User login
Managing pain expectations is key to enhanced recovery
Planning for reduced use of opioids in pain management involves identifying appropriate patients and managing their expectations, according to according to Timothy E. Miller, MB, ChB, FRCA, of Duke University, Durham, N.C., who is president of the American Society for Enhanced Recovery.
, he said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education.
Dr. Miller shared a treatment algorithm for achieving optimal analgesia in patients after colorectal surgery that combines intravenous or oral analgesia with local anesthetics and additional nonopioid options. The algorithm involves choosing NSAIDs, acetaminophen, or gabapentin for IV/oral use. In addition, options for local anesthetic include with a choice of single-shot transversus abdominis plane (TAP) block.
Careful patient selection is key to an opioid-free or opioid reduced anesthetic strategy, Dr. Miller said. The appropriate patients have “no chronic opioids, no anxiety, and the desire to avoid opioid side effects,” he said.
Opioid-free or opioid-reduced strategies include realigning patient expectations to prepare for pain at a level of 2-4 on a scale of 10 as “expected and reasonable,” he said. Patients given no opioids or reduced opioids may report cramping after laparoscopic surgery, as well as shoulder pain that is referred from the CO2 bubble under the diaphragm, he said. However, opioids don’t treat the shoulder pain well, and “walking or changing position usually relieves this pain,” and it usually resolves within 24 hours, Dr. Miller noted. “Just letting the patient know what is expected in terms of pain relief in their recovery is hugely important,” he said.
The optimal analgesia after surgery is a plan that combines optimized patient comfort with the fastest functional recovery and the fewest side effects, he emphasized.
Optimized patient comfort includes optimal pain ratings at rest and with movement, a decreasing impact of pain on emotion, function, and sleep disruption, and an improvement in the patient experience, he said. The fastest functional recovery is defined as a return to drinking liquids, eating solid foods, performing activities of daily living, and maintaining normal bladder, bowel, and cognitive function. Side effects to be considered in analgesia included nausea, vomiting, sedation, ileus, itching, dizziness, and delirium, he said.
In an unpublished study, Dr. Miller and colleagues eliminated opioids intraoperatively in a series of 56 cases of laparoscopic cholecystectomy and found significantly less opioids needed in the postanesthesia care unit (PACU). In addition, opioid-free patients had significantly shorter length of stay in the PACU, he said. “We are writing this up for publication and looking into doing larger studies,” Dr. Miller said.
Questions include whether the opioid-free technique translates more broadly, he said.
In addition, it is important to continue to collect data and study methods to treat pain and reduce opioid use perioperatively, Dr. Miller said. Some ongoing concerns include data surrounding the use of gabapentin and possible association with respiratory depression, he noted. Several meta-analyses have suggested that “gabapentinoids (gabapentin, pregabalin) when given as a single dose preoperatively are associated with a decrease in postoperative pain and opioid consumption at 24 hours,” said Dr. Miller. “When gabapentinoids are included in multimodal analgesic regimens, intraoperative opioids must be reduced, and increased vigilance for respiratory depression may be warranted, especially in elderly patients,” he said.
Overall, opioid-free anesthesia is both feasible and appropriate in certain patient populations, Dr. Miller concluded. “Implement your pathway and measure your outcomes with timely feedback so you can revise your protocol based on data,” he emphasized.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Miller disclosed relationships with Edwards Lifesciences, and serving as a board member for the Perioperative Quality Initiative and as a founding member of the Morpheus Consortium.
Planning for reduced use of opioids in pain management involves identifying appropriate patients and managing their expectations, according to according to Timothy E. Miller, MB, ChB, FRCA, of Duke University, Durham, N.C., who is president of the American Society for Enhanced Recovery.
, he said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education.
Dr. Miller shared a treatment algorithm for achieving optimal analgesia in patients after colorectal surgery that combines intravenous or oral analgesia with local anesthetics and additional nonopioid options. The algorithm involves choosing NSAIDs, acetaminophen, or gabapentin for IV/oral use. In addition, options for local anesthetic include with a choice of single-shot transversus abdominis plane (TAP) block.
Careful patient selection is key to an opioid-free or opioid reduced anesthetic strategy, Dr. Miller said. The appropriate patients have “no chronic opioids, no anxiety, and the desire to avoid opioid side effects,” he said.
Opioid-free or opioid-reduced strategies include realigning patient expectations to prepare for pain at a level of 2-4 on a scale of 10 as “expected and reasonable,” he said. Patients given no opioids or reduced opioids may report cramping after laparoscopic surgery, as well as shoulder pain that is referred from the CO2 bubble under the diaphragm, he said. However, opioids don’t treat the shoulder pain well, and “walking or changing position usually relieves this pain,” and it usually resolves within 24 hours, Dr. Miller noted. “Just letting the patient know what is expected in terms of pain relief in their recovery is hugely important,” he said.
The optimal analgesia after surgery is a plan that combines optimized patient comfort with the fastest functional recovery and the fewest side effects, he emphasized.
Optimized patient comfort includes optimal pain ratings at rest and with movement, a decreasing impact of pain on emotion, function, and sleep disruption, and an improvement in the patient experience, he said. The fastest functional recovery is defined as a return to drinking liquids, eating solid foods, performing activities of daily living, and maintaining normal bladder, bowel, and cognitive function. Side effects to be considered in analgesia included nausea, vomiting, sedation, ileus, itching, dizziness, and delirium, he said.
In an unpublished study, Dr. Miller and colleagues eliminated opioids intraoperatively in a series of 56 cases of laparoscopic cholecystectomy and found significantly less opioids needed in the postanesthesia care unit (PACU). In addition, opioid-free patients had significantly shorter length of stay in the PACU, he said. “We are writing this up for publication and looking into doing larger studies,” Dr. Miller said.
Questions include whether the opioid-free technique translates more broadly, he said.
In addition, it is important to continue to collect data and study methods to treat pain and reduce opioid use perioperatively, Dr. Miller said. Some ongoing concerns include data surrounding the use of gabapentin and possible association with respiratory depression, he noted. Several meta-analyses have suggested that “gabapentinoids (gabapentin, pregabalin) when given as a single dose preoperatively are associated with a decrease in postoperative pain and opioid consumption at 24 hours,” said Dr. Miller. “When gabapentinoids are included in multimodal analgesic regimens, intraoperative opioids must be reduced, and increased vigilance for respiratory depression may be warranted, especially in elderly patients,” he said.
Overall, opioid-free anesthesia is both feasible and appropriate in certain patient populations, Dr. Miller concluded. “Implement your pathway and measure your outcomes with timely feedback so you can revise your protocol based on data,” he emphasized.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Miller disclosed relationships with Edwards Lifesciences, and serving as a board member for the Perioperative Quality Initiative and as a founding member of the Morpheus Consortium.
Planning for reduced use of opioids in pain management involves identifying appropriate patients and managing their expectations, according to according to Timothy E. Miller, MB, ChB, FRCA, of Duke University, Durham, N.C., who is president of the American Society for Enhanced Recovery.
, he said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education.
Dr. Miller shared a treatment algorithm for achieving optimal analgesia in patients after colorectal surgery that combines intravenous or oral analgesia with local anesthetics and additional nonopioid options. The algorithm involves choosing NSAIDs, acetaminophen, or gabapentin for IV/oral use. In addition, options for local anesthetic include with a choice of single-shot transversus abdominis plane (TAP) block.
Careful patient selection is key to an opioid-free or opioid reduced anesthetic strategy, Dr. Miller said. The appropriate patients have “no chronic opioids, no anxiety, and the desire to avoid opioid side effects,” he said.
Opioid-free or opioid-reduced strategies include realigning patient expectations to prepare for pain at a level of 2-4 on a scale of 10 as “expected and reasonable,” he said. Patients given no opioids or reduced opioids may report cramping after laparoscopic surgery, as well as shoulder pain that is referred from the CO2 bubble under the diaphragm, he said. However, opioids don’t treat the shoulder pain well, and “walking or changing position usually relieves this pain,” and it usually resolves within 24 hours, Dr. Miller noted. “Just letting the patient know what is expected in terms of pain relief in their recovery is hugely important,” he said.
The optimal analgesia after surgery is a plan that combines optimized patient comfort with the fastest functional recovery and the fewest side effects, he emphasized.
Optimized patient comfort includes optimal pain ratings at rest and with movement, a decreasing impact of pain on emotion, function, and sleep disruption, and an improvement in the patient experience, he said. The fastest functional recovery is defined as a return to drinking liquids, eating solid foods, performing activities of daily living, and maintaining normal bladder, bowel, and cognitive function. Side effects to be considered in analgesia included nausea, vomiting, sedation, ileus, itching, dizziness, and delirium, he said.
In an unpublished study, Dr. Miller and colleagues eliminated opioids intraoperatively in a series of 56 cases of laparoscopic cholecystectomy and found significantly less opioids needed in the postanesthesia care unit (PACU). In addition, opioid-free patients had significantly shorter length of stay in the PACU, he said. “We are writing this up for publication and looking into doing larger studies,” Dr. Miller said.
Questions include whether the opioid-free technique translates more broadly, he said.
In addition, it is important to continue to collect data and study methods to treat pain and reduce opioid use perioperatively, Dr. Miller said. Some ongoing concerns include data surrounding the use of gabapentin and possible association with respiratory depression, he noted. Several meta-analyses have suggested that “gabapentinoids (gabapentin, pregabalin) when given as a single dose preoperatively are associated with a decrease in postoperative pain and opioid consumption at 24 hours,” said Dr. Miller. “When gabapentinoids are included in multimodal analgesic regimens, intraoperative opioids must be reduced, and increased vigilance for respiratory depression may be warranted, especially in elderly patients,” he said.
Overall, opioid-free anesthesia is both feasible and appropriate in certain patient populations, Dr. Miller concluded. “Implement your pathway and measure your outcomes with timely feedback so you can revise your protocol based on data,” he emphasized.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Miller disclosed relationships with Edwards Lifesciences, and serving as a board member for the Perioperative Quality Initiative and as a founding member of the Morpheus Consortium.
FROM MISS
Pursue multimodal pain management in patients taking opioids
For surgical patients on chronic opioid therapy, , according to Stephanie B. Jones, MD, professor and chair of anesthesiology at Albany Medical College, New York.
“[With] any patient coming in for any sort of surgery, you should be considering multimodal pain management. That applies to the opioid use disorder patient as well,” Dr. Jones said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education.
“The challenge of opioid-tolerant patients or opioid abuse patients is twofold – tolerance and hyperalgesia,” Dr. Jones said. Patient tolerance changes how patients perceive pain and respond to medication. Clinicians need to consider the “opioid debt,” defined as the daily amount of opioid medication required by opioid-dependent patients to maintain their usual prehospitalization opioid levels, she explained. Also consider hyperalgesia, a change in pain perception “resulting in an increase in pain sensitivity to painful stimuli, thereby decreasing the analgesic effects of opioids,” Dr. Jones added.
A multimodal approach to pain management in patients on chronic opioids can include some opioids as appropriate, Dr. Jones said. Modulation of pain may draw on epidurals and nerve blocks, as well as managing CNS perception of pain through opioids or acetaminophen, and also using systemic options such as alpha-2 agonists and tramadol, she said.
Studies have shown that opioid abuse or dependence were associated with increased readmission rates, length of stay, and health care costs in surgery patients, said Dr. Jones. However, switching opioids and managing equivalents is complex, and “equianalgesic conversions serve only as a general guide to estimate opioid dose equivalents,” according to UpToDate’s, “Management of acute pain in the patient chronically using opioids,” she said.
Dr. Jones also addressed the issue of using hospitalization as an opportunity to help patients with untreated opioid use disorder. Medication-assisted options include methadone, buprenorphine, and naltrexone.
“One problem with methadone is that there are a lot of medications interactions,” she said. Buprenorphine has the advantage of being long-lasting, and is formulated with naloxone which deters injection. “Because it is a partial agonist, there is a lower risk of overdose and sedation,” and it has fewer medication interactions. However, some doctors are reluctant to prescribe it and there is some risk of medication diversion, she said.
Naltrexone is newer to the role of treating opioid use disorder, Dr. Jones said. “It can cause acute withdrawal because it is a full opioid antagonist,” she noted. However, naltrexone itself causes no withdrawal if stopped, and no respiratory depression or sedation, said Dr. Jones.
“Utilize addiction services in your hospital if you suspect a patient may be at risk for opioid use disorder,” and engage these services early, she emphasized.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Jones had no financial conflicts to disclose.
For surgical patients on chronic opioid therapy, , according to Stephanie B. Jones, MD, professor and chair of anesthesiology at Albany Medical College, New York.
“[With] any patient coming in for any sort of surgery, you should be considering multimodal pain management. That applies to the opioid use disorder patient as well,” Dr. Jones said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education.
“The challenge of opioid-tolerant patients or opioid abuse patients is twofold – tolerance and hyperalgesia,” Dr. Jones said. Patient tolerance changes how patients perceive pain and respond to medication. Clinicians need to consider the “opioid debt,” defined as the daily amount of opioid medication required by opioid-dependent patients to maintain their usual prehospitalization opioid levels, she explained. Also consider hyperalgesia, a change in pain perception “resulting in an increase in pain sensitivity to painful stimuli, thereby decreasing the analgesic effects of opioids,” Dr. Jones added.
A multimodal approach to pain management in patients on chronic opioids can include some opioids as appropriate, Dr. Jones said. Modulation of pain may draw on epidurals and nerve blocks, as well as managing CNS perception of pain through opioids or acetaminophen, and also using systemic options such as alpha-2 agonists and tramadol, she said.
Studies have shown that opioid abuse or dependence were associated with increased readmission rates, length of stay, and health care costs in surgery patients, said Dr. Jones. However, switching opioids and managing equivalents is complex, and “equianalgesic conversions serve only as a general guide to estimate opioid dose equivalents,” according to UpToDate’s, “Management of acute pain in the patient chronically using opioids,” she said.
Dr. Jones also addressed the issue of using hospitalization as an opportunity to help patients with untreated opioid use disorder. Medication-assisted options include methadone, buprenorphine, and naltrexone.
“One problem with methadone is that there are a lot of medications interactions,” she said. Buprenorphine has the advantage of being long-lasting, and is formulated with naloxone which deters injection. “Because it is a partial agonist, there is a lower risk of overdose and sedation,” and it has fewer medication interactions. However, some doctors are reluctant to prescribe it and there is some risk of medication diversion, she said.
Naltrexone is newer to the role of treating opioid use disorder, Dr. Jones said. “It can cause acute withdrawal because it is a full opioid antagonist,” she noted. However, naltrexone itself causes no withdrawal if stopped, and no respiratory depression or sedation, said Dr. Jones.
“Utilize addiction services in your hospital if you suspect a patient may be at risk for opioid use disorder,” and engage these services early, she emphasized.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Jones had no financial conflicts to disclose.
For surgical patients on chronic opioid therapy, , according to Stephanie B. Jones, MD, professor and chair of anesthesiology at Albany Medical College, New York.
“[With] any patient coming in for any sort of surgery, you should be considering multimodal pain management. That applies to the opioid use disorder patient as well,” Dr. Jones said in a presentation at the virtual Annual Minimally Invasive Surgery Symposium sponsored by Global Academy for Medical Education.
“The challenge of opioid-tolerant patients or opioid abuse patients is twofold – tolerance and hyperalgesia,” Dr. Jones said. Patient tolerance changes how patients perceive pain and respond to medication. Clinicians need to consider the “opioid debt,” defined as the daily amount of opioid medication required by opioid-dependent patients to maintain their usual prehospitalization opioid levels, she explained. Also consider hyperalgesia, a change in pain perception “resulting in an increase in pain sensitivity to painful stimuli, thereby decreasing the analgesic effects of opioids,” Dr. Jones added.
A multimodal approach to pain management in patients on chronic opioids can include some opioids as appropriate, Dr. Jones said. Modulation of pain may draw on epidurals and nerve blocks, as well as managing CNS perception of pain through opioids or acetaminophen, and also using systemic options such as alpha-2 agonists and tramadol, she said.
Studies have shown that opioid abuse or dependence were associated with increased readmission rates, length of stay, and health care costs in surgery patients, said Dr. Jones. However, switching opioids and managing equivalents is complex, and “equianalgesic conversions serve only as a general guide to estimate opioid dose equivalents,” according to UpToDate’s, “Management of acute pain in the patient chronically using opioids,” she said.
Dr. Jones also addressed the issue of using hospitalization as an opportunity to help patients with untreated opioid use disorder. Medication-assisted options include methadone, buprenorphine, and naltrexone.
“One problem with methadone is that there are a lot of medications interactions,” she said. Buprenorphine has the advantage of being long-lasting, and is formulated with naloxone which deters injection. “Because it is a partial agonist, there is a lower risk of overdose and sedation,” and it has fewer medication interactions. However, some doctors are reluctant to prescribe it and there is some risk of medication diversion, she said.
Naltrexone is newer to the role of treating opioid use disorder, Dr. Jones said. “It can cause acute withdrawal because it is a full opioid antagonist,” she noted. However, naltrexone itself causes no withdrawal if stopped, and no respiratory depression or sedation, said Dr. Jones.
“Utilize addiction services in your hospital if you suspect a patient may be at risk for opioid use disorder,” and engage these services early, she emphasized.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Jones had no financial conflicts to disclose.
FROM MISS
EULAR gives pointers on intra-articular injection best practices
New EULAR recommendations for the intra-articular (IA) treatment of arthropathies aim to facilitate uniformity and quality of care for this mainstay of rheumatologic practice, according to a report on the new guidance that was presented at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Until now there were no official recommendations on how best to use it in everyday practice. “This is the first time that there’s been a joint effort to develop evidence-based recommendations,” Jacqueline Usón, MD, PhD, associate professor medicine at Rey Juan Carlos University in Madrid, said in an interview. “Everything that we are saying is pretty logical, but it’s nice to see it put in recommendations based on evidence.”
IA therapy has been around for decades and is key for treating adults with a number of different conditions where synovitis, effusion, pain, or all three, are present, such as inflammatory arthritis and osteoarthritis, Dr. Usón observed during her presentation.
“Today, commonly used injectables are not only corticosteroids but also local anesthetics, hyaluronic acid, blood products, and maybe pharmaceuticals,” she said, adding that “there is a wide variation in the way intra-articular therapies are used and delivered to patients.” Health professionals also have very different views and habits depending on geographic locations and health care systems, she observed. Ironing out the variation was one of the main objectives of the recommendations.
As one of the two conveners of the EULAR task force behind the recommendations, Dr. Usón, herself a rheumatologist at University Hospital of Móstoles, pointed out that the task force brought together a range of specialties – rheumatologists, orthopedic surgeons, radiologists, nuclear medicine specialists, among others, as well as patients – to ensure that the best advice could be given.
The task force followed EULAR standard operating procedures for developing recommendations, with discussion groups, systematic literature reviews, and Delphi technique-based consensus all being employed. The literature search considered publications from 1946 up until 2019.
“We agreed on the need for more background information from health professionals and patients, so we developed two surveys: One for health professionals with 160 items, [for which] we obtained 186 responses from 26 countries; and the patient survey was made up of 44 items, translated into 10 different languages, and we obtained 200 responses,” she said.
The results of the systematic literature review and surveys were used to help form expert consensus, leading to 5 overarching principles and 11 recommendations that look at before, during, and after intra-articular therapy.
Five overarching principles
The first overarching principle recognizes the widespread use of IA therapies and that their use is specific to the disease that is being treated and “may not be interchangeable across indications,” Dr. Usón said. The second principle concerns improving patient-centered outcomes, which are “those that are relevant to the patient,” and include the benefits, harms, preferences, or implications for self-management.
“Contextual factors are important and contribute to the effect of IAT [intra-articular treatment],” she said, discussing the third principle. “These include effective communication, patient expectations, or settings [where the procedure takes place]. In addition, one should take into account that the route of delivery has in itself a placebo effect. We found that in different RCTs [randomized controlled trials], the pooled placebo effect of IA saline is moderate to large.”
The fourth principle looks at ensuring that patients and clinicians make an informed and shared decision, which is again highlighted by the first recommendation. The fifth, and last, overarching principle acknowledges that IA injections may be given by a range of health care professionals.
Advice for before, during, and after injection
Patients need to be “fully informed of the nature of the procedure, the injectable used, and potential effects – benefits and risks – [and] informed consent should be obtained and documented,” said Dr. Usón, outlining the first recommendation. “That seems common,” she said in the interview, “but when we did the survey, we realize that many patients didn’t [give consent], and the doctors didn’t even ask for it. This is why it’s a very general statement, and it’s our first recommendation. The agreement was 99%!”
The recommendations also look at the optimal settings for performing injections, such as providing a professional and private, well-lighted room, and having a resuscitation kit nearby in case patients faint. Accuracy is important, Dr. Usón said, and imaging, such as ultrasound, should be used where available to ensure accurate injection into the joint. This is an area where further research could be performed, she said, urging young rheumatologists and health professionals to consider this. “Intra-articular therapy is something that you learn and do, but you never really investigate in it,” she said.
One recommendation states that when intra-articular injections are being given to pregnant patients, the safety of injected compound must be considered, both for the mother and for the fetus. There is another recommendation on the need to perform IA injections under aseptic conditions, and another stating that patients should be offered local anesthetics, after explaining the pros and cons.
Special populations of patients are also considered, Dr. Usón said. For example, the guidance advises warning patients with diabetes of the risk of transient glycemia after IA glucocorticoids and the need to monitor their blood glucose levels carefully for a couple of days afterward.
As a rule, “IAT is not a contraindication to people with clotting or bleeding disorders, or taking antithrombotic medications,” she said, unless they are at a high risk of bleeding.
Importantly, the recommendations cover when IAT can be performed after joint replacement surgery (after at least 3 months), and the need to “avoid overuse of injected joints” while also avoiding complete immobilization for at least 24 hours afterward. The recommendations very generally cover re-injections, but not how long intervals between injections should be. When asked about interval duration after her presentation, Dr. Usón said that the usual advice is to give IA injections no more than 2-3 times a year, but it depends on the injectable.
“It wasn’t our intention to review the efficacy and the safety of the different injectables, nor to review the use of IAT in different types of joint diseases,” she said. “We do lack a lot of information, a lot of evidence in this, and I really would hope that new rheumatologists start looking into and start investigating in this topic,” she added.
Recommendations will increase awareness of good clinical practice
“IA injections are commonly administered in the rheumatology setting. This is because [IA injection] is often a useful treatment for acute flare of arthritis, particularly when it is limited to a few joints,” observed Ai Lyn Tan, MD, associate professor and honorary consultant rheumatologist at the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine.
IA injection “also relieves symptoms relatively quickly for patients; however, the response can be variable, and there are side effects associated with IA injections,” Dr. Tan added in an interview.
There is a lack of universally accepted recommendations, Dr. Tan observed, noting that while there might be some local guidelines on how to safely perform IA injections these were often not standardized and were subject to being continually updated to try to improve the experience for patients.
“It is therefore timely to learn about the new EULAR recommendations for IA injections. The advantage of this will be to increase awareness of good clinical practice for performing IA injections.”
Dr. Tan had no relevant conflicts of interest.
SOURCE: EULAR COVID-19 Recommendations. E-congress content available until Sept. 1, 2020.
New EULAR recommendations for the intra-articular (IA) treatment of arthropathies aim to facilitate uniformity and quality of care for this mainstay of rheumatologic practice, according to a report on the new guidance that was presented at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Until now there were no official recommendations on how best to use it in everyday practice. “This is the first time that there’s been a joint effort to develop evidence-based recommendations,” Jacqueline Usón, MD, PhD, associate professor medicine at Rey Juan Carlos University in Madrid, said in an interview. “Everything that we are saying is pretty logical, but it’s nice to see it put in recommendations based on evidence.”
IA therapy has been around for decades and is key for treating adults with a number of different conditions where synovitis, effusion, pain, or all three, are present, such as inflammatory arthritis and osteoarthritis, Dr. Usón observed during her presentation.
“Today, commonly used injectables are not only corticosteroids but also local anesthetics, hyaluronic acid, blood products, and maybe pharmaceuticals,” she said, adding that “there is a wide variation in the way intra-articular therapies are used and delivered to patients.” Health professionals also have very different views and habits depending on geographic locations and health care systems, she observed. Ironing out the variation was one of the main objectives of the recommendations.
As one of the two conveners of the EULAR task force behind the recommendations, Dr. Usón, herself a rheumatologist at University Hospital of Móstoles, pointed out that the task force brought together a range of specialties – rheumatologists, orthopedic surgeons, radiologists, nuclear medicine specialists, among others, as well as patients – to ensure that the best advice could be given.
The task force followed EULAR standard operating procedures for developing recommendations, with discussion groups, systematic literature reviews, and Delphi technique-based consensus all being employed. The literature search considered publications from 1946 up until 2019.
“We agreed on the need for more background information from health professionals and patients, so we developed two surveys: One for health professionals with 160 items, [for which] we obtained 186 responses from 26 countries; and the patient survey was made up of 44 items, translated into 10 different languages, and we obtained 200 responses,” she said.
The results of the systematic literature review and surveys were used to help form expert consensus, leading to 5 overarching principles and 11 recommendations that look at before, during, and after intra-articular therapy.
Five overarching principles
The first overarching principle recognizes the widespread use of IA therapies and that their use is specific to the disease that is being treated and “may not be interchangeable across indications,” Dr. Usón said. The second principle concerns improving patient-centered outcomes, which are “those that are relevant to the patient,” and include the benefits, harms, preferences, or implications for self-management.
“Contextual factors are important and contribute to the effect of IAT [intra-articular treatment],” she said, discussing the third principle. “These include effective communication, patient expectations, or settings [where the procedure takes place]. In addition, one should take into account that the route of delivery has in itself a placebo effect. We found that in different RCTs [randomized controlled trials], the pooled placebo effect of IA saline is moderate to large.”
The fourth principle looks at ensuring that patients and clinicians make an informed and shared decision, which is again highlighted by the first recommendation. The fifth, and last, overarching principle acknowledges that IA injections may be given by a range of health care professionals.
Advice for before, during, and after injection
Patients need to be “fully informed of the nature of the procedure, the injectable used, and potential effects – benefits and risks – [and] informed consent should be obtained and documented,” said Dr. Usón, outlining the first recommendation. “That seems common,” she said in the interview, “but when we did the survey, we realize that many patients didn’t [give consent], and the doctors didn’t even ask for it. This is why it’s a very general statement, and it’s our first recommendation. The agreement was 99%!”
The recommendations also look at the optimal settings for performing injections, such as providing a professional and private, well-lighted room, and having a resuscitation kit nearby in case patients faint. Accuracy is important, Dr. Usón said, and imaging, such as ultrasound, should be used where available to ensure accurate injection into the joint. This is an area where further research could be performed, she said, urging young rheumatologists and health professionals to consider this. “Intra-articular therapy is something that you learn and do, but you never really investigate in it,” she said.
One recommendation states that when intra-articular injections are being given to pregnant patients, the safety of injected compound must be considered, both for the mother and for the fetus. There is another recommendation on the need to perform IA injections under aseptic conditions, and another stating that patients should be offered local anesthetics, after explaining the pros and cons.
Special populations of patients are also considered, Dr. Usón said. For example, the guidance advises warning patients with diabetes of the risk of transient glycemia after IA glucocorticoids and the need to monitor their blood glucose levels carefully for a couple of days afterward.
As a rule, “IAT is not a contraindication to people with clotting or bleeding disorders, or taking antithrombotic medications,” she said, unless they are at a high risk of bleeding.
Importantly, the recommendations cover when IAT can be performed after joint replacement surgery (after at least 3 months), and the need to “avoid overuse of injected joints” while also avoiding complete immobilization for at least 24 hours afterward. The recommendations very generally cover re-injections, but not how long intervals between injections should be. When asked about interval duration after her presentation, Dr. Usón said that the usual advice is to give IA injections no more than 2-3 times a year, but it depends on the injectable.
“It wasn’t our intention to review the efficacy and the safety of the different injectables, nor to review the use of IAT in different types of joint diseases,” she said. “We do lack a lot of information, a lot of evidence in this, and I really would hope that new rheumatologists start looking into and start investigating in this topic,” she added.
Recommendations will increase awareness of good clinical practice
“IA injections are commonly administered in the rheumatology setting. This is because [IA injection] is often a useful treatment for acute flare of arthritis, particularly when it is limited to a few joints,” observed Ai Lyn Tan, MD, associate professor and honorary consultant rheumatologist at the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine.
IA injection “also relieves symptoms relatively quickly for patients; however, the response can be variable, and there are side effects associated with IA injections,” Dr. Tan added in an interview.
There is a lack of universally accepted recommendations, Dr. Tan observed, noting that while there might be some local guidelines on how to safely perform IA injections these were often not standardized and were subject to being continually updated to try to improve the experience for patients.
“It is therefore timely to learn about the new EULAR recommendations for IA injections. The advantage of this will be to increase awareness of good clinical practice for performing IA injections.”
Dr. Tan had no relevant conflicts of interest.
SOURCE: EULAR COVID-19 Recommendations. E-congress content available until Sept. 1, 2020.
New EULAR recommendations for the intra-articular (IA) treatment of arthropathies aim to facilitate uniformity and quality of care for this mainstay of rheumatologic practice, according to a report on the new guidance that was presented at the annual European Congress of Rheumatology, held online this year due to COVID-19.
Until now there were no official recommendations on how best to use it in everyday practice. “This is the first time that there’s been a joint effort to develop evidence-based recommendations,” Jacqueline Usón, MD, PhD, associate professor medicine at Rey Juan Carlos University in Madrid, said in an interview. “Everything that we are saying is pretty logical, but it’s nice to see it put in recommendations based on evidence.”
IA therapy has been around for decades and is key for treating adults with a number of different conditions where synovitis, effusion, pain, or all three, are present, such as inflammatory arthritis and osteoarthritis, Dr. Usón observed during her presentation.
“Today, commonly used injectables are not only corticosteroids but also local anesthetics, hyaluronic acid, blood products, and maybe pharmaceuticals,” she said, adding that “there is a wide variation in the way intra-articular therapies are used and delivered to patients.” Health professionals also have very different views and habits depending on geographic locations and health care systems, she observed. Ironing out the variation was one of the main objectives of the recommendations.
As one of the two conveners of the EULAR task force behind the recommendations, Dr. Usón, herself a rheumatologist at University Hospital of Móstoles, pointed out that the task force brought together a range of specialties – rheumatologists, orthopedic surgeons, radiologists, nuclear medicine specialists, among others, as well as patients – to ensure that the best advice could be given.
The task force followed EULAR standard operating procedures for developing recommendations, with discussion groups, systematic literature reviews, and Delphi technique-based consensus all being employed. The literature search considered publications from 1946 up until 2019.
“We agreed on the need for more background information from health professionals and patients, so we developed two surveys: One for health professionals with 160 items, [for which] we obtained 186 responses from 26 countries; and the patient survey was made up of 44 items, translated into 10 different languages, and we obtained 200 responses,” she said.
The results of the systematic literature review and surveys were used to help form expert consensus, leading to 5 overarching principles and 11 recommendations that look at before, during, and after intra-articular therapy.
Five overarching principles
The first overarching principle recognizes the widespread use of IA therapies and that their use is specific to the disease that is being treated and “may not be interchangeable across indications,” Dr. Usón said. The second principle concerns improving patient-centered outcomes, which are “those that are relevant to the patient,” and include the benefits, harms, preferences, or implications for self-management.
“Contextual factors are important and contribute to the effect of IAT [intra-articular treatment],” she said, discussing the third principle. “These include effective communication, patient expectations, or settings [where the procedure takes place]. In addition, one should take into account that the route of delivery has in itself a placebo effect. We found that in different RCTs [randomized controlled trials], the pooled placebo effect of IA saline is moderate to large.”
The fourth principle looks at ensuring that patients and clinicians make an informed and shared decision, which is again highlighted by the first recommendation. The fifth, and last, overarching principle acknowledges that IA injections may be given by a range of health care professionals.
Advice for before, during, and after injection
Patients need to be “fully informed of the nature of the procedure, the injectable used, and potential effects – benefits and risks – [and] informed consent should be obtained and documented,” said Dr. Usón, outlining the first recommendation. “That seems common,” she said in the interview, “but when we did the survey, we realize that many patients didn’t [give consent], and the doctors didn’t even ask for it. This is why it’s a very general statement, and it’s our first recommendation. The agreement was 99%!”
The recommendations also look at the optimal settings for performing injections, such as providing a professional and private, well-lighted room, and having a resuscitation kit nearby in case patients faint. Accuracy is important, Dr. Usón said, and imaging, such as ultrasound, should be used where available to ensure accurate injection into the joint. This is an area where further research could be performed, she said, urging young rheumatologists and health professionals to consider this. “Intra-articular therapy is something that you learn and do, but you never really investigate in it,” she said.
One recommendation states that when intra-articular injections are being given to pregnant patients, the safety of injected compound must be considered, both for the mother and for the fetus. There is another recommendation on the need to perform IA injections under aseptic conditions, and another stating that patients should be offered local anesthetics, after explaining the pros and cons.
Special populations of patients are also considered, Dr. Usón said. For example, the guidance advises warning patients with diabetes of the risk of transient glycemia after IA glucocorticoids and the need to monitor their blood glucose levels carefully for a couple of days afterward.
As a rule, “IAT is not a contraindication to people with clotting or bleeding disorders, or taking antithrombotic medications,” she said, unless they are at a high risk of bleeding.
Importantly, the recommendations cover when IAT can be performed after joint replacement surgery (after at least 3 months), and the need to “avoid overuse of injected joints” while also avoiding complete immobilization for at least 24 hours afterward. The recommendations very generally cover re-injections, but not how long intervals between injections should be. When asked about interval duration after her presentation, Dr. Usón said that the usual advice is to give IA injections no more than 2-3 times a year, but it depends on the injectable.
“It wasn’t our intention to review the efficacy and the safety of the different injectables, nor to review the use of IAT in different types of joint diseases,” she said. “We do lack a lot of information, a lot of evidence in this, and I really would hope that new rheumatologists start looking into and start investigating in this topic,” she added.
Recommendations will increase awareness of good clinical practice
“IA injections are commonly administered in the rheumatology setting. This is because [IA injection] is often a useful treatment for acute flare of arthritis, particularly when it is limited to a few joints,” observed Ai Lyn Tan, MD, associate professor and honorary consultant rheumatologist at the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine.
IA injection “also relieves symptoms relatively quickly for patients; however, the response can be variable, and there are side effects associated with IA injections,” Dr. Tan added in an interview.
There is a lack of universally accepted recommendations, Dr. Tan observed, noting that while there might be some local guidelines on how to safely perform IA injections these were often not standardized and were subject to being continually updated to try to improve the experience for patients.
“It is therefore timely to learn about the new EULAR recommendations for IA injections. The advantage of this will be to increase awareness of good clinical practice for performing IA injections.”
Dr. Tan had no relevant conflicts of interest.
SOURCE: EULAR COVID-19 Recommendations. E-congress content available until Sept. 1, 2020.
FROM THE EULAR 2020 E-CONGRESS
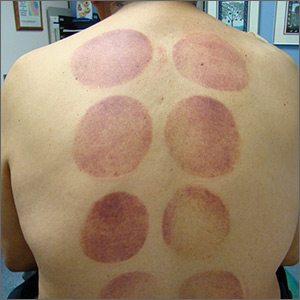
Ecchymotic patches
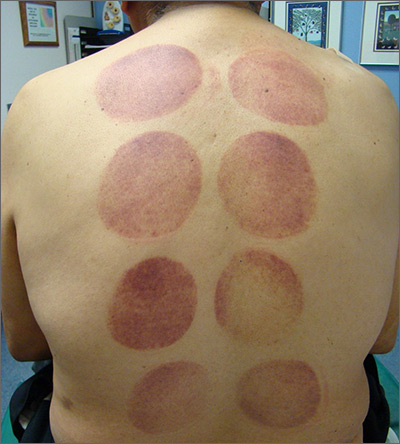
This patient’s circular ecchymotic patches were due to cupping. One of the clues that this was iatrogenic was the regular and repeated pattern on the skin.
Cupping is a centuries old treatment for pain relief (among other things) that involves applying glass globes or other hollow materials to the skin to create a vacuum. Traditionally, this vacuum is created by heating the air inside the vessel and then holding the vessel in place as the air cools. Practitioners may also use more modern instruments to induce the vacuum that are similar to those used to assist in vaginal deliveries. The mechanical devices leave these circular ecchymotic marks. The ecchymosis fades over time, and this procedure has been shown to significantly reduce myofascial neck and back pain in small trials.
It is important to recognize geometric patterns that are iatrogenic or due to abuse when evaluating skin findings. If skin findings do not follow dermatomal distributions, typical exanthem, or other classic patterns or presentations, there is the possibility that the pattern may be the result of neglect or abuse. On inspection, consider whether an odd pattern may have been caused from a belt buckle, striking instrument, furniture, medical equipment, or a hand strike.
This patient’s findings were consistent with his history of visiting a physical therapist for cupping. No treatment was required; the patient’s back pain from his car accident was improving, and the cupping marks were not troubling him.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Wang YT, Qi Y, Tang FY, et al. The effect of cupping therapy for low back pain: a meta-analysis based on existing randomized controlled trials. J Back Musculoskelet Rehabil. 2017;30:1187-1195.

