User login
Is cannabis gaining acceptance as a treatment for neuropathic pain?
, a recent debate on the topic suggests. During the debate, one expert argued for, and another against, there being sufficient evidence for the use of cannabis to treat neuropathic pain, but in the end, they agreed that some patients do benefit.
The discussion took place at the Congress of the European Academy of Neurology (EAN) 2020, which transitioned to a virtual online meeting because of the COVID-19 pandemic.
The cannabis plant has 460 constituents. The two main components are tetrahydrocannabinol (THC) and cannabidiol (CBD). It can be consumed by swallowing oil extracts, by the sublingual route, or by smoking or eating the plant. Cannabis medications already in use include oral THC (nabilone, dronabinol) and an oral mucosal spray, nabiximols (Sativex).
Arguing that therapeutic cannabis is helpful for neuropathic pain, Elon Eisenberg, MD, professor of neurology and pain medicine, Israel Institute of Technology, Haifa, cited a number of encouraging randomized, controlled trials and meta-analyses of studies on the subject.
Opioid substitute
Dr. Eisenberg discussed three relevant articles. One was a 2016 viewpoint article published in JAMA that concluded that “cannabis seems to be a substitute, a rather good one, for opioids,” said Dr. Eisenberg.
A “comprehensive” 440-page review, published by the National Academies Press in 2017, evaluated the evidence to that point and “came to the conclusion there is substantial evidence that cannabis is an effective treatment for chronic pain in adults,” said Dr. Eisenberg.
And a 2018 position paper from the European Pain Federation determined that “the quantity and quality of evidence is such that cannabis-based medicines may be reasonably considered for chronic neuropathic pain,” he said.
He noted that the most recent results from an Israeli prospective cohort registry study that is following more than 851 patients who are taking cannabis over 1 year are positive. Analyses show a steady reduction in pain intensity and improvements in catastrophizing and disability. Importantly, he said, participants are using fewer opioids. However, about 40% of patients in that registry study experienced some adverse event, although most were not serious, said Dr. Eisenberg.
Not convinced
Arguing on the other side – that therapeutic cannabis is not helpful for neuropathic pain – was Nadine Attal, MD, PhD, professor of therapeutics and pain at the University Versailles Saint Quentin, France. She questioned the quality of some of the research to date and stressed that studies should consider neuropathic pain as a primary outcome – not spasticity or pain in general. They should also be double-blind, randomized, and placebo controlled, she said.
In addition, she said these studies should enroll at least 10 patients per group and should continue for 3 weeks or longer.
Dr. Attal wondered which of the many plant derivatives (phytocannabinoids) are used in cannabis studies.
She discussed four meta-analyses or reviews on the topic, some of which she said are “heterogeneous” and don’t provide convincing evidence for cannabis use in neuropathic pain.
For example, one review examined only marijuana, and all studies in it were short term. One of the studies in this review was of spasticity. Another review included two studies of cancer pain, and the most positive study in NP used short-term inhaled THC.
“There is no evidence to date that cannabinoids, including nabiximols or oral THC, administered for at least 3 weeks are more effective than placebo in neuropathic pain,” she concluded.
Some responders
However, Dr. Attal acknowledged that cannabis might be effective for some patients. In her experience, which has been borne out by some observational studies, patients with paroxysmal pain, or sudden stabbing pain, seem to get more relief from cannabis. “It’s absolutely possible that there’s a subgroup of symptoms or a subgroup of patients with specific symptoms who are much better responders to cannabis than others,” she said.
Asked if patients experience increased pain after withdrawing from cannabis, Dr. Eisenberg said he has observed that many patients stop taking cannabis when they start feeling better, but he hasn’t seen severe withdrawal symptoms.
However, there are other concerns related to cannabis use, said Dr. Eisenberg. A major concern regards driving a vehicle. In Israel, getting behind the wheel is prohibited within 6 hours of using cannabis.
But Dr. Eisenberg pointed out that published data on the safety of cannabis and driving were based on recreational users. “We need to keep in mind that recreational users typically use other substances, so we’re not sure the data is accurate,” he said.
There are increasing reports of stroke, transient ischemic attack, and MI among cannabis users. This is especially concerning because many of these cases involve young male adults who have no risk factors, said Dr. Eisenberg.
One conference delegate asked whether legal issues make it difficult to properly investigate cannabis in large studies. Dr. Eisenberg noted that legal concerns may help explain why there have not been any new randomized, controlled trials for about 2 years. “In the U.S., you can’t do clinical trials; cannabis is still regarded as schedule I substance,” he said.
Some physicians “are reluctant to deal with cannabis unless they get better data,” he said. “Doing research on cannabis seems to be somehow out of the mainstream.” Moreover, the research is difficult to carry out, owing to the complexity of the cannabis plant, which has many constituents. Perhaps it’s a matter of identifying and adding particular components to better demonstrate reduced pain, said Dr. Eisenberg.
Another complicating factor is that bioavailability differs considerably from one patient to another, “sometimes even by 10-fold,” he said.
Dr. Attal’s group will be starting a study next January that will enroll a large sample of patients with neuropathic pain or spasticity. In that study, cannabis will be dispensed through pharmacies and primary care. The aim of the study is “to see how it works in a real-life setting,” she said
Those participating in the virtual session were asked to vote on which side they agreed with. About 57% voted in favor of cannabis use, 14% voted against, and 28% had no opinion.
Dr. Eisenberg has received research grants from Rafa Laboratories, Saga Medical Ltd., Israel Pain Association, and Teva Israel. Dr. Attal has received support from Merck Sharp & Dohme, Sanofi, Ipsen, Novartis, Aptinyx, Air Liquide, Lilly, and Grunenthal.
A version of this article originally appeared on Medscape.com.
, a recent debate on the topic suggests. During the debate, one expert argued for, and another against, there being sufficient evidence for the use of cannabis to treat neuropathic pain, but in the end, they agreed that some patients do benefit.
The discussion took place at the Congress of the European Academy of Neurology (EAN) 2020, which transitioned to a virtual online meeting because of the COVID-19 pandemic.
The cannabis plant has 460 constituents. The two main components are tetrahydrocannabinol (THC) and cannabidiol (CBD). It can be consumed by swallowing oil extracts, by the sublingual route, or by smoking or eating the plant. Cannabis medications already in use include oral THC (nabilone, dronabinol) and an oral mucosal spray, nabiximols (Sativex).
Arguing that therapeutic cannabis is helpful for neuropathic pain, Elon Eisenberg, MD, professor of neurology and pain medicine, Israel Institute of Technology, Haifa, cited a number of encouraging randomized, controlled trials and meta-analyses of studies on the subject.
Opioid substitute
Dr. Eisenberg discussed three relevant articles. One was a 2016 viewpoint article published in JAMA that concluded that “cannabis seems to be a substitute, a rather good one, for opioids,” said Dr. Eisenberg.
A “comprehensive” 440-page review, published by the National Academies Press in 2017, evaluated the evidence to that point and “came to the conclusion there is substantial evidence that cannabis is an effective treatment for chronic pain in adults,” said Dr. Eisenberg.
And a 2018 position paper from the European Pain Federation determined that “the quantity and quality of evidence is such that cannabis-based medicines may be reasonably considered for chronic neuropathic pain,” he said.
He noted that the most recent results from an Israeli prospective cohort registry study that is following more than 851 patients who are taking cannabis over 1 year are positive. Analyses show a steady reduction in pain intensity and improvements in catastrophizing and disability. Importantly, he said, participants are using fewer opioids. However, about 40% of patients in that registry study experienced some adverse event, although most were not serious, said Dr. Eisenberg.
Not convinced
Arguing on the other side – that therapeutic cannabis is not helpful for neuropathic pain – was Nadine Attal, MD, PhD, professor of therapeutics and pain at the University Versailles Saint Quentin, France. She questioned the quality of some of the research to date and stressed that studies should consider neuropathic pain as a primary outcome – not spasticity or pain in general. They should also be double-blind, randomized, and placebo controlled, she said.
In addition, she said these studies should enroll at least 10 patients per group and should continue for 3 weeks or longer.
Dr. Attal wondered which of the many plant derivatives (phytocannabinoids) are used in cannabis studies.
She discussed four meta-analyses or reviews on the topic, some of which she said are “heterogeneous” and don’t provide convincing evidence for cannabis use in neuropathic pain.
For example, one review examined only marijuana, and all studies in it were short term. One of the studies in this review was of spasticity. Another review included two studies of cancer pain, and the most positive study in NP used short-term inhaled THC.
“There is no evidence to date that cannabinoids, including nabiximols or oral THC, administered for at least 3 weeks are more effective than placebo in neuropathic pain,” she concluded.
Some responders
However, Dr. Attal acknowledged that cannabis might be effective for some patients. In her experience, which has been borne out by some observational studies, patients with paroxysmal pain, or sudden stabbing pain, seem to get more relief from cannabis. “It’s absolutely possible that there’s a subgroup of symptoms or a subgroup of patients with specific symptoms who are much better responders to cannabis than others,” she said.
Asked if patients experience increased pain after withdrawing from cannabis, Dr. Eisenberg said he has observed that many patients stop taking cannabis when they start feeling better, but he hasn’t seen severe withdrawal symptoms.
However, there are other concerns related to cannabis use, said Dr. Eisenberg. A major concern regards driving a vehicle. In Israel, getting behind the wheel is prohibited within 6 hours of using cannabis.
But Dr. Eisenberg pointed out that published data on the safety of cannabis and driving were based on recreational users. “We need to keep in mind that recreational users typically use other substances, so we’re not sure the data is accurate,” he said.
There are increasing reports of stroke, transient ischemic attack, and MI among cannabis users. This is especially concerning because many of these cases involve young male adults who have no risk factors, said Dr. Eisenberg.
One conference delegate asked whether legal issues make it difficult to properly investigate cannabis in large studies. Dr. Eisenberg noted that legal concerns may help explain why there have not been any new randomized, controlled trials for about 2 years. “In the U.S., you can’t do clinical trials; cannabis is still regarded as schedule I substance,” he said.
Some physicians “are reluctant to deal with cannabis unless they get better data,” he said. “Doing research on cannabis seems to be somehow out of the mainstream.” Moreover, the research is difficult to carry out, owing to the complexity of the cannabis plant, which has many constituents. Perhaps it’s a matter of identifying and adding particular components to better demonstrate reduced pain, said Dr. Eisenberg.
Another complicating factor is that bioavailability differs considerably from one patient to another, “sometimes even by 10-fold,” he said.
Dr. Attal’s group will be starting a study next January that will enroll a large sample of patients with neuropathic pain or spasticity. In that study, cannabis will be dispensed through pharmacies and primary care. The aim of the study is “to see how it works in a real-life setting,” she said
Those participating in the virtual session were asked to vote on which side they agreed with. About 57% voted in favor of cannabis use, 14% voted against, and 28% had no opinion.
Dr. Eisenberg has received research grants from Rafa Laboratories, Saga Medical Ltd., Israel Pain Association, and Teva Israel. Dr. Attal has received support from Merck Sharp & Dohme, Sanofi, Ipsen, Novartis, Aptinyx, Air Liquide, Lilly, and Grunenthal.
A version of this article originally appeared on Medscape.com.
, a recent debate on the topic suggests. During the debate, one expert argued for, and another against, there being sufficient evidence for the use of cannabis to treat neuropathic pain, but in the end, they agreed that some patients do benefit.
The discussion took place at the Congress of the European Academy of Neurology (EAN) 2020, which transitioned to a virtual online meeting because of the COVID-19 pandemic.
The cannabis plant has 460 constituents. The two main components are tetrahydrocannabinol (THC) and cannabidiol (CBD). It can be consumed by swallowing oil extracts, by the sublingual route, or by smoking or eating the plant. Cannabis medications already in use include oral THC (nabilone, dronabinol) and an oral mucosal spray, nabiximols (Sativex).
Arguing that therapeutic cannabis is helpful for neuropathic pain, Elon Eisenberg, MD, professor of neurology and pain medicine, Israel Institute of Technology, Haifa, cited a number of encouraging randomized, controlled trials and meta-analyses of studies on the subject.
Opioid substitute
Dr. Eisenberg discussed three relevant articles. One was a 2016 viewpoint article published in JAMA that concluded that “cannabis seems to be a substitute, a rather good one, for opioids,” said Dr. Eisenberg.
A “comprehensive” 440-page review, published by the National Academies Press in 2017, evaluated the evidence to that point and “came to the conclusion there is substantial evidence that cannabis is an effective treatment for chronic pain in adults,” said Dr. Eisenberg.
And a 2018 position paper from the European Pain Federation determined that “the quantity and quality of evidence is such that cannabis-based medicines may be reasonably considered for chronic neuropathic pain,” he said.
He noted that the most recent results from an Israeli prospective cohort registry study that is following more than 851 patients who are taking cannabis over 1 year are positive. Analyses show a steady reduction in pain intensity and improvements in catastrophizing and disability. Importantly, he said, participants are using fewer opioids. However, about 40% of patients in that registry study experienced some adverse event, although most were not serious, said Dr. Eisenberg.
Not convinced
Arguing on the other side – that therapeutic cannabis is not helpful for neuropathic pain – was Nadine Attal, MD, PhD, professor of therapeutics and pain at the University Versailles Saint Quentin, France. She questioned the quality of some of the research to date and stressed that studies should consider neuropathic pain as a primary outcome – not spasticity or pain in general. They should also be double-blind, randomized, and placebo controlled, she said.
In addition, she said these studies should enroll at least 10 patients per group and should continue for 3 weeks or longer.
Dr. Attal wondered which of the many plant derivatives (phytocannabinoids) are used in cannabis studies.
She discussed four meta-analyses or reviews on the topic, some of which she said are “heterogeneous” and don’t provide convincing evidence for cannabis use in neuropathic pain.
For example, one review examined only marijuana, and all studies in it were short term. One of the studies in this review was of spasticity. Another review included two studies of cancer pain, and the most positive study in NP used short-term inhaled THC.
“There is no evidence to date that cannabinoids, including nabiximols or oral THC, administered for at least 3 weeks are more effective than placebo in neuropathic pain,” she concluded.
Some responders
However, Dr. Attal acknowledged that cannabis might be effective for some patients. In her experience, which has been borne out by some observational studies, patients with paroxysmal pain, or sudden stabbing pain, seem to get more relief from cannabis. “It’s absolutely possible that there’s a subgroup of symptoms or a subgroup of patients with specific symptoms who are much better responders to cannabis than others,” she said.
