User login
CDC finds that too little naloxone is dispensed
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).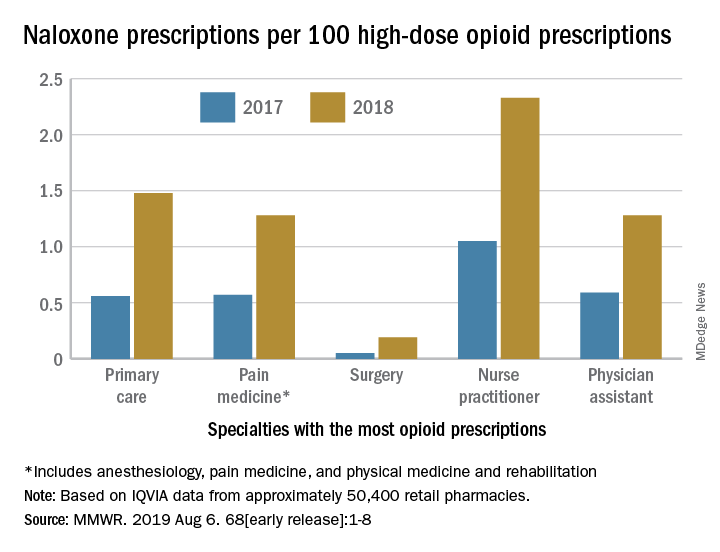
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
Although the CDC recommends that clinicians consider prescribing naloxone, which can reverse the effects of an opioid overdose, to patients who receive high-dose opioid prescriptions, one naloxone prescription was dispensed in 2018 for every 69 such patients, according to a Vital Signs investigation published Aug. 6 in the Morbidity and Mortality Weekly Report.
Approximately 9 million more naloxone prescriptions could have been dispensed in 2018 if every patient with a high-dose opioid prescription were offered the drug, according to the agency. In addition, the rate at which naloxone is dispensed varies significantly according to region.
“Thousands of Americans are alive today thanks to the use of naloxone,” said Alex M. Azar, secretary of Health and Human Services, in a press release. “Giving people a chance to survive an opioid overdose and safely enter recovery is one of the five key pillars of our HHS strategy for ending the overdose epidemic. With help from Congress, the private sector, state, and local governments and communities, targeted access to naloxone has expanded dramatically over the last several years, but today’s CDC report is a reminder that there is much more all of us need to do to save lives.”
Investigators examined retail pharmacy data
In 2017, 47,600 (67.8%) drug overdose deaths in the United States involved opioids. For decades, emergency medical service providers have administered naloxone to patients with suspected drug overdose. A major focus of public health initiatives intended to address the opioid overdose crisis has been to increase access to naloxone through clinician prescribing and pharmacy dispensing. The CDC recommends considering prescribing naloxone to patients with a history of overdose or substance use disorder, those receiving opioid dosages of 50 morphine milligram equivalents per day or greater (that is, high-dose prescriptions), and those who are using benzodiazepines concurrently.
Investigators at the CDC examined retail pharmacy data from IQVIA, a company that maintains information on prescriptions from approximately 50,400 retail pharmacies. They extracted data from 2012 through 2018 to analyze naloxone dispensing by region, urban versus rural status, prescriber specialty, and recipient characteristics (for example, age group, sex, out-of-pocket costs, and method of payment).
Dispensations doubled from 2017 to 2018
Naloxone dispensing from retail pharmacies increased from 0.4 prescriptions per 100,000 in 2012 to 170.2 prescriptions per 100,000 in 2018. From 2017 to 2018 alone, the number of prescriptions dispensed increased by 106%.
Despite consistency among state laws, naloxone dispensation varied by region. The average rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 0.2 in the lowest quartile to 2.9 in the highest quartile. In 2018, the rate of naloxone prescriptions per 100 high-dose opioid prescriptions ranged from 1.5 in metropolitan counties and 1.6 in the Northeast to 1.2 in rural counties and 1.3 in the Midwest. Rural counties were nearly three times more likely to be low-dispensing counties, compared with metropolitan counties.
The rate of naloxone prescriptions per 100 high-dose opioid prescriptions also varied by provider specialty. This rate was lowest among surgeons (0.2) and highest among psychiatrists (12.9).
Most naloxone prescriptions entailed out-of-pocket costs. About 71% of prescriptions paid for by Medicare entailed out-of-pocket costs, compared with 43.8% of prescriptions paid for by Medicaid, and 41.5% of prescriptions paid for by commercial insurance.
 Centers for Disease Control and Prevention
Centers for Disease Control and Prevention
More can be done

“It is clear from the data that there is still much needed education around the important role naloxone plays in reducing overdose deaths,” said Robert R. Redfield, MD, director of the CDC, in a press release. “The time is now to ensure all individuals who are prescribed high-dose opioids also receive naloxone as a potential life-saving intervention. As we aggressively confront what is the public health crisis of our time, CDC will continue to stress with health care providers the benefit of making this overdose-reversing medicine available to patients.”
“While we’ve seen these important increases [in naloxone prescriptions], we are not as far along as we’d like to be,” said Anne Schuchat, MD, principal deputy director of the CDC, during a press conference. “Cost is one of the issues, but I think awareness is another.” These data should prompt pharmacies to make sure that they stock naloxone and remind clinicians to consider naloxone when they prescribe opioids, she added. Patients and their family members should be aware of naloxone and ask their health care providers about it. “We’d really like to see the increase [in naloxone prescriptions] move much more rapidly,” she concluded.
The investigators disclosed no potential conflicts of interest.
SOURCE: Guy GP et al. MMWR Morb Mortal Wkly Rep. 2019 Aug 6.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Preoperative tramadol fails to improve function after knee surgery
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
according to findings of a study based on pre- and postsurgery data.
Tramadol has become a popular choice for nonoperative knee pain relief because of its low potential for abuse and favorable safety profile, but its impact on postoperative outcomes when given before knee surgery has not been well studied, wrote Adam Driesman, MD, of the New York University Langone Orthopedic Hospital and colleagues.
In a study published in the Journal of Arthroplasty, the researchers compared patient-reported outcomes (PRO) after total knee arthroplasty among 136 patients who received no opiates, 21 who received tramadol, and 42 who received other opiates. All patients who did not have preoperative and postoperative PRO scores were excluded
All patients received the same multimodal perioperative pain protocol, and all were placed on oxycodone postoperatively for maintenance and breakthrough pain as needed, with discharge prescriptions for acetaminophen/oxycodone combination (Percocet) for breakthrough pain.
Patients preoperative assessment using the Knee Disability and Osteoarthritis Outcome Score Jr. (KOOS, JR.) were similar among the groups prior to surgery; baseline scores for the groups receiving either tramadol, no opiates, or other opiates were 49.95, 50.4, and 48.0, respectively. Demographics also were not significantly different among the groups.
At 3 months, the average KOOS, JR., score for the tramadol group (62.4) was significantly lower, compared with the other-opiate group (67.1) and treatment-naive group (70.1). In addition, patients in the tramadol group had the least change in scores on KOOS, JR., with an average of 12.5 points, compared with 19.1-point and 20.1-point improvements, respectively, in the alternate-opiate group and opiate-naive group.
The data expand on previous findings that patients given preoperative opioids had proportionally less postoperative pain relief than those not on opioids, the researchers said, but noted that they were surprised by the worse outcomes in the tramadol group given its demonstrated side-effect profile.
The study findings were limited by several factors including the retrospective design and relatively short follow-up period, as well as the inability to accurately determine outpatient medication use, not only of opioids, but of nonopioid postoperative pain medications that could have affected the results, the researchers said.
“However, given the conflicting evidence presented in this study and despite the 2013 American Academy of Orthopedic Surgeons Clinical Practice Guidelines, it is recommended providers remain very conservative in their administration of outpatient narcotics including tramadol prior to surgery,” they concluded.
SOURCE: Driesman A et al. J Arthroplasty. 2019;34(8):1662-66.
FROM THE JOURNAL OF ARTHROPLASTY
Sacroiliac Joint Dysfunction in Patients With Low Back Pain
Patients experiencing sacroiliac joint (SIJ) dysfunction might show symptoms that overlap with those seen in lumbar spine pathology. This article reviews diagnostic tools that assist practitioners to discern the true pain generator in patients with low back pain (LBP) and therapeutic approaches when the cause is SIJ dysfunction.
Prevalence
Most of the US population will experience LBP at some point in their lives. A 2002 National Health Interview survey found that more than one-quarter (26.4%) of 31 044 respondents had complained of LBP in the previous 3 months.1 About 74 million individuals in the US experienced LBP in the past 3 months.1 A full 10% of the US population is expected to suffer from chronic LBP, and it is estimated that 2.3% of all visits to physicians are related to LBP.1
The etiology of LBP often is unclear even after thorough clinical and radiographic evaluation because of the myriad possible mechanisms. Degenerative disc disease, facet arthropathy, ligamentous hypertrophy, muscle spasm, hip arthropathy, and SIJ dysfunction are potential pain generators and exact clinical and radiographic correlation is not always possible. Compounding this difficulty is the lack of specificity with current diagnostic techniques. For example, many patients will have disc desiccation or herniation without any LBP or radicular symptoms on radiographic studies, such as X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). As such, providers of patients with diffuse radiographic abnormalities often have to identify a specific pain generator, which might not have any role in the patient’s pain.
Other tests, such as electromyographic studies, positron emission tomography (PET) scans, discography, and epidural steroid injections, can help pinpoint a specific pain generator. These tests might help determine whether the patient has a surgically treatable condition and could help predict whether a patient’s symptoms will respond to surgery.
However, the standard spine surgery workup often fails to identify an obvious pain generator in many individuals. The significant number of patients that fall into this category has prompted spine surgeons to consider other potential etiologies for LBP, and SIJ dysfunction has become a rapidly developing field of research.
Sacroiliac Joint Dysfunction
The SIJ is a bilateral, C-shaped synovial joint surrounded by a fibrous capsule and affixes the sacrum to the ilia. Several sacral ligaments and pelvic muscles support the SIJ. The L5 nerve ventral ramus and lumbosacral trunk pass anteriorly and the S1 nerve ventral ramus passes inferiorly to the joint capsule. The SIJ is innervated by the dorsal rami of L4-S3 nerve roots, transmitting nociception and temperature. Mechanisms of injury to the SIJ could arise from intra- and extra-articular etiologies, including capsular disruption, ligamentous tension, muscular inflammation, shearing, fractures, arthritis, and infection.2 Patients could develop SIJ pain spontaneously or after a traumatic event or repetitive shear.3 Risk factors for developing SIJ dysfunction include a history of lumbar fusion, scoliosis, leg length discrepancies, sustained athletic activity, pregnancy, seronegative HLA-B27 spondyloarthropathies, or gait abnormalities. Inflammation of the SIJ and surrounding structures secondary to an environmental insult in susceptible individuals is a common theme among these etiologies.2
Pain from the SIJ is localized to an area of approximately 3 cm × 10 cm that is inferior to the ipsilateral posterior superior iliac spine.4 Referred pain maps from SIJ dysfunction extend in the L5-S1 nerve distributions, commonly seen in the buttocks, groin, posterior thigh, and lower leg with radicular symptoms. However, this pain distribution demonstrates extensive variability among patients and bears strong similarities to discogenic or facet joint sources of LBP.5-7 Direct communication has been shown between the SIJ and adjacent neural structures, namely the L5 nerve, sacral foramina, and the lumbosacral plexus. These direct pathways could explain an inflammatory mechanism for lower extremity symptoms seen in SIJ dysfunction.8
The prevalence of SIJ dysfunction among patients with LBP is estimated to be 15% to 30%, an extraordinary number given the total number of patients presenting with LBP every year.9 These patients might represent a significant segment of patients with an unrevealing standard spine evaluation. Despite the large number of patients who experience SIJ dysfunction, there is disagreement about optimal methods for diagnosis and treatment.
Diagnosis
The International Association for the Study of Pain has proposed criteria for evaluating patients who have suspected SIJ dysfunction: Pain must be in the SIJ area, should be reproducible by performing specific provocative maneuvers, and must be relieved by injection of local anesthetic into the SIJ.10 These criteria provide a sound foundation, but in clinical practice, patients often defy categorization.
The presence of pain in the area inferior to the posterior superior iliac spine and lateral to the gluteal fold with pain referral patterns in the L5-S1 nerve distributions is highly sensitive for identifying patients with SIJ dysfunction. Furthermore, pain arising from the SIJ will not be above the level of the L5 nerve sensory distribution. However, this diagnostic finding alone is not specific and might represent other etiologies known to produce similar pain, such as intervertebral discs and facet joints. Patients with SIJ dysfunction often describe their pain as sciatica-like, recurrent, and triggered with bending or twisting motions. It is worsened with any activity loading the SIJ, such as walking, climbing stairs, standing, or sitting upright. SIJ pain might be accompanied by dyspareunia and changes in bladder function because of the nerves involved.11
The use of provocative maneuvers for testing SIJ dysfunction is controversial because of the high rate of false positives and the inability to distinguish whether the SIJ or an adjacent structure is affected. However, the diagnostic utility of specific stress tests has been studied, and clusters of tests are recommended if a health care provider (HCP) suspects SIJ dysfunction. A diagnostic algorithm should first focus on using the distraction test and the thigh thrust test. Distraction is done by applying vertically oriented pressure to the anterior superior iliac spine while aiming posteriorly, therefore distracting the SIJ. During the thigh thrust test the examiner fixates the patient’s sacrum against the table with the left hand and applies a vertical force through the line of the femur aiming posteriorly, producing a posterior shearing force at the SIJ. Studies show that the thigh thrust test is the most sensitive, and the distraction test is the most specific. If both tests are positive, there is reasonable evidence to suggest SIJ dysfunction as the source of LBP.
If there are not 2 positive results, the addition of the compression test, followed by the sacral thrust test also can point to the diagnosis. The compression test is performed with vertical downward force applied to the iliac crest with the patient lying on each side, compressing the SIJ by transverse pressure across the pelvis. The sacral thrust test is performed with vertical force applied to the midline posterior sacrum at its apex directed anteriorly with the patient lying prone, producing a shearing force at the SIJs. The Gaenslen test uses a torsion force by applying a superior and posterior force to the right knee and posteriorly directed force to the left knee. Omitting the Gaenslen test has not been shown to compromise diagnostic efficacy of the other tests and can be safely excluded.12
A HCP can rule out SIJ dysfunction if these provocation tests are negative. However, the diagnostic predictive value of these tests is subject to variability among HCPs, and their reliability is increased when used in clusters.9,13
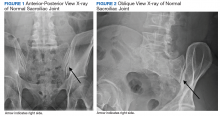
Imaging for the SIJ should begin with anterior/posterior, oblique, and lateral view plain X-rays of the pelvis (Figures 1 and 2), which will rule out other pathologies by identifying other sources of LBP, such as spondylolisthesis or hip osteoarthritis. HCPs should obtain lumbar and pelvis CT images to identify inflammatory or degenerative changes within the SIJ. CT images provide the high resolution that is needed to identify pathologies, such as fractures and tumors within the pelvic ring that could cause similar pain. MRI does not reliably depict a dysfunctional ligamentous apparatus within the SIJ; however, it can help identify inflammatory sacroiliitis, such as is seen in the spondyloarthropathies.11,14 Recent studies show combined single photon emission tomography and CT (SPECT-CT) might be the most promising imaging modality to reveal mechanical failure of load transfer with increased scintigraphic uptake in the posterior and superior SIJ ligamentous attachments. The joint loses its characteristic “dumbbell” shape in affected patients with about 50% higher uptake than unaffected joints. These findings were evident in patients who experienced pelvic trauma or during the peripartum period.15,16
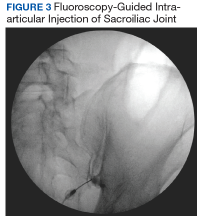
Fluoroscopy-guided intra-articular injection of a local anesthetic (lidocaine) and/or a corticosteroid (triamcinolone) has the dual functionality of diagnosis and treatment (Figure 3). It often is considered the most reliable method to diagnose SIJ dysfunction and has the benefit of pain relief for up to 1 year. However, intra-articular injections lack diagnostic validity because the solution often extravasates to extracapsular structures. This confounds the source of the pain and makes it difficult to interpret these diagnostic injections. In addition, the injection might not reach the entire SIJ capsule and could result in a false-negative diagnosis.17,18 Periarticular injections have been shown to result in better pain relief in patients diagnosed with SIJ dysfunction than intra-articular injections. Periarticular injections also are easier to perform and could be a first-step option for these patients.19
Treatment
Nonoperative management of SIJ dysfunction includes exercise programs, physical therapy, manual manipulation therapy, sacroiliac belts, and periodic articular injections. Efficacy of these methods is variable, and analgesics often do not significantly benefit this type of pain. Another nonoperative approach is radiofrequency ablation (RFA) of the lumbar dorsal rami and lateral sacral branches, which can vary based on the number of rami treated as well as the technique used. About two-thirds of patients report pain relief after RFA.2 When successful, pain is relieved for 6 to 12 months, which is a temporary yet effective option for patients experiencing SIJ dysfunction.14,20
Fusion Surgery
Cadaver studies show that biomechanical stabilization of the SIJ leads to decreased range of motion in flexion/extension, lateral bending, and axial rotation. This results in a decreased need for periarticular muscular and ligamentous support, therefore facilitating load transfer across the SIJ.21,22 Patients undergoing minimally invasive surgery report better pain relief compared with those receiving open surgery at 12 months postoperatively.23 The 2 main SIJ fusion approaches used are the lateral transarticular and the dorsal approaches. In the dorsal approach, the SIJ is distracted and allograft dowels or titanium cages with graft are inserted into the joint space posteriorly through the back. When approaching laterally, hollow screw implants filled with graft or triangular titanium implants are placed across the joint, accessing the SIJ through the iliac bones using imaging guidance. This lateral transiliac approach using porous titanium triangular rods currently is the most studied technique.24
A recent prospective, multicenter trial included 423 patients with SIJ dysfunction who were randomized to receive SIJ fusion with triangular titanium implants vs a control group who received nonoperative management. Patients in the SIJ fusion group showed substantially greater improvement in pain (81.4%) compared with that of the nonoperative group (26.1%) 6 months after surgery. Pain relief in the SIJ fusion group was maintained at > 80% at 1 and 2 year follow-up, while the nonoperative group’s pain relief decreased to < 10% at the follow-ups. Measures of quality of life and disability also improved for the SIJ fusion group compared with that of the nonoperative group. Patients who were crossed over from conservative management to SIJ fusion after 6 months demonstrated improvements that were similar to those in the SIJ fusion group by the end of the study. Only 3% of patients required surgical revision. The strongest predictor of pain relief after surgery was a diagnostic SIJ anesthetic block of 30 to 60 minutes, which resulted in > 75% pain reduction.21,25 Additional predictors of successful SIJ fusion include nonsmokers, nonopioid users, and older patients who have a longer time course of SIJ pain.26
Another study investigating the outcomes of SIJ fusion, RFA, and conservative management with a 6-year follow-up demonstrated similar results.27 This further confirms the durability of the surgical group’s outcome, which sustained significant improvement compared with RFA and conservative management group in pain relief, daily function, and opioid use.