This patient’s circular ecchymotic patches were due to cupping. One of the clues that this was iatrogenic was the regular and repeated pattern on the skin.
Cupping is a centuries old treatment for pain relief (among other things) that involves applying glass globes or other hollow materials to the skin to create a vacuum. Traditionally, this vacuum is created by heating the air inside the vessel and then holding the vessel in place as the air cools. Practitioners may also use more modern instruments to induce the vacuum that are similar to those used to assist in vaginal deliveries. The mechanical devices leave these circular ecchymotic marks. The ecchymosis fades over time, and this procedure has been shown to significantly reduce myofascial neck and back pain in small trials.
It is important to recognize geometric patterns that are iatrogenic or due to abuse when evaluating skin findings. If skin findings do not follow dermatomal distributions, typical exanthem, or other classic patterns or presentations, there is the possibility that the pattern may be the result of neglect or abuse. On inspection, consider whether an odd pattern may have been caused from a belt buckle, striking instrument, furniture, medical equipment, or a hand strike.
This patient’s findings were consistent with his history of visiting a physical therapist for cupping. No treatment was required; the patient’s back pain from his car accident was improving, and the cupping marks were not troubling him.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This patient’s circular ecchymotic patches were due to cupping. One of the clues that this was iatrogenic was the regular and repeated pattern on the skin.
Cupping is a centuries old treatment for pain relief (among other things) that involves applying glass globes or other hollow materials to the skin to create a vacuum. Traditionally, this vacuum is created by heating the air inside the vessel and then holding the vessel in place as the air cools. Practitioners may also use more modern instruments to induce the vacuum that are similar to those used to assist in vaginal deliveries. The mechanical devices leave these circular ecchymotic marks. The ecchymosis fades over time, and this procedure has been shown to significantly reduce myofascial neck and back pain in small trials.
It is important to recognize geometric patterns that are iatrogenic or due to abuse when evaluating skin findings. If skin findings do not follow dermatomal distributions, typical exanthem, or other classic patterns or presentations, there is the possibility that the pattern may be the result of neglect or abuse. On inspection, consider whether an odd pattern may have been caused from a belt buckle, striking instrument, furniture, medical equipment, or a hand strike.
This patient’s findings were consistent with his history of visiting a physical therapist for cupping. No treatment was required; the patient’s back pain from his car accident was improving, and the cupping marks were not troubling him.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Wang YT, Qi Y, Tang FY, et al. The effect of cupping therapy for low back pain: a meta-analysis based on existing randomized controlled trials. J Back Musculoskelet Rehabil. 2017;30:1187-1195.
Wang YT, Qi Y, Tang FY, et al. The effect of cupping therapy for low back pain: a meta-analysis based on existing randomized controlled trials. J Back Musculoskelet Rehabil. 2017;30:1187-1195.
Liposomal bupivacaine excreted in breast milk, but levels appear safe
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
based on a prospective cohort study.
Over the course of 4 days, relative neonatal dosages of bupivacaine were less than 1%, remaining below the 10% threshold of concern, reported Hiba J. Mustafa, MD, of the University of Minnesota, Minneapolis, and colleagues.
Liposomal bupivacaine can achieve up to 4 days of postcesarean pain control, which is significantly longer than the 8 hours provided by standard bupivacaine, the investigators wrote in Obstetrics & Gynecology. But usage of the liposomal formulation has not been widespread, they noted, partly because of a lack of clinical studies evaluating breast milk transfer and neonatal safety.
To address this knowledge gap, Dr. Mustafa and colleagues enrolled 30 healthy pregnant women scheduled to undergo cesarean birth at full term. All patients were aged 18-40 years, with an American Society of Anesthesiologists physical status of I or II. Exclusion criteria included a number of maternal and neonatal health concerns, such as sensitivity to local anesthetics, metabolic disorders, fetal anomaly, fetal growth restriction, and others.
The day of surgery, before the procedure, maternal blood samples were collected and used for baseline measurements.
Each woman received a spinal anesthetic including 150 mcg of morphine, 15 mcg of intrathecal fentanyl, and 1.4-1.6 mL of 0.75% hyperbaric bupivacaine hydrochloride. Within 30 minutes after birth, a bilateral transversus abdominus plane block was performed using 266 mg of 1.3% liposomal bupivacaine and 52 mg of 0.25% bupivacaine hydrochloride.
Using the block as time point zero, maternal blood and breast milk samples were collected at hour 2, 6, 12, 24, 48, 72, and 96. Sparse sampling was employed, such that participants were randomly assigned in a 1:1 ratio to provide paired blood and milk samples at hour 2, 12, and 48; or hour 6, 24, 72, and 96. Bupivacaine was quantified in samples by liquid chromatography–tandem mass spectrometry.
Using these data, the investigators determined bupivacaine concentrations in plasma and milk, milk/plasma area under the curve (AUC) ratios, neonatal dosage, and relative neonatal dosage. In addition, adverse events in both mothers and neonates were recorded for 2 weeks post partum.
Mean bupivacaine concentrations peaked in breast milk at 6 hours, at 58 ng/mL. This peak was followed by a steady reduction to an “almost undetectable” level of 5.2 ng/mL at 96 hours. Maternal plasma levels peaked first at hour 6 (155.9 ng/mL), then again at hour 48 (225.8 ng/mL), followed by a steady decline until hour 96, when the level reached 80.6 ng/mL.
Relative mean concentrations of milk to plasma were 44%, 36%, 28%, and 18% at hour 2, 6, 12, and 24, respectively. AUC ratios were used to represent exposure across various time intervals. For instance, the AUC ratio for milk/plasma from hour 0 to hour 2 was 0.45. The AUC findings declined steadily until the final ratio, which spanned hour 0 to hour 96, at 0.15.
These AUC ratios allowed for calculation of neonatal dosage and relative neonatal dosage using an average daily milk intake of 150 mL/kg per day. For the longest range, spanning from hour 0 to hour 96, the neonatal dosage was 15,155.4 ng/kg, which translated to a relative neonatal dosage of 0.396%.
No mothers or neonates experienced adverse events.
“Bupivacaine was transferred into mother’s milk such that an exclusively breastfeeding neonate would ingest less than 1% (relative neonatal dosage) of the maternal dose,” the investigators wrote, noting that this falls safely below the acceptable threshold of 10%.
“Because bupivacaine is metabolized primarily in the liver, a neonate’s absorption will likely be even lower [than modeled] given the first-pass effect,” they added.
Based on these findings, Dr. Mustafa and colleagues concluded that “the level of bupivacaine ingested by the sucking neonate is acceptable and compatible with breastfeeding.”
Michael G. Ross MD, MPH, Distinguished Professor of Obstetrics and Gynecology and Public Health at Geffen School of Medicine at the University of California, Los Angeles, commented that, this study adds to the literature of drug excretion into breast milk. “For the vast majority of drugs with passive transfer from maternal plasma to breast milk, the effective dosages of exclusive breastfeeding neonates are approximately 5% of the maternal (oral) dose. In the present study, the authors demonstrated a relative neonatal dosage of less than 1%. This low value results from consequences of minimal maternal plasma absorption (in the present case from transversus abdominis injection), maternal volume of distribution, transfer into breast milk, and the volume of milk ingestion. These results should provide reassurance for the safety of breastfeeding term infants under the conditions of the study.
“There are a number of study concerns, including the inability to differentiate absorption of the spinal bupivacaine from the liposomal bupivacaine, the lack of paired maternal plasma and breast milk sample, and the lack of detail as to how much milk was expressed for each sample. Importantly, breast milk composition varies from foremilk to hindmilk. Thus, a single sample may not accurately reflect the composition ingested by the infant. The suggestion of two peaks in maternal plasma concentration was not demonstrated statistically and may be an artifact of the timing of spinal and liposomal injections, or the fact that different patients were studied at each time period.
“Most importantly, despite the demonstrated safety, the authors acknowledge conflicting results of clinical benefits of liposomal bupivacaine injection. As such, I recommend that postcesarean transversus abdominis blocks be performed only under institutional review board-approved study protocols,” said Dr. Ross, codirector of the Institute for Women’ and Children’s Health at the Lundquist Institute, Torrance, Calif.*
The study was funded by the Thrasher Research Fund. The investigators reported no conflicts of interest. Dr. Ross had no relevant financial disclosures.
SOURCE: Mustafa et al. Obstet Gynecol. 2020 Jun 6. doi: 10.1097/AOG.0000000000003886.
*This article was updated 6/16/2020.
FROM OBSTETRICS & GYNECOLOGY
Opioid use up after TNF inhibitor for inflammatory arthritis
Opioid use does not decline after patients with inflammatory arthritis start TNF inhibitor therapy; in fact, average use appears to increase, results from a new study show.
“Starting a TNF inhibitor, you would think the pain would go down, and we were hoping the dose of opioids would go down with it,” said investigator Olafur Palsson, MD, from the University of Iceland in Reykjavik and Lund University in Sweden.
“But this research shows that the insertion of a TNF inhibitor has only a minor effect on that,” he told Medscape Medical News.
The findings are an “important reminder” to rheumatologists that they should broaden their consideration of other pain treatments and techniques for patients with inflammatory arthritis, Dr. Palsson said. “They should focus on trying other tactics to get patients’ pain and stiffness under control; there may be some underlying factors.”
The investigators compared opioid prescription rates in 940 patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and undifferentiated arthritis with a control group of 4,700 matched subjects. Dr. Palsson presented the findings at the virtual European League Against Rheumatism (EULAR) 2020 Congress.
The team assessed nationwide databases that capture all patients taking biologics for rheumatic diseases and more than 90% of all drug prescriptions. They found that patients with inflammatory arthritis in Iceland were more likely to have received at least one opioid prescription than control subjects (75% vs. 43%).
During the study period, average yearly opioid dose rose much more in the patient group than in the control group. And 2 years after the initiation of TNF inhibitors, the number of patients taking opioids was unchanged from baseline, at about 40%.
Overall, the patient group was prescribed nearly six times more opioids than the control group. The investigators used a bootstrapping analysis to obtain a reliable confidence interval.
“In a way, the data are extremely skewed,” Dr. Palsson explained. “Most patients were taking very low doses of opioids and a few were taking extremely high doses. It’s hard to do a statistical analysis.”
“With bootstrapping, you don’t detect small fluctuations in data,” he said, acknowledging this study limitation. Also, “prescription data don’t necessarily reflect consumption” of a drug. People prescribed high doses may not necessarily be consuming high doses.”
Additionally, the risk for addiction is low when opioids are used as intended, said John Isaacs, MBBS, PhD, from Newcastle University in Newcastle Upon Tyne, United Kingdom, who is chair of the EULAR scientific program committee.
To alleviate chronic pain, opioids “should, in any case, only be part of a comprehensive therapy program in which doctors, psychologists, and physiotherapists work together,” Dr. Isaacs said in a EULAR news release.
Dr. Palsson has disclosed no relevant financial relationships. Dr. Isaacs is a consultant or has received honoraria or grants from Pfizer, AbbVie, Amgen, Merck, Roche, and UCB.
This article first appeared on Medscape.com.
Opioid use does not decline after patients with inflammatory arthritis start TNF inhibitor therapy; in fact, average use appears to increase, results from a new study show.
“Starting a TNF inhibitor, you would think the pain would go down, and we were hoping the dose of opioids would go down with it,” said investigator Olafur Palsson, MD, from the University of Iceland in Reykjavik and Lund University in Sweden.
“But this research shows that the insertion of a TNF inhibitor has only a minor effect on that,” he told Medscape Medical News.
The findings are an “important reminder” to rheumatologists that they should broaden their consideration of other pain treatments and techniques for patients with inflammatory arthritis, Dr. Palsson said. “They should focus on trying other tactics to get patients’ pain and stiffness under control; there may be some underlying factors.”
The investigators compared opioid prescription rates in 940 patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and undifferentiated arthritis with a control group of 4,700 matched subjects. Dr. Palsson presented the findings at the virtual European League Against Rheumatism (EULAR) 2020 Congress.
The team assessed nationwide databases that capture all patients taking biologics for rheumatic diseases and more than 90% of all drug prescriptions. They found that patients with inflammatory arthritis in Iceland were more likely to have received at least one opioid prescription than control subjects (75% vs. 43%).
During the study period, average yearly opioid dose rose much more in the patient group than in the control group. And 2 years after the initiation of TNF inhibitors, the number of patients taking opioids was unchanged from baseline, at about 40%.
Overall, the patient group was prescribed nearly six times more opioids than the control group. The investigators used a bootstrapping analysis to obtain a reliable confidence interval.
“In a way, the data are extremely skewed,” Dr. Palsson explained. “Most patients were taking very low doses of opioids and a few were taking extremely high doses. It’s hard to do a statistical analysis.”
“With bootstrapping, you don’t detect small fluctuations in data,” he said, acknowledging this study limitation. Also, “prescription data don’t necessarily reflect consumption” of a drug. People prescribed high doses may not necessarily be consuming high doses.”
Additionally, the risk for addiction is low when opioids are used as intended, said John Isaacs, MBBS, PhD, from Newcastle University in Newcastle Upon Tyne, United Kingdom, who is chair of the EULAR scientific program committee.
To alleviate chronic pain, opioids “should, in any case, only be part of a comprehensive therapy program in which doctors, psychologists, and physiotherapists work together,” Dr. Isaacs said in a EULAR news release.
Dr. Palsson has disclosed no relevant financial relationships. Dr. Isaacs is a consultant or has received honoraria or grants from Pfizer, AbbVie, Amgen, Merck, Roche, and UCB.
This article first appeared on Medscape.com.
Opioid use does not decline after patients with inflammatory arthritis start TNF inhibitor therapy; in fact, average use appears to increase, results from a new study show.
“Starting a TNF inhibitor, you would think the pain would go down, and we were hoping the dose of opioids would go down with it,” said investigator Olafur Palsson, MD, from the University of Iceland in Reykjavik and Lund University in Sweden.
“But this research shows that the insertion of a TNF inhibitor has only a minor effect on that,” he told Medscape Medical News.
The findings are an “important reminder” to rheumatologists that they should broaden their consideration of other pain treatments and techniques for patients with inflammatory arthritis, Dr. Palsson said. “They should focus on trying other tactics to get patients’ pain and stiffness under control; there may be some underlying factors.”
The investigators compared opioid prescription rates in 940 patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and undifferentiated arthritis with a control group of 4,700 matched subjects. Dr. Palsson presented the findings at the virtual European League Against Rheumatism (EULAR) 2020 Congress.
The team assessed nationwide databases that capture all patients taking biologics for rheumatic diseases and more than 90% of all drug prescriptions. They found that patients with inflammatory arthritis in Iceland were more likely to have received at least one opioid prescription than control subjects (75% vs. 43%).
During the study period, average yearly opioid dose rose much more in the patient group than in the control group. And 2 years after the initiation of TNF inhibitors, the number of patients taking opioids was unchanged from baseline, at about 40%.
Overall, the patient group was prescribed nearly six times more opioids than the control group. The investigators used a bootstrapping analysis to obtain a reliable confidence interval.
“In a way, the data are extremely skewed,” Dr. Palsson explained. “Most patients were taking very low doses of opioids and a few were taking extremely high doses. It’s hard to do a statistical analysis.”
“With bootstrapping, you don’t detect small fluctuations in data,” he said, acknowledging this study limitation. Also, “prescription data don’t necessarily reflect consumption” of a drug. People prescribed high doses may not necessarily be consuming high doses.”
Additionally, the risk for addiction is low when opioids are used as intended, said John Isaacs, MBBS, PhD, from Newcastle University in Newcastle Upon Tyne, United Kingdom, who is chair of the EULAR scientific program committee.
To alleviate chronic pain, opioids “should, in any case, only be part of a comprehensive therapy program in which doctors, psychologists, and physiotherapists work together,” Dr. Isaacs said in a EULAR news release.
Dr. Palsson has disclosed no relevant financial relationships. Dr. Isaacs is a consultant or has received honoraria or grants from Pfizer, AbbVie, Amgen, Merck, Roche, and UCB.
This article first appeared on Medscape.com.
I’m getting old (and it’s costing me)
The inevitable consequences of aging finally hit me last year, at age 64. Before then, I was a (reasonably) healthy, active person. I exercised a little, ate reasonably healthy meals, and took no medications. My only visits to my doctor were for annual (sort of) exams. That all changed when I began to have neurogenic claudication in both legs. I had no history of back injury but, with worsening pain, I sought the opinion of my physician.
It turned out that I had a dynamic spondylolisthesis and disc herniation that could only be fixed with a single-level fusion. From a neurologic perspective, the procedure was an unequivocal success. However, my recovery (with lack of exercise) had the unintended “side effect” of a 25-pound weight gain. As a family doctor, I know that the best way to reverse this gain is by increasing my exercise. However, I also know that, at my age, many specialty organizations recommend a cardiac evaluation before beginning strenuous exercise.1
So, I set up a routine treadmill test. Although I exercised to a moderate level of intensity, the interpreting cardiologist was unwilling to call my test “totally normal” and recommended further evaluation. (One of the “unwritten rules” I’ve discovered during my career is that adverse outcomes are far more likely in medical personnel than in nonmedical personnel!)
He recommended undergoing coronary artery computed tomography angiography with coronary artery calcium (CAC) scoring. The result? A left anterior descending artery CAC score of 22, which placed me at a slightly increased risk of an adverse event over the next 10 years. (The benefit of exercise, however, far outweighed the risk.) I’m happy to report that I have lost five pounds with only mildly intensive exercise.
Along with facing the health aspects of aging, I am also faced with the economic realities. I have carried group term life insurance throughout my career. My 10-year term just happened to expire when I turned 65. I have always been insured as a “Tier 1” customer, meaning that I qualified for the best premiums due to my “healthy” status. That said, the transition to age 65 carries with it a significant premium increase.
Imagine my shock, though, when I was told that my premium would jump to MORE THAN 4 TIMES the previous premium for ONE-THIRD of my previous coverage! The culprit? The CAC score of 22!
It turns out that the insurance industry has adopted an underwriting standard that uses CAC—measured over a broad population, rather than a more age-confined one—to determine actuarial risk when rating life insurance policies.2 As a result, my underwriting profile went all the way to “Tier 3.”
Continue to: We're used to medical consequences...
We’re used to medical consequences for tests that we order—whether a prostate biopsy for an elevated prostate-specific antigen test result, breast biopsy after abnormal mammogram, or a hemoglobin A1C test after an elevated fasting blood sugar. We can handle discussions with patients about potential diagnostic paths and readily include that information as part of shared decision-making with patients. Unfortunately, many entities are increasingly using medical information to make nonmedical decisions.
Using the CAC score to discuss the risk of adverse coronary events with my patients may be appropriate. In nonmedical settings, however, this data may be incorrectly, unfairly, or dangerously applied to our patients. I’ve begun thinking about these nonmedical applications as part of the shared decision-making process with my patients. It’s making these conversations more complicated, but life and life events for our patients take place far beyond the walls of our exam rooms.
1. Garner KK, Pomeroy W, Arnold JJ. Exercise stress testing: indications and common questions. Am Fam Physician. 2017;96:293-299A.
2. Rose J. It’s possible to get life insurance with a high calcium score. Good Financial Cents 2019. www.goodfinancialcents.com/life-insurance-with-a-high-calcium-score/. Last modified Febuary 20, 2019. Accessed May 27, 2020.
The inevitable consequences of aging finally hit me last year, at age 64. Before then, I was a (reasonably) healthy, active person. I exercised a little, ate reasonably healthy meals, and took no medications. My only visits to my doctor were for annual (sort of) exams. That all changed when I began to have neurogenic claudication in both legs. I had no history of back injury but, with worsening pain, I sought the opinion of my physician.
It turned out that I had a dynamic spondylolisthesis and disc herniation that could only be fixed with a single-level fusion. From a neurologic perspective, the procedure was an unequivocal success. However, my recovery (with lack of exercise) had the unintended “side effect” of a 25-pound weight gain. As a family doctor, I know that the best way to reverse this gain is by increasing my exercise. However, I also know that, at my age, many specialty organizations recommend a cardiac evaluation before beginning strenuous exercise.1
So, I set up a routine treadmill test. Although I exercised to a moderate level of intensity, the interpreting cardiologist was unwilling to call my test “totally normal” and recommended further evaluation. (One of the “unwritten rules” I’ve discovered during my career is that adverse outcomes are far more likely in medical personnel than in nonmedical personnel!)
He recommended undergoing coronary artery computed tomography angiography with coronary artery calcium (CAC) scoring. The result? A left anterior descending artery CAC score of 22, which placed me at a slightly increased risk of an adverse event over the next 10 years. (The benefit of exercise, however, far outweighed the risk.) I’m happy to report that I have lost five pounds with only mildly intensive exercise.
Along with facing the health aspects of aging, I am also faced with the economic realities. I have carried group term life insurance throughout my career. My 10-year term just happened to expire when I turned 65. I have always been insured as a “Tier 1” customer, meaning that I qualified for the best premiums due to my “healthy” status. That said, the transition to age 65 carries with it a significant premium increase.
Imagine my shock, though, when I was told that my premium would jump to MORE THAN 4 TIMES the previous premium for ONE-THIRD of my previous coverage! The culprit? The CAC score of 22!
It turns out that the insurance industry has adopted an underwriting standard that uses CAC—measured over a broad population, rather than a more age-confined one—to determine actuarial risk when rating life insurance policies.2 As a result, my underwriting profile went all the way to “Tier 3.”
Continue to: We're used to medical consequences...
We’re used to medical consequences for tests that we order—whether a prostate biopsy for an elevated prostate-specific antigen test result, breast biopsy after abnormal mammogram, or a hemoglobin A1C test after an elevated fasting blood sugar. We can handle discussions with patients about potential diagnostic paths and readily include that information as part of shared decision-making with patients. Unfortunately, many entities are increasingly using medical information to make nonmedical decisions.
Using the CAC score to discuss the risk of adverse coronary events with my patients may be appropriate. In nonmedical settings, however, this data may be incorrectly, unfairly, or dangerously applied to our patients. I’ve begun thinking about these nonmedical applications as part of the shared decision-making process with my patients. It’s making these conversations more complicated, but life and life events for our patients take place far beyond the walls of our exam rooms.
The inevitable consequences of aging finally hit me last year, at age 64. Before then, I was a (reasonably) healthy, active person. I exercised a little, ate reasonably healthy meals, and took no medications. My only visits to my doctor were for annual (sort of) exams. That all changed when I began to have neurogenic claudication in both legs. I had no history of back injury but, with worsening pain, I sought the opinion of my physician.
It turned out that I had a dynamic spondylolisthesis and disc herniation that could only be fixed with a single-level fusion. From a neurologic perspective, the procedure was an unequivocal success. However, my recovery (with lack of exercise) had the unintended “side effect” of a 25-pound weight gain. As a family doctor, I know that the best way to reverse this gain is by increasing my exercise. However, I also know that, at my age, many specialty organizations recommend a cardiac evaluation before beginning strenuous exercise.1
So, I set up a routine treadmill test. Although I exercised to a moderate level of intensity, the interpreting cardiologist was unwilling to call my test “totally normal” and recommended further evaluation. (One of the “unwritten rules” I’ve discovered during my career is that adverse outcomes are far more likely in medical personnel than in nonmedical personnel!)
He recommended undergoing coronary artery computed tomography angiography with coronary artery calcium (CAC) scoring. The result? A left anterior descending artery CAC score of 22, which placed me at a slightly increased risk of an adverse event over the next 10 years. (The benefit of exercise, however, far outweighed the risk.) I’m happy to report that I have lost five pounds with only mildly intensive exercise.
Along with facing the health aspects of aging, I am also faced with the economic realities. I have carried group term life insurance throughout my career. My 10-year term just happened to expire when I turned 65. I have always been insured as a “Tier 1” customer, meaning that I qualified for the best premiums due to my “healthy” status. That said, the transition to age 65 carries with it a significant premium increase.
Imagine my shock, though, when I was told that my premium would jump to MORE THAN 4 TIMES the previous premium for ONE-THIRD of my previous coverage! The culprit? The CAC score of 22!
It turns out that the insurance industry has adopted an underwriting standard that uses CAC—measured over a broad population, rather than a more age-confined one—to determine actuarial risk when rating life insurance policies.2 As a result, my underwriting profile went all the way to “Tier 3.”
Continue to: We're used to medical consequences...
We’re used to medical consequences for tests that we order—whether a prostate biopsy for an elevated prostate-specific antigen test result, breast biopsy after abnormal mammogram, or a hemoglobin A1C test after an elevated fasting blood sugar. We can handle discussions with patients about potential diagnostic paths and readily include that information as part of shared decision-making with patients. Unfortunately, many entities are increasingly using medical information to make nonmedical decisions.
Using the CAC score to discuss the risk of adverse coronary events with my patients may be appropriate. In nonmedical settings, however, this data may be incorrectly, unfairly, or dangerously applied to our patients. I’ve begun thinking about these nonmedical applications as part of the shared decision-making process with my patients. It’s making these conversations more complicated, but life and life events for our patients take place far beyond the walls of our exam rooms.
1. Garner KK, Pomeroy W, Arnold JJ. Exercise stress testing: indications and common questions. Am Fam Physician. 2017;96:293-299A.
2. Rose J. It’s possible to get life insurance with a high calcium score. Good Financial Cents 2019. www.goodfinancialcents.com/life-insurance-with-a-high-calcium-score/. Last modified Febuary 20, 2019. Accessed May 27, 2020.
1. Garner KK, Pomeroy W, Arnold JJ. Exercise stress testing: indications and common questions. Am Fam Physician. 2017;96:293-299A.
2. Rose J. It’s possible to get life insurance with a high calcium score. Good Financial Cents 2019. www.goodfinancialcents.com/life-insurance-with-a-high-calcium-score/. Last modified Febuary 20, 2019. Accessed May 27, 2020.
Acute rhinosinusitis: When to prescribe an antibiotic
An estimated 30 million cases of acute rhinosinusitis (ARS) occur every year in the United States.1 More than 80% of people with ARS are prescribed antibiotics in North America, accounting for 15% to 20% of all antibiotic prescriptions in the adult outpatient setting.2,3 Many of these prescriptions are unnecessary, as the most common cause of ARS is a virus.4,5 Evidence consistently shows that symptoms of ARS will resolve spontaneously in most patients and that only those patients with severe or prolonged symptoms require consideration of antibiotic therapy.1,2,4,6 Nearly half of all patients will improve within 1 week and two-thirds of patients will improve within 2 weeks without the use of antibiotics.7 In children, only about 6% to 7% presenting with upper respiratory symptoms meet the criteria for acute bacterial rhinosinusitis (ABRS),8 which we’ll detail in a bit. For most patients, treatment should consist of symptom management.5
But what about the minority who require antibiotic therapy? This article reviews how to evaluate patients with ARS, identify those who require antibiotics, and prescribe the most appropriate antibiotic treatment regimens.
Diagnosis: Distinguishing viral from bacterial disease
ARS is defined as the sudden onset of purulent nasal discharge plus either nasal blockage or facial pressure/pain lasting < 4 weeks.3,9 Additional signs and symptoms may include postnasal drip, a reduced sense of smell, sinus tenderness to palpation, and maxillary toothaches.10,11
ARS may be viral or bacterial in etiology, with the most common bacterial organisms being Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1,3,5 The most common viral causes are influenza, parainfluenza, and rhinovirus. Approximately 90% to 98% of cases of ARS are viral6,11; only about 0.5% to 2% of viral rhinosinusitis episodes are complicated by bacterial infection.1,10-12
Diagnose ABRS when symptoms of ARS fail to improve after 10 days or symptoms of ARS worsen within 10 days after initial improvement (“double sickening”).1,11 Symptoms that are significantly associated with ABRS are unilateral sinus pain and reported maxillary pain. The presence of facial or dental pain correlates with ABRS but does not identify the specific sinus involved.1
There isn’t good correlation between patients saying they have sinusitis and actually having it.13 A 2019 meta-analysis by Ebell et al14 reported that based on limited data, the overall clinical impression, fetid odor on the breath, and pain in the teeth are the best individual clinical predictors of ABRS.
As recommended by the Infectious Disease Society of America (IDSA), a diagnosis of ABRS is also reasonable in patients who present with severe symptoms at the onset.6 Although there is no consensus about what constitutes “severe symptoms,” they are often described as a temperature ≥ 102°F (39°C) plus 3 to 4 days of purulent nasal drainage.1,4,6
Continue to: Additional symptoms of ABRS may include...
Additional symptoms of ABRS may include cough, fatigue, decreased or lack of sense of smell (hyposmia or anosmia), and ear pressure.10 Another sign of “double sickening” is the development of a fever after several days of symptoms.1,9,15 Viral sinusitis typically lasts 5 to 7 days with a peak at days 2 to 3.1,15 If symptoms continue for 10 days, there is a 60% chance of bacterial sinusitis, although some viral rhinosinusitis symptoms persist for > 14 days.1,5 Beyond 4 to 12 weeks, sinusitis is classified as subacute or chronic.3
Physical exam findings and the limited roles of imaging and labs
Common physical exam findings associated with the diagnosis of ABRS include altered speech indicating nasal obstruction; edema or erythema of the skin indicating congested capillaries; tenderness to palpation over the cheeks or upper teeth; odorous breath; and purulent drainage from the nose or in the posterior pharynx.
In a study by Hansen et al13 (N = 174), the only sign that showed significant association with ABRS (diagnosed by sinus aspiration or lavage) was unilateral tenderness of the maxillary sinuses. The presence of purulent drainage in the nose or posterior pharynx also has significant diagnostic value, as it predicts the presence of bacteria on antral aspiration.1 Purulent discharge in the pharynx is associated with a higher likelihood of benefit from antibiotic therapy compared to placebo (number needed to treat [NNT] = 8).16 However, colored nasal discharge indicates the presence of neutrophils—not bacteria—and does not predict the likelihood of bacterial sinus infection.14,17 Therefore, the history and physical exam should focus on location of pain (sinus and/or teeth), duration of symptoms, presence of fever, change in symptom severity, attempted home therapies, sinus tenderness on exam, breath odor, and purulent drainage seen in the nasal cavity or posterior pharynx.13,14
Radiographic imaging has no role in the diagnosis or treatment of uncomplicated ABRS because viral and bacterial etiologies have similar radiographic appearances. Additionally, employing radiologic imaging would increase health care costs by at least 4-fold.5,6,8,17 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) clinical practice guidelines recommend against radiographic imaging for patients who meet the diagnostic criteria for ABRS unless concern exists for a complication or an alternate diagnosis is suspected.1 Computed tomography (CT) imaging of the sinuses may be warranted in patients with severe headaches, facial swelling, cranial nerve palsies, or bulging of the eye (proptosis), all of which indicate a potential complication of ABRS.1
Laboratory evaluations. ABRS is a clinical diagnosis; therefore, routine lab work, such as a white blood cell count, C-reactive protein (CRP) level, and/or erythrocyte sedimentation rate (ESR), are not indicated unless an alternate diagnosis is suspected.1,5,13,18,19
Continue to: In one study...
In one study, CRP > 10 mg/L and ESR > 10 mm/h were the strongest individual predictors of purulent antral puncture aspirate or positive bacterial culture of aspirate, which is considered diagnostic for ABRS. 20 However, CRP and ESR by themselves are not adequate to diagnose ABRS.20 This study developed a clinical decision rule that used symptoms, signs, and laboratory values to rate the likelihood of ABRS as being either low, moderate, or high. However, this clinical decision rule has not been prospectively validated.
Thus, CRP and ESR elevations can support the diagnosis of ABRS, but the low sensitivity of these tests precludes their use as a screening tool for ABRS.14,18 Studies by Ebell19 and Huang21 have shown some benefit to dipstick assay of nasal secretions for the diagnosis of ABRS, but this method is not validated or widely used.19,21
Treatment: From managing symptoms to prescribing antibiotics
Overprescribing antibiotics for ARS is a prominent health care issue. In fact, 5 of 9 placebo-controlled studies showed that most people improve within 2 weeks regardless of antibiotic use (N = 1058).3 Therefore, weigh the decision to treat ABRS with antibiotics against the risk for potential adverse reactions and within the context of antibiotic stewardship.2,9,12,22-24 Consider antibiotics only if patients meet the diagnostic criteria for ABRS (TABLE 11,6) or, occasionally, for patients with severe symptoms upon presentation, such as a temperature ≥ 102°F (39°C) plus purulent nasal discharge for 3 to 4 days.1 The most commonly reported adverse effects of antibiotics are gastrointestinal in nature and include nausea, vomiting, and diarrhea.2,9