Asked if patients experience increased pain after withdrawing from cannabis, Dr. Eisenberg said he has observed that many patients stop taking cannabis when they start feeling better, but he hasn’t seen severe withdrawal symptoms.
However, there are other concerns related to cannabis use, said Dr. Eisenberg. A major concern regards driving a vehicle. In Israel, getting behind the wheel is prohibited within 6 hours of using cannabis.
But Dr. Eisenberg pointed out that published data on the safety of cannabis and driving were based on recreational users. “We need to keep in mind that recreational users typically use other substances, so we’re not sure the data is accurate,” he said.
There are increasing reports of stroke, transient ischemic attack, and MI among cannabis users. This is especially concerning because many of these cases involve young male adults who have no risk factors, said Dr. Eisenberg.
One conference delegate asked whether legal issues make it difficult to properly investigate cannabis in large studies. Dr. Eisenberg noted that legal concerns may help explain why there have not been any new randomized, controlled trials for about 2 years. “In the U.S., you can’t do clinical trials; cannabis is still regarded as schedule I substance,” he said.
Some physicians “are reluctant to deal with cannabis unless they get better data,” he said. “Doing research on cannabis seems to be somehow out of the mainstream.” Moreover, the research is difficult to carry out, owing to the complexity of the cannabis plant, which has many constituents. Perhaps it’s a matter of identifying and adding particular components to better demonstrate reduced pain, said Dr. Eisenberg.
Another complicating factor is that bioavailability differs considerably from one patient to another, “sometimes even by 10-fold,” he said.
Dr. Attal’s group will be starting a study next January that will enroll a large sample of patients with neuropathic pain or spasticity. In that study, cannabis will be dispensed through pharmacies and primary care. The aim of the study is “to see how it works in a real-life setting,” she said
Those participating in the virtual session were asked to vote on which side they agreed with. About 57% voted in favor of cannabis use, 14% voted against, and 28% had no opinion.
Dr. Eisenberg has received research grants from Rafa Laboratories, Saga Medical Ltd., Israel Pain Association, and Teva Israel. Dr. Attal has received support from Merck Sharp & Dohme, Sanofi, Ipsen, Novartis, Aptinyx, Air Liquide, Lilly, and Grunenthal.
A version of this article originally appeared on Medscape.com.
FROM EAN 2020
Unacceptable RA pain may drop with TNFi treatment but still lingers in many patients
according to findings from 21 months of follow-up in a post hoc analysis of data from the randomized, controlled Swedish Farmacotherapy (SWEFOT) trial.
Although RA patients who took biologic combination therapy had 32% lower risk for unacceptable pain (rated at >40 mm on a 0- to 100-mm visual analog scale) at 21 months, they still had no difference from patients taking triple therapy in the rate of pain described as refractory, or unacceptable despite inflammation control (C-reactive protein <10 mg/L).
While these results lend “some support to a better effect on long-term pain for the biological treatment, compared with triple therapy ... our findings are also in line with insufficient effects of current treatment strategies to prevent development of inflammation-independent pain components, warranting early alternative treatment approaches in affected patients,” Tor Olofsson, MD, PhD, of Lund (Sweden) University, and colleagues wrote in Arthritis Care & Research.
The pain outcomes analyzed in this post hoc study were all secondary outcomes of the original open-label SWEFOT trial, which during 2002-2005 enrolled 258 RA patients with less than a year of symptoms who did not reach low disease activity (28-joint Disease Activity Score ≤3.2) after 3 months of methotrexate and randomized them to an addition of either infliximab (3 mg/kg rounded up to nearest 100-mg increment) or sulfasalazine 1,000 mg twice daily plus hydroxychloroquine 400 mg once daily.
Overall, 90 of 128 patients in the infliximab group and 74 of 130 in the triple-therapy group continued the protocol until the 21-month follow-up. Patients in the infliximab group had a significantly lower area under the curve for visual analog scale for pain, most of which was accounted for during months 9-21. The percentage of patients in the infliximab group with unacceptable pain also dropped significantly from 57% at randomization to 32% at 21 months, while no difference was seen for triple therapy patients, of whom 45% had unacceptable pain at 21 months.
While patients in the infliximab group had a significantly lower risk of unacceptable pain without inflammatory control at 21 months, neither treatment arm showed a within-group difference in refractory pain from randomization to the 21-month follow-up.
Nearly one-third of patients overall still reported unacceptable pain 21 months after addition of either infliximab or sulfasalazine plus hydroxychloroquine. And at that time point, refractory pain constituted 82% of all unacceptable pain. “Notably, this pattern – with a domination of refractory pain – was evident already 3 months after starting combination therapy,” Dr. Olofsson and colleagues wrote.
The original SWEFOT study was supported in part by a grant from the Swedish Rheumatism Association, and in part by an annual unrestricted grant from Schering-Plough Sweden (now Merck Sharp & Dohme). The post hoc analysis was supported by Lund University and the Kockska Foundation, the Swedish Research Council, and the Stockholm County Council. Two authors disclosed financial relationships with multiple pharmaceutical companies.
SOURCE: Olofsson T et al. Arthritis Care Res. 2020 May 20. doi: 10.1002/acr.24264.
according to findings from 21 months of follow-up in a post hoc analysis of data from the randomized, controlled Swedish Farmacotherapy (SWEFOT) trial.
Although RA patients who took biologic combination therapy had 32% lower risk for unacceptable pain (rated at >40 mm on a 0- to 100-mm visual analog scale) at 21 months, they still had no difference from patients taking triple therapy in the rate of pain described as refractory, or unacceptable despite inflammation control (C-reactive protein <10 mg/L).
While these results lend “some support to a better effect on long-term pain for the biological treatment, compared with triple therapy ... our findings are also in line with insufficient effects of current treatment strategies to prevent development of inflammation-independent pain components, warranting early alternative treatment approaches in affected patients,” Tor Olofsson, MD, PhD, of Lund (Sweden) University, and colleagues wrote in Arthritis Care & Research.
The pain outcomes analyzed in this post hoc study were all secondary outcomes of the original open-label SWEFOT trial, which during 2002-2005 enrolled 258 RA patients with less than a year of symptoms who did not reach low disease activity (28-joint Disease Activity Score ≤3.2) after 3 months of methotrexate and randomized them to an addition of either infliximab (3 mg/kg rounded up to nearest 100-mg increment) or sulfasalazine 1,000 mg twice daily plus hydroxychloroquine 400 mg once daily.
Overall, 90 of 128 patients in the infliximab group and 74 of 130 in the triple-therapy group continued the protocol until the 21-month follow-up. Patients in the infliximab group had a significantly lower area under the curve for visual analog scale for pain, most of which was accounted for during months 9-21. The percentage of patients in the infliximab group with unacceptable pain also dropped significantly from 57% at randomization to 32% at 21 months, while no difference was seen for triple therapy patients, of whom 45% had unacceptable pain at 21 months.
While patients in the infliximab group had a significantly lower risk of unacceptable pain without inflammatory control at 21 months, neither treatment arm showed a within-group difference in refractory pain from randomization to the 21-month follow-up.
Nearly one-third of patients overall still reported unacceptable pain 21 months after addition of either infliximab or sulfasalazine plus hydroxychloroquine. And at that time point, refractory pain constituted 82% of all unacceptable pain. “Notably, this pattern – with a domination of refractory pain – was evident already 3 months after starting combination therapy,” Dr. Olofsson and colleagues wrote.
The original SWEFOT study was supported in part by a grant from the Swedish Rheumatism Association, and in part by an annual unrestricted grant from Schering-Plough Sweden (now Merck Sharp & Dohme). The post hoc analysis was supported by Lund University and the Kockska Foundation, the Swedish Research Council, and the Stockholm County Council. Two authors disclosed financial relationships with multiple pharmaceutical companies.
SOURCE: Olofsson T et al. Arthritis Care Res. 2020 May 20. doi: 10.1002/acr.24264.
according to findings from 21 months of follow-up in a post hoc analysis of data from the randomized, controlled Swedish Farmacotherapy (SWEFOT) trial.
Although RA patients who took biologic combination therapy had 32% lower risk for unacceptable pain (rated at >40 mm on a 0- to 100-mm visual analog scale) at 21 months, they still had no difference from patients taking triple therapy in the rate of pain described as refractory, or unacceptable despite inflammation control (C-reactive protein <10 mg/L).
While these results lend “some support to a better effect on long-term pain for the biological treatment, compared with triple therapy ... our findings are also in line with insufficient effects of current treatment strategies to prevent development of inflammation-independent pain components, warranting early alternative treatment approaches in affected patients,” Tor Olofsson, MD, PhD, of Lund (Sweden) University, and colleagues wrote in Arthritis Care & Research.
The pain outcomes analyzed in this post hoc study were all secondary outcomes of the original open-label SWEFOT trial, which during 2002-2005 enrolled 258 RA patients with less than a year of symptoms who did not reach low disease activity (28-joint Disease Activity Score ≤3.2) after 3 months of methotrexate and randomized them to an addition of either infliximab (3 mg/kg rounded up to nearest 100-mg increment) or sulfasalazine 1,000 mg twice daily plus hydroxychloroquine 400 mg once daily.
Overall, 90 of 128 patients in the infliximab group and 74 of 130 in the triple-therapy group continued the protocol until the 21-month follow-up. Patients in the infliximab group had a significantly lower area under the curve for visual analog scale for pain, most of which was accounted for during months 9-21. The percentage of patients in the infliximab group with unacceptable pain also dropped significantly from 57% at randomization to 32% at 21 months, while no difference was seen for triple therapy patients, of whom 45% had unacceptable pain at 21 months.
While patients in the infliximab group had a significantly lower risk of unacceptable pain without inflammatory control at 21 months, neither treatment arm showed a within-group difference in refractory pain from randomization to the 21-month follow-up.
Nearly one-third of patients overall still reported unacceptable pain 21 months after addition of either infliximab or sulfasalazine plus hydroxychloroquine. And at that time point, refractory pain constituted 82% of all unacceptable pain. “Notably, this pattern – with a domination of refractory pain – was evident already 3 months after starting combination therapy,” Dr. Olofsson and colleagues wrote.
The original SWEFOT study was supported in part by a grant from the Swedish Rheumatism Association, and in part by an annual unrestricted grant from Schering-Plough Sweden (now Merck Sharp & Dohme). The post hoc analysis was supported by Lund University and the Kockska Foundation, the Swedish Research Council, and the Stockholm County Council. Two authors disclosed financial relationships with multiple pharmaceutical companies.
SOURCE: Olofsson T et al. Arthritis Care Res. 2020 May 20. doi: 10.1002/acr.24264.
FROM ARTHRITIS CARE & RESEARCH
Yoga is a good adjunct to migraine therapy
in Neurology.
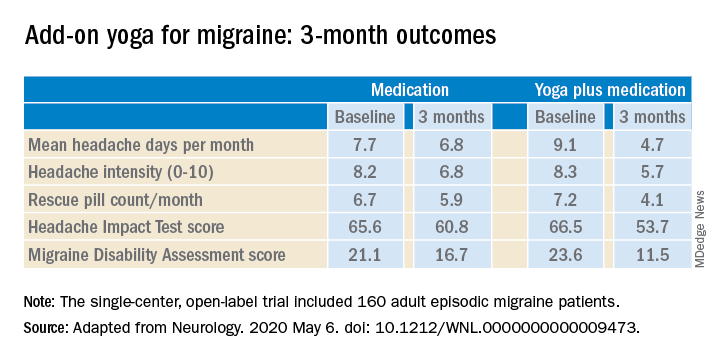
The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
FROM NEUROLOGY
Advice on treating rheumatic diseases from a COVID-19 epicenter
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic continues to pose an unprecedented challenge to health care systems worldwide. In addition to the direct impact of the disease itself, there is a growing concern related to ensuring adequate health care utilization and addressing the needs of vulnerable populations, such as those with chronic illness.
Emanuel et al. have advocated a framework of fair allocation of resources, led by the principles of equity, maximizing benefits, and prioritizing the vulnerable. In these uncertain times, patients with rheumatic diseases represent a vulnerable population whose health and wellness are particularly threatened, not only by the risk of COVID-19, but also by reduced access to usual medical care (e.g., in-person clinic visits), potential treatment interruptions (e.g., planned infusion therapies), and the ongoing shortage of hydroxychloroquine, to name a few.
As rheumatologists, we are now tasked with the development of best practices for caring for patients with rheumatic conditions in this uncertain, evolving, and nearly data-free landscape. We also must maintain an active role as advocates for our patients to help them navigate this pandemic. Herein, we discuss our approach to caring for patients with rheumatic diseases within our practice in New York City, an epicenter of the COVID-19 pandemic.
Communication with patients
Maintaining an open line of communication with our patients (by phone, patient portal, telemedicine, and so on) has become more essential than ever. It is through these communications that we best understand our patients’ concerns and provide support and personalized treatment decisions. The most common questions we have received during recent weeks are:
- Should I stop my medication to lower my risk for infection?
- Are my current symptoms caused by coronavirus, and what should I do next?
- Where can I fill my hydroxychloroquine prescription?
The American College of Rheumatology has deployed a number of task forces aimed at advocating for rheumatologists and patients with rheumatic diseases and is doing an exemplary job guiding us. For patients, several other organizations (e.g., CreakyJoints, Arthritis Foundation, Lupus Research Alliance, Vasculitis Foundation, and Scleroderma Foundation) are also providing accurate information regarding hygiene practices, social distancing, management of medications, and other guidance related to specific rheumatic diseases. In line with ACR recommendations, we encourage a personalized, shared decision-making process with each of our patients.
Patients with rheumatic disease at risk for COVID-19 infection
First, for rheumatology patients who have no COVID-19 symptoms, our management approach is individualized. For patients who are able to maintain social distancing, we have not routinely stopped immunosuppressive medications, including disease-modifying antirheumatic drugs (DMARDs) and biologic agents. However, we discuss the risks and benefits of continuing immunosuppressive therapy during this time with all of our patients.
In certain cases of stable, non–life-threatening disease, we may consider spacing or temporarily interrupting immunosuppressive therapy, using individualized, shared decision making. Yet, it is important to recognize that, for some patients, achieving adequate disease control can require a substantial amount of time.
Furthermore, it is important to acknowledge that disease flares requiring steroid therapy may increase the risk for infection even more, keeping in mind that, in some rheumatic diseases, high disease activity itself can increase infection risk. We advise patients who are continuing therapy to maintain at least a 1-month supply of their medications.