HCPs should consider SIJ fusion for patients who have at least 6 months of unsuccessful nonoperative management, significant SIJ pain (> 5 in a 10-point scale), ≥ 3 positive provocation tests, and at least 50% pain relief (> 75% preferred) with diagnostic intra-articular anesthetic injection.14 It is reasonable for primary care providers to refer these patients to a neurosurgeon or orthopedic spine surgeon for possible fusion. Patients with earlier lumbar/lumbosacral spinal fusions and persistent LBP should be evaluated for potential SIJ dysfunction. SIJ dysfunction after lumbosacral fusion could be considered a form of distal pseudarthrosis resulting from increased motion at the joint. One study found its incidence correlated with the number of segments fused in the lumbar spine.28 Another study found that about one-third of patients with persistent LBP after lumbosacral fusion could be attributed to SIJ dysfunction.29
Case Presentation
A 27-year-old female army veteran presented with bilateral buttock pain, which she described as a dull, aching pain across her sacral region, 8 out of 10 in severity. The pain was in a L5-S1 pattern. The pain was bilateral, with the right side worse than the left, and worsened with lateral bending and load transferring. She reported no numbness, tingling, or weakness.
On physical examination, she had full strength in her lower extremities and intact sensation. She reported tenderness to palpation of the sacrum and SIJ. Her gait was normal. The patient had positive thigh thrust and distraction tests. Lumbar spine X-ray, CT, MRI, and electromyographic studies did not show any pathology. She described little or no relief with analgesics or physical therapy. Previous L4-L5 and L5-S1 facet anesthetic injections and transforaminal epidural steroid injections provided minimal pain relief immediately after the procedures. Bilateral SIJ anesthetic injections under fluoroscopic guidance decreased her pain severity from a 7 to 3 out of 10 for 2 to 3 months before returning to her baseline. Radiofrequency ablation of the right SIJ under fluoroscopy provided moderate relief for about 4 months.
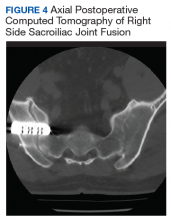
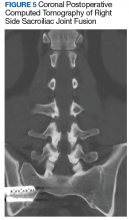
After exhausting nonoperative management for SIJ dysfunction without adequate pain control, the patient was referred to neurosurgery for surgical fusion. The patient was deemed an appropriate surgical candidate and underwent a right-sided SIJ fusion (Figures 4 and 5). At her 6-month and 1-year follow-up appointments, she had lasting pain relief, 2 out of 10.
Conclusion
SIJ dysfunction is widely overlooked because of the difficulty in distinguishing it from other similarly presenting syndromes. However, with a detailed history, appropriate physical maneuvers, imaging, and adequate response to intra-articular anesthetic, providers can reach an accurate diagnosis that will inform subsequent treatments. After failure of nonsurgical methods, patients with SIJ dysfunction should be considered for minimally invasive fusion techniques, which have proven to be a safe, effective, and viable treatment option.
1. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59-66.
2. Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
3. Chou LH, Slipman CW, Bhagia SM, et al. Inciting events initiating injection‐proven sacroiliac joint syndrome. Pain Med. 2004;5(1):26-32.
4. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
5. Buijs E, Visser L, Groen G. Sciatica and the sacroiliac joint: a forgotten concept. Br J Anaesth. 2007;99(5):713-716.
6. Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
7. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
8. Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. ANJR Am J Neuroradiol. 1999;20(8):1429-1434.
9. Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
10. Merskey H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
11. Cusi MF. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J Bodyw Mov Ther. 2010;14(2):152-161.
12. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
13. Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16(3):142-152.
14. Polly DW Jr. The sacroiliac joint. Neurosurg Clin N Am. 2017;28(3):301-312.
15. Cusi M, Van Der Wall H, Saunders J, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674-1682.
16. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24(4):859-863.
17. Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra‐articular sacroiliac joint injections: a systematic review. Pain Med. 2015;16(8):1500-1518.
18. Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does immediate pain relief after an injection into the sacroiliac joint with anesthetic and corticosteroid predict subsequent pain relief? Pain Med. 2018;19(2):244-251.
19. Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12(3):274-280.
20. Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
21. Polly DW, Cher DJ, Wine KD, et al; INSITE Study Group. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-690.
22. Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine. 2015;40(9):E525-E530.
23. Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
24. Rashbaum RF, Ohnmeiss DD, Lindley EM, Kitchel SH, Patel VV. Sacroiliac joint pain and its treatment. Clin Spine Surg. 2016;29(2):42-48.
25. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
26. Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine (Phila Pa 1976). 2017;42(21):1664-1673.
27. Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48-55.
28. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine (Phila Pa 1976). 2016;41(12):999-1005.
29. Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96-99.
Patients experiencing sacroiliac joint (SIJ) dysfunction might show symptoms that overlap with those seen in lumbar spine pathology. This article reviews diagnostic tools that assist practitioners to discern the true pain generator in patients with low back pain (LBP) and therapeutic approaches when the cause is SIJ dysfunction.
Prevalence
Most of the US population will experience LBP at some point in their lives. A 2002 National Health Interview survey found that more than one-quarter (26.4%) of 31 044 respondents had complained of LBP in the previous 3 months.1 About 74 million individuals in the US experienced LBP in the past 3 months.1 A full 10% of the US population is expected to suffer from chronic LBP, and it is estimated that 2.3% of all visits to physicians are related to LBP.1
The etiology of LBP often is unclear even after thorough clinical and radiographic evaluation because of the myriad possible mechanisms. Degenerative disc disease, facet arthropathy, ligamentous hypertrophy, muscle spasm, hip arthropathy, and SIJ dysfunction are potential pain generators and exact clinical and radiographic correlation is not always possible. Compounding this difficulty is the lack of specificity with current diagnostic techniques. For example, many patients will have disc desiccation or herniation without any LBP or radicular symptoms on radiographic studies, such as X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). As such, providers of patients with diffuse radiographic abnormalities often have to identify a specific pain generator, which might not have any role in the patient’s pain.
Other tests, such as electromyographic studies, positron emission tomography (PET) scans, discography, and epidural steroid injections, can help pinpoint a specific pain generator. These tests might help determine whether the patient has a surgically treatable condition and could help predict whether a patient’s symptoms will respond to surgery.
However, the standard spine surgery workup often fails to identify an obvious pain generator in many individuals. The significant number of patients that fall into this category has prompted spine surgeons to consider other potential etiologies for LBP, and SIJ dysfunction has become a rapidly developing field of research.
Sacroiliac Joint Dysfunction
The SIJ is a bilateral, C-shaped synovial joint surrounded by a fibrous capsule and affixes the sacrum to the ilia. Several sacral ligaments and pelvic muscles support the SIJ. The L5 nerve ventral ramus and lumbosacral trunk pass anteriorly and the S1 nerve ventral ramus passes inferiorly to the joint capsule. The SIJ is innervated by the dorsal rami of L4-S3 nerve roots, transmitting nociception and temperature. Mechanisms of injury to the SIJ could arise from intra- and extra-articular etiologies, including capsular disruption, ligamentous tension, muscular inflammation, shearing, fractures, arthritis, and infection.2 Patients could develop SIJ pain spontaneously or after a traumatic event or repetitive shear.3 Risk factors for developing SIJ dysfunction include a history of lumbar fusion, scoliosis, leg length discrepancies, sustained athletic activity, pregnancy, seronegative HLA-B27 spondyloarthropathies, or gait abnormalities. Inflammation of the SIJ and surrounding structures secondary to an environmental insult in susceptible individuals is a common theme among these etiologies.2
Pain from the SIJ is localized to an area of approximately 3 cm × 10 cm that is inferior to the ipsilateral posterior superior iliac spine.4 Referred pain maps from SIJ dysfunction extend in the L5-S1 nerve distributions, commonly seen in the buttocks, groin, posterior thigh, and lower leg with radicular symptoms. However, this pain distribution demonstrates extensive variability among patients and bears strong similarities to discogenic or facet joint sources of LBP.5-7 Direct communication has been shown between the SIJ and adjacent neural structures, namely the L5 nerve, sacral foramina, and the lumbosacral plexus. These direct pathways could explain an inflammatory mechanism for lower extremity symptoms seen in SIJ dysfunction.8
The prevalence of SIJ dysfunction among patients with LBP is estimated to be 15% to 30%, an extraordinary number given the total number of patients presenting with LBP every year.9 These patients might represent a significant segment of patients with an unrevealing standard spine evaluation. Despite the large number of patients who experience SIJ dysfunction, there is disagreement about optimal methods for diagnosis and treatment.
Diagnosis
The International Association for the Study of Pain has proposed criteria for evaluating patients who have suspected SIJ dysfunction: Pain must be in the SIJ area, should be reproducible by performing specific provocative maneuvers, and must be relieved by injection of local anesthetic into the SIJ.10 These criteria provide a sound foundation, but in clinical practice, patients often defy categorization.
The presence of pain in the area inferior to the posterior superior iliac spine and lateral to the gluteal fold with pain referral patterns in the L5-S1 nerve distributions is highly sensitive for identifying patients with SIJ dysfunction. Furthermore, pain arising from the SIJ will not be above the level of the L5 nerve sensory distribution. However, this diagnostic finding alone is not specific and might represent other etiologies known to produce similar pain, such as intervertebral discs and facet joints. Patients with SIJ dysfunction often describe their pain as sciatica-like, recurrent, and triggered with bending or twisting motions. It is worsened with any activity loading the SIJ, such as walking, climbing stairs, standing, or sitting upright. SIJ pain might be accompanied by dyspareunia and changes in bladder function because of the nerves involved.11
The use of provocative maneuvers for testing SIJ dysfunction is controversial because of the high rate of false positives and the inability to distinguish whether the SIJ or an adjacent structure is affected. However, the diagnostic utility of specific stress tests has been studied, and clusters of tests are recommended if a health care provider (HCP) suspects SIJ dysfunction. A diagnostic algorithm should first focus on using the distraction test and the thigh thrust test. Distraction is done by applying vertically oriented pressure to the anterior superior iliac spine while aiming posteriorly, therefore distracting the SIJ. During the thigh thrust test the examiner fixates the patient’s sacrum against the table with the left hand and applies a vertical force through the line of the femur aiming posteriorly, producing a posterior shearing force at the SIJ. Studies show that the thigh thrust test is the most sensitive, and the distraction test is the most specific. If both tests are positive, there is reasonable evidence to suggest SIJ dysfunction as the source of LBP.
If there are not 2 positive results, the addition of the compression test, followed by the sacral thrust test also can point to the diagnosis. The compression test is performed with vertical downward force applied to the iliac crest with the patient lying on each side, compressing the SIJ by transverse pressure across the pelvis. The sacral thrust test is performed with vertical force applied to the midline posterior sacrum at its apex directed anteriorly with the patient lying prone, producing a shearing force at the SIJs. The Gaenslen test uses a torsion force by applying a superior and posterior force to the right knee and posteriorly directed force to the left knee. Omitting the Gaenslen test has not been shown to compromise diagnostic efficacy of the other tests and can be safely excluded.12
A HCP can rule out SIJ dysfunction if these provocation tests are negative. However, the diagnostic predictive value of these tests is subject to variability among HCPs, and their reliability is increased when used in clusters.9,13
Imaging for the SIJ should begin with anterior/posterior, oblique, and lateral view plain X-rays of the pelvis (Figures 1 and 2), which will rule out other pathologies by identifying other sources of LBP, such as spondylolisthesis or hip osteoarthritis. HCPs should obtain lumbar and pelvis CT images to identify inflammatory or degenerative changes within the SIJ. CT images provide the high resolution that is needed to identify pathologies, such as fractures and tumors within the pelvic ring that could cause similar pain. MRI does not reliably depict a dysfunctional ligamentous apparatus within the SIJ; however, it can help identify inflammatory sacroiliitis, such as is seen in the spondyloarthropathies.11,14 Recent studies show combined single photon emission tomography and CT (SPECT-CT) might be the most promising imaging modality to reveal mechanical failure of load transfer with increased scintigraphic uptake in the posterior and superior SIJ ligamentous attachments. The joint loses its characteristic “dumbbell” shape in affected patients with about 50% higher uptake than unaffected joints. These findings were evident in patients who experienced pelvic trauma or during the peripartum period.15,16
Fluoroscopy-guided intra-articular injection of a local anesthetic (lidocaine) and/or a corticosteroid (triamcinolone) has the dual functionality of diagnosis and treatment (Figure 3). It often is considered the most reliable method to diagnose SIJ dysfunction and has the benefit of pain relief for up to 1 year. However, intra-articular injections lack diagnostic validity because the solution often extravasates to extracapsular structures. This confounds the source of the pain and makes it difficult to interpret these diagnostic injections. In addition, the injection might not reach the entire SIJ capsule and could result in a false-negative diagnosis.17,18 Periarticular injections have been shown to result in better pain relief in patients diagnosed with SIJ dysfunction than intra-articular injections. Periarticular injections also are easier to perform and could be a first-step option for these patients.19
Treatment
Nonoperative management of SIJ dysfunction includes exercise programs, physical therapy, manual manipulation therapy, sacroiliac belts, and periodic articular injections. Efficacy of these methods is variable, and analgesics often do not significantly benefit this type of pain. Another nonoperative approach is radiofrequency ablation (RFA) of the lumbar dorsal rami and lateral sacral branches, which can vary based on the number of rami treated as well as the technique used. About two-thirds of patients report pain relief after RFA.2 When successful, pain is relieved for 6 to 12 months, which is a temporary yet effective option for patients experiencing SIJ dysfunction.14,20
Fusion Surgery
Cadaver studies show that biomechanical stabilization of the SIJ leads to decreased range of motion in flexion/extension, lateral bending, and axial rotation. This results in a decreased need for periarticular muscular and ligamentous support, therefore facilitating load transfer across the SIJ.21,22 Patients undergoing minimally invasive surgery report better pain relief compared with those receiving open surgery at 12 months postoperatively.23 The 2 main SIJ fusion approaches used are the lateral transarticular and the dorsal approaches. In the dorsal approach, the SIJ is distracted and allograft dowels or titanium cages with graft are inserted into the joint space posteriorly through the back. When approaching laterally, hollow screw implants filled with graft or triangular titanium implants are placed across the joint, accessing the SIJ through the iliac bones using imaging guidance. This lateral transiliac approach using porous titanium triangular rods currently is the most studied technique.24
A recent prospective, multicenter trial included 423 patients with SIJ dysfunction who were randomized to receive SIJ fusion with triangular titanium implants vs a control group who received nonoperative management. Patients in the SIJ fusion group showed substantially greater improvement in pain (81.4%) compared with that of the nonoperative group (26.1%) 6 months after surgery. Pain relief in the SIJ fusion group was maintained at > 80% at 1 and 2 year follow-up, while the nonoperative group’s pain relief decreased to < 10% at the follow-ups. Measures of quality of life and disability also improved for the SIJ fusion group compared with that of the nonoperative group. Patients who were crossed over from conservative management to SIJ fusion after 6 months demonstrated improvements that were similar to those in the SIJ fusion group by the end of the study. Only 3% of patients required surgical revision. The strongest predictor of pain relief after surgery was a diagnostic SIJ anesthetic block of 30 to 60 minutes, which resulted in > 75% pain reduction.21,25 Additional predictors of successful SIJ fusion include nonsmokers, nonopioid users, and older patients who have a longer time course of SIJ pain.26
Another study investigating the outcomes of SIJ fusion, RFA, and conservative management with a 6-year follow-up demonstrated similar results.27 This further confirms the durability of the surgical group’s outcome, which sustained significant improvement compared with RFA and conservative management group in pain relief, daily function, and opioid use.
