User login
Is carpal tunnel syndrome the tip of the iceberg?
He takes the following medications: felodipine and atorvastatin. On exam, his blood pressure is 110/60 mm Hg, and his pulse is 90 beats per minute.
A cardiac examination found normal heart sounds with no murmurs.
A chest examination found dullness to percussion at both bases and rales.
A chest x-ray showed bilateral effusions and mild pulmonary edema.
The brain natriuretic peptide test found a level of 1,300 picograms/mL.
An ECG found increased ventricular wall thickness, an ejection fraction of 32%, and normal aortic and mitral valves.
What history would be the most helpful in making a diagnosis?
A. History of prostate cancer
B. History of carpal tunnel syndrome
C. History of playing professional football
D. History of hyperlipidemia
E. History of ulcerative colitis
The correct answer here would be B. history of carpal tunnel syndrome (CTS). This patient has clinical heart failure, without a history of clinical ischemic disease. The differential diagnosis for causes of heart failure is long, with the most common causes being chronic hypertension and ischemic heart disease. Other common causes include chronic untreated sleep apnea and valvular heart disease.
This patient really does not have clear reasons for having clinical heart failure. His cardiovascular risk factors have been well controlled, and no valvular disease was found on ECG.
Several recent reports have raised the importance of a history of CTS significantly increasing the likelihood of amyloidosis being the cause of underlying heart failure.
CTS is such a common clinical entity that it is easy to not appreciate its presence as a clue to possible amyloid cardiomyopathy. Fosbøl et al. reported that a diagnosis of CTS was associated with a higher incidence of heart failure (hazard ratio, 1.54; CI, 1.45-1.64).1 They found a highly increased risk of amyloid (HR, 12.2) in patients who had surgery for CTS.
Sperry et al. found that over 10% of patients who underwent carpal tunnel release stained for amyloid on biopsy specimens, and that concomitant cardiac evaluation identified patients with cardiac involvement.2
Pinney et al. found that 48% of patients with transthyretin amyloidosis had a history of CTS.3
In a retrospective study of patients with wild-type transthyretin amyloid (253), patients with hereditary transthyretin amyloid (136), and asymptomatic gene carriers (77), participants were screened for a history of spinal stenosis and CTS.4 Almost 60% of the patients with amyloid had a history of CTS, and 11% had a history of spinal stenosis. Patients with CTS and hereditary amyloid had thicker interventricular septums, higher left ventricular mass, and lower Karnovsky index than those without CTS.
The diagnosis of CTS, especially in those who need surgery for treatment or have bilateral disease, should make us consider the possibility of underlying amyloidosis.
Pearl: In patients who have heart failure and a history of CTS, amyloidosis should be considered as a cause.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Fosbøl EL et al. J Am Coll Cardiol. 2019;74:15-23.
2. Sperry BW et al. J Am Coll Cardiol. 2018 Oct 23;72(17):2040-50.
3. Pinney JH et al. J Am Heart Assoc. 2013 Apr 22;2(2):e000098.
4. Aus dem Siepen F et al. Clin Res Cardiol. 2019 Apr 5. doi: 10.1007/s00392-019-01467-1.
He takes the following medications: felodipine and atorvastatin. On exam, his blood pressure is 110/60 mm Hg, and his pulse is 90 beats per minute.
A cardiac examination found normal heart sounds with no murmurs.
A chest examination found dullness to percussion at both bases and rales.
A chest x-ray showed bilateral effusions and mild pulmonary edema.
The brain natriuretic peptide test found a level of 1,300 picograms/mL.
An ECG found increased ventricular wall thickness, an ejection fraction of 32%, and normal aortic and mitral valves.
What history would be the most helpful in making a diagnosis?
A. History of prostate cancer
B. History of carpal tunnel syndrome
C. History of playing professional football
D. History of hyperlipidemia
E. History of ulcerative colitis
The correct answer here would be B. history of carpal tunnel syndrome (CTS). This patient has clinical heart failure, without a history of clinical ischemic disease. The differential diagnosis for causes of heart failure is long, with the most common causes being chronic hypertension and ischemic heart disease. Other common causes include chronic untreated sleep apnea and valvular heart disease.
This patient really does not have clear reasons for having clinical heart failure. His cardiovascular risk factors have been well controlled, and no valvular disease was found on ECG.
Several recent reports have raised the importance of a history of CTS significantly increasing the likelihood of amyloidosis being the cause of underlying heart failure.
CTS is such a common clinical entity that it is easy to not appreciate its presence as a clue to possible amyloid cardiomyopathy. Fosbøl et al. reported that a diagnosis of CTS was associated with a higher incidence of heart failure (hazard ratio, 1.54; CI, 1.45-1.64).1 They found a highly increased risk of amyloid (HR, 12.2) in patients who had surgery for CTS.
Sperry et al. found that over 10% of patients who underwent carpal tunnel release stained for amyloid on biopsy specimens, and that concomitant cardiac evaluation identified patients with cardiac involvement.2
Pinney et al. found that 48% of patients with transthyretin amyloidosis had a history of CTS.3
In a retrospective study of patients with wild-type transthyretin amyloid (253), patients with hereditary transthyretin amyloid (136), and asymptomatic gene carriers (77), participants were screened for a history of spinal stenosis and CTS.4 Almost 60% of the patients with amyloid had a history of CTS, and 11% had a history of spinal stenosis. Patients with CTS and hereditary amyloid had thicker interventricular septums, higher left ventricular mass, and lower Karnovsky index than those without CTS.
The diagnosis of CTS, especially in those who need surgery for treatment or have bilateral disease, should make us consider the possibility of underlying amyloidosis.
Pearl: In patients who have heart failure and a history of CTS, amyloidosis should be considered as a cause.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Fosbøl EL et al. J Am Coll Cardiol. 2019;74:15-23.
2. Sperry BW et al. J Am Coll Cardiol. 2018 Oct 23;72(17):2040-50.
3. Pinney JH et al. J Am Heart Assoc. 2013 Apr 22;2(2):e000098.
4. Aus dem Siepen F et al. Clin Res Cardiol. 2019 Apr 5. doi: 10.1007/s00392-019-01467-1.
He takes the following medications: felodipine and atorvastatin. On exam, his blood pressure is 110/60 mm Hg, and his pulse is 90 beats per minute.
A cardiac examination found normal heart sounds with no murmurs.
A chest examination found dullness to percussion at both bases and rales.
A chest x-ray showed bilateral effusions and mild pulmonary edema.
The brain natriuretic peptide test found a level of 1,300 picograms/mL.
An ECG found increased ventricular wall thickness, an ejection fraction of 32%, and normal aortic and mitral valves.
What history would be the most helpful in making a diagnosis?
A. History of prostate cancer
B. History of carpal tunnel syndrome
C. History of playing professional football
D. History of hyperlipidemia
E. History of ulcerative colitis
The correct answer here would be B. history of carpal tunnel syndrome (CTS). This patient has clinical heart failure, without a history of clinical ischemic disease. The differential diagnosis for causes of heart failure is long, with the most common causes being chronic hypertension and ischemic heart disease. Other common causes include chronic untreated sleep apnea and valvular heart disease.
This patient really does not have clear reasons for having clinical heart failure. His cardiovascular risk factors have been well controlled, and no valvular disease was found on ECG.
Several recent reports have raised the importance of a history of CTS significantly increasing the likelihood of amyloidosis being the cause of underlying heart failure.
CTS is such a common clinical entity that it is easy to not appreciate its presence as a clue to possible amyloid cardiomyopathy. Fosbøl et al. reported that a diagnosis of CTS was associated with a higher incidence of heart failure (hazard ratio, 1.54; CI, 1.45-1.64).1 They found a highly increased risk of amyloid (HR, 12.2) in patients who had surgery for CTS.
Sperry et al. found that over 10% of patients who underwent carpal tunnel release stained for amyloid on biopsy specimens, and that concomitant cardiac evaluation identified patients with cardiac involvement.2
Pinney et al. found that 48% of patients with transthyretin amyloidosis had a history of CTS.3
In a retrospective study of patients with wild-type transthyretin amyloid (253), patients with hereditary transthyretin amyloid (136), and asymptomatic gene carriers (77), participants were screened for a history of spinal stenosis and CTS.4 Almost 60% of the patients with amyloid had a history of CTS, and 11% had a history of spinal stenosis. Patients with CTS and hereditary amyloid had thicker interventricular septums, higher left ventricular mass, and lower Karnovsky index than those without CTS.
The diagnosis of CTS, especially in those who need surgery for treatment or have bilateral disease, should make us consider the possibility of underlying amyloidosis.
Pearl: In patients who have heart failure and a history of CTS, amyloidosis should be considered as a cause.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at that university. Contact Dr. Paauw at [email protected].
References
1. Fosbøl EL et al. J Am Coll Cardiol. 2019;74:15-23.
2. Sperry BW et al. J Am Coll Cardiol. 2018 Oct 23;72(17):2040-50.
3. Pinney JH et al. J Am Heart Assoc. 2013 Apr 22;2(2):e000098.
4. Aus dem Siepen F et al. Clin Res Cardiol. 2019 Apr 5. doi: 10.1007/s00392-019-01467-1.
Drug crisis continues to evolve beyond opioids
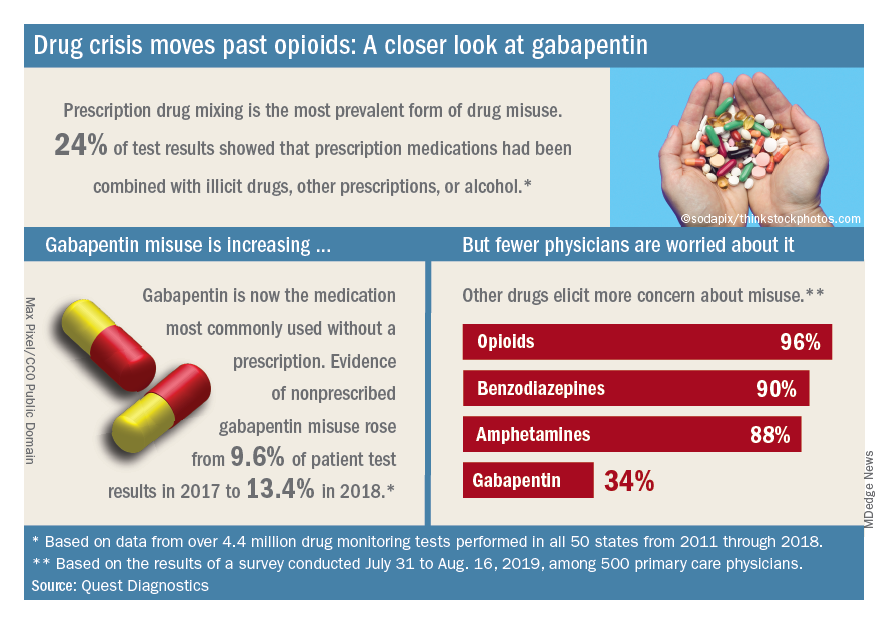
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.
“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.

“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
Almost three-quarters of primary care physicians believe that their patients will take their controlled medications as prescribed, but more than half of drug-monitoring lab tests show signs of misuse, according to a new report from Quest Diagnostics.

“ and may miss some of the drug misuse risks affecting their patients,” report coauthor Harvey W. Kaufman, MD, Quest’s senior medical director, said in a written statement.
Analysis of more than 4.4 million drug-monitoring tests showed that 51% involved an inconsistent result, such as detection of a nonprescribed drug or nondetection of a drug that was prescribed. The report also included a survey of 500 primary care physicians, of whom 72% said they trusted their patents to properly use opioids and other controlled substances.
“The intersection of these two data sets reveals, for the first time, the contrast between physician expectations about patient drug use and the evolution of the drug epidemic and actual patient behavior, as revealed by objective lab data, amid a national drug crisis that claimed an estimated 68,500 lives last year,” the report said.
A majority (62%) of the physicians surveyed also said that the opioid crisis will evolve into a new prescription drug crisis, and even more (72%) think that patients with chronic pain will use illicit drugs if they cannot get prescription opioids. Evidence from the drug test dataset suggests that “misuse of nonprescribed fentanyl and nonprescribed gabapentin warrant[s] a closer look,” the report said. In the survey, 78% of respondents reported prescribing gabapentin as an alternative to opioids for patients with chronic pain.
Those two drugs, along with alcohol, are the only three drug groups for which misuse increased from 2017 to 2018, and both are frequently involved in drug mixing, which is the most common form of misuse. Gabapentin went from 9.6% of all nonprescribed misuse in 2017 to 13.4% in 2018, an increase of 40%. Nonprescribed fentanyl was found in 64% of test results that were positive for heroin and 24% that were positive for cocaine, the Quest data showed.
The survey results, however, suggest that gabapentin is not on physicians’ radar, as only 34% said that they were concerned about its misuse, compared with 96% for opioids and 90% for benzodiazepines, according to the report.
“While gabapentin may not have opioids’ addictive potential, it can exaggerate euphoric effects when combined with opioids or anxiety medications. This drug mixing is dangerous,” said report coauthor Jeffrey Gudin, MD, senior medical advisor, prescription drug monitoring, for Quest Diagnostics.
The survey was conducted online among family physicians, general practitioners, and internists from July 31 to Aug. 16, 2019, by the Harris Poll on behalf of Quest and Center for Addiction. The test result data were collected in all 50 states and Washington, D.C., from 2011 to 2018, and results from drug rehabilitation clinics and addiction specialists were excluded from the analysis, so actual misuse rates are probably higher than reported.
FDA approves Reyvow for acute migraine treatment
The Food and Drug Administration has approved lasmiditan (Reyvow) for acute treatment of migraines with and without auras in adults.
The agency’s Oct. 11 announcement said the approval is based on results from a pair of randomized, double-blind, placebo-controlled trials that included 3,177 adult patients with a history of migraine with and without aura. The percentage of patients whose pain and most bothersome migraine symptom (nausea, light sensitivity, or sound sensitivity) resolved after 2 hours was higher in patients receiving lasmiditan than in patients receiving placebo.
Lasmiditan is a serotonin 5-hydroxytryptamine1F–receptor agonist, giving it a unique mechanism of action as compared with other migraine treatments.
The most common adverse events associated with lasmiditan include dizziness, fatigue, paresthesia, and sedation. There is a risk of driving impairment while taking the medication, and patients are advised not to operate or drive machinery for 8 hours after taking lasmiditan.
“Reyvow is a new option for the acute treatment of migraine, a painful condition that affects one in seven Americans. We know that the migraine community is keenly interested in additional treatment options, and we remain committed to continuing to work with stakeholders to promote the development of new therapies for the acute and preventive treatment of migraine,” said Nick Kozauer, MD, acting deputy director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research.
Eli Lilly, the drug’s manufacturer, said in a news release that “the recommended controlled substance classification for Reyvow is currently under review by the Drug Enforcement Administration and is expected within 90 days of today’s FDA approval, after which Reyvow will be available to patients in retail pharmacies” in oral doses of 50 mg, 100 mg, and 200 mg.
The Food and Drug Administration has approved lasmiditan (Reyvow) for acute treatment of migraines with and without auras in adults.
The agency’s Oct. 11 announcement said the approval is based on results from a pair of randomized, double-blind, placebo-controlled trials that included 3,177 adult patients with a history of migraine with and without aura. The percentage of patients whose pain and most bothersome migraine symptom (nausea, light sensitivity, or sound sensitivity) resolved after 2 hours was higher in patients receiving lasmiditan than in patients receiving placebo.
Lasmiditan is a serotonin 5-hydroxytryptamine1F–receptor agonist, giving it a unique mechanism of action as compared with other migraine treatments.
The most common adverse events associated with lasmiditan include dizziness, fatigue, paresthesia, and sedation. There is a risk of driving impairment while taking the medication, and patients are advised not to operate or drive machinery for 8 hours after taking lasmiditan.
“Reyvow is a new option for the acute treatment of migraine, a painful condition that affects one in seven Americans. We know that the migraine community is keenly interested in additional treatment options, and we remain committed to continuing to work with stakeholders to promote the development of new therapies for the acute and preventive treatment of migraine,” said Nick Kozauer, MD, acting deputy director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research.
Eli Lilly, the drug’s manufacturer, said in a news release that “the recommended controlled substance classification for Reyvow is currently under review by the Drug Enforcement Administration and is expected within 90 days of today’s FDA approval, after which Reyvow will be available to patients in retail pharmacies” in oral doses of 50 mg, 100 mg, and 200 mg.
The Food and Drug Administration has approved lasmiditan (Reyvow) for acute treatment of migraines with and without auras in adults.
The agency’s Oct. 11 announcement said the approval is based on results from a pair of randomized, double-blind, placebo-controlled trials that included 3,177 adult patients with a history of migraine with and without aura. The percentage of patients whose pain and most bothersome migraine symptom (nausea, light sensitivity, or sound sensitivity) resolved after 2 hours was higher in patients receiving lasmiditan than in patients receiving placebo.
Lasmiditan is a serotonin 5-hydroxytryptamine1F–receptor agonist, giving it a unique mechanism of action as compared with other migraine treatments.
The most common adverse events associated with lasmiditan include dizziness, fatigue, paresthesia, and sedation. There is a risk of driving impairment while taking the medication, and patients are advised not to operate or drive machinery for 8 hours after taking lasmiditan.
“Reyvow is a new option for the acute treatment of migraine, a painful condition that affects one in seven Americans. We know that the migraine community is keenly interested in additional treatment options, and we remain committed to continuing to work with stakeholders to promote the development of new therapies for the acute and preventive treatment of migraine,” said Nick Kozauer, MD, acting deputy director of the division of neurology products in the FDA’s Center for Drug Evaluation and Research.
Eli Lilly, the drug’s manufacturer, said in a news release that “the recommended controlled substance classification for Reyvow is currently under review by the Drug Enforcement Administration and is expected within 90 days of today’s FDA approval, after which Reyvow will be available to patients in retail pharmacies” in oral doses of 50 mg, 100 mg, and 200 mg.
Preop IV dexamethasone conveys relief after total knee surgery
Patients given a single preoperative dose of intravenous dexamethasone had significantly less pain after total knee arthroplasty than did those given a placebo in a randomized controlled study of 100 adults.
“Corticosteroids were introduced several years ago for relieving postoperative pain in total joint replacement but, unfortunately, are not widely used due to surgeons’ concerns and the limited supporting evidence,” wrote Nattapol Tammachote, MD, of Thammasat University, Khlong Luang, Pathumthani, Thailand, and colleagues.
