dr. Aurora.
Dr. Aurora and coinvestigators are employees of Eli Lilly, which developed galcanezumab and funded the EVOLVE and REGAIN studies.
SOURCE: Aurora S et al. AHS 2019. Abstract IOR07.
PHILADELPHIA –
“When you have targeted preventive treatment and you reduce the burden of illness, medication overuse seems to be reduced as well,” said Sheena Aurora, MD, adjunct clinical associate professor of anesthesiology and perioperative and pain medicine at Stanford (Calif.) Health Care. Dr. Aurora also is a medical fellow and global launch leader for galcanezumab at Eli Lilly, which has developed the treatment.
Galcanezumab is a humanized monoclonal antibody that selectively binds to the calcitonin gene-related peptide. The phase 3 EVOLVE-1, EVOLVE-2, and REGAIN studies indicated galcanezumab’s superiority to placebo in preventing episodic and chronic migraine.
A post hoc analysis of phase 3 data
Dr. Aurora and colleagues conducted a post hoc analysis of data from the three phase 3 studies to examine galcanezumab’s effect in patients with medication overuse. EVOLVE-1 and EVOLVE-2 included patients with episodic migraine, and REGAIN included patients with chronic migraine. All participants were randomized to monthly subcutaneous injections of placebo or galcanezumab (120 mg/month or 240 mg/month) for 3-6 months. Based on information obtained through electronic patient-reported outcome diaries, investigators determined headache medication overuse using criteria adapted from the International Classification of Headache Disorders, third edition. They estimated mean changes in monthly headache days and the proportion of patients with medication overuse after randomization using mixed modeling.
The demographic characteristics of the three study populations were similar to those reported in epidemiologic studies of migraine, said Dr. Aurora. Most participants were women, and most patients were between ages 40 and 49 years. At baseline, the mean number of monthly migraine headache days was 20 among patients with chronic migraine and 9 among patients with episodic migraine.
The rate of medication overuse was higher in the combined study population than in the literature. Patients with medication overuse had greater disability and greater health care resource utilization, compared with patients without medication overuse. Among patients with chronic migraine, participants who overused medication were not significantly different from those who did not. But among patients with episodic migraine, participants who overused medication had higher headache frequency than those who did not.
In the EVOLVE trials, the proportion of patients with baseline medication overuse was 19.3% in the placebo arm, 17.0% in the galcanezumab 120-mg arm, and 19.2% in the galcanezumab 240-mg arm. In REGAIN, the proportion of patients with baseline medication overuse was 63.4% in the placebo arm, 64.3% in the galcanezumab 120-mg arm, and 64.1% in the galcanezumab 240-mg arm.
Galcanezumab reduced medication overuse
Compared with placebo, both doses of galcanezumab significantly decreased mean monthly migraine headache days in patients with baseline medication overuse. In the EVOLVE studies, this endpoint decreased by 2.71 in the placebo group, 6.26 in the galcanezumab 120-mg group, and 5.77 in the galcanezumab 240-mg group. In REGAIN, the reductions were 2.25 in the placebo group, 4.78 in the galcanezumab 120-mg group, and 4.51 in the galcanezumab 240-mg group. The effect size was higher in patients who were overusing medications, compared with those who were not, said Dr. Aurora. “This is clinically relevant, because most of us ... had this belief that patients who were overusing medications may be more treatment-resistant to prevention.”
In addition, galcanezumab was associated with significantly lower rates of average monthly medication overuse, compared with placebo. In the EVOLVE studies, the average rate of monthly medication overuse was 15.9% for the placebo group, 6.2% for the galcanezumab 120-mg group, and 7.9% for the galcanezumab 240-mg group. In REGAIN, the average rate of monthly medication overuse was 40.6% in the placebo group, 24.3% in the galcanezumab 120-mg group, and 23.1% in the galcanezumab 240-mg group. About 85% of patients with episodic migraine and medication overuse had a reduction in medication overuse, and approximately 50% of patients with chronic migraine and medication overuse had a reduction in medication overuse, said Dr. Aurora.
Dr. Aurora and coinvestigators are employees of Eli Lilly, which developed galcanezumab and funded the EVOLVE and REGAIN studies.
SOURCE: Aurora S et al. AHS 2019. Abstract IOR07.