Decisions regarding infusions in the hospital and outpatient settings are similarly made on an individual basis, weighing the risk for virus exposure against that of disease flare. The more limited availability of appropriately distanced infusion chairs in some already overburdened systems must be considered in this discussion. We agree with the ACR, whose infusion guidance recommends that “possible changes might include temporary interruption of therapy, temporary initiation of a bridge therapy such as a less potent anti-inflammatory or immune-modulating agent, or temporary change to an alternative therapy.”
We also reinforce recommended behaviors for preventing infection, including social distancing, frequent handwashing, and avoiding touching one’s face.
Patients with rheumatic disease and confirmed or suspected COVID-19 infection
With the worldwide spread of COVID-19, patients with rheumatic diseases will undoubtedly be among those exposed and infected. Though current data are limited, within a cohort from China, 1% had an autoimmune disease. Testing recommendations to confirm COVID-19 and decision guidelines for outpatient versus inpatient management are evolving, and we consult the most up-to-date, local information regarding testing as individual potential cases arise.
For patients who develop COVID-19 and are currently taking DMARDs and biologics, we recommend that they discontinue these medications, with the exception of hydroxychloroquine (HCQ). HCQ may be continued because its mechanism is not expected to worsen infection, and it plays a key role in the management of patients with systemic lupus erythematosus (SLE). In addition, in vitro antiviral effects have been reported and there is growing interest for its use in the management of COVID-19. However, there are conflicting data and methodological concerns about the nonrandomized human studies that suggest a benefit of HCQ against COVID-19.
The decision regarding management of glucocorticoids in the setting of new COVID-19 infection is challenging and should be individualized. At present, expert panels recommend against the use of glucocorticoids among individuals with COVID-19 who do not have acute respiratory distress syndrome. However, adrenal insufficiency must be considered among patients with COVID-19 who are treated with chronic glucocorticoids. Again, these decisions should be made on an individual, case-by-case basis.
Implications of a hydroxychloroquine shortage
The use of HCQ in rheumatology is supported by years of research. Particularly in SLE, HCQ has been shown to reduce disease activity and damage and to improve survival. Furthermore, for pregnant patients with SLE, numerous studies have demonstrated the safety and benefit of HCQ for both the mother and fetus; thus, it is strongly recommended. By contrast, despite the growing interest for HCQ in patients with COVID-19, the evidence is inconclusive and limited.
The ACR suggests that decisions regarding HCQ dose reductions to extend individual patients supplies should be tailored to each patient’s need and risk in the unfortunate setting of medication shortages. Even in patients with stable SLE, however, disease flares at 6 months are more common among individuals who discontinue HCQ. Of note, these flares may incorporate novel and severe disease manifestations.
Unfortunately, other therapeutic options for SLE are associated with more adverse effects (including increased susceptibility to infection) or are largely unavailable (e.g., quinacrine). Thus, we strive to continue standard dosing of HCQ for patients who are currently flaring or recently flared, and we make shared, individualized decisions for those patients with stable disease as the HCQ shortage evolves.
Future research on COVID-19 and rheumatic disease
While we might expect that an underlying rheumatic disease and associated treatments may predispose individuals to developing COVID-19, current data do not indicate which, if any, rheumatic diseases and associated therapies convey the greatest risk.
To address this uncertainty, the rheumatology community created the COVID-19 Global Rheumatology Alliance, an international effort to initiate and maintain a deidentified patient registry for individuals with rheumatic disease who develop COVID-19. These efforts will allow us to gain essential insights regarding which patient demographics, underlying diseases, and medications are most common among patients who develop COVID-19.
This alliance encourages rheumatologists and those caring for patients with rheumatic diseases to report their patient cases to this registry. As we are confronted with making management decisions with a scarcity of supporting data, efforts like these will improve our ability to make individualized treatment recommendations.
The COVID-19 pandemic has presented us all with unprecedented challenges. As rheumatologists, it is our duty to lead our patients through this uncharted territory with close communication, information, advocacy, and personalized treatment decisions. Each of these is central to the management of rheumatology patients during the COVID-19 pandemic.
With the growing interest in immunomodulatory therapies for the complications of this infection, we have the unique opportunity to share our expertise, recommendations, and caution with our colleagues. As clinicians and scientists, we must advocate for data collection and studies that will allow us to develop novel, data-driven disease management approaches while providing the best care possible for our patients.
Stephen Paget, MD, is physician in chief emeritus for the Center for Rheumatology at Hospital for Special Surgery in New York. Kimberly Showalter, MD, is a third-year rheumatology fellow at Hospital for Special Surgery. Sebastian E. Sattui, MD, is a third-year rheumatology and 1-year vasculitis fellow at Hospital for Special Surgery.
A version of this article originally appeared on Medscape.com.
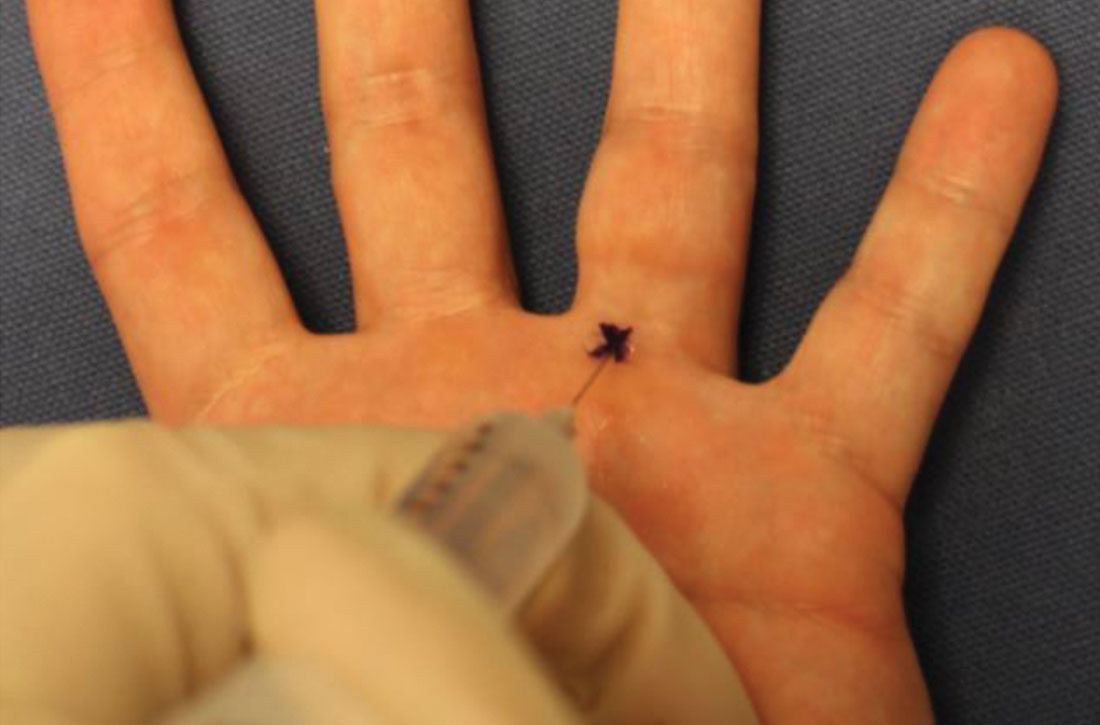
How to minimize the pain of local anesthetic administration
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
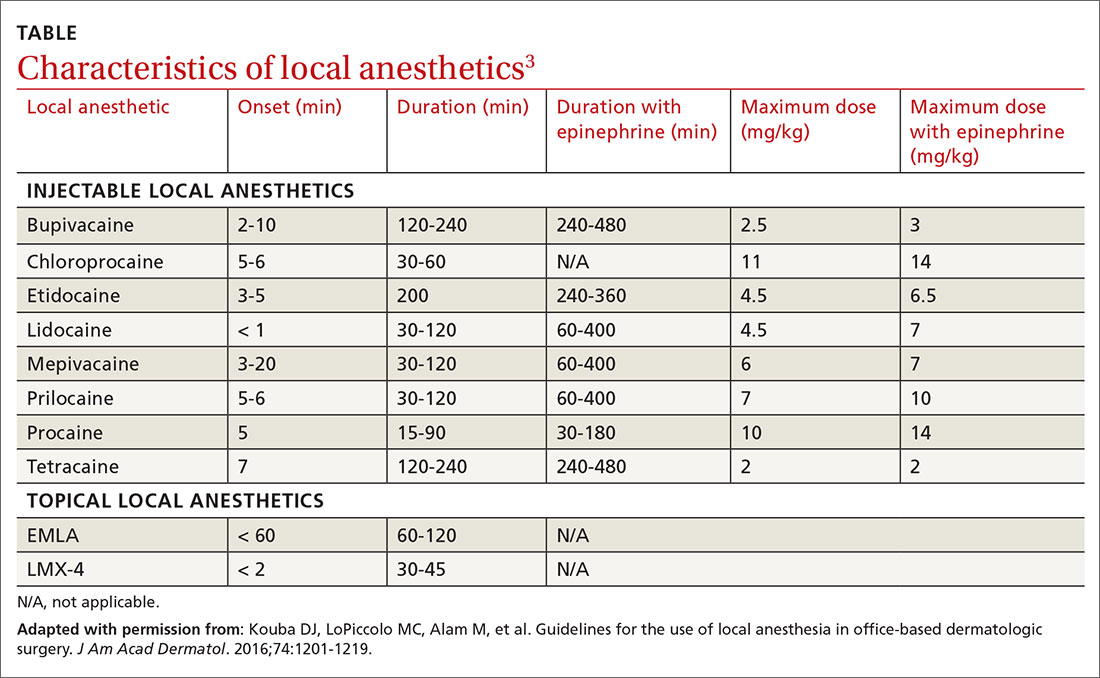
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
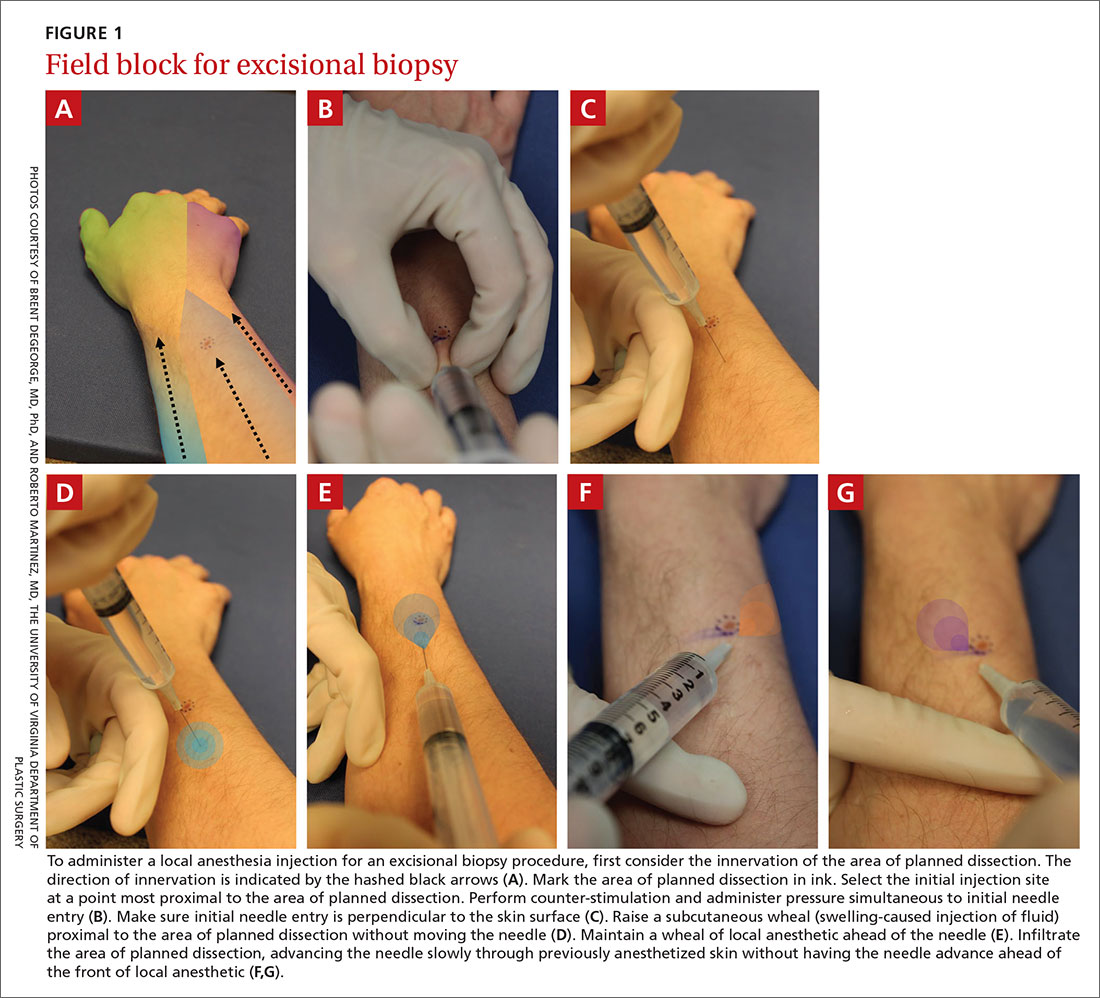
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
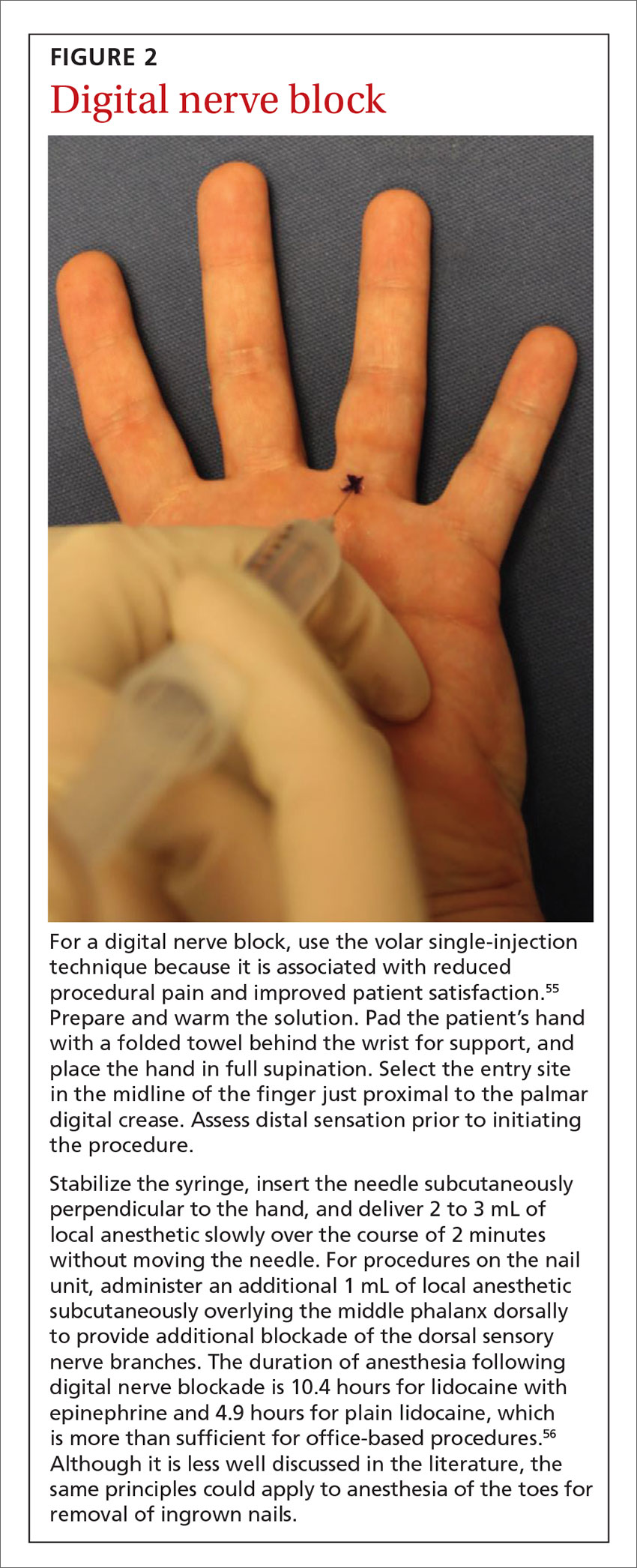
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.

Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42

Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.

CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.

Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42

Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.

CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
PRACTICE RECOMMENDATIONS
› Add epinephrine and sodium bicarbonate buffer to local anesthetic solution to reduce pain and procedural blood loss. A
› Use such techniques as counter-stimulation, a perpendicular angle of injection, a subcutaneous depth of injection, and a slow rate of injection to minimize patient discomfort. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How do neurologists choose an acute treatment for migraine?
STOWE, VT. – A large and growing number of medications is available for the acute treatment of migraine. Effective acute treatment enables patients to re-engage in their work and other daily activities, as well as reducing the likelihood that their disease will progress from episodic to chronic migraine. efficacy and tolerability according to Barbara L. Nye, MD, assistant professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H.. Dr. Nye discussed the acute treatment of migraine at the annual meeting of the Headache Cooperative of New England.
Choosing an initial treatment
Nonspecific medications are perhaps the first treatments to consider for a patient with acute migraine. This class includes NSAIDs such as naproxen sodium, piroxicam, diclofenac, celecoxib, and indomethacin. Emerging data indicate that some NSAIDs are associated with an increased risk of stroke, which is an important consideration as the population ages, said Dr. Nye. Other nonspecific options are neuroleptics such as prochlorperazine, metoclopramide, promethazine, and chlorpromazine. Many neuroleptics have sedative effects, however, so they do not necessarily help a patient return to function. Nevertheless, these drugs can be good rescue medications, said Dr. Nye.
Triptans are effective in the acute treatment of migraine, and seven drugs in this class are available. Most, such as rizatriptan, almotriptan, eletriptan, naratriptan, and frovatriptan, are available only as tablets. Other routes of delivery are available, however. Sumatriptan, for example, is available in injectable and intranasal formulations, and zolmitriptan is available as an orally dissolving tablet.
Another option to consider is dihydroergotamine (DHE), which has long been used for migraine. The injectable formulation of DHE can be cumbersome because it requires the patients with a headache to open a vial, draw the medication into a filter needle, and inject themselves, said Dr. Nye. “The nasal sprays that are available right now aren’t as effective as we’d like them to be,” she added. But overall, DHE is effective. Associated adverse events include flushing, nausea, and diarrhea.
Lasmiditan received approval from the Food and Drug Administration for the acute treatment of migraine in October 2019. Compared with placebo, the drug increases the likelihood of pain freedom and freedom from the most bothersome symptom at 2 hours. Driving tests indicated that patients were impaired for about 8 hours after treatment, and lasmiditan is a Schedule V drug. It is available in doses of 50 mg/day, 100 mg/day, and 200 mg/day.
The class of drugs known as the “gepants” provides further options. The most recently approved therapy in this class, which targets calcitonin gene–related peptide, is ubrogepant. Because the drug is metabolized through the CYP3A4 system, they are not appropriate for patients who use strong CYP3A4 inhibitors. The most common side effects are nausea, hypersensitivity reaction, and somnolence.
Neuromodulation can provide effective treatment without provoking side effects, said Dr. Nye. Options include transcutaneous supraorbital stimulation, single-pulse transcutaneous magnetic stimulation, noninvasive vagal nerve stimulation, and remote nonpainful stimulation.
If a patient presents during an acute attack, neurologists could consider using a nerve block. The latter may administer occipital nerve blocks, trigger point injections, auriculotemporal nerve blocks, and supraorbital and supratrochlear nerve blocks. This treatment can bring immediate relief, which is gratifying for patients and neurologists. But no consensus about which medications to use or how to administer them has been established. Neurologists most often use a combination of bupivacaine and lidocaine. Another possibility is a sphenopalatine ganglion nerve block, which requires treatment to be inserted through the nose. This treatment can be delivered in the office using the Sphenocath device or the Allevio device. Another device, the Tx360, is intended to enable patient self-administration.
Addressing treatment failure
If a patient returns and reports that the current treatment is ineffective, the neurologist must reevaluate the therapy. A helpful way to conduct this reassessment is to administer the Migraine Treatment Optimization Questionnaire (MTOQ), which was developed by Lipton et al., to the patient. Neurologists ask whether the patient can function normally 2 hours after treatment or whether the medication is, for example, causing a side effect that makes this outcome less likely. Other questions for the patient are whether the headache pain disappears within 2 hours and whether the medication provides consistent relief. Finally, the neurologist can ask whether the patient is comfortable taking the medication. A score lower than 2 on the MTOQ indicates that the acute treatment should be changed, said Dr. Nye.
Gastroparesis is common during migraine attacks. It is inadvisable to give an oral medication to a patient who vomits within 20 minutes of attack onset, said Dr. Nye. “It’s a little less intuitive for those people who are nauseous immediately to think that that oral tablet is probably going to sit in their stomach and not get absorbed in the intestines as intended.” Nasal sprays, injectable medicines, and oral dissolving tablets are appropriate options for patients with gastroparesis.
Treating migraine during pregnancy
Special consideration must be given to treatment when the patient is pregnant. Decreased headache frequency is common in pregnancy, but not universal. Occipital nerve blocks are a good option for prevention and acute management in pregnant patients. They may be administered every 2 weeks. Sphenopalatine ganglion nerve block is another option, and it can be administered several times per week. Data “suggest that stacking the injections 2 or 3 days per week for up to 6 weeks can eliminate headaches for up to 6 months,” said Dr. Nye.
Tylenol is appropriate for acute headache in pregnant patients, “but we do warn about medication overuse headache and limiting its use.” Ondansetron and promethazine are acceptable treatments for nausea. Although ondansetron has less central activity than promethazine, and thus does not reduce the headache, it lessens nausea, said Dr. Nye.
Triptan exposure during the first trimester is not significantly associated with major congenital malformations, which is reassuring, given that many patients take triptans before they realize that they are pregnant. During the second and third trimesters, triptan exposure is significantly associated with atonic uterus and increased blood loss during labor. In a 16-year registry, sumatriptan, naratriptan, and treximet were not associated with teratogenicity.
Nonpharmacological treatments, too, may help pregnant patients. Lifestyle management, including a regular sleep schedule, exercise routine, and diet, can be beneficial. Massage therapy may reduce stress, and cognitive-behavioral therapy and biofeedback are additional options. Behavioral therapy, however, should be initiated before the patient plans the pregnancy, said Dr. Nye. These therapies require training that a patient having an exacerbation of migraine is less likely to have the motivation to begin.
Many medications are transferred to infants through breast milk. The American Pediatric Association considers a relative infant dosing of less than 10% to be safe. A clinician or patient can look up a medication on websites such as LactMed to understand the relative infant dose and possible effects. Another helpful reference is Medications and Mothers’ Milk, said Dr. Nye. Acetaminophen, steroids, ibuprofen, riboflavin, indomethacin, ketorolac, and naproxen are generally safe during lactation. “Eletriptan is the triptan that’s least likely to be in the breast milk,” said Dr. Nye. Aspirin, atenolol, ergotamine, and lithium, however, should be given with caution. The safety of amitriptyline, nortriptyline, and SSRIs during lactation is unknown.
Dr. Nye is on advisory boards for Alder, Allergan, Biohaven, electroCore, Pernix, and Xoc.
STOWE, VT. – A large and growing number of medications is available for the acute treatment of migraine. Effective acute treatment enables patients to re-engage in their work and other daily activities, as well as reducing the likelihood that their disease will progress from episodic to chronic migraine. efficacy and tolerability according to Barbara L. Nye, MD, assistant professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H.. Dr. Nye discussed the acute treatment of migraine at the annual meeting of the Headache Cooperative of New England.
Choosing an initial treatment
Nonspecific medications are perhaps the first treatments to consider for a patient with acute migraine. This class includes NSAIDs such as naproxen sodium, piroxicam, diclofenac, celecoxib, and indomethacin. Emerging data indicate that some NSAIDs are associated with an increased risk of stroke, which is an important consideration as the population ages, said Dr. Nye. Other nonspecific options are neuroleptics such as prochlorperazine, metoclopramide, promethazine, and chlorpromazine. Many neuroleptics have sedative effects, however, so they do not necessarily help a patient return to function. Nevertheless, these drugs can be good rescue medications, said Dr. Nye.
Triptans are effective in the acute treatment of migraine, and seven drugs in this class are available. Most, such as rizatriptan, almotriptan, eletriptan, naratriptan, and frovatriptan, are available only as tablets. Other routes of delivery are available, however. Sumatriptan, for example, is available in injectable and intranasal formulations, and zolmitriptan is available as an orally dissolving tablet.
Another option to consider is dihydroergotamine (DHE), which has long been used for migraine. The injectable formulation of DHE can be cumbersome because it requires the patients with a headache to open a vial, draw the medication into a filter needle, and inject themselves, said Dr. Nye. “The nasal sprays that are available right now aren’t as effective as we’d like them to be,” she added. But overall, DHE is effective. Associated adverse events include flushing, nausea, and diarrhea.
Lasmiditan received approval from the Food and Drug Administration for the acute treatment of migraine in October 2019. Compared with placebo, the drug increases the likelihood of pain freedom and freedom from the most bothersome symptom at 2 hours. Driving tests indicated that patients were impaired for about 8 hours after treatment, and lasmiditan is a Schedule V drug. It is available in doses of 50 mg/day, 100 mg/day, and 200 mg/day.
The class of drugs known as the “gepants” provides further options. The most recently approved therapy in this class, which targets calcitonin gene–related peptide, is ubrogepant. Because the drug is metabolized through the CYP3A4 system, they are not appropriate for patients who use strong CYP3A4 inhibitors. The most common side effects are nausea, hypersensitivity reaction, and somnolence.
Neuromodulation can provide effective treatment without provoking side effects, said Dr. Nye. Options include transcutaneous supraorbital stimulation, single-pulse transcutaneous magnetic stimulation, noninvasive vagal nerve stimulation, and remote nonpainful stimulation.
If a patient presents during an acute attack, neurologists could consider using a nerve block. The latter may administer occipital nerve blocks, trigger point injections, auriculotemporal nerve blocks, and supraorbital and supratrochlear nerve blocks. This treatment can bring immediate relief, which is gratifying for patients and neurologists. But no consensus about which medications to use or how to administer them has been established. Neurologists most often use a combination of bupivacaine and lidocaine. Another possibility is a sphenopalatine ganglion nerve block, which requires treatment to be inserted through the nose. This treatment can be delivered in the office using the Sphenocath device or the Allevio device. Another device, the Tx360, is intended to enable patient self-administration.
Addressing treatment failure
If a patient returns and reports that the current treatment is ineffective, the neurologist must reevaluate the therapy. A helpful way to conduct this reassessment is to administer the Migraine Treatment Optimization Questionnaire (MTOQ), which was developed by Lipton et al., to the patient. Neurologists ask whether the patient can function normally 2 hours after treatment or whether the medication is, for example, causing a side effect that makes this outcome less likely. Other questions for the patient are whether the headache pain disappears within 2 hours and whether the medication provides consistent relief. Finally, the neurologist can ask whether the patient is comfortable taking the medication. A score lower than 2 on the MTOQ indicates that the acute treatment should be changed, said Dr. Nye.
Gastroparesis is common during migraine attacks. It is inadvisable to give an oral medication to a patient who vomits within 20 minutes of attack onset, said Dr. Nye. “It’s a little less intuitive for those people who are nauseous immediately to think that that oral tablet is probably going to sit in their stomach and not get absorbed in the intestines as intended.” Nasal sprays, injectable medicines, and oral dissolving tablets are appropriate options for patients with gastroparesis.
Treating migraine during pregnancy
Special consideration must be given to treatment when the patient is pregnant. Decreased headache frequency is common in pregnancy, but not universal. Occipital nerve blocks are a good option for prevention and acute management in pregnant patients. They may be administered every 2 weeks. Sphenopalatine ganglion nerve block is another option, and it can be administered several times per week. Data “suggest that stacking the injections 2 or 3 days per week for up to 6 weeks can eliminate headaches for up to 6 months,” said Dr. Nye.
Tylenol is appropriate for acute headache in pregnant patients, “but we do warn about medication overuse headache and limiting its use.” Ondansetron and promethazine are acceptable treatments for nausea. Although ondansetron has less central activity than promethazine, and thus does not reduce the headache, it lessens nausea, said Dr. Nye.
Triptan exposure during the first trimester is not significantly associated with major congenital malformations, which is reassuring, given that many patients take triptans before they realize that they are pregnant. During the second and third trimesters, triptan exposure is significantly associated with atonic uterus and increased blood loss during labor. In a 16-year registry, sumatriptan, naratriptan, and treximet were not associated with teratogenicity.