In a study published in the Journal of Arthroplasty, the researchers randomized 50 adults undergoing unilateral total knee surgery to a preoperative IV dexamethasone dose of 0.15 mg/kg diluted with normal saline or saline placebo. Patients, who were aged 50-85 years, were assessed every 3 hours after surgery, up to 48 hours; the primary outcomes were pain level, using the visual analog pain scale (VAS), and morphine use.
Overall, patients in the treatment group reported significant reductions on the VAS in mean pain scores of 11 points at rest and 15 points with knee movement. No significant differences in morphine use were noted between groups overall or at 12-hour intervals post-surgery.
In the first 24-48 hours after surgery dexamethasone was associated with a significantly lower rate of nausea and vomiting vs. placebo (58% vs. 84%), and a lower average C-reactive protein level (89 mg/L vs. 167 mg/L) at 48 hours after surgery. Hospital stays averaged 3 days for both groups, and no wound infections were reported.
Scores on tests of knee function using the modified Western Ontario and McMaster University Osteoarthritis Index scores and range of motion of the knee at three months were similar between the groups.
The study findings were limited by several factors, including the small sample size and use of multimodal pain control that may have impacted morphine use, a lack of data on hyperglycemia, and variation in doses of ketorolac given to patients in both groups, the researchers noted.
The results nevertheless support the potential of preoperative dexamethasone as “a promising approach in postoperative pain management and may be suitable for patients with contraindication to multimodal pain regimens,” they concluded.
The researchers reported no financial conflicts.
SOURCE: Tammachote N et al. J Arthroplasty. 2019. doi: https://doi.org/10.1016/ j.arth.2019.09.002.
Patients given a single preoperative dose of intravenous dexamethasone had significantly less pain after total knee arthroplasty than did those given a placebo in a randomized controlled study of 100 adults.
“Corticosteroids were introduced several years ago for relieving postoperative pain in total joint replacement but, unfortunately, are not widely used due to surgeons’ concerns and the limited supporting evidence,” wrote Nattapol Tammachote, MD, of Thammasat University, Khlong Luang, Pathumthani, Thailand, and colleagues.
In a study published in the Journal of Arthroplasty, the researchers randomized 50 adults undergoing unilateral total knee surgery to a preoperative IV dexamethasone dose of 0.15 mg/kg diluted with normal saline or saline placebo. Patients, who were aged 50-85 years, were assessed every 3 hours after surgery, up to 48 hours; the primary outcomes were pain level, using the visual analog pain scale (VAS), and morphine use.
Overall, patients in the treatment group reported significant reductions on the VAS in mean pain scores of 11 points at rest and 15 points with knee movement. No significant differences in morphine use were noted between groups overall or at 12-hour intervals post-surgery.
In the first 24-48 hours after surgery dexamethasone was associated with a significantly lower rate of nausea and vomiting vs. placebo (58% vs. 84%), and a lower average C-reactive protein level (89 mg/L vs. 167 mg/L) at 48 hours after surgery. Hospital stays averaged 3 days for both groups, and no wound infections were reported.
Scores on tests of knee function using the modified Western Ontario and McMaster University Osteoarthritis Index scores and range of motion of the knee at three months were similar between the groups.
The study findings were limited by several factors, including the small sample size and use of multimodal pain control that may have impacted morphine use, a lack of data on hyperglycemia, and variation in doses of ketorolac given to patients in both groups, the researchers noted.
The results nevertheless support the potential of preoperative dexamethasone as “a promising approach in postoperative pain management and may be suitable for patients with contraindication to multimodal pain regimens,” they concluded.
The researchers reported no financial conflicts.
SOURCE: Tammachote N et al. J Arthroplasty. 2019. doi: https://doi.org/10.1016/ j.arth.2019.09.002.
Patients given a single preoperative dose of intravenous dexamethasone had significantly less pain after total knee arthroplasty than did those given a placebo in a randomized controlled study of 100 adults.
“Corticosteroids were introduced several years ago for relieving postoperative pain in total joint replacement but, unfortunately, are not widely used due to surgeons’ concerns and the limited supporting evidence,” wrote Nattapol Tammachote, MD, of Thammasat University, Khlong Luang, Pathumthani, Thailand, and colleagues.
In a study published in the Journal of Arthroplasty, the researchers randomized 50 adults undergoing unilateral total knee surgery to a preoperative IV dexamethasone dose of 0.15 mg/kg diluted with normal saline or saline placebo. Patients, who were aged 50-85 years, were assessed every 3 hours after surgery, up to 48 hours; the primary outcomes were pain level, using the visual analog pain scale (VAS), and morphine use.
Overall, patients in the treatment group reported significant reductions on the VAS in mean pain scores of 11 points at rest and 15 points with knee movement. No significant differences in morphine use were noted between groups overall or at 12-hour intervals post-surgery.
In the first 24-48 hours after surgery dexamethasone was associated with a significantly lower rate of nausea and vomiting vs. placebo (58% vs. 84%), and a lower average C-reactive protein level (89 mg/L vs. 167 mg/L) at 48 hours after surgery. Hospital stays averaged 3 days for both groups, and no wound infections were reported.
Scores on tests of knee function using the modified Western Ontario and McMaster University Osteoarthritis Index scores and range of motion of the knee at three months were similar between the groups.
The study findings were limited by several factors, including the small sample size and use of multimodal pain control that may have impacted morphine use, a lack of data on hyperglycemia, and variation in doses of ketorolac given to patients in both groups, the researchers noted.
The results nevertheless support the potential of preoperative dexamethasone as “a promising approach in postoperative pain management and may be suitable for patients with contraindication to multimodal pain regimens,” they concluded.
The researchers reported no financial conflicts.
SOURCE: Tammachote N et al. J Arthroplasty. 2019. doi: https://doi.org/10.1016/ j.arth.2019.09.002.
FROM THE JOURNAL OF ARTHROPLASTY
Preop pain perceptions drive outcomes after knee surgery
Adult athletes who underwent knee surgery and had higher levels of preoperative pain catastrophizing were significantly less likely to return to preinjury activity, based on data from 101 individuals.
Pain is highly subjective, and pain perception can play a role in postsurgical outcomes, but the relationships among preoperative pain perception and short-term outcomes including returning to sports have not been well-studied, wrote Joshua S. Everhart, MD, of The Ohio State University Wexner Medical Center, Columbus, and colleagues.
In a study published in the Journal of Science and Medicine in Sport, the researchers assessed 101 adult athletes who underwent knee surgery at a single center. The average age of the patients was 33 years, and 49 were women.
Pain perception and coping were assessed via the McGill Pain questionnaire (SF-MPQ), Pain Catastrophizing Scale (PCS), Pain Coping Measure (PCM), and the brief COPE subscales of acceptance, denial, positive reframing, and use of instrumental support.
Patients who were severe pain catastrophizers (defined as scores greater than 36 on the Pain Catastrophizing Scale) had increased odds of not returning to a similar level of sport (OR 11.3).
Higher scores on the brief COPE subscale of “use of instrumental support” (instruments designed to help patients cope with pain) had a protective effect on returning to preinjury activity (OR 0.72 per point increase). However, higher COPE-denial scores were significantly associated with lower odds of improvement in kinesiophobia (OR 0.43).
Patients with greater levels of problem-focused coping had significantly greater improvement in International Knee Documentation Committee (IKDC) scores, as did patients who were older and more active.
“Specific coping strategies appear to moderate the effect of pain perceptions on postoperative outcomes, with some coping strategies being protective and others being harmful,” the researchers said.
The findings were limited by several factors including the use of multiple comparisons, the inability to assess the impact of pain perception after knee rehabilitation independent of surgery, and the small number of some uncommon procedures, the researchers noted.
However, the results suggest that “recognition of pain perception and coping styles early on in treatment may help sports medicine providers identify patients at risk for an unsatisfactory subjective outcome,” they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Everhart JS et al. J Sci Med Sport. 2019. doi: 10.1016/j.jsams.2019.09.011.
Adult athletes who underwent knee surgery and had higher levels of preoperative pain catastrophizing were significantly less likely to return to preinjury activity, based on data from 101 individuals.
Pain is highly subjective, and pain perception can play a role in postsurgical outcomes, but the relationships among preoperative pain perception and short-term outcomes including returning to sports have not been well-studied, wrote Joshua S. Everhart, MD, of The Ohio State University Wexner Medical Center, Columbus, and colleagues.
In a study published in the Journal of Science and Medicine in Sport, the researchers assessed 101 adult athletes who underwent knee surgery at a single center. The average age of the patients was 33 years, and 49 were women.
Pain perception and coping were assessed via the McGill Pain questionnaire (SF-MPQ), Pain Catastrophizing Scale (PCS), Pain Coping Measure (PCM), and the brief COPE subscales of acceptance, denial, positive reframing, and use of instrumental support.
Patients who were severe pain catastrophizers (defined as scores greater than 36 on the Pain Catastrophizing Scale) had increased odds of not returning to a similar level of sport (OR 11.3).
Higher scores on the brief COPE subscale of “use of instrumental support” (instruments designed to help patients cope with pain) had a protective effect on returning to preinjury activity (OR 0.72 per point increase). However, higher COPE-denial scores were significantly associated with lower odds of improvement in kinesiophobia (OR 0.43).
Patients with greater levels of problem-focused coping had significantly greater improvement in International Knee Documentation Committee (IKDC) scores, as did patients who were older and more active.
“Specific coping strategies appear to moderate the effect of pain perceptions on postoperative outcomes, with some coping strategies being protective and others being harmful,” the researchers said.
The findings were limited by several factors including the use of multiple comparisons, the inability to assess the impact of pain perception after knee rehabilitation independent of surgery, and the small number of some uncommon procedures, the researchers noted.
However, the results suggest that “recognition of pain perception and coping styles early on in treatment may help sports medicine providers identify patients at risk for an unsatisfactory subjective outcome,” they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Everhart JS et al. J Sci Med Sport. 2019. doi: 10.1016/j.jsams.2019.09.011.
Adult athletes who underwent knee surgery and had higher levels of preoperative pain catastrophizing were significantly less likely to return to preinjury activity, based on data from 101 individuals.
Pain is highly subjective, and pain perception can play a role in postsurgical outcomes, but the relationships among preoperative pain perception and short-term outcomes including returning to sports have not been well-studied, wrote Joshua S. Everhart, MD, of The Ohio State University Wexner Medical Center, Columbus, and colleagues.
In a study published in the Journal of Science and Medicine in Sport, the researchers assessed 101 adult athletes who underwent knee surgery at a single center. The average age of the patients was 33 years, and 49 were women.
Pain perception and coping were assessed via the McGill Pain questionnaire (SF-MPQ), Pain Catastrophizing Scale (PCS), Pain Coping Measure (PCM), and the brief COPE subscales of acceptance, denial, positive reframing, and use of instrumental support.
Patients who were severe pain catastrophizers (defined as scores greater than 36 on the Pain Catastrophizing Scale) had increased odds of not returning to a similar level of sport (OR 11.3).
Higher scores on the brief COPE subscale of “use of instrumental support” (instruments designed to help patients cope with pain) had a protective effect on returning to preinjury activity (OR 0.72 per point increase). However, higher COPE-denial scores were significantly associated with lower odds of improvement in kinesiophobia (OR 0.43).
Patients with greater levels of problem-focused coping had significantly greater improvement in International Knee Documentation Committee (IKDC) scores, as did patients who were older and more active.
“Specific coping strategies appear to moderate the effect of pain perceptions on postoperative outcomes, with some coping strategies being protective and others being harmful,” the researchers said.
The findings were limited by several factors including the use of multiple comparisons, the inability to assess the impact of pain perception after knee rehabilitation independent of surgery, and the small number of some uncommon procedures, the researchers noted.
However, the results suggest that “recognition of pain perception and coping styles early on in treatment may help sports medicine providers identify patients at risk for an unsatisfactory subjective outcome,” they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Everhart JS et al. J Sci Med Sport. 2019. doi: 10.1016/j.jsams.2019.09.011.
FROM THE JOURNAL OF SCIENCE AND MEDICINE IN SPORT
FDA approves afamelanotide for treatment of rare condition with light-induced pain
The Food and Drug Administration has approved , a rare condition that causes extremely painful reactions when skin is exposed to light, according to an FDA announcement.
This is the first treatment approved to help patients with this condition increase their exposure to light, according to the release.
Afamelanotide, administered in a subcutaneous implant, is a melanocortin-1 receptor (MC1-R) agonist, which “increases the production of eumelanin in the skin independent of exposure to sunlight or artificial light sources,” the release says.
Approval is based on a pair of parallel-group clinical trials that compared the number of hours spent in sunlight in the treatment and placebo groups. The first trial enrolled 93 patients; 48 received afamelanotide. The treated patients spent a median of 61 hours in total over 180 days in direct sunlight between 10 a.m. and 6 p.m. on days with no pain, compared with 41 hours for patients taking placebo.
The second trial assessed the total number of hours over 270 days spent outdoors between 10 a.m. and 3 p.m. on days with no pain for which “most of the day” was spent in direct sunlight. In this study, 38 patients treated with afamelanotide spent a median total of 6 hours, compared with 0.75 hours among the remaining 36 who were taking a placebo.
The most common side effects include implant site reaction, nausea, and oropharyngeal pain. The implant should be administered only by trained professionals. Because afamelanotide may cause skin darkening, it’s recommended that patients should undergo twice-yearly skin examinations. Patients are also encouraged to maintain sun protection measures to help prevent phototoxic reactions.
“Today’s approval is one example of the FDA’s ongoing commitment to encourage industry innovation of therapies to treat rare diseases, and work with drug developers to make promising new therapies available to patients as safely and efficiently as possible,” said Julie Beitz, MD, director of FDA’s Center for Drug Evaluation and Research Office of Drug Evaluation III in the FDA release.
The Food and Drug Administration has approved , a rare condition that causes extremely painful reactions when skin is exposed to light, according to an FDA announcement.
This is the first treatment approved to help patients with this condition increase their exposure to light, according to the release.
Afamelanotide, administered in a subcutaneous implant, is a melanocortin-1 receptor (MC1-R) agonist, which “increases the production of eumelanin in the skin independent of exposure to sunlight or artificial light sources,” the release says.
Approval is based on a pair of parallel-group clinical trials that compared the number of hours spent in sunlight in the treatment and placebo groups. The first trial enrolled 93 patients; 48 received afamelanotide. The treated patients spent a median of 61 hours in total over 180 days in direct sunlight between 10 a.m. and 6 p.m. on days with no pain, compared with 41 hours for patients taking placebo.
The second trial assessed the total number of hours over 270 days spent outdoors between 10 a.m. and 3 p.m. on days with no pain for which “most of the day” was spent in direct sunlight. In this study, 38 patients treated with afamelanotide spent a median total of 6 hours, compared with 0.75 hours among the remaining 36 who were taking a placebo.
The most common side effects include implant site reaction, nausea, and oropharyngeal pain. The implant should be administered only by trained professionals. Because afamelanotide may cause skin darkening, it’s recommended that patients should undergo twice-yearly skin examinations. Patients are also encouraged to maintain sun protection measures to help prevent phototoxic reactions.
“Today’s approval is one example of the FDA’s ongoing commitment to encourage industry innovation of therapies to treat rare diseases, and work with drug developers to make promising new therapies available to patients as safely and efficiently as possible,” said Julie Beitz, MD, director of FDA’s Center for Drug Evaluation and Research Office of Drug Evaluation III in the FDA release.
The Food and Drug Administration has approved , a rare condition that causes extremely painful reactions when skin is exposed to light, according to an FDA announcement.
This is the first treatment approved to help patients with this condition increase their exposure to light, according to the release.
Afamelanotide, administered in a subcutaneous implant, is a melanocortin-1 receptor (MC1-R) agonist, which “increases the production of eumelanin in the skin independent of exposure to sunlight or artificial light sources,” the release says.
Approval is based on a pair of parallel-group clinical trials that compared the number of hours spent in sunlight in the treatment and placebo groups. The first trial enrolled 93 patients; 48 received afamelanotide. The treated patients spent a median of 61 hours in total over 180 days in direct sunlight between 10 a.m. and 6 p.m. on days with no pain, compared with 41 hours for patients taking placebo.
The second trial assessed the total number of hours over 270 days spent outdoors between 10 a.m. and 3 p.m. on days with no pain for which “most of the day” was spent in direct sunlight. In this study, 38 patients treated with afamelanotide spent a median total of 6 hours, compared with 0.75 hours among the remaining 36 who were taking a placebo.
The most common side effects include implant site reaction, nausea, and oropharyngeal pain. The implant should be administered only by trained professionals. Because afamelanotide may cause skin darkening, it’s recommended that patients should undergo twice-yearly skin examinations. Patients are also encouraged to maintain sun protection measures to help prevent phototoxic reactions.
“Today’s approval is one example of the FDA’s ongoing commitment to encourage industry innovation of therapies to treat rare diseases, and work with drug developers to make promising new therapies available to patients as safely and efficiently as possible,” said Julie Beitz, MD, director of FDA’s Center for Drug Evaluation and Research Office of Drug Evaluation III in the FDA release.
Consider centralized pain in patients with rheumatic disease
Las Vegas – A fibromyalgia survey may provide important information about the degree to which patients with rheumatic disease experience centralized pain. This information may guide treatment decisions, said Daniel J. Clauw, MD, professor of anesthesiology, rheumatology, and psychiatry and director of the Chronic Pain and Fatigue Research Center at the University of Michigan in Ann Arbor.
The questionnaire that Dr. Clauw uses is a patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. In it, he asks patients to report where they experience pain throughout the body and symptoms such as fatigue, sleep problems, and memory problems. The survey predicts outcomes of surgery for osteoarthritis better than x-rays, MRI scans, or psychological factors do, he said.
Physicians should ask every patient with chronic pain, including patients with OA, rheumatoid arthritis, or lupus, to complete the survey, Dr. Clauw said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “This score will tell you the degree to which their central nervous system is augmenting or amplifying what is going on in their body,” he said. “And the higher their score is, the more you should treat them like you would someone with fibromyalgia, even if their underlying disease might be an autoimmune disease.”
Physicians should not use a cutoff of 13 points on the fibromyalgia measure to define whether a patient has the disease, as has been done in the past, he said. The threshold is arbitrary, he said. “We should not think about fibromyalgia as ‘yes’ or ‘no.’ We should think of the degree of fibromyalgia that people have.”