PHILADELPHIA –
“When you have targeted preventive treatment and you reduce the burden of illness, medication overuse seems to be reduced as well,” said Sheena Aurora, MD, adjunct clinical associate professor of anesthesiology and perioperative and pain medicine at Stanford (Calif.) Health Care. Dr. Aurora also is a medical fellow and global launch leader for galcanezumab at Eli Lilly, which has developed the treatment.
Galcanezumab is a humanized monoclonal antibody that selectively binds to the calcitonin gene-related peptide. The phase 3 EVOLVE-1, EVOLVE-2, and REGAIN studies indicated galcanezumab’s superiority to placebo in preventing episodic and chronic migraine.
A post hoc analysis of phase 3 data
Dr. Aurora and colleagues conducted a post hoc analysis of data from the three phase 3 studies to examine galcanezumab’s effect in patients with medication overuse. EVOLVE-1 and EVOLVE-2 included patients with episodic migraine, and REGAIN included patients with chronic migraine. All participants were randomized to monthly subcutaneous injections of placebo or galcanezumab (120 mg/month or 240 mg/month) for 3-6 months. Based on information obtained through electronic patient-reported outcome diaries, investigators determined headache medication overuse using criteria adapted from the International Classification of Headache Disorders, third edition. They estimated mean changes in monthly headache days and the proportion of patients with medication overuse after randomization using mixed modeling.
The demographic characteristics of the three study populations were similar to those reported in epidemiologic studies of migraine, said Dr. Aurora. Most participants were women, and most patients were between ages 40 and 49 years. At baseline, the mean number of monthly migraine headache days was 20 among patients with chronic migraine and 9 among patients with episodic migraine.
The rate of medication overuse was higher in the combined study population than in the literature. Patients with medication overuse had greater disability and greater health care resource utilization, compared with patients without medication overuse. Among patients with chronic migraine, participants who overused medication were not significantly different from those who did not. But among patients with episodic migraine, participants who overused medication had higher headache frequency than those who did not.
In the EVOLVE trials, the proportion of patients with baseline medication overuse was 19.3% in the placebo arm, 17.0% in the galcanezumab 120-mg arm, and 19.2% in the galcanezumab 240-mg arm. In REGAIN, the proportion of patients with baseline medication overuse was 63.4% in the placebo arm, 64.3% in the galcanezumab 120-mg arm, and 64.1% in the galcanezumab 240-mg arm.
Galcanezumab reduced medication overuse
Compared with placebo, both doses of galcanezumab significantly decreased mean monthly migraine headache days in patients with baseline medication overuse. In the EVOLVE studies, this endpoint decreased by 2.71 in the placebo group, 6.26 in the galcanezumab 120-mg group, and 5.77 in the galcanezumab 240-mg group. In REGAIN, the reductions were 2.25 in the placebo group, 4.78 in the galcanezumab 120-mg group, and 4.51 in the galcanezumab 240-mg group. The effect size was higher in patients who were overusing medications, compared with those who were not, said Dr. Aurora. “This is clinically relevant, because most of us ... had this belief that patients who were overusing medications may be more treatment-resistant to prevention.”
In addition, galcanezumab was associated with significantly lower rates of average monthly medication overuse, compared with placebo. In the EVOLVE studies, the average rate of monthly medication overuse was 15.9% for the placebo group, 6.2% for the galcanezumab 120-mg group, and 7.9% for the galcanezumab 240-mg group. In REGAIN, the average rate of monthly medication overuse was 40.6% in the placebo group, 24.3% in the galcanezumab 120-mg group, and 23.1% in the galcanezumab 240-mg group. About 85% of patients with episodic migraine and medication overuse had a reduction in medication overuse, and approximately 50% of patients with chronic migraine and medication overuse had a reduction in medication overuse, said Dr. Aurora.
Dr. Aurora and coinvestigators are employees of Eli Lilly, which developed galcanezumab and funded the EVOLVE and REGAIN studies.
SOURCE: Aurora S et al. AHS 2019. Abstract IOR07.
REPORTING FROM AHS 2019
Peripheral nervous system events have lasting impact on SLE patients
Peripheral nervous system disease, predominantly neuropathies, constitutes a substantial proportion of the manifestations of neuropsychiatric systemic lupus erythematosus (SLE) and has a lasting negative impact on health-related quality of life, John G. Hanly, MD, of Queen Elizabeth II Health Sciences Center and Dalhousie University, Halifax, N.S., and associates reported in Arthritis & Rheumatology.