Nonpharmacological treatments, too, may help pregnant patients. Lifestyle management, including a regular sleep schedule, exercise routine, and diet, can be beneficial. Massage therapy may reduce stress, and cognitive-behavioral therapy and biofeedback are additional options. Behavioral therapy, however, should be initiated before the patient plans the pregnancy, said Dr. Nye. These therapies require training that a patient having an exacerbation of migraine is less likely to have the motivation to begin.
Many medications are transferred to infants through breast milk. The American Pediatric Association considers a relative infant dosing of less than 10% to be safe. A clinician or patient can look up a medication on websites such as LactMed to understand the relative infant dose and possible effects. Another helpful reference is Medications and Mothers’ Milk, said Dr. Nye. Acetaminophen, steroids, ibuprofen, riboflavin, indomethacin, ketorolac, and naproxen are generally safe during lactation. “Eletriptan is the triptan that’s least likely to be in the breast milk,” said Dr. Nye. Aspirin, atenolol, ergotamine, and lithium, however, should be given with caution. The safety of amitriptyline, nortriptyline, and SSRIs during lactation is unknown.
Dr. Nye is on advisory boards for Alder, Allergan, Biohaven, electroCore, Pernix, and Xoc.
STOWE, VT. – A large and growing number of medications is available for the acute treatment of migraine. Effective acute treatment enables patients to re-engage in their work and other daily activities, as well as reducing the likelihood that their disease will progress from episodic to chronic migraine. efficacy and tolerability according to Barbara L. Nye, MD, assistant professor of neurology at the Geisel School of Medicine at Dartmouth, Hanover, N.H.. Dr. Nye discussed the acute treatment of migraine at the annual meeting of the Headache Cooperative of New England.
Choosing an initial treatment
Nonspecific medications are perhaps the first treatments to consider for a patient with acute migraine. This class includes NSAIDs such as naproxen sodium, piroxicam, diclofenac, celecoxib, and indomethacin. Emerging data indicate that some NSAIDs are associated with an increased risk of stroke, which is an important consideration as the population ages, said Dr. Nye. Other nonspecific options are neuroleptics such as prochlorperazine, metoclopramide, promethazine, and chlorpromazine. Many neuroleptics have sedative effects, however, so they do not necessarily help a patient return to function. Nevertheless, these drugs can be good rescue medications, said Dr. Nye.
Triptans are effective in the acute treatment of migraine, and seven drugs in this class are available. Most, such as rizatriptan, almotriptan, eletriptan, naratriptan, and frovatriptan, are available only as tablets. Other routes of delivery are available, however. Sumatriptan, for example, is available in injectable and intranasal formulations, and zolmitriptan is available as an orally dissolving tablet.
Another option to consider is dihydroergotamine (DHE), which has long been used for migraine. The injectable formulation of DHE can be cumbersome because it requires the patients with a headache to open a vial, draw the medication into a filter needle, and inject themselves, said Dr. Nye. “The nasal sprays that are available right now aren’t as effective as we’d like them to be,” she added. But overall, DHE is effective. Associated adverse events include flushing, nausea, and diarrhea.
Lasmiditan received approval from the Food and Drug Administration for the acute treatment of migraine in October 2019. Compared with placebo, the drug increases the likelihood of pain freedom and freedom from the most bothersome symptom at 2 hours. Driving tests indicated that patients were impaired for about 8 hours after treatment, and lasmiditan is a Schedule V drug. It is available in doses of 50 mg/day, 100 mg/day, and 200 mg/day.
The class of drugs known as the “gepants” provides further options. The most recently approved therapy in this class, which targets calcitonin gene–related peptide, is ubrogepant. Because the drug is metabolized through the CYP3A4 system, they are not appropriate for patients who use strong CYP3A4 inhibitors. The most common side effects are nausea, hypersensitivity reaction, and somnolence.
Neuromodulation can provide effective treatment without provoking side effects, said Dr. Nye. Options include transcutaneous supraorbital stimulation, single-pulse transcutaneous magnetic stimulation, noninvasive vagal nerve stimulation, and remote nonpainful stimulation.
If a patient presents during an acute attack, neurologists could consider using a nerve block. The latter may administer occipital nerve blocks, trigger point injections, auriculotemporal nerve blocks, and supraorbital and supratrochlear nerve blocks. This treatment can bring immediate relief, which is gratifying for patients and neurologists. But no consensus about which medications to use or how to administer them has been established. Neurologists most often use a combination of bupivacaine and lidocaine. Another possibility is a sphenopalatine ganglion nerve block, which requires treatment to be inserted through the nose. This treatment can be delivered in the office using the Sphenocath device or the Allevio device. Another device, the Tx360, is intended to enable patient self-administration.
Addressing treatment failure
If a patient returns and reports that the current treatment is ineffective, the neurologist must reevaluate the therapy. A helpful way to conduct this reassessment is to administer the Migraine Treatment Optimization Questionnaire (MTOQ), which was developed by Lipton et al., to the patient. Neurologists ask whether the patient can function normally 2 hours after treatment or whether the medication is, for example, causing a side effect that makes this outcome less likely. Other questions for the patient are whether the headache pain disappears within 2 hours and whether the medication provides consistent relief. Finally, the neurologist can ask whether the patient is comfortable taking the medication. A score lower than 2 on the MTOQ indicates that the acute treatment should be changed, said Dr. Nye.
Gastroparesis is common during migraine attacks. It is inadvisable to give an oral medication to a patient who vomits within 20 minutes of attack onset, said Dr. Nye. “It’s a little less intuitive for those people who are nauseous immediately to think that that oral tablet is probably going to sit in their stomach and not get absorbed in the intestines as intended.” Nasal sprays, injectable medicines, and oral dissolving tablets are appropriate options for patients with gastroparesis.
Treating migraine during pregnancy
Special consideration must be given to treatment when the patient is pregnant. Decreased headache frequency is common in pregnancy, but not universal. Occipital nerve blocks are a good option for prevention and acute management in pregnant patients. They may be administered every 2 weeks. Sphenopalatine ganglion nerve block is another option, and it can be administered several times per week. Data “suggest that stacking the injections 2 or 3 days per week for up to 6 weeks can eliminate headaches for up to 6 months,” said Dr. Nye.
Tylenol is appropriate for acute headache in pregnant patients, “but we do warn about medication overuse headache and limiting its use.” Ondansetron and promethazine are acceptable treatments for nausea. Although ondansetron has less central activity than promethazine, and thus does not reduce the headache, it lessens nausea, said Dr. Nye.
Triptan exposure during the first trimester is not significantly associated with major congenital malformations, which is reassuring, given that many patients take triptans before they realize that they are pregnant. During the second and third trimesters, triptan exposure is significantly associated with atonic uterus and increased blood loss during labor. In a 16-year registry, sumatriptan, naratriptan, and treximet were not associated with teratogenicity.
Nonpharmacological treatments, too, may help pregnant patients. Lifestyle management, including a regular sleep schedule, exercise routine, and diet, can be beneficial. Massage therapy may reduce stress, and cognitive-behavioral therapy and biofeedback are additional options. Behavioral therapy, however, should be initiated before the patient plans the pregnancy, said Dr. Nye. These therapies require training that a patient having an exacerbation of migraine is less likely to have the motivation to begin.
Many medications are transferred to infants through breast milk. The American Pediatric Association considers a relative infant dosing of less than 10% to be safe. A clinician or patient can look up a medication on websites such as LactMed to understand the relative infant dose and possible effects. Another helpful reference is Medications and Mothers’ Milk, said Dr. Nye. Acetaminophen, steroids, ibuprofen, riboflavin, indomethacin, ketorolac, and naproxen are generally safe during lactation. “Eletriptan is the triptan that’s least likely to be in the breast milk,” said Dr. Nye. Aspirin, atenolol, ergotamine, and lithium, however, should be given with caution. The safety of amitriptyline, nortriptyline, and SSRIs during lactation is unknown.
Dr. Nye is on advisory boards for Alder, Allergan, Biohaven, electroCore, Pernix, and Xoc.
REPORTING FROM HCNE 2020
When is preventive treatment of migraine appropriate?
STOWE, VT – , said Rebecca Burch, MD, staff attending neurologist at Brigham and Women’s Hospital in Boston. Clinical observation suggests that preventive treatment provides benefits for appropriately selected migraineurs, although few data confirm a modifying effect on disease course, she said at the Stowe Headache Symposium sponsored by the Headache Cooperative of New England. In her overview, Dr. Burch discussed when preventive treatment is appropriate, which patients are candidates for preventive therapy, and what the levels of evidence are for the preventive therapies.
Identifying candidates for preventive treatment
Migraine is the second most disabling condition worldwide and imposes a large social and economic burden, said Dr. Burch. Preventive therapy reduces the disability associated with migraine. It reduces headache frequency and, thus, the risk that episodic migraine will transform into chronic migraine. By reducing the number of headache days, preventive treatment also may reduce the overuse of acute medication, which is a risk factor for migraine chronification.
Neurologists can consider preventive therapy for migraineurs with frequent headaches, but the term “frequent” is not clearly defined. Common definitions include one headache per week and two headaches per month with significant disability. These definitions are based on expert consensus and do not have strong evidential support, said Dr. Burch. Preventive therapy also may be appropriate for migraineurs who overuse acute medication or who have failed acute medications. Special cases, such as patients with exceptional anxiety or disability, may also call for preventive treatment, said Dr. Burch.
Data suggest that preventive treatment for migraine is underused. The American Migraine Prevalence and Prevention study of 2007 found that half of patients who should be offered preventive treatment are currently receiving it. In 2016, the Chronic Migraine Epidemiology and Outcomes study found that 4.5% of chronic migraineurs take both acute and preventive treatment.
Other data published in Cephalalgia in 2015 indicate that adherence to migraine preventive treatment is approximately 20%. About 45% of patients discontinue medication because of side effects, and 45% cite lack of efficacy as their reason for discontinuation. Patients also mentioned cost, interactions with other medications, and the inconvenience of daily medication as other reasons for discontinuation.
Neurologists can take several steps to increase adherence to preventive treatment, said Dr. Burch. First, neurologists should confirm that patients want preventive medication. A clear discussion of the goals of preventive treatment is helpful as well. Furthermore, neurologists should explain that they are offering patients a trial, said Dr. Burch. The medication can be titrated slowly from a low dose to minimize side effects. Patients can be reassured that ineffective medications will be stopped. Neurologists can emphasize that their relationship with the patient is a partnership and that the treatment strategy will be improved over time.
Examining the evidence on treatments’ efficacy
Many drug classes, such as antiepileptics, antidepressants, beta blockers, neurotoxins, and calcitonin gene-related peptide (CGRP) antibodies, include therapies that are used as preventive treatments for migraine. When selecting a medication, a neurologist should start with one that is supported by Level A or Level B evidence, said Dr. Burch. Medications with Level A evidence include divalproex, topiramate, metoprolol, propranolol, erenumab, galcanezumab, fremanezumab, eptinezumab, and onabotulinumtoxinA. Medications with Level B evidence include amitriptyline, venlafaxine, memantine, lisinopril, and candesartan. Neurologists sometimes prescribe gabapentin and verapamil, although the evidence for them is Level U. Duloxetine, nortriptyline, and pregabalin also are used, but the evidence for them has not been evaluated. “We need more evidence in these areas,” said Dr. Burch.
Neurologists should consider access (e.g., cost and insurance coverage), efficacy, side effects, and comorbidities and contraindications when choosing a preventive therapy, she added. Verapamil and memantine are well tolerated and appropriate choices if the goal is to avoid side effects in general. If weight gain or fatigue is a concern, then topiramate and venlafaxine should be considered. Neurologists should avoid prescribing antiepileptic drugs if cognitive symptoms are a concern, said Dr. Burch. Beta blockers and venlafaxine would be better options in this case.
In clinical trials of CGRP therapies, the rates of adverse events were similar between the active and control arms. “But it’s become fairly clear that the clinical trials did not fully capture the side-effect profile that we are seeing in clinical practice,” said Dr. Burch. In a paper currently in review, she and her colleagues retrospectively studied 241 patients that they had treated with CGRP monoclonal antibodies at their headache center. The most common adverse events were constipation (43%), injection-site reaction (24%), muscle or joint pain (17%), and fatigue (15%). Furthermore, CGRP antagonists were associated with maternal hypertension, fetal growth restriction, and fetal mortality in animal studies. The current recommendation is to avoid CGRP monoclonal antibodies during pregnancy or in any patient who is at risk of becoming pregnant, said Dr. Burch.
How should neurologists assess preventive efficacy?
The assessment of a medication’s preventive efficacy “is a moving target in the headache world,” said Dr. Burch. “Historically, we have used headache days per month, and that is still, according to the International Headache Society clinical trials guidelines, how we should be judging whether a medication is working or not. But that doesn’t necessarily tell us what’s going to happen to an individual patient in front of us.”
In 2017, the Institute for Clinical Effectiveness Research compared data for old and new migraine treatments in a network meta-analysis. They all tended to reduce the number of monthly migraine days by one to two, compared with placebo. When one analyzes clinical trials of the drugs using this criterion, “most of these treatments come out about the same,” said Dr. Burch.
More recently, investigators have examined responder rates. They commonly report the proportions of patients who had a reduction in headache days of 50%, 75%, or 100%, for example. To extrapolate responder rates from the trial participants to the general population, a neurologist must know which groups of patients got worse on treatment, said Dr. Burch. Furthermore, the responder rates for older medications are unknown, because they were not examined. This situation makes comparisons of newer and older therapies more complicated.
Phase 3 trials of the CGRP drugs included analyses of the therapies’ 50% responder rates. This rate was about 42% for the 70-mg dose of erenumab and 50% for the 140-mg dose. The 50% responder rates for fremanezumab were 47.7% for the 225-mg dose and 44.4% for the 675-mg dose. In two trials of galcanezumab, the 50% responder rate for the 120-mg dose was approximately 60%, and the rate for the 240-mg dose was about 59%. The 50% responder rates for eptinezumab were 50% for the 100-mg dose and 56% for the 300-mg dose. The 50% responder rate across all trials was around 50%-60% in the active group, which is roughly 25% over the placebo group, said Dr. Burch.
Another measurement of efficacy is the efficacy-to-harm ratio, which is derived from the number needed to treat and the number needed to harm. To calculate this ratio, however, harm needs to be assessed adequately during a clinical trial. Although the ratio can provide a clinically relevant overview of a drug’s effects, patients may differ from each other in the way they evaluate efficacy and harm.