A poor relationship between pain and imaging
Some patients who have severe knee OA on imaging walk without pain. Other patients have normal x-rays, but severe pain. “There is a terrible relationship between what you see on a knee x-ray or an MRI and whether someone has pain,” Dr. Clauw said. Furthermore, the poor relationship between imaging and pain is common across chronic pain conditions, he said.
This phenomenon may occur because pain manifests in different ways, similar to there being multiple ways to adjust the volume of an electric guitar, he said. How hard the strings are strummed affects the volume. But so does the amplifier setting. “In these centralized pain conditions, the problem is an amplifier problem, not a guitar problem,” he said. “The amplifier, i.e., the central nervous system, is set too high.”
Researchers have found that people who have severe OA of the knee on x-ray but do not experience pain “have a very low amplifier setting,” he said. That is, they are nontender and less sensitive to pain. Most of these patients are men. “On average, men have a much lower amplifier setting than women,” he said. “This is also why ... women have 1.5 to 2 times the rate of any type of chronic pain than men, because on average women have a higher amplifier setting. ... In OA, at any given age, men and women have the exact same percentage of radiographic OA. But if you look at the clinical condition of OA, it is always two-thirds women, one-third men.”
Opioid responsiveness
To examine whether fibromyalgia survey results correlate with outcomes after knee and hip arthroplasty, Dr. Clauw and colleagues conducted a prospective, observational cohort study that included approximately 500 people. Patients completed the questionnaire on the day of surgery.
Patients with higher levels of fibromyalgia were less responsive to opioids. “For each 1-point increase in the fibromyalgia score, people needed about one more hydrocodone tablet in the first 24-48 hours to control their pain,” he said (Anesthesiology. 2013 Dec;119[6]:1434-43). In addition, each 1-point increase in the fibromyalgia score made people about 25% less likely to have a 50% improvement in knee pain level after 6 months (Arthritis Rheumatol. 2015 May;67[5]:1386-94). The correlations were independent of psychological factors. In addition, the associations were linear. “There was nothing magical about a fibromyalgia score of 13,” Dr. Clauw said.
Dr. Clauw is a coauthor of a study to be presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting that found pain centralization in patients with RA is associated with poor response to disease-modifying antirheumatic drugs (DMARDs).
Prior studies in patients with RA have found that the degree of fibromyalgia is a better predictor of pain and disability than erythrocyte sedimentation rate or the number of swollen joints.
Diagnosed cases are the “tip of the iceberg”
Researchers at Dr. Clauw’s institution have identified dozens of patients undergoing knee surgery who met criteria for fibromyalgia but had not received the diagnosis. “This is at the University of Michigan, which is the epicenter for fibromyalgia research. If we are not seeing fibromyalgia superimposed on OA in our patients, no one is seeing it,” he said.
Patients with diagnosed fibromyalgia are “the tip of the iceberg,” he said. “There are far greater numbers of individuals whose primary diagnosis is OA, RA, lupus, ankylosing spondylitis, cancer pain, or sickle cell disease that have the same fundamental problem as fibromyalgia patients. But you do not see it because you label them as having an autoimmune disease or osteoarthritis. And that is at your peril and at their peril. Because treating that individual as if all of their pain and other symptoms are due to a problem out on the periphery will not make that person better.”
Patients with high levels of centralized pain may be less responsive to peripherally directed therapies such as surgery or injections, Dr. Clauw said. Pharmacologic options for patients with centralized pain include gabapentinoids (e.g., pregabalin and gabapentin), serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and milnacipran), and tricyclic compounds (e.g., amitriptyline and cyclobenzaprine), he said. “Opioids are going to be quite unlikely to help these individuals,” he said. “In fact, it is likely that opioids will make this kind of pain worse.”
Dr. Clauw is a consultant for Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Pfizer, Samumed, Theravance, Tonix, and Zynerba Pharma. He has received grant or research support from Aptinyx and Pfizer and is an expert witness.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Las Vegas – A fibromyalgia survey may provide important information about the degree to which patients with rheumatic disease experience centralized pain. This information may guide treatment decisions, said Daniel J. Clauw, MD, professor of anesthesiology, rheumatology, and psychiatry and director of the Chronic Pain and Fatigue Research Center at the University of Michigan in Ann Arbor.
The questionnaire that Dr. Clauw uses is a patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. In it, he asks patients to report where they experience pain throughout the body and symptoms such as fatigue, sleep problems, and memory problems. The survey predicts outcomes of surgery for osteoarthritis better than x-rays, MRI scans, or psychological factors do, he said.
Physicians should ask every patient with chronic pain, including patients with OA, rheumatoid arthritis, or lupus, to complete the survey, Dr. Clauw said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “This score will tell you the degree to which their central nervous system is augmenting or amplifying what is going on in their body,” he said. “And the higher their score is, the more you should treat them like you would someone with fibromyalgia, even if their underlying disease might be an autoimmune disease.”
Physicians should not use a cutoff of 13 points on the fibromyalgia measure to define whether a patient has the disease, as has been done in the past, he said. The threshold is arbitrary, he said. “We should not think about fibromyalgia as ‘yes’ or ‘no.’ We should think of the degree of fibromyalgia that people have.”
A poor relationship between pain and imaging
Some patients who have severe knee OA on imaging walk without pain. Other patients have normal x-rays, but severe pain. “There is a terrible relationship between what you see on a knee x-ray or an MRI and whether someone has pain,” Dr. Clauw said. Furthermore, the poor relationship between imaging and pain is common across chronic pain conditions, he said.
This phenomenon may occur because pain manifests in different ways, similar to there being multiple ways to adjust the volume of an electric guitar, he said. How hard the strings are strummed affects the volume. But so does the amplifier setting. “In these centralized pain conditions, the problem is an amplifier problem, not a guitar problem,” he said. “The amplifier, i.e., the central nervous system, is set too high.”
Researchers have found that people who have severe OA of the knee on x-ray but do not experience pain “have a very low amplifier setting,” he said. That is, they are nontender and less sensitive to pain. Most of these patients are men. “On average, men have a much lower amplifier setting than women,” he said. “This is also why ... women have 1.5 to 2 times the rate of any type of chronic pain than men, because on average women have a higher amplifier setting. ... In OA, at any given age, men and women have the exact same percentage of radiographic OA. But if you look at the clinical condition of OA, it is always two-thirds women, one-third men.”
Opioid responsiveness
To examine whether fibromyalgia survey results correlate with outcomes after knee and hip arthroplasty, Dr. Clauw and colleagues conducted a prospective, observational cohort study that included approximately 500 people. Patients completed the questionnaire on the day of surgery.
Patients with higher levels of fibromyalgia were less responsive to opioids. “For each 1-point increase in the fibromyalgia score, people needed about one more hydrocodone tablet in the first 24-48 hours to control their pain,” he said (Anesthesiology. 2013 Dec;119[6]:1434-43). In addition, each 1-point increase in the fibromyalgia score made people about 25% less likely to have a 50% improvement in knee pain level after 6 months (Arthritis Rheumatol. 2015 May;67[5]:1386-94). The correlations were independent of psychological factors. In addition, the associations were linear. “There was nothing magical about a fibromyalgia score of 13,” Dr. Clauw said.
Dr. Clauw is a coauthor of a study to be presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting that found pain centralization in patients with RA is associated with poor response to disease-modifying antirheumatic drugs (DMARDs).
Prior studies in patients with RA have found that the degree of fibromyalgia is a better predictor of pain and disability than erythrocyte sedimentation rate or the number of swollen joints.
Diagnosed cases are the “tip of the iceberg”
Researchers at Dr. Clauw’s institution have identified dozens of patients undergoing knee surgery who met criteria for fibromyalgia but had not received the diagnosis. “This is at the University of Michigan, which is the epicenter for fibromyalgia research. If we are not seeing fibromyalgia superimposed on OA in our patients, no one is seeing it,” he said.
Patients with diagnosed fibromyalgia are “the tip of the iceberg,” he said. “There are far greater numbers of individuals whose primary diagnosis is OA, RA, lupus, ankylosing spondylitis, cancer pain, or sickle cell disease that have the same fundamental problem as fibromyalgia patients. But you do not see it because you label them as having an autoimmune disease or osteoarthritis. And that is at your peril and at their peril. Because treating that individual as if all of their pain and other symptoms are due to a problem out on the periphery will not make that person better.”
Patients with high levels of centralized pain may be less responsive to peripherally directed therapies such as surgery or injections, Dr. Clauw said. Pharmacologic options for patients with centralized pain include gabapentinoids (e.g., pregabalin and gabapentin), serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and milnacipran), and tricyclic compounds (e.g., amitriptyline and cyclobenzaprine), he said. “Opioids are going to be quite unlikely to help these individuals,” he said. “In fact, it is likely that opioids will make this kind of pain worse.”
Dr. Clauw is a consultant for Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Pfizer, Samumed, Theravance, Tonix, and Zynerba Pharma. He has received grant or research support from Aptinyx and Pfizer and is an expert witness.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Las Vegas – A fibromyalgia survey may provide important information about the degree to which patients with rheumatic disease experience centralized pain. This information may guide treatment decisions, said Daniel J. Clauw, MD, professor of anesthesiology, rheumatology, and psychiatry and director of the Chronic Pain and Fatigue Research Center at the University of Michigan in Ann Arbor.
The questionnaire that Dr. Clauw uses is a patient self-report survey for the assessment of fibromyalgia based on criteria in the 2011 modification of the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia. In it, he asks patients to report where they experience pain throughout the body and symptoms such as fatigue, sleep problems, and memory problems. The survey predicts outcomes of surgery for osteoarthritis better than x-rays, MRI scans, or psychological factors do, he said.
Physicians should ask every patient with chronic pain, including patients with OA, rheumatoid arthritis, or lupus, to complete the survey, Dr. Clauw said at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “This score will tell you the degree to which their central nervous system is augmenting or amplifying what is going on in their body,” he said. “And the higher their score is, the more you should treat them like you would someone with fibromyalgia, even if their underlying disease might be an autoimmune disease.”
Physicians should not use a cutoff of 13 points on the fibromyalgia measure to define whether a patient has the disease, as has been done in the past, he said. The threshold is arbitrary, he said. “We should not think about fibromyalgia as ‘yes’ or ‘no.’ We should think of the degree of fibromyalgia that people have.”
A poor relationship between pain and imaging
Some patients who have severe knee OA on imaging walk without pain. Other patients have normal x-rays, but severe pain. “There is a terrible relationship between what you see on a knee x-ray or an MRI and whether someone has pain,” Dr. Clauw said. Furthermore, the poor relationship between imaging and pain is common across chronic pain conditions, he said.
This phenomenon may occur because pain manifests in different ways, similar to there being multiple ways to adjust the volume of an electric guitar, he said. How hard the strings are strummed affects the volume. But so does the amplifier setting. “In these centralized pain conditions, the problem is an amplifier problem, not a guitar problem,” he said. “The amplifier, i.e., the central nervous system, is set too high.”
Researchers have found that people who have severe OA of the knee on x-ray but do not experience pain “have a very low amplifier setting,” he said. That is, they are nontender and less sensitive to pain. Most of these patients are men. “On average, men have a much lower amplifier setting than women,” he said. “This is also why ... women have 1.5 to 2 times the rate of any type of chronic pain than men, because on average women have a higher amplifier setting. ... In OA, at any given age, men and women have the exact same percentage of radiographic OA. But if you look at the clinical condition of OA, it is always two-thirds women, one-third men.”
Opioid responsiveness
To examine whether fibromyalgia survey results correlate with outcomes after knee and hip arthroplasty, Dr. Clauw and colleagues conducted a prospective, observational cohort study that included approximately 500 people. Patients completed the questionnaire on the day of surgery.
Patients with higher levels of fibromyalgia were less responsive to opioids. “For each 1-point increase in the fibromyalgia score, people needed about one more hydrocodone tablet in the first 24-48 hours to control their pain,” he said (Anesthesiology. 2013 Dec;119[6]:1434-43). In addition, each 1-point increase in the fibromyalgia score made people about 25% less likely to have a 50% improvement in knee pain level after 6 months (Arthritis Rheumatol. 2015 May;67[5]:1386-94). The correlations were independent of psychological factors. In addition, the associations were linear. “There was nothing magical about a fibromyalgia score of 13,” Dr. Clauw said.
Dr. Clauw is a coauthor of a study to be presented at the 2019 American College of Rheumatology/Association of Rheumatology Professionals annual meeting that found pain centralization in patients with RA is associated with poor response to disease-modifying antirheumatic drugs (DMARDs).
Prior studies in patients with RA have found that the degree of fibromyalgia is a better predictor of pain and disability than erythrocyte sedimentation rate or the number of swollen joints.
Diagnosed cases are the “tip of the iceberg”
Researchers at Dr. Clauw’s institution have identified dozens of patients undergoing knee surgery who met criteria for fibromyalgia but had not received the diagnosis. “This is at the University of Michigan, which is the epicenter for fibromyalgia research. If we are not seeing fibromyalgia superimposed on OA in our patients, no one is seeing it,” he said.
Patients with diagnosed fibromyalgia are “the tip of the iceberg,” he said. “There are far greater numbers of individuals whose primary diagnosis is OA, RA, lupus, ankylosing spondylitis, cancer pain, or sickle cell disease that have the same fundamental problem as fibromyalgia patients. But you do not see it because you label them as having an autoimmune disease or osteoarthritis. And that is at your peril and at their peril. Because treating that individual as if all of their pain and other symptoms are due to a problem out on the periphery will not make that person better.”
Patients with high levels of centralized pain may be less responsive to peripherally directed therapies such as surgery or injections, Dr. Clauw said. Pharmacologic options for patients with centralized pain include gabapentinoids (e.g., pregabalin and gabapentin), serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and milnacipran), and tricyclic compounds (e.g., amitriptyline and cyclobenzaprine), he said. “Opioids are going to be quite unlikely to help these individuals,” he said. “In fact, it is likely that opioids will make this kind of pain worse.”
Dr. Clauw is a consultant for Aptinyx, Daiichi Sankyo, Eli Lilly, Intec Pharma, Pfizer, Samumed, Theravance, Tonix, and Zynerba Pharma. He has received grant or research support from Aptinyx and Pfizer and is an expert witness.
Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM PRD 2019
Persistent rash on the sole
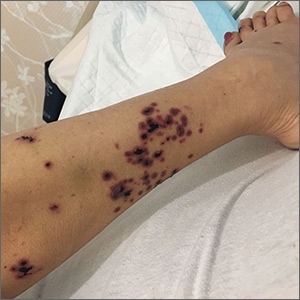
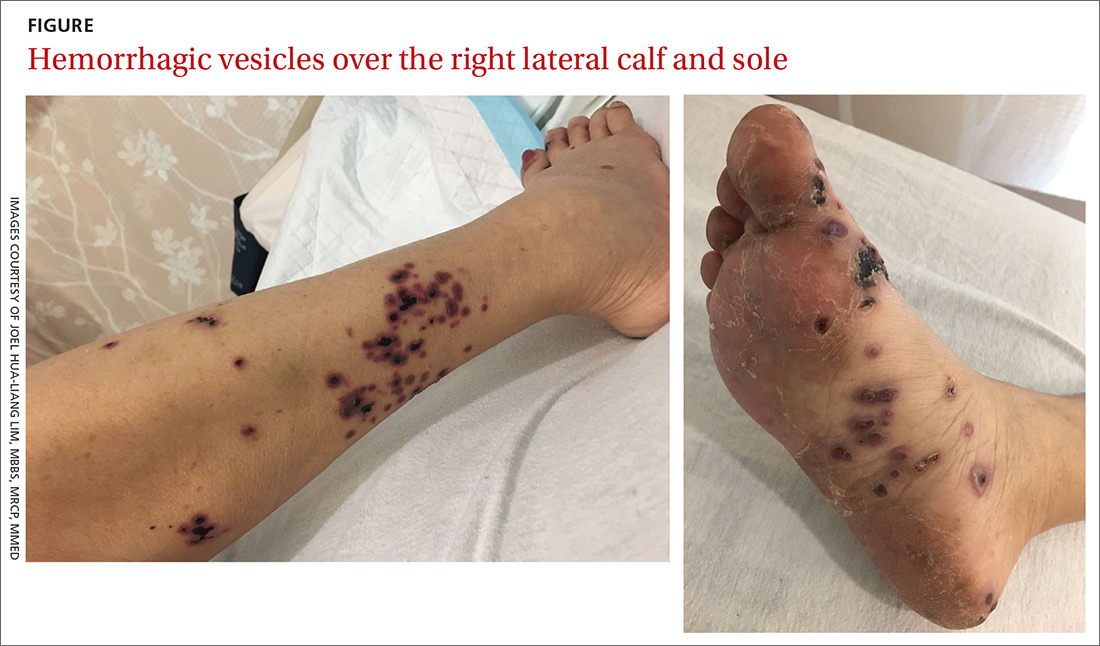
A 52-year-old Chinese woman presented to a tertiary hospital in Singapore with a 3-month history of persistent and intermittently painful rashes over her right calf and foot (FIGURE). The patient had pancytopenia due to ongoing chemotherapy for metastatic nasopharyngeal carcinoma. She was systemically well and denied other dermatoses. Examination demonstrated scattered crops of tense hemorrhagic vesicles, each surrounded by a livid purpuric base, over the right plantar aspect of the foot, with areas of eschar over the right medial hallux. No allodynia, hyperaesthesia, or lymphadenopathy was noted.
A punch biopsy of an intact vesicle was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis:
Herpes zoster
Histopathologic examination showed full-thickness epidermal necrosis with ballooning degeneration resulting in an intra-epidermal blister. Multinucleated keratinocytes with nuclear moulding were seen within the blister cavity. Grocott-Gomori methenamine-silver (GMS), acid-fast, and Gram stains were negative. Granular immunoglobulin (Ig) G, IgM, and C3 were seen intramurally. DNA analysis of vesicular fluid was positive for varicella zoster virus (VZV). A diagnosis of herpes zoster (HZ) of the right S1 dermatome with primary obliterative vasculitis was established.