According to the study of 1,827 SLE patients who had been recently diagnosed and enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network at sites in Europe, Asia, and North America during 1999-2011, 161 peripheral nervous system (PNS) events occurred in 139 of the patients (8%) over a mean 7.6 years of follow-up.
Using the seven American College of Rheumatology case definitions for PNS disease in neuropsychiatric SLE, most of the events were peripheral neuropathy (41%), mononeuropathy (27%), and cranial neuropathy (24%). For 110 with peripheral neuropathy or mononeuropathy who underwent electrophysiologic testing, axonal damage was often present (42%), followed by demyelination (22%).
The PNS events were attributed to SLE in about 58%-75% of the patients. Based on these data the investigators estimated that after 10 years the cumulative incidence of any PNS event regardless of its attribution was about 9%, and it was nearly 7% for events attributed to SLE.
The probability that the neuropathies would not resolve over time was estimated at about 43% for peripheral neuropathy, 29% for mononeuropathy, and 30% for cranial neuropathy. Resolution of neuropathy was most rapid for cranial neuropathy, followed by mononeuropathy and peripheral neuropathy.
Patients with PNS events had significantly lower physical and mental health component scores on the 36-item Short Form Health Survey than did patients without a neuropsychiatric event up to the study assessment, and these differences persisted for 10 years of follow-up.
These “findings provide a benchmark for the assessment of future treatment modalities,” the investigators concluded.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41070.
Peripheral nervous system disease, predominantly neuropathies, constitutes a substantial proportion of the manifestations of neuropsychiatric systemic lupus erythematosus (SLE) and has a lasting negative impact on health-related quality of life, John G. Hanly, MD, of Queen Elizabeth II Health Sciences Center and Dalhousie University, Halifax, N.S., and associates reported in Arthritis & Rheumatology.
According to the study of 1,827 SLE patients who had been recently diagnosed and enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network at sites in Europe, Asia, and North America during 1999-2011, 161 peripheral nervous system (PNS) events occurred in 139 of the patients (8%) over a mean 7.6 years of follow-up.
Using the seven American College of Rheumatology case definitions for PNS disease in neuropsychiatric SLE, most of the events were peripheral neuropathy (41%), mononeuropathy (27%), and cranial neuropathy (24%). For 110 with peripheral neuropathy or mononeuropathy who underwent electrophysiologic testing, axonal damage was often present (42%), followed by demyelination (22%).
The PNS events were attributed to SLE in about 58%-75% of the patients. Based on these data the investigators estimated that after 10 years the cumulative incidence of any PNS event regardless of its attribution was about 9%, and it was nearly 7% for events attributed to SLE.
The probability that the neuropathies would not resolve over time was estimated at about 43% for peripheral neuropathy, 29% for mononeuropathy, and 30% for cranial neuropathy. Resolution of neuropathy was most rapid for cranial neuropathy, followed by mononeuropathy and peripheral neuropathy.
Patients with PNS events had significantly lower physical and mental health component scores on the 36-item Short Form Health Survey than did patients without a neuropsychiatric event up to the study assessment, and these differences persisted for 10 years of follow-up.
These “findings provide a benchmark for the assessment of future treatment modalities,” the investigators concluded.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41070.
Peripheral nervous system disease, predominantly neuropathies, constitutes a substantial proportion of the manifestations of neuropsychiatric systemic lupus erythematosus (SLE) and has a lasting negative impact on health-related quality of life, John G. Hanly, MD, of Queen Elizabeth II Health Sciences Center and Dalhousie University, Halifax, N.S., and associates reported in Arthritis & Rheumatology.
According to the study of 1,827 SLE patients who had been recently diagnosed and enrolled in the Systemic Lupus International Collaborating Clinics (SLICC) network at sites in Europe, Asia, and North America during 1999-2011, 161 peripheral nervous system (PNS) events occurred in 139 of the patients (8%) over a mean 7.6 years of follow-up.
Using the seven American College of Rheumatology case definitions for PNS disease in neuropsychiatric SLE, most of the events were peripheral neuropathy (41%), mononeuropathy (27%), and cranial neuropathy (24%). For 110 with peripheral neuropathy or mononeuropathy who underwent electrophysiologic testing, axonal damage was often present (42%), followed by demyelination (22%).