Symptomatic management for both ARS and ABRS is recommended as first-line therapy; it should be offered to patients before making a diagnosis of ABRS.1,5,9,25 Consider using analgesics, topical intranasal steroids, and/or nasal saline irrigation to alleviate symptoms and improve quality of life.1,5,25 Interventions with questionable or unproven efficacy include the use of antihistamines, systemic steroids, decongestants, and mucolytics, but they may be considered on an individual basis.1 A systematic review found that topical nasal steroids relieved facial pain and nasal congestion in patients with rhinitis and acute sinusitis (NNT = 14).1,26
Even after diagnosing ABRS, clinicians should offer watchful waiting and symptomatic therapies as long as patients have adequate access to follow-up (TABLE 2,1,15FIGURE1,6). Antibiotic therapy can then be initiated if symptoms do not improve after an additional 7 days of watchful waiting or if symptoms worsen at any time. It is reasonable to give patients a prescription to keep on hand to be used if symptoms worsen, with instructions to notify the provider if antibiotics are started.1
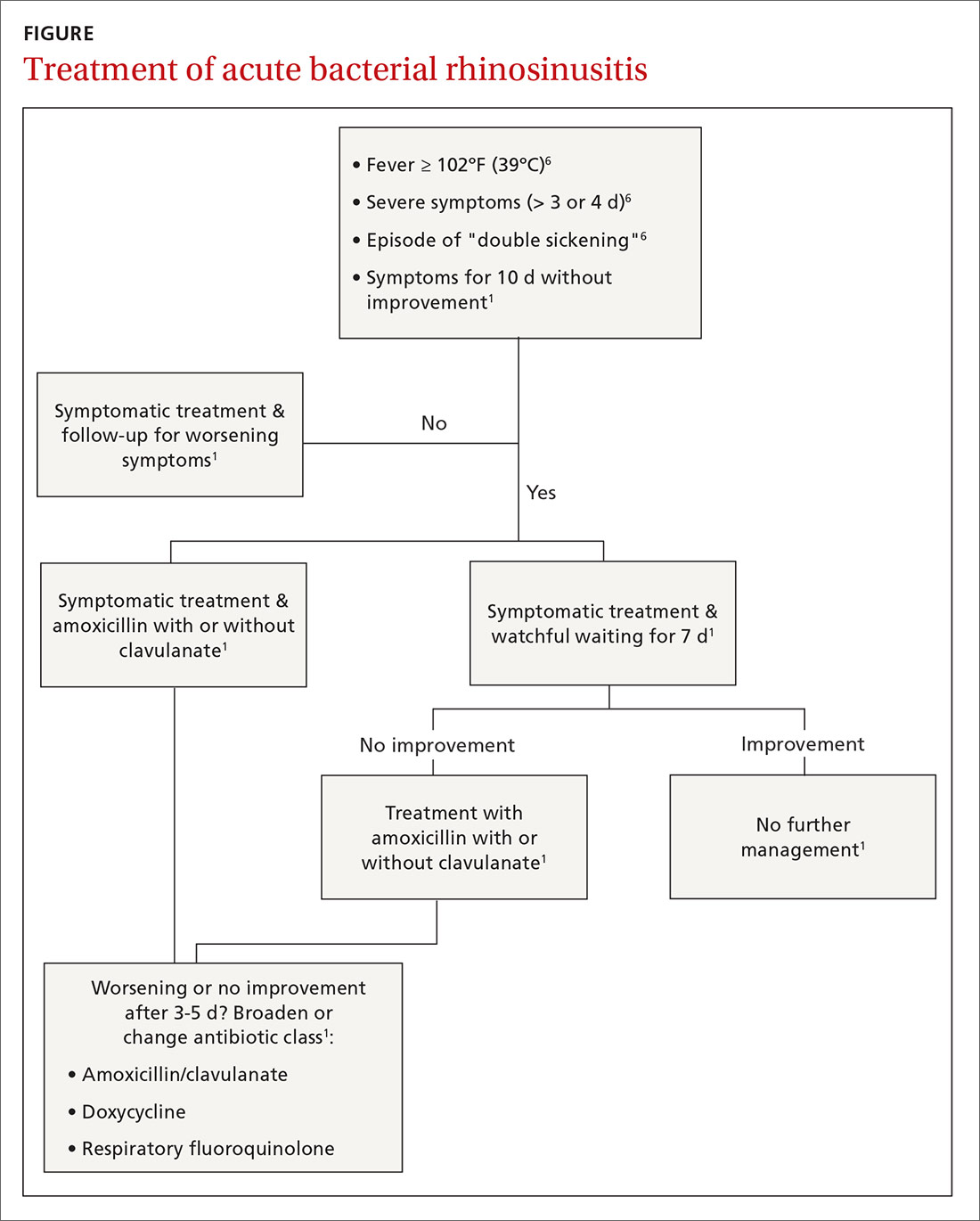
Continue to: Antibiotic therapy
Antibiotic therapy. The rationale for treating ABRS with antibiotics is to expedite recovery and prevent complications such as periorbital or orbital cellulitis, meningitis, frontal osteomyelitis, cavernous sinus thrombosis, and other serious illness.27 Antibiotic treatment is associated with a shorter duration of symptoms (NNT = 19) but an increased risk of adverse events (NNH = 8).7,19
Amoxicillin with or without clavulanate for 5 to 10 days is first-line antibiotic therapy for most adults with ABRS.1,3,5,8,9,11 Per AAO-HNS, the “justification for amoxicillin as first-line treatment relates to its safety, efficacy, low cost, and narrow microbiologic spectrum.”1 Amoxicillin may be dosed 500 mg tid for 5 to 10 days. Amoxicillin/clavulanate (Augmentin) is recommended for patients with comorbid conditions or with increased risk of bacterial resistance. Dosing for amoxicillin/clavulanate is 500/125 mg tid or 875/125 mg bid for 5 to 10 days. Duration of therapy should be determined by the severity of symptoms.5
For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is considered first-line treatment.1,6 Doxycycline is preferred because of its narrower spectrum and fewer adverse effects than the fluoroquinolones. Fluoroquinolones should be reserved for patients who fail first-line treatment and are penicillin allergic.1 Because of the high rates of resistance among S pneumoniae and H influenzae, macrolides, trimethoprim/sulfamethoxazole (TMP/SMX), and cephalosporins are not recommended as first-line therapy.1,5
How antibiotic options compare. A Cochrane review of 54 studies comparing different antibiotics showed no antibiotic was superior.3 Of the 54 studies, 6 studies (N = 1887) were pooled to compare cephalosporins to amoxicillin/clavulanate at 7 to 15 days. The findings indicated a statistically significant difference for amoxicillin/clavulanate with a relative risk (RR) of 1.37 (confidence interval [CI], 1.04-1.8).3 However, none of these 6 studies were graded as having a low risk of bias; therefore, confidence in this finding was deemed limited due to the quality of included studies. The failure rate for cephalosporins was 12% vs 8% for amoxicillin/clavulanate.3
Treatment failure is considered when a patient has not improved by Day 7 after ABRS diagnosis (with or without medication) or when symptoms worsen at any time. If watchful waiting was chosen and a safety net prescription was provided, the antibiotics should be filled and started. If no antibiotic was prescribed at the time watchful waiting commenced, the patient should return for further evaluation and be started on antibiotics. If antibiotics were prescribed initially for severe symptoms, a change in antibiotic therapy is indicated, and a broader-spectrum antibiotic should be chosen. If amoxicillin was prescribed, the patient should be switched to amoxicillin/clavulanate, doxycycline, a respiratory fluoroquinolone, or a combination of clindamycin plus a third-generation cephalosporin.1
Continue to: Diagnosis and management of pediatric patients
Diagnosis and management of pediatric patients
Diagnosis of ABRS in children is defined as an acute upper respiratory infection (URI) accompanied by persistent nasal discharge, daytime cough for ≥ 10 days without improvement, an episode of “double sickening,” or severe onset with a temperature ≥ 102°F and purulent nasal discharge for 3 days.15
Initial presentations of viral URIs and ABRS are almost identical; thus, persistence of symptoms is key to diagnosis.6 Nasal discharge tends to appear several days after initial symptoms manifest for viral infections including influenza. In children < 5 years of age, the most common complication involves the orbit.15 Orbital complications generally manifest with eye pain and/or periorbital swelling and may be accompanied by proptosis or decreased functioning of extraocular musculature. The differential diagnosis for orbital complications includes cavernous sinus thrombosis, orbital cellulitis/abscess, subperiosteal abscess, and inflammatory edema.27,28 Intracranial complications are also possible with severe ABRS.12
Radiology studies are not recommended for the initial diagnosis of ABRS in children, as again, imaging does not differentiate between viral and bacterial etiologies. However, in children with complications such as orbital or cerebral involvement, a contrast-enhanced CT scan of the paranasal sinuses is indicated.15
Antibiotic therapy is indicated in children with a diagnosis of severe ABRS or in cases of “double sickening.” Clinicians may consider watchful waiting for 3 additional days before initiating antibiotics in patients meeting criteria for ABRS.Amoxicillin with or without clavulanate is the antibiotic of choice.15
For penicillin-allergic children without a history of anaphylactoid reaction, treatment with cefpodoxime, cefdinir, or cefuroxime is appropriate. For children with a history of anaphylaxis, treatment with a combination of clindamycin (or linezolid) and cefixime is indicated. Alternatively, a fluoroquinolone such as levofloxacin may be used, but adverse effects and emerging resistance limit its use.15
Continue to: Specialist referral
Specialist referral
Referral to Otolaryngology is indicated for patients with > 3 episodes of clinically diagnosed bacterial sinusitis in 1 year, evidence of fungal disease (which is outside the scope of this article), immunocompromised status, or a persistent temperature ≥ 102°F despite antibiotic therapy. Also consider otolaryngology referral for patients with a history of sinus surgery.2,5,6
CORRESPONDENCE
Pamela R. Hughes, Family Medicine Residency Clinic, Mike O’Callaghan Military Medical Center, 4700 Las Vegas Boulevard North, Nellis AFB, NV 89191; [email protected].
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
2. Fokkens WJ, Hoffmans R, Thomas M. Avoid prescribing antibiotics in acute rhinosinusitis. BMJ. 2014;349:g5703.
3. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev. 2014:CD000243.
4. Burgstaller, JM, Steurer J, Holzmann D, et al. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. Eur Arch Otorhinolaryngol. 2016;273:1067-1077.
5. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94:97-105.
6. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
7. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018:CD006089.
8. Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
9. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. 2015;53:3-9.
10. Benninger M, Segreti J. Is it bacterial or viral? Criteria for distinguishing bacterial and viral infections. J Fam Pract. 2008;57(2 suppl):S5-S11.
11. Sharma P, Finley R, Weese S, et al. Antibiotic prescriptions for outpatient acute rhinosinusitis in Canada, 2007-2013. PLoS One. 2017;12:e0181957.
12. Pynnonen MA, Lynn S, Kern HE, et al. Diagnosis and treatment of acute sinusitis in the primary care setting: a retrospective cohort. Laryngoscope. 2015;125:2266-2272.
13. Hansen JG, Schmidt H, Rosborg J, et al. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
14. Ebell MH, McKay B, Dale, A, et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164-172.
15. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280.
16. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
17. Smith SS, Ference EH, Evan CT, et al. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and meta-analysis. Laryngoscope. 2015;125:57-69.
18. Autio TJ, Koskenkorva T, Koivunen P, et al. Inflammatory biomarkers during bacterial acute rhinosinusitis. Curr Allergy Asthma Rep. 2018;18:13.