Immunocompromised people—those who have impaired T-cell immunity (eg, recipients of organ or hematopoietic stem-cell transplants), take immunosuppressive therapy, or have lymphoma, leukemia, or human immunodeficiency virus (HIV) infection—have an increased risk for HZ. For example, in patients with acquired immunodeficiency syndrome (AIDS), HZ uniquely manifests as recurrent shingles. An estimated 20% to 30% of HIV-infected patients will have more than 1 episode of HZ, which may involve the same or different dermatomes.1,2 Furthermore, HZ in this population is more commonly associated with atypical presentations.3
What an atypical presentation may look like
In immunocompromised patients, HZ may present with atypical cutaneous manifestations or with atypical generalized symptoms.
Atypical cutaneous manifestations, as in disseminated zoster, manifest with multiple hyperkeratotic papules (3-20 mm in diameter) that follow no dermatomal pattern. These lesions may be chronic, persisting for months or years, and may be associated with acyclovir-resistant strains of VZV.2,3 Another dermatologic variant is ecthymatous VZV, which manifests with multiple large (10-30 mm) punched-out ulcerations with a central black eschar and a peripheral rim of vesicles.4 Viral folliculitis—in which infection is limited to the hair follicle, with no associated blisters—has also been reported in atypical HZ.5
Our patient presented with hemorrhagic vesicles mimicking vasculitic lesions, which had persisted over a 3-month period with intermittent localized pain. It has been proposed that in atypical presentations, the reactivated VZV spreads transaxonally from adjacent nerves to the outermost adventitial layer of the arterial wall, leading to a vasculitic appearance of the vesicles.6 Viral-induced vasculitis may also result either directly from infection of the blood vessels or secondary to vascular damage from an inflammatory immune complex–mediated reaction, cell-mediated hypersensitivity, or inflammation due to immune dysregulation.7,8
Continue to: Differential includes vesiculobullous conditions
Differential includes vesiculobullous conditions
There are several important items to consider in the differential.
Cutaneous vasculitis, in severe cases, may manifest with vesicles or bullae that resemble the lesions seen in HZ. However, its unilateral nature and distribution distinguish it.
Angioinvasive fungal infections in immunocompromised patients may manifest with scattered ulceronecrotic lesions to purpuric vesiculobullous dermatoses.9 However, no fungal organisms were seen on GMS staining of the biopsied tissue.
Atypical hand-foot-and-mouth disease tends to affect adults and is associated with Coxsackievirus A6 infection.10 It may manifest as generalized vesiculobullous exanthem resembling varicella. The chronic nature and restricted extent of the patient’s rash made this diagnosis unlikely.
Successful management depends on timely identification
Although most cases of HZ can be diagnosed clinically, atypical rashes may require a biopsy and direct immunofluorescence assay for VZV antigen or a polymerase-chain-reaction (PCR) assay for VZV DNA in cells from the base of blisters. Therefore, it is important to consider the diagnosis of HZ in immunocompromised patients presenting with an atypical rash to avoid misdiagnosis and costly testing.
Continue to: Our patient was treated...
Our patient was treated with oral acyclovir 800 mg 5 times/day for 10 days, with prompt resolution of her rash.
CORRESPONDENCE
Joel Hua-Liang Lim, MBBS, MRCP, MMed, 1 Mandalay Road, Singapore 308205; [email protected]
1. LeBoit PE, Limova M, Yen TS, et al. Chronic verrucous varicella-zoster virus infection in patients with the acquired immunodeficiency syndrome (AIDS): histologic and molecular biologic findings. Am J Dermatopathol. 1992;14:1-7.
2. Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
3. Weinberg JM, Mysliwiec A, Turiansky GW, et al. Viral folliculitis: atypical presentations of herpes simplex, herpes zoster, and molluscum contagiosum. Arch Dermatol. 1997;133:983-986.
4. Gilson IH, Barnett JH, Conant MA, et al. Disseminated ecthymatous herpes varicella zoster virus infection in patients with acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20:637-642.
5. Løkke BJ, Weismann K, Mathiesen L, et al. Atypical varicella-zoster infection in AIDS. Acta Derm Venereol. 1993;73:123-125.
6. Uhoda I, Piérard-Franchimont C, Piérard GE. Varicella-zoster virus vasculitis: a case of recurrent varicella without epidermal involvement. Dermatology. 2000;200:173-175.
7. Teng GG, Chatham WW. Vasculitis related to viral and other microbial agents. Best Pract Res Clin Rheumatol. 2015;29:226-243.
8. Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16:12.
9. Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1-53.
10. Lott JP, Liu K, Landry M-L, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
A 52-year-old Chinese woman presented to a tertiary hospital in Singapore with a 3-month history of persistent and intermittently painful rashes over her right calf and foot (FIGURE). The patient had pancytopenia due to ongoing chemotherapy for metastatic nasopharyngeal carcinoma. She was systemically well and denied other dermatoses. Examination demonstrated scattered crops of tense hemorrhagic vesicles, each surrounded by a livid purpuric base, over the right plantar aspect of the foot, with areas of eschar over the right medial hallux. No allodynia, hyperaesthesia, or lymphadenopathy was noted.
A punch biopsy of an intact vesicle was performed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis:
Herpes zoster
Histopathologic examination showed full-thickness epidermal necrosis with ballooning degeneration resulting in an intra-epidermal blister. Multinucleated keratinocytes with nuclear moulding were seen within the blister cavity. Grocott-Gomori methenamine-silver (GMS), acid-fast, and Gram stains were negative. Granular immunoglobulin (Ig) G, IgM, and C3 were seen intramurally. DNA analysis of vesicular fluid was positive for varicella zoster virus (VZV). A diagnosis of herpes zoster (HZ) of the right S1 dermatome with primary obliterative vasculitis was established.
Immunocompromised people—those who have impaired T-cell immunity (eg, recipients of organ or hematopoietic stem-cell transplants), take immunosuppressive therapy, or have lymphoma, leukemia, or human immunodeficiency virus (HIV) infection—have an increased risk for HZ. For example, in patients with acquired immunodeficiency syndrome (AIDS), HZ uniquely manifests as recurrent shingles. An estimated 20% to 30% of HIV-infected patients will have more than 1 episode of HZ, which may involve the same or different dermatomes.1,2 Furthermore, HZ in this population is more commonly associated with atypical presentations.3
What an atypical presentation may look like
In immunocompromised patients, HZ may present with atypical cutaneous manifestations or with atypical generalized symptoms.
Atypical cutaneous manifestations, as in disseminated zoster, manifest with multiple hyperkeratotic papules (3-20 mm in diameter) that follow no dermatomal pattern. These lesions may be chronic, persisting for months or years, and may be associated with acyclovir-resistant strains of VZV.2,3 Another dermatologic variant is ecthymatous VZV, which manifests with multiple large (10-30 mm) punched-out ulcerations with a central black eschar and a peripheral rim of vesicles.4 Viral folliculitis—in which infection is limited to the hair follicle, with no associated blisters—has also been reported in atypical HZ.5
Our patient presented with hemorrhagic vesicles mimicking vasculitic lesions, which had persisted over a 3-month period with intermittent localized pain. It has been proposed that in atypical presentations, the reactivated VZV spreads transaxonally from adjacent nerves to the outermost adventitial layer of the arterial wall, leading to a vasculitic appearance of the vesicles.6 Viral-induced vasculitis may also result either directly from infection of the blood vessels or secondary to vascular damage from an inflammatory immune complex–mediated reaction, cell-mediated hypersensitivity, or inflammation due to immune dysregulation.7,8
Continue to: Differential includes vesiculobullous conditions
Differential includes vesiculobullous conditions
There are several important items to consider in the differential.
Cutaneous vasculitis, in severe cases, may manifest with vesicles or bullae that resemble the lesions seen in HZ. However, its unilateral nature and distribution distinguish it.
Angioinvasive fungal infections in immunocompromised patients may manifest with scattered ulceronecrotic lesions to purpuric vesiculobullous dermatoses.9 However, no fungal organisms were seen on GMS staining of the biopsied tissue.
Atypical hand-foot-and-mouth disease tends to affect adults and is associated with Coxsackievirus A6 infection.10 It may manifest as generalized vesiculobullous exanthem resembling varicella. The chronic nature and restricted extent of the patient’s rash made this diagnosis unlikely.
Successful management depends on timely identification
Although most cases of HZ can be diagnosed clinically, atypical rashes may require a biopsy and direct immunofluorescence assay for VZV antigen or a polymerase-chain-reaction (PCR) assay for VZV DNA in cells from the base of blisters. Therefore, it is important to consider the diagnosis of HZ in immunocompromised patients presenting with an atypical rash to avoid misdiagnosis and costly testing.
Continue to: Our patient was treated...
Our patient was treated with oral acyclovir 800 mg 5 times/day for 10 days, with prompt resolution of her rash.
CORRESPONDENCE
Joel Hua-Liang Lim, MBBS, MRCP, MMed, 1 Mandalay Road, Singapore 308205; [email protected]
A 52-year-old Chinese woman presented to a tertiary hospital in Singapore with a 3-month history of persistent and intermittently painful rashes over her right calf and foot (FIGURE). The patient had pancytopenia due to ongoing chemotherapy for metastatic nasopharyngeal carcinoma. She was systemically well and denied other dermatoses. Examination demonstrated scattered crops of tense hemorrhagic vesicles, each surrounded by a livid purpuric base, over the right plantar aspect of the foot, with areas of eschar over the right medial hallux. No allodynia, hyperaesthesia, or lymphadenopathy was noted.
A punch biopsy of an intact vesicle was performed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis:
Herpes zoster
Histopathologic examination showed full-thickness epidermal necrosis with ballooning degeneration resulting in an intra-epidermal blister. Multinucleated keratinocytes with nuclear moulding were seen within the blister cavity. Grocott-Gomori methenamine-silver (GMS), acid-fast, and Gram stains were negative. Granular immunoglobulin (Ig) G, IgM, and C3 were seen intramurally. DNA analysis of vesicular fluid was positive for varicella zoster virus (VZV). A diagnosis of herpes zoster (HZ) of the right S1 dermatome with primary obliterative vasculitis was established.
Immunocompromised people—those who have impaired T-cell immunity (eg, recipients of organ or hematopoietic stem-cell transplants), take immunosuppressive therapy, or have lymphoma, leukemia, or human immunodeficiency virus (HIV) infection—have an increased risk for HZ. For example, in patients with acquired immunodeficiency syndrome (AIDS), HZ uniquely manifests as recurrent shingles. An estimated 20% to 30% of HIV-infected patients will have more than 1 episode of HZ, which may involve the same or different dermatomes.1,2 Furthermore, HZ in this population is more commonly associated with atypical presentations.3
What an atypical presentation may look like
In immunocompromised patients, HZ may present with atypical cutaneous manifestations or with atypical generalized symptoms.
Atypical cutaneous manifestations, as in disseminated zoster, manifest with multiple hyperkeratotic papules (3-20 mm in diameter) that follow no dermatomal pattern. These lesions may be chronic, persisting for months or years, and may be associated with acyclovir-resistant strains of VZV.2,3 Another dermatologic variant is ecthymatous VZV, which manifests with multiple large (10-30 mm) punched-out ulcerations with a central black eschar and a peripheral rim of vesicles.4 Viral folliculitis—in which infection is limited to the hair follicle, with no associated blisters—has also been reported in atypical HZ.5
Our patient presented with hemorrhagic vesicles mimicking vasculitic lesions, which had persisted over a 3-month period with intermittent localized pain. It has been proposed that in atypical presentations, the reactivated VZV spreads transaxonally from adjacent nerves to the outermost adventitial layer of the arterial wall, leading to a vasculitic appearance of the vesicles.6 Viral-induced vasculitis may also result either directly from infection of the blood vessels or secondary to vascular damage from an inflammatory immune complex–mediated reaction, cell-mediated hypersensitivity, or inflammation due to immune dysregulation.7,8
Continue to: Differential includes vesiculobullous conditions
Differential includes vesiculobullous conditions
There are several important items to consider in the differential.
Cutaneous vasculitis, in severe cases, may manifest with vesicles or bullae that resemble the lesions seen in HZ. However, its unilateral nature and distribution distinguish it.
Angioinvasive fungal infections in immunocompromised patients may manifest with scattered ulceronecrotic lesions to purpuric vesiculobullous dermatoses.9 However, no fungal organisms were seen on GMS staining of the biopsied tissue.
Atypical hand-foot-and-mouth disease tends to affect adults and is associated with Coxsackievirus A6 infection.10 It may manifest as generalized vesiculobullous exanthem resembling varicella. The chronic nature and restricted extent of the patient’s rash made this diagnosis unlikely.
Successful management depends on timely identification
Although most cases of HZ can be diagnosed clinically, atypical rashes may require a biopsy and direct immunofluorescence assay for VZV antigen or a polymerase-chain-reaction (PCR) assay for VZV DNA in cells from the base of blisters. Therefore, it is important to consider the diagnosis of HZ in immunocompromised patients presenting with an atypical rash to avoid misdiagnosis and costly testing.
Continue to: Our patient was treated...
Our patient was treated with oral acyclovir 800 mg 5 times/day for 10 days, with prompt resolution of her rash.
CORRESPONDENCE
Joel Hua-Liang Lim, MBBS, MRCP, MMed, 1 Mandalay Road, Singapore 308205; [email protected]
1. LeBoit PE, Limova M, Yen TS, et al. Chronic verrucous varicella-zoster virus infection in patients with the acquired immunodeficiency syndrome (AIDS): histologic and molecular biologic findings. Am J Dermatopathol. 1992;14:1-7.
2. Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
3. Weinberg JM, Mysliwiec A, Turiansky GW, et al. Viral folliculitis: atypical presentations of herpes simplex, herpes zoster, and molluscum contagiosum. Arch Dermatol. 1997;133:983-986.
4. Gilson IH, Barnett JH, Conant MA, et al. Disseminated ecthymatous herpes varicella zoster virus infection in patients with acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20:637-642.
5. Løkke BJ, Weismann K, Mathiesen L, et al. Atypical varicella-zoster infection in AIDS. Acta Derm Venereol. 1993;73:123-125.
6. Uhoda I, Piérard-Franchimont C, Piérard GE. Varicella-zoster virus vasculitis: a case of recurrent varicella without epidermal involvement. Dermatology. 2000;200:173-175.
7. Teng GG, Chatham WW. Vasculitis related to viral and other microbial agents. Best Pract Res Clin Rheumatol. 2015;29:226-243.
8. Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16:12.
9. Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1-53.
10. Lott JP, Liu K, Landry M-L, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
1. LeBoit PE, Limova M, Yen TS, et al. Chronic verrucous varicella-zoster virus infection in patients with the acquired immunodeficiency syndrome (AIDS): histologic and molecular biologic findings. Am J Dermatopathol. 1992;14:1-7.
2. Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
3. Weinberg JM, Mysliwiec A, Turiansky GW, et al. Viral folliculitis: atypical presentations of herpes simplex, herpes zoster, and molluscum contagiosum. Arch Dermatol. 1997;133:983-986.
4. Gilson IH, Barnett JH, Conant MA, et al. Disseminated ecthymatous herpes varicella zoster virus infection in patients with acquired immunodeficiency syndrome. J Am Acad Dermatol. 1989;20:637-642.
5. Løkke BJ, Weismann K, Mathiesen L, et al. Atypical varicella-zoster infection in AIDS. Acta Derm Venereol. 1993;73:123-125.
6. Uhoda I, Piérard-Franchimont C, Piérard GE. Varicella-zoster virus vasculitis: a case of recurrent varicella without epidermal involvement. Dermatology. 2000;200:173-175.
7. Teng GG, Chatham WW. Vasculitis related to viral and other microbial agents. Best Pract Res Clin Rheumatol. 2015;29:226-243.
8. Nagel MA, Gilden D. Developments in varicella zoster virus vasculopathy. Curr Neurol Neurosci Rep. 2016;16:12.
9. Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1-53.
10. Lott JP, Liu K, Landry M-L, et al. Atypical hand-foot-and-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736-741.
Presentation is key to diagnosing salivary gland disorders
Making a diagnosis of a salivary gland disorder can be difficult. Common presentations, such as a painful or swollen gland, can be caused by numerous disorders of strikingly variable severity and consequences, including inflammatory, infectious, and neoplastic conditions, for which treatment can differ significantly, and referral for specialty care is sometimes necessary.
Yet it is the patient’s presentation that can aid you in making the diagnosis that will guide management. Consider that acute symptoms often result from infection, for example, and chronic or recurrent symptoms are caused more often by obstructive or nonobstructive inflammatory conditions and neoplasms. Diagnosis of an apparent neoplasm, prompted by clinical findings, is made using imaging and fine-needle aspiration (FNA) biopsy. Acute infection usually resolves with antibiotics and supportive management; calculi that cause persistent symptoms warrant referral for consideration of stone or gland removal; and malignant neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both—calling for multidisciplinary care.
In this article, we clarify what can be an imprecise and perplexing path from the presentation to diagnosis to treatment of disorders of the salivary glands. To begin, see “Geography of the salivary glands,” for an overview of the location, structure, and corresponding ducts of the component salivary glands (parotid, submandibular, sublingual, and minor glands).
SIDEBAR
Geography of the salivary glands
The salivary glands comprise the major paired parotid, submandibular, and sublingual glands, as well as minor salivary glands that line the oropharyngeal mucosa. Secretion of saliva is modulated by both autonomic and humoral factors.
The parotid gland sits between the mastoid process, the ramus of the mandible, and the styloid process, extend- ing from the external auditory meatus superiorly to below the angle of the mandible and into the neck inferiorly. The gland is surrounded by a tough capsule. Embedded within the gland is the facial nerve, which divides into its 5 branches within the substance of the gland. The parotid (Stensen’s) duct passes anteriorly before turning medially to pierce the buccinator muscle, opening onto the mucous membrane of the cheek opposite the second upper molar.
The submandibular gland comprises (1) a large superficial part that fills the space between the mandible and the floor of the mouth and (2) a small deep part that wraps around the posterior border of the mylohyoid muscle. The submandibular (Wharton’s) duct runs anteriorly to open onto the floor of the mouth, alongside the frenulum.
The sublingual gland, the smallest of the major salivary glands, lies anteriorly in the floor of the mouth, with many small ducts opening either into the submandibular duct or directly into the mouth.
Basic secretory units of salivary glands are clusters of cells, each called an acinus. These cells secrete a fluid that contains water, electrolytes, mucous, and enzymes, all of which flow out of the acini into collecting ducts. The saliva produced by the parotid is mainly serous; by the submandibular gland, mixed; and by the sublingual and minor salivary glands, mucoid.