The PNS events were attributed to SLE in about 58%-75% of the patients. Based on these data the investigators estimated that after 10 years the cumulative incidence of any PNS event regardless of its attribution was about 9%, and it was nearly 7% for events attributed to SLE.
The probability that the neuropathies would not resolve over time was estimated at about 43% for peripheral neuropathy, 29% for mononeuropathy, and 30% for cranial neuropathy. Resolution of neuropathy was most rapid for cranial neuropathy, followed by mononeuropathy and peripheral neuropathy.
Patients with PNS events had significantly lower physical and mental health component scores on the 36-item Short Form Health Survey than did patients without a neuropsychiatric event up to the study assessment, and these differences persisted for 10 years of follow-up.
These “findings provide a benchmark for the assessment of future treatment modalities,” the investigators concluded.
SOURCE: Hanly JG et al. Arthritis Rheumatol. 2019 Aug 7. doi: 10.1002/art.41070.
REPORTING FROM ARTHRITIS & RHEUMATOLOGY
USPSTF draft guidance calls for drug use screening
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
according to a draft recommendation statement now available for public comment.
The statement defines illicit drug use as “use of illegal drugs and the nonmedical use of prescription psychoactive medications (i.e., use for reasons, for duration, in amounts, or with frequency other than prescribed or use by persons other than the prescribed individual).”
The guidelines do not apply to individuals younger than 18 years, for whom the USPSTF found insufficient evidence to recommend routine screening, or to adults currently diagnosed or in treatment for a drug use disorder.
In the draft recommendation statement, available online, the USPSTF noted that several screening tools are available for use in primary care practices, including the BSTAD (Brief Screener for Tobacco, Alcohol, and Other Drugs) that consists of six questions. The task force noted that they have found “adequate evidence” that these screening tools can detect illicit drug use. In addition, they wrote that no studies offer evidence of benefits versus harms of these screening tools, and evidence of harms associated with screening are limited.
Screening intervals can be simplified by screening young adults whenever they seek medical services and when clinicians suspect illicit drug use, the USPSTF said.
When the draft recommendation is finalized, it will replace the 2008 recommendation, which found insufficient evidence for screening in adults, as well as in adolescents. New evidence since 2008 supports the value of screening for adults aged 18 years and older, including pregnant and postpartum women.
The draft recommendations are based on the results of two systematic evidence reviews that assessed the accuracy and harms of routine illicit drug use screening. The USPSTF’s review included 12 studies on the accuracy of 15 screening tools. Overall, the sensitivity of direct screening tools to identify “unhealthy use of ‘any drug’ (including illegal drugs and nonmedical use of prescription drugs) in the past month or year” ranged from 0.71 to 0.94, and the specificity ranged from 0.87 to 0.97.
Based on the current evidence, the USPSTF assigned drug screening for adults a grade B recommendation, defined as “high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial.”
For treatment, the Task Force found evidence to support strategies including pharmacotherapy with naltrexone, buprenorphine, and methadone, as well as for psychosocial interventions.
The USPSTF acknowledged that many factors may affect a clinicians’ decision of whether to implement the drug screening recommendation. “In many communities, affordable, accessible, and timely services for diagnostic assessment and treatment for patients with positive screening results are in limited supply or unaffordable. Providers should be aware of any state requirements for mandatory screening or reporting of screening results to medicolegal authorities and understand the positive and negative implications of reporting,” they wrote.
The draft recommendations also identified several research gaps including the effectiveness of screening for illicit drug use in adolescents, the optimal screening interval for all patients, the accuracy of screening tools for detecting opioids, the accuracy of screening within the same population, the benefits of naloxone as rescue therapy, and nonmedical use of other prescription drugs, as well as ways to improve access to care for those diagnosed with drug use disorders.
The draft recommendation is available for public comment until Sept. 9, 2019, at 8 p.m. EST.
The USPSTF is supported by the Agency for Healthcare Research and Quality. The researchers had no financial conflicts to disclose.
FROM THE USPSTF
Treatment of episodic cluster headache deviates from recommendations
PHILADELPHIA – , according to an analysis presented at the annual meeting of the American Headache Society.