HCPs should consider SIJ fusion for patients who have at least 6 months of unsuccessful nonoperative management, significant SIJ pain (> 5 in a 10-point scale), ≥ 3 positive provocation tests, and at least 50% pain relief (> 75% preferred) with diagnostic intra-articular anesthetic injection.14 It is reasonable for primary care providers to refer these patients to a neurosurgeon or orthopedic spine surgeon for possible fusion. Patients with earlier lumbar/lumbosacral spinal fusions and persistent LBP should be evaluated for potential SIJ dysfunction. SIJ dysfunction after lumbosacral fusion could be considered a form of distal pseudarthrosis resulting from increased motion at the joint. One study found its incidence correlated with the number of segments fused in the lumbar spine.28 Another study found that about one-third of patients with persistent LBP after lumbosacral fusion could be attributed to SIJ dysfunction.29
Case Presentation
A 27-year-old female army veteran presented with bilateral buttock pain, which she described as a dull, aching pain across her sacral region, 8 out of 10 in severity. The pain was in a L5-S1 pattern. The pain was bilateral, with the right side worse than the left, and worsened with lateral bending and load transferring. She reported no numbness, tingling, or weakness.
On physical examination, she had full strength in her lower extremities and intact sensation. She reported tenderness to palpation of the sacrum and SIJ. Her gait was normal. The patient had positive thigh thrust and distraction tests. Lumbar spine X-ray, CT, MRI, and electromyographic studies did not show any pathology. She described little or no relief with analgesics or physical therapy. Previous L4-L5 and L5-S1 facet anesthetic injections and transforaminal epidural steroid injections provided minimal pain relief immediately after the procedures. Bilateral SIJ anesthetic injections under fluoroscopic guidance decreased her pain severity from a 7 to 3 out of 10 for 2 to 3 months before returning to her baseline. Radiofrequency ablation of the right SIJ under fluoroscopy provided moderate relief for about 4 months.
After exhausting nonoperative management for SIJ dysfunction without adequate pain control, the patient was referred to neurosurgery for surgical fusion. The patient was deemed an appropriate surgical candidate and underwent a right-sided SIJ fusion (Figures 4 and 5). At her 6-month and 1-year follow-up appointments, she had lasting pain relief, 2 out of 10.
Conclusion
SIJ dysfunction is widely overlooked because of the difficulty in distinguishing it from other similarly presenting syndromes. However, with a detailed history, appropriate physical maneuvers, imaging, and adequate response to intra-articular anesthetic, providers can reach an accurate diagnosis that will inform subsequent treatments. After failure of nonsurgical methods, patients with SIJ dysfunction should be considered for minimally invasive fusion techniques, which have proven to be a safe, effective, and viable treatment option.
Patients experiencing sacroiliac joint (SIJ) dysfunction might show symptoms that overlap with those seen in lumbar spine pathology. This article reviews diagnostic tools that assist practitioners to discern the true pain generator in patients with low back pain (LBP) and therapeutic approaches when the cause is SIJ dysfunction.
Prevalence
Most of the US population will experience LBP at some point in their lives. A 2002 National Health Interview survey found that more than one-quarter (26.4%) of 31 044 respondents had complained of LBP in the previous 3 months.1 About 74 million individuals in the US experienced LBP in the past 3 months.1 A full 10% of the US population is expected to suffer from chronic LBP, and it is estimated that 2.3% of all visits to physicians are related to LBP.1
The etiology of LBP often is unclear even after thorough clinical and radiographic evaluation because of the myriad possible mechanisms. Degenerative disc disease, facet arthropathy, ligamentous hypertrophy, muscle spasm, hip arthropathy, and SIJ dysfunction are potential pain generators and exact clinical and radiographic correlation is not always possible. Compounding this difficulty is the lack of specificity with current diagnostic techniques. For example, many patients will have disc desiccation or herniation without any LBP or radicular symptoms on radiographic studies, such as X-rays, computed tomography (CT), and magnetic resonance imaging (MRI). As such, providers of patients with diffuse radiographic abnormalities often have to identify a specific pain generator, which might not have any role in the patient’s pain.
Other tests, such as electromyographic studies, positron emission tomography (PET) scans, discography, and epidural steroid injections, can help pinpoint a specific pain generator. These tests might help determine whether the patient has a surgically treatable condition and could help predict whether a patient’s symptoms will respond to surgery.
However, the standard spine surgery workup often fails to identify an obvious pain generator in many individuals. The significant number of patients that fall into this category has prompted spine surgeons to consider other potential etiologies for LBP, and SIJ dysfunction has become a rapidly developing field of research.
Sacroiliac Joint Dysfunction
The SIJ is a bilateral, C-shaped synovial joint surrounded by a fibrous capsule and affixes the sacrum to the ilia. Several sacral ligaments and pelvic muscles support the SIJ. The L5 nerve ventral ramus and lumbosacral trunk pass anteriorly and the S1 nerve ventral ramus passes inferiorly to the joint capsule. The SIJ is innervated by the dorsal rami of L4-S3 nerve roots, transmitting nociception and temperature. Mechanisms of injury to the SIJ could arise from intra- and extra-articular etiologies, including capsular disruption, ligamentous tension, muscular inflammation, shearing, fractures, arthritis, and infection.2 Patients could develop SIJ pain spontaneously or after a traumatic event or repetitive shear.3 Risk factors for developing SIJ dysfunction include a history of lumbar fusion, scoliosis, leg length discrepancies, sustained athletic activity, pregnancy, seronegative HLA-B27 spondyloarthropathies, or gait abnormalities. Inflammation of the SIJ and surrounding structures secondary to an environmental insult in susceptible individuals is a common theme among these etiologies.2
Pain from the SIJ is localized to an area of approximately 3 cm × 10 cm that is inferior to the ipsilateral posterior superior iliac spine.4 Referred pain maps from SIJ dysfunction extend in the L5-S1 nerve distributions, commonly seen in the buttocks, groin, posterior thigh, and lower leg with radicular symptoms. However, this pain distribution demonstrates extensive variability among patients and bears strong similarities to discogenic or facet joint sources of LBP.5-7 Direct communication has been shown between the SIJ and adjacent neural structures, namely the L5 nerve, sacral foramina, and the lumbosacral plexus. These direct pathways could explain an inflammatory mechanism for lower extremity symptoms seen in SIJ dysfunction.8
The prevalence of SIJ dysfunction among patients with LBP is estimated to be 15% to 30%, an extraordinary number given the total number of patients presenting with LBP every year.9 These patients might represent a significant segment of patients with an unrevealing standard spine evaluation. Despite the large number of patients who experience SIJ dysfunction, there is disagreement about optimal methods for diagnosis and treatment.
Diagnosis
The International Association for the Study of Pain has proposed criteria for evaluating patients who have suspected SIJ dysfunction: Pain must be in the SIJ area, should be reproducible by performing specific provocative maneuvers, and must be relieved by injection of local anesthetic into the SIJ.10 These criteria provide a sound foundation, but in clinical practice, patients often defy categorization.
The presence of pain in the area inferior to the posterior superior iliac spine and lateral to the gluteal fold with pain referral patterns in the L5-S1 nerve distributions is highly sensitive for identifying patients with SIJ dysfunction. Furthermore, pain arising from the SIJ will not be above the level of the L5 nerve sensory distribution. However, this diagnostic finding alone is not specific and might represent other etiologies known to produce similar pain, such as intervertebral discs and facet joints. Patients with SIJ dysfunction often describe their pain as sciatica-like, recurrent, and triggered with bending or twisting motions. It is worsened with any activity loading the SIJ, such as walking, climbing stairs, standing, or sitting upright. SIJ pain might be accompanied by dyspareunia and changes in bladder function because of the nerves involved.11
The use of provocative maneuvers for testing SIJ dysfunction is controversial because of the high rate of false positives and the inability to distinguish whether the SIJ or an adjacent structure is affected. However, the diagnostic utility of specific stress tests has been studied, and clusters of tests are recommended if a health care provider (HCP) suspects SIJ dysfunction. A diagnostic algorithm should first focus on using the distraction test and the thigh thrust test. Distraction is done by applying vertically oriented pressure to the anterior superior iliac spine while aiming posteriorly, therefore distracting the SIJ. During the thigh thrust test the examiner fixates the patient’s sacrum against the table with the left hand and applies a vertical force through the line of the femur aiming posteriorly, producing a posterior shearing force at the SIJ. Studies show that the thigh thrust test is the most sensitive, and the distraction test is the most specific. If both tests are positive, there is reasonable evidence to suggest SIJ dysfunction as the source of LBP.
If there are not 2 positive results, the addition of the compression test, followed by the sacral thrust test also can point to the diagnosis. The compression test is performed with vertical downward force applied to the iliac crest with the patient lying on each side, compressing the SIJ by transverse pressure across the pelvis. The sacral thrust test is performed with vertical force applied to the midline posterior sacrum at its apex directed anteriorly with the patient lying prone, producing a shearing force at the SIJs. The Gaenslen test uses a torsion force by applying a superior and posterior force to the right knee and posteriorly directed force to the left knee. Omitting the Gaenslen test has not been shown to compromise diagnostic efficacy of the other tests and can be safely excluded.12
A HCP can rule out SIJ dysfunction if these provocation tests are negative. However, the diagnostic predictive value of these tests is subject to variability among HCPs, and their reliability is increased when used in clusters.9,13
Imaging for the SIJ should begin with anterior/posterior, oblique, and lateral view plain X-rays of the pelvis (Figures 1 and 2), which will rule out other pathologies by identifying other sources of LBP, such as spondylolisthesis or hip osteoarthritis. HCPs should obtain lumbar and pelvis CT images to identify inflammatory or degenerative changes within the SIJ. CT images provide the high resolution that is needed to identify pathologies, such as fractures and tumors within the pelvic ring that could cause similar pain. MRI does not reliably depict a dysfunctional ligamentous apparatus within the SIJ; however, it can help identify inflammatory sacroiliitis, such as is seen in the spondyloarthropathies.11,14 Recent studies show combined single photon emission tomography and CT (SPECT-CT) might be the most promising imaging modality to reveal mechanical failure of load transfer with increased scintigraphic uptake in the posterior and superior SIJ ligamentous attachments. The joint loses its characteristic “dumbbell” shape in affected patients with about 50% higher uptake than unaffected joints. These findings were evident in patients who experienced pelvic trauma or during the peripartum period.15,16
Fluoroscopy-guided intra-articular injection of a local anesthetic (lidocaine) and/or a corticosteroid (triamcinolone) has the dual functionality of diagnosis and treatment (Figure 3). It often is considered the most reliable method to diagnose SIJ dysfunction and has the benefit of pain relief for up to 1 year. However, intra-articular injections lack diagnostic validity because the solution often extravasates to extracapsular structures. This confounds the source of the pain and makes it difficult to interpret these diagnostic injections. In addition, the injection might not reach the entire SIJ capsule and could result in a false-negative diagnosis.17,18 Periarticular injections have been shown to result in better pain relief in patients diagnosed with SIJ dysfunction than intra-articular injections. Periarticular injections also are easier to perform and could be a first-step option for these patients.19
Treatment
Nonoperative management of SIJ dysfunction includes exercise programs, physical therapy, manual manipulation therapy, sacroiliac belts, and periodic articular injections. Efficacy of these methods is variable, and analgesics often do not significantly benefit this type of pain. Another nonoperative approach is radiofrequency ablation (RFA) of the lumbar dorsal rami and lateral sacral branches, which can vary based on the number of rami treated as well as the technique used. About two-thirds of patients report pain relief after RFA.2 When successful, pain is relieved for 6 to 12 months, which is a temporary yet effective option for patients experiencing SIJ dysfunction.14,20
Fusion Surgery
Cadaver studies show that biomechanical stabilization of the SIJ leads to decreased range of motion in flexion/extension, lateral bending, and axial rotation. This results in a decreased need for periarticular muscular and ligamentous support, therefore facilitating load transfer across the SIJ.21,22 Patients undergoing minimally invasive surgery report better pain relief compared with those receiving open surgery at 12 months postoperatively.23 The 2 main SIJ fusion approaches used are the lateral transarticular and the dorsal approaches. In the dorsal approach, the SIJ is distracted and allograft dowels or titanium cages with graft are inserted into the joint space posteriorly through the back. When approaching laterally, hollow screw implants filled with graft or triangular titanium implants are placed across the joint, accessing the SIJ through the iliac bones using imaging guidance. This lateral transiliac approach using porous titanium triangular rods currently is the most studied technique.24
A recent prospective, multicenter trial included 423 patients with SIJ dysfunction who were randomized to receive SIJ fusion with triangular titanium implants vs a control group who received nonoperative management. Patients in the SIJ fusion group showed substantially greater improvement in pain (81.4%) compared with that of the nonoperative group (26.1%) 6 months after surgery. Pain relief in the SIJ fusion group was maintained at > 80% at 1 and 2 year follow-up, while the nonoperative group’s pain relief decreased to < 10% at the follow-ups. Measures of quality of life and disability also improved for the SIJ fusion group compared with that of the nonoperative group. Patients who were crossed over from conservative management to SIJ fusion after 6 months demonstrated improvements that were similar to those in the SIJ fusion group by the end of the study. Only 3% of patients required surgical revision. The strongest predictor of pain relief after surgery was a diagnostic SIJ anesthetic block of 30 to 60 minutes, which resulted in > 75% pain reduction.21,25 Additional predictors of successful SIJ fusion include nonsmokers, nonopioid users, and older patients who have a longer time course of SIJ pain.26
Another study investigating the outcomes of SIJ fusion, RFA, and conservative management with a 6-year follow-up demonstrated similar results.27 This further confirms the durability of the surgical group’s outcome, which sustained significant improvement compared with RFA and conservative management group in pain relief, daily function, and opioid use.
HCPs should consider SIJ fusion for patients who have at least 6 months of unsuccessful nonoperative management, significant SIJ pain (> 5 in a 10-point scale), ≥ 3 positive provocation tests, and at least 50% pain relief (> 75% preferred) with diagnostic intra-articular anesthetic injection.14 It is reasonable for primary care providers to refer these patients to a neurosurgeon or orthopedic spine surgeon for possible fusion. Patients with earlier lumbar/lumbosacral spinal fusions and persistent LBP should be evaluated for potential SIJ dysfunction. SIJ dysfunction after lumbosacral fusion could be considered a form of distal pseudarthrosis resulting from increased motion at the joint. One study found its incidence correlated with the number of segments fused in the lumbar spine.28 Another study found that about one-third of patients with persistent LBP after lumbosacral fusion could be attributed to SIJ dysfunction.29
Case Presentation
A 27-year-old female army veteran presented with bilateral buttock pain, which she described as a dull, aching pain across her sacral region, 8 out of 10 in severity. The pain was in a L5-S1 pattern. The pain was bilateral, with the right side worse than the left, and worsened with lateral bending and load transferring. She reported no numbness, tingling, or weakness.
On physical examination, she had full strength in her lower extremities and intact sensation. She reported tenderness to palpation of the sacrum and SIJ. Her gait was normal. The patient had positive thigh thrust and distraction tests. Lumbar spine X-ray, CT, MRI, and electromyographic studies did not show any pathology. She described little or no relief with analgesics or physical therapy. Previous L4-L5 and L5-S1 facet anesthetic injections and transforaminal epidural steroid injections provided minimal pain relief immediately after the procedures. Bilateral SIJ anesthetic injections under fluoroscopic guidance decreased her pain severity from a 7 to 3 out of 10 for 2 to 3 months before returning to her baseline. Radiofrequency ablation of the right SIJ under fluoroscopy provided moderate relief for about 4 months.
After exhausting nonoperative management for SIJ dysfunction without adequate pain control, the patient was referred to neurosurgery for surgical fusion. The patient was deemed an appropriate surgical candidate and underwent a right-sided SIJ fusion (Figures 4 and 5). At her 6-month and 1-year follow-up appointments, she had lasting pain relief, 2 out of 10.
Conclusion
SIJ dysfunction is widely overlooked because of the difficulty in distinguishing it from other similarly presenting syndromes. However, with a detailed history, appropriate physical maneuvers, imaging, and adequate response to intra-articular anesthetic, providers can reach an accurate diagnosis that will inform subsequent treatments. After failure of nonsurgical methods, patients with SIJ dysfunction should be considered for minimally invasive fusion techniques, which have proven to be a safe, effective, and viable treatment option.
1. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59-66.
2. Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
3. Chou LH, Slipman CW, Bhagia SM, et al. Inciting events initiating injection‐proven sacroiliac joint syndrome. Pain Med. 2004;5(1):26-32.
4. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
5. Buijs E, Visser L, Groen G. Sciatica and the sacroiliac joint: a forgotten concept. Br J Anaesth. 2007;99(5):713-716.
6. Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
7. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
8. Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. ANJR Am J Neuroradiol. 1999;20(8):1429-1434.
9. Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
10. Merskey H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
11. Cusi MF. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J Bodyw Mov Ther. 2010;14(2):152-161.
12. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
13. Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16(3):142-152.
14. Polly DW Jr. The sacroiliac joint. Neurosurg Clin N Am. 2017;28(3):301-312.
15. Cusi M, Van Der Wall H, Saunders J, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674-1682.
16. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24(4):859-863.
17. Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra‐articular sacroiliac joint injections: a systematic review. Pain Med. 2015;16(8):1500-1518.
18. Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does immediate pain relief after an injection into the sacroiliac joint with anesthetic and corticosteroid predict subsequent pain relief? Pain Med. 2018;19(2):244-251.
19. Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12(3):274-280.
20. Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
21. Polly DW, Cher DJ, Wine KD, et al; INSITE Study Group. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-690.
22. Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine. 2015;40(9):E525-E530.
23. Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
24. Rashbaum RF, Ohnmeiss DD, Lindley EM, Kitchel SH, Patel VV. Sacroiliac joint pain and its treatment. Clin Spine Surg. 2016;29(2):42-48.
25. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
26. Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine (Phila Pa 1976). 2017;42(21):1664-1673.
27. Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48-55.
28. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine (Phila Pa 1976). 2016;41(12):999-1005.
29. Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96-99.
1. Zaidi HA, Montoure AJ, Dickman CA. Surgical and clinical efficacy of sacroiliac joint fusion: a systematic review of the literature. J Neurosurg Spine. 2015;23(1):59-66.
2. Cohen SP. Sacroiliac joint pain: a comprehensive review of anatomy, diagnosis, and treatment. Anesth Analg. 2005;101(5):1440-1453.
3. Chou LH, Slipman CW, Bhagia SM, et al. Inciting events initiating injection‐proven sacroiliac joint syndrome. Pain Med. 2004;5(1):26-32.
4. Dreyfuss P, Dreyer SJ, Cole A, Mayo K. Sacroiliac joint pain. J Am Acad Orthop Surg. 2004;12(4):255-265.
5. Buijs E, Visser L, Groen G. Sciatica and the sacroiliac joint: a forgotten concept. Br J Anaesth. 2007;99(5):713-716.
6. Fortin JD, Dwyer AP, West S, Pier J. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: asymptomatic volunteers. Spine (Phila Pa 1976). 1994;19(13):1475-1482.
7. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine (Phila Pa 1976). 1995;20(1):31-37.
8. Fortin JD, Washington WJ, Falco FJ. Three pathways between the sacroiliac joint and neural structures. ANJR Am J Neuroradiol. 1999;20(8):1429-1434.
9. Szadek KM, van der Wurff P, van Tulder MW, Zuurmond WW, Perez RS. Diagnostic validity of criteria for sacroiliac joint pain: a systematic review. J Pain. 2009;10(4):354-368.
10. Merskey H, Bogduk N, eds. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
11. Cusi MF. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J Bodyw Mov Ther. 2010;14(2):152-161.
12. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207-218.
13. Laslett M. Evidence-based diagnosis and treatment of the painful sacroiliac joint. J Man Manip Ther. 2008;16(3):142-152.
14. Polly DW Jr. The sacroiliac joint. Neurosurg Clin N Am. 2017;28(3):301-312.
15. Cusi M, Van Der Wall H, Saunders J, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674-1682.
16. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24(4):859-863.
17. Kennedy DJ, Engel A, Kreiner DS, Nampiaparampil D, Duszynski B, MacVicar J. Fluoroscopically guided diagnostic and therapeutic intra‐articular sacroiliac joint injections: a systematic review. Pain Med. 2015;16(8):1500-1518.
18. Schneider BJ, Huynh L, Levin J, Rinkaekan P, Kordi R, Kennedy DJ. Does immediate pain relief after an injection into the sacroiliac joint with anesthetic and corticosteroid predict subsequent pain relief? Pain Med. 2018;19(2):244-251.
19. Murakami E, Tanaka Y, Aizawa T, Ishizuka M, Kokubun S. Effect of periarticular and intraarticular lidocaine injections for sacroiliac joint pain: prospective comparative study. J Orthop Sci. 2007;12(3):274-280.
20. Cohen SP, Hurley RW, Buckenmaier CC 3rd, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279-288.
21. Polly DW, Cher DJ, Wine KD, et al; INSITE Study Group. Randomized controlled trial of minimally invasive sacroiliac joint fusion using triangular titanium implants vs nonsurgical management for sacroiliac joint dysfunction: 12-month outcomes. Neurosurgery. 2015;77(5):674-690.
22. Soriano-Baron H, Lindsey DP, Rodriguez-Martinez N, et al. The effect of implant placement on sacroiliac joint range of motion: posterior versus transarticular. Spine. 2015;40(9):E525-E530.
23. Smith AG, Capobianco R, Cher D, et al. Open versus minimally invasive sacroiliac joint fusion: a multi-center comparison of perioperative measures and clinical outcomes. Ann Surg Innov Res. 2013;7(1):14.
24. Rashbaum RF, Ohnmeiss DD, Lindley EM, Kitchel SH, Patel VV. Sacroiliac joint pain and its treatment. Clin Spine Surg. 2016;29(2):42-48.
25. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28.
26. Dengler J, Duhon B, Whang P, et al. Predictors of outcome in conservative and minimally invasive surgical management of pain originating from the sacroiliac joint: a pooled analysis. Spine (Phila Pa 1976). 2017;42(21):1664-1673.
27. Vanaclocha V, Herrera JM, Sáiz-Sapena N, Rivera-Paz M, Verdú-López F. Minimally invasive sacroiliac joint fusion, radiofrequency denervation, and conservative management for sacroiliac joint pain: 6-year comparative case series. Neurosurgery. 2018;82(1):48-55.
28. Unoki E, Abe E, Murai H, Kobayashi T, Abe T. Fusion of multiple segments can increase the incidence of sacroiliac joint pain after lumbar or lumbosacral fusion. Spine (Phila Pa 1976). 2016;41(12):999-1005.
29. Katz V, Schofferman J, Reynolds J. The sacroiliac joint: a potential cause of pain after lumbar fusion to the sacrum. J Spinal Disord Tech. 2003;16(1):96-99.
COPD adds complexity to shared decision making for LDCT lung cancer screening
research suggests.
Jonathan M. Iaccarino, MD, of the pulmonary center at the Boston University, and coauthors reported the results of a secondary analysis of patient-level outcomes from 75,138 low-dose CT (LDCT) scans in 26,453 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016).
Currently, LDCT screening is recommended annually for high-risk smokers aged 55-80 years. The National Lung Screening Trial showed that this screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in this group. The guidelines stress the importance of shared decision making, with discussion of the risks and benefits of screening.
Dr. Iaccarino and colleagues point out that decision aids for shared decision making need to include important baseline characteristics, such as the presence of COPD, as these can complicate the risk and benefit analysis.
In this study, they found that 14.2% of LDCT scans performed led to a subsequent diagnostic study and 1.5% resulted in an invasive procedure. In addition, 0.3% of scans resulted in a procedure-related complication, and in 89 cases (0.1%), this procedure-related complication was serious.
At the patient level, nearly one-third (30.5%) received a diagnostic study, 4.2% underwent an invasive procedure – 41% of whom ultimately were found not to have lung cancer – 0.9% had a procedure-related complication, and 0.3% had a serious procedure related complication. Furthermore, among those who experienced a serious complication, 12.5% were found not to have lung cancer.
“Our study analyzes cumulative outcomes at the level of the individual patient over the three years of LDCT screening during the NLST, showing higher rates of diagnostic procedures, invasive procedures, complications and serious complications than apparent when data is presented at the level of the individual test,” the authors wrote.
The 4,632 participants with COPD were significantly more likely to undergo diagnostic studies (36.2%), have an invasive procedure (6%), experience a procedure-related complication (1.5%) and experience a serious procedure-related complication (0.6%) than were participants without COPD. However, they also had a significantly higher incidence of lung cancer diagnosis than did participants without COPD (6.1% vs. 3.6%).
“While most decision aids note the risks of screening may be increased in those with COPD, our study helps quantify these increased risks as well as the increased likelihood of a lung cancer diagnosis, a critical advance given that providing personalized (rather than generic) information results in more accurate risk perception and more informed choices among individuals considering screening,” the authors wrote. “With the significant change in the balance of benefits and risks of screening in patients with COPD, it is critical to adjust the shared decision-making discussions accordingly.”
They also noted that other comorbidities, such as heart disease, vascular disease, and other lung diseases, would likely affect the balance of risk and benefit of LDCT screening, and that there was a need for further exploration of screening in these patients.
Noting the study’s limitations, the authors pointed that their analysis focused on outcomes that were not the primary outcomes of the National Lung Screening trial, and that they relied on self-reported COPD diagnoses.
The study was supported by the American Society of Clinical Oncology, the Charles A. King Trust, and Edith Nourse Rogers Memorial Veterans Hospital. No conflicts of interest were declared.
SOURCE: Iaccarino JM et al. CHEST 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016.
research suggests.
Jonathan M. Iaccarino, MD, of the pulmonary center at the Boston University, and coauthors reported the results of a secondary analysis of patient-level outcomes from 75,138 low-dose CT (LDCT) scans in 26,453 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016).
Currently, LDCT screening is recommended annually for high-risk smokers aged 55-80 years. The National Lung Screening Trial showed that this screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in this group. The guidelines stress the importance of shared decision making, with discussion of the risks and benefits of screening.
Dr. Iaccarino and colleagues point out that decision aids for shared decision making need to include important baseline characteristics, such as the presence of COPD, as these can complicate the risk and benefit analysis.
In this study, they found that 14.2% of LDCT scans performed led to a subsequent diagnostic study and 1.5% resulted in an invasive procedure. In addition, 0.3% of scans resulted in a procedure-related complication, and in 89 cases (0.1%), this procedure-related complication was serious.
At the patient level, nearly one-third (30.5%) received a diagnostic study, 4.2% underwent an invasive procedure – 41% of whom ultimately were found not to have lung cancer – 0.9% had a procedure-related complication, and 0.3% had a serious procedure related complication. Furthermore, among those who experienced a serious complication, 12.5% were found not to have lung cancer.
“Our study analyzes cumulative outcomes at the level of the individual patient over the three years of LDCT screening during the NLST, showing higher rates of diagnostic procedures, invasive procedures, complications and serious complications than apparent when data is presented at the level of the individual test,” the authors wrote.
The 4,632 participants with COPD were significantly more likely to undergo diagnostic studies (36.2%), have an invasive procedure (6%), experience a procedure-related complication (1.5%) and experience a serious procedure-related complication (0.6%) than were participants without COPD. However, they also had a significantly higher incidence of lung cancer diagnosis than did participants without COPD (6.1% vs. 3.6%).
“While most decision aids note the risks of screening may be increased in those with COPD, our study helps quantify these increased risks as well as the increased likelihood of a lung cancer diagnosis, a critical advance given that providing personalized (rather than generic) information results in more accurate risk perception and more informed choices among individuals considering screening,” the authors wrote. “With the significant change in the balance of benefits and risks of screening in patients with COPD, it is critical to adjust the shared decision-making discussions accordingly.”
They also noted that other comorbidities, such as heart disease, vascular disease, and other lung diseases, would likely affect the balance of risk and benefit of LDCT screening, and that there was a need for further exploration of screening in these patients.
Noting the study’s limitations, the authors pointed that their analysis focused on outcomes that were not the primary outcomes of the National Lung Screening trial, and that they relied on self-reported COPD diagnoses.
The study was supported by the American Society of Clinical Oncology, the Charles A. King Trust, and Edith Nourse Rogers Memorial Veterans Hospital. No conflicts of interest were declared.
SOURCE: Iaccarino JM et al. CHEST 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016.
research suggests.
Jonathan M. Iaccarino, MD, of the pulmonary center at the Boston University, and coauthors reported the results of a secondary analysis of patient-level outcomes from 75,138 low-dose CT (LDCT) scans in 26,453 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016).
Currently, LDCT screening is recommended annually for high-risk smokers aged 55-80 years. The National Lung Screening Trial showed that this screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in this group. The guidelines stress the importance of shared decision making, with discussion of the risks and benefits of screening.
Dr. Iaccarino and colleagues point out that decision aids for shared decision making need to include important baseline characteristics, such as the presence of COPD, as these can complicate the risk and benefit analysis.
In this study, they found that 14.2% of LDCT scans performed led to a subsequent diagnostic study and 1.5% resulted in an invasive procedure. In addition, 0.3% of scans resulted in a procedure-related complication, and in 89 cases (0.1%), this procedure-related complication was serious.
At the patient level, nearly one-third (30.5%) received a diagnostic study, 4.2% underwent an invasive procedure – 41% of whom ultimately were found not to have lung cancer – 0.9% had a procedure-related complication, and 0.3% had a serious procedure related complication. Furthermore, among those who experienced a serious complication, 12.5% were found not to have lung cancer.
“Our study analyzes cumulative outcomes at the level of the individual patient over the three years of LDCT screening during the NLST, showing higher rates of diagnostic procedures, invasive procedures, complications and serious complications than apparent when data is presented at the level of the individual test,” the authors wrote.
The 4,632 participants with COPD were significantly more likely to undergo diagnostic studies (36.2%), have an invasive procedure (6%), experience a procedure-related complication (1.5%) and experience a serious procedure-related complication (0.6%) than were participants without COPD. However, they also had a significantly higher incidence of lung cancer diagnosis than did participants without COPD (6.1% vs. 3.6%).
“While most decision aids note the risks of screening may be increased in those with COPD, our study helps quantify these increased risks as well as the increased likelihood of a lung cancer diagnosis, a critical advance given that providing personalized (rather than generic) information results in more accurate risk perception and more informed choices among individuals considering screening,” the authors wrote. “With the significant change in the balance of benefits and risks of screening in patients with COPD, it is critical to adjust the shared decision-making discussions accordingly.”
They also noted that other comorbidities, such as heart disease, vascular disease, and other lung diseases, would likely affect the balance of risk and benefit of LDCT screening, and that there was a need for further exploration of screening in these patients.
Noting the study’s limitations, the authors pointed that their analysis focused on outcomes that were not the primary outcomes of the National Lung Screening trial, and that they relied on self-reported COPD diagnoses.
The study was supported by the American Society of Clinical Oncology, the Charles A. King Trust, and Edith Nourse Rogers Memorial Veterans Hospital. No conflicts of interest were declared.
SOURCE: Iaccarino JM et al. CHEST 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016.
FROM CHEST
Posttraumatic headache may be associated with reduced pain thresholds
PHILADELPHIA – , according to results of a pilot study presented at the annual meeting of the American Headache Society. The findings suggest that patients with posttraumatic headache have abnormal, multimodal sensory processing, said Amaal J. Starling, MD, a neurologist at Mayo Clinic in Phoenix.
Mild traumatic brain injury (TBI) is a growing public health problem. Headache is the most common symptom after mild TBI, and often the most debilitating symptom for these patients. No Food and Drug Administration–approved treatments are available for patients with posttraumatic headache, and about three-quarters of these patients report that current treatments bring them no relief.
Identifying novel targets and developing new treatment options will require a deeper understanding of the pathophysiology of posttraumatic headache, said Dr. Starling. She and her colleagues conducted a pilot study to characterize allodynia, cutaneous heat pain thresholds, photophobia, and light-induced pain thresholds objectively in patients with posttraumatic headache, compared with healthy controls.
Participants were exposed to a bright-light stressor
The researchers enrolled 20 patients between ages 18 years and 65 years with posttraumatic headache attributed to mild TBI in their study. They matched these patients by age with 20 healthy controls. Dr. Starling and colleagues evaluated all participants prospectively using the Allodynia Symptom Checklist (ASC-12), Photosensitivity Assessment Questionnaire (PAQ), State-Trait Anxiety Inventory (STAI), and Beck Depression Inventory (BDI).
The investigators performed quantitative sensory testing to measure each participant’s cutaneous forearm heat pain threshold. Using a progressive light stimulation device, they quantified each participant’s light-induced pain threshold. Finally, Dr. Starling and colleagues obtained participants’ cutaneous heat pain thresholds immediately after, 10 minutes after, and 40 minutes after exposing them to a bright-light stressor.
The researchers found no significant differences between groups in age, gender, or race. The population’s average age was 41 years. Approximately 70% of the sample was female. Among participants with posttraumatic headache, the average time since the onset of posttraumatic headache was 46 months. The average number of headache days per month in that group was 17.2, which represented “a significantly high headache burden,” said Dr. Starling. Approximately 80% of patients with posttraumatic headache had headaches with a migraine phenotype.
Patients’ pain thresholds were lower
STAI and BDI scores were significantly higher among patients with posttraumatic headache, compared with controls. Mean PAQ score was 0.62 among patients and 0.24 among controls, representing significantly greater photophobia symptom severity among patients, said Dr. Starling.