19. Ebell MH, McKay B, Guilbault R, et al. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612-e632.
20. Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann Fam Med. 2017;15:347-354.
21. Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008;29:640-643.
22. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132.
23. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
24. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
25. Garbutt JM, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
26. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013:CD005149.
27. Abzug MJ. Acute sinusitis in children: do antibiotics have any role? J Infect. 2014;68 (suppl 1):S33-S37.
28. Williams JW Jr, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710.
An estimated 30 million cases of acute rhinosinusitis (ARS) occur every year in the United States.1 More than 80% of people with ARS are prescribed antibiotics in North America, accounting for 15% to 20% of all antibiotic prescriptions in the adult outpatient setting.2,3 Many of these prescriptions are unnecessary, as the most common cause of ARS is a virus.4,5 Evidence consistently shows that symptoms of ARS will resolve spontaneously in most patients and that only those patients with severe or prolonged symptoms require consideration of antibiotic therapy.1,2,4,6 Nearly half of all patients will improve within 1 week and two-thirds of patients will improve within 2 weeks without the use of antibiotics.7 In children, only about 6% to 7% presenting with upper respiratory symptoms meet the criteria for acute bacterial rhinosinusitis (ABRS),8 which we’ll detail in a bit. For most patients, treatment should consist of symptom management.5
But what about the minority who require antibiotic therapy? This article reviews how to evaluate patients with ARS, identify those who require antibiotics, and prescribe the most appropriate antibiotic treatment regimens.
Diagnosis: Distinguishing viral from bacterial disease
ARS is defined as the sudden onset of purulent nasal discharge plus either nasal blockage or facial pressure/pain lasting < 4 weeks.3,9 Additional signs and symptoms may include postnasal drip, a reduced sense of smell, sinus tenderness to palpation, and maxillary toothaches.10,11
ARS may be viral or bacterial in etiology, with the most common bacterial organisms being Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1,3,5 The most common viral causes are influenza, parainfluenza, and rhinovirus. Approximately 90% to 98% of cases of ARS are viral6,11; only about 0.5% to 2% of viral rhinosinusitis episodes are complicated by bacterial infection.1,10-12
Diagnose ABRS when symptoms of ARS fail to improve after 10 days or symptoms of ARS worsen within 10 days after initial improvement (“double sickening”).1,11 Symptoms that are significantly associated with ABRS are unilateral sinus pain and reported maxillary pain. The presence of facial or dental pain correlates with ABRS but does not identify the specific sinus involved.1
There isn’t good correlation between patients saying they have sinusitis and actually having it.13 A 2019 meta-analysis by Ebell et al14 reported that based on limited data, the overall clinical impression, fetid odor on the breath, and pain in the teeth are the best individual clinical predictors of ABRS.
As recommended by the Infectious Disease Society of America (IDSA), a diagnosis of ABRS is also reasonable in patients who present with severe symptoms at the onset.6 Although there is no consensus about what constitutes “severe symptoms,” they are often described as a temperature ≥ 102°F (39°C) plus 3 to 4 days of purulent nasal drainage.1,4,6
Continue to: Additional symptoms of ABRS may include...
Additional symptoms of ABRS may include cough, fatigue, decreased or lack of sense of smell (hyposmia or anosmia), and ear pressure.10 Another sign of “double sickening” is the development of a fever after several days of symptoms.1,9,15 Viral sinusitis typically lasts 5 to 7 days with a peak at days 2 to 3.1,15 If symptoms continue for 10 days, there is a 60% chance of bacterial sinusitis, although some viral rhinosinusitis symptoms persist for > 14 days.1,5 Beyond 4 to 12 weeks, sinusitis is classified as subacute or chronic.3
Physical exam findings and the limited roles of imaging and labs
Common physical exam findings associated with the diagnosis of ABRS include altered speech indicating nasal obstruction; edema or erythema of the skin indicating congested capillaries; tenderness to palpation over the cheeks or upper teeth; odorous breath; and purulent drainage from the nose or in the posterior pharynx.
In a study by Hansen et al13 (N = 174), the only sign that showed significant association with ABRS (diagnosed by sinus aspiration or lavage) was unilateral tenderness of the maxillary sinuses. The presence of purulent drainage in the nose or posterior pharynx also has significant diagnostic value, as it predicts the presence of bacteria on antral aspiration.1 Purulent discharge in the pharynx is associated with a higher likelihood of benefit from antibiotic therapy compared to placebo (number needed to treat [NNT] = 8).16 However, colored nasal discharge indicates the presence of neutrophils—not bacteria—and does not predict the likelihood of bacterial sinus infection.14,17 Therefore, the history and physical exam should focus on location of pain (sinus and/or teeth), duration of symptoms, presence of fever, change in symptom severity, attempted home therapies, sinus tenderness on exam, breath odor, and purulent drainage seen in the nasal cavity or posterior pharynx.13,14
Radiographic imaging has no role in the diagnosis or treatment of uncomplicated ABRS because viral and bacterial etiologies have similar radiographic appearances. Additionally, employing radiologic imaging would increase health care costs by at least 4-fold.5,6,8,17 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) clinical practice guidelines recommend against radiographic imaging for patients who meet the diagnostic criteria for ABRS unless concern exists for a complication or an alternate diagnosis is suspected.1 Computed tomography (CT) imaging of the sinuses may be warranted in patients with severe headaches, facial swelling, cranial nerve palsies, or bulging of the eye (proptosis), all of which indicate a potential complication of ABRS.1
Laboratory evaluations. ABRS is a clinical diagnosis; therefore, routine lab work, such as a white blood cell count, C-reactive protein (CRP) level, and/or erythrocyte sedimentation rate (ESR), are not indicated unless an alternate diagnosis is suspected.1,5,13,18,19
Continue to: In one study...
In one study, CRP > 10 mg/L and ESR > 10 mm/h were the strongest individual predictors of purulent antral puncture aspirate or positive bacterial culture of aspirate, which is considered diagnostic for ABRS. 20 However, CRP and ESR by themselves are not adequate to diagnose ABRS.20 This study developed a clinical decision rule that used symptoms, signs, and laboratory values to rate the likelihood of ABRS as being either low, moderate, or high. However, this clinical decision rule has not been prospectively validated.
Thus, CRP and ESR elevations can support the diagnosis of ABRS, but the low sensitivity of these tests precludes their use as a screening tool for ABRS.14,18 Studies by Ebell19 and Huang21 have shown some benefit to dipstick assay of nasal secretions for the diagnosis of ABRS, but this method is not validated or widely used.19,21
Treatment: From managing symptoms to prescribing antibiotics
Overprescribing antibiotics for ARS is a prominent health care issue. In fact, 5 of 9 placebo-controlled studies showed that most people improve within 2 weeks regardless of antibiotic use (N = 1058).3 Therefore, weigh the decision to treat ABRS with antibiotics against the risk for potential adverse reactions and within the context of antibiotic stewardship.2,9,12,22-24 Consider antibiotics only if patients meet the diagnostic criteria for ABRS (TABLE 11,6) or, occasionally, for patients with severe symptoms upon presentation, such as a temperature ≥ 102°F (39°C) plus purulent nasal discharge for 3 to 4 days.1 The most commonly reported adverse effects of antibiotics are gastrointestinal in nature and include nausea, vomiting, and diarrhea.2,9

Symptomatic management for both ARS and ABRS is recommended as first-line therapy; it should be offered to patients before making a diagnosis of ABRS.1,5,9,25 Consider using analgesics, topical intranasal steroids, and/or nasal saline irrigation to alleviate symptoms and improve quality of life.1,5,25 Interventions with questionable or unproven efficacy include the use of antihistamines, systemic steroids, decongestants, and mucolytics, but they may be considered on an individual basis.1 A systematic review found that topical nasal steroids relieved facial pain and nasal congestion in patients with rhinitis and acute sinusitis (NNT = 14).1,26

Even after diagnosing ABRS, clinicians should offer watchful waiting and symptomatic therapies as long as patients have adequate access to follow-up (TABLE 2,1,15FIGURE1,6). Antibiotic therapy can then be initiated if symptoms do not improve after an additional 7 days of watchful waiting or if symptoms worsen at any time. It is reasonable to give patients a prescription to keep on hand to be used if symptoms worsen, with instructions to notify the provider if antibiotics are started.1

Continue to: Antibiotic therapy
Antibiotic therapy. The rationale for treating ABRS with antibiotics is to expedite recovery and prevent complications such as periorbital or orbital cellulitis, meningitis, frontal osteomyelitis, cavernous sinus thrombosis, and other serious illness.27 Antibiotic treatment is associated with a shorter duration of symptoms (NNT = 19) but an increased risk of adverse events (NNH = 8).7,19
Amoxicillin with or without clavulanate for 5 to 10 days is first-line antibiotic therapy for most adults with ABRS.1,3,5,8,9,11 Per AAO-HNS, the “justification for amoxicillin as first-line treatment relates to its safety, efficacy, low cost, and narrow microbiologic spectrum.”1 Amoxicillin may be dosed 500 mg tid for 5 to 10 days. Amoxicillin/clavulanate (Augmentin) is recommended for patients with comorbid conditions or with increased risk of bacterial resistance. Dosing for amoxicillin/clavulanate is 500/125 mg tid or 875/125 mg bid for 5 to 10 days. Duration of therapy should be determined by the severity of symptoms.5
For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is considered first-line treatment.1,6 Doxycycline is preferred because of its narrower spectrum and fewer adverse effects than the fluoroquinolones. Fluoroquinolones should be reserved for patients who fail first-line treatment and are penicillin allergic.1 Because of the high rates of resistance among S pneumoniae and H influenzae, macrolides, trimethoprim/sulfamethoxazole (TMP/SMX), and cephalosporins are not recommended as first-line therapy.1,5
How antibiotic options compare. A Cochrane review of 54 studies comparing different antibiotics showed no antibiotic was superior.3 Of the 54 studies, 6 studies (N = 1887) were pooled to compare cephalosporins to amoxicillin/clavulanate at 7 to 15 days. The findings indicated a statistically significant difference for amoxicillin/clavulanate with a relative risk (RR) of 1.37 (confidence interval [CI], 1.04-1.8).3 However, none of these 6 studies were graded as having a low risk of bias; therefore, confidence in this finding was deemed limited due to the quality of included studies. The failure rate for cephalosporins was 12% vs 8% for amoxicillin/clavulanate.3
Treatment failure is considered when a patient has not improved by Day 7 after ABRS diagnosis (with or without medication) or when symptoms worsen at any time. If watchful waiting was chosen and a safety net prescription was provided, the antibiotics should be filled and started. If no antibiotic was prescribed at the time watchful waiting commenced, the patient should return for further evaluation and be started on antibiotics. If antibiotics were prescribed initially for severe symptoms, a change in antibiotic therapy is indicated, and a broader-spectrum antibiotic should be chosen. If amoxicillin was prescribed, the patient should be switched to amoxicillin/clavulanate, doxycycline, a respiratory fluoroquinolone, or a combination of clindamycin plus a third-generation cephalosporin.1
Continue to: Diagnosis and management of pediatric patients
Diagnosis and management of pediatric patients
Diagnosis of ABRS in children is defined as an acute upper respiratory infection (URI) accompanied by persistent nasal discharge, daytime cough for ≥ 10 days without improvement, an episode of “double sickening,” or severe onset with a temperature ≥ 102°F and purulent nasal discharge for 3 days.15
Initial presentations of viral URIs and ABRS are almost identical; thus, persistence of symptoms is key to diagnosis.6 Nasal discharge tends to appear several days after initial symptoms manifest for viral infections including influenza. In children < 5 years of age, the most common complication involves the orbit.15 Orbital complications generally manifest with eye pain and/or periorbital swelling and may be accompanied by proptosis or decreased functioning of extraocular musculature. The differential diagnosis for orbital complications includes cavernous sinus thrombosis, orbital cellulitis/abscess, subperiosteal abscess, and inflammatory edema.27,28 Intracranial complications are also possible with severe ABRS.12
Radiology studies are not recommended for the initial diagnosis of ABRS in children, as again, imaging does not differentiate between viral and bacterial etiologies. However, in children with complications such as orbital or cerebral involvement, a contrast-enhanced CT scan of the paranasal sinuses is indicated.15
Antibiotic therapy is indicated in children with a diagnosis of severe ABRS or in cases of “double sickening.” Clinicians may consider watchful waiting for 3 additional days before initiating antibiotics in patients meeting criteria for ABRS.Amoxicillin with or without clavulanate is the antibiotic of choice.15
For penicillin-allergic children without a history of anaphylactoid reaction, treatment with cefpodoxime, cefdinir, or cefuroxime is appropriate. For children with a history of anaphylaxis, treatment with a combination of clindamycin (or linezolid) and cefixime is indicated. Alternatively, a fluoroquinolone such as levofloxacin may be used, but adverse effects and emerging resistance limit its use.15
Continue to: Specialist referral
Specialist referral
Referral to Otolaryngology is indicated for patients with > 3 episodes of clinically diagnosed bacterial sinusitis in 1 year, evidence of fungal disease (which is outside the scope of this article), immunocompromised status, or a persistent temperature ≥ 102°F despite antibiotic therapy. Also consider otolaryngology referral for patients with a history of sinus surgery.2,5,6
CORRESPONDENCE
Pamela R. Hughes, Family Medicine Residency Clinic, Mike O’Callaghan Military Medical Center, 4700 Las Vegas Boulevard North, Nellis AFB, NV 89191; [email protected].
An estimated 30 million cases of acute rhinosinusitis (ARS) occur every year in the United States.1 More than 80% of people with ARS are prescribed antibiotics in North America, accounting for 15% to 20% of all antibiotic prescriptions in the adult outpatient setting.2,3 Many of these prescriptions are unnecessary, as the most common cause of ARS is a virus.4,5 Evidence consistently shows that symptoms of ARS will resolve spontaneously in most patients and that only those patients with severe or prolonged symptoms require consideration of antibiotic therapy.1,2,4,6 Nearly half of all patients will improve within 1 week and two-thirds of patients will improve within 2 weeks without the use of antibiotics.7 In children, only about 6% to 7% presenting with upper respiratory symptoms meet the criteria for acute bacterial rhinosinusitis (ABRS),8 which we’ll detail in a bit. For most patients, treatment should consist of symptom management.5
But what about the minority who require antibiotic therapy? This article reviews how to evaluate patients with ARS, identify those who require antibiotics, and prescribe the most appropriate antibiotic treatment regimens.
Diagnosis: Distinguishing viral from bacterial disease
ARS is defined as the sudden onset of purulent nasal discharge plus either nasal blockage or facial pressure/pain lasting < 4 weeks.3,9 Additional signs and symptoms may include postnasal drip, a reduced sense of smell, sinus tenderness to palpation, and maxillary toothaches.10,11
ARS may be viral or bacterial in etiology, with the most common bacterial organisms being Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.1,3,5 The most common viral causes are influenza, parainfluenza, and rhinovirus. Approximately 90% to 98% of cases of ARS are viral6,11; only about 0.5% to 2% of viral rhinosinusitis episodes are complicated by bacterial infection.1,10-12
Diagnose ABRS when symptoms of ARS fail to improve after 10 days or symptoms of ARS worsen within 10 days after initial improvement (“double sickening”).1,11 Symptoms that are significantly associated with ABRS are unilateral sinus pain and reported maxillary pain. The presence of facial or dental pain correlates with ABRS but does not identify the specific sinus involved.1
There isn’t good correlation between patients saying they have sinusitis and actually having it.13 A 2019 meta-analysis by Ebell et al14 reported that based on limited data, the overall clinical impression, fetid odor on the breath, and pain in the teeth are the best individual clinical predictors of ABRS.
As recommended by the Infectious Disease Society of America (IDSA), a diagnosis of ABRS is also reasonable in patients who present with severe symptoms at the onset.6 Although there is no consensus about what constitutes “severe symptoms,” they are often described as a temperature ≥ 102°F (39°C) plus 3 to 4 days of purulent nasal drainage.1,4,6
Continue to: Additional symptoms of ABRS may include...
Additional symptoms of ABRS may include cough, fatigue, decreased or lack of sense of smell (hyposmia or anosmia), and ear pressure.10 Another sign of “double sickening” is the development of a fever after several days of symptoms.1,9,15 Viral sinusitis typically lasts 5 to 7 days with a peak at days 2 to 3.1,15 If symptoms continue for 10 days, there is a 60% chance of bacterial sinusitis, although some viral rhinosinusitis symptoms persist for > 14 days.1,5 Beyond 4 to 12 weeks, sinusitis is classified as subacute or chronic.3
Physical exam findings and the limited roles of imaging and labs
Common physical exam findings associated with the diagnosis of ABRS include altered speech indicating nasal obstruction; edema or erythema of the skin indicating congested capillaries; tenderness to palpation over the cheeks or upper teeth; odorous breath; and purulent drainage from the nose or in the posterior pharynx.
In a study by Hansen et al13 (N = 174), the only sign that showed significant association with ABRS (diagnosed by sinus aspiration or lavage) was unilateral tenderness of the maxillary sinuses. The presence of purulent drainage in the nose or posterior pharynx also has significant diagnostic value, as it predicts the presence of bacteria on antral aspiration.1 Purulent discharge in the pharynx is associated with a higher likelihood of benefit from antibiotic therapy compared to placebo (number needed to treat [NNT] = 8).16 However, colored nasal discharge indicates the presence of neutrophils—not bacteria—and does not predict the likelihood of bacterial sinus infection.14,17 Therefore, the history and physical exam should focus on location of pain (sinus and/or teeth), duration of symptoms, presence of fever, change in symptom severity, attempted home therapies, sinus tenderness on exam, breath odor, and purulent drainage seen in the nasal cavity or posterior pharynx.13,14
Radiographic imaging has no role in the diagnosis or treatment of uncomplicated ABRS because viral and bacterial etiologies have similar radiographic appearances. Additionally, employing radiologic imaging would increase health care costs by at least 4-fold.5,6,8,17 The American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) clinical practice guidelines recommend against radiographic imaging for patients who meet the diagnostic criteria for ABRS unless concern exists for a complication or an alternate diagnosis is suspected.1 Computed tomography (CT) imaging of the sinuses may be warranted in patients with severe headaches, facial swelling, cranial nerve palsies, or bulging of the eye (proptosis), all of which indicate a potential complication of ABRS.1
Laboratory evaluations. ABRS is a clinical diagnosis; therefore, routine lab work, such as a white blood cell count, C-reactive protein (CRP) level, and/or erythrocyte sedimentation rate (ESR), are not indicated unless an alternate diagnosis is suspected.1,5,13,18,19
Continue to: In one study...
In one study, CRP > 10 mg/L and ESR > 10 mm/h were the strongest individual predictors of purulent antral puncture aspirate or positive bacterial culture of aspirate, which is considered diagnostic for ABRS. 20 However, CRP and ESR by themselves are not adequate to diagnose ABRS.20 This study developed a clinical decision rule that used symptoms, signs, and laboratory values to rate the likelihood of ABRS as being either low, moderate, or high. However, this clinical decision rule has not been prospectively validated.
Thus, CRP and ESR elevations can support the diagnosis of ABRS, but the low sensitivity of these tests precludes their use as a screening tool for ABRS.14,18 Studies by Ebell19 and Huang21 have shown some benefit to dipstick assay of nasal secretions for the diagnosis of ABRS, but this method is not validated or widely used.19,21
Treatment: From managing symptoms to prescribing antibiotics
Overprescribing antibiotics for ARS is a prominent health care issue. In fact, 5 of 9 placebo-controlled studies showed that most people improve within 2 weeks regardless of antibiotic use (N = 1058).3 Therefore, weigh the decision to treat ABRS with antibiotics against the risk for potential adverse reactions and within the context of antibiotic stewardship.2,9,12,22-24 Consider antibiotics only if patients meet the diagnostic criteria for ABRS (TABLE 11,6) or, occasionally, for patients with severe symptoms upon presentation, such as a temperature ≥ 102°F (39°C) plus purulent nasal discharge for 3 to 4 days.1 The most commonly reported adverse effects of antibiotics are gastrointestinal in nature and include nausea, vomiting, and diarrhea.2,9