Although consensus treatment guidelines do not exist for episodic cluster headache, treatment of this disorder did not follow many established recommendations that call for the use of preventive medications (e.g., MacGregor et al., 2010; Sarchielli et al., 2012; and May et al., 2006). Additional preventive medication options may be needed.
Patients with episodic cluster headache have several unilateral headache attacks per day. Little information is available to guide the selection of treatments for this population, and little is known about how available treatments are used in routine practice.
Analyzing cross-sectional survey data
To address this paucity of evidence, Jeffrey Scott Andrews, PharmD, a senior research scientist at Eli Lilly in Indianapolis, and colleagues examined data from the Adelphi 2017 Cluster Headache Disease Specific Programme, a large, international, cross-sectional survey. Physicians and patients in Germany, the United Kingdom, and the United States responded to the survey. Eligible physicians consulted with at least four patients with cluster headache per month, and eligible patients had a diagnosis of episodic cluster headache that was consistent with ICHD-3 beta criteria. Additional data were collected from all participants through questionnaires.
The analysis included 309 patients in Germany, 328 in the United Kingdom, and 375 in the United States. The average age of the patients was 40 years, and most of the patients were male. Less than 70% of patients reported working full time, which may indicate “the impact of this condition on work status,” said Dr. Andrews. Patients’ average number of attacks per day within an active period was 2.4. The two most commonly reported comorbidities were anxiety and depression. About 40% of cases of depression were reported to have occurred after the receipt of a diagnosis of cluster headache.
Use of inhaled oxygen was low
Most patients received acute treatments. The proportion of patients who received acute therapy only was 53% in Germany, 48% in the United Kingdom, and 43% in the United States. Approximately 34% of patients in Germany received a combination of acute and preventive therapy, compared with 37% in the United Kingdom and 42% in the United States. The proportion of patients who received preventive therapy only was 10% in Germany, 8% in the United Kingdom, and 12% in the United States.
The most commonly prescribed acute treatment, regardless of formulation, was sumatriptan. About 60% of patients received this medication. Less than one-third of patients used inhaled oxygen. Oxygen was prescribed more often in Germany (45%) and the United Kingdom (33%), compared with the United States (19%). U.S. patients face well-known obstacles in getting access to, and reimbursement for, oxygen, said Dr. Andrews. “That’s an area that deserves increased attention.” Zolmitriptan was the third most commonly prescribed acute medication.
Among prescriptions for sumatriptan, oral and injectable formulations were approximately equally common. Recommendations, however, indicate formulations with potentially fast onset of action. “The average duration of one of these attacks is between 15 and 180 minutes, so that certainly suggests that a formulation that gives you a faster onset of action might improve outcomes,” said Dr. Andrews. The use of injectable sumatriptan was lowest in the United States and highest in the United Kingdom.
“The most common decision regarding preventive treatment was [to give] no preventive treatment,” said Dr. Andrews. Verapamil was the most commonly prescribed preventive therapy (34% in Germany, 29% in the United States, and 25% in the United Kingdom), followed by topiramate, lithium, and valproate.
Nonadherence and noncompliance was common
Fewer U.K. patients (32%) reported taking their preventive therapy as advised, compared with German patients (60%) and U.S. patients (80%). Common reasons for noncompliance, regardless of location, were forgetfulness, the belief that a dose was not needed, and side effects. Most patients in the United Kingdom (60%) and the United States (54%) reported the need to take an extra dose of their acute medication to relieve pain symptoms, compared with 30% in Germany. Furthermore, 13% of U.S. patients indicated that they took extra doses all the time or nearly all the time, compared with 2% in Germany and 7% in the United Kingdom. Among patients who had discontinued a preventive treatment in the past, the most common reasons for discontinuation were lack of efficacy and problems with tolerability.
One limitation of the study was that the survey was not designed to represent the general cluster headache or treating physician populations fully. The data may reflect selection bias in favor of physicians who treat high volumes of patients and in favor of patients who frequently seek health care. In addition, the data were based on self-reports.
“Increased awareness and educational efforts that aim at promoting the need and benefit of the preventive treatment for these patients is warranted,” Dr. Andrews concluded.
Dr. Andrews is an employee of Eli Lilly, which funded the study.
SOURCE: Nichols R et al. AHS 2019. Abstract OR04.
PHILADELPHIA – , according to an analysis presented at the annual meeting of the American Headache Society.