In addition, many questions about preventive treatment of migraine have no clear answers yet. It is uncertain, for example, how long a patient should receive preventive treatment and when treatment should be withdrawn, said Dr. Burch. “Can we expect that a lot of people are going to need to be on it for life, or is there a subpopulation who will get better and [for whom] we can withdraw [treatment]?” she asked. “How do we identify them?” Also, more data are needed before neurologists can understand why a given patient responds to one treatment, but not to another. It is difficult to predict which patients will respond to which treatments. Finally, it remains unclear how much of patients’ improvement can be attributed to regression to the mean, rather than preventive treatment.
STOWE, VT – , said Rebecca Burch, MD, staff attending neurologist at Brigham and Women’s Hospital in Boston. Clinical observation suggests that preventive treatment provides benefits for appropriately selected migraineurs, although few data confirm a modifying effect on disease course, she said at the Stowe Headache Symposium sponsored by the Headache Cooperative of New England. In her overview, Dr. Burch discussed when preventive treatment is appropriate, which patients are candidates for preventive therapy, and what the levels of evidence are for the preventive therapies.
Identifying candidates for preventive treatment
Migraine is the second most disabling condition worldwide and imposes a large social and economic burden, said Dr. Burch. Preventive therapy reduces the disability associated with migraine. It reduces headache frequency and, thus, the risk that episodic migraine will transform into chronic migraine. By reducing the number of headache days, preventive treatment also may reduce the overuse of acute medication, which is a risk factor for migraine chronification.
Neurologists can consider preventive therapy for migraineurs with frequent headaches, but the term “frequent” is not clearly defined. Common definitions include one headache per week and two headaches per month with significant disability. These definitions are based on expert consensus and do not have strong evidential support, said Dr. Burch. Preventive therapy also may be appropriate for migraineurs who overuse acute medication or who have failed acute medications. Special cases, such as patients with exceptional anxiety or disability, may also call for preventive treatment, said Dr. Burch.
Data suggest that preventive treatment for migraine is underused. The American Migraine Prevalence and Prevention study of 2007 found that half of patients who should be offered preventive treatment are currently receiving it. In 2016, the Chronic Migraine Epidemiology and Outcomes study found that 4.5% of chronic migraineurs take both acute and preventive treatment.
Other data published in Cephalalgia in 2015 indicate that adherence to migraine preventive treatment is approximately 20%. About 45% of patients discontinue medication because of side effects, and 45% cite lack of efficacy as their reason for discontinuation. Patients also mentioned cost, interactions with other medications, and the inconvenience of daily medication as other reasons for discontinuation.
Neurologists can take several steps to increase adherence to preventive treatment, said Dr. Burch. First, neurologists should confirm that patients want preventive medication. A clear discussion of the goals of preventive treatment is helpful as well. Furthermore, neurologists should explain that they are offering patients a trial, said Dr. Burch. The medication can be titrated slowly from a low dose to minimize side effects. Patients can be reassured that ineffective medications will be stopped. Neurologists can emphasize that their relationship with the patient is a partnership and that the treatment strategy will be improved over time.
Examining the evidence on treatments’ efficacy
Many drug classes, such as antiepileptics, antidepressants, beta blockers, neurotoxins, and calcitonin gene-related peptide (CGRP) antibodies, include therapies that are used as preventive treatments for migraine. When selecting a medication, a neurologist should start with one that is supported by Level A or Level B evidence, said Dr. Burch. Medications with Level A evidence include divalproex, topiramate, metoprolol, propranolol, erenumab, galcanezumab, fremanezumab, eptinezumab, and onabotulinumtoxinA. Medications with Level B evidence include amitriptyline, venlafaxine, memantine, lisinopril, and candesartan. Neurologists sometimes prescribe gabapentin and verapamil, although the evidence for them is Level U. Duloxetine, nortriptyline, and pregabalin also are used, but the evidence for them has not been evaluated. “We need more evidence in these areas,” said Dr. Burch.
Neurologists should consider access (e.g., cost and insurance coverage), efficacy, side effects, and comorbidities and contraindications when choosing a preventive therapy, she added. Verapamil and memantine are well tolerated and appropriate choices if the goal is to avoid side effects in general. If weight gain or fatigue is a concern, then topiramate and venlafaxine should be considered. Neurologists should avoid prescribing antiepileptic drugs if cognitive symptoms are a concern, said Dr. Burch. Beta blockers and venlafaxine would be better options in this case.
In clinical trials of CGRP therapies, the rates of adverse events were similar between the active and control arms. “But it’s become fairly clear that the clinical trials did not fully capture the side-effect profile that we are seeing in clinical practice,” said Dr. Burch. In a paper currently in review, she and her colleagues retrospectively studied 241 patients that they had treated with CGRP monoclonal antibodies at their headache center. The most common adverse events were constipation (43%), injection-site reaction (24%), muscle or joint pain (17%), and fatigue (15%). Furthermore, CGRP antagonists were associated with maternal hypertension, fetal growth restriction, and fetal mortality in animal studies. The current recommendation is to avoid CGRP monoclonal antibodies during pregnancy or in any patient who is at risk of becoming pregnant, said Dr. Burch.
How should neurologists assess preventive efficacy?
The assessment of a medication’s preventive efficacy “is a moving target in the headache world,” said Dr. Burch. “Historically, we have used headache days per month, and that is still, according to the International Headache Society clinical trials guidelines, how we should be judging whether a medication is working or not. But that doesn’t necessarily tell us what’s going to happen to an individual patient in front of us.”
In 2017, the Institute for Clinical Effectiveness Research compared data for old and new migraine treatments in a network meta-analysis. They all tended to reduce the number of monthly migraine days by one to two, compared with placebo. When one analyzes clinical trials of the drugs using this criterion, “most of these treatments come out about the same,” said Dr. Burch.
More recently, investigators have examined responder rates. They commonly report the proportions of patients who had a reduction in headache days of 50%, 75%, or 100%, for example. To extrapolate responder rates from the trial participants to the general population, a neurologist must know which groups of patients got worse on treatment, said Dr. Burch. Furthermore, the responder rates for older medications are unknown, because they were not examined. This situation makes comparisons of newer and older therapies more complicated.
Phase 3 trials of the CGRP drugs included analyses of the therapies’ 50% responder rates. This rate was about 42% for the 70-mg dose of erenumab and 50% for the 140-mg dose. The 50% responder rates for fremanezumab were 47.7% for the 225-mg dose and 44.4% for the 675-mg dose. In two trials of galcanezumab, the 50% responder rate for the 120-mg dose was approximately 60%, and the rate for the 240-mg dose was about 59%. The 50% responder rates for eptinezumab were 50% for the 100-mg dose and 56% for the 300-mg dose. The 50% responder rate across all trials was around 50%-60% in the active group, which is roughly 25% over the placebo group, said Dr. Burch.
Another measurement of efficacy is the efficacy-to-harm ratio, which is derived from the number needed to treat and the number needed to harm. To calculate this ratio, however, harm needs to be assessed adequately during a clinical trial. Although the ratio can provide a clinically relevant overview of a drug’s effects, patients may differ from each other in the way they evaluate efficacy and harm.
In addition, many questions about preventive treatment of migraine have no clear answers yet. It is uncertain, for example, how long a patient should receive preventive treatment and when treatment should be withdrawn, said Dr. Burch. “Can we expect that a lot of people are going to need to be on it for life, or is there a subpopulation who will get better and [for whom] we can withdraw [treatment]?” she asked. “How do we identify them?” Also, more data are needed before neurologists can understand why a given patient responds to one treatment, but not to another. It is difficult to predict which patients will respond to which treatments. Finally, it remains unclear how much of patients’ improvement can be attributed to regression to the mean, rather than preventive treatment.
STOWE, VT – , said Rebecca Burch, MD, staff attending neurologist at Brigham and Women’s Hospital in Boston. Clinical observation suggests that preventive treatment provides benefits for appropriately selected migraineurs, although few data confirm a modifying effect on disease course, she said at the Stowe Headache Symposium sponsored by the Headache Cooperative of New England. In her overview, Dr. Burch discussed when preventive treatment is appropriate, which patients are candidates for preventive therapy, and what the levels of evidence are for the preventive therapies.
Identifying candidates for preventive treatment
Migraine is the second most disabling condition worldwide and imposes a large social and economic burden, said Dr. Burch. Preventive therapy reduces the disability associated with migraine. It reduces headache frequency and, thus, the risk that episodic migraine will transform into chronic migraine. By reducing the number of headache days, preventive treatment also may reduce the overuse of acute medication, which is a risk factor for migraine chronification.
Neurologists can consider preventive therapy for migraineurs with frequent headaches, but the term “frequent” is not clearly defined. Common definitions include one headache per week and two headaches per month with significant disability. These definitions are based on expert consensus and do not have strong evidential support, said Dr. Burch. Preventive therapy also may be appropriate for migraineurs who overuse acute medication or who have failed acute medications. Special cases, such as patients with exceptional anxiety or disability, may also call for preventive treatment, said Dr. Burch.
Data suggest that preventive treatment for migraine is underused. The American Migraine Prevalence and Prevention study of 2007 found that half of patients who should be offered preventive treatment are currently receiving it. In 2016, the Chronic Migraine Epidemiology and Outcomes study found that 4.5% of chronic migraineurs take both acute and preventive treatment.
Other data published in Cephalalgia in 2015 indicate that adherence to migraine preventive treatment is approximately 20%. About 45% of patients discontinue medication because of side effects, and 45% cite lack of efficacy as their reason for discontinuation. Patients also mentioned cost, interactions with other medications, and the inconvenience of daily medication as other reasons for discontinuation.
Neurologists can take several steps to increase adherence to preventive treatment, said Dr. Burch. First, neurologists should confirm that patients want preventive medication. A clear discussion of the goals of preventive treatment is helpful as well. Furthermore, neurologists should explain that they are offering patients a trial, said Dr. Burch. The medication can be titrated slowly from a low dose to minimize side effects. Patients can be reassured that ineffective medications will be stopped. Neurologists can emphasize that their relationship with the patient is a partnership and that the treatment strategy will be improved over time.
Examining the evidence on treatments’ efficacy
Many drug classes, such as antiepileptics, antidepressants, beta blockers, neurotoxins, and calcitonin gene-related peptide (CGRP) antibodies, include therapies that are used as preventive treatments for migraine. When selecting a medication, a neurologist should start with one that is supported by Level A or Level B evidence, said Dr. Burch. Medications with Level A evidence include divalproex, topiramate, metoprolol, propranolol, erenumab, galcanezumab, fremanezumab, eptinezumab, and onabotulinumtoxinA. Medications with Level B evidence include amitriptyline, venlafaxine, memantine, lisinopril, and candesartan. Neurologists sometimes prescribe gabapentin and verapamil, although the evidence for them is Level U. Duloxetine, nortriptyline, and pregabalin also are used, but the evidence for them has not been evaluated. “We need more evidence in these areas,” said Dr. Burch.
Neurologists should consider access (e.g., cost and insurance coverage), efficacy, side effects, and comorbidities and contraindications when choosing a preventive therapy, she added. Verapamil and memantine are well tolerated and appropriate choices if the goal is to avoid side effects in general. If weight gain or fatigue is a concern, then topiramate and venlafaxine should be considered. Neurologists should avoid prescribing antiepileptic drugs if cognitive symptoms are a concern, said Dr. Burch. Beta blockers and venlafaxine would be better options in this case.
In clinical trials of CGRP therapies, the rates of adverse events were similar between the active and control arms. “But it’s become fairly clear that the clinical trials did not fully capture the side-effect profile that we are seeing in clinical practice,” said Dr. Burch. In a paper currently in review, she and her colleagues retrospectively studied 241 patients that they had treated with CGRP monoclonal antibodies at their headache center. The most common adverse events were constipation (43%), injection-site reaction (24%), muscle or joint pain (17%), and fatigue (15%). Furthermore, CGRP antagonists were associated with maternal hypertension, fetal growth restriction, and fetal mortality in animal studies. The current recommendation is to avoid CGRP monoclonal antibodies during pregnancy or in any patient who is at risk of becoming pregnant, said Dr. Burch.
How should neurologists assess preventive efficacy?
The assessment of a medication’s preventive efficacy “is a moving target in the headache world,” said Dr. Burch. “Historically, we have used headache days per month, and that is still, according to the International Headache Society clinical trials guidelines, how we should be judging whether a medication is working or not. But that doesn’t necessarily tell us what’s going to happen to an individual patient in front of us.”
In 2017, the Institute for Clinical Effectiveness Research compared data for old and new migraine treatments in a network meta-analysis. They all tended to reduce the number of monthly migraine days by one to two, compared with placebo. When one analyzes clinical trials of the drugs using this criterion, “most of these treatments come out about the same,” said Dr. Burch.
More recently, investigators have examined responder rates. They commonly report the proportions of patients who had a reduction in headache days of 50%, 75%, or 100%, for example. To extrapolate responder rates from the trial participants to the general population, a neurologist must know which groups of patients got worse on treatment, said Dr. Burch. Furthermore, the responder rates for older medications are unknown, because they were not examined. This situation makes comparisons of newer and older therapies more complicated.
Phase 3 trials of the CGRP drugs included analyses of the therapies’ 50% responder rates. This rate was about 42% for the 70-mg dose of erenumab and 50% for the 140-mg dose. The 50% responder rates for fremanezumab were 47.7% for the 225-mg dose and 44.4% for the 675-mg dose. In two trials of galcanezumab, the 50% responder rate for the 120-mg dose was approximately 60%, and the rate for the 240-mg dose was about 59%. The 50% responder rates for eptinezumab were 50% for the 100-mg dose and 56% for the 300-mg dose. The 50% responder rate across all trials was around 50%-60% in the active group, which is roughly 25% over the placebo group, said Dr. Burch.
Another measurement of efficacy is the efficacy-to-harm ratio, which is derived from the number needed to treat and the number needed to harm. To calculate this ratio, however, harm needs to be assessed adequately during a clinical trial. Although the ratio can provide a clinically relevant overview of a drug’s effects, patients may differ from each other in the way they evaluate efficacy and harm.
In addition, many questions about preventive treatment of migraine have no clear answers yet. It is uncertain, for example, how long a patient should receive preventive treatment and when treatment should be withdrawn, said Dr. Burch. “Can we expect that a lot of people are going to need to be on it for life, or is there a subpopulation who will get better and [for whom] we can withdraw [treatment]?” she asked. “How do we identify them?” Also, more data are needed before neurologists can understand why a given patient responds to one treatment, but not to another. It is difficult to predict which patients will respond to which treatments. Finally, it remains unclear how much of patients’ improvement can be attributed to regression to the mean, rather than preventive treatment.