Presentation helps establish the differential Dx
Ask: Are the glands swollen?
Painless salivary gland swelling has a variety of causes, including neoplasm, sialadenosis, and the eating disorders bulimia and anorexia nervosa. There is significant overlap of presentations among those causes (FIGURE). Pain accompanying swelling is uncommon but not unheard of.
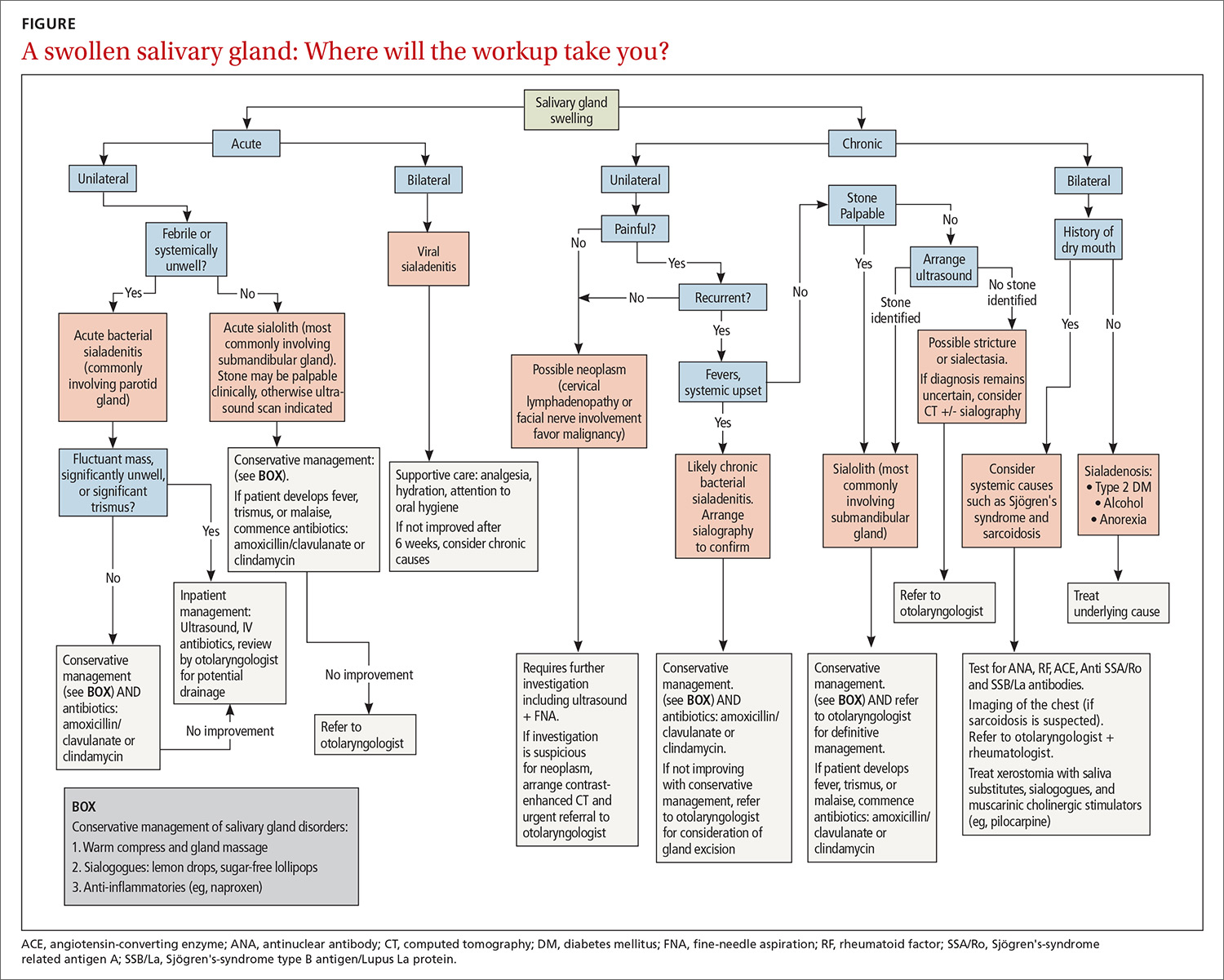
Neoplasms. Tumors of the salivary gland are relatively uncommon, constituting approximately 2% of head and neck neoplasms; most (80%) occur in the parotid gland, and most of those are benign.1 Although benign and malignant salivary gland neoplasms do not usually present with pain, pain can be associated with a neoplasm secondary to suppuration, hemorrhage into a mass, or infiltration of a malignancy into adjacent tissue.
Benign tumors. The majority of benign tumors are pleomorphic adenomas of the parotid, accounting for approximately 60% of salivary gland neoplasms.1,2 Tumors localized to the submandibular gland are often (in 50% of cases) malignant, however.3
Benign tumors are typically slow-growing and, generally, painless. On examination, they are well-circumscribed, mobile, and nontender. Patients presenting late with a large tumor might, however, experience pain secondary to stretching of the parotid capsule or compression of local structures.
Continue to: Ultrasonograhpy (US) is an excellent...
Ultrasonography (US) is an excellent initial imaging choice for investigating a possible salivary gland tumor; US is combined with FNA, which is safe and highly reliable for differentiating neoplastic and non-neoplastic disorders.4 (Avoid open biopsy of a neoplasm because of the risk of tumor spillage.) In patients with suspected neoplasm, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) should also be performed, because both modalities allow delineation of the tumor mass and demonstration of any infiltration of surrounding structures.
Treatment of benign neoplasms involves complete excision because, with some tumors, particularly pleomorphic adenomas, there is risk of malignant transformation over time. Superficial parotidectomy is the most common procedure, because most benign tumors occur in the superficial lobe of the parotid gland. Delicate dissection of the facial nerve is integral to the operation, although temporary facial nerve palsy will still occur in 5% to 10% of patients undergoing superficial parotidectomy for a benign tumor, with permanent injury occurring in fewer than 1%.5
Malignancy. Features of a tumor that raise concern of malignancy include6:
- rapid growth
- pain
- tethering to underlying structures or overlying skin
- firm mass
- associated cervical lymphadenopathy
- facial-nerve palsy.
The workup of a malignant tumor is the same as it is for a benign neoplasm: US-guided FNA, essential for diagnosis, and contrast-enhanced CT or MRI to delineate the tumor.
Malignant salivary gland neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both, necessitating a multidisciplinary approach. Also, there is potential for squamous-cell carcinoma and melanoma of the head to metastasize to salivary gland lymph nodes; it is important, therefore, to examine for, and elicit any history of, cutaneous malignancy of the scalp or face.
Continue to: Sialadenosis...
Sialadenosis presents with asymptomatic bilateral hypertrophy of the salivary glands—more commonly the parotids and rarely the submandibular glands. Swelling is persistent, symmetrical, painless, and of normal tone on palpation.
Causes of sialadenosis include alcoholism and, less commonly, diabetes mellitus and malnutrition; some cases are idiopathic. An autonomic neuropathy, causing excessive salivary acinar protein synthesis or failure of adequate secretion, or both, is common to alcoholism, diabetes, and malnutrition.7 Subsequent engorgement of acinar cells leads to clinical parotid hypertrophy.
Diagnosis is based on history and examination, as well as on the findings of US or CT, which will reveal bilateral gland enlargement and increased density. The glands appear dense because adipose cells are displaced by acinar cell hypertrophy; however, end-stage changes can result in the opposite appearance: a lucent enlargement caused by fatty infiltration.2 FNA is unnecessary, unless there is suspicion of neoplasm, as there would be in patients with asymmetrical parotid enlargement, pain, lymph node enlargement, or facial-nerve involvement. In patients with sialadenosis, in contrast, acinar cell hypertrophy alone will be present.
Treatment of sialadenosis is best aimed at rectifying the underlying medical condition, which might, over time, lead to some reduction in the size of the gland. There is no specific effective therapy for elimination of glandular swelling.
Bulimia and anorexia nervosa. Bulimia nervosa, the induction of vomiting after binge eating, can be associated with bilateral or occasionally unilateral parotid swelling. Anorexia, a form of self-starvation, can occur in association with bulimia, with patients also presenting with parotid swelling. Associated parotid swelling is similar to what is seen in sialadenosis: painless, persistent, and of nonpathologic consistency.
The pathophysiology of bulimia- and anorexia-associated parotid-gland swelling is identical to what is seen in sialadenosis: dysregulation of acinar cell sympathetic nerve supply that leads to enlargement of individual parenchymal cells.8 Contrast-enhanced CT can reveal increased vascularity associated with active bulimia. FNA and CT, however, are required only in patients in whom the diagnosis is not clear and when neoplasm is suspected.
Continue to: Treatment includes...
Treatment includes correcting electrolyte abnormalities and, more importantly, addressing underlying emotional issues to stop purging episodes. Psychiatric input and social support are invaluable. Parotid gland swelling generally improves with cessation of vomiting episodes.
Ask: Is the patient in pain?
Causes of salivary gland pain include sialolithiasis, sialadenitis, and recurrent parotitis of childhood. Pain occurs secondary to stretching of the fibrous capsule in which the parotid or submandibular gland is surrounded, compression of pain fibers by an expanding mass, or infiltration of nerves by neoplasia.
Sialolithiasis. Sialolithiasis, or salivary stones, are primarily calcium carbonate concentrations within the salivary ductal system. More than 80% occur in the submandibular gland or duct9 as a result of production of mixed mucoid and serous saliva and a tortuous duct path.
Patients usually present with a history of intermittent swelling and pain of the involved gland associated with eating. Increased production of saliva during meals, which then passes through a partially obstructed salivary duct, leads to salivary retention and glandular swelling. Thus, a recurring pattern can develop, with varying periods of remission,7 eventually leading to an acute suppurative process or sialadenitis (described below). Chronic salivary disease can also be caused by stricture of a duct or, rarely, external compression by a tumor mass.
Examination often reveals an enlarged and often tender gland; conversely, chronic disease can lead to gland atrophy. Usually, only minimal saliva is able to be expressed from an obstructed duct. For a submandibular duct stone, bimanual palpation might reveal its position if it is located distally in the floor of the mouth; a proximal stone might not be palpable.
Continue to: Although US is operator-dependent...
Although US is operator-dependent, it is the imaging modality of choice for identifying sialolithiasis10 because it can identify gland architecture, duct dilation, and both radiolucent and radiopaque stones. For patients in whom US findings are normal despite a convincing clinical presentation of sialolithiasis, CT should be performed because small stones can be missed on US.11
Supportive measures for sialolithiasis are listed in the TABLE. Reserve antibiotics for patients who have signs or symptoms of infection, including pyrexia, trismus, and malaise. A beta-lactam antibiotic, such as amoxicillin–clavulanate, 875 mg orally bid, or a cephalosporin, such as cephalexin, 500 mg orally qid, are appropriate first-line options. Clindamycin, 300 mg orally tid, or metronidazole, 500 mg orally tid, are acceptable alternatives. When signs or symptoms are persistent or recurrent, refer the patient for a surgical opinion.

Stones located in the floor of the mouth are usually excised through an intraoral approach. In the past, gland excision was advocated when a sialolith was found more proximally within the gland parenchyma. More recently, however, sialendoscopy, involving insertion of a small, semirigid endoscope into the salivary duct, has been shown safe and effective for removing a stone; successful removal, in as many as 80% of cases, increases to 90% when performed using a minimally invasive surgical technique.12 Although sialendoscopy is effective, the technique cannot always treat the underlying abnormality of the salivary gland; gland excision is therefore warranted in some cases.
Last, extracorporeal shock wave therapy is aimed at fragmenting salivary stones before retrieval. Results are variable, however, and treatment should be guided by an otolaryngologist.13,14
Sialadenitis (bacterial and viral infection). Acute suppurative sialadenitis occurs secondary to retrograde ductal bacterial infection. The parotid gland is most frequently involved,15 although submandibular sialadenitis is not uncommon. Patients usually present with sudden-onset unilateral, painful swelling.
Continue to: Pathophysiology involves...
Pathophysiology involves dehydration or decreased oral intake leading to salivary stasis and subsequent bacterial migration into the gland. Medically debilitated and postoperative patients are therefore at greater risk; so are patients with diabetes mellitus, poor oral hygiene, Sjögren’s syndrome, hypothyroidism, or renal failure.16 Certain medications, including anticholinergics, can also predispose to hyposalivation.17
(As discussed, sialolithiasis and stricture of salivary ducts can also cause acute bacterial infection; in such cases, however, the typical presentation is one of chronic or recurrent infection.)
Examination might reveal an exquisitely tender, indurated, and inflamed gland; pus can often be expressed from the respective intraoral orifice. Any expressed pus should be sent for culture to guide antibiotic therapy.
Treatment should focus on hydration, oral hygiene, and antibiotics, while reversing or minimizing any underlying contributing medical condition. Warm compresses applied to the involved gland, massage, and sialagogues, such as lemon drops or sugar-free lollipops, can stimulate salivary flow and prevent stasis.
More than 80% of infections are caused by Staphylococcus aureus17; anaerobic and mixed infections have also been recognized.A beta-lactam penicillin, such as amoxicillin-clavulanate, is the antibiotic of choice. A patient who is systemically unwell should be treated as an inpatient with nafcillin and metronidazole. Methicillin-resistant S aureus must also be considered in patients with comorbid disease, such as diabetes mellitus or intravenous drug use, or in patients residing in an area of substantial incidence of methicillin-resistant S aureus. In those cases, substitute vancomycin or linezolid for nafcillin.18
Continue to: Less commonly...
Less commonly, abscess can form, with the patient presenting as systemically unwell with a fluctuant mass. If the diagnosis is unclear or the patient does not improve, abscess can be confirmed by US. Expedient surgical review and inpatient admission can then be arranged.
Unlike bacterial sialadenitis, causes of viral sialadenitis are often bilateral. Mumps (a paramyxovirus) is the most common viral cause, affecting primarily children < 15 years.19 The parotid glands are most often involved, with inflammation and edema causing significant pain because of increasing intraparotid pressure as expansion of the gland is limited by its tense fibrous capsule. Complications of mumps include orchitis, meningitis, pancreatitis, and oophoritis.
Mumps is highly contagious; it is spread through contact with airborne saliva droplets, with viral entry through the nose or mouth, followed by proliferation in the salivary glands or on surface epithelium of the respiratory tract.7 Diagnosis is confirmed by viral serology. A positive test of serum immunoglobulin M confirms the diagnosis, but this test should not be performed until 3 days after onset of symptoms because a false-negative result is otherwise possible.20 Immunoglobulin G serologic testing can further aid diagnosis; the titer is measured approximately 4 days after onset of symptoms and again 2 to 3 weeks later. A 4-fold rise in titer confirms mumps.
Other viral infections that can cause sialadenitis include Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, coxsackievirus, and influenza. Treatment is supportive: analgesia, hydration, oral hygiene, and rest. Inflammation might take weeks to resolve, but expect complete resolution. For a patient who has significant trismus, poor oral intake, or a potentially threatened airway, inpatient care should be provided.
Recurrent parotitis of childhood is an inflammatory condition that usually affects one, but at times both, parotid glands. It is characterized by episodes of painful swelling. Incidence peaks at 3 to 6 years of age.7 Episodes can be frequent, occurring 1 to 5 times a year and lasting 3 to 7 days—sometimes longer—and usually resolving without treatment.
Continue to: The precise etiology...
The precise etiology of recurrent parotitis of childhood is unclear; possibly, saliva aggregates to form obstructive mucous plugs, thus causing stasis and swelling of the gland. As pressure builds, spontaneous plug extrusion occurs and symptoms resolve, provided infection is not a factor. US demonstrates multiple round, hypoechoic areas consistent with duct dilation, and surrounding infiltration by lymphocytes.1
Supportive care—adequate hydration, gland massage, warm compresses, and sialogogues—are mainstays of treatment. Fever and malaise warrant treatment with oral antibiotics. Sialadenoscopy, which can be considered in children with frequent episodes, can decrease the frequency and severity of episodes.21 The condition usually resolves spontaneously at puberty.
Ask: Does the patient have dry mouth?
In-depth review of xerostomia is beyond the scope of this article. Causes include Sjögren's syndrome, immunoglobulin G4-related sialadenitis, sarcoidosis, radiation therapy, diabetes, chronic infection, and medications—in particular those with anticholinergic effects.
Treatment of xerostomia includes saliva substitutes, sialagogues, and, for oral candidiasis, antifungals. Muscarinic cholinergic stimulators, such as pilocarpine, 5 mg qid have been used with some success22; patients should be advised of potential adverse effects with these agents, including sweating, urinary frequency, flushing, and chills.
CORRESPONDENCE
Shankar Haran, MBBS, ENT Department, Townsville Hospital, 100 Angus Smith Dr, Douglas, Queensland, Australia 4814; [email protected].
1. de Oliveira FA, Duarte EC, Taveira CT, et al. Salivary gland tumor: a review of 599 cases in a Brazilian population. Head Neck Pathol. 2009;3:271-275.
2. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
3. Bova R. A guide to salivary gland disorders. Medicine Today. 2006;7:44-48.
4. Zhang S, Bao R, Bagby J, et al. Fine needle aspiration of salivary glands: 5-year experience from a single academic center. Acta Cytol. 2009;53:375-382.
5. Bova R, Saylor A, Coman WB. Parotidectomy: review of treatment and outcomes. ANZ J Surg. 2004;74:563-568.
6. Sood S, McGurk M, Vaz F. Management of salivary gland tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S142-S149.
7. Mandel L. Salivary gland disorders. Med Clin North Am. 2014;98:1407-1449.
8. Mandel L, Abai S. Diagnosing bulimia nervosa with parotid gland swelling. J Am Dent Assoc. 2004;135:613–616.
9. Lustmann J, Regev E, Melamed Y. Sialolithiasis. A survey on 245 patients and a review of literature. Int J Oral Maxillofac Surg. 1990;19:135–138.
10. Vogl TJ, Al-Nawas B, Beutner D, et al. Updated S2K AWMF guideline for the diagnosis and follow-up of obstructive sialadenitis—relevance for radiologic imaging. Rofo. 2014;186:843-846.
11. Schwarz D, Kabbasch C, Scheer M, et al. Comparative analysis of sialendoscopy, sonography, and CBCT in the detection of sialolithiasis. Laryngoscope. 2015;125:1098–1101.