Light-induced pain thresholds were significantly lower in patients with posttraumatic headache (median, 90.5 lux), compared with healthy controls (median, 863.5 lux), independent of depression and anxiety. Allodynia symptom severity was significantly higher in patients with posttraumatic headache (mean ASC-12 score, 5.7), compared with controls (mean ASC-12 score, 0.98).
In addition, the mean baseline cutaneous heat pain threshold was 40.8° C in patients with posttraumatic headache and 44.4° C in healthy controls. When participants were subjected to the bright-light stressor, the immediate change in heat pain threshold was significant in patients with posttraumatic headache (−1.9° C), compared with healthy controls. The difference between groups was not significant at 10 and 40 minutes after exposure to the stressor, however. The light intensity inducing moderate pain was 688 lux in patients with posttraumatic headache, compared with 6,000 lux in healthy controls.
“Our next steps are going to be replicating this [study] in a larger population, as well as determining whether any type of intervention would change these different types of sensory sensitivities and thresholds,” said Dr. Starling. She and her colleagues will use this human research model to examine whether posttraumatic headache differs from other headache disorders such as migraine and to examine potential differences between acute and persistent posttraumatic headache.
The study was funded through an intramural Mayo Clinic early career research award.
SOURCE: Starling AJ et al. AHS 2019. Abstract OR14.
PHILADELPHIA – , according to results of a pilot study presented at the annual meeting of the American Headache Society. The findings suggest that patients with posttraumatic headache have abnormal, multimodal sensory processing, said Amaal J. Starling, MD, a neurologist at Mayo Clinic in Phoenix.
Mild traumatic brain injury (TBI) is a growing public health problem. Headache is the most common symptom after mild TBI, and often the most debilitating symptom for these patients. No Food and Drug Administration–approved treatments are available for patients with posttraumatic headache, and about three-quarters of these patients report that current treatments bring them no relief.
Identifying novel targets and developing new treatment options will require a deeper understanding of the pathophysiology of posttraumatic headache, said Dr. Starling. She and her colleagues conducted a pilot study to characterize allodynia, cutaneous heat pain thresholds, photophobia, and light-induced pain thresholds objectively in patients with posttraumatic headache, compared with healthy controls.
Participants were exposed to a bright-light stressor
The researchers enrolled 20 patients between ages 18 years and 65 years with posttraumatic headache attributed to mild TBI in their study. They matched these patients by age with 20 healthy controls. Dr. Starling and colleagues evaluated all participants prospectively using the Allodynia Symptom Checklist (ASC-12), Photosensitivity Assessment Questionnaire (PAQ), State-Trait Anxiety Inventory (STAI), and Beck Depression Inventory (BDI).
The investigators performed quantitative sensory testing to measure each participant’s cutaneous forearm heat pain threshold. Using a progressive light stimulation device, they quantified each participant’s light-induced pain threshold. Finally, Dr. Starling and colleagues obtained participants’ cutaneous heat pain thresholds immediately after, 10 minutes after, and 40 minutes after exposing them to a bright-light stressor.
The researchers found no significant differences between groups in age, gender, or race. The population’s average age was 41 years. Approximately 70% of the sample was female. Among participants with posttraumatic headache, the average time since the onset of posttraumatic headache was 46 months. The average number of headache days per month in that group was 17.2, which represented “a significantly high headache burden,” said Dr. Starling. Approximately 80% of patients with posttraumatic headache had headaches with a migraine phenotype.
Patients’ pain thresholds were lower
STAI and BDI scores were significantly higher among patients with posttraumatic headache, compared with controls. Mean PAQ score was 0.62 among patients and 0.24 among controls, representing significantly greater photophobia symptom severity among patients, said Dr. Starling.
Light-induced pain thresholds were significantly lower in patients with posttraumatic headache (median, 90.5 lux), compared with healthy controls (median, 863.5 lux), independent of depression and anxiety. Allodynia symptom severity was significantly higher in patients with posttraumatic headache (mean ASC-12 score, 5.7), compared with controls (mean ASC-12 score, 0.98).
In addition, the mean baseline cutaneous heat pain threshold was 40.8° C in patients with posttraumatic headache and 44.4° C in healthy controls. When participants were subjected to the bright-light stressor, the immediate change in heat pain threshold was significant in patients with posttraumatic headache (−1.9° C), compared with healthy controls. The difference between groups was not significant at 10 and 40 minutes after exposure to the stressor, however. The light intensity inducing moderate pain was 688 lux in patients with posttraumatic headache, compared with 6,000 lux in healthy controls.
“Our next steps are going to be replicating this [study] in a larger population, as well as determining whether any type of intervention would change these different types of sensory sensitivities and thresholds,” said Dr. Starling. She and her colleagues will use this human research model to examine whether posttraumatic headache differs from other headache disorders such as migraine and to examine potential differences between acute and persistent posttraumatic headache.
The study was funded through an intramural Mayo Clinic early career research award.
SOURCE: Starling AJ et al. AHS 2019. Abstract OR14.
PHILADELPHIA – , according to results of a pilot study presented at the annual meeting of the American Headache Society. The findings suggest that patients with posttraumatic headache have abnormal, multimodal sensory processing, said Amaal J. Starling, MD, a neurologist at Mayo Clinic in Phoenix.
Mild traumatic brain injury (TBI) is a growing public health problem. Headache is the most common symptom after mild TBI, and often the most debilitating symptom for these patients. No Food and Drug Administration–approved treatments are available for patients with posttraumatic headache, and about three-quarters of these patients report that current treatments bring them no relief.
Identifying novel targets and developing new treatment options will require a deeper understanding of the pathophysiology of posttraumatic headache, said Dr. Starling. She and her colleagues conducted a pilot study to characterize allodynia, cutaneous heat pain thresholds, photophobia, and light-induced pain thresholds objectively in patients with posttraumatic headache, compared with healthy controls.
Participants were exposed to a bright-light stressor
The researchers enrolled 20 patients between ages 18 years and 65 years with posttraumatic headache attributed to mild TBI in their study. They matched these patients by age with 20 healthy controls. Dr. Starling and colleagues evaluated all participants prospectively using the Allodynia Symptom Checklist (ASC-12), Photosensitivity Assessment Questionnaire (PAQ), State-Trait Anxiety Inventory (STAI), and Beck Depression Inventory (BDI).
The investigators performed quantitative sensory testing to measure each participant’s cutaneous forearm heat pain threshold. Using a progressive light stimulation device, they quantified each participant’s light-induced pain threshold. Finally, Dr. Starling and colleagues obtained participants’ cutaneous heat pain thresholds immediately after, 10 minutes after, and 40 minutes after exposing them to a bright-light stressor.
The researchers found no significant differences between groups in age, gender, or race. The population’s average age was 41 years. Approximately 70% of the sample was female. Among participants with posttraumatic headache, the average time since the onset of posttraumatic headache was 46 months. The average number of headache days per month in that group was 17.2, which represented “a significantly high headache burden,” said Dr. Starling. Approximately 80% of patients with posttraumatic headache had headaches with a migraine phenotype.
Patients’ pain thresholds were lower
STAI and BDI scores were significantly higher among patients with posttraumatic headache, compared with controls. Mean PAQ score was 0.62 among patients and 0.24 among controls, representing significantly greater photophobia symptom severity among patients, said Dr. Starling.
Light-induced pain thresholds were significantly lower in patients with posttraumatic headache (median, 90.5 lux), compared with healthy controls (median, 863.5 lux), independent of depression and anxiety. Allodynia symptom severity was significantly higher in patients with posttraumatic headache (mean ASC-12 score, 5.7), compared with controls (mean ASC-12 score, 0.98).
In addition, the mean baseline cutaneous heat pain threshold was 40.8° C in patients with posttraumatic headache and 44.4° C in healthy controls. When participants were subjected to the bright-light stressor, the immediate change in heat pain threshold was significant in patients with posttraumatic headache (−1.9° C), compared with healthy controls. The difference between groups was not significant at 10 and 40 minutes after exposure to the stressor, however. The light intensity inducing moderate pain was 688 lux in patients with posttraumatic headache, compared with 6,000 lux in healthy controls.
“Our next steps are going to be replicating this [study] in a larger population, as well as determining whether any type of intervention would change these different types of sensory sensitivities and thresholds,” said Dr. Starling. She and her colleagues will use this human research model to examine whether posttraumatic headache differs from other headache disorders such as migraine and to examine potential differences between acute and persistent posttraumatic headache.
The study was funded through an intramural Mayo Clinic early career research award.
SOURCE: Starling AJ et al. AHS 2019. Abstract OR14.
REPORTING FROM AHS 2019
Aspirin: 4,000 years and still learning
Aspirin (acetylsalicylic acid) and its progenitors are valuable medications with a history spanning at least 4 millennia. An enormous number of patients take aspirin for a variety of reasons, and managing their therapy around the time of surgery can be challenging, as Drs. Prabhakaran and Whinney discuss in this issue.1 Even after 4,000 years, we are still learning about these remarkable drugs.
LEARNING WHAT SALICYLATES ARE
LEARNING (AND IGNORING) WHAT ASPIRIN CAN DO
In the 1940s, a general practitioner in California named Lawrence Craven recognized that many of his post-tonsillectomy patients had to be hospitalized for bleeding after he started recommending they use aspirin-containing chewing gum for pain relief.4 Under the then-debated hypothesis that myocardial infarction (MI) involves thrombosis, he recommended that adult men should take aspirin daily. He believed that women had lower rates of MI because they were more likely to take aspirin, something that men did not view as a “masculine” thing to do.
In a series of letters in journals such as the Mississippi Valley Medical Journal,5 Craven reported his observations of very low rates of MI and no strokes in aspirin users. Given the nonrigorous nature of his research and the obscure journals in which he published, his findings languished for many years. Ironically, he died of an MI in 1957.
LEARNING HOW ASPIRIN WORKS (AND A FEW OTHER THINGS)
The history of aspirin research illustrates how the fields of hemostasis and inflammation are now linked.
In the late 1960s, Weiss et al6 reported that aspirin rapidly and irreversibly inhibits platelet aggregation. In parallel, using biological assays in work that eventually led to the Nobel Prize, Vane7 discovered that inflammation involves the de novo synthesis of prostaglandins and that aspirin directly inhibits this synthesis. Further work connecting these lines of investigation led us to understand that platelet aggregation is enhanced by the prostaglandin derivative thromboxane A2, produced by cyclooxygenase-1, and that aspirin irreversibly inhibits this enzyme by acetylation.
LEARNING WHEN TO USE ASPIRIN
After decades of research ranging from the Physicians’ Health Study to well-named trials such as ARRIVE, ASCEND, and ASPREE, we now know that taking daily low doses of aspirin for primary prevention can reduce the risk of cardiovascular events and may reduce the risk of colorectal cancer—but at the cost of an increased risk of bleeding.8
Which patients will gain the most benefit and incur the least risk is still debated. What is certain, however, is that aspirin has an important role in acute coronary syndromes, secondary prevention of MI and stroke, and prevention of thrombosis after coronary stent placement. In the perioperative setting, we are learning that aspirin may benefit patients with myocardial injury after noncardiac surgery, a recently described clinical entity associated with surprisingly high mortality rates.9,10
LEARNING WHEN NOT TO USE ASPIRIN
The perioperative period is a dangerous time—surgical stress, hypercoagulability, inflammation, pain, and hemodynamic changes predispose to plaque rupture and supply-demand imbalance. It is therefore logical to hope aspirin would provide protection for at-risk patients in this context.
Unfortunately, results from the second Perioperative Ischemic Evaluation trial have dampened enthusiasm.11 Aspirin has now joined clonidine and beta-blockers on the list of interventions that probably do not reduce perioperative cardiovascular mortality rates. Other than protecting against stent thrombosis, aspirin’s main perioperative effect is to increase bleeding. Consequently, some surgical procedures mandate withdrawal of aspirin.
WHAT WE STILL NEED TO LEARN
Over the years, we have learned the broad outlines of using aspirin to prevent and treat cardiovascular disease, to relieve pain and inflammation (its original purpose), and to prevent stent thrombosis.
However, many details remain to be filled in. We need to better define groups who should and should not take aspirin for primary prevention. We also need to understand aspirin’s role in cancer chemoprevention, to find better ways to mitigate its undesirable effects, and to study its role in treating myocardial injury after noncardiac surgery.
Finally, we need to determine which (if any) patients without coronary stents will benefit from continuing their aspirin perioperatively or even initiating aspirin therapy preoperatively.
Will humanity still be using salicylates 4,000 years from now? Probably not. But what we have learned and will continue to learn from this remarkable group of medications will certainly inform new and better therapies in the years to come.
- Prabhakaran A, Whinney C. Should we stop aspirin before noncardiac surgery? Cleve Clin J Med 2019; 86(8):518–521. doi:10.3949/ccjm.86a.19036
- Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York: Bloomsbury; 2008.
- Mann CC, Plummer ML. The Aspirin Wars: Money, Medicine, and 100 Years of Rampant Competition. New York: Alfred A. Knopf; 1991.
- Miner J, Hoffhines A. The discovery of aspirin's antithrombotic effects. Tex Heart Inst J 2007; 34(2):179–186. pmid:17622365
- Craven LL. Prevention of coronary and cerebral thrombosis. Miss Valley Med J 1956; 78(5):213–215. pmid:13358612
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968; 47(9):2169–2180. doi:10.1172/JCI105903
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 231(25):232–235. pmid:5284360
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150(6):396–404. pmid:19293072
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
Aspirin (acetylsalicylic acid) and its progenitors are valuable medications with a history spanning at least 4 millennia. An enormous number of patients take aspirin for a variety of reasons, and managing their therapy around the time of surgery can be challenging, as Drs. Prabhakaran and Whinney discuss in this issue.1 Even after 4,000 years, we are still learning about these remarkable drugs.
LEARNING WHAT SALICYLATES ARE
LEARNING (AND IGNORING) WHAT ASPIRIN CAN DO
In the 1940s, a general practitioner in California named Lawrence Craven recognized that many of his post-tonsillectomy patients had to be hospitalized for bleeding after he started recommending they use aspirin-containing chewing gum for pain relief.4 Under the then-debated hypothesis that myocardial infarction (MI) involves thrombosis, he recommended that adult men should take aspirin daily. He believed that women had lower rates of MI because they were more likely to take aspirin, something that men did not view as a “masculine” thing to do.
In a series of letters in journals such as the Mississippi Valley Medical Journal,5 Craven reported his observations of very low rates of MI and no strokes in aspirin users. Given the nonrigorous nature of his research and the obscure journals in which he published, his findings languished for many years. Ironically, he died of an MI in 1957.
LEARNING HOW ASPIRIN WORKS (AND A FEW OTHER THINGS)
The history of aspirin research illustrates how the fields of hemostasis and inflammation are now linked.
In the late 1960s, Weiss et al6 reported that aspirin rapidly and irreversibly inhibits platelet aggregation. In parallel, using biological assays in work that eventually led to the Nobel Prize, Vane7 discovered that inflammation involves the de novo synthesis of prostaglandins and that aspirin directly inhibits this synthesis. Further work connecting these lines of investigation led us to understand that platelet aggregation is enhanced by the prostaglandin derivative thromboxane A2, produced by cyclooxygenase-1, and that aspirin irreversibly inhibits this enzyme by acetylation.
LEARNING WHEN TO USE ASPIRIN
After decades of research ranging from the Physicians’ Health Study to well-named trials such as ARRIVE, ASCEND, and ASPREE, we now know that taking daily low doses of aspirin for primary prevention can reduce the risk of cardiovascular events and may reduce the risk of colorectal cancer—but at the cost of an increased risk of bleeding.8
Which patients will gain the most benefit and incur the least risk is still debated. What is certain, however, is that aspirin has an important role in acute coronary syndromes, secondary prevention of MI and stroke, and prevention of thrombosis after coronary stent placement. In the perioperative setting, we are learning that aspirin may benefit patients with myocardial injury after noncardiac surgery, a recently described clinical entity associated with surprisingly high mortality rates.9,10
LEARNING WHEN NOT TO USE ASPIRIN
The perioperative period is a dangerous time—surgical stress, hypercoagulability, inflammation, pain, and hemodynamic changes predispose to plaque rupture and supply-demand imbalance. It is therefore logical to hope aspirin would provide protection for at-risk patients in this context.
Unfortunately, results from the second Perioperative Ischemic Evaluation trial have dampened enthusiasm.11 Aspirin has now joined clonidine and beta-blockers on the list of interventions that probably do not reduce perioperative cardiovascular mortality rates. Other than protecting against stent thrombosis, aspirin’s main perioperative effect is to increase bleeding. Consequently, some surgical procedures mandate withdrawal of aspirin.
WHAT WE STILL NEED TO LEARN
Over the years, we have learned the broad outlines of using aspirin to prevent and treat cardiovascular disease, to relieve pain and inflammation (its original purpose), and to prevent stent thrombosis.
However, many details remain to be filled in. We need to better define groups who should and should not take aspirin for primary prevention. We also need to understand aspirin’s role in cancer chemoprevention, to find better ways to mitigate its undesirable effects, and to study its role in treating myocardial injury after noncardiac surgery.
Finally, we need to determine which (if any) patients without coronary stents will benefit from continuing their aspirin perioperatively or even initiating aspirin therapy preoperatively.