Symptomatic management for both ARS and ABRS is recommended as first-line therapy; it should be offered to patients before making a diagnosis of ABRS.1,5,9,25 Consider using analgesics, topical intranasal steroids, and/or nasal saline irrigation to alleviate symptoms and improve quality of life.1,5,25 Interventions with questionable or unproven efficacy include the use of antihistamines, systemic steroids, decongestants, and mucolytics, but they may be considered on an individual basis.1 A systematic review found that topical nasal steroids relieved facial pain and nasal congestion in patients with rhinitis and acute sinusitis (NNT = 14).1,26

Even after diagnosing ABRS, clinicians should offer watchful waiting and symptomatic therapies as long as patients have adequate access to follow-up (TABLE 2,1,15FIGURE1,6). Antibiotic therapy can then be initiated if symptoms do not improve after an additional 7 days of watchful waiting or if symptoms worsen at any time. It is reasonable to give patients a prescription to keep on hand to be used if symptoms worsen, with instructions to notify the provider if antibiotics are started.1

Continue to: Antibiotic therapy
Antibiotic therapy. The rationale for treating ABRS with antibiotics is to expedite recovery and prevent complications such as periorbital or orbital cellulitis, meningitis, frontal osteomyelitis, cavernous sinus thrombosis, and other serious illness.27 Antibiotic treatment is associated with a shorter duration of symptoms (NNT = 19) but an increased risk of adverse events (NNH = 8).7,19
Amoxicillin with or without clavulanate for 5 to 10 days is first-line antibiotic therapy for most adults with ABRS.1,3,5,8,9,11 Per AAO-HNS, the “justification for amoxicillin as first-line treatment relates to its safety, efficacy, low cost, and narrow microbiologic spectrum.”1 Amoxicillin may be dosed 500 mg tid for 5 to 10 days. Amoxicillin/clavulanate (Augmentin) is recommended for patients with comorbid conditions or with increased risk of bacterial resistance. Dosing for amoxicillin/clavulanate is 500/125 mg tid or 875/125 mg bid for 5 to 10 days. Duration of therapy should be determined by the severity of symptoms.5
For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) is considered first-line treatment.1,6 Doxycycline is preferred because of its narrower spectrum and fewer adverse effects than the fluoroquinolones. Fluoroquinolones should be reserved for patients who fail first-line treatment and are penicillin allergic.1 Because of the high rates of resistance among S pneumoniae and H influenzae, macrolides, trimethoprim/sulfamethoxazole (TMP/SMX), and cephalosporins are not recommended as first-line therapy.1,5
How antibiotic options compare. A Cochrane review of 54 studies comparing different antibiotics showed no antibiotic was superior.3 Of the 54 studies, 6 studies (N = 1887) were pooled to compare cephalosporins to amoxicillin/clavulanate at 7 to 15 days. The findings indicated a statistically significant difference for amoxicillin/clavulanate with a relative risk (RR) of 1.37 (confidence interval [CI], 1.04-1.8).3 However, none of these 6 studies were graded as having a low risk of bias; therefore, confidence in this finding was deemed limited due to the quality of included studies. The failure rate for cephalosporins was 12% vs 8% for amoxicillin/clavulanate.3
Treatment failure is considered when a patient has not improved by Day 7 after ABRS diagnosis (with or without medication) or when symptoms worsen at any time. If watchful waiting was chosen and a safety net prescription was provided, the antibiotics should be filled and started. If no antibiotic was prescribed at the time watchful waiting commenced, the patient should return for further evaluation and be started on antibiotics. If antibiotics were prescribed initially for severe symptoms, a change in antibiotic therapy is indicated, and a broader-spectrum antibiotic should be chosen. If amoxicillin was prescribed, the patient should be switched to amoxicillin/clavulanate, doxycycline, a respiratory fluoroquinolone, or a combination of clindamycin plus a third-generation cephalosporin.1
Continue to: Diagnosis and management of pediatric patients
Diagnosis and management of pediatric patients
Diagnosis of ABRS in children is defined as an acute upper respiratory infection (URI) accompanied by persistent nasal discharge, daytime cough for ≥ 10 days without improvement, an episode of “double sickening,” or severe onset with a temperature ≥ 102°F and purulent nasal discharge for 3 days.15
Initial presentations of viral URIs and ABRS are almost identical; thus, persistence of symptoms is key to diagnosis.6 Nasal discharge tends to appear several days after initial symptoms manifest for viral infections including influenza. In children < 5 years of age, the most common complication involves the orbit.15 Orbital complications generally manifest with eye pain and/or periorbital swelling and may be accompanied by proptosis or decreased functioning of extraocular musculature. The differential diagnosis for orbital complications includes cavernous sinus thrombosis, orbital cellulitis/abscess, subperiosteal abscess, and inflammatory edema.27,28 Intracranial complications are also possible with severe ABRS.12
Radiology studies are not recommended for the initial diagnosis of ABRS in children, as again, imaging does not differentiate between viral and bacterial etiologies. However, in children with complications such as orbital or cerebral involvement, a contrast-enhanced CT scan of the paranasal sinuses is indicated.15
Antibiotic therapy is indicated in children with a diagnosis of severe ABRS or in cases of “double sickening.” Clinicians may consider watchful waiting for 3 additional days before initiating antibiotics in patients meeting criteria for ABRS.Amoxicillin with or without clavulanate is the antibiotic of choice.15
For penicillin-allergic children without a history of anaphylactoid reaction, treatment with cefpodoxime, cefdinir, or cefuroxime is appropriate. For children with a history of anaphylaxis, treatment with a combination of clindamycin (or linezolid) and cefixime is indicated. Alternatively, a fluoroquinolone such as levofloxacin may be used, but adverse effects and emerging resistance limit its use.15
Continue to: Specialist referral
Specialist referral
Referral to Otolaryngology is indicated for patients with > 3 episodes of clinically diagnosed bacterial sinusitis in 1 year, evidence of fungal disease (which is outside the scope of this article), immunocompromised status, or a persistent temperature ≥ 102°F despite antibiotic therapy. Also consider otolaryngology referral for patients with a history of sinus surgery.2,5,6
CORRESPONDENCE
Pamela R. Hughes, Family Medicine Residency Clinic, Mike O’Callaghan Military Medical Center, 4700 Las Vegas Boulevard North, Nellis AFB, NV 89191; [email protected].
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
2. Fokkens WJ, Hoffmans R, Thomas M. Avoid prescribing antibiotics in acute rhinosinusitis. BMJ. 2014;349:g5703.
3. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev. 2014:CD000243.
4. Burgstaller, JM, Steurer J, Holzmann D, et al. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. Eur Arch Otorhinolaryngol. 2016;273:1067-1077.
5. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94:97-105.
6. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
7. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018:CD006089.
8. Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
9. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. 2015;53:3-9.
10. Benninger M, Segreti J. Is it bacterial or viral? Criteria for distinguishing bacterial and viral infections. J Fam Pract. 2008;57(2 suppl):S5-S11.
11. Sharma P, Finley R, Weese S, et al. Antibiotic prescriptions for outpatient acute rhinosinusitis in Canada, 2007-2013. PLoS One. 2017;12:e0181957.
12. Pynnonen MA, Lynn S, Kern HE, et al. Diagnosis and treatment of acute sinusitis in the primary care setting: a retrospective cohort. Laryngoscope. 2015;125:2266-2272.
13. Hansen JG, Schmidt H, Rosborg J, et al. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
14. Ebell MH, McKay B, Dale, A, et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164-172.
15. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280.
16. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
17. Smith SS, Ference EH, Evan CT, et al. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and meta-analysis. Laryngoscope. 2015;125:57-69.
18. Autio TJ, Koskenkorva T, Koivunen P, et al. Inflammatory biomarkers during bacterial acute rhinosinusitis. Curr Allergy Asthma Rep. 2018;18:13.

19. Ebell MH, McKay B, Guilbault R, et al. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612-e632.
20. Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann Fam Med. 2017;15:347-354.
21. Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008;29:640-643.
22. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132.
23. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
24. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
25. Garbutt JM, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
26. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013:CD005149.
27. Abzug MJ. Acute sinusitis in children: do antibiotics have any role? J Infect. 2014;68 (suppl 1):S33-S37.
28. Williams JW Jr, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710.
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
2. Fokkens WJ, Hoffmans R, Thomas M. Avoid prescribing antibiotics in acute rhinosinusitis. BMJ. 2014;349:g5703.
3. Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, et al. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database Syst Rev. 2014:CD000243.
4. Burgstaller, JM, Steurer J, Holzmann D, et al. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. Eur Arch Otorhinolaryngol. 2016;273:1067-1077.
5. Aring AM, Chan MM. Current concepts in adult acute rhinosinusitis. Am Fam Physician. 2016;94:97-105.
6. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72-e112.
7. Lemiengre MB, van Driel ML, Merenstein D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. 2018:CD006089.
8. Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
9. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. 2015;53:3-9.
10. Benninger M, Segreti J. Is it bacterial or viral? Criteria for distinguishing bacterial and viral infections. J Fam Pract. 2008;57(2 suppl):S5-S11.
11. Sharma P, Finley R, Weese S, et al. Antibiotic prescriptions for outpatient acute rhinosinusitis in Canada, 2007-2013. PLoS One. 2017;12:e0181957.
12. Pynnonen MA, Lynn S, Kern HE, et al. Diagnosis and treatment of acute sinusitis in the primary care setting: a retrospective cohort. Laryngoscope. 2015;125:2266-2272.
13. Hansen JG, Schmidt H, Rosborg J, et al. Predicting acute maxillary sinusitis in a general practice population. BMJ 1995;311:233-236.
14. Ebell MH, McKay B, Dale, A, et al. Accuracy of signs and symptoms for the diagnosis of acute rhinosinusitis and acute bacterial rhinosinusitis. Ann Fam Med. 2019;17:164-172.
15. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262-e280.
16. Young J, De Sutter A, Merenstein D, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta-analysis of individual patient data. Lancet. 2008;371:908-914.
17. Smith SS, Ference EH, Evan CT, et al. The prevalence of bacterial infection in acute rhinosinusitis: a systematic review and meta-analysis. Laryngoscope. 2015;125:57-69.
18. Autio TJ, Koskenkorva T, Koivunen P, et al. Inflammatory biomarkers during bacterial acute rhinosinusitis. Curr Allergy Asthma Rep. 2018;18:13.