Although consensus treatment guidelines do not exist for episodic cluster headache, treatment of this disorder did not follow many established recommendations that call for the use of preventive medications (e.g., MacGregor et al., 2010; Sarchielli et al., 2012; and May et al., 2006). Additional preventive medication options may be needed.
Patients with episodic cluster headache have several unilateral headache attacks per day. Little information is available to guide the selection of treatments for this population, and little is known about how available treatments are used in routine practice.
Analyzing cross-sectional survey data
To address this paucity of evidence, Jeffrey Scott Andrews, PharmD, a senior research scientist at Eli Lilly in Indianapolis, and colleagues examined data from the Adelphi 2017 Cluster Headache Disease Specific Programme, a large, international, cross-sectional survey. Physicians and patients in Germany, the United Kingdom, and the United States responded to the survey. Eligible physicians consulted with at least four patients with cluster headache per month, and eligible patients had a diagnosis of episodic cluster headache that was consistent with ICHD-3 beta criteria. Additional data were collected from all participants through questionnaires.
The analysis included 309 patients in Germany, 328 in the United Kingdom, and 375 in the United States. The average age of the patients was 40 years, and most of the patients were male. Less than 70% of patients reported working full time, which may indicate “the impact of this condition on work status,” said Dr. Andrews. Patients’ average number of attacks per day within an active period was 2.4. The two most commonly reported comorbidities were anxiety and depression. About 40% of cases of depression were reported to have occurred after the receipt of a diagnosis of cluster headache.
Use of inhaled oxygen was low
Most patients received acute treatments. The proportion of patients who received acute therapy only was 53% in Germany, 48% in the United Kingdom, and 43% in the United States. Approximately 34% of patients in Germany received a combination of acute and preventive therapy, compared with 37% in the United Kingdom and 42% in the United States. The proportion of patients who received preventive therapy only was 10% in Germany, 8% in the United Kingdom, and 12% in the United States.
The most commonly prescribed acute treatment, regardless of formulation, was sumatriptan. About 60% of patients received this medication. Less than one-third of patients used inhaled oxygen. Oxygen was prescribed more often in Germany (45%) and the United Kingdom (33%), compared with the United States (19%). U.S. patients face well-known obstacles in getting access to, and reimbursement for, oxygen, said Dr. Andrews. “That’s an area that deserves increased attention.” Zolmitriptan was the third most commonly prescribed acute medication.
Among prescriptions for sumatriptan, oral and injectable formulations were approximately equally common. Recommendations, however, indicate formulations with potentially fast onset of action. “The average duration of one of these attacks is between 15 and 180 minutes, so that certainly suggests that a formulation that gives you a faster onset of action might improve outcomes,” said Dr. Andrews. The use of injectable sumatriptan was lowest in the United States and highest in the United Kingdom.
“The most common decision regarding preventive treatment was [to give] no preventive treatment,” said Dr. Andrews. Verapamil was the most commonly prescribed preventive therapy (34% in Germany, 29% in the United States, and 25% in the United Kingdom), followed by topiramate, lithium, and valproate.
Nonadherence and noncompliance was common
Fewer U.K. patients (32%) reported taking their preventive therapy as advised, compared with German patients (60%) and U.S. patients (80%). Common reasons for noncompliance, regardless of location, were forgetfulness, the belief that a dose was not needed, and side effects. Most patients in the United Kingdom (60%) and the United States (54%) reported the need to take an extra dose of their acute medication to relieve pain symptoms, compared with 30% in Germany. Furthermore, 13% of U.S. patients indicated that they took extra doses all the time or nearly all the time, compared with 2% in Germany and 7% in the United Kingdom. Among patients who had discontinued a preventive treatment in the past, the most common reasons for discontinuation were lack of efficacy and problems with tolerability.
One limitation of the study was that the survey was not designed to represent the general cluster headache or treating physician populations fully. The data may reflect selection bias in favor of physicians who treat high volumes of patients and in favor of patients who frequently seek health care. In addition, the data were based on self-reports.
“Increased awareness and educational efforts that aim at promoting the need and benefit of the preventive treatment for these patients is warranted,” Dr. Andrews concluded.
Dr. Andrews is an employee of Eli Lilly, which funded the study.
SOURCE: Nichols R et al. AHS 2019. Abstract OR04.
PHILADELPHIA – , according to an analysis presented at the annual meeting of the American Headache Society.