12. Atienza G, López-Cedrún JL. Management of obstructive salivary disorders by sialendoscopy: a systematic review. Br J Oral Maxillofac Surg. 2015;53:507-519.
13. Escudier MP, Brown JE, Putcha V, et al. Factors influencing the outcome of extracorporeal shock wave lithotripsy in the management of salivary calculi. Laryngoscope. 2010;120:1545-1549.
14. Koch M, Schapher M, Mantsopoulos K, et al. Multimodal treatment in difficult sialolithiasis: Role of extracorporeal shock-wave lithotripsy and intraductal pneumatic lithotripsy. Laryngoscope. 2018;128:E332-E338.
15. McQuone SJ. Acute viral and bacterial infections of the salivary glands. Otolaryngol Clin North Am. 1999;32:793-811.
16. O’Neil C, Sidhu S. Salivary gland disorders. Australian Doctor. 2011;28:19-25.
17. Mandel L. Differentiating acute suppurative parotitis from acute exacerbation of a chronic parotitis: case reports. J Oral Maxillofac Surg. 2008;66:1964-1968.
18. Chow AW. Suppurative parotitis in adults. UpToDate.com. www.uptodate.com/contents/suppurative-parotitis-in-adults. Accessed September 25, 2019.
19. Katz SL, Gershon AA, Hotez PJ. Infectious Diseases of Children. New York, NY: Mosby Year Book; 1998:280-289.
20. Krause CH, Molyneaux PJ, Ho-Yen DO, et al. Comparison of mumps-IgM ELISAs in acute infection. J Clin Virol. 2007;38:153-156.
21. Quenin S, Plouin-Gaudon I, Marchal F, et al. Juvenile recurrent parotitis: sialendoscopic approach. Arch Otolaryngol Head Neck Surg. 2008;134:715-719.
22. Papas AS, Sherrer YS, Charney M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169-177.
Making a diagnosis of a salivary gland disorder can be difficult. Common presentations, such as a painful or swollen gland, can be caused by numerous disorders of strikingly variable severity and consequences, including inflammatory, infectious, and neoplastic conditions, for which treatment can differ significantly, and referral for specialty care is sometimes necessary.
Yet it is the patient’s presentation that can aid you in making the diagnosis that will guide management. Consider that acute symptoms often result from infection, for example, and chronic or recurrent symptoms are caused more often by obstructive or nonobstructive inflammatory conditions and neoplasms. Diagnosis of an apparent neoplasm, prompted by clinical findings, is made using imaging and fine-needle aspiration (FNA) biopsy. Acute infection usually resolves with antibiotics and supportive management; calculi that cause persistent symptoms warrant referral for consideration of stone or gland removal; and malignant neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both—calling for multidisciplinary care.
In this article, we clarify what can be an imprecise and perplexing path from the presentation to diagnosis to treatment of disorders of the salivary glands. To begin, see “Geography of the salivary glands,” for an overview of the location, structure, and corresponding ducts of the component salivary glands (parotid, submandibular, sublingual, and minor glands).
SIDEBAR
Geography of the salivary glands

The salivary glands comprise the major paired parotid, submandibular, and sublingual glands, as well as minor salivary glands that line the oropharyngeal mucosa. Secretion of saliva is modulated by both autonomic and humoral factors.
The parotid gland sits between the mastoid process, the ramus of the mandible, and the styloid process, extend- ing from the external auditory meatus superiorly to below the angle of the mandible and into the neck inferiorly. The gland is surrounded by a tough capsule. Embedded within the gland is the facial nerve, which divides into its 5 branches within the substance of the gland. The parotid (Stensen’s) duct passes anteriorly before turning medially to pierce the buccinator muscle, opening onto the mucous membrane of the cheek opposite the second upper molar.
The submandibular gland comprises (1) a large superficial part that fills the space between the mandible and the floor of the mouth and (2) a small deep part that wraps around the posterior border of the mylohyoid muscle. The submandibular (Wharton’s) duct runs anteriorly to open onto the floor of the mouth, alongside the frenulum.
The sublingual gland, the smallest of the major salivary glands, lies anteriorly in the floor of the mouth, with many small ducts opening either into the submandibular duct or directly into the mouth.
Basic secretory units of salivary glands are clusters of cells, each called an acinus. These cells secrete a fluid that contains water, electrolytes, mucous, and enzymes, all of which flow out of the acini into collecting ducts. The saliva produced by the parotid is mainly serous; by the submandibular gland, mixed; and by the sublingual and minor salivary glands, mucoid.
Presentation helps establish the differential Dx
Ask: Are the glands swollen?
Painless salivary gland swelling has a variety of causes, including neoplasm, sialadenosis, and the eating disorders bulimia and anorexia nervosa. There is significant overlap of presentations among those causes (FIGURE). Pain accompanying swelling is uncommon but not unheard of.

Neoplasms. Tumors of the salivary gland are relatively uncommon, constituting approximately 2% of head and neck neoplasms; most (80%) occur in the parotid gland, and most of those are benign.1 Although benign and malignant salivary gland neoplasms do not usually present with pain, pain can be associated with a neoplasm secondary to suppuration, hemorrhage into a mass, or infiltration of a malignancy into adjacent tissue.
Benign tumors. The majority of benign tumors are pleomorphic adenomas of the parotid, accounting for approximately 60% of salivary gland neoplasms.1,2 Tumors localized to the submandibular gland are often (in 50% of cases) malignant, however.3
Benign tumors are typically slow-growing and, generally, painless. On examination, they are well-circumscribed, mobile, and nontender. Patients presenting late with a large tumor might, however, experience pain secondary to stretching of the parotid capsule or compression of local structures.
Continue to: Ultrasonograhpy (US) is an excellent...
Ultrasonography (US) is an excellent initial imaging choice for investigating a possible salivary gland tumor; US is combined with FNA, which is safe and highly reliable for differentiating neoplastic and non-neoplastic disorders.4 (Avoid open biopsy of a neoplasm because of the risk of tumor spillage.) In patients with suspected neoplasm, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) should also be performed, because both modalities allow delineation of the tumor mass and demonstration of any infiltration of surrounding structures.
Treatment of benign neoplasms involves complete excision because, with some tumors, particularly pleomorphic adenomas, there is risk of malignant transformation over time. Superficial parotidectomy is the most common procedure, because most benign tumors occur in the superficial lobe of the parotid gland. Delicate dissection of the facial nerve is integral to the operation, although temporary facial nerve palsy will still occur in 5% to 10% of patients undergoing superficial parotidectomy for a benign tumor, with permanent injury occurring in fewer than 1%.5
Malignancy. Features of a tumor that raise concern of malignancy include6:
- rapid growth
- pain
- tethering to underlying structures or overlying skin
- firm mass
- associated cervical lymphadenopathy
- facial-nerve palsy.
The workup of a malignant tumor is the same as it is for a benign neoplasm: US-guided FNA, essential for diagnosis, and contrast-enhanced CT or MRI to delineate the tumor.
Malignant salivary gland neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both, necessitating a multidisciplinary approach. Also, there is potential for squamous-cell carcinoma and melanoma of the head to metastasize to salivary gland lymph nodes; it is important, therefore, to examine for, and elicit any history of, cutaneous malignancy of the scalp or face.
Continue to: Sialadenosis...
Sialadenosis presents with asymptomatic bilateral hypertrophy of the salivary glands—more commonly the parotids and rarely the submandibular glands. Swelling is persistent, symmetrical, painless, and of normal tone on palpation.
Causes of sialadenosis include alcoholism and, less commonly, diabetes mellitus and malnutrition; some cases are idiopathic. An autonomic neuropathy, causing excessive salivary acinar protein synthesis or failure of adequate secretion, or both, is common to alcoholism, diabetes, and malnutrition.7 Subsequent engorgement of acinar cells leads to clinical parotid hypertrophy.
Diagnosis is based on history and examination, as well as on the findings of US or CT, which will reveal bilateral gland enlargement and increased density. The glands appear dense because adipose cells are displaced by acinar cell hypertrophy; however, end-stage changes can result in the opposite appearance: a lucent enlargement caused by fatty infiltration.2 FNA is unnecessary, unless there is suspicion of neoplasm, as there would be in patients with asymmetrical parotid enlargement, pain, lymph node enlargement, or facial-nerve involvement. In patients with sialadenosis, in contrast, acinar cell hypertrophy alone will be present.
Treatment of sialadenosis is best aimed at rectifying the underlying medical condition, which might, over time, lead to some reduction in the size of the gland. There is no specific effective therapy for elimination of glandular swelling.
Bulimia and anorexia nervosa. Bulimia nervosa, the induction of vomiting after binge eating, can be associated with bilateral or occasionally unilateral parotid swelling. Anorexia, a form of self-starvation, can occur in association with bulimia, with patients also presenting with parotid swelling. Associated parotid swelling is similar to what is seen in sialadenosis: painless, persistent, and of nonpathologic consistency.
The pathophysiology of bulimia- and anorexia-associated parotid-gland swelling is identical to what is seen in sialadenosis: dysregulation of acinar cell sympathetic nerve supply that leads to enlargement of individual parenchymal cells.8 Contrast-enhanced CT can reveal increased vascularity associated with active bulimia. FNA and CT, however, are required only in patients in whom the diagnosis is not clear and when neoplasm is suspected.
Continue to: Treatment includes...
Treatment includes correcting electrolyte abnormalities and, more importantly, addressing underlying emotional issues to stop purging episodes. Psychiatric input and social support are invaluable. Parotid gland swelling generally improves with cessation of vomiting episodes.
Ask: Is the patient in pain?
Causes of salivary gland pain include sialolithiasis, sialadenitis, and recurrent parotitis of childhood. Pain occurs secondary to stretching of the fibrous capsule in which the parotid or submandibular gland is surrounded, compression of pain fibers by an expanding mass, or infiltration of nerves by neoplasia.
Sialolithiasis. Sialolithiasis, or salivary stones, are primarily calcium carbonate concentrations within the salivary ductal system. More than 80% occur in the submandibular gland or duct9 as a result of production of mixed mucoid and serous saliva and a tortuous duct path.
Patients usually present with a history of intermittent swelling and pain of the involved gland associated with eating. Increased production of saliva during meals, which then passes through a partially obstructed salivary duct, leads to salivary retention and glandular swelling. Thus, a recurring pattern can develop, with varying periods of remission,7 eventually leading to an acute suppurative process or sialadenitis (described below). Chronic salivary disease can also be caused by stricture of a duct or, rarely, external compression by a tumor mass.
Examination often reveals an enlarged and often tender gland; conversely, chronic disease can lead to gland atrophy. Usually, only minimal saliva is able to be expressed from an obstructed duct. For a submandibular duct stone, bimanual palpation might reveal its position if it is located distally in the floor of the mouth; a proximal stone might not be palpable.
Continue to: Although US is operator-dependent...
Although US is operator-dependent, it is the imaging modality of choice for identifying sialolithiasis10 because it can identify gland architecture, duct dilation, and both radiolucent and radiopaque stones. For patients in whom US findings are normal despite a convincing clinical presentation of sialolithiasis, CT should be performed because small stones can be missed on US.11
Supportive measures for sialolithiasis are listed in the TABLE. Reserve antibiotics for patients who have signs or symptoms of infection, including pyrexia, trismus, and malaise. A beta-lactam antibiotic, such as amoxicillin–clavulanate, 875 mg orally bid, or a cephalosporin, such as cephalexin, 500 mg orally qid, are appropriate first-line options. Clindamycin, 300 mg orally tid, or metronidazole, 500 mg orally tid, are acceptable alternatives. When signs or symptoms are persistent or recurrent, refer the patient for a surgical opinion.

Stones located in the floor of the mouth are usually excised through an intraoral approach. In the past, gland excision was advocated when a sialolith was found more proximally within the gland parenchyma. More recently, however, sialendoscopy, involving insertion of a small, semirigid endoscope into the salivary duct, has been shown safe and effective for removing a stone; successful removal, in as many as 80% of cases, increases to 90% when performed using a minimally invasive surgical technique.12 Although sialendoscopy is effective, the technique cannot always treat the underlying abnormality of the salivary gland; gland excision is therefore warranted in some cases.
Last, extracorporeal shock wave therapy is aimed at fragmenting salivary stones before retrieval. Results are variable, however, and treatment should be guided by an otolaryngologist.13,14
Sialadenitis (bacterial and viral infection). Acute suppurative sialadenitis occurs secondary to retrograde ductal bacterial infection. The parotid gland is most frequently involved,15 although submandibular sialadenitis is not uncommon. Patients usually present with sudden-onset unilateral, painful swelling.
Continue to: Pathophysiology involves...
Pathophysiology involves dehydration or decreased oral intake leading to salivary stasis and subsequent bacterial migration into the gland. Medically debilitated and postoperative patients are therefore at greater risk; so are patients with diabetes mellitus, poor oral hygiene, Sjögren’s syndrome, hypothyroidism, or renal failure.16 Certain medications, including anticholinergics, can also predispose to hyposalivation.17
(As discussed, sialolithiasis and stricture of salivary ducts can also cause acute bacterial infection; in such cases, however, the typical presentation is one of chronic or recurrent infection.)
Examination might reveal an exquisitely tender, indurated, and inflamed gland; pus can often be expressed from the respective intraoral orifice. Any expressed pus should be sent for culture to guide antibiotic therapy.
Treatment should focus on hydration, oral hygiene, and antibiotics, while reversing or minimizing any underlying contributing medical condition. Warm compresses applied to the involved gland, massage, and sialagogues, such as lemon drops or sugar-free lollipops, can stimulate salivary flow and prevent stasis.
More than 80% of infections are caused by Staphylococcus aureus17; anaerobic and mixed infections have also been recognized.A beta-lactam penicillin, such as amoxicillin-clavulanate, is the antibiotic of choice. A patient who is systemically unwell should be treated as an inpatient with nafcillin and metronidazole. Methicillin-resistant S aureus must also be considered in patients with comorbid disease, such as diabetes mellitus or intravenous drug use, or in patients residing in an area of substantial incidence of methicillin-resistant S aureus. In those cases, substitute vancomycin or linezolid for nafcillin.18
Continue to: Less commonly...
Less commonly, abscess can form, with the patient presenting as systemically unwell with a fluctuant mass. If the diagnosis is unclear or the patient does not improve, abscess can be confirmed by US. Expedient surgical review and inpatient admission can then be arranged.
Unlike bacterial sialadenitis, causes of viral sialadenitis are often bilateral. Mumps (a paramyxovirus) is the most common viral cause, affecting primarily children < 15 years.19 The parotid glands are most often involved, with inflammation and edema causing significant pain because of increasing intraparotid pressure as expansion of the gland is limited by its tense fibrous capsule. Complications of mumps include orchitis, meningitis, pancreatitis, and oophoritis.
Mumps is highly contagious; it is spread through contact with airborne saliva droplets, with viral entry through the nose or mouth, followed by proliferation in the salivary glands or on surface epithelium of the respiratory tract.7 Diagnosis is confirmed by viral serology. A positive test of serum immunoglobulin M confirms the diagnosis, but this test should not be performed until 3 days after onset of symptoms because a false-negative result is otherwise possible.20 Immunoglobulin G serologic testing can further aid diagnosis; the titer is measured approximately 4 days after onset of symptoms and again 2 to 3 weeks later. A 4-fold rise in titer confirms mumps.
Other viral infections that can cause sialadenitis include Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, coxsackievirus, and influenza. Treatment is supportive: analgesia, hydration, oral hygiene, and rest. Inflammation might take weeks to resolve, but expect complete resolution. For a patient who has significant trismus, poor oral intake, or a potentially threatened airway, inpatient care should be provided.
Recurrent parotitis of childhood is an inflammatory condition that usually affects one, but at times both, parotid glands. It is characterized by episodes of painful swelling. Incidence peaks at 3 to 6 years of age.7 Episodes can be frequent, occurring 1 to 5 times a year and lasting 3 to 7 days—sometimes longer—and usually resolving without treatment.
Continue to: The precise etiology...
The precise etiology of recurrent parotitis of childhood is unclear; possibly, saliva aggregates to form obstructive mucous plugs, thus causing stasis and swelling of the gland. As pressure builds, spontaneous plug extrusion occurs and symptoms resolve, provided infection is not a factor. US demonstrates multiple round, hypoechoic areas consistent with duct dilation, and surrounding infiltration by lymphocytes.1
Supportive care—adequate hydration, gland massage, warm compresses, and sialogogues—are mainstays of treatment. Fever and malaise warrant treatment with oral antibiotics. Sialadenoscopy, which can be considered in children with frequent episodes, can decrease the frequency and severity of episodes.21 The condition usually resolves spontaneously at puberty.
Ask: Does the patient have dry mouth?
In-depth review of xerostomia is beyond the scope of this article. Causes include Sjögren's syndrome, immunoglobulin G4-related sialadenitis, sarcoidosis, radiation therapy, diabetes, chronic infection, and medications—in particular those with anticholinergic effects.
Treatment of xerostomia includes saliva substitutes, sialagogues, and, for oral candidiasis, antifungals. Muscarinic cholinergic stimulators, such as pilocarpine, 5 mg qid have been used with some success22; patients should be advised of potential adverse effects with these agents, including sweating, urinary frequency, flushing, and chills.
CORRESPONDENCE
Shankar Haran, MBBS, ENT Department, Townsville Hospital, 100 Angus Smith Dr, Douglas, Queensland, Australia 4814; [email protected].
Making a diagnosis of a salivary gland disorder can be difficult. Common presentations, such as a painful or swollen gland, can be caused by numerous disorders of strikingly variable severity and consequences, including inflammatory, infectious, and neoplastic conditions, for which treatment can differ significantly, and referral for specialty care is sometimes necessary.
Yet it is the patient’s presentation that can aid you in making the diagnosis that will guide management. Consider that acute symptoms often result from infection, for example, and chronic or recurrent symptoms are caused more often by obstructive or nonobstructive inflammatory conditions and neoplasms. Diagnosis of an apparent neoplasm, prompted by clinical findings, is made using imaging and fine-needle aspiration (FNA) biopsy. Acute infection usually resolves with antibiotics and supportive management; calculi that cause persistent symptoms warrant referral for consideration of stone or gland removal; and malignant neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both—calling for multidisciplinary care.
In this article, we clarify what can be an imprecise and perplexing path from the presentation to diagnosis to treatment of disorders of the salivary glands. To begin, see “Geography of the salivary glands,” for an overview of the location, structure, and corresponding ducts of the component salivary glands (parotid, submandibular, sublingual, and minor glands).