REPORTING FROM HCNE STOWE 2020
Incidence of Chronic Opioid Use in Previously Opioid-Naïve Patients Receiving Opioids for Analgesia in the Intensive Care Unit
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
Red painful nodules in a hospitalized patient
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.
Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).
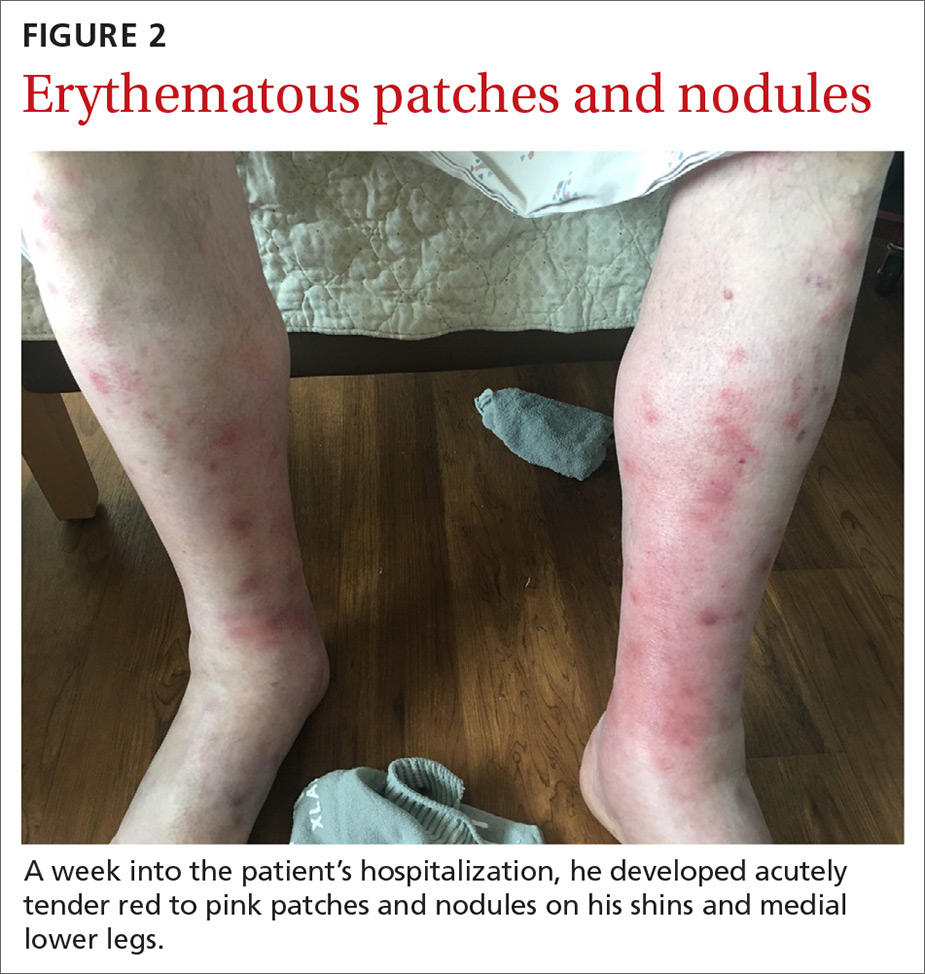
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).
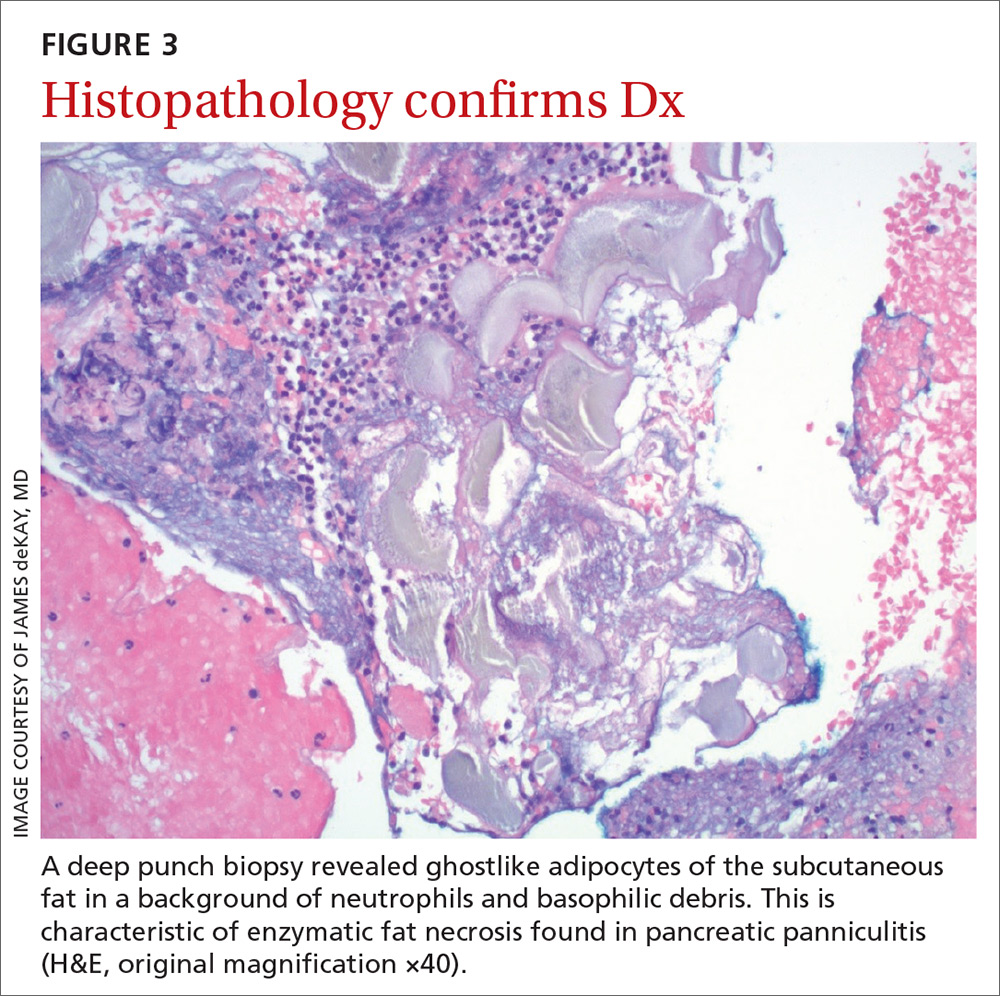
Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).
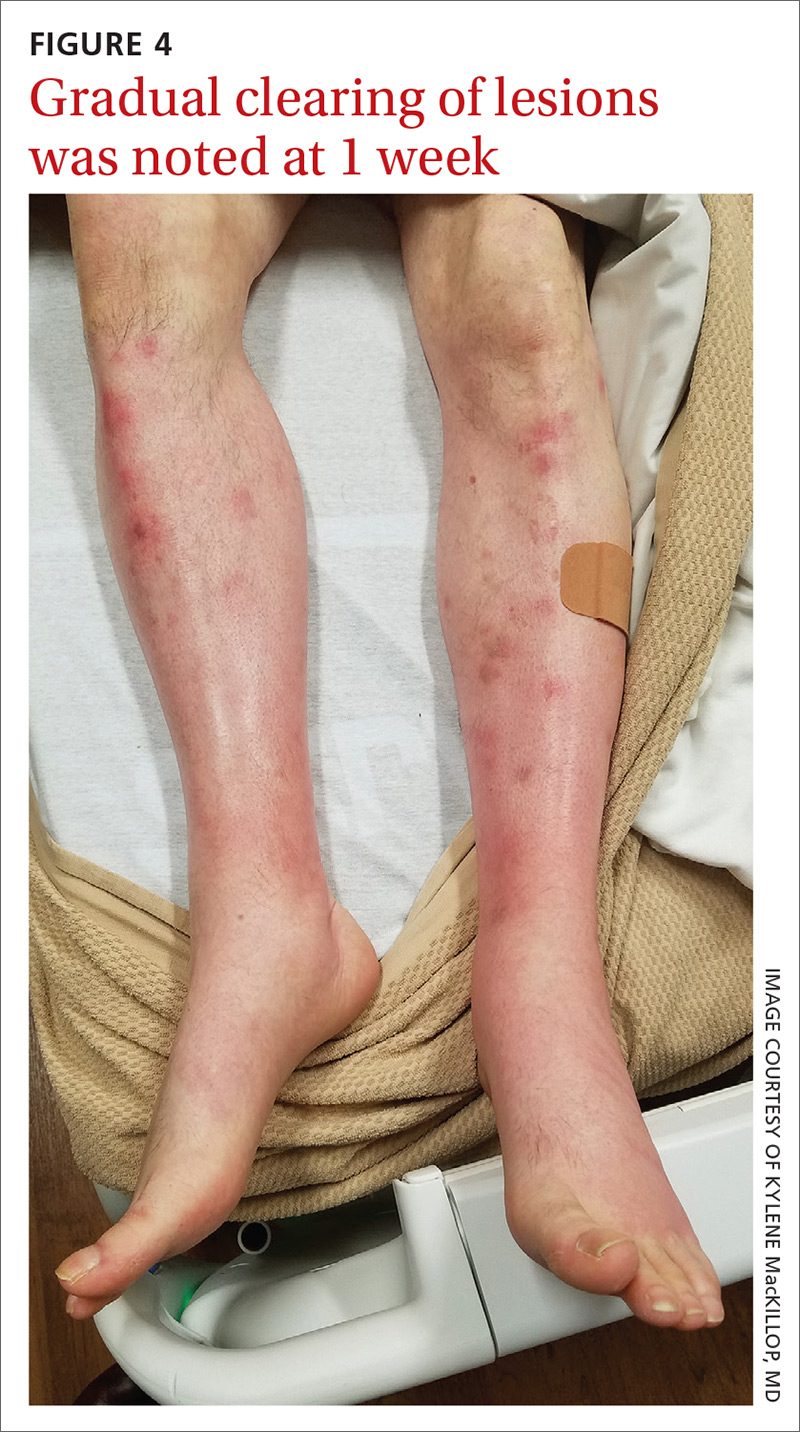
His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; [email protected]
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.

Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).

Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).

His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; [email protected]
A 58-year-old white man with a history of alcoholism presented to the emergency department with epigastric and right upper quadrant pain radiating to the back, as well as emesis and anorexia. An elevated lipase of 16,609 U/L (reference range, 31–186 U/L) and pathognomonic abdominal computed tomography (CT) findings (FIGURE 1) led to the diagnosis of acute pancreatitis, for which he was admitted.

Fluid resuscitation and pain management were implemented, and over 3 days his diet was advanced from NPO to clear fluids to a full diet. On the sixth day of hospitalization, the patient developed increasing abdominal pain and worsening leukocytosis (white blood cell count, 16.6–22 K/mcL [reference range, 4.5–11 K/mcL]). Repeat CT and blood cultures were obtained, and the patient was started on intravenous meropenem 1 g every 8 hours for presumed necrotizing pancreatitis. The next day he developed acutely tender red to pink patches and nodules on his shins and medial lower legs (FIGURE 2).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pancreatic panniculitis
It’s theorized that the systemic release of trypsin from pancreatic cell destruction causes increased capillary permeability and subsequent escape of lipase from the circulation into the subcutaneous fat. This causes fat necrosis, saponification, and inflammation.3,4 Pancreatic panniculitis is demonstrated histologically as hollowed-out adipocytes with granular basophilic cytoplasm and displaced or absent nuclei—aptly named “ghostlike” adipocytes.3-6
Painful, erythematous nodules most commonly present on the distal lower extremities. Nodules may be found over the shins, posterior calves, and periarticular skin. Rarely, nodules may occur on the buttocks, abdomen, or intramedullary bone.7 In severe cases, nodules spontaneously may ulcerate and drain an oily brown, viscous material formed from necrotic adipocytes.1
Timing of the eruption of skin lesions is varied and may even precede abdominal pain. Lesions can involute and regress several weeks after the underlying etiology improves. With pancreatic carcinoma, there is a greater likelihood of persistence, atypical locations of involvement, ulcerations, and recurrences.7
The histologic features of pancreatic panniculitis and the assessment of the subcutaneous fat are paramount in diagnosis. A deep punch biopsy or incisional biopsy is necessary to reliably reach the depth of the subcutaneous tissue. In our patient, a deep punch biopsy from the lateral calf was performed at the suggestion of Dermatology, and histopathology revealed necrosis of fat lobules with calcium soap around necrotic lipocytes, consistent with pancreatic panniculitis (FIGURE 3).

Continue to: Differential was complicated by antibiotic use
Differential was complicated by antibiotic use
The differential diagnosis was broad due to the confounding factors of recent antibiotic use and worsening pancreatitis.
Cellulitis may present as a red patch and is common on the lower legs; it often is associated with skin pathogens including Staphylococcus and Streptococcus. Usually, symptoms are unilateral and associated with warmth to the touch, expanding borders, leukocytosis, and systemic symptoms.
Vasculitis, which is an inflammation of various sized vessels through immunologic or infectious processes, often manifests on the lower legs. The characteristic sign of small vessel vasculitis is nonblanching purpura or petechiae. There often is a preceding illness or medication that triggers immunoglobulin proliferation and off-target inflammation of the vessels. Associated symptoms include pain and pruritus.
Drug eruptions may present as red patches on the skin. Often the patches are scaly and red and have more widespread distribution than the lower legs. A history of exposure is important, but common inciting drugs include nonsteroidal anti-inflammatory drugs that may be used only occasionally and are challenging to elicit in the history. Our patient did have known drug changes (ie, the introduction of meropenem) while hospitalized, but the morphology was not consistent with this diagnosis.
Treatment is directed to underlying disease
Treatment of pancreatic panniculitis primarily is supportive and directed toward treating the underlying pancreatic disease. Depending upon the underlying pancreatic diagnosis, surgical correction of anatomic or ductal anomalies or pseudocysts may lead to resolution of panniculitis.3,7,8
Continue to: In this case
In this case, our patient had already received fluid resuscitation and pain management, and his diet had been advanced. In addition, his antibiotics were changed to exclude drug eruption as a cause. Over the course of a week, our patient saw a reduction in his pain level and an improvement in the appearance of his legs (FIGURE 4).