Will humanity still be using salicylates 4,000 years from now? Probably not. But what we have learned and will continue to learn from this remarkable group of medications will certainly inform new and better therapies in the years to come.
Aspirin (acetylsalicylic acid) and its progenitors are valuable medications with a history spanning at least 4 millennia. An enormous number of patients take aspirin for a variety of reasons, and managing their therapy around the time of surgery can be challenging, as Drs. Prabhakaran and Whinney discuss in this issue.1 Even after 4,000 years, we are still learning about these remarkable drugs.
LEARNING WHAT SALICYLATES ARE
LEARNING (AND IGNORING) WHAT ASPIRIN CAN DO
In the 1940s, a general practitioner in California named Lawrence Craven recognized that many of his post-tonsillectomy patients had to be hospitalized for bleeding after he started recommending they use aspirin-containing chewing gum for pain relief.4 Under the then-debated hypothesis that myocardial infarction (MI) involves thrombosis, he recommended that adult men should take aspirin daily. He believed that women had lower rates of MI because they were more likely to take aspirin, something that men did not view as a “masculine” thing to do.
In a series of letters in journals such as the Mississippi Valley Medical Journal,5 Craven reported his observations of very low rates of MI and no strokes in aspirin users. Given the nonrigorous nature of his research and the obscure journals in which he published, his findings languished for many years. Ironically, he died of an MI in 1957.
LEARNING HOW ASPIRIN WORKS (AND A FEW OTHER THINGS)
The history of aspirin research illustrates how the fields of hemostasis and inflammation are now linked.
In the late 1960s, Weiss et al6 reported that aspirin rapidly and irreversibly inhibits platelet aggregation. In parallel, using biological assays in work that eventually led to the Nobel Prize, Vane7 discovered that inflammation involves the de novo synthesis of prostaglandins and that aspirin directly inhibits this synthesis. Further work connecting these lines of investigation led us to understand that platelet aggregation is enhanced by the prostaglandin derivative thromboxane A2, produced by cyclooxygenase-1, and that aspirin irreversibly inhibits this enzyme by acetylation.
LEARNING WHEN TO USE ASPIRIN
After decades of research ranging from the Physicians’ Health Study to well-named trials such as ARRIVE, ASCEND, and ASPREE, we now know that taking daily low doses of aspirin for primary prevention can reduce the risk of cardiovascular events and may reduce the risk of colorectal cancer—but at the cost of an increased risk of bleeding.8
Which patients will gain the most benefit and incur the least risk is still debated. What is certain, however, is that aspirin has an important role in acute coronary syndromes, secondary prevention of MI and stroke, and prevention of thrombosis after coronary stent placement. In the perioperative setting, we are learning that aspirin may benefit patients with myocardial injury after noncardiac surgery, a recently described clinical entity associated with surprisingly high mortality rates.9,10
LEARNING WHEN NOT TO USE ASPIRIN
The perioperative period is a dangerous time—surgical stress, hypercoagulability, inflammation, pain, and hemodynamic changes predispose to plaque rupture and supply-demand imbalance. It is therefore logical to hope aspirin would provide protection for at-risk patients in this context.
Unfortunately, results from the second Perioperative Ischemic Evaluation trial have dampened enthusiasm.11 Aspirin has now joined clonidine and beta-blockers on the list of interventions that probably do not reduce perioperative cardiovascular mortality rates. Other than protecting against stent thrombosis, aspirin’s main perioperative effect is to increase bleeding. Consequently, some surgical procedures mandate withdrawal of aspirin.
WHAT WE STILL NEED TO LEARN
Over the years, we have learned the broad outlines of using aspirin to prevent and treat cardiovascular disease, to relieve pain and inflammation (its original purpose), and to prevent stent thrombosis.
However, many details remain to be filled in. We need to better define groups who should and should not take aspirin for primary prevention. We also need to understand aspirin’s role in cancer chemoprevention, to find better ways to mitigate its undesirable effects, and to study its role in treating myocardial injury after noncardiac surgery.
Finally, we need to determine which (if any) patients without coronary stents will benefit from continuing their aspirin perioperatively or even initiating aspirin therapy preoperatively.
Will humanity still be using salicylates 4,000 years from now? Probably not. But what we have learned and will continue to learn from this remarkable group of medications will certainly inform new and better therapies in the years to come.
- Prabhakaran A, Whinney C. Should we stop aspirin before noncardiac surgery? Cleve Clin J Med 2019; 86(8):518–521. doi:10.3949/ccjm.86a.19036
- Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York: Bloomsbury; 2008.
- Mann CC, Plummer ML. The Aspirin Wars: Money, Medicine, and 100 Years of Rampant Competition. New York: Alfred A. Knopf; 1991.
- Miner J, Hoffhines A. The discovery of aspirin's antithrombotic effects. Tex Heart Inst J 2007; 34(2):179–186. pmid:17622365
- Craven LL. Prevention of coronary and cerebral thrombosis. Miss Valley Med J 1956; 78(5):213–215. pmid:13358612
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968; 47(9):2169–2180. doi:10.1172/JCI105903
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 231(25):232–235. pmid:5284360
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150(6):396–404. pmid:19293072
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
- Prabhakaran A, Whinney C. Should we stop aspirin before noncardiac surgery? Cleve Clin J Med 2019; 86(8):518–521. doi:10.3949/ccjm.86a.19036
- Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York: Bloomsbury; 2008.
- Mann CC, Plummer ML. The Aspirin Wars: Money, Medicine, and 100 Years of Rampant Competition. New York: Alfred A. Knopf; 1991.
- Miner J, Hoffhines A. The discovery of aspirin's antithrombotic effects. Tex Heart Inst J 2007; 34(2):179–186. pmid:17622365
- Craven LL. Prevention of coronary and cerebral thrombosis. Miss Valley Med J 1956; 78(5):213–215. pmid:13358612
- Weiss HJ, Aledort LM, Kochwa S. The effect of salicylates on the hemostatic properties of platelets in man. J Clin Invest 1968; 47(9):2169–2180. doi:10.1172/JCI105903
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol 1971; 231(25):232–235. pmid:5284360
- US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009; 150(6):396–404. pmid:19293072
- Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014; 120(3):564–578. doi:10.1097/ALN.0000000000000113
- George R, Menon VP, Edathadathil F, et al. Myocardial injury after noncardiac surgery—incidence and predictors from a prospective observational cohort study at an Indian tertiary care centre. Medicine (Baltimore) 2018; 97(19):e0402. doi:10.1097/MD.0000000000010402
- Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014; 370(16):1494–1503. doi:10.1056/NEJMoa1401105
Doing our part to dismantle the opioid crisis
When the Joint Commission dubbed pain assessment the “fifth vital sign” in 2001 and insisted that all outpatients be assessed for pain at each office visit, they had no idea of the unintended consequences that would result.
The problem they wanted to solve was undertreatment of postoperative pain, but the problem they helped create far outweighed any benefit to hospitalized patients. They would have been wise to listen to R.E.M.’s song “Everybody Hurts” and recognize that pain is a fact of life that doesn’t always require medical intervention. Combined with aggressive marketing of opioids by pharmaceutical companies, these 2 factors led to the opioid epidemic we currently find ourselves in.
The good news is that there has been a significant drop in opioid prescribing in recent years. Between 2014 and 2017, opioid prescriptions declined from 7.4% to 6.4%, based on a national electronic health record review.1 Reducing opioid prescribing for patients with chronic noncancer pain, however, is difficult. Although there are no truly evidence-based methods, the Centers for Disease Control and Prevention has provided expert advice on improving opioid prescribing, and Drs. Mendoza and Russell provide thoughtful recommendations for tapering opioids in patients on chronic therapy in this issue of JFP.
In addition, Patchett et al describe their experience with a practice-wide approach to reducing chronic opioid prescribing in their practice at Mayo Clinic in Scottsdale, Ariz. Using a systematic approach, they were able to reduce the number of patients on chronic opioid therapy by 22%.
And there is more good news from a 2018 JAMA study.2
All family physicians should share the results of this study with their chronic pain patients and follow Pachett’s lead in a practice-wide approach to reducing opioid prescribing. We were part of the problem and must be part of the solution.
1. García MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68:25–30.
2. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. The SPACE randomized clinical trial. JAMA. 2018;319:872-882.
When the Joint Commission dubbed pain assessment the “fifth vital sign” in 2001 and insisted that all outpatients be assessed for pain at each office visit, they had no idea of the unintended consequences that would result.
The problem they wanted to solve was undertreatment of postoperative pain, but the problem they helped create far outweighed any benefit to hospitalized patients. They would have been wise to listen to R.E.M.’s song “Everybody Hurts” and recognize that pain is a fact of life that doesn’t always require medical intervention. Combined with aggressive marketing of opioids by pharmaceutical companies, these 2 factors led to the opioid epidemic we currently find ourselves in.
The good news is that there has been a significant drop in opioid prescribing in recent years. Between 2014 and 2017, opioid prescriptions declined from 7.4% to 6.4%, based on a national electronic health record review.1 Reducing opioid prescribing for patients with chronic noncancer pain, however, is difficult. Although there are no truly evidence-based methods, the Centers for Disease Control and Prevention has provided expert advice on improving opioid prescribing, and Drs. Mendoza and Russell provide thoughtful recommendations for tapering opioids in patients on chronic therapy in this issue of JFP.
In addition, Patchett et al describe their experience with a practice-wide approach to reducing chronic opioid prescribing in their practice at Mayo Clinic in Scottsdale, Ariz. Using a systematic approach, they were able to reduce the number of patients on chronic opioid therapy by 22%.
And there is more good news from a 2018 JAMA study.2
All family physicians should share the results of this study with their chronic pain patients and follow Pachett’s lead in a practice-wide approach to reducing opioid prescribing. We were part of the problem and must be part of the solution.
When the Joint Commission dubbed pain assessment the “fifth vital sign” in 2001 and insisted that all outpatients be assessed for pain at each office visit, they had no idea of the unintended consequences that would result.
The problem they wanted to solve was undertreatment of postoperative pain, but the problem they helped create far outweighed any benefit to hospitalized patients. They would have been wise to listen to R.E.M.’s song “Everybody Hurts” and recognize that pain is a fact of life that doesn’t always require medical intervention. Combined with aggressive marketing of opioids by pharmaceutical companies, these 2 factors led to the opioid epidemic we currently find ourselves in.
The good news is that there has been a significant drop in opioid prescribing in recent years. Between 2014 and 2017, opioid prescriptions declined from 7.4% to 6.4%, based on a national electronic health record review.1 Reducing opioid prescribing for patients with chronic noncancer pain, however, is difficult. Although there are no truly evidence-based methods, the Centers for Disease Control and Prevention has provided expert advice on improving opioid prescribing, and Drs. Mendoza and Russell provide thoughtful recommendations for tapering opioids in patients on chronic therapy in this issue of JFP.
In addition, Patchett et al describe their experience with a practice-wide approach to reducing chronic opioid prescribing in their practice at Mayo Clinic in Scottsdale, Ariz. Using a systematic approach, they were able to reduce the number of patients on chronic opioid therapy by 22%.
And there is more good news from a 2018 JAMA study.2
All family physicians should share the results of this study with their chronic pain patients and follow Pachett’s lead in a practice-wide approach to reducing opioid prescribing. We were part of the problem and must be part of the solution.
1. García MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68:25–30.
2. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. The SPACE randomized clinical trial. JAMA. 2018;319:872-882.
1. García MC, Heilig CM, Lee SH, et al. Opioid prescribing rates in nonmetropolitan and metropolitan counties among primary care providers using an electronic health record system — United States, 2014–2017. MMWR Morb Mortal Wkly Rep. 2019;68:25–30.
2. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain. The SPACE randomized clinical trial. JAMA. 2018;319:872-882.
Is it time to taper that opioid? (And how best to do it)
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29
Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.
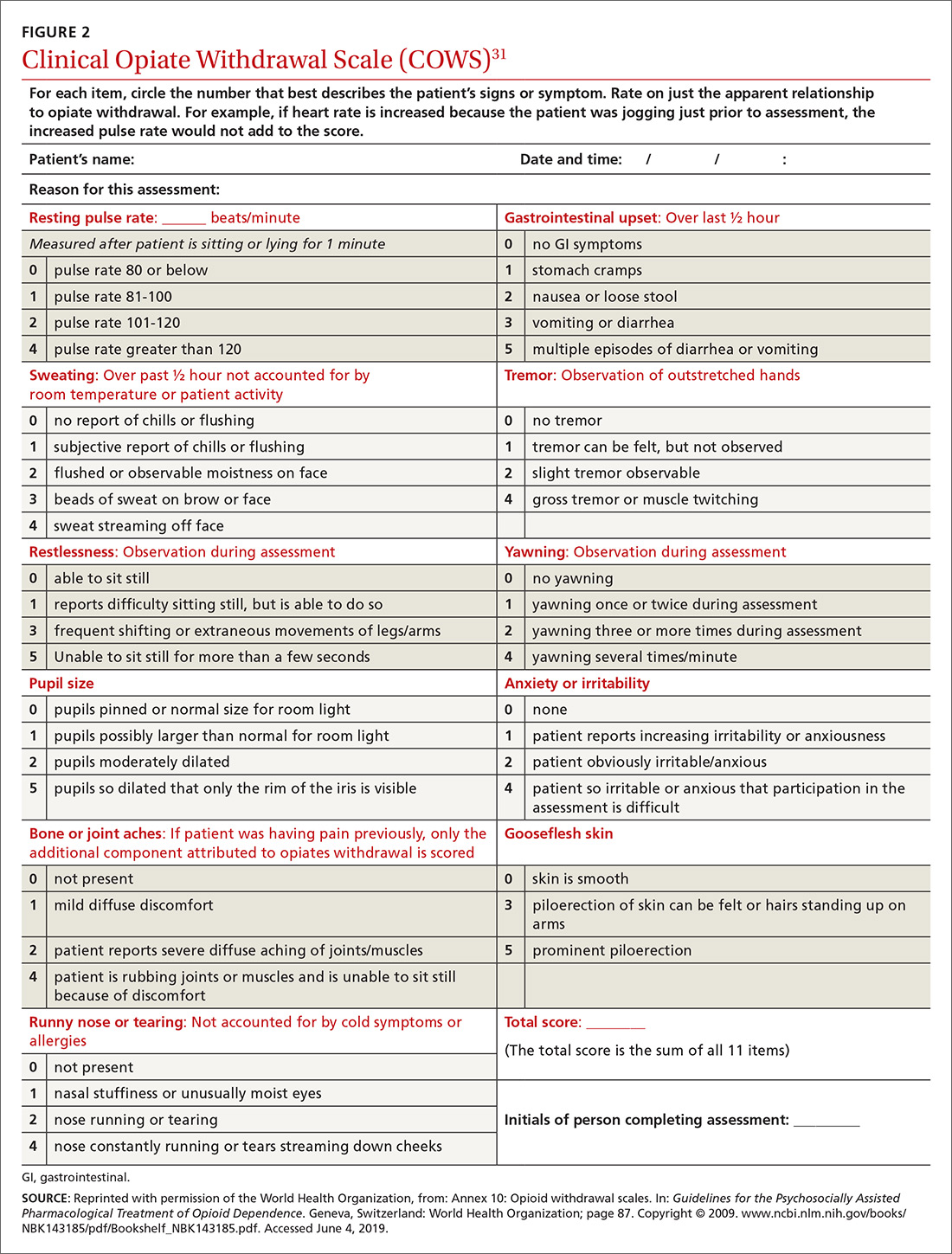
Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
The opioid crisis has brought added scrutiny to opioid prescribing, particularly to health care providers, whom many blame for the genesis of the opioid overdose epidemic. Family physicians are acutely aware of these complexities: By sheer volume, family physicians prescribe more opioid analgesics than any other subspecialist.1
Overwhelmed by opioid prescriptions
Because of a complexity of factors (notably, the influence of the US pharmaceutical industry), the quantity of opioid prescriptions has risen substantially—enough so that, in 2010, opioids were prescribed in great enough quantity to medicate every American around the clock for a month.2 Among people who began abusing opioids in the 2000s, 75% reported that their first opioid was a prescription drug; this is a shift from prior decades, when heroin was the gateway to opioid addiction.3 As the reality of the size of the opioid problem sunk in, many were hopeful that the epidemic would reverse itself as quickly as it began if the medical community would simply prescribe fewer opioids.

Since 2010, the opioid overdose fatality rate has risen dramatically, even though prescription opioid overdose mortality has leveled off, or even declined. 2 One explanation for this paradox? As availability of prescription opioids declined, people suffering from an underlying opioid use disorder (OUD) turned instead first to heroin, then later to potent fentanyl analogues to fuel their addiction. In most communities, the prevalence of fentanyl analogues—alone or more commonly mixed with other opioids—has driven the staggering rise in opioid-related fatalities in recent years.
No question: Prescription opioids played a critical role in the origins of this epidemic, but just withdrawing prescriptions will not result in marked reduction in the epidemic. This quandary is no more apparent than in primary care, where the considerable risk of continuing opioids—especially at high dosages—must be weighed against the potential risks of discontinuation. Adding to this dilemma are lack of access to treatment for patients with an OUD and the continued stigma and misunderstanding of substance use disorders.