19. Ebell MH, McKay B, Guilbault R, et al. Diagnosis of acute rhinosinusitis in primary care: a systematic review of test accuracy. Br J Gen Pract. 2016;66:e612-e632.
20. Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Ann Fam Med. 2017;15:347-354.
21. Huang SW, Small PA. Rapid diagnosis of bacterial sinusitis in patients using a simple test of nasal secretions. Allergy Asthma Proc. 2008;29:640-643.
22. Smith SS, Evans CT, Tan BK, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132.
23. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
24. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
25. Garbutt JM, Banister C, Spitznagel E, et al. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA. 2012;307:685-692.
26. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013:CD005149.
27. Abzug MJ. Acute sinusitis in children: do antibiotics have any role? J Infect. 2014;68 (suppl 1):S33-S37.
28. Williams JW Jr, Simel DL, Roberts L, et al. Clinical evaluation for sinusitis. Making the diagnosis by history and physical examination. Ann Intern Med. 1992;117:705-710.
PRACTICE RECOMMENDATIONS
› Reserve antibiotics for patients who meet diagnostic criteria for acute bacterial rhinosinusitis (ABRS). Patients must have purulent nasal drainage that is accompanied by either nasal obstruction or facial pain/pressure/fullness and EITHER symptoms that persist without improvement for at least 10 days OR symptoms that worsen within 10 days of initial improvement (“double sickening”). A
› Offer watchful waiting and delay antibiotics for up to 7 days after diagnosing ABRS in a patient if adequate access to follow-up is available; otherwise, treat with amoxicillin (with or without clavulanate) for 5 to 10 days. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Painful foot or ankle? Don't overlook these 5 injuries
Foot and ankle injuries are among the most common conditions evaluated at primary care visits; the differential diagnosis of such injury is broad.1 Although many of these injuries are easily identified on imaging studies, a number of subtle, yet important, conditions can be easily missed, especially if you do not routinely encounter them. Given that broad differential, a high degree of suspicion is required to make an accurate diagnosis, which allows appropriate treatment within a reasonable time frame and minimizes the risk of long-term morbidity.
This article outlines the diagnosis and initial management of 5 important, yet often elusive, types of foot and ankle conditions: Achilles tendon rupture, injury to the syndesmosis, ankle fracture, Lisfranc injury, and proximal fracture of the fifth metatarsal.
Achilles tendon rupture
The Achilles tendon is the most frequently ruptured tendon in the body (approximately 20% of all large-tendon injuries)2; as many as 25% of cases are initially misdiagnosed.3
Presentation. Patients frequently present with pain at the Achilles tendon—2 to 6 cm above the insertion into the calcaneus—and an inability to fully bear weight.4,5 A small percentage of patients are able to ambulate on the affected side, albeit with minor pain, which likely contributes to the rate of missed diagnosis. Absence of difficulty bearing weight is due to the presence of secondary plantar flexors, which can compensate for loss of chief plantar flexor function by the Achilles tendon.2
Examination of a patient with an Achilles tendon rupture typically reveals edema, bruising, and a palpable gap within the tendon, 2 to 6 cm proximal to insertion.3,4 The Thompson test—squeezing the calf with the patient prone and the knee on the affected side flexed—can aid in diagnosis. When the Achilles tendon is intact, plantar flexion occurs at the ankle; when the tendon is ruptured, plantar flexion is absent.5 The test can be modified when examining a patient who is unable to lie prone by having them rest the flexed knee on a chair while standing on the unaffected leg.
A diagnosis of Achilles tendon rupture is supported when at least 2 of the following conditions are met4,5:
- positive Thompson test
- decreased strength during plantar flexion of the ankle
- palpable gap or pain at the typical location (2-6 cm above insertion)
- increased passive ankle dorsiflexion upon gentle ranging of the ankle joint.
Imaging has a limited role in the diagnosis of Achilles tendon rupture; because the findings of the physical examination are reliable, reserve x-rays for cases in which the diagnosis remains uncertain after examination.2 Consider ordering plain x-rays to rule out an avulsion fracture at the insertion of the Achilles tendon; ultrasonography or magnetic resonance imaging (MRI) might assist you in detecting the rupture proper, along with the location of the tear for surgical planning, if surgery is deemed necessary by an orthopedic surgeon.3-5
Continue to: Management
Management. Some degree of controversy surrounds preferred treatment of Achilles tendon rupture, although available evidence demonstrates that these injuries can be effectively managed by surgical repair or nonoperative treatment, as outcomes are comparable.3,5 Operative management tends to reduce the risk of repeat rupture, compared to nonoperative treatment; however, the potential for surgical complications, including wound infection, sensory disturbance, and adhesions favors nonoperative treatment.3,4,6
Nonoperative treatment consists of referral to a functional rehabilitation program, without which outcomes are, on the whole, less favorable than with surgery.3,6 Surgery is preferred if functional rehabilitation is unavailable, 6 months of conservative management fails, or there is avulsion injury.3,4,6
Injury to the syndesmosis
A complex of ligaments that provide dynamic stability to the ankle joint, the tibiofibular syndesmosis comprises:
- the anterior inferior tibiofibular ligament
- the posterior inferior tibiofibular ligament
- the inferior transverse tibiofibular ligament
- the interosseous membrane.
These structures are further supported by the deltoid ligament.7,8
Commonly referred to as a “high ankle sprain,” a syndesmotic injury is present in as many as 20% of ankle fractures and 5% to 10% of ankle sprains. Injury typically results from external rotation with hyperdorsiflexion of the ankle. Recovery is typically prolonged (ie, twice as long as recovery from a lateral ankle sprain). The diagnosis is missed in as many as 20% of patients; failure to recognize and treat syndesmotic instability appropriately can lead to posttraumatic arthritis.7,9
Continue to: Presentation
Presentation. Patients generally present with ankle pain, swelling, instability, pain when walking on uneven terrain, and pain upon push-off.9
Examination reveals reduced passive ankle dorsiflexion and tenderness upon palpation of individual ligaments. Several clinical tests have been described to aid in detecting this often-elusive diagnosis7,9,10,11:
- Squeeze test. The patient sits with the knee on the affected side bent at a 90° degree angle while the examiner applies compression, with one or both hands, to the tibia and fibula at midcalf. The test is positive when pain is elicited at the level of the syndesmosis just above the ankle joint.9,11
- External rotation test. External rotation of the foot and ankle relative to the tibia reproduces pain.
- Crossed leg test. The affected ankle is crossed over the opposite knee in a figure-4 position. The test is positive when pain is elicited at the syndesmosis.10
- Cotton test. The proximal lower leg is steadied with 1 hand and the plantar heel grasped with the other hand. Pain when the heel is externally rotated (and radiographic widening of the syndesmosis under fluoroscopy) signal syndesmotic instability.
- Fibular translation test. When anterior or posterior drawer force is applied to the fibula, pain and increased translation of the fibula (compared to the contralateral side) suggest instability.
With the Cotton and fibular translation tests, interexaminer technique is more variable and findings are less reproducible.8 Taken alone, none of the above-listed tests are diagnostic; they can, however, assist in making a diagnosis of an injury to the syndesmosis.11
Imaging typically involves anteroposterior [AP], lateral, and mortise plain films of the ankle and weight-bearing AP and lateral views of the tibia and fibula.9 Important measures on weight-bearing AP x-rays are the tibiofibular clear space (abnormal, > 6 mm) and the tibiofibular overlap (abnormal, < 6 mm) (both abnormalities shown in FIGURE 1). Comparing films of the affected ankle with views of the contralateral ankle is often useful.
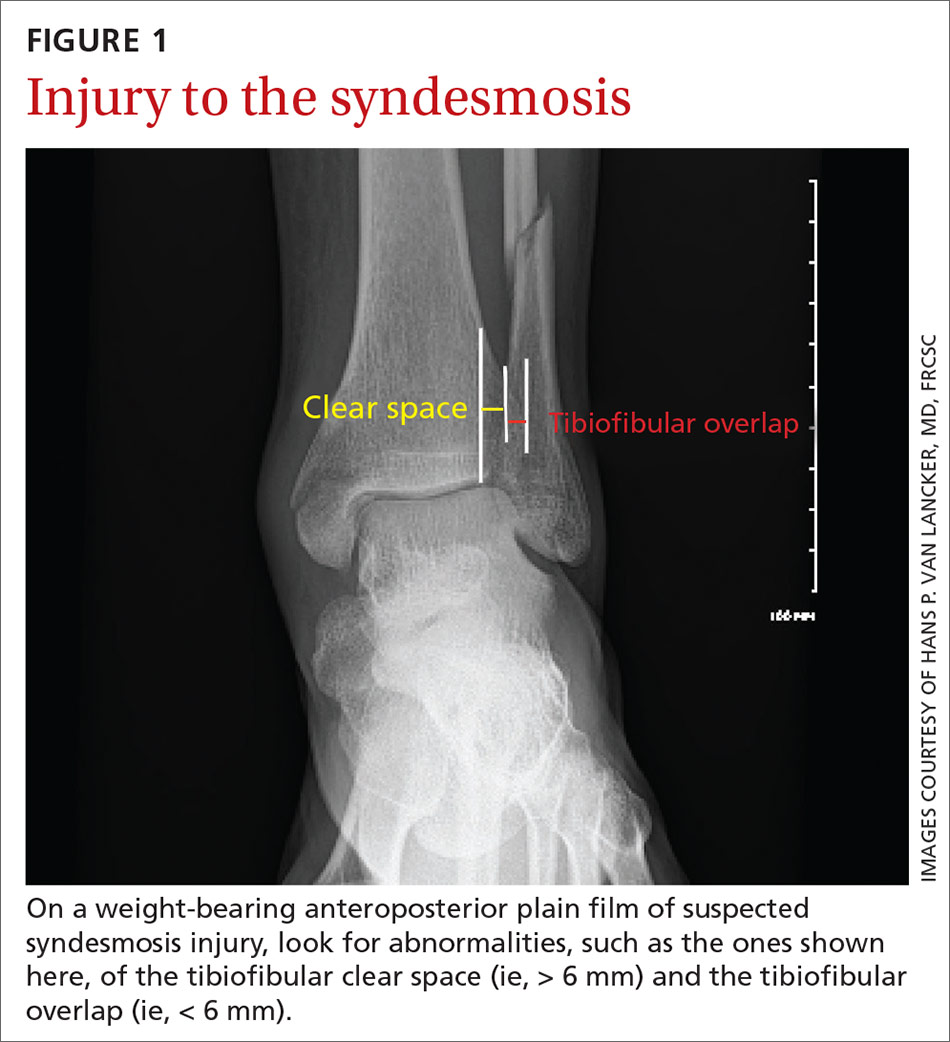
Management of syndesmotic injuries depends on degree of disruption:
- Grade 1 injury is a sprain without diastasis on imaging. Management is conservative, with immobilization in a splint or boot for 1 to 3 weeks, followed by functional rehabilitation over 3 to 6 weeks.10
- Grade 2 injury is demonstrated by diastasis on a stress radiograph. Although evidence to guide successful identification of a grade 2 injury is lacking, it is clinically important to make that identification because these injuries might require surgical intervention, due to instability. Because the diagnosis of this injury can be challenging in primary care, high clinical suspicion of a grade 2 injury makes it appropriate to defer further evaluation to an orthopedic surgeon. On the other hand, if suspicion of a grade 2 injury is low, a trial of conservative management, with weekly clinical assessment, can be considered. A diagnosis of grade 2 injury can be inferred when a patient is unable to perform a single-leg hop after 3 weeks of immobilization; referral to an orthopedic surgeon is then indicated.12
- Grade 3 injury is frank separation at the distal tibiofibular joint that is detectable on a routine plain film. Management—surgical intervention to address instability—is often provided concurrently with the treatment for a Danis-Weber B or C fracture, which tends to coexist with grade 3 syndesmotic injury. (The Danis-Weber A–B–C classification of lateral ankle fracture will be discussed in a bit.)
Continue to: Ankle fracture
Ankle fracture
Fracture of the ankle joint is among the more common fractures in adults, comprising 10% of all fractures.13,14 The ankle joint is defined as the junction of 3 bony structures: (1) the distal ends of the tibia and fibula and (2) the trochlea of the talus, all stabilized by (3) the collateral ligament complex. Appropriate diagnosis and timely intervention are needed to prevent long-term posttraumatic joint degeneration.
Presentation, examination, and imaging. In addition to difficulty bearing (or inability to bear) weight, patients with suspected ankle fracture can present with tenderness or pain, swelling (generally, the more severe the injury, the more severe the swelling, although this finding is time-dependent), and ecchymosis. However, distinguishing fracture from a ligamentous injury is often difficult by physical examination alone; the evidence-based Ottawa Ankle Rules can guide determination of the need for radiographic imaging, although this tool is less reliable in certain patient populations (TABLE15-17).13,15-17
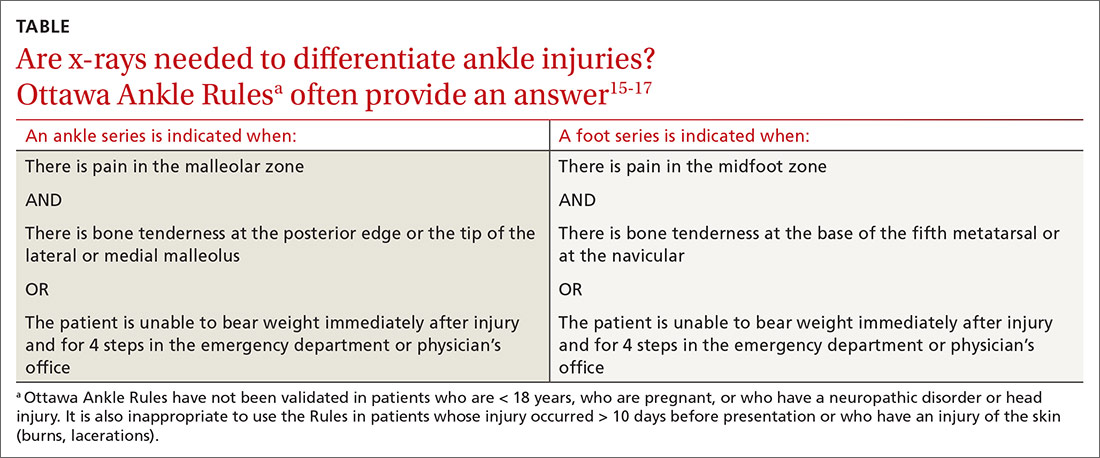
Management. A widely used classification system for guiding ankle fracture management is the Danis-Weber classification (FIGURE 2). In this scheme, type A fractures (distal to the level of the tibial plafond) are managed with ankle stabilization bracing without immobilization. Nondisplaced type B and C fractures (at the level of the tibial plafond and proximal to it, respectively) should be treated with 6 weeks of immobilization in a walking boot; close follow-up within 1 week of injury is recommended to ensure that no displacement of fragments has occurred. Type B and C fractures need to be followed until bony union is achieved. If there is radiologic evidence of a fracture line after 3 months, referral to an orthopedic surgeon is indicated for management of delayed union.
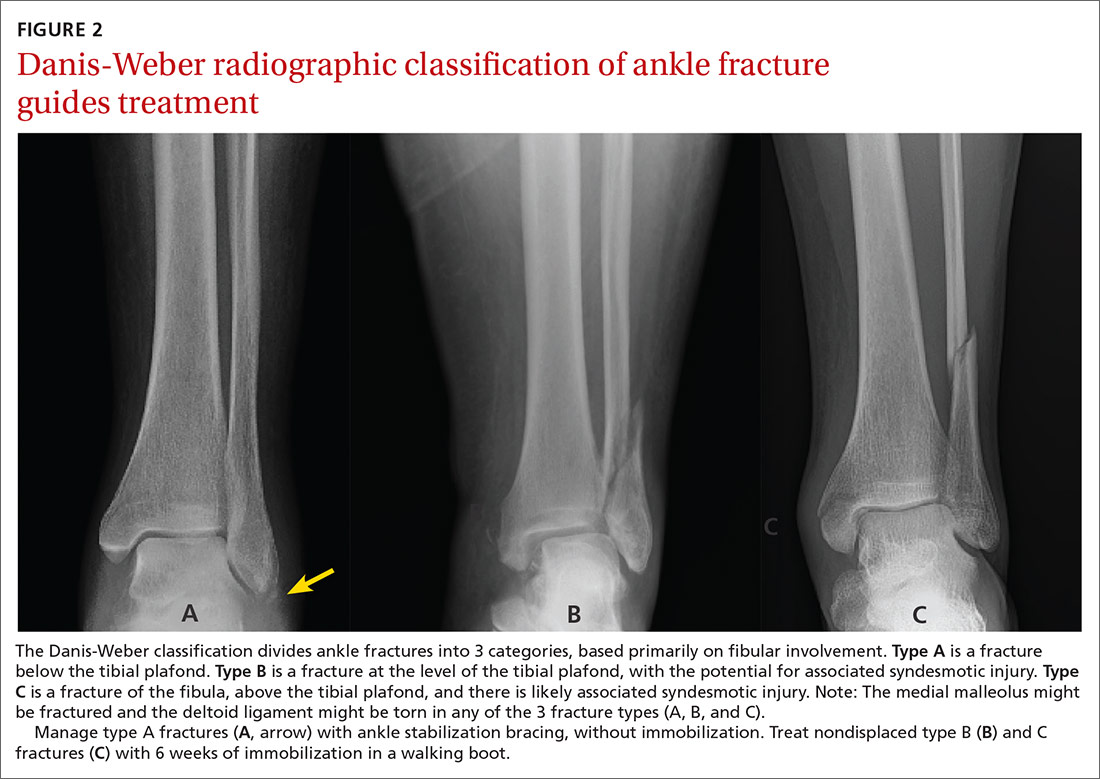
Common indications for referral to Orthopedics for surgical intervention of ankle fracture include open fracture, bimalleolar and trimalleolar fracture, posterior malleolar fracture, medial malleolar displacement > 2 mm, and lateral malleolar displacement > 3 mm.18
Special concern: Talar fracture. Although talar fracture is rare, the injury is important to detect because a limited blood supply places fragments at risk of avascular necrosis.19 Talus fracture is frequently confused with ankle sprain because initial x-rays are not always revelatory.20 A high index of suspicion is required to make the diagnosis, which should be suspected in high-energy injuries that result in pain and swelling of the ankle accompanied by difficulty weight-bearing, severely reduced range of motion, and tenderness to palpation at different areas of the talus.1 Computed tomography (CT) or MRI might be necessary to detect a talar fracture if initial x-rays are negative. A low threshold for surgical management of talar fracture means that referral to Orthopedics is indicated once this injury is diagnosed.21
Continue to: Other frequently missed types of ankle fracture
Other frequently missed types of ankle fracture are shown in FIGURE 3.22 These are relatively uncommon injuries that can be missed for a number of reasons, alone or in combination, including their subtlety on radiography, their often vague clinical presentation, and providers’ lack of awareness of these types. Identification or strong suspicion of fracture at any of these sites (ie, in a patient who is persistently unable to bear weight) should prompt orthopedic referral.
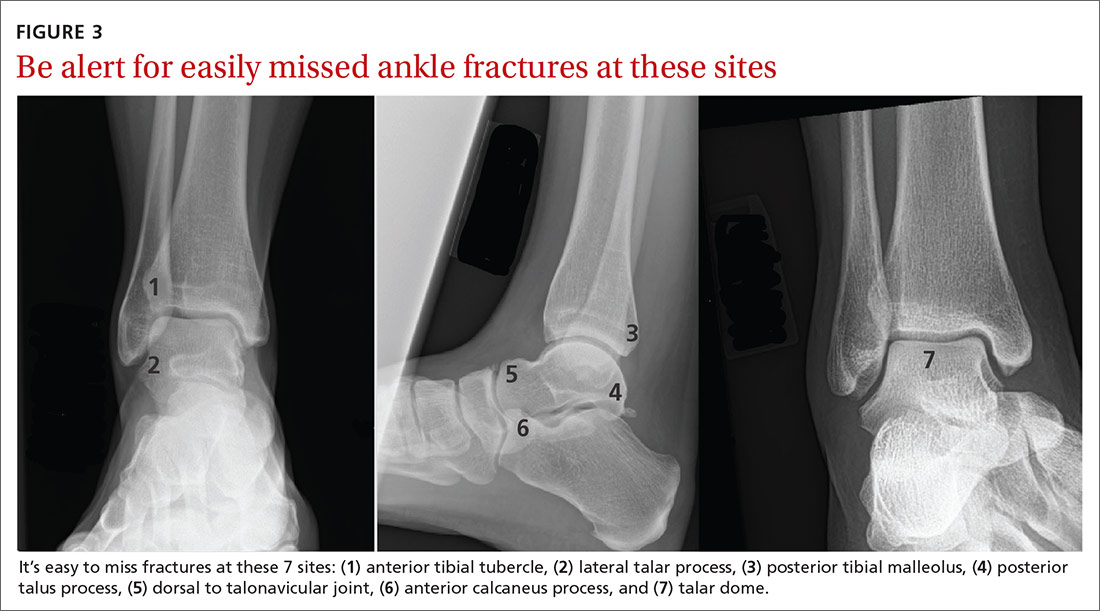
Lisfranc injury
The tarsometatarsal joint comprises 3 cuneiforms, the cuboid, and 5 metatarsals. Stability is maintained by an intricate ligamentous complex. Lisfranc injury comprises a spectrum of midfoot injuries in which 1 or more metatarsals are displaced from the tarsus. These injuries are both rare and notoriously difficult to diagnose: As many as 20% of cases are missed on initial assessment. Without proper treatment, long-term disability and deformity, such as pes planus, can result.22-24 Lisfranc injuries typically result from a direct blow to the midfoot or excessive pronation or supination in a plantarflexed foot.23
Presentation. A historical clue to Lisfranc injury is a report of pain while walking down stairs. Patients can present with pain, swelling, and tenderness to palpation over the dorsal aspect of the Lisfranc joint. Weight-bearing on the injured foot frequently cannot be tolerated but is occasionally possible in some patients, especially those who have diabetes or other baseline neuropathy.23
Examination. Physical examination can also reveal plantar ecchymosis, which is considered pathognomonic. Another highly supportive maneuver is passive abduction and pronation of the forefoot, which can elicit pain.25,26
Imaging. Lisfranc injury can be diagnosed on weight-bearing x-rays; as many as one-half of cases are missed when only non-weight-bearing films are obtained. If initial weight-bearing cannot be tolerated by the patient, another attempt at imaging can be made after 1 week of rest.24
Continue to: Distance > 2 mm between the base...
Distance > 2 mm between the base of the first and second metatarsals (FIGURE 4) or an avulsion fracture at the medial base of the second metatarsal or distal lateral corner of the medial cuneiform (the “fleck sign”) supports a disturbance of the Lisfranc joint complex.24 Imaging of the contralateral foot might highlight the injury in subtle cases, followed by CT when diagnostic uncertainty persists.24,25
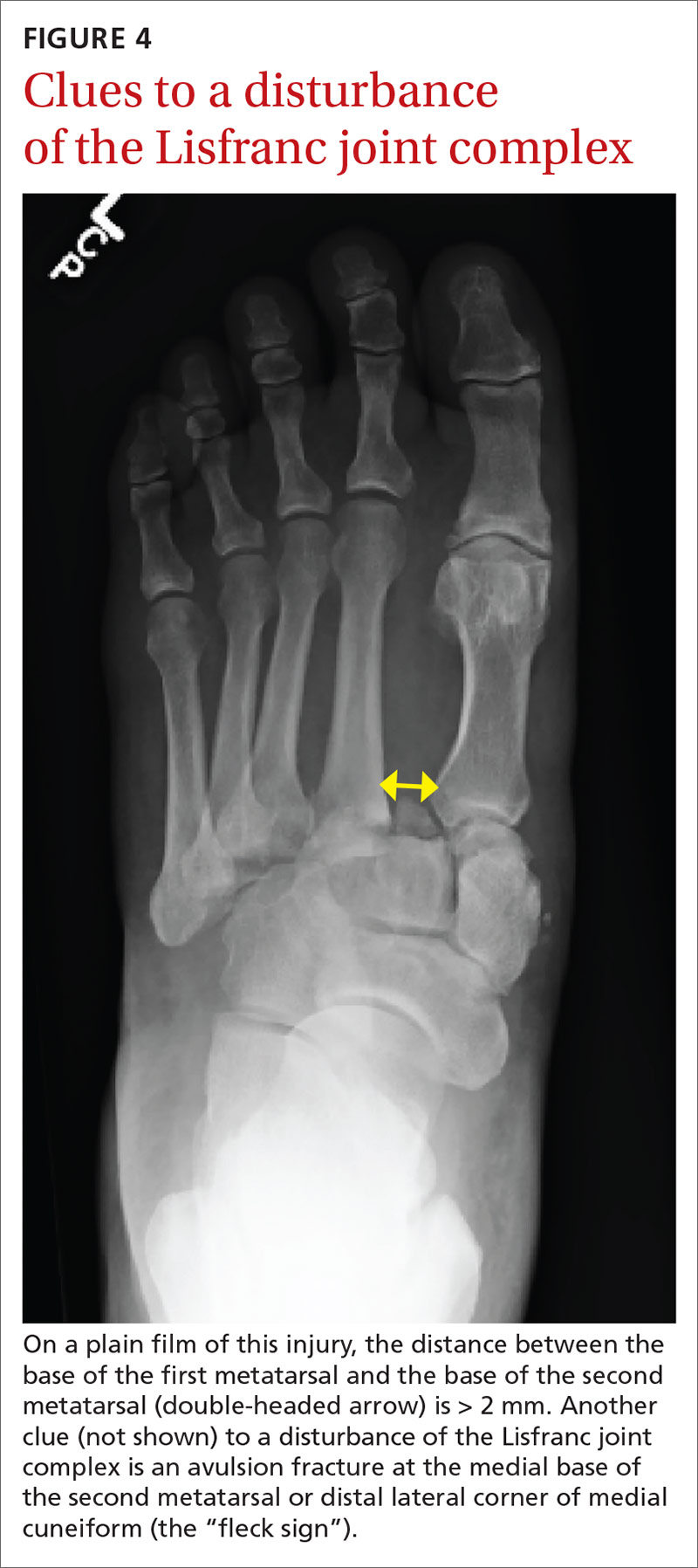
Management of Lisfranc injury depends on the stability of the joint complex. Stable injury without diastasis can be managed conservatively with immobilization in a short walker boot and limited weight-bearing for 2 weeks, followed by weight-bearing as tolerated in the boot if tenderness has improved.24 After 6 to 8 weeks, if the patient is pain-free with abduction stress, weight-bearing without the boot (but with a rigid-sole shoe) is permissible for an additional 6 months. Sport-specific rehabilitation for an athlete can begin once the patient can walk down multiple flights of stairs without pain.24
Orthopedic referral for surgical evaluation is recommended for all patients who have any radiographic evidence of dynamic instability, indicated by the fleck sign; displacement; or obvious diastasis between the metatarsals on imaging. A delay of 1 to 2 weeks from injury to fixation has not been associated with a negative outcome; delay as long as 6 weeks is permissible in some cases. Longer delay in surgical treatment (≥ 6 months) can be associated with posttraumatic arthritis and the need for Lisfranc fusion.24-26
Proximal fifth-metatarsal fractures
These common fractures are classified in 3 broad categories: tuberosity avulsion fracture, proximal diaphyseal (Jones) fracture, and stress fractures of the diaphysis (immediately distal to the site of the Jones fracture zone).27-29 Differentiating an acute Jones fracture and other fracture types is clinically important because the watershed area at the metaphysis–diaphysis junction results in a higher risk of delayed union and nonunion of Jones fractures, compared to other fractures in this region (FIGURE 5).28,29

Presentation. Proximal fifth-metatarsal fractures generally present with lateral foot pain and tenderness at the base of the fifth metatarsal, made worse by inversion of the foot, and inability to bear weight on the lateral aspect of the foot. Acute pain can follow a more insidious course of lateral foot pain in stress fracture.
Continue to: Examination
Examination. On exam, there might be swelling and ecchymosis over the lateral foot, with sharp tenderness to palpation at the base of the fifth metatarsal.
Imaging. Most fractures are revealed on standing AP, oblique, and lateral x-rays. Plain films are often falsely negative early in stress fracture; MRI is the gold standard of diagnosis.27,30
Management. Preferred treatment for a nondisplaced tuberosity avulsion fracture is typically 2-pronged: compressive dressings or casting for pain control and weight-bearing and range-of-motion exercises as tolerated.1 Follow-up every 2 to 3 weeks is recommended to ensure appropriate healing—ie, pain nearly resolved by 3 weeks post-injury and radiographic union evident at 8 weeks. If displacement is > 3 mm, > 60% of the metatarsal–cuboid joint surface is affected, or there is a 1 to 2 mm step-off on the cuboid articular surface, consider referral to an orthopedist.1,29
Jones fractures can be managed initially with posterior splinting, non-weight-bearing, and close follow-up. When radiographic healing has not been achieved by 6 to 8 weeks, non-weight-bearing status can be extended by another 4 weeks. When displacement is > 2 mm, or there is no healing after 12 weeks of immobilization and delayed union on x-rays, referral for surgical management is indicated.1 In select cases, when earlier return to activity is desired, referral for early surgical fixation is appropriate.27
Surgical referral is indicated in all cases of diaphysial stress fracture because of the high rate of nonunion and refracture. Conservative management, based on the orthopedic surgeon’s assessment, might be an option in a minority of patients.29
CORRESPONDENCE
Aileen Roman, MD, Boston University Medical School, Department of Family Medicine, 11 Melnea Cass Boulevard, Boston MA, 02119; [email protected]
1. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
2. Gross CE, Nunley JA 2nd. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233-239.
3. Cooper MT. Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med. 2015;34:595-606.
4. Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34:607-624.
5. Hutchison A-M, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19:112-117.
6. Kadakia AR, Dekker RG 2nd, Ho BS. Acute Achilles tendon ruptures: an update on treatment. Am Acad Orthop Surg. 2017;25:23-31.
7. van Zuuren WJ, Schepers T, Beumer A, et al. Acute syndesmotic instability in ankle fractures: a review. Foot Ankle Surg. 2017;23:135-141.
8. van Dijk CN, Longo UG, Loppini M, et al. Classification and diagnosis of acute isolated syndesmotic injuries: ESSKA–AFAS consensus and guidelines. Knee Surg Sports Traumatol Arthrosc. 2016;24:1200-1216.
9. Fort NM, Aiyer AA, Kaplan JR, et al. Management of acute injuries of the tibiofibular syndesmosis. Eur J Orthop Surg Traumatol. 2017;27:449-459.
10. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
11. Hunt KJ, Phisitkul P, Pirolo J, et al. High ankle sprains and syndesmotic injuries in athletes. J Am Acad Orthop Surg. 2015;23:661-673.
12. DeWeber K. Syndesmotic ankle injury (high ankle sprain). UpToDate. September 17, 2019. www.uptodate.com/contents/syndesmotic-ankle-injury-high-ankle-sprain. Accessed May 26, 2020.
13. Goost H, Wimmer MD, Barg A, et al. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377-388.
14. Qin C, Dekker RG, Helfrich MM, et al. Outpatient management of ankle fractures. Orthop Clin North Am. 2018;49:103-108.
15. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127-1132.
16. Jenkin M, Sitler MR, Kelly JD. Clinical usefulness of the Ottawa Ankle Rules for detecting fractures of the ankle and midfoot. J Athl Train. 2010;45:480-482.
17. Glas AS, Pijnenburg BACM, Lijmer JG, et al. Comparison of diagnostic decision rules and structured data collection in assessment of acute ankle injury. CMAJ. 2002;166:727-733.
18. Leduc S, Nault M-L, Rouleau DM, et al. My experience as a foot and ankle trauma surgeon in Montreal, Canada: what’s not in the books. Foot Ankle Clin. 2016;21:297-334.
19. Ibrahim MS, Jordan R, Lotfi N, et al. Talar head fracture: a case report, systematic review and suggested algorithm of treatment. Foot (Edinb). 2015;25:258-264.
20. Shank JR, Benirschke SK, Swords MP. Treatment of peripheral talus fractures. Foot Ankle Clin. 2017;22:181-192.
21. Kwaadu KY. Management of talar fractures. Clin Podiatr Med Sur. 2018;35:161-173.
22. Yu JS. Easily missed fractures in the lower extremity. Radiol Clin North Am. 2015;53:737-755.
23. Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536-541.
24. Seybold JD, Coetzee JC. Lisfranc injuries: when to observe, fix, or fuse. Clin Sports Med. 2015;34:705-723.
25. Puna RA, Tomlinson MPW. The role of percutaneous reduction and fixation of lisfranc injuries. Foot Ankle Clin. 2017;22:15-34.
26. Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1-14.
27. Porter DA. Fifth metatarsal Jones fractures in the athlete. Foot Ankle Int. 2018;39:250-258.
28. Cheung CN, Lui TH. Proximal fifth metatarsal fractures: anatomy, classification, treatment and complications. Arch Trauma Res. 2016;5:e32298.
29. Alsobrook J, Hatch RL. Proximal fifth metatarsal fractures. UpToDate. January 31, 2020. www.uptodate.com/contents/proximal-fifth-metatarsal-fractures. Accessed May 26, 2020.
30. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
Foot and ankle injuries are among the most common conditions evaluated at primary care visits; the differential diagnosis of such injury is broad.1 Although many of these injuries are easily identified on imaging studies, a number of subtle, yet important, conditions can be easily missed, especially if you do not routinely encounter them. Given that broad differential, a high degree of suspicion is required to make an accurate diagnosis, which allows appropriate treatment within a reasonable time frame and minimizes the risk of long-term morbidity.
This article outlines the diagnosis and initial management of 5 important, yet often elusive, types of foot and ankle conditions: Achilles tendon rupture, injury to the syndesmosis, ankle fracture, Lisfranc injury, and proximal fracture of the fifth metatarsal.

Achilles tendon rupture
The Achilles tendon is the most frequently ruptured tendon in the body (approximately 20% of all large-tendon injuries)2; as many as 25% of cases are initially misdiagnosed.3
Presentation. Patients frequently present with pain at the Achilles tendon—2 to 6 cm above the insertion into the calcaneus—and an inability to fully bear weight.4,5 A small percentage of patients are able to ambulate on the affected side, albeit with minor pain, which likely contributes to the rate of missed diagnosis. Absence of difficulty bearing weight is due to the presence of secondary plantar flexors, which can compensate for loss of chief plantar flexor function by the Achilles tendon.2
Examination of a patient with an Achilles tendon rupture typically reveals edema, bruising, and a palpable gap within the tendon, 2 to 6 cm proximal to insertion.3,4 The Thompson test—squeezing the calf with the patient prone and the knee on the affected side flexed—can aid in diagnosis. When the Achilles tendon is intact, plantar flexion occurs at the ankle; when the tendon is ruptured, plantar flexion is absent.5 The test can be modified when examining a patient who is unable to lie prone by having them rest the flexed knee on a chair while standing on the unaffected leg.
A diagnosis of Achilles tendon rupture is supported when at least 2 of the following conditions are met4,5:
- positive Thompson test
- decreased strength during plantar flexion of the ankle
- palpable gap or pain at the typical location (2-6 cm above insertion)
- increased passive ankle dorsiflexion upon gentle ranging of the ankle joint.
Imaging has a limited role in the diagnosis of Achilles tendon rupture; because the findings of the physical examination are reliable, reserve x-rays for cases in which the diagnosis remains uncertain after examination.2 Consider ordering plain x-rays to rule out an avulsion fracture at the insertion of the Achilles tendon; ultrasonography or magnetic resonance imaging (MRI) might assist you in detecting the rupture proper, along with the location of the tear for surgical planning, if surgery is deemed necessary by an orthopedic surgeon.3-5
Continue to: Management
Management. Some degree of controversy surrounds preferred treatment of Achilles tendon rupture, although available evidence demonstrates that these injuries can be effectively managed by surgical repair or nonoperative treatment, as outcomes are comparable.3,5 Operative management tends to reduce the risk of repeat rupture, compared to nonoperative treatment; however, the potential for surgical complications, including wound infection, sensory disturbance, and adhesions favors nonoperative treatment.3,4,6
Nonoperative treatment consists of referral to a functional rehabilitation program, without which outcomes are, on the whole, less favorable than with surgery.3,6 Surgery is preferred if functional rehabilitation is unavailable, 6 months of conservative management fails, or there is avulsion injury.3,4,6
Injury to the syndesmosis
A complex of ligaments that provide dynamic stability to the ankle joint, the tibiofibular syndesmosis comprises:
- the anterior inferior tibiofibular ligament
- the posterior inferior tibiofibular ligament
- the inferior transverse tibiofibular ligament
- the interosseous membrane.
These structures are further supported by the deltoid ligament.7,8
Commonly referred to as a “high ankle sprain,” a syndesmotic injury is present in as many as 20% of ankle fractures and 5% to 10% of ankle sprains. Injury typically results from external rotation with hyperdorsiflexion of the ankle. Recovery is typically prolonged (ie, twice as long as recovery from a lateral ankle sprain). The diagnosis is missed in as many as 20% of patients; failure to recognize and treat syndesmotic instability appropriately can lead to posttraumatic arthritis.7,9
Continue to: Presentation
Presentation. Patients generally present with ankle pain, swelling, instability, pain when walking on uneven terrain, and pain upon push-off.9
Examination reveals reduced passive ankle dorsiflexion and tenderness upon palpation of individual ligaments. Several clinical tests have been described to aid in detecting this often-elusive diagnosis7,9,10,11:
- Squeeze test. The patient sits with the knee on the affected side bent at a 90° degree angle while the examiner applies compression, with one or both hands, to the tibia and fibula at midcalf. The test is positive when pain is elicited at the level of the syndesmosis just above the ankle joint.9,11
- External rotation test. External rotation of the foot and ankle relative to the tibia reproduces pain.
- Crossed leg test. The affected ankle is crossed over the opposite knee in a figure-4 position. The test is positive when pain is elicited at the syndesmosis.10
- Cotton test. The proximal lower leg is steadied with 1 hand and the plantar heel grasped with the other hand. Pain when the heel is externally rotated (and radiographic widening of the syndesmosis under fluoroscopy) signal syndesmotic instability.
- Fibular translation test. When anterior or posterior drawer force is applied to the fibula, pain and increased translation of the fibula (compared to the contralateral side) suggest instability.
With the Cotton and fibular translation tests, interexaminer technique is more variable and findings are less reproducible.8 Taken alone, none of the above-listed tests are diagnostic; they can, however, assist in making a diagnosis of an injury to the syndesmosis.11
Imaging typically involves anteroposterior [AP], lateral, and mortise plain films of the ankle and weight-bearing AP and lateral views of the tibia and fibula.9 Important measures on weight-bearing AP x-rays are the tibiofibular clear space (abnormal, > 6 mm) and the tibiofibular overlap (abnormal, < 6 mm) (both abnormalities shown in FIGURE 1). Comparing films of the affected ankle with views of the contralateral ankle is often useful.