Although consensus treatment guidelines do not exist for episodic cluster headache, treatment of this disorder did not follow many established recommendations that call for the use of preventive medications (e.g., MacGregor et al., 2010; Sarchielli et al., 2012; and May et al., 2006). Additional preventive medication options may be needed.
Patients with episodic cluster headache have several unilateral headache attacks per day. Little information is available to guide the selection of treatments for this population, and little is known about how available treatments are used in routine practice.
Analyzing cross-sectional survey data
To address this paucity of evidence, Jeffrey Scott Andrews, PharmD, a senior research scientist at Eli Lilly in Indianapolis, and colleagues examined data from the Adelphi 2017 Cluster Headache Disease Specific Programme, a large, international, cross-sectional survey. Physicians and patients in Germany, the United Kingdom, and the United States responded to the survey. Eligible physicians consulted with at least four patients with cluster headache per month, and eligible patients had a diagnosis of episodic cluster headache that was consistent with ICHD-3 beta criteria. Additional data were collected from all participants through questionnaires.
The analysis included 309 patients in Germany, 328 in the United Kingdom, and 375 in the United States. The average age of the patients was 40 years, and most of the patients were male. Less than 70% of patients reported working full time, which may indicate “the impact of this condition on work status,” said Dr. Andrews. Patients’ average number of attacks per day within an active period was 2.4. The two most commonly reported comorbidities were anxiety and depression. About 40% of cases of depression were reported to have occurred after the receipt of a diagnosis of cluster headache.
Use of inhaled oxygen was low
Most patients received acute treatments. The proportion of patients who received acute therapy only was 53% in Germany, 48% in the United Kingdom, and 43% in the United States. Approximately 34% of patients in Germany received a combination of acute and preventive therapy, compared with 37% in the United Kingdom and 42% in the United States. The proportion of patients who received preventive therapy only was 10% in Germany, 8% in the United Kingdom, and 12% in the United States.
The most commonly prescribed acute treatment, regardless of formulation, was sumatriptan. About 60% of patients received this medication. Less than one-third of patients used inhaled oxygen. Oxygen was prescribed more often in Germany (45%) and the United Kingdom (33%), compared with the United States (19%). U.S. patients face well-known obstacles in getting access to, and reimbursement for, oxygen, said Dr. Andrews. “That’s an area that deserves increased attention.” Zolmitriptan was the third most commonly prescribed acute medication.
Among prescriptions for sumatriptan, oral and injectable formulations were approximately equally common. Recommendations, however, indicate formulations with potentially fast onset of action. “The average duration of one of these attacks is between 15 and 180 minutes, so that certainly suggests that a formulation that gives you a faster onset of action might improve outcomes,” said Dr. Andrews. The use of injectable sumatriptan was lowest in the United States and highest in the United Kingdom.
“The most common decision regarding preventive treatment was [to give] no preventive treatment,” said Dr. Andrews. Verapamil was the most commonly prescribed preventive therapy (34% in Germany, 29% in the United States, and 25% in the United Kingdom), followed by topiramate, lithium, and valproate.
Nonadherence and noncompliance was common
Fewer U.K. patients (32%) reported taking their preventive therapy as advised, compared with German patients (60%) and U.S. patients (80%). Common reasons for noncompliance, regardless of location, were forgetfulness, the belief that a dose was not needed, and side effects. Most patients in the United Kingdom (60%) and the United States (54%) reported the need to take an extra dose of their acute medication to relieve pain symptoms, compared with 30% in Germany. Furthermore, 13% of U.S. patients indicated that they took extra doses all the time or nearly all the time, compared with 2% in Germany and 7% in the United Kingdom. Among patients who had discontinued a preventive treatment in the past, the most common reasons for discontinuation were lack of efficacy and problems with tolerability.
One limitation of the study was that the survey was not designed to represent the general cluster headache or treating physician populations fully. The data may reflect selection bias in favor of physicians who treat high volumes of patients and in favor of patients who frequently seek health care. In addition, the data were based on self-reports.
“Increased awareness and educational efforts that aim at promoting the need and benefit of the preventive treatment for these patients is warranted,” Dr. Andrews concluded.
Dr. Andrews is an employee of Eli Lilly, which funded the study.
SOURCE: Nichols R et al. AHS 2019. Abstract OR04.
REPORTING FROM AHS 2019