SIDEBAR
Geography of the salivary glands

The salivary glands comprise the major paired parotid, submandibular, and sublingual glands, as well as minor salivary glands that line the oropharyngeal mucosa. Secretion of saliva is modulated by both autonomic and humoral factors.
The parotid gland sits between the mastoid process, the ramus of the mandible, and the styloid process, extend- ing from the external auditory meatus superiorly to below the angle of the mandible and into the neck inferiorly. The gland is surrounded by a tough capsule. Embedded within the gland is the facial nerve, which divides into its 5 branches within the substance of the gland. The parotid (Stensen’s) duct passes anteriorly before turning medially to pierce the buccinator muscle, opening onto the mucous membrane of the cheek opposite the second upper molar.
The submandibular gland comprises (1) a large superficial part that fills the space between the mandible and the floor of the mouth and (2) a small deep part that wraps around the posterior border of the mylohyoid muscle. The submandibular (Wharton’s) duct runs anteriorly to open onto the floor of the mouth, alongside the frenulum.
The sublingual gland, the smallest of the major salivary glands, lies anteriorly in the floor of the mouth, with many small ducts opening either into the submandibular duct or directly into the mouth.
Basic secretory units of salivary glands are clusters of cells, each called an acinus. These cells secrete a fluid that contains water, electrolytes, mucous, and enzymes, all of which flow out of the acini into collecting ducts. The saliva produced by the parotid is mainly serous; by the submandibular gland, mixed; and by the sublingual and minor salivary glands, mucoid.
Presentation helps establish the differential Dx
Ask: Are the glands swollen?
Painless salivary gland swelling has a variety of causes, including neoplasm, sialadenosis, and the eating disorders bulimia and anorexia nervosa. There is significant overlap of presentations among those causes (FIGURE). Pain accompanying swelling is uncommon but not unheard of.

Neoplasms. Tumors of the salivary gland are relatively uncommon, constituting approximately 2% of head and neck neoplasms; most (80%) occur in the parotid gland, and most of those are benign.1 Although benign and malignant salivary gland neoplasms do not usually present with pain, pain can be associated with a neoplasm secondary to suppuration, hemorrhage into a mass, or infiltration of a malignancy into adjacent tissue.
Benign tumors. The majority of benign tumors are pleomorphic adenomas of the parotid, accounting for approximately 60% of salivary gland neoplasms.1,2 Tumors localized to the submandibular gland are often (in 50% of cases) malignant, however.3
Benign tumors are typically slow-growing and, generally, painless. On examination, they are well-circumscribed, mobile, and nontender. Patients presenting late with a large tumor might, however, experience pain secondary to stretching of the parotid capsule or compression of local structures.
Continue to: Ultrasonograhpy (US) is an excellent...
Ultrasonography (US) is an excellent initial imaging choice for investigating a possible salivary gland tumor; US is combined with FNA, which is safe and highly reliable for differentiating neoplastic and non-neoplastic disorders.4 (Avoid open biopsy of a neoplasm because of the risk of tumor spillage.) In patients with suspected neoplasm, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) should also be performed, because both modalities allow delineation of the tumor mass and demonstration of any infiltration of surrounding structures.
Treatment of benign neoplasms involves complete excision because, with some tumors, particularly pleomorphic adenomas, there is risk of malignant transformation over time. Superficial parotidectomy is the most common procedure, because most benign tumors occur in the superficial lobe of the parotid gland. Delicate dissection of the facial nerve is integral to the operation, although temporary facial nerve palsy will still occur in 5% to 10% of patients undergoing superficial parotidectomy for a benign tumor, with permanent injury occurring in fewer than 1%.5
Malignancy. Features of a tumor that raise concern of malignancy include6:
- rapid growth
- pain
- tethering to underlying structures or overlying skin
- firm mass
- associated cervical lymphadenopathy
- facial-nerve palsy.
The workup of a malignant tumor is the same as it is for a benign neoplasm: US-guided FNA, essential for diagnosis, and contrast-enhanced CT or MRI to delineate the tumor.
Malignant salivary gland neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both, necessitating a multidisciplinary approach. Also, there is potential for squamous-cell carcinoma and melanoma of the head to metastasize to salivary gland lymph nodes; it is important, therefore, to examine for, and elicit any history of, cutaneous malignancy of the scalp or face.
Continue to: Sialadenosis...
Sialadenosis presents with asymptomatic bilateral hypertrophy of the salivary glands—more commonly the parotids and rarely the submandibular glands. Swelling is persistent, symmetrical, painless, and of normal tone on palpation.
Causes of sialadenosis include alcoholism and, less commonly, diabetes mellitus and malnutrition; some cases are idiopathic. An autonomic neuropathy, causing excessive salivary acinar protein synthesis or failure of adequate secretion, or both, is common to alcoholism, diabetes, and malnutrition.7 Subsequent engorgement of acinar cells leads to clinical parotid hypertrophy.
Diagnosis is based on history and examination, as well as on the findings of US or CT, which will reveal bilateral gland enlargement and increased density. The glands appear dense because adipose cells are displaced by acinar cell hypertrophy; however, end-stage changes can result in the opposite appearance: a lucent enlargement caused by fatty infiltration.2 FNA is unnecessary, unless there is suspicion of neoplasm, as there would be in patients with asymmetrical parotid enlargement, pain, lymph node enlargement, or facial-nerve involvement. In patients with sialadenosis, in contrast, acinar cell hypertrophy alone will be present.
Treatment of sialadenosis is best aimed at rectifying the underlying medical condition, which might, over time, lead to some reduction in the size of the gland. There is no specific effective therapy for elimination of glandular swelling.
Bulimia and anorexia nervosa. Bulimia nervosa, the induction of vomiting after binge eating, can be associated with bilateral or occasionally unilateral parotid swelling. Anorexia, a form of self-starvation, can occur in association with bulimia, with patients also presenting with parotid swelling. Associated parotid swelling is similar to what is seen in sialadenosis: painless, persistent, and of nonpathologic consistency.
The pathophysiology of bulimia- and anorexia-associated parotid-gland swelling is identical to what is seen in sialadenosis: dysregulation of acinar cell sympathetic nerve supply that leads to enlargement of individual parenchymal cells.8 Contrast-enhanced CT can reveal increased vascularity associated with active bulimia. FNA and CT, however, are required only in patients in whom the diagnosis is not clear and when neoplasm is suspected.
Continue to: Treatment includes...
Treatment includes correcting electrolyte abnormalities and, more importantly, addressing underlying emotional issues to stop purging episodes. Psychiatric input and social support are invaluable. Parotid gland swelling generally improves with cessation of vomiting episodes.
Ask: Is the patient in pain?
Causes of salivary gland pain include sialolithiasis, sialadenitis, and recurrent parotitis of childhood. Pain occurs secondary to stretching of the fibrous capsule in which the parotid or submandibular gland is surrounded, compression of pain fibers by an expanding mass, or infiltration of nerves by neoplasia.
Sialolithiasis. Sialolithiasis, or salivary stones, are primarily calcium carbonate concentrations within the salivary ductal system. More than 80% occur in the submandibular gland or duct9 as a result of production of mixed mucoid and serous saliva and a tortuous duct path.
Patients usually present with a history of intermittent swelling and pain of the involved gland associated with eating. Increased production of saliva during meals, which then passes through a partially obstructed salivary duct, leads to salivary retention and glandular swelling. Thus, a recurring pattern can develop, with varying periods of remission,7 eventually leading to an acute suppurative process or sialadenitis (described below). Chronic salivary disease can also be caused by stricture of a duct or, rarely, external compression by a tumor mass.
Examination often reveals an enlarged and often tender gland; conversely, chronic disease can lead to gland atrophy. Usually, only minimal saliva is able to be expressed from an obstructed duct. For a submandibular duct stone, bimanual palpation might reveal its position if it is located distally in the floor of the mouth; a proximal stone might not be palpable.
Continue to: Although US is operator-dependent...
Although US is operator-dependent, it is the imaging modality of choice for identifying sialolithiasis10 because it can identify gland architecture, duct dilation, and both radiolucent and radiopaque stones. For patients in whom US findings are normal despite a convincing clinical presentation of sialolithiasis, CT should be performed because small stones can be missed on US.11
Supportive measures for sialolithiasis are listed in the TABLE. Reserve antibiotics for patients who have signs or symptoms of infection, including pyrexia, trismus, and malaise. A beta-lactam antibiotic, such as amoxicillin–clavulanate, 875 mg orally bid, or a cephalosporin, such as cephalexin, 500 mg orally qid, are appropriate first-line options. Clindamycin, 300 mg orally tid, or metronidazole, 500 mg orally tid, are acceptable alternatives. When signs or symptoms are persistent or recurrent, refer the patient for a surgical opinion.

Stones located in the floor of the mouth are usually excised through an intraoral approach. In the past, gland excision was advocated when a sialolith was found more proximally within the gland parenchyma. More recently, however, sialendoscopy, involving insertion of a small, semirigid endoscope into the salivary duct, has been shown safe and effective for removing a stone; successful removal, in as many as 80% of cases, increases to 90% when performed using a minimally invasive surgical technique.12 Although sialendoscopy is effective, the technique cannot always treat the underlying abnormality of the salivary gland; gland excision is therefore warranted in some cases.
Last, extracorporeal shock wave therapy is aimed at fragmenting salivary stones before retrieval. Results are variable, however, and treatment should be guided by an otolaryngologist.13,14
Sialadenitis (bacterial and viral infection). Acute suppurative sialadenitis occurs secondary to retrograde ductal bacterial infection. The parotid gland is most frequently involved,15 although submandibular sialadenitis is not uncommon. Patients usually present with sudden-onset unilateral, painful swelling.
Continue to: Pathophysiology involves...
Pathophysiology involves dehydration or decreased oral intake leading to salivary stasis and subsequent bacterial migration into the gland. Medically debilitated and postoperative patients are therefore at greater risk; so are patients with diabetes mellitus, poor oral hygiene, Sjögren’s syndrome, hypothyroidism, or renal failure.16 Certain medications, including anticholinergics, can also predispose to hyposalivation.17
(As discussed, sialolithiasis and stricture of salivary ducts can also cause acute bacterial infection; in such cases, however, the typical presentation is one of chronic or recurrent infection.)
Examination might reveal an exquisitely tender, indurated, and inflamed gland; pus can often be expressed from the respective intraoral orifice. Any expressed pus should be sent for culture to guide antibiotic therapy.
Treatment should focus on hydration, oral hygiene, and antibiotics, while reversing or minimizing any underlying contributing medical condition. Warm compresses applied to the involved gland, massage, and sialagogues, such as lemon drops or sugar-free lollipops, can stimulate salivary flow and prevent stasis.
More than 80% of infections are caused by Staphylococcus aureus17; anaerobic and mixed infections have also been recognized.A beta-lactam penicillin, such as amoxicillin-clavulanate, is the antibiotic of choice. A patient who is systemically unwell should be treated as an inpatient with nafcillin and metronidazole. Methicillin-resistant S aureus must also be considered in patients with comorbid disease, such as diabetes mellitus or intravenous drug use, or in patients residing in an area of substantial incidence of methicillin-resistant S aureus. In those cases, substitute vancomycin or linezolid for nafcillin.18
Continue to: Less commonly...
Less commonly, abscess can form, with the patient presenting as systemically unwell with a fluctuant mass. If the diagnosis is unclear or the patient does not improve, abscess can be confirmed by US. Expedient surgical review and inpatient admission can then be arranged.
Unlike bacterial sialadenitis, causes of viral sialadenitis are often bilateral. Mumps (a paramyxovirus) is the most common viral cause, affecting primarily children < 15 years.19 The parotid glands are most often involved, with inflammation and edema causing significant pain because of increasing intraparotid pressure as expansion of the gland is limited by its tense fibrous capsule. Complications of mumps include orchitis, meningitis, pancreatitis, and oophoritis.
Mumps is highly contagious; it is spread through contact with airborne saliva droplets, with viral entry through the nose or mouth, followed by proliferation in the salivary glands or on surface epithelium of the respiratory tract.7 Diagnosis is confirmed by viral serology. A positive test of serum immunoglobulin M confirms the diagnosis, but this test should not be performed until 3 days after onset of symptoms because a false-negative result is otherwise possible.20 Immunoglobulin G serologic testing can further aid diagnosis; the titer is measured approximately 4 days after onset of symptoms and again 2 to 3 weeks later. A 4-fold rise in titer confirms mumps.
Other viral infections that can cause sialadenitis include Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, coxsackievirus, and influenza. Treatment is supportive: analgesia, hydration, oral hygiene, and rest. Inflammation might take weeks to resolve, but expect complete resolution. For a patient who has significant trismus, poor oral intake, or a potentially threatened airway, inpatient care should be provided.
Recurrent parotitis of childhood is an inflammatory condition that usually affects one, but at times both, parotid glands. It is characterized by episodes of painful swelling. Incidence peaks at 3 to 6 years of age.7 Episodes can be frequent, occurring 1 to 5 times a year and lasting 3 to 7 days—sometimes longer—and usually resolving without treatment.
Continue to: The precise etiology...
The precise etiology of recurrent parotitis of childhood is unclear; possibly, saliva aggregates to form obstructive mucous plugs, thus causing stasis and swelling of the gland. As pressure builds, spontaneous plug extrusion occurs and symptoms resolve, provided infection is not a factor. US demonstrates multiple round, hypoechoic areas consistent with duct dilation, and surrounding infiltration by lymphocytes.1
Supportive care—adequate hydration, gland massage, warm compresses, and sialogogues—are mainstays of treatment. Fever and malaise warrant treatment with oral antibiotics. Sialadenoscopy, which can be considered in children with frequent episodes, can decrease the frequency and severity of episodes.21 The condition usually resolves spontaneously at puberty.
Ask: Does the patient have dry mouth?
In-depth review of xerostomia is beyond the scope of this article. Causes include Sjögren's syndrome, immunoglobulin G4-related sialadenitis, sarcoidosis, radiation therapy, diabetes, chronic infection, and medications—in particular those with anticholinergic effects.
Treatment of xerostomia includes saliva substitutes, sialagogues, and, for oral candidiasis, antifungals. Muscarinic cholinergic stimulators, such as pilocarpine, 5 mg qid have been used with some success22; patients should be advised of potential adverse effects with these agents, including sweating, urinary frequency, flushing, and chills.
CORRESPONDENCE
Shankar Haran, MBBS, ENT Department, Townsville Hospital, 100 Angus Smith Dr, Douglas, Queensland, Australia 4814; [email protected].
1. de Oliveira FA, Duarte EC, Taveira CT, et al. Salivary gland tumor: a review of 599 cases in a Brazilian population. Head Neck Pathol. 2009;3:271-275.
2. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
3. Bova R. A guide to salivary gland disorders. Medicine Today. 2006;7:44-48.
4. Zhang S, Bao R, Bagby J, et al. Fine needle aspiration of salivary glands: 5-year experience from a single academic center. Acta Cytol. 2009;53:375-382.
5. Bova R, Saylor A, Coman WB. Parotidectomy: review of treatment and outcomes. ANZ J Surg. 2004;74:563-568.
6. Sood S, McGurk M, Vaz F. Management of salivary gland tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S142-S149.
7. Mandel L. Salivary gland disorders. Med Clin North Am. 2014;98:1407-1449.
8. Mandel L, Abai S. Diagnosing bulimia nervosa with parotid gland swelling. J Am Dent Assoc. 2004;135:613–616.
9. Lustmann J, Regev E, Melamed Y. Sialolithiasis. A survey on 245 patients and a review of literature. Int J Oral Maxillofac Surg. 1990;19:135–138.
10. Vogl TJ, Al-Nawas B, Beutner D, et al. Updated S2K AWMF guideline for the diagnosis and follow-up of obstructive sialadenitis—relevance for radiologic imaging. Rofo. 2014;186:843-846.
11. Schwarz D, Kabbasch C, Scheer M, et al. Comparative analysis of sialendoscopy, sonography, and CBCT in the detection of sialolithiasis. Laryngoscope. 2015;125:1098–1101.
12. Atienza G, López-Cedrún JL. Management of obstructive salivary disorders by sialendoscopy: a systematic review. Br J Oral Maxillofac Surg. 2015;53:507-519.
13. Escudier MP, Brown JE, Putcha V, et al. Factors influencing the outcome of extracorporeal shock wave lithotripsy in the management of salivary calculi. Laryngoscope. 2010;120:1545-1549.
14. Koch M, Schapher M, Mantsopoulos K, et al. Multimodal treatment in difficult sialolithiasis: Role of extracorporeal shock-wave lithotripsy and intraductal pneumatic lithotripsy. Laryngoscope. 2018;128:E332-E338.
15. McQuone SJ. Acute viral and bacterial infections of the salivary glands. Otolaryngol Clin North Am. 1999;32:793-811.
16. O’Neil C, Sidhu S. Salivary gland disorders. Australian Doctor. 2011;28:19-25.
17. Mandel L. Differentiating acute suppurative parotitis from acute exacerbation of a chronic parotitis: case reports. J Oral Maxillofac Surg. 2008;66:1964-1968.
18. Chow AW. Suppurative parotitis in adults. UpToDate.com. www.uptodate.com/contents/suppurative-parotitis-in-adults. Accessed September 25, 2019.
19. Katz SL, Gershon AA, Hotez PJ. Infectious Diseases of Children. New York, NY: Mosby Year Book; 1998:280-289.
20. Krause CH, Molyneaux PJ, Ho-Yen DO, et al. Comparison of mumps-IgM ELISAs in acute infection. J Clin Virol. 2007;38:153-156.
21. Quenin S, Plouin-Gaudon I, Marchal F, et al. Juvenile recurrent parotitis: sialendoscopic approach. Arch Otolaryngol Head Neck Surg. 2008;134:715-719.
22. Papas AS, Sherrer YS, Charney M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169-177.
1. de Oliveira FA, Duarte EC, Taveira CT, et al. Salivary gland tumor: a review of 599 cases in a Brazilian population. Head Neck Pathol. 2009;3:271-275.
2. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
3. Bova R. A guide to salivary gland disorders. Medicine Today. 2006;7:44-48.
4. Zhang S, Bao R, Bagby J, et al. Fine needle aspiration of salivary glands: 5-year experience from a single academic center. Acta Cytol. 2009;53:375-382.