Management of syndesmotic injuries depends on degree of disruption:
- Grade 1 injury is a sprain without diastasis on imaging. Management is conservative, with immobilization in a splint or boot for 1 to 3 weeks, followed by functional rehabilitation over 3 to 6 weeks.10
- Grade 2 injury is demonstrated by diastasis on a stress radiograph. Although evidence to guide successful identification of a grade 2 injury is lacking, it is clinically important to make that identification because these injuries might require surgical intervention, due to instability. Because the diagnosis of this injury can be challenging in primary care, high clinical suspicion of a grade 2 injury makes it appropriate to defer further evaluation to an orthopedic surgeon. On the other hand, if suspicion of a grade 2 injury is low, a trial of conservative management, with weekly clinical assessment, can be considered. A diagnosis of grade 2 injury can be inferred when a patient is unable to perform a single-leg hop after 3 weeks of immobilization; referral to an orthopedic surgeon is then indicated.12
- Grade 3 injury is frank separation at the distal tibiofibular joint that is detectable on a routine plain film. Management—surgical intervention to address instability—is often provided concurrently with the treatment for a Danis-Weber B or C fracture, which tends to coexist with grade 3 syndesmotic injury. (The Danis-Weber A–B–C classification of lateral ankle fracture will be discussed in a bit.)
Continue to: Ankle fracture
Ankle fracture
Fracture of the ankle joint is among the more common fractures in adults, comprising 10% of all fractures.13,14 The ankle joint is defined as the junction of 3 bony structures: (1) the distal ends of the tibia and fibula and (2) the trochlea of the talus, all stabilized by (3) the collateral ligament complex. Appropriate diagnosis and timely intervention are needed to prevent long-term posttraumatic joint degeneration.
Presentation, examination, and imaging. In addition to difficulty bearing (or inability to bear) weight, patients with suspected ankle fracture can present with tenderness or pain, swelling (generally, the more severe the injury, the more severe the swelling, although this finding is time-dependent), and ecchymosis. However, distinguishing fracture from a ligamentous injury is often difficult by physical examination alone; the evidence-based Ottawa Ankle Rules can guide determination of the need for radiographic imaging, although this tool is less reliable in certain patient populations (TABLE15-17).13,15-17

Management. A widely used classification system for guiding ankle fracture management is the Danis-Weber classification (FIGURE 2). In this scheme, type A fractures (distal to the level of the tibial plafond) are managed with ankle stabilization bracing without immobilization. Nondisplaced type B and C fractures (at the level of the tibial plafond and proximal to it, respectively) should be treated with 6 weeks of immobilization in a walking boot; close follow-up within 1 week of injury is recommended to ensure that no displacement of fragments has occurred. Type B and C fractures need to be followed until bony union is achieved. If there is radiologic evidence of a fracture line after 3 months, referral to an orthopedic surgeon is indicated for management of delayed union.

Common indications for referral to Orthopedics for surgical intervention of ankle fracture include open fracture, bimalleolar and trimalleolar fracture, posterior malleolar fracture, medial malleolar displacement > 2 mm, and lateral malleolar displacement > 3 mm.18
Special concern: Talar fracture. Although talar fracture is rare, the injury is important to detect because a limited blood supply places fragments at risk of avascular necrosis.19 Talus fracture is frequently confused with ankle sprain because initial x-rays are not always revelatory.20 A high index of suspicion is required to make the diagnosis, which should be suspected in high-energy injuries that result in pain and swelling of the ankle accompanied by difficulty weight-bearing, severely reduced range of motion, and tenderness to palpation at different areas of the talus.1 Computed tomography (CT) or MRI might be necessary to detect a talar fracture if initial x-rays are negative. A low threshold for surgical management of talar fracture means that referral to Orthopedics is indicated once this injury is diagnosed.21
Continue to: Other frequently missed types of ankle fracture
Other frequently missed types of ankle fracture are shown in FIGURE 3.22 These are relatively uncommon injuries that can be missed for a number of reasons, alone or in combination, including their subtlety on radiography, their often vague clinical presentation, and providers’ lack of awareness of these types. Identification or strong suspicion of fracture at any of these sites (ie, in a patient who is persistently unable to bear weight) should prompt orthopedic referral.

Lisfranc injury
The tarsometatarsal joint comprises 3 cuneiforms, the cuboid, and 5 metatarsals. Stability is maintained by an intricate ligamentous complex. Lisfranc injury comprises a spectrum of midfoot injuries in which 1 or more metatarsals are displaced from the tarsus. These injuries are both rare and notoriously difficult to diagnose: As many as 20% of cases are missed on initial assessment. Without proper treatment, long-term disability and deformity, such as pes planus, can result.22-24 Lisfranc injuries typically result from a direct blow to the midfoot or excessive pronation or supination in a plantarflexed foot.23
Presentation. A historical clue to Lisfranc injury is a report of pain while walking down stairs. Patients can present with pain, swelling, and tenderness to palpation over the dorsal aspect of the Lisfranc joint. Weight-bearing on the injured foot frequently cannot be tolerated but is occasionally possible in some patients, especially those who have diabetes or other baseline neuropathy.23
Examination. Physical examination can also reveal plantar ecchymosis, which is considered pathognomonic. Another highly supportive maneuver is passive abduction and pronation of the forefoot, which can elicit pain.25,26
Imaging. Lisfranc injury can be diagnosed on weight-bearing x-rays; as many as one-half of cases are missed when only non-weight-bearing films are obtained. If initial weight-bearing cannot be tolerated by the patient, another attempt at imaging can be made after 1 week of rest.24
Continue to: Distance > 2 mm between the base...
Distance > 2 mm between the base of the first and second metatarsals (FIGURE 4) or an avulsion fracture at the medial base of the second metatarsal or distal lateral corner of the medial cuneiform (the “fleck sign”) supports a disturbance of the Lisfranc joint complex.24 Imaging of the contralateral foot might highlight the injury in subtle cases, followed by CT when diagnostic uncertainty persists.24,25

Management of Lisfranc injury depends on the stability of the joint complex. Stable injury without diastasis can be managed conservatively with immobilization in a short walker boot and limited weight-bearing for 2 weeks, followed by weight-bearing as tolerated in the boot if tenderness has improved.24 After 6 to 8 weeks, if the patient is pain-free with abduction stress, weight-bearing without the boot (but with a rigid-sole shoe) is permissible for an additional 6 months. Sport-specific rehabilitation for an athlete can begin once the patient can walk down multiple flights of stairs without pain.24
Orthopedic referral for surgical evaluation is recommended for all patients who have any radiographic evidence of dynamic instability, indicated by the fleck sign; displacement; or obvious diastasis between the metatarsals on imaging. A delay of 1 to 2 weeks from injury to fixation has not been associated with a negative outcome; delay as long as 6 weeks is permissible in some cases. Longer delay in surgical treatment (≥ 6 months) can be associated with posttraumatic arthritis and the need for Lisfranc fusion.24-26
Proximal fifth-metatarsal fractures
These common fractures are classified in 3 broad categories: tuberosity avulsion fracture, proximal diaphyseal (Jones) fracture, and stress fractures of the diaphysis (immediately distal to the site of the Jones fracture zone).27-29 Differentiating an acute Jones fracture and other fracture types is clinically important because the watershed area at the metaphysis–diaphysis junction results in a higher risk of delayed union and nonunion of Jones fractures, compared to other fractures in this region (FIGURE 5).28,29

Presentation. Proximal fifth-metatarsal fractures generally present with lateral foot pain and tenderness at the base of the fifth metatarsal, made worse by inversion of the foot, and inability to bear weight on the lateral aspect of the foot. Acute pain can follow a more insidious course of lateral foot pain in stress fracture.
Continue to: Examination
Examination. On exam, there might be swelling and ecchymosis over the lateral foot, with sharp tenderness to palpation at the base of the fifth metatarsal.
Imaging. Most fractures are revealed on standing AP, oblique, and lateral x-rays. Plain films are often falsely negative early in stress fracture; MRI is the gold standard of diagnosis.27,30
Management. Preferred treatment for a nondisplaced tuberosity avulsion fracture is typically 2-pronged: compressive dressings or casting for pain control and weight-bearing and range-of-motion exercises as tolerated.1 Follow-up every 2 to 3 weeks is recommended to ensure appropriate healing—ie, pain nearly resolved by 3 weeks post-injury and radiographic union evident at 8 weeks. If displacement is > 3 mm, > 60% of the metatarsal–cuboid joint surface is affected, or there is a 1 to 2 mm step-off on the cuboid articular surface, consider referral to an orthopedist.1,29
Jones fractures can be managed initially with posterior splinting, non-weight-bearing, and close follow-up. When radiographic healing has not been achieved by 6 to 8 weeks, non-weight-bearing status can be extended by another 4 weeks. When displacement is > 2 mm, or there is no healing after 12 weeks of immobilization and delayed union on x-rays, referral for surgical management is indicated.1 In select cases, when earlier return to activity is desired, referral for early surgical fixation is appropriate.27
Surgical referral is indicated in all cases of diaphysial stress fracture because of the high rate of nonunion and refracture. Conservative management, based on the orthopedic surgeon’s assessment, might be an option in a minority of patients.29
CORRESPONDENCE
Aileen Roman, MD, Boston University Medical School, Department of Family Medicine, 11 Melnea Cass Boulevard, Boston MA, 02119; [email protected]
Foot and ankle injuries are among the most common conditions evaluated at primary care visits; the differential diagnosis of such injury is broad.1 Although many of these injuries are easily identified on imaging studies, a number of subtle, yet important, conditions can be easily missed, especially if you do not routinely encounter them. Given that broad differential, a high degree of suspicion is required to make an accurate diagnosis, which allows appropriate treatment within a reasonable time frame and minimizes the risk of long-term morbidity.
This article outlines the diagnosis and initial management of 5 important, yet often elusive, types of foot and ankle conditions: Achilles tendon rupture, injury to the syndesmosis, ankle fracture, Lisfranc injury, and proximal fracture of the fifth metatarsal.

Achilles tendon rupture
The Achilles tendon is the most frequently ruptured tendon in the body (approximately 20% of all large-tendon injuries)2; as many as 25% of cases are initially misdiagnosed.3
Presentation. Patients frequently present with pain at the Achilles tendon—2 to 6 cm above the insertion into the calcaneus—and an inability to fully bear weight.4,5 A small percentage of patients are able to ambulate on the affected side, albeit with minor pain, which likely contributes to the rate of missed diagnosis. Absence of difficulty bearing weight is due to the presence of secondary plantar flexors, which can compensate for loss of chief plantar flexor function by the Achilles tendon.2
Examination of a patient with an Achilles tendon rupture typically reveals edema, bruising, and a palpable gap within the tendon, 2 to 6 cm proximal to insertion.3,4 The Thompson test—squeezing the calf with the patient prone and the knee on the affected side flexed—can aid in diagnosis. When the Achilles tendon is intact, plantar flexion occurs at the ankle; when the tendon is ruptured, plantar flexion is absent.5 The test can be modified when examining a patient who is unable to lie prone by having them rest the flexed knee on a chair while standing on the unaffected leg.
A diagnosis of Achilles tendon rupture is supported when at least 2 of the following conditions are met4,5:
- positive Thompson test
- decreased strength during plantar flexion of the ankle
- palpable gap or pain at the typical location (2-6 cm above insertion)
- increased passive ankle dorsiflexion upon gentle ranging of the ankle joint.
Imaging has a limited role in the diagnosis of Achilles tendon rupture; because the findings of the physical examination are reliable, reserve x-rays for cases in which the diagnosis remains uncertain after examination.2 Consider ordering plain x-rays to rule out an avulsion fracture at the insertion of the Achilles tendon; ultrasonography or magnetic resonance imaging (MRI) might assist you in detecting the rupture proper, along with the location of the tear for surgical planning, if surgery is deemed necessary by an orthopedic surgeon.3-5
Continue to: Management
Management. Some degree of controversy surrounds preferred treatment of Achilles tendon rupture, although available evidence demonstrates that these injuries can be effectively managed by surgical repair or nonoperative treatment, as outcomes are comparable.3,5 Operative management tends to reduce the risk of repeat rupture, compared to nonoperative treatment; however, the potential for surgical complications, including wound infection, sensory disturbance, and adhesions favors nonoperative treatment.3,4,6
Nonoperative treatment consists of referral to a functional rehabilitation program, without which outcomes are, on the whole, less favorable than with surgery.3,6 Surgery is preferred if functional rehabilitation is unavailable, 6 months of conservative management fails, or there is avulsion injury.3,4,6
Injury to the syndesmosis
A complex of ligaments that provide dynamic stability to the ankle joint, the tibiofibular syndesmosis comprises:
- the anterior inferior tibiofibular ligament
- the posterior inferior tibiofibular ligament
- the inferior transverse tibiofibular ligament
- the interosseous membrane.
These structures are further supported by the deltoid ligament.7,8
Commonly referred to as a “high ankle sprain,” a syndesmotic injury is present in as many as 20% of ankle fractures and 5% to 10% of ankle sprains. Injury typically results from external rotation with hyperdorsiflexion of the ankle. Recovery is typically prolonged (ie, twice as long as recovery from a lateral ankle sprain). The diagnosis is missed in as many as 20% of patients; failure to recognize and treat syndesmotic instability appropriately can lead to posttraumatic arthritis.7,9
Continue to: Presentation
Presentation. Patients generally present with ankle pain, swelling, instability, pain when walking on uneven terrain, and pain upon push-off.9
Examination reveals reduced passive ankle dorsiflexion and tenderness upon palpation of individual ligaments. Several clinical tests have been described to aid in detecting this often-elusive diagnosis7,9,10,11:
- Squeeze test. The patient sits with the knee on the affected side bent at a 90° degree angle while the examiner applies compression, with one or both hands, to the tibia and fibula at midcalf. The test is positive when pain is elicited at the level of the syndesmosis just above the ankle joint.9,11
- External rotation test. External rotation of the foot and ankle relative to the tibia reproduces pain.
- Crossed leg test. The affected ankle is crossed over the opposite knee in a figure-4 position. The test is positive when pain is elicited at the syndesmosis.10
- Cotton test. The proximal lower leg is steadied with 1 hand and the plantar heel grasped with the other hand. Pain when the heel is externally rotated (and radiographic widening of the syndesmosis under fluoroscopy) signal syndesmotic instability.
- Fibular translation test. When anterior or posterior drawer force is applied to the fibula, pain and increased translation of the fibula (compared to the contralateral side) suggest instability.
With the Cotton and fibular translation tests, interexaminer technique is more variable and findings are less reproducible.8 Taken alone, none of the above-listed tests are diagnostic; they can, however, assist in making a diagnosis of an injury to the syndesmosis.11
Imaging typically involves anteroposterior [AP], lateral, and mortise plain films of the ankle and weight-bearing AP and lateral views of the tibia and fibula.9 Important measures on weight-bearing AP x-rays are the tibiofibular clear space (abnormal, > 6 mm) and the tibiofibular overlap (abnormal, < 6 mm) (both abnormalities shown in FIGURE 1). Comparing films of the affected ankle with views of the contralateral ankle is often useful.

Management of syndesmotic injuries depends on degree of disruption:
- Grade 1 injury is a sprain without diastasis on imaging. Management is conservative, with immobilization in a splint or boot for 1 to 3 weeks, followed by functional rehabilitation over 3 to 6 weeks.10
- Grade 2 injury is demonstrated by diastasis on a stress radiograph. Although evidence to guide successful identification of a grade 2 injury is lacking, it is clinically important to make that identification because these injuries might require surgical intervention, due to instability. Because the diagnosis of this injury can be challenging in primary care, high clinical suspicion of a grade 2 injury makes it appropriate to defer further evaluation to an orthopedic surgeon. On the other hand, if suspicion of a grade 2 injury is low, a trial of conservative management, with weekly clinical assessment, can be considered. A diagnosis of grade 2 injury can be inferred when a patient is unable to perform a single-leg hop after 3 weeks of immobilization; referral to an orthopedic surgeon is then indicated.12
- Grade 3 injury is frank separation at the distal tibiofibular joint that is detectable on a routine plain film. Management—surgical intervention to address instability—is often provided concurrently with the treatment for a Danis-Weber B or C fracture, which tends to coexist with grade 3 syndesmotic injury. (The Danis-Weber A–B–C classification of lateral ankle fracture will be discussed in a bit.)
Continue to: Ankle fracture
Ankle fracture
Fracture of the ankle joint is among the more common fractures in adults, comprising 10% of all fractures.13,14 The ankle joint is defined as the junction of 3 bony structures: (1) the distal ends of the tibia and fibula and (2) the trochlea of the talus, all stabilized by (3) the collateral ligament complex. Appropriate diagnosis and timely intervention are needed to prevent long-term posttraumatic joint degeneration.
Presentation, examination, and imaging. In addition to difficulty bearing (or inability to bear) weight, patients with suspected ankle fracture can present with tenderness or pain, swelling (generally, the more severe the injury, the more severe the swelling, although this finding is time-dependent), and ecchymosis. However, distinguishing fracture from a ligamentous injury is often difficult by physical examination alone; the evidence-based Ottawa Ankle Rules can guide determination of the need for radiographic imaging, although this tool is less reliable in certain patient populations (TABLE15-17).13,15-17

Management. A widely used classification system for guiding ankle fracture management is the Danis-Weber classification (FIGURE 2). In this scheme, type A fractures (distal to the level of the tibial plafond) are managed with ankle stabilization bracing without immobilization. Nondisplaced type B and C fractures (at the level of the tibial plafond and proximal to it, respectively) should be treated with 6 weeks of immobilization in a walking boot; close follow-up within 1 week of injury is recommended to ensure that no displacement of fragments has occurred. Type B and C fractures need to be followed until bony union is achieved. If there is radiologic evidence of a fracture line after 3 months, referral to an orthopedic surgeon is indicated for management of delayed union.

Common indications for referral to Orthopedics for surgical intervention of ankle fracture include open fracture, bimalleolar and trimalleolar fracture, posterior malleolar fracture, medial malleolar displacement > 2 mm, and lateral malleolar displacement > 3 mm.18
Special concern: Talar fracture. Although talar fracture is rare, the injury is important to detect because a limited blood supply places fragments at risk of avascular necrosis.19 Talus fracture is frequently confused with ankle sprain because initial x-rays are not always revelatory.20 A high index of suspicion is required to make the diagnosis, which should be suspected in high-energy injuries that result in pain and swelling of the ankle accompanied by difficulty weight-bearing, severely reduced range of motion, and tenderness to palpation at different areas of the talus.1 Computed tomography (CT) or MRI might be necessary to detect a talar fracture if initial x-rays are negative. A low threshold for surgical management of talar fracture means that referral to Orthopedics is indicated once this injury is diagnosed.21
Continue to: Other frequently missed types of ankle fracture
Other frequently missed types of ankle fracture are shown in FIGURE 3.22 These are relatively uncommon injuries that can be missed for a number of reasons, alone or in combination, including their subtlety on radiography, their often vague clinical presentation, and providers’ lack of awareness of these types. Identification or strong suspicion of fracture at any of these sites (ie, in a patient who is persistently unable to bear weight) should prompt orthopedic referral.

Lisfranc injury
The tarsometatarsal joint comprises 3 cuneiforms, the cuboid, and 5 metatarsals. Stability is maintained by an intricate ligamentous complex. Lisfranc injury comprises a spectrum of midfoot injuries in which 1 or more metatarsals are displaced from the tarsus. These injuries are both rare and notoriously difficult to diagnose: As many as 20% of cases are missed on initial assessment. Without proper treatment, long-term disability and deformity, such as pes planus, can result.22-24 Lisfranc injuries typically result from a direct blow to the midfoot or excessive pronation or supination in a plantarflexed foot.23
Presentation. A historical clue to Lisfranc injury is a report of pain while walking down stairs. Patients can present with pain, swelling, and tenderness to palpation over the dorsal aspect of the Lisfranc joint. Weight-bearing on the injured foot frequently cannot be tolerated but is occasionally possible in some patients, especially those who have diabetes or other baseline neuropathy.23
Examination. Physical examination can also reveal plantar ecchymosis, which is considered pathognomonic. Another highly supportive maneuver is passive abduction and pronation of the forefoot, which can elicit pain.25,26
Imaging. Lisfranc injury can be diagnosed on weight-bearing x-rays; as many as one-half of cases are missed when only non-weight-bearing films are obtained. If initial weight-bearing cannot be tolerated by the patient, another attempt at imaging can be made after 1 week of rest.24
Continue to: Distance > 2 mm between the base...
Distance > 2 mm between the base of the first and second metatarsals (FIGURE 4) or an avulsion fracture at the medial base of the second metatarsal or distal lateral corner of the medial cuneiform (the “fleck sign”) supports a disturbance of the Lisfranc joint complex.24 Imaging of the contralateral foot might highlight the injury in subtle cases, followed by CT when diagnostic uncertainty persists.24,25

Management of Lisfranc injury depends on the stability of the joint complex. Stable injury without diastasis can be managed conservatively with immobilization in a short walker boot and limited weight-bearing for 2 weeks, followed by weight-bearing as tolerated in the boot if tenderness has improved.24 After 6 to 8 weeks, if the patient is pain-free with abduction stress, weight-bearing without the boot (but with a rigid-sole shoe) is permissible for an additional 6 months. Sport-specific rehabilitation for an athlete can begin once the patient can walk down multiple flights of stairs without pain.24
Orthopedic referral for surgical evaluation is recommended for all patients who have any radiographic evidence of dynamic instability, indicated by the fleck sign; displacement; or obvious diastasis between the metatarsals on imaging. A delay of 1 to 2 weeks from injury to fixation has not been associated with a negative outcome; delay as long as 6 weeks is permissible in some cases. Longer delay in surgical treatment (≥ 6 months) can be associated with posttraumatic arthritis and the need for Lisfranc fusion.24-26
Proximal fifth-metatarsal fractures
These common fractures are classified in 3 broad categories: tuberosity avulsion fracture, proximal diaphyseal (Jones) fracture, and stress fractures of the diaphysis (immediately distal to the site of the Jones fracture zone).27-29 Differentiating an acute Jones fracture and other fracture types is clinically important because the watershed area at the metaphysis–diaphysis junction results in a higher risk of delayed union and nonunion of Jones fractures, compared to other fractures in this region (FIGURE 5).28,29