In this article, we describe the challenges of long-term opioid use and review necessary protocols and precautions for maintaining or tapering an opioid regimen in patients who suffer chronic pain.
Managing chronic pain is fraught with complexity
Chronic pain is both real and a disease in its own right. Although definitions of chronic pain vary, pain that lasts > 3 months or past the duration of normal tissue healing is typically considered chronic.4 Approximations of prevalence vary, but in 1 study that examined a representative sample, it was estimated that 14.6% of US adults experience chronic pain.5
Patients who report symptoms or a history of chronic pain can elicit negative reactions from physicians—stemming from our biases, which can inadvertently provoke emotions on our part.6 Unflattering portrayals of patients in the media can further fuel unwarranted biases and prejudices.7
Continue to: Preventing, assessing, and treating...
Preventing, assessing, and treating chronic pain can be difficult, at the level of both the individual physician and the larger system of care, even without adding in complications of the opioid epidemic. For racial and ethnic minority groups, women, older people, and people with cognitive impairment or cancer, pain can be underrecognized and go inadequately treated.
Chronic pain itself has clinical, psychological, and social consequences and is associated with limitations in activity, work productivity, quality of life, and stigma.8 Treatment of chronic pain—with opioids or other modalities—remains an important component of patient-centered primary care. Interestingly, however, many patients struggling through chronic pain report that efforts to curb the opioid epidemic have inadvertently led to lower-quality pain management and, therefore, understandable concern among patients whose chronic pain is well managed with opioid pain medications.9,10
When is it appropriate to continue opioids for chronic pain?
Apart from the treatment of active cancer, palliative care, and end-of-life care, the appropriate use of opioids for chronic and acute pain has become clouded in recent years. To assist with this problem, the Centers for Disease Control and Prevention issued guidelines in 2016 for primary care physicians who are faced with this clinical dilemma.11 The guidelines (1) address circumstances in which it is safe to consider opioid prescribing and (2) provide ongoing reassessment of indications for chronic opioid prescribing within the context of potential risk to the patient and society. Because appropriate use of opioids has grown murky, nonpharmacotherapeutic management and nonopioid pharmacotherapy are preferred for chronic pain.
Plan ahead. Establish goals of treatment that focus on both pain and function when starting opioid therapy. This will facilitate decision-making when it comes time to continue—or discontinue—opioids down the road. Opioids should be prescribed at the lowest effective dosage; ongoing reassessment of benefit should be made, and particular caution should be exercised, if the daily opioid dosage reaches ≥ 50 morphine milligram equivalents (MME) and especially as the dosage approaches ≥ 90 MME/d. Prescribers should ensure that patients are educated about known risks and the limited evidence of benefit of opioid therapy.
An age-related concern. Special consideration is warranted in older patients, who might have reduced renal function even in the absence of renal disease; this can lead to a reduction in clearance of pain medication. Because of that increased risk of drug accumulation, the therapeutic window—between safe dosages and those that could lead to respiratory depression or overdose—is narrow for these patients.11
Continue to: Use in pregnancy
Use in pregnancy. Treatment with opioid medication in pregnancy warrants special consideration. In general, it’s wise to avoid opioid use in pregnant women because data on long- and short-term safety are limited.12 In 2015, the US Food and Drug Administration issued a safety announcement that further investigation is needed to determine whether the fetus is at increased risk of a neural tube defect related to opioid exposure during the first trimester.13 In women with an OUD, both methadone and buprenorphine are safe to use. Buprenorphine is associated with slightly better outcomes for neonatal abstinence syndrome and length of hospital stay.14
Ongoing monitoring of risk. Periodically assessing risk factors for opioid-related harm during continuation of opioid treatment is important. Tools such as the Opioid Risk Tool (ORT) or the Screener and Opioid Assessment for Patients with Pain-Revised, or SOAPP-R, can be used to evaluate the risk of misuse in adults who are prescribed opioids for chronic pain,15 although the evidence for utilizing these tools is inconclusive.11
Offering naloxone should be considered when factors that increase the risk of opioid overdose are present, such as a history of substance use disorder, a daily opioid dosage > 50 MME, concurrent use of benzodiazepines, and medical comorbidities that increase the risk of overdose (eg, sleep apnea, pulmonary disease, heart failure).16 Prescribers should review prescription drug monitoring program data, when available, to assess treatment adherence and to obtain a collateral history that might suggest abuse or diversion. Urine drug testing can be a useful adjunct to ongoing therapy—again, to assess treatment adherence and look for evidence of other substance use disorders.
Watchfulness for misuse and OUD. Opioid misuse—the nontherapeutic use of opioids—includes taking opioids in amounts other than prescribed, for indications other than prescribed, and administering by alternative routes other than prescribed (eg, crushing and snorting, rather than ingesting). The presence of opioid misuse does not always signify OUD. However, The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.,17 defines OUD as out-of-control use; devoting increasing mental and physical resources to obtaining, using, and recovering from substances; and continued use despite adverse consequences.
Behaviors that increase the risk of, and might signal, opioid misuse and OUD include18
- seeking early refills
- obtaining opioids from the emergency room
- using medications prescribed to others
- using opioids to treat symptoms other than pain, such as anxiety or insomnia
- “doctor-shopping.”
Continue to: Furthermore...
Furthermore, psychiatric comorbidities,19 a personal or family history of substance use disorder,20 and a preadolescent history of sexual abuse21 are associated with a higher risk of a substance use disorder.
If OUD is identified, remain nonjudgmental and acknowledge that addiction is a chronic disease. Assumptions about a patient’s character or morality have no place in the appropriate management of OUD; remain mindful of your own implicit biases.
When is it appropriateto start an opioid taper?
The decision to taper opioids is difficult and can provoke anxiety for both prescriber and patient. Complicating matters is that there is insufficient evidence to evaluate opioid dosage-reduction interventions for patients with chronic noncancer pain.22
Safety concerns. Even in patients who are taking opioids as prescribed and for whom no red flags have been raised, the long-term safety of high-dosage opioids remains unclear. There is no “safe” dosage of opioids; however, evidence is clear that the risk of death from overdose increases with dosage. Compared with patients taking a dosage anywhere from 1 to 20 MME/d, those taking 50 to 99 MME/d have a 3.7-fold increased risk of overdose; patients taking ≥ 100 MME/d had an 8.9-fold increased risk.23 Patients for whom concomitant benzodiazepines are prescribed are also at higher risk of overdose and death. In studies of opioid overdose deaths, there was evidence of concurrent benzodiazepine use in 31% to 61% of cases.11
Inadequate analgesia. Given the well-established risk of drug tolerance, the inability to achieve or maintain pain relief or functional improvement can still occur—even when the opioid dosage is escalated reasonably. It might be prudent in that situation to taper opioids while also considering alternative modalities, including ones that were deferred previously.
Continue to: Intolerable adverse effects
Intolerable adverse effects. Adverse effects are common. Constipation has a reported prevalence of 15% to 90% among patients on long-term opioid treatment.24 Short-term, mild constipation is often manageable; long-term opioid use, however, can produce constipation refractory to bowel regimens and, in rare cases, lead to bowel obstruction, perforation, and even death. Other adverse effects include25
- sedation and drowsiness
- impaired memory or concentration
- mood changes
- dry mouth
- abdominal pain and nausea
- sexual dysfunction.
When these effects limit the tolerability of treatment, tapering might be indicated.
How are opioids tapered?
There is no definitive evidence of an optimal rate of taper or frequency of follow-up. Most guidelines suggest tapering opioids at 10% of the dosage each week; patients who have been taking opioids for many years, however, might require a slower taper (eg, a dosage decrease of 5%-20% every 2-4 weeks).11
Psychosocial support and maximizing nonopioid pain management techniques are critical to successful opioid tapering. When tapering is part of a comprehensive pain and rehabilitative plan, patients might find their symptoms alleviated.26 Given the potential risks in patients taking both short- and long-acting opioids, tapering the long-acting opioid should be the initial priority.
A more rapid taper—eg, a 20% reduction each week or even abrupt discontinuation of opioids—might be necessary if diversion is suspected or if there is concern that continued use of the medication presents high risk. In such cases, consultation with an addiction medicine specialist can be helpful—to assess whether medication-assisted therapy for OUD would be appropriate and how to support patients who are having withdrawal symptoms.
Continue to: For all patients...
For all patients, frequent follow-up visits with their primary care clinician, as well as referrals to mental health, physical therapy, and pain or rehabilitation services, can promote a successful taper. It is advised that, before beginning a taper, a treatment plan should be written out with the patient so that expectations are shared by physician and patient for the goals of the taper, the speed of dosage decreases, and the frequency of follow-up after each dosage change. At each follow-up visit, education regarding self-management and individualized recommendations for psychosocial support, mental health services, and substance use disorder services should be updated.
Assessing risk when tapering chronic opioid therapy
The goals of tapering should be to (1) reduce adverse effects of treatment and (2) mitigate short- and long-term risks.
Three short-term risks
Unmasking OUD. Tapering prescribed opioids, or even just discussing tapering, can unmask OUD in some patients. Follow-up visits during the tapering schedule should include frequent screening for OUD. If OUD is diagnosed, we recommend beginning medication-assisted treatment or referring the patient to a substance use treatment center. There is strong evidence of the safety and efficacy of medication-assisted treatment, even with a coexisting chronic pain disorder.27

Withdrawal syndrome. Opioid withdrawal syndrome is characterized by signs and symptoms of sympathetic stimulation, resulting from decreased sympathetic blockade by opioids (TABLE).28 (See “Changes in the locus ceruleus lead to withdrawal.”29) Symptoms start 2 to 3 half-lives after the last dose of opioid. Oxycodone, for example, has a half-life of 3 to 4 hours; withdrawal symptoms should therefore be anticipated in 6 to 12 hours. Because mixing opioids is commonplace, it can be difficult to predict exactly when withdrawal symptoms will begin. Patients are often most helpful in predicting the onset and severity of withdrawal symptoms.
SIDEBAR
Changes in the locus ceruleus lead to withdrawal
Normally, the locus ceruleus (LC), a pontine nucleus within the brainstem, produces noradrenaline (NA), which stimulates alertness, breathing, and blood pressure, among other physiologic functions. When opioids bind to the mu-opioid receptors in the LC and decrease the release of NA, the result is diminished alertness, lower blood pressure, and slower respiration.
With chronic exposure to opioids, the LC acts to increase levels of NA to counteract suppression. When a patient stops taking opioids, the increased NA levels become excessive and produce symptoms of opioid withdrawal. 29

Withdrawal can be measured using any of a number of validated tools, including
- the Subjective Opiate Withdrawal Scale, or SOWS30 (FIGURE 1), which utilizes a patient self-report
- the Clinical Opiate Withdrawal Scale, or COWS31 (FIGURE 2), which relies on assessment made by the physician.

Continue to: Although withdrawal...
Although withdrawal is generally not considered life-threatening in patients without significant comorbidities, do not underestimate the severity of withdrawal symptoms. Often, the desire to avoid these intense symptoms drives patients with OUD to continue to overuse.
Increased pain. Patients might fear that pain will become worse if opioids are tapered. Although it is important to acknowledge this fear, studies of patients undergoing a long-term opioid taper report improvements in function without loss of adequate pain control; some even report that pain control improves.32
Three long-term risks
Relapse. The most dangerous risk of tapering opioids is use of illicit opioids, a danger made worse by the increasing presence of highly lethal synthetic fentanyl analogues in the community. Risk factors for relapse following a full taper include the presence of depressive symptoms at initiation of tapering and higher pain scores at initiation and conclusion of the taper.33 Having low pain at the end of an opioid taper, on the other hand, is predictive of long-term abstinence from opioids.32
Declining function. As is the case while prescribing opioids for pain, maintenance of function remains a priority when tapering opioids. Function can be difficult to assess, given the many variables that can influence an individual’s function. Psychosocial factors, such as coping strategies and mood, strongly influence function; so do psychiatric morbidities, which are more prevalent in patients with chronic pain and disability, compared with the general population.34
Medicolegal matters. Although difficult to characterize, medicolegal risk is an inevitable consideration when tapering opioids:
- In a study of closed malpractice claims involving all medical specialties, narcotic pain medications were the most common drug class involved, representing 1% of claims.35
- In a study of closed malpractice claims involving pain medicine specialists, 3% were related to medication management. Most claims arose following death from opioid overdose.36
Continue to: What else is needed in this area of practice?
What else is needed in this area of practice?
Increasingly, family physicians face the inherent tension of wanting to provide patient-centered, compassionate care for patients in pain while being mindful of opioid prescription stewardship. To support their work and help allay this tension, clinical research on this topic in the future should focus on
- new options for nonopioid pharmacotherapy for pain
- best practices for using opioids in noncancer chronic pain.
In addition, health care systems can help—by providing insurance coverage of nonpharmacotherapeutic options for treating pain.
CORRESPONDENCE
Michael Mendoza, MD, MPH, MS, FAAFP, 111 Westfall Road, Room 952, Rochester, NY 14620; MichaelMendoza@ monroecounty.gov
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
1. Chen J, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
2. Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006-2015. MMWR Morb Mortal Wkly Rep. 2017;66:697-704.
3. Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826.
4. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1-S226.
5. Hardt J, Jacobsen C, Goldberg J, et al. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803-812.
6. Wilson HD, Dansie EJ, Kim MS, et al. Clinicians’ attitudes and beliefs about opioids survey (CAOS): instrument development and results of a national physician survey. J Pain. 2013;14:613-627.
7. Peppin JF. The marginalization of chronic pain patients on chronic opioid therapy. Pain Physician. 2009;12:493-498.
8. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011.
9. Bonnie RJ. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington, DC: The National Academies Press; 2017.
10. Sherman KJ, Walker RL, Saunders K, et al. Doctor-patient trust among chronic pain patients on chronic opioid therapy after opioid risk reduction initiatives: a survey. J Am Board Fam Med. 2018;31:578-587.
11. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
12. Broussard CS, Rasmussen SA, Reefhuis J, et al; National Birth Defects Prevention Study. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314.e1-e11.
13. FDA Drug Safety Communication: FDA has reviewed possible risks of pain medicine use during pregnancy. US Food and Drug Administration website. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-has-reviewed-possible-risks-pain-medicine-use-during-pregnancy. Published January 9, 2015. Accessed May 27, 2019.
14. Tran TH, Griffin BL, Stone RH, et al. Methadone, buprenorphine, and naltrexone for the treatment of opioid use disorder in pregnant women. Pharmacotherapy. 2017;37:824-839.
15. Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131-146.
16. Kuryshev YA, Bruening-Wright A, Brown AM, et al. Increased cardiac risk in concomitant methadone and diazepam treatment: pharmacodynamic interactions in cardiac ion channels. J Cardiovasc Pharmacol. 2010;56:420-430.
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
18. Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355-363.
19. Brooner RK, King VL, Kidorf M, et al. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71-80.
20. Merikangas KR, Stolar M, Stevens DE, et al. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973-979.
21. Kendler KS, Bulik CM, Silberg J, et al. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953-959.
22. Eccleston C, Fisher E, Thomas KH, et al. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst Rev. 2017;11:CD010323.
23. Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686-691.
24. Holzer P. Opioid antagonists for prevention and treatment of opioid-induced gastrointestinal effects. Curr Opin Anaesthesiol. 2010;23:616-622.
25. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;1:CD006605.
26. Murphy JL, Clark ME, Banou E. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain. 2013;29:109-117.
27. Dennis BB, Bawor M, Naji L, et al. Impact of chronic pain on treatment prognosis for patients with opioid use disorder: a systematic review and meta-analysis. Subst Abuse. 2015;9:59-80.
28. Farrell M. Opiate withdrawal. Addiction. 1994;89:1471-1475.
29. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20.
30. Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293-308.
31. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs. 2003;35:253-259.
32. Baron MJ, McDonald PW. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J Opioid Manag. 2006;2:277-282.
33. Heiwe S, Lönnquist I, Källmén H. Potential risk factors associated with risk for drop-out and relapse during and following withdrawal of opioid prescription medication. Eur J Pain. 2011;15:966-970.
34. Dersh J, Gatchel RJ, Polatin P, et al. Prevalence of psychiatric disorders in patients with chronic work-related musculoskeletal pain disability. J Occup Environ Med. 2002;44:459-468.
35. Troxel DB. REMS: Opioid-Related Patient Safety and Liability. Richardson, TX: The Doctors Company; 2012.
36. Fitzgibbon DR, Rathmell JP, Michna E, et al. Malpractice claims associated with medication management for chronic pain. Anesthesiology. 2010;112:948-956.
PRACTICE RECOMMENDATIONS
› Continue opioid therapy only when it has brought clinically meaningful improvement in pain and function and when the benefits outweigh adverse events or risks. C
› Review the selected opioid tapering plan in detail with the patient and provide close follow-up monitoring of ongoing or emerging risks. C
› Be vigilant: Enacting an opioid-tapering plan can unmask opioid use disorder, which can cause the patient to seek alternative forms of opioids, including illicit, potentially lethal fentanyl analogues. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Exposure to patients with migraine increases likelihood of stigmatizing attitudes
Philadelphia – , according to an analysis presented at the annual meeting of the American Headache Society.