5. Bova R, Saylor A, Coman WB. Parotidectomy: review of treatment and outcomes. ANZ J Surg. 2004;74:563-568.
6. Sood S, McGurk M, Vaz F. Management of salivary gland tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S142-S149.
7. Mandel L. Salivary gland disorders. Med Clin North Am. 2014;98:1407-1449.
8. Mandel L, Abai S. Diagnosing bulimia nervosa with parotid gland swelling. J Am Dent Assoc. 2004;135:613–616.
9. Lustmann J, Regev E, Melamed Y. Sialolithiasis. A survey on 245 patients and a review of literature. Int J Oral Maxillofac Surg. 1990;19:135–138.
10. Vogl TJ, Al-Nawas B, Beutner D, et al. Updated S2K AWMF guideline for the diagnosis and follow-up of obstructive sialadenitis—relevance for radiologic imaging. Rofo. 2014;186:843-846.
11. Schwarz D, Kabbasch C, Scheer M, et al. Comparative analysis of sialendoscopy, sonography, and CBCT in the detection of sialolithiasis. Laryngoscope. 2015;125:1098–1101.
12. Atienza G, López-Cedrún JL. Management of obstructive salivary disorders by sialendoscopy: a systematic review. Br J Oral Maxillofac Surg. 2015;53:507-519.
13. Escudier MP, Brown JE, Putcha V, et al. Factors influencing the outcome of extracorporeal shock wave lithotripsy in the management of salivary calculi. Laryngoscope. 2010;120:1545-1549.
14. Koch M, Schapher M, Mantsopoulos K, et al. Multimodal treatment in difficult sialolithiasis: Role of extracorporeal shock-wave lithotripsy and intraductal pneumatic lithotripsy. Laryngoscope. 2018;128:E332-E338.
15. McQuone SJ. Acute viral and bacterial infections of the salivary glands. Otolaryngol Clin North Am. 1999;32:793-811.
16. O’Neil C, Sidhu S. Salivary gland disorders. Australian Doctor. 2011;28:19-25.
17. Mandel L. Differentiating acute suppurative parotitis from acute exacerbation of a chronic parotitis: case reports. J Oral Maxillofac Surg. 2008;66:1964-1968.
18. Chow AW. Suppurative parotitis in adults. UpToDate.com. www.uptodate.com/contents/suppurative-parotitis-in-adults. Accessed September 25, 2019.
19. Katz SL, Gershon AA, Hotez PJ. Infectious Diseases of Children. New York, NY: Mosby Year Book; 1998:280-289.
20. Krause CH, Molyneaux PJ, Ho-Yen DO, et al. Comparison of mumps-IgM ELISAs in acute infection. J Clin Virol. 2007;38:153-156.
21. Quenin S, Plouin-Gaudon I, Marchal F, et al. Juvenile recurrent parotitis: sialendoscopic approach. Arch Otolaryngol Head Neck Surg. 2008;134:715-719.
22. Papas AS, Sherrer YS, Charney M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169-177.
PRACTICE RECOMMENDATIONS
› Use ultrasonography for initial imaging of a salivary gland. A
› Refer patients with the following findings for further specialty evaluation: abscess, inflammation unresponsive to medical care, recurrent or chronic symptoms, suspected neoplasm (for excision), and suspected sialolithiasis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Painful ulcers on gingiva, tongue, and buccal mucosa
A 29-year-old man with no prior history of mouth sores abruptly developed many 1- to 1.5-mm blisters on the gingiva (FIGURE 1A),tongue (FIGURE 1B), and buccal mucosa (FIGURE 1C), which evolved into small erosions accompanied by a low-grade fever 5 days prior to presentation. The patient had no history of any dermatologic conditions or systemic illnesses and was taking no medication.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute primary herpetic gingivostomatitis
Herpes simplex virus (HSV) is the causative agent for acute primary herpetic gingivostomatitis.1 HSV-1 is primarily responsible for oral mucosal infections, while HSV-2 is implicated in most genital and cutaneous lower body lesions.1 Herpetic gingivostomatitis often presents as a sudden vesiculoulcerative eruption anywhere in the mouth, including the perioral skin, vermillion border, gingiva, tongue, or buccal mucosa.2 Associated symptoms include malaise, headache, fever, and cervical lymphadenopathy; however, most occurrences are subclinical or asymptomatic.2
A diagnosis that’s more common in children. Primary HSV occurs in people who have not previously been exposed to the virus. While it is an infection that classically presents in childhood, it is not limited to this group. Manifestations often are more severe in adults.1
Following an incubation period of a few days to 3 weeks, the primary infection typically lasts 10 to 14 days.1,2 Recurrence is highly variable and generally less severe than primary infection, with grouped vesicles often recurring in the same spot with each recurrence on the vermillion border of the lip. Triggers for reactivation include immunosuppression, pregnancy, fever, UV radiation, or trauma.1,2
Differential includes other conditions with mucosal lesions
Acute herpetic gingivostomatitis must be distinguished from other disease processes that cause ulcerative mucosal lesions.
Aphthous stomatitis (canker sores) is the most common ulcerative disease of the oral mucosa.3 It presents as painful, punched-out, shallow ulcers with a yellowish gray pseudomembranous center and surrounding erythema.3 No definitive etiology has been established; however, aphthae often occur after trauma.
Continue to: Herpangina...
Herpangina is caused by coxsackie A virus and primarily is seen in infants and children younger than 5.4 The papulovesicular lesions primarily affect the posterior oral cavity, including the soft palate, anterior tonsillar pillars, and uvula.4
Allergic contact dermatitis is precipitated by contact with an allergen and presents with pain or pruritus. Lesions are erythematous with vesicles, erosions, ulcers, or hyperkeratosis that gradually resolve after withdrawal of the causative allergen.5
Pemphigus vulgaris. Oral ulcerations of the buccal mucosa and gingiva are the first manifestation of pemphigus vulgaris in the majority of patients, with skin blisters occurring months to years later over areas exposed to frictional stress.6 Skin sloughs may be seen in response to frictional stress (Nikolsky sign).6
The new Dx gold standard is PCR
Acute herpetic gingivostomatitis usually is diagnosed by history and hallmark clinical signs and symptoms.1 In this case, our patient presented with a sudden eruption of painful blisters on multiple areas of the oral mucosa associated with fever. The diagnosis can be confirmed by viral culture, serology with anti-HSV IgM and IgG, Tzanck preparation, immunofluorescence, and polymerase chain reaction (PCR).1 Viral culture has been the gold standard for mucosal HSV diagnosis; however, PCR is emerging as the new gold standard because of its unrivaled sensitivity, specificity, and rapid turnaround time.7,8 Specimens for PCR are submitted using a swab of infected cells placed in the same viral transport medium used for HSV cultures.
Our patient’s culture was positive for HSV-1.
Continue to: Prompt use of antivirals is key
Prompt use of antivirals is key
Treatment of acute HSV gingivostomatitis involves symptomatic management with topical anesthetics, oral analgesics, and normal saline rinses.1 Acyclovir is an established therapy; however, it has poor bioavailability and gastrointestinal absorption.1 Valacyclovir has improved bioavailability and is well tolerated.1 For primary herpes gingivostomatitis, we favor 1 g twice daily for 7 days.1 Our patient responded well to this valacyclovir regimen and healed completely in 1 week.
CORRESPONDENCE
Robert T. Brodell, MD, 2500 N State St, Jackson, MS 39216; [email protected]
1. Ajar AH, Chauvin PJ. Acute herpetic gingivostomatitis in adults: a review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68:247-251.
2. George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: report of a case. J Int Oral Health. 2014;6:99-102.
3. Akintoye SO, Greenburg MS. Recurrent aphthous stomatitis. Dent Clin N Am. 2014;58:281-297.
4. Scott LA, Stone MS. Viral exanthems. Dermatol Online J. 2003;9:4.
5. Feller L, Wood NH, Khammissa RA, et al. Review: allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-565.
6. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain C, et al. Oral manifestations of pemphigus vulgaris: clinical presentation, differential diagnosis and management. J Clin Exp Dermatol Res. 2010;1:112.
7. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
8. Centers for Disease Control and Prevention. Genital HSV infections. www.cdc.gov/std/tg2015/herpes.htm. Updated June 4, 2015. Accessed September 26, 2019.
A 29-year-old man with no prior history of mouth sores abruptly developed many 1- to 1.5-mm blisters on the gingiva (FIGURE 1A),tongue (FIGURE 1B), and buccal mucosa (FIGURE 1C), which evolved into small erosions accompanied by a low-grade fever 5 days prior to presentation. The patient had no history of any dermatologic conditions or systemic illnesses and was taking no medication.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute primary herpetic gingivostomatitis
Herpes simplex virus (HSV) is the causative agent for acute primary herpetic gingivostomatitis.1 HSV-1 is primarily responsible for oral mucosal infections, while HSV-2 is implicated in most genital and cutaneous lower body lesions.1 Herpetic gingivostomatitis often presents as a sudden vesiculoulcerative eruption anywhere in the mouth, including the perioral skin, vermillion border, gingiva, tongue, or buccal mucosa.2 Associated symptoms include malaise, headache, fever, and cervical lymphadenopathy; however, most occurrences are subclinical or asymptomatic.2
A diagnosis that’s more common in children. Primary HSV occurs in people who have not previously been exposed to the virus. While it is an infection that classically presents in childhood, it is not limited to this group. Manifestations often are more severe in adults.1
Following an incubation period of a few days to 3 weeks, the primary infection typically lasts 10 to 14 days.1,2 Recurrence is highly variable and generally less severe than primary infection, with grouped vesicles often recurring in the same spot with each recurrence on the vermillion border of the lip. Triggers for reactivation include immunosuppression, pregnancy, fever, UV radiation, or trauma.1,2
Differential includes other conditions with mucosal lesions
Acute herpetic gingivostomatitis must be distinguished from other disease processes that cause ulcerative mucosal lesions.
Aphthous stomatitis (canker sores) is the most common ulcerative disease of the oral mucosa.3 It presents as painful, punched-out, shallow ulcers with a yellowish gray pseudomembranous center and surrounding erythema.3 No definitive etiology has been established; however, aphthae often occur after trauma.
Continue to: Herpangina...
Herpangina is caused by coxsackie A virus and primarily is seen in infants and children younger than 5.4 The papulovesicular lesions primarily affect the posterior oral cavity, including the soft palate, anterior tonsillar pillars, and uvula.4
Allergic contact dermatitis is precipitated by contact with an allergen and presents with pain or pruritus. Lesions are erythematous with vesicles, erosions, ulcers, or hyperkeratosis that gradually resolve after withdrawal of the causative allergen.5
Pemphigus vulgaris. Oral ulcerations of the buccal mucosa and gingiva are the first manifestation of pemphigus vulgaris in the majority of patients, with skin blisters occurring months to years later over areas exposed to frictional stress.6 Skin sloughs may be seen in response to frictional stress (Nikolsky sign).6
The new Dx gold standard is PCR
Acute herpetic gingivostomatitis usually is diagnosed by history and hallmark clinical signs and symptoms.1 In this case, our patient presented with a sudden eruption of painful blisters on multiple areas of the oral mucosa associated with fever. The diagnosis can be confirmed by viral culture, serology with anti-HSV IgM and IgG, Tzanck preparation, immunofluorescence, and polymerase chain reaction (PCR).1 Viral culture has been the gold standard for mucosal HSV diagnosis; however, PCR is emerging as the new gold standard because of its unrivaled sensitivity, specificity, and rapid turnaround time.7,8 Specimens for PCR are submitted using a swab of infected cells placed in the same viral transport medium used for HSV cultures.
Our patient’s culture was positive for HSV-1.
Continue to: Prompt use of antivirals is key
Prompt use of antivirals is key
Treatment of acute HSV gingivostomatitis involves symptomatic management with topical anesthetics, oral analgesics, and normal saline rinses.1 Acyclovir is an established therapy; however, it has poor bioavailability and gastrointestinal absorption.1 Valacyclovir has improved bioavailability and is well tolerated.1 For primary herpes gingivostomatitis, we favor 1 g twice daily for 7 days.1 Our patient responded well to this valacyclovir regimen and healed completely in 1 week.
CORRESPONDENCE
Robert T. Brodell, MD, 2500 N State St, Jackson, MS 39216; [email protected]
A 29-year-old man with no prior history of mouth sores abruptly developed many 1- to 1.5-mm blisters on the gingiva (FIGURE 1A),tongue (FIGURE 1B), and buccal mucosa (FIGURE 1C), which evolved into small erosions accompanied by a low-grade fever 5 days prior to presentation. The patient had no history of any dermatologic conditions or systemic illnesses and was taking no medication.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute primary herpetic gingivostomatitis
Herpes simplex virus (HSV) is the causative agent for acute primary herpetic gingivostomatitis.1 HSV-1 is primarily responsible for oral mucosal infections, while HSV-2 is implicated in most genital and cutaneous lower body lesions.1 Herpetic gingivostomatitis often presents as a sudden vesiculoulcerative eruption anywhere in the mouth, including the perioral skin, vermillion border, gingiva, tongue, or buccal mucosa.2 Associated symptoms include malaise, headache, fever, and cervical lymphadenopathy; however, most occurrences are subclinical or asymptomatic.2
A diagnosis that’s more common in children. Primary HSV occurs in people who have not previously been exposed to the virus. While it is an infection that classically presents in childhood, it is not limited to this group. Manifestations often are more severe in adults.1
Following an incubation period of a few days to 3 weeks, the primary infection typically lasts 10 to 14 days.1,2 Recurrence is highly variable and generally less severe than primary infection, with grouped vesicles often recurring in the same spot with each recurrence on the vermillion border of the lip. Triggers for reactivation include immunosuppression, pregnancy, fever, UV radiation, or trauma.1,2
Differential includes other conditions with mucosal lesions
Acute herpetic gingivostomatitis must be distinguished from other disease processes that cause ulcerative mucosal lesions.
Aphthous stomatitis (canker sores) is the most common ulcerative disease of the oral mucosa.3 It presents as painful, punched-out, shallow ulcers with a yellowish gray pseudomembranous center and surrounding erythema.3 No definitive etiology has been established; however, aphthae often occur after trauma.
Continue to: Herpangina...
Herpangina is caused by coxsackie A virus and primarily is seen in infants and children younger than 5.4 The papulovesicular lesions primarily affect the posterior oral cavity, including the soft palate, anterior tonsillar pillars, and uvula.4
Allergic contact dermatitis is precipitated by contact with an allergen and presents with pain or pruritus. Lesions are erythematous with vesicles, erosions, ulcers, or hyperkeratosis that gradually resolve after withdrawal of the causative allergen.5
Pemphigus vulgaris. Oral ulcerations of the buccal mucosa and gingiva are the first manifestation of pemphigus vulgaris in the majority of patients, with skin blisters occurring months to years later over areas exposed to frictional stress.6 Skin sloughs may be seen in response to frictional stress (Nikolsky sign).6
The new Dx gold standard is PCR
Acute herpetic gingivostomatitis usually is diagnosed by history and hallmark clinical signs and symptoms.1 In this case, our patient presented with a sudden eruption of painful blisters on multiple areas of the oral mucosa associated with fever. The diagnosis can be confirmed by viral culture, serology with anti-HSV IgM and IgG, Tzanck preparation, immunofluorescence, and polymerase chain reaction (PCR).1 Viral culture has been the gold standard for mucosal HSV diagnosis; however, PCR is emerging as the new gold standard because of its unrivaled sensitivity, specificity, and rapid turnaround time.7,8 Specimens for PCR are submitted using a swab of infected cells placed in the same viral transport medium used for HSV cultures.
Our patient’s culture was positive for HSV-1.
Continue to: Prompt use of antivirals is key
Prompt use of antivirals is key
Treatment of acute HSV gingivostomatitis involves symptomatic management with topical anesthetics, oral analgesics, and normal saline rinses.1 Acyclovir is an established therapy; however, it has poor bioavailability and gastrointestinal absorption.1 Valacyclovir has improved bioavailability and is well tolerated.1 For primary herpes gingivostomatitis, we favor 1 g twice daily for 7 days.1 Our patient responded well to this valacyclovir regimen and healed completely in 1 week.
CORRESPONDENCE
Robert T. Brodell, MD, 2500 N State St, Jackson, MS 39216; [email protected]
1. Ajar AH, Chauvin PJ. Acute herpetic gingivostomatitis in adults: a review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68:247-251.
2. George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: report of a case. J Int Oral Health. 2014;6:99-102.
3. Akintoye SO, Greenburg MS. Recurrent aphthous stomatitis. Dent Clin N Am. 2014;58:281-297.
4. Scott LA, Stone MS. Viral exanthems. Dermatol Online J. 2003;9:4.
5. Feller L, Wood NH, Khammissa RA, et al. Review: allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-565.
6. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain C, et al. Oral manifestations of pemphigus vulgaris: clinical presentation, differential diagnosis and management. J Clin Exp Dermatol Res. 2010;1:112.
7. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
8. Centers for Disease Control and Prevention. Genital HSV infections. www.cdc.gov/std/tg2015/herpes.htm. Updated June 4, 2015. Accessed September 26, 2019.
1. Ajar AH, Chauvin PJ. Acute herpetic gingivostomatitis in adults: a review of 13 cases, including diagnosis and management. J Can Dent Assoc. 2002;68:247-251.
2. George AK, Anil S. Acute herpetic gingivostomatitis associated with herpes simplex virus 2: report of a case. J Int Oral Health. 2014;6:99-102.
3. Akintoye SO, Greenburg MS. Recurrent aphthous stomatitis. Dent Clin N Am. 2014;58:281-297.
4. Scott LA, Stone MS. Viral exanthems. Dermatol Online J. 2003;9:4.
5. Feller L, Wood NH, Khammissa RA, et al. Review: allergic contact stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123:559-565.
6. Bascones-Martinez A, Munoz-Corcuera M, Bascones-Ilundain C, et al. Oral manifestations of pemphigus vulgaris: clinical presentation, differential diagnosis and management. J Clin Exp Dermatol Res. 2010;1:112.
7. LeGoff J, Péré H, Bélec L. Diagnosis of genital herpes simplex virus infection in the clinical laboratory. Virol J. 2014;11:83.
8. Centers for Disease Control and Prevention. Genital HSV infections. www.cdc.gov/std/tg2015/herpes.htm. Updated June 4, 2015. Accessed September 26, 2019.