Presentation. Proximal fifth-metatarsal fractures generally present with lateral foot pain and tenderness at the base of the fifth metatarsal, made worse by inversion of the foot, and inability to bear weight on the lateral aspect of the foot. Acute pain can follow a more insidious course of lateral foot pain in stress fracture.
Continue to: Examination
Examination. On exam, there might be swelling and ecchymosis over the lateral foot, with sharp tenderness to palpation at the base of the fifth metatarsal.
Imaging. Most fractures are revealed on standing AP, oblique, and lateral x-rays. Plain films are often falsely negative early in stress fracture; MRI is the gold standard of diagnosis.27,30
Management. Preferred treatment for a nondisplaced tuberosity avulsion fracture is typically 2-pronged: compressive dressings or casting for pain control and weight-bearing and range-of-motion exercises as tolerated.1 Follow-up every 2 to 3 weeks is recommended to ensure appropriate healing—ie, pain nearly resolved by 3 weeks post-injury and radiographic union evident at 8 weeks. If displacement is > 3 mm, > 60% of the metatarsal–cuboid joint surface is affected, or there is a 1 to 2 mm step-off on the cuboid articular surface, consider referral to an orthopedist.1,29
Jones fractures can be managed initially with posterior splinting, non-weight-bearing, and close follow-up. When radiographic healing has not been achieved by 6 to 8 weeks, non-weight-bearing status can be extended by another 4 weeks. When displacement is > 2 mm, or there is no healing after 12 weeks of immobilization and delayed union on x-rays, referral for surgical management is indicated.1 In select cases, when earlier return to activity is desired, referral for early surgical fixation is appropriate.27
Surgical referral is indicated in all cases of diaphysial stress fracture because of the high rate of nonunion and refracture. Conservative management, based on the orthopedic surgeon’s assessment, might be an option in a minority of patients.29
CORRESPONDENCE
Aileen Roman, MD, Boston University Medical School, Department of Family Medicine, 11 Melnea Cass Boulevard, Boston MA, 02119; [email protected]
1. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
2. Gross CE, Nunley JA 2nd. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233-239.
3. Cooper MT. Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med. 2015;34:595-606.
4. Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34:607-624.
5. Hutchison A-M, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19:112-117.
6. Kadakia AR, Dekker RG 2nd, Ho BS. Acute Achilles tendon ruptures: an update on treatment. Am Acad Orthop Surg. 2017;25:23-31.
7. van Zuuren WJ, Schepers T, Beumer A, et al. Acute syndesmotic instability in ankle fractures: a review. Foot Ankle Surg. 2017;23:135-141.
8. van Dijk CN, Longo UG, Loppini M, et al. Classification and diagnosis of acute isolated syndesmotic injuries: ESSKA–AFAS consensus and guidelines. Knee Surg Sports Traumatol Arthrosc. 2016;24:1200-1216.
9. Fort NM, Aiyer AA, Kaplan JR, et al. Management of acute injuries of the tibiofibular syndesmosis. Eur J Orthop Surg Traumatol. 2017;27:449-459.
10. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
11. Hunt KJ, Phisitkul P, Pirolo J, et al. High ankle sprains and syndesmotic injuries in athletes. J Am Acad Orthop Surg. 2015;23:661-673.
12. DeWeber K. Syndesmotic ankle injury (high ankle sprain). UpToDate. September 17, 2019. www.uptodate.com/contents/syndesmotic-ankle-injury-high-ankle-sprain. Accessed May 26, 2020.
13. Goost H, Wimmer MD, Barg A, et al. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377-388.
14. Qin C, Dekker RG, Helfrich MM, et al. Outpatient management of ankle fractures. Orthop Clin North Am. 2018;49:103-108.
15. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127-1132.
16. Jenkin M, Sitler MR, Kelly JD. Clinical usefulness of the Ottawa Ankle Rules for detecting fractures of the ankle and midfoot. J Athl Train. 2010;45:480-482.
17. Glas AS, Pijnenburg BACM, Lijmer JG, et al. Comparison of diagnostic decision rules and structured data collection in assessment of acute ankle injury. CMAJ. 2002;166:727-733.
18. Leduc S, Nault M-L, Rouleau DM, et al. My experience as a foot and ankle trauma surgeon in Montreal, Canada: what’s not in the books. Foot Ankle Clin. 2016;21:297-334.
19. Ibrahim MS, Jordan R, Lotfi N, et al. Talar head fracture: a case report, systematic review and suggested algorithm of treatment. Foot (Edinb). 2015;25:258-264.
20. Shank JR, Benirschke SK, Swords MP. Treatment of peripheral talus fractures. Foot Ankle Clin. 2017;22:181-192.
21. Kwaadu KY. Management of talar fractures. Clin Podiatr Med Sur. 2018;35:161-173.
22. Yu JS. Easily missed fractures in the lower extremity. Radiol Clin North Am. 2015;53:737-755.
23. Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536-541.
24. Seybold JD, Coetzee JC. Lisfranc injuries: when to observe, fix, or fuse. Clin Sports Med. 2015;34:705-723.
25. Puna RA, Tomlinson MPW. The role of percutaneous reduction and fixation of lisfranc injuries. Foot Ankle Clin. 2017;22:15-34.
26. Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1-14.
27. Porter DA. Fifth metatarsal Jones fractures in the athlete. Foot Ankle Int. 2018;39:250-258.
28. Cheung CN, Lui TH. Proximal fifth metatarsal fractures: anatomy, classification, treatment and complications. Arch Trauma Res. 2016;5:e32298.
29. Alsobrook J, Hatch RL. Proximal fifth metatarsal fractures. UpToDate. January 31, 2020. www.uptodate.com/contents/proximal-fifth-metatarsal-fractures. Accessed May 26, 2020.
30. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
1. Bica D, Sprouse RA, Armen J. Diagnosis and management of common foot fractures. Am Fam Physician. 2016;93:183-191.
2. Gross CE, Nunley JA 2nd. Acute Achilles tendon ruptures. Foot Ankle Int. 2016;37:233-239.
3. Cooper MT. Acute Achilles tendon ruptures: does surgery offer superior results (and other confusing issues)? Clin Sports Med. 2015;34:595-606.
4. Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34:607-624.
5. Hutchison A-M, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19:112-117.
6. Kadakia AR, Dekker RG 2nd, Ho BS. Acute Achilles tendon ruptures: an update on treatment. Am Acad Orthop Surg. 2017;25:23-31.
7. van Zuuren WJ, Schepers T, Beumer A, et al. Acute syndesmotic instability in ankle fractures: a review. Foot Ankle Surg. 2017;23:135-141.
8. van Dijk CN, Longo UG, Loppini M, et al. Classification and diagnosis of acute isolated syndesmotic injuries: ESSKA–AFAS consensus and guidelines. Knee Surg Sports Traumatol Arthrosc. 2016;24:1200-1216.
9. Fort NM, Aiyer AA, Kaplan JR, et al. Management of acute injuries of the tibiofibular syndesmosis. Eur J Orthop Surg Traumatol. 2017;27:449-459.
10. Miller TL, Skalak T. Evaluation and treatment recommendations for acute injuries to the ankle syndesmosis without associated fracture. Sports Med. 2014;44:179-188.
11. Hunt KJ, Phisitkul P, Pirolo J, et al. High ankle sprains and syndesmotic injuries in athletes. J Am Acad Orthop Surg. 2015;23:661-673.
12. DeWeber K. Syndesmotic ankle injury (high ankle sprain). UpToDate. September 17, 2019. www.uptodate.com/contents/syndesmotic-ankle-injury-high-ankle-sprain. Accessed May 26, 2020.
13. Goost H, Wimmer MD, Barg A, et al. Fractures of the ankle joint: investigation and treatment options. Dtsch Arztebl Int. 2014;111:377-388.
14. Qin C, Dekker RG, Helfrich MM, et al. Outpatient management of ankle fractures. Orthop Clin North Am. 2018;49:103-108.
15. Stiell IG, Greenberg GH, McKnight RD, et al. Decision rules for the use of radiography in acute ankle injuries. Refinement and prospective validation. JAMA. 1993;269:1127-1132.
16. Jenkin M, Sitler MR, Kelly JD. Clinical usefulness of the Ottawa Ankle Rules for detecting fractures of the ankle and midfoot. J Athl Train. 2010;45:480-482.
17. Glas AS, Pijnenburg BACM, Lijmer JG, et al. Comparison of diagnostic decision rules and structured data collection in assessment of acute ankle injury. CMAJ. 2002;166:727-733.
18. Leduc S, Nault M-L, Rouleau DM, et al. My experience as a foot and ankle trauma surgeon in Montreal, Canada: what’s not in the books. Foot Ankle Clin. 2016;21:297-334.
19. Ibrahim MS, Jordan R, Lotfi N, et al. Talar head fracture: a case report, systematic review and suggested algorithm of treatment. Foot (Edinb). 2015;25:258-264.
20. Shank JR, Benirschke SK, Swords MP. Treatment of peripheral talus fractures. Foot Ankle Clin. 2017;22:181-192.
21. Kwaadu KY. Management of talar fractures. Clin Podiatr Med Sur. 2018;35:161-173.
22. Yu JS. Easily missed fractures in the lower extremity. Radiol Clin North Am. 2015;53:737-755.
23. Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536-541.
24. Seybold JD, Coetzee JC. Lisfranc injuries: when to observe, fix, or fuse. Clin Sports Med. 2015;34:705-723.
25. Puna RA, Tomlinson MPW. The role of percutaneous reduction and fixation of lisfranc injuries. Foot Ankle Clin. 2017;22:15-34.
26. Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1-14.
27. Porter DA. Fifth metatarsal Jones fractures in the athlete. Foot Ankle Int. 2018;39:250-258.
28. Cheung CN, Lui TH. Proximal fifth metatarsal fractures: anatomy, classification, treatment and complications. Arch Trauma Res. 2016;5:e32298.
29. Alsobrook J, Hatch RL. Proximal fifth metatarsal fractures. UpToDate. January 31, 2020. www.uptodate.com/contents/proximal-fifth-metatarsal-fractures. Accessed May 26, 2020.
30. Welck MJ, Hayes T, Pastides P, et al. Stress fractures of the foot and ankle. Injury. 2017;48:1722-1726.
PRACTICE RECOMMENDATIONS
› Suspect higher-grade syndesmotic disruption (which typically requires surgical intervention) in patients whose ankle pain persists after 3 weeks of immobilization or who have a tibial or fibular diastasis on a plain film. C
› Order weight-bearing x-rays to make an accurate diagnosis of Lisfranc injury. Refer for potential surgical intervention if diastasis is evident at the base between the first and second metatarsals. C
› Distinguish between proximal diaphysial (Jones) fracture of the fifth metatarsal, diaphysial stress fracture, and avulsion fracture—essential because avulsion fracture can be treated nonoperatively but the other 2 require surgical intervention. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Tramadol mortality risk in osteoarthritis could outweigh benefits
Patients with OA treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs, according to the results of a large, population-based study performed in British Columbia.
Within 1 year of starting treatment, 296 of 13,798 patients treated with tramadol had died, compared with 246 of 13,798 treated with naproxen, giving a death rate of 21.5 versus 17.8 per 1,000 person-years, and representing a 20% increase in all-cause mortality versus the NSAID (hazard ratio, 1.2).
Similar results were seen comparing tramadol with diclofenac and tramadol with cyclooxygenase (COX)-2 inhibitors, but with increasing death rates of 24.8 versus 19.5 per 1,000 person-years (HR, 1.3) and 23.6 versus 15.7 per 1,000 person-years (HR, 1.5), respectively.
However, all-cause mortality was lower with tramadol than with the opiate painkiller, codeine (21.5 vs. 25.5 per 1,000 person-years; HR, 0.8), reported Ms. Lingyi Li, a PhD student from the University of British Columbia, Vancouver, at the annual European Congress of Rheumatology, held online this year due to COVID-19.
This is not the first time that tramadol’s excess mortality risk has been highlighted. Indeed, just last year (JAMA. 2019;321[10]:969-82), researchers using The Health Improvement Network database reported found that tramadol was associated with higher all-cause mortality than two COX-2 inhibitors, celecoxib (31.2 versus 18.4 per 1,000 person-years) and etoricoxib (25.7 versus 12.8 per 1,000 person-years).
Ms. Li and associates’ data not only now add further weight to those findings, but also go a step further by also looking at other serious risks associated with tramadol’s use among patients with OA. “The objective of this study is to compare tramadol with other commonly prescribed pain relief medications on the risk of several severe outcomes, including mortality, cardiovascular diseases [CVD], venous thromboembolism [VTE], and hip fracture,” Ms. Li said during her virtual presentation.
Using sequential propensity score matching, the researchers compared data on patients in British Columbia during 2005-2014 with a first prescription of tramadol (56,325), the NSAIDs naproxen (n = 13,798) or diclofenac (n = 17,675), COX-2 inhibitors (17,039), or codeine (n = 7,813).
“For CVD, we found that there is a higher risk among tramadol users, compared with diclofenac [HR, 1.2] and COX-2 inhibitors [HR, 1.2], but not with naproxen [HR, 1.0] and codeine [HR, 0.9] users,” Ms. Li reported.
Similarly, the 1-year risk of VTE was significantly higher among tramadol users only when compared with diclofenac (HR, 1.5) and COX-2 inhibitors (HR, 1.7).
“For hip fractures, tramadol initiation was associated with an increased risk of hip fractures, compared with all NSAIDs, but not with codeine,” Ms. Li said. The risk of hip fractures was 40%-50% higher with tramadol versus naproxen (HR, 1.4), diclofenac and COX-2 inhibitors (both HR, 1.5).
“Our results suggest an unfavorable safety profile of tramadol use,” Ms. Li said, suggesting that “several guidelines on tramadol use in clinical practice might need to be revisited.”
According to a recent Cochrane review there is “moderate-quality evidence” that tramadol “has no important benefit on mean pain or function in people with osteoarthritis.” The authors of the review wrote that, while some patients might glean a benefit from treatment, the evidence suggests that “adverse events probably cause substantially more participants to stop taking tramadol.”
Current guidance on the use of tramadol varies. The American Academy of Orthopaedic Surgeons guidelines recommend its use in patients with symptomatic knee OA on a par with NSAIDs while the American College of Rheumatology guidance (Arthritis Care Res. 2020;72[2]:149-62) conditionally recommends that it be used only if there is no real alternative, such as a contraindication to NSAIDs or pain relief is ineffective.
Patients with rheumatic disease are increasingly taking opioid painkillers such as tramadol, with other data reported at the EULAR 2020 E-Congress showing a rise from 15% in 2007 to 25% in 2016 in the Catalonia region of Spain alone. A rise from 5% to 10% has previously been reported in the United States from 2003 to 2009.
With increasing rates of tramadol prescribing, the worry is that perhaps tramadol is not as safe a people think it is, as Thomas Schwenk, MD, pointed out when he reviewed the previous research showing excess mortality with tramadol (NEJM Journal Watch, March 2019).
“The opioid agonist tramadol often is prescribed for patients with osteoarthritis pain because it is thought to be safer than opioids or nonsteroidal anti-inflammatory drugs,” he observed. Dr. Schwenk, who is dean of the University of Nevada, Reno, added that the “results [of that study] suggest that tramadol is not as safe as some people believe.”
He suggested cautious prescribing: “Tramadol might be an option for patients in whom NSAIDs are contraindicated, but it should be prescribed as judiciously as traditional opioids.”
Responsible prescribing to avoid opioid misuse in patients with rheumatic diseases was also advocated in a EULAR press release from the congress. A study from Iceland was highlighted that found patients with inflammatory arthritis frequently did not stop taking opioids after the source of their pain had gone; in fact, their use went up despite being treated with tumor necrosis factor inhibitors.
“We would like to raise awareness of a responsible approach both by the prescribers and also the patients,” said John Isaacs, PhD, of the University of Newcastle (England). “In order to alleviate chronic pain, medications should in any case only be part of a comprehensive therapy program, in which doctors, psychologists, and physiotherapists work together.”
The study authors had no conflicts of interest.
SOURCE: Li L et al. Ann Rheum Dis. 2020;79[suppl 1]:118, Abstract OP0191.
Patients with OA treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs, according to the results of a large, population-based study performed in British Columbia.
Within 1 year of starting treatment, 296 of 13,798 patients treated with tramadol had died, compared with 246 of 13,798 treated with naproxen, giving a death rate of 21.5 versus 17.8 per 1,000 person-years, and representing a 20% increase in all-cause mortality versus the NSAID (hazard ratio, 1.2).
Similar results were seen comparing tramadol with diclofenac and tramadol with cyclooxygenase (COX)-2 inhibitors, but with increasing death rates of 24.8 versus 19.5 per 1,000 person-years (HR, 1.3) and 23.6 versus 15.7 per 1,000 person-years (HR, 1.5), respectively.
However, all-cause mortality was lower with tramadol than with the opiate painkiller, codeine (21.5 vs. 25.5 per 1,000 person-years; HR, 0.8), reported Ms. Lingyi Li, a PhD student from the University of British Columbia, Vancouver, at the annual European Congress of Rheumatology, held online this year due to COVID-19.
This is not the first time that tramadol’s excess mortality risk has been highlighted. Indeed, just last year (JAMA. 2019;321[10]:969-82), researchers using The Health Improvement Network database reported found that tramadol was associated with higher all-cause mortality than two COX-2 inhibitors, celecoxib (31.2 versus 18.4 per 1,000 person-years) and etoricoxib (25.7 versus 12.8 per 1,000 person-years).
Ms. Li and associates’ data not only now add further weight to those findings, but also go a step further by also looking at other serious risks associated with tramadol’s use among patients with OA. “The objective of this study is to compare tramadol with other commonly prescribed pain relief medications on the risk of several severe outcomes, including mortality, cardiovascular diseases [CVD], venous thromboembolism [VTE], and hip fracture,” Ms. Li said during her virtual presentation.
Using sequential propensity score matching, the researchers compared data on patients in British Columbia during 2005-2014 with a first prescription of tramadol (56,325), the NSAIDs naproxen (n = 13,798) or diclofenac (n = 17,675), COX-2 inhibitors (17,039), or codeine (n = 7,813).
“For CVD, we found that there is a higher risk among tramadol users, compared with diclofenac [HR, 1.2] and COX-2 inhibitors [HR, 1.2], but not with naproxen [HR, 1.0] and codeine [HR, 0.9] users,” Ms. Li reported.
Similarly, the 1-year risk of VTE was significantly higher among tramadol users only when compared with diclofenac (HR, 1.5) and COX-2 inhibitors (HR, 1.7).
“For hip fractures, tramadol initiation was associated with an increased risk of hip fractures, compared with all NSAIDs, but not with codeine,” Ms. Li said. The risk of hip fractures was 40%-50% higher with tramadol versus naproxen (HR, 1.4), diclofenac and COX-2 inhibitors (both HR, 1.5).
“Our results suggest an unfavorable safety profile of tramadol use,” Ms. Li said, suggesting that “several guidelines on tramadol use in clinical practice might need to be revisited.”
According to a recent Cochrane review there is “moderate-quality evidence” that tramadol “has no important benefit on mean pain or function in people with osteoarthritis.” The authors of the review wrote that, while some patients might glean a benefit from treatment, the evidence suggests that “adverse events probably cause substantially more participants to stop taking tramadol.”
Current guidance on the use of tramadol varies. The American Academy of Orthopaedic Surgeons guidelines recommend its use in patients with symptomatic knee OA on a par with NSAIDs while the American College of Rheumatology guidance (Arthritis Care Res. 2020;72[2]:149-62) conditionally recommends that it be used only if there is no real alternative, such as a contraindication to NSAIDs or pain relief is ineffective.
Patients with rheumatic disease are increasingly taking opioid painkillers such as tramadol, with other data reported at the EULAR 2020 E-Congress showing a rise from 15% in 2007 to 25% in 2016 in the Catalonia region of Spain alone. A rise from 5% to 10% has previously been reported in the United States from 2003 to 2009.
With increasing rates of tramadol prescribing, the worry is that perhaps tramadol is not as safe a people think it is, as Thomas Schwenk, MD, pointed out when he reviewed the previous research showing excess mortality with tramadol (NEJM Journal Watch, March 2019).
“The opioid agonist tramadol often is prescribed for patients with osteoarthritis pain because it is thought to be safer than opioids or nonsteroidal anti-inflammatory drugs,” he observed. Dr. Schwenk, who is dean of the University of Nevada, Reno, added that the “results [of that study] suggest that tramadol is not as safe as some people believe.”
He suggested cautious prescribing: “Tramadol might be an option for patients in whom NSAIDs are contraindicated, but it should be prescribed as judiciously as traditional opioids.”
Responsible prescribing to avoid opioid misuse in patients with rheumatic diseases was also advocated in a EULAR press release from the congress. A study from Iceland was highlighted that found patients with inflammatory arthritis frequently did not stop taking opioids after the source of their pain had gone; in fact, their use went up despite being treated with tumor necrosis factor inhibitors.
“We would like to raise awareness of a responsible approach both by the prescribers and also the patients,” said John Isaacs, PhD, of the University of Newcastle (England). “In order to alleviate chronic pain, medications should in any case only be part of a comprehensive therapy program, in which doctors, psychologists, and physiotherapists work together.”
The study authors had no conflicts of interest.
SOURCE: Li L et al. Ann Rheum Dis. 2020;79[suppl 1]:118, Abstract OP0191.
Patients with OA treated with tramadol had a 20%-50% higher risk of dying during the first year of treatment than did patients who were treated with NSAIDs, according to the results of a large, population-based study performed in British Columbia.
Within 1 year of starting treatment, 296 of 13,798 patients treated with tramadol had died, compared with 246 of 13,798 treated with naproxen, giving a death rate of 21.5 versus 17.8 per 1,000 person-years, and representing a 20% increase in all-cause mortality versus the NSAID (hazard ratio, 1.2).
Similar results were seen comparing tramadol with diclofenac and tramadol with cyclooxygenase (COX)-2 inhibitors, but with increasing death rates of 24.8 versus 19.5 per 1,000 person-years (HR, 1.3) and 23.6 versus 15.7 per 1,000 person-years (HR, 1.5), respectively.
However, all-cause mortality was lower with tramadol than with the opiate painkiller, codeine (21.5 vs. 25.5 per 1,000 person-years; HR, 0.8), reported Ms. Lingyi Li, a PhD student from the University of British Columbia, Vancouver, at the annual European Congress of Rheumatology, held online this year due to COVID-19.
This is not the first time that tramadol’s excess mortality risk has been highlighted. Indeed, just last year (JAMA. 2019;321[10]:969-82), researchers using The Health Improvement Network database reported found that tramadol was associated with higher all-cause mortality than two COX-2 inhibitors, celecoxib (31.2 versus 18.4 per 1,000 person-years) and etoricoxib (25.7 versus 12.8 per 1,000 person-years).
Ms. Li and associates’ data not only now add further weight to those findings, but also go a step further by also looking at other serious risks associated with tramadol’s use among patients with OA. “The objective of this study is to compare tramadol with other commonly prescribed pain relief medications on the risk of several severe outcomes, including mortality, cardiovascular diseases [CVD], venous thromboembolism [VTE], and hip fracture,” Ms. Li said during her virtual presentation.
Using sequential propensity score matching, the researchers compared data on patients in British Columbia during 2005-2014 with a first prescription of tramadol (56,325), the NSAIDs naproxen (n = 13,798) or diclofenac (n = 17,675), COX-2 inhibitors (17,039), or codeine (n = 7,813).
“For CVD, we found that there is a higher risk among tramadol users, compared with diclofenac [HR, 1.2] and COX-2 inhibitors [HR, 1.2], but not with naproxen [HR, 1.0] and codeine [HR, 0.9] users,” Ms. Li reported.
Similarly, the 1-year risk of VTE was significantly higher among tramadol users only when compared with diclofenac (HR, 1.5) and COX-2 inhibitors (HR, 1.7).
“For hip fractures, tramadol initiation was associated with an increased risk of hip fractures, compared with all NSAIDs, but not with codeine,” Ms. Li said. The risk of hip fractures was 40%-50% higher with tramadol versus naproxen (HR, 1.4), diclofenac and COX-2 inhibitors (both HR, 1.5).
“Our results suggest an unfavorable safety profile of tramadol use,” Ms. Li said, suggesting that “several guidelines on tramadol use in clinical practice might need to be revisited.”
According to a recent Cochrane review there is “moderate-quality evidence” that tramadol “has no important benefit on mean pain or function in people with osteoarthritis.” The authors of the review wrote that, while some patients might glean a benefit from treatment, the evidence suggests that “adverse events probably cause substantially more participants to stop taking tramadol.”
Current guidance on the use of tramadol varies. The American Academy of Orthopaedic Surgeons guidelines recommend its use in patients with symptomatic knee OA on a par with NSAIDs while the American College of Rheumatology guidance (Arthritis Care Res. 2020;72[2]:149-62) conditionally recommends that it be used only if there is no real alternative, such as a contraindication to NSAIDs or pain relief is ineffective.
Patients with rheumatic disease are increasingly taking opioid painkillers such as tramadol, with other data reported at the EULAR 2020 E-Congress showing a rise from 15% in 2007 to 25% in 2016 in the Catalonia region of Spain alone. A rise from 5% to 10% has previously been reported in the United States from 2003 to 2009.
With increasing rates of tramadol prescribing, the worry is that perhaps tramadol is not as safe a people think it is, as Thomas Schwenk, MD, pointed out when he reviewed the previous research showing excess mortality with tramadol (NEJM Journal Watch, March 2019).
“The opioid agonist tramadol often is prescribed for patients with osteoarthritis pain because it is thought to be safer than opioids or nonsteroidal anti-inflammatory drugs,” he observed. Dr. Schwenk, who is dean of the University of Nevada, Reno, added that the “results [of that study] suggest that tramadol is not as safe as some people believe.”
He suggested cautious prescribing: “Tramadol might be an option for patients in whom NSAIDs are contraindicated, but it should be prescribed as judiciously as traditional opioids.”
Responsible prescribing to avoid opioid misuse in patients with rheumatic diseases was also advocated in a EULAR press release from the congress. A study from Iceland was highlighted that found patients with inflammatory arthritis frequently did not stop taking opioids after the source of their pain had gone; in fact, their use went up despite being treated with tumor necrosis factor inhibitors.
“We would like to raise awareness of a responsible approach both by the prescribers and also the patients,” said John Isaacs, PhD, of the University of Newcastle (England). “In order to alleviate chronic pain, medications should in any case only be part of a comprehensive therapy program, in which doctors, psychologists, and physiotherapists work together.”
The study authors had no conflicts of interest.
SOURCE: Li L et al. Ann Rheum Dis. 2020;79[suppl 1]:118, Abstract OP0191.
FROM EULAR 2020 E-CONGRESS