“We need to understand why this is true,” said Robert Shapiro, MD, PhD, professor of neurological sciences at the University of Vermont in Burlington. The finding also raises questions about which measures could successfully mitigate these stigmatizing attitudes.
An examination of data from OVERCOME
Stigma is a social process by which people are excluded from society because of particular traits that they have. The process encompasses stereotypes, prejudice, and discrimination. Data suggest that the level of stigma that people with migraine experience is similar to that experienced by people with epilepsy. Other data indicate that people without migraine are equally likely to hold stigmatizing attitudes toward people with migraine and people with epilepsy.
Dr. Shapiro and colleagues examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study to better understand the attitudes that people without migraine have toward those who have the disorder. The data were gathered in fall 2018 through a web-based survey of a representative U.S. sample population. The researchers focused on a random sample of 2,000 people without migraine who responded to 11 questions about their attitudes toward patients with migraine. Responses described the frequency of holding attitudes and were scored on a 5-point Likert scale. The researchers categorized the responses “don’t know,” “never,” and “rarely” as “no” answers, and “sometimes,” “often,” and “very often” as “yes” answers. In addition, Dr. Shapiro and colleagues characterized each responder’s proximity to migraine according to the number of people with migraine that he or she knew (0, 1, or 2 or more) and the type of relationship (none, coworker, friend, or family member).
Sample was demographically representative
The demographic and socioeconomic characteristics of the study sample were similar to those of the most recent U.S. census data. The population’s mean age was 48, and 51% were female. Approximately 65% of respondents were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, 5% were Asian, and 5% were “other.” Approximately 45% of respondents reported that they had never known anyone with migraine. “Given the prevalence of migraine, it’s extraordinary that only 13% acknowledged that they had known two or more people with migraine,” said Dr. Shapiro. The finding raises questions about whether people with migraine have received adequate diagnoses and are aware of their disorder, he added. About 5% of the sample reported knowing only a coworker with migraine, and 37% reported knowing only one person with migraine.
About 31% of respondents thought that people with migraine use the disorder to avoid school or work commitments, and 33% thought that patients used migraine to avoid family or social commitments. Approximately 27% of respondents thought that people with migraine used it to get attention. About 45% of respondents thought that migraine should be treated easily, and 36% thought that people have migraine because of their own unhealthy behavior.
Individuals who knew people with migraine consistently held more negative attitudes toward those people, compared with those who did not know anyone with migraine. “These data are a little alarming,” said Dr. Shapiro. “They point to the difficulties that people with disabling migraine often encounter in having their experiences with the disease receive validation and understanding.”
Among the study’s strengths is the fact that it examined a large, population-based sample. The survey was conducted before many of the newer medications for migraine were available, and respondents were not likely to have been influenced by commercials that raised awareness of migraine, said Dr. Shapiro. The sample was not random, however, and the survey questions were based on the investigators’ interests, rather than on objective data. The generalizability of the results is in question, he added.
Dr. Shapiro consults for Eli Lilly, which sponsored the OVERCOME study.
SOURCE: Shapiro R et al. AHS 2019. Abstract OR15.
Philadelphia – , according to an analysis presented at the annual meeting of the American Headache Society.
“We need to understand why this is true,” said Robert Shapiro, MD, PhD, professor of neurological sciences at the University of Vermont in Burlington. The finding also raises questions about which measures could successfully mitigate these stigmatizing attitudes.
An examination of data from OVERCOME
Stigma is a social process by which people are excluded from society because of particular traits that they have. The process encompasses stereotypes, prejudice, and discrimination. Data suggest that the level of stigma that people with migraine experience is similar to that experienced by people with epilepsy. Other data indicate that people without migraine are equally likely to hold stigmatizing attitudes toward people with migraine and people with epilepsy.
Dr. Shapiro and colleagues examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study to better understand the attitudes that people without migraine have toward those who have the disorder. The data were gathered in fall 2018 through a web-based survey of a representative U.S. sample population. The researchers focused on a random sample of 2,000 people without migraine who responded to 11 questions about their attitudes toward patients with migraine. Responses described the frequency of holding attitudes and were scored on a 5-point Likert scale. The researchers categorized the responses “don’t know,” “never,” and “rarely” as “no” answers, and “sometimes,” “often,” and “very often” as “yes” answers. In addition, Dr. Shapiro and colleagues characterized each responder’s proximity to migraine according to the number of people with migraine that he or she knew (0, 1, or 2 or more) and the type of relationship (none, coworker, friend, or family member).
Sample was demographically representative
The demographic and socioeconomic characteristics of the study sample were similar to those of the most recent U.S. census data. The population’s mean age was 48, and 51% were female. Approximately 65% of respondents were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, 5% were Asian, and 5% were “other.” Approximately 45% of respondents reported that they had never known anyone with migraine. “Given the prevalence of migraine, it’s extraordinary that only 13% acknowledged that they had known two or more people with migraine,” said Dr. Shapiro. The finding raises questions about whether people with migraine have received adequate diagnoses and are aware of their disorder, he added. About 5% of the sample reported knowing only a coworker with migraine, and 37% reported knowing only one person with migraine.
About 31% of respondents thought that people with migraine use the disorder to avoid school or work commitments, and 33% thought that patients used migraine to avoid family or social commitments. Approximately 27% of respondents thought that people with migraine used it to get attention. About 45% of respondents thought that migraine should be treated easily, and 36% thought that people have migraine because of their own unhealthy behavior.
Individuals who knew people with migraine consistently held more negative attitudes toward those people, compared with those who did not know anyone with migraine. “These data are a little alarming,” said Dr. Shapiro. “They point to the difficulties that people with disabling migraine often encounter in having their experiences with the disease receive validation and understanding.”
Among the study’s strengths is the fact that it examined a large, population-based sample. The survey was conducted before many of the newer medications for migraine were available, and respondents were not likely to have been influenced by commercials that raised awareness of migraine, said Dr. Shapiro. The sample was not random, however, and the survey questions were based on the investigators’ interests, rather than on objective data. The generalizability of the results is in question, he added.
Dr. Shapiro consults for Eli Lilly, which sponsored the OVERCOME study.
SOURCE: Shapiro R et al. AHS 2019. Abstract OR15.
Philadelphia – , according to an analysis presented at the annual meeting of the American Headache Society.
“We need to understand why this is true,” said Robert Shapiro, MD, PhD, professor of neurological sciences at the University of Vermont in Burlington. The finding also raises questions about which measures could successfully mitigate these stigmatizing attitudes.
An examination of data from OVERCOME
Stigma is a social process by which people are excluded from society because of particular traits that they have. The process encompasses stereotypes, prejudice, and discrimination. Data suggest that the level of stigma that people with migraine experience is similar to that experienced by people with epilepsy. Other data indicate that people without migraine are equally likely to hold stigmatizing attitudes toward people with migraine and people with epilepsy.
Dr. Shapiro and colleagues examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study to better understand the attitudes that people without migraine have toward those who have the disorder. The data were gathered in fall 2018 through a web-based survey of a representative U.S. sample population. The researchers focused on a random sample of 2,000 people without migraine who responded to 11 questions about their attitudes toward patients with migraine. Responses described the frequency of holding attitudes and were scored on a 5-point Likert scale. The researchers categorized the responses “don’t know,” “never,” and “rarely” as “no” answers, and “sometimes,” “often,” and “very often” as “yes” answers. In addition, Dr. Shapiro and colleagues characterized each responder’s proximity to migraine according to the number of people with migraine that he or she knew (0, 1, or 2 or more) and the type of relationship (none, coworker, friend, or family member).
Sample was demographically representative
The demographic and socioeconomic characteristics of the study sample were similar to those of the most recent U.S. census data. The population’s mean age was 48, and 51% were female. Approximately 65% of respondents were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, 5% were Asian, and 5% were “other.” Approximately 45% of respondents reported that they had never known anyone with migraine. “Given the prevalence of migraine, it’s extraordinary that only 13% acknowledged that they had known two or more people with migraine,” said Dr. Shapiro. The finding raises questions about whether people with migraine have received adequate diagnoses and are aware of their disorder, he added. About 5% of the sample reported knowing only a coworker with migraine, and 37% reported knowing only one person with migraine.
About 31% of respondents thought that people with migraine use the disorder to avoid school or work commitments, and 33% thought that patients used migraine to avoid family or social commitments. Approximately 27% of respondents thought that people with migraine used it to get attention. About 45% of respondents thought that migraine should be treated easily, and 36% thought that people have migraine because of their own unhealthy behavior.
Individuals who knew people with migraine consistently held more negative attitudes toward those people, compared with those who did not know anyone with migraine. “These data are a little alarming,” said Dr. Shapiro. “They point to the difficulties that people with disabling migraine often encounter in having their experiences with the disease receive validation and understanding.”
Among the study’s strengths is the fact that it examined a large, population-based sample. The survey was conducted before many of the newer medications for migraine were available, and respondents were not likely to have been influenced by commercials that raised awareness of migraine, said Dr. Shapiro. The sample was not random, however, and the survey questions were based on the investigators’ interests, rather than on objective data. The generalizability of the results is in question, he added.
Dr. Shapiro consults for Eli Lilly, which sponsored the OVERCOME study.
SOURCE: Shapiro R et al. AHS 2019. Abstract OR15.
REPORTING FROM AHS 2019
Depression, anxiety among elderly breast cancer survivors linked to increased opioid use, death
Mental health comorbidities increase the rates of opioid use and mortality among breast cancer survivors on endocrine therapy, based on a retrospective study of more than 10,000 patients in a Medicare-linked database.
Screen for mental health conditions in the early stages of cancer care and lean toward opioid alternatives for pain management, advised lead author Raj Desai, MS, of the University of Florida, Gainesville, and colleagues.
“The complex relationship among breast cancer, mental health problems, and the use of opioids is not well understood, despite the high prevalence of mental health comorbidities like depression and anxiety in breast cancer survivors, and the high rate of opioid use in those on AET [adjuvant endocrine therapy],” the investigators wrote in the Journal of Oncology Practice.
“Therefore, this study aimed to determine whether breast cancer survivors with varying levels of mental health comorbidities, such as depression and anxiety, are more likely to use opioids for AET-related pain,” they added.
The study involved 10,452 breast cancer survivors who first filled an AET prescription from 2006 to 2012 and had follow-up records available for at least 2 years. All patients had a diagnosis of incident, primary, hormone receptor–positive, stage I-III breast cancer. Data were drawn from the Surveillance, Epidemiology, and End Results–Medicare linked database. Records were evaluated for diagnoses of mental health conditions such as depression and anxiety, opioid use, and survival.
Analysis showed that the most common mental health conditions were depression and anxiety, diagnosed in 554 and 246 women, respectively. Patients with mental health comorbidities were compared with patients who did not have such problems, using both unmatched and matched cohorts. While unmatched comparison for opioid use was not statistically significant, matched comparison showed that survivors with mental health comorbidities were 33% more likely to use opioids than those without mental health comorbidities (95% confidence interval, 1.06-1.68). Similarly, greater adjusted probabilities of opioid use were reported in the mental health comorbidity cohort (72.5% vs. 66.9%; P = .01).
Concerning survival, unmatched comparison revealed a 44% higher risk of death among women with depression and a 32% increase associated with anxiety. Matched comparison showed an even higher increased risk of mortality among women with any mental health comorbidity (49%; P less than .05).
The investigators concluded that opioid use among breast cancer survivors with mental health comorbidities “remains a significant problem.”
“A need exists for collaborative care in the management of mental health comorbidities in women with breast cancer, which could improve symptoms, adherence to treatment, and recovery from these mental conditions,” the investigators wrote. “Mental health treatments also are recommended to be offered in primary care, which not only would be convenient for patients, but also would reduce the stigma associated with treatments for mental health comorbidities and improve the patient-provider relationship.”
The investigators reported financial relationships with Merck.
SOURCE: Desai R et al. J Oncol Pract. 2019 Jul 19. doi: 10.1200/JOP.18.00781.
Mental health comorbidities increase the rates of opioid use and mortality among breast cancer survivors on endocrine therapy, based on a retrospective study of more than 10,000 patients in a Medicare-linked database.
Screen for mental health conditions in the early stages of cancer care and lean toward opioid alternatives for pain management, advised lead author Raj Desai, MS, of the University of Florida, Gainesville, and colleagues.
“The complex relationship among breast cancer, mental health problems, and the use of opioids is not well understood, despite the high prevalence of mental health comorbidities like depression and anxiety in breast cancer survivors, and the high rate of opioid use in those on AET [adjuvant endocrine therapy],” the investigators wrote in the Journal of Oncology Practice.
“Therefore, this study aimed to determine whether breast cancer survivors with varying levels of mental health comorbidities, such as depression and anxiety, are more likely to use opioids for AET-related pain,” they added.
The study involved 10,452 breast cancer survivors who first filled an AET prescription from 2006 to 2012 and had follow-up records available for at least 2 years. All patients had a diagnosis of incident, primary, hormone receptor–positive, stage I-III breast cancer. Data were drawn from the Surveillance, Epidemiology, and End Results–Medicare linked database. Records were evaluated for diagnoses of mental health conditions such as depression and anxiety, opioid use, and survival.
Analysis showed that the most common mental health conditions were depression and anxiety, diagnosed in 554 and 246 women, respectively. Patients with mental health comorbidities were compared with patients who did not have such problems, using both unmatched and matched cohorts. While unmatched comparison for opioid use was not statistically significant, matched comparison showed that survivors with mental health comorbidities were 33% more likely to use opioids than those without mental health comorbidities (95% confidence interval, 1.06-1.68). Similarly, greater adjusted probabilities of opioid use were reported in the mental health comorbidity cohort (72.5% vs. 66.9%; P = .01).
Concerning survival, unmatched comparison revealed a 44% higher risk of death among women with depression and a 32% increase associated with anxiety. Matched comparison showed an even higher increased risk of mortality among women with any mental health comorbidity (49%; P less than .05).
The investigators concluded that opioid use among breast cancer survivors with mental health comorbidities “remains a significant problem.”
“A need exists for collaborative care in the management of mental health comorbidities in women with breast cancer, which could improve symptoms, adherence to treatment, and recovery from these mental conditions,” the investigators wrote. “Mental health treatments also are recommended to be offered in primary care, which not only would be convenient for patients, but also would reduce the stigma associated with treatments for mental health comorbidities and improve the patient-provider relationship.”
The investigators reported financial relationships with Merck.
SOURCE: Desai R et al. J Oncol Pract. 2019 Jul 19. doi: 10.1200/JOP.18.00781.
Mental health comorbidities increase the rates of opioid use and mortality among breast cancer survivors on endocrine therapy, based on a retrospective study of more than 10,000 patients in a Medicare-linked database.
Screen for mental health conditions in the early stages of cancer care and lean toward opioid alternatives for pain management, advised lead author Raj Desai, MS, of the University of Florida, Gainesville, and colleagues.
“The complex relationship among breast cancer, mental health problems, and the use of opioids is not well understood, despite the high prevalence of mental health comorbidities like depression and anxiety in breast cancer survivors, and the high rate of opioid use in those on AET [adjuvant endocrine therapy],” the investigators wrote in the Journal of Oncology Practice.
“Therefore, this study aimed to determine whether breast cancer survivors with varying levels of mental health comorbidities, such as depression and anxiety, are more likely to use opioids for AET-related pain,” they added.
The study involved 10,452 breast cancer survivors who first filled an AET prescription from 2006 to 2012 and had follow-up records available for at least 2 years. All patients had a diagnosis of incident, primary, hormone receptor–positive, stage I-III breast cancer. Data were drawn from the Surveillance, Epidemiology, and End Results–Medicare linked database. Records were evaluated for diagnoses of mental health conditions such as depression and anxiety, opioid use, and survival.
Analysis showed that the most common mental health conditions were depression and anxiety, diagnosed in 554 and 246 women, respectively. Patients with mental health comorbidities were compared with patients who did not have such problems, using both unmatched and matched cohorts. While unmatched comparison for opioid use was not statistically significant, matched comparison showed that survivors with mental health comorbidities were 33% more likely to use opioids than those without mental health comorbidities (95% confidence interval, 1.06-1.68). Similarly, greater adjusted probabilities of opioid use were reported in the mental health comorbidity cohort (72.5% vs. 66.9%; P = .01).
Concerning survival, unmatched comparison revealed a 44% higher risk of death among women with depression and a 32% increase associated with anxiety. Matched comparison showed an even higher increased risk of mortality among women with any mental health comorbidity (49%; P less than .05).
The investigators concluded that opioid use among breast cancer survivors with mental health comorbidities “remains a significant problem.”
“A need exists for collaborative care in the management of mental health comorbidities in women with breast cancer, which could improve symptoms, adherence to treatment, and recovery from these mental conditions,” the investigators wrote. “Mental health treatments also are recommended to be offered in primary care, which not only would be convenient for patients, but also would reduce the stigma associated with treatments for mental health comorbidities and improve the patient-provider relationship.”
The investigators reported financial relationships with Merck.
SOURCE: Desai R et al. J Oncol Pract. 2019 Jul 19. doi: 10.1200/JOP.18.00781.
FROM THE JOURNAL OF ONCOLOGY PRACTICE