His pancreatitis, however, continued to persist and resist increases in his diet. He ultimately required transfer to a tertiary care center for consideration of interventional options including stenting. The patient ultimately recovered, after stenting of the main pancreatic duct, and was discharged home.
CORRESPONDENCE
Jonathan Karnes, MD, 6 East Chestnut Street, Augusta, ME 04330; [email protected]
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
1. Madarasingha NP, Satgurunathan K, Fernando R. Pancreatic panniculitis: a rare form of panniculitis. Dermatol Online J. 2009;15:17.
2. Haber RM, Assaad DM. Panniculitis associated with a pancreas divisum. J Am Acad Dermatol. 1986;14(2 pt 2):331-334.
3. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
4. Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419-425.
5. Förström TL, Winkelmann RK. Acute, generalized panniculitis with amylase and lipase in skin. Arch Dermatol. 1975;111:497-502.
6. Hughes SH, Apisarnthanarax P, Mullins F. Subcutaneous fat necrosis associated with pancreatic disease. Arch Dermatol. 1975;111:506-510.
7. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
8. Lambiase P, Seery JP, Taylor-Robinson SD, et al. Resolution of panniculitis after placement of pancreatic duct stent in chro nic pancreatitis. Am J Gastroenterol. 1996;91:1835-1837.
Sharp lower back pain • left-side paraspinal tenderness • anterior thigh sensory loss • Dx?
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction
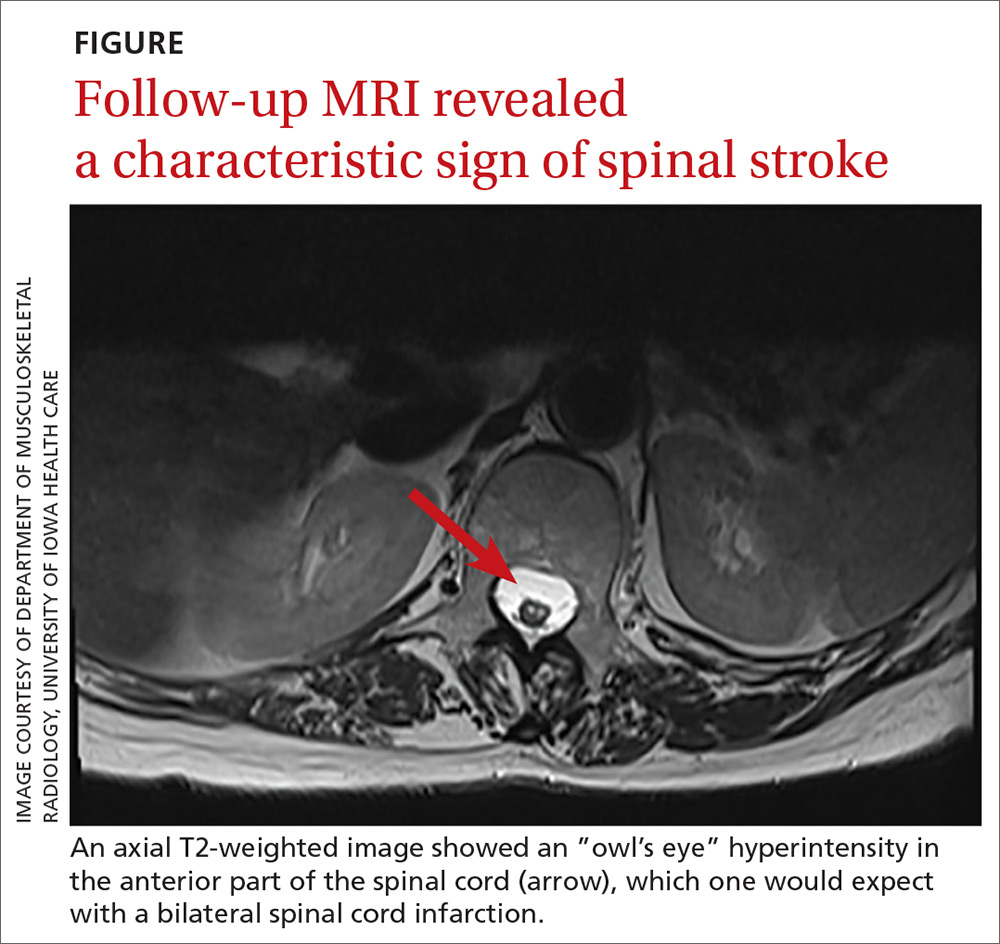
THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; [email protected].
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction

THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; [email protected].
THE CASE
A 64-year-old woman with a history of late-onset type 1 diabetes mellitus, Hashimoto thyroiditis, and scoliosis presented to the sports medicine clinic with acute-onset, sharp, nonradiating right lower back pain that began when she bent forward to apply lotion. At presentation, she denied fever, chills, numbness, tingling, aggravation of pain with movement, weakness, and incontinence. Her neuromuscular examination was unremarkable except for left-side paraspinal tenderness. She was prescribed cyclobenzaprine for symptomatic relief.
Two days later, she was seen for worsening pain. Her physical exam was unchanged. She was prescribed tramadol and advised to start physical therapy gradually. As the day progressed, however, she developed anterior thigh sensory loss, which gradually extended distally.
The following day, she was brought to the emergency department with severe left-side weakness without urinary incontinence. Her mental status and cranial nerve exams were normal. On examination, strength of the iliopsoas and quadriceps was 1/5 bilaterally, and of the peroneal tendon and gastrocnemius, 3/5 bilaterally. Reflexes of triceps, biceps, knee, and Achilles tendon were symmetric and 3+ with bilateral clonus of the ankle. The Babinski sign was positive bilaterally. The patient had diminished pain sensation bilaterally, extending down from the T11 dermatome (left more than right side) with diminished vibration sensation at the left ankle. Her perianal sensation, bilateral temperature sensation, and cerebellar examination were normal.
Magnetic resonance imaging (MRI) without contrast of the lumbar spine demonstrated ischemia findings corresponding to T12-L1. Degenerative changes from L1-S1 were noted, with multiple osteophytes impinging on the neural foramina without cord compression.
THE DIAGNOSIS
The initial presentation was consistent with mechanical low back pain with signs of anterior spinal artery infarction and medial lemniscus pathway involvement 48 hours after initial presentation. Spinal cord infarction occurs more commonly in women and in the young than does cerebral infarction,1 with better reemployment rates.1,2 Similar to other strokes, long-term prognosis is primarily determined by the initial severity of motor impairment, which is linked to long-term immobility and need for bladder catheterization.3
Neurogenic pain developing years after spinal cord infarction is most often observed in anterior spinal artery infarction4 without functional limitations.
Initial treatment. Our patient was started on aspirin 325 mg/d and clopidogrel 75 mg/d. Her mean arterial blood pressure was maintained above 80 mm Hg. Computed tomography angiography of the abdomen and pelvis was negative for aortic dissection. Lumbar puncture for cerebrospinal fluid analysis was unremarkable. Results of antineutrophil cytoplasmic antibody testing, antinuclear antibody testing, a hepatitis panel, and an antiphospholipid panel were all negative. The patient was started on IV steroids with a plan for gradual tapering. The neurosurgical team agreed with medical management.
Continue to: DISCUSSION
DISCUSSION
Possible etiologies for acute spinal cord infarction include spinal cord ischemia from compression of the vessels, fibrocartilaginous embolism, and arterial thrombosis or atherosclerosis, especially in patients with diabetes.5
The majority (86%) of spinal strokes are due to spontaneous occlusion of the vessels with no identifiable cause; much less frequently (9% of cases), hemorrhage is the causative factor.1 A retrospective study demonstrated that 10 of 27 patients with spinal stroke had an anterior spinal infarct. Of those 10 patients, 6 reported a mechanical triggering movement (similar to this case), indicating potential compression of the radicular arteries due to said movement.4
Fibrocartilaginous embolism (FCE) is worth considering as a possible cause, because it accounts for 5.5% of all cases of acute spinal cord infarction.3 FCE is thought to arise after a precipitating event such as minor trauma, heavy lifting, physical exertion, or Valsalva maneuver causing embolization of the fragments of nucleus pulposus to the arterial system. In a case series of 8 patients, 2 had possible FCE with precipitating events occurring within the prior 24 hours. This was also demonstrated in another case series6 in which 7 of 9 patients had precipitating events.
Although FCE can only definitively be diagnosed postmortem, the researchers6 proposed clinical criteria for its diagnosis in living patients, based on 40 postmortem and 11 suspected antemortem cases of FCE. These criteria include a rapid evolution of symptoms consistent with vascular etiology, with or without preceding minor trauma or Valsalva maneuver; MRI changes consistent with ischemic myelopathy, with or without evidence of disc herniation; and no more than 2 vascular risk factors.
Our patient had no trauma (although there was a triggering movement), no signs of disc herniation, and 2 risk factors (> 60 years and diabetes mellitus). Also, a neurologically symptom-free interval between the painful movement and the onset of neurologic manifestations in our case parallels the clinical picture of FCE.
Continue to: The role of factor V Leiden (FVL) mutation
The role of factor V Leiden (FVL) mutation in arterial thrombosis is questionable. Previous reports demonstrate a risk for venous thrombosis 7 to 10 times higher with heterozygous FVL mutation and 100 times higher with homozygous mutation, with a less established role in arterial thrombosis.7 A retrospective Turkish study compared the incidence of FVL mutation in patients with arterial thrombosis vs healthy subjects; incidence was significantly higher in female patients than female controls (37.5% vs. 2%).7 A meta-analysis of published studies showed an association between arterial ischemic events and FVL mutation to be modest, with an odds ratio of 1.21 (95% CI, 0.99-1.49).8
In contrast, a 3.4-year longitudinal health study of patients ages 65 and older found no significant difference in the occurrence of myocardial infarction, transient ischemic attack, stroke, or angina for more than 5000 patients with heterozygous FVL mutation compared to fewer than 500 controls.9 The case patient’s clinical course did not fit a thrombotic clinical picture.
Evaluating for “red flags” is crucial in any case of low back pain to exclude serious pathologies. Red flag symptoms include signs of myelopathy, signs of infection, history of trauma with focal tenderness to palpation, and steroid or anticoagulant use (to rule out medication adverse effects).10 Our patient lacked these classical signs, but she did have subjective pain out of proportion to the clinical exam findings.
Of note: The above red flags for low back pain are all based on expert opinion,11 and the positive predictive value of a red flag is always low because of the low prevalence of serious spinal pathologies.12
Striking a proper balance. This case emphasizes the necessity to keep uncommon causes—such as nontraumatic spinal stroke, which has a prevalence of about 5% to 8% of all acute myelopathies—in the differential diagnosis.3
Continue to: We recommend watchful...
We recommend watchful waiting coupled with communication with the patient regarding monitoring for changes in symptoms over time.11 Any changes in symptoms concerning for underlying spinal cord injury indicate necessity for transfer to a tertiary care center (if possible), along with immediate evaluation with imaging—including computed tomography angiography of the abdomen to rule out aortic dissection (1%-2% of all spinal cord infarcts), followed by a specialist consultation based on the findings.3
Our patient
Our patient was discharged to rehabilitation on hospital Day 5, after progressive return of lower extremity strength. At the 2-month follow-up visit, she demonstrated grade 4+ strength throughout her lower extremities bilaterally. Weakness was predominant at the hip flexors and ankle dorsiflexors, which was consistent with her status at discharge. She had burning pain in the distribution of the L1 dermatome that responded to ibuprofen.
Hypercoagulability work-up was positive for heterozygous FVL mutation without any previous history of venous thromboembolic disease. She was continued on aspirin 325 mg/d, as per American College of Chest Physicians antithrombotic guidelines.13
One year later, our patient underwent a follow-up MRI of the thoracic spine, which showed an “owl’s eye” hyperintensity in the anterior cord (FIGURE), a sign that’s often seen in bilateral spinal cord infarction

THE TAKEAWAY
Spinal stroke is rare, but a missed diagnosis and lack of treatment can result in long-term morbidity. Therefore, it is prudent to consider this diagnosis in the differential—especially when the patient’s subjective back pain is out of proportion to the clinical examination findings.
CORRESPONDENCE
Srikanth Nithyanandam, MBBS, MS, University of Kentucky Family and Community Medicine, 2195 Harrodsburg Road, Suite 125, Lexington, KY 40504-3504; [email protected].
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.
1. Romi F, Naess H. Spinal cord infarction in clinical neurology: a review of characteristics and long-term prognosis in comparison to cerebral infarction. Eur Neurol. 2016;76:95-98.
2. Hanson SR, Romi F, Rekand T, et al. Long-term outcome after spinal cord infarctions. Acta Neurol Scand. 2015;131:253-257.
3. Rigney L, Cappelen-Smith C, Sebire D, et al. Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci. 2015;22:1544-1549.
4. Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120.
5. Goldstein LB, Adams R, Alberts MJ, et al; American Heart Association; American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873-e923.
6. Mateen FJ, Monrad PA, Hunderfund AN, et al. Clinically suspected fibrocartilaginous embolism: clinical characteristics, treatments, and outcomes. Eur J Neurol. 2011;18:218-225.
7. Ozmen F, Ozmen MM, Ozalp N, et al. The prevalence of factor V (G1691A), MTHFR (C677T) and PT (G20210A) gene mutations in arterial thrombosis. Ulus Travma Acil Cerrahi Derg. 2009;15:113-119.
8. Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146:948-957.
9. Cushman M, Rosendaal FR, Psaty BM, et al. Factor V Leiden is not a risk factor for arterial vascular disease in the elderly: results from the Cardiovascular Health Study. Thromb Haemost. 1998;79:912-915.
10. Strudwick K, McPhee M, Bell A, et al. Review article: best practice management of low back pain in the emergency department (part 1 of the musculoskeletal injuries rapid review series). Emerg Med Australas. 2018;30:18-35.
11. Cook CE, George SZ, Reiman MP. Red flag screening for low back pain: nothing to see here, move along: a narrative review. Br J Sports Med. 2018;52:493-496.
12. Grunau GL, Darlow B, Flynn T, et al. Red flags or red herrings? Redefining the role of red flags in low back pain to reduce overimaging. Br J Sports Med. 2018;52:488-489.
13. Lansberg MG, O’Donnell MJ, Khatri P, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e601S-e636S.
14. Pikija S, Mutzenbach JS, Kunz AB, et al. Delayed hospital presentation and neuroimaging in non-surgical spinal cord infarction. Front Neurol. 2017;8:143.